User login
67-year-old woman • excessive flatulence • persistent heartburn • chronic cough • Dx?
THE CASE
A 67-year-old woman with type 2 diabetes mellitus and hypertension presented to our family medicine office for evaluation of excessive flatulence, belching, and bloating that had worsened over the previous 6 months. The patient said the symptoms occurred throughout the day but were most noticeable after eating meals. She had a 5-year history of heartburn and chronic cough. We initially suspected gastroesophageal reflux disease (GERD). However, trials with several different proton pump inhibitors (PPIs) over a 3-year period did not provide any relief. Lifestyle modifications such as losing weight; remaining upright for at least 3 hours after eating; and eliminating gluten, dairy, soy, and alcohol from her diet did not alleviate her symptoms.
At the current presentation, the physical examination was normal, and an upper endoscopy was unremarkable except for some mild gastric irritation. A urea breath test was negative for Helicobacter pylori, and a chest radiograph to investigate the cause of the chronic cough was normal. The patient’s increased symptoms after eating indicated that a sensitivity to food antibodies might be at work. The absence of urticaria and anaphylaxis correlated with an IgG-mediated rather than an IgE-mediated reaction.
Due to the high cost of IgG testing, we recommended that the patient start a 6-week elimination diet that excluded the most common culprits for food allergies: dairy, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soy.1 We also recommended that she eliminate alcohol (because of its role in exacerbating GERD); however, excluding these foods from her diet did not provide sufficient relief of her symptoms. We subsequently recommended a serum IgG food antibody test.
THE DIAGNOSIS
The results of the test were positive for IgG-mediated allergy to vegetables in the onion family, as indicated by a high (3+) antibody presence. The patient told us she consumed onions up to 3 times daily in her meals. We recommended that she eliminate onions from her diet. At a follow-up appointment 3 months later, the patient reported that the flatulence, belching, and bloating after eating had resolved and her heartburn had decreased. When we asked about her chronic cough, the patient mentioned she had not experienced it for a few months and had forgotten about it.
DISCUSSION
The most common food sensitivity test is the scratch test, which only measures IgE antibodies. However, past studies have suggested that IgE is not the only mediator in certain symptoms related to food allergy. It is thought that these symptoms may instead be IgG mediated.2 Normally, IgG antibodies do not form in the digestive tract because the epithelium creates a barrier that is impermeable to antigens. However, antigens can bypass the epithelium and reach immune cells in states of inflammation where the epithelium is damaged. This contact with immune cells provides an opportunity for development of IgG antibodies.3 Successive interactions with these antigens leads to defensive and inflammatory processes that manifest as food allergies.
Rather than the typical IgE-mediated presentations (eg, urticaria, anaphylaxis), patients with IgG-mediated allergies experience more subtle symptoms, such as nausea, abdominal pain, diarrhea, flatulence, cramping, bloating, heartburn, cough, bronchoconstriction, eczema, stiff joints, headache, and/or increased risk of infection.4 One study showed that eliminating IgG-sensitive foods (eg, dairy, eggs) improved symptoms in migraine patients.5 Likewise, a separate study showed that patients with irritable bowel syndrome experienced improved symptoms after eliminating foods for which they had high IgG sensitivity.6
Casting a wider net. Whereas scratch testing only looks at IgE-mediated allergies, serum IgG food antibody testing looks for both IgE- and IgG-mediated reactions. IgE-mediated food allergies are monitored via the scratch test as a visual expression of a histamine reaction on the skin. However, serum IgG food antibody testing identifies culprit foods via enzyme-linked immunosorbent assay.
Continue to: Furthermore, the serum antibody test...
Furthermore, the serum antibody test also identifies allergenic foods whose symptoms have a delayed onset of 4 to 72 hours.7 Without this test, those symptoms may be wrongfully attributed to other conditions, and prescribed treatments will not treat the root cause of the reaction.8 The information provided in the serum antibody test allows the patient to develop a tailored elimination diet and eliminate causative food(s) faster. Without this test, we may not have identified onions as the allergenic food in our patient.
THE TAKEAWAY
Recent guidelines emphasize that IgG testing plays no role in the diagnosis of food allergies or intolerance.1 This may indeed be true for the general population, but other studies have shown IgG testing to be of value for specific diagnoses such as migraines or irritable bowel syndrome.5,6 Given our patient’s unique presentation and lack of response to traditional treatments, IgG testing was warranted. This case demonstrates the importance of IgG food antibody testing as part of a second-tier diagnostic workup when a patient’s gastrointestinal symptoms are not alleviated by traditional interventions.
CORRESPONDENCE
Elizabeth A. Khan, MD, Personalized Longevity Medical Center, 1146 South Cedar Crest Boulevard, Allentown, PA 18103; [email protected].
1. Boyce JA, Assa’ad A, Burks AW, et al; NIAID-sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored Expert Panel report. J Allergy Clin Immunol. 2010;126:1105-1118.
2. Kemeny DM, Urbanek R, Amlot PL, et al. Sub-class of IgG in an allergic disease. I. IgG sub-class antibodies in immediate and non-immediate food allergies. Clin Allergy. 1986;16:571-581.
3. Gocki J, Zbigniew B. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol. 2016;33:253-256.
4. Shaw W. Clinical usefulness of IgG food allergy testing. Integrative Medicine for Mental Health Web site. www.immh.org/article-source/2016/6/29/clinical-usefulness-of-igg-food-allergy-testing. Published November 16, 2015. Accessed June 29, 2020.
5. Arroyave Hernández CM, Echavarría Pinto M, Hernández Montiel HL. Food allergy mediated by IgG antibodies associated with migraine in adults. Rev Alerg Mex. 2007;54:162-168.
6. Guo H, Jiang T, Wang J, et al. The value of eliminating foods according to food-specific immunoglobulin G antibodies in irritable bowel syndrome with diarrhoea. J Int Med Res. 2012;40:204-210.
7. IgG food antibodies. Genova Diagnostics Web site. www.gdx.net/product/igg-food-antibodies-food-sensitivity-test-blood. Accessed June 29, 2020.
8. Atkinson W, Sheldon TA, Shaath N, et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464.
THE CASE
A 67-year-old woman with type 2 diabetes mellitus and hypertension presented to our family medicine office for evaluation of excessive flatulence, belching, and bloating that had worsened over the previous 6 months. The patient said the symptoms occurred throughout the day but were most noticeable after eating meals. She had a 5-year history of heartburn and chronic cough. We initially suspected gastroesophageal reflux disease (GERD). However, trials with several different proton pump inhibitors (PPIs) over a 3-year period did not provide any relief. Lifestyle modifications such as losing weight; remaining upright for at least 3 hours after eating; and eliminating gluten, dairy, soy, and alcohol from her diet did not alleviate her symptoms.
At the current presentation, the physical examination was normal, and an upper endoscopy was unremarkable except for some mild gastric irritation. A urea breath test was negative for Helicobacter pylori, and a chest radiograph to investigate the cause of the chronic cough was normal. The patient’s increased symptoms after eating indicated that a sensitivity to food antibodies might be at work. The absence of urticaria and anaphylaxis correlated with an IgG-mediated rather than an IgE-mediated reaction.
Due to the high cost of IgG testing, we recommended that the patient start a 6-week elimination diet that excluded the most common culprits for food allergies: dairy, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soy.1 We also recommended that she eliminate alcohol (because of its role in exacerbating GERD); however, excluding these foods from her diet did not provide sufficient relief of her symptoms. We subsequently recommended a serum IgG food antibody test.
THE DIAGNOSIS
The results of the test were positive for IgG-mediated allergy to vegetables in the onion family, as indicated by a high (3+) antibody presence. The patient told us she consumed onions up to 3 times daily in her meals. We recommended that she eliminate onions from her diet. At a follow-up appointment 3 months later, the patient reported that the flatulence, belching, and bloating after eating had resolved and her heartburn had decreased. When we asked about her chronic cough, the patient mentioned she had not experienced it for a few months and had forgotten about it.
DISCUSSION
The most common food sensitivity test is the scratch test, which only measures IgE antibodies. However, past studies have suggested that IgE is not the only mediator in certain symptoms related to food allergy. It is thought that these symptoms may instead be IgG mediated.2 Normally, IgG antibodies do not form in the digestive tract because the epithelium creates a barrier that is impermeable to antigens. However, antigens can bypass the epithelium and reach immune cells in states of inflammation where the epithelium is damaged. This contact with immune cells provides an opportunity for development of IgG antibodies.3 Successive interactions with these antigens leads to defensive and inflammatory processes that manifest as food allergies.
Rather than the typical IgE-mediated presentations (eg, urticaria, anaphylaxis), patients with IgG-mediated allergies experience more subtle symptoms, such as nausea, abdominal pain, diarrhea, flatulence, cramping, bloating, heartburn, cough, bronchoconstriction, eczema, stiff joints, headache, and/or increased risk of infection.4 One study showed that eliminating IgG-sensitive foods (eg, dairy, eggs) improved symptoms in migraine patients.5 Likewise, a separate study showed that patients with irritable bowel syndrome experienced improved symptoms after eliminating foods for which they had high IgG sensitivity.6
Casting a wider net. Whereas scratch testing only looks at IgE-mediated allergies, serum IgG food antibody testing looks for both IgE- and IgG-mediated reactions. IgE-mediated food allergies are monitored via the scratch test as a visual expression of a histamine reaction on the skin. However, serum IgG food antibody testing identifies culprit foods via enzyme-linked immunosorbent assay.
Continue to: Furthermore, the serum antibody test...
Furthermore, the serum antibody test also identifies allergenic foods whose symptoms have a delayed onset of 4 to 72 hours.7 Without this test, those symptoms may be wrongfully attributed to other conditions, and prescribed treatments will not treat the root cause of the reaction.8 The information provided in the serum antibody test allows the patient to develop a tailored elimination diet and eliminate causative food(s) faster. Without this test, we may not have identified onions as the allergenic food in our patient.
THE TAKEAWAY
Recent guidelines emphasize that IgG testing plays no role in the diagnosis of food allergies or intolerance.1 This may indeed be true for the general population, but other studies have shown IgG testing to be of value for specific diagnoses such as migraines or irritable bowel syndrome.5,6 Given our patient’s unique presentation and lack of response to traditional treatments, IgG testing was warranted. This case demonstrates the importance of IgG food antibody testing as part of a second-tier diagnostic workup when a patient’s gastrointestinal symptoms are not alleviated by traditional interventions.
CORRESPONDENCE
Elizabeth A. Khan, MD, Personalized Longevity Medical Center, 1146 South Cedar Crest Boulevard, Allentown, PA 18103; [email protected].
THE CASE
A 67-year-old woman with type 2 diabetes mellitus and hypertension presented to our family medicine office for evaluation of excessive flatulence, belching, and bloating that had worsened over the previous 6 months. The patient said the symptoms occurred throughout the day but were most noticeable after eating meals. She had a 5-year history of heartburn and chronic cough. We initially suspected gastroesophageal reflux disease (GERD). However, trials with several different proton pump inhibitors (PPIs) over a 3-year period did not provide any relief. Lifestyle modifications such as losing weight; remaining upright for at least 3 hours after eating; and eliminating gluten, dairy, soy, and alcohol from her diet did not alleviate her symptoms.
At the current presentation, the physical examination was normal, and an upper endoscopy was unremarkable except for some mild gastric irritation. A urea breath test was negative for Helicobacter pylori, and a chest radiograph to investigate the cause of the chronic cough was normal. The patient’s increased symptoms after eating indicated that a sensitivity to food antibodies might be at work. The absence of urticaria and anaphylaxis correlated with an IgG-mediated rather than an IgE-mediated reaction.
Due to the high cost of IgG testing, we recommended that the patient start a 6-week elimination diet that excluded the most common culprits for food allergies: dairy, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soy.1 We also recommended that she eliminate alcohol (because of its role in exacerbating GERD); however, excluding these foods from her diet did not provide sufficient relief of her symptoms. We subsequently recommended a serum IgG food antibody test.
THE DIAGNOSIS
The results of the test were positive for IgG-mediated allergy to vegetables in the onion family, as indicated by a high (3+) antibody presence. The patient told us she consumed onions up to 3 times daily in her meals. We recommended that she eliminate onions from her diet. At a follow-up appointment 3 months later, the patient reported that the flatulence, belching, and bloating after eating had resolved and her heartburn had decreased. When we asked about her chronic cough, the patient mentioned she had not experienced it for a few months and had forgotten about it.
DISCUSSION
The most common food sensitivity test is the scratch test, which only measures IgE antibodies. However, past studies have suggested that IgE is not the only mediator in certain symptoms related to food allergy. It is thought that these symptoms may instead be IgG mediated.2 Normally, IgG antibodies do not form in the digestive tract because the epithelium creates a barrier that is impermeable to antigens. However, antigens can bypass the epithelium and reach immune cells in states of inflammation where the epithelium is damaged. This contact with immune cells provides an opportunity for development of IgG antibodies.3 Successive interactions with these antigens leads to defensive and inflammatory processes that manifest as food allergies.
Rather than the typical IgE-mediated presentations (eg, urticaria, anaphylaxis), patients with IgG-mediated allergies experience more subtle symptoms, such as nausea, abdominal pain, diarrhea, flatulence, cramping, bloating, heartburn, cough, bronchoconstriction, eczema, stiff joints, headache, and/or increased risk of infection.4 One study showed that eliminating IgG-sensitive foods (eg, dairy, eggs) improved symptoms in migraine patients.5 Likewise, a separate study showed that patients with irritable bowel syndrome experienced improved symptoms after eliminating foods for which they had high IgG sensitivity.6
Casting a wider net. Whereas scratch testing only looks at IgE-mediated allergies, serum IgG food antibody testing looks for both IgE- and IgG-mediated reactions. IgE-mediated food allergies are monitored via the scratch test as a visual expression of a histamine reaction on the skin. However, serum IgG food antibody testing identifies culprit foods via enzyme-linked immunosorbent assay.
Continue to: Furthermore, the serum antibody test...
Furthermore, the serum antibody test also identifies allergenic foods whose symptoms have a delayed onset of 4 to 72 hours.7 Without this test, those symptoms may be wrongfully attributed to other conditions, and prescribed treatments will not treat the root cause of the reaction.8 The information provided in the serum antibody test allows the patient to develop a tailored elimination diet and eliminate causative food(s) faster. Without this test, we may not have identified onions as the allergenic food in our patient.
THE TAKEAWAY
Recent guidelines emphasize that IgG testing plays no role in the diagnosis of food allergies or intolerance.1 This may indeed be true for the general population, but other studies have shown IgG testing to be of value for specific diagnoses such as migraines or irritable bowel syndrome.5,6 Given our patient’s unique presentation and lack of response to traditional treatments, IgG testing was warranted. This case demonstrates the importance of IgG food antibody testing as part of a second-tier diagnostic workup when a patient’s gastrointestinal symptoms are not alleviated by traditional interventions.
CORRESPONDENCE
Elizabeth A. Khan, MD, Personalized Longevity Medical Center, 1146 South Cedar Crest Boulevard, Allentown, PA 18103; [email protected].
1. Boyce JA, Assa’ad A, Burks AW, et al; NIAID-sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored Expert Panel report. J Allergy Clin Immunol. 2010;126:1105-1118.
2. Kemeny DM, Urbanek R, Amlot PL, et al. Sub-class of IgG in an allergic disease. I. IgG sub-class antibodies in immediate and non-immediate food allergies. Clin Allergy. 1986;16:571-581.
3. Gocki J, Zbigniew B. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol. 2016;33:253-256.
4. Shaw W. Clinical usefulness of IgG food allergy testing. Integrative Medicine for Mental Health Web site. www.immh.org/article-source/2016/6/29/clinical-usefulness-of-igg-food-allergy-testing. Published November 16, 2015. Accessed June 29, 2020.
5. Arroyave Hernández CM, Echavarría Pinto M, Hernández Montiel HL. Food allergy mediated by IgG antibodies associated with migraine in adults. Rev Alerg Mex. 2007;54:162-168.
6. Guo H, Jiang T, Wang J, et al. The value of eliminating foods according to food-specific immunoglobulin G antibodies in irritable bowel syndrome with diarrhoea. J Int Med Res. 2012;40:204-210.
7. IgG food antibodies. Genova Diagnostics Web site. www.gdx.net/product/igg-food-antibodies-food-sensitivity-test-blood. Accessed June 29, 2020.
8. Atkinson W, Sheldon TA, Shaath N, et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464.
1. Boyce JA, Assa’ad A, Burks AW, et al; NIAID-sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored Expert Panel report. J Allergy Clin Immunol. 2010;126:1105-1118.
2. Kemeny DM, Urbanek R, Amlot PL, et al. Sub-class of IgG in an allergic disease. I. IgG sub-class antibodies in immediate and non-immediate food allergies. Clin Allergy. 1986;16:571-581.
3. Gocki J, Zbigniew B. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol. 2016;33:253-256.
4. Shaw W. Clinical usefulness of IgG food allergy testing. Integrative Medicine for Mental Health Web site. www.immh.org/article-source/2016/6/29/clinical-usefulness-of-igg-food-allergy-testing. Published November 16, 2015. Accessed June 29, 2020.
5. Arroyave Hernández CM, Echavarría Pinto M, Hernández Montiel HL. Food allergy mediated by IgG antibodies associated with migraine in adults. Rev Alerg Mex. 2007;54:162-168.
6. Guo H, Jiang T, Wang J, et al. The value of eliminating foods according to food-specific immunoglobulin G antibodies in irritable bowel syndrome with diarrhoea. J Int Med Res. 2012;40:204-210.
7. IgG food antibodies. Genova Diagnostics Web site. www.gdx.net/product/igg-food-antibodies-food-sensitivity-test-blood. Accessed June 29, 2020.
8. Atkinson W, Sheldon TA, Shaath N, et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464.
COVID vaccine tested in people shows early promise
the company says in a news release.
Researchers also reported some side effects in the 45 people in the phase I study, but no significant safety issues, the news release says.
The vaccine is among hundreds being tested worldwide in an effort to halt the pandemic that has killed nearly 600,000 worldwide.
A researcher testing the vaccine called the results encouraging but cautioned more study is needed. “Importantly, the vaccine resulted in a robust immune response,” Evan Anderson, MD, principal investigator for the trial at Emory University, says in a news release. Emory and Kaiser Permanente Washington Health Research Institute were the two sites for the study.
The company is already testing the vaccine in a larger group of people, known as a phase II trial. It plans to begin phase III trials in late July. Phase III trials involve testing the vaccine on an even larger group and are the final step before FDA approval.
The study results are published in The New England Journal of Medicine. The study was led by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Moderna’s vaccine uses messenger RNA, also called mRNA. It carries the instruction for making the spike protein, a key protein on the surface of the virus that allows it to enter cells when a person is infected. After it’s injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the actual coronavirus. This allows other immune cells to develop immunity.
In the study, participants were divided into three groups of 15 people each. All groups received two vaccinations 28 days apart. Each group received a different strength of the vaccine – either 25, 100, or 250 micrograms.
Every person in the study developed antibodies that can block the infection. Most commonly reported side effects after the second vaccination in the 100-microgram group were fatigue, chills, headache, and muscle pains, ranging from mild to moderately severe.
The phase II study has 300 heathy adults ages 18-55, along with another 300 ages 55 and older
Moderna says it hopes to include about 30,000 participants at the 100-microgram dose level in the U.S. for the phase III trial. The estimated start date is July 27.
This article first appeared on WebMD.com.
the company says in a news release.
Researchers also reported some side effects in the 45 people in the phase I study, but no significant safety issues, the news release says.
The vaccine is among hundreds being tested worldwide in an effort to halt the pandemic that has killed nearly 600,000 worldwide.
A researcher testing the vaccine called the results encouraging but cautioned more study is needed. “Importantly, the vaccine resulted in a robust immune response,” Evan Anderson, MD, principal investigator for the trial at Emory University, says in a news release. Emory and Kaiser Permanente Washington Health Research Institute were the two sites for the study.
The company is already testing the vaccine in a larger group of people, known as a phase II trial. It plans to begin phase III trials in late July. Phase III trials involve testing the vaccine on an even larger group and are the final step before FDA approval.
The study results are published in The New England Journal of Medicine. The study was led by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Moderna’s vaccine uses messenger RNA, also called mRNA. It carries the instruction for making the spike protein, a key protein on the surface of the virus that allows it to enter cells when a person is infected. After it’s injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the actual coronavirus. This allows other immune cells to develop immunity.
In the study, participants were divided into three groups of 15 people each. All groups received two vaccinations 28 days apart. Each group received a different strength of the vaccine – either 25, 100, or 250 micrograms.
Every person in the study developed antibodies that can block the infection. Most commonly reported side effects after the second vaccination in the 100-microgram group were fatigue, chills, headache, and muscle pains, ranging from mild to moderately severe.
The phase II study has 300 heathy adults ages 18-55, along with another 300 ages 55 and older
Moderna says it hopes to include about 30,000 participants at the 100-microgram dose level in the U.S. for the phase III trial. The estimated start date is July 27.
This article first appeared on WebMD.com.
the company says in a news release.
Researchers also reported some side effects in the 45 people in the phase I study, but no significant safety issues, the news release says.
The vaccine is among hundreds being tested worldwide in an effort to halt the pandemic that has killed nearly 600,000 worldwide.
A researcher testing the vaccine called the results encouraging but cautioned more study is needed. “Importantly, the vaccine resulted in a robust immune response,” Evan Anderson, MD, principal investigator for the trial at Emory University, says in a news release. Emory and Kaiser Permanente Washington Health Research Institute were the two sites for the study.
The company is already testing the vaccine in a larger group of people, known as a phase II trial. It plans to begin phase III trials in late July. Phase III trials involve testing the vaccine on an even larger group and are the final step before FDA approval.
The study results are published in The New England Journal of Medicine. The study was led by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Moderna’s vaccine uses messenger RNA, also called mRNA. It carries the instruction for making the spike protein, a key protein on the surface of the virus that allows it to enter cells when a person is infected. After it’s injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the actual coronavirus. This allows other immune cells to develop immunity.
In the study, participants were divided into three groups of 15 people each. All groups received two vaccinations 28 days apart. Each group received a different strength of the vaccine – either 25, 100, or 250 micrograms.
Every person in the study developed antibodies that can block the infection. Most commonly reported side effects after the second vaccination in the 100-microgram group were fatigue, chills, headache, and muscle pains, ranging from mild to moderately severe.
The phase II study has 300 heathy adults ages 18-55, along with another 300 ages 55 and older
Moderna says it hopes to include about 30,000 participants at the 100-microgram dose level in the U.S. for the phase III trial. The estimated start date is July 27.
This article first appeared on WebMD.com.
Taking steps to slow the upswing in oral and pharyngeal cancers
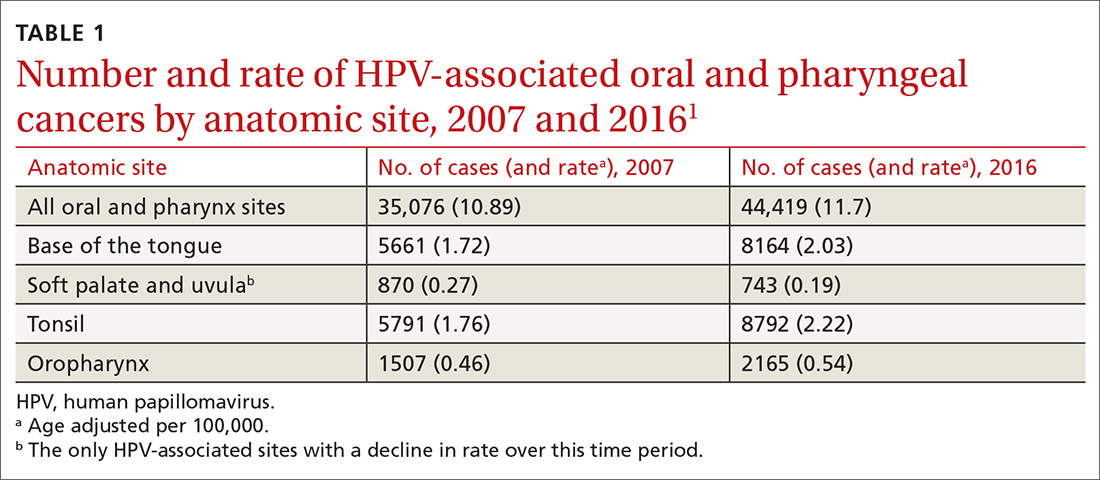
A recent report by the Centers for Disease Control and Prevention (CDC) documents the trends in oral and pharyngeal cancers (OPC) in the United States over a 10-year period, 2007-2016.1 The rate of OPC began to increase in 1999 and has been increasing ever since. The age-adjusted rate in 2007 was 10.89/100,000 compared with 11.7/100,000 in 2016 (TABLE 11). This is an annual relative increase of about 6% per year. In absolute numbers, there were 35,076 cases in 2007 and 44,419 in 2016.1 The trends in incidence of OPC vary by anatomical site, with some increasing and others declining.
There are 3 known causal factors related to OPC: tobacco use, alcohol use, and human papillomavirus (HPV) infection. The CDC estimates that, overall, 70% of OPCs are caused by HPV.2 However, while cancers at some oropharyngeal sites are likely related to HPV infection, cancers at other sites are not. The rising overall incidence of OPC is being driven by increases in HPV-related cancers at an average rate of 2.1% per year, while the rates at non-HPV-associated sites have been declining by 0.4% per year.1 It is also important to appreciate that HPV causes cancer at other anatomical sites (TABLE 22) and is responsible for an estimated 35,000 cancers per year.2

Other trends of note in all OPCs combined are increasing rates among non-Hispanic whites and Asian-Pacific Islanders; decreasing rates among Hispanics and African Americans; increasing rates among males with no real change in rates among females; increasing rates in those 50 to 79 years of age; decreasing rates among those 40 to 49 years of age; and unchanged rates in other age groups.1
The role of the family physician
Preventing OPC and all HPV-related cancers begins by encouraging patients to reduce alcohol and tobacco use and by emphasizing the importance of HPV vaccination. Educate teens and parents/guardians about HPV vaccine and its safety. Screen for tobacco and alcohol use, and offer brief clinical interventions as needed to decrease usage.
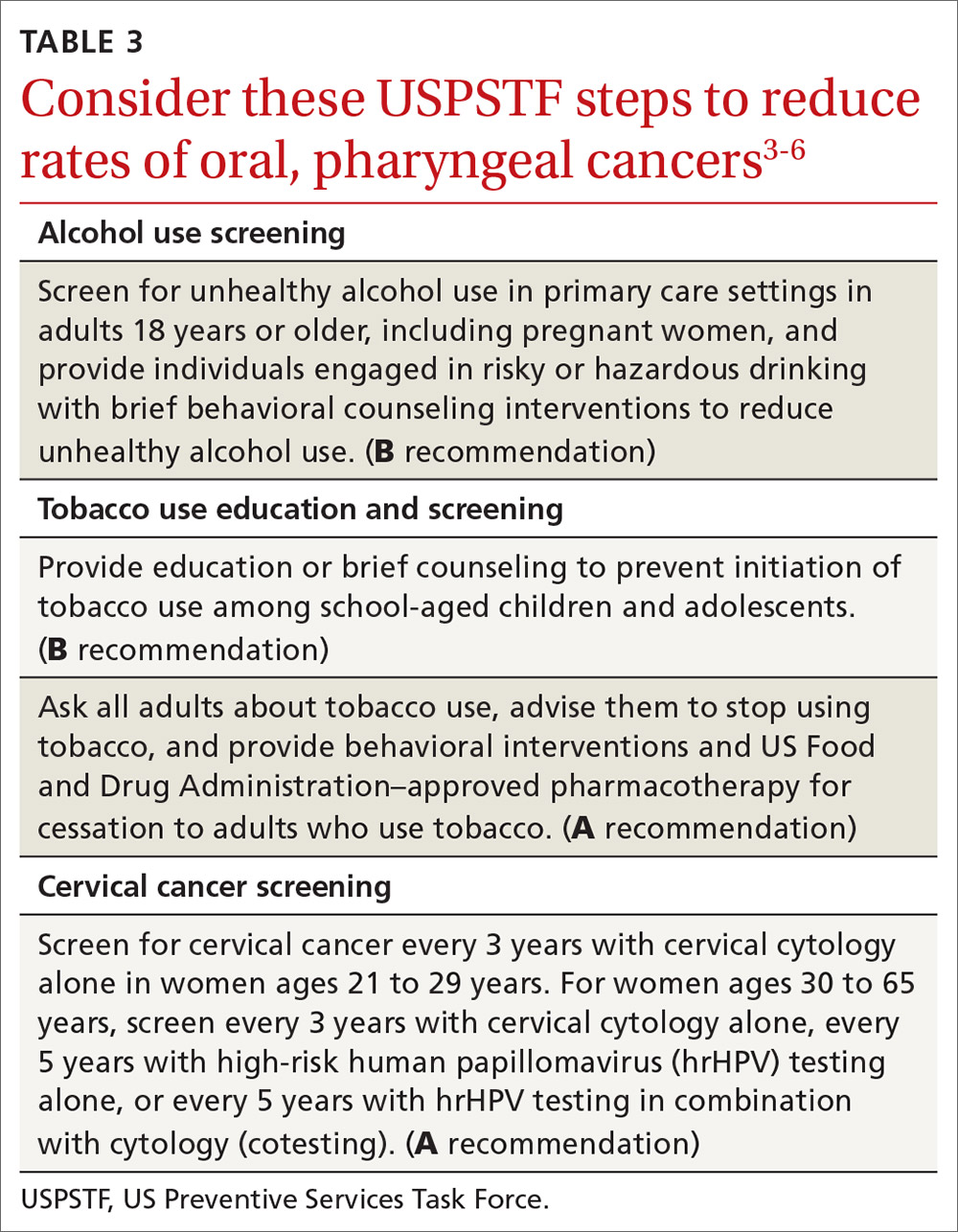
Recommendations by the US Preventive Services Task Force regarding screening for, and reducing use of, tobacco and alcohol, as well as screening for cervical cancer, are listed in TABLE 3.3-6 Remember that cervical cancer screening is both a primary and secondary intervention: It can reduce mortality by preventing cervical cancer (via treatment of precancerous lesions) and by detecting cervical cancer early at more treatable stages.
HPV vaccination essentials. CDC recommendations for the use of HPV vaccine and the vaccine dosing schedule appear in TABLE 4.7 While it is true that the best evidence for HPV vaccine’s prevention of cancer comes from the study of cervical and anal cancers, it is reasonable to expect that it will also be proven over time to prevent other HPV-caused cancers as the rate of HPV infections declines.
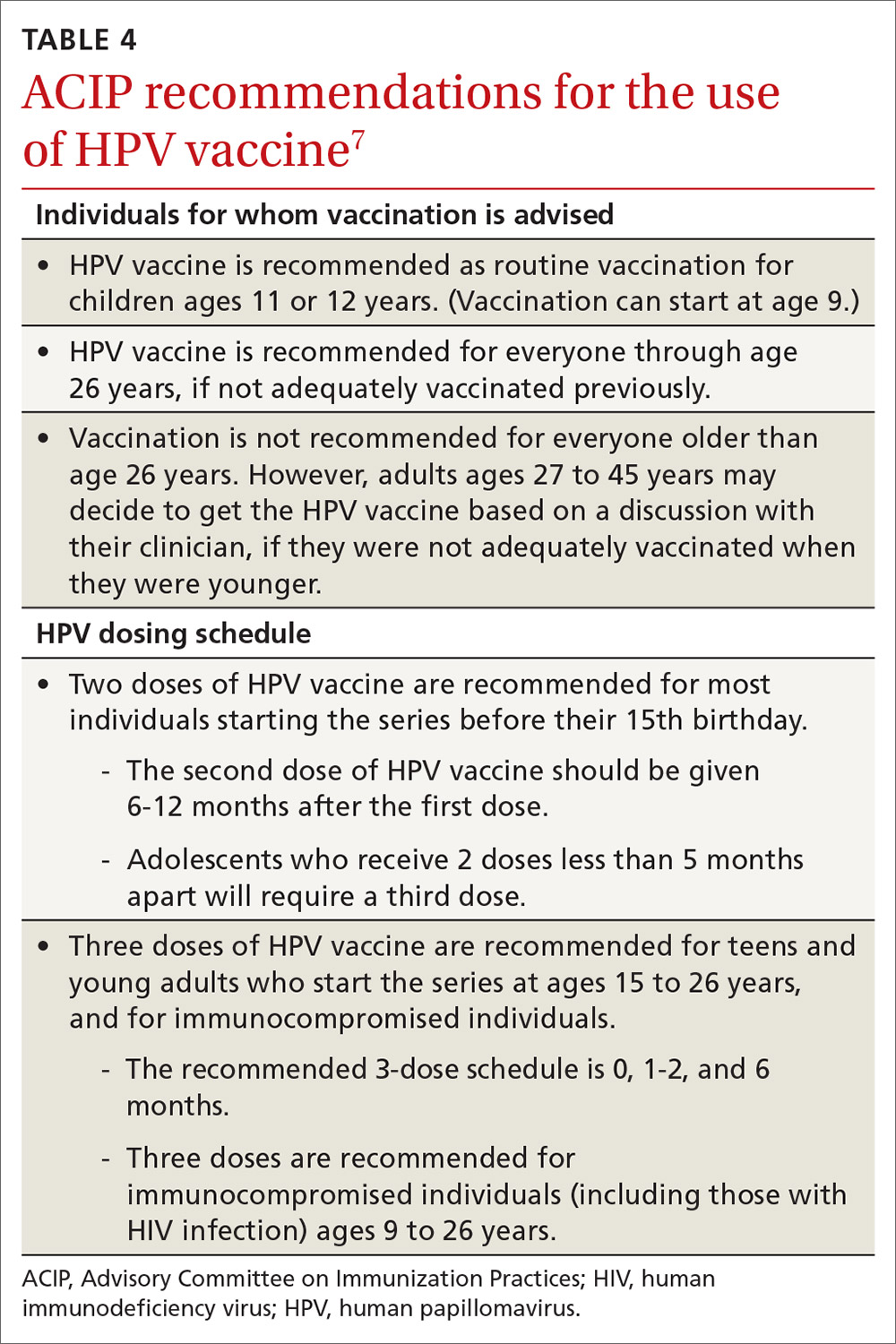
HPV vaccine is underused. In a 2018 survey, only 68.1% of adolescents had received 1 or more doses of HPV vaccine, and only 51.1% were up to date.8 In contrast, 86.6% had received 1 or more doses of quadrivalent meningococcal vaccine; 88.9% had received 1 or more doses of tetanus, diphtheria & acellular pertussis vaccine; 91.9% were up to date with 2 or more doses of measles, mumps & rubella vaccine; and 92.1% were up to date with hepatitis B vaccine, with 3 or more doses.8
Continue to: Address parental concerns, including these 5 false beliefs
Address parental concerns, including these 5 false beliefs
One study found 5 major false beliefs parents hold about HPV vaccine9:
- Vaccination is not effective at preventing cancer.
- Pap smears are sufficient to prevent cervical cancer.
- HPV vaccination is not safe.
- HPV vaccination is not needed since most infections are naturally cleared by the immune system.
- Eleven to 12 years of age is too young to vaccinate.
There is some evidence that if clinicians actively engage with parents about these concerns and address them head on, same-day vaccination rates can improve.10
We can expect to see HPV-associated OPC decline in the coming years due to the delayed effects on cancer incidence by the HPV vaccine. These anticipated declines will be more dramatic if we can increase the uptake of the HPV vaccine.
1. Ellington TD, Henley SJ, Senkomago V, et al. Trends in the incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433-438.
2. CDC. HPV and cancer. 2019. https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed June 29, 2020.
3. USPSTF. Unhealthy alcohol use in adolescents and adults: screening and behavioral counseling interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Accessed June 29, 2020.
4. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed June 29, 2020.
5. USPSTF. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed June 29, 2020.
6. USPSTF. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Accessed June 29, 2020.
7. CDC. Vaccines and preventable diseases. HPV vaccine recommendations. 2020. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed June 29, 2020.
8. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019:68:718-723.
9. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15:1628-1638.
10. Shay LA, Baldwin AS, Betts AC, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312.
A recent report by the Centers for Disease Control and Prevention (CDC) documents the trends in oral and pharyngeal cancers (OPC) in the United States over a 10-year period, 2007-2016.1 The rate of OPC began to increase in 1999 and has been increasing ever since. The age-adjusted rate in 2007 was 10.89/100,000 compared with 11.7/100,000 in 2016 (TABLE 11). This is an annual relative increase of about 6% per year. In absolute numbers, there were 35,076 cases in 2007 and 44,419 in 2016.1 The trends in incidence of OPC vary by anatomical site, with some increasing and others declining.

There are 3 known causal factors related to OPC: tobacco use, alcohol use, and human papillomavirus (HPV) infection. The CDC estimates that, overall, 70% of OPCs are caused by HPV.2 However, while cancers at some oropharyngeal sites are likely related to HPV infection, cancers at other sites are not. The rising overall incidence of OPC is being driven by increases in HPV-related cancers at an average rate of 2.1% per year, while the rates at non-HPV-associated sites have been declining by 0.4% per year.1 It is also important to appreciate that HPV causes cancer at other anatomical sites (TABLE 22) and is responsible for an estimated 35,000 cancers per year.2

Other trends of note in all OPCs combined are increasing rates among non-Hispanic whites and Asian-Pacific Islanders; decreasing rates among Hispanics and African Americans; increasing rates among males with no real change in rates among females; increasing rates in those 50 to 79 years of age; decreasing rates among those 40 to 49 years of age; and unchanged rates in other age groups.1
The role of the family physician
Preventing OPC and all HPV-related cancers begins by encouraging patients to reduce alcohol and tobacco use and by emphasizing the importance of HPV vaccination. Educate teens and parents/guardians about HPV vaccine and its safety. Screen for tobacco and alcohol use, and offer brief clinical interventions as needed to decrease usage.
Recommendations by the US Preventive Services Task Force regarding screening for, and reducing use of, tobacco and alcohol, as well as screening for cervical cancer, are listed in TABLE 3.3-6 Remember that cervical cancer screening is both a primary and secondary intervention: It can reduce mortality by preventing cervical cancer (via treatment of precancerous lesions) and by detecting cervical cancer early at more treatable stages.

HPV vaccination essentials. CDC recommendations for the use of HPV vaccine and the vaccine dosing schedule appear in TABLE 4.7 While it is true that the best evidence for HPV vaccine’s prevention of cancer comes from the study of cervical and anal cancers, it is reasonable to expect that it will also be proven over time to prevent other HPV-caused cancers as the rate of HPV infections declines.

HPV vaccine is underused. In a 2018 survey, only 68.1% of adolescents had received 1 or more doses of HPV vaccine, and only 51.1% were up to date.8 In contrast, 86.6% had received 1 or more doses of quadrivalent meningococcal vaccine; 88.9% had received 1 or more doses of tetanus, diphtheria & acellular pertussis vaccine; 91.9% were up to date with 2 or more doses of measles, mumps & rubella vaccine; and 92.1% were up to date with hepatitis B vaccine, with 3 or more doses.8
Continue to: Address parental concerns, including these 5 false beliefs
Address parental concerns, including these 5 false beliefs
One study found 5 major false beliefs parents hold about HPV vaccine9:
- Vaccination is not effective at preventing cancer.
- Pap smears are sufficient to prevent cervical cancer.
- HPV vaccination is not safe.
- HPV vaccination is not needed since most infections are naturally cleared by the immune system.
- Eleven to 12 years of age is too young to vaccinate.
There is some evidence that if clinicians actively engage with parents about these concerns and address them head on, same-day vaccination rates can improve.10
We can expect to see HPV-associated OPC decline in the coming years due to the delayed effects on cancer incidence by the HPV vaccine. These anticipated declines will be more dramatic if we can increase the uptake of the HPV vaccine.
A recent report by the Centers for Disease Control and Prevention (CDC) documents the trends in oral and pharyngeal cancers (OPC) in the United States over a 10-year period, 2007-2016.1 The rate of OPC began to increase in 1999 and has been increasing ever since. The age-adjusted rate in 2007 was 10.89/100,000 compared with 11.7/100,000 in 2016 (TABLE 11). This is an annual relative increase of about 6% per year. In absolute numbers, there were 35,076 cases in 2007 and 44,419 in 2016.1 The trends in incidence of OPC vary by anatomical site, with some increasing and others declining.

There are 3 known causal factors related to OPC: tobacco use, alcohol use, and human papillomavirus (HPV) infection. The CDC estimates that, overall, 70% of OPCs are caused by HPV.2 However, while cancers at some oropharyngeal sites are likely related to HPV infection, cancers at other sites are not. The rising overall incidence of OPC is being driven by increases in HPV-related cancers at an average rate of 2.1% per year, while the rates at non-HPV-associated sites have been declining by 0.4% per year.1 It is also important to appreciate that HPV causes cancer at other anatomical sites (TABLE 22) and is responsible for an estimated 35,000 cancers per year.2

Other trends of note in all OPCs combined are increasing rates among non-Hispanic whites and Asian-Pacific Islanders; decreasing rates among Hispanics and African Americans; increasing rates among males with no real change in rates among females; increasing rates in those 50 to 79 years of age; decreasing rates among those 40 to 49 years of age; and unchanged rates in other age groups.1
The role of the family physician
Preventing OPC and all HPV-related cancers begins by encouraging patients to reduce alcohol and tobacco use and by emphasizing the importance of HPV vaccination. Educate teens and parents/guardians about HPV vaccine and its safety. Screen for tobacco and alcohol use, and offer brief clinical interventions as needed to decrease usage.
Recommendations by the US Preventive Services Task Force regarding screening for, and reducing use of, tobacco and alcohol, as well as screening for cervical cancer, are listed in TABLE 3.3-6 Remember that cervical cancer screening is both a primary and secondary intervention: It can reduce mortality by preventing cervical cancer (via treatment of precancerous lesions) and by detecting cervical cancer early at more treatable stages.

HPV vaccination essentials. CDC recommendations for the use of HPV vaccine and the vaccine dosing schedule appear in TABLE 4.7 While it is true that the best evidence for HPV vaccine’s prevention of cancer comes from the study of cervical and anal cancers, it is reasonable to expect that it will also be proven over time to prevent other HPV-caused cancers as the rate of HPV infections declines.

HPV vaccine is underused. In a 2018 survey, only 68.1% of adolescents had received 1 or more doses of HPV vaccine, and only 51.1% were up to date.8 In contrast, 86.6% had received 1 or more doses of quadrivalent meningococcal vaccine; 88.9% had received 1 or more doses of tetanus, diphtheria & acellular pertussis vaccine; 91.9% were up to date with 2 or more doses of measles, mumps & rubella vaccine; and 92.1% were up to date with hepatitis B vaccine, with 3 or more doses.8
Continue to: Address parental concerns, including these 5 false beliefs
Address parental concerns, including these 5 false beliefs
One study found 5 major false beliefs parents hold about HPV vaccine9:
- Vaccination is not effective at preventing cancer.
- Pap smears are sufficient to prevent cervical cancer.
- HPV vaccination is not safe.
- HPV vaccination is not needed since most infections are naturally cleared by the immune system.
- Eleven to 12 years of age is too young to vaccinate.
There is some evidence that if clinicians actively engage with parents about these concerns and address them head on, same-day vaccination rates can improve.10
We can expect to see HPV-associated OPC decline in the coming years due to the delayed effects on cancer incidence by the HPV vaccine. These anticipated declines will be more dramatic if we can increase the uptake of the HPV vaccine.
1. Ellington TD, Henley SJ, Senkomago V, et al. Trends in the incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433-438.
2. CDC. HPV and cancer. 2019. https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed June 29, 2020.
3. USPSTF. Unhealthy alcohol use in adolescents and adults: screening and behavioral counseling interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Accessed June 29, 2020.
4. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed June 29, 2020.
5. USPSTF. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed June 29, 2020.
6. USPSTF. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Accessed June 29, 2020.
7. CDC. Vaccines and preventable diseases. HPV vaccine recommendations. 2020. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed June 29, 2020.
8. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019:68:718-723.
9. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15:1628-1638.
10. Shay LA, Baldwin AS, Betts AC, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312.
1. Ellington TD, Henley SJ, Senkomago V, et al. Trends in the incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433-438.
2. CDC. HPV and cancer. 2019. https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed June 29, 2020.
3. USPSTF. Unhealthy alcohol use in adolescents and adults: screening and behavioral counseling interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Accessed June 29, 2020.
4. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed June 29, 2020.
5. USPSTF. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed June 29, 2020.
6. USPSTF. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Accessed June 29, 2020.
7. CDC. Vaccines and preventable diseases. HPV vaccine recommendations. 2020. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed June 29, 2020.
8. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019:68:718-723.
9. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15:1628-1638.
10. Shay LA, Baldwin AS, Betts AC, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312.
Even mild obesity raises severe COVID-19 risks
People with a body mass index of 30 kg/m2 or above are at significantly increased risk for severe COVID-19, while a BMI of 35 and higher dramatically increases the risk for death, new research suggests.
The data, from nearly 500 patients hospitalized with COVID-19 in March and April 2020, were published in the European Journal of Endocrinology by Matteo Rottoli, MD, of the Alma Mater Studiorum, University of Bologna (Italy), and colleagues.
The data support the recent change by the Centers for Disease Control and Prevention to lower the cutoff for categorizing a person at increased risk from COVID-19 from a BMI of 40 down to 30. However, in the United Kingdom, the National Health Service still lists only a BMI of 40 or above as placing a person at “moderate risk (clinically vulnerable).”
“This finding calls for prevention and treatment strategies to reduce the risk of infection and hospitalization in patients with relevant degrees of obesity, supporting a revision of the BMI cutoff of 40 kg/m2, which was proposed as an independent risk factor for an adverse outcome of COVID-19 in the ... guidelines for social distancing in the United Kingdom: It may be appropriate to include patients with BMI >30 among those at higher risk for COVID-19 severe progression,” the authors wrote.
The study included 482 adults admitted with confirmed COVID-19 to a single Italian hospital between March 1 and April 20, 2020. Of those, 41.9% had a BMI of less than 25 (normal weight), 36.5% had a BMI of 25-29.9 (overweight), and 21.6% had BMI of at least 30 (obese). Of the obese group, 20 (4.1%) had BMIs of at least 35, while 18 patients (3.7%) had BMIs of less than 20 (underweight).
Among those with obesity, 51.9% experienced respiratory failure, 36.4% were admitted to the ICU, 25% required mechanical ventilation, and 29.8% died within 30 days of symptom onset.
Patients with BMIs of at least 30 had significantly increased risks for respiratory failure (odds ratio, 2.48; P = .001), ICU admission (OR, 5.28; P < .001), and death (2.35, P = .017), compared with those with lower BMIs. Within the group classified as obese, the risks of respiratory failure and ICU admission were higher, with BMIs of 30-34.9 (OR, 2.32; P = .004 and OR, 4.96; P < .001, respectively) and for BMIs of at least 35 (OR, 3.24; P = .019 and OR, 6.58; P < .001, respectively).
The risk of death was significantly higher among patients with a BMI of at least 35 (OR, 12.1; P < .001).
Every 1-unit increase in BMI was significantly associated with all outcomes, but there was no significant difference in any outcome between the 25-29.9 BMI category and normal weight. In all models, the BMI cutoff for increased risk was 30.
The authors reported no disclosures.
SOURCE: Rottoli M et al. Eur J Endocrinol. 2020 Jul 1. doi: 10.1530/EJE-20-054.
People with a body mass index of 30 kg/m2 or above are at significantly increased risk for severe COVID-19, while a BMI of 35 and higher dramatically increases the risk for death, new research suggests.
The data, from nearly 500 patients hospitalized with COVID-19 in March and April 2020, were published in the European Journal of Endocrinology by Matteo Rottoli, MD, of the Alma Mater Studiorum, University of Bologna (Italy), and colleagues.
The data support the recent change by the Centers for Disease Control and Prevention to lower the cutoff for categorizing a person at increased risk from COVID-19 from a BMI of 40 down to 30. However, in the United Kingdom, the National Health Service still lists only a BMI of 40 or above as placing a person at “moderate risk (clinically vulnerable).”
“This finding calls for prevention and treatment strategies to reduce the risk of infection and hospitalization in patients with relevant degrees of obesity, supporting a revision of the BMI cutoff of 40 kg/m2, which was proposed as an independent risk factor for an adverse outcome of COVID-19 in the ... guidelines for social distancing in the United Kingdom: It may be appropriate to include patients with BMI >30 among those at higher risk for COVID-19 severe progression,” the authors wrote.
The study included 482 adults admitted with confirmed COVID-19 to a single Italian hospital between March 1 and April 20, 2020. Of those, 41.9% had a BMI of less than 25 (normal weight), 36.5% had a BMI of 25-29.9 (overweight), and 21.6% had BMI of at least 30 (obese). Of the obese group, 20 (4.1%) had BMIs of at least 35, while 18 patients (3.7%) had BMIs of less than 20 (underweight).
Among those with obesity, 51.9% experienced respiratory failure, 36.4% were admitted to the ICU, 25% required mechanical ventilation, and 29.8% died within 30 days of symptom onset.
Patients with BMIs of at least 30 had significantly increased risks for respiratory failure (odds ratio, 2.48; P = .001), ICU admission (OR, 5.28; P < .001), and death (2.35, P = .017), compared with those with lower BMIs. Within the group classified as obese, the risks of respiratory failure and ICU admission were higher, with BMIs of 30-34.9 (OR, 2.32; P = .004 and OR, 4.96; P < .001, respectively) and for BMIs of at least 35 (OR, 3.24; P = .019 and OR, 6.58; P < .001, respectively).
The risk of death was significantly higher among patients with a BMI of at least 35 (OR, 12.1; P < .001).
Every 1-unit increase in BMI was significantly associated with all outcomes, but there was no significant difference in any outcome between the 25-29.9 BMI category and normal weight. In all models, the BMI cutoff for increased risk was 30.
The authors reported no disclosures.
SOURCE: Rottoli M et al. Eur J Endocrinol. 2020 Jul 1. doi: 10.1530/EJE-20-054.
People with a body mass index of 30 kg/m2 or above are at significantly increased risk for severe COVID-19, while a BMI of 35 and higher dramatically increases the risk for death, new research suggests.
The data, from nearly 500 patients hospitalized with COVID-19 in March and April 2020, were published in the European Journal of Endocrinology by Matteo Rottoli, MD, of the Alma Mater Studiorum, University of Bologna (Italy), and colleagues.
The data support the recent change by the Centers for Disease Control and Prevention to lower the cutoff for categorizing a person at increased risk from COVID-19 from a BMI of 40 down to 30. However, in the United Kingdom, the National Health Service still lists only a BMI of 40 or above as placing a person at “moderate risk (clinically vulnerable).”
“This finding calls for prevention and treatment strategies to reduce the risk of infection and hospitalization in patients with relevant degrees of obesity, supporting a revision of the BMI cutoff of 40 kg/m2, which was proposed as an independent risk factor for an adverse outcome of COVID-19 in the ... guidelines for social distancing in the United Kingdom: It may be appropriate to include patients with BMI >30 among those at higher risk for COVID-19 severe progression,” the authors wrote.
The study included 482 adults admitted with confirmed COVID-19 to a single Italian hospital between March 1 and April 20, 2020. Of those, 41.9% had a BMI of less than 25 (normal weight), 36.5% had a BMI of 25-29.9 (overweight), and 21.6% had BMI of at least 30 (obese). Of the obese group, 20 (4.1%) had BMIs of at least 35, while 18 patients (3.7%) had BMIs of less than 20 (underweight).
Among those with obesity, 51.9% experienced respiratory failure, 36.4% were admitted to the ICU, 25% required mechanical ventilation, and 29.8% died within 30 days of symptom onset.
Patients with BMIs of at least 30 had significantly increased risks for respiratory failure (odds ratio, 2.48; P = .001), ICU admission (OR, 5.28; P < .001), and death (2.35, P = .017), compared with those with lower BMIs. Within the group classified as obese, the risks of respiratory failure and ICU admission were higher, with BMIs of 30-34.9 (OR, 2.32; P = .004 and OR, 4.96; P < .001, respectively) and for BMIs of at least 35 (OR, 3.24; P = .019 and OR, 6.58; P < .001, respectively).
The risk of death was significantly higher among patients with a BMI of at least 35 (OR, 12.1; P < .001).
Every 1-unit increase in BMI was significantly associated with all outcomes, but there was no significant difference in any outcome between the 25-29.9 BMI category and normal weight. In all models, the BMI cutoff for increased risk was 30.
The authors reported no disclosures.
SOURCE: Rottoli M et al. Eur J Endocrinol. 2020 Jul 1. doi: 10.1530/EJE-20-054.
FROM THE EUROPEAN JOURNAL OF ENDOCRINOLOGY
Consider adverse childhood experiences during the pandemic
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at [email protected].
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
ECT more effective for psychotic vs. nonpsychotic depression?
For patients with psychotic depression, response to treatment, remission rates, and cognitive improvement are better following electroconvulsive therapy (ECT) than for patients with nonpsychotic depression, results from a new study suggest.
However, findings from another study suggest that at least some of these differences may be because psychotic patients are referred for ECT earlier in the disease course.
Both studies were presented at the European Psychiatric Association 2020 Congress, which was held online this year because of the COVID-19 pandemic.
Limited, old evidence
The first study was led by Christopher Yi Wen Chan, MD, Institute of Mental Health, Singapore. The investigators stated that they have “often observed” superior remission rates with ECT in psychotic versus nonpsychotic depression. However, the evidence base is “limited and mostly more than 10 years old.”
They conducted a retrospective case-control study that included 160 patients – 50 with psychotic depression, and 110 with nonpsychotic depression. All patients had a primary diagnosis of unipolar major depressive disorder and underwent ECT at a tertiary psychiatric institute between January 2016 and January 2018.
Baseline characteristics of the two groups were similar, although patients with psychosis were more likely to have had an involuntary hospital admission and to have had higher baseline scores on the Montreal Cognitive Assessment (MoCA) and Clinical Global Impression–Severity scale (CGI-S) than nonpsychotic patients.
Response rates to ECT were significantly higher for the patients with psychotic depression than for those with nonpsychotic depression (79% vs. 51%; P = .009), as were remission rates (71% vs. 36%; P = .001).
Both groups showed significant improvement following ECT in Montgomery-Åsberg Depression Rating Scale, CGI, and quality-of-life scores.
However, only the participants with psychotic depression showed a significant improvement in MoCA total score (P = .038), as well as on attention (P = .024), language (P = .008), and orientation (P = .021) subdomains.
Psychotic depression markers?
For the second study, a team led by Aida De Arriba Arnau, MD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, retrospectively analyzed 66 patients with depression who had received ECT. Of these, 26 had psychotic depression, and 40 had nonpsychotic depression.
Response rates were again higher in patients with psychotic vs nonpsychotic depression (92.3% vs. 85.0%). A similar number of sessions was needed to achieve a response.
Improvements in Hamilton Depression Rating Scale scores were significant between the two groups from the start of treatment, although the difference became nonsignificant at week 6.
Arriba Arnau said that there were some notable differences between patients with psychotic depression and those with nonpsychotic depression. For example, the former had “poor functionality, shorter episode duration, and less pharmacological resistance before receiving ECT,” she said.
“So we hypothesized that they might be referred more promptly to ECT treatment,” she added.
The psychotic depression group was significantly older than the group with nonpsychotic depression, at an average of 67.81 years vs 58.96 years.
They also “showed more illness severity and cognitive disturbances at baseline and ... required less anesthetic doses and higher initial stimulus intensity,» Arriba Arnau noted.
“All these features could be the markers of psychotic depression as an entity,” she said. However, the potential impact of age on these differences should be “further studied.”
She added that other aspects, such as age at onset and number of previous episodes, were similar between the groups.
Confirmatory data
Commenting on the findings for Medscape Medical News, Georgios Petrides, MD, associate professor of psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, East Garden City, New York, noted that differences in response to ECT between patients with psychotic depression and those with nonpsychotic depression are “well known.”
However, “it’s actually good to present more data that confirm what people are doing in clinical practice,” said Petrides, who was not involved with the research.
Petrides noted that some guidelines recommend ECT as first-line treatment for psychotic depression.
“For nonpsychotic depression, we’d try medications, psychotherapy, and everything else first,” he said. He noted that the current results are “a good replication of what is known so far.”
As to why ECT should be more effective for patients with psychotic depression, he said, “A lot of people think that the biology of psychotic depression is different from the biology of nonpsychotic depression.”
Petrides added.
That ECT is more effective in psychotic depression is an “indirect point of evidence” to support that theory.
One aspect that has traditionally dogged the use of ECT has been the stigma that surrounds the procedure, Petrides noted. That’s “always an issue, but it’s getting less and less over time,” he said.
He added that ECT is extremely safe and that it is associated with the “lowest mortality for any procedure performed under general anesthesia,” which helps to reduce the stigma around it, he noted.
The study authors and Petrides have reported no relevant financial relationships.
This article first appeared on Medscape.com.
For patients with psychotic depression, response to treatment, remission rates, and cognitive improvement are better following electroconvulsive therapy (ECT) than for patients with nonpsychotic depression, results from a new study suggest.
However, findings from another study suggest that at least some of these differences may be because psychotic patients are referred for ECT earlier in the disease course.
Both studies were presented at the European Psychiatric Association 2020 Congress, which was held online this year because of the COVID-19 pandemic.
Limited, old evidence
The first study was led by Christopher Yi Wen Chan, MD, Institute of Mental Health, Singapore. The investigators stated that they have “often observed” superior remission rates with ECT in psychotic versus nonpsychotic depression. However, the evidence base is “limited and mostly more than 10 years old.”
They conducted a retrospective case-control study that included 160 patients – 50 with psychotic depression, and 110 with nonpsychotic depression. All patients had a primary diagnosis of unipolar major depressive disorder and underwent ECT at a tertiary psychiatric institute between January 2016 and January 2018.
Baseline characteristics of the two groups were similar, although patients with psychosis were more likely to have had an involuntary hospital admission and to have had higher baseline scores on the Montreal Cognitive Assessment (MoCA) and Clinical Global Impression–Severity scale (CGI-S) than nonpsychotic patients.
Response rates to ECT were significantly higher for the patients with psychotic depression than for those with nonpsychotic depression (79% vs. 51%; P = .009), as were remission rates (71% vs. 36%; P = .001).
Both groups showed significant improvement following ECT in Montgomery-Åsberg Depression Rating Scale, CGI, and quality-of-life scores.
However, only the participants with psychotic depression showed a significant improvement in MoCA total score (P = .038), as well as on attention (P = .024), language (P = .008), and orientation (P = .021) subdomains.
Psychotic depression markers?
For the second study, a team led by Aida De Arriba Arnau, MD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, retrospectively analyzed 66 patients with depression who had received ECT. Of these, 26 had psychotic depression, and 40 had nonpsychotic depression.
Response rates were again higher in patients with psychotic vs nonpsychotic depression (92.3% vs. 85.0%). A similar number of sessions was needed to achieve a response.
Improvements in Hamilton Depression Rating Scale scores were significant between the two groups from the start of treatment, although the difference became nonsignificant at week 6.
Arriba Arnau said that there were some notable differences between patients with psychotic depression and those with nonpsychotic depression. For example, the former had “poor functionality, shorter episode duration, and less pharmacological resistance before receiving ECT,” she said.
“So we hypothesized that they might be referred more promptly to ECT treatment,” she added.
The psychotic depression group was significantly older than the group with nonpsychotic depression, at an average of 67.81 years vs 58.96 years.
They also “showed more illness severity and cognitive disturbances at baseline and ... required less anesthetic doses and higher initial stimulus intensity,» Arriba Arnau noted.
“All these features could be the markers of psychotic depression as an entity,” she said. However, the potential impact of age on these differences should be “further studied.”
She added that other aspects, such as age at onset and number of previous episodes, were similar between the groups.
Confirmatory data
Commenting on the findings for Medscape Medical News, Georgios Petrides, MD, associate professor of psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, East Garden City, New York, noted that differences in response to ECT between patients with psychotic depression and those with nonpsychotic depression are “well known.”
However, “it’s actually good to present more data that confirm what people are doing in clinical practice,” said Petrides, who was not involved with the research.
Petrides noted that some guidelines recommend ECT as first-line treatment for psychotic depression.
“For nonpsychotic depression, we’d try medications, psychotherapy, and everything else first,” he said. He noted that the current results are “a good replication of what is known so far.”
As to why ECT should be more effective for patients with psychotic depression, he said, “A lot of people think that the biology of psychotic depression is different from the biology of nonpsychotic depression.”
Petrides added.
That ECT is more effective in psychotic depression is an “indirect point of evidence” to support that theory.
One aspect that has traditionally dogged the use of ECT has been the stigma that surrounds the procedure, Petrides noted. That’s “always an issue, but it’s getting less and less over time,” he said.
He added that ECT is extremely safe and that it is associated with the “lowest mortality for any procedure performed under general anesthesia,” which helps to reduce the stigma around it, he noted.
The study authors and Petrides have reported no relevant financial relationships.
This article first appeared on Medscape.com.
For patients with psychotic depression, response to treatment, remission rates, and cognitive improvement are better following electroconvulsive therapy (ECT) than for patients with nonpsychotic depression, results from a new study suggest.
However, findings from another study suggest that at least some of these differences may be because psychotic patients are referred for ECT earlier in the disease course.
Both studies were presented at the European Psychiatric Association 2020 Congress, which was held online this year because of the COVID-19 pandemic.
Limited, old evidence
The first study was led by Christopher Yi Wen Chan, MD, Institute of Mental Health, Singapore. The investigators stated that they have “often observed” superior remission rates with ECT in psychotic versus nonpsychotic depression. However, the evidence base is “limited and mostly more than 10 years old.”
They conducted a retrospective case-control study that included 160 patients – 50 with psychotic depression, and 110 with nonpsychotic depression. All patients had a primary diagnosis of unipolar major depressive disorder and underwent ECT at a tertiary psychiatric institute between January 2016 and January 2018.
Baseline characteristics of the two groups were similar, although patients with psychosis were more likely to have had an involuntary hospital admission and to have had higher baseline scores on the Montreal Cognitive Assessment (MoCA) and Clinical Global Impression–Severity scale (CGI-S) than nonpsychotic patients.
Response rates to ECT were significantly higher for the patients with psychotic depression than for those with nonpsychotic depression (79% vs. 51%; P = .009), as were remission rates (71% vs. 36%; P = .001).
Both groups showed significant improvement following ECT in Montgomery-Åsberg Depression Rating Scale, CGI, and quality-of-life scores.
However, only the participants with psychotic depression showed a significant improvement in MoCA total score (P = .038), as well as on attention (P = .024), language (P = .008), and orientation (P = .021) subdomains.
Psychotic depression markers?
For the second study, a team led by Aida De Arriba Arnau, MD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, retrospectively analyzed 66 patients with depression who had received ECT. Of these, 26 had psychotic depression, and 40 had nonpsychotic depression.
Response rates were again higher in patients with psychotic vs nonpsychotic depression (92.3% vs. 85.0%). A similar number of sessions was needed to achieve a response.
Improvements in Hamilton Depression Rating Scale scores were significant between the two groups from the start of treatment, although the difference became nonsignificant at week 6.
Arriba Arnau said that there were some notable differences between patients with psychotic depression and those with nonpsychotic depression. For example, the former had “poor functionality, shorter episode duration, and less pharmacological resistance before receiving ECT,” she said.
“So we hypothesized that they might be referred more promptly to ECT treatment,” she added.
The psychotic depression group was significantly older than the group with nonpsychotic depression, at an average of 67.81 years vs 58.96 years.
They also “showed more illness severity and cognitive disturbances at baseline and ... required less anesthetic doses and higher initial stimulus intensity,» Arriba Arnau noted.
“All these features could be the markers of psychotic depression as an entity,” she said. However, the potential impact of age on these differences should be “further studied.”
She added that other aspects, such as age at onset and number of previous episodes, were similar between the groups.
Confirmatory data
Commenting on the findings for Medscape Medical News, Georgios Petrides, MD, associate professor of psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, East Garden City, New York, noted that differences in response to ECT between patients with psychotic depression and those with nonpsychotic depression are “well known.”
However, “it’s actually good to present more data that confirm what people are doing in clinical practice,” said Petrides, who was not involved with the research.
Petrides noted that some guidelines recommend ECT as first-line treatment for psychotic depression.
“For nonpsychotic depression, we’d try medications, psychotherapy, and everything else first,” he said. He noted that the current results are “a good replication of what is known so far.”
As to why ECT should be more effective for patients with psychotic depression, he said, “A lot of people think that the biology of psychotic depression is different from the biology of nonpsychotic depression.”
Petrides added.
That ECT is more effective in psychotic depression is an “indirect point of evidence” to support that theory.
One aspect that has traditionally dogged the use of ECT has been the stigma that surrounds the procedure, Petrides noted. That’s “always an issue, but it’s getting less and less over time,” he said.
He added that ECT is extremely safe and that it is associated with the “lowest mortality for any procedure performed under general anesthesia,” which helps to reduce the stigma around it, he noted.
The study authors and Petrides have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Some women use prescription opioids during pregnancy
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.
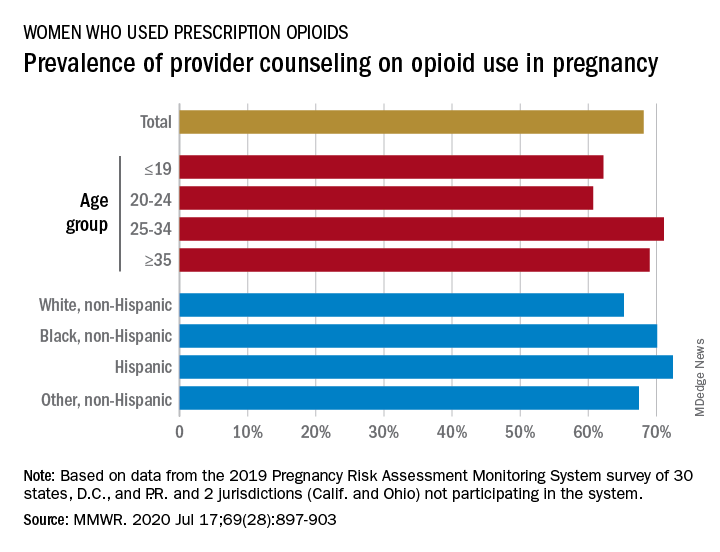
Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
FROM MMWR
Limit customized compounded hormones to special circumstances
The use of compounded bioidentical hormone therapies should be limited to patients who are not able to use a hormone therapy product approved by the Food and Drug Administration for reasons of allergy or dosage, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
In recent years, compounded bioidentical hormone therapies (cBHTs) have been “marketed as a personalized and natural approach to enhanced wellness using tailored preparations that address a myriad of symptoms, including those associated with menopause and aging,” wrote Donald R. Mattison, MD, of the University of Ottawa, and chair of the committee charged with producing the report, and colleagues.
Although both cBHTs and bioidentical hormone therapies (BHTs) contain hormones that are structurally and chemically identical to those in the human body, cBHTs have not undergone the safety, efficacy, and quality control tests of approved FDA products, according to the report.
In addition, cBHTs have no standardization when it comes to medication doses, and the products often are available in topicals such as creams or ointments, as well as pills or pellets. The lack of standards in dosing or form can contribute to the risk of overdose, the report emphasized.
Various cBTH products continue to be marketed to the public for age-related hormone symptoms including hot flashes associated with menopause and decreased muscle mass associated with decreased testosterone. However, cBHTs are not approved by the FDA in part because the individually mixed products are not tested to verify the amount of hormone that may be absorbed.
In response to the increased use of cBHTs, the National Academies convened a Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy and commissioned a report.
The two typical reasons to prescribe cBHT are either to provide a medication in an alternate dose not available in approved products or to omit components of a medication to which a patient is allergic, according to the report.
The report includes an algorithm to help guide clinicians in prescribing FDA-approved products, including off-label use of approved products, before cBHT products. “There is a dearth of high-quality evidence ... available to establish whether cBHT preparations are safe or efficacious for their prescribed uses,” the report states.
Of note, the committee also found no guidelines to recommend the use of cBHT products as a substitute for off-label use of FDA-approved BHT products for patients with female sexual dysfunction or gender dysphoria, two conditions for which no FDA-approved BHT products exist.
“The North American Menopause Society applauds the efforts of the National Academies of Sciences, Engineering, and Medicine (NASEM) and endorses their recommendations on compounded bioidentical hormone therapy,” Stephanie S. Faubion, MD, medical director of The North American Menopause Society, wrote in a statement. “As a society, we remain committed to improving the care of midlife women through the promotion of evidence-based research, education, and clinical care.”
A report on the use of cBHTs was important at this time because of the widespread and largely unregulated use of these products with little data to support their safety and efficacy, Dr. Faubion said in an interview.
“There are no indications for use of custom compounded hormone therapy aside from an allergy to a component in the FDA-approved products or lack of availability of the needed dose, which would be exceedingly rare given the variety of forms and doses available with FDA-approved products,” she said.
Main concerns regarding the use of cBHTs are the lack of safety and efficacy data, Dr. Faubion emphasized. “Women believe these products are safer than FDA-approved products because they do not receive a package insert outlining potential risks as they do with FDA-approved products.” A lack of data and safety monitoring of cBHTs means that adverse effects are not monitored and reported, she said. Also, safety concerns persist regarding some forms of cBHTs such as pellets, which were specifically highlighted in the report.
Dr. Faubion said that she “absolutely” agrees with the report’s limited circumstances in which the used of cBHTs would be appropriate. “There are very few reasons why women would need to use compounded hormones instead of the FDA-approved versions, which are regulated for quality, efficacy and safety, readily available in the local pharmacy, and often covered by insurance.”
In terms of the future, “we need more education for women as consumers and for medical providers on this topic,” Dr. Faubion noted. Also, “clearly, there is a dearth of research on the true efficacy and safety of these compounded hormone therapy products.”
The statement from the National Academies crystallizes what experts have been saying for decades, according to Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn.
The formal recommendations to limit the use of cBHTs “are not novel, but certainly needed,” and the statement “offers guidance regardless of your specialty,” Dr. Pal said in an interview.
There is often a disconnect between consumers’ understanding of compounding and the reality of safety concerns, she said. “We are in a tabloid era,” and education is key to guiding patients toward the FDA-approved treatments with safety data and demonstrated effectiveness, she said. “Safety should be the driving factor.” In compounded products, “there is no consistency that what you get today is the same as what you get tomorrow,” and the lack of standardization of cBHTs increases the risk for adverse events, she emphasized.
For patients with special needs such as allergies or other specialized dosing requirements, as noted in the National Academies statement, clinicians should discuss the options with patients and monitor them regularly to head off potential adverse events such as the development of uterine cancer, said Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board.
The research involved in creating the report was supported by the Food and Drug Administration.
Dr. Faubion had no financial conflicts to disclose. Dr. Pal had no relevant financial disclosures.
SOURCE: Mattison DR et al.; National Academies of Sciences, Engineering, and Medicine. The clinical utility of compounded bioidentical hormone therapy: A review of safety, effectiveness, and use. (Washington, DC: The National Academies Press. 2020.)
The use of compounded bioidentical hormone therapies should be limited to patients who are not able to use a hormone therapy product approved by the Food and Drug Administration for reasons of allergy or dosage, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
In recent years, compounded bioidentical hormone therapies (cBHTs) have been “marketed as a personalized and natural approach to enhanced wellness using tailored preparations that address a myriad of symptoms, including those associated with menopause and aging,” wrote Donald R. Mattison, MD, of the University of Ottawa, and chair of the committee charged with producing the report, and colleagues.
Although both cBHTs and bioidentical hormone therapies (BHTs) contain hormones that are structurally and chemically identical to those in the human body, cBHTs have not undergone the safety, efficacy, and quality control tests of approved FDA products, according to the report.
In addition, cBHTs have no standardization when it comes to medication doses, and the products often are available in topicals such as creams or ointments, as well as pills or pellets. The lack of standards in dosing or form can contribute to the risk of overdose, the report emphasized.
Various cBTH products continue to be marketed to the public for age-related hormone symptoms including hot flashes associated with menopause and decreased muscle mass associated with decreased testosterone. However, cBHTs are not approved by the FDA in part because the individually mixed products are not tested to verify the amount of hormone that may be absorbed.
In response to the increased use of cBHTs, the National Academies convened a Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy and commissioned a report.
The two typical reasons to prescribe cBHT are either to provide a medication in an alternate dose not available in approved products or to omit components of a medication to which a patient is allergic, according to the report.
The report includes an algorithm to help guide clinicians in prescribing FDA-approved products, including off-label use of approved products, before cBHT products. “There is a dearth of high-quality evidence ... available to establish whether cBHT preparations are safe or efficacious for their prescribed uses,” the report states.
Of note, the committee also found no guidelines to recommend the use of cBHT products as a substitute for off-label use of FDA-approved BHT products for patients with female sexual dysfunction or gender dysphoria, two conditions for which no FDA-approved BHT products exist.
“The North American Menopause Society applauds the efforts of the National Academies of Sciences, Engineering, and Medicine (NASEM) and endorses their recommendations on compounded bioidentical hormone therapy,” Stephanie S. Faubion, MD, medical director of The North American Menopause Society, wrote in a statement. “As a society, we remain committed to improving the care of midlife women through the promotion of evidence-based research, education, and clinical care.”
A report on the use of cBHTs was important at this time because of the widespread and largely unregulated use of these products with little data to support their safety and efficacy, Dr. Faubion said in an interview.
“There are no indications for use of custom compounded hormone therapy aside from an allergy to a component in the FDA-approved products or lack of availability of the needed dose, which would be exceedingly rare given the variety of forms and doses available with FDA-approved products,” she said.
Main concerns regarding the use of cBHTs are the lack of safety and efficacy data, Dr. Faubion emphasized. “Women believe these products are safer than FDA-approved products because they do not receive a package insert outlining potential risks as they do with FDA-approved products.” A lack of data and safety monitoring of cBHTs means that adverse effects are not monitored and reported, she said. Also, safety concerns persist regarding some forms of cBHTs such as pellets, which were specifically highlighted in the report.
Dr. Faubion said that she “absolutely” agrees with the report’s limited circumstances in which the used of cBHTs would be appropriate. “There are very few reasons why women would need to use compounded hormones instead of the FDA-approved versions, which are regulated for quality, efficacy and safety, readily available in the local pharmacy, and often covered by insurance.”
In terms of the future, “we need more education for women as consumers and for medical providers on this topic,” Dr. Faubion noted. Also, “clearly, there is a dearth of research on the true efficacy and safety of these compounded hormone therapy products.”
The statement from the National Academies crystallizes what experts have been saying for decades, according to Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn.
The formal recommendations to limit the use of cBHTs “are not novel, but certainly needed,” and the statement “offers guidance regardless of your specialty,” Dr. Pal said in an interview.
There is often a disconnect between consumers’ understanding of compounding and the reality of safety concerns, she said. “We are in a tabloid era,” and education is key to guiding patients toward the FDA-approved treatments with safety data and demonstrated effectiveness, she said. “Safety should be the driving factor.” In compounded products, “there is no consistency that what you get today is the same as what you get tomorrow,” and the lack of standardization of cBHTs increases the risk for adverse events, she emphasized.
For patients with special needs such as allergies or other specialized dosing requirements, as noted in the National Academies statement, clinicians should discuss the options with patients and monitor them regularly to head off potential adverse events such as the development of uterine cancer, said Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board.
The research involved in creating the report was supported by the Food and Drug Administration.
Dr. Faubion had no financial conflicts to disclose. Dr. Pal had no relevant financial disclosures.
SOURCE: Mattison DR et al.; National Academies of Sciences, Engineering, and Medicine. The clinical utility of compounded bioidentical hormone therapy: A review of safety, effectiveness, and use. (Washington, DC: The National Academies Press. 2020.)
The use of compounded bioidentical hormone therapies should be limited to patients who are not able to use a hormone therapy product approved by the Food and Drug Administration for reasons of allergy or dosage, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
In recent years, compounded bioidentical hormone therapies (cBHTs) have been “marketed as a personalized and natural approach to enhanced wellness using tailored preparations that address a myriad of symptoms, including those associated with menopause and aging,” wrote Donald R. Mattison, MD, of the University of Ottawa, and chair of the committee charged with producing the report, and colleagues.
Although both cBHTs and bioidentical hormone therapies (BHTs) contain hormones that are structurally and chemically identical to those in the human body, cBHTs have not undergone the safety, efficacy, and quality control tests of approved FDA products, according to the report.
In addition, cBHTs have no standardization when it comes to medication doses, and the products often are available in topicals such as creams or ointments, as well as pills or pellets. The lack of standards in dosing or form can contribute to the risk of overdose, the report emphasized.
Various cBTH products continue to be marketed to the public for age-related hormone symptoms including hot flashes associated with menopause and decreased muscle mass associated with decreased testosterone. However, cBHTs are not approved by the FDA in part because the individually mixed products are not tested to verify the amount of hormone that may be absorbed.
In response to the increased use of cBHTs, the National Academies convened a Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy and commissioned a report.
The two typical reasons to prescribe cBHT are either to provide a medication in an alternate dose not available in approved products or to omit components of a medication to which a patient is allergic, according to the report.
The report includes an algorithm to help guide clinicians in prescribing FDA-approved products, including off-label use of approved products, before cBHT products. “There is a dearth of high-quality evidence ... available to establish whether cBHT preparations are safe or efficacious for their prescribed uses,” the report states.
Of note, the committee also found no guidelines to recommend the use of cBHT products as a substitute for off-label use of FDA-approved BHT products for patients with female sexual dysfunction or gender dysphoria, two conditions for which no FDA-approved BHT products exist.
“The North American Menopause Society applauds the efforts of the National Academies of Sciences, Engineering, and Medicine (NASEM) and endorses their recommendations on compounded bioidentical hormone therapy,” Stephanie S. Faubion, MD, medical director of The North American Menopause Society, wrote in a statement. “As a society, we remain committed to improving the care of midlife women through the promotion of evidence-based research, education, and clinical care.”
A report on the use of cBHTs was important at this time because of the widespread and largely unregulated use of these products with little data to support their safety and efficacy, Dr. Faubion said in an interview.
“There are no indications for use of custom compounded hormone therapy aside from an allergy to a component in the FDA-approved products or lack of availability of the needed dose, which would be exceedingly rare given the variety of forms and doses available with FDA-approved products,” she said.
Main concerns regarding the use of cBHTs are the lack of safety and efficacy data, Dr. Faubion emphasized. “Women believe these products are safer than FDA-approved products because they do not receive a package insert outlining potential risks as they do with FDA-approved products.” A lack of data and safety monitoring of cBHTs means that adverse effects are not monitored and reported, she said. Also, safety concerns persist regarding some forms of cBHTs such as pellets, which were specifically highlighted in the report.
Dr. Faubion said that she “absolutely” agrees with the report’s limited circumstances in which the used of cBHTs would be appropriate. “There are very few reasons why women would need to use compounded hormones instead of the FDA-approved versions, which are regulated for quality, efficacy and safety, readily available in the local pharmacy, and often covered by insurance.”
In terms of the future, “we need more education for women as consumers and for medical providers on this topic,” Dr. Faubion noted. Also, “clearly, there is a dearth of research on the true efficacy and safety of these compounded hormone therapy products.”
The statement from the National Academies crystallizes what experts have been saying for decades, according to Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn.
The formal recommendations to limit the use of cBHTs “are not novel, but certainly needed,” and the statement “offers guidance regardless of your specialty,” Dr. Pal said in an interview.
There is often a disconnect between consumers’ understanding of compounding and the reality of safety concerns, she said. “We are in a tabloid era,” and education is key to guiding patients toward the FDA-approved treatments with safety data and demonstrated effectiveness, she said. “Safety should be the driving factor.” In compounded products, “there is no consistency that what you get today is the same as what you get tomorrow,” and the lack of standardization of cBHTs increases the risk for adverse events, she emphasized.
For patients with special needs such as allergies or other specialized dosing requirements, as noted in the National Academies statement, clinicians should discuss the options with patients and monitor them regularly to head off potential adverse events such as the development of uterine cancer, said Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board.
The research involved in creating the report was supported by the Food and Drug Administration.
Dr. Faubion had no financial conflicts to disclose. Dr. Pal had no relevant financial disclosures.
SOURCE: Mattison DR et al.; National Academies of Sciences, Engineering, and Medicine. The clinical utility of compounded bioidentical hormone therapy: A review of safety, effectiveness, and use. (Washington, DC: The National Academies Press. 2020.)
PSMA PET/CT may be new ‘gold standard’ for prostate cancer staging
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
FROM EAU20
It’s time to rethink your approach to C diff infection
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
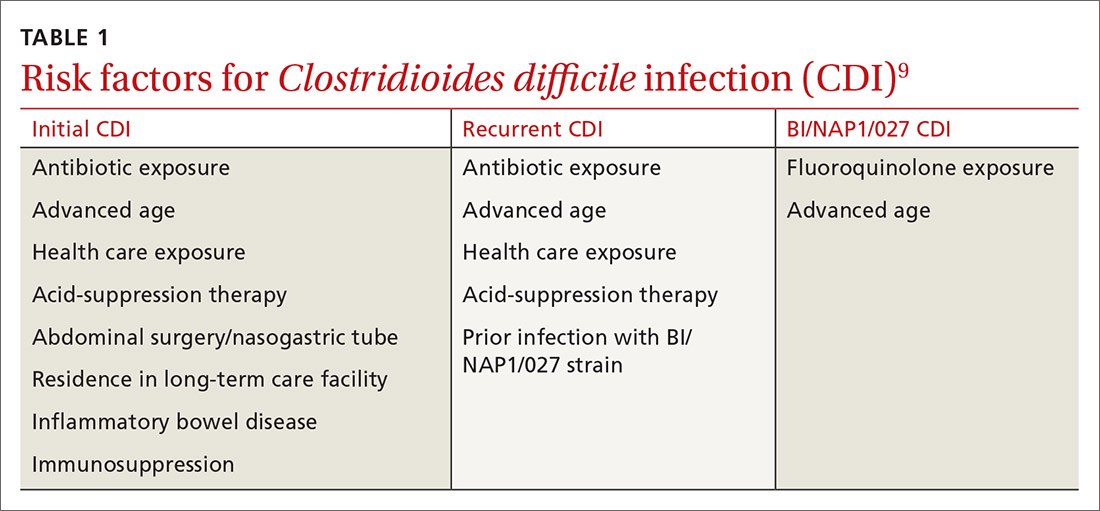
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
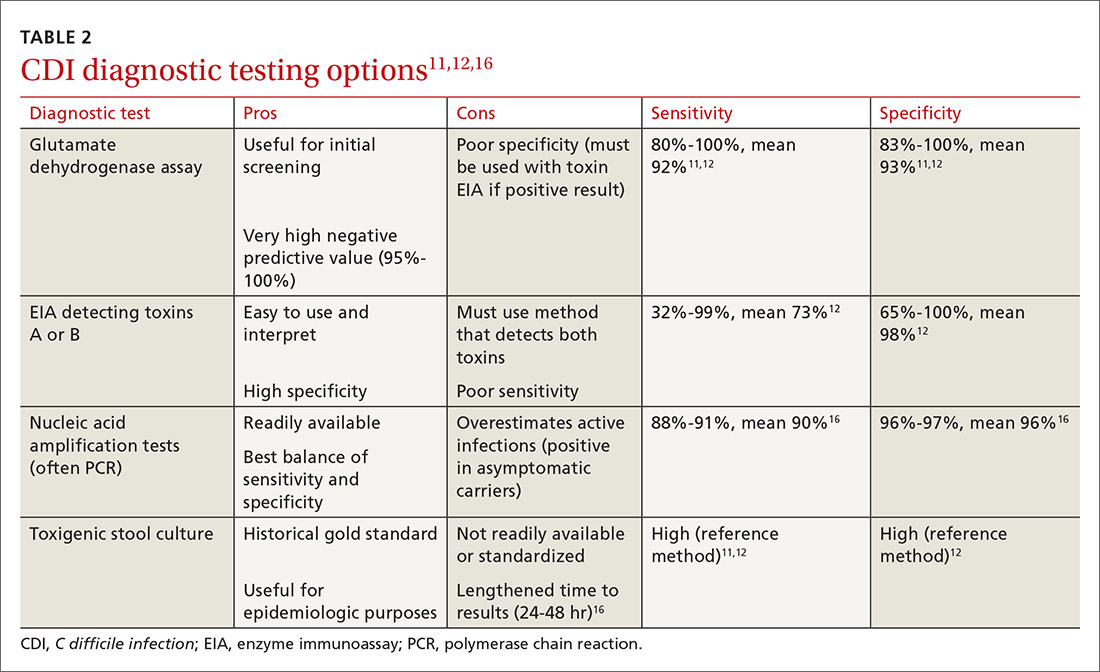
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; [email protected]
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11

Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.

The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; [email protected]
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11

Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.

The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; [email protected]
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
PRACTICE RECOMMENDATIONS
› Keep in mind that previous exposure to antibiotics is the most important risk factor for initial and recurrent Clostridioides difficile infection (CDI). Thus, appropriate antimicrobial stewardship is key to prevention. C
› Begin with vancomycin or fidaxomicin (over metronidazole) for first-line treatment of CDI in adults. A
› Consider fecal microbiota transplantation in high-risk patients with recurrent CDI for whom antimicrobial therapy has failed. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series