User login
ESD vs. cEMR: Rates of complete remission in Barrett’s compared
Treatment with endoscopic submucosal dissection (ESD) is associated with higher rates of complete remission of dysplasia at 2 years compared with cap-assisted endoscopic mucosal resection (cEMR) in patients with Barrett’s esophagus with dysplasia or early-stage intramucosal esophageal adenocarcinoma (EAC), according to study findings.
Despite the seeming advantage of ESD over cEMR, the study found similar rates of complete remission of intestinal metaplasia (CRIM) between the treatment groups at 2 years.
The study authors explained that ESD, a recent development in endoscopic resection, allows for en bloc resection of larger lesions in dysplastic Barrett’s and EAC and features less diagnostic uncertainty compared with cEMR. Findings from the study highlight the importance of this newer technique but also emphasize the utility of both treatments. “In expert hands both sets of procedures appear to be safe and well tolerated,” wrote study authors Don Codipilly, MD, of the Mayo Clinic in Rochester, Minn., and colleagues in Clinical Gastroenterology and Hepatology.
Given the lack of comparative data on the long-term outcomes of cEMR versus ESD in patients with neoplasia associated with Barrett’s esophagus, Dr. Codipilly and colleagues examined histologic outcomes in a prospectively maintained database of 537 patients who underwent endoscopic eradication therapy for Barrett’s esophagus or EAC at the Mayo Clinic between 2006 and 2020. Only patients who had undergone either cEMR (n = 456) or ESD (n = 81) followed by endoscopic ablation were included in the analysis.
The primary endpoint of the study was the rate and time to complete remission of dysplasia (CRD), which was defined by the absence of dysplasia on biopsy from the gastroesophageal junction and tubular esophagus during ≥1 surveillance endoscopy. Researchers also examined the rates of complications, such as clinically significant intraprocedural or post-procedural bleeding that required hospitalization, perforation, receipt of red blood cells within 30 days of the initial procedure, and stricture formation that required dilation within 120 days of the index procedure.
Patients in the ESD group had a longer mean length of resected specimens (23.9 vs. 10.9 mm; P <.01) as well as higher rates of en bloc (97.5% vs. 41.9%; P <.01) and R0 resection (58% vs. 20.2%; P <.01). Patients were generally balanced on other basic baseline demographics, including age, sex distribution, and smoking status.
Over a median 11.2-year follow-up period, a total of 420 patients in the cEMR group achieved CRD. In the ESD group, 48 patients achieved CRD over a median 1.4-year follow-up period. The 2-year cumulative probability of CRD was lower in patients who received cEMR versus those who received ESD (75.8% vs. 85.6%, respectively). In a univariate analysis, the odds of achieving CRD were lower in cEMR versus ESD (hazard ratio, 0.41; 95% CI, 0.31-0.54; P <.01).
According to multivariate analysis, 2 independent predictors of CRD included ESD (hazard ratio, 2.38; P <.01) and shorter Barrett’s segment length (HR, 1.11; P <.01).
The investigators also assessed whether advancements made in cEMR technique have contributed to the findings in an analysis of patients who underwent cEMR (n = 48) with ESD (n = 80) from 2015 to 2019. In this analysis, the researchers found that the odds of CRD were lower than that of ESD (HR, 0.67; 95% CI, 0.45-0.99). Additionally, higher odds of achieving CRD in the cEMR group were observed in years between 2013 and 2019 (n = 129) compared with years 2006 and 2012 (n = 112) (HR, 2.09; 95% CI, 1.59-2.75; P <.01).
Demographic and clinical variables were incorporated into a Cox proportional hazard model to identify factors associated with decreased odds of CRD. This analysis found that decreased odds of CRD were associated with longer Barrett’s esophagus segment length (HR, 0.90; P <.01) and treatment with cEMR versus ESD (HR, 0.42; P <.01).
Over median follow-up periods of 7.8 years in the cEMR group and 1.1 years in the ESD group, approximately 78.5% and 40.7% of patients, respectively, achieved CRIM. While those in the ESD group achieved CRIM earlier, the cumulative probabilities of CRIM were similar by 2 years (59.3% vs. 50.6%; HR, 0.74; 95% CI, 0.52-1.07; P =.11). Shorter Barrett’s esophagus segment was the only independent predictor of CRIM (HR, 1.16; P <.01).
The researchers noted that the study population may have included patients with more severe disease than that in the general population, which may limit the generalizability of the findings. Additionally, the lack of a randomized design was cited as an additional study limitation.
In spite of their findings, the researchers explained that “continued monitoring for additional outcomes such as recurrence are required for further elucidation of the optimal role of these procedures in the management of” neoplasia associated with Barrett’s esophagus.”
The study was funded by the National Cancer Institute and the Freeman Foundation. The researchers reported no conflicts of interest with any pharmaceutical companies.
Treatment with endoscopic submucosal dissection (ESD) is associated with higher rates of complete remission of dysplasia at 2 years compared with cap-assisted endoscopic mucosal resection (cEMR) in patients with Barrett’s esophagus with dysplasia or early-stage intramucosal esophageal adenocarcinoma (EAC), according to study findings.
Despite the seeming advantage of ESD over cEMR, the study found similar rates of complete remission of intestinal metaplasia (CRIM) between the treatment groups at 2 years.
The study authors explained that ESD, a recent development in endoscopic resection, allows for en bloc resection of larger lesions in dysplastic Barrett’s and EAC and features less diagnostic uncertainty compared with cEMR. Findings from the study highlight the importance of this newer technique but also emphasize the utility of both treatments. “In expert hands both sets of procedures appear to be safe and well tolerated,” wrote study authors Don Codipilly, MD, of the Mayo Clinic in Rochester, Minn., and colleagues in Clinical Gastroenterology and Hepatology.
Given the lack of comparative data on the long-term outcomes of cEMR versus ESD in patients with neoplasia associated with Barrett’s esophagus, Dr. Codipilly and colleagues examined histologic outcomes in a prospectively maintained database of 537 patients who underwent endoscopic eradication therapy for Barrett’s esophagus or EAC at the Mayo Clinic between 2006 and 2020. Only patients who had undergone either cEMR (n = 456) or ESD (n = 81) followed by endoscopic ablation were included in the analysis.
The primary endpoint of the study was the rate and time to complete remission of dysplasia (CRD), which was defined by the absence of dysplasia on biopsy from the gastroesophageal junction and tubular esophagus during ≥1 surveillance endoscopy. Researchers also examined the rates of complications, such as clinically significant intraprocedural or post-procedural bleeding that required hospitalization, perforation, receipt of red blood cells within 30 days of the initial procedure, and stricture formation that required dilation within 120 days of the index procedure.
Patients in the ESD group had a longer mean length of resected specimens (23.9 vs. 10.9 mm; P <.01) as well as higher rates of en bloc (97.5% vs. 41.9%; P <.01) and R0 resection (58% vs. 20.2%; P <.01). Patients were generally balanced on other basic baseline demographics, including age, sex distribution, and smoking status.
Over a median 11.2-year follow-up period, a total of 420 patients in the cEMR group achieved CRD. In the ESD group, 48 patients achieved CRD over a median 1.4-year follow-up period. The 2-year cumulative probability of CRD was lower in patients who received cEMR versus those who received ESD (75.8% vs. 85.6%, respectively). In a univariate analysis, the odds of achieving CRD were lower in cEMR versus ESD (hazard ratio, 0.41; 95% CI, 0.31-0.54; P <.01).
According to multivariate analysis, 2 independent predictors of CRD included ESD (hazard ratio, 2.38; P <.01) and shorter Barrett’s segment length (HR, 1.11; P <.01).
The investigators also assessed whether advancements made in cEMR technique have contributed to the findings in an analysis of patients who underwent cEMR (n = 48) with ESD (n = 80) from 2015 to 2019. In this analysis, the researchers found that the odds of CRD were lower than that of ESD (HR, 0.67; 95% CI, 0.45-0.99). Additionally, higher odds of achieving CRD in the cEMR group were observed in years between 2013 and 2019 (n = 129) compared with years 2006 and 2012 (n = 112) (HR, 2.09; 95% CI, 1.59-2.75; P <.01).
Demographic and clinical variables were incorporated into a Cox proportional hazard model to identify factors associated with decreased odds of CRD. This analysis found that decreased odds of CRD were associated with longer Barrett’s esophagus segment length (HR, 0.90; P <.01) and treatment with cEMR versus ESD (HR, 0.42; P <.01).
Over median follow-up periods of 7.8 years in the cEMR group and 1.1 years in the ESD group, approximately 78.5% and 40.7% of patients, respectively, achieved CRIM. While those in the ESD group achieved CRIM earlier, the cumulative probabilities of CRIM were similar by 2 years (59.3% vs. 50.6%; HR, 0.74; 95% CI, 0.52-1.07; P =.11). Shorter Barrett’s esophagus segment was the only independent predictor of CRIM (HR, 1.16; P <.01).
The researchers noted that the study population may have included patients with more severe disease than that in the general population, which may limit the generalizability of the findings. Additionally, the lack of a randomized design was cited as an additional study limitation.
In spite of their findings, the researchers explained that “continued monitoring for additional outcomes such as recurrence are required for further elucidation of the optimal role of these procedures in the management of” neoplasia associated with Barrett’s esophagus.”
The study was funded by the National Cancer Institute and the Freeman Foundation. The researchers reported no conflicts of interest with any pharmaceutical companies.
Treatment with endoscopic submucosal dissection (ESD) is associated with higher rates of complete remission of dysplasia at 2 years compared with cap-assisted endoscopic mucosal resection (cEMR) in patients with Barrett’s esophagus with dysplasia or early-stage intramucosal esophageal adenocarcinoma (EAC), according to study findings.
Despite the seeming advantage of ESD over cEMR, the study found similar rates of complete remission of intestinal metaplasia (CRIM) between the treatment groups at 2 years.
The study authors explained that ESD, a recent development in endoscopic resection, allows for en bloc resection of larger lesions in dysplastic Barrett’s and EAC and features less diagnostic uncertainty compared with cEMR. Findings from the study highlight the importance of this newer technique but also emphasize the utility of both treatments. “In expert hands both sets of procedures appear to be safe and well tolerated,” wrote study authors Don Codipilly, MD, of the Mayo Clinic in Rochester, Minn., and colleagues in Clinical Gastroenterology and Hepatology.
Given the lack of comparative data on the long-term outcomes of cEMR versus ESD in patients with neoplasia associated with Barrett’s esophagus, Dr. Codipilly and colleagues examined histologic outcomes in a prospectively maintained database of 537 patients who underwent endoscopic eradication therapy for Barrett’s esophagus or EAC at the Mayo Clinic between 2006 and 2020. Only patients who had undergone either cEMR (n = 456) or ESD (n = 81) followed by endoscopic ablation were included in the analysis.
The primary endpoint of the study was the rate and time to complete remission of dysplasia (CRD), which was defined by the absence of dysplasia on biopsy from the gastroesophageal junction and tubular esophagus during ≥1 surveillance endoscopy. Researchers also examined the rates of complications, such as clinically significant intraprocedural or post-procedural bleeding that required hospitalization, perforation, receipt of red blood cells within 30 days of the initial procedure, and stricture formation that required dilation within 120 days of the index procedure.
Patients in the ESD group had a longer mean length of resected specimens (23.9 vs. 10.9 mm; P <.01) as well as higher rates of en bloc (97.5% vs. 41.9%; P <.01) and R0 resection (58% vs. 20.2%; P <.01). Patients were generally balanced on other basic baseline demographics, including age, sex distribution, and smoking status.
Over a median 11.2-year follow-up period, a total of 420 patients in the cEMR group achieved CRD. In the ESD group, 48 patients achieved CRD over a median 1.4-year follow-up period. The 2-year cumulative probability of CRD was lower in patients who received cEMR versus those who received ESD (75.8% vs. 85.6%, respectively). In a univariate analysis, the odds of achieving CRD were lower in cEMR versus ESD (hazard ratio, 0.41; 95% CI, 0.31-0.54; P <.01).
According to multivariate analysis, 2 independent predictors of CRD included ESD (hazard ratio, 2.38; P <.01) and shorter Barrett’s segment length (HR, 1.11; P <.01).
The investigators also assessed whether advancements made in cEMR technique have contributed to the findings in an analysis of patients who underwent cEMR (n = 48) with ESD (n = 80) from 2015 to 2019. In this analysis, the researchers found that the odds of CRD were lower than that of ESD (HR, 0.67; 95% CI, 0.45-0.99). Additionally, higher odds of achieving CRD in the cEMR group were observed in years between 2013 and 2019 (n = 129) compared with years 2006 and 2012 (n = 112) (HR, 2.09; 95% CI, 1.59-2.75; P <.01).
Demographic and clinical variables were incorporated into a Cox proportional hazard model to identify factors associated with decreased odds of CRD. This analysis found that decreased odds of CRD were associated with longer Barrett’s esophagus segment length (HR, 0.90; P <.01) and treatment with cEMR versus ESD (HR, 0.42; P <.01).
Over median follow-up periods of 7.8 years in the cEMR group and 1.1 years in the ESD group, approximately 78.5% and 40.7% of patients, respectively, achieved CRIM. While those in the ESD group achieved CRIM earlier, the cumulative probabilities of CRIM were similar by 2 years (59.3% vs. 50.6%; HR, 0.74; 95% CI, 0.52-1.07; P =.11). Shorter Barrett’s esophagus segment was the only independent predictor of CRIM (HR, 1.16; P <.01).
The researchers noted that the study population may have included patients with more severe disease than that in the general population, which may limit the generalizability of the findings. Additionally, the lack of a randomized design was cited as an additional study limitation.
In spite of their findings, the researchers explained that “continued monitoring for additional outcomes such as recurrence are required for further elucidation of the optimal role of these procedures in the management of” neoplasia associated with Barrett’s esophagus.”
The study was funded by the National Cancer Institute and the Freeman Foundation. The researchers reported no conflicts of interest with any pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Human CRP protects against acetaminophen-induced liver injury in mice
While often linked to deleterious outcomes in certain disease states, the hepatocyte-produced inflammatory marker C-reactive protein (CRP) may be a checkpoint that protects against acetaminophen-induced acute liver injury, according to research findings.
Based on the study findings, researchers believe long-term suppression of CRP function or expression may increase an individual’s susceptibility to acetaminophen-induced liver injury. In contrast, CRP “could be exploited as a promising therapeutic approach to treat hepatotoxicity caused by drug overdose” wrote study authors Hai-Yun Li, MD, of the Xi’an Jiaotong University in Shaanxi, China, and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
According to Dr. Li and colleagues, a major cause of acute liver failure is acetaminophen-induced liver injury, but despite this risk, very few treatment options for this condition exist. The only approved treatment for this complication is N-acetyl cysteine (NAC).
Although CRP represents a marker for inflammation following tissue injury, a study from 2020 and one from 2018 suggest the protein regulates complement activation and may modulate responses of immune cells. The authors of the current study noted that few studies have explored what roles complement activation and modulated immune cell responses via CRP play in acetaminophen-induced acute liver injury.
To further elucidate the role of CRP in this setting, Dr. Li and researchers assessed the mechanisms of CRP action both in vitro as well as in CRP mice with Fcy receptor 2B knockout. The researchers suggested CRP may modulate immune cell responses via these receptors. Additionally, the investigators assessed CRP action in mice with C3 knockout, given previous studies suggesting C3 knockout may alleviate acetaminophen-induced liver injury in mice. The researchers also investigated hepatic expression of CRP mutants that were defective in complement interaction. Finally, the researchers sought to understand the therapeutic potential of the inflammatory marker by performing intraperitoneal administration of human CRP at 2 or 6 hours after induction of acetaminophen-induced acute liver injury in wild-type mice.
Injection of 300 mg/kg acetaminophen over 24 hours led to overt liver injury in wild-type mice, which was characterized by increased levels of circulating alanine transaminase (ALT) and aspartate transaminase (AST) as well as massive necrosis of hepatocytes. The researchers noted that these manifestations were exacerbated significantly in the CRP knockout mice.
The intravenous administration of human CRP in the mice with the drug-induced liver injury rescued defects caused by mouse CRP knockout. Additionally, human CRP administration alleviated acetaminophen-induced acute liver injury in the wild-type mice. The researchers wrote that these findings demonstrate that endogenous and human CRP “are both protective,” at least in mouse models of acetaminophen-induced liver injury.
In a second experiment, the researchers examined the mechanisms involved in CRP protection in early phases of drug-induced liver injury. Based on the experiment, the researchers found that the knockout of an inhibitory Fcy receptor mediating the anti-inflammatory activities of CRP demonstrated only “marginal effects” on the protection of the protein in acetaminophen-induced liver injury. Overall, the investigators suggested that the inflammatory marker does not likely act via the cellular Fcy receptor 2B to inhibit early phases of acetaminophen-induced hepatocyte injury. Rather, the investigators explained that CRP may act via factor H, which is recruited by CRP in regulating complement activation, to inhibit overactivation of complement on injured hepatocytes. Ultimately, the researchers explained, this results in suppression of the late phase amplification of inflammation that is mediated by neutrophils’ C3a-dependent actions.
Finally, the researchers found that intraperitoneal administration of human CRP at 2.5 mg/kg in wild-type mice at 2 hours following induction of acetaminophen-induced liver injury led to “markedly reduced liver injury,” with an efficacy that was similar to that of 500 mg/kg N-acetylcysteine, the only available treatment approved for acetaminophen-induced liver injury.
The researchers noted that N-acetylcysteine is only effective during the early phases of the acetaminophen-induced liver injury and loses effectiveness at 6 hours following injury. In contrast, human CRP in this study was still highly effective at this time point. “Given that people can tolerate high levels of circulating CRP, the administration of this protein might be a promising option to treat [acetaminophen-induced liver injury] with minimal side effects,” the researchers wrote.
The study was funded by the National Natural Science Foundation of China. The researchers reported no conflicts of interest with any pharmaceutical companies.
This article was updated on Sep. 20, 2022.
While often linked to deleterious outcomes in certain disease states, the hepatocyte-produced inflammatory marker C-reactive protein (CRP) may be a checkpoint that protects against acetaminophen-induced acute liver injury, according to research findings.
Based on the study findings, researchers believe long-term suppression of CRP function or expression may increase an individual’s susceptibility to acetaminophen-induced liver injury. In contrast, CRP “could be exploited as a promising therapeutic approach to treat hepatotoxicity caused by drug overdose” wrote study authors Hai-Yun Li, MD, of the Xi’an Jiaotong University in Shaanxi, China, and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
According to Dr. Li and colleagues, a major cause of acute liver failure is acetaminophen-induced liver injury, but despite this risk, very few treatment options for this condition exist. The only approved treatment for this complication is N-acetyl cysteine (NAC).
Although CRP represents a marker for inflammation following tissue injury, a study from 2020 and one from 2018 suggest the protein regulates complement activation and may modulate responses of immune cells. The authors of the current study noted that few studies have explored what roles complement activation and modulated immune cell responses via CRP play in acetaminophen-induced acute liver injury.
To further elucidate the role of CRP in this setting, Dr. Li and researchers assessed the mechanisms of CRP action both in vitro as well as in CRP mice with Fcy receptor 2B knockout. The researchers suggested CRP may modulate immune cell responses via these receptors. Additionally, the investigators assessed CRP action in mice with C3 knockout, given previous studies suggesting C3 knockout may alleviate acetaminophen-induced liver injury in mice. The researchers also investigated hepatic expression of CRP mutants that were defective in complement interaction. Finally, the researchers sought to understand the therapeutic potential of the inflammatory marker by performing intraperitoneal administration of human CRP at 2 or 6 hours after induction of acetaminophen-induced acute liver injury in wild-type mice.
Injection of 300 mg/kg acetaminophen over 24 hours led to overt liver injury in wild-type mice, which was characterized by increased levels of circulating alanine transaminase (ALT) and aspartate transaminase (AST) as well as massive necrosis of hepatocytes. The researchers noted that these manifestations were exacerbated significantly in the CRP knockout mice.
The intravenous administration of human CRP in the mice with the drug-induced liver injury rescued defects caused by mouse CRP knockout. Additionally, human CRP administration alleviated acetaminophen-induced acute liver injury in the wild-type mice. The researchers wrote that these findings demonstrate that endogenous and human CRP “are both protective,” at least in mouse models of acetaminophen-induced liver injury.
In a second experiment, the researchers examined the mechanisms involved in CRP protection in early phases of drug-induced liver injury. Based on the experiment, the researchers found that the knockout of an inhibitory Fcy receptor mediating the anti-inflammatory activities of CRP demonstrated only “marginal effects” on the protection of the protein in acetaminophen-induced liver injury. Overall, the investigators suggested that the inflammatory marker does not likely act via the cellular Fcy receptor 2B to inhibit early phases of acetaminophen-induced hepatocyte injury. Rather, the investigators explained that CRP may act via factor H, which is recruited by CRP in regulating complement activation, to inhibit overactivation of complement on injured hepatocytes. Ultimately, the researchers explained, this results in suppression of the late phase amplification of inflammation that is mediated by neutrophils’ C3a-dependent actions.
Finally, the researchers found that intraperitoneal administration of human CRP at 2.5 mg/kg in wild-type mice at 2 hours following induction of acetaminophen-induced liver injury led to “markedly reduced liver injury,” with an efficacy that was similar to that of 500 mg/kg N-acetylcysteine, the only available treatment approved for acetaminophen-induced liver injury.
The researchers noted that N-acetylcysteine is only effective during the early phases of the acetaminophen-induced liver injury and loses effectiveness at 6 hours following injury. In contrast, human CRP in this study was still highly effective at this time point. “Given that people can tolerate high levels of circulating CRP, the administration of this protein might be a promising option to treat [acetaminophen-induced liver injury] with minimal side effects,” the researchers wrote.
The study was funded by the National Natural Science Foundation of China. The researchers reported no conflicts of interest with any pharmaceutical companies.
This article was updated on Sep. 20, 2022.
While often linked to deleterious outcomes in certain disease states, the hepatocyte-produced inflammatory marker C-reactive protein (CRP) may be a checkpoint that protects against acetaminophen-induced acute liver injury, according to research findings.
Based on the study findings, researchers believe long-term suppression of CRP function or expression may increase an individual’s susceptibility to acetaminophen-induced liver injury. In contrast, CRP “could be exploited as a promising therapeutic approach to treat hepatotoxicity caused by drug overdose” wrote study authors Hai-Yun Li, MD, of the Xi’an Jiaotong University in Shaanxi, China, and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
According to Dr. Li and colleagues, a major cause of acute liver failure is acetaminophen-induced liver injury, but despite this risk, very few treatment options for this condition exist. The only approved treatment for this complication is N-acetyl cysteine (NAC).
Although CRP represents a marker for inflammation following tissue injury, a study from 2020 and one from 2018 suggest the protein regulates complement activation and may modulate responses of immune cells. The authors of the current study noted that few studies have explored what roles complement activation and modulated immune cell responses via CRP play in acetaminophen-induced acute liver injury.
To further elucidate the role of CRP in this setting, Dr. Li and researchers assessed the mechanisms of CRP action both in vitro as well as in CRP mice with Fcy receptor 2B knockout. The researchers suggested CRP may modulate immune cell responses via these receptors. Additionally, the investigators assessed CRP action in mice with C3 knockout, given previous studies suggesting C3 knockout may alleviate acetaminophen-induced liver injury in mice. The researchers also investigated hepatic expression of CRP mutants that were defective in complement interaction. Finally, the researchers sought to understand the therapeutic potential of the inflammatory marker by performing intraperitoneal administration of human CRP at 2 or 6 hours after induction of acetaminophen-induced acute liver injury in wild-type mice.
Injection of 300 mg/kg acetaminophen over 24 hours led to overt liver injury in wild-type mice, which was characterized by increased levels of circulating alanine transaminase (ALT) and aspartate transaminase (AST) as well as massive necrosis of hepatocytes. The researchers noted that these manifestations were exacerbated significantly in the CRP knockout mice.
The intravenous administration of human CRP in the mice with the drug-induced liver injury rescued defects caused by mouse CRP knockout. Additionally, human CRP administration alleviated acetaminophen-induced acute liver injury in the wild-type mice. The researchers wrote that these findings demonstrate that endogenous and human CRP “are both protective,” at least in mouse models of acetaminophen-induced liver injury.
In a second experiment, the researchers examined the mechanisms involved in CRP protection in early phases of drug-induced liver injury. Based on the experiment, the researchers found that the knockout of an inhibitory Fcy receptor mediating the anti-inflammatory activities of CRP demonstrated only “marginal effects” on the protection of the protein in acetaminophen-induced liver injury. Overall, the investigators suggested that the inflammatory marker does not likely act via the cellular Fcy receptor 2B to inhibit early phases of acetaminophen-induced hepatocyte injury. Rather, the investigators explained that CRP may act via factor H, which is recruited by CRP in regulating complement activation, to inhibit overactivation of complement on injured hepatocytes. Ultimately, the researchers explained, this results in suppression of the late phase amplification of inflammation that is mediated by neutrophils’ C3a-dependent actions.
Finally, the researchers found that intraperitoneal administration of human CRP at 2.5 mg/kg in wild-type mice at 2 hours following induction of acetaminophen-induced liver injury led to “markedly reduced liver injury,” with an efficacy that was similar to that of 500 mg/kg N-acetylcysteine, the only available treatment approved for acetaminophen-induced liver injury.
The researchers noted that N-acetylcysteine is only effective during the early phases of the acetaminophen-induced liver injury and loses effectiveness at 6 hours following injury. In contrast, human CRP in this study was still highly effective at this time point. “Given that people can tolerate high levels of circulating CRP, the administration of this protein might be a promising option to treat [acetaminophen-induced liver injury] with minimal side effects,” the researchers wrote.
The study was funded by the National Natural Science Foundation of China. The researchers reported no conflicts of interest with any pharmaceutical companies.
This article was updated on Sep. 20, 2022.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Medical technology should keep patient in mind
Indeed, science and technology provide opportunities to improve outcomes in ways not even imagined 100 years ago, yet we must acknowledge that technology also threatens to erect barriers between us and our patients. We can be easily tempted to confuse new care delivery tools with the actual care itself.
Threats to the physician-patient relationship
Medical history provides many examples of how our zeal to innovate can have untoward consequences to the physician-patient relationship.
In the late 1800s, for example, to convey a sense of science, purity of intent, and trust, the medical community began wearing white coats. Those white coats have been discussed as creating emotional distance between physicians and their patients.1
Even when we in the medical community are slow and reluctant to change, the external forces propelling us forward often seem unstoppable; kinetic aspirations to innovate electronic information systems and new applications seem suddenly to revolutionize care delivery when we least expect it. The rapidity of change in technology can sometimes be dizzying but can at the same time can occur so swiftly we don’t even notice it.
After René Laennec invented the stethoscope in the early 1800s, clinicians no longer needed to physically lean in and place an ear directly onto patients to hear their hearts beating. This created a distance from patients that was still lamented 50 years later, when a professor of medicine is reported to have said, “he that hath ears to hear, let him use his ears and not a stethoscope.” Still, while the stethoscope has literally distanced us from patients, it is such an important tool that we no longer think about this distancing. We have adapted over time to remain close to our patients, to sincerely listen to their thoughts and reassure them that we hear them without the need to feel our ears on their chests.
Francis Peabody, the eminent Harvard physician, wrote an essay in 1927 titled, “The Care of the Patient.” At the end of the first paragraph, he states: “The most common criticism made at present by older practitioners is that young graduates ... are too “scientific” and do not know how to take care of patients.” He goes on to say that “one of the essential qualities of the clinician is interest in humanity, for the secret of the care of the patient is in caring for the patient.”2
We agree with Dr. Peabody. As we embrace science and technology that can change health outcomes, our patients’ needs to feel understood and cared for will not diminish. Instead, that need will continue to be an important aspect of our struggle and joy in providing holistic, humane, competent care into the future.
Twenty-first century physicians have access to an ever-growing trove of data, yet our ability to truly know our patients seems somehow less accessible. Home health devices have begun to provide a flow of information about parameters, ranging from continuous glucose readings to home blood pressures, weights, and inspiratory flow readings. These data can provide much more accurate insight into patients than what we can glean from one point in time during an office visit. Yet we need to remember that behind the data are people with dreams and desires, not just table entries in an electronic health record.
In 1923, the German philosopher Martin Buber published the book for which he is best known, “I and Thou.” In that book, Mr. Buber says that there are two ways we can approach relationships: “I-Thou” or “I-It.” In I-It relationships, we view the other person as an “it” to be used to accomplish a purpose, or to be experienced without his or her full involvement. In an I-Thou relationship, we appreciate the other people for all their complexity, in their full humanness. We must consciously remind ourselves amid the rush of technology that there are real people behind those data. We must acknowledge and approach each person as a unique individual who has dreams, goals, fears, and wishes that may be different from ours but to which we can still relate.
‘From the Beating End of the Stethoscope’
John Ciardi, an American poet, said the following in a poem titled, “Lines From the Beating End of the Stethoscope”:
I speak, as I say, the patient’s point of view.
But, given time, doctors are patients, too.
And there’s our bond: beyond anatomy,
Or in it, through it, to the mystery
Medicine takes the pulse of and lets go
Forever unexplained. It’s art, we know,
Not science at the heart. Doctor be whole,
I won’t insist the patient is a soul,
But he’s a something, possibly laughable,
Or possibly sublime, but not quite graphable.
Not quite containable on a bed chart.
Where science touches man it turns to art.3
This poem is a reminder of the subtle needs of patients during their encounters with doctors, especially around many of the most important decisions and events in their lives. Patients’ needs are varied, complex, difficult to discern, and not able to be fully explained or understood through math and science.
Einstein warned us that the modern age would be characterized by a perfection of means and a confusion of goals.4 As clinicians, we should strive to clarify and align our goals with those of our patients, providing care that is real, compassionate, and personal, not just an optimized means to achieve standardized metrics. While technology can assist us in this pursuit, we’ll need be careful that our enchantment with innovation does not cloud our actual goal: truly caring for our patients.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
References
1. Jones VA. The white coat: Why not follow suit? JAMA. 1999;281(5):478. doi: 10.1001/jama.281.5.478-JMS0203-5-1
2. Peabody, Francis (1927). “The care of the patient.” JAMA. 88(12):877-82. doi: 10.1001/jama.1927.02680380001001.
3. Ciardi, John. Lines from the Beating End of the Stethoscope. Saturday Review, Nov. 18, 1968.
4. Albert Einstein, Out of My Later Years, 1950.
Indeed, science and technology provide opportunities to improve outcomes in ways not even imagined 100 years ago, yet we must acknowledge that technology also threatens to erect barriers between us and our patients. We can be easily tempted to confuse new care delivery tools with the actual care itself.
Threats to the physician-patient relationship
Medical history provides many examples of how our zeal to innovate can have untoward consequences to the physician-patient relationship.
In the late 1800s, for example, to convey a sense of science, purity of intent, and trust, the medical community began wearing white coats. Those white coats have been discussed as creating emotional distance between physicians and their patients.1
Even when we in the medical community are slow and reluctant to change, the external forces propelling us forward often seem unstoppable; kinetic aspirations to innovate electronic information systems and new applications seem suddenly to revolutionize care delivery when we least expect it. The rapidity of change in technology can sometimes be dizzying but can at the same time can occur so swiftly we don’t even notice it.
After René Laennec invented the stethoscope in the early 1800s, clinicians no longer needed to physically lean in and place an ear directly onto patients to hear their hearts beating. This created a distance from patients that was still lamented 50 years later, when a professor of medicine is reported to have said, “he that hath ears to hear, let him use his ears and not a stethoscope.” Still, while the stethoscope has literally distanced us from patients, it is such an important tool that we no longer think about this distancing. We have adapted over time to remain close to our patients, to sincerely listen to their thoughts and reassure them that we hear them without the need to feel our ears on their chests.
Francis Peabody, the eminent Harvard physician, wrote an essay in 1927 titled, “The Care of the Patient.” At the end of the first paragraph, he states: “The most common criticism made at present by older practitioners is that young graduates ... are too “scientific” and do not know how to take care of patients.” He goes on to say that “one of the essential qualities of the clinician is interest in humanity, for the secret of the care of the patient is in caring for the patient.”2
We agree with Dr. Peabody. As we embrace science and technology that can change health outcomes, our patients’ needs to feel understood and cared for will not diminish. Instead, that need will continue to be an important aspect of our struggle and joy in providing holistic, humane, competent care into the future.
Twenty-first century physicians have access to an ever-growing trove of data, yet our ability to truly know our patients seems somehow less accessible. Home health devices have begun to provide a flow of information about parameters, ranging from continuous glucose readings to home blood pressures, weights, and inspiratory flow readings. These data can provide much more accurate insight into patients than what we can glean from one point in time during an office visit. Yet we need to remember that behind the data are people with dreams and desires, not just table entries in an electronic health record.
In 1923, the German philosopher Martin Buber published the book for which he is best known, “I and Thou.” In that book, Mr. Buber says that there are two ways we can approach relationships: “I-Thou” or “I-It.” In I-It relationships, we view the other person as an “it” to be used to accomplish a purpose, or to be experienced without his or her full involvement. In an I-Thou relationship, we appreciate the other people for all their complexity, in their full humanness. We must consciously remind ourselves amid the rush of technology that there are real people behind those data. We must acknowledge and approach each person as a unique individual who has dreams, goals, fears, and wishes that may be different from ours but to which we can still relate.
‘From the Beating End of the Stethoscope’
John Ciardi, an American poet, said the following in a poem titled, “Lines From the Beating End of the Stethoscope”:
I speak, as I say, the patient’s point of view.
But, given time, doctors are patients, too.
And there’s our bond: beyond anatomy,
Or in it, through it, to the mystery
Medicine takes the pulse of and lets go
Forever unexplained. It’s art, we know,
Not science at the heart. Doctor be whole,
I won’t insist the patient is a soul,
But he’s a something, possibly laughable,
Or possibly sublime, but not quite graphable.
Not quite containable on a bed chart.
Where science touches man it turns to art.3
This poem is a reminder of the subtle needs of patients during their encounters with doctors, especially around many of the most important decisions and events in their lives. Patients’ needs are varied, complex, difficult to discern, and not able to be fully explained or understood through math and science.
Einstein warned us that the modern age would be characterized by a perfection of means and a confusion of goals.4 As clinicians, we should strive to clarify and align our goals with those of our patients, providing care that is real, compassionate, and personal, not just an optimized means to achieve standardized metrics. While technology can assist us in this pursuit, we’ll need be careful that our enchantment with innovation does not cloud our actual goal: truly caring for our patients.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
References
1. Jones VA. The white coat: Why not follow suit? JAMA. 1999;281(5):478. doi: 10.1001/jama.281.5.478-JMS0203-5-1
2. Peabody, Francis (1927). “The care of the patient.” JAMA. 88(12):877-82. doi: 10.1001/jama.1927.02680380001001.
3. Ciardi, John. Lines from the Beating End of the Stethoscope. Saturday Review, Nov. 18, 1968.
4. Albert Einstein, Out of My Later Years, 1950.
Indeed, science and technology provide opportunities to improve outcomes in ways not even imagined 100 years ago, yet we must acknowledge that technology also threatens to erect barriers between us and our patients. We can be easily tempted to confuse new care delivery tools with the actual care itself.
Threats to the physician-patient relationship
Medical history provides many examples of how our zeal to innovate can have untoward consequences to the physician-patient relationship.
In the late 1800s, for example, to convey a sense of science, purity of intent, and trust, the medical community began wearing white coats. Those white coats have been discussed as creating emotional distance between physicians and their patients.1
Even when we in the medical community are slow and reluctant to change, the external forces propelling us forward often seem unstoppable; kinetic aspirations to innovate electronic information systems and new applications seem suddenly to revolutionize care delivery when we least expect it. The rapidity of change in technology can sometimes be dizzying but can at the same time can occur so swiftly we don’t even notice it.
After René Laennec invented the stethoscope in the early 1800s, clinicians no longer needed to physically lean in and place an ear directly onto patients to hear their hearts beating. This created a distance from patients that was still lamented 50 years later, when a professor of medicine is reported to have said, “he that hath ears to hear, let him use his ears and not a stethoscope.” Still, while the stethoscope has literally distanced us from patients, it is such an important tool that we no longer think about this distancing. We have adapted over time to remain close to our patients, to sincerely listen to their thoughts and reassure them that we hear them without the need to feel our ears on their chests.
Francis Peabody, the eminent Harvard physician, wrote an essay in 1927 titled, “The Care of the Patient.” At the end of the first paragraph, he states: “The most common criticism made at present by older practitioners is that young graduates ... are too “scientific” and do not know how to take care of patients.” He goes on to say that “one of the essential qualities of the clinician is interest in humanity, for the secret of the care of the patient is in caring for the patient.”2
We agree with Dr. Peabody. As we embrace science and technology that can change health outcomes, our patients’ needs to feel understood and cared for will not diminish. Instead, that need will continue to be an important aspect of our struggle and joy in providing holistic, humane, competent care into the future.
Twenty-first century physicians have access to an ever-growing trove of data, yet our ability to truly know our patients seems somehow less accessible. Home health devices have begun to provide a flow of information about parameters, ranging from continuous glucose readings to home blood pressures, weights, and inspiratory flow readings. These data can provide much more accurate insight into patients than what we can glean from one point in time during an office visit. Yet we need to remember that behind the data are people with dreams and desires, not just table entries in an electronic health record.
In 1923, the German philosopher Martin Buber published the book for which he is best known, “I and Thou.” In that book, Mr. Buber says that there are two ways we can approach relationships: “I-Thou” or “I-It.” In I-It relationships, we view the other person as an “it” to be used to accomplish a purpose, or to be experienced without his or her full involvement. In an I-Thou relationship, we appreciate the other people for all their complexity, in their full humanness. We must consciously remind ourselves amid the rush of technology that there are real people behind those data. We must acknowledge and approach each person as a unique individual who has dreams, goals, fears, and wishes that may be different from ours but to which we can still relate.
‘From the Beating End of the Stethoscope’
John Ciardi, an American poet, said the following in a poem titled, “Lines From the Beating End of the Stethoscope”:
I speak, as I say, the patient’s point of view.
But, given time, doctors are patients, too.
And there’s our bond: beyond anatomy,
Or in it, through it, to the mystery
Medicine takes the pulse of and lets go
Forever unexplained. It’s art, we know,
Not science at the heart. Doctor be whole,
I won’t insist the patient is a soul,
But he’s a something, possibly laughable,
Or possibly sublime, but not quite graphable.
Not quite containable on a bed chart.
Where science touches man it turns to art.3
This poem is a reminder of the subtle needs of patients during their encounters with doctors, especially around many of the most important decisions and events in their lives. Patients’ needs are varied, complex, difficult to discern, and not able to be fully explained or understood through math and science.
Einstein warned us that the modern age would be characterized by a perfection of means and a confusion of goals.4 As clinicians, we should strive to clarify and align our goals with those of our patients, providing care that is real, compassionate, and personal, not just an optimized means to achieve standardized metrics. While technology can assist us in this pursuit, we’ll need be careful that our enchantment with innovation does not cloud our actual goal: truly caring for our patients.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
References
1. Jones VA. The white coat: Why not follow suit? JAMA. 1999;281(5):478. doi: 10.1001/jama.281.5.478-JMS0203-5-1
2. Peabody, Francis (1927). “The care of the patient.” JAMA. 88(12):877-82. doi: 10.1001/jama.1927.02680380001001.
3. Ciardi, John. Lines from the Beating End of the Stethoscope. Saturday Review, Nov. 18, 1968.
4. Albert Einstein, Out of My Later Years, 1950.
Many breast cancer patients use cannabis for symptom relief
Most (75%) of the patients who reported using cannabis said it was extremely helpful or very helpful in alleviating symptoms.
The authors warn of potential safety concerns with cannabis, especially with the use of unregulated products.
In addition, the survey found that physicians were not highly regarded as a source of information about cannabis use. Only 39% of patients said that they discussed cannabis with their physicians; 28% reported feeling uncomfortable when broaching the topic. Only 4% indicated that physicians were the most helpful source of information about cannabis.
The survey involved 612 patients with breast cancer. The results were published online Oct. 12 in Cancer.
“Our study highlights an important opportunity for providers to initiate informed conversations about medical cannabis with their patients, as the evidence shows that many are using medical cannabis without our knowledge or guidance,” said lead author Marisa Weiss, MD, of Breastcancer.org and the Lankenau Medical Center, near Philadelphia. “Not knowing whether or not our cancer patients are using cannabis is a major blind spot in our ability to provide optimal care,” she said.
Cannabis in one form or another has been legalized in many states across America, and even in states where it hasn’t been legalized, people are using it.
“Even though many states have relaxed their laws on cannabis, it remains a Schedule I drug on the federal level and is essentially still considered illegal,” commented Donald I. Abrams, MD, professor of medicine at the University of California, San Francisco, and an integrative oncologist at the UCSF Osher Center for Integrative Medicine. “This is why many physicians are uncomfortable discussing it with patients,” he said.
“Cannabis use isn’t taught in medical school, and until that changes, I don’t know how physicians are going to be advisers for this,” said Dr. Abrams, who was approached by this news organization for comment.
This “is a really nice study in that it looks at a large group of breast cancer patients from the community ... It’s not from a single institution [such as this previous study] and so a more representative mix,” Dr. Abrams said.
However, he also commented that the article had a “scent of ‘reefer madness’” about it, given its emphasis on potential harms and safety concerns.
“It’s interesting how alcohol is considered mainstream but cannabis has been demonized,” he said. “Especially for women with breast cancer, it’s so clear that alcohol is related to the development of postmenopausal breast cancer. As a recreational intervention, cannabis in my mind appears to be much safer for women for relaxation.”
“The one thing I worry about are patients who take highly concentrated CBD [cannabidiol] oil, as it can block the metabolism of prescription drugs and allow them to build up in the blood,” Dr. Abrams said. “I advise people against using these products.”
Cannabis to relieve symptoms
Previous studies have noted widespread use of cannabis among patients with cancer. For example, a large study from Israel that included nearly 3,000 participants found that cannabis use improved a variety of cancer-related symptoms, including nausea and vomiting, sleep disorders, pain, anxiety, and depression. Among those with cancer who survived to 6 months and who finished the study protocol, 60% achieved “treatment success.” Of note, at 6 months, 36% of patients had stopped taking opioids, and for 9.9%, the dose of opioids had decreased.
In the current study, dubbed the Coala-T-Cannabis study, the investigators approached U.S. members of the Breastcancer.org and Healthline.com communities who self-reported that they had been diagnosed with breast cancer within the past 5 years; 612 surveys were completed.
Half of all respondents said they had looked for information on medical cannabis, but most were unsatisfied with the information that they had received. Only 6% were extremely satisfied; 25% were very satisfied with the information.
Most patients (39%) did not discuss cannabis use with their physicians. Of those who did, 28% reported feeling uncomfortable discussing the topic. Only 4% of survey respondents indicated that physicians were the most helpful source of cannabis information.
Regarding which source of information was most helpful, 22% said websites, 18% said family members or friends, 12% said staffers and pharmacists in dispensaries, and 7% said other patients with breast cancer.
Forty-two percent of the survey respondents said they used cannabis for medical purposes and for relief of symptoms, which included pain (78%), insomnia (70%), anxiety (57%), stress (51%), and nausea/vomiting (46%).
In addition, 49% believed that medical cannabis could be used to treat the cancer itself.
A fair number were also using cannabis for recreational purposes. Of those who used cannabis, only 23% reported that they used it for medical purposes only.
Participants used cannabis in a variety of forms. The most popular form of consumption was as edibles (70%), followed by liquids/tinctures (65%), smoking (51%), topicals (46%), and vape pens (45%). Participants reported using an average of 3.7 different products.
Safety concerns?
The authors caution about the use of cannabis while receiving anticancer therapies because such use “raises important efficacy and safety concerns.”
“Many chemotherapy agents as well as cannabinoids are metabolized in the liver’s p450 cytochrome system,” Dr. Weiss and colleagues note, and the mechanism by which cannabinoids interact with particular CYP450 isoenzymes “has the potential to alter the metabolism of other medications and lead to adverse side effects.”
They also question the safety of some of the cannabis products that are being used. Participants reported receiving cannabis from a variety of sources, which included state-regulated dispensaries, “dealers,” and family/friends.
Three-quarters of respondents believed that cannabis was better than “chemicals” and that the benefits outweighed the risks. But many of the products used are unregulated, the authors point out.
“Providers should communicate clearly about the health and safety concerns associated with certain cannabis products and methods of delivery,” they conclude. “Without these measures, patients may make these decisions without qualified medical guidance, obtain poor-quality cannabis products, and consume them through potentially hazardous delivery methods during various types of cancer therapies.”
The study was supported by research grants from Ananda Health/Ecofibre and the Dr. Philip Reeves Legacy Fund. Several coauthors reported relationships with industry, as noted in the article. Dr. Abrams owns stock in Cannformatics and Lumen; he has received honorarium from Clever Leaves and Maui Grown Therapies and speaker honorarium from GW Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Most (75%) of the patients who reported using cannabis said it was extremely helpful or very helpful in alleviating symptoms.
The authors warn of potential safety concerns with cannabis, especially with the use of unregulated products.
In addition, the survey found that physicians were not highly regarded as a source of information about cannabis use. Only 39% of patients said that they discussed cannabis with their physicians; 28% reported feeling uncomfortable when broaching the topic. Only 4% indicated that physicians were the most helpful source of information about cannabis.
The survey involved 612 patients with breast cancer. The results were published online Oct. 12 in Cancer.
“Our study highlights an important opportunity for providers to initiate informed conversations about medical cannabis with their patients, as the evidence shows that many are using medical cannabis without our knowledge or guidance,” said lead author Marisa Weiss, MD, of Breastcancer.org and the Lankenau Medical Center, near Philadelphia. “Not knowing whether or not our cancer patients are using cannabis is a major blind spot in our ability to provide optimal care,” she said.
Cannabis in one form or another has been legalized in many states across America, and even in states where it hasn’t been legalized, people are using it.
“Even though many states have relaxed their laws on cannabis, it remains a Schedule I drug on the federal level and is essentially still considered illegal,” commented Donald I. Abrams, MD, professor of medicine at the University of California, San Francisco, and an integrative oncologist at the UCSF Osher Center for Integrative Medicine. “This is why many physicians are uncomfortable discussing it with patients,” he said.
“Cannabis use isn’t taught in medical school, and until that changes, I don’t know how physicians are going to be advisers for this,” said Dr. Abrams, who was approached by this news organization for comment.
This “is a really nice study in that it looks at a large group of breast cancer patients from the community ... It’s not from a single institution [such as this previous study] and so a more representative mix,” Dr. Abrams said.
However, he also commented that the article had a “scent of ‘reefer madness’” about it, given its emphasis on potential harms and safety concerns.
“It’s interesting how alcohol is considered mainstream but cannabis has been demonized,” he said. “Especially for women with breast cancer, it’s so clear that alcohol is related to the development of postmenopausal breast cancer. As a recreational intervention, cannabis in my mind appears to be much safer for women for relaxation.”
“The one thing I worry about are patients who take highly concentrated CBD [cannabidiol] oil, as it can block the metabolism of prescription drugs and allow them to build up in the blood,” Dr. Abrams said. “I advise people against using these products.”
Cannabis to relieve symptoms
Previous studies have noted widespread use of cannabis among patients with cancer. For example, a large study from Israel that included nearly 3,000 participants found that cannabis use improved a variety of cancer-related symptoms, including nausea and vomiting, sleep disorders, pain, anxiety, and depression. Among those with cancer who survived to 6 months and who finished the study protocol, 60% achieved “treatment success.” Of note, at 6 months, 36% of patients had stopped taking opioids, and for 9.9%, the dose of opioids had decreased.
In the current study, dubbed the Coala-T-Cannabis study, the investigators approached U.S. members of the Breastcancer.org and Healthline.com communities who self-reported that they had been diagnosed with breast cancer within the past 5 years; 612 surveys were completed.
Half of all respondents said they had looked for information on medical cannabis, but most were unsatisfied with the information that they had received. Only 6% were extremely satisfied; 25% were very satisfied with the information.
Most patients (39%) did not discuss cannabis use with their physicians. Of those who did, 28% reported feeling uncomfortable discussing the topic. Only 4% of survey respondents indicated that physicians were the most helpful source of cannabis information.
Regarding which source of information was most helpful, 22% said websites, 18% said family members or friends, 12% said staffers and pharmacists in dispensaries, and 7% said other patients with breast cancer.
Forty-two percent of the survey respondents said they used cannabis for medical purposes and for relief of symptoms, which included pain (78%), insomnia (70%), anxiety (57%), stress (51%), and nausea/vomiting (46%).
In addition, 49% believed that medical cannabis could be used to treat the cancer itself.
A fair number were also using cannabis for recreational purposes. Of those who used cannabis, only 23% reported that they used it for medical purposes only.
Participants used cannabis in a variety of forms. The most popular form of consumption was as edibles (70%), followed by liquids/tinctures (65%), smoking (51%), topicals (46%), and vape pens (45%). Participants reported using an average of 3.7 different products.
Safety concerns?
The authors caution about the use of cannabis while receiving anticancer therapies because such use “raises important efficacy and safety concerns.”
“Many chemotherapy agents as well as cannabinoids are metabolized in the liver’s p450 cytochrome system,” Dr. Weiss and colleagues note, and the mechanism by which cannabinoids interact with particular CYP450 isoenzymes “has the potential to alter the metabolism of other medications and lead to adverse side effects.”
They also question the safety of some of the cannabis products that are being used. Participants reported receiving cannabis from a variety of sources, which included state-regulated dispensaries, “dealers,” and family/friends.
Three-quarters of respondents believed that cannabis was better than “chemicals” and that the benefits outweighed the risks. But many of the products used are unregulated, the authors point out.
“Providers should communicate clearly about the health and safety concerns associated with certain cannabis products and methods of delivery,” they conclude. “Without these measures, patients may make these decisions without qualified medical guidance, obtain poor-quality cannabis products, and consume them through potentially hazardous delivery methods during various types of cancer therapies.”
The study was supported by research grants from Ananda Health/Ecofibre and the Dr. Philip Reeves Legacy Fund. Several coauthors reported relationships with industry, as noted in the article. Dr. Abrams owns stock in Cannformatics and Lumen; he has received honorarium from Clever Leaves and Maui Grown Therapies and speaker honorarium from GW Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Most (75%) of the patients who reported using cannabis said it was extremely helpful or very helpful in alleviating symptoms.
The authors warn of potential safety concerns with cannabis, especially with the use of unregulated products.
In addition, the survey found that physicians were not highly regarded as a source of information about cannabis use. Only 39% of patients said that they discussed cannabis with their physicians; 28% reported feeling uncomfortable when broaching the topic. Only 4% indicated that physicians were the most helpful source of information about cannabis.
The survey involved 612 patients with breast cancer. The results were published online Oct. 12 in Cancer.
“Our study highlights an important opportunity for providers to initiate informed conversations about medical cannabis with their patients, as the evidence shows that many are using medical cannabis without our knowledge or guidance,” said lead author Marisa Weiss, MD, of Breastcancer.org and the Lankenau Medical Center, near Philadelphia. “Not knowing whether or not our cancer patients are using cannabis is a major blind spot in our ability to provide optimal care,” she said.
Cannabis in one form or another has been legalized in many states across America, and even in states where it hasn’t been legalized, people are using it.
“Even though many states have relaxed their laws on cannabis, it remains a Schedule I drug on the federal level and is essentially still considered illegal,” commented Donald I. Abrams, MD, professor of medicine at the University of California, San Francisco, and an integrative oncologist at the UCSF Osher Center for Integrative Medicine. “This is why many physicians are uncomfortable discussing it with patients,” he said.
“Cannabis use isn’t taught in medical school, and until that changes, I don’t know how physicians are going to be advisers for this,” said Dr. Abrams, who was approached by this news organization for comment.
This “is a really nice study in that it looks at a large group of breast cancer patients from the community ... It’s not from a single institution [such as this previous study] and so a more representative mix,” Dr. Abrams said.
However, he also commented that the article had a “scent of ‘reefer madness’” about it, given its emphasis on potential harms and safety concerns.
“It’s interesting how alcohol is considered mainstream but cannabis has been demonized,” he said. “Especially for women with breast cancer, it’s so clear that alcohol is related to the development of postmenopausal breast cancer. As a recreational intervention, cannabis in my mind appears to be much safer for women for relaxation.”
“The one thing I worry about are patients who take highly concentrated CBD [cannabidiol] oil, as it can block the metabolism of prescription drugs and allow them to build up in the blood,” Dr. Abrams said. “I advise people against using these products.”
Cannabis to relieve symptoms
Previous studies have noted widespread use of cannabis among patients with cancer. For example, a large study from Israel that included nearly 3,000 participants found that cannabis use improved a variety of cancer-related symptoms, including nausea and vomiting, sleep disorders, pain, anxiety, and depression. Among those with cancer who survived to 6 months and who finished the study protocol, 60% achieved “treatment success.” Of note, at 6 months, 36% of patients had stopped taking opioids, and for 9.9%, the dose of opioids had decreased.
In the current study, dubbed the Coala-T-Cannabis study, the investigators approached U.S. members of the Breastcancer.org and Healthline.com communities who self-reported that they had been diagnosed with breast cancer within the past 5 years; 612 surveys were completed.
Half of all respondents said they had looked for information on medical cannabis, but most were unsatisfied with the information that they had received. Only 6% were extremely satisfied; 25% were very satisfied with the information.
Most patients (39%) did not discuss cannabis use with their physicians. Of those who did, 28% reported feeling uncomfortable discussing the topic. Only 4% of survey respondents indicated that physicians were the most helpful source of cannabis information.
Regarding which source of information was most helpful, 22% said websites, 18% said family members or friends, 12% said staffers and pharmacists in dispensaries, and 7% said other patients with breast cancer.
Forty-two percent of the survey respondents said they used cannabis for medical purposes and for relief of symptoms, which included pain (78%), insomnia (70%), anxiety (57%), stress (51%), and nausea/vomiting (46%).
In addition, 49% believed that medical cannabis could be used to treat the cancer itself.
A fair number were also using cannabis for recreational purposes. Of those who used cannabis, only 23% reported that they used it for medical purposes only.
Participants used cannabis in a variety of forms. The most popular form of consumption was as edibles (70%), followed by liquids/tinctures (65%), smoking (51%), topicals (46%), and vape pens (45%). Participants reported using an average of 3.7 different products.
Safety concerns?
The authors caution about the use of cannabis while receiving anticancer therapies because such use “raises important efficacy and safety concerns.”
“Many chemotherapy agents as well as cannabinoids are metabolized in the liver’s p450 cytochrome system,” Dr. Weiss and colleagues note, and the mechanism by which cannabinoids interact with particular CYP450 isoenzymes “has the potential to alter the metabolism of other medications and lead to adverse side effects.”
They also question the safety of some of the cannabis products that are being used. Participants reported receiving cannabis from a variety of sources, which included state-regulated dispensaries, “dealers,” and family/friends.
Three-quarters of respondents believed that cannabis was better than “chemicals” and that the benefits outweighed the risks. But many of the products used are unregulated, the authors point out.
“Providers should communicate clearly about the health and safety concerns associated with certain cannabis products and methods of delivery,” they conclude. “Without these measures, patients may make these decisions without qualified medical guidance, obtain poor-quality cannabis products, and consume them through potentially hazardous delivery methods during various types of cancer therapies.”
The study was supported by research grants from Ananda Health/Ecofibre and the Dr. Philip Reeves Legacy Fund. Several coauthors reported relationships with industry, as noted in the article. Dr. Abrams owns stock in Cannformatics and Lumen; he has received honorarium from Clever Leaves and Maui Grown Therapies and speaker honorarium from GW Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Moms’ cannabis use in pregnancy tied to anxiety and hyperactivity in offspring
Mothers who use cannabis during pregnancy risk disrupting immune gene networks in the placenta and potentially increasing the risk of anxiety and hyperactivity in their children.
These findings emerged from a study led by Yasmin Hurd, PhD, a professor of psychiatry and director of the Addiction Institute at the Icahn School of Medicine at Mount Sinai, New York, and Yoko Nomura, PhD, a professor of behavioral neuroscience at Queen’s College, City University of New York, that was published online in Proceedings of the National Academy of Sciences.
The analysis assessed the effects of gestational maternal cannabis use on psychosocial and physiological measures in young children as well as its potentially immunomodulatory effect on the in utero environment as reflected in the placental transcriptome.
Participants were drawn from a larger cohort in a study launched in 2012; the investigators evaluated offspring aged 3-6 years for hair hormone levels, neurobehavioral traits on the Behavioral Assessment System for Children survey, and heart rate variability (HRV) at rest and during auditory startle.
The cohort consisted of 322 mother-child dyads and children with prenatal exposure to cannabis were compared with those having no exposure. The cohort consisted of 251 non–cannabis-using mothers and 71 cannabis-using mothers, with mean maternal ages in the two groups of 28.46 years and 25.91 years, respectively, The mothers gave birth at Mount Sinai and they and their children were assessed annually at affiliated medical centers in Mount Sinai’s catchment area.
For a subset of children with behavioral assessments, placental specimens collected at birth were processed for RNA sequencing.
Among the findings:
- Maternal cannabis use was associated with reduced maternal and paternal age, more single-mother pregnancies, state anxiety, trait anxiety, depression, cigarette smoking, and African American race.
- Hair hormone analysis revealed increased cortisol levels in the children of cannabis-using mothers, and was associated with greater anxiety, aggression, and hyperactivity.
- Affected children showed a reduction in the high-frequency component of HRV at baseline, reflecting reduced vagal tone.
- In the placenta, there was reduced expression of many genes involved in immune system function. These included genes for type I interferon, neutrophil, and cytokine-signaling pathways.
Several of these genes organized into coexpression networks that correlated with child anxiety and hyperactivity.
The principal active component of cannabis, tetrahydrocannabinol (THC), targets the endocannabinoid system in placental tissue and the developing brain, the authors noted. Exposure during pregnancy is associated with a range of adverse outcomes from fetal growth restriction to low birth weight and preterm birth.
“There are cannabinoid receptors on immune cells, and it is known that cannabinoids can alter immune function, which is important for maintaining maternal tolerance and protecting the fetus,” Dr. Hurd said. “It’s not surprising that something that affects the immune cells can have an impact on the developing fetus.”
“Overall, our findings reveal a relationship between [maternal cannabis use] and immune response gene networks in the placenta as a potential mediator of risk for anxiety-related problems in early childhood,” Dr. Hurd and colleagues wrote, adding that the results have significant implications for defining mental health issues in the children gestated by cannabis-smoking mothers.
Their results align with previous research indicating a greater risk for psychiatric illness in children with prenatal cannabis exposure from maternal use.
“While data are pretty limited in this realm, there are other studies that demonstrate a relationship between early child developmental and behavioral measures and cannabis use during pregnancy,” Camille Hoffman, MD, MSc, a high-risk obstetrics specialist and an associate professor at the University of Colorado at Denver, Aurora, said in an interview. “Our research group found children exposed to cannabis in utero at 10 weeks’ gestation and beyond were less interactive and more withdrawn than children who were not exposed.”
And THC remains in maternal breast milk even 6 weeks after usage stops.
The long-term effects of prenatal cannabis exposure remain to be determined and it is unknown whether the effects of gestational THC might attenuate as a child grows older. “We use early childhood measures in research as a proxy for the later development of diagnosed mental health conditions or behavioral problems,” Dr. Hoffman explained. “We know when we do this that not every child with an abnormal score early will go on to develop an actual condition. Fortunately, or unfortunately, other factors and exposures during childhood can change the trajectory for the better or worse.”
According to Dr. Hurd, child development is a dynamic process and epigenetic events in utero need not be deterministic. “The important thing is to identify children at risk early and to be able to go in and try to improve the environment they’re being raised in – not in terms of impoverishment but in terms of positive nurturing and giving the mother and family support.”
At the prenatal level, what’s the best advice for cannabis-using mothers-to-be? “If a woman doesn’t know she’s pregnant and has been using cannabis, taking extra choline for the remainder of the pregnancy can help buffer the potential negative impact of the cannabis exposure,” Dr. Hoffman said. The Food and Drug Administration and the American Medical Association recommend a dose of 550 mg daily. “The same is true for alcohol, which we know is also very bad for fetal brain development. This is not to say go ahead and use these substances and just take choline. The choline is more to try and salvage damage to the fetal brain that may have already occurred.”
This study was supported by the National Institute of Mental Health and the National Institute on Drug Abuse. The authors declared no competing interests. Dr. Hoffman disclosed no conflicts of interest with respect to her comments.
Mothers who use cannabis during pregnancy risk disrupting immune gene networks in the placenta and potentially increasing the risk of anxiety and hyperactivity in their children.
These findings emerged from a study led by Yasmin Hurd, PhD, a professor of psychiatry and director of the Addiction Institute at the Icahn School of Medicine at Mount Sinai, New York, and Yoko Nomura, PhD, a professor of behavioral neuroscience at Queen’s College, City University of New York, that was published online in Proceedings of the National Academy of Sciences.
The analysis assessed the effects of gestational maternal cannabis use on psychosocial and physiological measures in young children as well as its potentially immunomodulatory effect on the in utero environment as reflected in the placental transcriptome.
Participants were drawn from a larger cohort in a study launched in 2012; the investigators evaluated offspring aged 3-6 years for hair hormone levels, neurobehavioral traits on the Behavioral Assessment System for Children survey, and heart rate variability (HRV) at rest and during auditory startle.
The cohort consisted of 322 mother-child dyads and children with prenatal exposure to cannabis were compared with those having no exposure. The cohort consisted of 251 non–cannabis-using mothers and 71 cannabis-using mothers, with mean maternal ages in the two groups of 28.46 years and 25.91 years, respectively, The mothers gave birth at Mount Sinai and they and their children were assessed annually at affiliated medical centers in Mount Sinai’s catchment area.
For a subset of children with behavioral assessments, placental specimens collected at birth were processed for RNA sequencing.
Among the findings:
- Maternal cannabis use was associated with reduced maternal and paternal age, more single-mother pregnancies, state anxiety, trait anxiety, depression, cigarette smoking, and African American race.
- Hair hormone analysis revealed increased cortisol levels in the children of cannabis-using mothers, and was associated with greater anxiety, aggression, and hyperactivity.
- Affected children showed a reduction in the high-frequency component of HRV at baseline, reflecting reduced vagal tone.
- In the placenta, there was reduced expression of many genes involved in immune system function. These included genes for type I interferon, neutrophil, and cytokine-signaling pathways.
Several of these genes organized into coexpression networks that correlated with child anxiety and hyperactivity.
The principal active component of cannabis, tetrahydrocannabinol (THC), targets the endocannabinoid system in placental tissue and the developing brain, the authors noted. Exposure during pregnancy is associated with a range of adverse outcomes from fetal growth restriction to low birth weight and preterm birth.
“There are cannabinoid receptors on immune cells, and it is known that cannabinoids can alter immune function, which is important for maintaining maternal tolerance and protecting the fetus,” Dr. Hurd said. “It’s not surprising that something that affects the immune cells can have an impact on the developing fetus.”
“Overall, our findings reveal a relationship between [maternal cannabis use] and immune response gene networks in the placenta as a potential mediator of risk for anxiety-related problems in early childhood,” Dr. Hurd and colleagues wrote, adding that the results have significant implications for defining mental health issues in the children gestated by cannabis-smoking mothers.
Their results align with previous research indicating a greater risk for psychiatric illness in children with prenatal cannabis exposure from maternal use.
“While data are pretty limited in this realm, there are other studies that demonstrate a relationship between early child developmental and behavioral measures and cannabis use during pregnancy,” Camille Hoffman, MD, MSc, a high-risk obstetrics specialist and an associate professor at the University of Colorado at Denver, Aurora, said in an interview. “Our research group found children exposed to cannabis in utero at 10 weeks’ gestation and beyond were less interactive and more withdrawn than children who were not exposed.”
And THC remains in maternal breast milk even 6 weeks after usage stops.
The long-term effects of prenatal cannabis exposure remain to be determined and it is unknown whether the effects of gestational THC might attenuate as a child grows older. “We use early childhood measures in research as a proxy for the later development of diagnosed mental health conditions or behavioral problems,” Dr. Hoffman explained. “We know when we do this that not every child with an abnormal score early will go on to develop an actual condition. Fortunately, or unfortunately, other factors and exposures during childhood can change the trajectory for the better or worse.”
According to Dr. Hurd, child development is a dynamic process and epigenetic events in utero need not be deterministic. “The important thing is to identify children at risk early and to be able to go in and try to improve the environment they’re being raised in – not in terms of impoverishment but in terms of positive nurturing and giving the mother and family support.”
At the prenatal level, what’s the best advice for cannabis-using mothers-to-be? “If a woman doesn’t know she’s pregnant and has been using cannabis, taking extra choline for the remainder of the pregnancy can help buffer the potential negative impact of the cannabis exposure,” Dr. Hoffman said. The Food and Drug Administration and the American Medical Association recommend a dose of 550 mg daily. “The same is true for alcohol, which we know is also very bad for fetal brain development. This is not to say go ahead and use these substances and just take choline. The choline is more to try and salvage damage to the fetal brain that may have already occurred.”
This study was supported by the National Institute of Mental Health and the National Institute on Drug Abuse. The authors declared no competing interests. Dr. Hoffman disclosed no conflicts of interest with respect to her comments.
Mothers who use cannabis during pregnancy risk disrupting immune gene networks in the placenta and potentially increasing the risk of anxiety and hyperactivity in their children.
These findings emerged from a study led by Yasmin Hurd, PhD, a professor of psychiatry and director of the Addiction Institute at the Icahn School of Medicine at Mount Sinai, New York, and Yoko Nomura, PhD, a professor of behavioral neuroscience at Queen’s College, City University of New York, that was published online in Proceedings of the National Academy of Sciences.
The analysis assessed the effects of gestational maternal cannabis use on psychosocial and physiological measures in young children as well as its potentially immunomodulatory effect on the in utero environment as reflected in the placental transcriptome.
Participants were drawn from a larger cohort in a study launched in 2012; the investigators evaluated offspring aged 3-6 years for hair hormone levels, neurobehavioral traits on the Behavioral Assessment System for Children survey, and heart rate variability (HRV) at rest and during auditory startle.
The cohort consisted of 322 mother-child dyads and children with prenatal exposure to cannabis were compared with those having no exposure. The cohort consisted of 251 non–cannabis-using mothers and 71 cannabis-using mothers, with mean maternal ages in the two groups of 28.46 years and 25.91 years, respectively, The mothers gave birth at Mount Sinai and they and their children were assessed annually at affiliated medical centers in Mount Sinai’s catchment area.
For a subset of children with behavioral assessments, placental specimens collected at birth were processed for RNA sequencing.
Among the findings:
- Maternal cannabis use was associated with reduced maternal and paternal age, more single-mother pregnancies, state anxiety, trait anxiety, depression, cigarette smoking, and African American race.
- Hair hormone analysis revealed increased cortisol levels in the children of cannabis-using mothers, and was associated with greater anxiety, aggression, and hyperactivity.
- Affected children showed a reduction in the high-frequency component of HRV at baseline, reflecting reduced vagal tone.
- In the placenta, there was reduced expression of many genes involved in immune system function. These included genes for type I interferon, neutrophil, and cytokine-signaling pathways.
Several of these genes organized into coexpression networks that correlated with child anxiety and hyperactivity.
The principal active component of cannabis, tetrahydrocannabinol (THC), targets the endocannabinoid system in placental tissue and the developing brain, the authors noted. Exposure during pregnancy is associated with a range of adverse outcomes from fetal growth restriction to low birth weight and preterm birth.
“There are cannabinoid receptors on immune cells, and it is known that cannabinoids can alter immune function, which is important for maintaining maternal tolerance and protecting the fetus,” Dr. Hurd said. “It’s not surprising that something that affects the immune cells can have an impact on the developing fetus.”
“Overall, our findings reveal a relationship between [maternal cannabis use] and immune response gene networks in the placenta as a potential mediator of risk for anxiety-related problems in early childhood,” Dr. Hurd and colleagues wrote, adding that the results have significant implications for defining mental health issues in the children gestated by cannabis-smoking mothers.
Their results align with previous research indicating a greater risk for psychiatric illness in children with prenatal cannabis exposure from maternal use.
“While data are pretty limited in this realm, there are other studies that demonstrate a relationship between early child developmental and behavioral measures and cannabis use during pregnancy,” Camille Hoffman, MD, MSc, a high-risk obstetrics specialist and an associate professor at the University of Colorado at Denver, Aurora, said in an interview. “Our research group found children exposed to cannabis in utero at 10 weeks’ gestation and beyond were less interactive and more withdrawn than children who were not exposed.”
And THC remains in maternal breast milk even 6 weeks after usage stops.
The long-term effects of prenatal cannabis exposure remain to be determined and it is unknown whether the effects of gestational THC might attenuate as a child grows older. “We use early childhood measures in research as a proxy for the later development of diagnosed mental health conditions or behavioral problems,” Dr. Hoffman explained. “We know when we do this that not every child with an abnormal score early will go on to develop an actual condition. Fortunately, or unfortunately, other factors and exposures during childhood can change the trajectory for the better or worse.”
According to Dr. Hurd, child development is a dynamic process and epigenetic events in utero need not be deterministic. “The important thing is to identify children at risk early and to be able to go in and try to improve the environment they’re being raised in – not in terms of impoverishment but in terms of positive nurturing and giving the mother and family support.”
At the prenatal level, what’s the best advice for cannabis-using mothers-to-be? “If a woman doesn’t know she’s pregnant and has been using cannabis, taking extra choline for the remainder of the pregnancy can help buffer the potential negative impact of the cannabis exposure,” Dr. Hoffman said. The Food and Drug Administration and the American Medical Association recommend a dose of 550 mg daily. “The same is true for alcohol, which we know is also very bad for fetal brain development. This is not to say go ahead and use these substances and just take choline. The choline is more to try and salvage damage to the fetal brain that may have already occurred.”
This study was supported by the National Institute of Mental Health and the National Institute on Drug Abuse. The authors declared no competing interests. Dr. Hoffman disclosed no conflicts of interest with respect to her comments.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Erenumab beats topiramate for migraine in first head-to-head trial
, according to data from almost 800 patients in the first head-to-head trial of its kind.
The findings suggest that erenumab may help overcome longstanding issues with migraine medication adherence, and additional supportive data may alter treatment sequencing, reported lead author Uwe Reuter, MD, professor at Charité Universitätsmedizin Berlin, and colleagues.
“So far, no study has been done in order to compare the efficacy of a monoclonal antibody targeting the CGRP pathway to that of a standard of care oral preventive drug,” the investigators wrote in Cephalalgia.
The phase 4 HER-MES trial aimed to address this knowledge gap by enrolling 777 adult patients with a history of migraine. All patients reported migraine with or without aura for at least 1 year prior to screening. At baseline, most patients (65%) reported 8-14 migraine days per months, followed by 4-7 days (24.0%), and at least 15 days (11.0%). No patients had previously received topiramate or a CGRP-targeting agent.
“HER-MES includes a broad migraine population with two-thirds of the patients in the high-frequency migraine spectrum,” the investigators noted. “Despite a mean disease duration of about 20 years, almost 60% of the patients had not received previous prophylactic treatment, which underlines the long-standing problem of undertreatment in migraine.”
The trial had a double-dummy design; patients were randomized in a 1:1 ratio to receive either subcutaneous erenumab (70 or 140 mg/month) plus oral placebo, or oral topiramate (50-100 mg/day) plus subcutaneous placebo. The topiramate dose was uptitrated over the first 6 weeks. Treatments were given for a total of 24 weeks or until discontinuation due to adverse events, which was the primary endpoint. The secondary endpoint was efficacy over months 4-6, defined as at least 50% reduction in monthly migraine days, compared with baseline. Other patient-reported outcomes were also evaluated.
After 24 weeks, 95.1% of patients were still enrolled in the trial. Discontinuations due to adverse events were almost four times as common in the topiramate group than the erenumab group (38.9% vs. 10.6%; odds ratio [OR], 0.19; confidence interval, 0.13-0.27; P less than .001). Efficacy findings followed suit, with 55.4% of patients in the erenumab group reporting at least 50% reduction in monthly migraine days, compared with 31.2% of patients in the topiramate group (OR, 2.76; 95% CI, 2.06-3.71; P less than.001).
Erenumab significantly improved monthly migraine days, headache impact test (HIT-6) scores, and short form health survey version (SF-35v2) scores, including physical and mental components (P less than .001 for all).
Safety profiles aligned with previous findings.
“Compared to topiramate, treatment with erenumab has a superior tolerability profile and a significantly higher efficacy,” the investigators concluded. “HER-MES supports the potential of erenumab in overcoming issues of low adherence in clinical practice observed with topiramate, lessening migraine burden, and improving quality of life in a broad migraine population.”
Superior tolerability
Commenting on the study, Alan Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews, said this is “a very important, very well conducted trial that documents what many of us already suspected; erenumab clearly has better tolerability than topiramate as well as better efficacy.”
Dr. Rapoport, a past president of the International Headache Society, said the study highlights an area of unmet need in neurology practice.
“Despite most patients in the trial having chronic headaches for 20 years, 60% of them had never received preventive treatment,” he said, noting that this reflects current practice in the United States.
Dr. Rapoport said primary care providers in the United States prescribe preventive migraine medications to 10%-15% of eligible patients. Prescribing rates for general neurologists are slightly higher, he said, ranging from 35% to 40%, while headache specialists prescribe 70%-90% of the time.
“How can we improve this situation?” Dr. Rapoport asked. “For years we have tried to improve it with education, but we need to do a better job. We need to educate our primary care physicians in more practical ways. We have to teach them how to make a diagnosis of high frequency migraine and chronic migraine and strongly suggest that those patients be put on appropriate preventive medications.”
Barriers to care may be systemic, according to Dr. Rapoport.
“One issue in the U.S. is that patients with commercial insurance are almost always required to fail two or three categories of older oral preventive migraine medications before they can get a monoclonal antibody or gepants for prevention,” he said. “It would be good if we could change that system so that patients that absolutely need the better tolerated, more effective preventive medications could get them sooner rather than later. This will help them feel and function better, with less pain, and eventually bring down the cost of migraine therapy.”
While Dr. Reuter and colleagues concluded that revised treatment sequencing may be warranted after more trials show similar results, Dr. Rapoport suggested that “this was such a large, well-performed, 6-month study with few dropouts, that further trials to confirm these findings are unnecessary, in my opinion.”
The HER-MES trial was funded by Novartis. Dr. Reuter and colleagues disclosed additional relationships with Eli Lilly, Teva Pharmaceutical, Allergan, and others. Dr. Rapoport was involved in early topiramate trials for prevention and migraine, and is a speaker for Amgen.
, according to data from almost 800 patients in the first head-to-head trial of its kind.
The findings suggest that erenumab may help overcome longstanding issues with migraine medication adherence, and additional supportive data may alter treatment sequencing, reported lead author Uwe Reuter, MD, professor at Charité Universitätsmedizin Berlin, and colleagues.
“So far, no study has been done in order to compare the efficacy of a monoclonal antibody targeting the CGRP pathway to that of a standard of care oral preventive drug,” the investigators wrote in Cephalalgia.
The phase 4 HER-MES trial aimed to address this knowledge gap by enrolling 777 adult patients with a history of migraine. All patients reported migraine with or without aura for at least 1 year prior to screening. At baseline, most patients (65%) reported 8-14 migraine days per months, followed by 4-7 days (24.0%), and at least 15 days (11.0%). No patients had previously received topiramate or a CGRP-targeting agent.
“HER-MES includes a broad migraine population with two-thirds of the patients in the high-frequency migraine spectrum,” the investigators noted. “Despite a mean disease duration of about 20 years, almost 60% of the patients had not received previous prophylactic treatment, which underlines the long-standing problem of undertreatment in migraine.”
The trial had a double-dummy design; patients were randomized in a 1:1 ratio to receive either subcutaneous erenumab (70 or 140 mg/month) plus oral placebo, or oral topiramate (50-100 mg/day) plus subcutaneous placebo. The topiramate dose was uptitrated over the first 6 weeks. Treatments were given for a total of 24 weeks or until discontinuation due to adverse events, which was the primary endpoint. The secondary endpoint was efficacy over months 4-6, defined as at least 50% reduction in monthly migraine days, compared with baseline. Other patient-reported outcomes were also evaluated.
After 24 weeks, 95.1% of patients were still enrolled in the trial. Discontinuations due to adverse events were almost four times as common in the topiramate group than the erenumab group (38.9% vs. 10.6%; odds ratio [OR], 0.19; confidence interval, 0.13-0.27; P less than .001). Efficacy findings followed suit, with 55.4% of patients in the erenumab group reporting at least 50% reduction in monthly migraine days, compared with 31.2% of patients in the topiramate group (OR, 2.76; 95% CI, 2.06-3.71; P less than.001).
Erenumab significantly improved monthly migraine days, headache impact test (HIT-6) scores, and short form health survey version (SF-35v2) scores, including physical and mental components (P less than .001 for all).
Safety profiles aligned with previous findings.
“Compared to topiramate, treatment with erenumab has a superior tolerability profile and a significantly higher efficacy,” the investigators concluded. “HER-MES supports the potential of erenumab in overcoming issues of low adherence in clinical practice observed with topiramate, lessening migraine burden, and improving quality of life in a broad migraine population.”
Superior tolerability
Commenting on the study, Alan Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews, said this is “a very important, very well conducted trial that documents what many of us already suspected; erenumab clearly has better tolerability than topiramate as well as better efficacy.”
Dr. Rapoport, a past president of the International Headache Society, said the study highlights an area of unmet need in neurology practice.
“Despite most patients in the trial having chronic headaches for 20 years, 60% of them had never received preventive treatment,” he said, noting that this reflects current practice in the United States.
Dr. Rapoport said primary care providers in the United States prescribe preventive migraine medications to 10%-15% of eligible patients. Prescribing rates for general neurologists are slightly higher, he said, ranging from 35% to 40%, while headache specialists prescribe 70%-90% of the time.
“How can we improve this situation?” Dr. Rapoport asked. “For years we have tried to improve it with education, but we need to do a better job. We need to educate our primary care physicians in more practical ways. We have to teach them how to make a diagnosis of high frequency migraine and chronic migraine and strongly suggest that those patients be put on appropriate preventive medications.”
Barriers to care may be systemic, according to Dr. Rapoport.
“One issue in the U.S. is that patients with commercial insurance are almost always required to fail two or three categories of older oral preventive migraine medications before they can get a monoclonal antibody or gepants for prevention,” he said. “It would be good if we could change that system so that patients that absolutely need the better tolerated, more effective preventive medications could get them sooner rather than later. This will help them feel and function better, with less pain, and eventually bring down the cost of migraine therapy.”
While Dr. Reuter and colleagues concluded that revised treatment sequencing may be warranted after more trials show similar results, Dr. Rapoport suggested that “this was such a large, well-performed, 6-month study with few dropouts, that further trials to confirm these findings are unnecessary, in my opinion.”
The HER-MES trial was funded by Novartis. Dr. Reuter and colleagues disclosed additional relationships with Eli Lilly, Teva Pharmaceutical, Allergan, and others. Dr. Rapoport was involved in early topiramate trials for prevention and migraine, and is a speaker for Amgen.
, according to data from almost 800 patients in the first head-to-head trial of its kind.
The findings suggest that erenumab may help overcome longstanding issues with migraine medication adherence, and additional supportive data may alter treatment sequencing, reported lead author Uwe Reuter, MD, professor at Charité Universitätsmedizin Berlin, and colleagues.
“So far, no study has been done in order to compare the efficacy of a monoclonal antibody targeting the CGRP pathway to that of a standard of care oral preventive drug,” the investigators wrote in Cephalalgia.
The phase 4 HER-MES trial aimed to address this knowledge gap by enrolling 777 adult patients with a history of migraine. All patients reported migraine with or without aura for at least 1 year prior to screening. At baseline, most patients (65%) reported 8-14 migraine days per months, followed by 4-7 days (24.0%), and at least 15 days (11.0%). No patients had previously received topiramate or a CGRP-targeting agent.
“HER-MES includes a broad migraine population with two-thirds of the patients in the high-frequency migraine spectrum,” the investigators noted. “Despite a mean disease duration of about 20 years, almost 60% of the patients had not received previous prophylactic treatment, which underlines the long-standing problem of undertreatment in migraine.”
The trial had a double-dummy design; patients were randomized in a 1:1 ratio to receive either subcutaneous erenumab (70 or 140 mg/month) plus oral placebo, or oral topiramate (50-100 mg/day) plus subcutaneous placebo. The topiramate dose was uptitrated over the first 6 weeks. Treatments were given for a total of 24 weeks or until discontinuation due to adverse events, which was the primary endpoint. The secondary endpoint was efficacy over months 4-6, defined as at least 50% reduction in monthly migraine days, compared with baseline. Other patient-reported outcomes were also evaluated.
After 24 weeks, 95.1% of patients were still enrolled in the trial. Discontinuations due to adverse events were almost four times as common in the topiramate group than the erenumab group (38.9% vs. 10.6%; odds ratio [OR], 0.19; confidence interval, 0.13-0.27; P less than .001). Efficacy findings followed suit, with 55.4% of patients in the erenumab group reporting at least 50% reduction in monthly migraine days, compared with 31.2% of patients in the topiramate group (OR, 2.76; 95% CI, 2.06-3.71; P less than.001).
Erenumab significantly improved monthly migraine days, headache impact test (HIT-6) scores, and short form health survey version (SF-35v2) scores, including physical and mental components (P less than .001 for all).
Safety profiles aligned with previous findings.
“Compared to topiramate, treatment with erenumab has a superior tolerability profile and a significantly higher efficacy,” the investigators concluded. “HER-MES supports the potential of erenumab in overcoming issues of low adherence in clinical practice observed with topiramate, lessening migraine burden, and improving quality of life in a broad migraine population.”
Superior tolerability
Commenting on the study, Alan Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews, said this is “a very important, very well conducted trial that documents what many of us already suspected; erenumab clearly has better tolerability than topiramate as well as better efficacy.”
Dr. Rapoport, a past president of the International Headache Society, said the study highlights an area of unmet need in neurology practice.
“Despite most patients in the trial having chronic headaches for 20 years, 60% of them had never received preventive treatment,” he said, noting that this reflects current practice in the United States.
Dr. Rapoport said primary care providers in the United States prescribe preventive migraine medications to 10%-15% of eligible patients. Prescribing rates for general neurologists are slightly higher, he said, ranging from 35% to 40%, while headache specialists prescribe 70%-90% of the time.
“How can we improve this situation?” Dr. Rapoport asked. “For years we have tried to improve it with education, but we need to do a better job. We need to educate our primary care physicians in more practical ways. We have to teach them how to make a diagnosis of high frequency migraine and chronic migraine and strongly suggest that those patients be put on appropriate preventive medications.”
Barriers to care may be systemic, according to Dr. Rapoport.
“One issue in the U.S. is that patients with commercial insurance are almost always required to fail two or three categories of older oral preventive migraine medications before they can get a monoclonal antibody or gepants for prevention,” he said. “It would be good if we could change that system so that patients that absolutely need the better tolerated, more effective preventive medications could get them sooner rather than later. This will help them feel and function better, with less pain, and eventually bring down the cost of migraine therapy.”
While Dr. Reuter and colleagues concluded that revised treatment sequencing may be warranted after more trials show similar results, Dr. Rapoport suggested that “this was such a large, well-performed, 6-month study with few dropouts, that further trials to confirm these findings are unnecessary, in my opinion.”
The HER-MES trial was funded by Novartis. Dr. Reuter and colleagues disclosed additional relationships with Eli Lilly, Teva Pharmaceutical, Allergan, and others. Dr. Rapoport was involved in early topiramate trials for prevention and migraine, and is a speaker for Amgen.
FROM CEPHALALGIA
Hospitalists helped plan COVID-19 field hospitals
‘It’s a great thing to be overprepared’
At the height of the COVID-19 pandemic’s terrifying first wave in the spring of 2020, dozens of hospitals in high-incidence areas either planned or opened temporary, emergency field hospitals to cover anticipated demand for beds beyond the capacity of local permanent hospitals.
Chastened by images of overwhelmed health care systems in Northern Italy and other hard-hit areas,1 the planners used available modeling tools and estimates for projecting maximum potential need in worst-case scenarios. Some of these temporary hospitals never opened. Others opened in convention centers, parking garages, or parking lot tents, and ended up being used to a lesser degree than the worst-case scenarios.
But those who participated in the planning – including, in many cases, hospitalists – believe they created alternate care site manuals that could be quickly revived in the event of future COVID surges or other, similar crises. Better to plan for too much, they say, than not plan for enough.
Field hospitals or alternate care sites are defined in a recent journal article in Prehospital Disaster Medicine as “locations that can be converted to provide either inpatient and/or outpatient health services when existing facilities are compromised by a hazard impact or the volume of patients exceeds available capacity and/or capabilities.”2
The lead author of that report, Sue Anne Bell, PhD, FNP-BC, a disaster expert and assistant professor of nursing at the University of Michigan (UM), was one of five members of the leadership team for planning UM’s field hospital. They used an organizational unit structure based on the U.S. military’s staffing structure, with their work organized around six units of planning: personnel and labor, security, clinical operations, logistics and supply, planning and training, and communications. This team planned a 519-bed step-down care facility, the Michigan Medicine Field Hospital, for a 73,000-foot indoor track and performance facility at the university, three miles from UM’s main hospital. The aim was to provide safe care in a resource-limited environment.
“We were prepared, but the need never materialized as the peak of COVID cases started to subside,” Dr. Bell said. The team was ready to open within days using a “T-Minus” framework of days remaining on an official countdown clock. But when the need and deadlines kept getting pushed back, that gave them more time to develop clearer procedures.
Two Michigan Medicine hospitalists, Christopher Smith, MD, and David Paje, MD, MPH, both professors at UM’s medical school, were intimately involved in the process. “I was the medical director for the respiratory care unit that was opened for COVID patients, so I was pulled in to assist in the field hospital planning,” said Dr. Smith.
Dr. Paje was director of the short-stay unit and had been a medical officer in the U.S. Army, with training in how to set up military field hospitals. He credits that background as helpful for UM’s COVID field hospital planning, along with his experience in hospital medicine operations.
“We expected that these patients would need the expertise of hospitalists, who had quickly become familiar with the peculiarities of the new disease. That played a role in the decisions we made. Hospitalists were at the front lines of COVID care and had unique clinical insights about managing those with severe disease,” Dr. Paje added.
“When we started, the projections were dire. You don’t want to believe something like that is going to happen. When COVID started to cool off, it was more of a relief to us than anything else,” Dr. Smith said. “Still, it was a very worthwhile exercise. At the end of the day, we put together a comprehensive guide, which is ready for the next crisis.”
Baltimore builds a convention center hospital
A COVID-19 field hospital was planned and executed at an exhibit hall in the Baltimore Convention Center, starting in March 2020 under the leadership of Johns Hopkins Bayview hospitalist Eric Howell, MD, MHM, who eventually handed over responsibilities as chief medical officer when he assumed the position of CEO for the Society of Hospital Medicine in July of that year.
Hopkins collaborated with the University of Maryland health system and state leaders, including the Secretary of Health, to open a 252-bed temporary facility, which at its peak carried a census of 48 patients, with no on-site mortality or cardiac arrests, before it was closed in June 2021 – ready to reopen if necessary. It also served as Baltimore’s major site for polymerase chain reaction COVID-19 testing, vaccinations, and monoclonal antibody infusions, along with medical research.
“My belief at the time we started was that my entire 20-year career as a hospitalist had prepared me for the challenge of opening a COVID field hospital,” Dr. Howell said. “I had learned how to build clinical programs. The difference was that instead of months and years to build a program, we only had a few weeks.”
His first request was to bring on an associate medical director for the field hospital, Melinda E. Kantsiper, MD, a hospitalist and director of clinical operations in the Division of Hospital Medicine at Johns Hopkins Bayview. She became the field hospital’s CMO when Dr. Howell moved to SHM. “As hospitalists, we are trained to care for the patient in front of us while at the same time creating systems that can adjust to rapidly changing circumstances,” Dr. Kantsiper said. “We did what was asked and set up a field hospital that cared for a total of 1,500 COVID patients.”
Hospitalists have the tools that are needed for this work, and shouldn’t be reluctant to contribute to field hospital planning, she said. “This was a real eye-opener for me. Eric explained to me that hospitalists really practice acute care medicine, which doesn’t have to be within the four walls of a hospital.”
The Baltimore field hospital has been a fantastic experience, Dr. Kantsiper added. “But it’s not a building designed for health care delivery.” For the right group of providers, the experience of working in a temporary facility such as this can be positive and exhilarating. “But we need to make sure we take care of our staff. It takes a toll. How we keep them safe – physically and emotionally – has to be top of mind,” she said.
The leaders at Hopkins Medicine and their collaborators truly engaged with the field hospital’s mission, Dr. Howell added.
“They gave us a lot of autonomy and helped us break down barriers. They gave us the political capital to say proper PPE was absolutely essential. As hard and devastating as the pandemic has been, one take-away is that we showed that we can be more flexible and elastic in response to actual needs than we used to think.”
Range of challenges
Among the questions that need to be answered by a field hospital’s planners, the first is ‘where to put it?’ The answer is, hopefully, someplace not too far away, large enough, with ready access to supplies and intake. The next question is ‘who is the patient?’ Clinicians must determine who goes to the field hospital versus who stays at the standing hospital. How sick should these patients be? And when do they need to go back to the permanent hospital? Can staff be trained to recognize when patients in the field hospital are starting to decompensate? The EPIC Deterioration Index3 is a proprietary prediction model that was used by more than a hundred hospitals during the pandemic.
The hospitalist team may develop specific inclusion and exclusion criteria – for example, don’t admit patients who are receiving oxygen therapy above a certain threshold or who are hemodynamically unstable. These criteria should reflect the capacity of the field hospital and the needs of the permanent hospital. At Michigan, as at other field hospital sites, the goal was to offer a step-down or postacute setting for patients with COVID-19 who were too sick to return home but didn’t need acute or ICU-level care, thereby freeing up beds at the permanent hospital for patients who were sicker.
Other questions: What is the process for admissions and discharges? How will patients be transported? What kind of staffing is needed, and what levels of care will be provided? What about rehabilitation services, or palliative care? What about patients with substance abuse or psychiatric comorbidities?
“Are we going to do paper charting? How will that work out for long-term documentation and billing?” Dr. Bell said. A clear reporting structure and communication pathways are essential. Among the other operational processes to address, outlined in Dr. Bell’s article, are orientation and training, PPE donning and doffing procedures, the code or rapid response team, patient and staff food and nutrition, infection control protocols, pharmacy services, access to radiology, rounding procedures, staff support, and the morgue.
One other issue that shouldn’t be overlooked is health equity in the field hospital. “Providing safe and equitable care should be the focus. Thinking who goes to the field hospital should be done within a health equity framework,” Dr. Bell said.4 She also wonders if field hospital planners are sharing their experience with colleagues across the country and developing more collaborative relationships with other hospitals in their communities.
“Field hospitals can be different things,” Dr. Bell said. “The important take-home is it doesn’t have to be in a tent or a parking garage, which can be suboptimal.” In many cases, it may be better to focus on finding unused space within the hospital – whether a lobby, staff lounge, or unoccupied unit – closer to personnel, supplies, pharmacy, and the like. “I think the pandemic showed us how unprepared we were as a health care system, and how much more we need to do in preparation for future crises.”
Limits to the temporary hospital
In New York City, which had the country’s worst COVID-19 outbreak during the first surge in the spring of 2020, a 1,000-bed field hospital was opened at the Jacob Javits Center in March 2020 and closed that June. “I was in the field hospital early, in March and April, when our hospitals were temporarily overrun,” said hospitalist Mona Krouss, MD, FACP, CPPS, NYC Health + Hospitals’ director of patient safety. “My role was to figure out how to get patients on our medical floors into these field hospitals, with responsibility for helping to revise admission criteria,” she said.
“No one knew how horrible it would become. This was so unanticipated, so difficult to operationalize. What they were able to create was amazing, but there were just too many barriers to have it work smoothly,” Dr. Krouss said.
“The military stepped in, and they helped us so much. We wouldn’t have been able to survive without their help.” But there is only so much a field hospital can do to provide acute medical care. Later, military medical teams shifted to roles in temporary units inside the permanent hospitals. “They came to the hospital wanting to be deployed,” she said.
“We could only send patients [to the field hospital] who were fairly stable, and choosing the right ones was difficult.” Dr. Krouss said. In the end, not a lot of COVID-19 patients from NYC Health + Hospitals ended up going to the Javits Center, in part because the paperwork and logistics of getting someone in was a barrier, Dr. Krouss said. A process was established for referring doctors to call a phone number and speak with a New York City Department of Health employee to go through the criteria for admission to the field hospital.
“That could take up to 30 minutes before getting approval. Then you had to go through the same process all over again for sign-out to another physician, and then register the patient with a special bar code. Then you had to arrange ambulance transfer. Doctors didn’t want to go through all of that – everybody was too busy,” she explained. Hospitalists have since worked on streamlining the criteria. “Now we have a good process for the future. We made it more seamless,” she noted.
Susan Lee, DO, MBA, hospitalist and chief medical officer for Renown Regional Medical Center in Reno, Nev., helped to plan an alternate care site in anticipation of up to a thousand COVID patients in her community – far beyond the scope of the existing hospitals. Hospitalists were involved the entire time in planning, design of the unit, design of staffing models, care protocols, and the like, working through an evidence-based medical committee and a COVID-19 provider task force for the Renown Health System.
“Because of a history of fires and earthquakes in this region, we had an emergency planning infrastructure in place. We put the field hospital on the first and second floors of a parking garage, with built-in negative pressure capacity. We also built space for staff break rooms and desk space. It took 10 days to build the hospital, thanks to some very talented people in management and facility design,” Dr. Lee said.
Then, the hospital was locked up and sat empty for 7 months, until the surge in December 2020, when Reno was hit by a bigger wave – this time exceeding the hospitals’ capacity. Through mid-January of 2021, clinicians cared for approximately 240 COVID-19 patients, up to 47 at a time, in the field hospital. A third wave in the autumn of 2021 plateaued at a level lower than the previous fall, so the field hospital is not currently needed.
Replicating hospital work flows
“We ensured that everybody who needed to be within the walls of the permanent hospitals was able to stay there,” said Dr. Lee’s colleague, hospitalist Adnan (Eddy) Akbar, MD. “The postacute system we ordinarily rely on was no longer accepting patients. Other hospitals in the area were able to manage within their capacity because Renown’s field hospital could admit excess patients. We tried to replicate in the field hospital, as much as possible, the work flows and systems of our main hospital.”
When the field hospital finally opened, Dr. Akbar said, “we had a good feeling. We were ready. If something more catastrophic had come down, we were ready to care for more patients. In the field hospital you have to keep monitoring your work flow – almost on a daily basis. But we felt privileged to be working for a system where you knew you can go and care for everyone who needed care.”
One upside of the field hospital experience for participating clinicians, Dr. Lee added, is the opportunity to practice creatively. “The downside is it’s extremely expensive, and has consequences for the mental health of staff. Like so many of these things, it wore on people over time – such as all the time spent donning and doffing protective equipment. And recently the patients have become a lot less gracious.”
Amy Baughman, MD, a hospitalist at Massachusetts General Hospital in Boston, was co-medical director of the postacute care section of a 1,000-bed field hospital, Boston Hope Medical Center, opened in April 2020 at the Boston Convention and Exhibition Center. The other half of the facility was dedicated to undomiciled COVID-19 patients who had no place else to go. Peak census was around 100 patients, housed on four units, each with a clinical team led by a physician.
Dr. Baughman’s field hospital experience has taught her the importance of “staying within your domain of expertise. Physicians are attracted to difficult problems and want to do everything themselves. Next time I won’t be the one installing hand sanitizer dispensers.” A big part of running a field hospital is logistics, she said, and physicians are trained clinicians, not necessarily logistics engineers.
“So it’s important to partner with logistics experts. A huge part of our success in building a facility in 9 days of almost continuous construction was the involvement of the National Guard,” she said. An incident command system was led by an experienced military general incident commander, with two clinical codirectors. The army also sent in full teams of health professionals.
The facility admitted a lot fewer patients than the worst-case projections before it closed in June 2020. “But at the end of the day, we provided a lot of excellent care,” Dr. Baughman said. “This was about preparing for a disaster. It was all hands on deck, and the hands were health professionals. We spent a lot of money for the patients we took care of, but we had no choice, based on what we believed could happen. At that time, so many nursing facilities and homeless shelters were closed to us. It was impossible to predict what utilization would be.”
Subsequent experience has taught that a lot of even seriously ill COVID-19 patients can be managed safely at home, for example, using accelerated home oxygen monitoring with telelinked pulse oximeters. But in the beginning, Dr. Baughman said, “it was a new situation for us. We had seen what happened in Europe and China. It’s a great thing to be overprepared.”
References
1. Horowitz J. Italy’s health care system groans under coronavirus – a warning to the world. New York Times. 2020 Mar 12.
2. Bell SA et al. T-Minus 10 days: The role of an academic medical institution in field hospital planning. Prehosp Disaster Med. 2021 Feb 18:1-6. doi: 10.1017/S1049023X21000224.
3. Singh K et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized patients with COVID-19. Ann Am Thorac Soc. 2021 Jul;18(7):1129-37. doi: 10.1513/AnnalsATS.202006-698OC.
4. Bell SA et al. Alternate care sites during COVID-19 pandemic: Policy implications for pandemic surge planning. Disaster Med Public Health Prep. 2021 Jul 23;1-3. doi: 10.1017/dmp.2021.241.
‘It’s a great thing to be overprepared’
‘It’s a great thing to be overprepared’
At the height of the COVID-19 pandemic’s terrifying first wave in the spring of 2020, dozens of hospitals in high-incidence areas either planned or opened temporary, emergency field hospitals to cover anticipated demand for beds beyond the capacity of local permanent hospitals.
Chastened by images of overwhelmed health care systems in Northern Italy and other hard-hit areas,1 the planners used available modeling tools and estimates for projecting maximum potential need in worst-case scenarios. Some of these temporary hospitals never opened. Others opened in convention centers, parking garages, or parking lot tents, and ended up being used to a lesser degree than the worst-case scenarios.
But those who participated in the planning – including, in many cases, hospitalists – believe they created alternate care site manuals that could be quickly revived in the event of future COVID surges or other, similar crises. Better to plan for too much, they say, than not plan for enough.
Field hospitals or alternate care sites are defined in a recent journal article in Prehospital Disaster Medicine as “locations that can be converted to provide either inpatient and/or outpatient health services when existing facilities are compromised by a hazard impact or the volume of patients exceeds available capacity and/or capabilities.”2
The lead author of that report, Sue Anne Bell, PhD, FNP-BC, a disaster expert and assistant professor of nursing at the University of Michigan (UM), was one of five members of the leadership team for planning UM’s field hospital. They used an organizational unit structure based on the U.S. military’s staffing structure, with their work organized around six units of planning: personnel and labor, security, clinical operations, logistics and supply, planning and training, and communications. This team planned a 519-bed step-down care facility, the Michigan Medicine Field Hospital, for a 73,000-foot indoor track and performance facility at the university, three miles from UM’s main hospital. The aim was to provide safe care in a resource-limited environment.
“We were prepared, but the need never materialized as the peak of COVID cases started to subside,” Dr. Bell said. The team was ready to open within days using a “T-Minus” framework of days remaining on an official countdown clock. But when the need and deadlines kept getting pushed back, that gave them more time to develop clearer procedures.
Two Michigan Medicine hospitalists, Christopher Smith, MD, and David Paje, MD, MPH, both professors at UM’s medical school, were intimately involved in the process. “I was the medical director for the respiratory care unit that was opened for COVID patients, so I was pulled in to assist in the field hospital planning,” said Dr. Smith.
Dr. Paje was director of the short-stay unit and had been a medical officer in the U.S. Army, with training in how to set up military field hospitals. He credits that background as helpful for UM’s COVID field hospital planning, along with his experience in hospital medicine operations.
“We expected that these patients would need the expertise of hospitalists, who had quickly become familiar with the peculiarities of the new disease. That played a role in the decisions we made. Hospitalists were at the front lines of COVID care and had unique clinical insights about managing those with severe disease,” Dr. Paje added.
“When we started, the projections were dire. You don’t want to believe something like that is going to happen. When COVID started to cool off, it was more of a relief to us than anything else,” Dr. Smith said. “Still, it was a very worthwhile exercise. At the end of the day, we put together a comprehensive guide, which is ready for the next crisis.”
Baltimore builds a convention center hospital
A COVID-19 field hospital was planned and executed at an exhibit hall in the Baltimore Convention Center, starting in March 2020 under the leadership of Johns Hopkins Bayview hospitalist Eric Howell, MD, MHM, who eventually handed over responsibilities as chief medical officer when he assumed the position of CEO for the Society of Hospital Medicine in July of that year.
Hopkins collaborated with the University of Maryland health system and state leaders, including the Secretary of Health, to open a 252-bed temporary facility, which at its peak carried a census of 48 patients, with no on-site mortality or cardiac arrests, before it was closed in June 2021 – ready to reopen if necessary. It also served as Baltimore’s major site for polymerase chain reaction COVID-19 testing, vaccinations, and monoclonal antibody infusions, along with medical research.
“My belief at the time we started was that my entire 20-year career as a hospitalist had prepared me for the challenge of opening a COVID field hospital,” Dr. Howell said. “I had learned how to build clinical programs. The difference was that instead of months and years to build a program, we only had a few weeks.”
His first request was to bring on an associate medical director for the field hospital, Melinda E. Kantsiper, MD, a hospitalist and director of clinical operations in the Division of Hospital Medicine at Johns Hopkins Bayview. She became the field hospital’s CMO when Dr. Howell moved to SHM. “As hospitalists, we are trained to care for the patient in front of us while at the same time creating systems that can adjust to rapidly changing circumstances,” Dr. Kantsiper said. “We did what was asked and set up a field hospital that cared for a total of 1,500 COVID patients.”
Hospitalists have the tools that are needed for this work, and shouldn’t be reluctant to contribute to field hospital planning, she said. “This was a real eye-opener for me. Eric explained to me that hospitalists really practice acute care medicine, which doesn’t have to be within the four walls of a hospital.”
The Baltimore field hospital has been a fantastic experience, Dr. Kantsiper added. “But it’s not a building designed for health care delivery.” For the right group of providers, the experience of working in a temporary facility such as this can be positive and exhilarating. “But we need to make sure we take care of our staff. It takes a toll. How we keep them safe – physically and emotionally – has to be top of mind,” she said.
The leaders at Hopkins Medicine and their collaborators truly engaged with the field hospital’s mission, Dr. Howell added.
“They gave us a lot of autonomy and helped us break down barriers. They gave us the political capital to say proper PPE was absolutely essential. As hard and devastating as the pandemic has been, one take-away is that we showed that we can be more flexible and elastic in response to actual needs than we used to think.”
Range of challenges
Among the questions that need to be answered by a field hospital’s planners, the first is ‘where to put it?’ The answer is, hopefully, someplace not too far away, large enough, with ready access to supplies and intake. The next question is ‘who is the patient?’ Clinicians must determine who goes to the field hospital versus who stays at the standing hospital. How sick should these patients be? And when do they need to go back to the permanent hospital? Can staff be trained to recognize when patients in the field hospital are starting to decompensate? The EPIC Deterioration Index3 is a proprietary prediction model that was used by more than a hundred hospitals during the pandemic.
The hospitalist team may develop specific inclusion and exclusion criteria – for example, don’t admit patients who are receiving oxygen therapy above a certain threshold or who are hemodynamically unstable. These criteria should reflect the capacity of the field hospital and the needs of the permanent hospital. At Michigan, as at other field hospital sites, the goal was to offer a step-down or postacute setting for patients with COVID-19 who were too sick to return home but didn’t need acute or ICU-level care, thereby freeing up beds at the permanent hospital for patients who were sicker.
Other questions: What is the process for admissions and discharges? How will patients be transported? What kind of staffing is needed, and what levels of care will be provided? What about rehabilitation services, or palliative care? What about patients with substance abuse or psychiatric comorbidities?
“Are we going to do paper charting? How will that work out for long-term documentation and billing?” Dr. Bell said. A clear reporting structure and communication pathways are essential. Among the other operational processes to address, outlined in Dr. Bell’s article, are orientation and training, PPE donning and doffing procedures, the code or rapid response team, patient and staff food and nutrition, infection control protocols, pharmacy services, access to radiology, rounding procedures, staff support, and the morgue.
One other issue that shouldn’t be overlooked is health equity in the field hospital. “Providing safe and equitable care should be the focus. Thinking who goes to the field hospital should be done within a health equity framework,” Dr. Bell said.4 She also wonders if field hospital planners are sharing their experience with colleagues across the country and developing more collaborative relationships with other hospitals in their communities.
“Field hospitals can be different things,” Dr. Bell said. “The important take-home is it doesn’t have to be in a tent or a parking garage, which can be suboptimal.” In many cases, it may be better to focus on finding unused space within the hospital – whether a lobby, staff lounge, or unoccupied unit – closer to personnel, supplies, pharmacy, and the like. “I think the pandemic showed us how unprepared we were as a health care system, and how much more we need to do in preparation for future crises.”
Limits to the temporary hospital
In New York City, which had the country’s worst COVID-19 outbreak during the first surge in the spring of 2020, a 1,000-bed field hospital was opened at the Jacob Javits Center in March 2020 and closed that June. “I was in the field hospital early, in March and April, when our hospitals were temporarily overrun,” said hospitalist Mona Krouss, MD, FACP, CPPS, NYC Health + Hospitals’ director of patient safety. “My role was to figure out how to get patients on our medical floors into these field hospitals, with responsibility for helping to revise admission criteria,” she said.
“No one knew how horrible it would become. This was so unanticipated, so difficult to operationalize. What they were able to create was amazing, but there were just too many barriers to have it work smoothly,” Dr. Krouss said.
“The military stepped in, and they helped us so much. We wouldn’t have been able to survive without their help.” But there is only so much a field hospital can do to provide acute medical care. Later, military medical teams shifted to roles in temporary units inside the permanent hospitals. “They came to the hospital wanting to be deployed,” she said.
“We could only send patients [to the field hospital] who were fairly stable, and choosing the right ones was difficult.” Dr. Krouss said. In the end, not a lot of COVID-19 patients from NYC Health + Hospitals ended up going to the Javits Center, in part because the paperwork and logistics of getting someone in was a barrier, Dr. Krouss said. A process was established for referring doctors to call a phone number and speak with a New York City Department of Health employee to go through the criteria for admission to the field hospital.
“That could take up to 30 minutes before getting approval. Then you had to go through the same process all over again for sign-out to another physician, and then register the patient with a special bar code. Then you had to arrange ambulance transfer. Doctors didn’t want to go through all of that – everybody was too busy,” she explained. Hospitalists have since worked on streamlining the criteria. “Now we have a good process for the future. We made it more seamless,” she noted.
Susan Lee, DO, MBA, hospitalist and chief medical officer for Renown Regional Medical Center in Reno, Nev., helped to plan an alternate care site in anticipation of up to a thousand COVID patients in her community – far beyond the scope of the existing hospitals. Hospitalists were involved the entire time in planning, design of the unit, design of staffing models, care protocols, and the like, working through an evidence-based medical committee and a COVID-19 provider task force for the Renown Health System.
“Because of a history of fires and earthquakes in this region, we had an emergency planning infrastructure in place. We put the field hospital on the first and second floors of a parking garage, with built-in negative pressure capacity. We also built space for staff break rooms and desk space. It took 10 days to build the hospital, thanks to some very talented people in management and facility design,” Dr. Lee said.
Then, the hospital was locked up and sat empty for 7 months, until the surge in December 2020, when Reno was hit by a bigger wave – this time exceeding the hospitals’ capacity. Through mid-January of 2021, clinicians cared for approximately 240 COVID-19 patients, up to 47 at a time, in the field hospital. A third wave in the autumn of 2021 plateaued at a level lower than the previous fall, so the field hospital is not currently needed.
Replicating hospital work flows
“We ensured that everybody who needed to be within the walls of the permanent hospitals was able to stay there,” said Dr. Lee’s colleague, hospitalist Adnan (Eddy) Akbar, MD. “The postacute system we ordinarily rely on was no longer accepting patients. Other hospitals in the area were able to manage within their capacity because Renown’s field hospital could admit excess patients. We tried to replicate in the field hospital, as much as possible, the work flows and systems of our main hospital.”
When the field hospital finally opened, Dr. Akbar said, “we had a good feeling. We were ready. If something more catastrophic had come down, we were ready to care for more patients. In the field hospital you have to keep monitoring your work flow – almost on a daily basis. But we felt privileged to be working for a system where you knew you can go and care for everyone who needed care.”
One upside of the field hospital experience for participating clinicians, Dr. Lee added, is the opportunity to practice creatively. “The downside is it’s extremely expensive, and has consequences for the mental health of staff. Like so many of these things, it wore on people over time – such as all the time spent donning and doffing protective equipment. And recently the patients have become a lot less gracious.”
Amy Baughman, MD, a hospitalist at Massachusetts General Hospital in Boston, was co-medical director of the postacute care section of a 1,000-bed field hospital, Boston Hope Medical Center, opened in April 2020 at the Boston Convention and Exhibition Center. The other half of the facility was dedicated to undomiciled COVID-19 patients who had no place else to go. Peak census was around 100 patients, housed on four units, each with a clinical team led by a physician.
Dr. Baughman’s field hospital experience has taught her the importance of “staying within your domain of expertise. Physicians are attracted to difficult problems and want to do everything themselves. Next time I won’t be the one installing hand sanitizer dispensers.” A big part of running a field hospital is logistics, she said, and physicians are trained clinicians, not necessarily logistics engineers.
“So it’s important to partner with logistics experts. A huge part of our success in building a facility in 9 days of almost continuous construction was the involvement of the National Guard,” she said. An incident command system was led by an experienced military general incident commander, with two clinical codirectors. The army also sent in full teams of health professionals.
The facility admitted a lot fewer patients than the worst-case projections before it closed in June 2020. “But at the end of the day, we provided a lot of excellent care,” Dr. Baughman said. “This was about preparing for a disaster. It was all hands on deck, and the hands were health professionals. We spent a lot of money for the patients we took care of, but we had no choice, based on what we believed could happen. At that time, so many nursing facilities and homeless shelters were closed to us. It was impossible to predict what utilization would be.”
Subsequent experience has taught that a lot of even seriously ill COVID-19 patients can be managed safely at home, for example, using accelerated home oxygen monitoring with telelinked pulse oximeters. But in the beginning, Dr. Baughman said, “it was a new situation for us. We had seen what happened in Europe and China. It’s a great thing to be overprepared.”
References
1. Horowitz J. Italy’s health care system groans under coronavirus – a warning to the world. New York Times. 2020 Mar 12.
2. Bell SA et al. T-Minus 10 days: The role of an academic medical institution in field hospital planning. Prehosp Disaster Med. 2021 Feb 18:1-6. doi: 10.1017/S1049023X21000224.
3. Singh K et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized patients with COVID-19. Ann Am Thorac Soc. 2021 Jul;18(7):1129-37. doi: 10.1513/AnnalsATS.202006-698OC.
4. Bell SA et al. Alternate care sites during COVID-19 pandemic: Policy implications for pandemic surge planning. Disaster Med Public Health Prep. 2021 Jul 23;1-3. doi: 10.1017/dmp.2021.241.
At the height of the COVID-19 pandemic’s terrifying first wave in the spring of 2020, dozens of hospitals in high-incidence areas either planned or opened temporary, emergency field hospitals to cover anticipated demand for beds beyond the capacity of local permanent hospitals.
Chastened by images of overwhelmed health care systems in Northern Italy and other hard-hit areas,1 the planners used available modeling tools and estimates for projecting maximum potential need in worst-case scenarios. Some of these temporary hospitals never opened. Others opened in convention centers, parking garages, or parking lot tents, and ended up being used to a lesser degree than the worst-case scenarios.
But those who participated in the planning – including, in many cases, hospitalists – believe they created alternate care site manuals that could be quickly revived in the event of future COVID surges or other, similar crises. Better to plan for too much, they say, than not plan for enough.
Field hospitals or alternate care sites are defined in a recent journal article in Prehospital Disaster Medicine as “locations that can be converted to provide either inpatient and/or outpatient health services when existing facilities are compromised by a hazard impact or the volume of patients exceeds available capacity and/or capabilities.”2
The lead author of that report, Sue Anne Bell, PhD, FNP-BC, a disaster expert and assistant professor of nursing at the University of Michigan (UM), was one of five members of the leadership team for planning UM’s field hospital. They used an organizational unit structure based on the U.S. military’s staffing structure, with their work organized around six units of planning: personnel and labor, security, clinical operations, logistics and supply, planning and training, and communications. This team planned a 519-bed step-down care facility, the Michigan Medicine Field Hospital, for a 73,000-foot indoor track and performance facility at the university, three miles from UM’s main hospital. The aim was to provide safe care in a resource-limited environment.
“We were prepared, but the need never materialized as the peak of COVID cases started to subside,” Dr. Bell said. The team was ready to open within days using a “T-Minus” framework of days remaining on an official countdown clock. But when the need and deadlines kept getting pushed back, that gave them more time to develop clearer procedures.
Two Michigan Medicine hospitalists, Christopher Smith, MD, and David Paje, MD, MPH, both professors at UM’s medical school, were intimately involved in the process. “I was the medical director for the respiratory care unit that was opened for COVID patients, so I was pulled in to assist in the field hospital planning,” said Dr. Smith.
Dr. Paje was director of the short-stay unit and had been a medical officer in the U.S. Army, with training in how to set up military field hospitals. He credits that background as helpful for UM’s COVID field hospital planning, along with his experience in hospital medicine operations.
“We expected that these patients would need the expertise of hospitalists, who had quickly become familiar with the peculiarities of the new disease. That played a role in the decisions we made. Hospitalists were at the front lines of COVID care and had unique clinical insights about managing those with severe disease,” Dr. Paje added.
“When we started, the projections were dire. You don’t want to believe something like that is going to happen. When COVID started to cool off, it was more of a relief to us than anything else,” Dr. Smith said. “Still, it was a very worthwhile exercise. At the end of the day, we put together a comprehensive guide, which is ready for the next crisis.”
Baltimore builds a convention center hospital
A COVID-19 field hospital was planned and executed at an exhibit hall in the Baltimore Convention Center, starting in March 2020 under the leadership of Johns Hopkins Bayview hospitalist Eric Howell, MD, MHM, who eventually handed over responsibilities as chief medical officer when he assumed the position of CEO for the Society of Hospital Medicine in July of that year.
Hopkins collaborated with the University of Maryland health system and state leaders, including the Secretary of Health, to open a 252-bed temporary facility, which at its peak carried a census of 48 patients, with no on-site mortality or cardiac arrests, before it was closed in June 2021 – ready to reopen if necessary. It also served as Baltimore’s major site for polymerase chain reaction COVID-19 testing, vaccinations, and monoclonal antibody infusions, along with medical research.
“My belief at the time we started was that my entire 20-year career as a hospitalist had prepared me for the challenge of opening a COVID field hospital,” Dr. Howell said. “I had learned how to build clinical programs. The difference was that instead of months and years to build a program, we only had a few weeks.”
His first request was to bring on an associate medical director for the field hospital, Melinda E. Kantsiper, MD, a hospitalist and director of clinical operations in the Division of Hospital Medicine at Johns Hopkins Bayview. She became the field hospital’s CMO when Dr. Howell moved to SHM. “As hospitalists, we are trained to care for the patient in front of us while at the same time creating systems that can adjust to rapidly changing circumstances,” Dr. Kantsiper said. “We did what was asked and set up a field hospital that cared for a total of 1,500 COVID patients.”
Hospitalists have the tools that are needed for this work, and shouldn’t be reluctant to contribute to field hospital planning, she said. “This was a real eye-opener for me. Eric explained to me that hospitalists really practice acute care medicine, which doesn’t have to be within the four walls of a hospital.”
The Baltimore field hospital has been a fantastic experience, Dr. Kantsiper added. “But it’s not a building designed for health care delivery.” For the right group of providers, the experience of working in a temporary facility such as this can be positive and exhilarating. “But we need to make sure we take care of our staff. It takes a toll. How we keep them safe – physically and emotionally – has to be top of mind,” she said.
The leaders at Hopkins Medicine and their collaborators truly engaged with the field hospital’s mission, Dr. Howell added.
“They gave us a lot of autonomy and helped us break down barriers. They gave us the political capital to say proper PPE was absolutely essential. As hard and devastating as the pandemic has been, one take-away is that we showed that we can be more flexible and elastic in response to actual needs than we used to think.”
Range of challenges
Among the questions that need to be answered by a field hospital’s planners, the first is ‘where to put it?’ The answer is, hopefully, someplace not too far away, large enough, with ready access to supplies and intake. The next question is ‘who is the patient?’ Clinicians must determine who goes to the field hospital versus who stays at the standing hospital. How sick should these patients be? And when do they need to go back to the permanent hospital? Can staff be trained to recognize when patients in the field hospital are starting to decompensate? The EPIC Deterioration Index3 is a proprietary prediction model that was used by more than a hundred hospitals during the pandemic.
The hospitalist team may develop specific inclusion and exclusion criteria – for example, don’t admit patients who are receiving oxygen therapy above a certain threshold or who are hemodynamically unstable. These criteria should reflect the capacity of the field hospital and the needs of the permanent hospital. At Michigan, as at other field hospital sites, the goal was to offer a step-down or postacute setting for patients with COVID-19 who were too sick to return home but didn’t need acute or ICU-level care, thereby freeing up beds at the permanent hospital for patients who were sicker.
Other questions: What is the process for admissions and discharges? How will patients be transported? What kind of staffing is needed, and what levels of care will be provided? What about rehabilitation services, or palliative care? What about patients with substance abuse or psychiatric comorbidities?
“Are we going to do paper charting? How will that work out for long-term documentation and billing?” Dr. Bell said. A clear reporting structure and communication pathways are essential. Among the other operational processes to address, outlined in Dr. Bell’s article, are orientation and training, PPE donning and doffing procedures, the code or rapid response team, patient and staff food and nutrition, infection control protocols, pharmacy services, access to radiology, rounding procedures, staff support, and the morgue.
One other issue that shouldn’t be overlooked is health equity in the field hospital. “Providing safe and equitable care should be the focus. Thinking who goes to the field hospital should be done within a health equity framework,” Dr. Bell said.4 She also wonders if field hospital planners are sharing their experience with colleagues across the country and developing more collaborative relationships with other hospitals in their communities.
“Field hospitals can be different things,” Dr. Bell said. “The important take-home is it doesn’t have to be in a tent or a parking garage, which can be suboptimal.” In many cases, it may be better to focus on finding unused space within the hospital – whether a lobby, staff lounge, or unoccupied unit – closer to personnel, supplies, pharmacy, and the like. “I think the pandemic showed us how unprepared we were as a health care system, and how much more we need to do in preparation for future crises.”
Limits to the temporary hospital
In New York City, which had the country’s worst COVID-19 outbreak during the first surge in the spring of 2020, a 1,000-bed field hospital was opened at the Jacob Javits Center in March 2020 and closed that June. “I was in the field hospital early, in March and April, when our hospitals were temporarily overrun,” said hospitalist Mona Krouss, MD, FACP, CPPS, NYC Health + Hospitals’ director of patient safety. “My role was to figure out how to get patients on our medical floors into these field hospitals, with responsibility for helping to revise admission criteria,” she said.
“No one knew how horrible it would become. This was so unanticipated, so difficult to operationalize. What they were able to create was amazing, but there were just too many barriers to have it work smoothly,” Dr. Krouss said.
“The military stepped in, and they helped us so much. We wouldn’t have been able to survive without their help.” But there is only so much a field hospital can do to provide acute medical care. Later, military medical teams shifted to roles in temporary units inside the permanent hospitals. “They came to the hospital wanting to be deployed,” she said.
“We could only send patients [to the field hospital] who were fairly stable, and choosing the right ones was difficult.” Dr. Krouss said. In the end, not a lot of COVID-19 patients from NYC Health + Hospitals ended up going to the Javits Center, in part because the paperwork and logistics of getting someone in was a barrier, Dr. Krouss said. A process was established for referring doctors to call a phone number and speak with a New York City Department of Health employee to go through the criteria for admission to the field hospital.
“That could take up to 30 minutes before getting approval. Then you had to go through the same process all over again for sign-out to another physician, and then register the patient with a special bar code. Then you had to arrange ambulance transfer. Doctors didn’t want to go through all of that – everybody was too busy,” she explained. Hospitalists have since worked on streamlining the criteria. “Now we have a good process for the future. We made it more seamless,” she noted.
Susan Lee, DO, MBA, hospitalist and chief medical officer for Renown Regional Medical Center in Reno, Nev., helped to plan an alternate care site in anticipation of up to a thousand COVID patients in her community – far beyond the scope of the existing hospitals. Hospitalists were involved the entire time in planning, design of the unit, design of staffing models, care protocols, and the like, working through an evidence-based medical committee and a COVID-19 provider task force for the Renown Health System.
“Because of a history of fires and earthquakes in this region, we had an emergency planning infrastructure in place. We put the field hospital on the first and second floors of a parking garage, with built-in negative pressure capacity. We also built space for staff break rooms and desk space. It took 10 days to build the hospital, thanks to some very talented people in management and facility design,” Dr. Lee said.
Then, the hospital was locked up and sat empty for 7 months, until the surge in December 2020, when Reno was hit by a bigger wave – this time exceeding the hospitals’ capacity. Through mid-January of 2021, clinicians cared for approximately 240 COVID-19 patients, up to 47 at a time, in the field hospital. A third wave in the autumn of 2021 plateaued at a level lower than the previous fall, so the field hospital is not currently needed.
Replicating hospital work flows
“We ensured that everybody who needed to be within the walls of the permanent hospitals was able to stay there,” said Dr. Lee’s colleague, hospitalist Adnan (Eddy) Akbar, MD. “The postacute system we ordinarily rely on was no longer accepting patients. Other hospitals in the area were able to manage within their capacity because Renown’s field hospital could admit excess patients. We tried to replicate in the field hospital, as much as possible, the work flows and systems of our main hospital.”
When the field hospital finally opened, Dr. Akbar said, “we had a good feeling. We were ready. If something more catastrophic had come down, we were ready to care for more patients. In the field hospital you have to keep monitoring your work flow – almost on a daily basis. But we felt privileged to be working for a system where you knew you can go and care for everyone who needed care.”
One upside of the field hospital experience for participating clinicians, Dr. Lee added, is the opportunity to practice creatively. “The downside is it’s extremely expensive, and has consequences for the mental health of staff. Like so many of these things, it wore on people over time – such as all the time spent donning and doffing protective equipment. And recently the patients have become a lot less gracious.”
Amy Baughman, MD, a hospitalist at Massachusetts General Hospital in Boston, was co-medical director of the postacute care section of a 1,000-bed field hospital, Boston Hope Medical Center, opened in April 2020 at the Boston Convention and Exhibition Center. The other half of the facility was dedicated to undomiciled COVID-19 patients who had no place else to go. Peak census was around 100 patients, housed on four units, each with a clinical team led by a physician.
Dr. Baughman’s field hospital experience has taught her the importance of “staying within your domain of expertise. Physicians are attracted to difficult problems and want to do everything themselves. Next time I won’t be the one installing hand sanitizer dispensers.” A big part of running a field hospital is logistics, she said, and physicians are trained clinicians, not necessarily logistics engineers.
“So it’s important to partner with logistics experts. A huge part of our success in building a facility in 9 days of almost continuous construction was the involvement of the National Guard,” she said. An incident command system was led by an experienced military general incident commander, with two clinical codirectors. The army also sent in full teams of health professionals.
The facility admitted a lot fewer patients than the worst-case projections before it closed in June 2020. “But at the end of the day, we provided a lot of excellent care,” Dr. Baughman said. “This was about preparing for a disaster. It was all hands on deck, and the hands were health professionals. We spent a lot of money for the patients we took care of, but we had no choice, based on what we believed could happen. At that time, so many nursing facilities and homeless shelters were closed to us. It was impossible to predict what utilization would be.”
Subsequent experience has taught that a lot of even seriously ill COVID-19 patients can be managed safely at home, for example, using accelerated home oxygen monitoring with telelinked pulse oximeters. But in the beginning, Dr. Baughman said, “it was a new situation for us. We had seen what happened in Europe and China. It’s a great thing to be overprepared.”
References
1. Horowitz J. Italy’s health care system groans under coronavirus – a warning to the world. New York Times. 2020 Mar 12.
2. Bell SA et al. T-Minus 10 days: The role of an academic medical institution in field hospital planning. Prehosp Disaster Med. 2021 Feb 18:1-6. doi: 10.1017/S1049023X21000224.
3. Singh K et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized patients with COVID-19. Ann Am Thorac Soc. 2021 Jul;18(7):1129-37. doi: 10.1513/AnnalsATS.202006-698OC.
4. Bell SA et al. Alternate care sites during COVID-19 pandemic: Policy implications for pandemic surge planning. Disaster Med Public Health Prep. 2021 Jul 23;1-3. doi: 10.1017/dmp.2021.241.
More tools for the COVID toolbox
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.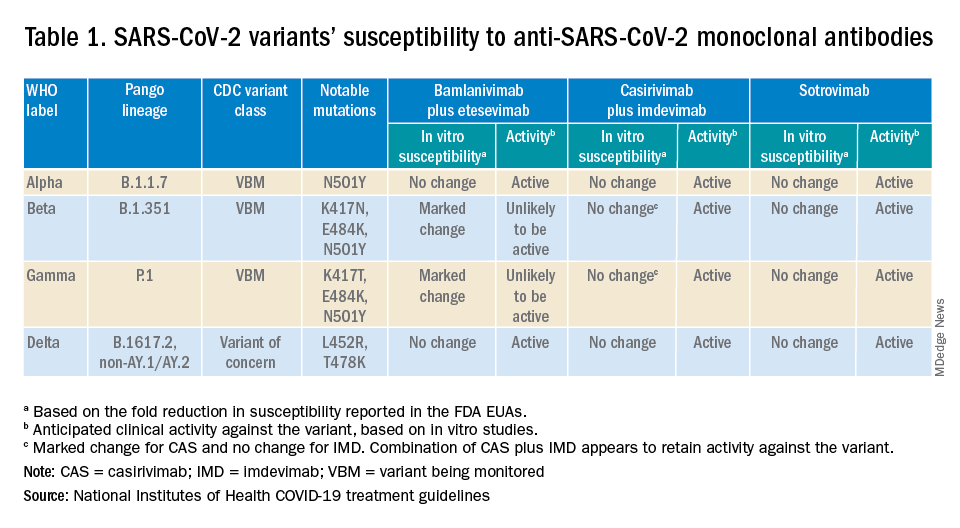
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.
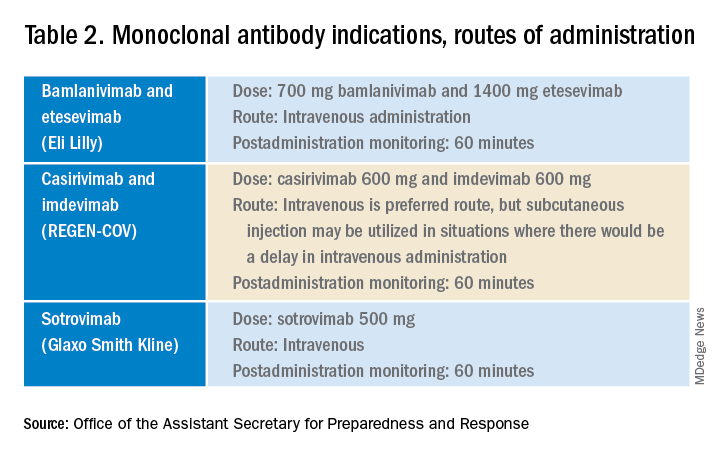
Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
With COVID-19 prevalent in deer, experts urge precautions
With deer hunting season underway or starting in states across the United States, people should wear a mask and gloves when handling deer to prevent coronavirus transmission, experts say.
A recent study by researchers at Penn State University found that more than 80% of the deer sampled during last year’s hunting season in counties across Iowa tested positive for COVID-19. Overall, a third of the deer sampled between September 2020 and January 2021 tested positive.
“The findings suggest that white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans,” the researchers said in a statement.
The deer were likely infected due to “multiple human-to-deer spillover events and deer-to-deer transmission,” they noted.
Studies haven’t shown whether deer have infected humans, but public health experts are recommending that hunters and deer handlers consider possible transmission avenues, The Plain Dealer, a Cleveland newspaper, reported.
To limit deer-to-deer transmission, homeowners and hunters should avoid concentrating deer at backyard feeders or in hunting situations, according to the Ohio Department of Natural Resources Division of Wildlife. The department also urged people to not allow contact between wildlife and domestic animals, including pets and hunting dogs.
Eating venison shouldn’t be a concern if people cook the meat thoroughly, the newspaper reported. Until more is known, people should wear a mask and gloves when handling deer.
In the study, Penn State researchers examined 283 deer between December 2020 and January 2021. They took samples from lymph nodes in the head and neck as part of Iowa’s chronic wasting disease surveillance program.
“This is the first direct evidence of SARS-CoV-2 virus in any free-living species, and our findings have important implications for the ecology and long-term persistence of the virus,” Suresh Kuchipudi, PhD, associate director of the Animal Diagnostics Laboratory at Penn State, said in the statement.
“These include spillover to other free-living or captive animals and potential spillback to human hosts,” he said. “Of course, this highlights that many urgent steps are needed to monitor the spread of the virus in deer and prevent spillback to humans.”
The research team sequenced genomes from all the positive samples and identified 12 coronavirus lineages. The prominent ones corresponded to the same lineages found in humans at the time.
The U.S. Department of Agriculture has also inspected 480 samples this year from white-tailed deer in Illinois, Michigan, New York, and Pennsylvania. Researchers detected virus antibodies in 33% of samples, according to a statement from the department. The department has confirmed the virus in deer in Ohio as well.
Health officials have recommended that hunters also take precautions while around other people by getting vaccinated and wearing a mask, according to WMTV in Wisconsin.
“If someone comes to deer camp and they have COVID and other folks aren’t vaccinated, in that enclosed space with the laughing and good times that are had, the likelihood that those other hunters would be infected is pretty high,” Jeff Pothof, MD, an emergency medicine doctor at UW Health, told the news outlet.
“I think the biggest risk to deer hunters is going to be other hunters, not so much the deer,” he said.
A version of this article first appeared on Medscape.com.
With deer hunting season underway or starting in states across the United States, people should wear a mask and gloves when handling deer to prevent coronavirus transmission, experts say.
A recent study by researchers at Penn State University found that more than 80% of the deer sampled during last year’s hunting season in counties across Iowa tested positive for COVID-19. Overall, a third of the deer sampled between September 2020 and January 2021 tested positive.
“The findings suggest that white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans,” the researchers said in a statement.
The deer were likely infected due to “multiple human-to-deer spillover events and deer-to-deer transmission,” they noted.
Studies haven’t shown whether deer have infected humans, but public health experts are recommending that hunters and deer handlers consider possible transmission avenues, The Plain Dealer, a Cleveland newspaper, reported.
To limit deer-to-deer transmission, homeowners and hunters should avoid concentrating deer at backyard feeders or in hunting situations, according to the Ohio Department of Natural Resources Division of Wildlife. The department also urged people to not allow contact between wildlife and domestic animals, including pets and hunting dogs.
Eating venison shouldn’t be a concern if people cook the meat thoroughly, the newspaper reported. Until more is known, people should wear a mask and gloves when handling deer.
In the study, Penn State researchers examined 283 deer between December 2020 and January 2021. They took samples from lymph nodes in the head and neck as part of Iowa’s chronic wasting disease surveillance program.
“This is the first direct evidence of SARS-CoV-2 virus in any free-living species, and our findings have important implications for the ecology and long-term persistence of the virus,” Suresh Kuchipudi, PhD, associate director of the Animal Diagnostics Laboratory at Penn State, said in the statement.
“These include spillover to other free-living or captive animals and potential spillback to human hosts,” he said. “Of course, this highlights that many urgent steps are needed to monitor the spread of the virus in deer and prevent spillback to humans.”
The research team sequenced genomes from all the positive samples and identified 12 coronavirus lineages. The prominent ones corresponded to the same lineages found in humans at the time.
The U.S. Department of Agriculture has also inspected 480 samples this year from white-tailed deer in Illinois, Michigan, New York, and Pennsylvania. Researchers detected virus antibodies in 33% of samples, according to a statement from the department. The department has confirmed the virus in deer in Ohio as well.
Health officials have recommended that hunters also take precautions while around other people by getting vaccinated and wearing a mask, according to WMTV in Wisconsin.
“If someone comes to deer camp and they have COVID and other folks aren’t vaccinated, in that enclosed space with the laughing and good times that are had, the likelihood that those other hunters would be infected is pretty high,” Jeff Pothof, MD, an emergency medicine doctor at UW Health, told the news outlet.
“I think the biggest risk to deer hunters is going to be other hunters, not so much the deer,” he said.
A version of this article first appeared on Medscape.com.
With deer hunting season underway or starting in states across the United States, people should wear a mask and gloves when handling deer to prevent coronavirus transmission, experts say.
A recent study by researchers at Penn State University found that more than 80% of the deer sampled during last year’s hunting season in counties across Iowa tested positive for COVID-19. Overall, a third of the deer sampled between September 2020 and January 2021 tested positive.
“The findings suggest that white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans,” the researchers said in a statement.
The deer were likely infected due to “multiple human-to-deer spillover events and deer-to-deer transmission,” they noted.
Studies haven’t shown whether deer have infected humans, but public health experts are recommending that hunters and deer handlers consider possible transmission avenues, The Plain Dealer, a Cleveland newspaper, reported.
To limit deer-to-deer transmission, homeowners and hunters should avoid concentrating deer at backyard feeders or in hunting situations, according to the Ohio Department of Natural Resources Division of Wildlife. The department also urged people to not allow contact between wildlife and domestic animals, including pets and hunting dogs.
Eating venison shouldn’t be a concern if people cook the meat thoroughly, the newspaper reported. Until more is known, people should wear a mask and gloves when handling deer.
In the study, Penn State researchers examined 283 deer between December 2020 and January 2021. They took samples from lymph nodes in the head and neck as part of Iowa’s chronic wasting disease surveillance program.
“This is the first direct evidence of SARS-CoV-2 virus in any free-living species, and our findings have important implications for the ecology and long-term persistence of the virus,” Suresh Kuchipudi, PhD, associate director of the Animal Diagnostics Laboratory at Penn State, said in the statement.
“These include spillover to other free-living or captive animals and potential spillback to human hosts,” he said. “Of course, this highlights that many urgent steps are needed to monitor the spread of the virus in deer and prevent spillback to humans.”
The research team sequenced genomes from all the positive samples and identified 12 coronavirus lineages. The prominent ones corresponded to the same lineages found in humans at the time.
The U.S. Department of Agriculture has also inspected 480 samples this year from white-tailed deer in Illinois, Michigan, New York, and Pennsylvania. Researchers detected virus antibodies in 33% of samples, according to a statement from the department. The department has confirmed the virus in deer in Ohio as well.
Health officials have recommended that hunters also take precautions while around other people by getting vaccinated and wearing a mask, according to WMTV in Wisconsin.
“If someone comes to deer camp and they have COVID and other folks aren’t vaccinated, in that enclosed space with the laughing and good times that are had, the likelihood that those other hunters would be infected is pretty high,” Jeff Pothof, MD, an emergency medicine doctor at UW Health, told the news outlet.
“I think the biggest risk to deer hunters is going to be other hunters, not so much the deer,” he said.
A version of this article first appeared on Medscape.com.
Should you worry about picking up COVID or other infections from public bathrooms?
but some experts disagree with the study’s conclusions. The study was published in Science of the Total Environment.
Sotiris Vardoulakis, PhD, of the Australian National University, Canberra, and colleagues reviewed studies of infections associated with public washrooms.
The researchers used keywords to identify potential articles. After screening study abstracts to ensure that only publicly available washrooms with toilets, sinks, and hand dryers were included, 65 studies remained. The investigators excluded washrooms on public transportation (ships, planes, trains, and buses).
“What most of the studies concluded was that what’s really important is to have good hand hygiene and proper maintenance and ventilation of washrooms,” Dr. Vardoulakis said in an interview. “So if the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
There has been ongoing debate on whether electric hand dryers or paper towels are better. Some studies focused on hygiene. Others focused on the environmental cost of paper towels. One concern is that air dryers might spread germs further.
One study focused on the idea that the air recirculation from electric dryers may spread infective aerosols. Another study determined that the Airblade filters in some electric dryers clean more than 99% of the bacteria. The first study, published in Mayo Clinic Proceedings by Cunrui Huang, MMed, MSPH, and colleagues, concluded that “drying hands thoroughly with single-use, disposable paper towels is the preferred method of hand drying in terms of hand hygiene.” Many people prefer to use paper towels because they can be used as a barrier when opening the washroom door.
Dr. Vardoulakis dismissed the air-versus-paper debate, saying, “If the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
Although Dr. Vardoulakis’ review did not find that public washrooms pose a risk for infection, other researchers have shown that some settings do pose problems. For example, toilet plumes are thought to have contributed to the 2003 outbreak of severe acute respiratory syndrome at the Amoy Gardens housing complex in Hong Kong and nearby buildings by aerosolization of fecal waste. Also, norovirus has long been shown to be transmitted by aerosolized particles in vomitus or stool.
Rodney E. Rohde, PhD, professor and chair, clinical lab science program, Texas State University, San Marcos, expressed concern about this systematic review in an interview with this news organization. “I believe one of the major limitations is that studies which involved restrooms on planes, hotels, camping (those camp kids are nasty), and other similar public-access restrooms MUST be included in this type of review. I also believe they excluded restrooms from low-income/rural areas. WHAT? Their ultimate conclusions seem to be in line with the most current understanding about hand hygiene (including drying without devices that create strong air currents, which may create widespread emission of microbes).”
In an interview, Emanuel Goldman, PhD, professor of microbiology, biochemistry, and molecular genetics, New Jersey Medical School, Newark, focused on the COVID-specific aspects of the review. “The chances are less than 1 in 10,000 of getting COVID from a fomite, and that’s very conservative,” he said. “I think it’s a lot lower than that. The virus is fragile. It dies very quickly outside of a human host.” He emphasized, “virtually no infectious virus has been found on fomites over the last 2 years. ... A big mistake in a lot of papers is they confuse viral RNA with the virus. It’s not the same. Viral RNA is the genetic material of the virus, but it also is the ghost of the virus after the virus is dead, and that’s what people are finding. They’re finding the ghost of the virus.”
Because “studies show that the transfer from a surface to fingers is in the neighborhood of 10% efficiency” and one’s fingers also kill the virus, “transmission through your fingers is not easy,” Dr. Goldman said. “You’ve got to really work at it to deliberately infect yourself” with COVID from a fomite.
Dr. Rohde’s conclusion about Dr. Vardoulakis’s review? “So, the question may be, have there been enough studies, in general, of these other areas to include in a review? Otherwise, can we really generalize from this study? I don’t think so.”
Dr. Goldman is not worried about COVID transmission in public bathrooms. His summation: “I think indoor dining is more risky than anything else right now.”
The study was funded by Dyson Technology. Dr. Vardoulakis is a member of the Dyson scientific advisory board.
A version of this article first appeared on Medscape.com.
but some experts disagree with the study’s conclusions. The study was published in Science of the Total Environment.
Sotiris Vardoulakis, PhD, of the Australian National University, Canberra, and colleagues reviewed studies of infections associated with public washrooms.
The researchers used keywords to identify potential articles. After screening study abstracts to ensure that only publicly available washrooms with toilets, sinks, and hand dryers were included, 65 studies remained. The investigators excluded washrooms on public transportation (ships, planes, trains, and buses).
“What most of the studies concluded was that what’s really important is to have good hand hygiene and proper maintenance and ventilation of washrooms,” Dr. Vardoulakis said in an interview. “So if the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
There has been ongoing debate on whether electric hand dryers or paper towels are better. Some studies focused on hygiene. Others focused on the environmental cost of paper towels. One concern is that air dryers might spread germs further.
One study focused on the idea that the air recirculation from electric dryers may spread infective aerosols. Another study determined that the Airblade filters in some electric dryers clean more than 99% of the bacteria. The first study, published in Mayo Clinic Proceedings by Cunrui Huang, MMed, MSPH, and colleagues, concluded that “drying hands thoroughly with single-use, disposable paper towels is the preferred method of hand drying in terms of hand hygiene.” Many people prefer to use paper towels because they can be used as a barrier when opening the washroom door.
Dr. Vardoulakis dismissed the air-versus-paper debate, saying, “If the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
Although Dr. Vardoulakis’ review did not find that public washrooms pose a risk for infection, other researchers have shown that some settings do pose problems. For example, toilet plumes are thought to have contributed to the 2003 outbreak of severe acute respiratory syndrome at the Amoy Gardens housing complex in Hong Kong and nearby buildings by aerosolization of fecal waste. Also, norovirus has long been shown to be transmitted by aerosolized particles in vomitus or stool.
Rodney E. Rohde, PhD, professor and chair, clinical lab science program, Texas State University, San Marcos, expressed concern about this systematic review in an interview with this news organization. “I believe one of the major limitations is that studies which involved restrooms on planes, hotels, camping (those camp kids are nasty), and other similar public-access restrooms MUST be included in this type of review. I also believe they excluded restrooms from low-income/rural areas. WHAT? Their ultimate conclusions seem to be in line with the most current understanding about hand hygiene (including drying without devices that create strong air currents, which may create widespread emission of microbes).”
In an interview, Emanuel Goldman, PhD, professor of microbiology, biochemistry, and molecular genetics, New Jersey Medical School, Newark, focused on the COVID-specific aspects of the review. “The chances are less than 1 in 10,000 of getting COVID from a fomite, and that’s very conservative,” he said. “I think it’s a lot lower than that. The virus is fragile. It dies very quickly outside of a human host.” He emphasized, “virtually no infectious virus has been found on fomites over the last 2 years. ... A big mistake in a lot of papers is they confuse viral RNA with the virus. It’s not the same. Viral RNA is the genetic material of the virus, but it also is the ghost of the virus after the virus is dead, and that’s what people are finding. They’re finding the ghost of the virus.”
Because “studies show that the transfer from a surface to fingers is in the neighborhood of 10% efficiency” and one’s fingers also kill the virus, “transmission through your fingers is not easy,” Dr. Goldman said. “You’ve got to really work at it to deliberately infect yourself” with COVID from a fomite.
Dr. Rohde’s conclusion about Dr. Vardoulakis’s review? “So, the question may be, have there been enough studies, in general, of these other areas to include in a review? Otherwise, can we really generalize from this study? I don’t think so.”
Dr. Goldman is not worried about COVID transmission in public bathrooms. His summation: “I think indoor dining is more risky than anything else right now.”
The study was funded by Dyson Technology. Dr. Vardoulakis is a member of the Dyson scientific advisory board.
A version of this article first appeared on Medscape.com.
but some experts disagree with the study’s conclusions. The study was published in Science of the Total Environment.
Sotiris Vardoulakis, PhD, of the Australian National University, Canberra, and colleagues reviewed studies of infections associated with public washrooms.
The researchers used keywords to identify potential articles. After screening study abstracts to ensure that only publicly available washrooms with toilets, sinks, and hand dryers were included, 65 studies remained. The investigators excluded washrooms on public transportation (ships, planes, trains, and buses).
“What most of the studies concluded was that what’s really important is to have good hand hygiene and proper maintenance and ventilation of washrooms,” Dr. Vardoulakis said in an interview. “So if the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
There has been ongoing debate on whether electric hand dryers or paper towels are better. Some studies focused on hygiene. Others focused on the environmental cost of paper towels. One concern is that air dryers might spread germs further.
One study focused on the idea that the air recirculation from electric dryers may spread infective aerosols. Another study determined that the Airblade filters in some electric dryers clean more than 99% of the bacteria. The first study, published in Mayo Clinic Proceedings by Cunrui Huang, MMed, MSPH, and colleagues, concluded that “drying hands thoroughly with single-use, disposable paper towels is the preferred method of hand drying in terms of hand hygiene.” Many people prefer to use paper towels because they can be used as a barrier when opening the washroom door.
Dr. Vardoulakis dismissed the air-versus-paper debate, saying, “If the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
Although Dr. Vardoulakis’ review did not find that public washrooms pose a risk for infection, other researchers have shown that some settings do pose problems. For example, toilet plumes are thought to have contributed to the 2003 outbreak of severe acute respiratory syndrome at the Amoy Gardens housing complex in Hong Kong and nearby buildings by aerosolization of fecal waste. Also, norovirus has long been shown to be transmitted by aerosolized particles in vomitus or stool.
Rodney E. Rohde, PhD, professor and chair, clinical lab science program, Texas State University, San Marcos, expressed concern about this systematic review in an interview with this news organization. “I believe one of the major limitations is that studies which involved restrooms on planes, hotels, camping (those camp kids are nasty), and other similar public-access restrooms MUST be included in this type of review. I also believe they excluded restrooms from low-income/rural areas. WHAT? Their ultimate conclusions seem to be in line with the most current understanding about hand hygiene (including drying without devices that create strong air currents, which may create widespread emission of microbes).”
In an interview, Emanuel Goldman, PhD, professor of microbiology, biochemistry, and molecular genetics, New Jersey Medical School, Newark, focused on the COVID-specific aspects of the review. “The chances are less than 1 in 10,000 of getting COVID from a fomite, and that’s very conservative,” he said. “I think it’s a lot lower than that. The virus is fragile. It dies very quickly outside of a human host.” He emphasized, “virtually no infectious virus has been found on fomites over the last 2 years. ... A big mistake in a lot of papers is they confuse viral RNA with the virus. It’s not the same. Viral RNA is the genetic material of the virus, but it also is the ghost of the virus after the virus is dead, and that’s what people are finding. They’re finding the ghost of the virus.”
Because “studies show that the transfer from a surface to fingers is in the neighborhood of 10% efficiency” and one’s fingers also kill the virus, “transmission through your fingers is not easy,” Dr. Goldman said. “You’ve got to really work at it to deliberately infect yourself” with COVID from a fomite.
Dr. Rohde’s conclusion about Dr. Vardoulakis’s review? “So, the question may be, have there been enough studies, in general, of these other areas to include in a review? Otherwise, can we really generalize from this study? I don’t think so.”
Dr. Goldman is not worried about COVID transmission in public bathrooms. His summation: “I think indoor dining is more risky than anything else right now.”
The study was funded by Dyson Technology. Dr. Vardoulakis is a member of the Dyson scientific advisory board.
A version of this article first appeared on Medscape.com.
FROM SCIENCE OF THE TOTAL ENVIRONMENT






















