User login
Extensive Purpura and Necrosis of the Leg
The Diagnosis: Disseminated Mucormycosis
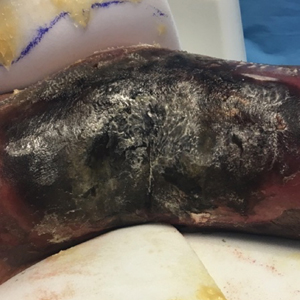
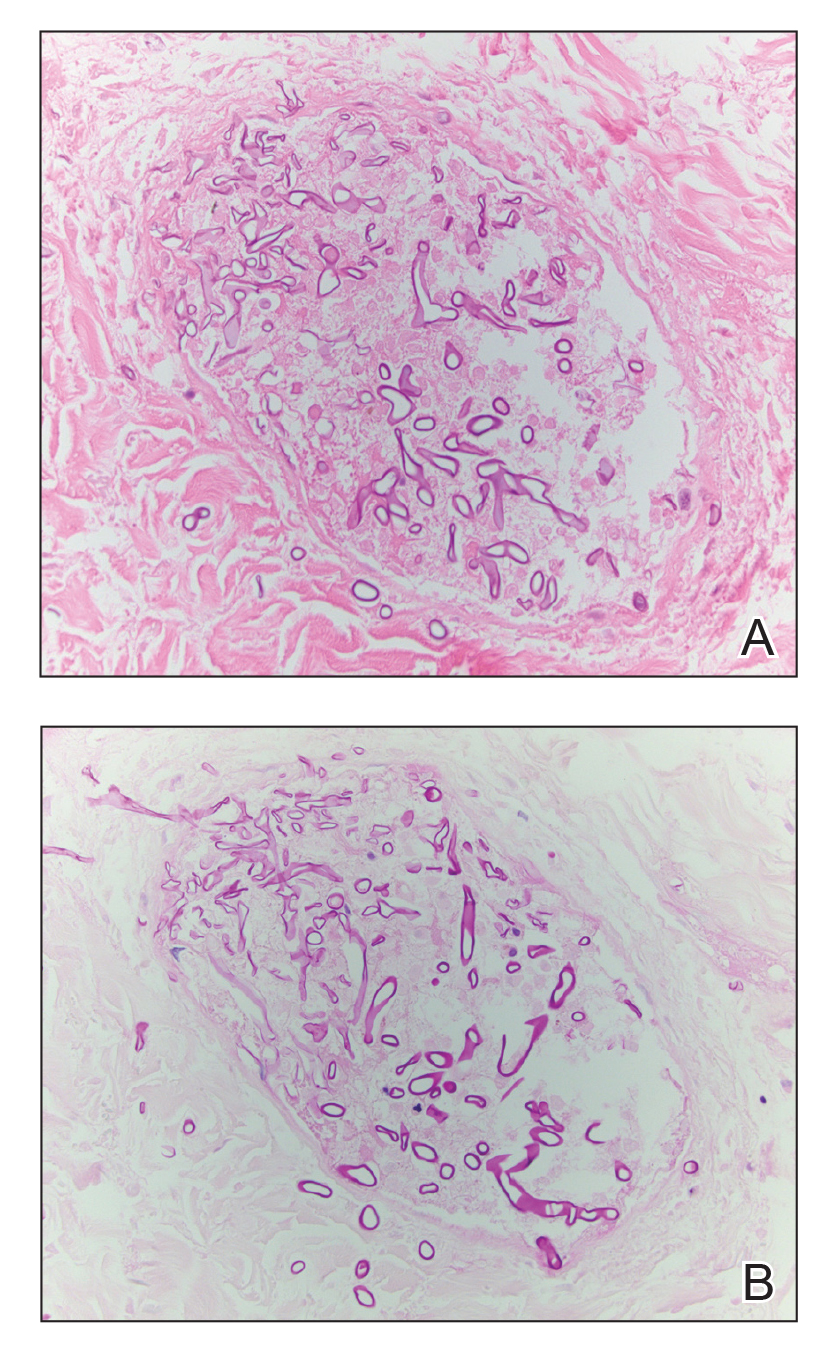
Histopathologic examination of a 6-mm punch biopsy of the edge of the lesion revealed numerous intravascular, broad, nonseptate hyphae in the deep vessels and perivascular dermis that stained bright red with periodic acid-Schiff (Figure). Acid-fast bacilli and Gram stains were negative. Tissue culture grew Rhizopus species. Given the patient's overall poor prognosis, her family decided to pursue hospice care following this diagnosis.
Mucormycosis (formerly zygomycosis) refers to infections from a variety of genera of fungi, most commonly Mucor and Rhizopus, that cause infections primarily in immunocompromised individuals.1 Mucormycosis infections are characterized by tissue necrosis that results from invasion of the vasculature and subsequent thrombosis. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration.2 Diagnosis is based on clinical suspicion, requiring additional testing with skin biopsy and tissue cultures for confirmation.
Cutaneous infection is the third most common presentation of mucormycosis, following rhinocerebral and pulmonary involvement.1 Although rhinocerebral and pulmonary infections normally are caused by inhalation of spores, cutaneous mucormycosis typically is caused by local inoculation, often following skin trauma.2 The skin is the most common location of iatrogenic mucormycosis, often from skin injury related to surgery, catheters, and adhesive tape.3 Most patients with cutaneous mucormycosis have underlying conditions such as hematologic malignancies, diabetes mellitus, or immunosuppression.1 However, outbreaks have occurred in immunocompetent patients following natural disasters.4 Cutaneous mucormycosis disseminates in 13% to 20% of cases in which mortality rates typically exceed 90%.1
Treatment consists of prompt surgical debridement and antifungal agents such as amphotericin B, posaconazole, and isavuconazonium sulfate.1 Our patient had multiple risk factors for infection, including hematopoietic stem cell transplantation, prolonged neutropenia, and treatment with eculizumab, a monoclonal antibody against C5 that blocks the terminal complement cascade. Eculizumab has been associated with increased risk for meningococcemia,5 but the association with mucormycosis is rare. We highlight the importance of recognizing and promptly diagnosing cutaneous mucormycosis given the difficulty of treating this disease and its poor prognosis.
Disseminated aspergillosis demonstrates septate rather than nonseptate hyphae on biopsy. Disseminated intravascular coagulation and purpura fulminans may be associated with thrombocytopenia but demonstrate thrombotic microangiopathy on biopsy. Pyoderma gangrenosum demonstrates neutrophilic infiltrate on biopsy.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S44-S54.
- Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-2225.
- McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734-737.
The Diagnosis: Disseminated Mucormycosis
Histopathologic examination of a 6-mm punch biopsy of the edge of the lesion revealed numerous intravascular, broad, nonseptate hyphae in the deep vessels and perivascular dermis that stained bright red with periodic acid-Schiff (Figure). Acid-fast bacilli and Gram stains were negative. Tissue culture grew Rhizopus species. Given the patient's overall poor prognosis, her family decided to pursue hospice care following this diagnosis.

Mucormycosis (formerly zygomycosis) refers to infections from a variety of genera of fungi, most commonly Mucor and Rhizopus, that cause infections primarily in immunocompromised individuals.1 Mucormycosis infections are characterized by tissue necrosis that results from invasion of the vasculature and subsequent thrombosis. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration.2 Diagnosis is based on clinical suspicion, requiring additional testing with skin biopsy and tissue cultures for confirmation.
Cutaneous infection is the third most common presentation of mucormycosis, following rhinocerebral and pulmonary involvement.1 Although rhinocerebral and pulmonary infections normally are caused by inhalation of spores, cutaneous mucormycosis typically is caused by local inoculation, often following skin trauma.2 The skin is the most common location of iatrogenic mucormycosis, often from skin injury related to surgery, catheters, and adhesive tape.3 Most patients with cutaneous mucormycosis have underlying conditions such as hematologic malignancies, diabetes mellitus, or immunosuppression.1 However, outbreaks have occurred in immunocompetent patients following natural disasters.4 Cutaneous mucormycosis disseminates in 13% to 20% of cases in which mortality rates typically exceed 90%.1
Treatment consists of prompt surgical debridement and antifungal agents such as amphotericin B, posaconazole, and isavuconazonium sulfate.1 Our patient had multiple risk factors for infection, including hematopoietic stem cell transplantation, prolonged neutropenia, and treatment with eculizumab, a monoclonal antibody against C5 that blocks the terminal complement cascade. Eculizumab has been associated with increased risk for meningococcemia,5 but the association with mucormycosis is rare. We highlight the importance of recognizing and promptly diagnosing cutaneous mucormycosis given the difficulty of treating this disease and its poor prognosis.
Disseminated aspergillosis demonstrates septate rather than nonseptate hyphae on biopsy. Disseminated intravascular coagulation and purpura fulminans may be associated with thrombocytopenia but demonstrate thrombotic microangiopathy on biopsy. Pyoderma gangrenosum demonstrates neutrophilic infiltrate on biopsy.
The Diagnosis: Disseminated Mucormycosis
Histopathologic examination of a 6-mm punch biopsy of the edge of the lesion revealed numerous intravascular, broad, nonseptate hyphae in the deep vessels and perivascular dermis that stained bright red with periodic acid-Schiff (Figure). Acid-fast bacilli and Gram stains were negative. Tissue culture grew Rhizopus species. Given the patient's overall poor prognosis, her family decided to pursue hospice care following this diagnosis.

Mucormycosis (formerly zygomycosis) refers to infections from a variety of genera of fungi, most commonly Mucor and Rhizopus, that cause infections primarily in immunocompromised individuals.1 Mucormycosis infections are characterized by tissue necrosis that results from invasion of the vasculature and subsequent thrombosis. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration.2 Diagnosis is based on clinical suspicion, requiring additional testing with skin biopsy and tissue cultures for confirmation.
Cutaneous infection is the third most common presentation of mucormycosis, following rhinocerebral and pulmonary involvement.1 Although rhinocerebral and pulmonary infections normally are caused by inhalation of spores, cutaneous mucormycosis typically is caused by local inoculation, often following skin trauma.2 The skin is the most common location of iatrogenic mucormycosis, often from skin injury related to surgery, catheters, and adhesive tape.3 Most patients with cutaneous mucormycosis have underlying conditions such as hematologic malignancies, diabetes mellitus, or immunosuppression.1 However, outbreaks have occurred in immunocompetent patients following natural disasters.4 Cutaneous mucormycosis disseminates in 13% to 20% of cases in which mortality rates typically exceed 90%.1
Treatment consists of prompt surgical debridement and antifungal agents such as amphotericin B, posaconazole, and isavuconazonium sulfate.1 Our patient had multiple risk factors for infection, including hematopoietic stem cell transplantation, prolonged neutropenia, and treatment with eculizumab, a monoclonal antibody against C5 that blocks the terminal complement cascade. Eculizumab has been associated with increased risk for meningococcemia,5 but the association with mucormycosis is rare. We highlight the importance of recognizing and promptly diagnosing cutaneous mucormycosis given the difficulty of treating this disease and its poor prognosis.
Disseminated aspergillosis demonstrates septate rather than nonseptate hyphae on biopsy. Disseminated intravascular coagulation and purpura fulminans may be associated with thrombocytopenia but demonstrate thrombotic microangiopathy on biopsy. Pyoderma gangrenosum demonstrates neutrophilic infiltrate on biopsy.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S44-S54.
- Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-2225.
- McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734-737.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S44-S54.
- Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-2225.
- McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734-737.
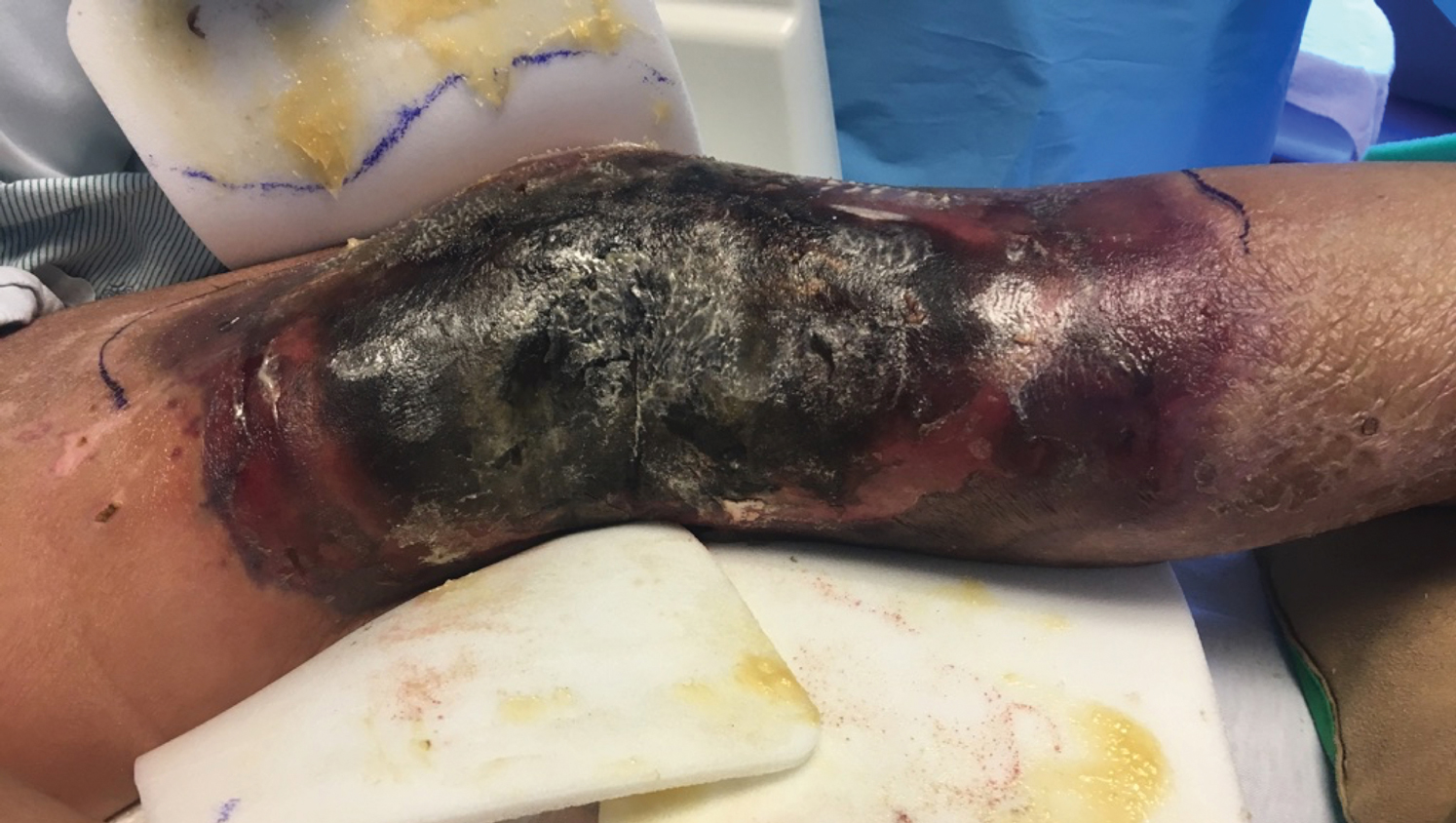
A 57-year-old woman presented with expanding purpura on the left leg of 2 weeks’ duration following a recent hematopoietic stem cell transplant for refractory diffuse large B-cell lymphoma. Prior to dermatologic consultation, the patient had been hospitalized for 2 months following the transplant due to Clostridium difficile colitis, Enterococcus faecium bacteremia, cardiac arrest, delayed engraftment with pancytopenia, and atypical hemolytic uremic syndrome with acute renal failure requiring hemodialysis and treatment with eculizumab. Her care team in the hospital initially noticed a small purpuric lesion on the posterior aspect of the left knee. The patient subsequently developed persistent fevers and expansion of the lesion, which prompted consultation of the dermatology service. Physical examination revealed a 22×10-cm, rectangular, indurated, purpuric plaque with central dusky, violaceous to black necrosis with superficial skin sloughing and peripheral dusky erythema extending from the inner thigh to the lower leg. The left distal leg felt cool, and both dorsalis pedis and posterior tibial pulses were absent. Laboratory test results revealed neutropenia and thrombocytopenia (white blood cell count, 0.2×103 /mm3 [reference range, 5–10×103 /mm3 ]; hematocrit, 23.2% [reference range, 41%–50%]; platelet count, 105×103 /µL [reference range, 150–350×103 /µL]). A punch biopsy was performed.
Chloroquine linked to serious psychiatric side effects
Chloroquine may be associated with serious psychiatric side effects, even in patients with no family or personal history of psychiatric disorders, a new review suggests.
In a letter to the editor published online July 28 in The Journal of Clinical Psychiatry, the authors summarize data from several studies published as far back as 1993 and as recently as May 2020.
“In addition to previously reported side effects, chloroquine could also induce psychiatric side effects which are polymorphic and can persist even after stopping the drug,” lead author Florence Gressier, MD, PhD, CESP, Inserm, department of psychiatry, Le Kremlin Bicêtre, France, said in an interview.
“In COVID-19 patients who may still be [undergoing treatment] with chloroquine, close psychiatric assessment and monitoring should be performed,” she said.
Heated controversy
Following findings of a small French study that suggested efficacy in lowering the viral load in patients with COVID-19, President Donald Trump expressed optimism regarding the role of hydroxychloroquine in treating COVID-19, calling it a “game changer”.
Other studies, however, have called into question both the efficacy and the safety of hydroxychloroquine in treating COVID-19. On June 15, the Food and Drug Administration revoked the emergency use authorization it had given in March to chloroquine and hydroxychloroquine for the treatment of COVID-19.
Nevertheless, hydroxychloroquine continues to be prescribed for COVID-19. For example, an article that appeared in Click2Houston on June 15 quoted the chief medical officer of Houston’s United Memorial Center as saying he plans to continue prescribing hydroxychloroquine for patients with COVID-19 until he finds a better alternative.
As discussed in a Medscape expert commentary, a group of physicians who held a “white coat summit” in front of the U.S. Supreme Court building promoted the use of hydroxychloroquine for the treatment of COVID-19. The video of their summit was retweeted by President Trump and garnered millions of views before it was taken down by Twitter, Facebook, and YouTube.
Sudden onset
For the new review, “we wanted to alert the public and practitioners on the potentially psychiatric risks induced by chloroquine, as it could be taken as self-medication or potentially still prescribed,” Dr. Gressier said.
“We think the format of the letter to the editor allows information to be provided in a concise and clear manner,” she added.
According to the FDA’s Adverse Event Reporting System database, 12% of reported adverse events (520 of 4,336) following the use of chloroquine that occurred between the fourth quarter of 2012 and the fourth quarter of 2019 were neuropsychiatric. These events included amnesia, delirium, hallucinations, depression, and loss of consciousness, the authors write.
The researchers acknowledged that the incidence of psychiatric adverse effects associated with the use of chloroquine is “unclear in the absence of high-quality, randomized placebo-controlled trials of its safety.” Nevertheless, they pointed out that there have been reports of insomnia and depression when the drug was used as prophylaxis against malaria .
Moreover, some case series or case reports describe symptoms such as depression, anxiety, agitation, violent outburst, suicidal ideation, and psychosis in patients who have been treated with chloroquine for malaria, lupus erythematosus, and rheumatoid arthritis .
“In contrast to many other psychoses, chloroquine psychosis may be more affective and include prominent visual hallucinations, symptoms of derealization, and disorders of thought, with preserved insight,” the authors wrote.
They noted that the frequency of symptoms does not appear to be connected to the cumulative dose or the duration of treatment, and the onset of psychosis or other adverse effects is usually “sudden.”
In addition, they warn that the drug’s psychiatric effects may go unnoticed, especially because COVID-19 itself has been associated with neuropsychiatric symptoms, making it hard to distinguish between symptoms caused by the illness and those caused by the drug.
Although the psychiatric symptoms typically occur early after treatment initiation, some “subtle” symptoms might persist after stopping the drug, possibly owing to its “extremely long” half-life, the authors stated.
Dr. Gressier noted that practicing clinicians should look up reports about self-medication with chloroquine “and warn their patients about the risk induced by chloroquine.”
Safe but ‘not benign’
Nilanjana Bose, MD, MBA, a rheumatologist at the Rheumatology Center of Houston, said she uses hydroxychloroquine “all the time” in clinical practice to treat patients with rheumatic conditions.
“I cannot comment on whether it [hydroxychloroquine or chloroquine] is a potential prophylactic or treatment for COVID-19, but I can say that, from a safety point of view, as a rheumatologist who uses hydroxychloroquine at a dose of 400 mg/day, I do not think we need to worry about serious [psychiatric] side effects,” Dr. Bose said in an interview.
Because clinicians are trying all types of possible treatments for COVID-19, “if this medication has possible efficacy, it is a great medicine from a rheumatologic perspective and is safe,” she added.
Nevertheless, the drug is “not benign, and regular side effects will be there, and of course, higher doses will cause more side effects,” said Dr. Bose, who was not involved in authoring the letter.
She counsels patients about potential psychiatric side effects of hydroxychloroquine because some of her patients have complained about irritability, worsening anxiety and depression, and difficulty sleeping.
Be wary
James “Jimmy” Potash, MD, MPH, Henry Phipps Professor of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, said in an interview that the “take-home message of this letter is that serious psychiatric effects, psychotic illness in particular,” can occur in individuals who take chloroquine and hydroxychloroquine.
In addition, “these are potentially very concerning side effects that psychiatrists should be aware of,” noted Dr. Potash, department director and psychiatrist-in-chief at Johns Hopkins.
He said that one of his patients who had been “completely psychiatrically healthy” took chloroquine prophylactically prior to traveling overseas. After she began taking the drug, she had an episode of mania that resolved once she discontinued the medication and received treatment for the mania.
“If you add potential psychiatric side effects to the other side effects that can result from these medications, that adds up to a pretty important reason to be wary of taking them, particularly for the indication of COVID-19, where the level of evidence that it helps in any way is still quite weak,” Dr. Potash said.
In an interview, Remington Nevin, MD, MPH, DrPH, executive director at the Quinism Foundation, White River Junction, Vt., a nonprofit organization that supports and promotes education and research on disorders caused by poisoning by quinoline drugs; and faculty associate in the department of mental health at Johns Hopkins Bloomberg School of Public Health, said that the authors of the letter “are to be commended for their efforts in raising awareness of the potentially lasting and disabling psychiatric effects of chloroquine and hydroxychloroquine, which, as with similar effects from other synthetic quinoline antimalarials, have occasionally been overlooked or misattributed to other conditions.”
He added: “I have proposed that the chronic neuropsychiatric effects of this class of drug are best considered not as side effects but as signs and symptoms of a disorder known as chronic quinoline encephalopathy caused by poisoning of the central nervous system.”
Dr. Gressier and the other letter authors, Dr. Bose, and Dr. Potash have reported no relevant financial relationships. Dr. Nevin has been retained as a consultant and expert witness in legal cases involving claims of adverse effects from quinoline antimalarial drugs.
A version of this article originally appeared on Medscape.com.
Chloroquine may be associated with serious psychiatric side effects, even in patients with no family or personal history of psychiatric disorders, a new review suggests.
In a letter to the editor published online July 28 in The Journal of Clinical Psychiatry, the authors summarize data from several studies published as far back as 1993 and as recently as May 2020.
“In addition to previously reported side effects, chloroquine could also induce psychiatric side effects which are polymorphic and can persist even after stopping the drug,” lead author Florence Gressier, MD, PhD, CESP, Inserm, department of psychiatry, Le Kremlin Bicêtre, France, said in an interview.
“In COVID-19 patients who may still be [undergoing treatment] with chloroquine, close psychiatric assessment and monitoring should be performed,” she said.
Heated controversy
Following findings of a small French study that suggested efficacy in lowering the viral load in patients with COVID-19, President Donald Trump expressed optimism regarding the role of hydroxychloroquine in treating COVID-19, calling it a “game changer”.
Other studies, however, have called into question both the efficacy and the safety of hydroxychloroquine in treating COVID-19. On June 15, the Food and Drug Administration revoked the emergency use authorization it had given in March to chloroquine and hydroxychloroquine for the treatment of COVID-19.
Nevertheless, hydroxychloroquine continues to be prescribed for COVID-19. For example, an article that appeared in Click2Houston on June 15 quoted the chief medical officer of Houston’s United Memorial Center as saying he plans to continue prescribing hydroxychloroquine for patients with COVID-19 until he finds a better alternative.
As discussed in a Medscape expert commentary, a group of physicians who held a “white coat summit” in front of the U.S. Supreme Court building promoted the use of hydroxychloroquine for the treatment of COVID-19. The video of their summit was retweeted by President Trump and garnered millions of views before it was taken down by Twitter, Facebook, and YouTube.
Sudden onset
For the new review, “we wanted to alert the public and practitioners on the potentially psychiatric risks induced by chloroquine, as it could be taken as self-medication or potentially still prescribed,” Dr. Gressier said.
“We think the format of the letter to the editor allows information to be provided in a concise and clear manner,” she added.
According to the FDA’s Adverse Event Reporting System database, 12% of reported adverse events (520 of 4,336) following the use of chloroquine that occurred between the fourth quarter of 2012 and the fourth quarter of 2019 were neuropsychiatric. These events included amnesia, delirium, hallucinations, depression, and loss of consciousness, the authors write.
The researchers acknowledged that the incidence of psychiatric adverse effects associated with the use of chloroquine is “unclear in the absence of high-quality, randomized placebo-controlled trials of its safety.” Nevertheless, they pointed out that there have been reports of insomnia and depression when the drug was used as prophylaxis against malaria .
Moreover, some case series or case reports describe symptoms such as depression, anxiety, agitation, violent outburst, suicidal ideation, and psychosis in patients who have been treated with chloroquine for malaria, lupus erythematosus, and rheumatoid arthritis .
“In contrast to many other psychoses, chloroquine psychosis may be more affective and include prominent visual hallucinations, symptoms of derealization, and disorders of thought, with preserved insight,” the authors wrote.
They noted that the frequency of symptoms does not appear to be connected to the cumulative dose or the duration of treatment, and the onset of psychosis or other adverse effects is usually “sudden.”
In addition, they warn that the drug’s psychiatric effects may go unnoticed, especially because COVID-19 itself has been associated with neuropsychiatric symptoms, making it hard to distinguish between symptoms caused by the illness and those caused by the drug.
Although the psychiatric symptoms typically occur early after treatment initiation, some “subtle” symptoms might persist after stopping the drug, possibly owing to its “extremely long” half-life, the authors stated.
Dr. Gressier noted that practicing clinicians should look up reports about self-medication with chloroquine “and warn their patients about the risk induced by chloroquine.”
Safe but ‘not benign’
Nilanjana Bose, MD, MBA, a rheumatologist at the Rheumatology Center of Houston, said she uses hydroxychloroquine “all the time” in clinical practice to treat patients with rheumatic conditions.
“I cannot comment on whether it [hydroxychloroquine or chloroquine] is a potential prophylactic or treatment for COVID-19, but I can say that, from a safety point of view, as a rheumatologist who uses hydroxychloroquine at a dose of 400 mg/day, I do not think we need to worry about serious [psychiatric] side effects,” Dr. Bose said in an interview.
Because clinicians are trying all types of possible treatments for COVID-19, “if this medication has possible efficacy, it is a great medicine from a rheumatologic perspective and is safe,” she added.
Nevertheless, the drug is “not benign, and regular side effects will be there, and of course, higher doses will cause more side effects,” said Dr. Bose, who was not involved in authoring the letter.
She counsels patients about potential psychiatric side effects of hydroxychloroquine because some of her patients have complained about irritability, worsening anxiety and depression, and difficulty sleeping.
Be wary
James “Jimmy” Potash, MD, MPH, Henry Phipps Professor of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, said in an interview that the “take-home message of this letter is that serious psychiatric effects, psychotic illness in particular,” can occur in individuals who take chloroquine and hydroxychloroquine.
In addition, “these are potentially very concerning side effects that psychiatrists should be aware of,” noted Dr. Potash, department director and psychiatrist-in-chief at Johns Hopkins.
He said that one of his patients who had been “completely psychiatrically healthy” took chloroquine prophylactically prior to traveling overseas. After she began taking the drug, she had an episode of mania that resolved once she discontinued the medication and received treatment for the mania.
“If you add potential psychiatric side effects to the other side effects that can result from these medications, that adds up to a pretty important reason to be wary of taking them, particularly for the indication of COVID-19, where the level of evidence that it helps in any way is still quite weak,” Dr. Potash said.
In an interview, Remington Nevin, MD, MPH, DrPH, executive director at the Quinism Foundation, White River Junction, Vt., a nonprofit organization that supports and promotes education and research on disorders caused by poisoning by quinoline drugs; and faculty associate in the department of mental health at Johns Hopkins Bloomberg School of Public Health, said that the authors of the letter “are to be commended for their efforts in raising awareness of the potentially lasting and disabling psychiatric effects of chloroquine and hydroxychloroquine, which, as with similar effects from other synthetic quinoline antimalarials, have occasionally been overlooked or misattributed to other conditions.”
He added: “I have proposed that the chronic neuropsychiatric effects of this class of drug are best considered not as side effects but as signs and symptoms of a disorder known as chronic quinoline encephalopathy caused by poisoning of the central nervous system.”
Dr. Gressier and the other letter authors, Dr. Bose, and Dr. Potash have reported no relevant financial relationships. Dr. Nevin has been retained as a consultant and expert witness in legal cases involving claims of adverse effects from quinoline antimalarial drugs.
A version of this article originally appeared on Medscape.com.
Chloroquine may be associated with serious psychiatric side effects, even in patients with no family or personal history of psychiatric disorders, a new review suggests.
In a letter to the editor published online July 28 in The Journal of Clinical Psychiatry, the authors summarize data from several studies published as far back as 1993 and as recently as May 2020.
“In addition to previously reported side effects, chloroquine could also induce psychiatric side effects which are polymorphic and can persist even after stopping the drug,” lead author Florence Gressier, MD, PhD, CESP, Inserm, department of psychiatry, Le Kremlin Bicêtre, France, said in an interview.
“In COVID-19 patients who may still be [undergoing treatment] with chloroquine, close psychiatric assessment and monitoring should be performed,” she said.
Heated controversy
Following findings of a small French study that suggested efficacy in lowering the viral load in patients with COVID-19, President Donald Trump expressed optimism regarding the role of hydroxychloroquine in treating COVID-19, calling it a “game changer”.
Other studies, however, have called into question both the efficacy and the safety of hydroxychloroquine in treating COVID-19. On June 15, the Food and Drug Administration revoked the emergency use authorization it had given in March to chloroquine and hydroxychloroquine for the treatment of COVID-19.
Nevertheless, hydroxychloroquine continues to be prescribed for COVID-19. For example, an article that appeared in Click2Houston on June 15 quoted the chief medical officer of Houston’s United Memorial Center as saying he plans to continue prescribing hydroxychloroquine for patients with COVID-19 until he finds a better alternative.
As discussed in a Medscape expert commentary, a group of physicians who held a “white coat summit” in front of the U.S. Supreme Court building promoted the use of hydroxychloroquine for the treatment of COVID-19. The video of their summit was retweeted by President Trump and garnered millions of views before it was taken down by Twitter, Facebook, and YouTube.
Sudden onset
For the new review, “we wanted to alert the public and practitioners on the potentially psychiatric risks induced by chloroquine, as it could be taken as self-medication or potentially still prescribed,” Dr. Gressier said.
“We think the format of the letter to the editor allows information to be provided in a concise and clear manner,” she added.
According to the FDA’s Adverse Event Reporting System database, 12% of reported adverse events (520 of 4,336) following the use of chloroquine that occurred between the fourth quarter of 2012 and the fourth quarter of 2019 were neuropsychiatric. These events included amnesia, delirium, hallucinations, depression, and loss of consciousness, the authors write.
The researchers acknowledged that the incidence of psychiatric adverse effects associated with the use of chloroquine is “unclear in the absence of high-quality, randomized placebo-controlled trials of its safety.” Nevertheless, they pointed out that there have been reports of insomnia and depression when the drug was used as prophylaxis against malaria .
Moreover, some case series or case reports describe symptoms such as depression, anxiety, agitation, violent outburst, suicidal ideation, and psychosis in patients who have been treated with chloroquine for malaria, lupus erythematosus, and rheumatoid arthritis .
“In contrast to many other psychoses, chloroquine psychosis may be more affective and include prominent visual hallucinations, symptoms of derealization, and disorders of thought, with preserved insight,” the authors wrote.
They noted that the frequency of symptoms does not appear to be connected to the cumulative dose or the duration of treatment, and the onset of psychosis or other adverse effects is usually “sudden.”
In addition, they warn that the drug’s psychiatric effects may go unnoticed, especially because COVID-19 itself has been associated with neuropsychiatric symptoms, making it hard to distinguish between symptoms caused by the illness and those caused by the drug.
Although the psychiatric symptoms typically occur early after treatment initiation, some “subtle” symptoms might persist after stopping the drug, possibly owing to its “extremely long” half-life, the authors stated.
Dr. Gressier noted that practicing clinicians should look up reports about self-medication with chloroquine “and warn their patients about the risk induced by chloroquine.”
Safe but ‘not benign’
Nilanjana Bose, MD, MBA, a rheumatologist at the Rheumatology Center of Houston, said she uses hydroxychloroquine “all the time” in clinical practice to treat patients with rheumatic conditions.
“I cannot comment on whether it [hydroxychloroquine or chloroquine] is a potential prophylactic or treatment for COVID-19, but I can say that, from a safety point of view, as a rheumatologist who uses hydroxychloroquine at a dose of 400 mg/day, I do not think we need to worry about serious [psychiatric] side effects,” Dr. Bose said in an interview.
Because clinicians are trying all types of possible treatments for COVID-19, “if this medication has possible efficacy, it is a great medicine from a rheumatologic perspective and is safe,” she added.
Nevertheless, the drug is “not benign, and regular side effects will be there, and of course, higher doses will cause more side effects,” said Dr. Bose, who was not involved in authoring the letter.
She counsels patients about potential psychiatric side effects of hydroxychloroquine because some of her patients have complained about irritability, worsening anxiety and depression, and difficulty sleeping.
Be wary
James “Jimmy” Potash, MD, MPH, Henry Phipps Professor of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, said in an interview that the “take-home message of this letter is that serious psychiatric effects, psychotic illness in particular,” can occur in individuals who take chloroquine and hydroxychloroquine.
In addition, “these are potentially very concerning side effects that psychiatrists should be aware of,” noted Dr. Potash, department director and psychiatrist-in-chief at Johns Hopkins.
He said that one of his patients who had been “completely psychiatrically healthy” took chloroquine prophylactically prior to traveling overseas. After she began taking the drug, she had an episode of mania that resolved once she discontinued the medication and received treatment for the mania.
“If you add potential psychiatric side effects to the other side effects that can result from these medications, that adds up to a pretty important reason to be wary of taking them, particularly for the indication of COVID-19, where the level of evidence that it helps in any way is still quite weak,” Dr. Potash said.
In an interview, Remington Nevin, MD, MPH, DrPH, executive director at the Quinism Foundation, White River Junction, Vt., a nonprofit organization that supports and promotes education and research on disorders caused by poisoning by quinoline drugs; and faculty associate in the department of mental health at Johns Hopkins Bloomberg School of Public Health, said that the authors of the letter “are to be commended for their efforts in raising awareness of the potentially lasting and disabling psychiatric effects of chloroquine and hydroxychloroquine, which, as with similar effects from other synthetic quinoline antimalarials, have occasionally been overlooked or misattributed to other conditions.”
He added: “I have proposed that the chronic neuropsychiatric effects of this class of drug are best considered not as side effects but as signs and symptoms of a disorder known as chronic quinoline encephalopathy caused by poisoning of the central nervous system.”
Dr. Gressier and the other letter authors, Dr. Bose, and Dr. Potash have reported no relevant financial relationships. Dr. Nevin has been retained as a consultant and expert witness in legal cases involving claims of adverse effects from quinoline antimalarial drugs.
A version of this article originally appeared on Medscape.com.
Botulinum toxin associated with antidepressant effects across indications, injection sites
, according to the study’s authors.
Their results show that the antidepressant effect of botulinum toxin “administered for various indications goes beyond the control of the intended disease states and does not depend on the location of the injection,” according to Tigran Makunts, PharmD, of the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego, and coauthors.
Previous high-quality studies have found botulinum toxin treatment has been associated with antidepressant effects when administered to the glabellar region of the face, they noted. The study was published in Scientific Reports.
The researchers evaluated adverse events reported to the Food and Drug Administration’s current adverse event reporting system (FAERS) between September 2012 and December 2019, and the FDA’s previous adverse event reporting system between January 2004 and August 2012. Overall, they analyzed 174,243 reports, which were divided into eight treatment-related groups based on the indication for botulinum toxin: Cosmetic use (20,684 patients), migraine (4,180 patients), spasms and spasticity not involving facial muscles (2,335 patients), neurological and urinary bladder disorders (915 patients), torticollis (1,360 patients), hyperhidrosis (601 patients), blepharospasm (487 patients), and sialorrhea (157 patients). Each group was matched to controls from the FAERS database, who had different treatments for the same indications. (Reports in which patients were on an antidepressant or where depression was listed as an indication were not included).
In nearly all treatment groups, reports of depression and depression-related adverse events were significantly lower among those who received botulinum toxin, compared with controls: For those who received botulinum toxin injections in facial muscles for cosmetic uses, the reporting odds ratio was 0.46 (95% confidence interval, 0.27-0.78). Significant effects were also see in the following groups: those who received injections into facial and head muscles for migraine (ROR, 0.60; 95% CI, 0.48-0.74), injections into the upper and lower limbs for spasms and spasticity (ROR, 0.28; 95% CI, 0.18-0.42), injections into neck muscles for torticollis and neck pain (ROR, 0.30; 95% CI, 0.20-0.44), injections into eyelid muscles for blepharospasm (ROR, 0.13; 95% CI, 0.05-0.39), and injections into the axilla and palm for hyperhidrosis (ROR, 0.12; 95% CI, 0.04-0.33).
There were no cases of depression or depression-related adverse event reports among those treated with botulinum toxin for sialorrhea with injections into the parotid and submandibular glands, and there were decreased reports of depression among those who received detrusor muscle injections for neurological and urinary bladder disorders, but the results in both groups were not statistically significant, according to the researchers.
In an interview, Ruben Abagyan, PhD, study coauthor and professor at Skaggs School of Pharmacy and Pharmaceutical Sciences, said the study’s finding go “beyond breaking a positive feedback loop between depression and the ‘frown’ wrinkles in the glabellar region of the forehead.” The data showing efficacy with botulinum toxin injected in other areas of the body can help clinicians “expand their search for the most effective injection location and dose beyond the facial injections to improve the depression-related therapeutic outcomes.”
Another takeaway from the study, he noted, is that botulinum toxin can have effects beyond the local effect seen near an injection site. Administering botulinum toxin for spasms and spasticity, excessive sweating, migraine, urinary bladder disorders, blepharospasm, or excessive salivation/drooling could result in reduced depression and improved systemic neurological effects.
“Severe depression remains a very difficult condition to treat. The existing drugs have dangerous side effects, the onset of the therapeutic action is delayed by at least a month, and the adherence to the medication is suboptimal. Therefore, finding new ways to treat depression is critical,” Dr. Abagyan said. “Botulinum toxin opens up a new physiological mechanism to be tried to reduce depression.”
Michelle Magid, MD, MBA, of the department of psychiatry at the University of Texas at Austin, said in an interview that, although the study was retrospective, “physicians can feel confident that botulinum toxin treatment will not cause depression; it may very well lead to improved mood in some of their patients.” Dr. Magid was not an author of this study, but has studied botulinum toxin as a possible treatment of depression.
“Previous studies have shown that botulinum toxin injected into the forehead region can improve symptoms of depression. The studies were small and confined to treating the glabellar region only,” she added. “This is a large retrospective study showing that botulinum toxin injected into other regions, such as the neck, underarms, bladder, hands, arms, and legs, can also have an antidepressant effect.”
Dr. Magid agreed that the use of botulinum toxin as an antidepressant should be investigated further, and could be a tool for patients who do not respond well to traditional antidepressant medications.
In their paper, the authors offered several plausible mechanisms for the antidepressant effects of botulinum toxin, including transneuronal transport to the parts of the central nervous system that regulate mood and emotion, systemic distribution, distributed muscle stress memory, and efficacy in the primary indication treatment. Although the mechanism of action is not well understood, Dr. Magid noted it could be the removal of somatic symptoms that contribute to an improvement in mood.
“It is possible that alleviating the psychological distress associated with neck spasms, excessive sweating [and so on] can be causing the antidepressive effects,” she said. “However, it is also possible that depression is actualized by a series of somatic symptoms – body aches, insomnia, sweating, for example. By removing somatic symptoms, one may also remove the correlating mood dysregulation.”
The study “certainly raises a lot of questions,” particularly about the “apparent multiple mechanisms of action of BoNT that we don’t understand yet,” Mark Rubin, MD, a cosmetic dermatologist who practices in Beverly Hills, Calif., said in an interview. “I believe it lends great deal of credence to the use of [botulinum toxin] for depression and certainly validates the need for more robust clinical trials for that indication,” he added.
“I think what we all as clinicians need to take away from this paper is that there is a great deal we don’t understand about the global pharmacologic effects of [botulinum toxin] and equally important, that there are apparently other pharmacologic pathways we need to explore in the treatment of depression, said Dr. Rubin, of the department of dermatology at the University of California, San Diego, who was not an investigator in the study.
One author reported being a consultant for Allergan. Dr. Makunts and the other author report no relevant conflicts of interest; Dr. Magid reported being a consultant for Allergan and a speaker for Ipsen. Dr. Rubin had no related disclosures.
SOURCE: Makunts T et al. Sci Rep. 2020 Jul 30;10(1):12851. doi: 10.1038/s41598-020-69773-7.
, according to the study’s authors.
Their results show that the antidepressant effect of botulinum toxin “administered for various indications goes beyond the control of the intended disease states and does not depend on the location of the injection,” according to Tigran Makunts, PharmD, of the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego, and coauthors.
Previous high-quality studies have found botulinum toxin treatment has been associated with antidepressant effects when administered to the glabellar region of the face, they noted. The study was published in Scientific Reports.
The researchers evaluated adverse events reported to the Food and Drug Administration’s current adverse event reporting system (FAERS) between September 2012 and December 2019, and the FDA’s previous adverse event reporting system between January 2004 and August 2012. Overall, they analyzed 174,243 reports, which were divided into eight treatment-related groups based on the indication for botulinum toxin: Cosmetic use (20,684 patients), migraine (4,180 patients), spasms and spasticity not involving facial muscles (2,335 patients), neurological and urinary bladder disorders (915 patients), torticollis (1,360 patients), hyperhidrosis (601 patients), blepharospasm (487 patients), and sialorrhea (157 patients). Each group was matched to controls from the FAERS database, who had different treatments for the same indications. (Reports in which patients were on an antidepressant or where depression was listed as an indication were not included).
In nearly all treatment groups, reports of depression and depression-related adverse events were significantly lower among those who received botulinum toxin, compared with controls: For those who received botulinum toxin injections in facial muscles for cosmetic uses, the reporting odds ratio was 0.46 (95% confidence interval, 0.27-0.78). Significant effects were also see in the following groups: those who received injections into facial and head muscles for migraine (ROR, 0.60; 95% CI, 0.48-0.74), injections into the upper and lower limbs for spasms and spasticity (ROR, 0.28; 95% CI, 0.18-0.42), injections into neck muscles for torticollis and neck pain (ROR, 0.30; 95% CI, 0.20-0.44), injections into eyelid muscles for blepharospasm (ROR, 0.13; 95% CI, 0.05-0.39), and injections into the axilla and palm for hyperhidrosis (ROR, 0.12; 95% CI, 0.04-0.33).
There were no cases of depression or depression-related adverse event reports among those treated with botulinum toxin for sialorrhea with injections into the parotid and submandibular glands, and there were decreased reports of depression among those who received detrusor muscle injections for neurological and urinary bladder disorders, but the results in both groups were not statistically significant, according to the researchers.
In an interview, Ruben Abagyan, PhD, study coauthor and professor at Skaggs School of Pharmacy and Pharmaceutical Sciences, said the study’s finding go “beyond breaking a positive feedback loop between depression and the ‘frown’ wrinkles in the glabellar region of the forehead.” The data showing efficacy with botulinum toxin injected in other areas of the body can help clinicians “expand their search for the most effective injection location and dose beyond the facial injections to improve the depression-related therapeutic outcomes.”
Another takeaway from the study, he noted, is that botulinum toxin can have effects beyond the local effect seen near an injection site. Administering botulinum toxin for spasms and spasticity, excessive sweating, migraine, urinary bladder disorders, blepharospasm, or excessive salivation/drooling could result in reduced depression and improved systemic neurological effects.
“Severe depression remains a very difficult condition to treat. The existing drugs have dangerous side effects, the onset of the therapeutic action is delayed by at least a month, and the adherence to the medication is suboptimal. Therefore, finding new ways to treat depression is critical,” Dr. Abagyan said. “Botulinum toxin opens up a new physiological mechanism to be tried to reduce depression.”
Michelle Magid, MD, MBA, of the department of psychiatry at the University of Texas at Austin, said in an interview that, although the study was retrospective, “physicians can feel confident that botulinum toxin treatment will not cause depression; it may very well lead to improved mood in some of their patients.” Dr. Magid was not an author of this study, but has studied botulinum toxin as a possible treatment of depression.
“Previous studies have shown that botulinum toxin injected into the forehead region can improve symptoms of depression. The studies were small and confined to treating the glabellar region only,” she added. “This is a large retrospective study showing that botulinum toxin injected into other regions, such as the neck, underarms, bladder, hands, arms, and legs, can also have an antidepressant effect.”
Dr. Magid agreed that the use of botulinum toxin as an antidepressant should be investigated further, and could be a tool for patients who do not respond well to traditional antidepressant medications.
In their paper, the authors offered several plausible mechanisms for the antidepressant effects of botulinum toxin, including transneuronal transport to the parts of the central nervous system that regulate mood and emotion, systemic distribution, distributed muscle stress memory, and efficacy in the primary indication treatment. Although the mechanism of action is not well understood, Dr. Magid noted it could be the removal of somatic symptoms that contribute to an improvement in mood.
“It is possible that alleviating the psychological distress associated with neck spasms, excessive sweating [and so on] can be causing the antidepressive effects,” she said. “However, it is also possible that depression is actualized by a series of somatic symptoms – body aches, insomnia, sweating, for example. By removing somatic symptoms, one may also remove the correlating mood dysregulation.”
The study “certainly raises a lot of questions,” particularly about the “apparent multiple mechanisms of action of BoNT that we don’t understand yet,” Mark Rubin, MD, a cosmetic dermatologist who practices in Beverly Hills, Calif., said in an interview. “I believe it lends great deal of credence to the use of [botulinum toxin] for depression and certainly validates the need for more robust clinical trials for that indication,” he added.
“I think what we all as clinicians need to take away from this paper is that there is a great deal we don’t understand about the global pharmacologic effects of [botulinum toxin] and equally important, that there are apparently other pharmacologic pathways we need to explore in the treatment of depression, said Dr. Rubin, of the department of dermatology at the University of California, San Diego, who was not an investigator in the study.
One author reported being a consultant for Allergan. Dr. Makunts and the other author report no relevant conflicts of interest; Dr. Magid reported being a consultant for Allergan and a speaker for Ipsen. Dr. Rubin had no related disclosures.
SOURCE: Makunts T et al. Sci Rep. 2020 Jul 30;10(1):12851. doi: 10.1038/s41598-020-69773-7.
, according to the study’s authors.
Their results show that the antidepressant effect of botulinum toxin “administered for various indications goes beyond the control of the intended disease states and does not depend on the location of the injection,” according to Tigran Makunts, PharmD, of the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego, and coauthors.
Previous high-quality studies have found botulinum toxin treatment has been associated with antidepressant effects when administered to the glabellar region of the face, they noted. The study was published in Scientific Reports.
The researchers evaluated adverse events reported to the Food and Drug Administration’s current adverse event reporting system (FAERS) between September 2012 and December 2019, and the FDA’s previous adverse event reporting system between January 2004 and August 2012. Overall, they analyzed 174,243 reports, which were divided into eight treatment-related groups based on the indication for botulinum toxin: Cosmetic use (20,684 patients), migraine (4,180 patients), spasms and spasticity not involving facial muscles (2,335 patients), neurological and urinary bladder disorders (915 patients), torticollis (1,360 patients), hyperhidrosis (601 patients), blepharospasm (487 patients), and sialorrhea (157 patients). Each group was matched to controls from the FAERS database, who had different treatments for the same indications. (Reports in which patients were on an antidepressant or where depression was listed as an indication were not included).
In nearly all treatment groups, reports of depression and depression-related adverse events were significantly lower among those who received botulinum toxin, compared with controls: For those who received botulinum toxin injections in facial muscles for cosmetic uses, the reporting odds ratio was 0.46 (95% confidence interval, 0.27-0.78). Significant effects were also see in the following groups: those who received injections into facial and head muscles for migraine (ROR, 0.60; 95% CI, 0.48-0.74), injections into the upper and lower limbs for spasms and spasticity (ROR, 0.28; 95% CI, 0.18-0.42), injections into neck muscles for torticollis and neck pain (ROR, 0.30; 95% CI, 0.20-0.44), injections into eyelid muscles for blepharospasm (ROR, 0.13; 95% CI, 0.05-0.39), and injections into the axilla and palm for hyperhidrosis (ROR, 0.12; 95% CI, 0.04-0.33).
There were no cases of depression or depression-related adverse event reports among those treated with botulinum toxin for sialorrhea with injections into the parotid and submandibular glands, and there were decreased reports of depression among those who received detrusor muscle injections for neurological and urinary bladder disorders, but the results in both groups were not statistically significant, according to the researchers.
In an interview, Ruben Abagyan, PhD, study coauthor and professor at Skaggs School of Pharmacy and Pharmaceutical Sciences, said the study’s finding go “beyond breaking a positive feedback loop between depression and the ‘frown’ wrinkles in the glabellar region of the forehead.” The data showing efficacy with botulinum toxin injected in other areas of the body can help clinicians “expand their search for the most effective injection location and dose beyond the facial injections to improve the depression-related therapeutic outcomes.”
Another takeaway from the study, he noted, is that botulinum toxin can have effects beyond the local effect seen near an injection site. Administering botulinum toxin for spasms and spasticity, excessive sweating, migraine, urinary bladder disorders, blepharospasm, or excessive salivation/drooling could result in reduced depression and improved systemic neurological effects.
“Severe depression remains a very difficult condition to treat. The existing drugs have dangerous side effects, the onset of the therapeutic action is delayed by at least a month, and the adherence to the medication is suboptimal. Therefore, finding new ways to treat depression is critical,” Dr. Abagyan said. “Botulinum toxin opens up a new physiological mechanism to be tried to reduce depression.”
Michelle Magid, MD, MBA, of the department of psychiatry at the University of Texas at Austin, said in an interview that, although the study was retrospective, “physicians can feel confident that botulinum toxin treatment will not cause depression; it may very well lead to improved mood in some of their patients.” Dr. Magid was not an author of this study, but has studied botulinum toxin as a possible treatment of depression.
“Previous studies have shown that botulinum toxin injected into the forehead region can improve symptoms of depression. The studies were small and confined to treating the glabellar region only,” she added. “This is a large retrospective study showing that botulinum toxin injected into other regions, such as the neck, underarms, bladder, hands, arms, and legs, can also have an antidepressant effect.”
Dr. Magid agreed that the use of botulinum toxin as an antidepressant should be investigated further, and could be a tool for patients who do not respond well to traditional antidepressant medications.
In their paper, the authors offered several plausible mechanisms for the antidepressant effects of botulinum toxin, including transneuronal transport to the parts of the central nervous system that regulate mood and emotion, systemic distribution, distributed muscle stress memory, and efficacy in the primary indication treatment. Although the mechanism of action is not well understood, Dr. Magid noted it could be the removal of somatic symptoms that contribute to an improvement in mood.
“It is possible that alleviating the psychological distress associated with neck spasms, excessive sweating [and so on] can be causing the antidepressive effects,” she said. “However, it is also possible that depression is actualized by a series of somatic symptoms – body aches, insomnia, sweating, for example. By removing somatic symptoms, one may also remove the correlating mood dysregulation.”
The study “certainly raises a lot of questions,” particularly about the “apparent multiple mechanisms of action of BoNT that we don’t understand yet,” Mark Rubin, MD, a cosmetic dermatologist who practices in Beverly Hills, Calif., said in an interview. “I believe it lends great deal of credence to the use of [botulinum toxin] for depression and certainly validates the need for more robust clinical trials for that indication,” he added.
“I think what we all as clinicians need to take away from this paper is that there is a great deal we don’t understand about the global pharmacologic effects of [botulinum toxin] and equally important, that there are apparently other pharmacologic pathways we need to explore in the treatment of depression, said Dr. Rubin, of the department of dermatology at the University of California, San Diego, who was not an investigator in the study.
One author reported being a consultant for Allergan. Dr. Makunts and the other author report no relevant conflicts of interest; Dr. Magid reported being a consultant for Allergan and a speaker for Ipsen. Dr. Rubin had no related disclosures.
SOURCE: Makunts T et al. Sci Rep. 2020 Jul 30;10(1):12851. doi: 10.1038/s41598-020-69773-7.
FROM SCIENTIFIC REPORTS
Rapid cycle pediatric simulation exercises promise improved readiness
Focused repetition builds sustained skill
A methodical, constructive, goal-oriented rapid repetition of emergency response simulations has emerged as a dominant strategy for pediatric readiness in the hospital setting, according to a detailed description of one such program at the virtual Pediatric Hospital Medicine.
Rather than a single run-through followed by a lengthy debriefing, which has been a traditional approach, short simulations done rapidly and repeatedly until skills are mastered improve skill development, according to Jeanmarie Schied, MD, of the department of pediatrics, University of Chicago Medicine.
“This method utilizes repetitions to develop muscle memory much like an athlete who ‘practices, practices, practices’ until it becomes second nature,” Dr. Schied explained.
Dr. Schied credited this approach to Elizabeth Hunt, MD, PhD, director of the Johns Hopkins Medicine Simulation Center. The method created by Dr. Hunt is called Rapid Cycle Deliberate Practice (RCDP). At the University of Chicago, where the same principles are being applied, “we have had great success,” Dr. Schied said.
Deficiencies in the traditional approach prompted the change. It has been shown that when experienced residents who have performed multiple simulations are compared to new residents with limited experience or when those certified in Pediatric Advanced Life Support (PAL) are compared to those who are not, they “do not necessarily do better” in the metrics used in simulations to measure competence, according to Dr. Schied.
With the RDCP, learners get multiple chances to master skills.
“Everyone makes mistakes, and letting the participants know this ahead of time puts people at ease,” Dr. Schied said. “People want to know they will have a chance to rewind and do it right.”
In setting up an effective simulation program, the first step is a needs assessment. By first gauging the skill and experience level of those scheduled to participate, Dr. Schied said the program can be tailored to the audience.
The next step is formulating learning objectives. Dr. Schied recommended creating these objectives for the case overall and for each phase of the simulation as it progresses from basic clinical assessments through the specific interventions appropriate for the diagnosis.
Within these objectives there are additional goals. For example, the team should work to administer care within prespecified benchmarks, such as an elapsed time of 60 seconds or less for oxygenation or a time of 180 seconds or less for defibrillation, according to Dr. Schied.
Yet, Dr. Schied suggested that enforcing these goals on initial run-throughs might not be appropriate.
“Let the scenario run longer so you can see the deficits,” Dr. Schied said. If, for example, chest compression is not being done correctly, she recommended interrupting the process to provide immediate and direct feedback. In critiquing the performance, Dr. Schied advised against a critical or punitive tone.
“Inform the learners that they are in a safe environment,” she said. It is essential to identify errors so that they can be corrected on the next run of the practice simulation, but Dr. Schied advised instructors to “be nonjudgmental.” Praise is appropriate when warranted, but she also warned, “don’t sugarcoat” a substandard performance.
During the simulation, team leaders should employ action phrases, meaning that the problem and the action needed are expressed at the same time, according to Dr. Schied. Examples include, “the patient is not breathing, start bagging,” or “there is no pulse, start compression.”
“When the team gets used to these action-linked phrases, studies show that they react in a more timely fashion,” Dr. Schied explained at the event sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
In the study by Dr. Hunt that established the effectiveness of RDCP, 51 pediatric residents who had previously participated in a cardiopulmonary arrest simulation were retested again after being retrained with the RDCP methodology (Resuscitation 2014;85:945-51).
RDCP “was associated with improvement in performance of key measures of quality life support and progressive acquisition of resuscitation skills,” according to Dr. Hunt, who has published frequently on resuscitation training in pediatrics.
Prior to RDCP, traditional methods produced “little improvement” in resuscitation skills when measured over the course of pediatric residency, according to Dr. Hunt. After RDCP, third-year residents were shown to be “significantly more likely than first-years to defibrillate within 2 minutes,” she reported.
However, there are other strategies to improve retention of skills, according to Dr. Schied. For example, it is important to conduct simulations when the staff can focus. Specifically, Dr. Schied recommended conducting simulations immediately after a staff meeting or before a scheduled shift so that clinical responsibilities will not interfere or divert the learner’s attention. She also recommended conducting key simulations quarterly.
“Studies have shown that knowledge deterioration related to resuscitation begins about 4 months after the last simulation,” she said.
In addition to building the skills of individual participants, Dr. Schied emphasized the importance of also developing effective team dynamics and active communication. In the debriefing that should follow every simulation, she recommended encouraging a discussion of strengths and weaknesses of the team response.
Pediatric emergency simulation scenarios are readily available on multiple sites found on the Internet,” Dr. Schied said. She recommended documenting performance so the data are available for subsequent analysis.
Focused repetition builds sustained skill
Focused repetition builds sustained skill
A methodical, constructive, goal-oriented rapid repetition of emergency response simulations has emerged as a dominant strategy for pediatric readiness in the hospital setting, according to a detailed description of one such program at the virtual Pediatric Hospital Medicine.
Rather than a single run-through followed by a lengthy debriefing, which has been a traditional approach, short simulations done rapidly and repeatedly until skills are mastered improve skill development, according to Jeanmarie Schied, MD, of the department of pediatrics, University of Chicago Medicine.
“This method utilizes repetitions to develop muscle memory much like an athlete who ‘practices, practices, practices’ until it becomes second nature,” Dr. Schied explained.
Dr. Schied credited this approach to Elizabeth Hunt, MD, PhD, director of the Johns Hopkins Medicine Simulation Center. The method created by Dr. Hunt is called Rapid Cycle Deliberate Practice (RCDP). At the University of Chicago, where the same principles are being applied, “we have had great success,” Dr. Schied said.
Deficiencies in the traditional approach prompted the change. It has been shown that when experienced residents who have performed multiple simulations are compared to new residents with limited experience or when those certified in Pediatric Advanced Life Support (PAL) are compared to those who are not, they “do not necessarily do better” in the metrics used in simulations to measure competence, according to Dr. Schied.
With the RDCP, learners get multiple chances to master skills.
“Everyone makes mistakes, and letting the participants know this ahead of time puts people at ease,” Dr. Schied said. “People want to know they will have a chance to rewind and do it right.”
In setting up an effective simulation program, the first step is a needs assessment. By first gauging the skill and experience level of those scheduled to participate, Dr. Schied said the program can be tailored to the audience.
The next step is formulating learning objectives. Dr. Schied recommended creating these objectives for the case overall and for each phase of the simulation as it progresses from basic clinical assessments through the specific interventions appropriate for the diagnosis.
Within these objectives there are additional goals. For example, the team should work to administer care within prespecified benchmarks, such as an elapsed time of 60 seconds or less for oxygenation or a time of 180 seconds or less for defibrillation, according to Dr. Schied.
Yet, Dr. Schied suggested that enforcing these goals on initial run-throughs might not be appropriate.
“Let the scenario run longer so you can see the deficits,” Dr. Schied said. If, for example, chest compression is not being done correctly, she recommended interrupting the process to provide immediate and direct feedback. In critiquing the performance, Dr. Schied advised against a critical or punitive tone.
“Inform the learners that they are in a safe environment,” she said. It is essential to identify errors so that they can be corrected on the next run of the practice simulation, but Dr. Schied advised instructors to “be nonjudgmental.” Praise is appropriate when warranted, but she also warned, “don’t sugarcoat” a substandard performance.
During the simulation, team leaders should employ action phrases, meaning that the problem and the action needed are expressed at the same time, according to Dr. Schied. Examples include, “the patient is not breathing, start bagging,” or “there is no pulse, start compression.”
“When the team gets used to these action-linked phrases, studies show that they react in a more timely fashion,” Dr. Schied explained at the event sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
In the study by Dr. Hunt that established the effectiveness of RDCP, 51 pediatric residents who had previously participated in a cardiopulmonary arrest simulation were retested again after being retrained with the RDCP methodology (Resuscitation 2014;85:945-51).
RDCP “was associated with improvement in performance of key measures of quality life support and progressive acquisition of resuscitation skills,” according to Dr. Hunt, who has published frequently on resuscitation training in pediatrics.
Prior to RDCP, traditional methods produced “little improvement” in resuscitation skills when measured over the course of pediatric residency, according to Dr. Hunt. After RDCP, third-year residents were shown to be “significantly more likely than first-years to defibrillate within 2 minutes,” she reported.
However, there are other strategies to improve retention of skills, according to Dr. Schied. For example, it is important to conduct simulations when the staff can focus. Specifically, Dr. Schied recommended conducting simulations immediately after a staff meeting or before a scheduled shift so that clinical responsibilities will not interfere or divert the learner’s attention. She also recommended conducting key simulations quarterly.
“Studies have shown that knowledge deterioration related to resuscitation begins about 4 months after the last simulation,” she said.
In addition to building the skills of individual participants, Dr. Schied emphasized the importance of also developing effective team dynamics and active communication. In the debriefing that should follow every simulation, she recommended encouraging a discussion of strengths and weaknesses of the team response.
Pediatric emergency simulation scenarios are readily available on multiple sites found on the Internet,” Dr. Schied said. She recommended documenting performance so the data are available for subsequent analysis.
A methodical, constructive, goal-oriented rapid repetition of emergency response simulations has emerged as a dominant strategy for pediatric readiness in the hospital setting, according to a detailed description of one such program at the virtual Pediatric Hospital Medicine.
Rather than a single run-through followed by a lengthy debriefing, which has been a traditional approach, short simulations done rapidly and repeatedly until skills are mastered improve skill development, according to Jeanmarie Schied, MD, of the department of pediatrics, University of Chicago Medicine.
“This method utilizes repetitions to develop muscle memory much like an athlete who ‘practices, practices, practices’ until it becomes second nature,” Dr. Schied explained.
Dr. Schied credited this approach to Elizabeth Hunt, MD, PhD, director of the Johns Hopkins Medicine Simulation Center. The method created by Dr. Hunt is called Rapid Cycle Deliberate Practice (RCDP). At the University of Chicago, where the same principles are being applied, “we have had great success,” Dr. Schied said.
Deficiencies in the traditional approach prompted the change. It has been shown that when experienced residents who have performed multiple simulations are compared to new residents with limited experience or when those certified in Pediatric Advanced Life Support (PAL) are compared to those who are not, they “do not necessarily do better” in the metrics used in simulations to measure competence, according to Dr. Schied.
With the RDCP, learners get multiple chances to master skills.
“Everyone makes mistakes, and letting the participants know this ahead of time puts people at ease,” Dr. Schied said. “People want to know they will have a chance to rewind and do it right.”
In setting up an effective simulation program, the first step is a needs assessment. By first gauging the skill and experience level of those scheduled to participate, Dr. Schied said the program can be tailored to the audience.
The next step is formulating learning objectives. Dr. Schied recommended creating these objectives for the case overall and for each phase of the simulation as it progresses from basic clinical assessments through the specific interventions appropriate for the diagnosis.
Within these objectives there are additional goals. For example, the team should work to administer care within prespecified benchmarks, such as an elapsed time of 60 seconds or less for oxygenation or a time of 180 seconds or less for defibrillation, according to Dr. Schied.
Yet, Dr. Schied suggested that enforcing these goals on initial run-throughs might not be appropriate.
“Let the scenario run longer so you can see the deficits,” Dr. Schied said. If, for example, chest compression is not being done correctly, she recommended interrupting the process to provide immediate and direct feedback. In critiquing the performance, Dr. Schied advised against a critical or punitive tone.
“Inform the learners that they are in a safe environment,” she said. It is essential to identify errors so that they can be corrected on the next run of the practice simulation, but Dr. Schied advised instructors to “be nonjudgmental.” Praise is appropriate when warranted, but she also warned, “don’t sugarcoat” a substandard performance.
During the simulation, team leaders should employ action phrases, meaning that the problem and the action needed are expressed at the same time, according to Dr. Schied. Examples include, “the patient is not breathing, start bagging,” or “there is no pulse, start compression.”
“When the team gets used to these action-linked phrases, studies show that they react in a more timely fashion,” Dr. Schied explained at the event sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
In the study by Dr. Hunt that established the effectiveness of RDCP, 51 pediatric residents who had previously participated in a cardiopulmonary arrest simulation were retested again after being retrained with the RDCP methodology (Resuscitation 2014;85:945-51).
RDCP “was associated with improvement in performance of key measures of quality life support and progressive acquisition of resuscitation skills,” according to Dr. Hunt, who has published frequently on resuscitation training in pediatrics.
Prior to RDCP, traditional methods produced “little improvement” in resuscitation skills when measured over the course of pediatric residency, according to Dr. Hunt. After RDCP, third-year residents were shown to be “significantly more likely than first-years to defibrillate within 2 minutes,” she reported.
However, there are other strategies to improve retention of skills, according to Dr. Schied. For example, it is important to conduct simulations when the staff can focus. Specifically, Dr. Schied recommended conducting simulations immediately after a staff meeting or before a scheduled shift so that clinical responsibilities will not interfere or divert the learner’s attention. She also recommended conducting key simulations quarterly.
“Studies have shown that knowledge deterioration related to resuscitation begins about 4 months after the last simulation,” she said.
In addition to building the skills of individual participants, Dr. Schied emphasized the importance of also developing effective team dynamics and active communication. In the debriefing that should follow every simulation, she recommended encouraging a discussion of strengths and weaknesses of the team response.
Pediatric emergency simulation scenarios are readily available on multiple sites found on the Internet,” Dr. Schied said. She recommended documenting performance so the data are available for subsequent analysis.
FROM PHM20 VIRTUAL
Critical care readiness. Coding for telemedicine. Physical therapy teleconsultations. Physical therapy teleconsultations.
Preparation is key for disaster management. It includes identifying heath-care worker capability, surge capacity, disposable medical resources, and expert consultation availability.
Staff
In disaster, the hospital transitions to a mass casualty strategy, repurposing noncritical care staff to a tiered critical care model focusing on disaster triage and mass critical care. The goal is to provide care to minimize mortality.
Stuff
Critical care supplies improve survival and are implemented quickly and easily. Essential supplies include personal protective equipment, basic modes of mechanical ventilation, hemodynamic support, antimicrobial therapy or other disease-specific countermeasures, oxygen, and prophylactic treatments.
Structure
Disaster critical care can be delivered in noncritical care areas. Hospital policies should establish surge capacity strategies.
System
Providing quality lifesaving care to appropriately triaged patients by utilizing minimal qualifications for survival, predetermined ICU admission criteria, and dynamic protocols using the highest level of evidence available scalable to local resources.
Inappropriate triage results in suboptimal care and can lead to increased mortality.
Virtual critical care can augment critical care capacity and capability.
The implementation of mass critical care requires hospitals to rapidly increase its patient volume above its normal capacity. The essential four components are staff, stuff, space, and structure. Effective mass critical care requires a different mindset than critical care in day-to-day operations.
Patrick Moon, MD; and Alexis MacDonald, MD
(Drs. Reed and Tripp's Fellows)
Mary Jane Reed, MD, FCCP, and Michael Tripp, MD, FCCP
Steering Committee Members
Practice Operations
Over the years since telemedicine (TM) was developed in the 1960s, it has transformed into more mobile, compact, and interconnected forms. However, its widespread adoption has been limited by the regulatory, compensatory, and licensing status quo. The emergence of the COVID-19 pandemic and its necessity for physical distancing has brought TM into the limelight. With restrictions on TM use lifted by CMS, the scope of TM could extend from outpatient to inpatient care to emergency triaging and management of chronic medical conditions.
In February 2020, the comprehensive 2020 COVID-19 ICD 10 coding guidelines were released. To date, CMS has approved approximately 80 codes, which can be used with telehealth and non face-face-to-face (NFTF) encounters. They include telephone calls, online digital E/M services, interprofessional telephone/internet/electric health record consultations, digitally stored data services/remote physiologic monitoring, remote reporting of self-measure blood pressure, and remote physiologic monitoring treatment management services. Some of the key "rules of the game" are highlighted below.
- For telephone visits in the outpatient setting use the codes 99441 (5-10 minutes), 99442 (11-20 minutes), and 99443 (21-30 minutes).
- For interactive real-time audio and video telecommunication (RAVT) in the outpatient setting, use the codes normally used for outpatient E/M: 99201-99215.
- For using RAVT to perform an initial visit for an inpatient, use the codes that are normally used for inpatient E/M: 99221-99223.
- For using RAVT to perform a subsequent visit for an inpatient, use the codes that are normally used for subsequent hospital care service E/M: 99231-99233.
- Seeing a critically ill patient without being in the patient's room is allowed, as a physical exam is not required for either 99291 or 99292. Be sure to use 99292 for each 30 minutes beyond the initial 74 minutes and document the time spent on the patient.
The details of the coding/billing guidelines are intricate and full of nuances and for a better understanding on how to utilize TM both in an inpatient and outpatient setting, consider the following resources:
1. CHEST Experience presentation entitled "TELE MEDICINE/TELE HEALTH IN THE ERA OF PANDEMIC" at the CHEST Annual Meeting 2020.
1. Coding and Billing Guidelines by ATS:
https://www.thoracic.org/about/newsroom/newsletters/coding-and-billing/resources/2020/mostrecentcbqapril.pdf
2. Coding specific for management of COVID patients by the AMA:
https://www.ama-assn.org/system/files/2020-05/covid-19-coding-advice.pdf
Humayun Anjum, MD, FCCP
Vice-Chair, Practice Operations
Haala Rokadia, MD, FCCP
Practice Operations NetWork Steering Committee Member
Transplant
Physical therapy teleconsultations
The COVID 19 pandemic led the health-care community to rapidly adopt telecommunication tools allowing provision of care equivalent to in-person visits. Implementation of telemedicine visits demonstrated that providers can simultaneously distance and connect with patients to provide expert care.
The University of Pennsylvania lung transplant team adapted video communications to provide individualized physical therapy (PT) recommendations for lung transplantation candidates. The evaluation includes a systems review, musculoskeletal screen, submaximal aerobic capacity testing, and performance of the short physical performance battery test (SPPBT), a frequently used frailty evaluation tool focused on lower extremity function and balance. In the era of social distancing, telemedicine capabilities have made this crucial aspect of pretransplant evaluation possible.
In advance, patients are emailed a document outlining the telemedicine PT assessment, including the SPPBT. Patients receive videos of the SPPBT to ensure they understand the test and can prepare their home to safely perform the tasks. We are able to highlight the patient's functional capabilities and detail accurate assessments of their deficits. Our teleconsultations utilize BlueJeans for connectivity and typically last about 30 minutes. At this time, we are billing for these pretransplant visits but not for posttransplant PT follow-up.
Patient experiences with the PT teleconsultations have been overwhelmingly positive. Patients and their families appreciate the uninterrupted evaluation time and the individualized recommendations for improving their deficits. The providers can devote their full attention to the patient directly in front of them. Importantly, patients and providers report they have never felt a stronger connection than through these telemedicine encounters. Longitudinal telemedicine PT assessments will enable us to better monitor our patients throughout the lung transplantation process.
Joshua Diamond, MD
Steering Committee Member
Derek Zaleski, PT, DPT
Women's Lung Health
SARS-COV-2 and pregnancy
The SARS-COV-2 pandemic has brought on many fears and uncertainties with new information emerging daily, including the effect during pregnancy. At the time of this article,however, data pertaining to COVID-19 and pregnancy remain limited. Pregnant women do not seem to have a higher infection rate than the general population. In a correspondence where pregnant women admitted for delivery underwent universal screening in NY, 1.9% of women were symptomatic and tested positive, and 13.7% of the asymptomatic patients were found to be SARS-COV-2 positive.1 Furthermore, unlike H1NI, data suggest that pregnant women infected with SARS-COV-2 currently do not seem to have worse outcomes than the average person.2,3 As of now, there have not been any reports of maternal fetal vertical transmission from COVID-19 or any other coronavirus variants.4 Postpartum testing of infants has yielded a very small number of babies who have tested positive for virus, but this more likely represents transmission after birth. There are currently no specific FDA-approved medications for the treatment of moderate-severe infections with COVID-19 in pregnant women, although there are several clinical trials underway. Patients with moderate to severe symptoms should seek medical attention, while those with mild symptoms should continue with conservative therapies, as well as maintaining proper hygiene.5 Delivery methods and timing remain unchanged with cesarean delivery as currently indicated per established guidelines.5
Mariam Louis, MD
Steering Committee Member
Jorge Trabanco, MD
1. N Engl J Med. 2020 Apr 13;382:2163-4. April 13, 2020, DOI: 10.1056/NEJMc2009316
2. N Engl J Med. 2020 Jun 18; 382:e100. April 17, 2020 DOI: 10.1056/NEJMc2009226
3. Acta Obstet Gynecol Scand. 2020 Jul;99(7):823-829. 2020 Apr 7. doi: 10.1111/aogs.13867. [Epub ahead of print]
4. Arch Pathol Lab Med. 2020 Apr 27. doi: 10.5858/arpa.2020-0211-SA. [Epub ahead of print]
5. ACOG practice advisory, Novel Coronavirus 2019 (COVID-19) April 23, 2020.
Preparation is key for disaster management. It includes identifying heath-care worker capability, surge capacity, disposable medical resources, and expert consultation availability.
Staff
In disaster, the hospital transitions to a mass casualty strategy, repurposing noncritical care staff to a tiered critical care model focusing on disaster triage and mass critical care. The goal is to provide care to minimize mortality.
Stuff
Critical care supplies improve survival and are implemented quickly and easily. Essential supplies include personal protective equipment, basic modes of mechanical ventilation, hemodynamic support, antimicrobial therapy or other disease-specific countermeasures, oxygen, and prophylactic treatments.
Structure
Disaster critical care can be delivered in noncritical care areas. Hospital policies should establish surge capacity strategies.
System
Providing quality lifesaving care to appropriately triaged patients by utilizing minimal qualifications for survival, predetermined ICU admission criteria, and dynamic protocols using the highest level of evidence available scalable to local resources.
Inappropriate triage results in suboptimal care and can lead to increased mortality.
Virtual critical care can augment critical care capacity and capability.
The implementation of mass critical care requires hospitals to rapidly increase its patient volume above its normal capacity. The essential four components are staff, stuff, space, and structure. Effective mass critical care requires a different mindset than critical care in day-to-day operations.
Patrick Moon, MD; and Alexis MacDonald, MD
(Drs. Reed and Tripp's Fellows)
Mary Jane Reed, MD, FCCP, and Michael Tripp, MD, FCCP
Steering Committee Members
Practice Operations
Over the years since telemedicine (TM) was developed in the 1960s, it has transformed into more mobile, compact, and interconnected forms. However, its widespread adoption has been limited by the regulatory, compensatory, and licensing status quo. The emergence of the COVID-19 pandemic and its necessity for physical distancing has brought TM into the limelight. With restrictions on TM use lifted by CMS, the scope of TM could extend from outpatient to inpatient care to emergency triaging and management of chronic medical conditions.
In February 2020, the comprehensive 2020 COVID-19 ICD 10 coding guidelines were released. To date, CMS has approved approximately 80 codes, which can be used with telehealth and non face-face-to-face (NFTF) encounters. They include telephone calls, online digital E/M services, interprofessional telephone/internet/electric health record consultations, digitally stored data services/remote physiologic monitoring, remote reporting of self-measure blood pressure, and remote physiologic monitoring treatment management services. Some of the key "rules of the game" are highlighted below.
- For telephone visits in the outpatient setting use the codes 99441 (5-10 minutes), 99442 (11-20 minutes), and 99443 (21-30 minutes).
- For interactive real-time audio and video telecommunication (RAVT) in the outpatient setting, use the codes normally used for outpatient E/M: 99201-99215.
- For using RAVT to perform an initial visit for an inpatient, use the codes that are normally used for inpatient E/M: 99221-99223.
- For using RAVT to perform a subsequent visit for an inpatient, use the codes that are normally used for subsequent hospital care service E/M: 99231-99233.
- Seeing a critically ill patient without being in the patient's room is allowed, as a physical exam is not required for either 99291 or 99292. Be sure to use 99292 for each 30 minutes beyond the initial 74 minutes and document the time spent on the patient.
The details of the coding/billing guidelines are intricate and full of nuances and for a better understanding on how to utilize TM both in an inpatient and outpatient setting, consider the following resources:
1. CHEST Experience presentation entitled "TELE MEDICINE/TELE HEALTH IN THE ERA OF PANDEMIC" at the CHEST Annual Meeting 2020.
1. Coding and Billing Guidelines by ATS:
https://www.thoracic.org/about/newsroom/newsletters/coding-and-billing/resources/2020/mostrecentcbqapril.pdf
2. Coding specific for management of COVID patients by the AMA:
https://www.ama-assn.org/system/files/2020-05/covid-19-coding-advice.pdf
Humayun Anjum, MD, FCCP
Vice-Chair, Practice Operations
Haala Rokadia, MD, FCCP
Practice Operations NetWork Steering Committee Member
Transplant
Physical therapy teleconsultations
The COVID 19 pandemic led the health-care community to rapidly adopt telecommunication tools allowing provision of care equivalent to in-person visits. Implementation of telemedicine visits demonstrated that providers can simultaneously distance and connect with patients to provide expert care.
The University of Pennsylvania lung transplant team adapted video communications to provide individualized physical therapy (PT) recommendations for lung transplantation candidates. The evaluation includes a systems review, musculoskeletal screen, submaximal aerobic capacity testing, and performance of the short physical performance battery test (SPPBT), a frequently used frailty evaluation tool focused on lower extremity function and balance. In the era of social distancing, telemedicine capabilities have made this crucial aspect of pretransplant evaluation possible.
In advance, patients are emailed a document outlining the telemedicine PT assessment, including the SPPBT. Patients receive videos of the SPPBT to ensure they understand the test and can prepare their home to safely perform the tasks. We are able to highlight the patient's functional capabilities and detail accurate assessments of their deficits. Our teleconsultations utilize BlueJeans for connectivity and typically last about 30 minutes. At this time, we are billing for these pretransplant visits but not for posttransplant PT follow-up.
Patient experiences with the PT teleconsultations have been overwhelmingly positive. Patients and their families appreciate the uninterrupted evaluation time and the individualized recommendations for improving their deficits. The providers can devote their full attention to the patient directly in front of them. Importantly, patients and providers report they have never felt a stronger connection than through these telemedicine encounters. Longitudinal telemedicine PT assessments will enable us to better monitor our patients throughout the lung transplantation process.
Joshua Diamond, MD
Steering Committee Member
Derek Zaleski, PT, DPT
Women's Lung Health
SARS-COV-2 and pregnancy
The SARS-COV-2 pandemic has brought on many fears and uncertainties with new information emerging daily, including the effect during pregnancy. At the time of this article,however, data pertaining to COVID-19 and pregnancy remain limited. Pregnant women do not seem to have a higher infection rate than the general population. In a correspondence where pregnant women admitted for delivery underwent universal screening in NY, 1.9% of women were symptomatic and tested positive, and 13.7% of the asymptomatic patients were found to be SARS-COV-2 positive.1 Furthermore, unlike H1NI, data suggest that pregnant women infected with SARS-COV-2 currently do not seem to have worse outcomes than the average person.2,3 As of now, there have not been any reports of maternal fetal vertical transmission from COVID-19 or any other coronavirus variants.4 Postpartum testing of infants has yielded a very small number of babies who have tested positive for virus, but this more likely represents transmission after birth. There are currently no specific FDA-approved medications for the treatment of moderate-severe infections with COVID-19 in pregnant women, although there are several clinical trials underway. Patients with moderate to severe symptoms should seek medical attention, while those with mild symptoms should continue with conservative therapies, as well as maintaining proper hygiene.5 Delivery methods and timing remain unchanged with cesarean delivery as currently indicated per established guidelines.5
Mariam Louis, MD
Steering Committee Member
Jorge Trabanco, MD
1. N Engl J Med. 2020 Apr 13;382:2163-4. April 13, 2020, DOI: 10.1056/NEJMc2009316
2. N Engl J Med. 2020 Jun 18; 382:e100. April 17, 2020 DOI: 10.1056/NEJMc2009226
3. Acta Obstet Gynecol Scand. 2020 Jul;99(7):823-829. 2020 Apr 7. doi: 10.1111/aogs.13867. [Epub ahead of print]
4. Arch Pathol Lab Med. 2020 Apr 27. doi: 10.5858/arpa.2020-0211-SA. [Epub ahead of print]
5. ACOG practice advisory, Novel Coronavirus 2019 (COVID-19) April 23, 2020.
Preparation is key for disaster management. It includes identifying heath-care worker capability, surge capacity, disposable medical resources, and expert consultation availability.
Staff
In disaster, the hospital transitions to a mass casualty strategy, repurposing noncritical care staff to a tiered critical care model focusing on disaster triage and mass critical care. The goal is to provide care to minimize mortality.
Stuff
Critical care supplies improve survival and are implemented quickly and easily. Essential supplies include personal protective equipment, basic modes of mechanical ventilation, hemodynamic support, antimicrobial therapy or other disease-specific countermeasures, oxygen, and prophylactic treatments.
Structure
Disaster critical care can be delivered in noncritical care areas. Hospital policies should establish surge capacity strategies.
System
Providing quality lifesaving care to appropriately triaged patients by utilizing minimal qualifications for survival, predetermined ICU admission criteria, and dynamic protocols using the highest level of evidence available scalable to local resources.
Inappropriate triage results in suboptimal care and can lead to increased mortality.
Virtual critical care can augment critical care capacity and capability.
The implementation of mass critical care requires hospitals to rapidly increase its patient volume above its normal capacity. The essential four components are staff, stuff, space, and structure. Effective mass critical care requires a different mindset than critical care in day-to-day operations.
Patrick Moon, MD; and Alexis MacDonald, MD
(Drs. Reed and Tripp's Fellows)
Mary Jane Reed, MD, FCCP, and Michael Tripp, MD, FCCP
Steering Committee Members
Practice Operations
Over the years since telemedicine (TM) was developed in the 1960s, it has transformed into more mobile, compact, and interconnected forms. However, its widespread adoption has been limited by the regulatory, compensatory, and licensing status quo. The emergence of the COVID-19 pandemic and its necessity for physical distancing has brought TM into the limelight. With restrictions on TM use lifted by CMS, the scope of TM could extend from outpatient to inpatient care to emergency triaging and management of chronic medical conditions.
In February 2020, the comprehensive 2020 COVID-19 ICD 10 coding guidelines were released. To date, CMS has approved approximately 80 codes, which can be used with telehealth and non face-face-to-face (NFTF) encounters. They include telephone calls, online digital E/M services, interprofessional telephone/internet/electric health record consultations, digitally stored data services/remote physiologic monitoring, remote reporting of self-measure blood pressure, and remote physiologic monitoring treatment management services. Some of the key "rules of the game" are highlighted below.
- For telephone visits in the outpatient setting use the codes 99441 (5-10 minutes), 99442 (11-20 minutes), and 99443 (21-30 minutes).
- For interactive real-time audio and video telecommunication (RAVT) in the outpatient setting, use the codes normally used for outpatient E/M: 99201-99215.
- For using RAVT to perform an initial visit for an inpatient, use the codes that are normally used for inpatient E/M: 99221-99223.
- For using RAVT to perform a subsequent visit for an inpatient, use the codes that are normally used for subsequent hospital care service E/M: 99231-99233.
- Seeing a critically ill patient without being in the patient's room is allowed, as a physical exam is not required for either 99291 or 99292. Be sure to use 99292 for each 30 minutes beyond the initial 74 minutes and document the time spent on the patient.
The details of the coding/billing guidelines are intricate and full of nuances and for a better understanding on how to utilize TM both in an inpatient and outpatient setting, consider the following resources:
1. CHEST Experience presentation entitled "TELE MEDICINE/TELE HEALTH IN THE ERA OF PANDEMIC" at the CHEST Annual Meeting 2020.
1. Coding and Billing Guidelines by ATS:
https://www.thoracic.org/about/newsroom/newsletters/coding-and-billing/resources/2020/mostrecentcbqapril.pdf
2. Coding specific for management of COVID patients by the AMA:
https://www.ama-assn.org/system/files/2020-05/covid-19-coding-advice.pdf
Humayun Anjum, MD, FCCP
Vice-Chair, Practice Operations
Haala Rokadia, MD, FCCP
Practice Operations NetWork Steering Committee Member
Transplant
Physical therapy teleconsultations
The COVID 19 pandemic led the health-care community to rapidly adopt telecommunication tools allowing provision of care equivalent to in-person visits. Implementation of telemedicine visits demonstrated that providers can simultaneously distance and connect with patients to provide expert care.
The University of Pennsylvania lung transplant team adapted video communications to provide individualized physical therapy (PT) recommendations for lung transplantation candidates. The evaluation includes a systems review, musculoskeletal screen, submaximal aerobic capacity testing, and performance of the short physical performance battery test (SPPBT), a frequently used frailty evaluation tool focused on lower extremity function and balance. In the era of social distancing, telemedicine capabilities have made this crucial aspect of pretransplant evaluation possible.
In advance, patients are emailed a document outlining the telemedicine PT assessment, including the SPPBT. Patients receive videos of the SPPBT to ensure they understand the test and can prepare their home to safely perform the tasks. We are able to highlight the patient's functional capabilities and detail accurate assessments of their deficits. Our teleconsultations utilize BlueJeans for connectivity and typically last about 30 minutes. At this time, we are billing for these pretransplant visits but not for posttransplant PT follow-up.
Patient experiences with the PT teleconsultations have been overwhelmingly positive. Patients and their families appreciate the uninterrupted evaluation time and the individualized recommendations for improving their deficits. The providers can devote their full attention to the patient directly in front of them. Importantly, patients and providers report they have never felt a stronger connection than through these telemedicine encounters. Longitudinal telemedicine PT assessments will enable us to better monitor our patients throughout the lung transplantation process.
Joshua Diamond, MD
Steering Committee Member
Derek Zaleski, PT, DPT
Women's Lung Health
SARS-COV-2 and pregnancy
The SARS-COV-2 pandemic has brought on many fears and uncertainties with new information emerging daily, including the effect during pregnancy. At the time of this article,however, data pertaining to COVID-19 and pregnancy remain limited. Pregnant women do not seem to have a higher infection rate than the general population. In a correspondence where pregnant women admitted for delivery underwent universal screening in NY, 1.9% of women were symptomatic and tested positive, and 13.7% of the asymptomatic patients were found to be SARS-COV-2 positive.1 Furthermore, unlike H1NI, data suggest that pregnant women infected with SARS-COV-2 currently do not seem to have worse outcomes than the average person.2,3 As of now, there have not been any reports of maternal fetal vertical transmission from COVID-19 or any other coronavirus variants.4 Postpartum testing of infants has yielded a very small number of babies who have tested positive for virus, but this more likely represents transmission after birth. There are currently no specific FDA-approved medications for the treatment of moderate-severe infections with COVID-19 in pregnant women, although there are several clinical trials underway. Patients with moderate to severe symptoms should seek medical attention, while those with mild symptoms should continue with conservative therapies, as well as maintaining proper hygiene.5 Delivery methods and timing remain unchanged with cesarean delivery as currently indicated per established guidelines.5
Mariam Louis, MD
Steering Committee Member
Jorge Trabanco, MD
1. N Engl J Med. 2020 Apr 13;382:2163-4. April 13, 2020, DOI: 10.1056/NEJMc2009316
2. N Engl J Med. 2020 Jun 18; 382:e100. April 17, 2020 DOI: 10.1056/NEJMc2009226
3. Acta Obstet Gynecol Scand. 2020 Jul;99(7):823-829. 2020 Apr 7. doi: 10.1111/aogs.13867. [Epub ahead of print]
4. Arch Pathol Lab Med. 2020 Apr 27. doi: 10.5858/arpa.2020-0211-SA. [Epub ahead of print]
5. ACOG practice advisory, Novel Coronavirus 2019 (COVID-19) April 23, 2020.
Telehealth in the COVID-19 era: The New York experience
Big data scientists and health-care experts have tried preparing physicians and patients for the arrival of telemedicine for years. Health tracking applications are on our smartphones. Compact ambulatory devices diagnose hypertension and atrial fibrillation. Advanced imaging modalities make the stethoscope more of a neck accessory than a practical tool. Despite these efficient technologic advancements, the idea of making the sacred in-person office visit remote and through a screen appealed to few. In fact, prior to the COVID-19 pandemic, only 15% of medical practices offered telehealth services and 8% of Americans joined in remote visits annually (Mann DM et al. J Am Med Inform Assoc. 2019 Feb 1;26[2]:106-114).
When the COVID-19 pandemic hit New York City and admissions for hypoxemic respiratory failure skyrocketed, ED and in-person clinic visits for other acute and chronic conditions plummeted. Prior to clinics officially closing their doors, doctors in New York City asked their patients to reserve office visits for emergency issues only ,with most patients willingly staying home to avoid exposure to the virus. Suddenly, after years of disinterest in adopting telehealth, hospitals and clinics were catapulted into a full-on need for this technology. Overnight, our division’s secretaries and medical assistants became IT support staff. We all learned together what worked, what didn’t work, and how to adapt our workflow to meet everyone’s needs.
Previously, longstanding issues with accessibility and reimbursement presented barriers to widespread adoption of telemedicine. Once the pandemic hit, though, many regulatory changes were quickly made to accommodate telehealth.
Three such changes are worth highlighting (Centers for Medicare and Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. March 30, 2020).
First, patient privacy rules became more lenient. Prior to the pandemic, HIPAA mandated that both doctor and patient use embedded video interfaces with high levels of security. Now, health-care providers can use commonplace video chat applications such as FaceTime, Google Hangouts, Zoom, or Skype to provide telehealth without risk of penalty for HIPAA noncompliance. When connectivity concerns arose with our EMR’s embedded telehealth application, a quick transition to one of these platforms mitigated patient and provider frustration.
Second, prior to the pandemic, some private insurance providers reimbursed for televisits, but there were stipulations on how the visit could be conducted. Now, many of the commercial insurers plus Medicare and Medicaid in New York State reimburse the same amount for televisits as in-person visits (fee-for-service rate). Reimbursement rates of audio-only encounters were increased. If these changes are continued postpandemic, it will have an expansive impact on the future of an outpatient practice.
Third, restrictive government regulations relaxed with regard to telehealth deployment. Gone are the demands on providers and patients to be physically face-to-face. Many colleagues worked from home, safely social distancing.
Even though remote medical visits were a crucial part of flattening the curve during the peak of the pandemic in New York City, the telehealth experience is not without flaws.
An informal survey of providers in our own division garnered diverse and spirited viewpoints about seeing patients remotely. Instead of using a stethoscope to pick up a subtle finding, telehealth visits require the use of our eyes to scan a patient’s home environment for insights explaining their chronic cough (Where is the mold? Where is the water damage? Where is the bird?). We use our ears to hear the intonation of our patient’s voice to know when he or she is concerned, anxious, or are at their baselines. We would implore patients to put on their pulse oximeter and perform activities of daily living and/or exertion. On multiple occasions, patients would perform their own, unsolicited walks about their home to show us what they could and couldn’t do, where they place their concentrators, and where they are likely to trip over oxygen tubing. We learned to depend on them to reach the conclusion that they were at their normal state of health.
For straight-forward encounters with existing patients, most of our colleagues appreciated the simplicity and efficiency of telemedicine. But when it came to new patients, some colleagues struggled with whether they should see them for the first time over video. Universally, providers felt feelings of inadequacy without an in-person examination and review of diagnostic information.
Along those lines, many of our colleagues worried about their ability to perform the most fundamental role of a physician over the phone/internet for all patients: building trust with a patient. Eye contact, the physical exam, and verbal and nonv
Providers also noted that telehealth implementation is not the same for all individuals. Just as COVID-19 disproportionately affects the most vulnerable populations (NYC Health. COVID-19: data. Accessed July 1, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page), practicing telehealth has uncovered more ways in which racial/ethnic minorities, low income communities, and older patients are at a disadvantage (Garg S, et al. MMWR Morb Mortal Wkly Rep. 2020;69[15]:458). The relatively quick transition to telemedicine revealed that many of our patients don’t have emails or home computers to connect with online platforms. Similarly, some do not have smart phones with internet capabilities. Many do not speak English and cannot partake in video visits since translators are not yet embedded into the EMR’s video system. Elderly patients were frequently very anxious with telemedicine because of unfamiliarity with the technology, and many preferred a phone conversation. Thus, while more fortunate patients get to use a video interface and its association with higher patient understanding and satisfaction, our most vulnerable populations are often denied the same access to such care (Voils CI et al. J Genet Couns. 2018;27[2]:339).
Telemedicine will continue to have a significant impact on the future of health care long after the COVID-19 pandemic abates. There will be growing pains, refinement of technology, improvements in policy, and an ongoing general evolution of the system. Patients and providers will grow together as its utilization continues. We suspect patient surveys about their attitudes and preferences for telemedicine will be as varied as the providers surveyed here. A recent survey of 1000 patients about their telehealth experiences during the pandemic reported that over 75% were very or completely satisfied with their virtual care experiences and over 50% indicated they would be willing to switch providers to have virtual visits on a regular basis (Patient Perspectives on Virtual Care Report, Accessed July 7, 2020, https://www.kyruus.com/2020-virtual-care-report).
One hopes that with time and on-going feedback, the fundamental purpose of the physician-patient relationship can be maintained and both sides can still appreciate the conveniences and power of telehealth technology.
Dr. Fedyna and Dr. McGroder are affiliated with the Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Medical Center, New York, NY.
Big data scientists and health-care experts have tried preparing physicians and patients for the arrival of telemedicine for years. Health tracking applications are on our smartphones. Compact ambulatory devices diagnose hypertension and atrial fibrillation. Advanced imaging modalities make the stethoscope more of a neck accessory than a practical tool. Despite these efficient technologic advancements, the idea of making the sacred in-person office visit remote and through a screen appealed to few. In fact, prior to the COVID-19 pandemic, only 15% of medical practices offered telehealth services and 8% of Americans joined in remote visits annually (Mann DM et al. J Am Med Inform Assoc. 2019 Feb 1;26[2]:106-114).
When the COVID-19 pandemic hit New York City and admissions for hypoxemic respiratory failure skyrocketed, ED and in-person clinic visits for other acute and chronic conditions plummeted. Prior to clinics officially closing their doors, doctors in New York City asked their patients to reserve office visits for emergency issues only ,with most patients willingly staying home to avoid exposure to the virus. Suddenly, after years of disinterest in adopting telehealth, hospitals and clinics were catapulted into a full-on need for this technology. Overnight, our division’s secretaries and medical assistants became IT support staff. We all learned together what worked, what didn’t work, and how to adapt our workflow to meet everyone’s needs.
Previously, longstanding issues with accessibility and reimbursement presented barriers to widespread adoption of telemedicine. Once the pandemic hit, though, many regulatory changes were quickly made to accommodate telehealth.
Three such changes are worth highlighting (Centers for Medicare and Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. March 30, 2020).
First, patient privacy rules became more lenient. Prior to the pandemic, HIPAA mandated that both doctor and patient use embedded video interfaces with high levels of security. Now, health-care providers can use commonplace video chat applications such as FaceTime, Google Hangouts, Zoom, or Skype to provide telehealth without risk of penalty for HIPAA noncompliance. When connectivity concerns arose with our EMR’s embedded telehealth application, a quick transition to one of these platforms mitigated patient and provider frustration.
Second, prior to the pandemic, some private insurance providers reimbursed for televisits, but there were stipulations on how the visit could be conducted. Now, many of the commercial insurers plus Medicare and Medicaid in New York State reimburse the same amount for televisits as in-person visits (fee-for-service rate). Reimbursement rates of audio-only encounters were increased. If these changes are continued postpandemic, it will have an expansive impact on the future of an outpatient practice.
Third, restrictive government regulations relaxed with regard to telehealth deployment. Gone are the demands on providers and patients to be physically face-to-face. Many colleagues worked from home, safely social distancing.
Even though remote medical visits were a crucial part of flattening the curve during the peak of the pandemic in New York City, the telehealth experience is not without flaws.
An informal survey of providers in our own division garnered diverse and spirited viewpoints about seeing patients remotely. Instead of using a stethoscope to pick up a subtle finding, telehealth visits require the use of our eyes to scan a patient’s home environment for insights explaining their chronic cough (Where is the mold? Where is the water damage? Where is the bird?). We use our ears to hear the intonation of our patient’s voice to know when he or she is concerned, anxious, or are at their baselines. We would implore patients to put on their pulse oximeter and perform activities of daily living and/or exertion. On multiple occasions, patients would perform their own, unsolicited walks about their home to show us what they could and couldn’t do, where they place their concentrators, and where they are likely to trip over oxygen tubing. We learned to depend on them to reach the conclusion that they were at their normal state of health.
For straight-forward encounters with existing patients, most of our colleagues appreciated the simplicity and efficiency of telemedicine. But when it came to new patients, some colleagues struggled with whether they should see them for the first time over video. Universally, providers felt feelings of inadequacy without an in-person examination and review of diagnostic information.
Along those lines, many of our colleagues worried about their ability to perform the most fundamental role of a physician over the phone/internet for all patients: building trust with a patient. Eye contact, the physical exam, and verbal and nonv
Providers also noted that telehealth implementation is not the same for all individuals. Just as COVID-19 disproportionately affects the most vulnerable populations (NYC Health. COVID-19: data. Accessed July 1, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page), practicing telehealth has uncovered more ways in which racial/ethnic minorities, low income communities, and older patients are at a disadvantage (Garg S, et al. MMWR Morb Mortal Wkly Rep. 2020;69[15]:458). The relatively quick transition to telemedicine revealed that many of our patients don’t have emails or home computers to connect with online platforms. Similarly, some do not have smart phones with internet capabilities. Many do not speak English and cannot partake in video visits since translators are not yet embedded into the EMR’s video system. Elderly patients were frequently very anxious with telemedicine because of unfamiliarity with the technology, and many preferred a phone conversation. Thus, while more fortunate patients get to use a video interface and its association with higher patient understanding and satisfaction, our most vulnerable populations are often denied the same access to such care (Voils CI et al. J Genet Couns. 2018;27[2]:339).
Telemedicine will continue to have a significant impact on the future of health care long after the COVID-19 pandemic abates. There will be growing pains, refinement of technology, improvements in policy, and an ongoing general evolution of the system. Patients and providers will grow together as its utilization continues. We suspect patient surveys about their attitudes and preferences for telemedicine will be as varied as the providers surveyed here. A recent survey of 1000 patients about their telehealth experiences during the pandemic reported that over 75% were very or completely satisfied with their virtual care experiences and over 50% indicated they would be willing to switch providers to have virtual visits on a regular basis (Patient Perspectives on Virtual Care Report, Accessed July 7, 2020, https://www.kyruus.com/2020-virtual-care-report).
One hopes that with time and on-going feedback, the fundamental purpose of the physician-patient relationship can be maintained and both sides can still appreciate the conveniences and power of telehealth technology.
Dr. Fedyna and Dr. McGroder are affiliated with the Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Medical Center, New York, NY.
Big data scientists and health-care experts have tried preparing physicians and patients for the arrival of telemedicine for years. Health tracking applications are on our smartphones. Compact ambulatory devices diagnose hypertension and atrial fibrillation. Advanced imaging modalities make the stethoscope more of a neck accessory than a practical tool. Despite these efficient technologic advancements, the idea of making the sacred in-person office visit remote and through a screen appealed to few. In fact, prior to the COVID-19 pandemic, only 15% of medical practices offered telehealth services and 8% of Americans joined in remote visits annually (Mann DM et al. J Am Med Inform Assoc. 2019 Feb 1;26[2]:106-114).
When the COVID-19 pandemic hit New York City and admissions for hypoxemic respiratory failure skyrocketed, ED and in-person clinic visits for other acute and chronic conditions plummeted. Prior to clinics officially closing their doors, doctors in New York City asked their patients to reserve office visits for emergency issues only ,with most patients willingly staying home to avoid exposure to the virus. Suddenly, after years of disinterest in adopting telehealth, hospitals and clinics were catapulted into a full-on need for this technology. Overnight, our division’s secretaries and medical assistants became IT support staff. We all learned together what worked, what didn’t work, and how to adapt our workflow to meet everyone’s needs.
Previously, longstanding issues with accessibility and reimbursement presented barriers to widespread adoption of telemedicine. Once the pandemic hit, though, many regulatory changes were quickly made to accommodate telehealth.
Three such changes are worth highlighting (Centers for Medicare and Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. March 30, 2020).
First, patient privacy rules became more lenient. Prior to the pandemic, HIPAA mandated that both doctor and patient use embedded video interfaces with high levels of security. Now, health-care providers can use commonplace video chat applications such as FaceTime, Google Hangouts, Zoom, or Skype to provide telehealth without risk of penalty for HIPAA noncompliance. When connectivity concerns arose with our EMR’s embedded telehealth application, a quick transition to one of these platforms mitigated patient and provider frustration.
Second, prior to the pandemic, some private insurance providers reimbursed for televisits, but there were stipulations on how the visit could be conducted. Now, many of the commercial insurers plus Medicare and Medicaid in New York State reimburse the same amount for televisits as in-person visits (fee-for-service rate). Reimbursement rates of audio-only encounters were increased. If these changes are continued postpandemic, it will have an expansive impact on the future of an outpatient practice.
Third, restrictive government regulations relaxed with regard to telehealth deployment. Gone are the demands on providers and patients to be physically face-to-face. Many colleagues worked from home, safely social distancing.
Even though remote medical visits were a crucial part of flattening the curve during the peak of the pandemic in New York City, the telehealth experience is not without flaws.
An informal survey of providers in our own division garnered diverse and spirited viewpoints about seeing patients remotely. Instead of using a stethoscope to pick up a subtle finding, telehealth visits require the use of our eyes to scan a patient’s home environment for insights explaining their chronic cough (Where is the mold? Where is the water damage? Where is the bird?). We use our ears to hear the intonation of our patient’s voice to know when he or she is concerned, anxious, or are at their baselines. We would implore patients to put on their pulse oximeter and perform activities of daily living and/or exertion. On multiple occasions, patients would perform their own, unsolicited walks about their home to show us what they could and couldn’t do, where they place their concentrators, and where they are likely to trip over oxygen tubing. We learned to depend on them to reach the conclusion that they were at their normal state of health.
For straight-forward encounters with existing patients, most of our colleagues appreciated the simplicity and efficiency of telemedicine. But when it came to new patients, some colleagues struggled with whether they should see them for the first time over video. Universally, providers felt feelings of inadequacy without an in-person examination and review of diagnostic information.
Along those lines, many of our colleagues worried about their ability to perform the most fundamental role of a physician over the phone/internet for all patients: building trust with a patient. Eye contact, the physical exam, and verbal and nonv
Providers also noted that telehealth implementation is not the same for all individuals. Just as COVID-19 disproportionately affects the most vulnerable populations (NYC Health. COVID-19: data. Accessed July 1, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page), practicing telehealth has uncovered more ways in which racial/ethnic minorities, low income communities, and older patients are at a disadvantage (Garg S, et al. MMWR Morb Mortal Wkly Rep. 2020;69[15]:458). The relatively quick transition to telemedicine revealed that many of our patients don’t have emails or home computers to connect with online platforms. Similarly, some do not have smart phones with internet capabilities. Many do not speak English and cannot partake in video visits since translators are not yet embedded into the EMR’s video system. Elderly patients were frequently very anxious with telemedicine because of unfamiliarity with the technology, and many preferred a phone conversation. Thus, while more fortunate patients get to use a video interface and its association with higher patient understanding and satisfaction, our most vulnerable populations are often denied the same access to such care (Voils CI et al. J Genet Couns. 2018;27[2]:339).
Telemedicine will continue to have a significant impact on the future of health care long after the COVID-19 pandemic abates. There will be growing pains, refinement of technology, improvements in policy, and an ongoing general evolution of the system. Patients and providers will grow together as its utilization continues. We suspect patient surveys about their attitudes and preferences for telemedicine will be as varied as the providers surveyed here. A recent survey of 1000 patients about their telehealth experiences during the pandemic reported that over 75% were very or completely satisfied with their virtual care experiences and over 50% indicated they would be willing to switch providers to have virtual visits on a regular basis (Patient Perspectives on Virtual Care Report, Accessed July 7, 2020, https://www.kyruus.com/2020-virtual-care-report).
One hopes that with time and on-going feedback, the fundamental purpose of the physician-patient relationship can be maintained and both sides can still appreciate the conveniences and power of telehealth technology.
Dr. Fedyna and Dr. McGroder are affiliated with the Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Medical Center, New York, NY.
Management of EVALI in the ICU
Since 2019, more than 2,700 individuals have been hospitalized with electronic cigarette- (e-cigarette), or vaping-associated lung injury (EVALI). This entity first reached clinical attention after a series of otherwise healthy young adults presented with dyspnea, severe hypoxia, and diffuse pulmonary infiltrates in the Midwest (Layden J, et al. N Engl J Med. 2020;382[10]:903). Investigation of these cases revealed an association with the use of e-cigarettes, or vaping. As cases continued to mount, the link between vaping and acute lung injury became increasingly apparent.
How it presents
EVALI can present in variable ways, ranging from mild cough or dyspnea without hypoxia to severe acute respiratory distress syndrome (ARDS), requiring advanced life support. Although challenging in the ICU setting, obtaining a detailed history of vaping is crucial to make the diagnosis. Collateral history can be helpful, but if unrevealing, it should not be considered sufficient to exclude vaping as potential etiology, particularly in adolescent e-cigarette users, where parental awareness of substance use history may be limited. If a vaping history is obtained, it is important to assess the substance(s) vaped, how these substances were obtained, and methods of inhalation. While e-cigarettes are the most commonly recognized method of vaping, alternate methods such as “dabbing” and “dripping,” are increasingly popular among vape users, often utilizing modified e-liquid components that may not be reported by patients unless specifically queried.
About 82% of patients hospitalized with EVALI reported vaping tetrahydrocannabinol- (THC) containing fluid. This is important because, unlike nicotine based e-liquids that are primarily purchased over the counter, more than 70% of THC-containing e-liquids are reportedly obtained through informal sources, including illegal distributors. In contrast, only 14% of patients hospitalized with EVALI reported vaping of commercial nicotine products alone. Nicotine-based e-liquids can also be modified, and informal purchasing sources remain a concern, particularly among younger users.
The onset of respiratory symptoms in EVALI is often preceded by several days of a systemic prodrome, including low-grade fevers, myalgia, gastrointestinal complaints, and fatigue (MacMurdo M, et al. Chest. 2020;157[6]:e181). The diagnosis of EVALI is made clinically, and alternative etiologies of lung injury (eg, infections) should be excluded. As there is significant overlap between the presenting symptoms of EVALI and COVID-19 infection, patients should be tested for COVID-19 before a diagnosis of EVALI can be made.
Imaging patterns of EVALI include diffuse alveolar damage (the most common), comprising of diffuse ground-glass opacities, septal thickening, and heterogeneous consolidation (MacMurdo M, et al. Chest. 2020;157[6]:e181). Bilateral ground glass opacities suggestive of organizing pneumonia have also been described. Atypical patterns of nodularity suggestive of hypersensitivity pneumonitis are significantly less common. Given the variety of imaging patterns, EVALI should be considered as a differential diagnosis in all patients presenting with new bilateral pulmonary infiltrates and severe hypoxia.
Early evaluation of these patients revealed lipid-laden macrophages in the bronchoalveolar lavage (BAL) fluid of these patients, raising concern for exogenous lipid inhalation resulting in the development of lipoid pneumonia (Maddock SD, et al. N Engl J Med. 2019;381[15]:1488). Analysis of BAL fluid revealed the presence of vitamin E acetate, a diluent utilized to cut, or dilute, e-liquid (Blount BC, et al. MMWR. 2019;68[45]:1040). This supported the hypothesis that the outbreak of EVALI was being driven, at least in part, by contaminated or self-modified e-liquid. Evaluation of lung biopsies revealed different pathologic patterns of acute lung injury, including diffuse alveolar damage and organizing pneumonia. Importantly, while lipid-laden macrophages were detected, other characteristics of lipoid pneumonia were absent (Mukhopadhyay S, et al. Am J Clin Path. 2019;153[1]30).
How to manage EVALI
Approximately half of patients hospitalized with EVALI required ICU admission. However, there is likely a substantial portion of patients with mild disease who may not be represented in the current registry since they did not require hospitalization. The management is primarily supportive and, in patients who require mechanical ventilation, following lung-protective ventilator strategies is of paramount importance. Steroids have been used in some case series, particularly for patients presenting with more severe disease, but data on benefit, optimal dose, and duration are limited.
Vaping cessation is crucial and should be aggressively encouraged. Newer generations of e-cigarettes contain comparatively higher nicotine concentrations, and likely have high potential for nicotine addiction. Treatment for nicotine dependence, including pharmacologic therapy, needs to be considered in all patients following recovery from EVALI.
With supportive care and removal of ongoing exposure, recovery is anticipated in most patients. Long-term outcomes in patients who develop EVALI remain unclear. Although early fibrosis was present in some patients who had transbronchial biopsies, the long-term effects on pulmonary function that may be seen in patients with a history of EVALI are yet to be determined.
What about policy?
New regulations related to e-cigarette use have been proposed in response to the increasing prevalence of vaping and the EVALI outbreak. These regulations center primarily on limiting adolescent e-cigarette usage. Tobacco 21, federal legislation passed in 2019, makes it illegal to sell tobacco products to those under the age of 21. The FDA also issued an enforcement policy on unauthorized flavored e-cigarette products. However, this has been criticized for not being comprehensive enough. For example, tobacco and menthol flavors were not included in the ban. Furthermore, THC-containing e-liquid remains largely unregulated at the federal level, and state-level regulation varies significantly by marijuana legalization status.
Policy initiatives that restrict sales without also addressing drivers of e-cigarette use, such as nicotine dependence and aggressive marketing campaigns, are of particular concern and are likely to disproportionately impact younger users. Another unintended effect of e-cigarette sales restrictions may result in a new wave of illegal product distribution and e-liquid modification. Supporting this hypothesis was the finding that the risk of EVALI was higher in states without legalized recreational marijuana, suggesting that users who obtained e-liquid through these informal sources were at greater risk of exposure to contaminated product (Wing C, et al. JAMA Netw Open. 2020;3[4]:e202187). While the CDC is no longer actively tracking EVALI cases, they continue to be reported, and vape use remains common (Armatas C, et al. MMWR. 69[25]:801). As long as e-cigarettes remain in use, another EVALI outbreak remains possible.
It remains important for the intensivist to be familiar with the full spectrum of vaping methods, and to report suspected cases when they arise. While treatable, much remains unknown about the long-term effects on this patient population. Further research is needed to better understand the long-term outcomes in patients with EVALI, in addition to the treatment of nicotine dependence and substance use associated with vaping. Finally, comprehensive regulation to curb e-cigarette usage is needed, particularly among adolescents. However, legislation that is too narrow in scope runs the risk of channeling adolescent e-cigarette users to obtain product through informal sources, further increasing their risk for EVALI. As clinicians, we cannot afford to drop our guard!
Dr. Macmurdo and Dr. Choi are with Cleveland Clinic, Respiratory Institute, Cleveland, Ohio.
Since 2019, more than 2,700 individuals have been hospitalized with electronic cigarette- (e-cigarette), or vaping-associated lung injury (EVALI). This entity first reached clinical attention after a series of otherwise healthy young adults presented with dyspnea, severe hypoxia, and diffuse pulmonary infiltrates in the Midwest (Layden J, et al. N Engl J Med. 2020;382[10]:903). Investigation of these cases revealed an association with the use of e-cigarettes, or vaping. As cases continued to mount, the link between vaping and acute lung injury became increasingly apparent.
How it presents
EVALI can present in variable ways, ranging from mild cough or dyspnea without hypoxia to severe acute respiratory distress syndrome (ARDS), requiring advanced life support. Although challenging in the ICU setting, obtaining a detailed history of vaping is crucial to make the diagnosis. Collateral history can be helpful, but if unrevealing, it should not be considered sufficient to exclude vaping as potential etiology, particularly in adolescent e-cigarette users, where parental awareness of substance use history may be limited. If a vaping history is obtained, it is important to assess the substance(s) vaped, how these substances were obtained, and methods of inhalation. While e-cigarettes are the most commonly recognized method of vaping, alternate methods such as “dabbing” and “dripping,” are increasingly popular among vape users, often utilizing modified e-liquid components that may not be reported by patients unless specifically queried.
About 82% of patients hospitalized with EVALI reported vaping tetrahydrocannabinol- (THC) containing fluid. This is important because, unlike nicotine based e-liquids that are primarily purchased over the counter, more than 70% of THC-containing e-liquids are reportedly obtained through informal sources, including illegal distributors. In contrast, only 14% of patients hospitalized with EVALI reported vaping of commercial nicotine products alone. Nicotine-based e-liquids can also be modified, and informal purchasing sources remain a concern, particularly among younger users.
The onset of respiratory symptoms in EVALI is often preceded by several days of a systemic prodrome, including low-grade fevers, myalgia, gastrointestinal complaints, and fatigue (MacMurdo M, et al. Chest. 2020;157[6]:e181). The diagnosis of EVALI is made clinically, and alternative etiologies of lung injury (eg, infections) should be excluded. As there is significant overlap between the presenting symptoms of EVALI and COVID-19 infection, patients should be tested for COVID-19 before a diagnosis of EVALI can be made.
Imaging patterns of EVALI include diffuse alveolar damage (the most common), comprising of diffuse ground-glass opacities, septal thickening, and heterogeneous consolidation (MacMurdo M, et al. Chest. 2020;157[6]:e181). Bilateral ground glass opacities suggestive of organizing pneumonia have also been described. Atypical patterns of nodularity suggestive of hypersensitivity pneumonitis are significantly less common. Given the variety of imaging patterns, EVALI should be considered as a differential diagnosis in all patients presenting with new bilateral pulmonary infiltrates and severe hypoxia.
Early evaluation of these patients revealed lipid-laden macrophages in the bronchoalveolar lavage (BAL) fluid of these patients, raising concern for exogenous lipid inhalation resulting in the development of lipoid pneumonia (Maddock SD, et al. N Engl J Med. 2019;381[15]:1488). Analysis of BAL fluid revealed the presence of vitamin E acetate, a diluent utilized to cut, or dilute, e-liquid (Blount BC, et al. MMWR. 2019;68[45]:1040). This supported the hypothesis that the outbreak of EVALI was being driven, at least in part, by contaminated or self-modified e-liquid. Evaluation of lung biopsies revealed different pathologic patterns of acute lung injury, including diffuse alveolar damage and organizing pneumonia. Importantly, while lipid-laden macrophages were detected, other characteristics of lipoid pneumonia were absent (Mukhopadhyay S, et al. Am J Clin Path. 2019;153[1]30).
How to manage EVALI
Approximately half of patients hospitalized with EVALI required ICU admission. However, there is likely a substantial portion of patients with mild disease who may not be represented in the current registry since they did not require hospitalization. The management is primarily supportive and, in patients who require mechanical ventilation, following lung-protective ventilator strategies is of paramount importance. Steroids have been used in some case series, particularly for patients presenting with more severe disease, but data on benefit, optimal dose, and duration are limited.
Vaping cessation is crucial and should be aggressively encouraged. Newer generations of e-cigarettes contain comparatively higher nicotine concentrations, and likely have high potential for nicotine addiction. Treatment for nicotine dependence, including pharmacologic therapy, needs to be considered in all patients following recovery from EVALI.
With supportive care and removal of ongoing exposure, recovery is anticipated in most patients. Long-term outcomes in patients who develop EVALI remain unclear. Although early fibrosis was present in some patients who had transbronchial biopsies, the long-term effects on pulmonary function that may be seen in patients with a history of EVALI are yet to be determined.
What about policy?
New regulations related to e-cigarette use have been proposed in response to the increasing prevalence of vaping and the EVALI outbreak. These regulations center primarily on limiting adolescent e-cigarette usage. Tobacco 21, federal legislation passed in 2019, makes it illegal to sell tobacco products to those under the age of 21. The FDA also issued an enforcement policy on unauthorized flavored e-cigarette products. However, this has been criticized for not being comprehensive enough. For example, tobacco and menthol flavors were not included in the ban. Furthermore, THC-containing e-liquid remains largely unregulated at the federal level, and state-level regulation varies significantly by marijuana legalization status.
Policy initiatives that restrict sales without also addressing drivers of e-cigarette use, such as nicotine dependence and aggressive marketing campaigns, are of particular concern and are likely to disproportionately impact younger users. Another unintended effect of e-cigarette sales restrictions may result in a new wave of illegal product distribution and e-liquid modification. Supporting this hypothesis was the finding that the risk of EVALI was higher in states without legalized recreational marijuana, suggesting that users who obtained e-liquid through these informal sources were at greater risk of exposure to contaminated product (Wing C, et al. JAMA Netw Open. 2020;3[4]:e202187). While the CDC is no longer actively tracking EVALI cases, they continue to be reported, and vape use remains common (Armatas C, et al. MMWR. 69[25]:801). As long as e-cigarettes remain in use, another EVALI outbreak remains possible.
It remains important for the intensivist to be familiar with the full spectrum of vaping methods, and to report suspected cases when they arise. While treatable, much remains unknown about the long-term effects on this patient population. Further research is needed to better understand the long-term outcomes in patients with EVALI, in addition to the treatment of nicotine dependence and substance use associated with vaping. Finally, comprehensive regulation to curb e-cigarette usage is needed, particularly among adolescents. However, legislation that is too narrow in scope runs the risk of channeling adolescent e-cigarette users to obtain product through informal sources, further increasing their risk for EVALI. As clinicians, we cannot afford to drop our guard!
Dr. Macmurdo and Dr. Choi are with Cleveland Clinic, Respiratory Institute, Cleveland, Ohio.
Since 2019, more than 2,700 individuals have been hospitalized with electronic cigarette- (e-cigarette), or vaping-associated lung injury (EVALI). This entity first reached clinical attention after a series of otherwise healthy young adults presented with dyspnea, severe hypoxia, and diffuse pulmonary infiltrates in the Midwest (Layden J, et al. N Engl J Med. 2020;382[10]:903). Investigation of these cases revealed an association with the use of e-cigarettes, or vaping. As cases continued to mount, the link between vaping and acute lung injury became increasingly apparent.
How it presents
EVALI can present in variable ways, ranging from mild cough or dyspnea without hypoxia to severe acute respiratory distress syndrome (ARDS), requiring advanced life support. Although challenging in the ICU setting, obtaining a detailed history of vaping is crucial to make the diagnosis. Collateral history can be helpful, but if unrevealing, it should not be considered sufficient to exclude vaping as potential etiology, particularly in adolescent e-cigarette users, where parental awareness of substance use history may be limited. If a vaping history is obtained, it is important to assess the substance(s) vaped, how these substances were obtained, and methods of inhalation. While e-cigarettes are the most commonly recognized method of vaping, alternate methods such as “dabbing” and “dripping,” are increasingly popular among vape users, often utilizing modified e-liquid components that may not be reported by patients unless specifically queried.
About 82% of patients hospitalized with EVALI reported vaping tetrahydrocannabinol- (THC) containing fluid. This is important because, unlike nicotine based e-liquids that are primarily purchased over the counter, more than 70% of THC-containing e-liquids are reportedly obtained through informal sources, including illegal distributors. In contrast, only 14% of patients hospitalized with EVALI reported vaping of commercial nicotine products alone. Nicotine-based e-liquids can also be modified, and informal purchasing sources remain a concern, particularly among younger users.
The onset of respiratory symptoms in EVALI is often preceded by several days of a systemic prodrome, including low-grade fevers, myalgia, gastrointestinal complaints, and fatigue (MacMurdo M, et al. Chest. 2020;157[6]:e181). The diagnosis of EVALI is made clinically, and alternative etiologies of lung injury (eg, infections) should be excluded. As there is significant overlap between the presenting symptoms of EVALI and COVID-19 infection, patients should be tested for COVID-19 before a diagnosis of EVALI can be made.
Imaging patterns of EVALI include diffuse alveolar damage (the most common), comprising of diffuse ground-glass opacities, septal thickening, and heterogeneous consolidation (MacMurdo M, et al. Chest. 2020;157[6]:e181). Bilateral ground glass opacities suggestive of organizing pneumonia have also been described. Atypical patterns of nodularity suggestive of hypersensitivity pneumonitis are significantly less common. Given the variety of imaging patterns, EVALI should be considered as a differential diagnosis in all patients presenting with new bilateral pulmonary infiltrates and severe hypoxia.
Early evaluation of these patients revealed lipid-laden macrophages in the bronchoalveolar lavage (BAL) fluid of these patients, raising concern for exogenous lipid inhalation resulting in the development of lipoid pneumonia (Maddock SD, et al. N Engl J Med. 2019;381[15]:1488). Analysis of BAL fluid revealed the presence of vitamin E acetate, a diluent utilized to cut, or dilute, e-liquid (Blount BC, et al. MMWR. 2019;68[45]:1040). This supported the hypothesis that the outbreak of EVALI was being driven, at least in part, by contaminated or self-modified e-liquid. Evaluation of lung biopsies revealed different pathologic patterns of acute lung injury, including diffuse alveolar damage and organizing pneumonia. Importantly, while lipid-laden macrophages were detected, other characteristics of lipoid pneumonia were absent (Mukhopadhyay S, et al. Am J Clin Path. 2019;153[1]30).
How to manage EVALI
Approximately half of patients hospitalized with EVALI required ICU admission. However, there is likely a substantial portion of patients with mild disease who may not be represented in the current registry since they did not require hospitalization. The management is primarily supportive and, in patients who require mechanical ventilation, following lung-protective ventilator strategies is of paramount importance. Steroids have been used in some case series, particularly for patients presenting with more severe disease, but data on benefit, optimal dose, and duration are limited.
Vaping cessation is crucial and should be aggressively encouraged. Newer generations of e-cigarettes contain comparatively higher nicotine concentrations, and likely have high potential for nicotine addiction. Treatment for nicotine dependence, including pharmacologic therapy, needs to be considered in all patients following recovery from EVALI.
With supportive care and removal of ongoing exposure, recovery is anticipated in most patients. Long-term outcomes in patients who develop EVALI remain unclear. Although early fibrosis was present in some patients who had transbronchial biopsies, the long-term effects on pulmonary function that may be seen in patients with a history of EVALI are yet to be determined.
What about policy?
New regulations related to e-cigarette use have been proposed in response to the increasing prevalence of vaping and the EVALI outbreak. These regulations center primarily on limiting adolescent e-cigarette usage. Tobacco 21, federal legislation passed in 2019, makes it illegal to sell tobacco products to those under the age of 21. The FDA also issued an enforcement policy on unauthorized flavored e-cigarette products. However, this has been criticized for not being comprehensive enough. For example, tobacco and menthol flavors were not included in the ban. Furthermore, THC-containing e-liquid remains largely unregulated at the federal level, and state-level regulation varies significantly by marijuana legalization status.
Policy initiatives that restrict sales without also addressing drivers of e-cigarette use, such as nicotine dependence and aggressive marketing campaigns, are of particular concern and are likely to disproportionately impact younger users. Another unintended effect of e-cigarette sales restrictions may result in a new wave of illegal product distribution and e-liquid modification. Supporting this hypothesis was the finding that the risk of EVALI was higher in states without legalized recreational marijuana, suggesting that users who obtained e-liquid through these informal sources were at greater risk of exposure to contaminated product (Wing C, et al. JAMA Netw Open. 2020;3[4]:e202187). While the CDC is no longer actively tracking EVALI cases, they continue to be reported, and vape use remains common (Armatas C, et al. MMWR. 69[25]:801). As long as e-cigarettes remain in use, another EVALI outbreak remains possible.
It remains important for the intensivist to be familiar with the full spectrum of vaping methods, and to report suspected cases when they arise. While treatable, much remains unknown about the long-term effects on this patient population. Further research is needed to better understand the long-term outcomes in patients with EVALI, in addition to the treatment of nicotine dependence and substance use associated with vaping. Finally, comprehensive regulation to curb e-cigarette usage is needed, particularly among adolescents. However, legislation that is too narrow in scope runs the risk of channeling adolescent e-cigarette users to obtain product through informal sources, further increasing their risk for EVALI. As clinicians, we cannot afford to drop our guard!
Dr. Macmurdo and Dr. Choi are with Cleveland Clinic, Respiratory Institute, Cleveland, Ohio.
NetWorks Challenge 2020
The CHEST Foundation is excited to announce that the NetWorks Challenge will be reinvented for 2020! Instead of raising funds to support travel grants to CHEST’s Annual Meeting as in previous years, the NetWorks Challenge will focus on raising funds to support COVID-19 community service grants. With so many people suffering due to the pandemic, we believe this change will make a tangible impact on the lives of people who need it most.
To date, the CHEST Foundation has dispersed over $60,000 in payments for patient support groups that provide services to those living with chronic lung disease, and we hope this year’s efforts will enable us to continue this work. For every $2,500 raised by a NetWork, the CHEST Foundation will provide a grant to a community support group in need.
While providing vulnerable populations with funds to purchase essential items (PPE, cleaning supplies, emergency food purchases, etc), each grant will be named in honor of the NetWork raising the funds, and all stories of impact will be shared with NetWorks’ members, once they are available.
The NetWorks Challenge spans from Monday, July 20, to the end of Board Review on August 22, and members can easily designate their donation to their NetWork on the CHEST Foundation’s donor page.
In addition to receiving named recognition of your NetWork, the NetWork that raises the most funds, along with the NetWork with the highest percentage of participation, will receive additional prizes, including two complimentary registrations to CHEST 2020. These registrations are specifically for early-career clinicians and fellows-in-training, which will be selected by each NetWorks’s steering committee.
For every $5,000 raised by a NetWork, that NetWork will receive one complimentary registration to CHEST 2020, which will be awarded to their early-career and fellows-in-training as selected by that NetWorks’s steering committee.
In addition to directly impacting patients across the United States, NetWorks members will have a chance to test their knowledge against their peers by participating in a NetWork Challenge Game Series, where they will be asked a series of hand-selected board review questions each week through the end of Board Review.
For additional Information about the NetWorks Challenge, visit the CHEST Foundation’s website.
The CHEST Foundation is excited to announce that the NetWorks Challenge will be reinvented for 2020! Instead of raising funds to support travel grants to CHEST’s Annual Meeting as in previous years, the NetWorks Challenge will focus on raising funds to support COVID-19 community service grants. With so many people suffering due to the pandemic, we believe this change will make a tangible impact on the lives of people who need it most.
To date, the CHEST Foundation has dispersed over $60,000 in payments for patient support groups that provide services to those living with chronic lung disease, and we hope this year’s efforts will enable us to continue this work. For every $2,500 raised by a NetWork, the CHEST Foundation will provide a grant to a community support group in need.
While providing vulnerable populations with funds to purchase essential items (PPE, cleaning supplies, emergency food purchases, etc), each grant will be named in honor of the NetWork raising the funds, and all stories of impact will be shared with NetWorks’ members, once they are available.
The NetWorks Challenge spans from Monday, July 20, to the end of Board Review on August 22, and members can easily designate their donation to their NetWork on the CHEST Foundation’s donor page.
In addition to receiving named recognition of your NetWork, the NetWork that raises the most funds, along with the NetWork with the highest percentage of participation, will receive additional prizes, including two complimentary registrations to CHEST 2020. These registrations are specifically for early-career clinicians and fellows-in-training, which will be selected by each NetWorks’s steering committee.
For every $5,000 raised by a NetWork, that NetWork will receive one complimentary registration to CHEST 2020, which will be awarded to their early-career and fellows-in-training as selected by that NetWorks’s steering committee.
In addition to directly impacting patients across the United States, NetWorks members will have a chance to test their knowledge against their peers by participating in a NetWork Challenge Game Series, where they will be asked a series of hand-selected board review questions each week through the end of Board Review.
For additional Information about the NetWorks Challenge, visit the CHEST Foundation’s website.
The CHEST Foundation is excited to announce that the NetWorks Challenge will be reinvented for 2020! Instead of raising funds to support travel grants to CHEST’s Annual Meeting as in previous years, the NetWorks Challenge will focus on raising funds to support COVID-19 community service grants. With so many people suffering due to the pandemic, we believe this change will make a tangible impact on the lives of people who need it most.
To date, the CHEST Foundation has dispersed over $60,000 in payments for patient support groups that provide services to those living with chronic lung disease, and we hope this year’s efforts will enable us to continue this work. For every $2,500 raised by a NetWork, the CHEST Foundation will provide a grant to a community support group in need.
While providing vulnerable populations with funds to purchase essential items (PPE, cleaning supplies, emergency food purchases, etc), each grant will be named in honor of the NetWork raising the funds, and all stories of impact will be shared with NetWorks’ members, once they are available.
The NetWorks Challenge spans from Monday, July 20, to the end of Board Review on August 22, and members can easily designate their donation to their NetWork on the CHEST Foundation’s donor page.
In addition to receiving named recognition of your NetWork, the NetWork that raises the most funds, along with the NetWork with the highest percentage of participation, will receive additional prizes, including two complimentary registrations to CHEST 2020. These registrations are specifically for early-career clinicians and fellows-in-training, which will be selected by each NetWorks’s steering committee.
For every $5,000 raised by a NetWork, that NetWork will receive one complimentary registration to CHEST 2020, which will be awarded to their early-career and fellows-in-training as selected by that NetWorks’s steering committee.
In addition to directly impacting patients across the United States, NetWorks members will have a chance to test their knowledge against their peers by participating in a NetWork Challenge Game Series, where they will be asked a series of hand-selected board review questions each week through the end of Board Review.
For additional Information about the NetWorks Challenge, visit the CHEST Foundation’s website.
News from the Board of Regents: Progress during a pandemic – June 2020
The Board of Regents met remotely in June because of ongoing travel restrictions and safety concerns for staff and board members.
• The meeting was opened with Stephanie Levine, President; Steve Simpson, President-Elect; and Robert Musacchio, CEO/EVP discussing the impacts of the COVID-19 pandemic and Business Continuity Planning. The COVID-19 Task Force, chaired by Steve Simpson, continues to meet weekly to identify emerging content needs toward supporting membership and their patients through the pandemic, connecting with the Education Committee and Foundation to ensure robust coverage, drawing on the expertise of the NetWorks for content development, and leveraging the Social Media Workgroup for dissemination. Key activities include: a regular Thursday webinar series at 3:00 pm CDT titled: “Advice From the Front Lines”; clinical resources in the form of infographics and guides are posted in the resource center and circulated through social media; Alex Niven, MD FCCP, led a team to develop a wellness curriculum and series; the CHEST Foundation developed patient education videos and guides, a public service announcement in partnership with the American Thoracic Society, and a pilot partnership with AMITA Health enabling access to telehealth.
• The Finance Committee, chaired by John Howington, reported that CHEST is on track to meet its budget and exceed its debt covenants and operating reserve policy for the current fiscal year. The record attendance at the October 2019 annual meeting, along with strong performance from our digital offerings offset the financial impacts of the global pandemic. Bob Musacchio, CEO/EVP, reminded the Board why CHEST is switching from a fiscal year to calendar year budget. A calendar year budget process creates better alignment with budgets of pharma, other clients, and vendors; facilitates various accruals that are based on the calendar year, such as benefits, vacation, sick, and PTO days; provides for greater continuity for doing business throughout the year, and permits more planning time for staff in setting individual goals related to the annual meeting.
• CHEST’S Digital Transformation strategy that kicked off in 2019 was timely considering the pandemic. With education as one of our main foci, CHEST has hired and onboarded a Chief Learning Officer, Jim Young, to actively examine how we develop and deploy our educational products and services. Our first movement toward remote meetings occurred on June 26 with the Virtual Congress originally slated for Bologna, Italy. Here, we piloted a new platform and brought to life the tenets established in the new learning strategy—providing choice, demonstrating responsiveness, and fostering connection.
• CHEST’s Governance Committee reviewed the College bylaws for revisions, as per the group’s practice every 2-3 years, and the Board approved the revisions to the bylaws as proposed by the committee.
• CHEST’s newly formed Health Policy and Advocacy Committee (HPAC), chaired by Neil Freedman, MD, FCCP, is holding monthly meetings with a goal of making a recommendation to the Board of Regents on CHEST’s regulatory and policy priorities during the August meeting. The HPAC assists CHEST leadership and the BOR in developing and implementing health policy positions, setting chest advocacy agendas in the legislative and regulatory arenas, engaging with policymakers as directed by the BOR, and educating CHEST members of government affairs relevant to CHEST’s mission. The HPAC is currently setting its priorities to bring to the BOR for approval later this summer. Areas of focus include home mechanical ventilation and competitive bidding access to in education four home auction therapy, only rehabilitation and tobacco vaping education,
• Peter Mazzone, MD, FCCP; Editor in Chief, CHEST journal, reviewed his editorial team, which now consists of three Deputy Editors, nine Associate Editors, an Assistant Editor, a Statistical Editor, and three Case Series Editors and the publishing staff and partners.
The Board’s next meetings will be a scheduled teleconference in August, followed by their meeting that will occur concomitantly with the CHEST meeting in October.
The Board of Regents met remotely in June because of ongoing travel restrictions and safety concerns for staff and board members.
• The meeting was opened with Stephanie Levine, President; Steve Simpson, President-Elect; and Robert Musacchio, CEO/EVP discussing the impacts of the COVID-19 pandemic and Business Continuity Planning. The COVID-19 Task Force, chaired by Steve Simpson, continues to meet weekly to identify emerging content needs toward supporting membership and their patients through the pandemic, connecting with the Education Committee and Foundation to ensure robust coverage, drawing on the expertise of the NetWorks for content development, and leveraging the Social Media Workgroup for dissemination. Key activities include: a regular Thursday webinar series at 3:00 pm CDT titled: “Advice From the Front Lines”; clinical resources in the form of infographics and guides are posted in the resource center and circulated through social media; Alex Niven, MD FCCP, led a team to develop a wellness curriculum and series; the CHEST Foundation developed patient education videos and guides, a public service announcement in partnership with the American Thoracic Society, and a pilot partnership with AMITA Health enabling access to telehealth.
• The Finance Committee, chaired by John Howington, reported that CHEST is on track to meet its budget and exceed its debt covenants and operating reserve policy for the current fiscal year. The record attendance at the October 2019 annual meeting, along with strong performance from our digital offerings offset the financial impacts of the global pandemic. Bob Musacchio, CEO/EVP, reminded the Board why CHEST is switching from a fiscal year to calendar year budget. A calendar year budget process creates better alignment with budgets of pharma, other clients, and vendors; facilitates various accruals that are based on the calendar year, such as benefits, vacation, sick, and PTO days; provides for greater continuity for doing business throughout the year, and permits more planning time for staff in setting individual goals related to the annual meeting.
• CHEST’S Digital Transformation strategy that kicked off in 2019 was timely considering the pandemic. With education as one of our main foci, CHEST has hired and onboarded a Chief Learning Officer, Jim Young, to actively examine how we develop and deploy our educational products and services. Our first movement toward remote meetings occurred on June 26 with the Virtual Congress originally slated for Bologna, Italy. Here, we piloted a new platform and brought to life the tenets established in the new learning strategy—providing choice, demonstrating responsiveness, and fostering connection.
• CHEST’s Governance Committee reviewed the College bylaws for revisions, as per the group’s practice every 2-3 years, and the Board approved the revisions to the bylaws as proposed by the committee.
• CHEST’s newly formed Health Policy and Advocacy Committee (HPAC), chaired by Neil Freedman, MD, FCCP, is holding monthly meetings with a goal of making a recommendation to the Board of Regents on CHEST’s regulatory and policy priorities during the August meeting. The HPAC assists CHEST leadership and the BOR in developing and implementing health policy positions, setting chest advocacy agendas in the legislative and regulatory arenas, engaging with policymakers as directed by the BOR, and educating CHEST members of government affairs relevant to CHEST’s mission. The HPAC is currently setting its priorities to bring to the BOR for approval later this summer. Areas of focus include home mechanical ventilation and competitive bidding access to in education four home auction therapy, only rehabilitation and tobacco vaping education,
• Peter Mazzone, MD, FCCP; Editor in Chief, CHEST journal, reviewed his editorial team, which now consists of three Deputy Editors, nine Associate Editors, an Assistant Editor, a Statistical Editor, and three Case Series Editors and the publishing staff and partners.
The Board’s next meetings will be a scheduled teleconference in August, followed by their meeting that will occur concomitantly with the CHEST meeting in October.
The Board of Regents met remotely in June because of ongoing travel restrictions and safety concerns for staff and board members.
• The meeting was opened with Stephanie Levine, President; Steve Simpson, President-Elect; and Robert Musacchio, CEO/EVP discussing the impacts of the COVID-19 pandemic and Business Continuity Planning. The COVID-19 Task Force, chaired by Steve Simpson, continues to meet weekly to identify emerging content needs toward supporting membership and their patients through the pandemic, connecting with the Education Committee and Foundation to ensure robust coverage, drawing on the expertise of the NetWorks for content development, and leveraging the Social Media Workgroup for dissemination. Key activities include: a regular Thursday webinar series at 3:00 pm CDT titled: “Advice From the Front Lines”; clinical resources in the form of infographics and guides are posted in the resource center and circulated through social media; Alex Niven, MD FCCP, led a team to develop a wellness curriculum and series; the CHEST Foundation developed patient education videos and guides, a public service announcement in partnership with the American Thoracic Society, and a pilot partnership with AMITA Health enabling access to telehealth.
• The Finance Committee, chaired by John Howington, reported that CHEST is on track to meet its budget and exceed its debt covenants and operating reserve policy for the current fiscal year. The record attendance at the October 2019 annual meeting, along with strong performance from our digital offerings offset the financial impacts of the global pandemic. Bob Musacchio, CEO/EVP, reminded the Board why CHEST is switching from a fiscal year to calendar year budget. A calendar year budget process creates better alignment with budgets of pharma, other clients, and vendors; facilitates various accruals that are based on the calendar year, such as benefits, vacation, sick, and PTO days; provides for greater continuity for doing business throughout the year, and permits more planning time for staff in setting individual goals related to the annual meeting.
• CHEST’S Digital Transformation strategy that kicked off in 2019 was timely considering the pandemic. With education as one of our main foci, CHEST has hired and onboarded a Chief Learning Officer, Jim Young, to actively examine how we develop and deploy our educational products and services. Our first movement toward remote meetings occurred on June 26 with the Virtual Congress originally slated for Bologna, Italy. Here, we piloted a new platform and brought to life the tenets established in the new learning strategy—providing choice, demonstrating responsiveness, and fostering connection.
• CHEST’s Governance Committee reviewed the College bylaws for revisions, as per the group’s practice every 2-3 years, and the Board approved the revisions to the bylaws as proposed by the committee.
• CHEST’s newly formed Health Policy and Advocacy Committee (HPAC), chaired by Neil Freedman, MD, FCCP, is holding monthly meetings with a goal of making a recommendation to the Board of Regents on CHEST’s regulatory and policy priorities during the August meeting. The HPAC assists CHEST leadership and the BOR in developing and implementing health policy positions, setting chest advocacy agendas in the legislative and regulatory arenas, engaging with policymakers as directed by the BOR, and educating CHEST members of government affairs relevant to CHEST’s mission. The HPAC is currently setting its priorities to bring to the BOR for approval later this summer. Areas of focus include home mechanical ventilation and competitive bidding access to in education four home auction therapy, only rehabilitation and tobacco vaping education,
• Peter Mazzone, MD, FCCP; Editor in Chief, CHEST journal, reviewed his editorial team, which now consists of three Deputy Editors, nine Associate Editors, an Assistant Editor, a Statistical Editor, and three Case Series Editors and the publishing staff and partners.
The Board’s next meetings will be a scheduled teleconference in August, followed by their meeting that will occur concomitantly with the CHEST meeting in October.
Artificial intelligence matches cancer genotypes to patient phenotypes
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR 2020