User login
FDA okays difelikefalin for dialysis-associated pruritus in patients with CKD
Some nephrologists welcomed the Aug. 23 approval of this new option for treating pruritus, a relatively common and often hard-to-resolve complication of dialysis in patients with chronic kidney disease (CKD) that can substantially impinge on quality of life for some patients, but also voiced uncertainty about the role of a new agent with a modest trial track record that may be expensive and face insurance-coverage hurdles.
“Uptake of difelikefalin will depend on awareness of itch among patients dependent on hemodialysis, and on payment policies,” predicted Daniel E. Weiner, MD, a nephrologist at Tufts Medical Center in Boston. “Pruritus is underdiagnosed among people with kidney failure, and in some patients ongoing pruritus can be highly impactful on sleep and quality of life. The clinical trial results were very encouraging that difelikefalin is effective and safe,” which makes recognition of pruritus as a significant issue for patients a key factor in uptake of the new drug, Dr. Weiner, an investigator in a difelikefalin clinical study, said in an interview.
Other nephrologists acknowledged the substantial problem that itch can pose for many patients with CKD on dialysis but questioned the weight of evidence behind difelikefalin’s approval.
Two pivotal trials with fewer than 900 total randomized patients
The data considered by the FDA primarily featured results from two pivotal trials, KALM-1 and KALM-2. KALM-1 randomized 378 patients with CKD and on hemodialysis and with moderate to severe pruritus to intravenous treatment with difelikefalin or placebo three times a week for 12 weeks with a primary endpoint of an improvement (decrease) of at least 3 points from baseline in their Worst Itching Intensity Numerical Rating Scale (WI-NRS) score, which averaged just over 7 points at baseline. After 12 weeks on treatment, 52% of patients who received difelikefalin had at least a 3-point drop, compared with 31% of patients who received placebo, a significant difference. The results appeared in a 2020 report in the New England Journal of Medicine.
Confirmatory results came in the second pivotal trial, KALM-2, a similarly designed, 12-week study that randomized 473 patients, with 54% of those in the active arm achieving at least a 3-point cut in their baseline WI-NRS score, compared with 42% of patients who received placebo, a significant difference. A report at the Kidney Week meeting sponsored by the National Kidney Foundation in October 2020 presented the KALM-2 results, but the findings have not yet appeared in a published article.
In sum, the data suggest that treatment with difelikefalin will, on average, produce a clinically meaningful effect on itch compared with placebo in about 20% of patients, with nearly half the patients who receive the active drug having a less robust response and many patients who receive no active treatment also show a meaningful cut in their pruritus severity in a trial setting, noted Paul Palevsky, MD, professor of medicine at the University of Pittsburgh and chief of the renal section at the Veterans Affairs Pittsburgh Healthcare System.
The upshot is that questions linger over which patients are the best candidates for this drug and how it might perform in real-world practice given difelikefalin’s limited track record, Dr. Palevsky said in an interview.
In addition, the labeling specifies the indication is for patients with moderate to severe pruritus, but itching severity is not routinely quantified in these patients in current practice, added Dr. Palevsky, who is also president of the National Kidney Foundation.
Dr. Weiner noted that another unknown is the appropriate duration of treatment in real-world use.
What will it cost, and will it be covered?
The drug’s price and insurance coverage will likely be a major factor in uptake of the new drug, agreed both Dr. Weiner and Dr. Palevsky, especially the coverage decision for Medicare patients by the Centers for Medicare & Medicaid Services. A corollary is whether or not coverage for difelikefalin, which patients receive as an intravenous infusion during each of their usual three-times-a-week dialysis sessions, will lie outside of the bundled dialysis reimbursement payment. If is no mechanism exists to pay for difelikefalin separately beyond the current bundled dialysis rate, “I suspect it will not get used very much unless it is very inexpensive,” predicted Dr. Weiner.
Another issue is where difelikefalin fits within the lineup of standard treatment options. “A lot of people receiving hemodialysis suffer from pruritus and have not been successfully treated. For these individuals difelikefalin could be a game changer,” Dr. Weiner said.
Other nephrologists have a more positive take on the existing treatment options.
“Start systemic therapy for patients with itch that is significantly affecting quality of life; stepping up from topical therapy just delays effective treatment,” advised Hugh C. Rayner, MD, a nephrologist affiliated with Birmingham (England) Heartland’s Hospital who was lead author on a review of pruritus treatments for patients with CKD on hemodialysis.
“Standard systemic therapy is gabapentin or pregabalin,” an approach “supported by robust evidence confirmed in a Cochrane review,” he said in an interview. The impact of difelikefalin “will be limited as its effectiveness in reducing itch is modest at best and far inferior to gabapentin and pregabalin,” Dr. Rayner added. Difelikefalin’s “main downsides will be its cost, compared with gabapentin, and its gastrointestinal side effects.”
Adverse-event profiles
In KALM-1, the most frequent adverse effects from difelikefalin treatment was diarrhea, in 10% of patients, compared with a 4% rate among patients who received placebo. Vomiting occurred at a 5% incidence on difelikefalin and in 3% of patients on placebo. All serious adverse events occurred in 26% of patients on difelikefalin and in 22% of those who received placebo. Discontinuations because of an adverse event occurred in 8% of patients on difelikefalin and in 5% of the placebo patients.
An editorial that accompanied the published KALM-1 report in 2020 said “the findings are compelling, although diarrhea, dizziness, and vomiting were frequent side effects.”
Both Dr. Weiner and Dr. Palevsky were more reserved than Dr. Rayner in their appraisal of gabapentin and pregabalin, although Dr. Palevsky admitted that he has prescribed one or the other of these two drugs to “lots of patients,” especially gabapentin. “But they are not completely benign drugs,” he cautioned, a concern echoed by Dr. Weiner.
“Antihistamines, gabapentin, and pregabalin have a high side-effect burden in patients on hemodialysis and limited efficacy, and are poor options for chronic pruritus management,” explained Dr. Weiner. “I would favor difelikefalin to chronic prescription of these other agents” because difelikefalin “appears effective and has a very low side effect burden. Very few effective treatments for pruritus do not have side effects.”
Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that exerts antipruritic effects by activating kappa opioid receptors on peripheral neurons and immune cells. The drug’s hydrophilic, small-peptide structure restricts passive diffusion across membranes, which limits the drug’s access to kappa opioid receptors in the central nervous system and hence reduces potential adverse effects.
The FDA made this approval decision without consulting an advisory committee. The companies that will market difelikefalin (Korsuva), Cara Therapeutics and Vifor Pharma, announced that their U.S. promotional launch of the drug starts early in 2022.
The KALM-1 and KALM-2 studies were sponsored by Cara Therapeutics and Vifor Pharma, the two companies that have been jointly developing difelikefalin. Dr. Pavelsky and Dr. Rayner had no relevant disclosures. Dr. Weiner was previously an adviser to Cara and Vifor and participated as an investigator in a difelikefalin clinical study, but more recently has had no relationships with the companies.
Some nephrologists welcomed the Aug. 23 approval of this new option for treating pruritus, a relatively common and often hard-to-resolve complication of dialysis in patients with chronic kidney disease (CKD) that can substantially impinge on quality of life for some patients, but also voiced uncertainty about the role of a new agent with a modest trial track record that may be expensive and face insurance-coverage hurdles.
“Uptake of difelikefalin will depend on awareness of itch among patients dependent on hemodialysis, and on payment policies,” predicted Daniel E. Weiner, MD, a nephrologist at Tufts Medical Center in Boston. “Pruritus is underdiagnosed among people with kidney failure, and in some patients ongoing pruritus can be highly impactful on sleep and quality of life. The clinical trial results were very encouraging that difelikefalin is effective and safe,” which makes recognition of pruritus as a significant issue for patients a key factor in uptake of the new drug, Dr. Weiner, an investigator in a difelikefalin clinical study, said in an interview.
Other nephrologists acknowledged the substantial problem that itch can pose for many patients with CKD on dialysis but questioned the weight of evidence behind difelikefalin’s approval.
Two pivotal trials with fewer than 900 total randomized patients
The data considered by the FDA primarily featured results from two pivotal trials, KALM-1 and KALM-2. KALM-1 randomized 378 patients with CKD and on hemodialysis and with moderate to severe pruritus to intravenous treatment with difelikefalin or placebo three times a week for 12 weeks with a primary endpoint of an improvement (decrease) of at least 3 points from baseline in their Worst Itching Intensity Numerical Rating Scale (WI-NRS) score, which averaged just over 7 points at baseline. After 12 weeks on treatment, 52% of patients who received difelikefalin had at least a 3-point drop, compared with 31% of patients who received placebo, a significant difference. The results appeared in a 2020 report in the New England Journal of Medicine.
Confirmatory results came in the second pivotal trial, KALM-2, a similarly designed, 12-week study that randomized 473 patients, with 54% of those in the active arm achieving at least a 3-point cut in their baseline WI-NRS score, compared with 42% of patients who received placebo, a significant difference. A report at the Kidney Week meeting sponsored by the National Kidney Foundation in October 2020 presented the KALM-2 results, but the findings have not yet appeared in a published article.
In sum, the data suggest that treatment with difelikefalin will, on average, produce a clinically meaningful effect on itch compared with placebo in about 20% of patients, with nearly half the patients who receive the active drug having a less robust response and many patients who receive no active treatment also show a meaningful cut in their pruritus severity in a trial setting, noted Paul Palevsky, MD, professor of medicine at the University of Pittsburgh and chief of the renal section at the Veterans Affairs Pittsburgh Healthcare System.
The upshot is that questions linger over which patients are the best candidates for this drug and how it might perform in real-world practice given difelikefalin’s limited track record, Dr. Palevsky said in an interview.
In addition, the labeling specifies the indication is for patients with moderate to severe pruritus, but itching severity is not routinely quantified in these patients in current practice, added Dr. Palevsky, who is also president of the National Kidney Foundation.
Dr. Weiner noted that another unknown is the appropriate duration of treatment in real-world use.
What will it cost, and will it be covered?
The drug’s price and insurance coverage will likely be a major factor in uptake of the new drug, agreed both Dr. Weiner and Dr. Palevsky, especially the coverage decision for Medicare patients by the Centers for Medicare & Medicaid Services. A corollary is whether or not coverage for difelikefalin, which patients receive as an intravenous infusion during each of their usual three-times-a-week dialysis sessions, will lie outside of the bundled dialysis reimbursement payment. If is no mechanism exists to pay for difelikefalin separately beyond the current bundled dialysis rate, “I suspect it will not get used very much unless it is very inexpensive,” predicted Dr. Weiner.
Another issue is where difelikefalin fits within the lineup of standard treatment options. “A lot of people receiving hemodialysis suffer from pruritus and have not been successfully treated. For these individuals difelikefalin could be a game changer,” Dr. Weiner said.
Other nephrologists have a more positive take on the existing treatment options.
“Start systemic therapy for patients with itch that is significantly affecting quality of life; stepping up from topical therapy just delays effective treatment,” advised Hugh C. Rayner, MD, a nephrologist affiliated with Birmingham (England) Heartland’s Hospital who was lead author on a review of pruritus treatments for patients with CKD on hemodialysis.
“Standard systemic therapy is gabapentin or pregabalin,” an approach “supported by robust evidence confirmed in a Cochrane review,” he said in an interview. The impact of difelikefalin “will be limited as its effectiveness in reducing itch is modest at best and far inferior to gabapentin and pregabalin,” Dr. Rayner added. Difelikefalin’s “main downsides will be its cost, compared with gabapentin, and its gastrointestinal side effects.”
Adverse-event profiles
In KALM-1, the most frequent adverse effects from difelikefalin treatment was diarrhea, in 10% of patients, compared with a 4% rate among patients who received placebo. Vomiting occurred at a 5% incidence on difelikefalin and in 3% of patients on placebo. All serious adverse events occurred in 26% of patients on difelikefalin and in 22% of those who received placebo. Discontinuations because of an adverse event occurred in 8% of patients on difelikefalin and in 5% of the placebo patients.
An editorial that accompanied the published KALM-1 report in 2020 said “the findings are compelling, although diarrhea, dizziness, and vomiting were frequent side effects.”
Both Dr. Weiner and Dr. Palevsky were more reserved than Dr. Rayner in their appraisal of gabapentin and pregabalin, although Dr. Palevsky admitted that he has prescribed one or the other of these two drugs to “lots of patients,” especially gabapentin. “But they are not completely benign drugs,” he cautioned, a concern echoed by Dr. Weiner.
“Antihistamines, gabapentin, and pregabalin have a high side-effect burden in patients on hemodialysis and limited efficacy, and are poor options for chronic pruritus management,” explained Dr. Weiner. “I would favor difelikefalin to chronic prescription of these other agents” because difelikefalin “appears effective and has a very low side effect burden. Very few effective treatments for pruritus do not have side effects.”
Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that exerts antipruritic effects by activating kappa opioid receptors on peripheral neurons and immune cells. The drug’s hydrophilic, small-peptide structure restricts passive diffusion across membranes, which limits the drug’s access to kappa opioid receptors in the central nervous system and hence reduces potential adverse effects.
The FDA made this approval decision without consulting an advisory committee. The companies that will market difelikefalin (Korsuva), Cara Therapeutics and Vifor Pharma, announced that their U.S. promotional launch of the drug starts early in 2022.
The KALM-1 and KALM-2 studies were sponsored by Cara Therapeutics and Vifor Pharma, the two companies that have been jointly developing difelikefalin. Dr. Pavelsky and Dr. Rayner had no relevant disclosures. Dr. Weiner was previously an adviser to Cara and Vifor and participated as an investigator in a difelikefalin clinical study, but more recently has had no relationships with the companies.
Some nephrologists welcomed the Aug. 23 approval of this new option for treating pruritus, a relatively common and often hard-to-resolve complication of dialysis in patients with chronic kidney disease (CKD) that can substantially impinge on quality of life for some patients, but also voiced uncertainty about the role of a new agent with a modest trial track record that may be expensive and face insurance-coverage hurdles.
“Uptake of difelikefalin will depend on awareness of itch among patients dependent on hemodialysis, and on payment policies,” predicted Daniel E. Weiner, MD, a nephrologist at Tufts Medical Center in Boston. “Pruritus is underdiagnosed among people with kidney failure, and in some patients ongoing pruritus can be highly impactful on sleep and quality of life. The clinical trial results were very encouraging that difelikefalin is effective and safe,” which makes recognition of pruritus as a significant issue for patients a key factor in uptake of the new drug, Dr. Weiner, an investigator in a difelikefalin clinical study, said in an interview.
Other nephrologists acknowledged the substantial problem that itch can pose for many patients with CKD on dialysis but questioned the weight of evidence behind difelikefalin’s approval.
Two pivotal trials with fewer than 900 total randomized patients
The data considered by the FDA primarily featured results from two pivotal trials, KALM-1 and KALM-2. KALM-1 randomized 378 patients with CKD and on hemodialysis and with moderate to severe pruritus to intravenous treatment with difelikefalin or placebo three times a week for 12 weeks with a primary endpoint of an improvement (decrease) of at least 3 points from baseline in their Worst Itching Intensity Numerical Rating Scale (WI-NRS) score, which averaged just over 7 points at baseline. After 12 weeks on treatment, 52% of patients who received difelikefalin had at least a 3-point drop, compared with 31% of patients who received placebo, a significant difference. The results appeared in a 2020 report in the New England Journal of Medicine.
Confirmatory results came in the second pivotal trial, KALM-2, a similarly designed, 12-week study that randomized 473 patients, with 54% of those in the active arm achieving at least a 3-point cut in their baseline WI-NRS score, compared with 42% of patients who received placebo, a significant difference. A report at the Kidney Week meeting sponsored by the National Kidney Foundation in October 2020 presented the KALM-2 results, but the findings have not yet appeared in a published article.
In sum, the data suggest that treatment with difelikefalin will, on average, produce a clinically meaningful effect on itch compared with placebo in about 20% of patients, with nearly half the patients who receive the active drug having a less robust response and many patients who receive no active treatment also show a meaningful cut in their pruritus severity in a trial setting, noted Paul Palevsky, MD, professor of medicine at the University of Pittsburgh and chief of the renal section at the Veterans Affairs Pittsburgh Healthcare System.
The upshot is that questions linger over which patients are the best candidates for this drug and how it might perform in real-world practice given difelikefalin’s limited track record, Dr. Palevsky said in an interview.
In addition, the labeling specifies the indication is for patients with moderate to severe pruritus, but itching severity is not routinely quantified in these patients in current practice, added Dr. Palevsky, who is also president of the National Kidney Foundation.
Dr. Weiner noted that another unknown is the appropriate duration of treatment in real-world use.
What will it cost, and will it be covered?
The drug’s price and insurance coverage will likely be a major factor in uptake of the new drug, agreed both Dr. Weiner and Dr. Palevsky, especially the coverage decision for Medicare patients by the Centers for Medicare & Medicaid Services. A corollary is whether or not coverage for difelikefalin, which patients receive as an intravenous infusion during each of their usual three-times-a-week dialysis sessions, will lie outside of the bundled dialysis reimbursement payment. If is no mechanism exists to pay for difelikefalin separately beyond the current bundled dialysis rate, “I suspect it will not get used very much unless it is very inexpensive,” predicted Dr. Weiner.
Another issue is where difelikefalin fits within the lineup of standard treatment options. “A lot of people receiving hemodialysis suffer from pruritus and have not been successfully treated. For these individuals difelikefalin could be a game changer,” Dr. Weiner said.
Other nephrologists have a more positive take on the existing treatment options.
“Start systemic therapy for patients with itch that is significantly affecting quality of life; stepping up from topical therapy just delays effective treatment,” advised Hugh C. Rayner, MD, a nephrologist affiliated with Birmingham (England) Heartland’s Hospital who was lead author on a review of pruritus treatments for patients with CKD on hemodialysis.
“Standard systemic therapy is gabapentin or pregabalin,” an approach “supported by robust evidence confirmed in a Cochrane review,” he said in an interview. The impact of difelikefalin “will be limited as its effectiveness in reducing itch is modest at best and far inferior to gabapentin and pregabalin,” Dr. Rayner added. Difelikefalin’s “main downsides will be its cost, compared with gabapentin, and its gastrointestinal side effects.”
Adverse-event profiles
In KALM-1, the most frequent adverse effects from difelikefalin treatment was diarrhea, in 10% of patients, compared with a 4% rate among patients who received placebo. Vomiting occurred at a 5% incidence on difelikefalin and in 3% of patients on placebo. All serious adverse events occurred in 26% of patients on difelikefalin and in 22% of those who received placebo. Discontinuations because of an adverse event occurred in 8% of patients on difelikefalin and in 5% of the placebo patients.
An editorial that accompanied the published KALM-1 report in 2020 said “the findings are compelling, although diarrhea, dizziness, and vomiting were frequent side effects.”
Both Dr. Weiner and Dr. Palevsky were more reserved than Dr. Rayner in their appraisal of gabapentin and pregabalin, although Dr. Palevsky admitted that he has prescribed one or the other of these two drugs to “lots of patients,” especially gabapentin. “But they are not completely benign drugs,” he cautioned, a concern echoed by Dr. Weiner.
“Antihistamines, gabapentin, and pregabalin have a high side-effect burden in patients on hemodialysis and limited efficacy, and are poor options for chronic pruritus management,” explained Dr. Weiner. “I would favor difelikefalin to chronic prescription of these other agents” because difelikefalin “appears effective and has a very low side effect burden. Very few effective treatments for pruritus do not have side effects.”
Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that exerts antipruritic effects by activating kappa opioid receptors on peripheral neurons and immune cells. The drug’s hydrophilic, small-peptide structure restricts passive diffusion across membranes, which limits the drug’s access to kappa opioid receptors in the central nervous system and hence reduces potential adverse effects.
The FDA made this approval decision without consulting an advisory committee. The companies that will market difelikefalin (Korsuva), Cara Therapeutics and Vifor Pharma, announced that their U.S. promotional launch of the drug starts early in 2022.
The KALM-1 and KALM-2 studies were sponsored by Cara Therapeutics and Vifor Pharma, the two companies that have been jointly developing difelikefalin. Dr. Pavelsky and Dr. Rayner had no relevant disclosures. Dr. Weiner was previously an adviser to Cara and Vifor and participated as an investigator in a difelikefalin clinical study, but more recently has had no relationships with the companies.
Two patients with metastatic lung cancer are disease-free for 1.5 years after TILs
Adoptive cell therapy using tumor-infiltrating lymphocytes (TILs) led to complete and long-lasting responses in 2 of 16 patients with metastatic non-small cell lung cancer (NSCLC) who fully underwent the therapy in a phase 1 study.
The results are “very impressive, with two complete responses that are ongoing 1.5 years later, so it’s durable, and that’s encouraging,” Fred Hirsch, MD, PhD, director of the Center of Excellence for Thoracic Oncology at Mount Sinai’s Tisch Cancer Institute, New York, told this news organization.
Dr. Hirsch, who wasn’t involved in the research, also noted that this study is in immunotherapy-resistant metastatic lung cancer – “a situation where we actually don’t know exactly how best to treat it today.”
The study was published online in Nature Medicine.
A form of immunotherapy, TILs have been studied extensively in melanoma, as reported by this news organization, but this is the first test of the TIL therapy in metastatic NSCLC.
The single-arm, open-label phase 1 trial involved 20 patients who had TILs collected, including 16 who eventually received TILs. Median age was 54 years; all patients had metastatic NSCLC and disease progression after nivolumab monotherapy.
TILs cultured from an individual patient’s tumor were expanded ex vivo from minced tumors cultured with interleukin-2 (IL-2). Via this method, “billions of activated T cells can be produced and infused back into a patient,” explain the authors.
The full treatment regimen comprised cyclophosphamide and fludarabine lymphodepletion, TIL infusion and IL-2, followed by maintenance nivolumab.
“We found that infusion of TILs in combination with lymphodepletion and IL-2 had manageable toxicity and mediated tumor regressions in several patients, including complete responses,” report Benjamin Creelan, MD, of the Moffitt Cancer Center & Research Institute, Tampa, and colleagues.
The endpoint of safety was met according to the prespecified criteria of a rate of severe toxicity of 17% or less.
Among 13 evaluable patients, three had confirmed responses and 11 had reduction in tumor burden. Two patients achieved complete responses that were ongoing 1.5 years following TIL treatment.
One durable complete response occurred in a PD-L1-negative never-smoker, who had a low tumor mutation burden and who was refractory to nivolumab.
“This may be particularly encouraging for the large subset of never-smoker patients, for whom immune-checkpoint inhibitors have historically had limited efficacy,” the investigators say.
This complete responder had “features where you wouldn’t expect to see a response for immunotherapy,” Dr. Hirsch told this news organization.
“Low tumor mutation burden, negative PDL-1, and never-smoker are three factors which indicate some kind of resistance to immunotherapy, and despite that, there was a complete response with this specific therapy. That is fascinating,” he said.
In exploratory analyses, T cells recognizing multiple types of cancer mutations were detected after TIL treatment and were enriched in patients who responded to treatment.
The researchers say these early data indicate that TILs can mediate effective responses in tumor subtypes that are not sensitive to traditional immune-checkpoint-targeted therapy.
“Therefore, therapy with TILs may extend the scope and impact of immunotherapy into wider populations,” they write.
‘Yeoman’s effort’ paving the way forward
Also weighing in on the study, Philip Greenberg, MD, professor and head of immunology, Fred Hutchinson Cancer Center, Seattle, said, “In some respects, it’s quite promising and in other respects, actually more limited than you would hope for.”
“I think it’s a great demonstration that there is activity here, and there’s a world of things that can be done to improve the activity and no doubt that will be done. After this trial, I’m sure we will see next-generation trials,” said Dr. Greenberg.
He said key issues going forward are how cells are selected and manufactured: “That’s going to be a critical piece for making it better.”
“There is now a world of data that says T cells that recognize mutations in cancers can be effective in solid tumors,” Dr. Greenberg said.
“Sustaining that response is still a huge obstacle for achieving the kinds of therapeutic benefits we’d like to be achieved. And having that response be broad enough, particularly in the setting where most of the mutations are just passenger mutations, not driver oncogenes, is going to require a way of generating a large polyspecific population of cells that can persist for a long time,” he further commented.
All in all, this study was a “yeoman’s effort” and the researchers “deserve a lot of credit for pushing it forward,” Dr. Greenberg said.
The study was supported in part by grants from Stand Up to Cancer Foundation, the Barbara Bauer Prelude to a Cure Foundation, Iovance Biotherapeutics, and a Young Investigator award from Adaptive Biotechnologies. Nivolumab was supplied by Bristol-Myers Squibb. Aldesleukin (IL-2) was supplied by Clinigen Group. A complete list of author disclosures is available with the original article. Dr. Hirsch and Dr. Greenberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adoptive cell therapy using tumor-infiltrating lymphocytes (TILs) led to complete and long-lasting responses in 2 of 16 patients with metastatic non-small cell lung cancer (NSCLC) who fully underwent the therapy in a phase 1 study.
The results are “very impressive, with two complete responses that are ongoing 1.5 years later, so it’s durable, and that’s encouraging,” Fred Hirsch, MD, PhD, director of the Center of Excellence for Thoracic Oncology at Mount Sinai’s Tisch Cancer Institute, New York, told this news organization.
Dr. Hirsch, who wasn’t involved in the research, also noted that this study is in immunotherapy-resistant metastatic lung cancer – “a situation where we actually don’t know exactly how best to treat it today.”
The study was published online in Nature Medicine.
A form of immunotherapy, TILs have been studied extensively in melanoma, as reported by this news organization, but this is the first test of the TIL therapy in metastatic NSCLC.
The single-arm, open-label phase 1 trial involved 20 patients who had TILs collected, including 16 who eventually received TILs. Median age was 54 years; all patients had metastatic NSCLC and disease progression after nivolumab monotherapy.
TILs cultured from an individual patient’s tumor were expanded ex vivo from minced tumors cultured with interleukin-2 (IL-2). Via this method, “billions of activated T cells can be produced and infused back into a patient,” explain the authors.
The full treatment regimen comprised cyclophosphamide and fludarabine lymphodepletion, TIL infusion and IL-2, followed by maintenance nivolumab.
“We found that infusion of TILs in combination with lymphodepletion and IL-2 had manageable toxicity and mediated tumor regressions in several patients, including complete responses,” report Benjamin Creelan, MD, of the Moffitt Cancer Center & Research Institute, Tampa, and colleagues.
The endpoint of safety was met according to the prespecified criteria of a rate of severe toxicity of 17% or less.
Among 13 evaluable patients, three had confirmed responses and 11 had reduction in tumor burden. Two patients achieved complete responses that were ongoing 1.5 years following TIL treatment.
One durable complete response occurred in a PD-L1-negative never-smoker, who had a low tumor mutation burden and who was refractory to nivolumab.
“This may be particularly encouraging for the large subset of never-smoker patients, for whom immune-checkpoint inhibitors have historically had limited efficacy,” the investigators say.
This complete responder had “features where you wouldn’t expect to see a response for immunotherapy,” Dr. Hirsch told this news organization.
“Low tumor mutation burden, negative PDL-1, and never-smoker are three factors which indicate some kind of resistance to immunotherapy, and despite that, there was a complete response with this specific therapy. That is fascinating,” he said.
In exploratory analyses, T cells recognizing multiple types of cancer mutations were detected after TIL treatment and were enriched in patients who responded to treatment.
The researchers say these early data indicate that TILs can mediate effective responses in tumor subtypes that are not sensitive to traditional immune-checkpoint-targeted therapy.
“Therefore, therapy with TILs may extend the scope and impact of immunotherapy into wider populations,” they write.
‘Yeoman’s effort’ paving the way forward
Also weighing in on the study, Philip Greenberg, MD, professor and head of immunology, Fred Hutchinson Cancer Center, Seattle, said, “In some respects, it’s quite promising and in other respects, actually more limited than you would hope for.”
“I think it’s a great demonstration that there is activity here, and there’s a world of things that can be done to improve the activity and no doubt that will be done. After this trial, I’m sure we will see next-generation trials,” said Dr. Greenberg.
He said key issues going forward are how cells are selected and manufactured: “That’s going to be a critical piece for making it better.”
“There is now a world of data that says T cells that recognize mutations in cancers can be effective in solid tumors,” Dr. Greenberg said.
“Sustaining that response is still a huge obstacle for achieving the kinds of therapeutic benefits we’d like to be achieved. And having that response be broad enough, particularly in the setting where most of the mutations are just passenger mutations, not driver oncogenes, is going to require a way of generating a large polyspecific population of cells that can persist for a long time,” he further commented.
All in all, this study was a “yeoman’s effort” and the researchers “deserve a lot of credit for pushing it forward,” Dr. Greenberg said.
The study was supported in part by grants from Stand Up to Cancer Foundation, the Barbara Bauer Prelude to a Cure Foundation, Iovance Biotherapeutics, and a Young Investigator award from Adaptive Biotechnologies. Nivolumab was supplied by Bristol-Myers Squibb. Aldesleukin (IL-2) was supplied by Clinigen Group. A complete list of author disclosures is available with the original article. Dr. Hirsch and Dr. Greenberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adoptive cell therapy using tumor-infiltrating lymphocytes (TILs) led to complete and long-lasting responses in 2 of 16 patients with metastatic non-small cell lung cancer (NSCLC) who fully underwent the therapy in a phase 1 study.
The results are “very impressive, with two complete responses that are ongoing 1.5 years later, so it’s durable, and that’s encouraging,” Fred Hirsch, MD, PhD, director of the Center of Excellence for Thoracic Oncology at Mount Sinai’s Tisch Cancer Institute, New York, told this news organization.
Dr. Hirsch, who wasn’t involved in the research, also noted that this study is in immunotherapy-resistant metastatic lung cancer – “a situation where we actually don’t know exactly how best to treat it today.”
The study was published online in Nature Medicine.
A form of immunotherapy, TILs have been studied extensively in melanoma, as reported by this news organization, but this is the first test of the TIL therapy in metastatic NSCLC.
The single-arm, open-label phase 1 trial involved 20 patients who had TILs collected, including 16 who eventually received TILs. Median age was 54 years; all patients had metastatic NSCLC and disease progression after nivolumab monotherapy.
TILs cultured from an individual patient’s tumor were expanded ex vivo from minced tumors cultured with interleukin-2 (IL-2). Via this method, “billions of activated T cells can be produced and infused back into a patient,” explain the authors.
The full treatment regimen comprised cyclophosphamide and fludarabine lymphodepletion, TIL infusion and IL-2, followed by maintenance nivolumab.
“We found that infusion of TILs in combination with lymphodepletion and IL-2 had manageable toxicity and mediated tumor regressions in several patients, including complete responses,” report Benjamin Creelan, MD, of the Moffitt Cancer Center & Research Institute, Tampa, and colleagues.
The endpoint of safety was met according to the prespecified criteria of a rate of severe toxicity of 17% or less.
Among 13 evaluable patients, three had confirmed responses and 11 had reduction in tumor burden. Two patients achieved complete responses that were ongoing 1.5 years following TIL treatment.
One durable complete response occurred in a PD-L1-negative never-smoker, who had a low tumor mutation burden and who was refractory to nivolumab.
“This may be particularly encouraging for the large subset of never-smoker patients, for whom immune-checkpoint inhibitors have historically had limited efficacy,” the investigators say.
This complete responder had “features where you wouldn’t expect to see a response for immunotherapy,” Dr. Hirsch told this news organization.
“Low tumor mutation burden, negative PDL-1, and never-smoker are three factors which indicate some kind of resistance to immunotherapy, and despite that, there was a complete response with this specific therapy. That is fascinating,” he said.
In exploratory analyses, T cells recognizing multiple types of cancer mutations were detected after TIL treatment and were enriched in patients who responded to treatment.
The researchers say these early data indicate that TILs can mediate effective responses in tumor subtypes that are not sensitive to traditional immune-checkpoint-targeted therapy.
“Therefore, therapy with TILs may extend the scope and impact of immunotherapy into wider populations,” they write.
‘Yeoman’s effort’ paving the way forward
Also weighing in on the study, Philip Greenberg, MD, professor and head of immunology, Fred Hutchinson Cancer Center, Seattle, said, “In some respects, it’s quite promising and in other respects, actually more limited than you would hope for.”
“I think it’s a great demonstration that there is activity here, and there’s a world of things that can be done to improve the activity and no doubt that will be done. After this trial, I’m sure we will see next-generation trials,” said Dr. Greenberg.
He said key issues going forward are how cells are selected and manufactured: “That’s going to be a critical piece for making it better.”
“There is now a world of data that says T cells that recognize mutations in cancers can be effective in solid tumors,” Dr. Greenberg said.
“Sustaining that response is still a huge obstacle for achieving the kinds of therapeutic benefits we’d like to be achieved. And having that response be broad enough, particularly in the setting where most of the mutations are just passenger mutations, not driver oncogenes, is going to require a way of generating a large polyspecific population of cells that can persist for a long time,” he further commented.
All in all, this study was a “yeoman’s effort” and the researchers “deserve a lot of credit for pushing it forward,” Dr. Greenberg said.
The study was supported in part by grants from Stand Up to Cancer Foundation, the Barbara Bauer Prelude to a Cure Foundation, Iovance Biotherapeutics, and a Young Investigator award from Adaptive Biotechnologies. Nivolumab was supplied by Bristol-Myers Squibb. Aldesleukin (IL-2) was supplied by Clinigen Group. A complete list of author disclosures is available with the original article. Dr. Hirsch and Dr. Greenberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Progressive disability in MS explained?
Results from a retrospective study show that complete resolution of brain lesions on MRI was more common among patients with myelin-oligodendrocyte-glycoprotein-IgG-associated disorder (MOGAD). Complete resolution occurred in 72% of the group with MOGAD, versus 17% of those with MS and 14% of those with aquaporin-4-positive neuromyelitis optica spectrum disorder (AQP4+ NMOSD).
“What we found was, with MOGAD in particular, many of the lesions resolved completely,” said co-investigator Eoin Flanagan, MBBCh, neurologist, Mayo Clinic, Rochester, Minn. “That fits with MOGAD having a fairly good prognosis and patients not developing much long-term disability with that disease,” he said.
The researchers also studied whether scarring may account for the absence of slowly progressive disability among patients with AQP4+ NMOSD and MOGAD compared with patients with MS. “The differences in scarring that we found will help physicians distinguish these three diseases more easily to aid in diagnosis. More importantly, our findings improve our understanding of the mechanisms of nerve damage in these three diseases and may suggest an important role of such scars in the development of long-term disability in MS,” Dr. Flanagan said in a statement.
The findings were published online July 14 in Neurology.
Lesion evolution
MOGAD, AQP4+ NMOSD, and MS are inflammatory demyelinating disorders that share certain manifestations. However, these disorders differ in important ways, including the severity of attacks and their clinical course.
Although patients with MOGAD and AQP4+ NMOSD generally have severe attacks that bring major disability, the clinical course of these disorders is better than initial attacks would suggest. In contrast, patients with MS have comparatively mild attacks that are associated with a high risk for progressive disability.
Previous studies of these demyelinating disorders have examined the shape and location of lesions but not change over time. Observing these lesions’ development and resolution could provide information about disease course and influence treatment and the monitoring of disease activity, the current researchers noted.
They retrospectively identified consecutive patients with MOGAD, AQP4+ NMOSD, or MS who presented to the Mayo Clinic between January 2000 and August 2019. Data from a cohort of patients with MS in Olmsted County, Minn., were also included.
Eligible participants had experienced a first brain or myelitis attack, had undergone MRI of the brain or spinal cord within 6 weeks of the attack nadir, and had undergone a follow-up MRI 6 months after the attack.
Patients who experienced a relapse during follow-up in the same region as the initial attack were excluded. Concomitant brain and myelitis attacks were analyzed separately.
An index lesion was identified for each patient. The index lesion was defined as an acute lesion that provided an anatomic explanation for the clinical symptoms. If multiple lesions were present, the largest of them was chosen as the index lesion. MRIs were examined by neuroradiologists who were blinded to patients’ diagnoses and serology results.
Among the 156 participants, 67 had MS (76% women), 51 had AQP4+ NMOSD (80% women), and 38 had MOGAD (45% women). The median age at first attack for the groups was 37, 53, and 25 years, respectively.
In addition, 63 patients had relapsing-remitting MS, two had a single attack of progressive MS, and two had clinically isolated syndrome. No patients with NMOSD or MOGAD had developed progressive disease at final follow-up.
Participants experienced a total of 81 brain attacks and 91 myelitis attacks. Sixteen patients had experienced both a brain attack and a myelitis attack.
Symptoms corresponding to the index brain lesions were brainstem or cerebellar syndrome (56), encephalopathy or focal symptoms (12), or combinations of these (13). Among patients with an index myelitis attack, 31 had cervical involvement, 21 had thoracic involvement, and 39 had involvement of both regions.
Complete resolution
Results showed that 72% of patients with MOGAD experienced complete resolution of the brain index lesion, compared with 17% of patients with MS and 14% of patients with NMOSD (P < .001).
Similarly, 79% of the MOGAD group experienced complete resolution of the myelitis index lesion, compared with no members of the MS or NMOSD groups (P < .001 for both comparisons).
Complete resolution of all T2-abnormalities at MRI follow-up was more common in the MOGAD group than in the other two groups.
For brain attacks, complete resolution occurred in 39% of patients with MOGAD, 10% of patients with NMOSD, and 5% of patients with MS. For spinal cord attacks, complete resolution occurred in 79% of patients with MOGAD, versus none of the patients with NMOSD or MS.
Median reduction in T2 lesion area on follow-up axial brain MRI was larger in patients with MOGAD (213 mm2) than in those with NMOSD (104 mm2; P = .02) or MS (36 mm2; P < .001).
Reductions in lesion size on sagittal spine MRI follow-up were similar between the MOGAD (262 mm2) and NMOSD (309 mm2) groups; both experienced greater reductions than the MS group (23 mm2; P < .001).
Lesion prevention
Dr. Flanagan noted that the diagnosis of MOGAD is based on a test for MOG antibody, but sometimes false positive results occur. “A single follow-up MRI can be useful, showing that if all the lesions went away, you would be more confident that it would be MOGAD,” he said.
Study participants with MS experienced less lesion healing than the patients with MOGAD or NMOSD.
“We now have very effective medications in MS to prevent new lesions from occurring,” Dr. Flanagan said. The study highlights the importance of lesion prevention, “because when you do get a lesion, it does tend to stay and not recover completely,” he added.
He noted that the resolution of lesions in the study population may reflect remyelination. Future research examining whether remyelination is more efficient in MOGAD than in the other disorders could possibly lead to new approaches for MS treatment, said Dr. Flanagan.
“Maybe some of the MOGAD lesions are from edema. When we use steroids, that tends to resolve and not leave a scar. So, that’s another possibility. We’d like to better understand that,” he said.
Differences in pathology
Commenting on the findings, Bruce Cree, MD, PhD, professor of neurology, Weill Institute for Neurosciences, University of California, San Francisco, noted that the study is one of the first to systematically examine and compare MRI lesion evolution across three disease states.
“What they put their finger on are differences in the fundamental pathology of these three different diseases,” said Dr. Cree, who was not involved with the research.
The study’s cross-sectional comparison was its main strength, he noted.
“The main weakness, from my point of view, is that in these three disorders, optic nerve involvement is very common,” Dr. Cree said. “In this paper, no analysis of optic nerve lesions by MRI was performed.”
The researchers acknowledge this limitation and explain that they did not have consistent, dedicated orbital imaging for such an analysis.
Dr. Cree noted that the findings also provide a reminder that the pathogenesis of MOGAD is not yet clear.
“We know that these anti-MOG antibodies are associated with this demyelinating disorder, but whether these antibodies have a pathogenic role has yet to be clearly demonstrated,” said Dr. Cree. “What is actually going on within these lesions [is also] not fully understood.”
The finding that MOGAD lesions can resolve completely suggests that repair mechanisms are at work within the brain and spinal cord, he noted.
Being able to understand and comprehend what those mechanisms at work are and why they occur in MOGAD but not in NMOSD or MS “would be of enormous clinical advantage,” he said.
The current study also highlights the importance of incorporating imaging into clinical trials that study these rare disorders, especially serial imaging for MOGAD, Dr. Cree added.
This imaging is vital not only for developing new treatments but also for understanding the clinical impact of a given medication. “We really need rigorous imaging to be applied to these rare disorders, just as was done with MS,” Dr. Cree concluded.
The study was funded by the National Institute of Neurological Disorders and Stroke. Dr. Flanagan has received research support from MedImmune/Viela Bio. Dr. Cree is working with two of the researchers on the steering committee for the N-MOmentum trial of inebilizumab in patients with NMOSD. He has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a retrospective study show that complete resolution of brain lesions on MRI was more common among patients with myelin-oligodendrocyte-glycoprotein-IgG-associated disorder (MOGAD). Complete resolution occurred in 72% of the group with MOGAD, versus 17% of those with MS and 14% of those with aquaporin-4-positive neuromyelitis optica spectrum disorder (AQP4+ NMOSD).
“What we found was, with MOGAD in particular, many of the lesions resolved completely,” said co-investigator Eoin Flanagan, MBBCh, neurologist, Mayo Clinic, Rochester, Minn. “That fits with MOGAD having a fairly good prognosis and patients not developing much long-term disability with that disease,” he said.
The researchers also studied whether scarring may account for the absence of slowly progressive disability among patients with AQP4+ NMOSD and MOGAD compared with patients with MS. “The differences in scarring that we found will help physicians distinguish these three diseases more easily to aid in diagnosis. More importantly, our findings improve our understanding of the mechanisms of nerve damage in these three diseases and may suggest an important role of such scars in the development of long-term disability in MS,” Dr. Flanagan said in a statement.
The findings were published online July 14 in Neurology.
Lesion evolution
MOGAD, AQP4+ NMOSD, and MS are inflammatory demyelinating disorders that share certain manifestations. However, these disorders differ in important ways, including the severity of attacks and their clinical course.
Although patients with MOGAD and AQP4+ NMOSD generally have severe attacks that bring major disability, the clinical course of these disorders is better than initial attacks would suggest. In contrast, patients with MS have comparatively mild attacks that are associated with a high risk for progressive disability.
Previous studies of these demyelinating disorders have examined the shape and location of lesions but not change over time. Observing these lesions’ development and resolution could provide information about disease course and influence treatment and the monitoring of disease activity, the current researchers noted.
They retrospectively identified consecutive patients with MOGAD, AQP4+ NMOSD, or MS who presented to the Mayo Clinic between January 2000 and August 2019. Data from a cohort of patients with MS in Olmsted County, Minn., were also included.
Eligible participants had experienced a first brain or myelitis attack, had undergone MRI of the brain or spinal cord within 6 weeks of the attack nadir, and had undergone a follow-up MRI 6 months after the attack.
Patients who experienced a relapse during follow-up in the same region as the initial attack were excluded. Concomitant brain and myelitis attacks were analyzed separately.
An index lesion was identified for each patient. The index lesion was defined as an acute lesion that provided an anatomic explanation for the clinical symptoms. If multiple lesions were present, the largest of them was chosen as the index lesion. MRIs were examined by neuroradiologists who were blinded to patients’ diagnoses and serology results.
Among the 156 participants, 67 had MS (76% women), 51 had AQP4+ NMOSD (80% women), and 38 had MOGAD (45% women). The median age at first attack for the groups was 37, 53, and 25 years, respectively.
In addition, 63 patients had relapsing-remitting MS, two had a single attack of progressive MS, and two had clinically isolated syndrome. No patients with NMOSD or MOGAD had developed progressive disease at final follow-up.
Participants experienced a total of 81 brain attacks and 91 myelitis attacks. Sixteen patients had experienced both a brain attack and a myelitis attack.
Symptoms corresponding to the index brain lesions were brainstem or cerebellar syndrome (56), encephalopathy or focal symptoms (12), or combinations of these (13). Among patients with an index myelitis attack, 31 had cervical involvement, 21 had thoracic involvement, and 39 had involvement of both regions.
Complete resolution
Results showed that 72% of patients with MOGAD experienced complete resolution of the brain index lesion, compared with 17% of patients with MS and 14% of patients with NMOSD (P < .001).
Similarly, 79% of the MOGAD group experienced complete resolution of the myelitis index lesion, compared with no members of the MS or NMOSD groups (P < .001 for both comparisons).
Complete resolution of all T2-abnormalities at MRI follow-up was more common in the MOGAD group than in the other two groups.
For brain attacks, complete resolution occurred in 39% of patients with MOGAD, 10% of patients with NMOSD, and 5% of patients with MS. For spinal cord attacks, complete resolution occurred in 79% of patients with MOGAD, versus none of the patients with NMOSD or MS.
Median reduction in T2 lesion area on follow-up axial brain MRI was larger in patients with MOGAD (213 mm2) than in those with NMOSD (104 mm2; P = .02) or MS (36 mm2; P < .001).
Reductions in lesion size on sagittal spine MRI follow-up were similar between the MOGAD (262 mm2) and NMOSD (309 mm2) groups; both experienced greater reductions than the MS group (23 mm2; P < .001).
Lesion prevention
Dr. Flanagan noted that the diagnosis of MOGAD is based on a test for MOG antibody, but sometimes false positive results occur. “A single follow-up MRI can be useful, showing that if all the lesions went away, you would be more confident that it would be MOGAD,” he said.
Study participants with MS experienced less lesion healing than the patients with MOGAD or NMOSD.
“We now have very effective medications in MS to prevent new lesions from occurring,” Dr. Flanagan said. The study highlights the importance of lesion prevention, “because when you do get a lesion, it does tend to stay and not recover completely,” he added.
He noted that the resolution of lesions in the study population may reflect remyelination. Future research examining whether remyelination is more efficient in MOGAD than in the other disorders could possibly lead to new approaches for MS treatment, said Dr. Flanagan.
“Maybe some of the MOGAD lesions are from edema. When we use steroids, that tends to resolve and not leave a scar. So, that’s another possibility. We’d like to better understand that,” he said.
Differences in pathology
Commenting on the findings, Bruce Cree, MD, PhD, professor of neurology, Weill Institute for Neurosciences, University of California, San Francisco, noted that the study is one of the first to systematically examine and compare MRI lesion evolution across three disease states.
“What they put their finger on are differences in the fundamental pathology of these three different diseases,” said Dr. Cree, who was not involved with the research.
The study’s cross-sectional comparison was its main strength, he noted.
“The main weakness, from my point of view, is that in these three disorders, optic nerve involvement is very common,” Dr. Cree said. “In this paper, no analysis of optic nerve lesions by MRI was performed.”
The researchers acknowledge this limitation and explain that they did not have consistent, dedicated orbital imaging for such an analysis.
Dr. Cree noted that the findings also provide a reminder that the pathogenesis of MOGAD is not yet clear.
“We know that these anti-MOG antibodies are associated with this demyelinating disorder, but whether these antibodies have a pathogenic role has yet to be clearly demonstrated,” said Dr. Cree. “What is actually going on within these lesions [is also] not fully understood.”
The finding that MOGAD lesions can resolve completely suggests that repair mechanisms are at work within the brain and spinal cord, he noted.
Being able to understand and comprehend what those mechanisms at work are and why they occur in MOGAD but not in NMOSD or MS “would be of enormous clinical advantage,” he said.
The current study also highlights the importance of incorporating imaging into clinical trials that study these rare disorders, especially serial imaging for MOGAD, Dr. Cree added.
This imaging is vital not only for developing new treatments but also for understanding the clinical impact of a given medication. “We really need rigorous imaging to be applied to these rare disorders, just as was done with MS,” Dr. Cree concluded.
The study was funded by the National Institute of Neurological Disorders and Stroke. Dr. Flanagan has received research support from MedImmune/Viela Bio. Dr. Cree is working with two of the researchers on the steering committee for the N-MOmentum trial of inebilizumab in patients with NMOSD. He has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a retrospective study show that complete resolution of brain lesions on MRI was more common among patients with myelin-oligodendrocyte-glycoprotein-IgG-associated disorder (MOGAD). Complete resolution occurred in 72% of the group with MOGAD, versus 17% of those with MS and 14% of those with aquaporin-4-positive neuromyelitis optica spectrum disorder (AQP4+ NMOSD).
“What we found was, with MOGAD in particular, many of the lesions resolved completely,” said co-investigator Eoin Flanagan, MBBCh, neurologist, Mayo Clinic, Rochester, Minn. “That fits with MOGAD having a fairly good prognosis and patients not developing much long-term disability with that disease,” he said.
The researchers also studied whether scarring may account for the absence of slowly progressive disability among patients with AQP4+ NMOSD and MOGAD compared with patients with MS. “The differences in scarring that we found will help physicians distinguish these three diseases more easily to aid in diagnosis. More importantly, our findings improve our understanding of the mechanisms of nerve damage in these three diseases and may suggest an important role of such scars in the development of long-term disability in MS,” Dr. Flanagan said in a statement.
The findings were published online July 14 in Neurology.
Lesion evolution
MOGAD, AQP4+ NMOSD, and MS are inflammatory demyelinating disorders that share certain manifestations. However, these disorders differ in important ways, including the severity of attacks and their clinical course.
Although patients with MOGAD and AQP4+ NMOSD generally have severe attacks that bring major disability, the clinical course of these disorders is better than initial attacks would suggest. In contrast, patients with MS have comparatively mild attacks that are associated with a high risk for progressive disability.
Previous studies of these demyelinating disorders have examined the shape and location of lesions but not change over time. Observing these lesions’ development and resolution could provide information about disease course and influence treatment and the monitoring of disease activity, the current researchers noted.
They retrospectively identified consecutive patients with MOGAD, AQP4+ NMOSD, or MS who presented to the Mayo Clinic between January 2000 and August 2019. Data from a cohort of patients with MS in Olmsted County, Minn., were also included.
Eligible participants had experienced a first brain or myelitis attack, had undergone MRI of the brain or spinal cord within 6 weeks of the attack nadir, and had undergone a follow-up MRI 6 months after the attack.
Patients who experienced a relapse during follow-up in the same region as the initial attack were excluded. Concomitant brain and myelitis attacks were analyzed separately.
An index lesion was identified for each patient. The index lesion was defined as an acute lesion that provided an anatomic explanation for the clinical symptoms. If multiple lesions were present, the largest of them was chosen as the index lesion. MRIs were examined by neuroradiologists who were blinded to patients’ diagnoses and serology results.
Among the 156 participants, 67 had MS (76% women), 51 had AQP4+ NMOSD (80% women), and 38 had MOGAD (45% women). The median age at first attack for the groups was 37, 53, and 25 years, respectively.
In addition, 63 patients had relapsing-remitting MS, two had a single attack of progressive MS, and two had clinically isolated syndrome. No patients with NMOSD or MOGAD had developed progressive disease at final follow-up.
Participants experienced a total of 81 brain attacks and 91 myelitis attacks. Sixteen patients had experienced both a brain attack and a myelitis attack.
Symptoms corresponding to the index brain lesions were brainstem or cerebellar syndrome (56), encephalopathy or focal symptoms (12), or combinations of these (13). Among patients with an index myelitis attack, 31 had cervical involvement, 21 had thoracic involvement, and 39 had involvement of both regions.
Complete resolution
Results showed that 72% of patients with MOGAD experienced complete resolution of the brain index lesion, compared with 17% of patients with MS and 14% of patients with NMOSD (P < .001).
Similarly, 79% of the MOGAD group experienced complete resolution of the myelitis index lesion, compared with no members of the MS or NMOSD groups (P < .001 for both comparisons).
Complete resolution of all T2-abnormalities at MRI follow-up was more common in the MOGAD group than in the other two groups.
For brain attacks, complete resolution occurred in 39% of patients with MOGAD, 10% of patients with NMOSD, and 5% of patients with MS. For spinal cord attacks, complete resolution occurred in 79% of patients with MOGAD, versus none of the patients with NMOSD or MS.
Median reduction in T2 lesion area on follow-up axial brain MRI was larger in patients with MOGAD (213 mm2) than in those with NMOSD (104 mm2; P = .02) or MS (36 mm2; P < .001).
Reductions in lesion size on sagittal spine MRI follow-up were similar between the MOGAD (262 mm2) and NMOSD (309 mm2) groups; both experienced greater reductions than the MS group (23 mm2; P < .001).
Lesion prevention
Dr. Flanagan noted that the diagnosis of MOGAD is based on a test for MOG antibody, but sometimes false positive results occur. “A single follow-up MRI can be useful, showing that if all the lesions went away, you would be more confident that it would be MOGAD,” he said.
Study participants with MS experienced less lesion healing than the patients with MOGAD or NMOSD.
“We now have very effective medications in MS to prevent new lesions from occurring,” Dr. Flanagan said. The study highlights the importance of lesion prevention, “because when you do get a lesion, it does tend to stay and not recover completely,” he added.
He noted that the resolution of lesions in the study population may reflect remyelination. Future research examining whether remyelination is more efficient in MOGAD than in the other disorders could possibly lead to new approaches for MS treatment, said Dr. Flanagan.
“Maybe some of the MOGAD lesions are from edema. When we use steroids, that tends to resolve and not leave a scar. So, that’s another possibility. We’d like to better understand that,” he said.
Differences in pathology
Commenting on the findings, Bruce Cree, MD, PhD, professor of neurology, Weill Institute for Neurosciences, University of California, San Francisco, noted that the study is one of the first to systematically examine and compare MRI lesion evolution across three disease states.
“What they put their finger on are differences in the fundamental pathology of these three different diseases,” said Dr. Cree, who was not involved with the research.
The study’s cross-sectional comparison was its main strength, he noted.
“The main weakness, from my point of view, is that in these three disorders, optic nerve involvement is very common,” Dr. Cree said. “In this paper, no analysis of optic nerve lesions by MRI was performed.”
The researchers acknowledge this limitation and explain that they did not have consistent, dedicated orbital imaging for such an analysis.
Dr. Cree noted that the findings also provide a reminder that the pathogenesis of MOGAD is not yet clear.
“We know that these anti-MOG antibodies are associated with this demyelinating disorder, but whether these antibodies have a pathogenic role has yet to be clearly demonstrated,” said Dr. Cree. “What is actually going on within these lesions [is also] not fully understood.”
The finding that MOGAD lesions can resolve completely suggests that repair mechanisms are at work within the brain and spinal cord, he noted.
Being able to understand and comprehend what those mechanisms at work are and why they occur in MOGAD but not in NMOSD or MS “would be of enormous clinical advantage,” he said.
The current study also highlights the importance of incorporating imaging into clinical trials that study these rare disorders, especially serial imaging for MOGAD, Dr. Cree added.
This imaging is vital not only for developing new treatments but also for understanding the clinical impact of a given medication. “We really need rigorous imaging to be applied to these rare disorders, just as was done with MS,” Dr. Cree concluded.
The study was funded by the National Institute of Neurological Disorders and Stroke. Dr. Flanagan has received research support from MedImmune/Viela Bio. Dr. Cree is working with two of the researchers on the steering committee for the N-MOmentum trial of inebilizumab in patients with NMOSD. He has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From Neurology
‘Countdown to zero’: Endocrine disruptors and worldwide sperm counts
In medical school, I remember thinking that telling a patient “you have cancer” would be the most professionally challenging phrase I would ever utter. And don’t get me wrong – it certainly isn’t easy; but, compared with telling someone “you are infertile,” it’s a cakewalk.
Maybe it’s because people “have” cancer and cancer is something you “fight.” Or maybe because, unlike infertility, cancer has become a part of public life (think lapel pins and support groups) and is now easier to accept. On the other hand, someone “is” infertile. The condition is a source of embarrassment for the couple and is often hidden from society.
Here’s another concerning point of contrast: While the overall rate of cancer death has declined since the early 1990s, infertility is increasing. Reports now show that one in six couples have problems conceiving and the use of assisted reproductive technologies is increasing by 5%-10% per year. Many theories exist to explain these trends, chief among them the rise in average maternal age and the increasing incidence of obesity, as well as various other male- and female-specific factors.
But interestingly, recent data suggest that the most male of all male-specific factors – total sperm count – may be specifically to blame.
According to a recent meta-analysis, the average total sperm count in men declined by 59.3% between 1973 and 2011. While these data certainly have limitations – including the exclusion of non-English publications, the reliance on total sperm count and not sperm motility, and the potential bias of those patients willing to give a semen sample – the overall trend nevertheless seems to be clearly downward. What’s more concerning, if you believe the data presented, is that there does not appear to be a leveling off of the downward curve in total sperm count.
Think about that last statement. At the current rate of decline, the average sperm count will be zero in 2045. One of the lead authors on the meta-analysis, Hagai Levine, MD, MPH, goes so far as to state, “We should hope for the best and prepare for the worst.”
As a matter of personal philosophy, I’m not a huge fan of end-of-the-world predictions because they tend not to come true (think Montanism back in the 2nd century; the 2012 Mayan calendar scare; or my personal favorite, the Prophet Hen of Leeds). On the other hand, the overall trend of decreased total sperm count in the English-speaking world seems to be true and it raises the interesting question of why.
According to the Mayo Clinic, causes of decreased sperm count include everything from anatomical factors (like varicoceles and ejaculatory issues) and lifestyle issues (such as recreational drugs, weight gain, and emotional stress) to environmental exposures (heavy metal or radiation). The senior author of the aforementioned meta-analysis, Shanna Swan, PhD, has championed another theory: the widespread exposure to endocrine-disrupting chemicals in everyday plastics.
It turns out that at least two chemicals used in the plastics industry, bisphenol A and phthalates, can mimic the effect of estrogen when ingested into the body. Even low levels of these chemicals in our bodies can lead to health problems.
Consider for a moment the presence of plastics in your life: the plastic wrappings on your food, plastic containers for shampoos and beauty products, and even the coatings of our oral supplements. A study by the Centers for Disease Control and Prevention looked at the urine of people participating in the National Health and Nutrition Examination Survey and found detectable concentrations of both of these chemicals in nearly all participants.
In 2045, I intend to be retired. But in the meantime, I think we all need to be aware of the potential impact that various endocrine-disrupting chemicals could be having on humanity. We need more research. If indeed the connection between endocrine disruptors and decreased sperm count is borne out, changes in our environmental exposure to these chemicals need to be made.
Henry Rosevear, MD, is a private-practice urologist based in Colorado Springs. He comes from a long line of doctors, but before entering medicine he served in the U.S. Navy as an officer aboard the USS Pittsburgh, a fast-attack submarine based out of New London, Conn. During his time in the Navy, he served in two deployments to the Persian Gulf, including combat experience as part of Operation Iraqi Freedom. Dr. Rosevear disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
In medical school, I remember thinking that telling a patient “you have cancer” would be the most professionally challenging phrase I would ever utter. And don’t get me wrong – it certainly isn’t easy; but, compared with telling someone “you are infertile,” it’s a cakewalk.
Maybe it’s because people “have” cancer and cancer is something you “fight.” Or maybe because, unlike infertility, cancer has become a part of public life (think lapel pins and support groups) and is now easier to accept. On the other hand, someone “is” infertile. The condition is a source of embarrassment for the couple and is often hidden from society.
Here’s another concerning point of contrast: While the overall rate of cancer death has declined since the early 1990s, infertility is increasing. Reports now show that one in six couples have problems conceiving and the use of assisted reproductive technologies is increasing by 5%-10% per year. Many theories exist to explain these trends, chief among them the rise in average maternal age and the increasing incidence of obesity, as well as various other male- and female-specific factors.
But interestingly, recent data suggest that the most male of all male-specific factors – total sperm count – may be specifically to blame.
According to a recent meta-analysis, the average total sperm count in men declined by 59.3% between 1973 and 2011. While these data certainly have limitations – including the exclusion of non-English publications, the reliance on total sperm count and not sperm motility, and the potential bias of those patients willing to give a semen sample – the overall trend nevertheless seems to be clearly downward. What’s more concerning, if you believe the data presented, is that there does not appear to be a leveling off of the downward curve in total sperm count.
Think about that last statement. At the current rate of decline, the average sperm count will be zero in 2045. One of the lead authors on the meta-analysis, Hagai Levine, MD, MPH, goes so far as to state, “We should hope for the best and prepare for the worst.”
As a matter of personal philosophy, I’m not a huge fan of end-of-the-world predictions because they tend not to come true (think Montanism back in the 2nd century; the 2012 Mayan calendar scare; or my personal favorite, the Prophet Hen of Leeds). On the other hand, the overall trend of decreased total sperm count in the English-speaking world seems to be true and it raises the interesting question of why.
According to the Mayo Clinic, causes of decreased sperm count include everything from anatomical factors (like varicoceles and ejaculatory issues) and lifestyle issues (such as recreational drugs, weight gain, and emotional stress) to environmental exposures (heavy metal or radiation). The senior author of the aforementioned meta-analysis, Shanna Swan, PhD, has championed another theory: the widespread exposure to endocrine-disrupting chemicals in everyday plastics.
It turns out that at least two chemicals used in the plastics industry, bisphenol A and phthalates, can mimic the effect of estrogen when ingested into the body. Even low levels of these chemicals in our bodies can lead to health problems.
Consider for a moment the presence of plastics in your life: the plastic wrappings on your food, plastic containers for shampoos and beauty products, and even the coatings of our oral supplements. A study by the Centers for Disease Control and Prevention looked at the urine of people participating in the National Health and Nutrition Examination Survey and found detectable concentrations of both of these chemicals in nearly all participants.
In 2045, I intend to be retired. But in the meantime, I think we all need to be aware of the potential impact that various endocrine-disrupting chemicals could be having on humanity. We need more research. If indeed the connection between endocrine disruptors and decreased sperm count is borne out, changes in our environmental exposure to these chemicals need to be made.
Henry Rosevear, MD, is a private-practice urologist based in Colorado Springs. He comes from a long line of doctors, but before entering medicine he served in the U.S. Navy as an officer aboard the USS Pittsburgh, a fast-attack submarine based out of New London, Conn. During his time in the Navy, he served in two deployments to the Persian Gulf, including combat experience as part of Operation Iraqi Freedom. Dr. Rosevear disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
In medical school, I remember thinking that telling a patient “you have cancer” would be the most professionally challenging phrase I would ever utter. And don’t get me wrong – it certainly isn’t easy; but, compared with telling someone “you are infertile,” it’s a cakewalk.
Maybe it’s because people “have” cancer and cancer is something you “fight.” Or maybe because, unlike infertility, cancer has become a part of public life (think lapel pins and support groups) and is now easier to accept. On the other hand, someone “is” infertile. The condition is a source of embarrassment for the couple and is often hidden from society.
Here’s another concerning point of contrast: While the overall rate of cancer death has declined since the early 1990s, infertility is increasing. Reports now show that one in six couples have problems conceiving and the use of assisted reproductive technologies is increasing by 5%-10% per year. Many theories exist to explain these trends, chief among them the rise in average maternal age and the increasing incidence of obesity, as well as various other male- and female-specific factors.
But interestingly, recent data suggest that the most male of all male-specific factors – total sperm count – may be specifically to blame.
According to a recent meta-analysis, the average total sperm count in men declined by 59.3% between 1973 and 2011. While these data certainly have limitations – including the exclusion of non-English publications, the reliance on total sperm count and not sperm motility, and the potential bias of those patients willing to give a semen sample – the overall trend nevertheless seems to be clearly downward. What’s more concerning, if you believe the data presented, is that there does not appear to be a leveling off of the downward curve in total sperm count.
Think about that last statement. At the current rate of decline, the average sperm count will be zero in 2045. One of the lead authors on the meta-analysis, Hagai Levine, MD, MPH, goes so far as to state, “We should hope for the best and prepare for the worst.”
As a matter of personal philosophy, I’m not a huge fan of end-of-the-world predictions because they tend not to come true (think Montanism back in the 2nd century; the 2012 Mayan calendar scare; or my personal favorite, the Prophet Hen of Leeds). On the other hand, the overall trend of decreased total sperm count in the English-speaking world seems to be true and it raises the interesting question of why.
According to the Mayo Clinic, causes of decreased sperm count include everything from anatomical factors (like varicoceles and ejaculatory issues) and lifestyle issues (such as recreational drugs, weight gain, and emotional stress) to environmental exposures (heavy metal or radiation). The senior author of the aforementioned meta-analysis, Shanna Swan, PhD, has championed another theory: the widespread exposure to endocrine-disrupting chemicals in everyday plastics.
It turns out that at least two chemicals used in the plastics industry, bisphenol A and phthalates, can mimic the effect of estrogen when ingested into the body. Even low levels of these chemicals in our bodies can lead to health problems.
Consider for a moment the presence of plastics in your life: the plastic wrappings on your food, plastic containers for shampoos and beauty products, and even the coatings of our oral supplements. A study by the Centers for Disease Control and Prevention looked at the urine of people participating in the National Health and Nutrition Examination Survey and found detectable concentrations of both of these chemicals in nearly all participants.
In 2045, I intend to be retired. But in the meantime, I think we all need to be aware of the potential impact that various endocrine-disrupting chemicals could be having on humanity. We need more research. If indeed the connection between endocrine disruptors and decreased sperm count is borne out, changes in our environmental exposure to these chemicals need to be made.
Henry Rosevear, MD, is a private-practice urologist based in Colorado Springs. He comes from a long line of doctors, but before entering medicine he served in the U.S. Navy as an officer aboard the USS Pittsburgh, a fast-attack submarine based out of New London, Conn. During his time in the Navy, he served in two deployments to the Persian Gulf, including combat experience as part of Operation Iraqi Freedom. Dr. Rosevear disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The Limited Academic Footprint of Hospital Medicine: Where Do We Go From Here?
What has been the scholarly output of academic hospital medicine faculty (AHMF) and what academic rank have they achieved at US academic medical centers (AMCs)? Sumarsono et al1 address these questions and add to the growing body of literature exposing the limited academic footprint of hospitalists.
The authors performed a cross-sectional analysis of AHMF affiliated with the top 25 internal medicine training programs (as determined by the physician networking service doximity.com) and used Scopus to determine number of publications, citations, and H-index (a metric of productivity) for each faculty member. They also evaluated predictors for promotion. In contrast, most prior research on this topic relies on data obtained by survey methodology.2-5
Among 1554 AHMF from 22 AMCs, 42 (2.7%) were full professors and 140 (9.0%) were associate professors. The number of publications per AHMF was noticeably low, with a mean of 6.3 and median of 0 (interquartile range, 0-4). The authors found that H-index, completion of chief residency, and graduation from a top 25 medical school were independently associated with promotion.
The authors only evaluated AHMF among the most academically rigorous AMCs, an approach that likely overestimates scholarly output of hospitalists across all US AMCs. Conversely, if we presume that promotion is more difficult at these major AMCs, the results may underestimate academic rank of AHMF nationally. Additionally, the authors did not distinguish faculty by tracks (eg, clinician-investigators, clinician-educators), which often have different criteria for academic promotion.
These findings are worrisomely consistent with prior reports, despite the tremendous expansion of the field.2-4 A 2008 survey of academic hospitalists found that 4% of respondents were full professors and 9% were associate professors, values nearly identical to the results in this current analysis,4 suggesting enduring barriers to academic advancement.
We are left with the following questions provoked by this body of literature: How can hospitalists increase their scholarly output and climb the promotional ladder? And how can we increase the academic footprint of hospital medicine? We recently proposed the following strategies based on a survey of academic groups participating in the Hospital Medicine Reengineering Network (HOMERuN) survey5: (1) expand hospital medicine research fellowships, which will provide graduates with research skills to justify dedicated time for research and aid their ability to obtain independent funding; (2) formalize mentorship between research faculty in hospital medicine and other internal medicine disciplines with robust track records for research; (3) invest in research infrastructure and data access within and between institutions; and (4) encourage hospital medicine group leaders to foster academic growth by incentivizing faculty to perform research, present their work at national conferences, and publish manuscripts with their findings.
Although an increase in scholarly output should contribute to higher academic rank, hospitalists routinely make other invaluable contributions beyond clinical care to AMCs, including medical education, hospital leadership, quality improvement, clinical innovation, and social justice advocacy. Also, hospitalists are increasingly disseminating their contributions via newer mediums (eg, social media, podcasts) that arguably have greater reach than traditional scholarship outlets. We believe that promotion committees should update their criteria to reflect the evolution of academic contribution and integrate these within traditional promotion pathways.
Finally, we must address federal funding mechanisms, which currently favor specialty-specific funding over funding that would be more applicable to hospital medicine researchers. Funding agencies are largely specialty- or disease-specific, with limited options for broader-based research.6 Additionally, grant-review committees are largely comprised of specialists, with few generalists and fewer hospitalists. These limitations make it difficult to “argue” the necessity of hospital medicine research. One concrete step would be for the National Institutes of Health (NIH) to create an Office for Hospital Medicine Research, analogous to the Office of Emergency Care Research, which works across NIH institutes and centers to foster research and research training for the emergency setting.
With these strategies, we are hopeful that hospital medicine will continue to expand its academic footprint and be recognized for its ever-growing contributions to the practice of medicine.
1. Sumarsono A, Keshvani N, Saleh SN, et al. Scholarly productivity and rank in academic hospital medicine. J Hosp Med. 2021;16(9):545-548. https://doi.org/10.12788/jhm.3631
2. Chopra V, Burden M, Jones CD, et al. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
3. Miller CS, Fogerty RL, Gann J, et al, the Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Shannon EM, Chopra V, Greysen SR, et al. Dearth of hospitalist investigators in academic medicine: a call to action. J Hosp Med. 2021;16(3):189-191. https://doi.org/10.12788/jhm.3536
6. Levinson W, Linzer M. What is an academic general internist? Career options and training pathways. JAMA. 2002;288(16):2045-2048. https://doi.org/10.1001/jama.288.16.2045
What has been the scholarly output of academic hospital medicine faculty (AHMF) and what academic rank have they achieved at US academic medical centers (AMCs)? Sumarsono et al1 address these questions and add to the growing body of literature exposing the limited academic footprint of hospitalists.
The authors performed a cross-sectional analysis of AHMF affiliated with the top 25 internal medicine training programs (as determined by the physician networking service doximity.com) and used Scopus to determine number of publications, citations, and H-index (a metric of productivity) for each faculty member. They also evaluated predictors for promotion. In contrast, most prior research on this topic relies on data obtained by survey methodology.2-5
Among 1554 AHMF from 22 AMCs, 42 (2.7%) were full professors and 140 (9.0%) were associate professors. The number of publications per AHMF was noticeably low, with a mean of 6.3 and median of 0 (interquartile range, 0-4). The authors found that H-index, completion of chief residency, and graduation from a top 25 medical school were independently associated with promotion.
The authors only evaluated AHMF among the most academically rigorous AMCs, an approach that likely overestimates scholarly output of hospitalists across all US AMCs. Conversely, if we presume that promotion is more difficult at these major AMCs, the results may underestimate academic rank of AHMF nationally. Additionally, the authors did not distinguish faculty by tracks (eg, clinician-investigators, clinician-educators), which often have different criteria for academic promotion.
These findings are worrisomely consistent with prior reports, despite the tremendous expansion of the field.2-4 A 2008 survey of academic hospitalists found that 4% of respondents were full professors and 9% were associate professors, values nearly identical to the results in this current analysis,4 suggesting enduring barriers to academic advancement.
We are left with the following questions provoked by this body of literature: How can hospitalists increase their scholarly output and climb the promotional ladder? And how can we increase the academic footprint of hospital medicine? We recently proposed the following strategies based on a survey of academic groups participating in the Hospital Medicine Reengineering Network (HOMERuN) survey5: (1) expand hospital medicine research fellowships, which will provide graduates with research skills to justify dedicated time for research and aid their ability to obtain independent funding; (2) formalize mentorship between research faculty in hospital medicine and other internal medicine disciplines with robust track records for research; (3) invest in research infrastructure and data access within and between institutions; and (4) encourage hospital medicine group leaders to foster academic growth by incentivizing faculty to perform research, present their work at national conferences, and publish manuscripts with their findings.
Although an increase in scholarly output should contribute to higher academic rank, hospitalists routinely make other invaluable contributions beyond clinical care to AMCs, including medical education, hospital leadership, quality improvement, clinical innovation, and social justice advocacy. Also, hospitalists are increasingly disseminating their contributions via newer mediums (eg, social media, podcasts) that arguably have greater reach than traditional scholarship outlets. We believe that promotion committees should update their criteria to reflect the evolution of academic contribution and integrate these within traditional promotion pathways.
Finally, we must address federal funding mechanisms, which currently favor specialty-specific funding over funding that would be more applicable to hospital medicine researchers. Funding agencies are largely specialty- or disease-specific, with limited options for broader-based research.6 Additionally, grant-review committees are largely comprised of specialists, with few generalists and fewer hospitalists. These limitations make it difficult to “argue” the necessity of hospital medicine research. One concrete step would be for the National Institutes of Health (NIH) to create an Office for Hospital Medicine Research, analogous to the Office of Emergency Care Research, which works across NIH institutes and centers to foster research and research training for the emergency setting.
With these strategies, we are hopeful that hospital medicine will continue to expand its academic footprint and be recognized for its ever-growing contributions to the practice of medicine.
What has been the scholarly output of academic hospital medicine faculty (AHMF) and what academic rank have they achieved at US academic medical centers (AMCs)? Sumarsono et al1 address these questions and add to the growing body of literature exposing the limited academic footprint of hospitalists.
The authors performed a cross-sectional analysis of AHMF affiliated with the top 25 internal medicine training programs (as determined by the physician networking service doximity.com) and used Scopus to determine number of publications, citations, and H-index (a metric of productivity) for each faculty member. They also evaluated predictors for promotion. In contrast, most prior research on this topic relies on data obtained by survey methodology.2-5
Among 1554 AHMF from 22 AMCs, 42 (2.7%) were full professors and 140 (9.0%) were associate professors. The number of publications per AHMF was noticeably low, with a mean of 6.3 and median of 0 (interquartile range, 0-4). The authors found that H-index, completion of chief residency, and graduation from a top 25 medical school were independently associated with promotion.
The authors only evaluated AHMF among the most academically rigorous AMCs, an approach that likely overestimates scholarly output of hospitalists across all US AMCs. Conversely, if we presume that promotion is more difficult at these major AMCs, the results may underestimate academic rank of AHMF nationally. Additionally, the authors did not distinguish faculty by tracks (eg, clinician-investigators, clinician-educators), which often have different criteria for academic promotion.
These findings are worrisomely consistent with prior reports, despite the tremendous expansion of the field.2-4 A 2008 survey of academic hospitalists found that 4% of respondents were full professors and 9% were associate professors, values nearly identical to the results in this current analysis,4 suggesting enduring barriers to academic advancement.
We are left with the following questions provoked by this body of literature: How can hospitalists increase their scholarly output and climb the promotional ladder? And how can we increase the academic footprint of hospital medicine? We recently proposed the following strategies based on a survey of academic groups participating in the Hospital Medicine Reengineering Network (HOMERuN) survey5: (1) expand hospital medicine research fellowships, which will provide graduates with research skills to justify dedicated time for research and aid their ability to obtain independent funding; (2) formalize mentorship between research faculty in hospital medicine and other internal medicine disciplines with robust track records for research; (3) invest in research infrastructure and data access within and between institutions; and (4) encourage hospital medicine group leaders to foster academic growth by incentivizing faculty to perform research, present their work at national conferences, and publish manuscripts with their findings.
Although an increase in scholarly output should contribute to higher academic rank, hospitalists routinely make other invaluable contributions beyond clinical care to AMCs, including medical education, hospital leadership, quality improvement, clinical innovation, and social justice advocacy. Also, hospitalists are increasingly disseminating their contributions via newer mediums (eg, social media, podcasts) that arguably have greater reach than traditional scholarship outlets. We believe that promotion committees should update their criteria to reflect the evolution of academic contribution and integrate these within traditional promotion pathways.
Finally, we must address federal funding mechanisms, which currently favor specialty-specific funding over funding that would be more applicable to hospital medicine researchers. Funding agencies are largely specialty- or disease-specific, with limited options for broader-based research.6 Additionally, grant-review committees are largely comprised of specialists, with few generalists and fewer hospitalists. These limitations make it difficult to “argue” the necessity of hospital medicine research. One concrete step would be for the National Institutes of Health (NIH) to create an Office for Hospital Medicine Research, analogous to the Office of Emergency Care Research, which works across NIH institutes and centers to foster research and research training for the emergency setting.
With these strategies, we are hopeful that hospital medicine will continue to expand its academic footprint and be recognized for its ever-growing contributions to the practice of medicine.
1. Sumarsono A, Keshvani N, Saleh SN, et al. Scholarly productivity and rank in academic hospital medicine. J Hosp Med. 2021;16(9):545-548. https://doi.org/10.12788/jhm.3631
2. Chopra V, Burden M, Jones CD, et al. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
3. Miller CS, Fogerty RL, Gann J, et al, the Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Shannon EM, Chopra V, Greysen SR, et al. Dearth of hospitalist investigators in academic medicine: a call to action. J Hosp Med. 2021;16(3):189-191. https://doi.org/10.12788/jhm.3536
6. Levinson W, Linzer M. What is an academic general internist? Career options and training pathways. JAMA. 2002;288(16):2045-2048. https://doi.org/10.1001/jama.288.16.2045
1. Sumarsono A, Keshvani N, Saleh SN, et al. Scholarly productivity and rank in academic hospital medicine. J Hosp Med. 2021;16(9):545-548. https://doi.org/10.12788/jhm.3631
2. Chopra V, Burden M, Jones CD, et al. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
3. Miller CS, Fogerty RL, Gann J, et al, the Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the society of general internal medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
4. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
5. Shannon EM, Chopra V, Greysen SR, et al. Dearth of hospitalist investigators in academic medicine: a call to action. J Hosp Med. 2021;16(3):189-191. https://doi.org/10.12788/jhm.3536
6. Levinson W, Linzer M. What is an academic general internist? Career options and training pathways. JAMA. 2002;288(16):2045-2048. https://doi.org/10.1001/jama.288.16.2045
© 2021 Society of Hospital Medicine
The Chronic Effects of COVID-19 Hospitalizations: Learning How Patients Can Get “Back to Normal”
As our understanding of SARS-CoV-2 has progressed, researchers, clinicians, and patients have learned that recovery from COVID-19 can last well beyond the acute phase of the illness. As we see fewer fatal cases and more survivors, studies that characterize the postacute sequelae of COVID-19 (PASC) are increasingly important for understanding how to help patients return to their normal lives, especially after hospitalization. Critical to investigating this is knowing patients’ burden of symptoms and disabilities prior to infection. In this issue, a study by Iwashyna et al1 helps us understand patients’ lives after COVID compared to their lives before COVID.
The study analyzed patients with SARS-CoV-2 infection admitted during the third wave of the pandemic to assess for new cardiopulmonary symptoms, new disability, and financial toxicity of hospitalization 1 month after discharge.1 Many patients had new cardiopulmonary symptoms and oxygen use, and a much larger number had new limitations in activities of daily living (ADLs) or instrumental activities of daily living (iADLs). The majority were discharged home without home care services, and new limitations in ADLs or iADLs were common in these cases. Most patients reported not having returned to their cardiopulmonary or functional baseline; however, new cough, shortness of breath, or oxygen use usually did not explain their new disabilities. Financial toxicity was also common, reflecting the effects of COVID-19 on both employment and family finances.
These results complement those of Chopra et al,2 who examined 60-day outcomes for patients hospitalized during the first wave of the pandemic. At 2 months from discharge, many patients had ongoing cough, shortness of breath, oxygen use, and disability, but at lower rates. This likely reflects continuing recovery during the extra 30 days, but other potential explanations deserve consideration. One possibility is improving survival over the course of the pandemic. Many patients who may have passed away earlier in the pandemic now survive to return home, albeit with a heavy burden of symptomatology. This raises the possibility that symptoms among survivors may continue to increase as survival of COVID-19 improves. However, it should be noted that neither study is representative of the national patterns of hospitalization by race or ethnicity.3 Iwashyna et al1 underrepresented Black patients, while Chopra et al2 underrepresented Hispanic patients. Given what we know about outcomes for these populations and their underrepresentation in PASC literature, the impact of COVID-19 for them is likely underestimated. As data from 3, 6, or 12 months become available, we may also see the effect sizes described in this early literature become even larger.
Consistent with the findings of Chopra et al,2 financial toxicity after COVID-19 hospitalization was high. The longer-term financial burden of COVID-19 will likely exceed what is described here, particularly for Black and Hispanic patients, who experienced a disproportionate drain on their savings. These populations are also more likely to be negatively impacted by the COVID economy4 and thus may suffer a “double hit” financially if hospitalized.
Iwashyna et al1 underscore the urgent need for progress in understanding COVID “long-haulers”5 and helping patients with physical and financial recovery. Whether the spectacular innovations identified by the medical community in COVID-19 prevention and treatment of acute illness can be found for long COVID remains to be seen. The fact that so many patients studied by Iwashyna et al did not receive home care services and experienced financial toxicity shows the importance of broader implementation of systems and services to support survivors of COVID-19 hospitalization. Developers of this support must emphasize the importance of physical and cardiopulmonary rehabilitation as well as financial relief, particularly for minorities. For our patients and their families, this may be the best strategy to get “back to normal.”
Acknowledgment
The authors thank Dr Vineet Arora for reviewing and advising on this manuscript.
1. Iwashyna TJ, Kamphuis LA, Gundel SJ, et al. Continuing cardiopulmonary symptoms, disability, and financial toxicity 1 month after hospitalization for third-wave COVID-19: early results from a US nationwide cohort. J Hosp Med. 2021;16(9):531-537. https://doi.org/10.12788/jhm.3660
2. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. https://doi.org/10.7326/M20-5661
3. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated July 16, 2021. Accessed August 19, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
4. Robert Wood Johnson Foundation, NPR, Harvard T.H. Chan School of Public Health. The impact of coronavirus on households by race/ethnicity. September 2020. Accessed July 28, 2021. https://www.rwjf.org/en/library/research/2020/09/the-impact-of-coronavirus-on-households-across-america.html
5. Barber C. The problem of ‘long haul’ COVID. December 29, 2020. Accessed July 28, 2021. https://www.scientificamerican.com/article/the-problem-of-long-haul-covid/
As our understanding of SARS-CoV-2 has progressed, researchers, clinicians, and patients have learned that recovery from COVID-19 can last well beyond the acute phase of the illness. As we see fewer fatal cases and more survivors, studies that characterize the postacute sequelae of COVID-19 (PASC) are increasingly important for understanding how to help patients return to their normal lives, especially after hospitalization. Critical to investigating this is knowing patients’ burden of symptoms and disabilities prior to infection. In this issue, a study by Iwashyna et al1 helps us understand patients’ lives after COVID compared to their lives before COVID.
The study analyzed patients with SARS-CoV-2 infection admitted during the third wave of the pandemic to assess for new cardiopulmonary symptoms, new disability, and financial toxicity of hospitalization 1 month after discharge.1 Many patients had new cardiopulmonary symptoms and oxygen use, and a much larger number had new limitations in activities of daily living (ADLs) or instrumental activities of daily living (iADLs). The majority were discharged home without home care services, and new limitations in ADLs or iADLs were common in these cases. Most patients reported not having returned to their cardiopulmonary or functional baseline; however, new cough, shortness of breath, or oxygen use usually did not explain their new disabilities. Financial toxicity was also common, reflecting the effects of COVID-19 on both employment and family finances.
These results complement those of Chopra et al,2 who examined 60-day outcomes for patients hospitalized during the first wave of the pandemic. At 2 months from discharge, many patients had ongoing cough, shortness of breath, oxygen use, and disability, but at lower rates. This likely reflects continuing recovery during the extra 30 days, but other potential explanations deserve consideration. One possibility is improving survival over the course of the pandemic. Many patients who may have passed away earlier in the pandemic now survive to return home, albeit with a heavy burden of symptomatology. This raises the possibility that symptoms among survivors may continue to increase as survival of COVID-19 improves. However, it should be noted that neither study is representative of the national patterns of hospitalization by race or ethnicity.3 Iwashyna et al1 underrepresented Black patients, while Chopra et al2 underrepresented Hispanic patients. Given what we know about outcomes for these populations and their underrepresentation in PASC literature, the impact of COVID-19 for them is likely underestimated. As data from 3, 6, or 12 months become available, we may also see the effect sizes described in this early literature become even larger.
Consistent with the findings of Chopra et al,2 financial toxicity after COVID-19 hospitalization was high. The longer-term financial burden of COVID-19 will likely exceed what is described here, particularly for Black and Hispanic patients, who experienced a disproportionate drain on their savings. These populations are also more likely to be negatively impacted by the COVID economy4 and thus may suffer a “double hit” financially if hospitalized.
Iwashyna et al1 underscore the urgent need for progress in understanding COVID “long-haulers”5 and helping patients with physical and financial recovery. Whether the spectacular innovations identified by the medical community in COVID-19 prevention and treatment of acute illness can be found for long COVID remains to be seen. The fact that so many patients studied by Iwashyna et al did not receive home care services and experienced financial toxicity shows the importance of broader implementation of systems and services to support survivors of COVID-19 hospitalization. Developers of this support must emphasize the importance of physical and cardiopulmonary rehabilitation as well as financial relief, particularly for minorities. For our patients and their families, this may be the best strategy to get “back to normal.”
Acknowledgment
The authors thank Dr Vineet Arora for reviewing and advising on this manuscript.
As our understanding of SARS-CoV-2 has progressed, researchers, clinicians, and patients have learned that recovery from COVID-19 can last well beyond the acute phase of the illness. As we see fewer fatal cases and more survivors, studies that characterize the postacute sequelae of COVID-19 (PASC) are increasingly important for understanding how to help patients return to their normal lives, especially after hospitalization. Critical to investigating this is knowing patients’ burden of symptoms and disabilities prior to infection. In this issue, a study by Iwashyna et al1 helps us understand patients’ lives after COVID compared to their lives before COVID.
The study analyzed patients with SARS-CoV-2 infection admitted during the third wave of the pandemic to assess for new cardiopulmonary symptoms, new disability, and financial toxicity of hospitalization 1 month after discharge.1 Many patients had new cardiopulmonary symptoms and oxygen use, and a much larger number had new limitations in activities of daily living (ADLs) or instrumental activities of daily living (iADLs). The majority were discharged home without home care services, and new limitations in ADLs or iADLs were common in these cases. Most patients reported not having returned to their cardiopulmonary or functional baseline; however, new cough, shortness of breath, or oxygen use usually did not explain their new disabilities. Financial toxicity was also common, reflecting the effects of COVID-19 on both employment and family finances.
These results complement those of Chopra et al,2 who examined 60-day outcomes for patients hospitalized during the first wave of the pandemic. At 2 months from discharge, many patients had ongoing cough, shortness of breath, oxygen use, and disability, but at lower rates. This likely reflects continuing recovery during the extra 30 days, but other potential explanations deserve consideration. One possibility is improving survival over the course of the pandemic. Many patients who may have passed away earlier in the pandemic now survive to return home, albeit with a heavy burden of symptomatology. This raises the possibility that symptoms among survivors may continue to increase as survival of COVID-19 improves. However, it should be noted that neither study is representative of the national patterns of hospitalization by race or ethnicity.3 Iwashyna et al1 underrepresented Black patients, while Chopra et al2 underrepresented Hispanic patients. Given what we know about outcomes for these populations and their underrepresentation in PASC literature, the impact of COVID-19 for them is likely underestimated. As data from 3, 6, or 12 months become available, we may also see the effect sizes described in this early literature become even larger.
Consistent with the findings of Chopra et al,2 financial toxicity after COVID-19 hospitalization was high. The longer-term financial burden of COVID-19 will likely exceed what is described here, particularly for Black and Hispanic patients, who experienced a disproportionate drain on their savings. These populations are also more likely to be negatively impacted by the COVID economy4 and thus may suffer a “double hit” financially if hospitalized.
Iwashyna et al1 underscore the urgent need for progress in understanding COVID “long-haulers”5 and helping patients with physical and financial recovery. Whether the spectacular innovations identified by the medical community in COVID-19 prevention and treatment of acute illness can be found for long COVID remains to be seen. The fact that so many patients studied by Iwashyna et al did not receive home care services and experienced financial toxicity shows the importance of broader implementation of systems and services to support survivors of COVID-19 hospitalization. Developers of this support must emphasize the importance of physical and cardiopulmonary rehabilitation as well as financial relief, particularly for minorities. For our patients and their families, this may be the best strategy to get “back to normal.”
Acknowledgment
The authors thank Dr Vineet Arora for reviewing and advising on this manuscript.
1. Iwashyna TJ, Kamphuis LA, Gundel SJ, et al. Continuing cardiopulmonary symptoms, disability, and financial toxicity 1 month after hospitalization for third-wave COVID-19: early results from a US nationwide cohort. J Hosp Med. 2021;16(9):531-537. https://doi.org/10.12788/jhm.3660
2. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. https://doi.org/10.7326/M20-5661
3. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated July 16, 2021. Accessed August 19, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
4. Robert Wood Johnson Foundation, NPR, Harvard T.H. Chan School of Public Health. The impact of coronavirus on households by race/ethnicity. September 2020. Accessed July 28, 2021. https://www.rwjf.org/en/library/research/2020/09/the-impact-of-coronavirus-on-households-across-america.html
5. Barber C. The problem of ‘long haul’ COVID. December 29, 2020. Accessed July 28, 2021. https://www.scientificamerican.com/article/the-problem-of-long-haul-covid/
1. Iwashyna TJ, Kamphuis LA, Gundel SJ, et al. Continuing cardiopulmonary symptoms, disability, and financial toxicity 1 month after hospitalization for third-wave COVID-19: early results from a US nationwide cohort. J Hosp Med. 2021;16(9):531-537. https://doi.org/10.12788/jhm.3660
2. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. https://doi.org/10.7326/M20-5661
3. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated July 16, 2021. Accessed August 19, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
4. Robert Wood Johnson Foundation, NPR, Harvard T.H. Chan School of Public Health. The impact of coronavirus on households by race/ethnicity. September 2020. Accessed July 28, 2021. https://www.rwjf.org/en/library/research/2020/09/the-impact-of-coronavirus-on-households-across-america.html
5. Barber C. The problem of ‘long haul’ COVID. December 29, 2020. Accessed July 28, 2021. https://www.scientificamerican.com/article/the-problem-of-long-haul-covid/
© 2021 Society of Hospital Medicine
Leveraging the Care Team to Optimize Disposition Planning
Is this patient a good candidate? In medicine, we subconsciously answer this question for every clinical decision we make. Occasionally, though, a clinical scenario is so complex that it cannot or should not be answered by a single individual. One example is the decision on whether a patient should receive an organ transplant. In this situation, a multidisciplinary committee weighs the complex ethical, clinical, and financial implications of the decision before coming to a verdict. Together, team members discuss the risks and benefits of each patient’s candidacy and, in a united fashion, decide the best course of care. For hospitalists, a far more common question occurs every day and is similarly fraught with multifaceted implications: Is my patient a good candidate for a skilled nursing facility (SNF)? We often rely on a single individual to make the final call, but should we instead be leveraging the expertise of other care team members to assist with this decision?
In this issue, Boyle et al1 describe the implementation of a multidisciplinary team consisting of physicians, case managers, social workers, physical and occupational therapists, and home-health representatives that reviewed all patients with an expected discharge to a SNF. Case managers or social workers began the process by referring eligible patients to the committee for review. If deemed appropriate, the committee discussed each case and reached a consensus recommendation as to whether a SNF was an appropriate discharge destination. The investigators used a matched, preintervention sample as a comparison group, with a primary outcome of total discharges to SNFs, and secondary outcomes consisting of readmissions, time to readmission, and median length of stay. The authors observed a 49.7% relative reduction in total SNF discharges (25.5% of preintervention patients discharged to a SNF vs 12.8% postintervention), as well as a 66.9% relative reduction in new SNF discharges. Despite the significant reduction in SNF utilization, no differences were noted in readmissions, time to readmission, or readmission length of stay.
While this study was performed during the COVID-19 pandemic, several characteristics make its findings applicable beyond this period. First, the structure and workflow of the team are extensively detailed and make the intervention easily generalizable to most hospitals. Second, while not specifically examined, the outcome of SNF reduction likely corresponds to an increase in the patient’s time at home—an important patient-centered target for most posthospitalization plans.2 Finally, the intervention used existing infrastructure and individuals, and did not require new resources to improve patient care, which increases the feasibility of implementation at other institutions.
These findings also reveal potential overutilization of SNFs in the discharge process. On average, a typical SNF stay costs the health system more than $11,000.3 A simple intervention could lead to substantial savings for individuals and the healthcare system. With a nearly 50% reduction in SNF use, understanding why patients who were eligible to go home were ultimately discharged to a SNF will be a crucial question to answer. Are there barriers to patient or family education? Is there a perceived safety difference between a SNF and home for nonskilled nursing needs? Additionally, care should be taken to ensure that decreases in SNF utilization do not disproportionately affect certain populations. Further work should assess the performance of similar models in a non-COVID era and among multiple institutions to verify potential scalability and generalizability.
Like organ transplant committees, Boyle et al’s multidisciplinary approach to reduce SNF discharges had to include thoughtful and intentional decisions. Perhaps it is time we use this same model to transplant patients back into their homes as safely and efficiently as possible.
1. Boyle CA, Ravichandran U, Hankamp V, et al. Safe transitions and congregate living in the age of COVID-19: a retrospective cohort study. J Hosp Med. 2021;16(9):524-530. https://doi.org/10.12788/jhm.3657
2. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. https://doi.org/10.1056/NEJMp1703423
3. Werner RM, Coe NB, Qi M, Konetzka RT. Patient outcomes after hospital discharge to home with home health care vs to a skilled nursing facility. JAMA Intern Med. 2019;179(5):617-623. https://doi.org/10.1001/jamainternmed.2018.7998
Is this patient a good candidate? In medicine, we subconsciously answer this question for every clinical decision we make. Occasionally, though, a clinical scenario is so complex that it cannot or should not be answered by a single individual. One example is the decision on whether a patient should receive an organ transplant. In this situation, a multidisciplinary committee weighs the complex ethical, clinical, and financial implications of the decision before coming to a verdict. Together, team members discuss the risks and benefits of each patient’s candidacy and, in a united fashion, decide the best course of care. For hospitalists, a far more common question occurs every day and is similarly fraught with multifaceted implications: Is my patient a good candidate for a skilled nursing facility (SNF)? We often rely on a single individual to make the final call, but should we instead be leveraging the expertise of other care team members to assist with this decision?
In this issue, Boyle et al1 describe the implementation of a multidisciplinary team consisting of physicians, case managers, social workers, physical and occupational therapists, and home-health representatives that reviewed all patients with an expected discharge to a SNF. Case managers or social workers began the process by referring eligible patients to the committee for review. If deemed appropriate, the committee discussed each case and reached a consensus recommendation as to whether a SNF was an appropriate discharge destination. The investigators used a matched, preintervention sample as a comparison group, with a primary outcome of total discharges to SNFs, and secondary outcomes consisting of readmissions, time to readmission, and median length of stay. The authors observed a 49.7% relative reduction in total SNF discharges (25.5% of preintervention patients discharged to a SNF vs 12.8% postintervention), as well as a 66.9% relative reduction in new SNF discharges. Despite the significant reduction in SNF utilization, no differences were noted in readmissions, time to readmission, or readmission length of stay.
While this study was performed during the COVID-19 pandemic, several characteristics make its findings applicable beyond this period. First, the structure and workflow of the team are extensively detailed and make the intervention easily generalizable to most hospitals. Second, while not specifically examined, the outcome of SNF reduction likely corresponds to an increase in the patient’s time at home—an important patient-centered target for most posthospitalization plans.2 Finally, the intervention used existing infrastructure and individuals, and did not require new resources to improve patient care, which increases the feasibility of implementation at other institutions.
These findings also reveal potential overutilization of SNFs in the discharge process. On average, a typical SNF stay costs the health system more than $11,000.3 A simple intervention could lead to substantial savings for individuals and the healthcare system. With a nearly 50% reduction in SNF use, understanding why patients who were eligible to go home were ultimately discharged to a SNF will be a crucial question to answer. Are there barriers to patient or family education? Is there a perceived safety difference between a SNF and home for nonskilled nursing needs? Additionally, care should be taken to ensure that decreases in SNF utilization do not disproportionately affect certain populations. Further work should assess the performance of similar models in a non-COVID era and among multiple institutions to verify potential scalability and generalizability.
Like organ transplant committees, Boyle et al’s multidisciplinary approach to reduce SNF discharges had to include thoughtful and intentional decisions. Perhaps it is time we use this same model to transplant patients back into their homes as safely and efficiently as possible.
Is this patient a good candidate? In medicine, we subconsciously answer this question for every clinical decision we make. Occasionally, though, a clinical scenario is so complex that it cannot or should not be answered by a single individual. One example is the decision on whether a patient should receive an organ transplant. In this situation, a multidisciplinary committee weighs the complex ethical, clinical, and financial implications of the decision before coming to a verdict. Together, team members discuss the risks and benefits of each patient’s candidacy and, in a united fashion, decide the best course of care. For hospitalists, a far more common question occurs every day and is similarly fraught with multifaceted implications: Is my patient a good candidate for a skilled nursing facility (SNF)? We often rely on a single individual to make the final call, but should we instead be leveraging the expertise of other care team members to assist with this decision?
In this issue, Boyle et al1 describe the implementation of a multidisciplinary team consisting of physicians, case managers, social workers, physical and occupational therapists, and home-health representatives that reviewed all patients with an expected discharge to a SNF. Case managers or social workers began the process by referring eligible patients to the committee for review. If deemed appropriate, the committee discussed each case and reached a consensus recommendation as to whether a SNF was an appropriate discharge destination. The investigators used a matched, preintervention sample as a comparison group, with a primary outcome of total discharges to SNFs, and secondary outcomes consisting of readmissions, time to readmission, and median length of stay. The authors observed a 49.7% relative reduction in total SNF discharges (25.5% of preintervention patients discharged to a SNF vs 12.8% postintervention), as well as a 66.9% relative reduction in new SNF discharges. Despite the significant reduction in SNF utilization, no differences were noted in readmissions, time to readmission, or readmission length of stay.
While this study was performed during the COVID-19 pandemic, several characteristics make its findings applicable beyond this period. First, the structure and workflow of the team are extensively detailed and make the intervention easily generalizable to most hospitals. Second, while not specifically examined, the outcome of SNF reduction likely corresponds to an increase in the patient’s time at home—an important patient-centered target for most posthospitalization plans.2 Finally, the intervention used existing infrastructure and individuals, and did not require new resources to improve patient care, which increases the feasibility of implementation at other institutions.
These findings also reveal potential overutilization of SNFs in the discharge process. On average, a typical SNF stay costs the health system more than $11,000.3 A simple intervention could lead to substantial savings for individuals and the healthcare system. With a nearly 50% reduction in SNF use, understanding why patients who were eligible to go home were ultimately discharged to a SNF will be a crucial question to answer. Are there barriers to patient or family education? Is there a perceived safety difference between a SNF and home for nonskilled nursing needs? Additionally, care should be taken to ensure that decreases in SNF utilization do not disproportionately affect certain populations. Further work should assess the performance of similar models in a non-COVID era and among multiple institutions to verify potential scalability and generalizability.
Like organ transplant committees, Boyle et al’s multidisciplinary approach to reduce SNF discharges had to include thoughtful and intentional decisions. Perhaps it is time we use this same model to transplant patients back into their homes as safely and efficiently as possible.
1. Boyle CA, Ravichandran U, Hankamp V, et al. Safe transitions and congregate living in the age of COVID-19: a retrospective cohort study. J Hosp Med. 2021;16(9):524-530. https://doi.org/10.12788/jhm.3657
2. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. https://doi.org/10.1056/NEJMp1703423
3. Werner RM, Coe NB, Qi M, Konetzka RT. Patient outcomes after hospital discharge to home with home health care vs to a skilled nursing facility. JAMA Intern Med. 2019;179(5):617-623. https://doi.org/10.1001/jamainternmed.2018.7998
1. Boyle CA, Ravichandran U, Hankamp V, et al. Safe transitions and congregate living in the age of COVID-19: a retrospective cohort study. J Hosp Med. 2021;16(9):524-530. https://doi.org/10.12788/jhm.3657
2. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. https://doi.org/10.1056/NEJMp1703423
3. Werner RM, Coe NB, Qi M, Konetzka RT. Patient outcomes after hospital discharge to home with home health care vs to a skilled nursing facility. JAMA Intern Med. 2019;179(5):617-623. https://doi.org/10.1001/jamainternmed.2018.7998
© 2021 Society of Hospital Medicine
A More Intentional Analysis of Race and Racism in Research
Earlier this year, the Journal of Hospital Medicine updated its author guidelines to include recommendations on addressing race and racism.1 These recommendations include explicitly naming racism (rather than race) as a determinant of health. Operationalizing these recommendations into manuscripts represents a fundamental shift in how we ask research questions, structure analyses, and interpret results.
In this issue, Maxwell et al2 illustrate how to disseminate research through this lens in their retrospective cohort study of children with type 1 diabetes hospitalized with diabetic ketoacidosis (DKA). Using 6 years of data from a major academic pediatric medical center, the authors examine the association between risk for DKA admission and three factors: neighborhood poverty level, race, and type of insurance (public or private). Secondary outcomes include DKA severity and length of stay. In their unadjusted model, poverty, race, and insurance were all associated with increased hospitalizations. However, following adjustment, the association between race and hospitalizations disappeared.In line with the journal’s new guidelines, the authors point out that the statistically significant associations of poverty and insurance type with clinical outcomes suggest that racism, rather than race, is a social factor at work in their population. The authors provide further context regarding structural racism in the United States and the history of redlining, which has helped shape a society in which Black individuals are more likely to live in areas of concentrated poverty and be publicly insured.
Two other findings related to the impact of racism are notable. First, in both their univariate and multivariate models, the authors found significant A1c differences between Black and White children—higher than those of previous reports.3 These findings suggest the existence of structural factors at work in the health of their patients. Second, Black patients had longer lengths of stay when compared to White patients with the same severity of DKA. Neither poverty level nor insurance status were significantly associated with length of stay. While the analysis was limited to detecting this difference, rather than identifying its causes, the authors suggest factors at both individual and structural levels that may be impacting outcomes. Specifically, care team bias may impact discharge decisions, and factors such as less flexible times to complete diabetes education, transportation barriers, and childcare challenges could also impact discharge timing.
This work provides a template for how to address the impact of racism on health with intentionality. Moreover, individuals’ lived environments should be considered through alternative economic measurements and neighborhood definitions. The proportion of people within a census tract living below the federal poverty line is just one measure of the complex dynamics that contribute to an individual’s socioeconomic status. An alternative measure is the area deprivation index, which incorporates 17 indicators at the more granular census block group level to describe an individual’s environment4 and could be useful in this area of research.
Perhaps most relevant is the use of public insurance as a marker of socioeconomic status. Medicaid, although not without its flaws, provides fairly comprehensive coverage. However, many Americans have incomes too high to qualify for public insurance but too low to afford adequate insurance coverage. Theoretically, these individuals qualify for subsidies through the Affordable Care Act, yet underinsurance remains a significant issue.5 Future analyses to further understand and describe clinical outcomes could include this population of underinsured children as a distinct at-risk group. Maxwell et al2 provide an excellent example of how we should address race and racism in disseminated literature. Although initially challenging, writing with intentionality regarding this fundamental determinant of health can provide rich and actionable information for practitioners and policy-makers.
1. Andrews AL, Unaka N, Shah SS. New author guidelines for addressing race and racism in the Journal of Hospital Medicine. J Hosp Med. 2021;16(4):197. https://doi.org/10.12788/jhm.3598
2. Maxwell AR, Jones NHY, Taylor S, et al. Socioeconomic and racial disparities in diabetic ketoacidosis admissions in youth with type 1 diabetes. J Hosp Med. 2021;16(9):517-523. https://doi.org/10.12788/jhm.3664
3. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. https://doi.org/10.7326/M16-2596
4. Kind AJH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30 day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014(11);161:765-774. https://doi.org/10.7326/M13-2946
5. Strane D, Rosenquist R, Rubin D. Leveraging health care reform to address underinsurance in working families. Health Affairs. June 15, 2021. Accessed August 23, 2021. www.healthaffairs.org/do/10.1377/hblog20210611.153918/full/
Earlier this year, the Journal of Hospital Medicine updated its author guidelines to include recommendations on addressing race and racism.1 These recommendations include explicitly naming racism (rather than race) as a determinant of health. Operationalizing these recommendations into manuscripts represents a fundamental shift in how we ask research questions, structure analyses, and interpret results.
In this issue, Maxwell et al2 illustrate how to disseminate research through this lens in their retrospective cohort study of children with type 1 diabetes hospitalized with diabetic ketoacidosis (DKA). Using 6 years of data from a major academic pediatric medical center, the authors examine the association between risk for DKA admission and three factors: neighborhood poverty level, race, and type of insurance (public or private). Secondary outcomes include DKA severity and length of stay. In their unadjusted model, poverty, race, and insurance were all associated with increased hospitalizations. However, following adjustment, the association between race and hospitalizations disappeared.In line with the journal’s new guidelines, the authors point out that the statistically significant associations of poverty and insurance type with clinical outcomes suggest that racism, rather than race, is a social factor at work in their population. The authors provide further context regarding structural racism in the United States and the history of redlining, which has helped shape a society in which Black individuals are more likely to live in areas of concentrated poverty and be publicly insured.
Two other findings related to the impact of racism are notable. First, in both their univariate and multivariate models, the authors found significant A1c differences between Black and White children—higher than those of previous reports.3 These findings suggest the existence of structural factors at work in the health of their patients. Second, Black patients had longer lengths of stay when compared to White patients with the same severity of DKA. Neither poverty level nor insurance status were significantly associated with length of stay. While the analysis was limited to detecting this difference, rather than identifying its causes, the authors suggest factors at both individual and structural levels that may be impacting outcomes. Specifically, care team bias may impact discharge decisions, and factors such as less flexible times to complete diabetes education, transportation barriers, and childcare challenges could also impact discharge timing.
This work provides a template for how to address the impact of racism on health with intentionality. Moreover, individuals’ lived environments should be considered through alternative economic measurements and neighborhood definitions. The proportion of people within a census tract living below the federal poverty line is just one measure of the complex dynamics that contribute to an individual’s socioeconomic status. An alternative measure is the area deprivation index, which incorporates 17 indicators at the more granular census block group level to describe an individual’s environment4 and could be useful in this area of research.
Perhaps most relevant is the use of public insurance as a marker of socioeconomic status. Medicaid, although not without its flaws, provides fairly comprehensive coverage. However, many Americans have incomes too high to qualify for public insurance but too low to afford adequate insurance coverage. Theoretically, these individuals qualify for subsidies through the Affordable Care Act, yet underinsurance remains a significant issue.5 Future analyses to further understand and describe clinical outcomes could include this population of underinsured children as a distinct at-risk group. Maxwell et al2 provide an excellent example of how we should address race and racism in disseminated literature. Although initially challenging, writing with intentionality regarding this fundamental determinant of health can provide rich and actionable information for practitioners and policy-makers.
Earlier this year, the Journal of Hospital Medicine updated its author guidelines to include recommendations on addressing race and racism.1 These recommendations include explicitly naming racism (rather than race) as a determinant of health. Operationalizing these recommendations into manuscripts represents a fundamental shift in how we ask research questions, structure analyses, and interpret results.
In this issue, Maxwell et al2 illustrate how to disseminate research through this lens in their retrospective cohort study of children with type 1 diabetes hospitalized with diabetic ketoacidosis (DKA). Using 6 years of data from a major academic pediatric medical center, the authors examine the association between risk for DKA admission and three factors: neighborhood poverty level, race, and type of insurance (public or private). Secondary outcomes include DKA severity and length of stay. In their unadjusted model, poverty, race, and insurance were all associated with increased hospitalizations. However, following adjustment, the association between race and hospitalizations disappeared.In line with the journal’s new guidelines, the authors point out that the statistically significant associations of poverty and insurance type with clinical outcomes suggest that racism, rather than race, is a social factor at work in their population. The authors provide further context regarding structural racism in the United States and the history of redlining, which has helped shape a society in which Black individuals are more likely to live in areas of concentrated poverty and be publicly insured.
Two other findings related to the impact of racism are notable. First, in both their univariate and multivariate models, the authors found significant A1c differences between Black and White children—higher than those of previous reports.3 These findings suggest the existence of structural factors at work in the health of their patients. Second, Black patients had longer lengths of stay when compared to White patients with the same severity of DKA. Neither poverty level nor insurance status were significantly associated with length of stay. While the analysis was limited to detecting this difference, rather than identifying its causes, the authors suggest factors at both individual and structural levels that may be impacting outcomes. Specifically, care team bias may impact discharge decisions, and factors such as less flexible times to complete diabetes education, transportation barriers, and childcare challenges could also impact discharge timing.
This work provides a template for how to address the impact of racism on health with intentionality. Moreover, individuals’ lived environments should be considered through alternative economic measurements and neighborhood definitions. The proportion of people within a census tract living below the federal poverty line is just one measure of the complex dynamics that contribute to an individual’s socioeconomic status. An alternative measure is the area deprivation index, which incorporates 17 indicators at the more granular census block group level to describe an individual’s environment4 and could be useful in this area of research.
Perhaps most relevant is the use of public insurance as a marker of socioeconomic status. Medicaid, although not without its flaws, provides fairly comprehensive coverage. However, many Americans have incomes too high to qualify for public insurance but too low to afford adequate insurance coverage. Theoretically, these individuals qualify for subsidies through the Affordable Care Act, yet underinsurance remains a significant issue.5 Future analyses to further understand and describe clinical outcomes could include this population of underinsured children as a distinct at-risk group. Maxwell et al2 provide an excellent example of how we should address race and racism in disseminated literature. Although initially challenging, writing with intentionality regarding this fundamental determinant of health can provide rich and actionable information for practitioners and policy-makers.
1. Andrews AL, Unaka N, Shah SS. New author guidelines for addressing race and racism in the Journal of Hospital Medicine. J Hosp Med. 2021;16(4):197. https://doi.org/10.12788/jhm.3598
2. Maxwell AR, Jones NHY, Taylor S, et al. Socioeconomic and racial disparities in diabetic ketoacidosis admissions in youth with type 1 diabetes. J Hosp Med. 2021;16(9):517-523. https://doi.org/10.12788/jhm.3664
3. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. https://doi.org/10.7326/M16-2596
4. Kind AJH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30 day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014(11);161:765-774. https://doi.org/10.7326/M13-2946
5. Strane D, Rosenquist R, Rubin D. Leveraging health care reform to address underinsurance in working families. Health Affairs. June 15, 2021. Accessed August 23, 2021. www.healthaffairs.org/do/10.1377/hblog20210611.153918/full/
1. Andrews AL, Unaka N, Shah SS. New author guidelines for addressing race and racism in the Journal of Hospital Medicine. J Hosp Med. 2021;16(4):197. https://doi.org/10.12788/jhm.3598
2. Maxwell AR, Jones NHY, Taylor S, et al. Socioeconomic and racial disparities in diabetic ketoacidosis admissions in youth with type 1 diabetes. J Hosp Med. 2021;16(9):517-523. https://doi.org/10.12788/jhm.3664
3. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. https://doi.org/10.7326/M16-2596
4. Kind AJH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30 day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014(11);161:765-774. https://doi.org/10.7326/M13-2946
5. Strane D, Rosenquist R, Rubin D. Leveraging health care reform to address underinsurance in working families. Health Affairs. June 15, 2021. Accessed August 23, 2021. www.healthaffairs.org/do/10.1377/hblog20210611.153918/full/
© 2021 Society of Hospital Medicine
A hot dog a day takes 36 minutes away
The death ‘dog’
Imagine you’re out in your backyard managing the grill for a big family barbecue. You’ve got a dazzling assortment of meat assorted on your fancy new propane grill, all charring nicely. Naturally, the hot dogs finish first, and as you pull them off, you figure you’ll help yourself to one now. After all, you are the chef, you deserve a reward. But, as you bite into your smoking hot sandwich, a cold, bony finger taps you on the shoulder. You turn and come face to face with the Grim Reaper. “YOU JUST LOST 36 MINUTES,” Death says. “ALSO, MAY I HAVE ONE OF THOSE? THEY LOOK DELICIOUS.”
Nonplussed and moving automatically, you scoop up another hot dog and place it in a bun. “WITH KETCHUP PLEASE,” Death says. “I NEVER CARED FOR MUSTARD.”
“I don’t understand,” you say. “Surely I won’t die at a family barbecue.”
“DO NOT CALL ME SHIRLEY,” Death says. “AND YOU WILL NOT. IT’S PART OF MY NEW CONTRACT.”
A new study, published in Nature Food, found that a person may lose up to 36 minutes for every hot dog consumed. Researchers from the University of Michigan analyzed nearly 6,000 different foods using a new nutritional index to quantify their health effects in minutes of healthy life lost or gained. Eating a serving of nuts adds an extra 26 minutes of life. The researchers determined that replacing just 10% of daily caloric intake from beef and processed foods with fruits, vegetables, and nuts can add 48 minutes per day. It would also reduce the daily carbon footprint by 33%.
“So you go around to everyone eating bad food and tell them how much life they’ve lost?” you ask when the Grim Reaper finishes his story. “Sounds like a drag.”
“IT IS. WE’VE HAD TO HIRE NEW BLOOD.” Death chuckles at its own bad pun. “NOW IF YOU’LL EXCUSE ME, I MUST CHASTISE A MAN IN FLORIDA FOR EATING A WELL-DONE STEAK.”
More stress, less sex
As the world becomes a more stressful place, the human population could face a 50% drop by the end of the century.
Think of stress as a one-two punch to the libido and human fertility. The more people are stressed out, the less likely they are to have quality interactions with others. Many of us would rather be alone with our wine and cheese to watch our favorite show.
Researchers have found that high stress levels have been known to drop sperm count, ovulation, and sexual activity. Guess what? There has been a 50% decrease in sperm counts over the last 50 years. That’s the second punch. But let’s not forget, the times are changing.
“Changes in reproductive behavior that contribute to the population drop include more young couples choosing to be ‘child-free,’ people having fewer children, and couples waiting longer to start families,” said Alexander Suvorov, PhD, of the University of Massachusetts, the paper’s author.
Let’s summarize: The more stress we’re dealing with, the less people want to deal with each other.
Who would have thought the future would be less fun?
‘You are not a horse. You are not a cow. Seriously, y’all. Stop it.’
WARNING: The following descriptions of COVID-19–related insanity may be offensive to some readers.
Greetings, ladies and gentlemen! Welcome to the first round of Pandemic Pandemonium. Let’s get right to the action.
This week’s preshow match-off involves face mask woes. The first comes to us from Alabama, where a woman wore a space helmet to a school board meeting to protest mask mandates. The second comes from Australia, in the form of mischievous magpies. We will explain.
It is not uncommon for magpies to attack those who come too close to their nests in the spring, or “swooping season,” as it’s affectionately called. The magpies are smart enough to recognize the faces of people they see regularly and not attack; however, it’s feared that mask wearing will change this.
While you’re chewing on that exciting appetizer, let’s take a look at our main course, which has a distinct governmental flavor. Jeff Landry is the attorney general of Louisiana, and, like our space-helmet wearer, he’s not a fan of mask mandates. According to Business Insider, Mr. Landry “drafted and distributed sample letters intended to help parents evade mask-wearing ordinances and COVID-19 vaccination requirements for their children in schools.”
Up against him is the Food and Drug Administration’s Twitter account. In an unrelated matter, the agency tweeted, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This was in response to people using the nonhuman forms of ivermectin to treat very human COVID-19.
Well, there you have it. Who will win tonight’s exciting edition of Pandemic Pandemonium? The first reader to contact us gets to decide the fate of these worthy contestants.
From venomous poison to heart drug
It’s not likely that anyone who sees a giant, venomous spider is thinking, “Hey! That thing could save my life!” It’s usually quite the opposite. Honestly, we would run away from just about any spider. But what if one of the deadliest spiders in the world could also save you from dying of a heart attack?
You probably don’t believe us, right? That’s fair, but the deadly Fraser Island (K’gari) funnel web spider, might also be the most helpful. Investigators from the University of Queensland in Australia have found a way to extract a molecule from the spider’s venom that might help stop damage from heart attacks and may even preserve hearts being used for transplants. “The Hi1a protein from spider venom blocks acid-sensing ion channels in the heart, so the death message is blocked, cell death is reduced, and we see improved heart cell survival,” Nathan Palpant, PhD, of the university, noted in a written statement.
No one has ever developed a drug to stop the “death signal,” so maybe it’s time to befriend spiders instead of running away from them in horror. Just leave the venom extraction to the professionals.
The death ‘dog’
Imagine you’re out in your backyard managing the grill for a big family barbecue. You’ve got a dazzling assortment of meat assorted on your fancy new propane grill, all charring nicely. Naturally, the hot dogs finish first, and as you pull them off, you figure you’ll help yourself to one now. After all, you are the chef, you deserve a reward. But, as you bite into your smoking hot sandwich, a cold, bony finger taps you on the shoulder. You turn and come face to face with the Grim Reaper. “YOU JUST LOST 36 MINUTES,” Death says. “ALSO, MAY I HAVE ONE OF THOSE? THEY LOOK DELICIOUS.”
Nonplussed and moving automatically, you scoop up another hot dog and place it in a bun. “WITH KETCHUP PLEASE,” Death says. “I NEVER CARED FOR MUSTARD.”
“I don’t understand,” you say. “Surely I won’t die at a family barbecue.”
“DO NOT CALL ME SHIRLEY,” Death says. “AND YOU WILL NOT. IT’S PART OF MY NEW CONTRACT.”
A new study, published in Nature Food, found that a person may lose up to 36 minutes for every hot dog consumed. Researchers from the University of Michigan analyzed nearly 6,000 different foods using a new nutritional index to quantify their health effects in minutes of healthy life lost or gained. Eating a serving of nuts adds an extra 26 minutes of life. The researchers determined that replacing just 10% of daily caloric intake from beef and processed foods with fruits, vegetables, and nuts can add 48 minutes per day. It would also reduce the daily carbon footprint by 33%.
“So you go around to everyone eating bad food and tell them how much life they’ve lost?” you ask when the Grim Reaper finishes his story. “Sounds like a drag.”
“IT IS. WE’VE HAD TO HIRE NEW BLOOD.” Death chuckles at its own bad pun. “NOW IF YOU’LL EXCUSE ME, I MUST CHASTISE A MAN IN FLORIDA FOR EATING A WELL-DONE STEAK.”
More stress, less sex
As the world becomes a more stressful place, the human population could face a 50% drop by the end of the century.
Think of stress as a one-two punch to the libido and human fertility. The more people are stressed out, the less likely they are to have quality interactions with others. Many of us would rather be alone with our wine and cheese to watch our favorite show.
Researchers have found that high stress levels have been known to drop sperm count, ovulation, and sexual activity. Guess what? There has been a 50% decrease in sperm counts over the last 50 years. That’s the second punch. But let’s not forget, the times are changing.
“Changes in reproductive behavior that contribute to the population drop include more young couples choosing to be ‘child-free,’ people having fewer children, and couples waiting longer to start families,” said Alexander Suvorov, PhD, of the University of Massachusetts, the paper’s author.
Let’s summarize: The more stress we’re dealing with, the less people want to deal with each other.
Who would have thought the future would be less fun?
‘You are not a horse. You are not a cow. Seriously, y’all. Stop it.’
WARNING: The following descriptions of COVID-19–related insanity may be offensive to some readers.
Greetings, ladies and gentlemen! Welcome to the first round of Pandemic Pandemonium. Let’s get right to the action.
This week’s preshow match-off involves face mask woes. The first comes to us from Alabama, where a woman wore a space helmet to a school board meeting to protest mask mandates. The second comes from Australia, in the form of mischievous magpies. We will explain.
It is not uncommon for magpies to attack those who come too close to their nests in the spring, or “swooping season,” as it’s affectionately called. The magpies are smart enough to recognize the faces of people they see regularly and not attack; however, it’s feared that mask wearing will change this.
While you’re chewing on that exciting appetizer, let’s take a look at our main course, which has a distinct governmental flavor. Jeff Landry is the attorney general of Louisiana, and, like our space-helmet wearer, he’s not a fan of mask mandates. According to Business Insider, Mr. Landry “drafted and distributed sample letters intended to help parents evade mask-wearing ordinances and COVID-19 vaccination requirements for their children in schools.”
Up against him is the Food and Drug Administration’s Twitter account. In an unrelated matter, the agency tweeted, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This was in response to people using the nonhuman forms of ivermectin to treat very human COVID-19.
Well, there you have it. Who will win tonight’s exciting edition of Pandemic Pandemonium? The first reader to contact us gets to decide the fate of these worthy contestants.
From venomous poison to heart drug
It’s not likely that anyone who sees a giant, venomous spider is thinking, “Hey! That thing could save my life!” It’s usually quite the opposite. Honestly, we would run away from just about any spider. But what if one of the deadliest spiders in the world could also save you from dying of a heart attack?
You probably don’t believe us, right? That’s fair, but the deadly Fraser Island (K’gari) funnel web spider, might also be the most helpful. Investigators from the University of Queensland in Australia have found a way to extract a molecule from the spider’s venom that might help stop damage from heart attacks and may even preserve hearts being used for transplants. “The Hi1a protein from spider venom blocks acid-sensing ion channels in the heart, so the death message is blocked, cell death is reduced, and we see improved heart cell survival,” Nathan Palpant, PhD, of the university, noted in a written statement.
No one has ever developed a drug to stop the “death signal,” so maybe it’s time to befriend spiders instead of running away from them in horror. Just leave the venom extraction to the professionals.
The death ‘dog’
Imagine you’re out in your backyard managing the grill for a big family barbecue. You’ve got a dazzling assortment of meat assorted on your fancy new propane grill, all charring nicely. Naturally, the hot dogs finish first, and as you pull them off, you figure you’ll help yourself to one now. After all, you are the chef, you deserve a reward. But, as you bite into your smoking hot sandwich, a cold, bony finger taps you on the shoulder. You turn and come face to face with the Grim Reaper. “YOU JUST LOST 36 MINUTES,” Death says. “ALSO, MAY I HAVE ONE OF THOSE? THEY LOOK DELICIOUS.”
Nonplussed and moving automatically, you scoop up another hot dog and place it in a bun. “WITH KETCHUP PLEASE,” Death says. “I NEVER CARED FOR MUSTARD.”
“I don’t understand,” you say. “Surely I won’t die at a family barbecue.”
“DO NOT CALL ME SHIRLEY,” Death says. “AND YOU WILL NOT. IT’S PART OF MY NEW CONTRACT.”
A new study, published in Nature Food, found that a person may lose up to 36 minutes for every hot dog consumed. Researchers from the University of Michigan analyzed nearly 6,000 different foods using a new nutritional index to quantify their health effects in minutes of healthy life lost or gained. Eating a serving of nuts adds an extra 26 minutes of life. The researchers determined that replacing just 10% of daily caloric intake from beef and processed foods with fruits, vegetables, and nuts can add 48 minutes per day. It would also reduce the daily carbon footprint by 33%.
“So you go around to everyone eating bad food and tell them how much life they’ve lost?” you ask when the Grim Reaper finishes his story. “Sounds like a drag.”
“IT IS. WE’VE HAD TO HIRE NEW BLOOD.” Death chuckles at its own bad pun. “NOW IF YOU’LL EXCUSE ME, I MUST CHASTISE A MAN IN FLORIDA FOR EATING A WELL-DONE STEAK.”
More stress, less sex
As the world becomes a more stressful place, the human population could face a 50% drop by the end of the century.
Think of stress as a one-two punch to the libido and human fertility. The more people are stressed out, the less likely they are to have quality interactions with others. Many of us would rather be alone with our wine and cheese to watch our favorite show.
Researchers have found that high stress levels have been known to drop sperm count, ovulation, and sexual activity. Guess what? There has been a 50% decrease in sperm counts over the last 50 years. That’s the second punch. But let’s not forget, the times are changing.
“Changes in reproductive behavior that contribute to the population drop include more young couples choosing to be ‘child-free,’ people having fewer children, and couples waiting longer to start families,” said Alexander Suvorov, PhD, of the University of Massachusetts, the paper’s author.
Let’s summarize: The more stress we’re dealing with, the less people want to deal with each other.
Who would have thought the future would be less fun?
‘You are not a horse. You are not a cow. Seriously, y’all. Stop it.’
WARNING: The following descriptions of COVID-19–related insanity may be offensive to some readers.
Greetings, ladies and gentlemen! Welcome to the first round of Pandemic Pandemonium. Let’s get right to the action.
This week’s preshow match-off involves face mask woes. The first comes to us from Alabama, where a woman wore a space helmet to a school board meeting to protest mask mandates. The second comes from Australia, in the form of mischievous magpies. We will explain.
It is not uncommon for magpies to attack those who come too close to their nests in the spring, or “swooping season,” as it’s affectionately called. The magpies are smart enough to recognize the faces of people they see regularly and not attack; however, it’s feared that mask wearing will change this.
While you’re chewing on that exciting appetizer, let’s take a look at our main course, which has a distinct governmental flavor. Jeff Landry is the attorney general of Louisiana, and, like our space-helmet wearer, he’s not a fan of mask mandates. According to Business Insider, Mr. Landry “drafted and distributed sample letters intended to help parents evade mask-wearing ordinances and COVID-19 vaccination requirements for their children in schools.”
Up against him is the Food and Drug Administration’s Twitter account. In an unrelated matter, the agency tweeted, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This was in response to people using the nonhuman forms of ivermectin to treat very human COVID-19.
Well, there you have it. Who will win tonight’s exciting edition of Pandemic Pandemonium? The first reader to contact us gets to decide the fate of these worthy contestants.
From venomous poison to heart drug
It’s not likely that anyone who sees a giant, venomous spider is thinking, “Hey! That thing could save my life!” It’s usually quite the opposite. Honestly, we would run away from just about any spider. But what if one of the deadliest spiders in the world could also save you from dying of a heart attack?
You probably don’t believe us, right? That’s fair, but the deadly Fraser Island (K’gari) funnel web spider, might also be the most helpful. Investigators from the University of Queensland in Australia have found a way to extract a molecule from the spider’s venom that might help stop damage from heart attacks and may even preserve hearts being used for transplants. “The Hi1a protein from spider venom blocks acid-sensing ion channels in the heart, so the death message is blocked, cell death is reduced, and we see improved heart cell survival,” Nathan Palpant, PhD, of the university, noted in a written statement.
No one has ever developed a drug to stop the “death signal,” so maybe it’s time to befriend spiders instead of running away from them in horror. Just leave the venom extraction to the professionals.
Arm and neck rash
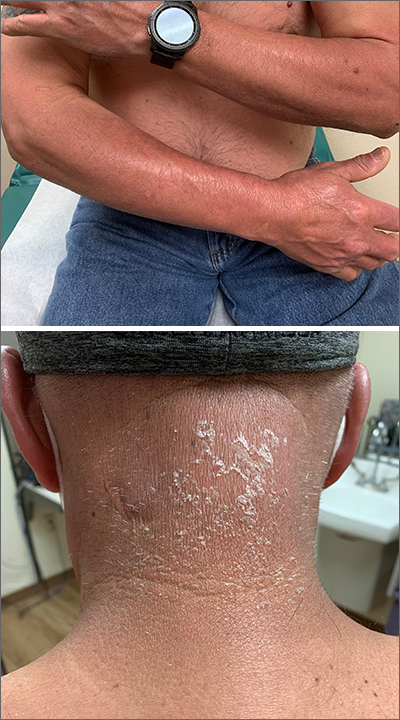
The manifestation of what appeared to be an exaggerated sunburn with associated pruritis and blistering (made worse after sun exposure), along with the recent initiation of HCTZ, led to the diagnosis of a phototoxic drug reaction.
There are 2 principal mechanisms of photosensitive drug reactions, phototoxic and photoallergic. Phototoxic reactions occur when the drug absorbs UVA light, causing a chemical reaction that produces either free radicals or stable photoproducts, both of which can cause DNA and tissue damage. This mechanism is more common; the time of onset between exposure and reaction is minutes to hours (as seen with this patient), and the reaction manifests with an exaggerated sunburn appearance.1
Photoallergic reactions typically occur by a delayed-immune response, mediated by photoallergic-specific T-cells reacting to photohaptens. This mechanism is less common, and the time of onset is greater than 24 hours. Photoallergic reactions often present as an eczematous dermatitis and can involve regions not directly exposed to sun.
In this case, the presumed offending agent—HCTZ—was discontinued, and the patient was advised to take sun-protective measures and use topical emollients, cool compresses, and oral analgesics for his acute symptoms. Photoallergic reactions look less like a discrete sunburn and can be treated symptomatically with topical or oral steroids. In addition, the implicated medication should be discontinued.
Text courtesy of Amanda Yaney, MD, and Daniel Stulberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Image courtesy of Daniel Stulberg, MD.
Lozzi F, Di Raimondo C, Lanna C, et al. Latest evidence regarding the effects of photosensitive drugs on the skin: pathogenic mechanisms and clinical manifestations. Pharmaceutics 2020;12:1104. doi: 10.3390/pharmaceutics12111104

The manifestation of what appeared to be an exaggerated sunburn with associated pruritis and blistering (made worse after sun exposure), along with the recent initiation of HCTZ, led to the diagnosis of a phototoxic drug reaction.
There are 2 principal mechanisms of photosensitive drug reactions, phototoxic and photoallergic. Phototoxic reactions occur when the drug absorbs UVA light, causing a chemical reaction that produces either free radicals or stable photoproducts, both of which can cause DNA and tissue damage. This mechanism is more common; the time of onset between exposure and reaction is minutes to hours (as seen with this patient), and the reaction manifests with an exaggerated sunburn appearance.1
Photoallergic reactions typically occur by a delayed-immune response, mediated by photoallergic-specific T-cells reacting to photohaptens. This mechanism is less common, and the time of onset is greater than 24 hours. Photoallergic reactions often present as an eczematous dermatitis and can involve regions not directly exposed to sun.
In this case, the presumed offending agent—HCTZ—was discontinued, and the patient was advised to take sun-protective measures and use topical emollients, cool compresses, and oral analgesics for his acute symptoms. Photoallergic reactions look less like a discrete sunburn and can be treated symptomatically with topical or oral steroids. In addition, the implicated medication should be discontinued.
Text courtesy of Amanda Yaney, MD, and Daniel Stulberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Image courtesy of Daniel Stulberg, MD.

The manifestation of what appeared to be an exaggerated sunburn with associated pruritis and blistering (made worse after sun exposure), along with the recent initiation of HCTZ, led to the diagnosis of a phototoxic drug reaction.
There are 2 principal mechanisms of photosensitive drug reactions, phototoxic and photoallergic. Phototoxic reactions occur when the drug absorbs UVA light, causing a chemical reaction that produces either free radicals or stable photoproducts, both of which can cause DNA and tissue damage. This mechanism is more common; the time of onset between exposure and reaction is minutes to hours (as seen with this patient), and the reaction manifests with an exaggerated sunburn appearance.1
Photoallergic reactions typically occur by a delayed-immune response, mediated by photoallergic-specific T-cells reacting to photohaptens. This mechanism is less common, and the time of onset is greater than 24 hours. Photoallergic reactions often present as an eczematous dermatitis and can involve regions not directly exposed to sun.
In this case, the presumed offending agent—HCTZ—was discontinued, and the patient was advised to take sun-protective measures and use topical emollients, cool compresses, and oral analgesics for his acute symptoms. Photoallergic reactions look less like a discrete sunburn and can be treated symptomatically with topical or oral steroids. In addition, the implicated medication should be discontinued.
Text courtesy of Amanda Yaney, MD, and Daniel Stulberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Image courtesy of Daniel Stulberg, MD.
Lozzi F, Di Raimondo C, Lanna C, et al. Latest evidence regarding the effects of photosensitive drugs on the skin: pathogenic mechanisms and clinical manifestations. Pharmaceutics 2020;12:1104. doi: 10.3390/pharmaceutics12111104
Lozzi F, Di Raimondo C, Lanna C, et al. Latest evidence regarding the effects of photosensitive drugs on the skin: pathogenic mechanisms and clinical manifestations. Pharmaceutics 2020;12:1104. doi: 10.3390/pharmaceutics12111104