User login
Clinical Edge Journal Scan Commentary: Atopic Dermatitis April 2022
Dupilumab is a subcutaneous injection therapy that inhibits the interleukin 4 receptor alpha subunit. It has been approved in the United States for the treatment of adults with moderate-to-severe AD since 2017 and has since been approved for children and adolescents older than 6 years. Many real-world studies of the effectiveness of dupilumab have been published over the past few years. Kojanova and colleagues reported findings from a retrospective, multicenter study of 360 adults with severe AD who received dupilumab. They found that a high proportion of patients achieved a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16 (66.6%), 1 year (89.5%), and 2 years (95.8%). Drug persistence rates were very high (> 90%) throughout the 2 years of therapy, suggesting that dupilumab was effective and well-tolerated.
Dupilumab was previously found to be associated with increased conjunctivitis in clinical trials and real-world studies. Schneeweiss and colleagues conducted a population-based longitudinal study of 5,004,117 patients with AD who newly initiated dupilumab, methotrexate, mycophenolate, and cyclosporine. They found that the risk of developing conjunctivitis diagnosed in clinical practice within 6 months of treatment initiation was approximately double with dupilumab compared with methotrexate, mycophenolate, or cyclosporine. Interestingly, comorbid asthma was found to be a risk factor for conjunctivitis in dupilumab initiators. This study provides insight into how commonly clinically significant conjunctivitis occurs with dupilumab treatment. Of note, the study results may underestimate the incidence of conjunctivitis because milder cases may go undetected.
Upadacitinib is an oral selective Janus kinase (JAK) 1 inhibitor that was approved in the United States in 2022 for the treatment of moderate-to-severe AD. Simpson and colleagues reported on the long-term efficacy and safety of oral upadacitinib from the phase 3 double-blind, randomized controlled trials Measure Up 1 and Measure Up 2 in adolescents and adults with moderate-to-severe atopic dermatitis ho had an inadequate response to topical therapy. The initial phase of the Measure Up 1 and Measure Up 2 studies was 16 weeks in duration, with a placebo control group. At week 16, patients who received 15 or 30 mg upadacitinib in the initial 16 weeks of the study continued to receive that dose. Patients who received placebo in the initial 16 weeks were randomly assigned to receive oral 15 mg or 30 mg upadacitinib daily. So, in the long run, all patients received upadacitinib in the second phase of the study but were blinded to the dose they were receiving. The results from the first 16 weeks of treatment were previously published. Simpson and colleagues reported on the results up to week 52 from the second phase of the study. They showed that at week 52, 82.0% and 79.1% of patients in Measure Up 1 and Measure Up 2 achieved a 75% improvement in EASI-75 when continuing on 15 mg upadacitinib and 84.9% and 84.3% in patients continuing 30 mg upadacitinib, respectively. More than 80% of patients who switched from placebo to upadacitinib at week 16 achieved EASI-75 at week 52. No safety signals were observed in this phase of the study that were not previously observed in other studies of upadacitinib for atopic dermatitis and other indications (rheumatoid arthritis and psoriatic arthritis). These results indicate that upadacitinib has durable efficacy with long-term treatment in moderate-to-severe AD. These studies are ongoing, and I look forward to future results reporting for even longer-term treatment.
It is an exciting time in dermatology with the arrival of multiple novel therapies for atopic dermatitis. The field has already benefited so much by the tremendous interest and research effort into understanding how to best manage this disease. With so many more treatments in the pipeline, perhaps the best is yet to come.
Dupilumab is a subcutaneous injection therapy that inhibits the interleukin 4 receptor alpha subunit. It has been approved in the United States for the treatment of adults with moderate-to-severe AD since 2017 and has since been approved for children and adolescents older than 6 years. Many real-world studies of the effectiveness of dupilumab have been published over the past few years. Kojanova and colleagues reported findings from a retrospective, multicenter study of 360 adults with severe AD who received dupilumab. They found that a high proportion of patients achieved a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16 (66.6%), 1 year (89.5%), and 2 years (95.8%). Drug persistence rates were very high (> 90%) throughout the 2 years of therapy, suggesting that dupilumab was effective and well-tolerated.
Dupilumab was previously found to be associated with increased conjunctivitis in clinical trials and real-world studies. Schneeweiss and colleagues conducted a population-based longitudinal study of 5,004,117 patients with AD who newly initiated dupilumab, methotrexate, mycophenolate, and cyclosporine. They found that the risk of developing conjunctivitis diagnosed in clinical practice within 6 months of treatment initiation was approximately double with dupilumab compared with methotrexate, mycophenolate, or cyclosporine. Interestingly, comorbid asthma was found to be a risk factor for conjunctivitis in dupilumab initiators. This study provides insight into how commonly clinically significant conjunctivitis occurs with dupilumab treatment. Of note, the study results may underestimate the incidence of conjunctivitis because milder cases may go undetected.
Upadacitinib is an oral selective Janus kinase (JAK) 1 inhibitor that was approved in the United States in 2022 for the treatment of moderate-to-severe AD. Simpson and colleagues reported on the long-term efficacy and safety of oral upadacitinib from the phase 3 double-blind, randomized controlled trials Measure Up 1 and Measure Up 2 in adolescents and adults with moderate-to-severe atopic dermatitis ho had an inadequate response to topical therapy. The initial phase of the Measure Up 1 and Measure Up 2 studies was 16 weeks in duration, with a placebo control group. At week 16, patients who received 15 or 30 mg upadacitinib in the initial 16 weeks of the study continued to receive that dose. Patients who received placebo in the initial 16 weeks were randomly assigned to receive oral 15 mg or 30 mg upadacitinib daily. So, in the long run, all patients received upadacitinib in the second phase of the study but were blinded to the dose they were receiving. The results from the first 16 weeks of treatment were previously published. Simpson and colleagues reported on the results up to week 52 from the second phase of the study. They showed that at week 52, 82.0% and 79.1% of patients in Measure Up 1 and Measure Up 2 achieved a 75% improvement in EASI-75 when continuing on 15 mg upadacitinib and 84.9% and 84.3% in patients continuing 30 mg upadacitinib, respectively. More than 80% of patients who switched from placebo to upadacitinib at week 16 achieved EASI-75 at week 52. No safety signals were observed in this phase of the study that were not previously observed in other studies of upadacitinib for atopic dermatitis and other indications (rheumatoid arthritis and psoriatic arthritis). These results indicate that upadacitinib has durable efficacy with long-term treatment in moderate-to-severe AD. These studies are ongoing, and I look forward to future results reporting for even longer-term treatment.
It is an exciting time in dermatology with the arrival of multiple novel therapies for atopic dermatitis. The field has already benefited so much by the tremendous interest and research effort into understanding how to best manage this disease. With so many more treatments in the pipeline, perhaps the best is yet to come.
Dupilumab is a subcutaneous injection therapy that inhibits the interleukin 4 receptor alpha subunit. It has been approved in the United States for the treatment of adults with moderate-to-severe AD since 2017 and has since been approved for children and adolescents older than 6 years. Many real-world studies of the effectiveness of dupilumab have been published over the past few years. Kojanova and colleagues reported findings from a retrospective, multicenter study of 360 adults with severe AD who received dupilumab. They found that a high proportion of patients achieved a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16 (66.6%), 1 year (89.5%), and 2 years (95.8%). Drug persistence rates were very high (> 90%) throughout the 2 years of therapy, suggesting that dupilumab was effective and well-tolerated.
Dupilumab was previously found to be associated with increased conjunctivitis in clinical trials and real-world studies. Schneeweiss and colleagues conducted a population-based longitudinal study of 5,004,117 patients with AD who newly initiated dupilumab, methotrexate, mycophenolate, and cyclosporine. They found that the risk of developing conjunctivitis diagnosed in clinical practice within 6 months of treatment initiation was approximately double with dupilumab compared with methotrexate, mycophenolate, or cyclosporine. Interestingly, comorbid asthma was found to be a risk factor for conjunctivitis in dupilumab initiators. This study provides insight into how commonly clinically significant conjunctivitis occurs with dupilumab treatment. Of note, the study results may underestimate the incidence of conjunctivitis because milder cases may go undetected.
Upadacitinib is an oral selective Janus kinase (JAK) 1 inhibitor that was approved in the United States in 2022 for the treatment of moderate-to-severe AD. Simpson and colleagues reported on the long-term efficacy and safety of oral upadacitinib from the phase 3 double-blind, randomized controlled trials Measure Up 1 and Measure Up 2 in adolescents and adults with moderate-to-severe atopic dermatitis ho had an inadequate response to topical therapy. The initial phase of the Measure Up 1 and Measure Up 2 studies was 16 weeks in duration, with a placebo control group. At week 16, patients who received 15 or 30 mg upadacitinib in the initial 16 weeks of the study continued to receive that dose. Patients who received placebo in the initial 16 weeks were randomly assigned to receive oral 15 mg or 30 mg upadacitinib daily. So, in the long run, all patients received upadacitinib in the second phase of the study but were blinded to the dose they were receiving. The results from the first 16 weeks of treatment were previously published. Simpson and colleagues reported on the results up to week 52 from the second phase of the study. They showed that at week 52, 82.0% and 79.1% of patients in Measure Up 1 and Measure Up 2 achieved a 75% improvement in EASI-75 when continuing on 15 mg upadacitinib and 84.9% and 84.3% in patients continuing 30 mg upadacitinib, respectively. More than 80% of patients who switched from placebo to upadacitinib at week 16 achieved EASI-75 at week 52. No safety signals were observed in this phase of the study that were not previously observed in other studies of upadacitinib for atopic dermatitis and other indications (rheumatoid arthritis and psoriatic arthritis). These results indicate that upadacitinib has durable efficacy with long-term treatment in moderate-to-severe AD. These studies are ongoing, and I look forward to future results reporting for even longer-term treatment.
It is an exciting time in dermatology with the arrival of multiple novel therapies for atopic dermatitis. The field has already benefited so much by the tremendous interest and research effort into understanding how to best manage this disease. With so many more treatments in the pipeline, perhaps the best is yet to come.
Gene therapy demonstrates modest success in genetic blindness
SEATTLE – The therapy, delivered by intravitreal injection, uses an adeno-associated virus vector to deliver a corrected copy of the mutated ND4 mitochondrial gene.
LHON is a rare, maternally inherited mitochondrial mutation that can cause blindness, most commonly in young men, though it does not happen in all individuals with the mutation. The condition often starts with blindness in one eye, accompanied or followed shortly by blindness in the second eye. Researchers believe that the injected viral vector gets taken up retinal ganglion cells, where the mutated gene interferes with vision. Once synthesized, a mitochondria-targeting sequence facilitates transport of the protein to the mitochondria.
The study protocol called for injection of the therapy into one eye and a placebo into the other, using the patient as his or own placebo control. The results in the treated eye were encouraging, though modest. “This is not hitting it out of the ballpark. But for people whose vision is devastated by this disease, it certainly is a first step,” said Nancy J. Newman, MD, during a press conference held March 29 in advance of the 2022 annual meeting of the American Academy of Neurology.
Dr. Newman also noted a surprise finding: Visual improvement also occurred in the placebo-control eye. This was noted in previous studies, called RESCUE and REVERSE, and follow-up studies in monkeys found viral vector in the unaffected eye 3-6 months after an injection. “This would imply some kind of transport within retrograde up the opposite optic nerve after crossing in the chiasm to the eye, but this is going to take a fair bit of work to know exactly how that happens,” said Dr. Newman
Unfortunately, the phase 3 REFLECT study was designed before that process was understood. “This was not a case-control study by person, it was by eye. And that was a mistake, because it turns out there is a does appear to be second eye effects. We do not have naive controls here that did not receive any injection at all in any eye. That’s something that we will [do going] forward,” said Dr. Newman.
Despite the problem with placebo, the results were encouraging. “Those patients who had both eyes injected with the drug did better than in those who had one eye injected with drug and one eye injected with placebo, suggesting some sort of dose effect. There were no adverse events other than what we would expect from injecting [into] eyes. Those treated with the drug had more ocular inflammation, as would also be expected, but all were easily treated with topical medications,” said Dr. Newman.
What are the long-term effects?
Natalia Rost, MD, who chairs the AAN Science Committee, commented after the presentation: “We’re quite impressed with advances in gene therapy. The question is, are there early indications that this improvement in vision will have a lasting effect?”
Dr. Newman responded that ongoing data from earlier studies are also encouraging regarding the long-term effect of the treatment. At 4 years, there was a difference of 16.5 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters equivalent between treated patients and natural history controls (P < .01), “which [does] suggest that this effect is maintained,” said Dr. Newman, who is a professor of ophthalmology and neurology at Emory University, Atlanta.
Dr. Rost also wondered if it would be possible to capture patients earlier in their disease process, in the hopes of countering degeneration before it becomes severe enough to impact vision. Dr. Newman answered by noting another surprise from the research. Previous studies had shown that intervention while only a single eye is affected had little impact on spread of the condition to the second eye, “which was very disappointing,” said Dr. Newman. When they stratified patients by time since vision loss, they found that those who received the therapy 6 months or later after vision loss had better responses than those who were treated earlier.
The mechanism of this counter-intuitive finding remains uncertain, “but we do know that acutely in this disease when people are just starting to lose this vision, during the first couple of months, they get swelling of the axons from these retinal ganglion cells. Our hypothesis is that swelling may actually act as a barrier for the drug to get into the retinal ganglion cell bodies themselves and be transfected. So it turns out that earlier may not be better,” said Dr. Newman.
The study included patients at 13 sites worldwide; 48 were treated bilaterally and 50 treated unilaterally. Just under 80% were male, the mean age was 31.5 years, and the mean duration of vision loss was 8.30 months.
After 1.5 years, the improvement in best-corrected visual acuity between second-affected eyes was stronger in the treatment eye, equivalent to +3 ETDRS letters. The first-affected eye improved by 19 ETDRS letters, and the second-affected eye improved by 16 (P < .0001). Improvement in placebo eyes was +13 ETDRS letters (P < .0001).
Dr. Rost has served on a scientific advisory board or data monitoring board for Omniox. Dr. Newman has consulted for GenSight, Santhera/Chiesi, and Neurophoenix, and has received research support from GenSight and Santhera/Chiesi.
SEATTLE – The therapy, delivered by intravitreal injection, uses an adeno-associated virus vector to deliver a corrected copy of the mutated ND4 mitochondrial gene.
LHON is a rare, maternally inherited mitochondrial mutation that can cause blindness, most commonly in young men, though it does not happen in all individuals with the mutation. The condition often starts with blindness in one eye, accompanied or followed shortly by blindness in the second eye. Researchers believe that the injected viral vector gets taken up retinal ganglion cells, where the mutated gene interferes with vision. Once synthesized, a mitochondria-targeting sequence facilitates transport of the protein to the mitochondria.
The study protocol called for injection of the therapy into one eye and a placebo into the other, using the patient as his or own placebo control. The results in the treated eye were encouraging, though modest. “This is not hitting it out of the ballpark. But for people whose vision is devastated by this disease, it certainly is a first step,” said Nancy J. Newman, MD, during a press conference held March 29 in advance of the 2022 annual meeting of the American Academy of Neurology.
Dr. Newman also noted a surprise finding: Visual improvement also occurred in the placebo-control eye. This was noted in previous studies, called RESCUE and REVERSE, and follow-up studies in monkeys found viral vector in the unaffected eye 3-6 months after an injection. “This would imply some kind of transport within retrograde up the opposite optic nerve after crossing in the chiasm to the eye, but this is going to take a fair bit of work to know exactly how that happens,” said Dr. Newman
Unfortunately, the phase 3 REFLECT study was designed before that process was understood. “This was not a case-control study by person, it was by eye. And that was a mistake, because it turns out there is a does appear to be second eye effects. We do not have naive controls here that did not receive any injection at all in any eye. That’s something that we will [do going] forward,” said Dr. Newman.
Despite the problem with placebo, the results were encouraging. “Those patients who had both eyes injected with the drug did better than in those who had one eye injected with drug and one eye injected with placebo, suggesting some sort of dose effect. There were no adverse events other than what we would expect from injecting [into] eyes. Those treated with the drug had more ocular inflammation, as would also be expected, but all were easily treated with topical medications,” said Dr. Newman.
What are the long-term effects?
Natalia Rost, MD, who chairs the AAN Science Committee, commented after the presentation: “We’re quite impressed with advances in gene therapy. The question is, are there early indications that this improvement in vision will have a lasting effect?”
Dr. Newman responded that ongoing data from earlier studies are also encouraging regarding the long-term effect of the treatment. At 4 years, there was a difference of 16.5 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters equivalent between treated patients and natural history controls (P < .01), “which [does] suggest that this effect is maintained,” said Dr. Newman, who is a professor of ophthalmology and neurology at Emory University, Atlanta.
Dr. Rost also wondered if it would be possible to capture patients earlier in their disease process, in the hopes of countering degeneration before it becomes severe enough to impact vision. Dr. Newman answered by noting another surprise from the research. Previous studies had shown that intervention while only a single eye is affected had little impact on spread of the condition to the second eye, “which was very disappointing,” said Dr. Newman. When they stratified patients by time since vision loss, they found that those who received the therapy 6 months or later after vision loss had better responses than those who were treated earlier.
The mechanism of this counter-intuitive finding remains uncertain, “but we do know that acutely in this disease when people are just starting to lose this vision, during the first couple of months, they get swelling of the axons from these retinal ganglion cells. Our hypothesis is that swelling may actually act as a barrier for the drug to get into the retinal ganglion cell bodies themselves and be transfected. So it turns out that earlier may not be better,” said Dr. Newman.
The study included patients at 13 sites worldwide; 48 were treated bilaterally and 50 treated unilaterally. Just under 80% were male, the mean age was 31.5 years, and the mean duration of vision loss was 8.30 months.
After 1.5 years, the improvement in best-corrected visual acuity between second-affected eyes was stronger in the treatment eye, equivalent to +3 ETDRS letters. The first-affected eye improved by 19 ETDRS letters, and the second-affected eye improved by 16 (P < .0001). Improvement in placebo eyes was +13 ETDRS letters (P < .0001).
Dr. Rost has served on a scientific advisory board or data monitoring board for Omniox. Dr. Newman has consulted for GenSight, Santhera/Chiesi, and Neurophoenix, and has received research support from GenSight and Santhera/Chiesi.
SEATTLE – The therapy, delivered by intravitreal injection, uses an adeno-associated virus vector to deliver a corrected copy of the mutated ND4 mitochondrial gene.
LHON is a rare, maternally inherited mitochondrial mutation that can cause blindness, most commonly in young men, though it does not happen in all individuals with the mutation. The condition often starts with blindness in one eye, accompanied or followed shortly by blindness in the second eye. Researchers believe that the injected viral vector gets taken up retinal ganglion cells, where the mutated gene interferes with vision. Once synthesized, a mitochondria-targeting sequence facilitates transport of the protein to the mitochondria.
The study protocol called for injection of the therapy into one eye and a placebo into the other, using the patient as his or own placebo control. The results in the treated eye were encouraging, though modest. “This is not hitting it out of the ballpark. But for people whose vision is devastated by this disease, it certainly is a first step,” said Nancy J. Newman, MD, during a press conference held March 29 in advance of the 2022 annual meeting of the American Academy of Neurology.
Dr. Newman also noted a surprise finding: Visual improvement also occurred in the placebo-control eye. This was noted in previous studies, called RESCUE and REVERSE, and follow-up studies in monkeys found viral vector in the unaffected eye 3-6 months after an injection. “This would imply some kind of transport within retrograde up the opposite optic nerve after crossing in the chiasm to the eye, but this is going to take a fair bit of work to know exactly how that happens,” said Dr. Newman
Unfortunately, the phase 3 REFLECT study was designed before that process was understood. “This was not a case-control study by person, it was by eye. And that was a mistake, because it turns out there is a does appear to be second eye effects. We do not have naive controls here that did not receive any injection at all in any eye. That’s something that we will [do going] forward,” said Dr. Newman.
Despite the problem with placebo, the results were encouraging. “Those patients who had both eyes injected with the drug did better than in those who had one eye injected with drug and one eye injected with placebo, suggesting some sort of dose effect. There were no adverse events other than what we would expect from injecting [into] eyes. Those treated with the drug had more ocular inflammation, as would also be expected, but all were easily treated with topical medications,” said Dr. Newman.
What are the long-term effects?
Natalia Rost, MD, who chairs the AAN Science Committee, commented after the presentation: “We’re quite impressed with advances in gene therapy. The question is, are there early indications that this improvement in vision will have a lasting effect?”
Dr. Newman responded that ongoing data from earlier studies are also encouraging regarding the long-term effect of the treatment. At 4 years, there was a difference of 16.5 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters equivalent between treated patients and natural history controls (P < .01), “which [does] suggest that this effect is maintained,” said Dr. Newman, who is a professor of ophthalmology and neurology at Emory University, Atlanta.
Dr. Rost also wondered if it would be possible to capture patients earlier in their disease process, in the hopes of countering degeneration before it becomes severe enough to impact vision. Dr. Newman answered by noting another surprise from the research. Previous studies had shown that intervention while only a single eye is affected had little impact on spread of the condition to the second eye, “which was very disappointing,” said Dr. Newman. When they stratified patients by time since vision loss, they found that those who received the therapy 6 months or later after vision loss had better responses than those who were treated earlier.
The mechanism of this counter-intuitive finding remains uncertain, “but we do know that acutely in this disease when people are just starting to lose this vision, during the first couple of months, they get swelling of the axons from these retinal ganglion cells. Our hypothesis is that swelling may actually act as a barrier for the drug to get into the retinal ganglion cell bodies themselves and be transfected. So it turns out that earlier may not be better,” said Dr. Newman.
The study included patients at 13 sites worldwide; 48 were treated bilaterally and 50 treated unilaterally. Just under 80% were male, the mean age was 31.5 years, and the mean duration of vision loss was 8.30 months.
After 1.5 years, the improvement in best-corrected visual acuity between second-affected eyes was stronger in the treatment eye, equivalent to +3 ETDRS letters. The first-affected eye improved by 19 ETDRS letters, and the second-affected eye improved by 16 (P < .0001). Improvement in placebo eyes was +13 ETDRS letters (P < .0001).
Dr. Rost has served on a scientific advisory board or data monitoring board for Omniox. Dr. Newman has consulted for GenSight, Santhera/Chiesi, and Neurophoenix, and has received research support from GenSight and Santhera/Chiesi.
AT AAN 2022
APOLLO: SLN360 clears first major hurdle, hammering Lp(a)
The short interfering RNA (siRNA) agent SLN360 was well tolerated and lowered lipoprotein(a) by up to 98% in volunteers without cardiovascular disease but with elevated Lp(a) in the small dose-ranging APOLLO trial.
Following a single subcutaneous dose of SLN360 (Silence Therapeutics), there was a dose-dependent reduction in Lp(a) plasma levels by a median of 46%, 86%, 96%, and 98% at about 45-60 days with 30-mg, 100-mg, 300-mg, and 600-mg doses, respectively.
Lp(a) levels at 150 days were 70% and 81% below baseline with the 300-and 600-mg doses.
In addition, for participants receiving the two highest doses, apolipoprotein B (apo B) was reduced was 21% and 24%, respectively, and LDL cholesterol (LDL-C), by 21% and 26%, respectively.
“The development of therapies targeting messenger RNA has made possible significant lowering of lipoprotein(a). Whether these reductions can impact on the incidence of ASCVD [atherosclerotic cardiovascular disease] or prevent progression of aortic stenosis remains to be determined but, we think, that optimism is warranted,” said principal investigator Steven E. Nissen, MD, Cleveland Clinic.
The results were presented in a late-breaking clinical trial session at the annual scientific sessions of the American College of Cardiology and published simultaneously in JAMA.
Elevated Lp(a) is a powerful genetic risk factor for ASCVD and aortic stenosis, which affects some 64 million Americans and 1.4 billion people globally. Although several experimental agents are under investigation, no currently approved drugs selectively lower Lp(a).
SLN360 is designed to lower Lp(a) production by using RNA interference to silence messenger RNA transcribed from the LPA gene in liver cells.
Testing vacuum
Dr. Nissen said in an interview that one of the big takeaways from the study is the need for greater testing of Lp(a). Automatic assays are available in almost every hospital, but two-unit systems (nmol/L and mg/dL) are used and thresholds for accelerated risk vary. The Cleveland Clinic currently tests all patients in its cardiac critical care unit and its prevention clinic.
“Someone comes in with an MI in their 40s and we measure it and it’s 100, 150 [mg/dL], clearly abnormal, and often these patients don’t have a lot of other risk factors,” Dr. Nissen said. “So the explanation very likely for their premature disease is this risk factor. We now have to educate everybody about the importance of getting it tested and finding out about it.”
During a media briefing, ACC 2022 program cochair Pamela B. Morris, MD, Medical University of South Carolina, Charleston, said testing for Lp(a) is not well reimbursed by insurance providers and that her patients will often cancel the test after learning it won’t be reimbursed because they don’t understand it.
“What Dr. Nissen is telling you: It should be measured in everyone at least once, we all believe that, but it hasn’t made it into the major guidelines,” she added. “I think what we’re going to have to do is have the guidelines mandate it and the insurers will follow.”
Guidelines currently list elevated Lp(a) as a “risk-enhancing factor,” which can help with at least recommending LDL-C treatment in patients with borderline risk and a sky-high Lp(a), noted Dr. Nissen. “But we need to go beyond that.”
Safety analyses
The first-in-human APOLLO trial evaluated 32 adults without known ASCVD and an Lp(a) concentration greater than 150 nmol/L (approximately 60 mg/dL) who received one of the four doses of SLN360 or placebo subcutaneously. Participants were monitored in a research unit for the first 24 hours and then followed periodically for up to 150 days. At baseline, their median Lp(a) level was 224 nmol/L, mean apo B level was 85 mg/dL, and mean LDL-C level was 108 mg/dL.
Treatment-emergent adverse events were generally mild, mostly grade 1 injection site reactions (83% at 30 mg, 100% at 100 mg, 67% at 300 mg, and 33% at 600 mg) and headache (33%, 17%, 0%, and 83%).
At the highest dose, C-reactive protein was increased in four patients and neutrophil counts in three. ALT and AST levels were elevated three times above the upper limit of normal in one patient at the lowest dose.
One participant in the lowest-dose group experienced two serious adverse events unrelated to SLN360 at day 45 after receiving a SARS-Co-V-2 vaccine.
Dr. Nissen noted that safety cannot be comprehensively assessed in a trial of this duration or size and that follow-up has been extended to 1 year in the two highest-dose groups.
Enrollment continues in the multiple-ascending dose portion of the study in patients with high Lp(a) and a history of stable ASCVD. A phase 2 study of SLN360 is also planned for the second half of 2022, pending regulatory discussions.
But will it reduce ASCVD events?
Study discussant Vera Bittner, MD, MSPH, University of Alabama at Birmingham, said that the development of Lp(a)-specific lowering agents has been a “holy grail” for years and congratulated the authors on a successful trial demonstrating very robust Lp(a) lowering.
She asked Dr. Nissen about the observation in proprotein convertase subtilisin/kexin type 9 inhibitor trials that absolute Lp(a) lowering is greater at higher baseline levels.
Dr. Nissen said this kind of analysis wasn’t possible because of the small sample size but “because these agents so effectively degrade messenger RNA, it’s very likely we will see robust suppression of plasma levels virtually regardless of the baseline level.”
Dr. Bittner also questioned if “LDL-C declined because of the cholesterol content in the lipoprotein(a) or is there some additional effect on LDL particles themselves?”
“It’s a really terrific question that will ultimately need to be answered,” Dr. Nissen replied. “There’s some controversy about the extent to which suppressing lipoprotein(a) will reduce LDL because the assays for LDL are measuring the LDL that’s in lipoprotein(a) and the LDL that is not. ... I think it’s probably a bystander effect, but it may also contribute to efficacy from a morbidity and mortality point of view, which is why we measured it.”
Dr. Bittner also called out the elevation in C-reactive protein and leukocytosis, which has not been seen in other siRNA studies. Dr. Nissen said the increases in C-reactive protein occurred in the first few days after administration and were gone after a week or so. “I don’t see it as a long-term limitation.”
In an accompanying editorial, Brian Ference, MD, MPhil, MSc, University of Cambridge (England), suggests that because circulating Lp(a) particles can progressively become trapped within the artery wall over time, it’s unlikely that lowering Lp(a) for only a few years starting later in life will eliminate the effect of lifelong exposure to Lp(a) and may only cut cardiovascular event risk by about 10%-15%.
He called for continued safety and efficacy evaluation of SLN360 and olpasiran, a similar siRNA agent in early development, and said further insights into whether large absolute reductions in Lp(a) can reduce the risk for major cardiovascular events will come from cardiovascular trials, such as the ongoing phase 3 Lp(a)HORIZON trial. It follows strong phase 2 results with the antisense agent AKCEA-APO(a)-LRx and has Dr. Nissen pulling double duty as study chair.
The study was funded by Silence Therapeutics. Dr. Nissen reported consulting for many pharmaceutical companies, which are directed to pay any renumeration directly to charity. Dr. Bittner reported consultant fees or honoraria from Pfizer; other from AstraZeneca, DalCor, Esperion, and Sanofi-Aventis; and research/research grants from Amgen and Novartis. Dr. Ference reported financial ties to Merck, Novartis, Amgen, Pfizer, Esperion Therapeutics, and numerous other companies.
A version of this article first appeared on Medscape.com.
The short interfering RNA (siRNA) agent SLN360 was well tolerated and lowered lipoprotein(a) by up to 98% in volunteers without cardiovascular disease but with elevated Lp(a) in the small dose-ranging APOLLO trial.
Following a single subcutaneous dose of SLN360 (Silence Therapeutics), there was a dose-dependent reduction in Lp(a) plasma levels by a median of 46%, 86%, 96%, and 98% at about 45-60 days with 30-mg, 100-mg, 300-mg, and 600-mg doses, respectively.
Lp(a) levels at 150 days were 70% and 81% below baseline with the 300-and 600-mg doses.
In addition, for participants receiving the two highest doses, apolipoprotein B (apo B) was reduced was 21% and 24%, respectively, and LDL cholesterol (LDL-C), by 21% and 26%, respectively.
“The development of therapies targeting messenger RNA has made possible significant lowering of lipoprotein(a). Whether these reductions can impact on the incidence of ASCVD [atherosclerotic cardiovascular disease] or prevent progression of aortic stenosis remains to be determined but, we think, that optimism is warranted,” said principal investigator Steven E. Nissen, MD, Cleveland Clinic.
The results were presented in a late-breaking clinical trial session at the annual scientific sessions of the American College of Cardiology and published simultaneously in JAMA.
Elevated Lp(a) is a powerful genetic risk factor for ASCVD and aortic stenosis, which affects some 64 million Americans and 1.4 billion people globally. Although several experimental agents are under investigation, no currently approved drugs selectively lower Lp(a).
SLN360 is designed to lower Lp(a) production by using RNA interference to silence messenger RNA transcribed from the LPA gene in liver cells.
Testing vacuum
Dr. Nissen said in an interview that one of the big takeaways from the study is the need for greater testing of Lp(a). Automatic assays are available in almost every hospital, but two-unit systems (nmol/L and mg/dL) are used and thresholds for accelerated risk vary. The Cleveland Clinic currently tests all patients in its cardiac critical care unit and its prevention clinic.
“Someone comes in with an MI in their 40s and we measure it and it’s 100, 150 [mg/dL], clearly abnormal, and often these patients don’t have a lot of other risk factors,” Dr. Nissen said. “So the explanation very likely for their premature disease is this risk factor. We now have to educate everybody about the importance of getting it tested and finding out about it.”
During a media briefing, ACC 2022 program cochair Pamela B. Morris, MD, Medical University of South Carolina, Charleston, said testing for Lp(a) is not well reimbursed by insurance providers and that her patients will often cancel the test after learning it won’t be reimbursed because they don’t understand it.
“What Dr. Nissen is telling you: It should be measured in everyone at least once, we all believe that, but it hasn’t made it into the major guidelines,” she added. “I think what we’re going to have to do is have the guidelines mandate it and the insurers will follow.”
Guidelines currently list elevated Lp(a) as a “risk-enhancing factor,” which can help with at least recommending LDL-C treatment in patients with borderline risk and a sky-high Lp(a), noted Dr. Nissen. “But we need to go beyond that.”
Safety analyses
The first-in-human APOLLO trial evaluated 32 adults without known ASCVD and an Lp(a) concentration greater than 150 nmol/L (approximately 60 mg/dL) who received one of the four doses of SLN360 or placebo subcutaneously. Participants were monitored in a research unit for the first 24 hours and then followed periodically for up to 150 days. At baseline, their median Lp(a) level was 224 nmol/L, mean apo B level was 85 mg/dL, and mean LDL-C level was 108 mg/dL.
Treatment-emergent adverse events were generally mild, mostly grade 1 injection site reactions (83% at 30 mg, 100% at 100 mg, 67% at 300 mg, and 33% at 600 mg) and headache (33%, 17%, 0%, and 83%).
At the highest dose, C-reactive protein was increased in four patients and neutrophil counts in three. ALT and AST levels were elevated three times above the upper limit of normal in one patient at the lowest dose.
One participant in the lowest-dose group experienced two serious adverse events unrelated to SLN360 at day 45 after receiving a SARS-Co-V-2 vaccine.
Dr. Nissen noted that safety cannot be comprehensively assessed in a trial of this duration or size and that follow-up has been extended to 1 year in the two highest-dose groups.
Enrollment continues in the multiple-ascending dose portion of the study in patients with high Lp(a) and a history of stable ASCVD. A phase 2 study of SLN360 is also planned for the second half of 2022, pending regulatory discussions.
But will it reduce ASCVD events?
Study discussant Vera Bittner, MD, MSPH, University of Alabama at Birmingham, said that the development of Lp(a)-specific lowering agents has been a “holy grail” for years and congratulated the authors on a successful trial demonstrating very robust Lp(a) lowering.
She asked Dr. Nissen about the observation in proprotein convertase subtilisin/kexin type 9 inhibitor trials that absolute Lp(a) lowering is greater at higher baseline levels.
Dr. Nissen said this kind of analysis wasn’t possible because of the small sample size but “because these agents so effectively degrade messenger RNA, it’s very likely we will see robust suppression of plasma levels virtually regardless of the baseline level.”
Dr. Bittner also questioned if “LDL-C declined because of the cholesterol content in the lipoprotein(a) or is there some additional effect on LDL particles themselves?”
“It’s a really terrific question that will ultimately need to be answered,” Dr. Nissen replied. “There’s some controversy about the extent to which suppressing lipoprotein(a) will reduce LDL because the assays for LDL are measuring the LDL that’s in lipoprotein(a) and the LDL that is not. ... I think it’s probably a bystander effect, but it may also contribute to efficacy from a morbidity and mortality point of view, which is why we measured it.”
Dr. Bittner also called out the elevation in C-reactive protein and leukocytosis, which has not been seen in other siRNA studies. Dr. Nissen said the increases in C-reactive protein occurred in the first few days after administration and were gone after a week or so. “I don’t see it as a long-term limitation.”
In an accompanying editorial, Brian Ference, MD, MPhil, MSc, University of Cambridge (England), suggests that because circulating Lp(a) particles can progressively become trapped within the artery wall over time, it’s unlikely that lowering Lp(a) for only a few years starting later in life will eliminate the effect of lifelong exposure to Lp(a) and may only cut cardiovascular event risk by about 10%-15%.
He called for continued safety and efficacy evaluation of SLN360 and olpasiran, a similar siRNA agent in early development, and said further insights into whether large absolute reductions in Lp(a) can reduce the risk for major cardiovascular events will come from cardiovascular trials, such as the ongoing phase 3 Lp(a)HORIZON trial. It follows strong phase 2 results with the antisense agent AKCEA-APO(a)-LRx and has Dr. Nissen pulling double duty as study chair.
The study was funded by Silence Therapeutics. Dr. Nissen reported consulting for many pharmaceutical companies, which are directed to pay any renumeration directly to charity. Dr. Bittner reported consultant fees or honoraria from Pfizer; other from AstraZeneca, DalCor, Esperion, and Sanofi-Aventis; and research/research grants from Amgen and Novartis. Dr. Ference reported financial ties to Merck, Novartis, Amgen, Pfizer, Esperion Therapeutics, and numerous other companies.
A version of this article first appeared on Medscape.com.
The short interfering RNA (siRNA) agent SLN360 was well tolerated and lowered lipoprotein(a) by up to 98% in volunteers without cardiovascular disease but with elevated Lp(a) in the small dose-ranging APOLLO trial.
Following a single subcutaneous dose of SLN360 (Silence Therapeutics), there was a dose-dependent reduction in Lp(a) plasma levels by a median of 46%, 86%, 96%, and 98% at about 45-60 days with 30-mg, 100-mg, 300-mg, and 600-mg doses, respectively.
Lp(a) levels at 150 days were 70% and 81% below baseline with the 300-and 600-mg doses.
In addition, for participants receiving the two highest doses, apolipoprotein B (apo B) was reduced was 21% and 24%, respectively, and LDL cholesterol (LDL-C), by 21% and 26%, respectively.
“The development of therapies targeting messenger RNA has made possible significant lowering of lipoprotein(a). Whether these reductions can impact on the incidence of ASCVD [atherosclerotic cardiovascular disease] or prevent progression of aortic stenosis remains to be determined but, we think, that optimism is warranted,” said principal investigator Steven E. Nissen, MD, Cleveland Clinic.
The results were presented in a late-breaking clinical trial session at the annual scientific sessions of the American College of Cardiology and published simultaneously in JAMA.
Elevated Lp(a) is a powerful genetic risk factor for ASCVD and aortic stenosis, which affects some 64 million Americans and 1.4 billion people globally. Although several experimental agents are under investigation, no currently approved drugs selectively lower Lp(a).
SLN360 is designed to lower Lp(a) production by using RNA interference to silence messenger RNA transcribed from the LPA gene in liver cells.
Testing vacuum
Dr. Nissen said in an interview that one of the big takeaways from the study is the need for greater testing of Lp(a). Automatic assays are available in almost every hospital, but two-unit systems (nmol/L and mg/dL) are used and thresholds for accelerated risk vary. The Cleveland Clinic currently tests all patients in its cardiac critical care unit and its prevention clinic.
“Someone comes in with an MI in their 40s and we measure it and it’s 100, 150 [mg/dL], clearly abnormal, and often these patients don’t have a lot of other risk factors,” Dr. Nissen said. “So the explanation very likely for their premature disease is this risk factor. We now have to educate everybody about the importance of getting it tested and finding out about it.”
During a media briefing, ACC 2022 program cochair Pamela B. Morris, MD, Medical University of South Carolina, Charleston, said testing for Lp(a) is not well reimbursed by insurance providers and that her patients will often cancel the test after learning it won’t be reimbursed because they don’t understand it.
“What Dr. Nissen is telling you: It should be measured in everyone at least once, we all believe that, but it hasn’t made it into the major guidelines,” she added. “I think what we’re going to have to do is have the guidelines mandate it and the insurers will follow.”
Guidelines currently list elevated Lp(a) as a “risk-enhancing factor,” which can help with at least recommending LDL-C treatment in patients with borderline risk and a sky-high Lp(a), noted Dr. Nissen. “But we need to go beyond that.”
Safety analyses
The first-in-human APOLLO trial evaluated 32 adults without known ASCVD and an Lp(a) concentration greater than 150 nmol/L (approximately 60 mg/dL) who received one of the four doses of SLN360 or placebo subcutaneously. Participants were monitored in a research unit for the first 24 hours and then followed periodically for up to 150 days. At baseline, their median Lp(a) level was 224 nmol/L, mean apo B level was 85 mg/dL, and mean LDL-C level was 108 mg/dL.
Treatment-emergent adverse events were generally mild, mostly grade 1 injection site reactions (83% at 30 mg, 100% at 100 mg, 67% at 300 mg, and 33% at 600 mg) and headache (33%, 17%, 0%, and 83%).
At the highest dose, C-reactive protein was increased in four patients and neutrophil counts in three. ALT and AST levels were elevated three times above the upper limit of normal in one patient at the lowest dose.
One participant in the lowest-dose group experienced two serious adverse events unrelated to SLN360 at day 45 after receiving a SARS-Co-V-2 vaccine.
Dr. Nissen noted that safety cannot be comprehensively assessed in a trial of this duration or size and that follow-up has been extended to 1 year in the two highest-dose groups.
Enrollment continues in the multiple-ascending dose portion of the study in patients with high Lp(a) and a history of stable ASCVD. A phase 2 study of SLN360 is also planned for the second half of 2022, pending regulatory discussions.
But will it reduce ASCVD events?
Study discussant Vera Bittner, MD, MSPH, University of Alabama at Birmingham, said that the development of Lp(a)-specific lowering agents has been a “holy grail” for years and congratulated the authors on a successful trial demonstrating very robust Lp(a) lowering.
She asked Dr. Nissen about the observation in proprotein convertase subtilisin/kexin type 9 inhibitor trials that absolute Lp(a) lowering is greater at higher baseline levels.
Dr. Nissen said this kind of analysis wasn’t possible because of the small sample size but “because these agents so effectively degrade messenger RNA, it’s very likely we will see robust suppression of plasma levels virtually regardless of the baseline level.”
Dr. Bittner also questioned if “LDL-C declined because of the cholesterol content in the lipoprotein(a) or is there some additional effect on LDL particles themselves?”
“It’s a really terrific question that will ultimately need to be answered,” Dr. Nissen replied. “There’s some controversy about the extent to which suppressing lipoprotein(a) will reduce LDL because the assays for LDL are measuring the LDL that’s in lipoprotein(a) and the LDL that is not. ... I think it’s probably a bystander effect, but it may also contribute to efficacy from a morbidity and mortality point of view, which is why we measured it.”
Dr. Bittner also called out the elevation in C-reactive protein and leukocytosis, which has not been seen in other siRNA studies. Dr. Nissen said the increases in C-reactive protein occurred in the first few days after administration and were gone after a week or so. “I don’t see it as a long-term limitation.”
In an accompanying editorial, Brian Ference, MD, MPhil, MSc, University of Cambridge (England), suggests that because circulating Lp(a) particles can progressively become trapped within the artery wall over time, it’s unlikely that lowering Lp(a) for only a few years starting later in life will eliminate the effect of lifelong exposure to Lp(a) and may only cut cardiovascular event risk by about 10%-15%.
He called for continued safety and efficacy evaluation of SLN360 and olpasiran, a similar siRNA agent in early development, and said further insights into whether large absolute reductions in Lp(a) can reduce the risk for major cardiovascular events will come from cardiovascular trials, such as the ongoing phase 3 Lp(a)HORIZON trial. It follows strong phase 2 results with the antisense agent AKCEA-APO(a)-LRx and has Dr. Nissen pulling double duty as study chair.
The study was funded by Silence Therapeutics. Dr. Nissen reported consulting for many pharmaceutical companies, which are directed to pay any renumeration directly to charity. Dr. Bittner reported consultant fees or honoraria from Pfizer; other from AstraZeneca, DalCor, Esperion, and Sanofi-Aventis; and research/research grants from Amgen and Novartis. Dr. Ference reported financial ties to Merck, Novartis, Amgen, Pfizer, Esperion Therapeutics, and numerous other companies.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
New HF guidelines feature ‘quad’ therapy, tweaked terminology
The new heart failure (HF) guidelines released by three North American societies had a lot of catching up to do given the significant, even paradigm-shifting, additions to available treatment options in the last few years.
The landscape now includes both new and repurposed drug therapies that benefit almost without regard to ejection fraction (EF), and evidence-based urgency to engage patients early on with at least four core medication classes, so-called quadruple therapy.
The guideline document offers a roadmap for navigating those key issues and many others and uses some creative tactics. They include the introduction of generalist-friendly labels for the traditional but obscurely named four stages of HF severity that, it is hoped, will have wider reach and expand the use of effective therapies.
It introduces additional disease-staging terminology that characterizes the syndrome as a continuum:
- “At risk for HF” for stage A, applied to asymptomatic patients with risk factors such as diabetes or hypertension but no known cardiac changes.
- “Pre-HF” for stage B, which adds cardiac structural changes or elevated natriuretic peptides, still in the absence of symptoms.
- “Symptomatic HF” for stage C, that is, structural disease with current or previous symptoms.
- “Advanced HF” for stage D, characterized by severe debilitating symptoms or repeated hospitalizations even with guideline-directed medical therapy (GDMT).
The new terms should be “easier for primary care physicians as well as nonspecialists” to remember and use effectively “and easier to translate to the patients,” compared with the solely alphabetical staging labels appearing in the guidelines for more than 15 years, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
An emphasis on “at risk for HF” and “pre-HF” in the new document may help efforts to expand primary prevention of HF and management of preclinical HF. The guideline, Dr. Bozkurt said, includes specific treatment recommendations for those early stages.
The document also updates and sometimes introduces “recommendations for advanced heart failure, acute heart failure, and comorbidities – specifically for atrial fibrillation, iron deficiency, sleep apnea, coronary artery disease, and valvular heart disease,” Dr. Bozkurt observed, as well as for cardiomyopathy and HF related to pregnancy and cancer chemotherapy. “So, it’s a very comprehensive guideline.”
Dr. Bozkurt is vice chair of the guideline writing committee and helped introduce the guideline at the annual scientific sessions of the American College of Cardiology. The document, developed by the ACC, the American Heart Association, and the Heart Failure Society of America, was published April 1, 2022, in the societies’ flagship journals, Journal of the American College of Cardiology, Circulation, and the Journal of Cardiac Failure, respectively. It replaces the 2013 guideline from the ACC and AHA and the ACC/AHA/HFSA–focused update from 2017.
“We really need to treat early, and then we need to treat appropriately,” Douglas L. Mann, MD, Washington University in St. Louis, said in an interview. Dr. Mann, who was not involved in development of the new guideline, said he is “enthusiastic” about the new staging terminology.
“I think it makes it easier to convey the message that these people do need medicines, will benefit from medicines, and in some cases heart failure can be preventable,” he said. “I’m in favor of anything that simplifies it and makes it more readily interpretable by busy doctors who aren’t specialists.”
With the new staging terminology and in other ways, the guideline seems to appreciate cardiomyopathy as a journey from preclinical to advanced symptomatic stages – the preclinical “at-risk” stage tightening focus on primary prevention – and updated thinking on classification of HF by EF.
For example, there is new consideration of “HF with improved ejection fraction” (HFimpEF), which suggests the patient may be evolving from HF with reduced EF (HFrEF) to HF with EF that is preserved or mildly reduced, or vice versa.
With HFimpEF, which identifies patients previously with an EF of 40% or lower that improves to beyond 40% at follow-up testing, patients should continue on the medications they had been previously taking for HFrEF, Dr. Bozkurt said.
Patients at risk for HF, in stage A by the older terminology, are characterized by one or more significant HF risk factors, such as hypertension, diabetes, or coronary disease, as they have been in prior guidelines. But the new document, Dr. Bozkurt observed, adds genetic cardiomyopathies and exposure to cardiotoxic agents to the list.
Perhaps surprisingly, the guideline also includes elevated natriuretic peptides as an indicator of “at risk for HF,” with implications for screening. The evidence suggests that, “for patients who are at risk for heart failure, natriuretic peptide-based screening, followed by team-based care, can prevent development of left ventricular dysfunction in heart failure,” Dr. Bozkurt said.
Persons at risk for HF realistically encompass a huge swath of the population given the world prevalence of high blood pressure, obesity, and diabetes. Management of stage A, therefore, focuses on established tenets of primary cardiovascular prevention, such as weight and BP control, exercise, and healthy dietary choices.
They may well be eligible for treatment with sodium-glucose transporter 2 (SGLT2) inhibitors, which have been “game changers,” Dr. Mann said. “Now you can give them to diabetics and it’s going to prevent heart failure and [cardiovascular] events. We didn’t have a drug like that before, so I think that places a lot of emphasis on aggressive treatment of diabetes.”
For patients with symptomatic HF, the document touts multidisciplinary care and early initiation of drugs from each of four drug classes. Such quadruple therapy includes an SGLT2 inhibitor along with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system (RAS) inhibitor: the “core foundational therapies” for patients with HFrEF, Dr. Bozkurt observed.
Of note, she said, the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan (Entresto, Novartis) is the preferred RAS inhibitor. But “if the ARNI cannot be used, then use ACE inhibitors.” If the patient is intolerant of ACE inhibitors because of cough or angioedema, then the choice should be an angiotensin-receptor blocker.
“We have very effective therapies offering survival and morbidity benefits as well as improvements in quality of life and reverse remodeling,” Dr. Bozkurt observed. “The most important message is that optimization of therapies, including all of these medication classes, saves lives.”
The guideline also includes, for the first time, a series of “value statements” on cost-effectiveness of different therapies that assign a “high-value” rating to MRAs, hydralazine, and isosorbide dinitrate in otherwise optimally treated self-identified African Americans, and device therapy in appropriately selected patients. The statements hold SGLT2 inhibitors in chronic symptomatic HF and cardiac transplantation in advanced GDMT-resistant HF to be of “intermediate” value.
The value statements, Dr. Bozkurt noted, “are included throughout the document when there is evidence; when there is a high-quality cost-effectiveness study published.”
Dr. Bozkurt disclosed receiving honoraria or consulting fees from Amgen, AstraZeneca, Baxter International, Bristol-Myers Squibb, Sanofi-Aventis, scPharmaceuticals, and Vifor Pharma; serving on a data safety monitoring board for LivaNova USA; and holding other relationships with Abbott Laboratories and Relypsa. Dr. Mann disclosed receiving honoraria or consulting fees from MyoKardia, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
The new heart failure (HF) guidelines released by three North American societies had a lot of catching up to do given the significant, even paradigm-shifting, additions to available treatment options in the last few years.
The landscape now includes both new and repurposed drug therapies that benefit almost without regard to ejection fraction (EF), and evidence-based urgency to engage patients early on with at least four core medication classes, so-called quadruple therapy.
The guideline document offers a roadmap for navigating those key issues and many others and uses some creative tactics. They include the introduction of generalist-friendly labels for the traditional but obscurely named four stages of HF severity that, it is hoped, will have wider reach and expand the use of effective therapies.
It introduces additional disease-staging terminology that characterizes the syndrome as a continuum:
- “At risk for HF” for stage A, applied to asymptomatic patients with risk factors such as diabetes or hypertension but no known cardiac changes.
- “Pre-HF” for stage B, which adds cardiac structural changes or elevated natriuretic peptides, still in the absence of symptoms.
- “Symptomatic HF” for stage C, that is, structural disease with current or previous symptoms.
- “Advanced HF” for stage D, characterized by severe debilitating symptoms or repeated hospitalizations even with guideline-directed medical therapy (GDMT).
The new terms should be “easier for primary care physicians as well as nonspecialists” to remember and use effectively “and easier to translate to the patients,” compared with the solely alphabetical staging labels appearing in the guidelines for more than 15 years, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
An emphasis on “at risk for HF” and “pre-HF” in the new document may help efforts to expand primary prevention of HF and management of preclinical HF. The guideline, Dr. Bozkurt said, includes specific treatment recommendations for those early stages.
The document also updates and sometimes introduces “recommendations for advanced heart failure, acute heart failure, and comorbidities – specifically for atrial fibrillation, iron deficiency, sleep apnea, coronary artery disease, and valvular heart disease,” Dr. Bozkurt observed, as well as for cardiomyopathy and HF related to pregnancy and cancer chemotherapy. “So, it’s a very comprehensive guideline.”
Dr. Bozkurt is vice chair of the guideline writing committee and helped introduce the guideline at the annual scientific sessions of the American College of Cardiology. The document, developed by the ACC, the American Heart Association, and the Heart Failure Society of America, was published April 1, 2022, in the societies’ flagship journals, Journal of the American College of Cardiology, Circulation, and the Journal of Cardiac Failure, respectively. It replaces the 2013 guideline from the ACC and AHA and the ACC/AHA/HFSA–focused update from 2017.
“We really need to treat early, and then we need to treat appropriately,” Douglas L. Mann, MD, Washington University in St. Louis, said in an interview. Dr. Mann, who was not involved in development of the new guideline, said he is “enthusiastic” about the new staging terminology.
“I think it makes it easier to convey the message that these people do need medicines, will benefit from medicines, and in some cases heart failure can be preventable,” he said. “I’m in favor of anything that simplifies it and makes it more readily interpretable by busy doctors who aren’t specialists.”
With the new staging terminology and in other ways, the guideline seems to appreciate cardiomyopathy as a journey from preclinical to advanced symptomatic stages – the preclinical “at-risk” stage tightening focus on primary prevention – and updated thinking on classification of HF by EF.
For example, there is new consideration of “HF with improved ejection fraction” (HFimpEF), which suggests the patient may be evolving from HF with reduced EF (HFrEF) to HF with EF that is preserved or mildly reduced, or vice versa.
With HFimpEF, which identifies patients previously with an EF of 40% or lower that improves to beyond 40% at follow-up testing, patients should continue on the medications they had been previously taking for HFrEF, Dr. Bozkurt said.
Patients at risk for HF, in stage A by the older terminology, are characterized by one or more significant HF risk factors, such as hypertension, diabetes, or coronary disease, as they have been in prior guidelines. But the new document, Dr. Bozkurt observed, adds genetic cardiomyopathies and exposure to cardiotoxic agents to the list.
Perhaps surprisingly, the guideline also includes elevated natriuretic peptides as an indicator of “at risk for HF,” with implications for screening. The evidence suggests that, “for patients who are at risk for heart failure, natriuretic peptide-based screening, followed by team-based care, can prevent development of left ventricular dysfunction in heart failure,” Dr. Bozkurt said.
Persons at risk for HF realistically encompass a huge swath of the population given the world prevalence of high blood pressure, obesity, and diabetes. Management of stage A, therefore, focuses on established tenets of primary cardiovascular prevention, such as weight and BP control, exercise, and healthy dietary choices.
They may well be eligible for treatment with sodium-glucose transporter 2 (SGLT2) inhibitors, which have been “game changers,” Dr. Mann said. “Now you can give them to diabetics and it’s going to prevent heart failure and [cardiovascular] events. We didn’t have a drug like that before, so I think that places a lot of emphasis on aggressive treatment of diabetes.”
For patients with symptomatic HF, the document touts multidisciplinary care and early initiation of drugs from each of four drug classes. Such quadruple therapy includes an SGLT2 inhibitor along with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system (RAS) inhibitor: the “core foundational therapies” for patients with HFrEF, Dr. Bozkurt observed.
Of note, she said, the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan (Entresto, Novartis) is the preferred RAS inhibitor. But “if the ARNI cannot be used, then use ACE inhibitors.” If the patient is intolerant of ACE inhibitors because of cough or angioedema, then the choice should be an angiotensin-receptor blocker.
“We have very effective therapies offering survival and morbidity benefits as well as improvements in quality of life and reverse remodeling,” Dr. Bozkurt observed. “The most important message is that optimization of therapies, including all of these medication classes, saves lives.”
The guideline also includes, for the first time, a series of “value statements” on cost-effectiveness of different therapies that assign a “high-value” rating to MRAs, hydralazine, and isosorbide dinitrate in otherwise optimally treated self-identified African Americans, and device therapy in appropriately selected patients. The statements hold SGLT2 inhibitors in chronic symptomatic HF and cardiac transplantation in advanced GDMT-resistant HF to be of “intermediate” value.
The value statements, Dr. Bozkurt noted, “are included throughout the document when there is evidence; when there is a high-quality cost-effectiveness study published.”
Dr. Bozkurt disclosed receiving honoraria or consulting fees from Amgen, AstraZeneca, Baxter International, Bristol-Myers Squibb, Sanofi-Aventis, scPharmaceuticals, and Vifor Pharma; serving on a data safety monitoring board for LivaNova USA; and holding other relationships with Abbott Laboratories and Relypsa. Dr. Mann disclosed receiving honoraria or consulting fees from MyoKardia, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
The new heart failure (HF) guidelines released by three North American societies had a lot of catching up to do given the significant, even paradigm-shifting, additions to available treatment options in the last few years.
The landscape now includes both new and repurposed drug therapies that benefit almost without regard to ejection fraction (EF), and evidence-based urgency to engage patients early on with at least four core medication classes, so-called quadruple therapy.
The guideline document offers a roadmap for navigating those key issues and many others and uses some creative tactics. They include the introduction of generalist-friendly labels for the traditional but obscurely named four stages of HF severity that, it is hoped, will have wider reach and expand the use of effective therapies.
It introduces additional disease-staging terminology that characterizes the syndrome as a continuum:
- “At risk for HF” for stage A, applied to asymptomatic patients with risk factors such as diabetes or hypertension but no known cardiac changes.
- “Pre-HF” for stage B, which adds cardiac structural changes or elevated natriuretic peptides, still in the absence of symptoms.
- “Symptomatic HF” for stage C, that is, structural disease with current or previous symptoms.
- “Advanced HF” for stage D, characterized by severe debilitating symptoms or repeated hospitalizations even with guideline-directed medical therapy (GDMT).
The new terms should be “easier for primary care physicians as well as nonspecialists” to remember and use effectively “and easier to translate to the patients,” compared with the solely alphabetical staging labels appearing in the guidelines for more than 15 years, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
An emphasis on “at risk for HF” and “pre-HF” in the new document may help efforts to expand primary prevention of HF and management of preclinical HF. The guideline, Dr. Bozkurt said, includes specific treatment recommendations for those early stages.
The document also updates and sometimes introduces “recommendations for advanced heart failure, acute heart failure, and comorbidities – specifically for atrial fibrillation, iron deficiency, sleep apnea, coronary artery disease, and valvular heart disease,” Dr. Bozkurt observed, as well as for cardiomyopathy and HF related to pregnancy and cancer chemotherapy. “So, it’s a very comprehensive guideline.”
Dr. Bozkurt is vice chair of the guideline writing committee and helped introduce the guideline at the annual scientific sessions of the American College of Cardiology. The document, developed by the ACC, the American Heart Association, and the Heart Failure Society of America, was published April 1, 2022, in the societies’ flagship journals, Journal of the American College of Cardiology, Circulation, and the Journal of Cardiac Failure, respectively. It replaces the 2013 guideline from the ACC and AHA and the ACC/AHA/HFSA–focused update from 2017.
“We really need to treat early, and then we need to treat appropriately,” Douglas L. Mann, MD, Washington University in St. Louis, said in an interview. Dr. Mann, who was not involved in development of the new guideline, said he is “enthusiastic” about the new staging terminology.
“I think it makes it easier to convey the message that these people do need medicines, will benefit from medicines, and in some cases heart failure can be preventable,” he said. “I’m in favor of anything that simplifies it and makes it more readily interpretable by busy doctors who aren’t specialists.”
With the new staging terminology and in other ways, the guideline seems to appreciate cardiomyopathy as a journey from preclinical to advanced symptomatic stages – the preclinical “at-risk” stage tightening focus on primary prevention – and updated thinking on classification of HF by EF.
For example, there is new consideration of “HF with improved ejection fraction” (HFimpEF), which suggests the patient may be evolving from HF with reduced EF (HFrEF) to HF with EF that is preserved or mildly reduced, or vice versa.
With HFimpEF, which identifies patients previously with an EF of 40% or lower that improves to beyond 40% at follow-up testing, patients should continue on the medications they had been previously taking for HFrEF, Dr. Bozkurt said.
Patients at risk for HF, in stage A by the older terminology, are characterized by one or more significant HF risk factors, such as hypertension, diabetes, or coronary disease, as they have been in prior guidelines. But the new document, Dr. Bozkurt observed, adds genetic cardiomyopathies and exposure to cardiotoxic agents to the list.
Perhaps surprisingly, the guideline also includes elevated natriuretic peptides as an indicator of “at risk for HF,” with implications for screening. The evidence suggests that, “for patients who are at risk for heart failure, natriuretic peptide-based screening, followed by team-based care, can prevent development of left ventricular dysfunction in heart failure,” Dr. Bozkurt said.
Persons at risk for HF realistically encompass a huge swath of the population given the world prevalence of high blood pressure, obesity, and diabetes. Management of stage A, therefore, focuses on established tenets of primary cardiovascular prevention, such as weight and BP control, exercise, and healthy dietary choices.
They may well be eligible for treatment with sodium-glucose transporter 2 (SGLT2) inhibitors, which have been “game changers,” Dr. Mann said. “Now you can give them to diabetics and it’s going to prevent heart failure and [cardiovascular] events. We didn’t have a drug like that before, so I think that places a lot of emphasis on aggressive treatment of diabetes.”
For patients with symptomatic HF, the document touts multidisciplinary care and early initiation of drugs from each of four drug classes. Such quadruple therapy includes an SGLT2 inhibitor along with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system (RAS) inhibitor: the “core foundational therapies” for patients with HFrEF, Dr. Bozkurt observed.
Of note, she said, the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan (Entresto, Novartis) is the preferred RAS inhibitor. But “if the ARNI cannot be used, then use ACE inhibitors.” If the patient is intolerant of ACE inhibitors because of cough or angioedema, then the choice should be an angiotensin-receptor blocker.
“We have very effective therapies offering survival and morbidity benefits as well as improvements in quality of life and reverse remodeling,” Dr. Bozkurt observed. “The most important message is that optimization of therapies, including all of these medication classes, saves lives.”
The guideline also includes, for the first time, a series of “value statements” on cost-effectiveness of different therapies that assign a “high-value” rating to MRAs, hydralazine, and isosorbide dinitrate in otherwise optimally treated self-identified African Americans, and device therapy in appropriately selected patients. The statements hold SGLT2 inhibitors in chronic symptomatic HF and cardiac transplantation in advanced GDMT-resistant HF to be of “intermediate” value.
The value statements, Dr. Bozkurt noted, “are included throughout the document when there is evidence; when there is a high-quality cost-effectiveness study published.”
Dr. Bozkurt disclosed receiving honoraria or consulting fees from Amgen, AstraZeneca, Baxter International, Bristol-Myers Squibb, Sanofi-Aventis, scPharmaceuticals, and Vifor Pharma; serving on a data safety monitoring board for LivaNova USA; and holding other relationships with Abbott Laboratories and Relypsa. Dr. Mann disclosed receiving honoraria or consulting fees from MyoKardia, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Supermarket diet advice improves DASH adherence: SuperWIN
People who received personalized nutrition education in a series of sessions at their regular grocery store significantly improved adherence to a healthy diet, in a new “first-of-its-kind” study in which scientific researchers partnered with a large supermarket company.
In the SuperWIN study, participants were given individualized advice from supermarket-based dietitians using data on their own buying habits recorded on their supermarket loyalty cards. This was associated with an increased adherence to the DASH (Dietary Approaches to Stop Hypertension) diet, which emphasizes vegetables, fruits and whole grains while limiting foods that are high in saturated fat, sugar, and sodium and has been shown to lower blood pressure and LDL cholesterol.
One group of patients also received additional education about healthy eating and meal planning through online technologies, and this group showed even better adherence to the DASH diet.
The study was presented at the annual scientific sessions of the American College of Cardiology by Dylan Steen, MD, adjunct associate professor of medicine at the University of Cincinnati.
“The SuperWIN study provides evidence for the benefit of delivering healthy-eating interventions at modern supermarkets and retail-based clinics,” Dr. Steen said. “It demonstrates the efficacy of dietary interventions harnessing the physical environment of the supermarket, the retail-based dietitians working within the store, and the purchasing data captured on the store’s loyalty cards.”
The study was conducted in partnership with Kroger, the largest supermarket chain in the United States, which also operates a large chain of pharmacies and health clinics.
Dr. Steen said the study was addressing one of the biggest public health problems – unhealthy eating – with an innovative approach. “We need to think about how we can extend the reach of modern health care systems into communities and better deliver services right where people are; meet them where they live,” he said at an ACC press conference.
Commenting on the study at the press conference, Eileen Handberg, PhD, professor of medicine at University of Florida, Gainesville, and immediate past chair of the ACC Cardiovascular Care Team Council, said: “I am amazingly excited about this. There is so much potential here. We have never really taken advantage of the current explosion in retail-based health care before.”
Dr. Handberg suggested the study had major implications for the primary prevention of cardiovascular disease. “Little kids go shopping with their parents, so you have the ability here to change behavior from children on up if you can change the dynamic of the choices they make in the grocery store.”
In his presentation, Dr. Steen noted that, despite many longstanding guidelines on healthy eating, about 75% of Americans still have a poor-quality diet. This trial was conducted to see if a new approach could improve that situation. “If we change the environment in which we deliver dietary education, we can make a difference.”
The SuperWIN trial was conducted in 13 Kroger stores in Ohio and Kentucky. The study enrolled 267 people with at least one cardiovascular risk factor from a primary care network who regularly shopped at one of the study stores. All participants also had to be willing to follow the DASH diet, which was taught at each educational session in the trial.
All participants received one “enhanced” medical nutrition therapy that was guided by the individual’s own dietary intake analytics.
They were then randomly assigned to one of three arms. The control group received no further education. The strategy 1 group received six additional teaching sessions in the supermarket aisles over a 3-month period. Each session was guided by updated individualized purchasing data provided to the dietitian and the participant.
The strategy 2 group received the same six additional teaching sessions as strategy 1, but they also had some additional teaching on healthy eating and meal planning from a variety of online shopping tools, and nutrition and health care apps.
“The supermarket analytics were automatically collected so the dietitians could tell what each person liked to eat, how much of each product they were buying and how much they were spending,” Dr. Steen explained.
COVID hit halfway through the trial, and 20 participants were withdrawn for their own safety as they could no longer visit the stores, but the trial continued with the rest of the participants with enhanced safety precautions. The overall analysis cohort was 247 participants.
The average age of the participants was mid-50s, around 70% were female, and most did not have a history of cardiovascular disease.
Eating habits were assessed by three 24-hour dietary recalls assessed at the start of the study and at 3 and 6 months. The DASH score, which is a measure of adherence to the DASH diet, was calculated from this information. The score can range from 0 to 90, with an increased score showing increased adherence.
In one analysis, the researchers compared the DASH scores from the two intervention groups together with the control group, and in a second analysis they compared the scores in the strategy 2 group with those in the strategy 1 group.
Before the pandemic there was “near 100%” attendance for the six visits over the 3-month study period, which Dr. Steen said he thought was “remarkable.” During the pandemic, attendance came down to around 80%.
Results showed that the DASH score increased in all three groups at 3 months, with stepwise increases corresponding to the intensity of the intervention. DASH scores increased by 5.8 points in the control group, by 8.6 points in the strategy 1 group, and by 12.4 points in the strategy 2 group.
DASH scores significantly differed between the two intervention groups and the control group (P = .02). “This shows that purchasing data–guided in-store tours do increase the efficacy of dietary education,” Dr. Steen said.
The difference in scores between the strategy 1 and strategy 2 groups was also significant (P = .01). “This shows online enhancements increase adherence to the DASH diet even further,” Dr. Steen commented
By 6 months, the scores had dropped off a little but were still increased from baseline: by 4.4 points in the control group, 6.6 points in the strategy 1 group, and 8.4 points in the strategy 2 group. “There was again a stepwise increase as the intervention intensified, but there was no longer a significant difference between the interventions and control,” Dr. Steen noted.
Secondary endpoints included blood pressure and body mass index. Systolic blood pressure decreased slightly in all three groups: by 2.8 mm Hg in the control group, 6.6 mm Hg in the strategy 1 group, and 5.7 mm Hg in the strategy 2 group. Body mass index was reduced by 0.2, 0.4 and 0.8, respectively, but the between-group differences were not significant.
Dr. Steen said this is the first study of its kind to date in which scientific researchers collaborated with a large supermarket chain. He explained they also involved a primary care network so that health care utilization information will be available.
“We can the integrate retail-based health care information with traditional health care information. And we can start to look at downstream health care utilization and cost outcomes as well, which will be important as we start to think how to evolve the health care system,” he commented. “The hope is that we can get more scientists working with more retailers to really drive the evidence to shape the evolution of our health care system.”
Challenges ahead
Dr. Handberg pointed out there would be challenges in reaching the underserved population who do not shop at the major supermarkets. “We need to figure out how to get partnerships across the whole spectrum of grocery stores.”
She also noted that 3 months (the duration of the study intervention) was not much time to change the eating habits of a family. “Interventions may have to be a bit more intensive to get the change in blood pressure and weight that we would want to see.”
Dr Handberg hoped the major grocery store companies will see the opportunities in this approach. “Changing behavior is very complicated, and the key will be how to make people stick with the changes. But grocery stores are smart. They have got us going to their pharmacies, so getting us to see a dietitian is not that much of a stretch.”
Moderator of the ACC late-breaker session at which the study was presented, Pamela Morris, MD, from the Medical University of South Carolina, Charleston, who is also ACC annual scientific session chair, asked whether the approach could be sustained.
“I am thinking back to the barber shop study of blood pressure treatment and to my knowledge those PharmDs are no longer in those barbershops, taking blood pressures, counseling patients, and prescribing antihypertensives. So is Kroger maintaining a long-term commitment to providing this education, or how can this be financed over the long term?” she asked.
Dr. Steen replied that he believed sustainability to be one of the key strengths of this model. “Retail-based health care is exploding in the U.S. The number of retail outlets offering a comprehensive list of services is going up all the time. These programs exist regardless of whether this trial was conducted or not.”
But Dr. Steen stressed that having an evidence base will be critically important.
“Validation is an enormous part of this evolution in retail-based health care – not only to figure out what works but also to engage payors and others in the process of supporting these interventions. I think the sustainability is there – it is sort of baked into the model – but research will be a huge part of cementing this in and helping us to understand what we should do.”
The study was funded by Kroger. Dr. Steen is a consultant for Sanofi and CEO and cofounder of High Enroll.
A version of this article first appeared on Medscape.com.
People who received personalized nutrition education in a series of sessions at their regular grocery store significantly improved adherence to a healthy diet, in a new “first-of-its-kind” study in which scientific researchers partnered with a large supermarket company.
In the SuperWIN study, participants were given individualized advice from supermarket-based dietitians using data on their own buying habits recorded on their supermarket loyalty cards. This was associated with an increased adherence to the DASH (Dietary Approaches to Stop Hypertension) diet, which emphasizes vegetables, fruits and whole grains while limiting foods that are high in saturated fat, sugar, and sodium and has been shown to lower blood pressure and LDL cholesterol.
One group of patients also received additional education about healthy eating and meal planning through online technologies, and this group showed even better adherence to the DASH diet.
The study was presented at the annual scientific sessions of the American College of Cardiology by Dylan Steen, MD, adjunct associate professor of medicine at the University of Cincinnati.
“The SuperWIN study provides evidence for the benefit of delivering healthy-eating interventions at modern supermarkets and retail-based clinics,” Dr. Steen said. “It demonstrates the efficacy of dietary interventions harnessing the physical environment of the supermarket, the retail-based dietitians working within the store, and the purchasing data captured on the store’s loyalty cards.”
The study was conducted in partnership with Kroger, the largest supermarket chain in the United States, which also operates a large chain of pharmacies and health clinics.
Dr. Steen said the study was addressing one of the biggest public health problems – unhealthy eating – with an innovative approach. “We need to think about how we can extend the reach of modern health care systems into communities and better deliver services right where people are; meet them where they live,” he said at an ACC press conference.
Commenting on the study at the press conference, Eileen Handberg, PhD, professor of medicine at University of Florida, Gainesville, and immediate past chair of the ACC Cardiovascular Care Team Council, said: “I am amazingly excited about this. There is so much potential here. We have never really taken advantage of the current explosion in retail-based health care before.”
Dr. Handberg suggested the study had major implications for the primary prevention of cardiovascular disease. “Little kids go shopping with their parents, so you have the ability here to change behavior from children on up if you can change the dynamic of the choices they make in the grocery store.”
In his presentation, Dr. Steen noted that, despite many longstanding guidelines on healthy eating, about 75% of Americans still have a poor-quality diet. This trial was conducted to see if a new approach could improve that situation. “If we change the environment in which we deliver dietary education, we can make a difference.”
The SuperWIN trial was conducted in 13 Kroger stores in Ohio and Kentucky. The study enrolled 267 people with at least one cardiovascular risk factor from a primary care network who regularly shopped at one of the study stores. All participants also had to be willing to follow the DASH diet, which was taught at each educational session in the trial.
All participants received one “enhanced” medical nutrition therapy that was guided by the individual’s own dietary intake analytics.
They were then randomly assigned to one of three arms. The control group received no further education. The strategy 1 group received six additional teaching sessions in the supermarket aisles over a 3-month period. Each session was guided by updated individualized purchasing data provided to the dietitian and the participant.
The strategy 2 group received the same six additional teaching sessions as strategy 1, but they also had some additional teaching on healthy eating and meal planning from a variety of online shopping tools, and nutrition and health care apps.
“The supermarket analytics were automatically collected so the dietitians could tell what each person liked to eat, how much of each product they were buying and how much they were spending,” Dr. Steen explained.
COVID hit halfway through the trial, and 20 participants were withdrawn for their own safety as they could no longer visit the stores, but the trial continued with the rest of the participants with enhanced safety precautions. The overall analysis cohort was 247 participants.
The average age of the participants was mid-50s, around 70% were female, and most did not have a history of cardiovascular disease.
Eating habits were assessed by three 24-hour dietary recalls assessed at the start of the study and at 3 and 6 months. The DASH score, which is a measure of adherence to the DASH diet, was calculated from this information. The score can range from 0 to 90, with an increased score showing increased adherence.
In one analysis, the researchers compared the DASH scores from the two intervention groups together with the control group, and in a second analysis they compared the scores in the strategy 2 group with those in the strategy 1 group.
Before the pandemic there was “near 100%” attendance for the six visits over the 3-month study period, which Dr. Steen said he thought was “remarkable.” During the pandemic, attendance came down to around 80%.
Results showed that the DASH score increased in all three groups at 3 months, with stepwise increases corresponding to the intensity of the intervention. DASH scores increased by 5.8 points in the control group, by 8.6 points in the strategy 1 group, and by 12.4 points in the strategy 2 group.
DASH scores significantly differed between the two intervention groups and the control group (P = .02). “This shows that purchasing data–guided in-store tours do increase the efficacy of dietary education,” Dr. Steen said.
The difference in scores between the strategy 1 and strategy 2 groups was also significant (P = .01). “This shows online enhancements increase adherence to the DASH diet even further,” Dr. Steen commented
By 6 months, the scores had dropped off a little but were still increased from baseline: by 4.4 points in the control group, 6.6 points in the strategy 1 group, and 8.4 points in the strategy 2 group. “There was again a stepwise increase as the intervention intensified, but there was no longer a significant difference between the interventions and control,” Dr. Steen noted.
Secondary endpoints included blood pressure and body mass index. Systolic blood pressure decreased slightly in all three groups: by 2.8 mm Hg in the control group, 6.6 mm Hg in the strategy 1 group, and 5.7 mm Hg in the strategy 2 group. Body mass index was reduced by 0.2, 0.4 and 0.8, respectively, but the between-group differences were not significant.
Dr. Steen said this is the first study of its kind to date in which scientific researchers collaborated with a large supermarket chain. He explained they also involved a primary care network so that health care utilization information will be available.
“We can the integrate retail-based health care information with traditional health care information. And we can start to look at downstream health care utilization and cost outcomes as well, which will be important as we start to think how to evolve the health care system,” he commented. “The hope is that we can get more scientists working with more retailers to really drive the evidence to shape the evolution of our health care system.”
Challenges ahead
Dr. Handberg pointed out there would be challenges in reaching the underserved population who do not shop at the major supermarkets. “We need to figure out how to get partnerships across the whole spectrum of grocery stores.”
She also noted that 3 months (the duration of the study intervention) was not much time to change the eating habits of a family. “Interventions may have to be a bit more intensive to get the change in blood pressure and weight that we would want to see.”
Dr Handberg hoped the major grocery store companies will see the opportunities in this approach. “Changing behavior is very complicated, and the key will be how to make people stick with the changes. But grocery stores are smart. They have got us going to their pharmacies, so getting us to see a dietitian is not that much of a stretch.”
Moderator of the ACC late-breaker session at which the study was presented, Pamela Morris, MD, from the Medical University of South Carolina, Charleston, who is also ACC annual scientific session chair, asked whether the approach could be sustained.
“I am thinking back to the barber shop study of blood pressure treatment and to my knowledge those PharmDs are no longer in those barbershops, taking blood pressures, counseling patients, and prescribing antihypertensives. So is Kroger maintaining a long-term commitment to providing this education, or how can this be financed over the long term?” she asked.
Dr. Steen replied that he believed sustainability to be one of the key strengths of this model. “Retail-based health care is exploding in the U.S. The number of retail outlets offering a comprehensive list of services is going up all the time. These programs exist regardless of whether this trial was conducted or not.”
But Dr. Steen stressed that having an evidence base will be critically important.
“Validation is an enormous part of this evolution in retail-based health care – not only to figure out what works but also to engage payors and others in the process of supporting these interventions. I think the sustainability is there – it is sort of baked into the model – but research will be a huge part of cementing this in and helping us to understand what we should do.”
The study was funded by Kroger. Dr. Steen is a consultant for Sanofi and CEO and cofounder of High Enroll.
A version of this article first appeared on Medscape.com.
People who received personalized nutrition education in a series of sessions at their regular grocery store significantly improved adherence to a healthy diet, in a new “first-of-its-kind” study in which scientific researchers partnered with a large supermarket company.
In the SuperWIN study, participants were given individualized advice from supermarket-based dietitians using data on their own buying habits recorded on their supermarket loyalty cards. This was associated with an increased adherence to the DASH (Dietary Approaches to Stop Hypertension) diet, which emphasizes vegetables, fruits and whole grains while limiting foods that are high in saturated fat, sugar, and sodium and has been shown to lower blood pressure and LDL cholesterol.
One group of patients also received additional education about healthy eating and meal planning through online technologies, and this group showed even better adherence to the DASH diet.
The study was presented at the annual scientific sessions of the American College of Cardiology by Dylan Steen, MD, adjunct associate professor of medicine at the University of Cincinnati.
“The SuperWIN study provides evidence for the benefit of delivering healthy-eating interventions at modern supermarkets and retail-based clinics,” Dr. Steen said. “It demonstrates the efficacy of dietary interventions harnessing the physical environment of the supermarket, the retail-based dietitians working within the store, and the purchasing data captured on the store’s loyalty cards.”
The study was conducted in partnership with Kroger, the largest supermarket chain in the United States, which also operates a large chain of pharmacies and health clinics.
Dr. Steen said the study was addressing one of the biggest public health problems – unhealthy eating – with an innovative approach. “We need to think about how we can extend the reach of modern health care systems into communities and better deliver services right where people are; meet them where they live,” he said at an ACC press conference.
Commenting on the study at the press conference, Eileen Handberg, PhD, professor of medicine at University of Florida, Gainesville, and immediate past chair of the ACC Cardiovascular Care Team Council, said: “I am amazingly excited about this. There is so much potential here. We have never really taken advantage of the current explosion in retail-based health care before.”
Dr. Handberg suggested the study had major implications for the primary prevention of cardiovascular disease. “Little kids go shopping with their parents, so you have the ability here to change behavior from children on up if you can change the dynamic of the choices they make in the grocery store.”
In his presentation, Dr. Steen noted that, despite many longstanding guidelines on healthy eating, about 75% of Americans still have a poor-quality diet. This trial was conducted to see if a new approach could improve that situation. “If we change the environment in which we deliver dietary education, we can make a difference.”
The SuperWIN trial was conducted in 13 Kroger stores in Ohio and Kentucky. The study enrolled 267 people with at least one cardiovascular risk factor from a primary care network who regularly shopped at one of the study stores. All participants also had to be willing to follow the DASH diet, which was taught at each educational session in the trial.
All participants received one “enhanced” medical nutrition therapy that was guided by the individual’s own dietary intake analytics.
They were then randomly assigned to one of three arms. The control group received no further education. The strategy 1 group received six additional teaching sessions in the supermarket aisles over a 3-month period. Each session was guided by updated individualized purchasing data provided to the dietitian and the participant.
The strategy 2 group received the same six additional teaching sessions as strategy 1, but they also had some additional teaching on healthy eating and meal planning from a variety of online shopping tools, and nutrition and health care apps.
“The supermarket analytics were automatically collected so the dietitians could tell what each person liked to eat, how much of each product they were buying and how much they were spending,” Dr. Steen explained.
COVID hit halfway through the trial, and 20 participants were withdrawn for their own safety as they could no longer visit the stores, but the trial continued with the rest of the participants with enhanced safety precautions. The overall analysis cohort was 247 participants.
The average age of the participants was mid-50s, around 70% were female, and most did not have a history of cardiovascular disease.
Eating habits were assessed by three 24-hour dietary recalls assessed at the start of the study and at 3 and 6 months. The DASH score, which is a measure of adherence to the DASH diet, was calculated from this information. The score can range from 0 to 90, with an increased score showing increased adherence.
In one analysis, the researchers compared the DASH scores from the two intervention groups together with the control group, and in a second analysis they compared the scores in the strategy 2 group with those in the strategy 1 group.
Before the pandemic there was “near 100%” attendance for the six visits over the 3-month study period, which Dr. Steen said he thought was “remarkable.” During the pandemic, attendance came down to around 80%.
Results showed that the DASH score increased in all three groups at 3 months, with stepwise increases corresponding to the intensity of the intervention. DASH scores increased by 5.8 points in the control group, by 8.6 points in the strategy 1 group, and by 12.4 points in the strategy 2 group.
DASH scores significantly differed between the two intervention groups and the control group (P = .02). “This shows that purchasing data–guided in-store tours do increase the efficacy of dietary education,” Dr. Steen said.
The difference in scores between the strategy 1 and strategy 2 groups was also significant (P = .01). “This shows online enhancements increase adherence to the DASH diet even further,” Dr. Steen commented
By 6 months, the scores had dropped off a little but were still increased from baseline: by 4.4 points in the control group, 6.6 points in the strategy 1 group, and 8.4 points in the strategy 2 group. “There was again a stepwise increase as the intervention intensified, but there was no longer a significant difference between the interventions and control,” Dr. Steen noted.
Secondary endpoints included blood pressure and body mass index. Systolic blood pressure decreased slightly in all three groups: by 2.8 mm Hg in the control group, 6.6 mm Hg in the strategy 1 group, and 5.7 mm Hg in the strategy 2 group. Body mass index was reduced by 0.2, 0.4 and 0.8, respectively, but the between-group differences were not significant.
Dr. Steen said this is the first study of its kind to date in which scientific researchers collaborated with a large supermarket chain. He explained they also involved a primary care network so that health care utilization information will be available.
“We can the integrate retail-based health care information with traditional health care information. And we can start to look at downstream health care utilization and cost outcomes as well, which will be important as we start to think how to evolve the health care system,” he commented. “The hope is that we can get more scientists working with more retailers to really drive the evidence to shape the evolution of our health care system.”
Challenges ahead
Dr. Handberg pointed out there would be challenges in reaching the underserved population who do not shop at the major supermarkets. “We need to figure out how to get partnerships across the whole spectrum of grocery stores.”
She also noted that 3 months (the duration of the study intervention) was not much time to change the eating habits of a family. “Interventions may have to be a bit more intensive to get the change in blood pressure and weight that we would want to see.”
Dr Handberg hoped the major grocery store companies will see the opportunities in this approach. “Changing behavior is very complicated, and the key will be how to make people stick with the changes. But grocery stores are smart. They have got us going to their pharmacies, so getting us to see a dietitian is not that much of a stretch.”
Moderator of the ACC late-breaker session at which the study was presented, Pamela Morris, MD, from the Medical University of South Carolina, Charleston, who is also ACC annual scientific session chair, asked whether the approach could be sustained.
“I am thinking back to the barber shop study of blood pressure treatment and to my knowledge those PharmDs are no longer in those barbershops, taking blood pressures, counseling patients, and prescribing antihypertensives. So is Kroger maintaining a long-term commitment to providing this education, or how can this be financed over the long term?” she asked.
Dr. Steen replied that he believed sustainability to be one of the key strengths of this model. “Retail-based health care is exploding in the U.S. The number of retail outlets offering a comprehensive list of services is going up all the time. These programs exist regardless of whether this trial was conducted or not.”
But Dr. Steen stressed that having an evidence base will be critically important.
“Validation is an enormous part of this evolution in retail-based health care – not only to figure out what works but also to engage payors and others in the process of supporting these interventions. I think the sustainability is there – it is sort of baked into the model – but research will be a huge part of cementing this in and helping us to understand what we should do.”
The study was funded by Kroger. Dr. Steen is a consultant for Sanofi and CEO and cofounder of High Enroll.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Mavacamten controlled hypertrophic cardiomyopathy for over 1 year
WASHINGTON – Treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy who remained on treatment with the investigational agent mavacamten for a median of 62 weeks continued to show the same level of safe response to the drug as seen after the first 30 weeks on treatment in the pivotal trial for this agent.
The new findings from longer-term treatment bode well for mavacamten. That’s because if the drug is used in routine practice to avoid the need for surgery or an invasive intervention to reduce blockage of a patient’s left ventricular outflow tract, the duration of mavacamten treatment will likely need to continue for many years and even for decades, said Florian Rader, MD, who presented the results at the annual scientific sessions of the American College of Cardiology.
“In practice, mavacamten will probably be used for many, many years, especially as it replaces septal-reduction therapy, so we need long-term data,” noted Dr. Rader during a press briefing on his report. “I’m very happy with the long-term data” in the follow-up study.
The Food and Drug Administration is currently considering whether to approve mavacamten for routine marketing to treat patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM), with a decision expected by the end of April 2022.
The study Dr. Rader reported followed 231 patients with symptomatic oHCM who had completed the 30-week pivotal trial of mavacamten, EXPLORER-HCM, and opted to continue on open-label extended treatment with mavacamten, either continuing the treatment they started during the trial or crossing over to receive mavacamten after receiving placebo during the trial.
The major findings from EXPLORER-LTE (long-term extension) were that continued treatment for a median of about 62 weeks maintained the safety and efficacy findings seen at the end of the blinded, randomized, initial 30-week phase, said Dr. Rader, codirector of the Clinic for Hypertrophic Cardiomyopathy and Aortopathies at Cedars-Sinai Medical Center in Los Angeles.
‘Almost revolutionary’
Mavacamten represents “an almost revolutionary change” for treating oHCM, commented Maya E. Guglin, MD, professor of clinical medicine and an advanced heart failure physician at Indiana University, Indianapolis. “Until now, there was no good medical treatment for symptomatic oHCM. This will change the landscape, and without question it will change guidelines for treating oHCM,” said Dr. Guglin during the press briefing.
“All of us who care for patients with oHCM have looked forward to having a disease-specific therapy. It is encouraging to see that the safety and efficacy remained high with long-term follow-up,” commented Kyle W. Klarich, MD, a professor and cardiologist who specializes in treating patients with HCM at the Mayo Clinic in Rochester, Minn.
Mavacamten is a direct myosin inhibitor that reduces the excess number of myosin-actin cross bridges that form in patients with oHCM, and thereby directly targets the pathophysiology that underlies the disorder, explained Dr. Rader.
The patients on mavacamten included in the long-term extension reported by Dr. Rader averaged 60 years of age, and 61% were men. They averaged a 35.6–mm Hg drop in their resting left ventricular outflow tract (LVOT) gradient after 48 weeks on treatment, and a 32.8–mm Hg reduction after 84 weeks. When the investigators measured their LVOT gradient during a valsalva maneuver, their reductions from baseline averaged 45.3 mm Hg after 48 weeks and 46.4 mm Hg after 84 weeks.
Resting left ventricular ejection fraction also fell, by an average of 7.0 percentage points from baseline after 48 weeks, and by an average of 9.0 percentage points after 84 weeks. After 48 weeks on treatment, 68% of patients had at least a one-class improvement from baseline in their New York Heart Association functional class.
The safety results showed that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten of the 231 patients discontinued mavacamten because of a treated-related adverse event.
EXPLORER-HCM and EXPLORER-LTE were sponsored by MyoKardia, the company that is developing mavacamten and which is now owned by Bristol-Myers Squibb. Dr. Rader has been a consultant to MyoKardia as well as to Medtronic and ReCor. Dr. Guglin and Dr. Klarich had no disclosures.
WASHINGTON – Treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy who remained on treatment with the investigational agent mavacamten for a median of 62 weeks continued to show the same level of safe response to the drug as seen after the first 30 weeks on treatment in the pivotal trial for this agent.
The new findings from longer-term treatment bode well for mavacamten. That’s because if the drug is used in routine practice to avoid the need for surgery or an invasive intervention to reduce blockage of a patient’s left ventricular outflow tract, the duration of mavacamten treatment will likely need to continue for many years and even for decades, said Florian Rader, MD, who presented the results at the annual scientific sessions of the American College of Cardiology.
“In practice, mavacamten will probably be used for many, many years, especially as it replaces septal-reduction therapy, so we need long-term data,” noted Dr. Rader during a press briefing on his report. “I’m very happy with the long-term data” in the follow-up study.
The Food and Drug Administration is currently considering whether to approve mavacamten for routine marketing to treat patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM), with a decision expected by the end of April 2022.
The study Dr. Rader reported followed 231 patients with symptomatic oHCM who had completed the 30-week pivotal trial of mavacamten, EXPLORER-HCM, and opted to continue on open-label extended treatment with mavacamten, either continuing the treatment they started during the trial or crossing over to receive mavacamten after receiving placebo during the trial.
The major findings from EXPLORER-LTE (long-term extension) were that continued treatment for a median of about 62 weeks maintained the safety and efficacy findings seen at the end of the blinded, randomized, initial 30-week phase, said Dr. Rader, codirector of the Clinic for Hypertrophic Cardiomyopathy and Aortopathies at Cedars-Sinai Medical Center in Los Angeles.
‘Almost revolutionary’
Mavacamten represents “an almost revolutionary change” for treating oHCM, commented Maya E. Guglin, MD, professor of clinical medicine and an advanced heart failure physician at Indiana University, Indianapolis. “Until now, there was no good medical treatment for symptomatic oHCM. This will change the landscape, and without question it will change guidelines for treating oHCM,” said Dr. Guglin during the press briefing.
“All of us who care for patients with oHCM have looked forward to having a disease-specific therapy. It is encouraging to see that the safety and efficacy remained high with long-term follow-up,” commented Kyle W. Klarich, MD, a professor and cardiologist who specializes in treating patients with HCM at the Mayo Clinic in Rochester, Minn.
Mavacamten is a direct myosin inhibitor that reduces the excess number of myosin-actin cross bridges that form in patients with oHCM, and thereby directly targets the pathophysiology that underlies the disorder, explained Dr. Rader.
The patients on mavacamten included in the long-term extension reported by Dr. Rader averaged 60 years of age, and 61% were men. They averaged a 35.6–mm Hg drop in their resting left ventricular outflow tract (LVOT) gradient after 48 weeks on treatment, and a 32.8–mm Hg reduction after 84 weeks. When the investigators measured their LVOT gradient during a valsalva maneuver, their reductions from baseline averaged 45.3 mm Hg after 48 weeks and 46.4 mm Hg after 84 weeks.
Resting left ventricular ejection fraction also fell, by an average of 7.0 percentage points from baseline after 48 weeks, and by an average of 9.0 percentage points after 84 weeks. After 48 weeks on treatment, 68% of patients had at least a one-class improvement from baseline in their New York Heart Association functional class.
The safety results showed that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten of the 231 patients discontinued mavacamten because of a treated-related adverse event.
EXPLORER-HCM and EXPLORER-LTE were sponsored by MyoKardia, the company that is developing mavacamten and which is now owned by Bristol-Myers Squibb. Dr. Rader has been a consultant to MyoKardia as well as to Medtronic and ReCor. Dr. Guglin and Dr. Klarich had no disclosures.
WASHINGTON – Treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy who remained on treatment with the investigational agent mavacamten for a median of 62 weeks continued to show the same level of safe response to the drug as seen after the first 30 weeks on treatment in the pivotal trial for this agent.
The new findings from longer-term treatment bode well for mavacamten. That’s because if the drug is used in routine practice to avoid the need for surgery or an invasive intervention to reduce blockage of a patient’s left ventricular outflow tract, the duration of mavacamten treatment will likely need to continue for many years and even for decades, said Florian Rader, MD, who presented the results at the annual scientific sessions of the American College of Cardiology.
“In practice, mavacamten will probably be used for many, many years, especially as it replaces septal-reduction therapy, so we need long-term data,” noted Dr. Rader during a press briefing on his report. “I’m very happy with the long-term data” in the follow-up study.
The Food and Drug Administration is currently considering whether to approve mavacamten for routine marketing to treat patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM), with a decision expected by the end of April 2022.
The study Dr. Rader reported followed 231 patients with symptomatic oHCM who had completed the 30-week pivotal trial of mavacamten, EXPLORER-HCM, and opted to continue on open-label extended treatment with mavacamten, either continuing the treatment they started during the trial or crossing over to receive mavacamten after receiving placebo during the trial.
The major findings from EXPLORER-LTE (long-term extension) were that continued treatment for a median of about 62 weeks maintained the safety and efficacy findings seen at the end of the blinded, randomized, initial 30-week phase, said Dr. Rader, codirector of the Clinic for Hypertrophic Cardiomyopathy and Aortopathies at Cedars-Sinai Medical Center in Los Angeles.
‘Almost revolutionary’
Mavacamten represents “an almost revolutionary change” for treating oHCM, commented Maya E. Guglin, MD, professor of clinical medicine and an advanced heart failure physician at Indiana University, Indianapolis. “Until now, there was no good medical treatment for symptomatic oHCM. This will change the landscape, and without question it will change guidelines for treating oHCM,” said Dr. Guglin during the press briefing.
“All of us who care for patients with oHCM have looked forward to having a disease-specific therapy. It is encouraging to see that the safety and efficacy remained high with long-term follow-up,” commented Kyle W. Klarich, MD, a professor and cardiologist who specializes in treating patients with HCM at the Mayo Clinic in Rochester, Minn.
Mavacamten is a direct myosin inhibitor that reduces the excess number of myosin-actin cross bridges that form in patients with oHCM, and thereby directly targets the pathophysiology that underlies the disorder, explained Dr. Rader.
The patients on mavacamten included in the long-term extension reported by Dr. Rader averaged 60 years of age, and 61% were men. They averaged a 35.6–mm Hg drop in their resting left ventricular outflow tract (LVOT) gradient after 48 weeks on treatment, and a 32.8–mm Hg reduction after 84 weeks. When the investigators measured their LVOT gradient during a valsalva maneuver, their reductions from baseline averaged 45.3 mm Hg after 48 weeks and 46.4 mm Hg after 84 weeks.
Resting left ventricular ejection fraction also fell, by an average of 7.0 percentage points from baseline after 48 weeks, and by an average of 9.0 percentage points after 84 weeks. After 48 weeks on treatment, 68% of patients had at least a one-class improvement from baseline in their New York Heart Association functional class.
The safety results showed that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten of the 231 patients discontinued mavacamten because of a treated-related adverse event.
EXPLORER-HCM and EXPLORER-LTE were sponsored by MyoKardia, the company that is developing mavacamten and which is now owned by Bristol-Myers Squibb. Dr. Rader has been a consultant to MyoKardia as well as to Medtronic and ReCor. Dr. Guglin and Dr. Klarich had no disclosures.
AT ACC 2022
Novel cholesterol drug disappoints: TRANSLATE-TIMI 70
An investigational drug targeting a novel cholesterol pathway has shown disappointing results in the TRANSLATE-TIMI 70 phase 2b study.
Vupanorsen is an antisense oligonucleotide targeting hepatic angiopoietin-like protein 3 (ANGPTL3), a protein that inhibits enzymes involved in the metabolism of triglyceride and cholesterol. Inhibition of ANGPTL3 is one of several novel targets for lowering triglycerides and non-HDL cholesterol.
Results of the TRANSLATE-TIMI 70 study were presented at the annual scientific sessions of the American College of Cardiology by Brian Bergmark, MD, a cardiologist at Brigham and Women’s Hospital, Boston. They were also simultaneously published online in Circulation.
“While vupanorsen significantly reduced triglycerides and non-HDL cholesterol, the reduction in non-HDL cholesterol of 22%-27% was not to a degree that was clinically meaningful for cardiovascular risk reduction, and there were also some potentially important safety issues,” Dr. Bergmark said in an interview.
Pfizer has announced that, after reviewing the results of this study, it is discontinuing development of vupanorsen and will return rights to Ionis, from which it licensed the investigational therapy in 2019.
In response to a question at an ACC press conference on whether there could be any future for the drug, Dr. Bergmark said that “the degree of lipid lowering was not as much as what had been suggested was potentially possible by acting on this pathway, and then there are the additional safety concerns. So, for the specific question of what we were looking at – cardiovascular risk reduction by impacting non-HDL cholesterol and apo [apolipoprotein] B – the modest efficacy paired with the safety concerns does not look favorable for future development of this drug.”
But he added: “Whether some other person or company wants to think about triglyceride lowering and try to find a dose that is a bit safer, that is not for me to say.”
In his ACC presentation, Dr. Bergmark explained that ANGPTL3 is a protein secreted by the liver that inhibits lipases, including lipoprotein lipase. Loss-of-function variants in ANGPTL3 are associated with lower levels of plasma lipids and a monoclonal antibody targeting ANGPTL3, evinacumab (Evkeeza, Regeneron), is approved as an intravenous infusion for the treatment of familial hypercholesterolemia. Vupanorsen is a second-generation antisense oligonucleotide targeting hepatic ANGPTL3 messenger RNA with a potential role for cardiovascular risk reduction.
A previous phase 2a study of vupanorsen in patients with hypertriglyceridemia, hepatic steatosis, and type 2 diabetes mellitus showed significant reductions in triglycerides at all doses studied, as well as reductions in non-HDL cholesterol at the highest doses (80 mg per month given by subcutaneous injection).
Dr. Bergmark noted that, because a potential cardiovascular benefit of vupanorsen would best be reflected by its effects on non-HDL cholesterol, the current TRANSLATE-TIMI 70 trial was designed to assess the effect of escalating doses of vupanorsen on non-HDL cholesterol levels in statin-treated adults with hyperlipidemia.
For the study, 286 adults with non-HDL cholesterol levels of 100 mg/dL or greater (median, 132 mg/dL) and triglyceride levels of 150-500 mg/dL (median, 216 mg/dL) who were receiving statin therapy were randomly assigned to placebo or one of seven vupanorsen dose regimens (80, 120, or 160 mg every 4 weeks or 60, 80, 120, or 160 mg every 2 weeks). All doses were given by subcutaneous injection.
The study population was said to reflect “a typical cohort intended for cardiovascular risk reduction, with type 2 diabetes in approximately one-half of patients and prevalent atherosclerotic cardiovascular disease in a substantial portion,” the researchers wrote in the published report.
The primary endpoint was placebo-adjusted percentage change from baseline in non-HDL cholesterol at 24 weeks. Secondary endpoints included placebo-adjusted percentage changes from baseline in triglycerides, LDL cholesterol, apo B, and ANGPTL3.
Vupanorsen resulted in significant decreases from baseline over placebo in non-HDL cholesterol ranging from 22.0% in the group receiving 60 mg every 2 weeks to 27.7% in the group receiving 80 mg every 2 weeks, but there did not appear to be a dose response.
Regarding additional lipid endpoints, vupanorsen reduced triglyceride levels in a dose-dependent manner, ranging from 41.3% in the group receiving 120 mg every 4 weeks to 56.8% in the group receiving 160 mg every 2 weeks.
The effects of vupanorsen on LDL cholesterol and apo B were more modest and without a clear dose response. Vupanorsen also lowered HDL cholesterol levels at all doses studied, and there was no significant change in high-sensitivity C-reactive protein at any dose.
Liver enzymes and hepatic fat increases of concern
In terms of safety, vupanorsen treatment was linked to liver enzyme elevations; more than three-times elevations of alanine aminotransferase or aspartate aminotransferase were more common at higher total monthly doses (up to 33.3% and 44.4%, respectively). Injection site reactions were also an issue, including recall reactions at sites of previous injections when subsequent injections were given. In addition, there was a dose-related increase (up to 76%) in hepatic fat fraction.
In the Circulation paper, the researchers say it is unclear whether the increases in hepatic fat fraction and liver enzymes reflect a metabolic effect of vupanorsen specifically or an off-target effect resulting from hepatic targeting of ANGPTL3. “Regardless, these are medically meaningful findings with important safety ramifications,” they wrote.
They pointed out that, whereas the reduction in ANGPTL3 levels increased with total monthly dose of vupanorsen, there was no clear dose-response reduction in LDL cholesterol, apo B, or non-HDL cholesterol.
In comparison, evinacumab, a monoclonal antibody against ANGPTL3 that is thought to cause near-total suppression of ANGPTL3 activity, reduces apo B levels by more than 40% in adults with refractory hypercholesterolemia or homozygous familial hypercholesterolemia.
Asked why vupanorsen showed less of an effect on non-HDL cholesterol than evinacumab, Dr. Bergmark suggested that the monoclonal antibody may achieve greater inhibition of ANGPTL3. “It may be that near complete suppression is needed to obtain clinically meaningful reductions in apo B and non-HDL cholesterol. That is a speculative and simplistic explanation,” he commented.
Conversely, reductions in triglycerides with vupanorsen showed a dose-response relationship, mirroring the reduction in ANGPTL3 and consistent with the expected increases in lipoprotein lipase activity, the researchers reported.
They note that the “relatively muted effect on apo B levels” suggests that vupanorsen is primarily decreasing the triglyceride and, to a lesser extent, cholesterol content of very low-density lipoprotein cholesterol particles rather than reducing the number of such particles.
“These observations have important implications for the potential ability of this mechanism to reduce lipid-mediated cardiovascular risk, which largely appears to be a function of the number of ApoB-containing lipoproteins,” they said.
Designated discussant of the study at the ACC late-breaking session, Pradeep Natarajan, MD, director of preventive cardiology at Massachusetts General Hospital in Boston, asked Dr. Bergmark what minimum degree of non-HDL cholesterol reduction would be compelling for a new drug to be considered for wide-scale use.
Dr. Bergmark replied there was no clear to answer to that question, as it would depend on many factors, including the risk of the population and the time horizon involved. But he added: “I think a minimum of at least a 30%-40% reduction in non-HDL cholesterol would be needed for a meaningful reduction in cardiovascular risk across a variety of settings.”
The TRANSLATE-TIMI 70 study was funded by Pfizer. Dr. Bergmark is a member of the TIMI Study Group, which has received institutional grant support through Brigham and Women’s Hospital from numerous pharmaceutical companies. Dr. Bergmark also reported receiving grant support through Brigham and Women’s Hospital from Pfizer, Ionis, AstraZeneca, and Abbott Vascular and consulting/personal fees from Abiomed, CSI, Philips, Abbott Vascular, Servier, DaiichiSankyo, Janssen, and Quark.
A version of this article first appeared on Medscape.com.
An investigational drug targeting a novel cholesterol pathway has shown disappointing results in the TRANSLATE-TIMI 70 phase 2b study.
Vupanorsen is an antisense oligonucleotide targeting hepatic angiopoietin-like protein 3 (ANGPTL3), a protein that inhibits enzymes involved in the metabolism of triglyceride and cholesterol. Inhibition of ANGPTL3 is one of several novel targets for lowering triglycerides and non-HDL cholesterol.
Results of the TRANSLATE-TIMI 70 study were presented at the annual scientific sessions of the American College of Cardiology by Brian Bergmark, MD, a cardiologist at Brigham and Women’s Hospital, Boston. They were also simultaneously published online in Circulation.
“While vupanorsen significantly reduced triglycerides and non-HDL cholesterol, the reduction in non-HDL cholesterol of 22%-27% was not to a degree that was clinically meaningful for cardiovascular risk reduction, and there were also some potentially important safety issues,” Dr. Bergmark said in an interview.
Pfizer has announced that, after reviewing the results of this study, it is discontinuing development of vupanorsen and will return rights to Ionis, from which it licensed the investigational therapy in 2019.
In response to a question at an ACC press conference on whether there could be any future for the drug, Dr. Bergmark said that “the degree of lipid lowering was not as much as what had been suggested was potentially possible by acting on this pathway, and then there are the additional safety concerns. So, for the specific question of what we were looking at – cardiovascular risk reduction by impacting non-HDL cholesterol and apo [apolipoprotein] B – the modest efficacy paired with the safety concerns does not look favorable for future development of this drug.”
But he added: “Whether some other person or company wants to think about triglyceride lowering and try to find a dose that is a bit safer, that is not for me to say.”
In his ACC presentation, Dr. Bergmark explained that ANGPTL3 is a protein secreted by the liver that inhibits lipases, including lipoprotein lipase. Loss-of-function variants in ANGPTL3 are associated with lower levels of plasma lipids and a monoclonal antibody targeting ANGPTL3, evinacumab (Evkeeza, Regeneron), is approved as an intravenous infusion for the treatment of familial hypercholesterolemia. Vupanorsen is a second-generation antisense oligonucleotide targeting hepatic ANGPTL3 messenger RNA with a potential role for cardiovascular risk reduction.
A previous phase 2a study of vupanorsen in patients with hypertriglyceridemia, hepatic steatosis, and type 2 diabetes mellitus showed significant reductions in triglycerides at all doses studied, as well as reductions in non-HDL cholesterol at the highest doses (80 mg per month given by subcutaneous injection).
Dr. Bergmark noted that, because a potential cardiovascular benefit of vupanorsen would best be reflected by its effects on non-HDL cholesterol, the current TRANSLATE-TIMI 70 trial was designed to assess the effect of escalating doses of vupanorsen on non-HDL cholesterol levels in statin-treated adults with hyperlipidemia.
For the study, 286 adults with non-HDL cholesterol levels of 100 mg/dL or greater (median, 132 mg/dL) and triglyceride levels of 150-500 mg/dL (median, 216 mg/dL) who were receiving statin therapy were randomly assigned to placebo or one of seven vupanorsen dose regimens (80, 120, or 160 mg every 4 weeks or 60, 80, 120, or 160 mg every 2 weeks). All doses were given by subcutaneous injection.
The study population was said to reflect “a typical cohort intended for cardiovascular risk reduction, with type 2 diabetes in approximately one-half of patients and prevalent atherosclerotic cardiovascular disease in a substantial portion,” the researchers wrote in the published report.
The primary endpoint was placebo-adjusted percentage change from baseline in non-HDL cholesterol at 24 weeks. Secondary endpoints included placebo-adjusted percentage changes from baseline in triglycerides, LDL cholesterol, apo B, and ANGPTL3.
Vupanorsen resulted in significant decreases from baseline over placebo in non-HDL cholesterol ranging from 22.0% in the group receiving 60 mg every 2 weeks to 27.7% in the group receiving 80 mg every 2 weeks, but there did not appear to be a dose response.
Regarding additional lipid endpoints, vupanorsen reduced triglyceride levels in a dose-dependent manner, ranging from 41.3% in the group receiving 120 mg every 4 weeks to 56.8% in the group receiving 160 mg every 2 weeks.
The effects of vupanorsen on LDL cholesterol and apo B were more modest and without a clear dose response. Vupanorsen also lowered HDL cholesterol levels at all doses studied, and there was no significant change in high-sensitivity C-reactive protein at any dose.
Liver enzymes and hepatic fat increases of concern
In terms of safety, vupanorsen treatment was linked to liver enzyme elevations; more than three-times elevations of alanine aminotransferase or aspartate aminotransferase were more common at higher total monthly doses (up to 33.3% and 44.4%, respectively). Injection site reactions were also an issue, including recall reactions at sites of previous injections when subsequent injections were given. In addition, there was a dose-related increase (up to 76%) in hepatic fat fraction.
In the Circulation paper, the researchers say it is unclear whether the increases in hepatic fat fraction and liver enzymes reflect a metabolic effect of vupanorsen specifically or an off-target effect resulting from hepatic targeting of ANGPTL3. “Regardless, these are medically meaningful findings with important safety ramifications,” they wrote.
They pointed out that, whereas the reduction in ANGPTL3 levels increased with total monthly dose of vupanorsen, there was no clear dose-response reduction in LDL cholesterol, apo B, or non-HDL cholesterol.
In comparison, evinacumab, a monoclonal antibody against ANGPTL3 that is thought to cause near-total suppression of ANGPTL3 activity, reduces apo B levels by more than 40% in adults with refractory hypercholesterolemia or homozygous familial hypercholesterolemia.
Asked why vupanorsen showed less of an effect on non-HDL cholesterol than evinacumab, Dr. Bergmark suggested that the monoclonal antibody may achieve greater inhibition of ANGPTL3. “It may be that near complete suppression is needed to obtain clinically meaningful reductions in apo B and non-HDL cholesterol. That is a speculative and simplistic explanation,” he commented.
Conversely, reductions in triglycerides with vupanorsen showed a dose-response relationship, mirroring the reduction in ANGPTL3 and consistent with the expected increases in lipoprotein lipase activity, the researchers reported.
They note that the “relatively muted effect on apo B levels” suggests that vupanorsen is primarily decreasing the triglyceride and, to a lesser extent, cholesterol content of very low-density lipoprotein cholesterol particles rather than reducing the number of such particles.
“These observations have important implications for the potential ability of this mechanism to reduce lipid-mediated cardiovascular risk, which largely appears to be a function of the number of ApoB-containing lipoproteins,” they said.
Designated discussant of the study at the ACC late-breaking session, Pradeep Natarajan, MD, director of preventive cardiology at Massachusetts General Hospital in Boston, asked Dr. Bergmark what minimum degree of non-HDL cholesterol reduction would be compelling for a new drug to be considered for wide-scale use.
Dr. Bergmark replied there was no clear to answer to that question, as it would depend on many factors, including the risk of the population and the time horizon involved. But he added: “I think a minimum of at least a 30%-40% reduction in non-HDL cholesterol would be needed for a meaningful reduction in cardiovascular risk across a variety of settings.”
The TRANSLATE-TIMI 70 study was funded by Pfizer. Dr. Bergmark is a member of the TIMI Study Group, which has received institutional grant support through Brigham and Women’s Hospital from numerous pharmaceutical companies. Dr. Bergmark also reported receiving grant support through Brigham and Women’s Hospital from Pfizer, Ionis, AstraZeneca, and Abbott Vascular and consulting/personal fees from Abiomed, CSI, Philips, Abbott Vascular, Servier, DaiichiSankyo, Janssen, and Quark.
A version of this article first appeared on Medscape.com.
An investigational drug targeting a novel cholesterol pathway has shown disappointing results in the TRANSLATE-TIMI 70 phase 2b study.
Vupanorsen is an antisense oligonucleotide targeting hepatic angiopoietin-like protein 3 (ANGPTL3), a protein that inhibits enzymes involved in the metabolism of triglyceride and cholesterol. Inhibition of ANGPTL3 is one of several novel targets for lowering triglycerides and non-HDL cholesterol.
Results of the TRANSLATE-TIMI 70 study were presented at the annual scientific sessions of the American College of Cardiology by Brian Bergmark, MD, a cardiologist at Brigham and Women’s Hospital, Boston. They were also simultaneously published online in Circulation.
“While vupanorsen significantly reduced triglycerides and non-HDL cholesterol, the reduction in non-HDL cholesterol of 22%-27% was not to a degree that was clinically meaningful for cardiovascular risk reduction, and there were also some potentially important safety issues,” Dr. Bergmark said in an interview.
Pfizer has announced that, after reviewing the results of this study, it is discontinuing development of vupanorsen and will return rights to Ionis, from which it licensed the investigational therapy in 2019.
In response to a question at an ACC press conference on whether there could be any future for the drug, Dr. Bergmark said that “the degree of lipid lowering was not as much as what had been suggested was potentially possible by acting on this pathway, and then there are the additional safety concerns. So, for the specific question of what we were looking at – cardiovascular risk reduction by impacting non-HDL cholesterol and apo [apolipoprotein] B – the modest efficacy paired with the safety concerns does not look favorable for future development of this drug.”
But he added: “Whether some other person or company wants to think about triglyceride lowering and try to find a dose that is a bit safer, that is not for me to say.”
In his ACC presentation, Dr. Bergmark explained that ANGPTL3 is a protein secreted by the liver that inhibits lipases, including lipoprotein lipase. Loss-of-function variants in ANGPTL3 are associated with lower levels of plasma lipids and a monoclonal antibody targeting ANGPTL3, evinacumab (Evkeeza, Regeneron), is approved as an intravenous infusion for the treatment of familial hypercholesterolemia. Vupanorsen is a second-generation antisense oligonucleotide targeting hepatic ANGPTL3 messenger RNA with a potential role for cardiovascular risk reduction.
A previous phase 2a study of vupanorsen in patients with hypertriglyceridemia, hepatic steatosis, and type 2 diabetes mellitus showed significant reductions in triglycerides at all doses studied, as well as reductions in non-HDL cholesterol at the highest doses (80 mg per month given by subcutaneous injection).
Dr. Bergmark noted that, because a potential cardiovascular benefit of vupanorsen would best be reflected by its effects on non-HDL cholesterol, the current TRANSLATE-TIMI 70 trial was designed to assess the effect of escalating doses of vupanorsen on non-HDL cholesterol levels in statin-treated adults with hyperlipidemia.
For the study, 286 adults with non-HDL cholesterol levels of 100 mg/dL or greater (median, 132 mg/dL) and triglyceride levels of 150-500 mg/dL (median, 216 mg/dL) who were receiving statin therapy were randomly assigned to placebo or one of seven vupanorsen dose regimens (80, 120, or 160 mg every 4 weeks or 60, 80, 120, or 160 mg every 2 weeks). All doses were given by subcutaneous injection.
The study population was said to reflect “a typical cohort intended for cardiovascular risk reduction, with type 2 diabetes in approximately one-half of patients and prevalent atherosclerotic cardiovascular disease in a substantial portion,” the researchers wrote in the published report.
The primary endpoint was placebo-adjusted percentage change from baseline in non-HDL cholesterol at 24 weeks. Secondary endpoints included placebo-adjusted percentage changes from baseline in triglycerides, LDL cholesterol, apo B, and ANGPTL3.
Vupanorsen resulted in significant decreases from baseline over placebo in non-HDL cholesterol ranging from 22.0% in the group receiving 60 mg every 2 weeks to 27.7% in the group receiving 80 mg every 2 weeks, but there did not appear to be a dose response.
Regarding additional lipid endpoints, vupanorsen reduced triglyceride levels in a dose-dependent manner, ranging from 41.3% in the group receiving 120 mg every 4 weeks to 56.8% in the group receiving 160 mg every 2 weeks.
The effects of vupanorsen on LDL cholesterol and apo B were more modest and without a clear dose response. Vupanorsen also lowered HDL cholesterol levels at all doses studied, and there was no significant change in high-sensitivity C-reactive protein at any dose.
Liver enzymes and hepatic fat increases of concern
In terms of safety, vupanorsen treatment was linked to liver enzyme elevations; more than three-times elevations of alanine aminotransferase or aspartate aminotransferase were more common at higher total monthly doses (up to 33.3% and 44.4%, respectively). Injection site reactions were also an issue, including recall reactions at sites of previous injections when subsequent injections were given. In addition, there was a dose-related increase (up to 76%) in hepatic fat fraction.
In the Circulation paper, the researchers say it is unclear whether the increases in hepatic fat fraction and liver enzymes reflect a metabolic effect of vupanorsen specifically or an off-target effect resulting from hepatic targeting of ANGPTL3. “Regardless, these are medically meaningful findings with important safety ramifications,” they wrote.
They pointed out that, whereas the reduction in ANGPTL3 levels increased with total monthly dose of vupanorsen, there was no clear dose-response reduction in LDL cholesterol, apo B, or non-HDL cholesterol.
In comparison, evinacumab, a monoclonal antibody against ANGPTL3 that is thought to cause near-total suppression of ANGPTL3 activity, reduces apo B levels by more than 40% in adults with refractory hypercholesterolemia or homozygous familial hypercholesterolemia.
Asked why vupanorsen showed less of an effect on non-HDL cholesterol than evinacumab, Dr. Bergmark suggested that the monoclonal antibody may achieve greater inhibition of ANGPTL3. “It may be that near complete suppression is needed to obtain clinically meaningful reductions in apo B and non-HDL cholesterol. That is a speculative and simplistic explanation,” he commented.
Conversely, reductions in triglycerides with vupanorsen showed a dose-response relationship, mirroring the reduction in ANGPTL3 and consistent with the expected increases in lipoprotein lipase activity, the researchers reported.
They note that the “relatively muted effect on apo B levels” suggests that vupanorsen is primarily decreasing the triglyceride and, to a lesser extent, cholesterol content of very low-density lipoprotein cholesterol particles rather than reducing the number of such particles.
“These observations have important implications for the potential ability of this mechanism to reduce lipid-mediated cardiovascular risk, which largely appears to be a function of the number of ApoB-containing lipoproteins,” they said.
Designated discussant of the study at the ACC late-breaking session, Pradeep Natarajan, MD, director of preventive cardiology at Massachusetts General Hospital in Boston, asked Dr. Bergmark what minimum degree of non-HDL cholesterol reduction would be compelling for a new drug to be considered for wide-scale use.
Dr. Bergmark replied there was no clear to answer to that question, as it would depend on many factors, including the risk of the population and the time horizon involved. But he added: “I think a minimum of at least a 30%-40% reduction in non-HDL cholesterol would be needed for a meaningful reduction in cardiovascular risk across a variety of settings.”
The TRANSLATE-TIMI 70 study was funded by Pfizer. Dr. Bergmark is a member of the TIMI Study Group, which has received institutional grant support through Brigham and Women’s Hospital from numerous pharmaceutical companies. Dr. Bergmark also reported receiving grant support through Brigham and Women’s Hospital from Pfizer, Ionis, AstraZeneca, and Abbott Vascular and consulting/personal fees from Abiomed, CSI, Philips, Abbott Vascular, Servier, DaiichiSankyo, Janssen, and Quark.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Diltiazem fails to improve vasomotor dysfunction, angina in ANOCA: EDIT-CMD
In a randomized trial of patients with angina and no obstructive coronary artery disease (ANOCA), 6 weeks of treatment with diltiazem did not improve coronary vasomotor dysfunction – apart from epicardial spasm – or angina symptoms and quality of life.
The trial investigated whether this therapy would improve these outcomes in patients with two mutually exclusive subgroups, or endotypes, of coronary vasomotor dysfunction: coronary artery spasm (epicardial spasm, microvascular spasm) or coronary microvascular dysfunction indicated by coronary flow reserve (CFR) and index of microvascular resistance (IMR) values.
Treatment success, the primary study endpoint – defined as normalization of one of the abnormal endotypes and no normal endotype becoming abnormal – was similar after treatment with diltiazem, compared with placebo. Nor were there significant differences for secondary endpoints apart from improvements in epicardial spasm in the two groups.
Tijn Jansen, MD, presented these findings from the EDIT-CMD trial in a featured clinical research session at the annual scientific sessions of the American College of Cardiology. The study was simultaneously published online April 2, 2022, in JACC: Cardiovascular Imaging.
“This first study using repeated coronary function testing provides a platform for future research,” concluded Dr. Jansen, a PhD candidate in the department of cardiology, Radboud University, Nijmegen, the Netherlands.
“We were surprised indeed” that diltiazem did not meet its primary endpoint for successful treatment and did not reduce symptoms or improve quality of life, compared with placebo, unlike results of the CorMicA trial, he said in an interview.
“We did find a treatment success, however, of 21%, which was slightly lower than expected, but it was not better than just giving placebo. This was similar regarding symptoms and quality of life, where we did find an overall improvement with diltiazem, but again not higher than using placebo,” he added. “It seems that giving the diagnosis to these patients itself creates a reduction in symptoms,” that might be caused by a reduction in stress, Dr. Jansen suggested.
The clinical implication, he said, is that more randomized controlled trials in this patient population are needed to permit evidence-based patient-tailored treatment, based on the different endotypes. “It might even be imaginable to test effectiveness in each individual patient using coronary function testing,” he said.
These tests are more and more commonly used in clinical practice, Dr. Jansen noted. “In the Netherlands, we recently launched the NL-CFT registry, which enables the participating centers to perform the CFT with a standardized protocol, with the goal to collect data and increase knowledge in this patient population.”
Heterogeneous population?
“I think probably the reason this trial was negative is [that coronary vasomotor dysfunction is] just too heterogeneous,” assigned discussant, C. Noel Bairey Merz, MD, commented.
“The problem with effectiveness trials is that you get a very heterogeneous population, and not everything works for everyone,” she said.
“This was a strategy trial – too heterogenous and too small to assess each endotype response,” Dr. Bairey Merz elaborated in an interview.
“Calcium channel blockers [CCBs] will not [effectively] treat all endotypes of coronary microvascular dysfunction,” she added, noting that the 6-month CorMIcA trial demonstrated in a larger, more rigorous trial design that CCBs are effective for epicardial and microvascular spasm.
“If you were going to do this study again, would you allow physicians to do up-titration and/or go a little bit longer?” Dr. Bairey Merz asked Dr. Jansen during the discussion.
“I do think this is a very heterogeneous group,” he agreed. However, the protocol allowed researchers to titrate diltiazem from 120 mg/day to 360 mg/day.
“If I were to do it again,” Dr. Jansen said, “I would focus on one specific endotype, probably epicardial spasm.”
First RCT of diltiazem in patients with ANOCA
Up to 40% of patients undergoing coronary angiography for stable angina do not have obstructive coronary artery disease (CAD), and 60%-90% of these patients have coronary vasomotor dysfunction, Dr. Jansen noted.
The landmark CorMicA trial showed that diagnosing the specific endotype of coronary vasomotor dysfunction using coronary function testing allows for tailored medication that decreased angina and improved quality of life, the researchers noted.
A recent European Society of Cardiology position paper on ANOCA “recommends the use of various pharmacological treatments including calcium-channel blockers, beta-blockers, ACE inhibitors, statins, and nitric oxide modulators, of which CCBs have the most prominent role in both endotypes of coronary vasospasms” and coronary microvascular dysfunction, they wrote.
“However, evidence substantiating these recommendations is lacking,” the researchers added, “since it is based on studies in a different population, with small sample sizes, or not placebo controlled.”
To investigate this, between 2019 and 2021, EDIT-CMD enrolled 126 adults aged 18 years and older who had two or more chronic angina episodes per week and no signs of obstructive CAD, who were seen at three hospitals specializing in ANOCA in the Netherlands.
The participants underwent coronary function testing that consisted of an acetylcholine spasm provocation test to evaluate for epicardial spasm and microvascular spasm, and a bolus thermodilution test with adenosine, to assess CFR and IMR. Coronary microvascular dysfunction was defined as CFR less than 2.0 and IMR of 25 or greater.
Of 99 patients with vasospasm or microvascular dysfunction, 85 patients were randomly assigned to receive diltiazem (n = 41) or placebo (n = 44) for 6 weeks.
The patients in both groups had a mean age of 58 years, and 29% were male; 22% had previously undergone percutaneous coronary intervention, and 48% had severe angina (Canadian Cardiovascular Society grade III/IV).
At baseline, about 50% had epicardial spasm, 25% had microvascular spasm and 25% had no spasm, and 54% in the diltiazem group and 73% in the placebo group had microvascular dysfunction.
After 6 weeks, 73 patients (35 in the placebo group and 38 in the diltiazem group) were available for repeat coronary function testing.
For the primary outcome, after 6 weeks of treatment, the proportion of patients with normalization of one abnormal parameter of coronary vasomotor dysfunction, without any normal parameter becoming abnormal, occurred in 8 patients (21%) in the diltiazem group versus 10 patients (29%) in the placebo group (P = .46)
In secondary outcomes, after 6 weeks of treatment, there were no significant differences in the prevalence of microvascular dysfunction, in Seattle Angina Questionnaire scores for angina symptoms, or RAND-36 scores for quality of life between patients who received diltiazem vs those who received placebo.
However, more patients in the diltiazem group than in the placebo group progressed from epicardial spasm to microvascular or no spasm (47% vs. 6%; P = .006).
The EDIT-CMD trial was sponsored by Abbott. Dr. Jansen has no relevant financial disclosures. Dr. Bairey Merz discloses having a fiduciary role and shares in iRhythm and being on the advisory board for Sanofi.
A version of this article first appeared on Medscape.com.
In a randomized trial of patients with angina and no obstructive coronary artery disease (ANOCA), 6 weeks of treatment with diltiazem did not improve coronary vasomotor dysfunction – apart from epicardial spasm – or angina symptoms and quality of life.
The trial investigated whether this therapy would improve these outcomes in patients with two mutually exclusive subgroups, or endotypes, of coronary vasomotor dysfunction: coronary artery spasm (epicardial spasm, microvascular spasm) or coronary microvascular dysfunction indicated by coronary flow reserve (CFR) and index of microvascular resistance (IMR) values.
Treatment success, the primary study endpoint – defined as normalization of one of the abnormal endotypes and no normal endotype becoming abnormal – was similar after treatment with diltiazem, compared with placebo. Nor were there significant differences for secondary endpoints apart from improvements in epicardial spasm in the two groups.
Tijn Jansen, MD, presented these findings from the EDIT-CMD trial in a featured clinical research session at the annual scientific sessions of the American College of Cardiology. The study was simultaneously published online April 2, 2022, in JACC: Cardiovascular Imaging.
“This first study using repeated coronary function testing provides a platform for future research,” concluded Dr. Jansen, a PhD candidate in the department of cardiology, Radboud University, Nijmegen, the Netherlands.
“We were surprised indeed” that diltiazem did not meet its primary endpoint for successful treatment and did not reduce symptoms or improve quality of life, compared with placebo, unlike results of the CorMicA trial, he said in an interview.
“We did find a treatment success, however, of 21%, which was slightly lower than expected, but it was not better than just giving placebo. This was similar regarding symptoms and quality of life, where we did find an overall improvement with diltiazem, but again not higher than using placebo,” he added. “It seems that giving the diagnosis to these patients itself creates a reduction in symptoms,” that might be caused by a reduction in stress, Dr. Jansen suggested.
The clinical implication, he said, is that more randomized controlled trials in this patient population are needed to permit evidence-based patient-tailored treatment, based on the different endotypes. “It might even be imaginable to test effectiveness in each individual patient using coronary function testing,” he said.
These tests are more and more commonly used in clinical practice, Dr. Jansen noted. “In the Netherlands, we recently launched the NL-CFT registry, which enables the participating centers to perform the CFT with a standardized protocol, with the goal to collect data and increase knowledge in this patient population.”
Heterogeneous population?
“I think probably the reason this trial was negative is [that coronary vasomotor dysfunction is] just too heterogeneous,” assigned discussant, C. Noel Bairey Merz, MD, commented.
“The problem with effectiveness trials is that you get a very heterogeneous population, and not everything works for everyone,” she said.
“This was a strategy trial – too heterogenous and too small to assess each endotype response,” Dr. Bairey Merz elaborated in an interview.
“Calcium channel blockers [CCBs] will not [effectively] treat all endotypes of coronary microvascular dysfunction,” she added, noting that the 6-month CorMIcA trial demonstrated in a larger, more rigorous trial design that CCBs are effective for epicardial and microvascular spasm.
“If you were going to do this study again, would you allow physicians to do up-titration and/or go a little bit longer?” Dr. Bairey Merz asked Dr. Jansen during the discussion.
“I do think this is a very heterogeneous group,” he agreed. However, the protocol allowed researchers to titrate diltiazem from 120 mg/day to 360 mg/day.
“If I were to do it again,” Dr. Jansen said, “I would focus on one specific endotype, probably epicardial spasm.”
First RCT of diltiazem in patients with ANOCA
Up to 40% of patients undergoing coronary angiography for stable angina do not have obstructive coronary artery disease (CAD), and 60%-90% of these patients have coronary vasomotor dysfunction, Dr. Jansen noted.
The landmark CorMicA trial showed that diagnosing the specific endotype of coronary vasomotor dysfunction using coronary function testing allows for tailored medication that decreased angina and improved quality of life, the researchers noted.
A recent European Society of Cardiology position paper on ANOCA “recommends the use of various pharmacological treatments including calcium-channel blockers, beta-blockers, ACE inhibitors, statins, and nitric oxide modulators, of which CCBs have the most prominent role in both endotypes of coronary vasospasms” and coronary microvascular dysfunction, they wrote.
“However, evidence substantiating these recommendations is lacking,” the researchers added, “since it is based on studies in a different population, with small sample sizes, or not placebo controlled.”
To investigate this, between 2019 and 2021, EDIT-CMD enrolled 126 adults aged 18 years and older who had two or more chronic angina episodes per week and no signs of obstructive CAD, who were seen at three hospitals specializing in ANOCA in the Netherlands.
The participants underwent coronary function testing that consisted of an acetylcholine spasm provocation test to evaluate for epicardial spasm and microvascular spasm, and a bolus thermodilution test with adenosine, to assess CFR and IMR. Coronary microvascular dysfunction was defined as CFR less than 2.0 and IMR of 25 or greater.
Of 99 patients with vasospasm or microvascular dysfunction, 85 patients were randomly assigned to receive diltiazem (n = 41) or placebo (n = 44) for 6 weeks.
The patients in both groups had a mean age of 58 years, and 29% were male; 22% had previously undergone percutaneous coronary intervention, and 48% had severe angina (Canadian Cardiovascular Society grade III/IV).
At baseline, about 50% had epicardial spasm, 25% had microvascular spasm and 25% had no spasm, and 54% in the diltiazem group and 73% in the placebo group had microvascular dysfunction.
After 6 weeks, 73 patients (35 in the placebo group and 38 in the diltiazem group) were available for repeat coronary function testing.
For the primary outcome, after 6 weeks of treatment, the proportion of patients with normalization of one abnormal parameter of coronary vasomotor dysfunction, without any normal parameter becoming abnormal, occurred in 8 patients (21%) in the diltiazem group versus 10 patients (29%) in the placebo group (P = .46)
In secondary outcomes, after 6 weeks of treatment, there were no significant differences in the prevalence of microvascular dysfunction, in Seattle Angina Questionnaire scores for angina symptoms, or RAND-36 scores for quality of life between patients who received diltiazem vs those who received placebo.
However, more patients in the diltiazem group than in the placebo group progressed from epicardial spasm to microvascular or no spasm (47% vs. 6%; P = .006).
The EDIT-CMD trial was sponsored by Abbott. Dr. Jansen has no relevant financial disclosures. Dr. Bairey Merz discloses having a fiduciary role and shares in iRhythm and being on the advisory board for Sanofi.
A version of this article first appeared on Medscape.com.
In a randomized trial of patients with angina and no obstructive coronary artery disease (ANOCA), 6 weeks of treatment with diltiazem did not improve coronary vasomotor dysfunction – apart from epicardial spasm – or angina symptoms and quality of life.
The trial investigated whether this therapy would improve these outcomes in patients with two mutually exclusive subgroups, or endotypes, of coronary vasomotor dysfunction: coronary artery spasm (epicardial spasm, microvascular spasm) or coronary microvascular dysfunction indicated by coronary flow reserve (CFR) and index of microvascular resistance (IMR) values.
Treatment success, the primary study endpoint – defined as normalization of one of the abnormal endotypes and no normal endotype becoming abnormal – was similar after treatment with diltiazem, compared with placebo. Nor were there significant differences for secondary endpoints apart from improvements in epicardial spasm in the two groups.
Tijn Jansen, MD, presented these findings from the EDIT-CMD trial in a featured clinical research session at the annual scientific sessions of the American College of Cardiology. The study was simultaneously published online April 2, 2022, in JACC: Cardiovascular Imaging.
“This first study using repeated coronary function testing provides a platform for future research,” concluded Dr. Jansen, a PhD candidate in the department of cardiology, Radboud University, Nijmegen, the Netherlands.
“We were surprised indeed” that diltiazem did not meet its primary endpoint for successful treatment and did not reduce symptoms or improve quality of life, compared with placebo, unlike results of the CorMicA trial, he said in an interview.
“We did find a treatment success, however, of 21%, which was slightly lower than expected, but it was not better than just giving placebo. This was similar regarding symptoms and quality of life, where we did find an overall improvement with diltiazem, but again not higher than using placebo,” he added. “It seems that giving the diagnosis to these patients itself creates a reduction in symptoms,” that might be caused by a reduction in stress, Dr. Jansen suggested.
The clinical implication, he said, is that more randomized controlled trials in this patient population are needed to permit evidence-based patient-tailored treatment, based on the different endotypes. “It might even be imaginable to test effectiveness in each individual patient using coronary function testing,” he said.
These tests are more and more commonly used in clinical practice, Dr. Jansen noted. “In the Netherlands, we recently launched the NL-CFT registry, which enables the participating centers to perform the CFT with a standardized protocol, with the goal to collect data and increase knowledge in this patient population.”
Heterogeneous population?
“I think probably the reason this trial was negative is [that coronary vasomotor dysfunction is] just too heterogeneous,” assigned discussant, C. Noel Bairey Merz, MD, commented.
“The problem with effectiveness trials is that you get a very heterogeneous population, and not everything works for everyone,” she said.
“This was a strategy trial – too heterogenous and too small to assess each endotype response,” Dr. Bairey Merz elaborated in an interview.
“Calcium channel blockers [CCBs] will not [effectively] treat all endotypes of coronary microvascular dysfunction,” she added, noting that the 6-month CorMIcA trial demonstrated in a larger, more rigorous trial design that CCBs are effective for epicardial and microvascular spasm.
“If you were going to do this study again, would you allow physicians to do up-titration and/or go a little bit longer?” Dr. Bairey Merz asked Dr. Jansen during the discussion.
“I do think this is a very heterogeneous group,” he agreed. However, the protocol allowed researchers to titrate diltiazem from 120 mg/day to 360 mg/day.
“If I were to do it again,” Dr. Jansen said, “I would focus on one specific endotype, probably epicardial spasm.”
First RCT of diltiazem in patients with ANOCA
Up to 40% of patients undergoing coronary angiography for stable angina do not have obstructive coronary artery disease (CAD), and 60%-90% of these patients have coronary vasomotor dysfunction, Dr. Jansen noted.
The landmark CorMicA trial showed that diagnosing the specific endotype of coronary vasomotor dysfunction using coronary function testing allows for tailored medication that decreased angina and improved quality of life, the researchers noted.
A recent European Society of Cardiology position paper on ANOCA “recommends the use of various pharmacological treatments including calcium-channel blockers, beta-blockers, ACE inhibitors, statins, and nitric oxide modulators, of which CCBs have the most prominent role in both endotypes of coronary vasospasms” and coronary microvascular dysfunction, they wrote.
“However, evidence substantiating these recommendations is lacking,” the researchers added, “since it is based on studies in a different population, with small sample sizes, or not placebo controlled.”
To investigate this, between 2019 and 2021, EDIT-CMD enrolled 126 adults aged 18 years and older who had two or more chronic angina episodes per week and no signs of obstructive CAD, who were seen at three hospitals specializing in ANOCA in the Netherlands.
The participants underwent coronary function testing that consisted of an acetylcholine spasm provocation test to evaluate for epicardial spasm and microvascular spasm, and a bolus thermodilution test with adenosine, to assess CFR and IMR. Coronary microvascular dysfunction was defined as CFR less than 2.0 and IMR of 25 or greater.
Of 99 patients with vasospasm or microvascular dysfunction, 85 patients were randomly assigned to receive diltiazem (n = 41) or placebo (n = 44) for 6 weeks.
The patients in both groups had a mean age of 58 years, and 29% were male; 22% had previously undergone percutaneous coronary intervention, and 48% had severe angina (Canadian Cardiovascular Society grade III/IV).
At baseline, about 50% had epicardial spasm, 25% had microvascular spasm and 25% had no spasm, and 54% in the diltiazem group and 73% in the placebo group had microvascular dysfunction.
After 6 weeks, 73 patients (35 in the placebo group and 38 in the diltiazem group) were available for repeat coronary function testing.
For the primary outcome, after 6 weeks of treatment, the proportion of patients with normalization of one abnormal parameter of coronary vasomotor dysfunction, without any normal parameter becoming abnormal, occurred in 8 patients (21%) in the diltiazem group versus 10 patients (29%) in the placebo group (P = .46)
In secondary outcomes, after 6 weeks of treatment, there were no significant differences in the prevalence of microvascular dysfunction, in Seattle Angina Questionnaire scores for angina symptoms, or RAND-36 scores for quality of life between patients who received diltiazem vs those who received placebo.
However, more patients in the diltiazem group than in the placebo group progressed from epicardial spasm to microvascular or no spasm (47% vs. 6%; P = .006).
The EDIT-CMD trial was sponsored by Abbott. Dr. Jansen has no relevant financial disclosures. Dr. Bairey Merz discloses having a fiduciary role and shares in iRhythm and being on the advisory board for Sanofi.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
VALOR-HCM: Novel drug may delay, avert invasive therapy in OHCM
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.
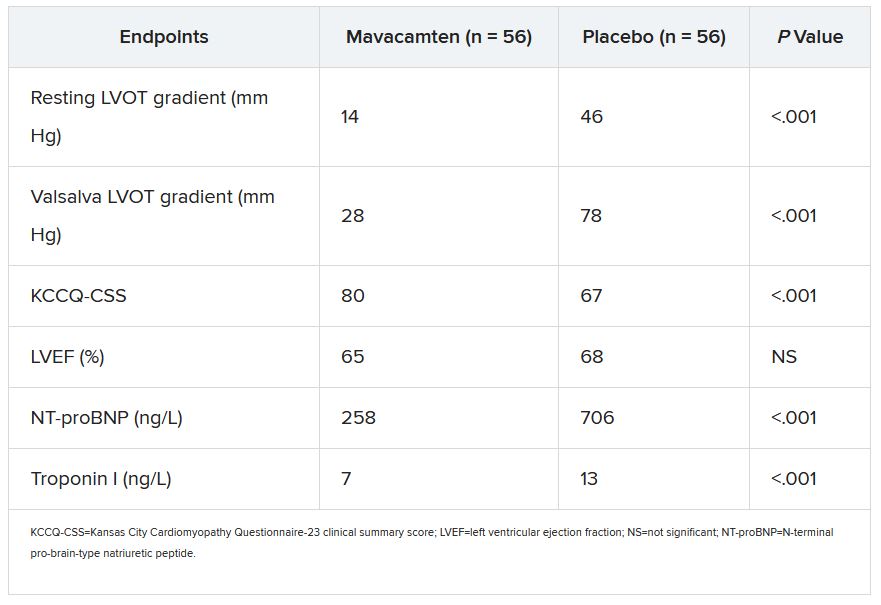
The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.

The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.

The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
FAME 3 subanalysis adds twist to negative primary results
A new subanalysis of the FAME 3 trial, which failed to show that percutaneous intervention (PCI) guided by fractional flow reserve (FFR) is noninferior to coronary artery bypass grafting (CABG) for treating three-vessel coronary artery disease, has associated PCI with early quality of life (QOL) advantages, according to findings presented at the annual scientific sessions of the American College of Cardiology.
Despite a modestly greater risk of major adverse cardiac events (MACE) at the end of 12 months’ follow-up among those treated with FFR-guided PCI, the greater QOL early after the procedure might be relevant to patients weighing these options, according to Frederik M. Zimmerman, MD, of Catharina Hospital in Eindhoven, the Netherlands.
“FFR-guided PCI results in a faster improvement in quality of life than CABG during the first year after revascularization, and it improved working status in patients younger than 65 years of age,” Dr. Zimmermann said.
The primary results of FAME 3 were presented at the 2021 Transcatheter Cardiovascular Therapeutics annual meeting by lead author William F. Fearon, MD, of Stanford (Calif.) University and published simultaneously in the New England Journal of Medicine.
Rather than confirming the hypothesis that FFR-guided PCI is comparable with CABG for the primary composite MACE outcome death from any cause, myocardial infarction, stroke, or revascularization, the incidence of MACE at 12 months was 10.6% in those randomized to PCI and 6.9% in the group assigned to CABG.
This translated into a hazard ratio for MACE of 1.5, signifying a 50% increase in risk for FFR-guided PCI relative to CABG for the primary outcome, a difference that negated the study definition of noninferiority (P = .35).
In this new health-related subanalysis, which was published simultaneously with his ACC presentation, the groups were compared over 12 months for QOL as measured with European Quality of Life–5 dimensions (EQ-5D) scale, angina as measured with the Canadian Cardiovascular Classification (CCC) system, and employment.
Outcomes data available in >85% of patients
Of the 1,500 patients enrolled and randomized in FAME 3 (757 to FFR-guided PCI and 743 to CABG), this health outcomes subanalysis was performed with complete data at 12 months from 89% of those in the PCI group and 88% of those in the CABG group.
Ultimately, the study did not show differences in any of these measures at the end of 12 months, but there were significant differences in QOL and employment at earlier time points. In particular, the significantly different (P < .001) trajectory for QOL improvement at 1 and 6 months favored FFR-guided PCI whether evaluated with the EQ-5D instrument or an EQ visual analog scale.
Rates of angina defined by as CCC class of at least 2 were low after revascularization in both arms of the study, negating any opportunity for differences, but patients aged younger than 65 years were almost twice as likely to have returned to full- or part-time work 1 month after revascularization (60.2% vs. 33.1%), and they remained at higher odds for working at 12 months (68.1% vs. 57.4%).
In patients aged older than 65 years, return-to-work rates did not differ significantly at any time point.
These results suggest potentially clinically meaningful early advantages for FFR-guided PCI, but some experts questioned the rationale for reporting positive secondary findings from a negative trial.
“This subanalysis is curious,” said Allen Jeremias, MD, director of interventional cardiology research, Saint Francis Hospital, Roslyn, N.Y. He pointed out that reporting these data is an anomaly.
Subanalyses uncommon in negative trials
“CABG was found to be better, so why look at QOL,” said Dr. Jeremias, who was an ACC-invited expert to discuss the results. However, he went on to say, “this could be an exception to the rule.”
The reason, according to Dr. Jeremias, is that the absolute difference at 12 months between FFR-guided PCI and CABG for the MACE events of greatest concern – death, MI, or stroke – was only about 2% greater in the FFR-guided PCI group (7.3% vs. 5.2%). The biggest contributor to the difference in MACE in FAME 3 at 12 months was the higher rate of repeat revascularization (5.9% vs. 3.9%).
Moreover, patients randomized to FFR-guided PCI had lower rates of many adverse events. This included risk of bleeding (1.6% vs. 3.8%; P = .009 as defined by type ≥3 Bleeding Academic Research Consortium , acute kidney injury (0.1% vs. 0.9%; P = .04), atrial fibrillation (2.4% vs. 14.1%; P < .001) and rehospitalization within 30 days (5.5% vs. 10.2%; P < .001).
In the context of a modest increase in risk of MACE and the lower rate of several important treatment-related adverse events, the QOL advantages identified in this subanalysis “might be a reasonable topic for patient-shared decision-making,” Dr. Jeremias suggested.
New data might inform patient decision-making
He granted the possibility that well-informed patients might accept the modestly increased risk of MACE for one or more of the outcomes, such as a higher likelihood of an early return to work, that favored FFR-guided PCI.
This is the point of this subanalysis, agreed Dr. Zimmermann.
“It is all about shared decision-making,” he said. Also emphasizing that the negative trial endpoint of FAME 3 “was driven largely by an increased risk of revascularization,” he believes that these new data might be a basis for discussions with patients weighing relative risks and benefits.
There are more data to come, according to Dr. Zimmermann, who said that follow-up of up to 5 years is planned. The 3-year data will be made available in 2023.
Dr. Zimmermann reported no potential conflicts of interest. Dr. Jeremias reported financial relationships with Abbott, ACIST, Boston Scientific, and Volcano. The investigator-initiated trial received research grants from Abbott Vascular and Medtronic.
A new subanalysis of the FAME 3 trial, which failed to show that percutaneous intervention (PCI) guided by fractional flow reserve (FFR) is noninferior to coronary artery bypass grafting (CABG) for treating three-vessel coronary artery disease, has associated PCI with early quality of life (QOL) advantages, according to findings presented at the annual scientific sessions of the American College of Cardiology.
Despite a modestly greater risk of major adverse cardiac events (MACE) at the end of 12 months’ follow-up among those treated with FFR-guided PCI, the greater QOL early after the procedure might be relevant to patients weighing these options, according to Frederik M. Zimmerman, MD, of Catharina Hospital in Eindhoven, the Netherlands.
“FFR-guided PCI results in a faster improvement in quality of life than CABG during the first year after revascularization, and it improved working status in patients younger than 65 years of age,” Dr. Zimmermann said.
The primary results of FAME 3 were presented at the 2021 Transcatheter Cardiovascular Therapeutics annual meeting by lead author William F. Fearon, MD, of Stanford (Calif.) University and published simultaneously in the New England Journal of Medicine.
Rather than confirming the hypothesis that FFR-guided PCI is comparable with CABG for the primary composite MACE outcome death from any cause, myocardial infarction, stroke, or revascularization, the incidence of MACE at 12 months was 10.6% in those randomized to PCI and 6.9% in the group assigned to CABG.
This translated into a hazard ratio for MACE of 1.5, signifying a 50% increase in risk for FFR-guided PCI relative to CABG for the primary outcome, a difference that negated the study definition of noninferiority (P = .35).
In this new health-related subanalysis, which was published simultaneously with his ACC presentation, the groups were compared over 12 months for QOL as measured with European Quality of Life–5 dimensions (EQ-5D) scale, angina as measured with the Canadian Cardiovascular Classification (CCC) system, and employment.
Outcomes data available in >85% of patients
Of the 1,500 patients enrolled and randomized in FAME 3 (757 to FFR-guided PCI and 743 to CABG), this health outcomes subanalysis was performed with complete data at 12 months from 89% of those in the PCI group and 88% of those in the CABG group.
Ultimately, the study did not show differences in any of these measures at the end of 12 months, but there were significant differences in QOL and employment at earlier time points. In particular, the significantly different (P < .001) trajectory for QOL improvement at 1 and 6 months favored FFR-guided PCI whether evaluated with the EQ-5D instrument or an EQ visual analog scale.
Rates of angina defined by as CCC class of at least 2 were low after revascularization in both arms of the study, negating any opportunity for differences, but patients aged younger than 65 years were almost twice as likely to have returned to full- or part-time work 1 month after revascularization (60.2% vs. 33.1%), and they remained at higher odds for working at 12 months (68.1% vs. 57.4%).
In patients aged older than 65 years, return-to-work rates did not differ significantly at any time point.
These results suggest potentially clinically meaningful early advantages for FFR-guided PCI, but some experts questioned the rationale for reporting positive secondary findings from a negative trial.
“This subanalysis is curious,” said Allen Jeremias, MD, director of interventional cardiology research, Saint Francis Hospital, Roslyn, N.Y. He pointed out that reporting these data is an anomaly.
Subanalyses uncommon in negative trials
“CABG was found to be better, so why look at QOL,” said Dr. Jeremias, who was an ACC-invited expert to discuss the results. However, he went on to say, “this could be an exception to the rule.”
The reason, according to Dr. Jeremias, is that the absolute difference at 12 months between FFR-guided PCI and CABG for the MACE events of greatest concern – death, MI, or stroke – was only about 2% greater in the FFR-guided PCI group (7.3% vs. 5.2%). The biggest contributor to the difference in MACE in FAME 3 at 12 months was the higher rate of repeat revascularization (5.9% vs. 3.9%).
Moreover, patients randomized to FFR-guided PCI had lower rates of many adverse events. This included risk of bleeding (1.6% vs. 3.8%; P = .009 as defined by type ≥3 Bleeding Academic Research Consortium , acute kidney injury (0.1% vs. 0.9%; P = .04), atrial fibrillation (2.4% vs. 14.1%; P < .001) and rehospitalization within 30 days (5.5% vs. 10.2%; P < .001).
In the context of a modest increase in risk of MACE and the lower rate of several important treatment-related adverse events, the QOL advantages identified in this subanalysis “might be a reasonable topic for patient-shared decision-making,” Dr. Jeremias suggested.
New data might inform patient decision-making
He granted the possibility that well-informed patients might accept the modestly increased risk of MACE for one or more of the outcomes, such as a higher likelihood of an early return to work, that favored FFR-guided PCI.
This is the point of this subanalysis, agreed Dr. Zimmermann.
“It is all about shared decision-making,” he said. Also emphasizing that the negative trial endpoint of FAME 3 “was driven largely by an increased risk of revascularization,” he believes that these new data might be a basis for discussions with patients weighing relative risks and benefits.
There are more data to come, according to Dr. Zimmermann, who said that follow-up of up to 5 years is planned. The 3-year data will be made available in 2023.
Dr. Zimmermann reported no potential conflicts of interest. Dr. Jeremias reported financial relationships with Abbott, ACIST, Boston Scientific, and Volcano. The investigator-initiated trial received research grants from Abbott Vascular and Medtronic.
A new subanalysis of the FAME 3 trial, which failed to show that percutaneous intervention (PCI) guided by fractional flow reserve (FFR) is noninferior to coronary artery bypass grafting (CABG) for treating three-vessel coronary artery disease, has associated PCI with early quality of life (QOL) advantages, according to findings presented at the annual scientific sessions of the American College of Cardiology.
Despite a modestly greater risk of major adverse cardiac events (MACE) at the end of 12 months’ follow-up among those treated with FFR-guided PCI, the greater QOL early after the procedure might be relevant to patients weighing these options, according to Frederik M. Zimmerman, MD, of Catharina Hospital in Eindhoven, the Netherlands.
“FFR-guided PCI results in a faster improvement in quality of life than CABG during the first year after revascularization, and it improved working status in patients younger than 65 years of age,” Dr. Zimmermann said.
The primary results of FAME 3 were presented at the 2021 Transcatheter Cardiovascular Therapeutics annual meeting by lead author William F. Fearon, MD, of Stanford (Calif.) University and published simultaneously in the New England Journal of Medicine.
Rather than confirming the hypothesis that FFR-guided PCI is comparable with CABG for the primary composite MACE outcome death from any cause, myocardial infarction, stroke, or revascularization, the incidence of MACE at 12 months was 10.6% in those randomized to PCI and 6.9% in the group assigned to CABG.
This translated into a hazard ratio for MACE of 1.5, signifying a 50% increase in risk for FFR-guided PCI relative to CABG for the primary outcome, a difference that negated the study definition of noninferiority (P = .35).
In this new health-related subanalysis, which was published simultaneously with his ACC presentation, the groups were compared over 12 months for QOL as measured with European Quality of Life–5 dimensions (EQ-5D) scale, angina as measured with the Canadian Cardiovascular Classification (CCC) system, and employment.
Outcomes data available in >85% of patients
Of the 1,500 patients enrolled and randomized in FAME 3 (757 to FFR-guided PCI and 743 to CABG), this health outcomes subanalysis was performed with complete data at 12 months from 89% of those in the PCI group and 88% of those in the CABG group.
Ultimately, the study did not show differences in any of these measures at the end of 12 months, but there were significant differences in QOL and employment at earlier time points. In particular, the significantly different (P < .001) trajectory for QOL improvement at 1 and 6 months favored FFR-guided PCI whether evaluated with the EQ-5D instrument or an EQ visual analog scale.
Rates of angina defined by as CCC class of at least 2 were low after revascularization in both arms of the study, negating any opportunity for differences, but patients aged younger than 65 years were almost twice as likely to have returned to full- or part-time work 1 month after revascularization (60.2% vs. 33.1%), and they remained at higher odds for working at 12 months (68.1% vs. 57.4%).
In patients aged older than 65 years, return-to-work rates did not differ significantly at any time point.
These results suggest potentially clinically meaningful early advantages for FFR-guided PCI, but some experts questioned the rationale for reporting positive secondary findings from a negative trial.
“This subanalysis is curious,” said Allen Jeremias, MD, director of interventional cardiology research, Saint Francis Hospital, Roslyn, N.Y. He pointed out that reporting these data is an anomaly.
Subanalyses uncommon in negative trials
“CABG was found to be better, so why look at QOL,” said Dr. Jeremias, who was an ACC-invited expert to discuss the results. However, he went on to say, “this could be an exception to the rule.”
The reason, according to Dr. Jeremias, is that the absolute difference at 12 months between FFR-guided PCI and CABG for the MACE events of greatest concern – death, MI, or stroke – was only about 2% greater in the FFR-guided PCI group (7.3% vs. 5.2%). The biggest contributor to the difference in MACE in FAME 3 at 12 months was the higher rate of repeat revascularization (5.9% vs. 3.9%).
Moreover, patients randomized to FFR-guided PCI had lower rates of many adverse events. This included risk of bleeding (1.6% vs. 3.8%; P = .009 as defined by type ≥3 Bleeding Academic Research Consortium , acute kidney injury (0.1% vs. 0.9%; P = .04), atrial fibrillation (2.4% vs. 14.1%; P < .001) and rehospitalization within 30 days (5.5% vs. 10.2%; P < .001).
In the context of a modest increase in risk of MACE and the lower rate of several important treatment-related adverse events, the QOL advantages identified in this subanalysis “might be a reasonable topic for patient-shared decision-making,” Dr. Jeremias suggested.
New data might inform patient decision-making
He granted the possibility that well-informed patients might accept the modestly increased risk of MACE for one or more of the outcomes, such as a higher likelihood of an early return to work, that favored FFR-guided PCI.
This is the point of this subanalysis, agreed Dr. Zimmermann.
“It is all about shared decision-making,” he said. Also emphasizing that the negative trial endpoint of FAME 3 “was driven largely by an increased risk of revascularization,” he believes that these new data might be a basis for discussions with patients weighing relative risks and benefits.
There are more data to come, according to Dr. Zimmermann, who said that follow-up of up to 5 years is planned. The 3-year data will be made available in 2023.
Dr. Zimmermann reported no potential conflicts of interest. Dr. Jeremias reported financial relationships with Abbott, ACIST, Boston Scientific, and Volcano. The investigator-initiated trial received research grants from Abbott Vascular and Medtronic.
FROM ACC 2021



















