User login
Aspirin and warfarin together leads to increased bleeding without reducing thrombotic events
Background: Current guidelines recommend against using aspirin in combination with warfarin for patients with AFib, unless the patient has another indication for aspirin such as recent percutaneous coronary intervention (PCI) or a mechanical heart valve. These recommendations are based on limited clinical trial data that showed an increased risk of adverse events with combination therapy without clinical benefit. Despite these recommendations, recent studies have shown that aspirin use without a clinical indication remains common in patients taking warfarin for AFib. The prevalence of aspirin use without a clinical indication in patients taking warfarin for VTE is less well studied.
Study design: Registry-based cohort study.
Setting: Six anticoagulation clinics in Michigan.
Synopsis: Of the 6,539 patients included in the study, 2,453 patients (37.5%) were taking both warfarin and aspirin without an indication for aspirin therapy; 3,688 propensity score–matched patients (1,844 in each group) were compared to assess rates of bleeding and rates of observed thrombosis at 1 year in patients taking warfarin alone versus warfarin plus aspirin. Patients treated with warfarin plus aspirin experienced more bleeding events than did patients on warfarin monotherapy (95% confidence interval, 23.8%-28.3% vs. 95% CI, 18.3%-22.3%; P less than .001). Rates of observed thrombosis were similar between the two groups (95% CI, 1.6%-3.1% vs. 95% CI, 2.0%-3.6%; P = .40). This study demonstrates that aspirin use without a clinical indication remains common in patients taking warfarin for AFib or VTE, and that reducing inappropriate aspirin use in this patient population may help prevent adverse outcomes.
Bottom line: Use of aspirin without a clinical indication in patients taking warfarin is common and is associated with an increased risk of bleeding without significant clinical benefit.
Citation: Schaefer JK et al. Association of adding aspirin to warfarin therapy without an apparent indication with bleeding and other adverse events. JAMA Intern Med. 2019 Mar 4;179(4):533-41.
Dr. Wachter is an associate medical director at Duke Regional Hospital and an assistant professor of medicine at Duke University.
Background: Current guidelines recommend against using aspirin in combination with warfarin for patients with AFib, unless the patient has another indication for aspirin such as recent percutaneous coronary intervention (PCI) or a mechanical heart valve. These recommendations are based on limited clinical trial data that showed an increased risk of adverse events with combination therapy without clinical benefit. Despite these recommendations, recent studies have shown that aspirin use without a clinical indication remains common in patients taking warfarin for AFib. The prevalence of aspirin use without a clinical indication in patients taking warfarin for VTE is less well studied.
Study design: Registry-based cohort study.
Setting: Six anticoagulation clinics in Michigan.
Synopsis: Of the 6,539 patients included in the study, 2,453 patients (37.5%) were taking both warfarin and aspirin without an indication for aspirin therapy; 3,688 propensity score–matched patients (1,844 in each group) were compared to assess rates of bleeding and rates of observed thrombosis at 1 year in patients taking warfarin alone versus warfarin plus aspirin. Patients treated with warfarin plus aspirin experienced more bleeding events than did patients on warfarin monotherapy (95% confidence interval, 23.8%-28.3% vs. 95% CI, 18.3%-22.3%; P less than .001). Rates of observed thrombosis were similar between the two groups (95% CI, 1.6%-3.1% vs. 95% CI, 2.0%-3.6%; P = .40). This study demonstrates that aspirin use without a clinical indication remains common in patients taking warfarin for AFib or VTE, and that reducing inappropriate aspirin use in this patient population may help prevent adverse outcomes.
Bottom line: Use of aspirin without a clinical indication in patients taking warfarin is common and is associated with an increased risk of bleeding without significant clinical benefit.
Citation: Schaefer JK et al. Association of adding aspirin to warfarin therapy without an apparent indication with bleeding and other adverse events. JAMA Intern Med. 2019 Mar 4;179(4):533-41.
Dr. Wachter is an associate medical director at Duke Regional Hospital and an assistant professor of medicine at Duke University.
Background: Current guidelines recommend against using aspirin in combination with warfarin for patients with AFib, unless the patient has another indication for aspirin such as recent percutaneous coronary intervention (PCI) or a mechanical heart valve. These recommendations are based on limited clinical trial data that showed an increased risk of adverse events with combination therapy without clinical benefit. Despite these recommendations, recent studies have shown that aspirin use without a clinical indication remains common in patients taking warfarin for AFib. The prevalence of aspirin use without a clinical indication in patients taking warfarin for VTE is less well studied.
Study design: Registry-based cohort study.
Setting: Six anticoagulation clinics in Michigan.
Synopsis: Of the 6,539 patients included in the study, 2,453 patients (37.5%) were taking both warfarin and aspirin without an indication for aspirin therapy; 3,688 propensity score–matched patients (1,844 in each group) were compared to assess rates of bleeding and rates of observed thrombosis at 1 year in patients taking warfarin alone versus warfarin plus aspirin. Patients treated with warfarin plus aspirin experienced more bleeding events than did patients on warfarin monotherapy (95% confidence interval, 23.8%-28.3% vs. 95% CI, 18.3%-22.3%; P less than .001). Rates of observed thrombosis were similar between the two groups (95% CI, 1.6%-3.1% vs. 95% CI, 2.0%-3.6%; P = .40). This study demonstrates that aspirin use without a clinical indication remains common in patients taking warfarin for AFib or VTE, and that reducing inappropriate aspirin use in this patient population may help prevent adverse outcomes.
Bottom line: Use of aspirin without a clinical indication in patients taking warfarin is common and is associated with an increased risk of bleeding without significant clinical benefit.
Citation: Schaefer JK et al. Association of adding aspirin to warfarin therapy without an apparent indication with bleeding and other adverse events. JAMA Intern Med. 2019 Mar 4;179(4):533-41.
Dr. Wachter is an associate medical director at Duke Regional Hospital and an assistant professor of medicine at Duke University.
SECTION 4: HEALTHCARE SYSTEMS: SUPPORTING AND ADVANCING CHILD HEALTH
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 4: Healthcare Systems: Supporting and Advancing Child Health. J Hosp Med. 2020;15(S1):xxx-xxx (insert page numbers). https://doi.org/jhm.3400
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 4: Healthcare Systems: Supporting and Advancing Child Health. J Hosp Med. 2020;15(S1):xxx-xxx (insert page numbers). https://doi.org/jhm.3400
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 4: Healthcare Systems: Supporting and Advancing Child Health. J Hosp Med. 2020;15(S1):xxx-xxx (insert page numbers). https://doi.org/jhm.3400
SECTION 3: SPECIALIZED SERVICES
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 3: Specialized Services. J Hosp Med. 2020;15(S1):xx-xxx (insert page numbers). https://doi.org/10.12788/jhm.3399
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 3: Specialized Services. J Hosp Med. 2020;15(S1):xx-xxx (insert page numbers). https://doi.org/10.12788/jhm.3399
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 3: Specialized Services. J Hosp Med. 2020;15(S1):xx-xxx (insert page numbers). https://doi.org/10.12788/jhm.3399
SECTION 2: CORE SKILLS
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 2: Core Skills. J Hosp Med. 2020;15(S1):XX-XX (insert page numbers). https://doi.org/10.12788/jhm.3398
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 2: Core Skills. J Hosp Med. 2020;15(S1):XX-XX (insert page numbers). https://doi.org/10.12788/jhm.3398
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 2: Core Skills. J Hosp Med. 2020;15(S1):XX-XX (insert page numbers). https://doi.org/10.12788/jhm.3398
SECTION 1: COMMON CLINICAL DIAGNOSES AND CONDITIONS
How to cite articles within Section 1
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 1: Common Clinical Diagnoses and Conditions. J Hosp Med. 2020;15(S1):xx-xx (insert page numbers). https://doi.org/10.12788/jhm.3397
How to cite articles within Section 1
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 1: Common Clinical Diagnoses and Conditions. J Hosp Med. 2020;15(S1):xx-xx (insert page numbers). https://doi.org/10.12788/jhm.3397
How to cite articles within Section 1
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 1: Common Clinical Diagnoses and Conditions. J Hosp Med. 2020;15(S1):xx-xx (insert page numbers). https://doi.org/10.12788/jhm.3397
Lessons From the COVID-19 Pandemic: It’s Time to Invest in Public Health
What have you been doing since you left the US Public Health Service?
RADM Boris D. Lushniak, MD, MPH. I retired in 2015 and spent a year at the Uniformed Services University for the Health Sciences in Bethesda, Maryland as the Chair of Preventive Medicine and Biostatistics before I took the opportunity to become the Dean of the School of Public Health at the University of Maryland in College Park. I was very intrigued with that position. It’s a large and young school of public health—just 13 years since its inception. And it functions at both the undergraduate and graduate school levels. We have 2,400 undergraduates in 4 different degree paths. The intriguing part of this is the ability to influence a young person’s educational pathway, and for them to look at all the opportunities in public health, and to focus on a mission, which falls into the mission of the US Public Health Service (PHS) Commissioned Corps: Protect, promote and advance the health and safety of our nation.
It has been a very intriguing transition; I have been the Dean there for 3 years. Who would have predicted that things would change drastically in that time, both at the academic level (ie, moving a school from being a normal college environment to an online environment) and now moving into the realm of preparing for the near future of that university in terms of a potential reopening. It is using all of my public health experiences and putting it at that culmination point, which is my community of 52,000 people—40,000 students at the University in College Park, and 12,000 faculty and staff members.
We are responsible for making sure that the return is as safe as possible. With so many unknowns in the world of COVID-19 and so many unpredictable components, it is quite an undertaking to be able to determine for that community of 52,000 whether it’s time to return, and under what circumstances do we return.
In addition, we’re part of a larger community. The University of Maryland in College Park is in Prince George’s County, which is the epicenter of disease and death in Maryland. The School of Public Health is working closely with county authorities. Some of our students are now contact tracers. It’s been interesting to see our faculty, staff, and students standing up as a volunteer support structure for Public Health.
We have incredible research going on at the school. One of my prime research physicians, Don Milton, MD, DrPH, has been studying the transmission of influenza. Now his work is priming on not just influenza, but also COVID-19. Our hope is to establish a community that will be safe and healthy for everyone, and so it’s been an incredible amount of work.
How would you describe the federal/ local public health cooperation?
RADM Lushniak. First and foremost, we have seen a major issue in terms of state and local response to the COVID-19 pandemic. I have to congratulate the state and the local officials for doing as best as they can under the strained circumstances that they’re in.
The first strained circumstance is that local and state health departments have lost nearly a quarter of their workforce: 50,000 jobs across the country since the recession of 2008. Part of the answer why it’s been such a struggle is that our nation as a whole hasn’t looked at public health and hasn’t looked at prevention as a key component of how our country works. We have seen a lack of support at the state and the local level, the shedding of jobs, and the lack of foresight in terms of saying that prevention works and public health is important for our cities, states, regions, and the nation. We need to reemphasize that in terms of public health.
In the State of Maryland, in general, the counties are doing as best as they can under the circumstances. They certainly started out with trying to do as much testing as possible. Testing is a critical component to this response, and obviously, we have a situation nationwide with the testing still trying to be put online to the extent that it needs to be. We need to be able to test more and more individuals to be able to determine the people who are positive. The curve ball that COVID-19 threw us is that 25 to 50% of individuals who may have a positive test may be asymptomatic. So, this isn’t simple. It’s not a matter of just saying, “Okay, you’re sick. You may then have it.” It may be: “Hey, you’re feeling healthy, you still may have it.”
But just as important as testing is what you do with those individuals who are tested. You need to have health departments turning to these individuals and providing them directions of what needs to be done. If one is COVID-19-positive, one goes into isolation for at least 14 days. And if ill, they need to be connected with a medical care system. That’s an important part of the state and local response is making sure the individuals are properly directed to the right pathway.
In addition, contact tracing is critical. The way we’re going to fight COVID-19 is the ability for us to go out there and determine if you are a positive, who did you come in contact with, and did you potentially spread this to others? You need to direct individuals who may have been in contact with the person who is now COVID- 19-positive, saying “You may have to quarantine yourself, watch out for symptoms, and you have to be really careful in the meantime.”
State and local officials took up the burden of making decisions in terms of communicating the directions given to the population. Is stay at home required? Is it the closure of businesses? Is it the wearing of masks? Certainly, the issue of physical distancing plays a role.
All that was implemented at the state and local level. Under the circumstances, it has been done as well as possible, but that now reflects on the issue of the federal response. And the federal response, I’ll admit, has been less than I had hoped for on several realms.
Number one, coordination and direction from the federal level has been rather piecemeal. State and local officials, I think, were waiting for further directions. What did federal officials think; what did they want us to do? State and local officials want independence to implement things, but what’s the right answer? I think this has been not handled well at the highest levels of the US government.
Secondly, obviously, there was an issue with testing, and the responsibility here lays with the Centers of Disease Control and Prevention (CDC), which had problems from the get-go with setting up their testing caches and getting them out. We’re still catching up from there. Now it’s unfolding that the tie in between the federal government and the private sector and academic centers are at least making some headway on that testing front.
Third, people rely on the federal officials not only for action but also for communication. It really boils down to: Who’s in charge, who’s telling me the information that I need to know, who’s honest with me and telling me what they don’t know, and who has the insight to say, “Here’s how we’re going to find out the things that we don’t know?” Who’s there empathizing with the population?
The reality is there’s been a mismatch between the communication channels for the federal government and getting down not just to the state and locals but, also, to the general population in this country.
How would you characterize the US Public Health Service Response?
RADM Lushniak. I’ll first start off with kudos and congratulations to the Commissioned Corps of the PHS for their response to date. I think the latest numbers that ADM Brett Giroir, MD, Assistant Secretary of Health, told Congress in May, was that at the time more than 3,100 of the 6,100 current officers at the PHS have been deployed over the last several months. The reality is that the Commissioned Corps is out there doing service to our nation and to the world. PHS teams were deployed initially to Japan and the Diamond Princess cruise ship. The Corps been out there internationally.
Nationally, the Corps was at the Javits Center in New York assisting in setting up that medical response. They have been assisting at the military bases initially where some of the individuals who were coming in from China and other places were being held in quarantine. They have been assisting with investigations at nursing homes across the country and meat packing plants where there have been outbreaks occurring. The Commissioned Corps has been out there, so that’s the good news.
The bad news is that the Corps is a small uniformed service. The reality is nobody still is seeing the Corps or knows about the Corps as they’re out there doing their thing. It was very nice that ADM Giroir put a plug in for them in his recent congressional testimony. That’s great that our leadership is out there acknowledging the Corps. But to a large extent, I still have an issue with the Commissioned Corps being an underfunded uniformed service of this country. The Commissioned Corps is the only uniformed service in the world whose only mission is public health. But, lack of support reflects the idea of the lack of importance that public health plays in the minds of policy makers.
To a large extent, we have had no dollars in the Corps recently for training of officers to prepare for this. For 10 years we’ve waited for a Ready Reserve to be set up. The Ready Reserve component was part of the Affordable Care Act. I was in the office of the Surgeon General as we were told to ramp this up. Now 10 years later, in the midst of this COVID-19 pandemic, Congress finally has passed legislation that sets a pathway for a Ready Reserve.
Why is the Ready Reserve important? In essence, we have incredible public health professionals out there in the civilian ranks who would be willing to assist the Commissioned Corps in their mission, either to backfill critical positions where Corps officers are currently stationed and need to be deployed, or as a Ready Reserve that’s ready to deploy itself. All this is happening right now. I hope for better days, and I hope this COVID-19 pandemic will wake our nation up to the need of a Public Health Service Commissioned Corps, a uniformed service, that's out there doing good.
What lessons are we learning about public health in this pandemic?
RADM Lushniak. We’ve just developed a new space force, the 8th uniformed US service. In reality they are talking about tens of thousands of people assigned to it. Excuse me if I’m going to be assertive. I’m a big fan of space exploration. I realize that space is the final frontier and that perhaps we have to be able to defend our country in that regard. But we’re already saying that space is worth investing in. Where is the wisdom that we’re not investing in battling on this planet against emerging threats like COVID-19? And why is it that to this date the Commissioned Corps of the Public Health Service does not have its own budget; does not have a line item anywhere; does not have money directed for training; and, in essence, only serves because its officers are stationed at other agencies who pay for these officers? It’s a personnel system and not really treated as a key and critical uniformed service of this country. That’s point number one in terms of lessons learned and what needs to be done.
In addition, it’s not just the people in uniform who serve at the federal level, civilians serve as well. These civilians work at the CDC, at the US Food and Drug Administration, at the National Institute of Health, at the Indian Health Service, and at many, many other agencies throughout the US government. Within those realms, we need to show support of those federal practitioners who are working very diligently and in a devoted fashion to fight this pandemic as well. Part of it is the moral support to recognize that there are multiple fronts to fighting this pandemic and the federal practitioner who is working out there, is a key component to this.
I don’t want everything to be money, money, money, but the fact is that CDC’s budget has been decreasing over the years. How are we supposed to set up the laboratories, how are we supposed to demand the high level of expertise when, in fact, everything has to be done on a shoestring?
Finally, we notice public health in the midst of a crisis, but public health matters each and every day. The idea that the pandemic certainly brings to light what needs to get done, but without a pandemic, what do we have? We still have cigarette smoking, the number 1 killer in this country. That’s a public health issue. We have cardiovascular diseases as an extreme killer in this country. That’s a public health issue. We have diabetes mellitus that is rampant. We have substance abuse, including the opioid epidemic. Those are public health issues. We have hypertension, we have overweight and obesity. Those are all public health issues that public health battles each and every day without the recognition.
What we need is a major shift in the philosophy of this country to really take the health and wellness of our society as a key component of how you’ll raise that on to a pedestal—the idea that health and wellness is critical to the functioning of this country.
How have recent public health emergencies influenced the Commissioned Corps?
RADM Lushniak. The key feature is that the Public Health Service Commissioned Corps has been growing in its mission over the years. The pre-9/11 Commissioned Corps, was a different life. The post-9/11 world is the first time that the Commissioned Corps really fell into this idea of being America’s public health responders. I think that we ramped it up; we started out strong.
This was shown not only in the World Trade Center and the 9/11 disasters that occurred, but in the anthrax scenario that unfolded shortly afterwards. We saw it further continue in Hurricane Katrina and the multiple hurricane responses.
Then the Ebola response, in my last year of serving in uniform, was another action of both the civilian sector of federal responders as well as the uniformed sector. The beauty of that in terms of what we learned from Ebola was that coordination is key. That was the first time that the PHS worked so closely with the US Department of Defense and our sister services to basically have an international mission unfold with that level of coordination.
We can use those changes that have gone on, the metamorphoses that have happened over the years, as a jumping off point, but they need to be fulfilled with further growth and support of the Commissioned Corps of the US Public Health Service. The numbers are the lowest they’ve been in recent times in terms of active duty officers. That’s not a good thing. As the mission expands, the idea of recruiting and retaining remains a problem. We have to deal with it.
Was your interest in taking the position at the University of Maryland in part to help build the future of public health?
RADM Lushniak. Certainly, I was so excited to be at the University of Maryland College Park exactly for that reason. The undergraduates are coming in from high school and their eyes are wide open. Two things are important at that stage. One is to teach them about the beauty of public health. That it’s a bold and noble mission. As I always tell our students, it’s about the 3 Ps: Promoting health and wellbeing, preventing disease and injury, and prolonging a high quality of life.
When you put all those things together, that’s an incredible mission. I want to tell them at that young age, “Be a part of this, figure out where you fit in.” But it’s not for everyone. I tell my students that one of the major attributes that I need to see in a student is optimism. Public health does not deal well with pessimism. If your character is pessimistic, I actually dissuade you from becoming a public health person because there are a lot of barriers in this incredible bold and noble mission, and optimism needs to be a key feature that keeps us all going.
Next is the realization that there’s so many different public health issues in our world, so many different problems to deal with. I mentioned some of them previously in terms of the public health issues we see each and every day.
Let me talk about one that’s, in particular, shining through in the midst of COVID-19, but also shines through each and every day. That’s the issue of health equity in our communities. A young person, who usually comes in and wants to help their community, needs to realize that part of the battle of public health is to make sure that we deal with the disparities that exist. We must make health equity a key component of our jobs. We are here to serve others.
There’s a saying at the University of Maryland College Park that we’re a “Do good university.” I would say that public health is a do-good profession. It is about compassion, it’s about love, it’s about caring. Those are the types of people that I try to bring into the school, and I try to mentor and support.
What have you been doing since you left the US Public Health Service?
RADM Boris D. Lushniak, MD, MPH. I retired in 2015 and spent a year at the Uniformed Services University for the Health Sciences in Bethesda, Maryland as the Chair of Preventive Medicine and Biostatistics before I took the opportunity to become the Dean of the School of Public Health at the University of Maryland in College Park. I was very intrigued with that position. It’s a large and young school of public health—just 13 years since its inception. And it functions at both the undergraduate and graduate school levels. We have 2,400 undergraduates in 4 different degree paths. The intriguing part of this is the ability to influence a young person’s educational pathway, and for them to look at all the opportunities in public health, and to focus on a mission, which falls into the mission of the US Public Health Service (PHS) Commissioned Corps: Protect, promote and advance the health and safety of our nation.
It has been a very intriguing transition; I have been the Dean there for 3 years. Who would have predicted that things would change drastically in that time, both at the academic level (ie, moving a school from being a normal college environment to an online environment) and now moving into the realm of preparing for the near future of that university in terms of a potential reopening. It is using all of my public health experiences and putting it at that culmination point, which is my community of 52,000 people—40,000 students at the University in College Park, and 12,000 faculty and staff members.
We are responsible for making sure that the return is as safe as possible. With so many unknowns in the world of COVID-19 and so many unpredictable components, it is quite an undertaking to be able to determine for that community of 52,000 whether it’s time to return, and under what circumstances do we return.
In addition, we’re part of a larger community. The University of Maryland in College Park is in Prince George’s County, which is the epicenter of disease and death in Maryland. The School of Public Health is working closely with county authorities. Some of our students are now contact tracers. It’s been interesting to see our faculty, staff, and students standing up as a volunteer support structure for Public Health.
We have incredible research going on at the school. One of my prime research physicians, Don Milton, MD, DrPH, has been studying the transmission of influenza. Now his work is priming on not just influenza, but also COVID-19. Our hope is to establish a community that will be safe and healthy for everyone, and so it’s been an incredible amount of work.
How would you describe the federal/ local public health cooperation?
RADM Lushniak. First and foremost, we have seen a major issue in terms of state and local response to the COVID-19 pandemic. I have to congratulate the state and the local officials for doing as best as they can under the strained circumstances that they’re in.
The first strained circumstance is that local and state health departments have lost nearly a quarter of their workforce: 50,000 jobs across the country since the recession of 2008. Part of the answer why it’s been such a struggle is that our nation as a whole hasn’t looked at public health and hasn’t looked at prevention as a key component of how our country works. We have seen a lack of support at the state and the local level, the shedding of jobs, and the lack of foresight in terms of saying that prevention works and public health is important for our cities, states, regions, and the nation. We need to reemphasize that in terms of public health.
In the State of Maryland, in general, the counties are doing as best as they can under the circumstances. They certainly started out with trying to do as much testing as possible. Testing is a critical component to this response, and obviously, we have a situation nationwide with the testing still trying to be put online to the extent that it needs to be. We need to be able to test more and more individuals to be able to determine the people who are positive. The curve ball that COVID-19 threw us is that 25 to 50% of individuals who may have a positive test may be asymptomatic. So, this isn’t simple. It’s not a matter of just saying, “Okay, you’re sick. You may then have it.” It may be: “Hey, you’re feeling healthy, you still may have it.”
But just as important as testing is what you do with those individuals who are tested. You need to have health departments turning to these individuals and providing them directions of what needs to be done. If one is COVID-19-positive, one goes into isolation for at least 14 days. And if ill, they need to be connected with a medical care system. That’s an important part of the state and local response is making sure the individuals are properly directed to the right pathway.
In addition, contact tracing is critical. The way we’re going to fight COVID-19 is the ability for us to go out there and determine if you are a positive, who did you come in contact with, and did you potentially spread this to others? You need to direct individuals who may have been in contact with the person who is now COVID- 19-positive, saying “You may have to quarantine yourself, watch out for symptoms, and you have to be really careful in the meantime.”
State and local officials took up the burden of making decisions in terms of communicating the directions given to the population. Is stay at home required? Is it the closure of businesses? Is it the wearing of masks? Certainly, the issue of physical distancing plays a role.
All that was implemented at the state and local level. Under the circumstances, it has been done as well as possible, but that now reflects on the issue of the federal response. And the federal response, I’ll admit, has been less than I had hoped for on several realms.
Number one, coordination and direction from the federal level has been rather piecemeal. State and local officials, I think, were waiting for further directions. What did federal officials think; what did they want us to do? State and local officials want independence to implement things, but what’s the right answer? I think this has been not handled well at the highest levels of the US government.
Secondly, obviously, there was an issue with testing, and the responsibility here lays with the Centers of Disease Control and Prevention (CDC), which had problems from the get-go with setting up their testing caches and getting them out. We’re still catching up from there. Now it’s unfolding that the tie in between the federal government and the private sector and academic centers are at least making some headway on that testing front.
Third, people rely on the federal officials not only for action but also for communication. It really boils down to: Who’s in charge, who’s telling me the information that I need to know, who’s honest with me and telling me what they don’t know, and who has the insight to say, “Here’s how we’re going to find out the things that we don’t know?” Who’s there empathizing with the population?
The reality is there’s been a mismatch between the communication channels for the federal government and getting down not just to the state and locals but, also, to the general population in this country.
How would you characterize the US Public Health Service Response?
RADM Lushniak. I’ll first start off with kudos and congratulations to the Commissioned Corps of the PHS for their response to date. I think the latest numbers that ADM Brett Giroir, MD, Assistant Secretary of Health, told Congress in May, was that at the time more than 3,100 of the 6,100 current officers at the PHS have been deployed over the last several months. The reality is that the Commissioned Corps is out there doing service to our nation and to the world. PHS teams were deployed initially to Japan and the Diamond Princess cruise ship. The Corps been out there internationally.
Nationally, the Corps was at the Javits Center in New York assisting in setting up that medical response. They have been assisting at the military bases initially where some of the individuals who were coming in from China and other places were being held in quarantine. They have been assisting with investigations at nursing homes across the country and meat packing plants where there have been outbreaks occurring. The Commissioned Corps has been out there, so that’s the good news.
The bad news is that the Corps is a small uniformed service. The reality is nobody still is seeing the Corps or knows about the Corps as they’re out there doing their thing. It was very nice that ADM Giroir put a plug in for them in his recent congressional testimony. That’s great that our leadership is out there acknowledging the Corps. But to a large extent, I still have an issue with the Commissioned Corps being an underfunded uniformed service of this country. The Commissioned Corps is the only uniformed service in the world whose only mission is public health. But, lack of support reflects the idea of the lack of importance that public health plays in the minds of policy makers.
To a large extent, we have had no dollars in the Corps recently for training of officers to prepare for this. For 10 years we’ve waited for a Ready Reserve to be set up. The Ready Reserve component was part of the Affordable Care Act. I was in the office of the Surgeon General as we were told to ramp this up. Now 10 years later, in the midst of this COVID-19 pandemic, Congress finally has passed legislation that sets a pathway for a Ready Reserve.
Why is the Ready Reserve important? In essence, we have incredible public health professionals out there in the civilian ranks who would be willing to assist the Commissioned Corps in their mission, either to backfill critical positions where Corps officers are currently stationed and need to be deployed, or as a Ready Reserve that’s ready to deploy itself. All this is happening right now. I hope for better days, and I hope this COVID-19 pandemic will wake our nation up to the need of a Public Health Service Commissioned Corps, a uniformed service, that's out there doing good.
What lessons are we learning about public health in this pandemic?
RADM Lushniak. We’ve just developed a new space force, the 8th uniformed US service. In reality they are talking about tens of thousands of people assigned to it. Excuse me if I’m going to be assertive. I’m a big fan of space exploration. I realize that space is the final frontier and that perhaps we have to be able to defend our country in that regard. But we’re already saying that space is worth investing in. Where is the wisdom that we’re not investing in battling on this planet against emerging threats like COVID-19? And why is it that to this date the Commissioned Corps of the Public Health Service does not have its own budget; does not have a line item anywhere; does not have money directed for training; and, in essence, only serves because its officers are stationed at other agencies who pay for these officers? It’s a personnel system and not really treated as a key and critical uniformed service of this country. That’s point number one in terms of lessons learned and what needs to be done.
In addition, it’s not just the people in uniform who serve at the federal level, civilians serve as well. These civilians work at the CDC, at the US Food and Drug Administration, at the National Institute of Health, at the Indian Health Service, and at many, many other agencies throughout the US government. Within those realms, we need to show support of those federal practitioners who are working very diligently and in a devoted fashion to fight this pandemic as well. Part of it is the moral support to recognize that there are multiple fronts to fighting this pandemic and the federal practitioner who is working out there, is a key component to this.
I don’t want everything to be money, money, money, but the fact is that CDC’s budget has been decreasing over the years. How are we supposed to set up the laboratories, how are we supposed to demand the high level of expertise when, in fact, everything has to be done on a shoestring?
Finally, we notice public health in the midst of a crisis, but public health matters each and every day. The idea that the pandemic certainly brings to light what needs to get done, but without a pandemic, what do we have? We still have cigarette smoking, the number 1 killer in this country. That’s a public health issue. We have cardiovascular diseases as an extreme killer in this country. That’s a public health issue. We have diabetes mellitus that is rampant. We have substance abuse, including the opioid epidemic. Those are public health issues. We have hypertension, we have overweight and obesity. Those are all public health issues that public health battles each and every day without the recognition.
What we need is a major shift in the philosophy of this country to really take the health and wellness of our society as a key component of how you’ll raise that on to a pedestal—the idea that health and wellness is critical to the functioning of this country.
How have recent public health emergencies influenced the Commissioned Corps?
RADM Lushniak. The key feature is that the Public Health Service Commissioned Corps has been growing in its mission over the years. The pre-9/11 Commissioned Corps, was a different life. The post-9/11 world is the first time that the Commissioned Corps really fell into this idea of being America’s public health responders. I think that we ramped it up; we started out strong.
This was shown not only in the World Trade Center and the 9/11 disasters that occurred, but in the anthrax scenario that unfolded shortly afterwards. We saw it further continue in Hurricane Katrina and the multiple hurricane responses.
Then the Ebola response, in my last year of serving in uniform, was another action of both the civilian sector of federal responders as well as the uniformed sector. The beauty of that in terms of what we learned from Ebola was that coordination is key. That was the first time that the PHS worked so closely with the US Department of Defense and our sister services to basically have an international mission unfold with that level of coordination.
We can use those changes that have gone on, the metamorphoses that have happened over the years, as a jumping off point, but they need to be fulfilled with further growth and support of the Commissioned Corps of the US Public Health Service. The numbers are the lowest they’ve been in recent times in terms of active duty officers. That’s not a good thing. As the mission expands, the idea of recruiting and retaining remains a problem. We have to deal with it.
Was your interest in taking the position at the University of Maryland in part to help build the future of public health?
RADM Lushniak. Certainly, I was so excited to be at the University of Maryland College Park exactly for that reason. The undergraduates are coming in from high school and their eyes are wide open. Two things are important at that stage. One is to teach them about the beauty of public health. That it’s a bold and noble mission. As I always tell our students, it’s about the 3 Ps: Promoting health and wellbeing, preventing disease and injury, and prolonging a high quality of life.
When you put all those things together, that’s an incredible mission. I want to tell them at that young age, “Be a part of this, figure out where you fit in.” But it’s not for everyone. I tell my students that one of the major attributes that I need to see in a student is optimism. Public health does not deal well with pessimism. If your character is pessimistic, I actually dissuade you from becoming a public health person because there are a lot of barriers in this incredible bold and noble mission, and optimism needs to be a key feature that keeps us all going.
Next is the realization that there’s so many different public health issues in our world, so many different problems to deal with. I mentioned some of them previously in terms of the public health issues we see each and every day.
Let me talk about one that’s, in particular, shining through in the midst of COVID-19, but also shines through each and every day. That’s the issue of health equity in our communities. A young person, who usually comes in and wants to help their community, needs to realize that part of the battle of public health is to make sure that we deal with the disparities that exist. We must make health equity a key component of our jobs. We are here to serve others.
There’s a saying at the University of Maryland College Park that we’re a “Do good university.” I would say that public health is a do-good profession. It is about compassion, it’s about love, it’s about caring. Those are the types of people that I try to bring into the school, and I try to mentor and support.
What have you been doing since you left the US Public Health Service?
RADM Boris D. Lushniak, MD, MPH. I retired in 2015 and spent a year at the Uniformed Services University for the Health Sciences in Bethesda, Maryland as the Chair of Preventive Medicine and Biostatistics before I took the opportunity to become the Dean of the School of Public Health at the University of Maryland in College Park. I was very intrigued with that position. It’s a large and young school of public health—just 13 years since its inception. And it functions at both the undergraduate and graduate school levels. We have 2,400 undergraduates in 4 different degree paths. The intriguing part of this is the ability to influence a young person’s educational pathway, and for them to look at all the opportunities in public health, and to focus on a mission, which falls into the mission of the US Public Health Service (PHS) Commissioned Corps: Protect, promote and advance the health and safety of our nation.
It has been a very intriguing transition; I have been the Dean there for 3 years. Who would have predicted that things would change drastically in that time, both at the academic level (ie, moving a school from being a normal college environment to an online environment) and now moving into the realm of preparing for the near future of that university in terms of a potential reopening. It is using all of my public health experiences and putting it at that culmination point, which is my community of 52,000 people—40,000 students at the University in College Park, and 12,000 faculty and staff members.
We are responsible for making sure that the return is as safe as possible. With so many unknowns in the world of COVID-19 and so many unpredictable components, it is quite an undertaking to be able to determine for that community of 52,000 whether it’s time to return, and under what circumstances do we return.
In addition, we’re part of a larger community. The University of Maryland in College Park is in Prince George’s County, which is the epicenter of disease and death in Maryland. The School of Public Health is working closely with county authorities. Some of our students are now contact tracers. It’s been interesting to see our faculty, staff, and students standing up as a volunteer support structure for Public Health.
We have incredible research going on at the school. One of my prime research physicians, Don Milton, MD, DrPH, has been studying the transmission of influenza. Now his work is priming on not just influenza, but also COVID-19. Our hope is to establish a community that will be safe and healthy for everyone, and so it’s been an incredible amount of work.
How would you describe the federal/ local public health cooperation?
RADM Lushniak. First and foremost, we have seen a major issue in terms of state and local response to the COVID-19 pandemic. I have to congratulate the state and the local officials for doing as best as they can under the strained circumstances that they’re in.
The first strained circumstance is that local and state health departments have lost nearly a quarter of their workforce: 50,000 jobs across the country since the recession of 2008. Part of the answer why it’s been such a struggle is that our nation as a whole hasn’t looked at public health and hasn’t looked at prevention as a key component of how our country works. We have seen a lack of support at the state and the local level, the shedding of jobs, and the lack of foresight in terms of saying that prevention works and public health is important for our cities, states, regions, and the nation. We need to reemphasize that in terms of public health.
In the State of Maryland, in general, the counties are doing as best as they can under the circumstances. They certainly started out with trying to do as much testing as possible. Testing is a critical component to this response, and obviously, we have a situation nationwide with the testing still trying to be put online to the extent that it needs to be. We need to be able to test more and more individuals to be able to determine the people who are positive. The curve ball that COVID-19 threw us is that 25 to 50% of individuals who may have a positive test may be asymptomatic. So, this isn’t simple. It’s not a matter of just saying, “Okay, you’re sick. You may then have it.” It may be: “Hey, you’re feeling healthy, you still may have it.”
But just as important as testing is what you do with those individuals who are tested. You need to have health departments turning to these individuals and providing them directions of what needs to be done. If one is COVID-19-positive, one goes into isolation for at least 14 days. And if ill, they need to be connected with a medical care system. That’s an important part of the state and local response is making sure the individuals are properly directed to the right pathway.
In addition, contact tracing is critical. The way we’re going to fight COVID-19 is the ability for us to go out there and determine if you are a positive, who did you come in contact with, and did you potentially spread this to others? You need to direct individuals who may have been in contact with the person who is now COVID- 19-positive, saying “You may have to quarantine yourself, watch out for symptoms, and you have to be really careful in the meantime.”
State and local officials took up the burden of making decisions in terms of communicating the directions given to the population. Is stay at home required? Is it the closure of businesses? Is it the wearing of masks? Certainly, the issue of physical distancing plays a role.
All that was implemented at the state and local level. Under the circumstances, it has been done as well as possible, but that now reflects on the issue of the federal response. And the federal response, I’ll admit, has been less than I had hoped for on several realms.
Number one, coordination and direction from the federal level has been rather piecemeal. State and local officials, I think, were waiting for further directions. What did federal officials think; what did they want us to do? State and local officials want independence to implement things, but what’s the right answer? I think this has been not handled well at the highest levels of the US government.
Secondly, obviously, there was an issue with testing, and the responsibility here lays with the Centers of Disease Control and Prevention (CDC), which had problems from the get-go with setting up their testing caches and getting them out. We’re still catching up from there. Now it’s unfolding that the tie in between the federal government and the private sector and academic centers are at least making some headway on that testing front.
Third, people rely on the federal officials not only for action but also for communication. It really boils down to: Who’s in charge, who’s telling me the information that I need to know, who’s honest with me and telling me what they don’t know, and who has the insight to say, “Here’s how we’re going to find out the things that we don’t know?” Who’s there empathizing with the population?
The reality is there’s been a mismatch between the communication channels for the federal government and getting down not just to the state and locals but, also, to the general population in this country.
How would you characterize the US Public Health Service Response?
RADM Lushniak. I’ll first start off with kudos and congratulations to the Commissioned Corps of the PHS for their response to date. I think the latest numbers that ADM Brett Giroir, MD, Assistant Secretary of Health, told Congress in May, was that at the time more than 3,100 of the 6,100 current officers at the PHS have been deployed over the last several months. The reality is that the Commissioned Corps is out there doing service to our nation and to the world. PHS teams were deployed initially to Japan and the Diamond Princess cruise ship. The Corps been out there internationally.
Nationally, the Corps was at the Javits Center in New York assisting in setting up that medical response. They have been assisting at the military bases initially where some of the individuals who were coming in from China and other places were being held in quarantine. They have been assisting with investigations at nursing homes across the country and meat packing plants where there have been outbreaks occurring. The Commissioned Corps has been out there, so that’s the good news.
The bad news is that the Corps is a small uniformed service. The reality is nobody still is seeing the Corps or knows about the Corps as they’re out there doing their thing. It was very nice that ADM Giroir put a plug in for them in his recent congressional testimony. That’s great that our leadership is out there acknowledging the Corps. But to a large extent, I still have an issue with the Commissioned Corps being an underfunded uniformed service of this country. The Commissioned Corps is the only uniformed service in the world whose only mission is public health. But, lack of support reflects the idea of the lack of importance that public health plays in the minds of policy makers.
To a large extent, we have had no dollars in the Corps recently for training of officers to prepare for this. For 10 years we’ve waited for a Ready Reserve to be set up. The Ready Reserve component was part of the Affordable Care Act. I was in the office of the Surgeon General as we were told to ramp this up. Now 10 years later, in the midst of this COVID-19 pandemic, Congress finally has passed legislation that sets a pathway for a Ready Reserve.
Why is the Ready Reserve important? In essence, we have incredible public health professionals out there in the civilian ranks who would be willing to assist the Commissioned Corps in their mission, either to backfill critical positions where Corps officers are currently stationed and need to be deployed, or as a Ready Reserve that’s ready to deploy itself. All this is happening right now. I hope for better days, and I hope this COVID-19 pandemic will wake our nation up to the need of a Public Health Service Commissioned Corps, a uniformed service, that's out there doing good.
What lessons are we learning about public health in this pandemic?
RADM Lushniak. We’ve just developed a new space force, the 8th uniformed US service. In reality they are talking about tens of thousands of people assigned to it. Excuse me if I’m going to be assertive. I’m a big fan of space exploration. I realize that space is the final frontier and that perhaps we have to be able to defend our country in that regard. But we’re already saying that space is worth investing in. Where is the wisdom that we’re not investing in battling on this planet against emerging threats like COVID-19? And why is it that to this date the Commissioned Corps of the Public Health Service does not have its own budget; does not have a line item anywhere; does not have money directed for training; and, in essence, only serves because its officers are stationed at other agencies who pay for these officers? It’s a personnel system and not really treated as a key and critical uniformed service of this country. That’s point number one in terms of lessons learned and what needs to be done.
In addition, it’s not just the people in uniform who serve at the federal level, civilians serve as well. These civilians work at the CDC, at the US Food and Drug Administration, at the National Institute of Health, at the Indian Health Service, and at many, many other agencies throughout the US government. Within those realms, we need to show support of those federal practitioners who are working very diligently and in a devoted fashion to fight this pandemic as well. Part of it is the moral support to recognize that there are multiple fronts to fighting this pandemic and the federal practitioner who is working out there, is a key component to this.
I don’t want everything to be money, money, money, but the fact is that CDC’s budget has been decreasing over the years. How are we supposed to set up the laboratories, how are we supposed to demand the high level of expertise when, in fact, everything has to be done on a shoestring?
Finally, we notice public health in the midst of a crisis, but public health matters each and every day. The idea that the pandemic certainly brings to light what needs to get done, but without a pandemic, what do we have? We still have cigarette smoking, the number 1 killer in this country. That’s a public health issue. We have cardiovascular diseases as an extreme killer in this country. That’s a public health issue. We have diabetes mellitus that is rampant. We have substance abuse, including the opioid epidemic. Those are public health issues. We have hypertension, we have overweight and obesity. Those are all public health issues that public health battles each and every day without the recognition.
What we need is a major shift in the philosophy of this country to really take the health and wellness of our society as a key component of how you’ll raise that on to a pedestal—the idea that health and wellness is critical to the functioning of this country.
How have recent public health emergencies influenced the Commissioned Corps?
RADM Lushniak. The key feature is that the Public Health Service Commissioned Corps has been growing in its mission over the years. The pre-9/11 Commissioned Corps, was a different life. The post-9/11 world is the first time that the Commissioned Corps really fell into this idea of being America’s public health responders. I think that we ramped it up; we started out strong.
This was shown not only in the World Trade Center and the 9/11 disasters that occurred, but in the anthrax scenario that unfolded shortly afterwards. We saw it further continue in Hurricane Katrina and the multiple hurricane responses.
Then the Ebola response, in my last year of serving in uniform, was another action of both the civilian sector of federal responders as well as the uniformed sector. The beauty of that in terms of what we learned from Ebola was that coordination is key. That was the first time that the PHS worked so closely with the US Department of Defense and our sister services to basically have an international mission unfold with that level of coordination.
We can use those changes that have gone on, the metamorphoses that have happened over the years, as a jumping off point, but they need to be fulfilled with further growth and support of the Commissioned Corps of the US Public Health Service. The numbers are the lowest they’ve been in recent times in terms of active duty officers. That’s not a good thing. As the mission expands, the idea of recruiting and retaining remains a problem. We have to deal with it.
Was your interest in taking the position at the University of Maryland in part to help build the future of public health?
RADM Lushniak. Certainly, I was so excited to be at the University of Maryland College Park exactly for that reason. The undergraduates are coming in from high school and their eyes are wide open. Two things are important at that stage. One is to teach them about the beauty of public health. That it’s a bold and noble mission. As I always tell our students, it’s about the 3 Ps: Promoting health and wellbeing, preventing disease and injury, and prolonging a high quality of life.
When you put all those things together, that’s an incredible mission. I want to tell them at that young age, “Be a part of this, figure out where you fit in.” But it’s not for everyone. I tell my students that one of the major attributes that I need to see in a student is optimism. Public health does not deal well with pessimism. If your character is pessimistic, I actually dissuade you from becoming a public health person because there are a lot of barriers in this incredible bold and noble mission, and optimism needs to be a key feature that keeps us all going.
Next is the realization that there’s so many different public health issues in our world, so many different problems to deal with. I mentioned some of them previously in terms of the public health issues we see each and every day.
Let me talk about one that’s, in particular, shining through in the midst of COVID-19, but also shines through each and every day. That’s the issue of health equity in our communities. A young person, who usually comes in and wants to help their community, needs to realize that part of the battle of public health is to make sure that we deal with the disparities that exist. We must make health equity a key component of our jobs. We are here to serve others.
There’s a saying at the University of Maryland College Park that we’re a “Do good university.” I would say that public health is a do-good profession. It is about compassion, it’s about love, it’s about caring. Those are the types of people that I try to bring into the school, and I try to mentor and support.
Gap Analysis for the Conversion to Area Under the Curve Vancomycin Monitoring in a Small Rural Hospital
The use of weight-based dosing with trough-based monitoring of vancomycin has been in clinical practice for more than a decade. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published the first guidelines for vancomycin monitoring in 2009.1 Although it has been well established that area under the curve (AUC) over the minimal inhibitory concentration (MIC) ratio > 400 mg.h/L is the best predictor of clinical efficacy, obtaining this value in clinical practice was not pragmatic. Therefore, the 2009 guidelines recommended a goal vancomycin trough of 15 to 20 mcg/ml as a surrogate marker for AUC/MIC > 400 mg.hr/L. This has since become a common practice despite little data that support this recommendation.
The efficacy and safety of trough-based monitoring has been evaluated extensively over the past several years and more recent data suggest that there is wide patient variability in AUC with this method and higher trough levels are associated with more nephrotoxicity.2,3 ASHP, IDSA, SIDP, and the Pediatric Infectious Diseases Society (PIDS) updated the consensus guidelines in 2020.4 Trough-based monitoring is no longer recommended. Instead AUC24 monitoring should be implemented with a goal range of 400 to 600 mg.h/L for efficacy and safety. Given concerns for vancomycin penetration into the central nervous system (CNS), many facility protocols utilize higher targets (> 600 mg.h/L) for CNS infections.
Some hospitals have been utilizing AUC-based monitoring for years. There are strategies from tertiary care centers that drive this practice change in the medical literature.5,6 However, it is important to reproduce these implementation practices in small, rural facilities that may face unique challenges with limited resources and may be slower to implement consensus guidelines.7,8 As this is a major practice change, it is imperative to evaluate the extent of transition and identify areas of needed improvement.
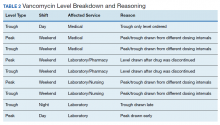
Accurate therapeutic drug monitoring ensures both the safety and efficacy of vancomycin therapy. Unfortunately, research shows that inappropriate laboratory tests are common in medical facilities.9 Drug levels taken inappropriately can lead to delays in therapeutic decision-making, inappropriate dosage adjustments and create a need for repeated drug levels, which increases the overall cost of admission.
Given the multiple affected services needed to make successful practice transitions, it is paramount that facilities evaluate progress during the transition phase. The Agency for Healthcare Research and Quality and the Institute for Healthcare Improvement provide guidance in the Plan-Do-Study-Act Cycle for quality assessment and improvement of new initiatives.10,11 A gap analysis can be used as a simple tool for evaluating the transition of research into practice and to identify areas of needed improvement.
The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas made the transition from trough-based monitoring to 2-level AUC-based monitoring on April 1, 2019. The purpose of this study was to evaluate the effectiveness of transition methods used to implement AUC-monitoring for vancomycin treated patients in a small, primary facility. A further goal of the study was to identify areas of needed improvement and education and whether the problems derived from deficiencies in knowledge and ordering (medical and pharmacy services) or execution (nursing and laboratory services).
Methods
VHSO is a 52-bed US Department of Veterans Affairs primary care hospital. The pharmacy and laboratory are staffed 24 hours each day. There is 1 clinical pharmacy specialist (CPS) available for therapeutic drug monitoring consults Monday through Friday between the hours of 7:30 AM and 4:00 PM. No partial full-time equivalent employees were added for this conversion. Pharmacy-driven vancomycin dosing and monitoring is conducted on a collaborative basis, with pharmacy managing the majority of vancomycin treated patients. Night and weekend pharmacy staff provide cross-coverage on vancomycin consultations. Laboratory orders and medication dosage adjustments fall within the CPS scope of practice. Nurses do not perform laboratory draws for therapeutic drug monitoring; this is done solely by phlebotomists. There is no infectious diseases specialist at the facility to champion antibiotic dosing initiatives.
The implementation strategy largely reflected those outlined from tertiary care centers.5,6 First, key personnel from the laboratory department met to discuss this practice change and to add vancomycin peaks to the ordering menu. A critical value was set at 40 mcg/ml. Vancomycin troughs and random levels already were orderable items. A comment field was added to all laboratory orders for further clarification. Verbiage was added to laboratory reports in the computerized medical record to assist clinicians in determining the appropriateness of the level. This was followed by an educational email to both the nursing and laboratory departments explaining the practice change and included a link to the Pharmacy Joe “Vancomycin Dosing by AUC:MIC Instead of Trough-level” podcast (www.pharmacyjoe.com episode 356).
The pharmacy department received an interactive 30-minute presentation, followed immediately by a group activity to discuss practice problems. This presentation was condensed, recorded, and emailed to all VHSO pharmacists. A shared folder contained pertinent material on AUC monitoring.
Finally, an interactive presentation was set up for hospitalists and a video teleconferencing was conducted for rotating medical residents. Both the podcast and recorded presentation were emailed to the entire medical staff with a brief introduction of the practice change. Additionally, the transition process was added as a standing item on the monthly antimicrobial stewardship meeting agenda.
The standardized pharmacokinetic model at the study facility consisted of a vancomycin volume of distribution of 0.7 mg/kg and elimination rate constant (Ke) by Matzke and colleagues for total daily dose calculations.12 Obese patients (BMI ≥ 30) undergo alternative clearance equations described by Crass and colleagues.13 Cockcroft-Gault methods using ideal body weight (or actual body weight if < ideal body weight) are used for determining creatinine clearance. In patients aged ≥ 65 years with a serum creatinine < 1.0 mg/dL, facility guidance was to round serum creatinine up to 1.0 mg/dL. Loading doses were determined on a case-by-case basis with a cap of 2,000 mg, maintenance doses were rounded to the nearest 250 mg.
Vancomycin levels typically are drawn at steady state and analyzed using the logarithmic trapezoidal rule.14 The pharmacy and medical staff were educated to provide details on timing and coordination in nursing and laboratory orders (Table 1). Two-level AUC monitoring typically is not performed in patients with acute renal failure, expected duration of therapy < 72 hours, urinary tract infections, skin and soft tissue infections, or in renal replacement therapy.5
This gap analysis consisted of a retrospective chart review of vancomycin levels ordered after the implementation of AUC-based monitoring to determine the effectiveness of the transition. Three months of data were collected between April 2019 and June 2019. Vancomycin levels were deemed either appropriate or inappropriate based on timing and type (peak, trough, or random) of the laboratory test in relation to the previously administered vancomycin dose. Appropriate peaks were drawn within 2 hours after the end of infusion and troughs at least 1 half-life after the dose or just prior to the next dose and within the same dosing interval as the peak. Tests drawn outside of the specified time range, trough-only laboratory tests, or those drawn after vancomycin had been discontinued were considered inappropriate. Peaks and troughs drawn from separate dosing intervals also were considered inappropriate. Random levels were considered appropriate only if they fit the clinical context in acute renal failure or renal replacement therapy. An effective transition was defined as ≥ 80% of all vancomycin treated patients monitored with AUC methods rather than trough-based methods.
Inclusion criteria included all vancomycin levels ordered during the study period with no exclusions. The primary endpoint was the proportion of vancomycin levels drawn appropriately. Secondary endpoints were the proportion of AUC24 calculations within therapeutic range and a stratification of reasons for inappropriate levels. Descriptive statistics were collected to describe the scope of the project. Levels drawn from various shifts were compared (ie, day, night, or weekend). Calculated AUC24 levels between 400 and 600 mg.h/L were considered therapeutic unless treating CNS infection (600-700 mg.h/L). Given the operational outcomes (rather than clinical outcomes) and no comparator group, patient specific data were not collected.
Descriptive statistics without further analysis were used to describe proportions. The goal level for compliance was set at 100%. These methods were reviewed by the VHSO Institutional Review Board and granted nonresearch status, waiving the requirement for informed consent.
Results
The transition was effective with 97% of all cases utilizing AUC-based methods for monitoring. A total of 65 vancomycin levels were drawn in the study period; 32 peaks, 32 troughs, and 1 random level (drawn appropriately during acute renal failure 24 hours after starting therapy). All shifts were affected proportionately; days (n = 26, 40%), nights (n = 18, 27.7%), and weekends (n = 21, 32.3%). Based on time of dosage administration and laboratory test, there were 9 levels (13.8%) deemed inappropriate, 56 levels (86.1%) were appropriate. Reasons for inappropriate levels gleaned from chart review are presented in Table 2. Four levels had to be repeated for accurate calculations.
From the peak/trough couplets drawn appropriately, calculated AUC24 fell with the desired range in 61% (n = 17) of cases. Of the 11 that fell outside of range, 8 were subtherapeutic (< 400 mg.h/L) and 3 were supratherapeutic (> 600 mg.h/L). All levels were drawn at steady state. Indications for vancomycin monitoring were osteomyelitis (n = 13, 43%), sepsis (n = 10, 33%), pneumonia (n = 6, 20%), and 1 case of meningitis (3%).
Discussion
To the author’s knowledge, this is the first report of a vancomycin AUC24 monitoring conversion in a rural facility. This study adds to the existing medical literature in that it demonstrates that: (1) implementation methods described in large, tertiary centers can be effectively utilized in primary care, rural facilities; (2) the gap analysis used can be duplicated with minimal personnel and resources to ensure effective implementation (Table 3); and (3) the reported improvement needs can serve as a model for preventative measures at other facilities. The incidence of appropriate vancomycin levels was notably better than those reported in other single center studies.15-17 However, given variations in study design and facility operating procedures, it would be difficult to compare incidence among medical facilities. As such, there are no consensus benchmarks for comparison. The majority of inappropriate levels occurred early in the study period and on weekends. Appropriateness of drug levels may have improved with continued feedback and familiarity.
The calculated AUC24 fell within predicted range in 61% of cases. For comparison, a recent study from a large academic medical center reported that 73.5% of 2-level AUC24 cases had initial values within the therapeutic range.18 Of note, the target range used was much wider (400 - 800 mg.h/L) than the present study. Another study reported dose adjustments for subtherapeutic AUC levels in 25% of cases and dose reductions for supratherapeutic levels in 33.3% of cases.19
Of the AUC24 calculations that fell outside of therapeutic range, the majority (n = 8, 73%) were subtherapeutic (< 400 mg.h/L), half of these were for patients who were obese. It was unclear in the medical record which equation was used for initial dosing (Matzke vs Crass), or whether more conservative AUCs were used for calculating the total daily dose. The VHSO policy limiting loading doses also may have played a role; indeed the updated guidelines recommend a maximum loading dose of 3,000 mg depending on the severity of infection.4 Two of the 3 supratherapeutic levels were thought to be due to accumulation with long-term therapy.
Given such a large change from long-standing practices, there was surprisingly little resistance from the various clinical services. A recent survey of academic medical centers reported that the majority (88%) of all respondents who did not currently utilize AUC24 monitoring did not plan on making this immediate transition, largely citing unfamiliarity and training requirements.20 It is conceivable that the transition to AUC monitoring in smaller facilities may have fewer barriers than those seen in tertiary care centers. There are fewer health care providers and pharmacists to educate with the primary responsibilities falling on relatively few clinicians. There is little question as to who will be conducting follow up or whom to contact for questions. A smaller patient load and lesser patient acuity may translate to fewer vancomycin cases that require monitoring.
The interactive meetings were an important element for facility implementation. Research shows that emails alone are not effective for health care provider education, and interactive methods are recommended over passive methods.21,22 Assessing and avoiding barriers up front such as unclear laboratory orders, or communication failures is paramount to successful implementation strategies.23 Additionally, the detailed written ordering communication may have contributed to a smoother transition. The educational recording proved to be helpful in educating new staff and residents. An identified logistical error was that laboratory orders entered while patients were enrolled in sham clinics for electronic workload capture (eg, Pharmacy Inpatient Clinic) created confusion on the physical location of the patient for the phlebotomists, potentially causing delays in specimen collection.
A major development that stemmed from this intervention was that the Medical Service asked that policy changes be made so that the Pharmacy Service take over all vancomycin dosing at the facility. Previously, this had been done on a collaborative basis. Similar facilities with a collaborative practice model may need to anticipate such a request as this may present a new set of challenges. Accordingly, the pharmacy department is in the process of establishing standing operating procedures, pharmacist competencies, and a facility memorandum. Future research should evaluate the safety and efficacy of vancomycin therapy after the switch to AUC-based monitoring.
Limitations
There are several limitations to consider with this study. Operating procedures and implementation processes may vary between facilities, which could limit the generalizability of these results. Given the small facility size, the overall number of laboratory tests drawn was much smaller than those seen in larger facilities. The time needed for AUC calculations is notably longer than older methods of monitoring; however, this was not objectively assessed. It is important to note that clinical outcomes were beyond the scope of this gap analysis and this is an area of future research at the study facility. Vancomycin laboratory tests that were missed due to procedures and subsequently rescheduled were occasionally observed but not accounted for in this analysis. Additionally, vancomycin courses without monitoring (appropriate or otherwise) when indicated were not assessed. However, anecdotally speaking, this would be a very unlikely occurrence.
Conclusion
Conversion to AUC-based vancomycin monitoring is feasible in primary, rural medical centers. Implementation strategies from tertiary facilities can be successfully utilized in smaller hospitals. Quality assessment strategies such as a gap analysis can be utilized with minimal resources for facility uptake of new clinical practices.
1. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [published correction appears in Am J Health Syst Pharm. 2009;66(10):887]. Am J Health Syst Pharm. 2009;66(1):82‐98. doi:10.2146/ajhp080434
2. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734‐744. doi:10.1128/AAC.01568-12
3. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. doi:10.1016/j.addr.2014.05.016
4. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists [published online ahead of print, 2020 Mar 19]. Am J Health Syst Pharm. 2020;zxaa036. doi:10.1093/ajhp/zxaa036
5. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986‐1995. doi:10.2146/ajhp180034
6. Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned [published online ahead of print, 2019 Mar 10]. J Pharm Pract. 2019;897190019834369. doi:10.1177/0897190019834369
7. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8‐S14. doi:10.1093/cid/cir363
8. Goldman LE, Dudley RA. United States rural hospital quality in the Hospital Compare database-accounting for hospital characteristics. Health Policy. 2008;87(1):112‐127. doi:10.1016/j.healthpol.2008.02.002
9. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:10.1371/journal.pone.0078962
10. Institute for Healthcare Improvement. Plan-do-study-act (PDSA) worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed May 13, 2020.
11. Agency for Healthcare Research and Quality. Plan-do-study-act (PDSA) cycle. https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle. Updated April 10, 2013. Accessed May 13, 2020.
12. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433‐437. doi:10.1128/aac.25.4.433
13. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081‐3086. doi:10.1093/jac/dky310
14. Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162‐3167. doi:10.1128/AAC.02355-14
15. Suryadevara M, Steidl KE, Probst LA, Shaw J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17(2):159‐165. doi:10.5863/1551-6776-17.2.159
16. Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472‐478. doi:10.1309/AJCPDSYS0DVLKFOH
17. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801‐806. doi:10.1309/AJCPKQ6EAH7OYQLB
18. Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;39(4):433‐442. doi:10.1002/phar.2234
19. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant staphylococcus aureus Infection. J Pharm Pract. 2019;32(4):442‐446. doi:10.1177/0897190018764564
20. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889‐894. doi:10.1093/ajhp/zxz070
21. Bluestone J, Johnson P, Fullerton J, Carr C, Alderman J, BonTempo J. Effective in-service training design and delivery: evidence from an integrative literature review. Hum Resour Health. 2013;11:51. doi:10.1186/1478-4491-11-51
22. Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi:10.1136/bmjopen-2017-017572
23. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). 2016;4(3):36. doi:10.3390/healthcare4030036
The use of weight-based dosing with trough-based monitoring of vancomycin has been in clinical practice for more than a decade. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published the first guidelines for vancomycin monitoring in 2009.1 Although it has been well established that area under the curve (AUC) over the minimal inhibitory concentration (MIC) ratio > 400 mg.h/L is the best predictor of clinical efficacy, obtaining this value in clinical practice was not pragmatic. Therefore, the 2009 guidelines recommended a goal vancomycin trough of 15 to 20 mcg/ml as a surrogate marker for AUC/MIC > 400 mg.hr/L. This has since become a common practice despite little data that support this recommendation.
The efficacy and safety of trough-based monitoring has been evaluated extensively over the past several years and more recent data suggest that there is wide patient variability in AUC with this method and higher trough levels are associated with more nephrotoxicity.2,3 ASHP, IDSA, SIDP, and the Pediatric Infectious Diseases Society (PIDS) updated the consensus guidelines in 2020.4 Trough-based monitoring is no longer recommended. Instead AUC24 monitoring should be implemented with a goal range of 400 to 600 mg.h/L for efficacy and safety. Given concerns for vancomycin penetration into the central nervous system (CNS), many facility protocols utilize higher targets (> 600 mg.h/L) for CNS infections.
Some hospitals have been utilizing AUC-based monitoring for years. There are strategies from tertiary care centers that drive this practice change in the medical literature.5,6 However, it is important to reproduce these implementation practices in small, rural facilities that may face unique challenges with limited resources and may be slower to implement consensus guidelines.7,8 As this is a major practice change, it is imperative to evaluate the extent of transition and identify areas of needed improvement.
Accurate therapeutic drug monitoring ensures both the safety and efficacy of vancomycin therapy. Unfortunately, research shows that inappropriate laboratory tests are common in medical facilities.9 Drug levels taken inappropriately can lead to delays in therapeutic decision-making, inappropriate dosage adjustments and create a need for repeated drug levels, which increases the overall cost of admission.
Given the multiple affected services needed to make successful practice transitions, it is paramount that facilities evaluate progress during the transition phase. The Agency for Healthcare Research and Quality and the Institute for Healthcare Improvement provide guidance in the Plan-Do-Study-Act Cycle for quality assessment and improvement of new initiatives.10,11 A gap analysis can be used as a simple tool for evaluating the transition of research into practice and to identify areas of needed improvement.
The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas made the transition from trough-based monitoring to 2-level AUC-based monitoring on April 1, 2019. The purpose of this study was to evaluate the effectiveness of transition methods used to implement AUC-monitoring for vancomycin treated patients in a small, primary facility. A further goal of the study was to identify areas of needed improvement and education and whether the problems derived from deficiencies in knowledge and ordering (medical and pharmacy services) or execution (nursing and laboratory services).
Methods
VHSO is a 52-bed US Department of Veterans Affairs primary care hospital. The pharmacy and laboratory are staffed 24 hours each day. There is 1 clinical pharmacy specialist (CPS) available for therapeutic drug monitoring consults Monday through Friday between the hours of 7:30 AM and 4:00 PM. No partial full-time equivalent employees were added for this conversion. Pharmacy-driven vancomycin dosing and monitoring is conducted on a collaborative basis, with pharmacy managing the majority of vancomycin treated patients. Night and weekend pharmacy staff provide cross-coverage on vancomycin consultations. Laboratory orders and medication dosage adjustments fall within the CPS scope of practice. Nurses do not perform laboratory draws for therapeutic drug monitoring; this is done solely by phlebotomists. There is no infectious diseases specialist at the facility to champion antibiotic dosing initiatives.
The implementation strategy largely reflected those outlined from tertiary care centers.5,6 First, key personnel from the laboratory department met to discuss this practice change and to add vancomycin peaks to the ordering menu. A critical value was set at 40 mcg/ml. Vancomycin troughs and random levels already were orderable items. A comment field was added to all laboratory orders for further clarification. Verbiage was added to laboratory reports in the computerized medical record to assist clinicians in determining the appropriateness of the level. This was followed by an educational email to both the nursing and laboratory departments explaining the practice change and included a link to the Pharmacy Joe “Vancomycin Dosing by AUC:MIC Instead of Trough-level” podcast (www.pharmacyjoe.com episode 356).
The pharmacy department received an interactive 30-minute presentation, followed immediately by a group activity to discuss practice problems. This presentation was condensed, recorded, and emailed to all VHSO pharmacists. A shared folder contained pertinent material on AUC monitoring.
Finally, an interactive presentation was set up for hospitalists and a video teleconferencing was conducted for rotating medical residents. Both the podcast and recorded presentation were emailed to the entire medical staff with a brief introduction of the practice change. Additionally, the transition process was added as a standing item on the monthly antimicrobial stewardship meeting agenda.
The standardized pharmacokinetic model at the study facility consisted of a vancomycin volume of distribution of 0.7 mg/kg and elimination rate constant (Ke) by Matzke and colleagues for total daily dose calculations.12 Obese patients (BMI ≥ 30) undergo alternative clearance equations described by Crass and colleagues.13 Cockcroft-Gault methods using ideal body weight (or actual body weight if < ideal body weight) are used for determining creatinine clearance. In patients aged ≥ 65 years with a serum creatinine < 1.0 mg/dL, facility guidance was to round serum creatinine up to 1.0 mg/dL. Loading doses were determined on a case-by-case basis with a cap of 2,000 mg, maintenance doses were rounded to the nearest 250 mg.
Vancomycin levels typically are drawn at steady state and analyzed using the logarithmic trapezoidal rule.14 The pharmacy and medical staff were educated to provide details on timing and coordination in nursing and laboratory orders (Table 1). Two-level AUC monitoring typically is not performed in patients with acute renal failure, expected duration of therapy < 72 hours, urinary tract infections, skin and soft tissue infections, or in renal replacement therapy.5
This gap analysis consisted of a retrospective chart review of vancomycin levels ordered after the implementation of AUC-based monitoring to determine the effectiveness of the transition. Three months of data were collected between April 2019 and June 2019. Vancomycin levels were deemed either appropriate or inappropriate based on timing and type (peak, trough, or random) of the laboratory test in relation to the previously administered vancomycin dose. Appropriate peaks were drawn within 2 hours after the end of infusion and troughs at least 1 half-life after the dose or just prior to the next dose and within the same dosing interval as the peak. Tests drawn outside of the specified time range, trough-only laboratory tests, or those drawn after vancomycin had been discontinued were considered inappropriate. Peaks and troughs drawn from separate dosing intervals also were considered inappropriate. Random levels were considered appropriate only if they fit the clinical context in acute renal failure or renal replacement therapy. An effective transition was defined as ≥ 80% of all vancomycin treated patients monitored with AUC methods rather than trough-based methods.
Inclusion criteria included all vancomycin levels ordered during the study period with no exclusions. The primary endpoint was the proportion of vancomycin levels drawn appropriately. Secondary endpoints were the proportion of AUC24 calculations within therapeutic range and a stratification of reasons for inappropriate levels. Descriptive statistics were collected to describe the scope of the project. Levels drawn from various shifts were compared (ie, day, night, or weekend). Calculated AUC24 levels between 400 and 600 mg.h/L were considered therapeutic unless treating CNS infection (600-700 mg.h/L). Given the operational outcomes (rather than clinical outcomes) and no comparator group, patient specific data were not collected.
Descriptive statistics without further analysis were used to describe proportions. The goal level for compliance was set at 100%. These methods were reviewed by the VHSO Institutional Review Board and granted nonresearch status, waiving the requirement for informed consent.
Results
The transition was effective with 97% of all cases utilizing AUC-based methods for monitoring. A total of 65 vancomycin levels were drawn in the study period; 32 peaks, 32 troughs, and 1 random level (drawn appropriately during acute renal failure 24 hours after starting therapy). All shifts were affected proportionately; days (n = 26, 40%), nights (n = 18, 27.7%), and weekends (n = 21, 32.3%). Based on time of dosage administration and laboratory test, there were 9 levels (13.8%) deemed inappropriate, 56 levels (86.1%) were appropriate. Reasons for inappropriate levels gleaned from chart review are presented in Table 2. Four levels had to be repeated for accurate calculations.
From the peak/trough couplets drawn appropriately, calculated AUC24 fell with the desired range in 61% (n = 17) of cases. Of the 11 that fell outside of range, 8 were subtherapeutic (< 400 mg.h/L) and 3 were supratherapeutic (> 600 mg.h/L). All levels were drawn at steady state. Indications for vancomycin monitoring were osteomyelitis (n = 13, 43%), sepsis (n = 10, 33%), pneumonia (n = 6, 20%), and 1 case of meningitis (3%).
Discussion
To the author’s knowledge, this is the first report of a vancomycin AUC24 monitoring conversion in a rural facility. This study adds to the existing medical literature in that it demonstrates that: (1) implementation methods described in large, tertiary centers can be effectively utilized in primary care, rural facilities; (2) the gap analysis used can be duplicated with minimal personnel and resources to ensure effective implementation (Table 3); and (3) the reported improvement needs can serve as a model for preventative measures at other facilities. The incidence of appropriate vancomycin levels was notably better than those reported in other single center studies.15-17 However, given variations in study design and facility operating procedures, it would be difficult to compare incidence among medical facilities. As such, there are no consensus benchmarks for comparison. The majority of inappropriate levels occurred early in the study period and on weekends. Appropriateness of drug levels may have improved with continued feedback and familiarity.
The calculated AUC24 fell within predicted range in 61% of cases. For comparison, a recent study from a large academic medical center reported that 73.5% of 2-level AUC24 cases had initial values within the therapeutic range.18 Of note, the target range used was much wider (400 - 800 mg.h/L) than the present study. Another study reported dose adjustments for subtherapeutic AUC levels in 25% of cases and dose reductions for supratherapeutic levels in 33.3% of cases.19
Of the AUC24 calculations that fell outside of therapeutic range, the majority (n = 8, 73%) were subtherapeutic (< 400 mg.h/L), half of these were for patients who were obese. It was unclear in the medical record which equation was used for initial dosing (Matzke vs Crass), or whether more conservative AUCs were used for calculating the total daily dose. The VHSO policy limiting loading doses also may have played a role; indeed the updated guidelines recommend a maximum loading dose of 3,000 mg depending on the severity of infection.4 Two of the 3 supratherapeutic levels were thought to be due to accumulation with long-term therapy.
Given such a large change from long-standing practices, there was surprisingly little resistance from the various clinical services. A recent survey of academic medical centers reported that the majority (88%) of all respondents who did not currently utilize AUC24 monitoring did not plan on making this immediate transition, largely citing unfamiliarity and training requirements.20 It is conceivable that the transition to AUC monitoring in smaller facilities may have fewer barriers than those seen in tertiary care centers. There are fewer health care providers and pharmacists to educate with the primary responsibilities falling on relatively few clinicians. There is little question as to who will be conducting follow up or whom to contact for questions. A smaller patient load and lesser patient acuity may translate to fewer vancomycin cases that require monitoring.
The interactive meetings were an important element for facility implementation. Research shows that emails alone are not effective for health care provider education, and interactive methods are recommended over passive methods.21,22 Assessing and avoiding barriers up front such as unclear laboratory orders, or communication failures is paramount to successful implementation strategies.23 Additionally, the detailed written ordering communication may have contributed to a smoother transition. The educational recording proved to be helpful in educating new staff and residents. An identified logistical error was that laboratory orders entered while patients were enrolled in sham clinics for electronic workload capture (eg, Pharmacy Inpatient Clinic) created confusion on the physical location of the patient for the phlebotomists, potentially causing delays in specimen collection.
A major development that stemmed from this intervention was that the Medical Service asked that policy changes be made so that the Pharmacy Service take over all vancomycin dosing at the facility. Previously, this had been done on a collaborative basis. Similar facilities with a collaborative practice model may need to anticipate such a request as this may present a new set of challenges. Accordingly, the pharmacy department is in the process of establishing standing operating procedures, pharmacist competencies, and a facility memorandum. Future research should evaluate the safety and efficacy of vancomycin therapy after the switch to AUC-based monitoring.
Limitations
There are several limitations to consider with this study. Operating procedures and implementation processes may vary between facilities, which could limit the generalizability of these results. Given the small facility size, the overall number of laboratory tests drawn was much smaller than those seen in larger facilities. The time needed for AUC calculations is notably longer than older methods of monitoring; however, this was not objectively assessed. It is important to note that clinical outcomes were beyond the scope of this gap analysis and this is an area of future research at the study facility. Vancomycin laboratory tests that were missed due to procedures and subsequently rescheduled were occasionally observed but not accounted for in this analysis. Additionally, vancomycin courses without monitoring (appropriate or otherwise) when indicated were not assessed. However, anecdotally speaking, this would be a very unlikely occurrence.
Conclusion
Conversion to AUC-based vancomycin monitoring is feasible in primary, rural medical centers. Implementation strategies from tertiary facilities can be successfully utilized in smaller hospitals. Quality assessment strategies such as a gap analysis can be utilized with minimal resources for facility uptake of new clinical practices.
The use of weight-based dosing with trough-based monitoring of vancomycin has been in clinical practice for more than a decade. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published the first guidelines for vancomycin monitoring in 2009.1 Although it has been well established that area under the curve (AUC) over the minimal inhibitory concentration (MIC) ratio > 400 mg.h/L is the best predictor of clinical efficacy, obtaining this value in clinical practice was not pragmatic. Therefore, the 2009 guidelines recommended a goal vancomycin trough of 15 to 20 mcg/ml as a surrogate marker for AUC/MIC > 400 mg.hr/L. This has since become a common practice despite little data that support this recommendation.
The efficacy and safety of trough-based monitoring has been evaluated extensively over the past several years and more recent data suggest that there is wide patient variability in AUC with this method and higher trough levels are associated with more nephrotoxicity.2,3 ASHP, IDSA, SIDP, and the Pediatric Infectious Diseases Society (PIDS) updated the consensus guidelines in 2020.4 Trough-based monitoring is no longer recommended. Instead AUC24 monitoring should be implemented with a goal range of 400 to 600 mg.h/L for efficacy and safety. Given concerns for vancomycin penetration into the central nervous system (CNS), many facility protocols utilize higher targets (> 600 mg.h/L) for CNS infections.
Some hospitals have been utilizing AUC-based monitoring for years. There are strategies from tertiary care centers that drive this practice change in the medical literature.5,6 However, it is important to reproduce these implementation practices in small, rural facilities that may face unique challenges with limited resources and may be slower to implement consensus guidelines.7,8 As this is a major practice change, it is imperative to evaluate the extent of transition and identify areas of needed improvement.
Accurate therapeutic drug monitoring ensures both the safety and efficacy of vancomycin therapy. Unfortunately, research shows that inappropriate laboratory tests are common in medical facilities.9 Drug levels taken inappropriately can lead to delays in therapeutic decision-making, inappropriate dosage adjustments and create a need for repeated drug levels, which increases the overall cost of admission.
Given the multiple affected services needed to make successful practice transitions, it is paramount that facilities evaluate progress during the transition phase. The Agency for Healthcare Research and Quality and the Institute for Healthcare Improvement provide guidance in the Plan-Do-Study-Act Cycle for quality assessment and improvement of new initiatives.10,11 A gap analysis can be used as a simple tool for evaluating the transition of research into practice and to identify areas of needed improvement.
The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas made the transition from trough-based monitoring to 2-level AUC-based monitoring on April 1, 2019. The purpose of this study was to evaluate the effectiveness of transition methods used to implement AUC-monitoring for vancomycin treated patients in a small, primary facility. A further goal of the study was to identify areas of needed improvement and education and whether the problems derived from deficiencies in knowledge and ordering (medical and pharmacy services) or execution (nursing and laboratory services).
Methods
VHSO is a 52-bed US Department of Veterans Affairs primary care hospital. The pharmacy and laboratory are staffed 24 hours each day. There is 1 clinical pharmacy specialist (CPS) available for therapeutic drug monitoring consults Monday through Friday between the hours of 7:30 AM and 4:00 PM. No partial full-time equivalent employees were added for this conversion. Pharmacy-driven vancomycin dosing and monitoring is conducted on a collaborative basis, with pharmacy managing the majority of vancomycin treated patients. Night and weekend pharmacy staff provide cross-coverage on vancomycin consultations. Laboratory orders and medication dosage adjustments fall within the CPS scope of practice. Nurses do not perform laboratory draws for therapeutic drug monitoring; this is done solely by phlebotomists. There is no infectious diseases specialist at the facility to champion antibiotic dosing initiatives.
The implementation strategy largely reflected those outlined from tertiary care centers.5,6 First, key personnel from the laboratory department met to discuss this practice change and to add vancomycin peaks to the ordering menu. A critical value was set at 40 mcg/ml. Vancomycin troughs and random levels already were orderable items. A comment field was added to all laboratory orders for further clarification. Verbiage was added to laboratory reports in the computerized medical record to assist clinicians in determining the appropriateness of the level. This was followed by an educational email to both the nursing and laboratory departments explaining the practice change and included a link to the Pharmacy Joe “Vancomycin Dosing by AUC:MIC Instead of Trough-level” podcast (www.pharmacyjoe.com episode 356).
The pharmacy department received an interactive 30-minute presentation, followed immediately by a group activity to discuss practice problems. This presentation was condensed, recorded, and emailed to all VHSO pharmacists. A shared folder contained pertinent material on AUC monitoring.
Finally, an interactive presentation was set up for hospitalists and a video teleconferencing was conducted for rotating medical residents. Both the podcast and recorded presentation were emailed to the entire medical staff with a brief introduction of the practice change. Additionally, the transition process was added as a standing item on the monthly antimicrobial stewardship meeting agenda.
The standardized pharmacokinetic model at the study facility consisted of a vancomycin volume of distribution of 0.7 mg/kg and elimination rate constant (Ke) by Matzke and colleagues for total daily dose calculations.12 Obese patients (BMI ≥ 30) undergo alternative clearance equations described by Crass and colleagues.13 Cockcroft-Gault methods using ideal body weight (or actual body weight if < ideal body weight) are used for determining creatinine clearance. In patients aged ≥ 65 years with a serum creatinine < 1.0 mg/dL, facility guidance was to round serum creatinine up to 1.0 mg/dL. Loading doses were determined on a case-by-case basis with a cap of 2,000 mg, maintenance doses were rounded to the nearest 250 mg.
Vancomycin levels typically are drawn at steady state and analyzed using the logarithmic trapezoidal rule.14 The pharmacy and medical staff were educated to provide details on timing and coordination in nursing and laboratory orders (Table 1). Two-level AUC monitoring typically is not performed in patients with acute renal failure, expected duration of therapy < 72 hours, urinary tract infections, skin and soft tissue infections, or in renal replacement therapy.5
This gap analysis consisted of a retrospective chart review of vancomycin levels ordered after the implementation of AUC-based monitoring to determine the effectiveness of the transition. Three months of data were collected between April 2019 and June 2019. Vancomycin levels were deemed either appropriate or inappropriate based on timing and type (peak, trough, or random) of the laboratory test in relation to the previously administered vancomycin dose. Appropriate peaks were drawn within 2 hours after the end of infusion and troughs at least 1 half-life after the dose or just prior to the next dose and within the same dosing interval as the peak. Tests drawn outside of the specified time range, trough-only laboratory tests, or those drawn after vancomycin had been discontinued were considered inappropriate. Peaks and troughs drawn from separate dosing intervals also were considered inappropriate. Random levels were considered appropriate only if they fit the clinical context in acute renal failure or renal replacement therapy. An effective transition was defined as ≥ 80% of all vancomycin treated patients monitored with AUC methods rather than trough-based methods.
Inclusion criteria included all vancomycin levels ordered during the study period with no exclusions. The primary endpoint was the proportion of vancomycin levels drawn appropriately. Secondary endpoints were the proportion of AUC24 calculations within therapeutic range and a stratification of reasons for inappropriate levels. Descriptive statistics were collected to describe the scope of the project. Levels drawn from various shifts were compared (ie, day, night, or weekend). Calculated AUC24 levels between 400 and 600 mg.h/L were considered therapeutic unless treating CNS infection (600-700 mg.h/L). Given the operational outcomes (rather than clinical outcomes) and no comparator group, patient specific data were not collected.
Descriptive statistics without further analysis were used to describe proportions. The goal level for compliance was set at 100%. These methods were reviewed by the VHSO Institutional Review Board and granted nonresearch status, waiving the requirement for informed consent.
Results
The transition was effective with 97% of all cases utilizing AUC-based methods for monitoring. A total of 65 vancomycin levels were drawn in the study period; 32 peaks, 32 troughs, and 1 random level (drawn appropriately during acute renal failure 24 hours after starting therapy). All shifts were affected proportionately; days (n = 26, 40%), nights (n = 18, 27.7%), and weekends (n = 21, 32.3%). Based on time of dosage administration and laboratory test, there were 9 levels (13.8%) deemed inappropriate, 56 levels (86.1%) were appropriate. Reasons for inappropriate levels gleaned from chart review are presented in Table 2. Four levels had to be repeated for accurate calculations.
From the peak/trough couplets drawn appropriately, calculated AUC24 fell with the desired range in 61% (n = 17) of cases. Of the 11 that fell outside of range, 8 were subtherapeutic (< 400 mg.h/L) and 3 were supratherapeutic (> 600 mg.h/L). All levels were drawn at steady state. Indications for vancomycin monitoring were osteomyelitis (n = 13, 43%), sepsis (n = 10, 33%), pneumonia (n = 6, 20%), and 1 case of meningitis (3%).
Discussion
To the author’s knowledge, this is the first report of a vancomycin AUC24 monitoring conversion in a rural facility. This study adds to the existing medical literature in that it demonstrates that: (1) implementation methods described in large, tertiary centers can be effectively utilized in primary care, rural facilities; (2) the gap analysis used can be duplicated with minimal personnel and resources to ensure effective implementation (Table 3); and (3) the reported improvement needs can serve as a model for preventative measures at other facilities. The incidence of appropriate vancomycin levels was notably better than those reported in other single center studies.15-17 However, given variations in study design and facility operating procedures, it would be difficult to compare incidence among medical facilities. As such, there are no consensus benchmarks for comparison. The majority of inappropriate levels occurred early in the study period and on weekends. Appropriateness of drug levels may have improved with continued feedback and familiarity.
The calculated AUC24 fell within predicted range in 61% of cases. For comparison, a recent study from a large academic medical center reported that 73.5% of 2-level AUC24 cases had initial values within the therapeutic range.18 Of note, the target range used was much wider (400 - 800 mg.h/L) than the present study. Another study reported dose adjustments for subtherapeutic AUC levels in 25% of cases and dose reductions for supratherapeutic levels in 33.3% of cases.19
Of the AUC24 calculations that fell outside of therapeutic range, the majority (n = 8, 73%) were subtherapeutic (< 400 mg.h/L), half of these were for patients who were obese. It was unclear in the medical record which equation was used for initial dosing (Matzke vs Crass), or whether more conservative AUCs were used for calculating the total daily dose. The VHSO policy limiting loading doses also may have played a role; indeed the updated guidelines recommend a maximum loading dose of 3,000 mg depending on the severity of infection.4 Two of the 3 supratherapeutic levels were thought to be due to accumulation with long-term therapy.
Given such a large change from long-standing practices, there was surprisingly little resistance from the various clinical services. A recent survey of academic medical centers reported that the majority (88%) of all respondents who did not currently utilize AUC24 monitoring did not plan on making this immediate transition, largely citing unfamiliarity and training requirements.20 It is conceivable that the transition to AUC monitoring in smaller facilities may have fewer barriers than those seen in tertiary care centers. There are fewer health care providers and pharmacists to educate with the primary responsibilities falling on relatively few clinicians. There is little question as to who will be conducting follow up or whom to contact for questions. A smaller patient load and lesser patient acuity may translate to fewer vancomycin cases that require monitoring.
The interactive meetings were an important element for facility implementation. Research shows that emails alone are not effective for health care provider education, and interactive methods are recommended over passive methods.21,22 Assessing and avoiding barriers up front such as unclear laboratory orders, or communication failures is paramount to successful implementation strategies.23 Additionally, the detailed written ordering communication may have contributed to a smoother transition. The educational recording proved to be helpful in educating new staff and residents. An identified logistical error was that laboratory orders entered while patients were enrolled in sham clinics for electronic workload capture (eg, Pharmacy Inpatient Clinic) created confusion on the physical location of the patient for the phlebotomists, potentially causing delays in specimen collection.
A major development that stemmed from this intervention was that the Medical Service asked that policy changes be made so that the Pharmacy Service take over all vancomycin dosing at the facility. Previously, this had been done on a collaborative basis. Similar facilities with a collaborative practice model may need to anticipate such a request as this may present a new set of challenges. Accordingly, the pharmacy department is in the process of establishing standing operating procedures, pharmacist competencies, and a facility memorandum. Future research should evaluate the safety and efficacy of vancomycin therapy after the switch to AUC-based monitoring.
Limitations
There are several limitations to consider with this study. Operating procedures and implementation processes may vary between facilities, which could limit the generalizability of these results. Given the small facility size, the overall number of laboratory tests drawn was much smaller than those seen in larger facilities. The time needed for AUC calculations is notably longer than older methods of monitoring; however, this was not objectively assessed. It is important to note that clinical outcomes were beyond the scope of this gap analysis and this is an area of future research at the study facility. Vancomycin laboratory tests that were missed due to procedures and subsequently rescheduled were occasionally observed but not accounted for in this analysis. Additionally, vancomycin courses without monitoring (appropriate or otherwise) when indicated were not assessed. However, anecdotally speaking, this would be a very unlikely occurrence.
Conclusion
Conversion to AUC-based vancomycin monitoring is feasible in primary, rural medical centers. Implementation strategies from tertiary facilities can be successfully utilized in smaller hospitals. Quality assessment strategies such as a gap analysis can be utilized with minimal resources for facility uptake of new clinical practices.
1. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [published correction appears in Am J Health Syst Pharm. 2009;66(10):887]. Am J Health Syst Pharm. 2009;66(1):82‐98. doi:10.2146/ajhp080434
2. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734‐744. doi:10.1128/AAC.01568-12
3. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. doi:10.1016/j.addr.2014.05.016
4. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists [published online ahead of print, 2020 Mar 19]. Am J Health Syst Pharm. 2020;zxaa036. doi:10.1093/ajhp/zxaa036
5. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986‐1995. doi:10.2146/ajhp180034
6. Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned [published online ahead of print, 2019 Mar 10]. J Pharm Pract. 2019;897190019834369. doi:10.1177/0897190019834369
7. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8‐S14. doi:10.1093/cid/cir363
8. Goldman LE, Dudley RA. United States rural hospital quality in the Hospital Compare database-accounting for hospital characteristics. Health Policy. 2008;87(1):112‐127. doi:10.1016/j.healthpol.2008.02.002
9. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:10.1371/journal.pone.0078962
10. Institute for Healthcare Improvement. Plan-do-study-act (PDSA) worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed May 13, 2020.
11. Agency for Healthcare Research and Quality. Plan-do-study-act (PDSA) cycle. https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle. Updated April 10, 2013. Accessed May 13, 2020.
12. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433‐437. doi:10.1128/aac.25.4.433
13. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081‐3086. doi:10.1093/jac/dky310
14. Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162‐3167. doi:10.1128/AAC.02355-14
15. Suryadevara M, Steidl KE, Probst LA, Shaw J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17(2):159‐165. doi:10.5863/1551-6776-17.2.159
16. Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472‐478. doi:10.1309/AJCPDSYS0DVLKFOH
17. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801‐806. doi:10.1309/AJCPKQ6EAH7OYQLB
18. Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;39(4):433‐442. doi:10.1002/phar.2234
19. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant staphylococcus aureus Infection. J Pharm Pract. 2019;32(4):442‐446. doi:10.1177/0897190018764564
20. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889‐894. doi:10.1093/ajhp/zxz070
21. Bluestone J, Johnson P, Fullerton J, Carr C, Alderman J, BonTempo J. Effective in-service training design and delivery: evidence from an integrative literature review. Hum Resour Health. 2013;11:51. doi:10.1186/1478-4491-11-51
22. Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi:10.1136/bmjopen-2017-017572
23. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). 2016;4(3):36. doi:10.3390/healthcare4030036
1. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [published correction appears in Am J Health Syst Pharm. 2009;66(10):887]. Am J Health Syst Pharm. 2009;66(1):82‐98. doi:10.2146/ajhp080434
2. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734‐744. doi:10.1128/AAC.01568-12
3. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. doi:10.1016/j.addr.2014.05.016
4. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists [published online ahead of print, 2020 Mar 19]. Am J Health Syst Pharm. 2020;zxaa036. doi:10.1093/ajhp/zxaa036
5. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986‐1995. doi:10.2146/ajhp180034
6. Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned [published online ahead of print, 2019 Mar 10]. J Pharm Pract. 2019;897190019834369. doi:10.1177/0897190019834369
7. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8‐S14. doi:10.1093/cid/cir363
8. Goldman LE, Dudley RA. United States rural hospital quality in the Hospital Compare database-accounting for hospital characteristics. Health Policy. 2008;87(1):112‐127. doi:10.1016/j.healthpol.2008.02.002
9. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:10.1371/journal.pone.0078962
10. Institute for Healthcare Improvement. Plan-do-study-act (PDSA) worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed May 13, 2020.
11. Agency for Healthcare Research and Quality. Plan-do-study-act (PDSA) cycle. https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle. Updated April 10, 2013. Accessed May 13, 2020.
12. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433‐437. doi:10.1128/aac.25.4.433
13. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081‐3086. doi:10.1093/jac/dky310
14. Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162‐3167. doi:10.1128/AAC.02355-14
15. Suryadevara M, Steidl KE, Probst LA, Shaw J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17(2):159‐165. doi:10.5863/1551-6776-17.2.159
16. Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472‐478. doi:10.1309/AJCPDSYS0DVLKFOH
17. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801‐806. doi:10.1309/AJCPKQ6EAH7OYQLB
18. Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;39(4):433‐442. doi:10.1002/phar.2234
19. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant staphylococcus aureus Infection. J Pharm Pract. 2019;32(4):442‐446. doi:10.1177/0897190018764564
20. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889‐894. doi:10.1093/ajhp/zxz070
21. Bluestone J, Johnson P, Fullerton J, Carr C, Alderman J, BonTempo J. Effective in-service training design and delivery: evidence from an integrative literature review. Hum Resour Health. 2013;11:51. doi:10.1186/1478-4491-11-51
22. Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi:10.1136/bmjopen-2017-017572
23. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). 2016;4(3):36. doi:10.3390/healthcare4030036
Open Clinical Trials for Patients With COVID-19
Finding effective treatment or a vaccine for COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has placed significant strains on the global health care system. The National Library of Medicine database lists > 1,800 trials that are aimed at addressing COVID-19-related health care. Already, trials developed by the US Department of Veterans Affairs (VA), US Department of Defense (DoD), and the National Institute of Allergy and Infectious Diseases have provided important data on effective treatment options. The clinical trials listed below are all open as of May 31, 2020 and have trial sites at VA and DoD facilities. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Adaptive COVID-19 Treatment Trial (ACTT)
This study is an adaptive, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of novel therapeutic agents in hospitalized adults diagnosed with COVID-19. The study will compare different investigational therapeutic agents to a control arm. ID: NCT04280705
Sponsor: National Institute of Allergy and Infectious Diseases
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19)
The primary objective of this study is to evaluate the efficacy of 2 remdesivir (RDV) regimens with respect to clinical status assessed by a 7-point ordinal scale on Day 11 (NCT04292730) or Day 14 (NCT04292899).
ID: NCT04292730/NCT04292899
Sponsor: Gilead Sciences
Contact: Gilead Clinical Study Information Center (833-445-3230)
Location: James J. Peters VA Medical Center, Bronx, New York
Expanded Access Remdesivir (RDV; GS-5734)
The treatment of communicable Novel Coronavirus of 2019 with Remdesivir (RDV; GS-5734) also known as severe acute respiratory syndrome coronavirus 2.
ID: NCT04302766
Sponsor: US Army Medical Research and Development Command
Contact: Sandi Parriott ([email protected])
A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA)
This study will evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of tocilizumab (TCZ) compared with a matching placebo in combination with standard of care (SOC) in hospitalized patients with severe COVID-19 pneumonia.
ID: NCT04320615
Sponsor: Hoffmann-La Roche
Location: James J Peters VA Medical Center, Bronx, New York
Administration of Intravenous Vitamin C in Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation (AVoCaDO)
Previous research has shown that high dose intravenous vitamin C (HDIVC) may benefit patients with sepsis, acute lung injury (ALI), and the acute respiratory distress syndrome (ARDS). However, it is not known if early administration of HDIVC could prevent progression to ARDS. We hypothesize that HDIVC is safe and tolerable in COVID-19 subjects given early or late in the disease course and may reduce the risk of respiratory failure requiring mechanical ventilation and development of ARDS along with reductions in supplemental oxygen demand and inflammatory markers.
ID: NCT04357782
Sponsor: Hunter Holmes Mcguire VA Medical CenterContact: Brian Davis ([email protected])
Location: Hunter Holmes Mcguire VA Medical Center, Richmond, Virginia
Treatment Of CORONAVIRUS DISEASE 2019 (COVID-19) With Anti-Sars-CoV-2 Convalescent Plasma (ASCoV2CP)
This is an expanded access open-label, single-arm, multi-site protocol to provide convalescent plasma as a treatment for patients diagnosed with severe, or life-threatening COVID-19.
ID: NCT04360486
Sponsor: US Army Medical Research and Development Command
Contact: Andrew Cap ([email protected])
VA Remote and Equitable Access to COVID-19 Healthcare Delivery (VA-REACH TRIAL) (VA-REACH)
We propose a 3-arm randomized control trial to determine the efficacy of hydroxychloroquine or azithromycin in treating mild to moderate COVID-19 among veterans in the outpatient setting.
ID: NCT04363203
Sponsor: Salomeh Keyhani
Location: San Francisco VA Health Care System, California
A Study to Evaluate the Safety and Efficacy of MSTT1041A (Astegolimab) or UTTR1147A in Patients With Severe COVID-19 Pneumonia (COVASTIL)
This is a Phase II, randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of MSTT1041A (astegolimab) or UTTR1147A in combination with standard of care (SOC) compared with matching placebo in combination with SOC in patients hospitalized with severe coronavirus disease 2019 (COVID-19) pneumonia.
ID: NCT04386616
Sponsor: Genentech
Contact: Study ID Number: GA42469 ([email protected])
Location: Southeast Louisiana Veterans Health Care System, New Orleans
Hormonal Intervention for the Treatment in Veterans With COVID-19 Requiring Hospitalization (HITCH)
The purpose of this study is to determine if temporary androgen suppression improves the clinical outcomes of veterans who are hospitalized to an acute care ward due to COVID-19.ID: NCT04397718
Sponsor: VA Office of Research and Development
Contact: Matthew B Rettig ([email protected]), Nicholas Nickols ([email protected])
Locations: VA Greater Los Angeles Healthcare System, California; VA NY Harbor Healthcare System, New York; VA Puget Sound Health Care System, Seattle, Washington
Adaptive COVID-19 Treatment Trial 2 (ACTT-II)
ACTT-II will evaluate the combination of baricitinib and remdesivir compared to remdesivir alone. Subjects will be assessed daily while hospitalized. If the subjects are discharged from the hospital, they will have a study visit at Days 15, 22, and 29.
ID: NCT04401579
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Rocky Mountain Regional Veteran Affairs Medical Center, Aurora, Colorado; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Finding effective treatment or a vaccine for COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has placed significant strains on the global health care system. The National Library of Medicine database lists > 1,800 trials that are aimed at addressing COVID-19-related health care. Already, trials developed by the US Department of Veterans Affairs (VA), US Department of Defense (DoD), and the National Institute of Allergy and Infectious Diseases have provided important data on effective treatment options. The clinical trials listed below are all open as of May 31, 2020 and have trial sites at VA and DoD facilities. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Adaptive COVID-19 Treatment Trial (ACTT)
This study is an adaptive, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of novel therapeutic agents in hospitalized adults diagnosed with COVID-19. The study will compare different investigational therapeutic agents to a control arm. ID: NCT04280705
Sponsor: National Institute of Allergy and Infectious Diseases
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19)
The primary objective of this study is to evaluate the efficacy of 2 remdesivir (RDV) regimens with respect to clinical status assessed by a 7-point ordinal scale on Day 11 (NCT04292730) or Day 14 (NCT04292899).
ID: NCT04292730/NCT04292899
Sponsor: Gilead Sciences
Contact: Gilead Clinical Study Information Center (833-445-3230)
Location: James J. Peters VA Medical Center, Bronx, New York
Expanded Access Remdesivir (RDV; GS-5734)
The treatment of communicable Novel Coronavirus of 2019 with Remdesivir (RDV; GS-5734) also known as severe acute respiratory syndrome coronavirus 2.
ID: NCT04302766
Sponsor: US Army Medical Research and Development Command
Contact: Sandi Parriott ([email protected])
A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA)
This study will evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of tocilizumab (TCZ) compared with a matching placebo in combination with standard of care (SOC) in hospitalized patients with severe COVID-19 pneumonia.
ID: NCT04320615
Sponsor: Hoffmann-La Roche
Location: James J Peters VA Medical Center, Bronx, New York
Administration of Intravenous Vitamin C in Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation (AVoCaDO)
Previous research has shown that high dose intravenous vitamin C (HDIVC) may benefit patients with sepsis, acute lung injury (ALI), and the acute respiratory distress syndrome (ARDS). However, it is not known if early administration of HDIVC could prevent progression to ARDS. We hypothesize that HDIVC is safe and tolerable in COVID-19 subjects given early or late in the disease course and may reduce the risk of respiratory failure requiring mechanical ventilation and development of ARDS along with reductions in supplemental oxygen demand and inflammatory markers.
ID: NCT04357782
Sponsor: Hunter Holmes Mcguire VA Medical CenterContact: Brian Davis ([email protected])
Location: Hunter Holmes Mcguire VA Medical Center, Richmond, Virginia
Treatment Of CORONAVIRUS DISEASE 2019 (COVID-19) With Anti-Sars-CoV-2 Convalescent Plasma (ASCoV2CP)
This is an expanded access open-label, single-arm, multi-site protocol to provide convalescent plasma as a treatment for patients diagnosed with severe, or life-threatening COVID-19.
ID: NCT04360486
Sponsor: US Army Medical Research and Development Command
Contact: Andrew Cap ([email protected])
VA Remote and Equitable Access to COVID-19 Healthcare Delivery (VA-REACH TRIAL) (VA-REACH)
We propose a 3-arm randomized control trial to determine the efficacy of hydroxychloroquine or azithromycin in treating mild to moderate COVID-19 among veterans in the outpatient setting.
ID: NCT04363203
Sponsor: Salomeh Keyhani
Location: San Francisco VA Health Care System, California
A Study to Evaluate the Safety and Efficacy of MSTT1041A (Astegolimab) or UTTR1147A in Patients With Severe COVID-19 Pneumonia (COVASTIL)
This is a Phase II, randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of MSTT1041A (astegolimab) or UTTR1147A in combination with standard of care (SOC) compared with matching placebo in combination with SOC in patients hospitalized with severe coronavirus disease 2019 (COVID-19) pneumonia.
ID: NCT04386616
Sponsor: Genentech
Contact: Study ID Number: GA42469 ([email protected])
Location: Southeast Louisiana Veterans Health Care System, New Orleans
Hormonal Intervention for the Treatment in Veterans With COVID-19 Requiring Hospitalization (HITCH)
The purpose of this study is to determine if temporary androgen suppression improves the clinical outcomes of veterans who are hospitalized to an acute care ward due to COVID-19.ID: NCT04397718
Sponsor: VA Office of Research and Development
Contact: Matthew B Rettig ([email protected]), Nicholas Nickols ([email protected])
Locations: VA Greater Los Angeles Healthcare System, California; VA NY Harbor Healthcare System, New York; VA Puget Sound Health Care System, Seattle, Washington
Adaptive COVID-19 Treatment Trial 2 (ACTT-II)
ACTT-II will evaluate the combination of baricitinib and remdesivir compared to remdesivir alone. Subjects will be assessed daily while hospitalized. If the subjects are discharged from the hospital, they will have a study visit at Days 15, 22, and 29.
ID: NCT04401579
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Rocky Mountain Regional Veteran Affairs Medical Center, Aurora, Colorado; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Finding effective treatment or a vaccine for COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has placed significant strains on the global health care system. The National Library of Medicine database lists > 1,800 trials that are aimed at addressing COVID-19-related health care. Already, trials developed by the US Department of Veterans Affairs (VA), US Department of Defense (DoD), and the National Institute of Allergy and Infectious Diseases have provided important data on effective treatment options. The clinical trials listed below are all open as of May 31, 2020 and have trial sites at VA and DoD facilities. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Adaptive COVID-19 Treatment Trial (ACTT)
This study is an adaptive, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of novel therapeutic agents in hospitalized adults diagnosed with COVID-19. The study will compare different investigational therapeutic agents to a control arm. ID: NCT04280705
Sponsor: National Institute of Allergy and Infectious Diseases
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19)
The primary objective of this study is to evaluate the efficacy of 2 remdesivir (RDV) regimens with respect to clinical status assessed by a 7-point ordinal scale on Day 11 (NCT04292730) or Day 14 (NCT04292899).
ID: NCT04292730/NCT04292899
Sponsor: Gilead Sciences
Contact: Gilead Clinical Study Information Center (833-445-3230)
Location: James J. Peters VA Medical Center, Bronx, New York
Expanded Access Remdesivir (RDV; GS-5734)
The treatment of communicable Novel Coronavirus of 2019 with Remdesivir (RDV; GS-5734) also known as severe acute respiratory syndrome coronavirus 2.
ID: NCT04302766
Sponsor: US Army Medical Research and Development Command
Contact: Sandi Parriott ([email protected])
A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA)
This study will evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of tocilizumab (TCZ) compared with a matching placebo in combination with standard of care (SOC) in hospitalized patients with severe COVID-19 pneumonia.
ID: NCT04320615
Sponsor: Hoffmann-La Roche
Location: James J Peters VA Medical Center, Bronx, New York
Administration of Intravenous Vitamin C in Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation (AVoCaDO)
Previous research has shown that high dose intravenous vitamin C (HDIVC) may benefit patients with sepsis, acute lung injury (ALI), and the acute respiratory distress syndrome (ARDS). However, it is not known if early administration of HDIVC could prevent progression to ARDS. We hypothesize that HDIVC is safe and tolerable in COVID-19 subjects given early or late in the disease course and may reduce the risk of respiratory failure requiring mechanical ventilation and development of ARDS along with reductions in supplemental oxygen demand and inflammatory markers.
ID: NCT04357782
Sponsor: Hunter Holmes Mcguire VA Medical CenterContact: Brian Davis ([email protected])
Location: Hunter Holmes Mcguire VA Medical Center, Richmond, Virginia
Treatment Of CORONAVIRUS DISEASE 2019 (COVID-19) With Anti-Sars-CoV-2 Convalescent Plasma (ASCoV2CP)
This is an expanded access open-label, single-arm, multi-site protocol to provide convalescent plasma as a treatment for patients diagnosed with severe, or life-threatening COVID-19.
ID: NCT04360486
Sponsor: US Army Medical Research and Development Command
Contact: Andrew Cap ([email protected])
VA Remote and Equitable Access to COVID-19 Healthcare Delivery (VA-REACH TRIAL) (VA-REACH)
We propose a 3-arm randomized control trial to determine the efficacy of hydroxychloroquine or azithromycin in treating mild to moderate COVID-19 among veterans in the outpatient setting.
ID: NCT04363203
Sponsor: Salomeh Keyhani
Location: San Francisco VA Health Care System, California
A Study to Evaluate the Safety and Efficacy of MSTT1041A (Astegolimab) or UTTR1147A in Patients With Severe COVID-19 Pneumonia (COVASTIL)
This is a Phase II, randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of MSTT1041A (astegolimab) or UTTR1147A in combination with standard of care (SOC) compared with matching placebo in combination with SOC in patients hospitalized with severe coronavirus disease 2019 (COVID-19) pneumonia.
ID: NCT04386616
Sponsor: Genentech
Contact: Study ID Number: GA42469 ([email protected])
Location: Southeast Louisiana Veterans Health Care System, New Orleans
Hormonal Intervention for the Treatment in Veterans With COVID-19 Requiring Hospitalization (HITCH)
The purpose of this study is to determine if temporary androgen suppression improves the clinical outcomes of veterans who are hospitalized to an acute care ward due to COVID-19.ID: NCT04397718
Sponsor: VA Office of Research and Development
Contact: Matthew B Rettig ([email protected]), Nicholas Nickols ([email protected])
Locations: VA Greater Los Angeles Healthcare System, California; VA NY Harbor Healthcare System, New York; VA Puget Sound Health Care System, Seattle, Washington
Adaptive COVID-19 Treatment Trial 2 (ACTT-II)
ACTT-II will evaluate the combination of baricitinib and remdesivir compared to remdesivir alone. Subjects will be assessed daily while hospitalized. If the subjects are discharged from the hospital, they will have a study visit at Days 15, 22, and 29.
ID: NCT04401579
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Contact: Central Contact ([email protected])
Locations: VA Palo Alto Health Care System, California; Naval Medical Center San Diego, California; Rocky Mountain Regional Veteran Affairs Medical Center, Aurora, Colorado; Southeast Louisiana Veterans Health Care System, New Orleans; Walter Reed National Military Medical Center, Bethesda, Maryland; National Institutes of Health - Clinical Center, National Institute of Allergy and Infectious Diseases Laboratory Of Immunoregulation, Bethesda, Maryland; Brooke Army Medical Center, Fort Sam Houston, Texas; Madigan Army Medical Center, Tacoma, Washington
Preliminary evidence indicates famotidine might improve COVID-19 symptoms
High-dose oral famotidine might improve cardinal symptoms of COVID-19 infection, according to the findings of a small outpatient case series and a subsequent retrospective study.
After developing COVID-19 symptoms, the 10 patients in the case series began self-medicating with 60-240 mg famotidine daily over a median of 11 days. “All patients reported marked improvements of disease-related symptoms after starting famotidine,” first author Tobias Janowitz, MD, PhD, of Cold Spring Harbor Laboratory, N.Y., and associates wrote in Gut.
Improvements began within 24-48 hours of starting on the histamine-2 receptor antagonist. By 14 days after treatment initiation, all patients reported near-normalization of both respiratory and systemic symptoms, the researchers reported.
The patients were 23-71 years old. Seven tested positive for COVID-19, two had antibodies to COVID-19, and one had a clinical diagnosis of COVID-19 without laboratory confirmation. Over a median of 11 days (range, 5-21 days), six patients self-administered 80 mg famotidine three times daily and four self-administered lower amounts – from 60 to 150 mg of famotidine daily, divided into two or three doses. Patients started on famotidine between 2 and 26 days after symptom onset.
Through phone interviews and questionnaires, the researchers ascertained changes in cough, dyspnea, fatigue, headache, anosmia, and general unwellness by using a modified four-point Eastern Cooperative Oncology Group (ECOG) performance status scale. Improvements were seen across all symptom categories, and respiratory symptoms improved faster than systemic symptoms. Apart from two cases of persistent anosmia, symptoms resolved completely within 14 days of starting famotidine.
Seven patients reported no side effects of famotidine; one reported grade 1 dizziness and infrequent perceptions of tachycardia; one reported grade 1 dizziness, dry skin, and insomnia; and one reported grade 1 gastrointestinal symptoms and temporary forgetfulness. “Other than forgetfulness, all of these side effects are listed in the prescription information for famotidine, and all side effects resolved on discontinuation of famotidine,” the investigators wrote.
While the findings are intriguing, Dr. Janowitz and associates cautioned against overinterpretation of them. Another expert agreed: “This is a preliminary study based on a hypothesized antiviral effect. It’s important to know that it doesn’t really prove it works,” said Amesh Adalja, MD, senior scholar at the Johns Hopkins University Center for Health Security, Baltimore, and a spokesperson for the Infectious Diseases Society of America, during an interview with MDedge.
These patients might have improved anyway, without self-administering famotidine, said Dr. Adalja, who was not involved in the study.
Furthermore, the mechanism by which famotidine might act on COVID-19 remains unclear. The drug “could have a viral target, for example, one of the viral proteases, or a host target, resulting, for example, in modulation of the immunological response to the virus,” Dr. Janowitz and associates wrote.
Dr. Adalja noted that many compounds show effects against COVID-19 that are not well understood. He called for randomized trials to evaluate the biological plausibility of famotidine use, and its potential efficacy.
“This is a cheap, over-the-counter drug, but no drug is without side effects,” he added. “We need to know whether it works.”
Based on the case series findings, researchers conducted another retrospective study of patients hospitalized with COVID-19 infection. Those who were incidentally taking famotidine before or at hospitalization had a significantly reduced risk of intubation or death, with a hazard ratio of 0.43 (Gastroenterology. 2020 May 22. doi: 10.1053/j.gastro.2020.05.053)
The National Institutes of Health provided partial support. The investigators reported having no conflicts of interest.
SOURCE: Janowitz T et al. Gut. 2020 Jun 4. doi: 10.1136/gutjnl-2020-321852.
High-dose oral famotidine might improve cardinal symptoms of COVID-19 infection, according to the findings of a small outpatient case series and a subsequent retrospective study.
After developing COVID-19 symptoms, the 10 patients in the case series began self-medicating with 60-240 mg famotidine daily over a median of 11 days. “All patients reported marked improvements of disease-related symptoms after starting famotidine,” first author Tobias Janowitz, MD, PhD, of Cold Spring Harbor Laboratory, N.Y., and associates wrote in Gut.
Improvements began within 24-48 hours of starting on the histamine-2 receptor antagonist. By 14 days after treatment initiation, all patients reported near-normalization of both respiratory and systemic symptoms, the researchers reported.
The patients were 23-71 years old. Seven tested positive for COVID-19, two had antibodies to COVID-19, and one had a clinical diagnosis of COVID-19 without laboratory confirmation. Over a median of 11 days (range, 5-21 days), six patients self-administered 80 mg famotidine three times daily and four self-administered lower amounts – from 60 to 150 mg of famotidine daily, divided into two or three doses. Patients started on famotidine between 2 and 26 days after symptom onset.
Through phone interviews and questionnaires, the researchers ascertained changes in cough, dyspnea, fatigue, headache, anosmia, and general unwellness by using a modified four-point Eastern Cooperative Oncology Group (ECOG) performance status scale. Improvements were seen across all symptom categories, and respiratory symptoms improved faster than systemic symptoms. Apart from two cases of persistent anosmia, symptoms resolved completely within 14 days of starting famotidine.
Seven patients reported no side effects of famotidine; one reported grade 1 dizziness and infrequent perceptions of tachycardia; one reported grade 1 dizziness, dry skin, and insomnia; and one reported grade 1 gastrointestinal symptoms and temporary forgetfulness. “Other than forgetfulness, all of these side effects are listed in the prescription information for famotidine, and all side effects resolved on discontinuation of famotidine,” the investigators wrote.
While the findings are intriguing, Dr. Janowitz and associates cautioned against overinterpretation of them. Another expert agreed: “This is a preliminary study based on a hypothesized antiviral effect. It’s important to know that it doesn’t really prove it works,” said Amesh Adalja, MD, senior scholar at the Johns Hopkins University Center for Health Security, Baltimore, and a spokesperson for the Infectious Diseases Society of America, during an interview with MDedge.
These patients might have improved anyway, without self-administering famotidine, said Dr. Adalja, who was not involved in the study.
Furthermore, the mechanism by which famotidine might act on COVID-19 remains unclear. The drug “could have a viral target, for example, one of the viral proteases, or a host target, resulting, for example, in modulation of the immunological response to the virus,” Dr. Janowitz and associates wrote.
Dr. Adalja noted that many compounds show effects against COVID-19 that are not well understood. He called for randomized trials to evaluate the biological plausibility of famotidine use, and its potential efficacy.
“This is a cheap, over-the-counter drug, but no drug is without side effects,” he added. “We need to know whether it works.”
Based on the case series findings, researchers conducted another retrospective study of patients hospitalized with COVID-19 infection. Those who were incidentally taking famotidine before or at hospitalization had a significantly reduced risk of intubation or death, with a hazard ratio of 0.43 (Gastroenterology. 2020 May 22. doi: 10.1053/j.gastro.2020.05.053)
The National Institutes of Health provided partial support. The investigators reported having no conflicts of interest.
SOURCE: Janowitz T et al. Gut. 2020 Jun 4. doi: 10.1136/gutjnl-2020-321852.
High-dose oral famotidine might improve cardinal symptoms of COVID-19 infection, according to the findings of a small outpatient case series and a subsequent retrospective study.
After developing COVID-19 symptoms, the 10 patients in the case series began self-medicating with 60-240 mg famotidine daily over a median of 11 days. “All patients reported marked improvements of disease-related symptoms after starting famotidine,” first author Tobias Janowitz, MD, PhD, of Cold Spring Harbor Laboratory, N.Y., and associates wrote in Gut.
Improvements began within 24-48 hours of starting on the histamine-2 receptor antagonist. By 14 days after treatment initiation, all patients reported near-normalization of both respiratory and systemic symptoms, the researchers reported.
The patients were 23-71 years old. Seven tested positive for COVID-19, two had antibodies to COVID-19, and one had a clinical diagnosis of COVID-19 without laboratory confirmation. Over a median of 11 days (range, 5-21 days), six patients self-administered 80 mg famotidine three times daily and four self-administered lower amounts – from 60 to 150 mg of famotidine daily, divided into two or three doses. Patients started on famotidine between 2 and 26 days after symptom onset.
Through phone interviews and questionnaires, the researchers ascertained changes in cough, dyspnea, fatigue, headache, anosmia, and general unwellness by using a modified four-point Eastern Cooperative Oncology Group (ECOG) performance status scale. Improvements were seen across all symptom categories, and respiratory symptoms improved faster than systemic symptoms. Apart from two cases of persistent anosmia, symptoms resolved completely within 14 days of starting famotidine.
Seven patients reported no side effects of famotidine; one reported grade 1 dizziness and infrequent perceptions of tachycardia; one reported grade 1 dizziness, dry skin, and insomnia; and one reported grade 1 gastrointestinal symptoms and temporary forgetfulness. “Other than forgetfulness, all of these side effects are listed in the prescription information for famotidine, and all side effects resolved on discontinuation of famotidine,” the investigators wrote.
While the findings are intriguing, Dr. Janowitz and associates cautioned against overinterpretation of them. Another expert agreed: “This is a preliminary study based on a hypothesized antiviral effect. It’s important to know that it doesn’t really prove it works,” said Amesh Adalja, MD, senior scholar at the Johns Hopkins University Center for Health Security, Baltimore, and a spokesperson for the Infectious Diseases Society of America, during an interview with MDedge.
These patients might have improved anyway, without self-administering famotidine, said Dr. Adalja, who was not involved in the study.
Furthermore, the mechanism by which famotidine might act on COVID-19 remains unclear. The drug “could have a viral target, for example, one of the viral proteases, or a host target, resulting, for example, in modulation of the immunological response to the virus,” Dr. Janowitz and associates wrote.
Dr. Adalja noted that many compounds show effects against COVID-19 that are not well understood. He called for randomized trials to evaluate the biological plausibility of famotidine use, and its potential efficacy.
“This is a cheap, over-the-counter drug, but no drug is without side effects,” he added. “We need to know whether it works.”
Based on the case series findings, researchers conducted another retrospective study of patients hospitalized with COVID-19 infection. Those who were incidentally taking famotidine before or at hospitalization had a significantly reduced risk of intubation or death, with a hazard ratio of 0.43 (Gastroenterology. 2020 May 22. doi: 10.1053/j.gastro.2020.05.053)
The National Institutes of Health provided partial support. The investigators reported having no conflicts of interest.
SOURCE: Janowitz T et al. Gut. 2020 Jun 4. doi: 10.1136/gutjnl-2020-321852.
FROM GUT
Can an app guide cancer treatment decisions during the pandemic?
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.