User login
Intravenous immunoglobulin controls dermatomyositis in phase 3 trial
Nearly 50% achieve moderate improvement or better
The first multinational, phase 3, placebo-controlled trial conducted with intravenous immunoglobulin therapy (IVIg) for dermatomyositis has confirmed significant efficacy and acceptable safety, according to data presented at the opening plenary abstract session of the annual European Congress of Rheumatology.
At the week 16 evaluation of the trial, called ProDERM, the response rates were 78.7% and 43.8% (P = .0008) for active therapy and placebo, respectively, reported Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh.
ProDERM is a “much-awaited study,” according to session moderator Hendrik Schulze-Koops, MD, PhD, of the division of rheumatology and clinical immunology at Ludwig Maximilian University of Munich (Germany). He was not involved in the study.
“We all have been doing what we have been doing,” Dr. Schulze-Koops said, referring to the use of IVIg for the control of dermatomyositis, “but we had no evidence for support.”
This statement could apply not only to IVIg, which has long been listed among treatment options by the Myositis Association despite the absence of controlled studies, but also to most immunosuppressive therapies and other options used for this challenging disease.
The proprietary IVIg employed in this study, Octagam 10%, has been approved in the United States for the treatment of chronic immune thrombocytopenic purpura. Its manufacturer, Octagam, plans to file a supplemental new drug application with the Food and Drug Administration for the treatment of dermatomyositis. The agent is already approved for dermatomyositis by the European Medicines Agency, according to Dr. Aggarwal.
Multiple response criteria favor IVIg
In the trial, 95 patients with dermatomyositis were randomized to 2 g/kg of IVIg (Octagam 10%) or placebo administered every 4 weeks. In a subsequent open-label extension study in which patients on placebo were switched to active therapy, the same every-4-week treatment schedule was used. The patients’ mean age was 53; 75% were women, and 92% were White.
The primary endpoint was at least minimal improvement on 2016 ACR/EULAR (American College of Rheumatology/European Alliance of Associations for Rheumatology) myositis response criteria, defined as a 20-point or greater gain in the Total Improvement Score (TIS) and no clinical worsening at two consecutive visits. But IVIg also provided a large relative benefit over placebo using more rigorous definitions of improvement. For moderate improvement, defined as at least a 40-point TIS improvement, there was a 45.2% relative advantage for IVIg over placebo (68.1% vs. 22.9%; P < .0001). For major improvement, defined as at least a 60-point TIS improvement, the relative advantage was 23.6% (31.9% vs. 8.3%; P < .0062).
At 16 weeks, the mean TIS score was more than twice as high in those receiving IVIg than in those randomized to placebo (48.4 vs. 21.6). At that point, an open-label extension was initiated. Those in the IVIg group were permitted to remain on therapy for an additional 24 weeks if they had not worsened in the blinded phase.
The mean TIS score in the IVIg group continued to rise during the extension phase. By 12 weeks in this phase, it reached 54.0. Over the same period, mean TIS scores climbed steeply among the placebo-treated patients who had switched to active therapy, reaching 44.4.
At the end of 24 weeks of the extension trial, when patients initiated on IVIg had been on active therapy for 40 weeks, the mean TIS score advantage of starting on IVIg rather than placebo was relatively modest (55.4 vs. 51.1).
Benefit is significant for skin and muscle
Changes in the two major components of dermatomyositis were tracked individually. For skin symptoms, patients were evaluated with the Cutaneous Dermatomyositis Disease Areas and Severity Index (CDASI). For muscle involvement, symptoms were evaluated with the 8-item Manual Muscle Testing (MMT-8) tool.
“The effects of IVIg on the muscle and the skin were both highly statistically significant,” Dr. Aggarwal reported. He said the CDASI score was reduced by almost half at the end of 16 weeks among those treated with IVIg relative to those treated with placebo. Improvement in MMT-8 scores were also clinically as well as statistically significant.
The IVIg therapy was well tolerated. The most common adverse effects in this study, like those reported with IVIg when used to treat other diseases, were headache, pyrexia, and nausea, but Dr. Aggarwal reported that these were generally mild.
Serious adverse events, particularly thromboembolism, did occur over the course of the study, but the rate of events was only slightly higher in the group receiving active therapy (5.8% vs. 4.2%).
Patients who entered the study were permitted to remain on most immunosuppressive therapies, such as methotrexate, mycophenolate, tacrolimus, and glucocorticoids. Dr. Aggarwal said that the majority of patients were taking a glucocorticoid and at least one nonglucocorticoid immunosuppressant.
Effect on associated conditions is planned
The data from this trial have not yet been analyzed for the impact of IVIg on conditions that occur frequently in association with dermatomyositis, such as interstitial lung disease (ILD) and dysphagia, but Dr. Aggarwal reported that there are plans to do so. Although severe ILD was a trial exclusion, the presence of mild to moderate ILD and dysphagia were evaluated at baseline, so the impact of treatment can be assessed.
There are also plans to evaluate how the presence or absence of myositis-specific antibodies, which were also evaluated at baseline, affected response to IVIg.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Dr. Schulze-Koops reported no relevant potential conflicts of interest.
Nearly 50% achieve moderate improvement or better
Nearly 50% achieve moderate improvement or better
The first multinational, phase 3, placebo-controlled trial conducted with intravenous immunoglobulin therapy (IVIg) for dermatomyositis has confirmed significant efficacy and acceptable safety, according to data presented at the opening plenary abstract session of the annual European Congress of Rheumatology.
At the week 16 evaluation of the trial, called ProDERM, the response rates were 78.7% and 43.8% (P = .0008) for active therapy and placebo, respectively, reported Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh.
ProDERM is a “much-awaited study,” according to session moderator Hendrik Schulze-Koops, MD, PhD, of the division of rheumatology and clinical immunology at Ludwig Maximilian University of Munich (Germany). He was not involved in the study.
“We all have been doing what we have been doing,” Dr. Schulze-Koops said, referring to the use of IVIg for the control of dermatomyositis, “but we had no evidence for support.”
This statement could apply not only to IVIg, which has long been listed among treatment options by the Myositis Association despite the absence of controlled studies, but also to most immunosuppressive therapies and other options used for this challenging disease.
The proprietary IVIg employed in this study, Octagam 10%, has been approved in the United States for the treatment of chronic immune thrombocytopenic purpura. Its manufacturer, Octagam, plans to file a supplemental new drug application with the Food and Drug Administration for the treatment of dermatomyositis. The agent is already approved for dermatomyositis by the European Medicines Agency, according to Dr. Aggarwal.
Multiple response criteria favor IVIg
In the trial, 95 patients with dermatomyositis were randomized to 2 g/kg of IVIg (Octagam 10%) or placebo administered every 4 weeks. In a subsequent open-label extension study in which patients on placebo were switched to active therapy, the same every-4-week treatment schedule was used. The patients’ mean age was 53; 75% were women, and 92% were White.
The primary endpoint was at least minimal improvement on 2016 ACR/EULAR (American College of Rheumatology/European Alliance of Associations for Rheumatology) myositis response criteria, defined as a 20-point or greater gain in the Total Improvement Score (TIS) and no clinical worsening at two consecutive visits. But IVIg also provided a large relative benefit over placebo using more rigorous definitions of improvement. For moderate improvement, defined as at least a 40-point TIS improvement, there was a 45.2% relative advantage for IVIg over placebo (68.1% vs. 22.9%; P < .0001). For major improvement, defined as at least a 60-point TIS improvement, the relative advantage was 23.6% (31.9% vs. 8.3%; P < .0062).
At 16 weeks, the mean TIS score was more than twice as high in those receiving IVIg than in those randomized to placebo (48.4 vs. 21.6). At that point, an open-label extension was initiated. Those in the IVIg group were permitted to remain on therapy for an additional 24 weeks if they had not worsened in the blinded phase.
The mean TIS score in the IVIg group continued to rise during the extension phase. By 12 weeks in this phase, it reached 54.0. Over the same period, mean TIS scores climbed steeply among the placebo-treated patients who had switched to active therapy, reaching 44.4.
At the end of 24 weeks of the extension trial, when patients initiated on IVIg had been on active therapy for 40 weeks, the mean TIS score advantage of starting on IVIg rather than placebo was relatively modest (55.4 vs. 51.1).
Benefit is significant for skin and muscle
Changes in the two major components of dermatomyositis were tracked individually. For skin symptoms, patients were evaluated with the Cutaneous Dermatomyositis Disease Areas and Severity Index (CDASI). For muscle involvement, symptoms were evaluated with the 8-item Manual Muscle Testing (MMT-8) tool.
“The effects of IVIg on the muscle and the skin were both highly statistically significant,” Dr. Aggarwal reported. He said the CDASI score was reduced by almost half at the end of 16 weeks among those treated with IVIg relative to those treated with placebo. Improvement in MMT-8 scores were also clinically as well as statistically significant.
The IVIg therapy was well tolerated. The most common adverse effects in this study, like those reported with IVIg when used to treat other diseases, were headache, pyrexia, and nausea, but Dr. Aggarwal reported that these were generally mild.
Serious adverse events, particularly thromboembolism, did occur over the course of the study, but the rate of events was only slightly higher in the group receiving active therapy (5.8% vs. 4.2%).
Patients who entered the study were permitted to remain on most immunosuppressive therapies, such as methotrexate, mycophenolate, tacrolimus, and glucocorticoids. Dr. Aggarwal said that the majority of patients were taking a glucocorticoid and at least one nonglucocorticoid immunosuppressant.
Effect on associated conditions is planned
The data from this trial have not yet been analyzed for the impact of IVIg on conditions that occur frequently in association with dermatomyositis, such as interstitial lung disease (ILD) and dysphagia, but Dr. Aggarwal reported that there are plans to do so. Although severe ILD was a trial exclusion, the presence of mild to moderate ILD and dysphagia were evaluated at baseline, so the impact of treatment can be assessed.
There are also plans to evaluate how the presence or absence of myositis-specific antibodies, which were also evaluated at baseline, affected response to IVIg.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Dr. Schulze-Koops reported no relevant potential conflicts of interest.
The first multinational, phase 3, placebo-controlled trial conducted with intravenous immunoglobulin therapy (IVIg) for dermatomyositis has confirmed significant efficacy and acceptable safety, according to data presented at the opening plenary abstract session of the annual European Congress of Rheumatology.
At the week 16 evaluation of the trial, called ProDERM, the response rates were 78.7% and 43.8% (P = .0008) for active therapy and placebo, respectively, reported Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh.
ProDERM is a “much-awaited study,” according to session moderator Hendrik Schulze-Koops, MD, PhD, of the division of rheumatology and clinical immunology at Ludwig Maximilian University of Munich (Germany). He was not involved in the study.
“We all have been doing what we have been doing,” Dr. Schulze-Koops said, referring to the use of IVIg for the control of dermatomyositis, “but we had no evidence for support.”
This statement could apply not only to IVIg, which has long been listed among treatment options by the Myositis Association despite the absence of controlled studies, but also to most immunosuppressive therapies and other options used for this challenging disease.
The proprietary IVIg employed in this study, Octagam 10%, has been approved in the United States for the treatment of chronic immune thrombocytopenic purpura. Its manufacturer, Octagam, plans to file a supplemental new drug application with the Food and Drug Administration for the treatment of dermatomyositis. The agent is already approved for dermatomyositis by the European Medicines Agency, according to Dr. Aggarwal.
Multiple response criteria favor IVIg
In the trial, 95 patients with dermatomyositis were randomized to 2 g/kg of IVIg (Octagam 10%) or placebo administered every 4 weeks. In a subsequent open-label extension study in which patients on placebo were switched to active therapy, the same every-4-week treatment schedule was used. The patients’ mean age was 53; 75% were women, and 92% were White.
The primary endpoint was at least minimal improvement on 2016 ACR/EULAR (American College of Rheumatology/European Alliance of Associations for Rheumatology) myositis response criteria, defined as a 20-point or greater gain in the Total Improvement Score (TIS) and no clinical worsening at two consecutive visits. But IVIg also provided a large relative benefit over placebo using more rigorous definitions of improvement. For moderate improvement, defined as at least a 40-point TIS improvement, there was a 45.2% relative advantage for IVIg over placebo (68.1% vs. 22.9%; P < .0001). For major improvement, defined as at least a 60-point TIS improvement, the relative advantage was 23.6% (31.9% vs. 8.3%; P < .0062).
At 16 weeks, the mean TIS score was more than twice as high in those receiving IVIg than in those randomized to placebo (48.4 vs. 21.6). At that point, an open-label extension was initiated. Those in the IVIg group were permitted to remain on therapy for an additional 24 weeks if they had not worsened in the blinded phase.
The mean TIS score in the IVIg group continued to rise during the extension phase. By 12 weeks in this phase, it reached 54.0. Over the same period, mean TIS scores climbed steeply among the placebo-treated patients who had switched to active therapy, reaching 44.4.
At the end of 24 weeks of the extension trial, when patients initiated on IVIg had been on active therapy for 40 weeks, the mean TIS score advantage of starting on IVIg rather than placebo was relatively modest (55.4 vs. 51.1).
Benefit is significant for skin and muscle
Changes in the two major components of dermatomyositis were tracked individually. For skin symptoms, patients were evaluated with the Cutaneous Dermatomyositis Disease Areas and Severity Index (CDASI). For muscle involvement, symptoms were evaluated with the 8-item Manual Muscle Testing (MMT-8) tool.
“The effects of IVIg on the muscle and the skin were both highly statistically significant,” Dr. Aggarwal reported. He said the CDASI score was reduced by almost half at the end of 16 weeks among those treated with IVIg relative to those treated with placebo. Improvement in MMT-8 scores were also clinically as well as statistically significant.
The IVIg therapy was well tolerated. The most common adverse effects in this study, like those reported with IVIg when used to treat other diseases, were headache, pyrexia, and nausea, but Dr. Aggarwal reported that these were generally mild.
Serious adverse events, particularly thromboembolism, did occur over the course of the study, but the rate of events was only slightly higher in the group receiving active therapy (5.8% vs. 4.2%).
Patients who entered the study were permitted to remain on most immunosuppressive therapies, such as methotrexate, mycophenolate, tacrolimus, and glucocorticoids. Dr. Aggarwal said that the majority of patients were taking a glucocorticoid and at least one nonglucocorticoid immunosuppressant.
Effect on associated conditions is planned
The data from this trial have not yet been analyzed for the impact of IVIg on conditions that occur frequently in association with dermatomyositis, such as interstitial lung disease (ILD) and dysphagia, but Dr. Aggarwal reported that there are plans to do so. Although severe ILD was a trial exclusion, the presence of mild to moderate ILD and dysphagia were evaluated at baseline, so the impact of treatment can be assessed.
There are also plans to evaluate how the presence or absence of myositis-specific antibodies, which were also evaluated at baseline, affected response to IVIg.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Dr. Schulze-Koops reported no relevant potential conflicts of interest.
FROM THE EULAR 2021 CONGRESS
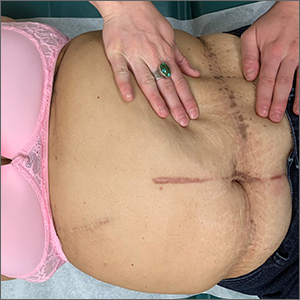
Asymptomatic hyperpigmented skin changes
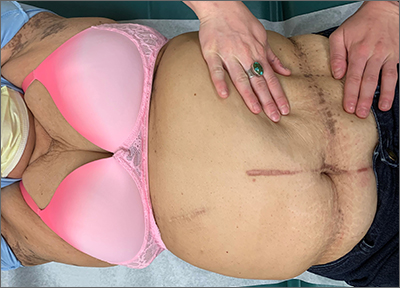
The intertriginous findings, along with results from a punch biopsy showing resolving lichenoid inflammation with post-inflammatory pigmentary alteration, indicated a diagnosis of lichen planus pigmentosus inversus (LPPI).
Insidious onset of usually asymptomatic, sometimes mildly pruritic, well-defined, hyperpigmented macules and patches with occasional Wickham striae of the intertriginous areas is characteristic of LPPI. It is a variant of lichen planus pigmentosus, which conversely occurs on sun-exposed areas. The etiology is unknown and there is no association with medications or sun exposure. Pathophysiology is thought to be chronic inflammation, mediated by T-lymphocyte cytotoxic activity against basal keratinocytes.1
At the time of this patient’s clinical presentation, the differential diagnoses for new onset hyperpigmentation included confluent and reticulated papillomatosis, post-inflammatory hyperpigmentation, and erythema dyschromicum perstans. However, further tests were ordered for diagnostic clarification.
The clinical course of LPPI is variable. In some cases, there is complete resolution of lesions without treatment, while in other cases, lesions may last for years despite treatment and the condition may recur. Current management options include topical calcineurin inhibitors (tacrolimus and pimecrolimus) and topical steroids. Response to these topical medications may be variable. The patient in this case was started on topical tacrolimus 0.1% twice daily, with follow-up in 3 months.
Text courtesy of Rachel Rose, BS, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Photo courtesy of Daniel Stulberg, MD, FAAFP.
Barros HR, de Almeida JRP, Mattos e Dinato SL, et al. Lichen planus pigmentosus inversus. An Bras Dermatol. 2013;88(6 suppl 1):146-149. doi:10.1590/abd1806-4841.20132599

The intertriginous findings, along with results from a punch biopsy showing resolving lichenoid inflammation with post-inflammatory pigmentary alteration, indicated a diagnosis of lichen planus pigmentosus inversus (LPPI).
Insidious onset of usually asymptomatic, sometimes mildly pruritic, well-defined, hyperpigmented macules and patches with occasional Wickham striae of the intertriginous areas is characteristic of LPPI. It is a variant of lichen planus pigmentosus, which conversely occurs on sun-exposed areas. The etiology is unknown and there is no association with medications or sun exposure. Pathophysiology is thought to be chronic inflammation, mediated by T-lymphocyte cytotoxic activity against basal keratinocytes.1
At the time of this patient’s clinical presentation, the differential diagnoses for new onset hyperpigmentation included confluent and reticulated papillomatosis, post-inflammatory hyperpigmentation, and erythema dyschromicum perstans. However, further tests were ordered for diagnostic clarification.
The clinical course of LPPI is variable. In some cases, there is complete resolution of lesions without treatment, while in other cases, lesions may last for years despite treatment and the condition may recur. Current management options include topical calcineurin inhibitors (tacrolimus and pimecrolimus) and topical steroids. Response to these topical medications may be variable. The patient in this case was started on topical tacrolimus 0.1% twice daily, with follow-up in 3 months.
Text courtesy of Rachel Rose, BS, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Photo courtesy of Daniel Stulberg, MD, FAAFP.

The intertriginous findings, along with results from a punch biopsy showing resolving lichenoid inflammation with post-inflammatory pigmentary alteration, indicated a diagnosis of lichen planus pigmentosus inversus (LPPI).
Insidious onset of usually asymptomatic, sometimes mildly pruritic, well-defined, hyperpigmented macules and patches with occasional Wickham striae of the intertriginous areas is characteristic of LPPI. It is a variant of lichen planus pigmentosus, which conversely occurs on sun-exposed areas. The etiology is unknown and there is no association with medications or sun exposure. Pathophysiology is thought to be chronic inflammation, mediated by T-lymphocyte cytotoxic activity against basal keratinocytes.1
At the time of this patient’s clinical presentation, the differential diagnoses for new onset hyperpigmentation included confluent and reticulated papillomatosis, post-inflammatory hyperpigmentation, and erythema dyschromicum perstans. However, further tests were ordered for diagnostic clarification.
The clinical course of LPPI is variable. In some cases, there is complete resolution of lesions without treatment, while in other cases, lesions may last for years despite treatment and the condition may recur. Current management options include topical calcineurin inhibitors (tacrolimus and pimecrolimus) and topical steroids. Response to these topical medications may be variable. The patient in this case was started on topical tacrolimus 0.1% twice daily, with follow-up in 3 months.
Text courtesy of Rachel Rose, BS, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Photo courtesy of Daniel Stulberg, MD, FAAFP.
Barros HR, de Almeida JRP, Mattos e Dinato SL, et al. Lichen planus pigmentosus inversus. An Bras Dermatol. 2013;88(6 suppl 1):146-149. doi:10.1590/abd1806-4841.20132599
Barros HR, de Almeida JRP, Mattos e Dinato SL, et al. Lichen planus pigmentosus inversus. An Bras Dermatol. 2013;88(6 suppl 1):146-149. doi:10.1590/abd1806-4841.20132599
Novel text-messaging program boosts ADHD treatment adherence
An innovative text-messaging program that reminds patients with attention-deficit/hyperactivity disorder to take their medication and warns them about the hazards of noncompliance significantly increases treatment adherence in children and adults, new research suggests.
In a pediatric study, 85% of participants who received a text message had their prescriptions refilled in a timely manner, compared with 62% of those who received treatment as usual and no text messaging. In a second study of adults, 81% of the group that received a text message refilled their prescriptions, versus 36% of those in the usual-treatment group.
“Patients are not going to be fully compliant if they do not understand what the implications are if they do not take their pills,” lead author Joseph Biederman, MD, chief of clinical and research programs in pediatric psychopharmacology and adult ADHD at the Massachusetts General Hospital and professor of psychiatry at Harvard Medical School, Boston, told this news organization.
He noted that the text-messaging program also provides information, support, encouragement, and guidance.
“We remind them to get in touch with their prescriber as renewals come due, and if they tell us no, we tell them how important it is” to do so, Dr. Biederman said.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 annual meeting.
Poor adherence
“Adherence to medications for ADHD is extremely poor, among the worst in medicine, despite the fact that ADHD is very morbid and we have excellent treatments people can take,” Dr. Biederman noted. “That’s the first tragedy, and it is totally unappreciated.”
He added that when patients require multiple prescriptions, he said.
Another contributor to medication nonadherence is the ongoing prejudice or stigma associated with ADHD, said Dr. Biederman.
“There is bad press about ADHD. There are no good comments, only disaster, doom and gloom, catastrophe, and so on. All people read in the available media are bad things about ADHD, and that only adds to stigma and misinformation,” he noted.
To combat these factors, Dr. Biederman and his team conducted two studies on the effectiveness of a novel ADHD-centric intervention based on text messaging.
One study included 87 children aged 6-12 years, and the other included 117 adults aged 18-55 years. Both groups were from primary care settings and were prescribed a stimulant medication for the treatment of ADHD.
As comparators, the researchers used age- and sex-matched pediatric patients and age-, race-, and sex-matched adult patients from the same primary care settings. They had also been prescribed stimulants but had not received the text messaging intervention.
Timely reminders
Results showed that 85% of the children who received text messages refilled their prescriptions vs. 65% of those who did not get the intervention (odds ratio, 3.46; 95% confidence interval, 1.82-6.58; P < .001).
Among adults, 81% of the intervention group refilled their prescriptions vs. 36% of the comparator group (OR, 7.54; 95% CI, 4.46-12.77; P < .001).
“In the number-needed-to-treat analysis, for every five pediatric patients who receive text messaging, we can keep one adherent with stimulant medication. In adults, that is one in every three who receive the text-messaging intervention,” Dr. Biederman said.
Text messaging reminds patients with ADHD to take their medications as prescribed, and it also reminds them of the consequences of not taking their medications, he added.
In another study presented at the ASCP meeting, Dr. Biederman introduced a new tool to help clinicians determine whether a patient with ADHD also has deficient emotional self-regulation (DESR).
ADHD has been associated with low frustration tolerance, impatience, and quickness to anger, he noted.
Emotional dysregulation, however, “is not a mood disorder,” said Dr. Biederman. “Some people use the term ‘hot tempered.’ These are people who overreact to things, and this is associated with a wide range of difficulties.”
Clinical guidance
The investigators operationalized DESR using the eight-item Emotional Dysregulation (ED) subscale of the Barkley Current Behavior Scale. They then used receiver operating characteristic curves to identify the optimal cutoff on the Barkley ED Scale that would categorize patients as having high- vs. low-level DESR.
“We wanted to give some guidance to clinicians, using a very simple rating scale that was developed by Dr. Barkley. It is one we think configures this syndrome of emotional dysregulation and emotional impulsivity,” Dr. Biederman said.
The study included 441 newly referred 18- to 55-year-old men and women who met DSM-5 diagnostic criteria for ADHD.
Using a cutoff score of 8 to represent high levels of DESR, the researchers identified 191 adults as having high-level DESR and the rest as having low-level DESR.
Those with high-level DESR had significantly more severe symptoms of ADHD, executive dysfunction, autistic traits, levels of psychopathology, and worse quality of life, compared with those with low-level DESR.
The problem of emotional dysregulation in ADHD is widespread and affects many people, Dr. Biederman noted.
“If you take 5% of adults at a minimum and 10% of children with ADHD [and] if 50% of those have emotional dysregulation, we’re talking about millions of people. And it is very morbid,” he said. “Having emotional dysregulation problems will get you in hot water.”
Promising results
Commenting on the findings for this news organization, Ira D. Glick, MD, professor emeritus of psychiatry and behavioral sciences, Stanford (Calif.) University, said the new studies are important.
He noted that, although ADHD has become more accepted as a “disease of the brain” over the past 20 years, patients with the disorder and their families often are not accepting of the diagnosis.
“Instead, they try to downplay it. They say this is just a ploy by psychiatrists to get business or this is just normal boys’ behavior, [and] they don’t need medicines,” said Dr. Glick, who was not involved in the current research.
“Biederman is trying to make clear that ADHD is a brain disease, and DESR symptoms are cardinal signs of a brain illness,” he said.
Dr. Glick also agreed that text messaging could be very useful for these patients.
“Text messaging might be helpful, especially in this population which can often be disorganized or forgetful. The results of that study were very promising,” he said.
Dr. Biederman is in the process of commercializing the text program used in the study. Dr. Glick reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An innovative text-messaging program that reminds patients with attention-deficit/hyperactivity disorder to take their medication and warns them about the hazards of noncompliance significantly increases treatment adherence in children and adults, new research suggests.
In a pediatric study, 85% of participants who received a text message had their prescriptions refilled in a timely manner, compared with 62% of those who received treatment as usual and no text messaging. In a second study of adults, 81% of the group that received a text message refilled their prescriptions, versus 36% of those in the usual-treatment group.
“Patients are not going to be fully compliant if they do not understand what the implications are if they do not take their pills,” lead author Joseph Biederman, MD, chief of clinical and research programs in pediatric psychopharmacology and adult ADHD at the Massachusetts General Hospital and professor of psychiatry at Harvard Medical School, Boston, told this news organization.
He noted that the text-messaging program also provides information, support, encouragement, and guidance.
“We remind them to get in touch with their prescriber as renewals come due, and if they tell us no, we tell them how important it is” to do so, Dr. Biederman said.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 annual meeting.
Poor adherence
“Adherence to medications for ADHD is extremely poor, among the worst in medicine, despite the fact that ADHD is very morbid and we have excellent treatments people can take,” Dr. Biederman noted. “That’s the first tragedy, and it is totally unappreciated.”
He added that when patients require multiple prescriptions, he said.
Another contributor to medication nonadherence is the ongoing prejudice or stigma associated with ADHD, said Dr. Biederman.
“There is bad press about ADHD. There are no good comments, only disaster, doom and gloom, catastrophe, and so on. All people read in the available media are bad things about ADHD, and that only adds to stigma and misinformation,” he noted.
To combat these factors, Dr. Biederman and his team conducted two studies on the effectiveness of a novel ADHD-centric intervention based on text messaging.
One study included 87 children aged 6-12 years, and the other included 117 adults aged 18-55 years. Both groups were from primary care settings and were prescribed a stimulant medication for the treatment of ADHD.
As comparators, the researchers used age- and sex-matched pediatric patients and age-, race-, and sex-matched adult patients from the same primary care settings. They had also been prescribed stimulants but had not received the text messaging intervention.
Timely reminders
Results showed that 85% of the children who received text messages refilled their prescriptions vs. 65% of those who did not get the intervention (odds ratio, 3.46; 95% confidence interval, 1.82-6.58; P < .001).
Among adults, 81% of the intervention group refilled their prescriptions vs. 36% of the comparator group (OR, 7.54; 95% CI, 4.46-12.77; P < .001).
“In the number-needed-to-treat analysis, for every five pediatric patients who receive text messaging, we can keep one adherent with stimulant medication. In adults, that is one in every three who receive the text-messaging intervention,” Dr. Biederman said.
Text messaging reminds patients with ADHD to take their medications as prescribed, and it also reminds them of the consequences of not taking their medications, he added.
In another study presented at the ASCP meeting, Dr. Biederman introduced a new tool to help clinicians determine whether a patient with ADHD also has deficient emotional self-regulation (DESR).
ADHD has been associated with low frustration tolerance, impatience, and quickness to anger, he noted.
Emotional dysregulation, however, “is not a mood disorder,” said Dr. Biederman. “Some people use the term ‘hot tempered.’ These are people who overreact to things, and this is associated with a wide range of difficulties.”
Clinical guidance
The investigators operationalized DESR using the eight-item Emotional Dysregulation (ED) subscale of the Barkley Current Behavior Scale. They then used receiver operating characteristic curves to identify the optimal cutoff on the Barkley ED Scale that would categorize patients as having high- vs. low-level DESR.
“We wanted to give some guidance to clinicians, using a very simple rating scale that was developed by Dr. Barkley. It is one we think configures this syndrome of emotional dysregulation and emotional impulsivity,” Dr. Biederman said.
The study included 441 newly referred 18- to 55-year-old men and women who met DSM-5 diagnostic criteria for ADHD.
Using a cutoff score of 8 to represent high levels of DESR, the researchers identified 191 adults as having high-level DESR and the rest as having low-level DESR.
Those with high-level DESR had significantly more severe symptoms of ADHD, executive dysfunction, autistic traits, levels of psychopathology, and worse quality of life, compared with those with low-level DESR.
The problem of emotional dysregulation in ADHD is widespread and affects many people, Dr. Biederman noted.
“If you take 5% of adults at a minimum and 10% of children with ADHD [and] if 50% of those have emotional dysregulation, we’re talking about millions of people. And it is very morbid,” he said. “Having emotional dysregulation problems will get you in hot water.”
Promising results
Commenting on the findings for this news organization, Ira D. Glick, MD, professor emeritus of psychiatry and behavioral sciences, Stanford (Calif.) University, said the new studies are important.
He noted that, although ADHD has become more accepted as a “disease of the brain” over the past 20 years, patients with the disorder and their families often are not accepting of the diagnosis.
“Instead, they try to downplay it. They say this is just a ploy by psychiatrists to get business or this is just normal boys’ behavior, [and] they don’t need medicines,” said Dr. Glick, who was not involved in the current research.
“Biederman is trying to make clear that ADHD is a brain disease, and DESR symptoms are cardinal signs of a brain illness,” he said.
Dr. Glick also agreed that text messaging could be very useful for these patients.
“Text messaging might be helpful, especially in this population which can often be disorganized or forgetful. The results of that study were very promising,” he said.
Dr. Biederman is in the process of commercializing the text program used in the study. Dr. Glick reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An innovative text-messaging program that reminds patients with attention-deficit/hyperactivity disorder to take their medication and warns them about the hazards of noncompliance significantly increases treatment adherence in children and adults, new research suggests.
In a pediatric study, 85% of participants who received a text message had their prescriptions refilled in a timely manner, compared with 62% of those who received treatment as usual and no text messaging. In a second study of adults, 81% of the group that received a text message refilled their prescriptions, versus 36% of those in the usual-treatment group.
“Patients are not going to be fully compliant if they do not understand what the implications are if they do not take their pills,” lead author Joseph Biederman, MD, chief of clinical and research programs in pediatric psychopharmacology and adult ADHD at the Massachusetts General Hospital and professor of psychiatry at Harvard Medical School, Boston, told this news organization.
He noted that the text-messaging program also provides information, support, encouragement, and guidance.
“We remind them to get in touch with their prescriber as renewals come due, and if they tell us no, we tell them how important it is” to do so, Dr. Biederman said.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 annual meeting.
Poor adherence
“Adherence to medications for ADHD is extremely poor, among the worst in medicine, despite the fact that ADHD is very morbid and we have excellent treatments people can take,” Dr. Biederman noted. “That’s the first tragedy, and it is totally unappreciated.”
He added that when patients require multiple prescriptions, he said.
Another contributor to medication nonadherence is the ongoing prejudice or stigma associated with ADHD, said Dr. Biederman.
“There is bad press about ADHD. There are no good comments, only disaster, doom and gloom, catastrophe, and so on. All people read in the available media are bad things about ADHD, and that only adds to stigma and misinformation,” he noted.
To combat these factors, Dr. Biederman and his team conducted two studies on the effectiveness of a novel ADHD-centric intervention based on text messaging.
One study included 87 children aged 6-12 years, and the other included 117 adults aged 18-55 years. Both groups were from primary care settings and were prescribed a stimulant medication for the treatment of ADHD.
As comparators, the researchers used age- and sex-matched pediatric patients and age-, race-, and sex-matched adult patients from the same primary care settings. They had also been prescribed stimulants but had not received the text messaging intervention.
Timely reminders
Results showed that 85% of the children who received text messages refilled their prescriptions vs. 65% of those who did not get the intervention (odds ratio, 3.46; 95% confidence interval, 1.82-6.58; P < .001).
Among adults, 81% of the intervention group refilled their prescriptions vs. 36% of the comparator group (OR, 7.54; 95% CI, 4.46-12.77; P < .001).
“In the number-needed-to-treat analysis, for every five pediatric patients who receive text messaging, we can keep one adherent with stimulant medication. In adults, that is one in every three who receive the text-messaging intervention,” Dr. Biederman said.
Text messaging reminds patients with ADHD to take their medications as prescribed, and it also reminds them of the consequences of not taking their medications, he added.
In another study presented at the ASCP meeting, Dr. Biederman introduced a new tool to help clinicians determine whether a patient with ADHD also has deficient emotional self-regulation (DESR).
ADHD has been associated with low frustration tolerance, impatience, and quickness to anger, he noted.
Emotional dysregulation, however, “is not a mood disorder,” said Dr. Biederman. “Some people use the term ‘hot tempered.’ These are people who overreact to things, and this is associated with a wide range of difficulties.”
Clinical guidance
The investigators operationalized DESR using the eight-item Emotional Dysregulation (ED) subscale of the Barkley Current Behavior Scale. They then used receiver operating characteristic curves to identify the optimal cutoff on the Barkley ED Scale that would categorize patients as having high- vs. low-level DESR.
“We wanted to give some guidance to clinicians, using a very simple rating scale that was developed by Dr. Barkley. It is one we think configures this syndrome of emotional dysregulation and emotional impulsivity,” Dr. Biederman said.
The study included 441 newly referred 18- to 55-year-old men and women who met DSM-5 diagnostic criteria for ADHD.
Using a cutoff score of 8 to represent high levels of DESR, the researchers identified 191 adults as having high-level DESR and the rest as having low-level DESR.
Those with high-level DESR had significantly more severe symptoms of ADHD, executive dysfunction, autistic traits, levels of psychopathology, and worse quality of life, compared with those with low-level DESR.
The problem of emotional dysregulation in ADHD is widespread and affects many people, Dr. Biederman noted.
“If you take 5% of adults at a minimum and 10% of children with ADHD [and] if 50% of those have emotional dysregulation, we’re talking about millions of people. And it is very morbid,” he said. “Having emotional dysregulation problems will get you in hot water.”
Promising results
Commenting on the findings for this news organization, Ira D. Glick, MD, professor emeritus of psychiatry and behavioral sciences, Stanford (Calif.) University, said the new studies are important.
He noted that, although ADHD has become more accepted as a “disease of the brain” over the past 20 years, patients with the disorder and their families often are not accepting of the diagnosis.
“Instead, they try to downplay it. They say this is just a ploy by psychiatrists to get business or this is just normal boys’ behavior, [and] they don’t need medicines,” said Dr. Glick, who was not involved in the current research.
“Biederman is trying to make clear that ADHD is a brain disease, and DESR symptoms are cardinal signs of a brain illness,” he said.
Dr. Glick also agreed that text messaging could be very useful for these patients.
“Text messaging might be helpful, especially in this population which can often be disorganized or forgetful. The results of that study were very promising,” he said.
Dr. Biederman is in the process of commercializing the text program used in the study. Dr. Glick reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sugar-sweetened beverage intake after breast cancer diagnosis may increase mortality
Key clinical point: Higher sugar-sweetened beverages (SSB) consumption after a breast cancer diagnosis was associated with greater breast cancer-specific and all-cause mortality.
Major finding: Compared with no consumption, the risk for breast cancer-specific and all-cause mortality increased with increasing consumption of SSB (Ptrend= .001 and .0001, respectively). The consumption of artificially sweetened beverages was not associated with higher breast cancer-specific or all-cause mortality.
Study details: A prospective cohort of 8,863 women with stages I-III breast cancer who completed a validated food frequency questionnaire every 4 years.
Disclosure: This study was supported by the National Institutes of Health, the American Institute for Cancer Research, and the Breast Cancer Research Foundation. Dr. Holmes received grants, personal fees, and nonfinancial support from various sources outside this work. The remaining authors made no disclosures.
Source: Farvid MS et al. Cancer. 2021 May 4. doi: 10.1002/cncr.33461.
Key clinical point: Higher sugar-sweetened beverages (SSB) consumption after a breast cancer diagnosis was associated with greater breast cancer-specific and all-cause mortality.
Major finding: Compared with no consumption, the risk for breast cancer-specific and all-cause mortality increased with increasing consumption of SSB (Ptrend= .001 and .0001, respectively). The consumption of artificially sweetened beverages was not associated with higher breast cancer-specific or all-cause mortality.
Study details: A prospective cohort of 8,863 women with stages I-III breast cancer who completed a validated food frequency questionnaire every 4 years.
Disclosure: This study was supported by the National Institutes of Health, the American Institute for Cancer Research, and the Breast Cancer Research Foundation. Dr. Holmes received grants, personal fees, and nonfinancial support from various sources outside this work. The remaining authors made no disclosures.
Source: Farvid MS et al. Cancer. 2021 May 4. doi: 10.1002/cncr.33461.
Key clinical point: Higher sugar-sweetened beverages (SSB) consumption after a breast cancer diagnosis was associated with greater breast cancer-specific and all-cause mortality.
Major finding: Compared with no consumption, the risk for breast cancer-specific and all-cause mortality increased with increasing consumption of SSB (Ptrend= .001 and .0001, respectively). The consumption of artificially sweetened beverages was not associated with higher breast cancer-specific or all-cause mortality.
Study details: A prospective cohort of 8,863 women with stages I-III breast cancer who completed a validated food frequency questionnaire every 4 years.
Disclosure: This study was supported by the National Institutes of Health, the American Institute for Cancer Research, and the Breast Cancer Research Foundation. Dr. Holmes received grants, personal fees, and nonfinancial support from various sources outside this work. The remaining authors made no disclosures.
Source: Farvid MS et al. Cancer. 2021 May 4. doi: 10.1002/cncr.33461.
Low risk for second breast cancer in older women with radiation alone
Key clinical point: In older women with stage I hormone receptor (HR)-positive breast cancer, radiation without endocrine therapy does not increase the risk for second breast cancer events (SBCE).
Major finding: Compared with endocrine therapy plus radiotherapy, radiotherapy alone was not associated with a higher risk for SBCE (P = .137), whereas no therapy (standardized hazard ratio [SHR], 3.7; P less than .001) or endocrine therapy (SHR, 2.2; P = .008) alone was associated with higher risk for SBCE.
Study details: A retrospective study of 13,321 women aged 66 years and older with stage I HR-positive breast cancer who underwent breast-conserving surgery between 2007 and 2012.
Disclosures: The study received funding from the Department of Radiation Oncology, NYU School of Medicine. Dr. Deb received personal fees from the NYU School of Medicine. The other authors did not disclose any conflicts of interest.
Source: Gerber NK et al. Int J Radiat Oncol Biol Phys. 2021 May 8. doi: 10.1016/j.ijrobp.2021.04.030.
Key clinical point: In older women with stage I hormone receptor (HR)-positive breast cancer, radiation without endocrine therapy does not increase the risk for second breast cancer events (SBCE).
Major finding: Compared with endocrine therapy plus radiotherapy, radiotherapy alone was not associated with a higher risk for SBCE (P = .137), whereas no therapy (standardized hazard ratio [SHR], 3.7; P less than .001) or endocrine therapy (SHR, 2.2; P = .008) alone was associated with higher risk for SBCE.
Study details: A retrospective study of 13,321 women aged 66 years and older with stage I HR-positive breast cancer who underwent breast-conserving surgery between 2007 and 2012.
Disclosures: The study received funding from the Department of Radiation Oncology, NYU School of Medicine. Dr. Deb received personal fees from the NYU School of Medicine. The other authors did not disclose any conflicts of interest.
Source: Gerber NK et al. Int J Radiat Oncol Biol Phys. 2021 May 8. doi: 10.1016/j.ijrobp.2021.04.030.
Key clinical point: In older women with stage I hormone receptor (HR)-positive breast cancer, radiation without endocrine therapy does not increase the risk for second breast cancer events (SBCE).
Major finding: Compared with endocrine therapy plus radiotherapy, radiotherapy alone was not associated with a higher risk for SBCE (P = .137), whereas no therapy (standardized hazard ratio [SHR], 3.7; P less than .001) or endocrine therapy (SHR, 2.2; P = .008) alone was associated with higher risk for SBCE.
Study details: A retrospective study of 13,321 women aged 66 years and older with stage I HR-positive breast cancer who underwent breast-conserving surgery between 2007 and 2012.
Disclosures: The study received funding from the Department of Radiation Oncology, NYU School of Medicine. Dr. Deb received personal fees from the NYU School of Medicine. The other authors did not disclose any conflicts of interest.
Source: Gerber NK et al. Int J Radiat Oncol Biol Phys. 2021 May 8. doi: 10.1016/j.ijrobp.2021.04.030.
Cochrane: PARP inhibitors improve survival in HER2-negative, BRCA-mutated breast cancer
Key clinical point: In patients with locally advanced or metastatic human epidermal growth factor receptor 2 (HER2)-negative, BRCA germline-mutated breast cancer, poly (ADP-ribose) polymerase (PARP) inhibitors improve progression-free survival (PFS), overall survival (OS), and tumor response rate.
Major findings: A PARP-containing regimen showed a small advantage in OS (hazard ratio, 0.87; 95% confidence interval, 0.76-1.00) vs. non-PARP regimen. PARP inhibitors improved PFS (hazard ratio, 0.63; P less than .00001) and tumor response rate (66.9% vs. 48.9%). The rate of grade 3 or higher adverse events was not significantly different in the PARP-containing vs. non-PARP regimen.
Study details: A meta-analysis of 5 randomized controlled trials comparing PARP-containing and non-PARP regimens in patients with locally advanced or metastatic breast cancer.
Disclosures: The funding source for this meta-analysis was not identified. Some of the authors received honoraria, research funding, compensation, financial support, consulting fees, and/or grants outside this work. Dr. Redfern is the Principal Investigator on the Brightness trial and served on the advisory board of AstraZeneca and Pfizer.
Source: Taylor AM. Cochrane Database Syst Rev. 2021 Apr 22. doi: 10.1002/14651858.CD011395.pub2.
Key clinical point: In patients with locally advanced or metastatic human epidermal growth factor receptor 2 (HER2)-negative, BRCA germline-mutated breast cancer, poly (ADP-ribose) polymerase (PARP) inhibitors improve progression-free survival (PFS), overall survival (OS), and tumor response rate.
Major findings: A PARP-containing regimen showed a small advantage in OS (hazard ratio, 0.87; 95% confidence interval, 0.76-1.00) vs. non-PARP regimen. PARP inhibitors improved PFS (hazard ratio, 0.63; P less than .00001) and tumor response rate (66.9% vs. 48.9%). The rate of grade 3 or higher adverse events was not significantly different in the PARP-containing vs. non-PARP regimen.
Study details: A meta-analysis of 5 randomized controlled trials comparing PARP-containing and non-PARP regimens in patients with locally advanced or metastatic breast cancer.
Disclosures: The funding source for this meta-analysis was not identified. Some of the authors received honoraria, research funding, compensation, financial support, consulting fees, and/or grants outside this work. Dr. Redfern is the Principal Investigator on the Brightness trial and served on the advisory board of AstraZeneca and Pfizer.
Source: Taylor AM. Cochrane Database Syst Rev. 2021 Apr 22. doi: 10.1002/14651858.CD011395.pub2.
Key clinical point: In patients with locally advanced or metastatic human epidermal growth factor receptor 2 (HER2)-negative, BRCA germline-mutated breast cancer, poly (ADP-ribose) polymerase (PARP) inhibitors improve progression-free survival (PFS), overall survival (OS), and tumor response rate.
Major findings: A PARP-containing regimen showed a small advantage in OS (hazard ratio, 0.87; 95% confidence interval, 0.76-1.00) vs. non-PARP regimen. PARP inhibitors improved PFS (hazard ratio, 0.63; P less than .00001) and tumor response rate (66.9% vs. 48.9%). The rate of grade 3 or higher adverse events was not significantly different in the PARP-containing vs. non-PARP regimen.
Study details: A meta-analysis of 5 randomized controlled trials comparing PARP-containing and non-PARP regimens in patients with locally advanced or metastatic breast cancer.
Disclosures: The funding source for this meta-analysis was not identified. Some of the authors received honoraria, research funding, compensation, financial support, consulting fees, and/or grants outside this work. Dr. Redfern is the Principal Investigator on the Brightness trial and served on the advisory board of AstraZeneca and Pfizer.
Source: Taylor AM. Cochrane Database Syst Rev. 2021 Apr 22. doi: 10.1002/14651858.CD011395.pub2.
Breast cancer: Fertility concerns affect endocrine therapy decisions in young survivors
Key clinical point: Fertility concerns affect adjuvant endocrine therapy (ET) decisions in one-third of young breast cancer survivors.
Major finding: Within 2 years after diagnosis, fertility concerns affected ET decisions in 33.12% of women. Parity at diagnosis showed a significant association with fertility concerns. The women who reported that fertility concerns affected their ET decisions showed a higher rate of noninitiation/nonpersistence with ET vs. those without fertility concerns (40% vs. 20%; P less than .0001).
Study details: An analysis of 643 hormone receptor-positive women (mean age, 36 years) who completed a survey from the Young Women’s Breast Cancer Study.
Disclosures: This study was funded by Susan G. Komen and the Breast Cancer Research Foundation. Dr. Rosenberg received a grant from the Agency for Healthcare Research and Quality. Dr. Sella was supported by the Pinchas Borenstein Talpiot Medical Leadership Program at Sheba Medical Center and the American Physicians Fellowship for Medicine in Israel. The authors reported receiving honorarium/research funding/consultancy fees/personal fees outside this study work. Dr. Peppercorn reported employment by/stocks in GlaxoSmithKline.
Source: Sella T. Cancer. 2021 Apr 22. doi: 10.1002/cncr.33596.
Key clinical point: Fertility concerns affect adjuvant endocrine therapy (ET) decisions in one-third of young breast cancer survivors.
Major finding: Within 2 years after diagnosis, fertility concerns affected ET decisions in 33.12% of women. Parity at diagnosis showed a significant association with fertility concerns. The women who reported that fertility concerns affected their ET decisions showed a higher rate of noninitiation/nonpersistence with ET vs. those without fertility concerns (40% vs. 20%; P less than .0001).
Study details: An analysis of 643 hormone receptor-positive women (mean age, 36 years) who completed a survey from the Young Women’s Breast Cancer Study.
Disclosures: This study was funded by Susan G. Komen and the Breast Cancer Research Foundation. Dr. Rosenberg received a grant from the Agency for Healthcare Research and Quality. Dr. Sella was supported by the Pinchas Borenstein Talpiot Medical Leadership Program at Sheba Medical Center and the American Physicians Fellowship for Medicine in Israel. The authors reported receiving honorarium/research funding/consultancy fees/personal fees outside this study work. Dr. Peppercorn reported employment by/stocks in GlaxoSmithKline.
Source: Sella T. Cancer. 2021 Apr 22. doi: 10.1002/cncr.33596.
Key clinical point: Fertility concerns affect adjuvant endocrine therapy (ET) decisions in one-third of young breast cancer survivors.
Major finding: Within 2 years after diagnosis, fertility concerns affected ET decisions in 33.12% of women. Parity at diagnosis showed a significant association with fertility concerns. The women who reported that fertility concerns affected their ET decisions showed a higher rate of noninitiation/nonpersistence with ET vs. those without fertility concerns (40% vs. 20%; P less than .0001).
Study details: An analysis of 643 hormone receptor-positive women (mean age, 36 years) who completed a survey from the Young Women’s Breast Cancer Study.
Disclosures: This study was funded by Susan G. Komen and the Breast Cancer Research Foundation. Dr. Rosenberg received a grant from the Agency for Healthcare Research and Quality. Dr. Sella was supported by the Pinchas Borenstein Talpiot Medical Leadership Program at Sheba Medical Center and the American Physicians Fellowship for Medicine in Israel. The authors reported receiving honorarium/research funding/consultancy fees/personal fees outside this study work. Dr. Peppercorn reported employment by/stocks in GlaxoSmithKline.
Source: Sella T. Cancer. 2021 Apr 22. doi: 10.1002/cncr.33596.
Breast cancer: Mortality rates remain high beyond 5 years of diagnosis
Key clinical point: The risk for breast cancer-specific mortality (BCSM) remains high beyond 5 years. The BCSM rate is significantly higher in hormone receptor (HR)-positive vs. HR-negative women.
Major finding: The overall BCSM rate was 14.9%. Of all BCSM, 54.2% of deaths occurred after 5 years and were significantly higher in HR-positive vs. HR-negative patients (P less than .001). Among patients with HR-positive and HR-negative breast cancer, independent risk factors for BCSM conditional on having survived 5 years were tumor size, nodal status, age, and year of diagnosis. Among patients with HR-positive status, tumor grade, marital status, and race were also independently associated with the risk for BCSM.
Study details: An observational study of 202,080 women with breast cancer with known HR status diagnosed between 1990 and 2005.
Disclosures: The study did not receive any funding. Some authors received research funding and/or advisory/consulting fees from various sources.
Source: Leone JP. Breast Cancer Res Treat. 2021 Apr 24. doi: 10.1007/s10549-021-06233-4.
Key clinical point: The risk for breast cancer-specific mortality (BCSM) remains high beyond 5 years. The BCSM rate is significantly higher in hormone receptor (HR)-positive vs. HR-negative women.
Major finding: The overall BCSM rate was 14.9%. Of all BCSM, 54.2% of deaths occurred after 5 years and were significantly higher in HR-positive vs. HR-negative patients (P less than .001). Among patients with HR-positive and HR-negative breast cancer, independent risk factors for BCSM conditional on having survived 5 years were tumor size, nodal status, age, and year of diagnosis. Among patients with HR-positive status, tumor grade, marital status, and race were also independently associated with the risk for BCSM.
Study details: An observational study of 202,080 women with breast cancer with known HR status diagnosed between 1990 and 2005.
Disclosures: The study did not receive any funding. Some authors received research funding and/or advisory/consulting fees from various sources.
Source: Leone JP. Breast Cancer Res Treat. 2021 Apr 24. doi: 10.1007/s10549-021-06233-4.
Key clinical point: The risk for breast cancer-specific mortality (BCSM) remains high beyond 5 years. The BCSM rate is significantly higher in hormone receptor (HR)-positive vs. HR-negative women.
Major finding: The overall BCSM rate was 14.9%. Of all BCSM, 54.2% of deaths occurred after 5 years and were significantly higher in HR-positive vs. HR-negative patients (P less than .001). Among patients with HR-positive and HR-negative breast cancer, independent risk factors for BCSM conditional on having survived 5 years were tumor size, nodal status, age, and year of diagnosis. Among patients with HR-positive status, tumor grade, marital status, and race were also independently associated with the risk for BCSM.
Study details: An observational study of 202,080 women with breast cancer with known HR status diagnosed between 1990 and 2005.
Disclosures: The study did not receive any funding. Some authors received research funding and/or advisory/consulting fees from various sources.
Source: Leone JP. Breast Cancer Res Treat. 2021 Apr 24. doi: 10.1007/s10549-021-06233-4.
Early breast cancer: Trastuzumab emtansine shows survival benefit regardless of NACT used
Key clinical point: In patients with human epidermal growth factor 2 (HER2)-positive early breast cancer, trastuzumab emtansine vs. trastuzumab is associated with longer invasive disease-free survival (DFS) regardless of the type of neoadjuvant chemotherapy (NACT) received.
Major finding: Trastuzumab emtansine was associated with longer invasive DFS vs. trastuzumab in high-risk patients (hazard ratio [HR], 0.50; 95% confidence interval [CI], 0.39-0.64), in patients who received anthracycline-based NACT (HR, 0.51; 95% CI, 0.38-0.67), and in those who received nonanthracycline-based NACT (HR, 0.43; 95% CI, 0.22-0.82).
Study details: This is a subgroup analysis of the KATHERINE trial comparing trastuzumab emtansine with trastuzumab in 1,486 HER2-positive patients with early breast cancer.
Disclosure: This study was funded by F. Hoffmann-La Roche Ltd. The authors received consulting fees, speaker bureau fees, honoraria, educational support, research funding, travel expenses, royalties from, and/or owned stocks in various organizations outside this work. Dr. Boulet was an employee of Parexel International GmbH contracted by F. Hoffmann-La Roche Ltd. Dr. Liu, Dr. Tesarowski, Dr. Lam, Dr. Song, and Dr. Smitt were/are employees of Genetech and/or owned stocks in Roche. All other authors declared no conflicts of interest.
Source: Mamounas EP et al. Ann Oncol. 2021 Apr 28. doi: 10.1016/j.annonc.2021.04.011.
Key clinical point: In patients with human epidermal growth factor 2 (HER2)-positive early breast cancer, trastuzumab emtansine vs. trastuzumab is associated with longer invasive disease-free survival (DFS) regardless of the type of neoadjuvant chemotherapy (NACT) received.
Major finding: Trastuzumab emtansine was associated with longer invasive DFS vs. trastuzumab in high-risk patients (hazard ratio [HR], 0.50; 95% confidence interval [CI], 0.39-0.64), in patients who received anthracycline-based NACT (HR, 0.51; 95% CI, 0.38-0.67), and in those who received nonanthracycline-based NACT (HR, 0.43; 95% CI, 0.22-0.82).
Study details: This is a subgroup analysis of the KATHERINE trial comparing trastuzumab emtansine with trastuzumab in 1,486 HER2-positive patients with early breast cancer.
Disclosure: This study was funded by F. Hoffmann-La Roche Ltd. The authors received consulting fees, speaker bureau fees, honoraria, educational support, research funding, travel expenses, royalties from, and/or owned stocks in various organizations outside this work. Dr. Boulet was an employee of Parexel International GmbH contracted by F. Hoffmann-La Roche Ltd. Dr. Liu, Dr. Tesarowski, Dr. Lam, Dr. Song, and Dr. Smitt were/are employees of Genetech and/or owned stocks in Roche. All other authors declared no conflicts of interest.
Source: Mamounas EP et al. Ann Oncol. 2021 Apr 28. doi: 10.1016/j.annonc.2021.04.011.
Key clinical point: In patients with human epidermal growth factor 2 (HER2)-positive early breast cancer, trastuzumab emtansine vs. trastuzumab is associated with longer invasive disease-free survival (DFS) regardless of the type of neoadjuvant chemotherapy (NACT) received.
Major finding: Trastuzumab emtansine was associated with longer invasive DFS vs. trastuzumab in high-risk patients (hazard ratio [HR], 0.50; 95% confidence interval [CI], 0.39-0.64), in patients who received anthracycline-based NACT (HR, 0.51; 95% CI, 0.38-0.67), and in those who received nonanthracycline-based NACT (HR, 0.43; 95% CI, 0.22-0.82).
Study details: This is a subgroup analysis of the KATHERINE trial comparing trastuzumab emtansine with trastuzumab in 1,486 HER2-positive patients with early breast cancer.
Disclosure: This study was funded by F. Hoffmann-La Roche Ltd. The authors received consulting fees, speaker bureau fees, honoraria, educational support, research funding, travel expenses, royalties from, and/or owned stocks in various organizations outside this work. Dr. Boulet was an employee of Parexel International GmbH contracted by F. Hoffmann-La Roche Ltd. Dr. Liu, Dr. Tesarowski, Dr. Lam, Dr. Song, and Dr. Smitt were/are employees of Genetech and/or owned stocks in Roche. All other authors declared no conflicts of interest.
Source: Mamounas EP et al. Ann Oncol. 2021 Apr 28. doi: 10.1016/j.annonc.2021.04.011.
Pertuzumab plus high-dose trastuzumab shows activity in HER-2-positive breast cancer
Key clinical point: In patients with human epidermal growth factor 2 (HER2)-positive metastatic breast cancer and progressive brain metastases despite radiotherapy, pertuzumab in combination with high-dose trastuzumab shows modest central nervous system (CNS) response and good clinical benefit.
Major finding: The confirmed CNS objective response rate was 11% with all partial responses. The clinical benefit rate at 4 months was 68% and at 6 months was 51%. The grade 3-4 adverse event rate was 44%. There were no new safety signals.
Study details: A phase 2 PATRICIA study evaluated pertuzumab in combination with high-dose trastuzumab in 39 patients with HER2-positive metastatic breast cancer and progressive brain metastases despite radiotherapy.
Disclosures: The study was sponsored by F. Hoffmann-La Roche/ Genentech. The authors received consulting/advisory fees, research funding, royalties, and travel/accommodation expenses from various sources. Dr. Fung, Dr. Cheng, and Dr. Kirschbrown reported employment by, stocks, and other ownership interests in Genentech/Roche.
Source: Lin NU et al. J Clin Oncol. 2021 May 4. doi: 10.1200/JCO.20.02822.
Key clinical point: In patients with human epidermal growth factor 2 (HER2)-positive metastatic breast cancer and progressive brain metastases despite radiotherapy, pertuzumab in combination with high-dose trastuzumab shows modest central nervous system (CNS) response and good clinical benefit.
Major finding: The confirmed CNS objective response rate was 11% with all partial responses. The clinical benefit rate at 4 months was 68% and at 6 months was 51%. The grade 3-4 adverse event rate was 44%. There were no new safety signals.
Study details: A phase 2 PATRICIA study evaluated pertuzumab in combination with high-dose trastuzumab in 39 patients with HER2-positive metastatic breast cancer and progressive brain metastases despite radiotherapy.
Disclosures: The study was sponsored by F. Hoffmann-La Roche/ Genentech. The authors received consulting/advisory fees, research funding, royalties, and travel/accommodation expenses from various sources. Dr. Fung, Dr. Cheng, and Dr. Kirschbrown reported employment by, stocks, and other ownership interests in Genentech/Roche.
Source: Lin NU et al. J Clin Oncol. 2021 May 4. doi: 10.1200/JCO.20.02822.
Key clinical point: In patients with human epidermal growth factor 2 (HER2)-positive metastatic breast cancer and progressive brain metastases despite radiotherapy, pertuzumab in combination with high-dose trastuzumab shows modest central nervous system (CNS) response and good clinical benefit.
Major finding: The confirmed CNS objective response rate was 11% with all partial responses. The clinical benefit rate at 4 months was 68% and at 6 months was 51%. The grade 3-4 adverse event rate was 44%. There were no new safety signals.
Study details: A phase 2 PATRICIA study evaluated pertuzumab in combination with high-dose trastuzumab in 39 patients with HER2-positive metastatic breast cancer and progressive brain metastases despite radiotherapy.
Disclosures: The study was sponsored by F. Hoffmann-La Roche/ Genentech. The authors received consulting/advisory fees, research funding, royalties, and travel/accommodation expenses from various sources. Dr. Fung, Dr. Cheng, and Dr. Kirschbrown reported employment by, stocks, and other ownership interests in Genentech/Roche.
Source: Lin NU et al. J Clin Oncol. 2021 May 4. doi: 10.1200/JCO.20.02822.