User login
Most addiction specialists support legalized therapeutic psychedelics
The majority of addiction specialists, including psychiatrists, believe psychedelics are promising for the treatment of substance use disorders (SUDs) and psychiatric illnesses and, with some caveats, support legalization of the substances for these indications, results of a new survey show.
This strong positive attitude is “a surprise” given previous wariness of addiction specialists regarding legalization of marijuana, noted study investigator Amanda Kim, MD, JD, of Brigham and Women’s Hospital and Harvard Medical School, Boston.
“We had hypothesized that addiction specialists would express more skepticism about psychedelics compared to nonaddiction specialists,” Dr. Kim said.
Instead, addiction experts who participated in the survey were very much in favor of psychedelics being legalized for therapeutic use, but only in a controlled setting.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry.
Growing interest
In recent years, there has been increased interest in the scientific community and among the general public in the therapeutic potential of psychedelics, said Dr. Kim. Previous research has shown growing positivity about psychedelics and support for their legalization among psychiatrists, she added.
Psychedelics have been decriminalized and/or legalized in several jurisdictions. The Food and Drug Administration has granted breakthrough therapy designation for 3,4-methylenedioxymethamphetamine (MDMA) in the treatment of posttraumatic stress disorder (PTSD) and has granted the same designation to psilocybin in the treatment of major depressive disorder.
“Despite psychedelics increasingly entering the mainstream, we are unaware of any studies specifically assessing the current attitudes of physicians specializing in addictions regarding psychedelics,” Dr. Kim said.
For the study, investigators identified prospective survey participants from the AAAP directory. They also reached out to program directors of addiction medicine and addiction psychiatry fellowships.
In the anonymous online survey, respondents were asked to rate their level of agreement with 30 statements.
The analysis included 145 respondents (59% men; mean age, 46.2 years). Psychiatrists made up about two-thirds of the sample. The remainder specialized in internal and family medicine.
Most respondents had some clinical exposure to psychedelics.
Positive attitudes, concerns
Overall, participants expressed very positive attitudes regarding the therapeutic use of psychedelics. About 64% strongly agreed or agreed psychedelics show promise in treating SUDs, and 82% agreed they show promise in treating psychiatric disorders.
However, more than one-third of respondents (37.9%) expressed concern about the addictive potential of psychedelics. This is more than in previous research polling psychiatrists, possibly because the study’s “broad” definition of psychedelics included “nonclassic, nonserotonergic hallucinogens,” such as ketamine and MDMA, Dr. Kim noted.
Because ketamine and MDMA are both lumped into the hallucinogen category in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), “and both are known to have addictive potential, this may have obscured participant responses,” she added.
Some 28% of participants expressed concern about psychedelic use increasing the risk for subsequent psychiatric disorders and long-term cognitive impairment.
Almost three-quarters (74.5%) believe the therapeutic use of psychedelics should be legalized. However, most wanted legal therapeutic psychedelics to be highly regulated and administered only in controlled settings with specially trained providers.
Almost half of the sample believed therapeutic psychedelics should be legal in a variety of different contexts and by non-Western providers, in accordance with indigenous and/or spiritual traditions.
One surprising finding was that most respondents believed patients would be keen on using psychedelics to treat SUDs, said Dr. Kim.
“This may reflect evolving attitudes of both providers and patients about psychedelics, and it will be interesting to further study attitudes of patients toward the use of psychedelics to treat SUD in the future,” she added.
Attitudes toward psychedelics were generally similar for psychiatrists and nonpsychiatrists; but psychiatrists expressed greater comfort in discussing them with patients and were more likely to have observed complications of psychedelics use in their practice.
Dr. Kim said the study’s limitations included the small sample size and possible selection bias, as those with more favorable views of psychedelics may have been more likely to respond.
The study was supported by the Source Research Foundation.
A version of this article first appeared on Medscape.com.
The majority of addiction specialists, including psychiatrists, believe psychedelics are promising for the treatment of substance use disorders (SUDs) and psychiatric illnesses and, with some caveats, support legalization of the substances for these indications, results of a new survey show.
This strong positive attitude is “a surprise” given previous wariness of addiction specialists regarding legalization of marijuana, noted study investigator Amanda Kim, MD, JD, of Brigham and Women’s Hospital and Harvard Medical School, Boston.
“We had hypothesized that addiction specialists would express more skepticism about psychedelics compared to nonaddiction specialists,” Dr. Kim said.
Instead, addiction experts who participated in the survey were very much in favor of psychedelics being legalized for therapeutic use, but only in a controlled setting.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry.
Growing interest
In recent years, there has been increased interest in the scientific community and among the general public in the therapeutic potential of psychedelics, said Dr. Kim. Previous research has shown growing positivity about psychedelics and support for their legalization among psychiatrists, she added.
Psychedelics have been decriminalized and/or legalized in several jurisdictions. The Food and Drug Administration has granted breakthrough therapy designation for 3,4-methylenedioxymethamphetamine (MDMA) in the treatment of posttraumatic stress disorder (PTSD) and has granted the same designation to psilocybin in the treatment of major depressive disorder.
“Despite psychedelics increasingly entering the mainstream, we are unaware of any studies specifically assessing the current attitudes of physicians specializing in addictions regarding psychedelics,” Dr. Kim said.
For the study, investigators identified prospective survey participants from the AAAP directory. They also reached out to program directors of addiction medicine and addiction psychiatry fellowships.
In the anonymous online survey, respondents were asked to rate their level of agreement with 30 statements.
The analysis included 145 respondents (59% men; mean age, 46.2 years). Psychiatrists made up about two-thirds of the sample. The remainder specialized in internal and family medicine.
Most respondents had some clinical exposure to psychedelics.
Positive attitudes, concerns
Overall, participants expressed very positive attitudes regarding the therapeutic use of psychedelics. About 64% strongly agreed or agreed psychedelics show promise in treating SUDs, and 82% agreed they show promise in treating psychiatric disorders.
However, more than one-third of respondents (37.9%) expressed concern about the addictive potential of psychedelics. This is more than in previous research polling psychiatrists, possibly because the study’s “broad” definition of psychedelics included “nonclassic, nonserotonergic hallucinogens,” such as ketamine and MDMA, Dr. Kim noted.
Because ketamine and MDMA are both lumped into the hallucinogen category in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), “and both are known to have addictive potential, this may have obscured participant responses,” she added.
Some 28% of participants expressed concern about psychedelic use increasing the risk for subsequent psychiatric disorders and long-term cognitive impairment.
Almost three-quarters (74.5%) believe the therapeutic use of psychedelics should be legalized. However, most wanted legal therapeutic psychedelics to be highly regulated and administered only in controlled settings with specially trained providers.
Almost half of the sample believed therapeutic psychedelics should be legal in a variety of different contexts and by non-Western providers, in accordance with indigenous and/or spiritual traditions.
One surprising finding was that most respondents believed patients would be keen on using psychedelics to treat SUDs, said Dr. Kim.
“This may reflect evolving attitudes of both providers and patients about psychedelics, and it will be interesting to further study attitudes of patients toward the use of psychedelics to treat SUD in the future,” she added.
Attitudes toward psychedelics were generally similar for psychiatrists and nonpsychiatrists; but psychiatrists expressed greater comfort in discussing them with patients and were more likely to have observed complications of psychedelics use in their practice.
Dr. Kim said the study’s limitations included the small sample size and possible selection bias, as those with more favorable views of psychedelics may have been more likely to respond.
The study was supported by the Source Research Foundation.
A version of this article first appeared on Medscape.com.
The majority of addiction specialists, including psychiatrists, believe psychedelics are promising for the treatment of substance use disorders (SUDs) and psychiatric illnesses and, with some caveats, support legalization of the substances for these indications, results of a new survey show.
This strong positive attitude is “a surprise” given previous wariness of addiction specialists regarding legalization of marijuana, noted study investigator Amanda Kim, MD, JD, of Brigham and Women’s Hospital and Harvard Medical School, Boston.
“We had hypothesized that addiction specialists would express more skepticism about psychedelics compared to nonaddiction specialists,” Dr. Kim said.
Instead, addiction experts who participated in the survey were very much in favor of psychedelics being legalized for therapeutic use, but only in a controlled setting.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry.
Growing interest
In recent years, there has been increased interest in the scientific community and among the general public in the therapeutic potential of psychedelics, said Dr. Kim. Previous research has shown growing positivity about psychedelics and support for their legalization among psychiatrists, she added.
Psychedelics have been decriminalized and/or legalized in several jurisdictions. The Food and Drug Administration has granted breakthrough therapy designation for 3,4-methylenedioxymethamphetamine (MDMA) in the treatment of posttraumatic stress disorder (PTSD) and has granted the same designation to psilocybin in the treatment of major depressive disorder.
“Despite psychedelics increasingly entering the mainstream, we are unaware of any studies specifically assessing the current attitudes of physicians specializing in addictions regarding psychedelics,” Dr. Kim said.
For the study, investigators identified prospective survey participants from the AAAP directory. They also reached out to program directors of addiction medicine and addiction psychiatry fellowships.
In the anonymous online survey, respondents were asked to rate their level of agreement with 30 statements.
The analysis included 145 respondents (59% men; mean age, 46.2 years). Psychiatrists made up about two-thirds of the sample. The remainder specialized in internal and family medicine.
Most respondents had some clinical exposure to psychedelics.
Positive attitudes, concerns
Overall, participants expressed very positive attitudes regarding the therapeutic use of psychedelics. About 64% strongly agreed or agreed psychedelics show promise in treating SUDs, and 82% agreed they show promise in treating psychiatric disorders.
However, more than one-third of respondents (37.9%) expressed concern about the addictive potential of psychedelics. This is more than in previous research polling psychiatrists, possibly because the study’s “broad” definition of psychedelics included “nonclassic, nonserotonergic hallucinogens,” such as ketamine and MDMA, Dr. Kim noted.
Because ketamine and MDMA are both lumped into the hallucinogen category in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), “and both are known to have addictive potential, this may have obscured participant responses,” she added.
Some 28% of participants expressed concern about psychedelic use increasing the risk for subsequent psychiatric disorders and long-term cognitive impairment.
Almost three-quarters (74.5%) believe the therapeutic use of psychedelics should be legalized. However, most wanted legal therapeutic psychedelics to be highly regulated and administered only in controlled settings with specially trained providers.
Almost half of the sample believed therapeutic psychedelics should be legal in a variety of different contexts and by non-Western providers, in accordance with indigenous and/or spiritual traditions.
One surprising finding was that most respondents believed patients would be keen on using psychedelics to treat SUDs, said Dr. Kim.
“This may reflect evolving attitudes of both providers and patients about psychedelics, and it will be interesting to further study attitudes of patients toward the use of psychedelics to treat SUD in the future,” she added.
Attitudes toward psychedelics were generally similar for psychiatrists and nonpsychiatrists; but psychiatrists expressed greater comfort in discussing them with patients and were more likely to have observed complications of psychedelics use in their practice.
Dr. Kim said the study’s limitations included the small sample size and possible selection bias, as those with more favorable views of psychedelics may have been more likely to respond.
The study was supported by the Source Research Foundation.
A version of this article first appeared on Medscape.com.
FROM AAAP 2021
COVID-19 interrupted global poliovirus surveillance and immunization
Most (86%) of these outbreaks were caused by cVDPV2 (circulating VDPV type 2 poliovirus, which originated with the vaccine), and most occurred in Africa, according to a new study of vaccine-derived poliovirus outbreaks between Jan. 2020 and June 2021 published in the CDC’s Morbidity and Mortality Weekly Report.
The Global Polio Eradication Initiative (GPEI) was launched in 1988 and used live attenuated oral poliovirus vaccine (OPV). Since then, cases of wild poliovirus have declined more than 99.99%.
The cVDPV2 likely originated among children born in areas with poor vaccine coverage. Jay Wenger, MD, director, Polio, at the Bill and Melinda Gates Foundation, told this news organization that “the inactivated vaccines that we give in most developed countries now are good in that they provide humoral immunity, the antibodies in the bloodstream. They don’t necessarily provide mucosal immunity. They don’t make the kid’s gut immune to getting reinfected or actually immune to reproducing the virus if they get it in their gut. So we could still have a situation where everybody was vaccinated with IPV [inactivated poliovirus], but the virus could still be transmitting around because kids’ guts would still be producing the virus and there will still be transmission in your population, probably without much or any paralysis because of the IPV. As soon as that virus hit a population that was not vaccinated, they would get paralyzed.”
Dr. Wenger added, “The ideal vaccine would be an oral vaccine that didn’t mutate back and couldn’t cause these VDPVs.” Scientists developed such a vaccine, approved by the World Health Organization last year under an Emergency Use Authorization. This nOPV2 (novel oral poliovirus type 2) vaccine has been given since March 2021 in areas with the VDPD2 outbreaks. The nOPV2 should allow them to “basically stamp out the outbreaks.”
The world had almost eradicated the disease, with the last cases of polio from wild virus occurring in Nigeria, Afghanistan, and Pakistan as of 2014. Africa was declared free of wild polio in 2020 after it had been eradicated from Nigeria, which accounted for more than half of the world’s cases only a decade earlier. Now cVDPV outbreaks affect 28 African countries, plus Iran, Yemen, Afghanistan, Pakistan, Tajikistan, Malaysia, the Philippines, and Indonesia. And there was also one case in China. Globally, there were 1,335 cases of cVDPV causing paralysis during the reporting period.
The COVID-19 pandemic has had a significant impact on polio, accounting for much of this year’s increase in cases. Dr. Wenger said, “We couldn’t do any campaigns. We pretty much stopped doing outbreak response campaigns in the middle of the year because of COVID.”
The CDC report notes that many of the supplementary immunizations in response to cVDPV2 outbreaks were of “poor quality,” and prolonged delays enabled geographically expanding cVDPV2 transmission.
Steve Wassilak, MD, chief coauthor of the CDC study, told this news organization that, because of COVID, “what we’ve been lacking is a rapid response for the most part. Some of that is due to laboratory delays and shipment because of COVID’s effect on international travel.” He noted, however, that there has been good recovery in surveillance and immunization activities despite COVID. And, he added, eradication “can be done, and many outbreaks have closed even during the [COVID] outbreak.”
Dr. Wassilak said that in Nigeria, “the face of the campaign became national.” In Pakistan, much of the work is done by national and international partners.
Dr. Wenger said that in Nigeria and other challenging areas, “the approach was essentially to make direct contact with the traditional leaders and the religious leaders and the local actors in each of these populations. So, it’s really getting down to the grassroots level.” Infectious disease officials send teams to speak with individuals in the “local, traditional leader system.”
“Just talking to them actually got us a long way and giving them the information that they need. In most cases, I mean, people want to do things to help their kids,” said Dr. Wenger.
For now, the initial plan, per the CDC, is to “initiate prompt and high coverage outbreak responses with available type 2 OPV to interrupt transmission” until a better supply of nOPV2 is available, then switch to IPVs.
Dr. Wenger and Dr. Wassilak report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most (86%) of these outbreaks were caused by cVDPV2 (circulating VDPV type 2 poliovirus, which originated with the vaccine), and most occurred in Africa, according to a new study of vaccine-derived poliovirus outbreaks between Jan. 2020 and June 2021 published in the CDC’s Morbidity and Mortality Weekly Report.
The Global Polio Eradication Initiative (GPEI) was launched in 1988 and used live attenuated oral poliovirus vaccine (OPV). Since then, cases of wild poliovirus have declined more than 99.99%.
The cVDPV2 likely originated among children born in areas with poor vaccine coverage. Jay Wenger, MD, director, Polio, at the Bill and Melinda Gates Foundation, told this news organization that “the inactivated vaccines that we give in most developed countries now are good in that they provide humoral immunity, the antibodies in the bloodstream. They don’t necessarily provide mucosal immunity. They don’t make the kid’s gut immune to getting reinfected or actually immune to reproducing the virus if they get it in their gut. So we could still have a situation where everybody was vaccinated with IPV [inactivated poliovirus], but the virus could still be transmitting around because kids’ guts would still be producing the virus and there will still be transmission in your population, probably without much or any paralysis because of the IPV. As soon as that virus hit a population that was not vaccinated, they would get paralyzed.”
Dr. Wenger added, “The ideal vaccine would be an oral vaccine that didn’t mutate back and couldn’t cause these VDPVs.” Scientists developed such a vaccine, approved by the World Health Organization last year under an Emergency Use Authorization. This nOPV2 (novel oral poliovirus type 2) vaccine has been given since March 2021 in areas with the VDPD2 outbreaks. The nOPV2 should allow them to “basically stamp out the outbreaks.”
The world had almost eradicated the disease, with the last cases of polio from wild virus occurring in Nigeria, Afghanistan, and Pakistan as of 2014. Africa was declared free of wild polio in 2020 after it had been eradicated from Nigeria, which accounted for more than half of the world’s cases only a decade earlier. Now cVDPV outbreaks affect 28 African countries, plus Iran, Yemen, Afghanistan, Pakistan, Tajikistan, Malaysia, the Philippines, and Indonesia. And there was also one case in China. Globally, there were 1,335 cases of cVDPV causing paralysis during the reporting period.
The COVID-19 pandemic has had a significant impact on polio, accounting for much of this year’s increase in cases. Dr. Wenger said, “We couldn’t do any campaigns. We pretty much stopped doing outbreak response campaigns in the middle of the year because of COVID.”
The CDC report notes that many of the supplementary immunizations in response to cVDPV2 outbreaks were of “poor quality,” and prolonged delays enabled geographically expanding cVDPV2 transmission.
Steve Wassilak, MD, chief coauthor of the CDC study, told this news organization that, because of COVID, “what we’ve been lacking is a rapid response for the most part. Some of that is due to laboratory delays and shipment because of COVID’s effect on international travel.” He noted, however, that there has been good recovery in surveillance and immunization activities despite COVID. And, he added, eradication “can be done, and many outbreaks have closed even during the [COVID] outbreak.”
Dr. Wassilak said that in Nigeria, “the face of the campaign became national.” In Pakistan, much of the work is done by national and international partners.
Dr. Wenger said that in Nigeria and other challenging areas, “the approach was essentially to make direct contact with the traditional leaders and the religious leaders and the local actors in each of these populations. So, it’s really getting down to the grassroots level.” Infectious disease officials send teams to speak with individuals in the “local, traditional leader system.”
“Just talking to them actually got us a long way and giving them the information that they need. In most cases, I mean, people want to do things to help their kids,” said Dr. Wenger.
For now, the initial plan, per the CDC, is to “initiate prompt and high coverage outbreak responses with available type 2 OPV to interrupt transmission” until a better supply of nOPV2 is available, then switch to IPVs.
Dr. Wenger and Dr. Wassilak report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most (86%) of these outbreaks were caused by cVDPV2 (circulating VDPV type 2 poliovirus, which originated with the vaccine), and most occurred in Africa, according to a new study of vaccine-derived poliovirus outbreaks between Jan. 2020 and June 2021 published in the CDC’s Morbidity and Mortality Weekly Report.
The Global Polio Eradication Initiative (GPEI) was launched in 1988 and used live attenuated oral poliovirus vaccine (OPV). Since then, cases of wild poliovirus have declined more than 99.99%.
The cVDPV2 likely originated among children born in areas with poor vaccine coverage. Jay Wenger, MD, director, Polio, at the Bill and Melinda Gates Foundation, told this news organization that “the inactivated vaccines that we give in most developed countries now are good in that they provide humoral immunity, the antibodies in the bloodstream. They don’t necessarily provide mucosal immunity. They don’t make the kid’s gut immune to getting reinfected or actually immune to reproducing the virus if they get it in their gut. So we could still have a situation where everybody was vaccinated with IPV [inactivated poliovirus], but the virus could still be transmitting around because kids’ guts would still be producing the virus and there will still be transmission in your population, probably without much or any paralysis because of the IPV. As soon as that virus hit a population that was not vaccinated, they would get paralyzed.”
Dr. Wenger added, “The ideal vaccine would be an oral vaccine that didn’t mutate back and couldn’t cause these VDPVs.” Scientists developed such a vaccine, approved by the World Health Organization last year under an Emergency Use Authorization. This nOPV2 (novel oral poliovirus type 2) vaccine has been given since March 2021 in areas with the VDPD2 outbreaks. The nOPV2 should allow them to “basically stamp out the outbreaks.”
The world had almost eradicated the disease, with the last cases of polio from wild virus occurring in Nigeria, Afghanistan, and Pakistan as of 2014. Africa was declared free of wild polio in 2020 after it had been eradicated from Nigeria, which accounted for more than half of the world’s cases only a decade earlier. Now cVDPV outbreaks affect 28 African countries, plus Iran, Yemen, Afghanistan, Pakistan, Tajikistan, Malaysia, the Philippines, and Indonesia. And there was also one case in China. Globally, there were 1,335 cases of cVDPV causing paralysis during the reporting period.
The COVID-19 pandemic has had a significant impact on polio, accounting for much of this year’s increase in cases. Dr. Wenger said, “We couldn’t do any campaigns. We pretty much stopped doing outbreak response campaigns in the middle of the year because of COVID.”
The CDC report notes that many of the supplementary immunizations in response to cVDPV2 outbreaks were of “poor quality,” and prolonged delays enabled geographically expanding cVDPV2 transmission.
Steve Wassilak, MD, chief coauthor of the CDC study, told this news organization that, because of COVID, “what we’ve been lacking is a rapid response for the most part. Some of that is due to laboratory delays and shipment because of COVID’s effect on international travel.” He noted, however, that there has been good recovery in surveillance and immunization activities despite COVID. And, he added, eradication “can be done, and many outbreaks have closed even during the [COVID] outbreak.”
Dr. Wassilak said that in Nigeria, “the face of the campaign became national.” In Pakistan, much of the work is done by national and international partners.
Dr. Wenger said that in Nigeria and other challenging areas, “the approach was essentially to make direct contact with the traditional leaders and the religious leaders and the local actors in each of these populations. So, it’s really getting down to the grassroots level.” Infectious disease officials send teams to speak with individuals in the “local, traditional leader system.”
“Just talking to them actually got us a long way and giving them the information that they need. In most cases, I mean, people want to do things to help their kids,” said Dr. Wenger.
For now, the initial plan, per the CDC, is to “initiate prompt and high coverage outbreak responses with available type 2 OPV to interrupt transmission” until a better supply of nOPV2 is available, then switch to IPVs.
Dr. Wenger and Dr. Wassilak report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular effects of breast cancer treatment vary based on weight, menopausal status
For example, certain chemotherapy drugs may confer higher risk in breast cancer survivors of normal weight, whereas they may lower stroke risk in those who are obese, according to Heather Greenlee, ND, PhD, a public health researcher and naturopathic physician with the Fred Hutchinson Cancer Research Center in Seattle.
In postmenopausal women with breast cancer, aromatase inhibitors may increase cardiovascular risk, while tamoxifen appears to reduce the risk of incident dyslipidemia, she said.
The findings are from separate analyses of data from studies presented during a poster discussion session at the symposium.
Breast cancer treatment and cardiovascular effects: The role of weight
In one analysis, Dr. Greenlee and colleagues examined outcomes in 13,582 breast cancer survivors with a median age of 60 years and median follow-up of 7 years to assess whether cardiovascular disease (CVD) risk associated with specific breast cancer therapies varies by body mass index (BMI) category at diagnosis.
Many routinely used breast cancer therapies are cardiotoxic, and being overweight or obese are known risk factors for CVD, but few studies have assessed whether BMI modifies the effect of these treatment on cardiovascular risk, Dr. Greenlee explained.
After adjusting for baseline demographic and health-related factors, and other breast cancer treatment, they found that:
- Ischemic heart disease risk was higher among normal-weight women who received anthracyclines, compared with those who did not (hazard ratio, 4.2). No other risk associations were observed for other breast cancer therapies and BMI groups.
- Heart failure/cardiomyopathy risk was higher among women with normal weight who received anthracyclines, cyclophosphamides, or left-sided radiation, compared with those who did not (HRs, 5.24, 3.27, and 2.05, respectively), and among overweight women who received anthracyclines, compared with those who did not (HR, 2.18). No risk associations were observed for women who received trastuzumab, taxanes, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were obese.
- Stroke risk was higher in normal-weight women who received taxanes, cyclophosphamides, or left-sided radiation versus those who did not (HRs, 2.14, 2.35, and 1.31, respectively), and stroke risk was lower in obese women who received anthracyclines, taxanes, or cyclophosphamide, compared with those who did not (HRs, 0.32, 0.41, and 0.29, respectively). No risk associations were observed for trastuzumab, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were overweight.
The lack of associations noted between treatments and heart failure risk among obese patients could be caused by the “obesity paradox” observed in prior obese populations, the investigators noted, adding that additional analyses are planned to “examine whether different dosage and duration of breast cancer therapy exposures across the BMI groups contributed to these risk associations.”
Breast cancer treatment and cardiometabolic effects: The role of menopausal status
In a separate analysis, Dr. Greenlee and colleagues looked at the association between endocrine therapies and cardiometabolic risk based on menopausal status.
Endocrine therapy is associated with CVD in breast cancer survivors and may be associated with developing cardiometabolic risk factors like diabetes, dyslipidemia, and hypertension, they noted, explaining that tamoxifen has mixed estrogenic and antiestrogenic activity, while aromatase inhibitors deplete endogenous estrogen.
Since most studies have compared tamoxifen with aromatase inhibitor use, it has been a challenge challenging to discern the effects of each, Dr. Greenlee said.
She and her colleagues reviewed records for 14,942 breast cancer survivors who were diagnosed between 2005 and 2013. The patients had a mean age of 61 years at baseline, and 24.9% were premenopausal at the time of diagnosis. Of the premenopausal women, 27.3% used tamoxifen, 19.2% used aromatase inhibitors, and 53.5% did not use endocrine therapy, and of the postmenopausal women, 6.6% used tamoxifen, 47.7% used aromatase inhibitors, and 45.7% did not use endocrine therapy.
After adjusting for baseline demographics and health factors, the investigators found that:
- The use of tamoxifen or aromatase inhibitors was not associated with a risk of developing diabetes, dyslipidemia, or hypertension in premenopausal women, or with a risk of developing diabetes or hypertension in postmenopausal women.
- The risk of dyslipidemia was higher in postmenopausal aromatase inhibitor users, and lower in postmenopausal tamoxifen users, compared with postmenopausal non-users of endocrine therapy (HRs, 1.15 and 0.75, respectively).
The lack of associations between endocrine therapy and CVD risk in premenopausal women may be from low power, Dr. Greenlee said, noting that analyses in larger sample sizes are needed.
She and her colleagues plan to conduct further analyses looking at treatment dosage and duration, and comparing steroidal versus nonsteroidal aromatase inhibitors.
Future studies should examine the implications of these associations on long-term CVD and how best to manage lipid profiles in postmenopausal breast cancer survivors who have a history of endocrine therapy treatment, they concluded.
This research was funded by grants from the National Cancer Institute.
For example, certain chemotherapy drugs may confer higher risk in breast cancer survivors of normal weight, whereas they may lower stroke risk in those who are obese, according to Heather Greenlee, ND, PhD, a public health researcher and naturopathic physician with the Fred Hutchinson Cancer Research Center in Seattle.
In postmenopausal women with breast cancer, aromatase inhibitors may increase cardiovascular risk, while tamoxifen appears to reduce the risk of incident dyslipidemia, she said.
The findings are from separate analyses of data from studies presented during a poster discussion session at the symposium.
Breast cancer treatment and cardiovascular effects: The role of weight
In one analysis, Dr. Greenlee and colleagues examined outcomes in 13,582 breast cancer survivors with a median age of 60 years and median follow-up of 7 years to assess whether cardiovascular disease (CVD) risk associated with specific breast cancer therapies varies by body mass index (BMI) category at diagnosis.
Many routinely used breast cancer therapies are cardiotoxic, and being overweight or obese are known risk factors for CVD, but few studies have assessed whether BMI modifies the effect of these treatment on cardiovascular risk, Dr. Greenlee explained.
After adjusting for baseline demographic and health-related factors, and other breast cancer treatment, they found that:
- Ischemic heart disease risk was higher among normal-weight women who received anthracyclines, compared with those who did not (hazard ratio, 4.2). No other risk associations were observed for other breast cancer therapies and BMI groups.
- Heart failure/cardiomyopathy risk was higher among women with normal weight who received anthracyclines, cyclophosphamides, or left-sided radiation, compared with those who did not (HRs, 5.24, 3.27, and 2.05, respectively), and among overweight women who received anthracyclines, compared with those who did not (HR, 2.18). No risk associations were observed for women who received trastuzumab, taxanes, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were obese.
- Stroke risk was higher in normal-weight women who received taxanes, cyclophosphamides, or left-sided radiation versus those who did not (HRs, 2.14, 2.35, and 1.31, respectively), and stroke risk was lower in obese women who received anthracyclines, taxanes, or cyclophosphamide, compared with those who did not (HRs, 0.32, 0.41, and 0.29, respectively). No risk associations were observed for trastuzumab, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were overweight.
The lack of associations noted between treatments and heart failure risk among obese patients could be caused by the “obesity paradox” observed in prior obese populations, the investigators noted, adding that additional analyses are planned to “examine whether different dosage and duration of breast cancer therapy exposures across the BMI groups contributed to these risk associations.”
Breast cancer treatment and cardiometabolic effects: The role of menopausal status
In a separate analysis, Dr. Greenlee and colleagues looked at the association between endocrine therapies and cardiometabolic risk based on menopausal status.
Endocrine therapy is associated with CVD in breast cancer survivors and may be associated with developing cardiometabolic risk factors like diabetes, dyslipidemia, and hypertension, they noted, explaining that tamoxifen has mixed estrogenic and antiestrogenic activity, while aromatase inhibitors deplete endogenous estrogen.
Since most studies have compared tamoxifen with aromatase inhibitor use, it has been a challenge challenging to discern the effects of each, Dr. Greenlee said.
She and her colleagues reviewed records for 14,942 breast cancer survivors who were diagnosed between 2005 and 2013. The patients had a mean age of 61 years at baseline, and 24.9% were premenopausal at the time of diagnosis. Of the premenopausal women, 27.3% used tamoxifen, 19.2% used aromatase inhibitors, and 53.5% did not use endocrine therapy, and of the postmenopausal women, 6.6% used tamoxifen, 47.7% used aromatase inhibitors, and 45.7% did not use endocrine therapy.
After adjusting for baseline demographics and health factors, the investigators found that:
- The use of tamoxifen or aromatase inhibitors was not associated with a risk of developing diabetes, dyslipidemia, or hypertension in premenopausal women, or with a risk of developing diabetes or hypertension in postmenopausal women.
- The risk of dyslipidemia was higher in postmenopausal aromatase inhibitor users, and lower in postmenopausal tamoxifen users, compared with postmenopausal non-users of endocrine therapy (HRs, 1.15 and 0.75, respectively).
The lack of associations between endocrine therapy and CVD risk in premenopausal women may be from low power, Dr. Greenlee said, noting that analyses in larger sample sizes are needed.
She and her colleagues plan to conduct further analyses looking at treatment dosage and duration, and comparing steroidal versus nonsteroidal aromatase inhibitors.
Future studies should examine the implications of these associations on long-term CVD and how best to manage lipid profiles in postmenopausal breast cancer survivors who have a history of endocrine therapy treatment, they concluded.
This research was funded by grants from the National Cancer Institute.
For example, certain chemotherapy drugs may confer higher risk in breast cancer survivors of normal weight, whereas they may lower stroke risk in those who are obese, according to Heather Greenlee, ND, PhD, a public health researcher and naturopathic physician with the Fred Hutchinson Cancer Research Center in Seattle.
In postmenopausal women with breast cancer, aromatase inhibitors may increase cardiovascular risk, while tamoxifen appears to reduce the risk of incident dyslipidemia, she said.
The findings are from separate analyses of data from studies presented during a poster discussion session at the symposium.
Breast cancer treatment and cardiovascular effects: The role of weight
In one analysis, Dr. Greenlee and colleagues examined outcomes in 13,582 breast cancer survivors with a median age of 60 years and median follow-up of 7 years to assess whether cardiovascular disease (CVD) risk associated with specific breast cancer therapies varies by body mass index (BMI) category at diagnosis.
Many routinely used breast cancer therapies are cardiotoxic, and being overweight or obese are known risk factors for CVD, but few studies have assessed whether BMI modifies the effect of these treatment on cardiovascular risk, Dr. Greenlee explained.
After adjusting for baseline demographic and health-related factors, and other breast cancer treatment, they found that:
- Ischemic heart disease risk was higher among normal-weight women who received anthracyclines, compared with those who did not (hazard ratio, 4.2). No other risk associations were observed for other breast cancer therapies and BMI groups.
- Heart failure/cardiomyopathy risk was higher among women with normal weight who received anthracyclines, cyclophosphamides, or left-sided radiation, compared with those who did not (HRs, 5.24, 3.27, and 2.05, respectively), and among overweight women who received anthracyclines, compared with those who did not (HR, 2.18). No risk associations were observed for women who received trastuzumab, taxanes, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were obese.
- Stroke risk was higher in normal-weight women who received taxanes, cyclophosphamides, or left-sided radiation versus those who did not (HRs, 2.14, 2.35, and 1.31, respectively), and stroke risk was lower in obese women who received anthracyclines, taxanes, or cyclophosphamide, compared with those who did not (HRs, 0.32, 0.41, and 0.29, respectively). No risk associations were observed for trastuzumab, endocrine therapy, or radiation on any side, and no risk associations were observed for women who were overweight.
The lack of associations noted between treatments and heart failure risk among obese patients could be caused by the “obesity paradox” observed in prior obese populations, the investigators noted, adding that additional analyses are planned to “examine whether different dosage and duration of breast cancer therapy exposures across the BMI groups contributed to these risk associations.”
Breast cancer treatment and cardiometabolic effects: The role of menopausal status
In a separate analysis, Dr. Greenlee and colleagues looked at the association between endocrine therapies and cardiometabolic risk based on menopausal status.
Endocrine therapy is associated with CVD in breast cancer survivors and may be associated with developing cardiometabolic risk factors like diabetes, dyslipidemia, and hypertension, they noted, explaining that tamoxifen has mixed estrogenic and antiestrogenic activity, while aromatase inhibitors deplete endogenous estrogen.
Since most studies have compared tamoxifen with aromatase inhibitor use, it has been a challenge challenging to discern the effects of each, Dr. Greenlee said.
She and her colleagues reviewed records for 14,942 breast cancer survivors who were diagnosed between 2005 and 2013. The patients had a mean age of 61 years at baseline, and 24.9% were premenopausal at the time of diagnosis. Of the premenopausal women, 27.3% used tamoxifen, 19.2% used aromatase inhibitors, and 53.5% did not use endocrine therapy, and of the postmenopausal women, 6.6% used tamoxifen, 47.7% used aromatase inhibitors, and 45.7% did not use endocrine therapy.
After adjusting for baseline demographics and health factors, the investigators found that:
- The use of tamoxifen or aromatase inhibitors was not associated with a risk of developing diabetes, dyslipidemia, or hypertension in premenopausal women, or with a risk of developing diabetes or hypertension in postmenopausal women.
- The risk of dyslipidemia was higher in postmenopausal aromatase inhibitor users, and lower in postmenopausal tamoxifen users, compared with postmenopausal non-users of endocrine therapy (HRs, 1.15 and 0.75, respectively).
The lack of associations between endocrine therapy and CVD risk in premenopausal women may be from low power, Dr. Greenlee said, noting that analyses in larger sample sizes are needed.
She and her colleagues plan to conduct further analyses looking at treatment dosage and duration, and comparing steroidal versus nonsteroidal aromatase inhibitors.
Future studies should examine the implications of these associations on long-term CVD and how best to manage lipid profiles in postmenopausal breast cancer survivors who have a history of endocrine therapy treatment, they concluded.
This research was funded by grants from the National Cancer Institute.
FROM SABCS 2021
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Overlap in a Pregnant Patient
To the Editor:
A 34-year-old pregnant woman at 5 weeks’ gestation was transferred to dermatology from an outside hospital with a full-body rash. Three days after noting a fever and generalized body aches, she developed a painful rash on the legs that had gradually spread to the arms, trunk, and face. Symptoms of eyelid pruritus and edema initially were improved with intravenous (IV) steroids at an emergency department visit, but they started to flare soon thereafter with worsening mucosal involvement and dysphagia. After a second visit to the emergency department and repeat treatment with IV steroids, she was transferred to our institution for a higher level of care.
The patient denied taking any new medications in the 2 months prior to the onset of the rash. Her medication history only consisted of over-the-counter prenatal vitamins, a single use of over-the-counter migraine medication (containing acetaminophen, aspirin, and caffeine as active ingredients), and a possible use of ibuprofen or acetaminophen separately. She reported ocular discomfort and blurriness, dysphagia, dysuria, and vaginal discomfort. Physical examination revealed dusky red to violaceous macules and patches that involved approximately 65% of the body surface area (BSA), with bullae involving approximately 10% BSA. The face was diffusely red and edematous with crusted erosions and scattered bullae on the cheeks. Mucosal involvement was notable for injected conjunctivae and erosions present on the upper hard palate of the mouth and lips (Figure, A). Erythematous macules with dusky centers coalescing into patches with overlying vesicles and bullae were scattered on the arms (Figure, B), hands, trunk (Figure, C), and legs. The Nikolsky sign was positive. The vulva was swollen and covered with erythematous macules with dusky centers.
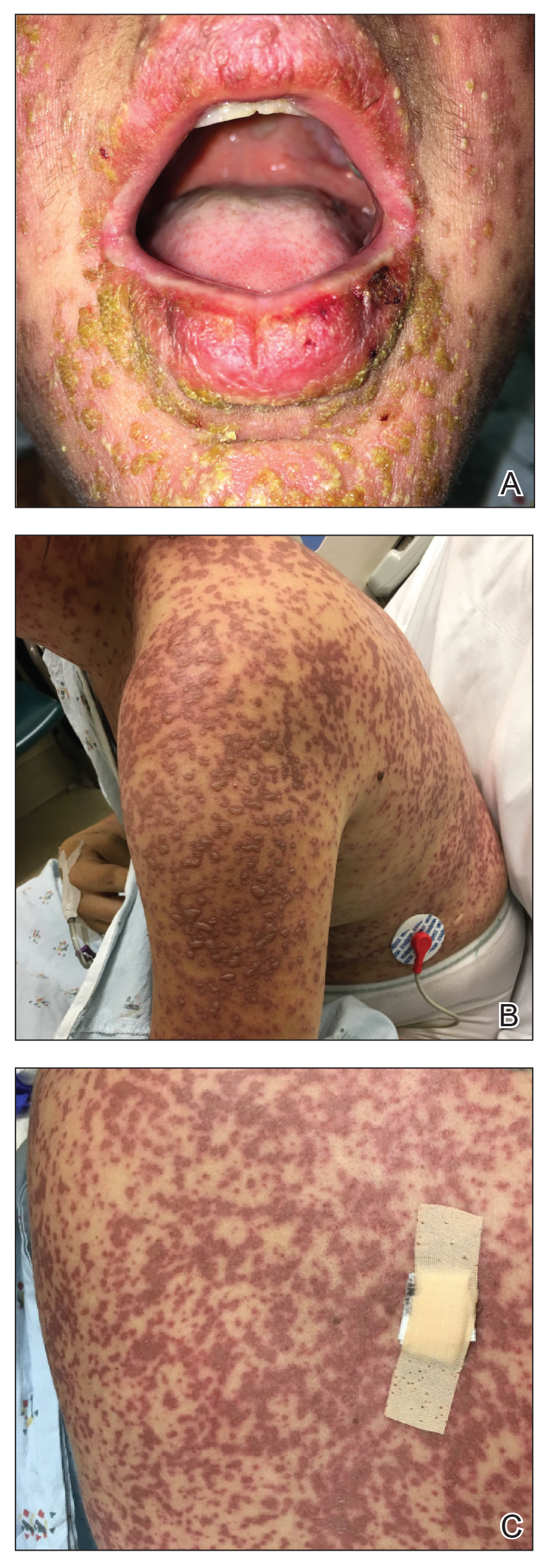
A biopsy from the upper back revealed a vacuolar interface with subepidermal bullae and confluent keratinocyte necrosis with many CD8+ cells and scattered granzyme B. Given these results in conjunction with the clinical findings, a diagnosis of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) overlap was made. In addition to providing supportive care, the patient was started on a 4-day course of IV immunoglobulin (IVIG)(3g/kg total) and prednisone 60 mg daily, tapered over several weeks with a good clinical response. At outpatient follow-up she was found to have postinflammatory hypopigmentation on the face, trunk, and extremities, as well as tear duct scarring, but she had no vulvovaginal scarring or stenosis. She was progressing well in her pregnancy with no serious complications for 4 months after admission, at which point she was lost to follow-up.
Stevens-Johnson syndrome and TEN represent a spectrum of severe mucocutaneous reactions with high morbidity and mortality. Medications are the leading trigger, followed by infection. The most common inciting medications include antibacterial sulfonamides, antiepileptics such as carbamazepine and lamotrigine, nonsteroidal anti-inflammatory drugs, nevirapine, and allopurinol. The onset of symptoms from 1 to 4 weeks combined with characteristic morphologic features helps distinguish SJS/TEN from other drug eruptions. The initial presentation classically consists of a flulike prodrome followed by mucocutaneous eruption. Skin lesions often present as a diffuse erythema or ill-defined, coalescing, erythematous macules with purpuric centers that may evolve into vesicles and bullae with sloughing of the skin. Histopathology reveals full-thickness epidermal necrosis with detachment.1
Erythema multiforme and Mycoplasma-induced rash and mucositis (MIRM) are high on the differential diagnosis. Distinguishing features of erythema multiforme include the morphology of targetoid lesions and a common distribution on the extremities, in addition to the limited bullae and epidermal detachment in comparison with SJS/TEN. In MIRM, mucositis often is more severe and extensive, with multiple mucosal surfaces affected. It typically has less cutaneous involvement than SJS/TEN, though clinical variants can include diffuse rash and affect fewer than 2 mucosal sites.2 Depending on the timing of rash onset, Mycoplasma IgM/IgG titers may be drawn to further support the diagnosis. A diagnosis of MIRM was not favored in our patient due to lack of respiratory symptoms, normal chest radiography, and negative Mycoplasma IgM and IgG titers.
Stevens-Johnson syndrome/toxic epidermal necrolysis overlap has been reported in pregnant patients, typically in association with HIV infection or new medication exposure.3 A combination of genetic susceptibility and an altered immune system during pregnancy may contribute to the pathogenesis, involving a cytotoxic T-cell mediated reaction with release of inflammatory cytokines.1 Interestingly, these factors that may predispose a patient to developing SJS/TEN may not pass on to the neonate, evidenced by a few cases that showed no reaction in the newborn when given the same offending drug.4
Stevens-Johnson syndrome/toxic epidermal necrolysis more frequently presents in the second or third trimester, with no increase in maternal mortality and an equally high survival rate of the fetus.1,5 Unique sequelae in pregnant patients may include vaginal stenosis, vulvar swelling, and postpartum sepsis. Fetal complications can include low birth weight, preterm delivery, and respiratory distress. The fetus rarely exhibits cutaneous manifestations of the disease.6
A multidisciplinary approach to the diagnosis and management of SJS/TEN overlap in special patient populations such as pregnant women is vital. Supportive measures consisting of wound care, fluid and electrolyte management, infection monitoring, and nutritional support have sufficed in treating SJS/TEN in pregnant patients.3 Although adjunctive therapy with systemic corticosteroids, IVIG, cyclosporine, and tumor necrosis factor inhibitors commonly are used in clinical practice, the safety of these treatments in pregnant patients affected by SJS/TEN has not been established. However, use of these medications for other indications, primarily rheumatologic diseases, has been reported to be safe in the pregnant population.7 If necessary, glucocorticoids should be used in the lowest effective dose to avoid complications such as premature rupture of membranes; intrauterine growth restriction; and increased risk for pregnancy-induced hypertension, gestational diabetes, osteoporosis, and infection. Little is known about IVIG use in pregnancy. While it has not been associated with increased risk of fetal malformations, it may cross the placenta in a notable amount when administered after 30 weeks’ gestation.7
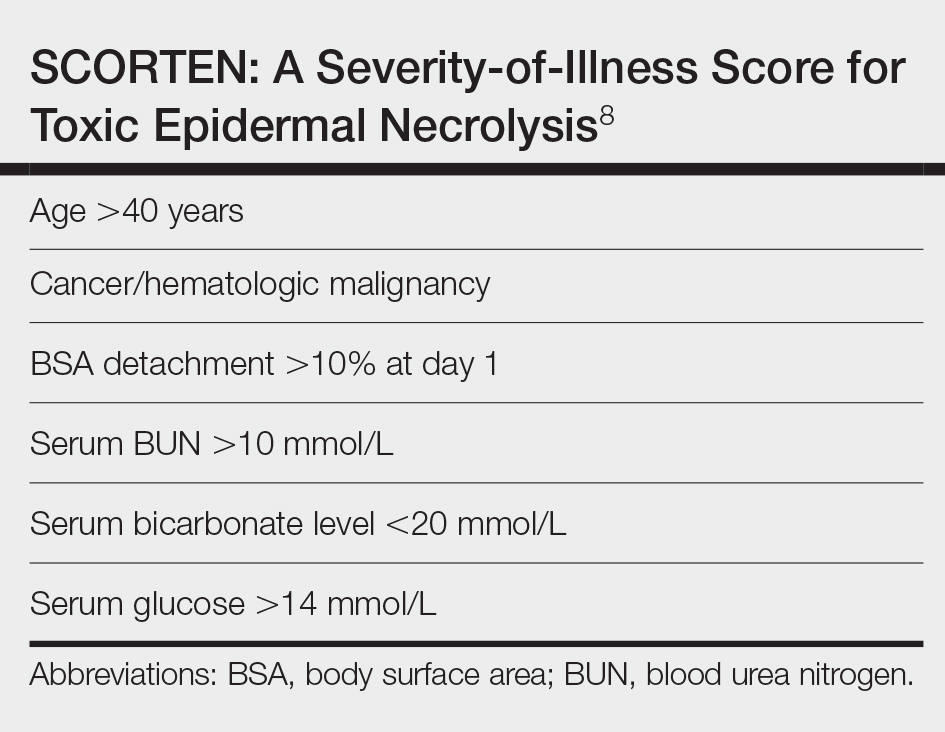
Unlike most cases of SJS/TEN in pregnancy that largely were associated with HIV infection or drug exposure, primarily antiretrovirals such as nevirapine or antiepileptics, our case is a rare incidence of SJS/TEN in a pregnant patient with no clear medication or infectious trigger. Although the causative drug was unclear, we suspected it was secondary to nonsteroidal anti-inflammatory drug use. The patient had a SCORTEN (SCORe of Toxic Epidermal Necrosis) of 0, which portends a relatively good prognosis with an estimated mortality rate of approximately 3% (Table).8 However, the large BSA involvement of the morbilliform rash warranted aggressive management to prevent the involved skin from fully detaching.
1. Struck MF, Illert T, Liss Y, et al. Toxic epidermal necrolysis in pregnancy: case report and review of the literature. J Burn Care Res. 2010;31:816-821. doi:10.1097/BCR.0b013e3181eed441
2. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245.e4. doi:10.1016/j.jaad.2014.06.026
3. Knight L, Todd G, Muloiwa R, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: maternal and foetal outcomes in twenty-two consecutive pregnant HIV infected women. PLoS One. 2015;10:1-11. doi:10.1371/journal.pone.0135501
4. Velter C, Hotz C, Ingen-Housz-Oro S. Stevens-Johnson syndrome during pregnancy: case report of a newborn treated with the culprit drug. JAMA Dermatol. 2018;154:224-225. doi:10.1001/jamadermatol.2017.4607
5. El Daief SG, Das S, Ekekwe G, et al. A successful pregnancy outcome after Stevens-Johnson syndrome. J Obstet Gynaecol (Lahore). 2014;34:445-446. doi:10.3109/01443615.2014.914897
6. Rodriguez G, Trent JT, Mirzabeigi M. Toxic epidermal necrolysis in a mother and fetus. J Am Acad Dermatol. 2006;55(5 suppl):96-98. doi:10.1016/j.jaad.2005.09.023
7. Bermas BL. Safety of rheumatic disease medication use during pregnancy and lactation. UptoDate website. Updated March 24, 2021. Accessed December 16, 2021. https://www.uptodate.com/contents/safety-of-rheumatic-disease-medication-use-during-pregnancy-and-lactation#H11
8. Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153. doi:10.1046/j.1523-1747.2000.00061.x
To the Editor:
A 34-year-old pregnant woman at 5 weeks’ gestation was transferred to dermatology from an outside hospital with a full-body rash. Three days after noting a fever and generalized body aches, she developed a painful rash on the legs that had gradually spread to the arms, trunk, and face. Symptoms of eyelid pruritus and edema initially were improved with intravenous (IV) steroids at an emergency department visit, but they started to flare soon thereafter with worsening mucosal involvement and dysphagia. After a second visit to the emergency department and repeat treatment with IV steroids, she was transferred to our institution for a higher level of care.
The patient denied taking any new medications in the 2 months prior to the onset of the rash. Her medication history only consisted of over-the-counter prenatal vitamins, a single use of over-the-counter migraine medication (containing acetaminophen, aspirin, and caffeine as active ingredients), and a possible use of ibuprofen or acetaminophen separately. She reported ocular discomfort and blurriness, dysphagia, dysuria, and vaginal discomfort. Physical examination revealed dusky red to violaceous macules and patches that involved approximately 65% of the body surface area (BSA), with bullae involving approximately 10% BSA. The face was diffusely red and edematous with crusted erosions and scattered bullae on the cheeks. Mucosal involvement was notable for injected conjunctivae and erosions present on the upper hard palate of the mouth and lips (Figure, A). Erythematous macules with dusky centers coalescing into patches with overlying vesicles and bullae were scattered on the arms (Figure, B), hands, trunk (Figure, C), and legs. The Nikolsky sign was positive. The vulva was swollen and covered with erythematous macules with dusky centers.

A biopsy from the upper back revealed a vacuolar interface with subepidermal bullae and confluent keratinocyte necrosis with many CD8+ cells and scattered granzyme B. Given these results in conjunction with the clinical findings, a diagnosis of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) overlap was made. In addition to providing supportive care, the patient was started on a 4-day course of IV immunoglobulin (IVIG)(3g/kg total) and prednisone 60 mg daily, tapered over several weeks with a good clinical response. At outpatient follow-up she was found to have postinflammatory hypopigmentation on the face, trunk, and extremities, as well as tear duct scarring, but she had no vulvovaginal scarring or stenosis. She was progressing well in her pregnancy with no serious complications for 4 months after admission, at which point she was lost to follow-up.
Stevens-Johnson syndrome and TEN represent a spectrum of severe mucocutaneous reactions with high morbidity and mortality. Medications are the leading trigger, followed by infection. The most common inciting medications include antibacterial sulfonamides, antiepileptics such as carbamazepine and lamotrigine, nonsteroidal anti-inflammatory drugs, nevirapine, and allopurinol. The onset of symptoms from 1 to 4 weeks combined with characteristic morphologic features helps distinguish SJS/TEN from other drug eruptions. The initial presentation classically consists of a flulike prodrome followed by mucocutaneous eruption. Skin lesions often present as a diffuse erythema or ill-defined, coalescing, erythematous macules with purpuric centers that may evolve into vesicles and bullae with sloughing of the skin. Histopathology reveals full-thickness epidermal necrosis with detachment.1
Erythema multiforme and Mycoplasma-induced rash and mucositis (MIRM) are high on the differential diagnosis. Distinguishing features of erythema multiforme include the morphology of targetoid lesions and a common distribution on the extremities, in addition to the limited bullae and epidermal detachment in comparison with SJS/TEN. In MIRM, mucositis often is more severe and extensive, with multiple mucosal surfaces affected. It typically has less cutaneous involvement than SJS/TEN, though clinical variants can include diffuse rash and affect fewer than 2 mucosal sites.2 Depending on the timing of rash onset, Mycoplasma IgM/IgG titers may be drawn to further support the diagnosis. A diagnosis of MIRM was not favored in our patient due to lack of respiratory symptoms, normal chest radiography, and negative Mycoplasma IgM and IgG titers.
Stevens-Johnson syndrome/toxic epidermal necrolysis overlap has been reported in pregnant patients, typically in association with HIV infection or new medication exposure.3 A combination of genetic susceptibility and an altered immune system during pregnancy may contribute to the pathogenesis, involving a cytotoxic T-cell mediated reaction with release of inflammatory cytokines.1 Interestingly, these factors that may predispose a patient to developing SJS/TEN may not pass on to the neonate, evidenced by a few cases that showed no reaction in the newborn when given the same offending drug.4
Stevens-Johnson syndrome/toxic epidermal necrolysis more frequently presents in the second or third trimester, with no increase in maternal mortality and an equally high survival rate of the fetus.1,5 Unique sequelae in pregnant patients may include vaginal stenosis, vulvar swelling, and postpartum sepsis. Fetal complications can include low birth weight, preterm delivery, and respiratory distress. The fetus rarely exhibits cutaneous manifestations of the disease.6
A multidisciplinary approach to the diagnosis and management of SJS/TEN overlap in special patient populations such as pregnant women is vital. Supportive measures consisting of wound care, fluid and electrolyte management, infection monitoring, and nutritional support have sufficed in treating SJS/TEN in pregnant patients.3 Although adjunctive therapy with systemic corticosteroids, IVIG, cyclosporine, and tumor necrosis factor inhibitors commonly are used in clinical practice, the safety of these treatments in pregnant patients affected by SJS/TEN has not been established. However, use of these medications for other indications, primarily rheumatologic diseases, has been reported to be safe in the pregnant population.7 If necessary, glucocorticoids should be used in the lowest effective dose to avoid complications such as premature rupture of membranes; intrauterine growth restriction; and increased risk for pregnancy-induced hypertension, gestational diabetes, osteoporosis, and infection. Little is known about IVIG use in pregnancy. While it has not been associated with increased risk of fetal malformations, it may cross the placenta in a notable amount when administered after 30 weeks’ gestation.7
Unlike most cases of SJS/TEN in pregnancy that largely were associated with HIV infection or drug exposure, primarily antiretrovirals such as nevirapine or antiepileptics, our case is a rare incidence of SJS/TEN in a pregnant patient with no clear medication or infectious trigger. Although the causative drug was unclear, we suspected it was secondary to nonsteroidal anti-inflammatory drug use. The patient had a SCORTEN (SCORe of Toxic Epidermal Necrosis) of 0, which portends a relatively good prognosis with an estimated mortality rate of approximately 3% (Table).8 However, the large BSA involvement of the morbilliform rash warranted aggressive management to prevent the involved skin from fully detaching.

To the Editor:
A 34-year-old pregnant woman at 5 weeks’ gestation was transferred to dermatology from an outside hospital with a full-body rash. Three days after noting a fever and generalized body aches, she developed a painful rash on the legs that had gradually spread to the arms, trunk, and face. Symptoms of eyelid pruritus and edema initially were improved with intravenous (IV) steroids at an emergency department visit, but they started to flare soon thereafter with worsening mucosal involvement and dysphagia. After a second visit to the emergency department and repeat treatment with IV steroids, she was transferred to our institution for a higher level of care.
The patient denied taking any new medications in the 2 months prior to the onset of the rash. Her medication history only consisted of over-the-counter prenatal vitamins, a single use of over-the-counter migraine medication (containing acetaminophen, aspirin, and caffeine as active ingredients), and a possible use of ibuprofen or acetaminophen separately. She reported ocular discomfort and blurriness, dysphagia, dysuria, and vaginal discomfort. Physical examination revealed dusky red to violaceous macules and patches that involved approximately 65% of the body surface area (BSA), with bullae involving approximately 10% BSA. The face was diffusely red and edematous with crusted erosions and scattered bullae on the cheeks. Mucosal involvement was notable for injected conjunctivae and erosions present on the upper hard palate of the mouth and lips (Figure, A). Erythematous macules with dusky centers coalescing into patches with overlying vesicles and bullae were scattered on the arms (Figure, B), hands, trunk (Figure, C), and legs. The Nikolsky sign was positive. The vulva was swollen and covered with erythematous macules with dusky centers.

A biopsy from the upper back revealed a vacuolar interface with subepidermal bullae and confluent keratinocyte necrosis with many CD8+ cells and scattered granzyme B. Given these results in conjunction with the clinical findings, a diagnosis of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) overlap was made. In addition to providing supportive care, the patient was started on a 4-day course of IV immunoglobulin (IVIG)(3g/kg total) and prednisone 60 mg daily, tapered over several weeks with a good clinical response. At outpatient follow-up she was found to have postinflammatory hypopigmentation on the face, trunk, and extremities, as well as tear duct scarring, but she had no vulvovaginal scarring or stenosis. She was progressing well in her pregnancy with no serious complications for 4 months after admission, at which point she was lost to follow-up.
Stevens-Johnson syndrome and TEN represent a spectrum of severe mucocutaneous reactions with high morbidity and mortality. Medications are the leading trigger, followed by infection. The most common inciting medications include antibacterial sulfonamides, antiepileptics such as carbamazepine and lamotrigine, nonsteroidal anti-inflammatory drugs, nevirapine, and allopurinol. The onset of symptoms from 1 to 4 weeks combined with characteristic morphologic features helps distinguish SJS/TEN from other drug eruptions. The initial presentation classically consists of a flulike prodrome followed by mucocutaneous eruption. Skin lesions often present as a diffuse erythema or ill-defined, coalescing, erythematous macules with purpuric centers that may evolve into vesicles and bullae with sloughing of the skin. Histopathology reveals full-thickness epidermal necrosis with detachment.1
Erythema multiforme and Mycoplasma-induced rash and mucositis (MIRM) are high on the differential diagnosis. Distinguishing features of erythema multiforme include the morphology of targetoid lesions and a common distribution on the extremities, in addition to the limited bullae and epidermal detachment in comparison with SJS/TEN. In MIRM, mucositis often is more severe and extensive, with multiple mucosal surfaces affected. It typically has less cutaneous involvement than SJS/TEN, though clinical variants can include diffuse rash and affect fewer than 2 mucosal sites.2 Depending on the timing of rash onset, Mycoplasma IgM/IgG titers may be drawn to further support the diagnosis. A diagnosis of MIRM was not favored in our patient due to lack of respiratory symptoms, normal chest radiography, and negative Mycoplasma IgM and IgG titers.
Stevens-Johnson syndrome/toxic epidermal necrolysis overlap has been reported in pregnant patients, typically in association with HIV infection or new medication exposure.3 A combination of genetic susceptibility and an altered immune system during pregnancy may contribute to the pathogenesis, involving a cytotoxic T-cell mediated reaction with release of inflammatory cytokines.1 Interestingly, these factors that may predispose a patient to developing SJS/TEN may not pass on to the neonate, evidenced by a few cases that showed no reaction in the newborn when given the same offending drug.4
Stevens-Johnson syndrome/toxic epidermal necrolysis more frequently presents in the second or third trimester, with no increase in maternal mortality and an equally high survival rate of the fetus.1,5 Unique sequelae in pregnant patients may include vaginal stenosis, vulvar swelling, and postpartum sepsis. Fetal complications can include low birth weight, preterm delivery, and respiratory distress. The fetus rarely exhibits cutaneous manifestations of the disease.6
A multidisciplinary approach to the diagnosis and management of SJS/TEN overlap in special patient populations such as pregnant women is vital. Supportive measures consisting of wound care, fluid and electrolyte management, infection monitoring, and nutritional support have sufficed in treating SJS/TEN in pregnant patients.3 Although adjunctive therapy with systemic corticosteroids, IVIG, cyclosporine, and tumor necrosis factor inhibitors commonly are used in clinical practice, the safety of these treatments in pregnant patients affected by SJS/TEN has not been established. However, use of these medications for other indications, primarily rheumatologic diseases, has been reported to be safe in the pregnant population.7 If necessary, glucocorticoids should be used in the lowest effective dose to avoid complications such as premature rupture of membranes; intrauterine growth restriction; and increased risk for pregnancy-induced hypertension, gestational diabetes, osteoporosis, and infection. Little is known about IVIG use in pregnancy. While it has not been associated with increased risk of fetal malformations, it may cross the placenta in a notable amount when administered after 30 weeks’ gestation.7
Unlike most cases of SJS/TEN in pregnancy that largely were associated with HIV infection or drug exposure, primarily antiretrovirals such as nevirapine or antiepileptics, our case is a rare incidence of SJS/TEN in a pregnant patient with no clear medication or infectious trigger. Although the causative drug was unclear, we suspected it was secondary to nonsteroidal anti-inflammatory drug use. The patient had a SCORTEN (SCORe of Toxic Epidermal Necrosis) of 0, which portends a relatively good prognosis with an estimated mortality rate of approximately 3% (Table).8 However, the large BSA involvement of the morbilliform rash warranted aggressive management to prevent the involved skin from fully detaching.

1. Struck MF, Illert T, Liss Y, et al. Toxic epidermal necrolysis in pregnancy: case report and review of the literature. J Burn Care Res. 2010;31:816-821. doi:10.1097/BCR.0b013e3181eed441
2. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245.e4. doi:10.1016/j.jaad.2014.06.026
3. Knight L, Todd G, Muloiwa R, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: maternal and foetal outcomes in twenty-two consecutive pregnant HIV infected women. PLoS One. 2015;10:1-11. doi:10.1371/journal.pone.0135501
4. Velter C, Hotz C, Ingen-Housz-Oro S. Stevens-Johnson syndrome during pregnancy: case report of a newborn treated with the culprit drug. JAMA Dermatol. 2018;154:224-225. doi:10.1001/jamadermatol.2017.4607
5. El Daief SG, Das S, Ekekwe G, et al. A successful pregnancy outcome after Stevens-Johnson syndrome. J Obstet Gynaecol (Lahore). 2014;34:445-446. doi:10.3109/01443615.2014.914897
6. Rodriguez G, Trent JT, Mirzabeigi M. Toxic epidermal necrolysis in a mother and fetus. J Am Acad Dermatol. 2006;55(5 suppl):96-98. doi:10.1016/j.jaad.2005.09.023
7. Bermas BL. Safety of rheumatic disease medication use during pregnancy and lactation. UptoDate website. Updated March 24, 2021. Accessed December 16, 2021. https://www.uptodate.com/contents/safety-of-rheumatic-disease-medication-use-during-pregnancy-and-lactation#H11
8. Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153. doi:10.1046/j.1523-1747.2000.00061.x
1. Struck MF, Illert T, Liss Y, et al. Toxic epidermal necrolysis in pregnancy: case report and review of the literature. J Burn Care Res. 2010;31:816-821. doi:10.1097/BCR.0b013e3181eed441
2. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245.e4. doi:10.1016/j.jaad.2014.06.026
3. Knight L, Todd G, Muloiwa R, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: maternal and foetal outcomes in twenty-two consecutive pregnant HIV infected women. PLoS One. 2015;10:1-11. doi:10.1371/journal.pone.0135501
4. Velter C, Hotz C, Ingen-Housz-Oro S. Stevens-Johnson syndrome during pregnancy: case report of a newborn treated with the culprit drug. JAMA Dermatol. 2018;154:224-225. doi:10.1001/jamadermatol.2017.4607
5. El Daief SG, Das S, Ekekwe G, et al. A successful pregnancy outcome after Stevens-Johnson syndrome. J Obstet Gynaecol (Lahore). 2014;34:445-446. doi:10.3109/01443615.2014.914897
6. Rodriguez G, Trent JT, Mirzabeigi M. Toxic epidermal necrolysis in a mother and fetus. J Am Acad Dermatol. 2006;55(5 suppl):96-98. doi:10.1016/j.jaad.2005.09.023
7. Bermas BL. Safety of rheumatic disease medication use during pregnancy and lactation. UptoDate website. Updated March 24, 2021. Accessed December 16, 2021. https://www.uptodate.com/contents/safety-of-rheumatic-disease-medication-use-during-pregnancy-and-lactation#H11
8. Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153. doi:10.1046/j.1523-1747.2000.00061.x
Practice Points
- Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of severe mucocutaneous reactions commonly presenting as drug eruptions.
- Pregnant patients affected by SJS/TEN represent a special patient population that requires a multidisciplinary approach for management and treatment.
- The rates of adverse outcomes for pregnant patients with SJS/TEN are low with timely diagnosis, removal of the offending agent, and supportive care as mainstays of treatment.
Don’t panic over lamotrigine, but beware of cardiac risks
New York University neurologist Jacqueline A. French, MD, told colleagues at the annual meeting of the American Epilepsy Society. But it’s now crucial to take special precautions in high-risk groups such as older people and heart patients.
“We need to plan more carefully when we use it, which we hate to do, as we know. But we’ve still got to do it,” said Dr. French, former president of the AES. “The risks are very small, but keep in mind that they’re not zero.”
In October 2020, the Food and Drug Administration added a warning to the lamotrigine label that said the drug “could slow ventricular conduction (widen QRS) and induce proarrhythmia, including sudden death, in patients with structural heart disease or myocardial ischemia.”
The FDA recommended avoiding the sodium channel blocker’s use “in patients who have cardiac conduction disorders (e.g., second- or third-degree heart block), ventricular arrhythmias, or cardiac disease or abnormality (e.g., myocardial ischemia, heart failure, structural heart disease, Brugada syndrome, or other sodium channelopathies). Concomitant use of other sodium channel blockers may increase the risk of proarrhythmia.”
Later, in March 2021, the FDA announced that a review of in vitro findings “showed a potential increased risk of heart rhythm problems.”
As Dr. French noted, lamotrigine remains widely prescribed even though there’s “no pharmaceutical company out there pushing [it].” It’s an especially beneficial drug for certain groups such as the elderly and women of child-bearing age, she said.
But older people are also at higher risk of drug-related heart complications because of the fact that many already have cardiac disease, Dr. French said. She highlighted a 2005 trial of lamotrigine that found 48% of 593 patients aged 60 years and older had cardiac disease.
Special precautions
So what should neurologists know about prescribing lamotrigine in light of the new warning? Dr. French recommended guidelines that she cowrote with the AES and International League Against Epilepsy.
- Prescribe as normal in patients under 60 with no cardiac risk factors. In patients older than 60, or younger with risk factors, consider an EKG before prescribing lamotrigine.
- “Nonspecific EKG abnormalities (e.g., nonspecific ST and T wave abnormalities) are not concerning, and should not preclude these individuals from being prescribed lamotrigine.”
- Beware of higher risk and consider consulting a cardiologist before starting treatment in patients with second- or third-degree heart block, Brugada syndrome, arrhythmogenic ventricular cardiomyopathy, left bundle branch block, and right bundle branch block with left anterior or posterior fascicular block.
- “In most cases the initial EKG can be obtained while titrating, mainly when the individual is at the first dose of 25 mg/day because lamotrigine must be titrated slowly, and because cardiac adverse events are dose related.”
- “Clinicians should consider obtaining an EKG and/or cardiology consultation in people on lamotrigine with sudden-onset syncope or presyncope with loss of muscular tone without a clear vasovagal or orthostatic cause.”
Dr. French cautioned colleagues that they shouldn’t assume that lamotrigine stands alone among sodium channel blockers in terms of cardiac risk. As she noted, the FDA is asking manufacturers of other drugs in that class to provide data. “At some point, maybe sometime in the near future, we are going to hear in this particular in vitro sense how the other sodium channel blockers do stack up, compared with lamotrigine. At presence, in the absence of the availability of all of the rest of the data, it would be incorrect to presume that lamotrigine has more cardiac effects than other sodium channel blocking antiseizure medicines or all antiseizure medicines.”
For now, she said, although the guidelines are for lamotrigine, it’s “prudent” to follow them for all sodium channel blockers.
Dr. French reported no disclosures.
New York University neurologist Jacqueline A. French, MD, told colleagues at the annual meeting of the American Epilepsy Society. But it’s now crucial to take special precautions in high-risk groups such as older people and heart patients.
“We need to plan more carefully when we use it, which we hate to do, as we know. But we’ve still got to do it,” said Dr. French, former president of the AES. “The risks are very small, but keep in mind that they’re not zero.”
In October 2020, the Food and Drug Administration added a warning to the lamotrigine label that said the drug “could slow ventricular conduction (widen QRS) and induce proarrhythmia, including sudden death, in patients with structural heart disease or myocardial ischemia.”
The FDA recommended avoiding the sodium channel blocker’s use “in patients who have cardiac conduction disorders (e.g., second- or third-degree heart block), ventricular arrhythmias, or cardiac disease or abnormality (e.g., myocardial ischemia, heart failure, structural heart disease, Brugada syndrome, or other sodium channelopathies). Concomitant use of other sodium channel blockers may increase the risk of proarrhythmia.”
Later, in March 2021, the FDA announced that a review of in vitro findings “showed a potential increased risk of heart rhythm problems.”
As Dr. French noted, lamotrigine remains widely prescribed even though there’s “no pharmaceutical company out there pushing [it].” It’s an especially beneficial drug for certain groups such as the elderly and women of child-bearing age, she said.
But older people are also at higher risk of drug-related heart complications because of the fact that many already have cardiac disease, Dr. French said. She highlighted a 2005 trial of lamotrigine that found 48% of 593 patients aged 60 years and older had cardiac disease.
Special precautions
So what should neurologists know about prescribing lamotrigine in light of the new warning? Dr. French recommended guidelines that she cowrote with the AES and International League Against Epilepsy.
- Prescribe as normal in patients under 60 with no cardiac risk factors. In patients older than 60, or younger with risk factors, consider an EKG before prescribing lamotrigine.
- “Nonspecific EKG abnormalities (e.g., nonspecific ST and T wave abnormalities) are not concerning, and should not preclude these individuals from being prescribed lamotrigine.”
- Beware of higher risk and consider consulting a cardiologist before starting treatment in patients with second- or third-degree heart block, Brugada syndrome, arrhythmogenic ventricular cardiomyopathy, left bundle branch block, and right bundle branch block with left anterior or posterior fascicular block.
- “In most cases the initial EKG can be obtained while titrating, mainly when the individual is at the first dose of 25 mg/day because lamotrigine must be titrated slowly, and because cardiac adverse events are dose related.”
- “Clinicians should consider obtaining an EKG and/or cardiology consultation in people on lamotrigine with sudden-onset syncope or presyncope with loss of muscular tone without a clear vasovagal or orthostatic cause.”
Dr. French cautioned colleagues that they shouldn’t assume that lamotrigine stands alone among sodium channel blockers in terms of cardiac risk. As she noted, the FDA is asking manufacturers of other drugs in that class to provide data. “At some point, maybe sometime in the near future, we are going to hear in this particular in vitro sense how the other sodium channel blockers do stack up, compared with lamotrigine. At presence, in the absence of the availability of all of the rest of the data, it would be incorrect to presume that lamotrigine has more cardiac effects than other sodium channel blocking antiseizure medicines or all antiseizure medicines.”
For now, she said, although the guidelines are for lamotrigine, it’s “prudent” to follow them for all sodium channel blockers.
Dr. French reported no disclosures.
New York University neurologist Jacqueline A. French, MD, told colleagues at the annual meeting of the American Epilepsy Society. But it’s now crucial to take special precautions in high-risk groups such as older people and heart patients.
“We need to plan more carefully when we use it, which we hate to do, as we know. But we’ve still got to do it,” said Dr. French, former president of the AES. “The risks are very small, but keep in mind that they’re not zero.”
In October 2020, the Food and Drug Administration added a warning to the lamotrigine label that said the drug “could slow ventricular conduction (widen QRS) and induce proarrhythmia, including sudden death, in patients with structural heart disease or myocardial ischemia.”
The FDA recommended avoiding the sodium channel blocker’s use “in patients who have cardiac conduction disorders (e.g., second- or third-degree heart block), ventricular arrhythmias, or cardiac disease or abnormality (e.g., myocardial ischemia, heart failure, structural heart disease, Brugada syndrome, or other sodium channelopathies). Concomitant use of other sodium channel blockers may increase the risk of proarrhythmia.”
Later, in March 2021, the FDA announced that a review of in vitro findings “showed a potential increased risk of heart rhythm problems.”
As Dr. French noted, lamotrigine remains widely prescribed even though there’s “no pharmaceutical company out there pushing [it].” It’s an especially beneficial drug for certain groups such as the elderly and women of child-bearing age, she said.
But older people are also at higher risk of drug-related heart complications because of the fact that many already have cardiac disease, Dr. French said. She highlighted a 2005 trial of lamotrigine that found 48% of 593 patients aged 60 years and older had cardiac disease.
Special precautions
So what should neurologists know about prescribing lamotrigine in light of the new warning? Dr. French recommended guidelines that she cowrote with the AES and International League Against Epilepsy.
- Prescribe as normal in patients under 60 with no cardiac risk factors. In patients older than 60, or younger with risk factors, consider an EKG before prescribing lamotrigine.
- “Nonspecific EKG abnormalities (e.g., nonspecific ST and T wave abnormalities) are not concerning, and should not preclude these individuals from being prescribed lamotrigine.”
- Beware of higher risk and consider consulting a cardiologist before starting treatment in patients with second- or third-degree heart block, Brugada syndrome, arrhythmogenic ventricular cardiomyopathy, left bundle branch block, and right bundle branch block with left anterior or posterior fascicular block.
- “In most cases the initial EKG can be obtained while titrating, mainly when the individual is at the first dose of 25 mg/day because lamotrigine must be titrated slowly, and because cardiac adverse events are dose related.”
- “Clinicians should consider obtaining an EKG and/or cardiology consultation in people on lamotrigine with sudden-onset syncope or presyncope with loss of muscular tone without a clear vasovagal or orthostatic cause.”
Dr. French cautioned colleagues that they shouldn’t assume that lamotrigine stands alone among sodium channel blockers in terms of cardiac risk. As she noted, the FDA is asking manufacturers of other drugs in that class to provide data. “At some point, maybe sometime in the near future, we are going to hear in this particular in vitro sense how the other sodium channel blockers do stack up, compared with lamotrigine. At presence, in the absence of the availability of all of the rest of the data, it would be incorrect to presume that lamotrigine has more cardiac effects than other sodium channel blocking antiseizure medicines or all antiseizure medicines.”
For now, she said, although the guidelines are for lamotrigine, it’s “prudent” to follow them for all sodium channel blockers.
Dr. French reported no disclosures.
FROM AES 2021
Genetic tests prompt therapy adjustments in children with epilepsy
Physicians at a Boston hospital adjusted medical management for nearly three-quarters of patients with infantile- or childhood-onset epilepsy who were diagnosed with genetic epilepsy, researchers reported at the annual meeting of the American Epilepsy Society. The findings provide new insight into the usefulness of genetic tests in children with epilepsy of unknown cause.
“. Genetic testing should be included as part of the standard evaluation of individuals with unexplained pediatric epilepsy as a means of achieving diagnostic precision and informing clinical management,” study lead author Isabel Haviland, MD, a neurologist with Boston Children’s Hospital/Harvard Medical School, said in an interview.
According to Dr. Haviland, the causes of epilepsy are unexplained in an estimated two-thirds of pediatric epilepsy cases. “Increasingly, when genetic testing is available, previously unexplained cases of pediatric epilepsy are being found to have single-gene etiologies,” she said. “Though a genetic diagnosis in this population has implications for medical care, the direct impact on medical management in a clinical setting has not been measured. We aimed to describe the impact of genetic diagnosis on medical management in a cohort of individuals with pediatric epilepsy.”
Researchers tracked 602 patients at Boston Children’s Hospital who received next-generation gene sequencing testing from 2012 to 2019. Of those, Dr. Haviland said, 152 (25%) had a positive result that indicated genetic epilepsy (46% female, median age of onset = 6 months [2-15 months]). These patients were included in the study.
“We documented an impact on medical management in nearly three-fourths of participants (72%),” Dr. Haviland said. “A genetic diagnosis affected at least one of four categories of medical management, including care coordination (48%), treatment (45%), counseling about a change in prognosis (28%), and change in diagnosis for a few individuals who had a prior established diagnosis (1%).”
As examples, she mentioned three cases:
- Testing revealed that a subject has a disease-causing genetic variant in a gene called PRRT2. “This gene is involved in the release of neurotransmitters in the brain,” Dr. Haviland said. “Thanks to his diagnosis, he was treated with the antiseizure medication oxcarbazepine, which is often effective for epilepsy caused by variants in this gene. He had excellent response to the medication and later became seizure free.”
- A subject had a variation in the SCN1A gene that causes types of epilepsy. “At the time of his diagnosis, there was a trial for a medication called fenfluramine being offered for individuals with SCN1A variants, and his family elected to participate,” she said. “This medication was later approved by the [Food and Drug Administration] for SCN1A-related epilepsy.”
- Testing identified disease-causing variant in the GRIN2A gene in another subject. “This gene is involved in brain cell communication,” Dr. Haviland said. “This individual was treated with memantine, which acts on the specific biological pathway affected by the gene. This treatment would not have been considered without the genetic diagnosis as it is currently only approved for Alzheimer’s disease.”
In addition, Dr. Haviland said, researchers found that “there was impact on medical management both in those with earlier age of epilepsy onset (under 2 years) and those with later age of onset, as well as both in those with developmental disorders (such as autism spectrum disorder, intellectual disability and developmental delay) and those with normal development.
As for the cost of genetic tests, Dr. Haviland pointed to a 2019 study that she said estimated epilepsy panel testing runs from $1,500 to $7,500, and the whole exome sequencing from $4,500 to $7,000. “Insurers sometimes cover testing, but not always,” she said. “In some cases, insurance will only cover testing if it is documented that results will directly alter medical management, which highlights the importance of our findings.”
No study funding was reported. Dr. Haviland and several other authors report no disclosures. One author reports consulting fees from Takeda, Zogenix, Marinus, and FOXG1 Research Foundation. Another author reports research support from the International Foundation for CDKL5 Research.
Physicians at a Boston hospital adjusted medical management for nearly three-quarters of patients with infantile- or childhood-onset epilepsy who were diagnosed with genetic epilepsy, researchers reported at the annual meeting of the American Epilepsy Society. The findings provide new insight into the usefulness of genetic tests in children with epilepsy of unknown cause.
“. Genetic testing should be included as part of the standard evaluation of individuals with unexplained pediatric epilepsy as a means of achieving diagnostic precision and informing clinical management,” study lead author Isabel Haviland, MD, a neurologist with Boston Children’s Hospital/Harvard Medical School, said in an interview.
According to Dr. Haviland, the causes of epilepsy are unexplained in an estimated two-thirds of pediatric epilepsy cases. “Increasingly, when genetic testing is available, previously unexplained cases of pediatric epilepsy are being found to have single-gene etiologies,” she said. “Though a genetic diagnosis in this population has implications for medical care, the direct impact on medical management in a clinical setting has not been measured. We aimed to describe the impact of genetic diagnosis on medical management in a cohort of individuals with pediatric epilepsy.”
Researchers tracked 602 patients at Boston Children’s Hospital who received next-generation gene sequencing testing from 2012 to 2019. Of those, Dr. Haviland said, 152 (25%) had a positive result that indicated genetic epilepsy (46% female, median age of onset = 6 months [2-15 months]). These patients were included in the study.
“We documented an impact on medical management in nearly three-fourths of participants (72%),” Dr. Haviland said. “A genetic diagnosis affected at least one of four categories of medical management, including care coordination (48%), treatment (45%), counseling about a change in prognosis (28%), and change in diagnosis for a few individuals who had a prior established diagnosis (1%).”
As examples, she mentioned three cases:
- Testing revealed that a subject has a disease-causing genetic variant in a gene called PRRT2. “This gene is involved in the release of neurotransmitters in the brain,” Dr. Haviland said. “Thanks to his diagnosis, he was treated with the antiseizure medication oxcarbazepine, which is often effective for epilepsy caused by variants in this gene. He had excellent response to the medication and later became seizure free.”
- A subject had a variation in the SCN1A gene that causes types of epilepsy. “At the time of his diagnosis, there was a trial for a medication called fenfluramine being offered for individuals with SCN1A variants, and his family elected to participate,” she said. “This medication was later approved by the [Food and Drug Administration] for SCN1A-related epilepsy.”
- Testing identified disease-causing variant in the GRIN2A gene in another subject. “This gene is involved in brain cell communication,” Dr. Haviland said. “This individual was treated with memantine, which acts on the specific biological pathway affected by the gene. This treatment would not have been considered without the genetic diagnosis as it is currently only approved for Alzheimer’s disease.”
In addition, Dr. Haviland said, researchers found that “there was impact on medical management both in those with earlier age of epilepsy onset (under 2 years) and those with later age of onset, as well as both in those with developmental disorders (such as autism spectrum disorder, intellectual disability and developmental delay) and those with normal development.
As for the cost of genetic tests, Dr. Haviland pointed to a 2019 study that she said estimated epilepsy panel testing runs from $1,500 to $7,500, and the whole exome sequencing from $4,500 to $7,000. “Insurers sometimes cover testing, but not always,” she said. “In some cases, insurance will only cover testing if it is documented that results will directly alter medical management, which highlights the importance of our findings.”
No study funding was reported. Dr. Haviland and several other authors report no disclosures. One author reports consulting fees from Takeda, Zogenix, Marinus, and FOXG1 Research Foundation. Another author reports research support from the International Foundation for CDKL5 Research.
Physicians at a Boston hospital adjusted medical management for nearly three-quarters of patients with infantile- or childhood-onset epilepsy who were diagnosed with genetic epilepsy, researchers reported at the annual meeting of the American Epilepsy Society. The findings provide new insight into the usefulness of genetic tests in children with epilepsy of unknown cause.
“. Genetic testing should be included as part of the standard evaluation of individuals with unexplained pediatric epilepsy as a means of achieving diagnostic precision and informing clinical management,” study lead author Isabel Haviland, MD, a neurologist with Boston Children’s Hospital/Harvard Medical School, said in an interview.
According to Dr. Haviland, the causes of epilepsy are unexplained in an estimated two-thirds of pediatric epilepsy cases. “Increasingly, when genetic testing is available, previously unexplained cases of pediatric epilepsy are being found to have single-gene etiologies,” she said. “Though a genetic diagnosis in this population has implications for medical care, the direct impact on medical management in a clinical setting has not been measured. We aimed to describe the impact of genetic diagnosis on medical management in a cohort of individuals with pediatric epilepsy.”
Researchers tracked 602 patients at Boston Children’s Hospital who received next-generation gene sequencing testing from 2012 to 2019. Of those, Dr. Haviland said, 152 (25%) had a positive result that indicated genetic epilepsy (46% female, median age of onset = 6 months [2-15 months]). These patients were included in the study.
“We documented an impact on medical management in nearly three-fourths of participants (72%),” Dr. Haviland said. “A genetic diagnosis affected at least one of four categories of medical management, including care coordination (48%), treatment (45%), counseling about a change in prognosis (28%), and change in diagnosis for a few individuals who had a prior established diagnosis (1%).”
As examples, she mentioned three cases:
- Testing revealed that a subject has a disease-causing genetic variant in a gene called PRRT2. “This gene is involved in the release of neurotransmitters in the brain,” Dr. Haviland said. “Thanks to his diagnosis, he was treated with the antiseizure medication oxcarbazepine, which is often effective for epilepsy caused by variants in this gene. He had excellent response to the medication and later became seizure free.”
- A subject had a variation in the SCN1A gene that causes types of epilepsy. “At the time of his diagnosis, there was a trial for a medication called fenfluramine being offered for individuals with SCN1A variants, and his family elected to participate,” she said. “This medication was later approved by the [Food and Drug Administration] for SCN1A-related epilepsy.”
- Testing identified disease-causing variant in the GRIN2A gene in another subject. “This gene is involved in brain cell communication,” Dr. Haviland said. “This individual was treated with memantine, which acts on the specific biological pathway affected by the gene. This treatment would not have been considered without the genetic diagnosis as it is currently only approved for Alzheimer’s disease.”
In addition, Dr. Haviland said, researchers found that “there was impact on medical management both in those with earlier age of epilepsy onset (under 2 years) and those with later age of onset, as well as both in those with developmental disorders (such as autism spectrum disorder, intellectual disability and developmental delay) and those with normal development.
As for the cost of genetic tests, Dr. Haviland pointed to a 2019 study that she said estimated epilepsy panel testing runs from $1,500 to $7,500, and the whole exome sequencing from $4,500 to $7,000. “Insurers sometimes cover testing, but not always,” she said. “In some cases, insurance will only cover testing if it is documented that results will directly alter medical management, which highlights the importance of our findings.”
No study funding was reported. Dr. Haviland and several other authors report no disclosures. One author reports consulting fees from Takeda, Zogenix, Marinus, and FOXG1 Research Foundation. Another author reports research support from the International Foundation for CDKL5 Research.
FROM AES 2021
COVID-19 hospital data: New-onset seizures more common than breakthrough seizures
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
FROM AES 2021
WPATH draft on gender dysphoria ‘skewed and misses urgent issues’
New draft guidance from the World Professional Association for Transgender Health (WPATH) is raising serious concerns among professionals caring for people with gender dysphoria, prompting claims that WPATH is an organization “captured by activists.”
Experts in adolescent and child psychology, as well as pediatric health, have expressed dismay that the WPATH Standards of Care (SOC) 8 appear to miss some of the most urgent issues in the field of transgender medicine and are considered to express a radical and unreserved leaning towards “gender-affirmation.”
The WPATH SOC 8 document is available for view and comment until Dec. 16 until 11.59 PM EST, after which time revisions will be made and the final version published.
Despite repeated attempts by this news organization to seek clarification on certain aspects of the guidance from members of the WPATH SOC 8 committee, requests were declined “until the guidance is finalized.”
According to the WPATH website, the SOC 8 aims to provide “clinical guidance for health professionals to assist transgender and gender diverse people with safe and effective pathways” to manage their gender dysphoria and potentially transition.
Such pathways may relate to primary care, gynecologic and urologic care, reproductive options, voice and communication therapy, mental health services, and hormonal or surgical treatments, among others.
WPATH adds that it was felt necessary to revise the existing SOC 7 (published in 2012) because of recent “globally unprecedented increase and visibility of transgender and gender-diverse people seeking support and gender-affirming medical treatment.”
Gender-affirming medical treatment means different things at different ages. In the case of kids with gender dysphoria who have not yet entered puberty associated with their birth sex, this might include prescribing so-called “puberty blockers” to delay natural puberty – gonadotrophin-releasing hormone analogs that are licensed for use in precocious puberty in children. Such agents have not been licensed for use in children with gender dysphoria, however, so any use for this purpose is off-label.
Following puberty blockade – or in cases where adolescents have already undergone natural puberty – the next step is to begin cross-sex hormones. So, for a female patient who wants to transition to male (FTM), that would be lifelong testosterone, and for a male who wants to be female (MTF), it involves lifelong estrogen. Again, use of such hormones in transgender individuals is entirely off-label.
Just last month, two of America’s leading experts on transgender medicine, both psychologists – including one who is transgender – told this news organization they were concerned that the quality of the evaluations of youth with gender dysphoria are being stifled by activists who are worried that open discussions will further stigmatize trans individuals.
They subsequently wrote an op-ed on the topic entitled, “The mental health establishment is failing trans kids,” which was finally published in the Washington Post on Nov. 24, after numerous other mainstream U.S. media outlets had rejected it.
New SOC 8 ‘is not evidence based,’ should not be new ‘gold standard’
One expert says the draft SOC 8 lacks balance and does not address certain issues, while paying undue attention to others that detract from real questions facing the field of transgender medicine, both in the United States and around the world.
Julia Mason, MD, is a pediatrician based in Gresham, Oregon, with a special interest in children and adolescents experiencing gender dysphoria. “The SOC 8 shows us that WPATH remains captured by activists,” she asserts.
Dr. Mason questions the integrity of WPATH based on what she has read in the draft SOC 8.
“We need a serious organization to take a sober look at the evidence, and that is why we have established the Society for Evidence-Based Gender Medicine [SEGM],” she noted. “This is what we do – we are looking at all of the evidence.”
Dr. Mason is a clinical advisor to SEGM, an organization set-up to evaluate current interventions and evidence on gender dysphoria.
The pediatrician has particular concerns regarding the child and adolescent chapters in the draft SOC 8. The adolescent chapter states: “Guidelines are meant to provide a gold standard based on the available evidence at this moment of time.”
Dr. Mason disputes this assertion. “This document should not be the new gold standard going forward, primarily because it is not evidence based.”
In an interview, Dr. Mason explained that WPATH say they used the “Delphi consensus process” to determine their recommendations, but “this process is designed for use with a panel of experts when evidence is lacking. I would say they didn’t have a panel of experts. They largely had a panel of activists, with a few experts.”
There is no mention, for example, of England’s National Institute for Health and Care Excellence (NICE) evidence reviews on puberty blockers and cross-sex hormones from earlier this year. These reviews determined that no studies have compared cross-sex hormones or puberty blockers with a control group and all follow-up periods for cross-sex hormones were relatively short.
This disappoints Dr. Mason: “These are significant; they are important documents.”
And much of the evidence quoted comes from the well-known and often-quoted “Dutch-protocol” study of 2011, in which the children studied were much younger at the time of their gender dysphoria, compared with the many adolescents who make up the current surge in presentation at gender clinics worldwide, she adds.
Rapid-onset GD: adolescents presenting late with little history
Dr. Mason also stresses that the SOC 8 does not address the most urgent issues in transgender medicine today, mainly because it does not address rapid-onset gender dysphoria (ROGD): “This is the dilemma of the 21st century; it’s new.”
ROGD – a term first coined in 2018 by researcher Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research (ICGDR) – refers to the phenomena of adolescents expressing a desire to transition from their birth sex after little or no apparent previous indication.
However, the SOC 8 does make reference to aspects of adolescent development that might impact their decision-making processes around gender identity during teen years. The chapter on adolescents reads: “... adolescence is also often associated with increased risk-taking behaviors. Along with these notable changes ... individuation from parents ... [there is] often a heightened focus on peer relationships, which can be both positive and detrimental.”
The guidance goes on to point out that “it is critical to understand how all of these aspects of development may impact the decision-making for a given young person within their specific cultural context.”
Desistance and detransitioning not adequately addressed
Dr. Mason also says there is little mention “about detransitioning in this SOC [8], and ‘gender dysphoria’ and ‘trans’ are terms that are not defined.”
Likewise, there is no mention of desistance, she highlights, which is when individuals naturally resolve their dysphoria around their birth sex as they grow older.
The most recent published data seen by this news organization relates to a study from March 2021 that showed nearly 88% of boys who struggled with gender identity in childhood (approximate mean age 8 years and follow-up at approximate mean age 20 years) desisted. It reads: “Of the 139 participants, 17 (12.2%) were classified as ‘persisters’ and the remaining 122 (87.8%) were classified as desisters.”
“Most children with gender dysphoria will desist and lose their concept of themselves as being the opposite gender,” Dr. Mason explains. “This is the safest path for a child – desistance.”
“Transition can turn a healthy young person into a lifelong medical patient and has significant health risks,” she emphasizes, stressing that transition has not been shown to decrease the probability of suicide, or attempts at suicide, despite myriad claims saying otherwise.
“Before we were routinely transitioning kids at school, the vast majority of children grew out of their gender dysphoria. This history is not recognized at all in these SOC [8],” she maintains.
Ken Zucker, PhD, CPsych, an author of the study of desistance in boys, says the terms desistence and persistence of gender dysphoria have caused some consternation in certain circles.
An editor of the Archives of Sexual Behavior and professor in the department of psychiatry, University of Toronto, Dr. Zucker has published widely on the topic.
He told this news organization: “The terms persistence and desistance have become verboten among the WPATH cognoscenti. Perhaps the contributors to SOC 8 have come up with alternative descriptors.”
“The term ‘desistance’ is particularly annoying to some of the gender-affirming clinicians, because they don’t believe that desistance is bona fide,” Dr. Zucker points out.
“The desistance resisters are like anti-vaxxers – nothing one can provide as evidence for the efficacy of vaccines is sufficient. There will always be a new objection.”
Other mental health issues, in particular ADHD and autism
It is also widely acknowledged that there is a higher rate of neurodevelopmental and psychiatric diagnoses in individuals with gender dysphoria. For example, one 2020 study found that transgender people were three to six times as likely to be autistic as cisgender people (those whose gender is aligned with their birth sex).
Statement one in the chapter on adolescents in draft WPATH SOC 8 does give a nod to this, pointing out that health professionals working with gender diverse adolescents “should receive training and develop expertise in autism spectrum disorders and other neurodiversity conditions.”
It also notes that in some cases “a more extended assessment process may be useful, such as for youth with more complex presentations (e.g., complicated mental health histories, co-occurring autism spectrum characteristics in particular) and an absence of experienced childhood gender incongruence.”
However, Dr. Mason stresses that underlying mental health issues are central to addressing how to manage a significant number of these patients.
“If a young person has ADHD or autism, they are not ready to make decisions about the rest of their life at age 18. Even a neurotypical young person is still developing their frontal cortex in their early 20s, and it takes longer for those with ADHD or on the autism spectrum.”
She firmly believes that the guidance does not give sufficient consideration to comorbidities in people over the age of 18.
According to their [SOC 8] guidelines, “once someone is 18 they are ready for anything,” says Dr. Mason.
Offering some explanation for the increased prevalence of ADHD and autism in those with gender dysphoria, Dr. Mason notes that children can have “hyperfocus” and those with autism will fixate on a particular area of interest. “If a child is unhappy in their life, and this can be more likely if someone is neuro-atypical, then it is likely that the individual might go online and find this one solution [for example, a transgender identity] that seems to fix everything.”
Perceptions of femininity and masculinity can also be extra challenging for a child with autism, Dr. Mason says. “It is relatively easy for an autistic girl to feel like she should be a boy because the rules of femininity are composed of nonverbal, subtle behaviors that can be difficult to pick up on,” she points out. “An autistic child who isn’t particularly good at nonverbal communication might not pick up on these and thus feel they are not very ‘female.’”
“There’s a whole lot of grass-is-greener-type thinking. Girls think boys have an easier life, and boys think girls have an easier life. I know some detransitioners who have spoken eloquently about realizing their mistake on this,” she adds.
Other parts of the SOC 8 that Dr. Mason disagrees with include the recommendation in the adolescent chapter that 14-year-olds are mature enough to start cross-sex hormones, that is, giving testosterone to a female who wants to transition to male or estrogen to a male who wishes to transition to female. “I think that’s far too young,” she asserts.
And she points out that the document states 17-year-olds are ready for genital reassignment surgery. Again, she believes this is far too young.
“Also, the SOC 8 document does not clarify who is appropriate for surgery. Whenever surgery is discussed, it becomes very vague,” she said.
A version of this article first appeared on Medscape.com.
New draft guidance from the World Professional Association for Transgender Health (WPATH) is raising serious concerns among professionals caring for people with gender dysphoria, prompting claims that WPATH is an organization “captured by activists.”
Experts in adolescent and child psychology, as well as pediatric health, have expressed dismay that the WPATH Standards of Care (SOC) 8 appear to miss some of the most urgent issues in the field of transgender medicine and are considered to express a radical and unreserved leaning towards “gender-affirmation.”
The WPATH SOC 8 document is available for view and comment until Dec. 16 until 11.59 PM EST, after which time revisions will be made and the final version published.
Despite repeated attempts by this news organization to seek clarification on certain aspects of the guidance from members of the WPATH SOC 8 committee, requests were declined “until the guidance is finalized.”
According to the WPATH website, the SOC 8 aims to provide “clinical guidance for health professionals to assist transgender and gender diverse people with safe and effective pathways” to manage their gender dysphoria and potentially transition.
Such pathways may relate to primary care, gynecologic and urologic care, reproductive options, voice and communication therapy, mental health services, and hormonal or surgical treatments, among others.
WPATH adds that it was felt necessary to revise the existing SOC 7 (published in 2012) because of recent “globally unprecedented increase and visibility of transgender and gender-diverse people seeking support and gender-affirming medical treatment.”
Gender-affirming medical treatment means different things at different ages. In the case of kids with gender dysphoria who have not yet entered puberty associated with their birth sex, this might include prescribing so-called “puberty blockers” to delay natural puberty – gonadotrophin-releasing hormone analogs that are licensed for use in precocious puberty in children. Such agents have not been licensed for use in children with gender dysphoria, however, so any use for this purpose is off-label.
Following puberty blockade – or in cases where adolescents have already undergone natural puberty – the next step is to begin cross-sex hormones. So, for a female patient who wants to transition to male (FTM), that would be lifelong testosterone, and for a male who wants to be female (MTF), it involves lifelong estrogen. Again, use of such hormones in transgender individuals is entirely off-label.
Just last month, two of America’s leading experts on transgender medicine, both psychologists – including one who is transgender – told this news organization they were concerned that the quality of the evaluations of youth with gender dysphoria are being stifled by activists who are worried that open discussions will further stigmatize trans individuals.
They subsequently wrote an op-ed on the topic entitled, “The mental health establishment is failing trans kids,” which was finally published in the Washington Post on Nov. 24, after numerous other mainstream U.S. media outlets had rejected it.
New SOC 8 ‘is not evidence based,’ should not be new ‘gold standard’
One expert says the draft SOC 8 lacks balance and does not address certain issues, while paying undue attention to others that detract from real questions facing the field of transgender medicine, both in the United States and around the world.
Julia Mason, MD, is a pediatrician based in Gresham, Oregon, with a special interest in children and adolescents experiencing gender dysphoria. “The SOC 8 shows us that WPATH remains captured by activists,” she asserts.
Dr. Mason questions the integrity of WPATH based on what she has read in the draft SOC 8.
“We need a serious organization to take a sober look at the evidence, and that is why we have established the Society for Evidence-Based Gender Medicine [SEGM],” she noted. “This is what we do – we are looking at all of the evidence.”
Dr. Mason is a clinical advisor to SEGM, an organization set-up to evaluate current interventions and evidence on gender dysphoria.
The pediatrician has particular concerns regarding the child and adolescent chapters in the draft SOC 8. The adolescent chapter states: “Guidelines are meant to provide a gold standard based on the available evidence at this moment of time.”
Dr. Mason disputes this assertion. “This document should not be the new gold standard going forward, primarily because it is not evidence based.”
In an interview, Dr. Mason explained that WPATH say they used the “Delphi consensus process” to determine their recommendations, but “this process is designed for use with a panel of experts when evidence is lacking. I would say they didn’t have a panel of experts. They largely had a panel of activists, with a few experts.”
There is no mention, for example, of England’s National Institute for Health and Care Excellence (NICE) evidence reviews on puberty blockers and cross-sex hormones from earlier this year. These reviews determined that no studies have compared cross-sex hormones or puberty blockers with a control group and all follow-up periods for cross-sex hormones were relatively short.
This disappoints Dr. Mason: “These are significant; they are important documents.”
And much of the evidence quoted comes from the well-known and often-quoted “Dutch-protocol” study of 2011, in which the children studied were much younger at the time of their gender dysphoria, compared with the many adolescents who make up the current surge in presentation at gender clinics worldwide, she adds.
Rapid-onset GD: adolescents presenting late with little history
Dr. Mason also stresses that the SOC 8 does not address the most urgent issues in transgender medicine today, mainly because it does not address rapid-onset gender dysphoria (ROGD): “This is the dilemma of the 21st century; it’s new.”
ROGD – a term first coined in 2018 by researcher Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research (ICGDR) – refers to the phenomena of adolescents expressing a desire to transition from their birth sex after little or no apparent previous indication.
However, the SOC 8 does make reference to aspects of adolescent development that might impact their decision-making processes around gender identity during teen years. The chapter on adolescents reads: “... adolescence is also often associated with increased risk-taking behaviors. Along with these notable changes ... individuation from parents ... [there is] often a heightened focus on peer relationships, which can be both positive and detrimental.”
The guidance goes on to point out that “it is critical to understand how all of these aspects of development may impact the decision-making for a given young person within their specific cultural context.”
Desistance and detransitioning not adequately addressed
Dr. Mason also says there is little mention “about detransitioning in this SOC [8], and ‘gender dysphoria’ and ‘trans’ are terms that are not defined.”
Likewise, there is no mention of desistance, she highlights, which is when individuals naturally resolve their dysphoria around their birth sex as they grow older.
The most recent published data seen by this news organization relates to a study from March 2021 that showed nearly 88% of boys who struggled with gender identity in childhood (approximate mean age 8 years and follow-up at approximate mean age 20 years) desisted. It reads: “Of the 139 participants, 17 (12.2%) were classified as ‘persisters’ and the remaining 122 (87.8%) were classified as desisters.”
“Most children with gender dysphoria will desist and lose their concept of themselves as being the opposite gender,” Dr. Mason explains. “This is the safest path for a child – desistance.”
“Transition can turn a healthy young person into a lifelong medical patient and has significant health risks,” she emphasizes, stressing that transition has not been shown to decrease the probability of suicide, or attempts at suicide, despite myriad claims saying otherwise.
“Before we were routinely transitioning kids at school, the vast majority of children grew out of their gender dysphoria. This history is not recognized at all in these SOC [8],” she maintains.
Ken Zucker, PhD, CPsych, an author of the study of desistance in boys, says the terms desistence and persistence of gender dysphoria have caused some consternation in certain circles.
An editor of the Archives of Sexual Behavior and professor in the department of psychiatry, University of Toronto, Dr. Zucker has published widely on the topic.
He told this news organization: “The terms persistence and desistance have become verboten among the WPATH cognoscenti. Perhaps the contributors to SOC 8 have come up with alternative descriptors.”
“The term ‘desistance’ is particularly annoying to some of the gender-affirming clinicians, because they don’t believe that desistance is bona fide,” Dr. Zucker points out.
“The desistance resisters are like anti-vaxxers – nothing one can provide as evidence for the efficacy of vaccines is sufficient. There will always be a new objection.”
Other mental health issues, in particular ADHD and autism
It is also widely acknowledged that there is a higher rate of neurodevelopmental and psychiatric diagnoses in individuals with gender dysphoria. For example, one 2020 study found that transgender people were three to six times as likely to be autistic as cisgender people (those whose gender is aligned with their birth sex).
Statement one in the chapter on adolescents in draft WPATH SOC 8 does give a nod to this, pointing out that health professionals working with gender diverse adolescents “should receive training and develop expertise in autism spectrum disorders and other neurodiversity conditions.”
It also notes that in some cases “a more extended assessment process may be useful, such as for youth with more complex presentations (e.g., complicated mental health histories, co-occurring autism spectrum characteristics in particular) and an absence of experienced childhood gender incongruence.”
However, Dr. Mason stresses that underlying mental health issues are central to addressing how to manage a significant number of these patients.
“If a young person has ADHD or autism, they are not ready to make decisions about the rest of their life at age 18. Even a neurotypical young person is still developing their frontal cortex in their early 20s, and it takes longer for those with ADHD or on the autism spectrum.”
She firmly believes that the guidance does not give sufficient consideration to comorbidities in people over the age of 18.
According to their [SOC 8] guidelines, “once someone is 18 they are ready for anything,” says Dr. Mason.
Offering some explanation for the increased prevalence of ADHD and autism in those with gender dysphoria, Dr. Mason notes that children can have “hyperfocus” and those with autism will fixate on a particular area of interest. “If a child is unhappy in their life, and this can be more likely if someone is neuro-atypical, then it is likely that the individual might go online and find this one solution [for example, a transgender identity] that seems to fix everything.”
Perceptions of femininity and masculinity can also be extra challenging for a child with autism, Dr. Mason says. “It is relatively easy for an autistic girl to feel like she should be a boy because the rules of femininity are composed of nonverbal, subtle behaviors that can be difficult to pick up on,” she points out. “An autistic child who isn’t particularly good at nonverbal communication might not pick up on these and thus feel they are not very ‘female.’”
“There’s a whole lot of grass-is-greener-type thinking. Girls think boys have an easier life, and boys think girls have an easier life. I know some detransitioners who have spoken eloquently about realizing their mistake on this,” she adds.
Other parts of the SOC 8 that Dr. Mason disagrees with include the recommendation in the adolescent chapter that 14-year-olds are mature enough to start cross-sex hormones, that is, giving testosterone to a female who wants to transition to male or estrogen to a male who wishes to transition to female. “I think that’s far too young,” she asserts.
And she points out that the document states 17-year-olds are ready for genital reassignment surgery. Again, she believes this is far too young.
“Also, the SOC 8 document does not clarify who is appropriate for surgery. Whenever surgery is discussed, it becomes very vague,” she said.
A version of this article first appeared on Medscape.com.
New draft guidance from the World Professional Association for Transgender Health (WPATH) is raising serious concerns among professionals caring for people with gender dysphoria, prompting claims that WPATH is an organization “captured by activists.”
Experts in adolescent and child psychology, as well as pediatric health, have expressed dismay that the WPATH Standards of Care (SOC) 8 appear to miss some of the most urgent issues in the field of transgender medicine and are considered to express a radical and unreserved leaning towards “gender-affirmation.”
The WPATH SOC 8 document is available for view and comment until Dec. 16 until 11.59 PM EST, after which time revisions will be made and the final version published.
Despite repeated attempts by this news organization to seek clarification on certain aspects of the guidance from members of the WPATH SOC 8 committee, requests were declined “until the guidance is finalized.”
According to the WPATH website, the SOC 8 aims to provide “clinical guidance for health professionals to assist transgender and gender diverse people with safe and effective pathways” to manage their gender dysphoria and potentially transition.
Such pathways may relate to primary care, gynecologic and urologic care, reproductive options, voice and communication therapy, mental health services, and hormonal or surgical treatments, among others.
WPATH adds that it was felt necessary to revise the existing SOC 7 (published in 2012) because of recent “globally unprecedented increase and visibility of transgender and gender-diverse people seeking support and gender-affirming medical treatment.”
Gender-affirming medical treatment means different things at different ages. In the case of kids with gender dysphoria who have not yet entered puberty associated with their birth sex, this might include prescribing so-called “puberty blockers” to delay natural puberty – gonadotrophin-releasing hormone analogs that are licensed for use in precocious puberty in children. Such agents have not been licensed for use in children with gender dysphoria, however, so any use for this purpose is off-label.
Following puberty blockade – or in cases where adolescents have already undergone natural puberty – the next step is to begin cross-sex hormones. So, for a female patient who wants to transition to male (FTM), that would be lifelong testosterone, and for a male who wants to be female (MTF), it involves lifelong estrogen. Again, use of such hormones in transgender individuals is entirely off-label.
Just last month, two of America’s leading experts on transgender medicine, both psychologists – including one who is transgender – told this news organization they were concerned that the quality of the evaluations of youth with gender dysphoria are being stifled by activists who are worried that open discussions will further stigmatize trans individuals.
They subsequently wrote an op-ed on the topic entitled, “The mental health establishment is failing trans kids,” which was finally published in the Washington Post on Nov. 24, after numerous other mainstream U.S. media outlets had rejected it.
New SOC 8 ‘is not evidence based,’ should not be new ‘gold standard’
One expert says the draft SOC 8 lacks balance and does not address certain issues, while paying undue attention to others that detract from real questions facing the field of transgender medicine, both in the United States and around the world.
Julia Mason, MD, is a pediatrician based in Gresham, Oregon, with a special interest in children and adolescents experiencing gender dysphoria. “The SOC 8 shows us that WPATH remains captured by activists,” she asserts.
Dr. Mason questions the integrity of WPATH based on what she has read in the draft SOC 8.
“We need a serious organization to take a sober look at the evidence, and that is why we have established the Society for Evidence-Based Gender Medicine [SEGM],” she noted. “This is what we do – we are looking at all of the evidence.”
Dr. Mason is a clinical advisor to SEGM, an organization set-up to evaluate current interventions and evidence on gender dysphoria.
The pediatrician has particular concerns regarding the child and adolescent chapters in the draft SOC 8. The adolescent chapter states: “Guidelines are meant to provide a gold standard based on the available evidence at this moment of time.”
Dr. Mason disputes this assertion. “This document should not be the new gold standard going forward, primarily because it is not evidence based.”
In an interview, Dr. Mason explained that WPATH say they used the “Delphi consensus process” to determine their recommendations, but “this process is designed for use with a panel of experts when evidence is lacking. I would say they didn’t have a panel of experts. They largely had a panel of activists, with a few experts.”
There is no mention, for example, of England’s National Institute for Health and Care Excellence (NICE) evidence reviews on puberty blockers and cross-sex hormones from earlier this year. These reviews determined that no studies have compared cross-sex hormones or puberty blockers with a control group and all follow-up periods for cross-sex hormones were relatively short.
This disappoints Dr. Mason: “These are significant; they are important documents.”
And much of the evidence quoted comes from the well-known and often-quoted “Dutch-protocol” study of 2011, in which the children studied were much younger at the time of their gender dysphoria, compared with the many adolescents who make up the current surge in presentation at gender clinics worldwide, she adds.
Rapid-onset GD: adolescents presenting late with little history
Dr. Mason also stresses that the SOC 8 does not address the most urgent issues in transgender medicine today, mainly because it does not address rapid-onset gender dysphoria (ROGD): “This is the dilemma of the 21st century; it’s new.”
ROGD – a term first coined in 2018 by researcher Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research (ICGDR) – refers to the phenomena of adolescents expressing a desire to transition from their birth sex after little or no apparent previous indication.
However, the SOC 8 does make reference to aspects of adolescent development that might impact their decision-making processes around gender identity during teen years. The chapter on adolescents reads: “... adolescence is also often associated with increased risk-taking behaviors. Along with these notable changes ... individuation from parents ... [there is] often a heightened focus on peer relationships, which can be both positive and detrimental.”
The guidance goes on to point out that “it is critical to understand how all of these aspects of development may impact the decision-making for a given young person within their specific cultural context.”
Desistance and detransitioning not adequately addressed
Dr. Mason also says there is little mention “about detransitioning in this SOC [8], and ‘gender dysphoria’ and ‘trans’ are terms that are not defined.”
Likewise, there is no mention of desistance, she highlights, which is when individuals naturally resolve their dysphoria around their birth sex as they grow older.
The most recent published data seen by this news organization relates to a study from March 2021 that showed nearly 88% of boys who struggled with gender identity in childhood (approximate mean age 8 years and follow-up at approximate mean age 20 years) desisted. It reads: “Of the 139 participants, 17 (12.2%) were classified as ‘persisters’ and the remaining 122 (87.8%) were classified as desisters.”
“Most children with gender dysphoria will desist and lose their concept of themselves as being the opposite gender,” Dr. Mason explains. “This is the safest path for a child – desistance.”
“Transition can turn a healthy young person into a lifelong medical patient and has significant health risks,” she emphasizes, stressing that transition has not been shown to decrease the probability of suicide, or attempts at suicide, despite myriad claims saying otherwise.
“Before we were routinely transitioning kids at school, the vast majority of children grew out of their gender dysphoria. This history is not recognized at all in these SOC [8],” she maintains.
Ken Zucker, PhD, CPsych, an author of the study of desistance in boys, says the terms desistence and persistence of gender dysphoria have caused some consternation in certain circles.
An editor of the Archives of Sexual Behavior and professor in the department of psychiatry, University of Toronto, Dr. Zucker has published widely on the topic.
He told this news organization: “The terms persistence and desistance have become verboten among the WPATH cognoscenti. Perhaps the contributors to SOC 8 have come up with alternative descriptors.”
“The term ‘desistance’ is particularly annoying to some of the gender-affirming clinicians, because they don’t believe that desistance is bona fide,” Dr. Zucker points out.
“The desistance resisters are like anti-vaxxers – nothing one can provide as evidence for the efficacy of vaccines is sufficient. There will always be a new objection.”
Other mental health issues, in particular ADHD and autism
It is also widely acknowledged that there is a higher rate of neurodevelopmental and psychiatric diagnoses in individuals with gender dysphoria. For example, one 2020 study found that transgender people were three to six times as likely to be autistic as cisgender people (those whose gender is aligned with their birth sex).
Statement one in the chapter on adolescents in draft WPATH SOC 8 does give a nod to this, pointing out that health professionals working with gender diverse adolescents “should receive training and develop expertise in autism spectrum disorders and other neurodiversity conditions.”
It also notes that in some cases “a more extended assessment process may be useful, such as for youth with more complex presentations (e.g., complicated mental health histories, co-occurring autism spectrum characteristics in particular) and an absence of experienced childhood gender incongruence.”
However, Dr. Mason stresses that underlying mental health issues are central to addressing how to manage a significant number of these patients.
“If a young person has ADHD or autism, they are not ready to make decisions about the rest of their life at age 18. Even a neurotypical young person is still developing their frontal cortex in their early 20s, and it takes longer for those with ADHD or on the autism spectrum.”
She firmly believes that the guidance does not give sufficient consideration to comorbidities in people over the age of 18.
According to their [SOC 8] guidelines, “once someone is 18 they are ready for anything,” says Dr. Mason.
Offering some explanation for the increased prevalence of ADHD and autism in those with gender dysphoria, Dr. Mason notes that children can have “hyperfocus” and those with autism will fixate on a particular area of interest. “If a child is unhappy in their life, and this can be more likely if someone is neuro-atypical, then it is likely that the individual might go online and find this one solution [for example, a transgender identity] that seems to fix everything.”
Perceptions of femininity and masculinity can also be extra challenging for a child with autism, Dr. Mason says. “It is relatively easy for an autistic girl to feel like she should be a boy because the rules of femininity are composed of nonverbal, subtle behaviors that can be difficult to pick up on,” she points out. “An autistic child who isn’t particularly good at nonverbal communication might not pick up on these and thus feel they are not very ‘female.’”
“There’s a whole lot of grass-is-greener-type thinking. Girls think boys have an easier life, and boys think girls have an easier life. I know some detransitioners who have spoken eloquently about realizing their mistake on this,” she adds.
Other parts of the SOC 8 that Dr. Mason disagrees with include the recommendation in the adolescent chapter that 14-year-olds are mature enough to start cross-sex hormones, that is, giving testosterone to a female who wants to transition to male or estrogen to a male who wishes to transition to female. “I think that’s far too young,” she asserts.
And she points out that the document states 17-year-olds are ready for genital reassignment surgery. Again, she believes this is far too young.
“Also, the SOC 8 document does not clarify who is appropriate for surgery. Whenever surgery is discussed, it becomes very vague,” she said.
A version of this article first appeared on Medscape.com.
Dermatologists take to TikTok to share their own ‘hacks’
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.

The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.

The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.

The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
DLBCL: PFS but no OS benefit with polatuzumab-vedotin add-on
Two-year progression free survival (PFS) was 76.7% for the 440 patients randomized to polatuzumab-vedotin (PV) add-on, versus 70.2% for the 439 randomized to R-CHOP, which translated to a 27% reduction in the risk of progression, relapse, or death (P = .02). However, overall survival (OS) at 2 years was just under 89% in both arms of the trial, dubbed POLARIX. Toxicity was comparable between the two arms.
The investigators swapped out the vincristine in R-CHOP for PV to avoid overlapping neurotoxic side effects and called their modified regimen “pola-R-CHP.”
“We believe these results support use of pola-R-CHP in the initial management of patients with DLBCL,” senior investigator Gilles Salles, MD, PhD, a hematologic oncologist at Memorial Sloan Cancer Center in New York, said at the American Society of Hematology annual meeting.
The study (ASH 2021 abstract LBA-1), was published simultaneously in the New England Journal of Medicine.
Worth the cost?
The investigators reported that the median follow up of 28.2 months may simply have been too short to see if the PFS benefit translates into better overall survival. Also, newer treatments for relapsed/refractory disease might have masked any OS benefit.
With the PFS benefit, however, “what we think we are seeing is a deeper, more profound complete remission that hopefully will translate into [better] overall survival, but it may be a while until that can be demonstrated,” said Jane N. Winter, MD, a hematologic oncologist at Northwestern University, Chicago, who moderated Dr. Salles’ presentation.
“If the improvement in PFS at 2 years represents a true higher cure rate and plateau rather than a simple delay in relapse,” the “results from the POLARIX trial are likely to be practice-changing,” blood cancer specialist Ajay K. Gopal, MD, professor of medicine at the University of Washington, Seattle, told this news organization when asked for comment.
With additional OS results pending, an audience member at ASH wondered if “the cost of this highly expensive monoclonal antibody drug conjugate is worth the small improvement in PFS.”
“We have to further study this point, but at this moment what is important is to have a treatment with better efficacy and no more toxicity” than R-CHOP, lead investigator Herve Tilly, MD, a hematologic oncologist at the University of Rouen, France, said at the meeting.
Dr. Gopal said the cost concerns are legitimate, but also pointed out that they “may be somewhat offset by the potential reduction in downstream use of expensive cellular therapies.”
The findings support his assertion. With reduced PFS, R-CHOP subjects were more likely than were pola-R-CHP subjects to go on to subsequent lines of therapy (30.3% versus 22.5%).
PV is already approved in the United States for relapsed or refractory DLBCL in combination with bendamustine and rituximab after failure of at least two previous regimens.
Defining a target population
R-CHOP – rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – has been the first-line standard of care for DLBCL for 2 decades, but it cures only about 60%-70% of patients. Researchers have tried for years to improve the cure rate by adding novel agents and other means, but outcomes haven’t been clinically meaningful, the investigators explained.
Polatuzumab, the antibody component of PV, zeroes in on a ubiquitous target on mature B-cell lymphomas, delivering vedotin, a potent microtubule inhibitor, directly to tumor cells.
Study subjects were treatment naive and a median of 65 years old with intermediate-risk or high-risk DLBCL. About a third had activated B-cell–like DLBCL, and almost two-thirds had baseline International Prognostic Index (IPI) scores between 3 and 5.
Each arm of the trial underwent six treatment cycles, plus two cycles of rituximab monotherapy.
On subgroup analysis, PFS benefit clustered among higher risk patients, namely patients older than 60 years, those with IPI scores between 3 and 5, and patients with the activated B-cell–like subtype.
Younger patients, subjects with lower IPI scores, patients with bulky disease, and those who had germinal-center B-cell–like DLBCL “did not show a clear [PFS] benefit,” the study team said.
Ongoing trial in the elderly
Adverse events in POLARIX were in line with the component drugs’ known toxicity profiles, with no new safety signals identified.
The most common grade 3/4 adverse events were neutropenia (28.3% in the pola-R-CHP group and 30.8% in the R-CHOP group), febrile neutropenia (13.8% and 8.0%, respectively), and anemia (12.0% and 8.4%). A bit over 6% of subjects in both arms discontinued because of adverse events.
The higher incidence of febrile neutropenia with pola-R-CHP “did not translate into a higher overall incidence of infection, treatment discontinuation, or dose reductions,” the investigators said.
They noted that patients with lymphoma arising from previously diagnosed indolent lymphoma, those with a primary mediastinal lymphoma, and people older than 80 years were not included in the study. A phase 3 trial in patients 75 years and up is recruiting.
The work was funded by PV maker Genentech/Roche. Many of the investigators disclosed ties to the companies, including Dr. Tilly, an adviser and speaker for Roche, and Dr. Salles, an adviser for Genentech. Three investigators were Genentech employees. Dr. Gopal is a consultant for Genentech/Roche. Dr. Winter did not have any ties to the companies.
Two-year progression free survival (PFS) was 76.7% for the 440 patients randomized to polatuzumab-vedotin (PV) add-on, versus 70.2% for the 439 randomized to R-CHOP, which translated to a 27% reduction in the risk of progression, relapse, or death (P = .02). However, overall survival (OS) at 2 years was just under 89% in both arms of the trial, dubbed POLARIX. Toxicity was comparable between the two arms.
The investigators swapped out the vincristine in R-CHOP for PV to avoid overlapping neurotoxic side effects and called their modified regimen “pola-R-CHP.”
“We believe these results support use of pola-R-CHP in the initial management of patients with DLBCL,” senior investigator Gilles Salles, MD, PhD, a hematologic oncologist at Memorial Sloan Cancer Center in New York, said at the American Society of Hematology annual meeting.
The study (ASH 2021 abstract LBA-1), was published simultaneously in the New England Journal of Medicine.
Worth the cost?
The investigators reported that the median follow up of 28.2 months may simply have been too short to see if the PFS benefit translates into better overall survival. Also, newer treatments for relapsed/refractory disease might have masked any OS benefit.
With the PFS benefit, however, “what we think we are seeing is a deeper, more profound complete remission that hopefully will translate into [better] overall survival, but it may be a while until that can be demonstrated,” said Jane N. Winter, MD, a hematologic oncologist at Northwestern University, Chicago, who moderated Dr. Salles’ presentation.
“If the improvement in PFS at 2 years represents a true higher cure rate and plateau rather than a simple delay in relapse,” the “results from the POLARIX trial are likely to be practice-changing,” blood cancer specialist Ajay K. Gopal, MD, professor of medicine at the University of Washington, Seattle, told this news organization when asked for comment.
With additional OS results pending, an audience member at ASH wondered if “the cost of this highly expensive monoclonal antibody drug conjugate is worth the small improvement in PFS.”
“We have to further study this point, but at this moment what is important is to have a treatment with better efficacy and no more toxicity” than R-CHOP, lead investigator Herve Tilly, MD, a hematologic oncologist at the University of Rouen, France, said at the meeting.
Dr. Gopal said the cost concerns are legitimate, but also pointed out that they “may be somewhat offset by the potential reduction in downstream use of expensive cellular therapies.”
The findings support his assertion. With reduced PFS, R-CHOP subjects were more likely than were pola-R-CHP subjects to go on to subsequent lines of therapy (30.3% versus 22.5%).
PV is already approved in the United States for relapsed or refractory DLBCL in combination with bendamustine and rituximab after failure of at least two previous regimens.
Defining a target population
R-CHOP – rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – has been the first-line standard of care for DLBCL for 2 decades, but it cures only about 60%-70% of patients. Researchers have tried for years to improve the cure rate by adding novel agents and other means, but outcomes haven’t been clinically meaningful, the investigators explained.
Polatuzumab, the antibody component of PV, zeroes in on a ubiquitous target on mature B-cell lymphomas, delivering vedotin, a potent microtubule inhibitor, directly to tumor cells.
Study subjects were treatment naive and a median of 65 years old with intermediate-risk or high-risk DLBCL. About a third had activated B-cell–like DLBCL, and almost two-thirds had baseline International Prognostic Index (IPI) scores between 3 and 5.
Each arm of the trial underwent six treatment cycles, plus two cycles of rituximab monotherapy.
On subgroup analysis, PFS benefit clustered among higher risk patients, namely patients older than 60 years, those with IPI scores between 3 and 5, and patients with the activated B-cell–like subtype.
Younger patients, subjects with lower IPI scores, patients with bulky disease, and those who had germinal-center B-cell–like DLBCL “did not show a clear [PFS] benefit,” the study team said.
Ongoing trial in the elderly
Adverse events in POLARIX were in line with the component drugs’ known toxicity profiles, with no new safety signals identified.
The most common grade 3/4 adverse events were neutropenia (28.3% in the pola-R-CHP group and 30.8% in the R-CHOP group), febrile neutropenia (13.8% and 8.0%, respectively), and anemia (12.0% and 8.4%). A bit over 6% of subjects in both arms discontinued because of adverse events.
The higher incidence of febrile neutropenia with pola-R-CHP “did not translate into a higher overall incidence of infection, treatment discontinuation, or dose reductions,” the investigators said.
They noted that patients with lymphoma arising from previously diagnosed indolent lymphoma, those with a primary mediastinal lymphoma, and people older than 80 years were not included in the study. A phase 3 trial in patients 75 years and up is recruiting.
The work was funded by PV maker Genentech/Roche. Many of the investigators disclosed ties to the companies, including Dr. Tilly, an adviser and speaker for Roche, and Dr. Salles, an adviser for Genentech. Three investigators were Genentech employees. Dr. Gopal is a consultant for Genentech/Roche. Dr. Winter did not have any ties to the companies.
Two-year progression free survival (PFS) was 76.7% for the 440 patients randomized to polatuzumab-vedotin (PV) add-on, versus 70.2% for the 439 randomized to R-CHOP, which translated to a 27% reduction in the risk of progression, relapse, or death (P = .02). However, overall survival (OS) at 2 years was just under 89% in both arms of the trial, dubbed POLARIX. Toxicity was comparable between the two arms.
The investigators swapped out the vincristine in R-CHOP for PV to avoid overlapping neurotoxic side effects and called their modified regimen “pola-R-CHP.”
“We believe these results support use of pola-R-CHP in the initial management of patients with DLBCL,” senior investigator Gilles Salles, MD, PhD, a hematologic oncologist at Memorial Sloan Cancer Center in New York, said at the American Society of Hematology annual meeting.
The study (ASH 2021 abstract LBA-1), was published simultaneously in the New England Journal of Medicine.
Worth the cost?
The investigators reported that the median follow up of 28.2 months may simply have been too short to see if the PFS benefit translates into better overall survival. Also, newer treatments for relapsed/refractory disease might have masked any OS benefit.
With the PFS benefit, however, “what we think we are seeing is a deeper, more profound complete remission that hopefully will translate into [better] overall survival, but it may be a while until that can be demonstrated,” said Jane N. Winter, MD, a hematologic oncologist at Northwestern University, Chicago, who moderated Dr. Salles’ presentation.
“If the improvement in PFS at 2 years represents a true higher cure rate and plateau rather than a simple delay in relapse,” the “results from the POLARIX trial are likely to be practice-changing,” blood cancer specialist Ajay K. Gopal, MD, professor of medicine at the University of Washington, Seattle, told this news organization when asked for comment.
With additional OS results pending, an audience member at ASH wondered if “the cost of this highly expensive monoclonal antibody drug conjugate is worth the small improvement in PFS.”
“We have to further study this point, but at this moment what is important is to have a treatment with better efficacy and no more toxicity” than R-CHOP, lead investigator Herve Tilly, MD, a hematologic oncologist at the University of Rouen, France, said at the meeting.
Dr. Gopal said the cost concerns are legitimate, but also pointed out that they “may be somewhat offset by the potential reduction in downstream use of expensive cellular therapies.”
The findings support his assertion. With reduced PFS, R-CHOP subjects were more likely than were pola-R-CHP subjects to go on to subsequent lines of therapy (30.3% versus 22.5%).
PV is already approved in the United States for relapsed or refractory DLBCL in combination with bendamustine and rituximab after failure of at least two previous regimens.
Defining a target population
R-CHOP – rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – has been the first-line standard of care for DLBCL for 2 decades, but it cures only about 60%-70% of patients. Researchers have tried for years to improve the cure rate by adding novel agents and other means, but outcomes haven’t been clinically meaningful, the investigators explained.
Polatuzumab, the antibody component of PV, zeroes in on a ubiquitous target on mature B-cell lymphomas, delivering vedotin, a potent microtubule inhibitor, directly to tumor cells.
Study subjects were treatment naive and a median of 65 years old with intermediate-risk or high-risk DLBCL. About a third had activated B-cell–like DLBCL, and almost two-thirds had baseline International Prognostic Index (IPI) scores between 3 and 5.
Each arm of the trial underwent six treatment cycles, plus two cycles of rituximab monotherapy.
On subgroup analysis, PFS benefit clustered among higher risk patients, namely patients older than 60 years, those with IPI scores between 3 and 5, and patients with the activated B-cell–like subtype.
Younger patients, subjects with lower IPI scores, patients with bulky disease, and those who had germinal-center B-cell–like DLBCL “did not show a clear [PFS] benefit,” the study team said.
Ongoing trial in the elderly
Adverse events in POLARIX were in line with the component drugs’ known toxicity profiles, with no new safety signals identified.
The most common grade 3/4 adverse events were neutropenia (28.3% in the pola-R-CHP group and 30.8% in the R-CHOP group), febrile neutropenia (13.8% and 8.0%, respectively), and anemia (12.0% and 8.4%). A bit over 6% of subjects in both arms discontinued because of adverse events.
The higher incidence of febrile neutropenia with pola-R-CHP “did not translate into a higher overall incidence of infection, treatment discontinuation, or dose reductions,” the investigators said.
They noted that patients with lymphoma arising from previously diagnosed indolent lymphoma, those with a primary mediastinal lymphoma, and people older than 80 years were not included in the study. A phase 3 trial in patients 75 years and up is recruiting.
The work was funded by PV maker Genentech/Roche. Many of the investigators disclosed ties to the companies, including Dr. Tilly, an adviser and speaker for Roche, and Dr. Salles, an adviser for Genentech. Three investigators were Genentech employees. Dr. Gopal is a consultant for Genentech/Roche. Dr. Winter did not have any ties to the companies.
REPORTING FROM ASH 2021










