User login
Judge tosses hospital staff suit over vaccine mandate
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
Is your patient having an existential crisis?
The news is portraying our modern time as an existential crisis as though our very existence is threatened. An existential crisis is a profound feeling of lack of meaning, choice, or freedom in one’s life that makes even existing seem worthless. It can emerge as early as 5 years old, especially in introspective, gifted children, when they realize that death is permanent and universal, after a real loss or a story of a loss or failure, or from a sense of guilt.
The past 18 months of COVID-19 have been a perfect storm for developing an existential crisis. One of the main sources of life meaning for children is friendships. COVID-19 has reduced or blocked access to old and new friends. Younger children, when asked what makes a friend, will say “we like to do the same things.” Virtual play dates help but don’t replace shared experiences.
School provides meaning for children not only from socializing but also from accomplishing academic tasks – fulfilling Erickson’s stages of “mastery” and “productivity.” Teachers were better able to carry out hands-on activities, group assignments, and field trips in person so that all children and learning styles were engaged and successful. Not having in-person school has also meant loss of extracurricular activities, sports, and clubs as sources of mastery.
Loss of the structure of daily life, common during COVID-19, for waking, dressing, meals, chores, homework time, bathing, or bedtime can be profoundly disorienting.
For adolescents, opportunities to contribute to society and become productive by volunteering or being employed have been stunted by quarantine and social distancing. Some teens have had to care for relatives at home so that parents can earn a living, which, while meaningful, blocks age-essential socializing.
Meaning can also be created at any age by community structures and agreed upon beliefs such as religion. While religious membership is low in the United States, members have been largely unable to attend services. Following sports teams, an alternate “religion” and source of identity, was on hold for many months.
Existential despair can also come from major life losses. COVID-19 has taken a terrible toll of lives, homes, and jobs for millions. As short-term thinkers, when children see so many of their plans and dreams for making the team, having a girlfriend, going to prom, attending summer camp, or graduating, it feels like the end of the world they had imagined. Even the most important source of meaning – connection to family – has been disrupted by lockdown, illness, or loss.
The loss of choice and freedom goes beyond being stuck indoors. Advanced classes and exams, as well as resume-building jobs or volunteering, which teens saw as essential to college, disappeared; sometimes also the money needed was exhausted by COVID-19 unemployment. Work-at-home parents supervising virtual school see their children’s malaise or panic and pressure them to work harder, which is impossible for despairing children. Observing a parent losing his or her job makes a teen’s own career aspirations uncertain. Teen depression and suicidal ideation/acts have shot up from hopelessness, with loss of meaning at the core.
A profound sense of powerlessness has taken over. COVID-19, an invisible threat, has taken down lives. Even with amazingly effective vaccines available, fear and helplessness have burned into our brains. Helplessness to stop structural racism and the arbitrary killings of our own Black citizens by police has finally registered. And climate change is now reported as an impending disaster that may not be stoppable.
So this must be the worst time in history, right? Actually, no. The past 60 years have been a period of historically remarkable stability of government, economy, and natural forces. Perhaps knowing no other world has made these problems appear unsolvable to the parents of our patients. Their own sense of meaning has been challenged in a way similar to that of their children. Perhaps from lack of privacy or peers, parents have been sharing their own sense of powerlessness with their children directly or indirectly, making it harder to reassure them.
With COVID-19 waning in the United States, many of the sources of meaning just discussed can be reinstated by way of in-person play dates, school, sports, socializing, practicing religion, volunteering, and getting jobs. Although there is “existential therapy,” what our children need most is adult leadership showing confidence in life’s meaning, even if we have to hide our own worries. Parents can point out that, even if it takes years, people have made it through difficult times in the past, and there are many positive alternatives for education and employment.
Children need to repeatedly hear about ways they are valued that are not dependent on accomplishments. Thanking them for and telling others about their effort, ideas, curiosity, integrity, love, and kindness point out meaning for their existence independent of world events. Parents need to establish routines and rules for children to demonstrate that life goes on as usual. Chores helpful to the family are a practical contribution. Family activities that are challenging and unpredictable set up for discussing, modeling, and building resilience; for example, visiting new places, camping, hiking, trying a new sport, or adopting a pet give opportunities to say: “Oh, well, we’ll find another way.”
Parents can share stories or books about people who made it through tougher times, such as Abraham Lincoln, or better, personal, or family experiences overcoming challenges. Recalling and nicknaming instances of the child’s own resilience is valuable. Books such as “The Little Engine That Could,” “Chicken Little,” and fairy tales of overcoming doubts when facing challenges can be helpful. “Stay calm and carry on,” a saying from the British when they were being bombed during World War II, has become a meme.
As clinicians we need to sort out significant complicated grief, anxiety, obsessive compulsive disorder, depression, or suicidal ideation, and provide assessment and treatment. But when children get stuck in existential futility, in addition to engaging them in meaningful activities, we can advise parents to coach them to distract themselves, “put the thoughts in a box in your head” to consider later, and/or write down or photograph things that make them grateful. Good lessons for us all to reinvent meaning in our lives.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
The news is portraying our modern time as an existential crisis as though our very existence is threatened. An existential crisis is a profound feeling of lack of meaning, choice, or freedom in one’s life that makes even existing seem worthless. It can emerge as early as 5 years old, especially in introspective, gifted children, when they realize that death is permanent and universal, after a real loss or a story of a loss or failure, or from a sense of guilt.
The past 18 months of COVID-19 have been a perfect storm for developing an existential crisis. One of the main sources of life meaning for children is friendships. COVID-19 has reduced or blocked access to old and new friends. Younger children, when asked what makes a friend, will say “we like to do the same things.” Virtual play dates help but don’t replace shared experiences.
School provides meaning for children not only from socializing but also from accomplishing academic tasks – fulfilling Erickson’s stages of “mastery” and “productivity.” Teachers were better able to carry out hands-on activities, group assignments, and field trips in person so that all children and learning styles were engaged and successful. Not having in-person school has also meant loss of extracurricular activities, sports, and clubs as sources of mastery.
Loss of the structure of daily life, common during COVID-19, for waking, dressing, meals, chores, homework time, bathing, or bedtime can be profoundly disorienting.
For adolescents, opportunities to contribute to society and become productive by volunteering or being employed have been stunted by quarantine and social distancing. Some teens have had to care for relatives at home so that parents can earn a living, which, while meaningful, blocks age-essential socializing.
Meaning can also be created at any age by community structures and agreed upon beliefs such as religion. While religious membership is low in the United States, members have been largely unable to attend services. Following sports teams, an alternate “religion” and source of identity, was on hold for many months.
Existential despair can also come from major life losses. COVID-19 has taken a terrible toll of lives, homes, and jobs for millions. As short-term thinkers, when children see so many of their plans and dreams for making the team, having a girlfriend, going to prom, attending summer camp, or graduating, it feels like the end of the world they had imagined. Even the most important source of meaning – connection to family – has been disrupted by lockdown, illness, or loss.
The loss of choice and freedom goes beyond being stuck indoors. Advanced classes and exams, as well as resume-building jobs or volunteering, which teens saw as essential to college, disappeared; sometimes also the money needed was exhausted by COVID-19 unemployment. Work-at-home parents supervising virtual school see their children’s malaise or panic and pressure them to work harder, which is impossible for despairing children. Observing a parent losing his or her job makes a teen’s own career aspirations uncertain. Teen depression and suicidal ideation/acts have shot up from hopelessness, with loss of meaning at the core.
A profound sense of powerlessness has taken over. COVID-19, an invisible threat, has taken down lives. Even with amazingly effective vaccines available, fear and helplessness have burned into our brains. Helplessness to stop structural racism and the arbitrary killings of our own Black citizens by police has finally registered. And climate change is now reported as an impending disaster that may not be stoppable.
So this must be the worst time in history, right? Actually, no. The past 60 years have been a period of historically remarkable stability of government, economy, and natural forces. Perhaps knowing no other world has made these problems appear unsolvable to the parents of our patients. Their own sense of meaning has been challenged in a way similar to that of their children. Perhaps from lack of privacy or peers, parents have been sharing their own sense of powerlessness with their children directly or indirectly, making it harder to reassure them.
With COVID-19 waning in the United States, many of the sources of meaning just discussed can be reinstated by way of in-person play dates, school, sports, socializing, practicing religion, volunteering, and getting jobs. Although there is “existential therapy,” what our children need most is adult leadership showing confidence in life’s meaning, even if we have to hide our own worries. Parents can point out that, even if it takes years, people have made it through difficult times in the past, and there are many positive alternatives for education and employment.
Children need to repeatedly hear about ways they are valued that are not dependent on accomplishments. Thanking them for and telling others about their effort, ideas, curiosity, integrity, love, and kindness point out meaning for their existence independent of world events. Parents need to establish routines and rules for children to demonstrate that life goes on as usual. Chores helpful to the family are a practical contribution. Family activities that are challenging and unpredictable set up for discussing, modeling, and building resilience; for example, visiting new places, camping, hiking, trying a new sport, or adopting a pet give opportunities to say: “Oh, well, we’ll find another way.”
Parents can share stories or books about people who made it through tougher times, such as Abraham Lincoln, or better, personal, or family experiences overcoming challenges. Recalling and nicknaming instances of the child’s own resilience is valuable. Books such as “The Little Engine That Could,” “Chicken Little,” and fairy tales of overcoming doubts when facing challenges can be helpful. “Stay calm and carry on,” a saying from the British when they were being bombed during World War II, has become a meme.
As clinicians we need to sort out significant complicated grief, anxiety, obsessive compulsive disorder, depression, or suicidal ideation, and provide assessment and treatment. But when children get stuck in existential futility, in addition to engaging them in meaningful activities, we can advise parents to coach them to distract themselves, “put the thoughts in a box in your head” to consider later, and/or write down or photograph things that make them grateful. Good lessons for us all to reinvent meaning in our lives.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
The news is portraying our modern time as an existential crisis as though our very existence is threatened. An existential crisis is a profound feeling of lack of meaning, choice, or freedom in one’s life that makes even existing seem worthless. It can emerge as early as 5 years old, especially in introspective, gifted children, when they realize that death is permanent and universal, after a real loss or a story of a loss or failure, or from a sense of guilt.
The past 18 months of COVID-19 have been a perfect storm for developing an existential crisis. One of the main sources of life meaning for children is friendships. COVID-19 has reduced or blocked access to old and new friends. Younger children, when asked what makes a friend, will say “we like to do the same things.” Virtual play dates help but don’t replace shared experiences.
School provides meaning for children not only from socializing but also from accomplishing academic tasks – fulfilling Erickson’s stages of “mastery” and “productivity.” Teachers were better able to carry out hands-on activities, group assignments, and field trips in person so that all children and learning styles were engaged and successful. Not having in-person school has also meant loss of extracurricular activities, sports, and clubs as sources of mastery.
Loss of the structure of daily life, common during COVID-19, for waking, dressing, meals, chores, homework time, bathing, or bedtime can be profoundly disorienting.
For adolescents, opportunities to contribute to society and become productive by volunteering or being employed have been stunted by quarantine and social distancing. Some teens have had to care for relatives at home so that parents can earn a living, which, while meaningful, blocks age-essential socializing.
Meaning can also be created at any age by community structures and agreed upon beliefs such as religion. While religious membership is low in the United States, members have been largely unable to attend services. Following sports teams, an alternate “religion” and source of identity, was on hold for many months.
Existential despair can also come from major life losses. COVID-19 has taken a terrible toll of lives, homes, and jobs for millions. As short-term thinkers, when children see so many of their plans and dreams for making the team, having a girlfriend, going to prom, attending summer camp, or graduating, it feels like the end of the world they had imagined. Even the most important source of meaning – connection to family – has been disrupted by lockdown, illness, or loss.
The loss of choice and freedom goes beyond being stuck indoors. Advanced classes and exams, as well as resume-building jobs or volunteering, which teens saw as essential to college, disappeared; sometimes also the money needed was exhausted by COVID-19 unemployment. Work-at-home parents supervising virtual school see their children’s malaise or panic and pressure them to work harder, which is impossible for despairing children. Observing a parent losing his or her job makes a teen’s own career aspirations uncertain. Teen depression and suicidal ideation/acts have shot up from hopelessness, with loss of meaning at the core.
A profound sense of powerlessness has taken over. COVID-19, an invisible threat, has taken down lives. Even with amazingly effective vaccines available, fear and helplessness have burned into our brains. Helplessness to stop structural racism and the arbitrary killings of our own Black citizens by police has finally registered. And climate change is now reported as an impending disaster that may not be stoppable.
So this must be the worst time in history, right? Actually, no. The past 60 years have been a period of historically remarkable stability of government, economy, and natural forces. Perhaps knowing no other world has made these problems appear unsolvable to the parents of our patients. Their own sense of meaning has been challenged in a way similar to that of their children. Perhaps from lack of privacy or peers, parents have been sharing their own sense of powerlessness with their children directly or indirectly, making it harder to reassure them.
With COVID-19 waning in the United States, many of the sources of meaning just discussed can be reinstated by way of in-person play dates, school, sports, socializing, practicing religion, volunteering, and getting jobs. Although there is “existential therapy,” what our children need most is adult leadership showing confidence in life’s meaning, even if we have to hide our own worries. Parents can point out that, even if it takes years, people have made it through difficult times in the past, and there are many positive alternatives for education and employment.
Children need to repeatedly hear about ways they are valued that are not dependent on accomplishments. Thanking them for and telling others about their effort, ideas, curiosity, integrity, love, and kindness point out meaning for their existence independent of world events. Parents need to establish routines and rules for children to demonstrate that life goes on as usual. Chores helpful to the family are a practical contribution. Family activities that are challenging and unpredictable set up for discussing, modeling, and building resilience; for example, visiting new places, camping, hiking, trying a new sport, or adopting a pet give opportunities to say: “Oh, well, we’ll find another way.”
Parents can share stories or books about people who made it through tougher times, such as Abraham Lincoln, or better, personal, or family experiences overcoming challenges. Recalling and nicknaming instances of the child’s own resilience is valuable. Books such as “The Little Engine That Could,” “Chicken Little,” and fairy tales of overcoming doubts when facing challenges can be helpful. “Stay calm and carry on,” a saying from the British when they were being bombed during World War II, has become a meme.
As clinicians we need to sort out significant complicated grief, anxiety, obsessive compulsive disorder, depression, or suicidal ideation, and provide assessment and treatment. But when children get stuck in existential futility, in addition to engaging them in meaningful activities, we can advise parents to coach them to distract themselves, “put the thoughts in a box in your head” to consider later, and/or write down or photograph things that make them grateful. Good lessons for us all to reinvent meaning in our lives.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
Conflicting medical opinions: Black lungs, Big Coal, and bias
In 2008, the U.S. Department of Labor (DOL) paid for Tony Adams, a 48-year-old coal miner, to have a chest x-ray. His doctor found stage I black lung disease. Yet Mr. Adams’ claim for medical benefits was denied. This was because the insurance group that represented his employer hired a different – more credentialed – doctor as its medical expert. That doctor said he saw no such evidence. The judge ruled in favor of the mining company on the basis of the latter’s “expertise.”
Before he died 5 years later, at age 53, Mr. Adams went through this process again. In fact, he did it four more times. Each time, his doctor found evidence of black lung, but the company’s medical expert did not. He died without receiving benefits. Among the causes of death listed on his autopsy were cardiopulmonary arrest and coal worker’s pneumoconiosis (CWP): black lung.
Since his death in 2013, two judges have awarded Mr. Adams’ benefits to his widow, Linda. Both times, the mining company appealed the decision, most recently in December 2020. She’s not giving up. “Two weeks before he died, he told me, ‘I’m going to die of black lung,’ ” Linda recalled. “‘But I don’t want you to give up on black lung. There are too many people screwing these miners out of what they deserve.’”
There has long been suspicion among miners and their advocates that doctors used by coal companies to fight claims like Mr. Adams’ are in the pocket of “Big Coal.” At the very least, some say these physicians are swayed by their client’s preference when reading a coal miner’s chest x-ray. A recent study published in Annals of the American Thoracic Society provides empirical evidence that these doctors’ conflict of interest – namely, that parties representing coal companies hired them – appears to influence their medical opinion.
Proof of a ‘broken system’
The Annals study examined 63,780 radiograph classifications made by 264 physicians – all certified as B-readers, a certification by the National Institute for Occupational Safety and Health (NIOSH) for physicians who demonstrate proficiency in classifying radiographs of pneumoconiosis. The results showed that doctors hired by miners identified black lung 49% of the time; those hired by coal companies identified black lung only 15% of the time.
The study also found that B-readers contracted by employers read results differently for different clients. The same doctors were significantly less likely to say a miner’s lungs were negative for CWP when they were hired by the DOL (77.2%) than when they were hired by a coal company or its insurers (90.2%).
The bias does appear to work both ways: B-readers hired by miners and miners’ attorneys were more likely to find evidence of black lung when they worked with plaintiffs. However, a much higher number of doctors appeared to be biased in favor of the companies. “There were 3X more B-readers providing 8X more classifications among those affiliated with employers compared to those affiliated with miners,” the study concluded.
The authors suggest that one reason for this was the difference in pay. Some company-hired doctors made as much as $750 per reading, about 10 times what miner-hired doctors were paid.
“We knew [about the potential bias] from our work over the decades taking care of these guys,” said Robert A. Cohen, MD, a pulmonologist and the study’s senior author. “But then you see it with P values that are incredibly statistically significant ...”
The study finally put numbers to a problem that many working with black lung claims had always assumed. Those within the system are accustomed to seeing names of the same doctors on documents and reports, with little to no overlap between those hired by the defense and the plaintiffs.
“The vast majority of the time, we know what a report will say based on the doctor’s name,” said Evan Smith, JD, advocacy director at AppalReD Legal Aid, in Prestonsburg, Ky.. It is far more surprising, he said, when a defense-hired doctor agrees with a miner-hired doctor.
Over the years, Katherine DePonte, MD, a radiologist and B-reader in West Virginia, has often seen an “almost textbook appearance” of CWP, only to later learn that “another radiologist read it as negative.” She explained, “They would use some other term, like ‘old granulomatous disease.’”
Employer-hired doctors often do acknowledge the same lung damage on the radiograph as miner-hired docs; they simply don’t attribute it to coal dust. Common “alternative diagnoses” include chronic obstructive pulmonary disease or histoplasmosis. “I know a number don’t believe this disease of coal worker pneumoconiosis exists [at all],” Dr. DePonte said.
What’s inarguable is that, even as coal mining in Appalachia is on the decline, black lung disease is on the rise. NIOSH now estimates that it affects over 20% of long-term (25+ years) coal workers in central Appalachia. That’s the highest prevalence in a quarter of a century.
Mr. Smith said that at its most basic level, these doctors’ conflicts of interest “lead to people who have the disease that these benefits are for, having them denied.” People like Tony Adams. Whether the doctors involved are complicit or just conservative, critics say they have become a fixture of a broken system.
Financial bias or difference of opinion?
Broken system or not, evidence suggests that the problem can’t be blamed solely on medical experts. Dr. DePonte primarily reads for the DOL and miners. “Not that I necessarily chose that,” she said. “You get pigeonholed.”
Some say that the bias demonstrated by the Annals study is at least partially driven by the litigation process itself. It is an adversarial system. As such, attorneys on both sides are naturally inclined to seek out doctors who will best support their clients’ cases. Doctors with a legitimately conservative perspective on what constitutes black lung are more sought after by the coal companies’ attorneys.
“It can often be impossible to tell whether the money is driving a change in the behavior or if the behavior is causing them to be sought out,” said Matt McCoy, PhD, a medical ethicist who specializes in conflicts of interest at the University of Pennsylvania, Philadelphia.
Although some believe that certain doctors are driven purely by financial incentive and offer a specific reading to secure repeat business, B-readers can end up working exclusively for companies because of other reasons. Wes Addington, JD, an attorney at the Appalachian Citizens’ Law Center, Whitesburg, Ky., said some doctors appear to have an authentically different – often antiquated – view of the disease.
Perhaps the most extreme example is Paul Wheeler, MD, a highly credentialed Johns Hopkins radiologist who was exposed for false medical testimony in Chris Hamby’s 2013 Pulitzer Prize reporting. In 1,500 readings, Dr. Wheeler never diagnosed a single case of severe black lung. And yet, Dr. Cohen, Mr. Addington, Mr. Smith, and other experts all agree that Dr. Wheeler appeared to wholeheartedly believe that his view of black lung was accurate. That made him a valuable asset to mining companies.
Since Dr. Wheeler’s exposure, there has been a greater sense of accountability among B-readers, said John Cline, JD, a West Virginia–based attorney who represents miners with federal black lung claims. “Radiologists were thinking, ‘Somebody could be watching me.’ Even if they thought they were doing this in the shadows, it made people more cautious,” he said.
The data used in the Annals study predate Mr. Hamby’s investigation, going back to 2000. Thus, it is possible that, as Mr. Cline argues, things may be different now. However, Lee S. Friedman, PhD, associate professor at the University of Illinois at Chicago, who is the lead author of the study, remains skeptical.
“While the Wheeler case might have dampened some physicians [who were] completely skewing their readings always negative, I think it’s premature or incorrect” to say it resolved the issue, he said. “Did they all change their behavior the morning after? It doesn’t seem likely, given the evidence of financial conflicts of interest and behavior that’s been demonstrated.”
Skewing the evidence?
Mr. Hamby’s 2013 reporting also revealed that even when company-hired doctors did diagnose CWP, law firms were burying those readings. In 2016, the DOL attempted to stop this practice. The agency made suppression of written evidence illegal – emphasis on written.
Law firms can’t hide positive reports, but they can prevent them. Dr. Cohen explained that now, “a doctor on the phone says, ‘I will read this as positive.’ Then the company says, ‘No, thank you,’ we will send you a check.”
This practice was confirmed by Kim Adcock, MD, a retired radiologist and B-reader in Littleton, Colo., who primarily reads for 26 law firms. Some of his clients want a report no matter how he reads the radiograph. However, some want him to call them first if he’s going to read the radiograph as positive. Dr. Adcock said this practice skews the dataset to make company-hired docs appear to read more negatively than they actually do.
Because the dataset used in the study is from the Federal Black Lung Program (FBLP), it includes only readings that made it to court. Dr. Adcock said he reads approximately 2,000 radiographs a year, although only a few of his readings appeared in the study’s dataset, according to a search by Dr. Friedman. This difference is likely because the study evaluated only readings between 2000 and 2013, the year Dr. Adcock started B-reading.
“I think it’s important to get a message that, to a certain extent, contravenes this paper. Yes, we should have some reservations about the conclusions,” Dr. Adcock explained. “There are people out there attempting to do the best job they could do.”
Law firms shopping for the reading they want and censoring the ones they don’t might alter the FBLP data, but experts say that doesn’t change the underlying problem. “In any case like this, where you’re looking at individuals going up against corporations,” Dr. McCoy said, “[corporations] are able to marshal their resources and hire more officials in a way claimants can’t, and that’s a baseline concern here.”
Battling bias
Admitting bias is notoriously difficult; thus, it isn’t surprising that many doctors involved refuse to believe they are influenced by money, incentives, or other biases. Dr. DePonte said she’s not swayed by money, nor does she actively take a pro-miner stance. She views herself as more of an advocate for accuracy. However, she did say that it has traditionally been far more difficult for miners to prove their cases, a problem that has improved with new regulations in recent years.
In Colorado, Dr. Adcock’s approach is to stay as far removed from the litigation process as possible. He said he has limited understanding of how his reports are used or how claims are filed and awarded. He leans heavily on his initial – almost instantaneous – impression of a chest x-ray.
Dr. DePonte and Dr. Adcock were both hired as experts on Tony Adams’ case. In 2008, Dr. DePonte read his chest x-ray as positive for early-stage black lung (1/0). Dr. Adcock also read two of Adams’ four chest x-rays, one in 2009 and the other in 2013. He read them as negative. When asked about the case, which autopsy confirmed as black lung, Dr. Adcock explained that positive histopathology doesn’t mean the radiograph reading was wrong, only that the disease didn’t show on that radiograph. He said his “highest ambition” is to be “an objective finder of fact” and that he trusts the process to work out the truth.
That process didn’t work in time for Tony Adams. Dr. Friedman argues that people who provide expert testimony have an ethical responsibility to know how their testimony is being used; to do otherwise, he says, is “willful ignorance.” Still, the Annals study authors, along with Dr. DePonte, Mr. Cline, and West Virginia attorney Sam Petsonk, say that the process is getting fairer, thanks to new policies developed over the past 5 years by the DOL.
“The DOL has worked very hard to reconcile the final award rate (around 30%) with the incidence of disease in the population (between 20% and 25%),” Mr. Petsonk said. Although the study calls into question the integrity of the system and the doctors within it, it’s critical for miners to know that the system is working and that they can get benefits, he explained. Many fear that cynicism about the system drives miners away and causes them to resort to Social Security or long-term disability.
Fixing what’s broken
The Annals study’s authors propose some solutions to the problems they quantified. The first is a sort of “super panel” that collectively evaluates readings. Although a completely unbiased panel would be nice, such impartiality is likely unsustainable, Mr. Smith said. He believes that over time, the panel would become vulnerable to politics and would work in favor of the companies.
Even without a panel, a method to provide greater transparency could be a great start, some suggest. The DOL could make the entire FBLP database public and analyze it annually. The authors also propose a flat fee for readings. Even now, Dr. Adcock said he doesn’t make anywhere close to the upper limit of $750 per readings. “My understanding is around $125 is a pretty characteristic fee [for reading a chest x-ray],” he elaborated. “Everyone I’ve had a conversation with is within 25 bucks [of that].”
That said, Dr. Adcock is not currently listed among the heavy readers who appear in the data used for the study; it’s possible that his experience is not representative. Some readers who were included in that dataset read more than 10 times the average number of classifications per reader – the average was 242 classifications – and read 95% of chest x-rays as negative, according to Dr. Friedman. This news organization obtained the names of two doctors whose readings were 95% negative on a high volume of cases. Neither agreed to an interview.
It’s possible that if the dataset had included readings from more recent years, Dr. Adcock would have appeared more frequently, given his personal estimates. That’s why the study authors recommend that the DOL conduct this kind of analysis annually in order to get an accurate picture of who is contributing to these cases, in what way, and how often. By doing so, readers who appear biased could be identified and addressed with more regularity, Dr. Friedman said.
Even if the rate were more consistent and the data were more frequently analyzed, the very nature of the adversarial system will put any potential solution at risk. “I’m not sure there’s a foolproof system that can be devised that can’t be corrupted in time,” Mr. Cline said.
A version of this article first appeared on Medscape.com.
In 2008, the U.S. Department of Labor (DOL) paid for Tony Adams, a 48-year-old coal miner, to have a chest x-ray. His doctor found stage I black lung disease. Yet Mr. Adams’ claim for medical benefits was denied. This was because the insurance group that represented his employer hired a different – more credentialed – doctor as its medical expert. That doctor said he saw no such evidence. The judge ruled in favor of the mining company on the basis of the latter’s “expertise.”
Before he died 5 years later, at age 53, Mr. Adams went through this process again. In fact, he did it four more times. Each time, his doctor found evidence of black lung, but the company’s medical expert did not. He died without receiving benefits. Among the causes of death listed on his autopsy were cardiopulmonary arrest and coal worker’s pneumoconiosis (CWP): black lung.
Since his death in 2013, two judges have awarded Mr. Adams’ benefits to his widow, Linda. Both times, the mining company appealed the decision, most recently in December 2020. She’s not giving up. “Two weeks before he died, he told me, ‘I’m going to die of black lung,’ ” Linda recalled. “‘But I don’t want you to give up on black lung. There are too many people screwing these miners out of what they deserve.’”
There has long been suspicion among miners and their advocates that doctors used by coal companies to fight claims like Mr. Adams’ are in the pocket of “Big Coal.” At the very least, some say these physicians are swayed by their client’s preference when reading a coal miner’s chest x-ray. A recent study published in Annals of the American Thoracic Society provides empirical evidence that these doctors’ conflict of interest – namely, that parties representing coal companies hired them – appears to influence their medical opinion.
Proof of a ‘broken system’
The Annals study examined 63,780 radiograph classifications made by 264 physicians – all certified as B-readers, a certification by the National Institute for Occupational Safety and Health (NIOSH) for physicians who demonstrate proficiency in classifying radiographs of pneumoconiosis. The results showed that doctors hired by miners identified black lung 49% of the time; those hired by coal companies identified black lung only 15% of the time.
The study also found that B-readers contracted by employers read results differently for different clients. The same doctors were significantly less likely to say a miner’s lungs were negative for CWP when they were hired by the DOL (77.2%) than when they were hired by a coal company or its insurers (90.2%).
The bias does appear to work both ways: B-readers hired by miners and miners’ attorneys were more likely to find evidence of black lung when they worked with plaintiffs. However, a much higher number of doctors appeared to be biased in favor of the companies. “There were 3X more B-readers providing 8X more classifications among those affiliated with employers compared to those affiliated with miners,” the study concluded.
The authors suggest that one reason for this was the difference in pay. Some company-hired doctors made as much as $750 per reading, about 10 times what miner-hired doctors were paid.
“We knew [about the potential bias] from our work over the decades taking care of these guys,” said Robert A. Cohen, MD, a pulmonologist and the study’s senior author. “But then you see it with P values that are incredibly statistically significant ...”
The study finally put numbers to a problem that many working with black lung claims had always assumed. Those within the system are accustomed to seeing names of the same doctors on documents and reports, with little to no overlap between those hired by the defense and the plaintiffs.
“The vast majority of the time, we know what a report will say based on the doctor’s name,” said Evan Smith, JD, advocacy director at AppalReD Legal Aid, in Prestonsburg, Ky.. It is far more surprising, he said, when a defense-hired doctor agrees with a miner-hired doctor.
Over the years, Katherine DePonte, MD, a radiologist and B-reader in West Virginia, has often seen an “almost textbook appearance” of CWP, only to later learn that “another radiologist read it as negative.” She explained, “They would use some other term, like ‘old granulomatous disease.’”
Employer-hired doctors often do acknowledge the same lung damage on the radiograph as miner-hired docs; they simply don’t attribute it to coal dust. Common “alternative diagnoses” include chronic obstructive pulmonary disease or histoplasmosis. “I know a number don’t believe this disease of coal worker pneumoconiosis exists [at all],” Dr. DePonte said.
What’s inarguable is that, even as coal mining in Appalachia is on the decline, black lung disease is on the rise. NIOSH now estimates that it affects over 20% of long-term (25+ years) coal workers in central Appalachia. That’s the highest prevalence in a quarter of a century.
Mr. Smith said that at its most basic level, these doctors’ conflicts of interest “lead to people who have the disease that these benefits are for, having them denied.” People like Tony Adams. Whether the doctors involved are complicit or just conservative, critics say they have become a fixture of a broken system.
Financial bias or difference of opinion?
Broken system or not, evidence suggests that the problem can’t be blamed solely on medical experts. Dr. DePonte primarily reads for the DOL and miners. “Not that I necessarily chose that,” she said. “You get pigeonholed.”
Some say that the bias demonstrated by the Annals study is at least partially driven by the litigation process itself. It is an adversarial system. As such, attorneys on both sides are naturally inclined to seek out doctors who will best support their clients’ cases. Doctors with a legitimately conservative perspective on what constitutes black lung are more sought after by the coal companies’ attorneys.
“It can often be impossible to tell whether the money is driving a change in the behavior or if the behavior is causing them to be sought out,” said Matt McCoy, PhD, a medical ethicist who specializes in conflicts of interest at the University of Pennsylvania, Philadelphia.
Although some believe that certain doctors are driven purely by financial incentive and offer a specific reading to secure repeat business, B-readers can end up working exclusively for companies because of other reasons. Wes Addington, JD, an attorney at the Appalachian Citizens’ Law Center, Whitesburg, Ky., said some doctors appear to have an authentically different – often antiquated – view of the disease.
Perhaps the most extreme example is Paul Wheeler, MD, a highly credentialed Johns Hopkins radiologist who was exposed for false medical testimony in Chris Hamby’s 2013 Pulitzer Prize reporting. In 1,500 readings, Dr. Wheeler never diagnosed a single case of severe black lung. And yet, Dr. Cohen, Mr. Addington, Mr. Smith, and other experts all agree that Dr. Wheeler appeared to wholeheartedly believe that his view of black lung was accurate. That made him a valuable asset to mining companies.
Since Dr. Wheeler’s exposure, there has been a greater sense of accountability among B-readers, said John Cline, JD, a West Virginia–based attorney who represents miners with federal black lung claims. “Radiologists were thinking, ‘Somebody could be watching me.’ Even if they thought they were doing this in the shadows, it made people more cautious,” he said.
The data used in the Annals study predate Mr. Hamby’s investigation, going back to 2000. Thus, it is possible that, as Mr. Cline argues, things may be different now. However, Lee S. Friedman, PhD, associate professor at the University of Illinois at Chicago, who is the lead author of the study, remains skeptical.
“While the Wheeler case might have dampened some physicians [who were] completely skewing their readings always negative, I think it’s premature or incorrect” to say it resolved the issue, he said. “Did they all change their behavior the morning after? It doesn’t seem likely, given the evidence of financial conflicts of interest and behavior that’s been demonstrated.”
Skewing the evidence?
Mr. Hamby’s 2013 reporting also revealed that even when company-hired doctors did diagnose CWP, law firms were burying those readings. In 2016, the DOL attempted to stop this practice. The agency made suppression of written evidence illegal – emphasis on written.
Law firms can’t hide positive reports, but they can prevent them. Dr. Cohen explained that now, “a doctor on the phone says, ‘I will read this as positive.’ Then the company says, ‘No, thank you,’ we will send you a check.”
This practice was confirmed by Kim Adcock, MD, a retired radiologist and B-reader in Littleton, Colo., who primarily reads for 26 law firms. Some of his clients want a report no matter how he reads the radiograph. However, some want him to call them first if he’s going to read the radiograph as positive. Dr. Adcock said this practice skews the dataset to make company-hired docs appear to read more negatively than they actually do.
Because the dataset used in the study is from the Federal Black Lung Program (FBLP), it includes only readings that made it to court. Dr. Adcock said he reads approximately 2,000 radiographs a year, although only a few of his readings appeared in the study’s dataset, according to a search by Dr. Friedman. This difference is likely because the study evaluated only readings between 2000 and 2013, the year Dr. Adcock started B-reading.
“I think it’s important to get a message that, to a certain extent, contravenes this paper. Yes, we should have some reservations about the conclusions,” Dr. Adcock explained. “There are people out there attempting to do the best job they could do.”
Law firms shopping for the reading they want and censoring the ones they don’t might alter the FBLP data, but experts say that doesn’t change the underlying problem. “In any case like this, where you’re looking at individuals going up against corporations,” Dr. McCoy said, “[corporations] are able to marshal their resources and hire more officials in a way claimants can’t, and that’s a baseline concern here.”
Battling bias
Admitting bias is notoriously difficult; thus, it isn’t surprising that many doctors involved refuse to believe they are influenced by money, incentives, or other biases. Dr. DePonte said she’s not swayed by money, nor does she actively take a pro-miner stance. She views herself as more of an advocate for accuracy. However, she did say that it has traditionally been far more difficult for miners to prove their cases, a problem that has improved with new regulations in recent years.
In Colorado, Dr. Adcock’s approach is to stay as far removed from the litigation process as possible. He said he has limited understanding of how his reports are used or how claims are filed and awarded. He leans heavily on his initial – almost instantaneous – impression of a chest x-ray.
Dr. DePonte and Dr. Adcock were both hired as experts on Tony Adams’ case. In 2008, Dr. DePonte read his chest x-ray as positive for early-stage black lung (1/0). Dr. Adcock also read two of Adams’ four chest x-rays, one in 2009 and the other in 2013. He read them as negative. When asked about the case, which autopsy confirmed as black lung, Dr. Adcock explained that positive histopathology doesn’t mean the radiograph reading was wrong, only that the disease didn’t show on that radiograph. He said his “highest ambition” is to be “an objective finder of fact” and that he trusts the process to work out the truth.
That process didn’t work in time for Tony Adams. Dr. Friedman argues that people who provide expert testimony have an ethical responsibility to know how their testimony is being used; to do otherwise, he says, is “willful ignorance.” Still, the Annals study authors, along with Dr. DePonte, Mr. Cline, and West Virginia attorney Sam Petsonk, say that the process is getting fairer, thanks to new policies developed over the past 5 years by the DOL.
“The DOL has worked very hard to reconcile the final award rate (around 30%) with the incidence of disease in the population (between 20% and 25%),” Mr. Petsonk said. Although the study calls into question the integrity of the system and the doctors within it, it’s critical for miners to know that the system is working and that they can get benefits, he explained. Many fear that cynicism about the system drives miners away and causes them to resort to Social Security or long-term disability.
Fixing what’s broken
The Annals study’s authors propose some solutions to the problems they quantified. The first is a sort of “super panel” that collectively evaluates readings. Although a completely unbiased panel would be nice, such impartiality is likely unsustainable, Mr. Smith said. He believes that over time, the panel would become vulnerable to politics and would work in favor of the companies.
Even without a panel, a method to provide greater transparency could be a great start, some suggest. The DOL could make the entire FBLP database public and analyze it annually. The authors also propose a flat fee for readings. Even now, Dr. Adcock said he doesn’t make anywhere close to the upper limit of $750 per readings. “My understanding is around $125 is a pretty characteristic fee [for reading a chest x-ray],” he elaborated. “Everyone I’ve had a conversation with is within 25 bucks [of that].”
That said, Dr. Adcock is not currently listed among the heavy readers who appear in the data used for the study; it’s possible that his experience is not representative. Some readers who were included in that dataset read more than 10 times the average number of classifications per reader – the average was 242 classifications – and read 95% of chest x-rays as negative, according to Dr. Friedman. This news organization obtained the names of two doctors whose readings were 95% negative on a high volume of cases. Neither agreed to an interview.
It’s possible that if the dataset had included readings from more recent years, Dr. Adcock would have appeared more frequently, given his personal estimates. That’s why the study authors recommend that the DOL conduct this kind of analysis annually in order to get an accurate picture of who is contributing to these cases, in what way, and how often. By doing so, readers who appear biased could be identified and addressed with more regularity, Dr. Friedman said.
Even if the rate were more consistent and the data were more frequently analyzed, the very nature of the adversarial system will put any potential solution at risk. “I’m not sure there’s a foolproof system that can be devised that can’t be corrupted in time,” Mr. Cline said.
A version of this article first appeared on Medscape.com.
In 2008, the U.S. Department of Labor (DOL) paid for Tony Adams, a 48-year-old coal miner, to have a chest x-ray. His doctor found stage I black lung disease. Yet Mr. Adams’ claim for medical benefits was denied. This was because the insurance group that represented his employer hired a different – more credentialed – doctor as its medical expert. That doctor said he saw no such evidence. The judge ruled in favor of the mining company on the basis of the latter’s “expertise.”
Before he died 5 years later, at age 53, Mr. Adams went through this process again. In fact, he did it four more times. Each time, his doctor found evidence of black lung, but the company’s medical expert did not. He died without receiving benefits. Among the causes of death listed on his autopsy were cardiopulmonary arrest and coal worker’s pneumoconiosis (CWP): black lung.
Since his death in 2013, two judges have awarded Mr. Adams’ benefits to his widow, Linda. Both times, the mining company appealed the decision, most recently in December 2020. She’s not giving up. “Two weeks before he died, he told me, ‘I’m going to die of black lung,’ ” Linda recalled. “‘But I don’t want you to give up on black lung. There are too many people screwing these miners out of what they deserve.’”
There has long been suspicion among miners and their advocates that doctors used by coal companies to fight claims like Mr. Adams’ are in the pocket of “Big Coal.” At the very least, some say these physicians are swayed by their client’s preference when reading a coal miner’s chest x-ray. A recent study published in Annals of the American Thoracic Society provides empirical evidence that these doctors’ conflict of interest – namely, that parties representing coal companies hired them – appears to influence their medical opinion.
Proof of a ‘broken system’
The Annals study examined 63,780 radiograph classifications made by 264 physicians – all certified as B-readers, a certification by the National Institute for Occupational Safety and Health (NIOSH) for physicians who demonstrate proficiency in classifying radiographs of pneumoconiosis. The results showed that doctors hired by miners identified black lung 49% of the time; those hired by coal companies identified black lung only 15% of the time.
The study also found that B-readers contracted by employers read results differently for different clients. The same doctors were significantly less likely to say a miner’s lungs were negative for CWP when they were hired by the DOL (77.2%) than when they were hired by a coal company or its insurers (90.2%).
The bias does appear to work both ways: B-readers hired by miners and miners’ attorneys were more likely to find evidence of black lung when they worked with plaintiffs. However, a much higher number of doctors appeared to be biased in favor of the companies. “There were 3X more B-readers providing 8X more classifications among those affiliated with employers compared to those affiliated with miners,” the study concluded.
The authors suggest that one reason for this was the difference in pay. Some company-hired doctors made as much as $750 per reading, about 10 times what miner-hired doctors were paid.
“We knew [about the potential bias] from our work over the decades taking care of these guys,” said Robert A. Cohen, MD, a pulmonologist and the study’s senior author. “But then you see it with P values that are incredibly statistically significant ...”
The study finally put numbers to a problem that many working with black lung claims had always assumed. Those within the system are accustomed to seeing names of the same doctors on documents and reports, with little to no overlap between those hired by the defense and the plaintiffs.
“The vast majority of the time, we know what a report will say based on the doctor’s name,” said Evan Smith, JD, advocacy director at AppalReD Legal Aid, in Prestonsburg, Ky.. It is far more surprising, he said, when a defense-hired doctor agrees with a miner-hired doctor.
Over the years, Katherine DePonte, MD, a radiologist and B-reader in West Virginia, has often seen an “almost textbook appearance” of CWP, only to later learn that “another radiologist read it as negative.” She explained, “They would use some other term, like ‘old granulomatous disease.’”
Employer-hired doctors often do acknowledge the same lung damage on the radiograph as miner-hired docs; they simply don’t attribute it to coal dust. Common “alternative diagnoses” include chronic obstructive pulmonary disease or histoplasmosis. “I know a number don’t believe this disease of coal worker pneumoconiosis exists [at all],” Dr. DePonte said.
What’s inarguable is that, even as coal mining in Appalachia is on the decline, black lung disease is on the rise. NIOSH now estimates that it affects over 20% of long-term (25+ years) coal workers in central Appalachia. That’s the highest prevalence in a quarter of a century.
Mr. Smith said that at its most basic level, these doctors’ conflicts of interest “lead to people who have the disease that these benefits are for, having them denied.” People like Tony Adams. Whether the doctors involved are complicit or just conservative, critics say they have become a fixture of a broken system.
Financial bias or difference of opinion?
Broken system or not, evidence suggests that the problem can’t be blamed solely on medical experts. Dr. DePonte primarily reads for the DOL and miners. “Not that I necessarily chose that,” she said. “You get pigeonholed.”
Some say that the bias demonstrated by the Annals study is at least partially driven by the litigation process itself. It is an adversarial system. As such, attorneys on both sides are naturally inclined to seek out doctors who will best support their clients’ cases. Doctors with a legitimately conservative perspective on what constitutes black lung are more sought after by the coal companies’ attorneys.
“It can often be impossible to tell whether the money is driving a change in the behavior or if the behavior is causing them to be sought out,” said Matt McCoy, PhD, a medical ethicist who specializes in conflicts of interest at the University of Pennsylvania, Philadelphia.
Although some believe that certain doctors are driven purely by financial incentive and offer a specific reading to secure repeat business, B-readers can end up working exclusively for companies because of other reasons. Wes Addington, JD, an attorney at the Appalachian Citizens’ Law Center, Whitesburg, Ky., said some doctors appear to have an authentically different – often antiquated – view of the disease.
Perhaps the most extreme example is Paul Wheeler, MD, a highly credentialed Johns Hopkins radiologist who was exposed for false medical testimony in Chris Hamby’s 2013 Pulitzer Prize reporting. In 1,500 readings, Dr. Wheeler never diagnosed a single case of severe black lung. And yet, Dr. Cohen, Mr. Addington, Mr. Smith, and other experts all agree that Dr. Wheeler appeared to wholeheartedly believe that his view of black lung was accurate. That made him a valuable asset to mining companies.
Since Dr. Wheeler’s exposure, there has been a greater sense of accountability among B-readers, said John Cline, JD, a West Virginia–based attorney who represents miners with federal black lung claims. “Radiologists were thinking, ‘Somebody could be watching me.’ Even if they thought they were doing this in the shadows, it made people more cautious,” he said.
The data used in the Annals study predate Mr. Hamby’s investigation, going back to 2000. Thus, it is possible that, as Mr. Cline argues, things may be different now. However, Lee S. Friedman, PhD, associate professor at the University of Illinois at Chicago, who is the lead author of the study, remains skeptical.
“While the Wheeler case might have dampened some physicians [who were] completely skewing their readings always negative, I think it’s premature or incorrect” to say it resolved the issue, he said. “Did they all change their behavior the morning after? It doesn’t seem likely, given the evidence of financial conflicts of interest and behavior that’s been demonstrated.”
Skewing the evidence?
Mr. Hamby’s 2013 reporting also revealed that even when company-hired doctors did diagnose CWP, law firms were burying those readings. In 2016, the DOL attempted to stop this practice. The agency made suppression of written evidence illegal – emphasis on written.
Law firms can’t hide positive reports, but they can prevent them. Dr. Cohen explained that now, “a doctor on the phone says, ‘I will read this as positive.’ Then the company says, ‘No, thank you,’ we will send you a check.”
This practice was confirmed by Kim Adcock, MD, a retired radiologist and B-reader in Littleton, Colo., who primarily reads for 26 law firms. Some of his clients want a report no matter how he reads the radiograph. However, some want him to call them first if he’s going to read the radiograph as positive. Dr. Adcock said this practice skews the dataset to make company-hired docs appear to read more negatively than they actually do.
Because the dataset used in the study is from the Federal Black Lung Program (FBLP), it includes only readings that made it to court. Dr. Adcock said he reads approximately 2,000 radiographs a year, although only a few of his readings appeared in the study’s dataset, according to a search by Dr. Friedman. This difference is likely because the study evaluated only readings between 2000 and 2013, the year Dr. Adcock started B-reading.
“I think it’s important to get a message that, to a certain extent, contravenes this paper. Yes, we should have some reservations about the conclusions,” Dr. Adcock explained. “There are people out there attempting to do the best job they could do.”
Law firms shopping for the reading they want and censoring the ones they don’t might alter the FBLP data, but experts say that doesn’t change the underlying problem. “In any case like this, where you’re looking at individuals going up against corporations,” Dr. McCoy said, “[corporations] are able to marshal their resources and hire more officials in a way claimants can’t, and that’s a baseline concern here.”
Battling bias
Admitting bias is notoriously difficult; thus, it isn’t surprising that many doctors involved refuse to believe they are influenced by money, incentives, or other biases. Dr. DePonte said she’s not swayed by money, nor does she actively take a pro-miner stance. She views herself as more of an advocate for accuracy. However, she did say that it has traditionally been far more difficult for miners to prove their cases, a problem that has improved with new regulations in recent years.
In Colorado, Dr. Adcock’s approach is to stay as far removed from the litigation process as possible. He said he has limited understanding of how his reports are used or how claims are filed and awarded. He leans heavily on his initial – almost instantaneous – impression of a chest x-ray.
Dr. DePonte and Dr. Adcock were both hired as experts on Tony Adams’ case. In 2008, Dr. DePonte read his chest x-ray as positive for early-stage black lung (1/0). Dr. Adcock also read two of Adams’ four chest x-rays, one in 2009 and the other in 2013. He read them as negative. When asked about the case, which autopsy confirmed as black lung, Dr. Adcock explained that positive histopathology doesn’t mean the radiograph reading was wrong, only that the disease didn’t show on that radiograph. He said his “highest ambition” is to be “an objective finder of fact” and that he trusts the process to work out the truth.
That process didn’t work in time for Tony Adams. Dr. Friedman argues that people who provide expert testimony have an ethical responsibility to know how their testimony is being used; to do otherwise, he says, is “willful ignorance.” Still, the Annals study authors, along with Dr. DePonte, Mr. Cline, and West Virginia attorney Sam Petsonk, say that the process is getting fairer, thanks to new policies developed over the past 5 years by the DOL.
“The DOL has worked very hard to reconcile the final award rate (around 30%) with the incidence of disease in the population (between 20% and 25%),” Mr. Petsonk said. Although the study calls into question the integrity of the system and the doctors within it, it’s critical for miners to know that the system is working and that they can get benefits, he explained. Many fear that cynicism about the system drives miners away and causes them to resort to Social Security or long-term disability.
Fixing what’s broken
The Annals study’s authors propose some solutions to the problems they quantified. The first is a sort of “super panel” that collectively evaluates readings. Although a completely unbiased panel would be nice, such impartiality is likely unsustainable, Mr. Smith said. He believes that over time, the panel would become vulnerable to politics and would work in favor of the companies.
Even without a panel, a method to provide greater transparency could be a great start, some suggest. The DOL could make the entire FBLP database public and analyze it annually. The authors also propose a flat fee for readings. Even now, Dr. Adcock said he doesn’t make anywhere close to the upper limit of $750 per readings. “My understanding is around $125 is a pretty characteristic fee [for reading a chest x-ray],” he elaborated. “Everyone I’ve had a conversation with is within 25 bucks [of that].”
That said, Dr. Adcock is not currently listed among the heavy readers who appear in the data used for the study; it’s possible that his experience is not representative. Some readers who were included in that dataset read more than 10 times the average number of classifications per reader – the average was 242 classifications – and read 95% of chest x-rays as negative, according to Dr. Friedman. This news organization obtained the names of two doctors whose readings were 95% negative on a high volume of cases. Neither agreed to an interview.
It’s possible that if the dataset had included readings from more recent years, Dr. Adcock would have appeared more frequently, given his personal estimates. That’s why the study authors recommend that the DOL conduct this kind of analysis annually in order to get an accurate picture of who is contributing to these cases, in what way, and how often. By doing so, readers who appear biased could be identified and addressed with more regularity, Dr. Friedman said.
Even if the rate were more consistent and the data were more frequently analyzed, the very nature of the adversarial system will put any potential solution at risk. “I’m not sure there’s a foolproof system that can be devised that can’t be corrupted in time,” Mr. Cline said.
A version of this article first appeared on Medscape.com.
NPs and PAs performing colonoscopies: Why not?
Highly trained nurse practitioners (NPs) and physician assistants (PAs) are just as capable of performing screening colonoscopies as gastroenterologists: This is the conclusion from a number of studies conducted across both the United States and Europe.
So, why aren’t more NPs and PAs doing them?
“We wanted it to take off, but we haven’t been able to do it,” said San Diego gastroenterologist Daniel “Stony” Anderson, MD. He spent decades working to expand access to colorectal cancer screening at Kaiser Permanente and other health care organizations, and he has now been doing the same as president of the California Colorectal Cancer Coalition.
Anderson told this news organization that he isn’t sure why the practice did not catch on but added, “I don’t see a groundswell for this.”
One explanation has been that the centers abandoned the practice because there were enough gastroenterologists to handle the demand.
Or perhaps it was one battle too many for NPs and PAs, who are fighting at the state level and at Veterans Affairs (VA) for permission to deliver more primary care and anesthesia services.
In addition, doctors are fighting back.
The American Medical Association runs a Stop Scope Creep campaign that opposes attempts by NPs and PAs “to inappropriately expand their scope of practice.” Along with anesthesiologists, the AMA is fighting the extension of a COVID-19 waiver at the VA that allows NPs and PAs to continue delivering anesthesia without a physician’s supervision. Other groups have joined in the battle against practice expansion via social media under the hashtags #stopscopecreep and #patientsafetymatters.
The battle is ongoing and ugly at times.
Proponents describe NPs and PAs as “advanced practice providers” but opponents call them “midlevel practitioners.” One website called Midlevel WTF argues that the health care system is declining, in part, because of “the proliferation of poorly supervised or completely unsupervised midlevels across the health care spectrum.”
NPs perform colonoscopies ‘safely and effectively’
One of the studies to examine the issue of NPs performing screening colonoscopies, and how this compares with gastroenterologists performing the procedure, was published in Endoscopy International Open in October 2020.
In a retrospective analysis from Johns Hopkins Hospital in Baltimore, the authors concluded that three fellowship-trained NPs satisfied the American College of Gastroenterology’s quality indicators and “demonstrated that adequately trained NPs can perform colonoscopy safely and effectively.”
There was little reaction to this conclusion when the study was published. But some months later, several gastroenterologists on social media began questioning the high percentage of African Americans in the study and suggested that the research exploited Black patients, according to a story in STAT, a national health news website.
In a written statement, Hopkins said in an interview, from 2010 to 2016, patients were given the option to have an NP or physician provide a screening colonoscopy. However, they are no longer offered that option. The project has been discontinued: The gastroenterologist who was overseeing the clinical program, Anthony Kalloo, MD, director of the division of gastroenterology and hepatology, left John Hopkins earlier this year, and two of the three NPs involved in the program have also left.
Dr. Kalloo declined to comment but was quoted at length in the STAT article. He noted that NPs regularly perform colonoscopies in the United Kingdom and that the John Hopkins study showed, for the first time, “that we could do this in the United States, and the implication of that is cost savings.”
Dr. Kalloo also defended his work against claims of racial exploitation. In fact, he said, “I found those comments to be amusing. ... Obviously, they saw that I was the lead author from Hopkins, but they obviously didn’t know what I look like.” Dr. Kalloo is Black.
Dr. Kalloo is now chair of the department of medicine at Maimonides Medical Center, New York City, and he told STAT that he was interested in starting up a similar project there to train NPs to perform colonoscopies.
Other centers that explored the practice have also not continued with it.
A 2008 study at the University of California, Davis, notes: “Several barriers to colorectal cancer screening have been identified, including limited access to trained endoscopists and highlight insufficient capacity to meet projected demand for colonoscopies. ... Training NPs to perform colonoscopy may be an effective strategy to increase access.”
The study compared 100 screening colonoscopies performed by board certified gastroenterologists (GI-MD) and 50 performed by a gastroenterology-trained NP (GI-NP). There were no complications reported among the 150 cases, and “the GI-NP in our study performed screening colonoscopy as safely, accurately, and satisfactorily as the GI-MDs,” the authors conclude.
But it’s not a strategy the hospital adopted. The nurse who conducted the study said in an interview that she is no longer doing them and declined further comment. The University of California, Davis could not confirm whether anyone else is.
An uncommon practice
The American Gastroenterological Association (AGA) referred questions from this news organization to the American Society for Gastrointestinal Endoscopy (ASGE).
It is very uncommon to have NPs do coloscopies, commented Douglas K. Rex, MD, director of endoscopy at Indiana University, Indianapolis, and incoming ASGE president.
“There was more of a movement the United Kingdom, but not in the United States,” he said. “I can’t tell you the reason. It’s a combination of minimal data and also the fact that there are plenty of gastroenterologists.”
The ASGE guidelines for endoscopy by nonphysicians concludes: “There are insufficient data to support nonphysician endoscopists to perform colonoscopy.”
Asked about concerns that the demand for colonoscopies will increase now that the recommendation is to start colorectal cancer screening at 45 years old, Dr. Rex said he thinks the system has enough capacity.
But will it continue to be sufficient? In a 2020 white paper on colorectal cancer screening, the AGA noted that lowering the starting age to 45 years will add 21 million people to the current pool of 94 million eligible for screening, an increase of 22%.
Lukejohn Day, MD, a gastroenterologist at the University of California, San Francisco, has reviewed the data collected on nonphysicians performing colorectal cancer screening. He led a meta-analysis of 24 studies conducted from 1997 to 2011, and the team concluded that “nonphysicians can safely perform endoscopic procedures with similar quality, especially with respect to screening flexible sigmoidoscopy. Far fewer data was reported for nonphysicians performing colonoscopy and upper endoscopy, but among this data nonphysicians perform both procedures within accepted national benchmarks for quality measures used in endoscopy.”
Dr. Day told this news organization that he had trained two NPs to do colonoscopies and did the study to get a better sense of the practice. Even still, he said the NPs required extensive training before they could perform the procedure.
“It’s not like someone could finish school and become an endoscopist. It requires a very rigorous training program – a lot of education and mentoring,” he said. “Their program is very similar to what our GI fellows go through.”
Dr. Day said they faced opposition from the gastroenterologists, but they “had enough guardrails” in place to convince skeptics.
One of those guardrails was the requirement that an attending physician be in the building. But when San Francisco General Hospital moved to a new facility, they were unable to guarantee that coverage and the program ended, Dr. Day said.
Quality and depth of training hard to replicate
One place where both NPs and PAs do colonoscopies is at the VA. However, the administration doesn’t keep track of how many work there, according to a VA spokesperson. Regulations on physician providers also vary from state to state, and that is reflected in its workforce.
At the St. Louis VA Medical Center, PAs have been performing diagnostic and colorectal cancer screening colonoscopies for nearly 20 years, according to a 2020 article on the quality of care delivered by PAs. The researchers looked at data from more than 700 patients treated over a year. They had colonoscopies performed by one of seven gastroenterologists, five PAs, or 32 GI fellows from two academic affiliates. The PAs performed better than the fellows and just as well as the gastroenterologists, they concluded.
Samir Gupta, MD, chief of gastroenterology at the VA San Diego Healthcare System, California, said in an email that he thinks a range of clinically licensed health care providers can be trained to do high-quality colonoscopy.
“The challenge has been ensuring training structure and volume of cases sufficient to consistently enable high-quality colonoscopy practice, including achieving adequate rates of polyp detection and removal and complete exams,” he wrote.
This is a challenge for gastroenterologists, but they receive ongoing medical education, and, in many settings, quality is closely monitored and managed, he wrote. He added that in a 3-year training program, most fellows do hundreds of colonoscopies.
“It is really hard to replicate this quality and depth of training outside of a GI fellowship,” Dr. Gupta said.
A version of this article first appeared on Medscape.com.
Highly trained nurse practitioners (NPs) and physician assistants (PAs) are just as capable of performing screening colonoscopies as gastroenterologists: This is the conclusion from a number of studies conducted across both the United States and Europe.
So, why aren’t more NPs and PAs doing them?
“We wanted it to take off, but we haven’t been able to do it,” said San Diego gastroenterologist Daniel “Stony” Anderson, MD. He spent decades working to expand access to colorectal cancer screening at Kaiser Permanente and other health care organizations, and he has now been doing the same as president of the California Colorectal Cancer Coalition.
Anderson told this news organization that he isn’t sure why the practice did not catch on but added, “I don’t see a groundswell for this.”
One explanation has been that the centers abandoned the practice because there were enough gastroenterologists to handle the demand.
Or perhaps it was one battle too many for NPs and PAs, who are fighting at the state level and at Veterans Affairs (VA) for permission to deliver more primary care and anesthesia services.
In addition, doctors are fighting back.
The American Medical Association runs a Stop Scope Creep campaign that opposes attempts by NPs and PAs “to inappropriately expand their scope of practice.” Along with anesthesiologists, the AMA is fighting the extension of a COVID-19 waiver at the VA that allows NPs and PAs to continue delivering anesthesia without a physician’s supervision. Other groups have joined in the battle against practice expansion via social media under the hashtags #stopscopecreep and #patientsafetymatters.
The battle is ongoing and ugly at times.
Proponents describe NPs and PAs as “advanced practice providers” but opponents call them “midlevel practitioners.” One website called Midlevel WTF argues that the health care system is declining, in part, because of “the proliferation of poorly supervised or completely unsupervised midlevels across the health care spectrum.”
NPs perform colonoscopies ‘safely and effectively’
One of the studies to examine the issue of NPs performing screening colonoscopies, and how this compares with gastroenterologists performing the procedure, was published in Endoscopy International Open in October 2020.
In a retrospective analysis from Johns Hopkins Hospital in Baltimore, the authors concluded that three fellowship-trained NPs satisfied the American College of Gastroenterology’s quality indicators and “demonstrated that adequately trained NPs can perform colonoscopy safely and effectively.”
There was little reaction to this conclusion when the study was published. But some months later, several gastroenterologists on social media began questioning the high percentage of African Americans in the study and suggested that the research exploited Black patients, according to a story in STAT, a national health news website.
In a written statement, Hopkins said in an interview, from 2010 to 2016, patients were given the option to have an NP or physician provide a screening colonoscopy. However, they are no longer offered that option. The project has been discontinued: The gastroenterologist who was overseeing the clinical program, Anthony Kalloo, MD, director of the division of gastroenterology and hepatology, left John Hopkins earlier this year, and two of the three NPs involved in the program have also left.
Dr. Kalloo declined to comment but was quoted at length in the STAT article. He noted that NPs regularly perform colonoscopies in the United Kingdom and that the John Hopkins study showed, for the first time, “that we could do this in the United States, and the implication of that is cost savings.”
Dr. Kalloo also defended his work against claims of racial exploitation. In fact, he said, “I found those comments to be amusing. ... Obviously, they saw that I was the lead author from Hopkins, but they obviously didn’t know what I look like.” Dr. Kalloo is Black.
Dr. Kalloo is now chair of the department of medicine at Maimonides Medical Center, New York City, and he told STAT that he was interested in starting up a similar project there to train NPs to perform colonoscopies.
Other centers that explored the practice have also not continued with it.
A 2008 study at the University of California, Davis, notes: “Several barriers to colorectal cancer screening have been identified, including limited access to trained endoscopists and highlight insufficient capacity to meet projected demand for colonoscopies. ... Training NPs to perform colonoscopy may be an effective strategy to increase access.”
The study compared 100 screening colonoscopies performed by board certified gastroenterologists (GI-MD) and 50 performed by a gastroenterology-trained NP (GI-NP). There were no complications reported among the 150 cases, and “the GI-NP in our study performed screening colonoscopy as safely, accurately, and satisfactorily as the GI-MDs,” the authors conclude.
But it’s not a strategy the hospital adopted. The nurse who conducted the study said in an interview that she is no longer doing them and declined further comment. The University of California, Davis could not confirm whether anyone else is.
An uncommon practice
The American Gastroenterological Association (AGA) referred questions from this news organization to the American Society for Gastrointestinal Endoscopy (ASGE).
It is very uncommon to have NPs do coloscopies, commented Douglas K. Rex, MD, director of endoscopy at Indiana University, Indianapolis, and incoming ASGE president.
“There was more of a movement the United Kingdom, but not in the United States,” he said. “I can’t tell you the reason. It’s a combination of minimal data and also the fact that there are plenty of gastroenterologists.”
The ASGE guidelines for endoscopy by nonphysicians concludes: “There are insufficient data to support nonphysician endoscopists to perform colonoscopy.”
Asked about concerns that the demand for colonoscopies will increase now that the recommendation is to start colorectal cancer screening at 45 years old, Dr. Rex said he thinks the system has enough capacity.
But will it continue to be sufficient? In a 2020 white paper on colorectal cancer screening, the AGA noted that lowering the starting age to 45 years will add 21 million people to the current pool of 94 million eligible for screening, an increase of 22%.
Lukejohn Day, MD, a gastroenterologist at the University of California, San Francisco, has reviewed the data collected on nonphysicians performing colorectal cancer screening. He led a meta-analysis of 24 studies conducted from 1997 to 2011, and the team concluded that “nonphysicians can safely perform endoscopic procedures with similar quality, especially with respect to screening flexible sigmoidoscopy. Far fewer data was reported for nonphysicians performing colonoscopy and upper endoscopy, but among this data nonphysicians perform both procedures within accepted national benchmarks for quality measures used in endoscopy.”
Dr. Day told this news organization that he had trained two NPs to do colonoscopies and did the study to get a better sense of the practice. Even still, he said the NPs required extensive training before they could perform the procedure.
“It’s not like someone could finish school and become an endoscopist. It requires a very rigorous training program – a lot of education and mentoring,” he said. “Their program is very similar to what our GI fellows go through.”
Dr. Day said they faced opposition from the gastroenterologists, but they “had enough guardrails” in place to convince skeptics.
One of those guardrails was the requirement that an attending physician be in the building. But when San Francisco General Hospital moved to a new facility, they were unable to guarantee that coverage and the program ended, Dr. Day said.
Quality and depth of training hard to replicate
One place where both NPs and PAs do colonoscopies is at the VA. However, the administration doesn’t keep track of how many work there, according to a VA spokesperson. Regulations on physician providers also vary from state to state, and that is reflected in its workforce.
At the St. Louis VA Medical Center, PAs have been performing diagnostic and colorectal cancer screening colonoscopies for nearly 20 years, according to a 2020 article on the quality of care delivered by PAs. The researchers looked at data from more than 700 patients treated over a year. They had colonoscopies performed by one of seven gastroenterologists, five PAs, or 32 GI fellows from two academic affiliates. The PAs performed better than the fellows and just as well as the gastroenterologists, they concluded.
Samir Gupta, MD, chief of gastroenterology at the VA San Diego Healthcare System, California, said in an email that he thinks a range of clinically licensed health care providers can be trained to do high-quality colonoscopy.
“The challenge has been ensuring training structure and volume of cases sufficient to consistently enable high-quality colonoscopy practice, including achieving adequate rates of polyp detection and removal and complete exams,” he wrote.
This is a challenge for gastroenterologists, but they receive ongoing medical education, and, in many settings, quality is closely monitored and managed, he wrote. He added that in a 3-year training program, most fellows do hundreds of colonoscopies.
“It is really hard to replicate this quality and depth of training outside of a GI fellowship,” Dr. Gupta said.
A version of this article first appeared on Medscape.com.
Highly trained nurse practitioners (NPs) and physician assistants (PAs) are just as capable of performing screening colonoscopies as gastroenterologists: This is the conclusion from a number of studies conducted across both the United States and Europe.
So, why aren’t more NPs and PAs doing them?
“We wanted it to take off, but we haven’t been able to do it,” said San Diego gastroenterologist Daniel “Stony” Anderson, MD. He spent decades working to expand access to colorectal cancer screening at Kaiser Permanente and other health care organizations, and he has now been doing the same as president of the California Colorectal Cancer Coalition.
Anderson told this news organization that he isn’t sure why the practice did not catch on but added, “I don’t see a groundswell for this.”
One explanation has been that the centers abandoned the practice because there were enough gastroenterologists to handle the demand.
Or perhaps it was one battle too many for NPs and PAs, who are fighting at the state level and at Veterans Affairs (VA) for permission to deliver more primary care and anesthesia services.
In addition, doctors are fighting back.
The American Medical Association runs a Stop Scope Creep campaign that opposes attempts by NPs and PAs “to inappropriately expand their scope of practice.” Along with anesthesiologists, the AMA is fighting the extension of a COVID-19 waiver at the VA that allows NPs and PAs to continue delivering anesthesia without a physician’s supervision. Other groups have joined in the battle against practice expansion via social media under the hashtags #stopscopecreep and #patientsafetymatters.
The battle is ongoing and ugly at times.
Proponents describe NPs and PAs as “advanced practice providers” but opponents call them “midlevel practitioners.” One website called Midlevel WTF argues that the health care system is declining, in part, because of “the proliferation of poorly supervised or completely unsupervised midlevels across the health care spectrum.”
NPs perform colonoscopies ‘safely and effectively’
One of the studies to examine the issue of NPs performing screening colonoscopies, and how this compares with gastroenterologists performing the procedure, was published in Endoscopy International Open in October 2020.
In a retrospective analysis from Johns Hopkins Hospital in Baltimore, the authors concluded that three fellowship-trained NPs satisfied the American College of Gastroenterology’s quality indicators and “demonstrated that adequately trained NPs can perform colonoscopy safely and effectively.”
There was little reaction to this conclusion when the study was published. But some months later, several gastroenterologists on social media began questioning the high percentage of African Americans in the study and suggested that the research exploited Black patients, according to a story in STAT, a national health news website.
In a written statement, Hopkins said in an interview, from 2010 to 2016, patients were given the option to have an NP or physician provide a screening colonoscopy. However, they are no longer offered that option. The project has been discontinued: The gastroenterologist who was overseeing the clinical program, Anthony Kalloo, MD, director of the division of gastroenterology and hepatology, left John Hopkins earlier this year, and two of the three NPs involved in the program have also left.
Dr. Kalloo declined to comment but was quoted at length in the STAT article. He noted that NPs regularly perform colonoscopies in the United Kingdom and that the John Hopkins study showed, for the first time, “that we could do this in the United States, and the implication of that is cost savings.”
Dr. Kalloo also defended his work against claims of racial exploitation. In fact, he said, “I found those comments to be amusing. ... Obviously, they saw that I was the lead author from Hopkins, but they obviously didn’t know what I look like.” Dr. Kalloo is Black.
Dr. Kalloo is now chair of the department of medicine at Maimonides Medical Center, New York City, and he told STAT that he was interested in starting up a similar project there to train NPs to perform colonoscopies.
Other centers that explored the practice have also not continued with it.
A 2008 study at the University of California, Davis, notes: “Several barriers to colorectal cancer screening have been identified, including limited access to trained endoscopists and highlight insufficient capacity to meet projected demand for colonoscopies. ... Training NPs to perform colonoscopy may be an effective strategy to increase access.”
The study compared 100 screening colonoscopies performed by board certified gastroenterologists (GI-MD) and 50 performed by a gastroenterology-trained NP (GI-NP). There were no complications reported among the 150 cases, and “the GI-NP in our study performed screening colonoscopy as safely, accurately, and satisfactorily as the GI-MDs,” the authors conclude.
But it’s not a strategy the hospital adopted. The nurse who conducted the study said in an interview that she is no longer doing them and declined further comment. The University of California, Davis could not confirm whether anyone else is.
An uncommon practice
The American Gastroenterological Association (AGA) referred questions from this news organization to the American Society for Gastrointestinal Endoscopy (ASGE).
It is very uncommon to have NPs do coloscopies, commented Douglas K. Rex, MD, director of endoscopy at Indiana University, Indianapolis, and incoming ASGE president.
“There was more of a movement the United Kingdom, but not in the United States,” he said. “I can’t tell you the reason. It’s a combination of minimal data and also the fact that there are plenty of gastroenterologists.”
The ASGE guidelines for endoscopy by nonphysicians concludes: “There are insufficient data to support nonphysician endoscopists to perform colonoscopy.”
Asked about concerns that the demand for colonoscopies will increase now that the recommendation is to start colorectal cancer screening at 45 years old, Dr. Rex said he thinks the system has enough capacity.
But will it continue to be sufficient? In a 2020 white paper on colorectal cancer screening, the AGA noted that lowering the starting age to 45 years will add 21 million people to the current pool of 94 million eligible for screening, an increase of 22%.
Lukejohn Day, MD, a gastroenterologist at the University of California, San Francisco, has reviewed the data collected on nonphysicians performing colorectal cancer screening. He led a meta-analysis of 24 studies conducted from 1997 to 2011, and the team concluded that “nonphysicians can safely perform endoscopic procedures with similar quality, especially with respect to screening flexible sigmoidoscopy. Far fewer data was reported for nonphysicians performing colonoscopy and upper endoscopy, but among this data nonphysicians perform both procedures within accepted national benchmarks for quality measures used in endoscopy.”
Dr. Day told this news organization that he had trained two NPs to do colonoscopies and did the study to get a better sense of the practice. Even still, he said the NPs required extensive training before they could perform the procedure.
“It’s not like someone could finish school and become an endoscopist. It requires a very rigorous training program – a lot of education and mentoring,” he said. “Their program is very similar to what our GI fellows go through.”
Dr. Day said they faced opposition from the gastroenterologists, but they “had enough guardrails” in place to convince skeptics.
One of those guardrails was the requirement that an attending physician be in the building. But when San Francisco General Hospital moved to a new facility, they were unable to guarantee that coverage and the program ended, Dr. Day said.
Quality and depth of training hard to replicate
One place where both NPs and PAs do colonoscopies is at the VA. However, the administration doesn’t keep track of how many work there, according to a VA spokesperson. Regulations on physician providers also vary from state to state, and that is reflected in its workforce.
At the St. Louis VA Medical Center, PAs have been performing diagnostic and colorectal cancer screening colonoscopies for nearly 20 years, according to a 2020 article on the quality of care delivered by PAs. The researchers looked at data from more than 700 patients treated over a year. They had colonoscopies performed by one of seven gastroenterologists, five PAs, or 32 GI fellows from two academic affiliates. The PAs performed better than the fellows and just as well as the gastroenterologists, they concluded.
Samir Gupta, MD, chief of gastroenterology at the VA San Diego Healthcare System, California, said in an email that he thinks a range of clinically licensed health care providers can be trained to do high-quality colonoscopy.
“The challenge has been ensuring training structure and volume of cases sufficient to consistently enable high-quality colonoscopy practice, including achieving adequate rates of polyp detection and removal and complete exams,” he wrote.
This is a challenge for gastroenterologists, but they receive ongoing medical education, and, in many settings, quality is closely monitored and managed, he wrote. He added that in a 3-year training program, most fellows do hundreds of colonoscopies.
“It is really hard to replicate this quality and depth of training outside of a GI fellowship,” Dr. Gupta said.
A version of this article first appeared on Medscape.com.
Atopic Dermatitis
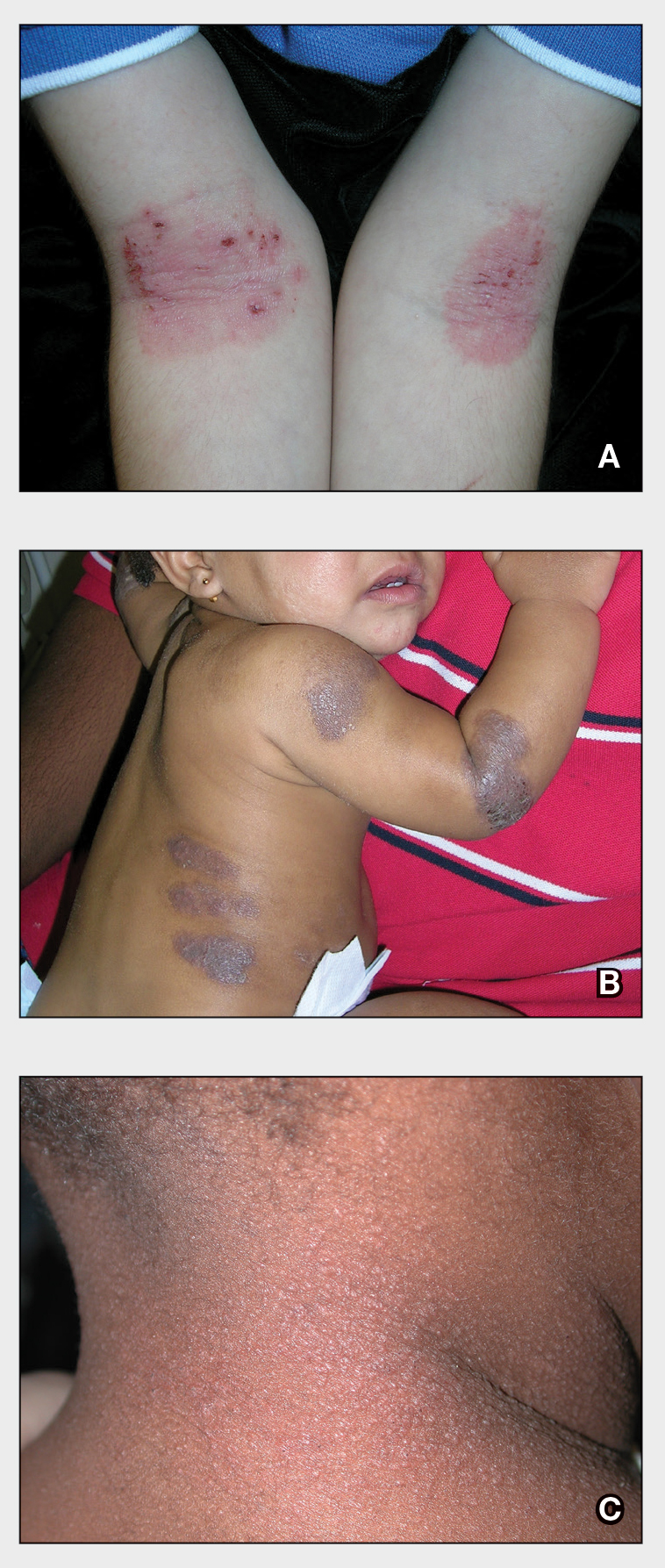
The Comparison
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-ofpocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016 /j.anai.2019.05.014
- Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016 /j.jid.2018.10.029
- Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432

The Comparison
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-ofpocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4

The Comparison
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-ofpocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016 /j.anai.2019.05.014
- Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016 /j.jid.2018.10.029
- Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016 /j.anai.2019.05.014
- Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016 /j.jid.2018.10.029
- Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
GLOW: Ibrutinib+venetoclax shines in first line for CLL/SLL
For older, unfit patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), first-line treatment with the all-oral combination of ibrutinib (Imbruvica) and venetoclax (Venclexta) was associated with superior progression-free survival, compared with chlorambucil and obinutuzumab (Gazyva), results of the phase 3 GLOW trial showed.
Among 211 patients with CLL/SLL who were 65 or older, or younger patients with a high comorbidity burden, the median progression-free survival (PFS) after 27.7 months of follow-up was not reached for patients treated with a fixed duration combination of ibrutinib plus venetoclax (I+V), compared with 21 months for patients who received chlorambucil plus obinutuzumab (Clb+O), reported Arnon P. Kater, MD, PhD, from Amsterdam University Medical Centers.
The oral combination was also associated with a higher rate of undetectable minimal residual disease (MRD) 3 months after the end of treatment, at 51.9% vs. 17.1% with Clb+O, he said in a late-breaking abstract presented during the European Hematology Association annual meeting (Abstract LB1902).
“Overall, the results from GLOW support a positive clinical profile of I+V as an all-oral, once-daily, fixed-duration regimen in older patients with newly diagnosed CLL,” he said.
A low bar
But the bar for success in the GLOW trial may have been set low, with the older combination of chlorambucil plus obinutuzumab used as the comparator, rather than a more contemporary regimen, said Clive S. Zent , MD, from the Wilmot Cancer Institute at the University of Rochester (New York) Medical Center, who was not involved in the study.
“Really, nobody in this country that I’m aware of, certainly not in academic medicine, would be using chlorambucil and obinutuzumab or rituximab for standard of care,” Dr. Zent said in an interview.
“This is like comparing a mule cart to a Tesla,” he said.
Apart from possibly providing a rationale for using ibrutinib and venetoclax in this population, the GLOW results do not add much beyond that already in the CAPTIVE MRD trial, he added.
“What we’re really interested in, is ibrutinib and venetoclax better than ibrutinib alone or venetoclax alone? And that has not been asked or answered yet,” he said.
Dr. Zent acknowledged that the combination works very well and is well tolerated, and the idea of fixed duration therapy is attractive, as are the long-term outcomes for many patients following cessation of therapy.
“But remember, if you take ibrutinib, you’ve got a 90% chance of going into remission, and an over 80% chance of being in remission 5 years later, so that’s pretty good as well,” he said.
Paolo P. Ghia, MD, from Univerista Vita-Salute San Raffaele in Milan, who has studied fixed-duration I+V in younger patients with CLL in the CAPTIVATE trial agreed that “in general, there is very little role for chemoimmunotherapy in CLL.”
But Dr. Ghia, who was not involved in the GLOW study, said in an interview that the results add to the growing body of evidence of the efficacy and safety of ibrutinib -venetoclax in a wide range of patients.
“Overall between the two studies [CAPTIVATE and GLOW] we have now over 400 patients who have been treated with the combination, and the message is rather similar in the two studies: you have a high frequency of undetectable MRD in peripheral blood and in the bone marrow, with a high concordance between the two tissues, and in particular we have a durability of the response,” he said.
GLOW details
Dr. Kater noted that ibrutinib and venetoclax have distinct and complementary modes of action, with ibrutinib mobilizing CLL cells out of their “protective lymphoid niches” and inhibiting their proliferation, as well as accelerating apoptosis by sensitizing cells to inhibition by the anti–B-cell lymphoma 2 (BCL-2) agent venetoclax. The combination leads to high levels of MRD negative by eliminating subpopulations of resting and dividing CLL cells.
The GLOW investigators enrolled 211 patients who were 65 or older, or were younger than 65 with a cumulative illness rating scale (CIRS) score of greater than 6, or creatinine clearance rate of less than 70 mL/min, and no known deletion 17p (del17p) or TP53 mutation.
The patients all had Eastern Cooperative Oncology Group performance status scores of 0-2.
After stratification by immunoglobulin heavy chain variable (IGHV) region genes and presence of deletion 11q (del11q), the patients were randomly assigned to either a three cycle run-in with ibrutinib 420 mg daily followed by ibrutinib plus venetoclax ramped up from 20 to 400 mg, or to chlorambucil 0.5 mg/kg on days and 15 for six cycles, and obinutuzumab 1,000 mg on days 1,2, 8, and 15 of cycle 1, and day 1 of cycles 2-6.
About one-third of patients in each study arm were 75 or older. Baseline characteristics were generally similar between the arms, except for a higher frequency of CIRS scores above 6 in the I+V arm, and a higher frequency of elevated lactate dehydrogenase in the Clb+O arm.
Superior PFS
As noted, the primary endpoint of PFS as assessed by independent review committee (IRC) after 27.7 months of follow-up had not been reached in I+V arm, compared with 21 months in the Clb+O arm, translating into a hazard ratio or progression with I+V of 0.21 (P < .0001). Investigator-assessed PFS was similar, with an HR of 2.07 (P < .0001).
PFS was superior with I+V across all subgroups, including age, baseline performance status. CIRS total score, Rai stage, bulky disease, elevated LDH at baseline, IGHV mutated or unmutated, and presence or absence of del(11q).
IRC-assessed combined complete response (CR) or CR with incomplete recovery of blood counts (CRi) rates were 38.7% with I+V vs. 11.4% with Clb+O (P < .0001).
Responses were also more durable with the oral combination, with 90% of patients having a sustained IRC-assessed response at 24 months, compared with 41% in the chemoimmunotherapy arm.
Rates of undetectable MRD by next-generation sequencing 3 months after the end of treatment were also significantly higher with I+V in both bone marrow (51.9% vs. 17.1%, respectively, P < .0001) and peripheral blood (54.7% vs. 39%, P = .0259).
One year post treatment 49% of patients assigned to I+V had undetectable MRD in peripheral blood, compared with 12% of patients assigned to Clb+O).
Safety
In all, 11 patients assigned to I+V discontinued treatment because of adverse events, compared with 2 in the Clb+O arm. Two patients in the I+V arm (1.9%) discontinued ibrutinib because of atrial fibrillation (AF). Serious adverse events in 5% or more of patients that were more frequent with I+V include infections (12.3% vs. 8.6% and AF (6.6% vs. o%). The tumor lysis syndrome (TLS) was not seen in the I+V arm, but occurred in 5.7% of patients in the Clb+O arm.
There were a total of 11 deaths in the I+V arm and 12 in the Clb+O arm during treatment or follow-up.
Causes of death were generally similar between the arms, with infections and cardiac events being the most common causes, Dr. Kater said.
Of the four deaths that occurred during ibrutinib lead-in, one was due to infection, one to metastatic carcinoma, and two due to cardiac disorders. Of the three that occurred in the I+V arm during treatment, two were from sudden death, and one from a nervous system disorder. Four patients in this arm died during follow-up, two from infections, one from sudden death, and one from progressive disease with Richter transformation.
In the Clb+O arm, one patient died during treatment from an infection and one died from hepatobiliary disease. Of the 10 that died during follow-up, 6 died from infections/infestations, 2 from cardiac disorders, and 1 each from nervous system and respiratory/thoracic/mediastinal disorder.
The study was supported by Janssen Research & Development. Dr. Kater disclosed advisory board activity, research committee, and steering committee participation for Janssen, and similar relationships with others. Dr. Zent disclosed research funding to the University of Rochester from AstraZenca/Acerta and TG Therpeutics. Dr. Ghia disclosed consultancy, honoraria, travel expenses, and research funding from Janssen and others.
For older, unfit patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), first-line treatment with the all-oral combination of ibrutinib (Imbruvica) and venetoclax (Venclexta) was associated with superior progression-free survival, compared with chlorambucil and obinutuzumab (Gazyva), results of the phase 3 GLOW trial showed.
Among 211 patients with CLL/SLL who were 65 or older, or younger patients with a high comorbidity burden, the median progression-free survival (PFS) after 27.7 months of follow-up was not reached for patients treated with a fixed duration combination of ibrutinib plus venetoclax (I+V), compared with 21 months for patients who received chlorambucil plus obinutuzumab (Clb+O), reported Arnon P. Kater, MD, PhD, from Amsterdam University Medical Centers.
The oral combination was also associated with a higher rate of undetectable minimal residual disease (MRD) 3 months after the end of treatment, at 51.9% vs. 17.1% with Clb+O, he said in a late-breaking abstract presented during the European Hematology Association annual meeting (Abstract LB1902).
“Overall, the results from GLOW support a positive clinical profile of I+V as an all-oral, once-daily, fixed-duration regimen in older patients with newly diagnosed CLL,” he said.
A low bar
But the bar for success in the GLOW trial may have been set low, with the older combination of chlorambucil plus obinutuzumab used as the comparator, rather than a more contemporary regimen, said Clive S. Zent , MD, from the Wilmot Cancer Institute at the University of Rochester (New York) Medical Center, who was not involved in the study.
“Really, nobody in this country that I’m aware of, certainly not in academic medicine, would be using chlorambucil and obinutuzumab or rituximab for standard of care,” Dr. Zent said in an interview.
“This is like comparing a mule cart to a Tesla,” he said.
Apart from possibly providing a rationale for using ibrutinib and venetoclax in this population, the GLOW results do not add much beyond that already in the CAPTIVE MRD trial, he added.
“What we’re really interested in, is ibrutinib and venetoclax better than ibrutinib alone or venetoclax alone? And that has not been asked or answered yet,” he said.
Dr. Zent acknowledged that the combination works very well and is well tolerated, and the idea of fixed duration therapy is attractive, as are the long-term outcomes for many patients following cessation of therapy.
“But remember, if you take ibrutinib, you’ve got a 90% chance of going into remission, and an over 80% chance of being in remission 5 years later, so that’s pretty good as well,” he said.
Paolo P. Ghia, MD, from Univerista Vita-Salute San Raffaele in Milan, who has studied fixed-duration I+V in younger patients with CLL in the CAPTIVATE trial agreed that “in general, there is very little role for chemoimmunotherapy in CLL.”
But Dr. Ghia, who was not involved in the GLOW study, said in an interview that the results add to the growing body of evidence of the efficacy and safety of ibrutinib -venetoclax in a wide range of patients.
“Overall between the two studies [CAPTIVATE and GLOW] we have now over 400 patients who have been treated with the combination, and the message is rather similar in the two studies: you have a high frequency of undetectable MRD in peripheral blood and in the bone marrow, with a high concordance between the two tissues, and in particular we have a durability of the response,” he said.
GLOW details
Dr. Kater noted that ibrutinib and venetoclax have distinct and complementary modes of action, with ibrutinib mobilizing CLL cells out of their “protective lymphoid niches” and inhibiting their proliferation, as well as accelerating apoptosis by sensitizing cells to inhibition by the anti–B-cell lymphoma 2 (BCL-2) agent venetoclax. The combination leads to high levels of MRD negative by eliminating subpopulations of resting and dividing CLL cells.
The GLOW investigators enrolled 211 patients who were 65 or older, or were younger than 65 with a cumulative illness rating scale (CIRS) score of greater than 6, or creatinine clearance rate of less than 70 mL/min, and no known deletion 17p (del17p) or TP53 mutation.
The patients all had Eastern Cooperative Oncology Group performance status scores of 0-2.
After stratification by immunoglobulin heavy chain variable (IGHV) region genes and presence of deletion 11q (del11q), the patients were randomly assigned to either a three cycle run-in with ibrutinib 420 mg daily followed by ibrutinib plus venetoclax ramped up from 20 to 400 mg, or to chlorambucil 0.5 mg/kg on days and 15 for six cycles, and obinutuzumab 1,000 mg on days 1,2, 8, and 15 of cycle 1, and day 1 of cycles 2-6.
About one-third of patients in each study arm were 75 or older. Baseline characteristics were generally similar between the arms, except for a higher frequency of CIRS scores above 6 in the I+V arm, and a higher frequency of elevated lactate dehydrogenase in the Clb+O arm.
Superior PFS
As noted, the primary endpoint of PFS as assessed by independent review committee (IRC) after 27.7 months of follow-up had not been reached in I+V arm, compared with 21 months in the Clb+O arm, translating into a hazard ratio or progression with I+V of 0.21 (P < .0001). Investigator-assessed PFS was similar, with an HR of 2.07 (P < .0001).
PFS was superior with I+V across all subgroups, including age, baseline performance status. CIRS total score, Rai stage, bulky disease, elevated LDH at baseline, IGHV mutated or unmutated, and presence or absence of del(11q).
IRC-assessed combined complete response (CR) or CR with incomplete recovery of blood counts (CRi) rates were 38.7% with I+V vs. 11.4% with Clb+O (P < .0001).
Responses were also more durable with the oral combination, with 90% of patients having a sustained IRC-assessed response at 24 months, compared with 41% in the chemoimmunotherapy arm.
Rates of undetectable MRD by next-generation sequencing 3 months after the end of treatment were also significantly higher with I+V in both bone marrow (51.9% vs. 17.1%, respectively, P < .0001) and peripheral blood (54.7% vs. 39%, P = .0259).
One year post treatment 49% of patients assigned to I+V had undetectable MRD in peripheral blood, compared with 12% of patients assigned to Clb+O).
Safety
In all, 11 patients assigned to I+V discontinued treatment because of adverse events, compared with 2 in the Clb+O arm. Two patients in the I+V arm (1.9%) discontinued ibrutinib because of atrial fibrillation (AF). Serious adverse events in 5% or more of patients that were more frequent with I+V include infections (12.3% vs. 8.6% and AF (6.6% vs. o%). The tumor lysis syndrome (TLS) was not seen in the I+V arm, but occurred in 5.7% of patients in the Clb+O arm.
There were a total of 11 deaths in the I+V arm and 12 in the Clb+O arm during treatment or follow-up.
Causes of death were generally similar between the arms, with infections and cardiac events being the most common causes, Dr. Kater said.
Of the four deaths that occurred during ibrutinib lead-in, one was due to infection, one to metastatic carcinoma, and two due to cardiac disorders. Of the three that occurred in the I+V arm during treatment, two were from sudden death, and one from a nervous system disorder. Four patients in this arm died during follow-up, two from infections, one from sudden death, and one from progressive disease with Richter transformation.
In the Clb+O arm, one patient died during treatment from an infection and one died from hepatobiliary disease. Of the 10 that died during follow-up, 6 died from infections/infestations, 2 from cardiac disorders, and 1 each from nervous system and respiratory/thoracic/mediastinal disorder.
The study was supported by Janssen Research & Development. Dr. Kater disclosed advisory board activity, research committee, and steering committee participation for Janssen, and similar relationships with others. Dr. Zent disclosed research funding to the University of Rochester from AstraZenca/Acerta and TG Therpeutics. Dr. Ghia disclosed consultancy, honoraria, travel expenses, and research funding from Janssen and others.
For older, unfit patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), first-line treatment with the all-oral combination of ibrutinib (Imbruvica) and venetoclax (Venclexta) was associated with superior progression-free survival, compared with chlorambucil and obinutuzumab (Gazyva), results of the phase 3 GLOW trial showed.
Among 211 patients with CLL/SLL who were 65 or older, or younger patients with a high comorbidity burden, the median progression-free survival (PFS) after 27.7 months of follow-up was not reached for patients treated with a fixed duration combination of ibrutinib plus venetoclax (I+V), compared with 21 months for patients who received chlorambucil plus obinutuzumab (Clb+O), reported Arnon P. Kater, MD, PhD, from Amsterdam University Medical Centers.
The oral combination was also associated with a higher rate of undetectable minimal residual disease (MRD) 3 months after the end of treatment, at 51.9% vs. 17.1% with Clb+O, he said in a late-breaking abstract presented during the European Hematology Association annual meeting (Abstract LB1902).
“Overall, the results from GLOW support a positive clinical profile of I+V as an all-oral, once-daily, fixed-duration regimen in older patients with newly diagnosed CLL,” he said.
A low bar
But the bar for success in the GLOW trial may have been set low, with the older combination of chlorambucil plus obinutuzumab used as the comparator, rather than a more contemporary regimen, said Clive S. Zent , MD, from the Wilmot Cancer Institute at the University of Rochester (New York) Medical Center, who was not involved in the study.
“Really, nobody in this country that I’m aware of, certainly not in academic medicine, would be using chlorambucil and obinutuzumab or rituximab for standard of care,” Dr. Zent said in an interview.
“This is like comparing a mule cart to a Tesla,” he said.
Apart from possibly providing a rationale for using ibrutinib and venetoclax in this population, the GLOW results do not add much beyond that already in the CAPTIVE MRD trial, he added.
“What we’re really interested in, is ibrutinib and venetoclax better than ibrutinib alone or venetoclax alone? And that has not been asked or answered yet,” he said.
Dr. Zent acknowledged that the combination works very well and is well tolerated, and the idea of fixed duration therapy is attractive, as are the long-term outcomes for many patients following cessation of therapy.
“But remember, if you take ibrutinib, you’ve got a 90% chance of going into remission, and an over 80% chance of being in remission 5 years later, so that’s pretty good as well,” he said.
Paolo P. Ghia, MD, from Univerista Vita-Salute San Raffaele in Milan, who has studied fixed-duration I+V in younger patients with CLL in the CAPTIVATE trial agreed that “in general, there is very little role for chemoimmunotherapy in CLL.”
But Dr. Ghia, who was not involved in the GLOW study, said in an interview that the results add to the growing body of evidence of the efficacy and safety of ibrutinib -venetoclax in a wide range of patients.
“Overall between the two studies [CAPTIVATE and GLOW] we have now over 400 patients who have been treated with the combination, and the message is rather similar in the two studies: you have a high frequency of undetectable MRD in peripheral blood and in the bone marrow, with a high concordance between the two tissues, and in particular we have a durability of the response,” he said.
GLOW details
Dr. Kater noted that ibrutinib and venetoclax have distinct and complementary modes of action, with ibrutinib mobilizing CLL cells out of their “protective lymphoid niches” and inhibiting their proliferation, as well as accelerating apoptosis by sensitizing cells to inhibition by the anti–B-cell lymphoma 2 (BCL-2) agent venetoclax. The combination leads to high levels of MRD negative by eliminating subpopulations of resting and dividing CLL cells.
The GLOW investigators enrolled 211 patients who were 65 or older, or were younger than 65 with a cumulative illness rating scale (CIRS) score of greater than 6, or creatinine clearance rate of less than 70 mL/min, and no known deletion 17p (del17p) or TP53 mutation.
The patients all had Eastern Cooperative Oncology Group performance status scores of 0-2.
After stratification by immunoglobulin heavy chain variable (IGHV) region genes and presence of deletion 11q (del11q), the patients were randomly assigned to either a three cycle run-in with ibrutinib 420 mg daily followed by ibrutinib plus venetoclax ramped up from 20 to 400 mg, or to chlorambucil 0.5 mg/kg on days and 15 for six cycles, and obinutuzumab 1,000 mg on days 1,2, 8, and 15 of cycle 1, and day 1 of cycles 2-6.
About one-third of patients in each study arm were 75 or older. Baseline characteristics were generally similar between the arms, except for a higher frequency of CIRS scores above 6 in the I+V arm, and a higher frequency of elevated lactate dehydrogenase in the Clb+O arm.
Superior PFS
As noted, the primary endpoint of PFS as assessed by independent review committee (IRC) after 27.7 months of follow-up had not been reached in I+V arm, compared with 21 months in the Clb+O arm, translating into a hazard ratio or progression with I+V of 0.21 (P < .0001). Investigator-assessed PFS was similar, with an HR of 2.07 (P < .0001).
PFS was superior with I+V across all subgroups, including age, baseline performance status. CIRS total score, Rai stage, bulky disease, elevated LDH at baseline, IGHV mutated or unmutated, and presence or absence of del(11q).
IRC-assessed combined complete response (CR) or CR with incomplete recovery of blood counts (CRi) rates were 38.7% with I+V vs. 11.4% with Clb+O (P < .0001).
Responses were also more durable with the oral combination, with 90% of patients having a sustained IRC-assessed response at 24 months, compared with 41% in the chemoimmunotherapy arm.
Rates of undetectable MRD by next-generation sequencing 3 months after the end of treatment were also significantly higher with I+V in both bone marrow (51.9% vs. 17.1%, respectively, P < .0001) and peripheral blood (54.7% vs. 39%, P = .0259).
One year post treatment 49% of patients assigned to I+V had undetectable MRD in peripheral blood, compared with 12% of patients assigned to Clb+O).
Safety
In all, 11 patients assigned to I+V discontinued treatment because of adverse events, compared with 2 in the Clb+O arm. Two patients in the I+V arm (1.9%) discontinued ibrutinib because of atrial fibrillation (AF). Serious adverse events in 5% or more of patients that were more frequent with I+V include infections (12.3% vs. 8.6% and AF (6.6% vs. o%). The tumor lysis syndrome (TLS) was not seen in the I+V arm, but occurred in 5.7% of patients in the Clb+O arm.
There were a total of 11 deaths in the I+V arm and 12 in the Clb+O arm during treatment or follow-up.
Causes of death were generally similar between the arms, with infections and cardiac events being the most common causes, Dr. Kater said.
Of the four deaths that occurred during ibrutinib lead-in, one was due to infection, one to metastatic carcinoma, and two due to cardiac disorders. Of the three that occurred in the I+V arm during treatment, two were from sudden death, and one from a nervous system disorder. Four patients in this arm died during follow-up, two from infections, one from sudden death, and one from progressive disease with Richter transformation.
In the Clb+O arm, one patient died during treatment from an infection and one died from hepatobiliary disease. Of the 10 that died during follow-up, 6 died from infections/infestations, 2 from cardiac disorders, and 1 each from nervous system and respiratory/thoracic/mediastinal disorder.
The study was supported by Janssen Research & Development. Dr. Kater disclosed advisory board activity, research committee, and steering committee participation for Janssen, and similar relationships with others. Dr. Zent disclosed research funding to the University of Rochester from AstraZenca/Acerta and TG Therpeutics. Dr. Ghia disclosed consultancy, honoraria, travel expenses, and research funding from Janssen and others.
FROM EHA 2021
OSHA issues new rules on COVID-19 safety for health care workers
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
Atrophic Lesions in a Pregnant Woman
The Diagnosis: Degos Disease
The pathophysiology of Degos disease (malignant atrophic papulosis) is unknown.1 Histopathology demonstrates a wedge-shaped area of dermal necrosis with edema and mucin deposition extending from the papillary dermis to the deep reticular dermis. Occluded vessels, thrombosis, and perivascular lymphocytic infiltrates also may be seen, particularly at the dermal subcutaneous junction and at the periphery of the wedge-shaped infarction. The vascular damage that occurs may be the result of vasculitis, coagulopathy, or endothelial cell dysfunction.1
Patients typically present with small, round, erythematous papules that eventually develop atrophic porcelain white centers and telangiectatic rims. These lesions most commonly occur on the trunk and arms. In the benign form of atrophic papulosis, only the skin is involved; however, systemic involvement of the gastrointestinal tract and central nervous system can occur, resulting in bowel perforation and stroke, respectively.1 Although there is no definitive treatment of Degos disease, successful therapy with aspirin or dipyridamole has been reported.1 Eculizumab, a monoclonal antibody that binds C5, and treprostinil, a prostacyclin analog, are emerging treatment options.2,3 The differential diagnosis of Degos disease may include granuloma annulare, guttate extragenital lichen sclerosus, livedoid vasculopathy, and lymphomatoid papulosis.
Granuloma annulare may clinically mimic the erythematous papules seen in early Degos disease, and histopathology can be used to distinguish between these two disease processes. Localized granuloma annulare is the most common variant and clinically presents as pink papules and plaques in an annular configuration.4 Histopathology demonstrates an unremarkable epidermis; however, the dermis contains degenerated collagen surrounded by palisading histiocytes as well as lymphocytes. Similar to Degos disease, increased mucin is seen within these areas of degeneration, but occluded vessels and thrombosis typically are not seen (Figure 1).4,5
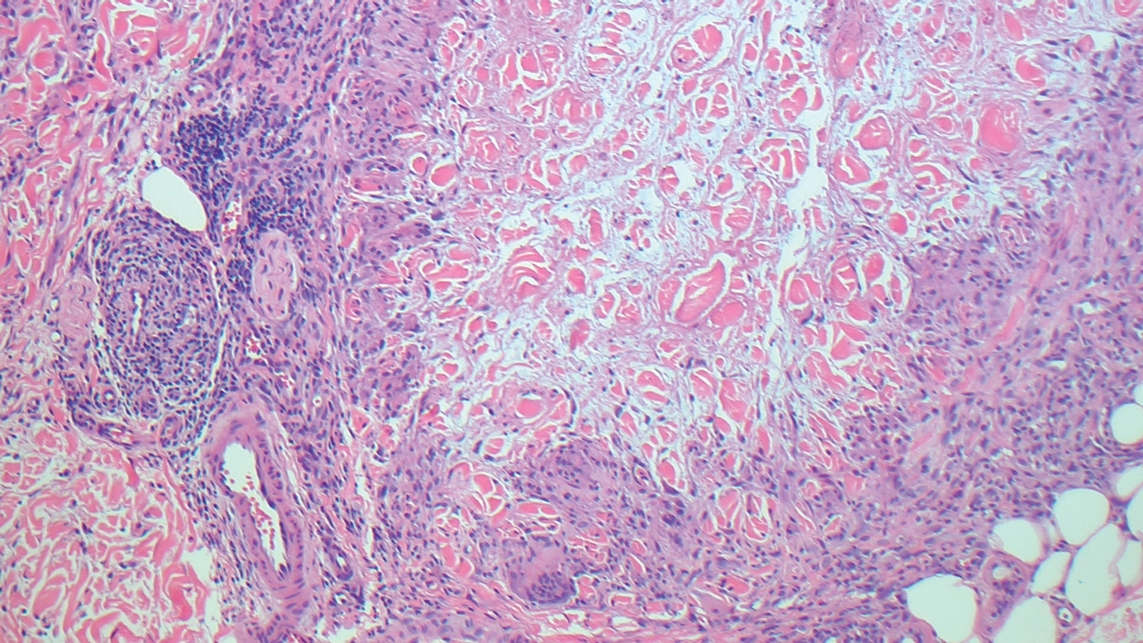
Guttate extragenital lichen sclerosus initially presents as polygonal, bluish white papules that coalesce into plaques.6 Over time, these lesions become more atrophic and may mimic Degos disease but appear differently on histopathology. Histopathology of lichen sclerosus classically demonstrates atrophy of the epidermis with loss of the rete ridges and vacuolar surface changes. Homogenization of the superficial/papillary dermis with an underlying bandlike lymphocytic infiltrate also is seen (Figure 2).6
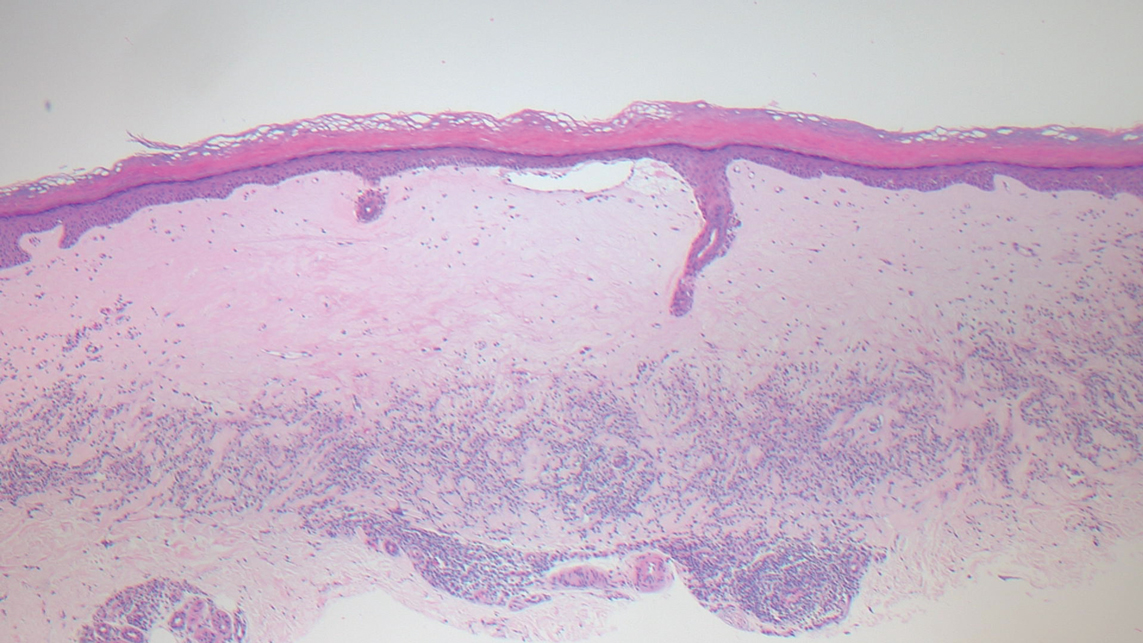
Livedoid vasculopathy is characterized by chronic recurrent ulceration of the legs secondary to thrombosis and subsequent ischemia. In the initial phase of this disease, livedo reticularis is seen followed by the development of ulcerations. As these ulcerations heal, they leave behind porcelain white scars referred to as atrophie blanche.7 The areas of scarring in livedoid vasculopathy are broad and angulated, differentiating them from the small, round, porcelain white macules in end-stage Degos disease. Histopathology demonstrates thrombosis and fibrin occlusion of the upper and mid dermal vessels. Very minimal perivascular infiltrate typically is seen, but when it is present, the infiltrate mostly is lymphocytic. Hyalinization of the vessel walls also is seen, particularly in the atrophie blanche stage (Figure 3).7
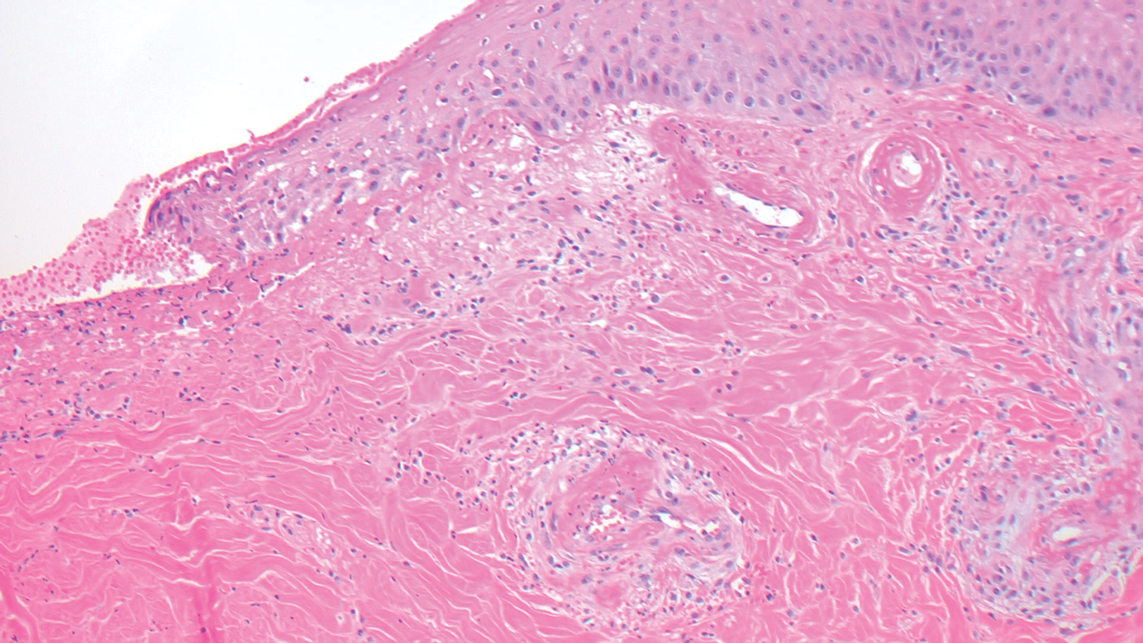
Lymphomatoid papulosis classically presents with pruritic red papules that often spontaneously involute. After resolution of the primary lesions, atrophic varioliform scars may be left behind that can resemble Degos disease.8 Classically, there are 5 histopathologic subtypes: A, B, C, D, and E. Type A is the most common type of lymphomatoid papulosis, and histopathology demonstrates a dermal lymphocytic infiltrate that consists of cells arranged in small clusters. Numerous medium- to large-sized atypical lymphocytes with prominent nucleoli and abundant cytoplasm are seen, and mitotic figures are common (Figure 4).8
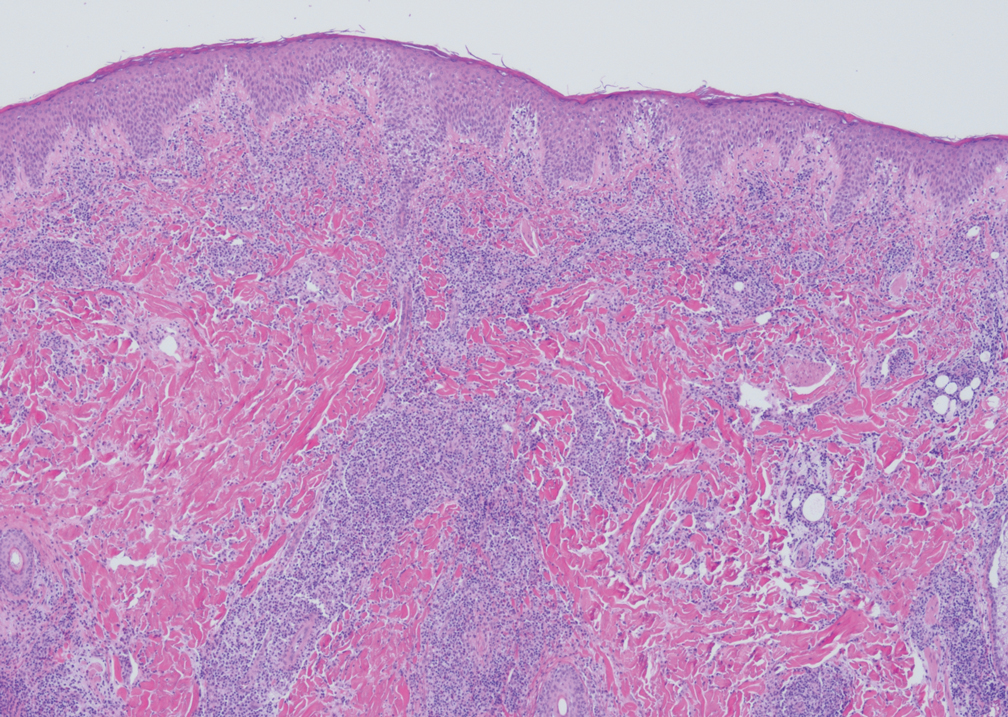
Our case was particularly interesting because the patient was 2 to 3 weeks pregnant. Degos disease in pregnancy appears to be quite exceptional. A PubMed search of articles indexed for MEDLINE using the terms Degos disease and pregnancy revealed only 4 other cases reported in the literature.9-12 With the exception of a single case that was complicated by severe abdominal pain requiring labor induction, the other reported cases resulted in uncomplicated pregnancies.9-12 Conversely, our patient's pregnancy was complicated by gestational hypertension and fetal hydrops requiring a preterm cesarean delivery. Furthermore, the infant had multiple complications, which were attributed to both placental insufficiency and a coagulopathic state.
Our patient also was found to have a heterozygous factor V Leiden mutation on workup. A PubMed search using the terms factor V Leiden mutation and Degos disease revealed 2 other cases of factor V Leiden mutation-associated Degos disease.13,14 The importance of factor V Leiden mutations in patients with Degos disease currently is unclear.
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)--a review. Orphanet J Rare Dis. 2013;8:10.
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277.
- Magro CM, Wang X, Garrett-Bakelman F, et al. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis. 2013;8:185.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Tronnier M, Mitteldorf C. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Dtsch Dermatol Ges. 2015;13:211-216.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478‐488.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73.
- Moulin G, Barrut D, Franc MP, et al. Familial Degos' atrophic papulosis (mother-daughter). Ann Dermatol Venereol. 1984;111:149-155.
- Bogenrieder T, Kuske M, Landthaler M, et al. Benign Degos' disease developing during pregnancy and followed for 10 years. Acta Derm Venereol. 2002;82:284-287.
- Sharma S, Brennan B, Naden R, et al. A case of Degos disease in pregnancy. Obstet Med. 2016;9:167-168.
- Zhao Q, Zhang S, Dong A. An unusual case of abdominal pain. Gastroenterology. 2018;154:E1-E2.
- Darwich E, Guilabert A, Mascaró JM Jr, et al. Dermoscopic description of a patient with thrombocythemia and factor V Leiden mutation-associated Degos' disease. Int J Dermatol. 2011;50:604-606.
- Hohwy T, Jensen MG, Tøttrup A, et al. A fatal case of malignant atrophic papulosis (Degos' disease) in a man with factor V Leiden mutation and lupus anticoagulant. Acta Derm Venereol. 2006;86:245-247.
The Diagnosis: Degos Disease
The pathophysiology of Degos disease (malignant atrophic papulosis) is unknown.1 Histopathology demonstrates a wedge-shaped area of dermal necrosis with edema and mucin deposition extending from the papillary dermis to the deep reticular dermis. Occluded vessels, thrombosis, and perivascular lymphocytic infiltrates also may be seen, particularly at the dermal subcutaneous junction and at the periphery of the wedge-shaped infarction. The vascular damage that occurs may be the result of vasculitis, coagulopathy, or endothelial cell dysfunction.1
Patients typically present with small, round, erythematous papules that eventually develop atrophic porcelain white centers and telangiectatic rims. These lesions most commonly occur on the trunk and arms. In the benign form of atrophic papulosis, only the skin is involved; however, systemic involvement of the gastrointestinal tract and central nervous system can occur, resulting in bowel perforation and stroke, respectively.1 Although there is no definitive treatment of Degos disease, successful therapy with aspirin or dipyridamole has been reported.1 Eculizumab, a monoclonal antibody that binds C5, and treprostinil, a prostacyclin analog, are emerging treatment options.2,3 The differential diagnosis of Degos disease may include granuloma annulare, guttate extragenital lichen sclerosus, livedoid vasculopathy, and lymphomatoid papulosis.
Granuloma annulare may clinically mimic the erythematous papules seen in early Degos disease, and histopathology can be used to distinguish between these two disease processes. Localized granuloma annulare is the most common variant and clinically presents as pink papules and plaques in an annular configuration.4 Histopathology demonstrates an unremarkable epidermis; however, the dermis contains degenerated collagen surrounded by palisading histiocytes as well as lymphocytes. Similar to Degos disease, increased mucin is seen within these areas of degeneration, but occluded vessels and thrombosis typically are not seen (Figure 1).4,5

Guttate extragenital lichen sclerosus initially presents as polygonal, bluish white papules that coalesce into plaques.6 Over time, these lesions become more atrophic and may mimic Degos disease but appear differently on histopathology. Histopathology of lichen sclerosus classically demonstrates atrophy of the epidermis with loss of the rete ridges and vacuolar surface changes. Homogenization of the superficial/papillary dermis with an underlying bandlike lymphocytic infiltrate also is seen (Figure 2).6

Livedoid vasculopathy is characterized by chronic recurrent ulceration of the legs secondary to thrombosis and subsequent ischemia. In the initial phase of this disease, livedo reticularis is seen followed by the development of ulcerations. As these ulcerations heal, they leave behind porcelain white scars referred to as atrophie blanche.7 The areas of scarring in livedoid vasculopathy are broad and angulated, differentiating them from the small, round, porcelain white macules in end-stage Degos disease. Histopathology demonstrates thrombosis and fibrin occlusion of the upper and mid dermal vessels. Very minimal perivascular infiltrate typically is seen, but when it is present, the infiltrate mostly is lymphocytic. Hyalinization of the vessel walls also is seen, particularly in the atrophie blanche stage (Figure 3).7

Lymphomatoid papulosis classically presents with pruritic red papules that often spontaneously involute. After resolution of the primary lesions, atrophic varioliform scars may be left behind that can resemble Degos disease.8 Classically, there are 5 histopathologic subtypes: A, B, C, D, and E. Type A is the most common type of lymphomatoid papulosis, and histopathology demonstrates a dermal lymphocytic infiltrate that consists of cells arranged in small clusters. Numerous medium- to large-sized atypical lymphocytes with prominent nucleoli and abundant cytoplasm are seen, and mitotic figures are common (Figure 4).8

Our case was particularly interesting because the patient was 2 to 3 weeks pregnant. Degos disease in pregnancy appears to be quite exceptional. A PubMed search of articles indexed for MEDLINE using the terms Degos disease and pregnancy revealed only 4 other cases reported in the literature.9-12 With the exception of a single case that was complicated by severe abdominal pain requiring labor induction, the other reported cases resulted in uncomplicated pregnancies.9-12 Conversely, our patient's pregnancy was complicated by gestational hypertension and fetal hydrops requiring a preterm cesarean delivery. Furthermore, the infant had multiple complications, which were attributed to both placental insufficiency and a coagulopathic state.
Our patient also was found to have a heterozygous factor V Leiden mutation on workup. A PubMed search using the terms factor V Leiden mutation and Degos disease revealed 2 other cases of factor V Leiden mutation-associated Degos disease.13,14 The importance of factor V Leiden mutations in patients with Degos disease currently is unclear.
The Diagnosis: Degos Disease
The pathophysiology of Degos disease (malignant atrophic papulosis) is unknown.1 Histopathology demonstrates a wedge-shaped area of dermal necrosis with edema and mucin deposition extending from the papillary dermis to the deep reticular dermis. Occluded vessels, thrombosis, and perivascular lymphocytic infiltrates also may be seen, particularly at the dermal subcutaneous junction and at the periphery of the wedge-shaped infarction. The vascular damage that occurs may be the result of vasculitis, coagulopathy, or endothelial cell dysfunction.1
Patients typically present with small, round, erythematous papules that eventually develop atrophic porcelain white centers and telangiectatic rims. These lesions most commonly occur on the trunk and arms. In the benign form of atrophic papulosis, only the skin is involved; however, systemic involvement of the gastrointestinal tract and central nervous system can occur, resulting in bowel perforation and stroke, respectively.1 Although there is no definitive treatment of Degos disease, successful therapy with aspirin or dipyridamole has been reported.1 Eculizumab, a monoclonal antibody that binds C5, and treprostinil, a prostacyclin analog, are emerging treatment options.2,3 The differential diagnosis of Degos disease may include granuloma annulare, guttate extragenital lichen sclerosus, livedoid vasculopathy, and lymphomatoid papulosis.
Granuloma annulare may clinically mimic the erythematous papules seen in early Degos disease, and histopathology can be used to distinguish between these two disease processes. Localized granuloma annulare is the most common variant and clinically presents as pink papules and plaques in an annular configuration.4 Histopathology demonstrates an unremarkable epidermis; however, the dermis contains degenerated collagen surrounded by palisading histiocytes as well as lymphocytes. Similar to Degos disease, increased mucin is seen within these areas of degeneration, but occluded vessels and thrombosis typically are not seen (Figure 1).4,5

Guttate extragenital lichen sclerosus initially presents as polygonal, bluish white papules that coalesce into plaques.6 Over time, these lesions become more atrophic and may mimic Degos disease but appear differently on histopathology. Histopathology of lichen sclerosus classically demonstrates atrophy of the epidermis with loss of the rete ridges and vacuolar surface changes. Homogenization of the superficial/papillary dermis with an underlying bandlike lymphocytic infiltrate also is seen (Figure 2).6

Livedoid vasculopathy is characterized by chronic recurrent ulceration of the legs secondary to thrombosis and subsequent ischemia. In the initial phase of this disease, livedo reticularis is seen followed by the development of ulcerations. As these ulcerations heal, they leave behind porcelain white scars referred to as atrophie blanche.7 The areas of scarring in livedoid vasculopathy are broad and angulated, differentiating them from the small, round, porcelain white macules in end-stage Degos disease. Histopathology demonstrates thrombosis and fibrin occlusion of the upper and mid dermal vessels. Very minimal perivascular infiltrate typically is seen, but when it is present, the infiltrate mostly is lymphocytic. Hyalinization of the vessel walls also is seen, particularly in the atrophie blanche stage (Figure 3).7

Lymphomatoid papulosis classically presents with pruritic red papules that often spontaneously involute. After resolution of the primary lesions, atrophic varioliform scars may be left behind that can resemble Degos disease.8 Classically, there are 5 histopathologic subtypes: A, B, C, D, and E. Type A is the most common type of lymphomatoid papulosis, and histopathology demonstrates a dermal lymphocytic infiltrate that consists of cells arranged in small clusters. Numerous medium- to large-sized atypical lymphocytes with prominent nucleoli and abundant cytoplasm are seen, and mitotic figures are common (Figure 4).8

Our case was particularly interesting because the patient was 2 to 3 weeks pregnant. Degos disease in pregnancy appears to be quite exceptional. A PubMed search of articles indexed for MEDLINE using the terms Degos disease and pregnancy revealed only 4 other cases reported in the literature.9-12 With the exception of a single case that was complicated by severe abdominal pain requiring labor induction, the other reported cases resulted in uncomplicated pregnancies.9-12 Conversely, our patient's pregnancy was complicated by gestational hypertension and fetal hydrops requiring a preterm cesarean delivery. Furthermore, the infant had multiple complications, which were attributed to both placental insufficiency and a coagulopathic state.
Our patient also was found to have a heterozygous factor V Leiden mutation on workup. A PubMed search using the terms factor V Leiden mutation and Degos disease revealed 2 other cases of factor V Leiden mutation-associated Degos disease.13,14 The importance of factor V Leiden mutations in patients with Degos disease currently is unclear.
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)--a review. Orphanet J Rare Dis. 2013;8:10.
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277.
- Magro CM, Wang X, Garrett-Bakelman F, et al. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis. 2013;8:185.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Tronnier M, Mitteldorf C. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Dtsch Dermatol Ges. 2015;13:211-216.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478‐488.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73.
- Moulin G, Barrut D, Franc MP, et al. Familial Degos' atrophic papulosis (mother-daughter). Ann Dermatol Venereol. 1984;111:149-155.
- Bogenrieder T, Kuske M, Landthaler M, et al. Benign Degos' disease developing during pregnancy and followed for 10 years. Acta Derm Venereol. 2002;82:284-287.
- Sharma S, Brennan B, Naden R, et al. A case of Degos disease in pregnancy. Obstet Med. 2016;9:167-168.
- Zhao Q, Zhang S, Dong A. An unusual case of abdominal pain. Gastroenterology. 2018;154:E1-E2.
- Darwich E, Guilabert A, Mascaró JM Jr, et al. Dermoscopic description of a patient with thrombocythemia and factor V Leiden mutation-associated Degos' disease. Int J Dermatol. 2011;50:604-606.
- Hohwy T, Jensen MG, Tøttrup A, et al. A fatal case of malignant atrophic papulosis (Degos' disease) in a man with factor V Leiden mutation and lupus anticoagulant. Acta Derm Venereol. 2006;86:245-247.
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)--a review. Orphanet J Rare Dis. 2013;8:10.
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277.
- Magro CM, Wang X, Garrett-Bakelman F, et al. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis. 2013;8:185.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Tronnier M, Mitteldorf C. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Dtsch Dermatol Ges. 2015;13:211-216.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478‐488.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73.
- Moulin G, Barrut D, Franc MP, et al. Familial Degos' atrophic papulosis (mother-daughter). Ann Dermatol Venereol. 1984;111:149-155.
- Bogenrieder T, Kuske M, Landthaler M, et al. Benign Degos' disease developing during pregnancy and followed for 10 years. Acta Derm Venereol. 2002;82:284-287.
- Sharma S, Brennan B, Naden R, et al. A case of Degos disease in pregnancy. Obstet Med. 2016;9:167-168.
- Zhao Q, Zhang S, Dong A. An unusual case of abdominal pain. Gastroenterology. 2018;154:E1-E2.
- Darwich E, Guilabert A, Mascaró JM Jr, et al. Dermoscopic description of a patient with thrombocythemia and factor V Leiden mutation-associated Degos' disease. Int J Dermatol. 2011;50:604-606.
- Hohwy T, Jensen MG, Tøttrup A, et al. A fatal case of malignant atrophic papulosis (Degos' disease) in a man with factor V Leiden mutation and lupus anticoagulant. Acta Derm Venereol. 2006;86:245-247.
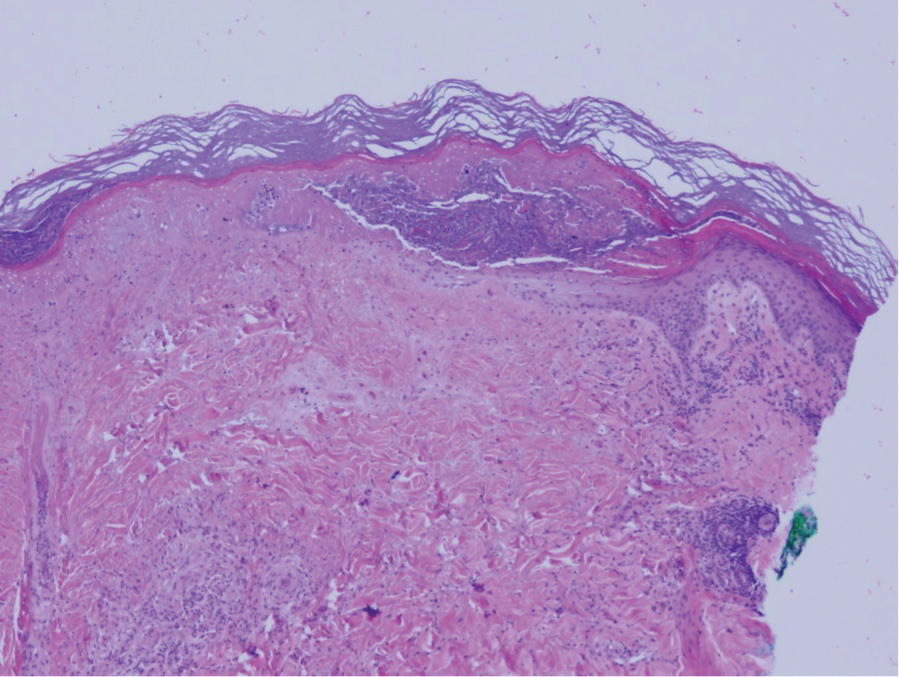
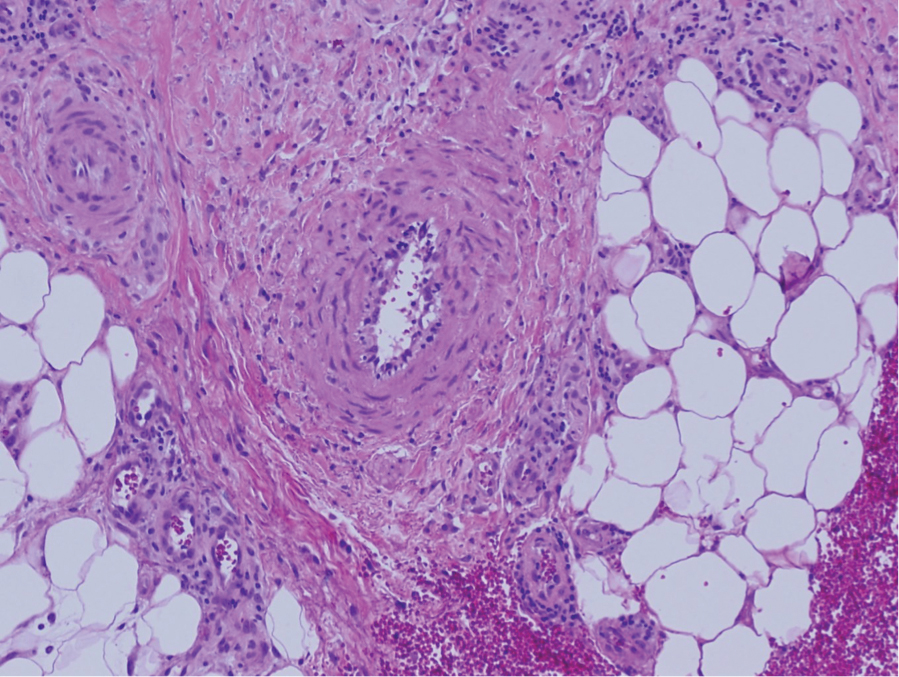
A 36-year-old pregnant woman presented with painful erythematous papules on the palms and fingers of 2 months’ duration. Similar lesions developed on the thighs and feet several weeks later. Two tender macules with central areas of porcelain white scarring rimmed by telangiectases on the right foot also were present. A punch biopsy of these lesions demonstrated a wedge-shaped area of ischemic necrosis associated with dermal mucin without associated necrobiosis. Fibrin thrombi were seen within several small dermal vessels and were associated with a perivascular lymphocytic infiltrate. Endotheliitis was observed within a deep dermal vessel. Laboratory workup including syphilis IgG, antinuclear antibodies, extractable nuclear antigen antibodies, anti–double-stranded DNA, antistreptolysin O antibodies, Russell viper venom time, cryoglobulin, hepatitis screening, perinuclear antineutrophil cytoplasmic antibodies (ANCA), and cytoplasmic ANCA was unremarkable. Hypercoagulable studies including prothrombin gene mutation, factor V Leiden, plasminogen, proteins C and S, antithrombin III, homocysteine, and antiphospholipid IgM and IgG antibodies were notable only for heterozygosity for factor V Leiden.
Moving more, sitting less vital for migraine patients
according to a presentation at the American Headache Society’s 2021 annual meeting.
Though reliable research is sparse overall on how much physical activity people with migraine get, enough exists to reveal the need for clinicians to help patients identify ways to increase their levels of physical activity and make it a habit, said Dale S. Bond, PhD, a professor of psychiatry and human behavior at the Miriam Hospital and Brown University, both in Providence, R.I.
He emphasized the need not only to replace sedentary time with physical activity but also to reduce sedentary time overall.
“It’s important to note that because active and sedentary represent different behavioral domains, people can still be active – that is, achieving recommended levels of moderate to vigorous physical activity [MVPA] – but still be highly sedentary because they sit for long hours throughout the day,” Dr. Bond said. “This is important because MVPA will not necessarily eliminate the health risks of long hours of sitting.”
Dr. Bond reviewed the existing literature on physical activity and sedentary behavior among patients with migraine. His presentation, “Move More, Sit Less,” aimed at finding ways to incorporate more physical activity into the daily lives of those with migraine. Dr. Bond began by briefly reviewing the well-established benefits of physical activity, including healthy sleep; cardiovascular, respiratory, musculoskeletal, mental, and cognitive health; and metabolic functioning.
“Physical activity and exercise in particular enhances the functioning of bodily systems, including those that have direct relevance to migraine in its comorbidities,” Dr. Bond said. “The positive systemic effects of exercise on bodily systems carries potential to reduce migraine severity and related disability and morbidity.”
He also explained the ways in which excessive sedentary time can exacerbate migraine triggers. “Long periods of interrupted sitting elevated levels of glucose and fat in the bloodstream, which in turn triggers the immune system to attack the body via inflammation,” Dr. Bond said. “Low grade chronic inflammation has long been hypothesized to play a role in migraine pathogenesis.”
Recommended levels of exercise
The World Health Organization and the U.S. Department of Health & Human Services recommends at least 150-300 minutes of moderate-intensity aerobic physical activity or 75-150 minutes of vigorous activity each week. An additional recommendation is at least 2 days per week of muscle strengthening activities that involve all major muscle groups.
While neither of those organizations has specific guidelines on how much reduction of sedentary time is recommended, the Canadian Society for Exercise Physiology recommends limiting sedentary time to 8 or fewer hours per day.
Exercise and migraine
“Unfortunately, at present, we have very few studies from which to draw conclusions about the extent to which individuals with migraine adhere to physical activity and sedentary guidelines,” Dr. Bond said. Existing studies vary widely in sample types, study design, physical activity measure and MVPA outcome, including the type or definition of MVPA. “This wide variability in measures and outcomes makes it challenging to draw any conclusions about adherence to guidelines among individuals with migraine,” he said.
Existing evidence suggests anywhere from 32% to 66% of migraine patients are at least moderately active, though it’s not clear what constitutes “moderately active” behavior. It appears that activity levels of patients with migraine are low overall, but it’s less clear the extent to which these levels are lower than in controls given the paucity of evidence.
In one of the few studies using objective measures to assess physical activity in migraine patients, the daily level of MVPA was significantly lower in 25 women with migraine than in 25 age- and body mass index–matched women without migraine (P <. 003). Both groups had obesity. The same study found that virtually no women with migraine adhered to the guidelines recommending less than 8 hours a day of sedentary time, compared with 30% of women without migraine.
“Also, low physical activity and high sedentary levels appear to be consistent across headache and nonheadache days,” Dr. Bond said. “This finding in particular raises an interesting question: If migraine severity is not related to physical activity and sedentary time, what is it about migraine that contributes to an inactive and sedentary lifestyle?”
Dr. Bond noted that future research needs to include reports of frequency, duration, and intensity of activities performed as well as the percentage of participants who meet guidelines for physical activity and sedentary time. Ideally, these studies should include not only self-report but also objective measures of activity as well as assess sleep and identify barriers and facilitators to physical activity in patients.
Exercise avoidance
Dr. Bond described findings from survey of 100 women he conducted to better understand potential barriers and reported that 78% of patients report intentionally avoiding physical activity. These patients typically avoided it an average of 4 days per week, regardless of intensity, and additional survey findings found “that participants who reported any avoidance had stronger beliefs that physical activity would both trigger and worsen a migraine attack, compared with participants who reported no avoidance,” he said.
That finding matches the clinical experience of Jennifer Robblee, MD, MSc, assistant professor of neurology at Barrow Neurological Institute in Phoenix, who viewed the presentation but was not involved with it.
“They often feel that it is a trigger for worsening an attack or, for some people, can actually trigger an attack, and that they feel worse in the midst of an attack when they’re exercising,” Dr. Robblee said in an interview regarding her patients who exercise less frequently. “Since so many of the patients I see have a daily and constant headache, it’s about how they can get themselves to start to exercise when something makes them feel worse, even if it makes them feel better in the long run.”
Yet, experimental research suggests that physical activity is not necessarily a reliable trigger of migraine attacks and only worsens migraine in a minority of attacks, Dr. Bond said, revealing an interesting paradox: “While engaging in regular physical activity is an important migraine management strategy, most individuals in the study reported doing the exact opposite – that is, avoiding physical activity as a management strategy – and this strategy was associated with higher frequency and duration of attacks. Research from our group and others also suggested individuals with migraine could be overestimating the role that physical activity hasn’t triggering or worsening of attacks.”
Encouraging patients to exercise
Since the benefits of physical activity and limiting sedentary time outweigh the potential harms, “some physical activity is better than none,” Dr. Bond said. To help patients begin increasing their physical activity, he recommended advising them to start with small amounts and then gradually increase frequency, intensity, and duration over time.
Dr. Robblee follows a similar approach, taking into account each patient’s particular circumstances and any medications they’re taking, including the side effects of those medications.
“It’s about starting where they are,” Dr. Robblee said. “Some patients, despite having severe migraine, have built themselves up so they’re doing exercise three or four times per week, or every day, and I have other people who never exercise,” she said. “For those patients who are very sedentary, if I can get them to start with 5 minutes per week so they have that sense of accomplishment, then that’s where I start. Then slowly build it up over time. Like most things in the migraine world, I individualize it for the person.”
Dr. Bond offered the following specific tips to clinicians in educating and encouraging patients to increase physical activity:
- Educate patients regarding the short-and long-term benefits of moving more and sitting less, both for their migraines and for overall health.
- Correct misconceptions about the negative effects of physical activity as it relates to migraines.
- Personalize the rationale for physical activity to that patient’s specific values and personal goals.
- Encourage patients to use an activity tracker, both for tracking physical activity and sedentary time, and to monitor migraine attacks, stress, energy levels, and fatigue on days they do and do not exercise.
- Help patients set goals for eventually meeting MVPA recommendations and interrupting prolonged periods of sitting with brief movement breaks.
- Help patients identify rewards for meeting goals that are tied to the activity, such as new exercise clothing.
- Encourage patients to identify a consistent time for physical activity each day to establish a habit, “ideally in the morning before barriers and life get in the way,” he said.
Eventually, physical activity itself should become intrinsically rewarding, Dr. Bond said.
“To limit sitting and encourage more movement throughout the day, we want to make the choice to engage in physical activity easier by adding environmental cues that encourage physical activity,” he said. “Conversely, we want to make the choice to engage in sedentary behavior more difficult by increasing the amount of effort that is required to engage in these behaviors.”
Dr. Robblee found Dr. Bond’s emphasis on sitting less – distinct from moving more – a helpful frame to consider with her patients. “I really like the approach of looking at it from that approach: in addition to how do we get you up and moving, how much time are you sitting, and how often can you break that up into smaller increments so that you’re up more often?” Dr. Robblee said. “That sometimes sounds less scary than ‘let’s get you exercising.’ So ‘let’s get you sitting a little bit less.’ I think that is something I might start to adopt.”
No external funding was noted. Dr. Robblee is a principal investigator for a study sponsored by Eli Lilly and receives stipends for MedLink Neurology and Neurodiem. Dr. Bond reported no disclosures.
according to a presentation at the American Headache Society’s 2021 annual meeting.
Though reliable research is sparse overall on how much physical activity people with migraine get, enough exists to reveal the need for clinicians to help patients identify ways to increase their levels of physical activity and make it a habit, said Dale S. Bond, PhD, a professor of psychiatry and human behavior at the Miriam Hospital and Brown University, both in Providence, R.I.
He emphasized the need not only to replace sedentary time with physical activity but also to reduce sedentary time overall.
“It’s important to note that because active and sedentary represent different behavioral domains, people can still be active – that is, achieving recommended levels of moderate to vigorous physical activity [MVPA] – but still be highly sedentary because they sit for long hours throughout the day,” Dr. Bond said. “This is important because MVPA will not necessarily eliminate the health risks of long hours of sitting.”
Dr. Bond reviewed the existing literature on physical activity and sedentary behavior among patients with migraine. His presentation, “Move More, Sit Less,” aimed at finding ways to incorporate more physical activity into the daily lives of those with migraine. Dr. Bond began by briefly reviewing the well-established benefits of physical activity, including healthy sleep; cardiovascular, respiratory, musculoskeletal, mental, and cognitive health; and metabolic functioning.
“Physical activity and exercise in particular enhances the functioning of bodily systems, including those that have direct relevance to migraine in its comorbidities,” Dr. Bond said. “The positive systemic effects of exercise on bodily systems carries potential to reduce migraine severity and related disability and morbidity.”
He also explained the ways in which excessive sedentary time can exacerbate migraine triggers. “Long periods of interrupted sitting elevated levels of glucose and fat in the bloodstream, which in turn triggers the immune system to attack the body via inflammation,” Dr. Bond said. “Low grade chronic inflammation has long been hypothesized to play a role in migraine pathogenesis.”
Recommended levels of exercise
The World Health Organization and the U.S. Department of Health & Human Services recommends at least 150-300 minutes of moderate-intensity aerobic physical activity or 75-150 minutes of vigorous activity each week. An additional recommendation is at least 2 days per week of muscle strengthening activities that involve all major muscle groups.
While neither of those organizations has specific guidelines on how much reduction of sedentary time is recommended, the Canadian Society for Exercise Physiology recommends limiting sedentary time to 8 or fewer hours per day.
Exercise and migraine
“Unfortunately, at present, we have very few studies from which to draw conclusions about the extent to which individuals with migraine adhere to physical activity and sedentary guidelines,” Dr. Bond said. Existing studies vary widely in sample types, study design, physical activity measure and MVPA outcome, including the type or definition of MVPA. “This wide variability in measures and outcomes makes it challenging to draw any conclusions about adherence to guidelines among individuals with migraine,” he said.
Existing evidence suggests anywhere from 32% to 66% of migraine patients are at least moderately active, though it’s not clear what constitutes “moderately active” behavior. It appears that activity levels of patients with migraine are low overall, but it’s less clear the extent to which these levels are lower than in controls given the paucity of evidence.
In one of the few studies using objective measures to assess physical activity in migraine patients, the daily level of MVPA was significantly lower in 25 women with migraine than in 25 age- and body mass index–matched women without migraine (P <. 003). Both groups had obesity. The same study found that virtually no women with migraine adhered to the guidelines recommending less than 8 hours a day of sedentary time, compared with 30% of women without migraine.
“Also, low physical activity and high sedentary levels appear to be consistent across headache and nonheadache days,” Dr. Bond said. “This finding in particular raises an interesting question: If migraine severity is not related to physical activity and sedentary time, what is it about migraine that contributes to an inactive and sedentary lifestyle?”
Dr. Bond noted that future research needs to include reports of frequency, duration, and intensity of activities performed as well as the percentage of participants who meet guidelines for physical activity and sedentary time. Ideally, these studies should include not only self-report but also objective measures of activity as well as assess sleep and identify barriers and facilitators to physical activity in patients.
Exercise avoidance
Dr. Bond described findings from survey of 100 women he conducted to better understand potential barriers and reported that 78% of patients report intentionally avoiding physical activity. These patients typically avoided it an average of 4 days per week, regardless of intensity, and additional survey findings found “that participants who reported any avoidance had stronger beliefs that physical activity would both trigger and worsen a migraine attack, compared with participants who reported no avoidance,” he said.
That finding matches the clinical experience of Jennifer Robblee, MD, MSc, assistant professor of neurology at Barrow Neurological Institute in Phoenix, who viewed the presentation but was not involved with it.
“They often feel that it is a trigger for worsening an attack or, for some people, can actually trigger an attack, and that they feel worse in the midst of an attack when they’re exercising,” Dr. Robblee said in an interview regarding her patients who exercise less frequently. “Since so many of the patients I see have a daily and constant headache, it’s about how they can get themselves to start to exercise when something makes them feel worse, even if it makes them feel better in the long run.”
Yet, experimental research suggests that physical activity is not necessarily a reliable trigger of migraine attacks and only worsens migraine in a minority of attacks, Dr. Bond said, revealing an interesting paradox: “While engaging in regular physical activity is an important migraine management strategy, most individuals in the study reported doing the exact opposite – that is, avoiding physical activity as a management strategy – and this strategy was associated with higher frequency and duration of attacks. Research from our group and others also suggested individuals with migraine could be overestimating the role that physical activity hasn’t triggering or worsening of attacks.”
Encouraging patients to exercise
Since the benefits of physical activity and limiting sedentary time outweigh the potential harms, “some physical activity is better than none,” Dr. Bond said. To help patients begin increasing their physical activity, he recommended advising them to start with small amounts and then gradually increase frequency, intensity, and duration over time.
Dr. Robblee follows a similar approach, taking into account each patient’s particular circumstances and any medications they’re taking, including the side effects of those medications.
“It’s about starting where they are,” Dr. Robblee said. “Some patients, despite having severe migraine, have built themselves up so they’re doing exercise three or four times per week, or every day, and I have other people who never exercise,” she said. “For those patients who are very sedentary, if I can get them to start with 5 minutes per week so they have that sense of accomplishment, then that’s where I start. Then slowly build it up over time. Like most things in the migraine world, I individualize it for the person.”
Dr. Bond offered the following specific tips to clinicians in educating and encouraging patients to increase physical activity:
- Educate patients regarding the short-and long-term benefits of moving more and sitting less, both for their migraines and for overall health.
- Correct misconceptions about the negative effects of physical activity as it relates to migraines.
- Personalize the rationale for physical activity to that patient’s specific values and personal goals.
- Encourage patients to use an activity tracker, both for tracking physical activity and sedentary time, and to monitor migraine attacks, stress, energy levels, and fatigue on days they do and do not exercise.
- Help patients set goals for eventually meeting MVPA recommendations and interrupting prolonged periods of sitting with brief movement breaks.
- Help patients identify rewards for meeting goals that are tied to the activity, such as new exercise clothing.
- Encourage patients to identify a consistent time for physical activity each day to establish a habit, “ideally in the morning before barriers and life get in the way,” he said.
Eventually, physical activity itself should become intrinsically rewarding, Dr. Bond said.
“To limit sitting and encourage more movement throughout the day, we want to make the choice to engage in physical activity easier by adding environmental cues that encourage physical activity,” he said. “Conversely, we want to make the choice to engage in sedentary behavior more difficult by increasing the amount of effort that is required to engage in these behaviors.”
Dr. Robblee found Dr. Bond’s emphasis on sitting less – distinct from moving more – a helpful frame to consider with her patients. “I really like the approach of looking at it from that approach: in addition to how do we get you up and moving, how much time are you sitting, and how often can you break that up into smaller increments so that you’re up more often?” Dr. Robblee said. “That sometimes sounds less scary than ‘let’s get you exercising.’ So ‘let’s get you sitting a little bit less.’ I think that is something I might start to adopt.”
No external funding was noted. Dr. Robblee is a principal investigator for a study sponsored by Eli Lilly and receives stipends for MedLink Neurology and Neurodiem. Dr. Bond reported no disclosures.
according to a presentation at the American Headache Society’s 2021 annual meeting.
Though reliable research is sparse overall on how much physical activity people with migraine get, enough exists to reveal the need for clinicians to help patients identify ways to increase their levels of physical activity and make it a habit, said Dale S. Bond, PhD, a professor of psychiatry and human behavior at the Miriam Hospital and Brown University, both in Providence, R.I.
He emphasized the need not only to replace sedentary time with physical activity but also to reduce sedentary time overall.
“It’s important to note that because active and sedentary represent different behavioral domains, people can still be active – that is, achieving recommended levels of moderate to vigorous physical activity [MVPA] – but still be highly sedentary because they sit for long hours throughout the day,” Dr. Bond said. “This is important because MVPA will not necessarily eliminate the health risks of long hours of sitting.”
Dr. Bond reviewed the existing literature on physical activity and sedentary behavior among patients with migraine. His presentation, “Move More, Sit Less,” aimed at finding ways to incorporate more physical activity into the daily lives of those with migraine. Dr. Bond began by briefly reviewing the well-established benefits of physical activity, including healthy sleep; cardiovascular, respiratory, musculoskeletal, mental, and cognitive health; and metabolic functioning.
“Physical activity and exercise in particular enhances the functioning of bodily systems, including those that have direct relevance to migraine in its comorbidities,” Dr. Bond said. “The positive systemic effects of exercise on bodily systems carries potential to reduce migraine severity and related disability and morbidity.”
He also explained the ways in which excessive sedentary time can exacerbate migraine triggers. “Long periods of interrupted sitting elevated levels of glucose and fat in the bloodstream, which in turn triggers the immune system to attack the body via inflammation,” Dr. Bond said. “Low grade chronic inflammation has long been hypothesized to play a role in migraine pathogenesis.”
Recommended levels of exercise
The World Health Organization and the U.S. Department of Health & Human Services recommends at least 150-300 minutes of moderate-intensity aerobic physical activity or 75-150 minutes of vigorous activity each week. An additional recommendation is at least 2 days per week of muscle strengthening activities that involve all major muscle groups.
While neither of those organizations has specific guidelines on how much reduction of sedentary time is recommended, the Canadian Society for Exercise Physiology recommends limiting sedentary time to 8 or fewer hours per day.
Exercise and migraine
“Unfortunately, at present, we have very few studies from which to draw conclusions about the extent to which individuals with migraine adhere to physical activity and sedentary guidelines,” Dr. Bond said. Existing studies vary widely in sample types, study design, physical activity measure and MVPA outcome, including the type or definition of MVPA. “This wide variability in measures and outcomes makes it challenging to draw any conclusions about adherence to guidelines among individuals with migraine,” he said.
Existing evidence suggests anywhere from 32% to 66% of migraine patients are at least moderately active, though it’s not clear what constitutes “moderately active” behavior. It appears that activity levels of patients with migraine are low overall, but it’s less clear the extent to which these levels are lower than in controls given the paucity of evidence.
In one of the few studies using objective measures to assess physical activity in migraine patients, the daily level of MVPA was significantly lower in 25 women with migraine than in 25 age- and body mass index–matched women without migraine (P <. 003). Both groups had obesity. The same study found that virtually no women with migraine adhered to the guidelines recommending less than 8 hours a day of sedentary time, compared with 30% of women without migraine.
“Also, low physical activity and high sedentary levels appear to be consistent across headache and nonheadache days,” Dr. Bond said. “This finding in particular raises an interesting question: If migraine severity is not related to physical activity and sedentary time, what is it about migraine that contributes to an inactive and sedentary lifestyle?”
Dr. Bond noted that future research needs to include reports of frequency, duration, and intensity of activities performed as well as the percentage of participants who meet guidelines for physical activity and sedentary time. Ideally, these studies should include not only self-report but also objective measures of activity as well as assess sleep and identify barriers and facilitators to physical activity in patients.
Exercise avoidance
Dr. Bond described findings from survey of 100 women he conducted to better understand potential barriers and reported that 78% of patients report intentionally avoiding physical activity. These patients typically avoided it an average of 4 days per week, regardless of intensity, and additional survey findings found “that participants who reported any avoidance had stronger beliefs that physical activity would both trigger and worsen a migraine attack, compared with participants who reported no avoidance,” he said.
That finding matches the clinical experience of Jennifer Robblee, MD, MSc, assistant professor of neurology at Barrow Neurological Institute in Phoenix, who viewed the presentation but was not involved with it.
“They often feel that it is a trigger for worsening an attack or, for some people, can actually trigger an attack, and that they feel worse in the midst of an attack when they’re exercising,” Dr. Robblee said in an interview regarding her patients who exercise less frequently. “Since so many of the patients I see have a daily and constant headache, it’s about how they can get themselves to start to exercise when something makes them feel worse, even if it makes them feel better in the long run.”
Yet, experimental research suggests that physical activity is not necessarily a reliable trigger of migraine attacks and only worsens migraine in a minority of attacks, Dr. Bond said, revealing an interesting paradox: “While engaging in regular physical activity is an important migraine management strategy, most individuals in the study reported doing the exact opposite – that is, avoiding physical activity as a management strategy – and this strategy was associated with higher frequency and duration of attacks. Research from our group and others also suggested individuals with migraine could be overestimating the role that physical activity hasn’t triggering or worsening of attacks.”
Encouraging patients to exercise
Since the benefits of physical activity and limiting sedentary time outweigh the potential harms, “some physical activity is better than none,” Dr. Bond said. To help patients begin increasing their physical activity, he recommended advising them to start with small amounts and then gradually increase frequency, intensity, and duration over time.
Dr. Robblee follows a similar approach, taking into account each patient’s particular circumstances and any medications they’re taking, including the side effects of those medications.
“It’s about starting where they are,” Dr. Robblee said. “Some patients, despite having severe migraine, have built themselves up so they’re doing exercise three or four times per week, or every day, and I have other people who never exercise,” she said. “For those patients who are very sedentary, if I can get them to start with 5 minutes per week so they have that sense of accomplishment, then that’s where I start. Then slowly build it up over time. Like most things in the migraine world, I individualize it for the person.”
Dr. Bond offered the following specific tips to clinicians in educating and encouraging patients to increase physical activity:
- Educate patients regarding the short-and long-term benefits of moving more and sitting less, both for their migraines and for overall health.
- Correct misconceptions about the negative effects of physical activity as it relates to migraines.
- Personalize the rationale for physical activity to that patient’s specific values and personal goals.
- Encourage patients to use an activity tracker, both for tracking physical activity and sedentary time, and to monitor migraine attacks, stress, energy levels, and fatigue on days they do and do not exercise.
- Help patients set goals for eventually meeting MVPA recommendations and interrupting prolonged periods of sitting with brief movement breaks.
- Help patients identify rewards for meeting goals that are tied to the activity, such as new exercise clothing.
- Encourage patients to identify a consistent time for physical activity each day to establish a habit, “ideally in the morning before barriers and life get in the way,” he said.
Eventually, physical activity itself should become intrinsically rewarding, Dr. Bond said.
“To limit sitting and encourage more movement throughout the day, we want to make the choice to engage in physical activity easier by adding environmental cues that encourage physical activity,” he said. “Conversely, we want to make the choice to engage in sedentary behavior more difficult by increasing the amount of effort that is required to engage in these behaviors.”
Dr. Robblee found Dr. Bond’s emphasis on sitting less – distinct from moving more – a helpful frame to consider with her patients. “I really like the approach of looking at it from that approach: in addition to how do we get you up and moving, how much time are you sitting, and how often can you break that up into smaller increments so that you’re up more often?” Dr. Robblee said. “That sometimes sounds less scary than ‘let’s get you exercising.’ So ‘let’s get you sitting a little bit less.’ I think that is something I might start to adopt.”
No external funding was noted. Dr. Robblee is a principal investigator for a study sponsored by Eli Lilly and receives stipends for MedLink Neurology and Neurodiem. Dr. Bond reported no disclosures.
FROM AHS 2021
Topical histone deacetylase inhibitor reduced BCC size in phase 2 study
according to research presented at the annual meeting of the Society for Investigative Dermatology.
“Our results demonstrate a clinically significant decrease in tumor size in response to 6 weeks of topical 1% remetinostat therapy in 70% of per-protocol tumors, with 55% reaching complete pathological resolution,” James M. Kilgour, MD, a postdoctoral research fellow at the Sarin Lab at Stanford (Calif.) University, said at the meeting.
Surgical excision is the preferred treatment for BCC, but there is still a need for noninvasive treatment options, Dr. Kilgour said. “Given the potential morbidity associated with excision, particularly for patients experiencing multiple or recurrent tumors, such as the immunosuppressed or patients with Gorlin syndrome, an effective and tolerable topical therapy would be a significant benefit,” he noted.
Previously, in an in silico screen experiment, Dr. Kilgour and colleagues identified HDAC inhibitors as a “top predicted therapeutic” for BCC treatment, and found that in mice studies, HDAC inhibitors were able to suppress the growth of BCC cell lines and BCC allografts.
Remetinostat, a pan-HDAC inhibitor, is being investigated as a treatment for cutaneous T-cell lymphoma.
HDAC inhibitors are thought to “alter expression of key oncogenes and tumor suppressors through epigenetic modification of histone and nonhistone proteins,” he noted.
To evaluate the efficacy of topical remetinostat for BCC, the investigators enrolled 30 patients with 49 BCC tumors in a phase 2, open-label, single-arm trial. Participants had tumors that were greater than 5 mm in diameter and had been referred to surgery at Stanford before enrollment. Patients were a mean of 59 years old, 63% were men, and 90% were White; 59.2% of participants had tumors with a diameter greater than 10 mm, the rest had tumors with a diameter of 10 mm or less.
After the tumors were photographed and measured, participants received 6 weeks of topical remetinostat therapy, followed by final measurement and photography of the tumors at 8 weeks and surgical excision. Topical remetinostat 1% gel was applied three times per day under bandage occlusion.
Overall, 25 participants with 33 tumors were included in the per-protocol analysis. At 8 weeks, there was at least a 30% decrease in diameter from baseline for 69.7% of tumors in these patients, with 17 of 33 tumors showing a complete response by week 8.
Regarding tumor subtypes, there was a 100% overall response rate for the 6 superficial BCC tumors (1 partial response, 5 complete responses), a 68.2% ORR for the 22 nodular tumors (5 partial responses, 10 complete responses), and a 66.7% ORR for the 3 infiltrative tumors (no partial responses, 2 complete responses). There were no partial or complete responses for the two micronodular tumors.
Most adverse events in the study were localized drug reactions, with no serious or systemic adverse events, Dr. Kilgour noted, with 10 tumors demonstrating either no reaction or a grade 1 reaction, and 23 tumors having a grade 2 or grade 3 response.
The investigators also used imaging (ImageJ) software to evaluate the average decrease in cross-sectional tumor area. The results of the analysis showed an average decrease at 8 weeks from baseline of 71.5%. In addition, histological assessment at 8 weeks demonstrated that 54.8% of tumors had complete pathological resolution.
“In the future, we advocate for a follow-up blinded, randomized, controlled trial of remetinostat with greater participant diversity,” Dr. Kilgour said. “Specifically, greater power is needed to understand which histological subtypes of BCC will respond best to the treatment and we need to understand the long-term durability of tumor resolution.”
Remetinostat promising as topical BCC therapy
In an interview, Beth G. Goldstein, MD, a dermatologist and Mohs surgeon in Chapel Hill, N.C., noted that the preliminary study was “an exciting report of a safe, well-tolerated nonsurgical option for patients with superficial BCCs on the trunk and extremities,” which has potential to be used in the future for nonsuperficial BCCs. The study shows that superficial BCCs can respond at a rate of 100% on nonfacial areas, said Dr. Goldstein, who was not involved in the research. “These lesions can be quite large with higher chances of recurrence and difficulty with wound care.
“This type of directed therapy hopefully continues to be perfected for treatment of BCC that avoids scarring and is well tolerated,” she added.
Dr. Goldstein commented that complete pathological resolution of 54.8% “was still unacceptably low for the remaining tumor types.” For those cases, she said remetinostat could possibly “provide a topical option as an adjunctive treatment for potentially reducing the size of the BCC prior to surgical removal,” as an alternative to a systemic therapy like vismodegib (Erivedge).
Dr. Goldstein said the strengths of the study were in the variety of tumors, close follow-up with histologic evaluation and safety signals. In terms of limitations, she said whether there were any cases of Gorlin syndrome was not clear. In addition, at least 30% of BCCs have a mixed tumor type on Mohs surgery that differs from the original biopsy, and “there was no mention if the residual tumor remained with the same histology,” she said.
In the future, a large, randomized trial is warranted to stratify for Gorlin syndrome, patients who are immunosuppressed, and additional tumor types that were underrepresented in this study, such as micronodular tumors, Dr. Goldstein said. Future studies also should examine how remetinostat impacts BCC in facial areas, the effect of multiple applications, and how the therapy performs as an adjunctive treatment before surgery.
This study was funded by Medivir, the Damon Runyon Foundation, National Cancer Institute, an American Skin Association Hambrick Medical Student grant, and Stanford Medical Scholars. Dr. Kilgour and Dr. Goldstein reported no relevant financial disclosures.
according to research presented at the annual meeting of the Society for Investigative Dermatology.
“Our results demonstrate a clinically significant decrease in tumor size in response to 6 weeks of topical 1% remetinostat therapy in 70% of per-protocol tumors, with 55% reaching complete pathological resolution,” James M. Kilgour, MD, a postdoctoral research fellow at the Sarin Lab at Stanford (Calif.) University, said at the meeting.
Surgical excision is the preferred treatment for BCC, but there is still a need for noninvasive treatment options, Dr. Kilgour said. “Given the potential morbidity associated with excision, particularly for patients experiencing multiple or recurrent tumors, such as the immunosuppressed or patients with Gorlin syndrome, an effective and tolerable topical therapy would be a significant benefit,” he noted.
Previously, in an in silico screen experiment, Dr. Kilgour and colleagues identified HDAC inhibitors as a “top predicted therapeutic” for BCC treatment, and found that in mice studies, HDAC inhibitors were able to suppress the growth of BCC cell lines and BCC allografts.
Remetinostat, a pan-HDAC inhibitor, is being investigated as a treatment for cutaneous T-cell lymphoma.
HDAC inhibitors are thought to “alter expression of key oncogenes and tumor suppressors through epigenetic modification of histone and nonhistone proteins,” he noted.
To evaluate the efficacy of topical remetinostat for BCC, the investigators enrolled 30 patients with 49 BCC tumors in a phase 2, open-label, single-arm trial. Participants had tumors that were greater than 5 mm in diameter and had been referred to surgery at Stanford before enrollment. Patients were a mean of 59 years old, 63% were men, and 90% were White; 59.2% of participants had tumors with a diameter greater than 10 mm, the rest had tumors with a diameter of 10 mm or less.
After the tumors were photographed and measured, participants received 6 weeks of topical remetinostat therapy, followed by final measurement and photography of the tumors at 8 weeks and surgical excision. Topical remetinostat 1% gel was applied three times per day under bandage occlusion.
Overall, 25 participants with 33 tumors were included in the per-protocol analysis. At 8 weeks, there was at least a 30% decrease in diameter from baseline for 69.7% of tumors in these patients, with 17 of 33 tumors showing a complete response by week 8.
Regarding tumor subtypes, there was a 100% overall response rate for the 6 superficial BCC tumors (1 partial response, 5 complete responses), a 68.2% ORR for the 22 nodular tumors (5 partial responses, 10 complete responses), and a 66.7% ORR for the 3 infiltrative tumors (no partial responses, 2 complete responses). There were no partial or complete responses for the two micronodular tumors.
Most adverse events in the study were localized drug reactions, with no serious or systemic adverse events, Dr. Kilgour noted, with 10 tumors demonstrating either no reaction or a grade 1 reaction, and 23 tumors having a grade 2 or grade 3 response.
The investigators also used imaging (ImageJ) software to evaluate the average decrease in cross-sectional tumor area. The results of the analysis showed an average decrease at 8 weeks from baseline of 71.5%. In addition, histological assessment at 8 weeks demonstrated that 54.8% of tumors had complete pathological resolution.
“In the future, we advocate for a follow-up blinded, randomized, controlled trial of remetinostat with greater participant diversity,” Dr. Kilgour said. “Specifically, greater power is needed to understand which histological subtypes of BCC will respond best to the treatment and we need to understand the long-term durability of tumor resolution.”
Remetinostat promising as topical BCC therapy
In an interview, Beth G. Goldstein, MD, a dermatologist and Mohs surgeon in Chapel Hill, N.C., noted that the preliminary study was “an exciting report of a safe, well-tolerated nonsurgical option for patients with superficial BCCs on the trunk and extremities,” which has potential to be used in the future for nonsuperficial BCCs. The study shows that superficial BCCs can respond at a rate of 100% on nonfacial areas, said Dr. Goldstein, who was not involved in the research. “These lesions can be quite large with higher chances of recurrence and difficulty with wound care.
“This type of directed therapy hopefully continues to be perfected for treatment of BCC that avoids scarring and is well tolerated,” she added.
Dr. Goldstein commented that complete pathological resolution of 54.8% “was still unacceptably low for the remaining tumor types.” For those cases, she said remetinostat could possibly “provide a topical option as an adjunctive treatment for potentially reducing the size of the BCC prior to surgical removal,” as an alternative to a systemic therapy like vismodegib (Erivedge).
Dr. Goldstein said the strengths of the study were in the variety of tumors, close follow-up with histologic evaluation and safety signals. In terms of limitations, she said whether there were any cases of Gorlin syndrome was not clear. In addition, at least 30% of BCCs have a mixed tumor type on Mohs surgery that differs from the original biopsy, and “there was no mention if the residual tumor remained with the same histology,” she said.
In the future, a large, randomized trial is warranted to stratify for Gorlin syndrome, patients who are immunosuppressed, and additional tumor types that were underrepresented in this study, such as micronodular tumors, Dr. Goldstein said. Future studies also should examine how remetinostat impacts BCC in facial areas, the effect of multiple applications, and how the therapy performs as an adjunctive treatment before surgery.
This study was funded by Medivir, the Damon Runyon Foundation, National Cancer Institute, an American Skin Association Hambrick Medical Student grant, and Stanford Medical Scholars. Dr. Kilgour and Dr. Goldstein reported no relevant financial disclosures.
according to research presented at the annual meeting of the Society for Investigative Dermatology.
“Our results demonstrate a clinically significant decrease in tumor size in response to 6 weeks of topical 1% remetinostat therapy in 70% of per-protocol tumors, with 55% reaching complete pathological resolution,” James M. Kilgour, MD, a postdoctoral research fellow at the Sarin Lab at Stanford (Calif.) University, said at the meeting.
Surgical excision is the preferred treatment for BCC, but there is still a need for noninvasive treatment options, Dr. Kilgour said. “Given the potential morbidity associated with excision, particularly for patients experiencing multiple or recurrent tumors, such as the immunosuppressed or patients with Gorlin syndrome, an effective and tolerable topical therapy would be a significant benefit,” he noted.
Previously, in an in silico screen experiment, Dr. Kilgour and colleagues identified HDAC inhibitors as a “top predicted therapeutic” for BCC treatment, and found that in mice studies, HDAC inhibitors were able to suppress the growth of BCC cell lines and BCC allografts.
Remetinostat, a pan-HDAC inhibitor, is being investigated as a treatment for cutaneous T-cell lymphoma.
HDAC inhibitors are thought to “alter expression of key oncogenes and tumor suppressors through epigenetic modification of histone and nonhistone proteins,” he noted.
To evaluate the efficacy of topical remetinostat for BCC, the investigators enrolled 30 patients with 49 BCC tumors in a phase 2, open-label, single-arm trial. Participants had tumors that were greater than 5 mm in diameter and had been referred to surgery at Stanford before enrollment. Patients were a mean of 59 years old, 63% were men, and 90% were White; 59.2% of participants had tumors with a diameter greater than 10 mm, the rest had tumors with a diameter of 10 mm or less.
After the tumors were photographed and measured, participants received 6 weeks of topical remetinostat therapy, followed by final measurement and photography of the tumors at 8 weeks and surgical excision. Topical remetinostat 1% gel was applied three times per day under bandage occlusion.
Overall, 25 participants with 33 tumors were included in the per-protocol analysis. At 8 weeks, there was at least a 30% decrease in diameter from baseline for 69.7% of tumors in these patients, with 17 of 33 tumors showing a complete response by week 8.
Regarding tumor subtypes, there was a 100% overall response rate for the 6 superficial BCC tumors (1 partial response, 5 complete responses), a 68.2% ORR for the 22 nodular tumors (5 partial responses, 10 complete responses), and a 66.7% ORR for the 3 infiltrative tumors (no partial responses, 2 complete responses). There were no partial or complete responses for the two micronodular tumors.
Most adverse events in the study were localized drug reactions, with no serious or systemic adverse events, Dr. Kilgour noted, with 10 tumors demonstrating either no reaction or a grade 1 reaction, and 23 tumors having a grade 2 or grade 3 response.
The investigators also used imaging (ImageJ) software to evaluate the average decrease in cross-sectional tumor area. The results of the analysis showed an average decrease at 8 weeks from baseline of 71.5%. In addition, histological assessment at 8 weeks demonstrated that 54.8% of tumors had complete pathological resolution.
“In the future, we advocate for a follow-up blinded, randomized, controlled trial of remetinostat with greater participant diversity,” Dr. Kilgour said. “Specifically, greater power is needed to understand which histological subtypes of BCC will respond best to the treatment and we need to understand the long-term durability of tumor resolution.”
Remetinostat promising as topical BCC therapy
In an interview, Beth G. Goldstein, MD, a dermatologist and Mohs surgeon in Chapel Hill, N.C., noted that the preliminary study was “an exciting report of a safe, well-tolerated nonsurgical option for patients with superficial BCCs on the trunk and extremities,” which has potential to be used in the future for nonsuperficial BCCs. The study shows that superficial BCCs can respond at a rate of 100% on nonfacial areas, said Dr. Goldstein, who was not involved in the research. “These lesions can be quite large with higher chances of recurrence and difficulty with wound care.
“This type of directed therapy hopefully continues to be perfected for treatment of BCC that avoids scarring and is well tolerated,” she added.
Dr. Goldstein commented that complete pathological resolution of 54.8% “was still unacceptably low for the remaining tumor types.” For those cases, she said remetinostat could possibly “provide a topical option as an adjunctive treatment for potentially reducing the size of the BCC prior to surgical removal,” as an alternative to a systemic therapy like vismodegib (Erivedge).
Dr. Goldstein said the strengths of the study were in the variety of tumors, close follow-up with histologic evaluation and safety signals. In terms of limitations, she said whether there were any cases of Gorlin syndrome was not clear. In addition, at least 30% of BCCs have a mixed tumor type on Mohs surgery that differs from the original biopsy, and “there was no mention if the residual tumor remained with the same histology,” she said.
In the future, a large, randomized trial is warranted to stratify for Gorlin syndrome, patients who are immunosuppressed, and additional tumor types that were underrepresented in this study, such as micronodular tumors, Dr. Goldstein said. Future studies also should examine how remetinostat impacts BCC in facial areas, the effect of multiple applications, and how the therapy performs as an adjunctive treatment before surgery.
This study was funded by Medivir, the Damon Runyon Foundation, National Cancer Institute, an American Skin Association Hambrick Medical Student grant, and Stanford Medical Scholars. Dr. Kilgour and Dr. Goldstein reported no relevant financial disclosures.
FROM SID 2021