User login
Promising HER2+/HR– breast cancer survival with de-escalated therapy
It may not be always necessary to approach the treatment of HER2-positive, hormone receptor–negative (HER2+/HR–) early breast cancer with added chemotherapy, survival results of a prospective multicenter randomized trial suggest.
In the ADAPT-HER2+/HR– trial, comparing a de-escalated 12-week neoadjuvant regimen consisting of dual HER2 blockade with trastuzumab (Herceptin) and pertuzumab (Perjeta) with or without weekly paclitaxel, the three-drug regimen was associated with high pathologic complete response(pCR) rates and excellent 5-year survival, irrespective of whether patients received additional chemotherapy, reported Nadia Harbeck, MD, PhD, of the University of Munich.
“Chemotherapy-free regimens are promising in highly sensitive tumors with early response, but future investigation of such chemotherapy-free regimens need to be focused on selected patients, like those with HER2 3+ tumors, non–basal-like tumors, those showing early response to the de-escalated therapy, and those with predictive RNA signatures such as immune signatures,” she said in an oral abstract session during the American Society of Clinical Oncology annual meeting (Abstract 503).
Under the WGS umbrella
The ADAPT HER2+/HR– trial (NCT01779206) is one of several conducted by the West German Study Group (WGS) on therapy for intrinsic breast cancer types.
In this study, 134 patients with HER2-positive, estrogen and progesterone receptor–negative tumors with no metastatic disease and good performance status were assigned on a 5:2 basis to neoadjuvant therapy with trastuzumab at a loading dose of 8 mg/kg for the first cycle followed by 6 mg/kg for subsequent cycles every 3 weeks x 4, plus pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks x 4 (92 patients), or to trastuzumab and pertuzumab at the same dose and schedule plus paclitaxel 80 mg/m2 once weekly for 12 weeks.
Patients had surgery within 3 weeks of the end of study therapy unless they did not have a histologically confirmed pCR, in which case they went on to receive standard neoadjuvant therapy prior to surgery.
Adjuvant therapy was performed according to national guidelines, although patients with a pCR after 12 weeks of study therapy could be spared from adjuvant chemotherapy at the investigator’s discretion.
Patients underwent biopsy at 3 weeks for therapy for early response assessment, defined as either a Ki67 decrease of at least 30% from baseline, or low cellularity (less than 500 invasive tumor cells).
First survival results
The investigators previously reported the primary pCR endpoint from the trial, which showed a rate of 90% after 12 weeks in the three-drug arm, and a “substantial and clinically meaningful” pCR rate of 34% after the trastuzumab plus pertuzumab alone.
At ASCO 2021, Dr. Harbeck reported the first survival data from the trial.
After a median follow-up of 59.9 months, there were no statistically significant differences between trial arms in either overall survival, invasive disease-free survival (iDFS), or distant disease-free survival (dDFS).
The 5-year iDFS rate in the three-drug arm was 98%, compared with 87% for the dual HER2 blockade-only arm, a difference that was not statistically significant.
The 5-year dDFS rates were 98% and 92% respectively. There were only seven dDFS events during follow-up, Dr. Harbeck noted.
There were only six deaths during follow-up, with overall survival rates of 98% in the paclitaxel-containing arm, and 94% in the anti-HER2 antibodies–only arm, a difference of one overall survival event, Dr. Harbeck said.
pCR counts
However, patients who did not have pathologic complete responses at the end of first-line de-escalated therapy had worse outcomes, with a 5-year iDFS rate of 82%, compared with 98% for patients who had achieved a pCR. This translated into a hazard ratio for invasive disease in patients with pCRs of 0.14 (P = .011).
This difference occurred despite the study requirement that all patients who did not have pCR after 12 weeks of initial therapy would receive additional chemotherapy.
Looking at the tumor subtype among patients in the paclitaxel-free arm to see whether they could identify predictors of early response, the researchers found a pCR rate of 36.5% among 85 patients with nonbasal tumors, but 0% among 7 patients with basal tumors.
The investigators identified a population of patients whose tumors could be considered nonsensitive to dual HER2 blockade alone: Those with basal tumors, those tumors with low immunohistochemical HER2 expression, and those without an early response to therapy on biopsy 3 weeks into initial therapy. Among 31 of the 92 patients in the dual HER2 arm who met this description, 2 had pCRs, Dr. Harbeck noted.
The 5-year iDFS rate among patients in the dual blockade–only arm with nonsensitive tumors was 79%, compared with 93% for patients with treatment-responsive types, although there were only 13 invasive events total in this arm.
“If we look at the whole trial population, the negative prognostic impact of what we termed nonsensitive tumors was even significant regarding dDFS, with a hazard ratio of about 5,” she said.
‘A consistent theme’
Invited discussant Lisa A. Carey, MD, ScM, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, noted that the trial was underpowered for outcomes, but that results nonetheless suggest that patients with strongly HER2-driven tumors might get comparable benefits from less chemotherapy.
“This trial included only hormone receptor–negative, HER2-positive tumors, and these we know are likely to be HER2-enriched in terms of subtype, about three-quarters of them,”she said.
The previously reported pCR rate of 90% in the paclitaxel-containing arm, with 80% of patients requiring no further chemotherapy, resulted in the excellent 5-year iDFS and dDFS in this group, despite the relatively highly clinical stage, with about 60% of patients having clinical stage 2 or higher tumors, and more than 40% being node positive.
The idea that pCR itself can predict which patients could be spared from more intensive chemotherapy “is starting to look like a consistent theme,” she said.
Dr. Carey pointed out that in the KRISTINE trial comparing the combination of trastuzumab emtansine (T-DM1) and pertuzumab with standard chemotherapy in patients with HER2-positive stage I-III breast cancer, although the experimental combination was associated with lower pCR rates and worse event-free survival, rates of iDFS/dDFS were virtually identical for patients in both arms who achieved a pCR.
“So the question is can pCR mean that we can either eliminate additional therapy,” she said, noting that the question is currently being addressed prospectively in two clinical trials, COMPASS-pCR and DECRESCENDO.
ADAPT HER2+/HR- is sponsored by F. Hoffman-La Roche. Dr. Harbeck disclosed institutional research funding from Roche/Genentech, as well as honoraria and consulting/advising for multiple companies. Dr. Carey disclosed institutional research funding and other relationships with various companies.
It may not be always necessary to approach the treatment of HER2-positive, hormone receptor–negative (HER2+/HR–) early breast cancer with added chemotherapy, survival results of a prospective multicenter randomized trial suggest.
In the ADAPT-HER2+/HR– trial, comparing a de-escalated 12-week neoadjuvant regimen consisting of dual HER2 blockade with trastuzumab (Herceptin) and pertuzumab (Perjeta) with or without weekly paclitaxel, the three-drug regimen was associated with high pathologic complete response(pCR) rates and excellent 5-year survival, irrespective of whether patients received additional chemotherapy, reported Nadia Harbeck, MD, PhD, of the University of Munich.
“Chemotherapy-free regimens are promising in highly sensitive tumors with early response, but future investigation of such chemotherapy-free regimens need to be focused on selected patients, like those with HER2 3+ tumors, non–basal-like tumors, those showing early response to the de-escalated therapy, and those with predictive RNA signatures such as immune signatures,” she said in an oral abstract session during the American Society of Clinical Oncology annual meeting (Abstract 503).
Under the WGS umbrella
The ADAPT HER2+/HR– trial (NCT01779206) is one of several conducted by the West German Study Group (WGS) on therapy for intrinsic breast cancer types.
In this study, 134 patients with HER2-positive, estrogen and progesterone receptor–negative tumors with no metastatic disease and good performance status were assigned on a 5:2 basis to neoadjuvant therapy with trastuzumab at a loading dose of 8 mg/kg for the first cycle followed by 6 mg/kg for subsequent cycles every 3 weeks x 4, plus pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks x 4 (92 patients), or to trastuzumab and pertuzumab at the same dose and schedule plus paclitaxel 80 mg/m2 once weekly for 12 weeks.
Patients had surgery within 3 weeks of the end of study therapy unless they did not have a histologically confirmed pCR, in which case they went on to receive standard neoadjuvant therapy prior to surgery.
Adjuvant therapy was performed according to national guidelines, although patients with a pCR after 12 weeks of study therapy could be spared from adjuvant chemotherapy at the investigator’s discretion.
Patients underwent biopsy at 3 weeks for therapy for early response assessment, defined as either a Ki67 decrease of at least 30% from baseline, or low cellularity (less than 500 invasive tumor cells).
First survival results
The investigators previously reported the primary pCR endpoint from the trial, which showed a rate of 90% after 12 weeks in the three-drug arm, and a “substantial and clinically meaningful” pCR rate of 34% after the trastuzumab plus pertuzumab alone.
At ASCO 2021, Dr. Harbeck reported the first survival data from the trial.
After a median follow-up of 59.9 months, there were no statistically significant differences between trial arms in either overall survival, invasive disease-free survival (iDFS), or distant disease-free survival (dDFS).
The 5-year iDFS rate in the three-drug arm was 98%, compared with 87% for the dual HER2 blockade-only arm, a difference that was not statistically significant.
The 5-year dDFS rates were 98% and 92% respectively. There were only seven dDFS events during follow-up, Dr. Harbeck noted.
There were only six deaths during follow-up, with overall survival rates of 98% in the paclitaxel-containing arm, and 94% in the anti-HER2 antibodies–only arm, a difference of one overall survival event, Dr. Harbeck said.
pCR counts
However, patients who did not have pathologic complete responses at the end of first-line de-escalated therapy had worse outcomes, with a 5-year iDFS rate of 82%, compared with 98% for patients who had achieved a pCR. This translated into a hazard ratio for invasive disease in patients with pCRs of 0.14 (P = .011).
This difference occurred despite the study requirement that all patients who did not have pCR after 12 weeks of initial therapy would receive additional chemotherapy.
Looking at the tumor subtype among patients in the paclitaxel-free arm to see whether they could identify predictors of early response, the researchers found a pCR rate of 36.5% among 85 patients with nonbasal tumors, but 0% among 7 patients with basal tumors.
The investigators identified a population of patients whose tumors could be considered nonsensitive to dual HER2 blockade alone: Those with basal tumors, those tumors with low immunohistochemical HER2 expression, and those without an early response to therapy on biopsy 3 weeks into initial therapy. Among 31 of the 92 patients in the dual HER2 arm who met this description, 2 had pCRs, Dr. Harbeck noted.
The 5-year iDFS rate among patients in the dual blockade–only arm with nonsensitive tumors was 79%, compared with 93% for patients with treatment-responsive types, although there were only 13 invasive events total in this arm.
“If we look at the whole trial population, the negative prognostic impact of what we termed nonsensitive tumors was even significant regarding dDFS, with a hazard ratio of about 5,” she said.
‘A consistent theme’
Invited discussant Lisa A. Carey, MD, ScM, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, noted that the trial was underpowered for outcomes, but that results nonetheless suggest that patients with strongly HER2-driven tumors might get comparable benefits from less chemotherapy.
“This trial included only hormone receptor–negative, HER2-positive tumors, and these we know are likely to be HER2-enriched in terms of subtype, about three-quarters of them,”she said.
The previously reported pCR rate of 90% in the paclitaxel-containing arm, with 80% of patients requiring no further chemotherapy, resulted in the excellent 5-year iDFS and dDFS in this group, despite the relatively highly clinical stage, with about 60% of patients having clinical stage 2 or higher tumors, and more than 40% being node positive.
The idea that pCR itself can predict which patients could be spared from more intensive chemotherapy “is starting to look like a consistent theme,” she said.
Dr. Carey pointed out that in the KRISTINE trial comparing the combination of trastuzumab emtansine (T-DM1) and pertuzumab with standard chemotherapy in patients with HER2-positive stage I-III breast cancer, although the experimental combination was associated with lower pCR rates and worse event-free survival, rates of iDFS/dDFS were virtually identical for patients in both arms who achieved a pCR.
“So the question is can pCR mean that we can either eliminate additional therapy,” she said, noting that the question is currently being addressed prospectively in two clinical trials, COMPASS-pCR and DECRESCENDO.
ADAPT HER2+/HR- is sponsored by F. Hoffman-La Roche. Dr. Harbeck disclosed institutional research funding from Roche/Genentech, as well as honoraria and consulting/advising for multiple companies. Dr. Carey disclosed institutional research funding and other relationships with various companies.
It may not be always necessary to approach the treatment of HER2-positive, hormone receptor–negative (HER2+/HR–) early breast cancer with added chemotherapy, survival results of a prospective multicenter randomized trial suggest.
In the ADAPT-HER2+/HR– trial, comparing a de-escalated 12-week neoadjuvant regimen consisting of dual HER2 blockade with trastuzumab (Herceptin) and pertuzumab (Perjeta) with or without weekly paclitaxel, the three-drug regimen was associated with high pathologic complete response(pCR) rates and excellent 5-year survival, irrespective of whether patients received additional chemotherapy, reported Nadia Harbeck, MD, PhD, of the University of Munich.
“Chemotherapy-free regimens are promising in highly sensitive tumors with early response, but future investigation of such chemotherapy-free regimens need to be focused on selected patients, like those with HER2 3+ tumors, non–basal-like tumors, those showing early response to the de-escalated therapy, and those with predictive RNA signatures such as immune signatures,” she said in an oral abstract session during the American Society of Clinical Oncology annual meeting (Abstract 503).
Under the WGS umbrella
The ADAPT HER2+/HR– trial (NCT01779206) is one of several conducted by the West German Study Group (WGS) on therapy for intrinsic breast cancer types.
In this study, 134 patients with HER2-positive, estrogen and progesterone receptor–negative tumors with no metastatic disease and good performance status were assigned on a 5:2 basis to neoadjuvant therapy with trastuzumab at a loading dose of 8 mg/kg for the first cycle followed by 6 mg/kg for subsequent cycles every 3 weeks x 4, plus pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks x 4 (92 patients), or to trastuzumab and pertuzumab at the same dose and schedule plus paclitaxel 80 mg/m2 once weekly for 12 weeks.
Patients had surgery within 3 weeks of the end of study therapy unless they did not have a histologically confirmed pCR, in which case they went on to receive standard neoadjuvant therapy prior to surgery.
Adjuvant therapy was performed according to national guidelines, although patients with a pCR after 12 weeks of study therapy could be spared from adjuvant chemotherapy at the investigator’s discretion.
Patients underwent biopsy at 3 weeks for therapy for early response assessment, defined as either a Ki67 decrease of at least 30% from baseline, or low cellularity (less than 500 invasive tumor cells).
First survival results
The investigators previously reported the primary pCR endpoint from the trial, which showed a rate of 90% after 12 weeks in the three-drug arm, and a “substantial and clinically meaningful” pCR rate of 34% after the trastuzumab plus pertuzumab alone.
At ASCO 2021, Dr. Harbeck reported the first survival data from the trial.
After a median follow-up of 59.9 months, there were no statistically significant differences between trial arms in either overall survival, invasive disease-free survival (iDFS), or distant disease-free survival (dDFS).
The 5-year iDFS rate in the three-drug arm was 98%, compared with 87% for the dual HER2 blockade-only arm, a difference that was not statistically significant.
The 5-year dDFS rates were 98% and 92% respectively. There were only seven dDFS events during follow-up, Dr. Harbeck noted.
There were only six deaths during follow-up, with overall survival rates of 98% in the paclitaxel-containing arm, and 94% in the anti-HER2 antibodies–only arm, a difference of one overall survival event, Dr. Harbeck said.
pCR counts
However, patients who did not have pathologic complete responses at the end of first-line de-escalated therapy had worse outcomes, with a 5-year iDFS rate of 82%, compared with 98% for patients who had achieved a pCR. This translated into a hazard ratio for invasive disease in patients with pCRs of 0.14 (P = .011).
This difference occurred despite the study requirement that all patients who did not have pCR after 12 weeks of initial therapy would receive additional chemotherapy.
Looking at the tumor subtype among patients in the paclitaxel-free arm to see whether they could identify predictors of early response, the researchers found a pCR rate of 36.5% among 85 patients with nonbasal tumors, but 0% among 7 patients with basal tumors.
The investigators identified a population of patients whose tumors could be considered nonsensitive to dual HER2 blockade alone: Those with basal tumors, those tumors with low immunohistochemical HER2 expression, and those without an early response to therapy on biopsy 3 weeks into initial therapy. Among 31 of the 92 patients in the dual HER2 arm who met this description, 2 had pCRs, Dr. Harbeck noted.
The 5-year iDFS rate among patients in the dual blockade–only arm with nonsensitive tumors was 79%, compared with 93% for patients with treatment-responsive types, although there were only 13 invasive events total in this arm.
“If we look at the whole trial population, the negative prognostic impact of what we termed nonsensitive tumors was even significant regarding dDFS, with a hazard ratio of about 5,” she said.
‘A consistent theme’
Invited discussant Lisa A. Carey, MD, ScM, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, noted that the trial was underpowered for outcomes, but that results nonetheless suggest that patients with strongly HER2-driven tumors might get comparable benefits from less chemotherapy.
“This trial included only hormone receptor–negative, HER2-positive tumors, and these we know are likely to be HER2-enriched in terms of subtype, about three-quarters of them,”she said.
The previously reported pCR rate of 90% in the paclitaxel-containing arm, with 80% of patients requiring no further chemotherapy, resulted in the excellent 5-year iDFS and dDFS in this group, despite the relatively highly clinical stage, with about 60% of patients having clinical stage 2 or higher tumors, and more than 40% being node positive.
The idea that pCR itself can predict which patients could be spared from more intensive chemotherapy “is starting to look like a consistent theme,” she said.
Dr. Carey pointed out that in the KRISTINE trial comparing the combination of trastuzumab emtansine (T-DM1) and pertuzumab with standard chemotherapy in patients with HER2-positive stage I-III breast cancer, although the experimental combination was associated with lower pCR rates and worse event-free survival, rates of iDFS/dDFS were virtually identical for patients in both arms who achieved a pCR.
“So the question is can pCR mean that we can either eliminate additional therapy,” she said, noting that the question is currently being addressed prospectively in two clinical trials, COMPASS-pCR and DECRESCENDO.
ADAPT HER2+/HR- is sponsored by F. Hoffman-La Roche. Dr. Harbeck disclosed institutional research funding from Roche/Genentech, as well as honoraria and consulting/advising for multiple companies. Dr. Carey disclosed institutional research funding and other relationships with various companies.
FROM ASCO 2021
How to choose the right vaginal moisturizer or lubricant for your patient
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.
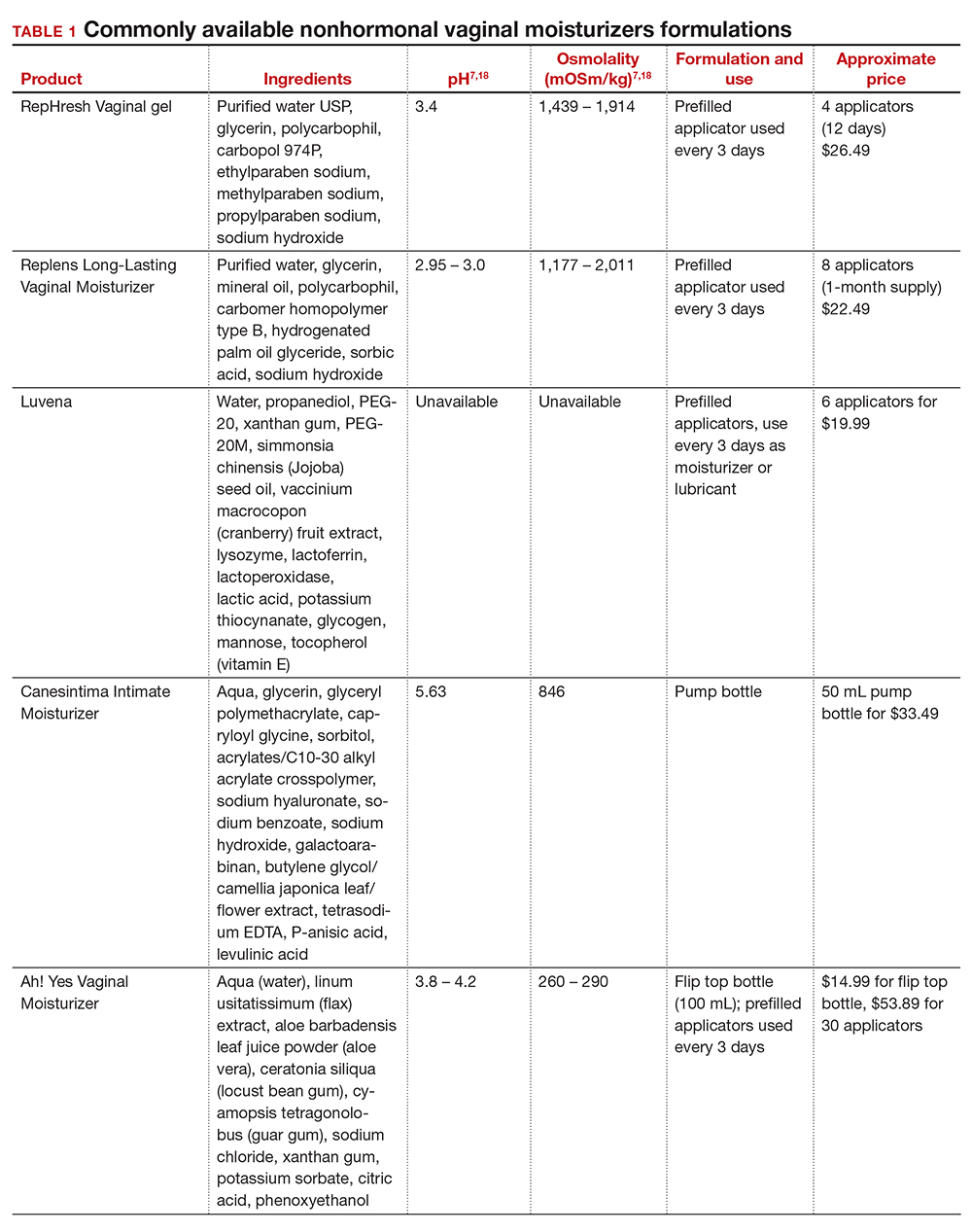
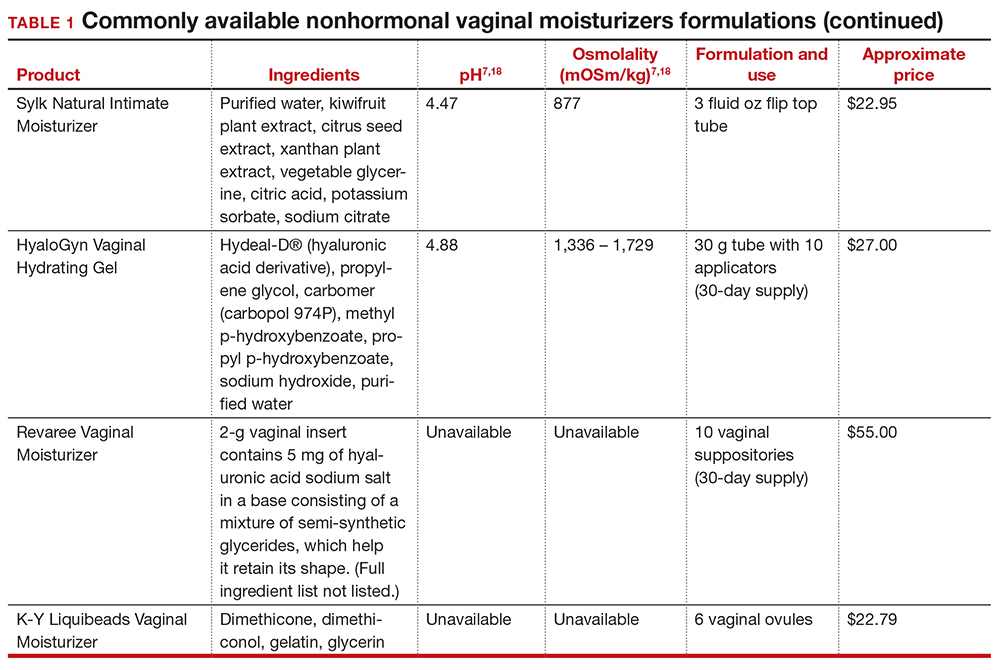
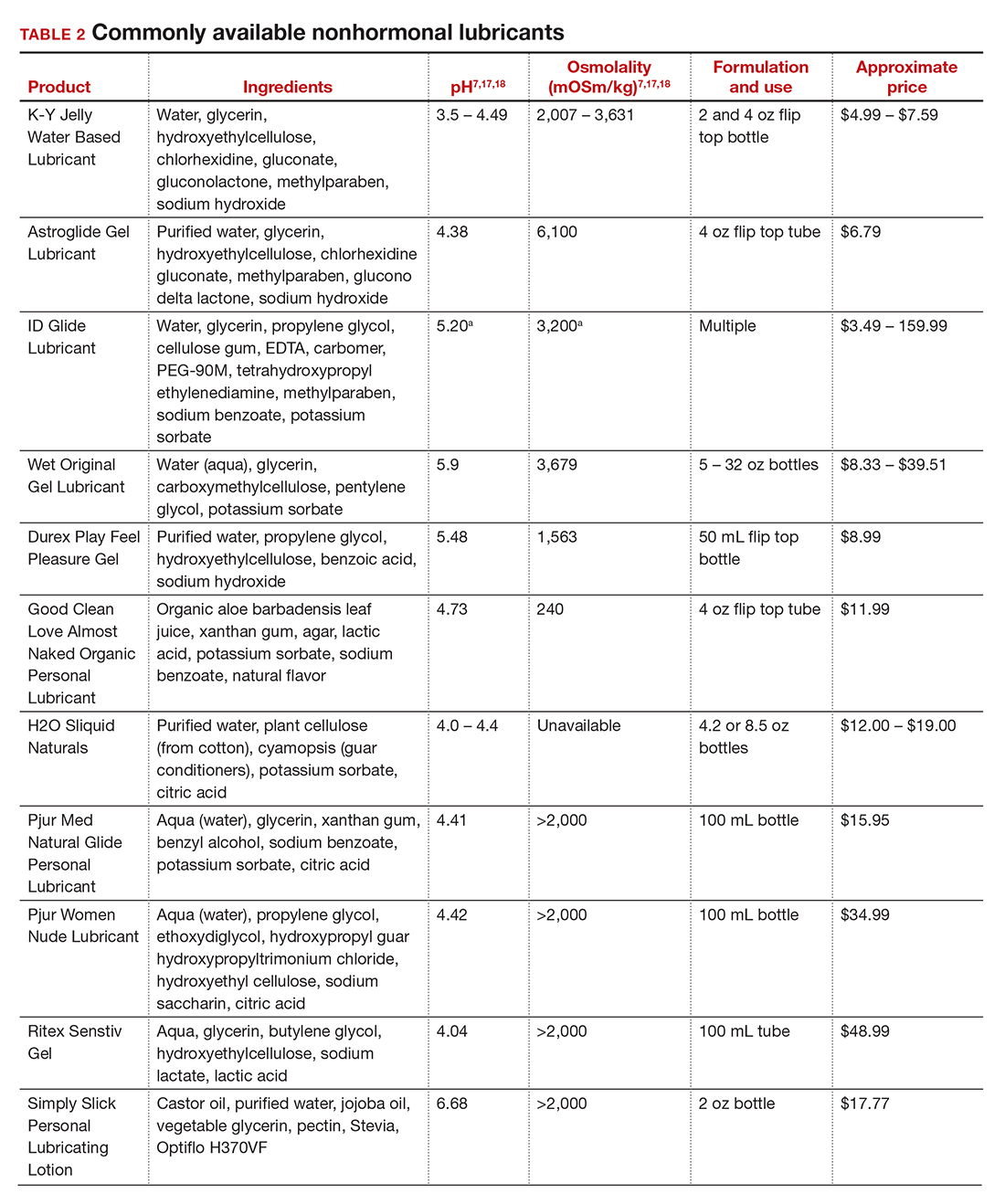
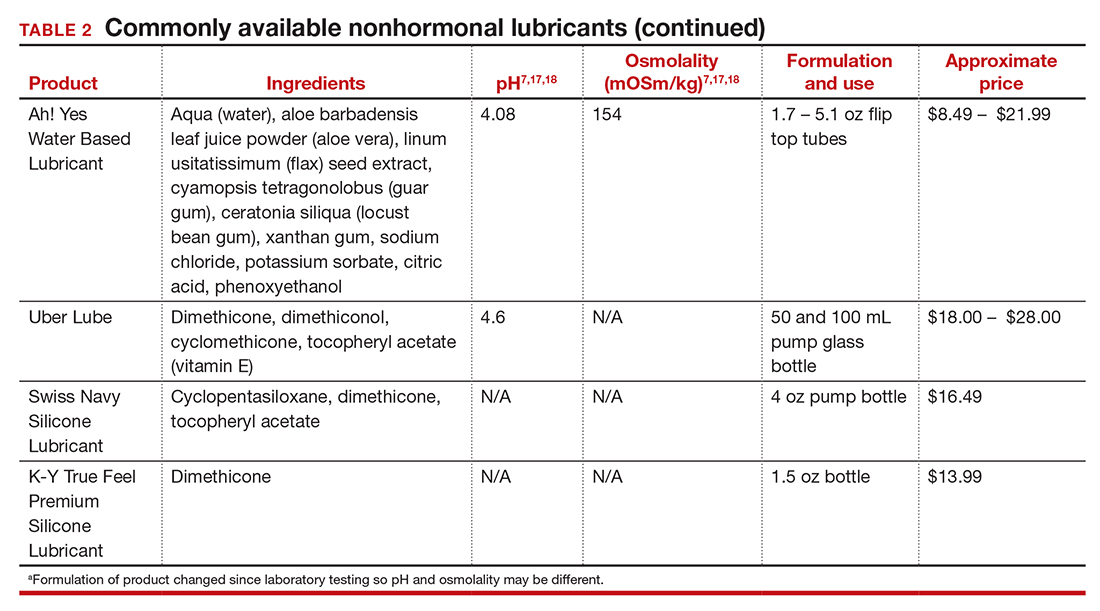
The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Adverse pregnancy outcomes and later cardiovascular disease
Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.
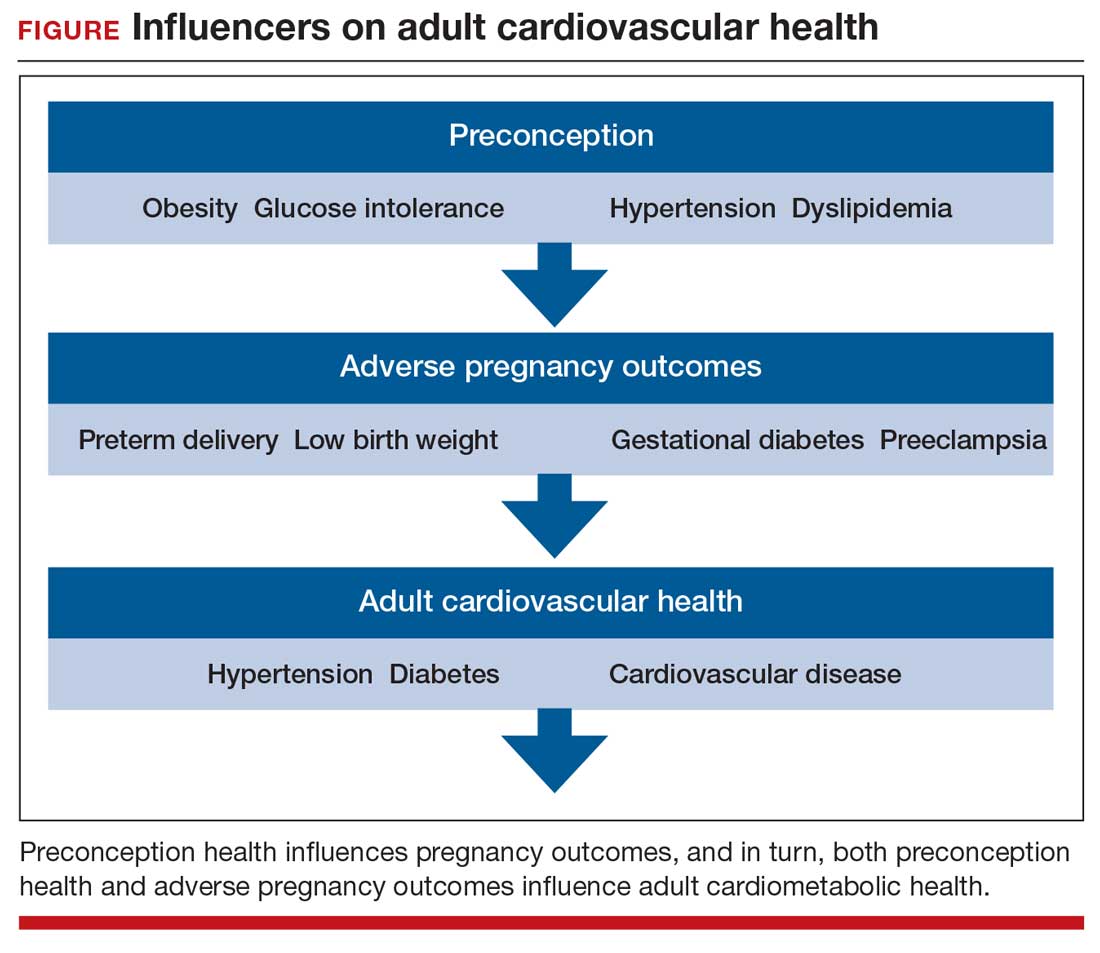
Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.
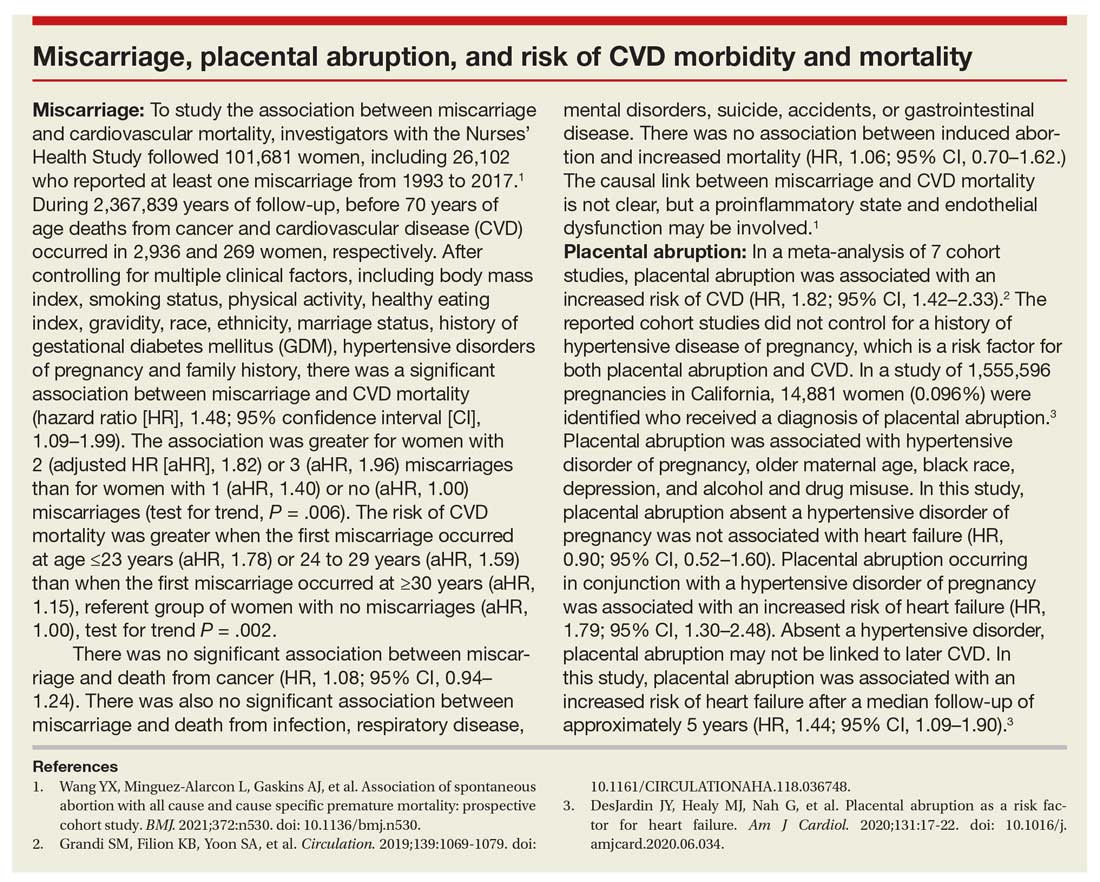
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.

Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.

Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.

Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.

- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
COVID-19 vaccination during pregnancy: Expert guidance on counseling your patients
Each clinician has had to develop his or her individual approach and follow their institutional guidance regarding counseling and managing all of their patients’ expectations in this quickly changing climate of vaccination availability and recommendations. For pregnant women specifically, who are at increased risk for severe SARS-COV-2 disease,1 the availability of varied types of vaccines for COVID-19 is promising, but with limited data available to discuss safety for the mother and the baby, what is the best discussion to guide decision making? OBG Management reached out to several experts, who share here what they do in their practices.
Addressing an uncharted front in the war on COVID-19
Ashley S. Roman, MD, MPH
In December 2020, the US Food and Drug Administration (FDA)’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.2 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.3 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.4 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.5,6
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- the presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes
- potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- the ability to take precautions (social distancing, wearing a mask, etc).
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
Continue to: Benefits outweigh the risks, for now...
Benefits outweigh the risks, for now
Ashley S. Coggins, MD, and Jeanne S. Sheffield, MD
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.7 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers— and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.8
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the FDA are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention (CDC)’s V-safe Vaccine Pregnancy Registry.9 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.10 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices. These organizations advise that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered. As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
- Zambrano LD, Ellington S, Strid S, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. 2020;69:1641–1647.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
- Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-datatracker/#pregnant-population. Accessed April 19, 2021.
- Centers for Disease Control and Prevention. V-safe COVID19 Vaccine Pregnancy Registry. https://www.cdc.gov /coronavirus/2019-ncov/vaccines/safety/vsafepregnancy registry.html. Updated May 3, 2021. Accessed April 19, 2021.
- Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j. ajog.2021.03.023.
Each clinician has had to develop his or her individual approach and follow their institutional guidance regarding counseling and managing all of their patients’ expectations in this quickly changing climate of vaccination availability and recommendations. For pregnant women specifically, who are at increased risk for severe SARS-COV-2 disease,1 the availability of varied types of vaccines for COVID-19 is promising, but with limited data available to discuss safety for the mother and the baby, what is the best discussion to guide decision making? OBG Management reached out to several experts, who share here what they do in their practices.
Addressing an uncharted front in the war on COVID-19
Ashley S. Roman, MD, MPH
In December 2020, the US Food and Drug Administration (FDA)’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.2 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.3 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.4 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.5,6
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- the presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes
- potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- the ability to take precautions (social distancing, wearing a mask, etc).
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
Continue to: Benefits outweigh the risks, for now...
Benefits outweigh the risks, for now
Ashley S. Coggins, MD, and Jeanne S. Sheffield, MD
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.7 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers— and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.8
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the FDA are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention (CDC)’s V-safe Vaccine Pregnancy Registry.9 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.10 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices. These organizations advise that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered. As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
Each clinician has had to develop his or her individual approach and follow their institutional guidance regarding counseling and managing all of their patients’ expectations in this quickly changing climate of vaccination availability and recommendations. For pregnant women specifically, who are at increased risk for severe SARS-COV-2 disease,1 the availability of varied types of vaccines for COVID-19 is promising, but with limited data available to discuss safety for the mother and the baby, what is the best discussion to guide decision making? OBG Management reached out to several experts, who share here what they do in their practices.
Addressing an uncharted front in the war on COVID-19
Ashley S. Roman, MD, MPH
In December 2020, the US Food and Drug Administration (FDA)’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.2 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.3 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.4 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.5,6
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- the presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes
- potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- the ability to take precautions (social distancing, wearing a mask, etc).
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
Continue to: Benefits outweigh the risks, for now...
Benefits outweigh the risks, for now
Ashley S. Coggins, MD, and Jeanne S. Sheffield, MD
Vaccines have been a lifesaving public health measure since 1000 CE, when the Chinese first used smallpox inoculations to induce immunity.7 Work by pioneers such as Edward Jenner, Louis Pasteur, and Maurice Hilleman has averted countless millions of vaccine-preventable illnesses and deaths, and vaccines have become a routine part of health maintenance throughout the human life cycle.
Pregnant patients who receive vaccines often have an added benefit of protection provided to their infants through passive transfer of antibodies. Several vaccine platforms have been utilized in pregnancy with well-documented improvements in maternal and obstetric outcomes as well as improved neonatal outcomes in the first several months of life.
Risks of COVID-19 in pregnancy
The COVID-19 pandemic placed a spotlight on medically at-risk groups. Pregnant women are 3 times more likely to require admission to the intensive care unit, have increased requirement for extracorporeal membrane oxygenation treatment, and are up to 70% more likely to die than nonpregnant peers— and this risk increases with the presence of additional comorbidities.
In the case of COVID-19, vaccination trials that have shaped worldwide clinical practice unfortunately followed the historical trend of excluding pregnant patients from participation. This has required clinicians to guide their patients through the decision of whether or not to accept vaccination without having the same reassurances regarding safety and effectiveness afforded to their nonpregnant counterparts. With more than 86,000 pregnant women infected with COVID-19 through April 19, 2021, this lack of information regarding vaccine safety in pregnancy is a significant public health gap.8
COVID-19 vaccines
The current COVID-19 vaccines approved for use in the United States under an Emergency Use Authorization issued by the FDA are nonreplicating and thus cannot cause infection in the mother or fetus. These are the Pfizer-BioNTech mRNA vaccine, the Moderna mRNA-1273 vaccine, and the Janssen Biotech Inc. monovalent vaccine. Furthermore, in animal studies that included the Pfizer-BioNTech, Moderna, or Janssen COVID-19 vaccines, no fetal, embryonal, female reproductive, or postnatal development safety concerns were demonstrated.
As of April 19, 2021, 94,335 pregnant women had received a COVID-19 vaccination, and 4,622 of these enrolled in the Centers for Disease Control and Prevention (CDC)’s V-safe Vaccine Pregnancy Registry.9 The data reported noted no unexpected pregnancy or infant outcomes related to COVID-19 vaccination in pregnancy. Adverse effects of the vaccine were similar to those in nonpregnant cohorts. Additionally, emerging data suggest passage of immunity to neonates, with maternal antibodies demonstrated in cord blood at time of delivery as well as in breast milk.10 To date, these data mainly have come from women immunized with the Moderna and Pfizer-BioNTech mRNA vaccines.
Counseling pregnant patients
Our counseling aligns with that of the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC’s Advisory Committee on Immunization Practices. These organizations advise that COVID-19 vaccination should not be withheld from pregnant patients or patients who want to become pregnant. In pregnant patients with comorbidities that place them at higher risk for severe COVID-19 infection, all available formulations of the COVID-19 vaccination should be strongly considered. As evidence for vaccination safety continues to emerge, patients should continue to discuss their individual needs for vaccination in a shared decision-making format with their obstetric providers.
- Zambrano LD, Ellington S, Strid S, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. 2020;69:1641–1647.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
- Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-datatracker/#pregnant-population. Accessed April 19, 2021.
- Centers for Disease Control and Prevention. V-safe COVID19 Vaccine Pregnancy Registry. https://www.cdc.gov /coronavirus/2019-ncov/vaccines/safety/vsafepregnancy registry.html. Updated May 3, 2021. Accessed April 19, 2021.
- Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j. ajog.2021.03.023.
- Zambrano LD, Ellington S, Strid S, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. 2020;69:1641–1647.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Boylston A. The origins of inoculation. J R Soc Med. 2012;105:309-313.
- Centers for Disease Control and Prevention. COVID data tracker. Data on COVID-19 during pregnancy: severity of maternal illness. https://covid.cdc.gov/covid-datatracker/#pregnant-population. Accessed April 19, 2021.
- Centers for Disease Control and Prevention. V-safe COVID19 Vaccine Pregnancy Registry. https://www.cdc.gov /coronavirus/2019-ncov/vaccines/safety/vsafepregnancy registry.html. Updated May 3, 2021. Accessed April 19, 2021.
- Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j. ajog.2021.03.023.
Many comatose TBI patients recover consciousness during rehab
according to a study of 3 decades of TBI survivors.
“Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DoC,” wrote Robert G. Kowalski, MBBCh, MS, of the department of neurology at the University of Colorado at Denver, Aurora, and colleagues. The study was published in JAMA Neurology.
To determine the likelihood of returning to consciousness in the weeks that follow a serious brain injury, along with any notable contributing factors, the researchers launched a retrospective analysis of 17,470 patients with moderate to severe TBI. All participants had been enrolled in the Traumatic Brain Injury Model Systems database from January 1989 to June 2019 after being admitted to any 1 of 23 inpatient rehabilitation centers. The cohort had a median age of 39 (interquartile range, 25-56), with 74% being male and 66% being white. Their median duration of acute hospital care was 16 days (IQR, 9-26).
Unconsciousness was defined by the researchers as not being able to follow commands or having a Glasgow Coma Scale motor score in the ED of lower than 6 or a Disability Rating Scale motor score greater than 0. Of the overall cohort, 7,547 (57%) patients initially lost consciousness and 2,058 (12%) remained unconscious as they were admitted to rehab. Of that subgroup, 1,674 (82%) recovered consciousness during rehab. The 414 patients who still had a DoC at completion of rehab had a longer median stay (37 days; IQR, 22-65), compared with the patients who recovered consciousness (19 days; IQR, 12-30; P < .001). After multivariable analysis, the factors most associated with recovery of consciousness were the absence of intraventricular hemorrhage (adjusted odds ratio, 0.678; 95% confidence interval, 0.532-0.863; P = .002) and the absence of intracranial mass effect (aOR, 0.759; 95% CI, 0.595-0.968; P = .03).
Though all patients experienced an improvement in functional status during rehabilitation, patients with DoC had an increase in median Functional Independence Measure total score from 19 to 71 while patients without DoC increased from 54 to 96 (change in total score, +43 versus +37; P = .002). After multivariate analysis, younger age and male sex were both associated with better functional outcomes during rehab and at discharge.
When it comes to TBI patients, don’t give up hope
The choice to withdraw care in TBI patients is a complicated and daunting one, and this study is further evidence that physicians should delay that decision in many scenarios, wrote Jennifer A. Kim, MD, PhD, and Kevin N. Sheth, MD, of Yale University, New Haven, Conn., in an accompanying editorial.
“By showing that a large proportion of patients with persistent DoC recover during acute rehabilitation, this article further challenges our potential toward overly nihilistic notions of who may or may not ultimately recover consciousness long term,” they added.
That said, they also recognized the questions that still persist: What are the reasons for late-stage withdrawal of lifesaving therapy? What is the recovery rate of all hospitalized patients with TBI, not just those in rehabilitation facilities? And is it possible to detect covert consciousness using MRI and electroencephalography, which this study did not include?
“Defining both good and poor prognostic risk factors is critical to portending recovery,” they wrote, emphasizing the need for physicians to rely on scientifically based predictions when making such important assessments.
Patience is a virtue for TBI specialists
“A lot of people write notes on hospital charts, ‘poor prognosis.’ You don’t know, that early in the game, in the acute care setting, how TBI patients are going to do,” said Jamie S. Ullman, MD, of the department of neurosurgery at Hofstra University, Hempstead, N.Y., in an interview. “It’s over the long term that we really have to judge that.”
“Of course, there may be some characteristics that patients might have that may portend for a worse outcome, like brain stem damage,” she added. “But in general, there is plenty of literature to suggest that not only can even the worst-looking patients have some kind of functional outcome but that it takes 18 months or more to actually realize an outcome from a traumatic brain injury.”
She emphasized that each patient with TBI is unique; beyond their current status, you have to consider the significance of their injury, the thoughts of their families or partner, and their own previously stated wishes and willingness to tolerate disability. Nonetheless, this study is another step toward distilling the “nihilistic thinking” that can lead physicians to expect the worst regarding patients who may still have a path toward a functional life.
“As traumatic brain injury specialists,” she said, “we need to see what we can do to give patients as good a chance as possible at a recovery.”
The authors acknowledged their study’s limitations, including an inability to account for 3 decades of variations in treatment regimens and its limited generalizability because of the cohort being composed of only TBI survivors admitted to inpatient rehab. In addition, they noted a possible referential bias for the study’s mostly young TBI patients in rehab facilities, another reason why these findings “may not be directly applicable to the overall population of patients with moderate or severe TBI.”
The study was funded by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research; the Department of Health & Human Services; and the Veterans Health Administration Central Office VA TBI Model Systems Program of Research. The authors reported several potential conflicts of interest, including receiving grants and support from various government agencies and pharmaceutical companies.
according to a study of 3 decades of TBI survivors.
“Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DoC,” wrote Robert G. Kowalski, MBBCh, MS, of the department of neurology at the University of Colorado at Denver, Aurora, and colleagues. The study was published in JAMA Neurology.
To determine the likelihood of returning to consciousness in the weeks that follow a serious brain injury, along with any notable contributing factors, the researchers launched a retrospective analysis of 17,470 patients with moderate to severe TBI. All participants had been enrolled in the Traumatic Brain Injury Model Systems database from January 1989 to June 2019 after being admitted to any 1 of 23 inpatient rehabilitation centers. The cohort had a median age of 39 (interquartile range, 25-56), with 74% being male and 66% being white. Their median duration of acute hospital care was 16 days (IQR, 9-26).
Unconsciousness was defined by the researchers as not being able to follow commands or having a Glasgow Coma Scale motor score in the ED of lower than 6 or a Disability Rating Scale motor score greater than 0. Of the overall cohort, 7,547 (57%) patients initially lost consciousness and 2,058 (12%) remained unconscious as they were admitted to rehab. Of that subgroup, 1,674 (82%) recovered consciousness during rehab. The 414 patients who still had a DoC at completion of rehab had a longer median stay (37 days; IQR, 22-65), compared with the patients who recovered consciousness (19 days; IQR, 12-30; P < .001). After multivariable analysis, the factors most associated with recovery of consciousness were the absence of intraventricular hemorrhage (adjusted odds ratio, 0.678; 95% confidence interval, 0.532-0.863; P = .002) and the absence of intracranial mass effect (aOR, 0.759; 95% CI, 0.595-0.968; P = .03).
Though all patients experienced an improvement in functional status during rehabilitation, patients with DoC had an increase in median Functional Independence Measure total score from 19 to 71 while patients without DoC increased from 54 to 96 (change in total score, +43 versus +37; P = .002). After multivariate analysis, younger age and male sex were both associated with better functional outcomes during rehab and at discharge.
When it comes to TBI patients, don’t give up hope
The choice to withdraw care in TBI patients is a complicated and daunting one, and this study is further evidence that physicians should delay that decision in many scenarios, wrote Jennifer A. Kim, MD, PhD, and Kevin N. Sheth, MD, of Yale University, New Haven, Conn., in an accompanying editorial.
“By showing that a large proportion of patients with persistent DoC recover during acute rehabilitation, this article further challenges our potential toward overly nihilistic notions of who may or may not ultimately recover consciousness long term,” they added.
That said, they also recognized the questions that still persist: What are the reasons for late-stage withdrawal of lifesaving therapy? What is the recovery rate of all hospitalized patients with TBI, not just those in rehabilitation facilities? And is it possible to detect covert consciousness using MRI and electroencephalography, which this study did not include?
“Defining both good and poor prognostic risk factors is critical to portending recovery,” they wrote, emphasizing the need for physicians to rely on scientifically based predictions when making such important assessments.
Patience is a virtue for TBI specialists
“A lot of people write notes on hospital charts, ‘poor prognosis.’ You don’t know, that early in the game, in the acute care setting, how TBI patients are going to do,” said Jamie S. Ullman, MD, of the department of neurosurgery at Hofstra University, Hempstead, N.Y., in an interview. “It’s over the long term that we really have to judge that.”
“Of course, there may be some characteristics that patients might have that may portend for a worse outcome, like brain stem damage,” she added. “But in general, there is plenty of literature to suggest that not only can even the worst-looking patients have some kind of functional outcome but that it takes 18 months or more to actually realize an outcome from a traumatic brain injury.”
She emphasized that each patient with TBI is unique; beyond their current status, you have to consider the significance of their injury, the thoughts of their families or partner, and their own previously stated wishes and willingness to tolerate disability. Nonetheless, this study is another step toward distilling the “nihilistic thinking” that can lead physicians to expect the worst regarding patients who may still have a path toward a functional life.
“As traumatic brain injury specialists,” she said, “we need to see what we can do to give patients as good a chance as possible at a recovery.”
The authors acknowledged their study’s limitations, including an inability to account for 3 decades of variations in treatment regimens and its limited generalizability because of the cohort being composed of only TBI survivors admitted to inpatient rehab. In addition, they noted a possible referential bias for the study’s mostly young TBI patients in rehab facilities, another reason why these findings “may not be directly applicable to the overall population of patients with moderate or severe TBI.”
The study was funded by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research; the Department of Health & Human Services; and the Veterans Health Administration Central Office VA TBI Model Systems Program of Research. The authors reported several potential conflicts of interest, including receiving grants and support from various government agencies and pharmaceutical companies.
according to a study of 3 decades of TBI survivors.
“Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DoC,” wrote Robert G. Kowalski, MBBCh, MS, of the department of neurology at the University of Colorado at Denver, Aurora, and colleagues. The study was published in JAMA Neurology.
To determine the likelihood of returning to consciousness in the weeks that follow a serious brain injury, along with any notable contributing factors, the researchers launched a retrospective analysis of 17,470 patients with moderate to severe TBI. All participants had been enrolled in the Traumatic Brain Injury Model Systems database from January 1989 to June 2019 after being admitted to any 1 of 23 inpatient rehabilitation centers. The cohort had a median age of 39 (interquartile range, 25-56), with 74% being male and 66% being white. Their median duration of acute hospital care was 16 days (IQR, 9-26).
Unconsciousness was defined by the researchers as not being able to follow commands or having a Glasgow Coma Scale motor score in the ED of lower than 6 or a Disability Rating Scale motor score greater than 0. Of the overall cohort, 7,547 (57%) patients initially lost consciousness and 2,058 (12%) remained unconscious as they were admitted to rehab. Of that subgroup, 1,674 (82%) recovered consciousness during rehab. The 414 patients who still had a DoC at completion of rehab had a longer median stay (37 days; IQR, 22-65), compared with the patients who recovered consciousness (19 days; IQR, 12-30; P < .001). After multivariable analysis, the factors most associated with recovery of consciousness were the absence of intraventricular hemorrhage (adjusted odds ratio, 0.678; 95% confidence interval, 0.532-0.863; P = .002) and the absence of intracranial mass effect (aOR, 0.759; 95% CI, 0.595-0.968; P = .03).
Though all patients experienced an improvement in functional status during rehabilitation, patients with DoC had an increase in median Functional Independence Measure total score from 19 to 71 while patients without DoC increased from 54 to 96 (change in total score, +43 versus +37; P = .002). After multivariate analysis, younger age and male sex were both associated with better functional outcomes during rehab and at discharge.
When it comes to TBI patients, don’t give up hope
The choice to withdraw care in TBI patients is a complicated and daunting one, and this study is further evidence that physicians should delay that decision in many scenarios, wrote Jennifer A. Kim, MD, PhD, and Kevin N. Sheth, MD, of Yale University, New Haven, Conn., in an accompanying editorial.
“By showing that a large proportion of patients with persistent DoC recover during acute rehabilitation, this article further challenges our potential toward overly nihilistic notions of who may or may not ultimately recover consciousness long term,” they added.
That said, they also recognized the questions that still persist: What are the reasons for late-stage withdrawal of lifesaving therapy? What is the recovery rate of all hospitalized patients with TBI, not just those in rehabilitation facilities? And is it possible to detect covert consciousness using MRI and electroencephalography, which this study did not include?
“Defining both good and poor prognostic risk factors is critical to portending recovery,” they wrote, emphasizing the need for physicians to rely on scientifically based predictions when making such important assessments.
Patience is a virtue for TBI specialists
“A lot of people write notes on hospital charts, ‘poor prognosis.’ You don’t know, that early in the game, in the acute care setting, how TBI patients are going to do,” said Jamie S. Ullman, MD, of the department of neurosurgery at Hofstra University, Hempstead, N.Y., in an interview. “It’s over the long term that we really have to judge that.”
“Of course, there may be some characteristics that patients might have that may portend for a worse outcome, like brain stem damage,” she added. “But in general, there is plenty of literature to suggest that not only can even the worst-looking patients have some kind of functional outcome but that it takes 18 months or more to actually realize an outcome from a traumatic brain injury.”
She emphasized that each patient with TBI is unique; beyond their current status, you have to consider the significance of their injury, the thoughts of their families or partner, and their own previously stated wishes and willingness to tolerate disability. Nonetheless, this study is another step toward distilling the “nihilistic thinking” that can lead physicians to expect the worst regarding patients who may still have a path toward a functional life.
“As traumatic brain injury specialists,” she said, “we need to see what we can do to give patients as good a chance as possible at a recovery.”
The authors acknowledged their study’s limitations, including an inability to account for 3 decades of variations in treatment regimens and its limited generalizability because of the cohort being composed of only TBI survivors admitted to inpatient rehab. In addition, they noted a possible referential bias for the study’s mostly young TBI patients in rehab facilities, another reason why these findings “may not be directly applicable to the overall population of patients with moderate or severe TBI.”
The study was funded by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research; the Department of Health & Human Services; and the Veterans Health Administration Central Office VA TBI Model Systems Program of Research. The authors reported several potential conflicts of interest, including receiving grants and support from various government agencies and pharmaceutical companies.
FROM JAMA NEUROLOGY
The Cures Act: Is the “cure” worse than the disease?
There is a sudden spill of icy anxiety down your spine as you pick up your phone in your shaking hands. It’s 6 p.m.; your doctor’s office is closed. You open the message, and your worst fears are confirmed ... the cancer is back.
Or is it? You’re not sure. The biopsy sure sounds bad. But you’re an English teacher, not a doctor, and you spend the rest of the night Googling words like “tubulovillous” and “high-grade dysplasia.” You sit awake, terrified in front of the computer screen desperately trying to make sense of the possibly life-changing results. You wish you knew someone who could help you understand; you consider calling your doctor’s emergency line, or your cousin who is an ophthalmologist – anybody who can help you make sense of the results.
Or imagine another scenario: you’re a trans teen who has asked your doctor to refer to you by your preferred pronouns. You’re still presenting as your birth sex, in part because your family would disown you if they knew, and you’re not financially or emotionally ready for that step. You feel proud of yourself for advocating for your needs to your long-time physician, and excited about the resources they’ve included in your after visit summary and the referrals they’d made to gender-confirming specialists.
When you get home, you are confronted with a terrible reality that your doctor’s notes, orders, and recommendations are immediately viewable to anybody with your MyChart login – your parents knew the second your doctor signed the note. They received the notification, logged on as your guardians, and you have effectively been “outed” by the physician who took and oath to care for you and who you trusted implicitly.
How the Cures Act is affecting patients
While these examples may sound extreme, they are becoming more and more commonplace thanks to a recently enacted 21st Century Cures Act. The act was originally written to improve communication between physicians and patients. Part of the act stipulates that nearly all medical information – from notes to biopsies to lab results – must be available within 24 hours, published to a patient portal and a notification be sent to the patient by phone.
Oftentimes, this occurs before the ordering physician has even seen the results, much less interpreted them and made a plan for the patient. What happens now, not long after its enactment date, when it has become clear that the Cures Act is causing extreme harm to our patients?
Take, for example, the real example of a physician whose patient found out about her own intrauterine fetal demise by way of an EMR text message alert of “new imaging results!” sent directly to her phone. Or a physician colleague who witnessed firsthand the intrusive unhelpfulness of the Cures Act when she was informed via patient portal releasing her imaging information that she had a large, possibly malignant breast mass. “No phone call,” she said. “No human being for questions or comfort. Just a notification on my phone.”
The stories about the impact of the Cures Act across the medical community are an endless stream of anxiety, hurt, and broken trust. The relationship between a physician and a patient should be sacred, bolstered by communication and mutual respect.
In many ways, the new act feels like a third party to the patient-physician relationship – a digital imposter, oftentimes blurting out personal and life-altering medical information without any of the finesse, context, and perspective of an experienced physician.
Breaking ‘bad news’ to a patient
In training, some residents are taught how to “break bad news” to a patient. Some good practices for doing this are to have information available for the patient, provide emotional support, have a plan for their next steps already formulated, and call the appropriate specialist ahead of time if you can.
Above all, it’s most important to let the patient be the one to direct their own care. Give them time to ask questions and answer them honestly and clearly. Ask them how much they want to know and help them to understand the complex change in their usual state of health.
Now, unless physicians are keeping a very close eye on their inbox, results are slipping out to patients in a void. The bad news conversations aren’t happening at all, or if they are, they’re happening at 8 p.m. on a phone call after an exhausted physician ends their shift but has to slog through their results bin, calling all the patients who shouldn’t have to find out their results in solitude.
Reaching out to these patients immediately is an honorable, kind thing to, but for a physician, knowing they need to beat the patient to opening an email creates anxiety. Plus, making these calls at whatever hour the results are released to a patient is another burden added to doctors’ already-full plates.
Interpreting results
None of us want to harm our patients. All of us want to be there for them. But this act stands in the way of delivering quality, humanizing medical care.
It is true that patients have a right to access their own medical information. It is also true that waiting anxiously on results can cause undue harm to a patient. But the across-the-board, breakneck speed of information release mandated in this act causes irreparable harm not only to patients, but to the patient-physician relationship.
No patient should find out their cancer recurred while checking their emails at their desk. No patient should first learn of a life-altering diagnosis by way of scrolling through their smartphone in bed. The role of a physician is more than just a healer – we should also be educators, interpreters, partners and, first and foremost, advocates for our patients’ needs.
Our patients are depending on us to stand up and speak out about necessary changes to this act. Result releases should be delayed until they are viewed by a physician. Our patients deserve the dignity and opportunity of a conversation with their medical provider about their test results, and physicians deserve the chance to interpret results and frame the conversation in a way which is conducive to patient understanding and healing.
Dr. Persampiere is a first-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece. You can contact them at [email protected].
There is a sudden spill of icy anxiety down your spine as you pick up your phone in your shaking hands. It’s 6 p.m.; your doctor’s office is closed. You open the message, and your worst fears are confirmed ... the cancer is back.
Or is it? You’re not sure. The biopsy sure sounds bad. But you’re an English teacher, not a doctor, and you spend the rest of the night Googling words like “tubulovillous” and “high-grade dysplasia.” You sit awake, terrified in front of the computer screen desperately trying to make sense of the possibly life-changing results. You wish you knew someone who could help you understand; you consider calling your doctor’s emergency line, or your cousin who is an ophthalmologist – anybody who can help you make sense of the results.
Or imagine another scenario: you’re a trans teen who has asked your doctor to refer to you by your preferred pronouns. You’re still presenting as your birth sex, in part because your family would disown you if they knew, and you’re not financially or emotionally ready for that step. You feel proud of yourself for advocating for your needs to your long-time physician, and excited about the resources they’ve included in your after visit summary and the referrals they’d made to gender-confirming specialists.
When you get home, you are confronted with a terrible reality that your doctor’s notes, orders, and recommendations are immediately viewable to anybody with your MyChart login – your parents knew the second your doctor signed the note. They received the notification, logged on as your guardians, and you have effectively been “outed” by the physician who took and oath to care for you and who you trusted implicitly.
How the Cures Act is affecting patients
While these examples may sound extreme, they are becoming more and more commonplace thanks to a recently enacted 21st Century Cures Act. The act was originally written to improve communication between physicians and patients. Part of the act stipulates that nearly all medical information – from notes to biopsies to lab results – must be available within 24 hours, published to a patient portal and a notification be sent to the patient by phone.
Oftentimes, this occurs before the ordering physician has even seen the results, much less interpreted them and made a plan for the patient. What happens now, not long after its enactment date, when it has become clear that the Cures Act is causing extreme harm to our patients?
Take, for example, the real example of a physician whose patient found out about her own intrauterine fetal demise by way of an EMR text message alert of “new imaging results!” sent directly to her phone. Or a physician colleague who witnessed firsthand the intrusive unhelpfulness of the Cures Act when she was informed via patient portal releasing her imaging information that she had a large, possibly malignant breast mass. “No phone call,” she said. “No human being for questions or comfort. Just a notification on my phone.”
The stories about the impact of the Cures Act across the medical community are an endless stream of anxiety, hurt, and broken trust. The relationship between a physician and a patient should be sacred, bolstered by communication and mutual respect.
In many ways, the new act feels like a third party to the patient-physician relationship – a digital imposter, oftentimes blurting out personal and life-altering medical information without any of the finesse, context, and perspective of an experienced physician.
Breaking ‘bad news’ to a patient
In training, some residents are taught how to “break bad news” to a patient. Some good practices for doing this are to have information available for the patient, provide emotional support, have a plan for their next steps already formulated, and call the appropriate specialist ahead of time if you can.
Above all, it’s most important to let the patient be the one to direct their own care. Give them time to ask questions and answer them honestly and clearly. Ask them how much they want to know and help them to understand the complex change in their usual state of health.
Now, unless physicians are keeping a very close eye on their inbox, results are slipping out to patients in a void. The bad news conversations aren’t happening at all, or if they are, they’re happening at 8 p.m. on a phone call after an exhausted physician ends their shift but has to slog through their results bin, calling all the patients who shouldn’t have to find out their results in solitude.
Reaching out to these patients immediately is an honorable, kind thing to, but for a physician, knowing they need to beat the patient to opening an email creates anxiety. Plus, making these calls at whatever hour the results are released to a patient is another burden added to doctors’ already-full plates.
Interpreting results
None of us want to harm our patients. All of us want to be there for them. But this act stands in the way of delivering quality, humanizing medical care.
It is true that patients have a right to access their own medical information. It is also true that waiting anxiously on results can cause undue harm to a patient. But the across-the-board, breakneck speed of information release mandated in this act causes irreparable harm not only to patients, but to the patient-physician relationship.
No patient should find out their cancer recurred while checking their emails at their desk. No patient should first learn of a life-altering diagnosis by way of scrolling through their smartphone in bed. The role of a physician is more than just a healer – we should also be educators, interpreters, partners and, first and foremost, advocates for our patients’ needs.
Our patients are depending on us to stand up and speak out about necessary changes to this act. Result releases should be delayed until they are viewed by a physician. Our patients deserve the dignity and opportunity of a conversation with their medical provider about their test results, and physicians deserve the chance to interpret results and frame the conversation in a way which is conducive to patient understanding and healing.
Dr. Persampiere is a first-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece. You can contact them at [email protected].
There is a sudden spill of icy anxiety down your spine as you pick up your phone in your shaking hands. It’s 6 p.m.; your doctor’s office is closed. You open the message, and your worst fears are confirmed ... the cancer is back.
Or is it? You’re not sure. The biopsy sure sounds bad. But you’re an English teacher, not a doctor, and you spend the rest of the night Googling words like “tubulovillous” and “high-grade dysplasia.” You sit awake, terrified in front of the computer screen desperately trying to make sense of the possibly life-changing results. You wish you knew someone who could help you understand; you consider calling your doctor’s emergency line, or your cousin who is an ophthalmologist – anybody who can help you make sense of the results.
Or imagine another scenario: you’re a trans teen who has asked your doctor to refer to you by your preferred pronouns. You’re still presenting as your birth sex, in part because your family would disown you if they knew, and you’re not financially or emotionally ready for that step. You feel proud of yourself for advocating for your needs to your long-time physician, and excited about the resources they’ve included in your after visit summary and the referrals they’d made to gender-confirming specialists.
When you get home, you are confronted with a terrible reality that your doctor’s notes, orders, and recommendations are immediately viewable to anybody with your MyChart login – your parents knew the second your doctor signed the note. They received the notification, logged on as your guardians, and you have effectively been “outed” by the physician who took and oath to care for you and who you trusted implicitly.
How the Cures Act is affecting patients
While these examples may sound extreme, they are becoming more and more commonplace thanks to a recently enacted 21st Century Cures Act. The act was originally written to improve communication between physicians and patients. Part of the act stipulates that nearly all medical information – from notes to biopsies to lab results – must be available within 24 hours, published to a patient portal and a notification be sent to the patient by phone.
Oftentimes, this occurs before the ordering physician has even seen the results, much less interpreted them and made a plan for the patient. What happens now, not long after its enactment date, when it has become clear that the Cures Act is causing extreme harm to our patients?
Take, for example, the real example of a physician whose patient found out about her own intrauterine fetal demise by way of an EMR text message alert of “new imaging results!” sent directly to her phone. Or a physician colleague who witnessed firsthand the intrusive unhelpfulness of the Cures Act when she was informed via patient portal releasing her imaging information that she had a large, possibly malignant breast mass. “No phone call,” she said. “No human being for questions or comfort. Just a notification on my phone.”
The stories about the impact of the Cures Act across the medical community are an endless stream of anxiety, hurt, and broken trust. The relationship between a physician and a patient should be sacred, bolstered by communication and mutual respect.
In many ways, the new act feels like a third party to the patient-physician relationship – a digital imposter, oftentimes blurting out personal and life-altering medical information without any of the finesse, context, and perspective of an experienced physician.
Breaking ‘bad news’ to a patient
In training, some residents are taught how to “break bad news” to a patient. Some good practices for doing this are to have information available for the patient, provide emotional support, have a plan for their next steps already formulated, and call the appropriate specialist ahead of time if you can.
Above all, it’s most important to let the patient be the one to direct their own care. Give them time to ask questions and answer them honestly and clearly. Ask them how much they want to know and help them to understand the complex change in their usual state of health.
Now, unless physicians are keeping a very close eye on their inbox, results are slipping out to patients in a void. The bad news conversations aren’t happening at all, or if they are, they’re happening at 8 p.m. on a phone call after an exhausted physician ends their shift but has to slog through their results bin, calling all the patients who shouldn’t have to find out their results in solitude.
Reaching out to these patients immediately is an honorable, kind thing to, but for a physician, knowing they need to beat the patient to opening an email creates anxiety. Plus, making these calls at whatever hour the results are released to a patient is another burden added to doctors’ already-full plates.
Interpreting results
None of us want to harm our patients. All of us want to be there for them. But this act stands in the way of delivering quality, humanizing medical care.
It is true that patients have a right to access their own medical information. It is also true that waiting anxiously on results can cause undue harm to a patient. But the across-the-board, breakneck speed of information release mandated in this act causes irreparable harm not only to patients, but to the patient-physician relationship.
No patient should find out their cancer recurred while checking their emails at their desk. No patient should first learn of a life-altering diagnosis by way of scrolling through their smartphone in bed. The role of a physician is more than just a healer – we should also be educators, interpreters, partners and, first and foremost, advocates for our patients’ needs.
Our patients are depending on us to stand up and speak out about necessary changes to this act. Result releases should be delayed until they are viewed by a physician. Our patients deserve the dignity and opportunity of a conversation with their medical provider about their test results, and physicians deserve the chance to interpret results and frame the conversation in a way which is conducive to patient understanding and healing.
Dr. Persampiere is a first-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece. You can contact them at [email protected].
A high-stakes numbers game
I’m not an academic. Never will be.
I’m also a crappy statistician. Neither my university nor medical school required statistics classes, so I never really learned them. In medicine you pick up an idea of how to interpret them as part of the job, but I’m certainly not a pro with numbers.
Which brings me to the word of the day, Aduhelm, AKA aducanumab.
A lot of drugs have come and gone in the 30 years since my medical school pharmacology class, but very few with this one’s degree of uncertainty.
Clearly its mechanism works: It removes amyloid from the brain. I don’t think anyone will argue that. But the real question is whether this translates into actual clinical benefit.
The water is murky here, and even its most ardent supporters admit the evidence isn’t exactly overwhelming. To some extent the approval basically puts it in a huge open-label clinical trial, with the Food and Drug Administration saying that it will be withdrawn if success isn’t seen in follow-up studies.
I’m not a statistics person, but I understand that, when numbers are marginal, they can be spun to mean whatever someone wants them to mean. And the stakes here, both medically and financially, are pretty high.
Alzheimer’s disease, unquestionably, is a devastating illness. The best treatments we have for it are modest at best. The demand for new treatments is huge.
But “new” doesn’t mean the same as “effective.” This is where the statistics, and their supporters and detractors, come in.
Patients and their families aren’t (usually) doctors. They want a treatment that’s both effective and reasonably safe, especially for a disease where a tragic prognosis is well established. With this drug (and similar ones in development) we face a balance between uncertain benefits and a clear risk of amyloid-related imaging abnormalities. The best we can do is explain these vagaries to people so they understand the uncertainties involved.
Perhaps more troubling is the possibility lurking in the background: The amyloid comes out, but the prognosis doesn’t improve. This brings us to the possibility (already voiced in journals) that the whole amyloid theory is wrong, and we’ve spent all this time and money chasing the wrong villain. As Morpheus, in The Matrix, implies, our whole reality on this may not be real.
Regrettably, in science (and medicine is a science) the only way to find out what works and what doesn’t is through trial and error. Computer modeling can take us only so far.
But if it (and similar agents) fail in the general population, then it may be time to accept that we’re chasing the wrong bad guy.
That’s what data and statistics do.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not an academic. Never will be.
I’m also a crappy statistician. Neither my university nor medical school required statistics classes, so I never really learned them. In medicine you pick up an idea of how to interpret them as part of the job, but I’m certainly not a pro with numbers.
Which brings me to the word of the day, Aduhelm, AKA aducanumab.
A lot of drugs have come and gone in the 30 years since my medical school pharmacology class, but very few with this one’s degree of uncertainty.
Clearly its mechanism works: It removes amyloid from the brain. I don’t think anyone will argue that. But the real question is whether this translates into actual clinical benefit.
The water is murky here, and even its most ardent supporters admit the evidence isn’t exactly overwhelming. To some extent the approval basically puts it in a huge open-label clinical trial, with the Food and Drug Administration saying that it will be withdrawn if success isn’t seen in follow-up studies.
I’m not a statistics person, but I understand that, when numbers are marginal, they can be spun to mean whatever someone wants them to mean. And the stakes here, both medically and financially, are pretty high.
Alzheimer’s disease, unquestionably, is a devastating illness. The best treatments we have for it are modest at best. The demand for new treatments is huge.
But “new” doesn’t mean the same as “effective.” This is where the statistics, and their supporters and detractors, come in.
Patients and their families aren’t (usually) doctors. They want a treatment that’s both effective and reasonably safe, especially for a disease where a tragic prognosis is well established. With this drug (and similar ones in development) we face a balance between uncertain benefits and a clear risk of amyloid-related imaging abnormalities. The best we can do is explain these vagaries to people so they understand the uncertainties involved.
Perhaps more troubling is the possibility lurking in the background: The amyloid comes out, but the prognosis doesn’t improve. This brings us to the possibility (already voiced in journals) that the whole amyloid theory is wrong, and we’ve spent all this time and money chasing the wrong villain. As Morpheus, in The Matrix, implies, our whole reality on this may not be real.
Regrettably, in science (and medicine is a science) the only way to find out what works and what doesn’t is through trial and error. Computer modeling can take us only so far.
But if it (and similar agents) fail in the general population, then it may be time to accept that we’re chasing the wrong bad guy.
That’s what data and statistics do.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not an academic. Never will be.
I’m also a crappy statistician. Neither my university nor medical school required statistics classes, so I never really learned them. In medicine you pick up an idea of how to interpret them as part of the job, but I’m certainly not a pro with numbers.
Which brings me to the word of the day, Aduhelm, AKA aducanumab.
A lot of drugs have come and gone in the 30 years since my medical school pharmacology class, but very few with this one’s degree of uncertainty.
Clearly its mechanism works: It removes amyloid from the brain. I don’t think anyone will argue that. But the real question is whether this translates into actual clinical benefit.
The water is murky here, and even its most ardent supporters admit the evidence isn’t exactly overwhelming. To some extent the approval basically puts it in a huge open-label clinical trial, with the Food and Drug Administration saying that it will be withdrawn if success isn’t seen in follow-up studies.
I’m not a statistics person, but I understand that, when numbers are marginal, they can be spun to mean whatever someone wants them to mean. And the stakes here, both medically and financially, are pretty high.
Alzheimer’s disease, unquestionably, is a devastating illness. The best treatments we have for it are modest at best. The demand for new treatments is huge.
But “new” doesn’t mean the same as “effective.” This is where the statistics, and their supporters and detractors, come in.
Patients and their families aren’t (usually) doctors. They want a treatment that’s both effective and reasonably safe, especially for a disease where a tragic prognosis is well established. With this drug (and similar ones in development) we face a balance between uncertain benefits and a clear risk of amyloid-related imaging abnormalities. The best we can do is explain these vagaries to people so they understand the uncertainties involved.
Perhaps more troubling is the possibility lurking in the background: The amyloid comes out, but the prognosis doesn’t improve. This brings us to the possibility (already voiced in journals) that the whole amyloid theory is wrong, and we’ve spent all this time and money chasing the wrong villain. As Morpheus, in The Matrix, implies, our whole reality on this may not be real.
Regrettably, in science (and medicine is a science) the only way to find out what works and what doesn’t is through trial and error. Computer modeling can take us only so far.
But if it (and similar agents) fail in the general population, then it may be time to accept that we’re chasing the wrong bad guy.
That’s what data and statistics do.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Professional versus facility billing: What hospitalists must know
Dramatic impact on hospital margins
Coding and billing for the professional services of physicians and other practitioners in the hospital and for the hospital’s facility costs are separate and distinct processes. But both reflect the totality of care given to patients in the complex, costly, heavily regulated setting of an acute care hospital. And both are essential to the financial well-being of the hospital and its providers, and to their mutual ability to survive current financial uncertainties imposed by the COVID pandemic.
“What hospitalists don’t realize is that your professional billing is a completely separate entity [from the facility’s billing],” said Aziz Ansari, DO, SFHM, hospitalist, professor of medicine, and associate chief medical officer for clinical optimization and revenue integrity at Loyola University Medical Center in Maywood, Ill. “Your E/M [Evaluation and Management] coding has a separate set of rules, which are not married at all to facility billing.”
Dr. Ansari presented a session at Converge – the annual conference of SHM – in May 2021, on the hospitalist’s role in “Piloting the Twin Engines of the Mid-Revenue Cycle Ship,” with a focus on how physician documentation can optimize both facility billing and quality of care. Hospitalists generally don’t realize how much impact they actually have on their hospital’s revenue cycle and quality, he said. Thorough documentation, accurately and specifically describing the patient’s severity of illness and complexity, affects both.
“When a utilization management nurse calls you about a case, you need to realize they are your partner in getting it right.” A simple documentation lapse that would change a case from observation to inpatient could cost the hospital $3,000 or more per case, and that can add up quickly, Dr. Ansari said. “We’ve seen what happened with COVID. We realized how fragile the system is, and how razor-thin hospital margins are.”
Distinction between professional and facility billing
Professional billing by hospitalist physicians and advanced practice providers is done for their individual encounters with patients and charged per visit for every day the patient is in the hospital based on the treatments, examinations, and medical decision-making required to care for that patient.
These are spelled out using E/M codes derived from Current Procedural Terminology, which is maintained by the American Medical Association for specifying what the provider did during the encounter. Other parameters of professional billing include complexity of decision-making versus amount of time spent, and a variety of modifiers.
By contrast, facility billing by hospitals is based on the complexity of the patient’s condition and is generally done whether the hospitalization is considered an inpatient hospitalization or an outpatient hospitalization such as an observation stay. Inpatient hospital stays are often paid using diagnosis-related groupings (DRGs), Medicare’s patient classification system for standardizing prospective payment to hospitals and encouraging cost-containment strategies.
DRGs, which represent about half of total hospital reimbursement, are a separate payment mechanism covering all facility charges associated with the inpatient stay from admission to discharge, incorporating the costs of providing hospital care, including but not limited to space, equipment, supplies, tests, and medications. Outpatient hospital stays, by contrast, are paid based on Ambulatory Payment Classifications.
A facility bill is submitted to the payer at the end of the hospital stay, describing the patient’s condition using ICD-10 diagnostic codes. All of the patient’s diagnoses and comorbidities contribute to the assignment of a DRG that best captures the total hospital stay. But to make the issue more complicated, the system is evolving toward models of bundled payment that will eventually phase out traditional DRGs in favor of new systems combining inpatient and outpatient reimbursement into a single bundled episode of care.
Professional and facility bills for a single hospitalization may be prepared by different personnel on separate teams following different rules, although they may both be housed in the hospital’s billing department. The differing rules for coding professional services versus facility services can be hard for hospitalists to appreciate, said Wendy Arafiles, MD, a pediatric hospitalist at Phoenix Children’s Hospital and medical director for its clinical documentation integrity (CDI) team. An example is for uncertain diagnoses. There may be a clinical suspicion of a diagnosis, and language such as “likely bacterial pneumonia” might be sufficient for facility coding but not for professional services coding.
Hospitalists, depending on their group’s size, structure, and relationship to the hospital, may be responsible for selecting the CPT codes or other parameters for the insurance claim and bill. Or these may be left to billing specialists. And those specialists could be employed by the hospital or by the hospitalist group or multispecialty medical group, or they could be contracted outside agencies that handle the billing for a fee.
The revenue cycle
The hospital revenue cycle has a lot of cogs in the machine, Dr. Arafiles said. “This is just one of the many nuances of our crazy system. I will go out on a limb and say it is not our job as clinicians to know all of those nuances.” The DRG assignment is dependent on how providers can describe the complexity of the patient and severity of the illness, even if it doesn’t impact professional billing, Dr. Arafiles added.
Hospitalists don’t want to think about money when providing patient care. “Our job is to provide the best care to our patients. We often utilize resources without thinking about how much they are going to cost, so that we can do what we think is necessary for our patients,” she explained. But accurate diagnosis codes can capture the complexity of the care. “Maybe we don’t take that part seriously enough. As long as I, as the provider, can accurately describe the complexity of my patient, I can justify why I spent all those resources and so many days caring for him or her.”
Charles Locke, MD, executive medical director of care management for LifeBridge Health and assistant professor of medicine at Johns Hopkins University, Baltimore, said hospitalists typically are paid set salaries directly by the hospital, in some cases with productivity bonuses based in part on their billing and posted RVUs (relative value units). RVUs are the cornerstone of Medicare’s reimbursement formula for physician services.
“Another thing to keep in mind, one might think in 2021 that the computer systems would be sophisticated enough to link up professional and facility billing to ensure that bills for each are concordant for services provided on a given day. But it turns out they are not yet well connected,” Dr. Locke said.
“These are issues that everybody struggles with. Hospitalists need to know and order the appropriate status, inpatient versus outpatient, and whether and when to order observation services, as this will affect hospital reimbursement and, potentially, patient liability,” he explained.1 If the hospital is denied its facility claim because of improper status, that denial doesn’t necessary extend to a denial for the doctor’s professional fee. “Hospitalists need to know these are often separated. Even though their professional fee is honored, the hospital’s service charges may not be.”
Dr. Locke said knowing the history of Medicare might help hospitalists to better appreciate the distinctions. When this federal entitlement was first proposed in the 1960s as a way to help older Americans in poverty obtain needed health care, organized medicine sought to be excluded from the program. “Nonhospital services and doctors’ service fees were not included in the original Medicare proposal,” he said. Medicare Part B was created to provide insurance for doctors’ professional fees, which are still handled separately under Medicare.
Many institutions use clinical documentation for multiple purposes. “There are so many masters for this one document,” Dr. Arafiles said. The information is also used for various quality and patient safety metrics and data gathering. “Every code we choose is used in many different ways by the institution. We don’t know where all it goes. But we need to know how to describe how complex the case was, and how much work it entailed. The more we know about how to describe that, the better for the institution.”
Dr. Arafiles views the clinical note, first and foremost, as clinical communication, so that one provider can seamlessly pick up where the previous left off. “If I use language in my note that is accurate and specific, it will be useful to all who later need it.” Building on metrics such as expected versus actual 30-day readmission rates, risk-adjusted mortality, and all the ways government agencies report hospital quality, she said, “what we document has lasting impact. That’s where the facility side of billing and coding is ever more important. You can’t just think about your professional billing and RVUs.”
Support from the hospital
Some hospitalists may think facility billing is not their concern. But consider this: The average support or subsidy paid by U.S. hospitals for a full-time equivalent hospitalist is estimated at $198,750, according to SHM’s 2020 State of Hospital Medicine.2 That support reflects the difference between the cost of employing a hospitalist in a competitive labor environment and what that provider is actually able to generate in billing income, said Hardik Vora, MD, MPH, SFHM, chair of SHM’s practice management committee.
With a lot of medical specialties, the physician’s salary is only or largely supported by professional billing, said Dr. Vora, who is medical director for Hospital Medicine and physician advisor for utilization management and CDI at Riverside Health System, Yorktown, Va.
“Hospital medicine is different in that aspect, regardless of employment model. And that’s where the concept of value comes in – how else do you bring value to the hospital that supports you,” said Dr. Vora.
Hospitalists often emphasize their contributions to quality improvement, patient safety, and hospital governance committees – all the ways they contribute to the health of the institution – as justification for their support from the hospital. But beneath all of that is the income the hospital generates from facility billing and from the hospitalist’s contributions to complete, accurate, and timely documentation that can support the hospital’s bills.
Typically, this hospital support to supplement hospitalist billing income is not directly tied to the income generated by facility billing or to the hospitalist’s contribution to its completeness. But between growing technological sophistication and greater belt-tightening, that link may get closer over time.
Other players
Because of the importance of complete and accurate billing to the hospital’s financial well-being, specialized supportive services have evolved, from traditional utilization review or utilization management to CDI services and the role of physician advisors – experienced doctors who know well how these processes work and are able to teach providers about regulatory compliance and medical necessity.
“One of my jobs as the medical director for our hospital’s CDI program is to educate residents, fellows, and newly onboarded providers to be descriptive enough in their charting to capture the complexity of the patient’s condition,” Dr. Arafiles said. Physician advisors and CDI programs can involve clinical providers in bringing value to the institution through their documentation. They serve as the intermediaries between the coders and the clinicians.
The CDI specialist’s job description focuses on diagnosis capture and associated reimbursement. But integrity broadly defined goes to the integrity of the medical record and its contribution to quality and patient safety as well as providing a medical record that is defensible to audits, physician revenue cycle expert Glenn Krauss noted in a recent post at ICD10 Monitor.3
Dr. Vora sees his role as physician advisor to be the link between the hospital’s executive team and the hospital’s medical providers. “Providers need help in understanding a complex set of ever-changing rules of facility billing and the frequently competing priorities between facility and professional billing. I tell my providers: The longer the patient stays in the hospital, you may be generating more RVUs, but our facility may be losing money.”
Hospital administrators are acutely aware of facility billing, but they don’t necessarily understand the nuances of professional billing, said Jay Weatherly, MS, the cofounder of Hospitalist Billing, a company that specializes in comprehensive billing and collection solutions for hospitalist groups that are employed directly by their hospitals. But he sees an essential symbiotic relationship between hospital administrators and clinicians.
“We rely on hospitalists’ record keeping to do our job. We rely on them to get it right,” he said. “We want to encourage doctors to cooperate with the process. Billing should never be a physician’s top priority, but it is important, nonetheless.”
HBI is relentless in pursuit of the information needed for its coding and billing, but does so gently, in a way not to put off doctors, Mr. Weatherly said. “There is an art and a science associated with securing the needed information. We have great respect for the doctors we work with, yet we’re all spokes in a bigger wheel, and we need to bill effectively in order to keep the wheel moving.”
What can hospitalists do?
Sources for this article say one of the best places for hospitalists to start improving their understanding of these distinctions is to ask the coders in their institution for advice on how to make the process run more smoothly.
“If you have a CDI team, they are there to help. Reach out to them,” Dr. Arafiles said. Generally, medical schools and residency programs fail to convey the complexities of contemporary hospital economics to future doctors.
Hospitalists have become indispensable, Dr. Vora said. But salaries for hospitalists are going up while hospital reimbursement is going down, and hospitalists are not seeing more patients. “At some point we will no longer be able to say financial support for hospital medicine groups is just a cost of doing business for the hospital. COVID tested us – and demonstrated how much hospital executives value us as part of the team. Our organization absolutely stood behind its physicians despite financially challenging times. Now we need to do what we can to support the organization,” he added.
Hospitalists can also continue to educate themselves on good documentation and coding practices, by finding programs like SHM’s Utilization Management and Clinical Documentation for Hospitalists.
“As we see a significant shift to value-based payment, with its focus on value, efficiency, quality – the best care at the lowest possible price – hospital medicine as a specialty will be best positioned to help with that. If the hospital does well, we do well. We should be building relationships with the hospital’s leadership team,” Dr. Vora said. “You always want to contribute to that partnership to the highest level possible. When they look at us, they should see their most reliable partner.”
References
1. Locke C, Hu E. Medicare’s two-midnight rule: What hospitalists must know. The Hospitalist. 2019 Feb 22.
2. Beresford L. Hospital medicine in a worldwide pandemic: State of Hospital Medicine 2020. The Hospitalist. 2020 Sep 20.
3. Krauss G. Clinical documentation integrity: rebranding and repurposing. ICD10 Monitor. March 16, 2020 Mar 16. https://www.icd10monitor.com/clinical-documentation-integrity-rebranding-and-repurposing.
Dramatic impact on hospital margins
Dramatic impact on hospital margins
Coding and billing for the professional services of physicians and other practitioners in the hospital and for the hospital’s facility costs are separate and distinct processes. But both reflect the totality of care given to patients in the complex, costly, heavily regulated setting of an acute care hospital. And both are essential to the financial well-being of the hospital and its providers, and to their mutual ability to survive current financial uncertainties imposed by the COVID pandemic.
“What hospitalists don’t realize is that your professional billing is a completely separate entity [from the facility’s billing],” said Aziz Ansari, DO, SFHM, hospitalist, professor of medicine, and associate chief medical officer for clinical optimization and revenue integrity at Loyola University Medical Center in Maywood, Ill. “Your E/M [Evaluation and Management] coding has a separate set of rules, which are not married at all to facility billing.”
Dr. Ansari presented a session at Converge – the annual conference of SHM – in May 2021, on the hospitalist’s role in “Piloting the Twin Engines of the Mid-Revenue Cycle Ship,” with a focus on how physician documentation can optimize both facility billing and quality of care. Hospitalists generally don’t realize how much impact they actually have on their hospital’s revenue cycle and quality, he said. Thorough documentation, accurately and specifically describing the patient’s severity of illness and complexity, affects both.
“When a utilization management nurse calls you about a case, you need to realize they are your partner in getting it right.” A simple documentation lapse that would change a case from observation to inpatient could cost the hospital $3,000 or more per case, and that can add up quickly, Dr. Ansari said. “We’ve seen what happened with COVID. We realized how fragile the system is, and how razor-thin hospital margins are.”
Distinction between professional and facility billing
Professional billing by hospitalist physicians and advanced practice providers is done for their individual encounters with patients and charged per visit for every day the patient is in the hospital based on the treatments, examinations, and medical decision-making required to care for that patient.
These are spelled out using E/M codes derived from Current Procedural Terminology, which is maintained by the American Medical Association for specifying what the provider did during the encounter. Other parameters of professional billing include complexity of decision-making versus amount of time spent, and a variety of modifiers.
By contrast, facility billing by hospitals is based on the complexity of the patient’s condition and is generally done whether the hospitalization is considered an inpatient hospitalization or an outpatient hospitalization such as an observation stay. Inpatient hospital stays are often paid using diagnosis-related groupings (DRGs), Medicare’s patient classification system for standardizing prospective payment to hospitals and encouraging cost-containment strategies.
DRGs, which represent about half of total hospital reimbursement, are a separate payment mechanism covering all facility charges associated with the inpatient stay from admission to discharge, incorporating the costs of providing hospital care, including but not limited to space, equipment, supplies, tests, and medications. Outpatient hospital stays, by contrast, are paid based on Ambulatory Payment Classifications.
A facility bill is submitted to the payer at the end of the hospital stay, describing the patient’s condition using ICD-10 diagnostic codes. All of the patient’s diagnoses and comorbidities contribute to the assignment of a DRG that best captures the total hospital stay. But to make the issue more complicated, the system is evolving toward models of bundled payment that will eventually phase out traditional DRGs in favor of new systems combining inpatient and outpatient reimbursement into a single bundled episode of care.
Professional and facility bills for a single hospitalization may be prepared by different personnel on separate teams following different rules, although they may both be housed in the hospital’s billing department. The differing rules for coding professional services versus facility services can be hard for hospitalists to appreciate, said Wendy Arafiles, MD, a pediatric hospitalist at Phoenix Children’s Hospital and medical director for its clinical documentation integrity (CDI) team. An example is for uncertain diagnoses. There may be a clinical suspicion of a diagnosis, and language such as “likely bacterial pneumonia” might be sufficient for facility coding but not for professional services coding.
Hospitalists, depending on their group’s size, structure, and relationship to the hospital, may be responsible for selecting the CPT codes or other parameters for the insurance claim and bill. Or these may be left to billing specialists. And those specialists could be employed by the hospital or by the hospitalist group or multispecialty medical group, or they could be contracted outside agencies that handle the billing for a fee.
The revenue cycle
The hospital revenue cycle has a lot of cogs in the machine, Dr. Arafiles said. “This is just one of the many nuances of our crazy system. I will go out on a limb and say it is not our job as clinicians to know all of those nuances.” The DRG assignment is dependent on how providers can describe the complexity of the patient and severity of the illness, even if it doesn’t impact professional billing, Dr. Arafiles added.
Hospitalists don’t want to think about money when providing patient care. “Our job is to provide the best care to our patients. We often utilize resources without thinking about how much they are going to cost, so that we can do what we think is necessary for our patients,” she explained. But accurate diagnosis codes can capture the complexity of the care. “Maybe we don’t take that part seriously enough. As long as I, as the provider, can accurately describe the complexity of my patient, I can justify why I spent all those resources and so many days caring for him or her.”
Charles Locke, MD, executive medical director of care management for LifeBridge Health and assistant professor of medicine at Johns Hopkins University, Baltimore, said hospitalists typically are paid set salaries directly by the hospital, in some cases with productivity bonuses based in part on their billing and posted RVUs (relative value units). RVUs are the cornerstone of Medicare’s reimbursement formula for physician services.
“Another thing to keep in mind, one might think in 2021 that the computer systems would be sophisticated enough to link up professional and facility billing to ensure that bills for each are concordant for services provided on a given day. But it turns out they are not yet well connected,” Dr. Locke said.
“These are issues that everybody struggles with. Hospitalists need to know and order the appropriate status, inpatient versus outpatient, and whether and when to order observation services, as this will affect hospital reimbursement and, potentially, patient liability,” he explained.1 If the hospital is denied its facility claim because of improper status, that denial doesn’t necessary extend to a denial for the doctor’s professional fee. “Hospitalists need to know these are often separated. Even though their professional fee is honored, the hospital’s service charges may not be.”
Dr. Locke said knowing the history of Medicare might help hospitalists to better appreciate the distinctions. When this federal entitlement was first proposed in the 1960s as a way to help older Americans in poverty obtain needed health care, organized medicine sought to be excluded from the program. “Nonhospital services and doctors’ service fees were not included in the original Medicare proposal,” he said. Medicare Part B was created to provide insurance for doctors’ professional fees, which are still handled separately under Medicare.
Many institutions use clinical documentation for multiple purposes. “There are so many masters for this one document,” Dr. Arafiles said. The information is also used for various quality and patient safety metrics and data gathering. “Every code we choose is used in many different ways by the institution. We don’t know where all it goes. But we need to know how to describe how complex the case was, and how much work it entailed. The more we know about how to describe that, the better for the institution.”
Dr. Arafiles views the clinical note, first and foremost, as clinical communication, so that one provider can seamlessly pick up where the previous left off. “If I use language in my note that is accurate and specific, it will be useful to all who later need it.” Building on metrics such as expected versus actual 30-day readmission rates, risk-adjusted mortality, and all the ways government agencies report hospital quality, she said, “what we document has lasting impact. That’s where the facility side of billing and coding is ever more important. You can’t just think about your professional billing and RVUs.”
Support from the hospital
Some hospitalists may think facility billing is not their concern. But consider this: The average support or subsidy paid by U.S. hospitals for a full-time equivalent hospitalist is estimated at $198,750, according to SHM’s 2020 State of Hospital Medicine.2 That support reflects the difference between the cost of employing a hospitalist in a competitive labor environment and what that provider is actually able to generate in billing income, said Hardik Vora, MD, MPH, SFHM, chair of SHM’s practice management committee.
With a lot of medical specialties, the physician’s salary is only or largely supported by professional billing, said Dr. Vora, who is medical director for Hospital Medicine and physician advisor for utilization management and CDI at Riverside Health System, Yorktown, Va.
“Hospital medicine is different in that aspect, regardless of employment model. And that’s where the concept of value comes in – how else do you bring value to the hospital that supports you,” said Dr. Vora.
Hospitalists often emphasize their contributions to quality improvement, patient safety, and hospital governance committees – all the ways they contribute to the health of the institution – as justification for their support from the hospital. But beneath all of that is the income the hospital generates from facility billing and from the hospitalist’s contributions to complete, accurate, and timely documentation that can support the hospital’s bills.
Typically, this hospital support to supplement hospitalist billing income is not directly tied to the income generated by facility billing or to the hospitalist’s contribution to its completeness. But between growing technological sophistication and greater belt-tightening, that link may get closer over time.
Other players
Because of the importance of complete and accurate billing to the hospital’s financial well-being, specialized supportive services have evolved, from traditional utilization review or utilization management to CDI services and the role of physician advisors – experienced doctors who know well how these processes work and are able to teach providers about regulatory compliance and medical necessity.
“One of my jobs as the medical director for our hospital’s CDI program is to educate residents, fellows, and newly onboarded providers to be descriptive enough in their charting to capture the complexity of the patient’s condition,” Dr. Arafiles said. Physician advisors and CDI programs can involve clinical providers in bringing value to the institution through their documentation. They serve as the intermediaries between the coders and the clinicians.
The CDI specialist’s job description focuses on diagnosis capture and associated reimbursement. But integrity broadly defined goes to the integrity of the medical record and its contribution to quality and patient safety as well as providing a medical record that is defensible to audits, physician revenue cycle expert Glenn Krauss noted in a recent post at ICD10 Monitor.3
Dr. Vora sees his role as physician advisor to be the link between the hospital’s executive team and the hospital’s medical providers. “Providers need help in understanding a complex set of ever-changing rules of facility billing and the frequently competing priorities between facility and professional billing. I tell my providers: The longer the patient stays in the hospital, you may be generating more RVUs, but our facility may be losing money.”
Hospital administrators are acutely aware of facility billing, but they don’t necessarily understand the nuances of professional billing, said Jay Weatherly, MS, the cofounder of Hospitalist Billing, a company that specializes in comprehensive billing and collection solutions for hospitalist groups that are employed directly by their hospitals. But he sees an essential symbiotic relationship between hospital administrators and clinicians.
“We rely on hospitalists’ record keeping to do our job. We rely on them to get it right,” he said. “We want to encourage doctors to cooperate with the process. Billing should never be a physician’s top priority, but it is important, nonetheless.”
HBI is relentless in pursuit of the information needed for its coding and billing, but does so gently, in a way not to put off doctors, Mr. Weatherly said. “There is an art and a science associated with securing the needed information. We have great respect for the doctors we work with, yet we’re all spokes in a bigger wheel, and we need to bill effectively in order to keep the wheel moving.”
What can hospitalists do?
Sources for this article say one of the best places for hospitalists to start improving their understanding of these distinctions is to ask the coders in their institution for advice on how to make the process run more smoothly.
“If you have a CDI team, they are there to help. Reach out to them,” Dr. Arafiles said. Generally, medical schools and residency programs fail to convey the complexities of contemporary hospital economics to future doctors.
Hospitalists have become indispensable, Dr. Vora said. But salaries for hospitalists are going up while hospital reimbursement is going down, and hospitalists are not seeing more patients. “At some point we will no longer be able to say financial support for hospital medicine groups is just a cost of doing business for the hospital. COVID tested us – and demonstrated how much hospital executives value us as part of the team. Our organization absolutely stood behind its physicians despite financially challenging times. Now we need to do what we can to support the organization,” he added.
Hospitalists can also continue to educate themselves on good documentation and coding practices, by finding programs like SHM’s Utilization Management and Clinical Documentation for Hospitalists.
“As we see a significant shift to value-based payment, with its focus on value, efficiency, quality – the best care at the lowest possible price – hospital medicine as a specialty will be best positioned to help with that. If the hospital does well, we do well. We should be building relationships with the hospital’s leadership team,” Dr. Vora said. “You always want to contribute to that partnership to the highest level possible. When they look at us, they should see their most reliable partner.”
References
1. Locke C, Hu E. Medicare’s two-midnight rule: What hospitalists must know. The Hospitalist. 2019 Feb 22.
2. Beresford L. Hospital medicine in a worldwide pandemic: State of Hospital Medicine 2020. The Hospitalist. 2020 Sep 20.
3. Krauss G. Clinical documentation integrity: rebranding and repurposing. ICD10 Monitor. March 16, 2020 Mar 16. https://www.icd10monitor.com/clinical-documentation-integrity-rebranding-and-repurposing.
Coding and billing for the professional services of physicians and other practitioners in the hospital and for the hospital’s facility costs are separate and distinct processes. But both reflect the totality of care given to patients in the complex, costly, heavily regulated setting of an acute care hospital. And both are essential to the financial well-being of the hospital and its providers, and to their mutual ability to survive current financial uncertainties imposed by the COVID pandemic.
“What hospitalists don’t realize is that your professional billing is a completely separate entity [from the facility’s billing],” said Aziz Ansari, DO, SFHM, hospitalist, professor of medicine, and associate chief medical officer for clinical optimization and revenue integrity at Loyola University Medical Center in Maywood, Ill. “Your E/M [Evaluation and Management] coding has a separate set of rules, which are not married at all to facility billing.”
Dr. Ansari presented a session at Converge – the annual conference of SHM – in May 2021, on the hospitalist’s role in “Piloting the Twin Engines of the Mid-Revenue Cycle Ship,” with a focus on how physician documentation can optimize both facility billing and quality of care. Hospitalists generally don’t realize how much impact they actually have on their hospital’s revenue cycle and quality, he said. Thorough documentation, accurately and specifically describing the patient’s severity of illness and complexity, affects both.
“When a utilization management nurse calls you about a case, you need to realize they are your partner in getting it right.” A simple documentation lapse that would change a case from observation to inpatient could cost the hospital $3,000 or more per case, and that can add up quickly, Dr. Ansari said. “We’ve seen what happened with COVID. We realized how fragile the system is, and how razor-thin hospital margins are.”
Distinction between professional and facility billing
Professional billing by hospitalist physicians and advanced practice providers is done for their individual encounters with patients and charged per visit for every day the patient is in the hospital based on the treatments, examinations, and medical decision-making required to care for that patient.
These are spelled out using E/M codes derived from Current Procedural Terminology, which is maintained by the American Medical Association for specifying what the provider did during the encounter. Other parameters of professional billing include complexity of decision-making versus amount of time spent, and a variety of modifiers.
By contrast, facility billing by hospitals is based on the complexity of the patient’s condition and is generally done whether the hospitalization is considered an inpatient hospitalization or an outpatient hospitalization such as an observation stay. Inpatient hospital stays are often paid using diagnosis-related groupings (DRGs), Medicare’s patient classification system for standardizing prospective payment to hospitals and encouraging cost-containment strategies.
DRGs, which represent about half of total hospital reimbursement, are a separate payment mechanism covering all facility charges associated with the inpatient stay from admission to discharge, incorporating the costs of providing hospital care, including but not limited to space, equipment, supplies, tests, and medications. Outpatient hospital stays, by contrast, are paid based on Ambulatory Payment Classifications.
A facility bill is submitted to the payer at the end of the hospital stay, describing the patient’s condition using ICD-10 diagnostic codes. All of the patient’s diagnoses and comorbidities contribute to the assignment of a DRG that best captures the total hospital stay. But to make the issue more complicated, the system is evolving toward models of bundled payment that will eventually phase out traditional DRGs in favor of new systems combining inpatient and outpatient reimbursement into a single bundled episode of care.
Professional and facility bills for a single hospitalization may be prepared by different personnel on separate teams following different rules, although they may both be housed in the hospital’s billing department. The differing rules for coding professional services versus facility services can be hard for hospitalists to appreciate, said Wendy Arafiles, MD, a pediatric hospitalist at Phoenix Children’s Hospital and medical director for its clinical documentation integrity (CDI) team. An example is for uncertain diagnoses. There may be a clinical suspicion of a diagnosis, and language such as “likely bacterial pneumonia” might be sufficient for facility coding but not for professional services coding.
Hospitalists, depending on their group’s size, structure, and relationship to the hospital, may be responsible for selecting the CPT codes or other parameters for the insurance claim and bill. Or these may be left to billing specialists. And those specialists could be employed by the hospital or by the hospitalist group or multispecialty medical group, or they could be contracted outside agencies that handle the billing for a fee.
The revenue cycle
The hospital revenue cycle has a lot of cogs in the machine, Dr. Arafiles said. “This is just one of the many nuances of our crazy system. I will go out on a limb and say it is not our job as clinicians to know all of those nuances.” The DRG assignment is dependent on how providers can describe the complexity of the patient and severity of the illness, even if it doesn’t impact professional billing, Dr. Arafiles added.
Hospitalists don’t want to think about money when providing patient care. “Our job is to provide the best care to our patients. We often utilize resources without thinking about how much they are going to cost, so that we can do what we think is necessary for our patients,” she explained. But accurate diagnosis codes can capture the complexity of the care. “Maybe we don’t take that part seriously enough. As long as I, as the provider, can accurately describe the complexity of my patient, I can justify why I spent all those resources and so many days caring for him or her.”
Charles Locke, MD, executive medical director of care management for LifeBridge Health and assistant professor of medicine at Johns Hopkins University, Baltimore, said hospitalists typically are paid set salaries directly by the hospital, in some cases with productivity bonuses based in part on their billing and posted RVUs (relative value units). RVUs are the cornerstone of Medicare’s reimbursement formula for physician services.
“Another thing to keep in mind, one might think in 2021 that the computer systems would be sophisticated enough to link up professional and facility billing to ensure that bills for each are concordant for services provided on a given day. But it turns out they are not yet well connected,” Dr. Locke said.
“These are issues that everybody struggles with. Hospitalists need to know and order the appropriate status, inpatient versus outpatient, and whether and when to order observation services, as this will affect hospital reimbursement and, potentially, patient liability,” he explained.1 If the hospital is denied its facility claim because of improper status, that denial doesn’t necessary extend to a denial for the doctor’s professional fee. “Hospitalists need to know these are often separated. Even though their professional fee is honored, the hospital’s service charges may not be.”
Dr. Locke said knowing the history of Medicare might help hospitalists to better appreciate the distinctions. When this federal entitlement was first proposed in the 1960s as a way to help older Americans in poverty obtain needed health care, organized medicine sought to be excluded from the program. “Nonhospital services and doctors’ service fees were not included in the original Medicare proposal,” he said. Medicare Part B was created to provide insurance for doctors’ professional fees, which are still handled separately under Medicare.
Many institutions use clinical documentation for multiple purposes. “There are so many masters for this one document,” Dr. Arafiles said. The information is also used for various quality and patient safety metrics and data gathering. “Every code we choose is used in many different ways by the institution. We don’t know where all it goes. But we need to know how to describe how complex the case was, and how much work it entailed. The more we know about how to describe that, the better for the institution.”
Dr. Arafiles views the clinical note, first and foremost, as clinical communication, so that one provider can seamlessly pick up where the previous left off. “If I use language in my note that is accurate and specific, it will be useful to all who later need it.” Building on metrics such as expected versus actual 30-day readmission rates, risk-adjusted mortality, and all the ways government agencies report hospital quality, she said, “what we document has lasting impact. That’s where the facility side of billing and coding is ever more important. You can’t just think about your professional billing and RVUs.”
Support from the hospital
Some hospitalists may think facility billing is not their concern. But consider this: The average support or subsidy paid by U.S. hospitals for a full-time equivalent hospitalist is estimated at $198,750, according to SHM’s 2020 State of Hospital Medicine.2 That support reflects the difference between the cost of employing a hospitalist in a competitive labor environment and what that provider is actually able to generate in billing income, said Hardik Vora, MD, MPH, SFHM, chair of SHM’s practice management committee.
With a lot of medical specialties, the physician’s salary is only or largely supported by professional billing, said Dr. Vora, who is medical director for Hospital Medicine and physician advisor for utilization management and CDI at Riverside Health System, Yorktown, Va.
“Hospital medicine is different in that aspect, regardless of employment model. And that’s where the concept of value comes in – how else do you bring value to the hospital that supports you,” said Dr. Vora.
Hospitalists often emphasize their contributions to quality improvement, patient safety, and hospital governance committees – all the ways they contribute to the health of the institution – as justification for their support from the hospital. But beneath all of that is the income the hospital generates from facility billing and from the hospitalist’s contributions to complete, accurate, and timely documentation that can support the hospital’s bills.
Typically, this hospital support to supplement hospitalist billing income is not directly tied to the income generated by facility billing or to the hospitalist’s contribution to its completeness. But between growing technological sophistication and greater belt-tightening, that link may get closer over time.
Other players
Because of the importance of complete and accurate billing to the hospital’s financial well-being, specialized supportive services have evolved, from traditional utilization review or utilization management to CDI services and the role of physician advisors – experienced doctors who know well how these processes work and are able to teach providers about regulatory compliance and medical necessity.
“One of my jobs as the medical director for our hospital’s CDI program is to educate residents, fellows, and newly onboarded providers to be descriptive enough in their charting to capture the complexity of the patient’s condition,” Dr. Arafiles said. Physician advisors and CDI programs can involve clinical providers in bringing value to the institution through their documentation. They serve as the intermediaries between the coders and the clinicians.
The CDI specialist’s job description focuses on diagnosis capture and associated reimbursement. But integrity broadly defined goes to the integrity of the medical record and its contribution to quality and patient safety as well as providing a medical record that is defensible to audits, physician revenue cycle expert Glenn Krauss noted in a recent post at ICD10 Monitor.3
Dr. Vora sees his role as physician advisor to be the link between the hospital’s executive team and the hospital’s medical providers. “Providers need help in understanding a complex set of ever-changing rules of facility billing and the frequently competing priorities between facility and professional billing. I tell my providers: The longer the patient stays in the hospital, you may be generating more RVUs, but our facility may be losing money.”
Hospital administrators are acutely aware of facility billing, but they don’t necessarily understand the nuances of professional billing, said Jay Weatherly, MS, the cofounder of Hospitalist Billing, a company that specializes in comprehensive billing and collection solutions for hospitalist groups that are employed directly by their hospitals. But he sees an essential symbiotic relationship between hospital administrators and clinicians.
“We rely on hospitalists’ record keeping to do our job. We rely on them to get it right,” he said. “We want to encourage doctors to cooperate with the process. Billing should never be a physician’s top priority, but it is important, nonetheless.”
HBI is relentless in pursuit of the information needed for its coding and billing, but does so gently, in a way not to put off doctors, Mr. Weatherly said. “There is an art and a science associated with securing the needed information. We have great respect for the doctors we work with, yet we’re all spokes in a bigger wheel, and we need to bill effectively in order to keep the wheel moving.”
What can hospitalists do?
Sources for this article say one of the best places for hospitalists to start improving their understanding of these distinctions is to ask the coders in their institution for advice on how to make the process run more smoothly.
“If you have a CDI team, they are there to help. Reach out to them,” Dr. Arafiles said. Generally, medical schools and residency programs fail to convey the complexities of contemporary hospital economics to future doctors.
Hospitalists have become indispensable, Dr. Vora said. But salaries for hospitalists are going up while hospital reimbursement is going down, and hospitalists are not seeing more patients. “At some point we will no longer be able to say financial support for hospital medicine groups is just a cost of doing business for the hospital. COVID tested us – and demonstrated how much hospital executives value us as part of the team. Our organization absolutely stood behind its physicians despite financially challenging times. Now we need to do what we can to support the organization,” he added.
Hospitalists can also continue to educate themselves on good documentation and coding practices, by finding programs like SHM’s Utilization Management and Clinical Documentation for Hospitalists.
“As we see a significant shift to value-based payment, with its focus on value, efficiency, quality – the best care at the lowest possible price – hospital medicine as a specialty will be best positioned to help with that. If the hospital does well, we do well. We should be building relationships with the hospital’s leadership team,” Dr. Vora said. “You always want to contribute to that partnership to the highest level possible. When they look at us, they should see their most reliable partner.”
References
1. Locke C, Hu E. Medicare’s two-midnight rule: What hospitalists must know. The Hospitalist. 2019 Feb 22.
2. Beresford L. Hospital medicine in a worldwide pandemic: State of Hospital Medicine 2020. The Hospitalist. 2020 Sep 20.
3. Krauss G. Clinical documentation integrity: rebranding and repurposing. ICD10 Monitor. March 16, 2020 Mar 16. https://www.icd10monitor.com/clinical-documentation-integrity-rebranding-and-repurposing.
Pyoderma gangrenosum: Understanding the difficult diagnosis
Pyoderma gangrenosum (PG), a rare and painful ulcerative skin disorder, requires special care because “it’s a challenging diagnosis to make” and is frequently linked to serious comorbid conditions, a dermatologist told colleagues.
PG is also challenging to manage, said Jeffrey Callen, MD, professor and chief of the division of dermatology at the University of Louisville (Ky.), who spoke at the Inaugural Symposium for Inflammatory Skin Disease. “There are multiple treatments, but few have a high level of evidence to document their efficacy.”
PG is a neutrophilic dermatosis that usually occurs with a small lesion, often pustular, that spreads, and is a diagnosis of exclusion. “There’s no way you can possibly exclude everything, but the major things that have to be excluded are infection and malignancies,” he said. “Doing a good history and physical examination is critical, and a biopsy should be done in the vast majority of patients.”
Cultures and routine labs should be obtained, said Dr. Callen, highlighting tests that measure immunofixation (IFE), antineutrophil cytoplasmic antibodies (ANCE), anticardiolipin antibody (aCL), and lupus anticoagulant (LA).
Bowel and bone marrow tests may be appropriate in some patients, he said, noting that about half of PG cases are linked to comorbid conditions such as inflammatory bowel disease (IBD), arthritis, and hematologic diseases.
Dr. Callen also made the following points about making the diagnosis:
- Several clinical variants exist: classic, peristomal, and atypical.
- Pathergy – hyperreactivity of skin to injury – occurs in about a third of patients.
- Neutrophilic infiltrates may occur in other organs.
- Numerous drugs, including isotretinoin, can cause PG.
- PG may be misdiagnosed as necrotizing fasciitis.
- Several diagnostic frameworks exist: the Su Criteria, the PARACELCUS Score, and the Delphi Consensus Criteria. The Delphi criteria identified the highest percentage of cases (89%) in a study comparing the three, published in 2020. The frameworks “are helpful in the clinic, but they are not to be used as criteria for diagnosis. They’re really for classification,” Dr. Callen said.
Once the diagnosis has been made, he said, focus on healing the wound, which he said “can be done as any other wound would be healed,” and calming the inflammation.
“Patients who have mild disease might be treated with lower doses of prednisone, topical medications, or intralesional injections,” he said. “Corticosteroids are never wrong in the beginning.” Some patients may have genetic abnormalities related to PG, he added, and medications that target them may be appropriate.
Antibiotics and biologic agents, particularly TNF-alpha inhibitors, are possible treatments, Dr. Callen said. He highlighted a 2018 systematic review that evaluated treatments and found the most evidence supported systemic corticosteroids, cyclosporine, and TNF-alpha inhibitors. However, the quality of studies was limited, and the authors noted that the lesions frequently failed to respond or recurred.
When appropriate, surgery can be performed, he said.
Dr. Callen reported no relevant disclosures.
Pyoderma gangrenosum (PG), a rare and painful ulcerative skin disorder, requires special care because “it’s a challenging diagnosis to make” and is frequently linked to serious comorbid conditions, a dermatologist told colleagues.
PG is also challenging to manage, said Jeffrey Callen, MD, professor and chief of the division of dermatology at the University of Louisville (Ky.), who spoke at the Inaugural Symposium for Inflammatory Skin Disease. “There are multiple treatments, but few have a high level of evidence to document their efficacy.”
PG is a neutrophilic dermatosis that usually occurs with a small lesion, often pustular, that spreads, and is a diagnosis of exclusion. “There’s no way you can possibly exclude everything, but the major things that have to be excluded are infection and malignancies,” he said. “Doing a good history and physical examination is critical, and a biopsy should be done in the vast majority of patients.”
Cultures and routine labs should be obtained, said Dr. Callen, highlighting tests that measure immunofixation (IFE), antineutrophil cytoplasmic antibodies (ANCE), anticardiolipin antibody (aCL), and lupus anticoagulant (LA).
Bowel and bone marrow tests may be appropriate in some patients, he said, noting that about half of PG cases are linked to comorbid conditions such as inflammatory bowel disease (IBD), arthritis, and hematologic diseases.
Dr. Callen also made the following points about making the diagnosis:
- Several clinical variants exist: classic, peristomal, and atypical.
- Pathergy – hyperreactivity of skin to injury – occurs in about a third of patients.
- Neutrophilic infiltrates may occur in other organs.
- Numerous drugs, including isotretinoin, can cause PG.
- PG may be misdiagnosed as necrotizing fasciitis.
- Several diagnostic frameworks exist: the Su Criteria, the PARACELCUS Score, and the Delphi Consensus Criteria. The Delphi criteria identified the highest percentage of cases (89%) in a study comparing the three, published in 2020. The frameworks “are helpful in the clinic, but they are not to be used as criteria for diagnosis. They’re really for classification,” Dr. Callen said.
Once the diagnosis has been made, he said, focus on healing the wound, which he said “can be done as any other wound would be healed,” and calming the inflammation.
“Patients who have mild disease might be treated with lower doses of prednisone, topical medications, or intralesional injections,” he said. “Corticosteroids are never wrong in the beginning.” Some patients may have genetic abnormalities related to PG, he added, and medications that target them may be appropriate.
Antibiotics and biologic agents, particularly TNF-alpha inhibitors, are possible treatments, Dr. Callen said. He highlighted a 2018 systematic review that evaluated treatments and found the most evidence supported systemic corticosteroids, cyclosporine, and TNF-alpha inhibitors. However, the quality of studies was limited, and the authors noted that the lesions frequently failed to respond or recurred.
When appropriate, surgery can be performed, he said.
Dr. Callen reported no relevant disclosures.
Pyoderma gangrenosum (PG), a rare and painful ulcerative skin disorder, requires special care because “it’s a challenging diagnosis to make” and is frequently linked to serious comorbid conditions, a dermatologist told colleagues.
PG is also challenging to manage, said Jeffrey Callen, MD, professor and chief of the division of dermatology at the University of Louisville (Ky.), who spoke at the Inaugural Symposium for Inflammatory Skin Disease. “There are multiple treatments, but few have a high level of evidence to document their efficacy.”
PG is a neutrophilic dermatosis that usually occurs with a small lesion, often pustular, that spreads, and is a diagnosis of exclusion. “There’s no way you can possibly exclude everything, but the major things that have to be excluded are infection and malignancies,” he said. “Doing a good history and physical examination is critical, and a biopsy should be done in the vast majority of patients.”
Cultures and routine labs should be obtained, said Dr. Callen, highlighting tests that measure immunofixation (IFE), antineutrophil cytoplasmic antibodies (ANCE), anticardiolipin antibody (aCL), and lupus anticoagulant (LA).
Bowel and bone marrow tests may be appropriate in some patients, he said, noting that about half of PG cases are linked to comorbid conditions such as inflammatory bowel disease (IBD), arthritis, and hematologic diseases.
Dr. Callen also made the following points about making the diagnosis:
- Several clinical variants exist: classic, peristomal, and atypical.
- Pathergy – hyperreactivity of skin to injury – occurs in about a third of patients.
- Neutrophilic infiltrates may occur in other organs.
- Numerous drugs, including isotretinoin, can cause PG.
- PG may be misdiagnosed as necrotizing fasciitis.
- Several diagnostic frameworks exist: the Su Criteria, the PARACELCUS Score, and the Delphi Consensus Criteria. The Delphi criteria identified the highest percentage of cases (89%) in a study comparing the three, published in 2020. The frameworks “are helpful in the clinic, but they are not to be used as criteria for diagnosis. They’re really for classification,” Dr. Callen said.
Once the diagnosis has been made, he said, focus on healing the wound, which he said “can be done as any other wound would be healed,” and calming the inflammation.
“Patients who have mild disease might be treated with lower doses of prednisone, topical medications, or intralesional injections,” he said. “Corticosteroids are never wrong in the beginning.” Some patients may have genetic abnormalities related to PG, he added, and medications that target them may be appropriate.
Antibiotics and biologic agents, particularly TNF-alpha inhibitors, are possible treatments, Dr. Callen said. He highlighted a 2018 systematic review that evaluated treatments and found the most evidence supported systemic corticosteroids, cyclosporine, and TNF-alpha inhibitors. However, the quality of studies was limited, and the authors noted that the lesions frequently failed to respond or recurred.
When appropriate, surgery can be performed, he said.
Dr. Callen reported no relevant disclosures.
FROM SISD 2021
Expert offers 10 ‘tips and tricks’ for everyday cosmetic practice
, based on nearly 10 years of experience treating patients on both coasts of the United States.
They are as follows:
1. Know your clinical endpoints. “One of the things that was drilled into me during my fellowship in lasers and cosmetics at Mass General was to know your clinical endpoints and to avoid a cookbook approach,” said Dr. Jalian, who practices dermatology in Los Angeles. “You should treat based on the pathology that you’re seeing on the skin and let the endpoints be your guide. The skin will tell you what you’re doing right, and the skin will tell you what you’re doing wrong. Picking up on these cues will allow you to deliver a safe and effective treatment to your patients.”
The selection of proper treatment parameters is driven by selective photothermolysis, a microsurgery technique that uses customized wavelengths, pulse durations, and fluences to target a chromophore. “Knowing the size and shape of your target allows you to pick the right pulse duration,” he said. “This is dictated by thermal relaxation time, which is proportional to the size and shape of the target. Smaller targets require a shorter pulse width, while larger targets require a longer pulse width.”
2. Do not perform a procedure for which you cannot recognize and treat the side effects. “I can’t tell you how many times I’ve gotten referrals from outside providers with a side effect that, if it had been recognized and treated, would have been inconsequential long-term for the patient,” Dr. Jalian said. “You can only avoid complications by not firing the laser. The more you practice, the more complications you’re going to have. This is inevitable, but good practice and common sense can reduce complications significantly.”
Even the most skilled clinicians encounter side effects from time to time. “The most important thing is to form a network of physicians you trust that you can call or text when you need help in managing a particular complication,” he continued. “This happens to all of us,” he said. Don’t be afraid to phone a colleague, he advised, “and get a fresh set of eyes because oftentimes they can provide insights, especially when you’re having tunnel vision during a complication, that can ultimately result in better patient care.”
3. Don’t forget your clinical training. “Trust your clinical judgment,” Dr. Jalian said. “If something doesn’t seem right, even if it was a case referred to you by experienced practitioners, you are a clinician first and foremost, and you are allowed to make a clinical judgment on lesions.” He referred to a 2011 report in which the authors described a series of four cases where patients presented for cosmetic evaluation of vascular lesions that turned out to be more significant pathologic disease. “Trust your clinical insight because this will serve you in the long term,” he said.
4. Set realistic expectations. Patients with unrealistically high expectations are likely to express dissatisfaction with their treatment results, “no matter how good of a job you do,” Dr. Jalian said. “In addition to safely treating the patient, we strive for patient satisfaction, because with these elective procedures we’re trying to give a patient a result they’re looking for. But our No. 1 job is also to be realistic about the results we can obtain. If someone comes in wanting treatment with a skin-tightening device but clearly needs a face-lift because they have too much laxity, your job is to tell them that this is not the appropriate device for them. Learn the art of saying no. If handled correctly, the patient will often thank you before she heads out the door. Ultimately, honesty is the best policy. I may say something like, ‘I’m not telling you the answer you want to hear, I’m telling you the truth.’ That often goes over well.”
5. Use proper anesthesia. Patients come to you for results, but they’re also likely to remember how well you controlled their pain during procedures. Strategies favored by Dr. Jalian include applying extra topical anesthetic to “hot spots” and splitting up treatment sessions when tackling a large area. “Consider using adjunctive analgesics such as oral medications and nitrous oxide,” he added. Other options, he said, are cooling techniques and distraction techniques, such as the use of a stress ball, consideration of the gate control theory of pain, and “talkesthesia”(using conversation to distract the patient).
6. Obtain proper informed consent. A lack of informed consent ranks as a common reason why doctors get sued. “This happens when a physician fails to inform the patient of all medically reasonable alternatives and their risks, even for noninvasive procedures prior to administering treatment,” Dr. Jalian said. “All patients have the right to an informed consent prior to any treatment. It doesn’t necessarily have to be in a written form, but it’s important to at least have a discussion and document it for all procedures, including medical and cosmetic procedures and oral and topical treatments. Keep it simple. A written consent is ineffective if the patient does not understand material about the procedure.” Avoid the use of excessive medical terms. For example, use bruising instead of purpura, redness instead of erythema, and drooping instead of ptosis.
7. Lower the laser treatment density for darker skin types. According to Dr. Jalian, several clinical studies have demonstrated that lower densities are associated with less postinflammatory hyperpigmentation in Asian and Black patients, without sacrificing clinical outcomes. “Density determines how ‘aggressive’ a treatment is,” he said. “The greater the density, the more downtime is required.”
8. Have a vascular occlusion emergency kit on hand. At a minimum, the kit should contain at least 1,500 units of hyaluronidase, aspirin, timolol/acetazolamide, a Snellen chart, steroids, and an EpiPen.
9. Use standardized photography. Even the slightest change in lighting can manipulate your results. According to Dr. Jalian, standardized photos enable you to monitor patient progress, minimize liability, and can serve as a marketing tool “so that you can capitalize on your talent.”
10. Consider combination treatments. He combines lasers based on target and depth. For example, prior to resurfacing he often performs a pass or two with a color laser such as intense pulsed light. “Depending on what’s being done, we’ll do soft-tissue augmentation before or after treatment with certain lasers,” Dr. Jalian added. “If you’re performing a toxin treatment on the same day as a laser procedure, do not treat the lower face or neck. Do the laser procedure first and limit that to the upper third of the face.”
He reported having no relevant financial disclosures.
, based on nearly 10 years of experience treating patients on both coasts of the United States.
They are as follows:
1. Know your clinical endpoints. “One of the things that was drilled into me during my fellowship in lasers and cosmetics at Mass General was to know your clinical endpoints and to avoid a cookbook approach,” said Dr. Jalian, who practices dermatology in Los Angeles. “You should treat based on the pathology that you’re seeing on the skin and let the endpoints be your guide. The skin will tell you what you’re doing right, and the skin will tell you what you’re doing wrong. Picking up on these cues will allow you to deliver a safe and effective treatment to your patients.”
The selection of proper treatment parameters is driven by selective photothermolysis, a microsurgery technique that uses customized wavelengths, pulse durations, and fluences to target a chromophore. “Knowing the size and shape of your target allows you to pick the right pulse duration,” he said. “This is dictated by thermal relaxation time, which is proportional to the size and shape of the target. Smaller targets require a shorter pulse width, while larger targets require a longer pulse width.”
2. Do not perform a procedure for which you cannot recognize and treat the side effects. “I can’t tell you how many times I’ve gotten referrals from outside providers with a side effect that, if it had been recognized and treated, would have been inconsequential long-term for the patient,” Dr. Jalian said. “You can only avoid complications by not firing the laser. The more you practice, the more complications you’re going to have. This is inevitable, but good practice and common sense can reduce complications significantly.”
Even the most skilled clinicians encounter side effects from time to time. “The most important thing is to form a network of physicians you trust that you can call or text when you need help in managing a particular complication,” he continued. “This happens to all of us,” he said. Don’t be afraid to phone a colleague, he advised, “and get a fresh set of eyes because oftentimes they can provide insights, especially when you’re having tunnel vision during a complication, that can ultimately result in better patient care.”
3. Don’t forget your clinical training. “Trust your clinical judgment,” Dr. Jalian said. “If something doesn’t seem right, even if it was a case referred to you by experienced practitioners, you are a clinician first and foremost, and you are allowed to make a clinical judgment on lesions.” He referred to a 2011 report in which the authors described a series of four cases where patients presented for cosmetic evaluation of vascular lesions that turned out to be more significant pathologic disease. “Trust your clinical insight because this will serve you in the long term,” he said.
4. Set realistic expectations. Patients with unrealistically high expectations are likely to express dissatisfaction with their treatment results, “no matter how good of a job you do,” Dr. Jalian said. “In addition to safely treating the patient, we strive for patient satisfaction, because with these elective procedures we’re trying to give a patient a result they’re looking for. But our No. 1 job is also to be realistic about the results we can obtain. If someone comes in wanting treatment with a skin-tightening device but clearly needs a face-lift because they have too much laxity, your job is to tell them that this is not the appropriate device for them. Learn the art of saying no. If handled correctly, the patient will often thank you before she heads out the door. Ultimately, honesty is the best policy. I may say something like, ‘I’m not telling you the answer you want to hear, I’m telling you the truth.’ That often goes over well.”
5. Use proper anesthesia. Patients come to you for results, but they’re also likely to remember how well you controlled their pain during procedures. Strategies favored by Dr. Jalian include applying extra topical anesthetic to “hot spots” and splitting up treatment sessions when tackling a large area. “Consider using adjunctive analgesics such as oral medications and nitrous oxide,” he added. Other options, he said, are cooling techniques and distraction techniques, such as the use of a stress ball, consideration of the gate control theory of pain, and “talkesthesia”(using conversation to distract the patient).
6. Obtain proper informed consent. A lack of informed consent ranks as a common reason why doctors get sued. “This happens when a physician fails to inform the patient of all medically reasonable alternatives and their risks, even for noninvasive procedures prior to administering treatment,” Dr. Jalian said. “All patients have the right to an informed consent prior to any treatment. It doesn’t necessarily have to be in a written form, but it’s important to at least have a discussion and document it for all procedures, including medical and cosmetic procedures and oral and topical treatments. Keep it simple. A written consent is ineffective if the patient does not understand material about the procedure.” Avoid the use of excessive medical terms. For example, use bruising instead of purpura, redness instead of erythema, and drooping instead of ptosis.
7. Lower the laser treatment density for darker skin types. According to Dr. Jalian, several clinical studies have demonstrated that lower densities are associated with less postinflammatory hyperpigmentation in Asian and Black patients, without sacrificing clinical outcomes. “Density determines how ‘aggressive’ a treatment is,” he said. “The greater the density, the more downtime is required.”
8. Have a vascular occlusion emergency kit on hand. At a minimum, the kit should contain at least 1,500 units of hyaluronidase, aspirin, timolol/acetazolamide, a Snellen chart, steroids, and an EpiPen.
9. Use standardized photography. Even the slightest change in lighting can manipulate your results. According to Dr. Jalian, standardized photos enable you to monitor patient progress, minimize liability, and can serve as a marketing tool “so that you can capitalize on your talent.”
10. Consider combination treatments. He combines lasers based on target and depth. For example, prior to resurfacing he often performs a pass or two with a color laser such as intense pulsed light. “Depending on what’s being done, we’ll do soft-tissue augmentation before or after treatment with certain lasers,” Dr. Jalian added. “If you’re performing a toxin treatment on the same day as a laser procedure, do not treat the lower face or neck. Do the laser procedure first and limit that to the upper third of the face.”
He reported having no relevant financial disclosures.
, based on nearly 10 years of experience treating patients on both coasts of the United States.
They are as follows:
1. Know your clinical endpoints. “One of the things that was drilled into me during my fellowship in lasers and cosmetics at Mass General was to know your clinical endpoints and to avoid a cookbook approach,” said Dr. Jalian, who practices dermatology in Los Angeles. “You should treat based on the pathology that you’re seeing on the skin and let the endpoints be your guide. The skin will tell you what you’re doing right, and the skin will tell you what you’re doing wrong. Picking up on these cues will allow you to deliver a safe and effective treatment to your patients.”
The selection of proper treatment parameters is driven by selective photothermolysis, a microsurgery technique that uses customized wavelengths, pulse durations, and fluences to target a chromophore. “Knowing the size and shape of your target allows you to pick the right pulse duration,” he said. “This is dictated by thermal relaxation time, which is proportional to the size and shape of the target. Smaller targets require a shorter pulse width, while larger targets require a longer pulse width.”
2. Do not perform a procedure for which you cannot recognize and treat the side effects. “I can’t tell you how many times I’ve gotten referrals from outside providers with a side effect that, if it had been recognized and treated, would have been inconsequential long-term for the patient,” Dr. Jalian said. “You can only avoid complications by not firing the laser. The more you practice, the more complications you’re going to have. This is inevitable, but good practice and common sense can reduce complications significantly.”
Even the most skilled clinicians encounter side effects from time to time. “The most important thing is to form a network of physicians you trust that you can call or text when you need help in managing a particular complication,” he continued. “This happens to all of us,” he said. Don’t be afraid to phone a colleague, he advised, “and get a fresh set of eyes because oftentimes they can provide insights, especially when you’re having tunnel vision during a complication, that can ultimately result in better patient care.”
3. Don’t forget your clinical training. “Trust your clinical judgment,” Dr. Jalian said. “If something doesn’t seem right, even if it was a case referred to you by experienced practitioners, you are a clinician first and foremost, and you are allowed to make a clinical judgment on lesions.” He referred to a 2011 report in which the authors described a series of four cases where patients presented for cosmetic evaluation of vascular lesions that turned out to be more significant pathologic disease. “Trust your clinical insight because this will serve you in the long term,” he said.
4. Set realistic expectations. Patients with unrealistically high expectations are likely to express dissatisfaction with their treatment results, “no matter how good of a job you do,” Dr. Jalian said. “In addition to safely treating the patient, we strive for patient satisfaction, because with these elective procedures we’re trying to give a patient a result they’re looking for. But our No. 1 job is also to be realistic about the results we can obtain. If someone comes in wanting treatment with a skin-tightening device but clearly needs a face-lift because they have too much laxity, your job is to tell them that this is not the appropriate device for them. Learn the art of saying no. If handled correctly, the patient will often thank you before she heads out the door. Ultimately, honesty is the best policy. I may say something like, ‘I’m not telling you the answer you want to hear, I’m telling you the truth.’ That often goes over well.”
5. Use proper anesthesia. Patients come to you for results, but they’re also likely to remember how well you controlled their pain during procedures. Strategies favored by Dr. Jalian include applying extra topical anesthetic to “hot spots” and splitting up treatment sessions when tackling a large area. “Consider using adjunctive analgesics such as oral medications and nitrous oxide,” he added. Other options, he said, are cooling techniques and distraction techniques, such as the use of a stress ball, consideration of the gate control theory of pain, and “talkesthesia”(using conversation to distract the patient).
6. Obtain proper informed consent. A lack of informed consent ranks as a common reason why doctors get sued. “This happens when a physician fails to inform the patient of all medically reasonable alternatives and their risks, even for noninvasive procedures prior to administering treatment,” Dr. Jalian said. “All patients have the right to an informed consent prior to any treatment. It doesn’t necessarily have to be in a written form, but it’s important to at least have a discussion and document it for all procedures, including medical and cosmetic procedures and oral and topical treatments. Keep it simple. A written consent is ineffective if the patient does not understand material about the procedure.” Avoid the use of excessive medical terms. For example, use bruising instead of purpura, redness instead of erythema, and drooping instead of ptosis.
7. Lower the laser treatment density for darker skin types. According to Dr. Jalian, several clinical studies have demonstrated that lower densities are associated with less postinflammatory hyperpigmentation in Asian and Black patients, without sacrificing clinical outcomes. “Density determines how ‘aggressive’ a treatment is,” he said. “The greater the density, the more downtime is required.”
8. Have a vascular occlusion emergency kit on hand. At a minimum, the kit should contain at least 1,500 units of hyaluronidase, aspirin, timolol/acetazolamide, a Snellen chart, steroids, and an EpiPen.
9. Use standardized photography. Even the slightest change in lighting can manipulate your results. According to Dr. Jalian, standardized photos enable you to monitor patient progress, minimize liability, and can serve as a marketing tool “so that you can capitalize on your talent.”
10. Consider combination treatments. He combines lasers based on target and depth. For example, prior to resurfacing he often performs a pass or two with a color laser such as intense pulsed light. “Depending on what’s being done, we’ll do soft-tissue augmentation before or after treatment with certain lasers,” Dr. Jalian added. “If you’re performing a toxin treatment on the same day as a laser procedure, do not treat the lower face or neck. Do the laser procedure first and limit that to the upper third of the face.”
He reported having no relevant financial disclosures.
FROM ASLMS 2021

















