User login
CDC supports ‘test-to-stay’ for COVID- exposed students
The Centers for Disease Control and Prevention has announced that in the following days.
The new guidance, known as the “test-to-stay” protocol, would reduce the number of children who are expected to stay home as a close contact to someone who tested positive for the virus.
“Test-to-stay is an encouraging public health practice to keep our children in schools,” Rochelle Walensky, MD, director of the CDC, said during a White House press briefing.
When a COVID-19 case is identified in a school, the test-to-stay strategy allows schools to implement regular testing rather than quarantine close contacts. If the contacts don’t experience symptoms and test negative at least twice in a seven-day period, they can continue in-person learning. If they test positive, then they are required to isolate.
In recent months, the CDC has collaborated with several school districts across the United States to evaluate test-to-stay programs. On Dec. 17, the CDC published two studies in its Morbidity and Mortality Weekly Report that demonstrated the effectiveness of these programs in limiting the spread of the virus while also keeping students in class.
“CDC is updating our materials to help schools and parents know how to best implement this promising and now-proven practice, along with our multi-layer prevention strategies that will help keep our children in the classroom safely,” Dr. Walensky said. “These studies demonstrated that test-to-stay works to keep unvaccinated children in school safely.”
In one study, researchers analyzed data for public schools in Los Angeles County between Aug. 16 and Oct. 31, where 432 schools implemented test-to-stay and 1,635 did not.
The Los Angeles County Department of Public Health found that COVID-19 cases did not increase among the schools that used the protocol, as compared with schools that didn’t.
Before test-to-stay was implemented, the average daily number of cases was 10 cases per 100,000 students in districts that later adopted the protocol and 20 cases per 100,000 students in districts that didn’t. After the program was implemented, average daily case rates declined in all school districts but remained lower in test-to-stay districts, with 6 cases per 100,000 students as compared with 11 cases per 100,000 students in districts that didn’t do the protocol.
In addition, schools that didn’t use the test-to-stay program “lost substantial in-person school days,” researchers wrote. At the same time, implementing the program “requires resources that might be currently unavailable for some schools,” they added, noting that “a higher percentage of disadvantaged schools” didn’t do the protocol.
The program requires personnel who can track which students need to be tested, their results and when they can come off the list of close contacts, officials told CNN. This can be a challenge for overstretched school nursing staff.
In another study published last week, researchers analyzed data between Aug. 9 and Oct. 29 for 90 schools across 31 districts in Lake County, Ill., that implemented test-to-stay programs. During that time, the schools reported 258 COVID-19 cases and 1,664 close contacts.
The Lake County Health Department examined the number of close contacts that later tested positive and whether the virus further spread from the close contacts to other people. They found that 16 of the close contacts tested positive and that these were all students. No one appeared to transmit the virus to others at school, but nine cases were identified among household contacts.
Overall, study authors wrote, the test-to-stay protocol preserved in-person learning days for students. In addition, regular testing, masking, and physical distancing led to lower virus transmission in school.
“The test-to-stay-programs are really good at balancing the costs and benefits,” Zoe McLaren, a health policy expert at the University of Maryland at Baltimore, told The New York Times.
“What the test-to-stay program does is help us keep COVID cases down, while also trying to make sure we keep kids in school as much as possible, which I think is really important,” she said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention has announced that in the following days.
The new guidance, known as the “test-to-stay” protocol, would reduce the number of children who are expected to stay home as a close contact to someone who tested positive for the virus.
“Test-to-stay is an encouraging public health practice to keep our children in schools,” Rochelle Walensky, MD, director of the CDC, said during a White House press briefing.
When a COVID-19 case is identified in a school, the test-to-stay strategy allows schools to implement regular testing rather than quarantine close contacts. If the contacts don’t experience symptoms and test negative at least twice in a seven-day period, they can continue in-person learning. If they test positive, then they are required to isolate.
In recent months, the CDC has collaborated with several school districts across the United States to evaluate test-to-stay programs. On Dec. 17, the CDC published two studies in its Morbidity and Mortality Weekly Report that demonstrated the effectiveness of these programs in limiting the spread of the virus while also keeping students in class.
“CDC is updating our materials to help schools and parents know how to best implement this promising and now-proven practice, along with our multi-layer prevention strategies that will help keep our children in the classroom safely,” Dr. Walensky said. “These studies demonstrated that test-to-stay works to keep unvaccinated children in school safely.”
In one study, researchers analyzed data for public schools in Los Angeles County between Aug. 16 and Oct. 31, where 432 schools implemented test-to-stay and 1,635 did not.
The Los Angeles County Department of Public Health found that COVID-19 cases did not increase among the schools that used the protocol, as compared with schools that didn’t.
Before test-to-stay was implemented, the average daily number of cases was 10 cases per 100,000 students in districts that later adopted the protocol and 20 cases per 100,000 students in districts that didn’t. After the program was implemented, average daily case rates declined in all school districts but remained lower in test-to-stay districts, with 6 cases per 100,000 students as compared with 11 cases per 100,000 students in districts that didn’t do the protocol.
In addition, schools that didn’t use the test-to-stay program “lost substantial in-person school days,” researchers wrote. At the same time, implementing the program “requires resources that might be currently unavailable for some schools,” they added, noting that “a higher percentage of disadvantaged schools” didn’t do the protocol.
The program requires personnel who can track which students need to be tested, their results and when they can come off the list of close contacts, officials told CNN. This can be a challenge for overstretched school nursing staff.
In another study published last week, researchers analyzed data between Aug. 9 and Oct. 29 for 90 schools across 31 districts in Lake County, Ill., that implemented test-to-stay programs. During that time, the schools reported 258 COVID-19 cases and 1,664 close contacts.
The Lake County Health Department examined the number of close contacts that later tested positive and whether the virus further spread from the close contacts to other people. They found that 16 of the close contacts tested positive and that these were all students. No one appeared to transmit the virus to others at school, but nine cases were identified among household contacts.
Overall, study authors wrote, the test-to-stay protocol preserved in-person learning days for students. In addition, regular testing, masking, and physical distancing led to lower virus transmission in school.
“The test-to-stay-programs are really good at balancing the costs and benefits,” Zoe McLaren, a health policy expert at the University of Maryland at Baltimore, told The New York Times.
“What the test-to-stay program does is help us keep COVID cases down, while also trying to make sure we keep kids in school as much as possible, which I think is really important,” she said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention has announced that in the following days.
The new guidance, known as the “test-to-stay” protocol, would reduce the number of children who are expected to stay home as a close contact to someone who tested positive for the virus.
“Test-to-stay is an encouraging public health practice to keep our children in schools,” Rochelle Walensky, MD, director of the CDC, said during a White House press briefing.
When a COVID-19 case is identified in a school, the test-to-stay strategy allows schools to implement regular testing rather than quarantine close contacts. If the contacts don’t experience symptoms and test negative at least twice in a seven-day period, they can continue in-person learning. If they test positive, then they are required to isolate.
In recent months, the CDC has collaborated with several school districts across the United States to evaluate test-to-stay programs. On Dec. 17, the CDC published two studies in its Morbidity and Mortality Weekly Report that demonstrated the effectiveness of these programs in limiting the spread of the virus while also keeping students in class.
“CDC is updating our materials to help schools and parents know how to best implement this promising and now-proven practice, along with our multi-layer prevention strategies that will help keep our children in the classroom safely,” Dr. Walensky said. “These studies demonstrated that test-to-stay works to keep unvaccinated children in school safely.”
In one study, researchers analyzed data for public schools in Los Angeles County between Aug. 16 and Oct. 31, where 432 schools implemented test-to-stay and 1,635 did not.
The Los Angeles County Department of Public Health found that COVID-19 cases did not increase among the schools that used the protocol, as compared with schools that didn’t.
Before test-to-stay was implemented, the average daily number of cases was 10 cases per 100,000 students in districts that later adopted the protocol and 20 cases per 100,000 students in districts that didn’t. After the program was implemented, average daily case rates declined in all school districts but remained lower in test-to-stay districts, with 6 cases per 100,000 students as compared with 11 cases per 100,000 students in districts that didn’t do the protocol.
In addition, schools that didn’t use the test-to-stay program “lost substantial in-person school days,” researchers wrote. At the same time, implementing the program “requires resources that might be currently unavailable for some schools,” they added, noting that “a higher percentage of disadvantaged schools” didn’t do the protocol.
The program requires personnel who can track which students need to be tested, their results and when they can come off the list of close contacts, officials told CNN. This can be a challenge for overstretched school nursing staff.
In another study published last week, researchers analyzed data between Aug. 9 and Oct. 29 for 90 schools across 31 districts in Lake County, Ill., that implemented test-to-stay programs. During that time, the schools reported 258 COVID-19 cases and 1,664 close contacts.
The Lake County Health Department examined the number of close contacts that later tested positive and whether the virus further spread from the close contacts to other people. They found that 16 of the close contacts tested positive and that these were all students. No one appeared to transmit the virus to others at school, but nine cases were identified among household contacts.
Overall, study authors wrote, the test-to-stay protocol preserved in-person learning days for students. In addition, regular testing, masking, and physical distancing led to lower virus transmission in school.
“The test-to-stay-programs are really good at balancing the costs and benefits,” Zoe McLaren, a health policy expert at the University of Maryland at Baltimore, told The New York Times.
“What the test-to-stay program does is help us keep COVID cases down, while also trying to make sure we keep kids in school as much as possible, which I think is really important,” she said.
A version of this article first appeared on WebMD.com.
Pill mill psychiatrist gets prison; must forfeit cash, luxury cars
Psychiatrist sentenced to 3 years in prison for running pill mill
, including a 2020 Porsche GT4 and a 2020 Aston Martin.
According to the U.S. Department of Justice, the U.S. Drug Enforcement Administration began investigating Gerald Michael Abraham, 76, after receiving a tip that he was prescribing opioids to patients who did not need the medication. The investigation, which began in October 2019, included 18 visits by undercover patients to Abraham’s cash-only clinic in Naples, Fla. Patients paid $400 per visit, according to court documents.
Even though the undercover patients pretended to have signs of drug abuse, Dr. Abraham still prescribed oxycodone, a strong opioid, on each visit without any physical examination, according to officials. He repeatedly increased the strength of the prescriptions when the patients asked. In one case, he told a patient that the patient’s medical paperwork “shows you are completely normal,” then went ahead to prescribe the oxycodone.
Dr. Abraham also gave Adderall to patients who had no legitimate medical need for the drug. As with the opioid, Dr. Abraham prescribed the frequently abused amphetamine (typically used to treat attention-deficit/hyperactivity disorder) after being asked for it, without performing an examination or asking questions to justify the prescription, according to the undercover law enforcement agents.
According to the Florida Department of Health, Dr. Abraham voluntarily relinquished his license in September pending board action. His license was set to expire in January 2022.
Pennsylvania physician admits to distributing drugs resulting in patient’s death
A Pennsylvania internist pleaded guilty to unlawful prescription of controlled substances, maintaining drug-involved premises, and healthcare fraud, as well as that the death of a patient resulted from the use of the controlled drugs.
When entering his plea, Dr.Kurt Moran, 69, acknowledged that the purpose of his Scranton-based pain management practice was to distribute high doses of opioids not for medical purposes, and that he often prescribed these drugs without examining the patient to verify the illness or condition the patient claimed to have. At times, he even prescribed the drugs without seeing the patient. He admitted that he knew that such practices could result in addiction and even death, according to federal officials.
The healthcare fraud charges resulted from a scheme that took place between 2014 and 2017 in which Dr. Moran received bribes in exchange for prescribing the drug Subsys (sublingual fentanyl), which is approved for use only in cancer patients who suffer from breakthrough pain. Court documents allege that Dr. Moran was paid approximately $140,000 over a 2-year period to prescribe Subsys to patients for whom the drug was not indicated.
In order to conceal the kickbacks and bribes, the company that paid Dr. Moran is alleged to have described the payments as honoraria for educational presentations about the drug. During that period, Dr. Moran prescribed millions of micrograms of the sublingual spray to patients with no cancer diagnosis.
Subsys is manufactured by Insys, a company whose founder and CEO, John Kapoor, was sentenced in 2019 to 5½ years in prison for his role in the bribery scheme involving Dr. Moran and other physicians.
Dr. Moran faces 12 years in prison. The maximum penalty for the crimes is 10 years for each charge. As a part of his plea deal, Dr. Moran agreed to forfeit $134,000. His license to practice medicine was suspended in October 2020 according to the Pennsylvania Bureau of Professional and Occupational Affairs.
Former pharmaceutical executive charged with embezzlement
The former owner and CEO of a New Jersey pharmaceutical company is accused of embezzling millions of dollars from his company by federal officials.
According to the charging documents, John Klein, 75, of Palisades Park, N.J., hired a chief financial officer in 2016 who then created a profit-and-loss statement showing the company’s sales and receivables. According to the CFO, Mr. Klein provided information that included an account receivable of approximately $3.9 million that had not been collected.
In December 2016 and January 2017, Mr. Klein approved a reserve against the uncollected receivable in the financial statements. However, back in May 2016, $3.9 million was transferred into a company bank account that Mr. Klein controlled. In a June 2016 email, Mr. Klein acknowledged that the invoices in question had been paid in full. A review of the company bank account showed that Mr. Klein used the money to pay personal debts, such as credit card payments, property taxes, and private-school tuition for his child.
Mr. Klein is the subject of several federal lawsuits from people who worked at Cambridge Therapeutic Technologies in Teaneck, N.J., as well as individuals he collected investment money from, according to media reports.
If convicted, Mr. Klein faces up to 20 years in prison and a fine of $250,000 or twice the gross profits or twice the gross loss suffered by the victims of the fraud, whichever is greatest.
Practice administrator sentenced for defrauding medical practice
An Indiana man was sentenced to 30 months in federal prison for stealing from the ophthalmology practice where he worked for 5 years as a practice administrator.
Joshua D. Millspaugh, 42, of Westfield, Ind., pled guilty to charges of wire fraud, according to federal officials.
Mr. Millspaugh, who earned an annual salary of over $100,000, was responsible for payroll processing, purchasing, and paying the practice’s bills. After less than a year on the job, he began taking advantage of his access to company money to use the practice’s funds for himself. During his 5 years of employment, he made over 500 such transactions, making purchases, paying personal bills, and sending payroll checks to his personal bank account. He concealed these transactions with false entries in the company’s bookkeeping system. When asked about expenditures, he made up false justifications.
During Mr. Millspaugh’s sentencing, William Whitson, MD, owner and director of the practice, Whitson Vision, told the court that the loss to him and the practice was about more than money. In addition to creating long-term credit and banking problems, the situation damaged both the company’s business reputation and the morale of its other employees.
“Fraud on a small business impacts every area of that business,” said U.S. Attorney for the Southern District of Indiana Zachary A. Myers. “It also breeds mistrust, especially if the fraud is perpetrated by a trusted employee. Mr. Millspaugh exploited his position of trust for purely personal gain, and he is now being held accountable for his actions.”
In addition to the prison sentence, Mr. Millspaugh was ordered to pay $270,000 in restitution and will be federally supervised for 3 years following his release from prison.
A version of this article first appeared on Medscape.com.
Psychiatrist sentenced to 3 years in prison for running pill mill
, including a 2020 Porsche GT4 and a 2020 Aston Martin.
According to the U.S. Department of Justice, the U.S. Drug Enforcement Administration began investigating Gerald Michael Abraham, 76, after receiving a tip that he was prescribing opioids to patients who did not need the medication. The investigation, which began in October 2019, included 18 visits by undercover patients to Abraham’s cash-only clinic in Naples, Fla. Patients paid $400 per visit, according to court documents.
Even though the undercover patients pretended to have signs of drug abuse, Dr. Abraham still prescribed oxycodone, a strong opioid, on each visit without any physical examination, according to officials. He repeatedly increased the strength of the prescriptions when the patients asked. In one case, he told a patient that the patient’s medical paperwork “shows you are completely normal,” then went ahead to prescribe the oxycodone.
Dr. Abraham also gave Adderall to patients who had no legitimate medical need for the drug. As with the opioid, Dr. Abraham prescribed the frequently abused amphetamine (typically used to treat attention-deficit/hyperactivity disorder) after being asked for it, without performing an examination or asking questions to justify the prescription, according to the undercover law enforcement agents.
According to the Florida Department of Health, Dr. Abraham voluntarily relinquished his license in September pending board action. His license was set to expire in January 2022.
Pennsylvania physician admits to distributing drugs resulting in patient’s death
A Pennsylvania internist pleaded guilty to unlawful prescription of controlled substances, maintaining drug-involved premises, and healthcare fraud, as well as that the death of a patient resulted from the use of the controlled drugs.
When entering his plea, Dr.Kurt Moran, 69, acknowledged that the purpose of his Scranton-based pain management practice was to distribute high doses of opioids not for medical purposes, and that he often prescribed these drugs without examining the patient to verify the illness or condition the patient claimed to have. At times, he even prescribed the drugs without seeing the patient. He admitted that he knew that such practices could result in addiction and even death, according to federal officials.
The healthcare fraud charges resulted from a scheme that took place between 2014 and 2017 in which Dr. Moran received bribes in exchange for prescribing the drug Subsys (sublingual fentanyl), which is approved for use only in cancer patients who suffer from breakthrough pain. Court documents allege that Dr. Moran was paid approximately $140,000 over a 2-year period to prescribe Subsys to patients for whom the drug was not indicated.
In order to conceal the kickbacks and bribes, the company that paid Dr. Moran is alleged to have described the payments as honoraria for educational presentations about the drug. During that period, Dr. Moran prescribed millions of micrograms of the sublingual spray to patients with no cancer diagnosis.
Subsys is manufactured by Insys, a company whose founder and CEO, John Kapoor, was sentenced in 2019 to 5½ years in prison for his role in the bribery scheme involving Dr. Moran and other physicians.
Dr. Moran faces 12 years in prison. The maximum penalty for the crimes is 10 years for each charge. As a part of his plea deal, Dr. Moran agreed to forfeit $134,000. His license to practice medicine was suspended in October 2020 according to the Pennsylvania Bureau of Professional and Occupational Affairs.
Former pharmaceutical executive charged with embezzlement
The former owner and CEO of a New Jersey pharmaceutical company is accused of embezzling millions of dollars from his company by federal officials.
According to the charging documents, John Klein, 75, of Palisades Park, N.J., hired a chief financial officer in 2016 who then created a profit-and-loss statement showing the company’s sales and receivables. According to the CFO, Mr. Klein provided information that included an account receivable of approximately $3.9 million that had not been collected.
In December 2016 and January 2017, Mr. Klein approved a reserve against the uncollected receivable in the financial statements. However, back in May 2016, $3.9 million was transferred into a company bank account that Mr. Klein controlled. In a June 2016 email, Mr. Klein acknowledged that the invoices in question had been paid in full. A review of the company bank account showed that Mr. Klein used the money to pay personal debts, such as credit card payments, property taxes, and private-school tuition for his child.
Mr. Klein is the subject of several federal lawsuits from people who worked at Cambridge Therapeutic Technologies in Teaneck, N.J., as well as individuals he collected investment money from, according to media reports.
If convicted, Mr. Klein faces up to 20 years in prison and a fine of $250,000 or twice the gross profits or twice the gross loss suffered by the victims of the fraud, whichever is greatest.
Practice administrator sentenced for defrauding medical practice
An Indiana man was sentenced to 30 months in federal prison for stealing from the ophthalmology practice where he worked for 5 years as a practice administrator.
Joshua D. Millspaugh, 42, of Westfield, Ind., pled guilty to charges of wire fraud, according to federal officials.
Mr. Millspaugh, who earned an annual salary of over $100,000, was responsible for payroll processing, purchasing, and paying the practice’s bills. After less than a year on the job, he began taking advantage of his access to company money to use the practice’s funds for himself. During his 5 years of employment, he made over 500 such transactions, making purchases, paying personal bills, and sending payroll checks to his personal bank account. He concealed these transactions with false entries in the company’s bookkeeping system. When asked about expenditures, he made up false justifications.
During Mr. Millspaugh’s sentencing, William Whitson, MD, owner and director of the practice, Whitson Vision, told the court that the loss to him and the practice was about more than money. In addition to creating long-term credit and banking problems, the situation damaged both the company’s business reputation and the morale of its other employees.
“Fraud on a small business impacts every area of that business,” said U.S. Attorney for the Southern District of Indiana Zachary A. Myers. “It also breeds mistrust, especially if the fraud is perpetrated by a trusted employee. Mr. Millspaugh exploited his position of trust for purely personal gain, and he is now being held accountable for his actions.”
In addition to the prison sentence, Mr. Millspaugh was ordered to pay $270,000 in restitution and will be federally supervised for 3 years following his release from prison.
A version of this article first appeared on Medscape.com.
Psychiatrist sentenced to 3 years in prison for running pill mill
, including a 2020 Porsche GT4 and a 2020 Aston Martin.
According to the U.S. Department of Justice, the U.S. Drug Enforcement Administration began investigating Gerald Michael Abraham, 76, after receiving a tip that he was prescribing opioids to patients who did not need the medication. The investigation, which began in October 2019, included 18 visits by undercover patients to Abraham’s cash-only clinic in Naples, Fla. Patients paid $400 per visit, according to court documents.
Even though the undercover patients pretended to have signs of drug abuse, Dr. Abraham still prescribed oxycodone, a strong opioid, on each visit without any physical examination, according to officials. He repeatedly increased the strength of the prescriptions when the patients asked. In one case, he told a patient that the patient’s medical paperwork “shows you are completely normal,” then went ahead to prescribe the oxycodone.
Dr. Abraham also gave Adderall to patients who had no legitimate medical need for the drug. As with the opioid, Dr. Abraham prescribed the frequently abused amphetamine (typically used to treat attention-deficit/hyperactivity disorder) after being asked for it, without performing an examination or asking questions to justify the prescription, according to the undercover law enforcement agents.
According to the Florida Department of Health, Dr. Abraham voluntarily relinquished his license in September pending board action. His license was set to expire in January 2022.
Pennsylvania physician admits to distributing drugs resulting in patient’s death
A Pennsylvania internist pleaded guilty to unlawful prescription of controlled substances, maintaining drug-involved premises, and healthcare fraud, as well as that the death of a patient resulted from the use of the controlled drugs.
When entering his plea, Dr.Kurt Moran, 69, acknowledged that the purpose of his Scranton-based pain management practice was to distribute high doses of opioids not for medical purposes, and that he often prescribed these drugs without examining the patient to verify the illness or condition the patient claimed to have. At times, he even prescribed the drugs without seeing the patient. He admitted that he knew that such practices could result in addiction and even death, according to federal officials.
The healthcare fraud charges resulted from a scheme that took place between 2014 and 2017 in which Dr. Moran received bribes in exchange for prescribing the drug Subsys (sublingual fentanyl), which is approved for use only in cancer patients who suffer from breakthrough pain. Court documents allege that Dr. Moran was paid approximately $140,000 over a 2-year period to prescribe Subsys to patients for whom the drug was not indicated.
In order to conceal the kickbacks and bribes, the company that paid Dr. Moran is alleged to have described the payments as honoraria for educational presentations about the drug. During that period, Dr. Moran prescribed millions of micrograms of the sublingual spray to patients with no cancer diagnosis.
Subsys is manufactured by Insys, a company whose founder and CEO, John Kapoor, was sentenced in 2019 to 5½ years in prison for his role in the bribery scheme involving Dr. Moran and other physicians.
Dr. Moran faces 12 years in prison. The maximum penalty for the crimes is 10 years for each charge. As a part of his plea deal, Dr. Moran agreed to forfeit $134,000. His license to practice medicine was suspended in October 2020 according to the Pennsylvania Bureau of Professional and Occupational Affairs.
Former pharmaceutical executive charged with embezzlement
The former owner and CEO of a New Jersey pharmaceutical company is accused of embezzling millions of dollars from his company by federal officials.
According to the charging documents, John Klein, 75, of Palisades Park, N.J., hired a chief financial officer in 2016 who then created a profit-and-loss statement showing the company’s sales and receivables. According to the CFO, Mr. Klein provided information that included an account receivable of approximately $3.9 million that had not been collected.
In December 2016 and January 2017, Mr. Klein approved a reserve against the uncollected receivable in the financial statements. However, back in May 2016, $3.9 million was transferred into a company bank account that Mr. Klein controlled. In a June 2016 email, Mr. Klein acknowledged that the invoices in question had been paid in full. A review of the company bank account showed that Mr. Klein used the money to pay personal debts, such as credit card payments, property taxes, and private-school tuition for his child.
Mr. Klein is the subject of several federal lawsuits from people who worked at Cambridge Therapeutic Technologies in Teaneck, N.J., as well as individuals he collected investment money from, according to media reports.
If convicted, Mr. Klein faces up to 20 years in prison and a fine of $250,000 or twice the gross profits or twice the gross loss suffered by the victims of the fraud, whichever is greatest.
Practice administrator sentenced for defrauding medical practice
An Indiana man was sentenced to 30 months in federal prison for stealing from the ophthalmology practice where he worked for 5 years as a practice administrator.
Joshua D. Millspaugh, 42, of Westfield, Ind., pled guilty to charges of wire fraud, according to federal officials.
Mr. Millspaugh, who earned an annual salary of over $100,000, was responsible for payroll processing, purchasing, and paying the practice’s bills. After less than a year on the job, he began taking advantage of his access to company money to use the practice’s funds for himself. During his 5 years of employment, he made over 500 such transactions, making purchases, paying personal bills, and sending payroll checks to his personal bank account. He concealed these transactions with false entries in the company’s bookkeeping system. When asked about expenditures, he made up false justifications.
During Mr. Millspaugh’s sentencing, William Whitson, MD, owner and director of the practice, Whitson Vision, told the court that the loss to him and the practice was about more than money. In addition to creating long-term credit and banking problems, the situation damaged both the company’s business reputation and the morale of its other employees.
“Fraud on a small business impacts every area of that business,” said U.S. Attorney for the Southern District of Indiana Zachary A. Myers. “It also breeds mistrust, especially if the fraud is perpetrated by a trusted employee. Mr. Millspaugh exploited his position of trust for purely personal gain, and he is now being held accountable for his actions.”
In addition to the prison sentence, Mr. Millspaugh was ordered to pay $270,000 in restitution and will be federally supervised for 3 years following his release from prison.
A version of this article first appeared on Medscape.com.
FDA approves tezepelumab-ekko (Tezspire) for severe asthma
The Food and Drug Administration has approved tezepelumab-ekko (Tezspire) as a first-in-class treatment for severe asthma in adults and pediatric patients aged 12 years and older. It is not recommended for the relief of acute bronchospasm or status asthmaticus.
Tezepelumab-ekko is a human monoclonal antibody that acts as a thymic stromal lymphopoietin (TSLP) blocker. TSLP is an epithelial cell–derived cytokine implicated in the pathogenesis of asthma. Tezepelumab-ekko is administered by subcutaneous injection at a recommended dosage of 210 mg given once every 4 weeks.
“Tezspire represents a much-needed new treatment for the many patients who remain underserved and continue to struggle with severe, uncontrolled asthma,” said professor Andrew Menzies-Gow, MD, PhD, director of the lung division, Royal Brompton Hospital, London, and the principal investigator of the pivotal NAVIGATOR trial, in a Dec. 17 Amgen press release.
Trial results
The early approval of the treatment was based on the results of various clinical trials, primarily the NAVIGATOR phase 3 trial, results of which were published in the New England Journal of Medicine in May 2021.
In the NAVIGATOR trial, a total of 1,061 patients were randomly assigned to receive tezepelumab (529 patients) or placebo (532 patients).
With tezepelumab, the annualized rate of asthma exacerbations was 0.93; with placebo, the rate was 2.10 (P < .001).
“Patients with severe, uncontrolled asthma who received tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life than those who received placebo,” according to the report of NAVIGATOR trial, which was funded by AstraZeneca and Amgen.
Tezepelumab details
The full prescribing information for tezepelumab-ekko is available, including specific warnings and areas of concern where information is not available. The drug should not be administered to individuals with known hypersensitivity to tezepelumab-ekko or excipients, and hypersensitivity reactions (e.g., rash and allergic conjunctivitis), can occur within hours of administration, but in some instances have a delayed onset (i.e., days).
The drug should not be used to treat acute asthma symptoms, acute exacerbations, acute bronchospasm, or status asthmaticus, and the use of live-attenuated vaccines in patients receiving tezepelumab-ekko should be avoided.
There is no available data regarding the use of tezepelumab-ekko in patients who are pregnant, although placental transfer of monoclonal antibodies such as tezepelumab-ekko is greater during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy, according to the company.
The most common adverse reactions for the drug, with a reported incidence of at least 3%, are pharyngitis, arthralgia, and back pain.
“The approval of Tezspire is long-awaited positive news for the asthma community,” said Tonya Winders, president and CEO at the Allergy & Asthma Network and president of the Global Allergy and Airways Patient Platform in the Amgen press release. “For the first time, many people living with severe asthma have the opportunity to receive treatment regardless of the cause of their inflammation.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved tezepelumab-ekko (Tezspire) as a first-in-class treatment for severe asthma in adults and pediatric patients aged 12 years and older. It is not recommended for the relief of acute bronchospasm or status asthmaticus.
Tezepelumab-ekko is a human monoclonal antibody that acts as a thymic stromal lymphopoietin (TSLP) blocker. TSLP is an epithelial cell–derived cytokine implicated in the pathogenesis of asthma. Tezepelumab-ekko is administered by subcutaneous injection at a recommended dosage of 210 mg given once every 4 weeks.
“Tezspire represents a much-needed new treatment for the many patients who remain underserved and continue to struggle with severe, uncontrolled asthma,” said professor Andrew Menzies-Gow, MD, PhD, director of the lung division, Royal Brompton Hospital, London, and the principal investigator of the pivotal NAVIGATOR trial, in a Dec. 17 Amgen press release.
Trial results
The early approval of the treatment was based on the results of various clinical trials, primarily the NAVIGATOR phase 3 trial, results of which were published in the New England Journal of Medicine in May 2021.
In the NAVIGATOR trial, a total of 1,061 patients were randomly assigned to receive tezepelumab (529 patients) or placebo (532 patients).
With tezepelumab, the annualized rate of asthma exacerbations was 0.93; with placebo, the rate was 2.10 (P < .001).
“Patients with severe, uncontrolled asthma who received tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life than those who received placebo,” according to the report of NAVIGATOR trial, which was funded by AstraZeneca and Amgen.
Tezepelumab details
The full prescribing information for tezepelumab-ekko is available, including specific warnings and areas of concern where information is not available. The drug should not be administered to individuals with known hypersensitivity to tezepelumab-ekko or excipients, and hypersensitivity reactions (e.g., rash and allergic conjunctivitis), can occur within hours of administration, but in some instances have a delayed onset (i.e., days).
The drug should not be used to treat acute asthma symptoms, acute exacerbations, acute bronchospasm, or status asthmaticus, and the use of live-attenuated vaccines in patients receiving tezepelumab-ekko should be avoided.
There is no available data regarding the use of tezepelumab-ekko in patients who are pregnant, although placental transfer of monoclonal antibodies such as tezepelumab-ekko is greater during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy, according to the company.
The most common adverse reactions for the drug, with a reported incidence of at least 3%, are pharyngitis, arthralgia, and back pain.
“The approval of Tezspire is long-awaited positive news for the asthma community,” said Tonya Winders, president and CEO at the Allergy & Asthma Network and president of the Global Allergy and Airways Patient Platform in the Amgen press release. “For the first time, many people living with severe asthma have the opportunity to receive treatment regardless of the cause of their inflammation.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved tezepelumab-ekko (Tezspire) as a first-in-class treatment for severe asthma in adults and pediatric patients aged 12 years and older. It is not recommended for the relief of acute bronchospasm or status asthmaticus.
Tezepelumab-ekko is a human monoclonal antibody that acts as a thymic stromal lymphopoietin (TSLP) blocker. TSLP is an epithelial cell–derived cytokine implicated in the pathogenesis of asthma. Tezepelumab-ekko is administered by subcutaneous injection at a recommended dosage of 210 mg given once every 4 weeks.
“Tezspire represents a much-needed new treatment for the many patients who remain underserved and continue to struggle with severe, uncontrolled asthma,” said professor Andrew Menzies-Gow, MD, PhD, director of the lung division, Royal Brompton Hospital, London, and the principal investigator of the pivotal NAVIGATOR trial, in a Dec. 17 Amgen press release.
Trial results
The early approval of the treatment was based on the results of various clinical trials, primarily the NAVIGATOR phase 3 trial, results of which were published in the New England Journal of Medicine in May 2021.
In the NAVIGATOR trial, a total of 1,061 patients were randomly assigned to receive tezepelumab (529 patients) or placebo (532 patients).
With tezepelumab, the annualized rate of asthma exacerbations was 0.93; with placebo, the rate was 2.10 (P < .001).
“Patients with severe, uncontrolled asthma who received tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life than those who received placebo,” according to the report of NAVIGATOR trial, which was funded by AstraZeneca and Amgen.
Tezepelumab details
The full prescribing information for tezepelumab-ekko is available, including specific warnings and areas of concern where information is not available. The drug should not be administered to individuals with known hypersensitivity to tezepelumab-ekko or excipients, and hypersensitivity reactions (e.g., rash and allergic conjunctivitis), can occur within hours of administration, but in some instances have a delayed onset (i.e., days).
The drug should not be used to treat acute asthma symptoms, acute exacerbations, acute bronchospasm, or status asthmaticus, and the use of live-attenuated vaccines in patients receiving tezepelumab-ekko should be avoided.
There is no available data regarding the use of tezepelumab-ekko in patients who are pregnant, although placental transfer of monoclonal antibodies such as tezepelumab-ekko is greater during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy, according to the company.
The most common adverse reactions for the drug, with a reported incidence of at least 3%, are pharyngitis, arthralgia, and back pain.
“The approval of Tezspire is long-awaited positive news for the asthma community,” said Tonya Winders, president and CEO at the Allergy & Asthma Network and president of the Global Allergy and Airways Patient Platform in the Amgen press release. “For the first time, many people living with severe asthma have the opportunity to receive treatment regardless of the cause of their inflammation.”
A version of this article first appeared on Medscape.com.
Blastomycosislike Pyoderma: Verrucous Hyperpigmented Plaques on the Pretibial Shins
To the Editor:
Blastomycosislike pyoderma (BLP), also commonly referred to as pyoderma vegetans, is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 It is characterized by a collection of neutrophilic abscesses with pseudoepitheliomatous hyperplasia that coalesce into crusted plaques.
A 15-year-old adolescent girl with a history of type 1 diabetes mellitus was admitted for diabetic ketoacidosis. The patient presented with bilateral pretibial lesions of 6 years’ duration that developed after swimming in a pool following reported trauma to the site. These pruritic plaques had grown slowly and were occasionally tender. Of note, with episodes of hyperglycemia, the lesions developed purulent drainage.
Upon admission to the hospital and subsequent dermatology consultation, physical examination revealed the right pretibial shin had a 15×5-cm, gray-brown, hyperpigmented, verrucous, tender plaque with purulent drainage and overlying crust (Figure 1). The left pretibial shin had a similar smaller lesion (Figure 2). Laboratory test results were notable for a white blood cell count of 41.84 cells/µL (reference range, 3.8–10.5 cells/µL), blood glucose level of 586 mg/dL (reference range, 70–99 mg/dL), and hemoglobin A1c of 11.7% (reference range, 4.0%–5.6%). A biopsy specimen from the right pretibial shin was stained with hematoxylin and eosin for dermatopathologic evaluation as well as sent for tissue culture. Tissue and wound cultures grew Staphylococcus aureus and group B Streptococcus with no fungal or acid-fast bacilli growth.
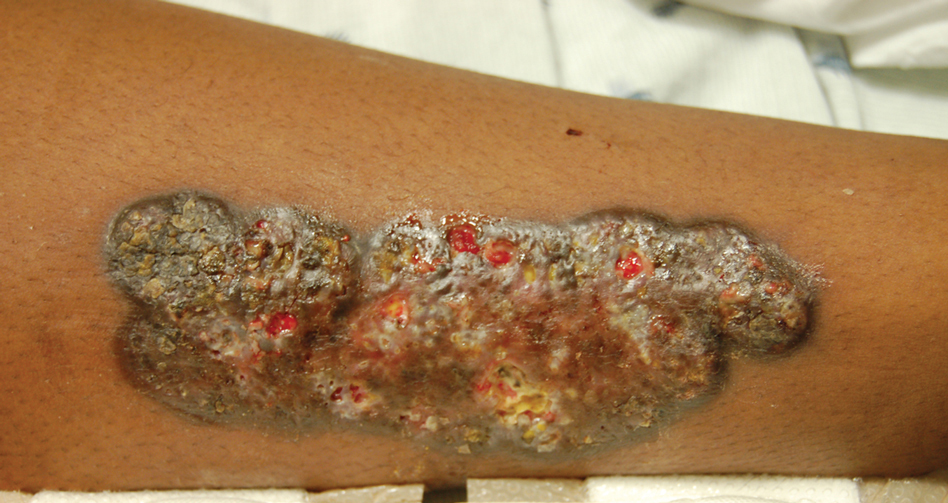
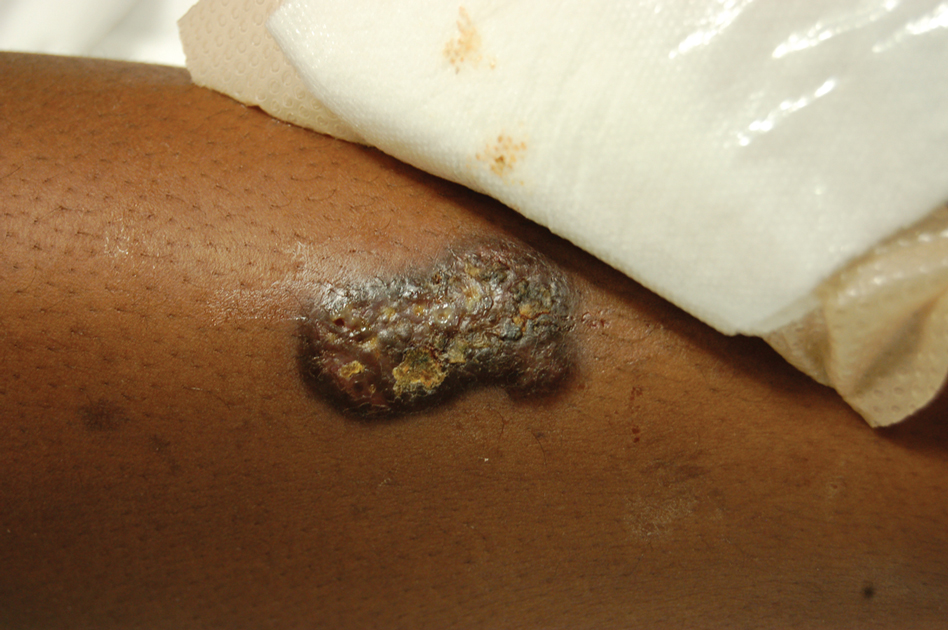
Blood cultures were negative for bacteria. Results of radiographic imaging were negative for osteomyelitis. Biopsy specimens from the right pretibial plaque showed a markedly inflamed, ruptured follicular unit with a dense dermal lympho-neutrophilic infiltrate and overlying pseudoepitheliomatous hyperplasia (Figure 3). Periodic acid–Schiff, Gomori methenamine-silver, acid-fast bacilli, and Giemsa stains were negative for organisms. No granules consistent with a Splendore-Hoeppli phenomenon were observed. These observations were consistent with a diagnosis of BLP.
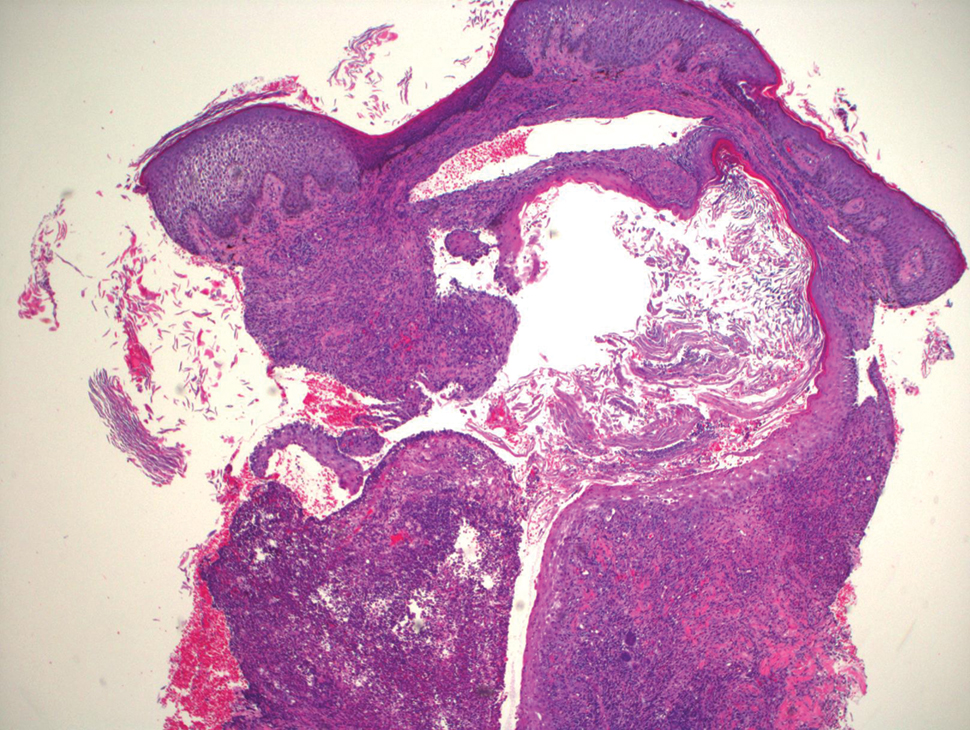
Blastomycosislike pyoderma is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 Pediatric cases also are uncommon. Blastomycosislike pyoderma most commonly is caused by infection with S aureus or group A streptococci, but several other organisms have been implicated.2 Clinically, BLP is similar to cutaneous botryomycosis, as both are caused by similar organisms.3 However, while BLP is limited to the skin, botryomycosis may involve visceral organs.
Blastomycosislike pyoderma typically presents as verrucous, hyperkeratotic, purulent plaques with raised borders. It most commonly occurs on the face, scalp, axillae, trunk, and distal extremities. Predisposing factors include immunosuppressed states such as poor nutrition, HIV, malignancy, alcoholism, and diabetes mellitus.3,4 Hyperglycemia is thought to suppress helper T cell (TH1)–dependent immunity, which may explain why our patient’s lesions worsened with hyperglycemic episodes.5Histopathology revealed pseudoepitheliomatous hyperplasia with neutrophilic abscesses.1 The distinguishing feature between botryomycosis and BLP is the development of grains known as the Splendore-Hoeppli phenomenon in botryomycosis.6 The grains are eosinophilic and contain the causative infectious agent. The presence of these grains is consistent with botryomycosis but is not pathognomonic, as it also can be found in several bacterial, fungal, and parasitic infections.3,6
The differential diagnosis of BLP includes atypical mycobacterial infection, pyoderma gangrenosum, fungal infection, and tuberculosis verrucosa cutis.7
Although BLP is caused by bacteria, response to systemic antibiotics is variable. Other treatment modalities include dapsone, systemic and intralesional corticosteroids, retinoids, debridement, CO2 laser, and excision.6,8 Lesions typically start out localized, but it is not uncommon for them to spread to distal or vulnerable tissue, such as sites of trauma or inflammation. Our patient was started on oral trimethoprim-sulfamethoxazole and showed improvement, but she worsened with subsequent hyperglycemic episodes when antibiotics were discontinued.
1. Adis¸en E, Tezel F, Gürer MA. Pyoderma vegetans: a case for discussion. Acta Derm Venereol. 2009;89:186-188.
2. Scuderi S, O’Brien B, Robertson I, et al. Heterogeneity of blastomycosis-like pyoderma: a selection of cases from the last 35 years. Australas J Dermatol. 2017;58:139-141.
3. Marschalko, M. Pyoderma vegetans: report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol Alp Pannonica Adriat. 1995;4:55-59.
4. Cerullo L, Zussman J, Young L. An unusual presentation of blastomycosislike pyoderma (pyoderma vegetans) and a review of the literature. Cutis. 2009;84:201-204.
5. Tanaka Y. Immunosuppressive mechanisms in diabetes mellitus [in Japanese]. Nihon Rinsho. 2008;66:2233-2237.
6. Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol. 2008;35:979-988.
7. Lee YS, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
8. Kobraei KB, Wesson SK. Blastomycosis-like pyoderma: response to systemic retinoid therapy. Int J Dermatol. 2010;49:1336-1338.
To the Editor:
Blastomycosislike pyoderma (BLP), also commonly referred to as pyoderma vegetans, is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 It is characterized by a collection of neutrophilic abscesses with pseudoepitheliomatous hyperplasia that coalesce into crusted plaques.
A 15-year-old adolescent girl with a history of type 1 diabetes mellitus was admitted for diabetic ketoacidosis. The patient presented with bilateral pretibial lesions of 6 years’ duration that developed after swimming in a pool following reported trauma to the site. These pruritic plaques had grown slowly and were occasionally tender. Of note, with episodes of hyperglycemia, the lesions developed purulent drainage.
Upon admission to the hospital and subsequent dermatology consultation, physical examination revealed the right pretibial shin had a 15×5-cm, gray-brown, hyperpigmented, verrucous, tender plaque with purulent drainage and overlying crust (Figure 1). The left pretibial shin had a similar smaller lesion (Figure 2). Laboratory test results were notable for a white blood cell count of 41.84 cells/µL (reference range, 3.8–10.5 cells/µL), blood glucose level of 586 mg/dL (reference range, 70–99 mg/dL), and hemoglobin A1c of 11.7% (reference range, 4.0%–5.6%). A biopsy specimen from the right pretibial shin was stained with hematoxylin and eosin for dermatopathologic evaluation as well as sent for tissue culture. Tissue and wound cultures grew Staphylococcus aureus and group B Streptococcus with no fungal or acid-fast bacilli growth.


Blood cultures were negative for bacteria. Results of radiographic imaging were negative for osteomyelitis. Biopsy specimens from the right pretibial plaque showed a markedly inflamed, ruptured follicular unit with a dense dermal lympho-neutrophilic infiltrate and overlying pseudoepitheliomatous hyperplasia (Figure 3). Periodic acid–Schiff, Gomori methenamine-silver, acid-fast bacilli, and Giemsa stains were negative for organisms. No granules consistent with a Splendore-Hoeppli phenomenon were observed. These observations were consistent with a diagnosis of BLP.

Blastomycosislike pyoderma is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 Pediatric cases also are uncommon. Blastomycosislike pyoderma most commonly is caused by infection with S aureus or group A streptococci, but several other organisms have been implicated.2 Clinically, BLP is similar to cutaneous botryomycosis, as both are caused by similar organisms.3 However, while BLP is limited to the skin, botryomycosis may involve visceral organs.
Blastomycosislike pyoderma typically presents as verrucous, hyperkeratotic, purulent plaques with raised borders. It most commonly occurs on the face, scalp, axillae, trunk, and distal extremities. Predisposing factors include immunosuppressed states such as poor nutrition, HIV, malignancy, alcoholism, and diabetes mellitus.3,4 Hyperglycemia is thought to suppress helper T cell (TH1)–dependent immunity, which may explain why our patient’s lesions worsened with hyperglycemic episodes.5Histopathology revealed pseudoepitheliomatous hyperplasia with neutrophilic abscesses.1 The distinguishing feature between botryomycosis and BLP is the development of grains known as the Splendore-Hoeppli phenomenon in botryomycosis.6 The grains are eosinophilic and contain the causative infectious agent. The presence of these grains is consistent with botryomycosis but is not pathognomonic, as it also can be found in several bacterial, fungal, and parasitic infections.3,6
The differential diagnosis of BLP includes atypical mycobacterial infection, pyoderma gangrenosum, fungal infection, and tuberculosis verrucosa cutis.7
Although BLP is caused by bacteria, response to systemic antibiotics is variable. Other treatment modalities include dapsone, systemic and intralesional corticosteroids, retinoids, debridement, CO2 laser, and excision.6,8 Lesions typically start out localized, but it is not uncommon for them to spread to distal or vulnerable tissue, such as sites of trauma or inflammation. Our patient was started on oral trimethoprim-sulfamethoxazole and showed improvement, but she worsened with subsequent hyperglycemic episodes when antibiotics were discontinued.
To the Editor:
Blastomycosislike pyoderma (BLP), also commonly referred to as pyoderma vegetans, is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 It is characterized by a collection of neutrophilic abscesses with pseudoepitheliomatous hyperplasia that coalesce into crusted plaques.
A 15-year-old adolescent girl with a history of type 1 diabetes mellitus was admitted for diabetic ketoacidosis. The patient presented with bilateral pretibial lesions of 6 years’ duration that developed after swimming in a pool following reported trauma to the site. These pruritic plaques had grown slowly and were occasionally tender. Of note, with episodes of hyperglycemia, the lesions developed purulent drainage.
Upon admission to the hospital and subsequent dermatology consultation, physical examination revealed the right pretibial shin had a 15×5-cm, gray-brown, hyperpigmented, verrucous, tender plaque with purulent drainage and overlying crust (Figure 1). The left pretibial shin had a similar smaller lesion (Figure 2). Laboratory test results were notable for a white blood cell count of 41.84 cells/µL (reference range, 3.8–10.5 cells/µL), blood glucose level of 586 mg/dL (reference range, 70–99 mg/dL), and hemoglobin A1c of 11.7% (reference range, 4.0%–5.6%). A biopsy specimen from the right pretibial shin was stained with hematoxylin and eosin for dermatopathologic evaluation as well as sent for tissue culture. Tissue and wound cultures grew Staphylococcus aureus and group B Streptococcus with no fungal or acid-fast bacilli growth.


Blood cultures were negative for bacteria. Results of radiographic imaging were negative for osteomyelitis. Biopsy specimens from the right pretibial plaque showed a markedly inflamed, ruptured follicular unit with a dense dermal lympho-neutrophilic infiltrate and overlying pseudoepitheliomatous hyperplasia (Figure 3). Periodic acid–Schiff, Gomori methenamine-silver, acid-fast bacilli, and Giemsa stains were negative for organisms. No granules consistent with a Splendore-Hoeppli phenomenon were observed. These observations were consistent with a diagnosis of BLP.

Blastomycosislike pyoderma is a rare cutaneous bacterial infection that often mimics other fungal, inflammatory, or neoplastic disorders.1 Pediatric cases also are uncommon. Blastomycosislike pyoderma most commonly is caused by infection with S aureus or group A streptococci, but several other organisms have been implicated.2 Clinically, BLP is similar to cutaneous botryomycosis, as both are caused by similar organisms.3 However, while BLP is limited to the skin, botryomycosis may involve visceral organs.
Blastomycosislike pyoderma typically presents as verrucous, hyperkeratotic, purulent plaques with raised borders. It most commonly occurs on the face, scalp, axillae, trunk, and distal extremities. Predisposing factors include immunosuppressed states such as poor nutrition, HIV, malignancy, alcoholism, and diabetes mellitus.3,4 Hyperglycemia is thought to suppress helper T cell (TH1)–dependent immunity, which may explain why our patient’s lesions worsened with hyperglycemic episodes.5Histopathology revealed pseudoepitheliomatous hyperplasia with neutrophilic abscesses.1 The distinguishing feature between botryomycosis and BLP is the development of grains known as the Splendore-Hoeppli phenomenon in botryomycosis.6 The grains are eosinophilic and contain the causative infectious agent. The presence of these grains is consistent with botryomycosis but is not pathognomonic, as it also can be found in several bacterial, fungal, and parasitic infections.3,6
The differential diagnosis of BLP includes atypical mycobacterial infection, pyoderma gangrenosum, fungal infection, and tuberculosis verrucosa cutis.7
Although BLP is caused by bacteria, response to systemic antibiotics is variable. Other treatment modalities include dapsone, systemic and intralesional corticosteroids, retinoids, debridement, CO2 laser, and excision.6,8 Lesions typically start out localized, but it is not uncommon for them to spread to distal or vulnerable tissue, such as sites of trauma or inflammation. Our patient was started on oral trimethoprim-sulfamethoxazole and showed improvement, but she worsened with subsequent hyperglycemic episodes when antibiotics were discontinued.
1. Adis¸en E, Tezel F, Gürer MA. Pyoderma vegetans: a case for discussion. Acta Derm Venereol. 2009;89:186-188.
2. Scuderi S, O’Brien B, Robertson I, et al. Heterogeneity of blastomycosis-like pyoderma: a selection of cases from the last 35 years. Australas J Dermatol. 2017;58:139-141.
3. Marschalko, M. Pyoderma vegetans: report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol Alp Pannonica Adriat. 1995;4:55-59.
4. Cerullo L, Zussman J, Young L. An unusual presentation of blastomycosislike pyoderma (pyoderma vegetans) and a review of the literature. Cutis. 2009;84:201-204.
5. Tanaka Y. Immunosuppressive mechanisms in diabetes mellitus [in Japanese]. Nihon Rinsho. 2008;66:2233-2237.
6. Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol. 2008;35:979-988.
7. Lee YS, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
8. Kobraei KB, Wesson SK. Blastomycosis-like pyoderma: response to systemic retinoid therapy. Int J Dermatol. 2010;49:1336-1338.
1. Adis¸en E, Tezel F, Gürer MA. Pyoderma vegetans: a case for discussion. Acta Derm Venereol. 2009;89:186-188.
2. Scuderi S, O’Brien B, Robertson I, et al. Heterogeneity of blastomycosis-like pyoderma: a selection of cases from the last 35 years. Australas J Dermatol. 2017;58:139-141.
3. Marschalko, M. Pyoderma vegetans: report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol Alp Pannonica Adriat. 1995;4:55-59.
4. Cerullo L, Zussman J, Young L. An unusual presentation of blastomycosislike pyoderma (pyoderma vegetans) and a review of the literature. Cutis. 2009;84:201-204.
5. Tanaka Y. Immunosuppressive mechanisms in diabetes mellitus [in Japanese]. Nihon Rinsho. 2008;66:2233-2237.
6. Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol. 2008;35:979-988.
7. Lee YS, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
8. Kobraei KB, Wesson SK. Blastomycosis-like pyoderma: response to systemic retinoid therapy. Int J Dermatol. 2010;49:1336-1338.
Practice Points
- Blastomycosislike pyoderma is a rare condition secondary to bacterial infection, but as the name suggests, it also can resemble cutaneous blastomycosis.
- Blastomycosislike pyoderma most commonly occurs in immunocompromised patients.
- The most common histologic findings include suppurative and neutrophilic inflammation with pseudoepitheliomatous hyperplasia.
FDA grants new indication to lumateperone (Caplyta) for bipolar depression
The Food and Drug Administration has expanded approval of lumateperone (Caplyta) to include treatment of adults with depressive episodes associated with bipolar I and II disorder, as monotherapy or adjunctive therapy with lithium or valproate.
This makes lumateperone the only FDA-approved drug for this indication.
“The efficacy, and favorable safety and tolerability profile, make Caplyta an important treatment option for the millions of patients living with bipolar I or II depression and represents a major development for these patients,” Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said in a company news release.
Lumateperone was first approved by the FDA in 2019 for the treatment of adults with schizophrenia.
‘Positioned to launch immediately’
that showed treatment with lumateperone, alone or with lithium or valproate, significantly improved depressive symptoms for patients with major depressive episodes associated with bipolar I and bipolar II disorders.
In these studies, treatment with a 42-mg once-daily dose was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale score versus placebo.
Lumateperone also showed a statistically significant improvement in the key secondary endpoint relating to clinical global impression of bipolar disorder.
Somnolence/sedation, dizziness, nausea, and dry mouth were the most commonly reported adverse events associated with the medication. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. Incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
Sharon Mates, PhD, chairman and CEO of Intra-Cellular Therapies, noted in the same press release that the company is “positioned to launch immediately and are excited to offer Caplyta to the millions of patients living with bipolar depression.”
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded approval of lumateperone (Caplyta) to include treatment of adults with depressive episodes associated with bipolar I and II disorder, as monotherapy or adjunctive therapy with lithium or valproate.
This makes lumateperone the only FDA-approved drug for this indication.
“The efficacy, and favorable safety and tolerability profile, make Caplyta an important treatment option for the millions of patients living with bipolar I or II depression and represents a major development for these patients,” Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said in a company news release.
Lumateperone was first approved by the FDA in 2019 for the treatment of adults with schizophrenia.
‘Positioned to launch immediately’
that showed treatment with lumateperone, alone or with lithium or valproate, significantly improved depressive symptoms for patients with major depressive episodes associated with bipolar I and bipolar II disorders.
In these studies, treatment with a 42-mg once-daily dose was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale score versus placebo.
Lumateperone also showed a statistically significant improvement in the key secondary endpoint relating to clinical global impression of bipolar disorder.
Somnolence/sedation, dizziness, nausea, and dry mouth were the most commonly reported adverse events associated with the medication. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. Incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
Sharon Mates, PhD, chairman and CEO of Intra-Cellular Therapies, noted in the same press release that the company is “positioned to launch immediately and are excited to offer Caplyta to the millions of patients living with bipolar depression.”
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded approval of lumateperone (Caplyta) to include treatment of adults with depressive episodes associated with bipolar I and II disorder, as monotherapy or adjunctive therapy with lithium or valproate.
This makes lumateperone the only FDA-approved drug for this indication.
“The efficacy, and favorable safety and tolerability profile, make Caplyta an important treatment option for the millions of patients living with bipolar I or II depression and represents a major development for these patients,” Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said in a company news release.
Lumateperone was first approved by the FDA in 2019 for the treatment of adults with schizophrenia.
‘Positioned to launch immediately’
that showed treatment with lumateperone, alone or with lithium or valproate, significantly improved depressive symptoms for patients with major depressive episodes associated with bipolar I and bipolar II disorders.
In these studies, treatment with a 42-mg once-daily dose was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale score versus placebo.
Lumateperone also showed a statistically significant improvement in the key secondary endpoint relating to clinical global impression of bipolar disorder.
Somnolence/sedation, dizziness, nausea, and dry mouth were the most commonly reported adverse events associated with the medication. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. Incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
Sharon Mates, PhD, chairman and CEO of Intra-Cellular Therapies, noted in the same press release that the company is “positioned to launch immediately and are excited to offer Caplyta to the millions of patients living with bipolar depression.”
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
Pfizer COVID vaccine for younger children hits snag
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
Class I recall of percutaneous thrombolytic device
Arrow International, a subsidiary of Teleflex, has recalled a total of 3,241 Arrow-Trerotola over-the-wire 7FR percutaneous thrombolytic device (PTD) kits because of the risk of the orange inner lumen of the catheter’s tip component separating from the basket.
The U.S. Food and Drug Administration has identified this as a Class I recall, the most serious type, because of the potential for serious injury or death.
The recalled kits include a rotatable catheter with an outer sheath and an inner cable with a self-expanding basket. The Arrow-Trerotola PTD catheter is used with the Arrow rotator drive unit to remove clots in patients with arteriovenous fistulas and synthetic dialysis grafts.
“If the orange inner lumen separates from the basket, it may fracture and detach and block the blood vessel(s),” the FDA says in the recall notice posted on the FDA website.
“If the orange inner lumen detaches from the basket, health consequences depend upon where the fractured tip component embolizes. If the embolization is local to the treatment target site, retrieval may be attempted, requiring an additional intervention and consequent delay of therapy,” the agency notes.
“In some cases, the embolization could be central or possibly even to the heart or pulmonary arteries. This may lead to serious adverse events such as vessel damage, need for additional medical procedures, or possibly death,” the agency says.
To date, there have been seven complaints and no injuries or deaths reported for this device.
The recalled devices were distributed in the United States between Nov. 1, 2019, and July 31, 2021. Product codes and lot numbers pertaining to the devices are listed on the FDA website.
Teleflex has sent an urgent field safety notice to customers requesting that they check inventory for affected product and remove and quarantine all recalled product.
Customers are also asked to complete the enclosed acknowledgement form and fax it to 1-855-419-8507 (attention: customer service) or e-mail the form to [email protected].
Customers with recalled product service will be contacted by a company representative with instructions for returning any recalled products.
Customers who have questions about this recall should contact Teleflex customer service by phone at 1-866-396-2111, by fax at 1-855-419-8507, or by email at [email protected].
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Arrow International, a subsidiary of Teleflex, has recalled a total of 3,241 Arrow-Trerotola over-the-wire 7FR percutaneous thrombolytic device (PTD) kits because of the risk of the orange inner lumen of the catheter’s tip component separating from the basket.
The U.S. Food and Drug Administration has identified this as a Class I recall, the most serious type, because of the potential for serious injury or death.
The recalled kits include a rotatable catheter with an outer sheath and an inner cable with a self-expanding basket. The Arrow-Trerotola PTD catheter is used with the Arrow rotator drive unit to remove clots in patients with arteriovenous fistulas and synthetic dialysis grafts.
“If the orange inner lumen separates from the basket, it may fracture and detach and block the blood vessel(s),” the FDA says in the recall notice posted on the FDA website.
“If the orange inner lumen detaches from the basket, health consequences depend upon where the fractured tip component embolizes. If the embolization is local to the treatment target site, retrieval may be attempted, requiring an additional intervention and consequent delay of therapy,” the agency notes.
“In some cases, the embolization could be central or possibly even to the heart or pulmonary arteries. This may lead to serious adverse events such as vessel damage, need for additional medical procedures, or possibly death,” the agency says.
To date, there have been seven complaints and no injuries or deaths reported for this device.
The recalled devices were distributed in the United States between Nov. 1, 2019, and July 31, 2021. Product codes and lot numbers pertaining to the devices are listed on the FDA website.
Teleflex has sent an urgent field safety notice to customers requesting that they check inventory for affected product and remove and quarantine all recalled product.
Customers are also asked to complete the enclosed acknowledgement form and fax it to 1-855-419-8507 (attention: customer service) or e-mail the form to [email protected].
Customers with recalled product service will be contacted by a company representative with instructions for returning any recalled products.
Customers who have questions about this recall should contact Teleflex customer service by phone at 1-866-396-2111, by fax at 1-855-419-8507, or by email at [email protected].
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Arrow International, a subsidiary of Teleflex, has recalled a total of 3,241 Arrow-Trerotola over-the-wire 7FR percutaneous thrombolytic device (PTD) kits because of the risk of the orange inner lumen of the catheter’s tip component separating from the basket.
The U.S. Food and Drug Administration has identified this as a Class I recall, the most serious type, because of the potential for serious injury or death.
The recalled kits include a rotatable catheter with an outer sheath and an inner cable with a self-expanding basket. The Arrow-Trerotola PTD catheter is used with the Arrow rotator drive unit to remove clots in patients with arteriovenous fistulas and synthetic dialysis grafts.
“If the orange inner lumen separates from the basket, it may fracture and detach and block the blood vessel(s),” the FDA says in the recall notice posted on the FDA website.
“If the orange inner lumen detaches from the basket, health consequences depend upon where the fractured tip component embolizes. If the embolization is local to the treatment target site, retrieval may be attempted, requiring an additional intervention and consequent delay of therapy,” the agency notes.
“In some cases, the embolization could be central or possibly even to the heart or pulmonary arteries. This may lead to serious adverse events such as vessel damage, need for additional medical procedures, or possibly death,” the agency says.
To date, there have been seven complaints and no injuries or deaths reported for this device.
The recalled devices were distributed in the United States between Nov. 1, 2019, and July 31, 2021. Product codes and lot numbers pertaining to the devices are listed on the FDA website.
Teleflex has sent an urgent field safety notice to customers requesting that they check inventory for affected product and remove and quarantine all recalled product.
Customers are also asked to complete the enclosed acknowledgement form and fax it to 1-855-419-8507 (attention: customer service) or e-mail the form to [email protected].
Customers with recalled product service will be contacted by a company representative with instructions for returning any recalled products.
Customers who have questions about this recall should contact Teleflex customer service by phone at 1-866-396-2111, by fax at 1-855-419-8507, or by email at [email protected].
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
COVID cases spike as questions remain about Omicron’s threat
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
FDA approves new myasthenia gravis drug
“There are significant unmet medical needs for people living with myasthenia gravis, as with many other rare diseases,” Billy Dunn, MD, director, office of neuroscience, FDA Center for Drug Evaluation and Research, said in a news release.
This approval represents “an important step in providing a novel therapy option for patients and underscores the agency’s commitment to help make new treatment options available for people living with rare diseases,” Dr. Dunn added.
Effective, well tolerated
The rare and chronic autoimmune neuromuscular disorder of gMG causes debilitating and potentially life-threatening muscle weakness and significantly impaired independence and quality of life. Most patients with gMG have IgG antibodies, which are most often directed against skeletal muscle nicotinic acetylcholine receptors.
Efgartigimod is an antibody fragment designed to reduce pathogenic IgG antibodies and block the IgG recycling process in patients with gMG.
The novel agent binds to the neonatal Fc receptor (FcRn), which is widely expressed throughout the body and plays a central role in rescuing IgG antibodies from degradation. Blocking FcRn reduces IgG antibody levels.
As previously reported, efgartigimod was effective and well tolerated in the phase 3, randomized, placebo-controlled ADAPT trial, which enrolled 187 adults with gMG regardless of acetylcholine receptor antibody status. All had a Myasthenia Gravis–Activities of Daily Living score of at least 5 (>50% nonocular) on a background of a stable dose of at least one MG drug.
For 26 weeks, 84 patients were randomly assigned to receive efgartigimod 10 mg/kg and 83 to receive matching placebo. Both treatments were administered as four infusions per cycle at one infusion per week. The process was repeated as needed, depending on clinical response no sooner than 8 weeks after initiation of the previous cycle.
Treatment with efgartigimod reduced disease burden and improved strength and quality of life in patients with gMG across four MG-specific scales. In addition, these benefits were observed early and were reproducible and durable.
The results were published in Lancet Neurology.
‘Important new advance’
Efgartigimod is a “very rapidly acting drug relative to other treatments that may take 4, 6, sometimes 10 months before they start to work; and the side-effect profile is much like placebo,” said principal investigator James Howard Jr., MD, department of neurology, University of North Carolina at Chapel Hill.
The FDA granted efgartigimod fast track and orphan drug designation.
“People living with gMG have been in need of new treatment options that are targeted to the underlying pathogenesis of the disease and supported by clinical data,” Dr. Howard said in a company news release issued upon approval.
This approval “represents an important new advance for gMG patients and families affected by this debilitating disease. This therapy has the potential to reduce the disease burden of gMG and transform the way we treat this disease,” Dr. Howard added.
A version of this article first appeared on Medscape.com.
“There are significant unmet medical needs for people living with myasthenia gravis, as with many other rare diseases,” Billy Dunn, MD, director, office of neuroscience, FDA Center for Drug Evaluation and Research, said in a news release.
This approval represents “an important step in providing a novel therapy option for patients and underscores the agency’s commitment to help make new treatment options available for people living with rare diseases,” Dr. Dunn added.
Effective, well tolerated
The rare and chronic autoimmune neuromuscular disorder of gMG causes debilitating and potentially life-threatening muscle weakness and significantly impaired independence and quality of life. Most patients with gMG have IgG antibodies, which are most often directed against skeletal muscle nicotinic acetylcholine receptors.
Efgartigimod is an antibody fragment designed to reduce pathogenic IgG antibodies and block the IgG recycling process in patients with gMG.
The novel agent binds to the neonatal Fc receptor (FcRn), which is widely expressed throughout the body and plays a central role in rescuing IgG antibodies from degradation. Blocking FcRn reduces IgG antibody levels.
As previously reported, efgartigimod was effective and well tolerated in the phase 3, randomized, placebo-controlled ADAPT trial, which enrolled 187 adults with gMG regardless of acetylcholine receptor antibody status. All had a Myasthenia Gravis–Activities of Daily Living score of at least 5 (>50% nonocular) on a background of a stable dose of at least one MG drug.
For 26 weeks, 84 patients were randomly assigned to receive efgartigimod 10 mg/kg and 83 to receive matching placebo. Both treatments were administered as four infusions per cycle at one infusion per week. The process was repeated as needed, depending on clinical response no sooner than 8 weeks after initiation of the previous cycle.
Treatment with efgartigimod reduced disease burden and improved strength and quality of life in patients with gMG across four MG-specific scales. In addition, these benefits were observed early and were reproducible and durable.
The results were published in Lancet Neurology.
‘Important new advance’
Efgartigimod is a “very rapidly acting drug relative to other treatments that may take 4, 6, sometimes 10 months before they start to work; and the side-effect profile is much like placebo,” said principal investigator James Howard Jr., MD, department of neurology, University of North Carolina at Chapel Hill.
The FDA granted efgartigimod fast track and orphan drug designation.
“People living with gMG have been in need of new treatment options that are targeted to the underlying pathogenesis of the disease and supported by clinical data,” Dr. Howard said in a company news release issued upon approval.
This approval “represents an important new advance for gMG patients and families affected by this debilitating disease. This therapy has the potential to reduce the disease burden of gMG and transform the way we treat this disease,” Dr. Howard added.
A version of this article first appeared on Medscape.com.
“There are significant unmet medical needs for people living with myasthenia gravis, as with many other rare diseases,” Billy Dunn, MD, director, office of neuroscience, FDA Center for Drug Evaluation and Research, said in a news release.
This approval represents “an important step in providing a novel therapy option for patients and underscores the agency’s commitment to help make new treatment options available for people living with rare diseases,” Dr. Dunn added.
Effective, well tolerated
The rare and chronic autoimmune neuromuscular disorder of gMG causes debilitating and potentially life-threatening muscle weakness and significantly impaired independence and quality of life. Most patients with gMG have IgG antibodies, which are most often directed against skeletal muscle nicotinic acetylcholine receptors.
Efgartigimod is an antibody fragment designed to reduce pathogenic IgG antibodies and block the IgG recycling process in patients with gMG.
The novel agent binds to the neonatal Fc receptor (FcRn), which is widely expressed throughout the body and plays a central role in rescuing IgG antibodies from degradation. Blocking FcRn reduces IgG antibody levels.
As previously reported, efgartigimod was effective and well tolerated in the phase 3, randomized, placebo-controlled ADAPT trial, which enrolled 187 adults with gMG regardless of acetylcholine receptor antibody status. All had a Myasthenia Gravis–Activities of Daily Living score of at least 5 (>50% nonocular) on a background of a stable dose of at least one MG drug.
For 26 weeks, 84 patients were randomly assigned to receive efgartigimod 10 mg/kg and 83 to receive matching placebo. Both treatments were administered as four infusions per cycle at one infusion per week. The process was repeated as needed, depending on clinical response no sooner than 8 weeks after initiation of the previous cycle.
Treatment with efgartigimod reduced disease burden and improved strength and quality of life in patients with gMG across four MG-specific scales. In addition, these benefits were observed early and were reproducible and durable.
The results were published in Lancet Neurology.
‘Important new advance’
Efgartigimod is a “very rapidly acting drug relative to other treatments that may take 4, 6, sometimes 10 months before they start to work; and the side-effect profile is much like placebo,” said principal investigator James Howard Jr., MD, department of neurology, University of North Carolina at Chapel Hill.
The FDA granted efgartigimod fast track and orphan drug designation.
“People living with gMG have been in need of new treatment options that are targeted to the underlying pathogenesis of the disease and supported by clinical data,” Dr. Howard said in a company news release issued upon approval.
This approval “represents an important new advance for gMG patients and families affected by this debilitating disease. This therapy has the potential to reduce the disease burden of gMG and transform the way we treat this disease,” Dr. Howard added.
A version of this article first appeared on Medscape.com.
Small myocarditis risk now seen for adenovirus-based COVID-19 vaccine
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE