User login
Celiac Disease: Five Things to Know
Celiac disease is a chronic, immune-mediated, systemic disorder caused by intolerance to gluten — a protein present in rye, barley, and wheat grains — that affects genetically predisposed individuals.
Due to its wide spectrum of clinical manifestations, celiac disease resembles a multisystemic disorder. Its most common gastrointestinal (GI) symptoms include chronic diarrhea, weight loss, and abdominal distention. However, celiac disease can also manifest in myriad extraintestinal symptoms, ranging from headache and fatigue to delayed puberty and psychiatric disorders, with differing presentations in children and adults.
To date, the only treatment is adopting a gluten-free diet (GFD). Although key to preventing persistent villous atrophy, the main cause of complications in celiac disease, lifelong adherence to GFD is challenging and may not resolve all clinical issues. These shortcomings have driven recent efforts to develop novel therapeutic options for patients with this disease.
Here are five things to know about celiac disease.
1. Rising Prevalence of Celiac Disease and Other Autoimmune Disorders Suggests Environmental Factors May Be at Play
Gluten was first identified as the cause of celiac disease in the 1950s. At that time, the condition was thought to be a relatively rare GI disease of childhood that primarily affected people of European descent, but it is now known to be a common disease affecting those of various ages, races, and ethnicities.
A 2018 meta-analysis found the pooled global prevalence of celiac disease was 1.4%. Incidence has increased by as much as 7.5% annually over the past several decades.
Increased awareness among clinicians and improved detection likely play a role in the trend. However, the growth in celiac disease is consistent with that seen for other autoimmune disorders, according to a 2024 update of evidence surrounding celiac disease. Shared environmental factors have been proposed as triggers for celiac disease and other autoimmune diseases and appear to be influencing their rise, the authors noted. These factors include migration and population growth, changing dietary patterns and food processing practices, and altered wheat consumption.
2. No-Biopsy Diagnosis Is Accepted for Children and Shows Promise for Adults
It is estimated that almost 60 million people worldwide have celiac disease, but most remain undiagnosed or misdiagnosed, or they experience significant diagnostic delays.
Prospective data indicate that children with first-degree relatives with celiac disease are at a significantly higher risk of developing the condition, which should prompt screening efforts in this population.
The 2023 updated guidelines from the American College of Gastroenterology (ACG) state that serology testing plays a central role in screening. This commonly involves serological testing for positive serological markers of the disease, including immunoglobulin A (IgA), anti-tissue transglutaminase IgA (tTG-IgA), anti-deamidated gliadin peptide, or endomysial antibodies.
To confirm diagnosis, clinicians have relied on intestinal biopsy since the late 1950s. The ACG still recommends esophagogastroduodenoscopy with multiple duodenal biopsies for confirmation of diagnosis in both children and adults with suspicion of celiac disease. However, recent years have seen a shift toward a no-biopsy approach.
For more than a decade in Europe, a no-biopsy approach has been established practice in pediatric patients, for whom the burden of obtaining a histological confirmation is understandably greater. Most guidelines now permit children to be diagnosed with celiac disease in the absence of a biopsy under specific circumstances (eg, characteristic symptoms of celiac disease and tTG-IgA levels > 10 times the upper limit of normal). The ACG guidelines state that “this approach is a reasonable alternative to the standard approach to a [celiac disease] diagnosis in selected children.”
The ACG does not recommend a no-biopsy approach in adults, noting that, in comparison with children, there is a relative lack of data indicating that serology is predictive in this population. However, it does recognize that physicians may encounter patients for whom a biopsy diagnosis may not be safe or practical. In such cases, an “after-the-fact” diagnosis of likely celiac disease can be given to symptomatic adult patients with a ≥ 10-fold elevation of tTG-IgA and a positive endomysial antibody in a second blood sample.
A 2024 meta-analysis of 18 studies involving over 12,103 adult patients from 15 countries concluded that a no-biopsy approach using tTG-IgA antibody levels ≥ 10 times the upper limit of normal was highly specific and predictive of celiac disease.
3. Celiac Disease Is Associated With Several Life-Threatening Conditions
Emerging data indicate that gastroenterologists should be vigilant in screening patients with celiac disease for several other GI conditions.
Inflammatory bowel disease and celiac disease have a strong bidirectional association, suggesting a possible genetic link between the conditions and indicating that physicians should consider the alternate diagnosis when symptoms persist after treatment.
Given the hypervigilance around food and diet inherent to celiac disease, patients are at an increased risk of developing avoidant/restrictive food intake disorder, according to a 2022 retrospective study.
In 2023, Italian investigators showed that children with celiac disease have an elevated prevalence of functional GI disorders even after adopting a GFD for a year, regardless of whether they consumed processed or natural foods. It was unclear whether this was due to a chronic inflammatory process or to nutritional factors.
Complications resulting from celiac disease are not limited to GI disorders. For a variety of underlying pathophysiological reasons, including intestinal permeability, hyposplenism, and malabsorption of nutrients, patients with celiac disease may be at a higher risk for non-GI conditions, such as osteopenia, women’s health disorders (eg, ovarian failure, endometriosis, or pregnancy loss), juvenile idiopathic arthritis in children and rheumatoid arthritis in adults, certain forms of cancer, infectious diseases, and cardiomyopathy.
4. GFD Is the Only Treatment, but It’s Imperfect and Frustrating for Patients
GFD is the only treatment for celiac disease and must be adhered to without deviation throughout a patient’s life.
Maintaining unwavering adherence reaps considerable benefits: Improved clinical symptoms, robust mucosal healing, and normalization of serological markers. Yet it also takes a considerable toll on patients. Patients with celiac disease struggle with a host of negative physical, psychological, and social impacts. They also report a higher treatment burden than those with gastroesophageal reflux disease or hypertension, and comparable with end-stage renal disease.
GFD also poses financial challenges. Although the price of gluten-free products has decreased in recent years, they still cost significantly more than items with gluten.
Adherence to GFD does not always equate to complete mucosal recovery. While mucosal recovery is achieved in 95% of children within 2 years of the diet’s adoption, only 34% and 66% of adults obtain it within 2 and 5 years, respectively.
GFD may lead to nutrient imbalances because gluten-free foods are typically low in alimentary fiber, micronutrients (eg, vitamin D, vitamin B12, or folate), and minerals (eg, iron, zinc, magnesium, or calcium). With higher sugar and fat content, GFD may leave patients susceptible to unwanted weight gain.
The pervasiveness of gluten in the food production system makes the risk for cross-contamination high. Gluten is often found in both naturally gluten-free foods and products labeled as such. Gluten-sensing technologies, some of which can be used via smartphone apps, have been developed to help patients identify possible cross-contamination. However, the ACG guidelines recommend against the use of these technologies until there is sufficient evidence supporting their ability to improve adherence and clinical outcomes.
5. Novel Therapies for Celiac Disease Are in the Pipeline
The limitations of GFD as the standard treatment for celiac disease have led to an increased focus on developing novel therapeutic interventions. They can be sorted into five key categories: Modulation of the immunostimulatory effects of toxic gluten peptides, elimination of toxic gluten peptides before they reach the intestine, induction of gluten tolerance, modulation of intestinal permeability, and restoration of gut microbiota balance.
Three therapies designed to block antigen presentation by HLA-DQ2/8, the gene alleles that predispose people to celiac disease, show promise: TPM502, an agent that contains three gluten-specific antigenic peptides with overlapping T-cell epitopes for the HLA-DQ2.5 gene; KAN-101, designed to induce gluten tolerance by targeting receptors on the liver; and DONQ52, a multi-specific antibody that targets HLA-DQ2. The KAN-101 therapy received Fast Track designation by the US Food and Drug Administration in 2022.
These and several other agents in clinical and preclinical development are discussed in detail in a 2024 review article. Although no therapies have reached phase 3 testing, when they do, it will undoubtedly be welcomed by those with celiac disease.
A version of this article first appeared on Medscape.com.
Celiac disease is a chronic, immune-mediated, systemic disorder caused by intolerance to gluten — a protein present in rye, barley, and wheat grains — that affects genetically predisposed individuals.
Due to its wide spectrum of clinical manifestations, celiac disease resembles a multisystemic disorder. Its most common gastrointestinal (GI) symptoms include chronic diarrhea, weight loss, and abdominal distention. However, celiac disease can also manifest in myriad extraintestinal symptoms, ranging from headache and fatigue to delayed puberty and psychiatric disorders, with differing presentations in children and adults.
To date, the only treatment is adopting a gluten-free diet (GFD). Although key to preventing persistent villous atrophy, the main cause of complications in celiac disease, lifelong adherence to GFD is challenging and may not resolve all clinical issues. These shortcomings have driven recent efforts to develop novel therapeutic options for patients with this disease.
Here are five things to know about celiac disease.
1. Rising Prevalence of Celiac Disease and Other Autoimmune Disorders Suggests Environmental Factors May Be at Play
Gluten was first identified as the cause of celiac disease in the 1950s. At that time, the condition was thought to be a relatively rare GI disease of childhood that primarily affected people of European descent, but it is now known to be a common disease affecting those of various ages, races, and ethnicities.
A 2018 meta-analysis found the pooled global prevalence of celiac disease was 1.4%. Incidence has increased by as much as 7.5% annually over the past several decades.
Increased awareness among clinicians and improved detection likely play a role in the trend. However, the growth in celiac disease is consistent with that seen for other autoimmune disorders, according to a 2024 update of evidence surrounding celiac disease. Shared environmental factors have been proposed as triggers for celiac disease and other autoimmune diseases and appear to be influencing their rise, the authors noted. These factors include migration and population growth, changing dietary patterns and food processing practices, and altered wheat consumption.
2. No-Biopsy Diagnosis Is Accepted for Children and Shows Promise for Adults
It is estimated that almost 60 million people worldwide have celiac disease, but most remain undiagnosed or misdiagnosed, or they experience significant diagnostic delays.
Prospective data indicate that children with first-degree relatives with celiac disease are at a significantly higher risk of developing the condition, which should prompt screening efforts in this population.
The 2023 updated guidelines from the American College of Gastroenterology (ACG) state that serology testing plays a central role in screening. This commonly involves serological testing for positive serological markers of the disease, including immunoglobulin A (IgA), anti-tissue transglutaminase IgA (tTG-IgA), anti-deamidated gliadin peptide, or endomysial antibodies.
To confirm diagnosis, clinicians have relied on intestinal biopsy since the late 1950s. The ACG still recommends esophagogastroduodenoscopy with multiple duodenal biopsies for confirmation of diagnosis in both children and adults with suspicion of celiac disease. However, recent years have seen a shift toward a no-biopsy approach.
For more than a decade in Europe, a no-biopsy approach has been established practice in pediatric patients, for whom the burden of obtaining a histological confirmation is understandably greater. Most guidelines now permit children to be diagnosed with celiac disease in the absence of a biopsy under specific circumstances (eg, characteristic symptoms of celiac disease and tTG-IgA levels > 10 times the upper limit of normal). The ACG guidelines state that “this approach is a reasonable alternative to the standard approach to a [celiac disease] diagnosis in selected children.”
The ACG does not recommend a no-biopsy approach in adults, noting that, in comparison with children, there is a relative lack of data indicating that serology is predictive in this population. However, it does recognize that physicians may encounter patients for whom a biopsy diagnosis may not be safe or practical. In such cases, an “after-the-fact” diagnosis of likely celiac disease can be given to symptomatic adult patients with a ≥ 10-fold elevation of tTG-IgA and a positive endomysial antibody in a second blood sample.
A 2024 meta-analysis of 18 studies involving over 12,103 adult patients from 15 countries concluded that a no-biopsy approach using tTG-IgA antibody levels ≥ 10 times the upper limit of normal was highly specific and predictive of celiac disease.
3. Celiac Disease Is Associated With Several Life-Threatening Conditions
Emerging data indicate that gastroenterologists should be vigilant in screening patients with celiac disease for several other GI conditions.
Inflammatory bowel disease and celiac disease have a strong bidirectional association, suggesting a possible genetic link between the conditions and indicating that physicians should consider the alternate diagnosis when symptoms persist after treatment.
Given the hypervigilance around food and diet inherent to celiac disease, patients are at an increased risk of developing avoidant/restrictive food intake disorder, according to a 2022 retrospective study.
In 2023, Italian investigators showed that children with celiac disease have an elevated prevalence of functional GI disorders even after adopting a GFD for a year, regardless of whether they consumed processed or natural foods. It was unclear whether this was due to a chronic inflammatory process or to nutritional factors.
Complications resulting from celiac disease are not limited to GI disorders. For a variety of underlying pathophysiological reasons, including intestinal permeability, hyposplenism, and malabsorption of nutrients, patients with celiac disease may be at a higher risk for non-GI conditions, such as osteopenia, women’s health disorders (eg, ovarian failure, endometriosis, or pregnancy loss), juvenile idiopathic arthritis in children and rheumatoid arthritis in adults, certain forms of cancer, infectious diseases, and cardiomyopathy.
4. GFD Is the Only Treatment, but It’s Imperfect and Frustrating for Patients
GFD is the only treatment for celiac disease and must be adhered to without deviation throughout a patient’s life.
Maintaining unwavering adherence reaps considerable benefits: Improved clinical symptoms, robust mucosal healing, and normalization of serological markers. Yet it also takes a considerable toll on patients. Patients with celiac disease struggle with a host of negative physical, psychological, and social impacts. They also report a higher treatment burden than those with gastroesophageal reflux disease or hypertension, and comparable with end-stage renal disease.
GFD also poses financial challenges. Although the price of gluten-free products has decreased in recent years, they still cost significantly more than items with gluten.
Adherence to GFD does not always equate to complete mucosal recovery. While mucosal recovery is achieved in 95% of children within 2 years of the diet’s adoption, only 34% and 66% of adults obtain it within 2 and 5 years, respectively.
GFD may lead to nutrient imbalances because gluten-free foods are typically low in alimentary fiber, micronutrients (eg, vitamin D, vitamin B12, or folate), and minerals (eg, iron, zinc, magnesium, or calcium). With higher sugar and fat content, GFD may leave patients susceptible to unwanted weight gain.
The pervasiveness of gluten in the food production system makes the risk for cross-contamination high. Gluten is often found in both naturally gluten-free foods and products labeled as such. Gluten-sensing technologies, some of which can be used via smartphone apps, have been developed to help patients identify possible cross-contamination. However, the ACG guidelines recommend against the use of these technologies until there is sufficient evidence supporting their ability to improve adherence and clinical outcomes.
5. Novel Therapies for Celiac Disease Are in the Pipeline
The limitations of GFD as the standard treatment for celiac disease have led to an increased focus on developing novel therapeutic interventions. They can be sorted into five key categories: Modulation of the immunostimulatory effects of toxic gluten peptides, elimination of toxic gluten peptides before they reach the intestine, induction of gluten tolerance, modulation of intestinal permeability, and restoration of gut microbiota balance.
Three therapies designed to block antigen presentation by HLA-DQ2/8, the gene alleles that predispose people to celiac disease, show promise: TPM502, an agent that contains three gluten-specific antigenic peptides with overlapping T-cell epitopes for the HLA-DQ2.5 gene; KAN-101, designed to induce gluten tolerance by targeting receptors on the liver; and DONQ52, a multi-specific antibody that targets HLA-DQ2. The KAN-101 therapy received Fast Track designation by the US Food and Drug Administration in 2022.
These and several other agents in clinical and preclinical development are discussed in detail in a 2024 review article. Although no therapies have reached phase 3 testing, when they do, it will undoubtedly be welcomed by those with celiac disease.
A version of this article first appeared on Medscape.com.
Celiac disease is a chronic, immune-mediated, systemic disorder caused by intolerance to gluten — a protein present in rye, barley, and wheat grains — that affects genetically predisposed individuals.
Due to its wide spectrum of clinical manifestations, celiac disease resembles a multisystemic disorder. Its most common gastrointestinal (GI) symptoms include chronic diarrhea, weight loss, and abdominal distention. However, celiac disease can also manifest in myriad extraintestinal symptoms, ranging from headache and fatigue to delayed puberty and psychiatric disorders, with differing presentations in children and adults.
To date, the only treatment is adopting a gluten-free diet (GFD). Although key to preventing persistent villous atrophy, the main cause of complications in celiac disease, lifelong adherence to GFD is challenging and may not resolve all clinical issues. These shortcomings have driven recent efforts to develop novel therapeutic options for patients with this disease.
Here are five things to know about celiac disease.
1. Rising Prevalence of Celiac Disease and Other Autoimmune Disorders Suggests Environmental Factors May Be at Play
Gluten was first identified as the cause of celiac disease in the 1950s. At that time, the condition was thought to be a relatively rare GI disease of childhood that primarily affected people of European descent, but it is now known to be a common disease affecting those of various ages, races, and ethnicities.
A 2018 meta-analysis found the pooled global prevalence of celiac disease was 1.4%. Incidence has increased by as much as 7.5% annually over the past several decades.
Increased awareness among clinicians and improved detection likely play a role in the trend. However, the growth in celiac disease is consistent with that seen for other autoimmune disorders, according to a 2024 update of evidence surrounding celiac disease. Shared environmental factors have been proposed as triggers for celiac disease and other autoimmune diseases and appear to be influencing their rise, the authors noted. These factors include migration and population growth, changing dietary patterns and food processing practices, and altered wheat consumption.
2. No-Biopsy Diagnosis Is Accepted for Children and Shows Promise for Adults
It is estimated that almost 60 million people worldwide have celiac disease, but most remain undiagnosed or misdiagnosed, or they experience significant diagnostic delays.
Prospective data indicate that children with first-degree relatives with celiac disease are at a significantly higher risk of developing the condition, which should prompt screening efforts in this population.
The 2023 updated guidelines from the American College of Gastroenterology (ACG) state that serology testing plays a central role in screening. This commonly involves serological testing for positive serological markers of the disease, including immunoglobulin A (IgA), anti-tissue transglutaminase IgA (tTG-IgA), anti-deamidated gliadin peptide, or endomysial antibodies.
To confirm diagnosis, clinicians have relied on intestinal biopsy since the late 1950s. The ACG still recommends esophagogastroduodenoscopy with multiple duodenal biopsies for confirmation of diagnosis in both children and adults with suspicion of celiac disease. However, recent years have seen a shift toward a no-biopsy approach.
For more than a decade in Europe, a no-biopsy approach has been established practice in pediatric patients, for whom the burden of obtaining a histological confirmation is understandably greater. Most guidelines now permit children to be diagnosed with celiac disease in the absence of a biopsy under specific circumstances (eg, characteristic symptoms of celiac disease and tTG-IgA levels > 10 times the upper limit of normal). The ACG guidelines state that “this approach is a reasonable alternative to the standard approach to a [celiac disease] diagnosis in selected children.”
The ACG does not recommend a no-biopsy approach in adults, noting that, in comparison with children, there is a relative lack of data indicating that serology is predictive in this population. However, it does recognize that physicians may encounter patients for whom a biopsy diagnosis may not be safe or practical. In such cases, an “after-the-fact” diagnosis of likely celiac disease can be given to symptomatic adult patients with a ≥ 10-fold elevation of tTG-IgA and a positive endomysial antibody in a second blood sample.
A 2024 meta-analysis of 18 studies involving over 12,103 adult patients from 15 countries concluded that a no-biopsy approach using tTG-IgA antibody levels ≥ 10 times the upper limit of normal was highly specific and predictive of celiac disease.
3. Celiac Disease Is Associated With Several Life-Threatening Conditions
Emerging data indicate that gastroenterologists should be vigilant in screening patients with celiac disease for several other GI conditions.
Inflammatory bowel disease and celiac disease have a strong bidirectional association, suggesting a possible genetic link between the conditions and indicating that physicians should consider the alternate diagnosis when symptoms persist after treatment.
Given the hypervigilance around food and diet inherent to celiac disease, patients are at an increased risk of developing avoidant/restrictive food intake disorder, according to a 2022 retrospective study.
In 2023, Italian investigators showed that children with celiac disease have an elevated prevalence of functional GI disorders even after adopting a GFD for a year, regardless of whether they consumed processed or natural foods. It was unclear whether this was due to a chronic inflammatory process or to nutritional factors.
Complications resulting from celiac disease are not limited to GI disorders. For a variety of underlying pathophysiological reasons, including intestinal permeability, hyposplenism, and malabsorption of nutrients, patients with celiac disease may be at a higher risk for non-GI conditions, such as osteopenia, women’s health disorders (eg, ovarian failure, endometriosis, or pregnancy loss), juvenile idiopathic arthritis in children and rheumatoid arthritis in adults, certain forms of cancer, infectious diseases, and cardiomyopathy.
4. GFD Is the Only Treatment, but It’s Imperfect and Frustrating for Patients
GFD is the only treatment for celiac disease and must be adhered to without deviation throughout a patient’s life.
Maintaining unwavering adherence reaps considerable benefits: Improved clinical symptoms, robust mucosal healing, and normalization of serological markers. Yet it also takes a considerable toll on patients. Patients with celiac disease struggle with a host of negative physical, psychological, and social impacts. They also report a higher treatment burden than those with gastroesophageal reflux disease or hypertension, and comparable with end-stage renal disease.
GFD also poses financial challenges. Although the price of gluten-free products has decreased in recent years, they still cost significantly more than items with gluten.
Adherence to GFD does not always equate to complete mucosal recovery. While mucosal recovery is achieved in 95% of children within 2 years of the diet’s adoption, only 34% and 66% of adults obtain it within 2 and 5 years, respectively.
GFD may lead to nutrient imbalances because gluten-free foods are typically low in alimentary fiber, micronutrients (eg, vitamin D, vitamin B12, or folate), and minerals (eg, iron, zinc, magnesium, or calcium). With higher sugar and fat content, GFD may leave patients susceptible to unwanted weight gain.
The pervasiveness of gluten in the food production system makes the risk for cross-contamination high. Gluten is often found in both naturally gluten-free foods and products labeled as such. Gluten-sensing technologies, some of which can be used via smartphone apps, have been developed to help patients identify possible cross-contamination. However, the ACG guidelines recommend against the use of these technologies until there is sufficient evidence supporting their ability to improve adherence and clinical outcomes.
5. Novel Therapies for Celiac Disease Are in the Pipeline
The limitations of GFD as the standard treatment for celiac disease have led to an increased focus on developing novel therapeutic interventions. They can be sorted into five key categories: Modulation of the immunostimulatory effects of toxic gluten peptides, elimination of toxic gluten peptides before they reach the intestine, induction of gluten tolerance, modulation of intestinal permeability, and restoration of gut microbiota balance.
Three therapies designed to block antigen presentation by HLA-DQ2/8, the gene alleles that predispose people to celiac disease, show promise: TPM502, an agent that contains three gluten-specific antigenic peptides with overlapping T-cell epitopes for the HLA-DQ2.5 gene; KAN-101, designed to induce gluten tolerance by targeting receptors on the liver; and DONQ52, a multi-specific antibody that targets HLA-DQ2. The KAN-101 therapy received Fast Track designation by the US Food and Drug Administration in 2022.
These and several other agents in clinical and preclinical development are discussed in detail in a 2024 review article. Although no therapies have reached phase 3 testing, when they do, it will undoubtedly be welcomed by those with celiac disease.
A version of this article first appeared on Medscape.com.
Clear Coverage Preference for Humira Over Biosimilars Seen in Most Medicare Part D Plans
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.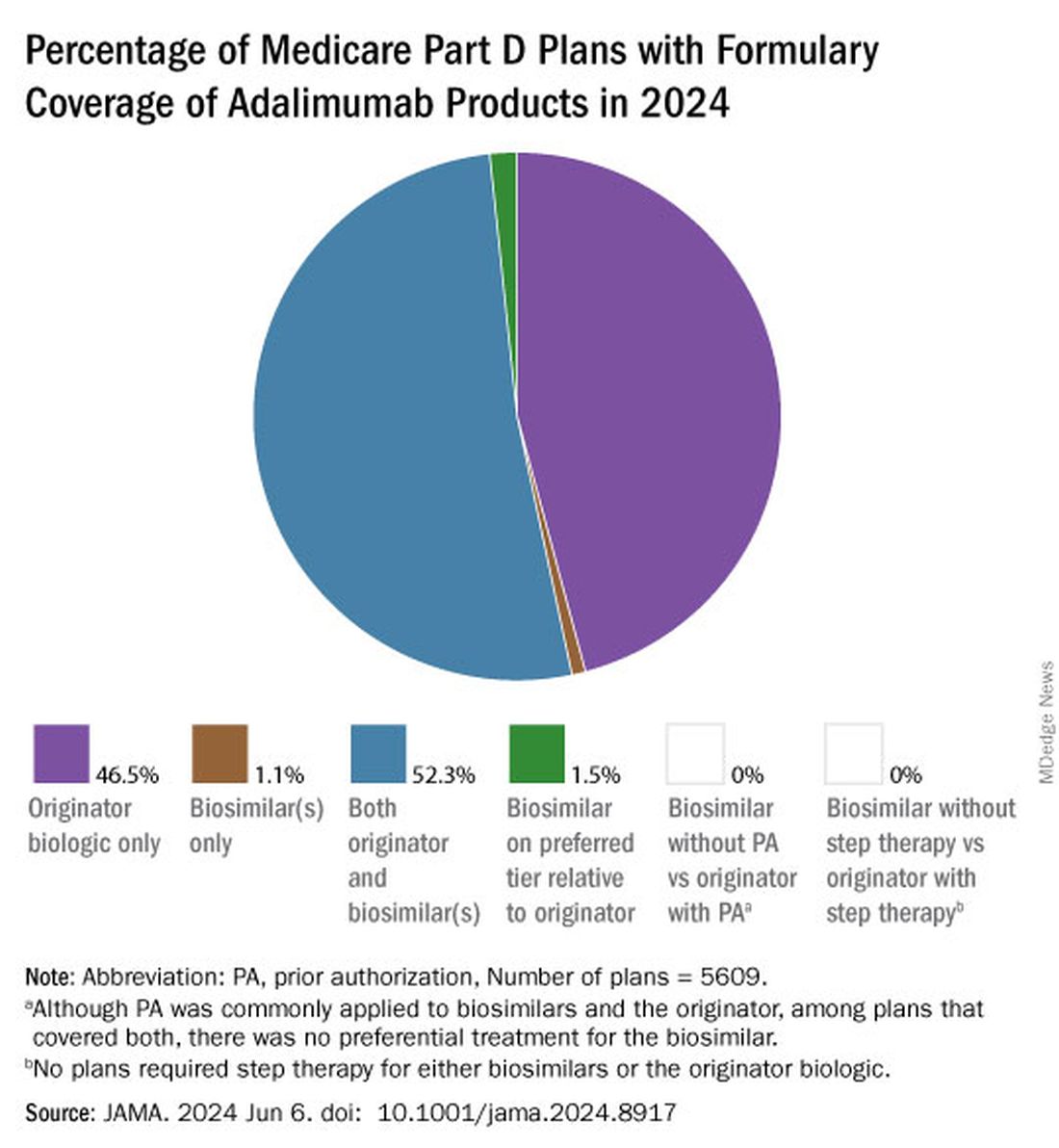
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
FROM JAMA
FDA Grants New Pediatric Arthritis Indications for Upadacitinib
Upadacitinib (Rinvoq) is now indicated for patients aged 2 years or older with active polyarticular juvenile idiopathic arthritis (pJIA) and psoriatic arthritis (PsA) who cannot tolerate or achieve adequate disease response with one or more tumor necrosis factor (TNF) blockers, according to a press release from manufacturer AbbVie.
For the youngest patients, upadacitinib is also available as a weight-based oral solution (Rinvoq LQ) in addition to the previously available tablets, according to the company. JIA, which includes pJIA and juvenile PsA, affects nearly 300,000 children and adolescents in the United States, and alternatives to TNF inhibitor (TNFi) therapy are limited, according to the company.
“Pediatric patients with pJIA and PsA can be severely limited in their ability to complete daily physical tasks and participate in everyday activities. Understanding their needs today and knowing the likelihood of disease in adulthood underscores the need for additional treatment options,” Aarat Patel, MD, a pediatric rheumatologist at Bon Secours Rheumatology Center, Richmond, Virginia, said in the press release. “Having a treatment option available for patients who do not respond well to a TNFi addresses a need for the healthcare community, patients, and their families,” he said.
Upadacitinib, a Janus kinase (JAK) inhibitor, is being studied for multiple immune-mediated inflammatory diseases. The new indication was supported by data from adults with rheumatoid arthritis (RA) and PsA, 51 pediatric patients with pJIA and active polyarthritis, and safety data from 83 pediatric patients aged 2 years to younger than 18 years with pJIA and active polyarthritis.
In the studies, the drug’s safety in pediatric patients was similar to the known safety profile in adults, which includes increased risk for serious infections such as tuberculosis, cancer, immune system problems, blood clots, and serious allergic reactions to components of the drug, according to the press release. However, the safety and effectiveness of upadacitinib for pJIA and PsA in patients younger than 2 years are unknown.
“Upadacitinib plasma exposures in pediatric patients with pJIA and PsA at the recommended dosage are predicted to be comparable to those observed in adults with RA and PsA based on population pharmacokinetic modeling and simulation,” according to the press release.
Currently, upadacitinib’s only other pediatric indication is for moderate to severe atopic dermatitis in children aged 12 years or older. Upadacitinib also is indicated for treatment of adults with moderate to severe RA, active PsA, active ankylosing spondylitis, active nonradiographic axial spondyloarthritis, and moderate to severe ulcerative colitis and Crohn’s disease, but safety and efficacy for its use in treatment of these conditions in children and adolescents is unknown.
Upadacitinib also is being studied in phase 3 trials for treatment of conditions including alopecia areata, ankylosing spondylitis, atopic dermatitis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, hidradenitis suppurativa, psoriatic arthritis, RA, systemic lupus erythematosus, Takayasu arteritis, ulcerative colitis, and vitiligo, according to the press release.
Full prescribing information and safety data for upadacitinib are available here.
A version of this article appeared on Medscape.com.
Upadacitinib (Rinvoq) is now indicated for patients aged 2 years or older with active polyarticular juvenile idiopathic arthritis (pJIA) and psoriatic arthritis (PsA) who cannot tolerate or achieve adequate disease response with one or more tumor necrosis factor (TNF) blockers, according to a press release from manufacturer AbbVie.
For the youngest patients, upadacitinib is also available as a weight-based oral solution (Rinvoq LQ) in addition to the previously available tablets, according to the company. JIA, which includes pJIA and juvenile PsA, affects nearly 300,000 children and adolescents in the United States, and alternatives to TNF inhibitor (TNFi) therapy are limited, according to the company.
“Pediatric patients with pJIA and PsA can be severely limited in their ability to complete daily physical tasks and participate in everyday activities. Understanding their needs today and knowing the likelihood of disease in adulthood underscores the need for additional treatment options,” Aarat Patel, MD, a pediatric rheumatologist at Bon Secours Rheumatology Center, Richmond, Virginia, said in the press release. “Having a treatment option available for patients who do not respond well to a TNFi addresses a need for the healthcare community, patients, and their families,” he said.
Upadacitinib, a Janus kinase (JAK) inhibitor, is being studied for multiple immune-mediated inflammatory diseases. The new indication was supported by data from adults with rheumatoid arthritis (RA) and PsA, 51 pediatric patients with pJIA and active polyarthritis, and safety data from 83 pediatric patients aged 2 years to younger than 18 years with pJIA and active polyarthritis.
In the studies, the drug’s safety in pediatric patients was similar to the known safety profile in adults, which includes increased risk for serious infections such as tuberculosis, cancer, immune system problems, blood clots, and serious allergic reactions to components of the drug, according to the press release. However, the safety and effectiveness of upadacitinib for pJIA and PsA in patients younger than 2 years are unknown.
“Upadacitinib plasma exposures in pediatric patients with pJIA and PsA at the recommended dosage are predicted to be comparable to those observed in adults with RA and PsA based on population pharmacokinetic modeling and simulation,” according to the press release.
Currently, upadacitinib’s only other pediatric indication is for moderate to severe atopic dermatitis in children aged 12 years or older. Upadacitinib also is indicated for treatment of adults with moderate to severe RA, active PsA, active ankylosing spondylitis, active nonradiographic axial spondyloarthritis, and moderate to severe ulcerative colitis and Crohn’s disease, but safety and efficacy for its use in treatment of these conditions in children and adolescents is unknown.
Upadacitinib also is being studied in phase 3 trials for treatment of conditions including alopecia areata, ankylosing spondylitis, atopic dermatitis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, hidradenitis suppurativa, psoriatic arthritis, RA, systemic lupus erythematosus, Takayasu arteritis, ulcerative colitis, and vitiligo, according to the press release.
Full prescribing information and safety data for upadacitinib are available here.
A version of this article appeared on Medscape.com.
Upadacitinib (Rinvoq) is now indicated for patients aged 2 years or older with active polyarticular juvenile idiopathic arthritis (pJIA) and psoriatic arthritis (PsA) who cannot tolerate or achieve adequate disease response with one or more tumor necrosis factor (TNF) blockers, according to a press release from manufacturer AbbVie.
For the youngest patients, upadacitinib is also available as a weight-based oral solution (Rinvoq LQ) in addition to the previously available tablets, according to the company. JIA, which includes pJIA and juvenile PsA, affects nearly 300,000 children and adolescents in the United States, and alternatives to TNF inhibitor (TNFi) therapy are limited, according to the company.
“Pediatric patients with pJIA and PsA can be severely limited in their ability to complete daily physical tasks and participate in everyday activities. Understanding their needs today and knowing the likelihood of disease in adulthood underscores the need for additional treatment options,” Aarat Patel, MD, a pediatric rheumatologist at Bon Secours Rheumatology Center, Richmond, Virginia, said in the press release. “Having a treatment option available for patients who do not respond well to a TNFi addresses a need for the healthcare community, patients, and their families,” he said.
Upadacitinib, a Janus kinase (JAK) inhibitor, is being studied for multiple immune-mediated inflammatory diseases. The new indication was supported by data from adults with rheumatoid arthritis (RA) and PsA, 51 pediatric patients with pJIA and active polyarthritis, and safety data from 83 pediatric patients aged 2 years to younger than 18 years with pJIA and active polyarthritis.
In the studies, the drug’s safety in pediatric patients was similar to the known safety profile in adults, which includes increased risk for serious infections such as tuberculosis, cancer, immune system problems, blood clots, and serious allergic reactions to components of the drug, according to the press release. However, the safety and effectiveness of upadacitinib for pJIA and PsA in patients younger than 2 years are unknown.
“Upadacitinib plasma exposures in pediatric patients with pJIA and PsA at the recommended dosage are predicted to be comparable to those observed in adults with RA and PsA based on population pharmacokinetic modeling and simulation,” according to the press release.
Currently, upadacitinib’s only other pediatric indication is for moderate to severe atopic dermatitis in children aged 12 years or older. Upadacitinib also is indicated for treatment of adults with moderate to severe RA, active PsA, active ankylosing spondylitis, active nonradiographic axial spondyloarthritis, and moderate to severe ulcerative colitis and Crohn’s disease, but safety and efficacy for its use in treatment of these conditions in children and adolescents is unknown.
Upadacitinib also is being studied in phase 3 trials for treatment of conditions including alopecia areata, ankylosing spondylitis, atopic dermatitis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, hidradenitis suppurativa, psoriatic arthritis, RA, systemic lupus erythematosus, Takayasu arteritis, ulcerative colitis, and vitiligo, according to the press release.
Full prescribing information and safety data for upadacitinib are available here.
A version of this article appeared on Medscape.com.
Inebilizumab ‘MITIGATES’ Flare Risk in IgG4-Related Disease
TOPLINE:
Inebilizumab-cdon, a monoclonal antibody that depletes B cells, reduces the risk for flares without showing any new safety signals in patients with immunoglobulin G4-related disease (IgG4-RD) who have multiorgan disease and are on glucocorticoid therapy.
METHODOLOGY:
- IgG4-RD is an immune-mediated, fibroinflammatory condition that affects multiple organs, causing irreversible organ damage. MITIGATE is the first multinational, placebo-controlled trial involving patients with IgG4-RD.
- Researchers evaluated the efficacy and safety of inebilizumab in 135 adult patients at risk for flares due to a history of multiorgan disease and active disease requiring treatment with glucocorticoids.
- The patients were randomly assigned to receive 300-mg intravenous inebilizumab or placebo on day 1, day 15, and week 26.
- The primary endpoint was the time to the first treated and adjudicated IgG4-RD flare within 52 weeks.
- The secondary endpoints included the annualized flare rate, flare-free and treatment-free complete remission, and flare-free and corticosteroid-free complete remission.
TAKEAWAY:
- Compared with the placebo, inebilizumab reduced the risk for IgG4-RD flares by 87% during the 52-week trial period (hazard ratio, 0.13; P < .0001).
- All the secondary endpoints showed improvement following treatment with inebilizumab.
- The most common adverse reactions with inebilizumab, as observed in a previous trial for neuromyelitis optica spectrum disorder, were urinary tract infection and arthralgia.
- There were no new safety signals in the MITIGATE trial.
IN PRACTICE:
“These data mark a major milestone for the IgG4-RD community and provide substantial insight into not only how inebilizumab can help manage IgG4-RD but also key insights into the nature of this condition,” John Stone, MD, MPH, principal investigator, said in a news release.
SOURCE:
Dr. Stone, a professor of medicine at the Harvard Medical School and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital, Boston, led this study.
LIMITATIONS:
This press release did not discuss any limitations of the current study.
DISCLOSURES:
This study was funded by Mitsubishi Tanabe Pharma and Hansoh Pharma and sponsored by Amgen. The author disclosures were not available.
A version of this article appeared on Medscape.com.
TOPLINE:
Inebilizumab-cdon, a monoclonal antibody that depletes B cells, reduces the risk for flares without showing any new safety signals in patients with immunoglobulin G4-related disease (IgG4-RD) who have multiorgan disease and are on glucocorticoid therapy.
METHODOLOGY:
- IgG4-RD is an immune-mediated, fibroinflammatory condition that affects multiple organs, causing irreversible organ damage. MITIGATE is the first multinational, placebo-controlled trial involving patients with IgG4-RD.
- Researchers evaluated the efficacy and safety of inebilizumab in 135 adult patients at risk for flares due to a history of multiorgan disease and active disease requiring treatment with glucocorticoids.
- The patients were randomly assigned to receive 300-mg intravenous inebilizumab or placebo on day 1, day 15, and week 26.
- The primary endpoint was the time to the first treated and adjudicated IgG4-RD flare within 52 weeks.
- The secondary endpoints included the annualized flare rate, flare-free and treatment-free complete remission, and flare-free and corticosteroid-free complete remission.
TAKEAWAY:
- Compared with the placebo, inebilizumab reduced the risk for IgG4-RD flares by 87% during the 52-week trial period (hazard ratio, 0.13; P < .0001).
- All the secondary endpoints showed improvement following treatment with inebilizumab.
- The most common adverse reactions with inebilizumab, as observed in a previous trial for neuromyelitis optica spectrum disorder, were urinary tract infection and arthralgia.
- There were no new safety signals in the MITIGATE trial.
IN PRACTICE:
“These data mark a major milestone for the IgG4-RD community and provide substantial insight into not only how inebilizumab can help manage IgG4-RD but also key insights into the nature of this condition,” John Stone, MD, MPH, principal investigator, said in a news release.
SOURCE:
Dr. Stone, a professor of medicine at the Harvard Medical School and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital, Boston, led this study.
LIMITATIONS:
This press release did not discuss any limitations of the current study.
DISCLOSURES:
This study was funded by Mitsubishi Tanabe Pharma and Hansoh Pharma and sponsored by Amgen. The author disclosures were not available.
A version of this article appeared on Medscape.com.
TOPLINE:
Inebilizumab-cdon, a monoclonal antibody that depletes B cells, reduces the risk for flares without showing any new safety signals in patients with immunoglobulin G4-related disease (IgG4-RD) who have multiorgan disease and are on glucocorticoid therapy.
METHODOLOGY:
- IgG4-RD is an immune-mediated, fibroinflammatory condition that affects multiple organs, causing irreversible organ damage. MITIGATE is the first multinational, placebo-controlled trial involving patients with IgG4-RD.
- Researchers evaluated the efficacy and safety of inebilizumab in 135 adult patients at risk for flares due to a history of multiorgan disease and active disease requiring treatment with glucocorticoids.
- The patients were randomly assigned to receive 300-mg intravenous inebilizumab or placebo on day 1, day 15, and week 26.
- The primary endpoint was the time to the first treated and adjudicated IgG4-RD flare within 52 weeks.
- The secondary endpoints included the annualized flare rate, flare-free and treatment-free complete remission, and flare-free and corticosteroid-free complete remission.
TAKEAWAY:
- Compared with the placebo, inebilizumab reduced the risk for IgG4-RD flares by 87% during the 52-week trial period (hazard ratio, 0.13; P < .0001).
- All the secondary endpoints showed improvement following treatment with inebilizumab.
- The most common adverse reactions with inebilizumab, as observed in a previous trial for neuromyelitis optica spectrum disorder, were urinary tract infection and arthralgia.
- There were no new safety signals in the MITIGATE trial.
IN PRACTICE:
“These data mark a major milestone for the IgG4-RD community and provide substantial insight into not only how inebilizumab can help manage IgG4-RD but also key insights into the nature of this condition,” John Stone, MD, MPH, principal investigator, said in a news release.
SOURCE:
Dr. Stone, a professor of medicine at the Harvard Medical School and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital, Boston, led this study.
LIMITATIONS:
This press release did not discuss any limitations of the current study.
DISCLOSURES:
This study was funded by Mitsubishi Tanabe Pharma and Hansoh Pharma and sponsored by Amgen. The author disclosures were not available.
A version of this article appeared on Medscape.com.
Commonly Used Meds Tied to Lower Risk for Brain Aneurysm Rupture
(aSAH), a drug-wide association study suggested.
The blood pressure drug lisinopril; the cholesterol drug simvastatin; the diabetes drug metformin; and the drug tamsulosin, prescribed for an enlarged prostate, were all associated with decreased aSAH risk, investigators found.
Conversely, four other drugs were associated with an increased risk for this severely morbid, often deadly, condition.
“The motivation for this study was the fact that we can currently prevent bleeding from intracranial aneurysms only by invasive treatment of those aneurysms with inherent complication risks,” said study investigator Ynte Ruigrok, MD, PhD, associate professor of neurology and neurosurgery, University Medical Center Utrecht, Utrecht, the Netherlands. “Drugs to reduce or eliminate this risk are not yet available. This study is a first step in identifying such drugs.”
The findings were published online in Neurology.
Surprising Results
For the study, the researchers used the Secure Anonymized Information Linkage data bank in Wales to identify 4879 patients with aSAH between January 2000 and December 2019 and 43,911 patients without aSAH matched on age, sex, and year of database entry. Clustering resulted in 2023 unique drugs, of which 205 were commonly prescribed.
After adjusting for other factors such as high blood pressure, alcohol abuse, smoking, and a total number of health conditions, the results yielded two surprises, Dr. Ruigrok observed.
The first was a significant decrease in aSAH risk for current use of lisinopril, compared with nonuse (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.44-0.90), and a nonsignificant decrease with current use of amlodipine (OR, 0.82; 95% CI, 0.65-1.04).
“Hypertension is a major risk factor for occurrence and bleeding from aneurysms. If there is indeed a specific blood pressure–lowering drug that not only has a blood pressure–lowering effect but also has additional protection against aSAH, then perhaps that drug should become the drug of choice in aneurysm patients in the future,” he said.
Notably, recent use of both drugs, defined as between 1 year and 3 months before the index date, was associated with an increased risk for aSAH. This trend was not found for other antihypertensives and was significant for amlodipine but not lisinopril.
The reasons are unclear, but “we trust the findings on lisinopril more,” Dr. Ruigrok said. “The findings on amlodipine may be due to confounding by indication, specifically caused by hypertension. Therefore, it is important to validate our findings in an independent research cohort, and we are in the process of doing so.”
The study’s second surprise was the antidiabetic drug metformin and cholesterol-lowering drug simvastatin were also associated with reduced aSAH risk, Dr. Ruigrok noted.
“We already knew from previous studies that diabetes and high cholesterol are protective factors for aSAH,” he said. “Our results suggest that perhaps not the conditions themselves are protective for aSAH but rather the drugs used to treat these conditions with are.”
The risk for a ruptured brain aneurysm among current users was 42% lower with metformin (OR, 0.58; 95% CI, 0.43-0.78), 22% lower with simvastatin (OR, 0.78; 95% CI, 0.64-0.96), and 45% lower with tamsulosin (OR, 0.55; 95% CI, 0.32-0.93).
An increased risk for aSAH was found only in current users of warfarin (OR, 1.35; 95% CI, 1.02-1.79), venlafaxine (OR, 1.67; 95% CI, 1.01-2.75), prochlorperazine (OR, 2.15; 95% CI, 1.45-3.18), and co-codamol (OR, 1.31; 95% CI, 1.10-1.56).
Other drugs within the classes of vitamin K antagonists, serotonin reuptake inhibitors, conventional antipsychotics, and compound analgesics did not show an association with aSAH.
The study was limited by the use of drug prescriptions, and patients may not take their drugs or use them incorrectly, noted the researchers, led by Jos P. Kanning, MSc, also with University Medical Center Utrecht.
The study was supported by the European Research Council. The authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
(aSAH), a drug-wide association study suggested.
The blood pressure drug lisinopril; the cholesterol drug simvastatin; the diabetes drug metformin; and the drug tamsulosin, prescribed for an enlarged prostate, were all associated with decreased aSAH risk, investigators found.
Conversely, four other drugs were associated with an increased risk for this severely morbid, often deadly, condition.
“The motivation for this study was the fact that we can currently prevent bleeding from intracranial aneurysms only by invasive treatment of those aneurysms with inherent complication risks,” said study investigator Ynte Ruigrok, MD, PhD, associate professor of neurology and neurosurgery, University Medical Center Utrecht, Utrecht, the Netherlands. “Drugs to reduce or eliminate this risk are not yet available. This study is a first step in identifying such drugs.”
The findings were published online in Neurology.
Surprising Results
For the study, the researchers used the Secure Anonymized Information Linkage data bank in Wales to identify 4879 patients with aSAH between January 2000 and December 2019 and 43,911 patients without aSAH matched on age, sex, and year of database entry. Clustering resulted in 2023 unique drugs, of which 205 were commonly prescribed.
After adjusting for other factors such as high blood pressure, alcohol abuse, smoking, and a total number of health conditions, the results yielded two surprises, Dr. Ruigrok observed.
The first was a significant decrease in aSAH risk for current use of lisinopril, compared with nonuse (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.44-0.90), and a nonsignificant decrease with current use of amlodipine (OR, 0.82; 95% CI, 0.65-1.04).
“Hypertension is a major risk factor for occurrence and bleeding from aneurysms. If there is indeed a specific blood pressure–lowering drug that not only has a blood pressure–lowering effect but also has additional protection against aSAH, then perhaps that drug should become the drug of choice in aneurysm patients in the future,” he said.
Notably, recent use of both drugs, defined as between 1 year and 3 months before the index date, was associated with an increased risk for aSAH. This trend was not found for other antihypertensives and was significant for amlodipine but not lisinopril.
The reasons are unclear, but “we trust the findings on lisinopril more,” Dr. Ruigrok said. “The findings on amlodipine may be due to confounding by indication, specifically caused by hypertension. Therefore, it is important to validate our findings in an independent research cohort, and we are in the process of doing so.”
The study’s second surprise was the antidiabetic drug metformin and cholesterol-lowering drug simvastatin were also associated with reduced aSAH risk, Dr. Ruigrok noted.
“We already knew from previous studies that diabetes and high cholesterol are protective factors for aSAH,” he said. “Our results suggest that perhaps not the conditions themselves are protective for aSAH but rather the drugs used to treat these conditions with are.”
The risk for a ruptured brain aneurysm among current users was 42% lower with metformin (OR, 0.58; 95% CI, 0.43-0.78), 22% lower with simvastatin (OR, 0.78; 95% CI, 0.64-0.96), and 45% lower with tamsulosin (OR, 0.55; 95% CI, 0.32-0.93).
An increased risk for aSAH was found only in current users of warfarin (OR, 1.35; 95% CI, 1.02-1.79), venlafaxine (OR, 1.67; 95% CI, 1.01-2.75), prochlorperazine (OR, 2.15; 95% CI, 1.45-3.18), and co-codamol (OR, 1.31; 95% CI, 1.10-1.56).
Other drugs within the classes of vitamin K antagonists, serotonin reuptake inhibitors, conventional antipsychotics, and compound analgesics did not show an association with aSAH.
The study was limited by the use of drug prescriptions, and patients may not take their drugs or use them incorrectly, noted the researchers, led by Jos P. Kanning, MSc, also with University Medical Center Utrecht.
The study was supported by the European Research Council. The authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
(aSAH), a drug-wide association study suggested.
The blood pressure drug lisinopril; the cholesterol drug simvastatin; the diabetes drug metformin; and the drug tamsulosin, prescribed for an enlarged prostate, were all associated with decreased aSAH risk, investigators found.
Conversely, four other drugs were associated with an increased risk for this severely morbid, often deadly, condition.
“The motivation for this study was the fact that we can currently prevent bleeding from intracranial aneurysms only by invasive treatment of those aneurysms with inherent complication risks,” said study investigator Ynte Ruigrok, MD, PhD, associate professor of neurology and neurosurgery, University Medical Center Utrecht, Utrecht, the Netherlands. “Drugs to reduce or eliminate this risk are not yet available. This study is a first step in identifying such drugs.”
The findings were published online in Neurology.
Surprising Results
For the study, the researchers used the Secure Anonymized Information Linkage data bank in Wales to identify 4879 patients with aSAH between January 2000 and December 2019 and 43,911 patients without aSAH matched on age, sex, and year of database entry. Clustering resulted in 2023 unique drugs, of which 205 were commonly prescribed.
After adjusting for other factors such as high blood pressure, alcohol abuse, smoking, and a total number of health conditions, the results yielded two surprises, Dr. Ruigrok observed.
The first was a significant decrease in aSAH risk for current use of lisinopril, compared with nonuse (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.44-0.90), and a nonsignificant decrease with current use of amlodipine (OR, 0.82; 95% CI, 0.65-1.04).
“Hypertension is a major risk factor for occurrence and bleeding from aneurysms. If there is indeed a specific blood pressure–lowering drug that not only has a blood pressure–lowering effect but also has additional protection against aSAH, then perhaps that drug should become the drug of choice in aneurysm patients in the future,” he said.
Notably, recent use of both drugs, defined as between 1 year and 3 months before the index date, was associated with an increased risk for aSAH. This trend was not found for other antihypertensives and was significant for amlodipine but not lisinopril.
The reasons are unclear, but “we trust the findings on lisinopril more,” Dr. Ruigrok said. “The findings on amlodipine may be due to confounding by indication, specifically caused by hypertension. Therefore, it is important to validate our findings in an independent research cohort, and we are in the process of doing so.”
The study’s second surprise was the antidiabetic drug metformin and cholesterol-lowering drug simvastatin were also associated with reduced aSAH risk, Dr. Ruigrok noted.
“We already knew from previous studies that diabetes and high cholesterol are protective factors for aSAH,” he said. “Our results suggest that perhaps not the conditions themselves are protective for aSAH but rather the drugs used to treat these conditions with are.”
The risk for a ruptured brain aneurysm among current users was 42% lower with metformin (OR, 0.58; 95% CI, 0.43-0.78), 22% lower with simvastatin (OR, 0.78; 95% CI, 0.64-0.96), and 45% lower with tamsulosin (OR, 0.55; 95% CI, 0.32-0.93).
An increased risk for aSAH was found only in current users of warfarin (OR, 1.35; 95% CI, 1.02-1.79), venlafaxine (OR, 1.67; 95% CI, 1.01-2.75), prochlorperazine (OR, 2.15; 95% CI, 1.45-3.18), and co-codamol (OR, 1.31; 95% CI, 1.10-1.56).
Other drugs within the classes of vitamin K antagonists, serotonin reuptake inhibitors, conventional antipsychotics, and compound analgesics did not show an association with aSAH.
The study was limited by the use of drug prescriptions, and patients may not take their drugs or use them incorrectly, noted the researchers, led by Jos P. Kanning, MSc, also with University Medical Center Utrecht.
The study was supported by the European Research Council. The authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM NEUROLOGY
High Sodium Intake Linked to Greater Risk for Eczema
In a study of adults, an increase of 1 g in estimated 24-hour urinary sodium excretion was associated with 11% higher odds of an atopic dermatitis (AD) diagnosis, 16% higher odds of having active AD, and 11% higher odds of increased severity of AD.
Those are key findings from a cross-sectional analysis of data from the United Kingdom.
“Excessive dietary sodium, common in fast food, may be associated with AD,” corresponding author Katrina Abuabara, MD, MA, MSCE, and colleagues wrote in the study, which was published online in JAMA Dermatology. They referred to recent research using sodium MRI, which showed that “the majority of the body’s exchangeable sodium is stored in the skin and that skin sodium is associated with autoimmune and chronic inflammatory conditions, including AD.” And in another study published in 2019, lesional skin sodium was 30-fold greater in patients with AD than in healthy controls.
To investigate whether there is an association between higher levels of sodium consumption and AD prevalence, activity, and severity at the population level, Dr. Abuabara, of the program for clinical research in the Department of Dermatology at the University of California, San Francisco, and coauthors drew from the UK Biobank, a population-based cohort of more than 500,000 individuals aged 37-73 years at the time of recruitment by the National Health Service. The primary exposure was 24-hour urinary sodium excretion, which was calculated by using the INTERSALT equation, a sex-specific estimation that incorporates body mass index; age; and urine concentrations of potassium, sodium, and creatinine. The primary study outcome was AD or active AD based on diagnostic and prescription codes from linked electronic medical records. The researchers used multivariable logistic regression models adjusted for age, sex, race and ethnicity, Townsend deprivation index, and education to measure the association.
Of the 215,832 Biobank participants included in the analysis, 54% were female, their mean age was 57 years, 95% were White, their mean estimated 24-hour urine sodium excretion was 3.01 g/day, and 10,839 (5%) had a diagnosis of AD. The researchers observed that on multivariable logistic regression, a 1-g increase in estimated 24-hour urine sodium excretion was associated with increased odds of AD (adjusted odds ratio [AOR], 1.11; 95% CI, 1.07-1.14), increased odds of active AD (AOR, 1.16; 95% CI, 1.05-1.28), and increased odds of increasing severity of AD (AOR, 1.11; 95% CI, 1.07-1.15).
Validating Results With US Data
To validate the findings, the researchers evaluated a cohort of 13,014 participants from the US-based National Health and Nutrition Examination Survey (NHANES), using pooled data from the 1999-2000, 2001-2002, and 2003-2004 samples. Of the 13,014 participants, 796 reported current AD, and 1493 reported AD in the past year. The mean dietary sodium intake of overall NHANES participants estimated with 24-hour dietary recall questionnaires was 3.45 g, with a mean of 3.47 g for those with current AD and a mean of 3.44 g for those without AD.
The researchers observed that a 1-g/day higher dietary sodium intake was associated with a higher risk for current AD (AOR, 1.22; 95%CI, 1.01-1.47) and a somewhat higher risk for AD in the past year (AOR, 1.14; 95% CI, 0.97-1.35).
“Future work should examine whether variation of sodium intake over time might trigger AD flares and whether it helps to explain heterogeneity in response to new immunomodulatory treatments for AD,” the authors wrote. “Reduced sodium intake was recommended as a treatment for AD more than a century ago, but there have yet to be studies examining the association of dietary sodium reduction with skin sodium concentration or AD severity,” they added. Noting that sodium reduction “has been shown to be a cost-effective intervention for hypertension and other cardiovascular disease outcomes,” they said that their data “support experimental studies of this approach in AD.”
They acknowledged certain limitations of the study, including the fact that a single spot urine sample was used in the UK Biobank cohort, “which only captures dietary intake of the last 24 hours and is not the best measure of usual or long-term intake of sodium.” They also noted that the findings may not be generalizable to other populations and that AD was based on self-report in the NHANES validation cohort.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the results, said the study by Dr. Abuabara and colleagues “gives us another reason to avoid salt, showing that 1 g/day of higher salt intake increases the risk of AD in an adult population and more severe AD.”
He added that, “Now, can you say that reducing salt intake will have a therapeutic effect or clinically relevant impact? No. [That is] certainly worth exploring but at a minimum, gives some more credibility to keeping it bland.”
The study was supported by a grant from the Medical Student in Aging Research Program, the National Institute on Aging, and the National Eczema Association. Dr. Abuabara reported receiving research funding for her institution from Pfizer and Cosmetique Internacional/La Roche-Posay and consulting fees from Target RWE, Sanofi, Nektar, and Amgen. No other disclosures were reported. Dr. Friedman had no relevant disclosures.
A version of this article appeared on Medscape.com.
In a study of adults, an increase of 1 g in estimated 24-hour urinary sodium excretion was associated with 11% higher odds of an atopic dermatitis (AD) diagnosis, 16% higher odds of having active AD, and 11% higher odds of increased severity of AD.
Those are key findings from a cross-sectional analysis of data from the United Kingdom.
“Excessive dietary sodium, common in fast food, may be associated with AD,” corresponding author Katrina Abuabara, MD, MA, MSCE, and colleagues wrote in the study, which was published online in JAMA Dermatology. They referred to recent research using sodium MRI, which showed that “the majority of the body’s exchangeable sodium is stored in the skin and that skin sodium is associated with autoimmune and chronic inflammatory conditions, including AD.” And in another study published in 2019, lesional skin sodium was 30-fold greater in patients with AD than in healthy controls.
To investigate whether there is an association between higher levels of sodium consumption and AD prevalence, activity, and severity at the population level, Dr. Abuabara, of the program for clinical research in the Department of Dermatology at the University of California, San Francisco, and coauthors drew from the UK Biobank, a population-based cohort of more than 500,000 individuals aged 37-73 years at the time of recruitment by the National Health Service. The primary exposure was 24-hour urinary sodium excretion, which was calculated by using the INTERSALT equation, a sex-specific estimation that incorporates body mass index; age; and urine concentrations of potassium, sodium, and creatinine. The primary study outcome was AD or active AD based on diagnostic and prescription codes from linked electronic medical records. The researchers used multivariable logistic regression models adjusted for age, sex, race and ethnicity, Townsend deprivation index, and education to measure the association.
Of the 215,832 Biobank participants included in the analysis, 54% were female, their mean age was 57 years, 95% were White, their mean estimated 24-hour urine sodium excretion was 3.01 g/day, and 10,839 (5%) had a diagnosis of AD. The researchers observed that on multivariable logistic regression, a 1-g increase in estimated 24-hour urine sodium excretion was associated with increased odds of AD (adjusted odds ratio [AOR], 1.11; 95% CI, 1.07-1.14), increased odds of active AD (AOR, 1.16; 95% CI, 1.05-1.28), and increased odds of increasing severity of AD (AOR, 1.11; 95% CI, 1.07-1.15).
Validating Results With US Data
To validate the findings, the researchers evaluated a cohort of 13,014 participants from the US-based National Health and Nutrition Examination Survey (NHANES), using pooled data from the 1999-2000, 2001-2002, and 2003-2004 samples. Of the 13,014 participants, 796 reported current AD, and 1493 reported AD in the past year. The mean dietary sodium intake of overall NHANES participants estimated with 24-hour dietary recall questionnaires was 3.45 g, with a mean of 3.47 g for those with current AD and a mean of 3.44 g for those without AD.
The researchers observed that a 1-g/day higher dietary sodium intake was associated with a higher risk for current AD (AOR, 1.22; 95%CI, 1.01-1.47) and a somewhat higher risk for AD in the past year (AOR, 1.14; 95% CI, 0.97-1.35).
“Future work should examine whether variation of sodium intake over time might trigger AD flares and whether it helps to explain heterogeneity in response to new immunomodulatory treatments for AD,” the authors wrote. “Reduced sodium intake was recommended as a treatment for AD more than a century ago, but there have yet to be studies examining the association of dietary sodium reduction with skin sodium concentration or AD severity,” they added. Noting that sodium reduction “has been shown to be a cost-effective intervention for hypertension and other cardiovascular disease outcomes,” they said that their data “support experimental studies of this approach in AD.”
They acknowledged certain limitations of the study, including the fact that a single spot urine sample was used in the UK Biobank cohort, “which only captures dietary intake of the last 24 hours and is not the best measure of usual or long-term intake of sodium.” They also noted that the findings may not be generalizable to other populations and that AD was based on self-report in the NHANES validation cohort.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the results, said the study by Dr. Abuabara and colleagues “gives us another reason to avoid salt, showing that 1 g/day of higher salt intake increases the risk of AD in an adult population and more severe AD.”
He added that, “Now, can you say that reducing salt intake will have a therapeutic effect or clinically relevant impact? No. [That is] certainly worth exploring but at a minimum, gives some more credibility to keeping it bland.”
The study was supported by a grant from the Medical Student in Aging Research Program, the National Institute on Aging, and the National Eczema Association. Dr. Abuabara reported receiving research funding for her institution from Pfizer and Cosmetique Internacional/La Roche-Posay and consulting fees from Target RWE, Sanofi, Nektar, and Amgen. No other disclosures were reported. Dr. Friedman had no relevant disclosures.
A version of this article appeared on Medscape.com.
In a study of adults, an increase of 1 g in estimated 24-hour urinary sodium excretion was associated with 11% higher odds of an atopic dermatitis (AD) diagnosis, 16% higher odds of having active AD, and 11% higher odds of increased severity of AD.
Those are key findings from a cross-sectional analysis of data from the United Kingdom.
“Excessive dietary sodium, common in fast food, may be associated with AD,” corresponding author Katrina Abuabara, MD, MA, MSCE, and colleagues wrote in the study, which was published online in JAMA Dermatology. They referred to recent research using sodium MRI, which showed that “the majority of the body’s exchangeable sodium is stored in the skin and that skin sodium is associated with autoimmune and chronic inflammatory conditions, including AD.” And in another study published in 2019, lesional skin sodium was 30-fold greater in patients with AD than in healthy controls.
To investigate whether there is an association between higher levels of sodium consumption and AD prevalence, activity, and severity at the population level, Dr. Abuabara, of the program for clinical research in the Department of Dermatology at the University of California, San Francisco, and coauthors drew from the UK Biobank, a population-based cohort of more than 500,000 individuals aged 37-73 years at the time of recruitment by the National Health Service. The primary exposure was 24-hour urinary sodium excretion, which was calculated by using the INTERSALT equation, a sex-specific estimation that incorporates body mass index; age; and urine concentrations of potassium, sodium, and creatinine. The primary study outcome was AD or active AD based on diagnostic and prescription codes from linked electronic medical records. The researchers used multivariable logistic regression models adjusted for age, sex, race and ethnicity, Townsend deprivation index, and education to measure the association.
Of the 215,832 Biobank participants included in the analysis, 54% were female, their mean age was 57 years, 95% were White, their mean estimated 24-hour urine sodium excretion was 3.01 g/day, and 10,839 (5%) had a diagnosis of AD. The researchers observed that on multivariable logistic regression, a 1-g increase in estimated 24-hour urine sodium excretion was associated with increased odds of AD (adjusted odds ratio [AOR], 1.11; 95% CI, 1.07-1.14), increased odds of active AD (AOR, 1.16; 95% CI, 1.05-1.28), and increased odds of increasing severity of AD (AOR, 1.11; 95% CI, 1.07-1.15).
Validating Results With US Data
To validate the findings, the researchers evaluated a cohort of 13,014 participants from the US-based National Health and Nutrition Examination Survey (NHANES), using pooled data from the 1999-2000, 2001-2002, and 2003-2004 samples. Of the 13,014 participants, 796 reported current AD, and 1493 reported AD in the past year. The mean dietary sodium intake of overall NHANES participants estimated with 24-hour dietary recall questionnaires was 3.45 g, with a mean of 3.47 g for those with current AD and a mean of 3.44 g for those without AD.
The researchers observed that a 1-g/day higher dietary sodium intake was associated with a higher risk for current AD (AOR, 1.22; 95%CI, 1.01-1.47) and a somewhat higher risk for AD in the past year (AOR, 1.14; 95% CI, 0.97-1.35).
“Future work should examine whether variation of sodium intake over time might trigger AD flares and whether it helps to explain heterogeneity in response to new immunomodulatory treatments for AD,” the authors wrote. “Reduced sodium intake was recommended as a treatment for AD more than a century ago, but there have yet to be studies examining the association of dietary sodium reduction with skin sodium concentration or AD severity,” they added. Noting that sodium reduction “has been shown to be a cost-effective intervention for hypertension and other cardiovascular disease outcomes,” they said that their data “support experimental studies of this approach in AD.”
They acknowledged certain limitations of the study, including the fact that a single spot urine sample was used in the UK Biobank cohort, “which only captures dietary intake of the last 24 hours and is not the best measure of usual or long-term intake of sodium.” They also noted that the findings may not be generalizable to other populations and that AD was based on self-report in the NHANES validation cohort.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the results, said the study by Dr. Abuabara and colleagues “gives us another reason to avoid salt, showing that 1 g/day of higher salt intake increases the risk of AD in an adult population and more severe AD.”
He added that, “Now, can you say that reducing salt intake will have a therapeutic effect or clinically relevant impact? No. [That is] certainly worth exploring but at a minimum, gives some more credibility to keeping it bland.”
The study was supported by a grant from the Medical Student in Aging Research Program, the National Institute on Aging, and the National Eczema Association. Dr. Abuabara reported receiving research funding for her institution from Pfizer and Cosmetique Internacional/La Roche-Posay and consulting fees from Target RWE, Sanofi, Nektar, and Amgen. No other disclosures were reported. Dr. Friedman had no relevant disclosures.
A version of this article appeared on Medscape.com.
Interictal Burden, Disability, Allodynia Linked to Increased Likelihood of Seeking Migraine Care
, according to recent research published in the journal Headache.
“[T]he burden and impact of migraine on the individual both during and between attacks were identified through supervised machine learning models to be strongly associated with seeking care,” Sait Ashina, MD, of the department of neurology at Harvard Medical School in Boston, and colleagues wrote in their study.
Dr. Ashina and colleagues performed a cross-sectional study of 61,826 patients from the web-based ObserVational survey of the Epidemiology, tReatment and Care Of MigrainE (OVERCOME) study with migraine who visited a primary care, specialty care, or urgent care, or emergency setting for headache between 2018 and 2020.
The patients recruited for OBSERVE were a mean of 41.7 years old and had experienced migraines for an average of 19.0 years; 59.4% had between 0 and 3 average headache days per month, 74.5% were women, 78.8% were White, and 85.4% had health insurance; and they were demographically representative of the US population.
Researchers used a machine learning model, which consisted of random forest and least absolute shrinkage and selection operator (LASSO) algorithms, to identify the relationship between patients who sought care for migraine and 54 different clinical, sociodemographic, and migraine-associated factors, which included age, years with migraine, symptom scores, pain intensity scores, disability score, comorbidities, vomiting, presence and severity of allodynia, and other factors.
The results showed 31,529 patients (51.0%) had an in-person or e-visit encounter with a primary care, specialty care, or urgent care, or emergency care location within 12 months of the survey, and were mostly White (76.5%) women (73.3%) with health insurance (88.9%). Of the patients who sought care, 52.8% had severe interictal burden measured by Migraine Interictal Burden Scale-4 score, compared with 23.1% of patients who did not seek care. Compared with patients who did not seek care, those who did visit a health care setting for migraine had a higher percentage of severe migraine-related disability as measured by the Migraine Disability Assessment Scale (36.7% vs 14.6%) and severe ictal cutaneous allodynia as measured by the Allodynia Symptom Checklist (21.0% vs 7.4%).
In a multivariable logistic regression model analysis, Dr. Ashina and colleagues said the factors most associated with seeking care included severe interictal burden (odds ratio [OR], 2.64; 95% confidence interval [CI], 2.5-2.8), severe migraine-related disability (OR, 2.2; 95% CI, 2.0-2.3), and severe ictal allodynia (OR, 1.7; 95% CI, 1.6-1.8), compared with less severe factors.
The researchers said their results have “significant implications for public health and advocacy efforts.”
“As seen through three decades of epidemiological research in the United States, rates of care-seeking have not improved dramatically over time despite significant additions to scientific knowledge and the therapeutic armamentarium, leaving a significant unmet need. This is also important from a clinical perspective,” they explained. “Health care professionals in primary care and internal medicine most likely see patients with migraine who do not discuss it during visits. This underscores the importance of maintaining vigilance for migraine, especially among those who may experience greater disability, impact, and interictal burden.”
Asking the Right Questions
Asked to comment on the research, Robert P. Cowan, MD, a neurologist and professor in the Stanford University School of Medicine department of neurology and neurological sciences in Palo Alto, California, said in an interview that the value of the paper is in what it does not say about the main reasons patients seek care.
“Most clinicians readily acknowledge that the average number of migraine headache days per month is, at best, a weak predictor of which patients seek care and when,” he said.
Dr. Cowan said that most patients are referred to him by other providers, and when he asks them why they did not seek care for migraine sooner, the answer is usually because the migraine was not severe enough or because over-the-counter medication had previously worked for them. He noted that change in frequency is, in his experience, a primary reason why patients will seek care. “[F]or new (or increasing) headache, it is the concern that the headaches are something more ‘serious,’ and once that is ruled out, the conversation often stops,” he said. “For long-standing migraine sufferers, it is the perception that the headache is a ‘fact of life’ and does not rise to the bar of seeking medical advice.”
The questions a survey or a provider asks matters, Dr. Cowan said. “Often, when we ask a patient how many headache (or migraine) days per month, the answer is in single digits. But if we follow-up with a question about the number of headache-free days [per] month, the answer is ‘never’ or ‘hardly ever,’” he explained. “The point here is that what questions a survey (or a provider) asks introduces a clear bias. The use of machine learning instruments, especially when utilizing supervised learning, only reinforces and amplifies the bias of the designers of the categories.”
Epidemiologic studies are interesting but “often ask the wrong questions,” Dr. Cowan said. “I am less worried about the ... 49% of migraine or possible migraine patients who do not seek care and do [not] progress to more disabling ‘chronic’ migraine than I am with identifying the subpopulations of migraine patients who seek care from providers who do not have adequate tools to match patients to the best treatments.”
The authors reported personal and institutional relationships in the form of advisory board memberships, consultancies, employment, honoraria, research support, speakers bureau positions, stock ownership, and teaching services with AbbVie, Aeon, Alder, Allay Lamp, Allergan, Amgen, Axon, Biohaven Pharmaceuticals, Collegium, CoolTech, Currax, Dr. Reddy’s Laboratories (Promius), electroCore, GlaxoSmithKline, Impel NeuroPharma, Informa, Eli Lilly and Company, Lundbeck, Mainistee, Merck, National Headache Foundation, National Institutes of Health, Novartis, Pfizer, Satsuma, Supernus, Percept, Teva, Theranica, UpsherSmith, the US Food and Drug Administration, Vector, Vedanta Research, and Wolff’s Headache. The study was supported by Eli Lilly. Dr. Cowan reports no relevant conflicts of interest.
, according to recent research published in the journal Headache.
“[T]he burden and impact of migraine on the individual both during and between attacks were identified through supervised machine learning models to be strongly associated with seeking care,” Sait Ashina, MD, of the department of neurology at Harvard Medical School in Boston, and colleagues wrote in their study.
Dr. Ashina and colleagues performed a cross-sectional study of 61,826 patients from the web-based ObserVational survey of the Epidemiology, tReatment and Care Of MigrainE (OVERCOME) study with migraine who visited a primary care, specialty care, or urgent care, or emergency setting for headache between 2018 and 2020.
The patients recruited for OBSERVE were a mean of 41.7 years old and had experienced migraines for an average of 19.0 years; 59.4% had between 0 and 3 average headache days per month, 74.5% were women, 78.8% were White, and 85.4% had health insurance; and they were demographically representative of the US population.
Researchers used a machine learning model, which consisted of random forest and least absolute shrinkage and selection operator (LASSO) algorithms, to identify the relationship between patients who sought care for migraine and 54 different clinical, sociodemographic, and migraine-associated factors, which included age, years with migraine, symptom scores, pain intensity scores, disability score, comorbidities, vomiting, presence and severity of allodynia, and other factors.
The results showed 31,529 patients (51.0%) had an in-person or e-visit encounter with a primary care, specialty care, or urgent care, or emergency care location within 12 months of the survey, and were mostly White (76.5%) women (73.3%) with health insurance (88.9%). Of the patients who sought care, 52.8% had severe interictal burden measured by Migraine Interictal Burden Scale-4 score, compared with 23.1% of patients who did not seek care. Compared with patients who did not seek care, those who did visit a health care setting for migraine had a higher percentage of severe migraine-related disability as measured by the Migraine Disability Assessment Scale (36.7% vs 14.6%) and severe ictal cutaneous allodynia as measured by the Allodynia Symptom Checklist (21.0% vs 7.4%).
In a multivariable logistic regression model analysis, Dr. Ashina and colleagues said the factors most associated with seeking care included severe interictal burden (odds ratio [OR], 2.64; 95% confidence interval [CI], 2.5-2.8), severe migraine-related disability (OR, 2.2; 95% CI, 2.0-2.3), and severe ictal allodynia (OR, 1.7; 95% CI, 1.6-1.8), compared with less severe factors.
The researchers said their results have “significant implications for public health and advocacy efforts.”
“As seen through three decades of epidemiological research in the United States, rates of care-seeking have not improved dramatically over time despite significant additions to scientific knowledge and the therapeutic armamentarium, leaving a significant unmet need. This is also important from a clinical perspective,” they explained. “Health care professionals in primary care and internal medicine most likely see patients with migraine who do not discuss it during visits. This underscores the importance of maintaining vigilance for migraine, especially among those who may experience greater disability, impact, and interictal burden.”
Asking the Right Questions
Asked to comment on the research, Robert P. Cowan, MD, a neurologist and professor in the Stanford University School of Medicine department of neurology and neurological sciences in Palo Alto, California, said in an interview that the value of the paper is in what it does not say about the main reasons patients seek care.
“Most clinicians readily acknowledge that the average number of migraine headache days per month is, at best, a weak predictor of which patients seek care and when,” he said.
Dr. Cowan said that most patients are referred to him by other providers, and when he asks them why they did not seek care for migraine sooner, the answer is usually because the migraine was not severe enough or because over-the-counter medication had previously worked for them. He noted that change in frequency is, in his experience, a primary reason why patients will seek care. “[F]or new (or increasing) headache, it is the concern that the headaches are something more ‘serious,’ and once that is ruled out, the conversation often stops,” he said. “For long-standing migraine sufferers, it is the perception that the headache is a ‘fact of life’ and does not rise to the bar of seeking medical advice.”
The questions a survey or a provider asks matters, Dr. Cowan said. “Often, when we ask a patient how many headache (or migraine) days per month, the answer is in single digits. But if we follow-up with a question about the number of headache-free days [per] month, the answer is ‘never’ or ‘hardly ever,’” he explained. “The point here is that what questions a survey (or a provider) asks introduces a clear bias. The use of machine learning instruments, especially when utilizing supervised learning, only reinforces and amplifies the bias of the designers of the categories.”
Epidemiologic studies are interesting but “often ask the wrong questions,” Dr. Cowan said. “I am less worried about the ... 49% of migraine or possible migraine patients who do not seek care and do [not] progress to more disabling ‘chronic’ migraine than I am with identifying the subpopulations of migraine patients who seek care from providers who do not have adequate tools to match patients to the best treatments.”
The authors reported personal and institutional relationships in the form of advisory board memberships, consultancies, employment, honoraria, research support, speakers bureau positions, stock ownership, and teaching services with AbbVie, Aeon, Alder, Allay Lamp, Allergan, Amgen, Axon, Biohaven Pharmaceuticals, Collegium, CoolTech, Currax, Dr. Reddy’s Laboratories (Promius), electroCore, GlaxoSmithKline, Impel NeuroPharma, Informa, Eli Lilly and Company, Lundbeck, Mainistee, Merck, National Headache Foundation, National Institutes of Health, Novartis, Pfizer, Satsuma, Supernus, Percept, Teva, Theranica, UpsherSmith, the US Food and Drug Administration, Vector, Vedanta Research, and Wolff’s Headache. The study was supported by Eli Lilly. Dr. Cowan reports no relevant conflicts of interest.
, according to recent research published in the journal Headache.
“[T]he burden and impact of migraine on the individual both during and between attacks were identified through supervised machine learning models to be strongly associated with seeking care,” Sait Ashina, MD, of the department of neurology at Harvard Medical School in Boston, and colleagues wrote in their study.
Dr. Ashina and colleagues performed a cross-sectional study of 61,826 patients from the web-based ObserVational survey of the Epidemiology, tReatment and Care Of MigrainE (OVERCOME) study with migraine who visited a primary care, specialty care, or urgent care, or emergency setting for headache between 2018 and 2020.
The patients recruited for OBSERVE were a mean of 41.7 years old and had experienced migraines for an average of 19.0 years; 59.4% had between 0 and 3 average headache days per month, 74.5% were women, 78.8% were White, and 85.4% had health insurance; and they were demographically representative of the US population.
Researchers used a machine learning model, which consisted of random forest and least absolute shrinkage and selection operator (LASSO) algorithms, to identify the relationship between patients who sought care for migraine and 54 different clinical, sociodemographic, and migraine-associated factors, which included age, years with migraine, symptom scores, pain intensity scores, disability score, comorbidities, vomiting, presence and severity of allodynia, and other factors.
The results showed 31,529 patients (51.0%) had an in-person or e-visit encounter with a primary care, specialty care, or urgent care, or emergency care location within 12 months of the survey, and were mostly White (76.5%) women (73.3%) with health insurance (88.9%). Of the patients who sought care, 52.8% had severe interictal burden measured by Migraine Interictal Burden Scale-4 score, compared with 23.1% of patients who did not seek care. Compared with patients who did not seek care, those who did visit a health care setting for migraine had a higher percentage of severe migraine-related disability as measured by the Migraine Disability Assessment Scale (36.7% vs 14.6%) and severe ictal cutaneous allodynia as measured by the Allodynia Symptom Checklist (21.0% vs 7.4%).
In a multivariable logistic regression model analysis, Dr. Ashina and colleagues said the factors most associated with seeking care included severe interictal burden (odds ratio [OR], 2.64; 95% confidence interval [CI], 2.5-2.8), severe migraine-related disability (OR, 2.2; 95% CI, 2.0-2.3), and severe ictal allodynia (OR, 1.7; 95% CI, 1.6-1.8), compared with less severe factors.
The researchers said their results have “significant implications for public health and advocacy efforts.”
“As seen through three decades of epidemiological research in the United States, rates of care-seeking have not improved dramatically over time despite significant additions to scientific knowledge and the therapeutic armamentarium, leaving a significant unmet need. This is also important from a clinical perspective,” they explained. “Health care professionals in primary care and internal medicine most likely see patients with migraine who do not discuss it during visits. This underscores the importance of maintaining vigilance for migraine, especially among those who may experience greater disability, impact, and interictal burden.”
Asking the Right Questions
Asked to comment on the research, Robert P. Cowan, MD, a neurologist and professor in the Stanford University School of Medicine department of neurology and neurological sciences in Palo Alto, California, said in an interview that the value of the paper is in what it does not say about the main reasons patients seek care.
“Most clinicians readily acknowledge that the average number of migraine headache days per month is, at best, a weak predictor of which patients seek care and when,” he said.
Dr. Cowan said that most patients are referred to him by other providers, and when he asks them why they did not seek care for migraine sooner, the answer is usually because the migraine was not severe enough or because over-the-counter medication had previously worked for them. He noted that change in frequency is, in his experience, a primary reason why patients will seek care. “[F]or new (or increasing) headache, it is the concern that the headaches are something more ‘serious,’ and once that is ruled out, the conversation often stops,” he said. “For long-standing migraine sufferers, it is the perception that the headache is a ‘fact of life’ and does not rise to the bar of seeking medical advice.”
The questions a survey or a provider asks matters, Dr. Cowan said. “Often, when we ask a patient how many headache (or migraine) days per month, the answer is in single digits. But if we follow-up with a question about the number of headache-free days [per] month, the answer is ‘never’ or ‘hardly ever,’” he explained. “The point here is that what questions a survey (or a provider) asks introduces a clear bias. The use of machine learning instruments, especially when utilizing supervised learning, only reinforces and amplifies the bias of the designers of the categories.”
Epidemiologic studies are interesting but “often ask the wrong questions,” Dr. Cowan said. “I am less worried about the ... 49% of migraine or possible migraine patients who do not seek care and do [not] progress to more disabling ‘chronic’ migraine than I am with identifying the subpopulations of migraine patients who seek care from providers who do not have adequate tools to match patients to the best treatments.”
The authors reported personal and institutional relationships in the form of advisory board memberships, consultancies, employment, honoraria, research support, speakers bureau positions, stock ownership, and teaching services with AbbVie, Aeon, Alder, Allay Lamp, Allergan, Amgen, Axon, Biohaven Pharmaceuticals, Collegium, CoolTech, Currax, Dr. Reddy’s Laboratories (Promius), electroCore, GlaxoSmithKline, Impel NeuroPharma, Informa, Eli Lilly and Company, Lundbeck, Mainistee, Merck, National Headache Foundation, National Institutes of Health, Novartis, Pfizer, Satsuma, Supernus, Percept, Teva, Theranica, UpsherSmith, the US Food and Drug Administration, Vector, Vedanta Research, and Wolff’s Headache. The study was supported by Eli Lilly. Dr. Cowan reports no relevant conflicts of interest.
FROM HEADACHE
Olive Oil Shows Promise for Wound Healing of Ulcers
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
Practice Points
- Interventions that effectively reduce excessive and prolonged inflammation can help promote timely wound healing. Consider integrating anti-inflammatory treatments into wound care protocols to enhance healing outcomes.
- Utilization of olive oil in wound therapy, particularly for conditions such as diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers, has shown promise for promoting healing.
- Regularly review and incorporate findings from recent studies on the use of olive oil and other novel interventions in wound therapy to ensure the application of the most current and effective treatment strategies.
Losing Weight, Decreasing Alcohol, and Improving Sex Life?
Richard* was a master-of-the-universe type. He went to Wharton, ran a large hedge fund, and lived in Greenwich, Connecticut. His three children attended Ivy League schools. He played golf on the weekends and ate three healthy meals per day. There was just one issue: He had gained 90 pounds since the 1990s from consuming six to seven alcoholic beverages per day. He already had one DUI under his belt, and his marriage was on shaky ground. He had tried to address his alcohol abuse disorder on multiple occasions: He went to a yearlong class on alcoholism, saw a psychologist for cognitive-behavioral therapy, and joined Alcoholics Anonymous, all to no avail.
When I met him in December 2023, he had hit rock bottom and was willing to try anything.
At our first visit, I prescribed him weekly tirzepatide (Zepbound) off label, along with a small dose of naltrexone.
Richard shared some feedback after his first 2 weeks:
The naltrexone works great and is strong ... small dose for me effective ... I haven’t wanted to drink and when I do I can’t finish a glass over 2 hours … went from 25 drinks a week to about 4 … don’t notice other side effects … sleeping better too.
And after 6 weeks:
Some more feedback … on week 6-7 and all going well ... drinking very little alcohol and still on half tab of naltrexone ... that works well and have no side effects ... the Zepbound works well too. I do get hungry a few days after the shot but still don’t crave sugar or bad snacks … weight down 21 pounds since started … 292 to 271.
And finally, after 8 weeks:
Looking at my last text to you I see the progress … been incredible ... now down 35 pounds and at 257 … continue to feel excellent with plenty of energy … want to exercise more ... and no temptation to eat or drink unhealthy stuff ... I’m very happy this has surpassed my expectations on how fast it’s worked and I don’t feel any side effects. Marriage has never been better … all thanks to you.
Tirzepatide contains two hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), that are naturally produced by our bodies after meals. Scientists recently learned that the GLP-1 system contributes to the feedback loop of addictive behaviors. Increasing synthetic GLP-1, through medications like tirzepatide, appears to minimize addictive behaviors by limiting their ability to upregulate the brain’s production of dopamine.
Dopamine is a neurotransmitter produced in the brain’s reward center, which regulates how people experience pleasure and control impulses. Dopamine reinforces the pleasure experienced by certain behaviors like drinking, smoking, and eating sweets. These new medications reduce the amount of dopamine released after these activities and thereby lower the motivation to repeat these behaviors.
Contrary to some reports in the news, the vast majority of my male patients using these medications for alcohol abuse disorder experience concurrent increases in testosterone, for two reasons: (1) testosterone increases as body mass index decreases and (2) chronic alcohol use can damage the cells in the testicles that produce testosterone and also decrease the brain’s ability to stimulate the testicles to produce testosterone.
At his most recent checkup last month, Richard’s testosterone had risen from borderline to robust levels, his libido and sleep had improved, and he reported never having felt so healthy or confident. Fingers crossed that the US Food and Drug Administration won’t wait too long before approving this class of medications for more than just diabetes, heart disease, and obesity.
*Patient’s name has been changed.
Dr. Messer is clinical assistant professor, Icahn School of Medicine at Mount Sinai, New York, and associate professor, Zucker School of Medicine at Hofstra University, Hempstead, New York. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Richard* was a master-of-the-universe type. He went to Wharton, ran a large hedge fund, and lived in Greenwich, Connecticut. His three children attended Ivy League schools. He played golf on the weekends and ate three healthy meals per day. There was just one issue: He had gained 90 pounds since the 1990s from consuming six to seven alcoholic beverages per day. He already had one DUI under his belt, and his marriage was on shaky ground. He had tried to address his alcohol abuse disorder on multiple occasions: He went to a yearlong class on alcoholism, saw a psychologist for cognitive-behavioral therapy, and joined Alcoholics Anonymous, all to no avail.
When I met him in December 2023, he had hit rock bottom and was willing to try anything.
At our first visit, I prescribed him weekly tirzepatide (Zepbound) off label, along with a small dose of naltrexone.
Richard shared some feedback after his first 2 weeks:
The naltrexone works great and is strong ... small dose for me effective ... I haven’t wanted to drink and when I do I can’t finish a glass over 2 hours … went from 25 drinks a week to about 4 … don’t notice other side effects … sleeping better too.
And after 6 weeks:
Some more feedback … on week 6-7 and all going well ... drinking very little alcohol and still on half tab of naltrexone ... that works well and have no side effects ... the Zepbound works well too. I do get hungry a few days after the shot but still don’t crave sugar or bad snacks … weight down 21 pounds since started … 292 to 271.
And finally, after 8 weeks:
Looking at my last text to you I see the progress … been incredible ... now down 35 pounds and at 257 … continue to feel excellent with plenty of energy … want to exercise more ... and no temptation to eat or drink unhealthy stuff ... I’m very happy this has surpassed my expectations on how fast it’s worked and I don’t feel any side effects. Marriage has never been better … all thanks to you.
Tirzepatide contains two hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), that are naturally produced by our bodies after meals. Scientists recently learned that the GLP-1 system contributes to the feedback loop of addictive behaviors. Increasing synthetic GLP-1, through medications like tirzepatide, appears to minimize addictive behaviors by limiting their ability to upregulate the brain’s production of dopamine.
Dopamine is a neurotransmitter produced in the brain’s reward center, which regulates how people experience pleasure and control impulses. Dopamine reinforces the pleasure experienced by certain behaviors like drinking, smoking, and eating sweets. These new medications reduce the amount of dopamine released after these activities and thereby lower the motivation to repeat these behaviors.
Contrary to some reports in the news, the vast majority of my male patients using these medications for alcohol abuse disorder experience concurrent increases in testosterone, for two reasons: (1) testosterone increases as body mass index decreases and (2) chronic alcohol use can damage the cells in the testicles that produce testosterone and also decrease the brain’s ability to stimulate the testicles to produce testosterone.
At his most recent checkup last month, Richard’s testosterone had risen from borderline to robust levels, his libido and sleep had improved, and he reported never having felt so healthy or confident. Fingers crossed that the US Food and Drug Administration won’t wait too long before approving this class of medications for more than just diabetes, heart disease, and obesity.
*Patient’s name has been changed.
Dr. Messer is clinical assistant professor, Icahn School of Medicine at Mount Sinai, New York, and associate professor, Zucker School of Medicine at Hofstra University, Hempstead, New York. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Richard* was a master-of-the-universe type. He went to Wharton, ran a large hedge fund, and lived in Greenwich, Connecticut. His three children attended Ivy League schools. He played golf on the weekends and ate three healthy meals per day. There was just one issue: He had gained 90 pounds since the 1990s from consuming six to seven alcoholic beverages per day. He already had one DUI under his belt, and his marriage was on shaky ground. He had tried to address his alcohol abuse disorder on multiple occasions: He went to a yearlong class on alcoholism, saw a psychologist for cognitive-behavioral therapy, and joined Alcoholics Anonymous, all to no avail.
When I met him in December 2023, he had hit rock bottom and was willing to try anything.
At our first visit, I prescribed him weekly tirzepatide (Zepbound) off label, along with a small dose of naltrexone.
Richard shared some feedback after his first 2 weeks:
The naltrexone works great and is strong ... small dose for me effective ... I haven’t wanted to drink and when I do I can’t finish a glass over 2 hours … went from 25 drinks a week to about 4 … don’t notice other side effects … sleeping better too.
And after 6 weeks:
Some more feedback … on week 6-7 and all going well ... drinking very little alcohol and still on half tab of naltrexone ... that works well and have no side effects ... the Zepbound works well too. I do get hungry a few days after the shot but still don’t crave sugar or bad snacks … weight down 21 pounds since started … 292 to 271.
And finally, after 8 weeks:
Looking at my last text to you I see the progress … been incredible ... now down 35 pounds and at 257 … continue to feel excellent with plenty of energy … want to exercise more ... and no temptation to eat or drink unhealthy stuff ... I’m very happy this has surpassed my expectations on how fast it’s worked and I don’t feel any side effects. Marriage has never been better … all thanks to you.
Tirzepatide contains two hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), that are naturally produced by our bodies after meals. Scientists recently learned that the GLP-1 system contributes to the feedback loop of addictive behaviors. Increasing synthetic GLP-1, through medications like tirzepatide, appears to minimize addictive behaviors by limiting their ability to upregulate the brain’s production of dopamine.
Dopamine is a neurotransmitter produced in the brain’s reward center, which regulates how people experience pleasure and control impulses. Dopamine reinforces the pleasure experienced by certain behaviors like drinking, smoking, and eating sweets. These new medications reduce the amount of dopamine released after these activities and thereby lower the motivation to repeat these behaviors.
Contrary to some reports in the news, the vast majority of my male patients using these medications for alcohol abuse disorder experience concurrent increases in testosterone, for two reasons: (1) testosterone increases as body mass index decreases and (2) chronic alcohol use can damage the cells in the testicles that produce testosterone and also decrease the brain’s ability to stimulate the testicles to produce testosterone.
At his most recent checkup last month, Richard’s testosterone had risen from borderline to robust levels, his libido and sleep had improved, and he reported never having felt so healthy or confident. Fingers crossed that the US Food and Drug Administration won’t wait too long before approving this class of medications for more than just diabetes, heart disease, and obesity.
*Patient’s name has been changed.
Dr. Messer is clinical assistant professor, Icahn School of Medicine at Mount Sinai, New York, and associate professor, Zucker School of Medicine at Hofstra University, Hempstead, New York. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Nonanemic Iron Deficiency Underdiagnosed in Women
Three different definitions of nonanemic iron deficiency (ID), a common disorder causing substantial morbidity in women, were significantly associated with different population prevalence estimates, a data analysis of the cross-sectional Hemochromatosis and Iron Overload Screening Study (HEIRS) study found.
These differences held, regardless of self-reported age, pregnancy, or race and ethnicity, according to HEIRS researchers led by James C. Barton, MD, professor of hematology at the University of Alabama at Birmingham.
“Using higher serum ferritin thresholds to define ID could lead to diagnosis and treatment of more women with ID and greater reduction of related morbidity,” the investigators wrote. The study was published in JAMA Network Open.
The authors noted that ID affects about 2 billion people worldwide, mainly women and children, increasing risks of fatigue, impaired muscular performance, cold intolerance, mucosal and epithelial abnormalities, pica, disturbances of menstruation, and adverse pregnancy outcomes.
Manifestations of ID, including anemia, are less prevalent or less severe in adults with higher serum ferritin (SF), and the three definitions correspond, in sequence, to ID of increasing prevalence and decreasing severity, they explained.
The Study
HEIRS conducted multiethnic, primary care–based screening for iron disorders during 2001-2003 at four field centers in the United States and one in Canada at primary care venues.
In data for the current study analyzed from June to December, 2023, the three ID definitions were: combined transferrin saturation less than 10% and SF less than 15 ng/mL (HEIRS); SF less than 15 ng/mL (World Health Organization [WHO]); and SF less than 25 ng/mL, the threshold for ID-deficient erythropoiesis [IDE).
Among the cohort’s 62,685 women (mean age, 49.58 years, 27,072 White, 17,272 Black), the estimated prevalence of ID emerged as follows across the different definitions:
- 1957 (3.12%) according to HEIRS
- 4659 (7.43%) according to WHO
- 9611 (15.33%) according to IDE
Those figures translated to an increased relative ID prevalence of 2.4-fold (95% CI, 2.3-2.5; P < .001) according to the WHO standard and 4.9-fold (95% CI, 4.7-5.2; P < .001) according IDEs.
In addition, prevalence was higher in younger women, and within each racial and ethnic subgroup of participants aged 25-54 years, prevalence rose significantly from the HEIRS definition to the WHO and IDE definitions.
Notably, ID was significantly higher among Black and Hispanic participants than Asian and White participants.
An accompanying editorial pointed to gender-based health equity issues raised by the HEIRS analysis and argued that a similar passive acceptance of laboratory definitions of a debilitating but correctable condition in White males would be “frankly unimaginable.”
“Iron deficiency is the leading cause of years lived with disability among women of reproductive age,” wrote hematologist Michelle Sholzberg, MDCM, MSc, and Grace H. Tang, MSc, of St. Michael’s Hospital in Toronto, Canada. “It is a factor clearly associated with maternal death and morbidity (including diminished IQ), and it is correctable, and, thus, unnecessary, in high-income, middle-income, and low-income geographic settings.”
The authors listed no specific funding for this analysis of HEIRS data. Dr. Barton reported contracts from the National Institutes of Health, National Human Genome Research Institute, outside of the submitted work. A coauthor reported grants from the National Heart, Lung, and Blood Institute and the National Human Genome Research Institute outside of the submitted work. Dr. Sholzberg reported unrestricted research funding to her institution from Octapharma and Pfizer and speakers’ honoraria from Takeda, Sobi, and Medison outside of the submitted work.
Three different definitions of nonanemic iron deficiency (ID), a common disorder causing substantial morbidity in women, were significantly associated with different population prevalence estimates, a data analysis of the cross-sectional Hemochromatosis and Iron Overload Screening Study (HEIRS) study found.
These differences held, regardless of self-reported age, pregnancy, or race and ethnicity, according to HEIRS researchers led by James C. Barton, MD, professor of hematology at the University of Alabama at Birmingham.
“Using higher serum ferritin thresholds to define ID could lead to diagnosis and treatment of more women with ID and greater reduction of related morbidity,” the investigators wrote. The study was published in JAMA Network Open.
The authors noted that ID affects about 2 billion people worldwide, mainly women and children, increasing risks of fatigue, impaired muscular performance, cold intolerance, mucosal and epithelial abnormalities, pica, disturbances of menstruation, and adverse pregnancy outcomes.
Manifestations of ID, including anemia, are less prevalent or less severe in adults with higher serum ferritin (SF), and the three definitions correspond, in sequence, to ID of increasing prevalence and decreasing severity, they explained.
The Study
HEIRS conducted multiethnic, primary care–based screening for iron disorders during 2001-2003 at four field centers in the United States and one in Canada at primary care venues.
In data for the current study analyzed from June to December, 2023, the three ID definitions were: combined transferrin saturation less than 10% and SF less than 15 ng/mL (HEIRS); SF less than 15 ng/mL (World Health Organization [WHO]); and SF less than 25 ng/mL, the threshold for ID-deficient erythropoiesis [IDE).
Among the cohort’s 62,685 women (mean age, 49.58 years, 27,072 White, 17,272 Black), the estimated prevalence of ID emerged as follows across the different definitions:
- 1957 (3.12%) according to HEIRS
- 4659 (7.43%) according to WHO
- 9611 (15.33%) according to IDE
Those figures translated to an increased relative ID prevalence of 2.4-fold (95% CI, 2.3-2.5; P < .001) according to the WHO standard and 4.9-fold (95% CI, 4.7-5.2; P < .001) according IDEs.
In addition, prevalence was higher in younger women, and within each racial and ethnic subgroup of participants aged 25-54 years, prevalence rose significantly from the HEIRS definition to the WHO and IDE definitions.
Notably, ID was significantly higher among Black and Hispanic participants than Asian and White participants.
An accompanying editorial pointed to gender-based health equity issues raised by the HEIRS analysis and argued that a similar passive acceptance of laboratory definitions of a debilitating but correctable condition in White males would be “frankly unimaginable.”
“Iron deficiency is the leading cause of years lived with disability among women of reproductive age,” wrote hematologist Michelle Sholzberg, MDCM, MSc, and Grace H. Tang, MSc, of St. Michael’s Hospital in Toronto, Canada. “It is a factor clearly associated with maternal death and morbidity (including diminished IQ), and it is correctable, and, thus, unnecessary, in high-income, middle-income, and low-income geographic settings.”
The authors listed no specific funding for this analysis of HEIRS data. Dr. Barton reported contracts from the National Institutes of Health, National Human Genome Research Institute, outside of the submitted work. A coauthor reported grants from the National Heart, Lung, and Blood Institute and the National Human Genome Research Institute outside of the submitted work. Dr. Sholzberg reported unrestricted research funding to her institution from Octapharma and Pfizer and speakers’ honoraria from Takeda, Sobi, and Medison outside of the submitted work.
Three different definitions of nonanemic iron deficiency (ID), a common disorder causing substantial morbidity in women, were significantly associated with different population prevalence estimates, a data analysis of the cross-sectional Hemochromatosis and Iron Overload Screening Study (HEIRS) study found.
These differences held, regardless of self-reported age, pregnancy, or race and ethnicity, according to HEIRS researchers led by James C. Barton, MD, professor of hematology at the University of Alabama at Birmingham.
“Using higher serum ferritin thresholds to define ID could lead to diagnosis and treatment of more women with ID and greater reduction of related morbidity,” the investigators wrote. The study was published in JAMA Network Open.
The authors noted that ID affects about 2 billion people worldwide, mainly women and children, increasing risks of fatigue, impaired muscular performance, cold intolerance, mucosal and epithelial abnormalities, pica, disturbances of menstruation, and adverse pregnancy outcomes.
Manifestations of ID, including anemia, are less prevalent or less severe in adults with higher serum ferritin (SF), and the three definitions correspond, in sequence, to ID of increasing prevalence and decreasing severity, they explained.
The Study
HEIRS conducted multiethnic, primary care–based screening for iron disorders during 2001-2003 at four field centers in the United States and one in Canada at primary care venues.
In data for the current study analyzed from June to December, 2023, the three ID definitions were: combined transferrin saturation less than 10% and SF less than 15 ng/mL (HEIRS); SF less than 15 ng/mL (World Health Organization [WHO]); and SF less than 25 ng/mL, the threshold for ID-deficient erythropoiesis [IDE).
Among the cohort’s 62,685 women (mean age, 49.58 years, 27,072 White, 17,272 Black), the estimated prevalence of ID emerged as follows across the different definitions:
- 1957 (3.12%) according to HEIRS
- 4659 (7.43%) according to WHO
- 9611 (15.33%) according to IDE
Those figures translated to an increased relative ID prevalence of 2.4-fold (95% CI, 2.3-2.5; P < .001) according to the WHO standard and 4.9-fold (95% CI, 4.7-5.2; P < .001) according IDEs.
In addition, prevalence was higher in younger women, and within each racial and ethnic subgroup of participants aged 25-54 years, prevalence rose significantly from the HEIRS definition to the WHO and IDE definitions.
Notably, ID was significantly higher among Black and Hispanic participants than Asian and White participants.
An accompanying editorial pointed to gender-based health equity issues raised by the HEIRS analysis and argued that a similar passive acceptance of laboratory definitions of a debilitating but correctable condition in White males would be “frankly unimaginable.”
“Iron deficiency is the leading cause of years lived with disability among women of reproductive age,” wrote hematologist Michelle Sholzberg, MDCM, MSc, and Grace H. Tang, MSc, of St. Michael’s Hospital in Toronto, Canada. “It is a factor clearly associated with maternal death and morbidity (including diminished IQ), and it is correctable, and, thus, unnecessary, in high-income, middle-income, and low-income geographic settings.”
The authors listed no specific funding for this analysis of HEIRS data. Dr. Barton reported contracts from the National Institutes of Health, National Human Genome Research Institute, outside of the submitted work. A coauthor reported grants from the National Heart, Lung, and Blood Institute and the National Human Genome Research Institute outside of the submitted work. Dr. Sholzberg reported unrestricted research funding to her institution from Octapharma and Pfizer and speakers’ honoraria from Takeda, Sobi, and Medison outside of the submitted work.
FROM JAMA NETWORK OPEN