User login
Health care on holidays
My office was open on Presidents Day this year. Granted, I’ve never closed for it.
We’re also open on Veteran’s Day, Columbus Day, and Martin Luther King Jr. Day.
Occasionally (usually MLK or Veteran’s days) we get a call from someone unhappy we’re open that day. Banks, government offices, and schools are closed, and they feel that, by not following suit, I’m insulting the memory of veterans and those who fought for civil rights.
Nothing could be farther from the truth. In fact, I don’t know any doctors’ offices that AREN’T open on those days.
Part of this is patient centered. When people need to see a doctor, they don’t want to wait too long. The emergency room isn’t where the majority of things should be handled. Besides, they’re already swamped with nonemergent cases.
Most practices work 8-5 on weekdays, and are booked out. Every additional weekday you’re closed only adds to the wait. So I try to be there enough days to care for people, but not enough so that I lose my sanity or family.
In my area, a fair number of my patients are schoolteachers, who work the same hours I do. So many of them come in on those days, and appreciate that I’m open when they’re off.
Another part is practical. In a small practice, cash flow is critical, and there are just so many days in a given year you can be closed without hurting your financial picture. So most practices are closed for the Big 6 (Memorial Day, Independence Day, Labor Day, Thanksgiving, Christmas, and New Years). Usually this also includes Black Friday and Christmas Eve. So a total of 8 days per year (in addition to vacations).
Unlike other businesses (such as stores and restaurants) most medical offices aren’t open on weekends and nights, so our entire revenue stream is dependent on weekdays from 8 to 5. In this day and age, with most practices running on razor-thin margins, every day off adds to the red line. I can’t take care of anyone if I can’t pay my rent and staff.
I mean no disrespect to anyone. Like other doctors I work hard to provide quality care to all. But So I try to be there for them as much as I can, without going overboard and at the same time keeping my small practice afloat.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My office was open on Presidents Day this year. Granted, I’ve never closed for it.
We’re also open on Veteran’s Day, Columbus Day, and Martin Luther King Jr. Day.
Occasionally (usually MLK or Veteran’s days) we get a call from someone unhappy we’re open that day. Banks, government offices, and schools are closed, and they feel that, by not following suit, I’m insulting the memory of veterans and those who fought for civil rights.
Nothing could be farther from the truth. In fact, I don’t know any doctors’ offices that AREN’T open on those days.
Part of this is patient centered. When people need to see a doctor, they don’t want to wait too long. The emergency room isn’t where the majority of things should be handled. Besides, they’re already swamped with nonemergent cases.
Most practices work 8-5 on weekdays, and are booked out. Every additional weekday you’re closed only adds to the wait. So I try to be there enough days to care for people, but not enough so that I lose my sanity or family.
In my area, a fair number of my patients are schoolteachers, who work the same hours I do. So many of them come in on those days, and appreciate that I’m open when they’re off.
Another part is practical. In a small practice, cash flow is critical, and there are just so many days in a given year you can be closed without hurting your financial picture. So most practices are closed for the Big 6 (Memorial Day, Independence Day, Labor Day, Thanksgiving, Christmas, and New Years). Usually this also includes Black Friday and Christmas Eve. So a total of 8 days per year (in addition to vacations).
Unlike other businesses (such as stores and restaurants) most medical offices aren’t open on weekends and nights, so our entire revenue stream is dependent on weekdays from 8 to 5. In this day and age, with most practices running on razor-thin margins, every day off adds to the red line. I can’t take care of anyone if I can’t pay my rent and staff.
I mean no disrespect to anyone. Like other doctors I work hard to provide quality care to all. But So I try to be there for them as much as I can, without going overboard and at the same time keeping my small practice afloat.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My office was open on Presidents Day this year. Granted, I’ve never closed for it.
We’re also open on Veteran’s Day, Columbus Day, and Martin Luther King Jr. Day.
Occasionally (usually MLK or Veteran’s days) we get a call from someone unhappy we’re open that day. Banks, government offices, and schools are closed, and they feel that, by not following suit, I’m insulting the memory of veterans and those who fought for civil rights.
Nothing could be farther from the truth. In fact, I don’t know any doctors’ offices that AREN’T open on those days.
Part of this is patient centered. When people need to see a doctor, they don’t want to wait too long. The emergency room isn’t where the majority of things should be handled. Besides, they’re already swamped with nonemergent cases.
Most practices work 8-5 on weekdays, and are booked out. Every additional weekday you’re closed only adds to the wait. So I try to be there enough days to care for people, but not enough so that I lose my sanity or family.
In my area, a fair number of my patients are schoolteachers, who work the same hours I do. So many of them come in on those days, and appreciate that I’m open when they’re off.
Another part is practical. In a small practice, cash flow is critical, and there are just so many days in a given year you can be closed without hurting your financial picture. So most practices are closed for the Big 6 (Memorial Day, Independence Day, Labor Day, Thanksgiving, Christmas, and New Years). Usually this also includes Black Friday and Christmas Eve. So a total of 8 days per year (in addition to vacations).
Unlike other businesses (such as stores and restaurants) most medical offices aren’t open on weekends and nights, so our entire revenue stream is dependent on weekdays from 8 to 5. In this day and age, with most practices running on razor-thin margins, every day off adds to the red line. I can’t take care of anyone if I can’t pay my rent and staff.
I mean no disrespect to anyone. Like other doctors I work hard to provide quality care to all. But So I try to be there for them as much as I can, without going overboard and at the same time keeping my small practice afloat.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Clinical Edge Journal Scan Commentary: PsA March 2022
The influence of sex and gender on psoriatic arthritis (PsA) continues to be of interest. Using data from the Dutch south-west Early Psoriatic Arthritis cohort (DEPAR), Passia et al1 assessed sex-related differences in demographics, disease characteristics, and evolution over 1 year in 273 men and 294 women newly diagnosed with PsA. They found that at baseline, women had a significantly longer duration of symptoms, higher tender joint count and enthesitis, higher disease activity, higher levels of pain, more severe limitations in function and worse quality of life. During the 1 year follow up, composite measures of disease activity declined in men and women, but women continued to have higher levels than men. At the end of 1 year, fewer women achieved the criteria for minimal disease activity (MDA). Thus, the disease burden of PsA was higher in women vs. men at all time points and even after 1 year of standard-of-care treatment. Sex-specific treatment strategies might help a higher proportion of women achieve MDA.
Although, enthesitis is believed to be a primary pathogenetic lesion in PsA, the relationship between active enthesitis and disease severity as measured by the presence of joint erosions is less well studied. In a cross-sectional study of 104 PsA patients, Smerilli et al2 explored the association between ultrasound (US) entheseal abnormalities and the presence of US detected bone erosions in PsA joints. At least 1 joint bone erosion was found in 45.2% of patients and was associated with power Doppler signal at enthesis (odds ratio [OR] 1.74; P < .01), entheseal bone erosions (OR 3.17; P = .01), and greyscale synovitis (OR 2.59; P = .02). Thus, Doppler signal and bone erosions at entheses indicate more severe PsA and patients with such abnormalities should therefore be treated aggressively.
Comorbidities and associated conditions were a focus of several publications last month. Venous thromboembolism (VTE) is associated with inflammatory diseases, including PsA. In a retrospective cohort study including 5,275 patients with newly diagnosed PsA, Gazitt et al3 assessed the association between PsA and VTE events using a large population-based database in Israel. During follow-up, 1.2% vs. 0.8% patients in the PsA vs. control group were diagnosed with VTE, but this association was not statistically significant after adjusting for demographic factors and comorbidities (adjusted hazard ratio [aHR] 1.27; P = .16) with only older age (aHR 1.08; P < .0001) and history of VTE (aHR 31.63; P < .0001) remaining associated with an increased risk for VTE. Thus, VTE in patients with PsA may be associated with underlying comorbidities rather than PsA per se. In another study, Harris et al4 demonstrated that PsA was associated with increased risk of endometriosis. In an analysis of 4112 patients with laparoscopically confirmed endometriosis from the Nurses’ Health Study II, they found that psoriasis with concomitant PsA was associated with increased risk for subsequent endometriosis (HR 2.01; 95% CI 1.23-3.30), which persisted even after adjusting for comorbidities. Finally, in a cross-sectional study using data from 1862 juvenile PsA (jPsA) patients (122 [6.6%] of whom developed uveitis) in the German National Pediatric Rheumatological Database, Walscheid et al5 showed that patients with jPsA were more likely to develop uveitis if they were diagnosed with PsA at a younger age or were antinuclear antibody positive, with higher disease activity being the only factor significantly associated with the presence of uveitis.
References
1. Passia E et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22 (Jan 11).
2. Smerilli G et al. Doppler signal and bone erosions at the enthesis are independently associated with ultrasound joint erosive damage in psoriatic arthritis. J Rheumatol. 2022 (Feb 1).
3. Gazitt T et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022;24(1):16 (Jan 7).
4. Harris HR et al. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 2022 (Jan 13).
5. Walscheid K et al. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J Rheumatol. 2022 (Jan 15).
The influence of sex and gender on psoriatic arthritis (PsA) continues to be of interest. Using data from the Dutch south-west Early Psoriatic Arthritis cohort (DEPAR), Passia et al1 assessed sex-related differences in demographics, disease characteristics, and evolution over 1 year in 273 men and 294 women newly diagnosed with PsA. They found that at baseline, women had a significantly longer duration of symptoms, higher tender joint count and enthesitis, higher disease activity, higher levels of pain, more severe limitations in function and worse quality of life. During the 1 year follow up, composite measures of disease activity declined in men and women, but women continued to have higher levels than men. At the end of 1 year, fewer women achieved the criteria for minimal disease activity (MDA). Thus, the disease burden of PsA was higher in women vs. men at all time points and even after 1 year of standard-of-care treatment. Sex-specific treatment strategies might help a higher proportion of women achieve MDA.
Although, enthesitis is believed to be a primary pathogenetic lesion in PsA, the relationship between active enthesitis and disease severity as measured by the presence of joint erosions is less well studied. In a cross-sectional study of 104 PsA patients, Smerilli et al2 explored the association between ultrasound (US) entheseal abnormalities and the presence of US detected bone erosions in PsA joints. At least 1 joint bone erosion was found in 45.2% of patients and was associated with power Doppler signal at enthesis (odds ratio [OR] 1.74; P < .01), entheseal bone erosions (OR 3.17; P = .01), and greyscale synovitis (OR 2.59; P = .02). Thus, Doppler signal and bone erosions at entheses indicate more severe PsA and patients with such abnormalities should therefore be treated aggressively.
Comorbidities and associated conditions were a focus of several publications last month. Venous thromboembolism (VTE) is associated with inflammatory diseases, including PsA. In a retrospective cohort study including 5,275 patients with newly diagnosed PsA, Gazitt et al3 assessed the association between PsA and VTE events using a large population-based database in Israel. During follow-up, 1.2% vs. 0.8% patients in the PsA vs. control group were diagnosed with VTE, but this association was not statistically significant after adjusting for demographic factors and comorbidities (adjusted hazard ratio [aHR] 1.27; P = .16) with only older age (aHR 1.08; P < .0001) and history of VTE (aHR 31.63; P < .0001) remaining associated with an increased risk for VTE. Thus, VTE in patients with PsA may be associated with underlying comorbidities rather than PsA per se. In another study, Harris et al4 demonstrated that PsA was associated with increased risk of endometriosis. In an analysis of 4112 patients with laparoscopically confirmed endometriosis from the Nurses’ Health Study II, they found that psoriasis with concomitant PsA was associated with increased risk for subsequent endometriosis (HR 2.01; 95% CI 1.23-3.30), which persisted even after adjusting for comorbidities. Finally, in a cross-sectional study using data from 1862 juvenile PsA (jPsA) patients (122 [6.6%] of whom developed uveitis) in the German National Pediatric Rheumatological Database, Walscheid et al5 showed that patients with jPsA were more likely to develop uveitis if they were diagnosed with PsA at a younger age or were antinuclear antibody positive, with higher disease activity being the only factor significantly associated with the presence of uveitis.
References
1. Passia E et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22 (Jan 11).
2. Smerilli G et al. Doppler signal and bone erosions at the enthesis are independently associated with ultrasound joint erosive damage in psoriatic arthritis. J Rheumatol. 2022 (Feb 1).
3. Gazitt T et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022;24(1):16 (Jan 7).
4. Harris HR et al. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 2022 (Jan 13).
5. Walscheid K et al. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J Rheumatol. 2022 (Jan 15).
The influence of sex and gender on psoriatic arthritis (PsA) continues to be of interest. Using data from the Dutch south-west Early Psoriatic Arthritis cohort (DEPAR), Passia et al1 assessed sex-related differences in demographics, disease characteristics, and evolution over 1 year in 273 men and 294 women newly diagnosed with PsA. They found that at baseline, women had a significantly longer duration of symptoms, higher tender joint count and enthesitis, higher disease activity, higher levels of pain, more severe limitations in function and worse quality of life. During the 1 year follow up, composite measures of disease activity declined in men and women, but women continued to have higher levels than men. At the end of 1 year, fewer women achieved the criteria for minimal disease activity (MDA). Thus, the disease burden of PsA was higher in women vs. men at all time points and even after 1 year of standard-of-care treatment. Sex-specific treatment strategies might help a higher proportion of women achieve MDA.
Although, enthesitis is believed to be a primary pathogenetic lesion in PsA, the relationship between active enthesitis and disease severity as measured by the presence of joint erosions is less well studied. In a cross-sectional study of 104 PsA patients, Smerilli et al2 explored the association between ultrasound (US) entheseal abnormalities and the presence of US detected bone erosions in PsA joints. At least 1 joint bone erosion was found in 45.2% of patients and was associated with power Doppler signal at enthesis (odds ratio [OR] 1.74; P < .01), entheseal bone erosions (OR 3.17; P = .01), and greyscale synovitis (OR 2.59; P = .02). Thus, Doppler signal and bone erosions at entheses indicate more severe PsA and patients with such abnormalities should therefore be treated aggressively.
Comorbidities and associated conditions were a focus of several publications last month. Venous thromboembolism (VTE) is associated with inflammatory diseases, including PsA. In a retrospective cohort study including 5,275 patients with newly diagnosed PsA, Gazitt et al3 assessed the association between PsA and VTE events using a large population-based database in Israel. During follow-up, 1.2% vs. 0.8% patients in the PsA vs. control group were diagnosed with VTE, but this association was not statistically significant after adjusting for demographic factors and comorbidities (adjusted hazard ratio [aHR] 1.27; P = .16) with only older age (aHR 1.08; P < .0001) and history of VTE (aHR 31.63; P < .0001) remaining associated with an increased risk for VTE. Thus, VTE in patients with PsA may be associated with underlying comorbidities rather than PsA per se. In another study, Harris et al4 demonstrated that PsA was associated with increased risk of endometriosis. In an analysis of 4112 patients with laparoscopically confirmed endometriosis from the Nurses’ Health Study II, they found that psoriasis with concomitant PsA was associated with increased risk for subsequent endometriosis (HR 2.01; 95% CI 1.23-3.30), which persisted even after adjusting for comorbidities. Finally, in a cross-sectional study using data from 1862 juvenile PsA (jPsA) patients (122 [6.6%] of whom developed uveitis) in the German National Pediatric Rheumatological Database, Walscheid et al5 showed that patients with jPsA were more likely to develop uveitis if they were diagnosed with PsA at a younger age or were antinuclear antibody positive, with higher disease activity being the only factor significantly associated with the presence of uveitis.
References
1. Passia E et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22 (Jan 11).
2. Smerilli G et al. Doppler signal and bone erosions at the enthesis are independently associated with ultrasound joint erosive damage in psoriatic arthritis. J Rheumatol. 2022 (Feb 1).
3. Gazitt T et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022;24(1):16 (Jan 7).
4. Harris HR et al. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 2022 (Jan 13).
5. Walscheid K et al. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J Rheumatol. 2022 (Jan 15).
Clinical Edge Journal Scan Commentary: RA March 2022
The recent ORAL Surveillance trial has raised concerns about the safety of tofacitinib (and potentially other JAK inhibitors) in the treatment of rheumatoid arthritis.1 JAK inhibitors are known to increase cholesterol levels; however, this was not previously known to increase cardiovascular risk. The ORAL Surveillance trial was an open-label randomized non-inferiority trial comparing 5mg or 10 mg tofacitinib twice daily with tumor necrosis factor (TNF) inhibitor use. Both adverse cardiac events and cancer were increased in the tofacitinib groups (with hazard ratios of 1.33 and 1.48, respectively). The study did not include a control group and so the impact of RA itself on cardiac events and cancer is not known. However, the apparent risk was greatest in patients over the age of 65, suggesting caution should be used in treating older patients with RA with JAK inhibitors. Interestingly, the STAR-RA study, an observational cohort study, looked at claims data from several sources to try to replicate trial results as well as provide real-world evidence on the topic of cardiovascular risk associated with tofacitinib use.2 The authors were able to replicate the inclusion/exclusion criteria from the ORAL Surveillance trial and found that the results were similar. However, on “relaxing” inclusion and exclusion criteria, ie, the “real-world evidence” cohort, there was no difference in incidence of cardiovascular outcomes between tofacitinib and TNF-inhibitor groups. Specifically, patients in the real-world cohort who had no cardiovascular risk factors or prior events were not found to have an increased cardiovascular risk with tofacitinib use, a reassuring finding supporting careful attention to cardiovascular risk in patients with RA when evaluating treatment options.
One consideration in the use of rituximab for treatment of RA is the possibility of changing or lowering subsequent or maintenance doses. Past studies have suggested that halving the dose of rituximab to a single 1000 mg infusion was tolerated by RA patients. The REDO trial, published in 2019, looked at even lower doses: 500 mg and 200 mg. The study did not establish non-inferiority of lower doses at 6 months in a per-protocol analysis, only in intention-to-treat. An extension study of REDO presented at the ACR Convergence in 2021 looked at a subset of those patients for up to 4 years and did find non-inferiority in terms of disease activity. Wientjes et al3 present further analysis of the REDO trial with 140 RA patients at 6 months, looking at the association of rituximab dosage with B cell counts as well as the predictive value of different patient characteristics in terms of response to lower dosages. Interestingly, serum drug levels and B cell counts at 3 and 6 months did not predict response to rituximab, nor did patient characteristics, such as age, smoking, disease, duration, or seropositivity for RF and CCP. Though the authors suggest that this implies even lower doses may be effective, it is not clear, and a similar analysis of the extension trial would be of interest.
Use of methotrexate as a first-line DMARD for RA is cost-effective but often limited by patient fears or intolerance due to adverse events (AE). This observational cohort study by Sherbini et al4 evaluates not only the prevalence of AE, but also baseline factors that may predict the development of AE. Over 1,000 patients with early RA initiating methotrexate were included in analysis. More than 75% reported at least one AE in 12 months, with gastrointestinal AE, such as nausea, being most prevalent (42%); 18% developed elevated liver enzyme tests (defined as a single reading above the upper limit of normal). Few strong predictors of AE were identified, though women were overall more likely than men to report AE. Reassuringly, combination conventional synthetic DMARD therapy did not generally lead to more reported AE. Given the few predictive findings from this study, analysis of a comparator group or comparison of AE at different starting and maintenance doses of methotrexate would be of interest.
References
- Ytterberg SR et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326 (Jan 27).
- Khosrow-Khavar F et al. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis. 2022 (Jan 13).
- Wientjes MHM et al. Drug levels, anti-drug antibodies and B-cell counts were not predictive of response in rheumatoid arthritis patients on (ultra-)low-dose rituximab. Rheumatology (Oxford). 2022 (Jan 12).
- Sherbini AA et al. Rates and predictors of methotrexate-related adverse events in patients with early rheumatoid arthritis: results from a nationwide UK study. Rheumatology (Oxford). 2022 (Jan 25).
The recent ORAL Surveillance trial has raised concerns about the safety of tofacitinib (and potentially other JAK inhibitors) in the treatment of rheumatoid arthritis.1 JAK inhibitors are known to increase cholesterol levels; however, this was not previously known to increase cardiovascular risk. The ORAL Surveillance trial was an open-label randomized non-inferiority trial comparing 5mg or 10 mg tofacitinib twice daily with tumor necrosis factor (TNF) inhibitor use. Both adverse cardiac events and cancer were increased in the tofacitinib groups (with hazard ratios of 1.33 and 1.48, respectively). The study did not include a control group and so the impact of RA itself on cardiac events and cancer is not known. However, the apparent risk was greatest in patients over the age of 65, suggesting caution should be used in treating older patients with RA with JAK inhibitors. Interestingly, the STAR-RA study, an observational cohort study, looked at claims data from several sources to try to replicate trial results as well as provide real-world evidence on the topic of cardiovascular risk associated with tofacitinib use.2 The authors were able to replicate the inclusion/exclusion criteria from the ORAL Surveillance trial and found that the results were similar. However, on “relaxing” inclusion and exclusion criteria, ie, the “real-world evidence” cohort, there was no difference in incidence of cardiovascular outcomes between tofacitinib and TNF-inhibitor groups. Specifically, patients in the real-world cohort who had no cardiovascular risk factors or prior events were not found to have an increased cardiovascular risk with tofacitinib use, a reassuring finding supporting careful attention to cardiovascular risk in patients with RA when evaluating treatment options.
One consideration in the use of rituximab for treatment of RA is the possibility of changing or lowering subsequent or maintenance doses. Past studies have suggested that halving the dose of rituximab to a single 1000 mg infusion was tolerated by RA patients. The REDO trial, published in 2019, looked at even lower doses: 500 mg and 200 mg. The study did not establish non-inferiority of lower doses at 6 months in a per-protocol analysis, only in intention-to-treat. An extension study of REDO presented at the ACR Convergence in 2021 looked at a subset of those patients for up to 4 years and did find non-inferiority in terms of disease activity. Wientjes et al3 present further analysis of the REDO trial with 140 RA patients at 6 months, looking at the association of rituximab dosage with B cell counts as well as the predictive value of different patient characteristics in terms of response to lower dosages. Interestingly, serum drug levels and B cell counts at 3 and 6 months did not predict response to rituximab, nor did patient characteristics, such as age, smoking, disease, duration, or seropositivity for RF and CCP. Though the authors suggest that this implies even lower doses may be effective, it is not clear, and a similar analysis of the extension trial would be of interest.
Use of methotrexate as a first-line DMARD for RA is cost-effective but often limited by patient fears or intolerance due to adverse events (AE). This observational cohort study by Sherbini et al4 evaluates not only the prevalence of AE, but also baseline factors that may predict the development of AE. Over 1,000 patients with early RA initiating methotrexate were included in analysis. More than 75% reported at least one AE in 12 months, with gastrointestinal AE, such as nausea, being most prevalent (42%); 18% developed elevated liver enzyme tests (defined as a single reading above the upper limit of normal). Few strong predictors of AE were identified, though women were overall more likely than men to report AE. Reassuringly, combination conventional synthetic DMARD therapy did not generally lead to more reported AE. Given the few predictive findings from this study, analysis of a comparator group or comparison of AE at different starting and maintenance doses of methotrexate would be of interest.
References
- Ytterberg SR et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326 (Jan 27).
- Khosrow-Khavar F et al. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis. 2022 (Jan 13).
- Wientjes MHM et al. Drug levels, anti-drug antibodies and B-cell counts were not predictive of response in rheumatoid arthritis patients on (ultra-)low-dose rituximab. Rheumatology (Oxford). 2022 (Jan 12).
- Sherbini AA et al. Rates and predictors of methotrexate-related adverse events in patients with early rheumatoid arthritis: results from a nationwide UK study. Rheumatology (Oxford). 2022 (Jan 25).
The recent ORAL Surveillance trial has raised concerns about the safety of tofacitinib (and potentially other JAK inhibitors) in the treatment of rheumatoid arthritis.1 JAK inhibitors are known to increase cholesterol levels; however, this was not previously known to increase cardiovascular risk. The ORAL Surveillance trial was an open-label randomized non-inferiority trial comparing 5mg or 10 mg tofacitinib twice daily with tumor necrosis factor (TNF) inhibitor use. Both adverse cardiac events and cancer were increased in the tofacitinib groups (with hazard ratios of 1.33 and 1.48, respectively). The study did not include a control group and so the impact of RA itself on cardiac events and cancer is not known. However, the apparent risk was greatest in patients over the age of 65, suggesting caution should be used in treating older patients with RA with JAK inhibitors. Interestingly, the STAR-RA study, an observational cohort study, looked at claims data from several sources to try to replicate trial results as well as provide real-world evidence on the topic of cardiovascular risk associated with tofacitinib use.2 The authors were able to replicate the inclusion/exclusion criteria from the ORAL Surveillance trial and found that the results were similar. However, on “relaxing” inclusion and exclusion criteria, ie, the “real-world evidence” cohort, there was no difference in incidence of cardiovascular outcomes between tofacitinib and TNF-inhibitor groups. Specifically, patients in the real-world cohort who had no cardiovascular risk factors or prior events were not found to have an increased cardiovascular risk with tofacitinib use, a reassuring finding supporting careful attention to cardiovascular risk in patients with RA when evaluating treatment options.
One consideration in the use of rituximab for treatment of RA is the possibility of changing or lowering subsequent or maintenance doses. Past studies have suggested that halving the dose of rituximab to a single 1000 mg infusion was tolerated by RA patients. The REDO trial, published in 2019, looked at even lower doses: 500 mg and 200 mg. The study did not establish non-inferiority of lower doses at 6 months in a per-protocol analysis, only in intention-to-treat. An extension study of REDO presented at the ACR Convergence in 2021 looked at a subset of those patients for up to 4 years and did find non-inferiority in terms of disease activity. Wientjes et al3 present further analysis of the REDO trial with 140 RA patients at 6 months, looking at the association of rituximab dosage with B cell counts as well as the predictive value of different patient characteristics in terms of response to lower dosages. Interestingly, serum drug levels and B cell counts at 3 and 6 months did not predict response to rituximab, nor did patient characteristics, such as age, smoking, disease, duration, or seropositivity for RF and CCP. Though the authors suggest that this implies even lower doses may be effective, it is not clear, and a similar analysis of the extension trial would be of interest.
Use of methotrexate as a first-line DMARD for RA is cost-effective but often limited by patient fears or intolerance due to adverse events (AE). This observational cohort study by Sherbini et al4 evaluates not only the prevalence of AE, but also baseline factors that may predict the development of AE. Over 1,000 patients with early RA initiating methotrexate were included in analysis. More than 75% reported at least one AE in 12 months, with gastrointestinal AE, such as nausea, being most prevalent (42%); 18% developed elevated liver enzyme tests (defined as a single reading above the upper limit of normal). Few strong predictors of AE were identified, though women were overall more likely than men to report AE. Reassuringly, combination conventional synthetic DMARD therapy did not generally lead to more reported AE. Given the few predictive findings from this study, analysis of a comparator group or comparison of AE at different starting and maintenance doses of methotrexate would be of interest.
References
- Ytterberg SR et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326 (Jan 27).
- Khosrow-Khavar F et al. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis. 2022 (Jan 13).
- Wientjes MHM et al. Drug levels, anti-drug antibodies and B-cell counts were not predictive of response in rheumatoid arthritis patients on (ultra-)low-dose rituximab. Rheumatology (Oxford). 2022 (Jan 12).
- Sherbini AA et al. Rates and predictors of methotrexate-related adverse events in patients with early rheumatoid arthritis: results from a nationwide UK study. Rheumatology (Oxford). 2022 (Jan 25).
Survey: Artificial intelligence finds support among dermatologists
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.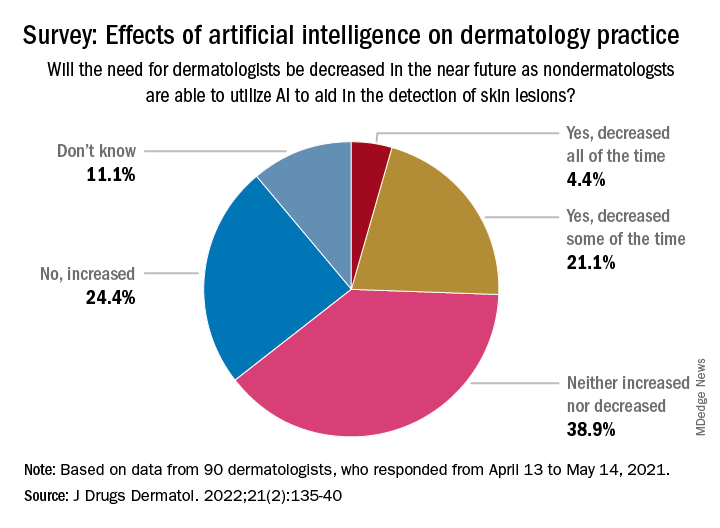
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
The importance of a post-COVID wellness program for medical staff
LAS VEGAS – , according to Jon A. Levenson, MD.
“We can learn from previous pandemics and epidemics, which will be important for us going forward from COVID-19,” Dr. Levenson, associate professor of psychiatry at Columbia University Irving Medical Center, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During the severe acute respiratory syndrome (SARS) epidemic in 2005, 68% of health care workers reported significant job-related stress, including increased workload, changing work duties, redeployment, shortage of medical supplies, concerns about insufficient personal protective equipment (PPE), lack of safety at work, absence of effective treatment protocols, inconsistent organizational support and information and misinformation from hospital management, and witnessing intense pain, isolation, and loss on a daily basis with few opportunities to take breaks (Psychiatr Serv. 2020 Oct 6. doi: 10.1176/appi.ps.202000274).
Personal concerns associated with psychopathological symptoms included spreading infection to family members; feeling responsibility for family members’ social isolation; self-isolating to avoid infecting family, which can lead to increased loneliness and sadness. “For those who were working remotely, this level of work is hard and challenging,” Dr. Levenson said. “For those who are parents, the 24-hour childcare responsibilities exist on top of work. They often found they can’t unwind with friends.”
Across SARS, MERS, Ebola, and swine flu, a wide range of prevalence in symptoms of distress, stress, anxiety, depressive symptoms, and substance use emerged, he continued. During COVID-19, at least three studies reported significant percentages of distress, depression, anxiety, insomnia, and PTSD among health care workers (JAMA Netw Open. 2020;3[3]:e203976, Front Psychol. 2020 Dec 8;11:608986., and Gen Hosp Psychiatry. Sep-Oct 2020;66:1-8).
“Who is at most-increased risk?” Dr. Levenson asked. “Women; those who are younger and have fewer years of work experience; those working on the front lines such as nurses and advanced practice professionals; and people with preexisting vulnerabilities to psychiatric disorders including anxiety, depression, obsessional symptoms, substance use, suicidal behavior, and impulse control disorders are likely to be especially vulnerable to stress-related symptoms.”
At CUIMC, there were certain “tipping points,” to the vulnerability of health care worker well-being in the early stage of the COVID-19 pandemic, he said, including the loss of an emergency medicine physician colleague from death by suicide. “On the national level there were so many other issues going on such as health care disparities of the COVID-19 infection itself, the murder of George Floyd in Minneapolis, other issues of racial injustice, a tense political climate with an upcoming election at the time, and other factors related to the natural climate concerns,” he said. This prompted several faculty members in the CUIMC department of psychiatry including Claude Ann Mellins, PhD, Laurel S. Mayer, MD, and Lourival Baptista-Neto, MD, to partner with ColumbiaDoctors and New York-Presbyterian Hospital and develop a model of care for health care workers known as CopeColumbia, a virtual program intended to address staff burnout and fatigue, with an emphasis on prevention and promotion of resilience.* It launched in March of 2020 and consists of 1:1 peer support, a peer support group program, town halls/webinars, and an active web site.
The 1:1 peer support sessions typically last 20-30 minutes and provide easy access for all distressed hospital and medical center staff. “We have a phone line staffed by Columbia psychiatrists and psychologists so that a distressed staff member can reach support directly,” he said. The format of these sessions includes a brief discussion of challenges and brainstorming around potential coping strategies. “This is not a psychotherapy session,” Dr. Levenson said. “Each session can be individualized to further assess the type of distress or to implement rating scales such as the Generalized Anxiety Disorder-7 scale to assess for signs and symptoms consistent with GAD. There are options to schedule a second or third peer support session, or a prompt referral within Columbia psychiatry when indicated.”
A typical peer support group meeting lasts about 30 minutes and comprises individual divisions or departments. Some goals of the peer groups are to discuss unique challenges of the work environment and to encourage the members of the group to come up with solutions; to promote team support and coping; to teach resilience-enhancing strategies from empirically based treatments such as CBT, “and to end each meeting with expressions of gratitude and of thanks within the group,” he said.
According to Dr. Levenson, sample questions CopeColumbia faculty use to facilitate coping, include “which coping skills are working for you?”; “Are you able to be present?”; “Have you honored loss with any specific ways or traditions?”; “Do you have any work buddies who support you and vice versa?”; “Can your work community build off each other’s individual strengths to help both the individual and the work group cope optimally?”; and “How can your work team help facilitate each other to best support each other?”
Other aspects of the CopeColumbia program include town halls/grand rounds that range from 30 to 60 minutes in length. “It may be a virtual presentation from a mental health professional on specific aspects of coping such as relaxation techniques,” he said. “The focus is how to manage stress, anxiety, trauma, loss, and grief. It also includes an active Q&A to engage staff participants. The advantage of this format is that you can reach many staff in an entire department.” The program also has an active web site for staff with both internal and external support links including mindfulness, meditation, exercise, parenting suggestions/caregiving, and other resources to promote well-being and resilience for staff and family.
To date, certain themes emerged from the 1:1 and peer support group sessions, including expressions of difficulty adapting to “such a new reality,” compared with the pre-COVID era. “Staff would often express anticipatory anxiety and uncertainty, such as is there going to be another surge of COVID-19 cases, and will there be a change in policies?” Dr. Levenson said. “There was a lot of expression of stress and frustration related to politicizing the virus and public containment strategies, both on a local and national level.”
Staff also mentioned the loss of usual coping strategies because of prolonged social isolation, especially for those doing remote work, and the loss of usual support resources that have helped them in the past. “They also reported delayed trauma and grief reactions, including symptoms of depression, anxiety, and posttraumatic stress,” he said. “Health care workers with children mentioned high levels of stress related to childcare, increased workload, and what seems like an impossible work-life balance.” Many reported exhaustion and irritability, “which could affect and cause tension within the work group and challenges to effective team cohesion,” he said. “There were also stressors related to the impact of racial injustices and the [presidential] election that could exacerbate the impact of COVID-19.”
Dr. Levenson hopes that CopeColumbia serves as a model for other health care systems looking for ways to support the mental well-being of their employees. “We want to promote the message that emotional health should have the same priority level as physical health,” he said. “The term that I like to use is total health. Addressing the well-being of health care workers is critical for a healthy workforce and for delivering high-quality patient care.”
He reported having no relevant financial disclosures related to his presentation.
Correction, 2/28/22: An earlier version of this article misstated Dr. Lourival Baptista-Neto's name.
LAS VEGAS – , according to Jon A. Levenson, MD.
“We can learn from previous pandemics and epidemics, which will be important for us going forward from COVID-19,” Dr. Levenson, associate professor of psychiatry at Columbia University Irving Medical Center, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During the severe acute respiratory syndrome (SARS) epidemic in 2005, 68% of health care workers reported significant job-related stress, including increased workload, changing work duties, redeployment, shortage of medical supplies, concerns about insufficient personal protective equipment (PPE), lack of safety at work, absence of effective treatment protocols, inconsistent organizational support and information and misinformation from hospital management, and witnessing intense pain, isolation, and loss on a daily basis with few opportunities to take breaks (Psychiatr Serv. 2020 Oct 6. doi: 10.1176/appi.ps.202000274).
Personal concerns associated with psychopathological symptoms included spreading infection to family members; feeling responsibility for family members’ social isolation; self-isolating to avoid infecting family, which can lead to increased loneliness and sadness. “For those who were working remotely, this level of work is hard and challenging,” Dr. Levenson said. “For those who are parents, the 24-hour childcare responsibilities exist on top of work. They often found they can’t unwind with friends.”
Across SARS, MERS, Ebola, and swine flu, a wide range of prevalence in symptoms of distress, stress, anxiety, depressive symptoms, and substance use emerged, he continued. During COVID-19, at least three studies reported significant percentages of distress, depression, anxiety, insomnia, and PTSD among health care workers (JAMA Netw Open. 2020;3[3]:e203976, Front Psychol. 2020 Dec 8;11:608986., and Gen Hosp Psychiatry. Sep-Oct 2020;66:1-8).
“Who is at most-increased risk?” Dr. Levenson asked. “Women; those who are younger and have fewer years of work experience; those working on the front lines such as nurses and advanced practice professionals; and people with preexisting vulnerabilities to psychiatric disorders including anxiety, depression, obsessional symptoms, substance use, suicidal behavior, and impulse control disorders are likely to be especially vulnerable to stress-related symptoms.”
At CUIMC, there were certain “tipping points,” to the vulnerability of health care worker well-being in the early stage of the COVID-19 pandemic, he said, including the loss of an emergency medicine physician colleague from death by suicide. “On the national level there were so many other issues going on such as health care disparities of the COVID-19 infection itself, the murder of George Floyd in Minneapolis, other issues of racial injustice, a tense political climate with an upcoming election at the time, and other factors related to the natural climate concerns,” he said. This prompted several faculty members in the CUIMC department of psychiatry including Claude Ann Mellins, PhD, Laurel S. Mayer, MD, and Lourival Baptista-Neto, MD, to partner with ColumbiaDoctors and New York-Presbyterian Hospital and develop a model of care for health care workers known as CopeColumbia, a virtual program intended to address staff burnout and fatigue, with an emphasis on prevention and promotion of resilience.* It launched in March of 2020 and consists of 1:1 peer support, a peer support group program, town halls/webinars, and an active web site.
The 1:1 peer support sessions typically last 20-30 minutes and provide easy access for all distressed hospital and medical center staff. “We have a phone line staffed by Columbia psychiatrists and psychologists so that a distressed staff member can reach support directly,” he said. The format of these sessions includes a brief discussion of challenges and brainstorming around potential coping strategies. “This is not a psychotherapy session,” Dr. Levenson said. “Each session can be individualized to further assess the type of distress or to implement rating scales such as the Generalized Anxiety Disorder-7 scale to assess for signs and symptoms consistent with GAD. There are options to schedule a second or third peer support session, or a prompt referral within Columbia psychiatry when indicated.”
A typical peer support group meeting lasts about 30 minutes and comprises individual divisions or departments. Some goals of the peer groups are to discuss unique challenges of the work environment and to encourage the members of the group to come up with solutions; to promote team support and coping; to teach resilience-enhancing strategies from empirically based treatments such as CBT, “and to end each meeting with expressions of gratitude and of thanks within the group,” he said.
According to Dr. Levenson, sample questions CopeColumbia faculty use to facilitate coping, include “which coping skills are working for you?”; “Are you able to be present?”; “Have you honored loss with any specific ways or traditions?”; “Do you have any work buddies who support you and vice versa?”; “Can your work community build off each other’s individual strengths to help both the individual and the work group cope optimally?”; and “How can your work team help facilitate each other to best support each other?”
Other aspects of the CopeColumbia program include town halls/grand rounds that range from 30 to 60 minutes in length. “It may be a virtual presentation from a mental health professional on specific aspects of coping such as relaxation techniques,” he said. “The focus is how to manage stress, anxiety, trauma, loss, and grief. It also includes an active Q&A to engage staff participants. The advantage of this format is that you can reach many staff in an entire department.” The program also has an active web site for staff with both internal and external support links including mindfulness, meditation, exercise, parenting suggestions/caregiving, and other resources to promote well-being and resilience for staff and family.
To date, certain themes emerged from the 1:1 and peer support group sessions, including expressions of difficulty adapting to “such a new reality,” compared with the pre-COVID era. “Staff would often express anticipatory anxiety and uncertainty, such as is there going to be another surge of COVID-19 cases, and will there be a change in policies?” Dr. Levenson said. “There was a lot of expression of stress and frustration related to politicizing the virus and public containment strategies, both on a local and national level.”
Staff also mentioned the loss of usual coping strategies because of prolonged social isolation, especially for those doing remote work, and the loss of usual support resources that have helped them in the past. “They also reported delayed trauma and grief reactions, including symptoms of depression, anxiety, and posttraumatic stress,” he said. “Health care workers with children mentioned high levels of stress related to childcare, increased workload, and what seems like an impossible work-life balance.” Many reported exhaustion and irritability, “which could affect and cause tension within the work group and challenges to effective team cohesion,” he said. “There were also stressors related to the impact of racial injustices and the [presidential] election that could exacerbate the impact of COVID-19.”
Dr. Levenson hopes that CopeColumbia serves as a model for other health care systems looking for ways to support the mental well-being of their employees. “We want to promote the message that emotional health should have the same priority level as physical health,” he said. “The term that I like to use is total health. Addressing the well-being of health care workers is critical for a healthy workforce and for delivering high-quality patient care.”
He reported having no relevant financial disclosures related to his presentation.
Correction, 2/28/22: An earlier version of this article misstated Dr. Lourival Baptista-Neto's name.
LAS VEGAS – , according to Jon A. Levenson, MD.
“We can learn from previous pandemics and epidemics, which will be important for us going forward from COVID-19,” Dr. Levenson, associate professor of psychiatry at Columbia University Irving Medical Center, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During the severe acute respiratory syndrome (SARS) epidemic in 2005, 68% of health care workers reported significant job-related stress, including increased workload, changing work duties, redeployment, shortage of medical supplies, concerns about insufficient personal protective equipment (PPE), lack of safety at work, absence of effective treatment protocols, inconsistent organizational support and information and misinformation from hospital management, and witnessing intense pain, isolation, and loss on a daily basis with few opportunities to take breaks (Psychiatr Serv. 2020 Oct 6. doi: 10.1176/appi.ps.202000274).
Personal concerns associated with psychopathological symptoms included spreading infection to family members; feeling responsibility for family members’ social isolation; self-isolating to avoid infecting family, which can lead to increased loneliness and sadness. “For those who were working remotely, this level of work is hard and challenging,” Dr. Levenson said. “For those who are parents, the 24-hour childcare responsibilities exist on top of work. They often found they can’t unwind with friends.”
Across SARS, MERS, Ebola, and swine flu, a wide range of prevalence in symptoms of distress, stress, anxiety, depressive symptoms, and substance use emerged, he continued. During COVID-19, at least three studies reported significant percentages of distress, depression, anxiety, insomnia, and PTSD among health care workers (JAMA Netw Open. 2020;3[3]:e203976, Front Psychol. 2020 Dec 8;11:608986., and Gen Hosp Psychiatry. Sep-Oct 2020;66:1-8).
“Who is at most-increased risk?” Dr. Levenson asked. “Women; those who are younger and have fewer years of work experience; those working on the front lines such as nurses and advanced practice professionals; and people with preexisting vulnerabilities to psychiatric disorders including anxiety, depression, obsessional symptoms, substance use, suicidal behavior, and impulse control disorders are likely to be especially vulnerable to stress-related symptoms.”
At CUIMC, there were certain “tipping points,” to the vulnerability of health care worker well-being in the early stage of the COVID-19 pandemic, he said, including the loss of an emergency medicine physician colleague from death by suicide. “On the national level there were so many other issues going on such as health care disparities of the COVID-19 infection itself, the murder of George Floyd in Minneapolis, other issues of racial injustice, a tense political climate with an upcoming election at the time, and other factors related to the natural climate concerns,” he said. This prompted several faculty members in the CUIMC department of psychiatry including Claude Ann Mellins, PhD, Laurel S. Mayer, MD, and Lourival Baptista-Neto, MD, to partner with ColumbiaDoctors and New York-Presbyterian Hospital and develop a model of care for health care workers known as CopeColumbia, a virtual program intended to address staff burnout and fatigue, with an emphasis on prevention and promotion of resilience.* It launched in March of 2020 and consists of 1:1 peer support, a peer support group program, town halls/webinars, and an active web site.
The 1:1 peer support sessions typically last 20-30 minutes and provide easy access for all distressed hospital and medical center staff. “We have a phone line staffed by Columbia psychiatrists and psychologists so that a distressed staff member can reach support directly,” he said. The format of these sessions includes a brief discussion of challenges and brainstorming around potential coping strategies. “This is not a psychotherapy session,” Dr. Levenson said. “Each session can be individualized to further assess the type of distress or to implement rating scales such as the Generalized Anxiety Disorder-7 scale to assess for signs and symptoms consistent with GAD. There are options to schedule a second or third peer support session, or a prompt referral within Columbia psychiatry when indicated.”
A typical peer support group meeting lasts about 30 minutes and comprises individual divisions or departments. Some goals of the peer groups are to discuss unique challenges of the work environment and to encourage the members of the group to come up with solutions; to promote team support and coping; to teach resilience-enhancing strategies from empirically based treatments such as CBT, “and to end each meeting with expressions of gratitude and of thanks within the group,” he said.
According to Dr. Levenson, sample questions CopeColumbia faculty use to facilitate coping, include “which coping skills are working for you?”; “Are you able to be present?”; “Have you honored loss with any specific ways or traditions?”; “Do you have any work buddies who support you and vice versa?”; “Can your work community build off each other’s individual strengths to help both the individual and the work group cope optimally?”; and “How can your work team help facilitate each other to best support each other?”
Other aspects of the CopeColumbia program include town halls/grand rounds that range from 30 to 60 minutes in length. “It may be a virtual presentation from a mental health professional on specific aspects of coping such as relaxation techniques,” he said. “The focus is how to manage stress, anxiety, trauma, loss, and grief. It also includes an active Q&A to engage staff participants. The advantage of this format is that you can reach many staff in an entire department.” The program also has an active web site for staff with both internal and external support links including mindfulness, meditation, exercise, parenting suggestions/caregiving, and other resources to promote well-being and resilience for staff and family.
To date, certain themes emerged from the 1:1 and peer support group sessions, including expressions of difficulty adapting to “such a new reality,” compared with the pre-COVID era. “Staff would often express anticipatory anxiety and uncertainty, such as is there going to be another surge of COVID-19 cases, and will there be a change in policies?” Dr. Levenson said. “There was a lot of expression of stress and frustration related to politicizing the virus and public containment strategies, both on a local and national level.”
Staff also mentioned the loss of usual coping strategies because of prolonged social isolation, especially for those doing remote work, and the loss of usual support resources that have helped them in the past. “They also reported delayed trauma and grief reactions, including symptoms of depression, anxiety, and posttraumatic stress,” he said. “Health care workers with children mentioned high levels of stress related to childcare, increased workload, and what seems like an impossible work-life balance.” Many reported exhaustion and irritability, “which could affect and cause tension within the work group and challenges to effective team cohesion,” he said. “There were also stressors related to the impact of racial injustices and the [presidential] election that could exacerbate the impact of COVID-19.”
Dr. Levenson hopes that CopeColumbia serves as a model for other health care systems looking for ways to support the mental well-being of their employees. “We want to promote the message that emotional health should have the same priority level as physical health,” he said. “The term that I like to use is total health. Addressing the well-being of health care workers is critical for a healthy workforce and for delivering high-quality patient care.”
He reported having no relevant financial disclosures related to his presentation.
Correction, 2/28/22: An earlier version of this article misstated Dr. Lourival Baptista-Neto's name.
FROM NPA 2022
Freiburg index accurately predicts survival in liver procedure
A new prognostic score is more accurate than the commonly used Model for End-Stage Liver Disease (MELD) in predicting post–transjugular intrahepatic portosystemic shunt (TIPS) survival, researchers say.
The Freiburg Index of Post-TIPS Survival (FIPS) could help patients and doctors weigh the benefits and risks of the procedure, said Chongtu Yang, MD, a postgraduate fellow at the Huazhong University of Science and Technology, Wuhan, China.
“For patients defined as high risk, the TIPS procedure may not be the optimal choice, and transplantation may be better,” Dr. Yang told this news organization. He cautioned that FIPS needs further validation before being applied in clinical practice.
The study by Dr. Yang and his colleagues was published online Feb. 9 in the American Journal of Roentgenology. To their knowledge, this is the first study to validate FIPS in a cohort of Asian patients.
Decompensated cirrhosis can cause variceal bleeding and refractory ascites and may be life threatening. TIPS can manage these complications but comes with its own risks.
To determine which patients can best benefit from the procedure, researchers have proposed a variety of prognostic scoring systems. Some were developed for other purposes, such as predicting survival following hospitalization, rather than specifically for TIPS. Additionally, few studies have compared these approaches to each other.
A four-way comparison
To fill that gap, Dr. Yang and his colleagues compared four predictive models: the MELD, the sodium MELD (MELD-Na), the Chronic Liver Failure–Consortium Acute Decompensation (CLIF-CAD), and FIPS.
The MELD score uses serum bilirubin, serum creatinine, and the international normalized ratio (INR) of prothrombin time. MELD-Na adds sodium to this algorithm. The CLIF-CAD score is calculated using age, serum creatinine, INR, white blood count, and sodium level. FIPS, which was recently devised to predict results with TIPS, uses age, bilirubin, albumin, and creatinine.
To see which yielded more accurate predictions, Dr. Yang and his colleagues followed 383 patients with cirrhosis (mean age, 55 years; 341 with variceal bleeding and 42 with refractory ascites) who underwent TIPS placement at Wuhan Union Hospital between January 2016 and August 2021.
The most common cause of cirrhosis was hepatitis B infection (58.2% of patients), followed by hepatitis C infection (11.7%) and alcohol use (13.6%).
The researchers followed the patients for a median of 23.4 months. They lost track of 31 patients over that time, and another 72 died. The survival rate after TIPS placement was 92.3% at 6 months, 87.8% at 12 months, and 81.2% at 24 months. Thirty-seven patients received a TIPS revision.
In their first measure of the models’ accuracy, the researchers used a concordance index, which compares actual results with predicted results. The number of concordant pairs are divided by the total number of possible evaluation pairs. A score of 1 represents 100% accuracy.
By this measure, the prediction of survival at 6 months was highest for FIPS followed by CLIF-CAD, MELD, and MELD-Na. However, the confidence intervals overlapped.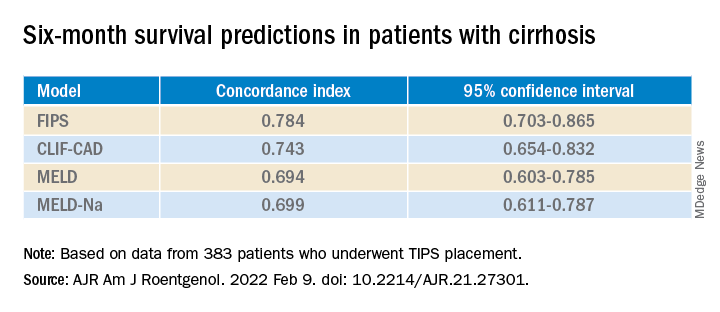
FIPS also scored highest in the concordance index at 12 and 24 months.
In a second measure of the models’ accuracy, the researchers used Brier scores, which calculate the mean squared error between predicted probabilities and actual values. Like the concordance index, Brier scores range from 0.0 to 1.0 but differ in that the lowest Brier score number represents the highest accuracy.
At 6 months, the CLIF-CAD score was the best, at 0.074. MELD and FIPS were equivalent at 0.075, with MELD-Na coming in at 0.077. However, FIPS attained slightly better scores than the other systems at 12 and 24 months.
Is FIPS worth implementing?
With scores this close, it may not be worth changing the predictive model clinicians use for choosing TIPS candidates, said Nancy Reau, MD, chief of hepatology at Rush University Medical Center, Chicago, who was not involved in the study.
MELD scores are already programmed into many electronic medical record systems in the United States, and clinicians are familiar with using that system to aid in further decisions, such as decisions regarding other kinds of surgery, she told this news organization.
“If you’re going to try to advocate for a new system, you really have to show that the performance of the predictive score is monumentally better than the tried and true,” she said.
Dr. Yang and Dr. Reau report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new prognostic score is more accurate than the commonly used Model for End-Stage Liver Disease (MELD) in predicting post–transjugular intrahepatic portosystemic shunt (TIPS) survival, researchers say.
The Freiburg Index of Post-TIPS Survival (FIPS) could help patients and doctors weigh the benefits and risks of the procedure, said Chongtu Yang, MD, a postgraduate fellow at the Huazhong University of Science and Technology, Wuhan, China.
“For patients defined as high risk, the TIPS procedure may not be the optimal choice, and transplantation may be better,” Dr. Yang told this news organization. He cautioned that FIPS needs further validation before being applied in clinical practice.
The study by Dr. Yang and his colleagues was published online Feb. 9 in the American Journal of Roentgenology. To their knowledge, this is the first study to validate FIPS in a cohort of Asian patients.
Decompensated cirrhosis can cause variceal bleeding and refractory ascites and may be life threatening. TIPS can manage these complications but comes with its own risks.
To determine which patients can best benefit from the procedure, researchers have proposed a variety of prognostic scoring systems. Some were developed for other purposes, such as predicting survival following hospitalization, rather than specifically for TIPS. Additionally, few studies have compared these approaches to each other.
A four-way comparison
To fill that gap, Dr. Yang and his colleagues compared four predictive models: the MELD, the sodium MELD (MELD-Na), the Chronic Liver Failure–Consortium Acute Decompensation (CLIF-CAD), and FIPS.
The MELD score uses serum bilirubin, serum creatinine, and the international normalized ratio (INR) of prothrombin time. MELD-Na adds sodium to this algorithm. The CLIF-CAD score is calculated using age, serum creatinine, INR, white blood count, and sodium level. FIPS, which was recently devised to predict results with TIPS, uses age, bilirubin, albumin, and creatinine.
To see which yielded more accurate predictions, Dr. Yang and his colleagues followed 383 patients with cirrhosis (mean age, 55 years; 341 with variceal bleeding and 42 with refractory ascites) who underwent TIPS placement at Wuhan Union Hospital between January 2016 and August 2021.
The most common cause of cirrhosis was hepatitis B infection (58.2% of patients), followed by hepatitis C infection (11.7%) and alcohol use (13.6%).
The researchers followed the patients for a median of 23.4 months. They lost track of 31 patients over that time, and another 72 died. The survival rate after TIPS placement was 92.3% at 6 months, 87.8% at 12 months, and 81.2% at 24 months. Thirty-seven patients received a TIPS revision.
In their first measure of the models’ accuracy, the researchers used a concordance index, which compares actual results with predicted results. The number of concordant pairs are divided by the total number of possible evaluation pairs. A score of 1 represents 100% accuracy.
By this measure, the prediction of survival at 6 months was highest for FIPS followed by CLIF-CAD, MELD, and MELD-Na. However, the confidence intervals overlapped.
FIPS also scored highest in the concordance index at 12 and 24 months.
In a second measure of the models’ accuracy, the researchers used Brier scores, which calculate the mean squared error between predicted probabilities and actual values. Like the concordance index, Brier scores range from 0.0 to 1.0 but differ in that the lowest Brier score number represents the highest accuracy.
At 6 months, the CLIF-CAD score was the best, at 0.074. MELD and FIPS were equivalent at 0.075, with MELD-Na coming in at 0.077. However, FIPS attained slightly better scores than the other systems at 12 and 24 months.
Is FIPS worth implementing?
With scores this close, it may not be worth changing the predictive model clinicians use for choosing TIPS candidates, said Nancy Reau, MD, chief of hepatology at Rush University Medical Center, Chicago, who was not involved in the study.
MELD scores are already programmed into many electronic medical record systems in the United States, and clinicians are familiar with using that system to aid in further decisions, such as decisions regarding other kinds of surgery, she told this news organization.
“If you’re going to try to advocate for a new system, you really have to show that the performance of the predictive score is monumentally better than the tried and true,” she said.
Dr. Yang and Dr. Reau report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new prognostic score is more accurate than the commonly used Model for End-Stage Liver Disease (MELD) in predicting post–transjugular intrahepatic portosystemic shunt (TIPS) survival, researchers say.
The Freiburg Index of Post-TIPS Survival (FIPS) could help patients and doctors weigh the benefits and risks of the procedure, said Chongtu Yang, MD, a postgraduate fellow at the Huazhong University of Science and Technology, Wuhan, China.
“For patients defined as high risk, the TIPS procedure may not be the optimal choice, and transplantation may be better,” Dr. Yang told this news organization. He cautioned that FIPS needs further validation before being applied in clinical practice.
The study by Dr. Yang and his colleagues was published online Feb. 9 in the American Journal of Roentgenology. To their knowledge, this is the first study to validate FIPS in a cohort of Asian patients.
Decompensated cirrhosis can cause variceal bleeding and refractory ascites and may be life threatening. TIPS can manage these complications but comes with its own risks.
To determine which patients can best benefit from the procedure, researchers have proposed a variety of prognostic scoring systems. Some were developed for other purposes, such as predicting survival following hospitalization, rather than specifically for TIPS. Additionally, few studies have compared these approaches to each other.
A four-way comparison
To fill that gap, Dr. Yang and his colleagues compared four predictive models: the MELD, the sodium MELD (MELD-Na), the Chronic Liver Failure–Consortium Acute Decompensation (CLIF-CAD), and FIPS.
The MELD score uses serum bilirubin, serum creatinine, and the international normalized ratio (INR) of prothrombin time. MELD-Na adds sodium to this algorithm. The CLIF-CAD score is calculated using age, serum creatinine, INR, white blood count, and sodium level. FIPS, which was recently devised to predict results with TIPS, uses age, bilirubin, albumin, and creatinine.
To see which yielded more accurate predictions, Dr. Yang and his colleagues followed 383 patients with cirrhosis (mean age, 55 years; 341 with variceal bleeding and 42 with refractory ascites) who underwent TIPS placement at Wuhan Union Hospital between January 2016 and August 2021.
The most common cause of cirrhosis was hepatitis B infection (58.2% of patients), followed by hepatitis C infection (11.7%) and alcohol use (13.6%).
The researchers followed the patients for a median of 23.4 months. They lost track of 31 patients over that time, and another 72 died. The survival rate after TIPS placement was 92.3% at 6 months, 87.8% at 12 months, and 81.2% at 24 months. Thirty-seven patients received a TIPS revision.
In their first measure of the models’ accuracy, the researchers used a concordance index, which compares actual results with predicted results. The number of concordant pairs are divided by the total number of possible evaluation pairs. A score of 1 represents 100% accuracy.
By this measure, the prediction of survival at 6 months was highest for FIPS followed by CLIF-CAD, MELD, and MELD-Na. However, the confidence intervals overlapped.
FIPS also scored highest in the concordance index at 12 and 24 months.
In a second measure of the models’ accuracy, the researchers used Brier scores, which calculate the mean squared error between predicted probabilities and actual values. Like the concordance index, Brier scores range from 0.0 to 1.0 but differ in that the lowest Brier score number represents the highest accuracy.
At 6 months, the CLIF-CAD score was the best, at 0.074. MELD and FIPS were equivalent at 0.075, with MELD-Na coming in at 0.077. However, FIPS attained slightly better scores than the other systems at 12 and 24 months.
Is FIPS worth implementing?
With scores this close, it may not be worth changing the predictive model clinicians use for choosing TIPS candidates, said Nancy Reau, MD, chief of hepatology at Rush University Medical Center, Chicago, who was not involved in the study.
MELD scores are already programmed into many electronic medical record systems in the United States, and clinicians are familiar with using that system to aid in further decisions, such as decisions regarding other kinds of surgery, she told this news organization.
“If you’re going to try to advocate for a new system, you really have to show that the performance of the predictive score is monumentally better than the tried and true,” she said.
Dr. Yang and Dr. Reau report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF ROENTGENOLOGY
Why pregnant people were left behind while vaccines moved at ‘warp speed’ to help the masses
Kia Slade was 7 months pregnant, unvaccinated, and fighting for breath, her oxygen levels plummeting, when her son came into the world last May.
A severe case of COVID-19 pneumonia had left Ms. Slade delirious. When the intensive care team tried to place an oxygen mask on her face, she snatched it away, she recalled. Her baby’s heart rate began to drop.
Ms. Slade’s doctor performed an emergency cesarean section at her bedside in the intensive care unit, delivering baby Tristan 10 weeks early. He weighed just 2 pounds, 14 ounces, about half the size of small full-term baby.
But Ms. Slade wouldn’t meet him until July. She was on a ventilator in a medically-induced coma for 8 weeks, and she developed a serious infection and blood clot while unconscious. It was only after a perilous 2½ months in the hospital, during which her heart stopped twice, that Ms. Slade was vaccinated against COVID-19.
“I wish I had gotten the vaccine earlier,” said Ms. Slade, 42, who remains too sick to return to work as a special education teacher in Baltimore. Doctors “kept pushing me to get vaccinated, but there just wasn’t enough information out there for me to do it.”
A year ago, there was little to no vaccine safety data for pregnant people like Ms. Slade, because they had been excluded from clinical trials run by Pfizer, Moderna, and other vaccine makers.
Lacking data, health experts were unsure and divided about how to advise expectant parents. Although U.S. health officials permitted pregnant people to be vaccinated, the World Health Organization in January 2021 actually discouraged them from doing so; it later reversed that recommendation.
The uncertainty led many women to delay vaccination, and only about two-thirds of the pregnant people who have been tracked by the Centers for Disease Control and Prevention were fully vaccinated as of Feb. 5, 2022, leaving many expectant moms at a high risk of infection and life-threatening complications.
More than 29,000 pregnant people have been hospitalized with COVID-19 and 274 have died, according to the CDC.
“There were surely women who were hospitalized because there wasn’t information available to them,” said Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Vaccine developers say that pregnant people – who have special health needs and risks – were excluded from clinical trials to protect them from potential side effects of novel technologies, including the Pfizer and Moderna mRNA vaccines and formulations made with cold viruses, such as the Johnson & Johnson vaccine.
But a KHN analysis also shows that pregnant people were left behind because including them in vaccine studies would have complicated and potentially delayed the delivery of COVID-19 vaccines to the broader population.
A growing number of women’s health researchers and advocates say that excluding pregnant people – and the months-long delay in recommending that they be immunized – helped fuel widespread vaccine hesitancy in this vulnerable group.
“Women and their unborn fetuses are dying of COVID infection,” said Jane Van Dis, MD, an ob.gyn. at the University of Rochester (N.Y.) Medical Center who has treated many patients like Ms. Slade. “Our failure as a society to vaccinate women in pregnancy will be remembered by the children and families who lost their mothers to this disease.”
New technology, uncertain risks
At the time COVID-19 vaccines were being developed, scientists had very little experience using mRNA vaccines in pregnant women, said Jacqueline Miller, MD, a senior vice president involved in vaccine research at Moderna.
“When you study anything in pregnant women, you have two patients, the mom and the unborn child,” Dr. Miller said. “Until we had more safety data on the platform, it wasn’t something we wanted to undertake.”
But Dr. Offit noted that vaccines have a strong record of safety in pregnancy and he sees no reason to have excluded pregnant people. None of the vaccines currently in use – including the chickenpox and rubella vaccines, which contain live viruses – have been shown to harm fetuses, he said. Doctors routinely recommend that pregnant people receive pertussis and flu vaccinations.
Dr. Offit, the coinventor of a rotavirus vaccine, said that some concerns about vaccines stem from commercial, not medical, interests. Drug makers don’t want to risk that their product will be blamed for any problems occurring in pregnant people, even if coincidental, he said.
“These companies don’t want bad news,” Dr. Offit said.
In the United States, health officials typically would have told expectant mothers not to take a vaccine that was untested during pregnancy, said Dr. Offit, a member of a committee that advises the Food and Drug Administration on vaccines.
Due to the urgency of the pandemic, health agencies instead permitted pregnant people to make up their own minds about vaccines without recommending them.
Women’s medical associations were also hampered by the lack of data. Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine actively encouraged pregnant people to be vaccinated until July 30, 2021, after the first real-world vaccine studies had been published. The CDC followed suit in August of 2021.
“If we had had this data in the beginning, we would have been able to vaccinate more women,” said Kelli Burroughs, MD, the department chair of obstetrics and gynecology at Memorial Hermann Sugar Land Hospital near Houston.
Yet anti-vaccine groups wasted no time in scaring pregnant people, flooding social media with misinformation about impaired fertility and harm to the fetus.
In the first few months after the COVID-19 vaccines were approved, some doctors were ambivalent about recommending them, and some still advise pregnant patients against vaccination.
An estimated 67% of pregnant people today are fully vaccinated, compared with about 89% of people 65 and older, another high-risk group, and 65% of Americans overall. Vaccination rates are lower among minorities, with 65% of expectant Hispanic mothers and 53% of pregnant African Americans fully vaccinated, according to the CDC.
Vaccination is especially important during pregnancy, because of increased risks of hospitalization, ICU admission, and mechanical ventilation, Dr. Burroughs said. A study released in February from the National Institutes of Health found that pregnant people with a moderate to severe COVID-19 infection also were more likely to have a C-section, deliver preterm, or develop a postpartum hemorrhage.
Black moms such as Ms. Slade were already at higher risk of maternal and infant mortality before the pandemic, because of higher underlying risks, unequal access to health care, and other factors. COVID-19 has magnified those risks, said Dr. Burroughs, who has persuaded reluctant patients by revealing that she had a healthy pregnancy and child after being vaccinated.
Ms. Slade said she has never opposed vaccines and had no hesitation about receiving other vaccines while pregnant. But she said she “just wasn’t comfortable” with COVID-19 shots.
“If there had been data out there saying the COVID shot was safe, and that nothing would happen to my baby and there was no risk of birth defects, I would have taken it,” said Ms. Slade, who has had type 2 diabetes for 12 years.
Working at warp speed
Government scientists at the NIH were concerned about the risk of COVID-19 to pregnant people from the very beginning and knew that expectant moms needed vaccines as much or more than anyone else, said Larry Corey, MD, a leader of the COVID-19 Prevention Network, which coordinated COVID-19 vaccine trials for the federal government.
But including pregnant volunteers in the larger vaccine trials could have led to interruptions and delays, Dr. Corey said. Researchers would have had to enroll thousands of pregnant volunteers to achieve statistically robust results that weren’t due to chance, he said.
Pregnancy can bring on a wide range of complications: gestational diabetes, hypertension, anemia, bleeding, blood clots, or problems with the placenta, for example. Up to 20% of people who know they’re pregnant miscarry. Because researchers would have been obliged to investigate any medical problem to make sure it wasn’t caused by one of the COVID-19 vaccines, including pregnant people might have meant having to hit pause on those trials, Dr. Corey said.
With death tolls from the pandemic mounting, “we had a mission to do this as quickly and as thoroughly as possible,” Dr. Corey said. Making COVID-19 vaccines available within a year “saved hundreds of thousands of lives.”
The first data on COVID-19 vaccine safety in pregnancy was published in April of 2021 when the CDC released an analysis of nearly 36,000 vaccinated pregnant people who had enrolled in a registry called V-safe, which allows users to log the dates of their vaccinations and any subsequent symptoms.
Later research showed that COVID-19 vaccines weren’t associated with increased risk of miscarriage or premature delivery.
Brenna Hughes, MD, a maternal-fetal medicine specialist and member of the American College of Obstetricians and Gynecologists’ COVID-19 expert group, agrees that adding pregnant people to large-scale COVID-19 vaccine and drug trials may have been impractical. But researchers could have launched parallel trials of pregnant women, once early studies showed the vaccines were safe in humans, she said.
“Would it have been hard? Everything with COVID is hard,” Dr. Hughes said. “But it would have been feasible.”
The FDA requires that researchers perform additional animal studies – called developmental and reproductive toxicity studies – before testing vaccines in pregnant people. Although these studies are essential, they take 5-6 months, and weren’t completed until late 2020, around the time the first COVID-19 vaccines were authorized for adults, said Emily Erbelding, MD, director of microbiology and infectious diseases at the National Institute of Allergy and Infectious Diseases, part of the NIH.
Pregnancy studies “were an afterthought,” said Irina Burd, MD, director of Johns Hopkins’ Integrated Research Center for Fetal Medicine and a professor of gynecology and obstetrics. “They should have been done sooner.”
The NIH is conducting a study of pregnant and postpartum people who decided on their own to be vaccinated, Dr. Erbelding said. The study is due to be completed by July 2023.
Janssen and Moderna are also conducting studies in pregnant people, both due to be completed in 2024.
Pfizer scientists encountered problems when they initiated a clinical trial, which would have randomly assigned pregnant people to receive either a vaccine or placebo. Once vaccines were widely available, many patients weren’t willing to take a chance on being unvaccinated until after delivery.
Pfizer has stopped recruiting patients and has not said whether it will publicly report any data from the trial.
Dr. Hughes said vaccine developers need to include pregnant people from the very beginning.
“There is this notion of protecting pregnant people from research,” Dr. Hughes said. “But we should be protecting patients through research, not from research.”
Recovering physically and emotionally
Ms. Slade still regrets being deprived of time with her children while she fought the disease.
Being on a ventilator kept her from spending those early weeks with her newborn, or from seeing her 9-year-old daughter, Zoe.
Even when Ms. Slade was finally able to see her son, she wasn’t able to tell him she loved him or sing a lullaby, or even talk at all, because of a breathing tube in her throat.
Today, Ms. Slade is a strong advocate of COVID-19 vaccinations, urging her friends and family to get their shots to avoid suffering the way she has.
Ms. Slade had to relearn to walk after being bedridden for weeks. Her many weeks on a ventilator may have contributed to her stomach paralysis, which often causes intense pain, nausea, and even vomiting when she eats or drinks. Ms. Slade weighs 50 pounds less today than before she became pregnant and has resorted to going to the emergency department when the pain is unbearable. “Most days, I’m just miserable,” she said.
Her family suffered as well. Like many babies born prematurely, Tristan, now nearly 9 months old and crawling, receives physical therapy to strengthen his muscles. At 15 pounds, Tristan is largely healthy, although his doctor said he has symptoms of asthma.
Ms. Slade said she would like to attend family counseling with Zoe, who rarely complains and tends to keep her feelings to herself. Ms. Slade said she knows her illness must have been terrifying for her little girl.
“The other day she was talking to me,” Ms. Slade said, “and she said, ‘You know, I almost had to bury you.’ ”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Kia Slade was 7 months pregnant, unvaccinated, and fighting for breath, her oxygen levels plummeting, when her son came into the world last May.
A severe case of COVID-19 pneumonia had left Ms. Slade delirious. When the intensive care team tried to place an oxygen mask on her face, she snatched it away, she recalled. Her baby’s heart rate began to drop.
Ms. Slade’s doctor performed an emergency cesarean section at her bedside in the intensive care unit, delivering baby Tristan 10 weeks early. He weighed just 2 pounds, 14 ounces, about half the size of small full-term baby.
But Ms. Slade wouldn’t meet him until July. She was on a ventilator in a medically-induced coma for 8 weeks, and she developed a serious infection and blood clot while unconscious. It was only after a perilous 2½ months in the hospital, during which her heart stopped twice, that Ms. Slade was vaccinated against COVID-19.
“I wish I had gotten the vaccine earlier,” said Ms. Slade, 42, who remains too sick to return to work as a special education teacher in Baltimore. Doctors “kept pushing me to get vaccinated, but there just wasn’t enough information out there for me to do it.”
A year ago, there was little to no vaccine safety data for pregnant people like Ms. Slade, because they had been excluded from clinical trials run by Pfizer, Moderna, and other vaccine makers.
Lacking data, health experts were unsure and divided about how to advise expectant parents. Although U.S. health officials permitted pregnant people to be vaccinated, the World Health Organization in January 2021 actually discouraged them from doing so; it later reversed that recommendation.
The uncertainty led many women to delay vaccination, and only about two-thirds of the pregnant people who have been tracked by the Centers for Disease Control and Prevention were fully vaccinated as of Feb. 5, 2022, leaving many expectant moms at a high risk of infection and life-threatening complications.
More than 29,000 pregnant people have been hospitalized with COVID-19 and 274 have died, according to the CDC.
“There were surely women who were hospitalized because there wasn’t information available to them,” said Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Vaccine developers say that pregnant people – who have special health needs and risks – were excluded from clinical trials to protect them from potential side effects of novel technologies, including the Pfizer and Moderna mRNA vaccines and formulations made with cold viruses, such as the Johnson & Johnson vaccine.
But a KHN analysis also shows that pregnant people were left behind because including them in vaccine studies would have complicated and potentially delayed the delivery of COVID-19 vaccines to the broader population.
A growing number of women’s health researchers and advocates say that excluding pregnant people – and the months-long delay in recommending that they be immunized – helped fuel widespread vaccine hesitancy in this vulnerable group.
“Women and their unborn fetuses are dying of COVID infection,” said Jane Van Dis, MD, an ob.gyn. at the University of Rochester (N.Y.) Medical Center who has treated many patients like Ms. Slade. “Our failure as a society to vaccinate women in pregnancy will be remembered by the children and families who lost their mothers to this disease.”
New technology, uncertain risks
At the time COVID-19 vaccines were being developed, scientists had very little experience using mRNA vaccines in pregnant women, said Jacqueline Miller, MD, a senior vice president involved in vaccine research at Moderna.
“When you study anything in pregnant women, you have two patients, the mom and the unborn child,” Dr. Miller said. “Until we had more safety data on the platform, it wasn’t something we wanted to undertake.”
But Dr. Offit noted that vaccines have a strong record of safety in pregnancy and he sees no reason to have excluded pregnant people. None of the vaccines currently in use – including the chickenpox and rubella vaccines, which contain live viruses – have been shown to harm fetuses, he said. Doctors routinely recommend that pregnant people receive pertussis and flu vaccinations.
Dr. Offit, the coinventor of a rotavirus vaccine, said that some concerns about vaccines stem from commercial, not medical, interests. Drug makers don’t want to risk that their product will be blamed for any problems occurring in pregnant people, even if coincidental, he said.
“These companies don’t want bad news,” Dr. Offit said.
In the United States, health officials typically would have told expectant mothers not to take a vaccine that was untested during pregnancy, said Dr. Offit, a member of a committee that advises the Food and Drug Administration on vaccines.
Due to the urgency of the pandemic, health agencies instead permitted pregnant people to make up their own minds about vaccines without recommending them.
Women’s medical associations were also hampered by the lack of data. Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine actively encouraged pregnant people to be vaccinated until July 30, 2021, after the first real-world vaccine studies had been published. The CDC followed suit in August of 2021.
“If we had had this data in the beginning, we would have been able to vaccinate more women,” said Kelli Burroughs, MD, the department chair of obstetrics and gynecology at Memorial Hermann Sugar Land Hospital near Houston.
Yet anti-vaccine groups wasted no time in scaring pregnant people, flooding social media with misinformation about impaired fertility and harm to the fetus.
In the first few months after the COVID-19 vaccines were approved, some doctors were ambivalent about recommending them, and some still advise pregnant patients against vaccination.
An estimated 67% of pregnant people today are fully vaccinated, compared with about 89% of people 65 and older, another high-risk group, and 65% of Americans overall. Vaccination rates are lower among minorities, with 65% of expectant Hispanic mothers and 53% of pregnant African Americans fully vaccinated, according to the CDC.
Vaccination is especially important during pregnancy, because of increased risks of hospitalization, ICU admission, and mechanical ventilation, Dr. Burroughs said. A study released in February from the National Institutes of Health found that pregnant people with a moderate to severe COVID-19 infection also were more likely to have a C-section, deliver preterm, or develop a postpartum hemorrhage.
Black moms such as Ms. Slade were already at higher risk of maternal and infant mortality before the pandemic, because of higher underlying risks, unequal access to health care, and other factors. COVID-19 has magnified those risks, said Dr. Burroughs, who has persuaded reluctant patients by revealing that she had a healthy pregnancy and child after being vaccinated.
Ms. Slade said she has never opposed vaccines and had no hesitation about receiving other vaccines while pregnant. But she said she “just wasn’t comfortable” with COVID-19 shots.
“If there had been data out there saying the COVID shot was safe, and that nothing would happen to my baby and there was no risk of birth defects, I would have taken it,” said Ms. Slade, who has had type 2 diabetes for 12 years.
Working at warp speed
Government scientists at the NIH were concerned about the risk of COVID-19 to pregnant people from the very beginning and knew that expectant moms needed vaccines as much or more than anyone else, said Larry Corey, MD, a leader of the COVID-19 Prevention Network, which coordinated COVID-19 vaccine trials for the federal government.
But including pregnant volunteers in the larger vaccine trials could have led to interruptions and delays, Dr. Corey said. Researchers would have had to enroll thousands of pregnant volunteers to achieve statistically robust results that weren’t due to chance, he said.
Pregnancy can bring on a wide range of complications: gestational diabetes, hypertension, anemia, bleeding, blood clots, or problems with the placenta, for example. Up to 20% of people who know they’re pregnant miscarry. Because researchers would have been obliged to investigate any medical problem to make sure it wasn’t caused by one of the COVID-19 vaccines, including pregnant people might have meant having to hit pause on those trials, Dr. Corey said.
With death tolls from the pandemic mounting, “we had a mission to do this as quickly and as thoroughly as possible,” Dr. Corey said. Making COVID-19 vaccines available within a year “saved hundreds of thousands of lives.”
The first data on COVID-19 vaccine safety in pregnancy was published in April of 2021 when the CDC released an analysis of nearly 36,000 vaccinated pregnant people who had enrolled in a registry called V-safe, which allows users to log the dates of their vaccinations and any subsequent symptoms.
Later research showed that COVID-19 vaccines weren’t associated with increased risk of miscarriage or premature delivery.
Brenna Hughes, MD, a maternal-fetal medicine specialist and member of the American College of Obstetricians and Gynecologists’ COVID-19 expert group, agrees that adding pregnant people to large-scale COVID-19 vaccine and drug trials may have been impractical. But researchers could have launched parallel trials of pregnant women, once early studies showed the vaccines were safe in humans, she said.
“Would it have been hard? Everything with COVID is hard,” Dr. Hughes said. “But it would have been feasible.”
The FDA requires that researchers perform additional animal studies – called developmental and reproductive toxicity studies – before testing vaccines in pregnant people. Although these studies are essential, they take 5-6 months, and weren’t completed until late 2020, around the time the first COVID-19 vaccines were authorized for adults, said Emily Erbelding, MD, director of microbiology and infectious diseases at the National Institute of Allergy and Infectious Diseases, part of the NIH.
Pregnancy studies “were an afterthought,” said Irina Burd, MD, director of Johns Hopkins’ Integrated Research Center for Fetal Medicine and a professor of gynecology and obstetrics. “They should have been done sooner.”
The NIH is conducting a study of pregnant and postpartum people who decided on their own to be vaccinated, Dr. Erbelding said. The study is due to be completed by July 2023.
Janssen and Moderna are also conducting studies in pregnant people, both due to be completed in 2024.
Pfizer scientists encountered problems when they initiated a clinical trial, which would have randomly assigned pregnant people to receive either a vaccine or placebo. Once vaccines were widely available, many patients weren’t willing to take a chance on being unvaccinated until after delivery.
Pfizer has stopped recruiting patients and has not said whether it will publicly report any data from the trial.
Dr. Hughes said vaccine developers need to include pregnant people from the very beginning.
“There is this notion of protecting pregnant people from research,” Dr. Hughes said. “But we should be protecting patients through research, not from research.”
Recovering physically and emotionally
Ms. Slade still regrets being deprived of time with her children while she fought the disease.
Being on a ventilator kept her from spending those early weeks with her newborn, or from seeing her 9-year-old daughter, Zoe.
Even when Ms. Slade was finally able to see her son, she wasn’t able to tell him she loved him or sing a lullaby, or even talk at all, because of a breathing tube in her throat.
Today, Ms. Slade is a strong advocate of COVID-19 vaccinations, urging her friends and family to get their shots to avoid suffering the way she has.
Ms. Slade had to relearn to walk after being bedridden for weeks. Her many weeks on a ventilator may have contributed to her stomach paralysis, which often causes intense pain, nausea, and even vomiting when she eats or drinks. Ms. Slade weighs 50 pounds less today than before she became pregnant and has resorted to going to the emergency department when the pain is unbearable. “Most days, I’m just miserable,” she said.
Her family suffered as well. Like many babies born prematurely, Tristan, now nearly 9 months old and crawling, receives physical therapy to strengthen his muscles. At 15 pounds, Tristan is largely healthy, although his doctor said he has symptoms of asthma.
Ms. Slade said she would like to attend family counseling with Zoe, who rarely complains and tends to keep her feelings to herself. Ms. Slade said she knows her illness must have been terrifying for her little girl.
“The other day she was talking to me,” Ms. Slade said, “and she said, ‘You know, I almost had to bury you.’ ”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Kia Slade was 7 months pregnant, unvaccinated, and fighting for breath, her oxygen levels plummeting, when her son came into the world last May.
A severe case of COVID-19 pneumonia had left Ms. Slade delirious. When the intensive care team tried to place an oxygen mask on her face, she snatched it away, she recalled. Her baby’s heart rate began to drop.
Ms. Slade’s doctor performed an emergency cesarean section at her bedside in the intensive care unit, delivering baby Tristan 10 weeks early. He weighed just 2 pounds, 14 ounces, about half the size of small full-term baby.
But Ms. Slade wouldn’t meet him until July. She was on a ventilator in a medically-induced coma for 8 weeks, and she developed a serious infection and blood clot while unconscious. It was only after a perilous 2½ months in the hospital, during which her heart stopped twice, that Ms. Slade was vaccinated against COVID-19.
“I wish I had gotten the vaccine earlier,” said Ms. Slade, 42, who remains too sick to return to work as a special education teacher in Baltimore. Doctors “kept pushing me to get vaccinated, but there just wasn’t enough information out there for me to do it.”
A year ago, there was little to no vaccine safety data for pregnant people like Ms. Slade, because they had been excluded from clinical trials run by Pfizer, Moderna, and other vaccine makers.
Lacking data, health experts were unsure and divided about how to advise expectant parents. Although U.S. health officials permitted pregnant people to be vaccinated, the World Health Organization in January 2021 actually discouraged them from doing so; it later reversed that recommendation.
The uncertainty led many women to delay vaccination, and only about two-thirds of the pregnant people who have been tracked by the Centers for Disease Control and Prevention were fully vaccinated as of Feb. 5, 2022, leaving many expectant moms at a high risk of infection and life-threatening complications.
More than 29,000 pregnant people have been hospitalized with COVID-19 and 274 have died, according to the CDC.
“There were surely women who were hospitalized because there wasn’t information available to them,” said Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Vaccine developers say that pregnant people – who have special health needs and risks – were excluded from clinical trials to protect them from potential side effects of novel technologies, including the Pfizer and Moderna mRNA vaccines and formulations made with cold viruses, such as the Johnson & Johnson vaccine.
But a KHN analysis also shows that pregnant people were left behind because including them in vaccine studies would have complicated and potentially delayed the delivery of COVID-19 vaccines to the broader population.
A growing number of women’s health researchers and advocates say that excluding pregnant people – and the months-long delay in recommending that they be immunized – helped fuel widespread vaccine hesitancy in this vulnerable group.
“Women and their unborn fetuses are dying of COVID infection,” said Jane Van Dis, MD, an ob.gyn. at the University of Rochester (N.Y.) Medical Center who has treated many patients like Ms. Slade. “Our failure as a society to vaccinate women in pregnancy will be remembered by the children and families who lost their mothers to this disease.”
New technology, uncertain risks
At the time COVID-19 vaccines were being developed, scientists had very little experience using mRNA vaccines in pregnant women, said Jacqueline Miller, MD, a senior vice president involved in vaccine research at Moderna.
“When you study anything in pregnant women, you have two patients, the mom and the unborn child,” Dr. Miller said. “Until we had more safety data on the platform, it wasn’t something we wanted to undertake.”
But Dr. Offit noted that vaccines have a strong record of safety in pregnancy and he sees no reason to have excluded pregnant people. None of the vaccines currently in use – including the chickenpox and rubella vaccines, which contain live viruses – have been shown to harm fetuses, he said. Doctors routinely recommend that pregnant people receive pertussis and flu vaccinations.
Dr. Offit, the coinventor of a rotavirus vaccine, said that some concerns about vaccines stem from commercial, not medical, interests. Drug makers don’t want to risk that their product will be blamed for any problems occurring in pregnant people, even if coincidental, he said.
“These companies don’t want bad news,” Dr. Offit said.
In the United States, health officials typically would have told expectant mothers not to take a vaccine that was untested during pregnancy, said Dr. Offit, a member of a committee that advises the Food and Drug Administration on vaccines.
Due to the urgency of the pandemic, health agencies instead permitted pregnant people to make up their own minds about vaccines without recommending them.
Women’s medical associations were also hampered by the lack of data. Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine actively encouraged pregnant people to be vaccinated until July 30, 2021, after the first real-world vaccine studies had been published. The CDC followed suit in August of 2021.
“If we had had this data in the beginning, we would have been able to vaccinate more women,” said Kelli Burroughs, MD, the department chair of obstetrics and gynecology at Memorial Hermann Sugar Land Hospital near Houston.
Yet anti-vaccine groups wasted no time in scaring pregnant people, flooding social media with misinformation about impaired fertility and harm to the fetus.
In the first few months after the COVID-19 vaccines were approved, some doctors were ambivalent about recommending them, and some still advise pregnant patients against vaccination.
An estimated 67% of pregnant people today are fully vaccinated, compared with about 89% of people 65 and older, another high-risk group, and 65% of Americans overall. Vaccination rates are lower among minorities, with 65% of expectant Hispanic mothers and 53% of pregnant African Americans fully vaccinated, according to the CDC.
Vaccination is especially important during pregnancy, because of increased risks of hospitalization, ICU admission, and mechanical ventilation, Dr. Burroughs said. A study released in February from the National Institutes of Health found that pregnant people with a moderate to severe COVID-19 infection also were more likely to have a C-section, deliver preterm, or develop a postpartum hemorrhage.
Black moms such as Ms. Slade were already at higher risk of maternal and infant mortality before the pandemic, because of higher underlying risks, unequal access to health care, and other factors. COVID-19 has magnified those risks, said Dr. Burroughs, who has persuaded reluctant patients by revealing that she had a healthy pregnancy and child after being vaccinated.
Ms. Slade said she has never opposed vaccines and had no hesitation about receiving other vaccines while pregnant. But she said she “just wasn’t comfortable” with COVID-19 shots.
“If there had been data out there saying the COVID shot was safe, and that nothing would happen to my baby and there was no risk of birth defects, I would have taken it,” said Ms. Slade, who has had type 2 diabetes for 12 years.
Working at warp speed
Government scientists at the NIH were concerned about the risk of COVID-19 to pregnant people from the very beginning and knew that expectant moms needed vaccines as much or more than anyone else, said Larry Corey, MD, a leader of the COVID-19 Prevention Network, which coordinated COVID-19 vaccine trials for the federal government.
But including pregnant volunteers in the larger vaccine trials could have led to interruptions and delays, Dr. Corey said. Researchers would have had to enroll thousands of pregnant volunteers to achieve statistically robust results that weren’t due to chance, he said.
Pregnancy can bring on a wide range of complications: gestational diabetes, hypertension, anemia, bleeding, blood clots, or problems with the placenta, for example. Up to 20% of people who know they’re pregnant miscarry. Because researchers would have been obliged to investigate any medical problem to make sure it wasn’t caused by one of the COVID-19 vaccines, including pregnant people might have meant having to hit pause on those trials, Dr. Corey said.
With death tolls from the pandemic mounting, “we had a mission to do this as quickly and as thoroughly as possible,” Dr. Corey said. Making COVID-19 vaccines available within a year “saved hundreds of thousands of lives.”
The first data on COVID-19 vaccine safety in pregnancy was published in April of 2021 when the CDC released an analysis of nearly 36,000 vaccinated pregnant people who had enrolled in a registry called V-safe, which allows users to log the dates of their vaccinations and any subsequent symptoms.
Later research showed that COVID-19 vaccines weren’t associated with increased risk of miscarriage or premature delivery.
Brenna Hughes, MD, a maternal-fetal medicine specialist and member of the American College of Obstetricians and Gynecologists’ COVID-19 expert group, agrees that adding pregnant people to large-scale COVID-19 vaccine and drug trials may have been impractical. But researchers could have launched parallel trials of pregnant women, once early studies showed the vaccines were safe in humans, she said.
“Would it have been hard? Everything with COVID is hard,” Dr. Hughes said. “But it would have been feasible.”
The FDA requires that researchers perform additional animal studies – called developmental and reproductive toxicity studies – before testing vaccines in pregnant people. Although these studies are essential, they take 5-6 months, and weren’t completed until late 2020, around the time the first COVID-19 vaccines were authorized for adults, said Emily Erbelding, MD, director of microbiology and infectious diseases at the National Institute of Allergy and Infectious Diseases, part of the NIH.
Pregnancy studies “were an afterthought,” said Irina Burd, MD, director of Johns Hopkins’ Integrated Research Center for Fetal Medicine and a professor of gynecology and obstetrics. “They should have been done sooner.”
The NIH is conducting a study of pregnant and postpartum people who decided on their own to be vaccinated, Dr. Erbelding said. The study is due to be completed by July 2023.
Janssen and Moderna are also conducting studies in pregnant people, both due to be completed in 2024.
Pfizer scientists encountered problems when they initiated a clinical trial, which would have randomly assigned pregnant people to receive either a vaccine or placebo. Once vaccines were widely available, many patients weren’t willing to take a chance on being unvaccinated until after delivery.
Pfizer has stopped recruiting patients and has not said whether it will publicly report any data from the trial.
Dr. Hughes said vaccine developers need to include pregnant people from the very beginning.
“There is this notion of protecting pregnant people from research,” Dr. Hughes said. “But we should be protecting patients through research, not from research.”
Recovering physically and emotionally
Ms. Slade still regrets being deprived of time with her children while she fought the disease.
Being on a ventilator kept her from spending those early weeks with her newborn, or from seeing her 9-year-old daughter, Zoe.
Even when Ms. Slade was finally able to see her son, she wasn’t able to tell him she loved him or sing a lullaby, or even talk at all, because of a breathing tube in her throat.
Today, Ms. Slade is a strong advocate of COVID-19 vaccinations, urging her friends and family to get their shots to avoid suffering the way she has.
Ms. Slade had to relearn to walk after being bedridden for weeks. Her many weeks on a ventilator may have contributed to her stomach paralysis, which often causes intense pain, nausea, and even vomiting when she eats or drinks. Ms. Slade weighs 50 pounds less today than before she became pregnant and has resorted to going to the emergency department when the pain is unbearable. “Most days, I’m just miserable,” she said.
Her family suffered as well. Like many babies born prematurely, Tristan, now nearly 9 months old and crawling, receives physical therapy to strengthen his muscles. At 15 pounds, Tristan is largely healthy, although his doctor said he has symptoms of asthma.
Ms. Slade said she would like to attend family counseling with Zoe, who rarely complains and tends to keep her feelings to herself. Ms. Slade said she knows her illness must have been terrifying for her little girl.
“The other day she was talking to me,” Ms. Slade said, “and she said, ‘You know, I almost had to bury you.’ ”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Epidural may lower odds of severe maternal birth complications
Use of neuraxial analgesia for vaginal delivery is associated with a 14% decreased risk for severe maternal morbidity, in part from a reduction in postpartum hemorrhage, new research shows.
The findings indicate that increasing the use of epidural or combined spinal-epidural analgesia may improve maternal health outcomes, especially for Hispanic, Black, and uninsured women who are less likely than White women to receive these interventions, according to the researchers, who published their findings online in JAMA Network Open.
About 80% of non-Hispanic White women receive neuraxial analgesia during labor in the United States, compared with 70% of non-Hispanic Black women and 65% of Hispanic women, according to birth certificate data. Among women without health insurance, half receive epidurals.
Programs that inform pregnant women about epidural use, expand Medicaid, and provide in-house obstetric anesthesia teams “may improve patient participation in clinical decision making and access to care,” study author Guohua Li, MD, DrPH, of Columbia University, New York, said in a statement about the research.
Earlier data from France showed that neuraxial analgesia is associated with reduced risk for severe postpartum hemorrhage. To examine the association between labor neuraxial analgesia and severe maternal morbidity in the United States, Dr. Li and colleagues analyzed more than 575,000 vaginal deliveries in New York hospitals between 2010 and 2017; about half (47.4%) of the women received epidurals during labor.
The researchers focused on severe maternal morbidity, including 16 complications, such as heart failure and sepsis, and five procedures, including hysterectomy and ventilation.
They also considered patient characteristics and comorbidities and hospital-related factors to identify patients who were at higher risk for injury or death.
Severe maternal morbidity occurred in 1.3% of the women. Of the 7,712 women with severe morbidity, more than one in three (35.6%) experienced postpartum hemorrhage.
The overall incidence of severe maternal morbidity was 1.3% among women who received an epidural injection and 1.4% among those who did not. In a weighted analysis, the adjusted odds ratio of severe maternal morbidity associated with epidurals was 0.86 (95% confidence interval, 0.82-0.90).
The study is limited by its observational design and does not prove causation, the authors acknowledged.
“Labor neuraxial analgesia may facilitate early evaluation and management of the third stage of labor to avoid escalation of postpartum hemorrhaging into grave complications and death,” study author Jean Guglielminotti, MD, PhD, an anesthesiologist at Columbia University, said in a statement.
Concerning trends
The Department of Health & Human Services has labeled severe maternal morbidity a public health priority. Recent data from the Centers for Disease Control and Prevention show an increase in maternal mortality rates and worsening disparities by race and ethnicity.
According to the CDC, 861 women died of maternal causes in 2020, up from 754 in 2019. The rate of maternal mortality increased from 20.1 to 23.8 deaths per 100,000 live births.
For Black women, however, the maternal mortality rate was far higher: 55.3 deaths per 100,000 live births – nearly triple the figure of 19.1 per 100,000 for White women. Between 2019 and 2020, the mortality rate increased significantly for Black and Hispanic women, but not White mothers.
Researchers affiliated with University of Toronto and the Hospital for Sick Children agreed in an accompanying editorial that more access to neuraxial labor analgesia for vaginal delivery might improve maternal health outcomes and “may be a strategy well worth pursuing in public health policy.”
The intervention is relatively safe and can “alleviate discomfort and distress,” they wrote.
Neuraxial anesthesia in surgical procedures has been shown to decrease the risk for complications like deep vein thrombosis, pulmonary embolus, transfusion requirements, and kidney failure, said editorialists Evelina Pankiv, MD; Alan Yang, MSc; and Kazuyoshi Aoyama, MD, PhD.
Benefits potentially could stem from improving blood flow, mitigating hypercoagulation, or reducing surgical stress response. But there are rare risks to consider as well, including hemorrhage, infection, and neurologic injury, they added.
Guglielminotti disclosed grants from the National Institute on Minority Health and Health Disparities. Dr. Aoyama reported receiving grants from the Perioperative Services Facilitator Grant Program and Hospital for Sick Children. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of neuraxial analgesia for vaginal delivery is associated with a 14% decreased risk for severe maternal morbidity, in part from a reduction in postpartum hemorrhage, new research shows.
The findings indicate that increasing the use of epidural or combined spinal-epidural analgesia may improve maternal health outcomes, especially for Hispanic, Black, and uninsured women who are less likely than White women to receive these interventions, according to the researchers, who published their findings online in JAMA Network Open.
About 80% of non-Hispanic White women receive neuraxial analgesia during labor in the United States, compared with 70% of non-Hispanic Black women and 65% of Hispanic women, according to birth certificate data. Among women without health insurance, half receive epidurals.
Programs that inform pregnant women about epidural use, expand Medicaid, and provide in-house obstetric anesthesia teams “may improve patient participation in clinical decision making and access to care,” study author Guohua Li, MD, DrPH, of Columbia University, New York, said in a statement about the research.
Earlier data from France showed that neuraxial analgesia is associated with reduced risk for severe postpartum hemorrhage. To examine the association between labor neuraxial analgesia and severe maternal morbidity in the United States, Dr. Li and colleagues analyzed more than 575,000 vaginal deliveries in New York hospitals between 2010 and 2017; about half (47.4%) of the women received epidurals during labor.
The researchers focused on severe maternal morbidity, including 16 complications, such as heart failure and sepsis, and five procedures, including hysterectomy and ventilation.
They also considered patient characteristics and comorbidities and hospital-related factors to identify patients who were at higher risk for injury or death.
Severe maternal morbidity occurred in 1.3% of the women. Of the 7,712 women with severe morbidity, more than one in three (35.6%) experienced postpartum hemorrhage.
The overall incidence of severe maternal morbidity was 1.3% among women who received an epidural injection and 1.4% among those who did not. In a weighted analysis, the adjusted odds ratio of severe maternal morbidity associated with epidurals was 0.86 (95% confidence interval, 0.82-0.90).
The study is limited by its observational design and does not prove causation, the authors acknowledged.
“Labor neuraxial analgesia may facilitate early evaluation and management of the third stage of labor to avoid escalation of postpartum hemorrhaging into grave complications and death,” study author Jean Guglielminotti, MD, PhD, an anesthesiologist at Columbia University, said in a statement.
Concerning trends
The Department of Health & Human Services has labeled severe maternal morbidity a public health priority. Recent data from the Centers for Disease Control and Prevention show an increase in maternal mortality rates and worsening disparities by race and ethnicity.
According to the CDC, 861 women died of maternal causes in 2020, up from 754 in 2019. The rate of maternal mortality increased from 20.1 to 23.8 deaths per 100,000 live births.
For Black women, however, the maternal mortality rate was far higher: 55.3 deaths per 100,000 live births – nearly triple the figure of 19.1 per 100,000 for White women. Between 2019 and 2020, the mortality rate increased significantly for Black and Hispanic women, but not White mothers.
Researchers affiliated with University of Toronto and the Hospital for Sick Children agreed in an accompanying editorial that more access to neuraxial labor analgesia for vaginal delivery might improve maternal health outcomes and “may be a strategy well worth pursuing in public health policy.”
The intervention is relatively safe and can “alleviate discomfort and distress,” they wrote.
Neuraxial anesthesia in surgical procedures has been shown to decrease the risk for complications like deep vein thrombosis, pulmonary embolus, transfusion requirements, and kidney failure, said editorialists Evelina Pankiv, MD; Alan Yang, MSc; and Kazuyoshi Aoyama, MD, PhD.
Benefits potentially could stem from improving blood flow, mitigating hypercoagulation, or reducing surgical stress response. But there are rare risks to consider as well, including hemorrhage, infection, and neurologic injury, they added.
Guglielminotti disclosed grants from the National Institute on Minority Health and Health Disparities. Dr. Aoyama reported receiving grants from the Perioperative Services Facilitator Grant Program and Hospital for Sick Children. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of neuraxial analgesia for vaginal delivery is associated with a 14% decreased risk for severe maternal morbidity, in part from a reduction in postpartum hemorrhage, new research shows.
The findings indicate that increasing the use of epidural or combined spinal-epidural analgesia may improve maternal health outcomes, especially for Hispanic, Black, and uninsured women who are less likely than White women to receive these interventions, according to the researchers, who published their findings online in JAMA Network Open.
About 80% of non-Hispanic White women receive neuraxial analgesia during labor in the United States, compared with 70% of non-Hispanic Black women and 65% of Hispanic women, according to birth certificate data. Among women without health insurance, half receive epidurals.
Programs that inform pregnant women about epidural use, expand Medicaid, and provide in-house obstetric anesthesia teams “may improve patient participation in clinical decision making and access to care,” study author Guohua Li, MD, DrPH, of Columbia University, New York, said in a statement about the research.
Earlier data from France showed that neuraxial analgesia is associated with reduced risk for severe postpartum hemorrhage. To examine the association between labor neuraxial analgesia and severe maternal morbidity in the United States, Dr. Li and colleagues analyzed more than 575,000 vaginal deliveries in New York hospitals between 2010 and 2017; about half (47.4%) of the women received epidurals during labor.
The researchers focused on severe maternal morbidity, including 16 complications, such as heart failure and sepsis, and five procedures, including hysterectomy and ventilation.
They also considered patient characteristics and comorbidities and hospital-related factors to identify patients who were at higher risk for injury or death.
Severe maternal morbidity occurred in 1.3% of the women. Of the 7,712 women with severe morbidity, more than one in three (35.6%) experienced postpartum hemorrhage.
The overall incidence of severe maternal morbidity was 1.3% among women who received an epidural injection and 1.4% among those who did not. In a weighted analysis, the adjusted odds ratio of severe maternal morbidity associated with epidurals was 0.86 (95% confidence interval, 0.82-0.90).
The study is limited by its observational design and does not prove causation, the authors acknowledged.
“Labor neuraxial analgesia may facilitate early evaluation and management of the third stage of labor to avoid escalation of postpartum hemorrhaging into grave complications and death,” study author Jean Guglielminotti, MD, PhD, an anesthesiologist at Columbia University, said in a statement.
Concerning trends
The Department of Health & Human Services has labeled severe maternal morbidity a public health priority. Recent data from the Centers for Disease Control and Prevention show an increase in maternal mortality rates and worsening disparities by race and ethnicity.
According to the CDC, 861 women died of maternal causes in 2020, up from 754 in 2019. The rate of maternal mortality increased from 20.1 to 23.8 deaths per 100,000 live births.
For Black women, however, the maternal mortality rate was far higher: 55.3 deaths per 100,000 live births – nearly triple the figure of 19.1 per 100,000 for White women. Between 2019 and 2020, the mortality rate increased significantly for Black and Hispanic women, but not White mothers.
Researchers affiliated with University of Toronto and the Hospital for Sick Children agreed in an accompanying editorial that more access to neuraxial labor analgesia for vaginal delivery might improve maternal health outcomes and “may be a strategy well worth pursuing in public health policy.”
The intervention is relatively safe and can “alleviate discomfort and distress,” they wrote.
Neuraxial anesthesia in surgical procedures has been shown to decrease the risk for complications like deep vein thrombosis, pulmonary embolus, transfusion requirements, and kidney failure, said editorialists Evelina Pankiv, MD; Alan Yang, MSc; and Kazuyoshi Aoyama, MD, PhD.
Benefits potentially could stem from improving blood flow, mitigating hypercoagulation, or reducing surgical stress response. But there are rare risks to consider as well, including hemorrhage, infection, and neurologic injury, they added.
Guglielminotti disclosed grants from the National Institute on Minority Health and Health Disparities. Dr. Aoyama reported receiving grants from the Perioperative Services Facilitator Grant Program and Hospital for Sick Children. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Stress and infertility – is it a proven cause and effect?
“Just relax, stop thinking about it and, more than likely, it will happen.” If ever there was a controversial subject in medicine, especially in reproduction, the relationship between stress and infertility would be high on the list. Who among us has not overheard or even personally shared with an infertility patient that they should try and reduce their stress to improve fertility? The theory is certainly not new. Hippocrates, back in the 5th century B.C., was one of the first to associate a woman’s psychological state with her reproductive potential. His contention was that a physical sign of psychological stress in women (which scholars later dubbed “hysteria”) could result in sterility. In medieval times, a German abbess and mystic named Hildegard of Bingen posited women suffering from melancholy – a condition that we today might call depression – were infertile as a result.
The deeper meaning behind the flippant advice to relax is implicit blame; that is, a woman interprets the link of stress and infertility as a declaration that she is sabotaging reproduction. Not only is this assumption flawed, but it does further damage to a woman’s emotional fragility. To provide the presumption of stress affecting reproduction, a recent survey of over 5,000 infertility patients found, remarkably, 98% considered emotional stress as either a cause or a contributor to infertility, and 31% believed stress was a cause of miscarriage, although racial differences existed (J Assist Reprod Genet. 2021 Apr;38[4]:877-87). This relationship was mostly seen in women who used complementary and alternative medicine, Black women, and those who frequented Internet search engines. Whereas women who had a professional degree, had more infertility insurance coverage, and were nonreligious were less likely to attribute stress to infertility. Intriguingly, the more engaged the physicians, the less patients linked stress with infertility, while the contrary also applied.
The power of stress can be exemplified by the pathophysiology of amenorrhea. Functional hypothalamic amenorrhea is the most common cause of the female athlete triad of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences (Endocrines. 2021;2:203-11). Stress of infertility has also been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities (J Psychosom Obstet Gynaecol. 1993;14[Suppl]:45-52).
A definitive link between stress and infertility is evasive because of the lack of controlled, prospective longitudinal studies and the challenge of reducing variables in the analysis. The question remains which developed initially – the stress or the infertility? Infertility treatment is a physical, emotional, and financial investment. Stress and the duration of infertility are correlative. The additive factor is that poor insurance coverage for costly fertility treatment can not only heighten stress but, concurrently, subject the patient to the risk of exploitation driven by desperation whereby they accept unproven “add-ons” offered with assisted reproductive technologies (ART).
Both acute and chronic stress affect the number of oocytes retrieved and fertilized with ART as well as live birth delivery and birth weights (Fertil Steril. 2001;76:675-87). Men are also affected by stress, which is manifested by decreased libido and impaired semen, further compromised as the duration of infertility continues. The gut-derived hormone ghrelin appears to play a role with stress and reproduction (Endocr Rev. 2017;38:432-67).
As the relationship between stress and infertility is far from proven, there are conflicting study results. Two meta-analyses failed to show any association between stress and the outcomes of ART cycles (Hum Reprod. 2011;26:2763-76; BMJ. 2011;342:d223). In contrast, a recent study suggested stress during infertility treatment was contributed by the variables of low spousal support, financial constraints, and social coercion in the early years of marriage (J Hum Reprod Sci. 2018;11:172-9). Emotional distress was found to be three times greater in women whose families had unrealistic expectations from treatments.
Fortunately, psychotherapy during the ART cycle has demonstrated a benefit in outcomes. Domar revealed psychological support and cognitive behavior therapy resulted in higher pregnancy rates than in the control group (Fertil Steril. 2000;73:805-12). Another recent study appears to support stress reduction improving reproductive potential (Dialogues Clin Neurosci. 2018;20[1]:41-7).
Given the evidence provided in this article, it behooves infertility clinics to address baseline (chronic) stress and acute stress (because of infertility) prior to initiating treatment (see Figure). While the definitive answer addressing the impact of stress on reproduction remains unknown, we may share with our patients a definition in which they may find enlightenment, “Stress is trying to control an event in which one is incapable.”
Dr. Mark P Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
“Just relax, stop thinking about it and, more than likely, it will happen.” If ever there was a controversial subject in medicine, especially in reproduction, the relationship between stress and infertility would be high on the list. Who among us has not overheard or even personally shared with an infertility patient that they should try and reduce their stress to improve fertility? The theory is certainly not new. Hippocrates, back in the 5th century B.C., was one of the first to associate a woman’s psychological state with her reproductive potential. His contention was that a physical sign of psychological stress in women (which scholars later dubbed “hysteria”) could result in sterility. In medieval times, a German abbess and mystic named Hildegard of Bingen posited women suffering from melancholy – a condition that we today might call depression – were infertile as a result.
The deeper meaning behind the flippant advice to relax is implicit blame; that is, a woman interprets the link of stress and infertility as a declaration that she is sabotaging reproduction. Not only is this assumption flawed, but it does further damage to a woman’s emotional fragility. To provide the presumption of stress affecting reproduction, a recent survey of over 5,000 infertility patients found, remarkably, 98% considered emotional stress as either a cause or a contributor to infertility, and 31% believed stress was a cause of miscarriage, although racial differences existed (J Assist Reprod Genet. 2021 Apr;38[4]:877-87). This relationship was mostly seen in women who used complementary and alternative medicine, Black women, and those who frequented Internet search engines. Whereas women who had a professional degree, had more infertility insurance coverage, and were nonreligious were less likely to attribute stress to infertility. Intriguingly, the more engaged the physicians, the less patients linked stress with infertility, while the contrary also applied.
The power of stress can be exemplified by the pathophysiology of amenorrhea. Functional hypothalamic amenorrhea is the most common cause of the female athlete triad of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences (Endocrines. 2021;2:203-11). Stress of infertility has also been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities (J Psychosom Obstet Gynaecol. 1993;14[Suppl]:45-52).
A definitive link between stress and infertility is evasive because of the lack of controlled, prospective longitudinal studies and the challenge of reducing variables in the analysis. The question remains which developed initially – the stress or the infertility? Infertility treatment is a physical, emotional, and financial investment. Stress and the duration of infertility are correlative. The additive factor is that poor insurance coverage for costly fertility treatment can not only heighten stress but, concurrently, subject the patient to the risk of exploitation driven by desperation whereby they accept unproven “add-ons” offered with assisted reproductive technologies (ART).
Both acute and chronic stress affect the number of oocytes retrieved and fertilized with ART as well as live birth delivery and birth weights (Fertil Steril. 2001;76:675-87). Men are also affected by stress, which is manifested by decreased libido and impaired semen, further compromised as the duration of infertility continues. The gut-derived hormone ghrelin appears to play a role with stress and reproduction (Endocr Rev. 2017;38:432-67).
As the relationship between stress and infertility is far from proven, there are conflicting study results. Two meta-analyses failed to show any association between stress and the outcomes of ART cycles (Hum Reprod. 2011;26:2763-76; BMJ. 2011;342:d223). In contrast, a recent study suggested stress during infertility treatment was contributed by the variables of low spousal support, financial constraints, and social coercion in the early years of marriage (J Hum Reprod Sci. 2018;11:172-9). Emotional distress was found to be three times greater in women whose families had unrealistic expectations from treatments.
Fortunately, psychotherapy during the ART cycle has demonstrated a benefit in outcomes. Domar revealed psychological support and cognitive behavior therapy resulted in higher pregnancy rates than in the control group (Fertil Steril. 2000;73:805-12). Another recent study appears to support stress reduction improving reproductive potential (Dialogues Clin Neurosci. 2018;20[1]:41-7).
Given the evidence provided in this article, it behooves infertility clinics to address baseline (chronic) stress and acute stress (because of infertility) prior to initiating treatment (see Figure). While the definitive answer addressing the impact of stress on reproduction remains unknown, we may share with our patients a definition in which they may find enlightenment, “Stress is trying to control an event in which one is incapable.”
Dr. Mark P Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
“Just relax, stop thinking about it and, more than likely, it will happen.” If ever there was a controversial subject in medicine, especially in reproduction, the relationship between stress and infertility would be high on the list. Who among us has not overheard or even personally shared with an infertility patient that they should try and reduce their stress to improve fertility? The theory is certainly not new. Hippocrates, back in the 5th century B.C., was one of the first to associate a woman’s psychological state with her reproductive potential. His contention was that a physical sign of psychological stress in women (which scholars later dubbed “hysteria”) could result in sterility. In medieval times, a German abbess and mystic named Hildegard of Bingen posited women suffering from melancholy – a condition that we today might call depression – were infertile as a result.
The deeper meaning behind the flippant advice to relax is implicit blame; that is, a woman interprets the link of stress and infertility as a declaration that she is sabotaging reproduction. Not only is this assumption flawed, but it does further damage to a woman’s emotional fragility. To provide the presumption of stress affecting reproduction, a recent survey of over 5,000 infertility patients found, remarkably, 98% considered emotional stress as either a cause or a contributor to infertility, and 31% believed stress was a cause of miscarriage, although racial differences existed (J Assist Reprod Genet. 2021 Apr;38[4]:877-87). This relationship was mostly seen in women who used complementary and alternative medicine, Black women, and those who frequented Internet search engines. Whereas women who had a professional degree, had more infertility insurance coverage, and were nonreligious were less likely to attribute stress to infertility. Intriguingly, the more engaged the physicians, the less patients linked stress with infertility, while the contrary also applied.
The power of stress can be exemplified by the pathophysiology of amenorrhea. Functional hypothalamic amenorrhea is the most common cause of the female athlete triad of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences (Endocrines. 2021;2:203-11). Stress of infertility has also been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities (J Psychosom Obstet Gynaecol. 1993;14[Suppl]:45-52).
A definitive link between stress and infertility is evasive because of the lack of controlled, prospective longitudinal studies and the challenge of reducing variables in the analysis. The question remains which developed initially – the stress or the infertility? Infertility treatment is a physical, emotional, and financial investment. Stress and the duration of infertility are correlative. The additive factor is that poor insurance coverage for costly fertility treatment can not only heighten stress but, concurrently, subject the patient to the risk of exploitation driven by desperation whereby they accept unproven “add-ons” offered with assisted reproductive technologies (ART).
Both acute and chronic stress affect the number of oocytes retrieved and fertilized with ART as well as live birth delivery and birth weights (Fertil Steril. 2001;76:675-87). Men are also affected by stress, which is manifested by decreased libido and impaired semen, further compromised as the duration of infertility continues. The gut-derived hormone ghrelin appears to play a role with stress and reproduction (Endocr Rev. 2017;38:432-67).
As the relationship between stress and infertility is far from proven, there are conflicting study results. Two meta-analyses failed to show any association between stress and the outcomes of ART cycles (Hum Reprod. 2011;26:2763-76; BMJ. 2011;342:d223). In contrast, a recent study suggested stress during infertility treatment was contributed by the variables of low spousal support, financial constraints, and social coercion in the early years of marriage (J Hum Reprod Sci. 2018;11:172-9). Emotional distress was found to be three times greater in women whose families had unrealistic expectations from treatments.
Fortunately, psychotherapy during the ART cycle has demonstrated a benefit in outcomes. Domar revealed psychological support and cognitive behavior therapy resulted in higher pregnancy rates than in the control group (Fertil Steril. 2000;73:805-12). Another recent study appears to support stress reduction improving reproductive potential (Dialogues Clin Neurosci. 2018;20[1]:41-7).
Given the evidence provided in this article, it behooves infertility clinics to address baseline (chronic) stress and acute stress (because of infertility) prior to initiating treatment (see Figure). While the definitive answer addressing the impact of stress on reproduction remains unknown, we may share with our patients a definition in which they may find enlightenment, “Stress is trying to control an event in which one is incapable.”
Dr. Mark P Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Novel scoring system emerges for alcoholic hepatitis mortality
A new scoring system proved more accurate than several other available models at predicting the 30-day mortality risk for patients with alcohol-associated hepatitis (AH), according to new data.
The system, called the Mortality Index for Alcohol-Associated Hepatitis (MIAAH), was created by a team of Mayo Clinic researchers, who published their results in Mayo Clinic Proceedings.
Among the currently available prognostic models for assessing AH severity are the Model for End-Stage Liver Disease (MELD); the Age, serum Bilirubin, International normalized ratio, and serum Creatinine (ABIC) score; the Maddrey Discriminant Function (MDF); and the Glasgow Alcoholic Hepatitis Score (GAHS). However, these models have poor accuracy, with the area under the curve between 0.71 and 0.77.
By comparison, the new model has an accuracy of 86% in predicting 30-day mortality for AH, said coauthor Ashwani K. Singal, MD, MS, professor of medicine at the University of South Dakota Sanford School of Medicine, Sioux Falls.
“It’s a better predictor of the outcome, and that’s what sets it apart,” he told this news organization. “I think providers and patients will benefit.”
He said accuracy in determining the likelihood of death can help clinicians better determine treatment options and prepare patients and their families.
Camille Kezer, MD, a Mayo Clinic resident internist and first author of the paper, said in a statement, “MIAAH also has the advantage of performing well in patients, regardless of whether they’ve been treated with steroids, which makes it generalizable.”
Creating and validating the MIAAH
Researchers analyzed the health records of 266 eligible patients diagnosed with AH between 1998 and 2018 at the Mayo Clinic, Rochester, Minn. The patients collectively had a 30-day mortality rate of 19.2%.
They then studied the effect of several variables, of which the following were found to be significantly associated with mortality: age (P = .002), blood urea nitrogen (P = .003), albumin (P = .01), bilirubin (P = .02), and international normalized ratio (P = .001).
Mayo researchers built the MIAAH model using these variables and found that it was able to achieve a C statistic of 0.86, which translates into its being able to accurately predict mortality more than 86% of the time. When tested in the initial cohort of 266 patients, MIAAH had a significantly superior C statistic compared with several other available models, such as the MELD, MDF, and GAHS, although not for the ABIC.
The researchers then tested the MIAAH model in a validation cohort of 249 patients from health care centers at the University of South Dakota, Sioux Falls, and the University of Kansas, Lawrence. In this cohort, the MIAAH’s C statistic decreased to 0.73, which remained significantly more accurate than the MDF but was comparable to that found with the MELD.
Helping with transplant decisions
There are no pharmacologic treatments that can reduce 90-day mortality in severe cases of AH, and only a small survival benefit at 30 days has been reported with prednisolone use.
With a shortage of liver donors, many centers still require 6 months of alcohol abstinence for transplant consideration, although exceptions are sometimes made for cases of early transplant.
A model that more accurately predicts who is at the highest risk of dying within a month can help clinicians decide how best to proceed, Dr. Singal said.
Paul Martin, MD, chief of the division of digestive health and liver diseases, Mandel Chair in gastroenterology, and professor of medicine at the University of Miami, told this news organization that the model is potentially important in light of the rising prevalence of AH.
“The numbers of patients with AH are unequivocally increasing and often in young patients,” he noted, presenting difficult choices of who to treat with steroids and who to refer for a transplant.
“This model is certainly timely,” he said. “We need more accuracy in predicting which patients will recover with medical therapy and which patients won’t in the absence of a liver transplant.”
He noted, however, that the study’s retrospective design requires that it’s validated prospectively: “not only looking at the outcome in terms of mortality of patients, but its potential utility in identifying candidates for liver transplantation who are not going to recover on their own.”
He said it was unlikely that the model would replace others, particularly MELD, which is ingrained in practices in the United States and other countries, but may instead have a complementary role.
Dr. Singal, Dr. Kezer, and Dr. Martin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new scoring system proved more accurate than several other available models at predicting the 30-day mortality risk for patients with alcohol-associated hepatitis (AH), according to new data.
The system, called the Mortality Index for Alcohol-Associated Hepatitis (MIAAH), was created by a team of Mayo Clinic researchers, who published their results in Mayo Clinic Proceedings.
Among the currently available prognostic models for assessing AH severity are the Model for End-Stage Liver Disease (MELD); the Age, serum Bilirubin, International normalized ratio, and serum Creatinine (ABIC) score; the Maddrey Discriminant Function (MDF); and the Glasgow Alcoholic Hepatitis Score (GAHS). However, these models have poor accuracy, with the area under the curve between 0.71 and 0.77.
By comparison, the new model has an accuracy of 86% in predicting 30-day mortality for AH, said coauthor Ashwani K. Singal, MD, MS, professor of medicine at the University of South Dakota Sanford School of Medicine, Sioux Falls.
“It’s a better predictor of the outcome, and that’s what sets it apart,” he told this news organization. “I think providers and patients will benefit.”
He said accuracy in determining the likelihood of death can help clinicians better determine treatment options and prepare patients and their families.
Camille Kezer, MD, a Mayo Clinic resident internist and first author of the paper, said in a statement, “MIAAH also has the advantage of performing well in patients, regardless of whether they’ve been treated with steroids, which makes it generalizable.”
Creating and validating the MIAAH
Researchers analyzed the health records of 266 eligible patients diagnosed with AH between 1998 and 2018 at the Mayo Clinic, Rochester, Minn. The patients collectively had a 30-day mortality rate of 19.2%.
They then studied the effect of several variables, of which the following were found to be significantly associated with mortality: age (P = .002), blood urea nitrogen (P = .003), albumin (P = .01), bilirubin (P = .02), and international normalized ratio (P = .001).
Mayo researchers built the MIAAH model using these variables and found that it was able to achieve a C statistic of 0.86, which translates into its being able to accurately predict mortality more than 86% of the time. When tested in the initial cohort of 266 patients, MIAAH had a significantly superior C statistic compared with several other available models, such as the MELD, MDF, and GAHS, although not for the ABIC.
The researchers then tested the MIAAH model in a validation cohort of 249 patients from health care centers at the University of South Dakota, Sioux Falls, and the University of Kansas, Lawrence. In this cohort, the MIAAH’s C statistic decreased to 0.73, which remained significantly more accurate than the MDF but was comparable to that found with the MELD.
Helping with transplant decisions
There are no pharmacologic treatments that can reduce 90-day mortality in severe cases of AH, and only a small survival benefit at 30 days has been reported with prednisolone use.
With a shortage of liver donors, many centers still require 6 months of alcohol abstinence for transplant consideration, although exceptions are sometimes made for cases of early transplant.
A model that more accurately predicts who is at the highest risk of dying within a month can help clinicians decide how best to proceed, Dr. Singal said.
Paul Martin, MD, chief of the division of digestive health and liver diseases, Mandel Chair in gastroenterology, and professor of medicine at the University of Miami, told this news organization that the model is potentially important in light of the rising prevalence of AH.
“The numbers of patients with AH are unequivocally increasing and often in young patients,” he noted, presenting difficult choices of who to treat with steroids and who to refer for a transplant.
“This model is certainly timely,” he said. “We need more accuracy in predicting which patients will recover with medical therapy and which patients won’t in the absence of a liver transplant.”
He noted, however, that the study’s retrospective design requires that it’s validated prospectively: “not only looking at the outcome in terms of mortality of patients, but its potential utility in identifying candidates for liver transplantation who are not going to recover on their own.”
He said it was unlikely that the model would replace others, particularly MELD, which is ingrained in practices in the United States and other countries, but may instead have a complementary role.
Dr. Singal, Dr. Kezer, and Dr. Martin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new scoring system proved more accurate than several other available models at predicting the 30-day mortality risk for patients with alcohol-associated hepatitis (AH), according to new data.
The system, called the Mortality Index for Alcohol-Associated Hepatitis (MIAAH), was created by a team of Mayo Clinic researchers, who published their results in Mayo Clinic Proceedings.
Among the currently available prognostic models for assessing AH severity are the Model for End-Stage Liver Disease (MELD); the Age, serum Bilirubin, International normalized ratio, and serum Creatinine (ABIC) score; the Maddrey Discriminant Function (MDF); and the Glasgow Alcoholic Hepatitis Score (GAHS). However, these models have poor accuracy, with the area under the curve between 0.71 and 0.77.
By comparison, the new model has an accuracy of 86% in predicting 30-day mortality for AH, said coauthor Ashwani K. Singal, MD, MS, professor of medicine at the University of South Dakota Sanford School of Medicine, Sioux Falls.
“It’s a better predictor of the outcome, and that’s what sets it apart,” he told this news organization. “I think providers and patients will benefit.”
He said accuracy in determining the likelihood of death can help clinicians better determine treatment options and prepare patients and their families.
Camille Kezer, MD, a Mayo Clinic resident internist and first author of the paper, said in a statement, “MIAAH also has the advantage of performing well in patients, regardless of whether they’ve been treated with steroids, which makes it generalizable.”
Creating and validating the MIAAH
Researchers analyzed the health records of 266 eligible patients diagnosed with AH between 1998 and 2018 at the Mayo Clinic, Rochester, Minn. The patients collectively had a 30-day mortality rate of 19.2%.
They then studied the effect of several variables, of which the following were found to be significantly associated with mortality: age (P = .002), blood urea nitrogen (P = .003), albumin (P = .01), bilirubin (P = .02), and international normalized ratio (P = .001).
Mayo researchers built the MIAAH model using these variables and found that it was able to achieve a C statistic of 0.86, which translates into its being able to accurately predict mortality more than 86% of the time. When tested in the initial cohort of 266 patients, MIAAH had a significantly superior C statistic compared with several other available models, such as the MELD, MDF, and GAHS, although not for the ABIC.
The researchers then tested the MIAAH model in a validation cohort of 249 patients from health care centers at the University of South Dakota, Sioux Falls, and the University of Kansas, Lawrence. In this cohort, the MIAAH’s C statistic decreased to 0.73, which remained significantly more accurate than the MDF but was comparable to that found with the MELD.
Helping with transplant decisions
There are no pharmacologic treatments that can reduce 90-day mortality in severe cases of AH, and only a small survival benefit at 30 days has been reported with prednisolone use.
With a shortage of liver donors, many centers still require 6 months of alcohol abstinence for transplant consideration, although exceptions are sometimes made for cases of early transplant.
A model that more accurately predicts who is at the highest risk of dying within a month can help clinicians decide how best to proceed, Dr. Singal said.
Paul Martin, MD, chief of the division of digestive health and liver diseases, Mandel Chair in gastroenterology, and professor of medicine at the University of Miami, told this news organization that the model is potentially important in light of the rising prevalence of AH.
“The numbers of patients with AH are unequivocally increasing and often in young patients,” he noted, presenting difficult choices of who to treat with steroids and who to refer for a transplant.
“This model is certainly timely,” he said. “We need more accuracy in predicting which patients will recover with medical therapy and which patients won’t in the absence of a liver transplant.”
He noted, however, that the study’s retrospective design requires that it’s validated prospectively: “not only looking at the outcome in terms of mortality of patients, but its potential utility in identifying candidates for liver transplantation who are not going to recover on their own.”
He said it was unlikely that the model would replace others, particularly MELD, which is ingrained in practices in the United States and other countries, but may instead have a complementary role.
Dr. Singal, Dr. Kezer, and Dr. Martin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MAYO CLINIC PROCEEDINGS





