User login
Reducing night-time checks is safe and helps patients sleep
Routine checks of vital signs during the night often prevent hospitalized patients from getting sufficient recuperative sleep. But patients who are judged to be clinically stable by an algorithm that uses real-time data can be safely spared these checks, according to a recent study published in JAMA Internal Medicine.
In their study,
“Sleep is crucial to health,” writes Hyung J. Cho, MD, from the New York University Grossman School of Medicine, in an accompanying editorial. “Ironically, hospitals, where people go to recover from illness, are among the most difficult places to sleep.”
Noise from the surrounding area, night-time examinations, multibed rooms, an unfamiliar environment, early morning blood sample collections, and frequent vital sign checks often prevent patients from sleeping through the night.
The goal of the study was to see if the elimination of one of these disrupting factors – the frequent checks of vital signs – would improve sleep and lead to a reduction in delirium, the primary endpoint.
To do this, the researchers incorporated a predictive algorithm they developed “to identify patients who are at low risk for abnormal night-time vital signs” into the hospitals electronic health records system. Attending physicians received a notification, based on real-time patient data, if it was predicted with a high degree of probability that a patient’s night-time vital signs would be within the normal range. Each physician was free to decide whether they would forgo night-time checks of the vital signs or whether they would turn off the notifications for a specific period.
The randomized clinical trial was conducted at a tertiary care academic teaching hospital from March to November 2019. Half the 1,930 patients were randomized to the algorithm group and half to standard care. None of the patients were receiving intensive care.
Number of night-time checks successfully reduced
The mean number of night-time checks was significantly lower in the algorithm group than in the standard-care group (0.97 vs. 1.41; P < .001).
The reduction in night-time checks had no effect on patient safety. There was no increase in transfers to the intensive care unit in the algorithm or standard-care groups (5% vs 5%; P = .92), and no difference between the number of heart alarms (0.2% vs. 0.9%; P = .07).
However, the reduction also had no effect on the incidence of episodes of delirium in the algorithm or standard-care groups (11% vs. 13%; P = .32).
“The reduction in vital signs checking, although statistically significant, was relatively small,” Dr. Cho explains. But the primary endpoint might have been different had the adherence to intervention been better, he notes.
In fact, the analysis confirmed that changes to routine daily practice in a hospital are not always easy to implement. In 35% of cases, the patients’ vital signs were checked at night, despite the physician’s order to the contrary.
“Busy patient-care assistants and nurses may check vital signs out of habit without noticing that the order has changed for some of the patients,” Dr. Najafi and his coauthors write. Many hospitals are used to thinking that regular measurements of the vital signs are part of good practice.
Include nursing staff
Future projects should use an interdisciplinary approach that includes nursing staff, Dr. Cho recommends. More user-friendly displays and optimized alerts in the electronic patient records could also encourage better implementation of the orders.
Less frequent checks of the vital signs would be welcomed by frontline staff because it would lighten their already heavy workload, he adds.
Although the study didn’t meet its primary endpoint, patients subjected to fewer night-time checks because of the algorithm were able to get a good night’s sleep. Other aspects of hospital care that are based on the patient’s stability, such as cardiac monitoring, could also potentially benefit from this type of intervention, Dr. Najafi and his colleagues suggest.
A version of this article first appeared on Medscape.com.
Routine checks of vital signs during the night often prevent hospitalized patients from getting sufficient recuperative sleep. But patients who are judged to be clinically stable by an algorithm that uses real-time data can be safely spared these checks, according to a recent study published in JAMA Internal Medicine.
In their study,
“Sleep is crucial to health,” writes Hyung J. Cho, MD, from the New York University Grossman School of Medicine, in an accompanying editorial. “Ironically, hospitals, where people go to recover from illness, are among the most difficult places to sleep.”
Noise from the surrounding area, night-time examinations, multibed rooms, an unfamiliar environment, early morning blood sample collections, and frequent vital sign checks often prevent patients from sleeping through the night.
The goal of the study was to see if the elimination of one of these disrupting factors – the frequent checks of vital signs – would improve sleep and lead to a reduction in delirium, the primary endpoint.
To do this, the researchers incorporated a predictive algorithm they developed “to identify patients who are at low risk for abnormal night-time vital signs” into the hospitals electronic health records system. Attending physicians received a notification, based on real-time patient data, if it was predicted with a high degree of probability that a patient’s night-time vital signs would be within the normal range. Each physician was free to decide whether they would forgo night-time checks of the vital signs or whether they would turn off the notifications for a specific period.
The randomized clinical trial was conducted at a tertiary care academic teaching hospital from March to November 2019. Half the 1,930 patients were randomized to the algorithm group and half to standard care. None of the patients were receiving intensive care.
Number of night-time checks successfully reduced
The mean number of night-time checks was significantly lower in the algorithm group than in the standard-care group (0.97 vs. 1.41; P < .001).
The reduction in night-time checks had no effect on patient safety. There was no increase in transfers to the intensive care unit in the algorithm or standard-care groups (5% vs 5%; P = .92), and no difference between the number of heart alarms (0.2% vs. 0.9%; P = .07).
However, the reduction also had no effect on the incidence of episodes of delirium in the algorithm or standard-care groups (11% vs. 13%; P = .32).
“The reduction in vital signs checking, although statistically significant, was relatively small,” Dr. Cho explains. But the primary endpoint might have been different had the adherence to intervention been better, he notes.
In fact, the analysis confirmed that changes to routine daily practice in a hospital are not always easy to implement. In 35% of cases, the patients’ vital signs were checked at night, despite the physician’s order to the contrary.
“Busy patient-care assistants and nurses may check vital signs out of habit without noticing that the order has changed for some of the patients,” Dr. Najafi and his coauthors write. Many hospitals are used to thinking that regular measurements of the vital signs are part of good practice.
Include nursing staff
Future projects should use an interdisciplinary approach that includes nursing staff, Dr. Cho recommends. More user-friendly displays and optimized alerts in the electronic patient records could also encourage better implementation of the orders.
Less frequent checks of the vital signs would be welcomed by frontline staff because it would lighten their already heavy workload, he adds.
Although the study didn’t meet its primary endpoint, patients subjected to fewer night-time checks because of the algorithm were able to get a good night’s sleep. Other aspects of hospital care that are based on the patient’s stability, such as cardiac monitoring, could also potentially benefit from this type of intervention, Dr. Najafi and his colleagues suggest.
A version of this article first appeared on Medscape.com.
Routine checks of vital signs during the night often prevent hospitalized patients from getting sufficient recuperative sleep. But patients who are judged to be clinically stable by an algorithm that uses real-time data can be safely spared these checks, according to a recent study published in JAMA Internal Medicine.
In their study,
“Sleep is crucial to health,” writes Hyung J. Cho, MD, from the New York University Grossman School of Medicine, in an accompanying editorial. “Ironically, hospitals, where people go to recover from illness, are among the most difficult places to sleep.”
Noise from the surrounding area, night-time examinations, multibed rooms, an unfamiliar environment, early morning blood sample collections, and frequent vital sign checks often prevent patients from sleeping through the night.
The goal of the study was to see if the elimination of one of these disrupting factors – the frequent checks of vital signs – would improve sleep and lead to a reduction in delirium, the primary endpoint.
To do this, the researchers incorporated a predictive algorithm they developed “to identify patients who are at low risk for abnormal night-time vital signs” into the hospitals electronic health records system. Attending physicians received a notification, based on real-time patient data, if it was predicted with a high degree of probability that a patient’s night-time vital signs would be within the normal range. Each physician was free to decide whether they would forgo night-time checks of the vital signs or whether they would turn off the notifications for a specific period.
The randomized clinical trial was conducted at a tertiary care academic teaching hospital from March to November 2019. Half the 1,930 patients were randomized to the algorithm group and half to standard care. None of the patients were receiving intensive care.
Number of night-time checks successfully reduced
The mean number of night-time checks was significantly lower in the algorithm group than in the standard-care group (0.97 vs. 1.41; P < .001).
The reduction in night-time checks had no effect on patient safety. There was no increase in transfers to the intensive care unit in the algorithm or standard-care groups (5% vs 5%; P = .92), and no difference between the number of heart alarms (0.2% vs. 0.9%; P = .07).
However, the reduction also had no effect on the incidence of episodes of delirium in the algorithm or standard-care groups (11% vs. 13%; P = .32).
“The reduction in vital signs checking, although statistically significant, was relatively small,” Dr. Cho explains. But the primary endpoint might have been different had the adherence to intervention been better, he notes.
In fact, the analysis confirmed that changes to routine daily practice in a hospital are not always easy to implement. In 35% of cases, the patients’ vital signs were checked at night, despite the physician’s order to the contrary.
“Busy patient-care assistants and nurses may check vital signs out of habit without noticing that the order has changed for some of the patients,” Dr. Najafi and his coauthors write. Many hospitals are used to thinking that regular measurements of the vital signs are part of good practice.
Include nursing staff
Future projects should use an interdisciplinary approach that includes nursing staff, Dr. Cho recommends. More user-friendly displays and optimized alerts in the electronic patient records could also encourage better implementation of the orders.
Less frequent checks of the vital signs would be welcomed by frontline staff because it would lighten their already heavy workload, he adds.
Although the study didn’t meet its primary endpoint, patients subjected to fewer night-time checks because of the algorithm were able to get a good night’s sleep. Other aspects of hospital care that are based on the patient’s stability, such as cardiac monitoring, could also potentially benefit from this type of intervention, Dr. Najafi and his colleagues suggest.
A version of this article first appeared on Medscape.com.
Epstein-Barr virus a likely leading cause of multiple sclerosis
This study is the first to provide compelling evidence of a causal link between EBV and MS, principal investigator Alberto Ascherio, MD, DrPH, professor of epidemiology, Harvard T. H. Chan School of Public Health, and professor of medicine, Harvard Medical School, Boston, told this news organization.
The “prevailing” view has been that MS is “an autoimmune disease of unknown etiology,” said Dr. Ascherio. “Now we know MS is a complication of a viral infection.” With this knowledge, he added, “we can redirect research” to find antiviral drugs to treat the disease.
The study was published online Jan. 13 in Science.
Unique dataset
A chronic disease of the central nervous system, MS involves an inflammatory attack on the myelin sheath and the axons it insulates. The disease affects 2.8 million people worldwide.
EBV is a human herpesvirus that can cause infectious mononucleosis. After infection, it persists in latent form in B-lymphocytes.
EBV is common and infects about 95% of adults. Most individuals are already infected with the virus by age 18 or 20 years, making it difficult to study uninfected populations, said Dr. Ascherio.
However, access to a “huge” database of more than 10 million active-duty U.S. service personnel made this possible, he said.
Service members are screened for HIV at the start of their service care and biennially thereafter. The investigators used stored blood samples to determine the relation between EBV infection and MS over a 20-year period from 1993 to 2013.
Researchers examined 801 MS case patients and 1,566 matched controls without MS. Most individuals were under 20 at the time of their first blood collection. Symptom onset for those who developed MS was a median of 10 years after the first sample was obtained.
Only one of the 801 MS case patients had no serologic evidence of EBV. This individual may have been infected with the virus after the last blood collection, failed to seroconvert in response to infection, or was misdiagnosed, the investigators note.
The hazard ratio for MS between EBV seroconversion versus persistent EBV seronegative was 32.4 (95% CI, 4.3-245.3; P < .001).
An MS vaccine?
MS risk was not increased after infection with cytomegalovirus, a herpesvirus that is transmitted through saliva, as is EBV.
Researchers measured serum concentrations of neurofilament light chain (sNflL), a biomarker of neuroaxonal degeneration, in samples from EBV-negative individuals at baseline. There were no signs of neuroaxonal degeneration before EBV seroconversion in subjects who later developed MS.
This indicates that “EBV infection preceded not only symptom onset but also the time of the first detectable pathological mechanisms underlying MS,” the investigators note.
The very magnitude of increased MS risk of MS observed EBV almost completely rules out confounding by known risk factors. Smoking and vitamin D deficiency double the risk, and genetic predisposition and childhood obesity also only raise the risks of MS to a “moderate” degree, said Dr. Ascherio.
It’s not clear why only some people infected with EBV go on to develop MS, he said.
The idea that reverse causation – that immune dysregulation during the preclinical phase of MS increases susceptibility to EBV infection – is unlikely, the investigators note. For instance, EBV seroconversion occurs before elevation of sNfL levels, an early marker of preclinical MS.
Since most MS cases appear to be caused by EBV, a suitable vaccine might thwart the disease. “A vaccine could, in theory, prevent infection and prevent MS,” said Dr. Ascherio, adding that there’s ongoing work to develop such a vaccine.
Another approach is to target the virus driving MS disease progression. Developing appropriate antivirals might treat and even cure MS, said Dr. Ascherio.
‘Compelling data’
In an accompanying commentary, William H. Robinson, MD, PhD, professor, Division of Immunology and Rheumatology, department of medicine, Stanford (Calif.) University, and a colleague said the study findings “provide compelling data that implicate EBV as the trigger for the development of MS.”
The mechanism or mechanisms by which EBV leads to MS “remain elusive,” the commentary authors write.
“Possibilities include molecular mimicry, through which EBV viral protein sequences mimic human myelin proteins and other CNS proteins and thereby induce autoimmunity against myelin and CNS antigens,” they note.
As other factors, including genetic susceptibility, are important to MS, EBV infection is likely necessary but not sufficient to trigger MS, said the commentary. “Infection with EBV is the initial pathogenic step in MS, but additional fuses must be ignited for the full pathophysiology.”
The commentary authors query whether there may be “new opportunities” for therapy with vaccines or antivirals. “Now that the initial trigger for MS has been identified, perhaps MS could be eradicated.”
In a statement from the Science Media Center, an independent venture promoting views from the scientific community, two other experts offered their take on the study.
Paul Farrell, PhD, professor of tumor virology, Imperial College London, said the paper “provides very clear confirmation of a causal role for EBV in most cases of MS.”
While there’s evidence that a vaccine can prevent the EBV disease infectious mononucleosis, no vaccine candidate has yet prevented the virus from infecting and establishing long-term persistence in people, noted Dr. Farrell.
“So, at this stage it is not clear whether a vaccine of the types currently being developed would be able to prevent the long-term effects of EBV in MS,” he said.
Daniel Davis, PhD, professor of immunology, University of Manchester, United Kingdom, commented that the value of this new discovery is not an immediate medical cure or treatment but is “a major step forward” in understanding MS.
The study “sets up new research working out the precise details of how this virus can sometimes lead to an autoimmune disease,” said Dr. Davis. “There is no shortage of ideas in how this might happen in principle and hopefully the correct details will emerge soon.”
The study received funding from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, National Multiple Sclerosis Society, the German Research Foundation, the National Institutes of Health, and the Howard Hughes Medical Institute. Dr. Ascherio reports no relevant financial relaitonships. Dr. Robinson is a coinventor on a patent application filed by Stanford University that includes antibodies to EBV. Dr. Farrell reports serving on an ad hoc review panel for GSK on EBV vaccines in 2019 as a one off. He has a current grant from MRC on EBV biology, including some EBV sequence variation, but the grant is not about MS. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This study is the first to provide compelling evidence of a causal link between EBV and MS, principal investigator Alberto Ascherio, MD, DrPH, professor of epidemiology, Harvard T. H. Chan School of Public Health, and professor of medicine, Harvard Medical School, Boston, told this news organization.
The “prevailing” view has been that MS is “an autoimmune disease of unknown etiology,” said Dr. Ascherio. “Now we know MS is a complication of a viral infection.” With this knowledge, he added, “we can redirect research” to find antiviral drugs to treat the disease.
The study was published online Jan. 13 in Science.
Unique dataset
A chronic disease of the central nervous system, MS involves an inflammatory attack on the myelin sheath and the axons it insulates. The disease affects 2.8 million people worldwide.
EBV is a human herpesvirus that can cause infectious mononucleosis. After infection, it persists in latent form in B-lymphocytes.
EBV is common and infects about 95% of adults. Most individuals are already infected with the virus by age 18 or 20 years, making it difficult to study uninfected populations, said Dr. Ascherio.
However, access to a “huge” database of more than 10 million active-duty U.S. service personnel made this possible, he said.
Service members are screened for HIV at the start of their service care and biennially thereafter. The investigators used stored blood samples to determine the relation between EBV infection and MS over a 20-year period from 1993 to 2013.
Researchers examined 801 MS case patients and 1,566 matched controls without MS. Most individuals were under 20 at the time of their first blood collection. Symptom onset for those who developed MS was a median of 10 years after the first sample was obtained.
Only one of the 801 MS case patients had no serologic evidence of EBV. This individual may have been infected with the virus after the last blood collection, failed to seroconvert in response to infection, or was misdiagnosed, the investigators note.
The hazard ratio for MS between EBV seroconversion versus persistent EBV seronegative was 32.4 (95% CI, 4.3-245.3; P < .001).
An MS vaccine?
MS risk was not increased after infection with cytomegalovirus, a herpesvirus that is transmitted through saliva, as is EBV.
Researchers measured serum concentrations of neurofilament light chain (sNflL), a biomarker of neuroaxonal degeneration, in samples from EBV-negative individuals at baseline. There were no signs of neuroaxonal degeneration before EBV seroconversion in subjects who later developed MS.
This indicates that “EBV infection preceded not only symptom onset but also the time of the first detectable pathological mechanisms underlying MS,” the investigators note.
The very magnitude of increased MS risk of MS observed EBV almost completely rules out confounding by known risk factors. Smoking and vitamin D deficiency double the risk, and genetic predisposition and childhood obesity also only raise the risks of MS to a “moderate” degree, said Dr. Ascherio.
It’s not clear why only some people infected with EBV go on to develop MS, he said.
The idea that reverse causation – that immune dysregulation during the preclinical phase of MS increases susceptibility to EBV infection – is unlikely, the investigators note. For instance, EBV seroconversion occurs before elevation of sNfL levels, an early marker of preclinical MS.
Since most MS cases appear to be caused by EBV, a suitable vaccine might thwart the disease. “A vaccine could, in theory, prevent infection and prevent MS,” said Dr. Ascherio, adding that there’s ongoing work to develop such a vaccine.
Another approach is to target the virus driving MS disease progression. Developing appropriate antivirals might treat and even cure MS, said Dr. Ascherio.
‘Compelling data’
In an accompanying commentary, William H. Robinson, MD, PhD, professor, Division of Immunology and Rheumatology, department of medicine, Stanford (Calif.) University, and a colleague said the study findings “provide compelling data that implicate EBV as the trigger for the development of MS.”
The mechanism or mechanisms by which EBV leads to MS “remain elusive,” the commentary authors write.
“Possibilities include molecular mimicry, through which EBV viral protein sequences mimic human myelin proteins and other CNS proteins and thereby induce autoimmunity against myelin and CNS antigens,” they note.
As other factors, including genetic susceptibility, are important to MS, EBV infection is likely necessary but not sufficient to trigger MS, said the commentary. “Infection with EBV is the initial pathogenic step in MS, but additional fuses must be ignited for the full pathophysiology.”
The commentary authors query whether there may be “new opportunities” for therapy with vaccines or antivirals. “Now that the initial trigger for MS has been identified, perhaps MS could be eradicated.”
In a statement from the Science Media Center, an independent venture promoting views from the scientific community, two other experts offered their take on the study.
Paul Farrell, PhD, professor of tumor virology, Imperial College London, said the paper “provides very clear confirmation of a causal role for EBV in most cases of MS.”
While there’s evidence that a vaccine can prevent the EBV disease infectious mononucleosis, no vaccine candidate has yet prevented the virus from infecting and establishing long-term persistence in people, noted Dr. Farrell.
“So, at this stage it is not clear whether a vaccine of the types currently being developed would be able to prevent the long-term effects of EBV in MS,” he said.
Daniel Davis, PhD, professor of immunology, University of Manchester, United Kingdom, commented that the value of this new discovery is not an immediate medical cure or treatment but is “a major step forward” in understanding MS.
The study “sets up new research working out the precise details of how this virus can sometimes lead to an autoimmune disease,” said Dr. Davis. “There is no shortage of ideas in how this might happen in principle and hopefully the correct details will emerge soon.”
The study received funding from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, National Multiple Sclerosis Society, the German Research Foundation, the National Institutes of Health, and the Howard Hughes Medical Institute. Dr. Ascherio reports no relevant financial relaitonships. Dr. Robinson is a coinventor on a patent application filed by Stanford University that includes antibodies to EBV. Dr. Farrell reports serving on an ad hoc review panel for GSK on EBV vaccines in 2019 as a one off. He has a current grant from MRC on EBV biology, including some EBV sequence variation, but the grant is not about MS. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This study is the first to provide compelling evidence of a causal link between EBV and MS, principal investigator Alberto Ascherio, MD, DrPH, professor of epidemiology, Harvard T. H. Chan School of Public Health, and professor of medicine, Harvard Medical School, Boston, told this news organization.
The “prevailing” view has been that MS is “an autoimmune disease of unknown etiology,” said Dr. Ascherio. “Now we know MS is a complication of a viral infection.” With this knowledge, he added, “we can redirect research” to find antiviral drugs to treat the disease.
The study was published online Jan. 13 in Science.
Unique dataset
A chronic disease of the central nervous system, MS involves an inflammatory attack on the myelin sheath and the axons it insulates. The disease affects 2.8 million people worldwide.
EBV is a human herpesvirus that can cause infectious mononucleosis. After infection, it persists in latent form in B-lymphocytes.
EBV is common and infects about 95% of adults. Most individuals are already infected with the virus by age 18 or 20 years, making it difficult to study uninfected populations, said Dr. Ascherio.
However, access to a “huge” database of more than 10 million active-duty U.S. service personnel made this possible, he said.
Service members are screened for HIV at the start of their service care and biennially thereafter. The investigators used stored blood samples to determine the relation between EBV infection and MS over a 20-year period from 1993 to 2013.
Researchers examined 801 MS case patients and 1,566 matched controls without MS. Most individuals were under 20 at the time of their first blood collection. Symptom onset for those who developed MS was a median of 10 years after the first sample was obtained.
Only one of the 801 MS case patients had no serologic evidence of EBV. This individual may have been infected with the virus after the last blood collection, failed to seroconvert in response to infection, or was misdiagnosed, the investigators note.
The hazard ratio for MS between EBV seroconversion versus persistent EBV seronegative was 32.4 (95% CI, 4.3-245.3; P < .001).
An MS vaccine?
MS risk was not increased after infection with cytomegalovirus, a herpesvirus that is transmitted through saliva, as is EBV.
Researchers measured serum concentrations of neurofilament light chain (sNflL), a biomarker of neuroaxonal degeneration, in samples from EBV-negative individuals at baseline. There were no signs of neuroaxonal degeneration before EBV seroconversion in subjects who later developed MS.
This indicates that “EBV infection preceded not only symptom onset but also the time of the first detectable pathological mechanisms underlying MS,” the investigators note.
The very magnitude of increased MS risk of MS observed EBV almost completely rules out confounding by known risk factors. Smoking and vitamin D deficiency double the risk, and genetic predisposition and childhood obesity also only raise the risks of MS to a “moderate” degree, said Dr. Ascherio.
It’s not clear why only some people infected with EBV go on to develop MS, he said.
The idea that reverse causation – that immune dysregulation during the preclinical phase of MS increases susceptibility to EBV infection – is unlikely, the investigators note. For instance, EBV seroconversion occurs before elevation of sNfL levels, an early marker of preclinical MS.
Since most MS cases appear to be caused by EBV, a suitable vaccine might thwart the disease. “A vaccine could, in theory, prevent infection and prevent MS,” said Dr. Ascherio, adding that there’s ongoing work to develop such a vaccine.
Another approach is to target the virus driving MS disease progression. Developing appropriate antivirals might treat and even cure MS, said Dr. Ascherio.
‘Compelling data’
In an accompanying commentary, William H. Robinson, MD, PhD, professor, Division of Immunology and Rheumatology, department of medicine, Stanford (Calif.) University, and a colleague said the study findings “provide compelling data that implicate EBV as the trigger for the development of MS.”
The mechanism or mechanisms by which EBV leads to MS “remain elusive,” the commentary authors write.
“Possibilities include molecular mimicry, through which EBV viral protein sequences mimic human myelin proteins and other CNS proteins and thereby induce autoimmunity against myelin and CNS antigens,” they note.
As other factors, including genetic susceptibility, are important to MS, EBV infection is likely necessary but not sufficient to trigger MS, said the commentary. “Infection with EBV is the initial pathogenic step in MS, but additional fuses must be ignited for the full pathophysiology.”
The commentary authors query whether there may be “new opportunities” for therapy with vaccines or antivirals. “Now that the initial trigger for MS has been identified, perhaps MS could be eradicated.”
In a statement from the Science Media Center, an independent venture promoting views from the scientific community, two other experts offered their take on the study.
Paul Farrell, PhD, professor of tumor virology, Imperial College London, said the paper “provides very clear confirmation of a causal role for EBV in most cases of MS.”
While there’s evidence that a vaccine can prevent the EBV disease infectious mononucleosis, no vaccine candidate has yet prevented the virus from infecting and establishing long-term persistence in people, noted Dr. Farrell.
“So, at this stage it is not clear whether a vaccine of the types currently being developed would be able to prevent the long-term effects of EBV in MS,” he said.
Daniel Davis, PhD, professor of immunology, University of Manchester, United Kingdom, commented that the value of this new discovery is not an immediate medical cure or treatment but is “a major step forward” in understanding MS.
The study “sets up new research working out the precise details of how this virus can sometimes lead to an autoimmune disease,” said Dr. Davis. “There is no shortage of ideas in how this might happen in principle and hopefully the correct details will emerge soon.”
The study received funding from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, National Multiple Sclerosis Society, the German Research Foundation, the National Institutes of Health, and the Howard Hughes Medical Institute. Dr. Ascherio reports no relevant financial relaitonships. Dr. Robinson is a coinventor on a patent application filed by Stanford University that includes antibodies to EBV. Dr. Farrell reports serving on an ad hoc review panel for GSK on EBV vaccines in 2019 as a one off. He has a current grant from MRC on EBV biology, including some EBV sequence variation, but the grant is not about MS. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCIENCE
Sometimes You Can’t Blame the Sun
ANSWER
The correct answer is all of the above (choice “e”).
DISCUSSION
Most cases of dermatomyositis, which the patient’s presentation and lab results suggested, require nerve conduction studies, a check of serum aldolase levels, and skin and muscle biopsies to complete the work-up. However, the arrival at a diagnosis is only the first step.
Patients with dermatomyositis, particularly those older than 60, require evaluation for occult malignancy. There is evidence that the body’s immune response to the cancer is what drives the disease process. Hence the need for the studies listed, looking for breast, lung, and gastrointestinal cancers especially.
Dermatomyositis is thought to be an inflammatory myopathy, possibly driven by autoimmune factors. It is rare (about 1 to 22 per 100,000) and affects women more than men.
The “sunburn” rash is typical, especially on the face, chest, and dorsal hands, and usually clears completely when the cancer is found and cured. Other common findings include elevated creatine kinase, hand rashes (known as Gottron’s papules), and dystrophic calcification in skin and/or joints.
TREATMENT
Aside from addressing a possible malignancy, treatment of dermatomyositis usually starts with glucocorticoids, eventually tapered and replaced by steroid-sparing agents such as azathioprine or cyclosporine. These drugs have dramatically increased the chances of survival and eventual cure.
It’s common for the photosensitivity to persist long after the myositis has resolved, which is why sunscreen and other sun-protective measures are advised.
ANSWER
The correct answer is all of the above (choice “e”).
DISCUSSION
Most cases of dermatomyositis, which the patient’s presentation and lab results suggested, require nerve conduction studies, a check of serum aldolase levels, and skin and muscle biopsies to complete the work-up. However, the arrival at a diagnosis is only the first step.
Patients with dermatomyositis, particularly those older than 60, require evaluation for occult malignancy. There is evidence that the body’s immune response to the cancer is what drives the disease process. Hence the need for the studies listed, looking for breast, lung, and gastrointestinal cancers especially.
Dermatomyositis is thought to be an inflammatory myopathy, possibly driven by autoimmune factors. It is rare (about 1 to 22 per 100,000) and affects women more than men.
The “sunburn” rash is typical, especially on the face, chest, and dorsal hands, and usually clears completely when the cancer is found and cured. Other common findings include elevated creatine kinase, hand rashes (known as Gottron’s papules), and dystrophic calcification in skin and/or joints.
TREATMENT
Aside from addressing a possible malignancy, treatment of dermatomyositis usually starts with glucocorticoids, eventually tapered and replaced by steroid-sparing agents such as azathioprine or cyclosporine. These drugs have dramatically increased the chances of survival and eventual cure.
It’s common for the photosensitivity to persist long after the myositis has resolved, which is why sunscreen and other sun-protective measures are advised.
ANSWER
The correct answer is all of the above (choice “e”).
DISCUSSION
Most cases of dermatomyositis, which the patient’s presentation and lab results suggested, require nerve conduction studies, a check of serum aldolase levels, and skin and muscle biopsies to complete the work-up. However, the arrival at a diagnosis is only the first step.
Patients with dermatomyositis, particularly those older than 60, require evaluation for occult malignancy. There is evidence that the body’s immune response to the cancer is what drives the disease process. Hence the need for the studies listed, looking for breast, lung, and gastrointestinal cancers especially.
Dermatomyositis is thought to be an inflammatory myopathy, possibly driven by autoimmune factors. It is rare (about 1 to 22 per 100,000) and affects women more than men.
The “sunburn” rash is typical, especially on the face, chest, and dorsal hands, and usually clears completely when the cancer is found and cured. Other common findings include elevated creatine kinase, hand rashes (known as Gottron’s papules), and dystrophic calcification in skin and/or joints.
TREATMENT
Aside from addressing a possible malignancy, treatment of dermatomyositis usually starts with glucocorticoids, eventually tapered and replaced by steroid-sparing agents such as azathioprine or cyclosporine. These drugs have dramatically increased the chances of survival and eventual cure.
It’s common for the photosensitivity to persist long after the myositis has resolved, which is why sunscreen and other sun-protective measures are advised.
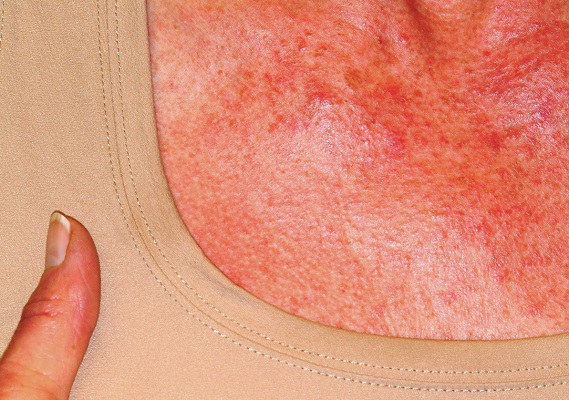
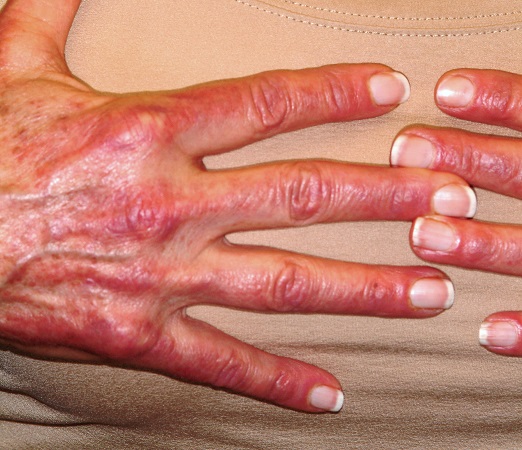
A 60-year-old woman was referred to dermatology for evaluation of “sunburn.” The rash was painful and unrelieved by topical medications, including class IV steroid creams. The redness was tender and warm to touch.
The rash had been present for months. During this period, the patient also had grown increasingly weak, leading her to quit her job. In the clinic, she was unable to stand from a seated position without difficulty. She reported no other health concerns and had quit smoking 5 years previously, after 30 years.
On examination, diffuse blanchable macular erythema on the patient’s face and chest was immediately observed. There was also an odd rash, composed of hundreds of tiny confluent papules, concentrated over the interphalangeal joints and dorsal hands. These too were warm and tender to touch. Most of her cuticles were peeling off; closer examination under magnification revealed tortuous capillaries on the distal cuticles of several fingers.
Bloodwork revealed a creatine kinase level slightly greater than 1000 U/L, and a positive antinuclear antibody, dilution unknown.
Infectious disease pop quiz: Clinical challenge #10 for the ObGyn
What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
Continue to answer...
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
Continue to answer...
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
Continue to answer...
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Hand eczema and atopic dermatitis closely linked
An estimated (AD), according to Jacob P. Thyssen, MD, PhD.
“If we look at individuals with AD, the lifetime prevalence of hand eczema reaches 50%, so we see a strong association between hand eczema and AD,” Dr. Thyssen, professor of dermatology at the University of Copenhagen, said at the Revolutionizing Atopic Dermatitis symposium.
Risk factors for hand eczema – defined as eczema on the hand and/or wrists – include AD, which increases the risk by two- to threefold, as well as genetic predisposition beyond AD, exposure to irritants and allergens, female gender, young age, low socioeconomic group, high risk occupations (including construction workers and hairdressers), and tobacco smoking.
“As clinicians, we sometimes need to rule out a few differentials, including psoriasis and T-cell lymphoma. As an example, 10% of T-cell lymphoma patients, a very rare condition, have first onset of the disease on their hands,” Dr. Thyssen said. “Once we see persistent hand eczema, we need to obtain a history of irritant exposure and allergen exposure, both at home and at work, perform a patch test, sometimes a skin prick test, and ask about personal and family history of AD and psoriasis.”
He noted that while formal classification of hand eczema has been a struggle for decades, he favors the “straightforward” clinical approach from the European Environmental and Contact Dermatitis Research Group. Atopic hand eczema, he said, “is very much characterized by dorsal involvement of the hands and fingers and sparse involvement of the palmar aspects of the hands.”
The cheeks and hands are predilection sites for AD in filaggrin mutation carriers (as they are sites of low filaggrin levels), and sometimes harsh environmental exposures, such as cold and dry air. In a study of 3,335 patients in Denmark, Dr. Thyssen and colleagues found that filaggrin mutations and AD were associated with early-onset and persistent hand eczema. In another study of 3,834 adults with AD or psoriasis, he and colleagues found that among those with AD, the wrists, back of the hands, and interdigital areas were often sites of severe eczema, while palmar involvement was more uncommon.
The same findings apply for the feet in filaggrin mutation carriers with AD; the dorsal aspect of the feet was more commonly affected, compared with plantar aspects of the feet.
Medical literature regarding foot eczema is scarce, but a retrospective cohort study from Germany found that foot eczema and hand eczema often co-occur. Among 723 hand eczema patients, 201 (28%) had concomitant foot eczema. The same morphological features were found on the hands and feet in 71% of patients. Foot eczema was significantly associated with male sex, atopic hand eczema, hyperhidrosis, wearing of safety shoes/boots at work, and tobacco smoking.
In addition, a systematic review and meta-analysis of studies of hand eczema and AD found that there was a 4.29-fold increased risk of hand eczema in individuals with AD, and the risk (lifetime prevalence) of occupational hand eczema was increased by more than twofold. “However, this study could not differentiate between irritant contact dermatitis on the hands and atopic dermatitis,” Dr. Thyssen said. “The studies were not accurate enough to allow for any conclusions.”
A multicenter study of adults with hand eczema in Italy found that the proportion of patients with AD was the highest among those with severe and refractory chronic hand eczema. In addition, certain professions, including those of hairdressers, health professionals, and those in trade work, such as plumbing, were more often associated with chronic hand eczema. “This teaches us that we should be very careful about steering these patients from at-risk occupations,” Dr. Thyssen said. “Also, we should remember to treat them aggressively in the beginning to reduce the risk of severe and refractory chronic hand eczema.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Asian, Arena, Almirall, AbbVie, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi Genzyme.
An estimated (AD), according to Jacob P. Thyssen, MD, PhD.
“If we look at individuals with AD, the lifetime prevalence of hand eczema reaches 50%, so we see a strong association between hand eczema and AD,” Dr. Thyssen, professor of dermatology at the University of Copenhagen, said at the Revolutionizing Atopic Dermatitis symposium.
Risk factors for hand eczema – defined as eczema on the hand and/or wrists – include AD, which increases the risk by two- to threefold, as well as genetic predisposition beyond AD, exposure to irritants and allergens, female gender, young age, low socioeconomic group, high risk occupations (including construction workers and hairdressers), and tobacco smoking.
“As clinicians, we sometimes need to rule out a few differentials, including psoriasis and T-cell lymphoma. As an example, 10% of T-cell lymphoma patients, a very rare condition, have first onset of the disease on their hands,” Dr. Thyssen said. “Once we see persistent hand eczema, we need to obtain a history of irritant exposure and allergen exposure, both at home and at work, perform a patch test, sometimes a skin prick test, and ask about personal and family history of AD and psoriasis.”
He noted that while formal classification of hand eczema has been a struggle for decades, he favors the “straightforward” clinical approach from the European Environmental and Contact Dermatitis Research Group. Atopic hand eczema, he said, “is very much characterized by dorsal involvement of the hands and fingers and sparse involvement of the palmar aspects of the hands.”
The cheeks and hands are predilection sites for AD in filaggrin mutation carriers (as they are sites of low filaggrin levels), and sometimes harsh environmental exposures, such as cold and dry air. In a study of 3,335 patients in Denmark, Dr. Thyssen and colleagues found that filaggrin mutations and AD were associated with early-onset and persistent hand eczema. In another study of 3,834 adults with AD or psoriasis, he and colleagues found that among those with AD, the wrists, back of the hands, and interdigital areas were often sites of severe eczema, while palmar involvement was more uncommon.
The same findings apply for the feet in filaggrin mutation carriers with AD; the dorsal aspect of the feet was more commonly affected, compared with plantar aspects of the feet.
Medical literature regarding foot eczema is scarce, but a retrospective cohort study from Germany found that foot eczema and hand eczema often co-occur. Among 723 hand eczema patients, 201 (28%) had concomitant foot eczema. The same morphological features were found on the hands and feet in 71% of patients. Foot eczema was significantly associated with male sex, atopic hand eczema, hyperhidrosis, wearing of safety shoes/boots at work, and tobacco smoking.
In addition, a systematic review and meta-analysis of studies of hand eczema and AD found that there was a 4.29-fold increased risk of hand eczema in individuals with AD, and the risk (lifetime prevalence) of occupational hand eczema was increased by more than twofold. “However, this study could not differentiate between irritant contact dermatitis on the hands and atopic dermatitis,” Dr. Thyssen said. “The studies were not accurate enough to allow for any conclusions.”
A multicenter study of adults with hand eczema in Italy found that the proportion of patients with AD was the highest among those with severe and refractory chronic hand eczema. In addition, certain professions, including those of hairdressers, health professionals, and those in trade work, such as plumbing, were more often associated with chronic hand eczema. “This teaches us that we should be very careful about steering these patients from at-risk occupations,” Dr. Thyssen said. “Also, we should remember to treat them aggressively in the beginning to reduce the risk of severe and refractory chronic hand eczema.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Asian, Arena, Almirall, AbbVie, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi Genzyme.
An estimated (AD), according to Jacob P. Thyssen, MD, PhD.
“If we look at individuals with AD, the lifetime prevalence of hand eczema reaches 50%, so we see a strong association between hand eczema and AD,” Dr. Thyssen, professor of dermatology at the University of Copenhagen, said at the Revolutionizing Atopic Dermatitis symposium.
Risk factors for hand eczema – defined as eczema on the hand and/or wrists – include AD, which increases the risk by two- to threefold, as well as genetic predisposition beyond AD, exposure to irritants and allergens, female gender, young age, low socioeconomic group, high risk occupations (including construction workers and hairdressers), and tobacco smoking.
“As clinicians, we sometimes need to rule out a few differentials, including psoriasis and T-cell lymphoma. As an example, 10% of T-cell lymphoma patients, a very rare condition, have first onset of the disease on their hands,” Dr. Thyssen said. “Once we see persistent hand eczema, we need to obtain a history of irritant exposure and allergen exposure, both at home and at work, perform a patch test, sometimes a skin prick test, and ask about personal and family history of AD and psoriasis.”
He noted that while formal classification of hand eczema has been a struggle for decades, he favors the “straightforward” clinical approach from the European Environmental and Contact Dermatitis Research Group. Atopic hand eczema, he said, “is very much characterized by dorsal involvement of the hands and fingers and sparse involvement of the palmar aspects of the hands.”
The cheeks and hands are predilection sites for AD in filaggrin mutation carriers (as they are sites of low filaggrin levels), and sometimes harsh environmental exposures, such as cold and dry air. In a study of 3,335 patients in Denmark, Dr. Thyssen and colleagues found that filaggrin mutations and AD were associated with early-onset and persistent hand eczema. In another study of 3,834 adults with AD or psoriasis, he and colleagues found that among those with AD, the wrists, back of the hands, and interdigital areas were often sites of severe eczema, while palmar involvement was more uncommon.
The same findings apply for the feet in filaggrin mutation carriers with AD; the dorsal aspect of the feet was more commonly affected, compared with plantar aspects of the feet.
Medical literature regarding foot eczema is scarce, but a retrospective cohort study from Germany found that foot eczema and hand eczema often co-occur. Among 723 hand eczema patients, 201 (28%) had concomitant foot eczema. The same morphological features were found on the hands and feet in 71% of patients. Foot eczema was significantly associated with male sex, atopic hand eczema, hyperhidrosis, wearing of safety shoes/boots at work, and tobacco smoking.
In addition, a systematic review and meta-analysis of studies of hand eczema and AD found that there was a 4.29-fold increased risk of hand eczema in individuals with AD, and the risk (lifetime prevalence) of occupational hand eczema was increased by more than twofold. “However, this study could not differentiate between irritant contact dermatitis on the hands and atopic dermatitis,” Dr. Thyssen said. “The studies were not accurate enough to allow for any conclusions.”
A multicenter study of adults with hand eczema in Italy found that the proportion of patients with AD was the highest among those with severe and refractory chronic hand eczema. In addition, certain professions, including those of hairdressers, health professionals, and those in trade work, such as plumbing, were more often associated with chronic hand eczema. “This teaches us that we should be very careful about steering these patients from at-risk occupations,” Dr. Thyssen said. “Also, we should remember to treat them aggressively in the beginning to reduce the risk of severe and refractory chronic hand eczema.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Asian, Arena, Almirall, AbbVie, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi Genzyme.
FROM REVOLUTIONIZING AD 2021
Cardiac inflammation can be present after mild COVID infection
Myocardial inflammation is present in a small proportion of patients who have recovered from relatively mild cases of COVID-19 infection, a new study shows.
“Our findings suggest that even in patients who have had relatively mild cases of COVID-19, some will have inflammatory changes to the heart, and these changes can be present without any cardiac symptoms,” senior author, Paaladinesh Thavendiranathan, MD, University of Toronto, told this news organization.
“While our data suggest that this inflammation improves over time, and the outcomes seem positive, we don’t know if there will be any long-term consequences,” he added.
Noting that even a short period of inflammation in the heart may be associated with symptoms or arrhythmias in the longer term, Dr. Thavendiranathan said: “I would recommend that it is best to avoid getting the infection if there is any chance of heart inflammation.”
The study was published online in JAMA Cardiology on Jan. 12.
The authors explain that among patients hospitalized with COVID, early studies suggested that approximately one in four experience cardiovascular injury, defined as an elevation in troponin levels, which was associated with a 5- to 10-fold increase in the risk for death. But there is limited information on cardiac injury in patients who do not require hospitalization.
Although a broad range of abnormal myocardial tissue has been reported in several cardiac MRI studies of patients recovered from COVID infection, there is little understanding of persistent changes in myocardial metabolism in recovered patients, which is a potential concern, given that COVID-19 is associated with systemic inflammation during the acute illness, they say.
For the current study, the researchers examined myocardial inflammation measured using two different methods – cardiac MRI and fluorodeoxyglucose–positron emission tomography (FDG-PET) – in individuals who had recovered from COVID-19 infection and looked at how this related to changes in inflammatory blood markers.
Lead author Kate Hanneman, MD, also from the University of Toronto, explained that FDG-PET imaging is more sensitive than MRI in detecting active inflammation. “Inflammatory cells have a higher uptake of glucose, and FDG-PET imaging is used to look for metabolically active inflammatory tissue that takes up glucose. It gives complementary information to MRI. Cardiac MRI shows structural or functional changes, such as scarring or edema, whereas FDG-PET imaging directly measures metabolic activity related to inflammatory cells.”
The study involved 47 individuals, 51% female, with a mean age of 43 years, who had recently recovered from COVID-19 infection. Of these, the majority had had relatively mild COVID disease, with 85% not requiring hospitalization.
Cardiac imaging was performed a mean of 67 days after the diagnosis of COVID-19. At the time of imaging, 19 participants (40%) reported at least one cardiac symptom, including palpitations, chest pain, and shortness of breath.
Results showed that eight patients (17%) had focal FDG uptake on PET consistent with myocardial inflammation. Compared with those without FDG uptake, patients with focal FDG uptake had higher regional T2, T1, and extracellular volume (colocalizing with focal FDG uptake), higher prevalence of late gadolinium enhancement indicating fibrosis, lower left ventricular ejection fraction, worse global longitudinal and circumferential strain, and higher systemic inflammatory blood markers, including interleukin (IL)-6, IL- 8, an high-sensitivity C-reactive protein.
Of the 47 patients in the study, 13 had received at least one dose of a COVID-19 vaccine. There was no significant difference in the proportion of patients who were PET-positive among those who had received a COVID-19 vaccine and those who had not.
There was also no difference in inflammation in patients who had been hospitalized with COVID-19 and those who had managed their infection at home.
Among patients with focal FDG uptake, PET, MRI, and inflammatory blood markers improved at follow-up imaging performed a mean of 52 days after the first imaging. The authors say this suggests that these abnormalities were not related to pre-existing cardiovascular disease.
Of the eight patients with positive FDG-PET results, two did not show any MRI abnormalities. These two patients also had elevated inflammatory biomarkers. “PET is a more sensitive method of measuring cardiac inflammation, and our results show that these changes may not always translate into functional changes seen on MRI,” Dr. Thavendiranathan noted.
The only cardiac risk factor that was more common in participants with FDG uptake was hypertension. Although cardiac symptoms were nearly twice as common in participants with focal FDG uptake, this difference was not statistically significant.
“Given the growing number of survivors with similar symptoms, these interesting findings warrant further investigation,” the authors say.
Noting that FDG uptake correlated with elevations in systemic inflammatory biomarkers, the researchers suggest that “a more intense systemic inflammatory process may be contributing to cardiac inflammation and the consequential alteration to regional and global myocardial function in PET-positive participants.”
On repeat imaging 2 months later, all eight patients who showed FDG uptake showed improvement or resolution of inflammation without any treatment, although two patients still had some signs of inflammation. Blood biomarkers also improved on follow-up.
“This is encouraging information, but we need longer-term data to see if there are any long-term repercussions of this inflammation,” Dr. Hanneman said.
“Overall, the study findings suggest an imaging phenotype that is expected to have good prognosis. However, longer-term follow-up studies are required to understand the need for ongoing cardiac surveillance, relationship to cardiac symptoms, guidance for safe return to exercise and sports participation, and long-term cardiovascular disease risk,” the researchers state.
This study was funded by grants from the Joint Department of Medical Imaging Academic Incentive Fund, Peter Munk Cardiac Center Innovation Committee, and Ted Rogers Center for Heart Research. Dr. Hanneman reports personal fees from Sanofi Genzyme, Amicus, and Medscape outside the submitted work.
A version of this article first appeared on Medscape.com.
Myocardial inflammation is present in a small proportion of patients who have recovered from relatively mild cases of COVID-19 infection, a new study shows.
“Our findings suggest that even in patients who have had relatively mild cases of COVID-19, some will have inflammatory changes to the heart, and these changes can be present without any cardiac symptoms,” senior author, Paaladinesh Thavendiranathan, MD, University of Toronto, told this news organization.
“While our data suggest that this inflammation improves over time, and the outcomes seem positive, we don’t know if there will be any long-term consequences,” he added.
Noting that even a short period of inflammation in the heart may be associated with symptoms or arrhythmias in the longer term, Dr. Thavendiranathan said: “I would recommend that it is best to avoid getting the infection if there is any chance of heart inflammation.”
The study was published online in JAMA Cardiology on Jan. 12.
The authors explain that among patients hospitalized with COVID, early studies suggested that approximately one in four experience cardiovascular injury, defined as an elevation in troponin levels, which was associated with a 5- to 10-fold increase in the risk for death. But there is limited information on cardiac injury in patients who do not require hospitalization.
Although a broad range of abnormal myocardial tissue has been reported in several cardiac MRI studies of patients recovered from COVID infection, there is little understanding of persistent changes in myocardial metabolism in recovered patients, which is a potential concern, given that COVID-19 is associated with systemic inflammation during the acute illness, they say.
For the current study, the researchers examined myocardial inflammation measured using two different methods – cardiac MRI and fluorodeoxyglucose–positron emission tomography (FDG-PET) – in individuals who had recovered from COVID-19 infection and looked at how this related to changes in inflammatory blood markers.
Lead author Kate Hanneman, MD, also from the University of Toronto, explained that FDG-PET imaging is more sensitive than MRI in detecting active inflammation. “Inflammatory cells have a higher uptake of glucose, and FDG-PET imaging is used to look for metabolically active inflammatory tissue that takes up glucose. It gives complementary information to MRI. Cardiac MRI shows structural or functional changes, such as scarring or edema, whereas FDG-PET imaging directly measures metabolic activity related to inflammatory cells.”
The study involved 47 individuals, 51% female, with a mean age of 43 years, who had recently recovered from COVID-19 infection. Of these, the majority had had relatively mild COVID disease, with 85% not requiring hospitalization.
Cardiac imaging was performed a mean of 67 days after the diagnosis of COVID-19. At the time of imaging, 19 participants (40%) reported at least one cardiac symptom, including palpitations, chest pain, and shortness of breath.
Results showed that eight patients (17%) had focal FDG uptake on PET consistent with myocardial inflammation. Compared with those without FDG uptake, patients with focal FDG uptake had higher regional T2, T1, and extracellular volume (colocalizing with focal FDG uptake), higher prevalence of late gadolinium enhancement indicating fibrosis, lower left ventricular ejection fraction, worse global longitudinal and circumferential strain, and higher systemic inflammatory blood markers, including interleukin (IL)-6, IL- 8, an high-sensitivity C-reactive protein.
Of the 47 patients in the study, 13 had received at least one dose of a COVID-19 vaccine. There was no significant difference in the proportion of patients who were PET-positive among those who had received a COVID-19 vaccine and those who had not.
There was also no difference in inflammation in patients who had been hospitalized with COVID-19 and those who had managed their infection at home.
Among patients with focal FDG uptake, PET, MRI, and inflammatory blood markers improved at follow-up imaging performed a mean of 52 days after the first imaging. The authors say this suggests that these abnormalities were not related to pre-existing cardiovascular disease.
Of the eight patients with positive FDG-PET results, two did not show any MRI abnormalities. These two patients also had elevated inflammatory biomarkers. “PET is a more sensitive method of measuring cardiac inflammation, and our results show that these changes may not always translate into functional changes seen on MRI,” Dr. Thavendiranathan noted.
The only cardiac risk factor that was more common in participants with FDG uptake was hypertension. Although cardiac symptoms were nearly twice as common in participants with focal FDG uptake, this difference was not statistically significant.
“Given the growing number of survivors with similar symptoms, these interesting findings warrant further investigation,” the authors say.
Noting that FDG uptake correlated with elevations in systemic inflammatory biomarkers, the researchers suggest that “a more intense systemic inflammatory process may be contributing to cardiac inflammation and the consequential alteration to regional and global myocardial function in PET-positive participants.”
On repeat imaging 2 months later, all eight patients who showed FDG uptake showed improvement or resolution of inflammation without any treatment, although two patients still had some signs of inflammation. Blood biomarkers also improved on follow-up.
“This is encouraging information, but we need longer-term data to see if there are any long-term repercussions of this inflammation,” Dr. Hanneman said.
“Overall, the study findings suggest an imaging phenotype that is expected to have good prognosis. However, longer-term follow-up studies are required to understand the need for ongoing cardiac surveillance, relationship to cardiac symptoms, guidance for safe return to exercise and sports participation, and long-term cardiovascular disease risk,” the researchers state.
This study was funded by grants from the Joint Department of Medical Imaging Academic Incentive Fund, Peter Munk Cardiac Center Innovation Committee, and Ted Rogers Center for Heart Research. Dr. Hanneman reports personal fees from Sanofi Genzyme, Amicus, and Medscape outside the submitted work.
A version of this article first appeared on Medscape.com.
Myocardial inflammation is present in a small proportion of patients who have recovered from relatively mild cases of COVID-19 infection, a new study shows.
“Our findings suggest that even in patients who have had relatively mild cases of COVID-19, some will have inflammatory changes to the heart, and these changes can be present without any cardiac symptoms,” senior author, Paaladinesh Thavendiranathan, MD, University of Toronto, told this news organization.
“While our data suggest that this inflammation improves over time, and the outcomes seem positive, we don’t know if there will be any long-term consequences,” he added.
Noting that even a short period of inflammation in the heart may be associated with symptoms or arrhythmias in the longer term, Dr. Thavendiranathan said: “I would recommend that it is best to avoid getting the infection if there is any chance of heart inflammation.”
The study was published online in JAMA Cardiology on Jan. 12.
The authors explain that among patients hospitalized with COVID, early studies suggested that approximately one in four experience cardiovascular injury, defined as an elevation in troponin levels, which was associated with a 5- to 10-fold increase in the risk for death. But there is limited information on cardiac injury in patients who do not require hospitalization.
Although a broad range of abnormal myocardial tissue has been reported in several cardiac MRI studies of patients recovered from COVID infection, there is little understanding of persistent changes in myocardial metabolism in recovered patients, which is a potential concern, given that COVID-19 is associated with systemic inflammation during the acute illness, they say.
For the current study, the researchers examined myocardial inflammation measured using two different methods – cardiac MRI and fluorodeoxyglucose–positron emission tomography (FDG-PET) – in individuals who had recovered from COVID-19 infection and looked at how this related to changes in inflammatory blood markers.
Lead author Kate Hanneman, MD, also from the University of Toronto, explained that FDG-PET imaging is more sensitive than MRI in detecting active inflammation. “Inflammatory cells have a higher uptake of glucose, and FDG-PET imaging is used to look for metabolically active inflammatory tissue that takes up glucose. It gives complementary information to MRI. Cardiac MRI shows structural or functional changes, such as scarring or edema, whereas FDG-PET imaging directly measures metabolic activity related to inflammatory cells.”
The study involved 47 individuals, 51% female, with a mean age of 43 years, who had recently recovered from COVID-19 infection. Of these, the majority had had relatively mild COVID disease, with 85% not requiring hospitalization.
Cardiac imaging was performed a mean of 67 days after the diagnosis of COVID-19. At the time of imaging, 19 participants (40%) reported at least one cardiac symptom, including palpitations, chest pain, and shortness of breath.
Results showed that eight patients (17%) had focal FDG uptake on PET consistent with myocardial inflammation. Compared with those without FDG uptake, patients with focal FDG uptake had higher regional T2, T1, and extracellular volume (colocalizing with focal FDG uptake), higher prevalence of late gadolinium enhancement indicating fibrosis, lower left ventricular ejection fraction, worse global longitudinal and circumferential strain, and higher systemic inflammatory blood markers, including interleukin (IL)-6, IL- 8, an high-sensitivity C-reactive protein.
Of the 47 patients in the study, 13 had received at least one dose of a COVID-19 vaccine. There was no significant difference in the proportion of patients who were PET-positive among those who had received a COVID-19 vaccine and those who had not.
There was also no difference in inflammation in patients who had been hospitalized with COVID-19 and those who had managed their infection at home.
Among patients with focal FDG uptake, PET, MRI, and inflammatory blood markers improved at follow-up imaging performed a mean of 52 days after the first imaging. The authors say this suggests that these abnormalities were not related to pre-existing cardiovascular disease.
Of the eight patients with positive FDG-PET results, two did not show any MRI abnormalities. These two patients also had elevated inflammatory biomarkers. “PET is a more sensitive method of measuring cardiac inflammation, and our results show that these changes may not always translate into functional changes seen on MRI,” Dr. Thavendiranathan noted.
The only cardiac risk factor that was more common in participants with FDG uptake was hypertension. Although cardiac symptoms were nearly twice as common in participants with focal FDG uptake, this difference was not statistically significant.
“Given the growing number of survivors with similar symptoms, these interesting findings warrant further investigation,” the authors say.
Noting that FDG uptake correlated with elevations in systemic inflammatory biomarkers, the researchers suggest that “a more intense systemic inflammatory process may be contributing to cardiac inflammation and the consequential alteration to regional and global myocardial function in PET-positive participants.”
On repeat imaging 2 months later, all eight patients who showed FDG uptake showed improvement or resolution of inflammation without any treatment, although two patients still had some signs of inflammation. Blood biomarkers also improved on follow-up.
“This is encouraging information, but we need longer-term data to see if there are any long-term repercussions of this inflammation,” Dr. Hanneman said.
“Overall, the study findings suggest an imaging phenotype that is expected to have good prognosis. However, longer-term follow-up studies are required to understand the need for ongoing cardiac surveillance, relationship to cardiac symptoms, guidance for safe return to exercise and sports participation, and long-term cardiovascular disease risk,” the researchers state.
This study was funded by grants from the Joint Department of Medical Imaging Academic Incentive Fund, Peter Munk Cardiac Center Innovation Committee, and Ted Rogers Center for Heart Research. Dr. Hanneman reports personal fees from Sanofi Genzyme, Amicus, and Medscape outside the submitted work.
A version of this article first appeared on Medscape.com.
Private insurers must cover follow-up colonoscopies
Private insurers are now required to cover the cost of follow-up colonoscopies after a positive stool-based test, according to updated guidance from the Biden administration cited in a press release from the American Gastroenterological Association.
“Now patients can choose the best colorectal cancer screening test for them without fear of a surprise bill. Patients have full coverage of the full screening continuum – from an initial stool or endoscopic test to a follow-up colonoscopy. Now that the financial barriers have been eliminated, we can focus on increasing screening so we can prevent cancer deaths,” John Inadomi, MD, president of the AGA, said in the press release.
The updated guidance, issued on Jan. 10, 2022, “will prevent patients from receiving surprise bills for a colonoscopy when they receive a positive result from a stool-based test,” according to the AGA press release.
In 2016, the U.S. Preventive Services Task Force recommended colorectal cancer screening for all adults starting at age 50 years and continuing to age 75 years, with an “A” rating. Because the Affordable Care Act (ACA) mandated coverage for preventive screenings without cost-sharing that receive an “A” or “B” grade from the USPSTF, previous statements have confirmed that cost sharing may not be imposed on patients for screening in accordance with the USPSTF recommendation, which included specialist consultation prior to the procedure, bowel prep medications, anesthesia services in conjunction with a preventive colonoscopy, polyp removal performed during the screening procedure, and any pathology exam on a polyp biopsy performed as part of the screening. By adding colonoscopies following positive stool tests to that list, the updated guidance means that all aspects of the screening procedure are now covered without cost sharing.
In May 2021, an update to the USPSTF recommendations called for a follow-up colonoscopy in the wake of a positive test: “Positive results on stool-based screening tests require follow-up with colonoscopy for the screening benefits to be achieved.” The 2021 update also extended the screening recommendation to adults aged 45-49 years with a “B” rating.
Private insurers must now pay for follow-up colonoscopy as needed in addition to the initial noninvasive screening, according to the guidance.
The updated guidance is presented as part of a series of frequently asked questions regarding implementation of the Families First Coronavirus Response Act, the Coronavirus Aid, Relief, and Economic Security Act, and the Affordable Care Act. The colonoscopy guidance falls under the heading of “Coverage of Preventive Services,” which includes evidence-based recommendations given an A or B rating by the USPSTF.
Coverage without cost sharing must begin on or after May 31, 2022, which is 1 year after the date of the latest recommendations, according to the FAQ.
Representatives of multiple organizations, including the AGA, American Cancer Society, American Cancer Society Cancer Action Network, and Fight CRC collaborated to promote the additional coverage. “We applaud the administration for supporting coverage of the full colorectal cancer screening continuum, which will improve access to lifesaving screening,” the collaborators said in the press release.
Colorectal cancer remains the second leading cancer killer in the United States, but only two-thirds of eligible individuals were screened in 2018, according to the AGA, and screening challenges were exacerbated by the arrival of the COVID-19 pandemic. The AGA estimates that colorectal cancer screening declined by 86% during the first few months of the COVID-19 pandemic in 2020.
The full Jan. 10 FAQ is available here.
This article was updated Jan. 14, 2022.
Private insurers are now required to cover the cost of follow-up colonoscopies after a positive stool-based test, according to updated guidance from the Biden administration cited in a press release from the American Gastroenterological Association.
“Now patients can choose the best colorectal cancer screening test for them without fear of a surprise bill. Patients have full coverage of the full screening continuum – from an initial stool or endoscopic test to a follow-up colonoscopy. Now that the financial barriers have been eliminated, we can focus on increasing screening so we can prevent cancer deaths,” John Inadomi, MD, president of the AGA, said in the press release.
The updated guidance, issued on Jan. 10, 2022, “will prevent patients from receiving surprise bills for a colonoscopy when they receive a positive result from a stool-based test,” according to the AGA press release.
In 2016, the U.S. Preventive Services Task Force recommended colorectal cancer screening for all adults starting at age 50 years and continuing to age 75 years, with an “A” rating. Because the Affordable Care Act (ACA) mandated coverage for preventive screenings without cost-sharing that receive an “A” or “B” grade from the USPSTF, previous statements have confirmed that cost sharing may not be imposed on patients for screening in accordance with the USPSTF recommendation, which included specialist consultation prior to the procedure, bowel prep medications, anesthesia services in conjunction with a preventive colonoscopy, polyp removal performed during the screening procedure, and any pathology exam on a polyp biopsy performed as part of the screening. By adding colonoscopies following positive stool tests to that list, the updated guidance means that all aspects of the screening procedure are now covered without cost sharing.
In May 2021, an update to the USPSTF recommendations called for a follow-up colonoscopy in the wake of a positive test: “Positive results on stool-based screening tests require follow-up with colonoscopy for the screening benefits to be achieved.” The 2021 update also extended the screening recommendation to adults aged 45-49 years with a “B” rating.
Private insurers must now pay for follow-up colonoscopy as needed in addition to the initial noninvasive screening, according to the guidance.
The updated guidance is presented as part of a series of frequently asked questions regarding implementation of the Families First Coronavirus Response Act, the Coronavirus Aid, Relief, and Economic Security Act, and the Affordable Care Act. The colonoscopy guidance falls under the heading of “Coverage of Preventive Services,” which includes evidence-based recommendations given an A or B rating by the USPSTF.
Coverage without cost sharing must begin on or after May 31, 2022, which is 1 year after the date of the latest recommendations, according to the FAQ.
Representatives of multiple organizations, including the AGA, American Cancer Society, American Cancer Society Cancer Action Network, and Fight CRC collaborated to promote the additional coverage. “We applaud the administration for supporting coverage of the full colorectal cancer screening continuum, which will improve access to lifesaving screening,” the collaborators said in the press release.
Colorectal cancer remains the second leading cancer killer in the United States, but only two-thirds of eligible individuals were screened in 2018, according to the AGA, and screening challenges were exacerbated by the arrival of the COVID-19 pandemic. The AGA estimates that colorectal cancer screening declined by 86% during the first few months of the COVID-19 pandemic in 2020.
The full Jan. 10 FAQ is available here.
This article was updated Jan. 14, 2022.
Private insurers are now required to cover the cost of follow-up colonoscopies after a positive stool-based test, according to updated guidance from the Biden administration cited in a press release from the American Gastroenterological Association.
“Now patients can choose the best colorectal cancer screening test for them without fear of a surprise bill. Patients have full coverage of the full screening continuum – from an initial stool or endoscopic test to a follow-up colonoscopy. Now that the financial barriers have been eliminated, we can focus on increasing screening so we can prevent cancer deaths,” John Inadomi, MD, president of the AGA, said in the press release.
The updated guidance, issued on Jan. 10, 2022, “will prevent patients from receiving surprise bills for a colonoscopy when they receive a positive result from a stool-based test,” according to the AGA press release.
In 2016, the U.S. Preventive Services Task Force recommended colorectal cancer screening for all adults starting at age 50 years and continuing to age 75 years, with an “A” rating. Because the Affordable Care Act (ACA) mandated coverage for preventive screenings without cost-sharing that receive an “A” or “B” grade from the USPSTF, previous statements have confirmed that cost sharing may not be imposed on patients for screening in accordance with the USPSTF recommendation, which included specialist consultation prior to the procedure, bowel prep medications, anesthesia services in conjunction with a preventive colonoscopy, polyp removal performed during the screening procedure, and any pathology exam on a polyp biopsy performed as part of the screening. By adding colonoscopies following positive stool tests to that list, the updated guidance means that all aspects of the screening procedure are now covered without cost sharing.
In May 2021, an update to the USPSTF recommendations called for a follow-up colonoscopy in the wake of a positive test: “Positive results on stool-based screening tests require follow-up with colonoscopy for the screening benefits to be achieved.” The 2021 update also extended the screening recommendation to adults aged 45-49 years with a “B” rating.
Private insurers must now pay for follow-up colonoscopy as needed in addition to the initial noninvasive screening, according to the guidance.
The updated guidance is presented as part of a series of frequently asked questions regarding implementation of the Families First Coronavirus Response Act, the Coronavirus Aid, Relief, and Economic Security Act, and the Affordable Care Act. The colonoscopy guidance falls under the heading of “Coverage of Preventive Services,” which includes evidence-based recommendations given an A or B rating by the USPSTF.
Coverage without cost sharing must begin on or after May 31, 2022, which is 1 year after the date of the latest recommendations, according to the FAQ.
Representatives of multiple organizations, including the AGA, American Cancer Society, American Cancer Society Cancer Action Network, and Fight CRC collaborated to promote the additional coverage. “We applaud the administration for supporting coverage of the full colorectal cancer screening continuum, which will improve access to lifesaving screening,” the collaborators said in the press release.
Colorectal cancer remains the second leading cancer killer in the United States, but only two-thirds of eligible individuals were screened in 2018, according to the AGA, and screening challenges were exacerbated by the arrival of the COVID-19 pandemic. The AGA estimates that colorectal cancer screening declined by 86% during the first few months of the COVID-19 pandemic in 2020.
The full Jan. 10 FAQ is available here.
This article was updated Jan. 14, 2022.
Virtual reality making progress as depression treatment
Virtual reality (VR) has been taking positive steps in a variety of treatment areas for some time. Now a Japanese company is asking the question: Can people with depression benefit from watching VR scenarios in which actors portray characters coping with the condition?
That’s the assertion of the Tokyo-based company Jolly Good, a VR start-up that introduced the U.S. version of its VRDTx program at the annual meeting of the Consumer Electronics Show.
“Using this as an adjunct for psychotherapy to help someone see an example of someone who’s struggling with depression can be a helpful tool,” said Katharine Larsson, PhD, RN, clinical director of Boston Behavioral Medicine in Brookline. Larsson and her BBM colleague, Amaro J. Laria, PhD, are helping Jolly Good to adapt the program for use in the United States.
VRDTx uses techniques from cognitive-behavioral therapy (CBT). Donning goggles, viewers watch people acting out situations common to depression.
One technique frequently used in CBT is to make a detailed plan, Dr. Laria said. For example, VRDTx users might watch a character with depression struggling to get out of bed but resolving to get up for at least 10 minutes one day, to go for a walk the next day, etc. “The virtual reality allows you to watch a person actually going through the process of applying the intervention,” he said.
In this way, the program could work like hypnotherapy or imaginal therapy where patients picture themselves in a situation that might trigger their depression and then picture themselves coping with that situation.
Dr. Larsson advised using the program primarily as a sort of homework. “ she said. “Using it to substitute or replace the time with a therapist, I don’t think it could begin to have any kind of real efficacy.”
Deploying virtual reality to treat mood disorders is not new, said Preethi Premkumar, PhD, a senior lecturer in psychology at London South Bank University, who has no relationship to Jolly Good.
Dr. Premkumar is first author of a study of a VR program used to treat people who have anxiety about speaking in public. The program depicts the user speaking before an audience and allows the user to vary the number of people in the audience and the audience’s reactions. The users gave it high marks, Dr. Premkumar said. “They felt that it encouraged them to take on public speaking more in reality.”
VR could work in a similar way for depressed people because they tend to catastrophize about specific situations. “Virtual reality can recreate those scenes and then make people confront it without overexposing them,” Dr. Premkumar said.
One recent review article found several studies on VR as a treatment for anxiety. While only a handful focused on depression, they had mostly favorable results.
Jolly Good sponsored one such study, presented Sept. 17, 2021, at the European Association for Behavioural and Cognitive Therapies. “Results indicate improvement in the scores of the targeted patients with depression,” according to an abstract the company published online. “Use of VR caused no adverse events, demonstrating that VR can be used safely in the CBT for of depression.” The company did not respond to a request for more details.
After viewing scenarios created for Japanese patients, Dr. Larsson and Dr. Laria offered Jolly Good several tips about making the transition to the United States. The actors should be more emotionally expressive. They should portray a more diverse cast of characters, including some female bosses. And not all scenes should be set in the workplace.
“In the U.S., at least in our experience, a lot of what depressed patients talk about is just their personal lives, their intimate relationship with a significant other, family relations, friends,” Dr. Laria said. “We gave them a whole list of topics that we felt would be more relevant for a U.S. audience.”
Dr. Larsson and Dr. Laria are consultants to Jolly Good. Dr. Premkumar reported no relevant financial interests.
A version of this article first appeared on Medscape.com.
Virtual reality (VR) has been taking positive steps in a variety of treatment areas for some time. Now a Japanese company is asking the question: Can people with depression benefit from watching VR scenarios in which actors portray characters coping with the condition?
That’s the assertion of the Tokyo-based company Jolly Good, a VR start-up that introduced the U.S. version of its VRDTx program at the annual meeting of the Consumer Electronics Show.
“Using this as an adjunct for psychotherapy to help someone see an example of someone who’s struggling with depression can be a helpful tool,” said Katharine Larsson, PhD, RN, clinical director of Boston Behavioral Medicine in Brookline. Larsson and her BBM colleague, Amaro J. Laria, PhD, are helping Jolly Good to adapt the program for use in the United States.
VRDTx uses techniques from cognitive-behavioral therapy (CBT). Donning goggles, viewers watch people acting out situations common to depression.
One technique frequently used in CBT is to make a detailed plan, Dr. Laria said. For example, VRDTx users might watch a character with depression struggling to get out of bed but resolving to get up for at least 10 minutes one day, to go for a walk the next day, etc. “The virtual reality allows you to watch a person actually going through the process of applying the intervention,” he said.
In this way, the program could work like hypnotherapy or imaginal therapy where patients picture themselves in a situation that might trigger their depression and then picture themselves coping with that situation.
Dr. Larsson advised using the program primarily as a sort of homework. “ she said. “Using it to substitute or replace the time with a therapist, I don’t think it could begin to have any kind of real efficacy.”
Deploying virtual reality to treat mood disorders is not new, said Preethi Premkumar, PhD, a senior lecturer in psychology at London South Bank University, who has no relationship to Jolly Good.
Dr. Premkumar is first author of a study of a VR program used to treat people who have anxiety about speaking in public. The program depicts the user speaking before an audience and allows the user to vary the number of people in the audience and the audience’s reactions. The users gave it high marks, Dr. Premkumar said. “They felt that it encouraged them to take on public speaking more in reality.”
VR could work in a similar way for depressed people because they tend to catastrophize about specific situations. “Virtual reality can recreate those scenes and then make people confront it without overexposing them,” Dr. Premkumar said.
One recent review article found several studies on VR as a treatment for anxiety. While only a handful focused on depression, they had mostly favorable results.
Jolly Good sponsored one such study, presented Sept. 17, 2021, at the European Association for Behavioural and Cognitive Therapies. “Results indicate improvement in the scores of the targeted patients with depression,” according to an abstract the company published online. “Use of VR caused no adverse events, demonstrating that VR can be used safely in the CBT for of depression.” The company did not respond to a request for more details.
After viewing scenarios created for Japanese patients, Dr. Larsson and Dr. Laria offered Jolly Good several tips about making the transition to the United States. The actors should be more emotionally expressive. They should portray a more diverse cast of characters, including some female bosses. And not all scenes should be set in the workplace.
“In the U.S., at least in our experience, a lot of what depressed patients talk about is just their personal lives, their intimate relationship with a significant other, family relations, friends,” Dr. Laria said. “We gave them a whole list of topics that we felt would be more relevant for a U.S. audience.”
Dr. Larsson and Dr. Laria are consultants to Jolly Good. Dr. Premkumar reported no relevant financial interests.
A version of this article first appeared on Medscape.com.
Virtual reality (VR) has been taking positive steps in a variety of treatment areas for some time. Now a Japanese company is asking the question: Can people with depression benefit from watching VR scenarios in which actors portray characters coping with the condition?
That’s the assertion of the Tokyo-based company Jolly Good, a VR start-up that introduced the U.S. version of its VRDTx program at the annual meeting of the Consumer Electronics Show.
“Using this as an adjunct for psychotherapy to help someone see an example of someone who’s struggling with depression can be a helpful tool,” said Katharine Larsson, PhD, RN, clinical director of Boston Behavioral Medicine in Brookline. Larsson and her BBM colleague, Amaro J. Laria, PhD, are helping Jolly Good to adapt the program for use in the United States.
VRDTx uses techniques from cognitive-behavioral therapy (CBT). Donning goggles, viewers watch people acting out situations common to depression.
One technique frequently used in CBT is to make a detailed plan, Dr. Laria said. For example, VRDTx users might watch a character with depression struggling to get out of bed but resolving to get up for at least 10 minutes one day, to go for a walk the next day, etc. “The virtual reality allows you to watch a person actually going through the process of applying the intervention,” he said.
In this way, the program could work like hypnotherapy or imaginal therapy where patients picture themselves in a situation that might trigger their depression and then picture themselves coping with that situation.
Dr. Larsson advised using the program primarily as a sort of homework. “ she said. “Using it to substitute or replace the time with a therapist, I don’t think it could begin to have any kind of real efficacy.”
Deploying virtual reality to treat mood disorders is not new, said Preethi Premkumar, PhD, a senior lecturer in psychology at London South Bank University, who has no relationship to Jolly Good.
Dr. Premkumar is first author of a study of a VR program used to treat people who have anxiety about speaking in public. The program depicts the user speaking before an audience and allows the user to vary the number of people in the audience and the audience’s reactions. The users gave it high marks, Dr. Premkumar said. “They felt that it encouraged them to take on public speaking more in reality.”
VR could work in a similar way for depressed people because they tend to catastrophize about specific situations. “Virtual reality can recreate those scenes and then make people confront it without overexposing them,” Dr. Premkumar said.
One recent review article found several studies on VR as a treatment for anxiety. While only a handful focused on depression, they had mostly favorable results.
Jolly Good sponsored one such study, presented Sept. 17, 2021, at the European Association for Behavioural and Cognitive Therapies. “Results indicate improvement in the scores of the targeted patients with depression,” according to an abstract the company published online. “Use of VR caused no adverse events, demonstrating that VR can be used safely in the CBT for of depression.” The company did not respond to a request for more details.
After viewing scenarios created for Japanese patients, Dr. Larsson and Dr. Laria offered Jolly Good several tips about making the transition to the United States. The actors should be more emotionally expressive. They should portray a more diverse cast of characters, including some female bosses. And not all scenes should be set in the workplace.
“In the U.S., at least in our experience, a lot of what depressed patients talk about is just their personal lives, their intimate relationship with a significant other, family relations, friends,” Dr. Laria said. “We gave them a whole list of topics that we felt would be more relevant for a U.S. audience.”
Dr. Larsson and Dr. Laria are consultants to Jolly Good. Dr. Premkumar reported no relevant financial interests.
A version of this article first appeared on Medscape.com.
Wilderness Medical Society issues clinical guidelines for tick-borne illness
The recently published “Clinical Practice Guidelines for the Prevention and Management of Tick-Borne Illness,” from the Wilderness Medical Society, are a good compilation of treatment suggestions but are not, in fact, new recommendations, lead author Benjamin Ho, MD, of Southern Wisconsin Emergency Associates in Janesville, acknowledged in an interview.
Dr. Ho emphasized that the focus of the report was on “practitioners who practice in resource-limited settings” and are “the group’s way of solidifying a ... standard of practice” for such physicians. Dr. Ho also said that, while “a lot of the recommendations aren’t well supported, the risk-benefit ratio, we believe, supports the recommendations.”
The article first reviewed the different types of ticks and their distribution in the United States, the specific pathogen associated with each, the disease it causes, and comments about seasonal variations in biting behavior. Another table outlines the most common clinical syndromes, typical lab findings, recommended diagnostic testing, and antibiotic treatments. A third section contains images of different types of ticks and photos of ticks in various life-cycle stages and different levels of engorgement.
The authors were careful to note: “Several tick species are able to carry multiple pathogens. In one study, nearly 25% of Ixodes were coinfected with some combination of the bacteria or parasites causing Lyme disease, anaplasmosis, or babesiosis. Although TBI [tick-borne illness] diagnosis is not the focus of this [clinical practice guideline], providers should be aware of high rates of coinfection; the presence of one TBI should in many instances prompt testing for others.”
In terms of recommendations for preventing TBIs, the authors challenge the suggestion of wearing light-colored clothing. For repellents, they recommend DEET, picaridin, and permethrin. And they also give instructions for laundering clothing and removing ticks.
One recommendation is controversial: that of providing single-dose doxycycline as prophylaxis against Lyme disease. Dr. Ho stresses that this was only for “high-risk” tick bites, defined as a tick bite from an identified Ixodes vector species in which the tick was attached for at least 36 hours and that occurred in an endemic area.
The recommendation for prophylactic doxycycline originated with an article by Robert Nadelman and colleagues in the New England Journal of Medicine and has been strongly challenged by ILADS (International Lyme and Associated Diseases Society) physicians, including Daniel Cameron, MD, and others.
Sam Donta, MD, a recent member of the Department of Health & Human Services Tick-borne Working Group and a member of the Infectious Disease Society of America, said in an interview: “The problem with the one-dose doxycycline is you may not begin to develop symptoms until 2 months later.” It might mask the early symptoms of Lyme. “My impression is that the doxycycline – even the single dose – might have abrogated the ability to see an immune response. The idea, though, if you’ve had a tick bite, is to do nothing and to wait for symptoms to develop. That becomes a little bit more complex. But even then, you could choose to follow the patient and see the patient in 2 weeks and then get blood testing.”
Dr. Donta added: “I think the screening test is inadequate. So you have to go directly to the Western blot. And you have to do both the IgM and IgG” and look for specific bands.
Dr. Donta emphasized that patients should be encouraged to save any ticks that were attached and that, if at all possible, ticks should be sent to a reference lab for testing before committing a patient to a course of antibiotics. There is no harm in that brief delay, he said, and most labs can identify an array of pathogens.
The Wilderness Society guidelines on TBIs provide a good overview for clinicians practicing in limited resource settings and mirror those from the IDSA.
Dr. Ho and Dr. Donta reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recently published “Clinical Practice Guidelines for the Prevention and Management of Tick-Borne Illness,” from the Wilderness Medical Society, are a good compilation of treatment suggestions but are not, in fact, new recommendations, lead author Benjamin Ho, MD, of Southern Wisconsin Emergency Associates in Janesville, acknowledged in an interview.
Dr. Ho emphasized that the focus of the report was on “practitioners who practice in resource-limited settings” and are “the group’s way of solidifying a ... standard of practice” for such physicians. Dr. Ho also said that, while “a lot of the recommendations aren’t well supported, the risk-benefit ratio, we believe, supports the recommendations.”
The article first reviewed the different types of ticks and their distribution in the United States, the specific pathogen associated with each, the disease it causes, and comments about seasonal variations in biting behavior. Another table outlines the most common clinical syndromes, typical lab findings, recommended diagnostic testing, and antibiotic treatments. A third section contains images of different types of ticks and photos of ticks in various life-cycle stages and different levels of engorgement.
The authors were careful to note: “Several tick species are able to carry multiple pathogens. In one study, nearly 25% of Ixodes were coinfected with some combination of the bacteria or parasites causing Lyme disease, anaplasmosis, or babesiosis. Although TBI [tick-borne illness] diagnosis is not the focus of this [clinical practice guideline], providers should be aware of high rates of coinfection; the presence of one TBI should in many instances prompt testing for others.”
In terms of recommendations for preventing TBIs, the authors challenge the suggestion of wearing light-colored clothing. For repellents, they recommend DEET, picaridin, and permethrin. And they also give instructions for laundering clothing and removing ticks.
One recommendation is controversial: that of providing single-dose doxycycline as prophylaxis against Lyme disease. Dr. Ho stresses that this was only for “high-risk” tick bites, defined as a tick bite from an identified Ixodes vector species in which the tick was attached for at least 36 hours and that occurred in an endemic area.
The recommendation for prophylactic doxycycline originated with an article by Robert Nadelman and colleagues in the New England Journal of Medicine and has been strongly challenged by ILADS (International Lyme and Associated Diseases Society) physicians, including Daniel Cameron, MD, and others.
Sam Donta, MD, a recent member of the Department of Health & Human Services Tick-borne Working Group and a member of the Infectious Disease Society of America, said in an interview: “The problem with the one-dose doxycycline is you may not begin to develop symptoms until 2 months later.” It might mask the early symptoms of Lyme. “My impression is that the doxycycline – even the single dose – might have abrogated the ability to see an immune response. The idea, though, if you’ve had a tick bite, is to do nothing and to wait for symptoms to develop. That becomes a little bit more complex. But even then, you could choose to follow the patient and see the patient in 2 weeks and then get blood testing.”
Dr. Donta added: “I think the screening test is inadequate. So you have to go directly to the Western blot. And you have to do both the IgM and IgG” and look for specific bands.
Dr. Donta emphasized that patients should be encouraged to save any ticks that were attached and that, if at all possible, ticks should be sent to a reference lab for testing before committing a patient to a course of antibiotics. There is no harm in that brief delay, he said, and most labs can identify an array of pathogens.
The Wilderness Society guidelines on TBIs provide a good overview for clinicians practicing in limited resource settings and mirror those from the IDSA.
Dr. Ho and Dr. Donta reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recently published “Clinical Practice Guidelines for the Prevention and Management of Tick-Borne Illness,” from the Wilderness Medical Society, are a good compilation of treatment suggestions but are not, in fact, new recommendations, lead author Benjamin Ho, MD, of Southern Wisconsin Emergency Associates in Janesville, acknowledged in an interview.
Dr. Ho emphasized that the focus of the report was on “practitioners who practice in resource-limited settings” and are “the group’s way of solidifying a ... standard of practice” for such physicians. Dr. Ho also said that, while “a lot of the recommendations aren’t well supported, the risk-benefit ratio, we believe, supports the recommendations.”
The article first reviewed the different types of ticks and their distribution in the United States, the specific pathogen associated with each, the disease it causes, and comments about seasonal variations in biting behavior. Another table outlines the most common clinical syndromes, typical lab findings, recommended diagnostic testing, and antibiotic treatments. A third section contains images of different types of ticks and photos of ticks in various life-cycle stages and different levels of engorgement.
The authors were careful to note: “Several tick species are able to carry multiple pathogens. In one study, nearly 25% of Ixodes were coinfected with some combination of the bacteria or parasites causing Lyme disease, anaplasmosis, or babesiosis. Although TBI [tick-borne illness] diagnosis is not the focus of this [clinical practice guideline], providers should be aware of high rates of coinfection; the presence of one TBI should in many instances prompt testing for others.”
In terms of recommendations for preventing TBIs, the authors challenge the suggestion of wearing light-colored clothing. For repellents, they recommend DEET, picaridin, and permethrin. And they also give instructions for laundering clothing and removing ticks.
One recommendation is controversial: that of providing single-dose doxycycline as prophylaxis against Lyme disease. Dr. Ho stresses that this was only for “high-risk” tick bites, defined as a tick bite from an identified Ixodes vector species in which the tick was attached for at least 36 hours and that occurred in an endemic area.
The recommendation for prophylactic doxycycline originated with an article by Robert Nadelman and colleagues in the New England Journal of Medicine and has been strongly challenged by ILADS (International Lyme and Associated Diseases Society) physicians, including Daniel Cameron, MD, and others.
Sam Donta, MD, a recent member of the Department of Health & Human Services Tick-borne Working Group and a member of the Infectious Disease Society of America, said in an interview: “The problem with the one-dose doxycycline is you may not begin to develop symptoms until 2 months later.” It might mask the early symptoms of Lyme. “My impression is that the doxycycline – even the single dose – might have abrogated the ability to see an immune response. The idea, though, if you’ve had a tick bite, is to do nothing and to wait for symptoms to develop. That becomes a little bit more complex. But even then, you could choose to follow the patient and see the patient in 2 weeks and then get blood testing.”
Dr. Donta added: “I think the screening test is inadequate. So you have to go directly to the Western blot. And you have to do both the IgM and IgG” and look for specific bands.
Dr. Donta emphasized that patients should be encouraged to save any ticks that were attached and that, if at all possible, ticks should be sent to a reference lab for testing before committing a patient to a course of antibiotics. There is no harm in that brief delay, he said, and most labs can identify an array of pathogens.
The Wilderness Society guidelines on TBIs provide a good overview for clinicians practicing in limited resource settings and mirror those from the IDSA.
Dr. Ho and Dr. Donta reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM WILDERNESS ENVIRONMENTAL MEDICINE
Semaglutide tops sibling liraglutide for weight loss
A study showing that once-weekly subcutaneous semaglutide 2.4 mg (Wegovy, Novo Nordisk) produces greater long-term weight loss than once-daily injected liraglutide 3.0 mg (Saxenda, Novo Nordisk) among adults with overweight or obesity without diabetes has now been published.
The data, previously reported at Obesity Week 2021, were published online Jan. 11 in JAMA.
The findings are from the phase 3 Semaglutide Treatment Effect in People with Obesity (STEP) 8 trial by Domenica M. Rubino, MD, of the Washington Center for Weight Management and Research, Arlington, Virginia, and colleagues.
Semaglutide and liraglutide, subcutaneously injectable glucagon-like peptide-1 (GLP-1) agonists, were both first approved for the treatment of type 2 diabetes in the United States and elsewhere, but are now also approved, in different doses, for chronic weight management and in people with obesity or overweight and comorbidities. A phase 2 trial demonstrated that once-daily semaglutide 0.4 mg produced significantly more weight loss than liraglutide 3.0 mg.
“Semaglutide and liraglutide induce weight loss by lowering energy intake. However, the reduction in caloric intake versus placebo appears to be larger with semaglutide (35%) than liraglutide (approximately 16%),” say Dr. Rubino and colleagues.
“Semaglutide has also been associated with reductions in food cravings, which is less evident with liraglutide, suggesting different mechanisms of energy intake regulation,” they add.
Novo Nordisk has recently reported that there may be supply problems with Wegovy, as a contract manufacturer that fills syringes for pens to inject the drug temporarily halted deliveries and manufacturing after issues related to good manufacturing practice.
The company is also developing an oral form of semaglutide for weight loss. The oral form has already been approved in doses of 7 or 14 mg/day for the treatment of type 2 diabetes in the United States as Rybelsus.
Individualize treatment for those with obesity
STEP 8 was a randomized, open-label, 68-week phase 3b trial of 338 adults randomized to once-weekly semaglutide 2.4 mg (n = 126), once-daily liraglutide 3.0 mg (n = 127), or matched injected placebo (n = 85) for 68 weeks, all provided with counseling on diet and physical activity.
The primary outcome – estimated mean change in body weight at week 68 – was –15.8% with semaglutide versus –6.4% with liraglutide, a significant difference (P < .001). The proportions of patients achieving loss of body weight of 10%, 15%, or 20% were 70.9%, 55.6%, and 38.5% with semaglutide versus 25.6%, 12.0%, and 6.0% with liraglutide, respectively.
Significantly greater reductions were also seen at 68 weeks for weekly semaglutide versus daily liraglutide in absolute body weight, waist circumference, diastolic blood pressure, total cholesterol, very low-density cholesterol, triglycerides, A1c, fasting plasma glucose, and C-reactive protein. Differences in systolic blood pressure, LDL and HDL cholesterol, free fatty acids, and fasting serum insulin did not achieve significance.
Overall, 19.8% of patients permanently discontinued treatment, with the most discontinuations in the liraglutide group (27.6%), followed by placebo (17.6%) and semaglutide (3.5%). Time to first and permanent discontinuation were shorter with liraglutide than with semaglutide or placebo.
Adverse events were reported by 95.2% of patients with semaglutide, 96.1% with liraglutide, and 95.3% with placebo. Gastrointestinal disorders were the most common with the two active drugs, reported by 84.1% with semaglutide and 82.7% with liraglutide versus 55.3% with placebo.
Most side effects were mild to moderate and resolved without treatment discontinuation. Severe gastrointestinal adverse events were reported by only 3.2%, 2.4%, and 3.5% of patients with semaglutide, liraglutide, and placebo, respectively.
“This trial found weight loss with semaglutide was significantly greater than with liraglutide. However, the variability in treatment response means an individual’s tolerance and sensitivity to a specific treatment is important for obesity management,” the researchers observe.
“Therefore, having multiple antiobesity medications proven to lower body weight through different mechanisms, with different adverse effect profiles and dosing regimens, can only benefit clinicians and patients,” they conclude.
The trial was funded by Novo Nordisk. Dr. Rubino has reported being a clinical investigator for Boehringer Ingelheim, AstraZeneca, and Novo Nordisk; receiving honoraria from WebMD; receiving speaker fees, consulting fees, scientific advisory fees, and honoraria from Novo Nordisk; receiving grants from SARL and personal fees from Medscape, PeerView, and the Endocrine Society; and being a shareholder in Novo Nordisk.
A version of this article first appeared on Medscape.com.
A study showing that once-weekly subcutaneous semaglutide 2.4 mg (Wegovy, Novo Nordisk) produces greater long-term weight loss than once-daily injected liraglutide 3.0 mg (Saxenda, Novo Nordisk) among adults with overweight or obesity without diabetes has now been published.
The data, previously reported at Obesity Week 2021, were published online Jan. 11 in JAMA.
The findings are from the phase 3 Semaglutide Treatment Effect in People with Obesity (STEP) 8 trial by Domenica M. Rubino, MD, of the Washington Center for Weight Management and Research, Arlington, Virginia, and colleagues.
Semaglutide and liraglutide, subcutaneously injectable glucagon-like peptide-1 (GLP-1) agonists, were both first approved for the treatment of type 2 diabetes in the United States and elsewhere, but are now also approved, in different doses, for chronic weight management and in people with obesity or overweight and comorbidities. A phase 2 trial demonstrated that once-daily semaglutide 0.4 mg produced significantly more weight loss than liraglutide 3.0 mg.
“Semaglutide and liraglutide induce weight loss by lowering energy intake. However, the reduction in caloric intake versus placebo appears to be larger with semaglutide (35%) than liraglutide (approximately 16%),” say Dr. Rubino and colleagues.
“Semaglutide has also been associated with reductions in food cravings, which is less evident with liraglutide, suggesting different mechanisms of energy intake regulation,” they add.
Novo Nordisk has recently reported that there may be supply problems with Wegovy, as a contract manufacturer that fills syringes for pens to inject the drug temporarily halted deliveries and manufacturing after issues related to good manufacturing practice.
The company is also developing an oral form of semaglutide for weight loss. The oral form has already been approved in doses of 7 or 14 mg/day for the treatment of type 2 diabetes in the United States as Rybelsus.
Individualize treatment for those with obesity
STEP 8 was a randomized, open-label, 68-week phase 3b trial of 338 adults randomized to once-weekly semaglutide 2.4 mg (n = 126), once-daily liraglutide 3.0 mg (n = 127), or matched injected placebo (n = 85) for 68 weeks, all provided with counseling on diet and physical activity.
The primary outcome – estimated mean change in body weight at week 68 – was –15.8% with semaglutide versus –6.4% with liraglutide, a significant difference (P < .001). The proportions of patients achieving loss of body weight of 10%, 15%, or 20% were 70.9%, 55.6%, and 38.5% with semaglutide versus 25.6%, 12.0%, and 6.0% with liraglutide, respectively.
Significantly greater reductions were also seen at 68 weeks for weekly semaglutide versus daily liraglutide in absolute body weight, waist circumference, diastolic blood pressure, total cholesterol, very low-density cholesterol, triglycerides, A1c, fasting plasma glucose, and C-reactive protein. Differences in systolic blood pressure, LDL and HDL cholesterol, free fatty acids, and fasting serum insulin did not achieve significance.
Overall, 19.8% of patients permanently discontinued treatment, with the most discontinuations in the liraglutide group (27.6%), followed by placebo (17.6%) and semaglutide (3.5%). Time to first and permanent discontinuation were shorter with liraglutide than with semaglutide or placebo.
Adverse events were reported by 95.2% of patients with semaglutide, 96.1% with liraglutide, and 95.3% with placebo. Gastrointestinal disorders were the most common with the two active drugs, reported by 84.1% with semaglutide and 82.7% with liraglutide versus 55.3% with placebo.
Most side effects were mild to moderate and resolved without treatment discontinuation. Severe gastrointestinal adverse events were reported by only 3.2%, 2.4%, and 3.5% of patients with semaglutide, liraglutide, and placebo, respectively.
“This trial found weight loss with semaglutide was significantly greater than with liraglutide. However, the variability in treatment response means an individual’s tolerance and sensitivity to a specific treatment is important for obesity management,” the researchers observe.
“Therefore, having multiple antiobesity medications proven to lower body weight through different mechanisms, with different adverse effect profiles and dosing regimens, can only benefit clinicians and patients,” they conclude.
The trial was funded by Novo Nordisk. Dr. Rubino has reported being a clinical investigator for Boehringer Ingelheim, AstraZeneca, and Novo Nordisk; receiving honoraria from WebMD; receiving speaker fees, consulting fees, scientific advisory fees, and honoraria from Novo Nordisk; receiving grants from SARL and personal fees from Medscape, PeerView, and the Endocrine Society; and being a shareholder in Novo Nordisk.
A version of this article first appeared on Medscape.com.
A study showing that once-weekly subcutaneous semaglutide 2.4 mg (Wegovy, Novo Nordisk) produces greater long-term weight loss than once-daily injected liraglutide 3.0 mg (Saxenda, Novo Nordisk) among adults with overweight or obesity without diabetes has now been published.
The data, previously reported at Obesity Week 2021, were published online Jan. 11 in JAMA.
The findings are from the phase 3 Semaglutide Treatment Effect in People with Obesity (STEP) 8 trial by Domenica M. Rubino, MD, of the Washington Center for Weight Management and Research, Arlington, Virginia, and colleagues.
Semaglutide and liraglutide, subcutaneously injectable glucagon-like peptide-1 (GLP-1) agonists, were both first approved for the treatment of type 2 diabetes in the United States and elsewhere, but are now also approved, in different doses, for chronic weight management and in people with obesity or overweight and comorbidities. A phase 2 trial demonstrated that once-daily semaglutide 0.4 mg produced significantly more weight loss than liraglutide 3.0 mg.
“Semaglutide and liraglutide induce weight loss by lowering energy intake. However, the reduction in caloric intake versus placebo appears to be larger with semaglutide (35%) than liraglutide (approximately 16%),” say Dr. Rubino and colleagues.
“Semaglutide has also been associated with reductions in food cravings, which is less evident with liraglutide, suggesting different mechanisms of energy intake regulation,” they add.
Novo Nordisk has recently reported that there may be supply problems with Wegovy, as a contract manufacturer that fills syringes for pens to inject the drug temporarily halted deliveries and manufacturing after issues related to good manufacturing practice.
The company is also developing an oral form of semaglutide for weight loss. The oral form has already been approved in doses of 7 or 14 mg/day for the treatment of type 2 diabetes in the United States as Rybelsus.
Individualize treatment for those with obesity
STEP 8 was a randomized, open-label, 68-week phase 3b trial of 338 adults randomized to once-weekly semaglutide 2.4 mg (n = 126), once-daily liraglutide 3.0 mg (n = 127), or matched injected placebo (n = 85) for 68 weeks, all provided with counseling on diet and physical activity.
The primary outcome – estimated mean change in body weight at week 68 – was –15.8% with semaglutide versus –6.4% with liraglutide, a significant difference (P < .001). The proportions of patients achieving loss of body weight of 10%, 15%, or 20% were 70.9%, 55.6%, and 38.5% with semaglutide versus 25.6%, 12.0%, and 6.0% with liraglutide, respectively.
Significantly greater reductions were also seen at 68 weeks for weekly semaglutide versus daily liraglutide in absolute body weight, waist circumference, diastolic blood pressure, total cholesterol, very low-density cholesterol, triglycerides, A1c, fasting plasma glucose, and C-reactive protein. Differences in systolic blood pressure, LDL and HDL cholesterol, free fatty acids, and fasting serum insulin did not achieve significance.
Overall, 19.8% of patients permanently discontinued treatment, with the most discontinuations in the liraglutide group (27.6%), followed by placebo (17.6%) and semaglutide (3.5%). Time to first and permanent discontinuation were shorter with liraglutide than with semaglutide or placebo.
Adverse events were reported by 95.2% of patients with semaglutide, 96.1% with liraglutide, and 95.3% with placebo. Gastrointestinal disorders were the most common with the two active drugs, reported by 84.1% with semaglutide and 82.7% with liraglutide versus 55.3% with placebo.
Most side effects were mild to moderate and resolved without treatment discontinuation. Severe gastrointestinal adverse events were reported by only 3.2%, 2.4%, and 3.5% of patients with semaglutide, liraglutide, and placebo, respectively.
“This trial found weight loss with semaglutide was significantly greater than with liraglutide. However, the variability in treatment response means an individual’s tolerance and sensitivity to a specific treatment is important for obesity management,” the researchers observe.
“Therefore, having multiple antiobesity medications proven to lower body weight through different mechanisms, with different adverse effect profiles and dosing regimens, can only benefit clinicians and patients,” they conclude.
The trial was funded by Novo Nordisk. Dr. Rubino has reported being a clinical investigator for Boehringer Ingelheim, AstraZeneca, and Novo Nordisk; receiving honoraria from WebMD; receiving speaker fees, consulting fees, scientific advisory fees, and honoraria from Novo Nordisk; receiving grants from SARL and personal fees from Medscape, PeerView, and the Endocrine Society; and being a shareholder in Novo Nordisk.
A version of this article first appeared on Medscape.com.
FROM JAMA








