User login
OSA diagnoses not carried forward to the inpatient setting
Obstructive sleep apnea diagnoses may not be carried over to the inpatient setting, with potentially negative consequences for clinical outcomes, quality of life, and health care costs, an investigator said at the virtual meeting of the American College of Chest Physicians.
In a retrospective, single-center study, nearly 40% of patients with obstructive sleep apnea (OSA) diagnosed in the outpatient setting did not have a corresponding diagnosis during hospitalization, according to researcher Nitasa Sahu, MD.*
The missed OSA diagnoses could have especially negative implications for patients who don’t continue on positive airway pressure (PAP) therapy during the hospital stay, said Dr. Sahu, a fellow in pulmonary/critical care at St. Luke’s University Health Network in Bethlehem, Pa.
The finding indicates a large-magnitude opportunity to improve health care through better communication and optimized care, according to the researcher.
“Obstructive sleep apnea is underrecognized, it’s underdiagnosed, and it has a lot of implications for a patient’s hospitalization,” she said in interview
Clinical pathways should be set up to ensure that patients with OSA are properly identified and use their prescribed treatment, according to Dr. Sahu.
“I think that should, and would, reduce overall health care costs, with better outcomes as well,” she said.
Pulmonologist Saadia A. Faiz, MD, FCCP, said she hoped this study, presented at a late-breaking abstract at the virtual meeting, would highlight the importance of OSA screening and call attention to barriers to screening that may be in place in the inpatient setting.
That’s especially important because, after admission, the focus is often on the cause of admission rather than underlying comorbidities such as OSA, said Dr. Faiz, professor in the department of pulmonary medicine at the University of Texas MD Anderson Cancer Center in Houston.
“Working in a cancer hospital, the focus is always on the cancer, so sometimes even the patient will dismiss issues with their sleep,” Dr. Faiz said of her own experience in an interview.
“Often with sleep apnea, for people in the general population, the reason they seek medical attention is because their spouse notices that they’re snoring, so it is something that is not as emphasized,” added Dr. Faiz, who was not involved in the study.
In their study, Dr. Sahu and coauthors reviewed electronic health record data for adults hospitalized on the general internal medicine service at Penn State Hershey Medical Center from January 2017 through 2018. They restricted their search to first admissions.
The researchers looked for ICD-9 codes indicating an OSA diagnosis during their inpatient admission. They looked for the same codes in the preceding 5 years to see if the patients had a prior outpatient OSA diagnosis.
The inpatient cohort included 13,067 patients, of whom 53% were male, 87% were White, and 77% were over 50 years of age. Comorbidities included hypertension in 42%, atrial fibrillation in 21%, type 2 diabetes mellitus in 14%, congestive heart failure in 15%, and prior stroke in 0.5%.
A total of 991 individuals in the inpatient cohort had a prior outpatient OSA diagnosis. Of that group, 376 patients (38%) did not have an inpatient OSA diagnosis on inpatient record, according to the reported study data.
That large proportion of discordant diagnoses suggests a lot of missed opportunities to provide OSA therapy in the inpatient setting and to reinforce chronic disease state management, according to Dr. Sahu and colleagues.
How those discordant OSA diagnoses impact length of stay, cost of care, and readmissions are unanswered questions that deserve further study, Dr. Sahu said.
Among patients who did not have outpatient OSA diagnoses, another 804 patients, or about 6%, ended up with an inpatient diagnosis during their hospitalization, the researchers also reported.
While a number of those inpatient OSA diagnoses could have been coded in error, it’s also possible that they were indeed cases of OSA that went unrecognized until the individuals were hospitalized, Dr. Sahu said.
Dr. Sahu had no relevant relationships to report related to the study. One of four study coauthors reported relationships with Boehringer-Ingelheim, Nitto Denko, and Galapagos.
SOURCE: Sahu N. CHEST 2020. Abstract.
*Correction, 11/3/20: An earlier version of this article misstated the name of Nitasa Sahu, MD.
Obstructive sleep apnea diagnoses may not be carried over to the inpatient setting, with potentially negative consequences for clinical outcomes, quality of life, and health care costs, an investigator said at the virtual meeting of the American College of Chest Physicians.
In a retrospective, single-center study, nearly 40% of patients with obstructive sleep apnea (OSA) diagnosed in the outpatient setting did not have a corresponding diagnosis during hospitalization, according to researcher Nitasa Sahu, MD.*
The missed OSA diagnoses could have especially negative implications for patients who don’t continue on positive airway pressure (PAP) therapy during the hospital stay, said Dr. Sahu, a fellow in pulmonary/critical care at St. Luke’s University Health Network in Bethlehem, Pa.
The finding indicates a large-magnitude opportunity to improve health care through better communication and optimized care, according to the researcher.
“Obstructive sleep apnea is underrecognized, it’s underdiagnosed, and it has a lot of implications for a patient’s hospitalization,” she said in interview
Clinical pathways should be set up to ensure that patients with OSA are properly identified and use their prescribed treatment, according to Dr. Sahu.
“I think that should, and would, reduce overall health care costs, with better outcomes as well,” she said.
Pulmonologist Saadia A. Faiz, MD, FCCP, said she hoped this study, presented at a late-breaking abstract at the virtual meeting, would highlight the importance of OSA screening and call attention to barriers to screening that may be in place in the inpatient setting.
That’s especially important because, after admission, the focus is often on the cause of admission rather than underlying comorbidities such as OSA, said Dr. Faiz, professor in the department of pulmonary medicine at the University of Texas MD Anderson Cancer Center in Houston.
“Working in a cancer hospital, the focus is always on the cancer, so sometimes even the patient will dismiss issues with their sleep,” Dr. Faiz said of her own experience in an interview.
“Often with sleep apnea, for people in the general population, the reason they seek medical attention is because their spouse notices that they’re snoring, so it is something that is not as emphasized,” added Dr. Faiz, who was not involved in the study.
In their study, Dr. Sahu and coauthors reviewed electronic health record data for adults hospitalized on the general internal medicine service at Penn State Hershey Medical Center from January 2017 through 2018. They restricted their search to first admissions.
The researchers looked for ICD-9 codes indicating an OSA diagnosis during their inpatient admission. They looked for the same codes in the preceding 5 years to see if the patients had a prior outpatient OSA diagnosis.
The inpatient cohort included 13,067 patients, of whom 53% were male, 87% were White, and 77% were over 50 years of age. Comorbidities included hypertension in 42%, atrial fibrillation in 21%, type 2 diabetes mellitus in 14%, congestive heart failure in 15%, and prior stroke in 0.5%.
A total of 991 individuals in the inpatient cohort had a prior outpatient OSA diagnosis. Of that group, 376 patients (38%) did not have an inpatient OSA diagnosis on inpatient record, according to the reported study data.
That large proportion of discordant diagnoses suggests a lot of missed opportunities to provide OSA therapy in the inpatient setting and to reinforce chronic disease state management, according to Dr. Sahu and colleagues.
How those discordant OSA diagnoses impact length of stay, cost of care, and readmissions are unanswered questions that deserve further study, Dr. Sahu said.
Among patients who did not have outpatient OSA diagnoses, another 804 patients, or about 6%, ended up with an inpatient diagnosis during their hospitalization, the researchers also reported.
While a number of those inpatient OSA diagnoses could have been coded in error, it’s also possible that they were indeed cases of OSA that went unrecognized until the individuals were hospitalized, Dr. Sahu said.
Dr. Sahu had no relevant relationships to report related to the study. One of four study coauthors reported relationships with Boehringer-Ingelheim, Nitto Denko, and Galapagos.
SOURCE: Sahu N. CHEST 2020. Abstract.
*Correction, 11/3/20: An earlier version of this article misstated the name of Nitasa Sahu, MD.
Obstructive sleep apnea diagnoses may not be carried over to the inpatient setting, with potentially negative consequences for clinical outcomes, quality of life, and health care costs, an investigator said at the virtual meeting of the American College of Chest Physicians.
In a retrospective, single-center study, nearly 40% of patients with obstructive sleep apnea (OSA) diagnosed in the outpatient setting did not have a corresponding diagnosis during hospitalization, according to researcher Nitasa Sahu, MD.*
The missed OSA diagnoses could have especially negative implications for patients who don’t continue on positive airway pressure (PAP) therapy during the hospital stay, said Dr. Sahu, a fellow in pulmonary/critical care at St. Luke’s University Health Network in Bethlehem, Pa.
The finding indicates a large-magnitude opportunity to improve health care through better communication and optimized care, according to the researcher.
“Obstructive sleep apnea is underrecognized, it’s underdiagnosed, and it has a lot of implications for a patient’s hospitalization,” she said in interview
Clinical pathways should be set up to ensure that patients with OSA are properly identified and use their prescribed treatment, according to Dr. Sahu.
“I think that should, and would, reduce overall health care costs, with better outcomes as well,” she said.
Pulmonologist Saadia A. Faiz, MD, FCCP, said she hoped this study, presented at a late-breaking abstract at the virtual meeting, would highlight the importance of OSA screening and call attention to barriers to screening that may be in place in the inpatient setting.
That’s especially important because, after admission, the focus is often on the cause of admission rather than underlying comorbidities such as OSA, said Dr. Faiz, professor in the department of pulmonary medicine at the University of Texas MD Anderson Cancer Center in Houston.
“Working in a cancer hospital, the focus is always on the cancer, so sometimes even the patient will dismiss issues with their sleep,” Dr. Faiz said of her own experience in an interview.
“Often with sleep apnea, for people in the general population, the reason they seek medical attention is because their spouse notices that they’re snoring, so it is something that is not as emphasized,” added Dr. Faiz, who was not involved in the study.
In their study, Dr. Sahu and coauthors reviewed electronic health record data for adults hospitalized on the general internal medicine service at Penn State Hershey Medical Center from January 2017 through 2018. They restricted their search to first admissions.
The researchers looked for ICD-9 codes indicating an OSA diagnosis during their inpatient admission. They looked for the same codes in the preceding 5 years to see if the patients had a prior outpatient OSA diagnosis.
The inpatient cohort included 13,067 patients, of whom 53% were male, 87% were White, and 77% were over 50 years of age. Comorbidities included hypertension in 42%, atrial fibrillation in 21%, type 2 diabetes mellitus in 14%, congestive heart failure in 15%, and prior stroke in 0.5%.
A total of 991 individuals in the inpatient cohort had a prior outpatient OSA diagnosis. Of that group, 376 patients (38%) did not have an inpatient OSA diagnosis on inpatient record, according to the reported study data.
That large proportion of discordant diagnoses suggests a lot of missed opportunities to provide OSA therapy in the inpatient setting and to reinforce chronic disease state management, according to Dr. Sahu and colleagues.
How those discordant OSA diagnoses impact length of stay, cost of care, and readmissions are unanswered questions that deserve further study, Dr. Sahu said.
Among patients who did not have outpatient OSA diagnoses, another 804 patients, or about 6%, ended up with an inpatient diagnosis during their hospitalization, the researchers also reported.
While a number of those inpatient OSA diagnoses could have been coded in error, it’s also possible that they were indeed cases of OSA that went unrecognized until the individuals were hospitalized, Dr. Sahu said.
Dr. Sahu had no relevant relationships to report related to the study. One of four study coauthors reported relationships with Boehringer-Ingelheim, Nitto Denko, and Galapagos.
SOURCE: Sahu N. CHEST 2020. Abstract.
*Correction, 11/3/20: An earlier version of this article misstated the name of Nitasa Sahu, MD.
FROM CHEST 2020
Health care workers implore OSHA for more oversight on COVID-19 safety
Last spring, when Cliff Willmeng, RN, was working at United Hospital in St. Paul, Minnesota, he’d take off his personal protective equipment (PPE) in the same hallway where children were transported from ambulances to the neighboring Children’s Hospital emergency department. Stretchers would roll across red tape on the floor that designated the area as a “hot zone.” The door from a break room was about 10 feet away.
Willmeng has been a union activist all his life, but he’d never filed a complaint with the Occupational Safety and Health Administration (OSHA) until the COVID-19 pandemic hit.
Concerned about the inadequate space for doffing PPE and other situations in which the spread of SARS-CoV-2 seemed possible, Willmeng and other colleagues filed multiple OSHA complaints with the Minnesota Department of Labor in March and April. Willmeng was also worried about bringing SARS-CoV-2 on his scrubs home to his wife and kids, and he started wearing hospital-supplied scrubs that were meant for doctors and that were washed on site, which was against hospital policy. The hospital fired Willmeng on May 8, citing code of conduct and respectful workplace violations arising from the uniform dispute.
In August, the state agency issued Willmeng’s hospital a $2,100 fine for failure to comply with guidance regarding “respiratory protection” in response to worker complaints over the fact that they were instructed to restaple elastic bands on N95 masks early in the pandemic. In a statement, United Hospital said it contested the citation, and it is in discussions with Minnesota OSHA. “We have and continue to instruct employees not to alter N95 respirators or reuse damaged or soiled N95 respirators,” such as when the straps are broken, the statement says.
Minnesota OSHA has received three times as many emails and phone calls from workers and employers requesting information and assistance during the pandemic, compared with last year, said spokesperson James Honerman. “If Minnesota OSHA is made aware of a workplace safety or health issue, it assesses the situation and determines how best to respond, including conducting a workplace investigation.”
But Willmeng, who has been out of work since he was fired, says that without a receipt or confirmation from OSHA, he has no way of knowing whether there has been any follow-up regarding his complaints. Minnesota OSHA said workers should receive a letter once a case is resolved.
Like Willmeng’s case, none of the more than 10,000 COVID-related complaints the federal OSHA office has received from across the country have resulted in meaningful sanctions. Unions have picketed local OSHA offices and publicized complaints on behalf of their members to protest what they see as a lack of oversight. Legislators have called on US Department of Labor Secretary Eugene Scalia to step up enforcement.
For many health care workers, complaining to OSHA is a last resort after failing to get satisfactory responses from supervisors and appealing to unions for help. But with such minimal oversight from OSHA, some union leaders and legislators say it’s actually more dangerous than not having workplace safety enforcement at all. Lack of directives from the Trump administration has left the agency without the teeth it has cut under previous administrations, and recent changes to the agency’s rules raise questions about whether companies are ever required to report workers’ hospitalizations due to COVID-19.
“It’s so ineffective that it’s more dangerous to workers,” said Kim Cordova, president of United Food and Commercial Workers (UFCW) Local 7, which represents 22,000 health care and other workers in Colorado and Wyoming. “Employers only do what they’re forced to do.” Instead of deterring a multi-billion-dollar company, she said, such low fines signal that a company doesn’t need to worry about COVID-related safety.
“OSHA is doing a lamentably poor job protecting workers during the pandemic,” said James Brudney, JD, a professor at Fordham Law School, in New York, and former chief counsel of the U.S. Senate Subcommittee on Labor. “I’m not alone in saying that the agency has performed so badly.”
Former government officials writing in JAMA were similarly critical: “In the face of the greatest worker health crisis in recent history, OSHA, the lead government agency responsible for worker health and safety, has not fulfilled its responsibilities.”
What could have been
There were early signs that the agency wouldn’t be heavy-handed about COVID-19 safety concerns, Brudney said.
The agency could have issued Emergency Temporary Standards, rules it can put in place during pandemics that address specific short-term concerns. These rules could have required employers to take infection-control measures to protect workers, including mask wearing, providing proper PPE, and screening for COVID-19 symptoms. “That’s what the agency is supposed to do. They’re supposed to respond to an emergency with emergency measures,” Brudney said.
But despite legislative pressure and a court case, Secretary of Labor Eugene Scalia has declined to do so, saying that the agency would instead rely on its regular general duty clause, which is always in place to keep workplaces free from hazards that “cause death or serious physical harm.” The agency invoked the general duty clause for COVID-19–related violations for the first time in September to levy modest fines.
In response to a request for an interview, a Department of Labor spokesperson said that preexisting OSHA requirements apply to workers during the pandemic, including providing PPE for workers and assessing sanitation and cleanliness standards. The agency has issued specific guidance to companies on pandemic preparedness, she said, and that it responds to all complaints. Additionally, she cited whistleblower laws that make it illegal for employers to retaliate against employees for making safety and health complaints.
The federal OSHA office received 10,868 COVID-related complaints from Feb. 1 through Oct. 20, citing issues ranging from failure to provide proper PPE to not informing workers about exposures. As of Oct. 22, a total of 2,349 of the complaints involved healthcare workers. This count doesn’t include the untold number of “informal” complaints handled by state OSHA offices.
In a recent JAMA opinion piece, two former government officials agreed that “the federal government has not fully utilized OSHA’s public safety authority” and called the issuing of an Emergency Temporary Standard that would require employers to develop and implement infection control plans “the most important action the federal government could take” to protect workers.
“Employers are more likely to implement these controls if they are mandated by a government agency that has adequate enforcement tools to ensure compliance,” wrote former Assistant Secretary of Labor David Michaels, PhD, MPH, now at the Milken Institute School of Public Health of the George Washington University, Washington, and Gregory Wagner, MD, a former senior adviser at the National Institute for Occupational Safety and Health at the Centers for Disease Control and Prevention, now at the Harvard T.H. Chan School of Public Health, Boston.
They cited the success of a standard that OSHA issued in 1991 in response to the HIV/AIDS crisis. “The bloodborne pathogens standard has contributed to a substantial decline in health care worker risk for bloodborne diseases like HIV and hepatitis B and C,” they wrote. In a new report for the Century Foundation, the pair offered recommendations to the federal government for controlling the spread of the disease by ramping up OSHA’s role.
OSHA did issue a response plan that requires employers to report in regard to employees who experienced workplace exposures to SARS-CoV-2 and who were hospitalized with COVID-19 or died of the disease within certain time frames, but recent changes to these rules make experts question whether companies are in fact required to report hospitalizations.
In its second revision of guidelines, added to its FAQ page on Sept. 30, the agency said that, in order to be reportable, “an in-patient hospitalization due to COVID-19 must occur within 24 hours of an exposure to SARS-CoV-2 at work” and that the employer must report the hospitalization within 24 hours of learning both that the employee has been hospitalized and that the reason for the hospitalization was a work-related case of COVID-19. Previously, the 24-hour hospitalization window started at the time of diagnosis of the disease, rather than the work-related exposure.
The agency subsequently dropped the first citation it had issued for a COVID-related violation, even though the company, a nursing home, had already agreed to pay $3,904 for reporting employee hospitalizations late.
“It’s a step backwards from an important workplace and public health function that OSHA should be doing,” said Wagner, coauthor of the JAMA opinion piece.
Even without issuing Emergency Temporary Standards, critics say OSHA could have acted much earlier. OSHA issued its first COVID-related federal citation, the one against the nursing home that was dropped, in May for events that occurred in mid-April. The second COVID-related federal citation came in July.
The agency could also charge much more substantial fines for the citations it has issued. If a medical facility was cited for a PPE violation, such as the Minnesota hospital where workers were told to restaple the elastic bands on N95s, the agency could have cited the hospital for one violation per employee. Such fines based on multiple violations could add up to the hundreds of thousands to millions of dollars.
“It would send a signal to the highest-risk employers that these are violations that need to be addressed immediately,” Brudney said.
Many of the 22 state OSHA offices appear to be more responsive to COVID-related complaints than the federal agency, creating a system in which health care workers have substantially different rights from one state to the next. The governor of California, for example, recently authorized California’s OSHA division to consider COVID-19 an imminent hazard, to prohibit workers from entering areas where the hazard exists, and to require employers to disclose exposures. The state also recently issued large fines for COVID safety issues: $222,075 to frozen food manufacturer Overhill Farms and $214,080 to employment agency Jobsource North America.
Elsewhere, state laws such as New Jersey’s Conscientious Employee Protection Act give workers the right to refuse to work in unsafe situations, Brudney said. “A lot more action is going on at the state level because so little is being done at the federal level,” he said. “Some of it is governors committed to protecting essential workers and their families.”
Unions call for sanctions
Unions are both decrying the lack of enforcement thus far and seeking more oversight going forward.
In August, the National Nurses’ United (NNU) union filed a complaint to implore OSHA to investigate the country’s biggest hospital systems, HCA Healthcare, which operates 184 hospitals and about 2,000 other care sites in 21 states and the United Kingdom. The union describes how, throughout HCA hospitals, there is an environment conducive to the spread of coronavirus. Nurses share space and equipment, such as computers, desks, phones, bathrooms, and break rooms, where staff take off masks to eat and drink. The complaint also describes how there is resistance to testing nurses and a lack of communication about infections among colleagues.
“When they have total disregard for safety, they should be punished to the utmost,” said Markowitz, noting that HCA Healthcare is worth $40 billion. “They can penalize them, but if it’s unsafe conditions for RNs and healthcare workers, we know it’s unsafe for the patients. There needs to be drastic measures to prevent hospital corporations from behaving that way.”
In a statement, HCA spokesman Harlow Sumerford said the company has followed CDC guidance for protecting frontline caregivers. “We’re proud of our response and the significant resources we’ve deployed to help protect our colleagues. Meanwhile, the NNU has chosen to use this pandemic as an opportunity to gain publicity by attacking hospitals across the country,” Sumerford said.
Members of the union recently protested in front of the federal OSHA offices in Denver.
After several months, OSHA finally penalized a meat packing plant where eight workers (six union members) had died of COVID-19 last spring. But the amount – $15,615 – was so low that Cordova worries it will actually have a worse impact than no fine.
“It’s more dangerous to workers because now employers know [they won’t be punished meaningfully],” she said. “During the pandemic, OSHA has been absolutely absent.”
Thus, the recent picketing outside the offices in Denver. But, Cordova noted, it’s unlikely OSHA employees saw them. Their own offices were deemed too risky to stay open during the pandemic. They were vacant.
A version of this article originally appeared on Medscape.com.
Last spring, when Cliff Willmeng, RN, was working at United Hospital in St. Paul, Minnesota, he’d take off his personal protective equipment (PPE) in the same hallway where children were transported from ambulances to the neighboring Children’s Hospital emergency department. Stretchers would roll across red tape on the floor that designated the area as a “hot zone.” The door from a break room was about 10 feet away.
Willmeng has been a union activist all his life, but he’d never filed a complaint with the Occupational Safety and Health Administration (OSHA) until the COVID-19 pandemic hit.
Concerned about the inadequate space for doffing PPE and other situations in which the spread of SARS-CoV-2 seemed possible, Willmeng and other colleagues filed multiple OSHA complaints with the Minnesota Department of Labor in March and April. Willmeng was also worried about bringing SARS-CoV-2 on his scrubs home to his wife and kids, and he started wearing hospital-supplied scrubs that were meant for doctors and that were washed on site, which was against hospital policy. The hospital fired Willmeng on May 8, citing code of conduct and respectful workplace violations arising from the uniform dispute.
In August, the state agency issued Willmeng’s hospital a $2,100 fine for failure to comply with guidance regarding “respiratory protection” in response to worker complaints over the fact that they were instructed to restaple elastic bands on N95 masks early in the pandemic. In a statement, United Hospital said it contested the citation, and it is in discussions with Minnesota OSHA. “We have and continue to instruct employees not to alter N95 respirators or reuse damaged or soiled N95 respirators,” such as when the straps are broken, the statement says.
Minnesota OSHA has received three times as many emails and phone calls from workers and employers requesting information and assistance during the pandemic, compared with last year, said spokesperson James Honerman. “If Minnesota OSHA is made aware of a workplace safety or health issue, it assesses the situation and determines how best to respond, including conducting a workplace investigation.”
But Willmeng, who has been out of work since he was fired, says that without a receipt or confirmation from OSHA, he has no way of knowing whether there has been any follow-up regarding his complaints. Minnesota OSHA said workers should receive a letter once a case is resolved.
Like Willmeng’s case, none of the more than 10,000 COVID-related complaints the federal OSHA office has received from across the country have resulted in meaningful sanctions. Unions have picketed local OSHA offices and publicized complaints on behalf of their members to protest what they see as a lack of oversight. Legislators have called on US Department of Labor Secretary Eugene Scalia to step up enforcement.
For many health care workers, complaining to OSHA is a last resort after failing to get satisfactory responses from supervisors and appealing to unions for help. But with such minimal oversight from OSHA, some union leaders and legislators say it’s actually more dangerous than not having workplace safety enforcement at all. Lack of directives from the Trump administration has left the agency without the teeth it has cut under previous administrations, and recent changes to the agency’s rules raise questions about whether companies are ever required to report workers’ hospitalizations due to COVID-19.
“It’s so ineffective that it’s more dangerous to workers,” said Kim Cordova, president of United Food and Commercial Workers (UFCW) Local 7, which represents 22,000 health care and other workers in Colorado and Wyoming. “Employers only do what they’re forced to do.” Instead of deterring a multi-billion-dollar company, she said, such low fines signal that a company doesn’t need to worry about COVID-related safety.
“OSHA is doing a lamentably poor job protecting workers during the pandemic,” said James Brudney, JD, a professor at Fordham Law School, in New York, and former chief counsel of the U.S. Senate Subcommittee on Labor. “I’m not alone in saying that the agency has performed so badly.”
Former government officials writing in JAMA were similarly critical: “In the face of the greatest worker health crisis in recent history, OSHA, the lead government agency responsible for worker health and safety, has not fulfilled its responsibilities.”
What could have been
There were early signs that the agency wouldn’t be heavy-handed about COVID-19 safety concerns, Brudney said.
The agency could have issued Emergency Temporary Standards, rules it can put in place during pandemics that address specific short-term concerns. These rules could have required employers to take infection-control measures to protect workers, including mask wearing, providing proper PPE, and screening for COVID-19 symptoms. “That’s what the agency is supposed to do. They’re supposed to respond to an emergency with emergency measures,” Brudney said.
But despite legislative pressure and a court case, Secretary of Labor Eugene Scalia has declined to do so, saying that the agency would instead rely on its regular general duty clause, which is always in place to keep workplaces free from hazards that “cause death or serious physical harm.” The agency invoked the general duty clause for COVID-19–related violations for the first time in September to levy modest fines.
In response to a request for an interview, a Department of Labor spokesperson said that preexisting OSHA requirements apply to workers during the pandemic, including providing PPE for workers and assessing sanitation and cleanliness standards. The agency has issued specific guidance to companies on pandemic preparedness, she said, and that it responds to all complaints. Additionally, she cited whistleblower laws that make it illegal for employers to retaliate against employees for making safety and health complaints.
The federal OSHA office received 10,868 COVID-related complaints from Feb. 1 through Oct. 20, citing issues ranging from failure to provide proper PPE to not informing workers about exposures. As of Oct. 22, a total of 2,349 of the complaints involved healthcare workers. This count doesn’t include the untold number of “informal” complaints handled by state OSHA offices.
In a recent JAMA opinion piece, two former government officials agreed that “the federal government has not fully utilized OSHA’s public safety authority” and called the issuing of an Emergency Temporary Standard that would require employers to develop and implement infection control plans “the most important action the federal government could take” to protect workers.
“Employers are more likely to implement these controls if they are mandated by a government agency that has adequate enforcement tools to ensure compliance,” wrote former Assistant Secretary of Labor David Michaels, PhD, MPH, now at the Milken Institute School of Public Health of the George Washington University, Washington, and Gregory Wagner, MD, a former senior adviser at the National Institute for Occupational Safety and Health at the Centers for Disease Control and Prevention, now at the Harvard T.H. Chan School of Public Health, Boston.
They cited the success of a standard that OSHA issued in 1991 in response to the HIV/AIDS crisis. “The bloodborne pathogens standard has contributed to a substantial decline in health care worker risk for bloodborne diseases like HIV and hepatitis B and C,” they wrote. In a new report for the Century Foundation, the pair offered recommendations to the federal government for controlling the spread of the disease by ramping up OSHA’s role.
OSHA did issue a response plan that requires employers to report in regard to employees who experienced workplace exposures to SARS-CoV-2 and who were hospitalized with COVID-19 or died of the disease within certain time frames, but recent changes to these rules make experts question whether companies are in fact required to report hospitalizations.
In its second revision of guidelines, added to its FAQ page on Sept. 30, the agency said that, in order to be reportable, “an in-patient hospitalization due to COVID-19 must occur within 24 hours of an exposure to SARS-CoV-2 at work” and that the employer must report the hospitalization within 24 hours of learning both that the employee has been hospitalized and that the reason for the hospitalization was a work-related case of COVID-19. Previously, the 24-hour hospitalization window started at the time of diagnosis of the disease, rather than the work-related exposure.
The agency subsequently dropped the first citation it had issued for a COVID-related violation, even though the company, a nursing home, had already agreed to pay $3,904 for reporting employee hospitalizations late.
“It’s a step backwards from an important workplace and public health function that OSHA should be doing,” said Wagner, coauthor of the JAMA opinion piece.
Even without issuing Emergency Temporary Standards, critics say OSHA could have acted much earlier. OSHA issued its first COVID-related federal citation, the one against the nursing home that was dropped, in May for events that occurred in mid-April. The second COVID-related federal citation came in July.
The agency could also charge much more substantial fines for the citations it has issued. If a medical facility was cited for a PPE violation, such as the Minnesota hospital where workers were told to restaple the elastic bands on N95s, the agency could have cited the hospital for one violation per employee. Such fines based on multiple violations could add up to the hundreds of thousands to millions of dollars.
“It would send a signal to the highest-risk employers that these are violations that need to be addressed immediately,” Brudney said.
Many of the 22 state OSHA offices appear to be more responsive to COVID-related complaints than the federal agency, creating a system in which health care workers have substantially different rights from one state to the next. The governor of California, for example, recently authorized California’s OSHA division to consider COVID-19 an imminent hazard, to prohibit workers from entering areas where the hazard exists, and to require employers to disclose exposures. The state also recently issued large fines for COVID safety issues: $222,075 to frozen food manufacturer Overhill Farms and $214,080 to employment agency Jobsource North America.
Elsewhere, state laws such as New Jersey’s Conscientious Employee Protection Act give workers the right to refuse to work in unsafe situations, Brudney said. “A lot more action is going on at the state level because so little is being done at the federal level,” he said. “Some of it is governors committed to protecting essential workers and their families.”
Unions call for sanctions
Unions are both decrying the lack of enforcement thus far and seeking more oversight going forward.
In August, the National Nurses’ United (NNU) union filed a complaint to implore OSHA to investigate the country’s biggest hospital systems, HCA Healthcare, which operates 184 hospitals and about 2,000 other care sites in 21 states and the United Kingdom. The union describes how, throughout HCA hospitals, there is an environment conducive to the spread of coronavirus. Nurses share space and equipment, such as computers, desks, phones, bathrooms, and break rooms, where staff take off masks to eat and drink. The complaint also describes how there is resistance to testing nurses and a lack of communication about infections among colleagues.
“When they have total disregard for safety, they should be punished to the utmost,” said Markowitz, noting that HCA Healthcare is worth $40 billion. “They can penalize them, but if it’s unsafe conditions for RNs and healthcare workers, we know it’s unsafe for the patients. There needs to be drastic measures to prevent hospital corporations from behaving that way.”
In a statement, HCA spokesman Harlow Sumerford said the company has followed CDC guidance for protecting frontline caregivers. “We’re proud of our response and the significant resources we’ve deployed to help protect our colleagues. Meanwhile, the NNU has chosen to use this pandemic as an opportunity to gain publicity by attacking hospitals across the country,” Sumerford said.
Members of the union recently protested in front of the federal OSHA offices in Denver.
After several months, OSHA finally penalized a meat packing plant where eight workers (six union members) had died of COVID-19 last spring. But the amount – $15,615 – was so low that Cordova worries it will actually have a worse impact than no fine.
“It’s more dangerous to workers because now employers know [they won’t be punished meaningfully],” she said. “During the pandemic, OSHA has been absolutely absent.”
Thus, the recent picketing outside the offices in Denver. But, Cordova noted, it’s unlikely OSHA employees saw them. Their own offices were deemed too risky to stay open during the pandemic. They were vacant.
A version of this article originally appeared on Medscape.com.
Last spring, when Cliff Willmeng, RN, was working at United Hospital in St. Paul, Minnesota, he’d take off his personal protective equipment (PPE) in the same hallway where children were transported from ambulances to the neighboring Children’s Hospital emergency department. Stretchers would roll across red tape on the floor that designated the area as a “hot zone.” The door from a break room was about 10 feet away.
Willmeng has been a union activist all his life, but he’d never filed a complaint with the Occupational Safety and Health Administration (OSHA) until the COVID-19 pandemic hit.
Concerned about the inadequate space for doffing PPE and other situations in which the spread of SARS-CoV-2 seemed possible, Willmeng and other colleagues filed multiple OSHA complaints with the Minnesota Department of Labor in March and April. Willmeng was also worried about bringing SARS-CoV-2 on his scrubs home to his wife and kids, and he started wearing hospital-supplied scrubs that were meant for doctors and that were washed on site, which was against hospital policy. The hospital fired Willmeng on May 8, citing code of conduct and respectful workplace violations arising from the uniform dispute.
In August, the state agency issued Willmeng’s hospital a $2,100 fine for failure to comply with guidance regarding “respiratory protection” in response to worker complaints over the fact that they were instructed to restaple elastic bands on N95 masks early in the pandemic. In a statement, United Hospital said it contested the citation, and it is in discussions with Minnesota OSHA. “We have and continue to instruct employees not to alter N95 respirators or reuse damaged or soiled N95 respirators,” such as when the straps are broken, the statement says.
Minnesota OSHA has received three times as many emails and phone calls from workers and employers requesting information and assistance during the pandemic, compared with last year, said spokesperson James Honerman. “If Minnesota OSHA is made aware of a workplace safety or health issue, it assesses the situation and determines how best to respond, including conducting a workplace investigation.”
But Willmeng, who has been out of work since he was fired, says that without a receipt or confirmation from OSHA, he has no way of knowing whether there has been any follow-up regarding his complaints. Minnesota OSHA said workers should receive a letter once a case is resolved.
Like Willmeng’s case, none of the more than 10,000 COVID-related complaints the federal OSHA office has received from across the country have resulted in meaningful sanctions. Unions have picketed local OSHA offices and publicized complaints on behalf of their members to protest what they see as a lack of oversight. Legislators have called on US Department of Labor Secretary Eugene Scalia to step up enforcement.
For many health care workers, complaining to OSHA is a last resort after failing to get satisfactory responses from supervisors and appealing to unions for help. But with such minimal oversight from OSHA, some union leaders and legislators say it’s actually more dangerous than not having workplace safety enforcement at all. Lack of directives from the Trump administration has left the agency without the teeth it has cut under previous administrations, and recent changes to the agency’s rules raise questions about whether companies are ever required to report workers’ hospitalizations due to COVID-19.
“It’s so ineffective that it’s more dangerous to workers,” said Kim Cordova, president of United Food and Commercial Workers (UFCW) Local 7, which represents 22,000 health care and other workers in Colorado and Wyoming. “Employers only do what they’re forced to do.” Instead of deterring a multi-billion-dollar company, she said, such low fines signal that a company doesn’t need to worry about COVID-related safety.
“OSHA is doing a lamentably poor job protecting workers during the pandemic,” said James Brudney, JD, a professor at Fordham Law School, in New York, and former chief counsel of the U.S. Senate Subcommittee on Labor. “I’m not alone in saying that the agency has performed so badly.”
Former government officials writing in JAMA were similarly critical: “In the face of the greatest worker health crisis in recent history, OSHA, the lead government agency responsible for worker health and safety, has not fulfilled its responsibilities.”
What could have been
There were early signs that the agency wouldn’t be heavy-handed about COVID-19 safety concerns, Brudney said.
The agency could have issued Emergency Temporary Standards, rules it can put in place during pandemics that address specific short-term concerns. These rules could have required employers to take infection-control measures to protect workers, including mask wearing, providing proper PPE, and screening for COVID-19 symptoms. “That’s what the agency is supposed to do. They’re supposed to respond to an emergency with emergency measures,” Brudney said.
But despite legislative pressure and a court case, Secretary of Labor Eugene Scalia has declined to do so, saying that the agency would instead rely on its regular general duty clause, which is always in place to keep workplaces free from hazards that “cause death or serious physical harm.” The agency invoked the general duty clause for COVID-19–related violations for the first time in September to levy modest fines.
In response to a request for an interview, a Department of Labor spokesperson said that preexisting OSHA requirements apply to workers during the pandemic, including providing PPE for workers and assessing sanitation and cleanliness standards. The agency has issued specific guidance to companies on pandemic preparedness, she said, and that it responds to all complaints. Additionally, she cited whistleblower laws that make it illegal for employers to retaliate against employees for making safety and health complaints.
The federal OSHA office received 10,868 COVID-related complaints from Feb. 1 through Oct. 20, citing issues ranging from failure to provide proper PPE to not informing workers about exposures. As of Oct. 22, a total of 2,349 of the complaints involved healthcare workers. This count doesn’t include the untold number of “informal” complaints handled by state OSHA offices.
In a recent JAMA opinion piece, two former government officials agreed that “the federal government has not fully utilized OSHA’s public safety authority” and called the issuing of an Emergency Temporary Standard that would require employers to develop and implement infection control plans “the most important action the federal government could take” to protect workers.
“Employers are more likely to implement these controls if they are mandated by a government agency that has adequate enforcement tools to ensure compliance,” wrote former Assistant Secretary of Labor David Michaels, PhD, MPH, now at the Milken Institute School of Public Health of the George Washington University, Washington, and Gregory Wagner, MD, a former senior adviser at the National Institute for Occupational Safety and Health at the Centers for Disease Control and Prevention, now at the Harvard T.H. Chan School of Public Health, Boston.
They cited the success of a standard that OSHA issued in 1991 in response to the HIV/AIDS crisis. “The bloodborne pathogens standard has contributed to a substantial decline in health care worker risk for bloodborne diseases like HIV and hepatitis B and C,” they wrote. In a new report for the Century Foundation, the pair offered recommendations to the federal government for controlling the spread of the disease by ramping up OSHA’s role.
OSHA did issue a response plan that requires employers to report in regard to employees who experienced workplace exposures to SARS-CoV-2 and who were hospitalized with COVID-19 or died of the disease within certain time frames, but recent changes to these rules make experts question whether companies are in fact required to report hospitalizations.
In its second revision of guidelines, added to its FAQ page on Sept. 30, the agency said that, in order to be reportable, “an in-patient hospitalization due to COVID-19 must occur within 24 hours of an exposure to SARS-CoV-2 at work” and that the employer must report the hospitalization within 24 hours of learning both that the employee has been hospitalized and that the reason for the hospitalization was a work-related case of COVID-19. Previously, the 24-hour hospitalization window started at the time of diagnosis of the disease, rather than the work-related exposure.
The agency subsequently dropped the first citation it had issued for a COVID-related violation, even though the company, a nursing home, had already agreed to pay $3,904 for reporting employee hospitalizations late.
“It’s a step backwards from an important workplace and public health function that OSHA should be doing,” said Wagner, coauthor of the JAMA opinion piece.
Even without issuing Emergency Temporary Standards, critics say OSHA could have acted much earlier. OSHA issued its first COVID-related federal citation, the one against the nursing home that was dropped, in May for events that occurred in mid-April. The second COVID-related federal citation came in July.
The agency could also charge much more substantial fines for the citations it has issued. If a medical facility was cited for a PPE violation, such as the Minnesota hospital where workers were told to restaple the elastic bands on N95s, the agency could have cited the hospital for one violation per employee. Such fines based on multiple violations could add up to the hundreds of thousands to millions of dollars.
“It would send a signal to the highest-risk employers that these are violations that need to be addressed immediately,” Brudney said.
Many of the 22 state OSHA offices appear to be more responsive to COVID-related complaints than the federal agency, creating a system in which health care workers have substantially different rights from one state to the next. The governor of California, for example, recently authorized California’s OSHA division to consider COVID-19 an imminent hazard, to prohibit workers from entering areas where the hazard exists, and to require employers to disclose exposures. The state also recently issued large fines for COVID safety issues: $222,075 to frozen food manufacturer Overhill Farms and $214,080 to employment agency Jobsource North America.
Elsewhere, state laws such as New Jersey’s Conscientious Employee Protection Act give workers the right to refuse to work in unsafe situations, Brudney said. “A lot more action is going on at the state level because so little is being done at the federal level,” he said. “Some of it is governors committed to protecting essential workers and their families.”
Unions call for sanctions
Unions are both decrying the lack of enforcement thus far and seeking more oversight going forward.
In August, the National Nurses’ United (NNU) union filed a complaint to implore OSHA to investigate the country’s biggest hospital systems, HCA Healthcare, which operates 184 hospitals and about 2,000 other care sites in 21 states and the United Kingdom. The union describes how, throughout HCA hospitals, there is an environment conducive to the spread of coronavirus. Nurses share space and equipment, such as computers, desks, phones, bathrooms, and break rooms, where staff take off masks to eat and drink. The complaint also describes how there is resistance to testing nurses and a lack of communication about infections among colleagues.
“When they have total disregard for safety, they should be punished to the utmost,” said Markowitz, noting that HCA Healthcare is worth $40 billion. “They can penalize them, but if it’s unsafe conditions for RNs and healthcare workers, we know it’s unsafe for the patients. There needs to be drastic measures to prevent hospital corporations from behaving that way.”
In a statement, HCA spokesman Harlow Sumerford said the company has followed CDC guidance for protecting frontline caregivers. “We’re proud of our response and the significant resources we’ve deployed to help protect our colleagues. Meanwhile, the NNU has chosen to use this pandemic as an opportunity to gain publicity by attacking hospitals across the country,” Sumerford said.
Members of the union recently protested in front of the federal OSHA offices in Denver.
After several months, OSHA finally penalized a meat packing plant where eight workers (six union members) had died of COVID-19 last spring. But the amount – $15,615 – was so low that Cordova worries it will actually have a worse impact than no fine.
“It’s more dangerous to workers because now employers know [they won’t be punished meaningfully],” she said. “During the pandemic, OSHA has been absolutely absent.”
Thus, the recent picketing outside the offices in Denver. But, Cordova noted, it’s unlikely OSHA employees saw them. Their own offices were deemed too risky to stay open during the pandemic. They were vacant.
A version of this article originally appeared on Medscape.com.
How do you manage common inpatient oncologic emergencies?
Three routinely encountered emergencies in the inpatient setting
In 2016, there were an estimated 15,338,988 people living with cancer in the United States.1 As such, it is important that hospitalists be proficient in managing oncologic emergencies that can arise during the natural history of cancer or from its treatment. This article will review three emergencies that are routinely encountered in the inpatient setting: malignant spinal cord compression (MSCC), hypercalcemia of malignancy (HCM), and febrile neutropenia (FN).
Case
Mr. Williams is a 56-year-old man with newly diagnosed metastatic prostate cancer, diabetes mellitus, peptic ulcer disease, and hypertension. He is admitted with back pain and lower extremity weakness worsening over 2 weeks. He denies loss of sensation or bowel and bladder incontinence and can walk. MRI confirms cord compression at T10. What initial and subsequent steroid doses would be of most benefit to administer?
Malignant spinal cord compression
Treatment of MSCC usually aims to preserve function rather than reverse established deficits. MSCC from epidural tumor metastasis develops in 5%-14% of all cancer cases,2 with back pain as the most common symptom. Nearly 60%-85% of patients have weakness at the time of diagnosis,3 and unfortunately, nearly two-thirds of patients will be nonambulatory at presentation.
While timely steroid administration in addition to definitive treatment may maintain ambulatory capacity at 1 year after therapy,4 there is no consensus on the optimal loading and maintenance dose and duration of steroids.
Overview of the data
Although there are no formal guidelines on optimal steroid dosing for MSCC, it is common practice for dexamethasone to be initially dosed at 10 mg followed by 4 mg every 4-6 hours.5 The use of higher doses of dexamethasone may result in improvement in neurologic deficits, but has higher risks for toxicity and is not universally supported in the literature.
A study conducted by Vecht and colleagues demonstrated few differences between initial high-dose and low-dose dexamethasone.6 Intravenous administration of either 10 mg or 100 mg dexamethasone, both followed by total 16 mg of dexamethasone orally per day, showed no significant difference in mobility or survival between the groups.
In a prospective study by Heimdal and colleagues that evaluated the relationship between dexamethasone dose and toxicity, higher doses of steroids had no meaningful impact on neurological symptoms and resulted in more severe side effects.7 Patients were either given 96-mg IV loading dose, gradually tapered over 2 weeks, or enrolled in the low-dose group in which they received 4-mg IV dexamethasone four times per day with a taper over 2 weeks. The high-dose group experienced side effects in 28.6% of patients, with 14.3% experiencing serious side effects. Meanwhile, 7.9% of the low-dose group exhibited some side effects, with none experiencing serious adverse effects.The high-dose group did not experience a significant increase in mobility (57.1 vs. 57.9%).
Key takeaways
Dexamthasone 10-mg oral or IV followed by 4 mg every 4-6 hours until definitive treatment is started is associated with improved neurologic outcomes and minimal adverse side effects. Higher doses of steroids are unlikely to offer more benefit. In patients with paraplegia or autonomic dysfunction, the ability to restore neurologic function is reduced and the burdens of steroid treatment may outweigh its benefits.5
Case continued
Mr. Williams completed treatment for MSCC but was still complaining of extreme lethargy and noticed an increase in thirst and no bowel movement in 5 days. His serum calcium was 14 mg/dL.
Hypercalcemia of malignancy
HCM is the most common paraneoplastic syndrome, observed in nearly 30% of patients with advanced cancer. It is a poor prognostic indicator, and approximately half of all patients with HCM will die within 30 days.8 Cancer is the most common reason for hypercalcemia in the inpatient setting9 and is most often associated with multiple myeloma, non–small cell lung cancer, breast cancer, renal cell carcinoma, non-Hodgkins lymphoma, and leukemia.
Hypercalcemia most often presents with cognitive changes and lethargy, anorexia, nausea, constipation, polyuria and polydipsia, and renal failure. Bradycardia and shortened QT interval are seen more with severe hypercalcemia.
Management of hypercalcemia of malignancy
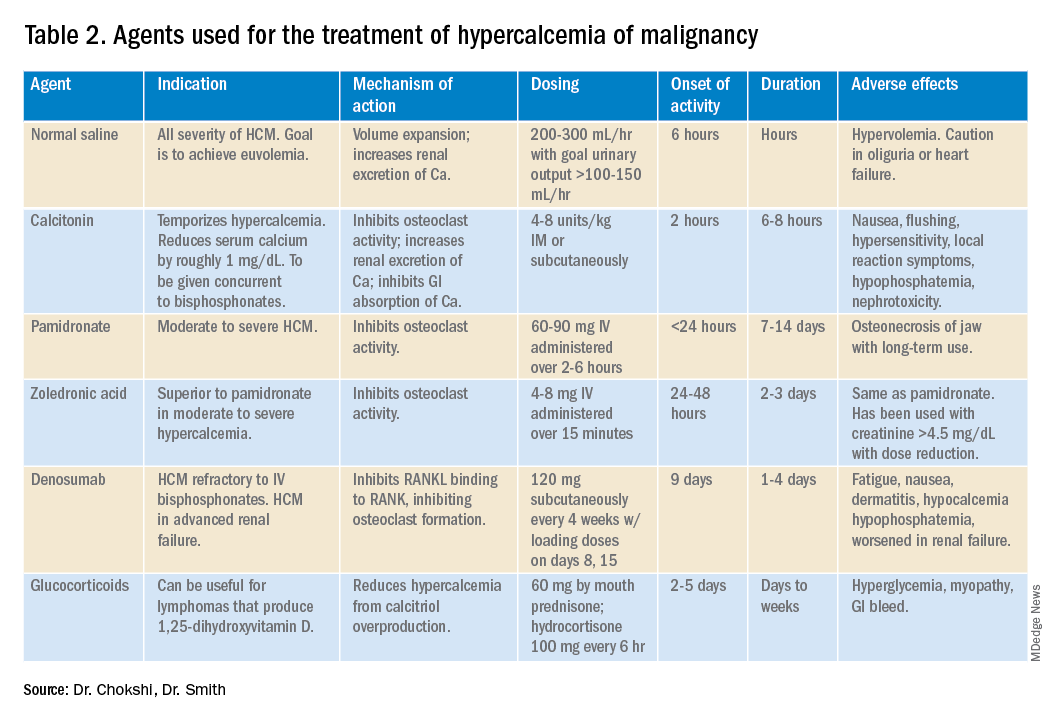
Management of HCM depends on corrected calcium or ionized calcium levels, chronicity, degree of symptoms, and presence of renal failure. In general, mild asymptomatic hypercalcemia can be managed with outpatient care. Serum calcium greater than 14 mg/dL should be treated regardless of symptoms (Table 1).
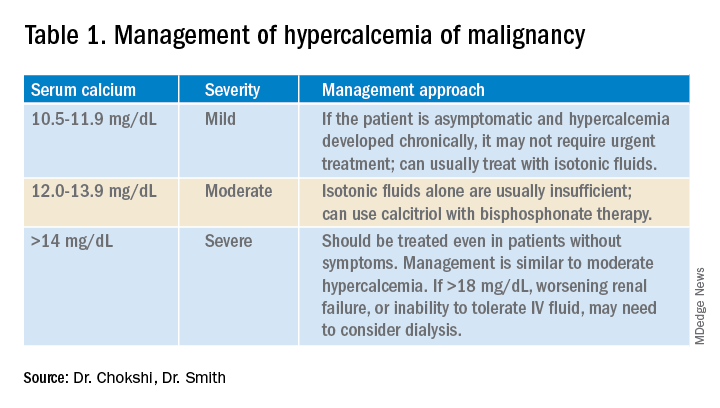
For mild to moderate HCM, management involves saline administration to achieve euvolemia and calcitonin, which has temporizing effects. Early administration of IV bisphosphonates for moderate to severe HCM is beneficial because onset of action is 24-48 hours. Furosemide for management of HCM has fallen out of favor unless the patient develops hypervolemia. Denosumab has been Food and Drug Administration–approved for HCM refractory to bisphosphonate therapy and can manage HCM in 64% of patients who did not respond adequately to bisphosphonate therapy.10 Because it can be used in advanced renal failure without dose adjustment, it is first-line therapy in this population, although the risk for hypocalcemia is increased in renal failure. For patients with serum calcium greater than 18 mg/dL, worsening renal failure, or inability to tolerate IV fluids, dialysis with a low-calcium bath should be considered (Table 2).
Zoledronic acid versus pamidronate
A single dose of zoledronic acid normalizes the serum calcium concentration in 88% of patients, compared with 70% of those who received pamidronate, in a pooled analysis of two phase 3 trials.11 The median duration of normocalcemia was longer for those receiving zoledronic acid (32-43 days vs. 18 days). The efficacy of the 4-mg and 8-mg zoledronic acid doses were similar, but the 4-mg dose was recommended because of renal toxicity and increased mortality associated with the higher dose.Despite this data, many specialists maintain that pamidronate, which is less expensive, is of similar clinical efficacy to ZA.12
Key takeaways
Management of HCM should be determined by the severity of the calcium level. The mainstay of treatment includes hydration with normal saline, calcitonin ,and bisphosphonate therapy; zoledronic acid is preferred over pamidronate. For patients refractory to bisphosphonates or patients with renal insufficiency, denosumab should be used.
Case continued: Febrile neutropenia
Febrile neutropenia is defined as a single oral temperature of 100.9° F or a temperature of 100.4° F sustained over a 1-hour period in a patient with absolute neutrophil count (ANC) less than 1,000 cells/mL or ANC expected to decrease to less than 500 cells/mL within a 48-hour period.13 Up to 30% of patients with solid tumors develop febrile neutropenia after chemotherapy, and nearly 80% of patients with hematologic malignancy or after hematopoietic stem cell therapy (HSCT) experience it.
Even though an infectious etiology is identified in only 30%-40% of cases, all patients with febrile neutropenia should initially receive at least empiric gram-negative coverage. The mortality rate is nearly 70% in neutropenic patients who do not receive empiric antibiotics and is reduced to 4%-20% with antibiotics.14
Risk stratification for febrile neutropenia and early discharge
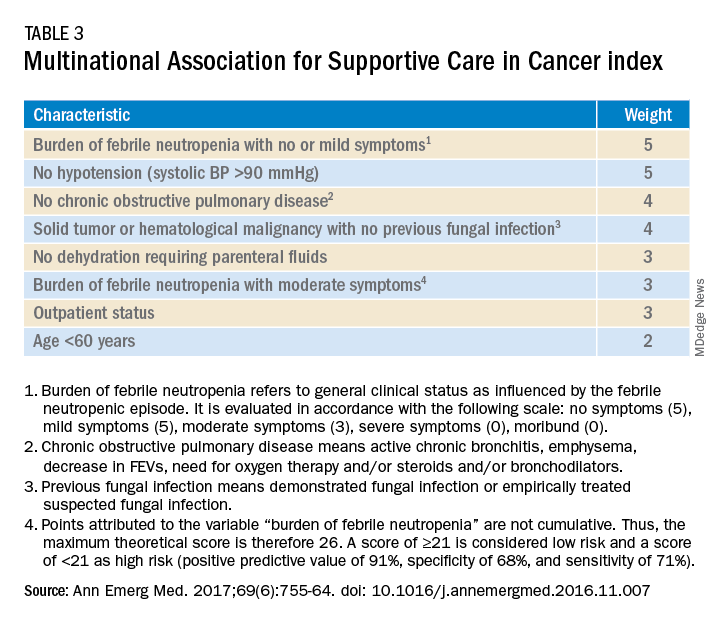
Talcott’s Rules, the Multinational Association for Supportive Care in Cancer (MASCC) score, and the Clinical Index of Stable Febrile Neutropenia (CISNE) are validated tools to determine low-risk febrile neutropenia patients (Tables 3 and 4). The Infectious Diseases Society of America guidelines validated the use of MASCC in 2002 but found that CISNE had better performance than other tools. Coyne and colleagues conducted a retrospective cohort study to assess these two risk stratification tools in the ED and found that the CISNE was 98.3% specific for identifying adverse outcomes, whereas the MASCC was 54.2% specific.15
A study by Talcott and colleagues used Talcott’s Rules to identify low-risk febrile neutropenia patients, who were randomized to early discharge with home intravenous antibiotics versus continued inpatient management. There were no significant differences in the primary outcomes, defined as any change in clinical status requiring medical evaluation.16 Another study suggested that discharge after 24 hours based on clinical stability with outpatient oral antibiotics were noninferior to standard inpatient and intravenous antibiotic therapy.17 A Cochrane review in 2013 of 22 randomized controlled trials determined that oral antibiotics were an acceptable treatment for low-risk patients.18
Key takeaways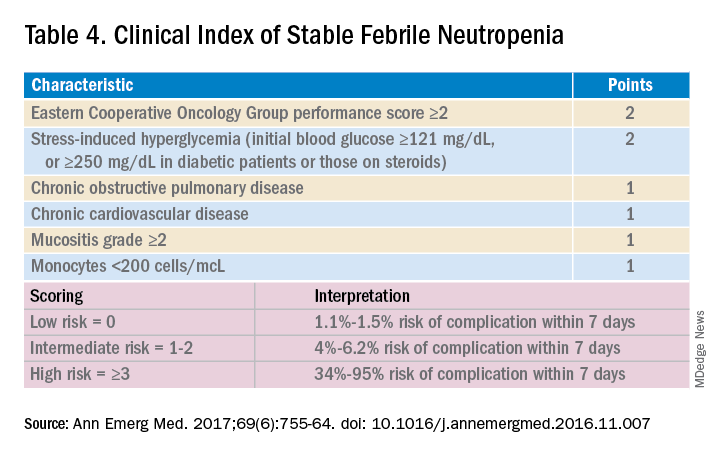
Though the MASCC is highly sensitive in identifying low-risk febrile neutropenia patients, it should be used with clinical caution because up to 11% of patients characterized as low risk developed severe complications.19 If a low-risk patient with solid tumor malignancy has adequate home support, lives within an hour of the hospital, and has access to follow-up within 72 hours, oral antibiotics and early discharge can be considered.
Dr. Chokshi is assistant professor in the division of hospital medicine at Mount Sinai Hospital, New York. Dr. Smith is associate professor in the division of hematology/oncology at Mount Sinai Hospital.
QUIZ
Mrs. Smith is a 64-year-old woman with endometrial cancer with temperature of 100.4° F at home. She takes no antibiotics, has no other medical history, and was sent in from clinic and admitted for further management. She feels well, and preliminary infectious workup is negative. She has been afebrile for more than 24 hours, and her ANC is 600 cells/mL.
Her son’s soccer game is tomorrow, and she would like to be present. Her family is involved in her care. Under what conditions can she be discharged?
A. She should not be discharged until full course of empiric intravenous antibiotics is completed.
B. Consider discharge in another 24 hours if she remains afebrile.
C. Discharge if low risk by MASCC or CISNE, with oral doses of levofloxacin or moxifloxacin or oral ciprofloxacin and amoxicillin/clavulanic acid.
Answer: C. The patient has a solid tumor malignancy, is low risk by both MASCC and CISNE, and can most likely be discharged if she is clinically stable or improved. A 7-day course of antibiotics is recommended with close follow-up.
References
1. SEER. Cancer of Any Site - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/all.html. Accessed 2019 Jul 17.
2. Kwok Y et al. Clinical Approach to Metastatic Epidural Spinal Cord Compression. Hematol Oncol Clin North Am. 2006;20(6):1297-305.
3. Helweg-Larsen S et al. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163-9.
4. Sørensen P et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30(1):22-7.
5. Skeoch G et al. Corticosteroid treatment for metastatic spinal cord compression: A review. Global Spine J. 2017;7(3):272-9.
6. Vecht C et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39(9):1255-7.
7. Heimdal K et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-4.
8. Ralston S et al. Cancer-associated hypercalcemia: Morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499-504.
9. Lindner G et al. Hypercalcemia in the ED: Prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657-60.
10. Hu M et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst. 2013;105(18):1417-20.
11. Major P et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558-67.
12. Stewart A. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-9.
13. Freifeld A et al. Executive summary: Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427-31.
14. Baden L et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
15. Coyne C et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med. 2017;69(6):755-64.
16. Talcott J et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977-83.
17. Innes H et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89(1):43-9.
18. Vidal L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst. Rev. 2013.
19. Taplitz RA et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-53.
Three routinely encountered emergencies in the inpatient setting
Three routinely encountered emergencies in the inpatient setting
In 2016, there were an estimated 15,338,988 people living with cancer in the United States.1 As such, it is important that hospitalists be proficient in managing oncologic emergencies that can arise during the natural history of cancer or from its treatment. This article will review three emergencies that are routinely encountered in the inpatient setting: malignant spinal cord compression (MSCC), hypercalcemia of malignancy (HCM), and febrile neutropenia (FN).
Case
Mr. Williams is a 56-year-old man with newly diagnosed metastatic prostate cancer, diabetes mellitus, peptic ulcer disease, and hypertension. He is admitted with back pain and lower extremity weakness worsening over 2 weeks. He denies loss of sensation or bowel and bladder incontinence and can walk. MRI confirms cord compression at T10. What initial and subsequent steroid doses would be of most benefit to administer?
Malignant spinal cord compression
Treatment of MSCC usually aims to preserve function rather than reverse established deficits. MSCC from epidural tumor metastasis develops in 5%-14% of all cancer cases,2 with back pain as the most common symptom. Nearly 60%-85% of patients have weakness at the time of diagnosis,3 and unfortunately, nearly two-thirds of patients will be nonambulatory at presentation.
While timely steroid administration in addition to definitive treatment may maintain ambulatory capacity at 1 year after therapy,4 there is no consensus on the optimal loading and maintenance dose and duration of steroids.
Overview of the data
Although there are no formal guidelines on optimal steroid dosing for MSCC, it is common practice for dexamethasone to be initially dosed at 10 mg followed by 4 mg every 4-6 hours.5 The use of higher doses of dexamethasone may result in improvement in neurologic deficits, but has higher risks for toxicity and is not universally supported in the literature.
A study conducted by Vecht and colleagues demonstrated few differences between initial high-dose and low-dose dexamethasone.6 Intravenous administration of either 10 mg or 100 mg dexamethasone, both followed by total 16 mg of dexamethasone orally per day, showed no significant difference in mobility or survival between the groups.
In a prospective study by Heimdal and colleagues that evaluated the relationship between dexamethasone dose and toxicity, higher doses of steroids had no meaningful impact on neurological symptoms and resulted in more severe side effects.7 Patients were either given 96-mg IV loading dose, gradually tapered over 2 weeks, or enrolled in the low-dose group in which they received 4-mg IV dexamethasone four times per day with a taper over 2 weeks. The high-dose group experienced side effects in 28.6% of patients, with 14.3% experiencing serious side effects. Meanwhile, 7.9% of the low-dose group exhibited some side effects, with none experiencing serious adverse effects.The high-dose group did not experience a significant increase in mobility (57.1 vs. 57.9%).
Key takeaways
Dexamthasone 10-mg oral or IV followed by 4 mg every 4-6 hours until definitive treatment is started is associated with improved neurologic outcomes and minimal adverse side effects. Higher doses of steroids are unlikely to offer more benefit. In patients with paraplegia or autonomic dysfunction, the ability to restore neurologic function is reduced and the burdens of steroid treatment may outweigh its benefits.5
Case continued
Mr. Williams completed treatment for MSCC but was still complaining of extreme lethargy and noticed an increase in thirst and no bowel movement in 5 days. His serum calcium was 14 mg/dL.
Hypercalcemia of malignancy
HCM is the most common paraneoplastic syndrome, observed in nearly 30% of patients with advanced cancer. It is a poor prognostic indicator, and approximately half of all patients with HCM will die within 30 days.8 Cancer is the most common reason for hypercalcemia in the inpatient setting9 and is most often associated with multiple myeloma, non–small cell lung cancer, breast cancer, renal cell carcinoma, non-Hodgkins lymphoma, and leukemia.
Hypercalcemia most often presents with cognitive changes and lethargy, anorexia, nausea, constipation, polyuria and polydipsia, and renal failure. Bradycardia and shortened QT interval are seen more with severe hypercalcemia.
Management of hypercalcemia of malignancy
Management of HCM depends on corrected calcium or ionized calcium levels, chronicity, degree of symptoms, and presence of renal failure. In general, mild asymptomatic hypercalcemia can be managed with outpatient care. Serum calcium greater than 14 mg/dL should be treated regardless of symptoms (Table 1).

For mild to moderate HCM, management involves saline administration to achieve euvolemia and calcitonin, which has temporizing effects. Early administration of IV bisphosphonates for moderate to severe HCM is beneficial because onset of action is 24-48 hours. Furosemide for management of HCM has fallen out of favor unless the patient develops hypervolemia. Denosumab has been Food and Drug Administration–approved for HCM refractory to bisphosphonate therapy and can manage HCM in 64% of patients who did not respond adequately to bisphosphonate therapy.10 Because it can be used in advanced renal failure without dose adjustment, it is first-line therapy in this population, although the risk for hypocalcemia is increased in renal failure. For patients with serum calcium greater than 18 mg/dL, worsening renal failure, or inability to tolerate IV fluids, dialysis with a low-calcium bath should be considered (Table 2).
Zoledronic acid versus pamidronate
A single dose of zoledronic acid normalizes the serum calcium concentration in 88% of patients, compared with 70% of those who received pamidronate, in a pooled analysis of two phase 3 trials.11 The median duration of normocalcemia was longer for those receiving zoledronic acid (32-43 days vs. 18 days). The efficacy of the 4-mg and 8-mg zoledronic acid doses were similar, but the 4-mg dose was recommended because of renal toxicity and increased mortality associated with the higher dose.Despite this data, many specialists maintain that pamidronate, which is less expensive, is of similar clinical efficacy to ZA.12

Key takeaways
Management of HCM should be determined by the severity of the calcium level. The mainstay of treatment includes hydration with normal saline, calcitonin ,and bisphosphonate therapy; zoledronic acid is preferred over pamidronate. For patients refractory to bisphosphonates or patients with renal insufficiency, denosumab should be used.
Case continued: Febrile neutropenia
Febrile neutropenia is defined as a single oral temperature of 100.9° F or a temperature of 100.4° F sustained over a 1-hour period in a patient with absolute neutrophil count (ANC) less than 1,000 cells/mL or ANC expected to decrease to less than 500 cells/mL within a 48-hour period.13 Up to 30% of patients with solid tumors develop febrile neutropenia after chemotherapy, and nearly 80% of patients with hematologic malignancy or after hematopoietic stem cell therapy (HSCT) experience it.
Even though an infectious etiology is identified in only 30%-40% of cases, all patients with febrile neutropenia should initially receive at least empiric gram-negative coverage. The mortality rate is nearly 70% in neutropenic patients who do not receive empiric antibiotics and is reduced to 4%-20% with antibiotics.14
Risk stratification for febrile neutropenia and early discharge

Talcott’s Rules, the Multinational Association for Supportive Care in Cancer (MASCC) score, and the Clinical Index of Stable Febrile Neutropenia (CISNE) are validated tools to determine low-risk febrile neutropenia patients (Tables 3 and 4). The Infectious Diseases Society of America guidelines validated the use of MASCC in 2002 but found that CISNE had better performance than other tools. Coyne and colleagues conducted a retrospective cohort study to assess these two risk stratification tools in the ED and found that the CISNE was 98.3% specific for identifying adverse outcomes, whereas the MASCC was 54.2% specific.15
A study by Talcott and colleagues used Talcott’s Rules to identify low-risk febrile neutropenia patients, who were randomized to early discharge with home intravenous antibiotics versus continued inpatient management. There were no significant differences in the primary outcomes, defined as any change in clinical status requiring medical evaluation.16 Another study suggested that discharge after 24 hours based on clinical stability with outpatient oral antibiotics were noninferior to standard inpatient and intravenous antibiotic therapy.17 A Cochrane review in 2013 of 22 randomized controlled trials determined that oral antibiotics were an acceptable treatment for low-risk patients.18
Key takeaways
Though the MASCC is highly sensitive in identifying low-risk febrile neutropenia patients, it should be used with clinical caution because up to 11% of patients characterized as low risk developed severe complications.19 If a low-risk patient with solid tumor malignancy has adequate home support, lives within an hour of the hospital, and has access to follow-up within 72 hours, oral antibiotics and early discharge can be considered.
Dr. Chokshi is assistant professor in the division of hospital medicine at Mount Sinai Hospital, New York. Dr. Smith is associate professor in the division of hematology/oncology at Mount Sinai Hospital.
QUIZ
Mrs. Smith is a 64-year-old woman with endometrial cancer with temperature of 100.4° F at home. She takes no antibiotics, has no other medical history, and was sent in from clinic and admitted for further management. She feels well, and preliminary infectious workup is negative. She has been afebrile for more than 24 hours, and her ANC is 600 cells/mL.
Her son’s soccer game is tomorrow, and she would like to be present. Her family is involved in her care. Under what conditions can she be discharged?
A. She should not be discharged until full course of empiric intravenous antibiotics is completed.
B. Consider discharge in another 24 hours if she remains afebrile.
C. Discharge if low risk by MASCC or CISNE, with oral doses of levofloxacin or moxifloxacin or oral ciprofloxacin and amoxicillin/clavulanic acid.
Answer: C. The patient has a solid tumor malignancy, is low risk by both MASCC and CISNE, and can most likely be discharged if she is clinically stable or improved. A 7-day course of antibiotics is recommended with close follow-up.
References
1. SEER. Cancer of Any Site - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/all.html. Accessed 2019 Jul 17.
2. Kwok Y et al. Clinical Approach to Metastatic Epidural Spinal Cord Compression. Hematol Oncol Clin North Am. 2006;20(6):1297-305.
3. Helweg-Larsen S et al. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163-9.
4. Sørensen P et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30(1):22-7.
5. Skeoch G et al. Corticosteroid treatment for metastatic spinal cord compression: A review. Global Spine J. 2017;7(3):272-9.
6. Vecht C et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39(9):1255-7.
7. Heimdal K et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-4.
8. Ralston S et al. Cancer-associated hypercalcemia: Morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499-504.
9. Lindner G et al. Hypercalcemia in the ED: Prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657-60.
10. Hu M et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst. 2013;105(18):1417-20.
11. Major P et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558-67.
12. Stewart A. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-9.
13. Freifeld A et al. Executive summary: Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427-31.
14. Baden L et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
15. Coyne C et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med. 2017;69(6):755-64.
16. Talcott J et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977-83.
17. Innes H et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89(1):43-9.
18. Vidal L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst. Rev. 2013.
19. Taplitz RA et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-53.
In 2016, there were an estimated 15,338,988 people living with cancer in the United States.1 As such, it is important that hospitalists be proficient in managing oncologic emergencies that can arise during the natural history of cancer or from its treatment. This article will review three emergencies that are routinely encountered in the inpatient setting: malignant spinal cord compression (MSCC), hypercalcemia of malignancy (HCM), and febrile neutropenia (FN).
Case
Mr. Williams is a 56-year-old man with newly diagnosed metastatic prostate cancer, diabetes mellitus, peptic ulcer disease, and hypertension. He is admitted with back pain and lower extremity weakness worsening over 2 weeks. He denies loss of sensation or bowel and bladder incontinence and can walk. MRI confirms cord compression at T10. What initial and subsequent steroid doses would be of most benefit to administer?
Malignant spinal cord compression
Treatment of MSCC usually aims to preserve function rather than reverse established deficits. MSCC from epidural tumor metastasis develops in 5%-14% of all cancer cases,2 with back pain as the most common symptom. Nearly 60%-85% of patients have weakness at the time of diagnosis,3 and unfortunately, nearly two-thirds of patients will be nonambulatory at presentation.
While timely steroid administration in addition to definitive treatment may maintain ambulatory capacity at 1 year after therapy,4 there is no consensus on the optimal loading and maintenance dose and duration of steroids.
Overview of the data
Although there are no formal guidelines on optimal steroid dosing for MSCC, it is common practice for dexamethasone to be initially dosed at 10 mg followed by 4 mg every 4-6 hours.5 The use of higher doses of dexamethasone may result in improvement in neurologic deficits, but has higher risks for toxicity and is not universally supported in the literature.
A study conducted by Vecht and colleagues demonstrated few differences between initial high-dose and low-dose dexamethasone.6 Intravenous administration of either 10 mg or 100 mg dexamethasone, both followed by total 16 mg of dexamethasone orally per day, showed no significant difference in mobility or survival between the groups.
In a prospective study by Heimdal and colleagues that evaluated the relationship between dexamethasone dose and toxicity, higher doses of steroids had no meaningful impact on neurological symptoms and resulted in more severe side effects.7 Patients were either given 96-mg IV loading dose, gradually tapered over 2 weeks, or enrolled in the low-dose group in which they received 4-mg IV dexamethasone four times per day with a taper over 2 weeks. The high-dose group experienced side effects in 28.6% of patients, with 14.3% experiencing serious side effects. Meanwhile, 7.9% of the low-dose group exhibited some side effects, with none experiencing serious adverse effects.The high-dose group did not experience a significant increase in mobility (57.1 vs. 57.9%).
Key takeaways
Dexamthasone 10-mg oral or IV followed by 4 mg every 4-6 hours until definitive treatment is started is associated with improved neurologic outcomes and minimal adverse side effects. Higher doses of steroids are unlikely to offer more benefit. In patients with paraplegia or autonomic dysfunction, the ability to restore neurologic function is reduced and the burdens of steroid treatment may outweigh its benefits.5
Case continued
Mr. Williams completed treatment for MSCC but was still complaining of extreme lethargy and noticed an increase in thirst and no bowel movement in 5 days. His serum calcium was 14 mg/dL.
Hypercalcemia of malignancy
HCM is the most common paraneoplastic syndrome, observed in nearly 30% of patients with advanced cancer. It is a poor prognostic indicator, and approximately half of all patients with HCM will die within 30 days.8 Cancer is the most common reason for hypercalcemia in the inpatient setting9 and is most often associated with multiple myeloma, non–small cell lung cancer, breast cancer, renal cell carcinoma, non-Hodgkins lymphoma, and leukemia.
Hypercalcemia most often presents with cognitive changes and lethargy, anorexia, nausea, constipation, polyuria and polydipsia, and renal failure. Bradycardia and shortened QT interval are seen more with severe hypercalcemia.
Management of hypercalcemia of malignancy
Management of HCM depends on corrected calcium or ionized calcium levels, chronicity, degree of symptoms, and presence of renal failure. In general, mild asymptomatic hypercalcemia can be managed with outpatient care. Serum calcium greater than 14 mg/dL should be treated regardless of symptoms (Table 1).

For mild to moderate HCM, management involves saline administration to achieve euvolemia and calcitonin, which has temporizing effects. Early administration of IV bisphosphonates for moderate to severe HCM is beneficial because onset of action is 24-48 hours. Furosemide for management of HCM has fallen out of favor unless the patient develops hypervolemia. Denosumab has been Food and Drug Administration–approved for HCM refractory to bisphosphonate therapy and can manage HCM in 64% of patients who did not respond adequately to bisphosphonate therapy.10 Because it can be used in advanced renal failure without dose adjustment, it is first-line therapy in this population, although the risk for hypocalcemia is increased in renal failure. For patients with serum calcium greater than 18 mg/dL, worsening renal failure, or inability to tolerate IV fluids, dialysis with a low-calcium bath should be considered (Table 2).
Zoledronic acid versus pamidronate
A single dose of zoledronic acid normalizes the serum calcium concentration in 88% of patients, compared with 70% of those who received pamidronate, in a pooled analysis of two phase 3 trials.11 The median duration of normocalcemia was longer for those receiving zoledronic acid (32-43 days vs. 18 days). The efficacy of the 4-mg and 8-mg zoledronic acid doses were similar, but the 4-mg dose was recommended because of renal toxicity and increased mortality associated with the higher dose.Despite this data, many specialists maintain that pamidronate, which is less expensive, is of similar clinical efficacy to ZA.12

Key takeaways
Management of HCM should be determined by the severity of the calcium level. The mainstay of treatment includes hydration with normal saline, calcitonin ,and bisphosphonate therapy; zoledronic acid is preferred over pamidronate. For patients refractory to bisphosphonates or patients with renal insufficiency, denosumab should be used.
Case continued: Febrile neutropenia
Febrile neutropenia is defined as a single oral temperature of 100.9° F or a temperature of 100.4° F sustained over a 1-hour period in a patient with absolute neutrophil count (ANC) less than 1,000 cells/mL or ANC expected to decrease to less than 500 cells/mL within a 48-hour period.13 Up to 30% of patients with solid tumors develop febrile neutropenia after chemotherapy, and nearly 80% of patients with hematologic malignancy or after hematopoietic stem cell therapy (HSCT) experience it.
Even though an infectious etiology is identified in only 30%-40% of cases, all patients with febrile neutropenia should initially receive at least empiric gram-negative coverage. The mortality rate is nearly 70% in neutropenic patients who do not receive empiric antibiotics and is reduced to 4%-20% with antibiotics.14
Risk stratification for febrile neutropenia and early discharge

Talcott’s Rules, the Multinational Association for Supportive Care in Cancer (MASCC) score, and the Clinical Index of Stable Febrile Neutropenia (CISNE) are validated tools to determine low-risk febrile neutropenia patients (Tables 3 and 4). The Infectious Diseases Society of America guidelines validated the use of MASCC in 2002 but found that CISNE had better performance than other tools. Coyne and colleagues conducted a retrospective cohort study to assess these two risk stratification tools in the ED and found that the CISNE was 98.3% specific for identifying adverse outcomes, whereas the MASCC was 54.2% specific.15
A study by Talcott and colleagues used Talcott’s Rules to identify low-risk febrile neutropenia patients, who were randomized to early discharge with home intravenous antibiotics versus continued inpatient management. There were no significant differences in the primary outcomes, defined as any change in clinical status requiring medical evaluation.16 Another study suggested that discharge after 24 hours based on clinical stability with outpatient oral antibiotics were noninferior to standard inpatient and intravenous antibiotic therapy.17 A Cochrane review in 2013 of 22 randomized controlled trials determined that oral antibiotics were an acceptable treatment for low-risk patients.18
Key takeaways
Though the MASCC is highly sensitive in identifying low-risk febrile neutropenia patients, it should be used with clinical caution because up to 11% of patients characterized as low risk developed severe complications.19 If a low-risk patient with solid tumor malignancy has adequate home support, lives within an hour of the hospital, and has access to follow-up within 72 hours, oral antibiotics and early discharge can be considered.
Dr. Chokshi is assistant professor in the division of hospital medicine at Mount Sinai Hospital, New York. Dr. Smith is associate professor in the division of hematology/oncology at Mount Sinai Hospital.
QUIZ
Mrs. Smith is a 64-year-old woman with endometrial cancer with temperature of 100.4° F at home. She takes no antibiotics, has no other medical history, and was sent in from clinic and admitted for further management. She feels well, and preliminary infectious workup is negative. She has been afebrile for more than 24 hours, and her ANC is 600 cells/mL.
Her son’s soccer game is tomorrow, and she would like to be present. Her family is involved in her care. Under what conditions can she be discharged?
A. She should not be discharged until full course of empiric intravenous antibiotics is completed.
B. Consider discharge in another 24 hours if she remains afebrile.
C. Discharge if low risk by MASCC or CISNE, with oral doses of levofloxacin or moxifloxacin or oral ciprofloxacin and amoxicillin/clavulanic acid.
Answer: C. The patient has a solid tumor malignancy, is low risk by both MASCC and CISNE, and can most likely be discharged if she is clinically stable or improved. A 7-day course of antibiotics is recommended with close follow-up.
References
1. SEER. Cancer of Any Site - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/all.html. Accessed 2019 Jul 17.
2. Kwok Y et al. Clinical Approach to Metastatic Epidural Spinal Cord Compression. Hematol Oncol Clin North Am. 2006;20(6):1297-305.
3. Helweg-Larsen S et al. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163-9.
4. Sørensen P et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30(1):22-7.
5. Skeoch G et al. Corticosteroid treatment for metastatic spinal cord compression: A review. Global Spine J. 2017;7(3):272-9.
6. Vecht C et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39(9):1255-7.
7. Heimdal K et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-4.
8. Ralston S et al. Cancer-associated hypercalcemia: Morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499-504.
9. Lindner G et al. Hypercalcemia in the ED: Prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657-60.
10. Hu M et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst. 2013;105(18):1417-20.
11. Major P et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558-67.
12. Stewart A. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-9.
13. Freifeld A et al. Executive summary: Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427-31.
14. Baden L et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
15. Coyne C et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med. 2017;69(6):755-64.
16. Talcott J et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977-83.
17. Innes H et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89(1):43-9.
18. Vidal L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst. Rev. 2013.
19. Taplitz RA et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-53.
Thermography plus software shows efficacy for breast cancer screening
Sensitivity and area under the curve (AUC) analyses of thermography that is combined with diagnostic software demonstrate “the efficacy of the tool for breast cancer screening,” concludes an observational, comparative study from India published online Oct. 1 in JCO Global Oncology, a publication of the American Society of Clinical Oncology.
Siva Teja Kakileti of Niramai Health Analytix, Koramangala, Bangalore, India, and colleagues said that the product, Thermalytix, is potentially a good fit for low- and middle-income countries because it is portable and provides automated quantitative analysis of thermal images – and thus can be conducted by technicians with “minimal training.”
Conventional thermography involves manual interpretation of complex thermal images, which “often results in erroneous results owing to subjectivity,” said the study authors.
That manual interpretation of thermal images might involve looking at 200 color shades, which is “high cognitive overload for the thermographer,” explained Mr. Kakileti in an interview.
However, an American mammography expert who was approached for comment dismissed thermography – even with the new twist of software-aided diagnostic scoring by Thermalytix – as wholly inappropriate for the detection of early breast cancer, owing to inherent limitations.
“Thermal imaging of any type has no value in finding early breast cancer,” Daniel Kopans, MD, of Harvard University and Massachusetts General Hospital, both in Boston, said in an interview. He said that thermal imaging only detects heat on the skin and perhaps a few millimeters beneath the skin and thus misses deeper cancers, the heat from which is carried away by the vascular system.
The new study included 470 women who presented for breast screening at two centers in Bangalore, India. A total of 238 women had symptoms such as breast lump, nipple discharge, skin changes, or breast pain; the remaining 232 women were asymptomatic.
All participants underwent a Thermalytix test and one or more standard-of-care tests for breast cancer screening (such as mammography, ultrasonography, biopsy, fine-needle aspiration, or elastography). A total of 78 women, or 16.6% of the group overall, were diagnosed with a malignancy. For the overall group of 470 women, Thermalytix had a sensitivity of 91.02% (symptomatic, 89.85%; asymptomatic,100%) and a specificity of 82.39% (symptomatic, 69.04%; asymptomatic, 92.41%) in detection of breast malignancy. Thermalytix showed an overall AUC of 0.90, with an AUC of 0.82 for symptomatic and 0.98 for asymptomatic women.
The study authors characterized both the sensitivity and AUC as “high.”
The results from the study, which the authors characterized as preliminary, encouraged the study sponsor, Niramai, to start planning a large-scale, multicountry trial.
But Dr. Kopans, who serves as a consultant to DART, which produces digital breast tomosynthesis units in China, suggested that this research will be fruitless. “Thermal imaging seems to raise its head every few years since it is passive, but it does not work and is a waste of money,” Dr. Kopans reiterated.
“Its use can be dangerous by dissuading women from being screened with mammography, which has been proven to save lives,” he stressed.
Thermalytix compared with mammography
Investigators also compared screening results in the subset of 242 women who underwent both Thermalytix and mammography. Results showed that Thermalytix had a higher sensitivity than did mammography (91.23% vs. 85.96%), but mammography had a higher specificity than Thermalytix did (94.05% vs. 68.65%).
In the asymptomatic group who underwent both tests (n = 95), four cancers were detected, and Thermalytix demonstrated superior sensitivity than mammography (100% vs. 50%), Mr. Kakileti and colleagues state.
Thermalytix evaluates vascularity variations too
In the subset of 228 women who did not undergo mammography (owing to dense breasts, younger age, or other reasons), Thermalytix detected tumors in all but 3 of 21 patients who went on to be diagnosed with breast cancer. The authors state that, because their artificial intelligence–based analysis uses vascularity, as well as temperature variations on the skin, to complement hot-spot detection, it is able to detect small lesions.
In the current study, 24 malignant tumors were less than 2 cm in diameter, and Thermalytix was able to identify 17 of the tumors as positive, for a 71% sensitivity rate for T1 tumors. This compared with a 68% sensitivity rate for mammography for detecting the same T1 tumors. Thermalytix also showed promising results in women younger than 40 years, for whom screening mammography is not usually recommended. The automated test picked up all 11 tumors eventually diagnosed in this younger cohort.
“Thermalytix is a portable, noninvasive, radiation-free test that has shown promising results in this preliminary study,” the investigators wrote, “[and] it can be an affordable and scalable method of screening in remote areas,” they added.
“We believe that Thermalytix ... is poised to be a promising modality for breast cancer screening,” Mr. Kakileti and colleagues summarized.
The FDA warns about thermography in place of mammography
The US Food and Drug Administration fairly recently warned against the use of thermography as an alternative to mammography for breast cancer screening or diagnosis, noting that it has received reports that facilities where thermography is offered often provide false information about the technology that can mislead patients into believing that it is either an alternative to or a better option than mammography.
Dr. Kopans says that other groups have invested in thermography research. “The Israelis spent millions working on a similar approach that didn’t work,” he commented.
The new software from Thermalytix, which is derived from artificial intelligence, is a “gimmick,” says the Boston radiologist. “If the basic information is not there, a computer cannot find it,” he stated, referring to what he believes are deeper-tissue tumors that are inaccessible to heat-detecting technology.
Mr. Kakileti is an employee of Nirami Health Analytix and owns stock and has filed patents with the company. Other investigators are also employed by the same company or receive research and other funding or have patents filed by the company as well. Dr. Kopans serves as a consultant to DART, which produces digital breast tomosynthesis units in China.
A version of this article originally appeared on Medscape.com.
Sensitivity and area under the curve (AUC) analyses of thermography that is combined with diagnostic software demonstrate “the efficacy of the tool for breast cancer screening,” concludes an observational, comparative study from India published online Oct. 1 in JCO Global Oncology, a publication of the American Society of Clinical Oncology.
Siva Teja Kakileti of Niramai Health Analytix, Koramangala, Bangalore, India, and colleagues said that the product, Thermalytix, is potentially a good fit for low- and middle-income countries because it is portable and provides automated quantitative analysis of thermal images – and thus can be conducted by technicians with “minimal training.”
Conventional thermography involves manual interpretation of complex thermal images, which “often results in erroneous results owing to subjectivity,” said the study authors.
That manual interpretation of thermal images might involve looking at 200 color shades, which is “high cognitive overload for the thermographer,” explained Mr. Kakileti in an interview.
However, an American mammography expert who was approached for comment dismissed thermography – even with the new twist of software-aided diagnostic scoring by Thermalytix – as wholly inappropriate for the detection of early breast cancer, owing to inherent limitations.
“Thermal imaging of any type has no value in finding early breast cancer,” Daniel Kopans, MD, of Harvard University and Massachusetts General Hospital, both in Boston, said in an interview. He said that thermal imaging only detects heat on the skin and perhaps a few millimeters beneath the skin and thus misses deeper cancers, the heat from which is carried away by the vascular system.
The new study included 470 women who presented for breast screening at two centers in Bangalore, India. A total of 238 women had symptoms such as breast lump, nipple discharge, skin changes, or breast pain; the remaining 232 women were asymptomatic.
All participants underwent a Thermalytix test and one or more standard-of-care tests for breast cancer screening (such as mammography, ultrasonography, biopsy, fine-needle aspiration, or elastography). A total of 78 women, or 16.6% of the group overall, were diagnosed with a malignancy. For the overall group of 470 women, Thermalytix had a sensitivity of 91.02% (symptomatic, 89.85%; asymptomatic,100%) and a specificity of 82.39% (symptomatic, 69.04%; asymptomatic, 92.41%) in detection of breast malignancy. Thermalytix showed an overall AUC of 0.90, with an AUC of 0.82 for symptomatic and 0.98 for asymptomatic women.
The study authors characterized both the sensitivity and AUC as “high.”
The results from the study, which the authors characterized as preliminary, encouraged the study sponsor, Niramai, to start planning a large-scale, multicountry trial.
But Dr. Kopans, who serves as a consultant to DART, which produces digital breast tomosynthesis units in China, suggested that this research will be fruitless. “Thermal imaging seems to raise its head every few years since it is passive, but it does not work and is a waste of money,” Dr. Kopans reiterated.
“Its use can be dangerous by dissuading women from being screened with mammography, which has been proven to save lives,” he stressed.
Thermalytix compared with mammography
Investigators also compared screening results in the subset of 242 women who underwent both Thermalytix and mammography. Results showed that Thermalytix had a higher sensitivity than did mammography (91.23% vs. 85.96%), but mammography had a higher specificity than Thermalytix did (94.05% vs. 68.65%).
In the asymptomatic group who underwent both tests (n = 95), four cancers were detected, and Thermalytix demonstrated superior sensitivity than mammography (100% vs. 50%), Mr. Kakileti and colleagues state.
Thermalytix evaluates vascularity variations too
In the subset of 228 women who did not undergo mammography (owing to dense breasts, younger age, or other reasons), Thermalytix detected tumors in all but 3 of 21 patients who went on to be diagnosed with breast cancer. The authors state that, because their artificial intelligence–based analysis uses vascularity, as well as temperature variations on the skin, to complement hot-spot detection, it is able to detect small lesions.
In the current study, 24 malignant tumors were less than 2 cm in diameter, and Thermalytix was able to identify 17 of the tumors as positive, for a 71% sensitivity rate for T1 tumors. This compared with a 68% sensitivity rate for mammography for detecting the same T1 tumors. Thermalytix also showed promising results in women younger than 40 years, for whom screening mammography is not usually recommended. The automated test picked up all 11 tumors eventually diagnosed in this younger cohort.
“Thermalytix is a portable, noninvasive, radiation-free test that has shown promising results in this preliminary study,” the investigators wrote, “[and] it can be an affordable and scalable method of screening in remote areas,” they added.
“We believe that Thermalytix ... is poised to be a promising modality for breast cancer screening,” Mr. Kakileti and colleagues summarized.
The FDA warns about thermography in place of mammography
The US Food and Drug Administration fairly recently warned against the use of thermography as an alternative to mammography for breast cancer screening or diagnosis, noting that it has received reports that facilities where thermography is offered often provide false information about the technology that can mislead patients into believing that it is either an alternative to or a better option than mammography.
Dr. Kopans says that other groups have invested in thermography research. “The Israelis spent millions working on a similar approach that didn’t work,” he commented.
The new software from Thermalytix, which is derived from artificial intelligence, is a “gimmick,” says the Boston radiologist. “If the basic information is not there, a computer cannot find it,” he stated, referring to what he believes are deeper-tissue tumors that are inaccessible to heat-detecting technology.
Mr. Kakileti is an employee of Nirami Health Analytix and owns stock and has filed patents with the company. Other investigators are also employed by the same company or receive research and other funding or have patents filed by the company as well. Dr. Kopans serves as a consultant to DART, which produces digital breast tomosynthesis units in China.
A version of this article originally appeared on Medscape.com.
Sensitivity and area under the curve (AUC) analyses of thermography that is combined with diagnostic software demonstrate “the efficacy of the tool for breast cancer screening,” concludes an observational, comparative study from India published online Oct. 1 in JCO Global Oncology, a publication of the American Society of Clinical Oncology.
Siva Teja Kakileti of Niramai Health Analytix, Koramangala, Bangalore, India, and colleagues said that the product, Thermalytix, is potentially a good fit for low- and middle-income countries because it is portable and provides automated quantitative analysis of thermal images – and thus can be conducted by technicians with “minimal training.”
Conventional thermography involves manual interpretation of complex thermal images, which “often results in erroneous results owing to subjectivity,” said the study authors.
That manual interpretation of thermal images might involve looking at 200 color shades, which is “high cognitive overload for the thermographer,” explained Mr. Kakileti in an interview.
However, an American mammography expert who was approached for comment dismissed thermography – even with the new twist of software-aided diagnostic scoring by Thermalytix – as wholly inappropriate for the detection of early breast cancer, owing to inherent limitations.
“Thermal imaging of any type has no value in finding early breast cancer,” Daniel Kopans, MD, of Harvard University and Massachusetts General Hospital, both in Boston, said in an interview. He said that thermal imaging only detects heat on the skin and perhaps a few millimeters beneath the skin and thus misses deeper cancers, the heat from which is carried away by the vascular system.
The new study included 470 women who presented for breast screening at two centers in Bangalore, India. A total of 238 women had symptoms such as breast lump, nipple discharge, skin changes, or breast pain; the remaining 232 women were asymptomatic.
All participants underwent a Thermalytix test and one or more standard-of-care tests for breast cancer screening (such as mammography, ultrasonography, biopsy, fine-needle aspiration, or elastography). A total of 78 women, or 16.6% of the group overall, were diagnosed with a malignancy. For the overall group of 470 women, Thermalytix had a sensitivity of 91.02% (symptomatic, 89.85%; asymptomatic,100%) and a specificity of 82.39% (symptomatic, 69.04%; asymptomatic, 92.41%) in detection of breast malignancy. Thermalytix showed an overall AUC of 0.90, with an AUC of 0.82 for symptomatic and 0.98 for asymptomatic women.
The study authors characterized both the sensitivity and AUC as “high.”
The results from the study, which the authors characterized as preliminary, encouraged the study sponsor, Niramai, to start planning a large-scale, multicountry trial.
But Dr. Kopans, who serves as a consultant to DART, which produces digital breast tomosynthesis units in China, suggested that this research will be fruitless. “Thermal imaging seems to raise its head every few years since it is passive, but it does not work and is a waste of money,” Dr. Kopans reiterated.
“Its use can be dangerous by dissuading women from being screened with mammography, which has been proven to save lives,” he stressed.
Thermalytix compared with mammography
Investigators also compared screening results in the subset of 242 women who underwent both Thermalytix and mammography. Results showed that Thermalytix had a higher sensitivity than did mammography (91.23% vs. 85.96%), but mammography had a higher specificity than Thermalytix did (94.05% vs. 68.65%).
In the asymptomatic group who underwent both tests (n = 95), four cancers were detected, and Thermalytix demonstrated superior sensitivity than mammography (100% vs. 50%), Mr. Kakileti and colleagues state.
Thermalytix evaluates vascularity variations too
In the subset of 228 women who did not undergo mammography (owing to dense breasts, younger age, or other reasons), Thermalytix detected tumors in all but 3 of 21 patients who went on to be diagnosed with breast cancer. The authors state that, because their artificial intelligence–based analysis uses vascularity, as well as temperature variations on the skin, to complement hot-spot detection, it is able to detect small lesions.
In the current study, 24 malignant tumors were less than 2 cm in diameter, and Thermalytix was able to identify 17 of the tumors as positive, for a 71% sensitivity rate for T1 tumors. This compared with a 68% sensitivity rate for mammography for detecting the same T1 tumors. Thermalytix also showed promising results in women younger than 40 years, for whom screening mammography is not usually recommended. The automated test picked up all 11 tumors eventually diagnosed in this younger cohort.
“Thermalytix is a portable, noninvasive, radiation-free test that has shown promising results in this preliminary study,” the investigators wrote, “[and] it can be an affordable and scalable method of screening in remote areas,” they added.
“We believe that Thermalytix ... is poised to be a promising modality for breast cancer screening,” Mr. Kakileti and colleagues summarized.
The FDA warns about thermography in place of mammography
The US Food and Drug Administration fairly recently warned against the use of thermography as an alternative to mammography for breast cancer screening or diagnosis, noting that it has received reports that facilities where thermography is offered often provide false information about the technology that can mislead patients into believing that it is either an alternative to or a better option than mammography.
Dr. Kopans says that other groups have invested in thermography research. “The Israelis spent millions working on a similar approach that didn’t work,” he commented.
The new software from Thermalytix, which is derived from artificial intelligence, is a “gimmick,” says the Boston radiologist. “If the basic information is not there, a computer cannot find it,” he stated, referring to what he believes are deeper-tissue tumors that are inaccessible to heat-detecting technology.
Mr. Kakileti is an employee of Nirami Health Analytix and owns stock and has filed patents with the company. Other investigators are also employed by the same company or receive research and other funding or have patents filed by the company as well. Dr. Kopans serves as a consultant to DART, which produces digital breast tomosynthesis units in China.
A version of this article originally appeared on Medscape.com.
COVID spikes exacerbate health worker shortages in Rocky Mountains, Great Plains
In Montana, pandemic-induced staffing shortages have shuttered a clinic in the state’s capital, led a northwestern regional hospital to ask employees exposed to COVID-19 to continue to work and emptied a health department 400 miles to the east.
“Just one more person out and we wouldn’t be able to keep the surgeries going,” said Dr. Shelly Harkins, MD, chief medical officer of St. Peter’s Health in Helena, a city of roughly 32,000 where cases continue to spread. “When the virus is just all around you, it’s almost impossible to not be deemed a contact at some point. One case can take out a whole team of people in a blink of an eye.”
In North Dakota, where cases per resident are growing faster than any other state, hospitals may once again curtail elective surgeries and possibly seek government aid to hire more nurses if the situation gets worse, North Dakota Hospital Association President Tim Blasl said.
“How long can we run at this rate with the workforce that we have?” Blasl said. “You can have all the licensed beds you want, but if you don’t have anybody to staff those beds, it doesn’t do you any good.”
The northern Rocky Mountains, Great Plains and Upper Midwest are seeing the highest surge of COVID-19 cases in the nation, as some residents have ignored recommendations for curtailing the virus, such as wearing masks and avoiding large gatherings. Montana, Idaho, Utah, Wyoming, North Dakota, South Dakota, Nebraska, Iowa, and Wisconsin have recently ranked among the top 10 U.S. states in confirmed cases per 100,000 residents over a 7-day period, according to an analysis by the New York Times.
Such coronavirus infections – and the quarantines that occur because of them – are exacerbating the health care worker shortage that existed in these states well before the pandemic. Unlike in the nation’s metropolitan hubs, these outbreaks are scattered across hundreds of miles. And even in these states’ biggest cities, the ranks of medical professionals are in short supply. Specialists and registered nurses are sometimes harder to track down than ventilators, N95 masks or hospital beds. Without enough care providers, patients may not be able to get the medical attention they need.
Hospitals have asked staffers to cover extra shifts and learn new skills. They have brought in temporary workers from other parts of the country and transferred some patients to less-crowded hospitals. But, at St. Peter’s Health, if the hospital’s one kidney doctor gets sick or is told to quarantine, Dr. Harkins doesn’t expect to find a backup.
“We make a point to not have excessive staff because we have an obligation to keep the cost of health care down for a community – we just don’t have a lot of slack in our rope,” Dr. Harkins said. “What we don’t account for is a mass exodus of staff for 14 days.”
Some hospitals are already at patient capacity or are nearly there. That’s not just because of the growing number of COVID-19 patients. Elective surgeries have resumed, and medical emergencies don’t pause for a pandemic.
Some Montana hospitals formed agreements with local affiliates early in the pandemic to share staff if one came up short. But now that the disease is spreading fast – and widely – the hope is that their needs don’t peak all at once.
Montana state officials keep a list of primarily in-state volunteer workers ready to travel to towns with shortages of contact tracers, nurses and more. But during a press conference on Oct. 15, Democratic Gov. Steve Bullock said the state had exhausted that database, and its nationwide request for National Guard medical staffing hadn’t brought in new workers.
“If you are a registered nurse, licensed practical nurse, paramedic, EMT, CNA or contact tracer, and are able to join our workforce, please do consider joining our team,” Gov. Bullock said.
This month, Kalispell Regional Medical Center in northwestern Montana even stopped quarantining COVID-exposed staff who remain asymptomatic, a change allowed by Centers for Disease Control and Prevention guidelines for health facilities facing staffing shortages.
“That’s very telling for what staffing is going through right now,” said Andrea Lueck, a registered nurse at the center. “We’re so tight that employees are called off of quarantine.”
Financial pressure early in the pandemic led the hospital to furlough staff, but it had to bring most of them back to work because it needs those bodies more than ever. The regional hub is based in Flathead County, which has recorded the state’s second-highest number of active COVID-19 cases.
Mellody Sharpton, a hospital spokesperson, said hospital workers who are exposed to someone infected with the virus are tested within three to five days and monitored for symptoms. The hospital is also pulling in new workers, with 25 traveling health professionals on hand and another 25 temporary ones on the way.
But Ms. Sharpton said the best way to conserve the hospital’s workforce is to stop the disease surge in the community.
Earlier in the pandemic, Central Montana Medical Center in Lewistown, a town of fewer than 6,000, experienced an exodus of part-time workers or those close to retirement who decided their jobs weren’t worth the risk. The facility recently secured two traveling workers, but both backed out because they couldn’t find housing. And, so far, roughly 40 of the hospital’s 322 employees have missed work for reasons connected to COVID-19.
“We’re at a critical staffing shortage and have been since the beginning of COVID,” said Joanie Slaybaugh, Central Montana Medical Center’s director of human resources. “We’re small enough, everybody feels an obligation to protect themselves and to protect each other. But it doesn’t take much to take out our staff.”
Roosevelt County, where roughly 11,000 live on the northeastern edge of Montana, had one of the nation’s highest rates of new cases as of Oct. 15. But by the end of the month, the county health department will lose half of its registered nurses as one person is about to retire and another was hired through a grant that’s ending. That leaves only one registered nurse aside from its director, Patty Presser. The health department already had to close earlier during the pandemic because of COVID exposure and not enough staffers to cover the gap. Now, if Ms. Presser can’t find nurse replacements in time, she hopes volunteers will step in, though she added they typically stay for only a few weeks.
“I need someone to do immunizations for my community, and you don’t become an immunization nurse in 14 days,” she said. “We don’t have the workforce here to deal with this virus, not even right now, and then I’m going to have my best two people go.”
Back in Helena, Dr. Harkins said St. Peter’s Health had to close a specialty outpatient clinic that treats chronic diseases for two weeks at the end of September because the entire staff had to quarantine.
Now the hospital is considering having doctors take turns spending a week working from home, so that if another wave of quarantines hits in the hospital, at least one untainted person can be brought back to work. But that won’t help for some specialties, like the hospital’s sole kidney doctor.
Every time Dr. Harkins’ phone rings, she said, she takes a breath and hopes it’s not another case that will force a whole division to close.
“Because I think immediately of the hundreds of people that need that service and won’t have it for 14 days,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In Montana, pandemic-induced staffing shortages have shuttered a clinic in the state’s capital, led a northwestern regional hospital to ask employees exposed to COVID-19 to continue to work and emptied a health department 400 miles to the east.
“Just one more person out and we wouldn’t be able to keep the surgeries going,” said Dr. Shelly Harkins, MD, chief medical officer of St. Peter’s Health in Helena, a city of roughly 32,000 where cases continue to spread. “When the virus is just all around you, it’s almost impossible to not be deemed a contact at some point. One case can take out a whole team of people in a blink of an eye.”
In North Dakota, where cases per resident are growing faster than any other state, hospitals may once again curtail elective surgeries and possibly seek government aid to hire more nurses if the situation gets worse, North Dakota Hospital Association President Tim Blasl said.
“How long can we run at this rate with the workforce that we have?” Blasl said. “You can have all the licensed beds you want, but if you don’t have anybody to staff those beds, it doesn’t do you any good.”
The northern Rocky Mountains, Great Plains and Upper Midwest are seeing the highest surge of COVID-19 cases in the nation, as some residents have ignored recommendations for curtailing the virus, such as wearing masks and avoiding large gatherings. Montana, Idaho, Utah, Wyoming, North Dakota, South Dakota, Nebraska, Iowa, and Wisconsin have recently ranked among the top 10 U.S. states in confirmed cases per 100,000 residents over a 7-day period, according to an analysis by the New York Times.
Such coronavirus infections – and the quarantines that occur because of them – are exacerbating the health care worker shortage that existed in these states well before the pandemic. Unlike in the nation’s metropolitan hubs, these outbreaks are scattered across hundreds of miles. And even in these states’ biggest cities, the ranks of medical professionals are in short supply. Specialists and registered nurses are sometimes harder to track down than ventilators, N95 masks or hospital beds. Without enough care providers, patients may not be able to get the medical attention they need.
Hospitals have asked staffers to cover extra shifts and learn new skills. They have brought in temporary workers from other parts of the country and transferred some patients to less-crowded hospitals. But, at St. Peter’s Health, if the hospital’s one kidney doctor gets sick or is told to quarantine, Dr. Harkins doesn’t expect to find a backup.
“We make a point to not have excessive staff because we have an obligation to keep the cost of health care down for a community – we just don’t have a lot of slack in our rope,” Dr. Harkins said. “What we don’t account for is a mass exodus of staff for 14 days.”
Some hospitals are already at patient capacity or are nearly there. That’s not just because of the growing number of COVID-19 patients. Elective surgeries have resumed, and medical emergencies don’t pause for a pandemic.
Some Montana hospitals formed agreements with local affiliates early in the pandemic to share staff if one came up short. But now that the disease is spreading fast – and widely – the hope is that their needs don’t peak all at once.
Montana state officials keep a list of primarily in-state volunteer workers ready to travel to towns with shortages of contact tracers, nurses and more. But during a press conference on Oct. 15, Democratic Gov. Steve Bullock said the state had exhausted that database, and its nationwide request for National Guard medical staffing hadn’t brought in new workers.
“If you are a registered nurse, licensed practical nurse, paramedic, EMT, CNA or contact tracer, and are able to join our workforce, please do consider joining our team,” Gov. Bullock said.
This month, Kalispell Regional Medical Center in northwestern Montana even stopped quarantining COVID-exposed staff who remain asymptomatic, a change allowed by Centers for Disease Control and Prevention guidelines for health facilities facing staffing shortages.
“That’s very telling for what staffing is going through right now,” said Andrea Lueck, a registered nurse at the center. “We’re so tight that employees are called off of quarantine.”
Financial pressure early in the pandemic led the hospital to furlough staff, but it had to bring most of them back to work because it needs those bodies more than ever. The regional hub is based in Flathead County, which has recorded the state’s second-highest number of active COVID-19 cases.
Mellody Sharpton, a hospital spokesperson, said hospital workers who are exposed to someone infected with the virus are tested within three to five days and monitored for symptoms. The hospital is also pulling in new workers, with 25 traveling health professionals on hand and another 25 temporary ones on the way.
But Ms. Sharpton said the best way to conserve the hospital’s workforce is to stop the disease surge in the community.
Earlier in the pandemic, Central Montana Medical Center in Lewistown, a town of fewer than 6,000, experienced an exodus of part-time workers or those close to retirement who decided their jobs weren’t worth the risk. The facility recently secured two traveling workers, but both backed out because they couldn’t find housing. And, so far, roughly 40 of the hospital’s 322 employees have missed work for reasons connected to COVID-19.
“We’re at a critical staffing shortage and have been since the beginning of COVID,” said Joanie Slaybaugh, Central Montana Medical Center’s director of human resources. “We’re small enough, everybody feels an obligation to protect themselves and to protect each other. But it doesn’t take much to take out our staff.”
Roosevelt County, where roughly 11,000 live on the northeastern edge of Montana, had one of the nation’s highest rates of new cases as of Oct. 15. But by the end of the month, the county health department will lose half of its registered nurses as one person is about to retire and another was hired through a grant that’s ending. That leaves only one registered nurse aside from its director, Patty Presser. The health department already had to close earlier during the pandemic because of COVID exposure and not enough staffers to cover the gap. Now, if Ms. Presser can’t find nurse replacements in time, she hopes volunteers will step in, though she added they typically stay for only a few weeks.
“I need someone to do immunizations for my community, and you don’t become an immunization nurse in 14 days,” she said. “We don’t have the workforce here to deal with this virus, not even right now, and then I’m going to have my best two people go.”
Back in Helena, Dr. Harkins said St. Peter’s Health had to close a specialty outpatient clinic that treats chronic diseases for two weeks at the end of September because the entire staff had to quarantine.
Now the hospital is considering having doctors take turns spending a week working from home, so that if another wave of quarantines hits in the hospital, at least one untainted person can be brought back to work. But that won’t help for some specialties, like the hospital’s sole kidney doctor.
Every time Dr. Harkins’ phone rings, she said, she takes a breath and hopes it’s not another case that will force a whole division to close.
“Because I think immediately of the hundreds of people that need that service and won’t have it for 14 days,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In Montana, pandemic-induced staffing shortages have shuttered a clinic in the state’s capital, led a northwestern regional hospital to ask employees exposed to COVID-19 to continue to work and emptied a health department 400 miles to the east.
“Just one more person out and we wouldn’t be able to keep the surgeries going,” said Dr. Shelly Harkins, MD, chief medical officer of St. Peter’s Health in Helena, a city of roughly 32,000 where cases continue to spread. “When the virus is just all around you, it’s almost impossible to not be deemed a contact at some point. One case can take out a whole team of people in a blink of an eye.”
In North Dakota, where cases per resident are growing faster than any other state, hospitals may once again curtail elective surgeries and possibly seek government aid to hire more nurses if the situation gets worse, North Dakota Hospital Association President Tim Blasl said.
“How long can we run at this rate with the workforce that we have?” Blasl said. “You can have all the licensed beds you want, but if you don’t have anybody to staff those beds, it doesn’t do you any good.”
The northern Rocky Mountains, Great Plains and Upper Midwest are seeing the highest surge of COVID-19 cases in the nation, as some residents have ignored recommendations for curtailing the virus, such as wearing masks and avoiding large gatherings. Montana, Idaho, Utah, Wyoming, North Dakota, South Dakota, Nebraska, Iowa, and Wisconsin have recently ranked among the top 10 U.S. states in confirmed cases per 100,000 residents over a 7-day period, according to an analysis by the New York Times.
Such coronavirus infections – and the quarantines that occur because of them – are exacerbating the health care worker shortage that existed in these states well before the pandemic. Unlike in the nation’s metropolitan hubs, these outbreaks are scattered across hundreds of miles. And even in these states’ biggest cities, the ranks of medical professionals are in short supply. Specialists and registered nurses are sometimes harder to track down than ventilators, N95 masks or hospital beds. Without enough care providers, patients may not be able to get the medical attention they need.
Hospitals have asked staffers to cover extra shifts and learn new skills. They have brought in temporary workers from other parts of the country and transferred some patients to less-crowded hospitals. But, at St. Peter’s Health, if the hospital’s one kidney doctor gets sick or is told to quarantine, Dr. Harkins doesn’t expect to find a backup.
“We make a point to not have excessive staff because we have an obligation to keep the cost of health care down for a community – we just don’t have a lot of slack in our rope,” Dr. Harkins said. “What we don’t account for is a mass exodus of staff for 14 days.”
Some hospitals are already at patient capacity or are nearly there. That’s not just because of the growing number of COVID-19 patients. Elective surgeries have resumed, and medical emergencies don’t pause for a pandemic.
Some Montana hospitals formed agreements with local affiliates early in the pandemic to share staff if one came up short. But now that the disease is spreading fast – and widely – the hope is that their needs don’t peak all at once.
Montana state officials keep a list of primarily in-state volunteer workers ready to travel to towns with shortages of contact tracers, nurses and more. But during a press conference on Oct. 15, Democratic Gov. Steve Bullock said the state had exhausted that database, and its nationwide request for National Guard medical staffing hadn’t brought in new workers.
“If you are a registered nurse, licensed practical nurse, paramedic, EMT, CNA or contact tracer, and are able to join our workforce, please do consider joining our team,” Gov. Bullock said.
This month, Kalispell Regional Medical Center in northwestern Montana even stopped quarantining COVID-exposed staff who remain asymptomatic, a change allowed by Centers for Disease Control and Prevention guidelines for health facilities facing staffing shortages.
“That’s very telling for what staffing is going through right now,” said Andrea Lueck, a registered nurse at the center. “We’re so tight that employees are called off of quarantine.”
Financial pressure early in the pandemic led the hospital to furlough staff, but it had to bring most of them back to work because it needs those bodies more than ever. The regional hub is based in Flathead County, which has recorded the state’s second-highest number of active COVID-19 cases.
Mellody Sharpton, a hospital spokesperson, said hospital workers who are exposed to someone infected with the virus are tested within three to five days and monitored for symptoms. The hospital is also pulling in new workers, with 25 traveling health professionals on hand and another 25 temporary ones on the way.
But Ms. Sharpton said the best way to conserve the hospital’s workforce is to stop the disease surge in the community.
Earlier in the pandemic, Central Montana Medical Center in Lewistown, a town of fewer than 6,000, experienced an exodus of part-time workers or those close to retirement who decided their jobs weren’t worth the risk. The facility recently secured two traveling workers, but both backed out because they couldn’t find housing. And, so far, roughly 40 of the hospital’s 322 employees have missed work for reasons connected to COVID-19.
“We’re at a critical staffing shortage and have been since the beginning of COVID,” said Joanie Slaybaugh, Central Montana Medical Center’s director of human resources. “We’re small enough, everybody feels an obligation to protect themselves and to protect each other. But it doesn’t take much to take out our staff.”
Roosevelt County, where roughly 11,000 live on the northeastern edge of Montana, had one of the nation’s highest rates of new cases as of Oct. 15. But by the end of the month, the county health department will lose half of its registered nurses as one person is about to retire and another was hired through a grant that’s ending. That leaves only one registered nurse aside from its director, Patty Presser. The health department already had to close earlier during the pandemic because of COVID exposure and not enough staffers to cover the gap. Now, if Ms. Presser can’t find nurse replacements in time, she hopes volunteers will step in, though she added they typically stay for only a few weeks.
“I need someone to do immunizations for my community, and you don’t become an immunization nurse in 14 days,” she said. “We don’t have the workforce here to deal with this virus, not even right now, and then I’m going to have my best two people go.”
Back in Helena, Dr. Harkins said St. Peter’s Health had to close a specialty outpatient clinic that treats chronic diseases for two weeks at the end of September because the entire staff had to quarantine.
Now the hospital is considering having doctors take turns spending a week working from home, so that if another wave of quarantines hits in the hospital, at least one untainted person can be brought back to work. But that won’t help for some specialties, like the hospital’s sole kidney doctor.
Every time Dr. Harkins’ phone rings, she said, she takes a breath and hopes it’s not another case that will force a whole division to close.
“Because I think immediately of the hundreds of people that need that service and won’t have it for 14 days,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
AHA adds recovery, emotional support to CPR guidelines
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
FROM CIRCULATION
Gastroenterology Data Trends 2020
Topics include:
Long-Term Impact of COVID-19 on the Practice of Gastroenterology and Endoscopy
John I. Allen, MD, MBA, AGAF
Consolidation Trends in Gastroenterology
Michael Weinstein, MD, AGAF
IBD Management Amid COVID-19
Kim L. Isaacs, MD, AGAF
Racial and Social Disparities in Gastroenterology
Sandra M. Quezada, MD, MS
Impending Physician Shortages in Gastroenterology
Michael Weinstein, MD, AGAF
Recommendations for Colonoscopy and the Management of Large Polyps
Aasma Shaukat, MD, MPH, AGAF
Nonalcoholic Steatohepatitis (NASH): Identifying Patients with High-Risk Disease
Brent Tetri, MD, AGAF
Esophageal Adenocarcinoma: Geographical Clustering and Time Trends
Joel Rubenstein, MD, MSc
Topics include:
Long-Term Impact of COVID-19 on the Practice of Gastroenterology and Endoscopy
John I. Allen, MD, MBA, AGAF
Consolidation Trends in Gastroenterology
Michael Weinstein, MD, AGAF
IBD Management Amid COVID-19
Kim L. Isaacs, MD, AGAF
Racial and Social Disparities in Gastroenterology
Sandra M. Quezada, MD, MS
Impending Physician Shortages in Gastroenterology
Michael Weinstein, MD, AGAF
Recommendations for Colonoscopy and the Management of Large Polyps
Aasma Shaukat, MD, MPH, AGAF
Nonalcoholic Steatohepatitis (NASH): Identifying Patients with High-Risk Disease
Brent Tetri, MD, AGAF
Esophageal Adenocarcinoma: Geographical Clustering and Time Trends
Joel Rubenstein, MD, MSc
Topics include:
Long-Term Impact of COVID-19 on the Practice of Gastroenterology and Endoscopy
John I. Allen, MD, MBA, AGAF
Consolidation Trends in Gastroenterology
Michael Weinstein, MD, AGAF
IBD Management Amid COVID-19
Kim L. Isaacs, MD, AGAF
Racial and Social Disparities in Gastroenterology
Sandra M. Quezada, MD, MS
Impending Physician Shortages in Gastroenterology
Michael Weinstein, MD, AGAF
Recommendations for Colonoscopy and the Management of Large Polyps
Aasma Shaukat, MD, MPH, AGAF
Nonalcoholic Steatohepatitis (NASH): Identifying Patients with High-Risk Disease
Brent Tetri, MD, AGAF
Esophageal Adenocarcinoma: Geographical Clustering and Time Trends
Joel Rubenstein, MD, MSc
Artificially sweetened drinks add to CVD risk
Sugary and artificially sweetened drinks are each associated with an increased risk of developing cardiovascular disease, according to results from a large prospective cohort study.
However, the design of that study fails to take into account other sources of dietary sugar, according to one expert.
In a research letter published online Oct. 26 in the Journal of the American College of Cardiology, Eloi Chazelas, a PhD candidate at Sorbonne Paris Nord University in Paris, and colleagues, shared results from nearly 105,000 subjects (79% women, mean age 43 at baseline, median follow up 6.6 years) enrolled in the NutriNet-Santé cohort study.
In this observational study, which began recruiting in 2009, dietary patterns are self-reported by subjects, while health outcomes are validated by investigators.
Mr. Chazelas and his colleagues identified 1,379 first incident cases of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, and angioplasty in the cohort during 2009-2019. Cases that occurred during the first 3 years’ follow up were excluded from the analysis, to avoid potential reverse causality bias.
After adjustment for a wide range of dietary, demographic and health confounders, the investigators found that high consumers of sugary drinks or artificially sweetened drinks saw 20% and 32% higher risk of such events, respectively, compared with people who reported drinking neither beverage type (hazard ratio: 1.20; 95% confidence interval 1.04-1.40, P for trend < .0009 and HR: 1.32; 95% CI, 1.00-1.73, P for trend < .03).
Sugary drinks were defined as containing 5% or more of sugars, including natural fruit juices. The high consumers in the study had a median intake of 185 mL per day of sugary drinks, or 176 mL per day for artificially sweetened drinks. Natural noncaloric sweeteners such as Stevia were included in the artificially sweetened group.
The findings, Mr. Chazelas and colleagues wrote in their analysis, add to evidence that artificially sweetened beverages “might not be a healthy substitute for sugary drinks.” While research has suggested that artificial sweeteners induce glucose intolerance by disturbing gut microbiota, they noted, more and bigger studies are needed to understand the mechanisms by which they might bear on cardiovascular disease risk.
Robert A. Vogel, MD, of the University of Colorado Denver, urged caution in interpreting the researchers’ results. In an interview, Dr. Vogel, a preventive cardiologist, said that it is “notoriously difficult” to evaluate what a food or food group does to the body outside of a carefully controlled trial. What little randomized trial evidence exists comparing the health effects of artificially sweetened and sugary drinks includes a 2012 trial in children that found diet drinks associated with reductions in body fat – if anything a positive indication for heart health.
With adults enrolled in an observational study, things are much more easily confounded, Dr. Vogel said. “So subjects self-report that they’re not consuming one thing – sugary or sweetened beverages. What else are they putting into their diet? Maybe they’re eating dessert and consuming sugar that way. Try as you will to unconfound, to do a multivariate correction for all these factors is just very difficult.”
In addition, Dr. Vogel noted, the investigators made no attempt to discern among the different sweeteners consumed. “Stevia, saccharine, Sucralose – it’s highly unlikely that each of these agents has the same effect on gut microbiota.”
In 2019, researchers led by Mr. Chazelas looked at cancer risk in high consumers of the sugary and artificially sweetened drinks in some 107,000 patients from the cohort, and reported that sugary drinks were significantly associated with the risk of overall cancer. They saw no similar association for artificially sweetened drinks.
The NutriNet-Santé study is funded by the French government, and the investigators disclosed no financial support from commercial entities. Dr. Vogel has received research support from Sanofi and speaking fees from Regeneron.
Sugary and artificially sweetened drinks are each associated with an increased risk of developing cardiovascular disease, according to results from a large prospective cohort study.
However, the design of that study fails to take into account other sources of dietary sugar, according to one expert.
In a research letter published online Oct. 26 in the Journal of the American College of Cardiology, Eloi Chazelas, a PhD candidate at Sorbonne Paris Nord University in Paris, and colleagues, shared results from nearly 105,000 subjects (79% women, mean age 43 at baseline, median follow up 6.6 years) enrolled in the NutriNet-Santé cohort study.
In this observational study, which began recruiting in 2009, dietary patterns are self-reported by subjects, while health outcomes are validated by investigators.
Mr. Chazelas and his colleagues identified 1,379 first incident cases of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, and angioplasty in the cohort during 2009-2019. Cases that occurred during the first 3 years’ follow up were excluded from the analysis, to avoid potential reverse causality bias.
After adjustment for a wide range of dietary, demographic and health confounders, the investigators found that high consumers of sugary drinks or artificially sweetened drinks saw 20% and 32% higher risk of such events, respectively, compared with people who reported drinking neither beverage type (hazard ratio: 1.20; 95% confidence interval 1.04-1.40, P for trend < .0009 and HR: 1.32; 95% CI, 1.00-1.73, P for trend < .03).
Sugary drinks were defined as containing 5% or more of sugars, including natural fruit juices. The high consumers in the study had a median intake of 185 mL per day of sugary drinks, or 176 mL per day for artificially sweetened drinks. Natural noncaloric sweeteners such as Stevia were included in the artificially sweetened group.
The findings, Mr. Chazelas and colleagues wrote in their analysis, add to evidence that artificially sweetened beverages “might not be a healthy substitute for sugary drinks.” While research has suggested that artificial sweeteners induce glucose intolerance by disturbing gut microbiota, they noted, more and bigger studies are needed to understand the mechanisms by which they might bear on cardiovascular disease risk.
Robert A. Vogel, MD, of the University of Colorado Denver, urged caution in interpreting the researchers’ results. In an interview, Dr. Vogel, a preventive cardiologist, said that it is “notoriously difficult” to evaluate what a food or food group does to the body outside of a carefully controlled trial. What little randomized trial evidence exists comparing the health effects of artificially sweetened and sugary drinks includes a 2012 trial in children that found diet drinks associated with reductions in body fat – if anything a positive indication for heart health.
With adults enrolled in an observational study, things are much more easily confounded, Dr. Vogel said. “So subjects self-report that they’re not consuming one thing – sugary or sweetened beverages. What else are they putting into their diet? Maybe they’re eating dessert and consuming sugar that way. Try as you will to unconfound, to do a multivariate correction for all these factors is just very difficult.”
In addition, Dr. Vogel noted, the investigators made no attempt to discern among the different sweeteners consumed. “Stevia, saccharine, Sucralose – it’s highly unlikely that each of these agents has the same effect on gut microbiota.”
In 2019, researchers led by Mr. Chazelas looked at cancer risk in high consumers of the sugary and artificially sweetened drinks in some 107,000 patients from the cohort, and reported that sugary drinks were significantly associated with the risk of overall cancer. They saw no similar association for artificially sweetened drinks.
The NutriNet-Santé study is funded by the French government, and the investigators disclosed no financial support from commercial entities. Dr. Vogel has received research support from Sanofi and speaking fees from Regeneron.
Sugary and artificially sweetened drinks are each associated with an increased risk of developing cardiovascular disease, according to results from a large prospective cohort study.
However, the design of that study fails to take into account other sources of dietary sugar, according to one expert.
In a research letter published online Oct. 26 in the Journal of the American College of Cardiology, Eloi Chazelas, a PhD candidate at Sorbonne Paris Nord University in Paris, and colleagues, shared results from nearly 105,000 subjects (79% women, mean age 43 at baseline, median follow up 6.6 years) enrolled in the NutriNet-Santé cohort study.
In this observational study, which began recruiting in 2009, dietary patterns are self-reported by subjects, while health outcomes are validated by investigators.
Mr. Chazelas and his colleagues identified 1,379 first incident cases of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, and angioplasty in the cohort during 2009-2019. Cases that occurred during the first 3 years’ follow up were excluded from the analysis, to avoid potential reverse causality bias.
After adjustment for a wide range of dietary, demographic and health confounders, the investigators found that high consumers of sugary drinks or artificially sweetened drinks saw 20% and 32% higher risk of such events, respectively, compared with people who reported drinking neither beverage type (hazard ratio: 1.20; 95% confidence interval 1.04-1.40, P for trend < .0009 and HR: 1.32; 95% CI, 1.00-1.73, P for trend < .03).
Sugary drinks were defined as containing 5% or more of sugars, including natural fruit juices. The high consumers in the study had a median intake of 185 mL per day of sugary drinks, or 176 mL per day for artificially sweetened drinks. Natural noncaloric sweeteners such as Stevia were included in the artificially sweetened group.
The findings, Mr. Chazelas and colleagues wrote in their analysis, add to evidence that artificially sweetened beverages “might not be a healthy substitute for sugary drinks.” While research has suggested that artificial sweeteners induce glucose intolerance by disturbing gut microbiota, they noted, more and bigger studies are needed to understand the mechanisms by which they might bear on cardiovascular disease risk.
Robert A. Vogel, MD, of the University of Colorado Denver, urged caution in interpreting the researchers’ results. In an interview, Dr. Vogel, a preventive cardiologist, said that it is “notoriously difficult” to evaluate what a food or food group does to the body outside of a carefully controlled trial. What little randomized trial evidence exists comparing the health effects of artificially sweetened and sugary drinks includes a 2012 trial in children that found diet drinks associated with reductions in body fat – if anything a positive indication for heart health.
With adults enrolled in an observational study, things are much more easily confounded, Dr. Vogel said. “So subjects self-report that they’re not consuming one thing – sugary or sweetened beverages. What else are they putting into their diet? Maybe they’re eating dessert and consuming sugar that way. Try as you will to unconfound, to do a multivariate correction for all these factors is just very difficult.”
In addition, Dr. Vogel noted, the investigators made no attempt to discern among the different sweeteners consumed. “Stevia, saccharine, Sucralose – it’s highly unlikely that each of these agents has the same effect on gut microbiota.”
In 2019, researchers led by Mr. Chazelas looked at cancer risk in high consumers of the sugary and artificially sweetened drinks in some 107,000 patients from the cohort, and reported that sugary drinks were significantly associated with the risk of overall cancer. They saw no similar association for artificially sweetened drinks.
The NutriNet-Santé study is funded by the French government, and the investigators disclosed no financial support from commercial entities. Dr. Vogel has received research support from Sanofi and speaking fees from Regeneron.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Top AGA Community patient cases
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community (https://community.gastro.org) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. The upgraded networking platform now features a newsfeed for difficult patient scenarios and regularly scheduled Roundtable discussions with experts in the field.
In case you missed it, here are some clinical discussions and Roundtables in the newsfeed this month:
- Initial results from the FMT National Registry (https://community.gastro.org/posts/22693)
- Practice update: Reducing rates of post endoscopy esophageal adenocarcinoma (https://community.gastro.org/posts/22805)
- Patient case: Eosinophilic gastrointestinal disease (https://community.gastro.org/posts/22743)
- Patient case: Repeat colonoscopy following splenic injury (https://community.gastro.org/posts/22739)
- Windows on Clinical GI Roundtables: Crohn’s disease and gastroparesis
View upcoming Roundtables in the community at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community (https://community.gastro.org) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. The upgraded networking platform now features a newsfeed for difficult patient scenarios and regularly scheduled Roundtable discussions with experts in the field.
In case you missed it, here are some clinical discussions and Roundtables in the newsfeed this month:
- Initial results from the FMT National Registry (https://community.gastro.org/posts/22693)
- Practice update: Reducing rates of post endoscopy esophageal adenocarcinoma (https://community.gastro.org/posts/22805)
- Patient case: Eosinophilic gastrointestinal disease (https://community.gastro.org/posts/22743)
- Patient case: Repeat colonoscopy following splenic injury (https://community.gastro.org/posts/22739)
- Windows on Clinical GI Roundtables: Crohn’s disease and gastroparesis
View upcoming Roundtables in the community at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community (https://community.gastro.org) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. The upgraded networking platform now features a newsfeed for difficult patient scenarios and regularly scheduled Roundtable discussions with experts in the field.
In case you missed it, here are some clinical discussions and Roundtables in the newsfeed this month:
- Initial results from the FMT National Registry (https://community.gastro.org/posts/22693)
- Practice update: Reducing rates of post endoscopy esophageal adenocarcinoma (https://community.gastro.org/posts/22805)
- Patient case: Eosinophilic gastrointestinal disease (https://community.gastro.org/posts/22743)
- Patient case: Repeat colonoscopy following splenic injury (https://community.gastro.org/posts/22739)
- Windows on Clinical GI Roundtables: Crohn’s disease and gastroparesis
View upcoming Roundtables in the community at https://community.gastro.org/discussions.
Rituximab vs. glatiramer acetate for secondary progressive multiple sclerosis
Key clinical point: Both rituximab and glatiramer acetate failed to stop disability progression in patients with secondary progressive multiple sclerosis (SPMS). They were equally effective in relapse control.
Major finding: The mean Expanded Disability Status Scale (EDSS) score increased from 3.05 to 4.14 in the rituximab group and from 3.22 to 4.60 in the glatiramer acetate group (P less than .001 for both). EDSS score showed no statistically significant difference between 2 groups (P = .071). Annualized relapse rate decreased in both groups with no significant difference between them (P = .534).
Study details: An open randomized clinical trial of 84 patients with SPMS assigned to receive rituximab (n = 43) or glatiramer acetate (n = 41) for 12 months.
Disclosures: The study was funded by vice-chancellor for research and technology of Isfahan University of Medical Sciences. The authors declared no conflicts of interest.
Citation: Cheshmavar M et al. Acta Neurol Scand. 2020 Sep 8. doi: 10.1111/ane.13344.
Key clinical point: Both rituximab and glatiramer acetate failed to stop disability progression in patients with secondary progressive multiple sclerosis (SPMS). They were equally effective in relapse control.
Major finding: The mean Expanded Disability Status Scale (EDSS) score increased from 3.05 to 4.14 in the rituximab group and from 3.22 to 4.60 in the glatiramer acetate group (P less than .001 for both). EDSS score showed no statistically significant difference between 2 groups (P = .071). Annualized relapse rate decreased in both groups with no significant difference between them (P = .534).
Study details: An open randomized clinical trial of 84 patients with SPMS assigned to receive rituximab (n = 43) or glatiramer acetate (n = 41) for 12 months.
Disclosures: The study was funded by vice-chancellor for research and technology of Isfahan University of Medical Sciences. The authors declared no conflicts of interest.
Citation: Cheshmavar M et al. Acta Neurol Scand. 2020 Sep 8. doi: 10.1111/ane.13344.
Key clinical point: Both rituximab and glatiramer acetate failed to stop disability progression in patients with secondary progressive multiple sclerosis (SPMS). They were equally effective in relapse control.
Major finding: The mean Expanded Disability Status Scale (EDSS) score increased from 3.05 to 4.14 in the rituximab group and from 3.22 to 4.60 in the glatiramer acetate group (P less than .001 for both). EDSS score showed no statistically significant difference between 2 groups (P = .071). Annualized relapse rate decreased in both groups with no significant difference between them (P = .534).
Study details: An open randomized clinical trial of 84 patients with SPMS assigned to receive rituximab (n = 43) or glatiramer acetate (n = 41) for 12 months.
Disclosures: The study was funded by vice-chancellor for research and technology of Isfahan University of Medical Sciences. The authors declared no conflicts of interest.
Citation: Cheshmavar M et al. Acta Neurol Scand. 2020 Sep 8. doi: 10.1111/ane.13344.