User login
BEAT-LUPUS: Belimumab after rituximab delays severe flares
Using belimumab after rituximab to treat patients with systemic lupus erythematosus (SLE) refractory to conventional therapy not only significantly decreased levels of serum IgG anti-dsDNA antibody levels but also prolonged the time before severe flares of disease occurred in the phase 2b BEAT-LUPUS (Belimumab after B cell depletion in SLE) study.
The trial’s primary outcome of serum IgG anti-dsDNA antibody levels showed a decline from a geometric mean of 162 IU/mL at baseline to 69 IU/mL at 24 weeks and 47 IU/mL at 1 year in patients treated with belimumab (Benlysta) after rituximab (Rituxan and biosimilars). These reductions were significantly lower than the values seen in the placebo after rituximab arm (a respective 121 IU/mL, 99 IU/mL, and 103 IU/mL; P < .001).
Just 3 patients who received belimumab versus 10 who received placebo after rituximab experienced a severe BILAG (British Isles Lupus Assessment Group) index A flare by the end of the study at 52 weeks. The hazard ratio (HR) for the flare reduction was 0.27 (P = .03), indicating a 73% reduction.
The use of belimumab rather than a placebo also led to a small reduction in total serum IgG, and significantly suppressed B-cell repopulation (P = .03).
These results need confirming in a larger, phase 3 trial, the trial’s principal investigator, Michael Ehrenstein, PhD, said at the annual European Congress of Rheumatology. They are “clearly encouraging” and “support the hypothesis that BAFF [B-cell–activating factor] can drive flares after rituximab,” he said.
Although B-cell depletion with rituximab is recommended by national and international guidelines to treat some patients with SLE who are refractory to conventional therapy, its use is not licensed.
“Certainly, rituximab is a controversial drug in lupus,” Dr. Ehrenstein, a consultant rheumatologist based at University College London, said in an interview. Although there is real-world evidence from registries and open-label studies suggesting that it is widely used and effective in some patients, the randomized, controlled trials conducted with rituximab about 10 years ago failed to meet their primary endpoints.
“A lot has been written about why that was, but probably the biggest reason was the high dose of steroids in both groups,” Dr. Ehrenstein said. To try to avoid muddying the waters of the BEAT-LUPUS trial findings, the maximum dose of prednisolone allowed to be used as background therapy was 20 mg/day. The trial’s investigators were also encouraged to reduce the baseline steroid dose to at least 50% by the trial’s 6-month halfway point.
“We tried to reflect what was going on in the U.K.,” Dr. Ehrenstein said, noting that the inspiration for the trial was a patient who had received sequential rituximab treatment. Although she got better with each cycle of rituximab, when her disease flared it would be worse than the time before, with increasingly higher anti-dsDNA levels recorded. The reason for this seemed to be because of increasing BAFF levels, and so the hypothesis was that if rituximab was associated with increased BAFF levels, then co-targeting BAFF with belimumab should be able to prevent those flares from happening.
The BEAT-LUPUS trial has been a huge collaborative effort and was conducted across 16 U.K. centers. From initial funding to the data analysis, it has taken 6 years to complete and was made possible by a unique partnership between Versus Arthritis, University College London Hospitals Biomedical Research Center, the National Institute for Health Research UK Musculoskeletal Translational Research Collaboration, and GlaxoSmithKline (GSK). GSK provided belimumab free of charge, as well as additional funding, but had no role in the design of the study and will not have any role going forward.
From an initial 172 patients assessed for eligibility, 52 patients were finally enrolled into the trial and received rituximab as two infusions given 2 weeks apart. Patients were then randomized in a double-blind manner to receive either belimumab (n = 26) or placebo (n = 26) 4-8 weeks after their first dose of rituximab. The intention-to-treat population consisted of 43 patients.
The use of belimumab after rituximab did not increase the risk for infection – serious or otherwise – or adverse effects, Dr. Ehrenstein reported. Serious adverse events were reported in six (23%) patients in each arm, and serious infections were seen in two (8%) of the belimumab- and four (15%) of the placebo-treated patients.
“I think the take-home message is that it seems that belimumab can reduce the number of severe flares that occur after rituximab therapy,” Dr. Ehrenstein said. “It’s promising, but not definitive,” he added. The next step is of course to publish these data and to perform a phase 3 trial.
In the discussion time following the presentation, session moderator Xavier Mariette, MD, PhD, of Bicêtre Hospital, Paris-Saclay University, asked why not give belimumab first before rituximab if using belimumab afterward works?
“Our strategy was driven by the observation that BAFF levels surged after rituximab, and therefore it’s logical to give the belimumab to block that BAFF surge,” he answered.
“Certainly, there are ideas that belimumab releases mature B cells into the circulation and rituximab can target that,” he added. That strategy is under investigation in the BLISS-BELIEVE trial, which should also report by the end of this year. It’s a much larger, phase 3 trial, involving nearly 300 patients and is sponsored by GSK.
“Clearly, this is a combination treatment [but] whether you give one before the other is uncertain,” Dr. Ehrenstein observed.
Another member of the viewing audience asked whether it would have been a fairer comparison if another dose of rituximab had been given to patients at week 24 instead of no treatment. Dr. Ehrenstein noted that it was a “good point” to make, but the investigators mainly wanted to answer whether giving belimumab after rituximab would target BAFF and thereby drop serum anti-dsDNA antibody levels. He said that a full trial of rituximab for patients with SLE, perhaps adding this extra dose, needs to be conducted.
Dr. Ehrenstein disclosed receiving research funding and educational grants from GSK and participating in advisory panels for the company.
Using belimumab after rituximab to treat patients with systemic lupus erythematosus (SLE) refractory to conventional therapy not only significantly decreased levels of serum IgG anti-dsDNA antibody levels but also prolonged the time before severe flares of disease occurred in the phase 2b BEAT-LUPUS (Belimumab after B cell depletion in SLE) study.
The trial’s primary outcome of serum IgG anti-dsDNA antibody levels showed a decline from a geometric mean of 162 IU/mL at baseline to 69 IU/mL at 24 weeks and 47 IU/mL at 1 year in patients treated with belimumab (Benlysta) after rituximab (Rituxan and biosimilars). These reductions were significantly lower than the values seen in the placebo after rituximab arm (a respective 121 IU/mL, 99 IU/mL, and 103 IU/mL; P < .001).
Just 3 patients who received belimumab versus 10 who received placebo after rituximab experienced a severe BILAG (British Isles Lupus Assessment Group) index A flare by the end of the study at 52 weeks. The hazard ratio (HR) for the flare reduction was 0.27 (P = .03), indicating a 73% reduction.
The use of belimumab rather than a placebo also led to a small reduction in total serum IgG, and significantly suppressed B-cell repopulation (P = .03).
These results need confirming in a larger, phase 3 trial, the trial’s principal investigator, Michael Ehrenstein, PhD, said at the annual European Congress of Rheumatology. They are “clearly encouraging” and “support the hypothesis that BAFF [B-cell–activating factor] can drive flares after rituximab,” he said.
Although B-cell depletion with rituximab is recommended by national and international guidelines to treat some patients with SLE who are refractory to conventional therapy, its use is not licensed.
“Certainly, rituximab is a controversial drug in lupus,” Dr. Ehrenstein, a consultant rheumatologist based at University College London, said in an interview. Although there is real-world evidence from registries and open-label studies suggesting that it is widely used and effective in some patients, the randomized, controlled trials conducted with rituximab about 10 years ago failed to meet their primary endpoints.
“A lot has been written about why that was, but probably the biggest reason was the high dose of steroids in both groups,” Dr. Ehrenstein said. To try to avoid muddying the waters of the BEAT-LUPUS trial findings, the maximum dose of prednisolone allowed to be used as background therapy was 20 mg/day. The trial’s investigators were also encouraged to reduce the baseline steroid dose to at least 50% by the trial’s 6-month halfway point.
“We tried to reflect what was going on in the U.K.,” Dr. Ehrenstein said, noting that the inspiration for the trial was a patient who had received sequential rituximab treatment. Although she got better with each cycle of rituximab, when her disease flared it would be worse than the time before, with increasingly higher anti-dsDNA levels recorded. The reason for this seemed to be because of increasing BAFF levels, and so the hypothesis was that if rituximab was associated with increased BAFF levels, then co-targeting BAFF with belimumab should be able to prevent those flares from happening.
The BEAT-LUPUS trial has been a huge collaborative effort and was conducted across 16 U.K. centers. From initial funding to the data analysis, it has taken 6 years to complete and was made possible by a unique partnership between Versus Arthritis, University College London Hospitals Biomedical Research Center, the National Institute for Health Research UK Musculoskeletal Translational Research Collaboration, and GlaxoSmithKline (GSK). GSK provided belimumab free of charge, as well as additional funding, but had no role in the design of the study and will not have any role going forward.
From an initial 172 patients assessed for eligibility, 52 patients were finally enrolled into the trial and received rituximab as two infusions given 2 weeks apart. Patients were then randomized in a double-blind manner to receive either belimumab (n = 26) or placebo (n = 26) 4-8 weeks after their first dose of rituximab. The intention-to-treat population consisted of 43 patients.
The use of belimumab after rituximab did not increase the risk for infection – serious or otherwise – or adverse effects, Dr. Ehrenstein reported. Serious adverse events were reported in six (23%) patients in each arm, and serious infections were seen in two (8%) of the belimumab- and four (15%) of the placebo-treated patients.
“I think the take-home message is that it seems that belimumab can reduce the number of severe flares that occur after rituximab therapy,” Dr. Ehrenstein said. “It’s promising, but not definitive,” he added. The next step is of course to publish these data and to perform a phase 3 trial.
In the discussion time following the presentation, session moderator Xavier Mariette, MD, PhD, of Bicêtre Hospital, Paris-Saclay University, asked why not give belimumab first before rituximab if using belimumab afterward works?
“Our strategy was driven by the observation that BAFF levels surged after rituximab, and therefore it’s logical to give the belimumab to block that BAFF surge,” he answered.
“Certainly, there are ideas that belimumab releases mature B cells into the circulation and rituximab can target that,” he added. That strategy is under investigation in the BLISS-BELIEVE trial, which should also report by the end of this year. It’s a much larger, phase 3 trial, involving nearly 300 patients and is sponsored by GSK.
“Clearly, this is a combination treatment [but] whether you give one before the other is uncertain,” Dr. Ehrenstein observed.
Another member of the viewing audience asked whether it would have been a fairer comparison if another dose of rituximab had been given to patients at week 24 instead of no treatment. Dr. Ehrenstein noted that it was a “good point” to make, but the investigators mainly wanted to answer whether giving belimumab after rituximab would target BAFF and thereby drop serum anti-dsDNA antibody levels. He said that a full trial of rituximab for patients with SLE, perhaps adding this extra dose, needs to be conducted.
Dr. Ehrenstein disclosed receiving research funding and educational grants from GSK and participating in advisory panels for the company.
Using belimumab after rituximab to treat patients with systemic lupus erythematosus (SLE) refractory to conventional therapy not only significantly decreased levels of serum IgG anti-dsDNA antibody levels but also prolonged the time before severe flares of disease occurred in the phase 2b BEAT-LUPUS (Belimumab after B cell depletion in SLE) study.
The trial’s primary outcome of serum IgG anti-dsDNA antibody levels showed a decline from a geometric mean of 162 IU/mL at baseline to 69 IU/mL at 24 weeks and 47 IU/mL at 1 year in patients treated with belimumab (Benlysta) after rituximab (Rituxan and biosimilars). These reductions were significantly lower than the values seen in the placebo after rituximab arm (a respective 121 IU/mL, 99 IU/mL, and 103 IU/mL; P < .001).
Just 3 patients who received belimumab versus 10 who received placebo after rituximab experienced a severe BILAG (British Isles Lupus Assessment Group) index A flare by the end of the study at 52 weeks. The hazard ratio (HR) for the flare reduction was 0.27 (P = .03), indicating a 73% reduction.
The use of belimumab rather than a placebo also led to a small reduction in total serum IgG, and significantly suppressed B-cell repopulation (P = .03).
These results need confirming in a larger, phase 3 trial, the trial’s principal investigator, Michael Ehrenstein, PhD, said at the annual European Congress of Rheumatology. They are “clearly encouraging” and “support the hypothesis that BAFF [B-cell–activating factor] can drive flares after rituximab,” he said.
Although B-cell depletion with rituximab is recommended by national and international guidelines to treat some patients with SLE who are refractory to conventional therapy, its use is not licensed.
“Certainly, rituximab is a controversial drug in lupus,” Dr. Ehrenstein, a consultant rheumatologist based at University College London, said in an interview. Although there is real-world evidence from registries and open-label studies suggesting that it is widely used and effective in some patients, the randomized, controlled trials conducted with rituximab about 10 years ago failed to meet their primary endpoints.
“A lot has been written about why that was, but probably the biggest reason was the high dose of steroids in both groups,” Dr. Ehrenstein said. To try to avoid muddying the waters of the BEAT-LUPUS trial findings, the maximum dose of prednisolone allowed to be used as background therapy was 20 mg/day. The trial’s investigators were also encouraged to reduce the baseline steroid dose to at least 50% by the trial’s 6-month halfway point.
“We tried to reflect what was going on in the U.K.,” Dr. Ehrenstein said, noting that the inspiration for the trial was a patient who had received sequential rituximab treatment. Although she got better with each cycle of rituximab, when her disease flared it would be worse than the time before, with increasingly higher anti-dsDNA levels recorded. The reason for this seemed to be because of increasing BAFF levels, and so the hypothesis was that if rituximab was associated with increased BAFF levels, then co-targeting BAFF with belimumab should be able to prevent those flares from happening.
The BEAT-LUPUS trial has been a huge collaborative effort and was conducted across 16 U.K. centers. From initial funding to the data analysis, it has taken 6 years to complete and was made possible by a unique partnership between Versus Arthritis, University College London Hospitals Biomedical Research Center, the National Institute for Health Research UK Musculoskeletal Translational Research Collaboration, and GlaxoSmithKline (GSK). GSK provided belimumab free of charge, as well as additional funding, but had no role in the design of the study and will not have any role going forward.
From an initial 172 patients assessed for eligibility, 52 patients were finally enrolled into the trial and received rituximab as two infusions given 2 weeks apart. Patients were then randomized in a double-blind manner to receive either belimumab (n = 26) or placebo (n = 26) 4-8 weeks after their first dose of rituximab. The intention-to-treat population consisted of 43 patients.
The use of belimumab after rituximab did not increase the risk for infection – serious or otherwise – or adverse effects, Dr. Ehrenstein reported. Serious adverse events were reported in six (23%) patients in each arm, and serious infections were seen in two (8%) of the belimumab- and four (15%) of the placebo-treated patients.
“I think the take-home message is that it seems that belimumab can reduce the number of severe flares that occur after rituximab therapy,” Dr. Ehrenstein said. “It’s promising, but not definitive,” he added. The next step is of course to publish these data and to perform a phase 3 trial.
In the discussion time following the presentation, session moderator Xavier Mariette, MD, PhD, of Bicêtre Hospital, Paris-Saclay University, asked why not give belimumab first before rituximab if using belimumab afterward works?
“Our strategy was driven by the observation that BAFF levels surged after rituximab, and therefore it’s logical to give the belimumab to block that BAFF surge,” he answered.
“Certainly, there are ideas that belimumab releases mature B cells into the circulation and rituximab can target that,” he added. That strategy is under investigation in the BLISS-BELIEVE trial, which should also report by the end of this year. It’s a much larger, phase 3 trial, involving nearly 300 patients and is sponsored by GSK.
“Clearly, this is a combination treatment [but] whether you give one before the other is uncertain,” Dr. Ehrenstein observed.
Another member of the viewing audience asked whether it would have been a fairer comparison if another dose of rituximab had been given to patients at week 24 instead of no treatment. Dr. Ehrenstein noted that it was a “good point” to make, but the investigators mainly wanted to answer whether giving belimumab after rituximab would target BAFF and thereby drop serum anti-dsDNA antibody levels. He said that a full trial of rituximab for patients with SLE, perhaps adding this extra dose, needs to be conducted.
Dr. Ehrenstein disclosed receiving research funding and educational grants from GSK and participating in advisory panels for the company.
FROM THE EULAR 2021 CONGRESS
Report shows decline in Black ob.gyn. residents from 2014 to 2019
There has been a steady decline in the proportion of Black ob.gyn. residents from 2014 to 2019, according to new research published in JAMA Network Open.
Researchers found that Black residents made up 10.2% of ob.gyn. residents during the 2014-2015 academic year, compared with 7.9% in 2018-2019. Meanwhile, Native American or Alaskan Native and Native Hawaiian or Pacific Islander residents were the least represented in the field, making up just 0.2% of residents in 2014 and 0.1% in 2015.
“When we look at the trend [of Black residents] across several years, it’s surprising that not only is the proportion of [ob.gyn.] Black residents [decreasing], but it was going down at a faster rate than other specialties,” study author Claudia Lopez, MD, said in an interview.
The ob.gyn. specialty tends to have the highest proportion of underrepresented physicians, especially Black and Latino physicians, compared with other specialties, according to a 2016 study published in Obstetrics & Gynecology. This study also found that underrepresented minority ob.gyns. were more likely than White or Asian physicians to practice in underserved areas. However, researchers of the current study found that the decline in Black residents in this field is surprising.
“I do think that ob.gyn. is very unique in that it’s surgical but also has a lot of primary care elements,” Dr. Lopez said. “I think that’s probably why initially our specialty historically has more underrepresented minorities because it combines all those things and [physicians are] so intimate with their patient population.”
Dr. Lopez, resident physician at the University of California, Davis, and colleagues analyzed deidentified data on the race and ethnicity of more than 520,000 residents in ob.gyn., surgical, and nonsurgical specialties from JAMA Medical Education reports from 2014 to 2019.
They found that ob.gyn., surgical, and nonsurgical residents most commonly identified as White, followed by Asian. In addition to the decline in Black ob.gyn. residents, researchers noticed that the proportion of Latino residents remained relatively unchanged. Furthermore, while the racial and ethnic composition of residents varied each year, higher proportions of ob.gyn. residents identified as Black or Latino, compared with those in surgical and nonsurgical specialties.
Researchers noted that, although their findings suggest ob.gyn. residencies have higher proportions of Black and Latino residents, compared with surgical and nonsurgical specialties, the diversity of the ob.gyn. programs lag behind the United States’ changing demographics.
“Medicine in general has a lot to do to match the [U.S. demographic] population,” Dr. Lopez said. “But at least the trend should hopefully be matching, showing some type of progression toward matching our population.”
Gnankang Sarah Napoe, MD, who was not involved in the study, said in an interview that she was saddened by the new findings and believes that if the decline in Black residents continues it would exacerbate racial disparities in obstetric and gynecological care.
“I think recruitment should focus more on specifically recruiting [underrepresented] populations of students into our field, because we know that they are a crucial part of narrowing the health disparities,” said Dr. Napoe, assistant professor* in the department of obstetrics and gynecology and reproductive sciences at the University of Pittsburgh.
Significant health disparities exist within women’s health and ob.gyn. care, with Black, American Indian, and Alaska Native women being two to three times more likely to have a pregnancy-related death than White women, according to the Centers for Disease Control and Prevention.
In an solicited commentary on the study, ob.gyns. from the University of Southern California, Los Angeles, referred to the declining population of Black ob.gyn. residents as “a failure of the medical education system to adapt to the changing demographic needs of its patients and cultivate diversity within the academic pipeline.”
One approach to addressing these health disparities is by increasing the diversity among health care practitioners. A 2020 study published in JAMA Network Open found that a shared identity between the physician and patient is linked to increased patient satisfaction and higher levels of trust.
“We know that, within ob.gyn., there are higher proportions of minority physicians, but just because we know that doesn’t mean that we’re doing everything right,” Dr. Lopez said. “When we look at the bigger picture,we’re not actually seeing the change we want to see. We need to not be complacent and keep evaluating ourselves, because I think that’s how you change.”
The authors and editorialists disclosed no relevant financial relationships.
*This article has been updated to reflect the correct title for Dr. Sarah Napoe.
There has been a steady decline in the proportion of Black ob.gyn. residents from 2014 to 2019, according to new research published in JAMA Network Open.
Researchers found that Black residents made up 10.2% of ob.gyn. residents during the 2014-2015 academic year, compared with 7.9% in 2018-2019. Meanwhile, Native American or Alaskan Native and Native Hawaiian or Pacific Islander residents were the least represented in the field, making up just 0.2% of residents in 2014 and 0.1% in 2015.
“When we look at the trend [of Black residents] across several years, it’s surprising that not only is the proportion of [ob.gyn.] Black residents [decreasing], but it was going down at a faster rate than other specialties,” study author Claudia Lopez, MD, said in an interview.
The ob.gyn. specialty tends to have the highest proportion of underrepresented physicians, especially Black and Latino physicians, compared with other specialties, according to a 2016 study published in Obstetrics & Gynecology. This study also found that underrepresented minority ob.gyns. were more likely than White or Asian physicians to practice in underserved areas. However, researchers of the current study found that the decline in Black residents in this field is surprising.
“I do think that ob.gyn. is very unique in that it’s surgical but also has a lot of primary care elements,” Dr. Lopez said. “I think that’s probably why initially our specialty historically has more underrepresented minorities because it combines all those things and [physicians are] so intimate with their patient population.”
Dr. Lopez, resident physician at the University of California, Davis, and colleagues analyzed deidentified data on the race and ethnicity of more than 520,000 residents in ob.gyn., surgical, and nonsurgical specialties from JAMA Medical Education reports from 2014 to 2019.
They found that ob.gyn., surgical, and nonsurgical residents most commonly identified as White, followed by Asian. In addition to the decline in Black ob.gyn. residents, researchers noticed that the proportion of Latino residents remained relatively unchanged. Furthermore, while the racial and ethnic composition of residents varied each year, higher proportions of ob.gyn. residents identified as Black or Latino, compared with those in surgical and nonsurgical specialties.
Researchers noted that, although their findings suggest ob.gyn. residencies have higher proportions of Black and Latino residents, compared with surgical and nonsurgical specialties, the diversity of the ob.gyn. programs lag behind the United States’ changing demographics.
“Medicine in general has a lot to do to match the [U.S. demographic] population,” Dr. Lopez said. “But at least the trend should hopefully be matching, showing some type of progression toward matching our population.”
Gnankang Sarah Napoe, MD, who was not involved in the study, said in an interview that she was saddened by the new findings and believes that if the decline in Black residents continues it would exacerbate racial disparities in obstetric and gynecological care.
“I think recruitment should focus more on specifically recruiting [underrepresented] populations of students into our field, because we know that they are a crucial part of narrowing the health disparities,” said Dr. Napoe, assistant professor* in the department of obstetrics and gynecology and reproductive sciences at the University of Pittsburgh.
Significant health disparities exist within women’s health and ob.gyn. care, with Black, American Indian, and Alaska Native women being two to three times more likely to have a pregnancy-related death than White women, according to the Centers for Disease Control and Prevention.
In an solicited commentary on the study, ob.gyns. from the University of Southern California, Los Angeles, referred to the declining population of Black ob.gyn. residents as “a failure of the medical education system to adapt to the changing demographic needs of its patients and cultivate diversity within the academic pipeline.”
One approach to addressing these health disparities is by increasing the diversity among health care practitioners. A 2020 study published in JAMA Network Open found that a shared identity between the physician and patient is linked to increased patient satisfaction and higher levels of trust.
“We know that, within ob.gyn., there are higher proportions of minority physicians, but just because we know that doesn’t mean that we’re doing everything right,” Dr. Lopez said. “When we look at the bigger picture,we’re not actually seeing the change we want to see. We need to not be complacent and keep evaluating ourselves, because I think that’s how you change.”
The authors and editorialists disclosed no relevant financial relationships.
*This article has been updated to reflect the correct title for Dr. Sarah Napoe.
There has been a steady decline in the proportion of Black ob.gyn. residents from 2014 to 2019, according to new research published in JAMA Network Open.
Researchers found that Black residents made up 10.2% of ob.gyn. residents during the 2014-2015 academic year, compared with 7.9% in 2018-2019. Meanwhile, Native American or Alaskan Native and Native Hawaiian or Pacific Islander residents were the least represented in the field, making up just 0.2% of residents in 2014 and 0.1% in 2015.
“When we look at the trend [of Black residents] across several years, it’s surprising that not only is the proportion of [ob.gyn.] Black residents [decreasing], but it was going down at a faster rate than other specialties,” study author Claudia Lopez, MD, said in an interview.
The ob.gyn. specialty tends to have the highest proportion of underrepresented physicians, especially Black and Latino physicians, compared with other specialties, according to a 2016 study published in Obstetrics & Gynecology. This study also found that underrepresented minority ob.gyns. were more likely than White or Asian physicians to practice in underserved areas. However, researchers of the current study found that the decline in Black residents in this field is surprising.
“I do think that ob.gyn. is very unique in that it’s surgical but also has a lot of primary care elements,” Dr. Lopez said. “I think that’s probably why initially our specialty historically has more underrepresented minorities because it combines all those things and [physicians are] so intimate with their patient population.”
Dr. Lopez, resident physician at the University of California, Davis, and colleagues analyzed deidentified data on the race and ethnicity of more than 520,000 residents in ob.gyn., surgical, and nonsurgical specialties from JAMA Medical Education reports from 2014 to 2019.
They found that ob.gyn., surgical, and nonsurgical residents most commonly identified as White, followed by Asian. In addition to the decline in Black ob.gyn. residents, researchers noticed that the proportion of Latino residents remained relatively unchanged. Furthermore, while the racial and ethnic composition of residents varied each year, higher proportions of ob.gyn. residents identified as Black or Latino, compared with those in surgical and nonsurgical specialties.
Researchers noted that, although their findings suggest ob.gyn. residencies have higher proportions of Black and Latino residents, compared with surgical and nonsurgical specialties, the diversity of the ob.gyn. programs lag behind the United States’ changing demographics.
“Medicine in general has a lot to do to match the [U.S. demographic] population,” Dr. Lopez said. “But at least the trend should hopefully be matching, showing some type of progression toward matching our population.”
Gnankang Sarah Napoe, MD, who was not involved in the study, said in an interview that she was saddened by the new findings and believes that if the decline in Black residents continues it would exacerbate racial disparities in obstetric and gynecological care.
“I think recruitment should focus more on specifically recruiting [underrepresented] populations of students into our field, because we know that they are a crucial part of narrowing the health disparities,” said Dr. Napoe, assistant professor* in the department of obstetrics and gynecology and reproductive sciences at the University of Pittsburgh.
Significant health disparities exist within women’s health and ob.gyn. care, with Black, American Indian, and Alaska Native women being two to three times more likely to have a pregnancy-related death than White women, according to the Centers for Disease Control and Prevention.
In an solicited commentary on the study, ob.gyns. from the University of Southern California, Los Angeles, referred to the declining population of Black ob.gyn. residents as “a failure of the medical education system to adapt to the changing demographic needs of its patients and cultivate diversity within the academic pipeline.”
One approach to addressing these health disparities is by increasing the diversity among health care practitioners. A 2020 study published in JAMA Network Open found that a shared identity between the physician and patient is linked to increased patient satisfaction and higher levels of trust.
“We know that, within ob.gyn., there are higher proportions of minority physicians, but just because we know that doesn’t mean that we’re doing everything right,” Dr. Lopez said. “When we look at the bigger picture,we’re not actually seeing the change we want to see. We need to not be complacent and keep evaluating ourselves, because I think that’s how you change.”
The authors and editorialists disclosed no relevant financial relationships.
*This article has been updated to reflect the correct title for Dr. Sarah Napoe.
FROM JAMA NETWORK OPEN
Sudden onset of severe pain in left thigh
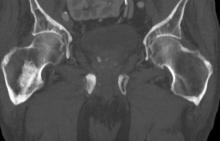
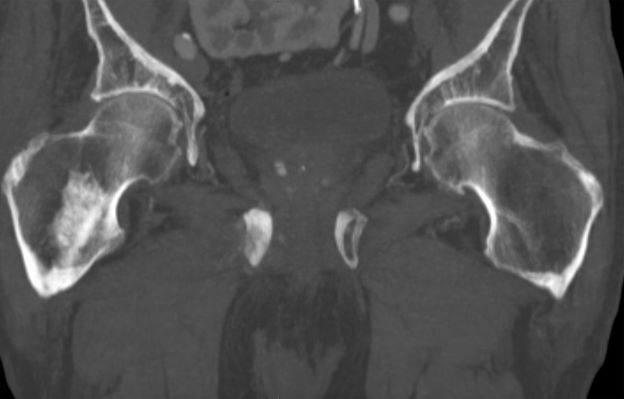
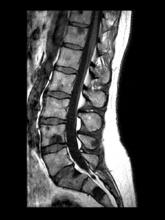
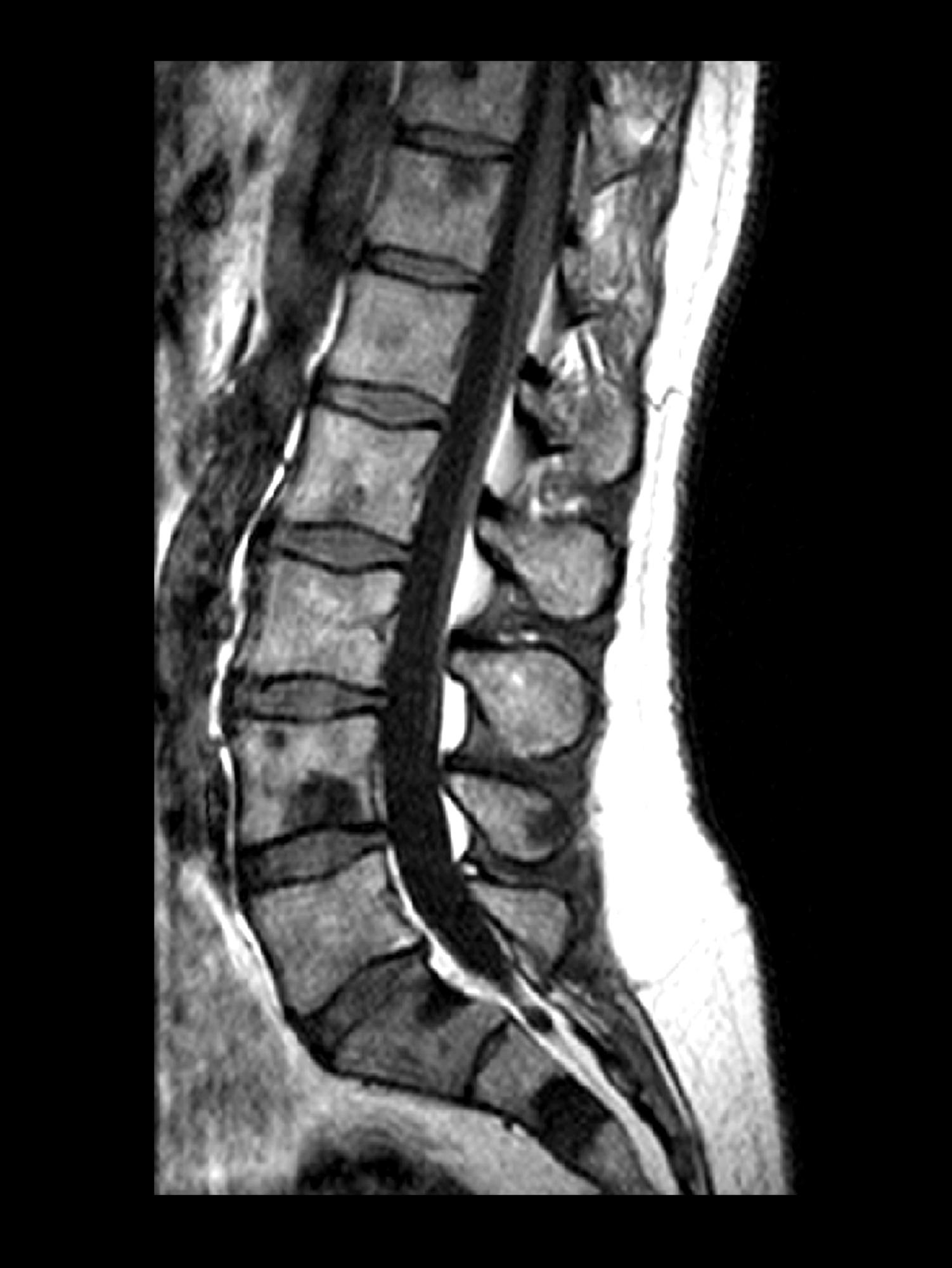
On the basis of the patient's physical examination, laboratory findings, and radiographic findings, a diagnosis of de novo metastatic prostate cancer is suspected and later confirmed by transrectal ultrasonography–guided needle biopsy of the prostate.
Prostate cancer is the most common cancer and the second most common cause of cancer-associated death in men in the United States. Among men diagnosed with prostate cancer in the United States, approximately three quarters have localized-stage disease at diagnosis; however, recent data show that an increasing number and percentage of men are being diagnosed with distant-stage prostate cancer. Despite advancements in treatment, less than one third of men survive 5 years after the diagnosis of distant-stage prostate cancer.
Prostate cancer frequently metastasizes to the bone. In fact, as many as 90% of patients with advanced prostate cancer have bone involvement. The morbidity from bone metastases is considerable and includes bone pain, immobility, pathologic fractures, hypercalcemia, hematologic disorders, and spinal cord compression. Bone metastases also have a considerable impact on mortality.
In patients with metastatic prostate cancer, determining the presence and extent of metastatic disease is essential for appropriate treatment to be selected. Studies have shown that the extent of metastatic disease affects treatment response. In a recent exploratory analysis of the STAMPEDE trial, survival benefit associated with prostate radiation therapy decreased continuously as the number of bone metastases rose, with the most benefit being seen in patients with up to three bone metastases.
Guidelines recommend that imaging studies be conducted in all patients with advanced prostate cancer. This may include conventional imaging (ie, CT, bone scan, and/or prostate MRI) and/or next-generation imaging (ie, PET, PET/CT, PET/MRI, whole-body MRI). In cases involving hormone-sensitive disease with obvious metastatic disease on conventional imaging at presentation, next-generation imaging may be useful for illuminating the disease burden and possibly shifting the treatment intent from multimodality management of oligometastatic disease to systemic anticancer therapy, either alone or in combination with targeted therapy for palliative purposes. However, prospective data on this are lacking.
Clinicians should also assess symptoms in patients with metastatic hormone-sensitive prostate cancer at presentation, because symptoms have been shown to have prognostic value. In addition, understanding symptoms related to cancer is essential for optimizing pain and other symptom management in addition to anticancer therapy.
Metastatic prostate cancer remains incurable. Immediate systemic treatment with androgen deprivation therapy (ADT) combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide should be offered to symptomatic patients who have distant metastases on diagnosis, both to alleviate symptoms and to lessen the risk for potential serious complications, such as spinal cord compression. ADT combined with docetaxel can also be offered to patients who are able to tolerate docetaxel.
ADT combined with prostate radiation therapy may be offered to patients with distant metastases and low-volume disease. However, when patients present with high-volume disease, referral to a clinical trial is recommended.
Surgery and/or local radiation therapy can be considered for patients with distant metastases and evidence of impending complications (eg, spinal cord compression or pathologic fracture).
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
On the basis of the patient's physical examination, laboratory findings, and radiographic findings, a diagnosis of de novo metastatic prostate cancer is suspected and later confirmed by transrectal ultrasonography–guided needle biopsy of the prostate.
Prostate cancer is the most common cancer and the second most common cause of cancer-associated death in men in the United States. Among men diagnosed with prostate cancer in the United States, approximately three quarters have localized-stage disease at diagnosis; however, recent data show that an increasing number and percentage of men are being diagnosed with distant-stage prostate cancer. Despite advancements in treatment, less than one third of men survive 5 years after the diagnosis of distant-stage prostate cancer.
Prostate cancer frequently metastasizes to the bone. In fact, as many as 90% of patients with advanced prostate cancer have bone involvement. The morbidity from bone metastases is considerable and includes bone pain, immobility, pathologic fractures, hypercalcemia, hematologic disorders, and spinal cord compression. Bone metastases also have a considerable impact on mortality.
In patients with metastatic prostate cancer, determining the presence and extent of metastatic disease is essential for appropriate treatment to be selected. Studies have shown that the extent of metastatic disease affects treatment response. In a recent exploratory analysis of the STAMPEDE trial, survival benefit associated with prostate radiation therapy decreased continuously as the number of bone metastases rose, with the most benefit being seen in patients with up to three bone metastases.
Guidelines recommend that imaging studies be conducted in all patients with advanced prostate cancer. This may include conventional imaging (ie, CT, bone scan, and/or prostate MRI) and/or next-generation imaging (ie, PET, PET/CT, PET/MRI, whole-body MRI). In cases involving hormone-sensitive disease with obvious metastatic disease on conventional imaging at presentation, next-generation imaging may be useful for illuminating the disease burden and possibly shifting the treatment intent from multimodality management of oligometastatic disease to systemic anticancer therapy, either alone or in combination with targeted therapy for palliative purposes. However, prospective data on this are lacking.
Clinicians should also assess symptoms in patients with metastatic hormone-sensitive prostate cancer at presentation, because symptoms have been shown to have prognostic value. In addition, understanding symptoms related to cancer is essential for optimizing pain and other symptom management in addition to anticancer therapy.
Metastatic prostate cancer remains incurable. Immediate systemic treatment with androgen deprivation therapy (ADT) combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide should be offered to symptomatic patients who have distant metastases on diagnosis, both to alleviate symptoms and to lessen the risk for potential serious complications, such as spinal cord compression. ADT combined with docetaxel can also be offered to patients who are able to tolerate docetaxel.
ADT combined with prostate radiation therapy may be offered to patients with distant metastases and low-volume disease. However, when patients present with high-volume disease, referral to a clinical trial is recommended.
Surgery and/or local radiation therapy can be considered for patients with distant metastases and evidence of impending complications (eg, spinal cord compression or pathologic fracture).
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
On the basis of the patient's physical examination, laboratory findings, and radiographic findings, a diagnosis of de novo metastatic prostate cancer is suspected and later confirmed by transrectal ultrasonography–guided needle biopsy of the prostate.
Prostate cancer is the most common cancer and the second most common cause of cancer-associated death in men in the United States. Among men diagnosed with prostate cancer in the United States, approximately three quarters have localized-stage disease at diagnosis; however, recent data show that an increasing number and percentage of men are being diagnosed with distant-stage prostate cancer. Despite advancements in treatment, less than one third of men survive 5 years after the diagnosis of distant-stage prostate cancer.
Prostate cancer frequently metastasizes to the bone. In fact, as many as 90% of patients with advanced prostate cancer have bone involvement. The morbidity from bone metastases is considerable and includes bone pain, immobility, pathologic fractures, hypercalcemia, hematologic disorders, and spinal cord compression. Bone metastases also have a considerable impact on mortality.
In patients with metastatic prostate cancer, determining the presence and extent of metastatic disease is essential for appropriate treatment to be selected. Studies have shown that the extent of metastatic disease affects treatment response. In a recent exploratory analysis of the STAMPEDE trial, survival benefit associated with prostate radiation therapy decreased continuously as the number of bone metastases rose, with the most benefit being seen in patients with up to three bone metastases.
Guidelines recommend that imaging studies be conducted in all patients with advanced prostate cancer. This may include conventional imaging (ie, CT, bone scan, and/or prostate MRI) and/or next-generation imaging (ie, PET, PET/CT, PET/MRI, whole-body MRI). In cases involving hormone-sensitive disease with obvious metastatic disease on conventional imaging at presentation, next-generation imaging may be useful for illuminating the disease burden and possibly shifting the treatment intent from multimodality management of oligometastatic disease to systemic anticancer therapy, either alone or in combination with targeted therapy for palliative purposes. However, prospective data on this are lacking.
Clinicians should also assess symptoms in patients with metastatic hormone-sensitive prostate cancer at presentation, because symptoms have been shown to have prognostic value. In addition, understanding symptoms related to cancer is essential for optimizing pain and other symptom management in addition to anticancer therapy.
Metastatic prostate cancer remains incurable. Immediate systemic treatment with androgen deprivation therapy (ADT) combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide should be offered to symptomatic patients who have distant metastases on diagnosis, both to alleviate symptoms and to lessen the risk for potential serious complications, such as spinal cord compression. ADT combined with docetaxel can also be offered to patients who are able to tolerate docetaxel.
ADT combined with prostate radiation therapy may be offered to patients with distant metastases and low-volume disease. However, when patients present with high-volume disease, referral to a clinical trial is recommended.
Surgery and/or local radiation therapy can be considered for patients with distant metastases and evidence of impending complications (eg, spinal cord compression or pathologic fracture).
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
A 74-year-old man presents to the emergency department with sudden onset of severe pain in his left thigh, a 3-month history of unexplained weight loss and general weakness, and progressive difficulty in walking for the past 3 weeks. The patient states that he has always been in excellent health and he has not seen a physician in at least 10 years. Cachexia is noted on physical examination. Laboratory findings include hemoglobin, 12.4 g/dL; white blood cells, 8.12 cells/µL; platelets, 310,000 cells/µL; creatinine, 1.4 mg/dL; sodium, 137 mmol/L; potassium, 4.4 mmol/L; calcium, 10.1 mg/dL; prostate-specific antigen, 31 ng/mL; aspartate aminotransferase, 37 IU/L; and gamma-glutamyltransferase, 16 IU/L. Proteinuria and hematuria are detected by urinalysis. CT reveals multiple diffuse osteoblastic lesions in the right proximal femur.
Patients and providers alike support virtual prenatal care
Obstetric patients and clinicians both overwhelmingly reported that telehealth was a safer way to receive ob.gyn. care and improve health care access during the COVID-19 pandemic, according to a survey at a single institution. The findings, from the Vanderbilt University Medical Center in Nashville, Tenn., were presented in a poster at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“The COVID-19 pandemic caused rapid and broad expansion of tele-obstetrics, warranting the need to assess patient and provider experiences and opinions about these services,” Karampreet Kaur, a 4th-year MD candidate at Vanderbilt University, and colleagues wrote in the poster. The group’s findings led them to conclude that virtual choices for prenatal care should be available independent of the pandemic.
Neel Shah, MD, assistant professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston, and founding director of the Delivery Decisions Initiative at Harvard’s Ariadne Labs, agreed that the study results supported continuation of telehealth even without COVID-19. Dr. Shah was not involved with the research.
“The fact that telehealth is broadly acceptable is not surprising but the magnitudes are striking,” Dr. Shah said in an interview. “Both providers and patients overwhelmingly see telehealth as a value-added fixture of obstetrical care that should be sustained beyond the pandemic.”
The researchers conducted an online survey of both obstetrical patients who received virtual prenatal care and ob.gyn. department providers, including MDs, DOs, advanced practice providers, genetic counselors, social workers, and registered dietitians.
Just over half (53%) of the 167 patients who completed the survey between June 2020 and April 2021 were between the ages 25 and 34. The remaining patients included 13% between ages 18 and 24 and 35% between ages 35 and 44. Most of these patients (84%) were at home for their telehealth appointment, but 16% were at a clinic for the telehealth appointment.
A quarter of the patients had a telehealth visit with a genetic counselor (26%) while 44% of patients saw an ob generalist and 28% saw a maternal fetal medicine specialist. Only 1% reported a social worker visit.
The majority of patients (75%) reported that they felt personally safer using telehealth rather than an in-person visit, and 18% said they would have forgone care if telehealth were not an option. Similarly, 74% of patients said the virtual care reduced their travel time, and 46% said they saved at least $35 in transportation, child care, or missed wages. More than half the patients surveyed were satisfied with their telehealth experience and believe Tennessee should have a tele-obstetrics program.
“The fact that a significant number of patients would have forgone care, and that nearly all providers observed improvements in access, makes widespread adoption of telehealth a moral imperative,” Dr. Shah said. “Telehealth and other forms of virtual care require rethinking our standard care models,” he added. “Traditional prenatal care for example is based on a model that is nearly a century old and may not meet the needs of many people. The experimentation with new ways of providing care that the pandemic forced should be an ongoing effort to ensure every person giving birth receives the care they deserve.”
Medical doctors (MD and DO) made up 53% of the 72 providers who completed the survey between June and August 2020, and a little over a third (36%) were advanced practice providers. Nearly all the providers (more than 95%) agreed with the statement that “telehealth was safer than in-clinic appointments for themselves, colleagues, and obstetrical patients.” Similar majorities felt telehealth was an acceptable way to provide health care (94%) and that virtual care improved access to health care (96%).
Most of the providers (85%) also felt that telehealth provided an opportunity for high-quality communication with their patients. More than half the providers said they would be willing to use telehealth outside of the pandemic, and a similar proportion felt that “Vanderbilt telehealth is a positive program for the state of Tennessee.”
Though not an author of the study, another Vanderbilt ob.gyn. also believes the findings support exploring continued telehealth options for the patients and providers interested in it.
“Health care providers and patients alike can attest to the benefits of telehealth utilization, Etoi A. Garrison, MD, PhD, associate professor of maternal-fetal medicine at Vanderbilt University, said in an interview. She was particularly struck by the savings reported by patients. “These costs are difficult to quantify but can have a significant impact on patients’ day-to-day quality of life,” she said.
A limitation of the study is the lack of information on how many were invited to complete it, so it’s not possible to know if the results are representative of the majority of people who used telehealth services, Dr. Garrison added. Dr. Shah agreed but didn’t think that limitation diminished the clinical implications of the study.
“A relatively small number of patients and providers are surveyed over a long period of time in which the context of the pandemic varied significantly,” he said. “Nonetheless, the findings show strong and internally consistent beliefs among those receiving and providing care that telehealth is valuable.”
The authors and Dr. Shah reported no disclosures. Dr. Garrison reported receiving a grant from the Tennessee Maternal Mortality Review committee to create an Unconscious Bias Faculty Train-the-Trainer program.
Obstetric patients and clinicians both overwhelmingly reported that telehealth was a safer way to receive ob.gyn. care and improve health care access during the COVID-19 pandemic, according to a survey at a single institution. The findings, from the Vanderbilt University Medical Center in Nashville, Tenn., were presented in a poster at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“The COVID-19 pandemic caused rapid and broad expansion of tele-obstetrics, warranting the need to assess patient and provider experiences and opinions about these services,” Karampreet Kaur, a 4th-year MD candidate at Vanderbilt University, and colleagues wrote in the poster. The group’s findings led them to conclude that virtual choices for prenatal care should be available independent of the pandemic.
Neel Shah, MD, assistant professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston, and founding director of the Delivery Decisions Initiative at Harvard’s Ariadne Labs, agreed that the study results supported continuation of telehealth even without COVID-19. Dr. Shah was not involved with the research.
“The fact that telehealth is broadly acceptable is not surprising but the magnitudes are striking,” Dr. Shah said in an interview. “Both providers and patients overwhelmingly see telehealth as a value-added fixture of obstetrical care that should be sustained beyond the pandemic.”
The researchers conducted an online survey of both obstetrical patients who received virtual prenatal care and ob.gyn. department providers, including MDs, DOs, advanced practice providers, genetic counselors, social workers, and registered dietitians.
Just over half (53%) of the 167 patients who completed the survey between June 2020 and April 2021 were between the ages 25 and 34. The remaining patients included 13% between ages 18 and 24 and 35% between ages 35 and 44. Most of these patients (84%) were at home for their telehealth appointment, but 16% were at a clinic for the telehealth appointment.
A quarter of the patients had a telehealth visit with a genetic counselor (26%) while 44% of patients saw an ob generalist and 28% saw a maternal fetal medicine specialist. Only 1% reported a social worker visit.
The majority of patients (75%) reported that they felt personally safer using telehealth rather than an in-person visit, and 18% said they would have forgone care if telehealth were not an option. Similarly, 74% of patients said the virtual care reduced their travel time, and 46% said they saved at least $35 in transportation, child care, or missed wages. More than half the patients surveyed were satisfied with their telehealth experience and believe Tennessee should have a tele-obstetrics program.
“The fact that a significant number of patients would have forgone care, and that nearly all providers observed improvements in access, makes widespread adoption of telehealth a moral imperative,” Dr. Shah said. “Telehealth and other forms of virtual care require rethinking our standard care models,” he added. “Traditional prenatal care for example is based on a model that is nearly a century old and may not meet the needs of many people. The experimentation with new ways of providing care that the pandemic forced should be an ongoing effort to ensure every person giving birth receives the care they deserve.”
Medical doctors (MD and DO) made up 53% of the 72 providers who completed the survey between June and August 2020, and a little over a third (36%) were advanced practice providers. Nearly all the providers (more than 95%) agreed with the statement that “telehealth was safer than in-clinic appointments for themselves, colleagues, and obstetrical patients.” Similar majorities felt telehealth was an acceptable way to provide health care (94%) and that virtual care improved access to health care (96%).
Most of the providers (85%) also felt that telehealth provided an opportunity for high-quality communication with their patients. More than half the providers said they would be willing to use telehealth outside of the pandemic, and a similar proportion felt that “Vanderbilt telehealth is a positive program for the state of Tennessee.”
Though not an author of the study, another Vanderbilt ob.gyn. also believes the findings support exploring continued telehealth options for the patients and providers interested in it.
“Health care providers and patients alike can attest to the benefits of telehealth utilization, Etoi A. Garrison, MD, PhD, associate professor of maternal-fetal medicine at Vanderbilt University, said in an interview. She was particularly struck by the savings reported by patients. “These costs are difficult to quantify but can have a significant impact on patients’ day-to-day quality of life,” she said.
A limitation of the study is the lack of information on how many were invited to complete it, so it’s not possible to know if the results are representative of the majority of people who used telehealth services, Dr. Garrison added. Dr. Shah agreed but didn’t think that limitation diminished the clinical implications of the study.
“A relatively small number of patients and providers are surveyed over a long period of time in which the context of the pandemic varied significantly,” he said. “Nonetheless, the findings show strong and internally consistent beliefs among those receiving and providing care that telehealth is valuable.”
The authors and Dr. Shah reported no disclosures. Dr. Garrison reported receiving a grant from the Tennessee Maternal Mortality Review committee to create an Unconscious Bias Faculty Train-the-Trainer program.
Obstetric patients and clinicians both overwhelmingly reported that telehealth was a safer way to receive ob.gyn. care and improve health care access during the COVID-19 pandemic, according to a survey at a single institution. The findings, from the Vanderbilt University Medical Center in Nashville, Tenn., were presented in a poster at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“The COVID-19 pandemic caused rapid and broad expansion of tele-obstetrics, warranting the need to assess patient and provider experiences and opinions about these services,” Karampreet Kaur, a 4th-year MD candidate at Vanderbilt University, and colleagues wrote in the poster. The group’s findings led them to conclude that virtual choices for prenatal care should be available independent of the pandemic.
Neel Shah, MD, assistant professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston, and founding director of the Delivery Decisions Initiative at Harvard’s Ariadne Labs, agreed that the study results supported continuation of telehealth even without COVID-19. Dr. Shah was not involved with the research.
“The fact that telehealth is broadly acceptable is not surprising but the magnitudes are striking,” Dr. Shah said in an interview. “Both providers and patients overwhelmingly see telehealth as a value-added fixture of obstetrical care that should be sustained beyond the pandemic.”
The researchers conducted an online survey of both obstetrical patients who received virtual prenatal care and ob.gyn. department providers, including MDs, DOs, advanced practice providers, genetic counselors, social workers, and registered dietitians.
Just over half (53%) of the 167 patients who completed the survey between June 2020 and April 2021 were between the ages 25 and 34. The remaining patients included 13% between ages 18 and 24 and 35% between ages 35 and 44. Most of these patients (84%) were at home for their telehealth appointment, but 16% were at a clinic for the telehealth appointment.
A quarter of the patients had a telehealth visit with a genetic counselor (26%) while 44% of patients saw an ob generalist and 28% saw a maternal fetal medicine specialist. Only 1% reported a social worker visit.
The majority of patients (75%) reported that they felt personally safer using telehealth rather than an in-person visit, and 18% said they would have forgone care if telehealth were not an option. Similarly, 74% of patients said the virtual care reduced their travel time, and 46% said they saved at least $35 in transportation, child care, or missed wages. More than half the patients surveyed were satisfied with their telehealth experience and believe Tennessee should have a tele-obstetrics program.
“The fact that a significant number of patients would have forgone care, and that nearly all providers observed improvements in access, makes widespread adoption of telehealth a moral imperative,” Dr. Shah said. “Telehealth and other forms of virtual care require rethinking our standard care models,” he added. “Traditional prenatal care for example is based on a model that is nearly a century old and may not meet the needs of many people. The experimentation with new ways of providing care that the pandemic forced should be an ongoing effort to ensure every person giving birth receives the care they deserve.”
Medical doctors (MD and DO) made up 53% of the 72 providers who completed the survey between June and August 2020, and a little over a third (36%) were advanced practice providers. Nearly all the providers (more than 95%) agreed with the statement that “telehealth was safer than in-clinic appointments for themselves, colleagues, and obstetrical patients.” Similar majorities felt telehealth was an acceptable way to provide health care (94%) and that virtual care improved access to health care (96%).
Most of the providers (85%) also felt that telehealth provided an opportunity for high-quality communication with their patients. More than half the providers said they would be willing to use telehealth outside of the pandemic, and a similar proportion felt that “Vanderbilt telehealth is a positive program for the state of Tennessee.”
Though not an author of the study, another Vanderbilt ob.gyn. also believes the findings support exploring continued telehealth options for the patients and providers interested in it.
“Health care providers and patients alike can attest to the benefits of telehealth utilization, Etoi A. Garrison, MD, PhD, associate professor of maternal-fetal medicine at Vanderbilt University, said in an interview. She was particularly struck by the savings reported by patients. “These costs are difficult to quantify but can have a significant impact on patients’ day-to-day quality of life,” she said.
A limitation of the study is the lack of information on how many were invited to complete it, so it’s not possible to know if the results are representative of the majority of people who used telehealth services, Dr. Garrison added. Dr. Shah agreed but didn’t think that limitation diminished the clinical implications of the study.
“A relatively small number of patients and providers are surveyed over a long period of time in which the context of the pandemic varied significantly,” he said. “Nonetheless, the findings show strong and internally consistent beliefs among those receiving and providing care that telehealth is valuable.”
The authors and Dr. Shah reported no disclosures. Dr. Garrison reported receiving a grant from the Tennessee Maternal Mortality Review committee to create an Unconscious Bias Faculty Train-the-Trainer program.
FROM ACOG 2021
Adding daily steps linked to longer life
Taking more steps each day, in short spurts or longer bouts, was associated with a longer life in women older than 60 years, according to data from more than 16,000 participants in the ongoing Women’s Health Study.
The American Heart Association recommends at least 150 minutes per week of moderate physical activity, 75 minutes of vigorous physical activity, or a combination of both as fitness guidelines for adults. Walking is a safe and easy way for many adults to follow these guidelines, according to Christopher C. Moore, MS, a PhD candidate at the University of North Carolina at Chapel Hill.
The popularity of step counts reflect that they are simple and objective, and “focusing on steps can help promote an active lifestyle,” he said. Data on the impact of sporadic steps accumulated outside of longer bouts of activity on health outcomes are limited; however, technology advances in the form of fitness apps and wearable devices make it possible for researchers to track and measure the benefits of short periods of activity as well as longer periods.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, sponsored by the AHA, Mr. Moore and colleagues assessed data from women older than 60 years who used wearable step-counting devices to measure their daily steps and walking patterns.
The study population included 16,732 women enrolled in the Women’s Health Study, a longstanding study of heart disease, cancer, and disease prevention among women in the United States. The participants wore waist step counters 4-7 days a week during 2011-2015. The average of the women was 72 years; 96% were non-Hispanic White, and the average BMI was 26 kg/m2.
The researchers divided the total number of steps for each study participant into two groups: “bouted” steps, defined as 10 minutes or longer bouts of walking with few interruptions; and “sporadic” steps, defined as short spurts of walking during regular daily activities such as housework, taking the stairs, or walking to or from a car.
A total of 804 deaths occurred during an average of 6 years of follow-up. Each initial increase of 1,000 steps including sporadic or bouted steps was associated with a 28% decrease in death, compared with no daily steps (hazard ratio, 0.72).
Each increasing quartile of sporadic steps was linked with higher total steps per day, Mr. Moore said. “Initial increase in sporadic steps corresponded to the greatest reductions in mortality,” with a HR of 0.69 per additional sporadic steps below 3,200 per day, and the impact on reduced mortality plateaued at about 4,500 sporadic steps per day.
In further analysis, the researchers also found a roughly 32% decrease in death in participants who took more than 2,000 steps daily in uninterrupted bouts (HR, 0.69).
The study findings were limited by several factors, including the relatively short follow-up period and number of events, the assessment of steps at a single time point, and the mostly homogeneous population, Mr. Moore noted. Additional research is needed to assess whether the results are generalizable to men, younger women, and diverse racial and ethnic groups.
However, the results may have implications for public health messaging, he emphasized. The message is that, to impact longevity, the total volume of steps is more important than the type of activity through which they are accumulated.
“You can accumulate your steps through longer bouts of purposeful activity or through everyday behaviors such as walking to your car, taking the stairs, and doing housework,” Mr. Moore concluded.
Find a friend, both of you benefit
On the basis of this study and other available evidence, more steps daily are recommended for everyone, Nieca Goldberg, MD, a cardiologist at New York University Langone Health, said in an interview.
“You can increase minutes of walking and frequency of walking,” she said.
Dr. Goldberg emphasized that you don’t need a fancy app or wearable device to up your steps. She offered some tips to help overcome barriers to putting one foot in front of the other. “Take the steps instead of the elevator. Park your car farther from your destination so you can walk.” Also, you can help yourself and help a friend to better health. “Get a walking buddy so you can encourage each other to walk,” Dr. Goldberg added.
Mr. Moore and Dr. Goldberg had no financial conflicts to disclose. The Women’s Health Study is funded by Brigham and Women’s Hospital; the National Heart, Lung, and Blood Institute; and the National Cancer Institute. Mr. Moore was funded by a grant from the NHLBI but had no other financial conflicts to disclose.
Taking more steps each day, in short spurts or longer bouts, was associated with a longer life in women older than 60 years, according to data from more than 16,000 participants in the ongoing Women’s Health Study.
The American Heart Association recommends at least 150 minutes per week of moderate physical activity, 75 minutes of vigorous physical activity, or a combination of both as fitness guidelines for adults. Walking is a safe and easy way for many adults to follow these guidelines, according to Christopher C. Moore, MS, a PhD candidate at the University of North Carolina at Chapel Hill.
The popularity of step counts reflect that they are simple and objective, and “focusing on steps can help promote an active lifestyle,” he said. Data on the impact of sporadic steps accumulated outside of longer bouts of activity on health outcomes are limited; however, technology advances in the form of fitness apps and wearable devices make it possible for researchers to track and measure the benefits of short periods of activity as well as longer periods.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, sponsored by the AHA, Mr. Moore and colleagues assessed data from women older than 60 years who used wearable step-counting devices to measure their daily steps and walking patterns.
The study population included 16,732 women enrolled in the Women’s Health Study, a longstanding study of heart disease, cancer, and disease prevention among women in the United States. The participants wore waist step counters 4-7 days a week during 2011-2015. The average of the women was 72 years; 96% were non-Hispanic White, and the average BMI was 26 kg/m2.
The researchers divided the total number of steps for each study participant into two groups: “bouted” steps, defined as 10 minutes or longer bouts of walking with few interruptions; and “sporadic” steps, defined as short spurts of walking during regular daily activities such as housework, taking the stairs, or walking to or from a car.
A total of 804 deaths occurred during an average of 6 years of follow-up. Each initial increase of 1,000 steps including sporadic or bouted steps was associated with a 28% decrease in death, compared with no daily steps (hazard ratio, 0.72).
Each increasing quartile of sporadic steps was linked with higher total steps per day, Mr. Moore said. “Initial increase in sporadic steps corresponded to the greatest reductions in mortality,” with a HR of 0.69 per additional sporadic steps below 3,200 per day, and the impact on reduced mortality plateaued at about 4,500 sporadic steps per day.
In further analysis, the researchers also found a roughly 32% decrease in death in participants who took more than 2,000 steps daily in uninterrupted bouts (HR, 0.69).
The study findings were limited by several factors, including the relatively short follow-up period and number of events, the assessment of steps at a single time point, and the mostly homogeneous population, Mr. Moore noted. Additional research is needed to assess whether the results are generalizable to men, younger women, and diverse racial and ethnic groups.
However, the results may have implications for public health messaging, he emphasized. The message is that, to impact longevity, the total volume of steps is more important than the type of activity through which they are accumulated.
“You can accumulate your steps through longer bouts of purposeful activity or through everyday behaviors such as walking to your car, taking the stairs, and doing housework,” Mr. Moore concluded.
Find a friend, both of you benefit
On the basis of this study and other available evidence, more steps daily are recommended for everyone, Nieca Goldberg, MD, a cardiologist at New York University Langone Health, said in an interview.
“You can increase minutes of walking and frequency of walking,” she said.
Dr. Goldberg emphasized that you don’t need a fancy app or wearable device to up your steps. She offered some tips to help overcome barriers to putting one foot in front of the other. “Take the steps instead of the elevator. Park your car farther from your destination so you can walk.” Also, you can help yourself and help a friend to better health. “Get a walking buddy so you can encourage each other to walk,” Dr. Goldberg added.
Mr. Moore and Dr. Goldberg had no financial conflicts to disclose. The Women’s Health Study is funded by Brigham and Women’s Hospital; the National Heart, Lung, and Blood Institute; and the National Cancer Institute. Mr. Moore was funded by a grant from the NHLBI but had no other financial conflicts to disclose.
Taking more steps each day, in short spurts or longer bouts, was associated with a longer life in women older than 60 years, according to data from more than 16,000 participants in the ongoing Women’s Health Study.
The American Heart Association recommends at least 150 minutes per week of moderate physical activity, 75 minutes of vigorous physical activity, or a combination of both as fitness guidelines for adults. Walking is a safe and easy way for many adults to follow these guidelines, according to Christopher C. Moore, MS, a PhD candidate at the University of North Carolina at Chapel Hill.
The popularity of step counts reflect that they are simple and objective, and “focusing on steps can help promote an active lifestyle,” he said. Data on the impact of sporadic steps accumulated outside of longer bouts of activity on health outcomes are limited; however, technology advances in the form of fitness apps and wearable devices make it possible for researchers to track and measure the benefits of short periods of activity as well as longer periods.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, sponsored by the AHA, Mr. Moore and colleagues assessed data from women older than 60 years who used wearable step-counting devices to measure their daily steps and walking patterns.
The study population included 16,732 women enrolled in the Women’s Health Study, a longstanding study of heart disease, cancer, and disease prevention among women in the United States. The participants wore waist step counters 4-7 days a week during 2011-2015. The average of the women was 72 years; 96% were non-Hispanic White, and the average BMI was 26 kg/m2.
The researchers divided the total number of steps for each study participant into two groups: “bouted” steps, defined as 10 minutes or longer bouts of walking with few interruptions; and “sporadic” steps, defined as short spurts of walking during regular daily activities such as housework, taking the stairs, or walking to or from a car.
A total of 804 deaths occurred during an average of 6 years of follow-up. Each initial increase of 1,000 steps including sporadic or bouted steps was associated with a 28% decrease in death, compared with no daily steps (hazard ratio, 0.72).
Each increasing quartile of sporadic steps was linked with higher total steps per day, Mr. Moore said. “Initial increase in sporadic steps corresponded to the greatest reductions in mortality,” with a HR of 0.69 per additional sporadic steps below 3,200 per day, and the impact on reduced mortality plateaued at about 4,500 sporadic steps per day.
In further analysis, the researchers also found a roughly 32% decrease in death in participants who took more than 2,000 steps daily in uninterrupted bouts (HR, 0.69).
The study findings were limited by several factors, including the relatively short follow-up period and number of events, the assessment of steps at a single time point, and the mostly homogeneous population, Mr. Moore noted. Additional research is needed to assess whether the results are generalizable to men, younger women, and diverse racial and ethnic groups.
However, the results may have implications for public health messaging, he emphasized. The message is that, to impact longevity, the total volume of steps is more important than the type of activity through which they are accumulated.
“You can accumulate your steps through longer bouts of purposeful activity or through everyday behaviors such as walking to your car, taking the stairs, and doing housework,” Mr. Moore concluded.
Find a friend, both of you benefit
On the basis of this study and other available evidence, more steps daily are recommended for everyone, Nieca Goldberg, MD, a cardiologist at New York University Langone Health, said in an interview.
“You can increase minutes of walking and frequency of walking,” she said.
Dr. Goldberg emphasized that you don’t need a fancy app or wearable device to up your steps. She offered some tips to help overcome barriers to putting one foot in front of the other. “Take the steps instead of the elevator. Park your car farther from your destination so you can walk.” Also, you can help yourself and help a friend to better health. “Get a walking buddy so you can encourage each other to walk,” Dr. Goldberg added.
Mr. Moore and Dr. Goldberg had no financial conflicts to disclose. The Women’s Health Study is funded by Brigham and Women’s Hospital; the National Heart, Lung, and Blood Institute; and the National Cancer Institute. Mr. Moore was funded by a grant from the NHLBI but had no other financial conflicts to disclose.
FROM EPI LIFESTYLE 2021
Do anti–apo A-I antibodies link fatty liver disease and CVD?
Anti–apolipoprotein A-I (apo A-I) antibodies are common in nonalcoholic fatty liver disease and may not only drive its development but also underlie the link between NAFLD and cardiovascular disease, suggests a novel analysis.
Conducting a clinical analysis and a series of experiments, Sabrina Pagano, PhD, diagnostic department, Geneva University Hospital, and colleagues looked for anti–apo A-I antibodies in patients with NAFLD and then examined their impact on hepatic cells and inflammatory markers.
They found that nearly half of 137 patients with NAFLD were seropositive, and that the antibodies were associated with increased lipid accumulation in the liver, altered triglyceride metabolism, and proinflammatory effects on liver cells.
“We hypothesize that anti–apo A-I IgG may be a potential driver in the development of NAFLD, and further studies are needed to support anti–apo A-I IgG as a possible link between NAFLD and cardiovascular disease,” Dr. Pagano said.
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress.
Asked whether anti–apo A-I antibodies could represent a potential treatment target for NAFLD, Dr. Pagano said in an interview that they have “already developed a peptide that is recognized by the antibodies in order to try to reverse the anti–apo A-I deleterious effect.”
While this was successful in vitro, “unfortunately we didn’t observe ... the peptide reverse of these anti–apo A-I effects in mice, so ... for the moment it’s a little early,” to say whether it represents a promising target.
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, said that the results are “very interesting and encouraging.”
He said that his own global burden of disease analysis, which is set to be published soon, showed that the worldwide prevalence of NAFLD is 11%, “representing almost 900 million cases,” and a more than 33% increase in prevalence in the past 30 years.
Consequently, any “attempt to have effective, especially early, diagnosis and treatment,” is highly anticipated.
Dr. Banach said the findings from the experimental analyses are “very interesting and promising,” especially regarding the proinflammatory effects of anti–apo A-I antibodies.
However, he underlined that the clinical part, looking at antibody seropositivity in patients with NAFLD, was limited by the lack of a control group, and there was no indication as to what treatment the patients received, despite it being clear that many were obese.
Dr. Banach also believes that, taking into account the patient characteristics, it is likely that most of the patients had the more severe nonalcoholic steatohepatitis, and “it would be additionally useful to see the autoantibodies levels both in NASH and NAFLD.”
Nevertheless, the clinical utility of measuring anti–apo A-I antibodies is limited at this stage.
He said that the lack of “good, easy, and cheap diagnostic methods based on both laboratory and imaging data” for NAFLD means it would be difficult to determine whether assessing antibody seropositivity “might be indeed an added value.”
Independent predictors
Dr. Pagano explained that anti–apo A-I antibodies, which target the major protein fraction of HDL cholesterol, are independent predictors of cardiovascular events in high-risk populations.
They are also independently associated with cardiovascular disease in the general population, as well as atherosclerotic plaque vulnerability in both mice and humans.
She said that apo A-I antibodies have a metabolic role in vivo, and have been shown in vitro to disrupt cholesterol metabolism, promoting foam cell formation.
Studies have also indicated they play a role in hepatic fibrosis, predicting the development of cirrhosis in individuals with chronic hepatitis C infection.
The team therefore set out to determine the presence of anti–apo A-I antibodies in individuals with NAFLD, defined here as fatty acid levels greater than 5% of liver weight, as well as their effect on hepatic cells.
Working with colleagues at Magna Græcia University of Catanzaro (Italy), they obtained serum samples from 137 patients with NAFLD confirmed on ultrasound.
The patients had an average age of 49 years, and 48.9% were male. The median body mass index was 31.8 kg/m2. Cholesterol levels were typically in the intermediate range.
They found that 46% of the participants had anti–apo A-I IgG antibodies, “which is quite high when compared with the 15%-20% positivity that we retrieved from the general population,” Dr. Pagano said.
To explore the link between high anti–apo A-I antibodies and NAFLD, the team studied hepatic cells, treating them with anti–apo A-I IgG antibodies or control IgG antibodies, or leaving them untreated, for 24 hours.
This revealed that anti–apo A-I IgG antibodies were associated with a significant increase in liquid droplet content in hepatic cells, compared with both cells treated with control IgG (P = .0008), and untreated cells (P = .0002).
Next, the team immunized apo E knockout mice with anti–apo A-I or control IgG antibodies. After 16 weeks, they found there was a significant increase in liver lipid content in mice given anti–apo A-I antibodies versus those treated with controls (P = .03).
They then asked whether anti–apo A-I antibodies could affect triglyceride metabolism. They examined the expression of the transcription factor sterol regulatory element binding protein (SREBP) and regulation of the triglyceride and cholesterol pathways.
Treating hepatic cells again for 24 hours with anti–apo A-I IgG antibodies or control IgG antibodies, or leaving them untreated, showed that anti–apo A-I antibodies were associated with “dramatic” increases in the active form of SREBP.
They also found that expression of two key enzymes in the triglyceride pathway, fatty acid synthetase and glycerol phosphate acyltransferase, was substantially decreased in the presence anti–apo A-I antibodies.
In both experiments, the untreated hepatic cells and those exposed to control IgG antibodies showed no significant changes.
“These results suggest that negative feedback ... turns off these enzymes, probably due to the lipid overload that is found in the cells after 24 hours of anti–apo A-I treatment,” Dr. Pagano said.
Finally, the researchers observed that anti–apo A-I, but not control antibodies, were associated with increases in inflammatory markers in liver cells.
Specifically, exposure to the antibodies was linked to an approximately 10-fold increase in interleukin-6 levels, as well as an approximate 25-fold increase in IL-8, and around a 7-fold increase in tumor necrosis factor–alpha.
Dr. Pagano suggested that the inflammatory effects are “probably mediated by binding anti–apo A-I antibodies to toll-like receptor 2, which has been previously described in macrophages.”
No funding was declared. The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anti–apolipoprotein A-I (apo A-I) antibodies are common in nonalcoholic fatty liver disease and may not only drive its development but also underlie the link between NAFLD and cardiovascular disease, suggests a novel analysis.
Conducting a clinical analysis and a series of experiments, Sabrina Pagano, PhD, diagnostic department, Geneva University Hospital, and colleagues looked for anti–apo A-I antibodies in patients with NAFLD and then examined their impact on hepatic cells and inflammatory markers.
They found that nearly half of 137 patients with NAFLD were seropositive, and that the antibodies were associated with increased lipid accumulation in the liver, altered triglyceride metabolism, and proinflammatory effects on liver cells.
“We hypothesize that anti–apo A-I IgG may be a potential driver in the development of NAFLD, and further studies are needed to support anti–apo A-I IgG as a possible link between NAFLD and cardiovascular disease,” Dr. Pagano said.
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress.
Asked whether anti–apo A-I antibodies could represent a potential treatment target for NAFLD, Dr. Pagano said in an interview that they have “already developed a peptide that is recognized by the antibodies in order to try to reverse the anti–apo A-I deleterious effect.”
While this was successful in vitro, “unfortunately we didn’t observe ... the peptide reverse of these anti–apo A-I effects in mice, so ... for the moment it’s a little early,” to say whether it represents a promising target.
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, said that the results are “very interesting and encouraging.”
He said that his own global burden of disease analysis, which is set to be published soon, showed that the worldwide prevalence of NAFLD is 11%, “representing almost 900 million cases,” and a more than 33% increase in prevalence in the past 30 years.
Consequently, any “attempt to have effective, especially early, diagnosis and treatment,” is highly anticipated.
Dr. Banach said the findings from the experimental analyses are “very interesting and promising,” especially regarding the proinflammatory effects of anti–apo A-I antibodies.
However, he underlined that the clinical part, looking at antibody seropositivity in patients with NAFLD, was limited by the lack of a control group, and there was no indication as to what treatment the patients received, despite it being clear that many were obese.
Dr. Banach also believes that, taking into account the patient characteristics, it is likely that most of the patients had the more severe nonalcoholic steatohepatitis, and “it would be additionally useful to see the autoantibodies levels both in NASH and NAFLD.”
Nevertheless, the clinical utility of measuring anti–apo A-I antibodies is limited at this stage.
He said that the lack of “good, easy, and cheap diagnostic methods based on both laboratory and imaging data” for NAFLD means it would be difficult to determine whether assessing antibody seropositivity “might be indeed an added value.”
Independent predictors
Dr. Pagano explained that anti–apo A-I antibodies, which target the major protein fraction of HDL cholesterol, are independent predictors of cardiovascular events in high-risk populations.
They are also independently associated with cardiovascular disease in the general population, as well as atherosclerotic plaque vulnerability in both mice and humans.
She said that apo A-I antibodies have a metabolic role in vivo, and have been shown in vitro to disrupt cholesterol metabolism, promoting foam cell formation.
Studies have also indicated they play a role in hepatic fibrosis, predicting the development of cirrhosis in individuals with chronic hepatitis C infection.
The team therefore set out to determine the presence of anti–apo A-I antibodies in individuals with NAFLD, defined here as fatty acid levels greater than 5% of liver weight, as well as their effect on hepatic cells.
Working with colleagues at Magna Græcia University of Catanzaro (Italy), they obtained serum samples from 137 patients with NAFLD confirmed on ultrasound.
The patients had an average age of 49 years, and 48.9% were male. The median body mass index was 31.8 kg/m2. Cholesterol levels were typically in the intermediate range.
They found that 46% of the participants had anti–apo A-I IgG antibodies, “which is quite high when compared with the 15%-20% positivity that we retrieved from the general population,” Dr. Pagano said.
To explore the link between high anti–apo A-I antibodies and NAFLD, the team studied hepatic cells, treating them with anti–apo A-I IgG antibodies or control IgG antibodies, or leaving them untreated, for 24 hours.
This revealed that anti–apo A-I IgG antibodies were associated with a significant increase in liquid droplet content in hepatic cells, compared with both cells treated with control IgG (P = .0008), and untreated cells (P = .0002).
Next, the team immunized apo E knockout mice with anti–apo A-I or control IgG antibodies. After 16 weeks, they found there was a significant increase in liver lipid content in mice given anti–apo A-I antibodies versus those treated with controls (P = .03).
They then asked whether anti–apo A-I antibodies could affect triglyceride metabolism. They examined the expression of the transcription factor sterol regulatory element binding protein (SREBP) and regulation of the triglyceride and cholesterol pathways.
Treating hepatic cells again for 24 hours with anti–apo A-I IgG antibodies or control IgG antibodies, or leaving them untreated, showed that anti–apo A-I antibodies were associated with “dramatic” increases in the active form of SREBP.
They also found that expression of two key enzymes in the triglyceride pathway, fatty acid synthetase and glycerol phosphate acyltransferase, was substantially decreased in the presence anti–apo A-I antibodies.
In both experiments, the untreated hepatic cells and those exposed to control IgG antibodies showed no significant changes.
“These results suggest that negative feedback ... turns off these enzymes, probably due to the lipid overload that is found in the cells after 24 hours of anti–apo A-I treatment,” Dr. Pagano said.
Finally, the researchers observed that anti–apo A-I, but not control antibodies, were associated with increases in inflammatory markers in liver cells.
Specifically, exposure to the antibodies was linked to an approximately 10-fold increase in interleukin-6 levels, as well as an approximate 25-fold increase in IL-8, and around a 7-fold increase in tumor necrosis factor–alpha.
Dr. Pagano suggested that the inflammatory effects are “probably mediated by binding anti–apo A-I antibodies to toll-like receptor 2, which has been previously described in macrophages.”
No funding was declared. The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anti–apolipoprotein A-I (apo A-I) antibodies are common in nonalcoholic fatty liver disease and may not only drive its development but also underlie the link between NAFLD and cardiovascular disease, suggests a novel analysis.
Conducting a clinical analysis and a series of experiments, Sabrina Pagano, PhD, diagnostic department, Geneva University Hospital, and colleagues looked for anti–apo A-I antibodies in patients with NAFLD and then examined their impact on hepatic cells and inflammatory markers.
They found that nearly half of 137 patients with NAFLD were seropositive, and that the antibodies were associated with increased lipid accumulation in the liver, altered triglyceride metabolism, and proinflammatory effects on liver cells.
“We hypothesize that anti–apo A-I IgG may be a potential driver in the development of NAFLD, and further studies are needed to support anti–apo A-I IgG as a possible link between NAFLD and cardiovascular disease,” Dr. Pagano said.
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress.
Asked whether anti–apo A-I antibodies could represent a potential treatment target for NAFLD, Dr. Pagano said in an interview that they have “already developed a peptide that is recognized by the antibodies in order to try to reverse the anti–apo A-I deleterious effect.”
While this was successful in vitro, “unfortunately we didn’t observe ... the peptide reverse of these anti–apo A-I effects in mice, so ... for the moment it’s a little early,” to say whether it represents a promising target.
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, said that the results are “very interesting and encouraging.”
He said that his own global burden of disease analysis, which is set to be published soon, showed that the worldwide prevalence of NAFLD is 11%, “representing almost 900 million cases,” and a more than 33% increase in prevalence in the past 30 years.
Consequently, any “attempt to have effective, especially early, diagnosis and treatment,” is highly anticipated.
Dr. Banach said the findings from the experimental analyses are “very interesting and promising,” especially regarding the proinflammatory effects of anti–apo A-I antibodies.
However, he underlined that the clinical part, looking at antibody seropositivity in patients with NAFLD, was limited by the lack of a control group, and there was no indication as to what treatment the patients received, despite it being clear that many were obese.
Dr. Banach also believes that, taking into account the patient characteristics, it is likely that most of the patients had the more severe nonalcoholic steatohepatitis, and “it would be additionally useful to see the autoantibodies levels both in NASH and NAFLD.”
Nevertheless, the clinical utility of measuring anti–apo A-I antibodies is limited at this stage.
He said that the lack of “good, easy, and cheap diagnostic methods based on both laboratory and imaging data” for NAFLD means it would be difficult to determine whether assessing antibody seropositivity “might be indeed an added value.”
Independent predictors
Dr. Pagano explained that anti–apo A-I antibodies, which target the major protein fraction of HDL cholesterol, are independent predictors of cardiovascular events in high-risk populations.
They are also independently associated with cardiovascular disease in the general population, as well as atherosclerotic plaque vulnerability in both mice and humans.
She said that apo A-I antibodies have a metabolic role in vivo, and have been shown in vitro to disrupt cholesterol metabolism, promoting foam cell formation.
Studies have also indicated they play a role in hepatic fibrosis, predicting the development of cirrhosis in individuals with chronic hepatitis C infection.
The team therefore set out to determine the presence of anti–apo A-I antibodies in individuals with NAFLD, defined here as fatty acid levels greater than 5% of liver weight, as well as their effect on hepatic cells.
Working with colleagues at Magna Græcia University of Catanzaro (Italy), they obtained serum samples from 137 patients with NAFLD confirmed on ultrasound.
The patients had an average age of 49 years, and 48.9% were male. The median body mass index was 31.8 kg/m2. Cholesterol levels were typically in the intermediate range.
They found that 46% of the participants had anti–apo A-I IgG antibodies, “which is quite high when compared with the 15%-20% positivity that we retrieved from the general population,” Dr. Pagano said.
To explore the link between high anti–apo A-I antibodies and NAFLD, the team studied hepatic cells, treating them with anti–apo A-I IgG antibodies or control IgG antibodies, or leaving them untreated, for 24 hours.
This revealed that anti–apo A-I IgG antibodies were associated with a significant increase in liquid droplet content in hepatic cells, compared with both cells treated with control IgG (P = .0008), and untreated cells (P = .0002).
Next, the team immunized apo E knockout mice with anti–apo A-I or control IgG antibodies. After 16 weeks, they found there was a significant increase in liver lipid content in mice given anti–apo A-I antibodies versus those treated with controls (P = .03).
They then asked whether anti–apo A-I antibodies could affect triglyceride metabolism. They examined the expression of the transcription factor sterol regulatory element binding protein (SREBP) and regulation of the triglyceride and cholesterol pathways.
Treating hepatic cells again for 24 hours with anti–apo A-I IgG antibodies or control IgG antibodies, or leaving them untreated, showed that anti–apo A-I antibodies were associated with “dramatic” increases in the active form of SREBP.
They also found that expression of two key enzymes in the triglyceride pathway, fatty acid synthetase and glycerol phosphate acyltransferase, was substantially decreased in the presence anti–apo A-I antibodies.
In both experiments, the untreated hepatic cells and those exposed to control IgG antibodies showed no significant changes.
“These results suggest that negative feedback ... turns off these enzymes, probably due to the lipid overload that is found in the cells after 24 hours of anti–apo A-I treatment,” Dr. Pagano said.
Finally, the researchers observed that anti–apo A-I, but not control antibodies, were associated with increases in inflammatory markers in liver cells.
Specifically, exposure to the antibodies was linked to an approximately 10-fold increase in interleukin-6 levels, as well as an approximate 25-fold increase in IL-8, and around a 7-fold increase in tumor necrosis factor–alpha.
Dr. Pagano suggested that the inflammatory effects are “probably mediated by binding anti–apo A-I antibodies to toll-like receptor 2, which has been previously described in macrophages.”
No funding was declared. The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine update: Uptake, effectiveness, and safety concerns
REFERENCES
- CDC. COVID Data Tracker. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- WHO Coronavirus (COVID-19) Dashboard. Accessed June 3, 2021. https://covid19.who.int/
- CDC. Demographic trends of people receiving COVID-19 vaccinations in the United States. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- Fleming-Dutra K. CDC COVID-19 vaccine effectiveness studies. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/09-COVID-Fleming-Dutra-508.pdf
- Scobie H. Update on emerging SARS-CoV-2 variants and vaccine considerations. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf
REFERENCES
- CDC. COVID Data Tracker. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- WHO Coronavirus (COVID-19) Dashboard. Accessed June 3, 2021. https://covid19.who.int/
- CDC. Demographic trends of people receiving COVID-19 vaccinations in the United States. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- Fleming-Dutra K. CDC COVID-19 vaccine effectiveness studies. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/09-COVID-Fleming-Dutra-508.pdf
- Scobie H. Update on emerging SARS-CoV-2 variants and vaccine considerations. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf
REFERENCES
- CDC. COVID Data Tracker. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- WHO Coronavirus (COVID-19) Dashboard. Accessed June 3, 2021. https://covid19.who.int/
- CDC. Demographic trends of people receiving COVID-19 vaccinations in the United States. Accessed June 3, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends
- Shimabukuro T. Update: thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf
- Fleming-Dutra K. CDC COVID-19 vaccine effectiveness studies. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/09-COVID-Fleming-Dutra-508.pdf
- Scobie H. Update on emerging SARS-CoV-2 variants and vaccine considerations. Presentation to the Advisory Committee on Immunization Practices, May 12, 2021. Accessed June 3, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/10-COVID-Scobie-508.pdf
Intermittent fever and gradually progressive low back pain
Transrectal ultrasonography–guided needle biopsy of the prostate confirms a diagnosis of metastatic prostate cancer.
With the exception of nonmelanoma skin cancer, prostate cancer is the most commonly occurring cancer and the second most common cause of cancer-associated mortality in men in the United States. Most patients have localized stage at diagnosis; however, the incidence of distant-stage prostate cancer at diagnosis is steadily increasing. Five-year survival for distant-stage prostate cancer is approximately 32%.
High serum levels of PSA have been associated with bone metastases in men with prostate cancer, and the presence of metastatic disease increases with rising PSA levels. Over the past several decades, PSA levels > 100 ng/mL have been used as a marker for metastatic prostate cancer. However, not all men with metastatic prostate cancer will have elevated PSA levels, and bone imaging is necessary for correct staging and treatment stratification.
Bone metastases occur in approximately 70% of men with advanced prostate cancer, most often in the spine, and are a leading cause of morbidity and mortality. Bone metastases can cause severe pain, particularly in the evening; decreased mobility; pathologic fractures; spinal cord compression; bone marrow aplasia; and hypercalcemia.
The bone marrow represents a fertile soil into which prostate tumors can colonize and proliferate. Such colonization by prostate tumor cells is commonly associated with tumor-induced bone lesions, which typically arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts generated by prostate cancer cells. Whereas most solid tumors, such as breast cancer and melanoma, have a propensity for causing osteolytic lesions with excessive bone resorption, bone lesions resulting from prostate cancer are largely osteoblastic and are associated with uncontrolled low-quality bone formation. The resultant metastases have a unique bone formation that can be detected by plain radiography, bone scan, bone biopsy, and increased serum alkaline phosphatase levels.
CT; skeletal scintigraphy and PET; and single-photon emission CT (SPECT)/CT, PET/CT, and PET/MRI are recommended diagnostics for men at risk for prostate cancer metastasis. Radiotracer-based PET, which mainly uses altered metabolic activity or explicitly overexpressed receptors, is a promising diagnostic modality. However, the choice of a respective radiotracer must be carefully considered because a single radiotracer is typically insufficient to visualize all clinical stages of prostate cancer. In addition, its use is reliant on the extent of malignant tissue, tumor heterogeneity, and previous treatments.
Systemic androgen-deprivation therapy, with or without docetaxel-based chemotherapy, is the standard of care for metastatic prostate cancer. Treatment is largely directed at preventing skeletal-related events and providing pain management.
Radium-223 is the only available therapy for castrate-resistant prostate cancer that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. Based on the results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for castrate-resistant prostate cancer with bone lesions.
The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
Transrectal ultrasonography–guided needle biopsy of the prostate confirms a diagnosis of metastatic prostate cancer.
With the exception of nonmelanoma skin cancer, prostate cancer is the most commonly occurring cancer and the second most common cause of cancer-associated mortality in men in the United States. Most patients have localized stage at diagnosis; however, the incidence of distant-stage prostate cancer at diagnosis is steadily increasing. Five-year survival for distant-stage prostate cancer is approximately 32%.
High serum levels of PSA have been associated with bone metastases in men with prostate cancer, and the presence of metastatic disease increases with rising PSA levels. Over the past several decades, PSA levels > 100 ng/mL have been used as a marker for metastatic prostate cancer. However, not all men with metastatic prostate cancer will have elevated PSA levels, and bone imaging is necessary for correct staging and treatment stratification.
Bone metastases occur in approximately 70% of men with advanced prostate cancer, most often in the spine, and are a leading cause of morbidity and mortality. Bone metastases can cause severe pain, particularly in the evening; decreased mobility; pathologic fractures; spinal cord compression; bone marrow aplasia; and hypercalcemia.
The bone marrow represents a fertile soil into which prostate tumors can colonize and proliferate. Such colonization by prostate tumor cells is commonly associated with tumor-induced bone lesions, which typically arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts generated by prostate cancer cells. Whereas most solid tumors, such as breast cancer and melanoma, have a propensity for causing osteolytic lesions with excessive bone resorption, bone lesions resulting from prostate cancer are largely osteoblastic and are associated with uncontrolled low-quality bone formation. The resultant metastases have a unique bone formation that can be detected by plain radiography, bone scan, bone biopsy, and increased serum alkaline phosphatase levels.
CT; skeletal scintigraphy and PET; and single-photon emission CT (SPECT)/CT, PET/CT, and PET/MRI are recommended diagnostics for men at risk for prostate cancer metastasis. Radiotracer-based PET, which mainly uses altered metabolic activity or explicitly overexpressed receptors, is a promising diagnostic modality. However, the choice of a respective radiotracer must be carefully considered because a single radiotracer is typically insufficient to visualize all clinical stages of prostate cancer. In addition, its use is reliant on the extent of malignant tissue, tumor heterogeneity, and previous treatments.
Systemic androgen-deprivation therapy, with or without docetaxel-based chemotherapy, is the standard of care for metastatic prostate cancer. Treatment is largely directed at preventing skeletal-related events and providing pain management.
Radium-223 is the only available therapy for castrate-resistant prostate cancer that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. Based on the results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for castrate-resistant prostate cancer with bone lesions.
The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
Transrectal ultrasonography–guided needle biopsy of the prostate confirms a diagnosis of metastatic prostate cancer.
With the exception of nonmelanoma skin cancer, prostate cancer is the most commonly occurring cancer and the second most common cause of cancer-associated mortality in men in the United States. Most patients have localized stage at diagnosis; however, the incidence of distant-stage prostate cancer at diagnosis is steadily increasing. Five-year survival for distant-stage prostate cancer is approximately 32%.
High serum levels of PSA have been associated with bone metastases in men with prostate cancer, and the presence of metastatic disease increases with rising PSA levels. Over the past several decades, PSA levels > 100 ng/mL have been used as a marker for metastatic prostate cancer. However, not all men with metastatic prostate cancer will have elevated PSA levels, and bone imaging is necessary for correct staging and treatment stratification.
Bone metastases occur in approximately 70% of men with advanced prostate cancer, most often in the spine, and are a leading cause of morbidity and mortality. Bone metastases can cause severe pain, particularly in the evening; decreased mobility; pathologic fractures; spinal cord compression; bone marrow aplasia; and hypercalcemia.
The bone marrow represents a fertile soil into which prostate tumors can colonize and proliferate. Such colonization by prostate tumor cells is commonly associated with tumor-induced bone lesions, which typically arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts generated by prostate cancer cells. Whereas most solid tumors, such as breast cancer and melanoma, have a propensity for causing osteolytic lesions with excessive bone resorption, bone lesions resulting from prostate cancer are largely osteoblastic and are associated with uncontrolled low-quality bone formation. The resultant metastases have a unique bone formation that can be detected by plain radiography, bone scan, bone biopsy, and increased serum alkaline phosphatase levels.
CT; skeletal scintigraphy and PET; and single-photon emission CT (SPECT)/CT, PET/CT, and PET/MRI are recommended diagnostics for men at risk for prostate cancer metastasis. Radiotracer-based PET, which mainly uses altered metabolic activity or explicitly overexpressed receptors, is a promising diagnostic modality. However, the choice of a respective radiotracer must be carefully considered because a single radiotracer is typically insufficient to visualize all clinical stages of prostate cancer. In addition, its use is reliant on the extent of malignant tissue, tumor heterogeneity, and previous treatments.
Systemic androgen-deprivation therapy, with or without docetaxel-based chemotherapy, is the standard of care for metastatic prostate cancer. Treatment is largely directed at preventing skeletal-related events and providing pain management.
Radium-223 is the only available therapy for castrate-resistant prostate cancer that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. Based on the results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for castrate-resistant prostate cancer with bone lesions.
The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
A 71-year-old homeless man presents to the emergency department (ED) with intermittent fever, gradually progressive low back pain restricting physical activities and movement, fatigue, exertional dyspnea, and poor appetite. The patient has been seen in the same ED sporadically over the years for various problems, and his medical history is notable for chronic obstructive pulmonary disease, tobacco use, alcoholism, and foot infections. Physical examination findings include tenderness to percussion over the thoracic and lumbar spine and a mildly enlarged prostate that appears to be smooth, normal in texture, and lacking nodules on digital rectal exam. Complete blood cell count and chemistry panel are normal. Both alkaline phosphatase and prostate-specific antigen (PSA) levels are elevated, at 240 U/L and 115 ng/mL, respectively. Urinalysis shows hematuria. CT shows osteolytic lesions in the patient's lumbar spine and femur.
Emotional support animals help lick symptoms of depression, anxiety in serious mental illness
Use of emotional support animals (ESAs) yields quantifiable reductions in depression, anxiety, and loneliness for patients with serious mental illness (SMI) who live alone, early research suggests.
Investigators followed 11 community-dwelling adults with SMI who were paired with a shelter dog or cat for 1 year. Participants’ depression, anxiety, and loneliness were assessed at baseline and 12 months after receipt of their ESAs.
At regular home visits during the study, participants also underwent saliva testing before playing with their pets and after 10 minutes of enjoyable pet interaction to assess levels of oxytocin – a biomarker associated with bonding – as well as cortisol and alpha amylase, which are markers of stress.
Significant reductions in measures of anxiety, depression, and loneliness were found between baseline and 12 months for all participants. Moreover, there was a pattern of an increase in levels of oxytocin and a decrease in levels of cortisol after 10 minutes of ESA interaction, but the degree of change did not reach statistical significance.
“Although this was a small pilot study and the findings are correlational, rather than causal, we can nevertheless say from the self-report of this group of participants and from the data collected that having an emotional support animal was beneficial to their mental health,” lead author Janet Hoy-Gerlach, PhD, professor of social work, University of Toledo (Ohio), said in an interview.
“We feel this data is a strong justification for additional study, and we hope that it will be a catalyst for future research with larger samples and more rigorous methodology,” said Dr. Hoy-Gerlach, author of “Human-Animal Interaction: A Social Work Guide,” published by NASW Press in 2017.
The study was published online May 20 in Human Animal Interaction Bulletin.
Everyday interactions
An ESA is a “companion animal (pet) who helps to reduce disability-related impairment for a particular person through the animal’s presence and everyday interactions,” the authors wrote.
Unlike service animals, which perform specific functions, ESAs “provide benefits that fall along the same dimensions as the benefits of pets – physical, social, emotional, and psychological – and there is research supporting the role that animals can play in each of these arenas,” Dr. Hoy-Gerlach said.
ESAs require no special training. All that is needed is a letter from a medical or mental health professional “that the individual meets the definition of ‘disability’ under the Fair Housing Act and a companion animal is a needed disability-ameliorating accommodation and should be allowed in buildings that don’t ordinarily permit pets,” she noted.
There is currently no peer-reviewed research that focuses explicitly on the impact of ESAs in individuals with SMI. To investigate, the researchers turned to the Hope and Recovery Pet Program (HARP) – a community partnership of the University of Toledo, the Toledo Humane Society, and ProMedica, a large regional nonprofit Toledo-based health care system – that pairs community-living individuals who have depression and/or anxiety with shelter animals that require adoption. The program pays for pet food, supplies, and veterinary care for those unable to afford these.
Participants (n = 11; mean age, 53.67 years; 78% women) were recruited from the HARP program. Participants were required to be psychiatrically stable, have stable housing, live alone, be at risk for social isolation, have low income, be sober, and have no history of violence. Their primary diagnoses were major depressive disorder, bipolar disorder, and schizoaffective disorder (63%, 18%, and 18%, respectively).
Six participants adopted a cat, and five adopted a dog.
Prior to ESA adoption and at 12 months, participants completed the Beck Depression Inventory (BDI), the Beck Anxiety Inventory (BAI), and the UCLA Loneliness Scale Version 3.
Prior to ESA adoption and at 1, 3, 6, 9, and 12 months, saliva samples were collected from participants by researchers at the beginning of a home visit and then after 10 minutes of “focused pleasant interaction” with the ESA. The saliva was tested for oxytocin, alpha amylase, and cortisol.
Motivation, comfort, calm
The researchers found statistically significant decreases in UCLA Loneliness Scale scores from pre-ESA (mean [SD],59.20 [9.47]) to 12 months (49.90 [13.66], P = .004). The eta-squared statistic (.62) indicated a large effect size.
For 18 of the 20 items on the loneliness scale, mean values were lower after the intervention than before the intervention. Of these, four were statistically significant.
A statistically significant decrease in BDI total scores was also seen from pre-ESA to 12 months (21.09 [8.43] to 14.64 [7.03], respectively; P = .03). The eta-squared statistic (.41) indicated a large effect size.
Of the 21 items on the BDI scale, the mean value was lower for 19 after the intervention. Of these, five were statistically significant.
Similarly, a statistically significant decrease in BAI score was found from pre-ESA to 12 months (23.55 [9.81] to 17.73 [11.79], P = .049). The eta-squared statistic (.36) indicated a large effect size, although there were no statistically significant changes in individual item scores.
The researchers found “observable patterns” of decreases in cortisol and increases in oxytocin after the 10-minute enjoyable ESA interactions. The highest oxytocin increase occurred at 12 months; however, these improvements did not reach statistical significance.
Participants offered open-ended statements about the positive impact of their ESA on their mental health, Dr. Hoy-Gerlach said. “For example, they described feeling motivated to take better care of themselves because their ESA needed them. Some described feeling ‘comforted,’ distracted from symptoms, soothed, and calmed.
“There is definitely a place for ESAs, especially with mental health post pandemic, when we need all the resources that we can for those who can benefit,” she added.
Postpandemic mental health
Commenting on the study for this news organization, Christine Crawford, MD, MPH, assistant professor of psychiatry, Boston University, observed that ESAs “are not on the radar for a lot of clinicians, and a lot of clinicians don’t know about the science [supporting their use] or what an emotional support pet entails.
“ besides traditional forms of treatment, such as medication and therapy. Even a little relief is important, and having an emotional support pet is a good option,” said Dr. Crawford, associate medical director of the National Alliance on Mental Illness. She was not involved with the study.
The Kenneth A. Scott Charitable Trust provided grant funding. Dr. Hoy-Gerlach, her coauthors, and Dr. Crawford have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of emotional support animals (ESAs) yields quantifiable reductions in depression, anxiety, and loneliness for patients with serious mental illness (SMI) who live alone, early research suggests.
Investigators followed 11 community-dwelling adults with SMI who were paired with a shelter dog or cat for 1 year. Participants’ depression, anxiety, and loneliness were assessed at baseline and 12 months after receipt of their ESAs.
At regular home visits during the study, participants also underwent saliva testing before playing with their pets and after 10 minutes of enjoyable pet interaction to assess levels of oxytocin – a biomarker associated with bonding – as well as cortisol and alpha amylase, which are markers of stress.
Significant reductions in measures of anxiety, depression, and loneliness were found between baseline and 12 months for all participants. Moreover, there was a pattern of an increase in levels of oxytocin and a decrease in levels of cortisol after 10 minutes of ESA interaction, but the degree of change did not reach statistical significance.
“Although this was a small pilot study and the findings are correlational, rather than causal, we can nevertheless say from the self-report of this group of participants and from the data collected that having an emotional support animal was beneficial to their mental health,” lead author Janet Hoy-Gerlach, PhD, professor of social work, University of Toledo (Ohio), said in an interview.
“We feel this data is a strong justification for additional study, and we hope that it will be a catalyst for future research with larger samples and more rigorous methodology,” said Dr. Hoy-Gerlach, author of “Human-Animal Interaction: A Social Work Guide,” published by NASW Press in 2017.
The study was published online May 20 in Human Animal Interaction Bulletin.
Everyday interactions
An ESA is a “companion animal (pet) who helps to reduce disability-related impairment for a particular person through the animal’s presence and everyday interactions,” the authors wrote.
Unlike service animals, which perform specific functions, ESAs “provide benefits that fall along the same dimensions as the benefits of pets – physical, social, emotional, and psychological – and there is research supporting the role that animals can play in each of these arenas,” Dr. Hoy-Gerlach said.
ESAs require no special training. All that is needed is a letter from a medical or mental health professional “that the individual meets the definition of ‘disability’ under the Fair Housing Act and a companion animal is a needed disability-ameliorating accommodation and should be allowed in buildings that don’t ordinarily permit pets,” she noted.
There is currently no peer-reviewed research that focuses explicitly on the impact of ESAs in individuals with SMI. To investigate, the researchers turned to the Hope and Recovery Pet Program (HARP) – a community partnership of the University of Toledo, the Toledo Humane Society, and ProMedica, a large regional nonprofit Toledo-based health care system – that pairs community-living individuals who have depression and/or anxiety with shelter animals that require adoption. The program pays for pet food, supplies, and veterinary care for those unable to afford these.
Participants (n = 11; mean age, 53.67 years; 78% women) were recruited from the HARP program. Participants were required to be psychiatrically stable, have stable housing, live alone, be at risk for social isolation, have low income, be sober, and have no history of violence. Their primary diagnoses were major depressive disorder, bipolar disorder, and schizoaffective disorder (63%, 18%, and 18%, respectively).
Six participants adopted a cat, and five adopted a dog.
Prior to ESA adoption and at 12 months, participants completed the Beck Depression Inventory (BDI), the Beck Anxiety Inventory (BAI), and the UCLA Loneliness Scale Version 3.
Prior to ESA adoption and at 1, 3, 6, 9, and 12 months, saliva samples were collected from participants by researchers at the beginning of a home visit and then after 10 minutes of “focused pleasant interaction” with the ESA. The saliva was tested for oxytocin, alpha amylase, and cortisol.
Motivation, comfort, calm
The researchers found statistically significant decreases in UCLA Loneliness Scale scores from pre-ESA (mean [SD],59.20 [9.47]) to 12 months (49.90 [13.66], P = .004). The eta-squared statistic (.62) indicated a large effect size.
For 18 of the 20 items on the loneliness scale, mean values were lower after the intervention than before the intervention. Of these, four were statistically significant.
A statistically significant decrease in BDI total scores was also seen from pre-ESA to 12 months (21.09 [8.43] to 14.64 [7.03], respectively; P = .03). The eta-squared statistic (.41) indicated a large effect size.
Of the 21 items on the BDI scale, the mean value was lower for 19 after the intervention. Of these, five were statistically significant.
Similarly, a statistically significant decrease in BAI score was found from pre-ESA to 12 months (23.55 [9.81] to 17.73 [11.79], P = .049). The eta-squared statistic (.36) indicated a large effect size, although there were no statistically significant changes in individual item scores.
The researchers found “observable patterns” of decreases in cortisol and increases in oxytocin after the 10-minute enjoyable ESA interactions. The highest oxytocin increase occurred at 12 months; however, these improvements did not reach statistical significance.
Participants offered open-ended statements about the positive impact of their ESA on their mental health, Dr. Hoy-Gerlach said. “For example, they described feeling motivated to take better care of themselves because their ESA needed them. Some described feeling ‘comforted,’ distracted from symptoms, soothed, and calmed.
“There is definitely a place for ESAs, especially with mental health post pandemic, when we need all the resources that we can for those who can benefit,” she added.
Postpandemic mental health
Commenting on the study for this news organization, Christine Crawford, MD, MPH, assistant professor of psychiatry, Boston University, observed that ESAs “are not on the radar for a lot of clinicians, and a lot of clinicians don’t know about the science [supporting their use] or what an emotional support pet entails.
“ besides traditional forms of treatment, such as medication and therapy. Even a little relief is important, and having an emotional support pet is a good option,” said Dr. Crawford, associate medical director of the National Alliance on Mental Illness. She was not involved with the study.
The Kenneth A. Scott Charitable Trust provided grant funding. Dr. Hoy-Gerlach, her coauthors, and Dr. Crawford have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of emotional support animals (ESAs) yields quantifiable reductions in depression, anxiety, and loneliness for patients with serious mental illness (SMI) who live alone, early research suggests.
Investigators followed 11 community-dwelling adults with SMI who were paired with a shelter dog or cat for 1 year. Participants’ depression, anxiety, and loneliness were assessed at baseline and 12 months after receipt of their ESAs.
At regular home visits during the study, participants also underwent saliva testing before playing with their pets and after 10 minutes of enjoyable pet interaction to assess levels of oxytocin – a biomarker associated with bonding – as well as cortisol and alpha amylase, which are markers of stress.
Significant reductions in measures of anxiety, depression, and loneliness were found between baseline and 12 months for all participants. Moreover, there was a pattern of an increase in levels of oxytocin and a decrease in levels of cortisol after 10 minutes of ESA interaction, but the degree of change did not reach statistical significance.
“Although this was a small pilot study and the findings are correlational, rather than causal, we can nevertheless say from the self-report of this group of participants and from the data collected that having an emotional support animal was beneficial to their mental health,” lead author Janet Hoy-Gerlach, PhD, professor of social work, University of Toledo (Ohio), said in an interview.
“We feel this data is a strong justification for additional study, and we hope that it will be a catalyst for future research with larger samples and more rigorous methodology,” said Dr. Hoy-Gerlach, author of “Human-Animal Interaction: A Social Work Guide,” published by NASW Press in 2017.
The study was published online May 20 in Human Animal Interaction Bulletin.
Everyday interactions
An ESA is a “companion animal (pet) who helps to reduce disability-related impairment for a particular person through the animal’s presence and everyday interactions,” the authors wrote.
Unlike service animals, which perform specific functions, ESAs “provide benefits that fall along the same dimensions as the benefits of pets – physical, social, emotional, and psychological – and there is research supporting the role that animals can play in each of these arenas,” Dr. Hoy-Gerlach said.
ESAs require no special training. All that is needed is a letter from a medical or mental health professional “that the individual meets the definition of ‘disability’ under the Fair Housing Act and a companion animal is a needed disability-ameliorating accommodation and should be allowed in buildings that don’t ordinarily permit pets,” she noted.
There is currently no peer-reviewed research that focuses explicitly on the impact of ESAs in individuals with SMI. To investigate, the researchers turned to the Hope and Recovery Pet Program (HARP) – a community partnership of the University of Toledo, the Toledo Humane Society, and ProMedica, a large regional nonprofit Toledo-based health care system – that pairs community-living individuals who have depression and/or anxiety with shelter animals that require adoption. The program pays for pet food, supplies, and veterinary care for those unable to afford these.
Participants (n = 11; mean age, 53.67 years; 78% women) were recruited from the HARP program. Participants were required to be psychiatrically stable, have stable housing, live alone, be at risk for social isolation, have low income, be sober, and have no history of violence. Their primary diagnoses were major depressive disorder, bipolar disorder, and schizoaffective disorder (63%, 18%, and 18%, respectively).
Six participants adopted a cat, and five adopted a dog.
Prior to ESA adoption and at 12 months, participants completed the Beck Depression Inventory (BDI), the Beck Anxiety Inventory (BAI), and the UCLA Loneliness Scale Version 3.
Prior to ESA adoption and at 1, 3, 6, 9, and 12 months, saliva samples were collected from participants by researchers at the beginning of a home visit and then after 10 minutes of “focused pleasant interaction” with the ESA. The saliva was tested for oxytocin, alpha amylase, and cortisol.
Motivation, comfort, calm
The researchers found statistically significant decreases in UCLA Loneliness Scale scores from pre-ESA (mean [SD],59.20 [9.47]) to 12 months (49.90 [13.66], P = .004). The eta-squared statistic (.62) indicated a large effect size.
For 18 of the 20 items on the loneliness scale, mean values were lower after the intervention than before the intervention. Of these, four were statistically significant.
A statistically significant decrease in BDI total scores was also seen from pre-ESA to 12 months (21.09 [8.43] to 14.64 [7.03], respectively; P = .03). The eta-squared statistic (.41) indicated a large effect size.
Of the 21 items on the BDI scale, the mean value was lower for 19 after the intervention. Of these, five were statistically significant.
Similarly, a statistically significant decrease in BAI score was found from pre-ESA to 12 months (23.55 [9.81] to 17.73 [11.79], P = .049). The eta-squared statistic (.36) indicated a large effect size, although there were no statistically significant changes in individual item scores.
The researchers found “observable patterns” of decreases in cortisol and increases in oxytocin after the 10-minute enjoyable ESA interactions. The highest oxytocin increase occurred at 12 months; however, these improvements did not reach statistical significance.
Participants offered open-ended statements about the positive impact of their ESA on their mental health, Dr. Hoy-Gerlach said. “For example, they described feeling motivated to take better care of themselves because their ESA needed them. Some described feeling ‘comforted,’ distracted from symptoms, soothed, and calmed.
“There is definitely a place for ESAs, especially with mental health post pandemic, when we need all the resources that we can for those who can benefit,” she added.
Postpandemic mental health
Commenting on the study for this news organization, Christine Crawford, MD, MPH, assistant professor of psychiatry, Boston University, observed that ESAs “are not on the radar for a lot of clinicians, and a lot of clinicians don’t know about the science [supporting their use] or what an emotional support pet entails.
“ besides traditional forms of treatment, such as medication and therapy. Even a little relief is important, and having an emotional support pet is a good option,” said Dr. Crawford, associate medical director of the National Alliance on Mental Illness. She was not involved with the study.
The Kenneth A. Scott Charitable Trust provided grant funding. Dr. Hoy-Gerlach, her coauthors, and Dr. Crawford have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postop palliative care may improve outcomes for those undergoing high-risk surgery
Background: In the final year before death, surgery is common for many patients. Prior studies have shown that fewer than 38% of surgical patients receive palliative care services before death. Palliative care involvement has been shown to improve quality of life and coordination of care in surgical patients.
Study design: Retrospective cross-sectional analysis of administrative data.
Setting: 129 Veteran Affairs medical centers.
Synopsis: In a retrospective review of 95,204 patients who underwent high-risk surgical procedures, the authors identified a 90-day mortality rate of 6.0%. Only 3.5% of patients received a perioperative palliative care consult. Multivariate analysis of bereaved family survey scores of patients who died within 90 days of surgery showed that families of patients who received a palliative care consult were significantly more likely to rate the care (odds ratio, 1.47), end-of-life communication (OR, 1.43), and support (OR, 1.31) as excellent, compared with those who did not. The use of survey responses and the Veteran Affairs population possibly introduces selection bias and limitations to the generalizability of the study.
Bottom line: Palliative care consultation for patients undergoing high-risk surgery remains underutilized but may be beneficial for patients.
Citation: Yefimova M et al. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020 Jan 2;155(2):138-46.
Dr. Halford is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: In the final year before death, surgery is common for many patients. Prior studies have shown that fewer than 38% of surgical patients receive palliative care services before death. Palliative care involvement has been shown to improve quality of life and coordination of care in surgical patients.
Study design: Retrospective cross-sectional analysis of administrative data.
Setting: 129 Veteran Affairs medical centers.
Synopsis: In a retrospective review of 95,204 patients who underwent high-risk surgical procedures, the authors identified a 90-day mortality rate of 6.0%. Only 3.5% of patients received a perioperative palliative care consult. Multivariate analysis of bereaved family survey scores of patients who died within 90 days of surgery showed that families of patients who received a palliative care consult were significantly more likely to rate the care (odds ratio, 1.47), end-of-life communication (OR, 1.43), and support (OR, 1.31) as excellent, compared with those who did not. The use of survey responses and the Veteran Affairs population possibly introduces selection bias and limitations to the generalizability of the study.
Bottom line: Palliative care consultation for patients undergoing high-risk surgery remains underutilized but may be beneficial for patients.
Citation: Yefimova M et al. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020 Jan 2;155(2):138-46.
Dr. Halford is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: In the final year before death, surgery is common for many patients. Prior studies have shown that fewer than 38% of surgical patients receive palliative care services before death. Palliative care involvement has been shown to improve quality of life and coordination of care in surgical patients.
Study design: Retrospective cross-sectional analysis of administrative data.
Setting: 129 Veteran Affairs medical centers.
Synopsis: In a retrospective review of 95,204 patients who underwent high-risk surgical procedures, the authors identified a 90-day mortality rate of 6.0%. Only 3.5% of patients received a perioperative palliative care consult. Multivariate analysis of bereaved family survey scores of patients who died within 90 days of surgery showed that families of patients who received a palliative care consult were significantly more likely to rate the care (odds ratio, 1.47), end-of-life communication (OR, 1.43), and support (OR, 1.31) as excellent, compared with those who did not. The use of survey responses and the Veteran Affairs population possibly introduces selection bias and limitations to the generalizability of the study.
Bottom line: Palliative care consultation for patients undergoing high-risk surgery remains underutilized but may be beneficial for patients.
Citation: Yefimova M et al. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020 Jan 2;155(2):138-46.
Dr. Halford is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.