User login
Racial/ethnic disparities in cesarean rates increase with greater maternal education
While the likelihood of a cesarean delivery usually drops as maternal education level increases, the disparities seen in cesarean rates between White and Black or Hispanic women actually increase with more maternal education, according to findings from a new study presented at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Typically, higher maternal education is associated with a lower likelihood of cesarean delivery, but this protective effect is much smaller for Black women and nonexistent for Hispanic women, leading to bigger gaps between these groups and White women, found Yael Eliner, MD, an ob.gyn. residency applicant at Boston University who conducted this research with her colleagues in the ob.gyn. department at Lenox Hill Hospital, New York, and Hofstra University, Hempstead, N.Y..
Researchers have previously identified racial and ethnic disparities in a wide range of maternal outcomes, including mortality, overall morbidity, preterm birth, low birth weight, fetal growth restriction, hypertensive disorders of pregnancy, diabetes, and cesarean deliveries. But the researchers wanted to know if the usual protective effects seen for cesarean deliveries existed in the racial and ethnic groups with these disparities. Past studies have already found that the protective effect of maternal education is greater for White women than Black women with infant mortality and overall self-rated health.
The researchers conducted a retrospective analysis of all low-risk nulliparous, term, singleton, vertex live births to U.S. residents from 2016 to 2019 by using the natality database of the Centers for Disease Control and Prevention. They looked only at women who were non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and Hispanic women. They excluded women with pregestational and gestational diabetes, chronic hypertension, and hypertensive disorders of pregnancy.
Maternal education levels were stratified into those without a high school diploma, high school graduates (including those with some college credit), college graduates, and those with advanced degrees. The total population included 2,969,207 mothers with a 23.4% cesarean delivery rate.
Before considering education or other potential confounders, the cesarean delivery rate was 27.4% in Black women and 25.6% in Asian women, compared with 22.4% in White women and 23% in Hispanic women (P < .001).
Among those with less than a high school education, Black (20.9%), Asian (23.1%), and Hispanic (17.9% cesarean delivery prevalence was greater than that among White women (17.2%) (P < .001). The same was true among those with a high school education (with or without some college): 22% of White women in this group had cesarean deliveries compared with 26.3% of Black women, 26.3% of Asian women, and 22.5% of Hispanic women (P < .001).
At higher levels of education, the disparities not only persisted but actually increased.
The prevalence of cesarean deliveries was 23% in White college graduates, compared with 32.5% of Black college graduates, 26.3% of Asian college graduates, and 27.7% of Hispanic college graduates (P < .001). Similarly, in those with an advanced degree, the prevalence of cesarean deliveries in their population set was 23.6% of Whites, 36.3% of Blacks, 26.1% of Asians, and 30.1% of Hispanics (P < .001).
After adjusting for maternal education as well as age, prepregnancy body mass index, weight gain during pregnancy, insurance type, and neonatal birth weight, the researchers still found substantial disparities in cesarean delivery rates. Black women had 1.54 times greater odds of cesarean delivery than White women (P < .001). Similarly, the odds were 1.45 times greater for Asian women and 1.24 times greater for Hispanic women (P < .001).
Controlling for race, ethnicity, and the other confounders, women with less than a high school education or a high school diploma had similar likelihoods of cesarean delivery. The likelihood of a cesarean delivery was slightly reduced for women with a college degree (odds ratio, 0.93) or advanced degree (OR, 0.88). But this protective effect did not dampen racial/ethnic disparities. In fact, even greater disparities were seen at higher levels of education.
“At each level of education, all the racial/ethnic groups had significantly higher odds of a cesarean delivery than White women,” Dr. Eliner said. “Additionally, the racial/ethnic disparity in cesarean delivery rates increased with increasing level of education, and we specifically see a meaningful jump in the odds ratio at the college graduate level.”
She pointed out that the OR for cesarean delivery in Black women was 1.4 times greater than White women in the group with less than a high school education and 1.44 times greater in those with high school diplomas. Then it jumped to 1.69 in the college graduates group and 1.7 in the advanced degree group.
Higher maternal education was associated with a lower likelihood of cesarean delivery in White women and Asian women. White women with advanced degrees were 17% less likely to have a cesarean than White women with less than a high school education, and the respective reduction in risk was 19% for Asian women.
In Black women, however, education has a much smaller protective effect: An advanced degree reduced the odds of a cesarean delivery by only 7% and no significant difference showed up between high school graduates and college graduates, Dr. Eliner reported.
In Hispanic women, no protective effect showed up, and the odds of a cesarean delivery actually increased slightly in high school and college graduates above those with less than a high school education.
Dr. Eliner discussed a couple possible reasons for a less protective effect from maternal education in Black and Hispanic groups, including higher levels of chronic stress found in past research among racial/ethnic minorities with higher levels of education.
“The impact of racism as a chronic stressor and its association with adverse obstetric and prenatal outcomes is an emerging theme in health disparity research and is yet to be fully understood,” Dr. Eliner said in an interview. “Nonetheless, there is some evidence suggesting that racial/ethnic minorities with higher levels of education suffer from higher levels of stress.”
Implicit and explicit interpersonal bias and institutional racism may also play a role in the disparities, she said, and these factors may disproportionately affect the quality of care for more educated women. She also suggested that White women may be more comfortable advocating for their care.
“While less educated women from all racial/ethnic groups may lack the self-advocacy skills to discuss their labor course, educated White women may be more confident than women from educated minority groups,” Dr. Eliner told attendees. “They may therefore be better equipped to discuss the need for a cesarean delivery with their provider.”
Dr. Eliner elaborated on this: “Given the historical and current disparities of the health care system, women in racial/ethnic minorities may potentially be guarded in their interaction with medical professionals, with a reduced trust in the health care system, and may thus not feel empowered to advocate for themselves in this setting,” she said.
Allison Bryant Mantha, MD, MPH, vice chair for quality, equity, and safety in the ob.gyn. department at Massachusetts General Hospital, Boston, suggested that bias and racism may play a role in this self-advocacy as well.
“I’m wondering if it might not be equally plausible that the advocacy might be met differently by who’s delivering the message,” Dr. Bryant Mantha said. “I think from the story of Dr. Susan Moore and patients who advocate for themselves, I think that we know there is probably some differential by who’s delivering the message.”
Finally, even though education is usually highly correlated with income and frequently used as a proxy for it, but the effect of education on income varies by race/ethnicity.
Since education alone is not sufficient to reduce these disparities, potential interventions should focus on increasing awareness of the disparities and the role of implicit bias, improving patients’ trust in the medical system, and training more doctors from underrepresented groups, Dr. Eliner said.
“I was also wondering about the overall patient choice,” said Sarahn M. Wheeler, MD, an assistant professor of ob.gyn. at Duke University Medical Center in Durham, N.C., who comoderated the session with Dr. Bryant Mantha. “Did we have any understanding of differences in patient values systems that might go into some of these differences in findings as well? There are lots of interesting concepts to explore and that this abstract brings up.”
Dr. Eliner, Dr. Wheeler, and Dr. Bryant Mantha had no disclosures.
While the likelihood of a cesarean delivery usually drops as maternal education level increases, the disparities seen in cesarean rates between White and Black or Hispanic women actually increase with more maternal education, according to findings from a new study presented at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Typically, higher maternal education is associated with a lower likelihood of cesarean delivery, but this protective effect is much smaller for Black women and nonexistent for Hispanic women, leading to bigger gaps between these groups and White women, found Yael Eliner, MD, an ob.gyn. residency applicant at Boston University who conducted this research with her colleagues in the ob.gyn. department at Lenox Hill Hospital, New York, and Hofstra University, Hempstead, N.Y..
Researchers have previously identified racial and ethnic disparities in a wide range of maternal outcomes, including mortality, overall morbidity, preterm birth, low birth weight, fetal growth restriction, hypertensive disorders of pregnancy, diabetes, and cesarean deliveries. But the researchers wanted to know if the usual protective effects seen for cesarean deliveries existed in the racial and ethnic groups with these disparities. Past studies have already found that the protective effect of maternal education is greater for White women than Black women with infant mortality and overall self-rated health.
The researchers conducted a retrospective analysis of all low-risk nulliparous, term, singleton, vertex live births to U.S. residents from 2016 to 2019 by using the natality database of the Centers for Disease Control and Prevention. They looked only at women who were non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and Hispanic women. They excluded women with pregestational and gestational diabetes, chronic hypertension, and hypertensive disorders of pregnancy.
Maternal education levels were stratified into those without a high school diploma, high school graduates (including those with some college credit), college graduates, and those with advanced degrees. The total population included 2,969,207 mothers with a 23.4% cesarean delivery rate.
Before considering education or other potential confounders, the cesarean delivery rate was 27.4% in Black women and 25.6% in Asian women, compared with 22.4% in White women and 23% in Hispanic women (P < .001).
Among those with less than a high school education, Black (20.9%), Asian (23.1%), and Hispanic (17.9% cesarean delivery prevalence was greater than that among White women (17.2%) (P < .001). The same was true among those with a high school education (with or without some college): 22% of White women in this group had cesarean deliveries compared with 26.3% of Black women, 26.3% of Asian women, and 22.5% of Hispanic women (P < .001).
At higher levels of education, the disparities not only persisted but actually increased.
The prevalence of cesarean deliveries was 23% in White college graduates, compared with 32.5% of Black college graduates, 26.3% of Asian college graduates, and 27.7% of Hispanic college graduates (P < .001). Similarly, in those with an advanced degree, the prevalence of cesarean deliveries in their population set was 23.6% of Whites, 36.3% of Blacks, 26.1% of Asians, and 30.1% of Hispanics (P < .001).
After adjusting for maternal education as well as age, prepregnancy body mass index, weight gain during pregnancy, insurance type, and neonatal birth weight, the researchers still found substantial disparities in cesarean delivery rates. Black women had 1.54 times greater odds of cesarean delivery than White women (P < .001). Similarly, the odds were 1.45 times greater for Asian women and 1.24 times greater for Hispanic women (P < .001).
Controlling for race, ethnicity, and the other confounders, women with less than a high school education or a high school diploma had similar likelihoods of cesarean delivery. The likelihood of a cesarean delivery was slightly reduced for women with a college degree (odds ratio, 0.93) or advanced degree (OR, 0.88). But this protective effect did not dampen racial/ethnic disparities. In fact, even greater disparities were seen at higher levels of education.
“At each level of education, all the racial/ethnic groups had significantly higher odds of a cesarean delivery than White women,” Dr. Eliner said. “Additionally, the racial/ethnic disparity in cesarean delivery rates increased with increasing level of education, and we specifically see a meaningful jump in the odds ratio at the college graduate level.”
She pointed out that the OR for cesarean delivery in Black women was 1.4 times greater than White women in the group with less than a high school education and 1.44 times greater in those with high school diplomas. Then it jumped to 1.69 in the college graduates group and 1.7 in the advanced degree group.
Higher maternal education was associated with a lower likelihood of cesarean delivery in White women and Asian women. White women with advanced degrees were 17% less likely to have a cesarean than White women with less than a high school education, and the respective reduction in risk was 19% for Asian women.
In Black women, however, education has a much smaller protective effect: An advanced degree reduced the odds of a cesarean delivery by only 7% and no significant difference showed up between high school graduates and college graduates, Dr. Eliner reported.
In Hispanic women, no protective effect showed up, and the odds of a cesarean delivery actually increased slightly in high school and college graduates above those with less than a high school education.
Dr. Eliner discussed a couple possible reasons for a less protective effect from maternal education in Black and Hispanic groups, including higher levels of chronic stress found in past research among racial/ethnic minorities with higher levels of education.
“The impact of racism as a chronic stressor and its association with adverse obstetric and prenatal outcomes is an emerging theme in health disparity research and is yet to be fully understood,” Dr. Eliner said in an interview. “Nonetheless, there is some evidence suggesting that racial/ethnic minorities with higher levels of education suffer from higher levels of stress.”
Implicit and explicit interpersonal bias and institutional racism may also play a role in the disparities, she said, and these factors may disproportionately affect the quality of care for more educated women. She also suggested that White women may be more comfortable advocating for their care.
“While less educated women from all racial/ethnic groups may lack the self-advocacy skills to discuss their labor course, educated White women may be more confident than women from educated minority groups,” Dr. Eliner told attendees. “They may therefore be better equipped to discuss the need for a cesarean delivery with their provider.”
Dr. Eliner elaborated on this: “Given the historical and current disparities of the health care system, women in racial/ethnic minorities may potentially be guarded in their interaction with medical professionals, with a reduced trust in the health care system, and may thus not feel empowered to advocate for themselves in this setting,” she said.
Allison Bryant Mantha, MD, MPH, vice chair for quality, equity, and safety in the ob.gyn. department at Massachusetts General Hospital, Boston, suggested that bias and racism may play a role in this self-advocacy as well.
“I’m wondering if it might not be equally plausible that the advocacy might be met differently by who’s delivering the message,” Dr. Bryant Mantha said. “I think from the story of Dr. Susan Moore and patients who advocate for themselves, I think that we know there is probably some differential by who’s delivering the message.”
Finally, even though education is usually highly correlated with income and frequently used as a proxy for it, but the effect of education on income varies by race/ethnicity.
Since education alone is not sufficient to reduce these disparities, potential interventions should focus on increasing awareness of the disparities and the role of implicit bias, improving patients’ trust in the medical system, and training more doctors from underrepresented groups, Dr. Eliner said.
“I was also wondering about the overall patient choice,” said Sarahn M. Wheeler, MD, an assistant professor of ob.gyn. at Duke University Medical Center in Durham, N.C., who comoderated the session with Dr. Bryant Mantha. “Did we have any understanding of differences in patient values systems that might go into some of these differences in findings as well? There are lots of interesting concepts to explore and that this abstract brings up.”
Dr. Eliner, Dr. Wheeler, and Dr. Bryant Mantha had no disclosures.
While the likelihood of a cesarean delivery usually drops as maternal education level increases, the disparities seen in cesarean rates between White and Black or Hispanic women actually increase with more maternal education, according to findings from a new study presented at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Typically, higher maternal education is associated with a lower likelihood of cesarean delivery, but this protective effect is much smaller for Black women and nonexistent for Hispanic women, leading to bigger gaps between these groups and White women, found Yael Eliner, MD, an ob.gyn. residency applicant at Boston University who conducted this research with her colleagues in the ob.gyn. department at Lenox Hill Hospital, New York, and Hofstra University, Hempstead, N.Y..
Researchers have previously identified racial and ethnic disparities in a wide range of maternal outcomes, including mortality, overall morbidity, preterm birth, low birth weight, fetal growth restriction, hypertensive disorders of pregnancy, diabetes, and cesarean deliveries. But the researchers wanted to know if the usual protective effects seen for cesarean deliveries existed in the racial and ethnic groups with these disparities. Past studies have already found that the protective effect of maternal education is greater for White women than Black women with infant mortality and overall self-rated health.
The researchers conducted a retrospective analysis of all low-risk nulliparous, term, singleton, vertex live births to U.S. residents from 2016 to 2019 by using the natality database of the Centers for Disease Control and Prevention. They looked only at women who were non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and Hispanic women. They excluded women with pregestational and gestational diabetes, chronic hypertension, and hypertensive disorders of pregnancy.
Maternal education levels were stratified into those without a high school diploma, high school graduates (including those with some college credit), college graduates, and those with advanced degrees. The total population included 2,969,207 mothers with a 23.4% cesarean delivery rate.
Before considering education or other potential confounders, the cesarean delivery rate was 27.4% in Black women and 25.6% in Asian women, compared with 22.4% in White women and 23% in Hispanic women (P < .001).
Among those with less than a high school education, Black (20.9%), Asian (23.1%), and Hispanic (17.9% cesarean delivery prevalence was greater than that among White women (17.2%) (P < .001). The same was true among those with a high school education (with or without some college): 22% of White women in this group had cesarean deliveries compared with 26.3% of Black women, 26.3% of Asian women, and 22.5% of Hispanic women (P < .001).
At higher levels of education, the disparities not only persisted but actually increased.
The prevalence of cesarean deliveries was 23% in White college graduates, compared with 32.5% of Black college graduates, 26.3% of Asian college graduates, and 27.7% of Hispanic college graduates (P < .001). Similarly, in those with an advanced degree, the prevalence of cesarean deliveries in their population set was 23.6% of Whites, 36.3% of Blacks, 26.1% of Asians, and 30.1% of Hispanics (P < .001).
After adjusting for maternal education as well as age, prepregnancy body mass index, weight gain during pregnancy, insurance type, and neonatal birth weight, the researchers still found substantial disparities in cesarean delivery rates. Black women had 1.54 times greater odds of cesarean delivery than White women (P < .001). Similarly, the odds were 1.45 times greater for Asian women and 1.24 times greater for Hispanic women (P < .001).
Controlling for race, ethnicity, and the other confounders, women with less than a high school education or a high school diploma had similar likelihoods of cesarean delivery. The likelihood of a cesarean delivery was slightly reduced for women with a college degree (odds ratio, 0.93) or advanced degree (OR, 0.88). But this protective effect did not dampen racial/ethnic disparities. In fact, even greater disparities were seen at higher levels of education.
“At each level of education, all the racial/ethnic groups had significantly higher odds of a cesarean delivery than White women,” Dr. Eliner said. “Additionally, the racial/ethnic disparity in cesarean delivery rates increased with increasing level of education, and we specifically see a meaningful jump in the odds ratio at the college graduate level.”
She pointed out that the OR for cesarean delivery in Black women was 1.4 times greater than White women in the group with less than a high school education and 1.44 times greater in those with high school diplomas. Then it jumped to 1.69 in the college graduates group and 1.7 in the advanced degree group.
Higher maternal education was associated with a lower likelihood of cesarean delivery in White women and Asian women. White women with advanced degrees were 17% less likely to have a cesarean than White women with less than a high school education, and the respective reduction in risk was 19% for Asian women.
In Black women, however, education has a much smaller protective effect: An advanced degree reduced the odds of a cesarean delivery by only 7% and no significant difference showed up between high school graduates and college graduates, Dr. Eliner reported.
In Hispanic women, no protective effect showed up, and the odds of a cesarean delivery actually increased slightly in high school and college graduates above those with less than a high school education.
Dr. Eliner discussed a couple possible reasons for a less protective effect from maternal education in Black and Hispanic groups, including higher levels of chronic stress found in past research among racial/ethnic minorities with higher levels of education.
“The impact of racism as a chronic stressor and its association with adverse obstetric and prenatal outcomes is an emerging theme in health disparity research and is yet to be fully understood,” Dr. Eliner said in an interview. “Nonetheless, there is some evidence suggesting that racial/ethnic minorities with higher levels of education suffer from higher levels of stress.”
Implicit and explicit interpersonal bias and institutional racism may also play a role in the disparities, she said, and these factors may disproportionately affect the quality of care for more educated women. She also suggested that White women may be more comfortable advocating for their care.
“While less educated women from all racial/ethnic groups may lack the self-advocacy skills to discuss their labor course, educated White women may be more confident than women from educated minority groups,” Dr. Eliner told attendees. “They may therefore be better equipped to discuss the need for a cesarean delivery with their provider.”
Dr. Eliner elaborated on this: “Given the historical and current disparities of the health care system, women in racial/ethnic minorities may potentially be guarded in their interaction with medical professionals, with a reduced trust in the health care system, and may thus not feel empowered to advocate for themselves in this setting,” she said.
Allison Bryant Mantha, MD, MPH, vice chair for quality, equity, and safety in the ob.gyn. department at Massachusetts General Hospital, Boston, suggested that bias and racism may play a role in this self-advocacy as well.
“I’m wondering if it might not be equally plausible that the advocacy might be met differently by who’s delivering the message,” Dr. Bryant Mantha said. “I think from the story of Dr. Susan Moore and patients who advocate for themselves, I think that we know there is probably some differential by who’s delivering the message.”
Finally, even though education is usually highly correlated with income and frequently used as a proxy for it, but the effect of education on income varies by race/ethnicity.
Since education alone is not sufficient to reduce these disparities, potential interventions should focus on increasing awareness of the disparities and the role of implicit bias, improving patients’ trust in the medical system, and training more doctors from underrepresented groups, Dr. Eliner said.
“I was also wondering about the overall patient choice,” said Sarahn M. Wheeler, MD, an assistant professor of ob.gyn. at Duke University Medical Center in Durham, N.C., who comoderated the session with Dr. Bryant Mantha. “Did we have any understanding of differences in patient values systems that might go into some of these differences in findings as well? There are lots of interesting concepts to explore and that this abstract brings up.”
Dr. Eliner, Dr. Wheeler, and Dr. Bryant Mantha had no disclosures.
FROM THE PREGNANCY MEETING
Placenta’s role in schizophrenia ‘bigger than we imagined'
Schizophrenia-related genes in the placenta are predictive of the size of a baby’s brain at birth and the rate of cognitive development. In a complicated pregnancy, such genes could raise the risk of developing schizophrenia later in life, new research suggests.
“This is further evidence that early life matters in schizophrenia, and the placenta plays a bigger role than we imagined,” Daniel R. Weinberger, MD, director and CEO, Lieber Institute for Brain Development, and professor of neurology, psychiatry, and neuroscience, Johns Hopkins University, Baltimore, said in a news release.
“The holy grail would be to identify, based by complicated pregnancies and placental risk scores, who is at maximum risk for schizophrenia from very early in life, and these individuals could be followed more carefully,” Dr. Weinberger said in an interview.
The study was published online Feb. 8 in Proceedings of the National Academy of Sciences.
A therapeutic target?
As reported by this news organization, in 2018, the same group of researchers reported that genes associated with schizophrenia are activated in the placenta during a complicated pregnancy, increasing a child’s risk of developing schizophrenia later in life.
In this latest study, they further explored the biological interplay between placental health and neurodevelopment.
They found that a higher placental genomic risk score for schizophrenia, in conjunction with early-life complications during pregnancy, at labor/delivery, and early in neonatal life, is associated with changes in early brain growth and function, particularly in males.
“, and this was associated with slower cognitive development over the first 2 years of life – particularly in the first year of life,” said Dr. Weinberger.
This research defines a “potentially reversible neurodevelopmental path of risk that may be unique to schizophrenia,” the researchers write.
Although most individuals on this altered neurodevelopmental path likely “canalize” back toward normal development, some may not be rescued and instead “decanalize” toward illness, they add.
To date, prevention of schizophrenia from early life has seemed “unapproachable if not unimaginable, but these new insights offer possibilities to change the paradigm,” Dr. Weinberger said in the news release.
“Measuring schizophrenia genetic scores in the placenta combined with studying the first 2 years of cognitive developmental patterns and early life complications could prove to be an important approach to identify those babies with increased risks,” he added.
Important research
Commenting on the study for this news organization, Christopher A. Ross, MD, PhD, professor of psychiatry and behavioral sciences, Johns Hopkins Medicine, Baltimore, said that this is “an interesting and important paper that replicates and extends previous findings of the relationship of placenta genes to schizophrenia in adults.”
“The hypothesis continues to be – and they are continuing to support it – that events in early development could set a person up for a risk of schizophrenia later in life,” said Dr. Ross.
This research, he added, also supports the concept that there are at least two broad classes of genetic risk for schizophrenia.
“One acts through genes that are expressed in the brain and doesn’t relate to early life events, and the other acts through genes expressed in the placenta in patients with these early life events,” said Dr. Ross.
The study had no specific funding. Dr. Weinberger and Dr. Ross have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Schizophrenia-related genes in the placenta are predictive of the size of a baby’s brain at birth and the rate of cognitive development. In a complicated pregnancy, such genes could raise the risk of developing schizophrenia later in life, new research suggests.
“This is further evidence that early life matters in schizophrenia, and the placenta plays a bigger role than we imagined,” Daniel R. Weinberger, MD, director and CEO, Lieber Institute for Brain Development, and professor of neurology, psychiatry, and neuroscience, Johns Hopkins University, Baltimore, said in a news release.
“The holy grail would be to identify, based by complicated pregnancies and placental risk scores, who is at maximum risk for schizophrenia from very early in life, and these individuals could be followed more carefully,” Dr. Weinberger said in an interview.
The study was published online Feb. 8 in Proceedings of the National Academy of Sciences.
A therapeutic target?
As reported by this news organization, in 2018, the same group of researchers reported that genes associated with schizophrenia are activated in the placenta during a complicated pregnancy, increasing a child’s risk of developing schizophrenia later in life.
In this latest study, they further explored the biological interplay between placental health and neurodevelopment.
They found that a higher placental genomic risk score for schizophrenia, in conjunction with early-life complications during pregnancy, at labor/delivery, and early in neonatal life, is associated with changes in early brain growth and function, particularly in males.
“, and this was associated with slower cognitive development over the first 2 years of life – particularly in the first year of life,” said Dr. Weinberger.
This research defines a “potentially reversible neurodevelopmental path of risk that may be unique to schizophrenia,” the researchers write.
Although most individuals on this altered neurodevelopmental path likely “canalize” back toward normal development, some may not be rescued and instead “decanalize” toward illness, they add.
To date, prevention of schizophrenia from early life has seemed “unapproachable if not unimaginable, but these new insights offer possibilities to change the paradigm,” Dr. Weinberger said in the news release.
“Measuring schizophrenia genetic scores in the placenta combined with studying the first 2 years of cognitive developmental patterns and early life complications could prove to be an important approach to identify those babies with increased risks,” he added.
Important research
Commenting on the study for this news organization, Christopher A. Ross, MD, PhD, professor of psychiatry and behavioral sciences, Johns Hopkins Medicine, Baltimore, said that this is “an interesting and important paper that replicates and extends previous findings of the relationship of placenta genes to schizophrenia in adults.”
“The hypothesis continues to be – and they are continuing to support it – that events in early development could set a person up for a risk of schizophrenia later in life,” said Dr. Ross.
This research, he added, also supports the concept that there are at least two broad classes of genetic risk for schizophrenia.
“One acts through genes that are expressed in the brain and doesn’t relate to early life events, and the other acts through genes expressed in the placenta in patients with these early life events,” said Dr. Ross.
The study had no specific funding. Dr. Weinberger and Dr. Ross have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Schizophrenia-related genes in the placenta are predictive of the size of a baby’s brain at birth and the rate of cognitive development. In a complicated pregnancy, such genes could raise the risk of developing schizophrenia later in life, new research suggests.
“This is further evidence that early life matters in schizophrenia, and the placenta plays a bigger role than we imagined,” Daniel R. Weinberger, MD, director and CEO, Lieber Institute for Brain Development, and professor of neurology, psychiatry, and neuroscience, Johns Hopkins University, Baltimore, said in a news release.
“The holy grail would be to identify, based by complicated pregnancies and placental risk scores, who is at maximum risk for schizophrenia from very early in life, and these individuals could be followed more carefully,” Dr. Weinberger said in an interview.
The study was published online Feb. 8 in Proceedings of the National Academy of Sciences.
A therapeutic target?
As reported by this news organization, in 2018, the same group of researchers reported that genes associated with schizophrenia are activated in the placenta during a complicated pregnancy, increasing a child’s risk of developing schizophrenia later in life.
In this latest study, they further explored the biological interplay between placental health and neurodevelopment.
They found that a higher placental genomic risk score for schizophrenia, in conjunction with early-life complications during pregnancy, at labor/delivery, and early in neonatal life, is associated with changes in early brain growth and function, particularly in males.
“, and this was associated with slower cognitive development over the first 2 years of life – particularly in the first year of life,” said Dr. Weinberger.
This research defines a “potentially reversible neurodevelopmental path of risk that may be unique to schizophrenia,” the researchers write.
Although most individuals on this altered neurodevelopmental path likely “canalize” back toward normal development, some may not be rescued and instead “decanalize” toward illness, they add.
To date, prevention of schizophrenia from early life has seemed “unapproachable if not unimaginable, but these new insights offer possibilities to change the paradigm,” Dr. Weinberger said in the news release.
“Measuring schizophrenia genetic scores in the placenta combined with studying the first 2 years of cognitive developmental patterns and early life complications could prove to be an important approach to identify those babies with increased risks,” he added.
Important research
Commenting on the study for this news organization, Christopher A. Ross, MD, PhD, professor of psychiatry and behavioral sciences, Johns Hopkins Medicine, Baltimore, said that this is “an interesting and important paper that replicates and extends previous findings of the relationship of placenta genes to schizophrenia in adults.”
“The hypothesis continues to be – and they are continuing to support it – that events in early development could set a person up for a risk of schizophrenia later in life,” said Dr. Ross.
This research, he added, also supports the concept that there are at least two broad classes of genetic risk for schizophrenia.
“One acts through genes that are expressed in the brain and doesn’t relate to early life events, and the other acts through genes expressed in the placenta in patients with these early life events,” said Dr. Ross.
The study had no specific funding. Dr. Weinberger and Dr. Ross have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Family medicine has grown; its composition has evolved
and the men and women who practice it are no exception.
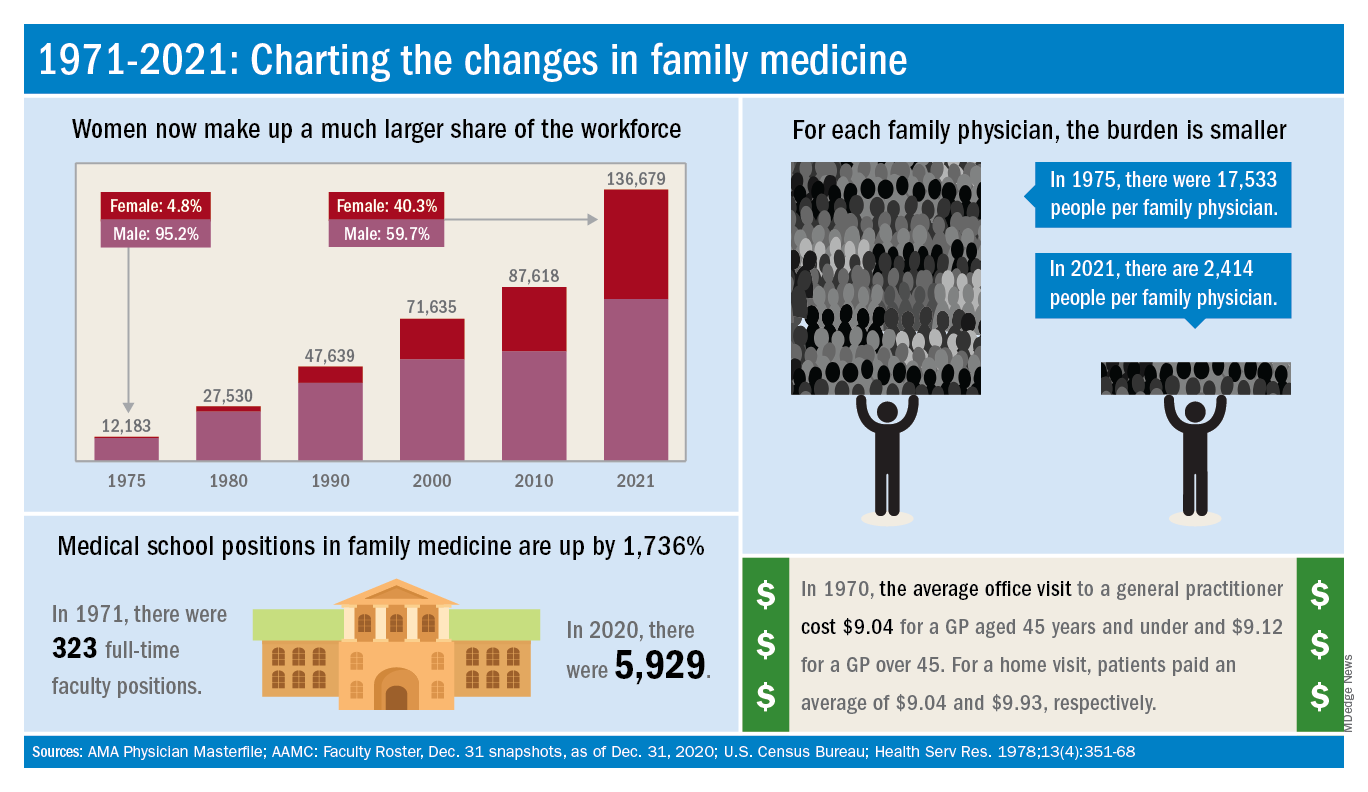
The family medicine workforce of 2021 is not the workforce of 1971. Not even close. Although we would like to give a huge shout-out to anyone who can claim to be a member of both.
Today’s FP workforce is, first of all, much larger than it was in 1971, although we can’t actually prove it because the American Medical Association’s data for that year are “only available in books that are locked away at the empty AMA headquarters,” according to a member of the AMA media relations staff who is, like so many people these days, working at home because of the pandemic.
The face of family medicine in 1975 vs. today
Today’s workforce is much larger than it was in 1975, when there were just over 12,000 family physicians in the United States. As of January 2021, the total was approaching 137,000, including all “physicians and residents in patient care, research, administration, teaching, retired, inactive, etc.,” the AMA explained.
Family physicians as a group are much more diverse than they were in 1975. That year, 8.3% of FPs were international medical graduates (IMGs). By 2010, IMGs made up almost 23% of the workforce, and in the 2020 resident match, 37% of the 4,662 available family medicine slots were filled by IMGs.
Women have made even greater inroads into the family physician ranks over the last 5 decades. In 1975, less than 5% of all FPs were females, but by 2021 the proportion of females in the specialty was just over 40%.
In the first 5 years of the family practice era, 1969-1973, only 12 women and 31 IMGs graduated from FP residency programs, those numbers representing 3.2% and 8.3%, respectively, of the total of 372, according to a 1996 study in JAMA. By 1990-1993, women made up 33% and IMGs 14% of the 9,400 graduates.
Another group that increased its presence in family medicine is doctors of osteopathy, who went from zero residency graduates in 1969-1973 to over 1,100 (11.8%) in 1990-1993, the JAMA report noted. By 2020, almost 1,400 osteopathic physicians entered family medicine residencies, filling 30% of all slots available, according to the National Resident Matching Program.
The medical schools producing all these new residents have raised their games since 1971: the number of full-time faculty in family medicine departments rose from 323 to 5,929 in 2020, based on data from the Association of American Medical Colleges (Faculty Roster, Dec. 31 snapshots, as of Dec. 31, 2020).
A shortage or a surplus of FPs?
It has been suggested, however, that all is not well in primary care land. A study conducted by the American Academy of Family Physicians in 2016 – a year after 2,463 graduates of MD- and DO-granting medical schools entered family medicine residencies – concluded “that the current medical school system is failing, collectively, to produce the primary care workforce that is needed to achieve optimal health.”
Warnings about physician shortages are nothing new, but how about the other side of the coin? The Jan. 15, 1981, issue of Family Practice News covered a somewhat controversial report from the Graduate Medical Education National Advisory Committee, which projected a surplus of 3,000 FPs, and as many as 70,000 physicians overall, by the year 1990.
Just a few months later, in the June 15, 1981, issue of FPN, an AAFP officer predicted that “the flood of new physicians in the next decade may affect family practice more than any other specialty.”
Mostly, though, the issue is shortages. In 2002, a status report on family practice from the Robert Graham Center acknowledged that “many centers of academic medicine continue to resist the development of family practice and primary care. ... Family medicine remains a true counterculture in these environments, and students may continue to face significant discouragement in response to interest they may express in becoming a family physician.”
and the men and women who practice it are no exception.

The family medicine workforce of 2021 is not the workforce of 1971. Not even close. Although we would like to give a huge shout-out to anyone who can claim to be a member of both.
Today’s FP workforce is, first of all, much larger than it was in 1971, although we can’t actually prove it because the American Medical Association’s data for that year are “only available in books that are locked away at the empty AMA headquarters,” according to a member of the AMA media relations staff who is, like so many people these days, working at home because of the pandemic.
The face of family medicine in 1975 vs. today
Today’s workforce is much larger than it was in 1975, when there were just over 12,000 family physicians in the United States. As of January 2021, the total was approaching 137,000, including all “physicians and residents in patient care, research, administration, teaching, retired, inactive, etc.,” the AMA explained.
Family physicians as a group are much more diverse than they were in 1975. That year, 8.3% of FPs were international medical graduates (IMGs). By 2010, IMGs made up almost 23% of the workforce, and in the 2020 resident match, 37% of the 4,662 available family medicine slots were filled by IMGs.
Women have made even greater inroads into the family physician ranks over the last 5 decades. In 1975, less than 5% of all FPs were females, but by 2021 the proportion of females in the specialty was just over 40%.
In the first 5 years of the family practice era, 1969-1973, only 12 women and 31 IMGs graduated from FP residency programs, those numbers representing 3.2% and 8.3%, respectively, of the total of 372, according to a 1996 study in JAMA. By 1990-1993, women made up 33% and IMGs 14% of the 9,400 graduates.
Another group that increased its presence in family medicine is doctors of osteopathy, who went from zero residency graduates in 1969-1973 to over 1,100 (11.8%) in 1990-1993, the JAMA report noted. By 2020, almost 1,400 osteopathic physicians entered family medicine residencies, filling 30% of all slots available, according to the National Resident Matching Program.
The medical schools producing all these new residents have raised their games since 1971: the number of full-time faculty in family medicine departments rose from 323 to 5,929 in 2020, based on data from the Association of American Medical Colleges (Faculty Roster, Dec. 31 snapshots, as of Dec. 31, 2020).
A shortage or a surplus of FPs?
It has been suggested, however, that all is not well in primary care land. A study conducted by the American Academy of Family Physicians in 2016 – a year after 2,463 graduates of MD- and DO-granting medical schools entered family medicine residencies – concluded “that the current medical school system is failing, collectively, to produce the primary care workforce that is needed to achieve optimal health.”
Warnings about physician shortages are nothing new, but how about the other side of the coin? The Jan. 15, 1981, issue of Family Practice News covered a somewhat controversial report from the Graduate Medical Education National Advisory Committee, which projected a surplus of 3,000 FPs, and as many as 70,000 physicians overall, by the year 1990.
Just a few months later, in the June 15, 1981, issue of FPN, an AAFP officer predicted that “the flood of new physicians in the next decade may affect family practice more than any other specialty.”
Mostly, though, the issue is shortages. In 2002, a status report on family practice from the Robert Graham Center acknowledged that “many centers of academic medicine continue to resist the development of family practice and primary care. ... Family medicine remains a true counterculture in these environments, and students may continue to face significant discouragement in response to interest they may express in becoming a family physician.”
and the men and women who practice it are no exception.

The family medicine workforce of 2021 is not the workforce of 1971. Not even close. Although we would like to give a huge shout-out to anyone who can claim to be a member of both.
Today’s FP workforce is, first of all, much larger than it was in 1971, although we can’t actually prove it because the American Medical Association’s data for that year are “only available in books that are locked away at the empty AMA headquarters,” according to a member of the AMA media relations staff who is, like so many people these days, working at home because of the pandemic.
The face of family medicine in 1975 vs. today
Today’s workforce is much larger than it was in 1975, when there were just over 12,000 family physicians in the United States. As of January 2021, the total was approaching 137,000, including all “physicians and residents in patient care, research, administration, teaching, retired, inactive, etc.,” the AMA explained.
Family physicians as a group are much more diverse than they were in 1975. That year, 8.3% of FPs were international medical graduates (IMGs). By 2010, IMGs made up almost 23% of the workforce, and in the 2020 resident match, 37% of the 4,662 available family medicine slots were filled by IMGs.
Women have made even greater inroads into the family physician ranks over the last 5 decades. In 1975, less than 5% of all FPs were females, but by 2021 the proportion of females in the specialty was just over 40%.
In the first 5 years of the family practice era, 1969-1973, only 12 women and 31 IMGs graduated from FP residency programs, those numbers representing 3.2% and 8.3%, respectively, of the total of 372, according to a 1996 study in JAMA. By 1990-1993, women made up 33% and IMGs 14% of the 9,400 graduates.
Another group that increased its presence in family medicine is doctors of osteopathy, who went from zero residency graduates in 1969-1973 to over 1,100 (11.8%) in 1990-1993, the JAMA report noted. By 2020, almost 1,400 osteopathic physicians entered family medicine residencies, filling 30% of all slots available, according to the National Resident Matching Program.
The medical schools producing all these new residents have raised their games since 1971: the number of full-time faculty in family medicine departments rose from 323 to 5,929 in 2020, based on data from the Association of American Medical Colleges (Faculty Roster, Dec. 31 snapshots, as of Dec. 31, 2020).
A shortage or a surplus of FPs?
It has been suggested, however, that all is not well in primary care land. A study conducted by the American Academy of Family Physicians in 2016 – a year after 2,463 graduates of MD- and DO-granting medical schools entered family medicine residencies – concluded “that the current medical school system is failing, collectively, to produce the primary care workforce that is needed to achieve optimal health.”
Warnings about physician shortages are nothing new, but how about the other side of the coin? The Jan. 15, 1981, issue of Family Practice News covered a somewhat controversial report from the Graduate Medical Education National Advisory Committee, which projected a surplus of 3,000 FPs, and as many as 70,000 physicians overall, by the year 1990.
Just a few months later, in the June 15, 1981, issue of FPN, an AAFP officer predicted that “the flood of new physicians in the next decade may affect family practice more than any other specialty.”
Mostly, though, the issue is shortages. In 2002, a status report on family practice from the Robert Graham Center acknowledged that “many centers of academic medicine continue to resist the development of family practice and primary care. ... Family medicine remains a true counterculture in these environments, and students may continue to face significant discouragement in response to interest they may express in becoming a family physician.”
Alien cells may explain COVID-19 brain fog
, a new report suggests.
The authors report five separate post-mortem cases from patients who died with COVID-19 in which large cells resembling megakaryocytes were identified in cortical capillaries. Immunohistochemistry subsequently confirmed their megakaryocyte identity.
They point out that the finding is of interest as – to their knowledge – megakaryocytes have not been found in the brain before.
The observations are described in a research letter published online Feb. 12 in JAMA Neurology.
Bone marrow cells in the brain
Lead author David Nauen, MD, PhD, a neuropathologist from Johns Hopkins University, Baltimore, reported that he identified these cells in the first analysis of post-mortem brain tissue from a patient who had COVID-19.
“Some other viruses cause changes in the brain such as encephalopathy, and as neurologic symptoms are often reported in COVID-19, I was curious to see if similar effects were seen in brain post-mortem samples from patients who had died with the infection,” Dr. Nauen said.
On his first analysis of the brain tissue of a patient who had COVID-19, Dr. Nauen saw no evidence of viral encephalitis, but he observed some “unusually large” cells in the brain capillaries.
“I was taken aback; I couldn’t figure out what they were. Then I realized these cells were megakaryocytes from the bone marrow. I have never seen these cells in the brain before. I asked several colleagues and none of them had either. After extensive literature searches, I could find no evidence of megakaryocytes being in the brain,” Dr. Nauen noted.
Megakaryocytes, he explained, are “very large cells, and the brain capillaries are very small – just large enough to let red blood cells and lymphocytes pass through. To see these very large cells in such vessels is extremely unusual. It looks like they are causing occlusions.”
By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment, the authors suggest.
“This might alter the hemodynamics and put pressure on other vessels, possibly contributing to the increased risk of stroke that has been reported in COVID-19,” Dr. Nauen said. None of the samples he examined came from patients with COVID-19 who had had a stroke, he reported.
Other than the presence of megakaryocytes in the capillaries, the brain looked normal, he said. He has now examined samples from 15 brains of patients who had COVID-19 and megakaryocytes have been found in the brain capillaries in five cases.
New neurologic complication
Classic encephalitis found with other viruses has not been reported in brain post-mortem examinations from patients who had COVID-19, Dr. Nauen noted. “The cognitive issues such as grogginess associated with COVID-19 would indicate problems with the cortex but that hasn’t been documented. This occlusion of a multitude of tiny vessels by megalokaryocytes may offer some explanation of the cognitive issues. This is a new kind of vascular insult seen on pathology, and suggests a new kind of neurologic complication,” he added.
The big question is what these megakaryocytes are doing in the brain.
“Megakaryocytes are bone marrow cells. They are not immune cells. Their job is to produce platelets to help the blood clot. They are not normally found outside the bone marrow, but they have been reported in other organs in COVID-19 patients.
“But the big puzzle associated with finding them in the brain is how they get through the very fine network of blood vessels in the lungs. The geometry just doesn’t work. We don’t know which part of the COVID inflammatory response makes this happen,” said Dr. Nauen.
The authors suggest one possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs.
“We need to try and understand if there is anything distinctive about these megakaryocytes – which proteins are they expressing that may explain why they are behaving in such an unusual way,” said Dr. Nauen.
Noting that many patients with severe COVID-19 have problems with clotting, and megakaryocytes are part of the clotting system, he speculated that some sort of aberrant message is being sent to these cells.
“It is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random [are] only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable,” the authors wrote.
Dr. Nauen added that to his knowledge, this is the first report of such observations, and the next step is to look for similar findings in larger sample sizes.
A version of this article first appeared on Medscape.com.
, a new report suggests.
The authors report five separate post-mortem cases from patients who died with COVID-19 in which large cells resembling megakaryocytes were identified in cortical capillaries. Immunohistochemistry subsequently confirmed their megakaryocyte identity.
They point out that the finding is of interest as – to their knowledge – megakaryocytes have not been found in the brain before.
The observations are described in a research letter published online Feb. 12 in JAMA Neurology.
Bone marrow cells in the brain
Lead author David Nauen, MD, PhD, a neuropathologist from Johns Hopkins University, Baltimore, reported that he identified these cells in the first analysis of post-mortem brain tissue from a patient who had COVID-19.
“Some other viruses cause changes in the brain such as encephalopathy, and as neurologic symptoms are often reported in COVID-19, I was curious to see if similar effects were seen in brain post-mortem samples from patients who had died with the infection,” Dr. Nauen said.
On his first analysis of the brain tissue of a patient who had COVID-19, Dr. Nauen saw no evidence of viral encephalitis, but he observed some “unusually large” cells in the brain capillaries.
“I was taken aback; I couldn’t figure out what they were. Then I realized these cells were megakaryocytes from the bone marrow. I have never seen these cells in the brain before. I asked several colleagues and none of them had either. After extensive literature searches, I could find no evidence of megakaryocytes being in the brain,” Dr. Nauen noted.
Megakaryocytes, he explained, are “very large cells, and the brain capillaries are very small – just large enough to let red blood cells and lymphocytes pass through. To see these very large cells in such vessels is extremely unusual. It looks like they are causing occlusions.”
By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment, the authors suggest.
“This might alter the hemodynamics and put pressure on other vessels, possibly contributing to the increased risk of stroke that has been reported in COVID-19,” Dr. Nauen said. None of the samples he examined came from patients with COVID-19 who had had a stroke, he reported.
Other than the presence of megakaryocytes in the capillaries, the brain looked normal, he said. He has now examined samples from 15 brains of patients who had COVID-19 and megakaryocytes have been found in the brain capillaries in five cases.
New neurologic complication
Classic encephalitis found with other viruses has not been reported in brain post-mortem examinations from patients who had COVID-19, Dr. Nauen noted. “The cognitive issues such as grogginess associated with COVID-19 would indicate problems with the cortex but that hasn’t been documented. This occlusion of a multitude of tiny vessels by megalokaryocytes may offer some explanation of the cognitive issues. This is a new kind of vascular insult seen on pathology, and suggests a new kind of neurologic complication,” he added.
The big question is what these megakaryocytes are doing in the brain.
“Megakaryocytes are bone marrow cells. They are not immune cells. Their job is to produce platelets to help the blood clot. They are not normally found outside the bone marrow, but they have been reported in other organs in COVID-19 patients.
“But the big puzzle associated with finding them in the brain is how they get through the very fine network of blood vessels in the lungs. The geometry just doesn’t work. We don’t know which part of the COVID inflammatory response makes this happen,” said Dr. Nauen.
The authors suggest one possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs.
“We need to try and understand if there is anything distinctive about these megakaryocytes – which proteins are they expressing that may explain why they are behaving in such an unusual way,” said Dr. Nauen.
Noting that many patients with severe COVID-19 have problems with clotting, and megakaryocytes are part of the clotting system, he speculated that some sort of aberrant message is being sent to these cells.
“It is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random [are] only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable,” the authors wrote.
Dr. Nauen added that to his knowledge, this is the first report of such observations, and the next step is to look for similar findings in larger sample sizes.
A version of this article first appeared on Medscape.com.
, a new report suggests.
The authors report five separate post-mortem cases from patients who died with COVID-19 in which large cells resembling megakaryocytes were identified in cortical capillaries. Immunohistochemistry subsequently confirmed their megakaryocyte identity.
They point out that the finding is of interest as – to their knowledge – megakaryocytes have not been found in the brain before.
The observations are described in a research letter published online Feb. 12 in JAMA Neurology.
Bone marrow cells in the brain
Lead author David Nauen, MD, PhD, a neuropathologist from Johns Hopkins University, Baltimore, reported that he identified these cells in the first analysis of post-mortem brain tissue from a patient who had COVID-19.
“Some other viruses cause changes in the brain such as encephalopathy, and as neurologic symptoms are often reported in COVID-19, I was curious to see if similar effects were seen in brain post-mortem samples from patients who had died with the infection,” Dr. Nauen said.
On his first analysis of the brain tissue of a patient who had COVID-19, Dr. Nauen saw no evidence of viral encephalitis, but he observed some “unusually large” cells in the brain capillaries.
“I was taken aback; I couldn’t figure out what they were. Then I realized these cells were megakaryocytes from the bone marrow. I have never seen these cells in the brain before. I asked several colleagues and none of them had either. After extensive literature searches, I could find no evidence of megakaryocytes being in the brain,” Dr. Nauen noted.
Megakaryocytes, he explained, are “very large cells, and the brain capillaries are very small – just large enough to let red blood cells and lymphocytes pass through. To see these very large cells in such vessels is extremely unusual. It looks like they are causing occlusions.”
By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment, the authors suggest.
“This might alter the hemodynamics and put pressure on other vessels, possibly contributing to the increased risk of stroke that has been reported in COVID-19,” Dr. Nauen said. None of the samples he examined came from patients with COVID-19 who had had a stroke, he reported.
Other than the presence of megakaryocytes in the capillaries, the brain looked normal, he said. He has now examined samples from 15 brains of patients who had COVID-19 and megakaryocytes have been found in the brain capillaries in five cases.
New neurologic complication
Classic encephalitis found with other viruses has not been reported in brain post-mortem examinations from patients who had COVID-19, Dr. Nauen noted. “The cognitive issues such as grogginess associated with COVID-19 would indicate problems with the cortex but that hasn’t been documented. This occlusion of a multitude of tiny vessels by megalokaryocytes may offer some explanation of the cognitive issues. This is a new kind of vascular insult seen on pathology, and suggests a new kind of neurologic complication,” he added.
The big question is what these megakaryocytes are doing in the brain.
“Megakaryocytes are bone marrow cells. They are not immune cells. Their job is to produce platelets to help the blood clot. They are not normally found outside the bone marrow, but they have been reported in other organs in COVID-19 patients.
“But the big puzzle associated with finding them in the brain is how they get through the very fine network of blood vessels in the lungs. The geometry just doesn’t work. We don’t know which part of the COVID inflammatory response makes this happen,” said Dr. Nauen.
The authors suggest one possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs.
“We need to try and understand if there is anything distinctive about these megakaryocytes – which proteins are they expressing that may explain why they are behaving in such an unusual way,” said Dr. Nauen.
Noting that many patients with severe COVID-19 have problems with clotting, and megakaryocytes are part of the clotting system, he speculated that some sort of aberrant message is being sent to these cells.
“It is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random [are] only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable,” the authors wrote.
Dr. Nauen added that to his knowledge, this is the first report of such observations, and the next step is to look for similar findings in larger sample sizes.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Medicaid and access to dermatologists
Recently, an interview titled “Dermatology a bellwether of health inequities during COVID-19,” was published by the AMA. In my opinion, the interview was largely accurate, but I took issue with the following statement in the article: “Dermatology is a lucrative specialty, and many dermatologists do not accept Medicaid.”
To me, this implies that physicians are to blame for poor health care access, which drives me insane. Dermatology is not a particularly lucrative specialty; it ranked 13th in a recent survey from the professional medical network Doximity. Furthermore, if payment for practice expense is removed, dermatology drops much further down, close to primary care.
There is a fundamental misunderstanding by the public and legislators about physician incomes. The reimbursements that are reported by Medicare for example, include the practice expense cost, which for dermatology is about 60% of the total remitted to the doctor, as I wrote in a 2015 column.
That is, the cost of providing the facility, supplies, staff, rent, and utilities are included in “reimbursement,” though this is money that goes out the door to pay the bills as quickly as it comes in. This is for overhead, nothing here for the practitioner’s time and work.
Even when dermatologists perform hospital consults, they usually bring their own supply kit from their office for skin biopsies, or other procedures since these are impossible to find in a hospital.
I also pointed out in my earlier column that most other specialties do not provide the majority of their procedures in the office, but instead, use the hospital, which provides supplies and staff for procedures. These other specialists are to be lauded for providing their services at charity rates, or for no pay at all, but at least they do not have to pay for the building, equipment, supplies, and staff out of pocket. Dermatologists do, since in a sense, they run their own “hospitals” as almost all of their procedures are based out of their offices.
The economics of a patient visit
I do not dispute that it is more difficult for a Medicaid patient to get an appointment with a dermatologist than it is for a patient with private insurance, but this is because Medicaid often pays less than the cost of supplies to see them. It is also easier to get an appointment for a cosmetic procedure than a rash because reimbursements in general are artificially suppressed, even for Medicare (which is also the benchmark for private insurers) by the federal government. Medicare reimbursements have not kept pace with inflation and are about 53% less than they were in 1992.
Let’s look at a skin biopsy. The supplies and equipment to perform a skin biopsy cost over $50. In Ohio, Medicare pays $96.19 for a skin biopsy. Medicaid pays $47.20. That’s correct: less than the cost of supplies and overhead. So, a private practitioner not only provides the service for free, but loses money on every visit that involves a skin biopsy. When I talk to legislators, I liken this to my standing in front of my office and handing out $5 bills. In Ohio, Medicare pays $105.04 for a level 3 office visit. Medicaid pays $57.76. Medicare overhead on a level 3 office visit is again about 50%, so the office visit is about a break-even proposition, if you donate your time.
Academic medical centers can charge additional facility fees, and some receive subsidies from the city and county to treat indigent patients, and are often obligated to see all. Most hospitals with high Medicaid and indigent patient loads pay their surgical specialists to take call at their emergency rooms and often subsidize their emergency room doctors as well.
I agree that dermatology is an important specialty to have access to in the COVID-19 pandemic. I agree that patients of color may be disproportionately impacted because they may be covered by Medicaid more often, or have no insurance at all. The finger of blame, however, should be squarely pointed at politicians who have woefully underfunded Medicaid reimbursement rates, as well as payments for physicians under the Affordable Care Act, while thumping their chests and boasting how they have provided health care to millions. I think this was eloquently demonstrated when as part of the “deal” Congress made with the AMA to get the ACA passed, Congress agreed to pay primary care physicians (but only primary care) Medicare rates for Medicaid patients for 2 years.
Some states have continued to pay enhanced Medicaid rates and have fewer Medicaid patient access issues.
Most convincing, perhaps, are the states that pay Medicare rates or better for their Medicaid enrollees, for example Alaska and Montana. In these states, you will not have access to care issues beyond the actual human shortage of physicians in remote areas.
So, in conclusion, I maintain that dermatology is not a particularly lucrative specialty, once the overhead expense payments are removed, and further argue, that even if it were, why does that obligate us to provide care to insurance plans at a loss? Medicaid access to dermatologists is a government economic issue, not a physician ethical one. Most Americans get to pick the charities they choose to donate to.
The federal government would love to force all physicians into a plan where you must see patients at their chosen rates or see no patients at all. Look no further than our Canadian neighbors, where long wait times to see specialists are legendary. It has been reported that there are only six to seven hundred dermatologists in all of Canada to serve 30 million people.
So, when the topic of poor patient access to care for the Medicaid enrollee or indigent comes up, stand tall and point your finger to your state capital. That is where the blame lies.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Recently, an interview titled “Dermatology a bellwether of health inequities during COVID-19,” was published by the AMA. In my opinion, the interview was largely accurate, but I took issue with the following statement in the article: “Dermatology is a lucrative specialty, and many dermatologists do not accept Medicaid.”
To me, this implies that physicians are to blame for poor health care access, which drives me insane. Dermatology is not a particularly lucrative specialty; it ranked 13th in a recent survey from the professional medical network Doximity. Furthermore, if payment for practice expense is removed, dermatology drops much further down, close to primary care.
There is a fundamental misunderstanding by the public and legislators about physician incomes. The reimbursements that are reported by Medicare for example, include the practice expense cost, which for dermatology is about 60% of the total remitted to the doctor, as I wrote in a 2015 column.
That is, the cost of providing the facility, supplies, staff, rent, and utilities are included in “reimbursement,” though this is money that goes out the door to pay the bills as quickly as it comes in. This is for overhead, nothing here for the practitioner’s time and work.
Even when dermatologists perform hospital consults, they usually bring their own supply kit from their office for skin biopsies, or other procedures since these are impossible to find in a hospital.
I also pointed out in my earlier column that most other specialties do not provide the majority of their procedures in the office, but instead, use the hospital, which provides supplies and staff for procedures. These other specialists are to be lauded for providing their services at charity rates, or for no pay at all, but at least they do not have to pay for the building, equipment, supplies, and staff out of pocket. Dermatologists do, since in a sense, they run their own “hospitals” as almost all of their procedures are based out of their offices.
The economics of a patient visit
I do not dispute that it is more difficult for a Medicaid patient to get an appointment with a dermatologist than it is for a patient with private insurance, but this is because Medicaid often pays less than the cost of supplies to see them. It is also easier to get an appointment for a cosmetic procedure than a rash because reimbursements in general are artificially suppressed, even for Medicare (which is also the benchmark for private insurers) by the federal government. Medicare reimbursements have not kept pace with inflation and are about 53% less than they were in 1992.
Let’s look at a skin biopsy. The supplies and equipment to perform a skin biopsy cost over $50. In Ohio, Medicare pays $96.19 for a skin biopsy. Medicaid pays $47.20. That’s correct: less than the cost of supplies and overhead. So, a private practitioner not only provides the service for free, but loses money on every visit that involves a skin biopsy. When I talk to legislators, I liken this to my standing in front of my office and handing out $5 bills. In Ohio, Medicare pays $105.04 for a level 3 office visit. Medicaid pays $57.76. Medicare overhead on a level 3 office visit is again about 50%, so the office visit is about a break-even proposition, if you donate your time.
Academic medical centers can charge additional facility fees, and some receive subsidies from the city and county to treat indigent patients, and are often obligated to see all. Most hospitals with high Medicaid and indigent patient loads pay their surgical specialists to take call at their emergency rooms and often subsidize their emergency room doctors as well.
I agree that dermatology is an important specialty to have access to in the COVID-19 pandemic. I agree that patients of color may be disproportionately impacted because they may be covered by Medicaid more often, or have no insurance at all. The finger of blame, however, should be squarely pointed at politicians who have woefully underfunded Medicaid reimbursement rates, as well as payments for physicians under the Affordable Care Act, while thumping their chests and boasting how they have provided health care to millions. I think this was eloquently demonstrated when as part of the “deal” Congress made with the AMA to get the ACA passed, Congress agreed to pay primary care physicians (but only primary care) Medicare rates for Medicaid patients for 2 years.
Some states have continued to pay enhanced Medicaid rates and have fewer Medicaid patient access issues.
Most convincing, perhaps, are the states that pay Medicare rates or better for their Medicaid enrollees, for example Alaska and Montana. In these states, you will not have access to care issues beyond the actual human shortage of physicians in remote areas.
So, in conclusion, I maintain that dermatology is not a particularly lucrative specialty, once the overhead expense payments are removed, and further argue, that even if it were, why does that obligate us to provide care to insurance plans at a loss? Medicaid access to dermatologists is a government economic issue, not a physician ethical one. Most Americans get to pick the charities they choose to donate to.
The federal government would love to force all physicians into a plan where you must see patients at their chosen rates or see no patients at all. Look no further than our Canadian neighbors, where long wait times to see specialists are legendary. It has been reported that there are only six to seven hundred dermatologists in all of Canada to serve 30 million people.
So, when the topic of poor patient access to care for the Medicaid enrollee or indigent comes up, stand tall and point your finger to your state capital. That is where the blame lies.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Recently, an interview titled “Dermatology a bellwether of health inequities during COVID-19,” was published by the AMA. In my opinion, the interview was largely accurate, but I took issue with the following statement in the article: “Dermatology is a lucrative specialty, and many dermatologists do not accept Medicaid.”
To me, this implies that physicians are to blame for poor health care access, which drives me insane. Dermatology is not a particularly lucrative specialty; it ranked 13th in a recent survey from the professional medical network Doximity. Furthermore, if payment for practice expense is removed, dermatology drops much further down, close to primary care.
There is a fundamental misunderstanding by the public and legislators about physician incomes. The reimbursements that are reported by Medicare for example, include the practice expense cost, which for dermatology is about 60% of the total remitted to the doctor, as I wrote in a 2015 column.
That is, the cost of providing the facility, supplies, staff, rent, and utilities are included in “reimbursement,” though this is money that goes out the door to pay the bills as quickly as it comes in. This is for overhead, nothing here for the practitioner’s time and work.
Even when dermatologists perform hospital consults, they usually bring their own supply kit from their office for skin biopsies, or other procedures since these are impossible to find in a hospital.
I also pointed out in my earlier column that most other specialties do not provide the majority of their procedures in the office, but instead, use the hospital, which provides supplies and staff for procedures. These other specialists are to be lauded for providing their services at charity rates, or for no pay at all, but at least they do not have to pay for the building, equipment, supplies, and staff out of pocket. Dermatologists do, since in a sense, they run their own “hospitals” as almost all of their procedures are based out of their offices.
The economics of a patient visit
I do not dispute that it is more difficult for a Medicaid patient to get an appointment with a dermatologist than it is for a patient with private insurance, but this is because Medicaid often pays less than the cost of supplies to see them. It is also easier to get an appointment for a cosmetic procedure than a rash because reimbursements in general are artificially suppressed, even for Medicare (which is also the benchmark for private insurers) by the federal government. Medicare reimbursements have not kept pace with inflation and are about 53% less than they were in 1992.
Let’s look at a skin biopsy. The supplies and equipment to perform a skin biopsy cost over $50. In Ohio, Medicare pays $96.19 for a skin biopsy. Medicaid pays $47.20. That’s correct: less than the cost of supplies and overhead. So, a private practitioner not only provides the service for free, but loses money on every visit that involves a skin biopsy. When I talk to legislators, I liken this to my standing in front of my office and handing out $5 bills. In Ohio, Medicare pays $105.04 for a level 3 office visit. Medicaid pays $57.76. Medicare overhead on a level 3 office visit is again about 50%, so the office visit is about a break-even proposition, if you donate your time.
Academic medical centers can charge additional facility fees, and some receive subsidies from the city and county to treat indigent patients, and are often obligated to see all. Most hospitals with high Medicaid and indigent patient loads pay their surgical specialists to take call at their emergency rooms and often subsidize their emergency room doctors as well.
I agree that dermatology is an important specialty to have access to in the COVID-19 pandemic. I agree that patients of color may be disproportionately impacted because they may be covered by Medicaid more often, or have no insurance at all. The finger of blame, however, should be squarely pointed at politicians who have woefully underfunded Medicaid reimbursement rates, as well as payments for physicians under the Affordable Care Act, while thumping their chests and boasting how they have provided health care to millions. I think this was eloquently demonstrated when as part of the “deal” Congress made with the AMA to get the ACA passed, Congress agreed to pay primary care physicians (but only primary care) Medicare rates for Medicaid patients for 2 years.
Some states have continued to pay enhanced Medicaid rates and have fewer Medicaid patient access issues.
Most convincing, perhaps, are the states that pay Medicare rates or better for their Medicaid enrollees, for example Alaska and Montana. In these states, you will not have access to care issues beyond the actual human shortage of physicians in remote areas.
So, in conclusion, I maintain that dermatology is not a particularly lucrative specialty, once the overhead expense payments are removed, and further argue, that even if it were, why does that obligate us to provide care to insurance plans at a loss? Medicaid access to dermatologists is a government economic issue, not a physician ethical one. Most Americans get to pick the charities they choose to donate to.
The federal government would love to force all physicians into a plan where you must see patients at their chosen rates or see no patients at all. Look no further than our Canadian neighbors, where long wait times to see specialists are legendary. It has been reported that there are only six to seven hundred dermatologists in all of Canada to serve 30 million people.
So, when the topic of poor patient access to care for the Medicaid enrollee or indigent comes up, stand tall and point your finger to your state capital. That is where the blame lies.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Victorious endurance: To pass the breaking point and not break
I’ve been thinking a lot about endurance recently.
COVID-19 is surging in the United States. Health care workers exhausted from the first and second waves are quickly reaching the verge of collapse. I’m seeing more and more heartbreaking articles about the bone-deep fatigue, fear, and frustration health care workers are facing, and I weep. As horrible as it is to be fighting this terrifying, little-understood, invisible virus, health care workers are also fighting an equally distressing war against misinformation, recklessness, apathy, and outright denial.
As if that wasn’t enough, we are also dealing with racial and social unrest not seen in decades. The most significant cultural divisions and political animosity perhaps since the Civil War. A contested election. The fraying of our democratic institutions and our standing in the global community. The weakest economy since the Great Depression. Record unemployment. Many individuals and families facing or already experiencing eviction and food insecurity. Record-setting fires, hurricanes, and other natural disasters that are only projected to intensify due to climate change.
That’s a lot to endure. And we don’t have much choice other than to live through it. Some of us will break under the strain; others will disengage by giving up clinical work or even leaving health care altogether. Some of us will pack it in and retire, walk away from relationships with family members or longtime friends, or even emigrate to another country (New Zealand, anyone?). Some of us will passively hunker down, letting the challenges of this time overwhelm us and just hoping we can hang on long enough to emerge, albeit beaten and scarred, on the other side.
But some of us will experience victorious endurance – the kind that doesn’t just accept suffering but finds a way to triumph over it. I came across the concept of victorious endurance in the Bible, but its origin is earlier, from classical Greece. It comes from the ancient Greek word hupomone, which literally means “abiding under” – as in disciplining oneself to bear up under a trial when one would more naturally rebel, or just give up. The ancient Greeks were big on virtues like self-control, long-suffering, and perseverance in the face of seemingly insurmountable difficulties; Odysseus was a poster child for hupomone. I believe the concept of victorious endurance can be applicable for people across many belief systems, philosophies, and ways of life.
The late William Barclay, former professor of divinity and biblical criticism at the University of Glasgow, Scotland, said of hupomone:
It is untranslatable. It does not describe the frame of mind which can sit down with folded hands and bowed head and let a torrent of troubles sweep over it in passive resignation. It describes the ability to bear things in such a triumphant way that it transfigures them. Chrysostom has a great panegyric on this hupomone. He calls it “the root of all goods, the mother of piety, the fruit that never withers, a fortress that is never taken, a harbour that knows no storms” and “the queen of virtues, the foundation of right actions, peace in war, calm in tempest, security in plots.” It is the courageous and triumphant ability to pass the breaking-point and not to break and always to greet the unseen with a cheer. It is the alchemy which transmutes tribulation into strength and glory.
Barclay further noted that “Cicero defines patientia, its Latin equivalent, as: ‘The voluntary and daily suffering of hard and difficult things, for the sake of honour and usefulness.”
In the midst of the most challenging public health emergency of our lifetimes, I am seeing hospitalists – and nurses, respiratory therapists, and countless other health care workers – doing exactly this, every day. I’m so incredibly proud of you all, and thankful beyond words.
I doubt that victorious endurance comes naturally to any of us; it’s something we work at, pursue and nurture. What’s the secret to cultivating victorious endurance in the midst of unimaginable stress? I’m pretty sure there’s no specific formula. I don’t mean to sound like a Pollyanna or to make light of the tumult and turmoil of these times, but here are a few things that, based on my own experiences, may help cultivate this valuable virtue.
Be part of a support network. In the midst of great stress, and especially during this time of social distancing, it’s especially tempting to just hunker down, close in on ourselves, and shut others out – sometimes even our closest friends and loved ones. Maintaining relationships is just too exhausting. But you need people who can come alongside you and offer words of encouragement when you are at your lowest. And there’s nothing that will bring out the best in you like being there to encourage and support someone else. We all need to both receive and to give emotional support at a time like this.
Take the long view. When we’re in the middle of a serious crisis, it seems like the problems we’re facing will last forever. There’s no light at the end of the tunnel, no port in the storm. But even this pandemic won’t last forever. If we can keep in mind the fact that things will eventually get better and that the current situation isn’t permanent, it can help us maintain our perspective and have more patience with the current dysfunction.
Focus on who you want to be in this moment. This is the hardest time most of us have ever lived through, both professionally and personally. But let me throw you a challenge. When you look back on this time from the perspective of five years from now, or maybe ten, how will you want to remember yourself? Who will you want to have been during this time? Looking back, what will make you proud of how you handled this challenge? Be that person.
Look for things to be thankful for. In the midst of the chaos that is our lives and our work right now, I believe we can still occasionally see moments of grace if we keep our eyes open for them. If we aren’t looking for them, we may miss them entirely. And those small moments of love, touches of compassion, displays of selflessness, and even flashes of victorious endurance in yourself or others are gifts to be treasured and held on to – to give thanks for.
Embrace a cause greater than yourself. May I suggest that one thing that might help our efforts to cultivate the virtue of victorious endurance during difficult times might be to embrace a cause that is bigger than yourself; that is, one that lures you to focus beyond your immediate circumstances? What are you passionate about, outside of your life’s normal routine?
If you don’t have a passion, consider what you might become passionate about, with a little effort. For some of us, like me, this will be our faith in God. For others it may be advocating for an end to racism or for broader social justice issues. Maybe it’s working to overcome our cultural and political divisions or to strengthen the institutions of our democracy. Perhaps it’s getting involved with efforts to mitigate climate change. Maybe it’s reaching out to the homeless or hungry in your own community or mentoring a child who is being left behind by the demands of remote learning.
Or perhaps what you embrace is even closer to home: maybe it’s working to eliminate health disparities in your institution or health system, or figuring out how to use technology and resources differently to improve how care is being delivered during or after this pandemic. Maybe it’s as simple as re-committing yourself to personally care for every patient you see today with the very best you have to offer, and with patience, compassion, and grace.
Find something that sets your heart on fire. Something that makes you want to take this difficult time and “transmute tribulation into strength and glory.” Something that, when you look back on these days, will make you thankful that you didn’t just hunker down and subsist through them. Instead, you accomplished great things; you learned; you contributed; and you grew stronger and better.
That’s victorious endurance.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey. This essay was published initially on The Hospital Leader, the official blog of SHM.
I’ve been thinking a lot about endurance recently.
COVID-19 is surging in the United States. Health care workers exhausted from the first and second waves are quickly reaching the verge of collapse. I’m seeing more and more heartbreaking articles about the bone-deep fatigue, fear, and frustration health care workers are facing, and I weep. As horrible as it is to be fighting this terrifying, little-understood, invisible virus, health care workers are also fighting an equally distressing war against misinformation, recklessness, apathy, and outright denial.
As if that wasn’t enough, we are also dealing with racial and social unrest not seen in decades. The most significant cultural divisions and political animosity perhaps since the Civil War. A contested election. The fraying of our democratic institutions and our standing in the global community. The weakest economy since the Great Depression. Record unemployment. Many individuals and families facing or already experiencing eviction and food insecurity. Record-setting fires, hurricanes, and other natural disasters that are only projected to intensify due to climate change.
That’s a lot to endure. And we don’t have much choice other than to live through it. Some of us will break under the strain; others will disengage by giving up clinical work or even leaving health care altogether. Some of us will pack it in and retire, walk away from relationships with family members or longtime friends, or even emigrate to another country (New Zealand, anyone?). Some of us will passively hunker down, letting the challenges of this time overwhelm us and just hoping we can hang on long enough to emerge, albeit beaten and scarred, on the other side.
But some of us will experience victorious endurance – the kind that doesn’t just accept suffering but finds a way to triumph over it. I came across the concept of victorious endurance in the Bible, but its origin is earlier, from classical Greece. It comes from the ancient Greek word hupomone, which literally means “abiding under” – as in disciplining oneself to bear up under a trial when one would more naturally rebel, or just give up. The ancient Greeks were big on virtues like self-control, long-suffering, and perseverance in the face of seemingly insurmountable difficulties; Odysseus was a poster child for hupomone. I believe the concept of victorious endurance can be applicable for people across many belief systems, philosophies, and ways of life.
The late William Barclay, former professor of divinity and biblical criticism at the University of Glasgow, Scotland, said of hupomone:
It is untranslatable. It does not describe the frame of mind which can sit down with folded hands and bowed head and let a torrent of troubles sweep over it in passive resignation. It describes the ability to bear things in such a triumphant way that it transfigures them. Chrysostom has a great panegyric on this hupomone. He calls it “the root of all goods, the mother of piety, the fruit that never withers, a fortress that is never taken, a harbour that knows no storms” and “the queen of virtues, the foundation of right actions, peace in war, calm in tempest, security in plots.” It is the courageous and triumphant ability to pass the breaking-point and not to break and always to greet the unseen with a cheer. It is the alchemy which transmutes tribulation into strength and glory.
Barclay further noted that “Cicero defines patientia, its Latin equivalent, as: ‘The voluntary and daily suffering of hard and difficult things, for the sake of honour and usefulness.”
In the midst of the most challenging public health emergency of our lifetimes, I am seeing hospitalists – and nurses, respiratory therapists, and countless other health care workers – doing exactly this, every day. I’m so incredibly proud of you all, and thankful beyond words.
I doubt that victorious endurance comes naturally to any of us; it’s something we work at, pursue and nurture. What’s the secret to cultivating victorious endurance in the midst of unimaginable stress? I’m pretty sure there’s no specific formula. I don’t mean to sound like a Pollyanna or to make light of the tumult and turmoil of these times, but here are a few things that, based on my own experiences, may help cultivate this valuable virtue.
Be part of a support network. In the midst of great stress, and especially during this time of social distancing, it’s especially tempting to just hunker down, close in on ourselves, and shut others out – sometimes even our closest friends and loved ones. Maintaining relationships is just too exhausting. But you need people who can come alongside you and offer words of encouragement when you are at your lowest. And there’s nothing that will bring out the best in you like being there to encourage and support someone else. We all need to both receive and to give emotional support at a time like this.
Take the long view. When we’re in the middle of a serious crisis, it seems like the problems we’re facing will last forever. There’s no light at the end of the tunnel, no port in the storm. But even this pandemic won’t last forever. If we can keep in mind the fact that things will eventually get better and that the current situation isn’t permanent, it can help us maintain our perspective and have more patience with the current dysfunction.
Focus on who you want to be in this moment. This is the hardest time most of us have ever lived through, both professionally and personally. But let me throw you a challenge. When you look back on this time from the perspective of five years from now, or maybe ten, how will you want to remember yourself? Who will you want to have been during this time? Looking back, what will make you proud of how you handled this challenge? Be that person.
Look for things to be thankful for. In the midst of the chaos that is our lives and our work right now, I believe we can still occasionally see moments of grace if we keep our eyes open for them. If we aren’t looking for them, we may miss them entirely. And those small moments of love, touches of compassion, displays of selflessness, and even flashes of victorious endurance in yourself or others are gifts to be treasured and held on to – to give thanks for.
Embrace a cause greater than yourself. May I suggest that one thing that might help our efforts to cultivate the virtue of victorious endurance during difficult times might be to embrace a cause that is bigger than yourself; that is, one that lures you to focus beyond your immediate circumstances? What are you passionate about, outside of your life’s normal routine?
If you don’t have a passion, consider what you might become passionate about, with a little effort. For some of us, like me, this will be our faith in God. For others it may be advocating for an end to racism or for broader social justice issues. Maybe it’s working to overcome our cultural and political divisions or to strengthen the institutions of our democracy. Perhaps it’s getting involved with efforts to mitigate climate change. Maybe it’s reaching out to the homeless or hungry in your own community or mentoring a child who is being left behind by the demands of remote learning.
Or perhaps what you embrace is even closer to home: maybe it’s working to eliminate health disparities in your institution or health system, or figuring out how to use technology and resources differently to improve how care is being delivered during or after this pandemic. Maybe it’s as simple as re-committing yourself to personally care for every patient you see today with the very best you have to offer, and with patience, compassion, and grace.
Find something that sets your heart on fire. Something that makes you want to take this difficult time and “transmute tribulation into strength and glory.” Something that, when you look back on these days, will make you thankful that you didn’t just hunker down and subsist through them. Instead, you accomplished great things; you learned; you contributed; and you grew stronger and better.
That’s victorious endurance.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey. This essay was published initially on The Hospital Leader, the official blog of SHM.
I’ve been thinking a lot about endurance recently.
COVID-19 is surging in the United States. Health care workers exhausted from the first and second waves are quickly reaching the verge of collapse. I’m seeing more and more heartbreaking articles about the bone-deep fatigue, fear, and frustration health care workers are facing, and I weep. As horrible as it is to be fighting this terrifying, little-understood, invisible virus, health care workers are also fighting an equally distressing war against misinformation, recklessness, apathy, and outright denial.
As if that wasn’t enough, we are also dealing with racial and social unrest not seen in decades. The most significant cultural divisions and political animosity perhaps since the Civil War. A contested election. The fraying of our democratic institutions and our standing in the global community. The weakest economy since the Great Depression. Record unemployment. Many individuals and families facing or already experiencing eviction and food insecurity. Record-setting fires, hurricanes, and other natural disasters that are only projected to intensify due to climate change.
That’s a lot to endure. And we don’t have much choice other than to live through it. Some of us will break under the strain; others will disengage by giving up clinical work or even leaving health care altogether. Some of us will pack it in and retire, walk away from relationships with family members or longtime friends, or even emigrate to another country (New Zealand, anyone?). Some of us will passively hunker down, letting the challenges of this time overwhelm us and just hoping we can hang on long enough to emerge, albeit beaten and scarred, on the other side.
But some of us will experience victorious endurance – the kind that doesn’t just accept suffering but finds a way to triumph over it. I came across the concept of victorious endurance in the Bible, but its origin is earlier, from classical Greece. It comes from the ancient Greek word hupomone, which literally means “abiding under” – as in disciplining oneself to bear up under a trial when one would more naturally rebel, or just give up. The ancient Greeks were big on virtues like self-control, long-suffering, and perseverance in the face of seemingly insurmountable difficulties; Odysseus was a poster child for hupomone. I believe the concept of victorious endurance can be applicable for people across many belief systems, philosophies, and ways of life.
The late William Barclay, former professor of divinity and biblical criticism at the University of Glasgow, Scotland, said of hupomone:
It is untranslatable. It does not describe the frame of mind which can sit down with folded hands and bowed head and let a torrent of troubles sweep over it in passive resignation. It describes the ability to bear things in such a triumphant way that it transfigures them. Chrysostom has a great panegyric on this hupomone. He calls it “the root of all goods, the mother of piety, the fruit that never withers, a fortress that is never taken, a harbour that knows no storms” and “the queen of virtues, the foundation of right actions, peace in war, calm in tempest, security in plots.” It is the courageous and triumphant ability to pass the breaking-point and not to break and always to greet the unseen with a cheer. It is the alchemy which transmutes tribulation into strength and glory.
Barclay further noted that “Cicero defines patientia, its Latin equivalent, as: ‘The voluntary and daily suffering of hard and difficult things, for the sake of honour and usefulness.”
In the midst of the most challenging public health emergency of our lifetimes, I am seeing hospitalists – and nurses, respiratory therapists, and countless other health care workers – doing exactly this, every day. I’m so incredibly proud of you all, and thankful beyond words.
I doubt that victorious endurance comes naturally to any of us; it’s something we work at, pursue and nurture. What’s the secret to cultivating victorious endurance in the midst of unimaginable stress? I’m pretty sure there’s no specific formula. I don’t mean to sound like a Pollyanna or to make light of the tumult and turmoil of these times, but here are a few things that, based on my own experiences, may help cultivate this valuable virtue.
Be part of a support network. In the midst of great stress, and especially during this time of social distancing, it’s especially tempting to just hunker down, close in on ourselves, and shut others out – sometimes even our closest friends and loved ones. Maintaining relationships is just too exhausting. But you need people who can come alongside you and offer words of encouragement when you are at your lowest. And there’s nothing that will bring out the best in you like being there to encourage and support someone else. We all need to both receive and to give emotional support at a time like this.
Take the long view. When we’re in the middle of a serious crisis, it seems like the problems we’re facing will last forever. There’s no light at the end of the tunnel, no port in the storm. But even this pandemic won’t last forever. If we can keep in mind the fact that things will eventually get better and that the current situation isn’t permanent, it can help us maintain our perspective and have more patience with the current dysfunction.
Focus on who you want to be in this moment. This is the hardest time most of us have ever lived through, both professionally and personally. But let me throw you a challenge. When you look back on this time from the perspective of five years from now, or maybe ten, how will you want to remember yourself? Who will you want to have been during this time? Looking back, what will make you proud of how you handled this challenge? Be that person.
Look for things to be thankful for. In the midst of the chaos that is our lives and our work right now, I believe we can still occasionally see moments of grace if we keep our eyes open for them. If we aren’t looking for them, we may miss them entirely. And those small moments of love, touches of compassion, displays of selflessness, and even flashes of victorious endurance in yourself or others are gifts to be treasured and held on to – to give thanks for.
Embrace a cause greater than yourself. May I suggest that one thing that might help our efforts to cultivate the virtue of victorious endurance during difficult times might be to embrace a cause that is bigger than yourself; that is, one that lures you to focus beyond your immediate circumstances? What are you passionate about, outside of your life’s normal routine?
If you don’t have a passion, consider what you might become passionate about, with a little effort. For some of us, like me, this will be our faith in God. For others it may be advocating for an end to racism or for broader social justice issues. Maybe it’s working to overcome our cultural and political divisions or to strengthen the institutions of our democracy. Perhaps it’s getting involved with efforts to mitigate climate change. Maybe it’s reaching out to the homeless or hungry in your own community or mentoring a child who is being left behind by the demands of remote learning.
Or perhaps what you embrace is even closer to home: maybe it’s working to eliminate health disparities in your institution or health system, or figuring out how to use technology and resources differently to improve how care is being delivered during or after this pandemic. Maybe it’s as simple as re-committing yourself to personally care for every patient you see today with the very best you have to offer, and with patience, compassion, and grace.
Find something that sets your heart on fire. Something that makes you want to take this difficult time and “transmute tribulation into strength and glory.” Something that, when you look back on these days, will make you thankful that you didn’t just hunker down and subsist through them. Instead, you accomplished great things; you learned; you contributed; and you grew stronger and better.
That’s victorious endurance.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey. This essay was published initially on The Hospital Leader, the official blog of SHM.
Patients with asthma and COPD lost ground in accessing care
Over the past 20 years, patients with asthma and chronic obstructive pulmonary disease (COPD) have seen next to no improvement in problems of delayed care because of cost or unaffordable medications, despite wider insurance coverage since the passage of the Affordable Care Act, a new analysis shows.
The long-view analysis illuminates the ongoing problem for people with these chronic diseases despite health care legislation that was considered historic.
“That long-term scope puts recent improvements in better context – whereas we have made improvements in coverage in recent years due to the Affordable Care Act, the longer-term picture is that people with asthma and COPD are struggling to obtain needed medical care and medications despite a substantial reduction in the uninsurance rate,” said Adam Gaffney, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston who authored the paper with David Himmelstein, MD, professor of public health at City University of New York–Hunter College. The findings were published in Chest.
Researchers examined data from 1997 to 2018 for 76,843 adults with asthma and 30,548 adults with COPD, from the National Health Interview Survey, an annual survey by the Centers for Disease Control that is based on in-person interviews and health questionnaires completed by an adult in each family.
Insurance coverage up, patients losing ground
During 1997 and 2018, there was an overall 9.3% decrease in the rate of adults with asthma who were uninsured, a significant improvement (P < .001). Between the pre- and post-ACA years, there was modest improvement in those putting off care because of cost, a drop of 3.8%, or going without prescriptions, a drop of 4.0%. But those improvements didn’t correspond to the 7.2% drop in the uninsured rate after the AC , contributing to the finding that there was no significant improvement over the 20 years.
For adults with COPD, it was a slightly different story. Over those 2 decades, the uninsured rate dropped by 9.5%. But the number of patients foregoing care due to cost actually rose by 3.4%, which wasn’t statistically significant, but the rate of those unable to afford needed medications rose significantly by 7.8%.
Researchers found there was improvement between the pre- and post-ACA years among COPD patients putting off care and going without medications (decreases of 6.9% and 4.5%, respectively). That adhered fairly closely with the improvement in the uninsured rate, which fell by 7.1%. But over the 20-year study period, the percentage of those needing medications they couldn’t afford increased significantly by 7.8%. The rate of those delaying or foregoing care also increased, though this amount was not statistically significant.
After the ACA was created, Blacks and Hispanics with asthma had greater improvement in obtaining insurance, compared with other racial and ethnic groups. But over the 20 years, like all racial and ethnic groups, they saw no statistically significant improvement in rates of “inadequate coverage,” defined in this study as either being uninsured, having to delay care because of cost, or being unable to afford needed medications.
For those with COPD, only Whites had statistically significant improvement in the number of patients with inadequate coverage after the ACA, researchers found.
So despite obtaining insurance, patients lost ground in managing their disease because of the growing cost of care and medication.
“Medication affordability has actually worsened for those with COPD – a worrisome development given that medication nonadherence worsens outcomes for these vulnerable patients,” Dr. Gaffney said. “Policy makers should return to the issue of national health care reform. Both uninsurance and underinsurance undermines pulmonologists’ ability to care for their patients with chronic disease. A health care system without financial barriers, in contrast, might well improve these patients’ outcomes, and advance health equity.”
Insurance is no guarantee to access
Daniel Ouellette, MD, FCCP, a pulmonary and critical care specialist at Henry Ford Health System in Detroit, said it’s not surprising that access to care remains a problem despite the Affordable Care Act.
“It covers the hospitalizations and ER visits – patients in this segment of society were getting cared for there anyway,” he said. “And what the ACA didn’t always do was provide adequate prescription coverage or cover these outpatient gaps. So even though the patients have the ACA they still have unaffordable prescriptions, they still can’t buy them, and they still can’t pay for their outpatient clinic if they have a $500 or $1,000 deductible.” These patients also continue to struggle with more fundamental issues that affect access to care, such as lack of transportation and poor health literacy.
At Henry Ford, pharmacists work with patients to identify medications covered by their insurance and work to find discounts and coupons, he said. As for the ACA, “it’s a good first start, but we really need to identify what its limitations are.” Locally driven, less expensive solutions might be a better way forward than costly federal initiatives.
Brandon M. Seay, MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, said the findings dovetail with what he has seen in the pediatric population.
“From my experience, the ACA has helped patients get their foot in the door and has helped patients decrease the possibility of serious financial burden in emergency situations, but the ability to afford medications has not changed very much,” he said. When patients struggle with sufficient prescription coverage, he helps patients fight for coverage and connects them with prescription assistance programs such as GoodRx.
“Instead of focusing on the access of insurance to patients, the goal of the system should be to make care as affordable as possible,” Dr. Seay said. “Access does not meet the needs of a patient if they cannot afford what they have access to. Transition to a nationalized health system where there is no question of access could help to drive down prescription drug prices by allowing the government to negotiate with pharmaceutical companies more adequately by removing the ‘middle man’ of the private insurance industry.”
The investigators reported no financial conflicts. Dr. Ouellette and Dr. Seay reported no financial conflicts.
Over the past 20 years, patients with asthma and chronic obstructive pulmonary disease (COPD) have seen next to no improvement in problems of delayed care because of cost or unaffordable medications, despite wider insurance coverage since the passage of the Affordable Care Act, a new analysis shows.
The long-view analysis illuminates the ongoing problem for people with these chronic diseases despite health care legislation that was considered historic.
“That long-term scope puts recent improvements in better context – whereas we have made improvements in coverage in recent years due to the Affordable Care Act, the longer-term picture is that people with asthma and COPD are struggling to obtain needed medical care and medications despite a substantial reduction in the uninsurance rate,” said Adam Gaffney, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston who authored the paper with David Himmelstein, MD, professor of public health at City University of New York–Hunter College. The findings were published in Chest.
Researchers examined data from 1997 to 2018 for 76,843 adults with asthma and 30,548 adults with COPD, from the National Health Interview Survey, an annual survey by the Centers for Disease Control that is based on in-person interviews and health questionnaires completed by an adult in each family.
Insurance coverage up, patients losing ground
During 1997 and 2018, there was an overall 9.3% decrease in the rate of adults with asthma who were uninsured, a significant improvement (P < .001). Between the pre- and post-ACA years, there was modest improvement in those putting off care because of cost, a drop of 3.8%, or going without prescriptions, a drop of 4.0%. But those improvements didn’t correspond to the 7.2% drop in the uninsured rate after the AC , contributing to the finding that there was no significant improvement over the 20 years.
For adults with COPD, it was a slightly different story. Over those 2 decades, the uninsured rate dropped by 9.5%. But the number of patients foregoing care due to cost actually rose by 3.4%, which wasn’t statistically significant, but the rate of those unable to afford needed medications rose significantly by 7.8%.
Researchers found there was improvement between the pre- and post-ACA years among COPD patients putting off care and going without medications (decreases of 6.9% and 4.5%, respectively). That adhered fairly closely with the improvement in the uninsured rate, which fell by 7.1%. But over the 20-year study period, the percentage of those needing medications they couldn’t afford increased significantly by 7.8%. The rate of those delaying or foregoing care also increased, though this amount was not statistically significant.
After the ACA was created, Blacks and Hispanics with asthma had greater improvement in obtaining insurance, compared with other racial and ethnic groups. But over the 20 years, like all racial and ethnic groups, they saw no statistically significant improvement in rates of “inadequate coverage,” defined in this study as either being uninsured, having to delay care because of cost, or being unable to afford needed medications.
For those with COPD, only Whites had statistically significant improvement in the number of patients with inadequate coverage after the ACA, researchers found.
So despite obtaining insurance, patients lost ground in managing their disease because of the growing cost of care and medication.
“Medication affordability has actually worsened for those with COPD – a worrisome development given that medication nonadherence worsens outcomes for these vulnerable patients,” Dr. Gaffney said. “Policy makers should return to the issue of national health care reform. Both uninsurance and underinsurance undermines pulmonologists’ ability to care for their patients with chronic disease. A health care system without financial barriers, in contrast, might well improve these patients’ outcomes, and advance health equity.”
Insurance is no guarantee to access
Daniel Ouellette, MD, FCCP, a pulmonary and critical care specialist at Henry Ford Health System in Detroit, said it’s not surprising that access to care remains a problem despite the Affordable Care Act.
“It covers the hospitalizations and ER visits – patients in this segment of society were getting cared for there anyway,” he said. “And what the ACA didn’t always do was provide adequate prescription coverage or cover these outpatient gaps. So even though the patients have the ACA they still have unaffordable prescriptions, they still can’t buy them, and they still can’t pay for their outpatient clinic if they have a $500 or $1,000 deductible.” These patients also continue to struggle with more fundamental issues that affect access to care, such as lack of transportation and poor health literacy.
At Henry Ford, pharmacists work with patients to identify medications covered by their insurance and work to find discounts and coupons, he said. As for the ACA, “it’s a good first start, but we really need to identify what its limitations are.” Locally driven, less expensive solutions might be a better way forward than costly federal initiatives.
Brandon M. Seay, MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, said the findings dovetail with what he has seen in the pediatric population.
“From my experience, the ACA has helped patients get their foot in the door and has helped patients decrease the possibility of serious financial burden in emergency situations, but the ability to afford medications has not changed very much,” he said. When patients struggle with sufficient prescription coverage, he helps patients fight for coverage and connects them with prescription assistance programs such as GoodRx.
“Instead of focusing on the access of insurance to patients, the goal of the system should be to make care as affordable as possible,” Dr. Seay said. “Access does not meet the needs of a patient if they cannot afford what they have access to. Transition to a nationalized health system where there is no question of access could help to drive down prescription drug prices by allowing the government to negotiate with pharmaceutical companies more adequately by removing the ‘middle man’ of the private insurance industry.”
The investigators reported no financial conflicts. Dr. Ouellette and Dr. Seay reported no financial conflicts.
Over the past 20 years, patients with asthma and chronic obstructive pulmonary disease (COPD) have seen next to no improvement in problems of delayed care because of cost or unaffordable medications, despite wider insurance coverage since the passage of the Affordable Care Act, a new analysis shows.
The long-view analysis illuminates the ongoing problem for people with these chronic diseases despite health care legislation that was considered historic.
“That long-term scope puts recent improvements in better context – whereas we have made improvements in coverage in recent years due to the Affordable Care Act, the longer-term picture is that people with asthma and COPD are struggling to obtain needed medical care and medications despite a substantial reduction in the uninsurance rate,” said Adam Gaffney, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston who authored the paper with David Himmelstein, MD, professor of public health at City University of New York–Hunter College. The findings were published in Chest.
Researchers examined data from 1997 to 2018 for 76,843 adults with asthma and 30,548 adults with COPD, from the National Health Interview Survey, an annual survey by the Centers for Disease Control that is based on in-person interviews and health questionnaires completed by an adult in each family.
Insurance coverage up, patients losing ground
During 1997 and 2018, there was an overall 9.3% decrease in the rate of adults with asthma who were uninsured, a significant improvement (P < .001). Between the pre- and post-ACA years, there was modest improvement in those putting off care because of cost, a drop of 3.8%, or going without prescriptions, a drop of 4.0%. But those improvements didn’t correspond to the 7.2% drop in the uninsured rate after the AC , contributing to the finding that there was no significant improvement over the 20 years.
For adults with COPD, it was a slightly different story. Over those 2 decades, the uninsured rate dropped by 9.5%. But the number of patients foregoing care due to cost actually rose by 3.4%, which wasn’t statistically significant, but the rate of those unable to afford needed medications rose significantly by 7.8%.
Researchers found there was improvement between the pre- and post-ACA years among COPD patients putting off care and going without medications (decreases of 6.9% and 4.5%, respectively). That adhered fairly closely with the improvement in the uninsured rate, which fell by 7.1%. But over the 20-year study period, the percentage of those needing medications they couldn’t afford increased significantly by 7.8%. The rate of those delaying or foregoing care also increased, though this amount was not statistically significant.
After the ACA was created, Blacks and Hispanics with asthma had greater improvement in obtaining insurance, compared with other racial and ethnic groups. But over the 20 years, like all racial and ethnic groups, they saw no statistically significant improvement in rates of “inadequate coverage,” defined in this study as either being uninsured, having to delay care because of cost, or being unable to afford needed medications.
For those with COPD, only Whites had statistically significant improvement in the number of patients with inadequate coverage after the ACA, researchers found.
So despite obtaining insurance, patients lost ground in managing their disease because of the growing cost of care and medication.
“Medication affordability has actually worsened for those with COPD – a worrisome development given that medication nonadherence worsens outcomes for these vulnerable patients,” Dr. Gaffney said. “Policy makers should return to the issue of national health care reform. Both uninsurance and underinsurance undermines pulmonologists’ ability to care for their patients with chronic disease. A health care system without financial barriers, in contrast, might well improve these patients’ outcomes, and advance health equity.”
Insurance is no guarantee to access
Daniel Ouellette, MD, FCCP, a pulmonary and critical care specialist at Henry Ford Health System in Detroit, said it’s not surprising that access to care remains a problem despite the Affordable Care Act.
“It covers the hospitalizations and ER visits – patients in this segment of society were getting cared for there anyway,” he said. “And what the ACA didn’t always do was provide adequate prescription coverage or cover these outpatient gaps. So even though the patients have the ACA they still have unaffordable prescriptions, they still can’t buy them, and they still can’t pay for their outpatient clinic if they have a $500 or $1,000 deductible.” These patients also continue to struggle with more fundamental issues that affect access to care, such as lack of transportation and poor health literacy.
At Henry Ford, pharmacists work with patients to identify medications covered by their insurance and work to find discounts and coupons, he said. As for the ACA, “it’s a good first start, but we really need to identify what its limitations are.” Locally driven, less expensive solutions might be a better way forward than costly federal initiatives.
Brandon M. Seay, MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, said the findings dovetail with what he has seen in the pediatric population.
“From my experience, the ACA has helped patients get their foot in the door and has helped patients decrease the possibility of serious financial burden in emergency situations, but the ability to afford medications has not changed very much,” he said. When patients struggle with sufficient prescription coverage, he helps patients fight for coverage and connects them with prescription assistance programs such as GoodRx.
“Instead of focusing on the access of insurance to patients, the goal of the system should be to make care as affordable as possible,” Dr. Seay said. “Access does not meet the needs of a patient if they cannot afford what they have access to. Transition to a nationalized health system where there is no question of access could help to drive down prescription drug prices by allowing the government to negotiate with pharmaceutical companies more adequately by removing the ‘middle man’ of the private insurance industry.”
The investigators reported no financial conflicts. Dr. Ouellette and Dr. Seay reported no financial conflicts.
FROM CHEST
Dried blood spot tests show sensitivity as cCMV screen
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
FROM JAMA PEDIATRICS
How has the pandemic affected rural and urban cancer patients?
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR: COVID-19 AND CANCER 2021
Cardiovascular trials lose more women than men
A new analysis of 11 phase 3/4 cardiovascular clinical trials conducted by the Thrombolysis in Myocardial Infarction (TIMI) group shows that women are more likely than men to discontinue study medications, and to withdraw from trials. The differences could not be explained by different frequencies of reporting adverse events, or by baseline differences.
The findings are significant, since cardiovascular drugs are routinely prescribed to women based on clinical trials that are populated largely by men, according to lead study author Emily Lau, MD, who is an advanced cardiology fellow at Massachusetts General Hospital, Boston. “It highlights an important disparity in clinical research in cardiology, because if women are already not represented well in clinical trials, and if once in clinical trials they don’t complete the study, it’s very hard to extrapolate the clinical trial findings to our female population in an accurate way,” Dr. Lau said in an interview. She also noted that sex-specific and reproductive factors are increasingly recognized as being important in the development and progression of cardiovascular disease.
The study was published in the journal Circulation.
The study refutes previously advanced explanations for higher withdrawal among women, including sex difference and comorbidities, according to an accompanying editorial by Sofia Sederholm Lawesson, MD, PhD, Eva Swahn, MD, PhD, and Joakim Alfredsson, MD, PhD, of Linköping University, Sweden. They also pointed out that the study found a larger between-sex difference in failure to adhere to study drug in North America (odds ratio, 1.35; 95% confidence interval, 1.30-1.41), but a more moderate difference among participants in Europe/Middle East/Africa (OR, 1.13; 95% CI, 1.09-1.17) and Asia/Pacific (OR, 1.13; 95% CI, 1.03-1.23) regions. And there were no sex differences at all among South/Central American populations.
They noted that high rates of nonadherence increase the chances of a false negative finding and overestimation of drug safety. “We know the associations between nonadherence and clinical outcomes. The next step should be to better understand the underlying reasons for, as well as consistent reporting of, nonadherence, and discontinuation in RCTs,” the editorial authors wrote.
Dr. Lau suggested a simple method to better understand reasons for withdrawal: Addition of questions to the case report form that asks about reasons for drug discontinuation or study withdrawal. “Was it an adverse event? Was it because I’m a mother of three and I can’t get to the clinical trial site after work and also pick up my kids? Are there societal barriers for women, or was it the experience of the clinical trial that was maybe less favorable for women compared to men? Or maybe there are medical reasons we simply don’t know. Something as simple as asking those questions can help us better understand the barriers to female retention,” said Dr. Lau.
The analysis included data from 135,879 men (72%) and 51,812 women (28%) enrolled in the trials. After adjustment for baseline differences, women were more likely than were men to permanently discontinue study drug (adjusted odds ratio [aOR], 1.22: P < .001), which did not vary by study duration. The finding was consistent regardless of the type of drug studied, as well as across placebo and active study arms.
Women also were more likely to prematurely discontinue study drug (trial-adjusted OR, 1.18; P < .001). The rate of drug discontinuation due to adverse event was identical in both men and women, at 36%.
Women were more likely to withdraw consent than were men in a meta-analysis and when individual patient-level results were pooled (aOR, 1.26; P < .001 for both).
Dr. Lau received funding from the National Institutes of Health and has no relevant financial disclosures. The editorial authors had various disclosures, including lecture fees from Bayer, Pfizer, and Boehringer Ingelheim, and they served on advisory boards for AstraZeneca and MSD.
A new analysis of 11 phase 3/4 cardiovascular clinical trials conducted by the Thrombolysis in Myocardial Infarction (TIMI) group shows that women are more likely than men to discontinue study medications, and to withdraw from trials. The differences could not be explained by different frequencies of reporting adverse events, or by baseline differences.
The findings are significant, since cardiovascular drugs are routinely prescribed to women based on clinical trials that are populated largely by men, according to lead study author Emily Lau, MD, who is an advanced cardiology fellow at Massachusetts General Hospital, Boston. “It highlights an important disparity in clinical research in cardiology, because if women are already not represented well in clinical trials, and if once in clinical trials they don’t complete the study, it’s very hard to extrapolate the clinical trial findings to our female population in an accurate way,” Dr. Lau said in an interview. She also noted that sex-specific and reproductive factors are increasingly recognized as being important in the development and progression of cardiovascular disease.
The study was published in the journal Circulation.
The study refutes previously advanced explanations for higher withdrawal among women, including sex difference and comorbidities, according to an accompanying editorial by Sofia Sederholm Lawesson, MD, PhD, Eva Swahn, MD, PhD, and Joakim Alfredsson, MD, PhD, of Linköping University, Sweden. They also pointed out that the study found a larger between-sex difference in failure to adhere to study drug in North America (odds ratio, 1.35; 95% confidence interval, 1.30-1.41), but a more moderate difference among participants in Europe/Middle East/Africa (OR, 1.13; 95% CI, 1.09-1.17) and Asia/Pacific (OR, 1.13; 95% CI, 1.03-1.23) regions. And there were no sex differences at all among South/Central American populations.
They noted that high rates of nonadherence increase the chances of a false negative finding and overestimation of drug safety. “We know the associations between nonadherence and clinical outcomes. The next step should be to better understand the underlying reasons for, as well as consistent reporting of, nonadherence, and discontinuation in RCTs,” the editorial authors wrote.
Dr. Lau suggested a simple method to better understand reasons for withdrawal: Addition of questions to the case report form that asks about reasons for drug discontinuation or study withdrawal. “Was it an adverse event? Was it because I’m a mother of three and I can’t get to the clinical trial site after work and also pick up my kids? Are there societal barriers for women, or was it the experience of the clinical trial that was maybe less favorable for women compared to men? Or maybe there are medical reasons we simply don’t know. Something as simple as asking those questions can help us better understand the barriers to female retention,” said Dr. Lau.
The analysis included data from 135,879 men (72%) and 51,812 women (28%) enrolled in the trials. After adjustment for baseline differences, women were more likely than were men to permanently discontinue study drug (adjusted odds ratio [aOR], 1.22: P < .001), which did not vary by study duration. The finding was consistent regardless of the type of drug studied, as well as across placebo and active study arms.
Women also were more likely to prematurely discontinue study drug (trial-adjusted OR, 1.18; P < .001). The rate of drug discontinuation due to adverse event was identical in both men and women, at 36%.
Women were more likely to withdraw consent than were men in a meta-analysis and when individual patient-level results were pooled (aOR, 1.26; P < .001 for both).
Dr. Lau received funding from the National Institutes of Health and has no relevant financial disclosures. The editorial authors had various disclosures, including lecture fees from Bayer, Pfizer, and Boehringer Ingelheim, and they served on advisory boards for AstraZeneca and MSD.
A new analysis of 11 phase 3/4 cardiovascular clinical trials conducted by the Thrombolysis in Myocardial Infarction (TIMI) group shows that women are more likely than men to discontinue study medications, and to withdraw from trials. The differences could not be explained by different frequencies of reporting adverse events, or by baseline differences.
The findings are significant, since cardiovascular drugs are routinely prescribed to women based on clinical trials that are populated largely by men, according to lead study author Emily Lau, MD, who is an advanced cardiology fellow at Massachusetts General Hospital, Boston. “It highlights an important disparity in clinical research in cardiology, because if women are already not represented well in clinical trials, and if once in clinical trials they don’t complete the study, it’s very hard to extrapolate the clinical trial findings to our female population in an accurate way,” Dr. Lau said in an interview. She also noted that sex-specific and reproductive factors are increasingly recognized as being important in the development and progression of cardiovascular disease.
The study was published in the journal Circulation.
The study refutes previously advanced explanations for higher withdrawal among women, including sex difference and comorbidities, according to an accompanying editorial by Sofia Sederholm Lawesson, MD, PhD, Eva Swahn, MD, PhD, and Joakim Alfredsson, MD, PhD, of Linköping University, Sweden. They also pointed out that the study found a larger between-sex difference in failure to adhere to study drug in North America (odds ratio, 1.35; 95% confidence interval, 1.30-1.41), but a more moderate difference among participants in Europe/Middle East/Africa (OR, 1.13; 95% CI, 1.09-1.17) and Asia/Pacific (OR, 1.13; 95% CI, 1.03-1.23) regions. And there were no sex differences at all among South/Central American populations.
They noted that high rates of nonadherence increase the chances of a false negative finding and overestimation of drug safety. “We know the associations between nonadherence and clinical outcomes. The next step should be to better understand the underlying reasons for, as well as consistent reporting of, nonadherence, and discontinuation in RCTs,” the editorial authors wrote.
Dr. Lau suggested a simple method to better understand reasons for withdrawal: Addition of questions to the case report form that asks about reasons for drug discontinuation or study withdrawal. “Was it an adverse event? Was it because I’m a mother of three and I can’t get to the clinical trial site after work and also pick up my kids? Are there societal barriers for women, or was it the experience of the clinical trial that was maybe less favorable for women compared to men? Or maybe there are medical reasons we simply don’t know. Something as simple as asking those questions can help us better understand the barriers to female retention,” said Dr. Lau.
The analysis included data from 135,879 men (72%) and 51,812 women (28%) enrolled in the trials. After adjustment for baseline differences, women were more likely than were men to permanently discontinue study drug (adjusted odds ratio [aOR], 1.22: P < .001), which did not vary by study duration. The finding was consistent regardless of the type of drug studied, as well as across placebo and active study arms.
Women also were more likely to prematurely discontinue study drug (trial-adjusted OR, 1.18; P < .001). The rate of drug discontinuation due to adverse event was identical in both men and women, at 36%.
Women were more likely to withdraw consent than were men in a meta-analysis and when individual patient-level results were pooled (aOR, 1.26; P < .001 for both).
Dr. Lau received funding from the National Institutes of Health and has no relevant financial disclosures. The editorial authors had various disclosures, including lecture fees from Bayer, Pfizer, and Boehringer Ingelheim, and they served on advisory boards for AstraZeneca and MSD.
FROM CIRCULATION
















