User login
Don’t Miss the Dx: A 24-Year-Old Man With Sudden-Onset Hematuria, Proteinuria, Edema, and Hypertension
Presentation
A 24-year-old man with no significant past medical history presents to urgent care with a 1-week history of sudden-onset dark urine, leg swelling, and unusually high blood pressure readings, with recent values around 160/100 mm Hg. Physical examination reveals pitting edema up to the mid-shins and mild periorbital edema, with an elevated blood pressure of 158/98 mm Hg. Past medical history was significant for frequent upper respiratory tract infections over the past year. Laboratory findings include hematuria, proteinuria, and a raised serum creatinine level at 1.8 mg/dL, indicating a reduced estimated glomerular filtration rate (eGFR) of 45 mL/min/1.73 m2. Other tests such as a complete blood count and comprehensive metabolic panel (except for creatinine and albumin) are within normal limits. Given these findings, the patient is referred to nephrology for further evaluation to determine the underlying cause of his renal symptoms.
Differential Diagnosis
A glomerular disease can be assumed to be present if the patient manifests glomerular hematuria, glomerular proteinuria, or both, such as in this patient.
Glomerulonephritis occurs due to inflammation in the glomeruli, which leads to blood in urine, variable degrees of protein in urine (sometimes in the nephrotic range), and white blood cells in urine without any urinary tract infection. Patients may also experience hypertension and kidney function impairment. Diagnoses to consider include:
- Postinfectious glomerulonephritis
- Crescentic glomerulonephritis
- Diffuse proliferative glomerulonephritis
- Glomerulonephritis associated with nonstreptococcal infection
- Membranoproliferative glomerulonephritis
- Membranous glomerulonephritis
- Poststreptococcal glomerulonephritis
- Rapidly Progressive glomerulonephritis
All patients presenting with proteinuria and hematuria should undergo a thorough evaluation for glomerular disease, which generally involves laboratory testing and, in most patients, a kidney biopsy to obtain a definitive diagnosis.
Diagnosis
This patient underwent a renal biopsy, which showed C3-dominant deposition by immunofluorescence; electron microscopy (EM) showed discontinuous, ill-defined intramembranous deposits; and mass spectrometry showed terminal complement components in C3 deposits. The patient was diagnosed with C3 glomerulonephritis (C3G).
The diagnosis of C3G is established by kidney biopsy demonstrating the characteristic findings on immunofluorescence microscopy or EM in a patient with suspected glomerulonephritis. In patients with biopsy-confirmed C3G, additional testing should be performed to help identify the underlying etiology of the glomerulopathy to help determine therapy.
For all patients diagnosed with C3G, especially those who are older than 50 years, it is important to rule out monoclonal gammopathy which can be done through various tests such as serum protein electrophoresis and immunofixation, serum free light chains, and urine protein electrophoresis and immunofixation. The presence of a paraprotein, including a monoclonal light chain, can activate the alternative complement cascade and may be responsible for the condition.
Expert opinion recommends a comprehensive complement evaluation for all C3 glomerulopathy patients, including overall complement activity assessment, serum levels measurement of complement proteins and their split products, and autoantibodies screening.
Complement evaluation may include:
- Serum C3 and C4
- Soluble C5b-9 (soluble membrane attack complex)
- Serum factor H
- Serum factor B, factor I, and membrane cofactor protein (MCP; CD46)
All patients with C3G should also undergo screening for autoantibodies:
- C3 nephritic factor (C3NeF)
- C5 nephritic factor (C5NeF)
- C4 nephritic factor (C4NeF)
- Other autoantibodies against factor H, factor B, and/or C3b
It is recommended that genetic testing be considered for patients with C3 glomerulopathy to screen for complement genes including C3, CFB, CFH, CFHR5, and CFI and copy number variations and rearrangements of the CFH-CFHR gene cluster. The value of genetic testing in the clinical setting is still being defined; however, it has been observed that patients with mutations in complement genes generally respond less favorably to mycophenolate mofetil (MMF) compared with those who are positive for nephritic factors.
Management
The patient was managed with an angiotensin-converting enzyme (ACE) inhibitor to treat proteinuria and hypertension and MMF for immunosuppression. Enrollment in a clinical trial of an investigational complement inhibitor was discussed with the patient.
Currently, there are no therapeutic agents specifically designed to target the underlying complement dysregulation that occurs in individuals with C3G, and an optimal treatment for C3 glomerulopathy has not been established.
Various nonspecific therapies have been used to treat C3G, including plasmapheresis, steroids, rituximab, cyclophosphamide, and MMF and have shown positive results. For patients with C3G who have a known genetic variant (eg, CFH mutation) or who have acute kidney injury, plasmapheresis and plasma exchange may be helpful. Using these agents judiciously and in conjunction with optimal blood pressure control is important for maximum benefit in treating C3G. When someone with end-stage renal disease (ESRD) caused by C3G chooses to have a kidney transplant, it is important to know that C3G is likely to return in almost all cases and is the leading cause of transplant failure in 50%-90% of recipients.
Prognosis
The prognosis of C3G varies and is affected by various clinical and histological factors. While some patients may have consistently low levels of protein in their urine and maintain stable kidney function over time, others may experience severe nephrotic syndrome or rapidly progressive glomerulonephritis, which often leads to a poor prognosis.
Progression to ESRD is a major complication of C3G, with approximately 70% of affected children and 30%-50% of adults reaching this stage. In addition, disease recurrence is common after kidney transplantation, with about 50% of patients experiencing allograft loss within 10 years. Predictive factors for disease progression, although not robustly established, include initial eGFR at diagnosis, percentage of tubular atrophy, and extent of interstitial fibrosis in the cortical area as observed on kidney biopsies.
Clinical Takeaways
For patients exhibiting symptoms like proteinuria and hematuria indicative of glomerulonephritis, a comprehensive evaluation including laboratory tests and a kidney biopsy is essential to confirm a C3G diagnosis through characteristic findings on immunofluorescence microscopy or electron microscopy.
Additional tests to rule out associated conditions like monoclonal gammopathy and comprehensive complement evaluation are also recommended to understand the underlying etiology and guide therapy.
Though there are no treatments specifically targeting the underlying complement dysregulation unique to C3G, nonspecific therapies like ACE inhibitors, immunosuppressants (eg, MMF), and plasmapheresis are commonly used.
Some anticomplement therapies are available or under investigation, which might offer more targeted intervention options.
The prognosis for patients with C3G can vary widely and factors such as initial eGFR, the extent of tubular atrophy, and interstitial fibrosis are important predictors of disease progression.
Dr. Alper is an associate professor, Nephrology, Tulane University School of Medicine, New Orleans, Louisiana. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Presentation
A 24-year-old man with no significant past medical history presents to urgent care with a 1-week history of sudden-onset dark urine, leg swelling, and unusually high blood pressure readings, with recent values around 160/100 mm Hg. Physical examination reveals pitting edema up to the mid-shins and mild periorbital edema, with an elevated blood pressure of 158/98 mm Hg. Past medical history was significant for frequent upper respiratory tract infections over the past year. Laboratory findings include hematuria, proteinuria, and a raised serum creatinine level at 1.8 mg/dL, indicating a reduced estimated glomerular filtration rate (eGFR) of 45 mL/min/1.73 m2. Other tests such as a complete blood count and comprehensive metabolic panel (except for creatinine and albumin) are within normal limits. Given these findings, the patient is referred to nephrology for further evaluation to determine the underlying cause of his renal symptoms.
Differential Diagnosis
A glomerular disease can be assumed to be present if the patient manifests glomerular hematuria, glomerular proteinuria, or both, such as in this patient.
Glomerulonephritis occurs due to inflammation in the glomeruli, which leads to blood in urine, variable degrees of protein in urine (sometimes in the nephrotic range), and white blood cells in urine without any urinary tract infection. Patients may also experience hypertension and kidney function impairment. Diagnoses to consider include:
- Postinfectious glomerulonephritis
- Crescentic glomerulonephritis
- Diffuse proliferative glomerulonephritis
- Glomerulonephritis associated with nonstreptococcal infection
- Membranoproliferative glomerulonephritis
- Membranous glomerulonephritis
- Poststreptococcal glomerulonephritis
- Rapidly Progressive glomerulonephritis
All patients presenting with proteinuria and hematuria should undergo a thorough evaluation for glomerular disease, which generally involves laboratory testing and, in most patients, a kidney biopsy to obtain a definitive diagnosis.
Diagnosis
This patient underwent a renal biopsy, which showed C3-dominant deposition by immunofluorescence; electron microscopy (EM) showed discontinuous, ill-defined intramembranous deposits; and mass spectrometry showed terminal complement components in C3 deposits. The patient was diagnosed with C3 glomerulonephritis (C3G).
The diagnosis of C3G is established by kidney biopsy demonstrating the characteristic findings on immunofluorescence microscopy or EM in a patient with suspected glomerulonephritis. In patients with biopsy-confirmed C3G, additional testing should be performed to help identify the underlying etiology of the glomerulopathy to help determine therapy.
For all patients diagnosed with C3G, especially those who are older than 50 years, it is important to rule out monoclonal gammopathy which can be done through various tests such as serum protein electrophoresis and immunofixation, serum free light chains, and urine protein electrophoresis and immunofixation. The presence of a paraprotein, including a monoclonal light chain, can activate the alternative complement cascade and may be responsible for the condition.
Expert opinion recommends a comprehensive complement evaluation for all C3 glomerulopathy patients, including overall complement activity assessment, serum levels measurement of complement proteins and their split products, and autoantibodies screening.
Complement evaluation may include:
- Serum C3 and C4
- Soluble C5b-9 (soluble membrane attack complex)
- Serum factor H
- Serum factor B, factor I, and membrane cofactor protein (MCP; CD46)
All patients with C3G should also undergo screening for autoantibodies:
- C3 nephritic factor (C3NeF)
- C5 nephritic factor (C5NeF)
- C4 nephritic factor (C4NeF)
- Other autoantibodies against factor H, factor B, and/or C3b
It is recommended that genetic testing be considered for patients with C3 glomerulopathy to screen for complement genes including C3, CFB, CFH, CFHR5, and CFI and copy number variations and rearrangements of the CFH-CFHR gene cluster. The value of genetic testing in the clinical setting is still being defined; however, it has been observed that patients with mutations in complement genes generally respond less favorably to mycophenolate mofetil (MMF) compared with those who are positive for nephritic factors.
Management
The patient was managed with an angiotensin-converting enzyme (ACE) inhibitor to treat proteinuria and hypertension and MMF for immunosuppression. Enrollment in a clinical trial of an investigational complement inhibitor was discussed with the patient.
Currently, there are no therapeutic agents specifically designed to target the underlying complement dysregulation that occurs in individuals with C3G, and an optimal treatment for C3 glomerulopathy has not been established.
Various nonspecific therapies have been used to treat C3G, including plasmapheresis, steroids, rituximab, cyclophosphamide, and MMF and have shown positive results. For patients with C3G who have a known genetic variant (eg, CFH mutation) or who have acute kidney injury, plasmapheresis and plasma exchange may be helpful. Using these agents judiciously and in conjunction with optimal blood pressure control is important for maximum benefit in treating C3G. When someone with end-stage renal disease (ESRD) caused by C3G chooses to have a kidney transplant, it is important to know that C3G is likely to return in almost all cases and is the leading cause of transplant failure in 50%-90% of recipients.
Prognosis
The prognosis of C3G varies and is affected by various clinical and histological factors. While some patients may have consistently low levels of protein in their urine and maintain stable kidney function over time, others may experience severe nephrotic syndrome or rapidly progressive glomerulonephritis, which often leads to a poor prognosis.
Progression to ESRD is a major complication of C3G, with approximately 70% of affected children and 30%-50% of adults reaching this stage. In addition, disease recurrence is common after kidney transplantation, with about 50% of patients experiencing allograft loss within 10 years. Predictive factors for disease progression, although not robustly established, include initial eGFR at diagnosis, percentage of tubular atrophy, and extent of interstitial fibrosis in the cortical area as observed on kidney biopsies.
Clinical Takeaways
For patients exhibiting symptoms like proteinuria and hematuria indicative of glomerulonephritis, a comprehensive evaluation including laboratory tests and a kidney biopsy is essential to confirm a C3G diagnosis through characteristic findings on immunofluorescence microscopy or electron microscopy.
Additional tests to rule out associated conditions like monoclonal gammopathy and comprehensive complement evaluation are also recommended to understand the underlying etiology and guide therapy.
Though there are no treatments specifically targeting the underlying complement dysregulation unique to C3G, nonspecific therapies like ACE inhibitors, immunosuppressants (eg, MMF), and plasmapheresis are commonly used.
Some anticomplement therapies are available or under investigation, which might offer more targeted intervention options.
The prognosis for patients with C3G can vary widely and factors such as initial eGFR, the extent of tubular atrophy, and interstitial fibrosis are important predictors of disease progression.
Dr. Alper is an associate professor, Nephrology, Tulane University School of Medicine, New Orleans, Louisiana. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Presentation
A 24-year-old man with no significant past medical history presents to urgent care with a 1-week history of sudden-onset dark urine, leg swelling, and unusually high blood pressure readings, with recent values around 160/100 mm Hg. Physical examination reveals pitting edema up to the mid-shins and mild periorbital edema, with an elevated blood pressure of 158/98 mm Hg. Past medical history was significant for frequent upper respiratory tract infections over the past year. Laboratory findings include hematuria, proteinuria, and a raised serum creatinine level at 1.8 mg/dL, indicating a reduced estimated glomerular filtration rate (eGFR) of 45 mL/min/1.73 m2. Other tests such as a complete blood count and comprehensive metabolic panel (except for creatinine and albumin) are within normal limits. Given these findings, the patient is referred to nephrology for further evaluation to determine the underlying cause of his renal symptoms.
Differential Diagnosis
A glomerular disease can be assumed to be present if the patient manifests glomerular hematuria, glomerular proteinuria, or both, such as in this patient.
Glomerulonephritis occurs due to inflammation in the glomeruli, which leads to blood in urine, variable degrees of protein in urine (sometimes in the nephrotic range), and white blood cells in urine without any urinary tract infection. Patients may also experience hypertension and kidney function impairment. Diagnoses to consider include:
- Postinfectious glomerulonephritis
- Crescentic glomerulonephritis
- Diffuse proliferative glomerulonephritis
- Glomerulonephritis associated with nonstreptococcal infection
- Membranoproliferative glomerulonephritis
- Membranous glomerulonephritis
- Poststreptococcal glomerulonephritis
- Rapidly Progressive glomerulonephritis
All patients presenting with proteinuria and hematuria should undergo a thorough evaluation for glomerular disease, which generally involves laboratory testing and, in most patients, a kidney biopsy to obtain a definitive diagnosis.
Diagnosis
This patient underwent a renal biopsy, which showed C3-dominant deposition by immunofluorescence; electron microscopy (EM) showed discontinuous, ill-defined intramembranous deposits; and mass spectrometry showed terminal complement components in C3 deposits. The patient was diagnosed with C3 glomerulonephritis (C3G).
The diagnosis of C3G is established by kidney biopsy demonstrating the characteristic findings on immunofluorescence microscopy or EM in a patient with suspected glomerulonephritis. In patients with biopsy-confirmed C3G, additional testing should be performed to help identify the underlying etiology of the glomerulopathy to help determine therapy.
For all patients diagnosed with C3G, especially those who are older than 50 years, it is important to rule out monoclonal gammopathy which can be done through various tests such as serum protein electrophoresis and immunofixation, serum free light chains, and urine protein electrophoresis and immunofixation. The presence of a paraprotein, including a monoclonal light chain, can activate the alternative complement cascade and may be responsible for the condition.
Expert opinion recommends a comprehensive complement evaluation for all C3 glomerulopathy patients, including overall complement activity assessment, serum levels measurement of complement proteins and their split products, and autoantibodies screening.
Complement evaluation may include:
- Serum C3 and C4
- Soluble C5b-9 (soluble membrane attack complex)
- Serum factor H
- Serum factor B, factor I, and membrane cofactor protein (MCP; CD46)
All patients with C3G should also undergo screening for autoantibodies:
- C3 nephritic factor (C3NeF)
- C5 nephritic factor (C5NeF)
- C4 nephritic factor (C4NeF)
- Other autoantibodies against factor H, factor B, and/or C3b
It is recommended that genetic testing be considered for patients with C3 glomerulopathy to screen for complement genes including C3, CFB, CFH, CFHR5, and CFI and copy number variations and rearrangements of the CFH-CFHR gene cluster. The value of genetic testing in the clinical setting is still being defined; however, it has been observed that patients with mutations in complement genes generally respond less favorably to mycophenolate mofetil (MMF) compared with those who are positive for nephritic factors.
Management
The patient was managed with an angiotensin-converting enzyme (ACE) inhibitor to treat proteinuria and hypertension and MMF for immunosuppression. Enrollment in a clinical trial of an investigational complement inhibitor was discussed with the patient.
Currently, there are no therapeutic agents specifically designed to target the underlying complement dysregulation that occurs in individuals with C3G, and an optimal treatment for C3 glomerulopathy has not been established.
Various nonspecific therapies have been used to treat C3G, including plasmapheresis, steroids, rituximab, cyclophosphamide, and MMF and have shown positive results. For patients with C3G who have a known genetic variant (eg, CFH mutation) or who have acute kidney injury, plasmapheresis and plasma exchange may be helpful. Using these agents judiciously and in conjunction with optimal blood pressure control is important for maximum benefit in treating C3G. When someone with end-stage renal disease (ESRD) caused by C3G chooses to have a kidney transplant, it is important to know that C3G is likely to return in almost all cases and is the leading cause of transplant failure in 50%-90% of recipients.
Prognosis
The prognosis of C3G varies and is affected by various clinical and histological factors. While some patients may have consistently low levels of protein in their urine and maintain stable kidney function over time, others may experience severe nephrotic syndrome or rapidly progressive glomerulonephritis, which often leads to a poor prognosis.
Progression to ESRD is a major complication of C3G, with approximately 70% of affected children and 30%-50% of adults reaching this stage. In addition, disease recurrence is common after kidney transplantation, with about 50% of patients experiencing allograft loss within 10 years. Predictive factors for disease progression, although not robustly established, include initial eGFR at diagnosis, percentage of tubular atrophy, and extent of interstitial fibrosis in the cortical area as observed on kidney biopsies.
Clinical Takeaways
For patients exhibiting symptoms like proteinuria and hematuria indicative of glomerulonephritis, a comprehensive evaluation including laboratory tests and a kidney biopsy is essential to confirm a C3G diagnosis through characteristic findings on immunofluorescence microscopy or electron microscopy.
Additional tests to rule out associated conditions like monoclonal gammopathy and comprehensive complement evaluation are also recommended to understand the underlying etiology and guide therapy.
Though there are no treatments specifically targeting the underlying complement dysregulation unique to C3G, nonspecific therapies like ACE inhibitors, immunosuppressants (eg, MMF), and plasmapheresis are commonly used.
Some anticomplement therapies are available or under investigation, which might offer more targeted intervention options.
The prognosis for patients with C3G can vary widely and factors such as initial eGFR, the extent of tubular atrophy, and interstitial fibrosis are important predictors of disease progression.
Dr. Alper is an associate professor, Nephrology, Tulane University School of Medicine, New Orleans, Louisiana. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three Keys to Longevity in Older Adults?
TOPLINE:
Physical performance significantly correlates with increased survival rates in individuals aged over 80 years.
METHODOLOGY:
- Researchers analyzed data from 195 participants in the ilSIRENTE study, a prospective cohort study in L’Aquila, Italy, that included men and women born before 1924. The analysis focused on participants aged < 85 years at the time of enrollment (mean age, 82 years).
- Physical performance was assessed via the Short Physical Performance Battery (SPPB), which tests balance, gait speed, and leg strength based on the ability to stand from a seated position in a chair.
- Based on SPPB scores, participants were classified as having severe, moderate, mild, or no functional impairment.
TAKEAWAY:
- About 21% of the participants lived to 95 years of age.
- Higher scores on the SPPB and faster gait speed were linked to a lower risk for mortality before that age.
- The average gait speed was 0.88 m/s among participants who lived to 95 years of age and 0.78 m/s for those who died at younger ages.
IN PRACTICE:
“Physical performance is ... a reliable metric for assessing mortality risk in octogenarians,” the authors of the study wrote. “Our findings, together with available evidence, support the view that physical performance is a primary target for interventions to enhance longevity and extend health span.”
SOURCE:
Stefano Cacciatore, MD, with Universita Cattolica del Sacro Cuore, in Rome, was the corresponding author on the paper. The study was published online on May 2, 2024, in Journal of the American Geriatrics Society.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Physical performance significantly correlates with increased survival rates in individuals aged over 80 years.
METHODOLOGY:
- Researchers analyzed data from 195 participants in the ilSIRENTE study, a prospective cohort study in L’Aquila, Italy, that included men and women born before 1924. The analysis focused on participants aged < 85 years at the time of enrollment (mean age, 82 years).
- Physical performance was assessed via the Short Physical Performance Battery (SPPB), which tests balance, gait speed, and leg strength based on the ability to stand from a seated position in a chair.
- Based on SPPB scores, participants were classified as having severe, moderate, mild, or no functional impairment.
TAKEAWAY:
- About 21% of the participants lived to 95 years of age.
- Higher scores on the SPPB and faster gait speed were linked to a lower risk for mortality before that age.
- The average gait speed was 0.88 m/s among participants who lived to 95 years of age and 0.78 m/s for those who died at younger ages.
IN PRACTICE:
“Physical performance is ... a reliable metric for assessing mortality risk in octogenarians,” the authors of the study wrote. “Our findings, together with available evidence, support the view that physical performance is a primary target for interventions to enhance longevity and extend health span.”
SOURCE:
Stefano Cacciatore, MD, with Universita Cattolica del Sacro Cuore, in Rome, was the corresponding author on the paper. The study was published online on May 2, 2024, in Journal of the American Geriatrics Society.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Physical performance significantly correlates with increased survival rates in individuals aged over 80 years.
METHODOLOGY:
- Researchers analyzed data from 195 participants in the ilSIRENTE study, a prospective cohort study in L’Aquila, Italy, that included men and women born before 1924. The analysis focused on participants aged < 85 years at the time of enrollment (mean age, 82 years).
- Physical performance was assessed via the Short Physical Performance Battery (SPPB), which tests balance, gait speed, and leg strength based on the ability to stand from a seated position in a chair.
- Based on SPPB scores, participants were classified as having severe, moderate, mild, or no functional impairment.
TAKEAWAY:
- About 21% of the participants lived to 95 years of age.
- Higher scores on the SPPB and faster gait speed were linked to a lower risk for mortality before that age.
- The average gait speed was 0.88 m/s among participants who lived to 95 years of age and 0.78 m/s for those who died at younger ages.
IN PRACTICE:
“Physical performance is ... a reliable metric for assessing mortality risk in octogenarians,” the authors of the study wrote. “Our findings, together with available evidence, support the view that physical performance is a primary target for interventions to enhance longevity and extend health span.”
SOURCE:
Stefano Cacciatore, MD, with Universita Cattolica del Sacro Cuore, in Rome, was the corresponding author on the paper. The study was published online on May 2, 2024, in Journal of the American Geriatrics Society.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Maternal Complication Risk Higher For Cesarean Deliveries With Low-Lying Placenta
SAN FRANCISCO — Patients with a low-lying placenta who underwent cesarean deliveries were at higher risk for multiple complications even if they did not have placenta previa, according to data presented at the annual meeting of the American College of Obstetricians and Gynecologists.
Rates of preterm delivery, postpartum hemorrhage, placenta accreta, and need for hysterectomy and transfusion were all significantly higher in patients with low-lying placenta than in patients without, Jacob Thomas, MD, of Advocate Aurora Health in Chicago, Illinois, and Ascension Illinois St. Alexius Medical Center in Hoffman Estates, reported at the meeting.
A low-lying placenta is defined as a placental edge less than 20 mm from the internal os but not covering it. Most studies looking at low-lying placentas, however, group them with placenta previa, making it difficult to know if there are differences in risk of adverse outcomes for those who don’t have placenta previa.
“These are not necessarily shocking findings, but it shows that even low-lying placentas have significant morbidity in and of themselves, not just when they’re lumped with placenta previas,” Dr. Thomas said in an interview. “This means, if you’re doing a C-section for a low-lying placenta, you probably want to treat it a lot like you would treat a placenta previa. You may have blood ready, whether or not you’re going to give it, and you’re going to be more prepared for those complications.”
Noting that approximately 30% of patients with low-lying placenta had preterm deliveries, Dr. Thomas added that these patients might need to be counseled differently as well. The researchers did not have data on how preterm the deliveries were — many could have been 35-37 weeks, for example — but “how you prepare those patients is different,” he said.
Breanna Bolivar, MD, MPH, an obgyn hospitalist at MAHEC Ob/Gyn Specialists in Asheville, North Carolina, said the findings confirm her experience in practice.
“Low-lying placentas are treated very similarly to placenta previas and the results seem similar to patients that have placenta previas,” Dr. Bolivar said in an interview. “In my practice, I treat patients with low-lying placenta the same as I do with placenta previa. I have the same risk factors in mind, and I prepare in the same way.”
The researchers conducted a retrospective analysis of all patients who underwent a cesarean delivery in the National Inpatient Sample from 2017 to 2019 through the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality. After excluding patients with placenta previa, the researchers compared outcomes among patients with ICD-10 codes for low-lying placenta to those of patients without low-lying placenta. The researchers specifically looked at preterm delivery, hemorrhage, hysterectomy, placenta accreta spectrum (PAS), sepsis, shock, disseminated intravascular coagulation, and blood transfusion.
Among 700,635 patients with cesarean deliveries in the database, 0.4% had low-lying placenta. These patients were more likely to be older, to be anemic, and to deliver at a large or urban teaching hospital. They were less likely to have public insurance or a previous cesarean.
After controlling for confounders that differed between the two populations, the researchers found a higher likelihood of all adverse maternal outcomes studied in patients with low-lying placenta (P < .05). These patients had three times greater risk for preterm delivery (adjusted odds ratio [aOR], 3.07; 95% CI, 2.81-3.35) and nearly three times greater risk for shock (aOR 2.55; 95% CI, 1.44-4.52), and transfusion (aOR, 2.56; 95% CI, 2.14-3.06).
Compared to those without low-lying placenta, risk for patients with low-lying placenta was even higher for hemorrhage (aOR, 8.87; 95% CI, 8.10-9.73), hysterectomy (aOR, 9.42; 95% CI, 7.11-12.47), and PAS (aOR, 13.41; 95% CI, 10.34-17.39).
Within the group with low-lying placenta, older patients were modestly, but significantly, more likely to have hemorrhage, hysterectomy, and PAS (aOR, 1.06 for all). The risk was more elevated and significant in patients with tobacco use for hemorrhage (aOR, 1.43), hysterectomy (aOR, 1.40), and PAS (aOR, 1.40). Patients with anemia were also significantly more likely to experience PAS (aOR, 1.34).
“Interestingly, in this population, prior cesarean was not associated with increased rates of hemorrhage or hysterectomy,” the researchers reported. The findings can also “help guide research in terms of questions for the future,” Dr. Thomas said, such as looking at complication rates for vaginal deliveries in people with low-lying placenta.
No external funding was noted, and the authors all had no disclosures. Dr. Bolivar had no disclosures.
SAN FRANCISCO — Patients with a low-lying placenta who underwent cesarean deliveries were at higher risk for multiple complications even if they did not have placenta previa, according to data presented at the annual meeting of the American College of Obstetricians and Gynecologists.
Rates of preterm delivery, postpartum hemorrhage, placenta accreta, and need for hysterectomy and transfusion were all significantly higher in patients with low-lying placenta than in patients without, Jacob Thomas, MD, of Advocate Aurora Health in Chicago, Illinois, and Ascension Illinois St. Alexius Medical Center in Hoffman Estates, reported at the meeting.
A low-lying placenta is defined as a placental edge less than 20 mm from the internal os but not covering it. Most studies looking at low-lying placentas, however, group them with placenta previa, making it difficult to know if there are differences in risk of adverse outcomes for those who don’t have placenta previa.
“These are not necessarily shocking findings, but it shows that even low-lying placentas have significant morbidity in and of themselves, not just when they’re lumped with placenta previas,” Dr. Thomas said in an interview. “This means, if you’re doing a C-section for a low-lying placenta, you probably want to treat it a lot like you would treat a placenta previa. You may have blood ready, whether or not you’re going to give it, and you’re going to be more prepared for those complications.”
Noting that approximately 30% of patients with low-lying placenta had preterm deliveries, Dr. Thomas added that these patients might need to be counseled differently as well. The researchers did not have data on how preterm the deliveries were — many could have been 35-37 weeks, for example — but “how you prepare those patients is different,” he said.
Breanna Bolivar, MD, MPH, an obgyn hospitalist at MAHEC Ob/Gyn Specialists in Asheville, North Carolina, said the findings confirm her experience in practice.
“Low-lying placentas are treated very similarly to placenta previas and the results seem similar to patients that have placenta previas,” Dr. Bolivar said in an interview. “In my practice, I treat patients with low-lying placenta the same as I do with placenta previa. I have the same risk factors in mind, and I prepare in the same way.”
The researchers conducted a retrospective analysis of all patients who underwent a cesarean delivery in the National Inpatient Sample from 2017 to 2019 through the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality. After excluding patients with placenta previa, the researchers compared outcomes among patients with ICD-10 codes for low-lying placenta to those of patients without low-lying placenta. The researchers specifically looked at preterm delivery, hemorrhage, hysterectomy, placenta accreta spectrum (PAS), sepsis, shock, disseminated intravascular coagulation, and blood transfusion.
Among 700,635 patients with cesarean deliveries in the database, 0.4% had low-lying placenta. These patients were more likely to be older, to be anemic, and to deliver at a large or urban teaching hospital. They were less likely to have public insurance or a previous cesarean.
After controlling for confounders that differed between the two populations, the researchers found a higher likelihood of all adverse maternal outcomes studied in patients with low-lying placenta (P < .05). These patients had three times greater risk for preterm delivery (adjusted odds ratio [aOR], 3.07; 95% CI, 2.81-3.35) and nearly three times greater risk for shock (aOR 2.55; 95% CI, 1.44-4.52), and transfusion (aOR, 2.56; 95% CI, 2.14-3.06).
Compared to those without low-lying placenta, risk for patients with low-lying placenta was even higher for hemorrhage (aOR, 8.87; 95% CI, 8.10-9.73), hysterectomy (aOR, 9.42; 95% CI, 7.11-12.47), and PAS (aOR, 13.41; 95% CI, 10.34-17.39).
Within the group with low-lying placenta, older patients were modestly, but significantly, more likely to have hemorrhage, hysterectomy, and PAS (aOR, 1.06 for all). The risk was more elevated and significant in patients with tobacco use for hemorrhage (aOR, 1.43), hysterectomy (aOR, 1.40), and PAS (aOR, 1.40). Patients with anemia were also significantly more likely to experience PAS (aOR, 1.34).
“Interestingly, in this population, prior cesarean was not associated with increased rates of hemorrhage or hysterectomy,” the researchers reported. The findings can also “help guide research in terms of questions for the future,” Dr. Thomas said, such as looking at complication rates for vaginal deliveries in people with low-lying placenta.
No external funding was noted, and the authors all had no disclosures. Dr. Bolivar had no disclosures.
SAN FRANCISCO — Patients with a low-lying placenta who underwent cesarean deliveries were at higher risk for multiple complications even if they did not have placenta previa, according to data presented at the annual meeting of the American College of Obstetricians and Gynecologists.
Rates of preterm delivery, postpartum hemorrhage, placenta accreta, and need for hysterectomy and transfusion were all significantly higher in patients with low-lying placenta than in patients without, Jacob Thomas, MD, of Advocate Aurora Health in Chicago, Illinois, and Ascension Illinois St. Alexius Medical Center in Hoffman Estates, reported at the meeting.
A low-lying placenta is defined as a placental edge less than 20 mm from the internal os but not covering it. Most studies looking at low-lying placentas, however, group them with placenta previa, making it difficult to know if there are differences in risk of adverse outcomes for those who don’t have placenta previa.
“These are not necessarily shocking findings, but it shows that even low-lying placentas have significant morbidity in and of themselves, not just when they’re lumped with placenta previas,” Dr. Thomas said in an interview. “This means, if you’re doing a C-section for a low-lying placenta, you probably want to treat it a lot like you would treat a placenta previa. You may have blood ready, whether or not you’re going to give it, and you’re going to be more prepared for those complications.”
Noting that approximately 30% of patients with low-lying placenta had preterm deliveries, Dr. Thomas added that these patients might need to be counseled differently as well. The researchers did not have data on how preterm the deliveries were — many could have been 35-37 weeks, for example — but “how you prepare those patients is different,” he said.
Breanna Bolivar, MD, MPH, an obgyn hospitalist at MAHEC Ob/Gyn Specialists in Asheville, North Carolina, said the findings confirm her experience in practice.
“Low-lying placentas are treated very similarly to placenta previas and the results seem similar to patients that have placenta previas,” Dr. Bolivar said in an interview. “In my practice, I treat patients with low-lying placenta the same as I do with placenta previa. I have the same risk factors in mind, and I prepare in the same way.”
The researchers conducted a retrospective analysis of all patients who underwent a cesarean delivery in the National Inpatient Sample from 2017 to 2019 through the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality. After excluding patients with placenta previa, the researchers compared outcomes among patients with ICD-10 codes for low-lying placenta to those of patients without low-lying placenta. The researchers specifically looked at preterm delivery, hemorrhage, hysterectomy, placenta accreta spectrum (PAS), sepsis, shock, disseminated intravascular coagulation, and blood transfusion.
Among 700,635 patients with cesarean deliveries in the database, 0.4% had low-lying placenta. These patients were more likely to be older, to be anemic, and to deliver at a large or urban teaching hospital. They were less likely to have public insurance or a previous cesarean.
After controlling for confounders that differed between the two populations, the researchers found a higher likelihood of all adverse maternal outcomes studied in patients with low-lying placenta (P < .05). These patients had three times greater risk for preterm delivery (adjusted odds ratio [aOR], 3.07; 95% CI, 2.81-3.35) and nearly three times greater risk for shock (aOR 2.55; 95% CI, 1.44-4.52), and transfusion (aOR, 2.56; 95% CI, 2.14-3.06).
Compared to those without low-lying placenta, risk for patients with low-lying placenta was even higher for hemorrhage (aOR, 8.87; 95% CI, 8.10-9.73), hysterectomy (aOR, 9.42; 95% CI, 7.11-12.47), and PAS (aOR, 13.41; 95% CI, 10.34-17.39).
Within the group with low-lying placenta, older patients were modestly, but significantly, more likely to have hemorrhage, hysterectomy, and PAS (aOR, 1.06 for all). The risk was more elevated and significant in patients with tobacco use for hemorrhage (aOR, 1.43), hysterectomy (aOR, 1.40), and PAS (aOR, 1.40). Patients with anemia were also significantly more likely to experience PAS (aOR, 1.34).
“Interestingly, in this population, prior cesarean was not associated with increased rates of hemorrhage or hysterectomy,” the researchers reported. The findings can also “help guide research in terms of questions for the future,” Dr. Thomas said, such as looking at complication rates for vaginal deliveries in people with low-lying placenta.
No external funding was noted, and the authors all had no disclosures. Dr. Bolivar had no disclosures.
FROM ACOG 2024
Treatments for Early HS Range From Topical Therapies to Laser Hair Removal
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
This can be challenging because to date, no Food and Drug Administration–approved treatments exist for early-stage HS and only two biologics exist for moderate to severe disease.
“For someone with occasional nodules and abscesses, we often use antibiotics and topical antiseptics,” Christopher Sayed, MD, a dermatologist at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill, told this news organization. “We may use these daily for weeks or months or just provide them to use for 1-2 weeks at a time for intermittent flares if a patient doesn’t want to take a pill every day,” he said. “For women, hormonal options like oral contraceptive pills and spironolactone can be a great option” if they don’t mind taking a daily pill.
Topical options that Jennifer L. Hsiao, MD, reaches for in her role as director of the HS clinic at the University of Southern California, Los Angeles, include chlorhexidine wash, topical clindamycin, and topical resorcinol. Systemic medications include oral antibiotics such as doxycycline or clindamycin, while hormonal options include oral contraceptives and/or spironolactone for women and finasteride for men.
Laser hair removal for both men and women can also help treat lesions and abscesses in the groin and axillae, since reducing hair follicles tends to result in fewer follicles that become inflamed and form nodules and abscesses over time, “but it requires multiple visits and not all patients have access to it,” Dr. Sayed said. “Once patients start to develop tunnels or scars or fail to respond to some of these other treatments, I am quick to open the conversation on biologics to help avoid progression and long-term need for surgery.”
Metformin Among Options to Consider
According to Dr. Hsiao, other treatment options to consider trying in patients with mild HS include metformin, “especially in patients who also have prediabetes, PCOS, or obesity;” isotretinoin if the patient has concomitant severe acne; botulinum toxin injections; apremilast or topical roflumilast, and antihyperhidrosis medications such as prescription aluminum chloride topicals, glycopyrronium wipes, and glycopyrrolate.
Recommending lifestyle modifications such as smoking cessation and weight loss for patients diagnosed with early-stage HS is “challenging,” Dr. Sayed said, “because the evidence on different triggers and lifestyle modifications isn’t very strong. There can also be a lot of stigmas around weight and smoking in HS, and it can alienate patients to go straight to these topics in the first visit.”
Many patients also ask what dietary changes they can make to improve their HS. “The most common things patients tend to bring up are dairy avoidance and reducing carbohydrates,” he said. “Supplements like zinc and turmeric are also frequently brought up by patients and some find them helpful. Once rapport is built, I may discuss smoking cessation as potentially helping prevent as much activity over time or weight loss as possibly helping improve response to treatments, but I don’t promise that these things always help since modifying them doesn’t always lead to improvement.”
Dr. Hsiao noted that existing research suggests that following a Mediterranean diet may benefit HS symptoms.
Early Data on Ruxolitinib Cream Promising
At the 2024 annual meeting of the American Academy of Dermatology, researchers reported on the results of a phase 2 study, which found that topical 1.5% ruxolitinib, a Janus kinase (JAK) inhibitor (currently FDA-approved for atopic dermatitis) was effective in reducing abscess and inflammatory nodule count in patients with mild HS. “There is a major need for this kind of option, and the early results are promising,” said Dr. Sayed, who was not involved with the study. “It’s very difficult to get this covered for patients currently since it is off label for HS. We’ve gotten it for a few patients, and one has really liked it, but it’s unclear how consistent the others were with their use, and their level of improvement was not clear to me.”
For mild HS, he added, “the most important area in which we’ve seen growing evidence is around hair removal lasers such as Nd:YAG and alexandrite lasers. Improving access for patients is a major priority in the coming years.”
According to Dr. Hsiao, other approaches being studied for treating mild HS include a topical aryl hydrocarbon receptor agonist known as AT193, and oral medications, such as phosphodiesterase-4 inhibitors. Laser therapies are also being studied, “such as fractional ablative CO2 laser therapy combined with topical triamcinolone,” she said. “However, the majority of ongoing HS trials are for moderate to severe disease, so there is certainly a need for more investigation into mild HS treatment approaches.”
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
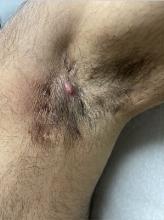
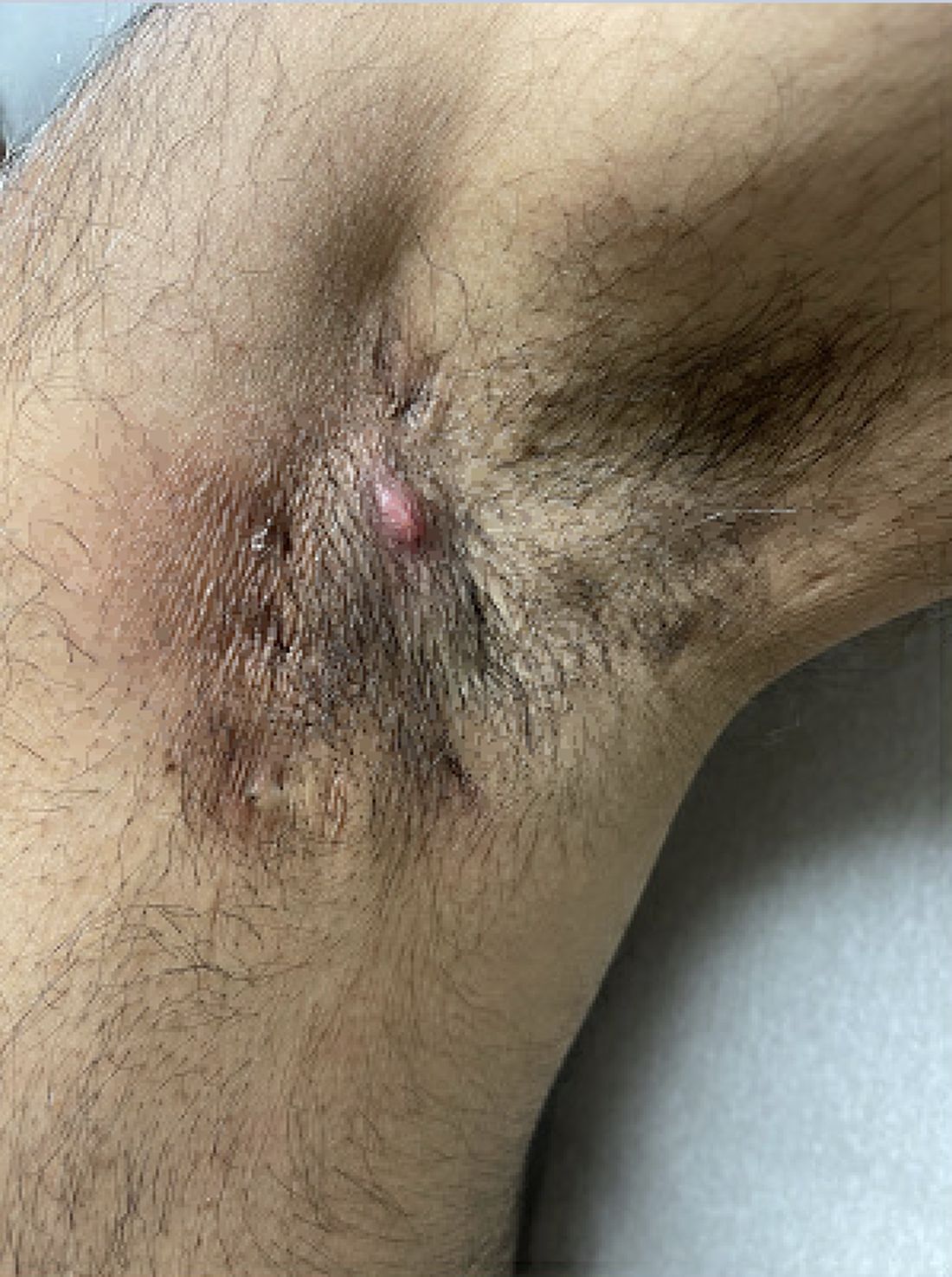
Diagnosing Mild Hidradenitis Suppurativa: Early Stage Can Mimic Other Diseases
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
, such as an infection, folliculitis, and acne.
According to 2019 guidelines from the United States and Canadian hidradenitis suppurativa foundations, the diagnostic criteria for HS in general are the presence of typical lesions such as abscesses, nodules, and tunnels in classic locations such as underarms, groins, and buttocks that recur over the course of at least 6 months. “There is no need for additional testing or imaging to make the diagnosis,” said Dr. Sayed, co-chair of the 2019 guidelines work group, who sees patients at the HS and Follicular Disorders Clinic at the University of North Carolina, Chapel Hill. “In many ways, the diagnosis should be very simple since the presentation is classic in most cases, though it can be confusing in the first 6 months or so.”
Persistence, Recurrence Major Clues
Prior to being diagnosed with Hurley stage I HS — characterized by recurrent nodules and abscesses with minimal scars, according to the guidelines — most people figure they’ve been getting recurrent Staphylococcus aureus infections or are having trouble with ingrown hairs from shaving, he continued. They may also say they get “boils” without an understanding of what has been causing them.
“Early HS can mimic an intense folliculitis or furuncles that can sometimes be caused by Staphylococcus infections, but the history of persistence or recurrence for months, despite treatment that should cover something like a Staph infection is a major clue,” Dr. Sayed said. “Thanks to improved resources on the internet, more patients, compared to several years ago, come in asking about HS after they’ve done their own research. As public awareness improves, hopefully this trend will grow, and patients will be diagnosed and treated earlier.” Family history is also a strong predictor of HS, since about half of patients have first-degree relatives who have a history of HS, he noted.
Clinicians can use the Hurley staging system to characterize the extent of disease and the Dermatology Life Quality Index to measure the impact of HS on quality of life. “We perform these assessments in our specialty clinic at each visit, but they are not necessary for diagnosis,” Dr. Sayed told this news organization.
The ‘2-2-6 Rule’
When she sees a patient who might have HS, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, follows the “2-2-6 rule,” which involves asking patients if they have had 2 episodes of 2 or more abscesses in 6 months. “If the patient answers yes, there’s a high likelihood that person has HS,” she said.
Hurley stage I HS is defined as nodules and abscesses without sinus tracts (tunnels) or scarring. But in Dr. Hsiao’s opinion, the Hurley staging system “is not the best way to characterize disease activity” because some patients meet criteria for Hurley stage I disease, meaning they do not have any scars or sinus tracts/tunnels, “but they have high disease activity with several inflammatory nodules and large painful abscesses that are limiting their quality of life and ability to function.”
Most cases of early-stage HS can be diagnosed in a single clinic visit, but some patients may present with a limited history of disease. For example, they may report having only had one episode of an axillary abscess or one episode of a few folliculitis-like papules in the groin. “In the absence of other physical exam findings suggestive of HS, such as open or double-headed comedones in flexural regions, I tell the patient that it is too early to call their condition HS, and I recommend that if they have another episode to call the office for an appointment for evaluation,” Dr. Hsiao said in an interview.
“What sets HS apart from an isolated incidence of a Staphylococcus aureus furuncle is the history of recurrence,” she added. To better characterize HS disease severity, she uses the six-point HS Physician Global Assessment score, a scale from 0 to 5, which classifies a patient as having moderate HS if they have five or more inflammatory nodules, or one abscess and one or more inflammatory nodule(s), without the requirement of demonstrating a scar or tunnel on a physical exam.
To help guide management decisions, Dr. Hsiao also considers asking patients with early-stage HS the following questions:
- Do you have a primary care provider (PCP)? PCPs are important care partners for patients with HS doctor to help screen for the comorbidities associated with the condition.
- What seems to make your HS worse? This can help identify potential triggers to avoid.
- What other medical conditions do you have?
- How would you describe the impact HS has on your quality of life?
- For women: Does your HS get worse around your period? “This can help to identify a potential hormonal trigger,” she said. “If the patient answers ‘yes,’ I would strongly consider a combined oral contraceptive pill and/or spironolactone as part of the patient’s treatment regimen.”
‘Window of Opportunity’ to Intervene
According to Dr. Hsiao, there has been a paradigm shift in the approach to HS management that emphasizes a “window of opportunity,” where earlier initiation of appropriate long-term immunomodulator therapy is recommended to try to mitigate disease progression. The development of tunnels and scars is a telltale sign that permanent tissue destruction is occurring, and the patient’s HS is no longer mild.
Ideally, a conversation about adalimumab, a tumor necrosis factor inhibitor, and secukinumab, an interleukin-17A antagonist (the two currently Food and Drug Administration–approved medications for HS, for moderate to severe disease/Hurley stage II/III) will have already been started with patients prior to development of a high tunnel or scar burden, signs of later-stage disease.
“Medications like this have the potential to slow and prevent that progression and reduce the surgical burden patients face over time, which is a major priority,” Dr. Sayed said. He noted that while comfort level with managing HS can vary among clinicians, “I’d encourage dermatologists to stay engaged with these patients because our training in the medical and surgical management of complex diseases like this is unmatched among other specialties,” he said. “Education of colleagues in other specialties should also be a big priority, especially for those in urgent care, emergency medicine, surgery, and ob.gyn. who often encounter these patients and may be less familiar” with HS.
Besides the North American clinical management guidelines for HS, which are expected to be updated in the next 18-24 months, as well as comorbidity screening recommendations for HS published in 2022, another resource Dr. Sayed and Dr. Hsiao recommend is the HS Foundation website, which features a link to Continuing Medical Education video lectures. The foundation also hosts an annual Symposium on HS Advances. This year’s event is scheduled in November in Austin, Texas.
Dr. Sayed disclosed that he is secretary of the HS Foundation and a member of the European HS Foundation. He has served as a consultant for AbbVie, Alumis, AstraZeneca, Incyte, InflaRx, Novartis, Sanofi, Sonoma Biotherapeutics, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Chemocentryx, Incyte, InflaRx, Novartis, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, Sanofi Regeneron, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
The Lure of Specialty Medicine Pulls Nurse Practitioners From Primary Care
For many patients, seeing a nurse practitioner has become a routine part of primary care, in which these “NPs” often perform the same tasks that patients have relied on doctors for.
But NPs in specialty care? That’s not routine, at least not yet. Increasingly, though, nurse practitioners and physician assistants are joining cardiology, dermatology, and other specialty practices, broadening their skills and increasing their income.
This development worries some people who track the health workforce, because current trends suggest primary care, which has counted on nurse practitioners to backstop physician shortages, soon might not be able to rely on them to the same extent.
“They’re succumbing to the same challenges that we have with physicians,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute at the Association of American Medical Colleges. The rates NPs can command in a specialty practice “are quite a bit higher” than practice salaries in primary care, he said.
When nurse practitioner programs began to proliferate in the 1970s, “at first it looked great, producing all these nurse practitioners that go to work with primary care physicians,” said Yalda Jabbarpour, MD, director of the American Academy of Family Physicians’ Robert Graham Center for Policy Studies. “But now only 30% are going into primary care.”
Dr. Jabbarpour was referring to the 2024 primary care scorecard by the Milbank Memorial Fund, which found that from 2016 to 2021 the proportion of nurse practitioners who worked in primary care practices hovered between 32% and 34%, even though their numbers grew rapidly. The proportion of physician assistants, also known as physician associates, in primary care ranged from 27% to 30%, the study found.
Both nurse practitioners and physician assistants are advanced practice clinicians who, in addition to graduate degrees, must complete distinct education, training, and certification steps. NPs can practice without a doctor’s supervision in more than two dozen states, while PAs have similar independence in only a handful of states.
About 88% of nurse practitioners are certified in an area of primary care, according to the American Association of Nurse Practitioners. But it is difficult to track exactly how many work in primary care or in specialty practices. Unlike physicians, they’re generally not required to be endorsed by a national standard-setting body to practice in specialties like oncology or cardiology, for example. The AANP declined to answer questions about its annual workforce survey or the extent to which primary care NPs are moving toward specialties.
Though data tracking the change is sparse, specialty practices are adding these advanced practice clinicians at almost the same rate as primary care practices, according to frequently cited research published in 2018.
The clearest evidence of the shift: From 2008 to 2016, there was a 22% increase in the number of specialty practices that employed nurse practitioners and physician assistants, according to that study. The increase in the number of primary care practices that employed these professionals was 24%.
Once more, the most recent projections by the Association of American Medical Colleges predict a dearth of at least 20,200 primary care physicians by 2036. There will also be a shortfall of non-primary care specialists, including a deficiency of at least 10,100 surgical physicians and up to 25,000 physicians in other specialties.
When it comes to the actual work performed, the lines between primary and specialty care are often blurred, said Candice Chen, MD, MPH, associate professor of health policy and management at George Washington University.
“You might be a nurse practitioner working in a gastroenterology clinic or cardiology clinic, but the scope of what you do is starting to overlap with primary care,” she said.
Nurse practitioners’ salaries vary widely by location, type of facility, and experience. Still, according to data from health care recruiter AMN Healthcare Physician Solutions, formerly known as Merritt Hawkins, the total annual average starting compensation, including signing bonus, for nurse practitioners and physician assistants in specialty practice was $172,544 in the year that ended March 31, slightly higher than the $166,544 for those in primary care.
According to forecasts from the federal Bureau of Labor Statistics, nurse practitioner jobs will increase faster than jobs in almost any other occupation in the decade leading up to 2032, growing by 123,600 jobs or 45%. (Wind turbine service technician is the only other occupation projected to grow as fast.) The growth rate for physician assistants is also much faster than average, at 27%. There are more than twice as many nurse practitioners as physician assistants, however: 323,900 versus 148,000, in 2022.
To Dr. Grover of the AAMC, numbers like this signal that there will probably be enough NPs, PAs, and physicians to meet primary care needs. At the same time, “expect more NPs and PAs to also flow out into other specialties,” he said.
When Pamela Ograbisz started working as a registered nurse 27 years ago, she worked in a cardiothoracic intensive care unit. After she became a family nurse practitioner a few years later, she found a job with a similar specialty practice, which trained her to take on a bigger role, first running their outpatient clinic, then working on the floor, and later in the intensive care unit.
If nurse practitioners want to specialize, often “the doctors mentor them just like they would with a physician residency,” said Ms. Ograbisz, now vice president of clinical operations at temporary placement recruiter LocumTenens.com.
If physician assistants want to specialize, they also can do so through mentoring, or they can receive “certificates of added qualifications” in 10 specialties to demonstrate their expertise. Most employers don’t “encourage or require” these certificates, however, said Jennifer Orozco, DMSc, PA-C, DFAAPA, chief medical officer at the American Academy of Physician Associates.
There are a number of training programs for family nurse practitioners who want to develop skills in other areas.
Raina Hoebelheinrich, 40, a family nurse practitioner at a regional medical center in Yankton, South Dakota, recently enrolled in a three-semester post-master’s endocrinology training program at Mount Marty University. She lives on a farm in nearby northeastern Nebraska with her husband and five sons.
Ms. Hoebelheinrich’s new skills could be helpful in her current hospital job, in which she sees a lot of patients with acute diabetes, or in a clinic setting like the one in Sioux Falls, South Dakota, where she is doing her clinical endocrinology training.
Lack of access to endocrinology care in rural areas is a real problem, and many people may travel hundreds of miles to see a specialist.
“There aren’t a lot of options,” she said.
A version of this article first appeared on Medscape.com.
For many patients, seeing a nurse practitioner has become a routine part of primary care, in which these “NPs” often perform the same tasks that patients have relied on doctors for.
But NPs in specialty care? That’s not routine, at least not yet. Increasingly, though, nurse practitioners and physician assistants are joining cardiology, dermatology, and other specialty practices, broadening their skills and increasing their income.
This development worries some people who track the health workforce, because current trends suggest primary care, which has counted on nurse practitioners to backstop physician shortages, soon might not be able to rely on them to the same extent.
“They’re succumbing to the same challenges that we have with physicians,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute at the Association of American Medical Colleges. The rates NPs can command in a specialty practice “are quite a bit higher” than practice salaries in primary care, he said.
When nurse practitioner programs began to proliferate in the 1970s, “at first it looked great, producing all these nurse practitioners that go to work with primary care physicians,” said Yalda Jabbarpour, MD, director of the American Academy of Family Physicians’ Robert Graham Center for Policy Studies. “But now only 30% are going into primary care.”
Dr. Jabbarpour was referring to the 2024 primary care scorecard by the Milbank Memorial Fund, which found that from 2016 to 2021 the proportion of nurse practitioners who worked in primary care practices hovered between 32% and 34%, even though their numbers grew rapidly. The proportion of physician assistants, also known as physician associates, in primary care ranged from 27% to 30%, the study found.
Both nurse practitioners and physician assistants are advanced practice clinicians who, in addition to graduate degrees, must complete distinct education, training, and certification steps. NPs can practice without a doctor’s supervision in more than two dozen states, while PAs have similar independence in only a handful of states.
About 88% of nurse practitioners are certified in an area of primary care, according to the American Association of Nurse Practitioners. But it is difficult to track exactly how many work in primary care or in specialty practices. Unlike physicians, they’re generally not required to be endorsed by a national standard-setting body to practice in specialties like oncology or cardiology, for example. The AANP declined to answer questions about its annual workforce survey or the extent to which primary care NPs are moving toward specialties.
Though data tracking the change is sparse, specialty practices are adding these advanced practice clinicians at almost the same rate as primary care practices, according to frequently cited research published in 2018.
The clearest evidence of the shift: From 2008 to 2016, there was a 22% increase in the number of specialty practices that employed nurse practitioners and physician assistants, according to that study. The increase in the number of primary care practices that employed these professionals was 24%.
Once more, the most recent projections by the Association of American Medical Colleges predict a dearth of at least 20,200 primary care physicians by 2036. There will also be a shortfall of non-primary care specialists, including a deficiency of at least 10,100 surgical physicians and up to 25,000 physicians in other specialties.
When it comes to the actual work performed, the lines between primary and specialty care are often blurred, said Candice Chen, MD, MPH, associate professor of health policy and management at George Washington University.
“You might be a nurse practitioner working in a gastroenterology clinic or cardiology clinic, but the scope of what you do is starting to overlap with primary care,” she said.
Nurse practitioners’ salaries vary widely by location, type of facility, and experience. Still, according to data from health care recruiter AMN Healthcare Physician Solutions, formerly known as Merritt Hawkins, the total annual average starting compensation, including signing bonus, for nurse practitioners and physician assistants in specialty practice was $172,544 in the year that ended March 31, slightly higher than the $166,544 for those in primary care.
According to forecasts from the federal Bureau of Labor Statistics, nurse practitioner jobs will increase faster than jobs in almost any other occupation in the decade leading up to 2032, growing by 123,600 jobs or 45%. (Wind turbine service technician is the only other occupation projected to grow as fast.) The growth rate for physician assistants is also much faster than average, at 27%. There are more than twice as many nurse practitioners as physician assistants, however: 323,900 versus 148,000, in 2022.
To Dr. Grover of the AAMC, numbers like this signal that there will probably be enough NPs, PAs, and physicians to meet primary care needs. At the same time, “expect more NPs and PAs to also flow out into other specialties,” he said.
When Pamela Ograbisz started working as a registered nurse 27 years ago, she worked in a cardiothoracic intensive care unit. After she became a family nurse practitioner a few years later, she found a job with a similar specialty practice, which trained her to take on a bigger role, first running their outpatient clinic, then working on the floor, and later in the intensive care unit.
If nurse practitioners want to specialize, often “the doctors mentor them just like they would with a physician residency,” said Ms. Ograbisz, now vice president of clinical operations at temporary placement recruiter LocumTenens.com.
If physician assistants want to specialize, they also can do so through mentoring, or they can receive “certificates of added qualifications” in 10 specialties to demonstrate their expertise. Most employers don’t “encourage or require” these certificates, however, said Jennifer Orozco, DMSc, PA-C, DFAAPA, chief medical officer at the American Academy of Physician Associates.
There are a number of training programs for family nurse practitioners who want to develop skills in other areas.
Raina Hoebelheinrich, 40, a family nurse practitioner at a regional medical center in Yankton, South Dakota, recently enrolled in a three-semester post-master’s endocrinology training program at Mount Marty University. She lives on a farm in nearby northeastern Nebraska with her husband and five sons.
Ms. Hoebelheinrich’s new skills could be helpful in her current hospital job, in which she sees a lot of patients with acute diabetes, or in a clinic setting like the one in Sioux Falls, South Dakota, where she is doing her clinical endocrinology training.
Lack of access to endocrinology care in rural areas is a real problem, and many people may travel hundreds of miles to see a specialist.
“There aren’t a lot of options,” she said.
A version of this article first appeared on Medscape.com.
For many patients, seeing a nurse practitioner has become a routine part of primary care, in which these “NPs” often perform the same tasks that patients have relied on doctors for.
But NPs in specialty care? That’s not routine, at least not yet. Increasingly, though, nurse practitioners and physician assistants are joining cardiology, dermatology, and other specialty practices, broadening their skills and increasing their income.
This development worries some people who track the health workforce, because current trends suggest primary care, which has counted on nurse practitioners to backstop physician shortages, soon might not be able to rely on them to the same extent.
“They’re succumbing to the same challenges that we have with physicians,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute at the Association of American Medical Colleges. The rates NPs can command in a specialty practice “are quite a bit higher” than practice salaries in primary care, he said.
When nurse practitioner programs began to proliferate in the 1970s, “at first it looked great, producing all these nurse practitioners that go to work with primary care physicians,” said Yalda Jabbarpour, MD, director of the American Academy of Family Physicians’ Robert Graham Center for Policy Studies. “But now only 30% are going into primary care.”
Dr. Jabbarpour was referring to the 2024 primary care scorecard by the Milbank Memorial Fund, which found that from 2016 to 2021 the proportion of nurse practitioners who worked in primary care practices hovered between 32% and 34%, even though their numbers grew rapidly. The proportion of physician assistants, also known as physician associates, in primary care ranged from 27% to 30%, the study found.
Both nurse practitioners and physician assistants are advanced practice clinicians who, in addition to graduate degrees, must complete distinct education, training, and certification steps. NPs can practice without a doctor’s supervision in more than two dozen states, while PAs have similar independence in only a handful of states.
About 88% of nurse practitioners are certified in an area of primary care, according to the American Association of Nurse Practitioners. But it is difficult to track exactly how many work in primary care or in specialty practices. Unlike physicians, they’re generally not required to be endorsed by a national standard-setting body to practice in specialties like oncology or cardiology, for example. The AANP declined to answer questions about its annual workforce survey or the extent to which primary care NPs are moving toward specialties.
Though data tracking the change is sparse, specialty practices are adding these advanced practice clinicians at almost the same rate as primary care practices, according to frequently cited research published in 2018.
The clearest evidence of the shift: From 2008 to 2016, there was a 22% increase in the number of specialty practices that employed nurse practitioners and physician assistants, according to that study. The increase in the number of primary care practices that employed these professionals was 24%.
Once more, the most recent projections by the Association of American Medical Colleges predict a dearth of at least 20,200 primary care physicians by 2036. There will also be a shortfall of non-primary care specialists, including a deficiency of at least 10,100 surgical physicians and up to 25,000 physicians in other specialties.
When it comes to the actual work performed, the lines between primary and specialty care are often blurred, said Candice Chen, MD, MPH, associate professor of health policy and management at George Washington University.
“You might be a nurse practitioner working in a gastroenterology clinic or cardiology clinic, but the scope of what you do is starting to overlap with primary care,” she said.
Nurse practitioners’ salaries vary widely by location, type of facility, and experience. Still, according to data from health care recruiter AMN Healthcare Physician Solutions, formerly known as Merritt Hawkins, the total annual average starting compensation, including signing bonus, for nurse practitioners and physician assistants in specialty practice was $172,544 in the year that ended March 31, slightly higher than the $166,544 for those in primary care.
According to forecasts from the federal Bureau of Labor Statistics, nurse practitioner jobs will increase faster than jobs in almost any other occupation in the decade leading up to 2032, growing by 123,600 jobs or 45%. (Wind turbine service technician is the only other occupation projected to grow as fast.) The growth rate for physician assistants is also much faster than average, at 27%. There are more than twice as many nurse practitioners as physician assistants, however: 323,900 versus 148,000, in 2022.
To Dr. Grover of the AAMC, numbers like this signal that there will probably be enough NPs, PAs, and physicians to meet primary care needs. At the same time, “expect more NPs and PAs to also flow out into other specialties,” he said.
When Pamela Ograbisz started working as a registered nurse 27 years ago, she worked in a cardiothoracic intensive care unit. After she became a family nurse practitioner a few years later, she found a job with a similar specialty practice, which trained her to take on a bigger role, first running their outpatient clinic, then working on the floor, and later in the intensive care unit.
If nurse practitioners want to specialize, often “the doctors mentor them just like they would with a physician residency,” said Ms. Ograbisz, now vice president of clinical operations at temporary placement recruiter LocumTenens.com.
If physician assistants want to specialize, they also can do so through mentoring, or they can receive “certificates of added qualifications” in 10 specialties to demonstrate their expertise. Most employers don’t “encourage or require” these certificates, however, said Jennifer Orozco, DMSc, PA-C, DFAAPA, chief medical officer at the American Academy of Physician Associates.
There are a number of training programs for family nurse practitioners who want to develop skills in other areas.
Raina Hoebelheinrich, 40, a family nurse practitioner at a regional medical center in Yankton, South Dakota, recently enrolled in a three-semester post-master’s endocrinology training program at Mount Marty University. She lives on a farm in nearby northeastern Nebraska with her husband and five sons.
Ms. Hoebelheinrich’s new skills could be helpful in her current hospital job, in which she sees a lot of patients with acute diabetes, or in a clinic setting like the one in Sioux Falls, South Dakota, where she is doing her clinical endocrinology training.
Lack of access to endocrinology care in rural areas is a real problem, and many people may travel hundreds of miles to see a specialist.
“There aren’t a lot of options,” she said.
A version of this article first appeared on Medscape.com.
Severe Maternal Morbidity Can Adversely Affect Mental Health
TOPLINE:
Individuals with severe maternal morbidity (SMM) are at an increased risk for mental health condition–related hospitalization or emergency department (ED) visits up to 13 years after delivery.
METHODOLOGY:
- This retrospective cohort study compared mental health hospitalizations and ED visits in postpartum individuals with and without SMM over 13 years after delivery from April 2008 to March 2021.
- The study analyzed 1,579,392 individuals aged 18-55 years with a first recorded liveborn or stillborn delivery from a pregnancy lasting 20-43 weeks, of which 35,825 (2.3%) had exposure to SMM.
- The SMM exposure was analyzed for events occurring after 20 weeks’ gestation and up to 42 days after delivery hospital discharge in the first recorded birth; those without SMM were considered unexposed.
- The main outcome was a combination of mental health hospitalizations or ED visits occurring at least 43 days after the index birth hospitalization.
TAKEAWAY:
- Individuals with SMM had a 1.3-fold increased risk of mental health hospitalizations or ED visits.
- The hospital or ED visits per 10,000 person-years were 59.2 for mood and anxiety disorders, 17.1 for substance abuse and related disorders, 4.8 for suicidality or self-harm, and 4.1 for schizophrenia spectrum or other psychotic disorders.
- Following SMM, an elevated risk was observed for all mental health outcomes except one (schizophrenia spectrum and other psychotic disorders), with the highest risk seen for suicidality and self-harm (aHR, 1.54).
IN PRACTICE:
“Knowledge of the short- and long-term risks of serious mental health conditions after SMM and its subtypes could inform the need for enhanced postpartum supportive resources,” the authors wrote.
SOURCE:
This study was led by Asia Blackman, MSc, Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Québec, Canada. It was published online in JAMA Network Open.
LIMITATIONS:
The study is limited by its observational design, missing data, and misclassification bias.
DISCLOSURES:
This study was supported by funding from the Canadian Institutes of Health Research. Three authors reported receiving personal fees or grants outside the submitted work. No other conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Individuals with severe maternal morbidity (SMM) are at an increased risk for mental health condition–related hospitalization or emergency department (ED) visits up to 13 years after delivery.
METHODOLOGY:
- This retrospective cohort study compared mental health hospitalizations and ED visits in postpartum individuals with and without SMM over 13 years after delivery from April 2008 to March 2021.
- The study analyzed 1,579,392 individuals aged 18-55 years with a first recorded liveborn or stillborn delivery from a pregnancy lasting 20-43 weeks, of which 35,825 (2.3%) had exposure to SMM.
- The SMM exposure was analyzed for events occurring after 20 weeks’ gestation and up to 42 days after delivery hospital discharge in the first recorded birth; those without SMM were considered unexposed.
- The main outcome was a combination of mental health hospitalizations or ED visits occurring at least 43 days after the index birth hospitalization.
TAKEAWAY:
- Individuals with SMM had a 1.3-fold increased risk of mental health hospitalizations or ED visits.
- The hospital or ED visits per 10,000 person-years were 59.2 for mood and anxiety disorders, 17.1 for substance abuse and related disorders, 4.8 for suicidality or self-harm, and 4.1 for schizophrenia spectrum or other psychotic disorders.
- Following SMM, an elevated risk was observed for all mental health outcomes except one (schizophrenia spectrum and other psychotic disorders), with the highest risk seen for suicidality and self-harm (aHR, 1.54).
IN PRACTICE:
“Knowledge of the short- and long-term risks of serious mental health conditions after SMM and its subtypes could inform the need for enhanced postpartum supportive resources,” the authors wrote.
SOURCE:
This study was led by Asia Blackman, MSc, Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Québec, Canada. It was published online in JAMA Network Open.
LIMITATIONS:
The study is limited by its observational design, missing data, and misclassification bias.
DISCLOSURES:
This study was supported by funding from the Canadian Institutes of Health Research. Three authors reported receiving personal fees or grants outside the submitted work. No other conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Individuals with severe maternal morbidity (SMM) are at an increased risk for mental health condition–related hospitalization or emergency department (ED) visits up to 13 years after delivery.
METHODOLOGY:
- This retrospective cohort study compared mental health hospitalizations and ED visits in postpartum individuals with and without SMM over 13 years after delivery from April 2008 to March 2021.
- The study analyzed 1,579,392 individuals aged 18-55 years with a first recorded liveborn or stillborn delivery from a pregnancy lasting 20-43 weeks, of which 35,825 (2.3%) had exposure to SMM.
- The SMM exposure was analyzed for events occurring after 20 weeks’ gestation and up to 42 days after delivery hospital discharge in the first recorded birth; those without SMM were considered unexposed.
- The main outcome was a combination of mental health hospitalizations or ED visits occurring at least 43 days after the index birth hospitalization.
TAKEAWAY:
- Individuals with SMM had a 1.3-fold increased risk of mental health hospitalizations or ED visits.
- The hospital or ED visits per 10,000 person-years were 59.2 for mood and anxiety disorders, 17.1 for substance abuse and related disorders, 4.8 for suicidality or self-harm, and 4.1 for schizophrenia spectrum or other psychotic disorders.
- Following SMM, an elevated risk was observed for all mental health outcomes except one (schizophrenia spectrum and other psychotic disorders), with the highest risk seen for suicidality and self-harm (aHR, 1.54).
IN PRACTICE:
“Knowledge of the short- and long-term risks of serious mental health conditions after SMM and its subtypes could inform the need for enhanced postpartum supportive resources,” the authors wrote.
SOURCE:
This study was led by Asia Blackman, MSc, Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Québec, Canada. It was published online in JAMA Network Open.
LIMITATIONS:
The study is limited by its observational design, missing data, and misclassification bias.
DISCLOSURES:
This study was supported by funding from the Canadian Institutes of Health Research. Three authors reported receiving personal fees or grants outside the submitted work. No other conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Statin Use May Extend Life for Early Breast Cancer Patients
Previous research examining the association between cholesterol and breast cancer metabolism suggests that cholesterol-lowering medications such as statins may improve outcomes in breast cancer patients, Sixten Harborg, a medical student and PhD student at Aarhus University, Denmark, said in a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
In addition, cardiovascular-related death is the second most common cause of death for breast cancer survivors, and given the survival rates in early breast cancer, there is a demand for cardioprotective initiatives and maintenance of cardioprotective drugs after diagnosis, he said in an interview.
What Is Known About Statins and Breast Cancer?
Statins are the most common drugs used to lower cholesterol and may deprive tumor cells of the cholesterol needed for cell membrane synthesis, Mr. Harborg said in his presentation.
Data from a randomized trial published in the Journal of Clinical Oncology in 2017 showed significantly improved disease-free survival, breast cancer–free interval, and distant recurrence–free interval in early stage breast cancer patients randomized to cholesterol-lowering medication vs. those who did not receive cholesterol-lowering medication.
The 2017 study prompted the creation of the MASTER study, a randomized, multicenter, double-blind, placebo-controlled trial comparing standard adjuvant therapy plus placebo to standard adjuvant therapy plus atorvastatin in patients with early breast cancer (NCT04601116), Mr. Harborg said. The MASTER trial is currently recruiting patients in Denmark.
How Was the Current Study Designed?
To provide preliminary analysis, Mr. Harborg and colleagues used an emulation trial design based on electronic health care data from 110,160 females with a diagnosis of stage I, II, or III breast cancer who were part of the Danish Breast Cancer Group, a national clinical registry in Denmark, between 2000 and 2020.
As defined in the European Journal of Epidemiology in 2017, target trial emulation involves application of randomized trial designs to observational data with the goal of improving the quality of observational epidemiology when a comparator trial is not yet available.
The researchers created a cohort of patients based on electronic health care data to simulate a target trial of the use of atorvastatin after breast cancer diagnosis. Patients were randomized to one of two treatment strategies: starting to use statins within 36 months of diagnosis, or not using statins. The primary outcome was death from breast cancer. The follow-up for the MASTER study starts with inclusion and ends with death, emigration from Denmark, end of clinical follow-up, or 10 years of follow-up (whichever comes first); the follow-up was the same in the current study.
The researchers calculated hazard ratios (HR) of breast cancer mortality in statin users vs. non–statin users and used a technique known as inverse-probability of censoring-weighting (IPCW) to estimate the effects of statin use based on prognostic factors.
What Did the Results Show?
The results favored statin use for improved survival in early breast cancer patients, Mr. Harborg said. Overall, the hazard ratio for breast cancer mortality was 0.96 in statin users compared with non–statin users, and was similar in both a Cox regression analysis (HR 0.81), and in a 10-year landmark analysis (HR 0.86).
The difference in mortality between statin and non–statin users was even stronger in patients who were receiving adjuvant chemotherapy (HR 0.94, 0.64, and 0.76 on the IPCW, Cox, and landmark analyses, respectively).
The results were in line with previous reports of statins’ effect on breast cancer survival, Mr. Harborg said in an interview.
“We believe the results encourage the continuous effort of the currently enrolling MASTER trial,” he said.
The results also suggest that deprescribing statins at the time of breast cancer diagnosis is not recommended, and that statin treatment can safely be prescribed to breast cancer patients with increased cardiovascular disease risk and/or dyslipidemia, Mr. Harborg said in the interview.
What Is the Takeaway Message for Clinical Practice?
“The clinical takeaway from our study is that statin use is associated with reduced risk of dying from breast cancer, but that it is not possible to determine the true effect of statins on breast cancer survival without a randomized, placebo-controlled trial,” Mr. Harborg told this publication. “Statins are inexpensive and well-tolerated drugs and may have a beneficial effect in terms of survival for breast cancer patients. However, with the current level of evidence [because the MASTER study is ongoing], we still cannot recommend that oncologists prescribe statins to prevent mortality from breast cancer,” he said.
What Are the Next Steps for Research?
The findings were limited by the study design, and real-world data are needed, Dr. Harborg said. Other limitations include the presence of residual bias, and the use of data based on prescription codes, but these were not considered to have an effect on the main conclusion of the study, Mr. Harborg said in the interview.
However, the results suggest that the addition of statins may improve outcomes for early breast cancer patients, especially when used with chemotherapy, and support the value of the ongoing MASTER study, he concluded.
Ultimately, the MASTER study will provide a more definitive answer to the question of whether statins should be added to the adjuvant treatment regimen of breast cancer to improve breast cancer outcomes, he said.
What Do Clinicians Think of the Study?
The current study is timely and highlights the need for phase 3 trials to examine the potential of statin use for breast cancer outcomes, Malinda T. West, MD, a medical oncologist and breast oncologist at the University of Wisconsin Carbone Cancer Center, Madison, said in an interview.
Questions for future research include whether statins can be used in combination with adjuvant abemaciclib if indicated, or how to best sequence these agents, said Dr. West, who was not involved in the study. Other questions raised by the current study include whether other cholesterol-lowering agents have a potential adjuvant benefit in reducing breast cancer recurrent and/or mortality, and whether the addition of statins would benefit subgroups such as HER2+ and triple negative breast cancer, she said.
“I was not surprised to see another study reporting benefit with statins and reduced risk of breast cancer recurrence and/or mortality, but I think the larger question is defining the subgroups who benefit the most, and identifying predictors for benefit or resistance,” Dr. West said in an interview.
Previous studies have shown that cholesterol elevation, specifically LDL levels, can be linked to increased tumor growth in breast cancer, so the lower mortality risk associated with lipid-lowering therapies in the current study was consistent, Peyton L. Reves, MD, a hematology/oncology fellow, also at the University of Wisconsin, said in an interview. In practice, data from the current study and previous research could be especially useful for patients with elevated LDL levels, said Dr. Reves, who was not involved in the study.
“These results could impact clinical practice in many ways, including leading to routine cholesterol monitoring in breast cancer patients on adjuvant therapy as well as the addition of lipid-lowering therapy with statins in these patients,” Dr. Reves said.
The findings showing particular benefit for patients on adjuvant chemotherapy highlight the need for more research on this specific population and the effect of statins on overall breast cancer mortality, to explore the extent to which the results of the current study were driven by the benefit seen in patients receiving adjuvant chemotherapy, Dr. Reves said.
The study was supported by Director Michael Hermann Nielsen’s Memorial Grant, Manufacturer Einar Willumsen’s Memorial Grant, Astrid Thaysen’s Grant for Medical Basic Research, Eva and Henry Fraenkel’s Memorial Fund, and the Novo Nordisk Foundation.
The researchers had no financial conflicts to disclose. Dr. West and Dr. Reves had no financial conflicts to disclose.
Previous research examining the association between cholesterol and breast cancer metabolism suggests that cholesterol-lowering medications such as statins may improve outcomes in breast cancer patients, Sixten Harborg, a medical student and PhD student at Aarhus University, Denmark, said in a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
In addition, cardiovascular-related death is the second most common cause of death for breast cancer survivors, and given the survival rates in early breast cancer, there is a demand for cardioprotective initiatives and maintenance of cardioprotective drugs after diagnosis, he said in an interview.
What Is Known About Statins and Breast Cancer?
Statins are the most common drugs used to lower cholesterol and may deprive tumor cells of the cholesterol needed for cell membrane synthesis, Mr. Harborg said in his presentation.
Data from a randomized trial published in the Journal of Clinical Oncology in 2017 showed significantly improved disease-free survival, breast cancer–free interval, and distant recurrence–free interval in early stage breast cancer patients randomized to cholesterol-lowering medication vs. those who did not receive cholesterol-lowering medication.
The 2017 study prompted the creation of the MASTER study, a randomized, multicenter, double-blind, placebo-controlled trial comparing standard adjuvant therapy plus placebo to standard adjuvant therapy plus atorvastatin in patients with early breast cancer (NCT04601116), Mr. Harborg said. The MASTER trial is currently recruiting patients in Denmark.
How Was the Current Study Designed?
To provide preliminary analysis, Mr. Harborg and colleagues used an emulation trial design based on electronic health care data from 110,160 females with a diagnosis of stage I, II, or III breast cancer who were part of the Danish Breast Cancer Group, a national clinical registry in Denmark, between 2000 and 2020.
As defined in the European Journal of Epidemiology in 2017, target trial emulation involves application of randomized trial designs to observational data with the goal of improving the quality of observational epidemiology when a comparator trial is not yet available.
The researchers created a cohort of patients based on electronic health care data to simulate a target trial of the use of atorvastatin after breast cancer diagnosis. Patients were randomized to one of two treatment strategies: starting to use statins within 36 months of diagnosis, or not using statins. The primary outcome was death from breast cancer. The follow-up for the MASTER study starts with inclusion and ends with death, emigration from Denmark, end of clinical follow-up, or 10 years of follow-up (whichever comes first); the follow-up was the same in the current study.
The researchers calculated hazard ratios (HR) of breast cancer mortality in statin users vs. non–statin users and used a technique known as inverse-probability of censoring-weighting (IPCW) to estimate the effects of statin use based on prognostic factors.
What Did the Results Show?
The results favored statin use for improved survival in early breast cancer patients, Mr. Harborg said. Overall, the hazard ratio for breast cancer mortality was 0.96 in statin users compared with non–statin users, and was similar in both a Cox regression analysis (HR 0.81), and in a 10-year landmark analysis (HR 0.86).
The difference in mortality between statin and non–statin users was even stronger in patients who were receiving adjuvant chemotherapy (HR 0.94, 0.64, and 0.76 on the IPCW, Cox, and landmark analyses, respectively).
The results were in line with previous reports of statins’ effect on breast cancer survival, Mr. Harborg said in an interview.
“We believe the results encourage the continuous effort of the currently enrolling MASTER trial,” he said.
The results also suggest that deprescribing statins at the time of breast cancer diagnosis is not recommended, and that statin treatment can safely be prescribed to breast cancer patients with increased cardiovascular disease risk and/or dyslipidemia, Mr. Harborg said in the interview.
What Is the Takeaway Message for Clinical Practice?
“The clinical takeaway from our study is that statin use is associated with reduced risk of dying from breast cancer, but that it is not possible to determine the true effect of statins on breast cancer survival without a randomized, placebo-controlled trial,” Mr. Harborg told this publication. “Statins are inexpensive and well-tolerated drugs and may have a beneficial effect in terms of survival for breast cancer patients. However, with the current level of evidence [because the MASTER study is ongoing], we still cannot recommend that oncologists prescribe statins to prevent mortality from breast cancer,” he said.
What Are the Next Steps for Research?
The findings were limited by the study design, and real-world data are needed, Dr. Harborg said. Other limitations include the presence of residual bias, and the use of data based on prescription codes, but these were not considered to have an effect on the main conclusion of the study, Mr. Harborg said in the interview.
However, the results suggest that the addition of statins may improve outcomes for early breast cancer patients, especially when used with chemotherapy, and support the value of the ongoing MASTER study, he concluded.
Ultimately, the MASTER study will provide a more definitive answer to the question of whether statins should be added to the adjuvant treatment regimen of breast cancer to improve breast cancer outcomes, he said.
What Do Clinicians Think of the Study?
The current study is timely and highlights the need for phase 3 trials to examine the potential of statin use for breast cancer outcomes, Malinda T. West, MD, a medical oncologist and breast oncologist at the University of Wisconsin Carbone Cancer Center, Madison, said in an interview.
Questions for future research include whether statins can be used in combination with adjuvant abemaciclib if indicated, or how to best sequence these agents, said Dr. West, who was not involved in the study. Other questions raised by the current study include whether other cholesterol-lowering agents have a potential adjuvant benefit in reducing breast cancer recurrent and/or mortality, and whether the addition of statins would benefit subgroups such as HER2+ and triple negative breast cancer, she said.
“I was not surprised to see another study reporting benefit with statins and reduced risk of breast cancer recurrence and/or mortality, but I think the larger question is defining the subgroups who benefit the most, and identifying predictors for benefit or resistance,” Dr. West said in an interview.
Previous studies have shown that cholesterol elevation, specifically LDL levels, can be linked to increased tumor growth in breast cancer, so the lower mortality risk associated with lipid-lowering therapies in the current study was consistent, Peyton L. Reves, MD, a hematology/oncology fellow, also at the University of Wisconsin, said in an interview. In practice, data from the current study and previous research could be especially useful for patients with elevated LDL levels, said Dr. Reves, who was not involved in the study.
“These results could impact clinical practice in many ways, including leading to routine cholesterol monitoring in breast cancer patients on adjuvant therapy as well as the addition of lipid-lowering therapy with statins in these patients,” Dr. Reves said.
The findings showing particular benefit for patients on adjuvant chemotherapy highlight the need for more research on this specific population and the effect of statins on overall breast cancer mortality, to explore the extent to which the results of the current study were driven by the benefit seen in patients receiving adjuvant chemotherapy, Dr. Reves said.
The study was supported by Director Michael Hermann Nielsen’s Memorial Grant, Manufacturer Einar Willumsen’s Memorial Grant, Astrid Thaysen’s Grant for Medical Basic Research, Eva and Henry Fraenkel’s Memorial Fund, and the Novo Nordisk Foundation.
The researchers had no financial conflicts to disclose. Dr. West and Dr. Reves had no financial conflicts to disclose.
Previous research examining the association between cholesterol and breast cancer metabolism suggests that cholesterol-lowering medications such as statins may improve outcomes in breast cancer patients, Sixten Harborg, a medical student and PhD student at Aarhus University, Denmark, said in a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
In addition, cardiovascular-related death is the second most common cause of death for breast cancer survivors, and given the survival rates in early breast cancer, there is a demand for cardioprotective initiatives and maintenance of cardioprotective drugs after diagnosis, he said in an interview.
What Is Known About Statins and Breast Cancer?
Statins are the most common drugs used to lower cholesterol and may deprive tumor cells of the cholesterol needed for cell membrane synthesis, Mr. Harborg said in his presentation.
Data from a randomized trial published in the Journal of Clinical Oncology in 2017 showed significantly improved disease-free survival, breast cancer–free interval, and distant recurrence–free interval in early stage breast cancer patients randomized to cholesterol-lowering medication vs. those who did not receive cholesterol-lowering medication.
The 2017 study prompted the creation of the MASTER study, a randomized, multicenter, double-blind, placebo-controlled trial comparing standard adjuvant therapy plus placebo to standard adjuvant therapy plus atorvastatin in patients with early breast cancer (NCT04601116), Mr. Harborg said. The MASTER trial is currently recruiting patients in Denmark.
How Was the Current Study Designed?
To provide preliminary analysis, Mr. Harborg and colleagues used an emulation trial design based on electronic health care data from 110,160 females with a diagnosis of stage I, II, or III breast cancer who were part of the Danish Breast Cancer Group, a national clinical registry in Denmark, between 2000 and 2020.
As defined in the European Journal of Epidemiology in 2017, target trial emulation involves application of randomized trial designs to observational data with the goal of improving the quality of observational epidemiology when a comparator trial is not yet available.
The researchers created a cohort of patients based on electronic health care data to simulate a target trial of the use of atorvastatin after breast cancer diagnosis. Patients were randomized to one of two treatment strategies: starting to use statins within 36 months of diagnosis, or not using statins. The primary outcome was death from breast cancer. The follow-up for the MASTER study starts with inclusion and ends with death, emigration from Denmark, end of clinical follow-up, or 10 years of follow-up (whichever comes first); the follow-up was the same in the current study.
The researchers calculated hazard ratios (HR) of breast cancer mortality in statin users vs. non–statin users and used a technique known as inverse-probability of censoring-weighting (IPCW) to estimate the effects of statin use based on prognostic factors.
What Did the Results Show?
The results favored statin use for improved survival in early breast cancer patients, Mr. Harborg said. Overall, the hazard ratio for breast cancer mortality was 0.96 in statin users compared with non–statin users, and was similar in both a Cox regression analysis (HR 0.81), and in a 10-year landmark analysis (HR 0.86).
The difference in mortality between statin and non–statin users was even stronger in patients who were receiving adjuvant chemotherapy (HR 0.94, 0.64, and 0.76 on the IPCW, Cox, and landmark analyses, respectively).
The results were in line with previous reports of statins’ effect on breast cancer survival, Mr. Harborg said in an interview.
“We believe the results encourage the continuous effort of the currently enrolling MASTER trial,” he said.
The results also suggest that deprescribing statins at the time of breast cancer diagnosis is not recommended, and that statin treatment can safely be prescribed to breast cancer patients with increased cardiovascular disease risk and/or dyslipidemia, Mr. Harborg said in the interview.
What Is the Takeaway Message for Clinical Practice?
“The clinical takeaway from our study is that statin use is associated with reduced risk of dying from breast cancer, but that it is not possible to determine the true effect of statins on breast cancer survival without a randomized, placebo-controlled trial,” Mr. Harborg told this publication. “Statins are inexpensive and well-tolerated drugs and may have a beneficial effect in terms of survival for breast cancer patients. However, with the current level of evidence [because the MASTER study is ongoing], we still cannot recommend that oncologists prescribe statins to prevent mortality from breast cancer,” he said.
What Are the Next Steps for Research?
The findings were limited by the study design, and real-world data are needed, Dr. Harborg said. Other limitations include the presence of residual bias, and the use of data based on prescription codes, but these were not considered to have an effect on the main conclusion of the study, Mr. Harborg said in the interview.
However, the results suggest that the addition of statins may improve outcomes for early breast cancer patients, especially when used with chemotherapy, and support the value of the ongoing MASTER study, he concluded.
Ultimately, the MASTER study will provide a more definitive answer to the question of whether statins should be added to the adjuvant treatment regimen of breast cancer to improve breast cancer outcomes, he said.
What Do Clinicians Think of the Study?
The current study is timely and highlights the need for phase 3 trials to examine the potential of statin use for breast cancer outcomes, Malinda T. West, MD, a medical oncologist and breast oncologist at the University of Wisconsin Carbone Cancer Center, Madison, said in an interview.
Questions for future research include whether statins can be used in combination with adjuvant abemaciclib if indicated, or how to best sequence these agents, said Dr. West, who was not involved in the study. Other questions raised by the current study include whether other cholesterol-lowering agents have a potential adjuvant benefit in reducing breast cancer recurrent and/or mortality, and whether the addition of statins would benefit subgroups such as HER2+ and triple negative breast cancer, she said.
“I was not surprised to see another study reporting benefit with statins and reduced risk of breast cancer recurrence and/or mortality, but I think the larger question is defining the subgroups who benefit the most, and identifying predictors for benefit or resistance,” Dr. West said in an interview.
Previous studies have shown that cholesterol elevation, specifically LDL levels, can be linked to increased tumor growth in breast cancer, so the lower mortality risk associated with lipid-lowering therapies in the current study was consistent, Peyton L. Reves, MD, a hematology/oncology fellow, also at the University of Wisconsin, said in an interview. In practice, data from the current study and previous research could be especially useful for patients with elevated LDL levels, said Dr. Reves, who was not involved in the study.
“These results could impact clinical practice in many ways, including leading to routine cholesterol monitoring in breast cancer patients on adjuvant therapy as well as the addition of lipid-lowering therapy with statins in these patients,” Dr. Reves said.
The findings showing particular benefit for patients on adjuvant chemotherapy highlight the need for more research on this specific population and the effect of statins on overall breast cancer mortality, to explore the extent to which the results of the current study were driven by the benefit seen in patients receiving adjuvant chemotherapy, Dr. Reves said.
The study was supported by Director Michael Hermann Nielsen’s Memorial Grant, Manufacturer Einar Willumsen’s Memorial Grant, Astrid Thaysen’s Grant for Medical Basic Research, Eva and Henry Fraenkel’s Memorial Fund, and the Novo Nordisk Foundation.
The researchers had no financial conflicts to disclose. Dr. West and Dr. Reves had no financial conflicts to disclose.
FROM ESMO BREAST CANCER 2024
Do You Really Know a UTI When You See It?
An updated clinical approach to diagnosing urinary tract infections (UTIs) that considers five potential phenotype categories instead of the usual three could aid clinical management and better center patient needs, according to the authors of a new study in The Journal of Urology.
The current diagnostic paradigm includes UTI, asymptomatic bacteriuria (ASB), or not UTI, but the researchers believe these categories exclude for more ambiguous clinical cases, such as patients whose bacteria counts are low but who are symptomatic, or when nonspecific symptoms make it difficult to determine whether treatment with antibiotics is appropriate.
“Our findings suggest the need to reframe our conceptual model of UTI vs ASB to recognize clinical uncertainty and reflect the full spectrum of clinical presentations,” Sonali D. Advani, MBBS, MPH, an associate professor of medicine in infectious disease at Duke University School of Medicine, in Durham, North Carolina, and her colleagues wrote. “Recent data suggest that UTI may present as a bidirectional continuum from asymptomatic bladder colonization to a symptomatic bladder infection,” and some populations may lack the signs or symptoms specific to urinary tract or have chronic lower urinary tract symptoms (LUTS) that make it difficult to distinguish between ASB and UTI, they wrote.
Nitya E. Abraham, MD, an associate professor of urology at Albert Einstein College of Medicine and Montefiore Einstein in New York City, agreed the current paradigm has room for refinement.
“The current classification system doesn’t account for certain patients such as patients who have bothersome urinary symptoms, but urine testing comes back negative, or patients with positive urine testing, but who aren’t able to report the presence or absence of symptoms,” Dr. Abraham, who was not involved in the new research, told this news organization.
Boback Berookhim, MD, a urologist at Northwell Health in New Hyde Park, New York, who was also not involved in the research, said the goal with this study appears to be better identifying who will need antibiotics.
“I think this is more of a forward-looking study in terms of trying to identify patients who currently may not be treated or may be over treated and better identifying subsets,” Dr. Berookhim told this news organization.
However, he said the relevance of the work is far greater in hospitals than in outpatient settings.
“I think it’s much more relevant in inpatient environments where a patient is in hospital and whatever antibiotics are being written are going to be overseen and you’re going to see higher resistance patterns,” Dr. Berookhim said. “For the average doctor who’s seeing patients in the office and writing them prescriptions in the office, this doesn’t really affect them.”
Antibiotic Dilemma
A key issue in determining the best approach to UTI diagnosis is assessing the appropriateness of antibiotic treatment. Up to half of hospitalized patients have ASB, for which current practice guidelines advise against antibiotics, Dr. Advani and her colleagues noted. Yet many of these patients receive antibiotics regardless, and research has shown links between treatment and longer length of stay, antibiotic resistance, and infection with Clostridioides difficile.
The challenge comes with patients who do not fit easily into the existing categories. One includes patients who have positive urine cultures but whose symptoms, such as hypotension or fever, are not specific to the genitourinary tract.
While current guidelines advise against treating these patients with antibiotics, the patients are often older adults with cognitive impairment or delirium, and frontline physicians may err on the side of prescribing antibiotics because of their clinical uncertainty. That treatment can lead to tension with hospital antibiotic stewardship teams that recommend withholding antibiotics for those patients.
“These clinical scenarios highlight differences between the frontline clinicians’ and antibiotic stewardship teams’ definitions of ‘asymptomatic,’ highlighting the ambiguity of the term ‘asymptomatic bacteriuria,’” Dr. Advani and her colleagues wrote.
A fever, for example, could signal a viral or bacterial infection or result from a nonurinary source, Dr. Abraham said. “The antibiotic stewardship team likely prefers to observe the clinical course and wait for more data to demonstrate need for antibiotics,” she said. “Hence, there are conflicting priorities and confusion of when to treat with antibiotics for this common dilemma in patients presenting to the ER or urgent care.”
Meanwhile, other patients, particularly women, may present with urinary symptoms and pyuria but have lab results revealing a colony count below the 100,000 CFU/mL threshold that would indicate antibiotic treatment.
“Some of these women are likely suffering from a UTI and may not receive treatment if clinicians focus primarily on the urine culture results,” Dr. Abraham said. She pointed out the existence of other options than urine culture for better identifying UTI, such as urinary cell-free DNA or next-generation DNA testing of the urine. But she also said the 100,000 CFU/mL threshold should not be absolute.
“For example, I will treat patients for UTI with 10,000-50,000 CFU/mL if they also have UTI symptoms like blood in the urine, burning with urination, bladder pain, increased urgency or frequency, and the urinalysis shows a high white blood cell count,” Dr. Abraham said.
Dr. Abraham also noted a third group outside the scope of the new study: People with urinary symptoms who don’t undergo urine tests or who are treated empirically with antibiotics. “It is unclear whether those in this group truly have a UTI, but it is a common scenario that patients are unable to get urine tests and are treated with over-the-phone prescriptions to expedite treatment,” she said.
Get on the BUS
The researchers conducted a retrospective study across one academic medical center and four community hospitals in three states to assess the feasibility of using five categories of UTI diagnosis: The three existing ones plus LUTS/other urologic symptoms (OUS) and bacteriuria of unclear significance (BUS). These additional categories arose out of an hour-long discussion with a focus group of experts across several disciplines.
The analysis covered the charts of 3392 randomly selected encounters out of 220,531 total inpatient or emergency department encounters between January 2017 and December 2019 in which adults received a urinalysis and urine culture order within the same 24-hour period. The patients’ median age was 67 years, over half (59.6%) were women, and nearly a quarter (24.2%) had an underlying immunocompromising condition.
Most of the cultures were obtained from inpatients. Nearly a third (30.6%) were negative for culture, while 42.1% grew at least 100,000 CFU/mL of bacteria and 17% grew mixed flora.
Based on current criteria, 21.3% of the patients had a UTI, 20.8% had ASB, and 47.6% had no UTI. The remaining 10.3% had culture growth under 100,000 CFU/mL and, therefore, did not fit in any of these categories, “as there is no consistent guidance on whether to classify them as no UTI or ASB or contamination,” the authors wrote.
When the researchers applied the new criteria, more than half of the cases of ASB (68%) were reclassified as BUS, and 28.9% of the no-UTI cases were reclassified as LUTS/OUS.
In a sensitivity analysis that examined samples with bacteriuria below the 100,000 CFU/mL threshold, nearly half the unclassified cases (43.3%) were reassigned as a UTI, increasing the proportion of patients with a diagnosed UTI from 21.3% to 25.8% of the total population. Of the remaining patients who had originally been unclassified, 14.2% were newly defined as ASB, and 42.5% became BUS.
Dr. Abraham said the addition of the BUS and LUTS/OUS categories has the potential to improve and individualize patient care. Clinicians can consider nonantibiotic therapies for the patients who had LUTS/OUS while they look into possible causes, while the BUS cases enable frontline clinicians and antibiotic stewardship teams to “meet in the middle” by monitoring those patients more closely in case symptoms worsen, she said.
The authors highlighted three key takeaways from their study, starting with the fact that nearly two thirds of patients who underwent testing for a UTI did not have signs or symptoms localized to the urinary tract — the ones reclassified as BUS.
“Hence, reclassifying patients as BUS may provide an opportunity to acknowledge diagnostic uncertainty and need for additional monitoring than ASB patients so as to promote a nuanced and patient-centered approach to diagnosis and management,” the authors wrote.
Second, a third of patients initially classified as not having a UTI were reclassified into the new LUTS/OUS category because of their symptoms, such as a poor or intermittent stream, dribbling, hesitancy, frequency, urge incontinence, and nocturia. These patients would need further workup to determine the best approach to management.
Finally, the sensitivity analysis “suggested that lowering the bacterial threshold in some symptomatic patients may capture additional patients with UTI whose symptoms may be dismissed due to concern for contamination or attributed to LUTS rather than infection.” Given that the 100,000 CFU/mL threshold is based on a single study in 1956, the authors suggested more research may help define better CFU thresholds to improve clinical care.
Dr. Berookhim said the study authors took a reasonable and thorough approach in how they tried to consider the best way to update the current diagnostic classification schema.
“I think using this as a jumping off point to look deeper is worthwhile,” such as conducting randomized controlled trials to assess the use of new categories, he said. “Getting more granular than this, I think, would just muddy the waters and make it more difficult to make clinical decisions.”
The research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Advani reported consulting fees from Locus Biosciences, Sysmex America, GlaxoSmithKline, and bioMérieux. Dr. Abraham and Dr. Berookhim reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
An updated clinical approach to diagnosing urinary tract infections (UTIs) that considers five potential phenotype categories instead of the usual three could aid clinical management and better center patient needs, according to the authors of a new study in The Journal of Urology.
The current diagnostic paradigm includes UTI, asymptomatic bacteriuria (ASB), or not UTI, but the researchers believe these categories exclude for more ambiguous clinical cases, such as patients whose bacteria counts are low but who are symptomatic, or when nonspecific symptoms make it difficult to determine whether treatment with antibiotics is appropriate.
“Our findings suggest the need to reframe our conceptual model of UTI vs ASB to recognize clinical uncertainty and reflect the full spectrum of clinical presentations,” Sonali D. Advani, MBBS, MPH, an associate professor of medicine in infectious disease at Duke University School of Medicine, in Durham, North Carolina, and her colleagues wrote. “Recent data suggest that UTI may present as a bidirectional continuum from asymptomatic bladder colonization to a symptomatic bladder infection,” and some populations may lack the signs or symptoms specific to urinary tract or have chronic lower urinary tract symptoms (LUTS) that make it difficult to distinguish between ASB and UTI, they wrote.
Nitya E. Abraham, MD, an associate professor of urology at Albert Einstein College of Medicine and Montefiore Einstein in New York City, agreed the current paradigm has room for refinement.
“The current classification system doesn’t account for certain patients such as patients who have bothersome urinary symptoms, but urine testing comes back negative, or patients with positive urine testing, but who aren’t able to report the presence or absence of symptoms,” Dr. Abraham, who was not involved in the new research, told this news organization.
Boback Berookhim, MD, a urologist at Northwell Health in New Hyde Park, New York, who was also not involved in the research, said the goal with this study appears to be better identifying who will need antibiotics.
“I think this is more of a forward-looking study in terms of trying to identify patients who currently may not be treated or may be over treated and better identifying subsets,” Dr. Berookhim told this news organization.
However, he said the relevance of the work is far greater in hospitals than in outpatient settings.
“I think it’s much more relevant in inpatient environments where a patient is in hospital and whatever antibiotics are being written are going to be overseen and you’re going to see higher resistance patterns,” Dr. Berookhim said. “For the average doctor who’s seeing patients in the office and writing them prescriptions in the office, this doesn’t really affect them.”
Antibiotic Dilemma
A key issue in determining the best approach to UTI diagnosis is assessing the appropriateness of antibiotic treatment. Up to half of hospitalized patients have ASB, for which current practice guidelines advise against antibiotics, Dr. Advani and her colleagues noted. Yet many of these patients receive antibiotics regardless, and research has shown links between treatment and longer length of stay, antibiotic resistance, and infection with Clostridioides difficile.
The challenge comes with patients who do not fit easily into the existing categories. One includes patients who have positive urine cultures but whose symptoms, such as hypotension or fever, are not specific to the genitourinary tract.
While current guidelines advise against treating these patients with antibiotics, the patients are often older adults with cognitive impairment or delirium, and frontline physicians may err on the side of prescribing antibiotics because of their clinical uncertainty. That treatment can lead to tension with hospital antibiotic stewardship teams that recommend withholding antibiotics for those patients.
“These clinical scenarios highlight differences between the frontline clinicians’ and antibiotic stewardship teams’ definitions of ‘asymptomatic,’ highlighting the ambiguity of the term ‘asymptomatic bacteriuria,’” Dr. Advani and her colleagues wrote.
A fever, for example, could signal a viral or bacterial infection or result from a nonurinary source, Dr. Abraham said. “The antibiotic stewardship team likely prefers to observe the clinical course and wait for more data to demonstrate need for antibiotics,” she said. “Hence, there are conflicting priorities and confusion of when to treat with antibiotics for this common dilemma in patients presenting to the ER or urgent care.”
Meanwhile, other patients, particularly women, may present with urinary symptoms and pyuria but have lab results revealing a colony count below the 100,000 CFU/mL threshold that would indicate antibiotic treatment.
“Some of these women are likely suffering from a UTI and may not receive treatment if clinicians focus primarily on the urine culture results,” Dr. Abraham said. She pointed out the existence of other options than urine culture for better identifying UTI, such as urinary cell-free DNA or next-generation DNA testing of the urine. But she also said the 100,000 CFU/mL threshold should not be absolute.
“For example, I will treat patients for UTI with 10,000-50,000 CFU/mL if they also have UTI symptoms like blood in the urine, burning with urination, bladder pain, increased urgency or frequency, and the urinalysis shows a high white blood cell count,” Dr. Abraham said.
Dr. Abraham also noted a third group outside the scope of the new study: People with urinary symptoms who don’t undergo urine tests or who are treated empirically with antibiotics. “It is unclear whether those in this group truly have a UTI, but it is a common scenario that patients are unable to get urine tests and are treated with over-the-phone prescriptions to expedite treatment,” she said.
Get on the BUS
The researchers conducted a retrospective study across one academic medical center and four community hospitals in three states to assess the feasibility of using five categories of UTI diagnosis: The three existing ones plus LUTS/other urologic symptoms (OUS) and bacteriuria of unclear significance (BUS). These additional categories arose out of an hour-long discussion with a focus group of experts across several disciplines.
The analysis covered the charts of 3392 randomly selected encounters out of 220,531 total inpatient or emergency department encounters between January 2017 and December 2019 in which adults received a urinalysis and urine culture order within the same 24-hour period. The patients’ median age was 67 years, over half (59.6%) were women, and nearly a quarter (24.2%) had an underlying immunocompromising condition.
Most of the cultures were obtained from inpatients. Nearly a third (30.6%) were negative for culture, while 42.1% grew at least 100,000 CFU/mL of bacteria and 17% grew mixed flora.
Based on current criteria, 21.3% of the patients had a UTI, 20.8% had ASB, and 47.6% had no UTI. The remaining 10.3% had culture growth under 100,000 CFU/mL and, therefore, did not fit in any of these categories, “as there is no consistent guidance on whether to classify them as no UTI or ASB or contamination,” the authors wrote.
When the researchers applied the new criteria, more than half of the cases of ASB (68%) were reclassified as BUS, and 28.9% of the no-UTI cases were reclassified as LUTS/OUS.
In a sensitivity analysis that examined samples with bacteriuria below the 100,000 CFU/mL threshold, nearly half the unclassified cases (43.3%) were reassigned as a UTI, increasing the proportion of patients with a diagnosed UTI from 21.3% to 25.8% of the total population. Of the remaining patients who had originally been unclassified, 14.2% were newly defined as ASB, and 42.5% became BUS.
Dr. Abraham said the addition of the BUS and LUTS/OUS categories has the potential to improve and individualize patient care. Clinicians can consider nonantibiotic therapies for the patients who had LUTS/OUS while they look into possible causes, while the BUS cases enable frontline clinicians and antibiotic stewardship teams to “meet in the middle” by monitoring those patients more closely in case symptoms worsen, she said.
The authors highlighted three key takeaways from their study, starting with the fact that nearly two thirds of patients who underwent testing for a UTI did not have signs or symptoms localized to the urinary tract — the ones reclassified as BUS.
“Hence, reclassifying patients as BUS may provide an opportunity to acknowledge diagnostic uncertainty and need for additional monitoring than ASB patients so as to promote a nuanced and patient-centered approach to diagnosis and management,” the authors wrote.
Second, a third of patients initially classified as not having a UTI were reclassified into the new LUTS/OUS category because of their symptoms, such as a poor or intermittent stream, dribbling, hesitancy, frequency, urge incontinence, and nocturia. These patients would need further workup to determine the best approach to management.
Finally, the sensitivity analysis “suggested that lowering the bacterial threshold in some symptomatic patients may capture additional patients with UTI whose symptoms may be dismissed due to concern for contamination or attributed to LUTS rather than infection.” Given that the 100,000 CFU/mL threshold is based on a single study in 1956, the authors suggested more research may help define better CFU thresholds to improve clinical care.
Dr. Berookhim said the study authors took a reasonable and thorough approach in how they tried to consider the best way to update the current diagnostic classification schema.
“I think using this as a jumping off point to look deeper is worthwhile,” such as conducting randomized controlled trials to assess the use of new categories, he said. “Getting more granular than this, I think, would just muddy the waters and make it more difficult to make clinical decisions.”
The research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Advani reported consulting fees from Locus Biosciences, Sysmex America, GlaxoSmithKline, and bioMérieux. Dr. Abraham and Dr. Berookhim reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
An updated clinical approach to diagnosing urinary tract infections (UTIs) that considers five potential phenotype categories instead of the usual three could aid clinical management and better center patient needs, according to the authors of a new study in The Journal of Urology.
The current diagnostic paradigm includes UTI, asymptomatic bacteriuria (ASB), or not UTI, but the researchers believe these categories exclude for more ambiguous clinical cases, such as patients whose bacteria counts are low but who are symptomatic, or when nonspecific symptoms make it difficult to determine whether treatment with antibiotics is appropriate.
“Our findings suggest the need to reframe our conceptual model of UTI vs ASB to recognize clinical uncertainty and reflect the full spectrum of clinical presentations,” Sonali D. Advani, MBBS, MPH, an associate professor of medicine in infectious disease at Duke University School of Medicine, in Durham, North Carolina, and her colleagues wrote. “Recent data suggest that UTI may present as a bidirectional continuum from asymptomatic bladder colonization to a symptomatic bladder infection,” and some populations may lack the signs or symptoms specific to urinary tract or have chronic lower urinary tract symptoms (LUTS) that make it difficult to distinguish between ASB and UTI, they wrote.
Nitya E. Abraham, MD, an associate professor of urology at Albert Einstein College of Medicine and Montefiore Einstein in New York City, agreed the current paradigm has room for refinement.
“The current classification system doesn’t account for certain patients such as patients who have bothersome urinary symptoms, but urine testing comes back negative, or patients with positive urine testing, but who aren’t able to report the presence or absence of symptoms,” Dr. Abraham, who was not involved in the new research, told this news organization.
Boback Berookhim, MD, a urologist at Northwell Health in New Hyde Park, New York, who was also not involved in the research, said the goal with this study appears to be better identifying who will need antibiotics.
“I think this is more of a forward-looking study in terms of trying to identify patients who currently may not be treated or may be over treated and better identifying subsets,” Dr. Berookhim told this news organization.
However, he said the relevance of the work is far greater in hospitals than in outpatient settings.
“I think it’s much more relevant in inpatient environments where a patient is in hospital and whatever antibiotics are being written are going to be overseen and you’re going to see higher resistance patterns,” Dr. Berookhim said. “For the average doctor who’s seeing patients in the office and writing them prescriptions in the office, this doesn’t really affect them.”
Antibiotic Dilemma
A key issue in determining the best approach to UTI diagnosis is assessing the appropriateness of antibiotic treatment. Up to half of hospitalized patients have ASB, for which current practice guidelines advise against antibiotics, Dr. Advani and her colleagues noted. Yet many of these patients receive antibiotics regardless, and research has shown links between treatment and longer length of stay, antibiotic resistance, and infection with Clostridioides difficile.
The challenge comes with patients who do not fit easily into the existing categories. One includes patients who have positive urine cultures but whose symptoms, such as hypotension or fever, are not specific to the genitourinary tract.
While current guidelines advise against treating these patients with antibiotics, the patients are often older adults with cognitive impairment or delirium, and frontline physicians may err on the side of prescribing antibiotics because of their clinical uncertainty. That treatment can lead to tension with hospital antibiotic stewardship teams that recommend withholding antibiotics for those patients.
“These clinical scenarios highlight differences between the frontline clinicians’ and antibiotic stewardship teams’ definitions of ‘asymptomatic,’ highlighting the ambiguity of the term ‘asymptomatic bacteriuria,’” Dr. Advani and her colleagues wrote.
A fever, for example, could signal a viral or bacterial infection or result from a nonurinary source, Dr. Abraham said. “The antibiotic stewardship team likely prefers to observe the clinical course and wait for more data to demonstrate need for antibiotics,” she said. “Hence, there are conflicting priorities and confusion of when to treat with antibiotics for this common dilemma in patients presenting to the ER or urgent care.”
Meanwhile, other patients, particularly women, may present with urinary symptoms and pyuria but have lab results revealing a colony count below the 100,000 CFU/mL threshold that would indicate antibiotic treatment.
“Some of these women are likely suffering from a UTI and may not receive treatment if clinicians focus primarily on the urine culture results,” Dr. Abraham said. She pointed out the existence of other options than urine culture for better identifying UTI, such as urinary cell-free DNA or next-generation DNA testing of the urine. But she also said the 100,000 CFU/mL threshold should not be absolute.
“For example, I will treat patients for UTI with 10,000-50,000 CFU/mL if they also have UTI symptoms like blood in the urine, burning with urination, bladder pain, increased urgency or frequency, and the urinalysis shows a high white blood cell count,” Dr. Abraham said.
Dr. Abraham also noted a third group outside the scope of the new study: People with urinary symptoms who don’t undergo urine tests or who are treated empirically with antibiotics. “It is unclear whether those in this group truly have a UTI, but it is a common scenario that patients are unable to get urine tests and are treated with over-the-phone prescriptions to expedite treatment,” she said.
Get on the BUS
The researchers conducted a retrospective study across one academic medical center and four community hospitals in three states to assess the feasibility of using five categories of UTI diagnosis: The three existing ones plus LUTS/other urologic symptoms (OUS) and bacteriuria of unclear significance (BUS). These additional categories arose out of an hour-long discussion with a focus group of experts across several disciplines.
The analysis covered the charts of 3392 randomly selected encounters out of 220,531 total inpatient or emergency department encounters between January 2017 and December 2019 in which adults received a urinalysis and urine culture order within the same 24-hour period. The patients’ median age was 67 years, over half (59.6%) were women, and nearly a quarter (24.2%) had an underlying immunocompromising condition.
Most of the cultures were obtained from inpatients. Nearly a third (30.6%) were negative for culture, while 42.1% grew at least 100,000 CFU/mL of bacteria and 17% grew mixed flora.
Based on current criteria, 21.3% of the patients had a UTI, 20.8% had ASB, and 47.6% had no UTI. The remaining 10.3% had culture growth under 100,000 CFU/mL and, therefore, did not fit in any of these categories, “as there is no consistent guidance on whether to classify them as no UTI or ASB or contamination,” the authors wrote.
When the researchers applied the new criteria, more than half of the cases of ASB (68%) were reclassified as BUS, and 28.9% of the no-UTI cases were reclassified as LUTS/OUS.
In a sensitivity analysis that examined samples with bacteriuria below the 100,000 CFU/mL threshold, nearly half the unclassified cases (43.3%) were reassigned as a UTI, increasing the proportion of patients with a diagnosed UTI from 21.3% to 25.8% of the total population. Of the remaining patients who had originally been unclassified, 14.2% were newly defined as ASB, and 42.5% became BUS.
Dr. Abraham said the addition of the BUS and LUTS/OUS categories has the potential to improve and individualize patient care. Clinicians can consider nonantibiotic therapies for the patients who had LUTS/OUS while they look into possible causes, while the BUS cases enable frontline clinicians and antibiotic stewardship teams to “meet in the middle” by monitoring those patients more closely in case symptoms worsen, she said.
The authors highlighted three key takeaways from their study, starting with the fact that nearly two thirds of patients who underwent testing for a UTI did not have signs or symptoms localized to the urinary tract — the ones reclassified as BUS.
“Hence, reclassifying patients as BUS may provide an opportunity to acknowledge diagnostic uncertainty and need for additional monitoring than ASB patients so as to promote a nuanced and patient-centered approach to diagnosis and management,” the authors wrote.
Second, a third of patients initially classified as not having a UTI were reclassified into the new LUTS/OUS category because of their symptoms, such as a poor or intermittent stream, dribbling, hesitancy, frequency, urge incontinence, and nocturia. These patients would need further workup to determine the best approach to management.
Finally, the sensitivity analysis “suggested that lowering the bacterial threshold in some symptomatic patients may capture additional patients with UTI whose symptoms may be dismissed due to concern for contamination or attributed to LUTS rather than infection.” Given that the 100,000 CFU/mL threshold is based on a single study in 1956, the authors suggested more research may help define better CFU thresholds to improve clinical care.
Dr. Berookhim said the study authors took a reasonable and thorough approach in how they tried to consider the best way to update the current diagnostic classification schema.
“I think using this as a jumping off point to look deeper is worthwhile,” such as conducting randomized controlled trials to assess the use of new categories, he said. “Getting more granular than this, I think, would just muddy the waters and make it more difficult to make clinical decisions.”
The research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Advani reported consulting fees from Locus Biosciences, Sysmex America, GlaxoSmithKline, and bioMérieux. Dr. Abraham and Dr. Berookhim reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF UROLOGY
Former UCLA Doctor Receives $14 Million in Gender Discrimination Retrial
A California jury has awarded $14 million to a former University of California, Los Angeles (UCLA) oncologist who claimed she was paid thousands less than her male colleagues and wrongfully terminated after her complaints of gender-based harassment and intimidation were ignored by program leadership.
The decision comes after a lengthy 8-year legal battle in which an appellate judge reversed a previous jury decision in her favor.
Lauren Pinter-Brown, MD, a hematologic oncologist, was hired in 2005 by the University of California, Los Angeles School of Medicine — now called UCLA’s David Geffen School of Medicine. As the school’s lymphoma program director, she conducted clinical research alongside other oncology doctors, including Sven de Vos, MD.
She claimed that her professional relationship with Dr. de Vos became contentious after he demonstrated “oppositional” and “disrespectful” behavior at team meetings, such as talking over her and turning his chair so Dr. Pinter-Brown faced his back. Court documents indicated that Dr. de Vos refused to use Dr. Pinter-Brown’s title in front of colleagues despite doing so for male counterparts.
Dr. Pinter-Brown argued that she was treated as the “butt of a joke” by Dr. de Vos and other male colleagues. In 2016, she sued Dr. de Vos, the university, and its governing body, the Board of Regents, for wrongful termination.
She was awarded a $13 million verdict in 2018. However, the California Court of Appeals overturned it in 2020 after concluding that several mistakes during the court proceedings impeded the school’s right to a fair and impartial trial. The case was retried, culminating in the even higher award of $14 million issued on May 9.
“Two juries have come to virtually identical findings showing multiple problems at UCLA involving gender discrimination,” Dr. Pinter-Brown’s attorney, Carney R. Shegerian, JD, told this news organization.
A spokesperson from UCLA’s David Geffen School of Medicine said administrators are carefully reviewing the new decision.
The spokesperson told this news organization that the medical school and its health system remain “deeply committed to maintaining a workplace free from discrimination, intimidation, retaliation, or harassment of any kind” and fostering a “respectful and inclusive environment ... in research, medical education, and patient care.”
Gender Pay Disparities Persist in Medicine
The gender pay gap in medicine is well documented. The 2024 Medscape Physician Compensation Report found that male doctors earn about 29% more than their female counterparts, with the disparity growing larger among specialists. In addition, a recent JAMA Health Forum study found that male physicians earned 21%-24% more per hour than female physicians.
Dr. Pinter-Brown, who now works at the University of California, Irvine, alleged that she was paid $200,000 less annually, on average, than her male colleagues.
That’s not surprising, says Martha Gulati, MD, professor and director of preventive cardiology at Cedars-Sinai Smidt Heart Institute, Los Angeles. She coauthored a commentary about gender disparities in JAMA Network Open. Dr. Gulati told this news organization that even a “small” pay disparity of $100,000 annually adds up.
“Let’s say the [male physician] invests it at 3% and adds to it yearly. Even without a raise, in 20 years, that is approximately $3 million,” Dr. Gulati explained. “Once you find out you are paid less than your male colleagues, you are upset. Your sense of value and self-worth disappears.”
Eileen Barrett, MD, MPH, president-elect of the American Medical Women’s Association, said that gender discrimination is likely more prevalent than research indicates. She told this news organization that self-doubt and fear of retaliation keep many from exposing the mistreatment.
Although more women are entering medicine, too few rise to the highest positions, Dr. Barrett said.
“Unfortunately, many are pulled and pushed into specialties and subspecialties that have lower compensation and are not promoted to leadership, so just having numbers isn’t enough to achieve equity,” Dr. Barrett said.
Dr. Pinter-Brown claimed she was repeatedly harassed and intimidated by Dr. de Vos from 2008 to 2015. Despite voicing concerns multiple times about the discriminatory behavior, the only resolutions offered by the male-dominated program leadership were for her to separate from the group and conduct lymphoma research independently or to avoid interacting with Dr. de Vos, court records said.
Even the school’s male Title IX officer, Jan Tillisch, MD, who handled gender-based discrimination complaints, reportedly made sexist comments. When Dr. Pinter-Brown sought his help, he allegedly told her that she had a reputation as an “angry woman” and “diva,” court records showed.
According to court documents, Dr. Pinter-Brown endured nitpicking and research audits as retaliation for speaking out, temporarily suspending her research privileges. She said she was subsequently removed from the director position and replaced by Dr. de Vos.
Female physicians who report discriminatory behavior often have unfavorable outcomes and risk future career prospects, Dr. Gulati said.
To shift this dynamic, she said institutions must increase transparency and practices that support female doctors receiving “equal pay for equal work.”
A version of this article appeared on Medscape.com.
A California jury has awarded $14 million to a former University of California, Los Angeles (UCLA) oncologist who claimed she was paid thousands less than her male colleagues and wrongfully terminated after her complaints of gender-based harassment and intimidation were ignored by program leadership.
The decision comes after a lengthy 8-year legal battle in which an appellate judge reversed a previous jury decision in her favor.
Lauren Pinter-Brown, MD, a hematologic oncologist, was hired in 2005 by the University of California, Los Angeles School of Medicine — now called UCLA’s David Geffen School of Medicine. As the school’s lymphoma program director, she conducted clinical research alongside other oncology doctors, including Sven de Vos, MD.
She claimed that her professional relationship with Dr. de Vos became contentious after he demonstrated “oppositional” and “disrespectful” behavior at team meetings, such as talking over her and turning his chair so Dr. Pinter-Brown faced his back. Court documents indicated that Dr. de Vos refused to use Dr. Pinter-Brown’s title in front of colleagues despite doing so for male counterparts.
Dr. Pinter-Brown argued that she was treated as the “butt of a joke” by Dr. de Vos and other male colleagues. In 2016, she sued Dr. de Vos, the university, and its governing body, the Board of Regents, for wrongful termination.
She was awarded a $13 million verdict in 2018. However, the California Court of Appeals overturned it in 2020 after concluding that several mistakes during the court proceedings impeded the school’s right to a fair and impartial trial. The case was retried, culminating in the even higher award of $14 million issued on May 9.
“Two juries have come to virtually identical findings showing multiple problems at UCLA involving gender discrimination,” Dr. Pinter-Brown’s attorney, Carney R. Shegerian, JD, told this news organization.
A spokesperson from UCLA’s David Geffen School of Medicine said administrators are carefully reviewing the new decision.
The spokesperson told this news organization that the medical school and its health system remain “deeply committed to maintaining a workplace free from discrimination, intimidation, retaliation, or harassment of any kind” and fostering a “respectful and inclusive environment ... in research, medical education, and patient care.”
Gender Pay Disparities Persist in Medicine
The gender pay gap in medicine is well documented. The 2024 Medscape Physician Compensation Report found that male doctors earn about 29% more than their female counterparts, with the disparity growing larger among specialists. In addition, a recent JAMA Health Forum study found that male physicians earned 21%-24% more per hour than female physicians.
Dr. Pinter-Brown, who now works at the University of California, Irvine, alleged that she was paid $200,000 less annually, on average, than her male colleagues.
That’s not surprising, says Martha Gulati, MD, professor and director of preventive cardiology at Cedars-Sinai Smidt Heart Institute, Los Angeles. She coauthored a commentary about gender disparities in JAMA Network Open. Dr. Gulati told this news organization that even a “small” pay disparity of $100,000 annually adds up.
“Let’s say the [male physician] invests it at 3% and adds to it yearly. Even without a raise, in 20 years, that is approximately $3 million,” Dr. Gulati explained. “Once you find out you are paid less than your male colleagues, you are upset. Your sense of value and self-worth disappears.”
Eileen Barrett, MD, MPH, president-elect of the American Medical Women’s Association, said that gender discrimination is likely more prevalent than research indicates. She told this news organization that self-doubt and fear of retaliation keep many from exposing the mistreatment.
Although more women are entering medicine, too few rise to the highest positions, Dr. Barrett said.
“Unfortunately, many are pulled and pushed into specialties and subspecialties that have lower compensation and are not promoted to leadership, so just having numbers isn’t enough to achieve equity,” Dr. Barrett said.
Dr. Pinter-Brown claimed she was repeatedly harassed and intimidated by Dr. de Vos from 2008 to 2015. Despite voicing concerns multiple times about the discriminatory behavior, the only resolutions offered by the male-dominated program leadership were for her to separate from the group and conduct lymphoma research independently or to avoid interacting with Dr. de Vos, court records said.
Even the school’s male Title IX officer, Jan Tillisch, MD, who handled gender-based discrimination complaints, reportedly made sexist comments. When Dr. Pinter-Brown sought his help, he allegedly told her that she had a reputation as an “angry woman” and “diva,” court records showed.
According to court documents, Dr. Pinter-Brown endured nitpicking and research audits as retaliation for speaking out, temporarily suspending her research privileges. She said she was subsequently removed from the director position and replaced by Dr. de Vos.
Female physicians who report discriminatory behavior often have unfavorable outcomes and risk future career prospects, Dr. Gulati said.
To shift this dynamic, she said institutions must increase transparency and practices that support female doctors receiving “equal pay for equal work.”
A version of this article appeared on Medscape.com.
A California jury has awarded $14 million to a former University of California, Los Angeles (UCLA) oncologist who claimed she was paid thousands less than her male colleagues and wrongfully terminated after her complaints of gender-based harassment and intimidation were ignored by program leadership.
The decision comes after a lengthy 8-year legal battle in which an appellate judge reversed a previous jury decision in her favor.
Lauren Pinter-Brown, MD, a hematologic oncologist, was hired in 2005 by the University of California, Los Angeles School of Medicine — now called UCLA’s David Geffen School of Medicine. As the school’s lymphoma program director, she conducted clinical research alongside other oncology doctors, including Sven de Vos, MD.
She claimed that her professional relationship with Dr. de Vos became contentious after he demonstrated “oppositional” and “disrespectful” behavior at team meetings, such as talking over her and turning his chair so Dr. Pinter-Brown faced his back. Court documents indicated that Dr. de Vos refused to use Dr. Pinter-Brown’s title in front of colleagues despite doing so for male counterparts.
Dr. Pinter-Brown argued that she was treated as the “butt of a joke” by Dr. de Vos and other male colleagues. In 2016, she sued Dr. de Vos, the university, and its governing body, the Board of Regents, for wrongful termination.
She was awarded a $13 million verdict in 2018. However, the California Court of Appeals overturned it in 2020 after concluding that several mistakes during the court proceedings impeded the school’s right to a fair and impartial trial. The case was retried, culminating in the even higher award of $14 million issued on May 9.
“Two juries have come to virtually identical findings showing multiple problems at UCLA involving gender discrimination,” Dr. Pinter-Brown’s attorney, Carney R. Shegerian, JD, told this news organization.
A spokesperson from UCLA’s David Geffen School of Medicine said administrators are carefully reviewing the new decision.
The spokesperson told this news organization that the medical school and its health system remain “deeply committed to maintaining a workplace free from discrimination, intimidation, retaliation, or harassment of any kind” and fostering a “respectful and inclusive environment ... in research, medical education, and patient care.”
Gender Pay Disparities Persist in Medicine
The gender pay gap in medicine is well documented. The 2024 Medscape Physician Compensation Report found that male doctors earn about 29% more than their female counterparts, with the disparity growing larger among specialists. In addition, a recent JAMA Health Forum study found that male physicians earned 21%-24% more per hour than female physicians.
Dr. Pinter-Brown, who now works at the University of California, Irvine, alleged that she was paid $200,000 less annually, on average, than her male colleagues.
That’s not surprising, says Martha Gulati, MD, professor and director of preventive cardiology at Cedars-Sinai Smidt Heart Institute, Los Angeles. She coauthored a commentary about gender disparities in JAMA Network Open. Dr. Gulati told this news organization that even a “small” pay disparity of $100,000 annually adds up.
“Let’s say the [male physician] invests it at 3% and adds to it yearly. Even without a raise, in 20 years, that is approximately $3 million,” Dr. Gulati explained. “Once you find out you are paid less than your male colleagues, you are upset. Your sense of value and self-worth disappears.”
Eileen Barrett, MD, MPH, president-elect of the American Medical Women’s Association, said that gender discrimination is likely more prevalent than research indicates. She told this news organization that self-doubt and fear of retaliation keep many from exposing the mistreatment.
Although more women are entering medicine, too few rise to the highest positions, Dr. Barrett said.
“Unfortunately, many are pulled and pushed into specialties and subspecialties that have lower compensation and are not promoted to leadership, so just having numbers isn’t enough to achieve equity,” Dr. Barrett said.
Dr. Pinter-Brown claimed she was repeatedly harassed and intimidated by Dr. de Vos from 2008 to 2015. Despite voicing concerns multiple times about the discriminatory behavior, the only resolutions offered by the male-dominated program leadership were for her to separate from the group and conduct lymphoma research independently or to avoid interacting with Dr. de Vos, court records said.
Even the school’s male Title IX officer, Jan Tillisch, MD, who handled gender-based discrimination complaints, reportedly made sexist comments. When Dr. Pinter-Brown sought his help, he allegedly told her that she had a reputation as an “angry woman” and “diva,” court records showed.
According to court documents, Dr. Pinter-Brown endured nitpicking and research audits as retaliation for speaking out, temporarily suspending her research privileges. She said she was subsequently removed from the director position and replaced by Dr. de Vos.
Female physicians who report discriminatory behavior often have unfavorable outcomes and risk future career prospects, Dr. Gulati said.
To shift this dynamic, she said institutions must increase transparency and practices that support female doctors receiving “equal pay for equal work.”
A version of this article appeared on Medscape.com.