User login
Prior biologic exposure no barrier for ozanimod in ulcerative colitis
The oral sphingosine-1-phosphate receptor modulator ozanimod is significantly more effective than placebo for treating patients with moderate to severe ulcerative colitis, regardless of prior biologic exposure, based on results of the phase 3 True North trial.
Although improvements were seen across all patients, those who had previously used biologics took slightly longer to respond to treatment, which suggests that any initial improvements with ozanimod warrant continuation of therapy to achieve full effect, reported to lead author Bruce E. Sands, MD, of Icahn School of Medicine at Mount Sinai, New York, and colleagues.
“[Ulcerative colitis] patients previously treated with a biologic agent may be less likely to respond to another advanced treatment,” Dr. Sands said during a presentation at the annual meeting of the American College of Gastroenterology.
Dr. Sands and colleagues evaluated this possibility in the True North trial, which previously demonstrated superior efficacy and safety of ozanimod over placebo through 1 year.
The present dataset included a double-blinded cohort of 639 patients (cohort 1), among whom 213 took placebo and 426 took ozanimod, and an open-label cohort of 353 patients who took ozanimod (cohort 2). Outcomes included clinical remission, clinical response, endoscopic improvement, and mucosal healing.
During induction (through week 10), biologic-naive patients in both cohorts generally responded better to ozanimod than those who had received at least one prior biologic. Patients who had received two or more biologics showed less improvement than those who had received only one prior biologic.
For example, in cohort 1, 53% of biologic-naive patients in the ozanimod group achieved a clinical response, compared with 50% of patients who had received one prior biologic, and just 27.2% of patients who had received two or more biologics.
With maintenance therapy, however, these differences faded. Among participants continuing ozanimod through week 52, 60.7% of biologic-naive patients achieved a clinical response, compared with 60.5% of those who had taken one prior biologic, and 55.3% of patients who had taken two or more prior biologics.
“Ozanimod treatment for up to 52 weeks in patients with moderate to severe ulcerative colitis improved clinical symptoms, mucosal ulcerations, and reduced cellular inflammation in both biologic-exposed and biologic-naive patients,” Dr. Sands concluded. “Greater efficacy was observed in biologic-naive patients, followed by patients with prior exposure to one biologic, at induction; however, all groups had benefits at end of maintenance. Patients with prior biologic use may require additional time to respond to treatment.”
Bradley Morganstern, MD, codirector of the IBD Center at Stony Brook (N.Y.) Medicine, called the study “a really important analysis” that addresses a common clinical decision.
“We often see a lower response rate among patients previously on biologics, but we have very limited data to guide us on how to choose a second-line agent,” Dr. Morganstern said during an interview.
He said the current findings encourage use of ozanimod for patients who didn’t respond to other biologics.
“The takeaway is that just because someone didn’t respond to a biologic in the past doesn’t mean they won’t respond to this,” Dr. Morganstern said. “They actually have a very good chance of responding – similar to the rest of the population that was never on medication.”
He went on to explain that exact treatment sequencing remains unclear, although ozanimod is a strong candidate in the second line.
“We can’t 100% say where it [ozanimod] fits in,” Dr. Morganstern said, “but we should certainly place it high up in the algorithm for patients who have failed a biologic. This should strongly be considered as a second-line agent.”
Dr. Morganstern said that he looks forward to long-term findings from True North beyond 1 year, “in terms of maintaining response,” and additional safety data.
The study was funded supported by Bristol-Myers Squibb. The investigators disclosed additional affiliations with AstraZeneca, AbbVie, Amgen, and others. Dr. Morganstern has previously spoken for Bristol-Myers Squibb.
The oral sphingosine-1-phosphate receptor modulator ozanimod is significantly more effective than placebo for treating patients with moderate to severe ulcerative colitis, regardless of prior biologic exposure, based on results of the phase 3 True North trial.
Although improvements were seen across all patients, those who had previously used biologics took slightly longer to respond to treatment, which suggests that any initial improvements with ozanimod warrant continuation of therapy to achieve full effect, reported to lead author Bruce E. Sands, MD, of Icahn School of Medicine at Mount Sinai, New York, and colleagues.
“[Ulcerative colitis] patients previously treated with a biologic agent may be less likely to respond to another advanced treatment,” Dr. Sands said during a presentation at the annual meeting of the American College of Gastroenterology.
Dr. Sands and colleagues evaluated this possibility in the True North trial, which previously demonstrated superior efficacy and safety of ozanimod over placebo through 1 year.
The present dataset included a double-blinded cohort of 639 patients (cohort 1), among whom 213 took placebo and 426 took ozanimod, and an open-label cohort of 353 patients who took ozanimod (cohort 2). Outcomes included clinical remission, clinical response, endoscopic improvement, and mucosal healing.
During induction (through week 10), biologic-naive patients in both cohorts generally responded better to ozanimod than those who had received at least one prior biologic. Patients who had received two or more biologics showed less improvement than those who had received only one prior biologic.
For example, in cohort 1, 53% of biologic-naive patients in the ozanimod group achieved a clinical response, compared with 50% of patients who had received one prior biologic, and just 27.2% of patients who had received two or more biologics.
With maintenance therapy, however, these differences faded. Among participants continuing ozanimod through week 52, 60.7% of biologic-naive patients achieved a clinical response, compared with 60.5% of those who had taken one prior biologic, and 55.3% of patients who had taken two or more prior biologics.
“Ozanimod treatment for up to 52 weeks in patients with moderate to severe ulcerative colitis improved clinical symptoms, mucosal ulcerations, and reduced cellular inflammation in both biologic-exposed and biologic-naive patients,” Dr. Sands concluded. “Greater efficacy was observed in biologic-naive patients, followed by patients with prior exposure to one biologic, at induction; however, all groups had benefits at end of maintenance. Patients with prior biologic use may require additional time to respond to treatment.”
Bradley Morganstern, MD, codirector of the IBD Center at Stony Brook (N.Y.) Medicine, called the study “a really important analysis” that addresses a common clinical decision.
“We often see a lower response rate among patients previously on biologics, but we have very limited data to guide us on how to choose a second-line agent,” Dr. Morganstern said during an interview.
He said the current findings encourage use of ozanimod for patients who didn’t respond to other biologics.
“The takeaway is that just because someone didn’t respond to a biologic in the past doesn’t mean they won’t respond to this,” Dr. Morganstern said. “They actually have a very good chance of responding – similar to the rest of the population that was never on medication.”
He went on to explain that exact treatment sequencing remains unclear, although ozanimod is a strong candidate in the second line.
“We can’t 100% say where it [ozanimod] fits in,” Dr. Morganstern said, “but we should certainly place it high up in the algorithm for patients who have failed a biologic. This should strongly be considered as a second-line agent.”
Dr. Morganstern said that he looks forward to long-term findings from True North beyond 1 year, “in terms of maintaining response,” and additional safety data.
The study was funded supported by Bristol-Myers Squibb. The investigators disclosed additional affiliations with AstraZeneca, AbbVie, Amgen, and others. Dr. Morganstern has previously spoken for Bristol-Myers Squibb.
The oral sphingosine-1-phosphate receptor modulator ozanimod is significantly more effective than placebo for treating patients with moderate to severe ulcerative colitis, regardless of prior biologic exposure, based on results of the phase 3 True North trial.
Although improvements were seen across all patients, those who had previously used biologics took slightly longer to respond to treatment, which suggests that any initial improvements with ozanimod warrant continuation of therapy to achieve full effect, reported to lead author Bruce E. Sands, MD, of Icahn School of Medicine at Mount Sinai, New York, and colleagues.
“[Ulcerative colitis] patients previously treated with a biologic agent may be less likely to respond to another advanced treatment,” Dr. Sands said during a presentation at the annual meeting of the American College of Gastroenterology.
Dr. Sands and colleagues evaluated this possibility in the True North trial, which previously demonstrated superior efficacy and safety of ozanimod over placebo through 1 year.
The present dataset included a double-blinded cohort of 639 patients (cohort 1), among whom 213 took placebo and 426 took ozanimod, and an open-label cohort of 353 patients who took ozanimod (cohort 2). Outcomes included clinical remission, clinical response, endoscopic improvement, and mucosal healing.
During induction (through week 10), biologic-naive patients in both cohorts generally responded better to ozanimod than those who had received at least one prior biologic. Patients who had received two or more biologics showed less improvement than those who had received only one prior biologic.
For example, in cohort 1, 53% of biologic-naive patients in the ozanimod group achieved a clinical response, compared with 50% of patients who had received one prior biologic, and just 27.2% of patients who had received two or more biologics.
With maintenance therapy, however, these differences faded. Among participants continuing ozanimod through week 52, 60.7% of biologic-naive patients achieved a clinical response, compared with 60.5% of those who had taken one prior biologic, and 55.3% of patients who had taken two or more prior biologics.
“Ozanimod treatment for up to 52 weeks in patients with moderate to severe ulcerative colitis improved clinical symptoms, mucosal ulcerations, and reduced cellular inflammation in both biologic-exposed and biologic-naive patients,” Dr. Sands concluded. “Greater efficacy was observed in biologic-naive patients, followed by patients with prior exposure to one biologic, at induction; however, all groups had benefits at end of maintenance. Patients with prior biologic use may require additional time to respond to treatment.”
Bradley Morganstern, MD, codirector of the IBD Center at Stony Brook (N.Y.) Medicine, called the study “a really important analysis” that addresses a common clinical decision.
“We often see a lower response rate among patients previously on biologics, but we have very limited data to guide us on how to choose a second-line agent,” Dr. Morganstern said during an interview.
He said the current findings encourage use of ozanimod for patients who didn’t respond to other biologics.
“The takeaway is that just because someone didn’t respond to a biologic in the past doesn’t mean they won’t respond to this,” Dr. Morganstern said. “They actually have a very good chance of responding – similar to the rest of the population that was never on medication.”
He went on to explain that exact treatment sequencing remains unclear, although ozanimod is a strong candidate in the second line.
“We can’t 100% say where it [ozanimod] fits in,” Dr. Morganstern said, “but we should certainly place it high up in the algorithm for patients who have failed a biologic. This should strongly be considered as a second-line agent.”
Dr. Morganstern said that he looks forward to long-term findings from True North beyond 1 year, “in terms of maintaining response,” and additional safety data.
The study was funded supported by Bristol-Myers Squibb. The investigators disclosed additional affiliations with AstraZeneca, AbbVie, Amgen, and others. Dr. Morganstern has previously spoken for Bristol-Myers Squibb.
FROM ACG 2021
Improving statewide reporting of melanoma cases
For years, . I have audited my melanoma cases (biopsies and excisions sent to me) and discovered that of the 240 cases confirmed over the past 5 years, only 41 were reported to the Ohio state health department and are in that database. That amounts to 199 unreported cases – nearly 83% of the total.
This raises the question as to who is responsible for reporting these cases. Dermatology is unique in that our pathology specimens are not routinely passed through a hospital pathology laboratory. The big difference in reporting is that hospital labs have trained data registrars to report all reportable cancers to state health departments. Therefore, in my case, only patients sent to a hospital-based surgeon for sentinel node biopsies or exceptionally large excisions get reported. When I have spoken about this to my dermatology lab and biopsying physicians, the discussion rapidly turns into a finger pointing game of who is responsible. No one, except perhaps the dermatologist who did the biopsy, has all the data.
Unfortunately, these cases are tedious and time consuming to report. Despite state laws requiring reporting, even with penalties for nonreporters, many small dermatology practices do not report these cases and expect their dermatopathology labs to do so, but the labs expect the biopsying dermatologist to report the cases. This is a classic case of an unfunded mandate since small dermatology practices do not have the time or resources for reporting.
I have worked with the Ohio Department of Health to remove any unnecessary data fields and they have managed to reduce the reporting fields (to 59!). This is the minimum amount required to be included in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database. Many of these fields are not applicable to thin melanomas and after reviewing the 1-hour online training course, each patient can be entered (once the necessary data are collected) in about 15 minutes. This is still a formidable task for small offices, which cannot be blamed for ducking and hoping someone else reports.
While there is controversy regarding the relevance of thin melanomas to overall survival, more accurate reporting can only bolster either argument.
A solution to underreporting
I believe we have developed a unique solution to this conundrum. Our office is partnering with the local melanoma support group (Melanoma Know More) to train volunteers to help with the data collection and reporting of these thin melanomas. We have also discovered that the local community college has students who are majoring in pathology data registry reporting and are happy to gain a little experience before graduating.
We eventually hope to become a clearinghouse for the entire state of Ohio. The state health department has agreed not to apply punitive measures to physicians who are new reporters. It is our plan to obtain melanoma pathology reports, run these past the state database, identify unreported cases, and obtain further data as needed from the biopsying physicians, and then complete the reporting.
I think dermatologic oncologists in every state should view this as an opportunity for a significant quality improvement project, and as a terrific service to the general dermatology community.
The ramifications of more comprehensive reporting of melanomas are great. I would expect more attention to the disease by researchers, and much more clout with state and national legislators. Think about increased funding for melanoma research, allowing sunscreen use for school children, sunshades for playgrounds, and more responsible tanning bed restrictions.
Now, I must inform you that this is my last column, but I plan to continue writing. Over the past 6 years, I have been able to cover a wide range of topics ranging from human trafficking and the American Medical Association, to the many problems faced by small practices. I have enjoyed myself hugely. To quote Douglas Adams, from The Hitchhiker’s Guide to the Galaxy, “So long and thanks for all the fish!” Keep in touch at [email protected].
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
For years, . I have audited my melanoma cases (biopsies and excisions sent to me) and discovered that of the 240 cases confirmed over the past 5 years, only 41 were reported to the Ohio state health department and are in that database. That amounts to 199 unreported cases – nearly 83% of the total.
This raises the question as to who is responsible for reporting these cases. Dermatology is unique in that our pathology specimens are not routinely passed through a hospital pathology laboratory. The big difference in reporting is that hospital labs have trained data registrars to report all reportable cancers to state health departments. Therefore, in my case, only patients sent to a hospital-based surgeon for sentinel node biopsies or exceptionally large excisions get reported. When I have spoken about this to my dermatology lab and biopsying physicians, the discussion rapidly turns into a finger pointing game of who is responsible. No one, except perhaps the dermatologist who did the biopsy, has all the data.
Unfortunately, these cases are tedious and time consuming to report. Despite state laws requiring reporting, even with penalties for nonreporters, many small dermatology practices do not report these cases and expect their dermatopathology labs to do so, but the labs expect the biopsying dermatologist to report the cases. This is a classic case of an unfunded mandate since small dermatology practices do not have the time or resources for reporting.
I have worked with the Ohio Department of Health to remove any unnecessary data fields and they have managed to reduce the reporting fields (to 59!). This is the minimum amount required to be included in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database. Many of these fields are not applicable to thin melanomas and after reviewing the 1-hour online training course, each patient can be entered (once the necessary data are collected) in about 15 minutes. This is still a formidable task for small offices, which cannot be blamed for ducking and hoping someone else reports.
While there is controversy regarding the relevance of thin melanomas to overall survival, more accurate reporting can only bolster either argument.
A solution to underreporting
I believe we have developed a unique solution to this conundrum. Our office is partnering with the local melanoma support group (Melanoma Know More) to train volunteers to help with the data collection and reporting of these thin melanomas. We have also discovered that the local community college has students who are majoring in pathology data registry reporting and are happy to gain a little experience before graduating.
We eventually hope to become a clearinghouse for the entire state of Ohio. The state health department has agreed not to apply punitive measures to physicians who are new reporters. It is our plan to obtain melanoma pathology reports, run these past the state database, identify unreported cases, and obtain further data as needed from the biopsying physicians, and then complete the reporting.
I think dermatologic oncologists in every state should view this as an opportunity for a significant quality improvement project, and as a terrific service to the general dermatology community.
The ramifications of more comprehensive reporting of melanomas are great. I would expect more attention to the disease by researchers, and much more clout with state and national legislators. Think about increased funding for melanoma research, allowing sunscreen use for school children, sunshades for playgrounds, and more responsible tanning bed restrictions.
Now, I must inform you that this is my last column, but I plan to continue writing. Over the past 6 years, I have been able to cover a wide range of topics ranging from human trafficking and the American Medical Association, to the many problems faced by small practices. I have enjoyed myself hugely. To quote Douglas Adams, from The Hitchhiker’s Guide to the Galaxy, “So long and thanks for all the fish!” Keep in touch at [email protected].
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
For years, . I have audited my melanoma cases (biopsies and excisions sent to me) and discovered that of the 240 cases confirmed over the past 5 years, only 41 were reported to the Ohio state health department and are in that database. That amounts to 199 unreported cases – nearly 83% of the total.
This raises the question as to who is responsible for reporting these cases. Dermatology is unique in that our pathology specimens are not routinely passed through a hospital pathology laboratory. The big difference in reporting is that hospital labs have trained data registrars to report all reportable cancers to state health departments. Therefore, in my case, only patients sent to a hospital-based surgeon for sentinel node biopsies or exceptionally large excisions get reported. When I have spoken about this to my dermatology lab and biopsying physicians, the discussion rapidly turns into a finger pointing game of who is responsible. No one, except perhaps the dermatologist who did the biopsy, has all the data.
Unfortunately, these cases are tedious and time consuming to report. Despite state laws requiring reporting, even with penalties for nonreporters, many small dermatology practices do not report these cases and expect their dermatopathology labs to do so, but the labs expect the biopsying dermatologist to report the cases. This is a classic case of an unfunded mandate since small dermatology practices do not have the time or resources for reporting.
I have worked with the Ohio Department of Health to remove any unnecessary data fields and they have managed to reduce the reporting fields (to 59!). This is the minimum amount required to be included in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database. Many of these fields are not applicable to thin melanomas and after reviewing the 1-hour online training course, each patient can be entered (once the necessary data are collected) in about 15 minutes. This is still a formidable task for small offices, which cannot be blamed for ducking and hoping someone else reports.
While there is controversy regarding the relevance of thin melanomas to overall survival, more accurate reporting can only bolster either argument.
A solution to underreporting
I believe we have developed a unique solution to this conundrum. Our office is partnering with the local melanoma support group (Melanoma Know More) to train volunteers to help with the data collection and reporting of these thin melanomas. We have also discovered that the local community college has students who are majoring in pathology data registry reporting and are happy to gain a little experience before graduating.
We eventually hope to become a clearinghouse for the entire state of Ohio. The state health department has agreed not to apply punitive measures to physicians who are new reporters. It is our plan to obtain melanoma pathology reports, run these past the state database, identify unreported cases, and obtain further data as needed from the biopsying physicians, and then complete the reporting.
I think dermatologic oncologists in every state should view this as an opportunity for a significant quality improvement project, and as a terrific service to the general dermatology community.
The ramifications of more comprehensive reporting of melanomas are great. I would expect more attention to the disease by researchers, and much more clout with state and national legislators. Think about increased funding for melanoma research, allowing sunscreen use for school children, sunshades for playgrounds, and more responsible tanning bed restrictions.
Now, I must inform you that this is my last column, but I plan to continue writing. Over the past 6 years, I have been able to cover a wide range of topics ranging from human trafficking and the American Medical Association, to the many problems faced by small practices. I have enjoyed myself hugely. To quote Douglas Adams, from The Hitchhiker’s Guide to the Galaxy, “So long and thanks for all the fish!” Keep in touch at [email protected].
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
FDA approves new interferon for polycythemia vera
, according to an agency press release.
Besremi has a longer half-life than do other pegylated interferon-alfas, allowing for dosing every 2 weeks instead of weekly. If red blood cell counts remain normal for a year, patients have the option of switching to once-monthly dosing. As with similar products, Besremi is self-administered as a subcutaneous injection.
It’s the first interferon approved in the United States specifically for polycythemia vera. Besremi is also approved for upfront therapy, unlike FDA’s first approval for the condition, the oral JAK inhibitor ruxolitinib (Jakafi), which is indicated only after hydroxyurea failure.
Taiwan-based maker PharmaEssentia said in another press release that it will roll Besremi out to the U.S. market in the coming weeks.
“As we begin working closely with the community to integrate this important treatment into clinical practice, we also continue to expand our scientific efforts to unlock the full potential of our pioneering molecule,” said Ko-Chung Lin, PhD, the company’s CEO.
As for unlocking the full potential, Besremi is under investigation for other interferon indications, including myelofibrosis, leukemia, and chronic hepatitis.
The FDA’s approval was based on results in 51 adults treated for an average of 5 years; 31 (61%) had a complete hematologic response, defined as a hematocrit below 45% with no phlebotomy for at least 2 months, plus normal platelet and white cell counts, normal spleen size, and no blood clots.
“Noninferiority to hydroxyurea regarding haematological response and normal spleen size was not shown at 12 months. However, response to ropeginterferon alfa-2b continued to increase over time with improved responses compared with hydroxyurea at 36 months,” investigators noted in an earlier report (Lancet Haematol. 2020 Mar;7[3]:e196-e208).
Besremi carries the same boxed warning as those of peginterferon alfa-2b (Pegintron) and peginterferon alfa-2a (Pegasys), which notes the risk of life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Related contraindications include severe depression and other psychiatric problems; liver impairment; serious or untreated autoimmune disease, and immunosuppression following organ transplant.
Influenza-like illness, arthralgia, fatigue, pruritis, nasopharyngitis, and musculoskeletal pain were the most common adverse events in studies, occurring in over 40% of subjects. Urinary tract infections, transient ischemic attacks, and depression were the most frequent serious complications, occurring in over 4%.
Labeling also notes the risk for fetal harm and the need for effective contraception.
Besremi was approved in Europe in 2019 and is approved in Taiwan and South Korea.
Polycythemia vera is a rare condition thought to be caused by acquired bone marrow stem cell mutations that trigger an overproduction of red blood cells. Patients are at increased risk of blood clots and emboli, and subsequent heart attacks, strokes, and other problems. There’s also the risk of transformation to secondary myelofibrosis or leukemia.
, according to an agency press release.
Besremi has a longer half-life than do other pegylated interferon-alfas, allowing for dosing every 2 weeks instead of weekly. If red blood cell counts remain normal for a year, patients have the option of switching to once-monthly dosing. As with similar products, Besremi is self-administered as a subcutaneous injection.
It’s the first interferon approved in the United States specifically for polycythemia vera. Besremi is also approved for upfront therapy, unlike FDA’s first approval for the condition, the oral JAK inhibitor ruxolitinib (Jakafi), which is indicated only after hydroxyurea failure.
Taiwan-based maker PharmaEssentia said in another press release that it will roll Besremi out to the U.S. market in the coming weeks.
“As we begin working closely with the community to integrate this important treatment into clinical practice, we also continue to expand our scientific efforts to unlock the full potential of our pioneering molecule,” said Ko-Chung Lin, PhD, the company’s CEO.
As for unlocking the full potential, Besremi is under investigation for other interferon indications, including myelofibrosis, leukemia, and chronic hepatitis.
The FDA’s approval was based on results in 51 adults treated for an average of 5 years; 31 (61%) had a complete hematologic response, defined as a hematocrit below 45% with no phlebotomy for at least 2 months, plus normal platelet and white cell counts, normal spleen size, and no blood clots.
“Noninferiority to hydroxyurea regarding haematological response and normal spleen size was not shown at 12 months. However, response to ropeginterferon alfa-2b continued to increase over time with improved responses compared with hydroxyurea at 36 months,” investigators noted in an earlier report (Lancet Haematol. 2020 Mar;7[3]:e196-e208).
Besremi carries the same boxed warning as those of peginterferon alfa-2b (Pegintron) and peginterferon alfa-2a (Pegasys), which notes the risk of life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Related contraindications include severe depression and other psychiatric problems; liver impairment; serious or untreated autoimmune disease, and immunosuppression following organ transplant.
Influenza-like illness, arthralgia, fatigue, pruritis, nasopharyngitis, and musculoskeletal pain were the most common adverse events in studies, occurring in over 40% of subjects. Urinary tract infections, transient ischemic attacks, and depression were the most frequent serious complications, occurring in over 4%.
Labeling also notes the risk for fetal harm and the need for effective contraception.
Besremi was approved in Europe in 2019 and is approved in Taiwan and South Korea.
Polycythemia vera is a rare condition thought to be caused by acquired bone marrow stem cell mutations that trigger an overproduction of red blood cells. Patients are at increased risk of blood clots and emboli, and subsequent heart attacks, strokes, and other problems. There’s also the risk of transformation to secondary myelofibrosis or leukemia.
, according to an agency press release.
Besremi has a longer half-life than do other pegylated interferon-alfas, allowing for dosing every 2 weeks instead of weekly. If red blood cell counts remain normal for a year, patients have the option of switching to once-monthly dosing. As with similar products, Besremi is self-administered as a subcutaneous injection.
It’s the first interferon approved in the United States specifically for polycythemia vera. Besremi is also approved for upfront therapy, unlike FDA’s first approval for the condition, the oral JAK inhibitor ruxolitinib (Jakafi), which is indicated only after hydroxyurea failure.
Taiwan-based maker PharmaEssentia said in another press release that it will roll Besremi out to the U.S. market in the coming weeks.
“As we begin working closely with the community to integrate this important treatment into clinical practice, we also continue to expand our scientific efforts to unlock the full potential of our pioneering molecule,” said Ko-Chung Lin, PhD, the company’s CEO.
As for unlocking the full potential, Besremi is under investigation for other interferon indications, including myelofibrosis, leukemia, and chronic hepatitis.
The FDA’s approval was based on results in 51 adults treated for an average of 5 years; 31 (61%) had a complete hematologic response, defined as a hematocrit below 45% with no phlebotomy for at least 2 months, plus normal platelet and white cell counts, normal spleen size, and no blood clots.
“Noninferiority to hydroxyurea regarding haematological response and normal spleen size was not shown at 12 months. However, response to ropeginterferon alfa-2b continued to increase over time with improved responses compared with hydroxyurea at 36 months,” investigators noted in an earlier report (Lancet Haematol. 2020 Mar;7[3]:e196-e208).
Besremi carries the same boxed warning as those of peginterferon alfa-2b (Pegintron) and peginterferon alfa-2a (Pegasys), which notes the risk of life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Related contraindications include severe depression and other psychiatric problems; liver impairment; serious or untreated autoimmune disease, and immunosuppression following organ transplant.
Influenza-like illness, arthralgia, fatigue, pruritis, nasopharyngitis, and musculoskeletal pain were the most common adverse events in studies, occurring in over 40% of subjects. Urinary tract infections, transient ischemic attacks, and depression were the most frequent serious complications, occurring in over 4%.
Labeling also notes the risk for fetal harm and the need for effective contraception.
Besremi was approved in Europe in 2019 and is approved in Taiwan and South Korea.
Polycythemia vera is a rare condition thought to be caused by acquired bone marrow stem cell mutations that trigger an overproduction of red blood cells. Patients are at increased risk of blood clots and emboli, and subsequent heart attacks, strokes, and other problems. There’s also the risk of transformation to secondary myelofibrosis or leukemia.
FDA flags cardiac perforation risks during leadless pacemaker implantation
The Food and Drug Administration is reminding health care providers about the risk of major complications if cardiac perforation occurs during leadless pacemaker implantation.
Cardiac perforation is a rare complication and the overall risk associated with leadless pacemaker implantation appears similar to that with traditional transvenous pacemakers, the agency says. However, premarket clinical studies of the Micra leadless pacemaker (Medtronic) suggested major complications related to cardiac perforation appear to be more severe for those receiving a leadless pacemaker.
“Information from real-world use suggests that cardiac perforations associated with Micra leadless pacemakers are more likely to be associated with serious complications, such as cardiac tamponade or death, than with traditional pacemakers,” the FDA said Nov. 17 in a letter to health care professionals.
“The FDA is bringing this information to your attention as a reminder and to encourage you to report leadless pacemaker cardiac perforations and complications related to perforation to the manufacturer and the FDA,” it notes.
The Micra Transcatheter Pacing System in 2015 was the first leadless pacemaker approved in Europe, and was approved in the United States the following year with a mandated postapproval study to help assess continued safety and efficacy. The Micra device is currently the only approved leadless pacemaker in the United States.
The FDA continues to evaluate outcomes in patients who receive leadless pacing systems and recommends that health care providers discuss the risks and benefits of available pacing system options with patients as part of shared clinical decision-making.
Providers are advised to read and carefully follow the instructions for use and training for Medtronic’s Micra pacemaker.
Any adverse events or suspected adverse events related to the Micra Transcatheter Pacing System or any other pacemaker systems should be reported to the FDA through MedWatch, its adverse-event reporting program.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is reminding health care providers about the risk of major complications if cardiac perforation occurs during leadless pacemaker implantation.
Cardiac perforation is a rare complication and the overall risk associated with leadless pacemaker implantation appears similar to that with traditional transvenous pacemakers, the agency says. However, premarket clinical studies of the Micra leadless pacemaker (Medtronic) suggested major complications related to cardiac perforation appear to be more severe for those receiving a leadless pacemaker.
“Information from real-world use suggests that cardiac perforations associated with Micra leadless pacemakers are more likely to be associated with serious complications, such as cardiac tamponade or death, than with traditional pacemakers,” the FDA said Nov. 17 in a letter to health care professionals.
“The FDA is bringing this information to your attention as a reminder and to encourage you to report leadless pacemaker cardiac perforations and complications related to perforation to the manufacturer and the FDA,” it notes.
The Micra Transcatheter Pacing System in 2015 was the first leadless pacemaker approved in Europe, and was approved in the United States the following year with a mandated postapproval study to help assess continued safety and efficacy. The Micra device is currently the only approved leadless pacemaker in the United States.
The FDA continues to evaluate outcomes in patients who receive leadless pacing systems and recommends that health care providers discuss the risks and benefits of available pacing system options with patients as part of shared clinical decision-making.
Providers are advised to read and carefully follow the instructions for use and training for Medtronic’s Micra pacemaker.
Any adverse events or suspected adverse events related to the Micra Transcatheter Pacing System or any other pacemaker systems should be reported to the FDA through MedWatch, its adverse-event reporting program.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is reminding health care providers about the risk of major complications if cardiac perforation occurs during leadless pacemaker implantation.
Cardiac perforation is a rare complication and the overall risk associated with leadless pacemaker implantation appears similar to that with traditional transvenous pacemakers, the agency says. However, premarket clinical studies of the Micra leadless pacemaker (Medtronic) suggested major complications related to cardiac perforation appear to be more severe for those receiving a leadless pacemaker.
“Information from real-world use suggests that cardiac perforations associated with Micra leadless pacemakers are more likely to be associated with serious complications, such as cardiac tamponade or death, than with traditional pacemakers,” the FDA said Nov. 17 in a letter to health care professionals.
“The FDA is bringing this information to your attention as a reminder and to encourage you to report leadless pacemaker cardiac perforations and complications related to perforation to the manufacturer and the FDA,” it notes.
The Micra Transcatheter Pacing System in 2015 was the first leadless pacemaker approved in Europe, and was approved in the United States the following year with a mandated postapproval study to help assess continued safety and efficacy. The Micra device is currently the only approved leadless pacemaker in the United States.
The FDA continues to evaluate outcomes in patients who receive leadless pacing systems and recommends that health care providers discuss the risks and benefits of available pacing system options with patients as part of shared clinical decision-making.
Providers are advised to read and carefully follow the instructions for use and training for Medtronic’s Micra pacemaker.
Any adverse events or suspected adverse events related to the Micra Transcatheter Pacing System or any other pacemaker systems should be reported to the FDA through MedWatch, its adverse-event reporting program.
A version of this article first appeared on Medscape.com.
The neurological super powers of grandma are real
Deer, COVID, how?
Usually humans cannot get close enough to a deer to really be face-to-face, so it’s easy to question how on Earth deer are contracting COVID-19. Well, stranger things have happened, and honestly, we’ve just stopped questioning most of them.
Exhibit A comes to us from a Penn State University study: Eighty percent of deer sampled in Iowa in December 2020 and January 2021 – as part of the state’s chronic wasting disease surveillance program – were found to be positive for COVID-19.
A statement from the university said that “white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans.” The investigators also suggested that deer probably caught the virus from humans and then transmitted it to other deer.
If you or someone you know is a hunter or a white-tailed deer, it’s best to proceed with caution. There’s no evidence that COVID-19 has jumped from deer to humans, but hunters should wear masks and gloves while working with deer, worrying not just about the deer’s face, but also … you know, the gastrointestinal parts, Robert Salata, MD, of University Hospitals Cleveland Medical Center, told Syracuse.com. It also shouldn’t be too risky to eat venison, he said, just make sure the meat is cooked thoroughly.
The more you know!
The neurological super powers of grandma are real
What is it about grandmothers that makes them seem almost magical at times? They somehow always know how you feel. And they can almost always tell when something is wrong. They also seem to be the biggest ally a child will have against his or her parents.
So what makes these super matriarchs? The answer is in the brain.
Apparently there’s a function in the brains of grandmothers geared toward “emotional empathy.” James Rilling, PhD, of Emory University, lead author of a recent study focused on looking at the brain function of grandmothers, suggested that they’re neurologically tapped into feeling how their grandchildren feel: “If their grandchild is smiling, they’re feeling the child’s joy. And if their grandchild is crying, they’re feeling the child’s pain and distress.”
And then there’s the cute factor. Never underestimate a child’s ability to manipulate his or her grandmother’s brain.
So how do the researchers know this? Functional MRI showed more brain activity in the parts of the brain that deal with emotional empathy and movement in the participating grandmas when shown pictures of their grandchildren. Images of their own adult children lit up areas more associated with cognitive empathy. So less emotional and more mental/logical understanding.
Kids, don’t tell Mom about the secret midnight snacks with grandma. She wouldn’t get it.
Then there’s the grandmother hypothesis, which suggests that women tend to live longer to provide some kind of evolutionary benefit to their children and grandchildren. Evidence also exists that children with positive engagement from their grandmothers tend to have better social and academic outcomes, behavior, and physical health.
A lot of credit on how children turn out, of course, goes to parents, but more can be said about grandmas. Don’t let the age and freshly baked cookies fool you. They have neurologic superpowers within.
Brain cleanup on aisle 5
You’ve got your local grocery store down. You know the ins and outs; you know where everything is. Last week you did your trip in record time. This week, however, you have to stop at a different store. Same chain, but a different location. You stroll in, confidently walk toward the first aisle for your fruits and veggies, and ... it’s all ice cream. Oops.
There’s a lot we don’t understand about the brain, including how it remembers familiar environments to avoid confusion. Or why it fails to do so, as with our grocery store example. However, thanks to a study from the University of Arizona, we may have an answer.
For the experiment, a group of participants watched a video tour of three virtual cities. Those cities were very similar, being laid out in basically identical fashion. Stores could be found in the same places, but the identity of those stores varied. Some stores were in all three cities, some were in two, and some were unique. Participants were asked to memorize the layouts, and those who got things more than 80% correct ran through the test again, only this time their brain activity was monitored through MRI.
In general, brain activity was similar for the participants; after all, they were recalling similar environments. However, when asked about stores that appeared in multiple cities, brain activity varied dramatically. This indicated to the researchers that the brain was recalling shared stores as if they were more dissimilar than two completely disparate and unique stores, a concept often known to brain scientists as “repulsion.” It also indicates that the memories regarding shared environments are stored in the prefrontal cortex, not the hippocampus, which typically handles memory.
The researchers plan to apply this information to questions about diseases such as Alzheimer’s, so the next time you get turned around in a weirdly unfamiliar grocery store, just think: “It’s okay, I’m helping to solve a terrible brain disease.”
The real endgame: Friction is the winner
Spoiler alert! If you haven’t seen “Avengers: Infinity War” yet, we’re about to ruin it for you.
For those still with us, here’s the spoiler: Thanos would not have been able to snap his fingers while wearing the Infinity Gauntlet.
Saad Bhamla, PhD, of Georgia Tech University’s school of chemical and biomolecular engineering, had been studying powerful and ultrafast motions in living organisms along with several colleagues before the movie came out in 2018, and when they saw the finger-snapping scene it got them wondering.
Being scientists of course, they had no choice. They got out their high-speed imaging equipment, automated image processing software, and dynamic force sensors and analyzed finger snaps, paying close attention to friction by covering fingers with “different materials, including metallic thimbles to simulate the effects of trying to snap while wearing a metallic gauntlet, much like Thanos,” according to a statement on Eurekalert.
With finger snaps, it’s all about the rotational velocity. The angular acceleration involved is the fastest ever measured in a human, with a professional baseball pitcher’s throwing arm a distant second.
Dr. Bhamla’s reaction to their work explains why scientists are the ones doing science. “When I first saw the data, I jumped out of my chair,” he said in the written statement.
Rotational velocities dropped dramatically when the friction-reducing thimbles were used, so there was no snap. Which means that billions and billions of fictional lives could have been saved if the filmmakers had just talked to the right scientist.
That scientist, clearly, is Dr. Bhamla, who said that “this is the only scientific project in my lab in which we could snap our fingers and get data.”
Deer, COVID, how?
Usually humans cannot get close enough to a deer to really be face-to-face, so it’s easy to question how on Earth deer are contracting COVID-19. Well, stranger things have happened, and honestly, we’ve just stopped questioning most of them.
Exhibit A comes to us from a Penn State University study: Eighty percent of deer sampled in Iowa in December 2020 and January 2021 – as part of the state’s chronic wasting disease surveillance program – were found to be positive for COVID-19.
A statement from the university said that “white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans.” The investigators also suggested that deer probably caught the virus from humans and then transmitted it to other deer.
If you or someone you know is a hunter or a white-tailed deer, it’s best to proceed with caution. There’s no evidence that COVID-19 has jumped from deer to humans, but hunters should wear masks and gloves while working with deer, worrying not just about the deer’s face, but also … you know, the gastrointestinal parts, Robert Salata, MD, of University Hospitals Cleveland Medical Center, told Syracuse.com. It also shouldn’t be too risky to eat venison, he said, just make sure the meat is cooked thoroughly.
The more you know!
The neurological super powers of grandma are real
What is it about grandmothers that makes them seem almost magical at times? They somehow always know how you feel. And they can almost always tell when something is wrong. They also seem to be the biggest ally a child will have against his or her parents.
So what makes these super matriarchs? The answer is in the brain.
Apparently there’s a function in the brains of grandmothers geared toward “emotional empathy.” James Rilling, PhD, of Emory University, lead author of a recent study focused on looking at the brain function of grandmothers, suggested that they’re neurologically tapped into feeling how their grandchildren feel: “If their grandchild is smiling, they’re feeling the child’s joy. And if their grandchild is crying, they’re feeling the child’s pain and distress.”
And then there’s the cute factor. Never underestimate a child’s ability to manipulate his or her grandmother’s brain.
So how do the researchers know this? Functional MRI showed more brain activity in the parts of the brain that deal with emotional empathy and movement in the participating grandmas when shown pictures of their grandchildren. Images of their own adult children lit up areas more associated with cognitive empathy. So less emotional and more mental/logical understanding.
Kids, don’t tell Mom about the secret midnight snacks with grandma. She wouldn’t get it.
Then there’s the grandmother hypothesis, which suggests that women tend to live longer to provide some kind of evolutionary benefit to their children and grandchildren. Evidence also exists that children with positive engagement from their grandmothers tend to have better social and academic outcomes, behavior, and physical health.
A lot of credit on how children turn out, of course, goes to parents, but more can be said about grandmas. Don’t let the age and freshly baked cookies fool you. They have neurologic superpowers within.
Brain cleanup on aisle 5
You’ve got your local grocery store down. You know the ins and outs; you know where everything is. Last week you did your trip in record time. This week, however, you have to stop at a different store. Same chain, but a different location. You stroll in, confidently walk toward the first aisle for your fruits and veggies, and ... it’s all ice cream. Oops.
There’s a lot we don’t understand about the brain, including how it remembers familiar environments to avoid confusion. Or why it fails to do so, as with our grocery store example. However, thanks to a study from the University of Arizona, we may have an answer.
For the experiment, a group of participants watched a video tour of three virtual cities. Those cities were very similar, being laid out in basically identical fashion. Stores could be found in the same places, but the identity of those stores varied. Some stores were in all three cities, some were in two, and some were unique. Participants were asked to memorize the layouts, and those who got things more than 80% correct ran through the test again, only this time their brain activity was monitored through MRI.
In general, brain activity was similar for the participants; after all, they were recalling similar environments. However, when asked about stores that appeared in multiple cities, brain activity varied dramatically. This indicated to the researchers that the brain was recalling shared stores as if they were more dissimilar than two completely disparate and unique stores, a concept often known to brain scientists as “repulsion.” It also indicates that the memories regarding shared environments are stored in the prefrontal cortex, not the hippocampus, which typically handles memory.
The researchers plan to apply this information to questions about diseases such as Alzheimer’s, so the next time you get turned around in a weirdly unfamiliar grocery store, just think: “It’s okay, I’m helping to solve a terrible brain disease.”
The real endgame: Friction is the winner
Spoiler alert! If you haven’t seen “Avengers: Infinity War” yet, we’re about to ruin it for you.
For those still with us, here’s the spoiler: Thanos would not have been able to snap his fingers while wearing the Infinity Gauntlet.
Saad Bhamla, PhD, of Georgia Tech University’s school of chemical and biomolecular engineering, had been studying powerful and ultrafast motions in living organisms along with several colleagues before the movie came out in 2018, and when they saw the finger-snapping scene it got them wondering.
Being scientists of course, they had no choice. They got out their high-speed imaging equipment, automated image processing software, and dynamic force sensors and analyzed finger snaps, paying close attention to friction by covering fingers with “different materials, including metallic thimbles to simulate the effects of trying to snap while wearing a metallic gauntlet, much like Thanos,” according to a statement on Eurekalert.
With finger snaps, it’s all about the rotational velocity. The angular acceleration involved is the fastest ever measured in a human, with a professional baseball pitcher’s throwing arm a distant second.
Dr. Bhamla’s reaction to their work explains why scientists are the ones doing science. “When I first saw the data, I jumped out of my chair,” he said in the written statement.
Rotational velocities dropped dramatically when the friction-reducing thimbles were used, so there was no snap. Which means that billions and billions of fictional lives could have been saved if the filmmakers had just talked to the right scientist.
That scientist, clearly, is Dr. Bhamla, who said that “this is the only scientific project in my lab in which we could snap our fingers and get data.”
Deer, COVID, how?
Usually humans cannot get close enough to a deer to really be face-to-face, so it’s easy to question how on Earth deer are contracting COVID-19. Well, stranger things have happened, and honestly, we’ve just stopped questioning most of them.
Exhibit A comes to us from a Penn State University study: Eighty percent of deer sampled in Iowa in December 2020 and January 2021 – as part of the state’s chronic wasting disease surveillance program – were found to be positive for COVID-19.
A statement from the university said that “white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans.” The investigators also suggested that deer probably caught the virus from humans and then transmitted it to other deer.
If you or someone you know is a hunter or a white-tailed deer, it’s best to proceed with caution. There’s no evidence that COVID-19 has jumped from deer to humans, but hunters should wear masks and gloves while working with deer, worrying not just about the deer’s face, but also … you know, the gastrointestinal parts, Robert Salata, MD, of University Hospitals Cleveland Medical Center, told Syracuse.com. It also shouldn’t be too risky to eat venison, he said, just make sure the meat is cooked thoroughly.
The more you know!
The neurological super powers of grandma are real
What is it about grandmothers that makes them seem almost magical at times? They somehow always know how you feel. And they can almost always tell when something is wrong. They also seem to be the biggest ally a child will have against his or her parents.
So what makes these super matriarchs? The answer is in the brain.
Apparently there’s a function in the brains of grandmothers geared toward “emotional empathy.” James Rilling, PhD, of Emory University, lead author of a recent study focused on looking at the brain function of grandmothers, suggested that they’re neurologically tapped into feeling how their grandchildren feel: “If their grandchild is smiling, they’re feeling the child’s joy. And if their grandchild is crying, they’re feeling the child’s pain and distress.”
And then there’s the cute factor. Never underestimate a child’s ability to manipulate his or her grandmother’s brain.
So how do the researchers know this? Functional MRI showed more brain activity in the parts of the brain that deal with emotional empathy and movement in the participating grandmas when shown pictures of their grandchildren. Images of their own adult children lit up areas more associated with cognitive empathy. So less emotional and more mental/logical understanding.
Kids, don’t tell Mom about the secret midnight snacks with grandma. She wouldn’t get it.
Then there’s the grandmother hypothesis, which suggests that women tend to live longer to provide some kind of evolutionary benefit to their children and grandchildren. Evidence also exists that children with positive engagement from their grandmothers tend to have better social and academic outcomes, behavior, and physical health.
A lot of credit on how children turn out, of course, goes to parents, but more can be said about grandmas. Don’t let the age and freshly baked cookies fool you. They have neurologic superpowers within.
Brain cleanup on aisle 5
You’ve got your local grocery store down. You know the ins and outs; you know where everything is. Last week you did your trip in record time. This week, however, you have to stop at a different store. Same chain, but a different location. You stroll in, confidently walk toward the first aisle for your fruits and veggies, and ... it’s all ice cream. Oops.
There’s a lot we don’t understand about the brain, including how it remembers familiar environments to avoid confusion. Or why it fails to do so, as with our grocery store example. However, thanks to a study from the University of Arizona, we may have an answer.
For the experiment, a group of participants watched a video tour of three virtual cities. Those cities were very similar, being laid out in basically identical fashion. Stores could be found in the same places, but the identity of those stores varied. Some stores were in all three cities, some were in two, and some were unique. Participants were asked to memorize the layouts, and those who got things more than 80% correct ran through the test again, only this time their brain activity was monitored through MRI.
In general, brain activity was similar for the participants; after all, they were recalling similar environments. However, when asked about stores that appeared in multiple cities, brain activity varied dramatically. This indicated to the researchers that the brain was recalling shared stores as if they were more dissimilar than two completely disparate and unique stores, a concept often known to brain scientists as “repulsion.” It also indicates that the memories regarding shared environments are stored in the prefrontal cortex, not the hippocampus, which typically handles memory.
The researchers plan to apply this information to questions about diseases such as Alzheimer’s, so the next time you get turned around in a weirdly unfamiliar grocery store, just think: “It’s okay, I’m helping to solve a terrible brain disease.”
The real endgame: Friction is the winner
Spoiler alert! If you haven’t seen “Avengers: Infinity War” yet, we’re about to ruin it for you.
For those still with us, here’s the spoiler: Thanos would not have been able to snap his fingers while wearing the Infinity Gauntlet.
Saad Bhamla, PhD, of Georgia Tech University’s school of chemical and biomolecular engineering, had been studying powerful and ultrafast motions in living organisms along with several colleagues before the movie came out in 2018, and when they saw the finger-snapping scene it got them wondering.
Being scientists of course, they had no choice. They got out their high-speed imaging equipment, automated image processing software, and dynamic force sensors and analyzed finger snaps, paying close attention to friction by covering fingers with “different materials, including metallic thimbles to simulate the effects of trying to snap while wearing a metallic gauntlet, much like Thanos,” according to a statement on Eurekalert.
With finger snaps, it’s all about the rotational velocity. The angular acceleration involved is the fastest ever measured in a human, with a professional baseball pitcher’s throwing arm a distant second.
Dr. Bhamla’s reaction to their work explains why scientists are the ones doing science. “When I first saw the data, I jumped out of my chair,” he said in the written statement.
Rotational velocities dropped dramatically when the friction-reducing thimbles were used, so there was no snap. Which means that billions and billions of fictional lives could have been saved if the filmmakers had just talked to the right scientist.
That scientist, clearly, is Dr. Bhamla, who said that “this is the only scientific project in my lab in which we could snap our fingers and get data.”
New wound over an old scar
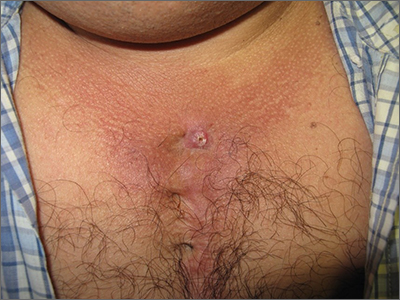
While a squamous cell carcinoma presenting this way is perhaps more common, this nonhealing draining papule over a sternal scar was actually a sternocutaneous fistula (SCF). Examination revealed multiple bound down pits along the sternotomy scar. Very gentle probing of the papule in consideration of biopsy revealed a wire foreign body—the end of a sternotomy wire. A culture of yellow discharge ultimately grew Staphylococcus aureus.
SCF is a rare, and sometimes devastating, complication of cardiac surgery that occurred in 0.23% of cases at 1-year in a single center study of 12,297 patients over 9 years.1 As in this case, it may also present distantly from the time of surgery. The risk of SCF increases with smoking, previous sternal wound infection, renal failure, and use of bone wax during surgery.1
As soon as there was concern for SCF as a possible diagnosis, the patient was referred to, and quickly evaluated by, Cardiothoracic Surgery. Ultrasound and computed tomography imaging did not reveal any osteomyelitis or deep mediastinal disease. He was treated with debridement and removal of the sternotomy wire. At the 1-year follow-up, he had no further episodes of skin infection in the area.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Steingrímsson S, Gustafsson R, Gudbjartsson T, et al. Sternocutaneous fistulas after cardiac surgery: incidence and late outcome during a ten-year follow-up. Ann Thorac Surg. 2009;88:1910-1915. doi: 10.1016/j.athoracsur.2009.07.012

While a squamous cell carcinoma presenting this way is perhaps more common, this nonhealing draining papule over a sternal scar was actually a sternocutaneous fistula (SCF). Examination revealed multiple bound down pits along the sternotomy scar. Very gentle probing of the papule in consideration of biopsy revealed a wire foreign body—the end of a sternotomy wire. A culture of yellow discharge ultimately grew Staphylococcus aureus.
SCF is a rare, and sometimes devastating, complication of cardiac surgery that occurred in 0.23% of cases at 1-year in a single center study of 12,297 patients over 9 years.1 As in this case, it may also present distantly from the time of surgery. The risk of SCF increases with smoking, previous sternal wound infection, renal failure, and use of bone wax during surgery.1
As soon as there was concern for SCF as a possible diagnosis, the patient was referred to, and quickly evaluated by, Cardiothoracic Surgery. Ultrasound and computed tomography imaging did not reveal any osteomyelitis or deep mediastinal disease. He was treated with debridement and removal of the sternotomy wire. At the 1-year follow-up, he had no further episodes of skin infection in the area.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

While a squamous cell carcinoma presenting this way is perhaps more common, this nonhealing draining papule over a sternal scar was actually a sternocutaneous fistula (SCF). Examination revealed multiple bound down pits along the sternotomy scar. Very gentle probing of the papule in consideration of biopsy revealed a wire foreign body—the end of a sternotomy wire. A culture of yellow discharge ultimately grew Staphylococcus aureus.
SCF is a rare, and sometimes devastating, complication of cardiac surgery that occurred in 0.23% of cases at 1-year in a single center study of 12,297 patients over 9 years.1 As in this case, it may also present distantly from the time of surgery. The risk of SCF increases with smoking, previous sternal wound infection, renal failure, and use of bone wax during surgery.1
As soon as there was concern for SCF as a possible diagnosis, the patient was referred to, and quickly evaluated by, Cardiothoracic Surgery. Ultrasound and computed tomography imaging did not reveal any osteomyelitis or deep mediastinal disease. He was treated with debridement and removal of the sternotomy wire. At the 1-year follow-up, he had no further episodes of skin infection in the area.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Steingrímsson S, Gustafsson R, Gudbjartsson T, et al. Sternocutaneous fistulas after cardiac surgery: incidence and late outcome during a ten-year follow-up. Ann Thorac Surg. 2009;88:1910-1915. doi: 10.1016/j.athoracsur.2009.07.012
1. Steingrímsson S, Gustafsson R, Gudbjartsson T, et al. Sternocutaneous fistulas after cardiac surgery: incidence and late outcome during a ten-year follow-up. Ann Thorac Surg. 2009;88:1910-1915. doi: 10.1016/j.athoracsur.2009.07.012
Asthma Highlights From ACAAI 2021
Asthma highlights from ACAAI 2021 range from efficacy of biologic therapies to the late effects of COVID-19 in asthma patients, as reported by Dr Sandhya Khurana from the University of Rochester, in Rochester, New York.
Dr Khurana opens by discussing a study that examined real-world data to assess the effect of COVID-19 infection in asthma patients. The study found that when adjusting for age, sex, BMI, use of inhaled corticosteroids, and atopy, Latino patients, when compared with non-Latino White and Black patients, were more susceptible to prolonged respiratory inflammation after COVID-19 infection.
She then reports on a study that examined potential long-term morbidities associated with systemic corticosteroid (SCS) therapy. The study, which drew from a large administrative claims database, found that high-risk SCS exposure was associated with lifelong adverse chronic health conditions, including type 2 diabetes, hypertension, osteoporosis, and depression. Children ages 4-11 are particularly at risk.
Next, Dr Khurana highlights studies evaluating the efficacy of dupilumab and tezepelumab, two novel biologics, in asthma patients who also have allergies. Both studies demonstrated a potential benefit for a broad population of patients with severe, uncontrolled asthma.
Finally, Dr Khurana comments on ZEPHYR 2, a retrospective cohort study that looked to quantify the real-world impact of switching between biologics.
--
Sandhya Khurana, MD, Professor, Department of Medicine, University of Rochester; Director, Mary Parkes Center for Asthma, Allergy & Pulmonary Care, Rochester, New York
Sandhya Khurana, MD, has disclosed the following relevant financial relationships:
Received research grant from: GlaxoSmithKline
Asthma highlights from ACAAI 2021 range from efficacy of biologic therapies to the late effects of COVID-19 in asthma patients, as reported by Dr Sandhya Khurana from the University of Rochester, in Rochester, New York.
Dr Khurana opens by discussing a study that examined real-world data to assess the effect of COVID-19 infection in asthma patients. The study found that when adjusting for age, sex, BMI, use of inhaled corticosteroids, and atopy, Latino patients, when compared with non-Latino White and Black patients, were more susceptible to prolonged respiratory inflammation after COVID-19 infection.
She then reports on a study that examined potential long-term morbidities associated with systemic corticosteroid (SCS) therapy. The study, which drew from a large administrative claims database, found that high-risk SCS exposure was associated with lifelong adverse chronic health conditions, including type 2 diabetes, hypertension, osteoporosis, and depression. Children ages 4-11 are particularly at risk.
Next, Dr Khurana highlights studies evaluating the efficacy of dupilumab and tezepelumab, two novel biologics, in asthma patients who also have allergies. Both studies demonstrated a potential benefit for a broad population of patients with severe, uncontrolled asthma.
Finally, Dr Khurana comments on ZEPHYR 2, a retrospective cohort study that looked to quantify the real-world impact of switching between biologics.
--
Sandhya Khurana, MD, Professor, Department of Medicine, University of Rochester; Director, Mary Parkes Center for Asthma, Allergy & Pulmonary Care, Rochester, New York
Sandhya Khurana, MD, has disclosed the following relevant financial relationships:
Received research grant from: GlaxoSmithKline
Asthma highlights from ACAAI 2021 range from efficacy of biologic therapies to the late effects of COVID-19 in asthma patients, as reported by Dr Sandhya Khurana from the University of Rochester, in Rochester, New York.
Dr Khurana opens by discussing a study that examined real-world data to assess the effect of COVID-19 infection in asthma patients. The study found that when adjusting for age, sex, BMI, use of inhaled corticosteroids, and atopy, Latino patients, when compared with non-Latino White and Black patients, were more susceptible to prolonged respiratory inflammation after COVID-19 infection.
She then reports on a study that examined potential long-term morbidities associated with systemic corticosteroid (SCS) therapy. The study, which drew from a large administrative claims database, found that high-risk SCS exposure was associated with lifelong adverse chronic health conditions, including type 2 diabetes, hypertension, osteoporosis, and depression. Children ages 4-11 are particularly at risk.
Next, Dr Khurana highlights studies evaluating the efficacy of dupilumab and tezepelumab, two novel biologics, in asthma patients who also have allergies. Both studies demonstrated a potential benefit for a broad population of patients with severe, uncontrolled asthma.
Finally, Dr Khurana comments on ZEPHYR 2, a retrospective cohort study that looked to quantify the real-world impact of switching between biologics.
--
Sandhya Khurana, MD, Professor, Department of Medicine, University of Rochester; Director, Mary Parkes Center for Asthma, Allergy & Pulmonary Care, Rochester, New York
Sandhya Khurana, MD, has disclosed the following relevant financial relationships:
Received research grant from: GlaxoSmithKline

Highlights on DMT Use in Progressive MS From CMSC 2021
Dr Mitzi Joi Williams, medical director of the Joi Life Wellness Group in Atlanta, Georgia, shares updates from the 2021 CMSC Annual Meeting on the use of disease-modifying therapies (DMTs) in progressive multiple sclerosis (MS).
Dr Williams begins with a review of findings from ACAPELLA, a prospective real-world study of ocrelizumab-associated adverse events. The various subanalyses found no higher rates of adverse events on the basis of age or EDSS scores, no downward trend in IgG levels, and mild B-cell repletion that had no significant correlation between disease activity or adverse events.
Next, she turns to several subanalyses from the EXPAND trial that looked at efficacy and safety of siponimod in patients with secondary progressive MS. Siponimod provided similar clinical benefits in all age groups and was well-tolerated at 3 and 6 months. Several MRI measures were found to be prognostic of disease worsening or improvement.
Dr Williams concludes with a first look at a new agent, ATA188, which is being studied in adults with progressive forms of MS. This phase 1/2 double-blind, placebo-controlled, dose-expansion trial aims to evaluate the effect of ATA188 on clinical disability, characterize the agent's safety and tolerability, and evaluate the impact of treatment on biological markers in progressive MS.
--
Mitzi Joi Williams, MD, Assistant Professor, Department of Neurology, Emory University; Medical Director, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Alexion; Genentech; EMD Serono; Novartis; Biogen Idec
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; Genentech; Novartis; Biogen; EMD Serono
Received research grant from: Novartis; Genentech
Dr Mitzi Joi Williams, medical director of the Joi Life Wellness Group in Atlanta, Georgia, shares updates from the 2021 CMSC Annual Meeting on the use of disease-modifying therapies (DMTs) in progressive multiple sclerosis (MS).
Dr Williams begins with a review of findings from ACAPELLA, a prospective real-world study of ocrelizumab-associated adverse events. The various subanalyses found no higher rates of adverse events on the basis of age or EDSS scores, no downward trend in IgG levels, and mild B-cell repletion that had no significant correlation between disease activity or adverse events.
Next, she turns to several subanalyses from the EXPAND trial that looked at efficacy and safety of siponimod in patients with secondary progressive MS. Siponimod provided similar clinical benefits in all age groups and was well-tolerated at 3 and 6 months. Several MRI measures were found to be prognostic of disease worsening or improvement.
Dr Williams concludes with a first look at a new agent, ATA188, which is being studied in adults with progressive forms of MS. This phase 1/2 double-blind, placebo-controlled, dose-expansion trial aims to evaluate the effect of ATA188 on clinical disability, characterize the agent's safety and tolerability, and evaluate the impact of treatment on biological markers in progressive MS.
--
Mitzi Joi Williams, MD, Assistant Professor, Department of Neurology, Emory University; Medical Director, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Alexion; Genentech; EMD Serono; Novartis; Biogen Idec
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; Genentech; Novartis; Biogen; EMD Serono
Received research grant from: Novartis; Genentech
Dr Mitzi Joi Williams, medical director of the Joi Life Wellness Group in Atlanta, Georgia, shares updates from the 2021 CMSC Annual Meeting on the use of disease-modifying therapies (DMTs) in progressive multiple sclerosis (MS).
Dr Williams begins with a review of findings from ACAPELLA, a prospective real-world study of ocrelizumab-associated adverse events. The various subanalyses found no higher rates of adverse events on the basis of age or EDSS scores, no downward trend in IgG levels, and mild B-cell repletion that had no significant correlation between disease activity or adverse events.
Next, she turns to several subanalyses from the EXPAND trial that looked at efficacy and safety of siponimod in patients with secondary progressive MS. Siponimod provided similar clinical benefits in all age groups and was well-tolerated at 3 and 6 months. Several MRI measures were found to be prognostic of disease worsening or improvement.
Dr Williams concludes with a first look at a new agent, ATA188, which is being studied in adults with progressive forms of MS. This phase 1/2 double-blind, placebo-controlled, dose-expansion trial aims to evaluate the effect of ATA188 on clinical disability, characterize the agent's safety and tolerability, and evaluate the impact of treatment on biological markers in progressive MS.
--
Mitzi Joi Williams, MD, Assistant Professor, Department of Neurology, Emory University; Medical Director, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Alexion; Genentech; EMD Serono; Novartis; Biogen Idec
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; Genentech; Novartis; Biogen; EMD Serono
Received research grant from: Novartis; Genentech

Update on Multiple Sclerosis Comorbidities From CMSC 2021
Dr Mitzi Joi Williams, medical director of Joi Life Wellness Group in Atlanta, Georgia, reviews updates from the 2021 CMSC Annual Meeting focusing on important considerations for patients with multiple sclerosis (MS) who have comorbid physical and mental health conditions.
She begins with a longitudinal mediation analysis that assessed how differences in socioeconomic status, lifestyle, and comorbidities may affect Black vs White patients with MS. Overall, Black patients had longer timed 25-foot walks than White patients, and it was concluded that elevated BMIs, higher rates of hypertension, and living in lower income neighborhoods all played partial roles in this disparity.
Dr Williams next discusses a study that examined the prevalence of depression and anxiety in patients with primary-progressive MS (PPMS), secondary-progressive MS (SPMS), and relapsing-remitting MS (RRMS). Rates of both conditions were lower in patients with PPMS than in those with SPMS and RRMS, but overall they were higher in patients with MS compared with the general population.
The final study she reports on looked at the relationships between cognitive, emotional, and physical factors and weekly engagement in physical activity among patients with MS. Unsurprisingly, meeting weekly physical exercise recommendations was associated with improvement in leg functioning, whereas decreased exercise was associated with increased symptoms of depression and with underweight and obese BMIs.
--
Mitzi Joi Williams, MD, Assistant Professor, Department of Neurology, Emory University; Medical Director, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Abbvie; Alexion; Genentech; EMD Serono; Novartis; Biogen Idec
Serve(d) as a speaker or a member of a speakers bureau for: Abbvie; Genentech; Novartis; Biogen; EMD Serono
Received research grant from: Novartis; Genentech
Dr Mitzi Joi Williams, medical director of Joi Life Wellness Group in Atlanta, Georgia, reviews updates from the 2021 CMSC Annual Meeting focusing on important considerations for patients with multiple sclerosis (MS) who have comorbid physical and mental health conditions.
She begins with a longitudinal mediation analysis that assessed how differences in socioeconomic status, lifestyle, and comorbidities may affect Black vs White patients with MS. Overall, Black patients had longer timed 25-foot walks than White patients, and it was concluded that elevated BMIs, higher rates of hypertension, and living in lower income neighborhoods all played partial roles in this disparity.
Dr Williams next discusses a study that examined the prevalence of depression and anxiety in patients with primary-progressive MS (PPMS), secondary-progressive MS (SPMS), and relapsing-remitting MS (RRMS). Rates of both conditions were lower in patients with PPMS than in those with SPMS and RRMS, but overall they were higher in patients with MS compared with the general population.
The final study she reports on looked at the relationships between cognitive, emotional, and physical factors and weekly engagement in physical activity among patients with MS. Unsurprisingly, meeting weekly physical exercise recommendations was associated with improvement in leg functioning, whereas decreased exercise was associated with increased symptoms of depression and with underweight and obese BMIs.
--
Mitzi Joi Williams, MD, Assistant Professor, Department of Neurology, Emory University; Medical Director, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Abbvie; Alexion; Genentech; EMD Serono; Novartis; Biogen Idec
Serve(d) as a speaker or a member of a speakers bureau for: Abbvie; Genentech; Novartis; Biogen; EMD Serono
Received research grant from: Novartis; Genentech
Dr Mitzi Joi Williams, medical director of Joi Life Wellness Group in Atlanta, Georgia, reviews updates from the 2021 CMSC Annual Meeting focusing on important considerations for patients with multiple sclerosis (MS) who have comorbid physical and mental health conditions.
She begins with a longitudinal mediation analysis that assessed how differences in socioeconomic status, lifestyle, and comorbidities may affect Black vs White patients with MS. Overall, Black patients had longer timed 25-foot walks than White patients, and it was concluded that elevated BMIs, higher rates of hypertension, and living in lower income neighborhoods all played partial roles in this disparity.
Dr Williams next discusses a study that examined the prevalence of depression and anxiety in patients with primary-progressive MS (PPMS), secondary-progressive MS (SPMS), and relapsing-remitting MS (RRMS). Rates of both conditions were lower in patients with PPMS than in those with SPMS and RRMS, but overall they were higher in patients with MS compared with the general population.
The final study she reports on looked at the relationships between cognitive, emotional, and physical factors and weekly engagement in physical activity among patients with MS. Unsurprisingly, meeting weekly physical exercise recommendations was associated with improvement in leg functioning, whereas decreased exercise was associated with increased symptoms of depression and with underweight and obese BMIs.
--
Mitzi Joi Williams, MD, Assistant Professor, Department of Neurology, Emory University; Medical Director, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Abbvie; Alexion; Genentech; EMD Serono; Novartis; Biogen Idec
Serve(d) as a speaker or a member of a speakers bureau for: Abbvie; Genentech; Novartis; Biogen; EMD Serono
Received research grant from: Novartis; Genentech

COPD Highlights From CHEST 2020
Dr Darcy Marciniuk, of the University of Saskatchewan in Saskatchewan, Canada, discusses essential abstracts in the management of patients with COPD presented at the American College of Chest Physicians' annual meeting, CHEST 2020, which was held virtually this year because of the coronavirus.
Dr Marciniuk reviews new data from a phase 3 ETHOS substudy evaluating lung function decline in patients receiving inhaled corticosteroid (ICS)-containing therapies vs non–ICS-containing therapies. He also discusses a retrospective cohort study using Medicare data from 2012-2017 evaluating the association of noninvasive ventilation at home with risk for death, hospitalizations, and emergency room visits.
Additionally, he highlights a multi-institutional, post hoc analysis of the phase 3 IMPACT trial to estimate cardiovascular event risk following acute exacerbation in patients with COPD.
From another post hoc analysis, this one from the SUMMIT trial comparing fluticasone, vilanterol, and ICS/LABA with placebo, Dr Marciniuk reports on an investigation of all-cause mortality and severe exacerbation risk in a subgroup of patients with a history of exacerbation.
Finally, he highlights a retrospective cohort study using data from the US 2015 Inpatient Sample, which compared outcomes of patients admitted to hospitals with COPD exacerbations with and without mobility impairment.
--
Darcy D. Marciniuk, MD, Master FCCP, Professor, Department of Medicine, Division of Respirology, Critical Care, and Sleep Medicine, University of Saskatoon, Saskatoon, Saskatchewan, Canada.
Darcy D. Marciniuk, MD, Master FCCP, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Alberta Lung Association; AstraZeneca; Boehringer Ingelheim; Canadian Foundation for Healthcare Improvement; GlaxoSmithKline; Heath Canada; Lung Association of Saskatchewan; Mylan; Novartis; Saskatchewan Ministry of Health; Saskatchewan Health Authority; Yukon Health and Social Services
Received research funding (managed by University of Saskatchewan) from: AstraZeneca; Boehringer Ingelheim; Canada Health Infoway; Canadian Institute of Health Research; GlaxoSmithKline; Grifols; Lung Association of Saskatchewan; Lung Health Institute of Canada; Novartis; Sanofi; Saskatchewan Health Research Foundation; Schering-Plough
Serve(s) as deputy editor of: CHEST Journal.
Dr Darcy Marciniuk, of the University of Saskatchewan in Saskatchewan, Canada, discusses essential abstracts in the management of patients with COPD presented at the American College of Chest Physicians' annual meeting, CHEST 2020, which was held virtually this year because of the coronavirus.
Dr Marciniuk reviews new data from a phase 3 ETHOS substudy evaluating lung function decline in patients receiving inhaled corticosteroid (ICS)-containing therapies vs non–ICS-containing therapies. He also discusses a retrospective cohort study using Medicare data from 2012-2017 evaluating the association of noninvasive ventilation at home with risk for death, hospitalizations, and emergency room visits.
Additionally, he highlights a multi-institutional, post hoc analysis of the phase 3 IMPACT trial to estimate cardiovascular event risk following acute exacerbation in patients with COPD.
From another post hoc analysis, this one from the SUMMIT trial comparing fluticasone, vilanterol, and ICS/LABA with placebo, Dr Marciniuk reports on an investigation of all-cause mortality and severe exacerbation risk in a subgroup of patients with a history of exacerbation.
Finally, he highlights a retrospective cohort study using data from the US 2015 Inpatient Sample, which compared outcomes of patients admitted to hospitals with COPD exacerbations with and without mobility impairment.
--
Darcy D. Marciniuk, MD, Master FCCP, Professor, Department of Medicine, Division of Respirology, Critical Care, and Sleep Medicine, University of Saskatoon, Saskatoon, Saskatchewan, Canada.
Darcy D. Marciniuk, MD, Master FCCP, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Alberta Lung Association; AstraZeneca; Boehringer Ingelheim; Canadian Foundation for Healthcare Improvement; GlaxoSmithKline; Heath Canada; Lung Association of Saskatchewan; Mylan; Novartis; Saskatchewan Ministry of Health; Saskatchewan Health Authority; Yukon Health and Social Services
Received research funding (managed by University of Saskatchewan) from: AstraZeneca; Boehringer Ingelheim; Canada Health Infoway; Canadian Institute of Health Research; GlaxoSmithKline; Grifols; Lung Association of Saskatchewan; Lung Health Institute of Canada; Novartis; Sanofi; Saskatchewan Health Research Foundation; Schering-Plough
Serve(s) as deputy editor of: CHEST Journal.
Dr Darcy Marciniuk, of the University of Saskatchewan in Saskatchewan, Canada, discusses essential abstracts in the management of patients with COPD presented at the American College of Chest Physicians' annual meeting, CHEST 2020, which was held virtually this year because of the coronavirus.
Dr Marciniuk reviews new data from a phase 3 ETHOS substudy evaluating lung function decline in patients receiving inhaled corticosteroid (ICS)-containing therapies vs non–ICS-containing therapies. He also discusses a retrospective cohort study using Medicare data from 2012-2017 evaluating the association of noninvasive ventilation at home with risk for death, hospitalizations, and emergency room visits.
Additionally, he highlights a multi-institutional, post hoc analysis of the phase 3 IMPACT trial to estimate cardiovascular event risk following acute exacerbation in patients with COPD.
From another post hoc analysis, this one from the SUMMIT trial comparing fluticasone, vilanterol, and ICS/LABA with placebo, Dr Marciniuk reports on an investigation of all-cause mortality and severe exacerbation risk in a subgroup of patients with a history of exacerbation.
Finally, he highlights a retrospective cohort study using data from the US 2015 Inpatient Sample, which compared outcomes of patients admitted to hospitals with COPD exacerbations with and without mobility impairment.
--
Darcy D. Marciniuk, MD, Master FCCP, Professor, Department of Medicine, Division of Respirology, Critical Care, and Sleep Medicine, University of Saskatoon, Saskatoon, Saskatchewan, Canada.
Darcy D. Marciniuk, MD, Master FCCP, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Alberta Lung Association; AstraZeneca; Boehringer Ingelheim; Canadian Foundation for Healthcare Improvement; GlaxoSmithKline; Heath Canada; Lung Association of Saskatchewan; Mylan; Novartis; Saskatchewan Ministry of Health; Saskatchewan Health Authority; Yukon Health and Social Services
Received research funding (managed by University of Saskatchewan) from: AstraZeneca; Boehringer Ingelheim; Canada Health Infoway; Canadian Institute of Health Research; GlaxoSmithKline; Grifols; Lung Association of Saskatchewan; Lung Health Institute of Canada; Novartis; Sanofi; Saskatchewan Health Research Foundation; Schering-Plough
Serve(s) as deputy editor of: CHEST Journal.











