User login
Hypertension often goes undertreated in patients with a history of stroke
A new study of hypertension treatment trends found that “To our knowledge, the present study is the first to analyze and report national antihypertensive medication trends exclusively among individuals with a history of stroke in the United States,” wrote Daniel Santos, MD, and Mandip S. Dhamoon, MD, DrPH, of the Icahn School of Medicine at Mount Sinai, New York. Their study was published in JAMA Neurology.
To examine blood pressure control and treatment trends among stroke survivors, the researchers examined more than a decade of data from the National Health and Nutrition Examination Survey (NHANES). The cross-sectional survey is conducted in 2-year cycles; the authors analyzed the results from 2005 to 2016 and uncovered a total of 4,971,136 eligible individuals with a history of both stroke and hypertension.
The mean age of the study population was 67.1 (95% confidence interval, 66.1-68.1), and 2,790,518 (56.1%) were women. Their mean blood pressure was 134/68 mm Hg (95% CI, 133/67–136/69), and the average number of antihypertensive medications they were taking was 1.8 (95% CI, 1.7-1.9). Of the 4,971,136 analyzed individuals, 4,721,409 (95%) were aware of their hypertension diagnosis yet more than 10% of that group had not previously been prescribed an antihypertensive medication.
More than 37% (n = 1,846,470) of the participants had uncontrolled high blood pressure upon examination (95% CI, 33.5%-40.8%), and 15.3% (95% CI, 12.5%-18.0%) were not taking any medication for it at all. The most commonly used antihypertensive medications included ACE inhibitors or angiotensin receptor blockers (59.2%; 95% CI, 54.9%-63.4%), beta-blockers (43.8%; 95% CI, 40.3%-47.3%), diuretics (41.6%; 95% CI, 37.3%-45.9%) and calcium-channel blockers (31.5%; 95% CI, 28.2%-34.8%).* Roughly 57% of the sample was taking more than one antihypertensive medication (95% CI, 52.8%-60.6%) while 28% (95% CI, 24.6%-31.5%) were taking only one.
Continued surveillance is key
“All the studies that have ever been done show that hypertension is inadequately treated,” Louis Caplan, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston, said in an interview. “One of the reasons is that it can be hard to get some of the patients to seek treatment, particularly Black Americans. Also, a lot of the medicines to treat high blood pressure have side effects, so many patients don’t want to take the pills.
“Treating hypertension really requires continued surveillance,” he added. “It’s not one visit where the doctor gives you a pill. It’s taking the pill, following your blood pressure, and seeing if it works. If it doesn’t, then maybe you change the dose, get another pill, and are followed once again. That doesn’t happen as often as it should.”
In regard to next steps, Dr. Caplan urged that hypertension “be evaluated more seriously. Even as home blood pressure kits and monitoring become increasingly available, many doctors are still going by a casual blood pressure test in the office, which doesn’t tell you how serious the problem is. There needs to be more use of technology and more conditioning of patients to monitor their own blood pressure as a guide, and then we go from there.”
The authors acknowledged their study’s limitations, including the NHANES’s reliance on self-reporting a history of stroke and the inability to distinguish between subtypes of stroke. In addition, they noted that many antihypertensive medications have uses beyond treating hypertension, which introduces “another confounding factor to medication trends.”
The authors and Dr. Caplan reported no conflicts of interest.
SOURCE: Santos D et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2499.
Correction, 8/20/20: An earlier version of this article misstated the confidence interval for diuretics.
A new study of hypertension treatment trends found that “To our knowledge, the present study is the first to analyze and report national antihypertensive medication trends exclusively among individuals with a history of stroke in the United States,” wrote Daniel Santos, MD, and Mandip S. Dhamoon, MD, DrPH, of the Icahn School of Medicine at Mount Sinai, New York. Their study was published in JAMA Neurology.
To examine blood pressure control and treatment trends among stroke survivors, the researchers examined more than a decade of data from the National Health and Nutrition Examination Survey (NHANES). The cross-sectional survey is conducted in 2-year cycles; the authors analyzed the results from 2005 to 2016 and uncovered a total of 4,971,136 eligible individuals with a history of both stroke and hypertension.
The mean age of the study population was 67.1 (95% confidence interval, 66.1-68.1), and 2,790,518 (56.1%) were women. Their mean blood pressure was 134/68 mm Hg (95% CI, 133/67–136/69), and the average number of antihypertensive medications they were taking was 1.8 (95% CI, 1.7-1.9). Of the 4,971,136 analyzed individuals, 4,721,409 (95%) were aware of their hypertension diagnosis yet more than 10% of that group had not previously been prescribed an antihypertensive medication.
More than 37% (n = 1,846,470) of the participants had uncontrolled high blood pressure upon examination (95% CI, 33.5%-40.8%), and 15.3% (95% CI, 12.5%-18.0%) were not taking any medication for it at all. The most commonly used antihypertensive medications included ACE inhibitors or angiotensin receptor blockers (59.2%; 95% CI, 54.9%-63.4%), beta-blockers (43.8%; 95% CI, 40.3%-47.3%), diuretics (41.6%; 95% CI, 37.3%-45.9%) and calcium-channel blockers (31.5%; 95% CI, 28.2%-34.8%).* Roughly 57% of the sample was taking more than one antihypertensive medication (95% CI, 52.8%-60.6%) while 28% (95% CI, 24.6%-31.5%) were taking only one.
Continued surveillance is key
“All the studies that have ever been done show that hypertension is inadequately treated,” Louis Caplan, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston, said in an interview. “One of the reasons is that it can be hard to get some of the patients to seek treatment, particularly Black Americans. Also, a lot of the medicines to treat high blood pressure have side effects, so many patients don’t want to take the pills.
“Treating hypertension really requires continued surveillance,” he added. “It’s not one visit where the doctor gives you a pill. It’s taking the pill, following your blood pressure, and seeing if it works. If it doesn’t, then maybe you change the dose, get another pill, and are followed once again. That doesn’t happen as often as it should.”
In regard to next steps, Dr. Caplan urged that hypertension “be evaluated more seriously. Even as home blood pressure kits and monitoring become increasingly available, many doctors are still going by a casual blood pressure test in the office, which doesn’t tell you how serious the problem is. There needs to be more use of technology and more conditioning of patients to monitor their own blood pressure as a guide, and then we go from there.”
The authors acknowledged their study’s limitations, including the NHANES’s reliance on self-reporting a history of stroke and the inability to distinguish between subtypes of stroke. In addition, they noted that many antihypertensive medications have uses beyond treating hypertension, which introduces “another confounding factor to medication trends.”
The authors and Dr. Caplan reported no conflicts of interest.
SOURCE: Santos D et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2499.
Correction, 8/20/20: An earlier version of this article misstated the confidence interval for diuretics.
A new study of hypertension treatment trends found that “To our knowledge, the present study is the first to analyze and report national antihypertensive medication trends exclusively among individuals with a history of stroke in the United States,” wrote Daniel Santos, MD, and Mandip S. Dhamoon, MD, DrPH, of the Icahn School of Medicine at Mount Sinai, New York. Their study was published in JAMA Neurology.
To examine blood pressure control and treatment trends among stroke survivors, the researchers examined more than a decade of data from the National Health and Nutrition Examination Survey (NHANES). The cross-sectional survey is conducted in 2-year cycles; the authors analyzed the results from 2005 to 2016 and uncovered a total of 4,971,136 eligible individuals with a history of both stroke and hypertension.
The mean age of the study population was 67.1 (95% confidence interval, 66.1-68.1), and 2,790,518 (56.1%) were women. Their mean blood pressure was 134/68 mm Hg (95% CI, 133/67–136/69), and the average number of antihypertensive medications they were taking was 1.8 (95% CI, 1.7-1.9). Of the 4,971,136 analyzed individuals, 4,721,409 (95%) were aware of their hypertension diagnosis yet more than 10% of that group had not previously been prescribed an antihypertensive medication.
More than 37% (n = 1,846,470) of the participants had uncontrolled high blood pressure upon examination (95% CI, 33.5%-40.8%), and 15.3% (95% CI, 12.5%-18.0%) were not taking any medication for it at all. The most commonly used antihypertensive medications included ACE inhibitors or angiotensin receptor blockers (59.2%; 95% CI, 54.9%-63.4%), beta-blockers (43.8%; 95% CI, 40.3%-47.3%), diuretics (41.6%; 95% CI, 37.3%-45.9%) and calcium-channel blockers (31.5%; 95% CI, 28.2%-34.8%).* Roughly 57% of the sample was taking more than one antihypertensive medication (95% CI, 52.8%-60.6%) while 28% (95% CI, 24.6%-31.5%) were taking only one.
Continued surveillance is key
“All the studies that have ever been done show that hypertension is inadequately treated,” Louis Caplan, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston, said in an interview. “One of the reasons is that it can be hard to get some of the patients to seek treatment, particularly Black Americans. Also, a lot of the medicines to treat high blood pressure have side effects, so many patients don’t want to take the pills.
“Treating hypertension really requires continued surveillance,” he added. “It’s not one visit where the doctor gives you a pill. It’s taking the pill, following your blood pressure, and seeing if it works. If it doesn’t, then maybe you change the dose, get another pill, and are followed once again. That doesn’t happen as often as it should.”
In regard to next steps, Dr. Caplan urged that hypertension “be evaluated more seriously. Even as home blood pressure kits and monitoring become increasingly available, many doctors are still going by a casual blood pressure test in the office, which doesn’t tell you how serious the problem is. There needs to be more use of technology and more conditioning of patients to monitor their own blood pressure as a guide, and then we go from there.”
The authors acknowledged their study’s limitations, including the NHANES’s reliance on self-reporting a history of stroke and the inability to distinguish between subtypes of stroke. In addition, they noted that many antihypertensive medications have uses beyond treating hypertension, which introduces “another confounding factor to medication trends.”
The authors and Dr. Caplan reported no conflicts of interest.
SOURCE: Santos D et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2499.
Correction, 8/20/20: An earlier version of this article misstated the confidence interval for diuretics.
FROM JAMA NEUROLOGY
Most clinicians undertreat childhood lichen sclerosus
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
FROM SPD 2020
Antibiotic resistance: Personal responsibility in somewhat short supply
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.
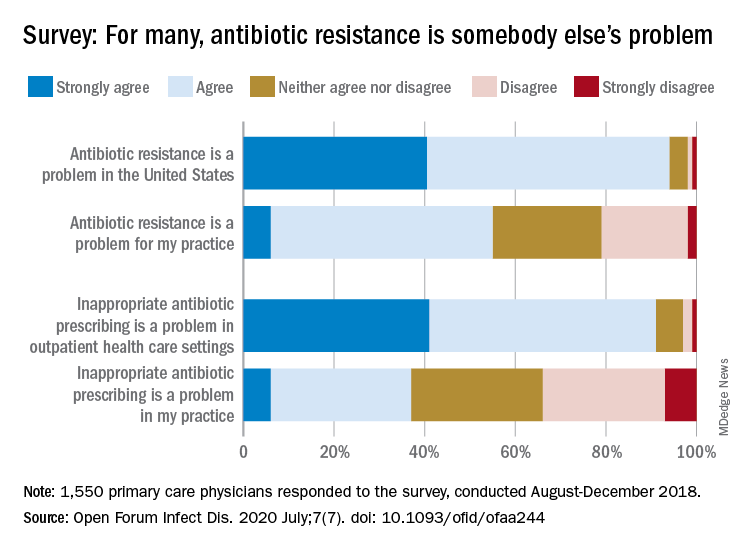
Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
FROM OPEN FORUM INFECTIOUS DISEASES
Pandemic hampers reopening of joint replacement gold mine
Dr. Ira Weintraub, a recently retired orthopedic surgeon who now works at a medical billing consultancy, saw a hip replacement bill for over $400,000 earlier this year.
“The patient stayed in the hospital 17 days, which is only 17 times normal. The bill got paid,” mused Weintraub, chief medical officer of Portland, Oregon-based WellRithms, which helps self-funded employers and workers’ compensation insurers make sense of large, complex medical bills and ensure they pay the fair amount.
Charges like that go a long way toward explaining why hospitals are eager to restore joint replacements to pre-COVID levels as quickly as possible – an eagerness tempered only by safety concerns amid a resurgence of the coronavirus in some regions of the country. Revenue losses at hospitals and outpatient surgery centers may have exceeded $5 billion from canceled knee and hip replacements alone during a roughly two-month hiatus on elective procedures earlier this year.
The cost of joint replacement surgery varies widely – though, on average, it is in the tens, not hundreds, of thousands of dollars. Still, given the high and rapidly growing volume, it’s easy to see why joint replacement operations have become a vital chunk of revenue at most U.S. hospitals.
The rate of knee and hip replacements more than doubled from 2000 to 2015, according to inpatient discharge data from the Agency for Healthcare Research and Quality. And that growth is likely to continue: Knee replacements are expected to triple between now and 2040, with hip replacements not far behind, according to projections published last year in the Journal of Rheumatology.
Joint procedures are usually not emergencies, and they were among the first to be scrubbed or delayed when hospitals froze elective surgeries in March – and again in July in some areas plagued by renewed COVID outbreaks.
“Without orthopedic volumes returning to something near their pre-pandemic levels, it will make it difficult for health systems to get back to anywhere near break-even from a bottom-line perspective,” said Stephen Thome, a principal in health care consulting at Grant Thornton, an advisory, audit and tax firm.
It’s impossible to know exactly how much knee and hip replacements are worth to hospitals, because no definitive data on total volume or price exists.
But using published estimates of volume, extrapolating average commercial payments from published Medicare rates based on a study, and making an educated guess of patient coinsurance, Thome helped KHN arrive at an annual market value for American hospitals and surgery centers of between $15.5 billion and $21.5 billion for knee replacements alone.
That suggests a revenue loss of $1.3 billion to $1.8 billion per month for the period the surgeries were shut down. These figures include ambulatory surgery centers not owned by hospitals, which also suspended most operations in late March, all of April and into May.
If you add hip replacements, which account for about half the volume of knees and are paid at similar rates, the total annual value rises to a range of $23 billion to $32 billion, with monthly revenue losses from $1.9 billion to $2.7 billion.
The American Hospital Association projects total revenue lost at U.S. hospitals will reach $323 billion by year’s end, not counting additional losses from surgeries canceled during the current coronavirus spike. That amount is partially offset by $69 billion in federal relief dollars hospitals have received so far, according to the association. The California Hospital Association puts the net revenue loss for hospitals in that state at about $10.5 billion, said spokesperson Jan Emerson-Shea.
Hospitals resumed joint replacement surgeries in early to mid-May, with the timing and ramp-up speed varying by region and hospital. Some hospitals restored volume quickly; others took a more cautious route and continue to lose revenue. Still others have had to shut down again.
At the NYU Langone Orthopedic Hospital in New York City, “people are starting to come in and you see the operating rooms full again,” said Dr. Claudette Lajam, chief orthopedic safety officer.
At St. Jude Medical Center in Fullerton, California, where the coronavirus is raging, inpatient joint replacements resumed in the second or third week of May – cautiously at first, but volume is “very close to pre-pandemic levels at this point,” said Dr. Kevin Khajavi, chairman of the hospital’s orthopedic surgery department. However, “we are constantly monitoring the situation to determine if we have to scale back once again,” he said.
In large swaths of Texas, elective surgeries were once again suspended in July because of the COVID-19 resurgence. The same is true at many hospitals in Florida, Alabama, South Carolina and Nevada.
The Mayo Clinic in Phoenix suspended nonemergency joint replacement surgeries in early July. It resumed outpatient replacement procedures the week of July 27, but still has not resumed nonemergency inpatient procedures, said Dr. Mark Spangehl, an orthopedic surgeon there. In terms of medical urgency, joint replacements are “at the bottom of the totem pole,” Spangehl said.
In terms of cash flow, however, joint replacements are decidedly not at the bottom of the totem pole. They have become a cash cow as the number of patients undergoing them has skyrocketed in recent decades.
The volume is being driven by an aging population, an epidemic of obesity and a significant rise in the number of younger people replacing joints worn out by years of sports and exercise.
It’s also being driven by the cash. Once only done in hospitals, the operations are now increasingly performed at ambulatory surgery centers – especially on younger, healthier patients who don’t require hospitalization.
The surgery centers are often physician-owned, but private equity groups such as Bain Capital and KKR & Co. have taken an interest in them, drawn by their high growth potential, robust financial returns and ability to offer competitive prices.
“[G]enerally the savings should be very good – but I do see a lot of outlier surgery centers where they are charging exorbitant amounts of money – $100,000 wouldn’t be too much,” said WellRithm’s Weintraub, who co-owned such a surgery center in Portland.
Fear of catching the coronavirus in a hospital is reinforcing the outpatient trend. Matthew Davis, a 58-year-old resident of Washington, was scheduled for a hip replacement on March 30 but got cold feet because of COVID-19, and canceled just before all elective surgeries were halted. When it came time to reschedule in June, he overcame his reservations in large part because the surgeon planned to perform the procedure at a free-standing surgery center.
“That was key to me – avoiding an overnight hospital stay to minimize my exposure,” Davis said. “These joint replacements are almost industrial-scale. They are cranking out joint replacements 9 to 5. I went in at 6:30 a.m. and I was walking out the door at 11:30.”
Acutely aware of the financial benefits, hospitals and surgery clinics have been marketing joint replacements for years, competing for coveted rankings and running ads that show healthy aging people, all smiles, engaged in vigorous activity.
However, a 2014 study concluded that one-third of knee replacements were not warranted, mainly because the symptoms of the patients were not severe enough to justify the procedures.
“The whole marketing of health care is so manipulative to the consuming public,” said Lisa McGiffert, a longtime consumer advocate and co-founder of the Patient Safety Action Network. “People might be encouraged to get a knee replacement, when in reality something less invasive could have improved their condition.”
McGiffert recounted a conversation with an orthopedic surgeon in Washington state who told her about a patient who requested a knee replacement, even though he had not tried any lower-impact treatments to fix the problem. “I asked the surgeon, ‘You didn’t do it, did you?’ And he said, ‘Of course I did. He would just have gone to somebody else.’ ”
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation.
Dr. Ira Weintraub, a recently retired orthopedic surgeon who now works at a medical billing consultancy, saw a hip replacement bill for over $400,000 earlier this year.
“The patient stayed in the hospital 17 days, which is only 17 times normal. The bill got paid,” mused Weintraub, chief medical officer of Portland, Oregon-based WellRithms, which helps self-funded employers and workers’ compensation insurers make sense of large, complex medical bills and ensure they pay the fair amount.
Charges like that go a long way toward explaining why hospitals are eager to restore joint replacements to pre-COVID levels as quickly as possible – an eagerness tempered only by safety concerns amid a resurgence of the coronavirus in some regions of the country. Revenue losses at hospitals and outpatient surgery centers may have exceeded $5 billion from canceled knee and hip replacements alone during a roughly two-month hiatus on elective procedures earlier this year.
The cost of joint replacement surgery varies widely – though, on average, it is in the tens, not hundreds, of thousands of dollars. Still, given the high and rapidly growing volume, it’s easy to see why joint replacement operations have become a vital chunk of revenue at most U.S. hospitals.
The rate of knee and hip replacements more than doubled from 2000 to 2015, according to inpatient discharge data from the Agency for Healthcare Research and Quality. And that growth is likely to continue: Knee replacements are expected to triple between now and 2040, with hip replacements not far behind, according to projections published last year in the Journal of Rheumatology.
Joint procedures are usually not emergencies, and they were among the first to be scrubbed or delayed when hospitals froze elective surgeries in March – and again in July in some areas plagued by renewed COVID outbreaks.
“Without orthopedic volumes returning to something near their pre-pandemic levels, it will make it difficult for health systems to get back to anywhere near break-even from a bottom-line perspective,” said Stephen Thome, a principal in health care consulting at Grant Thornton, an advisory, audit and tax firm.
It’s impossible to know exactly how much knee and hip replacements are worth to hospitals, because no definitive data on total volume or price exists.
But using published estimates of volume, extrapolating average commercial payments from published Medicare rates based on a study, and making an educated guess of patient coinsurance, Thome helped KHN arrive at an annual market value for American hospitals and surgery centers of between $15.5 billion and $21.5 billion for knee replacements alone.
That suggests a revenue loss of $1.3 billion to $1.8 billion per month for the period the surgeries were shut down. These figures include ambulatory surgery centers not owned by hospitals, which also suspended most operations in late March, all of April and into May.
If you add hip replacements, which account for about half the volume of knees and are paid at similar rates, the total annual value rises to a range of $23 billion to $32 billion, with monthly revenue losses from $1.9 billion to $2.7 billion.
The American Hospital Association projects total revenue lost at U.S. hospitals will reach $323 billion by year’s end, not counting additional losses from surgeries canceled during the current coronavirus spike. That amount is partially offset by $69 billion in federal relief dollars hospitals have received so far, according to the association. The California Hospital Association puts the net revenue loss for hospitals in that state at about $10.5 billion, said spokesperson Jan Emerson-Shea.
Hospitals resumed joint replacement surgeries in early to mid-May, with the timing and ramp-up speed varying by region and hospital. Some hospitals restored volume quickly; others took a more cautious route and continue to lose revenue. Still others have had to shut down again.
At the NYU Langone Orthopedic Hospital in New York City, “people are starting to come in and you see the operating rooms full again,” said Dr. Claudette Lajam, chief orthopedic safety officer.
At St. Jude Medical Center in Fullerton, California, where the coronavirus is raging, inpatient joint replacements resumed in the second or third week of May – cautiously at first, but volume is “very close to pre-pandemic levels at this point,” said Dr. Kevin Khajavi, chairman of the hospital’s orthopedic surgery department. However, “we are constantly monitoring the situation to determine if we have to scale back once again,” he said.
In large swaths of Texas, elective surgeries were once again suspended in July because of the COVID-19 resurgence. The same is true at many hospitals in Florida, Alabama, South Carolina and Nevada.
The Mayo Clinic in Phoenix suspended nonemergency joint replacement surgeries in early July. It resumed outpatient replacement procedures the week of July 27, but still has not resumed nonemergency inpatient procedures, said Dr. Mark Spangehl, an orthopedic surgeon there. In terms of medical urgency, joint replacements are “at the bottom of the totem pole,” Spangehl said.
In terms of cash flow, however, joint replacements are decidedly not at the bottom of the totem pole. They have become a cash cow as the number of patients undergoing them has skyrocketed in recent decades.
The volume is being driven by an aging population, an epidemic of obesity and a significant rise in the number of younger people replacing joints worn out by years of sports and exercise.
It’s also being driven by the cash. Once only done in hospitals, the operations are now increasingly performed at ambulatory surgery centers – especially on younger, healthier patients who don’t require hospitalization.
The surgery centers are often physician-owned, but private equity groups such as Bain Capital and KKR & Co. have taken an interest in them, drawn by their high growth potential, robust financial returns and ability to offer competitive prices.
“[G]enerally the savings should be very good – but I do see a lot of outlier surgery centers where they are charging exorbitant amounts of money – $100,000 wouldn’t be too much,” said WellRithm’s Weintraub, who co-owned such a surgery center in Portland.
Fear of catching the coronavirus in a hospital is reinforcing the outpatient trend. Matthew Davis, a 58-year-old resident of Washington, was scheduled for a hip replacement on March 30 but got cold feet because of COVID-19, and canceled just before all elective surgeries were halted. When it came time to reschedule in June, he overcame his reservations in large part because the surgeon planned to perform the procedure at a free-standing surgery center.
“That was key to me – avoiding an overnight hospital stay to minimize my exposure,” Davis said. “These joint replacements are almost industrial-scale. They are cranking out joint replacements 9 to 5. I went in at 6:30 a.m. and I was walking out the door at 11:30.”
Acutely aware of the financial benefits, hospitals and surgery clinics have been marketing joint replacements for years, competing for coveted rankings and running ads that show healthy aging people, all smiles, engaged in vigorous activity.
However, a 2014 study concluded that one-third of knee replacements were not warranted, mainly because the symptoms of the patients were not severe enough to justify the procedures.
“The whole marketing of health care is so manipulative to the consuming public,” said Lisa McGiffert, a longtime consumer advocate and co-founder of the Patient Safety Action Network. “People might be encouraged to get a knee replacement, when in reality something less invasive could have improved their condition.”
McGiffert recounted a conversation with an orthopedic surgeon in Washington state who told her about a patient who requested a knee replacement, even though he had not tried any lower-impact treatments to fix the problem. “I asked the surgeon, ‘You didn’t do it, did you?’ And he said, ‘Of course I did. He would just have gone to somebody else.’ ”
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation.
Dr. Ira Weintraub, a recently retired orthopedic surgeon who now works at a medical billing consultancy, saw a hip replacement bill for over $400,000 earlier this year.
“The patient stayed in the hospital 17 days, which is only 17 times normal. The bill got paid,” mused Weintraub, chief medical officer of Portland, Oregon-based WellRithms, which helps self-funded employers and workers’ compensation insurers make sense of large, complex medical bills and ensure they pay the fair amount.
Charges like that go a long way toward explaining why hospitals are eager to restore joint replacements to pre-COVID levels as quickly as possible – an eagerness tempered only by safety concerns amid a resurgence of the coronavirus in some regions of the country. Revenue losses at hospitals and outpatient surgery centers may have exceeded $5 billion from canceled knee and hip replacements alone during a roughly two-month hiatus on elective procedures earlier this year.
The cost of joint replacement surgery varies widely – though, on average, it is in the tens, not hundreds, of thousands of dollars. Still, given the high and rapidly growing volume, it’s easy to see why joint replacement operations have become a vital chunk of revenue at most U.S. hospitals.
The rate of knee and hip replacements more than doubled from 2000 to 2015, according to inpatient discharge data from the Agency for Healthcare Research and Quality. And that growth is likely to continue: Knee replacements are expected to triple between now and 2040, with hip replacements not far behind, according to projections published last year in the Journal of Rheumatology.
Joint procedures are usually not emergencies, and they were among the first to be scrubbed or delayed when hospitals froze elective surgeries in March – and again in July in some areas plagued by renewed COVID outbreaks.
“Without orthopedic volumes returning to something near their pre-pandemic levels, it will make it difficult for health systems to get back to anywhere near break-even from a bottom-line perspective,” said Stephen Thome, a principal in health care consulting at Grant Thornton, an advisory, audit and tax firm.
It’s impossible to know exactly how much knee and hip replacements are worth to hospitals, because no definitive data on total volume or price exists.
But using published estimates of volume, extrapolating average commercial payments from published Medicare rates based on a study, and making an educated guess of patient coinsurance, Thome helped KHN arrive at an annual market value for American hospitals and surgery centers of between $15.5 billion and $21.5 billion for knee replacements alone.
That suggests a revenue loss of $1.3 billion to $1.8 billion per month for the period the surgeries were shut down. These figures include ambulatory surgery centers not owned by hospitals, which also suspended most operations in late March, all of April and into May.
If you add hip replacements, which account for about half the volume of knees and are paid at similar rates, the total annual value rises to a range of $23 billion to $32 billion, with monthly revenue losses from $1.9 billion to $2.7 billion.
The American Hospital Association projects total revenue lost at U.S. hospitals will reach $323 billion by year’s end, not counting additional losses from surgeries canceled during the current coronavirus spike. That amount is partially offset by $69 billion in federal relief dollars hospitals have received so far, according to the association. The California Hospital Association puts the net revenue loss for hospitals in that state at about $10.5 billion, said spokesperson Jan Emerson-Shea.
Hospitals resumed joint replacement surgeries in early to mid-May, with the timing and ramp-up speed varying by region and hospital. Some hospitals restored volume quickly; others took a more cautious route and continue to lose revenue. Still others have had to shut down again.
At the NYU Langone Orthopedic Hospital in New York City, “people are starting to come in and you see the operating rooms full again,” said Dr. Claudette Lajam, chief orthopedic safety officer.
At St. Jude Medical Center in Fullerton, California, where the coronavirus is raging, inpatient joint replacements resumed in the second or third week of May – cautiously at first, but volume is “very close to pre-pandemic levels at this point,” said Dr. Kevin Khajavi, chairman of the hospital’s orthopedic surgery department. However, “we are constantly monitoring the situation to determine if we have to scale back once again,” he said.
In large swaths of Texas, elective surgeries were once again suspended in July because of the COVID-19 resurgence. The same is true at many hospitals in Florida, Alabama, South Carolina and Nevada.
The Mayo Clinic in Phoenix suspended nonemergency joint replacement surgeries in early July. It resumed outpatient replacement procedures the week of July 27, but still has not resumed nonemergency inpatient procedures, said Dr. Mark Spangehl, an orthopedic surgeon there. In terms of medical urgency, joint replacements are “at the bottom of the totem pole,” Spangehl said.
In terms of cash flow, however, joint replacements are decidedly not at the bottom of the totem pole. They have become a cash cow as the number of patients undergoing them has skyrocketed in recent decades.
The volume is being driven by an aging population, an epidemic of obesity and a significant rise in the number of younger people replacing joints worn out by years of sports and exercise.
It’s also being driven by the cash. Once only done in hospitals, the operations are now increasingly performed at ambulatory surgery centers – especially on younger, healthier patients who don’t require hospitalization.
The surgery centers are often physician-owned, but private equity groups such as Bain Capital and KKR & Co. have taken an interest in them, drawn by their high growth potential, robust financial returns and ability to offer competitive prices.
“[G]enerally the savings should be very good – but I do see a lot of outlier surgery centers where they are charging exorbitant amounts of money – $100,000 wouldn’t be too much,” said WellRithm’s Weintraub, who co-owned such a surgery center in Portland.
Fear of catching the coronavirus in a hospital is reinforcing the outpatient trend. Matthew Davis, a 58-year-old resident of Washington, was scheduled for a hip replacement on March 30 but got cold feet because of COVID-19, and canceled just before all elective surgeries were halted. When it came time to reschedule in June, he overcame his reservations in large part because the surgeon planned to perform the procedure at a free-standing surgery center.
“That was key to me – avoiding an overnight hospital stay to minimize my exposure,” Davis said. “These joint replacements are almost industrial-scale. They are cranking out joint replacements 9 to 5. I went in at 6:30 a.m. and I was walking out the door at 11:30.”
Acutely aware of the financial benefits, hospitals and surgery clinics have been marketing joint replacements for years, competing for coveted rankings and running ads that show healthy aging people, all smiles, engaged in vigorous activity.
However, a 2014 study concluded that one-third of knee replacements were not warranted, mainly because the symptoms of the patients were not severe enough to justify the procedures.
“The whole marketing of health care is so manipulative to the consuming public,” said Lisa McGiffert, a longtime consumer advocate and co-founder of the Patient Safety Action Network. “People might be encouraged to get a knee replacement, when in reality something less invasive could have improved their condition.”
McGiffert recounted a conversation with an orthopedic surgeon in Washington state who told her about a patient who requested a knee replacement, even though he had not tried any lower-impact treatments to fix the problem. “I asked the surgeon, ‘You didn’t do it, did you?’ And he said, ‘Of course I did. He would just have gone to somebody else.’ ”
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation.
FDA approves first liquid biopsy/NGS test for lung cancer
A new test, the first to combine liquid biopsy and next-generation sequencing (NGS), has been approved by the US Food and Drug Administration (FDA) for use in patients with metastatic non–small cell lung cancer (NSCLC) to identify tumors with specific mutation types of the epidermal growth factor receptor (EGFR) gene.
The Guardant360 CDx assay (Guardant Health) is the first to combine the two technologies into a diagnostic test to guide treatment decisions.
Liquid biopsy offers the advantage of obtaining genetic information on a tumor from a simple blood draw instead of a tissue biopsy, which requires fine-needle aspiration of the lung. “ It is less invasive and more easily repeatable in comparison to standard tissue biopsies ... and can be used in cases in which standard tissue biopsies are not feasible, for instance, due to the location of the tumor,” the FDA commented.
NGS offers the advantage of simultaneously detecting mutations in 55 tumor genes, as opposed to conducting a separate test for each gene.
However, although the assay can provide information on multiple solid tumor biomarkers, the approval is specific only to identifying EGFR mutations in patients who will benefit from treatment with osimertinib (Tagrisso, AstraZeneca).
The approval does not validate the test for use in detecting other biomarkers, the FDA noted.
As previously reported, the assay has a comprehensive NGS panel that identifies seven guideline-recommended predictive biomarkers (EGFR, ALK, ROS1, BRAF, RET, MET, ERBB2) — known as the G7 biomarkers — and one prognostic marker (KRAS).
But the FDA noted that “genomic findings for other biomarkers evaluated are not validated for choosing a particular corresponding treatment with this approval.
“If the specific NSCLC mutations associated with today’s approval are not detected in the blood, then a tumor biopsy should be performed to determine if the NSCLC mutations are present,” the agency emphasized.
Nevertheless, the FDA announcement highlights the potential of the test to identify these other biomarkers.
“Approval of a companion diagnostic that uses a liquid biopsy and leverages next-generation sequencing marks a new era for mutation testing,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, commented in a statement.
“In addition to benefiting from less invasive testing, patients are provided with a simultaneous mapping of multiple biomarkers of genomic alterations, rather than one biomarker at a time, which can translate to decreased wait times for starting treatment and provide insight into possible resistance mechanisms,” he noted.
The manufacturer also highlighted this potential. “We are confident that our FDA approval will help accelerate wider adoption of guideline-recommended genomic profiling, increase the number of advanced cancer patients who receive potentially life-changing treatments, and pave the way for new companion diagnostic developments for the Guardant360 CDx,” Helmy Eltoukhy, PhD, CEO of Guardant Health, said in a statement.
NILE Study
The FDA did not cite any specific trial of the assay in its announcement of the approval, but Medscape Medical News has previously reported results from the NILE study presented at the 2019 Annual Meeting of the American Association for Cancer Research (AACR).
The NILE trial was conducted in 282 patients with untreated nonsquamous NSCLC who underwent standard-of-care tissue genotyping and had a pretreatment blood sample for cell-free DNA (cfDNA) analysis.
Results showed that a G7 biomarker was identified in a significantly higher proportion of liquid biopsies compared with tissue genotyping (27.3% vs 21.3%; P < .0001).
The lower frequency of G7 biomarkers in tissue genotyping was due to insufficient tissue for sequential sequencing, the authors reported at that time.
Liquid biopsy improved G7 detection frequency by 48%, from 60 to 89 patients, which included samples that were negative by tissue testing (7), not tested (16), or lacked sufficient sample for a tissue-based test (6).
Of 193 patients without a G7 biomarker by tissue or cfDNA, 24 patients (12.4%) had an activating KRAS mutation identified in the tissue alone, and with cfDNA, KRAS-positivity increased from 24 to 92 patients.
The Guardant360 CDx assay has been granted a breakthrough device designation, whereby the FDA provides intensive interaction and guidance on efficient device development to the company.
This article first appeared on Medscape.com.
A new test, the first to combine liquid biopsy and next-generation sequencing (NGS), has been approved by the US Food and Drug Administration (FDA) for use in patients with metastatic non–small cell lung cancer (NSCLC) to identify tumors with specific mutation types of the epidermal growth factor receptor (EGFR) gene.
The Guardant360 CDx assay (Guardant Health) is the first to combine the two technologies into a diagnostic test to guide treatment decisions.
Liquid biopsy offers the advantage of obtaining genetic information on a tumor from a simple blood draw instead of a tissue biopsy, which requires fine-needle aspiration of the lung. “ It is less invasive and more easily repeatable in comparison to standard tissue biopsies ... and can be used in cases in which standard tissue biopsies are not feasible, for instance, due to the location of the tumor,” the FDA commented.
NGS offers the advantage of simultaneously detecting mutations in 55 tumor genes, as opposed to conducting a separate test for each gene.
However, although the assay can provide information on multiple solid tumor biomarkers, the approval is specific only to identifying EGFR mutations in patients who will benefit from treatment with osimertinib (Tagrisso, AstraZeneca).
The approval does not validate the test for use in detecting other biomarkers, the FDA noted.
As previously reported, the assay has a comprehensive NGS panel that identifies seven guideline-recommended predictive biomarkers (EGFR, ALK, ROS1, BRAF, RET, MET, ERBB2) — known as the G7 biomarkers — and one prognostic marker (KRAS).
But the FDA noted that “genomic findings for other biomarkers evaluated are not validated for choosing a particular corresponding treatment with this approval.
“If the specific NSCLC mutations associated with today’s approval are not detected in the blood, then a tumor biopsy should be performed to determine if the NSCLC mutations are present,” the agency emphasized.
Nevertheless, the FDA announcement highlights the potential of the test to identify these other biomarkers.
“Approval of a companion diagnostic that uses a liquid biopsy and leverages next-generation sequencing marks a new era for mutation testing,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, commented in a statement.
“In addition to benefiting from less invasive testing, patients are provided with a simultaneous mapping of multiple biomarkers of genomic alterations, rather than one biomarker at a time, which can translate to decreased wait times for starting treatment and provide insight into possible resistance mechanisms,” he noted.
The manufacturer also highlighted this potential. “We are confident that our FDA approval will help accelerate wider adoption of guideline-recommended genomic profiling, increase the number of advanced cancer patients who receive potentially life-changing treatments, and pave the way for new companion diagnostic developments for the Guardant360 CDx,” Helmy Eltoukhy, PhD, CEO of Guardant Health, said in a statement.
NILE Study
The FDA did not cite any specific trial of the assay in its announcement of the approval, but Medscape Medical News has previously reported results from the NILE study presented at the 2019 Annual Meeting of the American Association for Cancer Research (AACR).
The NILE trial was conducted in 282 patients with untreated nonsquamous NSCLC who underwent standard-of-care tissue genotyping and had a pretreatment blood sample for cell-free DNA (cfDNA) analysis.
Results showed that a G7 biomarker was identified in a significantly higher proportion of liquid biopsies compared with tissue genotyping (27.3% vs 21.3%; P < .0001).
The lower frequency of G7 biomarkers in tissue genotyping was due to insufficient tissue for sequential sequencing, the authors reported at that time.
Liquid biopsy improved G7 detection frequency by 48%, from 60 to 89 patients, which included samples that were negative by tissue testing (7), not tested (16), or lacked sufficient sample for a tissue-based test (6).
Of 193 patients without a G7 biomarker by tissue or cfDNA, 24 patients (12.4%) had an activating KRAS mutation identified in the tissue alone, and with cfDNA, KRAS-positivity increased from 24 to 92 patients.
The Guardant360 CDx assay has been granted a breakthrough device designation, whereby the FDA provides intensive interaction and guidance on efficient device development to the company.
This article first appeared on Medscape.com.
A new test, the first to combine liquid biopsy and next-generation sequencing (NGS), has been approved by the US Food and Drug Administration (FDA) for use in patients with metastatic non–small cell lung cancer (NSCLC) to identify tumors with specific mutation types of the epidermal growth factor receptor (EGFR) gene.
The Guardant360 CDx assay (Guardant Health) is the first to combine the two technologies into a diagnostic test to guide treatment decisions.
Liquid biopsy offers the advantage of obtaining genetic information on a tumor from a simple blood draw instead of a tissue biopsy, which requires fine-needle aspiration of the lung. “ It is less invasive and more easily repeatable in comparison to standard tissue biopsies ... and can be used in cases in which standard tissue biopsies are not feasible, for instance, due to the location of the tumor,” the FDA commented.
NGS offers the advantage of simultaneously detecting mutations in 55 tumor genes, as opposed to conducting a separate test for each gene.
However, although the assay can provide information on multiple solid tumor biomarkers, the approval is specific only to identifying EGFR mutations in patients who will benefit from treatment with osimertinib (Tagrisso, AstraZeneca).
The approval does not validate the test for use in detecting other biomarkers, the FDA noted.
As previously reported, the assay has a comprehensive NGS panel that identifies seven guideline-recommended predictive biomarkers (EGFR, ALK, ROS1, BRAF, RET, MET, ERBB2) — known as the G7 biomarkers — and one prognostic marker (KRAS).
But the FDA noted that “genomic findings for other biomarkers evaluated are not validated for choosing a particular corresponding treatment with this approval.
“If the specific NSCLC mutations associated with today’s approval are not detected in the blood, then a tumor biopsy should be performed to determine if the NSCLC mutations are present,” the agency emphasized.
Nevertheless, the FDA announcement highlights the potential of the test to identify these other biomarkers.
“Approval of a companion diagnostic that uses a liquid biopsy and leverages next-generation sequencing marks a new era for mutation testing,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, commented in a statement.
“In addition to benefiting from less invasive testing, patients are provided with a simultaneous mapping of multiple biomarkers of genomic alterations, rather than one biomarker at a time, which can translate to decreased wait times for starting treatment and provide insight into possible resistance mechanisms,” he noted.
The manufacturer also highlighted this potential. “We are confident that our FDA approval will help accelerate wider adoption of guideline-recommended genomic profiling, increase the number of advanced cancer patients who receive potentially life-changing treatments, and pave the way for new companion diagnostic developments for the Guardant360 CDx,” Helmy Eltoukhy, PhD, CEO of Guardant Health, said in a statement.
NILE Study
The FDA did not cite any specific trial of the assay in its announcement of the approval, but Medscape Medical News has previously reported results from the NILE study presented at the 2019 Annual Meeting of the American Association for Cancer Research (AACR).
The NILE trial was conducted in 282 patients with untreated nonsquamous NSCLC who underwent standard-of-care tissue genotyping and had a pretreatment blood sample for cell-free DNA (cfDNA) analysis.
Results showed that a G7 biomarker was identified in a significantly higher proportion of liquid biopsies compared with tissue genotyping (27.3% vs 21.3%; P < .0001).
The lower frequency of G7 biomarkers in tissue genotyping was due to insufficient tissue for sequential sequencing, the authors reported at that time.
Liquid biopsy improved G7 detection frequency by 48%, from 60 to 89 patients, which included samples that were negative by tissue testing (7), not tested (16), or lacked sufficient sample for a tissue-based test (6).
Of 193 patients without a G7 biomarker by tissue or cfDNA, 24 patients (12.4%) had an activating KRAS mutation identified in the tissue alone, and with cfDNA, KRAS-positivity increased from 24 to 92 patients.
The Guardant360 CDx assay has been granted a breakthrough device designation, whereby the FDA provides intensive interaction and guidance on efficient device development to the company.
This article first appeared on Medscape.com.
A dedicated mobility technician improves inpatient mobility
Background: Studies have shown improved hospital outcomes in patients who ambulate regularly. Many assisted mobility protocols aimed at ambulating patients multiple times daily are nurse centered. However, implementation is difficult because of the large number of nursing duties and difficulty finding time away from other competing responsibilities.
Study design: Single-blind randomized controlled trial.
Setting: Single-center 1,440-bed tertiary care hospital.
Synopsis: This study randomized 102 moderately impaired adult inpatients aged 60 years and older with Activity Measures for Post-Acute Care mobility scores of 16-20 to either dedicated regular ambulation sessions with mobility technicians or usual care with hospital nurse–driven protocol. Patients who achieved greater than 400 steps were more likely to discharge to home rather than post–acute care (71% vs. 46%; P = .01). Assisted ambulation did not decrease length of stay or affect the discharge disposition, but it did increase the total daily number of steps taken by patients (1,182 vs. 726; P = .02, per-protocol analysis) and the patients’ mobility scores (18.90 vs. 18.27, P = .04).
Bottom line: A dedicated mobility technician to provide assisted ambulation for older inpatients can improve patient mobility.
Citation: Hamilton AC et al. Increasing mobility via in-hospital ambulation protocol delivered by mobility technicians: A pilot randomized controlled trial. J Hosp Med. 2019;14:272-7.
Dr. Nelson is a hospitalist at Ochsner Health System, New Orleans.
Background: Studies have shown improved hospital outcomes in patients who ambulate regularly. Many assisted mobility protocols aimed at ambulating patients multiple times daily are nurse centered. However, implementation is difficult because of the large number of nursing duties and difficulty finding time away from other competing responsibilities.
Study design: Single-blind randomized controlled trial.
Setting: Single-center 1,440-bed tertiary care hospital.
Synopsis: This study randomized 102 moderately impaired adult inpatients aged 60 years and older with Activity Measures for Post-Acute Care mobility scores of 16-20 to either dedicated regular ambulation sessions with mobility technicians or usual care with hospital nurse–driven protocol. Patients who achieved greater than 400 steps were more likely to discharge to home rather than post–acute care (71% vs. 46%; P = .01). Assisted ambulation did not decrease length of stay or affect the discharge disposition, but it did increase the total daily number of steps taken by patients (1,182 vs. 726; P = .02, per-protocol analysis) and the patients’ mobility scores (18.90 vs. 18.27, P = .04).
Bottom line: A dedicated mobility technician to provide assisted ambulation for older inpatients can improve patient mobility.
Citation: Hamilton AC et al. Increasing mobility via in-hospital ambulation protocol delivered by mobility technicians: A pilot randomized controlled trial. J Hosp Med. 2019;14:272-7.
Dr. Nelson is a hospitalist at Ochsner Health System, New Orleans.
Background: Studies have shown improved hospital outcomes in patients who ambulate regularly. Many assisted mobility protocols aimed at ambulating patients multiple times daily are nurse centered. However, implementation is difficult because of the large number of nursing duties and difficulty finding time away from other competing responsibilities.
Study design: Single-blind randomized controlled trial.
Setting: Single-center 1,440-bed tertiary care hospital.
Synopsis: This study randomized 102 moderately impaired adult inpatients aged 60 years and older with Activity Measures for Post-Acute Care mobility scores of 16-20 to either dedicated regular ambulation sessions with mobility technicians or usual care with hospital nurse–driven protocol. Patients who achieved greater than 400 steps were more likely to discharge to home rather than post–acute care (71% vs. 46%; P = .01). Assisted ambulation did not decrease length of stay or affect the discharge disposition, but it did increase the total daily number of steps taken by patients (1,182 vs. 726; P = .02, per-protocol analysis) and the patients’ mobility scores (18.90 vs. 18.27, P = .04).
Bottom line: A dedicated mobility technician to provide assisted ambulation for older inpatients can improve patient mobility.
Citation: Hamilton AC et al. Increasing mobility via in-hospital ambulation protocol delivered by mobility technicians: A pilot randomized controlled trial. J Hosp Med. 2019;14:272-7.
Dr. Nelson is a hospitalist at Ochsner Health System, New Orleans.
Beyond PASI 100: striving for molecular clearance
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
FROM AAD 20
Experimental nonstimulant effective, fast-acting for ADHD
The experimental nonstimulant medication viloxazine extended-release, known as SPN-812, reduced symptoms of attention-deficit/hyperactivity disorder (ADHD) as soon as 1 week after dosing and was well tolerated in a randomized, placebo-controlled phase 3 study that included more than 400 children.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interviews.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The findings were published online July 25 in Clinical Therapeutics.
Novel modulating agent
Viloxazine extended-release is a novel multimodal serotonergic and noradrenergic modulating agent with activity at serotonin receptors and the norepinephrine transporter.
About two-thirds were boys.
All participants had an ADHD-Rating Scale–5 (ADHD-RS-5) score of at least 28 and a Clinical Global Impression–Severity score of at least 4. None had taken ADHD medication for at least 1 week prior to randomization.
The intent-to-treat population included 460 children. Of these, 155 were randomly assigned to receive placebo, 147 to receive viloxazine 100 mg, and 158 to receive viloxazine 200 mg.
The primary efficacy endpoint was change from baseline in ADHD-RS-5 total score at week 6. Score changes for both the 100-mg (P = .0004) and the 200-mg (P < .0001) viloxazine groups met statistical significance compared with the placebo group.
Change from baseline in both the ADHD-RS-5 inattention and hyperactivity/impulsivity subscale scores was also significantly reduced in the 100-mg (P = .0006 and .0026, respectively) and 200-mg (P < .0001 and P < .0001, respectively) treatment groups compared with the placebo group.
Improvements occurred after 1 week of treatment and were maintained throughout the 6-week trial, “indicating an early and sustained effect,” the investigators wrote.
FDA target action date
The Clinical Global Impression–Improvement (CGI-I) score at 6 weeks was significantly improved in those receiving 100 mg (P = .0020) and 200 mg (P < .0001) of the active treatment compared with placebo.
The CGI-I responder rate, the percentage of children with a CGI-I score of 1 (very much improved) or 2 (much improved), was significantly higher at 6 weeks with viloxazine 100 mg and 200 mg vs. placebo (45% and 51% vs. 30%, respectively; P = .0065 and P = .0002).
These standard investigator-rated assessments were supported by two parent self-rated assessments: the Conners 3–Parent Short Form and the Weiss Functional Impairment Rating Scale–Parent Form.
Parents noted improvement not only in their children’s ADHD symptoms but also in ADHD-associated learning problems, executive functioning, defiance/aggression, peer relations, and functioning in different settings.
At both doses, once-daily viloxazine was generally well tolerated, with a low rate of discontinuation because of adverse events (<5%). Most adverse events were characterized as mild or moderate in severity and included somnolence (8.9%), decreased appetite (6.0%), and headache (5.4%).
On the basis of results of this study and others, the Food and Drug Administration accepted the company’s new drug application for viloxazine extended-release for ADHD in children and adolescents. The application has a target action date of Nov. 8, 2020.
Potential advantages
Commenting on the study in an interview, Dean Elbe, PharmD, clinical pharmacy specialist, child and adolescent mental health, BC Children’s Hospital, Vancouver, B.C., said that use of viloxazine to treat ADHD is “interesting.”
Dr. Elbe, who was not involved with the current research, noted that “it is actually an old drug that has been around since the mid-1970s in Europe as an antidepressant. It was removed from the market due to poor sales, not safety issues.”
Overall, on the basis of this study, viloxazine has potential to offer “modest improvements” over atomoxetine (Strattera), and the dosing may be “more straightforward and somewhat less challenging than with atomoxetine, with no taper up and no adjustment for poor 2D6 metabolizers,” Dr. Elbe noted.
“The onset of action appears somewhat quicker than we typically see with atomoxetine, so that is also helpful for parent and clinician acceptance and partially overcomes a perceived barrier with atomoxetine,” he said.
Dr. Elbe said he wonders, however, whether viloxazine will show “real-world clinical utility for both hyperactive-impulsive as well as inattentive symptoms. Although the study shows efficacy in both symptom clusters, so did the atomoxetine RCTs, and this has not been the clinical impression for atomoxetine.”
The study was funded by Supernus Pharmaceuticals. Dr. Cutler is a consultant for Supernus, as well as for Adlon Therapeutics, Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, NLS Pharma, Otsuka, Purdue, Shire, Sunovion, Takeda, and Tris Pharma. He has received speaker/promotional honoraria from Adlon Therapeutics, Arbor Pharmaceuticals, Lundbeck, Neos Therapeutics, Otsuka, Shire, Sunovion, Takeda, and Tris Pharma and has received research grants from Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, Otsuka, Purdue, Shire, Sunovion, Supernus Pharmaceuticals, Takeda, and Tris Pharma. A complete list of disclosures for the other authors is available in the original article. Dr. Elbe has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The experimental nonstimulant medication viloxazine extended-release, known as SPN-812, reduced symptoms of attention-deficit/hyperactivity disorder (ADHD) as soon as 1 week after dosing and was well tolerated in a randomized, placebo-controlled phase 3 study that included more than 400 children.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interviews.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The findings were published online July 25 in Clinical Therapeutics.
Novel modulating agent
Viloxazine extended-release is a novel multimodal serotonergic and noradrenergic modulating agent with activity at serotonin receptors and the norepinephrine transporter.
About two-thirds were boys.
All participants had an ADHD-Rating Scale–5 (ADHD-RS-5) score of at least 28 and a Clinical Global Impression–Severity score of at least 4. None had taken ADHD medication for at least 1 week prior to randomization.
The intent-to-treat population included 460 children. Of these, 155 were randomly assigned to receive placebo, 147 to receive viloxazine 100 mg, and 158 to receive viloxazine 200 mg.
The primary efficacy endpoint was change from baseline in ADHD-RS-5 total score at week 6. Score changes for both the 100-mg (P = .0004) and the 200-mg (P < .0001) viloxazine groups met statistical significance compared with the placebo group.
Change from baseline in both the ADHD-RS-5 inattention and hyperactivity/impulsivity subscale scores was also significantly reduced in the 100-mg (P = .0006 and .0026, respectively) and 200-mg (P < .0001 and P < .0001, respectively) treatment groups compared with the placebo group.
Improvements occurred after 1 week of treatment and were maintained throughout the 6-week trial, “indicating an early and sustained effect,” the investigators wrote.
FDA target action date
The Clinical Global Impression–Improvement (CGI-I) score at 6 weeks was significantly improved in those receiving 100 mg (P = .0020) and 200 mg (P < .0001) of the active treatment compared with placebo.
The CGI-I responder rate, the percentage of children with a CGI-I score of 1 (very much improved) or 2 (much improved), was significantly higher at 6 weeks with viloxazine 100 mg and 200 mg vs. placebo (45% and 51% vs. 30%, respectively; P = .0065 and P = .0002).
These standard investigator-rated assessments were supported by two parent self-rated assessments: the Conners 3–Parent Short Form and the Weiss Functional Impairment Rating Scale–Parent Form.
Parents noted improvement not only in their children’s ADHD symptoms but also in ADHD-associated learning problems, executive functioning, defiance/aggression, peer relations, and functioning in different settings.
At both doses, once-daily viloxazine was generally well tolerated, with a low rate of discontinuation because of adverse events (<5%). Most adverse events were characterized as mild or moderate in severity and included somnolence (8.9%), decreased appetite (6.0%), and headache (5.4%).
On the basis of results of this study and others, the Food and Drug Administration accepted the company’s new drug application for viloxazine extended-release for ADHD in children and adolescents. The application has a target action date of Nov. 8, 2020.
Potential advantages
Commenting on the study in an interview, Dean Elbe, PharmD, clinical pharmacy specialist, child and adolescent mental health, BC Children’s Hospital, Vancouver, B.C., said that use of viloxazine to treat ADHD is “interesting.”
Dr. Elbe, who was not involved with the current research, noted that “it is actually an old drug that has been around since the mid-1970s in Europe as an antidepressant. It was removed from the market due to poor sales, not safety issues.”
Overall, on the basis of this study, viloxazine has potential to offer “modest improvements” over atomoxetine (Strattera), and the dosing may be “more straightforward and somewhat less challenging than with atomoxetine, with no taper up and no adjustment for poor 2D6 metabolizers,” Dr. Elbe noted.
“The onset of action appears somewhat quicker than we typically see with atomoxetine, so that is also helpful for parent and clinician acceptance and partially overcomes a perceived barrier with atomoxetine,” he said.
Dr. Elbe said he wonders, however, whether viloxazine will show “real-world clinical utility for both hyperactive-impulsive as well as inattentive symptoms. Although the study shows efficacy in both symptom clusters, so did the atomoxetine RCTs, and this has not been the clinical impression for atomoxetine.”
The study was funded by Supernus Pharmaceuticals. Dr. Cutler is a consultant for Supernus, as well as for Adlon Therapeutics, Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, NLS Pharma, Otsuka, Purdue, Shire, Sunovion, Takeda, and Tris Pharma. He has received speaker/promotional honoraria from Adlon Therapeutics, Arbor Pharmaceuticals, Lundbeck, Neos Therapeutics, Otsuka, Shire, Sunovion, Takeda, and Tris Pharma and has received research grants from Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, Otsuka, Purdue, Shire, Sunovion, Supernus Pharmaceuticals, Takeda, and Tris Pharma. A complete list of disclosures for the other authors is available in the original article. Dr. Elbe has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The experimental nonstimulant medication viloxazine extended-release, known as SPN-812, reduced symptoms of attention-deficit/hyperactivity disorder (ADHD) as soon as 1 week after dosing and was well tolerated in a randomized, placebo-controlled phase 3 study that included more than 400 children.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interviews.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The findings were published online July 25 in Clinical Therapeutics.
Novel modulating agent
Viloxazine extended-release is a novel multimodal serotonergic and noradrenergic modulating agent with activity at serotonin receptors and the norepinephrine transporter.
About two-thirds were boys.
All participants had an ADHD-Rating Scale–5 (ADHD-RS-5) score of at least 28 and a Clinical Global Impression–Severity score of at least 4. None had taken ADHD medication for at least 1 week prior to randomization.
The intent-to-treat population included 460 children. Of these, 155 were randomly assigned to receive placebo, 147 to receive viloxazine 100 mg, and 158 to receive viloxazine 200 mg.
The primary efficacy endpoint was change from baseline in ADHD-RS-5 total score at week 6. Score changes for both the 100-mg (P = .0004) and the 200-mg (P < .0001) viloxazine groups met statistical significance compared with the placebo group.
Change from baseline in both the ADHD-RS-5 inattention and hyperactivity/impulsivity subscale scores was also significantly reduced in the 100-mg (P = .0006 and .0026, respectively) and 200-mg (P < .0001 and P < .0001, respectively) treatment groups compared with the placebo group.
Improvements occurred after 1 week of treatment and were maintained throughout the 6-week trial, “indicating an early and sustained effect,” the investigators wrote.
FDA target action date
The Clinical Global Impression–Improvement (CGI-I) score at 6 weeks was significantly improved in those receiving 100 mg (P = .0020) and 200 mg (P < .0001) of the active treatment compared with placebo.
The CGI-I responder rate, the percentage of children with a CGI-I score of 1 (very much improved) or 2 (much improved), was significantly higher at 6 weeks with viloxazine 100 mg and 200 mg vs. placebo (45% and 51% vs. 30%, respectively; P = .0065 and P = .0002).
These standard investigator-rated assessments were supported by two parent self-rated assessments: the Conners 3–Parent Short Form and the Weiss Functional Impairment Rating Scale–Parent Form.
Parents noted improvement not only in their children’s ADHD symptoms but also in ADHD-associated learning problems, executive functioning, defiance/aggression, peer relations, and functioning in different settings.
At both doses, once-daily viloxazine was generally well tolerated, with a low rate of discontinuation because of adverse events (<5%). Most adverse events were characterized as mild or moderate in severity and included somnolence (8.9%), decreased appetite (6.0%), and headache (5.4%).
On the basis of results of this study and others, the Food and Drug Administration accepted the company’s new drug application for viloxazine extended-release for ADHD in children and adolescents. The application has a target action date of Nov. 8, 2020.
Potential advantages
Commenting on the study in an interview, Dean Elbe, PharmD, clinical pharmacy specialist, child and adolescent mental health, BC Children’s Hospital, Vancouver, B.C., said that use of viloxazine to treat ADHD is “interesting.”
Dr. Elbe, who was not involved with the current research, noted that “it is actually an old drug that has been around since the mid-1970s in Europe as an antidepressant. It was removed from the market due to poor sales, not safety issues.”
Overall, on the basis of this study, viloxazine has potential to offer “modest improvements” over atomoxetine (Strattera), and the dosing may be “more straightforward and somewhat less challenging than with atomoxetine, with no taper up and no adjustment for poor 2D6 metabolizers,” Dr. Elbe noted.
“The onset of action appears somewhat quicker than we typically see with atomoxetine, so that is also helpful for parent and clinician acceptance and partially overcomes a perceived barrier with atomoxetine,” he said.
Dr. Elbe said he wonders, however, whether viloxazine will show “real-world clinical utility for both hyperactive-impulsive as well as inattentive symptoms. Although the study shows efficacy in both symptom clusters, so did the atomoxetine RCTs, and this has not been the clinical impression for atomoxetine.”
The study was funded by Supernus Pharmaceuticals. Dr. Cutler is a consultant for Supernus, as well as for Adlon Therapeutics, Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, NLS Pharma, Otsuka, Purdue, Shire, Sunovion, Takeda, and Tris Pharma. He has received speaker/promotional honoraria from Adlon Therapeutics, Arbor Pharmaceuticals, Lundbeck, Neos Therapeutics, Otsuka, Shire, Sunovion, Takeda, and Tris Pharma and has received research grants from Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, Otsuka, Purdue, Shire, Sunovion, Supernus Pharmaceuticals, Takeda, and Tris Pharma. A complete list of disclosures for the other authors is available in the original article. Dr. Elbe has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Cachexia affects more than half of lupus patients
Cachexia developed in 56% of adults with systemic lupus erythematosus over a 5-year period, and 18% did not recover their weight, based on data from more than 2,000 patients.
Although weight loss is common in patients with systemic lupus erythematosus (SLE), cachexia, a disorder of involuntary weight loss, is largely undescribed in SLE patients, wrote George Stojan, MD, of Johns Hopkins University, Baltimore, and colleagues. Cachexia has been described in a range of disorders, including heart failure, renal disease, and rheumatoid arthritis, they said. “Cachexia has been shown to lead to progressive functional impairment, treatment-related complications, poor quality of life, and increased mortality,” they added.
In a study published in Arthritis Care & Research, the investigators reviewed data from the Hopkins Lupus Cohort, consisting of all SLE patients seen at a single center who are followed at least quarterly.
The study population included 2,452 SLE patients older than 18 years who had their weight assessed at each clinic visit. The average follow-up period was 7.75 years, and the average number of weight measurements per patient was nearly 24.
Cachexia was defined as a 5% stable weight loss in 6 months without starvation relative to the average weight in all prior cohort visits; and/or weight loss of more than 2% without starvation relative to the average weight in all prior cohort visits in addition to a body mass index less than 20 kg/m2.
Overall, the risk for cachexia within 5 years of entering the study was significantly higher in patients with a BMI less than 20, current steroid use, vasculitis, lupus nephritis, serositis, hematologic lupus, positive anti-double strand DNA (anti-dsDNA), anti-Smith (anti-Sm), and antiribonucleoprotein (anti-RNP), the researchers noted. After adjustment for prednisone use, cachexia remained significantly associated with lupus nephritis, vasculitis, serositis, and hematologic lupus.
Future organ damage including cataracts, retinal change or optic atrophy, cognitive impairment, cerebrovascular accidents, cranial or peripheral neuropathy, pulmonary hypertension, pleural fibrosis, angina or coronary bypass, bowel infarction or resection, osteoporosis, avascular necrosis, and premature gonadal failure were significantly more likely among patients with intermittent cachexia, compared with those with continuous or no cachexia. Patients with continuous cachexia were significantly more likely to experience an estimated glomerular filtration rate less than 50 mL/min/1.73 m2, proteinuria greater than 3.5 g/day, and end-stage renal disease.
The patients who never developed cachexia were significantly less likely to develop malignancies, diabetes, valvular disease, or cardiomyopathy than were those who did have cachexia, the researchers said.
The mechanisms of action for cachexia in SLE remain unclear, but studies in cancer patients may provide some guidance, the researchers noted. “Tumors secrete a range of procachexia factors thought to be unique to cancer-related cachexia, and colloquially termed the ‘tumor secretome,’ ” they said. “Every single proinflammatory cytokine mentioned as part of the tumor secretome has a role in lupus pathogenesis,” suggesting a possible common pathway to cachexia across different diseases, they said.
The study findings were limited by several factors, mainly the use of BMI to measure weight “since BMI is a rather poor indicator of percent of body fat,” the researchers noted. “Ideally, cachexia would be defined as sarcopenia based on body composition evaluation with a dual x-ray absorptiometry,” they wrote.
The study was supported by the National Institutes of Health and the NIH Roadmap for Medical Research. The researchers had no financial conflicts to disclose.
SOURCE: Stojan G et al. Arthritis Care Res. 2020 Aug 2. doi: 10.1002/acr.24395.
Cachexia developed in 56% of adults with systemic lupus erythematosus over a 5-year period, and 18% did not recover their weight, based on data from more than 2,000 patients.
Although weight loss is common in patients with systemic lupus erythematosus (SLE), cachexia, a disorder of involuntary weight loss, is largely undescribed in SLE patients, wrote George Stojan, MD, of Johns Hopkins University, Baltimore, and colleagues. Cachexia has been described in a range of disorders, including heart failure, renal disease, and rheumatoid arthritis, they said. “Cachexia has been shown to lead to progressive functional impairment, treatment-related complications, poor quality of life, and increased mortality,” they added.
In a study published in Arthritis Care & Research, the investigators reviewed data from the Hopkins Lupus Cohort, consisting of all SLE patients seen at a single center who are followed at least quarterly.
The study population included 2,452 SLE patients older than 18 years who had their weight assessed at each clinic visit. The average follow-up period was 7.75 years, and the average number of weight measurements per patient was nearly 24.
Cachexia was defined as a 5% stable weight loss in 6 months without starvation relative to the average weight in all prior cohort visits; and/or weight loss of more than 2% without starvation relative to the average weight in all prior cohort visits in addition to a body mass index less than 20 kg/m2.
Overall, the risk for cachexia within 5 years of entering the study was significantly higher in patients with a BMI less than 20, current steroid use, vasculitis, lupus nephritis, serositis, hematologic lupus, positive anti-double strand DNA (anti-dsDNA), anti-Smith (anti-Sm), and antiribonucleoprotein (anti-RNP), the researchers noted. After adjustment for prednisone use, cachexia remained significantly associated with lupus nephritis, vasculitis, serositis, and hematologic lupus.
Future organ damage including cataracts, retinal change or optic atrophy, cognitive impairment, cerebrovascular accidents, cranial or peripheral neuropathy, pulmonary hypertension, pleural fibrosis, angina or coronary bypass, bowel infarction or resection, osteoporosis, avascular necrosis, and premature gonadal failure were significantly more likely among patients with intermittent cachexia, compared with those with continuous or no cachexia. Patients with continuous cachexia were significantly more likely to experience an estimated glomerular filtration rate less than 50 mL/min/1.73 m2, proteinuria greater than 3.5 g/day, and end-stage renal disease.
The patients who never developed cachexia were significantly less likely to develop malignancies, diabetes, valvular disease, or cardiomyopathy than were those who did have cachexia, the researchers said.
The mechanisms of action for cachexia in SLE remain unclear, but studies in cancer patients may provide some guidance, the researchers noted. “Tumors secrete a range of procachexia factors thought to be unique to cancer-related cachexia, and colloquially termed the ‘tumor secretome,’ ” they said. “Every single proinflammatory cytokine mentioned as part of the tumor secretome has a role in lupus pathogenesis,” suggesting a possible common pathway to cachexia across different diseases, they said.
The study findings were limited by several factors, mainly the use of BMI to measure weight “since BMI is a rather poor indicator of percent of body fat,” the researchers noted. “Ideally, cachexia would be defined as sarcopenia based on body composition evaluation with a dual x-ray absorptiometry,” they wrote.
The study was supported by the National Institutes of Health and the NIH Roadmap for Medical Research. The researchers had no financial conflicts to disclose.
SOURCE: Stojan G et al. Arthritis Care Res. 2020 Aug 2. doi: 10.1002/acr.24395.
Cachexia developed in 56% of adults with systemic lupus erythematosus over a 5-year period, and 18% did not recover their weight, based on data from more than 2,000 patients.
Although weight loss is common in patients with systemic lupus erythematosus (SLE), cachexia, a disorder of involuntary weight loss, is largely undescribed in SLE patients, wrote George Stojan, MD, of Johns Hopkins University, Baltimore, and colleagues. Cachexia has been described in a range of disorders, including heart failure, renal disease, and rheumatoid arthritis, they said. “Cachexia has been shown to lead to progressive functional impairment, treatment-related complications, poor quality of life, and increased mortality,” they added.
In a study published in Arthritis Care & Research, the investigators reviewed data from the Hopkins Lupus Cohort, consisting of all SLE patients seen at a single center who are followed at least quarterly.
The study population included 2,452 SLE patients older than 18 years who had their weight assessed at each clinic visit. The average follow-up period was 7.75 years, and the average number of weight measurements per patient was nearly 24.
Cachexia was defined as a 5% stable weight loss in 6 months without starvation relative to the average weight in all prior cohort visits; and/or weight loss of more than 2% without starvation relative to the average weight in all prior cohort visits in addition to a body mass index less than 20 kg/m2.
Overall, the risk for cachexia within 5 years of entering the study was significantly higher in patients with a BMI less than 20, current steroid use, vasculitis, lupus nephritis, serositis, hematologic lupus, positive anti-double strand DNA (anti-dsDNA), anti-Smith (anti-Sm), and antiribonucleoprotein (anti-RNP), the researchers noted. After adjustment for prednisone use, cachexia remained significantly associated with lupus nephritis, vasculitis, serositis, and hematologic lupus.
Future organ damage including cataracts, retinal change or optic atrophy, cognitive impairment, cerebrovascular accidents, cranial or peripheral neuropathy, pulmonary hypertension, pleural fibrosis, angina or coronary bypass, bowel infarction or resection, osteoporosis, avascular necrosis, and premature gonadal failure were significantly more likely among patients with intermittent cachexia, compared with those with continuous or no cachexia. Patients with continuous cachexia were significantly more likely to experience an estimated glomerular filtration rate less than 50 mL/min/1.73 m2, proteinuria greater than 3.5 g/day, and end-stage renal disease.
The patients who never developed cachexia were significantly less likely to develop malignancies, diabetes, valvular disease, or cardiomyopathy than were those who did have cachexia, the researchers said.
The mechanisms of action for cachexia in SLE remain unclear, but studies in cancer patients may provide some guidance, the researchers noted. “Tumors secrete a range of procachexia factors thought to be unique to cancer-related cachexia, and colloquially termed the ‘tumor secretome,’ ” they said. “Every single proinflammatory cytokine mentioned as part of the tumor secretome has a role in lupus pathogenesis,” suggesting a possible common pathway to cachexia across different diseases, they said.
The study findings were limited by several factors, mainly the use of BMI to measure weight “since BMI is a rather poor indicator of percent of body fat,” the researchers noted. “Ideally, cachexia would be defined as sarcopenia based on body composition evaluation with a dual x-ray absorptiometry,” they wrote.
The study was supported by the National Institutes of Health and the NIH Roadmap for Medical Research. The researchers had no financial conflicts to disclose.
SOURCE: Stojan G et al. Arthritis Care Res. 2020 Aug 2. doi: 10.1002/acr.24395.
FROM ARTHRITIS CARE & RESEARCH
Taming a terrible illness
Darth Vader is, to me, one of the most intimidating villains in movie history. I was 11 when Star Wars came out. I even cleaned my room so my mother would take me to see it.
When Darth Vader first walked on screen, it was striking. A tall, imposing figure in black, with harsh mechanical respirations. There was no question of who the bad guy was. As the movie progressed his darkness became more frightening until, in the first lightsaber battle any of us had seen, he cut down the benevolent Obi-Wan Kenobi.
Last year my family went to Disneyland. While browsing the park’s stores we saw numerous Darth Vader items ... with him now available as a teddy bear, and on T-shirts riding carousels and the Dumbo ride.
From terrifying villain to cutesy toy in 43 years.* Quite the fall from glory.
Diseases are often (and hopefully) like that. Syphilis, once the most common, feared, and incurable neurologic disease is now, for most, just a nuisance. The butt of jokes and sexual innuendos, rendered harmless by Alexander Fleming’s discoveries.
Bit by bit we see other diseases tamed. Multiple sclerosis, though still serious, becomes better controlled every year as new agents come out. The cure for Parkinson’s disease remains elusive, but agents to control the symptoms and improve quality of life are available. Even HIV, the most feared disease of the 80s and 90s, has been beaten back from a terrible death sentence to one where patients lead normal lives with antiviral therapy.
Today we face a new enemy, the COVID-19 pandemic. So far we have no definite treatments, nor shortage of ideas. Many companies are racing to develop a vaccine, and will likely, at some point, find one, but what and when are still in the future. too.
Alzheimer’s disease, for all practical purposes, remains untreatable and rightfully feared. Perhaps the only ones more terrifying are those we’ve reduced to just three letters: ALS (amyotrophic lateral sclerosis) and GBM (glioblastoma multiforme). Both have terrible courses and, in spite of years of research, nothing even close to an effective treatment.
I hope that changes, and soon, for all those affected by these (and many other) terrible disorders.
Like the Darth Vader teddy bear, I’ll be happy to see them become shells of their former selves, with the dread they bring now reduced to the lesser trepidation seen when facing a serious, but treatable, illness.
*Correction, 8/11/20: An earlier version of this column misstated the number of years since Star Wars debuted.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Darth Vader is, to me, one of the most intimidating villains in movie history. I was 11 when Star Wars came out. I even cleaned my room so my mother would take me to see it.
When Darth Vader first walked on screen, it was striking. A tall, imposing figure in black, with harsh mechanical respirations. There was no question of who the bad guy was. As the movie progressed his darkness became more frightening until, in the first lightsaber battle any of us had seen, he cut down the benevolent Obi-Wan Kenobi.
Last year my family went to Disneyland. While browsing the park’s stores we saw numerous Darth Vader items ... with him now available as a teddy bear, and on T-shirts riding carousels and the Dumbo ride.
From terrifying villain to cutesy toy in 43 years.* Quite the fall from glory.
Diseases are often (and hopefully) like that. Syphilis, once the most common, feared, and incurable neurologic disease is now, for most, just a nuisance. The butt of jokes and sexual innuendos, rendered harmless by Alexander Fleming’s discoveries.
Bit by bit we see other diseases tamed. Multiple sclerosis, though still serious, becomes better controlled every year as new agents come out. The cure for Parkinson’s disease remains elusive, but agents to control the symptoms and improve quality of life are available. Even HIV, the most feared disease of the 80s and 90s, has been beaten back from a terrible death sentence to one where patients lead normal lives with antiviral therapy.
Today we face a new enemy, the COVID-19 pandemic. So far we have no definite treatments, nor shortage of ideas. Many companies are racing to develop a vaccine, and will likely, at some point, find one, but what and when are still in the future. too.
Alzheimer’s disease, for all practical purposes, remains untreatable and rightfully feared. Perhaps the only ones more terrifying are those we’ve reduced to just three letters: ALS (amyotrophic lateral sclerosis) and GBM (glioblastoma multiforme). Both have terrible courses and, in spite of years of research, nothing even close to an effective treatment.
I hope that changes, and soon, for all those affected by these (and many other) terrible disorders.
Like the Darth Vader teddy bear, I’ll be happy to see them become shells of their former selves, with the dread they bring now reduced to the lesser trepidation seen when facing a serious, but treatable, illness.
*Correction, 8/11/20: An earlier version of this column misstated the number of years since Star Wars debuted.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Darth Vader is, to me, one of the most intimidating villains in movie history. I was 11 when Star Wars came out. I even cleaned my room so my mother would take me to see it.
When Darth Vader first walked on screen, it was striking. A tall, imposing figure in black, with harsh mechanical respirations. There was no question of who the bad guy was. As the movie progressed his darkness became more frightening until, in the first lightsaber battle any of us had seen, he cut down the benevolent Obi-Wan Kenobi.
Last year my family went to Disneyland. While browsing the park’s stores we saw numerous Darth Vader items ... with him now available as a teddy bear, and on T-shirts riding carousels and the Dumbo ride.
From terrifying villain to cutesy toy in 43 years.* Quite the fall from glory.
Diseases are often (and hopefully) like that. Syphilis, once the most common, feared, and incurable neurologic disease is now, for most, just a nuisance. The butt of jokes and sexual innuendos, rendered harmless by Alexander Fleming’s discoveries.
Bit by bit we see other diseases tamed. Multiple sclerosis, though still serious, becomes better controlled every year as new agents come out. The cure for Parkinson’s disease remains elusive, but agents to control the symptoms and improve quality of life are available. Even HIV, the most feared disease of the 80s and 90s, has been beaten back from a terrible death sentence to one where patients lead normal lives with antiviral therapy.
Today we face a new enemy, the COVID-19 pandemic. So far we have no definite treatments, nor shortage of ideas. Many companies are racing to develop a vaccine, and will likely, at some point, find one, but what and when are still in the future. too.
Alzheimer’s disease, for all practical purposes, remains untreatable and rightfully feared. Perhaps the only ones more terrifying are those we’ve reduced to just three letters: ALS (amyotrophic lateral sclerosis) and GBM (glioblastoma multiforme). Both have terrible courses and, in spite of years of research, nothing even close to an effective treatment.
I hope that changes, and soon, for all those affected by these (and many other) terrible disorders.
Like the Darth Vader teddy bear, I’ll be happy to see them become shells of their former selves, with the dread they bring now reduced to the lesser trepidation seen when facing a serious, but treatable, illness.
*Correction, 8/11/20: An earlier version of this column misstated the number of years since Star Wars debuted.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.











