User login
Tofacitinib retreatment effective for ulcerative colitis
Retreatment with tofacitinib after a period of treatment interruption was well tolerated and effective in patients with ulcerative colitis who had shown a previous response to tofacitinib induction, according to an analysis of data from the OCTAVE extension trial.
“Clinical response was recaptured in most patients by month 2, and about half of patients by month 36, irrespective of prior anti–[tumor necrosis factor] status,” said lead researcher Edward V. Loftus Jr, MD, from the Mayo Medical School, Rochester, Minn.
A temporary suspension of treatment with the oral, small-molecule Janus kinase (JAK) inhibitor might be necessary for a number of reasons, such as if a patient has to undergo surgery, experiences adverse events, or becomes pregnant.
For their study, Dr. Loftus and colleagues set out to assess the safety and efficacy of retreatment after a period of interruption.
“The population we’re interested in are patients who received tofacitinib during induction and placebo during maintenance” in the original OCTAVE trials, said Dr. Loftus. “They then either completed the trial or flared and rolled over to the open-label extension.”
The researchers looked at the 100 patients who had achieved a clinical response after 8 weeks of treatment with tofacitinib 10 mg twice-daily in the OCTAVE Induction 1 and OCTAVE Induction 2 trials and then received placebo in the OCTAVE Sustain trial and experienced treatment failure between week 8 and week 52. These patients went on to receive tofacitinib 10 mg twice daily as part of the ongoing, open-label, long-term extension OCTAVE Open trial.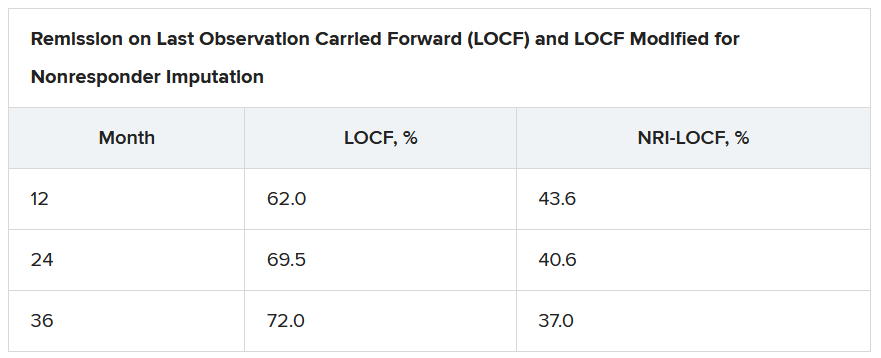
Treatment failure was defined as an increase of at least 3 points from the baseline total Mayo score achieved in OCTAVE Sustain, plus an increase of at least 1 point in rectal bleeding and endoscopic subscores and an absolute endoscopic subscore of at least 2 points after at least 8 weeks of treatment. Efficacy was evaluated for up to 36 months in the open-label extension; adverse events were assessed throughout the study period.
The median time to treatment failure was 135 days, Dr. Loftus reported during his award-winning presentation at the virtual annual meeting of the American College of Gastroenterology.
On last observation carried forward (LOCF) analysis, or observed data, 85.2% of the patients had recaptured clinical response by month 2. That rate fell to 74.3% when the analysis was modified for nonresponder imputation (NRI).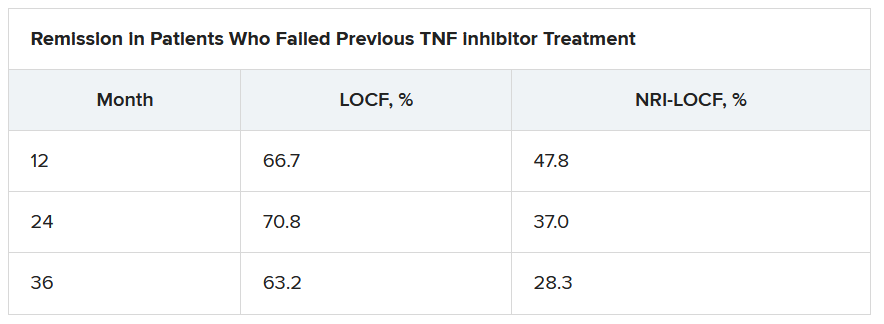
“The truth lies somewhere in between,” Dr. Loftus said.
Of interest, a clinical response to tofacitinib retreatment at month 2 was achieved by 92.5% (observed data) and 80.4% (NRI-LOCF) of patients who experienced treatment failure after tumor necrosis factor inhibitor therapy.
“Many patients were able to regain response with tofacitinib and then maintain that over time,” said Dr. Loftus.
Study supports retreatment, which is good news for patients
Incidence rates of adverse events were comparable in the retreatment population and in the overall extension cohort. “There are no signals jumping out, saying that safety events were higher or more frequent in this retreatment population, which is reassuring,” Dr. Loftus added.
Findings such as these are to be expected given the mechanism of action and pharmacologic features of tofacitinib, said Gionata Fiorino, MD, from Humanitas University in Milan, who was not involved in the study.
“I think this is important for patients who need to stop therapy for several reasons – pregnancy, adverse events that do not require permanent withdrawal of the drug, or surgical interventions – and experience a flare after drug withdrawal,” he said in an interview.
“There are several other therapeutic options for these patients, but I have experienced many patients who do not respond to other mechanisms of action apart from JAK [inhibitors],” he added. “And, in the case of a patient who has stopped the drug after having achieved remission, this study clearly supports retreatment, which is good news, especially for patients.”
This study was funded by Pfizer. Dr. Loftus reported financial relationships with AbbVie, Allergan, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Exact Sciences, Genentech, Gilead, Janssen, Pfizer, Robarts Clinical Trials, Takeda, and UCB. Dr. Fiorino reports financial relationships with MSD, Takeda, AbbVie, Janssen, Pfizer, Celltrion, Sandoz, AlfaSigma, Samsung, Amgen, Roche, and Ferring.
A version of this article originally appeared on Medscape.com.
Retreatment with tofacitinib after a period of treatment interruption was well tolerated and effective in patients with ulcerative colitis who had shown a previous response to tofacitinib induction, according to an analysis of data from the OCTAVE extension trial.
“Clinical response was recaptured in most patients by month 2, and about half of patients by month 36, irrespective of prior anti–[tumor necrosis factor] status,” said lead researcher Edward V. Loftus Jr, MD, from the Mayo Medical School, Rochester, Minn.
A temporary suspension of treatment with the oral, small-molecule Janus kinase (JAK) inhibitor might be necessary for a number of reasons, such as if a patient has to undergo surgery, experiences adverse events, or becomes pregnant.
For their study, Dr. Loftus and colleagues set out to assess the safety and efficacy of retreatment after a period of interruption.
“The population we’re interested in are patients who received tofacitinib during induction and placebo during maintenance” in the original OCTAVE trials, said Dr. Loftus. “They then either completed the trial or flared and rolled over to the open-label extension.”
The researchers looked at the 100 patients who had achieved a clinical response after 8 weeks of treatment with tofacitinib 10 mg twice-daily in the OCTAVE Induction 1 and OCTAVE Induction 2 trials and then received placebo in the OCTAVE Sustain trial and experienced treatment failure between week 8 and week 52. These patients went on to receive tofacitinib 10 mg twice daily as part of the ongoing, open-label, long-term extension OCTAVE Open trial.
Treatment failure was defined as an increase of at least 3 points from the baseline total Mayo score achieved in OCTAVE Sustain, plus an increase of at least 1 point in rectal bleeding and endoscopic subscores and an absolute endoscopic subscore of at least 2 points after at least 8 weeks of treatment. Efficacy was evaluated for up to 36 months in the open-label extension; adverse events were assessed throughout the study period.
The median time to treatment failure was 135 days, Dr. Loftus reported during his award-winning presentation at the virtual annual meeting of the American College of Gastroenterology.
On last observation carried forward (LOCF) analysis, or observed data, 85.2% of the patients had recaptured clinical response by month 2. That rate fell to 74.3% when the analysis was modified for nonresponder imputation (NRI).
“The truth lies somewhere in between,” Dr. Loftus said.
Of interest, a clinical response to tofacitinib retreatment at month 2 was achieved by 92.5% (observed data) and 80.4% (NRI-LOCF) of patients who experienced treatment failure after tumor necrosis factor inhibitor therapy.
“Many patients were able to regain response with tofacitinib and then maintain that over time,” said Dr. Loftus.
Study supports retreatment, which is good news for patients
Incidence rates of adverse events were comparable in the retreatment population and in the overall extension cohort. “There are no signals jumping out, saying that safety events were higher or more frequent in this retreatment population, which is reassuring,” Dr. Loftus added.
Findings such as these are to be expected given the mechanism of action and pharmacologic features of tofacitinib, said Gionata Fiorino, MD, from Humanitas University in Milan, who was not involved in the study.
“I think this is important for patients who need to stop therapy for several reasons – pregnancy, adverse events that do not require permanent withdrawal of the drug, or surgical interventions – and experience a flare after drug withdrawal,” he said in an interview.
“There are several other therapeutic options for these patients, but I have experienced many patients who do not respond to other mechanisms of action apart from JAK [inhibitors],” he added. “And, in the case of a patient who has stopped the drug after having achieved remission, this study clearly supports retreatment, which is good news, especially for patients.”
This study was funded by Pfizer. Dr. Loftus reported financial relationships with AbbVie, Allergan, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Exact Sciences, Genentech, Gilead, Janssen, Pfizer, Robarts Clinical Trials, Takeda, and UCB. Dr. Fiorino reports financial relationships with MSD, Takeda, AbbVie, Janssen, Pfizer, Celltrion, Sandoz, AlfaSigma, Samsung, Amgen, Roche, and Ferring.
A version of this article originally appeared on Medscape.com.
Retreatment with tofacitinib after a period of treatment interruption was well tolerated and effective in patients with ulcerative colitis who had shown a previous response to tofacitinib induction, according to an analysis of data from the OCTAVE extension trial.
“Clinical response was recaptured in most patients by month 2, and about half of patients by month 36, irrespective of prior anti–[tumor necrosis factor] status,” said lead researcher Edward V. Loftus Jr, MD, from the Mayo Medical School, Rochester, Minn.
A temporary suspension of treatment with the oral, small-molecule Janus kinase (JAK) inhibitor might be necessary for a number of reasons, such as if a patient has to undergo surgery, experiences adverse events, or becomes pregnant.
For their study, Dr. Loftus and colleagues set out to assess the safety and efficacy of retreatment after a period of interruption.
“The population we’re interested in are patients who received tofacitinib during induction and placebo during maintenance” in the original OCTAVE trials, said Dr. Loftus. “They then either completed the trial or flared and rolled over to the open-label extension.”
The researchers looked at the 100 patients who had achieved a clinical response after 8 weeks of treatment with tofacitinib 10 mg twice-daily in the OCTAVE Induction 1 and OCTAVE Induction 2 trials and then received placebo in the OCTAVE Sustain trial and experienced treatment failure between week 8 and week 52. These patients went on to receive tofacitinib 10 mg twice daily as part of the ongoing, open-label, long-term extension OCTAVE Open trial.
Treatment failure was defined as an increase of at least 3 points from the baseline total Mayo score achieved in OCTAVE Sustain, plus an increase of at least 1 point in rectal bleeding and endoscopic subscores and an absolute endoscopic subscore of at least 2 points after at least 8 weeks of treatment. Efficacy was evaluated for up to 36 months in the open-label extension; adverse events were assessed throughout the study period.
The median time to treatment failure was 135 days, Dr. Loftus reported during his award-winning presentation at the virtual annual meeting of the American College of Gastroenterology.
On last observation carried forward (LOCF) analysis, or observed data, 85.2% of the patients had recaptured clinical response by month 2. That rate fell to 74.3% when the analysis was modified for nonresponder imputation (NRI).
“The truth lies somewhere in between,” Dr. Loftus said.
Of interest, a clinical response to tofacitinib retreatment at month 2 was achieved by 92.5% (observed data) and 80.4% (NRI-LOCF) of patients who experienced treatment failure after tumor necrosis factor inhibitor therapy.
“Many patients were able to regain response with tofacitinib and then maintain that over time,” said Dr. Loftus.
Study supports retreatment, which is good news for patients
Incidence rates of adverse events were comparable in the retreatment population and in the overall extension cohort. “There are no signals jumping out, saying that safety events were higher or more frequent in this retreatment population, which is reassuring,” Dr. Loftus added.
Findings such as these are to be expected given the mechanism of action and pharmacologic features of tofacitinib, said Gionata Fiorino, MD, from Humanitas University in Milan, who was not involved in the study.
“I think this is important for patients who need to stop therapy for several reasons – pregnancy, adverse events that do not require permanent withdrawal of the drug, or surgical interventions – and experience a flare after drug withdrawal,” he said in an interview.
“There are several other therapeutic options for these patients, but I have experienced many patients who do not respond to other mechanisms of action apart from JAK [inhibitors],” he added. “And, in the case of a patient who has stopped the drug after having achieved remission, this study clearly supports retreatment, which is good news, especially for patients.”
This study was funded by Pfizer. Dr. Loftus reported financial relationships with AbbVie, Allergan, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Exact Sciences, Genentech, Gilead, Janssen, Pfizer, Robarts Clinical Trials, Takeda, and UCB. Dr. Fiorino reports financial relationships with MSD, Takeda, AbbVie, Janssen, Pfizer, Celltrion, Sandoz, AlfaSigma, Samsung, Amgen, Roche, and Ferring.
A version of this article originally appeared on Medscape.com.
Trump signs CR with Medicare loan relief
President Trump on Oct. 1 signed a bill to keep the federal government running through Dec. 11. This “continuing resolution” (CR), which was approved by the House by a 359-57 vote and the Senate by a 84-10 vote, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under Medicare’s Accelerated and Advance Payments (AAP) Program, the Centers for Medicare & Medicaid Services (CMS) advanced funds to physicians who were financially impacted by the pandemic.
Revisions were made under the Coronavirus Aid, Relief, and Economic Security (CARES) Act to broaden the existing program to supply provider relief related to the public health emergency. The program was revised in March but suspended accepting new applications related to the pandemic in late April.
Physicians who received APP loans were required to begin repayment within 120 days after the loan disbursement. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with a 10.25 % interest rate.
For practices that received these advances, their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
Under the new loan repayment terms in the CR, repayment of the disbursed funds is postponed until 365 days after the date on which a practice received the money. The balance is due by September 2022.
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with an interest rate of 4%.
More than 80% of the $100 billion that CMS loaned to health care providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
The American Gastroenterological Association has been advocating for more flexibility for the financial assistance programs, such as the Accelerated and Advanced Payment Program and the Paycheck Protection Program, that physicians have utilized. It is critical to give physicians leeway on these loans given that many practices are still not operating at full capacity.
Based on reporting from Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through Dec. 11. This “continuing resolution” (CR), which was approved by the House by a 359-57 vote and the Senate by a 84-10 vote, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under Medicare’s Accelerated and Advance Payments (AAP) Program, the Centers for Medicare & Medicaid Services (CMS) advanced funds to physicians who were financially impacted by the pandemic.
Revisions were made under the Coronavirus Aid, Relief, and Economic Security (CARES) Act to broaden the existing program to supply provider relief related to the public health emergency. The program was revised in March but suspended accepting new applications related to the pandemic in late April.
Physicians who received APP loans were required to begin repayment within 120 days after the loan disbursement. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with a 10.25 % interest rate.
For practices that received these advances, their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
Under the new loan repayment terms in the CR, repayment of the disbursed funds is postponed until 365 days after the date on which a practice received the money. The balance is due by September 2022.
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with an interest rate of 4%.
More than 80% of the $100 billion that CMS loaned to health care providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
The American Gastroenterological Association has been advocating for more flexibility for the financial assistance programs, such as the Accelerated and Advanced Payment Program and the Paycheck Protection Program, that physicians have utilized. It is critical to give physicians leeway on these loans given that many practices are still not operating at full capacity.
Based on reporting from Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through Dec. 11. This “continuing resolution” (CR), which was approved by the House by a 359-57 vote and the Senate by a 84-10 vote, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under Medicare’s Accelerated and Advance Payments (AAP) Program, the Centers for Medicare & Medicaid Services (CMS) advanced funds to physicians who were financially impacted by the pandemic.
Revisions were made under the Coronavirus Aid, Relief, and Economic Security (CARES) Act to broaden the existing program to supply provider relief related to the public health emergency. The program was revised in March but suspended accepting new applications related to the pandemic in late April.
Physicians who received APP loans were required to begin repayment within 120 days after the loan disbursement. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with a 10.25 % interest rate.
For practices that received these advances, their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
Under the new loan repayment terms in the CR, repayment of the disbursed funds is postponed until 365 days after the date on which a practice received the money. The balance is due by September 2022.
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with an interest rate of 4%.
More than 80% of the $100 billion that CMS loaned to health care providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
The American Gastroenterological Association has been advocating for more flexibility for the financial assistance programs, such as the Accelerated and Advanced Payment Program and the Paycheck Protection Program, that physicians have utilized. It is critical to give physicians leeway on these loans given that many practices are still not operating at full capacity.
Based on reporting from Medscape.com.
Cannabis may improve liver function in patients with obesity
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
FROM ACG 2020
Menstrual irregularity appears to be predictor of early death
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
FROM THE BMJ
‘Tour de force’ study reveals therapeutic targets in 38% of cancer patients
The effort is the National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) trial. For this study, researchers performed next-generation sequencing on tumor biopsy specimens to identify therapeutically actionable molecular alterations in patients with “underexplored” cancer types.
The trial included 5,954 patients with cancers that had progressed on standard treatments or rare cancers for which there is no standard treatment. If actionable alterations were found in these patients, they could receive new drugs in development that showed promise in other clinical trials or drugs that were approved by the Food and Drug Administration to treat at least one cancer type.
Data newly reported in the Journal of Clinical Oncology showed that 37.6% of patients had alterations that could be matched to targeted drugs, and 17.8% of patients were assigned to targeted treatment. Multiple actionable tumor mutations were seen in 11.9% of specimens, and resistance-conferring mutations were seen in 71.3% of specimens.
“The bottom line from this report is that next-generation sequencing is an efficient way to identify both approved and promising investigational therapies. For this reason, it should be considered standard of care for patients with advanced cancers,” said study chair Keith T. Flaherty, MD, director of the Henri and Belinda Termeer Center for Targeted Therapy at Massachusetts General Hospital Cancer Center in Boston.
“This study sets the benchmark for the ‘actionability’ of next-generation sequencing,” Dr. Flaherty added. “We expect this number [of actionable alterations] will continue to rise steadily as further advances are made in the development of therapies that target some of the genetic alterations for which we could not offer treatment options in NCI-MATCH.”
Relapsed/refractory vs. primary tumors
The NCI-MATCH researchers focused on the most commonly found genetic alterations and performed biopsies at study entry to provide the most accurate picture of the genetic landscape of relapsed/refractory cancer patients. That makes this cohort distinct from The Cancer Genome Atlas (TCGA), a database of patients with mostly untreated primary tumors, and other published cohorts that include genetic analysis of primary tumors and biopsies from the time of initial metastatic recurrence.
The researchers compared the tumor gene makeup of NCI-MATCH and TCGA patients with seven cancer types – breast, bile duct, cervix, colorectal, lung, pancreas, and prostate.
“Perhaps the biggest surprise was the relatively minimal change in the genetic alterations found in these relapsed/refractory patients, compared to primary tumors,” Dr. Flaherty said. “These findings suggest that it is very reasonable to perform next-generation sequencing at the time of initial metastatic cancer diagnosis and to rely on those findings for the purposes of considering FDA-approved therapies and clinical trial participation.”
Multiple alterations and resistance
The complex genetics of cancers has led researchers to explore combinations of targeted and other therapies to address multiple defects at the same time.
“Not surprisingly, the most common collision of multiple genetic alterations within the same tumor was in the commonly altered tumor suppressor genes: TP53, APC, and PTEN,” Dr. Flaherty said.
“An increasing body of evidence supports a role for loss-of-function alterations in these genes to confer resistance to many targeted therapies,” he added. “While we don’t have targeted therapies yet established to directly counter this form of therapeutic resistance, we hypothesize that various types of combination therapy may be able to indirectly undercut resistance and enhance the benefit of many targeted therapies.”
The NCI-MATCH researchers will continue to mine this large dataset to better understand the many small, genetically defined cancer subpopulations.
“We will continue to report the outcome of the individual treatment subprotocols, and combining this genetic analysis with those outcomes will likely inform the next clinical trials,” Dr. Flaherty said.
Actionable mutations make a difference
Precision oncology experts agree that NCI-MATCH results are impressive and add a fuller appreciation that actionable mutations make a clinical difference.
“This is a powerful, extremely well-designed study, a tour de force of collaborative science,” said Stephen Gruber, MD, PhD, director of the Center for Precision Medicine at City of Hope National Medical Center in Duarte, Calif.
“The future holds even more promise,” he added. “Our ability to interrogate the genomic landscape of cancer is improving rapidly. Tumor testing helps get the right drug to the right tumor faster than a guidelines-based approach from historical data of combination chemotherapy. This is a likely game changer for the way oncologists will practice in the future, especially as we learn more results of subset trials. The NCI-MATCH researchers have taken a laser-focused look at the current data, but we now know we can look far more comprehensively at genomic profiles of tumors.”
From the viewpoint of the practicing oncologist, co-occurring resistance mutations make a difference in defining what combinations are likely and, more importantly, less likely to be effective. “When we see two mutations and one is likely to confer resistance, we can make a choice to avoid a drug that is not likely to work,” Dr. Gruber said.
“The NCI-MATCH trial allows both approved and investigational agents, which expands the possibility of matching patients to newer agents. This is especially relevant if there are no FDA-approved drugs yet for some molecular aberrations,” said Lillian L. Siu, MD, a senior medical oncologist at the Princess Margaret Cancer Centre in Toronto. “This trial enables such evaluations under the auspice of a clinical trial to provide important knowledge.”
Both experts agree that in-depth biological interrogations of cancer will move the field of precision oncology forward. Dr. Gruber said that “studies have not yet fully addressed the power of germline genetic testing of DNA. Inherited susceptibility will drive therapeutic choices – for example, PARP inhibitors that access homologous recombination deficiency for breast, ovarian, and prostate cancer. We will learn more about treatment choices for those cancers.”
Dr. Siu added: “I truly believe that liquid biopsies [circulating tumor DNA] will help us perform target-drug matching in a less invasive way. We need to explore beyond the genome to look at the transcriptome, proteome, epigenome, and immunome, among others. It is likely that multiomic predictors are going to be able to identify more therapeutic options compared to single genomic predictors.”
Dr. Flaherty noted that all tumor samples from patients assigned to treatment are being subjected to whole-exome sequencing to further the discovery of the genetic features of responsive and nonresponsive tumors.
NCI-MATCH was funded by the National Cancer Institute. Dr. Flaherty disclosed relationships with Clovis Oncology, Loxo, X4 Pharma, and many other companies. His coauthors disclosed many conflicts as well. Dr. Gruber is cofounder of Brogent International. Dr. Siu disclosed relationships with Agios, Treadwell Therapeutics, Merck, Pfizer, and many other companies.
SOURCE: Flaherty KT et al. J Clin Oncol. 2020 Oct 13. doi: 10.1200/JCO.19.03010.
The effort is the National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) trial. For this study, researchers performed next-generation sequencing on tumor biopsy specimens to identify therapeutically actionable molecular alterations in patients with “underexplored” cancer types.
The trial included 5,954 patients with cancers that had progressed on standard treatments or rare cancers for which there is no standard treatment. If actionable alterations were found in these patients, they could receive new drugs in development that showed promise in other clinical trials or drugs that were approved by the Food and Drug Administration to treat at least one cancer type.
Data newly reported in the Journal of Clinical Oncology showed that 37.6% of patients had alterations that could be matched to targeted drugs, and 17.8% of patients were assigned to targeted treatment. Multiple actionable tumor mutations were seen in 11.9% of specimens, and resistance-conferring mutations were seen in 71.3% of specimens.
“The bottom line from this report is that next-generation sequencing is an efficient way to identify both approved and promising investigational therapies. For this reason, it should be considered standard of care for patients with advanced cancers,” said study chair Keith T. Flaherty, MD, director of the Henri and Belinda Termeer Center for Targeted Therapy at Massachusetts General Hospital Cancer Center in Boston.
“This study sets the benchmark for the ‘actionability’ of next-generation sequencing,” Dr. Flaherty added. “We expect this number [of actionable alterations] will continue to rise steadily as further advances are made in the development of therapies that target some of the genetic alterations for which we could not offer treatment options in NCI-MATCH.”
Relapsed/refractory vs. primary tumors
The NCI-MATCH researchers focused on the most commonly found genetic alterations and performed biopsies at study entry to provide the most accurate picture of the genetic landscape of relapsed/refractory cancer patients. That makes this cohort distinct from The Cancer Genome Atlas (TCGA), a database of patients with mostly untreated primary tumors, and other published cohorts that include genetic analysis of primary tumors and biopsies from the time of initial metastatic recurrence.
The researchers compared the tumor gene makeup of NCI-MATCH and TCGA patients with seven cancer types – breast, bile duct, cervix, colorectal, lung, pancreas, and prostate.
“Perhaps the biggest surprise was the relatively minimal change in the genetic alterations found in these relapsed/refractory patients, compared to primary tumors,” Dr. Flaherty said. “These findings suggest that it is very reasonable to perform next-generation sequencing at the time of initial metastatic cancer diagnosis and to rely on those findings for the purposes of considering FDA-approved therapies and clinical trial participation.”
Multiple alterations and resistance
The complex genetics of cancers has led researchers to explore combinations of targeted and other therapies to address multiple defects at the same time.
“Not surprisingly, the most common collision of multiple genetic alterations within the same tumor was in the commonly altered tumor suppressor genes: TP53, APC, and PTEN,” Dr. Flaherty said.
“An increasing body of evidence supports a role for loss-of-function alterations in these genes to confer resistance to many targeted therapies,” he added. “While we don’t have targeted therapies yet established to directly counter this form of therapeutic resistance, we hypothesize that various types of combination therapy may be able to indirectly undercut resistance and enhance the benefit of many targeted therapies.”
The NCI-MATCH researchers will continue to mine this large dataset to better understand the many small, genetically defined cancer subpopulations.
“We will continue to report the outcome of the individual treatment subprotocols, and combining this genetic analysis with those outcomes will likely inform the next clinical trials,” Dr. Flaherty said.
Actionable mutations make a difference
Precision oncology experts agree that NCI-MATCH results are impressive and add a fuller appreciation that actionable mutations make a clinical difference.
“This is a powerful, extremely well-designed study, a tour de force of collaborative science,” said Stephen Gruber, MD, PhD, director of the Center for Precision Medicine at City of Hope National Medical Center in Duarte, Calif.
“The future holds even more promise,” he added. “Our ability to interrogate the genomic landscape of cancer is improving rapidly. Tumor testing helps get the right drug to the right tumor faster than a guidelines-based approach from historical data of combination chemotherapy. This is a likely game changer for the way oncologists will practice in the future, especially as we learn more results of subset trials. The NCI-MATCH researchers have taken a laser-focused look at the current data, but we now know we can look far more comprehensively at genomic profiles of tumors.”
From the viewpoint of the practicing oncologist, co-occurring resistance mutations make a difference in defining what combinations are likely and, more importantly, less likely to be effective. “When we see two mutations and one is likely to confer resistance, we can make a choice to avoid a drug that is not likely to work,” Dr. Gruber said.
“The NCI-MATCH trial allows both approved and investigational agents, which expands the possibility of matching patients to newer agents. This is especially relevant if there are no FDA-approved drugs yet for some molecular aberrations,” said Lillian L. Siu, MD, a senior medical oncologist at the Princess Margaret Cancer Centre in Toronto. “This trial enables such evaluations under the auspice of a clinical trial to provide important knowledge.”
Both experts agree that in-depth biological interrogations of cancer will move the field of precision oncology forward. Dr. Gruber said that “studies have not yet fully addressed the power of germline genetic testing of DNA. Inherited susceptibility will drive therapeutic choices – for example, PARP inhibitors that access homologous recombination deficiency for breast, ovarian, and prostate cancer. We will learn more about treatment choices for those cancers.”
Dr. Siu added: “I truly believe that liquid biopsies [circulating tumor DNA] will help us perform target-drug matching in a less invasive way. We need to explore beyond the genome to look at the transcriptome, proteome, epigenome, and immunome, among others. It is likely that multiomic predictors are going to be able to identify more therapeutic options compared to single genomic predictors.”
Dr. Flaherty noted that all tumor samples from patients assigned to treatment are being subjected to whole-exome sequencing to further the discovery of the genetic features of responsive and nonresponsive tumors.
NCI-MATCH was funded by the National Cancer Institute. Dr. Flaherty disclosed relationships with Clovis Oncology, Loxo, X4 Pharma, and many other companies. His coauthors disclosed many conflicts as well. Dr. Gruber is cofounder of Brogent International. Dr. Siu disclosed relationships with Agios, Treadwell Therapeutics, Merck, Pfizer, and many other companies.
SOURCE: Flaherty KT et al. J Clin Oncol. 2020 Oct 13. doi: 10.1200/JCO.19.03010.
The effort is the National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) trial. For this study, researchers performed next-generation sequencing on tumor biopsy specimens to identify therapeutically actionable molecular alterations in patients with “underexplored” cancer types.
The trial included 5,954 patients with cancers that had progressed on standard treatments or rare cancers for which there is no standard treatment. If actionable alterations were found in these patients, they could receive new drugs in development that showed promise in other clinical trials or drugs that were approved by the Food and Drug Administration to treat at least one cancer type.
Data newly reported in the Journal of Clinical Oncology showed that 37.6% of patients had alterations that could be matched to targeted drugs, and 17.8% of patients were assigned to targeted treatment. Multiple actionable tumor mutations were seen in 11.9% of specimens, and resistance-conferring mutations were seen in 71.3% of specimens.
“The bottom line from this report is that next-generation sequencing is an efficient way to identify both approved and promising investigational therapies. For this reason, it should be considered standard of care for patients with advanced cancers,” said study chair Keith T. Flaherty, MD, director of the Henri and Belinda Termeer Center for Targeted Therapy at Massachusetts General Hospital Cancer Center in Boston.
“This study sets the benchmark for the ‘actionability’ of next-generation sequencing,” Dr. Flaherty added. “We expect this number [of actionable alterations] will continue to rise steadily as further advances are made in the development of therapies that target some of the genetic alterations for which we could not offer treatment options in NCI-MATCH.”
Relapsed/refractory vs. primary tumors
The NCI-MATCH researchers focused on the most commonly found genetic alterations and performed biopsies at study entry to provide the most accurate picture of the genetic landscape of relapsed/refractory cancer patients. That makes this cohort distinct from The Cancer Genome Atlas (TCGA), a database of patients with mostly untreated primary tumors, and other published cohorts that include genetic analysis of primary tumors and biopsies from the time of initial metastatic recurrence.
The researchers compared the tumor gene makeup of NCI-MATCH and TCGA patients with seven cancer types – breast, bile duct, cervix, colorectal, lung, pancreas, and prostate.
“Perhaps the biggest surprise was the relatively minimal change in the genetic alterations found in these relapsed/refractory patients, compared to primary tumors,” Dr. Flaherty said. “These findings suggest that it is very reasonable to perform next-generation sequencing at the time of initial metastatic cancer diagnosis and to rely on those findings for the purposes of considering FDA-approved therapies and clinical trial participation.”
Multiple alterations and resistance
The complex genetics of cancers has led researchers to explore combinations of targeted and other therapies to address multiple defects at the same time.
“Not surprisingly, the most common collision of multiple genetic alterations within the same tumor was in the commonly altered tumor suppressor genes: TP53, APC, and PTEN,” Dr. Flaherty said.
“An increasing body of evidence supports a role for loss-of-function alterations in these genes to confer resistance to many targeted therapies,” he added. “While we don’t have targeted therapies yet established to directly counter this form of therapeutic resistance, we hypothesize that various types of combination therapy may be able to indirectly undercut resistance and enhance the benefit of many targeted therapies.”
The NCI-MATCH researchers will continue to mine this large dataset to better understand the many small, genetically defined cancer subpopulations.
“We will continue to report the outcome of the individual treatment subprotocols, and combining this genetic analysis with those outcomes will likely inform the next clinical trials,” Dr. Flaherty said.
Actionable mutations make a difference
Precision oncology experts agree that NCI-MATCH results are impressive and add a fuller appreciation that actionable mutations make a clinical difference.
“This is a powerful, extremely well-designed study, a tour de force of collaborative science,” said Stephen Gruber, MD, PhD, director of the Center for Precision Medicine at City of Hope National Medical Center in Duarte, Calif.
“The future holds even more promise,” he added. “Our ability to interrogate the genomic landscape of cancer is improving rapidly. Tumor testing helps get the right drug to the right tumor faster than a guidelines-based approach from historical data of combination chemotherapy. This is a likely game changer for the way oncologists will practice in the future, especially as we learn more results of subset trials. The NCI-MATCH researchers have taken a laser-focused look at the current data, but we now know we can look far more comprehensively at genomic profiles of tumors.”
From the viewpoint of the practicing oncologist, co-occurring resistance mutations make a difference in defining what combinations are likely and, more importantly, less likely to be effective. “When we see two mutations and one is likely to confer resistance, we can make a choice to avoid a drug that is not likely to work,” Dr. Gruber said.
“The NCI-MATCH trial allows both approved and investigational agents, which expands the possibility of matching patients to newer agents. This is especially relevant if there are no FDA-approved drugs yet for some molecular aberrations,” said Lillian L. Siu, MD, a senior medical oncologist at the Princess Margaret Cancer Centre in Toronto. “This trial enables such evaluations under the auspice of a clinical trial to provide important knowledge.”
Both experts agree that in-depth biological interrogations of cancer will move the field of precision oncology forward. Dr. Gruber said that “studies have not yet fully addressed the power of germline genetic testing of DNA. Inherited susceptibility will drive therapeutic choices – for example, PARP inhibitors that access homologous recombination deficiency for breast, ovarian, and prostate cancer. We will learn more about treatment choices for those cancers.”
Dr. Siu added: “I truly believe that liquid biopsies [circulating tumor DNA] will help us perform target-drug matching in a less invasive way. We need to explore beyond the genome to look at the transcriptome, proteome, epigenome, and immunome, among others. It is likely that multiomic predictors are going to be able to identify more therapeutic options compared to single genomic predictors.”
Dr. Flaherty noted that all tumor samples from patients assigned to treatment are being subjected to whole-exome sequencing to further the discovery of the genetic features of responsive and nonresponsive tumors.
NCI-MATCH was funded by the National Cancer Institute. Dr. Flaherty disclosed relationships with Clovis Oncology, Loxo, X4 Pharma, and many other companies. His coauthors disclosed many conflicts as well. Dr. Gruber is cofounder of Brogent International. Dr. Siu disclosed relationships with Agios, Treadwell Therapeutics, Merck, Pfizer, and many other companies.
SOURCE: Flaherty KT et al. J Clin Oncol. 2020 Oct 13. doi: 10.1200/JCO.19.03010.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
POP surgeries not tied to decreased sexual functioning
Danielle D. Antosh, MD, of the Houston Methodist Hospital and colleagues reported in a systematic review of prospective comparative studies on pelvic organ prolapse surgery, which was published in Obstetrics & Gynecology.
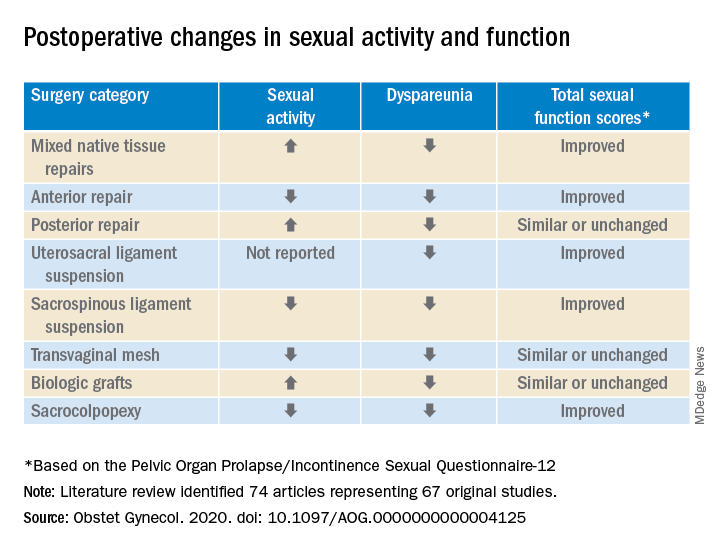
In a preliminary search of 3,124 citations, Dr. Antosh and her colleagues, who are members of the Society of Gynecologic Surgeons Systematic Review Group responsible for the study, identified and accepted 74 articles representing 67 original studies. Ten of these were ancillary studies with different reported outcomes or follow-up times, and 44 were randomized control trials. They compared the pre- and postoperative effects of pelvic organ prolapse (POP) surgery on sexual function for changes in sexual activity and function across eight different prolapse surgery categories: mixed native tissue repairs, anterior repair, posterior repair, uterosacral ligament suspension, sacrospinous ligament suspension, transvaginal mesh, biologic grafts, and sacrocolpopexy. In only three categories – posterior repair, transvaginal mesh, and biological grafts – postoperative changes in sexual function scores were similar or remained unchanged. In all other categories, total sexual function scores improved. Dyspareunia was lower after surgery for all prolapse surgery types.
“Although sexual function improves in the majority of women, it is important to note that a small proportion of women can develop de novo (new onset) dyspareunia after surgery. The rate of this ranges from 0%-9% for prolapse surgeries. However, there is limited data on posterior repairs,” Dr. Antosh said in a later interview.*
POP surgeries positively impact sexual function, dyspareunia outcomes
The researchers determined that there is “moderate to high quality data” supporting the influence of POP on sexual activity and function. They also observed a lower prevalence of dyspareunia postoperatively across all surgery types, compared with baseline. Additionally, no decrease in sexual function was reported across surgical categories; in fact, sexual activity and function improved or stayed the same after POP surgery in this review.
Across most POP surgery groups, Dr. Antosh and colleagues found that with the exception of the sacrospinous ligament suspension, transvaginal mesh, and sacrocolpopexy groups, the rate of postoperative sexual activity was modestly higher. Several studies attributed this finding to a lack of partner or partner-related issues. Another 16 studies (7.7%) cited pain as the primary factor for postoperative sexual inactivity.
Few studies included in the review “reported both preoperative and postoperative rates of sexual activity and dyspareunia, and no study reported patient-level changes in sexual activity or dyspareunia (except occasionally, for de novo activity or dyspareunia),” Dr. Antosh and associates clarified. As a result, they concluded that their findings are based primarily on qualitative comparisons of events reported pre- and postoperatively from different but overlapping sets of studies.
The finding that the prevalence of dyspareunia decreased following all types of POP surgery is consistent with previous reviews. Because the studies did not account for minimally important differences in sexual function scores, it is important to consider this when interpreting results of the review. Dr. Antosh and colleagues also noted that some studies did not define dyspareunia, and those that did frequently used measures that were not validated. They also were unable to identify the persistence of dyspareunia following surgery as this was not recorded in the literature.
Menopausal status and other considerations
Also worth noting, the mean age of women in the studies were postmenopausal, yet the “studies did not stratify sexual function outcomes based on premenopause compared with postmenopause status.”
The researchers advised that future studies using validated definitions of sexual activity, function, and dyspareunia, as well as reporting both their preoperative and postoperative measures would do much to improve the quality of research reported.
It is widely recognized that women with pelvic floor disorders experience a high rate of sexual dysfunction, so the need to achieve optimum outcomes that at least maintain if not improve sexual function postoperatively should be of key concern when planning POP surgery for patients, they cautioned. Previous studies have observed that women experiencing POP rated the need for improved sexual function second only to resolved bulge symptoms and improvement in overall function. The women also classified sexual dysfunction in the same category of adversity as having chronic pain or having to be admitted to an intensive care unit.
Study provides preoperative counseling help
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology and Natasha Abdullah, MD, obstetrics and gynecology resident, both of Einstein Medical Center, Philadelphia, collaboratively commented on the study findings: “This review provides physicians with data to add to the preoperative counseling for patients undergoing pelvic organ prolapse surgery.”
In particular, they noted that, while the article “is a review of previous literature, Table 1 provides an opportunity for physicians to share with their patients the important answers to their concerns surrounding postoperative sexual activity, dyspareunia, and total changes in sexual function scores based on the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire 12 (PISQ-12).”
Noting the clinical usefulness of the questionnaire, they added: “The PISQ-12 is a validated and reliable short form that evaluates sexual function in women with urinary incontinence and/or pelvic organ prolapse and predicts PISQ-31 scores. It was developed from the data of 99 of 182 women surveyed to create the long form (PISQ-31); 46 patients were recruited for further validation. Test-retest reliability was checked with a subset of 20 patients. All subsets regression analysis with R greater than 0.92 identified 12 items that predicted PISQ-31 scores. The PISQ-12 covers three domains of function: Behavioral/Emotive, Physical, and Partner-Related.”
Because ob.gyns. are trained to recognize the “multifactorial reasons (age, partner relationship, other health conditions etc.) that surround sexual activity,” Dr. Jaspan and Dr. Abdullah cautioned against prematurely concluding that the lower sexual activity score is directly related to POP surgery.
“Because sexual function is such an important postsurgical outcome for patients, this article provides significant preoperative counseling data for patients on all POP repair options,” they observed. “No surgical option worsens sexual function. The article concludes that individually validated definitions of sexual activity, function, and dyspareunia in a measuring instrument would improve the quality of data for future studies.”
The study authors reported no relevant financial disclosures. Although no direct funding was provided by the Society of Gynecologic Surgeons, they did provide meeting space, oversight, stipends for expert and statistical support, and aid in disseminating findings. Dr. Abdullah and Dr. Jaspan had no relevant financial disclosures.
SOURCE: Antosh DD et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004125.
*This article was updated on 10/28.
Danielle D. Antosh, MD, of the Houston Methodist Hospital and colleagues reported in a systematic review of prospective comparative studies on pelvic organ prolapse surgery, which was published in Obstetrics & Gynecology.

In a preliminary search of 3,124 citations, Dr. Antosh and her colleagues, who are members of the Society of Gynecologic Surgeons Systematic Review Group responsible for the study, identified and accepted 74 articles representing 67 original studies. Ten of these were ancillary studies with different reported outcomes or follow-up times, and 44 were randomized control trials. They compared the pre- and postoperative effects of pelvic organ prolapse (POP) surgery on sexual function for changes in sexual activity and function across eight different prolapse surgery categories: mixed native tissue repairs, anterior repair, posterior repair, uterosacral ligament suspension, sacrospinous ligament suspension, transvaginal mesh, biologic grafts, and sacrocolpopexy. In only three categories – posterior repair, transvaginal mesh, and biological grafts – postoperative changes in sexual function scores were similar or remained unchanged. In all other categories, total sexual function scores improved. Dyspareunia was lower after surgery for all prolapse surgery types.
“Although sexual function improves in the majority of women, it is important to note that a small proportion of women can develop de novo (new onset) dyspareunia after surgery. The rate of this ranges from 0%-9% for prolapse surgeries. However, there is limited data on posterior repairs,” Dr. Antosh said in a later interview.*
POP surgeries positively impact sexual function, dyspareunia outcomes
The researchers determined that there is “moderate to high quality data” supporting the influence of POP on sexual activity and function. They also observed a lower prevalence of dyspareunia postoperatively across all surgery types, compared with baseline. Additionally, no decrease in sexual function was reported across surgical categories; in fact, sexual activity and function improved or stayed the same after POP surgery in this review.
Across most POP surgery groups, Dr. Antosh and colleagues found that with the exception of the sacrospinous ligament suspension, transvaginal mesh, and sacrocolpopexy groups, the rate of postoperative sexual activity was modestly higher. Several studies attributed this finding to a lack of partner or partner-related issues. Another 16 studies (7.7%) cited pain as the primary factor for postoperative sexual inactivity.
Few studies included in the review “reported both preoperative and postoperative rates of sexual activity and dyspareunia, and no study reported patient-level changes in sexual activity or dyspareunia (except occasionally, for de novo activity or dyspareunia),” Dr. Antosh and associates clarified. As a result, they concluded that their findings are based primarily on qualitative comparisons of events reported pre- and postoperatively from different but overlapping sets of studies.
The finding that the prevalence of dyspareunia decreased following all types of POP surgery is consistent with previous reviews. Because the studies did not account for minimally important differences in sexual function scores, it is important to consider this when interpreting results of the review. Dr. Antosh and colleagues also noted that some studies did not define dyspareunia, and those that did frequently used measures that were not validated. They also were unable to identify the persistence of dyspareunia following surgery as this was not recorded in the literature.
Menopausal status and other considerations
Also worth noting, the mean age of women in the studies were postmenopausal, yet the “studies did not stratify sexual function outcomes based on premenopause compared with postmenopause status.”
The researchers advised that future studies using validated definitions of sexual activity, function, and dyspareunia, as well as reporting both their preoperative and postoperative measures would do much to improve the quality of research reported.
It is widely recognized that women with pelvic floor disorders experience a high rate of sexual dysfunction, so the need to achieve optimum outcomes that at least maintain if not improve sexual function postoperatively should be of key concern when planning POP surgery for patients, they cautioned. Previous studies have observed that women experiencing POP rated the need for improved sexual function second only to resolved bulge symptoms and improvement in overall function. The women also classified sexual dysfunction in the same category of adversity as having chronic pain or having to be admitted to an intensive care unit.
Study provides preoperative counseling help
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology and Natasha Abdullah, MD, obstetrics and gynecology resident, both of Einstein Medical Center, Philadelphia, collaboratively commented on the study findings: “This review provides physicians with data to add to the preoperative counseling for patients undergoing pelvic organ prolapse surgery.”
In particular, they noted that, while the article “is a review of previous literature, Table 1 provides an opportunity for physicians to share with their patients the important answers to their concerns surrounding postoperative sexual activity, dyspareunia, and total changes in sexual function scores based on the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire 12 (PISQ-12).”
Noting the clinical usefulness of the questionnaire, they added: “The PISQ-12 is a validated and reliable short form that evaluates sexual function in women with urinary incontinence and/or pelvic organ prolapse and predicts PISQ-31 scores. It was developed from the data of 99 of 182 women surveyed to create the long form (PISQ-31); 46 patients were recruited for further validation. Test-retest reliability was checked with a subset of 20 patients. All subsets regression analysis with R greater than 0.92 identified 12 items that predicted PISQ-31 scores. The PISQ-12 covers three domains of function: Behavioral/Emotive, Physical, and Partner-Related.”
Because ob.gyns. are trained to recognize the “multifactorial reasons (age, partner relationship, other health conditions etc.) that surround sexual activity,” Dr. Jaspan and Dr. Abdullah cautioned against prematurely concluding that the lower sexual activity score is directly related to POP surgery.
“Because sexual function is such an important postsurgical outcome for patients, this article provides significant preoperative counseling data for patients on all POP repair options,” they observed. “No surgical option worsens sexual function. The article concludes that individually validated definitions of sexual activity, function, and dyspareunia in a measuring instrument would improve the quality of data for future studies.”
The study authors reported no relevant financial disclosures. Although no direct funding was provided by the Society of Gynecologic Surgeons, they did provide meeting space, oversight, stipends for expert and statistical support, and aid in disseminating findings. Dr. Abdullah and Dr. Jaspan had no relevant financial disclosures.
SOURCE: Antosh DD et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004125.
*This article was updated on 10/28.
Danielle D. Antosh, MD, of the Houston Methodist Hospital and colleagues reported in a systematic review of prospective comparative studies on pelvic organ prolapse surgery, which was published in Obstetrics & Gynecology.

In a preliminary search of 3,124 citations, Dr. Antosh and her colleagues, who are members of the Society of Gynecologic Surgeons Systematic Review Group responsible for the study, identified and accepted 74 articles representing 67 original studies. Ten of these were ancillary studies with different reported outcomes or follow-up times, and 44 were randomized control trials. They compared the pre- and postoperative effects of pelvic organ prolapse (POP) surgery on sexual function for changes in sexual activity and function across eight different prolapse surgery categories: mixed native tissue repairs, anterior repair, posterior repair, uterosacral ligament suspension, sacrospinous ligament suspension, transvaginal mesh, biologic grafts, and sacrocolpopexy. In only three categories – posterior repair, transvaginal mesh, and biological grafts – postoperative changes in sexual function scores were similar or remained unchanged. In all other categories, total sexual function scores improved. Dyspareunia was lower after surgery for all prolapse surgery types.
“Although sexual function improves in the majority of women, it is important to note that a small proportion of women can develop de novo (new onset) dyspareunia after surgery. The rate of this ranges from 0%-9% for prolapse surgeries. However, there is limited data on posterior repairs,” Dr. Antosh said in a later interview.*
POP surgeries positively impact sexual function, dyspareunia outcomes
The researchers determined that there is “moderate to high quality data” supporting the influence of POP on sexual activity and function. They also observed a lower prevalence of dyspareunia postoperatively across all surgery types, compared with baseline. Additionally, no decrease in sexual function was reported across surgical categories; in fact, sexual activity and function improved or stayed the same after POP surgery in this review.
Across most POP surgery groups, Dr. Antosh and colleagues found that with the exception of the sacrospinous ligament suspension, transvaginal mesh, and sacrocolpopexy groups, the rate of postoperative sexual activity was modestly higher. Several studies attributed this finding to a lack of partner or partner-related issues. Another 16 studies (7.7%) cited pain as the primary factor for postoperative sexual inactivity.
Few studies included in the review “reported both preoperative and postoperative rates of sexual activity and dyspareunia, and no study reported patient-level changes in sexual activity or dyspareunia (except occasionally, for de novo activity or dyspareunia),” Dr. Antosh and associates clarified. As a result, they concluded that their findings are based primarily on qualitative comparisons of events reported pre- and postoperatively from different but overlapping sets of studies.
The finding that the prevalence of dyspareunia decreased following all types of POP surgery is consistent with previous reviews. Because the studies did not account for minimally important differences in sexual function scores, it is important to consider this when interpreting results of the review. Dr. Antosh and colleagues also noted that some studies did not define dyspareunia, and those that did frequently used measures that were not validated. They also were unable to identify the persistence of dyspareunia following surgery as this was not recorded in the literature.
Menopausal status and other considerations
Also worth noting, the mean age of women in the studies were postmenopausal, yet the “studies did not stratify sexual function outcomes based on premenopause compared with postmenopause status.”
The researchers advised that future studies using validated definitions of sexual activity, function, and dyspareunia, as well as reporting both their preoperative and postoperative measures would do much to improve the quality of research reported.
It is widely recognized that women with pelvic floor disorders experience a high rate of sexual dysfunction, so the need to achieve optimum outcomes that at least maintain if not improve sexual function postoperatively should be of key concern when planning POP surgery for patients, they cautioned. Previous studies have observed that women experiencing POP rated the need for improved sexual function second only to resolved bulge symptoms and improvement in overall function. The women also classified sexual dysfunction in the same category of adversity as having chronic pain or having to be admitted to an intensive care unit.
Study provides preoperative counseling help
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology and Natasha Abdullah, MD, obstetrics and gynecology resident, both of Einstein Medical Center, Philadelphia, collaboratively commented on the study findings: “This review provides physicians with data to add to the preoperative counseling for patients undergoing pelvic organ prolapse surgery.”
In particular, they noted that, while the article “is a review of previous literature, Table 1 provides an opportunity for physicians to share with their patients the important answers to their concerns surrounding postoperative sexual activity, dyspareunia, and total changes in sexual function scores based on the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire 12 (PISQ-12).”
Noting the clinical usefulness of the questionnaire, they added: “The PISQ-12 is a validated and reliable short form that evaluates sexual function in women with urinary incontinence and/or pelvic organ prolapse and predicts PISQ-31 scores. It was developed from the data of 99 of 182 women surveyed to create the long form (PISQ-31); 46 patients were recruited for further validation. Test-retest reliability was checked with a subset of 20 patients. All subsets regression analysis with R greater than 0.92 identified 12 items that predicted PISQ-31 scores. The PISQ-12 covers three domains of function: Behavioral/Emotive, Physical, and Partner-Related.”
Because ob.gyns. are trained to recognize the “multifactorial reasons (age, partner relationship, other health conditions etc.) that surround sexual activity,” Dr. Jaspan and Dr. Abdullah cautioned against prematurely concluding that the lower sexual activity score is directly related to POP surgery.
“Because sexual function is such an important postsurgical outcome for patients, this article provides significant preoperative counseling data for patients on all POP repair options,” they observed. “No surgical option worsens sexual function. The article concludes that individually validated definitions of sexual activity, function, and dyspareunia in a measuring instrument would improve the quality of data for future studies.”
The study authors reported no relevant financial disclosures. Although no direct funding was provided by the Society of Gynecologic Surgeons, they did provide meeting space, oversight, stipends for expert and statistical support, and aid in disseminating findings. Dr. Abdullah and Dr. Jaspan had no relevant financial disclosures.
SOURCE: Antosh DD et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004125.
*This article was updated on 10/28.
FROM OBSTETRICS & GYNECOLOGY
COVID-19: Thromboembolic events high despite prophylaxis
in a new large observational U.S. study.
“Despite very high rate of antithrombotic prophylaxis there were a high rate of thromboembolic events suggesting that we are probably not providing enough thromboprophylaxis,” lead author Gregory Piazza, MD, Brigham and Women’s Hospital, Boston, said in an interview.
“Standard prophylaxis as recommended in the guidelines is a low dose of low-molecular-weight heparin once daily, but these results suggest [patients] probably need higher doses,” he added.
However, Dr. Piazza cautioned that this is an observational study and randomized trials are needed to make changes in treatment strategies. Several such trials are currently underway.
The current study was published online ahead of print in the Nov. 3 issue of the Journal of the American College of Cardiology.
Rates similar to other very sick patients
The study showed that while thromboembolic complications were high, they were not as high as seen in some of the earlier studies from Asia and Europe, Dr. Piazza noted.
“The numbers we were seeing in early reports were so high we couldn’t figure out how that was possible,” he said. “Our study suggests that, in a U.S. population receiving thromboprophylaxis, the rate of thromboembolic complications [are] more in line with what we would expect to see in other very sick patients who end up in ICU.”
He suggested that the very high rates of thromboembolic complications in the early studies from Asia may have been because of the lack of thromboprophylaxis, which is not routine in hospitalized patients there. “Some of the earlier studies also used routine ultrasound and so picked up asymptomatic thrombotic events, which was not the case in our study. So our results are more representative of the U.S. population.”
Dr. Piazza attributed the high rate of thromboembolic complications being reported with COVID-19 to the sheer number of very sick patients being admitted to the hospital.
“We are accustomed to seeing a rare case of thrombosis despite prophylaxis in hospitalized patients, but we are seeing more in COVID patients. This is probably just because we have more critically ill patients,” he said.
“We are seeing an incredible influx of patients to the ICU that we have never experienced before, so the increase in thromboembolic complications is more obvious. In prior years we probably haven’t had enough critically ill patients at any one time to raise the flag about thromboprophylaxis,” he commented.
The study also found a high rate of cardiovascular complications. They are seeing an increase in the risk of MI, which is to be expected in such sick patients, but they also see quite a bit of new atrial fibrillation, myocarditis, and heart failure in patients who don’t always have underlying cardiovascular disease, he said.
“So this virus does appear to have a predilection to causing cardiovascular complications, but this is probably because it is making patients so sick,” Dr. Piazza said. “If flu was this virulent and resulted in such high rates of acute respiratory distress syndrome (ARDS), we would probably see similar cardiovascular complication rates.”
For the current report, the researchers analyzed a retrospective cohort of 1,114 patients with COVID-19 diagnosed through the Mass General Brigham integrated health network. Of these, 170 had been admitted to the ICU, 229 had been hospitalized but not treated in ICU, and 715 were outpatients. In terms of ethnicity, 22% were Hispanic/Latino and 44% were non-White.
Cardiovascular risk factors were common, with 36% of patients having hypertension, 29% hyperlipidemia, and 18% diabetes. Prophylactic anticoagulation was prescribed in 89% of patients with COVID-19 in the intensive care cohort and 85% of those in the hospitalized non–intensive care setting.
Results showed that major arterial or venous thromboembolism (VTE) occurred in 35% of the intensive care cohort, 2.6% of those hospitalized but not treated in ICU, and 0% of outpatients.
Major adverse cardiovascular events occurred in 46% of the intensive care cohort, 6.1% of those hospitalized but non-ICU, and 0% of outpatients.
Symptomatic VTE occurred in 27% of those admitted to ICU, 2.2% of those hospitalized but non-ICU, and 0% of outpatients.
“We found that outpatients had a very low rate of thromboembolic complications, with the vast majority of the risk being in hospitalized patients, especially those in ICU,” Dr. Piazza said.
“These results suggest that we don’t need routine thromboprophylaxis for all outpatients with COVID-19, but there will probably be some patients who need it – those with risk factors for thromboembolism.”
Catheter- and device-associated deep vein thrombosis accounted for 76.9% of the DVTs observed in the study.
“Our finding of high frequency of catheter-associated DVT supports the judicious use of central venous catheters that have been widely implemented, especially in the ICU, to minimize recurrent health care team exposure and facilitate monitoring,” the researchers wrote.
ARDS biggest risk factor
Of all the markers of disease severity, the presence of ARDS had the strongest association with adverse outcomes, including major arterial or VTE, major adverse cardiovascular events, symptomatic VTE, and death.
“The severe inflammatory state associated with ARDS and other complications of COVID-19 and its resultant hypercoagulability may explain, at least in part, the high frequency of thromboembolic events. Improved risk stratification, utilizing biochemical markers of inflammation and activated coagulation as well as clinical indicators, such as ARDS, may play an important role in the early identification of patients with an increased likelihood of developing symptomatic VTE or arterial thrombosis,” the researchers wrote. “They may benefit from full- or intermediate-intensity antithrombotic therapy rather than prophylactic anticoagulation.”
They point out that this study provides a cross-sectional view of the cardiovascular complications of COVID-19 in a large health care network, consisting of two academic medical centers serving the greater Boston area, several community hospitals, and numerous outpatient care sites.
“The study incorporates a wide scope of clinically meaningful cardiovascular endpoints and utilizes a rigorous process of event adjudication. Although data on patients with COVID-19 in the ICU have been the subject of most reports, our study provides insights into the broad spectrum of all hospitalized and outpatient populations,” the authors noted.
“The high frequency of arterial or venous thromboembolism in hospitalized patients despite routine thromboprophylaxis suggests the need for improved risk stratification and enhanced preventive efforts,” they concluded.
The study is continuing, and the researchers expect to have data on 10,000 patients by the end of winter.
Wait for randomized trials
In an accompanying editorial, Robert McBane, MD, Mayo Clinic, Rochester, Minn., said that these data provide important real-world arterial and venous thrombotic event rates across a large, integrated health care network and an experienced roster of clinician-scientists devoted to thrombosis research.
Noting that whether to interpret these results as alarming or reassuring requires a comparison of expected thromboembolic event rates separate from the pandemic, he pointed out that, while the overall VTE rate among ICU patients was high, the vast majority of these events were attributable to central venous lines, and apart from these, the event rates do not appear inflated relative to prior published incidence rates from the pre–COVID-19 era.
“It is therefore important to resist the urge to overprevent or overtreat patients and expose them to the serious risks of major bleeding,” Dr. McBane wrote, adding that “the systematized approach to delivery of guideline-driven VTE prophylaxis across this large, integrated health network likely contributed to the relatively low rates of serious thrombotic outcomes reported.”
He further noted that, as the majority of VTE events were related to central venous lines in ICU patients, “this underscores the importance of a bundled care approach to central venous line management with daily assessment of the continued necessity of central access.
“A number of important clinical trials aimed at optimizing thromboprophylaxis during hospitalization, following hospital dismissal, and in ambulatory settings are underway. Until available, the lessons of thoughtful anticoagulant prophylaxis and treatment guidelines harvested from years of clinical research appear to apply,” he concluded.
This study was funded, in part, by a research grant from Janssen Pharmaceuticals. Dr. Piazza has received research grant support from EKOS Corporation, Bayer, Bristol-Myers Squibb/Pfizer, Portola Pharmaceuticals, and Janssen Pharmaceuticals; and has received consulting fees from Amgen, Pfizer, Boston Scientific, Agile, and Thrombolex. Dr. McBane reported no relevant disclosures.
A version of this article originally appeared on Medscape.com.
in a new large observational U.S. study.
“Despite very high rate of antithrombotic prophylaxis there were a high rate of thromboembolic events suggesting that we are probably not providing enough thromboprophylaxis,” lead author Gregory Piazza, MD, Brigham and Women’s Hospital, Boston, said in an interview.
“Standard prophylaxis as recommended in the guidelines is a low dose of low-molecular-weight heparin once daily, but these results suggest [patients] probably need higher doses,” he added.
However, Dr. Piazza cautioned that this is an observational study and randomized trials are needed to make changes in treatment strategies. Several such trials are currently underway.
The current study was published online ahead of print in the Nov. 3 issue of the Journal of the American College of Cardiology.
Rates similar to other very sick patients
The study showed that while thromboembolic complications were high, they were not as high as seen in some of the earlier studies from Asia and Europe, Dr. Piazza noted.
“The numbers we were seeing in early reports were so high we couldn’t figure out how that was possible,” he said. “Our study suggests that, in a U.S. population receiving thromboprophylaxis, the rate of thromboembolic complications [are] more in line with what we would expect to see in other very sick patients who end up in ICU.”
He suggested that the very high rates of thromboembolic complications in the early studies from Asia may have been because of the lack of thromboprophylaxis, which is not routine in hospitalized patients there. “Some of the earlier studies also used routine ultrasound and so picked up asymptomatic thrombotic events, which was not the case in our study. So our results are more representative of the U.S. population.”
Dr. Piazza attributed the high rate of thromboembolic complications being reported with COVID-19 to the sheer number of very sick patients being admitted to the hospital.
“We are accustomed to seeing a rare case of thrombosis despite prophylaxis in hospitalized patients, but we are seeing more in COVID patients. This is probably just because we have more critically ill patients,” he said.
“We are seeing an incredible influx of patients to the ICU that we have never experienced before, so the increase in thromboembolic complications is more obvious. In prior years we probably haven’t had enough critically ill patients at any one time to raise the flag about thromboprophylaxis,” he commented.
The study also found a high rate of cardiovascular complications. They are seeing an increase in the risk of MI, which is to be expected in such sick patients, but they also see quite a bit of new atrial fibrillation, myocarditis, and heart failure in patients who don’t always have underlying cardiovascular disease, he said.
“So this virus does appear to have a predilection to causing cardiovascular complications, but this is probably because it is making patients so sick,” Dr. Piazza said. “If flu was this virulent and resulted in such high rates of acute respiratory distress syndrome (ARDS), we would probably see similar cardiovascular complication rates.”
For the current report, the researchers analyzed a retrospective cohort of 1,114 patients with COVID-19 diagnosed through the Mass General Brigham integrated health network. Of these, 170 had been admitted to the ICU, 229 had been hospitalized but not treated in ICU, and 715 were outpatients. In terms of ethnicity, 22% were Hispanic/Latino and 44% were non-White.
Cardiovascular risk factors were common, with 36% of patients having hypertension, 29% hyperlipidemia, and 18% diabetes. Prophylactic anticoagulation was prescribed in 89% of patients with COVID-19 in the intensive care cohort and 85% of those in the hospitalized non–intensive care setting.
Results showed that major arterial or venous thromboembolism (VTE) occurred in 35% of the intensive care cohort, 2.6% of those hospitalized but not treated in ICU, and 0% of outpatients.
Major adverse cardiovascular events occurred in 46% of the intensive care cohort, 6.1% of those hospitalized but non-ICU, and 0% of outpatients.
Symptomatic VTE occurred in 27% of those admitted to ICU, 2.2% of those hospitalized but non-ICU, and 0% of outpatients.
“We found that outpatients had a very low rate of thromboembolic complications, with the vast majority of the risk being in hospitalized patients, especially those in ICU,” Dr. Piazza said.
“These results suggest that we don’t need routine thromboprophylaxis for all outpatients with COVID-19, but there will probably be some patients who need it – those with risk factors for thromboembolism.”
Catheter- and device-associated deep vein thrombosis accounted for 76.9% of the DVTs observed in the study.
“Our finding of high frequency of catheter-associated DVT supports the judicious use of central venous catheters that have been widely implemented, especially in the ICU, to minimize recurrent health care team exposure and facilitate monitoring,” the researchers wrote.
ARDS biggest risk factor
Of all the markers of disease severity, the presence of ARDS had the strongest association with adverse outcomes, including major arterial or VTE, major adverse cardiovascular events, symptomatic VTE, and death.
“The severe inflammatory state associated with ARDS and other complications of COVID-19 and its resultant hypercoagulability may explain, at least in part, the high frequency of thromboembolic events. Improved risk stratification, utilizing biochemical markers of inflammation and activated coagulation as well as clinical indicators, such as ARDS, may play an important role in the early identification of patients with an increased likelihood of developing symptomatic VTE or arterial thrombosis,” the researchers wrote. “They may benefit from full- or intermediate-intensity antithrombotic therapy rather than prophylactic anticoagulation.”
They point out that this study provides a cross-sectional view of the cardiovascular complications of COVID-19 in a large health care network, consisting of two academic medical centers serving the greater Boston area, several community hospitals, and numerous outpatient care sites.
“The study incorporates a wide scope of clinically meaningful cardiovascular endpoints and utilizes a rigorous process of event adjudication. Although data on patients with COVID-19 in the ICU have been the subject of most reports, our study provides insights into the broad spectrum of all hospitalized and outpatient populations,” the authors noted.
“The high frequency of arterial or venous thromboembolism in hospitalized patients despite routine thromboprophylaxis suggests the need for improved risk stratification and enhanced preventive efforts,” they concluded.
The study is continuing, and the researchers expect to have data on 10,000 patients by the end of winter.
Wait for randomized trials
In an accompanying editorial, Robert McBane, MD, Mayo Clinic, Rochester, Minn., said that these data provide important real-world arterial and venous thrombotic event rates across a large, integrated health care network and an experienced roster of clinician-scientists devoted to thrombosis research.
Noting that whether to interpret these results as alarming or reassuring requires a comparison of expected thromboembolic event rates separate from the pandemic, he pointed out that, while the overall VTE rate among ICU patients was high, the vast majority of these events were attributable to central venous lines, and apart from these, the event rates do not appear inflated relative to prior published incidence rates from the pre–COVID-19 era.
“It is therefore important to resist the urge to overprevent or overtreat patients and expose them to the serious risks of major bleeding,” Dr. McBane wrote, adding that “the systematized approach to delivery of guideline-driven VTE prophylaxis across this large, integrated health network likely contributed to the relatively low rates of serious thrombotic outcomes reported.”
He further noted that, as the majority of VTE events were related to central venous lines in ICU patients, “this underscores the importance of a bundled care approach to central venous line management with daily assessment of the continued necessity of central access.
“A number of important clinical trials aimed at optimizing thromboprophylaxis during hospitalization, following hospital dismissal, and in ambulatory settings are underway. Until available, the lessons of thoughtful anticoagulant prophylaxis and treatment guidelines harvested from years of clinical research appear to apply,” he concluded.
This study was funded, in part, by a research grant from Janssen Pharmaceuticals. Dr. Piazza has received research grant support from EKOS Corporation, Bayer, Bristol-Myers Squibb/Pfizer, Portola Pharmaceuticals, and Janssen Pharmaceuticals; and has received consulting fees from Amgen, Pfizer, Boston Scientific, Agile, and Thrombolex. Dr. McBane reported no relevant disclosures.
A version of this article originally appeared on Medscape.com.
in a new large observational U.S. study.
“Despite very high rate of antithrombotic prophylaxis there were a high rate of thromboembolic events suggesting that we are probably not providing enough thromboprophylaxis,” lead author Gregory Piazza, MD, Brigham and Women’s Hospital, Boston, said in an interview.
“Standard prophylaxis as recommended in the guidelines is a low dose of low-molecular-weight heparin once daily, but these results suggest [patients] probably need higher doses,” he added.
However, Dr. Piazza cautioned that this is an observational study and randomized trials are needed to make changes in treatment strategies. Several such trials are currently underway.
The current study was published online ahead of print in the Nov. 3 issue of the Journal of the American College of Cardiology.
Rates similar to other very sick patients
The study showed that while thromboembolic complications were high, they were not as high as seen in some of the earlier studies from Asia and Europe, Dr. Piazza noted.
“The numbers we were seeing in early reports were so high we couldn’t figure out how that was possible,” he said. “Our study suggests that, in a U.S. population receiving thromboprophylaxis, the rate of thromboembolic complications [are] more in line with what we would expect to see in other very sick patients who end up in ICU.”
He suggested that the very high rates of thromboembolic complications in the early studies from Asia may have been because of the lack of thromboprophylaxis, which is not routine in hospitalized patients there. “Some of the earlier studies also used routine ultrasound and so picked up asymptomatic thrombotic events, which was not the case in our study. So our results are more representative of the U.S. population.”
Dr. Piazza attributed the high rate of thromboembolic complications being reported with COVID-19 to the sheer number of very sick patients being admitted to the hospital.
“We are accustomed to seeing a rare case of thrombosis despite prophylaxis in hospitalized patients, but we are seeing more in COVID patients. This is probably just because we have more critically ill patients,” he said.
“We are seeing an incredible influx of patients to the ICU that we have never experienced before, so the increase in thromboembolic complications is more obvious. In prior years we probably haven’t had enough critically ill patients at any one time to raise the flag about thromboprophylaxis,” he commented.
The study also found a high rate of cardiovascular complications. They are seeing an increase in the risk of MI, which is to be expected in such sick patients, but they also see quite a bit of new atrial fibrillation, myocarditis, and heart failure in patients who don’t always have underlying cardiovascular disease, he said.
“So this virus does appear to have a predilection to causing cardiovascular complications, but this is probably because it is making patients so sick,” Dr. Piazza said. “If flu was this virulent and resulted in such high rates of acute respiratory distress syndrome (ARDS), we would probably see similar cardiovascular complication rates.”
For the current report, the researchers analyzed a retrospective cohort of 1,114 patients with COVID-19 diagnosed through the Mass General Brigham integrated health network. Of these, 170 had been admitted to the ICU, 229 had been hospitalized but not treated in ICU, and 715 were outpatients. In terms of ethnicity, 22% were Hispanic/Latino and 44% were non-White.
Cardiovascular risk factors were common, with 36% of patients having hypertension, 29% hyperlipidemia, and 18% diabetes. Prophylactic anticoagulation was prescribed in 89% of patients with COVID-19 in the intensive care cohort and 85% of those in the hospitalized non–intensive care setting.
Results showed that major arterial or venous thromboembolism (VTE) occurred in 35% of the intensive care cohort, 2.6% of those hospitalized but not treated in ICU, and 0% of outpatients.
Major adverse cardiovascular events occurred in 46% of the intensive care cohort, 6.1% of those hospitalized but non-ICU, and 0% of outpatients.
Symptomatic VTE occurred in 27% of those admitted to ICU, 2.2% of those hospitalized but non-ICU, and 0% of outpatients.
“We found that outpatients had a very low rate of thromboembolic complications, with the vast majority of the risk being in hospitalized patients, especially those in ICU,” Dr. Piazza said.
“These results suggest that we don’t need routine thromboprophylaxis for all outpatients with COVID-19, but there will probably be some patients who need it – those with risk factors for thromboembolism.”
Catheter- and device-associated deep vein thrombosis accounted for 76.9% of the DVTs observed in the study.
“Our finding of high frequency of catheter-associated DVT supports the judicious use of central venous catheters that have been widely implemented, especially in the ICU, to minimize recurrent health care team exposure and facilitate monitoring,” the researchers wrote.
ARDS biggest risk factor
Of all the markers of disease severity, the presence of ARDS had the strongest association with adverse outcomes, including major arterial or VTE, major adverse cardiovascular events, symptomatic VTE, and death.
“The severe inflammatory state associated with ARDS and other complications of COVID-19 and its resultant hypercoagulability may explain, at least in part, the high frequency of thromboembolic events. Improved risk stratification, utilizing biochemical markers of inflammation and activated coagulation as well as clinical indicators, such as ARDS, may play an important role in the early identification of patients with an increased likelihood of developing symptomatic VTE or arterial thrombosis,” the researchers wrote. “They may benefit from full- or intermediate-intensity antithrombotic therapy rather than prophylactic anticoagulation.”
They point out that this study provides a cross-sectional view of the cardiovascular complications of COVID-19 in a large health care network, consisting of two academic medical centers serving the greater Boston area, several community hospitals, and numerous outpatient care sites.
“The study incorporates a wide scope of clinically meaningful cardiovascular endpoints and utilizes a rigorous process of event adjudication. Although data on patients with COVID-19 in the ICU have been the subject of most reports, our study provides insights into the broad spectrum of all hospitalized and outpatient populations,” the authors noted.
“The high frequency of arterial or venous thromboembolism in hospitalized patients despite routine thromboprophylaxis suggests the need for improved risk stratification and enhanced preventive efforts,” they concluded.
The study is continuing, and the researchers expect to have data on 10,000 patients by the end of winter.
Wait for randomized trials
In an accompanying editorial, Robert McBane, MD, Mayo Clinic, Rochester, Minn., said that these data provide important real-world arterial and venous thrombotic event rates across a large, integrated health care network and an experienced roster of clinician-scientists devoted to thrombosis research.
Noting that whether to interpret these results as alarming or reassuring requires a comparison of expected thromboembolic event rates separate from the pandemic, he pointed out that, while the overall VTE rate among ICU patients was high, the vast majority of these events were attributable to central venous lines, and apart from these, the event rates do not appear inflated relative to prior published incidence rates from the pre–COVID-19 era.
“It is therefore important to resist the urge to overprevent or overtreat patients and expose them to the serious risks of major bleeding,” Dr. McBane wrote, adding that “the systematized approach to delivery of guideline-driven VTE prophylaxis across this large, integrated health network likely contributed to the relatively low rates of serious thrombotic outcomes reported.”
He further noted that, as the majority of VTE events were related to central venous lines in ICU patients, “this underscores the importance of a bundled care approach to central venous line management with daily assessment of the continued necessity of central access.
“A number of important clinical trials aimed at optimizing thromboprophylaxis during hospitalization, following hospital dismissal, and in ambulatory settings are underway. Until available, the lessons of thoughtful anticoagulant prophylaxis and treatment guidelines harvested from years of clinical research appear to apply,” he concluded.
This study was funded, in part, by a research grant from Janssen Pharmaceuticals. Dr. Piazza has received research grant support from EKOS Corporation, Bayer, Bristol-Myers Squibb/Pfizer, Portola Pharmaceuticals, and Janssen Pharmaceuticals; and has received consulting fees from Amgen, Pfizer, Boston Scientific, Agile, and Thrombolex. Dr. McBane reported no relevant disclosures.
A version of this article originally appeared on Medscape.com.
ASSET extension supports abatacept as treatment for diffuse cutaneous systemic sclerosis
“Exploratory outcome measures during the open-label extension, including the composite ACR CRISS [American College of Rheumatology Combined Response Index in diffuse cutaneous Systemic Sclerosis] score, indicate that abatacept [Orencia] might promote overall global improvement in these participants,” Lorinda Chung, MD, of Stanford (Calif.) University, and colleagues wrote in the Lancet Rheumatology.
To continue to determine the safety and efficacy of abatacept as a treatment for dcSSc, the researchers launched the open-label extension of the randomized, double-blind ASSET trial. After patients in ASSET completed 12 months of treatment with either 125 mg weekly of subcutaneous abatacept or placebo, they were invited to join the extension period. ASSET’s primary endpoint was the change in modified Rodnan Skin Score (mRSS) from baseline to 12 months.
Of the 88 patients who began the ASSET trial – half of which were assigned to the abatacept group and the other half to the placebo group – 33 and 34 transitioned to weekly open-label treatment with 125 mg of abatacept, respectively. In the trial’s primary endpoint at 12 months, mean improvement in mRSS with abatacept was –6.6 (standard deviation, 6.4), compared to –3.7 (SD, 7.6) with placebo.
All told, 32 patients in each group completed 18 months of treatment. Patients who received abatacept in both periods had even more improvement in mRSS score (–9.8 [SD, 8.1]), as did patients who received placebo and then abatacept (–6.3 [SD, 9.3]). After 12 months, 68% of patients (23 of 34) in the abatacept group had a 5-unit or greater improvement in mRSS, compared with 50% of patients (19 of 38) in the placebo group. After 18 months, that percentage went up to 72% (23 of 32) among patients who took abatacept in both periods and 65% (20 of 31) for those who received it only in the extension.
Although the median ACR CRISS score was significantly greater at 12 months with abatacept, compared with placebo, both groups improved during the open-label period. After 12 months, an ACR CRISS score of 0.60 or higher was achieved by 55% (18 of 33) of the abatacept group and 36% (13 of 36) of the placebo group. In the extension, those percentages leapt to 66% (19 of 29) and 50% (13 of 26), respectively.
Throughout the double-blind phase, adverse events – including infectious events, serious events, and those that led to study withdrawal – were more frequent with placebo than with abatacept. During the extension period, although adverse events occurred slightly more among 18-month abatacept users, they ultimately occurred in fewer participants than in the double-blind phase.
Time to push forward with additional studies on abatacept
The results fortified by this open-label extension from Dr. Chung and colleagues should lay the groundwork for a phase 3 study on treatment with abatacept, wrote Francesco Del Galdo, MD, PhD, of the University of Leeds (England), in an accompanying editorial.
Along with clinically important improvements in several key measures, Dr. Del Galdo restated the value of abatacept’s “very benign” safety profile over the 18-month study period. However, he also acknowledged the “absence of concurrent immunosuppression” in the ASSET trial, noting that there is not yet a record of combination therapy safety across the board.
Beyond safety, he wondered if perhaps the time has passed for a placebo arm in dcSSc patients. He noted that participants in the initial placebo group were more likely to have “clinically relevant worsening of disease that required escape treatment,” which could make it ethically and analytically difficult to justify placebo.
The authors acknowledged the extension’s limitations, including the possibility that the survivors of the 12-month trial who joined the extension were more responsive to treatment or suffered from less severe disease. In addition, they noted that the study “was not powered for formal statistical comparison of the two treatment arms,” meaning all open-label results are considered exploratory. Finally, the number of participants in the extension period was small and likely contributed to low rates of adverse events, such as infection.
The trial was funded by Bristol-Myers Squibb, which markets abatacept, and the National Institutes of Health. The authors reported numerous potential conflicts of interest, including receiving grants, clinical trial support, and personal fees from various organizations and pharmaceutical companies, as well as serving on the advisory boards for such companies. Dr. Del Galdo reported receiving consultancy fees and research grants from several pharmaceutical companies.
SOURCE: Chung L et al. Lancet Rheumatol. 2020 Oct 19. doi: 10.1016/S2665-9913(20)30237-X.
“Exploratory outcome measures during the open-label extension, including the composite ACR CRISS [American College of Rheumatology Combined Response Index in diffuse cutaneous Systemic Sclerosis] score, indicate that abatacept [Orencia] might promote overall global improvement in these participants,” Lorinda Chung, MD, of Stanford (Calif.) University, and colleagues wrote in the Lancet Rheumatology.
To continue to determine the safety and efficacy of abatacept as a treatment for dcSSc, the researchers launched the open-label extension of the randomized, double-blind ASSET trial. After patients in ASSET completed 12 months of treatment with either 125 mg weekly of subcutaneous abatacept or placebo, they were invited to join the extension period. ASSET’s primary endpoint was the change in modified Rodnan Skin Score (mRSS) from baseline to 12 months.
Of the 88 patients who began the ASSET trial – half of which were assigned to the abatacept group and the other half to the placebo group – 33 and 34 transitioned to weekly open-label treatment with 125 mg of abatacept, respectively. In the trial’s primary endpoint at 12 months, mean improvement in mRSS with abatacept was –6.6 (standard deviation, 6.4), compared to –3.7 (SD, 7.6) with placebo.
All told, 32 patients in each group completed 18 months of treatment. Patients who received abatacept in both periods had even more improvement in mRSS score (–9.8 [SD, 8.1]), as did patients who received placebo and then abatacept (–6.3 [SD, 9.3]). After 12 months, 68% of patients (23 of 34) in the abatacept group had a 5-unit or greater improvement in mRSS, compared with 50% of patients (19 of 38) in the placebo group. After 18 months, that percentage went up to 72% (23 of 32) among patients who took abatacept in both periods and 65% (20 of 31) for those who received it only in the extension.
Although the median ACR CRISS score was significantly greater at 12 months with abatacept, compared with placebo, both groups improved during the open-label period. After 12 months, an ACR CRISS score of 0.60 or higher was achieved by 55% (18 of 33) of the abatacept group and 36% (13 of 36) of the placebo group. In the extension, those percentages leapt to 66% (19 of 29) and 50% (13 of 26), respectively.
Throughout the double-blind phase, adverse events – including infectious events, serious events, and those that led to study withdrawal – were more frequent with placebo than with abatacept. During the extension period, although adverse events occurred slightly more among 18-month abatacept users, they ultimately occurred in fewer participants than in the double-blind phase.
Time to push forward with additional studies on abatacept
The results fortified by this open-label extension from Dr. Chung and colleagues should lay the groundwork for a phase 3 study on treatment with abatacept, wrote Francesco Del Galdo, MD, PhD, of the University of Leeds (England), in an accompanying editorial.
Along with clinically important improvements in several key measures, Dr. Del Galdo restated the value of abatacept’s “very benign” safety profile over the 18-month study period. However, he also acknowledged the “absence of concurrent immunosuppression” in the ASSET trial, noting that there is not yet a record of combination therapy safety across the board.
Beyond safety, he wondered if perhaps the time has passed for a placebo arm in dcSSc patients. He noted that participants in the initial placebo group were more likely to have “clinically relevant worsening of disease that required escape treatment,” which could make it ethically and analytically difficult to justify placebo.
The authors acknowledged the extension’s limitations, including the possibility that the survivors of the 12-month trial who joined the extension were more responsive to treatment or suffered from less severe disease. In addition, they noted that the study “was not powered for formal statistical comparison of the two treatment arms,” meaning all open-label results are considered exploratory. Finally, the number of participants in the extension period was small and likely contributed to low rates of adverse events, such as infection.
The trial was funded by Bristol-Myers Squibb, which markets abatacept, and the National Institutes of Health. The authors reported numerous potential conflicts of interest, including receiving grants, clinical trial support, and personal fees from various organizations and pharmaceutical companies, as well as serving on the advisory boards for such companies. Dr. Del Galdo reported receiving consultancy fees and research grants from several pharmaceutical companies.
SOURCE: Chung L et al. Lancet Rheumatol. 2020 Oct 19. doi: 10.1016/S2665-9913(20)30237-X.
“Exploratory outcome measures during the open-label extension, including the composite ACR CRISS [American College of Rheumatology Combined Response Index in diffuse cutaneous Systemic Sclerosis] score, indicate that abatacept [Orencia] might promote overall global improvement in these participants,” Lorinda Chung, MD, of Stanford (Calif.) University, and colleagues wrote in the Lancet Rheumatology.
To continue to determine the safety and efficacy of abatacept as a treatment for dcSSc, the researchers launched the open-label extension of the randomized, double-blind ASSET trial. After patients in ASSET completed 12 months of treatment with either 125 mg weekly of subcutaneous abatacept or placebo, they were invited to join the extension period. ASSET’s primary endpoint was the change in modified Rodnan Skin Score (mRSS) from baseline to 12 months.
Of the 88 patients who began the ASSET trial – half of which were assigned to the abatacept group and the other half to the placebo group – 33 and 34 transitioned to weekly open-label treatment with 125 mg of abatacept, respectively. In the trial’s primary endpoint at 12 months, mean improvement in mRSS with abatacept was –6.6 (standard deviation, 6.4), compared to –3.7 (SD, 7.6) with placebo.
All told, 32 patients in each group completed 18 months of treatment. Patients who received abatacept in both periods had even more improvement in mRSS score (–9.8 [SD, 8.1]), as did patients who received placebo and then abatacept (–6.3 [SD, 9.3]). After 12 months, 68% of patients (23 of 34) in the abatacept group had a 5-unit or greater improvement in mRSS, compared with 50% of patients (19 of 38) in the placebo group. After 18 months, that percentage went up to 72% (23 of 32) among patients who took abatacept in both periods and 65% (20 of 31) for those who received it only in the extension.
Although the median ACR CRISS score was significantly greater at 12 months with abatacept, compared with placebo, both groups improved during the open-label period. After 12 months, an ACR CRISS score of 0.60 or higher was achieved by 55% (18 of 33) of the abatacept group and 36% (13 of 36) of the placebo group. In the extension, those percentages leapt to 66% (19 of 29) and 50% (13 of 26), respectively.
Throughout the double-blind phase, adverse events – including infectious events, serious events, and those that led to study withdrawal – were more frequent with placebo than with abatacept. During the extension period, although adverse events occurred slightly more among 18-month abatacept users, they ultimately occurred in fewer participants than in the double-blind phase.
Time to push forward with additional studies on abatacept
The results fortified by this open-label extension from Dr. Chung and colleagues should lay the groundwork for a phase 3 study on treatment with abatacept, wrote Francesco Del Galdo, MD, PhD, of the University of Leeds (England), in an accompanying editorial.
Along with clinically important improvements in several key measures, Dr. Del Galdo restated the value of abatacept’s “very benign” safety profile over the 18-month study period. However, he also acknowledged the “absence of concurrent immunosuppression” in the ASSET trial, noting that there is not yet a record of combination therapy safety across the board.
Beyond safety, he wondered if perhaps the time has passed for a placebo arm in dcSSc patients. He noted that participants in the initial placebo group were more likely to have “clinically relevant worsening of disease that required escape treatment,” which could make it ethically and analytically difficult to justify placebo.
The authors acknowledged the extension’s limitations, including the possibility that the survivors of the 12-month trial who joined the extension were more responsive to treatment or suffered from less severe disease. In addition, they noted that the study “was not powered for formal statistical comparison of the two treatment arms,” meaning all open-label results are considered exploratory. Finally, the number of participants in the extension period was small and likely contributed to low rates of adverse events, such as infection.
The trial was funded by Bristol-Myers Squibb, which markets abatacept, and the National Institutes of Health. The authors reported numerous potential conflicts of interest, including receiving grants, clinical trial support, and personal fees from various organizations and pharmaceutical companies, as well as serving on the advisory boards for such companies. Dr. Del Galdo reported receiving consultancy fees and research grants from several pharmaceutical companies.
SOURCE: Chung L et al. Lancet Rheumatol. 2020 Oct 19. doi: 10.1016/S2665-9913(20)30237-X.
FROM THE LANCET RHEUMATOLOGY
An assessment of asthma drugs in pregnancy
Asthma effects about 10% of pregnant women worldwide. About 10% of these will have severe disease requiring oral corticosteroids. Brief reviews of asthma drugs are shown below.
The trade names (if available) and molecular weights (rounded to the nearest whole number) are shown in parentheses. Nearly all of these drugs will cross the placenta.
Beclomethasone (Beconase AQ) (539)
Either beclomethasone or budesonide was considered the inhaled steroids of choice for use during pregnancy, according to a position statement from a joint committee of the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma, and Immunology published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Benralizumab (Fasenra) (150,000)
There is no published human pregnancy data. Based on studies in monkeys, the drug crosses the placenta in the third trimester. It caused no fetal harm in monkeys when given throughout pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the drug during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting mothertobaby.org/Fasenra.
Budesonide (Rhinocort) (431)
Either budesonide or beclomethasone was considered the inhaled steroids of choice for use during pregnancy in a position statement from a joint committee of ACOG and ACAAI published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Caffeine (194)
Although the amount of caffeine in commonly used beverages varies widely, caffeine consumption in pregnancy in moderate amounts does not pose a risk to the fetus. When used in moderation, no association with congenital malformations, spontaneous abortions, preterm birth, and low birth weight have been proven.
Ciclesonide (Alvesco) (541)
Ciclesonide is an inhaled corticosteroid. There is no published human pregnancy data but the molecular weight suggests that it will cross the placenta throughout pregnancy. The drug produced no defects in rats but caused fetal toxicity in rabbits. Although the risk may be low because it is inhaled, avoiding it in the first trimester should be considered (see dexamethasone).
Cromolyn sodium (490)
Cromolyn was available as a nasal spray and oral solution, but it is no longer available in the United States. It is poorly absorbed into the systemic circulation. Neither the human nor the animal data suggest a risk of embryo-fetal harm.
Dexamethasone (392)
This is a corticosteroid with potency similar to betamethasone. Because large epidemiologic studies have found positive associations between systemic corticosteroids and nonsyndromic orofacial clefts, it is best to avoid this agent in the first trimester. However, when used for the treatment of asthma, other studies have not found a significantly increased risk of maternal or fetal complications. The difference in these outcomes may be related to the systemic concentrations of the drug.
Dyphylline (254) + guaifenesin (198) (Difil-G Forte) (Dilex-G 400) (Dy-G)
This is an OTC liquid drug taken orally. It has not been studied in pregnant animals, and there is no published human pregnancy data. However, these bronchodilator agents probably can be classified as low risk for the embryo and fetus. Dyphylline alone has been removed from the market.
Fluticasone (539) + vilanterol (Breo Ellipta) (775)
Fluticasone is a corticosteroid and vilanterol is a long acting beta2-adrenergic agonist that are given by inhalation. The molecular weights suggest that the two agents will cross the placenta throughout pregnancy. The drug did not cause fetal harm in animals. There is no published human pregnancy data for this fixed combination.
Fluticasone (539) + umeclidinium (509) + vilanterol (Trelegy Ellipta) (776)
The combination of fluticasone (glucocorticoid), umeclidinium, and vilanterol (long-acting beta2-adrenergic agonists) is given by inhalation. The molecular weights suggest that the three agents will cross the placenta throughout pregnancy. Although the three-drug combination has not been studied in pregnant rats and rabbits, the individual agents did not cause embryo-fetal harm in these species. There is no evidence that these agents, when given by inhalation, will harm the human embryo and/or fetus. No published human pregnancy reports for this fixed combination have been located.
Formoterol + mometasone (Dulera Aerosol) (841 / 521)
This combination is an aerosol product. Formoterol is a long-acting beta2-adrenergic agonist and mometasone is a topical corticosteroid. There is no published human pregnancy data for this fixed combination. The molecular weights suggest that both drugs will cross the placenta throughout pregnancy. In animals given high oral doses, both were teratogenic.
Ipratropium (Atrovent) (430)
Inhaled ipratropium, an anticholinergic bronchodilator, is recommended for asthma in patients not responding adequately to other therapy. It was not teratogenic mice, rats, and rabbits. Although the human pregnancy data is limited, there is no evidence that the drug is hazardous to the fetus. It produces fewer systemic effects then atropine and may have an additive bronchodilatory effect to beta2 agonists.
Isoproterenol (211)
Isoproterenol is a sympathomimetic (bronchodilator) with beta-adrenergic effects that is given intravenously. No reports linking this agent with congenital defects have been located. The drug was not teratogenic in rats and rabbits but was in hamsters.
Levalbuterol (Xopenex HFA) (240)
Levalbuterol is the (R)-enantiomer of racemic albuterol. It is given by inhalation. No reports of its use in human pregnancy have been located. However, racemic albuterol is considered compatible in pregnancy, and there is no apparent reason not to classify levalbuterol the same way. The drug, when given orally, is teratogenic in animals. If levalbuterol is used in pregnancy for the treatment of asthma, health care professionals are encouraged to call the toll-free number (1-877-311-8972) for information about patient enrollment in an Organization of Teratology Specialists study.
Mepolizumab (Nucala) (149,000)
Mepolizumab is given by subcutaneous injection. It is not indicated for status asthmaticus. There is no published human pregnancy data but the molecular weight suggests that it will not cross the placenta in the first half of pregnancy. The drug did not cause defects in monkeys and mice. There is a pregnancy exposure registry that monitors pregnancy outcomes in women with asthma exposed to Nucala during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting www.mothertobaby.org/asthma.
Metaproterenol (521)
Metaproterenol, a selective beta2-adrenergic agonist, is a respiratory (bronchodilator) that is given orally. Use of this agent in pregnancy has not been linked with congenital defects. However, the drug is teratogenic in animals.
Methylprednisolone (Medrol) (374)
This is an oral glucocorticoid. The molecular weight suggests that it will cross the placenta throughout pregnancy. No reports relating to its use in human pregnancy or in pregnant animals have been located. However, teratogenicity is a potential problem (see below). If high doses of the drug are used in pregnancy, the newborn infants should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Methylprednisolone acetate (Depo-Medrol) (417)
This is an injectable glucocorticoid. See below.
Methylprednisolone sodium succinate (Solu-Medrol) (497)
Methylprednisolone is a glucocorticoid given parenterally. The molecular weight suggests that it will cross the placenta throughout pregnancy. As with other corticosteroids, the drug was teratogenic, at doses equivalent to the human dose, in mice, rats, and rabbits. If the drug is used in pregnancy, the newborn infant should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Mometasone + formoterol (Dulera) (321 + 841)
Dulera is a combination product of mometasone (corticosteroid) and formoterol (beta2-adrenergic agonist). There is no published human data for Dulera but the molecular weights suggest that the drugs will cross the placenta. Oral doses of formoterol were not teratogenic in animals but were with mometasone. The limited human pregnancy data with formoterol did not suggest a risk of embryo/fetal harm, but there is no human pregnancy data for mometasone.
Montelukast (Singulair) (608)
Montelukast is a leukotriene receptor antagonist that is given orally. Although the human data are limited, the drug does not appear to cause harm to the embryo and/or fetus. The drug was not teratogenic in rats and rabbits. The manufacturer maintains a pregnancy registry for women exposed to montelukast. Health care professionals are encouraged to report pregnancy exposures to the registry by calling the toll-free number 1-800-986-8999.
Omalizumab (Xolair) (149,000)
Omalizumab is a recombinant DNA–derived humanized immunoglobulin (IgG1k) monoclonal antibody that is administered subcutaneously for patients with moderate to severe persistent asthma. In monkeys, the drug did not cause embryotoxicity or teratogenicity. The human pregnancy data is very limited but does not suggest an increased embryo-fetal risk.
Prednisone (Rayos) (358)
The use of oral prednisone appears to represent a small risk to the developing fetus. One of these risks appears to be orofacial clefts. The drug causes birth defects in rats, mice, rabbits, and hamsters. However, the available evidence supports its use to control various maternal diseases, one of which is asthma.
Reslizumab (Cinqair) (147,000)
Reslizumab is given intravenously. Even though the molecular weight is high, the drug crosses the placenta during pregnancy. In placebo-controlled studies, anaphylaxis occurred in 0.3% of patients receiving the drug. No adverse effects were observed when the drug was given to pregnant mice and rabbits.
Salmeterol (Serevent Diskus) (416)
Salmeterol is a long-acting beta2-adrenergic agonist that is given as an aerosol or dry powder for oral inhalation. Because the drug acts locally in the lung, plasma levels are very low or undetectable and are a result of swallowed salmeterol. The limited human pregnancy data does not suggest risk of embryo-fetal harm. High oral doses in animals were not teratogenic.
Theophylline (180)
Oral theophylline is a methylxanthine that is indicated for the treatment of symptoms of chronic asthma and other chronic lung diseases. According to ACOG, theophylline is not a preferred asthma therapy but considered an alternative agent. No published reports linking the use of theophylline with congenital defects have been located. However, the drug is teratogenic in mice, rats, and rabbits at doses close to the human dose.
Tiotropium (Spiriva Respimat) (490)
Tiotropium, an anticholinergic bronchodilator, is given by oral inhalation only. No reports describing the use of tiotropium during human pregnancy have been located. The animal data suggest low risk. However, because of its long elimination half-life (about 25 hours), use of tiotropium immediately before the diagnosis of an inadvertent pregnancy would most likely result in the exposure of a portion of organogenesis.
Triamcinolone (Kenalog-40) (435)
Triamcinolone is an inhaled corticosteroid with potency slightly greater than prednisone. Although the systemic use of the drug has a small absolute risk of oral clefts and fetal growth restriction, inhaled triamcinolone does not appear to cause embryo-fetal harm. The drug is teratogenic when given orally to animals.
Breastfeeding
It is not known if the above drugs are excreted into breast milk. Agents with relatively low molecular weights will probably be in milk. However, if the maternal levels are low, the amount in milk will probably be very small, if at all. Nevertheless, it is doubtful if any of these agents, even if they are excreted into milk, will have a harmful effect on a nursing infant.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Asthma effects about 10% of pregnant women worldwide. About 10% of these will have severe disease requiring oral corticosteroids. Brief reviews of asthma drugs are shown below.
The trade names (if available) and molecular weights (rounded to the nearest whole number) are shown in parentheses. Nearly all of these drugs will cross the placenta.
Beclomethasone (Beconase AQ) (539)
Either beclomethasone or budesonide was considered the inhaled steroids of choice for use during pregnancy, according to a position statement from a joint committee of the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma, and Immunology published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Benralizumab (Fasenra) (150,000)
There is no published human pregnancy data. Based on studies in monkeys, the drug crosses the placenta in the third trimester. It caused no fetal harm in monkeys when given throughout pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the drug during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting mothertobaby.org/Fasenra.
Budesonide (Rhinocort) (431)
Either budesonide or beclomethasone was considered the inhaled steroids of choice for use during pregnancy in a position statement from a joint committee of ACOG and ACAAI published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Caffeine (194)
Although the amount of caffeine in commonly used beverages varies widely, caffeine consumption in pregnancy in moderate amounts does not pose a risk to the fetus. When used in moderation, no association with congenital malformations, spontaneous abortions, preterm birth, and low birth weight have been proven.
Ciclesonide (Alvesco) (541)
Ciclesonide is an inhaled corticosteroid. There is no published human pregnancy data but the molecular weight suggests that it will cross the placenta throughout pregnancy. The drug produced no defects in rats but caused fetal toxicity in rabbits. Although the risk may be low because it is inhaled, avoiding it in the first trimester should be considered (see dexamethasone).
Cromolyn sodium (490)
Cromolyn was available as a nasal spray and oral solution, but it is no longer available in the United States. It is poorly absorbed into the systemic circulation. Neither the human nor the animal data suggest a risk of embryo-fetal harm.
Dexamethasone (392)
This is a corticosteroid with potency similar to betamethasone. Because large epidemiologic studies have found positive associations between systemic corticosteroids and nonsyndromic orofacial clefts, it is best to avoid this agent in the first trimester. However, when used for the treatment of asthma, other studies have not found a significantly increased risk of maternal or fetal complications. The difference in these outcomes may be related to the systemic concentrations of the drug.
Dyphylline (254) + guaifenesin (198) (Difil-G Forte) (Dilex-G 400) (Dy-G)
This is an OTC liquid drug taken orally. It has not been studied in pregnant animals, and there is no published human pregnancy data. However, these bronchodilator agents probably can be classified as low risk for the embryo and fetus. Dyphylline alone has been removed from the market.
Fluticasone (539) + vilanterol (Breo Ellipta) (775)
Fluticasone is a corticosteroid and vilanterol is a long acting beta2-adrenergic agonist that are given by inhalation. The molecular weights suggest that the two agents will cross the placenta throughout pregnancy. The drug did not cause fetal harm in animals. There is no published human pregnancy data for this fixed combination.
Fluticasone (539) + umeclidinium (509) + vilanterol (Trelegy Ellipta) (776)
The combination of fluticasone (glucocorticoid), umeclidinium, and vilanterol (long-acting beta2-adrenergic agonists) is given by inhalation. The molecular weights suggest that the three agents will cross the placenta throughout pregnancy. Although the three-drug combination has not been studied in pregnant rats and rabbits, the individual agents did not cause embryo-fetal harm in these species. There is no evidence that these agents, when given by inhalation, will harm the human embryo and/or fetus. No published human pregnancy reports for this fixed combination have been located.
Formoterol + mometasone (Dulera Aerosol) (841 / 521)
This combination is an aerosol product. Formoterol is a long-acting beta2-adrenergic agonist and mometasone is a topical corticosteroid. There is no published human pregnancy data for this fixed combination. The molecular weights suggest that both drugs will cross the placenta throughout pregnancy. In animals given high oral doses, both were teratogenic.
Ipratropium (Atrovent) (430)
Inhaled ipratropium, an anticholinergic bronchodilator, is recommended for asthma in patients not responding adequately to other therapy. It was not teratogenic mice, rats, and rabbits. Although the human pregnancy data is limited, there is no evidence that the drug is hazardous to the fetus. It produces fewer systemic effects then atropine and may have an additive bronchodilatory effect to beta2 agonists.
Isoproterenol (211)
Isoproterenol is a sympathomimetic (bronchodilator) with beta-adrenergic effects that is given intravenously. No reports linking this agent with congenital defects have been located. The drug was not teratogenic in rats and rabbits but was in hamsters.
Levalbuterol (Xopenex HFA) (240)
Levalbuterol is the (R)-enantiomer of racemic albuterol. It is given by inhalation. No reports of its use in human pregnancy have been located. However, racemic albuterol is considered compatible in pregnancy, and there is no apparent reason not to classify levalbuterol the same way. The drug, when given orally, is teratogenic in animals. If levalbuterol is used in pregnancy for the treatment of asthma, health care professionals are encouraged to call the toll-free number (1-877-311-8972) for information about patient enrollment in an Organization of Teratology Specialists study.
Mepolizumab (Nucala) (149,000)
Mepolizumab is given by subcutaneous injection. It is not indicated for status asthmaticus. There is no published human pregnancy data but the molecular weight suggests that it will not cross the placenta in the first half of pregnancy. The drug did not cause defects in monkeys and mice. There is a pregnancy exposure registry that monitors pregnancy outcomes in women with asthma exposed to Nucala during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting www.mothertobaby.org/asthma.
Metaproterenol (521)
Metaproterenol, a selective beta2-adrenergic agonist, is a respiratory (bronchodilator) that is given orally. Use of this agent in pregnancy has not been linked with congenital defects. However, the drug is teratogenic in animals.
Methylprednisolone (Medrol) (374)
This is an oral glucocorticoid. The molecular weight suggests that it will cross the placenta throughout pregnancy. No reports relating to its use in human pregnancy or in pregnant animals have been located. However, teratogenicity is a potential problem (see below). If high doses of the drug are used in pregnancy, the newborn infants should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Methylprednisolone acetate (Depo-Medrol) (417)
This is an injectable glucocorticoid. See below.
Methylprednisolone sodium succinate (Solu-Medrol) (497)
Methylprednisolone is a glucocorticoid given parenterally. The molecular weight suggests that it will cross the placenta throughout pregnancy. As with other corticosteroids, the drug was teratogenic, at doses equivalent to the human dose, in mice, rats, and rabbits. If the drug is used in pregnancy, the newborn infant should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Mometasone + formoterol (Dulera) (321 + 841)
Dulera is a combination product of mometasone (corticosteroid) and formoterol (beta2-adrenergic agonist). There is no published human data for Dulera but the molecular weights suggest that the drugs will cross the placenta. Oral doses of formoterol were not teratogenic in animals but were with mometasone. The limited human pregnancy data with formoterol did not suggest a risk of embryo/fetal harm, but there is no human pregnancy data for mometasone.
Montelukast (Singulair) (608)
Montelukast is a leukotriene receptor antagonist that is given orally. Although the human data are limited, the drug does not appear to cause harm to the embryo and/or fetus. The drug was not teratogenic in rats and rabbits. The manufacturer maintains a pregnancy registry for women exposed to montelukast. Health care professionals are encouraged to report pregnancy exposures to the registry by calling the toll-free number 1-800-986-8999.
Omalizumab (Xolair) (149,000)
Omalizumab is a recombinant DNA–derived humanized immunoglobulin (IgG1k) monoclonal antibody that is administered subcutaneously for patients with moderate to severe persistent asthma. In monkeys, the drug did not cause embryotoxicity or teratogenicity. The human pregnancy data is very limited but does not suggest an increased embryo-fetal risk.
Prednisone (Rayos) (358)
The use of oral prednisone appears to represent a small risk to the developing fetus. One of these risks appears to be orofacial clefts. The drug causes birth defects in rats, mice, rabbits, and hamsters. However, the available evidence supports its use to control various maternal diseases, one of which is asthma.
Reslizumab (Cinqair) (147,000)
Reslizumab is given intravenously. Even though the molecular weight is high, the drug crosses the placenta during pregnancy. In placebo-controlled studies, anaphylaxis occurred in 0.3% of patients receiving the drug. No adverse effects were observed when the drug was given to pregnant mice and rabbits.
Salmeterol (Serevent Diskus) (416)
Salmeterol is a long-acting beta2-adrenergic agonist that is given as an aerosol or dry powder for oral inhalation. Because the drug acts locally in the lung, plasma levels are very low or undetectable and are a result of swallowed salmeterol. The limited human pregnancy data does not suggest risk of embryo-fetal harm. High oral doses in animals were not teratogenic.
Theophylline (180)
Oral theophylline is a methylxanthine that is indicated for the treatment of symptoms of chronic asthma and other chronic lung diseases. According to ACOG, theophylline is not a preferred asthma therapy but considered an alternative agent. No published reports linking the use of theophylline with congenital defects have been located. However, the drug is teratogenic in mice, rats, and rabbits at doses close to the human dose.
Tiotropium (Spiriva Respimat) (490)
Tiotropium, an anticholinergic bronchodilator, is given by oral inhalation only. No reports describing the use of tiotropium during human pregnancy have been located. The animal data suggest low risk. However, because of its long elimination half-life (about 25 hours), use of tiotropium immediately before the diagnosis of an inadvertent pregnancy would most likely result in the exposure of a portion of organogenesis.
Triamcinolone (Kenalog-40) (435)
Triamcinolone is an inhaled corticosteroid with potency slightly greater than prednisone. Although the systemic use of the drug has a small absolute risk of oral clefts and fetal growth restriction, inhaled triamcinolone does not appear to cause embryo-fetal harm. The drug is teratogenic when given orally to animals.
Breastfeeding
It is not known if the above drugs are excreted into breast milk. Agents with relatively low molecular weights will probably be in milk. However, if the maternal levels are low, the amount in milk will probably be very small, if at all. Nevertheless, it is doubtful if any of these agents, even if they are excreted into milk, will have a harmful effect on a nursing infant.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Asthma effects about 10% of pregnant women worldwide. About 10% of these will have severe disease requiring oral corticosteroids. Brief reviews of asthma drugs are shown below.
The trade names (if available) and molecular weights (rounded to the nearest whole number) are shown in parentheses. Nearly all of these drugs will cross the placenta.
Beclomethasone (Beconase AQ) (539)
Either beclomethasone or budesonide was considered the inhaled steroids of choice for use during pregnancy, according to a position statement from a joint committee of the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma, and Immunology published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Benralizumab (Fasenra) (150,000)
There is no published human pregnancy data. Based on studies in monkeys, the drug crosses the placenta in the third trimester. It caused no fetal harm in monkeys when given throughout pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the drug during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting mothertobaby.org/Fasenra.
Budesonide (Rhinocort) (431)
Either budesonide or beclomethasone was considered the inhaled steroids of choice for use during pregnancy in a position statement from a joint committee of ACOG and ACAAI published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Caffeine (194)
Although the amount of caffeine in commonly used beverages varies widely, caffeine consumption in pregnancy in moderate amounts does not pose a risk to the fetus. When used in moderation, no association with congenital malformations, spontaneous abortions, preterm birth, and low birth weight have been proven.
Ciclesonide (Alvesco) (541)
Ciclesonide is an inhaled corticosteroid. There is no published human pregnancy data but the molecular weight suggests that it will cross the placenta throughout pregnancy. The drug produced no defects in rats but caused fetal toxicity in rabbits. Although the risk may be low because it is inhaled, avoiding it in the first trimester should be considered (see dexamethasone).
Cromolyn sodium (490)
Cromolyn was available as a nasal spray and oral solution, but it is no longer available in the United States. It is poorly absorbed into the systemic circulation. Neither the human nor the animal data suggest a risk of embryo-fetal harm.
Dexamethasone (392)
This is a corticosteroid with potency similar to betamethasone. Because large epidemiologic studies have found positive associations between systemic corticosteroids and nonsyndromic orofacial clefts, it is best to avoid this agent in the first trimester. However, when used for the treatment of asthma, other studies have not found a significantly increased risk of maternal or fetal complications. The difference in these outcomes may be related to the systemic concentrations of the drug.
Dyphylline (254) + guaifenesin (198) (Difil-G Forte) (Dilex-G 400) (Dy-G)
This is an OTC liquid drug taken orally. It has not been studied in pregnant animals, and there is no published human pregnancy data. However, these bronchodilator agents probably can be classified as low risk for the embryo and fetus. Dyphylline alone has been removed from the market.
Fluticasone (539) + vilanterol (Breo Ellipta) (775)
Fluticasone is a corticosteroid and vilanterol is a long acting beta2-adrenergic agonist that are given by inhalation. The molecular weights suggest that the two agents will cross the placenta throughout pregnancy. The drug did not cause fetal harm in animals. There is no published human pregnancy data for this fixed combination.
Fluticasone (539) + umeclidinium (509) + vilanterol (Trelegy Ellipta) (776)
The combination of fluticasone (glucocorticoid), umeclidinium, and vilanterol (long-acting beta2-adrenergic agonists) is given by inhalation. The molecular weights suggest that the three agents will cross the placenta throughout pregnancy. Although the three-drug combination has not been studied in pregnant rats and rabbits, the individual agents did not cause embryo-fetal harm in these species. There is no evidence that these agents, when given by inhalation, will harm the human embryo and/or fetus. No published human pregnancy reports for this fixed combination have been located.
Formoterol + mometasone (Dulera Aerosol) (841 / 521)
This combination is an aerosol product. Formoterol is a long-acting beta2-adrenergic agonist and mometasone is a topical corticosteroid. There is no published human pregnancy data for this fixed combination. The molecular weights suggest that both drugs will cross the placenta throughout pregnancy. In animals given high oral doses, both were teratogenic.
Ipratropium (Atrovent) (430)
Inhaled ipratropium, an anticholinergic bronchodilator, is recommended for asthma in patients not responding adequately to other therapy. It was not teratogenic mice, rats, and rabbits. Although the human pregnancy data is limited, there is no evidence that the drug is hazardous to the fetus. It produces fewer systemic effects then atropine and may have an additive bronchodilatory effect to beta2 agonists.
Isoproterenol (211)
Isoproterenol is a sympathomimetic (bronchodilator) with beta-adrenergic effects that is given intravenously. No reports linking this agent with congenital defects have been located. The drug was not teratogenic in rats and rabbits but was in hamsters.
Levalbuterol (Xopenex HFA) (240)
Levalbuterol is the (R)-enantiomer of racemic albuterol. It is given by inhalation. No reports of its use in human pregnancy have been located. However, racemic albuterol is considered compatible in pregnancy, and there is no apparent reason not to classify levalbuterol the same way. The drug, when given orally, is teratogenic in animals. If levalbuterol is used in pregnancy for the treatment of asthma, health care professionals are encouraged to call the toll-free number (1-877-311-8972) for information about patient enrollment in an Organization of Teratology Specialists study.
Mepolizumab (Nucala) (149,000)
Mepolizumab is given by subcutaneous injection. It is not indicated for status asthmaticus. There is no published human pregnancy data but the molecular weight suggests that it will not cross the placenta in the first half of pregnancy. The drug did not cause defects in monkeys and mice. There is a pregnancy exposure registry that monitors pregnancy outcomes in women with asthma exposed to Nucala during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting www.mothertobaby.org/asthma.
Metaproterenol (521)
Metaproterenol, a selective beta2-adrenergic agonist, is a respiratory (bronchodilator) that is given orally. Use of this agent in pregnancy has not been linked with congenital defects. However, the drug is teratogenic in animals.
Methylprednisolone (Medrol) (374)
This is an oral glucocorticoid. The molecular weight suggests that it will cross the placenta throughout pregnancy. No reports relating to its use in human pregnancy or in pregnant animals have been located. However, teratogenicity is a potential problem (see below). If high doses of the drug are used in pregnancy, the newborn infants should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Methylprednisolone acetate (Depo-Medrol) (417)
This is an injectable glucocorticoid. See below.
Methylprednisolone sodium succinate (Solu-Medrol) (497)
Methylprednisolone is a glucocorticoid given parenterally. The molecular weight suggests that it will cross the placenta throughout pregnancy. As with other corticosteroids, the drug was teratogenic, at doses equivalent to the human dose, in mice, rats, and rabbits. If the drug is used in pregnancy, the newborn infant should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Mometasone + formoterol (Dulera) (321 + 841)
Dulera is a combination product of mometasone (corticosteroid) and formoterol (beta2-adrenergic agonist). There is no published human data for Dulera but the molecular weights suggest that the drugs will cross the placenta. Oral doses of formoterol were not teratogenic in animals but were with mometasone. The limited human pregnancy data with formoterol did not suggest a risk of embryo/fetal harm, but there is no human pregnancy data for mometasone.
Montelukast (Singulair) (608)
Montelukast is a leukotriene receptor antagonist that is given orally. Although the human data are limited, the drug does not appear to cause harm to the embryo and/or fetus. The drug was not teratogenic in rats and rabbits. The manufacturer maintains a pregnancy registry for women exposed to montelukast. Health care professionals are encouraged to report pregnancy exposures to the registry by calling the toll-free number 1-800-986-8999.
Omalizumab (Xolair) (149,000)
Omalizumab is a recombinant DNA–derived humanized immunoglobulin (IgG1k) monoclonal antibody that is administered subcutaneously for patients with moderate to severe persistent asthma. In monkeys, the drug did not cause embryotoxicity or teratogenicity. The human pregnancy data is very limited but does not suggest an increased embryo-fetal risk.
Prednisone (Rayos) (358)
The use of oral prednisone appears to represent a small risk to the developing fetus. One of these risks appears to be orofacial clefts. The drug causes birth defects in rats, mice, rabbits, and hamsters. However, the available evidence supports its use to control various maternal diseases, one of which is asthma.
Reslizumab (Cinqair) (147,000)
Reslizumab is given intravenously. Even though the molecular weight is high, the drug crosses the placenta during pregnancy. In placebo-controlled studies, anaphylaxis occurred in 0.3% of patients receiving the drug. No adverse effects were observed when the drug was given to pregnant mice and rabbits.
Salmeterol (Serevent Diskus) (416)
Salmeterol is a long-acting beta2-adrenergic agonist that is given as an aerosol or dry powder for oral inhalation. Because the drug acts locally in the lung, plasma levels are very low or undetectable and are a result of swallowed salmeterol. The limited human pregnancy data does not suggest risk of embryo-fetal harm. High oral doses in animals were not teratogenic.
Theophylline (180)
Oral theophylline is a methylxanthine that is indicated for the treatment of symptoms of chronic asthma and other chronic lung diseases. According to ACOG, theophylline is not a preferred asthma therapy but considered an alternative agent. No published reports linking the use of theophylline with congenital defects have been located. However, the drug is teratogenic in mice, rats, and rabbits at doses close to the human dose.
Tiotropium (Spiriva Respimat) (490)
Tiotropium, an anticholinergic bronchodilator, is given by oral inhalation only. No reports describing the use of tiotropium during human pregnancy have been located. The animal data suggest low risk. However, because of its long elimination half-life (about 25 hours), use of tiotropium immediately before the diagnosis of an inadvertent pregnancy would most likely result in the exposure of a portion of organogenesis.
Triamcinolone (Kenalog-40) (435)
Triamcinolone is an inhaled corticosteroid with potency slightly greater than prednisone. Although the systemic use of the drug has a small absolute risk of oral clefts and fetal growth restriction, inhaled triamcinolone does not appear to cause embryo-fetal harm. The drug is teratogenic when given orally to animals.
Breastfeeding
It is not known if the above drugs are excreted into breast milk. Agents with relatively low molecular weights will probably be in milk. However, if the maternal levels are low, the amount in milk will probably be very small, if at all. Nevertheless, it is doubtful if any of these agents, even if they are excreted into milk, will have a harmful effect on a nursing infant.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Novel treatments under study for chronic graft-versus-host disease in allo-HCT
Physicians are gaining a greater understanding of the pathophysiology of chronic graft-versus-host disease (cGVHD) in allo-hematopoietic cell transplantation (allo-HCT), a hematologist/oncologist told colleagues, and novel treatments are being tested.
However, options remain limited. There’s only one Food and Drug Administration–approved therapy for cGVHD that’s failed one or more treatments, and clinical trials remain a crucial option in some cases, said Mary E.D. Flowers, MD, professor of medicine at the University of Washington’s Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
According to Dr. Flowers, cGVHD – a product of a graft’s “immunological assault” against the person receiving a transplant – occurs in 40% of patients within a year after allo-HCT. The disorder “is associated with a poor quality of life, disability, and increased mortality after allo-transplantation,” she said. “It’s a syndrome that can be inflammatory and fibrotic. It involves several organs – the skin, the mouth, the eyes, the lungs, the GI tract.”
The median length of treatment after peripheral blood stem cell transplant is 3.5 years, Dr. Flowers said. Seven years after treatment, 10% of those who are alive – and have avoided relapse – will still need treatment. “Corticosteroids remain the first-line [treatment], at 0.5-1.0 [mg/kg], but they do not control at least 40% of the patients with cGVHD.”
In regard to pathophysiology, she highlighted a 2017 report that presented findings about the pathophysiology of cGVHD. The findings, the report authors wrote, “have yielded a raft of potential new therapeutics, centered on naive T-cell depletion, interleukin-17/21 inhibition, kinase inhibition, regulatory T-cell restoration, and CSF-1 inhibition.”
For now, no agents other than corticosteroids have shown benefit in cGVHD as initial therapy, Dr. Flowers said. In fact, several trials closed early from lack of benefit. But trials continue, she said: Results are pending for a completed phase 3 trial of ibrutinib, a Bruton tyrosine kinase inhibitor, plus steroids for initial treatment of cGVHD. Nearly 500 patients were enrolled, she said. And there’s an ongoing phase 2/3 trial of itacitinib, a Janus kinase 1 inhibitor plus steroids as initial treatment.
Dr. Flowers highlighted the case of a patient with moderate cGVHD. The patient was treated with infection prophylaxis, supportive care for oral and eyes manifestations, and prednisone 0.5 mg/kg (at a lower dose because of diabetes) plus a substitution of tacrolimus with sirolimus, a calcineurin inhibitor.
Why sirolimus? At this early point in progression, she said, the patient didn’t necessarily need systemwide chemo-suppression, and calcineurin inhibitors can be “quite effective” in management of inflammation in the liver. “It would be a completely different story once the patient develops severe cGVHR.” In that case, she said, calcineurin inhibitors wouldn’t be appropriate.
The patient’s status deteriorated to severe cGVHD, and sirolimus was replaced with ibrutinib. Other drugs were added to prevent infection and treat bronchiolitis obliterans syndrome.
In general, “the goal of the treatment is get adequate control of clinical manifestations and prevent more severe disease from developing,” Dr. Flowers said.
In response to a question about polypharmacy in patient with advanced disease – “we tend not to peel those drugs off” – Dr. Flowers said she does see new patients who appear to be taking too many medications. “They are on five drugs, and I say, ‘What are we doing?’ ”
Quite often, Dr. Flowers said, she doesn’t add therapies to existing ones but instead looks for substitutes. “A clinical lesson that I feel that I learned over time is: Ask your questions first. What would you like to see in 3 months? Or 6 months? Before you just add another therapy, do you really know what the trajectory of a disease might be?”
Dr. Flowers discloses research support (Pharmacyclics, Incyte), speaker honorarium (Janssen, Johnson & Johnson, Astellas, Mallinckrodt), and consulting relationships (Pharmacyclics, CSL Behring, Fresenius Kabi).
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Physicians are gaining a greater understanding of the pathophysiology of chronic graft-versus-host disease (cGVHD) in allo-hematopoietic cell transplantation (allo-HCT), a hematologist/oncologist told colleagues, and novel treatments are being tested.
However, options remain limited. There’s only one Food and Drug Administration–approved therapy for cGVHD that’s failed one or more treatments, and clinical trials remain a crucial option in some cases, said Mary E.D. Flowers, MD, professor of medicine at the University of Washington’s Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
According to Dr. Flowers, cGVHD – a product of a graft’s “immunological assault” against the person receiving a transplant – occurs in 40% of patients within a year after allo-HCT. The disorder “is associated with a poor quality of life, disability, and increased mortality after allo-transplantation,” she said. “It’s a syndrome that can be inflammatory and fibrotic. It involves several organs – the skin, the mouth, the eyes, the lungs, the GI tract.”
The median length of treatment after peripheral blood stem cell transplant is 3.5 years, Dr. Flowers said. Seven years after treatment, 10% of those who are alive – and have avoided relapse – will still need treatment. “Corticosteroids remain the first-line [treatment], at 0.5-1.0 [mg/kg], but they do not control at least 40% of the patients with cGVHD.”
In regard to pathophysiology, she highlighted a 2017 report that presented findings about the pathophysiology of cGVHD. The findings, the report authors wrote, “have yielded a raft of potential new therapeutics, centered on naive T-cell depletion, interleukin-17/21 inhibition, kinase inhibition, regulatory T-cell restoration, and CSF-1 inhibition.”
For now, no agents other than corticosteroids have shown benefit in cGVHD as initial therapy, Dr. Flowers said. In fact, several trials closed early from lack of benefit. But trials continue, she said: Results are pending for a completed phase 3 trial of ibrutinib, a Bruton tyrosine kinase inhibitor, plus steroids for initial treatment of cGVHD. Nearly 500 patients were enrolled, she said. And there’s an ongoing phase 2/3 trial of itacitinib, a Janus kinase 1 inhibitor plus steroids as initial treatment.
Dr. Flowers highlighted the case of a patient with moderate cGVHD. The patient was treated with infection prophylaxis, supportive care for oral and eyes manifestations, and prednisone 0.5 mg/kg (at a lower dose because of diabetes) plus a substitution of tacrolimus with sirolimus, a calcineurin inhibitor.
Why sirolimus? At this early point in progression, she said, the patient didn’t necessarily need systemwide chemo-suppression, and calcineurin inhibitors can be “quite effective” in management of inflammation in the liver. “It would be a completely different story once the patient develops severe cGVHR.” In that case, she said, calcineurin inhibitors wouldn’t be appropriate.
The patient’s status deteriorated to severe cGVHD, and sirolimus was replaced with ibrutinib. Other drugs were added to prevent infection and treat bronchiolitis obliterans syndrome.
In general, “the goal of the treatment is get adequate control of clinical manifestations and prevent more severe disease from developing,” Dr. Flowers said.
In response to a question about polypharmacy in patient with advanced disease – “we tend not to peel those drugs off” – Dr. Flowers said she does see new patients who appear to be taking too many medications. “They are on five drugs, and I say, ‘What are we doing?’ ”
Quite often, Dr. Flowers said, she doesn’t add therapies to existing ones but instead looks for substitutes. “A clinical lesson that I feel that I learned over time is: Ask your questions first. What would you like to see in 3 months? Or 6 months? Before you just add another therapy, do you really know what the trajectory of a disease might be?”
Dr. Flowers discloses research support (Pharmacyclics, Incyte), speaker honorarium (Janssen, Johnson & Johnson, Astellas, Mallinckrodt), and consulting relationships (Pharmacyclics, CSL Behring, Fresenius Kabi).
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Physicians are gaining a greater understanding of the pathophysiology of chronic graft-versus-host disease (cGVHD) in allo-hematopoietic cell transplantation (allo-HCT), a hematologist/oncologist told colleagues, and novel treatments are being tested.
However, options remain limited. There’s only one Food and Drug Administration–approved therapy for cGVHD that’s failed one or more treatments, and clinical trials remain a crucial option in some cases, said Mary E.D. Flowers, MD, professor of medicine at the University of Washington’s Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
According to Dr. Flowers, cGVHD – a product of a graft’s “immunological assault” against the person receiving a transplant – occurs in 40% of patients within a year after allo-HCT. The disorder “is associated with a poor quality of life, disability, and increased mortality after allo-transplantation,” she said. “It’s a syndrome that can be inflammatory and fibrotic. It involves several organs – the skin, the mouth, the eyes, the lungs, the GI tract.”
The median length of treatment after peripheral blood stem cell transplant is 3.5 years, Dr. Flowers said. Seven years after treatment, 10% of those who are alive – and have avoided relapse – will still need treatment. “Corticosteroids remain the first-line [treatment], at 0.5-1.0 [mg/kg], but they do not control at least 40% of the patients with cGVHD.”
In regard to pathophysiology, she highlighted a 2017 report that presented findings about the pathophysiology of cGVHD. The findings, the report authors wrote, “have yielded a raft of potential new therapeutics, centered on naive T-cell depletion, interleukin-17/21 inhibition, kinase inhibition, regulatory T-cell restoration, and CSF-1 inhibition.”
For now, no agents other than corticosteroids have shown benefit in cGVHD as initial therapy, Dr. Flowers said. In fact, several trials closed early from lack of benefit. But trials continue, she said: Results are pending for a completed phase 3 trial of ibrutinib, a Bruton tyrosine kinase inhibitor, plus steroids for initial treatment of cGVHD. Nearly 500 patients were enrolled, she said. And there’s an ongoing phase 2/3 trial of itacitinib, a Janus kinase 1 inhibitor plus steroids as initial treatment.
Dr. Flowers highlighted the case of a patient with moderate cGVHD. The patient was treated with infection prophylaxis, supportive care for oral and eyes manifestations, and prednisone 0.5 mg/kg (at a lower dose because of diabetes) plus a substitution of tacrolimus with sirolimus, a calcineurin inhibitor.
Why sirolimus? At this early point in progression, she said, the patient didn’t necessarily need systemwide chemo-suppression, and calcineurin inhibitors can be “quite effective” in management of inflammation in the liver. “It would be a completely different story once the patient develops severe cGVHR.” In that case, she said, calcineurin inhibitors wouldn’t be appropriate.
The patient’s status deteriorated to severe cGVHD, and sirolimus was replaced with ibrutinib. Other drugs were added to prevent infection and treat bronchiolitis obliterans syndrome.
In general, “the goal of the treatment is get adequate control of clinical manifestations and prevent more severe disease from developing,” Dr. Flowers said.
In response to a question about polypharmacy in patient with advanced disease – “we tend not to peel those drugs off” – Dr. Flowers said she does see new patients who appear to be taking too many medications. “They are on five drugs, and I say, ‘What are we doing?’ ”
Quite often, Dr. Flowers said, she doesn’t add therapies to existing ones but instead looks for substitutes. “A clinical lesson that I feel that I learned over time is: Ask your questions first. What would you like to see in 3 months? Or 6 months? Before you just add another therapy, do you really know what the trajectory of a disease might be?”
Dr. Flowers discloses research support (Pharmacyclics, Incyte), speaker honorarium (Janssen, Johnson & Johnson, Astellas, Mallinckrodt), and consulting relationships (Pharmacyclics, CSL Behring, Fresenius Kabi).
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
FROM ALF 2020