User login
Online Information About Hydroquinone: An Assessment of Accuracy and Readability
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
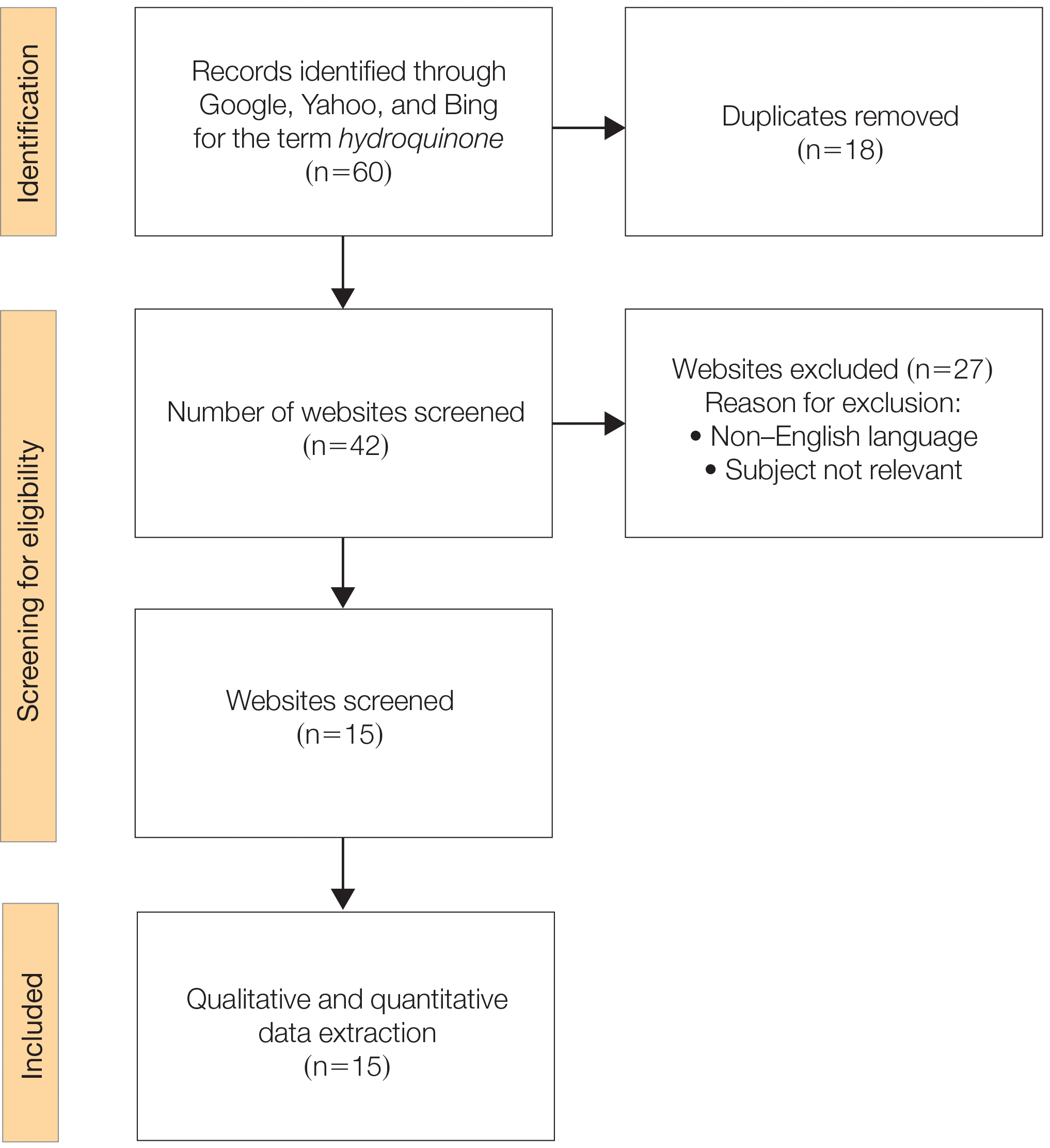
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

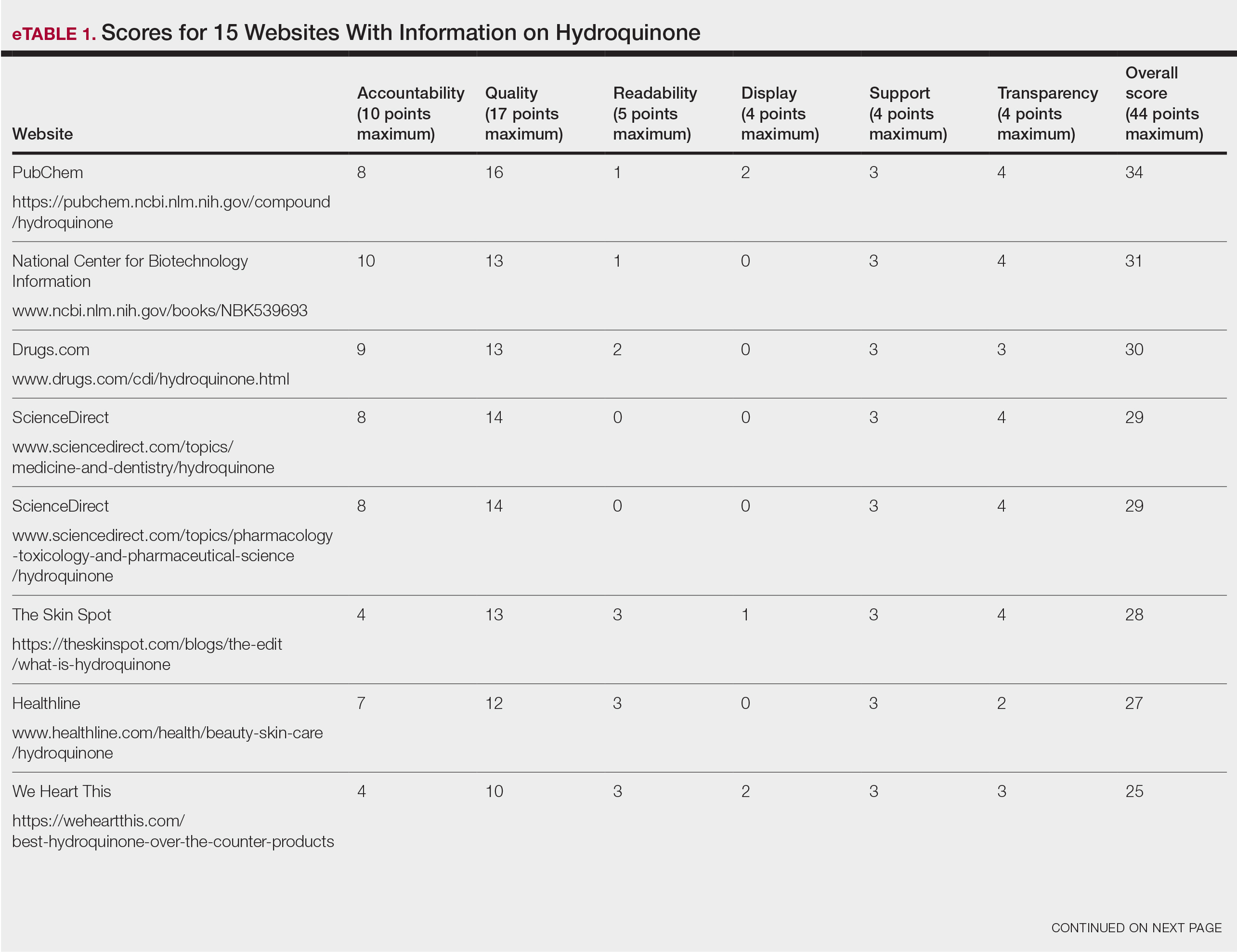
Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
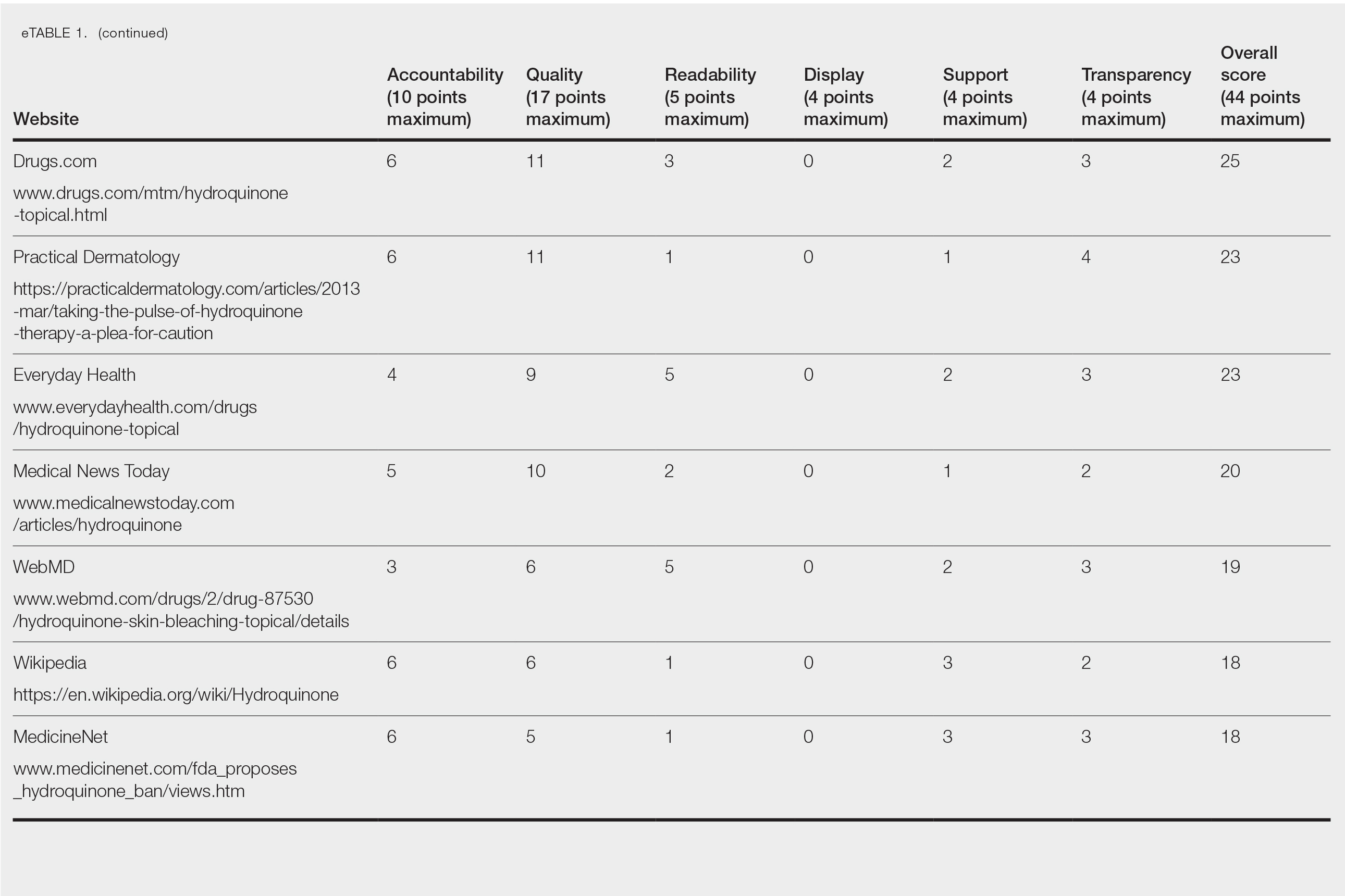
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
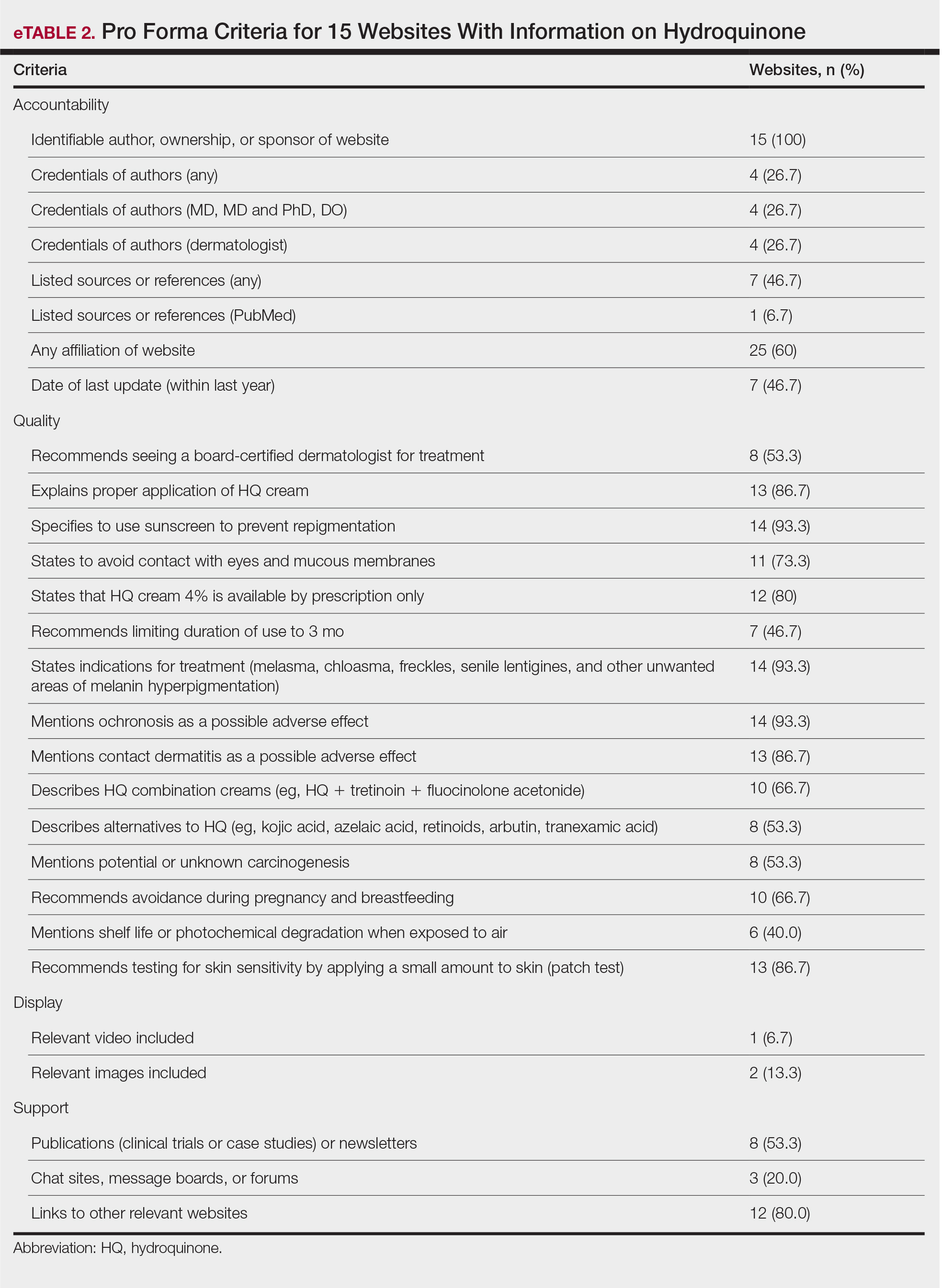
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
Practice Points
- Hydroquinone (HQ) 4% is US Food and Drug Administration (FDA) approved for skin hyperpigmentation including melasma.
- In September 2020, the FDA enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform, announcing that HQ is not classified as Category II/not generally recognized as safe and effective, thus prohibiting the distribution of OTC HQ products.
- Exogenous ochronosis is a potential side effect associated with HQ.
- There is a need for dermatologists to develop online patient education materials on HQ that are characterized by high-quality and up-to-date medical information.
California doctor to pay $9.5 million in Medicare, Medi-Cal fraud scheme
Part of the payment was a settlement in a civil case in which Minas Kochumian, MD, an internist who ran a solo practice in Northridge, Calif., was accused of submitting claims to Medicare and Medi-Cal for procedures, services, and tests that were never performed. The procedures he falsely billed for included injecting a medication for treating osteoarthritis and osteoporosis, draining tailbone cysts, and removal of various growths.
As part of the settlement, Dr. Kochumian admitted that he intentionally submitted false claims with the intent to deceive the United States and the State of California. The damages and penalties were possible under the federal False Claims Act and the California False Claims Act.
According to the Medical Board of California, Dr. Kochumian’s license is current and set to expire next July.
The allegations against Dr. Kochumian were first brought to the attention of authorities in a whistleblower lawsuit filed by Elize Oganesyan, Dr. Kochumian’s former medical assistant, and Damon Davies, former information technology consultant for the practice. Among her other duties, Ms. Oganesyan was responsible for verifying insurance eligibility and obtaining authorization for drugs, procedures, services, and tests.
The medical assistant first realized that something was amiss when a patient brought her a Medicare Explanation of Benefits document that included charges for an injection the practice had not administered, according to court records. Ms. Oganesyan then realized the clinic was filing claims for other services that were never provided. She stated in the original complaint that the clinic did not even have the necessary equipment for providing some of these tests — skin allergy tests, for example.
The False Claims Act permits private parties to sue on behalf of the government for false claims for government funds and to receive a share of any recovery. Ms. Oganesyan and Davies will receive more than $1.75 million as their share of the recovery. The whistleblowers’ claims for attorneys’ fees are not resolved by this settlement, according to a statement from the U.S. Attorney’s Office, Eastern District of California.
The $9.5 million payment includes $5.4 million owed by Dr. Kochumian as criminal restitution following his guilty plea to one count of healthcare fraud in a separate criminal case filed in the Central District of California. In addition to the fine, Dr. Kochumian was sentenced to 41 months in prison, according to a statement by California Attorney General Rob Bonta.
“When doctors misuse the state’s Medi-Cal funds, they violate their Hippocratic Oath by harming a program which exists to help California’s Medi-Cal population, including the elderly, the sick, and the vulnerable,” said Mr. Bonta. “Dr. Kochumian’s alleged misconduct violated the trust of the patients in his care, and he selfishly pocketed funds that would otherwise have gone toward critical publicly funded healthcare services.”
A version of this article first appeared on Medscape.com.
Part of the payment was a settlement in a civil case in which Minas Kochumian, MD, an internist who ran a solo practice in Northridge, Calif., was accused of submitting claims to Medicare and Medi-Cal for procedures, services, and tests that were never performed. The procedures he falsely billed for included injecting a medication for treating osteoarthritis and osteoporosis, draining tailbone cysts, and removal of various growths.
As part of the settlement, Dr. Kochumian admitted that he intentionally submitted false claims with the intent to deceive the United States and the State of California. The damages and penalties were possible under the federal False Claims Act and the California False Claims Act.
According to the Medical Board of California, Dr. Kochumian’s license is current and set to expire next July.
The allegations against Dr. Kochumian were first brought to the attention of authorities in a whistleblower lawsuit filed by Elize Oganesyan, Dr. Kochumian’s former medical assistant, and Damon Davies, former information technology consultant for the practice. Among her other duties, Ms. Oganesyan was responsible for verifying insurance eligibility and obtaining authorization for drugs, procedures, services, and tests.
The medical assistant first realized that something was amiss when a patient brought her a Medicare Explanation of Benefits document that included charges for an injection the practice had not administered, according to court records. Ms. Oganesyan then realized the clinic was filing claims for other services that were never provided. She stated in the original complaint that the clinic did not even have the necessary equipment for providing some of these tests — skin allergy tests, for example.
The False Claims Act permits private parties to sue on behalf of the government for false claims for government funds and to receive a share of any recovery. Ms. Oganesyan and Davies will receive more than $1.75 million as their share of the recovery. The whistleblowers’ claims for attorneys’ fees are not resolved by this settlement, according to a statement from the U.S. Attorney’s Office, Eastern District of California.
The $9.5 million payment includes $5.4 million owed by Dr. Kochumian as criminal restitution following his guilty plea to one count of healthcare fraud in a separate criminal case filed in the Central District of California. In addition to the fine, Dr. Kochumian was sentenced to 41 months in prison, according to a statement by California Attorney General Rob Bonta.
“When doctors misuse the state’s Medi-Cal funds, they violate their Hippocratic Oath by harming a program which exists to help California’s Medi-Cal population, including the elderly, the sick, and the vulnerable,” said Mr. Bonta. “Dr. Kochumian’s alleged misconduct violated the trust of the patients in his care, and he selfishly pocketed funds that would otherwise have gone toward critical publicly funded healthcare services.”
A version of this article first appeared on Medscape.com.
Part of the payment was a settlement in a civil case in which Minas Kochumian, MD, an internist who ran a solo practice in Northridge, Calif., was accused of submitting claims to Medicare and Medi-Cal for procedures, services, and tests that were never performed. The procedures he falsely billed for included injecting a medication for treating osteoarthritis and osteoporosis, draining tailbone cysts, and removal of various growths.
As part of the settlement, Dr. Kochumian admitted that he intentionally submitted false claims with the intent to deceive the United States and the State of California. The damages and penalties were possible under the federal False Claims Act and the California False Claims Act.
According to the Medical Board of California, Dr. Kochumian’s license is current and set to expire next July.
The allegations against Dr. Kochumian were first brought to the attention of authorities in a whistleblower lawsuit filed by Elize Oganesyan, Dr. Kochumian’s former medical assistant, and Damon Davies, former information technology consultant for the practice. Among her other duties, Ms. Oganesyan was responsible for verifying insurance eligibility and obtaining authorization for drugs, procedures, services, and tests.
The medical assistant first realized that something was amiss when a patient brought her a Medicare Explanation of Benefits document that included charges for an injection the practice had not administered, according to court records. Ms. Oganesyan then realized the clinic was filing claims for other services that were never provided. She stated in the original complaint that the clinic did not even have the necessary equipment for providing some of these tests — skin allergy tests, for example.
The False Claims Act permits private parties to sue on behalf of the government for false claims for government funds and to receive a share of any recovery. Ms. Oganesyan and Davies will receive more than $1.75 million as their share of the recovery. The whistleblowers’ claims for attorneys’ fees are not resolved by this settlement, according to a statement from the U.S. Attorney’s Office, Eastern District of California.
The $9.5 million payment includes $5.4 million owed by Dr. Kochumian as criminal restitution following his guilty plea to one count of healthcare fraud in a separate criminal case filed in the Central District of California. In addition to the fine, Dr. Kochumian was sentenced to 41 months in prison, according to a statement by California Attorney General Rob Bonta.
“When doctors misuse the state’s Medi-Cal funds, they violate their Hippocratic Oath by harming a program which exists to help California’s Medi-Cal population, including the elderly, the sick, and the vulnerable,” said Mr. Bonta. “Dr. Kochumian’s alleged misconduct violated the trust of the patients in his care, and he selfishly pocketed funds that would otherwise have gone toward critical publicly funded healthcare services.”
A version of this article first appeared on Medscape.com.
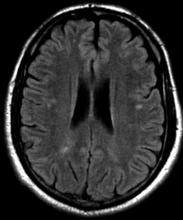
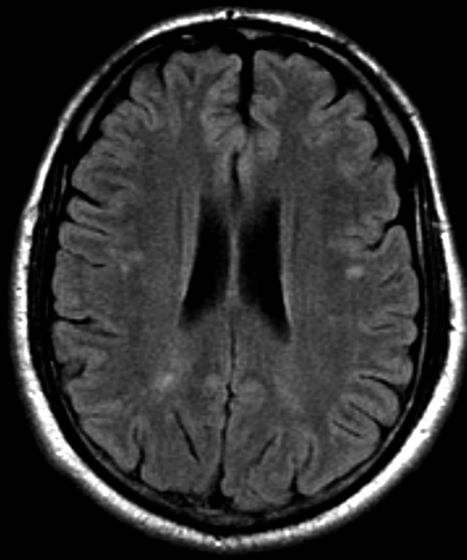
Severe ipsilateral headache
On the basis of the patient's presentation, family history, and personal history of headache, she seems to be presenting with hemiplegic migraine, an uncommon migraine subtype characterized by recurrent headaches associated with temporary unilateral hemiparesis or hemiplegia. The hemiparesis may resolve before the headache, as seen in the present case, or it may persist for days to weeks. These episodes are sometimes accompanied by ipsilateral numbness, tingling, or paresthesia, with or without a speech disturbance. Visual defects (ie, scintillating scotoma and hemianopia) and aphasia may occur.
Hemiplegic migraine can be sporadic or familial. Familial hemiplegic migraine is the only migraine subtype for which an autosomal dominant mode of inheritance has been identified. The onset is generally in adolescence between 12 and 17 years of age, with an estimated prevalence of 0.01%. Female patients are more likely to have these types of migraines.
Diagnosis of hemiplegic migraine is centered on exclusion of other possible causes of headache with motor weakness. When a patient presents with motor deficit, these symptoms can also be the result of a secondary headache rather than a primary headache disorder. Because of this neurologic aspect of presentation, the differential diagnosis is broad and should span other migraine subtypes, inflammatory or metabolic disorders, and mitochondrial diseases, as well as any condition that shows neurologic deficits without radiologic alterations. Pediatric patients with hemiplegic migraine are often misdiagnosed with epilepsy. Compared with hemiplegic migraine, seizures are much more brief, and any associated hemiparesis is usually characterized by limb jerking, head turning, and loss of consciousness. Of note, up to 7% of patients with familial hemiplegic migraine do eventually develop epilepsy. Although there are no telltale pathognomonic clinical, laboratory, or radiologic findings of hemiplegic migraine, electroencephalography may show asymmetric slow-wave activity contralateral to the hemiparesis.
In hemiplegic migraine, acute treatment options include antiemetics, NSAIDs, and nonnarcotic pain relievers; triptans and ergotamine preparations are contraindicated in this setting because of their potential vasoconstrictive effects. Even if episode frequency is low, the American Headache Society advises that prophylactic treatment should also be considered in the management of uncommon migraine subtypes such as this one.
Jasmin Harpe, MD, MPH, Headache Fellow, Department of Neurology, Harvard University, John R. Graham Headache Center, Mass General Brigham, Boston, MA
Jasmin Harpe, MD, MPH, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's presentation, family history, and personal history of headache, she seems to be presenting with hemiplegic migraine, an uncommon migraine subtype characterized by recurrent headaches associated with temporary unilateral hemiparesis or hemiplegia. The hemiparesis may resolve before the headache, as seen in the present case, or it may persist for days to weeks. These episodes are sometimes accompanied by ipsilateral numbness, tingling, or paresthesia, with or without a speech disturbance. Visual defects (ie, scintillating scotoma and hemianopia) and aphasia may occur.
Hemiplegic migraine can be sporadic or familial. Familial hemiplegic migraine is the only migraine subtype for which an autosomal dominant mode of inheritance has been identified. The onset is generally in adolescence between 12 and 17 years of age, with an estimated prevalence of 0.01%. Female patients are more likely to have these types of migraines.
Diagnosis of hemiplegic migraine is centered on exclusion of other possible causes of headache with motor weakness. When a patient presents with motor deficit, these symptoms can also be the result of a secondary headache rather than a primary headache disorder. Because of this neurologic aspect of presentation, the differential diagnosis is broad and should span other migraine subtypes, inflammatory or metabolic disorders, and mitochondrial diseases, as well as any condition that shows neurologic deficits without radiologic alterations. Pediatric patients with hemiplegic migraine are often misdiagnosed with epilepsy. Compared with hemiplegic migraine, seizures are much more brief, and any associated hemiparesis is usually characterized by limb jerking, head turning, and loss of consciousness. Of note, up to 7% of patients with familial hemiplegic migraine do eventually develop epilepsy. Although there are no telltale pathognomonic clinical, laboratory, or radiologic findings of hemiplegic migraine, electroencephalography may show asymmetric slow-wave activity contralateral to the hemiparesis.
In hemiplegic migraine, acute treatment options include antiemetics, NSAIDs, and nonnarcotic pain relievers; triptans and ergotamine preparations are contraindicated in this setting because of their potential vasoconstrictive effects. Even if episode frequency is low, the American Headache Society advises that prophylactic treatment should also be considered in the management of uncommon migraine subtypes such as this one.
Jasmin Harpe, MD, MPH, Headache Fellow, Department of Neurology, Harvard University, John R. Graham Headache Center, Mass General Brigham, Boston, MA
Jasmin Harpe, MD, MPH, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's presentation, family history, and personal history of headache, she seems to be presenting with hemiplegic migraine, an uncommon migraine subtype characterized by recurrent headaches associated with temporary unilateral hemiparesis or hemiplegia. The hemiparesis may resolve before the headache, as seen in the present case, or it may persist for days to weeks. These episodes are sometimes accompanied by ipsilateral numbness, tingling, or paresthesia, with or without a speech disturbance. Visual defects (ie, scintillating scotoma and hemianopia) and aphasia may occur.
Hemiplegic migraine can be sporadic or familial. Familial hemiplegic migraine is the only migraine subtype for which an autosomal dominant mode of inheritance has been identified. The onset is generally in adolescence between 12 and 17 years of age, with an estimated prevalence of 0.01%. Female patients are more likely to have these types of migraines.
Diagnosis of hemiplegic migraine is centered on exclusion of other possible causes of headache with motor weakness. When a patient presents with motor deficit, these symptoms can also be the result of a secondary headache rather than a primary headache disorder. Because of this neurologic aspect of presentation, the differential diagnosis is broad and should span other migraine subtypes, inflammatory or metabolic disorders, and mitochondrial diseases, as well as any condition that shows neurologic deficits without radiologic alterations. Pediatric patients with hemiplegic migraine are often misdiagnosed with epilepsy. Compared with hemiplegic migraine, seizures are much more brief, and any associated hemiparesis is usually characterized by limb jerking, head turning, and loss of consciousness. Of note, up to 7% of patients with familial hemiplegic migraine do eventually develop epilepsy. Although there are no telltale pathognomonic clinical, laboratory, or radiologic findings of hemiplegic migraine, electroencephalography may show asymmetric slow-wave activity contralateral to the hemiparesis.
In hemiplegic migraine, acute treatment options include antiemetics, NSAIDs, and nonnarcotic pain relievers; triptans and ergotamine preparations are contraindicated in this setting because of their potential vasoconstrictive effects. Even if episode frequency is low, the American Headache Society advises that prophylactic treatment should also be considered in the management of uncommon migraine subtypes such as this one.
Jasmin Harpe, MD, MPH, Headache Fellow, Department of Neurology, Harvard University, John R. Graham Headache Center, Mass General Brigham, Boston, MA
Jasmin Harpe, MD, MPH, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 16-year-old female patient presents with a severe ipsilateral headache. She describes that before the onset of head pain, she felt like she could not control her facial muscles on one side, and she was unable to speak in full sentences. She reports that these symptoms probably lasted an hour or so, and she was worried that she was experiencing an allergic reaction, though she reports no known allergies. In terms of family history, the patient explains that she does not have a close relationship with her father, but she recalls that he experienced similar episodes. She notes a history of frequent and recurrent headaches, varying in severity, for which she usually takes a high dose of nonsteroidal anti-inflammatory drugs (NSAIDs).
Cutaneous Body Image: How the Mental Health Benefits of Treating Dermatologic Disease Support Military Readiness in Service Members
According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
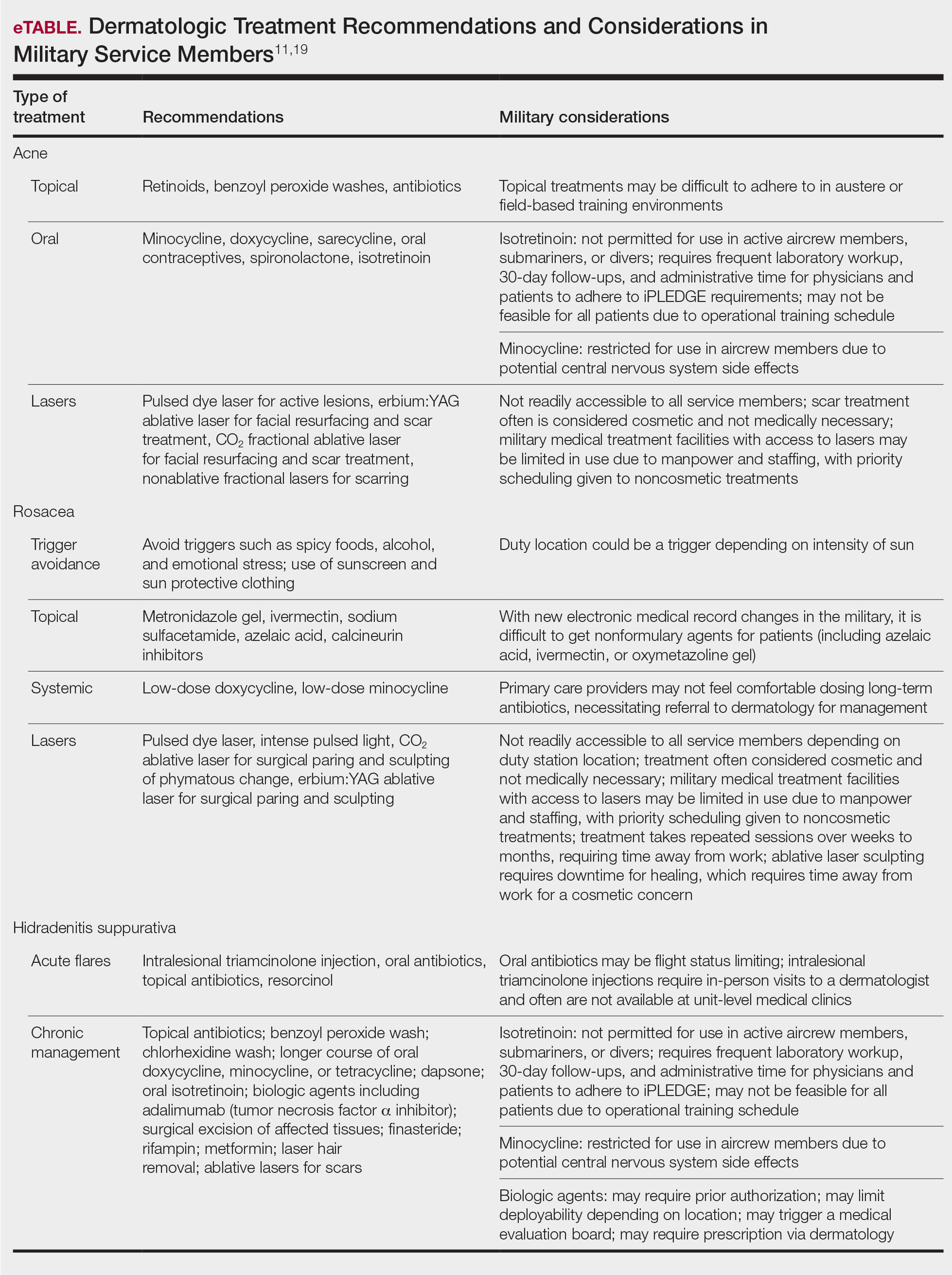
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.
Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.

- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.

Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.

According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.

Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.

- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
Practice Points
- The term readiness refers to the ability to recruit, train, deploy, and sustain military forces that are ready to “fight tonight” and succeed in combat.
- Maintaining readiness requires a holistic approach, as it is directly affected by physical and mental health outcomes.
- Cutaneous body image (CBI) refers to an individual’s mental perception of the condition of their hair, nails, and skin. Positive CBI is related to increased quality of life, while negative CBI, which often is associated with dermatologic disease, is associated with poorer health outcomes and even self-injury.
- Treatment of dermatologic disease in the context of active-duty military members can positively influence CBI, which may in turn increase service members’ quality of life and overall military readiness.
Assessing Treatment Delays for Vitiligo Patients: A Retrospective Chart Review
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.
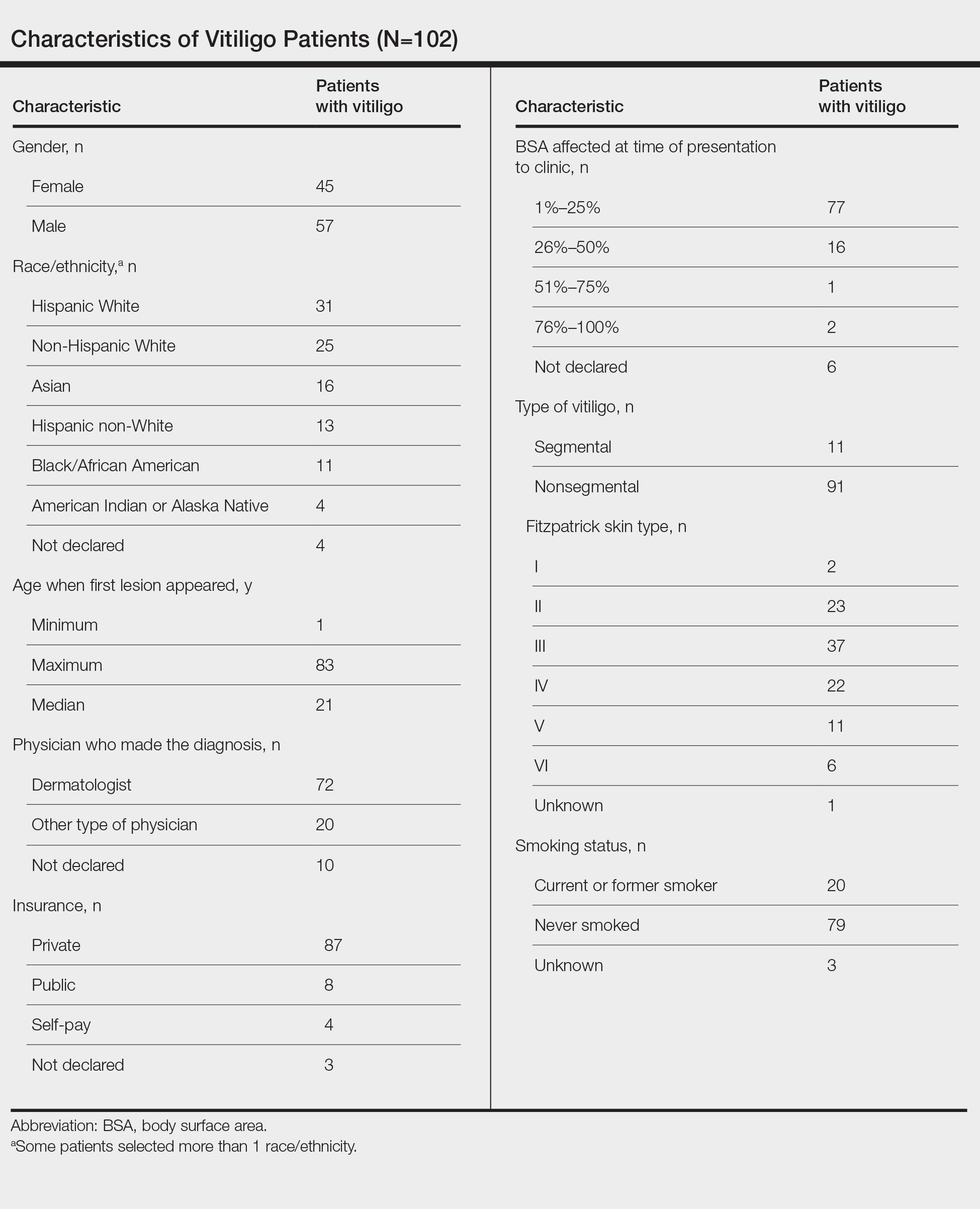
Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.

Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.

Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
Practice Points
- The medical community should be aware of factors associated with delay to treatment in patients with vitiligo, such as the diagnosing physician’s specialty and patient age at disease onset.
- Race and percentage of body surface area affected at time of presentation also demonstrate trends regarding treatment delays in patients with vitiligo.
When suffering defies diagnosis
I still remember the woman who came to my office that day, years ago. She was struggling and uncomfortable, and she wanted “something” for stress. She described her life, and to me, it sounded stressful. She lived in a blended family and she described the chaos that one might expect to find in a household with four teens, their friends, their activities, and all it took to keep the household going. I spent 2 hours evaluating the patient, and I could not find a diagnosis that fit this problem nor – I believed – a pill that would fix it. She didn’t “meet criteria” for a psychiatric disorder, but she insisted she was uncomfortable and she wanted to try medication. I admit, I relented and I gave her a prescription for fluoxetine.
When she returned a few weeks later, my patient said she felt better, and what I remember decades later was her statement: “Now I can see dishes in the sink and be okay with it.” Perhaps she had downplayed her anxiety during our first meeting, but what I took from this was that some people are uncomfortable in ways that our lexicon does not capture, and sometimes medication helps with this discomfort.
The APA’s Diagnostic and Statistical Manual of Mental Disorders attempts to capture the problems of emotional and behavioral distress and classify them into discrete syndromes that can be validated and reliably diagnosed by different evaluators. Our disorders are syndromic; they are defined by clusters of symptoms that occur together, and not by a single symptom, lab value, or radiologic finding. The DSM is rewritten periodically so that what is or is not a disorder can bend with new discoveries and with a changing culture. And for better or for worse, when there is an available medication that can alleviate a problem, this may influence what once was a variant of normal into becoming a disorder.
Our illnesses often lie along a spectrum, so there is no precise point where someone who is easily distracted is a person with attention deficit disorder as opposed to being a mentally healthy person who is easily distracted, or a shy person is someone with social anxiety disorder. whether they want to address this with medications, and whether their distress warrants taking a chance that they might have side effects or an adverse reaction to a medication.
When we look at our criteria, sometimes we fall short. One needs to have at least five symptoms out of nine options, to be present for 2 weeks to be diagnosed with major depression, yet I don’t know a single psychiatrist who would not offer medication to a patient who ascribed to feeling profoundly sad with thoughts of suicide in the absence of other symptoms of depression. These issues have come to the forefront with the recent inclusion of prolonged grief in the DSM, as a disorder that is distinct from both normal grieving and from major depression.
In recent weeks, mass murder has been on everyone’s mind as we mourn those lost in Uvalde, Buffalo, and unfortunately, in so many other places. Absolutely no one thinks that someone who shoots strangers is “normal” or emotionally well. Yet psychiatry is often tasked with figuring out if someone is mad (mentally ill), bad (evil), or both. We don’t have a clear path for how to treat and manage people who commit horrendous acts of violence unless they meet criteria for another illness. Yet no one would argue that a person who informs others that he is thinking of killing strangers is in need of some type of intervention, regardless of his motive. We struggle too, with how to manage people who have more regular angry outbursts or emotional dysregulation. Perhaps we diagnose intermittent explosive disorder, or irritability caused by a mood disorder, but we don’t always know how to help people to control their tempers and modulate their emotions. And our semantics to describe psychic pain and anguish are surprisingly limited – sometimes we can only assume that someone who lashes out must be in turmoil.
Psychiatry continues to struggle with our relationship with human suffering. Suffering is part of life, not necessarily a sign of illness, and in his iconic memoir, “Man’s Search for Meaning,” psychiatrist Viktor Frankl, MD, wrote of the atrocities he endured in a Nazi concentration camp. It was through his suffering that Dr. Frankl found meaning and he used these harrowing experiences to fuel positive emotions later in life. Dr. Frankl wrote: “If there is a meaning in life at all, then there must be a meaning in suffering. Suffering is an ineradicable part of life, even as fate and death. Without suffering and death, human life cannot be complete.”
Suffering may be the kindling for acts of violence, or for profound creativity. Would we have music, art, cinema, poetry, or fiction if no one ever suffered? Yet suffering and emotional torment are often what leads people to seek treatment, and what leads us, as healers, to offer any range of therapies. For years, suicide rates have been rising, as have overdose death. And now, in addition to these “deaths of despair,” we are hearing about skyrocketing rates of depression and anxiety in our world that is so full of reasons to be sad and anxious. Access to treatment is limited by so many things, and it is not always clear when one needs psychiatric interventions or when problems will heal on their own, leaving scars or not.
I wrote this article in response to the hundreds of comments that were placed on an article I wrote after the horrors at Uvalde and Buffalo: “Don’t Equate Mass Shootings with Mental Illness.” Many of the commenters suggested I believe the shooter was perfectly sane, and that I am naive (or worse). Many wrote in with their own thoughts about what causes people to become mass murderers. One commenter wrote: “To suggest that random killers do not have mental health issues and their behavior is normal is ridiculous.” I don’t believe that I ever suggested that such behavior was normal, but – for many of these crimes – we as a society have decided to treat the behavior as criminal and not as the product of our current concept of mental disorders. Obviously, people who are well, who are emotionally at peace and comfortable in their own skin, don’t kill strangers.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore.
I still remember the woman who came to my office that day, years ago. She was struggling and uncomfortable, and she wanted “something” for stress. She described her life, and to me, it sounded stressful. She lived in a blended family and she described the chaos that one might expect to find in a household with four teens, their friends, their activities, and all it took to keep the household going. I spent 2 hours evaluating the patient, and I could not find a diagnosis that fit this problem nor – I believed – a pill that would fix it. She didn’t “meet criteria” for a psychiatric disorder, but she insisted she was uncomfortable and she wanted to try medication. I admit, I relented and I gave her a prescription for fluoxetine.
When she returned a few weeks later, my patient said she felt better, and what I remember decades later was her statement: “Now I can see dishes in the sink and be okay with it.” Perhaps she had downplayed her anxiety during our first meeting, but what I took from this was that some people are uncomfortable in ways that our lexicon does not capture, and sometimes medication helps with this discomfort.
The APA’s Diagnostic and Statistical Manual of Mental Disorders attempts to capture the problems of emotional and behavioral distress and classify them into discrete syndromes that can be validated and reliably diagnosed by different evaluators. Our disorders are syndromic; they are defined by clusters of symptoms that occur together, and not by a single symptom, lab value, or radiologic finding. The DSM is rewritten periodically so that what is or is not a disorder can bend with new discoveries and with a changing culture. And for better or for worse, when there is an available medication that can alleviate a problem, this may influence what once was a variant of normal into becoming a disorder.
Our illnesses often lie along a spectrum, so there is no precise point where someone who is easily distracted is a person with attention deficit disorder as opposed to being a mentally healthy person who is easily distracted, or a shy person is someone with social anxiety disorder. whether they want to address this with medications, and whether their distress warrants taking a chance that they might have side effects or an adverse reaction to a medication.
When we look at our criteria, sometimes we fall short. One needs to have at least five symptoms out of nine options, to be present for 2 weeks to be diagnosed with major depression, yet I don’t know a single psychiatrist who would not offer medication to a patient who ascribed to feeling profoundly sad with thoughts of suicide in the absence of other symptoms of depression. These issues have come to the forefront with the recent inclusion of prolonged grief in the DSM, as a disorder that is distinct from both normal grieving and from major depression.
In recent weeks, mass murder has been on everyone’s mind as we mourn those lost in Uvalde, Buffalo, and unfortunately, in so many other places. Absolutely no one thinks that someone who shoots strangers is “normal” or emotionally well. Yet psychiatry is often tasked with figuring out if someone is mad (mentally ill), bad (evil), or both. We don’t have a clear path for how to treat and manage people who commit horrendous acts of violence unless they meet criteria for another illness. Yet no one would argue that a person who informs others that he is thinking of killing strangers is in need of some type of intervention, regardless of his motive. We struggle too, with how to manage people who have more regular angry outbursts or emotional dysregulation. Perhaps we diagnose intermittent explosive disorder, or irritability caused by a mood disorder, but we don’t always know how to help people to control their tempers and modulate their emotions. And our semantics to describe psychic pain and anguish are surprisingly limited – sometimes we can only assume that someone who lashes out must be in turmoil.
Psychiatry continues to struggle with our relationship with human suffering. Suffering is part of life, not necessarily a sign of illness, and in his iconic memoir, “Man’s Search for Meaning,” psychiatrist Viktor Frankl, MD, wrote of the atrocities he endured in a Nazi concentration camp. It was through his suffering that Dr. Frankl found meaning and he used these harrowing experiences to fuel positive emotions later in life. Dr. Frankl wrote: “If there is a meaning in life at all, then there must be a meaning in suffering. Suffering is an ineradicable part of life, even as fate and death. Without suffering and death, human life cannot be complete.”
Suffering may be the kindling for acts of violence, or for profound creativity. Would we have music, art, cinema, poetry, or fiction if no one ever suffered? Yet suffering and emotional torment are often what leads people to seek treatment, and what leads us, as healers, to offer any range of therapies. For years, suicide rates have been rising, as have overdose death. And now, in addition to these “deaths of despair,” we are hearing about skyrocketing rates of depression and anxiety in our world that is so full of reasons to be sad and anxious. Access to treatment is limited by so many things, and it is not always clear when one needs psychiatric interventions or when problems will heal on their own, leaving scars or not.
I wrote this article in response to the hundreds of comments that were placed on an article I wrote after the horrors at Uvalde and Buffalo: “Don’t Equate Mass Shootings with Mental Illness.” Many of the commenters suggested I believe the shooter was perfectly sane, and that I am naive (or worse). Many wrote in with their own thoughts about what causes people to become mass murderers. One commenter wrote: “To suggest that random killers do not have mental health issues and their behavior is normal is ridiculous.” I don’t believe that I ever suggested that such behavior was normal, but – for many of these crimes – we as a society have decided to treat the behavior as criminal and not as the product of our current concept of mental disorders. Obviously, people who are well, who are emotionally at peace and comfortable in their own skin, don’t kill strangers.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore.
I still remember the woman who came to my office that day, years ago. She was struggling and uncomfortable, and she wanted “something” for stress. She described her life, and to me, it sounded stressful. She lived in a blended family and she described the chaos that one might expect to find in a household with four teens, their friends, their activities, and all it took to keep the household going. I spent 2 hours evaluating the patient, and I could not find a diagnosis that fit this problem nor – I believed – a pill that would fix it. She didn’t “meet criteria” for a psychiatric disorder, but she insisted she was uncomfortable and she wanted to try medication. I admit, I relented and I gave her a prescription for fluoxetine.
When she returned a few weeks later, my patient said she felt better, and what I remember decades later was her statement: “Now I can see dishes in the sink and be okay with it.” Perhaps she had downplayed her anxiety during our first meeting, but what I took from this was that some people are uncomfortable in ways that our lexicon does not capture, and sometimes medication helps with this discomfort.
The APA’s Diagnostic and Statistical Manual of Mental Disorders attempts to capture the problems of emotional and behavioral distress and classify them into discrete syndromes that can be validated and reliably diagnosed by different evaluators. Our disorders are syndromic; they are defined by clusters of symptoms that occur together, and not by a single symptom, lab value, or radiologic finding. The DSM is rewritten periodically so that what is or is not a disorder can bend with new discoveries and with a changing culture. And for better or for worse, when there is an available medication that can alleviate a problem, this may influence what once was a variant of normal into becoming a disorder.
Our illnesses often lie along a spectrum, so there is no precise point where someone who is easily distracted is a person with attention deficit disorder as opposed to being a mentally healthy person who is easily distracted, or a shy person is someone with social anxiety disorder. whether they want to address this with medications, and whether their distress warrants taking a chance that they might have side effects or an adverse reaction to a medication.
When we look at our criteria, sometimes we fall short. One needs to have at least five symptoms out of nine options, to be present for 2 weeks to be diagnosed with major depression, yet I don’t know a single psychiatrist who would not offer medication to a patient who ascribed to feeling profoundly sad with thoughts of suicide in the absence of other symptoms of depression. These issues have come to the forefront with the recent inclusion of prolonged grief in the DSM, as a disorder that is distinct from both normal grieving and from major depression.
In recent weeks, mass murder has been on everyone’s mind as we mourn those lost in Uvalde, Buffalo, and unfortunately, in so many other places. Absolutely no one thinks that someone who shoots strangers is “normal” or emotionally well. Yet psychiatry is often tasked with figuring out if someone is mad (mentally ill), bad (evil), or both. We don’t have a clear path for how to treat and manage people who commit horrendous acts of violence unless they meet criteria for another illness. Yet no one would argue that a person who informs others that he is thinking of killing strangers is in need of some type of intervention, regardless of his motive. We struggle too, with how to manage people who have more regular angry outbursts or emotional dysregulation. Perhaps we diagnose intermittent explosive disorder, or irritability caused by a mood disorder, but we don’t always know how to help people to control their tempers and modulate their emotions. And our semantics to describe psychic pain and anguish are surprisingly limited – sometimes we can only assume that someone who lashes out must be in turmoil.
Psychiatry continues to struggle with our relationship with human suffering. Suffering is part of life, not necessarily a sign of illness, and in his iconic memoir, “Man’s Search for Meaning,” psychiatrist Viktor Frankl, MD, wrote of the atrocities he endured in a Nazi concentration camp. It was through his suffering that Dr. Frankl found meaning and he used these harrowing experiences to fuel positive emotions later in life. Dr. Frankl wrote: “If there is a meaning in life at all, then there must be a meaning in suffering. Suffering is an ineradicable part of life, even as fate and death. Without suffering and death, human life cannot be complete.”
Suffering may be the kindling for acts of violence, or for profound creativity. Would we have music, art, cinema, poetry, or fiction if no one ever suffered? Yet suffering and emotional torment are often what leads people to seek treatment, and what leads us, as healers, to offer any range of therapies. For years, suicide rates have been rising, as have overdose death. And now, in addition to these “deaths of despair,” we are hearing about skyrocketing rates of depression and anxiety in our world that is so full of reasons to be sad and anxious. Access to treatment is limited by so many things, and it is not always clear when one needs psychiatric interventions or when problems will heal on their own, leaving scars or not.
I wrote this article in response to the hundreds of comments that were placed on an article I wrote after the horrors at Uvalde and Buffalo: “Don’t Equate Mass Shootings with Mental Illness.” Many of the commenters suggested I believe the shooter was perfectly sane, and that I am naive (or worse). Many wrote in with their own thoughts about what causes people to become mass murderers. One commenter wrote: “To suggest that random killers do not have mental health issues and their behavior is normal is ridiculous.” I don’t believe that I ever suggested that such behavior was normal, but – for many of these crimes – we as a society have decided to treat the behavior as criminal and not as the product of our current concept of mental disorders. Obviously, people who are well, who are emotionally at peace and comfortable in their own skin, don’t kill strangers.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore.
Avexitide promising for hypoglycemia after weight-loss surgery
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022
Surgeons may underestimate recovery from incontinence operation
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AUGS 2022
In utero COVID exposure tied to neurodevelopmental disorders at 1 year
Infants exposed to SARS-CoV-2 in utero are at increased risk for neurodevelopmental disorders in the first year of life, new research suggests.
But whether it is exposure to the pandemic or maternal exposure to the virus itself that may harm early childhood neurodevelopment is unclear, caution investigators, led by Roy Perlis, MD, MSc, with Massachusetts General Hospital, Boston.
“In this analysis of 222 offspring of mothers infected with SARS-CoV-2, compared with the offspring of 7,550 mothers in the control group (not infected) delivered during the same period, we observed neurodevelopmental diagnoses to be significantly more common among exposed offspring, particularly those exposed to third-trimester maternal infection,” they write.
The study was published online in JAMA Network Open.
Speech and language disorders
The study included 7,772 mostly singleton live births across six hospitals in Massachusetts between March and September 2020, including 222 (2.9%) births to mothers with SARS-CoV-2 infection confirmed by polymerase chain reaction testing during pregnancy.
In all, 14 of 222 children born to SARS-CoV-2–infected mothers (6.3%) were diagnosed with a neurodevelopmental disorder in the first year of life versus 227 of 7,550 unexposed offspring (3%) (unadjusted odds ratio, 2.17; 95% confidence interval, 1.24-3.79; P = .006).
In models adjusted for preterm delivery, as well as race, ethnicity, insurance status, child sex, and maternal age, COVID-exposed offspring were significantly more likely to receive a neurodevelopmental diagnosis in the first year of life (adjusted OR, 1.86; 95% CI, 1.03-3.36; P = .04).
The magnitude of the association with neurodevelopmental disorders was greater with third-trimester SARS-CoV-2 infection (aOR, 2.34; 95% CI, 1.23-4.44; P = .01).
The majority of these diagnoses reflected developmental disorders of motor function or speech and language.
The researchers noted that the finding of an association between prenatal SARS-CoV-2 exposure and neurodevelopmental diagnoses at 12 months is in line with a “large body of literature” linking maternal viral infection and maternal immune activation with offspring neurodevelopmental disorders later in life.
They cautioned, however, that whether a definitive connection exists between prenatal SARS-CoV-2 exposure and adverse neurodevelopment in offspring is not yet known, in part because children born to women infected in the first wave of the pandemic haven’t reached their second birthday – a time when neurodevelopment disorders such as autism are typically diagnosed.
There is also the risk for ascertainment bias arising from greater concern for offspring of infected mothers who were ill during pregnancy. These parents may be more inclined to seek evaluation, and clinicians may be more inclined to diagnose or refer for evaluation, the researchers noted.
Nonetheless, as reported by this news organization, the study results support those of research released at the European Psychiatric Association 2022 Congress; those results also showed an association between maternal SARS-CoV-2 infection and impaired neurodevelopment in 6-week-old infants.
Hypothesis generating
In an accompanying commentary, Torri D. Metz, MD, MS, with University of Utah Health, Salt Lake City, said the preliminary findings of Dr. Perlis and colleagues are “critically important, yet many questions remain.”
“Essentially all of what we know now about the effects of in utero exposure to maternal SARS-CoV-2 infection is from children who were exposed to the early and Alpha variants of SARS-CoV-2, as those are the only children now old enough to undergo rigorous neurodevelopmental assessments,” Dr. Metz pointed out.
Ultimately, Dr. Metz said it’s not surprising that the pandemic and in utero exposure to maternal SARS-CoV-2 infection may adversely affect neurodevelopmental outcomes in young children.
Yet, as a retrospective cohort study, the study can only demonstrate associations, not causality.
“This type of work is intended to be hypothesis generating, and that goal has been accomplished as these preliminary findings generate numerous additional research questions to explore,” Dr. Metz wrote.
Among them: Are there genetic predispositions to adverse outcomes? Will we observe differential effects by SARS-CoV-2 variant, by severity of infection, and by trimester of infection? Is it the virus itself or all of the societal changes that occurred during this period, including differences in how those changes were experienced among those with and without SARS-CoV-2?
“Perhaps the most important question is how do we intervene to help mitigate the adverse effects of the pandemic on young children,” Dr. Metz noted.
“Prospective studies to validate these findings, tease out some of the nuance, and identify those at highest risk will help health care practitioners appropriately dedicate resources to improve outcomes as we follow the life course of this generation of children born during the COVID-19 pandemic,” she added.
The study was supported by the National Institute of Mental Health and the National Institute of Child Health and Human Development. Dr. Perlis is an associate editor for JAMA Network Open but was not involved in the editorial review or decision for the study. Dr. Metz reported receiving personal fees and grants from Pfizer and grants from GestVision.
A version of this article first appeared on Medscape.com.
Infants exposed to SARS-CoV-2 in utero are at increased risk for neurodevelopmental disorders in the first year of life, new research suggests.
But whether it is exposure to the pandemic or maternal exposure to the virus itself that may harm early childhood neurodevelopment is unclear, caution investigators, led by Roy Perlis, MD, MSc, with Massachusetts General Hospital, Boston.
“In this analysis of 222 offspring of mothers infected with SARS-CoV-2, compared with the offspring of 7,550 mothers in the control group (not infected) delivered during the same period, we observed neurodevelopmental diagnoses to be significantly more common among exposed offspring, particularly those exposed to third-trimester maternal infection,” they write.
The study was published online in JAMA Network Open.
Speech and language disorders
The study included 7,772 mostly singleton live births across six hospitals in Massachusetts between March and September 2020, including 222 (2.9%) births to mothers with SARS-CoV-2 infection confirmed by polymerase chain reaction testing during pregnancy.
In all, 14 of 222 children born to SARS-CoV-2–infected mothers (6.3%) were diagnosed with a neurodevelopmental disorder in the first year of life versus 227 of 7,550 unexposed offspring (3%) (unadjusted odds ratio, 2.17; 95% confidence interval, 1.24-3.79; P = .006).
In models adjusted for preterm delivery, as well as race, ethnicity, insurance status, child sex, and maternal age, COVID-exposed offspring were significantly more likely to receive a neurodevelopmental diagnosis in the first year of life (adjusted OR, 1.86; 95% CI, 1.03-3.36; P = .04).
The magnitude of the association with neurodevelopmental disorders was greater with third-trimester SARS-CoV-2 infection (aOR, 2.34; 95% CI, 1.23-4.44; P = .01).
The majority of these diagnoses reflected developmental disorders of motor function or speech and language.
The researchers noted that the finding of an association between prenatal SARS-CoV-2 exposure and neurodevelopmental diagnoses at 12 months is in line with a “large body of literature” linking maternal viral infection and maternal immune activation with offspring neurodevelopmental disorders later in life.
They cautioned, however, that whether a definitive connection exists between prenatal SARS-CoV-2 exposure and adverse neurodevelopment in offspring is not yet known, in part because children born to women infected in the first wave of the pandemic haven’t reached their second birthday – a time when neurodevelopment disorders such as autism are typically diagnosed.
There is also the risk for ascertainment bias arising from greater concern for offspring of infected mothers who were ill during pregnancy. These parents may be more inclined to seek evaluation, and clinicians may be more inclined to diagnose or refer for evaluation, the researchers noted.
Nonetheless, as reported by this news organization, the study results support those of research released at the European Psychiatric Association 2022 Congress; those results also showed an association between maternal SARS-CoV-2 infection and impaired neurodevelopment in 6-week-old infants.
Hypothesis generating
In an accompanying commentary, Torri D. Metz, MD, MS, with University of Utah Health, Salt Lake City, said the preliminary findings of Dr. Perlis and colleagues are “critically important, yet many questions remain.”
“Essentially all of what we know now about the effects of in utero exposure to maternal SARS-CoV-2 infection is from children who were exposed to the early and Alpha variants of SARS-CoV-2, as those are the only children now old enough to undergo rigorous neurodevelopmental assessments,” Dr. Metz pointed out.
Ultimately, Dr. Metz said it’s not surprising that the pandemic and in utero exposure to maternal SARS-CoV-2 infection may adversely affect neurodevelopmental outcomes in young children.
Yet, as a retrospective cohort study, the study can only demonstrate associations, not causality.
“This type of work is intended to be hypothesis generating, and that goal has been accomplished as these preliminary findings generate numerous additional research questions to explore,” Dr. Metz wrote.
Among them: Are there genetic predispositions to adverse outcomes? Will we observe differential effects by SARS-CoV-2 variant, by severity of infection, and by trimester of infection? Is it the virus itself or all of the societal changes that occurred during this period, including differences in how those changes were experienced among those with and without SARS-CoV-2?
“Perhaps the most important question is how do we intervene to help mitigate the adverse effects of the pandemic on young children,” Dr. Metz noted.
“Prospective studies to validate these findings, tease out some of the nuance, and identify those at highest risk will help health care practitioners appropriately dedicate resources to improve outcomes as we follow the life course of this generation of children born during the COVID-19 pandemic,” she added.
The study was supported by the National Institute of Mental Health and the National Institute of Child Health and Human Development. Dr. Perlis is an associate editor for JAMA Network Open but was not involved in the editorial review or decision for the study. Dr. Metz reported receiving personal fees and grants from Pfizer and grants from GestVision.
A version of this article first appeared on Medscape.com.
Infants exposed to SARS-CoV-2 in utero are at increased risk for neurodevelopmental disorders in the first year of life, new research suggests.
But whether it is exposure to the pandemic or maternal exposure to the virus itself that may harm early childhood neurodevelopment is unclear, caution investigators, led by Roy Perlis, MD, MSc, with Massachusetts General Hospital, Boston.
“In this analysis of 222 offspring of mothers infected with SARS-CoV-2, compared with the offspring of 7,550 mothers in the control group (not infected) delivered during the same period, we observed neurodevelopmental diagnoses to be significantly more common among exposed offspring, particularly those exposed to third-trimester maternal infection,” they write.
The study was published online in JAMA Network Open.
Speech and language disorders
The study included 7,772 mostly singleton live births across six hospitals in Massachusetts between March and September 2020, including 222 (2.9%) births to mothers with SARS-CoV-2 infection confirmed by polymerase chain reaction testing during pregnancy.
In all, 14 of 222 children born to SARS-CoV-2–infected mothers (6.3%) were diagnosed with a neurodevelopmental disorder in the first year of life versus 227 of 7,550 unexposed offspring (3%) (unadjusted odds ratio, 2.17; 95% confidence interval, 1.24-3.79; P = .006).
In models adjusted for preterm delivery, as well as race, ethnicity, insurance status, child sex, and maternal age, COVID-exposed offspring were significantly more likely to receive a neurodevelopmental diagnosis in the first year of life (adjusted OR, 1.86; 95% CI, 1.03-3.36; P = .04).
The magnitude of the association with neurodevelopmental disorders was greater with third-trimester SARS-CoV-2 infection (aOR, 2.34; 95% CI, 1.23-4.44; P = .01).
The majority of these diagnoses reflected developmental disorders of motor function or speech and language.
The researchers noted that the finding of an association between prenatal SARS-CoV-2 exposure and neurodevelopmental diagnoses at 12 months is in line with a “large body of literature” linking maternal viral infection and maternal immune activation with offspring neurodevelopmental disorders later in life.
They cautioned, however, that whether a definitive connection exists between prenatal SARS-CoV-2 exposure and adverse neurodevelopment in offspring is not yet known, in part because children born to women infected in the first wave of the pandemic haven’t reached their second birthday – a time when neurodevelopment disorders such as autism are typically diagnosed.
There is also the risk for ascertainment bias arising from greater concern for offspring of infected mothers who were ill during pregnancy. These parents may be more inclined to seek evaluation, and clinicians may be more inclined to diagnose or refer for evaluation, the researchers noted.
Nonetheless, as reported by this news organization, the study results support those of research released at the European Psychiatric Association 2022 Congress; those results also showed an association between maternal SARS-CoV-2 infection and impaired neurodevelopment in 6-week-old infants.
Hypothesis generating
In an accompanying commentary, Torri D. Metz, MD, MS, with University of Utah Health, Salt Lake City, said the preliminary findings of Dr. Perlis and colleagues are “critically important, yet many questions remain.”
“Essentially all of what we know now about the effects of in utero exposure to maternal SARS-CoV-2 infection is from children who were exposed to the early and Alpha variants of SARS-CoV-2, as those are the only children now old enough to undergo rigorous neurodevelopmental assessments,” Dr. Metz pointed out.
Ultimately, Dr. Metz said it’s not surprising that the pandemic and in utero exposure to maternal SARS-CoV-2 infection may adversely affect neurodevelopmental outcomes in young children.
Yet, as a retrospective cohort study, the study can only demonstrate associations, not causality.
“This type of work is intended to be hypothesis generating, and that goal has been accomplished as these preliminary findings generate numerous additional research questions to explore,” Dr. Metz wrote.
Among them: Are there genetic predispositions to adverse outcomes? Will we observe differential effects by SARS-CoV-2 variant, by severity of infection, and by trimester of infection? Is it the virus itself or all of the societal changes that occurred during this period, including differences in how those changes were experienced among those with and without SARS-CoV-2?
“Perhaps the most important question is how do we intervene to help mitigate the adverse effects of the pandemic on young children,” Dr. Metz noted.
“Prospective studies to validate these findings, tease out some of the nuance, and identify those at highest risk will help health care practitioners appropriately dedicate resources to improve outcomes as we follow the life course of this generation of children born during the COVID-19 pandemic,” she added.
The study was supported by the National Institute of Mental Health and the National Institute of Child Health and Human Development. Dr. Perlis is an associate editor for JAMA Network Open but was not involved in the editorial review or decision for the study. Dr. Metz reported receiving personal fees and grants from Pfizer and grants from GestVision.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
FDA OKs first systemic treatment for alopecia areata
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.