User login
All’s Well That Ends Swell(ing)
ANSWER
The correct answer is elephantiasis nostras verrucosa (ENV; choice “d”).
DISCUSSION
ENV is a rare condition of advanced cutaneous hypertrophy secondary to a combination of contributing factors including: a sedentary lifestyle, obesity, chronic venous stasis, repeated bouts of lymphangitis, cellulitis, and congestive heart failure (CHF). Most commonly affecting the lower extremities, it is occasionally seen in other dependent areas such as the scrotum and the abdominal pannus. It is, essentially, an exaggerated form of cutaneous lymphedema that causes the skin to become increasingly thick and fibrotic, changes which also reduce blood flow to or from the area.
Despite its name, ENV is not associated with elephantiasis, more commonly known as lymphatic filariasis (choice “b”). Although that condition manifests with similar skin changes, it is typically seen only in those who live in tropical areas where these organisms are endemic—places this patient has never visited.
There was no reason to believe that these skin changes were attributable to warts (choice “a”). Biopsy would have settled that question but also would have run the risk of creating a nonhealing wound, which could easily turn into an ulcer.
Lymphedema (choice “c”) was clearly present, but it was quite advanced—far beyond what is usually seen in venous insufficiency. This diagnosis would not, by itself, explain the nodules or extreme fibrosis.
Other potential causes for these skin changes include postradiation and pretibial myxedema, which had been ruled out prior to the dermatology consult.
TREATMENT
As with simple venous insufficiency, treatment of ENV consists of compression, elevation, and weight loss. For this patient, the diuretics prescribed as part of her CHF treatment might help a bit, but her prognosis is guarded at best.
ANSWER
The correct answer is elephantiasis nostras verrucosa (ENV; choice “d”).
DISCUSSION
ENV is a rare condition of advanced cutaneous hypertrophy secondary to a combination of contributing factors including: a sedentary lifestyle, obesity, chronic venous stasis, repeated bouts of lymphangitis, cellulitis, and congestive heart failure (CHF). Most commonly affecting the lower extremities, it is occasionally seen in other dependent areas such as the scrotum and the abdominal pannus. It is, essentially, an exaggerated form of cutaneous lymphedema that causes the skin to become increasingly thick and fibrotic, changes which also reduce blood flow to or from the area.
Despite its name, ENV is not associated with elephantiasis, more commonly known as lymphatic filariasis (choice “b”). Although that condition manifests with similar skin changes, it is typically seen only in those who live in tropical areas where these organisms are endemic—places this patient has never visited.
There was no reason to believe that these skin changes were attributable to warts (choice “a”). Biopsy would have settled that question but also would have run the risk of creating a nonhealing wound, which could easily turn into an ulcer.
Lymphedema (choice “c”) was clearly present, but it was quite advanced—far beyond what is usually seen in venous insufficiency. This diagnosis would not, by itself, explain the nodules or extreme fibrosis.
Other potential causes for these skin changes include postradiation and pretibial myxedema, which had been ruled out prior to the dermatology consult.
TREATMENT
As with simple venous insufficiency, treatment of ENV consists of compression, elevation, and weight loss. For this patient, the diuretics prescribed as part of her CHF treatment might help a bit, but her prognosis is guarded at best.
ANSWER
The correct answer is elephantiasis nostras verrucosa (ENV; choice “d”).
DISCUSSION
ENV is a rare condition of advanced cutaneous hypertrophy secondary to a combination of contributing factors including: a sedentary lifestyle, obesity, chronic venous stasis, repeated bouts of lymphangitis, cellulitis, and congestive heart failure (CHF). Most commonly affecting the lower extremities, it is occasionally seen in other dependent areas such as the scrotum and the abdominal pannus. It is, essentially, an exaggerated form of cutaneous lymphedema that causes the skin to become increasingly thick and fibrotic, changes which also reduce blood flow to or from the area.
Despite its name, ENV is not associated with elephantiasis, more commonly known as lymphatic filariasis (choice “b”). Although that condition manifests with similar skin changes, it is typically seen only in those who live in tropical areas where these organisms are endemic—places this patient has never visited.
There was no reason to believe that these skin changes were attributable to warts (choice “a”). Biopsy would have settled that question but also would have run the risk of creating a nonhealing wound, which could easily turn into an ulcer.
Lymphedema (choice “c”) was clearly present, but it was quite advanced—far beyond what is usually seen in venous insufficiency. This diagnosis would not, by itself, explain the nodules or extreme fibrosis.
Other potential causes for these skin changes include postradiation and pretibial myxedema, which had been ruled out prior to the dermatology consult.
TREATMENT
As with simple venous insufficiency, treatment of ENV consists of compression, elevation, and weight loss. For this patient, the diuretics prescribed as part of her CHF treatment might help a bit, but her prognosis is guarded at best.
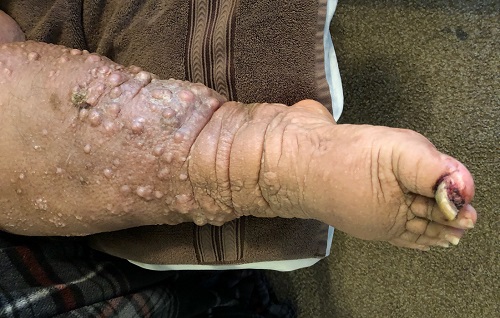
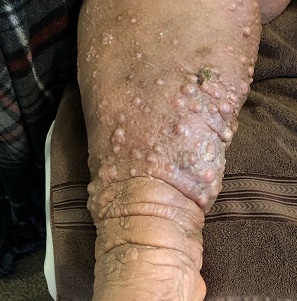
A 61-year-old black woman presents for worrisome skin changes on her lower extremities. She reports that the condition is generally uncomfortable, but during occasional flares, it causes serious pain. She’s been affected for “many years” without diagnosis or resolution. It was her new primary care provider who, after seeing the lesions, sent her to dermatology.
The patient’s medical history includes diabetes, congestive heart failure, and obesity. All are being managed reasonably well.
Examination, performed while she is in a recumbent position, reveals legs swollen out of proportion to the rest of her body. Little or no erythema is noted. Both legs are affected equally, but only from just below the knees down to and including the feet. These areas, including her feet, are quite swollen, though no pitting edema can be provoked. The skin is quite firm and studded with multiple discrete and confluent 1-2 cm firm nodules. The skin around her ankles feels almost “woody” to the touch. There is no tenderness or increased warmth on palpation, nor is any drainage noted. (She also has a dystrophic great toenail that was partially avulsed by recent trauma.)
Ubrogepant effective for acute migraine even with preventive monoclonal antibody therapy
according to preliminary findings presented at the American Headache Society’s 2021 annual meeting.
“Because prevention [with mAbs] is rarely 100% effective, virtually everyone on preventive treatment needs to also take acute treatment,” presenter Richard B. Lipton, MD, a professor of neurology and director of the Montefiore Headache Center at Albert Einstein College of Medicine, New York, said in an interview after his presentation. He explained that ubrogepant, a small-molecule CGRP receptor blocker, is approved for acute treatment of migraine, while mAbs, which block the CGRP receptor or CGRP itself, are approved for prevention. “Many people predicted that gepants would not work in people on CGRP-targeted mAbs because of overlapping mechanisms.”
Dr. Lipton himself was not surprised by the findings, however. “For me, the surprise was that ubrogepant worked so well,” he said.
Novel data collection
Uniquely, his study used an entirely remote design with mobile applications to safely evaluate the drug’s real-world effectiveness in the midst of the COVID-19 pandemic. The prospective, observational study used the mobile app Migraine Buddy to collect data and assess outcomes from the use of 50 mg or 100 mg of ubrogepant along with a mAb, onabotA, or both.
In most migraine trials, researchers ask patients to track their symptoms in electronic diaries they learn how to use in the clinic.
“One disadvantage of this approach is that people usually need to carry two devices, the study device and their smartphone,” Dr. Lipton said in an interview. “In this study, people download an app at home to their smartphone and only need to carry one device. Though remote studies are particularly valuable in the time of pandemic, I believe that apps like Migraine Buddy are and will remain a valuable tool for addressing many research questions.”
Jennifer Robblee, MD, MSc, an assistant professor of neurology at Barrow Neurological Institute in Phoenix, viewed the presentation and was also impressed with the novel use of a smartphone app to conduct the study. “I think that was a unique and cool demonstration of what can be done with the apps out there now,” Dr. Robblee said in an interview. “If you want to have really good tracking and more through tracking, apps like this are fabulous and are very patient forward and patient friendly.”
Combination therapy
The researchers invited 4,541 adults to participate in the study if they had previously reported at least three migraine attacks in the past 30 days and if they had treated at least three prior attacks with ubrogepant. The 483 participants who enrolled after consent and screening included 272 taking ubrogepant with mAb, 132 participants taking ubrogepant with onabotA, and 79 taking ubrogepant with both onabotA and mAb.
For 30 days, participants reported in the app’s diary their pain relief and the time elapsed since taking ubrogepant until they returned to normal functioning. Endpoints included meaningful pain relief – defined as “a level of pain relief that is meaningful to you” – and return to normal function at 2 and 4 hours.
During the study, 352 participants reported treating a migraine attack with a single dose of ubrogepant, and 78 participants treated migraine with two doses. The former group included 193 patients in the ubrogepant plus mAb group, 102 patients in the ubrogepant plus onabotA group, and 57 patients in the ubrogepant plus both group. Because of the limited enrollment in the second two arms, the data Dr. Lipton presented data only on the ubrogepant with mAb arm.
Most of this group (89.1%) was female, with an average age of 40 years and an average Migraine Disability Assessment score of 72.2. Most of the patients were taking erenumab (44.6%) or galcanezumab (34.2%) with the remaining patients taking fremanezumab (17.6%), eptinezumab (3.1%) or multiple mAbs (0.5%). Most participants (59.6%) were prescribed 100-mg ubrogepant dose while the remaining participants took 50 mg.
The analysis of the ubrogepant plus mAb group revealed that 64.2% of patients reported meaningful pain relief at 2 hours, and 84.5% had meaningful pain relief 4 hours after taking ubrogepant. The odds of achieving meaningful pain relief were statistically significant at both time points and remained significant after adjustment for participants’ age, Migraine Disability Assessment score and self-reported prescribed ubrogepant dose (P < .001).
“This study shows that in patients with migraine on CGRP-targeted monoclonal antibodies, ubrogepant is an acute treatment to consider for breakthrough headaches,” Dr. Lipton said. He added that they have now completed the study with more participants and begun analyzing all three groups.
“Full analyses will include data from multiple attacks, attacks treated with a second dose of ubrogepant, additional daily and 30-day effectiveness measures for use of ubrogepant with onabotA and use of ubrogepant with both onabotA and CGRP mAbs,” Dr. Lipton said.
While the findings did not surprise Dr. Robblee, she was happy to see a study that explicitly testing the combination of these treatments, especially given access challenges. “Right now, because treatments are new, we get a lot of insurance denials,” Dr. Robblee said in an interview. “It’s great to have a study out there that we can turn to and say, ‘hey, look, they had all these patients safely using these together.’ It’s going to help us improve access for patients.”
Though Dr. Robblee typically uses old-school pen-and-calendar diaries with her patients, she also sees potential for the use of apps going forward, just as she sees for virtual health care.
“I’ve found telemedicine in general to be a really great addition to the migraine world, and this plays into our ability to use telemedicine paired with tracking,” Dr. Robblee said. “In so many studies, we’re doing a diary anyway, so if there are standard diaries and programs we’re all using, that would be a nice way to do these.”
She notes that most symptom tracking for pain is subjective already, and these apps often include the options to print out the data or to export or transfer it electronically to physicians. “It’s giving us meaningful data,” she said.
The research was funded by AbbVie. Dr. Lipton has received honoraria or research support from AbbVie, Amgen, Biohaven, Dr. Reddy’s Laboratories, electroCore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, Teva, Vector and Vedanta Research. He holds stock options in Biohaven and Ctrl M. Dr. Robblee is a principal investigator for a study sponsored by Eli Lilly and receives stipends for MedLink Neurology and Neurodiem.
according to preliminary findings presented at the American Headache Society’s 2021 annual meeting.
“Because prevention [with mAbs] is rarely 100% effective, virtually everyone on preventive treatment needs to also take acute treatment,” presenter Richard B. Lipton, MD, a professor of neurology and director of the Montefiore Headache Center at Albert Einstein College of Medicine, New York, said in an interview after his presentation. He explained that ubrogepant, a small-molecule CGRP receptor blocker, is approved for acute treatment of migraine, while mAbs, which block the CGRP receptor or CGRP itself, are approved for prevention. “Many people predicted that gepants would not work in people on CGRP-targeted mAbs because of overlapping mechanisms.”
Dr. Lipton himself was not surprised by the findings, however. “For me, the surprise was that ubrogepant worked so well,” he said.
Novel data collection
Uniquely, his study used an entirely remote design with mobile applications to safely evaluate the drug’s real-world effectiveness in the midst of the COVID-19 pandemic. The prospective, observational study used the mobile app Migraine Buddy to collect data and assess outcomes from the use of 50 mg or 100 mg of ubrogepant along with a mAb, onabotA, or both.
In most migraine trials, researchers ask patients to track their symptoms in electronic diaries they learn how to use in the clinic.
“One disadvantage of this approach is that people usually need to carry two devices, the study device and their smartphone,” Dr. Lipton said in an interview. “In this study, people download an app at home to their smartphone and only need to carry one device. Though remote studies are particularly valuable in the time of pandemic, I believe that apps like Migraine Buddy are and will remain a valuable tool for addressing many research questions.”
Jennifer Robblee, MD, MSc, an assistant professor of neurology at Barrow Neurological Institute in Phoenix, viewed the presentation and was also impressed with the novel use of a smartphone app to conduct the study. “I think that was a unique and cool demonstration of what can be done with the apps out there now,” Dr. Robblee said in an interview. “If you want to have really good tracking and more through tracking, apps like this are fabulous and are very patient forward and patient friendly.”
Combination therapy
The researchers invited 4,541 adults to participate in the study if they had previously reported at least three migraine attacks in the past 30 days and if they had treated at least three prior attacks with ubrogepant. The 483 participants who enrolled after consent and screening included 272 taking ubrogepant with mAb, 132 participants taking ubrogepant with onabotA, and 79 taking ubrogepant with both onabotA and mAb.
For 30 days, participants reported in the app’s diary their pain relief and the time elapsed since taking ubrogepant until they returned to normal functioning. Endpoints included meaningful pain relief – defined as “a level of pain relief that is meaningful to you” – and return to normal function at 2 and 4 hours.
During the study, 352 participants reported treating a migraine attack with a single dose of ubrogepant, and 78 participants treated migraine with two doses. The former group included 193 patients in the ubrogepant plus mAb group, 102 patients in the ubrogepant plus onabotA group, and 57 patients in the ubrogepant plus both group. Because of the limited enrollment in the second two arms, the data Dr. Lipton presented data only on the ubrogepant with mAb arm.
Most of this group (89.1%) was female, with an average age of 40 years and an average Migraine Disability Assessment score of 72.2. Most of the patients were taking erenumab (44.6%) or galcanezumab (34.2%) with the remaining patients taking fremanezumab (17.6%), eptinezumab (3.1%) or multiple mAbs (0.5%). Most participants (59.6%) were prescribed 100-mg ubrogepant dose while the remaining participants took 50 mg.
The analysis of the ubrogepant plus mAb group revealed that 64.2% of patients reported meaningful pain relief at 2 hours, and 84.5% had meaningful pain relief 4 hours after taking ubrogepant. The odds of achieving meaningful pain relief were statistically significant at both time points and remained significant after adjustment for participants’ age, Migraine Disability Assessment score and self-reported prescribed ubrogepant dose (P < .001).
“This study shows that in patients with migraine on CGRP-targeted monoclonal antibodies, ubrogepant is an acute treatment to consider for breakthrough headaches,” Dr. Lipton said. He added that they have now completed the study with more participants and begun analyzing all three groups.
“Full analyses will include data from multiple attacks, attacks treated with a second dose of ubrogepant, additional daily and 30-day effectiveness measures for use of ubrogepant with onabotA and use of ubrogepant with both onabotA and CGRP mAbs,” Dr. Lipton said.
While the findings did not surprise Dr. Robblee, she was happy to see a study that explicitly testing the combination of these treatments, especially given access challenges. “Right now, because treatments are new, we get a lot of insurance denials,” Dr. Robblee said in an interview. “It’s great to have a study out there that we can turn to and say, ‘hey, look, they had all these patients safely using these together.’ It’s going to help us improve access for patients.”
Though Dr. Robblee typically uses old-school pen-and-calendar diaries with her patients, she also sees potential for the use of apps going forward, just as she sees for virtual health care.
“I’ve found telemedicine in general to be a really great addition to the migraine world, and this plays into our ability to use telemedicine paired with tracking,” Dr. Robblee said. “In so many studies, we’re doing a diary anyway, so if there are standard diaries and programs we’re all using, that would be a nice way to do these.”
She notes that most symptom tracking for pain is subjective already, and these apps often include the options to print out the data or to export or transfer it electronically to physicians. “It’s giving us meaningful data,” she said.
The research was funded by AbbVie. Dr. Lipton has received honoraria or research support from AbbVie, Amgen, Biohaven, Dr. Reddy’s Laboratories, electroCore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, Teva, Vector and Vedanta Research. He holds stock options in Biohaven and Ctrl M. Dr. Robblee is a principal investigator for a study sponsored by Eli Lilly and receives stipends for MedLink Neurology and Neurodiem.
according to preliminary findings presented at the American Headache Society’s 2021 annual meeting.
“Because prevention [with mAbs] is rarely 100% effective, virtually everyone on preventive treatment needs to also take acute treatment,” presenter Richard B. Lipton, MD, a professor of neurology and director of the Montefiore Headache Center at Albert Einstein College of Medicine, New York, said in an interview after his presentation. He explained that ubrogepant, a small-molecule CGRP receptor blocker, is approved for acute treatment of migraine, while mAbs, which block the CGRP receptor or CGRP itself, are approved for prevention. “Many people predicted that gepants would not work in people on CGRP-targeted mAbs because of overlapping mechanisms.”
Dr. Lipton himself was not surprised by the findings, however. “For me, the surprise was that ubrogepant worked so well,” he said.
Novel data collection
Uniquely, his study used an entirely remote design with mobile applications to safely evaluate the drug’s real-world effectiveness in the midst of the COVID-19 pandemic. The prospective, observational study used the mobile app Migraine Buddy to collect data and assess outcomes from the use of 50 mg or 100 mg of ubrogepant along with a mAb, onabotA, or both.
In most migraine trials, researchers ask patients to track their symptoms in electronic diaries they learn how to use in the clinic.
“One disadvantage of this approach is that people usually need to carry two devices, the study device and their smartphone,” Dr. Lipton said in an interview. “In this study, people download an app at home to their smartphone and only need to carry one device. Though remote studies are particularly valuable in the time of pandemic, I believe that apps like Migraine Buddy are and will remain a valuable tool for addressing many research questions.”
Jennifer Robblee, MD, MSc, an assistant professor of neurology at Barrow Neurological Institute in Phoenix, viewed the presentation and was also impressed with the novel use of a smartphone app to conduct the study. “I think that was a unique and cool demonstration of what can be done with the apps out there now,” Dr. Robblee said in an interview. “If you want to have really good tracking and more through tracking, apps like this are fabulous and are very patient forward and patient friendly.”
Combination therapy
The researchers invited 4,541 adults to participate in the study if they had previously reported at least three migraine attacks in the past 30 days and if they had treated at least three prior attacks with ubrogepant. The 483 participants who enrolled after consent and screening included 272 taking ubrogepant with mAb, 132 participants taking ubrogepant with onabotA, and 79 taking ubrogepant with both onabotA and mAb.
For 30 days, participants reported in the app’s diary their pain relief and the time elapsed since taking ubrogepant until they returned to normal functioning. Endpoints included meaningful pain relief – defined as “a level of pain relief that is meaningful to you” – and return to normal function at 2 and 4 hours.
During the study, 352 participants reported treating a migraine attack with a single dose of ubrogepant, and 78 participants treated migraine with two doses. The former group included 193 patients in the ubrogepant plus mAb group, 102 patients in the ubrogepant plus onabotA group, and 57 patients in the ubrogepant plus both group. Because of the limited enrollment in the second two arms, the data Dr. Lipton presented data only on the ubrogepant with mAb arm.
Most of this group (89.1%) was female, with an average age of 40 years and an average Migraine Disability Assessment score of 72.2. Most of the patients were taking erenumab (44.6%) or galcanezumab (34.2%) with the remaining patients taking fremanezumab (17.6%), eptinezumab (3.1%) or multiple mAbs (0.5%). Most participants (59.6%) were prescribed 100-mg ubrogepant dose while the remaining participants took 50 mg.
The analysis of the ubrogepant plus mAb group revealed that 64.2% of patients reported meaningful pain relief at 2 hours, and 84.5% had meaningful pain relief 4 hours after taking ubrogepant. The odds of achieving meaningful pain relief were statistically significant at both time points and remained significant after adjustment for participants’ age, Migraine Disability Assessment score and self-reported prescribed ubrogepant dose (P < .001).
“This study shows that in patients with migraine on CGRP-targeted monoclonal antibodies, ubrogepant is an acute treatment to consider for breakthrough headaches,” Dr. Lipton said. He added that they have now completed the study with more participants and begun analyzing all three groups.
“Full analyses will include data from multiple attacks, attacks treated with a second dose of ubrogepant, additional daily and 30-day effectiveness measures for use of ubrogepant with onabotA and use of ubrogepant with both onabotA and CGRP mAbs,” Dr. Lipton said.
While the findings did not surprise Dr. Robblee, she was happy to see a study that explicitly testing the combination of these treatments, especially given access challenges. “Right now, because treatments are new, we get a lot of insurance denials,” Dr. Robblee said in an interview. “It’s great to have a study out there that we can turn to and say, ‘hey, look, they had all these patients safely using these together.’ It’s going to help us improve access for patients.”
Though Dr. Robblee typically uses old-school pen-and-calendar diaries with her patients, she also sees potential for the use of apps going forward, just as she sees for virtual health care.
“I’ve found telemedicine in general to be a really great addition to the migraine world, and this plays into our ability to use telemedicine paired with tracking,” Dr. Robblee said. “In so many studies, we’re doing a diary anyway, so if there are standard diaries and programs we’re all using, that would be a nice way to do these.”
She notes that most symptom tracking for pain is subjective already, and these apps often include the options to print out the data or to export or transfer it electronically to physicians. “It’s giving us meaningful data,” she said.
The research was funded by AbbVie. Dr. Lipton has received honoraria or research support from AbbVie, Amgen, Biohaven, Dr. Reddy’s Laboratories, electroCore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, Teva, Vector and Vedanta Research. He holds stock options in Biohaven and Ctrl M. Dr. Robblee is a principal investigator for a study sponsored by Eli Lilly and receives stipends for MedLink Neurology and Neurodiem.
FROM AHS 2021
Reporting Biopsy Margin Status for Cutaneous Basal Cell Carcinoma: To Do or Not to Do
To the Editor:
In an interesting analysis, Brady and Hossler1 (Cutis. 2020;106:315-317) highlighted the limitations of histopathologic biopsy margin evaluation for cutaneous basal cell carcinoma (BCC). Taking into consideration the high prevalence of BCC and its medical and economic impact on the health care system, the issue raised by the authors is an important one. They proposed that pathologists may omit reporting margins or clarify the limitations in their reports. It is a valid suggestion; however, in practice, margin evaluation is not always a simple process and is influenced by a number of factors.
The subject of optimum margins for BCC has been debated over decades now; however, ambiguity and lack of definitive guidelines on certain aspects still remain, leading to a lack of standardization and variability in reporting, which opens potential for error. In anatomical pathology, the biopsies for malignancies are interpreted to confirm diagnosis and perform risk assessment, with evaluation of margins generally reserved for subsequent definitive resections. Typically, margins are not required by clinicians or reported by pathologists in common endoscopic (eg, stomach, colon) or needle core (eg, prostate, breast) biopsies. Skin holds a rather unique position in which margin evaluation is not just limited to excisions. With the exception of samples generated from electrodesiccation and curettage, it is common practice by some laboratories to report margins on most specimens of cutaneous malignancies.
In simple terms, when margins are labeled negative there should be no residual disease, and when they are deemed positive there should be disease still persisting in the patient. Margin evaluation for BCC on biopsies falls short on both fronts. In one analysis, 24% (34/143) of shave biopsies reported with negative margins displayed residual BCC in ensuing re-excisions (negative predictive value: 76%).2 Standard bread-loafing, en-face margins and inking for orientation utilized to provide a thorough margin evaluation of excisions cannot be optimally achieved on small skin biopsies. Microscopic sections for analysis are 2-dimensional representations of 3-dimensional structures. Slides prepared can miss deeply embedded outermost margins, positioned parallel to the plane of sectioning, thereby creating blind spots where margins cannot be precisely assessed and generating an inherent limitation in evaluation. Exhaustive deeper levels done routinely can address this issue to a certain degree; however, it can be an impractical solution with cost implications and delay in turnaround time.
Conversely, it also is common to encounter absence of residual BCC in re-excisions in which the original biopsy margins were labeled positive. In one analysis, 49% of BCC patients (n=100) with positive biopsy margins did not display residual neoplasm on following re-excisions.3 Localized biopsy site immune response as a cause of postbiopsy regression of residual tumor has been hypothesized to produce this phenomenon. Moreover, initial biopsies may eliminate the majority of the tumor with only minimal disease persisting. Re-excisions submitted in toto allow for a systematic examination; however, areas in between sections still remain where minute residual tumor may hide. Searching for such occult foci generally is not aggressively pursued via deeper levels unless the margins of re-excision are in question.
Superficial-type BCC (or superficial multifocal BCC) is a major factor in precluding precise biopsy margin evaluation. In a study where initial biopsies reported with negative margins displayed residual BCC in subsequent re-excisions, 91% (31/34) of residual BCCs were of superficial variety.2 Clinically, superficial BCC frequently has indistinct borders with subtle subclinical peripheral progression. It has a tendency to expand radially, with the clinical appearance deceptively smaller than its true extent. In a plane of histopathologic section, superficial BCC may exhibit skip zones within the epidermis. Even though the margin may seem uninvolved on the slide, a noncontiguous focus may still emerge beyond the “negative” margin. Because superficial pattern is not unusual as one of the components of mixed histology (composite) BCC, this issue is not just limited to tumors specifically designated as superficial type.4
The intent of a procedure is important to recognize. If a biopsy is done with the intention of diagnosis only, the pathologic assessment can be limited to tumor identification and core data elements, with margin evaluation reserved for excisions done with therapeutic intent. However, the intent is not always clear, which adds to ambiguity on when to report margins. It is not uncommon to find saucerization shaves or large punch biopsies for BCC carried out with a therapeutic intent. The status of margin is desired in such samples; however, the intent is not always clearly communicated on requisitions. To avoid any gaps in communication, some pathologists may err on the side of caution and start routinely reporting margins on biopsies.
Taking into account the inaccuracy of margin assessment in biopsies, an argument for omitting margin reporting is plausible. Although dermatologists are the major contributors of skin samples, pathology laboratories cater to a broader clientele. Other physicians from different surgical and medical specialities also perform skin biopsies, and catering to a variety of specialities adds another layer of complexity. A dermatologist may appreciate the debate regarding reliability of margins; however, a physician from another speciality who is not as familiar with the diseases of the integument may lack proper understanding. Omitting margin reporting may lead to misinterpretations or false assumptions, such as, “The margins must be uninvolved, otherwise the pathologist would have said something.” This also can generate additional phone or email inquiries and second review requests. Rather than completely omitting them, another strategy can be to report margins in more quantitative terms. One reporting approach is to have 3 categories of involved, uninvolved, and uninvolved but close for margins less than 1 mm. The cases in the third category may require greater scrutiny by deeper levels or an added caveat in the comment addressing the limitation. If the status of margins is not reported due to a certain reason, a short comment can be added to explain the reason.
In sum, clinicians should recognize that “margin negative” on skin biopsy does not always equate to “completely excised.” Margin status on biopsies is a data item that essentially provides a probability of margin clearance. Completely omitting the margin status on all biopsies may not be the most prudent approach; however, improved guidelines and modifications to enhance the reporting are definitely required.
References
- Brady MC, Hossler EW. Reliability of biopsy margin status for basal cell carcinoma: a retrospective study. Cutis. 2020;106:315-317.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
- Yuan Y, Duff ML, Sammons DL, et al. Retrospective chart review of skin cancer presence in the wide excisions. World J Clin Cases. 2014;2:52-56.
- Cohen PR, Schulze KE, Nelson BR. Basal cell carcinoma with mixed histology: a possible pathogenesis for recurrent skin cancer. Dermatol Surg. 2006;32:542-551.
Continue to: Author's Response...
Authors’ Response
We appreciate the thorough and thoughtful comments in the Letter to the Editor. We agree with the author’s assertion that negative margins on skin specimens does not equate to “completely excised” and that the intent of the clinician is not always clear, even when the pathologist has ready access to the clinician’s notes, as was the case for the majority of specimens included in our study.
There is already variability in how pathologists report margins, including the specific verbiage used, at least for melanocytic lesions.1 The choice of whether or not to report margins and the meaning of those margins is complex due to the uncertainty inherent in margin assessment. Quantifying this uncertainty was the main reason for our study. Ultimately, the pathologist’s decision on whether and how to report margins should be focused on improving patient outcomes. There are benefits and drawbacks to all approaches, and our goal is to provide more information for clinicians and pathologists so that they may better care for their patients. Understanding the limitations of margins on submitted skin specimens—whether margins are reported or not—can only serve to guide improve clinical decision-making.
We also agree that the breadth of specialties of submitting clinicians make reporting of margins difficult, and there is likely similar breadth in their understanding of the nuances of margin assessment and reports. The solution to this problem is adequate education regarding the limitations of a pathology report, and specifically what is meant when margins are (or are not) reported on skin specimens. How to best educate the myriad clinicians who submit biopsies is, of course, the ultimate challenge.
We hope that our study adds information to this ongoing debate regarding margin status reporting, and we appreciate the discussion points raised by the author.
Eric Hossler, MD; Mary Brady, MD
From the Department of Dermatology, Geisinger Health System, Danville, Pennsylvania.
The authors report no conflict of interest.
Reference
- Sellheyer K, Bergfeld WF, Stewart E, et al. Evaluation of surgical margins in melanocytic lesions: a survey among 152 dermatopathologists.J Cutan Pathol. 2005;32:293-299.
To the Editor:
In an interesting analysis, Brady and Hossler1 (Cutis. 2020;106:315-317) highlighted the limitations of histopathologic biopsy margin evaluation for cutaneous basal cell carcinoma (BCC). Taking into consideration the high prevalence of BCC and its medical and economic impact on the health care system, the issue raised by the authors is an important one. They proposed that pathologists may omit reporting margins or clarify the limitations in their reports. It is a valid suggestion; however, in practice, margin evaluation is not always a simple process and is influenced by a number of factors.
The subject of optimum margins for BCC has been debated over decades now; however, ambiguity and lack of definitive guidelines on certain aspects still remain, leading to a lack of standardization and variability in reporting, which opens potential for error. In anatomical pathology, the biopsies for malignancies are interpreted to confirm diagnosis and perform risk assessment, with evaluation of margins generally reserved for subsequent definitive resections. Typically, margins are not required by clinicians or reported by pathologists in common endoscopic (eg, stomach, colon) or needle core (eg, prostate, breast) biopsies. Skin holds a rather unique position in which margin evaluation is not just limited to excisions. With the exception of samples generated from electrodesiccation and curettage, it is common practice by some laboratories to report margins on most specimens of cutaneous malignancies.
In simple terms, when margins are labeled negative there should be no residual disease, and when they are deemed positive there should be disease still persisting in the patient. Margin evaluation for BCC on biopsies falls short on both fronts. In one analysis, 24% (34/143) of shave biopsies reported with negative margins displayed residual BCC in ensuing re-excisions (negative predictive value: 76%).2 Standard bread-loafing, en-face margins and inking for orientation utilized to provide a thorough margin evaluation of excisions cannot be optimally achieved on small skin biopsies. Microscopic sections for analysis are 2-dimensional representations of 3-dimensional structures. Slides prepared can miss deeply embedded outermost margins, positioned parallel to the plane of sectioning, thereby creating blind spots where margins cannot be precisely assessed and generating an inherent limitation in evaluation. Exhaustive deeper levels done routinely can address this issue to a certain degree; however, it can be an impractical solution with cost implications and delay in turnaround time.
Conversely, it also is common to encounter absence of residual BCC in re-excisions in which the original biopsy margins were labeled positive. In one analysis, 49% of BCC patients (n=100) with positive biopsy margins did not display residual neoplasm on following re-excisions.3 Localized biopsy site immune response as a cause of postbiopsy regression of residual tumor has been hypothesized to produce this phenomenon. Moreover, initial biopsies may eliminate the majority of the tumor with only minimal disease persisting. Re-excisions submitted in toto allow for a systematic examination; however, areas in between sections still remain where minute residual tumor may hide. Searching for such occult foci generally is not aggressively pursued via deeper levels unless the margins of re-excision are in question.
Superficial-type BCC (or superficial multifocal BCC) is a major factor in precluding precise biopsy margin evaluation. In a study where initial biopsies reported with negative margins displayed residual BCC in subsequent re-excisions, 91% (31/34) of residual BCCs were of superficial variety.2 Clinically, superficial BCC frequently has indistinct borders with subtle subclinical peripheral progression. It has a tendency to expand radially, with the clinical appearance deceptively smaller than its true extent. In a plane of histopathologic section, superficial BCC may exhibit skip zones within the epidermis. Even though the margin may seem uninvolved on the slide, a noncontiguous focus may still emerge beyond the “negative” margin. Because superficial pattern is not unusual as one of the components of mixed histology (composite) BCC, this issue is not just limited to tumors specifically designated as superficial type.4
The intent of a procedure is important to recognize. If a biopsy is done with the intention of diagnosis only, the pathologic assessment can be limited to tumor identification and core data elements, with margin evaluation reserved for excisions done with therapeutic intent. However, the intent is not always clear, which adds to ambiguity on when to report margins. It is not uncommon to find saucerization shaves or large punch biopsies for BCC carried out with a therapeutic intent. The status of margin is desired in such samples; however, the intent is not always clearly communicated on requisitions. To avoid any gaps in communication, some pathologists may err on the side of caution and start routinely reporting margins on biopsies.
Taking into account the inaccuracy of margin assessment in biopsies, an argument for omitting margin reporting is plausible. Although dermatologists are the major contributors of skin samples, pathology laboratories cater to a broader clientele. Other physicians from different surgical and medical specialities also perform skin biopsies, and catering to a variety of specialities adds another layer of complexity. A dermatologist may appreciate the debate regarding reliability of margins; however, a physician from another speciality who is not as familiar with the diseases of the integument may lack proper understanding. Omitting margin reporting may lead to misinterpretations or false assumptions, such as, “The margins must be uninvolved, otherwise the pathologist would have said something.” This also can generate additional phone or email inquiries and second review requests. Rather than completely omitting them, another strategy can be to report margins in more quantitative terms. One reporting approach is to have 3 categories of involved, uninvolved, and uninvolved but close for margins less than 1 mm. The cases in the third category may require greater scrutiny by deeper levels or an added caveat in the comment addressing the limitation. If the status of margins is not reported due to a certain reason, a short comment can be added to explain the reason.
In sum, clinicians should recognize that “margin negative” on skin biopsy does not always equate to “completely excised.” Margin status on biopsies is a data item that essentially provides a probability of margin clearance. Completely omitting the margin status on all biopsies may not be the most prudent approach; however, improved guidelines and modifications to enhance the reporting are definitely required.
References
- Brady MC, Hossler EW. Reliability of biopsy margin status for basal cell carcinoma: a retrospective study. Cutis. 2020;106:315-317.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
- Yuan Y, Duff ML, Sammons DL, et al. Retrospective chart review of skin cancer presence in the wide excisions. World J Clin Cases. 2014;2:52-56.
- Cohen PR, Schulze KE, Nelson BR. Basal cell carcinoma with mixed histology: a possible pathogenesis for recurrent skin cancer. Dermatol Surg. 2006;32:542-551.
Continue to: Author's Response...
Authors’ Response
We appreciate the thorough and thoughtful comments in the Letter to the Editor. We agree with the author’s assertion that negative margins on skin specimens does not equate to “completely excised” and that the intent of the clinician is not always clear, even when the pathologist has ready access to the clinician’s notes, as was the case for the majority of specimens included in our study.
There is already variability in how pathologists report margins, including the specific verbiage used, at least for melanocytic lesions.1 The choice of whether or not to report margins and the meaning of those margins is complex due to the uncertainty inherent in margin assessment. Quantifying this uncertainty was the main reason for our study. Ultimately, the pathologist’s decision on whether and how to report margins should be focused on improving patient outcomes. There are benefits and drawbacks to all approaches, and our goal is to provide more information for clinicians and pathologists so that they may better care for their patients. Understanding the limitations of margins on submitted skin specimens—whether margins are reported or not—can only serve to guide improve clinical decision-making.
We also agree that the breadth of specialties of submitting clinicians make reporting of margins difficult, and there is likely similar breadth in their understanding of the nuances of margin assessment and reports. The solution to this problem is adequate education regarding the limitations of a pathology report, and specifically what is meant when margins are (or are not) reported on skin specimens. How to best educate the myriad clinicians who submit biopsies is, of course, the ultimate challenge.
We hope that our study adds information to this ongoing debate regarding margin status reporting, and we appreciate the discussion points raised by the author.
Eric Hossler, MD; Mary Brady, MD
From the Department of Dermatology, Geisinger Health System, Danville, Pennsylvania.
The authors report no conflict of interest.
Reference
- Sellheyer K, Bergfeld WF, Stewart E, et al. Evaluation of surgical margins in melanocytic lesions: a survey among 152 dermatopathologists.J Cutan Pathol. 2005;32:293-299.
To the Editor:
In an interesting analysis, Brady and Hossler1 (Cutis. 2020;106:315-317) highlighted the limitations of histopathologic biopsy margin evaluation for cutaneous basal cell carcinoma (BCC). Taking into consideration the high prevalence of BCC and its medical and economic impact on the health care system, the issue raised by the authors is an important one. They proposed that pathologists may omit reporting margins or clarify the limitations in their reports. It is a valid suggestion; however, in practice, margin evaluation is not always a simple process and is influenced by a number of factors.
The subject of optimum margins for BCC has been debated over decades now; however, ambiguity and lack of definitive guidelines on certain aspects still remain, leading to a lack of standardization and variability in reporting, which opens potential for error. In anatomical pathology, the biopsies for malignancies are interpreted to confirm diagnosis and perform risk assessment, with evaluation of margins generally reserved for subsequent definitive resections. Typically, margins are not required by clinicians or reported by pathologists in common endoscopic (eg, stomach, colon) or needle core (eg, prostate, breast) biopsies. Skin holds a rather unique position in which margin evaluation is not just limited to excisions. With the exception of samples generated from electrodesiccation and curettage, it is common practice by some laboratories to report margins on most specimens of cutaneous malignancies.
In simple terms, when margins are labeled negative there should be no residual disease, and when they are deemed positive there should be disease still persisting in the patient. Margin evaluation for BCC on biopsies falls short on both fronts. In one analysis, 24% (34/143) of shave biopsies reported with negative margins displayed residual BCC in ensuing re-excisions (negative predictive value: 76%).2 Standard bread-loafing, en-face margins and inking for orientation utilized to provide a thorough margin evaluation of excisions cannot be optimally achieved on small skin biopsies. Microscopic sections for analysis are 2-dimensional representations of 3-dimensional structures. Slides prepared can miss deeply embedded outermost margins, positioned parallel to the plane of sectioning, thereby creating blind spots where margins cannot be precisely assessed and generating an inherent limitation in evaluation. Exhaustive deeper levels done routinely can address this issue to a certain degree; however, it can be an impractical solution with cost implications and delay in turnaround time.
Conversely, it also is common to encounter absence of residual BCC in re-excisions in which the original biopsy margins were labeled positive. In one analysis, 49% of BCC patients (n=100) with positive biopsy margins did not display residual neoplasm on following re-excisions.3 Localized biopsy site immune response as a cause of postbiopsy regression of residual tumor has been hypothesized to produce this phenomenon. Moreover, initial biopsies may eliminate the majority of the tumor with only minimal disease persisting. Re-excisions submitted in toto allow for a systematic examination; however, areas in between sections still remain where minute residual tumor may hide. Searching for such occult foci generally is not aggressively pursued via deeper levels unless the margins of re-excision are in question.
Superficial-type BCC (or superficial multifocal BCC) is a major factor in precluding precise biopsy margin evaluation. In a study where initial biopsies reported with negative margins displayed residual BCC in subsequent re-excisions, 91% (31/34) of residual BCCs were of superficial variety.2 Clinically, superficial BCC frequently has indistinct borders with subtle subclinical peripheral progression. It has a tendency to expand radially, with the clinical appearance deceptively smaller than its true extent. In a plane of histopathologic section, superficial BCC may exhibit skip zones within the epidermis. Even though the margin may seem uninvolved on the slide, a noncontiguous focus may still emerge beyond the “negative” margin. Because superficial pattern is not unusual as one of the components of mixed histology (composite) BCC, this issue is not just limited to tumors specifically designated as superficial type.4
The intent of a procedure is important to recognize. If a biopsy is done with the intention of diagnosis only, the pathologic assessment can be limited to tumor identification and core data elements, with margin evaluation reserved for excisions done with therapeutic intent. However, the intent is not always clear, which adds to ambiguity on when to report margins. It is not uncommon to find saucerization shaves or large punch biopsies for BCC carried out with a therapeutic intent. The status of margin is desired in such samples; however, the intent is not always clearly communicated on requisitions. To avoid any gaps in communication, some pathologists may err on the side of caution and start routinely reporting margins on biopsies.
Taking into account the inaccuracy of margin assessment in biopsies, an argument for omitting margin reporting is plausible. Although dermatologists are the major contributors of skin samples, pathology laboratories cater to a broader clientele. Other physicians from different surgical and medical specialities also perform skin biopsies, and catering to a variety of specialities adds another layer of complexity. A dermatologist may appreciate the debate regarding reliability of margins; however, a physician from another speciality who is not as familiar with the diseases of the integument may lack proper understanding. Omitting margin reporting may lead to misinterpretations or false assumptions, such as, “The margins must be uninvolved, otherwise the pathologist would have said something.” This also can generate additional phone or email inquiries and second review requests. Rather than completely omitting them, another strategy can be to report margins in more quantitative terms. One reporting approach is to have 3 categories of involved, uninvolved, and uninvolved but close for margins less than 1 mm. The cases in the third category may require greater scrutiny by deeper levels or an added caveat in the comment addressing the limitation. If the status of margins is not reported due to a certain reason, a short comment can be added to explain the reason.
In sum, clinicians should recognize that “margin negative” on skin biopsy does not always equate to “completely excised.” Margin status on biopsies is a data item that essentially provides a probability of margin clearance. Completely omitting the margin status on all biopsies may not be the most prudent approach; however, improved guidelines and modifications to enhance the reporting are definitely required.
References
- Brady MC, Hossler EW. Reliability of biopsy margin status for basal cell carcinoma: a retrospective study. Cutis. 2020;106:315-317.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
- Yuan Y, Duff ML, Sammons DL, et al. Retrospective chart review of skin cancer presence in the wide excisions. World J Clin Cases. 2014;2:52-56.
- Cohen PR, Schulze KE, Nelson BR. Basal cell carcinoma with mixed histology: a possible pathogenesis for recurrent skin cancer. Dermatol Surg. 2006;32:542-551.
Continue to: Author's Response...
Authors’ Response
We appreciate the thorough and thoughtful comments in the Letter to the Editor. We agree with the author’s assertion that negative margins on skin specimens does not equate to “completely excised” and that the intent of the clinician is not always clear, even when the pathologist has ready access to the clinician’s notes, as was the case for the majority of specimens included in our study.
There is already variability in how pathologists report margins, including the specific verbiage used, at least for melanocytic lesions.1 The choice of whether or not to report margins and the meaning of those margins is complex due to the uncertainty inherent in margin assessment. Quantifying this uncertainty was the main reason for our study. Ultimately, the pathologist’s decision on whether and how to report margins should be focused on improving patient outcomes. There are benefits and drawbacks to all approaches, and our goal is to provide more information for clinicians and pathologists so that they may better care for their patients. Understanding the limitations of margins on submitted skin specimens—whether margins are reported or not—can only serve to guide improve clinical decision-making.
We also agree that the breadth of specialties of submitting clinicians make reporting of margins difficult, and there is likely similar breadth in their understanding of the nuances of margin assessment and reports. The solution to this problem is adequate education regarding the limitations of a pathology report, and specifically what is meant when margins are (or are not) reported on skin specimens. How to best educate the myriad clinicians who submit biopsies is, of course, the ultimate challenge.
We hope that our study adds information to this ongoing debate regarding margin status reporting, and we appreciate the discussion points raised by the author.
Eric Hossler, MD; Mary Brady, MD
From the Department of Dermatology, Geisinger Health System, Danville, Pennsylvania.
The authors report no conflict of interest.
Reference
- Sellheyer K, Bergfeld WF, Stewart E, et al. Evaluation of surgical margins in melanocytic lesions: a survey among 152 dermatopathologists.J Cutan Pathol. 2005;32:293-299.
USMLE Step 1 Changes: Dermatology Program Director Perspectives and Implications
To the Editor:
With a trend toward increasing pass/fail medical school curricula, residency program directors (PDs) have relied on the US Medical Licensing Examination (USMLE) Step 1 as an objective measurement of applicant achievement, which is particularly true in competitive subspecialties such as dermatology, plastic surgery, orthopedic surgery, ophthalmology, and neurosurgery, in which reported Step 1 scores are consistently the highest among matched applicants.1 Program directors in dermatology have indicated that Step 1 scores are a priority when considering an applicant.2 However, among PDs, the general perception of plans to change Step 1 scores to pass/fail has largely been negative.3 Although the impact of this change on the dermatology residency selection process remains unknown, we undertook a study to determine dermatology PDs’ perspectives on the scoring change and discuss its potential implications among all competitive specialties.
A 19-question survey was designed that assessed PD demographics and opinions of the changes and potential implications of the Step 1 scoring change (eTable). A list of current US dermatology PDs at osteopathic and allopathic programs was obtained through the 2019-2020 Accreditation Council for Graduate Medical Education list of accredited programs. Surveys were piloted at our institution to assess for internal validity and misleading questions, and then were distributed electronically through REDCap software (https://www.project-redcap.org/). All responses were kept anonymous. Institutional review board approval was obtained. Variables were assessed with means, proportions, and CIs. Results were deemed statistically significant with nonoverlapping 99% CIs (P<.01).
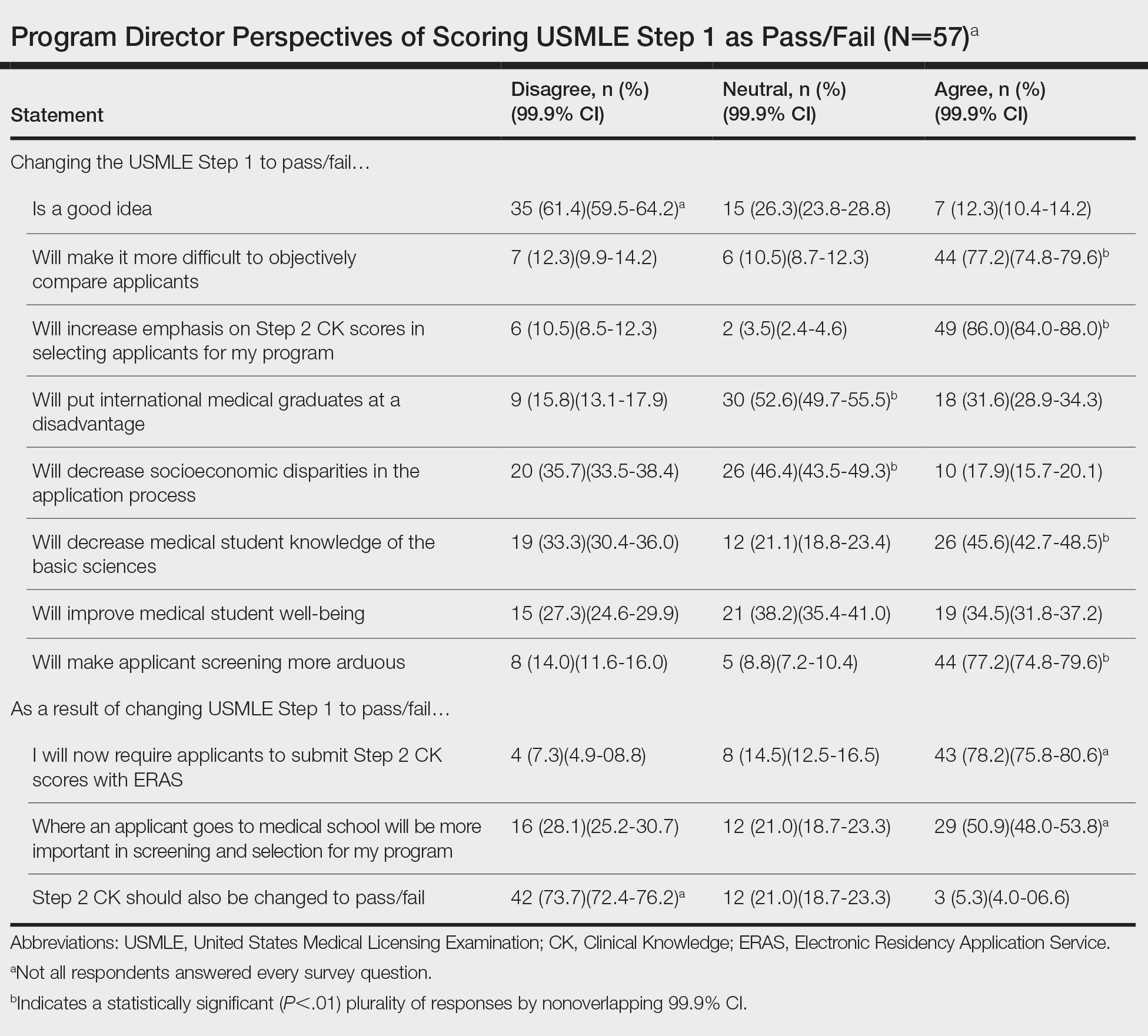
Of 139 surveys, 57 (41.0%) were completed. Most PDs (54.4% [31/57]) were women. The average years of service as a PD was 8.5 years. Most PDs (61.4% [35/57]) disagreed with the scoring change; 77.2% (44/57) of PDs noted that it would make it difficult to objectively assess candidates. Program directors indicated that this change would increase the emphasis they place on USMLE Step 2 Clinical Knowledge (CK) scores (86.0% [49/57]); 78.2% (43/55) reported that they would start requiring Step 2 CK results with submitted applications.
Meanwhile, 73.7% (42/57) of PDs disagreed that Step 2 CK should be changed to pass/fail. Most PDs (50.9% [29/57]) thought that binary Step 1 scoring would increase the importance of medical school reputation in application decisions. The percentage of PDs who were neutral (eTable) on whether pass/fail scoring would place international graduates at a disadvantage was 52.6% (30/57), decrease socioeconomic disparities in the application process was 46.4% (26/56), and improve student well-being was 38.2% (21/55).
Results of our survey indicate generally negative perceptions by dermatology PDs to pass/fail scoring of the USMLE Step 1. A primary goal of introducing binary scoring in both medical school grading and the USMLE was to improve student well-being, as traditional grading systems have been associated with a higher rate of medical student burnout.4-6 However, PDs were equivocal about such an impact on student well-being. Furthermore, PDs indicated that the importance of objective measures would merely shift to the USMLE Step 2 CK, which will still be graded with a 3-digit numeric score. Therefore, Step 2 likely will become the source of anxiety for medical students that was once synonymous with Step 1.
Another goal of the scoring change was to encourage a more holistic approach to applicant review, rather than focusing on numerical metrics. However, with most curricula adopting pass/fail models, there is already a lack of objective measures. Although removal of USMLE Step 1 scores could increase the focus on subjective measures, such as letters of recommendation and rank in medical school class (as indicated by our survey), these are susceptible to bias and may not be the best indicators of applicant suitability. This finding also is concerning for maintaining an equitable application process: PDs indicated that the USMLE Step 1 scoring change would not decrease socioeconomic disparities within the selection process.
In dermatology and other competitive specialties, in which USMLE Step 1 scores have become an important consideration, PDs and residency programs will need to identify additional metrics to compare applicants. Examples include research productivity, grades on relevant rotations, and shelf examination scores. Although more reliable subjective measures, such as interviews and performance on away rotations, are already important, they may become of greater significance.
The findings of our survey suggest that PDs are skeptical about changes to Step 1 and more diligence is necessary to maintain a fair and impartial selection process. Increased emphasis on other objective measurements, such as shelf examination scores, graded curricular components, and research productivity, could help maintain an unbiased approach. With changes to USMLE Step 1 expected to be implemented in the 2022 application cycle, programs may need to explore additional options to maintain reliable and transparent applicant review practices.
- National Resident Matching Program. Charting Outcomes in the Match: U.S Allopathic Seniors, 2018. 2nd ed. National Resident Matching Program; July 2018. Accessed May 12, 2021. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf
- Grading systems use by US medical schools. Association of American Medical Colleges. Accessed May 12, 2021. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/grading-systems-use-us-medical-schools
- Makhoul AT, Pontell ME, Ganesh Kumar N, et al. Objective measures needed—program directors’ perspectives on a pass/fail USMLE Step 1. N Engl J Med; 2020;382:2389-2392. doi:10.1056/NEJMp2006148
- Change to pass/fail score reporting for Step 1. United States Medical Licensing Examination. Accessed May 12, 2021. https://www.usmle.org/incus/
- Reed DA, Shanafelt TD, Satele DW, et al. Relationship of pass/fail grading and curriculum structure with well-being among preclinical medical students: a multi-institutional study. Acad Med. 2011;86:1367-1373. doi:10.1097/ACM.0b013e3182305d81
- Summary report and preliminary recommendations from the Invitational Conference on USMLE Scoring (InCUS). United States Medical Licensing Examination. March 11-12, 2019. Accessed May 12, 2021. https://www.usmle.org/pdfs/incus/incus_summary_report.pdf
To the Editor:
With a trend toward increasing pass/fail medical school curricula, residency program directors (PDs) have relied on the US Medical Licensing Examination (USMLE) Step 1 as an objective measurement of applicant achievement, which is particularly true in competitive subspecialties such as dermatology, plastic surgery, orthopedic surgery, ophthalmology, and neurosurgery, in which reported Step 1 scores are consistently the highest among matched applicants.1 Program directors in dermatology have indicated that Step 1 scores are a priority when considering an applicant.2 However, among PDs, the general perception of plans to change Step 1 scores to pass/fail has largely been negative.3 Although the impact of this change on the dermatology residency selection process remains unknown, we undertook a study to determine dermatology PDs’ perspectives on the scoring change and discuss its potential implications among all competitive specialties.
A 19-question survey was designed that assessed PD demographics and opinions of the changes and potential implications of the Step 1 scoring change (eTable). A list of current US dermatology PDs at osteopathic and allopathic programs was obtained through the 2019-2020 Accreditation Council for Graduate Medical Education list of accredited programs. Surveys were piloted at our institution to assess for internal validity and misleading questions, and then were distributed electronically through REDCap software (https://www.project-redcap.org/). All responses were kept anonymous. Institutional review board approval was obtained. Variables were assessed with means, proportions, and CIs. Results were deemed statistically significant with nonoverlapping 99% CIs (P<.01).

Of 139 surveys, 57 (41.0%) were completed. Most PDs (54.4% [31/57]) were women. The average years of service as a PD was 8.5 years. Most PDs (61.4% [35/57]) disagreed with the scoring change; 77.2% (44/57) of PDs noted that it would make it difficult to objectively assess candidates. Program directors indicated that this change would increase the emphasis they place on USMLE Step 2 Clinical Knowledge (CK) scores (86.0% [49/57]); 78.2% (43/55) reported that they would start requiring Step 2 CK results with submitted applications.
Meanwhile, 73.7% (42/57) of PDs disagreed that Step 2 CK should be changed to pass/fail. Most PDs (50.9% [29/57]) thought that binary Step 1 scoring would increase the importance of medical school reputation in application decisions. The percentage of PDs who were neutral (eTable) on whether pass/fail scoring would place international graduates at a disadvantage was 52.6% (30/57), decrease socioeconomic disparities in the application process was 46.4% (26/56), and improve student well-being was 38.2% (21/55).
Results of our survey indicate generally negative perceptions by dermatology PDs to pass/fail scoring of the USMLE Step 1. A primary goal of introducing binary scoring in both medical school grading and the USMLE was to improve student well-being, as traditional grading systems have been associated with a higher rate of medical student burnout.4-6 However, PDs were equivocal about such an impact on student well-being. Furthermore, PDs indicated that the importance of objective measures would merely shift to the USMLE Step 2 CK, which will still be graded with a 3-digit numeric score. Therefore, Step 2 likely will become the source of anxiety for medical students that was once synonymous with Step 1.
Another goal of the scoring change was to encourage a more holistic approach to applicant review, rather than focusing on numerical metrics. However, with most curricula adopting pass/fail models, there is already a lack of objective measures. Although removal of USMLE Step 1 scores could increase the focus on subjective measures, such as letters of recommendation and rank in medical school class (as indicated by our survey), these are susceptible to bias and may not be the best indicators of applicant suitability. This finding also is concerning for maintaining an equitable application process: PDs indicated that the USMLE Step 1 scoring change would not decrease socioeconomic disparities within the selection process.
In dermatology and other competitive specialties, in which USMLE Step 1 scores have become an important consideration, PDs and residency programs will need to identify additional metrics to compare applicants. Examples include research productivity, grades on relevant rotations, and shelf examination scores. Although more reliable subjective measures, such as interviews and performance on away rotations, are already important, they may become of greater significance.
The findings of our survey suggest that PDs are skeptical about changes to Step 1 and more diligence is necessary to maintain a fair and impartial selection process. Increased emphasis on other objective measurements, such as shelf examination scores, graded curricular components, and research productivity, could help maintain an unbiased approach. With changes to USMLE Step 1 expected to be implemented in the 2022 application cycle, programs may need to explore additional options to maintain reliable and transparent applicant review practices.
To the Editor:
With a trend toward increasing pass/fail medical school curricula, residency program directors (PDs) have relied on the US Medical Licensing Examination (USMLE) Step 1 as an objective measurement of applicant achievement, which is particularly true in competitive subspecialties such as dermatology, plastic surgery, orthopedic surgery, ophthalmology, and neurosurgery, in which reported Step 1 scores are consistently the highest among matched applicants.1 Program directors in dermatology have indicated that Step 1 scores are a priority when considering an applicant.2 However, among PDs, the general perception of plans to change Step 1 scores to pass/fail has largely been negative.3 Although the impact of this change on the dermatology residency selection process remains unknown, we undertook a study to determine dermatology PDs’ perspectives on the scoring change and discuss its potential implications among all competitive specialties.
A 19-question survey was designed that assessed PD demographics and opinions of the changes and potential implications of the Step 1 scoring change (eTable). A list of current US dermatology PDs at osteopathic and allopathic programs was obtained through the 2019-2020 Accreditation Council for Graduate Medical Education list of accredited programs. Surveys were piloted at our institution to assess for internal validity and misleading questions, and then were distributed electronically through REDCap software (https://www.project-redcap.org/). All responses were kept anonymous. Institutional review board approval was obtained. Variables were assessed with means, proportions, and CIs. Results were deemed statistically significant with nonoverlapping 99% CIs (P<.01).

Of 139 surveys, 57 (41.0%) were completed. Most PDs (54.4% [31/57]) were women. The average years of service as a PD was 8.5 years. Most PDs (61.4% [35/57]) disagreed with the scoring change; 77.2% (44/57) of PDs noted that it would make it difficult to objectively assess candidates. Program directors indicated that this change would increase the emphasis they place on USMLE Step 2 Clinical Knowledge (CK) scores (86.0% [49/57]); 78.2% (43/55) reported that they would start requiring Step 2 CK results with submitted applications.
Meanwhile, 73.7% (42/57) of PDs disagreed that Step 2 CK should be changed to pass/fail. Most PDs (50.9% [29/57]) thought that binary Step 1 scoring would increase the importance of medical school reputation in application decisions. The percentage of PDs who were neutral (eTable) on whether pass/fail scoring would place international graduates at a disadvantage was 52.6% (30/57), decrease socioeconomic disparities in the application process was 46.4% (26/56), and improve student well-being was 38.2% (21/55).
Results of our survey indicate generally negative perceptions by dermatology PDs to pass/fail scoring of the USMLE Step 1. A primary goal of introducing binary scoring in both medical school grading and the USMLE was to improve student well-being, as traditional grading systems have been associated with a higher rate of medical student burnout.4-6 However, PDs were equivocal about such an impact on student well-being. Furthermore, PDs indicated that the importance of objective measures would merely shift to the USMLE Step 2 CK, which will still be graded with a 3-digit numeric score. Therefore, Step 2 likely will become the source of anxiety for medical students that was once synonymous with Step 1.
Another goal of the scoring change was to encourage a more holistic approach to applicant review, rather than focusing on numerical metrics. However, with most curricula adopting pass/fail models, there is already a lack of objective measures. Although removal of USMLE Step 1 scores could increase the focus on subjective measures, such as letters of recommendation and rank in medical school class (as indicated by our survey), these are susceptible to bias and may not be the best indicators of applicant suitability. This finding also is concerning for maintaining an equitable application process: PDs indicated that the USMLE Step 1 scoring change would not decrease socioeconomic disparities within the selection process.
In dermatology and other competitive specialties, in which USMLE Step 1 scores have become an important consideration, PDs and residency programs will need to identify additional metrics to compare applicants. Examples include research productivity, grades on relevant rotations, and shelf examination scores. Although more reliable subjective measures, such as interviews and performance on away rotations, are already important, they may become of greater significance.
The findings of our survey suggest that PDs are skeptical about changes to Step 1 and more diligence is necessary to maintain a fair and impartial selection process. Increased emphasis on other objective measurements, such as shelf examination scores, graded curricular components, and research productivity, could help maintain an unbiased approach. With changes to USMLE Step 1 expected to be implemented in the 2022 application cycle, programs may need to explore additional options to maintain reliable and transparent applicant review practices.
- National Resident Matching Program. Charting Outcomes in the Match: U.S Allopathic Seniors, 2018. 2nd ed. National Resident Matching Program; July 2018. Accessed May 12, 2021. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf
- Grading systems use by US medical schools. Association of American Medical Colleges. Accessed May 12, 2021. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/grading-systems-use-us-medical-schools
- Makhoul AT, Pontell ME, Ganesh Kumar N, et al. Objective measures needed—program directors’ perspectives on a pass/fail USMLE Step 1. N Engl J Med; 2020;382:2389-2392. doi:10.1056/NEJMp2006148
- Change to pass/fail score reporting for Step 1. United States Medical Licensing Examination. Accessed May 12, 2021. https://www.usmle.org/incus/
- Reed DA, Shanafelt TD, Satele DW, et al. Relationship of pass/fail grading and curriculum structure with well-being among preclinical medical students: a multi-institutional study. Acad Med. 2011;86:1367-1373. doi:10.1097/ACM.0b013e3182305d81
- Summary report and preliminary recommendations from the Invitational Conference on USMLE Scoring (InCUS). United States Medical Licensing Examination. March 11-12, 2019. Accessed May 12, 2021. https://www.usmle.org/pdfs/incus/incus_summary_report.pdf
- National Resident Matching Program. Charting Outcomes in the Match: U.S Allopathic Seniors, 2018. 2nd ed. National Resident Matching Program; July 2018. Accessed May 12, 2021. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf
- Grading systems use by US medical schools. Association of American Medical Colleges. Accessed May 12, 2021. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/grading-systems-use-us-medical-schools
- Makhoul AT, Pontell ME, Ganesh Kumar N, et al. Objective measures needed—program directors’ perspectives on a pass/fail USMLE Step 1. N Engl J Med; 2020;382:2389-2392. doi:10.1056/NEJMp2006148
- Change to pass/fail score reporting for Step 1. United States Medical Licensing Examination. Accessed May 12, 2021. https://www.usmle.org/incus/
- Reed DA, Shanafelt TD, Satele DW, et al. Relationship of pass/fail grading and curriculum structure with well-being among preclinical medical students: a multi-institutional study. Acad Med. 2011;86:1367-1373. doi:10.1097/ACM.0b013e3182305d81
- Summary report and preliminary recommendations from the Invitational Conference on USMLE Scoring (InCUS). United States Medical Licensing Examination. March 11-12, 2019. Accessed May 12, 2021. https://www.usmle.org/pdfs/incus/incus_summary_report.pdf
Practice Points
- The changes to US Medical Licensing Examination (USMLE) Step 1 were met with mixed reactions from dermatology program directors.
- These changes likely will increase the emphasis on USMLE Step 2 and other objective measures.
Infections in infants: An update
Converge 2021 session
Febrile Infant Update
Presenter
Russell J. McCulloh, MD
Session summary
Infections in infants aged younger than 90 days have been the subject of intense study in pediatric hospital medicine for many years. With the guidance of our talented presenter Dr. Russell McCulloh of Children’s Hospital & Medical Center in Omaha, Neb., the audience explored the historical perspective and evolution of this scientific question, including successes, special situations, newer screening tests, and description of cutting-edge scoring tools and platforms.
The challenge – Tens of thousands of infants present for care in the setting of fever each year. We know that our physical exam and history-taking skills are unlikely to be helpful in risk stratification. We have been guided by the desire to separate serious bacterial infection (SBI: bone infection, meningitis, pneumonia, urinary tract infection, bacteremia, enteritis) from invasive bacterial infection (IBI: meningitis and bacteremia). Data has shown that no test is 100% sensitive or specific, therefore we have to balance risk of disease to cost and invasiveness of tests. Important questions include whether to test and how to stratify by age, who to admit, and who to provide antibiotics.
The wins and exceptions – Fortunately, the early Boston, Philadelphia, and Rochester criteria set the stage for safely reducing testing. The current American College of Emergency Physicians guidelines for infants aged 29-90 days allows for lumbar puncture to be optional knowing that a look back using prior criteria identified no cases of meningitis in the low risk group. Similarly, in low-risk infants aged less that 29 days in nearly 4,000 cases there were just 2 infants with meningitis. Universal screening of moms for Group B Streptococcus with delivery of antibiotics in appropriate cases has dramatically decreased incidence of SBI. The Hib and pneumovax vaccines have likewise decreased incidence of SBI. Exceptions persist, including knowledge that infants with herpes simplex virus disease will not have fever in 50% of cases and that risk of HSV transmission is highest (25%-60% transmission) in mothers with primary disease. Given risk of HSV CNS disease after 1 week of age, in any high-risk infant less than 21 days, the mantra remains to test and treat.
The cutting edge – Thanks to ongoing research, we now have the PECARN and REVISE study groups to further aid decision-making. With PECARN we know that SBI in infants is extremely unlikely (negative predictive value, 99.7%) with a negative urinalysis , absolute neutrophil count less than 4,090, and procalcitonin less than 1.71. REVISE has revealed that infants with positive viral testing are unlikely to have SBI (7%-12%), particularly with influenza and RSV disease. Procalcitonin has also recently been shown to be an effective tool to rule out disease with the highest negative predictive value among available inflammatory markers. The just-published Aronson rule identifies a scoring system for IBI (using age less than 21 days/1pt; temp 38-38.4° C/2pt; >38.5° C/4pt; abnormal urinalysis/3pt; and absolute neutrophil count >5185/2pt) where any score greater than2 provides a sensitivity of 98.8% and NPV in validation studies of 99.4%. Likewise, multiplex polymerase chain reaction testing of spinal fluid has allowed for additional insight in pretreated cases and has helped us to remove antibiotic treatment from cases where parechovirus and enterovirus are positive because of the low risk for concomitant bacterial meningitis. As we await the release of revised national American Academy of Pediatrics guidelines, it is safe to say great progress has been made in the care of young febrile infants with shorter length of stay and fewer tests for all.
Key takeaways
- Numerous screening tests, rules, and scoring tools have been created to improve identification of infants with IBI, a low-frequency, high-morbidity event. The most recent with negative predictive values of 99.7% and 99.4% are the PECARN and Aronson scoring tools.
- Recent studies of the febrile infant population indicate that the odds of UTI or bacteremia in infants with respiratory symptoms is low, particularly for RSV and influenza.
- Among newer tests developed, a negative procalcitonin has the highest negative predictive value.
- Viral pathogens identified on cerebrospinal fluid molecular testing can be helpful in pretreated cases and indicative of low likelihood of bacterial meningitis allowing for observation off of antibiotics.
Dr. King is a hospitalist, associate director for medical education and associate program director for the pediatrics residency program at Children’s Minnesota in Minneapolis. She has shared some of her resident teaching, presentation skills, and peer-coaching work on a national level.
Converge 2021 session
Febrile Infant Update
Presenter
Russell J. McCulloh, MD
Session summary
Infections in infants aged younger than 90 days have been the subject of intense study in pediatric hospital medicine for many years. With the guidance of our talented presenter Dr. Russell McCulloh of Children’s Hospital & Medical Center in Omaha, Neb., the audience explored the historical perspective and evolution of this scientific question, including successes, special situations, newer screening tests, and description of cutting-edge scoring tools and platforms.
The challenge – Tens of thousands of infants present for care in the setting of fever each year. We know that our physical exam and history-taking skills are unlikely to be helpful in risk stratification. We have been guided by the desire to separate serious bacterial infection (SBI: bone infection, meningitis, pneumonia, urinary tract infection, bacteremia, enteritis) from invasive bacterial infection (IBI: meningitis and bacteremia). Data has shown that no test is 100% sensitive or specific, therefore we have to balance risk of disease to cost and invasiveness of tests. Important questions include whether to test and how to stratify by age, who to admit, and who to provide antibiotics.
The wins and exceptions – Fortunately, the early Boston, Philadelphia, and Rochester criteria set the stage for safely reducing testing. The current American College of Emergency Physicians guidelines for infants aged 29-90 days allows for lumbar puncture to be optional knowing that a look back using prior criteria identified no cases of meningitis in the low risk group. Similarly, in low-risk infants aged less that 29 days in nearly 4,000 cases there were just 2 infants with meningitis. Universal screening of moms for Group B Streptococcus with delivery of antibiotics in appropriate cases has dramatically decreased incidence of SBI. The Hib and pneumovax vaccines have likewise decreased incidence of SBI. Exceptions persist, including knowledge that infants with herpes simplex virus disease will not have fever in 50% of cases and that risk of HSV transmission is highest (25%-60% transmission) in mothers with primary disease. Given risk of HSV CNS disease after 1 week of age, in any high-risk infant less than 21 days, the mantra remains to test and treat.
The cutting edge – Thanks to ongoing research, we now have the PECARN and REVISE study groups to further aid decision-making. With PECARN we know that SBI in infants is extremely unlikely (negative predictive value, 99.7%) with a negative urinalysis , absolute neutrophil count less than 4,090, and procalcitonin less than 1.71. REVISE has revealed that infants with positive viral testing are unlikely to have SBI (7%-12%), particularly with influenza and RSV disease. Procalcitonin has also recently been shown to be an effective tool to rule out disease with the highest negative predictive value among available inflammatory markers. The just-published Aronson rule identifies a scoring system for IBI (using age less than 21 days/1pt; temp 38-38.4° C/2pt; >38.5° C/4pt; abnormal urinalysis/3pt; and absolute neutrophil count >5185/2pt) where any score greater than2 provides a sensitivity of 98.8% and NPV in validation studies of 99.4%. Likewise, multiplex polymerase chain reaction testing of spinal fluid has allowed for additional insight in pretreated cases and has helped us to remove antibiotic treatment from cases where parechovirus and enterovirus are positive because of the low risk for concomitant bacterial meningitis. As we await the release of revised national American Academy of Pediatrics guidelines, it is safe to say great progress has been made in the care of young febrile infants with shorter length of stay and fewer tests for all.
Key takeaways
- Numerous screening tests, rules, and scoring tools have been created to improve identification of infants with IBI, a low-frequency, high-morbidity event. The most recent with negative predictive values of 99.7% and 99.4% are the PECARN and Aronson scoring tools.
- Recent studies of the febrile infant population indicate that the odds of UTI or bacteremia in infants with respiratory symptoms is low, particularly for RSV and influenza.
- Among newer tests developed, a negative procalcitonin has the highest negative predictive value.
- Viral pathogens identified on cerebrospinal fluid molecular testing can be helpful in pretreated cases and indicative of low likelihood of bacterial meningitis allowing for observation off of antibiotics.
Dr. King is a hospitalist, associate director for medical education and associate program director for the pediatrics residency program at Children’s Minnesota in Minneapolis. She has shared some of her resident teaching, presentation skills, and peer-coaching work on a national level.
Converge 2021 session
Febrile Infant Update
Presenter
Russell J. McCulloh, MD
Session summary
Infections in infants aged younger than 90 days have been the subject of intense study in pediatric hospital medicine for many years. With the guidance of our talented presenter Dr. Russell McCulloh of Children’s Hospital & Medical Center in Omaha, Neb., the audience explored the historical perspective and evolution of this scientific question, including successes, special situations, newer screening tests, and description of cutting-edge scoring tools and platforms.
The challenge – Tens of thousands of infants present for care in the setting of fever each year. We know that our physical exam and history-taking skills are unlikely to be helpful in risk stratification. We have been guided by the desire to separate serious bacterial infection (SBI: bone infection, meningitis, pneumonia, urinary tract infection, bacteremia, enteritis) from invasive bacterial infection (IBI: meningitis and bacteremia). Data has shown that no test is 100% sensitive or specific, therefore we have to balance risk of disease to cost and invasiveness of tests. Important questions include whether to test and how to stratify by age, who to admit, and who to provide antibiotics.
The wins and exceptions – Fortunately, the early Boston, Philadelphia, and Rochester criteria set the stage for safely reducing testing. The current American College of Emergency Physicians guidelines for infants aged 29-90 days allows for lumbar puncture to be optional knowing that a look back using prior criteria identified no cases of meningitis in the low risk group. Similarly, in low-risk infants aged less that 29 days in nearly 4,000 cases there were just 2 infants with meningitis. Universal screening of moms for Group B Streptococcus with delivery of antibiotics in appropriate cases has dramatically decreased incidence of SBI. The Hib and pneumovax vaccines have likewise decreased incidence of SBI. Exceptions persist, including knowledge that infants with herpes simplex virus disease will not have fever in 50% of cases and that risk of HSV transmission is highest (25%-60% transmission) in mothers with primary disease. Given risk of HSV CNS disease after 1 week of age, in any high-risk infant less than 21 days, the mantra remains to test and treat.
The cutting edge – Thanks to ongoing research, we now have the PECARN and REVISE study groups to further aid decision-making. With PECARN we know that SBI in infants is extremely unlikely (negative predictive value, 99.7%) with a negative urinalysis , absolute neutrophil count less than 4,090, and procalcitonin less than 1.71. REVISE has revealed that infants with positive viral testing are unlikely to have SBI (7%-12%), particularly with influenza and RSV disease. Procalcitonin has also recently been shown to be an effective tool to rule out disease with the highest negative predictive value among available inflammatory markers. The just-published Aronson rule identifies a scoring system for IBI (using age less than 21 days/1pt; temp 38-38.4° C/2pt; >38.5° C/4pt; abnormal urinalysis/3pt; and absolute neutrophil count >5185/2pt) where any score greater than2 provides a sensitivity of 98.8% and NPV in validation studies of 99.4%. Likewise, multiplex polymerase chain reaction testing of spinal fluid has allowed for additional insight in pretreated cases and has helped us to remove antibiotic treatment from cases where parechovirus and enterovirus are positive because of the low risk for concomitant bacterial meningitis. As we await the release of revised national American Academy of Pediatrics guidelines, it is safe to say great progress has been made in the care of young febrile infants with shorter length of stay and fewer tests for all.
Key takeaways
- Numerous screening tests, rules, and scoring tools have been created to improve identification of infants with IBI, a low-frequency, high-morbidity event. The most recent with negative predictive values of 99.7% and 99.4% are the PECARN and Aronson scoring tools.
- Recent studies of the febrile infant population indicate that the odds of UTI or bacteremia in infants with respiratory symptoms is low, particularly for RSV and influenza.
- Among newer tests developed, a negative procalcitonin has the highest negative predictive value.
- Viral pathogens identified on cerebrospinal fluid molecular testing can be helpful in pretreated cases and indicative of low likelihood of bacterial meningitis allowing for observation off of antibiotics.
Dr. King is a hospitalist, associate director for medical education and associate program director for the pediatrics residency program at Children’s Minnesota in Minneapolis. She has shared some of her resident teaching, presentation skills, and peer-coaching work on a national level.
FROM SHM CONVERGE 2021
Wound Healing on the Dorsal Hands: An Intrapatient Comparison of Primary Closure, Purse-String Closure, and Secondary Intention
Practice Gap
Many cutaneous surgery wounds can be closed primarily; however, in certain cases, other repair options might be appropriate and should be evaluated on a case-by-case basis with input from the patient. Defects on the dorsal aspect of the hands—where nonmelanoma skin cancer is common and reserve tissue is limited—often heal by secondary intention with good cosmetic and functional results. Patients often express a desire to reduce the time spent in the surgical suite and restrictions on postoperative activity, making secondary intention healing more appealing. An additional advantage is obviation of the need to remove additional tissue in the form of Burow triangles, which would lead to a longer wound. The major disadvantage of secondary intention healing is longer time to wound maturity; we often minimize this disadvantage with purse-string closure to decrease the size of the wound defect, which can be done quickly and without removing additional tissue.
The Technique
An elderly man had 3 nonmelanoma skin cancers—all on the dorsal aspect of the left hand—that were treated on the same day, leaving 3 similar wound defects after Mohs micrographic surgery. The wound defects (distal to proximal) measured 12 mm, 12 mm, and 10 mm in diameter (Figure 1) and were repaired by primary closure, secondary intention, and purse-string circumferential closure, respectively. Purse-string closure1 was performed with a 4-0 polyglactin 901 suture and left to heal without external sutures (Figure 2). Figure 3 shows the 3 types of repairs immediately following closure. All wounds healed with excellent and essentially equivalent cosmetic results, with excellent patient satisfaction at 6-month follow-up (Figure 4).



Practical Implications
Our case illustrates different modalities of wound repair during precisely the same time frame and essentially on the same location. Skin of the dorsal hand often is tight; depending on the size of the defect, large primary closure can be tedious to perform, can lead to increased wound tension and risk of dehiscence, and can be uncomfortable for the patient during healing. However, primary closure typically will lead to faster healing.
Secondary intention healing and purse-string closure require less surgery and therefore cost less; these modalities yield similar cosmesis and satisfaction. In the appropriate context, secondary intention has been highlighted as a suitable alternative to primary closure2-4; in our experience (and that of others5), patient satisfaction is not diminished with healing by secondary intention. Purse-string closure also can minimize wound size and healing time.
For small shallow wounds on the dorsal hand, dermatologic surgeons should have confidence that secondary intention healing, with or without wound reduction using purse-string repair, likely will lead to acceptable cosmetic and functional results. Of course, repair should be tailored to the circumstances and wishes of the individual patient.
- Peled IJ, Zagher U, Wexler MR. Purse-string suture for reduction and closure of skin defects. Ann Plast Surg. 1985;14:465-469. doi:10.1097/00000637-198505000-00012
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106. doi:10.1016/0738-081x(84)90031-2
- Fazio MJ, Zitelli JA. Principles of reconstruction following excision of nonmelanoma skin cancer. Clin Dermatol. 1995;13:601-616. doi:10.1016/0738-081x(95)00099-2
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38:647-653. doi:10.1111/j.1524-4725.2011.02258.x
- Stebbins WG, Gusev J, Higgins HW 2nd, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol. 2015;73:865-867.e1. doi:10.1016/j.jaad.2015.07.019
Practice Gap
Many cutaneous surgery wounds can be closed primarily; however, in certain cases, other repair options might be appropriate and should be evaluated on a case-by-case basis with input from the patient. Defects on the dorsal aspect of the hands—where nonmelanoma skin cancer is common and reserve tissue is limited—often heal by secondary intention with good cosmetic and functional results. Patients often express a desire to reduce the time spent in the surgical suite and restrictions on postoperative activity, making secondary intention healing more appealing. An additional advantage is obviation of the need to remove additional tissue in the form of Burow triangles, which would lead to a longer wound. The major disadvantage of secondary intention healing is longer time to wound maturity; we often minimize this disadvantage with purse-string closure to decrease the size of the wound defect, which can be done quickly and without removing additional tissue.
The Technique
An elderly man had 3 nonmelanoma skin cancers—all on the dorsal aspect of the left hand—that were treated on the same day, leaving 3 similar wound defects after Mohs micrographic surgery. The wound defects (distal to proximal) measured 12 mm, 12 mm, and 10 mm in diameter (Figure 1) and were repaired by primary closure, secondary intention, and purse-string circumferential closure, respectively. Purse-string closure1 was performed with a 4-0 polyglactin 901 suture and left to heal without external sutures (Figure 2). Figure 3 shows the 3 types of repairs immediately following closure. All wounds healed with excellent and essentially equivalent cosmetic results, with excellent patient satisfaction at 6-month follow-up (Figure 4).




Practical Implications
Our case illustrates different modalities of wound repair during precisely the same time frame and essentially on the same location. Skin of the dorsal hand often is tight; depending on the size of the defect, large primary closure can be tedious to perform, can lead to increased wound tension and risk of dehiscence, and can be uncomfortable for the patient during healing. However, primary closure typically will lead to faster healing.
Secondary intention healing and purse-string closure require less surgery and therefore cost less; these modalities yield similar cosmesis and satisfaction. In the appropriate context, secondary intention has been highlighted as a suitable alternative to primary closure2-4; in our experience (and that of others5), patient satisfaction is not diminished with healing by secondary intention. Purse-string closure also can minimize wound size and healing time.
For small shallow wounds on the dorsal hand, dermatologic surgeons should have confidence that secondary intention healing, with or without wound reduction using purse-string repair, likely will lead to acceptable cosmetic and functional results. Of course, repair should be tailored to the circumstances and wishes of the individual patient.
Practice Gap
Many cutaneous surgery wounds can be closed primarily; however, in certain cases, other repair options might be appropriate and should be evaluated on a case-by-case basis with input from the patient. Defects on the dorsal aspect of the hands—where nonmelanoma skin cancer is common and reserve tissue is limited—often heal by secondary intention with good cosmetic and functional results. Patients often express a desire to reduce the time spent in the surgical suite and restrictions on postoperative activity, making secondary intention healing more appealing. An additional advantage is obviation of the need to remove additional tissue in the form of Burow triangles, which would lead to a longer wound. The major disadvantage of secondary intention healing is longer time to wound maturity; we often minimize this disadvantage with purse-string closure to decrease the size of the wound defect, which can be done quickly and without removing additional tissue.
The Technique
An elderly man had 3 nonmelanoma skin cancers—all on the dorsal aspect of the left hand—that were treated on the same day, leaving 3 similar wound defects after Mohs micrographic surgery. The wound defects (distal to proximal) measured 12 mm, 12 mm, and 10 mm in diameter (Figure 1) and were repaired by primary closure, secondary intention, and purse-string circumferential closure, respectively. Purse-string closure1 was performed with a 4-0 polyglactin 901 suture and left to heal without external sutures (Figure 2). Figure 3 shows the 3 types of repairs immediately following closure. All wounds healed with excellent and essentially equivalent cosmetic results, with excellent patient satisfaction at 6-month follow-up (Figure 4).




Practical Implications
Our case illustrates different modalities of wound repair during precisely the same time frame and essentially on the same location. Skin of the dorsal hand often is tight; depending on the size of the defect, large primary closure can be tedious to perform, can lead to increased wound tension and risk of dehiscence, and can be uncomfortable for the patient during healing. However, primary closure typically will lead to faster healing.
Secondary intention healing and purse-string closure require less surgery and therefore cost less; these modalities yield similar cosmesis and satisfaction. In the appropriate context, secondary intention has been highlighted as a suitable alternative to primary closure2-4; in our experience (and that of others5), patient satisfaction is not diminished with healing by secondary intention. Purse-string closure also can minimize wound size and healing time.
For small shallow wounds on the dorsal hand, dermatologic surgeons should have confidence that secondary intention healing, with or without wound reduction using purse-string repair, likely will lead to acceptable cosmetic and functional results. Of course, repair should be tailored to the circumstances and wishes of the individual patient.
- Peled IJ, Zagher U, Wexler MR. Purse-string suture for reduction and closure of skin defects. Ann Plast Surg. 1985;14:465-469. doi:10.1097/00000637-198505000-00012
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106. doi:10.1016/0738-081x(84)90031-2
- Fazio MJ, Zitelli JA. Principles of reconstruction following excision of nonmelanoma skin cancer. Clin Dermatol. 1995;13:601-616. doi:10.1016/0738-081x(95)00099-2
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38:647-653. doi:10.1111/j.1524-4725.2011.02258.x
- Stebbins WG, Gusev J, Higgins HW 2nd, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol. 2015;73:865-867.e1. doi:10.1016/j.jaad.2015.07.019
- Peled IJ, Zagher U, Wexler MR. Purse-string suture for reduction and closure of skin defects. Ann Plast Surg. 1985;14:465-469. doi:10.1097/00000637-198505000-00012
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106. doi:10.1016/0738-081x(84)90031-2
- Fazio MJ, Zitelli JA. Principles of reconstruction following excision of nonmelanoma skin cancer. Clin Dermatol. 1995;13:601-616. doi:10.1016/0738-081x(95)00099-2
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38:647-653. doi:10.1111/j.1524-4725.2011.02258.x
- Stebbins WG, Gusev J, Higgins HW 2nd, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol. 2015;73:865-867.e1. doi:10.1016/j.jaad.2015.07.019
Cross-sectional study finds chronic skin conditions have highest opioid prescribing rates
at dermatology visits.
“Overall, opioid prescribing rates among dermatologists were low. However, dermatologists should remain aware of risk factors for long-term opioid use and consider using nonnarcotic or nonpharmacologic interventions when possible,” Sarah P. Pourali, a medical student at Vanderbilt University, Nashville, said at the annual meeting of the Society for Investigative Dermatology, where she presented the results.
Ms. Pourali said that although Mohs surgery and dermatologic procedures are the focus of “much of the literature” concerning opioid use in dermatology, there are limited data on medication prescribing patterns for other skin conditions treated by dermatologists.
She and her colleagues performed a cross-sectional study using data from the National Ambulatory Medical Care Survey (NAMCS) from 2009 to 2016 on 288,462,610 weighted dermatology visits. The researchers used International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to identify dermatologic diseases. They also identified and grouped oral pain medication into the following categories: opiate analgesics, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentin. A linear regression analysis was used to evaluate pain medicine prescribing each year, and the researchers used a logistic regression analysis to explore how opiate prescriptions were connected to patient clinical characteristics. The analysis was adjusted for age, gender, race, ethnicity, and region.
Overall, most dermatology visits were for patients older than 65 years (36.2%) and 45-64 years old (32.1%). Over half of the dermatologist visits were for women (56.4%) and most (92.2%) visits were for patients who were White (5.1 % were for patients who were Black); most were non-Hispanic or Latino (93.5%). Most dermatology visits were in the South (35.4%) and West (25.2%), followed by the Northeast (21.9%) and Midwest (17.5%).
Opioids were prescribed in 1.3% of the visits, Ms. Pourali said. In addition, 4.7% of visits included an NSAID prescription, 0.7% an acetaminophen prescription, and 0.6% a gabapentin prescription.
Dermatologic procedure visits accounted for 43.1% of opioid prescriptions, she noted. The most common skin conditions for which opioids were prescribed included vitiligo (10.3%), hemangioma (3.8%), pemphigus (3.6%), atopic dermatitis (3.4%), and psoriasis (2.5%).
Although patients older than 65 years accounted for 36.2% of visits to dermatologists, 58.5% of opioids prescribed by dermatologists were for patients in this age group. “We hypothesize that this may be due to a higher proportion of older patients requiring skin cancer surgeries where a lot of opioids are prescribed within dermatology,” Ms. Pourali said.
The highest population-adjusted prescription rates for opiates were in the Northeast and Western regions of the United States, which “partially corroborates” previous studies that have found “higher rates of opioid prescribing in the southern and western U.S.,” she noted.
When evaluating risk-factors for long-term opiate use, Ms. Pourali and colleagues found opioids were also prescribed in 13.2% of visits where a benzodiazepine was prescribed (adjusted odds ratio, 8.17; 95% confidence interval, 5.3-12.7), 8.4% of visits where the patient had a substance abuse disorder (adjusted OR, 9.40; 95% CI, 2.0-44.4), 5.2% of visits with a patient who had depression (adjusted OR, 3.28; 95% CI, 2.0-5.4), and 2.4% of visits with a patient who used tobacco (adjusted OR, 1.09; 95% CI, 1.0-1.1).
Consider nonopioid postoperative pain management options
In an interview, Sailesh Konda, MD, associate clinical professor of dermatology and director of Mohs surgery and surgical dermatology at the University of Florida, Gainesville, who was not involved with the research, noted the finding in the study that vitiligo, hemangioma, pemphigus, AD, and psoriasis were diagnoses with the highest rates of opioid prescription was surprising. “In general, these are conditions that are not routinely managed with opioids,” he said.
NAMCS contains a primary diagnosis field and space for four additional diagnoses such as chronic conditions, as well as thirty fields for medications. “If an opioid was prescribed at a visit, it could have been prescribed for any of the diagnoses related to the visit,” Dr. Konda said. “Additionally, for those opioid prescriptions associated with dermatologic procedures, it would have been helpful to have a breakdown of the specific procedures.”
Dr. Konda compared these results to a recent study of opioid prescribing patterns in the dermatology Medicare population, which found that 93.9% of the top 1% of opioid prescribers were dermatologists working in a surgical practice.
He said that recommendations for opioid prescribing should be developed for general dermatology as they have been for Mohs surgery and dermatologic surgery. For dermatologists currently prescribing opioids, he recommended monitoring prescribing patterns and to “consider nonopioid interventions, such as acetaminophen plus ibuprofen, which has been found to effectively control postoperative pain with fewer complications.”
Ms. Pourali reports no relevant financial disclosures. Her coauthors included the principal investigator, April Armstrong, MD, MPH, professor of dermatology, University of Southern California, Los Angeles. Dr. Konda reports no relevant financial disclosures.
at dermatology visits.
“Overall, opioid prescribing rates among dermatologists were low. However, dermatologists should remain aware of risk factors for long-term opioid use and consider using nonnarcotic or nonpharmacologic interventions when possible,” Sarah P. Pourali, a medical student at Vanderbilt University, Nashville, said at the annual meeting of the Society for Investigative Dermatology, where she presented the results.
Ms. Pourali said that although Mohs surgery and dermatologic procedures are the focus of “much of the literature” concerning opioid use in dermatology, there are limited data on medication prescribing patterns for other skin conditions treated by dermatologists.
She and her colleagues performed a cross-sectional study using data from the National Ambulatory Medical Care Survey (NAMCS) from 2009 to 2016 on 288,462,610 weighted dermatology visits. The researchers used International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to identify dermatologic diseases. They also identified and grouped oral pain medication into the following categories: opiate analgesics, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentin. A linear regression analysis was used to evaluate pain medicine prescribing each year, and the researchers used a logistic regression analysis to explore how opiate prescriptions were connected to patient clinical characteristics. The analysis was adjusted for age, gender, race, ethnicity, and region.
Overall, most dermatology visits were for patients older than 65 years (36.2%) and 45-64 years old (32.1%). Over half of the dermatologist visits were for women (56.4%) and most (92.2%) visits were for patients who were White (5.1 % were for patients who were Black); most were non-Hispanic or Latino (93.5%). Most dermatology visits were in the South (35.4%) and West (25.2%), followed by the Northeast (21.9%) and Midwest (17.5%).
Opioids were prescribed in 1.3% of the visits, Ms. Pourali said. In addition, 4.7% of visits included an NSAID prescription, 0.7% an acetaminophen prescription, and 0.6% a gabapentin prescription.
Dermatologic procedure visits accounted for 43.1% of opioid prescriptions, she noted. The most common skin conditions for which opioids were prescribed included vitiligo (10.3%), hemangioma (3.8%), pemphigus (3.6%), atopic dermatitis (3.4%), and psoriasis (2.5%).
Although patients older than 65 years accounted for 36.2% of visits to dermatologists, 58.5% of opioids prescribed by dermatologists were for patients in this age group. “We hypothesize that this may be due to a higher proportion of older patients requiring skin cancer surgeries where a lot of opioids are prescribed within dermatology,” Ms. Pourali said.
The highest population-adjusted prescription rates for opiates were in the Northeast and Western regions of the United States, which “partially corroborates” previous studies that have found “higher rates of opioid prescribing in the southern and western U.S.,” she noted.
When evaluating risk-factors for long-term opiate use, Ms. Pourali and colleagues found opioids were also prescribed in 13.2% of visits where a benzodiazepine was prescribed (adjusted odds ratio, 8.17; 95% confidence interval, 5.3-12.7), 8.4% of visits where the patient had a substance abuse disorder (adjusted OR, 9.40; 95% CI, 2.0-44.4), 5.2% of visits with a patient who had depression (adjusted OR, 3.28; 95% CI, 2.0-5.4), and 2.4% of visits with a patient who used tobacco (adjusted OR, 1.09; 95% CI, 1.0-1.1).
Consider nonopioid postoperative pain management options
In an interview, Sailesh Konda, MD, associate clinical professor of dermatology and director of Mohs surgery and surgical dermatology at the University of Florida, Gainesville, who was not involved with the research, noted the finding in the study that vitiligo, hemangioma, pemphigus, AD, and psoriasis were diagnoses with the highest rates of opioid prescription was surprising. “In general, these are conditions that are not routinely managed with opioids,” he said.
NAMCS contains a primary diagnosis field and space for four additional diagnoses such as chronic conditions, as well as thirty fields for medications. “If an opioid was prescribed at a visit, it could have been prescribed for any of the diagnoses related to the visit,” Dr. Konda said. “Additionally, for those opioid prescriptions associated with dermatologic procedures, it would have been helpful to have a breakdown of the specific procedures.”
Dr. Konda compared these results to a recent study of opioid prescribing patterns in the dermatology Medicare population, which found that 93.9% of the top 1% of opioid prescribers were dermatologists working in a surgical practice.
He said that recommendations for opioid prescribing should be developed for general dermatology as they have been for Mohs surgery and dermatologic surgery. For dermatologists currently prescribing opioids, he recommended monitoring prescribing patterns and to “consider nonopioid interventions, such as acetaminophen plus ibuprofen, which has been found to effectively control postoperative pain with fewer complications.”
Ms. Pourali reports no relevant financial disclosures. Her coauthors included the principal investigator, April Armstrong, MD, MPH, professor of dermatology, University of Southern California, Los Angeles. Dr. Konda reports no relevant financial disclosures.
at dermatology visits.
“Overall, opioid prescribing rates among dermatologists were low. However, dermatologists should remain aware of risk factors for long-term opioid use and consider using nonnarcotic or nonpharmacologic interventions when possible,” Sarah P. Pourali, a medical student at Vanderbilt University, Nashville, said at the annual meeting of the Society for Investigative Dermatology, where she presented the results.
Ms. Pourali said that although Mohs surgery and dermatologic procedures are the focus of “much of the literature” concerning opioid use in dermatology, there are limited data on medication prescribing patterns for other skin conditions treated by dermatologists.
She and her colleagues performed a cross-sectional study using data from the National Ambulatory Medical Care Survey (NAMCS) from 2009 to 2016 on 288,462,610 weighted dermatology visits. The researchers used International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to identify dermatologic diseases. They also identified and grouped oral pain medication into the following categories: opiate analgesics, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentin. A linear regression analysis was used to evaluate pain medicine prescribing each year, and the researchers used a logistic regression analysis to explore how opiate prescriptions were connected to patient clinical characteristics. The analysis was adjusted for age, gender, race, ethnicity, and region.
Overall, most dermatology visits were for patients older than 65 years (36.2%) and 45-64 years old (32.1%). Over half of the dermatologist visits were for women (56.4%) and most (92.2%) visits were for patients who were White (5.1 % were for patients who were Black); most were non-Hispanic or Latino (93.5%). Most dermatology visits were in the South (35.4%) and West (25.2%), followed by the Northeast (21.9%) and Midwest (17.5%).
Opioids were prescribed in 1.3% of the visits, Ms. Pourali said. In addition, 4.7% of visits included an NSAID prescription, 0.7% an acetaminophen prescription, and 0.6% a gabapentin prescription.
Dermatologic procedure visits accounted for 43.1% of opioid prescriptions, she noted. The most common skin conditions for which opioids were prescribed included vitiligo (10.3%), hemangioma (3.8%), pemphigus (3.6%), atopic dermatitis (3.4%), and psoriasis (2.5%).
Although patients older than 65 years accounted for 36.2% of visits to dermatologists, 58.5% of opioids prescribed by dermatologists were for patients in this age group. “We hypothesize that this may be due to a higher proportion of older patients requiring skin cancer surgeries where a lot of opioids are prescribed within dermatology,” Ms. Pourali said.
The highest population-adjusted prescription rates for opiates were in the Northeast and Western regions of the United States, which “partially corroborates” previous studies that have found “higher rates of opioid prescribing in the southern and western U.S.,” she noted.
When evaluating risk-factors for long-term opiate use, Ms. Pourali and colleagues found opioids were also prescribed in 13.2% of visits where a benzodiazepine was prescribed (adjusted odds ratio, 8.17; 95% confidence interval, 5.3-12.7), 8.4% of visits where the patient had a substance abuse disorder (adjusted OR, 9.40; 95% CI, 2.0-44.4), 5.2% of visits with a patient who had depression (adjusted OR, 3.28; 95% CI, 2.0-5.4), and 2.4% of visits with a patient who used tobacco (adjusted OR, 1.09; 95% CI, 1.0-1.1).
Consider nonopioid postoperative pain management options
In an interview, Sailesh Konda, MD, associate clinical professor of dermatology and director of Mohs surgery and surgical dermatology at the University of Florida, Gainesville, who was not involved with the research, noted the finding in the study that vitiligo, hemangioma, pemphigus, AD, and psoriasis were diagnoses with the highest rates of opioid prescription was surprising. “In general, these are conditions that are not routinely managed with opioids,” he said.
NAMCS contains a primary diagnosis field and space for four additional diagnoses such as chronic conditions, as well as thirty fields for medications. “If an opioid was prescribed at a visit, it could have been prescribed for any of the diagnoses related to the visit,” Dr. Konda said. “Additionally, for those opioid prescriptions associated with dermatologic procedures, it would have been helpful to have a breakdown of the specific procedures.”
Dr. Konda compared these results to a recent study of opioid prescribing patterns in the dermatology Medicare population, which found that 93.9% of the top 1% of opioid prescribers were dermatologists working in a surgical practice.
He said that recommendations for opioid prescribing should be developed for general dermatology as they have been for Mohs surgery and dermatologic surgery. For dermatologists currently prescribing opioids, he recommended monitoring prescribing patterns and to “consider nonopioid interventions, such as acetaminophen plus ibuprofen, which has been found to effectively control postoperative pain with fewer complications.”
Ms. Pourali reports no relevant financial disclosures. Her coauthors included the principal investigator, April Armstrong, MD, MPH, professor of dermatology, University of Southern California, Los Angeles. Dr. Konda reports no relevant financial disclosures.
FROM SID 2021
‘Twincretin’ meets primary endpoints in five pivotal diabetes trials
The investigational, novel, injected once-weekly “twincretin” tirzepatide met its primary efficacy endpoint of significantly cutting hemoglobin A1c as well as its secondary weight-loss endpoint in patients with type 2 diabetes when compared with control patients in top-line results from each of five discrete pivotal trials.
The company developing tirzepatide, Lilly, announced these results in a series of four press releases issued during December 2020–May 2021. Scientific reports on the outcomes from four of these trials are scheduled during the American Diabetes Association’s Scientific Sessions being held virtually in late June 2021, with results from the fifth on track for a report during the annual meeting of the European Association for the Study of Diabetes in September 2021.
Tirzepatide is a “twincretin” because it combines in a single molecule two different gut-hormone activities. It works as both a glucagonlike peptide–1 receptor agonist (GLP-1 RA) and as an agent that mimics the glucose-dependent insulinotropic polypeptide (GIP).
While diabetologists qualified their comments on these results because of the limited scope and format of the five reports to date, they also expressed enthusiasm over what the press releases said.
Results give hope
“It’s quite exciting, but of course we would like to go by the data that’s presented” at upcoming meetings, commented Robert A. Gabbay, MD, PhD, chief science and medical officer of the American Diabetes Association in Arlington, Va. “The idea of GLP-1 and GIP activities working together has been out there for a while, but without any therapeutic options that leverage this,” he said in an interview.
“The preliminary results give us hope that tirzepatide will be a very effective glucose-lowering agent, perhaps the most effective among all options currently available, including insulin,” commented Ildiko Lingvay, MD, a diabetologist and professor at the University of Texas Southwestern Medical Center, Dallas. “Tirzepatide might have the added benefit of clinically meaningful weight loss,” and “the adverse event profile seems to be in line with what we are accustomed to with the GLP-1 RA class. I look forward to seeing the full results. Tirzepatide promises to be a great addition for type 2 diabetes,” Dr. Lingvay said in an interview.
A rare head-to-head against semaglutide
The five phase 3, randomized controlled trials described by Lilly in its four press releases all belong to the SURPASS series of studies for this agent. Perhaps the most intriguing of the five were results from SURPASS-2, announced in a release on March 4. This trial randomized 1,879 patients from the United States or any of seven other countries to 40 weeks of open-label treatment with one of three different dosages of tirzepatide administered by injection once weekly, or to the control group that received a weekly 1-mg injection of semaglutide (Ozempic), the highest dosage approved for controlling glycemia in patients with type 2 diabetes at the time the study launched.
In SURPASS-2 all three tested dosages of tirzepatide led to a significantly larger reduction, from baseline in A1c, compared with semaglutide, after 40 weeks, according to the Lilly release. Each of the three tirzepatide dosages also led to significantly greater weight loss from baseline, compared with semaglutide, and significantly greater percentages of patients who achieved an A1c of less than 7%, compared with semaglutide.
As an example, the highest tested tirzepatide dosage of 15 mg weekly led to an average A1c reduction from baseline of 2.46% and an average weight loss from baseline of 12.4 kg; 92% of patients achieved an A1c of less than 7%, and 51% had their A1c fall below 5.7% which indicates completely normalization of glycemic control. By comparison, the patients randomized to treatment with semaglutide had an average 1.86% reduction in their A1c level from baseline and a 6.2-kg average cut in body weight from baseline; 81% achieved an A1c of less than 7%, and 20% reached an A1c of less than 5.7%.
There are caveats
While these findings are notable as a rare example of an industry-sponsored head-to-head comparison of two new agents, the study comes with a few important asterisks.
First, it was open label, a curious limitation given that both agents are delivered by the same delivery method and schedule. “I cannot conclude based on this study that tirzepatide is superior because it was open label,” commented Anastassia Amaro, MD, medical director of Penn Metabolic Medicine at the University of Pennsylvania, Philadelphia.
“The gold standard is the double-blind study. An open-label design is a limitation,” agreed Dr. Gabbay.
A second caveat is that the Food and Drug Administration recently approved a higher dosage of semaglutide (2.4 g once/week) for treating overweight or obesity in patients with type 2 diabetes and in those without diabetes but a different weight-related condition such as hypertension of hypercholesterolemia. This means that the tested comparator dosage of 1 mg/week is no longer the maximum that most patients treated with semaglutide for glycemic control can receive.
“The inevitable question” about this comparison study is “what about a higher semaglutide dose,” and how might tirzepatide perform relative to that, said Dr. Gabbay. The recently approved higher dosage of semaglutide “adds an interesting wrinkle.”
Lilly has launched a series of studies testing tirzepatide as a treatment for overweight or obesity in people without diabetes, but the results are not expected until sometime in 2022 or 2023.
And there’s a third caveat: Semaglutide has already shown its value for cardiovascular risk reduction in patients with type 2 diabetes in the SUSTAIN 6 trial with nearly 3,300 randomized patients followed for 2 years and reported in 2016. The cardiovascular outcomes trial for tirzepatide, SURPASS-CVOT with more than 12,000 patients with type 2 diabetes, is underway but its results are not expected until 2024.
Despite these important limitations, a blinded comparison of tirzepatide and higher-dose semaglutide is unlikely, Dr. Amaro predicted. “It’s not worth the expense,” she said in an interview. A more likely scenario will be that, if tirzepatide enters the U.S. market, decisions on whether to treat patients with it or semaglutide will pivot on factors like the cost for treatment to individual patients based on their insurance coverage and tolerability, suggested both Dr. Amaro and Dr. Gabbay. “Physicians will need to develop a sense for tirzepatide: Do patients tolerate it and are they happy using it?” Dr. Amaro said.
Tirzepatide versus insulin, or on top of insulin
The other four trials in patients with type 2 diabetes reported by Lilly in releases included SURPASS-1, which randomized 478 patients to treatment with tirzepatide or placebo as monotherapy; SURPASS-3, which randomized 1,444 patients to tirzepatide or insulin degludec (Tresiba) on top of background treatment with metformin; SURPASS-4, which randomized 2,002 patients with high cardiovascular disease risk to treatment with tirzepatide or insulin glargine (Lantus) on top of background treatment with one to three different oral drugs; and SURPASS-5, which randomized 475 patients to treatment with tirzepatide or placebo on top of background treatment with insulin glargine and optional addition of metformin. Altogether, the five trials randomized nearly 6,300 patients.
The studies that compared tirzepatide against two different types of insulin, and the third that tested tirzepatide on top of insulin glargine, are especially notable. “It’s good to see that the combination [of tirzepatide and insulin glargine] works without causing major adverse events,” said Dr. Amaro.
“These are fair and helpful comparisons. I applaud Lilly for doing the right kind of comparisons,” said Dr. Gabbay.
In total, the five studies “provide evidence that tirzepatide will be effective at all stages of type 2 diabetes and can safely be used in combination with other glucose-lowering agents, including insulin,” said Dr. Lingvay. The studies with active comparator agents “allow us to compare tirzepatide’s efficacy against established therapies.”
The SURPASS trials were sponsored by Lilly, which is developing tirzepatide. Dr. Gabbay had no relevant disclosures. Dr. Lingvay has received research funds, consulting and advisory fees, or other support from Lilly as well as from several other companies including Novo Nordisk, which markets semaglutide (Ozempic) and insulin degludec (Tresiba), and Sanofi, which markets insulin glargine (Lantus). Dr. Amaro has received research funding from Lilly and from Fractyl, and has been a consultant to and received research funding from Novo Nordisk.
The investigational, novel, injected once-weekly “twincretin” tirzepatide met its primary efficacy endpoint of significantly cutting hemoglobin A1c as well as its secondary weight-loss endpoint in patients with type 2 diabetes when compared with control patients in top-line results from each of five discrete pivotal trials.
The company developing tirzepatide, Lilly, announced these results in a series of four press releases issued during December 2020–May 2021. Scientific reports on the outcomes from four of these trials are scheduled during the American Diabetes Association’s Scientific Sessions being held virtually in late June 2021, with results from the fifth on track for a report during the annual meeting of the European Association for the Study of Diabetes in September 2021.
Tirzepatide is a “twincretin” because it combines in a single molecule two different gut-hormone activities. It works as both a glucagonlike peptide–1 receptor agonist (GLP-1 RA) and as an agent that mimics the glucose-dependent insulinotropic polypeptide (GIP).
While diabetologists qualified their comments on these results because of the limited scope and format of the five reports to date, they also expressed enthusiasm over what the press releases said.
Results give hope
“It’s quite exciting, but of course we would like to go by the data that’s presented” at upcoming meetings, commented Robert A. Gabbay, MD, PhD, chief science and medical officer of the American Diabetes Association in Arlington, Va. “The idea of GLP-1 and GIP activities working together has been out there for a while, but without any therapeutic options that leverage this,” he said in an interview.
“The preliminary results give us hope that tirzepatide will be a very effective glucose-lowering agent, perhaps the most effective among all options currently available, including insulin,” commented Ildiko Lingvay, MD, a diabetologist and professor at the University of Texas Southwestern Medical Center, Dallas. “Tirzepatide might have the added benefit of clinically meaningful weight loss,” and “the adverse event profile seems to be in line with what we are accustomed to with the GLP-1 RA class. I look forward to seeing the full results. Tirzepatide promises to be a great addition for type 2 diabetes,” Dr. Lingvay said in an interview.
A rare head-to-head against semaglutide
The five phase 3, randomized controlled trials described by Lilly in its four press releases all belong to the SURPASS series of studies for this agent. Perhaps the most intriguing of the five were results from SURPASS-2, announced in a release on March 4. This trial randomized 1,879 patients from the United States or any of seven other countries to 40 weeks of open-label treatment with one of three different dosages of tirzepatide administered by injection once weekly, or to the control group that received a weekly 1-mg injection of semaglutide (Ozempic), the highest dosage approved for controlling glycemia in patients with type 2 diabetes at the time the study launched.
In SURPASS-2 all three tested dosages of tirzepatide led to a significantly larger reduction, from baseline in A1c, compared with semaglutide, after 40 weeks, according to the Lilly release. Each of the three tirzepatide dosages also led to significantly greater weight loss from baseline, compared with semaglutide, and significantly greater percentages of patients who achieved an A1c of less than 7%, compared with semaglutide.
As an example, the highest tested tirzepatide dosage of 15 mg weekly led to an average A1c reduction from baseline of 2.46% and an average weight loss from baseline of 12.4 kg; 92% of patients achieved an A1c of less than 7%, and 51% had their A1c fall below 5.7% which indicates completely normalization of glycemic control. By comparison, the patients randomized to treatment with semaglutide had an average 1.86% reduction in their A1c level from baseline and a 6.2-kg average cut in body weight from baseline; 81% achieved an A1c of less than 7%, and 20% reached an A1c of less than 5.7%.
There are caveats
While these findings are notable as a rare example of an industry-sponsored head-to-head comparison of two new agents, the study comes with a few important asterisks.
First, it was open label, a curious limitation given that both agents are delivered by the same delivery method and schedule. “I cannot conclude based on this study that tirzepatide is superior because it was open label,” commented Anastassia Amaro, MD, medical director of Penn Metabolic Medicine at the University of Pennsylvania, Philadelphia.
“The gold standard is the double-blind study. An open-label design is a limitation,” agreed Dr. Gabbay.
A second caveat is that the Food and Drug Administration recently approved a higher dosage of semaglutide (2.4 g once/week) for treating overweight or obesity in patients with type 2 diabetes and in those without diabetes but a different weight-related condition such as hypertension of hypercholesterolemia. This means that the tested comparator dosage of 1 mg/week is no longer the maximum that most patients treated with semaglutide for glycemic control can receive.
“The inevitable question” about this comparison study is “what about a higher semaglutide dose,” and how might tirzepatide perform relative to that, said Dr. Gabbay. The recently approved higher dosage of semaglutide “adds an interesting wrinkle.”
Lilly has launched a series of studies testing tirzepatide as a treatment for overweight or obesity in people without diabetes, but the results are not expected until sometime in 2022 or 2023.
And there’s a third caveat: Semaglutide has already shown its value for cardiovascular risk reduction in patients with type 2 diabetes in the SUSTAIN 6 trial with nearly 3,300 randomized patients followed for 2 years and reported in 2016. The cardiovascular outcomes trial for tirzepatide, SURPASS-CVOT with more than 12,000 patients with type 2 diabetes, is underway but its results are not expected until 2024.
Despite these important limitations, a blinded comparison of tirzepatide and higher-dose semaglutide is unlikely, Dr. Amaro predicted. “It’s not worth the expense,” she said in an interview. A more likely scenario will be that, if tirzepatide enters the U.S. market, decisions on whether to treat patients with it or semaglutide will pivot on factors like the cost for treatment to individual patients based on their insurance coverage and tolerability, suggested both Dr. Amaro and Dr. Gabbay. “Physicians will need to develop a sense for tirzepatide: Do patients tolerate it and are they happy using it?” Dr. Amaro said.
Tirzepatide versus insulin, or on top of insulin
The other four trials in patients with type 2 diabetes reported by Lilly in releases included SURPASS-1, which randomized 478 patients to treatment with tirzepatide or placebo as monotherapy; SURPASS-3, which randomized 1,444 patients to tirzepatide or insulin degludec (Tresiba) on top of background treatment with metformin; SURPASS-4, which randomized 2,002 patients with high cardiovascular disease risk to treatment with tirzepatide or insulin glargine (Lantus) on top of background treatment with one to three different oral drugs; and SURPASS-5, which randomized 475 patients to treatment with tirzepatide or placebo on top of background treatment with insulin glargine and optional addition of metformin. Altogether, the five trials randomized nearly 6,300 patients.
The studies that compared tirzepatide against two different types of insulin, and the third that tested tirzepatide on top of insulin glargine, are especially notable. “It’s good to see that the combination [of tirzepatide and insulin glargine] works without causing major adverse events,” said Dr. Amaro.
“These are fair and helpful comparisons. I applaud Lilly for doing the right kind of comparisons,” said Dr. Gabbay.
In total, the five studies “provide evidence that tirzepatide will be effective at all stages of type 2 diabetes and can safely be used in combination with other glucose-lowering agents, including insulin,” said Dr. Lingvay. The studies with active comparator agents “allow us to compare tirzepatide’s efficacy against established therapies.”
The SURPASS trials were sponsored by Lilly, which is developing tirzepatide. Dr. Gabbay had no relevant disclosures. Dr. Lingvay has received research funds, consulting and advisory fees, or other support from Lilly as well as from several other companies including Novo Nordisk, which markets semaglutide (Ozempic) and insulin degludec (Tresiba), and Sanofi, which markets insulin glargine (Lantus). Dr. Amaro has received research funding from Lilly and from Fractyl, and has been a consultant to and received research funding from Novo Nordisk.
The investigational, novel, injected once-weekly “twincretin” tirzepatide met its primary efficacy endpoint of significantly cutting hemoglobin A1c as well as its secondary weight-loss endpoint in patients with type 2 diabetes when compared with control patients in top-line results from each of five discrete pivotal trials.
The company developing tirzepatide, Lilly, announced these results in a series of four press releases issued during December 2020–May 2021. Scientific reports on the outcomes from four of these trials are scheduled during the American Diabetes Association’s Scientific Sessions being held virtually in late June 2021, with results from the fifth on track for a report during the annual meeting of the European Association for the Study of Diabetes in September 2021.
Tirzepatide is a “twincretin” because it combines in a single molecule two different gut-hormone activities. It works as both a glucagonlike peptide–1 receptor agonist (GLP-1 RA) and as an agent that mimics the glucose-dependent insulinotropic polypeptide (GIP).
While diabetologists qualified their comments on these results because of the limited scope and format of the five reports to date, they also expressed enthusiasm over what the press releases said.
Results give hope
“It’s quite exciting, but of course we would like to go by the data that’s presented” at upcoming meetings, commented Robert A. Gabbay, MD, PhD, chief science and medical officer of the American Diabetes Association in Arlington, Va. “The idea of GLP-1 and GIP activities working together has been out there for a while, but without any therapeutic options that leverage this,” he said in an interview.
“The preliminary results give us hope that tirzepatide will be a very effective glucose-lowering agent, perhaps the most effective among all options currently available, including insulin,” commented Ildiko Lingvay, MD, a diabetologist and professor at the University of Texas Southwestern Medical Center, Dallas. “Tirzepatide might have the added benefit of clinically meaningful weight loss,” and “the adverse event profile seems to be in line with what we are accustomed to with the GLP-1 RA class. I look forward to seeing the full results. Tirzepatide promises to be a great addition for type 2 diabetes,” Dr. Lingvay said in an interview.
A rare head-to-head against semaglutide
The five phase 3, randomized controlled trials described by Lilly in its four press releases all belong to the SURPASS series of studies for this agent. Perhaps the most intriguing of the five were results from SURPASS-2, announced in a release on March 4. This trial randomized 1,879 patients from the United States or any of seven other countries to 40 weeks of open-label treatment with one of three different dosages of tirzepatide administered by injection once weekly, or to the control group that received a weekly 1-mg injection of semaglutide (Ozempic), the highest dosage approved for controlling glycemia in patients with type 2 diabetes at the time the study launched.
In SURPASS-2 all three tested dosages of tirzepatide led to a significantly larger reduction, from baseline in A1c, compared with semaglutide, after 40 weeks, according to the Lilly release. Each of the three tirzepatide dosages also led to significantly greater weight loss from baseline, compared with semaglutide, and significantly greater percentages of patients who achieved an A1c of less than 7%, compared with semaglutide.
As an example, the highest tested tirzepatide dosage of 15 mg weekly led to an average A1c reduction from baseline of 2.46% and an average weight loss from baseline of 12.4 kg; 92% of patients achieved an A1c of less than 7%, and 51% had their A1c fall below 5.7% which indicates completely normalization of glycemic control. By comparison, the patients randomized to treatment with semaglutide had an average 1.86% reduction in their A1c level from baseline and a 6.2-kg average cut in body weight from baseline; 81% achieved an A1c of less than 7%, and 20% reached an A1c of less than 5.7%.
There are caveats
While these findings are notable as a rare example of an industry-sponsored head-to-head comparison of two new agents, the study comes with a few important asterisks.
First, it was open label, a curious limitation given that both agents are delivered by the same delivery method and schedule. “I cannot conclude based on this study that tirzepatide is superior because it was open label,” commented Anastassia Amaro, MD, medical director of Penn Metabolic Medicine at the University of Pennsylvania, Philadelphia.
“The gold standard is the double-blind study. An open-label design is a limitation,” agreed Dr. Gabbay.
A second caveat is that the Food and Drug Administration recently approved a higher dosage of semaglutide (2.4 g once/week) for treating overweight or obesity in patients with type 2 diabetes and in those without diabetes but a different weight-related condition such as hypertension of hypercholesterolemia. This means that the tested comparator dosage of 1 mg/week is no longer the maximum that most patients treated with semaglutide for glycemic control can receive.
“The inevitable question” about this comparison study is “what about a higher semaglutide dose,” and how might tirzepatide perform relative to that, said Dr. Gabbay. The recently approved higher dosage of semaglutide “adds an interesting wrinkle.”
Lilly has launched a series of studies testing tirzepatide as a treatment for overweight or obesity in people without diabetes, but the results are not expected until sometime in 2022 or 2023.
And there’s a third caveat: Semaglutide has already shown its value for cardiovascular risk reduction in patients with type 2 diabetes in the SUSTAIN 6 trial with nearly 3,300 randomized patients followed for 2 years and reported in 2016. The cardiovascular outcomes trial for tirzepatide, SURPASS-CVOT with more than 12,000 patients with type 2 diabetes, is underway but its results are not expected until 2024.
Despite these important limitations, a blinded comparison of tirzepatide and higher-dose semaglutide is unlikely, Dr. Amaro predicted. “It’s not worth the expense,” she said in an interview. A more likely scenario will be that, if tirzepatide enters the U.S. market, decisions on whether to treat patients with it or semaglutide will pivot on factors like the cost for treatment to individual patients based on their insurance coverage and tolerability, suggested both Dr. Amaro and Dr. Gabbay. “Physicians will need to develop a sense for tirzepatide: Do patients tolerate it and are they happy using it?” Dr. Amaro said.
Tirzepatide versus insulin, or on top of insulin
The other four trials in patients with type 2 diabetes reported by Lilly in releases included SURPASS-1, which randomized 478 patients to treatment with tirzepatide or placebo as monotherapy; SURPASS-3, which randomized 1,444 patients to tirzepatide or insulin degludec (Tresiba) on top of background treatment with metformin; SURPASS-4, which randomized 2,002 patients with high cardiovascular disease risk to treatment with tirzepatide or insulin glargine (Lantus) on top of background treatment with one to three different oral drugs; and SURPASS-5, which randomized 475 patients to treatment with tirzepatide or placebo on top of background treatment with insulin glargine and optional addition of metformin. Altogether, the five trials randomized nearly 6,300 patients.
The studies that compared tirzepatide against two different types of insulin, and the third that tested tirzepatide on top of insulin glargine, are especially notable. “It’s good to see that the combination [of tirzepatide and insulin glargine] works without causing major adverse events,” said Dr. Amaro.
“These are fair and helpful comparisons. I applaud Lilly for doing the right kind of comparisons,” said Dr. Gabbay.
In total, the five studies “provide evidence that tirzepatide will be effective at all stages of type 2 diabetes and can safely be used in combination with other glucose-lowering agents, including insulin,” said Dr. Lingvay. The studies with active comparator agents “allow us to compare tirzepatide’s efficacy against established therapies.”
The SURPASS trials were sponsored by Lilly, which is developing tirzepatide. Dr. Gabbay had no relevant disclosures. Dr. Lingvay has received research funds, consulting and advisory fees, or other support from Lilly as well as from several other companies including Novo Nordisk, which markets semaglutide (Ozempic) and insulin degludec (Tresiba), and Sanofi, which markets insulin glargine (Lantus). Dr. Amaro has received research funding from Lilly and from Fractyl, and has been a consultant to and received research funding from Novo Nordisk.
Oral Verrucous Plaques in a Patient With Urothelial Cancer
The Diagnosis: Paraneoplastic Acanthosis Nigricans
Histopathologic examination demonstrated verrucous epidermal hyperplasia (Figure, A). Fungal organisms were identified with an Alcian blue and periodic acid-Schiff stain (Figure, B). The organisms demonstrated a vertical orientation in relation to the mucosal surface, which was consistent with candidal organisms.
Given the rapid eruption of these plaques, the distribution on the oral and palmar surfaces (tripe palms), and the minimal improvement with both systemic steroids and antifungal treatment, a diagnosis of paraneoplastic acanthosis nigricans with secondary candidal infection was made. Drug-induced cheilitis was considered; however, improvement with discontinuation of the suspected offending drug would have been expected. Although chronic mucocutaneous candidiasis was possible, more prompt improvement upon initiation of systemic antifungal therapy would have been observed. Oral Crohn disease should be included in the differential, but it was unlikely given the lack of granulomas on pathology and absence of history of gastrointestinal tract symptoms. Melkersson-Rosenthal syndrome also was unlikely given the lack of facial nerve palsy as well as the lack of granulomas on pathology. Furthermore, none of these options would be associated with tripe palms, as seen in our patient.
Acanthosis nigricans is a localized skin disorder characterized by hyperpigmented velvety plaques arising in flexural and intertriginous regions. Although most cases (80%) are associated with idiopathic or benign conditions, the link between acanthosis nigricans and an underlying malignancy has been well documented.1-3 Most commonly associated with an underlying intra-abdominal malignancy (often gastric carcinoma), the lesions of paraneoplastic acanthosis nigricans are indistinguishable from their benign counterparts.1,4 When the condition presents abruptly and extensively in a nonobese patient, prompt workup for malignancy should be initiated. Rapid onset and atypical distribution (ie, palmar, perioral, or mucosal) more commonly is associated with a paraneoplastic etiology.5,6
Histopathology for acanthosis nigricans shows hyperkeratosis and epidermal papillomatosis. Horn pseudocyst formation is possible, but usually no hyperpigmentation is observed. The findings typically are indistinguishable from seborrheic keratoses, epidermal nevi, or lesions of confluent and reticulated papillomatosis of Gougerot and Carteaud.2
The underlying pathogenesis of acanthosis nigricans is poorly understood. In the benign subtype, insulin resistance commonly has been described. In the paraneoplastic subtype, it is proposed that the tumor produces a transforming growth factor that mimics epidermal growth factor and leads to keratinocyte proliferation.7,8 Paraneoplastic acanthosis nigricans has the potential to arise at any point of tumor development, further contributing to the diagnostic challenge. Treatment of the skin lesions involves management of the underlying malignancy. Unfortunately, many such malignancies often are at an advanced stage, and subsequent prognosis is poor.2
- Shah A, Jack A, Liu H, et al. Neoplastic/paraneoplastic dermatitis, fasciitis, and panniculitis. Rheum Dis Clin North Am. 2011;37:573-592.
- Chairatchaneeboon M, Kim EJ. Cutaneous paraneoplastic syndromes. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick's Dermatology. 9th ed. McGraw-Hill Education; 2019:2441-2464.
- Lee HC, Ker KJ, Chong WS. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. 2017;15:208.
- Mohrenschlager M, Vocks E, Wessner DB, et al. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001;165:1629-1630.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101.
The Diagnosis: Paraneoplastic Acanthosis Nigricans
Histopathologic examination demonstrated verrucous epidermal hyperplasia (Figure, A). Fungal organisms were identified with an Alcian blue and periodic acid-Schiff stain (Figure, B). The organisms demonstrated a vertical orientation in relation to the mucosal surface, which was consistent with candidal organisms.

Given the rapid eruption of these plaques, the distribution on the oral and palmar surfaces (tripe palms), and the minimal improvement with both systemic steroids and antifungal treatment, a diagnosis of paraneoplastic acanthosis nigricans with secondary candidal infection was made. Drug-induced cheilitis was considered; however, improvement with discontinuation of the suspected offending drug would have been expected. Although chronic mucocutaneous candidiasis was possible, more prompt improvement upon initiation of systemic antifungal therapy would have been observed. Oral Crohn disease should be included in the differential, but it was unlikely given the lack of granulomas on pathology and absence of history of gastrointestinal tract symptoms. Melkersson-Rosenthal syndrome also was unlikely given the lack of facial nerve palsy as well as the lack of granulomas on pathology. Furthermore, none of these options would be associated with tripe palms, as seen in our patient.
Acanthosis nigricans is a localized skin disorder characterized by hyperpigmented velvety plaques arising in flexural and intertriginous regions. Although most cases (80%) are associated with idiopathic or benign conditions, the link between acanthosis nigricans and an underlying malignancy has been well documented.1-3 Most commonly associated with an underlying intra-abdominal malignancy (often gastric carcinoma), the lesions of paraneoplastic acanthosis nigricans are indistinguishable from their benign counterparts.1,4 When the condition presents abruptly and extensively in a nonobese patient, prompt workup for malignancy should be initiated. Rapid onset and atypical distribution (ie, palmar, perioral, or mucosal) more commonly is associated with a paraneoplastic etiology.5,6
Histopathology for acanthosis nigricans shows hyperkeratosis and epidermal papillomatosis. Horn pseudocyst formation is possible, but usually no hyperpigmentation is observed. The findings typically are indistinguishable from seborrheic keratoses, epidermal nevi, or lesions of confluent and reticulated papillomatosis of Gougerot and Carteaud.2
The underlying pathogenesis of acanthosis nigricans is poorly understood. In the benign subtype, insulin resistance commonly has been described. In the paraneoplastic subtype, it is proposed that the tumor produces a transforming growth factor that mimics epidermal growth factor and leads to keratinocyte proliferation.7,8 Paraneoplastic acanthosis nigricans has the potential to arise at any point of tumor development, further contributing to the diagnostic challenge. Treatment of the skin lesions involves management of the underlying malignancy. Unfortunately, many such malignancies often are at an advanced stage, and subsequent prognosis is poor.2
The Diagnosis: Paraneoplastic Acanthosis Nigricans
Histopathologic examination demonstrated verrucous epidermal hyperplasia (Figure, A). Fungal organisms were identified with an Alcian blue and periodic acid-Schiff stain (Figure, B). The organisms demonstrated a vertical orientation in relation to the mucosal surface, which was consistent with candidal organisms.

Given the rapid eruption of these plaques, the distribution on the oral and palmar surfaces (tripe palms), and the minimal improvement with both systemic steroids and antifungal treatment, a diagnosis of paraneoplastic acanthosis nigricans with secondary candidal infection was made. Drug-induced cheilitis was considered; however, improvement with discontinuation of the suspected offending drug would have been expected. Although chronic mucocutaneous candidiasis was possible, more prompt improvement upon initiation of systemic antifungal therapy would have been observed. Oral Crohn disease should be included in the differential, but it was unlikely given the lack of granulomas on pathology and absence of history of gastrointestinal tract symptoms. Melkersson-Rosenthal syndrome also was unlikely given the lack of facial nerve palsy as well as the lack of granulomas on pathology. Furthermore, none of these options would be associated with tripe palms, as seen in our patient.
Acanthosis nigricans is a localized skin disorder characterized by hyperpigmented velvety plaques arising in flexural and intertriginous regions. Although most cases (80%) are associated with idiopathic or benign conditions, the link between acanthosis nigricans and an underlying malignancy has been well documented.1-3 Most commonly associated with an underlying intra-abdominal malignancy (often gastric carcinoma), the lesions of paraneoplastic acanthosis nigricans are indistinguishable from their benign counterparts.1,4 When the condition presents abruptly and extensively in a nonobese patient, prompt workup for malignancy should be initiated. Rapid onset and atypical distribution (ie, palmar, perioral, or mucosal) more commonly is associated with a paraneoplastic etiology.5,6
Histopathology for acanthosis nigricans shows hyperkeratosis and epidermal papillomatosis. Horn pseudocyst formation is possible, but usually no hyperpigmentation is observed. The findings typically are indistinguishable from seborrheic keratoses, epidermal nevi, or lesions of confluent and reticulated papillomatosis of Gougerot and Carteaud.2
The underlying pathogenesis of acanthosis nigricans is poorly understood. In the benign subtype, insulin resistance commonly has been described. In the paraneoplastic subtype, it is proposed that the tumor produces a transforming growth factor that mimics epidermal growth factor and leads to keratinocyte proliferation.7,8 Paraneoplastic acanthosis nigricans has the potential to arise at any point of tumor development, further contributing to the diagnostic challenge. Treatment of the skin lesions involves management of the underlying malignancy. Unfortunately, many such malignancies often are at an advanced stage, and subsequent prognosis is poor.2
- Shah A, Jack A, Liu H, et al. Neoplastic/paraneoplastic dermatitis, fasciitis, and panniculitis. Rheum Dis Clin North Am. 2011;37:573-592.
- Chairatchaneeboon M, Kim EJ. Cutaneous paraneoplastic syndromes. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick's Dermatology. 9th ed. McGraw-Hill Education; 2019:2441-2464.
- Lee HC, Ker KJ, Chong WS. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. 2017;15:208.
- Mohrenschlager M, Vocks E, Wessner DB, et al. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001;165:1629-1630.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101.
- Shah A, Jack A, Liu H, et al. Neoplastic/paraneoplastic dermatitis, fasciitis, and panniculitis. Rheum Dis Clin North Am. 2011;37:573-592.
- Chairatchaneeboon M, Kim EJ. Cutaneous paraneoplastic syndromes. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick's Dermatology. 9th ed. McGraw-Hill Education; 2019:2441-2464.
- Lee HC, Ker KJ, Chong WS. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. 2017;15:208.
- Mohrenschlager M, Vocks E, Wessner DB, et al. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001;165:1629-1630.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101.
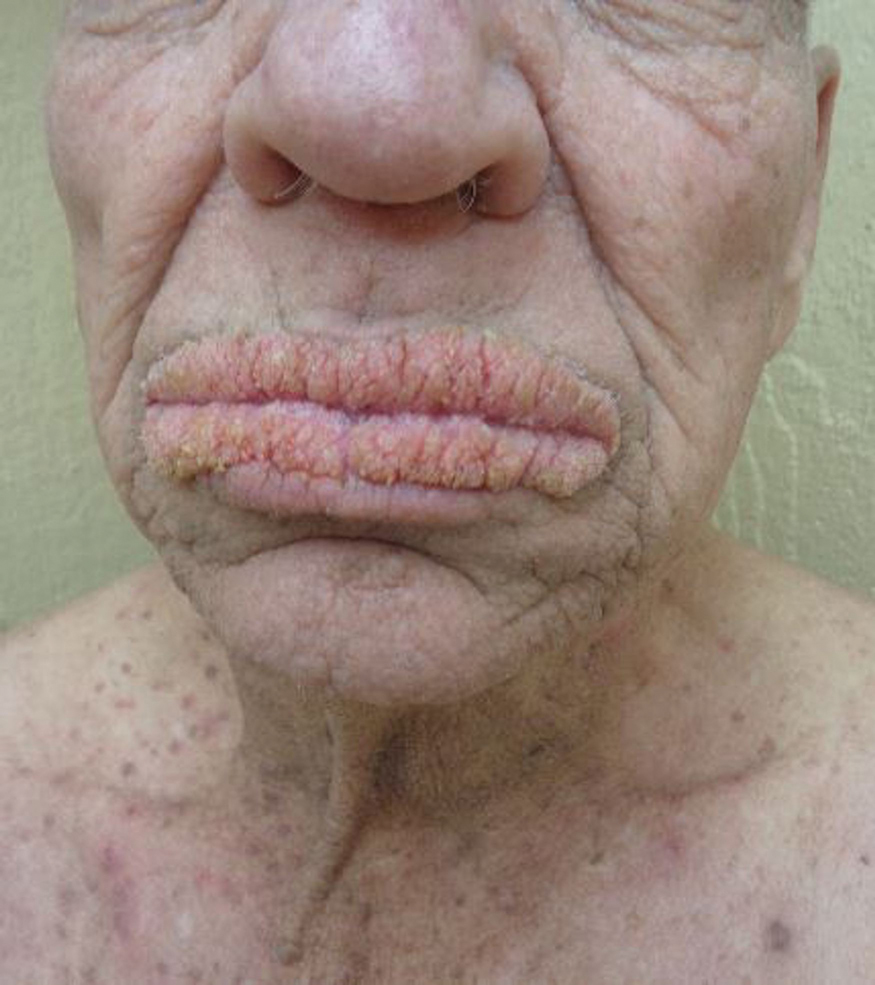
A 75-year-old nonobese man with metastatic urothelial carcinoma presented for evaluation and treatment of swollen lips. The patient stated that his lips began to swell and crack shortly after beginning pembrolizumab approximately 5 months prior. The swelling had progressively worsened, prompting discontinuation of the pembrolizumab by oncology about 2 months prior to presentation to our dermatology clinic. He reported slight improvement after the discontinuation of pembrolizumab, and he had since been started on carboplatin and gemcitabine. He previously was treated with oral corticosteroids without improvement. His oncologist started him on oral fluconazole for treatment of oral thrush on the day of presentation to our clinic. Physical examination revealed diffuse papillomatous and verrucous plaques of the upper and lower lips with involvement of the buccal mucosa. He also had deep fissures and white plaques on the tongue. Velvety hyperpigmented plaques were noted in the axillae, and he had confluent thickening of the palms. A 3-mm punch biopsy from the lower lip was performed. The patient subsequently was evaluated 2 weeks after the initial appointment, and minor improvement in the oral verrucous hyperplasia was noted following antifungal therapy, with resolution of the candidiasis.
AGA Clinical Practice Update: Chemoprevention for colorectal neoplasia
Experts assessed different chemopreventive agents meant to reduce the incidence of colorectal neoplasia and associated mortality based on whether these agents were effective and safe, but they found few fit both criteria, according to a new clinical practice update from the American Gastroenterological Association.
That said, the update does advise that clinicians use low-dose aspirin therapy in patients who are younger than 70 years with at least a 10-year life expectancy, are not at high risk for bleeding, and have at least a 10% cardiovascular disease risk over the next decade.
This best practice advice statement reflects “high-quality trial data” for this patient population and also echoes U.S. Preventive Services Task Force recommendations, wrote Peter S. Liang, MD, MPH, and his associates on behalf of the American Gastroenterological Association. However, they note that low-dose aspirin therapy has shown inconsistent results for older patients and that its chemopreventive benefits always should be weighed against an individual’s bleeding risk.
Published in Clinical Gastroenterology and Hepatology, the clinical practice update also recommends considering low-dose aspirin therapy for patients with a history of colorectal neoplasia, based on data from several trials in which daily doses of 81-325 mg were associated with a significantly lower likelihood of recurrence of earlier-stage adenomas (the findings did not extend to patients with more advanced lesions). Evidence on sessile serrated polyps is sparser, but there is some indication for a benefit in this setting, the experts noted.
Their best-practice advice also covers nonaspirin nonsteroidal anti-inflammatory drugs, metformin, calcium, vitamin D, folic acid, and statins. Among these agents, only metformin receives even a conditional green light. “Because of the results of a large number of observational studies, a small adenoma trial, as well as a favorable safety profile, metformin may be considered for chemoprevention against colorectal neoplasia in individuals with diabetes,” the experts concluded. Support for this best-practice advice includes a meta-analysis of colorectal cancer–specific survival in 17 observational studies, a meta-analysis of colorectal cancer incidence in 14 observational studies, and a randomized, placebo-controlled trial in which 250 mg daily metformin was safe and associated with a 40% lower risk of recurrent adenoma.
For calcium, study findings have been mixed, and a recent large clinical trial found no overall benefit for adenoma prevention. Because high-dose calcium has been linked to kidney toxicity, hypercalcemia, and prostate cancer, its risks likely outweigh any benefits, the experts concluded. Vitamin D (as monotherapy or with calcium) also has shown no overall benefit for preventing adenomas or sessile serrated lesions. A large ongoing, randomized trial will examine colorectal cancer incidence among adults receiving 5 years of oral vitamin D or placebo, but its results will not be available until at least 2025.
Nonsteroidal anti-inflammatory drugs are not recommended to prevent colorectal neoplasia among average-risk individuals. Cyclooxygenase-2 inhibitors pose “substantial cardiovascular risks,” while nonselective NSAIDs are associated with a significantly increased risk for gastrointestinal bleeding. Meta-analyses have shown no benefit of folic acid for preventing colorectal neoplasia, and observational studies on statins have produced only mixed results.
No funding sources were reported. The experts reported having no conflicts of interest.
Experts assessed different chemopreventive agents meant to reduce the incidence of colorectal neoplasia and associated mortality based on whether these agents were effective and safe, but they found few fit both criteria, according to a new clinical practice update from the American Gastroenterological Association.
That said, the update does advise that clinicians use low-dose aspirin therapy in patients who are younger than 70 years with at least a 10-year life expectancy, are not at high risk for bleeding, and have at least a 10% cardiovascular disease risk over the next decade.
This best practice advice statement reflects “high-quality trial data” for this patient population and also echoes U.S. Preventive Services Task Force recommendations, wrote Peter S. Liang, MD, MPH, and his associates on behalf of the American Gastroenterological Association. However, they note that low-dose aspirin therapy has shown inconsistent results for older patients and that its chemopreventive benefits always should be weighed against an individual’s bleeding risk.
Published in Clinical Gastroenterology and Hepatology, the clinical practice update also recommends considering low-dose aspirin therapy for patients with a history of colorectal neoplasia, based on data from several trials in which daily doses of 81-325 mg were associated with a significantly lower likelihood of recurrence of earlier-stage adenomas (the findings did not extend to patients with more advanced lesions). Evidence on sessile serrated polyps is sparser, but there is some indication for a benefit in this setting, the experts noted.
Their best-practice advice also covers nonaspirin nonsteroidal anti-inflammatory drugs, metformin, calcium, vitamin D, folic acid, and statins. Among these agents, only metformin receives even a conditional green light. “Because of the results of a large number of observational studies, a small adenoma trial, as well as a favorable safety profile, metformin may be considered for chemoprevention against colorectal neoplasia in individuals with diabetes,” the experts concluded. Support for this best-practice advice includes a meta-analysis of colorectal cancer–specific survival in 17 observational studies, a meta-analysis of colorectal cancer incidence in 14 observational studies, and a randomized, placebo-controlled trial in which 250 mg daily metformin was safe and associated with a 40% lower risk of recurrent adenoma.
For calcium, study findings have been mixed, and a recent large clinical trial found no overall benefit for adenoma prevention. Because high-dose calcium has been linked to kidney toxicity, hypercalcemia, and prostate cancer, its risks likely outweigh any benefits, the experts concluded. Vitamin D (as monotherapy or with calcium) also has shown no overall benefit for preventing adenomas or sessile serrated lesions. A large ongoing, randomized trial will examine colorectal cancer incidence among adults receiving 5 years of oral vitamin D or placebo, but its results will not be available until at least 2025.
Nonsteroidal anti-inflammatory drugs are not recommended to prevent colorectal neoplasia among average-risk individuals. Cyclooxygenase-2 inhibitors pose “substantial cardiovascular risks,” while nonselective NSAIDs are associated with a significantly increased risk for gastrointestinal bleeding. Meta-analyses have shown no benefit of folic acid for preventing colorectal neoplasia, and observational studies on statins have produced only mixed results.
No funding sources were reported. The experts reported having no conflicts of interest.
Experts assessed different chemopreventive agents meant to reduce the incidence of colorectal neoplasia and associated mortality based on whether these agents were effective and safe, but they found few fit both criteria, according to a new clinical practice update from the American Gastroenterological Association.
That said, the update does advise that clinicians use low-dose aspirin therapy in patients who are younger than 70 years with at least a 10-year life expectancy, are not at high risk for bleeding, and have at least a 10% cardiovascular disease risk over the next decade.
This best practice advice statement reflects “high-quality trial data” for this patient population and also echoes U.S. Preventive Services Task Force recommendations, wrote Peter S. Liang, MD, MPH, and his associates on behalf of the American Gastroenterological Association. However, they note that low-dose aspirin therapy has shown inconsistent results for older patients and that its chemopreventive benefits always should be weighed against an individual’s bleeding risk.
Published in Clinical Gastroenterology and Hepatology, the clinical practice update also recommends considering low-dose aspirin therapy for patients with a history of colorectal neoplasia, based on data from several trials in which daily doses of 81-325 mg were associated with a significantly lower likelihood of recurrence of earlier-stage adenomas (the findings did not extend to patients with more advanced lesions). Evidence on sessile serrated polyps is sparser, but there is some indication for a benefit in this setting, the experts noted.
Their best-practice advice also covers nonaspirin nonsteroidal anti-inflammatory drugs, metformin, calcium, vitamin D, folic acid, and statins. Among these agents, only metformin receives even a conditional green light. “Because of the results of a large number of observational studies, a small adenoma trial, as well as a favorable safety profile, metformin may be considered for chemoprevention against colorectal neoplasia in individuals with diabetes,” the experts concluded. Support for this best-practice advice includes a meta-analysis of colorectal cancer–specific survival in 17 observational studies, a meta-analysis of colorectal cancer incidence in 14 observational studies, and a randomized, placebo-controlled trial in which 250 mg daily metformin was safe and associated with a 40% lower risk of recurrent adenoma.
For calcium, study findings have been mixed, and a recent large clinical trial found no overall benefit for adenoma prevention. Because high-dose calcium has been linked to kidney toxicity, hypercalcemia, and prostate cancer, its risks likely outweigh any benefits, the experts concluded. Vitamin D (as monotherapy or with calcium) also has shown no overall benefit for preventing adenomas or sessile serrated lesions. A large ongoing, randomized trial will examine colorectal cancer incidence among adults receiving 5 years of oral vitamin D or placebo, but its results will not be available until at least 2025.
Nonsteroidal anti-inflammatory drugs are not recommended to prevent colorectal neoplasia among average-risk individuals. Cyclooxygenase-2 inhibitors pose “substantial cardiovascular risks,” while nonselective NSAIDs are associated with a significantly increased risk for gastrointestinal bleeding. Meta-analyses have shown no benefit of folic acid for preventing colorectal neoplasia, and observational studies on statins have produced only mixed results.
No funding sources were reported. The experts reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY