User login
Influenza-related maternal morbidity has more than doubled over 15 years
Despite slightly decreasing numbers of pregnant women hospitalized with influenza, the rate of morbidity among those who do have influenza has substantially increased from 2000 to 2015, likely due in part to an increase in comorbidities.
, according to findings from a new study presented at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine.
Pregnant women were also at substantially greater risk of sepsis or shock, needing mechanical ventilation, and acute respiratory distress syndrome. In fact, rates of overall severe maternal morbidity and of influenza-related complications have increased in maternal patients with influenza by more than 200% from 2000 to 2015.
“It was striking to see how the rate of delivery hospitalizations complicated by influenza has remained relatively stable with a small decline, but the rates of severe maternal morbidity were increasing and so markedly among those with influenza,” Timothy Wen, MD, MPH, a maternal-fetal medicine clinical fellow at the University of California, San Francisco, said in an interview. “The findings suggest that influenza may either be a contributor to rising rates of severe maternal morbidity or synergistically amplifying existing comorbidities to worsen outcomes,” he said during his presentation.
The increased risk of influenza complications in pregnant women became particularly apparent during the 2009-2010 H1N1 influenza pandemic. “Physiologic and immunologic changes predispose pregnant patients to higher risk for complications such as pneumonia, intensive care unit admission, and inpatient mortality,” Dr. Wen told attendees. But data have been scarce since H1N1.
The researchers conducted a cross-sectional analysis of delivery hospitalizations from 2000 to 2015 using the Nationwide Inpatient Sample, which includes about 20% of all U.S. inpatient hospitalizations from all payers. They looked at all maternal patients aged 15-54 who had a diagnosis of influenza. In looking at potential associations between influenza and morbidity, they adjusted their calculations for maternal age, payer status, median income, and race/ethnicity as well as the hospital factors of location, teaching status, and region. They also adjusted for a dozen clinical factors.
Of 62.7 million hospitalizations, 0.67% involved severe maternal mortality, including the following influenza complications:
- 0.02% with shock/sepsis.
- 0.01% needing mechanical ventilation.
- 0.04% with acute respiratory distress syndrome.
The 182,228 patients with influenza represented a rate of 29 cases per 10,000 deliveries, and 2.09% of them involved severe maternal morbidity, compared to severe maternal morbidity in just 0.66% of deliveries without influenza.
When looking specifically at rates of shock/sepsis, mechanical ventilation, and acute respiratory distress syndrome, the data revealed similar trends, with substantially higher proportions of patients with influenza experiencing these complications compared to maternal patients without influenza. For example, 0.3% of patients with influenza developed shock/sepsis whereas only 0.04% of patients without influenza did. Acute respiratory distress syndrome was similarly more common in patients with flu (0.45% vs. 0.04%), as was the need for mechanical ventilation (0.09% vs. 0.01%).
During the 15-year study period, the rate of maternal hospitalizations with influenza infections declined about 1.5%, from 30 to 24 per 10,000 deliveries. But trends with severe maternal morbidity in patients with influenza went in the other direction, increasing more than 200% over 15 years, from 100 to 342 cases of severe maternal morbidity per 10,000 patients with influenza. An increase also occurred in patients without influenza, but it was more modest, a nearly 50% increase, from 53 to 79 cases per 10,000 hospitalizations.
From year to year, severe maternal morbidity increased 5.3% annually among hospitalizations with influenza – more than twice the rate of a 2.4% annual increase among hospitalizations without influenza.
The researchers found that influenza is linked to twice the risk of severe maternal morbidity (adjusted risk ratio [aRR] = 2.08, P < .01). There were similarly higher risks with influenza of sepsis/shock (aRR = 3.23), mechanical ventilation (aRR = 6.04), and acute respiratory distress syndrome (aRR = 5.76; all P < .01).
Among the possible reasons for the increase in influenza morbidity – despite a decrease in influenza infections in this population – is the increase in the medical complexity of the patient population, Dr. Wen said.
“Patients who are getting pregnant today likely have more comorbid conditions (chronic hypertension, obesity, pregestational diabetes mellitus, etc.) than they did decades prior,” Dr. Wen said. “Clinically, it means that we have a baseline patient population at a higher risk of susceptibility for influenza and its complications.”
Maternal influenza immunization rates have meanwhile stagnated, Dr. Wen added. Influenza “is something that we know is preventable, or at least mitigated, by a vaccine,” he said. “Our results serve as a reminder for clinicians to continue counseling on the importance of influenza vaccination among pregnant patients, and even in those who are planning to become pregnant.”
He said these findings suggest the need for a low threshold for treating pregnant patients who have influenza symptoms with over-the-counter therapies or closely monitoring them.
Adetola Louis-Jacques, MD, of the University of South Florida, Tampa, found the increase in morbidity in those with flu particularly unexpected and concerning.
“What surprised me was the big difference in how severe maternal morbidity rates increased over time in the influenza group compared to the group without influenza,” Dr. Louis-Jacques, who moderated the session, said in an interview. She agreed with Dr. Wen that the findings underscore the benefits of immunization.
“The study means we should reinforce to mothers how important the vaccine is. It’s critical,” Dr. Louis-Jacques said. “We should encourage mothers to get it and focus on educating women, trying to understand and allay [any concerns about the vaccine] and reinforce the importance of flu vaccination to decrease the likelihood of these mothers getting pretty sick during pregnancy.”
Dr. Wen and Dr. Louis-Jacques had no disclosures.
Despite slightly decreasing numbers of pregnant women hospitalized with influenza, the rate of morbidity among those who do have influenza has substantially increased from 2000 to 2015, likely due in part to an increase in comorbidities.
, according to findings from a new study presented at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine.
Pregnant women were also at substantially greater risk of sepsis or shock, needing mechanical ventilation, and acute respiratory distress syndrome. In fact, rates of overall severe maternal morbidity and of influenza-related complications have increased in maternal patients with influenza by more than 200% from 2000 to 2015.
“It was striking to see how the rate of delivery hospitalizations complicated by influenza has remained relatively stable with a small decline, but the rates of severe maternal morbidity were increasing and so markedly among those with influenza,” Timothy Wen, MD, MPH, a maternal-fetal medicine clinical fellow at the University of California, San Francisco, said in an interview. “The findings suggest that influenza may either be a contributor to rising rates of severe maternal morbidity or synergistically amplifying existing comorbidities to worsen outcomes,” he said during his presentation.
The increased risk of influenza complications in pregnant women became particularly apparent during the 2009-2010 H1N1 influenza pandemic. “Physiologic and immunologic changes predispose pregnant patients to higher risk for complications such as pneumonia, intensive care unit admission, and inpatient mortality,” Dr. Wen told attendees. But data have been scarce since H1N1.
The researchers conducted a cross-sectional analysis of delivery hospitalizations from 2000 to 2015 using the Nationwide Inpatient Sample, which includes about 20% of all U.S. inpatient hospitalizations from all payers. They looked at all maternal patients aged 15-54 who had a diagnosis of influenza. In looking at potential associations between influenza and morbidity, they adjusted their calculations for maternal age, payer status, median income, and race/ethnicity as well as the hospital factors of location, teaching status, and region. They also adjusted for a dozen clinical factors.
Of 62.7 million hospitalizations, 0.67% involved severe maternal mortality, including the following influenza complications:
- 0.02% with shock/sepsis.
- 0.01% needing mechanical ventilation.
- 0.04% with acute respiratory distress syndrome.
The 182,228 patients with influenza represented a rate of 29 cases per 10,000 deliveries, and 2.09% of them involved severe maternal morbidity, compared to severe maternal morbidity in just 0.66% of deliveries without influenza.
When looking specifically at rates of shock/sepsis, mechanical ventilation, and acute respiratory distress syndrome, the data revealed similar trends, with substantially higher proportions of patients with influenza experiencing these complications compared to maternal patients without influenza. For example, 0.3% of patients with influenza developed shock/sepsis whereas only 0.04% of patients without influenza did. Acute respiratory distress syndrome was similarly more common in patients with flu (0.45% vs. 0.04%), as was the need for mechanical ventilation (0.09% vs. 0.01%).
During the 15-year study period, the rate of maternal hospitalizations with influenza infections declined about 1.5%, from 30 to 24 per 10,000 deliveries. But trends with severe maternal morbidity in patients with influenza went in the other direction, increasing more than 200% over 15 years, from 100 to 342 cases of severe maternal morbidity per 10,000 patients with influenza. An increase also occurred in patients without influenza, but it was more modest, a nearly 50% increase, from 53 to 79 cases per 10,000 hospitalizations.
From year to year, severe maternal morbidity increased 5.3% annually among hospitalizations with influenza – more than twice the rate of a 2.4% annual increase among hospitalizations without influenza.
The researchers found that influenza is linked to twice the risk of severe maternal morbidity (adjusted risk ratio [aRR] = 2.08, P < .01). There were similarly higher risks with influenza of sepsis/shock (aRR = 3.23), mechanical ventilation (aRR = 6.04), and acute respiratory distress syndrome (aRR = 5.76; all P < .01).
Among the possible reasons for the increase in influenza morbidity – despite a decrease in influenza infections in this population – is the increase in the medical complexity of the patient population, Dr. Wen said.
“Patients who are getting pregnant today likely have more comorbid conditions (chronic hypertension, obesity, pregestational diabetes mellitus, etc.) than they did decades prior,” Dr. Wen said. “Clinically, it means that we have a baseline patient population at a higher risk of susceptibility for influenza and its complications.”
Maternal influenza immunization rates have meanwhile stagnated, Dr. Wen added. Influenza “is something that we know is preventable, or at least mitigated, by a vaccine,” he said. “Our results serve as a reminder for clinicians to continue counseling on the importance of influenza vaccination among pregnant patients, and even in those who are planning to become pregnant.”
He said these findings suggest the need for a low threshold for treating pregnant patients who have influenza symptoms with over-the-counter therapies or closely monitoring them.
Adetola Louis-Jacques, MD, of the University of South Florida, Tampa, found the increase in morbidity in those with flu particularly unexpected and concerning.
“What surprised me was the big difference in how severe maternal morbidity rates increased over time in the influenza group compared to the group without influenza,” Dr. Louis-Jacques, who moderated the session, said in an interview. She agreed with Dr. Wen that the findings underscore the benefits of immunization.
“The study means we should reinforce to mothers how important the vaccine is. It’s critical,” Dr. Louis-Jacques said. “We should encourage mothers to get it and focus on educating women, trying to understand and allay [any concerns about the vaccine] and reinforce the importance of flu vaccination to decrease the likelihood of these mothers getting pretty sick during pregnancy.”
Dr. Wen and Dr. Louis-Jacques had no disclosures.
Despite slightly decreasing numbers of pregnant women hospitalized with influenza, the rate of morbidity among those who do have influenza has substantially increased from 2000 to 2015, likely due in part to an increase in comorbidities.
, according to findings from a new study presented at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine.
Pregnant women were also at substantially greater risk of sepsis or shock, needing mechanical ventilation, and acute respiratory distress syndrome. In fact, rates of overall severe maternal morbidity and of influenza-related complications have increased in maternal patients with influenza by more than 200% from 2000 to 2015.
“It was striking to see how the rate of delivery hospitalizations complicated by influenza has remained relatively stable with a small decline, but the rates of severe maternal morbidity were increasing and so markedly among those with influenza,” Timothy Wen, MD, MPH, a maternal-fetal medicine clinical fellow at the University of California, San Francisco, said in an interview. “The findings suggest that influenza may either be a contributor to rising rates of severe maternal morbidity or synergistically amplifying existing comorbidities to worsen outcomes,” he said during his presentation.
The increased risk of influenza complications in pregnant women became particularly apparent during the 2009-2010 H1N1 influenza pandemic. “Physiologic and immunologic changes predispose pregnant patients to higher risk for complications such as pneumonia, intensive care unit admission, and inpatient mortality,” Dr. Wen told attendees. But data have been scarce since H1N1.
The researchers conducted a cross-sectional analysis of delivery hospitalizations from 2000 to 2015 using the Nationwide Inpatient Sample, which includes about 20% of all U.S. inpatient hospitalizations from all payers. They looked at all maternal patients aged 15-54 who had a diagnosis of influenza. In looking at potential associations between influenza and morbidity, they adjusted their calculations for maternal age, payer status, median income, and race/ethnicity as well as the hospital factors of location, teaching status, and region. They also adjusted for a dozen clinical factors.
Of 62.7 million hospitalizations, 0.67% involved severe maternal mortality, including the following influenza complications:
- 0.02% with shock/sepsis.
- 0.01% needing mechanical ventilation.
- 0.04% with acute respiratory distress syndrome.
The 182,228 patients with influenza represented a rate of 29 cases per 10,000 deliveries, and 2.09% of them involved severe maternal morbidity, compared to severe maternal morbidity in just 0.66% of deliveries without influenza.
When looking specifically at rates of shock/sepsis, mechanical ventilation, and acute respiratory distress syndrome, the data revealed similar trends, with substantially higher proportions of patients with influenza experiencing these complications compared to maternal patients without influenza. For example, 0.3% of patients with influenza developed shock/sepsis whereas only 0.04% of patients without influenza did. Acute respiratory distress syndrome was similarly more common in patients with flu (0.45% vs. 0.04%), as was the need for mechanical ventilation (0.09% vs. 0.01%).
During the 15-year study period, the rate of maternal hospitalizations with influenza infections declined about 1.5%, from 30 to 24 per 10,000 deliveries. But trends with severe maternal morbidity in patients with influenza went in the other direction, increasing more than 200% over 15 years, from 100 to 342 cases of severe maternal morbidity per 10,000 patients with influenza. An increase also occurred in patients without influenza, but it was more modest, a nearly 50% increase, from 53 to 79 cases per 10,000 hospitalizations.
From year to year, severe maternal morbidity increased 5.3% annually among hospitalizations with influenza – more than twice the rate of a 2.4% annual increase among hospitalizations without influenza.
The researchers found that influenza is linked to twice the risk of severe maternal morbidity (adjusted risk ratio [aRR] = 2.08, P < .01). There were similarly higher risks with influenza of sepsis/shock (aRR = 3.23), mechanical ventilation (aRR = 6.04), and acute respiratory distress syndrome (aRR = 5.76; all P < .01).
Among the possible reasons for the increase in influenza morbidity – despite a decrease in influenza infections in this population – is the increase in the medical complexity of the patient population, Dr. Wen said.
“Patients who are getting pregnant today likely have more comorbid conditions (chronic hypertension, obesity, pregestational diabetes mellitus, etc.) than they did decades prior,” Dr. Wen said. “Clinically, it means that we have a baseline patient population at a higher risk of susceptibility for influenza and its complications.”
Maternal influenza immunization rates have meanwhile stagnated, Dr. Wen added. Influenza “is something that we know is preventable, or at least mitigated, by a vaccine,” he said. “Our results serve as a reminder for clinicians to continue counseling on the importance of influenza vaccination among pregnant patients, and even in those who are planning to become pregnant.”
He said these findings suggest the need for a low threshold for treating pregnant patients who have influenza symptoms with over-the-counter therapies or closely monitoring them.
Adetola Louis-Jacques, MD, of the University of South Florida, Tampa, found the increase in morbidity in those with flu particularly unexpected and concerning.
“What surprised me was the big difference in how severe maternal morbidity rates increased over time in the influenza group compared to the group without influenza,” Dr. Louis-Jacques, who moderated the session, said in an interview. She agreed with Dr. Wen that the findings underscore the benefits of immunization.
“The study means we should reinforce to mothers how important the vaccine is. It’s critical,” Dr. Louis-Jacques said. “We should encourage mothers to get it and focus on educating women, trying to understand and allay [any concerns about the vaccine] and reinforce the importance of flu vaccination to decrease the likelihood of these mothers getting pretty sick during pregnancy.”
Dr. Wen and Dr. Louis-Jacques had no disclosures.
FROM THE PREGNANCY MEETING
Vibrio vulnificus: Review of Mild to Life-threatening Skin Infections
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
Practice Points
- Vibrio vulnificus infection should be high on the differential for patients who present with chronic liver disease and immunosuppression; a history of raw seafood consumption or exposure to brackish water; and bullae, cellulitis, necrotic lesions, or sepsis.
- Time to treatment is directly proportional to mortality rates in V vulnificus infections, and prompt treatment with antibiotics, wound care, debridement, and supportive measures is necessary to decrease mortality rates.
- The incidence of V vulnificus infection is rising in the United States, likely due to a combination of factors, including an aging population with multiple comorbidities, improvements in diagnosis, and climate change.
Improving Dermatologic Care for South Asian Patients: Understanding Religious and Cultural Practices
Traditional garments are particularly important in both Sikhism and Islam. Sikhs began wearing symbolic garments in the 16th century as markers of their identity during periods of religious persecution. Today, many Sikhs continue to maintain this tradition of wearing the Five Ks—kesh (uncut hair, often tied in a turban), kanga (wooden hair comb), kirpan (symbolic dagger), kachha (cotton underwear), and kara (steel bracelet).2 Similarly, Islamic traditions also provide guidance for clothing. Many Muslim women wear the hijab (headscarf), a garment that originated as protective headgear for nomadic desert cultures and has come to symbolize modest dress. Traditionally, the hijab is worn in the presence of all men who are not immediate relatives, although patients may make exceptions for medical care. Some Muslim men also may cover their heads with a skullcap and/or maintain long beards (occasionally dyed with henna pigment) as a way of keeping continuity with the tradition of the Prophet Muhammad and his companions.3
Certain styles of headwear can cause high tension on hair follicles and have been associated with traction alopecia.4 Persistent use of the same turban, hijab, or comb also may lead to seborrheic dermatitis or fungal scalp infections. Dermatologists should advise patients about these potential challenges and suggest modifications in accordance with the patient’s religious beliefs; for example, providers can suggest removing headwear at night, using prophylactic antifungal shampoos, and/or tying the hair in a ponytail or loosening the headgear to reduce traction.
Although Hinduism does not have a unifying garment or hair tradition in the vein of Sikhism or Islam, all 3 religions share a strong emphasis on bodily modesty, which may affect dermatologic examinations. Patients from all 3 religions may seek to expose as little skin as possible during a physical examination, and many patients may be uncomfortable with a physician of the opposite gender. Dermatologists may find the following practices to be helpful5:
• Talk through each aspect of the skin examination while it is being performed and expose the least amount of skin necessary during the process
• Offer the patient a chaperone or a same-gender provider, if possible
• Empower patients to assist in adjusting garments themselves to help the physician visualize all parts of the skin
Some Sikhs also may have specific concerns regarding cutting their hair. One aspect of kesh is that every hair is sacred, and thus, many Sikhs refrain from removing hair on any part of the body. As such, providers should carefully obtain the patient’s informed consent before performing any procedure or physical examination maneuvers (eg, hair pull test) that may result in loss of hair.2
Physicians of all disciplines can help address these challenges through increased outreach and cultural awareness; for example, dermatologists can create skin care pamphlets translated into various South Asian languages and distribute them at houses of worship or other community centers. This may help patients identify their skin needs and seek appropriate care. The onus must be on physicians to make these patients feel comfortable seeking care by creating nonjudgmental, culturally knowledgeable clinical environments. When asking about social history, the physician might consider asking an open-ended question such as, “What role does religion/spirituality play in your life?” They can then proceed to ask specific questions about practices that might affect the patient’s care.5
Given the current coronavirus disease 2019 pandemic, South Asian patients may be even further discouraged from seeking dermatologic care. By understanding religious traditions and taking steps to address biases, dermatologists can help mitigate health care disparities and provide culturally competent care to South Asian patients.
- Nadimpalli SB, Cleland CM, Hutchinson MK, et al. The association between discrimination and the health of Sikh Asian Indians. Health Psychol. 2016;35:351-355.
- The five Ks. BBC website. Updated September 29, 2009. Accessed February 4, 2021. https://www.bbc.co.uk/religion/religions/sikhism/customs/fiveks.shtml
- Islam. BBC website. Accessed February 2, 2021. https://www.bbc.co.uk/religion/religions/islam/
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498.
- Hussain A. Recommendations for culturally competent dermatology care of Muslim patients. J Am Acad Dermatol. 2017;77:388-389.
Traditional garments are particularly important in both Sikhism and Islam. Sikhs began wearing symbolic garments in the 16th century as markers of their identity during periods of religious persecution. Today, many Sikhs continue to maintain this tradition of wearing the Five Ks—kesh (uncut hair, often tied in a turban), kanga (wooden hair comb), kirpan (symbolic dagger), kachha (cotton underwear), and kara (steel bracelet).2 Similarly, Islamic traditions also provide guidance for clothing. Many Muslim women wear the hijab (headscarf), a garment that originated as protective headgear for nomadic desert cultures and has come to symbolize modest dress. Traditionally, the hijab is worn in the presence of all men who are not immediate relatives, although patients may make exceptions for medical care. Some Muslim men also may cover their heads with a skullcap and/or maintain long beards (occasionally dyed with henna pigment) as a way of keeping continuity with the tradition of the Prophet Muhammad and his companions.3
Certain styles of headwear can cause high tension on hair follicles and have been associated with traction alopecia.4 Persistent use of the same turban, hijab, or comb also may lead to seborrheic dermatitis or fungal scalp infections. Dermatologists should advise patients about these potential challenges and suggest modifications in accordance with the patient’s religious beliefs; for example, providers can suggest removing headwear at night, using prophylactic antifungal shampoos, and/or tying the hair in a ponytail or loosening the headgear to reduce traction.
Although Hinduism does not have a unifying garment or hair tradition in the vein of Sikhism or Islam, all 3 religions share a strong emphasis on bodily modesty, which may affect dermatologic examinations. Patients from all 3 religions may seek to expose as little skin as possible during a physical examination, and many patients may be uncomfortable with a physician of the opposite gender. Dermatologists may find the following practices to be helpful5:
• Talk through each aspect of the skin examination while it is being performed and expose the least amount of skin necessary during the process
• Offer the patient a chaperone or a same-gender provider, if possible
• Empower patients to assist in adjusting garments themselves to help the physician visualize all parts of the skin
Some Sikhs also may have specific concerns regarding cutting their hair. One aspect of kesh is that every hair is sacred, and thus, many Sikhs refrain from removing hair on any part of the body. As such, providers should carefully obtain the patient’s informed consent before performing any procedure or physical examination maneuvers (eg, hair pull test) that may result in loss of hair.2
Physicians of all disciplines can help address these challenges through increased outreach and cultural awareness; for example, dermatologists can create skin care pamphlets translated into various South Asian languages and distribute them at houses of worship or other community centers. This may help patients identify their skin needs and seek appropriate care. The onus must be on physicians to make these patients feel comfortable seeking care by creating nonjudgmental, culturally knowledgeable clinical environments. When asking about social history, the physician might consider asking an open-ended question such as, “What role does religion/spirituality play in your life?” They can then proceed to ask specific questions about practices that might affect the patient’s care.5
Given the current coronavirus disease 2019 pandemic, South Asian patients may be even further discouraged from seeking dermatologic care. By understanding religious traditions and taking steps to address biases, dermatologists can help mitigate health care disparities and provide culturally competent care to South Asian patients.
Traditional garments are particularly important in both Sikhism and Islam. Sikhs began wearing symbolic garments in the 16th century as markers of their identity during periods of religious persecution. Today, many Sikhs continue to maintain this tradition of wearing the Five Ks—kesh (uncut hair, often tied in a turban), kanga (wooden hair comb), kirpan (symbolic dagger), kachha (cotton underwear), and kara (steel bracelet).2 Similarly, Islamic traditions also provide guidance for clothing. Many Muslim women wear the hijab (headscarf), a garment that originated as protective headgear for nomadic desert cultures and has come to symbolize modest dress. Traditionally, the hijab is worn in the presence of all men who are not immediate relatives, although patients may make exceptions for medical care. Some Muslim men also may cover their heads with a skullcap and/or maintain long beards (occasionally dyed with henna pigment) as a way of keeping continuity with the tradition of the Prophet Muhammad and his companions.3
Certain styles of headwear can cause high tension on hair follicles and have been associated with traction alopecia.4 Persistent use of the same turban, hijab, or comb also may lead to seborrheic dermatitis or fungal scalp infections. Dermatologists should advise patients about these potential challenges and suggest modifications in accordance with the patient’s religious beliefs; for example, providers can suggest removing headwear at night, using prophylactic antifungal shampoos, and/or tying the hair in a ponytail or loosening the headgear to reduce traction.
Although Hinduism does not have a unifying garment or hair tradition in the vein of Sikhism or Islam, all 3 religions share a strong emphasis on bodily modesty, which may affect dermatologic examinations. Patients from all 3 religions may seek to expose as little skin as possible during a physical examination, and many patients may be uncomfortable with a physician of the opposite gender. Dermatologists may find the following practices to be helpful5:
• Talk through each aspect of the skin examination while it is being performed and expose the least amount of skin necessary during the process
• Offer the patient a chaperone or a same-gender provider, if possible
• Empower patients to assist in adjusting garments themselves to help the physician visualize all parts of the skin
Some Sikhs also may have specific concerns regarding cutting their hair. One aspect of kesh is that every hair is sacred, and thus, many Sikhs refrain from removing hair on any part of the body. As such, providers should carefully obtain the patient’s informed consent before performing any procedure or physical examination maneuvers (eg, hair pull test) that may result in loss of hair.2
Physicians of all disciplines can help address these challenges through increased outreach and cultural awareness; for example, dermatologists can create skin care pamphlets translated into various South Asian languages and distribute them at houses of worship or other community centers. This may help patients identify their skin needs and seek appropriate care. The onus must be on physicians to make these patients feel comfortable seeking care by creating nonjudgmental, culturally knowledgeable clinical environments. When asking about social history, the physician might consider asking an open-ended question such as, “What role does religion/spirituality play in your life?” They can then proceed to ask specific questions about practices that might affect the patient’s care.5
Given the current coronavirus disease 2019 pandemic, South Asian patients may be even further discouraged from seeking dermatologic care. By understanding religious traditions and taking steps to address biases, dermatologists can help mitigate health care disparities and provide culturally competent care to South Asian patients.
- Nadimpalli SB, Cleland CM, Hutchinson MK, et al. The association between discrimination and the health of Sikh Asian Indians. Health Psychol. 2016;35:351-355.
- The five Ks. BBC website. Updated September 29, 2009. Accessed February 4, 2021. https://www.bbc.co.uk/religion/religions/sikhism/customs/fiveks.shtml
- Islam. BBC website. Accessed February 2, 2021. https://www.bbc.co.uk/religion/religions/islam/
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498.
- Hussain A. Recommendations for culturally competent dermatology care of Muslim patients. J Am Acad Dermatol. 2017;77:388-389.
- Nadimpalli SB, Cleland CM, Hutchinson MK, et al. The association between discrimination and the health of Sikh Asian Indians. Health Psychol. 2016;35:351-355.
- The five Ks. BBC website. Updated September 29, 2009. Accessed February 4, 2021. https://www.bbc.co.uk/religion/religions/sikhism/customs/fiveks.shtml
- Islam. BBC website. Accessed February 2, 2021. https://www.bbc.co.uk/religion/religions/islam/
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498.
- Hussain A. Recommendations for culturally competent dermatology care of Muslim patients. J Am Acad Dermatol. 2017;77:388-389.
Practice Points
- Providers should familiarize themselves with traditional garments of Sikhism and Islam, including head coverings and other symbolic items.
- Inform patients about health-conscious methods of wearing traditional headwear, such as removing certain headwear at night and tying hair in methods to avoid causing traction alopecia.
- Talk through each aspect of the skin examination while it is being performed and expose the least amount of skin necessary during the process. Offer the patient a chaperone or a same-gender provider, if possible.
- Empower patients to assist in adjusting garments themselves to help the physician visualize all parts of the skin.
Short sleep predicts incident dementia and all-cause mortality
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).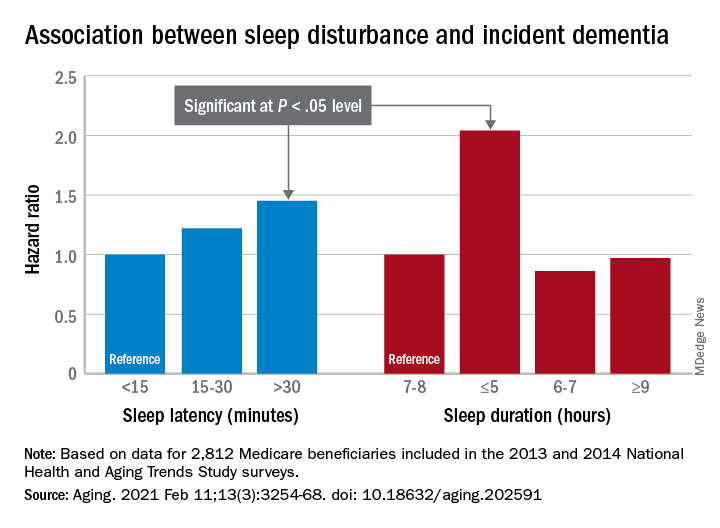
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
FROM AGING
COMMENT & CONTROVERSY
9vHPV VACCINE: PREVENTION OF OROPHARYNGEAL CANCER
ROBERT L. BARBIERI, MD (EDITORIAL; NOVEMBER 2020)
HPV vaccine for older ObGyns?
I am 67 years old and recently retired. I breathed in the smoke from laser conizations, LEEPs (loop electrosurgical excision procedures), and cautery of condyloma for 35 years. Am I a good candidate for the HPV vaccine?
Gus Barkett, DO
Muskegon, Michigan
Dr. Barbieri responds
I thank Dr. Barkett for his important question. As you know, the US Food and Drug Administration has approved 9vHPV vaccination for people 27 to 45 years of age. I do not believe there are sufficient data to provide an evidence-based answer for physicians with occupational exposure to HPV who are more than 45 years of age. My recommendation would be to have a consult with an otolaryngologist expert in HPV-induced oral-pharyngeal cancer.
EXAMINING THE EVIDENCE: HOW EFFECTIVE IS SCREENING MAMMOGRAPHY FOR PREVENTING BREAST CANCER MORTALITY?
ANDREW M. KAUNITZ, MD (AUGUST 2020)
Discordant results on screening mammography
In regard to the discussion on screening mammography for preventing breast cancer mortality, I would like to call attention to a more recent study than the ones referenced in the article. The study by Duffy and colleagues was from Sweden and included almost 550,000 women.1 Results of the study showed a statistically significant reduction of 41% in 10-year mortality and a 25% reduction in the incidence of advanced-stage disease at the time of diagnosis in women who underwent routine screening mammograms. In Sweden, routine screening is defined as a mammogram every 18 months for women aged 40 to 54 years and every 24 months after that, up to age 69.
I do not know if we will ever come to a consensus on the utility of mammograms or how often they should be done, but I wanted to illustrate this counterpoint.
Lisa Gennari, MD
Cincinnati, Ohio
Reference
1. Duffy SW, Tabar L, Yen AM, et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer. 2020;126:2971-2979.
Dr. Kaunitz responds
I thank Dr. Gennari for her interest in the Examining the Evidence discussion that summarized the findings of an article from Australia published in late summer of last year.1 That article indicated that as screening mammograms became common in the state of Victoria over several decades, the incidence of advanced breast cancer doubled, mirroring findings from the United States, Holland, and Norway. During the same time period, breast cancer mortality declined substantially. The authors concluded that all of the decline in breast cancer mortality that they observed since 1994 could be attributed not to screening mammography but rather to the introduction and uptake of adjuvant therapy (tamoxifen and chemotherapy).
In contrast, in the article Dr. Gennari cites, also published last summer, the authors found that the widespread uptake of screening mammograms among women residing in 9 counties in Sweden was associated with a decline in the incidence of advanced breast cancer. I am not able to explain these discrepant findings. However, as the authors pointed out, they employed a new strategy: measuring the incidence of breast cancer that proved fatal one decade after diagnosis.
Differing findings and interpretations of data that address benefits and risks of screening mammography lead to differing recommendations from professional societies and confusion among clinicians and our patients. Although it can be challenging in the constraints of time allotted for well-woman visits, I try to engage in shared decision making with my patients regarding when to start/stop mammography as well as frequency of screening.
Reference
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020:3:e208249.
Continue to: NEW HORMONAL MEDICAL TREATMENT...
NEW HORMONAL MEDICAL TREATMENT IS AN IMPORTANT ADVANCE FOR AUB CAUSED BY UTERINE FIBROIDS
ROBERT L. BARBIERI, MD (EDITORIAL; AUGUST 2020)
New AUB medical treatment
I appreciate Dr. Barbieri’s concise and pertinent review of myomatous disease etiology and treatments. I have a question regarding therapy with Oriahnn (elagolix, estradiol, and norethindrone acetate capsules). Most myomatous-related bleeding occurs in premenopausal women. The elagolix suppresses luteinizing hormone and follicle stimulating hormone, and the norethindrone is added to protect the endometrium from the estradiol. Do the elagolix and norethindrone also provide contraception?
Geoffrey J. Zann, MD, MBA
Boca Raton, Florida
Dr. Barbieri responds
Dr. Zann raises an important clinical question that arises often in practice. The US Food and Drug Administration (FDA) has not approved Oriahnn as a contraceptive. The FDA prescribing information recommends: Advise women to use non-hormonal contraception during treatment and for one week after discontinuing Oriahnn. Oriahnn may delay the ability to recognize the occurrence of a pregnancy because it alters menstrual bleeding. Perform pregnancy testing if pregnancy is suspected and discontinue Oriahnn if pregnancy is confirmed.
In Oriahnn, the elagolix dose is 300 mg twice daily. If a patient reliably takes 600 mg of elagolix daily, it is highly unlikely that she will ovulate. However, in practice, many patients miss doses of their medication, reducing the contraceptive effectiveness. For example, the combined estrogen-progestin contraceptive is highly effective at suppressing ovulation, but the Centers for Disease Control and Prevention (CDC) estimates that 9% of women taking an estrogen-progestin contraceptive will become pregnant each year.1,2
Oriahnn also contains norethindrone acetate at a dose of 0.5 mg daily. The FDA has approved norethindrone at a dose of 0.35 mg daily as a contraceptive. The CDC estimates that 9% of women prescribed a progestin-only pill will become pregnant each year with typical use.1,2
I counsel my patients that if they reliably take their prescribed Oriahnn medication as directed, they are unlikely to become pregnant, and a backup method of contraception will further help to reduce their risk of becoming pregnant.
References
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013. MMWR Morbid Mortal Weekly Rep. 2013;62(RR-5):1-59.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397-404.
9vHPV VACCINE: PREVENTION OF OROPHARYNGEAL CANCER
ROBERT L. BARBIERI, MD (EDITORIAL; NOVEMBER 2020)
HPV vaccine for older ObGyns?
I am 67 years old and recently retired. I breathed in the smoke from laser conizations, LEEPs (loop electrosurgical excision procedures), and cautery of condyloma for 35 years. Am I a good candidate for the HPV vaccine?
Gus Barkett, DO
Muskegon, Michigan
Dr. Barbieri responds
I thank Dr. Barkett for his important question. As you know, the US Food and Drug Administration has approved 9vHPV vaccination for people 27 to 45 years of age. I do not believe there are sufficient data to provide an evidence-based answer for physicians with occupational exposure to HPV who are more than 45 years of age. My recommendation would be to have a consult with an otolaryngologist expert in HPV-induced oral-pharyngeal cancer.
EXAMINING THE EVIDENCE: HOW EFFECTIVE IS SCREENING MAMMOGRAPHY FOR PREVENTING BREAST CANCER MORTALITY?
ANDREW M. KAUNITZ, MD (AUGUST 2020)
Discordant results on screening mammography
In regard to the discussion on screening mammography for preventing breast cancer mortality, I would like to call attention to a more recent study than the ones referenced in the article. The study by Duffy and colleagues was from Sweden and included almost 550,000 women.1 Results of the study showed a statistically significant reduction of 41% in 10-year mortality and a 25% reduction in the incidence of advanced-stage disease at the time of diagnosis in women who underwent routine screening mammograms. In Sweden, routine screening is defined as a mammogram every 18 months for women aged 40 to 54 years and every 24 months after that, up to age 69.
I do not know if we will ever come to a consensus on the utility of mammograms or how often they should be done, but I wanted to illustrate this counterpoint.
Lisa Gennari, MD
Cincinnati, Ohio
Reference
1. Duffy SW, Tabar L, Yen AM, et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer. 2020;126:2971-2979.
Dr. Kaunitz responds
I thank Dr. Gennari for her interest in the Examining the Evidence discussion that summarized the findings of an article from Australia published in late summer of last year.1 That article indicated that as screening mammograms became common in the state of Victoria over several decades, the incidence of advanced breast cancer doubled, mirroring findings from the United States, Holland, and Norway. During the same time period, breast cancer mortality declined substantially. The authors concluded that all of the decline in breast cancer mortality that they observed since 1994 could be attributed not to screening mammography but rather to the introduction and uptake of adjuvant therapy (tamoxifen and chemotherapy).
In contrast, in the article Dr. Gennari cites, also published last summer, the authors found that the widespread uptake of screening mammograms among women residing in 9 counties in Sweden was associated with a decline in the incidence of advanced breast cancer. I am not able to explain these discrepant findings. However, as the authors pointed out, they employed a new strategy: measuring the incidence of breast cancer that proved fatal one decade after diagnosis.
Differing findings and interpretations of data that address benefits and risks of screening mammography lead to differing recommendations from professional societies and confusion among clinicians and our patients. Although it can be challenging in the constraints of time allotted for well-woman visits, I try to engage in shared decision making with my patients regarding when to start/stop mammography as well as frequency of screening.
Reference
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020:3:e208249.
Continue to: NEW HORMONAL MEDICAL TREATMENT...
NEW HORMONAL MEDICAL TREATMENT IS AN IMPORTANT ADVANCE FOR AUB CAUSED BY UTERINE FIBROIDS
ROBERT L. BARBIERI, MD (EDITORIAL; AUGUST 2020)
New AUB medical treatment
I appreciate Dr. Barbieri’s concise and pertinent review of myomatous disease etiology and treatments. I have a question regarding therapy with Oriahnn (elagolix, estradiol, and norethindrone acetate capsules). Most myomatous-related bleeding occurs in premenopausal women. The elagolix suppresses luteinizing hormone and follicle stimulating hormone, and the norethindrone is added to protect the endometrium from the estradiol. Do the elagolix and norethindrone also provide contraception?
Geoffrey J. Zann, MD, MBA
Boca Raton, Florida
Dr. Barbieri responds
Dr. Zann raises an important clinical question that arises often in practice. The US Food and Drug Administration (FDA) has not approved Oriahnn as a contraceptive. The FDA prescribing information recommends: Advise women to use non-hormonal contraception during treatment and for one week after discontinuing Oriahnn. Oriahnn may delay the ability to recognize the occurrence of a pregnancy because it alters menstrual bleeding. Perform pregnancy testing if pregnancy is suspected and discontinue Oriahnn if pregnancy is confirmed.
In Oriahnn, the elagolix dose is 300 mg twice daily. If a patient reliably takes 600 mg of elagolix daily, it is highly unlikely that she will ovulate. However, in practice, many patients miss doses of their medication, reducing the contraceptive effectiveness. For example, the combined estrogen-progestin contraceptive is highly effective at suppressing ovulation, but the Centers for Disease Control and Prevention (CDC) estimates that 9% of women taking an estrogen-progestin contraceptive will become pregnant each year.1,2
Oriahnn also contains norethindrone acetate at a dose of 0.5 mg daily. The FDA has approved norethindrone at a dose of 0.35 mg daily as a contraceptive. The CDC estimates that 9% of women prescribed a progestin-only pill will become pregnant each year with typical use.1,2
I counsel my patients that if they reliably take their prescribed Oriahnn medication as directed, they are unlikely to become pregnant, and a backup method of contraception will further help to reduce their risk of becoming pregnant.
References
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013. MMWR Morbid Mortal Weekly Rep. 2013;62(RR-5):1-59.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397-404.
9vHPV VACCINE: PREVENTION OF OROPHARYNGEAL CANCER
ROBERT L. BARBIERI, MD (EDITORIAL; NOVEMBER 2020)
HPV vaccine for older ObGyns?
I am 67 years old and recently retired. I breathed in the smoke from laser conizations, LEEPs (loop electrosurgical excision procedures), and cautery of condyloma for 35 years. Am I a good candidate for the HPV vaccine?
Gus Barkett, DO
Muskegon, Michigan
Dr. Barbieri responds
I thank Dr. Barkett for his important question. As you know, the US Food and Drug Administration has approved 9vHPV vaccination for people 27 to 45 years of age. I do not believe there are sufficient data to provide an evidence-based answer for physicians with occupational exposure to HPV who are more than 45 years of age. My recommendation would be to have a consult with an otolaryngologist expert in HPV-induced oral-pharyngeal cancer.
EXAMINING THE EVIDENCE: HOW EFFECTIVE IS SCREENING MAMMOGRAPHY FOR PREVENTING BREAST CANCER MORTALITY?
ANDREW M. KAUNITZ, MD (AUGUST 2020)
Discordant results on screening mammography
In regard to the discussion on screening mammography for preventing breast cancer mortality, I would like to call attention to a more recent study than the ones referenced in the article. The study by Duffy and colleagues was from Sweden and included almost 550,000 women.1 Results of the study showed a statistically significant reduction of 41% in 10-year mortality and a 25% reduction in the incidence of advanced-stage disease at the time of diagnosis in women who underwent routine screening mammograms. In Sweden, routine screening is defined as a mammogram every 18 months for women aged 40 to 54 years and every 24 months after that, up to age 69.
I do not know if we will ever come to a consensus on the utility of mammograms or how often they should be done, but I wanted to illustrate this counterpoint.
Lisa Gennari, MD
Cincinnati, Ohio
Reference
1. Duffy SW, Tabar L, Yen AM, et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer. 2020;126:2971-2979.
Dr. Kaunitz responds
I thank Dr. Gennari for her interest in the Examining the Evidence discussion that summarized the findings of an article from Australia published in late summer of last year.1 That article indicated that as screening mammograms became common in the state of Victoria over several decades, the incidence of advanced breast cancer doubled, mirroring findings from the United States, Holland, and Norway. During the same time period, breast cancer mortality declined substantially. The authors concluded that all of the decline in breast cancer mortality that they observed since 1994 could be attributed not to screening mammography but rather to the introduction and uptake of adjuvant therapy (tamoxifen and chemotherapy).
In contrast, in the article Dr. Gennari cites, also published last summer, the authors found that the widespread uptake of screening mammograms among women residing in 9 counties in Sweden was associated with a decline in the incidence of advanced breast cancer. I am not able to explain these discrepant findings. However, as the authors pointed out, they employed a new strategy: measuring the incidence of breast cancer that proved fatal one decade after diagnosis.
Differing findings and interpretations of data that address benefits and risks of screening mammography lead to differing recommendations from professional societies and confusion among clinicians and our patients. Although it can be challenging in the constraints of time allotted for well-woman visits, I try to engage in shared decision making with my patients regarding when to start/stop mammography as well as frequency of screening.
Reference
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020:3:e208249.
Continue to: NEW HORMONAL MEDICAL TREATMENT...
NEW HORMONAL MEDICAL TREATMENT IS AN IMPORTANT ADVANCE FOR AUB CAUSED BY UTERINE FIBROIDS
ROBERT L. BARBIERI, MD (EDITORIAL; AUGUST 2020)
New AUB medical treatment
I appreciate Dr. Barbieri’s concise and pertinent review of myomatous disease etiology and treatments. I have a question regarding therapy with Oriahnn (elagolix, estradiol, and norethindrone acetate capsules). Most myomatous-related bleeding occurs in premenopausal women. The elagolix suppresses luteinizing hormone and follicle stimulating hormone, and the norethindrone is added to protect the endometrium from the estradiol. Do the elagolix and norethindrone also provide contraception?
Geoffrey J. Zann, MD, MBA
Boca Raton, Florida
Dr. Barbieri responds
Dr. Zann raises an important clinical question that arises often in practice. The US Food and Drug Administration (FDA) has not approved Oriahnn as a contraceptive. The FDA prescribing information recommends: Advise women to use non-hormonal contraception during treatment and for one week after discontinuing Oriahnn. Oriahnn may delay the ability to recognize the occurrence of a pregnancy because it alters menstrual bleeding. Perform pregnancy testing if pregnancy is suspected and discontinue Oriahnn if pregnancy is confirmed.
In Oriahnn, the elagolix dose is 300 mg twice daily. If a patient reliably takes 600 mg of elagolix daily, it is highly unlikely that she will ovulate. However, in practice, many patients miss doses of their medication, reducing the contraceptive effectiveness. For example, the combined estrogen-progestin contraceptive is highly effective at suppressing ovulation, but the Centers for Disease Control and Prevention (CDC) estimates that 9% of women taking an estrogen-progestin contraceptive will become pregnant each year.1,2
Oriahnn also contains norethindrone acetate at a dose of 0.5 mg daily. The FDA has approved norethindrone at a dose of 0.35 mg daily as a contraceptive. The CDC estimates that 9% of women prescribed a progestin-only pill will become pregnant each year with typical use.1,2
I counsel my patients that if they reliably take their prescribed Oriahnn medication as directed, they are unlikely to become pregnant, and a backup method of contraception will further help to reduce their risk of becoming pregnant.
References
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013. MMWR Morbid Mortal Weekly Rep. 2013;62(RR-5):1-59.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397-404.
Goldenseal may interfere with metformin absorption, jeopardizing glucose control
Goldenseal, a natural botanical product, may interfere with intestinal absorption of metformin, potentially compromising blood glucose control in patients with type 2 diabetes, according to investigators.
The study, which tested for interactions between goldenseal and several drugs in healthy volunteers, reveals that current models for predicting transporter-mediated drug-drug interactions may be insufficient to screen commonly used dietary supplements, reported lead investigator James T. Nguyen, PharmD, a PhD candidate at Washington State University, Spokane, and colleagues.
“Supplements containing goldenseal ... a perennial herb native to North America, have consistently ranked among the top 20 highest selling natural products during the last decade,” the investigators wrote in Clinical Pharmacology & Therapeutics . “As more patients continue to seek goldenseal and other natural products to self-treat their medical conditions, there is an increasing need to characterize their safety profiles, especially when co-consumed with prescribed medications, which can lead to adverse natural product-drug interactions.”
Previous clinical studies have shown that goldenseal inhibits cytochrome P450, with one study showing a roughly 40% increase in systemic midazolam exposure via CYP3A inhibition, “suggesting goldenseal could have prolonged inhibitory effects in vivo similar to grapefruit juice,” the investigators wrote.
Clinical and in vitro results for goldenseal-transporter interactions have been mixed, the investigators noted, specifically for P-glycoprotein, while other transporters remain clinically untested.
“Likewise, the effects of [goldenseal alkaloids], all of which are time-dependent inhibitors of CYP3A and/or CYP2D6, have not been tested on transporter function,” the investigators wrote.
To address this knowledge gap, the investigators first performed in vitro transporter inhibition assays and in vitro–in vivo predictions involving goldenseal, plus the alkaloids berberine, (−)-beta-hydrastine, and hydrastinine.
This analysis revealed that a number of transporters were sensitive to inhibition by goldenseal and its alkaloids.
“Using current [Food and Drug Administration]–recommended basic models, the goldenseal product was predicted to inhibit the intestinal efflux transporter BCRP [breast cancer resistance protein] and the hepatic uptake transporters OATP1B1 and OATP1B3,” the investigators wrote, which suggested that goldenseal would increase the area under the plasma concentration-time curve (AUC) of rosuvastatin acid and lactone.
This prediction was clinically tested in 16 healthy volunteers: 8 men and 8 nonpregnant women.
In the baseline portion of the study, each participant received an oral transporter probe cocktail consisting of 10 mg rosuvastatin (OATP1B1/3 and BCRP), 50 mg metformin (OCT1/2 and MATE1/2-K), 1 mg furosemide (OAT1/3), and 2.5 mg midazolam (CYP3A; positive control). Plasma and urine samples were collected before and after the cocktail, with urine collected up to 24 hours later, and plasma collected up to 96 hours later.
Following a minimum 9-day washout period, the same cohort received 1 gram of goldenseal every 8 hours for 5 days. On the day 6, the drug cocktail was given again, followed by two additional doses of goldenseal at 4-hour intervals. At the same time points used in the baseline protocol, urine and plasma samples were collected.
Plasma concentration vs. time profiles revealed that the model-based prediction was false, in that the presence of goldenseal did not alter the pharmacokinetics of rosuvastatin acid and lactone. The investigators suggested that this could be due to incomplete dissolution of goldenseal in the intestinal lumen, and/or low enterocyte concentrations of goldenseal stemming from “low permeability or extensive enterocyte metabolism or efflux.”
In contrast, and unpredicted by the basic model, goldenseal had a significant impact on apical efflux transporters MATE1 and MATE2-K, which mediate renal excretion of metformin. In consequence, AUC from zero to infinity and maximum plasma concentration of metformin were reduced by 23% and 27%, respectively.
“These observations, coupled with no change in half-life, suggested that goldenseal decreased metformin oral bioavailability by altering intestinal permeability, transport, and/or other processes involved in metformin absorption,” the investigators wrote.
According to principal author Mary Paine, PhD, of Washington State University, Spokane, this finding may have clinically significant implications for patients currently taking metformin for type 2 diabetes.
“Our study showed that goldenseal has an effect on the intestinal absorption of metformin, suggesting that the co-use of metformin and goldenseal may compromise blood glucose control in patients with type 2 diabetes and increase their risk of negative health outcomes,” Dr. Paine said. “While this finding warrants a degree of caution to be exercised among patients and their treating physicians, we have more work to do to confirm whether these findings in healthy volunteers in fact have clinical relevance in the management of diabetes. We are in the process of starting a follow-up study that should ultimately answer that question.”
The study was supported by the National Institutes of Health. The investigators reported no conflicts of interest.
Goldenseal, a natural botanical product, may interfere with intestinal absorption of metformin, potentially compromising blood glucose control in patients with type 2 diabetes, according to investigators.
The study, which tested for interactions between goldenseal and several drugs in healthy volunteers, reveals that current models for predicting transporter-mediated drug-drug interactions may be insufficient to screen commonly used dietary supplements, reported lead investigator James T. Nguyen, PharmD, a PhD candidate at Washington State University, Spokane, and colleagues.
“Supplements containing goldenseal ... a perennial herb native to North America, have consistently ranked among the top 20 highest selling natural products during the last decade,” the investigators wrote in Clinical Pharmacology & Therapeutics . “As more patients continue to seek goldenseal and other natural products to self-treat their medical conditions, there is an increasing need to characterize their safety profiles, especially when co-consumed with prescribed medications, which can lead to adverse natural product-drug interactions.”
Previous clinical studies have shown that goldenseal inhibits cytochrome P450, with one study showing a roughly 40% increase in systemic midazolam exposure via CYP3A inhibition, “suggesting goldenseal could have prolonged inhibitory effects in vivo similar to grapefruit juice,” the investigators wrote.
Clinical and in vitro results for goldenseal-transporter interactions have been mixed, the investigators noted, specifically for P-glycoprotein, while other transporters remain clinically untested.
“Likewise, the effects of [goldenseal alkaloids], all of which are time-dependent inhibitors of CYP3A and/or CYP2D6, have not been tested on transporter function,” the investigators wrote.
To address this knowledge gap, the investigators first performed in vitro transporter inhibition assays and in vitro–in vivo predictions involving goldenseal, plus the alkaloids berberine, (−)-beta-hydrastine, and hydrastinine.
This analysis revealed that a number of transporters were sensitive to inhibition by goldenseal and its alkaloids.
“Using current [Food and Drug Administration]–recommended basic models, the goldenseal product was predicted to inhibit the intestinal efflux transporter BCRP [breast cancer resistance protein] and the hepatic uptake transporters OATP1B1 and OATP1B3,” the investigators wrote, which suggested that goldenseal would increase the area under the plasma concentration-time curve (AUC) of rosuvastatin acid and lactone.
This prediction was clinically tested in 16 healthy volunteers: 8 men and 8 nonpregnant women.
In the baseline portion of the study, each participant received an oral transporter probe cocktail consisting of 10 mg rosuvastatin (OATP1B1/3 and BCRP), 50 mg metformin (OCT1/2 and MATE1/2-K), 1 mg furosemide (OAT1/3), and 2.5 mg midazolam (CYP3A; positive control). Plasma and urine samples were collected before and after the cocktail, with urine collected up to 24 hours later, and plasma collected up to 96 hours later.
Following a minimum 9-day washout period, the same cohort received 1 gram of goldenseal every 8 hours for 5 days. On the day 6, the drug cocktail was given again, followed by two additional doses of goldenseal at 4-hour intervals. At the same time points used in the baseline protocol, urine and plasma samples were collected.
Plasma concentration vs. time profiles revealed that the model-based prediction was false, in that the presence of goldenseal did not alter the pharmacokinetics of rosuvastatin acid and lactone. The investigators suggested that this could be due to incomplete dissolution of goldenseal in the intestinal lumen, and/or low enterocyte concentrations of goldenseal stemming from “low permeability or extensive enterocyte metabolism or efflux.”
In contrast, and unpredicted by the basic model, goldenseal had a significant impact on apical efflux transporters MATE1 and MATE2-K, which mediate renal excretion of metformin. In consequence, AUC from zero to infinity and maximum plasma concentration of metformin were reduced by 23% and 27%, respectively.
“These observations, coupled with no change in half-life, suggested that goldenseal decreased metformin oral bioavailability by altering intestinal permeability, transport, and/or other processes involved in metformin absorption,” the investigators wrote.
According to principal author Mary Paine, PhD, of Washington State University, Spokane, this finding may have clinically significant implications for patients currently taking metformin for type 2 diabetes.
“Our study showed that goldenseal has an effect on the intestinal absorption of metformin, suggesting that the co-use of metformin and goldenseal may compromise blood glucose control in patients with type 2 diabetes and increase their risk of negative health outcomes,” Dr. Paine said. “While this finding warrants a degree of caution to be exercised among patients and their treating physicians, we have more work to do to confirm whether these findings in healthy volunteers in fact have clinical relevance in the management of diabetes. We are in the process of starting a follow-up study that should ultimately answer that question.”
The study was supported by the National Institutes of Health. The investigators reported no conflicts of interest.
Goldenseal, a natural botanical product, may interfere with intestinal absorption of metformin, potentially compromising blood glucose control in patients with type 2 diabetes, according to investigators.
The study, which tested for interactions between goldenseal and several drugs in healthy volunteers, reveals that current models for predicting transporter-mediated drug-drug interactions may be insufficient to screen commonly used dietary supplements, reported lead investigator James T. Nguyen, PharmD, a PhD candidate at Washington State University, Spokane, and colleagues.
“Supplements containing goldenseal ... a perennial herb native to North America, have consistently ranked among the top 20 highest selling natural products during the last decade,” the investigators wrote in Clinical Pharmacology & Therapeutics . “As more patients continue to seek goldenseal and other natural products to self-treat their medical conditions, there is an increasing need to characterize their safety profiles, especially when co-consumed with prescribed medications, which can lead to adverse natural product-drug interactions.”
Previous clinical studies have shown that goldenseal inhibits cytochrome P450, with one study showing a roughly 40% increase in systemic midazolam exposure via CYP3A inhibition, “suggesting goldenseal could have prolonged inhibitory effects in vivo similar to grapefruit juice,” the investigators wrote.
Clinical and in vitro results for goldenseal-transporter interactions have been mixed, the investigators noted, specifically for P-glycoprotein, while other transporters remain clinically untested.
“Likewise, the effects of [goldenseal alkaloids], all of which are time-dependent inhibitors of CYP3A and/or CYP2D6, have not been tested on transporter function,” the investigators wrote.
To address this knowledge gap, the investigators first performed in vitro transporter inhibition assays and in vitro–in vivo predictions involving goldenseal, plus the alkaloids berberine, (−)-beta-hydrastine, and hydrastinine.
This analysis revealed that a number of transporters were sensitive to inhibition by goldenseal and its alkaloids.
“Using current [Food and Drug Administration]–recommended basic models, the goldenseal product was predicted to inhibit the intestinal efflux transporter BCRP [breast cancer resistance protein] and the hepatic uptake transporters OATP1B1 and OATP1B3,” the investigators wrote, which suggested that goldenseal would increase the area under the plasma concentration-time curve (AUC) of rosuvastatin acid and lactone.
This prediction was clinically tested in 16 healthy volunteers: 8 men and 8 nonpregnant women.
In the baseline portion of the study, each participant received an oral transporter probe cocktail consisting of 10 mg rosuvastatin (OATP1B1/3 and BCRP), 50 mg metformin (OCT1/2 and MATE1/2-K), 1 mg furosemide (OAT1/3), and 2.5 mg midazolam (CYP3A; positive control). Plasma and urine samples were collected before and after the cocktail, with urine collected up to 24 hours later, and plasma collected up to 96 hours later.
Following a minimum 9-day washout period, the same cohort received 1 gram of goldenseal every 8 hours for 5 days. On the day 6, the drug cocktail was given again, followed by two additional doses of goldenseal at 4-hour intervals. At the same time points used in the baseline protocol, urine and plasma samples were collected.
Plasma concentration vs. time profiles revealed that the model-based prediction was false, in that the presence of goldenseal did not alter the pharmacokinetics of rosuvastatin acid and lactone. The investigators suggested that this could be due to incomplete dissolution of goldenseal in the intestinal lumen, and/or low enterocyte concentrations of goldenseal stemming from “low permeability or extensive enterocyte metabolism or efflux.”
In contrast, and unpredicted by the basic model, goldenseal had a significant impact on apical efflux transporters MATE1 and MATE2-K, which mediate renal excretion of metformin. In consequence, AUC from zero to infinity and maximum plasma concentration of metformin were reduced by 23% and 27%, respectively.
“These observations, coupled with no change in half-life, suggested that goldenseal decreased metformin oral bioavailability by altering intestinal permeability, transport, and/or other processes involved in metformin absorption,” the investigators wrote.
According to principal author Mary Paine, PhD, of Washington State University, Spokane, this finding may have clinically significant implications for patients currently taking metformin for type 2 diabetes.
“Our study showed that goldenseal has an effect on the intestinal absorption of metformin, suggesting that the co-use of metformin and goldenseal may compromise blood glucose control in patients with type 2 diabetes and increase their risk of negative health outcomes,” Dr. Paine said. “While this finding warrants a degree of caution to be exercised among patients and their treating physicians, we have more work to do to confirm whether these findings in healthy volunteers in fact have clinical relevance in the management of diabetes. We are in the process of starting a follow-up study that should ultimately answer that question.”
The study was supported by the National Institutes of Health. The investigators reported no conflicts of interest.
FROM CLINICAL PHARMACOLOGY & THERAPEUTICS
Roots of physician burnout: It’s the work load
Work load, not personal vulnerability, may be at the root of the current physician burnout crisis, a recent study has concluded.
The cutting-edge research utilized cognitive theory and work load analysis to get at the source of burnout among practitioners. The findings indicate that, although some institutions continue to emphasize personal responsibility of physicians to address the issue, it may be the amount and structure of the work itself that triggers burnout in doctors.
“We evaluated the cognitive load of a clinical workday in a national sample of U.S. physicians and its relationship with burnout and professional satisfaction,” wrote Elizabeth Harry, MD, SFHM, a hospitalist at the University of Colorado at Denver, Aurora and coauthors. The results were reported in the Joint Commission Journal on Quality and Patient Safety.
The researchers investigated whether task load correlated with burnout scores in a large national study of U.S. physicians from October 2017 to March 2018.
As the delivery of health care becomes more complex, physicians are charged with ever-increasing amount of administrative and cognitive tasks. Recent evidence indicates that this growing complexity of work is tied to a greater risk of burnout in physicians, compared with workers in other fields. Cognitive load theory, pioneered by psychologist Jonathan Sweller, identified limitations in working memory that humans depend on to carry out cognitive tasks. Cognitive load refers to the amount of working memory used, which can be reduced in the presence of external emotional or physiological stressors. While a potential link between cognitive load and burnout may seem self-evident, the correlation between the cognitive load of physicians and burnout has not been evaluated in a large-scale study until recently.
Physician task load (PTL) was measured using the National Aeronautics and Space Administration Task Load Index (NASA-TLX), a validated questionnaire frequently used to evaluate the cognitive load of work environments, including health care environments. Four domains (perception of effort and mental, physical, and temporal demands) were used to calculate the total PTL score.
Burnout was evaluated using the Emotional Exhaustion and Depersonalization scales of the Maslach Burnout Inventory, a validated tool considered the gold standard for measurement.
The survey sample consisted of physicians of all specialties and was assembled using the American Medical Association Physician Masterfile, an almost complete record of all U.S. physicians independent of AMA membership. All responses were anonymous and participation was voluntary.
Results
Among 30,456 physicians who received the survey, 5,197 (17.1%) responded. In total, 5,276 physicians were included in the analysis.
The median age of respondents was 53 years, and 61.8% self-identified as male. Twenty-four specialties were identified: 23.8% were from a primary care discipline and internal medicine represented the largest respondent group (12.1%).
Almost half of respondents (49.7%) worked in private practice, and 44.8% had been in practice for 21 years or longer.
Overall, 44.0% had at least one symptom of burnout, 38.8% of participants scored in the high range for emotional exhaustion, and 27.4% scored in the high range for depersonalization. The mean score in task load dimension varied by specialty.
The mean PTL score was 260.9 (standard deviation, 71.4). The specialties with the highest PTL score were emergency medicine (369.8), urology (353.7), general surgery subspecialties (343.9), internal medicine subspecialties (342.2), and radiology (341.6).
Aside from specialty, PTL scores also varied by practice setting, gender, age, number of hours worked per week, number of nights on call per week, and years in practice.
The researchers observed a dose response relationship between PTL and risk of burnout. For every 40-point (10%) reduction in PTL, there was 33% lower odds of experiencing burnout (odds ratio, 0.67; 95% confidence interval, 0.65-0.70; P < .0001). Multivariable analyses also indicated that PTL was a significant predictor of burnout, independent of practice setting, specialty, age, gender, and hours worked.
Organizational strategies to reduce physician burnout
Coauthors of the study, Tait D. Shanafelt, MD, professor of medicine at Stanford (Calif.) University and Colin P. West, MD, PhD, of the Mayo Clinic in Rochester, Minn., are both experts on physician well-being and are passionate about finding new ways to reduce physician distress and improving health care delivery.
“Authentic efforts to address this problem must move beyond personal resilience,” Dr. Shanafelt said in an interview. “Organizations that fail to get serious about this issue are going to be left behind and struggle in the war for talent.
“Much like our efforts to improve quality, advancing clinician well-being requires organizations to make it a priority and establish the structure, process, and leadership to promote the desired outcomes,” said Dr. Shanafelt.
One potential strategy for improvement is appointing a chief wellness officer, a dedicated individual within the health care system that leads the organizational effort, explained Dr. Shanafelt. “Over 30 vanguard institutions across the United States have already taken this step.”
Dr. West, a coauthor of the study, explained that conducting an analysis of PTL is fairly straightforward for hospitals and individual institutions. “The NASA-TLX tool is widely available, free to use, and not overly complex, and it could be used to provide insight into physician effort and mental, physical, and temporal demand levels,” he said in an interview.
“Deeper evaluations could follow to identify specific potential solutions, particularly system-level approaches to alleviate PTL,” Dr. West explained. “In the short term, such analyses and solutions would have costs, but helping physicians work more optimally and with less chronic strain from excessive task load would save far more than these costs overall.”
Dr. West also noted that physician burnout is very expensive to a health care system, and strategies to promote physician well-being would be a prudent financial decision long term for health care organizations.
Dr. Harry, lead author of the study, agreed with Dr. West, noting that “quality improvement literature has demonstrated that improvements in inefficiencies that lead to increased demand in the workplace often has the benefit of reduced cost.
“Many studies have demonstrated the risk of turnover due to burnout and the significant cost of physician turn over,” she said in an interview. “This cost avoidance is well worth the investment in improved operations to minimize unnecessary task load.”
Dr. Harry also recommended the NASA-TLX tool as a free resource for health systems and organizations. She noted that future studies will further validate the reliability of the tool.
“At the core, we need to focus on system redesign at both the micro and the macro level,” Dr. Harry said. “Each health system will need to assess inefficiencies in their work flow, while regulatory bodies need to consider the downstream task load of mandates and reporting requirements, all of which contribute to more cognitive load.”
The study was supported by funding from the Stanford Medicine WellMD Center, the American Medical Association, and the Mayo Clinic department of medicine program on physician well-being. Coauthors Lotte N. Dyrbye, MD, and Dr. Shanafelt are coinventors of the Physician Well-being Index, Medical Student Well-Being Index, Nurse Well-Being, and Well-Being Index. Mayo Clinic holds the copyright to these instruments and has licensed them for external use. Dr. Dyrbye and Dr. Shanafelt receive a portion of any royalties paid to Mayo Clinic. All other authors reported no conflicts of interest.
Work load, not personal vulnerability, may be at the root of the current physician burnout crisis, a recent study has concluded.
The cutting-edge research utilized cognitive theory and work load analysis to get at the source of burnout among practitioners. The findings indicate that, although some institutions continue to emphasize personal responsibility of physicians to address the issue, it may be the amount and structure of the work itself that triggers burnout in doctors.
“We evaluated the cognitive load of a clinical workday in a national sample of U.S. physicians and its relationship with burnout and professional satisfaction,” wrote Elizabeth Harry, MD, SFHM, a hospitalist at the University of Colorado at Denver, Aurora and coauthors. The results were reported in the Joint Commission Journal on Quality and Patient Safety.
The researchers investigated whether task load correlated with burnout scores in a large national study of U.S. physicians from October 2017 to March 2018.
As the delivery of health care becomes more complex, physicians are charged with ever-increasing amount of administrative and cognitive tasks. Recent evidence indicates that this growing complexity of work is tied to a greater risk of burnout in physicians, compared with workers in other fields. Cognitive load theory, pioneered by psychologist Jonathan Sweller, identified limitations in working memory that humans depend on to carry out cognitive tasks. Cognitive load refers to the amount of working memory used, which can be reduced in the presence of external emotional or physiological stressors. While a potential link between cognitive load and burnout may seem self-evident, the correlation between the cognitive load of physicians and burnout has not been evaluated in a large-scale study until recently.
Physician task load (PTL) was measured using the National Aeronautics and Space Administration Task Load Index (NASA-TLX), a validated questionnaire frequently used to evaluate the cognitive load of work environments, including health care environments. Four domains (perception of effort and mental, physical, and temporal demands) were used to calculate the total PTL score.
Burnout was evaluated using the Emotional Exhaustion and Depersonalization scales of the Maslach Burnout Inventory, a validated tool considered the gold standard for measurement.
The survey sample consisted of physicians of all specialties and was assembled using the American Medical Association Physician Masterfile, an almost complete record of all U.S. physicians independent of AMA membership. All responses were anonymous and participation was voluntary.
Results
Among 30,456 physicians who received the survey, 5,197 (17.1%) responded. In total, 5,276 physicians were included in the analysis.
The median age of respondents was 53 years, and 61.8% self-identified as male. Twenty-four specialties were identified: 23.8% were from a primary care discipline and internal medicine represented the largest respondent group (12.1%).
Almost half of respondents (49.7%) worked in private practice, and 44.8% had been in practice for 21 years or longer.
Overall, 44.0% had at least one symptom of burnout, 38.8% of participants scored in the high range for emotional exhaustion, and 27.4% scored in the high range for depersonalization. The mean score in task load dimension varied by specialty.
The mean PTL score was 260.9 (standard deviation, 71.4). The specialties with the highest PTL score were emergency medicine (369.8), urology (353.7), general surgery subspecialties (343.9), internal medicine subspecialties (342.2), and radiology (341.6).
Aside from specialty, PTL scores also varied by practice setting, gender, age, number of hours worked per week, number of nights on call per week, and years in practice.
The researchers observed a dose response relationship between PTL and risk of burnout. For every 40-point (10%) reduction in PTL, there was 33% lower odds of experiencing burnout (odds ratio, 0.67; 95% confidence interval, 0.65-0.70; P < .0001). Multivariable analyses also indicated that PTL was a significant predictor of burnout, independent of practice setting, specialty, age, gender, and hours worked.
Organizational strategies to reduce physician burnout
Coauthors of the study, Tait D. Shanafelt, MD, professor of medicine at Stanford (Calif.) University and Colin P. West, MD, PhD, of the Mayo Clinic in Rochester, Minn., are both experts on physician well-being and are passionate about finding new ways to reduce physician distress and improving health care delivery.
“Authentic efforts to address this problem must move beyond personal resilience,” Dr. Shanafelt said in an interview. “Organizations that fail to get serious about this issue are going to be left behind and struggle in the war for talent.
“Much like our efforts to improve quality, advancing clinician well-being requires organizations to make it a priority and establish the structure, process, and leadership to promote the desired outcomes,” said Dr. Shanafelt.
One potential strategy for improvement is appointing a chief wellness officer, a dedicated individual within the health care system that leads the organizational effort, explained Dr. Shanafelt. “Over 30 vanguard institutions across the United States have already taken this step.”
Dr. West, a coauthor of the study, explained that conducting an analysis of PTL is fairly straightforward for hospitals and individual institutions. “The NASA-TLX tool is widely available, free to use, and not overly complex, and it could be used to provide insight into physician effort and mental, physical, and temporal demand levels,” he said in an interview.
“Deeper evaluations could follow to identify specific potential solutions, particularly system-level approaches to alleviate PTL,” Dr. West explained. “In the short term, such analyses and solutions would have costs, but helping physicians work more optimally and with less chronic strain from excessive task load would save far more than these costs overall.”
Dr. West also noted that physician burnout is very expensive to a health care system, and strategies to promote physician well-being would be a prudent financial decision long term for health care organizations.
Dr. Harry, lead author of the study, agreed with Dr. West, noting that “quality improvement literature has demonstrated that improvements in inefficiencies that lead to increased demand in the workplace often has the benefit of reduced cost.
“Many studies have demonstrated the risk of turnover due to burnout and the significant cost of physician turn over,” she said in an interview. “This cost avoidance is well worth the investment in improved operations to minimize unnecessary task load.”
Dr. Harry also recommended the NASA-TLX tool as a free resource for health systems and organizations. She noted that future studies will further validate the reliability of the tool.
“At the core, we need to focus on system redesign at both the micro and the macro level,” Dr. Harry said. “Each health system will need to assess inefficiencies in their work flow, while regulatory bodies need to consider the downstream task load of mandates and reporting requirements, all of which contribute to more cognitive load.”
The study was supported by funding from the Stanford Medicine WellMD Center, the American Medical Association, and the Mayo Clinic department of medicine program on physician well-being. Coauthors Lotte N. Dyrbye, MD, and Dr. Shanafelt are coinventors of the Physician Well-being Index, Medical Student Well-Being Index, Nurse Well-Being, and Well-Being Index. Mayo Clinic holds the copyright to these instruments and has licensed them for external use. Dr. Dyrbye and Dr. Shanafelt receive a portion of any royalties paid to Mayo Clinic. All other authors reported no conflicts of interest.
Work load, not personal vulnerability, may be at the root of the current physician burnout crisis, a recent study has concluded.
The cutting-edge research utilized cognitive theory and work load analysis to get at the source of burnout among practitioners. The findings indicate that, although some institutions continue to emphasize personal responsibility of physicians to address the issue, it may be the amount and structure of the work itself that triggers burnout in doctors.
“We evaluated the cognitive load of a clinical workday in a national sample of U.S. physicians and its relationship with burnout and professional satisfaction,” wrote Elizabeth Harry, MD, SFHM, a hospitalist at the University of Colorado at Denver, Aurora and coauthors. The results were reported in the Joint Commission Journal on Quality and Patient Safety.
The researchers investigated whether task load correlated with burnout scores in a large national study of U.S. physicians from October 2017 to March 2018.
As the delivery of health care becomes more complex, physicians are charged with ever-increasing amount of administrative and cognitive tasks. Recent evidence indicates that this growing complexity of work is tied to a greater risk of burnout in physicians, compared with workers in other fields. Cognitive load theory, pioneered by psychologist Jonathan Sweller, identified limitations in working memory that humans depend on to carry out cognitive tasks. Cognitive load refers to the amount of working memory used, which can be reduced in the presence of external emotional or physiological stressors. While a potential link between cognitive load and burnout may seem self-evident, the correlation between the cognitive load of physicians and burnout has not been evaluated in a large-scale study until recently.
Physician task load (PTL) was measured using the National Aeronautics and Space Administration Task Load Index (NASA-TLX), a validated questionnaire frequently used to evaluate the cognitive load of work environments, including health care environments. Four domains (perception of effort and mental, physical, and temporal demands) were used to calculate the total PTL score.
Burnout was evaluated using the Emotional Exhaustion and Depersonalization scales of the Maslach Burnout Inventory, a validated tool considered the gold standard for measurement.
The survey sample consisted of physicians of all specialties and was assembled using the American Medical Association Physician Masterfile, an almost complete record of all U.S. physicians independent of AMA membership. All responses were anonymous and participation was voluntary.
Results
Among 30,456 physicians who received the survey, 5,197 (17.1%) responded. In total, 5,276 physicians were included in the analysis.
The median age of respondents was 53 years, and 61.8% self-identified as male. Twenty-four specialties were identified: 23.8% were from a primary care discipline and internal medicine represented the largest respondent group (12.1%).
Almost half of respondents (49.7%) worked in private practice, and 44.8% had been in practice for 21 years or longer.
Overall, 44.0% had at least one symptom of burnout, 38.8% of participants scored in the high range for emotional exhaustion, and 27.4% scored in the high range for depersonalization. The mean score in task load dimension varied by specialty.
The mean PTL score was 260.9 (standard deviation, 71.4). The specialties with the highest PTL score were emergency medicine (369.8), urology (353.7), general surgery subspecialties (343.9), internal medicine subspecialties (342.2), and radiology (341.6).
Aside from specialty, PTL scores also varied by practice setting, gender, age, number of hours worked per week, number of nights on call per week, and years in practice.
The researchers observed a dose response relationship between PTL and risk of burnout. For every 40-point (10%) reduction in PTL, there was 33% lower odds of experiencing burnout (odds ratio, 0.67; 95% confidence interval, 0.65-0.70; P < .0001). Multivariable analyses also indicated that PTL was a significant predictor of burnout, independent of practice setting, specialty, age, gender, and hours worked.
Organizational strategies to reduce physician burnout
Coauthors of the study, Tait D. Shanafelt, MD, professor of medicine at Stanford (Calif.) University and Colin P. West, MD, PhD, of the Mayo Clinic in Rochester, Minn., are both experts on physician well-being and are passionate about finding new ways to reduce physician distress and improving health care delivery.
“Authentic efforts to address this problem must move beyond personal resilience,” Dr. Shanafelt said in an interview. “Organizations that fail to get serious about this issue are going to be left behind and struggle in the war for talent.
“Much like our efforts to improve quality, advancing clinician well-being requires organizations to make it a priority and establish the structure, process, and leadership to promote the desired outcomes,” said Dr. Shanafelt.
One potential strategy for improvement is appointing a chief wellness officer, a dedicated individual within the health care system that leads the organizational effort, explained Dr. Shanafelt. “Over 30 vanguard institutions across the United States have already taken this step.”
Dr. West, a coauthor of the study, explained that conducting an analysis of PTL is fairly straightforward for hospitals and individual institutions. “The NASA-TLX tool is widely available, free to use, and not overly complex, and it could be used to provide insight into physician effort and mental, physical, and temporal demand levels,” he said in an interview.
“Deeper evaluations could follow to identify specific potential solutions, particularly system-level approaches to alleviate PTL,” Dr. West explained. “In the short term, such analyses and solutions would have costs, but helping physicians work more optimally and with less chronic strain from excessive task load would save far more than these costs overall.”
Dr. West also noted that physician burnout is very expensive to a health care system, and strategies to promote physician well-being would be a prudent financial decision long term for health care organizations.
Dr. Harry, lead author of the study, agreed with Dr. West, noting that “quality improvement literature has demonstrated that improvements in inefficiencies that lead to increased demand in the workplace often has the benefit of reduced cost.
“Many studies have demonstrated the risk of turnover due to burnout and the significant cost of physician turn over,” she said in an interview. “This cost avoidance is well worth the investment in improved operations to minimize unnecessary task load.”
Dr. Harry also recommended the NASA-TLX tool as a free resource for health systems and organizations. She noted that future studies will further validate the reliability of the tool.
“At the core, we need to focus on system redesign at both the micro and the macro level,” Dr. Harry said. “Each health system will need to assess inefficiencies in their work flow, while regulatory bodies need to consider the downstream task load of mandates and reporting requirements, all of which contribute to more cognitive load.”
The study was supported by funding from the Stanford Medicine WellMD Center, the American Medical Association, and the Mayo Clinic department of medicine program on physician well-being. Coauthors Lotte N. Dyrbye, MD, and Dr. Shanafelt are coinventors of the Physician Well-being Index, Medical Student Well-Being Index, Nurse Well-Being, and Well-Being Index. Mayo Clinic holds the copyright to these instruments and has licensed them for external use. Dr. Dyrbye and Dr. Shanafelt receive a portion of any royalties paid to Mayo Clinic. All other authors reported no conflicts of interest.
FROM THE JOINT COMMISSION JOURNAL ON QUALITY AND PATIENT SAFETY
CDC chief lays out attack plan for COVID variants
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
CAR T-cell products shine in real-world setting, reveal new insights
Real-world experience with chimeric antigen receptor (CAR) T-cell therapies for large B-cell lymphomas compares favorably with experience in commercial and trial settings and provides new insights for predicting outcomes, according to Paolo Corradini, MD.
The 12-month duration of response (DOR) and progression-free survival (PFS) rates in 152 real-world patients treated with tisagenlecleucel (tisa-cel; Kymriah) for an approved indication were 48.4% and 26.4%, respectively, data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) and published in November 2020 in Blood Advances showed.
who relapsed or were refractory to at least two prior lines of therapy, Dr. Corradini said at the third European CAR T-cell Meeting, jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association.
A clinical update of the JULIET trial, as presented by Dr. Corradini and colleagues in a poster at the 2020 annual conference of the American Society of Hematology, showed a relapse-free probability of 60.4% at 24 and 30 months among 61 patients with an initial response.
The 12- and 36-month PFS rates as of February 2020, with median follow-up of 40.3 months, were 33% and 31%, respectively, and no new safety signals were identified, said Dr. Corradini, chair of hematology at the University of Milan.
Similarly, real-world data from the U.S. Lymphoma CAR T Consortium showing median PFS of 8.3 months at median follow-up of 12.9 months in 275 patients treated with axicabtagene ciloleucel (axi-cel; YESCARTA) were comparable with outcomes in the ZUMA-1 registrational trial, he noted.
An ongoing response was seen at 2 years in 39% of patients in ZUMA-1, and 3-year survival was 47%, according to an update reported at ASH 2019.
Of note, 43% of patients in the real-world study, which was published in the Journal of Clinical Oncology in September 2020, would not have met ZUMA-1 eligibility criteria because of comorbidities at the time of leukapheresis.
Predicting outcomes
The real-world data also demonstrated that performance status and lactate dehydrogenase (LDH) levels can predict outcomes: Patients with poor Eastern Cooperative Oncology Group performance status of 2-4 versus less than 2, and elevated LDH had shorter PFS and overall survival (OS) on both univariate and multivariate analysis, Dr. Corradini noted.
A subsequent multicenter study showed similar response rates of 70% and 68% in ZUMA-1-eligible and noneligible patients, but significantly improved DOR, PFS, and OS outcomes among the ZUMA-1-eligible patients.
The authors also looked for “clinical predictive factors or some easy clinical biomarkers to predict the outcomes in our patients receiving CAR T-cells,” and found that C-reactive protein levels of more than 30 mg at infusion were associated with poorer DOR, PFS, and OS, he said.
In 60 patients in another U.S. study of both tisa-cel- and axi-cel-treated patients at Memorial Sloan Kettering Cancer Center, 1-year event-free survival and OS were 40% and 69%, and Dr. Corradini’s experience with 55 patients at the University of Milan similarly showed 1-year PFS and OS of 40% and 70%, respectively.
“So all these studies support the notion that the results of CAR T-cells in real-world practice are durable for our patients, and are very similar to results obtained in the studies,” he said.
Other factors that have been shown to be associated with poor outcomes after CAR T-cell therapy include systemic bridging therapy, high metabolic tumor volume, and extranodal involvement; patients with these characteristics, along with those who have poor ECOG performance status or elevated LDH or CRP levels, do not comprise “a group to exclude from CAR T-cell therapy, but rather ... a group for whom there is an unmet need with our currently available treatments,” he said, adding: “So, it’s a group for which we have to do clinical trials and studies to improve the outcomes of our patient with large B-cell lymphomas.”
“These are all real-world data with commercially available products, he noted.
Product selection
Tisa-cel received Food and Drug Administration approval in 2017 and is used to treat relapsed or refractory acute lymphoblastic leukemia in those aged up to 25 years, and non-Hodgkin lymphoma that has relapsed or is refractory after at least two prior lines of therapy.
Axi-cel was also approved in 2017 for relapsed/refractory non-Hodgkin lymphoma, and in February 2021, after Dr. Corradini’s meeting presentation, the FDA granted a third approval to lisocabtagene maraleucel (liso-cel; Breyanzi) for this indication.
The information to date from both the trial and real-world settings are limited with respect to showing any differences in outcomes between the CAR T-cell products, but provide “an initial suggestion” that outcomes with tisa-cel and axi-cel are comparable, he said, adding that decisions should be strictly based on product registration data given the absence of reliable data for choosing one product over another.
Dr. Corradini reported honoraria and/or payment for travel and accommodations from Abbvie, Amgen, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, and a number of other pharmaceutical companies.
Real-world experience with chimeric antigen receptor (CAR) T-cell therapies for large B-cell lymphomas compares favorably with experience in commercial and trial settings and provides new insights for predicting outcomes, according to Paolo Corradini, MD.
The 12-month duration of response (DOR) and progression-free survival (PFS) rates in 152 real-world patients treated with tisagenlecleucel (tisa-cel; Kymriah) for an approved indication were 48.4% and 26.4%, respectively, data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) and published in November 2020 in Blood Advances showed.
who relapsed or were refractory to at least two prior lines of therapy, Dr. Corradini said at the third European CAR T-cell Meeting, jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association.
A clinical update of the JULIET trial, as presented by Dr. Corradini and colleagues in a poster at the 2020 annual conference of the American Society of Hematology, showed a relapse-free probability of 60.4% at 24 and 30 months among 61 patients with an initial response.
The 12- and 36-month PFS rates as of February 2020, with median follow-up of 40.3 months, were 33% and 31%, respectively, and no new safety signals were identified, said Dr. Corradini, chair of hematology at the University of Milan.
Similarly, real-world data from the U.S. Lymphoma CAR T Consortium showing median PFS of 8.3 months at median follow-up of 12.9 months in 275 patients treated with axicabtagene ciloleucel (axi-cel; YESCARTA) were comparable with outcomes in the ZUMA-1 registrational trial, he noted.
An ongoing response was seen at 2 years in 39% of patients in ZUMA-1, and 3-year survival was 47%, according to an update reported at ASH 2019.
Of note, 43% of patients in the real-world study, which was published in the Journal of Clinical Oncology in September 2020, would not have met ZUMA-1 eligibility criteria because of comorbidities at the time of leukapheresis.
Predicting outcomes
The real-world data also demonstrated that performance status and lactate dehydrogenase (LDH) levels can predict outcomes: Patients with poor Eastern Cooperative Oncology Group performance status of 2-4 versus less than 2, and elevated LDH had shorter PFS and overall survival (OS) on both univariate and multivariate analysis, Dr. Corradini noted.
A subsequent multicenter study showed similar response rates of 70% and 68% in ZUMA-1-eligible and noneligible patients, but significantly improved DOR, PFS, and OS outcomes among the ZUMA-1-eligible patients.
The authors also looked for “clinical predictive factors or some easy clinical biomarkers to predict the outcomes in our patients receiving CAR T-cells,” and found that C-reactive protein levels of more than 30 mg at infusion were associated with poorer DOR, PFS, and OS, he said.
In 60 patients in another U.S. study of both tisa-cel- and axi-cel-treated patients at Memorial Sloan Kettering Cancer Center, 1-year event-free survival and OS were 40% and 69%, and Dr. Corradini’s experience with 55 patients at the University of Milan similarly showed 1-year PFS and OS of 40% and 70%, respectively.
“So all these studies support the notion that the results of CAR T-cells in real-world practice are durable for our patients, and are very similar to results obtained in the studies,” he said.
Other factors that have been shown to be associated with poor outcomes after CAR T-cell therapy include systemic bridging therapy, high metabolic tumor volume, and extranodal involvement; patients with these characteristics, along with those who have poor ECOG performance status or elevated LDH or CRP levels, do not comprise “a group to exclude from CAR T-cell therapy, but rather ... a group for whom there is an unmet need with our currently available treatments,” he said, adding: “So, it’s a group for which we have to do clinical trials and studies to improve the outcomes of our patient with large B-cell lymphomas.”
“These are all real-world data with commercially available products, he noted.
Product selection
Tisa-cel received Food and Drug Administration approval in 2017 and is used to treat relapsed or refractory acute lymphoblastic leukemia in those aged up to 25 years, and non-Hodgkin lymphoma that has relapsed or is refractory after at least two prior lines of therapy.
Axi-cel was also approved in 2017 for relapsed/refractory non-Hodgkin lymphoma, and in February 2021, after Dr. Corradini’s meeting presentation, the FDA granted a third approval to lisocabtagene maraleucel (liso-cel; Breyanzi) for this indication.
The information to date from both the trial and real-world settings are limited with respect to showing any differences in outcomes between the CAR T-cell products, but provide “an initial suggestion” that outcomes with tisa-cel and axi-cel are comparable, he said, adding that decisions should be strictly based on product registration data given the absence of reliable data for choosing one product over another.
Dr. Corradini reported honoraria and/or payment for travel and accommodations from Abbvie, Amgen, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, and a number of other pharmaceutical companies.
Real-world experience with chimeric antigen receptor (CAR) T-cell therapies for large B-cell lymphomas compares favorably with experience in commercial and trial settings and provides new insights for predicting outcomes, according to Paolo Corradini, MD.
The 12-month duration of response (DOR) and progression-free survival (PFS) rates in 152 real-world patients treated with tisagenlecleucel (tisa-cel; Kymriah) for an approved indication were 48.4% and 26.4%, respectively, data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) and published in November 2020 in Blood Advances showed.
who relapsed or were refractory to at least two prior lines of therapy, Dr. Corradini said at the third European CAR T-cell Meeting, jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association.
A clinical update of the JULIET trial, as presented by Dr. Corradini and colleagues in a poster at the 2020 annual conference of the American Society of Hematology, showed a relapse-free probability of 60.4% at 24 and 30 months among 61 patients with an initial response.
The 12- and 36-month PFS rates as of February 2020, with median follow-up of 40.3 months, were 33% and 31%, respectively, and no new safety signals were identified, said Dr. Corradini, chair of hematology at the University of Milan.
Similarly, real-world data from the U.S. Lymphoma CAR T Consortium showing median PFS of 8.3 months at median follow-up of 12.9 months in 275 patients treated with axicabtagene ciloleucel (axi-cel; YESCARTA) were comparable with outcomes in the ZUMA-1 registrational trial, he noted.
An ongoing response was seen at 2 years in 39% of patients in ZUMA-1, and 3-year survival was 47%, according to an update reported at ASH 2019.
Of note, 43% of patients in the real-world study, which was published in the Journal of Clinical Oncology in September 2020, would not have met ZUMA-1 eligibility criteria because of comorbidities at the time of leukapheresis.
Predicting outcomes
The real-world data also demonstrated that performance status and lactate dehydrogenase (LDH) levels can predict outcomes: Patients with poor Eastern Cooperative Oncology Group performance status of 2-4 versus less than 2, and elevated LDH had shorter PFS and overall survival (OS) on both univariate and multivariate analysis, Dr. Corradini noted.
A subsequent multicenter study showed similar response rates of 70% and 68% in ZUMA-1-eligible and noneligible patients, but significantly improved DOR, PFS, and OS outcomes among the ZUMA-1-eligible patients.
The authors also looked for “clinical predictive factors or some easy clinical biomarkers to predict the outcomes in our patients receiving CAR T-cells,” and found that C-reactive protein levels of more than 30 mg at infusion were associated with poorer DOR, PFS, and OS, he said.
In 60 patients in another U.S. study of both tisa-cel- and axi-cel-treated patients at Memorial Sloan Kettering Cancer Center, 1-year event-free survival and OS were 40% and 69%, and Dr. Corradini’s experience with 55 patients at the University of Milan similarly showed 1-year PFS and OS of 40% and 70%, respectively.
“So all these studies support the notion that the results of CAR T-cells in real-world practice are durable for our patients, and are very similar to results obtained in the studies,” he said.
Other factors that have been shown to be associated with poor outcomes after CAR T-cell therapy include systemic bridging therapy, high metabolic tumor volume, and extranodal involvement; patients with these characteristics, along with those who have poor ECOG performance status or elevated LDH or CRP levels, do not comprise “a group to exclude from CAR T-cell therapy, but rather ... a group for whom there is an unmet need with our currently available treatments,” he said, adding: “So, it’s a group for which we have to do clinical trials and studies to improve the outcomes of our patient with large B-cell lymphomas.”
“These are all real-world data with commercially available products, he noted.
Product selection
Tisa-cel received Food and Drug Administration approval in 2017 and is used to treat relapsed or refractory acute lymphoblastic leukemia in those aged up to 25 years, and non-Hodgkin lymphoma that has relapsed or is refractory after at least two prior lines of therapy.
Axi-cel was also approved in 2017 for relapsed/refractory non-Hodgkin lymphoma, and in February 2021, after Dr. Corradini’s meeting presentation, the FDA granted a third approval to lisocabtagene maraleucel (liso-cel; Breyanzi) for this indication.
The information to date from both the trial and real-world settings are limited with respect to showing any differences in outcomes between the CAR T-cell products, but provide “an initial suggestion” that outcomes with tisa-cel and axi-cel are comparable, he said, adding that decisions should be strictly based on product registration data given the absence of reliable data for choosing one product over another.
Dr. Corradini reported honoraria and/or payment for travel and accommodations from Abbvie, Amgen, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, and a number of other pharmaceutical companies.
FROM CART21
Breast cancer surgeries deemed ‘low value’ continue, increase
“This is the first study to [evaluate] all four of the low-value breast cancer procedures at the same time and try to draw some conclusions on practice patterns across facilities,” said senior author Lesly A. Dossett, MD, MPH, Center for Health Outcomes and Policy, the University of Michigan, Ann Arbor.
The two low-value procedures that have increased in use are contralateral prophylactic mastectomy for average-risk women with unilateral cancer and sentinel lymph node biopsy for clinically node-negative women aged 70 years and older with hormone receptor–positive (HR+) cancer.
“This suggests that formal efforts to reduce low value care through dissemination of guidelines, education of patients or providers, or alignment of incentives will be necessary to achieve full deimplementation,” she told this news organization.
The researchers emphasize that the providing of services that have no clinically meaningful benefit is a national epidemic, costing the United States more than $100 billion dollars annually.
These trends are notable and likely reflect a broad range of factors, commented Katharine Yao, MD, chief of the division of surgical oncology at the NorthShore University HealthSystem, Evanston, Ill.
“I think the better message here is not so much that facilities are doing too many low-value procedures but more that these procedures are still being performed, and the trends show increased rates over the years – why is that?”
“Perhaps there are other factors here we need to explore: why do these procedures persist, and why, despite the Choosing Wisely campaign, [do] they continue to increase?” she said in an interview. “Maybe there is something we can learn here about patient and physician preferences that perhaps we should be paying more attention to.”
The study was published on Feb. 3 in JAMA Surgery.
For the analysis, Dr. Dossett and her colleagues evaluated surgical data from the National Cancer Database. They examined data from more than 1,500 surgical facilities and from surgeries involving 920,256 women in the United States who were diagnosed with breast cancer between 2004 and 2016.
The team focused on four procedures that have been determined to be of low value by Choosing Wisely, a campaign of the American Board of Internal Medicine Foundation, on the basis of recommendations of the American College of Surgeons, the Society for Surgical Oncology, and the American Society for Breast Surgeons.
The results show that, for two of the four low-value procedures, use declined significantly over the study period. These two procedures were axillary lymph node dissection for limited nodal disease, for patients undergoing lumpectomy and radiotherapy, and lumpectomy re-excision for patients whose surgical margins were close but were negative for invasive cancer.
Axillary lymph node dissection declined from 63% in 2004 to 14% in 2016. The steepest reduction was seen soon after data from the Z0011 study were published in 2010. The rates for this procedure halved in the following year, from 62% in 2010 to 31% in 2011 (P < .001).
Likewise, reoperation rates after lumpectomy dropped from 19% in 2004 to 15% in 2016. The sharpest decline, from 18% in 2013 to 16% in 2014, corresponded to the publishing of the SSO/ASTRO consensus statement, which designated a negative margin as having “no tumor on ink.”
Two of the four low-value procedures increased in use during the study period.
Rates of contralateral prophylactic mastectomy increased nearly 2.5-fold among women with unilateral breast cancer undergoing mastectomy, from 11% in 2004 to 26% in 2016, despite SSO guidelines issued in 2007 recommending that the procedure not be used for women at average risk.
In addition, rates of sentinel lymph node biopsy among women aged 70 years and older with clinically node-negative HR+ breast cancer increased from 78% in 2004 to 87% in 2012. There was no significant decline in the use of this procedure, even after the CALGB 9343 trial from the Cancer and Leukemia Group B showed no survival benefit in 2013.
Patterns at hospitals vary
The authors of the study also examined hospital factors, which can heavily influence choice of procedure.
These results showed that the greatest reductions of the low-value breast cancer procedures occurred at academic research programs and high-volume surgical facilities. Elsewhere, the rates varied widely.
Interfacility rates of axillary lymph node dissection ranged from 7% to 47%; lumpectomy reoperation rates ranged from 3% to 62%; contralateral prophylactic mastectomy rates ranged from 9% to 67%; and sentinel lymph node biopsy rates ranged from 25% to 97%.
Being an outlier for use of one procedure did not necessarily translate to nonconformity for others. Factors such as a hospital’s volume of breast cancer cases or the type of facility did not appear to influence rates of axillary lymph node dissection or lumpectomy reoperation.
However, the rates of contralateral prophylactic mastectomy were significantly higher in high-volume centers and integrated network cancer programs, compared with community cancer programs (23% vs. 2%; P < .001).
Dr. Dossett said the lack of consistency was somewhat unexpected.
“We expected we would find some facilities were constantly good or bad at deimplementation or that there would be stronger associations between certain facility characteristics and performance,” she said. “That really wasn’t the case, and most facilities had mixed performance.”
Evidence may or may not influence trends
The authors speculate on why the low-value designation is in some cases being ignored.
The evidence regarding the risk for lymphedema related to axillary lymph node dissection procedure appears to have helped reduce its use, they note.
However, surgeons have been much less convinced of benefits in omitting sentinel lymph node biopsy, either because they are unfamiliar with the recommendations to avoid the procedure, or they may feel the procedure adds only minimal time and risk to a patient’s operation, the authors explain.
Patients may be convinced to opt to omit sentinel lymph node biopsy if they are properly counseled regarding the risks and benefits of the procedure, Dr. Dossett commented.
Dr. Yao added that, for elderly patients, age can play an important role in sentinel node biopsy.
“Patients’ life expectancy has increased over the years, and node status may impact adjuvant therapy decisions for these patients, even chemotherapy decisions,” she said.
Pressure to continue to perform contralateral prophylactic mastectomy is believed to be significantly patient driven, Dr. Dossett noted.
“I ultimately think the best way to reduce contralateral prophylactic mastectomy is to encourage women with small cancers to undergo breast-conserving surgery, i.e., lumpectomy, instead of mastectomy,” she explained.
“Once the decision for mastectomy is made, there is often a great deal of momentum towards a contralateral prophylactic mastectomy.”
“Contralateral prophylactic mastectomy is a personal preference that many surgeons are willing to do for their patients,” Dr. Yao explained.
“Although no survival benefit has been demonstrated for this procedure, patients find many other benefits that have nothing to do with survival.”
The authors and Dr. Yao have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This is the first study to [evaluate] all four of the low-value breast cancer procedures at the same time and try to draw some conclusions on practice patterns across facilities,” said senior author Lesly A. Dossett, MD, MPH, Center for Health Outcomes and Policy, the University of Michigan, Ann Arbor.
The two low-value procedures that have increased in use are contralateral prophylactic mastectomy for average-risk women with unilateral cancer and sentinel lymph node biopsy for clinically node-negative women aged 70 years and older with hormone receptor–positive (HR+) cancer.
“This suggests that formal efforts to reduce low value care through dissemination of guidelines, education of patients or providers, or alignment of incentives will be necessary to achieve full deimplementation,” she told this news organization.
The researchers emphasize that the providing of services that have no clinically meaningful benefit is a national epidemic, costing the United States more than $100 billion dollars annually.
These trends are notable and likely reflect a broad range of factors, commented Katharine Yao, MD, chief of the division of surgical oncology at the NorthShore University HealthSystem, Evanston, Ill.
“I think the better message here is not so much that facilities are doing too many low-value procedures but more that these procedures are still being performed, and the trends show increased rates over the years – why is that?”
“Perhaps there are other factors here we need to explore: why do these procedures persist, and why, despite the Choosing Wisely campaign, [do] they continue to increase?” she said in an interview. “Maybe there is something we can learn here about patient and physician preferences that perhaps we should be paying more attention to.”
The study was published on Feb. 3 in JAMA Surgery.
For the analysis, Dr. Dossett and her colleagues evaluated surgical data from the National Cancer Database. They examined data from more than 1,500 surgical facilities and from surgeries involving 920,256 women in the United States who were diagnosed with breast cancer between 2004 and 2016.
The team focused on four procedures that have been determined to be of low value by Choosing Wisely, a campaign of the American Board of Internal Medicine Foundation, on the basis of recommendations of the American College of Surgeons, the Society for Surgical Oncology, and the American Society for Breast Surgeons.
The results show that, for two of the four low-value procedures, use declined significantly over the study period. These two procedures were axillary lymph node dissection for limited nodal disease, for patients undergoing lumpectomy and radiotherapy, and lumpectomy re-excision for patients whose surgical margins were close but were negative for invasive cancer.
Axillary lymph node dissection declined from 63% in 2004 to 14% in 2016. The steepest reduction was seen soon after data from the Z0011 study were published in 2010. The rates for this procedure halved in the following year, from 62% in 2010 to 31% in 2011 (P < .001).
Likewise, reoperation rates after lumpectomy dropped from 19% in 2004 to 15% in 2016. The sharpest decline, from 18% in 2013 to 16% in 2014, corresponded to the publishing of the SSO/ASTRO consensus statement, which designated a negative margin as having “no tumor on ink.”
Two of the four low-value procedures increased in use during the study period.
Rates of contralateral prophylactic mastectomy increased nearly 2.5-fold among women with unilateral breast cancer undergoing mastectomy, from 11% in 2004 to 26% in 2016, despite SSO guidelines issued in 2007 recommending that the procedure not be used for women at average risk.
In addition, rates of sentinel lymph node biopsy among women aged 70 years and older with clinically node-negative HR+ breast cancer increased from 78% in 2004 to 87% in 2012. There was no significant decline in the use of this procedure, even after the CALGB 9343 trial from the Cancer and Leukemia Group B showed no survival benefit in 2013.
Patterns at hospitals vary
The authors of the study also examined hospital factors, which can heavily influence choice of procedure.
These results showed that the greatest reductions of the low-value breast cancer procedures occurred at academic research programs and high-volume surgical facilities. Elsewhere, the rates varied widely.
Interfacility rates of axillary lymph node dissection ranged from 7% to 47%; lumpectomy reoperation rates ranged from 3% to 62%; contralateral prophylactic mastectomy rates ranged from 9% to 67%; and sentinel lymph node biopsy rates ranged from 25% to 97%.
Being an outlier for use of one procedure did not necessarily translate to nonconformity for others. Factors such as a hospital’s volume of breast cancer cases or the type of facility did not appear to influence rates of axillary lymph node dissection or lumpectomy reoperation.
However, the rates of contralateral prophylactic mastectomy were significantly higher in high-volume centers and integrated network cancer programs, compared with community cancer programs (23% vs. 2%; P < .001).
Dr. Dossett said the lack of consistency was somewhat unexpected.
“We expected we would find some facilities were constantly good or bad at deimplementation or that there would be stronger associations between certain facility characteristics and performance,” she said. “That really wasn’t the case, and most facilities had mixed performance.”
Evidence may or may not influence trends
The authors speculate on why the low-value designation is in some cases being ignored.
The evidence regarding the risk for lymphedema related to axillary lymph node dissection procedure appears to have helped reduce its use, they note.
However, surgeons have been much less convinced of benefits in omitting sentinel lymph node biopsy, either because they are unfamiliar with the recommendations to avoid the procedure, or they may feel the procedure adds only minimal time and risk to a patient’s operation, the authors explain.
Patients may be convinced to opt to omit sentinel lymph node biopsy if they are properly counseled regarding the risks and benefits of the procedure, Dr. Dossett commented.
Dr. Yao added that, for elderly patients, age can play an important role in sentinel node biopsy.
“Patients’ life expectancy has increased over the years, and node status may impact adjuvant therapy decisions for these patients, even chemotherapy decisions,” she said.
Pressure to continue to perform contralateral prophylactic mastectomy is believed to be significantly patient driven, Dr. Dossett noted.
“I ultimately think the best way to reduce contralateral prophylactic mastectomy is to encourage women with small cancers to undergo breast-conserving surgery, i.e., lumpectomy, instead of mastectomy,” she explained.
“Once the decision for mastectomy is made, there is often a great deal of momentum towards a contralateral prophylactic mastectomy.”
“Contralateral prophylactic mastectomy is a personal preference that many surgeons are willing to do for their patients,” Dr. Yao explained.
“Although no survival benefit has been demonstrated for this procedure, patients find many other benefits that have nothing to do with survival.”
The authors and Dr. Yao have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“This is the first study to [evaluate] all four of the low-value breast cancer procedures at the same time and try to draw some conclusions on practice patterns across facilities,” said senior author Lesly A. Dossett, MD, MPH, Center for Health Outcomes and Policy, the University of Michigan, Ann Arbor.
The two low-value procedures that have increased in use are contralateral prophylactic mastectomy for average-risk women with unilateral cancer and sentinel lymph node biopsy for clinically node-negative women aged 70 years and older with hormone receptor–positive (HR+) cancer.
“This suggests that formal efforts to reduce low value care through dissemination of guidelines, education of patients or providers, or alignment of incentives will be necessary to achieve full deimplementation,” she told this news organization.
The researchers emphasize that the providing of services that have no clinically meaningful benefit is a national epidemic, costing the United States more than $100 billion dollars annually.
These trends are notable and likely reflect a broad range of factors, commented Katharine Yao, MD, chief of the division of surgical oncology at the NorthShore University HealthSystem, Evanston, Ill.
“I think the better message here is not so much that facilities are doing too many low-value procedures but more that these procedures are still being performed, and the trends show increased rates over the years – why is that?”
“Perhaps there are other factors here we need to explore: why do these procedures persist, and why, despite the Choosing Wisely campaign, [do] they continue to increase?” she said in an interview. “Maybe there is something we can learn here about patient and physician preferences that perhaps we should be paying more attention to.”
The study was published on Feb. 3 in JAMA Surgery.
For the analysis, Dr. Dossett and her colleagues evaluated surgical data from the National Cancer Database. They examined data from more than 1,500 surgical facilities and from surgeries involving 920,256 women in the United States who were diagnosed with breast cancer between 2004 and 2016.
The team focused on four procedures that have been determined to be of low value by Choosing Wisely, a campaign of the American Board of Internal Medicine Foundation, on the basis of recommendations of the American College of Surgeons, the Society for Surgical Oncology, and the American Society for Breast Surgeons.
The results show that, for two of the four low-value procedures, use declined significantly over the study period. These two procedures were axillary lymph node dissection for limited nodal disease, for patients undergoing lumpectomy and radiotherapy, and lumpectomy re-excision for patients whose surgical margins were close but were negative for invasive cancer.
Axillary lymph node dissection declined from 63% in 2004 to 14% in 2016. The steepest reduction was seen soon after data from the Z0011 study were published in 2010. The rates for this procedure halved in the following year, from 62% in 2010 to 31% in 2011 (P < .001).
Likewise, reoperation rates after lumpectomy dropped from 19% in 2004 to 15% in 2016. The sharpest decline, from 18% in 2013 to 16% in 2014, corresponded to the publishing of the SSO/ASTRO consensus statement, which designated a negative margin as having “no tumor on ink.”
Two of the four low-value procedures increased in use during the study period.
Rates of contralateral prophylactic mastectomy increased nearly 2.5-fold among women with unilateral breast cancer undergoing mastectomy, from 11% in 2004 to 26% in 2016, despite SSO guidelines issued in 2007 recommending that the procedure not be used for women at average risk.
In addition, rates of sentinel lymph node biopsy among women aged 70 years and older with clinically node-negative HR+ breast cancer increased from 78% in 2004 to 87% in 2012. There was no significant decline in the use of this procedure, even after the CALGB 9343 trial from the Cancer and Leukemia Group B showed no survival benefit in 2013.
Patterns at hospitals vary
The authors of the study also examined hospital factors, which can heavily influence choice of procedure.
These results showed that the greatest reductions of the low-value breast cancer procedures occurred at academic research programs and high-volume surgical facilities. Elsewhere, the rates varied widely.
Interfacility rates of axillary lymph node dissection ranged from 7% to 47%; lumpectomy reoperation rates ranged from 3% to 62%; contralateral prophylactic mastectomy rates ranged from 9% to 67%; and sentinel lymph node biopsy rates ranged from 25% to 97%.
Being an outlier for use of one procedure did not necessarily translate to nonconformity for others. Factors such as a hospital’s volume of breast cancer cases or the type of facility did not appear to influence rates of axillary lymph node dissection or lumpectomy reoperation.
However, the rates of contralateral prophylactic mastectomy were significantly higher in high-volume centers and integrated network cancer programs, compared with community cancer programs (23% vs. 2%; P < .001).
Dr. Dossett said the lack of consistency was somewhat unexpected.
“We expected we would find some facilities were constantly good or bad at deimplementation or that there would be stronger associations between certain facility characteristics and performance,” she said. “That really wasn’t the case, and most facilities had mixed performance.”
Evidence may or may not influence trends
The authors speculate on why the low-value designation is in some cases being ignored.
The evidence regarding the risk for lymphedema related to axillary lymph node dissection procedure appears to have helped reduce its use, they note.
However, surgeons have been much less convinced of benefits in omitting sentinel lymph node biopsy, either because they are unfamiliar with the recommendations to avoid the procedure, or they may feel the procedure adds only minimal time and risk to a patient’s operation, the authors explain.
Patients may be convinced to opt to omit sentinel lymph node biopsy if they are properly counseled regarding the risks and benefits of the procedure, Dr. Dossett commented.
Dr. Yao added that, for elderly patients, age can play an important role in sentinel node biopsy.
“Patients’ life expectancy has increased over the years, and node status may impact adjuvant therapy decisions for these patients, even chemotherapy decisions,” she said.
Pressure to continue to perform contralateral prophylactic mastectomy is believed to be significantly patient driven, Dr. Dossett noted.
“I ultimately think the best way to reduce contralateral prophylactic mastectomy is to encourage women with small cancers to undergo breast-conserving surgery, i.e., lumpectomy, instead of mastectomy,” she explained.
“Once the decision for mastectomy is made, there is often a great deal of momentum towards a contralateral prophylactic mastectomy.”
“Contralateral prophylactic mastectomy is a personal preference that many surgeons are willing to do for their patients,” Dr. Yao explained.
“Although no survival benefit has been demonstrated for this procedure, patients find many other benefits that have nothing to do with survival.”
The authors and Dr. Yao have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.











