User login
Nanoparticle shows promise for ALS
SEATTLE – , which was the change in the summated motor unit index (MUNIX) from baseline to week 36.
The drug, CNM-Au8, is being developed by Clene, and would represent a novel mechanism of action. “This is a brand-new approach. We used it complementary with riluzole and it was well tolerated, so I see this as an add-on therapy. I think if we can show some more positivity and longer-term results, it’s going to be a game changer for ALS,” Matthew Kiernan, MBBS, PhD, said in an interview. Dr. Kiernan presented the results at the 2022 annual meeting of the American Academy of Neurology.
Riluzole (Rilutek), which received Food and Drug Administration approval in 1995, inhibits glutamate release to counter excitotoxicity, which is believed to play a role in ALS, Huntington’s disease, ischemia, and other acute and chronic neurodegenerative diseases. The other FDA-approved agent for ALS is the neuroprotective agent and free-radical scavenger edaravone (Radicava), approved in 2017.
CNM-Au8 is made up of catalytically active gold nanocrystals that cross the blood-brain barrier, but lacks the toxicity associated with other synthetic gold compounds, according to the company. The formulation is also being investigated for the treatment of Parkinson’s disease and multiple sclerosis. Basic research has shown that it stabilizes mitochondria and reduces accumulation of the TDP-43 protein, which is linked to spread of ALS through the brain, Dr. Kiernan said during his presentation.
The treatment is well tolerated. “Normally in an ALS trial, we see about a 25% dropout rate. There were no dropouts on the active compound in the clinical trial. There are less deaths, so improved survival,” said Dr. Kiernan, the Bushell chair of neurology at the University of Sydney and codirector of the Brain and Mind Center in Sydney.
Good safety signal
The fact that the trial missed its primary endpoint isn’t too concerning, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “ALS clinical trials are incredibly difficult to conduct, especially a phase 2 learning-phase clinical trial. At this phase, I’m much more buoyed by the fact that they have a good safety signal, and that they’re willing to move forward to that phase 3 clinical trial,” Dr. Johnson said in an interview. He is vice chair of research at Virginia Commonwealth University, Richmond.
A phase 3 clinical trial is in development in the United States and Europe. The drug also is included as part of the HEALEY ALS Platform Trial, which is testing multiple ALS therapies simultaneously. “The results from that should be available by the second half of this year and it will also inform us as to what the approach should be,” said Dr. Kiernan.
Dr. Johnson also was enthusiastic. “I’m excited to see the results in terms of the primary endpoints for that next phase 3 clinical trial,” he said.
Ongoing research
In September 2021, Clene announced a second expanded access program for people with ALS.
The study included a 36-week double-blind treatment period followed by long-term, open-label follow-up. Twenty-three patients received 30 mg CNM-Au8, and 22 received placebo. In the first 36 weeks, the treatment group was more likely to have no disease progression, defined as death, tracheostomy, noninvasive ventilation, or a gastronomy tube (P = .0125). The researchers compared the probability of experiencing a less than 6-point decline in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale. At 12 weeks, it was about 85% in both groups. At 24 weeks, it was about 60% to 50% in favor of the CNM-Au8 group, and at 36 weeks it was about 50% to 20% (P = .0350).
At 36 weeks, quality of life as measured by the ALS Specific Quality of Life–Short Form was better in the treatment group at 36 weeks (mean change, 0.9; P = .0177).
Survival was better in the treatment group at 96 weeks than the mortality derived from a European Network for the Cure of ALS prediction model (hazard ratio [HR], 0.2974; P = .0068). This benefit also was experienced by patients who received drug throughout the study (HR, 0.36; 95% confidence interval [CI], 0.12-1.1) and those who started out on placebo and converted to active drug during the open-label period (HR, 0.24; 95% CI, 0.064-0.88).
The study was funded by Clene and FightMND. Dr. Kiernan and Dr. Johnson have no relevant financial disclosures.
SEATTLE – , which was the change in the summated motor unit index (MUNIX) from baseline to week 36.
The drug, CNM-Au8, is being developed by Clene, and would represent a novel mechanism of action. “This is a brand-new approach. We used it complementary with riluzole and it was well tolerated, so I see this as an add-on therapy. I think if we can show some more positivity and longer-term results, it’s going to be a game changer for ALS,” Matthew Kiernan, MBBS, PhD, said in an interview. Dr. Kiernan presented the results at the 2022 annual meeting of the American Academy of Neurology.
Riluzole (Rilutek), which received Food and Drug Administration approval in 1995, inhibits glutamate release to counter excitotoxicity, which is believed to play a role in ALS, Huntington’s disease, ischemia, and other acute and chronic neurodegenerative diseases. The other FDA-approved agent for ALS is the neuroprotective agent and free-radical scavenger edaravone (Radicava), approved in 2017.
CNM-Au8 is made up of catalytically active gold nanocrystals that cross the blood-brain barrier, but lacks the toxicity associated with other synthetic gold compounds, according to the company. The formulation is also being investigated for the treatment of Parkinson’s disease and multiple sclerosis. Basic research has shown that it stabilizes mitochondria and reduces accumulation of the TDP-43 protein, which is linked to spread of ALS through the brain, Dr. Kiernan said during his presentation.
The treatment is well tolerated. “Normally in an ALS trial, we see about a 25% dropout rate. There were no dropouts on the active compound in the clinical trial. There are less deaths, so improved survival,” said Dr. Kiernan, the Bushell chair of neurology at the University of Sydney and codirector of the Brain and Mind Center in Sydney.
Good safety signal
The fact that the trial missed its primary endpoint isn’t too concerning, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “ALS clinical trials are incredibly difficult to conduct, especially a phase 2 learning-phase clinical trial. At this phase, I’m much more buoyed by the fact that they have a good safety signal, and that they’re willing to move forward to that phase 3 clinical trial,” Dr. Johnson said in an interview. He is vice chair of research at Virginia Commonwealth University, Richmond.
A phase 3 clinical trial is in development in the United States and Europe. The drug also is included as part of the HEALEY ALS Platform Trial, which is testing multiple ALS therapies simultaneously. “The results from that should be available by the second half of this year and it will also inform us as to what the approach should be,” said Dr. Kiernan.
Dr. Johnson also was enthusiastic. “I’m excited to see the results in terms of the primary endpoints for that next phase 3 clinical trial,” he said.
Ongoing research
In September 2021, Clene announced a second expanded access program for people with ALS.
The study included a 36-week double-blind treatment period followed by long-term, open-label follow-up. Twenty-three patients received 30 mg CNM-Au8, and 22 received placebo. In the first 36 weeks, the treatment group was more likely to have no disease progression, defined as death, tracheostomy, noninvasive ventilation, or a gastronomy tube (P = .0125). The researchers compared the probability of experiencing a less than 6-point decline in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale. At 12 weeks, it was about 85% in both groups. At 24 weeks, it was about 60% to 50% in favor of the CNM-Au8 group, and at 36 weeks it was about 50% to 20% (P = .0350).
At 36 weeks, quality of life as measured by the ALS Specific Quality of Life–Short Form was better in the treatment group at 36 weeks (mean change, 0.9; P = .0177).
Survival was better in the treatment group at 96 weeks than the mortality derived from a European Network for the Cure of ALS prediction model (hazard ratio [HR], 0.2974; P = .0068). This benefit also was experienced by patients who received drug throughout the study (HR, 0.36; 95% confidence interval [CI], 0.12-1.1) and those who started out on placebo and converted to active drug during the open-label period (HR, 0.24; 95% CI, 0.064-0.88).
The study was funded by Clene and FightMND. Dr. Kiernan and Dr. Johnson have no relevant financial disclosures.
SEATTLE – , which was the change in the summated motor unit index (MUNIX) from baseline to week 36.
The drug, CNM-Au8, is being developed by Clene, and would represent a novel mechanism of action. “This is a brand-new approach. We used it complementary with riluzole and it was well tolerated, so I see this as an add-on therapy. I think if we can show some more positivity and longer-term results, it’s going to be a game changer for ALS,” Matthew Kiernan, MBBS, PhD, said in an interview. Dr. Kiernan presented the results at the 2022 annual meeting of the American Academy of Neurology.
Riluzole (Rilutek), which received Food and Drug Administration approval in 1995, inhibits glutamate release to counter excitotoxicity, which is believed to play a role in ALS, Huntington’s disease, ischemia, and other acute and chronic neurodegenerative diseases. The other FDA-approved agent for ALS is the neuroprotective agent and free-radical scavenger edaravone (Radicava), approved in 2017.
CNM-Au8 is made up of catalytically active gold nanocrystals that cross the blood-brain barrier, but lacks the toxicity associated with other synthetic gold compounds, according to the company. The formulation is also being investigated for the treatment of Parkinson’s disease and multiple sclerosis. Basic research has shown that it stabilizes mitochondria and reduces accumulation of the TDP-43 protein, which is linked to spread of ALS through the brain, Dr. Kiernan said during his presentation.
The treatment is well tolerated. “Normally in an ALS trial, we see about a 25% dropout rate. There were no dropouts on the active compound in the clinical trial. There are less deaths, so improved survival,” said Dr. Kiernan, the Bushell chair of neurology at the University of Sydney and codirector of the Brain and Mind Center in Sydney.
Good safety signal
The fact that the trial missed its primary endpoint isn’t too concerning, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “ALS clinical trials are incredibly difficult to conduct, especially a phase 2 learning-phase clinical trial. At this phase, I’m much more buoyed by the fact that they have a good safety signal, and that they’re willing to move forward to that phase 3 clinical trial,” Dr. Johnson said in an interview. He is vice chair of research at Virginia Commonwealth University, Richmond.
A phase 3 clinical trial is in development in the United States and Europe. The drug also is included as part of the HEALEY ALS Platform Trial, which is testing multiple ALS therapies simultaneously. “The results from that should be available by the second half of this year and it will also inform us as to what the approach should be,” said Dr. Kiernan.
Dr. Johnson also was enthusiastic. “I’m excited to see the results in terms of the primary endpoints for that next phase 3 clinical trial,” he said.
Ongoing research
In September 2021, Clene announced a second expanded access program for people with ALS.
The study included a 36-week double-blind treatment period followed by long-term, open-label follow-up. Twenty-three patients received 30 mg CNM-Au8, and 22 received placebo. In the first 36 weeks, the treatment group was more likely to have no disease progression, defined as death, tracheostomy, noninvasive ventilation, or a gastronomy tube (P = .0125). The researchers compared the probability of experiencing a less than 6-point decline in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale. At 12 weeks, it was about 85% in both groups. At 24 weeks, it was about 60% to 50% in favor of the CNM-Au8 group, and at 36 weeks it was about 50% to 20% (P = .0350).
At 36 weeks, quality of life as measured by the ALS Specific Quality of Life–Short Form was better in the treatment group at 36 weeks (mean change, 0.9; P = .0177).
Survival was better in the treatment group at 96 weeks than the mortality derived from a European Network for the Cure of ALS prediction model (hazard ratio [HR], 0.2974; P = .0068). This benefit also was experienced by patients who received drug throughout the study (HR, 0.36; 95% confidence interval [CI], 0.12-1.1) and those who started out on placebo and converted to active drug during the open-label period (HR, 0.24; 95% CI, 0.064-0.88).
The study was funded by Clene and FightMND. Dr. Kiernan and Dr. Johnson have no relevant financial disclosures.
AT AAN 2022
Extraction of infected implanted cardiac devices rare, despite guidelines
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
FROM ACC 2022
Physical fitness tied to lower risk of Alzheimer’s disease
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.
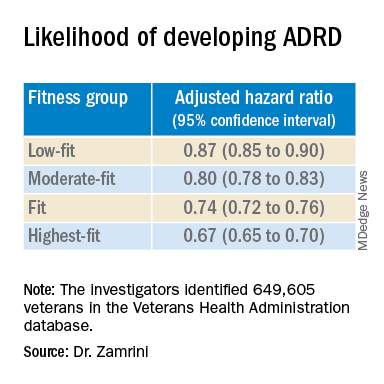
The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.

The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new findings suggest. “One exciting finding of this study is that as people’s fitness improved, their risk of Alzheimer’s disease decreased – it was not an all-or-nothing proposition,” study investigator Edward Zamrini, MD, of the Washington DC VA Medical Center, said in a news release.
The findings suggest that people can work toward making incremental changes and improvements in their physical fitness, which may help decrease their risk of dementia, Dr. Zamrini added.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Effective prevention strategy
Using the Veterans Health Administration database, Dr. Zamrini and colleagues identified 649,605 veterans (mean age, 61 years) free of Alzheimer’s disease and related disorders (ADRD) when they completed standardized exercise treadmill tests between 2000 and 2017.
They divided participants into five age-specific fitness groups, from least fit to most fit, based on peak metabolic equivalents (METs) achieved during the treadmill test: lowest-fit (METs, ±3.8), low-fit (METs, ±5.8), moderate-fit (METs, ±7.5), fit (METs, ±9.2), and highest-fit (METs, ±11.7).
In unadjusted analysis, veterans with the lowest cardiorespiratory fitness developed ADRD at a rate of 9.5 cases per 1,000 person-years, compared with a rate of 6.4 cases per 1,000 person-years for the most fit group (P < .001).
After adjusting for factors that could affect risk of ADRD, compared with the lowest-fit group, the highest-fit and fit groups were 33% and 26% less likely to develop ADRD, respectively, while the moderate-fit and low-fit groups were 20% and 13% less likely to develop the disease, respectively.

The findings suggest that the association between cardiorespiratory fitness and ADRD risk is “inverse, independent, and graded,” the researchers said in their conference abstract.
“The idea that you can reduce your risk for Alzheimer’s disease by simply increasing your activity is very promising, especially since there are no adequate treatments to prevent or stop the progression of the disease,” Dr. Zamrini added in the news release.
“We hope to develop a simple scale that can be individualized so people can see the benefits that even incremental improvements in fitness can deliver,” he said.
The next vital sign?
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Boston, noted that “for decades and with increasing body of support from studies like this, we have known that preventing dementia is based on healthy behaviors for the brain including a proper diet (NASH and/or Mediterranean), exercise regimen (aerobic/cardio more than anaerobic/weight-lifting), sleep hygiene, and social and intellectual engagements.”
“Frankly, what’s good for the body is good for the brain,” said Dr. Lakhan.
“It should be noted that the measure studied here is cardiorespiratory fitness, which has been associated with heart disease and resulting death, death from any cause, and now brain health,” Dr. Lakhan said.
“This powerful predictor may in fact be the next vital sign, after your heart rate and blood pressure, from which your primary care provider can make a personalized treatment plan,” he added.
“Accelerating this process, the ability to measure cardiorespiratory fitness traditionally from huge stationary machines down to wearables like a watch or ring, or even your iPhone or Android, is just on the horizon,” Dr. Lakhan said.
“Instead of tracking just your weight, shape, and BMI, personal fitness may be tailored to optimizing this indicator and further empowering individuals to take charge of their health,” he said.
The study was supported by the National Institute on Aging, the National Institutes of Health, the U.S. Department of Veterans Affairs, the Washington DC VA Medical Center, and George Washington University. Dr. Zamrini and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
More years of ice hockey play tied to higher CTE risk
new research suggests. Early results from a study that examined donor brains showed that each additional year of ice hockey play increased the risk for CTE by 23%.
This information should be on the “radar” of all clinicians, said coinvestigator Jesse Mez, MD, associate professor of neurology at Boston University. “When they’re talking to kids and families and parents about playing contact sports, they should discuss the benefits as well as the risks so all that information can be taken into consideration.”
Dr. Mez noted that clinicians should also consider the amount of hockey played when assessing patients for thinking and memory trouble later in life. “CTE could be in the differential diagnosis,” he said.
The study findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Football data
CTE is a neurodegenerative disease associated with repetitive hits to the head. In previous research, the investigators showed that the more that athletes play American football, the more likely they are to develop CTE.
“Hockey, like football, involves repetitive head impacts as part of the game,” said Dr. Mez. “So we hypothesized that we would see a similar type of dose-response relationship in hockey.”
From two brain banks – the Veterans Affairs–Boston University–Concussion Legacy Foundation and the Framingham Heart Study – the researchers accessed 74 consecutive brains from donors who had played ice hockey. They collected information about hockey play during “pretty comprehensive” interviews with next of kin, Dr. Mez reported.
The study participants ranged in age from 13 to 91 years. The cause of death varied; most died with end-stage dementia and neurodegenerative disease, but some died of cardiovascular disease, and others from accidents.
For 9% of the individuals, the highest level of play was a youth league; 34% had reached the high school level, 30% reached the juniors/college level, and 26% played professionally. In addition, 46% played another contact sport – including 43% who played American football.
Primary outcomes included evidence of CTE from stage 0 (no CTE) to stage IV and severity of CTE, which was defined by the amount of neurofibrillary tangle (NFT) burden in 11 brain regions. For this burden, the score ranged from 0 (absent) to 3 (severe) in each region for a total range of 0-33.
Dr. Mez noted that, in CTE, tau protein accumulates abnormally. “It typically begins in the cortex in the frontal lobe and then spreads to other parts of the brain, including to the medial temporal structures, and is widespread by stage IV.”
The researchers estimated the association of duration of ice hockey play in years with each neuropathologic outcome and adjusted for age at death and duration of football play.
Consistent findings
Results showed that, of the 74 donors, 40 (54%) had CTE. Each additional year of hockey play corresponded to increased chances for having CTE (odds ratio, 1.23; 95% confidence interval, 11%-36%; P < .01). This increase in risk is similar to that which was found with football players, Dr. Mez noted. This was somewhat surprising, as hockey involves fewer “hits” than football.
“Hits are not as quintessential to the game of hockey as they are in football, where contacts occur with nearly every play,” he said. “In football, you have several hundred impacts over the course of a season.”
Researchers also found a 15% increase in odds for increasing one CTE stage (95% CI, 8%-22%; P < .01), and a .03 standard deviation increase in cumulative NFT burden (95% CI, 0.01-0.05; P < .01).
Dr. Mez noted that the fact that the results were consistent across different outcomes “improves the validity” of the findings.
In a sensitivity analysis that excluded participants who also played football, estimates “were pretty similar” to those in the full analysis, said Dr. Mez.
The investigators have not yet examined the effect of level of hockey play, such as professionally or at the college level, on CTE risk. However, in football players, they found that level of play is another “valuable predictor of CTE pathology,” Dr. Mez said, adding that level of play, position played, and years of play “are all probably contributing” to CTE risk.
Asking about years of play is useful in a clinical setting. “It’s very easy for a clinician to ask patients how many years of hockey they played,” said Dr. Mez.
Overall, the new results are important, as “millions of individuals” play contact sports, whether that is hockey, football, or European soccer, he added. “And for all sports, there seems to be this relationship between more play and risk of this disease.”
‘Skewed’ population?
Commenting on the findings, Frank Conidi, MD, director, Florida Center for Health and Sports Neurology, Port St. Lucie, said he was surprised the investigators found a 23% per year increase in risk for CTE among hockey players.
Dr. Conidi has played hockey himself and works with the Florida Panthers of the National Hockey League. In his practice, he treats retired professional football players who have neurodegenerative disorders. From his experience, the number of repetitive direct head impacts in football is significantly higher than in hockey. “Most of the forces seen in hockey are from hits to the body, where the force is transferred to the head,” said Dr. Conidi, who was not involved with the research.
He noted differences in the way hockey is played around the world. In European countries, for example, the ice surface is relatively large and the emphasis tends to be more on skill than hitting.
“It would have been interesting to have the study group analyze the data based on where the athlete grew up,” he said. Dr. Conidi would also like to know when the participants played hockey. “The game is vastly different now than it was in the 1970s, ‘80s, and early ‘90s, when there was more fighting, less protective gear, and more hitting in general.”
As is the case for most studies of CTE in athletes, the study population is “skewed” because the participants likely had neurocognitive and other problems that led to their decision to donate their brain, said Dr. Conidi.
He also doesn’t believe the study should be the sole factor in a decision to continue or stop playing hockey. “We are still in the infancy stages of understanding the effects of high-impact sports on athletes’ brains.”
The study received funding from the National Institute of Neurological Diseases and Stroke and the National Institute on Aging. Dr. Mez and Dr. Conidi have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests. Early results from a study that examined donor brains showed that each additional year of ice hockey play increased the risk for CTE by 23%.
This information should be on the “radar” of all clinicians, said coinvestigator Jesse Mez, MD, associate professor of neurology at Boston University. “When they’re talking to kids and families and parents about playing contact sports, they should discuss the benefits as well as the risks so all that information can be taken into consideration.”
Dr. Mez noted that clinicians should also consider the amount of hockey played when assessing patients for thinking and memory trouble later in life. “CTE could be in the differential diagnosis,” he said.
The study findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Football data
CTE is a neurodegenerative disease associated with repetitive hits to the head. In previous research, the investigators showed that the more that athletes play American football, the more likely they are to develop CTE.
“Hockey, like football, involves repetitive head impacts as part of the game,” said Dr. Mez. “So we hypothesized that we would see a similar type of dose-response relationship in hockey.”
From two brain banks – the Veterans Affairs–Boston University–Concussion Legacy Foundation and the Framingham Heart Study – the researchers accessed 74 consecutive brains from donors who had played ice hockey. They collected information about hockey play during “pretty comprehensive” interviews with next of kin, Dr. Mez reported.
The study participants ranged in age from 13 to 91 years. The cause of death varied; most died with end-stage dementia and neurodegenerative disease, but some died of cardiovascular disease, and others from accidents.
For 9% of the individuals, the highest level of play was a youth league; 34% had reached the high school level, 30% reached the juniors/college level, and 26% played professionally. In addition, 46% played another contact sport – including 43% who played American football.
Primary outcomes included evidence of CTE from stage 0 (no CTE) to stage IV and severity of CTE, which was defined by the amount of neurofibrillary tangle (NFT) burden in 11 brain regions. For this burden, the score ranged from 0 (absent) to 3 (severe) in each region for a total range of 0-33.
Dr. Mez noted that, in CTE, tau protein accumulates abnormally. “It typically begins in the cortex in the frontal lobe and then spreads to other parts of the brain, including to the medial temporal structures, and is widespread by stage IV.”
The researchers estimated the association of duration of ice hockey play in years with each neuropathologic outcome and adjusted for age at death and duration of football play.
Consistent findings
Results showed that, of the 74 donors, 40 (54%) had CTE. Each additional year of hockey play corresponded to increased chances for having CTE (odds ratio, 1.23; 95% confidence interval, 11%-36%; P < .01). This increase in risk is similar to that which was found with football players, Dr. Mez noted. This was somewhat surprising, as hockey involves fewer “hits” than football.
“Hits are not as quintessential to the game of hockey as they are in football, where contacts occur with nearly every play,” he said. “In football, you have several hundred impacts over the course of a season.”
Researchers also found a 15% increase in odds for increasing one CTE stage (95% CI, 8%-22%; P < .01), and a .03 standard deviation increase in cumulative NFT burden (95% CI, 0.01-0.05; P < .01).
Dr. Mez noted that the fact that the results were consistent across different outcomes “improves the validity” of the findings.
In a sensitivity analysis that excluded participants who also played football, estimates “were pretty similar” to those in the full analysis, said Dr. Mez.
The investigators have not yet examined the effect of level of hockey play, such as professionally or at the college level, on CTE risk. However, in football players, they found that level of play is another “valuable predictor of CTE pathology,” Dr. Mez said, adding that level of play, position played, and years of play “are all probably contributing” to CTE risk.
Asking about years of play is useful in a clinical setting. “It’s very easy for a clinician to ask patients how many years of hockey they played,” said Dr. Mez.
Overall, the new results are important, as “millions of individuals” play contact sports, whether that is hockey, football, or European soccer, he added. “And for all sports, there seems to be this relationship between more play and risk of this disease.”
‘Skewed’ population?
Commenting on the findings, Frank Conidi, MD, director, Florida Center for Health and Sports Neurology, Port St. Lucie, said he was surprised the investigators found a 23% per year increase in risk for CTE among hockey players.
Dr. Conidi has played hockey himself and works with the Florida Panthers of the National Hockey League. In his practice, he treats retired professional football players who have neurodegenerative disorders. From his experience, the number of repetitive direct head impacts in football is significantly higher than in hockey. “Most of the forces seen in hockey are from hits to the body, where the force is transferred to the head,” said Dr. Conidi, who was not involved with the research.
He noted differences in the way hockey is played around the world. In European countries, for example, the ice surface is relatively large and the emphasis tends to be more on skill than hitting.
“It would have been interesting to have the study group analyze the data based on where the athlete grew up,” he said. Dr. Conidi would also like to know when the participants played hockey. “The game is vastly different now than it was in the 1970s, ‘80s, and early ‘90s, when there was more fighting, less protective gear, and more hitting in general.”
As is the case for most studies of CTE in athletes, the study population is “skewed” because the participants likely had neurocognitive and other problems that led to their decision to donate their brain, said Dr. Conidi.
He also doesn’t believe the study should be the sole factor in a decision to continue or stop playing hockey. “We are still in the infancy stages of understanding the effects of high-impact sports on athletes’ brains.”
The study received funding from the National Institute of Neurological Diseases and Stroke and the National Institute on Aging. Dr. Mez and Dr. Conidi have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests. Early results from a study that examined donor brains showed that each additional year of ice hockey play increased the risk for CTE by 23%.
This information should be on the “radar” of all clinicians, said coinvestigator Jesse Mez, MD, associate professor of neurology at Boston University. “When they’re talking to kids and families and parents about playing contact sports, they should discuss the benefits as well as the risks so all that information can be taken into consideration.”
Dr. Mez noted that clinicians should also consider the amount of hockey played when assessing patients for thinking and memory trouble later in life. “CTE could be in the differential diagnosis,” he said.
The study findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Football data
CTE is a neurodegenerative disease associated with repetitive hits to the head. In previous research, the investigators showed that the more that athletes play American football, the more likely they are to develop CTE.
“Hockey, like football, involves repetitive head impacts as part of the game,” said Dr. Mez. “So we hypothesized that we would see a similar type of dose-response relationship in hockey.”
From two brain banks – the Veterans Affairs–Boston University–Concussion Legacy Foundation and the Framingham Heart Study – the researchers accessed 74 consecutive brains from donors who had played ice hockey. They collected information about hockey play during “pretty comprehensive” interviews with next of kin, Dr. Mez reported.
The study participants ranged in age from 13 to 91 years. The cause of death varied; most died with end-stage dementia and neurodegenerative disease, but some died of cardiovascular disease, and others from accidents.
For 9% of the individuals, the highest level of play was a youth league; 34% had reached the high school level, 30% reached the juniors/college level, and 26% played professionally. In addition, 46% played another contact sport – including 43% who played American football.
Primary outcomes included evidence of CTE from stage 0 (no CTE) to stage IV and severity of CTE, which was defined by the amount of neurofibrillary tangle (NFT) burden in 11 brain regions. For this burden, the score ranged from 0 (absent) to 3 (severe) in each region for a total range of 0-33.
Dr. Mez noted that, in CTE, tau protein accumulates abnormally. “It typically begins in the cortex in the frontal lobe and then spreads to other parts of the brain, including to the medial temporal structures, and is widespread by stage IV.”
The researchers estimated the association of duration of ice hockey play in years with each neuropathologic outcome and adjusted for age at death and duration of football play.
Consistent findings
Results showed that, of the 74 donors, 40 (54%) had CTE. Each additional year of hockey play corresponded to increased chances for having CTE (odds ratio, 1.23; 95% confidence interval, 11%-36%; P < .01). This increase in risk is similar to that which was found with football players, Dr. Mez noted. This was somewhat surprising, as hockey involves fewer “hits” than football.
“Hits are not as quintessential to the game of hockey as they are in football, where contacts occur with nearly every play,” he said. “In football, you have several hundred impacts over the course of a season.”
Researchers also found a 15% increase in odds for increasing one CTE stage (95% CI, 8%-22%; P < .01), and a .03 standard deviation increase in cumulative NFT burden (95% CI, 0.01-0.05; P < .01).
Dr. Mez noted that the fact that the results were consistent across different outcomes “improves the validity” of the findings.
In a sensitivity analysis that excluded participants who also played football, estimates “were pretty similar” to those in the full analysis, said Dr. Mez.
The investigators have not yet examined the effect of level of hockey play, such as professionally or at the college level, on CTE risk. However, in football players, they found that level of play is another “valuable predictor of CTE pathology,” Dr. Mez said, adding that level of play, position played, and years of play “are all probably contributing” to CTE risk.
Asking about years of play is useful in a clinical setting. “It’s very easy for a clinician to ask patients how many years of hockey they played,” said Dr. Mez.
Overall, the new results are important, as “millions of individuals” play contact sports, whether that is hockey, football, or European soccer, he added. “And for all sports, there seems to be this relationship between more play and risk of this disease.”
‘Skewed’ population?
Commenting on the findings, Frank Conidi, MD, director, Florida Center for Health and Sports Neurology, Port St. Lucie, said he was surprised the investigators found a 23% per year increase in risk for CTE among hockey players.
Dr. Conidi has played hockey himself and works with the Florida Panthers of the National Hockey League. In his practice, he treats retired professional football players who have neurodegenerative disorders. From his experience, the number of repetitive direct head impacts in football is significantly higher than in hockey. “Most of the forces seen in hockey are from hits to the body, where the force is transferred to the head,” said Dr. Conidi, who was not involved with the research.
He noted differences in the way hockey is played around the world. In European countries, for example, the ice surface is relatively large and the emphasis tends to be more on skill than hitting.
“It would have been interesting to have the study group analyze the data based on where the athlete grew up,” he said. Dr. Conidi would also like to know when the participants played hockey. “The game is vastly different now than it was in the 1970s, ‘80s, and early ‘90s, when there was more fighting, less protective gear, and more hitting in general.”
As is the case for most studies of CTE in athletes, the study population is “skewed” because the participants likely had neurocognitive and other problems that led to their decision to donate their brain, said Dr. Conidi.
He also doesn’t believe the study should be the sole factor in a decision to continue or stop playing hockey. “We are still in the infancy stages of understanding the effects of high-impact sports on athletes’ brains.”
The study received funding from the National Institute of Neurological Diseases and Stroke and the National Institute on Aging. Dr. Mez and Dr. Conidi have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
First comprehensive guidelines for managing anorexia in pregnancy
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Ohio bill bans ‘co-pay accumulator’ practice by insurers
The Ohio House of Representatives recently passed a bill that would enable patients to use drug manufacturer coupons and other co-pay assistance as payment toward their annual deductible.
According to the Kaiser Family Foundation, approximately 1 in 4 Americans have difficulty paying for their prescription drugs, while almost half of U.S. adults report difficulty paying out-of-pocket costs not covered by their health insurance.
Supporting the bill that restricts co-pay accumulators are groups such as the Ohio State Medical Association, the Crohn’s and Colitis Foundation, Susan C. Komen, the National Multiple Sclerosis Society, and the American Diabetes Association. The bill faced opposition from health insurers and pharmacy benefit managers, reported The Columbus Dispatch.
“The debate on the management of rising drug costs between manufacturers and insurers unfortunately leaves patients caught in the middle, and practices like co-pay accumulators can have a devastating impact,” Monica Hueckel, senior director of government relations for the Ohio State Medical Association, told this news organization.
“Patients often do not even know about these policies until the coupons are no longer usable. As you can imagine, for patients with expensive medications and/or high deductible health plans, the impact is disastrous,” she said.
Ohio State Representative Susan Manchester, who co-sponsored the bill, told The Columbus Dispatch that the legislation “is needed to assist our constituents who find themselves increasingly subjected to more out-of-pocket costs as part of their insurance coverage.”
Other states blocking health insurers’ co-pay policies
With the passage of the bill, Ohio joins 12 states and Puerto Rico in preventing the use of health insurers’ co-pays to increase patients’ out-of-pocket costs, reported The Columbus Dispatch; 15 states are also considering this type of legislation.
Eighty-three percent of patients are in plans that include a co-pay accumulator, according to consulting firm Avalere, which wrote that, beginning in 2023, the Center for Medicare & Medicaid Services requires patients with Medicaid to receive “the full value of co-pay assistance” on drugs.
According to the National Conference of State Legislatures, co-pay adjustment programs present challenges for patients, with plans that include high cost sharing or co-insurance whereby a patient pays a percentage of the cost instead of a flat amount.
For example, with a co-pay adjustment policy, a patient with a $2,000 deductible plan couldn’t use a $500 coupon toward meeting the deductible, writes the National Conference of State Legislatures. Conversely, a patient in a plan without a co-pay adjustment policy could use the coupon to satisfy their annual deductible.
Patients with complex conditions, such as cancer, rheumatoid arthritis, and diabetes, which often require expensive medications, may have little choice but to fork over the unexpected co-pays, according to the organization that represents state legislatures in the United States.
The bill now moves to the Ohio Senate, reported The Columbus Dispatch.
A version of this article first appeared on Medscape.com.
The Ohio House of Representatives recently passed a bill that would enable patients to use drug manufacturer coupons and other co-pay assistance as payment toward their annual deductible.
According to the Kaiser Family Foundation, approximately 1 in 4 Americans have difficulty paying for their prescription drugs, while almost half of U.S. adults report difficulty paying out-of-pocket costs not covered by their health insurance.
Supporting the bill that restricts co-pay accumulators are groups such as the Ohio State Medical Association, the Crohn’s and Colitis Foundation, Susan C. Komen, the National Multiple Sclerosis Society, and the American Diabetes Association. The bill faced opposition from health insurers and pharmacy benefit managers, reported The Columbus Dispatch.
“The debate on the management of rising drug costs between manufacturers and insurers unfortunately leaves patients caught in the middle, and practices like co-pay accumulators can have a devastating impact,” Monica Hueckel, senior director of government relations for the Ohio State Medical Association, told this news organization.
“Patients often do not even know about these policies until the coupons are no longer usable. As you can imagine, for patients with expensive medications and/or high deductible health plans, the impact is disastrous,” she said.
Ohio State Representative Susan Manchester, who co-sponsored the bill, told The Columbus Dispatch that the legislation “is needed to assist our constituents who find themselves increasingly subjected to more out-of-pocket costs as part of their insurance coverage.”
Other states blocking health insurers’ co-pay policies
With the passage of the bill, Ohio joins 12 states and Puerto Rico in preventing the use of health insurers’ co-pays to increase patients’ out-of-pocket costs, reported The Columbus Dispatch; 15 states are also considering this type of legislation.
Eighty-three percent of patients are in plans that include a co-pay accumulator, according to consulting firm Avalere, which wrote that, beginning in 2023, the Center for Medicare & Medicaid Services requires patients with Medicaid to receive “the full value of co-pay assistance” on drugs.
According to the National Conference of State Legislatures, co-pay adjustment programs present challenges for patients, with plans that include high cost sharing or co-insurance whereby a patient pays a percentage of the cost instead of a flat amount.
For example, with a co-pay adjustment policy, a patient with a $2,000 deductible plan couldn’t use a $500 coupon toward meeting the deductible, writes the National Conference of State Legislatures. Conversely, a patient in a plan without a co-pay adjustment policy could use the coupon to satisfy their annual deductible.
Patients with complex conditions, such as cancer, rheumatoid arthritis, and diabetes, which often require expensive medications, may have little choice but to fork over the unexpected co-pays, according to the organization that represents state legislatures in the United States.
The bill now moves to the Ohio Senate, reported The Columbus Dispatch.
A version of this article first appeared on Medscape.com.
The Ohio House of Representatives recently passed a bill that would enable patients to use drug manufacturer coupons and other co-pay assistance as payment toward their annual deductible.
According to the Kaiser Family Foundation, approximately 1 in 4 Americans have difficulty paying for their prescription drugs, while almost half of U.S. adults report difficulty paying out-of-pocket costs not covered by their health insurance.
Supporting the bill that restricts co-pay accumulators are groups such as the Ohio State Medical Association, the Crohn’s and Colitis Foundation, Susan C. Komen, the National Multiple Sclerosis Society, and the American Diabetes Association. The bill faced opposition from health insurers and pharmacy benefit managers, reported The Columbus Dispatch.
“The debate on the management of rising drug costs between manufacturers and insurers unfortunately leaves patients caught in the middle, and practices like co-pay accumulators can have a devastating impact,” Monica Hueckel, senior director of government relations for the Ohio State Medical Association, told this news organization.
“Patients often do not even know about these policies until the coupons are no longer usable. As you can imagine, for patients with expensive medications and/or high deductible health plans, the impact is disastrous,” she said.
Ohio State Representative Susan Manchester, who co-sponsored the bill, told The Columbus Dispatch that the legislation “is needed to assist our constituents who find themselves increasingly subjected to more out-of-pocket costs as part of their insurance coverage.”
Other states blocking health insurers’ co-pay policies
With the passage of the bill, Ohio joins 12 states and Puerto Rico in preventing the use of health insurers’ co-pays to increase patients’ out-of-pocket costs, reported The Columbus Dispatch; 15 states are also considering this type of legislation.
Eighty-three percent of patients are in plans that include a co-pay accumulator, according to consulting firm Avalere, which wrote that, beginning in 2023, the Center for Medicare & Medicaid Services requires patients with Medicaid to receive “the full value of co-pay assistance” on drugs.
According to the National Conference of State Legislatures, co-pay adjustment programs present challenges for patients, with plans that include high cost sharing or co-insurance whereby a patient pays a percentage of the cost instead of a flat amount.
For example, with a co-pay adjustment policy, a patient with a $2,000 deductible plan couldn’t use a $500 coupon toward meeting the deductible, writes the National Conference of State Legislatures. Conversely, a patient in a plan without a co-pay adjustment policy could use the coupon to satisfy their annual deductible.
Patients with complex conditions, such as cancer, rheumatoid arthritis, and diabetes, which often require expensive medications, may have little choice but to fork over the unexpected co-pays, according to the organization that represents state legislatures in the United States.
The bill now moves to the Ohio Senate, reported The Columbus Dispatch.
A version of this article first appeared on Medscape.com.
Novel medication tied to better quality of life in major depression
DENVER –
In a phase 3 trial that included more than 500 adult patients with MDD, those who received zuranolone for 14 days showed greater improvement at day 15 across numerous QoL outcomes, compared with their counterparts in the placebo group.
In addition, combined analysis of four zuranolone clinical trials showed “mental well-being and functioning improved to near general population norm levels” for the active-treatment group, reported the researchers, led by Anita H. Clayton, MD, chair and professor of psychiatry, University of Virginia, Charlottesville.
“Based on these integrated analyses, the benefit of treatment with zuranolone may extend beyond reduction in depressive symptoms to include potential improvement in quality of life and overall health, as perceived by patients,” they add.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
First oral formulation
Zuranolone represents the second entry in the new class of neuroactive steroid drugs, which modulate GABA-A receptor activity – but it would be the first to have an oral formulation. Brexanolone, which was approved by the Food and Drug Administration in 2019 for postpartum depression, is administered through continuous IV infusion over 60 hours.
As previously reported by this news organization, zuranolone improved depressive symptoms as early as day 3, achieving the primary endpoint of significantly greater reduction in scores on the 17-item Hamilton Rating Scale for Depression from baseline to day 15 versus placebo (P = .014).
In the new analysis, patient-reported measures of functional health and well-being were assessed in the WATERFALL trial. It included 266 patients with MDD who were treated with zuranolone 50 mg daily for 2 weeks and 268 patients with MDD who were treated with placebo.
The study used the Short Form–36 (SF-36v2), which covers a wide range of patient-reported measures, including physical function, bodily pain, general health, vitality, social function, and “role-emotional” symptoms.
Results showed that although the treatment and placebo groups had similar baseline SF-36v2 scores, those receiving zuranolone reported significantly greater improvements at day 15 in almost all of the assessment’s domains, including physical function (treatment difference, 0.8), general health (1.0), vitality (3.1), social functioning (1.1), and role-emotional symptoms (1.5; for all comparisons, P < .05). The only exceptions were in role-physical symptoms and bodily pain.
In measures that included physical function, bodily pain, and general health, the patients achieved improvements at day 15 that were consistent with normal levels, with the improvement in vitality considered clinically meaningful versus placebo.
Integrated data
In further analysis of integrated data from four zuranolone clinical trials in the NEST and LANDSCAPE programs for patients with MDD and postpartum depression, results showed similar improvements at day 15 for zuranolone in QoL and overall health across all of the SF-36v2 functioning and well-being domains (P <.05), with the exceptions of physical measure and bodily pain.
By day 42, all of the domains showed significantly greater improvement with zuranolone versus placebo (all, P <.05).
Among the strongest score improvements in the integrated trials were measures in social functioning, which improved from baseline scores of 29.66 to 42.82 on day 15 and to 43.59 on day 42.
Emotional domain scores improved from 24.43 at baseline to 39.13 on day 15 and to 39.82 on day 42. For mental health, the integrated scores for the zuranolone group improved from 27.13 at baseline to 42.40 on day 15 and 42.62 on day 42.
Of note, the baseline scores for mental health represented just 54.3% of those in the normal population; with the increase at day 15, the level was 84.8% of the normal population.
“Across four completed placebo-controlled NEST and LANDSCAPE clinical trials, patient reports of functional health and well-being as assessed by the SF-36v2 indicated substantial impairment at baseline compared to the population norm,” the researchers reported.
The improvements are especially important in light of the fact that in some patients with MDD, functional improvement is a top priority.
“Patients have often prioritized returning to their usual level of functioning over reduction in depressive symptoms, and functional recovery has been associated with better prognosis of depression,” the investigators wrote.
Zuranolone trials have shown that treatment-emergent adverse events (AEs) occur among about 60% of patients, versus about 44% with placebo. The most common AEs are somnolence, dizziness, headache, sedation, and diarrhea, with no increases in suicidal ideation or withdrawal.
The rates of severe AEs are low, and they are observed in about 3% of patients, versus 1.1% with placebo, the researchers noted.
Further, as opposed to serotonergic antidepressants such as SNRIs and SSRIs, zuranolone does not appear to have the undesirable side effects of decreased libido and sexual dysfunction, they added.
Clinically meaningful?
Andrew J. Cutler, MD, clinical associate professor of psychiatry at State University of New York, Syracuse, said the data are “very significant” for a number of reasons.
“We need more options to treat depression, especially ones with novel mechanisms of action and faster onset of efficacy, such as zuranolone,” said Dr. Cutler, who was not involved in the current study. He has coauthored other studies on zuranolone.
Regarding the study’s QoL outcomes, “while improvement in depressive symptoms is very important, what really matters to patients is improvement in function and quality of life,” Dr. Cutler noted.
Also commenting on the study, Jonathan E. Alpert, MD, PhD, chair of the department of psychiatry and behavioral sciences and professor of psychiatry, neuroscience, and pediatrics at Albert Einstein College of Medicine, New York, said the investigational drug could represent an important addition to the armamentarium for treating depression.
“Zuranolone has good oral bioavailability and would represent the first neuroactive steroid antidepressant available in oral form and, indeed, the first non–monoamine-based antidepressant available in oral form,” he said in an interview.
Dr. Alpert was not involved in the research and has no relationship with the drug’s development.
He noted that although there are modest differences between the patients who received zuranolone and those who received placebo in the trials, “this may have been related to high placebo response rates, which often complicate antidepressant trials.
“Further research is needed to determine whether differences between zuranolone and placebo are clinically meaningful, though the separation between drug and placebo on the primary endpoint, as well as some other measures, such as quality of life measures, is promising,” Dr. Alpert said.
However, he added that comparisons with other active antidepressants in terms of efficacy and tolerability remain to be seen.
“Given the large number of individuals with major depressive disorder who have incomplete response to or do not tolerate monoaminergic antidepressants, the development of agents that leverage novel nonmonoaminergic mechanisms is important,” Dr. Alpert concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Cutler has been involved in research of zuranolone for Sage Therapeutics. Dr. Alpert has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DENVER –
In a phase 3 trial that included more than 500 adult patients with MDD, those who received zuranolone for 14 days showed greater improvement at day 15 across numerous QoL outcomes, compared with their counterparts in the placebo group.
In addition, combined analysis of four zuranolone clinical trials showed “mental well-being and functioning improved to near general population norm levels” for the active-treatment group, reported the researchers, led by Anita H. Clayton, MD, chair and professor of psychiatry, University of Virginia, Charlottesville.
“Based on these integrated analyses, the benefit of treatment with zuranolone may extend beyond reduction in depressive symptoms to include potential improvement in quality of life and overall health, as perceived by patients,” they add.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
First oral formulation
Zuranolone represents the second entry in the new class of neuroactive steroid drugs, which modulate GABA-A receptor activity – but it would be the first to have an oral formulation. Brexanolone, which was approved by the Food and Drug Administration in 2019 for postpartum depression, is administered through continuous IV infusion over 60 hours.
As previously reported by this news organization, zuranolone improved depressive symptoms as early as day 3, achieving the primary endpoint of significantly greater reduction in scores on the 17-item Hamilton Rating Scale for Depression from baseline to day 15 versus placebo (P = .014).
In the new analysis, patient-reported measures of functional health and well-being were assessed in the WATERFALL trial. It included 266 patients with MDD who were treated with zuranolone 50 mg daily for 2 weeks and 268 patients with MDD who were treated with placebo.
The study used the Short Form–36 (SF-36v2), which covers a wide range of patient-reported measures, including physical function, bodily pain, general health, vitality, social function, and “role-emotional” symptoms.
Results showed that although the treatment and placebo groups had similar baseline SF-36v2 scores, those receiving zuranolone reported significantly greater improvements at day 15 in almost all of the assessment’s domains, including physical function (treatment difference, 0.8), general health (1.0), vitality (3.1), social functioning (1.1), and role-emotional symptoms (1.5; for all comparisons, P < .05). The only exceptions were in role-physical symptoms and bodily pain.
In measures that included physical function, bodily pain, and general health, the patients achieved improvements at day 15 that were consistent with normal levels, with the improvement in vitality considered clinically meaningful versus placebo.
Integrated data
In further analysis of integrated data from four zuranolone clinical trials in the NEST and LANDSCAPE programs for patients with MDD and postpartum depression, results showed similar improvements at day 15 for zuranolone in QoL and overall health across all of the SF-36v2 functioning and well-being domains (P <.05), with the exceptions of physical measure and bodily pain.
By day 42, all of the domains showed significantly greater improvement with zuranolone versus placebo (all, P <.05).
Among the strongest score improvements in the integrated trials were measures in social functioning, which improved from baseline scores of 29.66 to 42.82 on day 15 and to 43.59 on day 42.
Emotional domain scores improved from 24.43 at baseline to 39.13 on day 15 and to 39.82 on day 42. For mental health, the integrated scores for the zuranolone group improved from 27.13 at baseline to 42.40 on day 15 and 42.62 on day 42.
Of note, the baseline scores for mental health represented just 54.3% of those in the normal population; with the increase at day 15, the level was 84.8% of the normal population.
“Across four completed placebo-controlled NEST and LANDSCAPE clinical trials, patient reports of functional health and well-being as assessed by the SF-36v2 indicated substantial impairment at baseline compared to the population norm,” the researchers reported.
The improvements are especially important in light of the fact that in some patients with MDD, functional improvement is a top priority.
“Patients have often prioritized returning to their usual level of functioning over reduction in depressive symptoms, and functional recovery has been associated with better prognosis of depression,” the investigators wrote.
Zuranolone trials have shown that treatment-emergent adverse events (AEs) occur among about 60% of patients, versus about 44% with placebo. The most common AEs are somnolence, dizziness, headache, sedation, and diarrhea, with no increases in suicidal ideation or withdrawal.
The rates of severe AEs are low, and they are observed in about 3% of patients, versus 1.1% with placebo, the researchers noted.
Further, as opposed to serotonergic antidepressants such as SNRIs and SSRIs, zuranolone does not appear to have the undesirable side effects of decreased libido and sexual dysfunction, they added.
Clinically meaningful?
Andrew J. Cutler, MD, clinical associate professor of psychiatry at State University of New York, Syracuse, said the data are “very significant” for a number of reasons.
“We need more options to treat depression, especially ones with novel mechanisms of action and faster onset of efficacy, such as zuranolone,” said Dr. Cutler, who was not involved in the current study. He has coauthored other studies on zuranolone.
Regarding the study’s QoL outcomes, “while improvement in depressive symptoms is very important, what really matters to patients is improvement in function and quality of life,” Dr. Cutler noted.
Also commenting on the study, Jonathan E. Alpert, MD, PhD, chair of the department of psychiatry and behavioral sciences and professor of psychiatry, neuroscience, and pediatrics at Albert Einstein College of Medicine, New York, said the investigational drug could represent an important addition to the armamentarium for treating depression.
“Zuranolone has good oral bioavailability and would represent the first neuroactive steroid antidepressant available in oral form and, indeed, the first non–monoamine-based antidepressant available in oral form,” he said in an interview.
Dr. Alpert was not involved in the research and has no relationship with the drug’s development.
He noted that although there are modest differences between the patients who received zuranolone and those who received placebo in the trials, “this may have been related to high placebo response rates, which often complicate antidepressant trials.
“Further research is needed to determine whether differences between zuranolone and placebo are clinically meaningful, though the separation between drug and placebo on the primary endpoint, as well as some other measures, such as quality of life measures, is promising,” Dr. Alpert said.
However, he added that comparisons with other active antidepressants in terms of efficacy and tolerability remain to be seen.
“Given the large number of individuals with major depressive disorder who have incomplete response to or do not tolerate monoaminergic antidepressants, the development of agents that leverage novel nonmonoaminergic mechanisms is important,” Dr. Alpert concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Cutler has been involved in research of zuranolone for Sage Therapeutics. Dr. Alpert has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DENVER –
In a phase 3 trial that included more than 500 adult patients with MDD, those who received zuranolone for 14 days showed greater improvement at day 15 across numerous QoL outcomes, compared with their counterparts in the placebo group.
In addition, combined analysis of four zuranolone clinical trials showed “mental well-being and functioning improved to near general population norm levels” for the active-treatment group, reported the researchers, led by Anita H. Clayton, MD, chair and professor of psychiatry, University of Virginia, Charlottesville.
“Based on these integrated analyses, the benefit of treatment with zuranolone may extend beyond reduction in depressive symptoms to include potential improvement in quality of life and overall health, as perceived by patients,” they add.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
First oral formulation
Zuranolone represents the second entry in the new class of neuroactive steroid drugs, which modulate GABA-A receptor activity – but it would be the first to have an oral formulation. Brexanolone, which was approved by the Food and Drug Administration in 2019 for postpartum depression, is administered through continuous IV infusion over 60 hours.
As previously reported by this news organization, zuranolone improved depressive symptoms as early as day 3, achieving the primary endpoint of significantly greater reduction in scores on the 17-item Hamilton Rating Scale for Depression from baseline to day 15 versus placebo (P = .014).
In the new analysis, patient-reported measures of functional health and well-being were assessed in the WATERFALL trial. It included 266 patients with MDD who were treated with zuranolone 50 mg daily for 2 weeks and 268 patients with MDD who were treated with placebo.
The study used the Short Form–36 (SF-36v2), which covers a wide range of patient-reported measures, including physical function, bodily pain, general health, vitality, social function, and “role-emotional” symptoms.
Results showed that although the treatment and placebo groups had similar baseline SF-36v2 scores, those receiving zuranolone reported significantly greater improvements at day 15 in almost all of the assessment’s domains, including physical function (treatment difference, 0.8), general health (1.0), vitality (3.1), social functioning (1.1), and role-emotional symptoms (1.5; for all comparisons, P < .05). The only exceptions were in role-physical symptoms and bodily pain.
In measures that included physical function, bodily pain, and general health, the patients achieved improvements at day 15 that were consistent with normal levels, with the improvement in vitality considered clinically meaningful versus placebo.
Integrated data
In further analysis of integrated data from four zuranolone clinical trials in the NEST and LANDSCAPE programs for patients with MDD and postpartum depression, results showed similar improvements at day 15 for zuranolone in QoL and overall health across all of the SF-36v2 functioning and well-being domains (P <.05), with the exceptions of physical measure and bodily pain.
By day 42, all of the domains showed significantly greater improvement with zuranolone versus placebo (all, P <.05).
Among the strongest score improvements in the integrated trials were measures in social functioning, which improved from baseline scores of 29.66 to 42.82 on day 15 and to 43.59 on day 42.
Emotional domain scores improved from 24.43 at baseline to 39.13 on day 15 and to 39.82 on day 42. For mental health, the integrated scores for the zuranolone group improved from 27.13 at baseline to 42.40 on day 15 and 42.62 on day 42.
Of note, the baseline scores for mental health represented just 54.3% of those in the normal population; with the increase at day 15, the level was 84.8% of the normal population.
“Across four completed placebo-controlled NEST and LANDSCAPE clinical trials, patient reports of functional health and well-being as assessed by the SF-36v2 indicated substantial impairment at baseline compared to the population norm,” the researchers reported.
The improvements are especially important in light of the fact that in some patients with MDD, functional improvement is a top priority.
“Patients have often prioritized returning to their usual level of functioning over reduction in depressive symptoms, and functional recovery has been associated with better prognosis of depression,” the investigators wrote.
Zuranolone trials have shown that treatment-emergent adverse events (AEs) occur among about 60% of patients, versus about 44% with placebo. The most common AEs are somnolence, dizziness, headache, sedation, and diarrhea, with no increases in suicidal ideation or withdrawal.
The rates of severe AEs are low, and they are observed in about 3% of patients, versus 1.1% with placebo, the researchers noted.
Further, as opposed to serotonergic antidepressants such as SNRIs and SSRIs, zuranolone does not appear to have the undesirable side effects of decreased libido and sexual dysfunction, they added.
Clinically meaningful?
Andrew J. Cutler, MD, clinical associate professor of psychiatry at State University of New York, Syracuse, said the data are “very significant” for a number of reasons.
“We need more options to treat depression, especially ones with novel mechanisms of action and faster onset of efficacy, such as zuranolone,” said Dr. Cutler, who was not involved in the current study. He has coauthored other studies on zuranolone.
Regarding the study’s QoL outcomes, “while improvement in depressive symptoms is very important, what really matters to patients is improvement in function and quality of life,” Dr. Cutler noted.
Also commenting on the study, Jonathan E. Alpert, MD, PhD, chair of the department of psychiatry and behavioral sciences and professor of psychiatry, neuroscience, and pediatrics at Albert Einstein College of Medicine, New York, said the investigational drug could represent an important addition to the armamentarium for treating depression.
“Zuranolone has good oral bioavailability and would represent the first neuroactive steroid antidepressant available in oral form and, indeed, the first non–monoamine-based antidepressant available in oral form,” he said in an interview.
Dr. Alpert was not involved in the research and has no relationship with the drug’s development.
He noted that although there are modest differences between the patients who received zuranolone and those who received placebo in the trials, “this may have been related to high placebo response rates, which often complicate antidepressant trials.
“Further research is needed to determine whether differences between zuranolone and placebo are clinically meaningful, though the separation between drug and placebo on the primary endpoint, as well as some other measures, such as quality of life measures, is promising,” Dr. Alpert said.
However, he added that comparisons with other active antidepressants in terms of efficacy and tolerability remain to be seen.
“Given the large number of individuals with major depressive disorder who have incomplete response to or do not tolerate monoaminergic antidepressants, the development of agents that leverage novel nonmonoaminergic mechanisms is important,” Dr. Alpert concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Cutler has been involved in research of zuranolone for Sage Therapeutics. Dr. Alpert has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADAA 2022
Clinician experience has been cited for declining operative vaginal delivery rates. Are you comfortable performing OVD as an alternative to cesarean?
[polldaddy:11086729]
[polldaddy:11086729]
[polldaddy:11086729]
Nerve-sparing laparoscopic low anterior resection for rectal endometriosis in ten steps
Bilateral palmar rash
A biopsy was performed and the pathology report showed ectatic, thin-walled vessels consistent with telangiectasias. There were no other inflammatory, infectious, or malignant changes.
Telangiectasias are caused by permanent dilatation of subpapillary plexus end vessels. Unlike petechiae and angiomata, telangiectasias blanch with pressure. They usually manifest as small, bright red, nonpulsatile vascular lesions with a fine, netlike pattern on the surface of the skin. Telangiectasis can affect many organs (eg, intestines, bladder, brain, eyes) and may occur in patients with certain genetic disorders and environmental exposures (eg, radiation).1
Palmar telangiectasias are specifically associated with hereditary hemorrhagic telangiectasia, dermatomyositis, Grave disease, CREST syndrome, systemic lupus erythematosus, and smoking.2 Sun exposure and smoking are the main risk factors for the development of telangiectasias.1
This patient had no history of autoimmune disease or hyperthyroidism, and no one in her family had telangiectasis. Thus, the likely cause of her lesions was smoking. While the pathophysiology is not fully understood, it is likely related to the vasoconstrictive quality of nicotine, causing ischemia in the dermis. This chronic, low-grade ischemia may trigger the compensatory development of telangiectasias.2
This patient was informed that her telangiectasias were most likely caused by her smoking and that the lesions themselves did not require treatment. She was encouraged to continue her smoking cessation efforts with her primary care provider.
Photos courtesy of Daniel Stulberg, MD. Text courtesy of Mia MJ Coleman, BA, BS, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Schieving JH, Shoenaker MHD, Weemaes CM, et al. Telangiectasias: Small lesions referring to serious disorders. Eur J Paediatr Neurol. 2017;21:807-815. doi: 10.1016/j.ejpn.2017.07.016
2. Levi A, Shechter R, Lapidoth M, et al. Palmar telangiectasias: a cutaneous sign for smoking. Dermatology. 2017;233:390-395. doi: 10.1159/000481855

A biopsy was performed and the pathology report showed ectatic, thin-walled vessels consistent with telangiectasias. There were no other inflammatory, infectious, or malignant changes.
Telangiectasias are caused by permanent dilatation of subpapillary plexus end vessels. Unlike petechiae and angiomata, telangiectasias blanch with pressure. They usually manifest as small, bright red, nonpulsatile vascular lesions with a fine, netlike pattern on the surface of the skin. Telangiectasis can affect many organs (eg, intestines, bladder, brain, eyes) and may occur in patients with certain genetic disorders and environmental exposures (eg, radiation).1
Palmar telangiectasias are specifically associated with hereditary hemorrhagic telangiectasia, dermatomyositis, Grave disease, CREST syndrome, systemic lupus erythematosus, and smoking.2 Sun exposure and smoking are the main risk factors for the development of telangiectasias.1
This patient had no history of autoimmune disease or hyperthyroidism, and no one in her family had telangiectasis. Thus, the likely cause of her lesions was smoking. While the pathophysiology is not fully understood, it is likely related to the vasoconstrictive quality of nicotine, causing ischemia in the dermis. This chronic, low-grade ischemia may trigger the compensatory development of telangiectasias.2
This patient was informed that her telangiectasias were most likely caused by her smoking and that the lesions themselves did not require treatment. She was encouraged to continue her smoking cessation efforts with her primary care provider.
Photos courtesy of Daniel Stulberg, MD. Text courtesy of Mia MJ Coleman, BA, BS, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A biopsy was performed and the pathology report showed ectatic, thin-walled vessels consistent with telangiectasias. There were no other inflammatory, infectious, or malignant changes.
Telangiectasias are caused by permanent dilatation of subpapillary plexus end vessels. Unlike petechiae and angiomata, telangiectasias blanch with pressure. They usually manifest as small, bright red, nonpulsatile vascular lesions with a fine, netlike pattern on the surface of the skin. Telangiectasis can affect many organs (eg, intestines, bladder, brain, eyes) and may occur in patients with certain genetic disorders and environmental exposures (eg, radiation).1
Palmar telangiectasias are specifically associated with hereditary hemorrhagic telangiectasia, dermatomyositis, Grave disease, CREST syndrome, systemic lupus erythematosus, and smoking.2 Sun exposure and smoking are the main risk factors for the development of telangiectasias.1
This patient had no history of autoimmune disease or hyperthyroidism, and no one in her family had telangiectasis. Thus, the likely cause of her lesions was smoking. While the pathophysiology is not fully understood, it is likely related to the vasoconstrictive quality of nicotine, causing ischemia in the dermis. This chronic, low-grade ischemia may trigger the compensatory development of telangiectasias.2
This patient was informed that her telangiectasias were most likely caused by her smoking and that the lesions themselves did not require treatment. She was encouraged to continue her smoking cessation efforts with her primary care provider.
Photos courtesy of Daniel Stulberg, MD. Text courtesy of Mia MJ Coleman, BA, BS, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Schieving JH, Shoenaker MHD, Weemaes CM, et al. Telangiectasias: Small lesions referring to serious disorders. Eur J Paediatr Neurol. 2017;21:807-815. doi: 10.1016/j.ejpn.2017.07.016
2. Levi A, Shechter R, Lapidoth M, et al. Palmar telangiectasias: a cutaneous sign for smoking. Dermatology. 2017;233:390-395. doi: 10.1159/000481855
1. Schieving JH, Shoenaker MHD, Weemaes CM, et al. Telangiectasias: Small lesions referring to serious disorders. Eur J Paediatr Neurol. 2017;21:807-815. doi: 10.1016/j.ejpn.2017.07.016
2. Levi A, Shechter R, Lapidoth M, et al. Palmar telangiectasias: a cutaneous sign for smoking. Dermatology. 2017;233:390-395. doi: 10.1159/000481855