User login
Empiric Therapies for COVID-19: Destined to Fail by Ignoring the Lessons of History
Manifestations of disease, as perceived by physicians, can shape conceptual views and favor specific therapeutic actions. Historically, these factors appear to have an outsized influence on medical thinking in general. Disease concepts derived from empirical observations during pandemics impose a trade-off. We obtain unparalleled insight into medical thought and practice, but risk incurring the cost of unfortunate mistakes. The psychologist and Nobel Prize winner in economics Daniel Kahneman describes two mental systems that shape our judgments and decision-making in his book, Thinking, Fast and Slow: System One is intuitive, emotional, and fast, whereas System Two is deliberative and logical and has slower onset.1 If we extrapolate these observations to clinical medicine, we often rely on either System One or System Two depending on particular situations. Errors can emerge when we default to fast and emotional responses in situations that instead require more deliberate and logical assessments. These include instances in which the desire to help—our humanitarian role as physicians, associated with an “adrenaline rush”—results from attempts to relieve human suffering. As mercenaries of misfortune, it is inevitable we engage medical interventions based on an incomplete understanding of the pathophysiology—in other words, without understanding the full risks and benefits.
During the ongoing COVID-19 pandemic, members of the medical community continue to provide care with the utmost nobility, empathy, and desire for action amid uncertainty. However, as the number of cases continues to increase worldwide, we urge caution in evaluating the current state of scientific understanding, our approaches to treatment, and the safety of empiric medical interventions targeting COVID-19. We are concerned that the extensive history of unintended adverse consequences of therapies for emerging infectious diseases in the past is being ignored in the development of approaches to COVID-19 treatment. It is likely harms will emerge from current empiric therapies for COVID-19 given what can be learned from history.
HISTORICAL EXAMPLES OF UNINTENDED ADVERSE CONSEQUENCES
Whereas influenza can be treated with neuraminidase inhibitors,2 there are currently no established effective antiviral therapies for COVID-19, which is similar to two other coronavirus diseases from the 21st century, SARS (Severe Acute Respiratory Syndrome) in 2003 and MERS (Middle-Eastern Respiratory Syndrome) in 2012.3 Even in times of global pandemic, we need to consider potential harms and adverse consequences of novel treatments and show justifiable ratio of risk versus benefit. With the absence of proven COVID-19 therapy and the desire to fulfill our oath of primum non nocere (first, do no harm) in mind, we review selected unintended adverse events of developing therapies for infectious diseases.
Two types of error in our decision-making strategies are errors of omission and errors of commission.4 Errors of omission, defined as instances in which a medical intervention was not carried out when there was a clear indication to do so, are less conspicuous in the history of infectious disease therapeutics. Errors of commission, in contrast, have become a more concerning component of our approach to COVID-19 therapy, perhaps prompted by our desire to act. Errors of commission are defined as instances in which a specific medical intervention that should have been avoided was instead performed. We will discuss historical examples of errors of commission to highlight parallels with the current pandemic (Appendix Figure).
During influenza epidemics in the 18th century, some physicians advocated the use of therapeutic lancet phlebotomies, while others recommended indiscriminate use of opium, which led to high rates of addiction.5 Neither intervention was supported by a reassuring body of evidence. Many recommended mercury-based preparations during major outbreaks of syphilis in medieval protestant Europe. Because of accumulated mercurial toxicity, many persons suffered long-term sequelae including chronic kidney injury and peripheral neuropathy.6 After the discovery of the tuberculous bacillus, Robert Koch attempted the inoculation of tuberculin as a curative intervention for tuberculosis.7 Under pressure from the king of Prussia to present his findings at the International Medical Meeting in Berlin, Germany, in 1890, Koch conducted a poorly executed clinical trial. Rudolf Virchow then demonstrated endobronchial spread of the infection with resultant clinical worsening in those who received Koch’s tuberculin. In 1905, Harold Wolfersan Thomas at the Liverpool School of Tropical Medicine treated cases of African trypanosomiasis with the arsenical drug Atoxyl (arsanilic acid), which demonstrated some efficacy but also caused optic nerve atrophy leading to blindness.8
There have also been errors of commission in the development of vaccines. One such event, known as the Cutter incident, followed from an incompletely inactivated batch of polio vaccine that caused 40,000 cases of abortive poliomyelitis and many cases of paralysis and death.9 In the early phases of the development of the yellow fever vaccine, Hideyo Noguchi tried to develop a vaccine based on the erroneous assumption that yellow fever was caused by Leptospira icteroides.10 In 1976, an error of commission occurred in response to an outbreak of a few dozen cases of Influenza A/H1N1 in Fort Dix, New Jersey: The accelerated implementation of a swine influenza–vaccination program led to many cases of Guillian-Barré Syndrome among recipients.11 Immunization experts defended this decision to vaccinate by arguing that “when lives are at risk, it’s better to err on the side of overreaction over underreaction.”11 However, this is a risk-perception versus risk-management concept that drives potential errors of commission.
A more recent error of commission involved the use of drotrecogin alfa (activated protein C) in the treatment of sepsis. This drug became the first and only Food and Drug Administration–approved drug for sepsis treatment. The approval process of this medication relied on one clinical trial, which was terminated early because of perceived overwhelming evidence of efficacy. Despite the initial high medical and financial expectations, Eli Lilly (Indianapolis) withdrew the drug when a larger, international clinical trial (PROWESS-SHOCK) did not show a similar benefit.12
THE COVID-19 ERA
The gravity of the COVID-19 pandemic has motivated the repurposing of previously available therapies. This explains the use of medications like hydroxychloroquine, interleukin-6 (IL-6) receptor antagonists, and remdesivir.13-15
Despite early authorization of emergency use for hydroxychloroquine by the FDA based on limited and poor-quality evidence,16 this drug has yet to demonstrate treatment efficacy for COVID-19. On the contrary, other, controlled, retrospective studies have shown that hydroxychloroquine might actually increase mortality, possibly through prolongation of the QT-interval.16,17 Also, diversion of this drug to treat COVID-19 raises the concern of hydroxychloroquine shortages for treatment of patients with autoimmune disease, in whom the drug has proven benefit. We question the hasty FDA authorization for emergency use of hydroxychloroquine for COVID-19.
There is also great enthusiasm among the medical community to administer IL-6 receptor antagonists as a COVID-19 treatment. The rationale for this approach includes observations in case series in which IL-6 levels correlated with adverse clinical outcomes.13 IL-6 antagonists have a proven track record of improving the outcome in autoimmune diseases. However, we must avoid the logical trap of post hoc, ergo propter hoc (after this, therefore because of this) dictum from which one would assume that, based on those observations of high IL-6 levels and adverse outcomes, lowering IL-6 levels will necessarily improve outcomes in COVID-19. The supposed role of IL-6 in causing COVID-19 is based on scant preliminary observations and on the yet unproven assumption that IL-6 association with disease severity is a cause-effect relationship and not an association separate from pathogenesis. Moreover, there is sufficient scientific evidence that, in the case of severe influenza infections, IL-6 limits inflammation and protects against severe and potentially life-threatening lung injury. The road ahead for IL-6 inhibition to treat COVID-19 is perilous and should be entered cautiously. One immediate concern of administering IL-6 receptor antagonists in this patient population is the potential reactivation of latent tuberculosis infection and hepatitis B, colonic perforation, and increased rate of infections in general.
The greatest hope at this early stage of the COVID-19 pandemic may be remdesivir, which is a direct-acting antiviral. Here again, initial case series in prestigious medical journals signaled the possibility of a morbidity and mortality benefit.14 Despite these encouraging signs, a recent clinical trial from China that was limited by incomplete patient enrollment demonstrated a lack of efficacy of remdesivir in accelerating clinical improvement or limiting mortality.18 In spite of these negative results, preliminary data from the Adaptive COVID-19 Treatment Trial (ACTT) has revealed a nonsignificant signal of reduced mortality and shorter time to recovery in the remdesivir group. In response to these reports, the FDA has now issued emergency use authorization of remdesivir for treating COVID-19. Given the precedence of conflicting study data in therapeutic development for infectious diseases, we urge caution in drawing interpretations of benefit based on these early reports. Early termination of clinical studies is often associated with a 30% overestimation of clinical benefit.19 Furthermore, the availability of remdesivir is limited, which raises substantial ethical concerns on the preferential allocation of the drug to selected populations in high-income countries. At the time of this report, uncertainty regarding the risk-benefit balance of remdesivir and other COVID-19 treatments should be emphasized among decision makers.
CONCLUSION
Errors of commission present particular concerns for risk in treating COVID-19 patients and suggest that sometimes inaction is preferable to action. With many pandemics, there is a history of repeating mistakes, and we believe this can be curtailed by heeding the lessons of history. In the end, we may learn that avoiding therapeutic interventions that are poorly supported may prove to be one of the most important legacies of the COVID-19 pandemic.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Shapiro is supported by The Emily Foundation, Boston, Massachusetts. For all other authors, no financial support was declared.
1. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
2. Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017;72(6):1556-1573. https://doi.org/10.1093/jac/dkx013.
3. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. https://doi.org/10.1038/nrmicro.2016.81.
4. Grober ED, Bohnen JM. Defining medical error. Can J Surg. 2005;48(1):39-44.
5. Schofield AT. Opium in influenza. Lancet. 1894;143(3676):372. https://doi.org/10.1016/S0140-6736(01)66349-9.
6. Abraham JJ. Some account of the history of the treatment of syphilis. Br J Vener Dis. 1948;24(4):153-161. https://doi.org/10.1136/sti.24.4.153.
7. Gradmann C. Laboratory Disease: Robert Koch’s Medical Bacteriology. Baltimore, MD: Johns Hopkins University Press; 2009. .
8. Steverding D. The history of African trypanosomiasis. Parasit Vectors. 2008;1(1):3. https://doi.org/10.1186/1756-3305-1-3
9. Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352(14):1411-1412. https://doi.org/10.1056/nejmp048180.
10. Frierson JG. The yellow fever vaccine: a history. Yale J Biol Med. 2010;83(2):77-85.
11. Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29-33. https://doi.org/10.3201/eid1201.051007.
12. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. https://doi.org/10.1056/nejmoa1202290.
13. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [online first]. ChinaXiv. 2020. https://doi.org/10.1073/pnas.2005615117.
14. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [online first]. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa2007016.
15. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [online first]. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949.
16. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20065920.
17. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [online first]. JAMA Cardiology. 2020. https://doi.org/10.1001/jamacardio.2020.1787.
18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. https://doi.org/10.1016/S0140-6736(20)31022-9.
19. Bassler D, Briel M, Montori VM, et al; STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects, systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-1187. https://doi.org/jama.2010.310.
Manifestations of disease, as perceived by physicians, can shape conceptual views and favor specific therapeutic actions. Historically, these factors appear to have an outsized influence on medical thinking in general. Disease concepts derived from empirical observations during pandemics impose a trade-off. We obtain unparalleled insight into medical thought and practice, but risk incurring the cost of unfortunate mistakes. The psychologist and Nobel Prize winner in economics Daniel Kahneman describes two mental systems that shape our judgments and decision-making in his book, Thinking, Fast and Slow: System One is intuitive, emotional, and fast, whereas System Two is deliberative and logical and has slower onset.1 If we extrapolate these observations to clinical medicine, we often rely on either System One or System Two depending on particular situations. Errors can emerge when we default to fast and emotional responses in situations that instead require more deliberate and logical assessments. These include instances in which the desire to help—our humanitarian role as physicians, associated with an “adrenaline rush”—results from attempts to relieve human suffering. As mercenaries of misfortune, it is inevitable we engage medical interventions based on an incomplete understanding of the pathophysiology—in other words, without understanding the full risks and benefits.
During the ongoing COVID-19 pandemic, members of the medical community continue to provide care with the utmost nobility, empathy, and desire for action amid uncertainty. However, as the number of cases continues to increase worldwide, we urge caution in evaluating the current state of scientific understanding, our approaches to treatment, and the safety of empiric medical interventions targeting COVID-19. We are concerned that the extensive history of unintended adverse consequences of therapies for emerging infectious diseases in the past is being ignored in the development of approaches to COVID-19 treatment. It is likely harms will emerge from current empiric therapies for COVID-19 given what can be learned from history.
HISTORICAL EXAMPLES OF UNINTENDED ADVERSE CONSEQUENCES
Whereas influenza can be treated with neuraminidase inhibitors,2 there are currently no established effective antiviral therapies for COVID-19, which is similar to two other coronavirus diseases from the 21st century, SARS (Severe Acute Respiratory Syndrome) in 2003 and MERS (Middle-Eastern Respiratory Syndrome) in 2012.3 Even in times of global pandemic, we need to consider potential harms and adverse consequences of novel treatments and show justifiable ratio of risk versus benefit. With the absence of proven COVID-19 therapy and the desire to fulfill our oath of primum non nocere (first, do no harm) in mind, we review selected unintended adverse events of developing therapies for infectious diseases.
Two types of error in our decision-making strategies are errors of omission and errors of commission.4 Errors of omission, defined as instances in which a medical intervention was not carried out when there was a clear indication to do so, are less conspicuous in the history of infectious disease therapeutics. Errors of commission, in contrast, have become a more concerning component of our approach to COVID-19 therapy, perhaps prompted by our desire to act. Errors of commission are defined as instances in which a specific medical intervention that should have been avoided was instead performed. We will discuss historical examples of errors of commission to highlight parallels with the current pandemic (Appendix Figure).
During influenza epidemics in the 18th century, some physicians advocated the use of therapeutic lancet phlebotomies, while others recommended indiscriminate use of opium, which led to high rates of addiction.5 Neither intervention was supported by a reassuring body of evidence. Many recommended mercury-based preparations during major outbreaks of syphilis in medieval protestant Europe. Because of accumulated mercurial toxicity, many persons suffered long-term sequelae including chronic kidney injury and peripheral neuropathy.6 After the discovery of the tuberculous bacillus, Robert Koch attempted the inoculation of tuberculin as a curative intervention for tuberculosis.7 Under pressure from the king of Prussia to present his findings at the International Medical Meeting in Berlin, Germany, in 1890, Koch conducted a poorly executed clinical trial. Rudolf Virchow then demonstrated endobronchial spread of the infection with resultant clinical worsening in those who received Koch’s tuberculin. In 1905, Harold Wolfersan Thomas at the Liverpool School of Tropical Medicine treated cases of African trypanosomiasis with the arsenical drug Atoxyl (arsanilic acid), which demonstrated some efficacy but also caused optic nerve atrophy leading to blindness.8
There have also been errors of commission in the development of vaccines. One such event, known as the Cutter incident, followed from an incompletely inactivated batch of polio vaccine that caused 40,000 cases of abortive poliomyelitis and many cases of paralysis and death.9 In the early phases of the development of the yellow fever vaccine, Hideyo Noguchi tried to develop a vaccine based on the erroneous assumption that yellow fever was caused by Leptospira icteroides.10 In 1976, an error of commission occurred in response to an outbreak of a few dozen cases of Influenza A/H1N1 in Fort Dix, New Jersey: The accelerated implementation of a swine influenza–vaccination program led to many cases of Guillian-Barré Syndrome among recipients.11 Immunization experts defended this decision to vaccinate by arguing that “when lives are at risk, it’s better to err on the side of overreaction over underreaction.”11 However, this is a risk-perception versus risk-management concept that drives potential errors of commission.
A more recent error of commission involved the use of drotrecogin alfa (activated protein C) in the treatment of sepsis. This drug became the first and only Food and Drug Administration–approved drug for sepsis treatment. The approval process of this medication relied on one clinical trial, which was terminated early because of perceived overwhelming evidence of efficacy. Despite the initial high medical and financial expectations, Eli Lilly (Indianapolis) withdrew the drug when a larger, international clinical trial (PROWESS-SHOCK) did not show a similar benefit.12
THE COVID-19 ERA
The gravity of the COVID-19 pandemic has motivated the repurposing of previously available therapies. This explains the use of medications like hydroxychloroquine, interleukin-6 (IL-6) receptor antagonists, and remdesivir.13-15
Despite early authorization of emergency use for hydroxychloroquine by the FDA based on limited and poor-quality evidence,16 this drug has yet to demonstrate treatment efficacy for COVID-19. On the contrary, other, controlled, retrospective studies have shown that hydroxychloroquine might actually increase mortality, possibly through prolongation of the QT-interval.16,17 Also, diversion of this drug to treat COVID-19 raises the concern of hydroxychloroquine shortages for treatment of patients with autoimmune disease, in whom the drug has proven benefit. We question the hasty FDA authorization for emergency use of hydroxychloroquine for COVID-19.
There is also great enthusiasm among the medical community to administer IL-6 receptor antagonists as a COVID-19 treatment. The rationale for this approach includes observations in case series in which IL-6 levels correlated with adverse clinical outcomes.13 IL-6 antagonists have a proven track record of improving the outcome in autoimmune diseases. However, we must avoid the logical trap of post hoc, ergo propter hoc (after this, therefore because of this) dictum from which one would assume that, based on those observations of high IL-6 levels and adverse outcomes, lowering IL-6 levels will necessarily improve outcomes in COVID-19. The supposed role of IL-6 in causing COVID-19 is based on scant preliminary observations and on the yet unproven assumption that IL-6 association with disease severity is a cause-effect relationship and not an association separate from pathogenesis. Moreover, there is sufficient scientific evidence that, in the case of severe influenza infections, IL-6 limits inflammation and protects against severe and potentially life-threatening lung injury. The road ahead for IL-6 inhibition to treat COVID-19 is perilous and should be entered cautiously. One immediate concern of administering IL-6 receptor antagonists in this patient population is the potential reactivation of latent tuberculosis infection and hepatitis B, colonic perforation, and increased rate of infections in general.
The greatest hope at this early stage of the COVID-19 pandemic may be remdesivir, which is a direct-acting antiviral. Here again, initial case series in prestigious medical journals signaled the possibility of a morbidity and mortality benefit.14 Despite these encouraging signs, a recent clinical trial from China that was limited by incomplete patient enrollment demonstrated a lack of efficacy of remdesivir in accelerating clinical improvement or limiting mortality.18 In spite of these negative results, preliminary data from the Adaptive COVID-19 Treatment Trial (ACTT) has revealed a nonsignificant signal of reduced mortality and shorter time to recovery in the remdesivir group. In response to these reports, the FDA has now issued emergency use authorization of remdesivir for treating COVID-19. Given the precedence of conflicting study data in therapeutic development for infectious diseases, we urge caution in drawing interpretations of benefit based on these early reports. Early termination of clinical studies is often associated with a 30% overestimation of clinical benefit.19 Furthermore, the availability of remdesivir is limited, which raises substantial ethical concerns on the preferential allocation of the drug to selected populations in high-income countries. At the time of this report, uncertainty regarding the risk-benefit balance of remdesivir and other COVID-19 treatments should be emphasized among decision makers.
CONCLUSION
Errors of commission present particular concerns for risk in treating COVID-19 patients and suggest that sometimes inaction is preferable to action. With many pandemics, there is a history of repeating mistakes, and we believe this can be curtailed by heeding the lessons of history. In the end, we may learn that avoiding therapeutic interventions that are poorly supported may prove to be one of the most important legacies of the COVID-19 pandemic.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Shapiro is supported by The Emily Foundation, Boston, Massachusetts. For all other authors, no financial support was declared.
Manifestations of disease, as perceived by physicians, can shape conceptual views and favor specific therapeutic actions. Historically, these factors appear to have an outsized influence on medical thinking in general. Disease concepts derived from empirical observations during pandemics impose a trade-off. We obtain unparalleled insight into medical thought and practice, but risk incurring the cost of unfortunate mistakes. The psychologist and Nobel Prize winner in economics Daniel Kahneman describes two mental systems that shape our judgments and decision-making in his book, Thinking, Fast and Slow: System One is intuitive, emotional, and fast, whereas System Two is deliberative and logical and has slower onset.1 If we extrapolate these observations to clinical medicine, we often rely on either System One or System Two depending on particular situations. Errors can emerge when we default to fast and emotional responses in situations that instead require more deliberate and logical assessments. These include instances in which the desire to help—our humanitarian role as physicians, associated with an “adrenaline rush”—results from attempts to relieve human suffering. As mercenaries of misfortune, it is inevitable we engage medical interventions based on an incomplete understanding of the pathophysiology—in other words, without understanding the full risks and benefits.
During the ongoing COVID-19 pandemic, members of the medical community continue to provide care with the utmost nobility, empathy, and desire for action amid uncertainty. However, as the number of cases continues to increase worldwide, we urge caution in evaluating the current state of scientific understanding, our approaches to treatment, and the safety of empiric medical interventions targeting COVID-19. We are concerned that the extensive history of unintended adverse consequences of therapies for emerging infectious diseases in the past is being ignored in the development of approaches to COVID-19 treatment. It is likely harms will emerge from current empiric therapies for COVID-19 given what can be learned from history.
HISTORICAL EXAMPLES OF UNINTENDED ADVERSE CONSEQUENCES
Whereas influenza can be treated with neuraminidase inhibitors,2 there are currently no established effective antiviral therapies for COVID-19, which is similar to two other coronavirus diseases from the 21st century, SARS (Severe Acute Respiratory Syndrome) in 2003 and MERS (Middle-Eastern Respiratory Syndrome) in 2012.3 Even in times of global pandemic, we need to consider potential harms and adverse consequences of novel treatments and show justifiable ratio of risk versus benefit. With the absence of proven COVID-19 therapy and the desire to fulfill our oath of primum non nocere (first, do no harm) in mind, we review selected unintended adverse events of developing therapies for infectious diseases.
Two types of error in our decision-making strategies are errors of omission and errors of commission.4 Errors of omission, defined as instances in which a medical intervention was not carried out when there was a clear indication to do so, are less conspicuous in the history of infectious disease therapeutics. Errors of commission, in contrast, have become a more concerning component of our approach to COVID-19 therapy, perhaps prompted by our desire to act. Errors of commission are defined as instances in which a specific medical intervention that should have been avoided was instead performed. We will discuss historical examples of errors of commission to highlight parallels with the current pandemic (Appendix Figure).
During influenza epidemics in the 18th century, some physicians advocated the use of therapeutic lancet phlebotomies, while others recommended indiscriminate use of opium, which led to high rates of addiction.5 Neither intervention was supported by a reassuring body of evidence. Many recommended mercury-based preparations during major outbreaks of syphilis in medieval protestant Europe. Because of accumulated mercurial toxicity, many persons suffered long-term sequelae including chronic kidney injury and peripheral neuropathy.6 After the discovery of the tuberculous bacillus, Robert Koch attempted the inoculation of tuberculin as a curative intervention for tuberculosis.7 Under pressure from the king of Prussia to present his findings at the International Medical Meeting in Berlin, Germany, in 1890, Koch conducted a poorly executed clinical trial. Rudolf Virchow then demonstrated endobronchial spread of the infection with resultant clinical worsening in those who received Koch’s tuberculin. In 1905, Harold Wolfersan Thomas at the Liverpool School of Tropical Medicine treated cases of African trypanosomiasis with the arsenical drug Atoxyl (arsanilic acid), which demonstrated some efficacy but also caused optic nerve atrophy leading to blindness.8
There have also been errors of commission in the development of vaccines. One such event, known as the Cutter incident, followed from an incompletely inactivated batch of polio vaccine that caused 40,000 cases of abortive poliomyelitis and many cases of paralysis and death.9 In the early phases of the development of the yellow fever vaccine, Hideyo Noguchi tried to develop a vaccine based on the erroneous assumption that yellow fever was caused by Leptospira icteroides.10 In 1976, an error of commission occurred in response to an outbreak of a few dozen cases of Influenza A/H1N1 in Fort Dix, New Jersey: The accelerated implementation of a swine influenza–vaccination program led to many cases of Guillian-Barré Syndrome among recipients.11 Immunization experts defended this decision to vaccinate by arguing that “when lives are at risk, it’s better to err on the side of overreaction over underreaction.”11 However, this is a risk-perception versus risk-management concept that drives potential errors of commission.
A more recent error of commission involved the use of drotrecogin alfa (activated protein C) in the treatment of sepsis. This drug became the first and only Food and Drug Administration–approved drug for sepsis treatment. The approval process of this medication relied on one clinical trial, which was terminated early because of perceived overwhelming evidence of efficacy. Despite the initial high medical and financial expectations, Eli Lilly (Indianapolis) withdrew the drug when a larger, international clinical trial (PROWESS-SHOCK) did not show a similar benefit.12
THE COVID-19 ERA
The gravity of the COVID-19 pandemic has motivated the repurposing of previously available therapies. This explains the use of medications like hydroxychloroquine, interleukin-6 (IL-6) receptor antagonists, and remdesivir.13-15
Despite early authorization of emergency use for hydroxychloroquine by the FDA based on limited and poor-quality evidence,16 this drug has yet to demonstrate treatment efficacy for COVID-19. On the contrary, other, controlled, retrospective studies have shown that hydroxychloroquine might actually increase mortality, possibly through prolongation of the QT-interval.16,17 Also, diversion of this drug to treat COVID-19 raises the concern of hydroxychloroquine shortages for treatment of patients with autoimmune disease, in whom the drug has proven benefit. We question the hasty FDA authorization for emergency use of hydroxychloroquine for COVID-19.
There is also great enthusiasm among the medical community to administer IL-6 receptor antagonists as a COVID-19 treatment. The rationale for this approach includes observations in case series in which IL-6 levels correlated with adverse clinical outcomes.13 IL-6 antagonists have a proven track record of improving the outcome in autoimmune diseases. However, we must avoid the logical trap of post hoc, ergo propter hoc (after this, therefore because of this) dictum from which one would assume that, based on those observations of high IL-6 levels and adverse outcomes, lowering IL-6 levels will necessarily improve outcomes in COVID-19. The supposed role of IL-6 in causing COVID-19 is based on scant preliminary observations and on the yet unproven assumption that IL-6 association with disease severity is a cause-effect relationship and not an association separate from pathogenesis. Moreover, there is sufficient scientific evidence that, in the case of severe influenza infections, IL-6 limits inflammation and protects against severe and potentially life-threatening lung injury. The road ahead for IL-6 inhibition to treat COVID-19 is perilous and should be entered cautiously. One immediate concern of administering IL-6 receptor antagonists in this patient population is the potential reactivation of latent tuberculosis infection and hepatitis B, colonic perforation, and increased rate of infections in general.
The greatest hope at this early stage of the COVID-19 pandemic may be remdesivir, which is a direct-acting antiviral. Here again, initial case series in prestigious medical journals signaled the possibility of a morbidity and mortality benefit.14 Despite these encouraging signs, a recent clinical trial from China that was limited by incomplete patient enrollment demonstrated a lack of efficacy of remdesivir in accelerating clinical improvement or limiting mortality.18 In spite of these negative results, preliminary data from the Adaptive COVID-19 Treatment Trial (ACTT) has revealed a nonsignificant signal of reduced mortality and shorter time to recovery in the remdesivir group. In response to these reports, the FDA has now issued emergency use authorization of remdesivir for treating COVID-19. Given the precedence of conflicting study data in therapeutic development for infectious diseases, we urge caution in drawing interpretations of benefit based on these early reports. Early termination of clinical studies is often associated with a 30% overestimation of clinical benefit.19 Furthermore, the availability of remdesivir is limited, which raises substantial ethical concerns on the preferential allocation of the drug to selected populations in high-income countries. At the time of this report, uncertainty regarding the risk-benefit balance of remdesivir and other COVID-19 treatments should be emphasized among decision makers.
CONCLUSION
Errors of commission present particular concerns for risk in treating COVID-19 patients and suggest that sometimes inaction is preferable to action. With many pandemics, there is a history of repeating mistakes, and we believe this can be curtailed by heeding the lessons of history. In the end, we may learn that avoiding therapeutic interventions that are poorly supported may prove to be one of the most important legacies of the COVID-19 pandemic.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Shapiro is supported by The Emily Foundation, Boston, Massachusetts. For all other authors, no financial support was declared.
1. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
2. Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017;72(6):1556-1573. https://doi.org/10.1093/jac/dkx013.
3. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. https://doi.org/10.1038/nrmicro.2016.81.
4. Grober ED, Bohnen JM. Defining medical error. Can J Surg. 2005;48(1):39-44.
5. Schofield AT. Opium in influenza. Lancet. 1894;143(3676):372. https://doi.org/10.1016/S0140-6736(01)66349-9.
6. Abraham JJ. Some account of the history of the treatment of syphilis. Br J Vener Dis. 1948;24(4):153-161. https://doi.org/10.1136/sti.24.4.153.
7. Gradmann C. Laboratory Disease: Robert Koch’s Medical Bacteriology. Baltimore, MD: Johns Hopkins University Press; 2009. .
8. Steverding D. The history of African trypanosomiasis. Parasit Vectors. 2008;1(1):3. https://doi.org/10.1186/1756-3305-1-3
9. Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352(14):1411-1412. https://doi.org/10.1056/nejmp048180.
10. Frierson JG. The yellow fever vaccine: a history. Yale J Biol Med. 2010;83(2):77-85.
11. Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29-33. https://doi.org/10.3201/eid1201.051007.
12. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. https://doi.org/10.1056/nejmoa1202290.
13. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [online first]. ChinaXiv. 2020. https://doi.org/10.1073/pnas.2005615117.
14. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [online first]. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa2007016.
15. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [online first]. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949.
16. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20065920.
17. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [online first]. JAMA Cardiology. 2020. https://doi.org/10.1001/jamacardio.2020.1787.
18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. https://doi.org/10.1016/S0140-6736(20)31022-9.
19. Bassler D, Briel M, Montori VM, et al; STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects, systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-1187. https://doi.org/jama.2010.310.
1. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
2. Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017;72(6):1556-1573. https://doi.org/10.1093/jac/dkx013.
3. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. https://doi.org/10.1038/nrmicro.2016.81.
4. Grober ED, Bohnen JM. Defining medical error. Can J Surg. 2005;48(1):39-44.
5. Schofield AT. Opium in influenza. Lancet. 1894;143(3676):372. https://doi.org/10.1016/S0140-6736(01)66349-9.
6. Abraham JJ. Some account of the history of the treatment of syphilis. Br J Vener Dis. 1948;24(4):153-161. https://doi.org/10.1136/sti.24.4.153.
7. Gradmann C. Laboratory Disease: Robert Koch’s Medical Bacteriology. Baltimore, MD: Johns Hopkins University Press; 2009. .
8. Steverding D. The history of African trypanosomiasis. Parasit Vectors. 2008;1(1):3. https://doi.org/10.1186/1756-3305-1-3
9. Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352(14):1411-1412. https://doi.org/10.1056/nejmp048180.
10. Frierson JG. The yellow fever vaccine: a history. Yale J Biol Med. 2010;83(2):77-85.
11. Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29-33. https://doi.org/10.3201/eid1201.051007.
12. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. https://doi.org/10.1056/nejmoa1202290.
13. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [online first]. ChinaXiv. 2020. https://doi.org/10.1073/pnas.2005615117.
14. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [online first]. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa2007016.
15. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [online first]. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949.
16. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20065920.
17. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [online first]. JAMA Cardiology. 2020. https://doi.org/10.1001/jamacardio.2020.1787.
18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. https://doi.org/10.1016/S0140-6736(20)31022-9.
19. Bassler D, Briel M, Montori VM, et al; STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects, systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-1187. https://doi.org/jama.2010.310.
© 2020 Society of Hospital Medicine
Compassionate Communication Amid the COVID-19 Pandemic
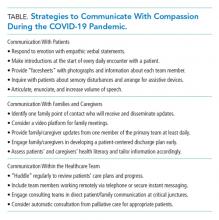
The coronavirus disease of 2019 (COVID-19) pandemic is the health crisis of our generation and will inevitably leave a lasting mark on how we practice medicine.1,2 It has already rapidly changed the way we communicate with patients, families, and colleagues. From the explosion of virtual care—which has been accelerated by need and new reimbursement policies3—to the physical barriers created by personal protective equipment (PPE) and no-visitor policies, the landscape of caring for hospitalized patients has seismically shifted in a few short months. At its core, the practice of medicine is about human connection—a connection between healers and the sick—and should remain as such to provide compassionate care to patients and their loved ones.4,5 In this perspective, we discuss challenges arising from communication barriers in the time of COVID-19 and opportunities to overcome them by preserving human connection to deliver high-quality care (Table).
COMMUNICATION WITH PATIENTS
While critically important to prevent transmission of the COVID-19 pathogen (ie, SARS-CoV-2), physical distancing and PPE create myriad challenges to achieving effective communication between healthcare providers and patients. Telemedicine has been leveraged to allow distanced communication between patients with COVID-19 and their providers from separate rooms. For face-to-face conversations, physical barriers, including distance between individuals and the wearing of face masks, impose new types of hindrances to nonverbal and verbal communication.
Challenges
Nonverbal communication helps build the therapeutic alliance and influences patient adherence to care plans, satisfaction, trust, and clinical outcomes.6,7 Expressions of emotion and reciprocity of nonverbal communication serve as important foundations for physician-patient encounters.6 Face masks, a necessity to reduce transmission of SARS-CoV-2, lead to fewer facial cues and may impede the ability to express and recognize emotional cues for patients and providers. A study of over 1,000 patients randomized to mask-wearing and non–mask-wearing physicians revealed a significant and negative effect on patient perception of physician empathy in consultations performed by mask-wearing physicians.8 Additionally, simple handshakes that convey respect and appreciation are no longer practiced.
Verbal communication is also affected by measures designed to reduce infection. The face mask and face shield worn by clinicians caring for patients with respiratory illnesses like COVID-19 diminish the volume and clarity of the spoken word. This is particularly problematic for patients who have sensory disturbances like hearing impairment. Additionally, these patients may rely on lipreading to effectively understand others, a strategy lost once the face mask is donned.
Opportunities
Healthcare providers may respond to nonverbal communication impediments by explicitly shifting nonverbal to verbal communication. For instance, when delivering serious news, a physician might previously have “mirrored” the patient’s sadness through a light touch on the hand and facial expressions congruent with that emotion. With physical distancing and PPE, the physician may instead express empathy through verbal statements such as acknowledging, validating, and respecting the patient’s emotions; making supportive statements; or exploring the patient’s feelings. The physician may also thank the patient for providing their input for the conversation.
Physicians should introduce themselves at the start of every daily encounter with a patient since there may be few distinct features above the face mask to distinguish the numerous individuals on a healthcare team. Some medical teams have provided “facesheets” with photographs and information about each member in an effort to humanize the team and connect more genuinely with the patient. In some cases, this may be the only way for a patient to see their healthcare providers’ faces.
To address obstacles to effective verbal communication, physicians should inquire about patients’ possible sensory disturbances on admission and, if necessary, arrange for hearing aids or other assistive devices. When communicating, physicians should articulate, enunciate, and increase volume to overcome the physical barrier created by the face mask. They should speak slowly, use plain language without jargon, and intentionally pause to check for understanding using the teach-back method.9
COMMUNICATION WITH FAMILIES AND CAREGIVERS
Challenges
With the aim of mitigating SARS-CoV-2 transmission, most healthcare systems have implemented no-visitor policies for hospitalized patients. This often leads to feelings of isolation among patients and their families. Goals-of-care discussions for COVID-19 and other serious diagnoses such as cancer can become even more difficult because family members often cannot witness how ill patients have become and clinicians cannot easily communicate virtually with multiple family members simultaneously.
Lack of family at the bedside also makes critical activities, such as discharge planning and education, more vulnerable to poor coordination and medical errors.10 Patients who are continuing to recover from acute illness may be expected to learn the details of home infusion for intravenous antibiotics, tracheostomy care, or specialized nutritional feeds. Without caregiver support, the patient may be at risk for readmission or other untoward safety events.
Opportunities
Several strategies may be used to improve virtual communication with families. The healthcare team should identify one family point of contact (ideally with the durable power of attorney for healthcare) who will receive and disseminate to others information about the patient’s status. This reduces the potential for multiple telephone conversations. We have witnessed some remarkable family points of contact call many family members to relay medical updates and moderate discussion. Care teams may decide to call the family contact during rounds so that they may listen in on the conversation with the patient or call after rounds to provide succinct updates. Family meetings may benefit greatly if conducted through a video platform, when possible, particularly if significant interval events have occurred. Connection through video allows eye contact and recognition of other nonverbal cues, as well as allowing findings like diagnostic images to be shared.
Because of increased anxiety associated with isolation, we recommend that one member of the primary healthcare team conduct telephone updates to the family point of contact on at least a daily basis. This simple act reduces potential for disjointed or discrepant messages from the healthcare team.11 It also demonstrates the value of keeping those individuals most important to the patient informed and has been shown to increase satisfaction with care and perceived effectiveness of meeting informational needs.12
Regarding discharge planning, physicians should engage the patient and family/caregivers in developing a patient-centered plan as early in the hospital stay as possible. The adage “discharge planning starts at admission” has never been more relevant. The team should avoid assumptions about patient/family sophistication for understanding complex healthcare concepts. Rather, physicians should assess patients’ and caregivers’ health literacy at the beginning of a hospital stay by asking simple, validated questions in a nonjudgmental way.13,14 This valuable information then allows the team to tailor medical information and discharge education appropriately for both patients and caregivers.
COMMUNICATION WITHIN THE HEALTHCARE TEAM
Challenges
As a result of the COVID-19 pandemic, various members of the healthcare team may be working remotely, and therefore, team members may feel less connected with each other. This could lead to a loss of camaraderie and fellowship within the team, as well as depersonalization, one of the main facets of burnout.15 Even if colocalized in the same area, those wearing face masks may experience disconnection and depersonalization. In an anecdote at our medical center, one clinician did not know what her team members’ faces looked like until they removed their masks for a moment to have a snack just before the end of the rotation.
In addition, healthcare systems have witnessed an increase in the volume of electronic consultations in which faculty and house staff review the patient’s medical record and render medical decision-making and recommendations without physically examining or interviewing the patient at the bedside. The purpose of this is twofold: to reduce the risk of transmitting SARS-CoV-2 and to conserve PPE. Electronic consultations could threaten to reduce collaborative communication and teaching among primary and consulting teams, which may lead to greater misunderstanding, less-effective patient care, and decreased satisfaction within the healthcare team.
Opportunities
Now more than ever, physicians should purposefully engage in regular communication with the multidisciplinary healthcare team that includes nurses, pharmacists, social workers, and other critical members. Because many of these individuals may now be working remotely or not joining in-person rounds, several strategies are needed to ensure care coordination within the primary healthcare team. For example, all members should “huddle” at least once daily to review each patient’s care and progress in meeting discharge goals. Team members who are working remotely should be dialed into these huddles and included in coordinating the plan for the day. While in-person multidisciplinary rounds may be temporarily halted to allow for physical distancing of staff, physician leaders can still encourage regular check-ins and updates throughout the day with multidisciplinary team members by other means, such as discussions by phone or a secure instant messenger, if available.
Another strategy to improve care coordination is to engage consulting teams in direct patient/family communication at critical junctures. For example, when a patient’s renal failure has gotten severe enough that dialysis is a consideration, the primary team may ask the nephrology consult service to participate in a joint telephone discussion with the family about risks, benefits, and alternatives to renal replacement therapy. Additionally, our palliative care consult service volunteered to be automatically consulted for all COVID-19 patients in the intensive care unit and high-risk COVID-19 patients on the acute care wards because of the disease’s high potential morbidity and mortality. Their roles included proactively confirming the patient’s surrogate decision maker, reviewing the patient’s decision-making capacity, eliciting specific goals of care and life-sustaining treatment preferences, and establishing relationships with the family. They also conducted daily huddles with the respective teams, another approach that fostered high-quality, collaborative care.
CONCLUSION
The COVID-19 pandemic has forced us to change the approaches we usually employ to interact with patients and their loved ones, as well as healthcare team members, but it has not changed the heart of medicine, which is to heal. Here we provide tangible and discrete strategies to achieve this goal through clear and compassionate communication, including shifting nonverbal to verbal communication with patients, speaking at least daily to one family point of contact, ensuring early and tailored discharge planning, emphasizing continued close care coordination among the multidisciplinary team, and thoughtfully engaging consultants in patient/family communication. We hope this guidance will assist us in striving to cultivate connection with our patients, their loved ones, and each other, just as we have always sought to do. With these strategies in mind, coupled with a continued focus on patient- and family-centered care for hospitalized patients, no amount of distance or PPE will diminish the power of human connection.
Acknowledgments
The authors wish to thank their colleagues—the physicians, nurses, respiratory therapists, clerks, custodial staff, security, and administrative professionals, to name a few—of the VA Ann Arbor Healthcare System for their collaboration, dedication, and grace in this time of crisis. The authors are indebted to the patients and their loved ones for putting their trust in their team, for teaching team members, and for providing the privilege of being a part of their lives.
Disclosures
The authors reported having nothing to disclose.
1. Ross JE. Resident response during pandemic: this is our time [online first]. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1240
2. Berwick DM. Choices for the “new normal” [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6949.
3. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. CMS.gov. Mar 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Accessed May 09, 2020.
4. Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70‐81. https://doi.org/10.1001/jama.2019.19003.
5. Haverfield MC, Tierney A, Schwartz R, et al. Can patient-provider interpersonal interventions achieve the quadruple aim of healthcare? a systematic review [online first]. J Gen Intern Med. 2020. https://doi.org/10.1007/s11606-019-05525-2.
6. Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits: mechanisms and outcomes. J Gen Intern Med. 2006;21(Suppl 1):S28-S34. https://doi.org/10.1111/j.1525-1497.2006.00306.x.
7. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005.
8. Wong CK, Yip BH, Mercer S, et al. Effect of facemasks on empathy and relational continuity: a randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200. https://doi.org/10.1186/1471-2296-14-200.
9. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. https://doi.org/10.1371/journal.pone.0231350.
10. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
11. Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12(4):317-324.
12. Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24-29.
13. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588-594.
14. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877. https://doi.org/10.1111/j.1525-1497.2006.00532.x.
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752.
The coronavirus disease of 2019 (COVID-19) pandemic is the health crisis of our generation and will inevitably leave a lasting mark on how we practice medicine.1,2 It has already rapidly changed the way we communicate with patients, families, and colleagues. From the explosion of virtual care—which has been accelerated by need and new reimbursement policies3—to the physical barriers created by personal protective equipment (PPE) and no-visitor policies, the landscape of caring for hospitalized patients has seismically shifted in a few short months. At its core, the practice of medicine is about human connection—a connection between healers and the sick—and should remain as such to provide compassionate care to patients and their loved ones.4,5 In this perspective, we discuss challenges arising from communication barriers in the time of COVID-19 and opportunities to overcome them by preserving human connection to deliver high-quality care (Table).
COMMUNICATION WITH PATIENTS
While critically important to prevent transmission of the COVID-19 pathogen (ie, SARS-CoV-2), physical distancing and PPE create myriad challenges to achieving effective communication between healthcare providers and patients. Telemedicine has been leveraged to allow distanced communication between patients with COVID-19 and their providers from separate rooms. For face-to-face conversations, physical barriers, including distance between individuals and the wearing of face masks, impose new types of hindrances to nonverbal and verbal communication.
Challenges
Nonverbal communication helps build the therapeutic alliance and influences patient adherence to care plans, satisfaction, trust, and clinical outcomes.6,7 Expressions of emotion and reciprocity of nonverbal communication serve as important foundations for physician-patient encounters.6 Face masks, a necessity to reduce transmission of SARS-CoV-2, lead to fewer facial cues and may impede the ability to express and recognize emotional cues for patients and providers. A study of over 1,000 patients randomized to mask-wearing and non–mask-wearing physicians revealed a significant and negative effect on patient perception of physician empathy in consultations performed by mask-wearing physicians.8 Additionally, simple handshakes that convey respect and appreciation are no longer practiced.
Verbal communication is also affected by measures designed to reduce infection. The face mask and face shield worn by clinicians caring for patients with respiratory illnesses like COVID-19 diminish the volume and clarity of the spoken word. This is particularly problematic for patients who have sensory disturbances like hearing impairment. Additionally, these patients may rely on lipreading to effectively understand others, a strategy lost once the face mask is donned.
Opportunities
Healthcare providers may respond to nonverbal communication impediments by explicitly shifting nonverbal to verbal communication. For instance, when delivering serious news, a physician might previously have “mirrored” the patient’s sadness through a light touch on the hand and facial expressions congruent with that emotion. With physical distancing and PPE, the physician may instead express empathy through verbal statements such as acknowledging, validating, and respecting the patient’s emotions; making supportive statements; or exploring the patient’s feelings. The physician may also thank the patient for providing their input for the conversation.
Physicians should introduce themselves at the start of every daily encounter with a patient since there may be few distinct features above the face mask to distinguish the numerous individuals on a healthcare team. Some medical teams have provided “facesheets” with photographs and information about each member in an effort to humanize the team and connect more genuinely with the patient. In some cases, this may be the only way for a patient to see their healthcare providers’ faces.
To address obstacles to effective verbal communication, physicians should inquire about patients’ possible sensory disturbances on admission and, if necessary, arrange for hearing aids or other assistive devices. When communicating, physicians should articulate, enunciate, and increase volume to overcome the physical barrier created by the face mask. They should speak slowly, use plain language without jargon, and intentionally pause to check for understanding using the teach-back method.9
COMMUNICATION WITH FAMILIES AND CAREGIVERS
Challenges
With the aim of mitigating SARS-CoV-2 transmission, most healthcare systems have implemented no-visitor policies for hospitalized patients. This often leads to feelings of isolation among patients and their families. Goals-of-care discussions for COVID-19 and other serious diagnoses such as cancer can become even more difficult because family members often cannot witness how ill patients have become and clinicians cannot easily communicate virtually with multiple family members simultaneously.
Lack of family at the bedside also makes critical activities, such as discharge planning and education, more vulnerable to poor coordination and medical errors.10 Patients who are continuing to recover from acute illness may be expected to learn the details of home infusion for intravenous antibiotics, tracheostomy care, or specialized nutritional feeds. Without caregiver support, the patient may be at risk for readmission or other untoward safety events.
Opportunities
Several strategies may be used to improve virtual communication with families. The healthcare team should identify one family point of contact (ideally with the durable power of attorney for healthcare) who will receive and disseminate to others information about the patient’s status. This reduces the potential for multiple telephone conversations. We have witnessed some remarkable family points of contact call many family members to relay medical updates and moderate discussion. Care teams may decide to call the family contact during rounds so that they may listen in on the conversation with the patient or call after rounds to provide succinct updates. Family meetings may benefit greatly if conducted through a video platform, when possible, particularly if significant interval events have occurred. Connection through video allows eye contact and recognition of other nonverbal cues, as well as allowing findings like diagnostic images to be shared.
Because of increased anxiety associated with isolation, we recommend that one member of the primary healthcare team conduct telephone updates to the family point of contact on at least a daily basis. This simple act reduces potential for disjointed or discrepant messages from the healthcare team.11 It also demonstrates the value of keeping those individuals most important to the patient informed and has been shown to increase satisfaction with care and perceived effectiveness of meeting informational needs.12
Regarding discharge planning, physicians should engage the patient and family/caregivers in developing a patient-centered plan as early in the hospital stay as possible. The adage “discharge planning starts at admission” has never been more relevant. The team should avoid assumptions about patient/family sophistication for understanding complex healthcare concepts. Rather, physicians should assess patients’ and caregivers’ health literacy at the beginning of a hospital stay by asking simple, validated questions in a nonjudgmental way.13,14 This valuable information then allows the team to tailor medical information and discharge education appropriately for both patients and caregivers.
COMMUNICATION WITHIN THE HEALTHCARE TEAM
Challenges
As a result of the COVID-19 pandemic, various members of the healthcare team may be working remotely, and therefore, team members may feel less connected with each other. This could lead to a loss of camaraderie and fellowship within the team, as well as depersonalization, one of the main facets of burnout.15 Even if colocalized in the same area, those wearing face masks may experience disconnection and depersonalization. In an anecdote at our medical center, one clinician did not know what her team members’ faces looked like until they removed their masks for a moment to have a snack just before the end of the rotation.
In addition, healthcare systems have witnessed an increase in the volume of electronic consultations in which faculty and house staff review the patient’s medical record and render medical decision-making and recommendations without physically examining or interviewing the patient at the bedside. The purpose of this is twofold: to reduce the risk of transmitting SARS-CoV-2 and to conserve PPE. Electronic consultations could threaten to reduce collaborative communication and teaching among primary and consulting teams, which may lead to greater misunderstanding, less-effective patient care, and decreased satisfaction within the healthcare team.
Opportunities
Now more than ever, physicians should purposefully engage in regular communication with the multidisciplinary healthcare team that includes nurses, pharmacists, social workers, and other critical members. Because many of these individuals may now be working remotely or not joining in-person rounds, several strategies are needed to ensure care coordination within the primary healthcare team. For example, all members should “huddle” at least once daily to review each patient’s care and progress in meeting discharge goals. Team members who are working remotely should be dialed into these huddles and included in coordinating the plan for the day. While in-person multidisciplinary rounds may be temporarily halted to allow for physical distancing of staff, physician leaders can still encourage regular check-ins and updates throughout the day with multidisciplinary team members by other means, such as discussions by phone or a secure instant messenger, if available.
Another strategy to improve care coordination is to engage consulting teams in direct patient/family communication at critical junctures. For example, when a patient’s renal failure has gotten severe enough that dialysis is a consideration, the primary team may ask the nephrology consult service to participate in a joint telephone discussion with the family about risks, benefits, and alternatives to renal replacement therapy. Additionally, our palliative care consult service volunteered to be automatically consulted for all COVID-19 patients in the intensive care unit and high-risk COVID-19 patients on the acute care wards because of the disease’s high potential morbidity and mortality. Their roles included proactively confirming the patient’s surrogate decision maker, reviewing the patient’s decision-making capacity, eliciting specific goals of care and life-sustaining treatment preferences, and establishing relationships with the family. They also conducted daily huddles with the respective teams, another approach that fostered high-quality, collaborative care.
CONCLUSION
The COVID-19 pandemic has forced us to change the approaches we usually employ to interact with patients and their loved ones, as well as healthcare team members, but it has not changed the heart of medicine, which is to heal. Here we provide tangible and discrete strategies to achieve this goal through clear and compassionate communication, including shifting nonverbal to verbal communication with patients, speaking at least daily to one family point of contact, ensuring early and tailored discharge planning, emphasizing continued close care coordination among the multidisciplinary team, and thoughtfully engaging consultants in patient/family communication. We hope this guidance will assist us in striving to cultivate connection with our patients, their loved ones, and each other, just as we have always sought to do. With these strategies in mind, coupled with a continued focus on patient- and family-centered care for hospitalized patients, no amount of distance or PPE will diminish the power of human connection.
Acknowledgments
The authors wish to thank their colleagues—the physicians, nurses, respiratory therapists, clerks, custodial staff, security, and administrative professionals, to name a few—of the VA Ann Arbor Healthcare System for their collaboration, dedication, and grace in this time of crisis. The authors are indebted to the patients and their loved ones for putting their trust in their team, for teaching team members, and for providing the privilege of being a part of their lives.
Disclosures
The authors reported having nothing to disclose.
The coronavirus disease of 2019 (COVID-19) pandemic is the health crisis of our generation and will inevitably leave a lasting mark on how we practice medicine.1,2 It has already rapidly changed the way we communicate with patients, families, and colleagues. From the explosion of virtual care—which has been accelerated by need and new reimbursement policies3—to the physical barriers created by personal protective equipment (PPE) and no-visitor policies, the landscape of caring for hospitalized patients has seismically shifted in a few short months. At its core, the practice of medicine is about human connection—a connection between healers and the sick—and should remain as such to provide compassionate care to patients and their loved ones.4,5 In this perspective, we discuss challenges arising from communication barriers in the time of COVID-19 and opportunities to overcome them by preserving human connection to deliver high-quality care (Table).
COMMUNICATION WITH PATIENTS
While critically important to prevent transmission of the COVID-19 pathogen (ie, SARS-CoV-2), physical distancing and PPE create myriad challenges to achieving effective communication between healthcare providers and patients. Telemedicine has been leveraged to allow distanced communication between patients with COVID-19 and their providers from separate rooms. For face-to-face conversations, physical barriers, including distance between individuals and the wearing of face masks, impose new types of hindrances to nonverbal and verbal communication.
Challenges
Nonverbal communication helps build the therapeutic alliance and influences patient adherence to care plans, satisfaction, trust, and clinical outcomes.6,7 Expressions of emotion and reciprocity of nonverbal communication serve as important foundations for physician-patient encounters.6 Face masks, a necessity to reduce transmission of SARS-CoV-2, lead to fewer facial cues and may impede the ability to express and recognize emotional cues for patients and providers. A study of over 1,000 patients randomized to mask-wearing and non–mask-wearing physicians revealed a significant and negative effect on patient perception of physician empathy in consultations performed by mask-wearing physicians.8 Additionally, simple handshakes that convey respect and appreciation are no longer practiced.
Verbal communication is also affected by measures designed to reduce infection. The face mask and face shield worn by clinicians caring for patients with respiratory illnesses like COVID-19 diminish the volume and clarity of the spoken word. This is particularly problematic for patients who have sensory disturbances like hearing impairment. Additionally, these patients may rely on lipreading to effectively understand others, a strategy lost once the face mask is donned.
Opportunities
Healthcare providers may respond to nonverbal communication impediments by explicitly shifting nonverbal to verbal communication. For instance, when delivering serious news, a physician might previously have “mirrored” the patient’s sadness through a light touch on the hand and facial expressions congruent with that emotion. With physical distancing and PPE, the physician may instead express empathy through verbal statements such as acknowledging, validating, and respecting the patient’s emotions; making supportive statements; or exploring the patient’s feelings. The physician may also thank the patient for providing their input for the conversation.
Physicians should introduce themselves at the start of every daily encounter with a patient since there may be few distinct features above the face mask to distinguish the numerous individuals on a healthcare team. Some medical teams have provided “facesheets” with photographs and information about each member in an effort to humanize the team and connect more genuinely with the patient. In some cases, this may be the only way for a patient to see their healthcare providers’ faces.
To address obstacles to effective verbal communication, physicians should inquire about patients’ possible sensory disturbances on admission and, if necessary, arrange for hearing aids or other assistive devices. When communicating, physicians should articulate, enunciate, and increase volume to overcome the physical barrier created by the face mask. They should speak slowly, use plain language without jargon, and intentionally pause to check for understanding using the teach-back method.9
COMMUNICATION WITH FAMILIES AND CAREGIVERS
Challenges
With the aim of mitigating SARS-CoV-2 transmission, most healthcare systems have implemented no-visitor policies for hospitalized patients. This often leads to feelings of isolation among patients and their families. Goals-of-care discussions for COVID-19 and other serious diagnoses such as cancer can become even more difficult because family members often cannot witness how ill patients have become and clinicians cannot easily communicate virtually with multiple family members simultaneously.
Lack of family at the bedside also makes critical activities, such as discharge planning and education, more vulnerable to poor coordination and medical errors.10 Patients who are continuing to recover from acute illness may be expected to learn the details of home infusion for intravenous antibiotics, tracheostomy care, or specialized nutritional feeds. Without caregiver support, the patient may be at risk for readmission or other untoward safety events.
Opportunities
Several strategies may be used to improve virtual communication with families. The healthcare team should identify one family point of contact (ideally with the durable power of attorney for healthcare) who will receive and disseminate to others information about the patient’s status. This reduces the potential for multiple telephone conversations. We have witnessed some remarkable family points of contact call many family members to relay medical updates and moderate discussion. Care teams may decide to call the family contact during rounds so that they may listen in on the conversation with the patient or call after rounds to provide succinct updates. Family meetings may benefit greatly if conducted through a video platform, when possible, particularly if significant interval events have occurred. Connection through video allows eye contact and recognition of other nonverbal cues, as well as allowing findings like diagnostic images to be shared.
Because of increased anxiety associated with isolation, we recommend that one member of the primary healthcare team conduct telephone updates to the family point of contact on at least a daily basis. This simple act reduces potential for disjointed or discrepant messages from the healthcare team.11 It also demonstrates the value of keeping those individuals most important to the patient informed and has been shown to increase satisfaction with care and perceived effectiveness of meeting informational needs.12
Regarding discharge planning, physicians should engage the patient and family/caregivers in developing a patient-centered plan as early in the hospital stay as possible. The adage “discharge planning starts at admission” has never been more relevant. The team should avoid assumptions about patient/family sophistication for understanding complex healthcare concepts. Rather, physicians should assess patients’ and caregivers’ health literacy at the beginning of a hospital stay by asking simple, validated questions in a nonjudgmental way.13,14 This valuable information then allows the team to tailor medical information and discharge education appropriately for both patients and caregivers.
COMMUNICATION WITHIN THE HEALTHCARE TEAM
Challenges
As a result of the COVID-19 pandemic, various members of the healthcare team may be working remotely, and therefore, team members may feel less connected with each other. This could lead to a loss of camaraderie and fellowship within the team, as well as depersonalization, one of the main facets of burnout.15 Even if colocalized in the same area, those wearing face masks may experience disconnection and depersonalization. In an anecdote at our medical center, one clinician did not know what her team members’ faces looked like until they removed their masks for a moment to have a snack just before the end of the rotation.
In addition, healthcare systems have witnessed an increase in the volume of electronic consultations in which faculty and house staff review the patient’s medical record and render medical decision-making and recommendations without physically examining or interviewing the patient at the bedside. The purpose of this is twofold: to reduce the risk of transmitting SARS-CoV-2 and to conserve PPE. Electronic consultations could threaten to reduce collaborative communication and teaching among primary and consulting teams, which may lead to greater misunderstanding, less-effective patient care, and decreased satisfaction within the healthcare team.
Opportunities
Now more than ever, physicians should purposefully engage in regular communication with the multidisciplinary healthcare team that includes nurses, pharmacists, social workers, and other critical members. Because many of these individuals may now be working remotely or not joining in-person rounds, several strategies are needed to ensure care coordination within the primary healthcare team. For example, all members should “huddle” at least once daily to review each patient’s care and progress in meeting discharge goals. Team members who are working remotely should be dialed into these huddles and included in coordinating the plan for the day. While in-person multidisciplinary rounds may be temporarily halted to allow for physical distancing of staff, physician leaders can still encourage regular check-ins and updates throughout the day with multidisciplinary team members by other means, such as discussions by phone or a secure instant messenger, if available.
Another strategy to improve care coordination is to engage consulting teams in direct patient/family communication at critical junctures. For example, when a patient’s renal failure has gotten severe enough that dialysis is a consideration, the primary team may ask the nephrology consult service to participate in a joint telephone discussion with the family about risks, benefits, and alternatives to renal replacement therapy. Additionally, our palliative care consult service volunteered to be automatically consulted for all COVID-19 patients in the intensive care unit and high-risk COVID-19 patients on the acute care wards because of the disease’s high potential morbidity and mortality. Their roles included proactively confirming the patient’s surrogate decision maker, reviewing the patient’s decision-making capacity, eliciting specific goals of care and life-sustaining treatment preferences, and establishing relationships with the family. They also conducted daily huddles with the respective teams, another approach that fostered high-quality, collaborative care.
CONCLUSION
The COVID-19 pandemic has forced us to change the approaches we usually employ to interact with patients and their loved ones, as well as healthcare team members, but it has not changed the heart of medicine, which is to heal. Here we provide tangible and discrete strategies to achieve this goal through clear and compassionate communication, including shifting nonverbal to verbal communication with patients, speaking at least daily to one family point of contact, ensuring early and tailored discharge planning, emphasizing continued close care coordination among the multidisciplinary team, and thoughtfully engaging consultants in patient/family communication. We hope this guidance will assist us in striving to cultivate connection with our patients, their loved ones, and each other, just as we have always sought to do. With these strategies in mind, coupled with a continued focus on patient- and family-centered care for hospitalized patients, no amount of distance or PPE will diminish the power of human connection.
Acknowledgments
The authors wish to thank their colleagues—the physicians, nurses, respiratory therapists, clerks, custodial staff, security, and administrative professionals, to name a few—of the VA Ann Arbor Healthcare System for their collaboration, dedication, and grace in this time of crisis. The authors are indebted to the patients and their loved ones for putting their trust in their team, for teaching team members, and for providing the privilege of being a part of their lives.
Disclosures
The authors reported having nothing to disclose.
1. Ross JE. Resident response during pandemic: this is our time [online first]. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1240
2. Berwick DM. Choices for the “new normal” [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6949.
3. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. CMS.gov. Mar 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Accessed May 09, 2020.
4. Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70‐81. https://doi.org/10.1001/jama.2019.19003.
5. Haverfield MC, Tierney A, Schwartz R, et al. Can patient-provider interpersonal interventions achieve the quadruple aim of healthcare? a systematic review [online first]. J Gen Intern Med. 2020. https://doi.org/10.1007/s11606-019-05525-2.
6. Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits: mechanisms and outcomes. J Gen Intern Med. 2006;21(Suppl 1):S28-S34. https://doi.org/10.1111/j.1525-1497.2006.00306.x.
7. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005.
8. Wong CK, Yip BH, Mercer S, et al. Effect of facemasks on empathy and relational continuity: a randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200. https://doi.org/10.1186/1471-2296-14-200.
9. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. https://doi.org/10.1371/journal.pone.0231350.
10. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
11. Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12(4):317-324.
12. Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24-29.
13. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588-594.
14. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877. https://doi.org/10.1111/j.1525-1497.2006.00532.x.
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752.
1. Ross JE. Resident response during pandemic: this is our time [online first]. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1240
2. Berwick DM. Choices for the “new normal” [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6949.
3. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. CMS.gov. Mar 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Accessed May 09, 2020.
4. Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70‐81. https://doi.org/10.1001/jama.2019.19003.
5. Haverfield MC, Tierney A, Schwartz R, et al. Can patient-provider interpersonal interventions achieve the quadruple aim of healthcare? a systematic review [online first]. J Gen Intern Med. 2020. https://doi.org/10.1007/s11606-019-05525-2.
6. Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits: mechanisms and outcomes. J Gen Intern Med. 2006;21(Suppl 1):S28-S34. https://doi.org/10.1111/j.1525-1497.2006.00306.x.
7. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005.
8. Wong CK, Yip BH, Mercer S, et al. Effect of facemasks on empathy and relational continuity: a randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200. https://doi.org/10.1186/1471-2296-14-200.
9. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. https://doi.org/10.1371/journal.pone.0231350.
10. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
11. Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12(4):317-324.
12. Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24-29.
13. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588-594.
14. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877. https://doi.org/10.1111/j.1525-1497.2006.00532.x.
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752.
© 2020 Society of Hospital Medicine
Few patients with migraine clear barriers to optimal care
, new research shows.
Results from a survey study showed less than 8% of patients with episodic migraine and less than 2% of patients with chronic migraine were able to overcome four key treatment barriers associated with optimal migraine management. These included current medical consultation, appropriate diagnosis, minimally adequate acute and preventive pharmacologic treatment (if indicated), and absence of acute medication overdose.
The researchers also evaluated any potential impact of race, ethnicity, and sociodemographic factors on these barriers.
“While chronic migraine was associated with higher rates of consulting, only 1.8% of respondents with chronic migraine traversed all four barriers compared with 8.5% of those with episodic migraine,” the investigators, led by Dawn C. Buse, PhD, clinical professor of neurology at Albert Einstein College of Medicine of Yeshiva University in New York City, noted.
The study was presented at the virtual annual meeting of the American Headache Society.
Ongoing challenges
Migraineurs’ challenges include receiving an appropriate diagnosis and finding effective acute and preventive treatments, the researchers noted. Many patients do not receive optimal care. Previous research by Dr. Buse and colleagues showed that general clinicians were less likely to provide an appropriate diagnosis of migraine compared with headache specialists.
Among patients with chronic migraine who consulted headache specialists, most did not receive an accurate diagnosis of chronic migraine. Data also indicate that a minority, approximately 34%, of patients with chronic migraine used preventive pharmacologic treatments.
The investigators analyzed data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study to determine the proportion of patients who overcame four prespecified barriers to good outcomes.
Eligible participants met modified International Classification of Headache Disorders (3rd edition) criteria for migraine, had Migraine Disability Assessment Scores (MIDAS) of grade II or higher, and provided data on health insurance status. In addition, all eligible participants had to be receiving appropriate treatment for either episodic or chronic migraine.
In all, 16,789 participants met criteria for migraine. Of this group, 9,184 patients had a MIDAS score of grade II or higher and reported health insurance status. In this subgroup, 7,930 (86.3%) patients had episodic migraine and 1,254 (13.7%) had chronic migraine.
A total of 2,187 (27.6%) patients with episodic migraine and 512 (40.8%) patients with chronic migraine were under the care of a healthcare professional. Of this group, 1,655 patients with episodic migraine (75.7%) and 168 with chronic migraine (32.8%) reported receiving an appropriate diagnosis.
Of participants who successfully overcame the first two optimal management barriers—a consultation with a healthcare professional and an appropriate diagnosis—1,133 (68.5%) episodic migraineurs and 113 (67.3%) chronic migraineurs reported receiving minimally adequate acute treatment.
Furthermore, 1,430 (86.4%) episodic migraineurs and 127 (75.6%) chronic migraineurs reported receiving minimally adequate preventive medication treatment. In addition, 982 (59.3%) episodic migraineurs and 88 (52.4%) chronic migraineurs received minimally adequate acute and preventive treatment.
Acute medication overuse was relatively common, the investigators reported. A total of 310 (31.6%) patients with episodic migraine and 66 (75%) patients with chronic migraine met criteria for acute medication overuse.
“Overuse of acute medication for migraine in people with chronic migraine is a serious concern and is associated with increased risks of migraine progression, headache-related disability, and anxiety and depression. Active patient management and education is important to reduce the likelihood of medication overuse,” said Dr. Buse.
Among all eligible respondents, only 672 (8.5%) patients with episodic migraine and 22 (1.8%) with chronic migraine overcame all four barriers to optimal care.
The researchers found no significant effect of ethnicity or race on the likelihood of overcoming any barrier, but they acknowledged that participation bias might have contributed to this lack of difference. Higher annual household income was significantly associated with high likelihood of surmounting all four barriers.
“The analysis of sociodemographics revealed that female sex and higher annual household income showed a strong relationship with likelihood of obtaining an accurate episodic migraine or chronic migraine diagnosis,” said Dr. Buse.
“Although the reasons for this are not clear, it may be that women are more likely to convey the full scope of their symptoms during consultation. Additionally, the known prevalence of migraine in women may influence healthcare providers by reducing suspicion of chronic migraine in men,” she added.
The CaMEO Study was funded by Allergan (now AbbVie). Dr. Buse reports receiving grant support and honoraria from Allergan, Amgen, Biohaven, Eli Lilly and Co, and Promius. She also receives compensation for work on the editorial board of Current Pain and Headache Reports.
This article first appeared on Medscape.com.
, new research shows.
Results from a survey study showed less than 8% of patients with episodic migraine and less than 2% of patients with chronic migraine were able to overcome four key treatment barriers associated with optimal migraine management. These included current medical consultation, appropriate diagnosis, minimally adequate acute and preventive pharmacologic treatment (if indicated), and absence of acute medication overdose.
The researchers also evaluated any potential impact of race, ethnicity, and sociodemographic factors on these barriers.
“While chronic migraine was associated with higher rates of consulting, only 1.8% of respondents with chronic migraine traversed all four barriers compared with 8.5% of those with episodic migraine,” the investigators, led by Dawn C. Buse, PhD, clinical professor of neurology at Albert Einstein College of Medicine of Yeshiva University in New York City, noted.
The study was presented at the virtual annual meeting of the American Headache Society.
Ongoing challenges
Migraineurs’ challenges include receiving an appropriate diagnosis and finding effective acute and preventive treatments, the researchers noted. Many patients do not receive optimal care. Previous research by Dr. Buse and colleagues showed that general clinicians were less likely to provide an appropriate diagnosis of migraine compared with headache specialists.
Among patients with chronic migraine who consulted headache specialists, most did not receive an accurate diagnosis of chronic migraine. Data also indicate that a minority, approximately 34%, of patients with chronic migraine used preventive pharmacologic treatments.
The investigators analyzed data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study to determine the proportion of patients who overcame four prespecified barriers to good outcomes.
Eligible participants met modified International Classification of Headache Disorders (3rd edition) criteria for migraine, had Migraine Disability Assessment Scores (MIDAS) of grade II or higher, and provided data on health insurance status. In addition, all eligible participants had to be receiving appropriate treatment for either episodic or chronic migraine.
In all, 16,789 participants met criteria for migraine. Of this group, 9,184 patients had a MIDAS score of grade II or higher and reported health insurance status. In this subgroup, 7,930 (86.3%) patients had episodic migraine and 1,254 (13.7%) had chronic migraine.
A total of 2,187 (27.6%) patients with episodic migraine and 512 (40.8%) patients with chronic migraine were under the care of a healthcare professional. Of this group, 1,655 patients with episodic migraine (75.7%) and 168 with chronic migraine (32.8%) reported receiving an appropriate diagnosis.
Of participants who successfully overcame the first two optimal management barriers—a consultation with a healthcare professional and an appropriate diagnosis—1,133 (68.5%) episodic migraineurs and 113 (67.3%) chronic migraineurs reported receiving minimally adequate acute treatment.
Furthermore, 1,430 (86.4%) episodic migraineurs and 127 (75.6%) chronic migraineurs reported receiving minimally adequate preventive medication treatment. In addition, 982 (59.3%) episodic migraineurs and 88 (52.4%) chronic migraineurs received minimally adequate acute and preventive treatment.
Acute medication overuse was relatively common, the investigators reported. A total of 310 (31.6%) patients with episodic migraine and 66 (75%) patients with chronic migraine met criteria for acute medication overuse.
“Overuse of acute medication for migraine in people with chronic migraine is a serious concern and is associated with increased risks of migraine progression, headache-related disability, and anxiety and depression. Active patient management and education is important to reduce the likelihood of medication overuse,” said Dr. Buse.
Among all eligible respondents, only 672 (8.5%) patients with episodic migraine and 22 (1.8%) with chronic migraine overcame all four barriers to optimal care.
The researchers found no significant effect of ethnicity or race on the likelihood of overcoming any barrier, but they acknowledged that participation bias might have contributed to this lack of difference. Higher annual household income was significantly associated with high likelihood of surmounting all four barriers.
“The analysis of sociodemographics revealed that female sex and higher annual household income showed a strong relationship with likelihood of obtaining an accurate episodic migraine or chronic migraine diagnosis,” said Dr. Buse.
“Although the reasons for this are not clear, it may be that women are more likely to convey the full scope of their symptoms during consultation. Additionally, the known prevalence of migraine in women may influence healthcare providers by reducing suspicion of chronic migraine in men,” she added.
The CaMEO Study was funded by Allergan (now AbbVie). Dr. Buse reports receiving grant support and honoraria from Allergan, Amgen, Biohaven, Eli Lilly and Co, and Promius. She also receives compensation for work on the editorial board of Current Pain and Headache Reports.
This article first appeared on Medscape.com.
, new research shows.
Results from a survey study showed less than 8% of patients with episodic migraine and less than 2% of patients with chronic migraine were able to overcome four key treatment barriers associated with optimal migraine management. These included current medical consultation, appropriate diagnosis, minimally adequate acute and preventive pharmacologic treatment (if indicated), and absence of acute medication overdose.
The researchers also evaluated any potential impact of race, ethnicity, and sociodemographic factors on these barriers.
“While chronic migraine was associated with higher rates of consulting, only 1.8% of respondents with chronic migraine traversed all four barriers compared with 8.5% of those with episodic migraine,” the investigators, led by Dawn C. Buse, PhD, clinical professor of neurology at Albert Einstein College of Medicine of Yeshiva University in New York City, noted.
The study was presented at the virtual annual meeting of the American Headache Society.
Ongoing challenges
Migraineurs’ challenges include receiving an appropriate diagnosis and finding effective acute and preventive treatments, the researchers noted. Many patients do not receive optimal care. Previous research by Dr. Buse and colleagues showed that general clinicians were less likely to provide an appropriate diagnosis of migraine compared with headache specialists.
Among patients with chronic migraine who consulted headache specialists, most did not receive an accurate diagnosis of chronic migraine. Data also indicate that a minority, approximately 34%, of patients with chronic migraine used preventive pharmacologic treatments.
The investigators analyzed data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study to determine the proportion of patients who overcame four prespecified barriers to good outcomes.
Eligible participants met modified International Classification of Headache Disorders (3rd edition) criteria for migraine, had Migraine Disability Assessment Scores (MIDAS) of grade II or higher, and provided data on health insurance status. In addition, all eligible participants had to be receiving appropriate treatment for either episodic or chronic migraine.
In all, 16,789 participants met criteria for migraine. Of this group, 9,184 patients had a MIDAS score of grade II or higher and reported health insurance status. In this subgroup, 7,930 (86.3%) patients had episodic migraine and 1,254 (13.7%) had chronic migraine.
A total of 2,187 (27.6%) patients with episodic migraine and 512 (40.8%) patients with chronic migraine were under the care of a healthcare professional. Of this group, 1,655 patients with episodic migraine (75.7%) and 168 with chronic migraine (32.8%) reported receiving an appropriate diagnosis.
Of participants who successfully overcame the first two optimal management barriers—a consultation with a healthcare professional and an appropriate diagnosis—1,133 (68.5%) episodic migraineurs and 113 (67.3%) chronic migraineurs reported receiving minimally adequate acute treatment.
Furthermore, 1,430 (86.4%) episodic migraineurs and 127 (75.6%) chronic migraineurs reported receiving minimally adequate preventive medication treatment. In addition, 982 (59.3%) episodic migraineurs and 88 (52.4%) chronic migraineurs received minimally adequate acute and preventive treatment.
Acute medication overuse was relatively common, the investigators reported. A total of 310 (31.6%) patients with episodic migraine and 66 (75%) patients with chronic migraine met criteria for acute medication overuse.
“Overuse of acute medication for migraine in people with chronic migraine is a serious concern and is associated with increased risks of migraine progression, headache-related disability, and anxiety and depression. Active patient management and education is important to reduce the likelihood of medication overuse,” said Dr. Buse.
Among all eligible respondents, only 672 (8.5%) patients with episodic migraine and 22 (1.8%) with chronic migraine overcame all four barriers to optimal care.
The researchers found no significant effect of ethnicity or race on the likelihood of overcoming any barrier, but they acknowledged that participation bias might have contributed to this lack of difference. Higher annual household income was significantly associated with high likelihood of surmounting all four barriers.
“The analysis of sociodemographics revealed that female sex and higher annual household income showed a strong relationship with likelihood of obtaining an accurate episodic migraine or chronic migraine diagnosis,” said Dr. Buse.
“Although the reasons for this are not clear, it may be that women are more likely to convey the full scope of their symptoms during consultation. Additionally, the known prevalence of migraine in women may influence healthcare providers by reducing suspicion of chronic migraine in men,” she added.
The CaMEO Study was funded by Allergan (now AbbVie). Dr. Buse reports receiving grant support and honoraria from Allergan, Amgen, Biohaven, Eli Lilly and Co, and Promius. She also receives compensation for work on the editorial board of Current Pain and Headache Reports.
This article first appeared on Medscape.com.
From AHS 2020
New guidance to help manage OCD during COVID-19
Two international specialty societies have jointly released new guidance on management of obsessive-compulsive disorder (OCD) during the COVID-19 pandemic.
“Individuals with OCD, particularly those with contamination concerns or hypochondriacal kinds of worries associated with OCD, people who have perfectionistic type of rituals, or who worry about transmitting COVID-19 [to others] might be particularly vulnerable to this pandemic,” statement coauthor Michael Van Ameringen, MD, professor, of the department of psychiatry and behavioral neurosciences, McMaster University, Hamilton, Ont., said in an interview.
The guidance, issued by the International College of Obsessive Compulsive Spectrum Disorders (ICOCS) and the Obsessive-Compulsive and Related Disorders Research Network (OCRN) of the European College of Neuropsychopharmacology, emphasizes the importance of using pharmacotherapy as a first-line approach, suspending or reducing exposure and response prevention (ERP), and offering psychoeducation.
The statement was published in the July issue of Comprehensive Psychiatry.
Confirm OCD diagnosis
A diagnosis of OCD should be confirmed, and it is important to clarify whether the current symptoms are a “rational or exaggerated response to recent highly stressful events” or a worsening of obsessive-compulsive symptomatology, the statement notes.
Some patients may experience an exacerbation of comorbid conditions such as anxiety disorder, depression, bipolar disorder, or posttraumatic stress disorder (PTSD), which may need to be managed separately.
The authors recommend consulting the World Health Organization (WHO) guidelines regarding mental health and psychosocial considerations during the COVID-19 outbreak.
“Several suicidal patients with OCD have come to the clinic during the pandemic,” reported Dr. Van Ameringen, director of the MacAnxiety Research Centre in Hamilton. “They felt overwhelmed and that they were contaminating themselves with everything they did, including breathing.”
The authors encourage clinicians to assess suicide risk using validated instruments, such as the Columbia Suicide Severity Rating Scale, and hospitalize patients if necessary.
Pharmacotherapy is “the most efficacious first-line treatment modality” for adults and children with OCD and contamination, washing, or cleaning symptoms during the pandemic, the authors note.
They recommend a stepwise pharmacotherapeutic approach:
Type of medication
- Selective serotonin reuptake inhibitor (SSRI) as first choice.
- Another SSRI if no response to first SSRI.
- Clomipramine as third choice.
Dosage
- Gradually increase suboptimal dose, paying attention to contraindications, adverse effects.
SSRI resistance
- Low-dose adjunctive antipsychotic (for example, aripiprazole, risperidone, quetiapine, olanzapine), for incomplete response, especially if tic is present.
Adherence
- Ensure patient can obtain an adequate supply of medication and is taking it regularly.
- Involve family/caregivers if adherence is problematic.
- Pill organizers and reminder apps may be helpful.
A role for CBT?
Under ordinary circumstances, CBT is considered a first-line intervention for OCD. However, there are risks associated specifically with ERP during the pandemic.
“In ERP, people are being exposed to things that trigger their OCD, so those with contamination fears may be asked to touch things in public places, then resist washing their hands, which would counter public health recommendations,” Dr. Van Ameringen said.
In vivo exposure should be paused, but some ERP interventions can be adapted or modified “on a case-by-case basis,” the authors state. For patients whose exposure is unrelated to contamination, other ERP treatment plans can be continued.
The authors recommend using therapy time to “prevent patients from deteriorating” by encouraging them to engage in activity scheduling and structuring the day to include physical activity, enjoyable activities, practices that enhance sleep, and mindfulness.
Limit news exposure
A central component of managing OCD during the pandemic is providing “balanced information” about the known risks and impact of COVID-19, the authors stated.
Dr. Van Ameringen recounted that he has seen patients who have washed their hands for hours and bleached or even boiled their hands.
“Some [patients with OCD] wonder if it’s safe to touch a newspaper or if they can catch the virus if they go outside, even if no one is around,” he reported. “Some wonder if they should ‘quarantine’ a package or wear gloves to bed.”
It has been helpful, for example, to show them the public health guidance of the WHO or CDC advising that 20 seconds of hand washing is adequate, he said.
“We have also seen that some of the sources of information about COVID-19 haven’t been factually correct and that people were watching the news all day and being bombarded with information from every source, which was making their symptoms a thousand times worse,” Dr. Van Ameringen reported.
Therefore, The authors also advise clinicians to “take a compassionate, calming,” and culturally sensitive approach to inform all interventions.
Unique anchor
Commenting on the statement in an interview, Debanjan Banerjee, MD, geriatric psychiatry senior resident, National Institute of Mental Health and Neurosciences, Bangalore, India, said that this “comprehensive guideline, based on expert experience, will serve as a guiding framework for physicians and psychiatrists globally.”
In the “absence of systemic data so far, this guideline can provide a unique anchor of a global consensus on how to take care of those with preexisting OCD or newly emergent cases” said Dr. Banerjee, who was not involved in authoring the statement.
Also commenting on the statement, Jonathan Abramowitz, PhD, professor of psychology and neuroscience, University of North Carolina at Chapel Hill, said that he “generally agrees” with these guidelines but disagrees with the “apparent recommendations to scale back” ERP.
“The fact is that effective and safe ERP is possible, even during this time, even following the scientific guidance,” stated Dr. Abramowitz, editor-in-chief of the Journal of Obsessive-Compulsive and Related Disorders. He was not involved in the statement.
He noted that the International OCD Foundation offers educational programs for clinicians regarding the safe use of ERP during this time.
The authors acknowledge that their guideline is “largely based on empirical evidence” and should be regarded as “preliminary.” The guidance “will be updated as new information arises.”
No specific source of funding for the statement is listed. Dr. Van Ameringen reports being on the advisory boards of Allergan, Almatica, Brainsway, Janssen, Lundbeck, Myriad Neuroscience, Otsuka, and Purdue Pharma (Canada); is on the speakers bureau for Allergan, Lundbeck, Otsuka, Pfizer, Purdue Pharma (Canada) and Takeda; and has received research support from Janssen, Purdue Pharma (Canada), the Canada Foundation for Innovation, and Hamilton Academic Health Sciences Organization. The other authors’ disclosures are listed on the original paper. Dr. Banerjee and Dr. Abramowitz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Two international specialty societies have jointly released new guidance on management of obsessive-compulsive disorder (OCD) during the COVID-19 pandemic.
“Individuals with OCD, particularly those with contamination concerns or hypochondriacal kinds of worries associated with OCD, people who have perfectionistic type of rituals, or who worry about transmitting COVID-19 [to others] might be particularly vulnerable to this pandemic,” statement coauthor Michael Van Ameringen, MD, professor, of the department of psychiatry and behavioral neurosciences, McMaster University, Hamilton, Ont., said in an interview.
The guidance, issued by the International College of Obsessive Compulsive Spectrum Disorders (ICOCS) and the Obsessive-Compulsive and Related Disorders Research Network (OCRN) of the European College of Neuropsychopharmacology, emphasizes the importance of using pharmacotherapy as a first-line approach, suspending or reducing exposure and response prevention (ERP), and offering psychoeducation.
The statement was published in the July issue of Comprehensive Psychiatry.
Confirm OCD diagnosis
A diagnosis of OCD should be confirmed, and it is important to clarify whether the current symptoms are a “rational or exaggerated response to recent highly stressful events” or a worsening of obsessive-compulsive symptomatology, the statement notes.
Some patients may experience an exacerbation of comorbid conditions such as anxiety disorder, depression, bipolar disorder, or posttraumatic stress disorder (PTSD), which may need to be managed separately.
The authors recommend consulting the World Health Organization (WHO) guidelines regarding mental health and psychosocial considerations during the COVID-19 outbreak.
“Several suicidal patients with OCD have come to the clinic during the pandemic,” reported Dr. Van Ameringen, director of the MacAnxiety Research Centre in Hamilton. “They felt overwhelmed and that they were contaminating themselves with everything they did, including breathing.”
The authors encourage clinicians to assess suicide risk using validated instruments, such as the Columbia Suicide Severity Rating Scale, and hospitalize patients if necessary.
Pharmacotherapy is “the most efficacious first-line treatment modality” for adults and children with OCD and contamination, washing, or cleaning symptoms during the pandemic, the authors note.
They recommend a stepwise pharmacotherapeutic approach:
Type of medication
- Selective serotonin reuptake inhibitor (SSRI) as first choice.
- Another SSRI if no response to first SSRI.
- Clomipramine as third choice.
Dosage
- Gradually increase suboptimal dose, paying attention to contraindications, adverse effects.
SSRI resistance
- Low-dose adjunctive antipsychotic (for example, aripiprazole, risperidone, quetiapine, olanzapine), for incomplete response, especially if tic is present.
Adherence
- Ensure patient can obtain an adequate supply of medication and is taking it regularly.
- Involve family/caregivers if adherence is problematic.
- Pill organizers and reminder apps may be helpful.
A role for CBT?
Under ordinary circumstances, CBT is considered a first-line intervention for OCD. However, there are risks associated specifically with ERP during the pandemic.
“In ERP, people are being exposed to things that trigger their OCD, so those with contamination fears may be asked to touch things in public places, then resist washing their hands, which would counter public health recommendations,” Dr. Van Ameringen said.
In vivo exposure should be paused, but some ERP interventions can be adapted or modified “on a case-by-case basis,” the authors state. For patients whose exposure is unrelated to contamination, other ERP treatment plans can be continued.
The authors recommend using therapy time to “prevent patients from deteriorating” by encouraging them to engage in activity scheduling and structuring the day to include physical activity, enjoyable activities, practices that enhance sleep, and mindfulness.
Limit news exposure
A central component of managing OCD during the pandemic is providing “balanced information” about the known risks and impact of COVID-19, the authors stated.
Dr. Van Ameringen recounted that he has seen patients who have washed their hands for hours and bleached or even boiled their hands.
“Some [patients with OCD] wonder if it’s safe to touch a newspaper or if they can catch the virus if they go outside, even if no one is around,” he reported. “Some wonder if they should ‘quarantine’ a package or wear gloves to bed.”
It has been helpful, for example, to show them the public health guidance of the WHO or CDC advising that 20 seconds of hand washing is adequate, he said.
“We have also seen that some of the sources of information about COVID-19 haven’t been factually correct and that people were watching the news all day and being bombarded with information from every source, which was making their symptoms a thousand times worse,” Dr. Van Ameringen reported.
Therefore, The authors also advise clinicians to “take a compassionate, calming,” and culturally sensitive approach to inform all interventions.
Unique anchor
Commenting on the statement in an interview, Debanjan Banerjee, MD, geriatric psychiatry senior resident, National Institute of Mental Health and Neurosciences, Bangalore, India, said that this “comprehensive guideline, based on expert experience, will serve as a guiding framework for physicians and psychiatrists globally.”
In the “absence of systemic data so far, this guideline can provide a unique anchor of a global consensus on how to take care of those with preexisting OCD or newly emergent cases” said Dr. Banerjee, who was not involved in authoring the statement.
Also commenting on the statement, Jonathan Abramowitz, PhD, professor of psychology and neuroscience, University of North Carolina at Chapel Hill, said that he “generally agrees” with these guidelines but disagrees with the “apparent recommendations to scale back” ERP.
“The fact is that effective and safe ERP is possible, even during this time, even following the scientific guidance,” stated Dr. Abramowitz, editor-in-chief of the Journal of Obsessive-Compulsive and Related Disorders. He was not involved in the statement.
He noted that the International OCD Foundation offers educational programs for clinicians regarding the safe use of ERP during this time.
The authors acknowledge that their guideline is “largely based on empirical evidence” and should be regarded as “preliminary.” The guidance “will be updated as new information arises.”
No specific source of funding for the statement is listed. Dr. Van Ameringen reports being on the advisory boards of Allergan, Almatica, Brainsway, Janssen, Lundbeck, Myriad Neuroscience, Otsuka, and Purdue Pharma (Canada); is on the speakers bureau for Allergan, Lundbeck, Otsuka, Pfizer, Purdue Pharma (Canada) and Takeda; and has received research support from Janssen, Purdue Pharma (Canada), the Canada Foundation for Innovation, and Hamilton Academic Health Sciences Organization. The other authors’ disclosures are listed on the original paper. Dr. Banerjee and Dr. Abramowitz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Two international specialty societies have jointly released new guidance on management of obsessive-compulsive disorder (OCD) during the COVID-19 pandemic.
“Individuals with OCD, particularly those with contamination concerns or hypochondriacal kinds of worries associated with OCD, people who have perfectionistic type of rituals, or who worry about transmitting COVID-19 [to others] might be particularly vulnerable to this pandemic,” statement coauthor Michael Van Ameringen, MD, professor, of the department of psychiatry and behavioral neurosciences, McMaster University, Hamilton, Ont., said in an interview.
The guidance, issued by the International College of Obsessive Compulsive Spectrum Disorders (ICOCS) and the Obsessive-Compulsive and Related Disorders Research Network (OCRN) of the European College of Neuropsychopharmacology, emphasizes the importance of using pharmacotherapy as a first-line approach, suspending or reducing exposure and response prevention (ERP), and offering psychoeducation.
The statement was published in the July issue of Comprehensive Psychiatry.
Confirm OCD diagnosis
A diagnosis of OCD should be confirmed, and it is important to clarify whether the current symptoms are a “rational or exaggerated response to recent highly stressful events” or a worsening of obsessive-compulsive symptomatology, the statement notes.
Some patients may experience an exacerbation of comorbid conditions such as anxiety disorder, depression, bipolar disorder, or posttraumatic stress disorder (PTSD), which may need to be managed separately.
The authors recommend consulting the World Health Organization (WHO) guidelines regarding mental health and psychosocial considerations during the COVID-19 outbreak.
“Several suicidal patients with OCD have come to the clinic during the pandemic,” reported Dr. Van Ameringen, director of the MacAnxiety Research Centre in Hamilton. “They felt overwhelmed and that they were contaminating themselves with everything they did, including breathing.”
The authors encourage clinicians to assess suicide risk using validated instruments, such as the Columbia Suicide Severity Rating Scale, and hospitalize patients if necessary.
Pharmacotherapy is “the most efficacious first-line treatment modality” for adults and children with OCD and contamination, washing, or cleaning symptoms during the pandemic, the authors note.
They recommend a stepwise pharmacotherapeutic approach:
Type of medication
- Selective serotonin reuptake inhibitor (SSRI) as first choice.
- Another SSRI if no response to first SSRI.
- Clomipramine as third choice.
Dosage
- Gradually increase suboptimal dose, paying attention to contraindications, adverse effects.
SSRI resistance
- Low-dose adjunctive antipsychotic (for example, aripiprazole, risperidone, quetiapine, olanzapine), for incomplete response, especially if tic is present.
Adherence
- Ensure patient can obtain an adequate supply of medication and is taking it regularly.
- Involve family/caregivers if adherence is problematic.
- Pill organizers and reminder apps may be helpful.
A role for CBT?
Under ordinary circumstances, CBT is considered a first-line intervention for OCD. However, there are risks associated specifically with ERP during the pandemic.
“In ERP, people are being exposed to things that trigger their OCD, so those with contamination fears may be asked to touch things in public places, then resist washing their hands, which would counter public health recommendations,” Dr. Van Ameringen said.
In vivo exposure should be paused, but some ERP interventions can be adapted or modified “on a case-by-case basis,” the authors state. For patients whose exposure is unrelated to contamination, other ERP treatment plans can be continued.
The authors recommend using therapy time to “prevent patients from deteriorating” by encouraging them to engage in activity scheduling and structuring the day to include physical activity, enjoyable activities, practices that enhance sleep, and mindfulness.
Limit news exposure
A central component of managing OCD during the pandemic is providing “balanced information” about the known risks and impact of COVID-19, the authors stated.
Dr. Van Ameringen recounted that he has seen patients who have washed their hands for hours and bleached or even boiled their hands.
“Some [patients with OCD] wonder if it’s safe to touch a newspaper or if they can catch the virus if they go outside, even if no one is around,” he reported. “Some wonder if they should ‘quarantine’ a package or wear gloves to bed.”
It has been helpful, for example, to show them the public health guidance of the WHO or CDC advising that 20 seconds of hand washing is adequate, he said.
“We have also seen that some of the sources of information about COVID-19 haven’t been factually correct and that people were watching the news all day and being bombarded with information from every source, which was making their symptoms a thousand times worse,” Dr. Van Ameringen reported.
Therefore, The authors also advise clinicians to “take a compassionate, calming,” and culturally sensitive approach to inform all interventions.
Unique anchor
Commenting on the statement in an interview, Debanjan Banerjee, MD, geriatric psychiatry senior resident, National Institute of Mental Health and Neurosciences, Bangalore, India, said that this “comprehensive guideline, based on expert experience, will serve as a guiding framework for physicians and psychiatrists globally.”
In the “absence of systemic data so far, this guideline can provide a unique anchor of a global consensus on how to take care of those with preexisting OCD or newly emergent cases” said Dr. Banerjee, who was not involved in authoring the statement.
Also commenting on the statement, Jonathan Abramowitz, PhD, professor of psychology and neuroscience, University of North Carolina at Chapel Hill, said that he “generally agrees” with these guidelines but disagrees with the “apparent recommendations to scale back” ERP.
“The fact is that effective and safe ERP is possible, even during this time, even following the scientific guidance,” stated Dr. Abramowitz, editor-in-chief of the Journal of Obsessive-Compulsive and Related Disorders. He was not involved in the statement.
He noted that the International OCD Foundation offers educational programs for clinicians regarding the safe use of ERP during this time.
The authors acknowledge that their guideline is “largely based on empirical evidence” and should be regarded as “preliminary.” The guidance “will be updated as new information arises.”
No specific source of funding for the statement is listed. Dr. Van Ameringen reports being on the advisory boards of Allergan, Almatica, Brainsway, Janssen, Lundbeck, Myriad Neuroscience, Otsuka, and Purdue Pharma (Canada); is on the speakers bureau for Allergan, Lundbeck, Otsuka, Pfizer, Purdue Pharma (Canada) and Takeda; and has received research support from Janssen, Purdue Pharma (Canada), the Canada Foundation for Innovation, and Hamilton Academic Health Sciences Organization. The other authors’ disclosures are listed on the original paper. Dr. Banerjee and Dr. Abramowitz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Robotics lightens load for bariatric surgeons in super obese
Use of a robotics platform provides a surgeon with more information so they can make better decisions, especially in challenging situations of primary and revisional bariatric surgery, according to Cheguevara Afaneh, MD, of New York–Presbyterian Hospital and Weill Cornell Medical Center, New York.
“The value of modern technology is to be able to do the most difficult cases in a much simpler format,” he said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Dr. Afaneh shared examples of how robotic assistance can help surgeons address challenges in bariatric surgery clinical practice, including managing super- and super-super-obese patients, dealing with gastroesophageal reflux disease (GERD), and negating the impact of surgical assistant experience.
“Super-obese patients pose more of a challenge interoperatively for both the surgeon and the assistant,” Dr. Afaneh noted. He and colleagues conducted a study of perioperative outcomes and found no significant differences between morbidly obese and super-obese patients in perioperative morbidity or operating time when a robotic platform was used.
The benefits to the surgeon when using robotic assistance in super-obese patients include effortless navigation of the abdominal wall, the ability to execute complex maneuvers in a challenging environment, and the security of a stable platform with no assistant fatigue, Dr. Afaneh emphasized. “When you are using the robotic platform, you are negating a lot of the patient factors” and fatigue factors that make bariatric surgery in super-obese and super-super-obese patients especially difficult, he said.
For example, in a patient who weighed nearly 500 pounds, pulling up on the stomach to get behind the actual stomach is easier because assistant fatigue is not a factor, so the surgeon can take more time and prevent a more difficult dissection, he said. In addition, Dr. Afaneh’s research showed no difference in operative time, and that the robot assistance outcomes were reproducible across a range of body mass index categories.
Robotic assistance also allows for comparable outcomes in surgeons with less experience, notably in revisional surgery, said Dr. Afaneh. He reviewed data from his first year of experience in revisional procedures using robotic assistance to his partners’ more than 20 years of laparoscopic experience. He found no significant differences in operative time, complications, or conversions to open procedures.
However, the more important message from the study was that less experienced surgeons were able to safely perform some of the most difficult revisional procedures without increasing morbidity, compared with more experienced surgeons. The data suggest that, with robotic assistance, surgeons early in their career can take on some of the bigger cases and expect outcomes similar to those of more experienced surgeons, Dr. Afaneh said.
Robotics has demonstrated improved outcomes in managing patients with GERD, which has become a common problem after bariatric surgery, noted Dr. Afaneh. When he and his colleagues reviewed data from their center on robotic-assisted approaches to GERD after bariatric surgery, they found that, even in primary magnetic sphincter operations, “robotics maintains comparable outcomes in revisional sleeve gastrectomy fields,” he said.
Another notable benefit of robotics in bariatric surgery is the negation of the “assistant effect,” said Dr. Afaneh. Often, less experienced surgeons are matched with less experienced assistants. “We took a look at the use of the robotic in cases of complex GI surgeries,” he said. They compared laparoscopic and robotic cases and stratified them by third-year assistant or fellow. “If you had a fellow, the operative time dropped by half an hour for laparoscopic cases, but the time was no different in robotics cases,” regardless of the use of fellow or third-year assistant, he said.
“The robotic platform allows you to assist yourself,” and allows for full surgeon autonomy, Dr. Afaneh emphasized. “You are the best person at predicting your next step.” The robotics platform also serves as a teaching tool. “For those who teach, there is no added morbidity based on the assistance of the trainee,” he said. In addition, the improved visualization of robotics “allows for better appreciation of scarred tissue planes and more precise suturing,” he noted.
Overall, “one of the values of the robotic platform is shortening the learning curve,” Dr. Afaneh said. He reviewed data from his fellows and himself, and found no difference in operative times. “My mentee was able to achieve operative times as good as my third year in practice. The robotic platform shaved off several years of learning experience,” he said. Dr. Afaneh’s operative times also decreased with robotics, which shows how experienced surgeons learn from this technology, he said.
Dr. Afaneh disclosed serving as a consultant for Intuitive Surgical.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Use of a robotics platform provides a surgeon with more information so they can make better decisions, especially in challenging situations of primary and revisional bariatric surgery, according to Cheguevara Afaneh, MD, of New York–Presbyterian Hospital and Weill Cornell Medical Center, New York.
“The value of modern technology is to be able to do the most difficult cases in a much simpler format,” he said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Dr. Afaneh shared examples of how robotic assistance can help surgeons address challenges in bariatric surgery clinical practice, including managing super- and super-super-obese patients, dealing with gastroesophageal reflux disease (GERD), and negating the impact of surgical assistant experience.
“Super-obese patients pose more of a challenge interoperatively for both the surgeon and the assistant,” Dr. Afaneh noted. He and colleagues conducted a study of perioperative outcomes and found no significant differences between morbidly obese and super-obese patients in perioperative morbidity or operating time when a robotic platform was used.
The benefits to the surgeon when using robotic assistance in super-obese patients include effortless navigation of the abdominal wall, the ability to execute complex maneuvers in a challenging environment, and the security of a stable platform with no assistant fatigue, Dr. Afaneh emphasized. “When you are using the robotic platform, you are negating a lot of the patient factors” and fatigue factors that make bariatric surgery in super-obese and super-super-obese patients especially difficult, he said.
For example, in a patient who weighed nearly 500 pounds, pulling up on the stomach to get behind the actual stomach is easier because assistant fatigue is not a factor, so the surgeon can take more time and prevent a more difficult dissection, he said. In addition, Dr. Afaneh’s research showed no difference in operative time, and that the robot assistance outcomes were reproducible across a range of body mass index categories.
Robotic assistance also allows for comparable outcomes in surgeons with less experience, notably in revisional surgery, said Dr. Afaneh. He reviewed data from his first year of experience in revisional procedures using robotic assistance to his partners’ more than 20 years of laparoscopic experience. He found no significant differences in operative time, complications, or conversions to open procedures.
However, the more important message from the study was that less experienced surgeons were able to safely perform some of the most difficult revisional procedures without increasing morbidity, compared with more experienced surgeons. The data suggest that, with robotic assistance, surgeons early in their career can take on some of the bigger cases and expect outcomes similar to those of more experienced surgeons, Dr. Afaneh said.
Robotics has demonstrated improved outcomes in managing patients with GERD, which has become a common problem after bariatric surgery, noted Dr. Afaneh. When he and his colleagues reviewed data from their center on robotic-assisted approaches to GERD after bariatric surgery, they found that, even in primary magnetic sphincter operations, “robotics maintains comparable outcomes in revisional sleeve gastrectomy fields,” he said.
Another notable benefit of robotics in bariatric surgery is the negation of the “assistant effect,” said Dr. Afaneh. Often, less experienced surgeons are matched with less experienced assistants. “We took a look at the use of the robotic in cases of complex GI surgeries,” he said. They compared laparoscopic and robotic cases and stratified them by third-year assistant or fellow. “If you had a fellow, the operative time dropped by half an hour for laparoscopic cases, but the time was no different in robotics cases,” regardless of the use of fellow or third-year assistant, he said.
“The robotic platform allows you to assist yourself,” and allows for full surgeon autonomy, Dr. Afaneh emphasized. “You are the best person at predicting your next step.” The robotics platform also serves as a teaching tool. “For those who teach, there is no added morbidity based on the assistance of the trainee,” he said. In addition, the improved visualization of robotics “allows for better appreciation of scarred tissue planes and more precise suturing,” he noted.
Overall, “one of the values of the robotic platform is shortening the learning curve,” Dr. Afaneh said. He reviewed data from his fellows and himself, and found no difference in operative times. “My mentee was able to achieve operative times as good as my third year in practice. The robotic platform shaved off several years of learning experience,” he said. Dr. Afaneh’s operative times also decreased with robotics, which shows how experienced surgeons learn from this technology, he said.
Dr. Afaneh disclosed serving as a consultant for Intuitive Surgical.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Use of a robotics platform provides a surgeon with more information so they can make better decisions, especially in challenging situations of primary and revisional bariatric surgery, according to Cheguevara Afaneh, MD, of New York–Presbyterian Hospital and Weill Cornell Medical Center, New York.
“The value of modern technology is to be able to do the most difficult cases in a much simpler format,” he said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Dr. Afaneh shared examples of how robotic assistance can help surgeons address challenges in bariatric surgery clinical practice, including managing super- and super-super-obese patients, dealing with gastroesophageal reflux disease (GERD), and negating the impact of surgical assistant experience.
“Super-obese patients pose more of a challenge interoperatively for both the surgeon and the assistant,” Dr. Afaneh noted. He and colleagues conducted a study of perioperative outcomes and found no significant differences between morbidly obese and super-obese patients in perioperative morbidity or operating time when a robotic platform was used.
The benefits to the surgeon when using robotic assistance in super-obese patients include effortless navigation of the abdominal wall, the ability to execute complex maneuvers in a challenging environment, and the security of a stable platform with no assistant fatigue, Dr. Afaneh emphasized. “When you are using the robotic platform, you are negating a lot of the patient factors” and fatigue factors that make bariatric surgery in super-obese and super-super-obese patients especially difficult, he said.
For example, in a patient who weighed nearly 500 pounds, pulling up on the stomach to get behind the actual stomach is easier because assistant fatigue is not a factor, so the surgeon can take more time and prevent a more difficult dissection, he said. In addition, Dr. Afaneh’s research showed no difference in operative time, and that the robot assistance outcomes were reproducible across a range of body mass index categories.
Robotic assistance also allows for comparable outcomes in surgeons with less experience, notably in revisional surgery, said Dr. Afaneh. He reviewed data from his first year of experience in revisional procedures using robotic assistance to his partners’ more than 20 years of laparoscopic experience. He found no significant differences in operative time, complications, or conversions to open procedures.
However, the more important message from the study was that less experienced surgeons were able to safely perform some of the most difficult revisional procedures without increasing morbidity, compared with more experienced surgeons. The data suggest that, with robotic assistance, surgeons early in their career can take on some of the bigger cases and expect outcomes similar to those of more experienced surgeons, Dr. Afaneh said.
Robotics has demonstrated improved outcomes in managing patients with GERD, which has become a common problem after bariatric surgery, noted Dr. Afaneh. When he and his colleagues reviewed data from their center on robotic-assisted approaches to GERD after bariatric surgery, they found that, even in primary magnetic sphincter operations, “robotics maintains comparable outcomes in revisional sleeve gastrectomy fields,” he said.
Another notable benefit of robotics in bariatric surgery is the negation of the “assistant effect,” said Dr. Afaneh. Often, less experienced surgeons are matched with less experienced assistants. “We took a look at the use of the robotic in cases of complex GI surgeries,” he said. They compared laparoscopic and robotic cases and stratified them by third-year assistant or fellow. “If you had a fellow, the operative time dropped by half an hour for laparoscopic cases, but the time was no different in robotics cases,” regardless of the use of fellow or third-year assistant, he said.
“The robotic platform allows you to assist yourself,” and allows for full surgeon autonomy, Dr. Afaneh emphasized. “You are the best person at predicting your next step.” The robotics platform also serves as a teaching tool. “For those who teach, there is no added morbidity based on the assistance of the trainee,” he said. In addition, the improved visualization of robotics “allows for better appreciation of scarred tissue planes and more precise suturing,” he noted.
Overall, “one of the values of the robotic platform is shortening the learning curve,” Dr. Afaneh said. He reviewed data from his fellows and himself, and found no difference in operative times. “My mentee was able to achieve operative times as good as my third year in practice. The robotic platform shaved off several years of learning experience,” he said. Dr. Afaneh’s operative times also decreased with robotics, which shows how experienced surgeons learn from this technology, he said.
Dr. Afaneh disclosed serving as a consultant for Intuitive Surgical.
Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM MISS
Comorbidities increase COVID-19 deaths by factor of 12
, compared with those who have no such condition, according to the Centers for Disease Control and Prevention.
Among those with underlying conditions such as cardiovascular disease or diabetes, 45.4% of patients with COVID-19 were hospitalized, versus 7.6% of patients without an underlying condition, said Erin K. Stokes, MPH, and associates of the CDC COVID-19 Emergency Response team.
The difference in deaths was even greater over the study period of Jan. 22–May 30, 2020: 19.5% of COVID-19 patients with underlying conditions died, compared with 1.6% of those with no underlying condition. The gap narrowed, however, for ICU admissions, with corresponding rates of 8.5% and 1.5%, the investigators reported June 15 in the Morbidity and Mortality Weekly Report.
“The COVID-19 pandemic continues to be severe, particularly in certain population groups,” they said.
The cumulative incidence of laboratory-confirmed cases up to May 30, for instance, was nearly twice as high for those aged 80 years and over (902 per 100,000 population) than for those aged 70-79 years (464.2 per 100,000). Those aged 50-59 years had the second-highest incidence, 550.5 per 100,000, Ms. Stokes and associates said.
“Among cases with known race and ethnicity, 33% of persons were Hispanic, 22% were black, and 1.3% were [American Indian/Alaska Native]. These findings suggest that persons in these groups, who account for 18%, 13%, and 0.7% of the U.S. population, respectively, are disproportionately affected by the COVID-19 pandemic,” they wrote.
Another source of disparity: “Incidence among males and females was similar overall, [but] severe outcomes were more commonly reported among males,” the investigators noted. Cumulative incidence was 401.1 per 100,000 for males and 406.0 for females, but 6.0% of male patients died, compared with 4.8% of females.
As of May 30, a total of 1,761,503 cases and 103,700 deaths had been reported to the CDC. Of those cases, approximately 1.3 million were included in the analysis, with data on individual underlying health conditions available for 287,320 (22%). The split on those cases was 198,879 with health conditions and 88,411 without, the CDC data show.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females, Ms. Stokes and associates said.
The pandemic “is an ongoing public health crisis in the United States that continues to affect all populations and result in severe outcomes including death,” they said, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.”
SOURCE: Stokes EK et al. MMWR. 2020 Jun 15;69(early release):1-7.
, compared with those who have no such condition, according to the Centers for Disease Control and Prevention.

Among those with underlying conditions such as cardiovascular disease or diabetes, 45.4% of patients with COVID-19 were hospitalized, versus 7.6% of patients without an underlying condition, said Erin K. Stokes, MPH, and associates of the CDC COVID-19 Emergency Response team.
The difference in deaths was even greater over the study period of Jan. 22–May 30, 2020: 19.5% of COVID-19 patients with underlying conditions died, compared with 1.6% of those with no underlying condition. The gap narrowed, however, for ICU admissions, with corresponding rates of 8.5% and 1.5%, the investigators reported June 15 in the Morbidity and Mortality Weekly Report.
“The COVID-19 pandemic continues to be severe, particularly in certain population groups,” they said.
The cumulative incidence of laboratory-confirmed cases up to May 30, for instance, was nearly twice as high for those aged 80 years and over (902 per 100,000 population) than for those aged 70-79 years (464.2 per 100,000). Those aged 50-59 years had the second-highest incidence, 550.5 per 100,000, Ms. Stokes and associates said.
“Among cases with known race and ethnicity, 33% of persons were Hispanic, 22% were black, and 1.3% were [American Indian/Alaska Native]. These findings suggest that persons in these groups, who account for 18%, 13%, and 0.7% of the U.S. population, respectively, are disproportionately affected by the COVID-19 pandemic,” they wrote.
Another source of disparity: “Incidence among males and females was similar overall, [but] severe outcomes were more commonly reported among males,” the investigators noted. Cumulative incidence was 401.1 per 100,000 for males and 406.0 for females, but 6.0% of male patients died, compared with 4.8% of females.
As of May 30, a total of 1,761,503 cases and 103,700 deaths had been reported to the CDC. Of those cases, approximately 1.3 million were included in the analysis, with data on individual underlying health conditions available for 287,320 (22%). The split on those cases was 198,879 with health conditions and 88,411 without, the CDC data show.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females, Ms. Stokes and associates said.
The pandemic “is an ongoing public health crisis in the United States that continues to affect all populations and result in severe outcomes including death,” they said, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.”
SOURCE: Stokes EK et al. MMWR. 2020 Jun 15;69(early release):1-7.
, compared with those who have no such condition, according to the Centers for Disease Control and Prevention.

Among those with underlying conditions such as cardiovascular disease or diabetes, 45.4% of patients with COVID-19 were hospitalized, versus 7.6% of patients without an underlying condition, said Erin K. Stokes, MPH, and associates of the CDC COVID-19 Emergency Response team.
The difference in deaths was even greater over the study period of Jan. 22–May 30, 2020: 19.5% of COVID-19 patients with underlying conditions died, compared with 1.6% of those with no underlying condition. The gap narrowed, however, for ICU admissions, with corresponding rates of 8.5% and 1.5%, the investigators reported June 15 in the Morbidity and Mortality Weekly Report.
“The COVID-19 pandemic continues to be severe, particularly in certain population groups,” they said.
The cumulative incidence of laboratory-confirmed cases up to May 30, for instance, was nearly twice as high for those aged 80 years and over (902 per 100,000 population) than for those aged 70-79 years (464.2 per 100,000). Those aged 50-59 years had the second-highest incidence, 550.5 per 100,000, Ms. Stokes and associates said.
“Among cases with known race and ethnicity, 33% of persons were Hispanic, 22% were black, and 1.3% were [American Indian/Alaska Native]. These findings suggest that persons in these groups, who account for 18%, 13%, and 0.7% of the U.S. population, respectively, are disproportionately affected by the COVID-19 pandemic,” they wrote.
Another source of disparity: “Incidence among males and females was similar overall, [but] severe outcomes were more commonly reported among males,” the investigators noted. Cumulative incidence was 401.1 per 100,000 for males and 406.0 for females, but 6.0% of male patients died, compared with 4.8% of females.
As of May 30, a total of 1,761,503 cases and 103,700 deaths had been reported to the CDC. Of those cases, approximately 1.3 million were included in the analysis, with data on individual underlying health conditions available for 287,320 (22%). The split on those cases was 198,879 with health conditions and 88,411 without, the CDC data show.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females, Ms. Stokes and associates said.
The pandemic “is an ongoing public health crisis in the United States that continues to affect all populations and result in severe outcomes including death,” they said, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.”
SOURCE: Stokes EK et al. MMWR. 2020 Jun 15;69(early release):1-7.
FROM MMWR
Weight loss stays consistent in one- and two-step in gastric band conversion
with either a one- or two-step procedure, a study of 78 patients showed.
“Laparoscopic adjustable gastric banding (LAGB) has largely fallen out of favor, likely related to variable efficacy in weight reduction coupled with poor effectiveness in reducing obesity related comorbidities like type 2 diabetes and hypercholesterolemia,” Vasu Chirumamilla, MD, of Westchester Medical Center, Valhalla, N.Y., and colleagues wrote in a poster presented at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education.
LAGB also can cause complications including, slippage, erosion, and gastric pouch dilation; subsequently many patients undergo conversion to laparoscopic sleeve gastrectomy (LSG). However, the impact of a one-step vs. two-step conversion procedure on patient weight loss remains unclear, the researchers said.
To compare weight loss after the two types of procedures, the researchers reviewed data from 78 patients (71 women) aged 15-74 years treated between 2013 and 2018 at a multi-surgeon, private practice bariatric surgery center. All patients had a history of LAGB; 31 underwent conversion to LSG in one stage, and 47 underwent conversion in two stages. Weight loss, defined as the percentage excess weight loss, was the primary endpoint.
The average excess weight loss was 44% for patients in both the one-stage and two-stage groups, and body mass index decreased by 8.9 points and 8.8 points, respectively, in the two groups, the researchers wrote.
Patients in the two-stage group experienced a significant increase in body mass index (P = .008) during the time between band removal to sleeve gastrectomy, which was an average of 207 days, they said.
The findings were limited in part by the small sample size and retrospective design, and more data are needed to compare complication rates in one-stage and two-stage procedures, the researchers noted. However, the results showed “no difference in excess weight loss in patients converted from laparoscopic adjustable gastric band to sleeve gastrectomy in one-stage versus a two-stage procedure,” they concluded.
“LAGB used to be a very popular weight loss procedure – bands were placed in a great deal of patients,” Dr. Chirumamilla said in an interview. “Now those patients are presenting with increasing frequency to bariatric surgeons with band complications or weight regain. The volume for LSG is increasing and results in percentage excess weight loss of approximately 65% versus approximately 42% for LAGB,” he said. A goal of the study was to provide patients and the surgeons with a more informed approach to performing and consenting to the particular operation, he added.
“The results have not surprised us, because as long as done by experienced surgeons on compliant patients the weight loss outcomes from the day of surgery onward should be equivalent,” Dr. Chirumamilla explained. “We were also not surprised to find that patients undergoing a two-stage conversion gained weight before their second-stage sleeve gastrectomy.”
The bottom line for clinicians is that “patients getting a conversion from band to sleeve in one-stage versus two-stages experience the same percentage excess body weight loss from time of surgery,” although two-stage patients do gain weight while awaiting their second-stage sleeve gastrectomy, Dr. Chirumamilla said.
“More research is needed to compare short- and long-term complications rates between one-stage and two-stage conversions. The ideal research situation would be a randomized, multicenter, large volume study to reduce bias,” he noted.
Dr. Chirumamilla’s collaborators included Akia Caine MD, Zachary Ballinger, Rebecca Castro, Thomas Cerabona MD, and Ashutosh Kaul MD, of the surgical group Advanced Surgeons at nygetfit.com.
Global Academy for Medical Education and this news organization are owned by the same parent company. The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Chirumamilla V et al. MISS 2020. Poster PA-14.
with either a one- or two-step procedure, a study of 78 patients showed.
“Laparoscopic adjustable gastric banding (LAGB) has largely fallen out of favor, likely related to variable efficacy in weight reduction coupled with poor effectiveness in reducing obesity related comorbidities like type 2 diabetes and hypercholesterolemia,” Vasu Chirumamilla, MD, of Westchester Medical Center, Valhalla, N.Y., and colleagues wrote in a poster presented at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education.
LAGB also can cause complications including, slippage, erosion, and gastric pouch dilation; subsequently many patients undergo conversion to laparoscopic sleeve gastrectomy (LSG). However, the impact of a one-step vs. two-step conversion procedure on patient weight loss remains unclear, the researchers said.
To compare weight loss after the two types of procedures, the researchers reviewed data from 78 patients (71 women) aged 15-74 years treated between 2013 and 2018 at a multi-surgeon, private practice bariatric surgery center. All patients had a history of LAGB; 31 underwent conversion to LSG in one stage, and 47 underwent conversion in two stages. Weight loss, defined as the percentage excess weight loss, was the primary endpoint.
The average excess weight loss was 44% for patients in both the one-stage and two-stage groups, and body mass index decreased by 8.9 points and 8.8 points, respectively, in the two groups, the researchers wrote.
Patients in the two-stage group experienced a significant increase in body mass index (P = .008) during the time between band removal to sleeve gastrectomy, which was an average of 207 days, they said.
The findings were limited in part by the small sample size and retrospective design, and more data are needed to compare complication rates in one-stage and two-stage procedures, the researchers noted. However, the results showed “no difference in excess weight loss in patients converted from laparoscopic adjustable gastric band to sleeve gastrectomy in one-stage versus a two-stage procedure,” they concluded.
“LAGB used to be a very popular weight loss procedure – bands were placed in a great deal of patients,” Dr. Chirumamilla said in an interview. “Now those patients are presenting with increasing frequency to bariatric surgeons with band complications or weight regain. The volume for LSG is increasing and results in percentage excess weight loss of approximately 65% versus approximately 42% for LAGB,” he said. A goal of the study was to provide patients and the surgeons with a more informed approach to performing and consenting to the particular operation, he added.
“The results have not surprised us, because as long as done by experienced surgeons on compliant patients the weight loss outcomes from the day of surgery onward should be equivalent,” Dr. Chirumamilla explained. “We were also not surprised to find that patients undergoing a two-stage conversion gained weight before their second-stage sleeve gastrectomy.”
The bottom line for clinicians is that “patients getting a conversion from band to sleeve in one-stage versus two-stages experience the same percentage excess body weight loss from time of surgery,” although two-stage patients do gain weight while awaiting their second-stage sleeve gastrectomy, Dr. Chirumamilla said.
“More research is needed to compare short- and long-term complications rates between one-stage and two-stage conversions. The ideal research situation would be a randomized, multicenter, large volume study to reduce bias,” he noted.
Dr. Chirumamilla’s collaborators included Akia Caine MD, Zachary Ballinger, Rebecca Castro, Thomas Cerabona MD, and Ashutosh Kaul MD, of the surgical group Advanced Surgeons at nygetfit.com.
Global Academy for Medical Education and this news organization are owned by the same parent company. The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Chirumamilla V et al. MISS 2020. Poster PA-14.
with either a one- or two-step procedure, a study of 78 patients showed.
“Laparoscopic adjustable gastric banding (LAGB) has largely fallen out of favor, likely related to variable efficacy in weight reduction coupled with poor effectiveness in reducing obesity related comorbidities like type 2 diabetes and hypercholesterolemia,” Vasu Chirumamilla, MD, of Westchester Medical Center, Valhalla, N.Y., and colleagues wrote in a poster presented at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education.
LAGB also can cause complications including, slippage, erosion, and gastric pouch dilation; subsequently many patients undergo conversion to laparoscopic sleeve gastrectomy (LSG). However, the impact of a one-step vs. two-step conversion procedure on patient weight loss remains unclear, the researchers said.
To compare weight loss after the two types of procedures, the researchers reviewed data from 78 patients (71 women) aged 15-74 years treated between 2013 and 2018 at a multi-surgeon, private practice bariatric surgery center. All patients had a history of LAGB; 31 underwent conversion to LSG in one stage, and 47 underwent conversion in two stages. Weight loss, defined as the percentage excess weight loss, was the primary endpoint.
The average excess weight loss was 44% for patients in both the one-stage and two-stage groups, and body mass index decreased by 8.9 points and 8.8 points, respectively, in the two groups, the researchers wrote.
Patients in the two-stage group experienced a significant increase in body mass index (P = .008) during the time between band removal to sleeve gastrectomy, which was an average of 207 days, they said.
The findings were limited in part by the small sample size and retrospective design, and more data are needed to compare complication rates in one-stage and two-stage procedures, the researchers noted. However, the results showed “no difference in excess weight loss in patients converted from laparoscopic adjustable gastric band to sleeve gastrectomy in one-stage versus a two-stage procedure,” they concluded.
“LAGB used to be a very popular weight loss procedure – bands were placed in a great deal of patients,” Dr. Chirumamilla said in an interview. “Now those patients are presenting with increasing frequency to bariatric surgeons with band complications or weight regain. The volume for LSG is increasing and results in percentage excess weight loss of approximately 65% versus approximately 42% for LAGB,” he said. A goal of the study was to provide patients and the surgeons with a more informed approach to performing and consenting to the particular operation, he added.
“The results have not surprised us, because as long as done by experienced surgeons on compliant patients the weight loss outcomes from the day of surgery onward should be equivalent,” Dr. Chirumamilla explained. “We were also not surprised to find that patients undergoing a two-stage conversion gained weight before their second-stage sleeve gastrectomy.”
The bottom line for clinicians is that “patients getting a conversion from band to sleeve in one-stage versus two-stages experience the same percentage excess body weight loss from time of surgery,” although two-stage patients do gain weight while awaiting their second-stage sleeve gastrectomy, Dr. Chirumamilla said.
“More research is needed to compare short- and long-term complications rates between one-stage and two-stage conversions. The ideal research situation would be a randomized, multicenter, large volume study to reduce bias,” he noted.
Dr. Chirumamilla’s collaborators included Akia Caine MD, Zachary Ballinger, Rebecca Castro, Thomas Cerabona MD, and Ashutosh Kaul MD, of the surgical group Advanced Surgeons at nygetfit.com.
Global Academy for Medical Education and this news organization are owned by the same parent company. The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Chirumamilla V et al. MISS 2020. Poster PA-14.
FROM MISS
Key clinical point: Weight loss was the same for patients after conversions from LAGB to LSG in both one-step and two-step procedures.
Major finding: The average excess weight loss was 44% for patients in both one-step and two-step conversion groups, and body mass index decreased by approximately 9 points in both groups.
Study details: The data come from a retrospective study of 78 adults who underwent conversion from LABG to LSG.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Chirumamilla V et al. MISS 2020. Poster PA-14.
Population study supports migraine–dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
“The findings of this study emphasize the need for studies in the migraine-dementia pathophysiology, in particular in migraine cases with aura,” said Sabrina Islamoska, MSc, PhD, a postdoctoral researcher in the department of public health at the University of Copenhagen. “This study highlights the importance of monitoring severe migraine to potentially prevent dementia.”
A national register-based study
The study used Danish national register–based data from 1988 to 2017 of 1.66 million individuals born between 1935 and 1956, retrieving exposure information until age 59 years and following individuals for dementia after age 60. The matched analysis included 18,135 people registered with migraine before age 59 and 1.38 million without migraine. The matched study population was 62,578.
A diagnosis of dementia or use of dementia medications after age 60 years was the main outcome. Covariates included socioeconomic factors, psychiatric comorbidities and other headache diagnoses.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Islamoska said.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“We also found a 20% higher but nonsignificant dementia rate in individuals who had migraine without aura,” she said. However, when the migraine-with-aura population was evaluated, it was found to have a dementia rate two times higher than people with no migraine. “The dementia rate was higher if individuals had more frequent hospital contacts with migraine.”
The findings support the hypothesis that migraine is a midlife risk factor for dementia later in life, she said.
“The findings underline the value of investigating the effect of migraine medications in dementia risk to assess the impact of mild to moderate migraines,” Dr. Islamoska said. “Therefore, the next step is to investigate the risk of dementia among users of migraine medications who are not diagnosed with migraines at hospitals.”
Strengths of the study, Dr. Islamoska noted, were its size and national nature of its population, that it included all migraine diagnoses at hospitals over a 29-year period, that it made adjustments for confounding of well-established dementia risk factors, and that it validated dementia diagnoses after age 60 years.
One limitation was that the study only included hospital-based diagnoses of dementia while 60% of cases in Denmark are undiagnosed, “thus our results only apply to migraine that is severe enough to require a hospital contact,” Dr. Islamoska said, while most migraine cases are treated in the primary care setting.
Also, the young study population may have a lower dementia risk. “We also know that age of migraine registration may not corresponded with the actual onset, since migraine is a complex disorder with individual variation in patient’s burden and course of disease,” Dr. Islamoska said.
“Future studies are needed to understand the pathological mechanisms underlying the relationship between migraine and dementia and to investigate whether proper prophylactic treatment of migraine can potentially prevent dementia,” Dr. Islamoska said. “In addition, when investigating the association between these two prevalent neurological disorders, the timing of migraine diagnosis and dementia onset is important to ensure temporality. We took this into account in our study to strengthen the validity of our results.”
‘Surprising’ findings
Andrew Charles, MD, director of the Goldberg Migraine Program at the University of California, Los Angeles, said the Danish study makes an important contribution to the literature on dementia risk factors. “Vanishingly small amounts of attention have been paid to migraine as a potential risk factor,” he said. However, he called the results “surprising” based on his own clinical experience. “I actually had a sense that migraine was somehow protective against Alzheimer’s or other kinds of dementias.”
He questioned if the migraine-dementia link could be a “reporting artifact” of migraine sufferers merely going to the neurologist, raising the likelihood of a positive migraine diagnosis. Nonetheless, the results are “intriguing” and raise important questions about migraine therapy and dementia risk.
“If it holds up, it really is something that behooves us to understand whether intervening in terms of therapy for migraine has even more consequences beyond just the immediate relief of symptoms,” Dr. Charles said. “It’s something we should be thinking about in terms of preventing longer-term consequences of this disorder.”
Dr. Islamoska disclosed that Veluxfondent funded the study as part of her PhD project. Dr. Charles disclosed he is a consultant to Amgen, Biohaven Pharmaceuticals, Eli Lilly, Lundbeck, and Novartis.
SOURCE: Islamoska S et al. AHS 2020, Submission 846214.
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
“The findings of this study emphasize the need for studies in the migraine-dementia pathophysiology, in particular in migraine cases with aura,” said Sabrina Islamoska, MSc, PhD, a postdoctoral researcher in the department of public health at the University of Copenhagen. “This study highlights the importance of monitoring severe migraine to potentially prevent dementia.”
A national register-based study
The study used Danish national register–based data from 1988 to 2017 of 1.66 million individuals born between 1935 and 1956, retrieving exposure information until age 59 years and following individuals for dementia after age 60. The matched analysis included 18,135 people registered with migraine before age 59 and 1.38 million without migraine. The matched study population was 62,578.
A diagnosis of dementia or use of dementia medications after age 60 years was the main outcome. Covariates included socioeconomic factors, psychiatric comorbidities and other headache diagnoses.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Islamoska said.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“We also found a 20% higher but nonsignificant dementia rate in individuals who had migraine without aura,” she said. However, when the migraine-with-aura population was evaluated, it was found to have a dementia rate two times higher than people with no migraine. “The dementia rate was higher if individuals had more frequent hospital contacts with migraine.”
The findings support the hypothesis that migraine is a midlife risk factor for dementia later in life, she said.
“The findings underline the value of investigating the effect of migraine medications in dementia risk to assess the impact of mild to moderate migraines,” Dr. Islamoska said. “Therefore, the next step is to investigate the risk of dementia among users of migraine medications who are not diagnosed with migraines at hospitals.”
Strengths of the study, Dr. Islamoska noted, were its size and national nature of its population, that it included all migraine diagnoses at hospitals over a 29-year period, that it made adjustments for confounding of well-established dementia risk factors, and that it validated dementia diagnoses after age 60 years.
One limitation was that the study only included hospital-based diagnoses of dementia while 60% of cases in Denmark are undiagnosed, “thus our results only apply to migraine that is severe enough to require a hospital contact,” Dr. Islamoska said, while most migraine cases are treated in the primary care setting.
Also, the young study population may have a lower dementia risk. “We also know that age of migraine registration may not corresponded with the actual onset, since migraine is a complex disorder with individual variation in patient’s burden and course of disease,” Dr. Islamoska said.
“Future studies are needed to understand the pathological mechanisms underlying the relationship between migraine and dementia and to investigate whether proper prophylactic treatment of migraine can potentially prevent dementia,” Dr. Islamoska said. “In addition, when investigating the association between these two prevalent neurological disorders, the timing of migraine diagnosis and dementia onset is important to ensure temporality. We took this into account in our study to strengthen the validity of our results.”
‘Surprising’ findings
Andrew Charles, MD, director of the Goldberg Migraine Program at the University of California, Los Angeles, said the Danish study makes an important contribution to the literature on dementia risk factors. “Vanishingly small amounts of attention have been paid to migraine as a potential risk factor,” he said. However, he called the results “surprising” based on his own clinical experience. “I actually had a sense that migraine was somehow protective against Alzheimer’s or other kinds of dementias.”
He questioned if the migraine-dementia link could be a “reporting artifact” of migraine sufferers merely going to the neurologist, raising the likelihood of a positive migraine diagnosis. Nonetheless, the results are “intriguing” and raise important questions about migraine therapy and dementia risk.
“If it holds up, it really is something that behooves us to understand whether intervening in terms of therapy for migraine has even more consequences beyond just the immediate relief of symptoms,” Dr. Charles said. “It’s something we should be thinking about in terms of preventing longer-term consequences of this disorder.”
Dr. Islamoska disclosed that Veluxfondent funded the study as part of her PhD project. Dr. Charles disclosed he is a consultant to Amgen, Biohaven Pharmaceuticals, Eli Lilly, Lundbeck, and Novartis.
SOURCE: Islamoska S et al. AHS 2020, Submission 846214.
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
“The findings of this study emphasize the need for studies in the migraine-dementia pathophysiology, in particular in migraine cases with aura,” said Sabrina Islamoska, MSc, PhD, a postdoctoral researcher in the department of public health at the University of Copenhagen. “This study highlights the importance of monitoring severe migraine to potentially prevent dementia.”
A national register-based study
The study used Danish national register–based data from 1988 to 2017 of 1.66 million individuals born between 1935 and 1956, retrieving exposure information until age 59 years and following individuals for dementia after age 60. The matched analysis included 18,135 people registered with migraine before age 59 and 1.38 million without migraine. The matched study population was 62,578.
A diagnosis of dementia or use of dementia medications after age 60 years was the main outcome. Covariates included socioeconomic factors, psychiatric comorbidities and other headache diagnoses.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Islamoska said.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“We also found a 20% higher but nonsignificant dementia rate in individuals who had migraine without aura,” she said. However, when the migraine-with-aura population was evaluated, it was found to have a dementia rate two times higher than people with no migraine. “The dementia rate was higher if individuals had more frequent hospital contacts with migraine.”
The findings support the hypothesis that migraine is a midlife risk factor for dementia later in life, she said.
“The findings underline the value of investigating the effect of migraine medications in dementia risk to assess the impact of mild to moderate migraines,” Dr. Islamoska said. “Therefore, the next step is to investigate the risk of dementia among users of migraine medications who are not diagnosed with migraines at hospitals.”
Strengths of the study, Dr. Islamoska noted, were its size and national nature of its population, that it included all migraine diagnoses at hospitals over a 29-year period, that it made adjustments for confounding of well-established dementia risk factors, and that it validated dementia diagnoses after age 60 years.
One limitation was that the study only included hospital-based diagnoses of dementia while 60% of cases in Denmark are undiagnosed, “thus our results only apply to migraine that is severe enough to require a hospital contact,” Dr. Islamoska said, while most migraine cases are treated in the primary care setting.
Also, the young study population may have a lower dementia risk. “We also know that age of migraine registration may not corresponded with the actual onset, since migraine is a complex disorder with individual variation in patient’s burden and course of disease,” Dr. Islamoska said.
“Future studies are needed to understand the pathological mechanisms underlying the relationship between migraine and dementia and to investigate whether proper prophylactic treatment of migraine can potentially prevent dementia,” Dr. Islamoska said. “In addition, when investigating the association between these two prevalent neurological disorders, the timing of migraine diagnosis and dementia onset is important to ensure temporality. We took this into account in our study to strengthen the validity of our results.”
‘Surprising’ findings
Andrew Charles, MD, director of the Goldberg Migraine Program at the University of California, Los Angeles, said the Danish study makes an important contribution to the literature on dementia risk factors. “Vanishingly small amounts of attention have been paid to migraine as a potential risk factor,” he said. However, he called the results “surprising” based on his own clinical experience. “I actually had a sense that migraine was somehow protective against Alzheimer’s or other kinds of dementias.”
He questioned if the migraine-dementia link could be a “reporting artifact” of migraine sufferers merely going to the neurologist, raising the likelihood of a positive migraine diagnosis. Nonetheless, the results are “intriguing” and raise important questions about migraine therapy and dementia risk.
“If it holds up, it really is something that behooves us to understand whether intervening in terms of therapy for migraine has even more consequences beyond just the immediate relief of symptoms,” Dr. Charles said. “It’s something we should be thinking about in terms of preventing longer-term consequences of this disorder.”
Dr. Islamoska disclosed that Veluxfondent funded the study as part of her PhD project. Dr. Charles disclosed he is a consultant to Amgen, Biohaven Pharmaceuticals, Eli Lilly, Lundbeck, and Novartis.
SOURCE: Islamoska S et al. AHS 2020, Submission 846214.
FROM AHS 2020
Omitting whole body irradiation before HSCT: Trial stopped early
Hematopoietic stem cell transplantation (HSCT) may offer the chance of a cure for patients with leukemia and other blood cancers, but the process of preparing the body to receive such a transplant can be brutal, involving whole body irradiation as well as chemotherapy conditioning. New results show that both steps are needed: a trial that omitted whole body irradiation in young patients with acute lymphoblastic leukemia (ALL) was stopped early because of significantly poorer outcomes.
The multicenter, global FORUM (For Omitting Radiation Under Majority Age) trial involved 75 centers in 17 countries between 2013 and 2018.
“Our study shows significantly better outcomes for total body irradiation compared to myeloablative chemo-conditioning arms, with no differences between the [two] chemo-conditioning groups,” concluded Christina Peters, MD, professor of pediatrics in the department of stem cell transplantation at St Anna Children’s Hospital in Vienna.
, she added.
Dr. Peters presented the findings as part of the virtual annual congress of the European Hematology Association.
Describing the results as “sobering,” session comoderator Shai Izraeli, MD, director of the department of hematology-oncology at Schneider Children’s Medical Center, in Petah Tikva, Israel, said an online comment from the virtual meeting audience reflected the reaction to these unwelcome results: “So we are still stuck with total body irradiation?”
Dr. Peters said the good news is that the number of patients needing to undergo stem cell transplants is low, and with research advances, may hopefully drop even further.
“Only 10% of patients under the age of 18 nowadays undergo allogeneic HSCT, and perhaps in the future that will become even less if we are able to rescue some of the groups with other immunological measures such as CAR-T cells and antibodies,” she said.
“I think it is very important to better identify those who really need total body irradiation in the future,” she added.
In an interview, Dr. Izraeli agreed.
“The prognosis of children after bone marrow transplantation is excellent – the majority are cured from their leukemia,” he said. “And we have to remember that those who undergo bone marrow transplant have the worst leukemias.”
He pointed out that, in fact, contemporary chemotherapy alone is effective in the treatment of more than 90% of patients with ALL younger than aged 18.
For the 10% of patients who do not respond to chemotherapy alone and undergo allogeneic HSCT, about 50%-80% of pediatric patients who have resistant leukemia are cured. However, the total body irradiation used to prepare the body to receive the transplant is linked to potentially serious consequences later in life, including sterility, lung problems, growth retardation, and secondary cancer.
To determine if the irradiation component could be safely replaced with a chemotherapy-based conditioning approach, Dr. Peters and colleagues conducted the FORUM trial.
In total 413 patients undergoing HSCT were enrolled and randomized to pretransplant conditioning with total body irradiation and etoposide (n = 202) or a chemotherapy-only approach with fludarabine/thiotepa/busulfan (flu/thio/bu; n = 99) or fludarabine/thiotepa/treosulfan (treo; n = 93).
Most patients (72%) had B-cell precursor ALL and 23% had T-cell ALL. Just over half (54%) were transplanted in first complete remission (CR1), 40% in CR2, and 4% in CR3.
The source of stem cells was bone marrow for most patients (82%); peripheral blood stem cell for 12%, and cord blood for 4%.
Study stopped early
The aim of the study was to demonstrate noninferiority with the chemotherapy approach.
However, the significantly inferior outcome observed in the chemotherapy-only group led to randomization being halted in March 2019.
The 2-year overall survival in the intent-to-treat (ITT) analysis, with a mean observation time of 2.1 years, was 0.75 ± 0.04 for chemo-conditioning versus 0.91 ± 0.02 for total body irradiation/etoposide (ITT P < .001).
The ITT analysis showed relapses were significantly higher in the chemo-conditioning group (2-year cumulative incidence of relapse [CIR], 0.33) compared with the total body irradiation group (CIR, 0.12; P < .001).
The 2-year event-free survival (EFS) rate was also significantly higher in the total body irradiation group (0.86 vs 0.58; P < .001), and transplant-related mortality over 2 years was lower with total body irradiation (0.02 vs 0.09; P = .02).
A per-protocol analysis showed the 2-year overall survival to be the same in the two chemotherapy groups (both 0.77 ± 0.05) compared with 0.91 ± 0.02 in the total body irradiation group (P = .003).
“In this cohort [the 91% overall survival rate] may even be lower than contemporary intensive frontline therapy results that are achieved nowadays,” Dr. Peters said.
In looking at subgroups, there were no significant differences according to age group or cancer phenotype, while MLL rearrangement was associated with higher relapse incidence.
Remission status was found to notably influence EFS, dropping from 0.91 in CR1 patients with total body irradiation to 0.76 in CR2 patients. However, total body irradiation remained significantly higher compared with the chemo-conditioning groups in CR1 (P = .004) and CR2 (P < .001).
Transplant-related mortality was not significantly different between the total body irradiation and chemo-conditioning groups in the CR1 or CR2 groups (P = .09 and P = .18, respectively), despite the significant difference when remission status was not included.
Overall, “we tried to identify subgroups in which total body irradiation might be eliminated, however in all analyses, total body irradiation was better than chemo-conditioning in all arms,” Dr. Peters said.
Meanwhile, the findings underscore that even when patients cannot receive total body irradiation, the alternative chemo-conditioning therapy in fact shows favorable efficacy on its own, Dr. Izraeli said.
“The prognosis of the chemotherapy group is also quite remarkably good, although less than the total body irradiation arm. This means that if for some reason total body irradiation cannot be given, the chemotherapy is a very reasonable alternative.”
Dr. Peters has reported relationships with Amgen, Novartis, Pfizer, Medac, Jazz, and Neovii. Dr. Izraeli has reported no relevant financial relationships.
SOURCE: EHA Congress. Abstract S102.
A version of this article originally appeared on Medscape.com.
Hematopoietic stem cell transplantation (HSCT) may offer the chance of a cure for patients with leukemia and other blood cancers, but the process of preparing the body to receive such a transplant can be brutal, involving whole body irradiation as well as chemotherapy conditioning. New results show that both steps are needed: a trial that omitted whole body irradiation in young patients with acute lymphoblastic leukemia (ALL) was stopped early because of significantly poorer outcomes.
The multicenter, global FORUM (For Omitting Radiation Under Majority Age) trial involved 75 centers in 17 countries between 2013 and 2018.
“Our study shows significantly better outcomes for total body irradiation compared to myeloablative chemo-conditioning arms, with no differences between the [two] chemo-conditioning groups,” concluded Christina Peters, MD, professor of pediatrics in the department of stem cell transplantation at St Anna Children’s Hospital in Vienna.
, she added.
Dr. Peters presented the findings as part of the virtual annual congress of the European Hematology Association.
Describing the results as “sobering,” session comoderator Shai Izraeli, MD, director of the department of hematology-oncology at Schneider Children’s Medical Center, in Petah Tikva, Israel, said an online comment from the virtual meeting audience reflected the reaction to these unwelcome results: “So we are still stuck with total body irradiation?”
Dr. Peters said the good news is that the number of patients needing to undergo stem cell transplants is low, and with research advances, may hopefully drop even further.
“Only 10% of patients under the age of 18 nowadays undergo allogeneic HSCT, and perhaps in the future that will become even less if we are able to rescue some of the groups with other immunological measures such as CAR-T cells and antibodies,” she said.
“I think it is very important to better identify those who really need total body irradiation in the future,” she added.
In an interview, Dr. Izraeli agreed.
“The prognosis of children after bone marrow transplantation is excellent – the majority are cured from their leukemia,” he said. “And we have to remember that those who undergo bone marrow transplant have the worst leukemias.”
He pointed out that, in fact, contemporary chemotherapy alone is effective in the treatment of more than 90% of patients with ALL younger than aged 18.
For the 10% of patients who do not respond to chemotherapy alone and undergo allogeneic HSCT, about 50%-80% of pediatric patients who have resistant leukemia are cured. However, the total body irradiation used to prepare the body to receive the transplant is linked to potentially serious consequences later in life, including sterility, lung problems, growth retardation, and secondary cancer.
To determine if the irradiation component could be safely replaced with a chemotherapy-based conditioning approach, Dr. Peters and colleagues conducted the FORUM trial.
In total 413 patients undergoing HSCT were enrolled and randomized to pretransplant conditioning with total body irradiation and etoposide (n = 202) or a chemotherapy-only approach with fludarabine/thiotepa/busulfan (flu/thio/bu; n = 99) or fludarabine/thiotepa/treosulfan (treo; n = 93).
Most patients (72%) had B-cell precursor ALL and 23% had T-cell ALL. Just over half (54%) were transplanted in first complete remission (CR1), 40% in CR2, and 4% in CR3.
The source of stem cells was bone marrow for most patients (82%); peripheral blood stem cell for 12%, and cord blood for 4%.
Study stopped early
The aim of the study was to demonstrate noninferiority with the chemotherapy approach.
However, the significantly inferior outcome observed in the chemotherapy-only group led to randomization being halted in March 2019.
The 2-year overall survival in the intent-to-treat (ITT) analysis, with a mean observation time of 2.1 years, was 0.75 ± 0.04 for chemo-conditioning versus 0.91 ± 0.02 for total body irradiation/etoposide (ITT P < .001).
The ITT analysis showed relapses were significantly higher in the chemo-conditioning group (2-year cumulative incidence of relapse [CIR], 0.33) compared with the total body irradiation group (CIR, 0.12; P < .001).
The 2-year event-free survival (EFS) rate was also significantly higher in the total body irradiation group (0.86 vs 0.58; P < .001), and transplant-related mortality over 2 years was lower with total body irradiation (0.02 vs 0.09; P = .02).
A per-protocol analysis showed the 2-year overall survival to be the same in the two chemotherapy groups (both 0.77 ± 0.05) compared with 0.91 ± 0.02 in the total body irradiation group (P = .003).
“In this cohort [the 91% overall survival rate] may even be lower than contemporary intensive frontline therapy results that are achieved nowadays,” Dr. Peters said.
In looking at subgroups, there were no significant differences according to age group or cancer phenotype, while MLL rearrangement was associated with higher relapse incidence.
Remission status was found to notably influence EFS, dropping from 0.91 in CR1 patients with total body irradiation to 0.76 in CR2 patients. However, total body irradiation remained significantly higher compared with the chemo-conditioning groups in CR1 (P = .004) and CR2 (P < .001).
Transplant-related mortality was not significantly different between the total body irradiation and chemo-conditioning groups in the CR1 or CR2 groups (P = .09 and P = .18, respectively), despite the significant difference when remission status was not included.
Overall, “we tried to identify subgroups in which total body irradiation might be eliminated, however in all analyses, total body irradiation was better than chemo-conditioning in all arms,” Dr. Peters said.
Meanwhile, the findings underscore that even when patients cannot receive total body irradiation, the alternative chemo-conditioning therapy in fact shows favorable efficacy on its own, Dr. Izraeli said.
“The prognosis of the chemotherapy group is also quite remarkably good, although less than the total body irradiation arm. This means that if for some reason total body irradiation cannot be given, the chemotherapy is a very reasonable alternative.”
Dr. Peters has reported relationships with Amgen, Novartis, Pfizer, Medac, Jazz, and Neovii. Dr. Izraeli has reported no relevant financial relationships.
SOURCE: EHA Congress. Abstract S102.
A version of this article originally appeared on Medscape.com.
Hematopoietic stem cell transplantation (HSCT) may offer the chance of a cure for patients with leukemia and other blood cancers, but the process of preparing the body to receive such a transplant can be brutal, involving whole body irradiation as well as chemotherapy conditioning. New results show that both steps are needed: a trial that omitted whole body irradiation in young patients with acute lymphoblastic leukemia (ALL) was stopped early because of significantly poorer outcomes.
The multicenter, global FORUM (For Omitting Radiation Under Majority Age) trial involved 75 centers in 17 countries between 2013 and 2018.
“Our study shows significantly better outcomes for total body irradiation compared to myeloablative chemo-conditioning arms, with no differences between the [two] chemo-conditioning groups,” concluded Christina Peters, MD, professor of pediatrics in the department of stem cell transplantation at St Anna Children’s Hospital in Vienna.
, she added.
Dr. Peters presented the findings as part of the virtual annual congress of the European Hematology Association.
Describing the results as “sobering,” session comoderator Shai Izraeli, MD, director of the department of hematology-oncology at Schneider Children’s Medical Center, in Petah Tikva, Israel, said an online comment from the virtual meeting audience reflected the reaction to these unwelcome results: “So we are still stuck with total body irradiation?”
Dr. Peters said the good news is that the number of patients needing to undergo stem cell transplants is low, and with research advances, may hopefully drop even further.
“Only 10% of patients under the age of 18 nowadays undergo allogeneic HSCT, and perhaps in the future that will become even less if we are able to rescue some of the groups with other immunological measures such as CAR-T cells and antibodies,” she said.
“I think it is very important to better identify those who really need total body irradiation in the future,” she added.
In an interview, Dr. Izraeli agreed.
“The prognosis of children after bone marrow transplantation is excellent – the majority are cured from their leukemia,” he said. “And we have to remember that those who undergo bone marrow transplant have the worst leukemias.”
He pointed out that, in fact, contemporary chemotherapy alone is effective in the treatment of more than 90% of patients with ALL younger than aged 18.
For the 10% of patients who do not respond to chemotherapy alone and undergo allogeneic HSCT, about 50%-80% of pediatric patients who have resistant leukemia are cured. However, the total body irradiation used to prepare the body to receive the transplant is linked to potentially serious consequences later in life, including sterility, lung problems, growth retardation, and secondary cancer.
To determine if the irradiation component could be safely replaced with a chemotherapy-based conditioning approach, Dr. Peters and colleagues conducted the FORUM trial.
In total 413 patients undergoing HSCT were enrolled and randomized to pretransplant conditioning with total body irradiation and etoposide (n = 202) or a chemotherapy-only approach with fludarabine/thiotepa/busulfan (flu/thio/bu; n = 99) or fludarabine/thiotepa/treosulfan (treo; n = 93).
Most patients (72%) had B-cell precursor ALL and 23% had T-cell ALL. Just over half (54%) were transplanted in first complete remission (CR1), 40% in CR2, and 4% in CR3.
The source of stem cells was bone marrow for most patients (82%); peripheral blood stem cell for 12%, and cord blood for 4%.
Study stopped early
The aim of the study was to demonstrate noninferiority with the chemotherapy approach.
However, the significantly inferior outcome observed in the chemotherapy-only group led to randomization being halted in March 2019.
The 2-year overall survival in the intent-to-treat (ITT) analysis, with a mean observation time of 2.1 years, was 0.75 ± 0.04 for chemo-conditioning versus 0.91 ± 0.02 for total body irradiation/etoposide (ITT P < .001).
The ITT analysis showed relapses were significantly higher in the chemo-conditioning group (2-year cumulative incidence of relapse [CIR], 0.33) compared with the total body irradiation group (CIR, 0.12; P < .001).
The 2-year event-free survival (EFS) rate was also significantly higher in the total body irradiation group (0.86 vs 0.58; P < .001), and transplant-related mortality over 2 years was lower with total body irradiation (0.02 vs 0.09; P = .02).
A per-protocol analysis showed the 2-year overall survival to be the same in the two chemotherapy groups (both 0.77 ± 0.05) compared with 0.91 ± 0.02 in the total body irradiation group (P = .003).
“In this cohort [the 91% overall survival rate] may even be lower than contemporary intensive frontline therapy results that are achieved nowadays,” Dr. Peters said.
In looking at subgroups, there were no significant differences according to age group or cancer phenotype, while MLL rearrangement was associated with higher relapse incidence.
Remission status was found to notably influence EFS, dropping from 0.91 in CR1 patients with total body irradiation to 0.76 in CR2 patients. However, total body irradiation remained significantly higher compared with the chemo-conditioning groups in CR1 (P = .004) and CR2 (P < .001).
Transplant-related mortality was not significantly different between the total body irradiation and chemo-conditioning groups in the CR1 or CR2 groups (P = .09 and P = .18, respectively), despite the significant difference when remission status was not included.
Overall, “we tried to identify subgroups in which total body irradiation might be eliminated, however in all analyses, total body irradiation was better than chemo-conditioning in all arms,” Dr. Peters said.
Meanwhile, the findings underscore that even when patients cannot receive total body irradiation, the alternative chemo-conditioning therapy in fact shows favorable efficacy on its own, Dr. Izraeli said.
“The prognosis of the chemotherapy group is also quite remarkably good, although less than the total body irradiation arm. This means that if for some reason total body irradiation cannot be given, the chemotherapy is a very reasonable alternative.”
Dr. Peters has reported relationships with Amgen, Novartis, Pfizer, Medac, Jazz, and Neovii. Dr. Izraeli has reported no relevant financial relationships.
SOURCE: EHA Congress. Abstract S102.
A version of this article originally appeared on Medscape.com.
FROM EHA CONGRESS
Studies give new insight on starting, stopping etanercept in nonradiographic axSpA
The results from a pair of clinical trials should help to take the guesswork out of starting and stopping the tumor necrosis factor inhibitor etanercept (Enbrel) in patients with nonradiographic axial spondyloarthritis (nr-axSpA). The trials were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Optimal use of etanercept in this disease is still being defined, according to the investigators. Its effects, if any, when given very early in the disease course is unclear, and guidance is conflicting when it comes to stopping the drug after inactive disease is achieved.
In the Dutch randomized controlled PrevAS trial of 80 patients with suspected very early nr-axSpA, initiating etanercept instead of placebo did not significantly improve the odds of achieving a 20% improvement in Assessment of Spondyloarthritis International Society (ASAS 20) response criteria at week 16.
And in the multinational, open-label, phase 4 RE-EMBARK trial, three-quarters of the 119 patients with nr-axSpA who achieved inactive disease on etanercept and stopped the drug experienced a flare within 40 weeks. However, the majority were able to regain disease inactivity after restarting the drug.
Findings in context
“We all have some patients like this [PrevAS population] where we strongly believe they have axial spondyloarthritis but do not fully qualify,” Nigil Haroon MD, PhD, said in an interview. “From a clinical decision-making process, we may diagnose these patients with axial spondyloarthritis, but due to restrictions in access to medications, we have difficulty accessing biologic medications for them. Hence, this study has practical implications.”
“It has already been shown in other, much larger studies that, even in patients who satisfy the criteria of axial spondyloarthritis, those who are MRI and CRP [C-reactive protein] negative are unlikely to respond, so the results are not surprising,” commented Dr. Haroon, who is codirector of the spondylitis program at the University Health Network and associate professor of medicine and rheumatology at the University of Toronto.
Although intended to be a population with suspected very early disease, several of the PrevAS patients would have met ASAS criteria for the disease at baseline, Dr. Haroon cautioned. In addition, the small sample size precluded subgroup analyses.
“The overall conclusion should be, this is a negative study, rather than state there was a trend to better improvement on etanercept. Although there are practical implications, as mentioned, I don’t think this study, with the numbers and the results presented, will change clinical practice,” he said.
The question of stopping biologics in nr-axSpA was previously addressed in the ABILITY-3 randomized trial of adalimumab (Humira), which found that flares were significantly more common with stopping versus continuing the drug and only about half of patients were able to get back in remission by restarting the drug, according to Dr. Haroon.
However, the RE-EMBARK and ABILITY-3 studies differed in both design and patient population, he noted. For example, the mean disease duration was only about 2 years in the former study, compared with 7 years in the latter.
The initial 59% rate of attaining inactive disease on etanercept in RE-EMBARK was “impressive,” Dr. Haroon said, “but as this was an open-label study, higher values are expected.”
“The message in both studies is that stopping biologics completely is not a good idea as the majority of patients, 70%-75%, will relapse within a short period,” he concluded. “However, it should be kept in mind that these [RE-EMBARK] patients received biologic only for a short 24-week period. This study does not answer the question of whether nonradiographic axial spondyloarthritis patients with sustained inactive disease can be taken off biologics abruptly without a taper over time.”
Details of the studies
In the PrevAS trial, Tamara Rusman, a PhD candidate in Rheumatology at the VU University Medical Center Amsterdam and coinvestigators studied patients meeting Calin criteria for inflammatory back pain who had high disease activity plus either HLA-B27 positivity with at least one feature of axial spondyloarthritis or HLA-B27 negativity with two features.
This population is of interest because “most studies have included only patients with nonradiographic axial spondyloarthritis with a positive MRI of the sacroiliac joints and/or an elevated C-reactive protein level,” she noted.
Results showed that, during 16 weeks of treatment, etanercept users had a nonsignficantly higher rate of achieving an ASAS 20 response with etanercept versus placebo users (17% vs. 11%; hazard ratio, 2.1; P = .2). The etanercept group also had a somewhat higher rate of response as defined by the Ankylosing Spondylitis Disease Activity Score CRP (ASDAS-CRP) criterion (25% vs. 13%; hazard ratio, 1.1; P = .8).
“Based on these data, early treatment in inflammatory back pain patients prone to develop axial spondyloarthritis seems not to be useful,” Ms. Rusman concluded. “However, monitoring of these patients should be continued since they remain a risk group for developing axial spondyloarthritis.”
In the RE-EMBARK trial, investigators led by Filip Van den Bosch, MD, PhD, Rheumatology Head-of-Clinic at Ghent (Belgium) University Hospital, started with a cohort of 208 patients with nr-axSpA who were given etanercept and background NSAIDs for 24 weeks.
“Current guidelines do not agree on whether a TNF-blocking agent or another biological DMARD should be tapered once a status of low disease activity or remission is achieved,” he noted.
Overall, 59% of the patients achieved inactive disease (defined as an ASDAS-CRP < 1.3) and discontinued etanercept.
During the next 40 weeks, 24% of these patients maintained inactive disease with only the background NSAID therapy. Among the 75% who experienced a flare, defined as an ASDAS with erythrocyte sedimentation rate (ASDAS-ESR) score of 2.1 or greater, the median time to flare was 16.1 weeks. Fully 62% of this group were able to regain disease inactivity within 12 weeks of restarting etanercept.
In a comparative analysis, relative to the RE-EMBARK patients discontinuing etanercept, similar patients who continued etanercept on the companion EMBARK trial had a longer time to flare (P < .0001) and an 85% lower risk of this outcome.
“There were no new safety signals identified, and as expected, the number of treatment-emergent adverse events dropped during the drug-free period and, interestingly, remained stable over retreatment,” Dr. Van den Bosch noted.
“Temporarily discontinuing etanercept may be an option for some patients with stable inactive nonradiographic axial spondyloarthritis,” he concluded.
The PrevAS trial was financially supported by Pfizer and ReumaNederland. Ms. Rusman declared no relevant conflicts of interest; four coauthors reported financial relationship(s) with Pfizer and other pharmaceutical companies. The RE-EMBARK trial was sponsored by Pfizer. Dr. Van den Bosch disclosed receiving grant/research support from AbbVie, Merck, and UCB, and consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Four coauthors reported financial ties to Pfizer and other pharmaceutical companies, and five coauthors were employees and shareholders of Pfizer.
SOURCES: Rusman T et al. Ann Rheum Dis. 2020;79[suppl 1]:72-3; and Van den Bosch F et al. Ann Rheum Dis. 2020;79[suppl 1]:70.
The results from a pair of clinical trials should help to take the guesswork out of starting and stopping the tumor necrosis factor inhibitor etanercept (Enbrel) in patients with nonradiographic axial spondyloarthritis (nr-axSpA). The trials were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Optimal use of etanercept in this disease is still being defined, according to the investigators. Its effects, if any, when given very early in the disease course is unclear, and guidance is conflicting when it comes to stopping the drug after inactive disease is achieved.
In the Dutch randomized controlled PrevAS trial of 80 patients with suspected very early nr-axSpA, initiating etanercept instead of placebo did not significantly improve the odds of achieving a 20% improvement in Assessment of Spondyloarthritis International Society (ASAS 20) response criteria at week 16.
And in the multinational, open-label, phase 4 RE-EMBARK trial, three-quarters of the 119 patients with nr-axSpA who achieved inactive disease on etanercept and stopped the drug experienced a flare within 40 weeks. However, the majority were able to regain disease inactivity after restarting the drug.
Findings in context
“We all have some patients like this [PrevAS population] where we strongly believe they have axial spondyloarthritis but do not fully qualify,” Nigil Haroon MD, PhD, said in an interview. “From a clinical decision-making process, we may diagnose these patients with axial spondyloarthritis, but due to restrictions in access to medications, we have difficulty accessing biologic medications for them. Hence, this study has practical implications.”
“It has already been shown in other, much larger studies that, even in patients who satisfy the criteria of axial spondyloarthritis, those who are MRI and CRP [C-reactive protein] negative are unlikely to respond, so the results are not surprising,” commented Dr. Haroon, who is codirector of the spondylitis program at the University Health Network and associate professor of medicine and rheumatology at the University of Toronto.
Although intended to be a population with suspected very early disease, several of the PrevAS patients would have met ASAS criteria for the disease at baseline, Dr. Haroon cautioned. In addition, the small sample size precluded subgroup analyses.
“The overall conclusion should be, this is a negative study, rather than state there was a trend to better improvement on etanercept. Although there are practical implications, as mentioned, I don’t think this study, with the numbers and the results presented, will change clinical practice,” he said.
The question of stopping biologics in nr-axSpA was previously addressed in the ABILITY-3 randomized trial of adalimumab (Humira), which found that flares were significantly more common with stopping versus continuing the drug and only about half of patients were able to get back in remission by restarting the drug, according to Dr. Haroon.
However, the RE-EMBARK and ABILITY-3 studies differed in both design and patient population, he noted. For example, the mean disease duration was only about 2 years in the former study, compared with 7 years in the latter.
The initial 59% rate of attaining inactive disease on etanercept in RE-EMBARK was “impressive,” Dr. Haroon said, “but as this was an open-label study, higher values are expected.”
“The message in both studies is that stopping biologics completely is not a good idea as the majority of patients, 70%-75%, will relapse within a short period,” he concluded. “However, it should be kept in mind that these [RE-EMBARK] patients received biologic only for a short 24-week period. This study does not answer the question of whether nonradiographic axial spondyloarthritis patients with sustained inactive disease can be taken off biologics abruptly without a taper over time.”
Details of the studies
In the PrevAS trial, Tamara Rusman, a PhD candidate in Rheumatology at the VU University Medical Center Amsterdam and coinvestigators studied patients meeting Calin criteria for inflammatory back pain who had high disease activity plus either HLA-B27 positivity with at least one feature of axial spondyloarthritis or HLA-B27 negativity with two features.
This population is of interest because “most studies have included only patients with nonradiographic axial spondyloarthritis with a positive MRI of the sacroiliac joints and/or an elevated C-reactive protein level,” she noted.
Results showed that, during 16 weeks of treatment, etanercept users had a nonsignficantly higher rate of achieving an ASAS 20 response with etanercept versus placebo users (17% vs. 11%; hazard ratio, 2.1; P = .2). The etanercept group also had a somewhat higher rate of response as defined by the Ankylosing Spondylitis Disease Activity Score CRP (ASDAS-CRP) criterion (25% vs. 13%; hazard ratio, 1.1; P = .8).
“Based on these data, early treatment in inflammatory back pain patients prone to develop axial spondyloarthritis seems not to be useful,” Ms. Rusman concluded. “However, monitoring of these patients should be continued since they remain a risk group for developing axial spondyloarthritis.”
In the RE-EMBARK trial, investigators led by Filip Van den Bosch, MD, PhD, Rheumatology Head-of-Clinic at Ghent (Belgium) University Hospital, started with a cohort of 208 patients with nr-axSpA who were given etanercept and background NSAIDs for 24 weeks.
“Current guidelines do not agree on whether a TNF-blocking agent or another biological DMARD should be tapered once a status of low disease activity or remission is achieved,” he noted.
Overall, 59% of the patients achieved inactive disease (defined as an ASDAS-CRP < 1.3) and discontinued etanercept.
During the next 40 weeks, 24% of these patients maintained inactive disease with only the background NSAID therapy. Among the 75% who experienced a flare, defined as an ASDAS with erythrocyte sedimentation rate (ASDAS-ESR) score of 2.1 or greater, the median time to flare was 16.1 weeks. Fully 62% of this group were able to regain disease inactivity within 12 weeks of restarting etanercept.
In a comparative analysis, relative to the RE-EMBARK patients discontinuing etanercept, similar patients who continued etanercept on the companion EMBARK trial had a longer time to flare (P < .0001) and an 85% lower risk of this outcome.
“There were no new safety signals identified, and as expected, the number of treatment-emergent adverse events dropped during the drug-free period and, interestingly, remained stable over retreatment,” Dr. Van den Bosch noted.
“Temporarily discontinuing etanercept may be an option for some patients with stable inactive nonradiographic axial spondyloarthritis,” he concluded.
The PrevAS trial was financially supported by Pfizer and ReumaNederland. Ms. Rusman declared no relevant conflicts of interest; four coauthors reported financial relationship(s) with Pfizer and other pharmaceutical companies. The RE-EMBARK trial was sponsored by Pfizer. Dr. Van den Bosch disclosed receiving grant/research support from AbbVie, Merck, and UCB, and consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Four coauthors reported financial ties to Pfizer and other pharmaceutical companies, and five coauthors were employees and shareholders of Pfizer.
SOURCES: Rusman T et al. Ann Rheum Dis. 2020;79[suppl 1]:72-3; and Van den Bosch F et al. Ann Rheum Dis. 2020;79[suppl 1]:70.
The results from a pair of clinical trials should help to take the guesswork out of starting and stopping the tumor necrosis factor inhibitor etanercept (Enbrel) in patients with nonradiographic axial spondyloarthritis (nr-axSpA). The trials were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Optimal use of etanercept in this disease is still being defined, according to the investigators. Its effects, if any, when given very early in the disease course is unclear, and guidance is conflicting when it comes to stopping the drug after inactive disease is achieved.
In the Dutch randomized controlled PrevAS trial of 80 patients with suspected very early nr-axSpA, initiating etanercept instead of placebo did not significantly improve the odds of achieving a 20% improvement in Assessment of Spondyloarthritis International Society (ASAS 20) response criteria at week 16.
And in the multinational, open-label, phase 4 RE-EMBARK trial, three-quarters of the 119 patients with nr-axSpA who achieved inactive disease on etanercept and stopped the drug experienced a flare within 40 weeks. However, the majority were able to regain disease inactivity after restarting the drug.
Findings in context
“We all have some patients like this [PrevAS population] where we strongly believe they have axial spondyloarthritis but do not fully qualify,” Nigil Haroon MD, PhD, said in an interview. “From a clinical decision-making process, we may diagnose these patients with axial spondyloarthritis, but due to restrictions in access to medications, we have difficulty accessing biologic medications for them. Hence, this study has practical implications.”
“It has already been shown in other, much larger studies that, even in patients who satisfy the criteria of axial spondyloarthritis, those who are MRI and CRP [C-reactive protein] negative are unlikely to respond, so the results are not surprising,” commented Dr. Haroon, who is codirector of the spondylitis program at the University Health Network and associate professor of medicine and rheumatology at the University of Toronto.
Although intended to be a population with suspected very early disease, several of the PrevAS patients would have met ASAS criteria for the disease at baseline, Dr. Haroon cautioned. In addition, the small sample size precluded subgroup analyses.
“The overall conclusion should be, this is a negative study, rather than state there was a trend to better improvement on etanercept. Although there are practical implications, as mentioned, I don’t think this study, with the numbers and the results presented, will change clinical practice,” he said.
The question of stopping biologics in nr-axSpA was previously addressed in the ABILITY-3 randomized trial of adalimumab (Humira), which found that flares were significantly more common with stopping versus continuing the drug and only about half of patients were able to get back in remission by restarting the drug, according to Dr. Haroon.
However, the RE-EMBARK and ABILITY-3 studies differed in both design and patient population, he noted. For example, the mean disease duration was only about 2 years in the former study, compared with 7 years in the latter.
The initial 59% rate of attaining inactive disease on etanercept in RE-EMBARK was “impressive,” Dr. Haroon said, “but as this was an open-label study, higher values are expected.”
“The message in both studies is that stopping biologics completely is not a good idea as the majority of patients, 70%-75%, will relapse within a short period,” he concluded. “However, it should be kept in mind that these [RE-EMBARK] patients received biologic only for a short 24-week period. This study does not answer the question of whether nonradiographic axial spondyloarthritis patients with sustained inactive disease can be taken off biologics abruptly without a taper over time.”
Details of the studies
In the PrevAS trial, Tamara Rusman, a PhD candidate in Rheumatology at the VU University Medical Center Amsterdam and coinvestigators studied patients meeting Calin criteria for inflammatory back pain who had high disease activity plus either HLA-B27 positivity with at least one feature of axial spondyloarthritis or HLA-B27 negativity with two features.
This population is of interest because “most studies have included only patients with nonradiographic axial spondyloarthritis with a positive MRI of the sacroiliac joints and/or an elevated C-reactive protein level,” she noted.
Results showed that, during 16 weeks of treatment, etanercept users had a nonsignficantly higher rate of achieving an ASAS 20 response with etanercept versus placebo users (17% vs. 11%; hazard ratio, 2.1; P = .2). The etanercept group also had a somewhat higher rate of response as defined by the Ankylosing Spondylitis Disease Activity Score CRP (ASDAS-CRP) criterion (25% vs. 13%; hazard ratio, 1.1; P = .8).
“Based on these data, early treatment in inflammatory back pain patients prone to develop axial spondyloarthritis seems not to be useful,” Ms. Rusman concluded. “However, monitoring of these patients should be continued since they remain a risk group for developing axial spondyloarthritis.”
In the RE-EMBARK trial, investigators led by Filip Van den Bosch, MD, PhD, Rheumatology Head-of-Clinic at Ghent (Belgium) University Hospital, started with a cohort of 208 patients with nr-axSpA who were given etanercept and background NSAIDs for 24 weeks.
“Current guidelines do not agree on whether a TNF-blocking agent or another biological DMARD should be tapered once a status of low disease activity or remission is achieved,” he noted.
Overall, 59% of the patients achieved inactive disease (defined as an ASDAS-CRP < 1.3) and discontinued etanercept.
During the next 40 weeks, 24% of these patients maintained inactive disease with only the background NSAID therapy. Among the 75% who experienced a flare, defined as an ASDAS with erythrocyte sedimentation rate (ASDAS-ESR) score of 2.1 or greater, the median time to flare was 16.1 weeks. Fully 62% of this group were able to regain disease inactivity within 12 weeks of restarting etanercept.
In a comparative analysis, relative to the RE-EMBARK patients discontinuing etanercept, similar patients who continued etanercept on the companion EMBARK trial had a longer time to flare (P < .0001) and an 85% lower risk of this outcome.
“There were no new safety signals identified, and as expected, the number of treatment-emergent adverse events dropped during the drug-free period and, interestingly, remained stable over retreatment,” Dr. Van den Bosch noted.
“Temporarily discontinuing etanercept may be an option for some patients with stable inactive nonradiographic axial spondyloarthritis,” he concluded.
The PrevAS trial was financially supported by Pfizer and ReumaNederland. Ms. Rusman declared no relevant conflicts of interest; four coauthors reported financial relationship(s) with Pfizer and other pharmaceutical companies. The RE-EMBARK trial was sponsored by Pfizer. Dr. Van den Bosch disclosed receiving grant/research support from AbbVie, Merck, and UCB, and consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Four coauthors reported financial ties to Pfizer and other pharmaceutical companies, and five coauthors were employees and shareholders of Pfizer.
SOURCES: Rusman T et al. Ann Rheum Dis. 2020;79[suppl 1]:72-3; and Van den Bosch F et al. Ann Rheum Dis. 2020;79[suppl 1]:70.
FROM THE EULAR 2020 E-CONGRESS
Key clinical point: In nonradiographic axial spondyloarthritis (nr-axSpA), etanercept does not have significant benefit by 16 weeks when started in very early disease, and the majority of patients who achieved inactive disease on the drug and then stopped it experienced a flare within 40 weeks.
Major finding: Patients with suspected very early disease who took etanercept did not have a significantly greater rate of achieving a 20% improvement in Assessment of Spondyloarthritis International Society (ASAS 20) response criteria at week 16 than did those taking placebo (17% vs. 11%; hazard ratio, 2.1; P = .2). In a separate trial, 75% of patients who achieved inactive disease with etanercept and then stopped the drug had a flare within 40 weeks, but 62% of this group were able to regain disease inactivity within 12 weeks of restarting etanercept.
Study details: A randomized, placebo-controlled PrevAS trial involved 80 patients with suspected very early nr-axSpA who started either etanercept or placebo, and the multicenter, open-label, phase 4 RE-EMBARK trial involved 119 patients achieving inactive nr-axSpA on etanercept.
Disclosures: The PrevAS trial was financially supported by Pfizer and ReumaNederland. Ms. Rusman declared no relevant conflicts of interest; four coauthors reported financial relationship(s) with Pfizer and other pharmaceutical companies. The RE-EMBARK trial was sponsored by Pfizer. Dr. Van den Bosch disclosed receiving grant/research support from AbbVie, Merck, and UCB and consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Four coauthors reported financial ties to Pfizer and other pharmaceutical companies, and five coauthors were employees and shareholders of Pfizer.
Sources: Rusman T et al. Ann Rheum Dis. 2020;79[suppl 1]:72-3; and Van den Bosch F et al. Ann Rheum Dis. 2020;79[suppl 1]:70.