User login
A ‘crisis’ of suicidal thoughts, attempts in transgender youth
Transgender youth are significantly more likely to consider suicide and attempt it, compared with their cisgender peers, new research shows.
In a large population-based study, investigators found the increased risk of suicidality is partly because of bullying and cyberbullying experienced by transgender teens.
The findings are “extremely concerning and should be a wake-up call,” Ian Colman, PhD, with the University of Ottawa School of Epidemiology and Public Health, said in an interview.
Young people who are exploring their sexual identities may suffer from depression and anxiety, both about the reactions of their peers and families, as well as their own sense of self.
“These youth are highly marginalized and stigmatized in many corners of our society, and these findings highlight just how distressing these experiences can be,” Dr. Colman said.
The study was published online in the Canadian Medical Association Journal.
Sevenfold increased risk of attempted suicide
The risk of suicidal thoughts and actions is not well studied in transgender and nonbinary youth.
To expand the evidence base, the researchers analyzed data for 6,800 adolescents aged 15-17 years from the 2019 Canadian Health Survey on Children and Youth.
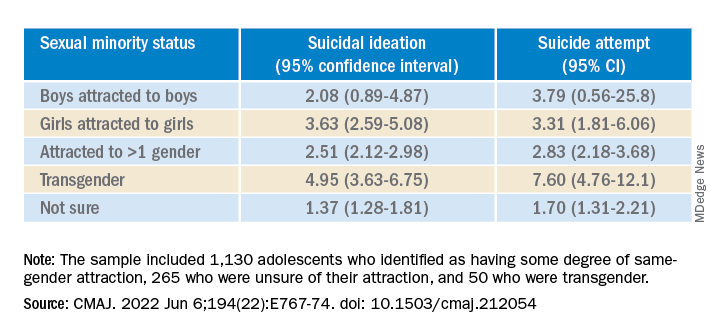
The sample included 1,130 (16.5%) adolescents who identified as having some degree of same-gender attraction, 265 (4.3%) who were unsure of their attraction (“questioning”), and 50 (0.6%) who were transgender, meaning they identified as being of a gender different from that assigned at birth.
Overall, 980 (14.0%) adolescents reported having thoughts of suicide in the prior year, and 480 (6.8%) had attempted suicide in their life.
Transgender youth were five times more likely to think about suicide and more than seven times more likely to have ever attempted suicide than cisgender, heterosexual peers.
Among cisgender adolescents, girls who were attracted to girls had 3.6 times the risk of suicidal ideation and 3.3 times the risk of having ever attempted suicide, compared with their heterosexual peers.
Teens attracted to multiple genders had more than twice the risk of suicidal ideation and suicide attempt. Youth who were questioning their sexual orientation had twice the risk of having attempted suicide in their lifetime.
A crisis – with reason for hope
“This is a crisis, and it shows just how much more needs to be done to support transgender young people,” co-author Fae Johnstone, MSW, executive director, Wisdom2Action, who is a trans woman herself, said in the news release.
“Suicide prevention programs specifically targeted to transgender, nonbinary, and sexual minority adolescents, as well as gender-affirming care for transgender adolescents, may help reduce the burden of suicidality among this group,” Ms. Johnstone added.
“The most important thing that parents, teachers, and health care providers can do is to be supportive of these youth,” Dr. Colman told this news organization.
“Providing a safe place where gender and sexual minorities can explore and express themselves is crucial. The first step is to listen and to be compassionate,” Dr. Colman added.
Reached for comment, Jess Ting, MD, director of surgery at the Mount Sinai Center for Transgender Medicine and Surgery, New York, said the data from this study on suicidal thoughts and actions among sexual minority and transgender adolescents “mirror what we see and what we know” about suicidality in trans and nonbinary adults.
“The reasons for this are complex, and it’s hard for someone who doesn’t have a lived experience as a trans or nonbinary person to understand the reasons for suicidality,” he told this news organization.
“But we also know that there are higher rates of anxiety and depression and self-image issues and posttraumatic stress disorder, not to mention outside factors – marginalization, discrimination, violence, abuse. When you add up all these intrinsic and extrinsic factors, it’s not hard to believe that there is a high rate of suicidality,” Dr. Ting said.
“There have been studies that have shown that in children who are supported in their gender identity, the rates of depression and anxiety decreased to almost the same levels as non-trans and nonbinary children, so I think that gives cause for hope,” Dr. Ting added.
The study was funded in part by the Research Council of Norway through its Centres of Excellence funding scheme and by a Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. Ms. Johnstone reports consulting fees from Spectrum Waterloo and volunteer participation with the Youth Suicide Prevention Leadership Committee of Ontario. No other competing interests were declared. Dr. Ting has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender youth are significantly more likely to consider suicide and attempt it, compared with their cisgender peers, new research shows.
In a large population-based study, investigators found the increased risk of suicidality is partly because of bullying and cyberbullying experienced by transgender teens.
The findings are “extremely concerning and should be a wake-up call,” Ian Colman, PhD, with the University of Ottawa School of Epidemiology and Public Health, said in an interview.
Young people who are exploring their sexual identities may suffer from depression and anxiety, both about the reactions of their peers and families, as well as their own sense of self.
“These youth are highly marginalized and stigmatized in many corners of our society, and these findings highlight just how distressing these experiences can be,” Dr. Colman said.
The study was published online in the Canadian Medical Association Journal.
Sevenfold increased risk of attempted suicide
The risk of suicidal thoughts and actions is not well studied in transgender and nonbinary youth.
To expand the evidence base, the researchers analyzed data for 6,800 adolescents aged 15-17 years from the 2019 Canadian Health Survey on Children and Youth.
The sample included 1,130 (16.5%) adolescents who identified as having some degree of same-gender attraction, 265 (4.3%) who were unsure of their attraction (“questioning”), and 50 (0.6%) who were transgender, meaning they identified as being of a gender different from that assigned at birth.
Overall, 980 (14.0%) adolescents reported having thoughts of suicide in the prior year, and 480 (6.8%) had attempted suicide in their life.
Transgender youth were five times more likely to think about suicide and more than seven times more likely to have ever attempted suicide than cisgender, heterosexual peers.
Among cisgender adolescents, girls who were attracted to girls had 3.6 times the risk of suicidal ideation and 3.3 times the risk of having ever attempted suicide, compared with their heterosexual peers.
Teens attracted to multiple genders had more than twice the risk of suicidal ideation and suicide attempt. Youth who were questioning their sexual orientation had twice the risk of having attempted suicide in their lifetime.

A crisis – with reason for hope
“This is a crisis, and it shows just how much more needs to be done to support transgender young people,” co-author Fae Johnstone, MSW, executive director, Wisdom2Action, who is a trans woman herself, said in the news release.
“Suicide prevention programs specifically targeted to transgender, nonbinary, and sexual minority adolescents, as well as gender-affirming care for transgender adolescents, may help reduce the burden of suicidality among this group,” Ms. Johnstone added.
“The most important thing that parents, teachers, and health care providers can do is to be supportive of these youth,” Dr. Colman told this news organization.
“Providing a safe place where gender and sexual minorities can explore and express themselves is crucial. The first step is to listen and to be compassionate,” Dr. Colman added.
Reached for comment, Jess Ting, MD, director of surgery at the Mount Sinai Center for Transgender Medicine and Surgery, New York, said the data from this study on suicidal thoughts and actions among sexual minority and transgender adolescents “mirror what we see and what we know” about suicidality in trans and nonbinary adults.
“The reasons for this are complex, and it’s hard for someone who doesn’t have a lived experience as a trans or nonbinary person to understand the reasons for suicidality,” he told this news organization.
“But we also know that there are higher rates of anxiety and depression and self-image issues and posttraumatic stress disorder, not to mention outside factors – marginalization, discrimination, violence, abuse. When you add up all these intrinsic and extrinsic factors, it’s not hard to believe that there is a high rate of suicidality,” Dr. Ting said.
“There have been studies that have shown that in children who are supported in their gender identity, the rates of depression and anxiety decreased to almost the same levels as non-trans and nonbinary children, so I think that gives cause for hope,” Dr. Ting added.
The study was funded in part by the Research Council of Norway through its Centres of Excellence funding scheme and by a Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. Ms. Johnstone reports consulting fees from Spectrum Waterloo and volunteer participation with the Youth Suicide Prevention Leadership Committee of Ontario. No other competing interests were declared. Dr. Ting has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender youth are significantly more likely to consider suicide and attempt it, compared with their cisgender peers, new research shows.
In a large population-based study, investigators found the increased risk of suicidality is partly because of bullying and cyberbullying experienced by transgender teens.
The findings are “extremely concerning and should be a wake-up call,” Ian Colman, PhD, with the University of Ottawa School of Epidemiology and Public Health, said in an interview.
Young people who are exploring their sexual identities may suffer from depression and anxiety, both about the reactions of their peers and families, as well as their own sense of self.
“These youth are highly marginalized and stigmatized in many corners of our society, and these findings highlight just how distressing these experiences can be,” Dr. Colman said.
The study was published online in the Canadian Medical Association Journal.
Sevenfold increased risk of attempted suicide
The risk of suicidal thoughts and actions is not well studied in transgender and nonbinary youth.
To expand the evidence base, the researchers analyzed data for 6,800 adolescents aged 15-17 years from the 2019 Canadian Health Survey on Children and Youth.
The sample included 1,130 (16.5%) adolescents who identified as having some degree of same-gender attraction, 265 (4.3%) who were unsure of their attraction (“questioning”), and 50 (0.6%) who were transgender, meaning they identified as being of a gender different from that assigned at birth.
Overall, 980 (14.0%) adolescents reported having thoughts of suicide in the prior year, and 480 (6.8%) had attempted suicide in their life.
Transgender youth were five times more likely to think about suicide and more than seven times more likely to have ever attempted suicide than cisgender, heterosexual peers.
Among cisgender adolescents, girls who were attracted to girls had 3.6 times the risk of suicidal ideation and 3.3 times the risk of having ever attempted suicide, compared with their heterosexual peers.
Teens attracted to multiple genders had more than twice the risk of suicidal ideation and suicide attempt. Youth who were questioning their sexual orientation had twice the risk of having attempted suicide in their lifetime.

A crisis – with reason for hope
“This is a crisis, and it shows just how much more needs to be done to support transgender young people,” co-author Fae Johnstone, MSW, executive director, Wisdom2Action, who is a trans woman herself, said in the news release.
“Suicide prevention programs specifically targeted to transgender, nonbinary, and sexual minority adolescents, as well as gender-affirming care for transgender adolescents, may help reduce the burden of suicidality among this group,” Ms. Johnstone added.
“The most important thing that parents, teachers, and health care providers can do is to be supportive of these youth,” Dr. Colman told this news organization.
“Providing a safe place where gender and sexual minorities can explore and express themselves is crucial. The first step is to listen and to be compassionate,” Dr. Colman added.
Reached for comment, Jess Ting, MD, director of surgery at the Mount Sinai Center for Transgender Medicine and Surgery, New York, said the data from this study on suicidal thoughts and actions among sexual minority and transgender adolescents “mirror what we see and what we know” about suicidality in trans and nonbinary adults.
“The reasons for this are complex, and it’s hard for someone who doesn’t have a lived experience as a trans or nonbinary person to understand the reasons for suicidality,” he told this news organization.
“But we also know that there are higher rates of anxiety and depression and self-image issues and posttraumatic stress disorder, not to mention outside factors – marginalization, discrimination, violence, abuse. When you add up all these intrinsic and extrinsic factors, it’s not hard to believe that there is a high rate of suicidality,” Dr. Ting said.
“There have been studies that have shown that in children who are supported in their gender identity, the rates of depression and anxiety decreased to almost the same levels as non-trans and nonbinary children, so I think that gives cause for hope,” Dr. Ting added.
The study was funded in part by the Research Council of Norway through its Centres of Excellence funding scheme and by a Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. Ms. Johnstone reports consulting fees from Spectrum Waterloo and volunteer participation with the Youth Suicide Prevention Leadership Committee of Ontario. No other competing interests were declared. Dr. Ting has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
New studies show growing number of trans, nonbinary youth in U.S.
Two new studies point to an ever-increasing number of young people in the United States who identify as transgender and nonbinary, with the figures doubling among 18- to 24-year-olds in one institute’s research – from 0.66% of the population in 2016 to 1.3% (398,900) in 2022.
In addition, 1.4% (300,100) of 13- to 17-year-olds identify as trans or nonbinary, according to the report from that group, the Williams Institute at the University of California, Los Angeles, School of Law.
Williams, which conducts independent research on sexual orientation and gender identity law and public policy, did not contain data on 13- to 17-year-olds in its 2016 study, so the growth in that group over the past 5+ years is not as well documented.
Overall, some 1.6 million Americans older than age 13 now identify as transgender, reported the Williams researchers.
And in a new Pew Research Center survey, 2% of adults aged 18-29 identify as transgender and 3% identify as nonbinary, a far greater number than in other age cohorts.
These reports are likely underestimates. The Human Rights Campaign estimates that some 2 million Americans of all ages identify as transgender.
The Pew survey is weighted to be representative but still has limitations, said the organization. The Williams analysis, based on responses to two CDC surveys – the Behavioral Risk Factor Surveillance System (BRFSS) and Youth Risk Behavior Survey (YRBS) – is incomplete, say researchers, because not every state collects data on gender identity.
Transgender identities more predominant among youth
The Williams researchers report that 18.3% of those who identified as trans were 13- to 17-year-olds; that age group makes up 7.6% of the United States population 13 and older.
And despite not having firm figures from earlier reports, they comment: “Youth ages 13-17 comprise a larger share of the transgender-identified population than we previously estimated, currently comprising about 18% of the transgender-identified population in the United State, up from 10% previously.”
About one-quarter of those who identified as trans in the new 2022 report were aged 18-24; that age cohort accounts for 11% of Americans.
The number of older Americans who identify as trans are more proportionate to their representation in the population, according to Williams. Overall, about half of those who said they were trans were aged 25-64; that group accounts for 62% of the overall American population. Some 10% of trans-identified individuals were over age 65. About 20% of Americans are 65 or older, said the researchers.
The Pew research – based on the responses of 10,188 individuals surveyed in May – also found growing numbers of young people who identify as trans. “The share of U.S. adults who are transgender is particularly high among adults younger than 25,” reported Pew in a blog post.
In the 18- to 25-year-old group, 3.1% identified as a trans man or a trans woman, compared with just 0.5% of those ages 25-29.
That compares to 0.3% of those aged 30-49 and 0.2% of those older than 50.
Racial and state-by-state variation
Similar percentages of youth aged 13-17 of all races and ethnicities in the Williams study report they are transgender, ranging from 1% of those who are Asian, to 1.3% of White youth, 1.4% of Black youth, 1.8% of American Indian or Alaska Native, and 1.8% of Latinx youth. The institute reported that 1.5% of biracial and multiracial youth identified as transgender.
The researchers said, however, that “transgender-identified youth and adults appear more likely to report being Latinx and less likely to report being White, as compared to the United States population.”
Transgender individuals live in every state, with the greatest percentage of both youth and adults in the Northeast and West, and lesser percentages in the Midwest and South, reported the Williams Institute.
Williams estimates as many as 3% of 13- to 17-year-olds in New York identify as trans, while just 0.6% of that age group in Wyoming is transgender. A total of 2%-2.5% of those aged 13-17 are transgender in Hawaii, New Mexico, Maryland, and Washington, D.C.
Among the states with higher percentages of trans-identifying 18- to 24-year-olds: Arizona (1.9%), Arkansas (3.6%), Colorado (2%), Delaware (2.4%), Illinois (1.9%), Maryland (1.9%), North Carolina (2.5%), Oklahoma (2.5%), Massachusetts (2.3%), Rhode Island (2.1%), and Washington (2%).
A version of this article first appeared on Medscape.com.
Two new studies point to an ever-increasing number of young people in the United States who identify as transgender and nonbinary, with the figures doubling among 18- to 24-year-olds in one institute’s research – from 0.66% of the population in 2016 to 1.3% (398,900) in 2022.
In addition, 1.4% (300,100) of 13- to 17-year-olds identify as trans or nonbinary, according to the report from that group, the Williams Institute at the University of California, Los Angeles, School of Law.
Williams, which conducts independent research on sexual orientation and gender identity law and public policy, did not contain data on 13- to 17-year-olds in its 2016 study, so the growth in that group over the past 5+ years is not as well documented.
Overall, some 1.6 million Americans older than age 13 now identify as transgender, reported the Williams researchers.
And in a new Pew Research Center survey, 2% of adults aged 18-29 identify as transgender and 3% identify as nonbinary, a far greater number than in other age cohorts.
These reports are likely underestimates. The Human Rights Campaign estimates that some 2 million Americans of all ages identify as transgender.
The Pew survey is weighted to be representative but still has limitations, said the organization. The Williams analysis, based on responses to two CDC surveys – the Behavioral Risk Factor Surveillance System (BRFSS) and Youth Risk Behavior Survey (YRBS) – is incomplete, say researchers, because not every state collects data on gender identity.
Transgender identities more predominant among youth
The Williams researchers report that 18.3% of those who identified as trans were 13- to 17-year-olds; that age group makes up 7.6% of the United States population 13 and older.
And despite not having firm figures from earlier reports, they comment: “Youth ages 13-17 comprise a larger share of the transgender-identified population than we previously estimated, currently comprising about 18% of the transgender-identified population in the United State, up from 10% previously.”
About one-quarter of those who identified as trans in the new 2022 report were aged 18-24; that age cohort accounts for 11% of Americans.
The number of older Americans who identify as trans are more proportionate to their representation in the population, according to Williams. Overall, about half of those who said they were trans were aged 25-64; that group accounts for 62% of the overall American population. Some 10% of trans-identified individuals were over age 65. About 20% of Americans are 65 or older, said the researchers.
The Pew research – based on the responses of 10,188 individuals surveyed in May – also found growing numbers of young people who identify as trans. “The share of U.S. adults who are transgender is particularly high among adults younger than 25,” reported Pew in a blog post.
In the 18- to 25-year-old group, 3.1% identified as a trans man or a trans woman, compared with just 0.5% of those ages 25-29.
That compares to 0.3% of those aged 30-49 and 0.2% of those older than 50.
Racial and state-by-state variation
Similar percentages of youth aged 13-17 of all races and ethnicities in the Williams study report they are transgender, ranging from 1% of those who are Asian, to 1.3% of White youth, 1.4% of Black youth, 1.8% of American Indian or Alaska Native, and 1.8% of Latinx youth. The institute reported that 1.5% of biracial and multiracial youth identified as transgender.
The researchers said, however, that “transgender-identified youth and adults appear more likely to report being Latinx and less likely to report being White, as compared to the United States population.”
Transgender individuals live in every state, with the greatest percentage of both youth and adults in the Northeast and West, and lesser percentages in the Midwest and South, reported the Williams Institute.
Williams estimates as many as 3% of 13- to 17-year-olds in New York identify as trans, while just 0.6% of that age group in Wyoming is transgender. A total of 2%-2.5% of those aged 13-17 are transgender in Hawaii, New Mexico, Maryland, and Washington, D.C.
Among the states with higher percentages of trans-identifying 18- to 24-year-olds: Arizona (1.9%), Arkansas (3.6%), Colorado (2%), Delaware (2.4%), Illinois (1.9%), Maryland (1.9%), North Carolina (2.5%), Oklahoma (2.5%), Massachusetts (2.3%), Rhode Island (2.1%), and Washington (2%).
A version of this article first appeared on Medscape.com.
Two new studies point to an ever-increasing number of young people in the United States who identify as transgender and nonbinary, with the figures doubling among 18- to 24-year-olds in one institute’s research – from 0.66% of the population in 2016 to 1.3% (398,900) in 2022.
In addition, 1.4% (300,100) of 13- to 17-year-olds identify as trans or nonbinary, according to the report from that group, the Williams Institute at the University of California, Los Angeles, School of Law.
Williams, which conducts independent research on sexual orientation and gender identity law and public policy, did not contain data on 13- to 17-year-olds in its 2016 study, so the growth in that group over the past 5+ years is not as well documented.
Overall, some 1.6 million Americans older than age 13 now identify as transgender, reported the Williams researchers.
And in a new Pew Research Center survey, 2% of adults aged 18-29 identify as transgender and 3% identify as nonbinary, a far greater number than in other age cohorts.
These reports are likely underestimates. The Human Rights Campaign estimates that some 2 million Americans of all ages identify as transgender.
The Pew survey is weighted to be representative but still has limitations, said the organization. The Williams analysis, based on responses to two CDC surveys – the Behavioral Risk Factor Surveillance System (BRFSS) and Youth Risk Behavior Survey (YRBS) – is incomplete, say researchers, because not every state collects data on gender identity.
Transgender identities more predominant among youth
The Williams researchers report that 18.3% of those who identified as trans were 13- to 17-year-olds; that age group makes up 7.6% of the United States population 13 and older.
And despite not having firm figures from earlier reports, they comment: “Youth ages 13-17 comprise a larger share of the transgender-identified population than we previously estimated, currently comprising about 18% of the transgender-identified population in the United State, up from 10% previously.”
About one-quarter of those who identified as trans in the new 2022 report were aged 18-24; that age cohort accounts for 11% of Americans.
The number of older Americans who identify as trans are more proportionate to their representation in the population, according to Williams. Overall, about half of those who said they were trans were aged 25-64; that group accounts for 62% of the overall American population. Some 10% of trans-identified individuals were over age 65. About 20% of Americans are 65 or older, said the researchers.
The Pew research – based on the responses of 10,188 individuals surveyed in May – also found growing numbers of young people who identify as trans. “The share of U.S. adults who are transgender is particularly high among adults younger than 25,” reported Pew in a blog post.
In the 18- to 25-year-old group, 3.1% identified as a trans man or a trans woman, compared with just 0.5% of those ages 25-29.
That compares to 0.3% of those aged 30-49 and 0.2% of those older than 50.
Racial and state-by-state variation
Similar percentages of youth aged 13-17 of all races and ethnicities in the Williams study report they are transgender, ranging from 1% of those who are Asian, to 1.3% of White youth, 1.4% of Black youth, 1.8% of American Indian or Alaska Native, and 1.8% of Latinx youth. The institute reported that 1.5% of biracial and multiracial youth identified as transgender.
The researchers said, however, that “transgender-identified youth and adults appear more likely to report being Latinx and less likely to report being White, as compared to the United States population.”
Transgender individuals live in every state, with the greatest percentage of both youth and adults in the Northeast and West, and lesser percentages in the Midwest and South, reported the Williams Institute.
Williams estimates as many as 3% of 13- to 17-year-olds in New York identify as trans, while just 0.6% of that age group in Wyoming is transgender. A total of 2%-2.5% of those aged 13-17 are transgender in Hawaii, New Mexico, Maryland, and Washington, D.C.
Among the states with higher percentages of trans-identifying 18- to 24-year-olds: Arizona (1.9%), Arkansas (3.6%), Colorado (2%), Delaware (2.4%), Illinois (1.9%), Maryland (1.9%), North Carolina (2.5%), Oklahoma (2.5%), Massachusetts (2.3%), Rhode Island (2.1%), and Washington (2%).
A version of this article first appeared on Medscape.com.
Physician convicted in $10M veteran insurance fraud scheme
A federal jury found Alexander, Ark., family physician Joe David May, MD, known locally as “Jay,” guilty on all 22 counts for which he was indicted in a multi-million-dollar conspiracy to defraud TRICARE, the federal insurance program for U.S. veterans.
. A pharmacy promoter paid recruiters to find TRICARE beneficiaries, then paid Dr. May and other medical professionals to rubber-stamp prescriptions for pain cream, whether or not the patients needed it.
As a result of this scheme, TRICARE paid more than $12 million for compounded drugs. Dr. May, according to evidence presented at the trial, wrote 226 prescriptions over the course of 10 months, costing TRICARE $4.63 million. All but one of these prescriptions were supplied by pharmaceutical sales representatives Glenn Hudson and Derek Clifton, according to federal officials. The sales reps passed the prescriptions to the providers, including Dr. May.
Dr. May accepted $15,000 in cash bribes and signed off on the prescriptions without consulting or examining patients to determine whether or not the prescriptions were needed. Mr. Hudson and Mr. Clifton have pleaded guilty in the scheme, according to the U.S. Department of Justice.
The conspiracy was complex and involved recruiting patients. According to prosecutors, one recruiter, for example, hosted a meeting at the Fisher Armory in North Little Rock, Ark., where he signed up patients for the drugs and offered to pay them $1,000. Thirteen of the patients from this meeting were sent to Dr. May, who signed the prescriptions. This group alone cost the veterans’ insurance program $370,000, say prosecutors. When the conspirators learned that reimbursements from TRICARE were set to decrease in May 2015, they rushed to profit from the scheme while there was still time. In April 2015 alone, Dr. May signed 59 prescriptions, contributing to the bilking of TRICARE for another $1.4 million.
According to a report in the Arkansas Democrat Gazette, Dr. May and Mr. Clifton, a former basketball coach, were longtime friends. Alexander Morgan, U.S. Attorney and one of the prosecutors in the case, said that Dr. May signed the prescriptions at the behest of Mr. Clifton. According to the Gazette report, Mr. Morgan said the scheme was successful for as long as it was because “TRICARE is not in the business of catching fraud. Tricare is in the business of delivering health care to our nation’s veterans. They trust the professionals to do the right thing.”
Dr. May is currently licensed in Arkansas to practice family medicine and emergency medicine and as a hospitalist. His license expires in September.
Dr. May is free pending a presentencing report. In addition to sentences imposed for fraud, mail fraud, falsifying records, and violation of anti kickback laws, for which he faces up to 20 years in prison, Dr. May will serve an additional 4 years for convictions on two counts of aggravated identity theft.
A version of this article first appeared on Medscape.com.
A federal jury found Alexander, Ark., family physician Joe David May, MD, known locally as “Jay,” guilty on all 22 counts for which he was indicted in a multi-million-dollar conspiracy to defraud TRICARE, the federal insurance program for U.S. veterans.
. A pharmacy promoter paid recruiters to find TRICARE beneficiaries, then paid Dr. May and other medical professionals to rubber-stamp prescriptions for pain cream, whether or not the patients needed it.
As a result of this scheme, TRICARE paid more than $12 million for compounded drugs. Dr. May, according to evidence presented at the trial, wrote 226 prescriptions over the course of 10 months, costing TRICARE $4.63 million. All but one of these prescriptions were supplied by pharmaceutical sales representatives Glenn Hudson and Derek Clifton, according to federal officials. The sales reps passed the prescriptions to the providers, including Dr. May.
Dr. May accepted $15,000 in cash bribes and signed off on the prescriptions without consulting or examining patients to determine whether or not the prescriptions were needed. Mr. Hudson and Mr. Clifton have pleaded guilty in the scheme, according to the U.S. Department of Justice.
The conspiracy was complex and involved recruiting patients. According to prosecutors, one recruiter, for example, hosted a meeting at the Fisher Armory in North Little Rock, Ark., where he signed up patients for the drugs and offered to pay them $1,000. Thirteen of the patients from this meeting were sent to Dr. May, who signed the prescriptions. This group alone cost the veterans’ insurance program $370,000, say prosecutors. When the conspirators learned that reimbursements from TRICARE were set to decrease in May 2015, they rushed to profit from the scheme while there was still time. In April 2015 alone, Dr. May signed 59 prescriptions, contributing to the bilking of TRICARE for another $1.4 million.
According to a report in the Arkansas Democrat Gazette, Dr. May and Mr. Clifton, a former basketball coach, were longtime friends. Alexander Morgan, U.S. Attorney and one of the prosecutors in the case, said that Dr. May signed the prescriptions at the behest of Mr. Clifton. According to the Gazette report, Mr. Morgan said the scheme was successful for as long as it was because “TRICARE is not in the business of catching fraud. Tricare is in the business of delivering health care to our nation’s veterans. They trust the professionals to do the right thing.”
Dr. May is currently licensed in Arkansas to practice family medicine and emergency medicine and as a hospitalist. His license expires in September.
Dr. May is free pending a presentencing report. In addition to sentences imposed for fraud, mail fraud, falsifying records, and violation of anti kickback laws, for which he faces up to 20 years in prison, Dr. May will serve an additional 4 years for convictions on two counts of aggravated identity theft.
A version of this article first appeared on Medscape.com.
A federal jury found Alexander, Ark., family physician Joe David May, MD, known locally as “Jay,” guilty on all 22 counts for which he was indicted in a multi-million-dollar conspiracy to defraud TRICARE, the federal insurance program for U.S. veterans.
. A pharmacy promoter paid recruiters to find TRICARE beneficiaries, then paid Dr. May and other medical professionals to rubber-stamp prescriptions for pain cream, whether or not the patients needed it.
As a result of this scheme, TRICARE paid more than $12 million for compounded drugs. Dr. May, according to evidence presented at the trial, wrote 226 prescriptions over the course of 10 months, costing TRICARE $4.63 million. All but one of these prescriptions were supplied by pharmaceutical sales representatives Glenn Hudson and Derek Clifton, according to federal officials. The sales reps passed the prescriptions to the providers, including Dr. May.
Dr. May accepted $15,000 in cash bribes and signed off on the prescriptions without consulting or examining patients to determine whether or not the prescriptions were needed. Mr. Hudson and Mr. Clifton have pleaded guilty in the scheme, according to the U.S. Department of Justice.
The conspiracy was complex and involved recruiting patients. According to prosecutors, one recruiter, for example, hosted a meeting at the Fisher Armory in North Little Rock, Ark., where he signed up patients for the drugs and offered to pay them $1,000. Thirteen of the patients from this meeting were sent to Dr. May, who signed the prescriptions. This group alone cost the veterans’ insurance program $370,000, say prosecutors. When the conspirators learned that reimbursements from TRICARE were set to decrease in May 2015, they rushed to profit from the scheme while there was still time. In April 2015 alone, Dr. May signed 59 prescriptions, contributing to the bilking of TRICARE for another $1.4 million.
According to a report in the Arkansas Democrat Gazette, Dr. May and Mr. Clifton, a former basketball coach, were longtime friends. Alexander Morgan, U.S. Attorney and one of the prosecutors in the case, said that Dr. May signed the prescriptions at the behest of Mr. Clifton. According to the Gazette report, Mr. Morgan said the scheme was successful for as long as it was because “TRICARE is not in the business of catching fraud. Tricare is in the business of delivering health care to our nation’s veterans. They trust the professionals to do the right thing.”
Dr. May is currently licensed in Arkansas to practice family medicine and emergency medicine and as a hospitalist. His license expires in September.
Dr. May is free pending a presentencing report. In addition to sentences imposed for fraud, mail fraud, falsifying records, and violation of anti kickback laws, for which he faces up to 20 years in prison, Dr. May will serve an additional 4 years for convictions on two counts of aggravated identity theft.
A version of this article first appeared on Medscape.com.
Surgery during a pandemic? COVID vaccination status matters – or not
An online survey captured mixed information about people’s willingness to undergo surgery during a viral pandemic in relation to the vaccine status of the patient and staff. The findings showcase opportunities for public education and “skillful messaging,” researchers report.
In survey scenarios that asked people to imagine their vaccination status, people were more willing to undergo surgery if it was lifesaving, rather than elective, especially if vaccinated. The prospect of no hospital stay tipped the scales further toward surgery. The vaccination status of hospital staff played only a minor role in decision making, according to the study, which was published in Vaccine.
But as a post hoc analysis revealed, it was participants who were not vaccinated against COVID-19 in real life who were more willing to undergo surgery, compared with those who had one or two shots.
In either case, too many people were unwilling to undergo lifesaving surgery, even though the risk of hospital-acquired COVID-19 is low. “Making this choice for an actual health problem would result in an unacceptably high rate of potential morbidity attributable to pandemic-related fears, the authors wrote.
In an unusual approach, the researchers used Amazon’s Mechanical Turk to electronically recruit 2,006 adults. The participants answered a 26-item survey about a hypothetical surgery in an unnamed pandemic with different combinations of vaccine status for patient and staff.
Coauthor and anesthesiologist Keith J. Ruskin, MD, of the University of Chicago, told this news organization that they “wanted to make this timeless” and independent of COVID “so that when the next thing came about, the paper would still be relevant.”
The researchers were surprised by the findings at the extreme ends of attitudes toward surgery. Some were still willing to have elective surgery with (hypothetically) unvaccinated patients and staff.
“And people at the other end, even though they are vaccinated, the hospital staff is vaccinated, and the surgery is lifesaving, they absolutely won’t have surgery,” Dr. Ruskin said.
He viewed these two groups as opportunities for education. “You can present information in the most positive light to get them to do the right thing with what’s best for themselves,” he said.
As an example, Dr. Ruskin pointed to an ad in Illinois. “It’s not only people saying I’m getting vaccinated for myself and my family, but there are people who said I got vaccinated and I still got COVID, but it could have been much worse. Please, if you’re on the fence, just get vaccinated,” he said.
Coauthor Anna Clebone Ruskin, MD, an anesthesiologist at the University of Chicago, said, “Humans are programmed to see things in extremes. With surgery, people tend to think of surgery as a monolith – surgery is all good, or surgery is all bad, where there is a huge in between. So we saw those extremes. ... Seeing that dichotomy with people on either end was pretty surprising.
“Getting surgery is not always good. Getting surgery is not always bad. It’s a risk-versus-benefit analysis and educating the public to consider the risks and benefits of medical decisions, in general, would be enormously beneficial,” she said.
A post hoc analysis found that “participants who were not actually vaccinated against COVID-19 were generally more willing to undergo surgery compared to those who had one vaccination or two vaccinations,” the authors wrote.
In a second post hoc finding, participants who reported high wariness of vaccines were generally more likely to be willing to undergo surgery. Notably, 15% of participants “were unwilling to undergo lifesaving surgery during a pandemic even when they and the health care staff were vaccinated,” the authors wrote.
Dr. Keith J. Ruskin hypothesized about this result, saying, “What we think is that potentially actually getting vaccinated against COVID-19 may indicate that you have a lower risk tolerance. So you may be less likely to do anything you perceive to be risky if you’re vaccinated against COVID-19.”
The authors stated that “the risk of hospital-acquired COVID-19 even prior to vaccination is vanishingly small.” The risk of nosocomial COVID varies among different studies. An EPIC-based study between April 2020 and October 2021 found the risk to be 1.8%; EPIC describes the fears of a patient catching COVID at a hospital as “likely unfounded.”
In the United Kingdom, the risk was as high as 24% earlier in the pandemic and then declined to approximately 5% a year ago. Omicron also brought more infections. Rates varied significantly among hospitals – and, notably, the risk of death from a nosocomial COVID infection was 21% in April-September 2020.
Emily Landon, MD, an epidemiologist and executive medical director for infection prevention and control at the University of Chicago Medicine, told this news organization that the study’s data were collected during Delta, a “time when we thought that this was a pandemic of the unvaccinated. But there was serious politicization of the vaccine.”
Dr. Landon said one of the study’s strengths was the large number of participants. A limitation was, “You’re going to have less participants who are generally poor and indigent, and fewer old participants, probably because they’re less likely to respond to an online survey.
“But the most interesting results are that people who were wary of vaccines or who hadn’t been vaccinated, were much more willing to undergo surgical procedures in the time of a pandemic, regardless of status, which reflects the fact that not being vaccinated correlates with not worrying much about COVID. Vaccinated individuals had a lot more wariness about undergoing surgical procedures during a pandemic.”
It appeared “individuals who were vaccinated in real life [were] worried about staff vaccination,” Dr. Landon noted. She concluded, “I think it supports the need for mandatory vaccinations in health care workers.”
The study has implications for hospital vaccination policies and practices. In Cumberland, Md., when COVID was high and vaccines first became available, the Maryland Hospital Association said that all health care staff should be vaccinated. The local hospital, UPMC–Western Maryland Hospital, refused.
Two months later, the local news reporter, Teresa McMinn, wrote, “While Maryland’s largest hospital systems have ‘led by example by mandating vaccines for all of their hospital staff,’ other facilities – including UPMC Western Maryland and Garrett Regional Medical Center – have taken no such action even though it’s been 8 months since vaccines were made available to health care workers.”
The hospital would not tell patients whether staff were vaccinated, either. An ongoing concern for members of the community is the lack of communication with UPMC, which erodes trust in the health system – the only hospital available in this rural community.
This vaccine study supports that the vaccination status of the staff may influence some patients’ decision on whether to have surgery.
The Ruskins and Dr. Landon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An online survey captured mixed information about people’s willingness to undergo surgery during a viral pandemic in relation to the vaccine status of the patient and staff. The findings showcase opportunities for public education and “skillful messaging,” researchers report.
In survey scenarios that asked people to imagine their vaccination status, people were more willing to undergo surgery if it was lifesaving, rather than elective, especially if vaccinated. The prospect of no hospital stay tipped the scales further toward surgery. The vaccination status of hospital staff played only a minor role in decision making, according to the study, which was published in Vaccine.
But as a post hoc analysis revealed, it was participants who were not vaccinated against COVID-19 in real life who were more willing to undergo surgery, compared with those who had one or two shots.
In either case, too many people were unwilling to undergo lifesaving surgery, even though the risk of hospital-acquired COVID-19 is low. “Making this choice for an actual health problem would result in an unacceptably high rate of potential morbidity attributable to pandemic-related fears, the authors wrote.
In an unusual approach, the researchers used Amazon’s Mechanical Turk to electronically recruit 2,006 adults. The participants answered a 26-item survey about a hypothetical surgery in an unnamed pandemic with different combinations of vaccine status for patient and staff.
Coauthor and anesthesiologist Keith J. Ruskin, MD, of the University of Chicago, told this news organization that they “wanted to make this timeless” and independent of COVID “so that when the next thing came about, the paper would still be relevant.”
The researchers were surprised by the findings at the extreme ends of attitudes toward surgery. Some were still willing to have elective surgery with (hypothetically) unvaccinated patients and staff.
“And people at the other end, even though they are vaccinated, the hospital staff is vaccinated, and the surgery is lifesaving, they absolutely won’t have surgery,” Dr. Ruskin said.
He viewed these two groups as opportunities for education. “You can present information in the most positive light to get them to do the right thing with what’s best for themselves,” he said.
As an example, Dr. Ruskin pointed to an ad in Illinois. “It’s not only people saying I’m getting vaccinated for myself and my family, but there are people who said I got vaccinated and I still got COVID, but it could have been much worse. Please, if you’re on the fence, just get vaccinated,” he said.
Coauthor Anna Clebone Ruskin, MD, an anesthesiologist at the University of Chicago, said, “Humans are programmed to see things in extremes. With surgery, people tend to think of surgery as a monolith – surgery is all good, or surgery is all bad, where there is a huge in between. So we saw those extremes. ... Seeing that dichotomy with people on either end was pretty surprising.
“Getting surgery is not always good. Getting surgery is not always bad. It’s a risk-versus-benefit analysis and educating the public to consider the risks and benefits of medical decisions, in general, would be enormously beneficial,” she said.
A post hoc analysis found that “participants who were not actually vaccinated against COVID-19 were generally more willing to undergo surgery compared to those who had one vaccination or two vaccinations,” the authors wrote.
In a second post hoc finding, participants who reported high wariness of vaccines were generally more likely to be willing to undergo surgery. Notably, 15% of participants “were unwilling to undergo lifesaving surgery during a pandemic even when they and the health care staff were vaccinated,” the authors wrote.
Dr. Keith J. Ruskin hypothesized about this result, saying, “What we think is that potentially actually getting vaccinated against COVID-19 may indicate that you have a lower risk tolerance. So you may be less likely to do anything you perceive to be risky if you’re vaccinated against COVID-19.”
The authors stated that “the risk of hospital-acquired COVID-19 even prior to vaccination is vanishingly small.” The risk of nosocomial COVID varies among different studies. An EPIC-based study between April 2020 and October 2021 found the risk to be 1.8%; EPIC describes the fears of a patient catching COVID at a hospital as “likely unfounded.”
In the United Kingdom, the risk was as high as 24% earlier in the pandemic and then declined to approximately 5% a year ago. Omicron also brought more infections. Rates varied significantly among hospitals – and, notably, the risk of death from a nosocomial COVID infection was 21% in April-September 2020.
Emily Landon, MD, an epidemiologist and executive medical director for infection prevention and control at the University of Chicago Medicine, told this news organization that the study’s data were collected during Delta, a “time when we thought that this was a pandemic of the unvaccinated. But there was serious politicization of the vaccine.”
Dr. Landon said one of the study’s strengths was the large number of participants. A limitation was, “You’re going to have less participants who are generally poor and indigent, and fewer old participants, probably because they’re less likely to respond to an online survey.
“But the most interesting results are that people who were wary of vaccines or who hadn’t been vaccinated, were much more willing to undergo surgical procedures in the time of a pandemic, regardless of status, which reflects the fact that not being vaccinated correlates with not worrying much about COVID. Vaccinated individuals had a lot more wariness about undergoing surgical procedures during a pandemic.”
It appeared “individuals who were vaccinated in real life [were] worried about staff vaccination,” Dr. Landon noted. She concluded, “I think it supports the need for mandatory vaccinations in health care workers.”
The study has implications for hospital vaccination policies and practices. In Cumberland, Md., when COVID was high and vaccines first became available, the Maryland Hospital Association said that all health care staff should be vaccinated. The local hospital, UPMC–Western Maryland Hospital, refused.
Two months later, the local news reporter, Teresa McMinn, wrote, “While Maryland’s largest hospital systems have ‘led by example by mandating vaccines for all of their hospital staff,’ other facilities – including UPMC Western Maryland and Garrett Regional Medical Center – have taken no such action even though it’s been 8 months since vaccines were made available to health care workers.”
The hospital would not tell patients whether staff were vaccinated, either. An ongoing concern for members of the community is the lack of communication with UPMC, which erodes trust in the health system – the only hospital available in this rural community.
This vaccine study supports that the vaccination status of the staff may influence some patients’ decision on whether to have surgery.
The Ruskins and Dr. Landon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An online survey captured mixed information about people’s willingness to undergo surgery during a viral pandemic in relation to the vaccine status of the patient and staff. The findings showcase opportunities for public education and “skillful messaging,” researchers report.
In survey scenarios that asked people to imagine their vaccination status, people were more willing to undergo surgery if it was lifesaving, rather than elective, especially if vaccinated. The prospect of no hospital stay tipped the scales further toward surgery. The vaccination status of hospital staff played only a minor role in decision making, according to the study, which was published in Vaccine.
But as a post hoc analysis revealed, it was participants who were not vaccinated against COVID-19 in real life who were more willing to undergo surgery, compared with those who had one or two shots.
In either case, too many people were unwilling to undergo lifesaving surgery, even though the risk of hospital-acquired COVID-19 is low. “Making this choice for an actual health problem would result in an unacceptably high rate of potential morbidity attributable to pandemic-related fears, the authors wrote.
In an unusual approach, the researchers used Amazon’s Mechanical Turk to electronically recruit 2,006 adults. The participants answered a 26-item survey about a hypothetical surgery in an unnamed pandemic with different combinations of vaccine status for patient and staff.
Coauthor and anesthesiologist Keith J. Ruskin, MD, of the University of Chicago, told this news organization that they “wanted to make this timeless” and independent of COVID “so that when the next thing came about, the paper would still be relevant.”
The researchers were surprised by the findings at the extreme ends of attitudes toward surgery. Some were still willing to have elective surgery with (hypothetically) unvaccinated patients and staff.
“And people at the other end, even though they are vaccinated, the hospital staff is vaccinated, and the surgery is lifesaving, they absolutely won’t have surgery,” Dr. Ruskin said.
He viewed these two groups as opportunities for education. “You can present information in the most positive light to get them to do the right thing with what’s best for themselves,” he said.
As an example, Dr. Ruskin pointed to an ad in Illinois. “It’s not only people saying I’m getting vaccinated for myself and my family, but there are people who said I got vaccinated and I still got COVID, but it could have been much worse. Please, if you’re on the fence, just get vaccinated,” he said.
Coauthor Anna Clebone Ruskin, MD, an anesthesiologist at the University of Chicago, said, “Humans are programmed to see things in extremes. With surgery, people tend to think of surgery as a monolith – surgery is all good, or surgery is all bad, where there is a huge in between. So we saw those extremes. ... Seeing that dichotomy with people on either end was pretty surprising.
“Getting surgery is not always good. Getting surgery is not always bad. It’s a risk-versus-benefit analysis and educating the public to consider the risks and benefits of medical decisions, in general, would be enormously beneficial,” she said.
A post hoc analysis found that “participants who were not actually vaccinated against COVID-19 were generally more willing to undergo surgery compared to those who had one vaccination or two vaccinations,” the authors wrote.
In a second post hoc finding, participants who reported high wariness of vaccines were generally more likely to be willing to undergo surgery. Notably, 15% of participants “were unwilling to undergo lifesaving surgery during a pandemic even when they and the health care staff were vaccinated,” the authors wrote.
Dr. Keith J. Ruskin hypothesized about this result, saying, “What we think is that potentially actually getting vaccinated against COVID-19 may indicate that you have a lower risk tolerance. So you may be less likely to do anything you perceive to be risky if you’re vaccinated against COVID-19.”
The authors stated that “the risk of hospital-acquired COVID-19 even prior to vaccination is vanishingly small.” The risk of nosocomial COVID varies among different studies. An EPIC-based study between April 2020 and October 2021 found the risk to be 1.8%; EPIC describes the fears of a patient catching COVID at a hospital as “likely unfounded.”
In the United Kingdom, the risk was as high as 24% earlier in the pandemic and then declined to approximately 5% a year ago. Omicron also brought more infections. Rates varied significantly among hospitals – and, notably, the risk of death from a nosocomial COVID infection was 21% in April-September 2020.
Emily Landon, MD, an epidemiologist and executive medical director for infection prevention and control at the University of Chicago Medicine, told this news organization that the study’s data were collected during Delta, a “time when we thought that this was a pandemic of the unvaccinated. But there was serious politicization of the vaccine.”
Dr. Landon said one of the study’s strengths was the large number of participants. A limitation was, “You’re going to have less participants who are generally poor and indigent, and fewer old participants, probably because they’re less likely to respond to an online survey.
“But the most interesting results are that people who were wary of vaccines or who hadn’t been vaccinated, were much more willing to undergo surgical procedures in the time of a pandemic, regardless of status, which reflects the fact that not being vaccinated correlates with not worrying much about COVID. Vaccinated individuals had a lot more wariness about undergoing surgical procedures during a pandemic.”
It appeared “individuals who were vaccinated in real life [were] worried about staff vaccination,” Dr. Landon noted. She concluded, “I think it supports the need for mandatory vaccinations in health care workers.”
The study has implications for hospital vaccination policies and practices. In Cumberland, Md., when COVID was high and vaccines first became available, the Maryland Hospital Association said that all health care staff should be vaccinated. The local hospital, UPMC–Western Maryland Hospital, refused.
Two months later, the local news reporter, Teresa McMinn, wrote, “While Maryland’s largest hospital systems have ‘led by example by mandating vaccines for all of their hospital staff,’ other facilities – including UPMC Western Maryland and Garrett Regional Medical Center – have taken no such action even though it’s been 8 months since vaccines were made available to health care workers.”
The hospital would not tell patients whether staff were vaccinated, either. An ongoing concern for members of the community is the lack of communication with UPMC, which erodes trust in the health system – the only hospital available in this rural community.
This vaccine study supports that the vaccination status of the staff may influence some patients’ decision on whether to have surgery.
The Ruskins and Dr. Landon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hormonal contraceptives protective against suicide?
Contrary to previous analyses, new research suggests.
In a study of more than 800 women younger than age 50 who attempted suicide and more than 3,000 age-matched peers, results showed those who took hormonal contraceptives had a 27% reduced risk for attempted suicide.
Further analysis showed this was confined to women without a history of psychiatric illness and the reduction in risk rose to 43% among those who took combined hormonal contraceptives rather than progestin-only versions.
The protective effect against attempted suicide increased further to 46% if ethinyl estradiol (EE)–containing preparations were used. Moreover, the beneficial effect of contraceptive use increased over time.
The main message is the “current use of hormonal contraceptives is not associated with an increased risk of attempted suicide in our population,” study presenter Elena Toffol, MD, PhD, department of public health, University of Helsinki, told meeting attendees at the European Psychiatric Association 2022 Congress.
Age range differences
Dr. Toffol said there could be “several reasons” why the results are different from those in previous studies, including that the researchers included a “larger age range.” She noted it is known that “older women have a lower rate of attempted suicide and use different types of contraceptives.”
Dr. Toffol said in an interview that, although it’s “hard to estimate any causality” because this is an observational study, it is “tempting to speculate, and it is plausible, that hormones partly play a role with some, but not all, women being more sensitive to hormonal influences.”
However, the results “may also reflect life choices or a protective life status; for example, more stable relationships or more conscious and health-focused behaviors,” she said.
“It may also be that the underlying characteristics of women who are prescribed or opt for certain types of contraceptives are somehow related to their suicidal risk,” she added.
In 2019, the global age-standardized suicide rate was 9.0 per 100,000, which translates into more than 700,000 deaths every year, Dr. Toffol noted.
However, she emphasized the World Health Organization has calculated that, for every adult who dies by suicide, more than 20 people attempt suicide. In addition, data from the U.S. Centers for Disease Control and Prevention indicate that attempted suicides are three times more common among young women than in men.
“What are the reasons for this gender gap?” Dr. Toffol asked during her presentation.
“It is known that the major risk factor for suicidal behavior is a psychiatric disorder, and in particular depression and mood disorders. And depression and mood disorders are more common in women than in men,” she said.
However, there is also “growing interest into the role of biological factors” in the risk for suicide, including hormones and hormonal contraception. Some studies have also suggested that there is an increased risk for depression and “both completed and attempted suicide” after starting hormonal contraception.
Dr. Toffol added that about 70% of European women use some form of contraception and, among Finnish women, 40% choose a hormonal contraceptive.
Nested analysis
The researchers conducted a nested case-control analysis combining 2017 national prescription data on 587,823 women aged 15-49 years with information from general and primary healthcare registers for the years 2018 to 2019.
They were able to identify 818 cases of attempted suicide among the women. These were matched 4:1 with 3,272 age-matched healthy women who acted as the control group. Use of hormonal contraceptives in the previous 180 days was determined for the whole cohort.
Among users of hormonal contraceptives, there were 344 attempted suicides in 2017, at an incidence rate of 0.59 per 1,000 person-years. This compared with 474 attempted suicides among nonusers, at an incidence rate of 0.81 per 1000 person-years.
Kaplan-Meier analysis showed there was a significant difference in rates for attempted suicide among hormonal contraceptive users versus nonusers, at an incidence rate ratio of 0.73 (P < .0001) – and the difference increased over time.
In addition, the incidence of attempted suicide decreased with increasing age, with the highest incidence rate in women aged 15-19 years (1.62 per 1,000 person-years).
Conditional logistic regression analysis that controlled for education, marital status, chronic disease, recent psychiatric hospitalization, and current use of psychotropic medication showed hormonal contraceptive use was not linked to an increased risk of attempted suicide overall, at an odds ratio of 0.79 (95% confidence interval, 0.56-1.11).
However, when they looked specifically at women without a history of psychiatric illness, the association became significant, at an OR of 0.73 for attempted suicide among hormonal contraceptive users (95% CI, 0.58-0.91), while the relationship remained nonsignificant in women with a history of psychiatric disorders.
Further analysis suggested the significant association was confined to women taking combined hormonal contraceptives, at an OR of 0.57 for suicide attempt versus nonusers (95% CI, 0.44-0.75), and those use EE-containing preparations (OR, 0.54; 95% CI, 0.40-0.73).
There was a suggestion in the data that hormonal contraceptives containing desogestrel or drospirenone alongside EE may offer the greatest reduction in attempted suicide risk, but that did not survive multivariate analysis.
Dr. Toffol also noted that they were not able to capture data on use of intrauterine devices in their analysis.
“There is a growing number of municipalities in Finland that are providing free-of-charge contraception to young women” that is often an intrauterine device, she said. The researchers hope to include these women in a future analysis.
‘Age matters’
Commenting on the findings, Alexis C. Edwards, PhD, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said the current study’s findings “made a lot of sense.” Dr. Edwards wasn’t involved with this study but conducted a previous study of 216,702 Swedish women aged 15-22 years that showed use of combination or progestin-only oral contraceptives was associated with an increased risk for suicidal behavior.
She agreed with Dr. Toffol that the “much larger age range” in the new study may have played a role in showing the opposite result.
“The trajectory that we saw if we had been able to continue following the women for longer – which we couldn’t, due to limitations of the registries – [was that] using hormonal contraceptives was going to end up being protective, so I do think that it matters what age you’re looking at,” she said.
Dr. Edwards noted the takeaway from both studies “is that, even if there is a slight increase in risk from using hormonal contraceptives, it’s short lived and it’s probably specific to young women, which is important.”
She suggested the hormonal benefit from extended contraceptive use could come from the regulation of mood, as it offers a “more stable hormonal course than what their body might be putting them through in the absence of using the pill.”
Overall, it is “really lovely to see very well-executed studies on this, providing more empirical evidence on this question, because it is something that’s relevant to anyone who’s potentially going to be using hormonal contraception,” Dr. Edwards said.
Clinical implications?
Andrea Fiorillo, MD, PhD, department of psychiatry, University of Campania “Luigi Vanvitelli,” Naples, Italy, said in a press release that the “striking” findings of the current study need “careful evaluation.”
They also need to be replicated in “different cohorts of women and controlled for the impact of several psychosocial stressors, such as economic upheavals, social insecurity, and uncertainty due to the COVID pandemic,” said Dr. Fiorillo, who was not involved with the research.
Nevertheless, she believes the “clinical implications of the study are obvious and may help to destigmatize the use of hormonal contraceptives.”
The study was funded by the Jane and Aatos Erkko Foundation, the Avohoidon Tsukimis äätiö (Foundation for Primary Care Research), the Yrj ö Jahnsson Foundation, and the Finnish Cultural Foundation. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Contrary to previous analyses, new research suggests.
In a study of more than 800 women younger than age 50 who attempted suicide and more than 3,000 age-matched peers, results showed those who took hormonal contraceptives had a 27% reduced risk for attempted suicide.
Further analysis showed this was confined to women without a history of psychiatric illness and the reduction in risk rose to 43% among those who took combined hormonal contraceptives rather than progestin-only versions.
The protective effect against attempted suicide increased further to 46% if ethinyl estradiol (EE)–containing preparations were used. Moreover, the beneficial effect of contraceptive use increased over time.
The main message is the “current use of hormonal contraceptives is not associated with an increased risk of attempted suicide in our population,” study presenter Elena Toffol, MD, PhD, department of public health, University of Helsinki, told meeting attendees at the European Psychiatric Association 2022 Congress.
Age range differences
Dr. Toffol said there could be “several reasons” why the results are different from those in previous studies, including that the researchers included a “larger age range.” She noted it is known that “older women have a lower rate of attempted suicide and use different types of contraceptives.”
Dr. Toffol said in an interview that, although it’s “hard to estimate any causality” because this is an observational study, it is “tempting to speculate, and it is plausible, that hormones partly play a role with some, but not all, women being more sensitive to hormonal influences.”
However, the results “may also reflect life choices or a protective life status; for example, more stable relationships or more conscious and health-focused behaviors,” she said.
“It may also be that the underlying characteristics of women who are prescribed or opt for certain types of contraceptives are somehow related to their suicidal risk,” she added.
In 2019, the global age-standardized suicide rate was 9.0 per 100,000, which translates into more than 700,000 deaths every year, Dr. Toffol noted.
However, she emphasized the World Health Organization has calculated that, for every adult who dies by suicide, more than 20 people attempt suicide. In addition, data from the U.S. Centers for Disease Control and Prevention indicate that attempted suicides are three times more common among young women than in men.
“What are the reasons for this gender gap?” Dr. Toffol asked during her presentation.
“It is known that the major risk factor for suicidal behavior is a psychiatric disorder, and in particular depression and mood disorders. And depression and mood disorders are more common in women than in men,” she said.
However, there is also “growing interest into the role of biological factors” in the risk for suicide, including hormones and hormonal contraception. Some studies have also suggested that there is an increased risk for depression and “both completed and attempted suicide” after starting hormonal contraception.
Dr. Toffol added that about 70% of European women use some form of contraception and, among Finnish women, 40% choose a hormonal contraceptive.
Nested analysis
The researchers conducted a nested case-control analysis combining 2017 national prescription data on 587,823 women aged 15-49 years with information from general and primary healthcare registers for the years 2018 to 2019.
They were able to identify 818 cases of attempted suicide among the women. These were matched 4:1 with 3,272 age-matched healthy women who acted as the control group. Use of hormonal contraceptives in the previous 180 days was determined for the whole cohort.
Among users of hormonal contraceptives, there were 344 attempted suicides in 2017, at an incidence rate of 0.59 per 1,000 person-years. This compared with 474 attempted suicides among nonusers, at an incidence rate of 0.81 per 1000 person-years.
Kaplan-Meier analysis showed there was a significant difference in rates for attempted suicide among hormonal contraceptive users versus nonusers, at an incidence rate ratio of 0.73 (P < .0001) – and the difference increased over time.
In addition, the incidence of attempted suicide decreased with increasing age, with the highest incidence rate in women aged 15-19 years (1.62 per 1,000 person-years).
Conditional logistic regression analysis that controlled for education, marital status, chronic disease, recent psychiatric hospitalization, and current use of psychotropic medication showed hormonal contraceptive use was not linked to an increased risk of attempted suicide overall, at an odds ratio of 0.79 (95% confidence interval, 0.56-1.11).
However, when they looked specifically at women without a history of psychiatric illness, the association became significant, at an OR of 0.73 for attempted suicide among hormonal contraceptive users (95% CI, 0.58-0.91), while the relationship remained nonsignificant in women with a history of psychiatric disorders.
Further analysis suggested the significant association was confined to women taking combined hormonal contraceptives, at an OR of 0.57 for suicide attempt versus nonusers (95% CI, 0.44-0.75), and those use EE-containing preparations (OR, 0.54; 95% CI, 0.40-0.73).
There was a suggestion in the data that hormonal contraceptives containing desogestrel or drospirenone alongside EE may offer the greatest reduction in attempted suicide risk, but that did not survive multivariate analysis.
Dr. Toffol also noted that they were not able to capture data on use of intrauterine devices in their analysis.
“There is a growing number of municipalities in Finland that are providing free-of-charge contraception to young women” that is often an intrauterine device, she said. The researchers hope to include these women in a future analysis.
‘Age matters’
Commenting on the findings, Alexis C. Edwards, PhD, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said the current study’s findings “made a lot of sense.” Dr. Edwards wasn’t involved with this study but conducted a previous study of 216,702 Swedish women aged 15-22 years that showed use of combination or progestin-only oral contraceptives was associated with an increased risk for suicidal behavior.
She agreed with Dr. Toffol that the “much larger age range” in the new study may have played a role in showing the opposite result.
“The trajectory that we saw if we had been able to continue following the women for longer – which we couldn’t, due to limitations of the registries – [was that] using hormonal contraceptives was going to end up being protective, so I do think that it matters what age you’re looking at,” she said.
Dr. Edwards noted the takeaway from both studies “is that, even if there is a slight increase in risk from using hormonal contraceptives, it’s short lived and it’s probably specific to young women, which is important.”
She suggested the hormonal benefit from extended contraceptive use could come from the regulation of mood, as it offers a “more stable hormonal course than what their body might be putting them through in the absence of using the pill.”
Overall, it is “really lovely to see very well-executed studies on this, providing more empirical evidence on this question, because it is something that’s relevant to anyone who’s potentially going to be using hormonal contraception,” Dr. Edwards said.
Clinical implications?
Andrea Fiorillo, MD, PhD, department of psychiatry, University of Campania “Luigi Vanvitelli,” Naples, Italy, said in a press release that the “striking” findings of the current study need “careful evaluation.”
They also need to be replicated in “different cohorts of women and controlled for the impact of several psychosocial stressors, such as economic upheavals, social insecurity, and uncertainty due to the COVID pandemic,” said Dr. Fiorillo, who was not involved with the research.
Nevertheless, she believes the “clinical implications of the study are obvious and may help to destigmatize the use of hormonal contraceptives.”
The study was funded by the Jane and Aatos Erkko Foundation, the Avohoidon Tsukimis äätiö (Foundation for Primary Care Research), the Yrj ö Jahnsson Foundation, and the Finnish Cultural Foundation. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Contrary to previous analyses, new research suggests.
In a study of more than 800 women younger than age 50 who attempted suicide and more than 3,000 age-matched peers, results showed those who took hormonal contraceptives had a 27% reduced risk for attempted suicide.
Further analysis showed this was confined to women without a history of psychiatric illness and the reduction in risk rose to 43% among those who took combined hormonal contraceptives rather than progestin-only versions.
The protective effect against attempted suicide increased further to 46% if ethinyl estradiol (EE)–containing preparations were used. Moreover, the beneficial effect of contraceptive use increased over time.
The main message is the “current use of hormonal contraceptives is not associated with an increased risk of attempted suicide in our population,” study presenter Elena Toffol, MD, PhD, department of public health, University of Helsinki, told meeting attendees at the European Psychiatric Association 2022 Congress.
Age range differences
Dr. Toffol said there could be “several reasons” why the results are different from those in previous studies, including that the researchers included a “larger age range.” She noted it is known that “older women have a lower rate of attempted suicide and use different types of contraceptives.”
Dr. Toffol said in an interview that, although it’s “hard to estimate any causality” because this is an observational study, it is “tempting to speculate, and it is plausible, that hormones partly play a role with some, but not all, women being more sensitive to hormonal influences.”
However, the results “may also reflect life choices or a protective life status; for example, more stable relationships or more conscious and health-focused behaviors,” she said.
“It may also be that the underlying characteristics of women who are prescribed or opt for certain types of contraceptives are somehow related to their suicidal risk,” she added.
In 2019, the global age-standardized suicide rate was 9.0 per 100,000, which translates into more than 700,000 deaths every year, Dr. Toffol noted.
However, she emphasized the World Health Organization has calculated that, for every adult who dies by suicide, more than 20 people attempt suicide. In addition, data from the U.S. Centers for Disease Control and Prevention indicate that attempted suicides are three times more common among young women than in men.
“What are the reasons for this gender gap?” Dr. Toffol asked during her presentation.
“It is known that the major risk factor for suicidal behavior is a psychiatric disorder, and in particular depression and mood disorders. And depression and mood disorders are more common in women than in men,” she said.
However, there is also “growing interest into the role of biological factors” in the risk for suicide, including hormones and hormonal contraception. Some studies have also suggested that there is an increased risk for depression and “both completed and attempted suicide” after starting hormonal contraception.
Dr. Toffol added that about 70% of European women use some form of contraception and, among Finnish women, 40% choose a hormonal contraceptive.
Nested analysis
The researchers conducted a nested case-control analysis combining 2017 national prescription data on 587,823 women aged 15-49 years with information from general and primary healthcare registers for the years 2018 to 2019.
They were able to identify 818 cases of attempted suicide among the women. These were matched 4:1 with 3,272 age-matched healthy women who acted as the control group. Use of hormonal contraceptives in the previous 180 days was determined for the whole cohort.
Among users of hormonal contraceptives, there were 344 attempted suicides in 2017, at an incidence rate of 0.59 per 1,000 person-years. This compared with 474 attempted suicides among nonusers, at an incidence rate of 0.81 per 1000 person-years.
Kaplan-Meier analysis showed there was a significant difference in rates for attempted suicide among hormonal contraceptive users versus nonusers, at an incidence rate ratio of 0.73 (P < .0001) – and the difference increased over time.
In addition, the incidence of attempted suicide decreased with increasing age, with the highest incidence rate in women aged 15-19 years (1.62 per 1,000 person-years).
Conditional logistic regression analysis that controlled for education, marital status, chronic disease, recent psychiatric hospitalization, and current use of psychotropic medication showed hormonal contraceptive use was not linked to an increased risk of attempted suicide overall, at an odds ratio of 0.79 (95% confidence interval, 0.56-1.11).
However, when they looked specifically at women without a history of psychiatric illness, the association became significant, at an OR of 0.73 for attempted suicide among hormonal contraceptive users (95% CI, 0.58-0.91), while the relationship remained nonsignificant in women with a history of psychiatric disorders.
Further analysis suggested the significant association was confined to women taking combined hormonal contraceptives, at an OR of 0.57 for suicide attempt versus nonusers (95% CI, 0.44-0.75), and those use EE-containing preparations (OR, 0.54; 95% CI, 0.40-0.73).
There was a suggestion in the data that hormonal contraceptives containing desogestrel or drospirenone alongside EE may offer the greatest reduction in attempted suicide risk, but that did not survive multivariate analysis.
Dr. Toffol also noted that they were not able to capture data on use of intrauterine devices in their analysis.
“There is a growing number of municipalities in Finland that are providing free-of-charge contraception to young women” that is often an intrauterine device, she said. The researchers hope to include these women in a future analysis.
‘Age matters’
Commenting on the findings, Alexis C. Edwards, PhD, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said the current study’s findings “made a lot of sense.” Dr. Edwards wasn’t involved with this study but conducted a previous study of 216,702 Swedish women aged 15-22 years that showed use of combination or progestin-only oral contraceptives was associated with an increased risk for suicidal behavior.
She agreed with Dr. Toffol that the “much larger age range” in the new study may have played a role in showing the opposite result.
“The trajectory that we saw if we had been able to continue following the women for longer – which we couldn’t, due to limitations of the registries – [was that] using hormonal contraceptives was going to end up being protective, so I do think that it matters what age you’re looking at,” she said.
Dr. Edwards noted the takeaway from both studies “is that, even if there is a slight increase in risk from using hormonal contraceptives, it’s short lived and it’s probably specific to young women, which is important.”
She suggested the hormonal benefit from extended contraceptive use could come from the regulation of mood, as it offers a “more stable hormonal course than what their body might be putting them through in the absence of using the pill.”
Overall, it is “really lovely to see very well-executed studies on this, providing more empirical evidence on this question, because it is something that’s relevant to anyone who’s potentially going to be using hormonal contraception,” Dr. Edwards said.
Clinical implications?
Andrea Fiorillo, MD, PhD, department of psychiatry, University of Campania “Luigi Vanvitelli,” Naples, Italy, said in a press release that the “striking” findings of the current study need “careful evaluation.”
They also need to be replicated in “different cohorts of women and controlled for the impact of several psychosocial stressors, such as economic upheavals, social insecurity, and uncertainty due to the COVID pandemic,” said Dr. Fiorillo, who was not involved with the research.
Nevertheless, she believes the “clinical implications of the study are obvious and may help to destigmatize the use of hormonal contraceptives.”
The study was funded by the Jane and Aatos Erkko Foundation, the Avohoidon Tsukimis äätiö (Foundation for Primary Care Research), the Yrj ö Jahnsson Foundation, and the Finnish Cultural Foundation. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM EPA 2022
Cause of death in pig heart recipient: New clues
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
A fish tale? More on that seafood, melanoma study
A recent study linking the consumption of fish to melanoma had sushi eaters looking up the number of the nearest dermatologist. But experts said the findings had several important limitations and that no one should change their diet based on the results.
“It wouldn’t impact my fish consumption at all,” said Sancy Leachman, MD, PhD, who directs the melanoma program at Oregon Health & Science University’s Knight Cancer Institute in Portland. “Fish are part of a healthy diet,” particularly if it replaces less healthy proteins such as beef.
Even the authors of the study advised caution when interpreting the findings.
“I wouldn’t encourage anyone to change their fish consumption habits just because of this paper,” said study leader Eunyoung Cho, ScD, an epidemiologist at Brown University, Providence, R.I. “Fish is cardioprotective and is related to reduced risk of developing certain cancers too.”
Solid findings or fishing expedition?
The study quickly generated headlines and was the most viewed article of the journal Cancer Causes & Control within a day of its publication.
Dr. Cho, who is on the editorial board of the journal, analyzed the results of a study funded by the National Institutes of Health and the Association for the Advancement of Retired Persons that began in the 1990s and explored possible links between fish consumption and developing different forms of cancer.
Previous research from this project had shown eating fish was associated with an increased risk of melanoma – but no other type of cancer.
Dr. Cho speculated that the risk is due to contaminants, such as mercury and arsenic, in fish. But she was curious to see if she could find a connection between the amount of fish consumed and the risk of developing skin cancer.
At the beginning of the study people reported how much fish they had consumed over the previous year, which varied widely by person. Then researchers tracked participants for 15 years, recording who developed cancer and who did not.
Dietary recall can be difficult; people often say they can’t remember what they ate yesterday, much less 1 year ago. Still, dietitian Clare Collins, PhD, of the University of Newcastle (Australia), noted that previous research showed that the dietary recall tool for this study is reliable.
NIH researchers never checked to see how their diet changed over time, as this was a study meant to observe changes in health status starting from a baseline point. The researchers assumed that the level of fish intake recorded at the beginning of the study remained steady.
Of 491,000 people tracked in the study, 5,000 developed malignant melanoma and another 3,300 developed melanoma cells on the skin surface. People who ate the most fish – an average of almost 43 g, or about 1.5 ounces, per day – were 23% more likely to develop melanoma than the lightest fish eaters, who averaged 3 g per day.
That risk is modest, Dr. Leachman said, especially for people with red hair who, she said, are 400% more likely to get melanoma than others. “A 23% increase, in the whole scheme of things, is not nearly as important as not getting sunburned if you’re a redhead,” Dr. Leachman said.
And what about the sun? Sun exposure is the principal risk factor for developing melanoma, and the researchers didn’t account for that factor. People who developed melanoma during the study may simply have spent more time in tanning beds or on the beach – or perhaps fishing and then eating their catch.
Dr. Cho and colleagues tried to consider the effects of solar radiation by looking at average sun exposure times in the places where the participants in the study were living when the research began. Using that framework, variation in sun exposure made no difference to melanoma risk, but Dr. Leachman said the technique isn’t foolproof.
“They assumed that they were going to get a certain amount of ultraviolet light just based on where they lived. We don’t know how long they lived there or if they really had ultraviolet exposure or not,” Dr. Leachman said. Someone in presumably less sunny Pennsylvania could get more sun than someone in sun-drenched Arizona depending on their lifestyle and habits.
The kind of study Dr. Cho published cannot account for individual behaviors related to sun exposure, a limitation her team readily acknowledges. Nor does it include information about moles or hair color – important considerations for skin cancers. It may be that redheads with moles who barely ate any fish developed melanoma at higher rates than anyone else, but the data don’t allow for that kind of analysis.
Dr. Cho’s group found that canned tuna and any type of fish that wasn’t fried were associated with a higher risk of developing melanoma, as people reported eating more of those products. However, people who said they ate greater quantities of fried fish had a lower risk of melanoma, a counterintuitive finding that she said warrants further research.
Given that the study showed only a modest chance of developing melanoma regardless of fish intake, and left so many questions unanswered, what was the point?
Other journals declined to publish this paper, Dr. Cho acknowledged, but she defended the article as a step toward better understanding the health impact of environmental contaminants in fish.
Dr. Leachman agreed. “These kinds of studies are very important to do. They have large data sets, where you can start to see trends that may be important,” she said. “They can help you identify things that might be related. These experiments are hypothesis generating.”
“I already published an article showing that total mercury level is related to skin cancer, and we know very well that in the U.S. fish consumption is the major source of mercury contamination,” Dr. Cho said. “So, I naturally thought that fish consumption may be associated with increased risk of skin cancer too.” Dr. Cho said she believed the findings confirm that hypothesis.
Dr. Cho said the next step would be to measure blood levels of different contaminants such as mercury and arsenic in people with melanoma, to determine which toxin is the biggest driver of melanoma. She said she plans to seek funding for that research.
Meanwhile, pass the salmon – but go light on the salt.
Dr. Cho and Dr. Leachman reported no relevant financial relationships. Dr. Cho is on the editorial board of Cancer Causes & Control.
A version of this article first appeared on Medscape.com.
A recent study linking the consumption of fish to melanoma had sushi eaters looking up the number of the nearest dermatologist. But experts said the findings had several important limitations and that no one should change their diet based on the results.
“It wouldn’t impact my fish consumption at all,” said Sancy Leachman, MD, PhD, who directs the melanoma program at Oregon Health & Science University’s Knight Cancer Institute in Portland. “Fish are part of a healthy diet,” particularly if it replaces less healthy proteins such as beef.
Even the authors of the study advised caution when interpreting the findings.
“I wouldn’t encourage anyone to change their fish consumption habits just because of this paper,” said study leader Eunyoung Cho, ScD, an epidemiologist at Brown University, Providence, R.I. “Fish is cardioprotective and is related to reduced risk of developing certain cancers too.”
Solid findings or fishing expedition?
The study quickly generated headlines and was the most viewed article of the journal Cancer Causes & Control within a day of its publication.
Dr. Cho, who is on the editorial board of the journal, analyzed the results of a study funded by the National Institutes of Health and the Association for the Advancement of Retired Persons that began in the 1990s and explored possible links between fish consumption and developing different forms of cancer.
Previous research from this project had shown eating fish was associated with an increased risk of melanoma – but no other type of cancer.
Dr. Cho speculated that the risk is due to contaminants, such as mercury and arsenic, in fish. But she was curious to see if she could find a connection between the amount of fish consumed and the risk of developing skin cancer.
At the beginning of the study people reported how much fish they had consumed over the previous year, which varied widely by person. Then researchers tracked participants for 15 years, recording who developed cancer and who did not.
Dietary recall can be difficult; people often say they can’t remember what they ate yesterday, much less 1 year ago. Still, dietitian Clare Collins, PhD, of the University of Newcastle (Australia), noted that previous research showed that the dietary recall tool for this study is reliable.
NIH researchers never checked to see how their diet changed over time, as this was a study meant to observe changes in health status starting from a baseline point. The researchers assumed that the level of fish intake recorded at the beginning of the study remained steady.
Of 491,000 people tracked in the study, 5,000 developed malignant melanoma and another 3,300 developed melanoma cells on the skin surface. People who ate the most fish – an average of almost 43 g, or about 1.5 ounces, per day – were 23% more likely to develop melanoma than the lightest fish eaters, who averaged 3 g per day.
That risk is modest, Dr. Leachman said, especially for people with red hair who, she said, are 400% more likely to get melanoma than others. “A 23% increase, in the whole scheme of things, is not nearly as important as not getting sunburned if you’re a redhead,” Dr. Leachman said.
And what about the sun? Sun exposure is the principal risk factor for developing melanoma, and the researchers didn’t account for that factor. People who developed melanoma during the study may simply have spent more time in tanning beds or on the beach – or perhaps fishing and then eating their catch.
Dr. Cho and colleagues tried to consider the effects of solar radiation by looking at average sun exposure times in the places where the participants in the study were living when the research began. Using that framework, variation in sun exposure made no difference to melanoma risk, but Dr. Leachman said the technique isn’t foolproof.
“They assumed that they were going to get a certain amount of ultraviolet light just based on where they lived. We don’t know how long they lived there or if they really had ultraviolet exposure or not,” Dr. Leachman said. Someone in presumably less sunny Pennsylvania could get more sun than someone in sun-drenched Arizona depending on their lifestyle and habits.
The kind of study Dr. Cho published cannot account for individual behaviors related to sun exposure, a limitation her team readily acknowledges. Nor does it include information about moles or hair color – important considerations for skin cancers. It may be that redheads with moles who barely ate any fish developed melanoma at higher rates than anyone else, but the data don’t allow for that kind of analysis.
Dr. Cho’s group found that canned tuna and any type of fish that wasn’t fried were associated with a higher risk of developing melanoma, as people reported eating more of those products. However, people who said they ate greater quantities of fried fish had a lower risk of melanoma, a counterintuitive finding that she said warrants further research.
Given that the study showed only a modest chance of developing melanoma regardless of fish intake, and left so many questions unanswered, what was the point?
Other journals declined to publish this paper, Dr. Cho acknowledged, but she defended the article as a step toward better understanding the health impact of environmental contaminants in fish.
Dr. Leachman agreed. “These kinds of studies are very important to do. They have large data sets, where you can start to see trends that may be important,” she said. “They can help you identify things that might be related. These experiments are hypothesis generating.”
“I already published an article showing that total mercury level is related to skin cancer, and we know very well that in the U.S. fish consumption is the major source of mercury contamination,” Dr. Cho said. “So, I naturally thought that fish consumption may be associated with increased risk of skin cancer too.” Dr. Cho said she believed the findings confirm that hypothesis.
Dr. Cho said the next step would be to measure blood levels of different contaminants such as mercury and arsenic in people with melanoma, to determine which toxin is the biggest driver of melanoma. She said she plans to seek funding for that research.
Meanwhile, pass the salmon – but go light on the salt.
Dr. Cho and Dr. Leachman reported no relevant financial relationships. Dr. Cho is on the editorial board of Cancer Causes & Control.
A version of this article first appeared on Medscape.com.
A recent study linking the consumption of fish to melanoma had sushi eaters looking up the number of the nearest dermatologist. But experts said the findings had several important limitations and that no one should change their diet based on the results.
“It wouldn’t impact my fish consumption at all,” said Sancy Leachman, MD, PhD, who directs the melanoma program at Oregon Health & Science University’s Knight Cancer Institute in Portland. “Fish are part of a healthy diet,” particularly if it replaces less healthy proteins such as beef.
Even the authors of the study advised caution when interpreting the findings.
“I wouldn’t encourage anyone to change their fish consumption habits just because of this paper,” said study leader Eunyoung Cho, ScD, an epidemiologist at Brown University, Providence, R.I. “Fish is cardioprotective and is related to reduced risk of developing certain cancers too.”
Solid findings or fishing expedition?
The study quickly generated headlines and was the most viewed article of the journal Cancer Causes & Control within a day of its publication.
Dr. Cho, who is on the editorial board of the journal, analyzed the results of a study funded by the National Institutes of Health and the Association for the Advancement of Retired Persons that began in the 1990s and explored possible links between fish consumption and developing different forms of cancer.
Previous research from this project had shown eating fish was associated with an increased risk of melanoma – but no other type of cancer.
Dr. Cho speculated that the risk is due to contaminants, such as mercury and arsenic, in fish. But she was curious to see if she could find a connection between the amount of fish consumed and the risk of developing skin cancer.
At the beginning of the study people reported how much fish they had consumed over the previous year, which varied widely by person. Then researchers tracked participants for 15 years, recording who developed cancer and who did not.
Dietary recall can be difficult; people often say they can’t remember what they ate yesterday, much less 1 year ago. Still, dietitian Clare Collins, PhD, of the University of Newcastle (Australia), noted that previous research showed that the dietary recall tool for this study is reliable.
NIH researchers never checked to see how their diet changed over time, as this was a study meant to observe changes in health status starting from a baseline point. The researchers assumed that the level of fish intake recorded at the beginning of the study remained steady.
Of 491,000 people tracked in the study, 5,000 developed malignant melanoma and another 3,300 developed melanoma cells on the skin surface. People who ate the most fish – an average of almost 43 g, or about 1.5 ounces, per day – were 23% more likely to develop melanoma than the lightest fish eaters, who averaged 3 g per day.
That risk is modest, Dr. Leachman said, especially for people with red hair who, she said, are 400% more likely to get melanoma than others. “A 23% increase, in the whole scheme of things, is not nearly as important as not getting sunburned if you’re a redhead,” Dr. Leachman said.
And what about the sun? Sun exposure is the principal risk factor for developing melanoma, and the researchers didn’t account for that factor. People who developed melanoma during the study may simply have spent more time in tanning beds or on the beach – or perhaps fishing and then eating their catch.
Dr. Cho and colleagues tried to consider the effects of solar radiation by looking at average sun exposure times in the places where the participants in the study were living when the research began. Using that framework, variation in sun exposure made no difference to melanoma risk, but Dr. Leachman said the technique isn’t foolproof.
“They assumed that they were going to get a certain amount of ultraviolet light just based on where they lived. We don’t know how long they lived there or if they really had ultraviolet exposure or not,” Dr. Leachman said. Someone in presumably less sunny Pennsylvania could get more sun than someone in sun-drenched Arizona depending on their lifestyle and habits.
The kind of study Dr. Cho published cannot account for individual behaviors related to sun exposure, a limitation her team readily acknowledges. Nor does it include information about moles or hair color – important considerations for skin cancers. It may be that redheads with moles who barely ate any fish developed melanoma at higher rates than anyone else, but the data don’t allow for that kind of analysis.
Dr. Cho’s group found that canned tuna and any type of fish that wasn’t fried were associated with a higher risk of developing melanoma, as people reported eating more of those products. However, people who said they ate greater quantities of fried fish had a lower risk of melanoma, a counterintuitive finding that she said warrants further research.
Given that the study showed only a modest chance of developing melanoma regardless of fish intake, and left so many questions unanswered, what was the point?
Other journals declined to publish this paper, Dr. Cho acknowledged, but she defended the article as a step toward better understanding the health impact of environmental contaminants in fish.
Dr. Leachman agreed. “These kinds of studies are very important to do. They have large data sets, where you can start to see trends that may be important,” she said. “They can help you identify things that might be related. These experiments are hypothesis generating.”
“I already published an article showing that total mercury level is related to skin cancer, and we know very well that in the U.S. fish consumption is the major source of mercury contamination,” Dr. Cho said. “So, I naturally thought that fish consumption may be associated with increased risk of skin cancer too.” Dr. Cho said she believed the findings confirm that hypothesis.
Dr. Cho said the next step would be to measure blood levels of different contaminants such as mercury and arsenic in people with melanoma, to determine which toxin is the biggest driver of melanoma. She said she plans to seek funding for that research.
Meanwhile, pass the salmon – but go light on the salt.
Dr. Cho and Dr. Leachman reported no relevant financial relationships. Dr. Cho is on the editorial board of Cancer Causes & Control.
A version of this article first appeared on Medscape.com.
Basal Cell Carcinoma
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
Basal cell carcinoma is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals.5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (Figure, A) as in non-Hispanic White individuals.7 Any skin cancer can present with ulcerations, so while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ ethnic variability. In my practice in San Antonio (R.P.U.), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (Figure, A). There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes. 1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and SCC found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non- Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (Figure, B) should prompt consideration of skin cancer regardless of skin color.
- Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. doi:10.1007/s40257-021-00662-z
- Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/S0190-9622(81)70067-7
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/S0190-9622(96)90007-9
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study [published online July 9, 2021]. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005

THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
Basal cell carcinoma is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals.5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (Figure, A) as in non-Hispanic White individuals.7 Any skin cancer can present with ulcerations, so while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ ethnic variability. In my practice in San Antonio (R.P.U.), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (Figure, A). There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes. 1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and SCC found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non- Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (Figure, B) should prompt consideration of skin cancer regardless of skin color.

THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
Basal cell carcinoma is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals.5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (Figure, A) as in non-Hispanic White individuals.7 Any skin cancer can present with ulcerations, so while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ ethnic variability. In my practice in San Antonio (R.P.U.), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (Figure, A). There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes. 1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and SCC found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non- Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (Figure, B) should prompt consideration of skin cancer regardless of skin color.
- Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. doi:10.1007/s40257-021-00662-z
- Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/S0190-9622(81)70067-7
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/S0190-9622(96)90007-9
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study [published online July 9, 2021]. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
- Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. doi:10.1007/s40257-021-00662-z
- Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/S0190-9622(81)70067-7
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/S0190-9622(96)90007-9
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study [published online July 9, 2021]. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
Prediabetes is linked independently to myocardial infarction
Prediabetes is not only a predictor of diabetes and the cardiovascular complications that ensue, but it is also a risk factor by itself for myocardial infarction, according to data drawn from almost 1.8 million patients hospitalized for MI.
“Our study serves as a wakeup call for clinicians and patients to shift the focus to preventing prediabetes, and not just diabetes, said Geethika Thota, MD, at the annual meeting of the Endocrine Society.
There are plenty of data suggesting that prediabetes places patients on a trajectory toward cardiovascular disease. In a meta-analysis of 129 studies published 2 years ago, prediabetes was not only associated with a statistically significant 16% increase in coronary heart disease, but also a 13% increased risk of all-cause mortality relative to those with normoglycemia.
Data drawn from 1.8 million patients
In this study, 1,794,149 weighted patient hospitalizations for MI were drawn from the National Inpatient Sample database. Excluding patients who eventually developed diabetes, roughly 1% of these patients had a history of prediabetes in the past, according to a search of ICD-10 codes.
Before adjustment for other risk factors, prediabetes was linked to a greater than 40% increased odds of MI (odds ratio, 1.41; P < .01). After adjustment for a large array of known MI risk factors – including prior history of MI, dyslipidemia, hypertension, nicotine dependence, and obesity – prediabetes remained an independent risk factor, corresponding with a 25% increased risk of MI (OR, 1.25; P < .01).
A history of prediabetes was also an independent risk factor for percutaneous intervention and coronary artery bypass grafting, with increased risk of 45% and 95%, respectively.
As a retrospective study looking at prediabetes as a risk factor in those who already had a MI, it is possible that not all patients with prediabetes were properly coded, but Dr. Thota said that was unlikely to have been an issue of sufficient magnitude to have affected the major conclusions.
Relevance seen for community care
Although the study was drawn from hospitalized patients, its relevance is for the community setting, where screening and intervention for prediabetes has the potential to alter the risk, according to Dr. Thota.
Most clinicians are likely aware of the value of screening for prediabetes, which was defined in this study as a hemoglobin A1c of 5.7%-6.4%, but Dr. Thota suggested that many might not fully grasp the full scope of goals. Early detection and prevention will prevent diabetes and, by extension, cardiovascular disease, but her data suggest that control of prediabetes with lower cardiovascular risk by a more direct route.
“Despite mounting evidence, many clinicians are unaware that prediabetes is also a major risk factor for atherosclerotic cardiovascular disease,” said Dr. Thota, an internal medicine resident at Saint Peter’s University Hospital, New Brunswick, N.J.
Like diabetes, the prevalence of prediabetes is growing rapidly, according to data from the Centers for Disease Control that Dr. Thota cited. In 2020, the Centers for Disease Control and Prevention estimated that 38% of the adult population have prediabetes. By 2030, one model predicts a further 25% growth.
Screening for hyperglycemia is part of routine patient evaluations at Dr. Thota’s center. In an interview, she said that once a diagnosis of prediabetes is entered in the electronic medical record, the history is carried forward so that changes in status are continually monitored.
Worsening prediabetes should be addressed
“Prediabetes is not treated with medication, at least initially,” Dr. Thota explained. Rather, patients are educated about important lifestyle changes, such as diet and physical activity, that can reverse the diagnosis. However, patients who remain on a path of worsening hyperglycemia are candidates for more intensive lifestyle intervention and might be considered selectively for metformin.
“Early recognition of prediabetes through screening is important,” Dr. Thota emphasized. The benefit for preventing patients from progressing to diabetes is well recognized, but these data provide the basis for incentivizing lifestyle changes in patients with prediabetes by telling them that it can reduce their risk for MI.
These data have an important message, but they are not surprising, according to Deepak L. Bhatt, MD, executive director, interventional cardiovascular programs, Brigham and Women’s Hospital Heart & Vascular Center, Boston.
“In fact, in daily practice we see a substantial percentage of patients with MI who have prediabetes that had not been previously recognized or formally diagnosed,” Dr. Bhatt said in an interview.
“Identifying these patients – preferably prior to coming in with cardiovascular complications – is important both to reduce cardiovascular risk but also to try and prevent progression at diabetes,” he added.
Dr. Bhatt went on to say that this large analysis, confirming that prediabetes is independently associated with MI, should prompt clinicians to screen patients rigorously for this condition.
“At a minimum, such patients would be candidates for intensive lifestyle modification aimed at weight loss and treatment of frequent coexistent conditions, such as hypertension and dyslipidemia,” Dr. Bhatt said.
Dr. Thota reports no potential conflicts of interest. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, many of which make products relevant to the management of diabetes and cardiovascular disease.
Prediabetes is not only a predictor of diabetes and the cardiovascular complications that ensue, but it is also a risk factor by itself for myocardial infarction, according to data drawn from almost 1.8 million patients hospitalized for MI.
“Our study serves as a wakeup call for clinicians and patients to shift the focus to preventing prediabetes, and not just diabetes, said Geethika Thota, MD, at the annual meeting of the Endocrine Society.
There are plenty of data suggesting that prediabetes places patients on a trajectory toward cardiovascular disease. In a meta-analysis of 129 studies published 2 years ago, prediabetes was not only associated with a statistically significant 16% increase in coronary heart disease, but also a 13% increased risk of all-cause mortality relative to those with normoglycemia.
Data drawn from 1.8 million patients
In this study, 1,794,149 weighted patient hospitalizations for MI were drawn from the National Inpatient Sample database. Excluding patients who eventually developed diabetes, roughly 1% of these patients had a history of prediabetes in the past, according to a search of ICD-10 codes.
Before adjustment for other risk factors, prediabetes was linked to a greater than 40% increased odds of MI (odds ratio, 1.41; P < .01). After adjustment for a large array of known MI risk factors – including prior history of MI, dyslipidemia, hypertension, nicotine dependence, and obesity – prediabetes remained an independent risk factor, corresponding with a 25% increased risk of MI (OR, 1.25; P < .01).
A history of prediabetes was also an independent risk factor for percutaneous intervention and coronary artery bypass grafting, with increased risk of 45% and 95%, respectively.
As a retrospective study looking at prediabetes as a risk factor in those who already had a MI, it is possible that not all patients with prediabetes were properly coded, but Dr. Thota said that was unlikely to have been an issue of sufficient magnitude to have affected the major conclusions.
Relevance seen for community care
Although the study was drawn from hospitalized patients, its relevance is for the community setting, where screening and intervention for prediabetes has the potential to alter the risk, according to Dr. Thota.
Most clinicians are likely aware of the value of screening for prediabetes, which was defined in this study as a hemoglobin A1c of 5.7%-6.4%, but Dr. Thota suggested that many might not fully grasp the full scope of goals. Early detection and prevention will prevent diabetes and, by extension, cardiovascular disease, but her data suggest that control of prediabetes with lower cardiovascular risk by a more direct route.
“Despite mounting evidence, many clinicians are unaware that prediabetes is also a major risk factor for atherosclerotic cardiovascular disease,” said Dr. Thota, an internal medicine resident at Saint Peter’s University Hospital, New Brunswick, N.J.
Like diabetes, the prevalence of prediabetes is growing rapidly, according to data from the Centers for Disease Control that Dr. Thota cited. In 2020, the Centers for Disease Control and Prevention estimated that 38% of the adult population have prediabetes. By 2030, one model predicts a further 25% growth.
Screening for hyperglycemia is part of routine patient evaluations at Dr. Thota’s center. In an interview, she said that once a diagnosis of prediabetes is entered in the electronic medical record, the history is carried forward so that changes in status are continually monitored.
Worsening prediabetes should be addressed
“Prediabetes is not treated with medication, at least initially,” Dr. Thota explained. Rather, patients are educated about important lifestyle changes, such as diet and physical activity, that can reverse the diagnosis. However, patients who remain on a path of worsening hyperglycemia are candidates for more intensive lifestyle intervention and might be considered selectively for metformin.
“Early recognition of prediabetes through screening is important,” Dr. Thota emphasized. The benefit for preventing patients from progressing to diabetes is well recognized, but these data provide the basis for incentivizing lifestyle changes in patients with prediabetes by telling them that it can reduce their risk for MI.
These data have an important message, but they are not surprising, according to Deepak L. Bhatt, MD, executive director, interventional cardiovascular programs, Brigham and Women’s Hospital Heart & Vascular Center, Boston.
“In fact, in daily practice we see a substantial percentage of patients with MI who have prediabetes that had not been previously recognized or formally diagnosed,” Dr. Bhatt said in an interview.
“Identifying these patients – preferably prior to coming in with cardiovascular complications – is important both to reduce cardiovascular risk but also to try and prevent progression at diabetes,” he added.
Dr. Bhatt went on to say that this large analysis, confirming that prediabetes is independently associated with MI, should prompt clinicians to screen patients rigorously for this condition.
“At a minimum, such patients would be candidates for intensive lifestyle modification aimed at weight loss and treatment of frequent coexistent conditions, such as hypertension and dyslipidemia,” Dr. Bhatt said.
Dr. Thota reports no potential conflicts of interest. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, many of which make products relevant to the management of diabetes and cardiovascular disease.
Prediabetes is not only a predictor of diabetes and the cardiovascular complications that ensue, but it is also a risk factor by itself for myocardial infarction, according to data drawn from almost 1.8 million patients hospitalized for MI.
“Our study serves as a wakeup call for clinicians and patients to shift the focus to preventing prediabetes, and not just diabetes, said Geethika Thota, MD, at the annual meeting of the Endocrine Society.
There are plenty of data suggesting that prediabetes places patients on a trajectory toward cardiovascular disease. In a meta-analysis of 129 studies published 2 years ago, prediabetes was not only associated with a statistically significant 16% increase in coronary heart disease, but also a 13% increased risk of all-cause mortality relative to those with normoglycemia.
Data drawn from 1.8 million patients
In this study, 1,794,149 weighted patient hospitalizations for MI were drawn from the National Inpatient Sample database. Excluding patients who eventually developed diabetes, roughly 1% of these patients had a history of prediabetes in the past, according to a search of ICD-10 codes.
Before adjustment for other risk factors, prediabetes was linked to a greater than 40% increased odds of MI (odds ratio, 1.41; P < .01). After adjustment for a large array of known MI risk factors – including prior history of MI, dyslipidemia, hypertension, nicotine dependence, and obesity – prediabetes remained an independent risk factor, corresponding with a 25% increased risk of MI (OR, 1.25; P < .01).
A history of prediabetes was also an independent risk factor for percutaneous intervention and coronary artery bypass grafting, with increased risk of 45% and 95%, respectively.
As a retrospective study looking at prediabetes as a risk factor in those who already had a MI, it is possible that not all patients with prediabetes were properly coded, but Dr. Thota said that was unlikely to have been an issue of sufficient magnitude to have affected the major conclusions.
Relevance seen for community care
Although the study was drawn from hospitalized patients, its relevance is for the community setting, where screening and intervention for prediabetes has the potential to alter the risk, according to Dr. Thota.
Most clinicians are likely aware of the value of screening for prediabetes, which was defined in this study as a hemoglobin A1c of 5.7%-6.4%, but Dr. Thota suggested that many might not fully grasp the full scope of goals. Early detection and prevention will prevent diabetes and, by extension, cardiovascular disease, but her data suggest that control of prediabetes with lower cardiovascular risk by a more direct route.
“Despite mounting evidence, many clinicians are unaware that prediabetes is also a major risk factor for atherosclerotic cardiovascular disease,” said Dr. Thota, an internal medicine resident at Saint Peter’s University Hospital, New Brunswick, N.J.
Like diabetes, the prevalence of prediabetes is growing rapidly, according to data from the Centers for Disease Control that Dr. Thota cited. In 2020, the Centers for Disease Control and Prevention estimated that 38% of the adult population have prediabetes. By 2030, one model predicts a further 25% growth.
Screening for hyperglycemia is part of routine patient evaluations at Dr. Thota’s center. In an interview, she said that once a diagnosis of prediabetes is entered in the electronic medical record, the history is carried forward so that changes in status are continually monitored.
Worsening prediabetes should be addressed
“Prediabetes is not treated with medication, at least initially,” Dr. Thota explained. Rather, patients are educated about important lifestyle changes, such as diet and physical activity, that can reverse the diagnosis. However, patients who remain on a path of worsening hyperglycemia are candidates for more intensive lifestyle intervention and might be considered selectively for metformin.
“Early recognition of prediabetes through screening is important,” Dr. Thota emphasized. The benefit for preventing patients from progressing to diabetes is well recognized, but these data provide the basis for incentivizing lifestyle changes in patients with prediabetes by telling them that it can reduce their risk for MI.
These data have an important message, but they are not surprising, according to Deepak L. Bhatt, MD, executive director, interventional cardiovascular programs, Brigham and Women’s Hospital Heart & Vascular Center, Boston.
“In fact, in daily practice we see a substantial percentage of patients with MI who have prediabetes that had not been previously recognized or formally diagnosed,” Dr. Bhatt said in an interview.
“Identifying these patients – preferably prior to coming in with cardiovascular complications – is important both to reduce cardiovascular risk but also to try and prevent progression at diabetes,” he added.
Dr. Bhatt went on to say that this large analysis, confirming that prediabetes is independently associated with MI, should prompt clinicians to screen patients rigorously for this condition.
“At a minimum, such patients would be candidates for intensive lifestyle modification aimed at weight loss and treatment of frequent coexistent conditions, such as hypertension and dyslipidemia,” Dr. Bhatt said.
Dr. Thota reports no potential conflicts of interest. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, many of which make products relevant to the management of diabetes and cardiovascular disease.
FROM ENDO 2022
Painless Vulvar Nodule
The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6
Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.
Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12
Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.
- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6

Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.

Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12

Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.

The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6

Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.

Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12

Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.

- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
A 45-year-old woman with no notable medical history presented with a small nodule in the left pubic region lateral to the left labia majora. The lesion grew to 8 cm over the course of several months, and she underwent a simple excision for what clinically appeared to be a cyst.