User login
Should you treat asymptomatic bacteriuria in an older adult with altered mental status?
THE CASE
A 78-year-old woman with a past medical history of hypertension, hyperlipidemia, osteoarthritis, and osteopenia was brought to the emergency department (ED) by her daughter. The woman had fallen 2 days earlier and had been experiencing a change in mental status (confusion) for the previous 4 days. Prior to her change in mental status, the patient had been independent in all activities of daily living and instrumental activities of daily living.
Her daughter could not recall any symptoms of illness; new or recently changed medications; complaints of pain, constipation, diarrhea, urinary frequency, or hematuria; or changes in continence prior to the onset of her mother’s confusion.
The patient’s medications included amlodipine, atorvastatin, calcium/vitamin D, and acetaminophen (as needed). In the ED, her vital signs were normal, and her cardiopulmonary and abdominal exams were unremarkable. A limited neurologic exam showed that the patient was oriented only to person and could not answer questions about her symptoms or follow commands. She could move all of her extremities equally and could ambulate; she had no facial asymmetry or slurred speech. Her exam was negative for orthostatic hypotension.
Her complete blood count, comprehensive metabolic panel, and troponin levels were normal. Her electrocardiogram showed normal sinus rhythm with no abnormalities. X-rays of her right hip and elbow were negative for fracture. Computed tomography of her head was negative for acute findings, and a chest x-ray was normal.
Her urinalysis showed many bacteria and large leukocyte esterase, and a urine culture was sent out. She was hemodynamically stable and there were no known urinary symptoms, so no empiric antibiotics were started. She was admitted for further evaluation of her altered mental status (AMS).
On our service, she was given intravenous fluids, and oral intake was encouraged. She had normal levels of B12, folic acid, and thyroid-stimulating hormone. She was negative for HIV and syphilis. Acute coronary syndrome was ruled out with normal electrocardiograms and troponin levels. Her telemetry showed a normal sinus rhythm.
After 2 days, her vital signs and labs remained stable and no other abnormalities were found; however, she had not returned to her baseline mental status. Then the urine culture returned with > 105 CFU/mL of Escherichia coli, prompting a resident to curbside me (AP) and ask: “I shouldn’t treat this patient based on her urine culture—she’s just colonized, right? Or should I treat her because she’s altered?”
Continue to: THE CHALLENGE
THE CHALLENGE
Identifying and managing urinary tract infections (UTIs) in older adults often presents a challenge, further complicated if patients have AMS or cognitive impairment and are unable to confirm or deny urinary symptoms.
Consider, for instance, the definition of symptomatic UTI: significant bacteriuria (≥ 105 CFU/mL) and pyuria (> 10 WBC/hpf) with UTI-specific symptoms (fever, acute dysuria, new or worsening urgency or frequency, new urinary incontinence, gross hematuria, and suprapubic or costovertebral angle pain or tenderness).1 In older adults, these parameters require a more careful look.
For instance, while we use the cutoff of ≥ 105 CFU/mL to define “significant” bacteriuria, the truth is that we don’t know the colony count threshold that can help identify patients who are at risk of serious illness and might benefit from antibiotic treatment.2
After reviewing the culture results, clinicians then face 2 specific challenges: differentiating between acute vs chronic symptoms and related vs unrelated symptoms in the older adult population.
Challenge 1: There is a high prevalence of chronic genitourinary symptoms in older adults that can sometimes make it hard to distinguish between an acute UTI and the acute recognition of a chronic, non-UTI problem.1
Continue to: Challenge 2
Challenge 2: There is a high prevalence of multimorbidity in older adults. For instance, diuretics for heart failure can cause UTI-specific symptoms such as urinary urgency, frequency, and even incontinence. Cognitive impairment can make it difficult to obtain the key components of the history needed to make a UTI diagnosis.1
Lastly, there are aspects of normal aging physiology that complicate the detection of infections, such as the fact that older adults may not mount a “true” fever to meet criteria for a symptomatic UTI. Therefore, fever in institutionalized or frail community-dwelling older adults has been redefined as an oral temperature ≥ 100 °F, 2 repeated oral temperatures > 99 °F, or an increase in temperature ≥ 2 °F from baseline.3
So how to proceed with our case patient? The following questions helped guide the approach to her care.
Is this patient asymptomatic?
Yes. The patient presented with nonspecific symptoms (falls and delirium) with bacteriuria suggesting asymptomatic bacteriuria (ASB). These symptoms are referred to as geriatric syndromes that, by definition, are “multifactorial health conditions that occur when the accumulated effects of impairments in multiple systems render an older person vulnerable to situational challenges.”4
As geriatric syndromes, falls and delirium are unlikely to be caused by one process, such as a UTI, but rather from multiple morbid processes. It is also important to note that there is no evidence to support a causal relationship between bacteriuria and delirium or that antibiotic treatment of bacteriuria improves delirium.2,5
Continue to: So, while we could...
So, while we could have diagnosed a UTI in this older adult with bacteriuria and delirium, it would have been premature closure and an incomplete assessment. We would have risked potentially missing other significant causes of her delirium and unnecessarily exposing the patient to antibiotics.
Are antibiotics generally useful in older adults who you believe to be asymptomatic with a urine culture showing bacteriuria?
No. The goal of antibiotic treatment for a symptomatic UTI is to ameliorate symptoms; therefore, there is no indication for antibiotics in ASB and no evidence of survival benefit.2 And, as noted earlier, there is no evidence to support a causal relationship between bacteriuria and delirium or that antibiotic treatment of bacteriuria improves delirium.2,5
The use of antibiotics in the asymptomatic setting will eradicate any bacteriuria but also increase the risk of reinfection, resistant organisms, antibiotic adverse reactions, and medication interactions.1
What is the recommendation for management of nonspecific symptoms, such as delirium and falls, in a geriatric patient such as this one with bacteriuria?
The Infectious Diseases Society of America (IDSA)’s 2019 Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria recommends a thorough assessment (for other causes) and careful observation, rather than immediate antimicrobial treatment and cessation of evaluation for other causes.5 (IDSA made this recommendation based on low-quality evidence.) The group found a high certainty of harm and low certainty of benefit in treating older adults with antibiotics for ASB.
This recommendation highlights the key geriatric principle of “geriatric syndromes” and the multifactorial nature of findings such as delirium and falls. It encourages clinicians to continue their thorough assessment for other causes in addition to bacteriuria.5 Even in the event that antibiotics are immediately initiated, we would recommend avoiding premature closure and continuing to evaluate for other causes.
Continue to: It is reasonable to...
It is reasonable to obtain a dipstick if, after the observation period (1-7 days, with earlier follow-up for frail patients), the patient continues to have the nonspecific symptoms.1 If the dipstick is negative, there is no need for further evaluation of UTI. If it’s positive, then it’s appropriate to send for urinalysis and urine culture.1
If the urine culture is negative, continue looking for other etiologies. If it’s positive, but there is resolution of symptoms, there is no need to treat. If it’s positive and symptoms persist, consider antibiotic treatment.1
CASE RESOLUTION
The team closely monitored the patient and delayed empiric antibiotics while continuing the AMS work-up. After 2 days in the hospital, her delirium persisted, but she had no UTI-specific symptoms and she remained hemodynamically stable.
I (AP) recommended antibiotic treatment guided by the urine culture sensitivity report: initially 1 g of ceftriaxone IV q24h with transition (after symptom improvement and prior to discharge) to oral trimethoprim/sulfamethoxazole 160 mg/800 mg q12h, for a total of 10 days of treatment. I emphasized that we were treating bacteriuria with persisting delirium without any other etiology identified. The patient returned to her baseline mental status after a few days of treatment and was discharged home.
THE TAKEAWAY
Avoid premature closure by stopping at the diagnosis of a “UTI” in an older adult with nonspecific symptoms and bacteriuria to avoid the risk of overlooking other important and potentially life-threatening causes of the patient’s signs and symptoms.
CORRESPONDENCE
L. Amanda Perry, MD, 1919 West Taylor Street, Mail Code 663, Chicago, IL 60612; [email protected]
1. Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA. 2014;311:844-854. doi: 10.1001/jama.2014.303
2. Finucane TE. “Urinary tract infection”- requiem for a heavyweight. J Am Geriatr Soc. 2017;65:1650-1655. doi: 10.1111/jgs.14907
3. Ashraf MS, Gaur S, Bushen OY, et al; Infection Advisory SubCommittee for AMDA—The Society of Post-Acute and Long-Term Care Medicine. Diagnosis, treatment, and prevention of urinary tract infections in post-acute and long-term care settings: a consensus statement from AMDA’s Infection Advisory Subcommittee. J Am Med Dir Assoc. 2020;21:12-24 e12. doi: 10.1016/j.jamda.2019.11.004
4. Inouye SK, Studenski S, Tinetti, ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780-791. doi: 10.1111/j.1532-5415.2007.01156.x
5. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83-e110. doi: 10.1093/cid/ciy1121
THE CASE
A 78-year-old woman with a past medical history of hypertension, hyperlipidemia, osteoarthritis, and osteopenia was brought to the emergency department (ED) by her daughter. The woman had fallen 2 days earlier and had been experiencing a change in mental status (confusion) for the previous 4 days. Prior to her change in mental status, the patient had been independent in all activities of daily living and instrumental activities of daily living.
Her daughter could not recall any symptoms of illness; new or recently changed medications; complaints of pain, constipation, diarrhea, urinary frequency, or hematuria; or changes in continence prior to the onset of her mother’s confusion.
The patient’s medications included amlodipine, atorvastatin, calcium/vitamin D, and acetaminophen (as needed). In the ED, her vital signs were normal, and her cardiopulmonary and abdominal exams were unremarkable. A limited neurologic exam showed that the patient was oriented only to person and could not answer questions about her symptoms or follow commands. She could move all of her extremities equally and could ambulate; she had no facial asymmetry or slurred speech. Her exam was negative for orthostatic hypotension.
Her complete blood count, comprehensive metabolic panel, and troponin levels were normal. Her electrocardiogram showed normal sinus rhythm with no abnormalities. X-rays of her right hip and elbow were negative for fracture. Computed tomography of her head was negative for acute findings, and a chest x-ray was normal.
Her urinalysis showed many bacteria and large leukocyte esterase, and a urine culture was sent out. She was hemodynamically stable and there were no known urinary symptoms, so no empiric antibiotics were started. She was admitted for further evaluation of her altered mental status (AMS).
On our service, she was given intravenous fluids, and oral intake was encouraged. She had normal levels of B12, folic acid, and thyroid-stimulating hormone. She was negative for HIV and syphilis. Acute coronary syndrome was ruled out with normal electrocardiograms and troponin levels. Her telemetry showed a normal sinus rhythm.
After 2 days, her vital signs and labs remained stable and no other abnormalities were found; however, she had not returned to her baseline mental status. Then the urine culture returned with > 105 CFU/mL of Escherichia coli, prompting a resident to curbside me (AP) and ask: “I shouldn’t treat this patient based on her urine culture—she’s just colonized, right? Or should I treat her because she’s altered?”
Continue to: THE CHALLENGE
THE CHALLENGE
Identifying and managing urinary tract infections (UTIs) in older adults often presents a challenge, further complicated if patients have AMS or cognitive impairment and are unable to confirm or deny urinary symptoms.
Consider, for instance, the definition of symptomatic UTI: significant bacteriuria (≥ 105 CFU/mL) and pyuria (> 10 WBC/hpf) with UTI-specific symptoms (fever, acute dysuria, new or worsening urgency or frequency, new urinary incontinence, gross hematuria, and suprapubic or costovertebral angle pain or tenderness).1 In older adults, these parameters require a more careful look.
For instance, while we use the cutoff of ≥ 105 CFU/mL to define “significant” bacteriuria, the truth is that we don’t know the colony count threshold that can help identify patients who are at risk of serious illness and might benefit from antibiotic treatment.2
After reviewing the culture results, clinicians then face 2 specific challenges: differentiating between acute vs chronic symptoms and related vs unrelated symptoms in the older adult population.
Challenge 1: There is a high prevalence of chronic genitourinary symptoms in older adults that can sometimes make it hard to distinguish between an acute UTI and the acute recognition of a chronic, non-UTI problem.1
Continue to: Challenge 2
Challenge 2: There is a high prevalence of multimorbidity in older adults. For instance, diuretics for heart failure can cause UTI-specific symptoms such as urinary urgency, frequency, and even incontinence. Cognitive impairment can make it difficult to obtain the key components of the history needed to make a UTI diagnosis.1
Lastly, there are aspects of normal aging physiology that complicate the detection of infections, such as the fact that older adults may not mount a “true” fever to meet criteria for a symptomatic UTI. Therefore, fever in institutionalized or frail community-dwelling older adults has been redefined as an oral temperature ≥ 100 °F, 2 repeated oral temperatures > 99 °F, or an increase in temperature ≥ 2 °F from baseline.3
So how to proceed with our case patient? The following questions helped guide the approach to her care.
Is this patient asymptomatic?
Yes. The patient presented with nonspecific symptoms (falls and delirium) with bacteriuria suggesting asymptomatic bacteriuria (ASB). These symptoms are referred to as geriatric syndromes that, by definition, are “multifactorial health conditions that occur when the accumulated effects of impairments in multiple systems render an older person vulnerable to situational challenges.”4
As geriatric syndromes, falls and delirium are unlikely to be caused by one process, such as a UTI, but rather from multiple morbid processes. It is also important to note that there is no evidence to support a causal relationship between bacteriuria and delirium or that antibiotic treatment of bacteriuria improves delirium.2,5
Continue to: So, while we could...
So, while we could have diagnosed a UTI in this older adult with bacteriuria and delirium, it would have been premature closure and an incomplete assessment. We would have risked potentially missing other significant causes of her delirium and unnecessarily exposing the patient to antibiotics.
Are antibiotics generally useful in older adults who you believe to be asymptomatic with a urine culture showing bacteriuria?
No. The goal of antibiotic treatment for a symptomatic UTI is to ameliorate symptoms; therefore, there is no indication for antibiotics in ASB and no evidence of survival benefit.2 And, as noted earlier, there is no evidence to support a causal relationship between bacteriuria and delirium or that antibiotic treatment of bacteriuria improves delirium.2,5
The use of antibiotics in the asymptomatic setting will eradicate any bacteriuria but also increase the risk of reinfection, resistant organisms, antibiotic adverse reactions, and medication interactions.1
What is the recommendation for management of nonspecific symptoms, such as delirium and falls, in a geriatric patient such as this one with bacteriuria?
The Infectious Diseases Society of America (IDSA)’s 2019 Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria recommends a thorough assessment (for other causes) and careful observation, rather than immediate antimicrobial treatment and cessation of evaluation for other causes.5 (IDSA made this recommendation based on low-quality evidence.) The group found a high certainty of harm and low certainty of benefit in treating older adults with antibiotics for ASB.
This recommendation highlights the key geriatric principle of “geriatric syndromes” and the multifactorial nature of findings such as delirium and falls. It encourages clinicians to continue their thorough assessment for other causes in addition to bacteriuria.5 Even in the event that antibiotics are immediately initiated, we would recommend avoiding premature closure and continuing to evaluate for other causes.
Continue to: It is reasonable to...
It is reasonable to obtain a dipstick if, after the observation period (1-7 days, with earlier follow-up for frail patients), the patient continues to have the nonspecific symptoms.1 If the dipstick is negative, there is no need for further evaluation of UTI. If it’s positive, then it’s appropriate to send for urinalysis and urine culture.1
If the urine culture is negative, continue looking for other etiologies. If it’s positive, but there is resolution of symptoms, there is no need to treat. If it’s positive and symptoms persist, consider antibiotic treatment.1
CASE RESOLUTION
The team closely monitored the patient and delayed empiric antibiotics while continuing the AMS work-up. After 2 days in the hospital, her delirium persisted, but she had no UTI-specific symptoms and she remained hemodynamically stable.
I (AP) recommended antibiotic treatment guided by the urine culture sensitivity report: initially 1 g of ceftriaxone IV q24h with transition (after symptom improvement and prior to discharge) to oral trimethoprim/sulfamethoxazole 160 mg/800 mg q12h, for a total of 10 days of treatment. I emphasized that we were treating bacteriuria with persisting delirium without any other etiology identified. The patient returned to her baseline mental status after a few days of treatment and was discharged home.
THE TAKEAWAY
Avoid premature closure by stopping at the diagnosis of a “UTI” in an older adult with nonspecific symptoms and bacteriuria to avoid the risk of overlooking other important and potentially life-threatening causes of the patient’s signs and symptoms.
CORRESPONDENCE
L. Amanda Perry, MD, 1919 West Taylor Street, Mail Code 663, Chicago, IL 60612; [email protected]
THE CASE
A 78-year-old woman with a past medical history of hypertension, hyperlipidemia, osteoarthritis, and osteopenia was brought to the emergency department (ED) by her daughter. The woman had fallen 2 days earlier and had been experiencing a change in mental status (confusion) for the previous 4 days. Prior to her change in mental status, the patient had been independent in all activities of daily living and instrumental activities of daily living.
Her daughter could not recall any symptoms of illness; new or recently changed medications; complaints of pain, constipation, diarrhea, urinary frequency, or hematuria; or changes in continence prior to the onset of her mother’s confusion.
The patient’s medications included amlodipine, atorvastatin, calcium/vitamin D, and acetaminophen (as needed). In the ED, her vital signs were normal, and her cardiopulmonary and abdominal exams were unremarkable. A limited neurologic exam showed that the patient was oriented only to person and could not answer questions about her symptoms or follow commands. She could move all of her extremities equally and could ambulate; she had no facial asymmetry or slurred speech. Her exam was negative for orthostatic hypotension.
Her complete blood count, comprehensive metabolic panel, and troponin levels were normal. Her electrocardiogram showed normal sinus rhythm with no abnormalities. X-rays of her right hip and elbow were negative for fracture. Computed tomography of her head was negative for acute findings, and a chest x-ray was normal.
Her urinalysis showed many bacteria and large leukocyte esterase, and a urine culture was sent out. She was hemodynamically stable and there were no known urinary symptoms, so no empiric antibiotics were started. She was admitted for further evaluation of her altered mental status (AMS).
On our service, she was given intravenous fluids, and oral intake was encouraged. She had normal levels of B12, folic acid, and thyroid-stimulating hormone. She was negative for HIV and syphilis. Acute coronary syndrome was ruled out with normal electrocardiograms and troponin levels. Her telemetry showed a normal sinus rhythm.
After 2 days, her vital signs and labs remained stable and no other abnormalities were found; however, she had not returned to her baseline mental status. Then the urine culture returned with > 105 CFU/mL of Escherichia coli, prompting a resident to curbside me (AP) and ask: “I shouldn’t treat this patient based on her urine culture—she’s just colonized, right? Or should I treat her because she’s altered?”
Continue to: THE CHALLENGE
THE CHALLENGE
Identifying and managing urinary tract infections (UTIs) in older adults often presents a challenge, further complicated if patients have AMS or cognitive impairment and are unable to confirm or deny urinary symptoms.
Consider, for instance, the definition of symptomatic UTI: significant bacteriuria (≥ 105 CFU/mL) and pyuria (> 10 WBC/hpf) with UTI-specific symptoms (fever, acute dysuria, new or worsening urgency or frequency, new urinary incontinence, gross hematuria, and suprapubic or costovertebral angle pain or tenderness).1 In older adults, these parameters require a more careful look.
For instance, while we use the cutoff of ≥ 105 CFU/mL to define “significant” bacteriuria, the truth is that we don’t know the colony count threshold that can help identify patients who are at risk of serious illness and might benefit from antibiotic treatment.2
After reviewing the culture results, clinicians then face 2 specific challenges: differentiating between acute vs chronic symptoms and related vs unrelated symptoms in the older adult population.
Challenge 1: There is a high prevalence of chronic genitourinary symptoms in older adults that can sometimes make it hard to distinguish between an acute UTI and the acute recognition of a chronic, non-UTI problem.1
Continue to: Challenge 2
Challenge 2: There is a high prevalence of multimorbidity in older adults. For instance, diuretics for heart failure can cause UTI-specific symptoms such as urinary urgency, frequency, and even incontinence. Cognitive impairment can make it difficult to obtain the key components of the history needed to make a UTI diagnosis.1
Lastly, there are aspects of normal aging physiology that complicate the detection of infections, such as the fact that older adults may not mount a “true” fever to meet criteria for a symptomatic UTI. Therefore, fever in institutionalized or frail community-dwelling older adults has been redefined as an oral temperature ≥ 100 °F, 2 repeated oral temperatures > 99 °F, or an increase in temperature ≥ 2 °F from baseline.3
So how to proceed with our case patient? The following questions helped guide the approach to her care.
Is this patient asymptomatic?
Yes. The patient presented with nonspecific symptoms (falls and delirium) with bacteriuria suggesting asymptomatic bacteriuria (ASB). These symptoms are referred to as geriatric syndromes that, by definition, are “multifactorial health conditions that occur when the accumulated effects of impairments in multiple systems render an older person vulnerable to situational challenges.”4
As geriatric syndromes, falls and delirium are unlikely to be caused by one process, such as a UTI, but rather from multiple morbid processes. It is also important to note that there is no evidence to support a causal relationship between bacteriuria and delirium or that antibiotic treatment of bacteriuria improves delirium.2,5
Continue to: So, while we could...
So, while we could have diagnosed a UTI in this older adult with bacteriuria and delirium, it would have been premature closure and an incomplete assessment. We would have risked potentially missing other significant causes of her delirium and unnecessarily exposing the patient to antibiotics.
Are antibiotics generally useful in older adults who you believe to be asymptomatic with a urine culture showing bacteriuria?
No. The goal of antibiotic treatment for a symptomatic UTI is to ameliorate symptoms; therefore, there is no indication for antibiotics in ASB and no evidence of survival benefit.2 And, as noted earlier, there is no evidence to support a causal relationship between bacteriuria and delirium or that antibiotic treatment of bacteriuria improves delirium.2,5
The use of antibiotics in the asymptomatic setting will eradicate any bacteriuria but also increase the risk of reinfection, resistant organisms, antibiotic adverse reactions, and medication interactions.1
What is the recommendation for management of nonspecific symptoms, such as delirium and falls, in a geriatric patient such as this one with bacteriuria?
The Infectious Diseases Society of America (IDSA)’s 2019 Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria recommends a thorough assessment (for other causes) and careful observation, rather than immediate antimicrobial treatment and cessation of evaluation for other causes.5 (IDSA made this recommendation based on low-quality evidence.) The group found a high certainty of harm and low certainty of benefit in treating older adults with antibiotics for ASB.
This recommendation highlights the key geriatric principle of “geriatric syndromes” and the multifactorial nature of findings such as delirium and falls. It encourages clinicians to continue their thorough assessment for other causes in addition to bacteriuria.5 Even in the event that antibiotics are immediately initiated, we would recommend avoiding premature closure and continuing to evaluate for other causes.
Continue to: It is reasonable to...
It is reasonable to obtain a dipstick if, after the observation period (1-7 days, with earlier follow-up for frail patients), the patient continues to have the nonspecific symptoms.1 If the dipstick is negative, there is no need for further evaluation of UTI. If it’s positive, then it’s appropriate to send for urinalysis and urine culture.1
If the urine culture is negative, continue looking for other etiologies. If it’s positive, but there is resolution of symptoms, there is no need to treat. If it’s positive and symptoms persist, consider antibiotic treatment.1
CASE RESOLUTION
The team closely monitored the patient and delayed empiric antibiotics while continuing the AMS work-up. After 2 days in the hospital, her delirium persisted, but she had no UTI-specific symptoms and she remained hemodynamically stable.
I (AP) recommended antibiotic treatment guided by the urine culture sensitivity report: initially 1 g of ceftriaxone IV q24h with transition (after symptom improvement and prior to discharge) to oral trimethoprim/sulfamethoxazole 160 mg/800 mg q12h, for a total of 10 days of treatment. I emphasized that we were treating bacteriuria with persisting delirium without any other etiology identified. The patient returned to her baseline mental status after a few days of treatment and was discharged home.
THE TAKEAWAY
Avoid premature closure by stopping at the diagnosis of a “UTI” in an older adult with nonspecific symptoms and bacteriuria to avoid the risk of overlooking other important and potentially life-threatening causes of the patient’s signs and symptoms.
CORRESPONDENCE
L. Amanda Perry, MD, 1919 West Taylor Street, Mail Code 663, Chicago, IL 60612; [email protected]
1. Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA. 2014;311:844-854. doi: 10.1001/jama.2014.303
2. Finucane TE. “Urinary tract infection”- requiem for a heavyweight. J Am Geriatr Soc. 2017;65:1650-1655. doi: 10.1111/jgs.14907
3. Ashraf MS, Gaur S, Bushen OY, et al; Infection Advisory SubCommittee for AMDA—The Society of Post-Acute and Long-Term Care Medicine. Diagnosis, treatment, and prevention of urinary tract infections in post-acute and long-term care settings: a consensus statement from AMDA’s Infection Advisory Subcommittee. J Am Med Dir Assoc. 2020;21:12-24 e12. doi: 10.1016/j.jamda.2019.11.004
4. Inouye SK, Studenski S, Tinetti, ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780-791. doi: 10.1111/j.1532-5415.2007.01156.x
5. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83-e110. doi: 10.1093/cid/ciy1121
1. Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA. 2014;311:844-854. doi: 10.1001/jama.2014.303
2. Finucane TE. “Urinary tract infection”- requiem for a heavyweight. J Am Geriatr Soc. 2017;65:1650-1655. doi: 10.1111/jgs.14907
3. Ashraf MS, Gaur S, Bushen OY, et al; Infection Advisory SubCommittee for AMDA—The Society of Post-Acute and Long-Term Care Medicine. Diagnosis, treatment, and prevention of urinary tract infections in post-acute and long-term care settings: a consensus statement from AMDA’s Infection Advisory Subcommittee. J Am Med Dir Assoc. 2020;21:12-24 e12. doi: 10.1016/j.jamda.2019.11.004
4. Inouye SK, Studenski S, Tinetti, ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780-791. doi: 10.1111/j.1532-5415.2007.01156.x
5. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83-e110. doi: 10.1093/cid/ciy1121
A guide to GERD, H pylori infection, and Barrett esophagus
Three conditions seen in primary care—gastroesophageal reflux disease (GERD), Helicobacter pylori (H pylori) infection, and Barrett esophagus (BE)—evolve in a gastric acid environment and are treated in part through gastric acid suppression. While GERD is a risk factor for the development of BE, H pylori is not associated with BE.1 Patients with H pylori are actually less likely to have GERD symptoms.2,3 In this article, we describe similarities and differences in patient presentations, diagnostic testing, and management, and review screening recommendations.
Gastroesophageal reflux disease
GERD is a clinical diagnosis based on symptoms of regurgitation and heartburn or the presence of one of its known complications (esophagitis, peptic strictures, or BE).2,4 Chest pain is also common. Atypical symptoms are dysphagia, bleeding, chronic cough, asthma, chronic laryngitis, hoarseness, wheezing, teeth erosions, belching, and bloating.2,5-7
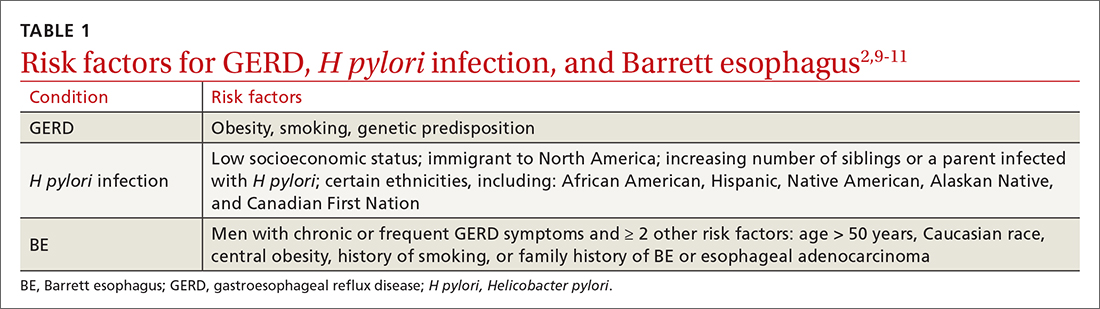
The worldwide prevalence of gastroesophageal reflux symptoms in adults is 14.8%.8 When using a stringent definition of GERD—weekly symptoms occurring for at least 3 months—prevalence drops to 9.4%.9 GERD symptoms vary markedly by geographic location; the highest rates are in Central America (19.6%) and the lowest rates are in Southeast Asia (7.4%).8TABLE 12,9-11 lists risk factors for GERD.
GERD results from dysfunction of the esophagogastric junction that permits regurgitation of acidic gastric contents into the esophagus. Normally, the lower esophageal sphincter (LES) relaxes temporarily with gastric distention; when this relaxation is frequent and prolonged, it causes GERD.2,12 Several medications, particularly those with anticholinergic effects (eg, tricyclic antidepressants) can decrease LES tone and contribute to symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often linked to dyspepsia and gastritis and should be avoided in patients who have symptoms of GERD. Pathologic reflux can also occur in conditions that increase intra-abdominal pressure, such as obesity and pregnancy, and with esophageal dysmotility, hiatal hernia, and delayed gastric emptying.5 When gastric contents travel proximally, this contributes to extraesophageal symptoms, such as chronic cough, asthma, laryngitis, dyspepsia, bloating, and belching.2,4
Treatment
Proton pump inhibitors (PPIs) are the most effective treatment for GERD, but lifestyle modifications are also recommended for all patients.2,6,13-16 Consider selective elimination of beverages and foods that are commonly associated with heartburn (eg, alcohol, caffeine, chocolate, citrus, and spicy foods) if patients note a correlation to symptoms.5,6,13 Also, advise weight loss and smoking cessation, as appropriate, and suggest that the patient elevate the head of their bed when sleeping.
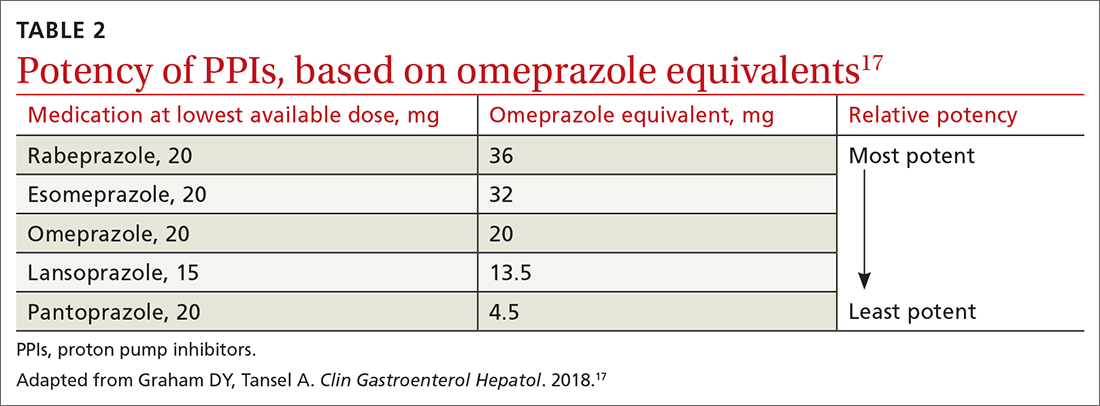
All PPIs are equally effective in suppressing acid when given at equivalent doses (TABLE 217), so they can be used interchangeably.17 Treat uncomplicated GERD with a once-daily PPI 30 to 60 minutes prior to a meal for 4 to 8 weeks. If treatment is effective, you’ll want to try to reduce or stop the medication after the 4- to 8-week period. (It’s worth noting that the benefits of treatment for those with extraesophageal GERD are less predictable than for those with heartburn or esophagitis symptoms.5)
If GERD symptoms reemerge after the PPI is stopped, the medication can be restarted but should be limited to the least potent effective dose, no matter if it is taken daily or only as needed.2,6,17 In patients with esophagitis, you may need to continue PPI treatment indefinitely at the lowest possible dose given the increased risk of recurrent esophagitis.2,13,16
Continue to: Keep in mind...
Keep in mind that the safety of long-term PPI use has not been fully established. While observational studies have shown that long-term PPI use may be associated with adverse events, including kidney damage, Clostridioides difficile infection, osteoporosis, and gastric cancer, subsequent prospective studies have not shown any significant risks with long-term PPI use.2,13,14,16,18,19 If a decision is made to discontinue PPIs after long-term use, the patient should be advised that rebound acid hypersecretion may occur, although this possibility can be mitigated by gradually tapering the PPI dose.
Another maintenance therapy option. Histamine-2 receptor antagonists (H2RAs) are a reasonable alternative to PPIs as maintenance therapy, but they are ineffective in healing esophagitis6,13 and may be best used as adjunctive therapy at bedtime for breakthrough symptoms while a patient is on maintenance PPIs.6,19 Antacids (eg, calcium carbonate, aluminum hydroxide, or magnesium hydroxide) and alginate may provide some symptomatic relief, as well.
When PPIs don’t work. If initial lifestyle changes and PPI treatment do not provide adequate relief, consider the possibility of nonadherence with medication or lifestyle directives. If nonadherence does not appear to be an issue, twice-daily PPI dosing is also an option. Recognize, though, that PPI treatment failure occurs in as many as 40% of patients and is much more common in those with atypical symptoms.6
Consider upper gastrointestinal (GI) endoscopy—and perhaps esophageal manometry or pH testing—if a patient does not respond to empiric treatment with a PPI for 4 to 8 weeks at a standard, once-daily dose.2,4,13 (Alternative diagnoses may also need to be considered.) Upper endoscopy is also appropriate for patients who have symptoms concerning for malignancy (progressive dysphagia, unintentional weight loss, or bleeding).
Esophagitis detected on endoscopy confirms GERD, although it is seen in only 18% to 25% of patients with GERD symptoms.2,4 (The absence of esophagitis only indicates a lack of mucosal injury and not the absence of GERD.4) Acid exposure can cause fibrotic scarring and, in turn, strictures visible on endoscopy.2 BE, the precursor to esophageal adenocarcinoma, is also a complication of GERD and is defined by columnar metaplasia replacing the normal squamous cell esophageal epithelium; it is detected on pathology review of biopsies.2
Continue to: GERD confirmed but PPIs aren't working?
GERD confirmed but PPIs aren’t working? Laparoscopic fundoplication is an effective treatment for GERD. However, due to its adverse effects (dysphagia, bloating, flatulence) and risk of treatment failure or breakdown within 5 to 10 years, it should be reserved for those poorly managed with PPIs.2,13,19
Considerations in pregnancy. GERD is reported by 40% to 85% of pregnant women,20,21 and its clinical presentation, diagnosis, and treatment are similar to that of nonpregnant adults.21 If lifestyle modification is not effective, pharmacologic therapy may be considered. Often, lifestyle modifications and antacids followed by the addition of sucralfate will be used first given the lack of systemic effects. H2RAs can be used next based on long-term historical use and reported safety.21 As with nonpregnant patients, PPIs are more effective than other medical therapies. If PPIs are used, dexlansoprazole, lansoprazole, pantoprazole, and rabeprazole are preferred. Omeprazole and esomeprazole are typically avoided due to findings of embryonic and fetal mortality in early animal studies, although subsequent human studies have noted no teratogenicity.2,20,21
Considerations in children. As with adults, findings in the history and exam are sufficient to diagnose and initiate treatment of GERD in children, provided there are no warning signs (eg, bilious vomiting, GI bleeding, consistent forceful vomiting, fever, lethargy, hepatosplenomegaly, bulging fontanelle, macro- or microcephaly, seizures, abdominal tenderness/distention, or genetic/metabolic syndromes). Lifestyle changes are first-line treatment, followed by medication. Acid suppressants are preferred, with PPIs showing superior efficacy compared with H2RAs.15 Some PPIs (omeprazole, lansoprazole, and esomeprazole) have US Food and Drug Administration (FDA) approval beginning at age 1 year, while rabeprazole has FDA approval beginning at age 12.22 As in adults, if PPIs are ineffective, consider alternative diagnoses.15,22
Helicobacter pylori infection
H pylori is a gram-negative spiral-shaped bacterium found in the stomach of humans and other mammals. It survives the acidic environment by metabolizing urea into alkaline ammonia and carbon dioxide. H pylori infection increases the risk of peptic ulcer disease, gastric cancer, iron deficiency anemia, and immune thrombocytopenia. It may be associated with dyspepsia, increased ulcer risk with use of an NSAID, and chronic gastritis.9 Infection with H pylori can decrease the risk of GERD.2 The bacterial infection causes atrophic gastritis and subsequent hypochlorhydria, which then diminishes the acidity of the reflux contents.19 There is no link between H pylori infection and BE.1
TABLE 12,9-11 shows those at highest risk of H pylori. The estimated prevalence of infection is 40% to 48%23 worldwide but lower in North America, at 32% to 42%.24H pylori is often acquired in childhood, and risk of infection is more likely if the parents (particularly mothers) are infected.9
Continue to: Whom to test, and how
Whom to test, and how
Test for H pylori in those with active peptic ulcer disease or a history of peptic ulcer disease that was not investigated for H pylori. Also test individuals who have gastric mucosa-associated lymphoid tissue lymphoma, have a history of gastric cancer or family history of gastric cancer, are scheduled for endoscopic evaluation for dyspepsia, or are starting chronic NSAID therapy. Patients with typical GERD symptoms do not need to be tested for H pylori.9,25
Means of testing for H pylori include the urea breath test, stool antigen studies, endoscopically obtained biopsies, or serum antibody tests. Antibody testing is discouraged because it has a lower diagnostic utility and cannot determine if the patient’s infection is current or past. Before undergoing urea breath tests, stool antigen tests, or biopsies for H pylori identification, patients should have abstained from taking the following agents for the time periods indicated: PPIs, 1 to 2 weeks; H2RAs, at least 1 day and preferably 2 weeks; and antibiotics, 4 weeks.9
The urea breath test and endoscopically obtained biopsies have the greatest diagnostic utility and, where available, should be the first-line tests. Stool antigen studies are useful for ruling out H pylori infection (very low negative likelihood ratio), but a positive test result is not as useful for confirming an infection, as false-positives do occur (moderate positive likelihood ratio).9,26,27 Stool antigen testing is less expensive and, in many cases, more convenient and readily available for patients than urea breath testing and endoscopic biopsies.
Treatment
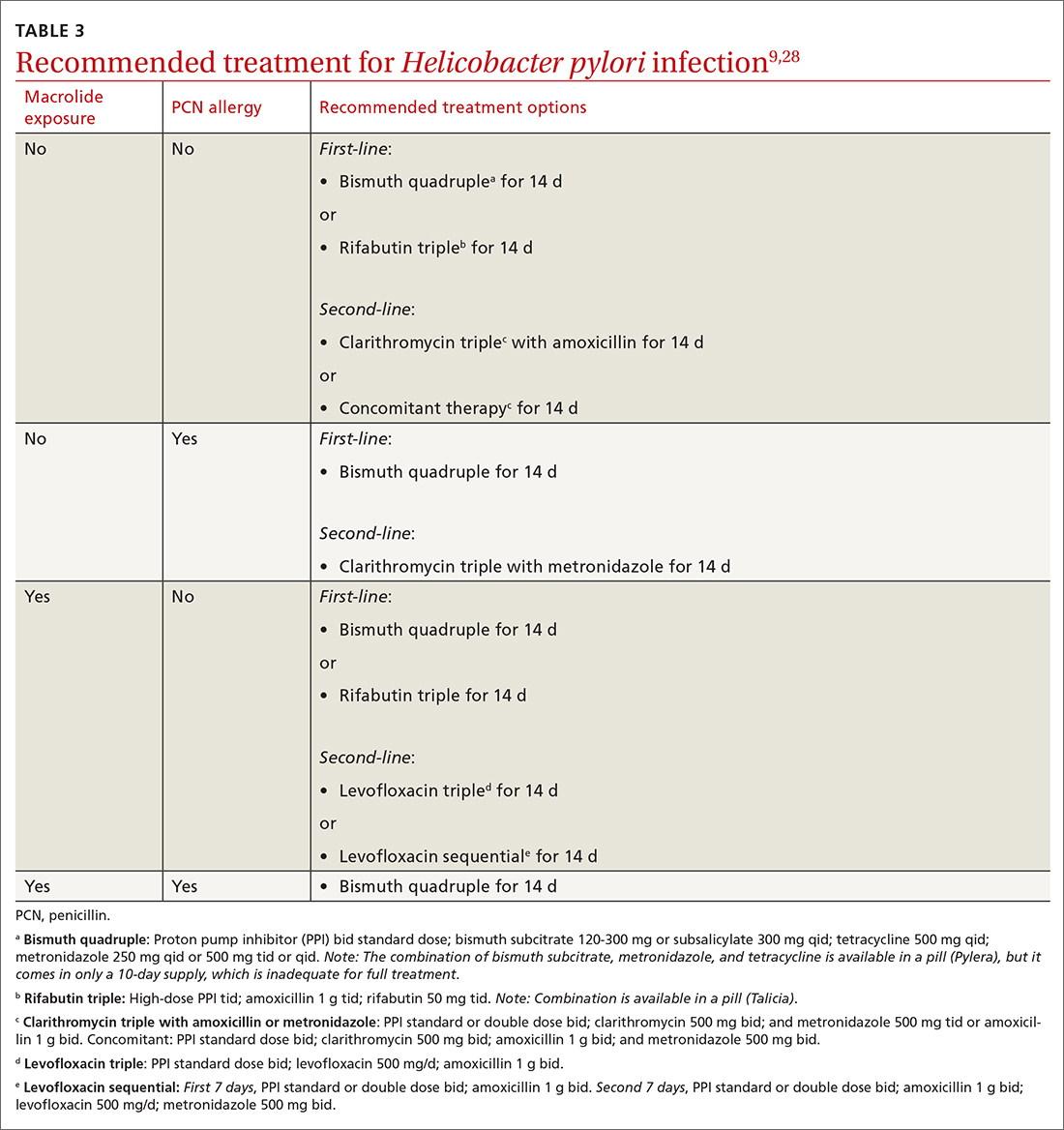
Offer treatment to all patients who test positive for H pylori. Eradication rates range from 70% to 91% using first-line treatment options.9 Treatment regimens consist of acid suppression and 2 to 3 antibiotics in combination (TABLE 39,28). The single greatest predictive factor for treatment failure is antibiotic resistance, so a detailed antibiotic history is essential. In particular, ask about macrolide antibiotic usage and penicillin allergies.
People living in areas with population macrolide resistance ≥ 15% should avoid clarithromycin-based regimens unless bacterial sensitivity testing has been done and shows sensitivity to these agents.9,28,29 For cases that do not resolve with a first-line treatment program, choose an alternative regimen with different antibiotics.9,29
Continue to: Additionally, adequate...
Additionally, adequate acid suppression is directly related to successful eradication. Thus, the likelihood of treatment success can be improved by using higher doses of PPIs and avoiding ones that are more likely to be metabolized quickly in some patients (lansoprazole, omeprazole). Patient adherence to the treatment regimen is an important determinant of effectiveness.9,29 Adding vitamin C 400 to 1000 mg/d, vitamin E 100 to 400 mg/d, and probiotics may improve the effectiveness of treatment.9,30
Duration of treatment is directly related to treatment effectiveness. Whenever possible, opt for 14 days of treatment instead of just 7.9
Test of cure. Patients treated for H pylori should be re-tested no sooner than 4 weeks after completion of therapy. Urea breath testing, stool antigen testing, and endoscopic biopsies (if endoscopy is indicated for some other reason) can all be used post treatment for test of cure.9
Barrett esophagus
Whom to screen
The American College of Gastroenterology recommends consideration of screening with upper endoscopy for men with chronic GERD (> 5 years) or frequent GERD symptoms (once weekly or more often), plus 2
Continue to: Not everyone with BE...
Not everyone with BE experiences GERD symptoms; sometimes BE may be diagnosed incidentally on upper endoscopy performed for unrelated symptoms.11 GERD patients who are currently asymptomatic and had a normal prior upper endoscopy do not require surveillance.
Diagnosis and management
BE is diagnosed based on specific endoscopic and histologic findings. The presence of dysplasia (either low grade or high grade) or its absence has important treatment implications. When histology is indefinite for dysplasia, treat reflux and, following acid suppression with PPIs for 3 to 6 months, repeat endoscopy (since reactive changes with reflux may obscure results).11
Nondysplastic BE has a risk of progressing to cancer in only 0.2% to 0.5% of affected patients per year.11 Guidelines for BE without dysplasia advise repeating surveillance endoscopy every 3 to 5 years after appropriate counseling regarding overall low risk of cancer progression.11,31 Surveillance endoscopy recommendations exist despite the lack of prospective randomized trials that demonstrate benefit. The rationale for surveillance is that survival in EAC is stage dependent and often EAC metastasizes prior to the development of symptoms from the tumor. Observational cohort studies in BE have demonstrated that surveillance endoscopy programs find EAC at earlier stages with improved survival; however, lead and length time bias may attenuate or eliminate these surveillance benefits.11,32
Risk for neoplastic progression increases with degree of dysplasia. BE with low-grade dysplasia and high-grade dysplasia have a risk of cancer progression of 0.7% per year and 7% per year, respectively.11
Historically, esophagectomy was the preferred treatment for BE with dysplasia. Now, endoscopic eradication therapies, including radiofrequency ablation and endoscopic mucosal resection for nodular BE, are the usual treatment for either low- or high-grade dysplasia.11
Chemoprophylaxis with PPIs. Most patients with BE have symptoms of GERD or reflux esophagitis, so treatment with a PPI is indicated for symptom control. In patients with BE without GERD, PPI use may still be indicated, although this is controversial. Current guidelines recommend once-daily PPI therapy for BE (twice daily only if needed for symptom control) to reduce reflux-associated inflammation and recommend against routine prescription of aspirin or NSAIDs for BE.11 In vitro and observational studies support PPI use to prevent progression to EAC11,33; however, data from randomized controlled trials to support their use are limited.34,35
CORRESPONDENCE
Megan Everson, MD, Medical College of Wisconsin, 229 South Morrison Street, Appleton WI, 54911; [email protected]
1. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500. doi: 10.1038/ajg.2008.37
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324:2536-2547. doi: 10.1001/jama.2020.21360
3. Scida S, Russo M, Miraglia C, et al. Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 2018;89:40-43. doi: 10.23750/abm.v89i8-S.7918
4. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. doi: 10.1111/j.1572-0241.2006.00630.x
5. Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. doi: 10.1016/j.suc.2015.02.006
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. doi: 10.1038/ajg.2012.444
7. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and treatment of the extraesophageal manifestations of gastroesophageal reflux disease. Ann Surg. 2017;265:63-67. doi: 10.1097/SLA.0000000000001907
8. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. doi: 10.1136/gutjnl-2016-313589
9. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. doi: 10.1038/ajg.2016.563
10. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. doi: 10.1136/gutjnl-2020-321365
11. Shaheen NJ, Falk GW, Iyer PG, et al; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30-50. doi: 10.1038/ajg.2015.322
12. Savarino E, Bredenoord AJ, Fox M, et al; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665-676. doi: 10.1038/nrgastro.2017.130
13. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. doi: 10.1056/NEJMcp0804684
14. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031
15. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. doi: 10.1097/MPG.0000000000001889
16. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391. doi: 10.1053/j.gastro.2008.08.045
17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808. doi: 10.1016/j.cgh.2017.09.033
18. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691. doi: 10.1053/j.gastro.2019.05.056
19. Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. doi: 10.1136/bmj.m3786
20. Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:793-806. doi: 10.1016/j.bpg.2007.05.006
21. Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. doi: 10.1016/j.gtc.2016.02.005
22. Lightdale JR, Gremse DA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131;e1684-e1695. doi: 10.1542/peds.2013-0421
23. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022
24. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. doi: 10.1111/apt.14561
25. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-436. doi: 10.1056/NEJMoa1909666
26. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. doi: 10.1111/j.1572-0241.2006.00528.x
27. Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2
28. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3. doi: 10.14309/ajg.0000000000001031
29. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831-1841. doi: 10.1053/j.gastro.2020.11.059
30. Yang-Ou YB, Hu Y, Zhu Y, et al. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535
31. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. doi: 10.1053/j.gastro.2011.01.030
32. Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068-2086. doi: 10.1053/j.gastro.2018.02.022
33. Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. doi: 10.1136/gutjnl-2013-305997
34. Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. doi: 10.1016/S0140-6736(18)31388-6
35. Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2017;12:e0169691. doi: 10.1371/journal.pone.0169691
Three conditions seen in primary care—gastroesophageal reflux disease (GERD), Helicobacter pylori (H pylori) infection, and Barrett esophagus (BE)—evolve in a gastric acid environment and are treated in part through gastric acid suppression. While GERD is a risk factor for the development of BE, H pylori is not associated with BE.1 Patients with H pylori are actually less likely to have GERD symptoms.2,3 In this article, we describe similarities and differences in patient presentations, diagnostic testing, and management, and review screening recommendations.
Gastroesophageal reflux disease
GERD is a clinical diagnosis based on symptoms of regurgitation and heartburn or the presence of one of its known complications (esophagitis, peptic strictures, or BE).2,4 Chest pain is also common. Atypical symptoms are dysphagia, bleeding, chronic cough, asthma, chronic laryngitis, hoarseness, wheezing, teeth erosions, belching, and bloating.2,5-7
The worldwide prevalence of gastroesophageal reflux symptoms in adults is 14.8%.8 When using a stringent definition of GERD—weekly symptoms occurring for at least 3 months—prevalence drops to 9.4%.9 GERD symptoms vary markedly by geographic location; the highest rates are in Central America (19.6%) and the lowest rates are in Southeast Asia (7.4%).8TABLE 12,9-11 lists risk factors for GERD.

GERD results from dysfunction of the esophagogastric junction that permits regurgitation of acidic gastric contents into the esophagus. Normally, the lower esophageal sphincter (LES) relaxes temporarily with gastric distention; when this relaxation is frequent and prolonged, it causes GERD.2,12 Several medications, particularly those with anticholinergic effects (eg, tricyclic antidepressants) can decrease LES tone and contribute to symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often linked to dyspepsia and gastritis and should be avoided in patients who have symptoms of GERD. Pathologic reflux can also occur in conditions that increase intra-abdominal pressure, such as obesity and pregnancy, and with esophageal dysmotility, hiatal hernia, and delayed gastric emptying.5 When gastric contents travel proximally, this contributes to extraesophageal symptoms, such as chronic cough, asthma, laryngitis, dyspepsia, bloating, and belching.2,4
Treatment
Proton pump inhibitors (PPIs) are the most effective treatment for GERD, but lifestyle modifications are also recommended for all patients.2,6,13-16 Consider selective elimination of beverages and foods that are commonly associated with heartburn (eg, alcohol, caffeine, chocolate, citrus, and spicy foods) if patients note a correlation to symptoms.5,6,13 Also, advise weight loss and smoking cessation, as appropriate, and suggest that the patient elevate the head of their bed when sleeping.
All PPIs are equally effective in suppressing acid when given at equivalent doses (TABLE 217), so they can be used interchangeably.17 Treat uncomplicated GERD with a once-daily PPI 30 to 60 minutes prior to a meal for 4 to 8 weeks. If treatment is effective, you’ll want to try to reduce or stop the medication after the 4- to 8-week period. (It’s worth noting that the benefits of treatment for those with extraesophageal GERD are less predictable than for those with heartburn or esophagitis symptoms.5)

If GERD symptoms reemerge after the PPI is stopped, the medication can be restarted but should be limited to the least potent effective dose, no matter if it is taken daily or only as needed.2,6,17 In patients with esophagitis, you may need to continue PPI treatment indefinitely at the lowest possible dose given the increased risk of recurrent esophagitis.2,13,16
Continue to: Keep in mind...
Keep in mind that the safety of long-term PPI use has not been fully established. While observational studies have shown that long-term PPI use may be associated with adverse events, including kidney damage, Clostridioides difficile infection, osteoporosis, and gastric cancer, subsequent prospective studies have not shown any significant risks with long-term PPI use.2,13,14,16,18,19 If a decision is made to discontinue PPIs after long-term use, the patient should be advised that rebound acid hypersecretion may occur, although this possibility can be mitigated by gradually tapering the PPI dose.
Another maintenance therapy option. Histamine-2 receptor antagonists (H2RAs) are a reasonable alternative to PPIs as maintenance therapy, but they are ineffective in healing esophagitis6,13 and may be best used as adjunctive therapy at bedtime for breakthrough symptoms while a patient is on maintenance PPIs.6,19 Antacids (eg, calcium carbonate, aluminum hydroxide, or magnesium hydroxide) and alginate may provide some symptomatic relief, as well.
When PPIs don’t work. If initial lifestyle changes and PPI treatment do not provide adequate relief, consider the possibility of nonadherence with medication or lifestyle directives. If nonadherence does not appear to be an issue, twice-daily PPI dosing is also an option. Recognize, though, that PPI treatment failure occurs in as many as 40% of patients and is much more common in those with atypical symptoms.6
Consider upper gastrointestinal (GI) endoscopy—and perhaps esophageal manometry or pH testing—if a patient does not respond to empiric treatment with a PPI for 4 to 8 weeks at a standard, once-daily dose.2,4,13 (Alternative diagnoses may also need to be considered.) Upper endoscopy is also appropriate for patients who have symptoms concerning for malignancy (progressive dysphagia, unintentional weight loss, or bleeding).
Esophagitis detected on endoscopy confirms GERD, although it is seen in only 18% to 25% of patients with GERD symptoms.2,4 (The absence of esophagitis only indicates a lack of mucosal injury and not the absence of GERD.4) Acid exposure can cause fibrotic scarring and, in turn, strictures visible on endoscopy.2 BE, the precursor to esophageal adenocarcinoma, is also a complication of GERD and is defined by columnar metaplasia replacing the normal squamous cell esophageal epithelium; it is detected on pathology review of biopsies.2
Continue to: GERD confirmed but PPIs aren't working?
GERD confirmed but PPIs aren’t working? Laparoscopic fundoplication is an effective treatment for GERD. However, due to its adverse effects (dysphagia, bloating, flatulence) and risk of treatment failure or breakdown within 5 to 10 years, it should be reserved for those poorly managed with PPIs.2,13,19
Considerations in pregnancy. GERD is reported by 40% to 85% of pregnant women,20,21 and its clinical presentation, diagnosis, and treatment are similar to that of nonpregnant adults.21 If lifestyle modification is not effective, pharmacologic therapy may be considered. Often, lifestyle modifications and antacids followed by the addition of sucralfate will be used first given the lack of systemic effects. H2RAs can be used next based on long-term historical use and reported safety.21 As with nonpregnant patients, PPIs are more effective than other medical therapies. If PPIs are used, dexlansoprazole, lansoprazole, pantoprazole, and rabeprazole are preferred. Omeprazole and esomeprazole are typically avoided due to findings of embryonic and fetal mortality in early animal studies, although subsequent human studies have noted no teratogenicity.2,20,21
Considerations in children. As with adults, findings in the history and exam are sufficient to diagnose and initiate treatment of GERD in children, provided there are no warning signs (eg, bilious vomiting, GI bleeding, consistent forceful vomiting, fever, lethargy, hepatosplenomegaly, bulging fontanelle, macro- or microcephaly, seizures, abdominal tenderness/distention, or genetic/metabolic syndromes). Lifestyle changes are first-line treatment, followed by medication. Acid suppressants are preferred, with PPIs showing superior efficacy compared with H2RAs.15 Some PPIs (omeprazole, lansoprazole, and esomeprazole) have US Food and Drug Administration (FDA) approval beginning at age 1 year, while rabeprazole has FDA approval beginning at age 12.22 As in adults, if PPIs are ineffective, consider alternative diagnoses.15,22
Helicobacter pylori infection
H pylori is a gram-negative spiral-shaped bacterium found in the stomach of humans and other mammals. It survives the acidic environment by metabolizing urea into alkaline ammonia and carbon dioxide. H pylori infection increases the risk of peptic ulcer disease, gastric cancer, iron deficiency anemia, and immune thrombocytopenia. It may be associated with dyspepsia, increased ulcer risk with use of an NSAID, and chronic gastritis.9 Infection with H pylori can decrease the risk of GERD.2 The bacterial infection causes atrophic gastritis and subsequent hypochlorhydria, which then diminishes the acidity of the reflux contents.19 There is no link between H pylori infection and BE.1
TABLE 12,9-11 shows those at highest risk of H pylori. The estimated prevalence of infection is 40% to 48%23 worldwide but lower in North America, at 32% to 42%.24H pylori is often acquired in childhood, and risk of infection is more likely if the parents (particularly mothers) are infected.9
Continue to: Whom to test, and how
Whom to test, and how
Test for H pylori in those with active peptic ulcer disease or a history of peptic ulcer disease that was not investigated for H pylori. Also test individuals who have gastric mucosa-associated lymphoid tissue lymphoma, have a history of gastric cancer or family history of gastric cancer, are scheduled for endoscopic evaluation for dyspepsia, or are starting chronic NSAID therapy. Patients with typical GERD symptoms do not need to be tested for H pylori.9,25
Means of testing for H pylori include the urea breath test, stool antigen studies, endoscopically obtained biopsies, or serum antibody tests. Antibody testing is discouraged because it has a lower diagnostic utility and cannot determine if the patient’s infection is current or past. Before undergoing urea breath tests, stool antigen tests, or biopsies for H pylori identification, patients should have abstained from taking the following agents for the time periods indicated: PPIs, 1 to 2 weeks; H2RAs, at least 1 day and preferably 2 weeks; and antibiotics, 4 weeks.9
The urea breath test and endoscopically obtained biopsies have the greatest diagnostic utility and, where available, should be the first-line tests. Stool antigen studies are useful for ruling out H pylori infection (very low negative likelihood ratio), but a positive test result is not as useful for confirming an infection, as false-positives do occur (moderate positive likelihood ratio).9,26,27 Stool antigen testing is less expensive and, in many cases, more convenient and readily available for patients than urea breath testing and endoscopic biopsies.
Treatment
Offer treatment to all patients who test positive for H pylori. Eradication rates range from 70% to 91% using first-line treatment options.9 Treatment regimens consist of acid suppression and 2 to 3 antibiotics in combination (TABLE 39,28). The single greatest predictive factor for treatment failure is antibiotic resistance, so a detailed antibiotic history is essential. In particular, ask about macrolide antibiotic usage and penicillin allergies.

People living in areas with population macrolide resistance ≥ 15% should avoid clarithromycin-based regimens unless bacterial sensitivity testing has been done and shows sensitivity to these agents.9,28,29 For cases that do not resolve with a first-line treatment program, choose an alternative regimen with different antibiotics.9,29
Continue to: Additionally, adequate...
Additionally, adequate acid suppression is directly related to successful eradication. Thus, the likelihood of treatment success can be improved by using higher doses of PPIs and avoiding ones that are more likely to be metabolized quickly in some patients (lansoprazole, omeprazole). Patient adherence to the treatment regimen is an important determinant of effectiveness.9,29 Adding vitamin C 400 to 1000 mg/d, vitamin E 100 to 400 mg/d, and probiotics may improve the effectiveness of treatment.9,30
Duration of treatment is directly related to treatment effectiveness. Whenever possible, opt for 14 days of treatment instead of just 7.9
Test of cure. Patients treated for H pylori should be re-tested no sooner than 4 weeks after completion of therapy. Urea breath testing, stool antigen testing, and endoscopic biopsies (if endoscopy is indicated for some other reason) can all be used post treatment for test of cure.9
Barrett esophagus
Whom to screen
The American College of Gastroenterology recommends consideration of screening with upper endoscopy for men with chronic GERD (> 5 years) or frequent GERD symptoms (once weekly or more often), plus 2
Continue to: Not everyone with BE...
Not everyone with BE experiences GERD symptoms; sometimes BE may be diagnosed incidentally on upper endoscopy performed for unrelated symptoms.11 GERD patients who are currently asymptomatic and had a normal prior upper endoscopy do not require surveillance.
Diagnosis and management
BE is diagnosed based on specific endoscopic and histologic findings. The presence of dysplasia (either low grade or high grade) or its absence has important treatment implications. When histology is indefinite for dysplasia, treat reflux and, following acid suppression with PPIs for 3 to 6 months, repeat endoscopy (since reactive changes with reflux may obscure results).11
Nondysplastic BE has a risk of progressing to cancer in only 0.2% to 0.5% of affected patients per year.11 Guidelines for BE without dysplasia advise repeating surveillance endoscopy every 3 to 5 years after appropriate counseling regarding overall low risk of cancer progression.11,31 Surveillance endoscopy recommendations exist despite the lack of prospective randomized trials that demonstrate benefit. The rationale for surveillance is that survival in EAC is stage dependent and often EAC metastasizes prior to the development of symptoms from the tumor. Observational cohort studies in BE have demonstrated that surveillance endoscopy programs find EAC at earlier stages with improved survival; however, lead and length time bias may attenuate or eliminate these surveillance benefits.11,32
Risk for neoplastic progression increases with degree of dysplasia. BE with low-grade dysplasia and high-grade dysplasia have a risk of cancer progression of 0.7% per year and 7% per year, respectively.11
Historically, esophagectomy was the preferred treatment for BE with dysplasia. Now, endoscopic eradication therapies, including radiofrequency ablation and endoscopic mucosal resection for nodular BE, are the usual treatment for either low- or high-grade dysplasia.11
Chemoprophylaxis with PPIs. Most patients with BE have symptoms of GERD or reflux esophagitis, so treatment with a PPI is indicated for symptom control. In patients with BE without GERD, PPI use may still be indicated, although this is controversial. Current guidelines recommend once-daily PPI therapy for BE (twice daily only if needed for symptom control) to reduce reflux-associated inflammation and recommend against routine prescription of aspirin or NSAIDs for BE.11 In vitro and observational studies support PPI use to prevent progression to EAC11,33; however, data from randomized controlled trials to support their use are limited.34,35
CORRESPONDENCE
Megan Everson, MD, Medical College of Wisconsin, 229 South Morrison Street, Appleton WI, 54911; [email protected]
Three conditions seen in primary care—gastroesophageal reflux disease (GERD), Helicobacter pylori (H pylori) infection, and Barrett esophagus (BE)—evolve in a gastric acid environment and are treated in part through gastric acid suppression. While GERD is a risk factor for the development of BE, H pylori is not associated with BE.1 Patients with H pylori are actually less likely to have GERD symptoms.2,3 In this article, we describe similarities and differences in patient presentations, diagnostic testing, and management, and review screening recommendations.
Gastroesophageal reflux disease
GERD is a clinical diagnosis based on symptoms of regurgitation and heartburn or the presence of one of its known complications (esophagitis, peptic strictures, or BE).2,4 Chest pain is also common. Atypical symptoms are dysphagia, bleeding, chronic cough, asthma, chronic laryngitis, hoarseness, wheezing, teeth erosions, belching, and bloating.2,5-7
The worldwide prevalence of gastroesophageal reflux symptoms in adults is 14.8%.8 When using a stringent definition of GERD—weekly symptoms occurring for at least 3 months—prevalence drops to 9.4%.9 GERD symptoms vary markedly by geographic location; the highest rates are in Central America (19.6%) and the lowest rates are in Southeast Asia (7.4%).8TABLE 12,9-11 lists risk factors for GERD.

GERD results from dysfunction of the esophagogastric junction that permits regurgitation of acidic gastric contents into the esophagus. Normally, the lower esophageal sphincter (LES) relaxes temporarily with gastric distention; when this relaxation is frequent and prolonged, it causes GERD.2,12 Several medications, particularly those with anticholinergic effects (eg, tricyclic antidepressants) can decrease LES tone and contribute to symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often linked to dyspepsia and gastritis and should be avoided in patients who have symptoms of GERD. Pathologic reflux can also occur in conditions that increase intra-abdominal pressure, such as obesity and pregnancy, and with esophageal dysmotility, hiatal hernia, and delayed gastric emptying.5 When gastric contents travel proximally, this contributes to extraesophageal symptoms, such as chronic cough, asthma, laryngitis, dyspepsia, bloating, and belching.2,4
Treatment
Proton pump inhibitors (PPIs) are the most effective treatment for GERD, but lifestyle modifications are also recommended for all patients.2,6,13-16 Consider selective elimination of beverages and foods that are commonly associated with heartburn (eg, alcohol, caffeine, chocolate, citrus, and spicy foods) if patients note a correlation to symptoms.5,6,13 Also, advise weight loss and smoking cessation, as appropriate, and suggest that the patient elevate the head of their bed when sleeping.
All PPIs are equally effective in suppressing acid when given at equivalent doses (TABLE 217), so they can be used interchangeably.17 Treat uncomplicated GERD with a once-daily PPI 30 to 60 minutes prior to a meal for 4 to 8 weeks. If treatment is effective, you’ll want to try to reduce or stop the medication after the 4- to 8-week period. (It’s worth noting that the benefits of treatment for those with extraesophageal GERD are less predictable than for those with heartburn or esophagitis symptoms.5)

If GERD symptoms reemerge after the PPI is stopped, the medication can be restarted but should be limited to the least potent effective dose, no matter if it is taken daily or only as needed.2,6,17 In patients with esophagitis, you may need to continue PPI treatment indefinitely at the lowest possible dose given the increased risk of recurrent esophagitis.2,13,16
Continue to: Keep in mind...
Keep in mind that the safety of long-term PPI use has not been fully established. While observational studies have shown that long-term PPI use may be associated with adverse events, including kidney damage, Clostridioides difficile infection, osteoporosis, and gastric cancer, subsequent prospective studies have not shown any significant risks with long-term PPI use.2,13,14,16,18,19 If a decision is made to discontinue PPIs after long-term use, the patient should be advised that rebound acid hypersecretion may occur, although this possibility can be mitigated by gradually tapering the PPI dose.
Another maintenance therapy option. Histamine-2 receptor antagonists (H2RAs) are a reasonable alternative to PPIs as maintenance therapy, but they are ineffective in healing esophagitis6,13 and may be best used as adjunctive therapy at bedtime for breakthrough symptoms while a patient is on maintenance PPIs.6,19 Antacids (eg, calcium carbonate, aluminum hydroxide, or magnesium hydroxide) and alginate may provide some symptomatic relief, as well.
When PPIs don’t work. If initial lifestyle changes and PPI treatment do not provide adequate relief, consider the possibility of nonadherence with medication or lifestyle directives. If nonadherence does not appear to be an issue, twice-daily PPI dosing is also an option. Recognize, though, that PPI treatment failure occurs in as many as 40% of patients and is much more common in those with atypical symptoms.6
Consider upper gastrointestinal (GI) endoscopy—and perhaps esophageal manometry or pH testing—if a patient does not respond to empiric treatment with a PPI for 4 to 8 weeks at a standard, once-daily dose.2,4,13 (Alternative diagnoses may also need to be considered.) Upper endoscopy is also appropriate for patients who have symptoms concerning for malignancy (progressive dysphagia, unintentional weight loss, or bleeding).
Esophagitis detected on endoscopy confirms GERD, although it is seen in only 18% to 25% of patients with GERD symptoms.2,4 (The absence of esophagitis only indicates a lack of mucosal injury and not the absence of GERD.4) Acid exposure can cause fibrotic scarring and, in turn, strictures visible on endoscopy.2 BE, the precursor to esophageal adenocarcinoma, is also a complication of GERD and is defined by columnar metaplasia replacing the normal squamous cell esophageal epithelium; it is detected on pathology review of biopsies.2
Continue to: GERD confirmed but PPIs aren't working?
GERD confirmed but PPIs aren’t working? Laparoscopic fundoplication is an effective treatment for GERD. However, due to its adverse effects (dysphagia, bloating, flatulence) and risk of treatment failure or breakdown within 5 to 10 years, it should be reserved for those poorly managed with PPIs.2,13,19
Considerations in pregnancy. GERD is reported by 40% to 85% of pregnant women,20,21 and its clinical presentation, diagnosis, and treatment are similar to that of nonpregnant adults.21 If lifestyle modification is not effective, pharmacologic therapy may be considered. Often, lifestyle modifications and antacids followed by the addition of sucralfate will be used first given the lack of systemic effects. H2RAs can be used next based on long-term historical use and reported safety.21 As with nonpregnant patients, PPIs are more effective than other medical therapies. If PPIs are used, dexlansoprazole, lansoprazole, pantoprazole, and rabeprazole are preferred. Omeprazole and esomeprazole are typically avoided due to findings of embryonic and fetal mortality in early animal studies, although subsequent human studies have noted no teratogenicity.2,20,21
Considerations in children. As with adults, findings in the history and exam are sufficient to diagnose and initiate treatment of GERD in children, provided there are no warning signs (eg, bilious vomiting, GI bleeding, consistent forceful vomiting, fever, lethargy, hepatosplenomegaly, bulging fontanelle, macro- or microcephaly, seizures, abdominal tenderness/distention, or genetic/metabolic syndromes). Lifestyle changes are first-line treatment, followed by medication. Acid suppressants are preferred, with PPIs showing superior efficacy compared with H2RAs.15 Some PPIs (omeprazole, lansoprazole, and esomeprazole) have US Food and Drug Administration (FDA) approval beginning at age 1 year, while rabeprazole has FDA approval beginning at age 12.22 As in adults, if PPIs are ineffective, consider alternative diagnoses.15,22
Helicobacter pylori infection
H pylori is a gram-negative spiral-shaped bacterium found in the stomach of humans and other mammals. It survives the acidic environment by metabolizing urea into alkaline ammonia and carbon dioxide. H pylori infection increases the risk of peptic ulcer disease, gastric cancer, iron deficiency anemia, and immune thrombocytopenia. It may be associated with dyspepsia, increased ulcer risk with use of an NSAID, and chronic gastritis.9 Infection with H pylori can decrease the risk of GERD.2 The bacterial infection causes atrophic gastritis and subsequent hypochlorhydria, which then diminishes the acidity of the reflux contents.19 There is no link between H pylori infection and BE.1
TABLE 12,9-11 shows those at highest risk of H pylori. The estimated prevalence of infection is 40% to 48%23 worldwide but lower in North America, at 32% to 42%.24H pylori is often acquired in childhood, and risk of infection is more likely if the parents (particularly mothers) are infected.9
Continue to: Whom to test, and how
Whom to test, and how
Test for H pylori in those with active peptic ulcer disease or a history of peptic ulcer disease that was not investigated for H pylori. Also test individuals who have gastric mucosa-associated lymphoid tissue lymphoma, have a history of gastric cancer or family history of gastric cancer, are scheduled for endoscopic evaluation for dyspepsia, or are starting chronic NSAID therapy. Patients with typical GERD symptoms do not need to be tested for H pylori.9,25
Means of testing for H pylori include the urea breath test, stool antigen studies, endoscopically obtained biopsies, or serum antibody tests. Antibody testing is discouraged because it has a lower diagnostic utility and cannot determine if the patient’s infection is current or past. Before undergoing urea breath tests, stool antigen tests, or biopsies for H pylori identification, patients should have abstained from taking the following agents for the time periods indicated: PPIs, 1 to 2 weeks; H2RAs, at least 1 day and preferably 2 weeks; and antibiotics, 4 weeks.9
The urea breath test and endoscopically obtained biopsies have the greatest diagnostic utility and, where available, should be the first-line tests. Stool antigen studies are useful for ruling out H pylori infection (very low negative likelihood ratio), but a positive test result is not as useful for confirming an infection, as false-positives do occur (moderate positive likelihood ratio).9,26,27 Stool antigen testing is less expensive and, in many cases, more convenient and readily available for patients than urea breath testing and endoscopic biopsies.
Treatment
Offer treatment to all patients who test positive for H pylori. Eradication rates range from 70% to 91% using first-line treatment options.9 Treatment regimens consist of acid suppression and 2 to 3 antibiotics in combination (TABLE 39,28). The single greatest predictive factor for treatment failure is antibiotic resistance, so a detailed antibiotic history is essential. In particular, ask about macrolide antibiotic usage and penicillin allergies.

People living in areas with population macrolide resistance ≥ 15% should avoid clarithromycin-based regimens unless bacterial sensitivity testing has been done and shows sensitivity to these agents.9,28,29 For cases that do not resolve with a first-line treatment program, choose an alternative regimen with different antibiotics.9,29
Continue to: Additionally, adequate...
Additionally, adequate acid suppression is directly related to successful eradication. Thus, the likelihood of treatment success can be improved by using higher doses of PPIs and avoiding ones that are more likely to be metabolized quickly in some patients (lansoprazole, omeprazole). Patient adherence to the treatment regimen is an important determinant of effectiveness.9,29 Adding vitamin C 400 to 1000 mg/d, vitamin E 100 to 400 mg/d, and probiotics may improve the effectiveness of treatment.9,30
Duration of treatment is directly related to treatment effectiveness. Whenever possible, opt for 14 days of treatment instead of just 7.9
Test of cure. Patients treated for H pylori should be re-tested no sooner than 4 weeks after completion of therapy. Urea breath testing, stool antigen testing, and endoscopic biopsies (if endoscopy is indicated for some other reason) can all be used post treatment for test of cure.9
Barrett esophagus
Whom to screen
The American College of Gastroenterology recommends consideration of screening with upper endoscopy for men with chronic GERD (> 5 years) or frequent GERD symptoms (once weekly or more often), plus 2
Continue to: Not everyone with BE...
Not everyone with BE experiences GERD symptoms; sometimes BE may be diagnosed incidentally on upper endoscopy performed for unrelated symptoms.11 GERD patients who are currently asymptomatic and had a normal prior upper endoscopy do not require surveillance.
Diagnosis and management
BE is diagnosed based on specific endoscopic and histologic findings. The presence of dysplasia (either low grade or high grade) or its absence has important treatment implications. When histology is indefinite for dysplasia, treat reflux and, following acid suppression with PPIs for 3 to 6 months, repeat endoscopy (since reactive changes with reflux may obscure results).11
Nondysplastic BE has a risk of progressing to cancer in only 0.2% to 0.5% of affected patients per year.11 Guidelines for BE without dysplasia advise repeating surveillance endoscopy every 3 to 5 years after appropriate counseling regarding overall low risk of cancer progression.11,31 Surveillance endoscopy recommendations exist despite the lack of prospective randomized trials that demonstrate benefit. The rationale for surveillance is that survival in EAC is stage dependent and often EAC metastasizes prior to the development of symptoms from the tumor. Observational cohort studies in BE have demonstrated that surveillance endoscopy programs find EAC at earlier stages with improved survival; however, lead and length time bias may attenuate or eliminate these surveillance benefits.11,32
Risk for neoplastic progression increases with degree of dysplasia. BE with low-grade dysplasia and high-grade dysplasia have a risk of cancer progression of 0.7% per year and 7% per year, respectively.11
Historically, esophagectomy was the preferred treatment for BE with dysplasia. Now, endoscopic eradication therapies, including radiofrequency ablation and endoscopic mucosal resection for nodular BE, are the usual treatment for either low- or high-grade dysplasia.11
Chemoprophylaxis with PPIs. Most patients with BE have symptoms of GERD or reflux esophagitis, so treatment with a PPI is indicated for symptom control. In patients with BE without GERD, PPI use may still be indicated, although this is controversial. Current guidelines recommend once-daily PPI therapy for BE (twice daily only if needed for symptom control) to reduce reflux-associated inflammation and recommend against routine prescription of aspirin or NSAIDs for BE.11 In vitro and observational studies support PPI use to prevent progression to EAC11,33; however, data from randomized controlled trials to support their use are limited.34,35
CORRESPONDENCE
Megan Everson, MD, Medical College of Wisconsin, 229 South Morrison Street, Appleton WI, 54911; [email protected]
1. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500. doi: 10.1038/ajg.2008.37
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324:2536-2547. doi: 10.1001/jama.2020.21360
3. Scida S, Russo M, Miraglia C, et al. Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 2018;89:40-43. doi: 10.23750/abm.v89i8-S.7918
4. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. doi: 10.1111/j.1572-0241.2006.00630.x
5. Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. doi: 10.1016/j.suc.2015.02.006
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. doi: 10.1038/ajg.2012.444
7. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and treatment of the extraesophageal manifestations of gastroesophageal reflux disease. Ann Surg. 2017;265:63-67. doi: 10.1097/SLA.0000000000001907
8. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. doi: 10.1136/gutjnl-2016-313589
9. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. doi: 10.1038/ajg.2016.563
10. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. doi: 10.1136/gutjnl-2020-321365
11. Shaheen NJ, Falk GW, Iyer PG, et al; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30-50. doi: 10.1038/ajg.2015.322
12. Savarino E, Bredenoord AJ, Fox M, et al; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665-676. doi: 10.1038/nrgastro.2017.130
13. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. doi: 10.1056/NEJMcp0804684
14. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031
15. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. doi: 10.1097/MPG.0000000000001889
16. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391. doi: 10.1053/j.gastro.2008.08.045
17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808. doi: 10.1016/j.cgh.2017.09.033
18. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691. doi: 10.1053/j.gastro.2019.05.056
19. Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. doi: 10.1136/bmj.m3786
20. Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:793-806. doi: 10.1016/j.bpg.2007.05.006
21. Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. doi: 10.1016/j.gtc.2016.02.005
22. Lightdale JR, Gremse DA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131;e1684-e1695. doi: 10.1542/peds.2013-0421
23. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022
24. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. doi: 10.1111/apt.14561
25. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-436. doi: 10.1056/NEJMoa1909666
26. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. doi: 10.1111/j.1572-0241.2006.00528.x
27. Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2
28. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3. doi: 10.14309/ajg.0000000000001031
29. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831-1841. doi: 10.1053/j.gastro.2020.11.059
30. Yang-Ou YB, Hu Y, Zhu Y, et al. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535
31. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. doi: 10.1053/j.gastro.2011.01.030
32. Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068-2086. doi: 10.1053/j.gastro.2018.02.022
33. Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. doi: 10.1136/gutjnl-2013-305997
34. Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. doi: 10.1016/S0140-6736(18)31388-6
35. Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2017;12:e0169691. doi: 10.1371/journal.pone.0169691
1. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500. doi: 10.1038/ajg.2008.37
2. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. 2020;324:2536-2547. doi: 10.1001/jama.2020.21360
3. Scida S, Russo M, Miraglia C, et al. Relationship between Helicobacter pylori infection and GERD. Acta Biomed. 2018;89:40-43. doi: 10.23750/abm.v89i8-S.7918
4. Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920. doi: 10.1111/j.1572-0241.2006.00630.x
5. Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515-525. doi: 10.1016/j.suc.2015.02.006
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328. doi: 10.1038/ajg.2012.444
7. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and treatment of the extraesophageal manifestations of gastroesophageal reflux disease. Ann Surg. 2017;265:63-67. doi: 10.1097/SLA.0000000000001907
8. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430-440. doi: 10.1136/gutjnl-2016-313589
9. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. doi: 10.1038/ajg.2016.563
10. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. doi: 10.1136/gutjnl-2020-321365
11. Shaheen NJ, Falk GW, Iyer PG, et al; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30-50. doi: 10.1038/ajg.2015.322
12. Savarino E, Bredenoord AJ, Fox M, et al; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2017;14:665-676. doi: 10.1038/nrgastro.2017.130
13. Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. doi: 10.1056/NEJMcp0804684
14. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031
15. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. doi: 10.1097/MPG.0000000000001889
16. Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391. doi: 10.1053/j.gastro.2008.08.045
17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808. doi: 10.1016/j.cgh.2017.09.033
18. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691. doi: 10.1053/j.gastro.2019.05.056
19. Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. doi: 10.1136/bmj.m3786
20. Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:793-806. doi: 10.1016/j.bpg.2007.05.006
21. Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. doi: 10.1016/j.gtc.2016.02.005
22. Lightdale JR, Gremse DA, et al. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131;e1684-e1695. doi: 10.1542/peds.2013-0421
23. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022
24. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. doi: 10.1111/apt.14561
25. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-436. doi: 10.1056/NEJMoa1909666
26. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. doi: 10.1111/j.1572-0241.2006.00528.x
27. Best LM, Takwoingi Y, Siddique S, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2
28. Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1-3. doi: 10.14309/ajg.0000000000001031
29. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160:1831-1841. doi: 10.1053/j.gastro.2020.11.059
30. Yang-Ou YB, Hu Y, Zhu Y, et al. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535
31. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. doi: 10.1053/j.gastro.2011.01.030
32. Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068-2086. doi: 10.1053/j.gastro.2018.02.022
33. Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. doi: 10.1136/gutjnl-2013-305997
34. Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. doi: 10.1016/S0140-6736(18)31388-6
35. Hu Q, Sun TT, Hong J, et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. PLoS One. 2017;12:e0169691. doi: 10.1371/journal.pone.0169691
PRACTICE RECOMMENDATIONS
› Recommend endoscopy for patients with gastroesophageal reflux disease (GERD) and red flag symptoms: dysphagia, unintentional weight loss, or bleeding. B
› Recommend long-term use of a proton pump inhibitor at the lowest tolerated dose in patients with esophagitis or Barrett esophagus. C
› Test for Helicobacter pylori in patients with peptic ulcer disease, in those with past ulcers not investigated for H pylori, and in those starting chronic nonsteroidal anti-inflammatory drug therapy. A
› Use a urea breath test, stool antigen study, or endoscopically obtained biopsy to test for H pylori. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Tips for managing 4 common soft-tissue finger and thumb injuries
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).
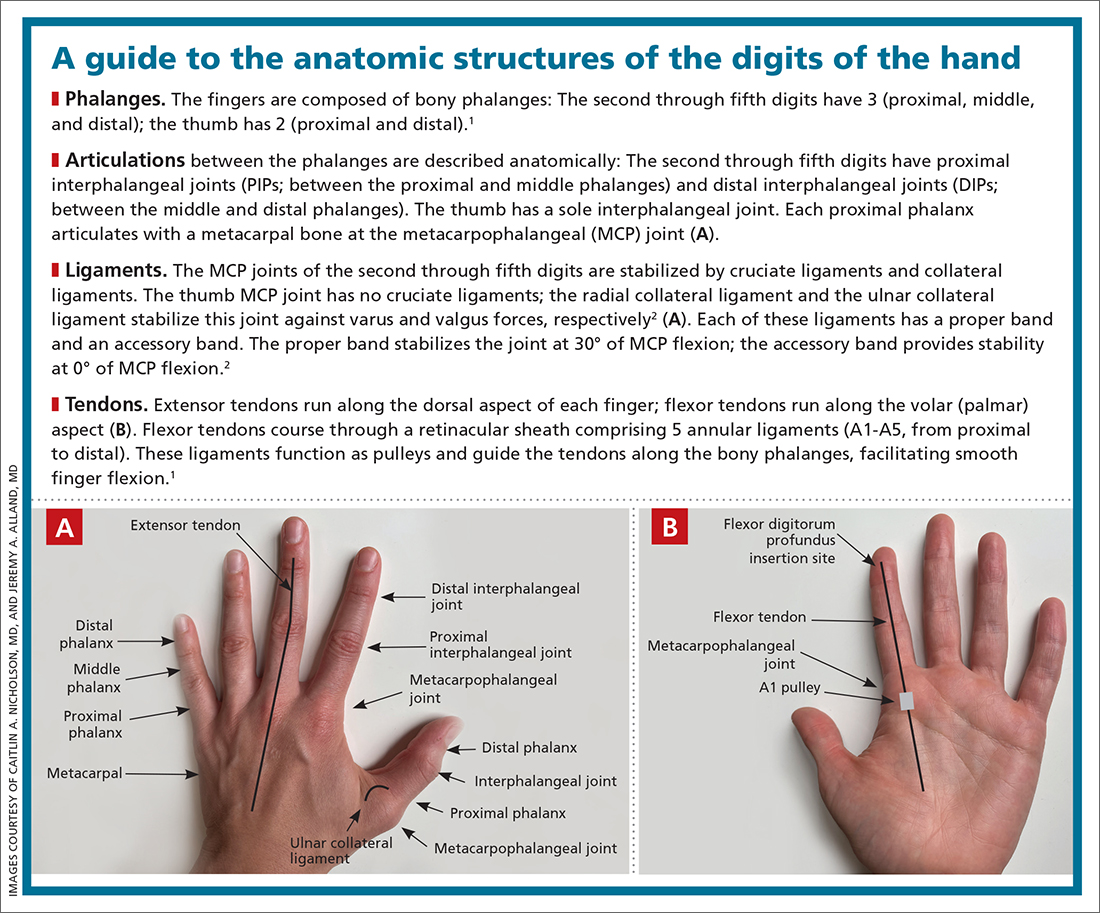
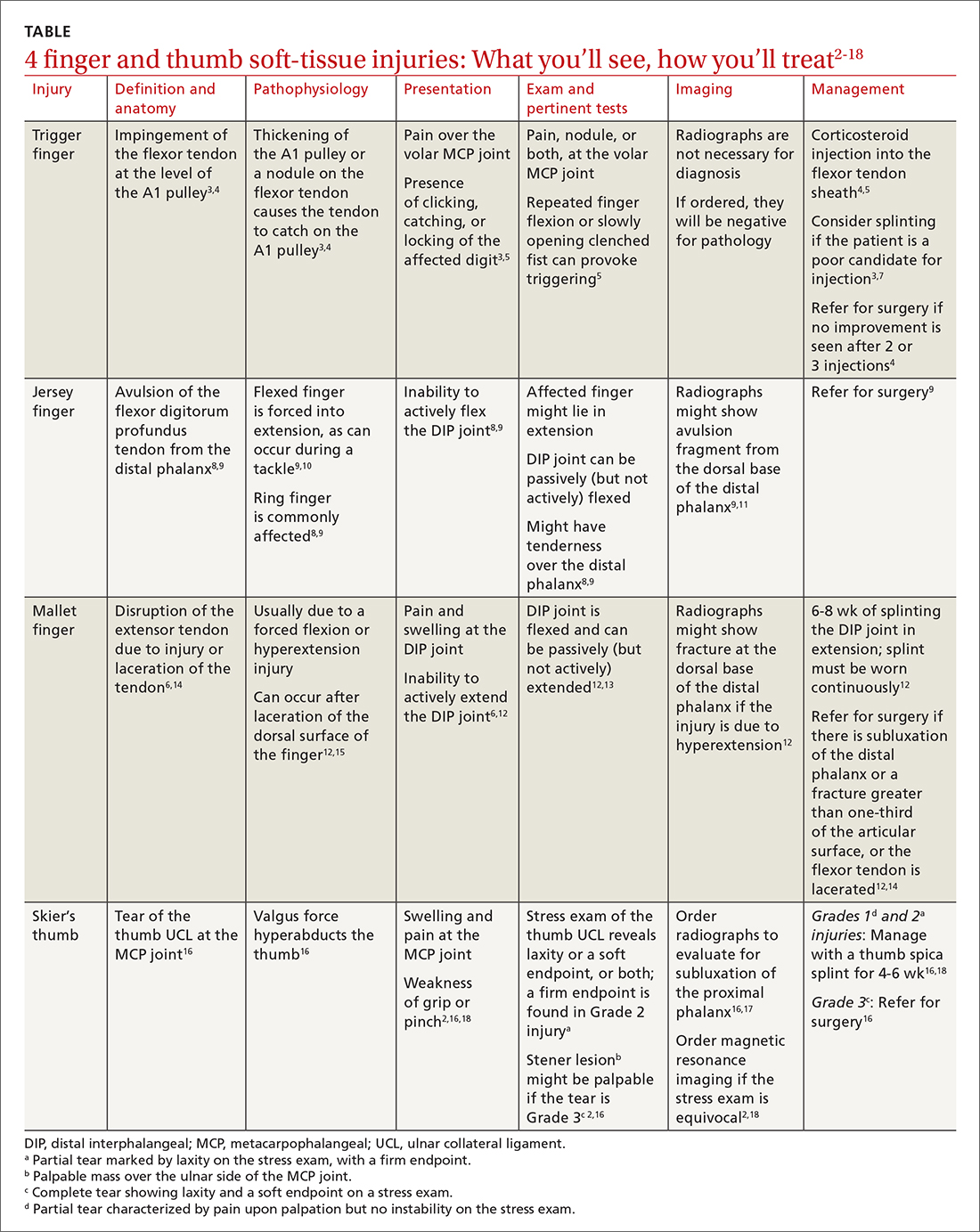
Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6
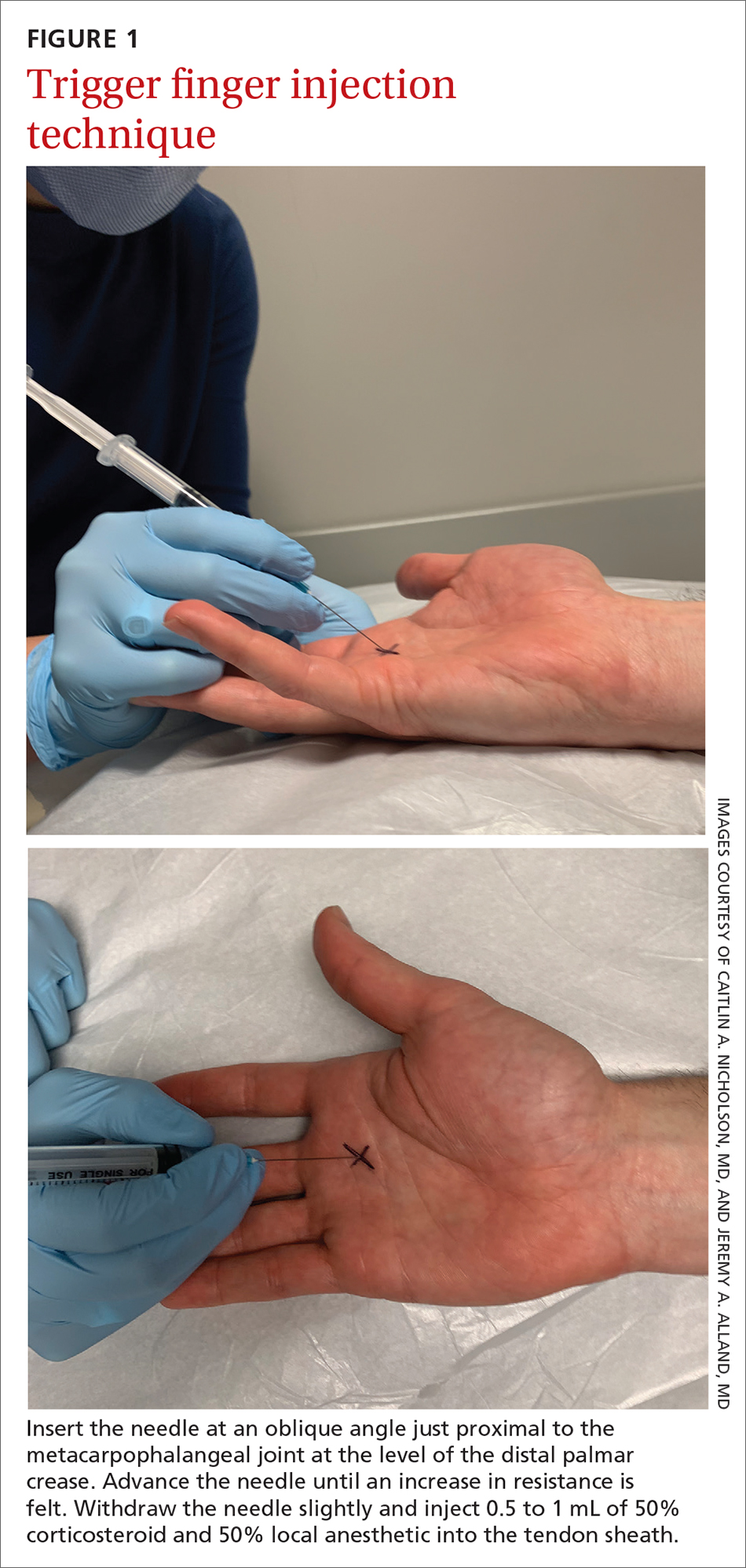
The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23
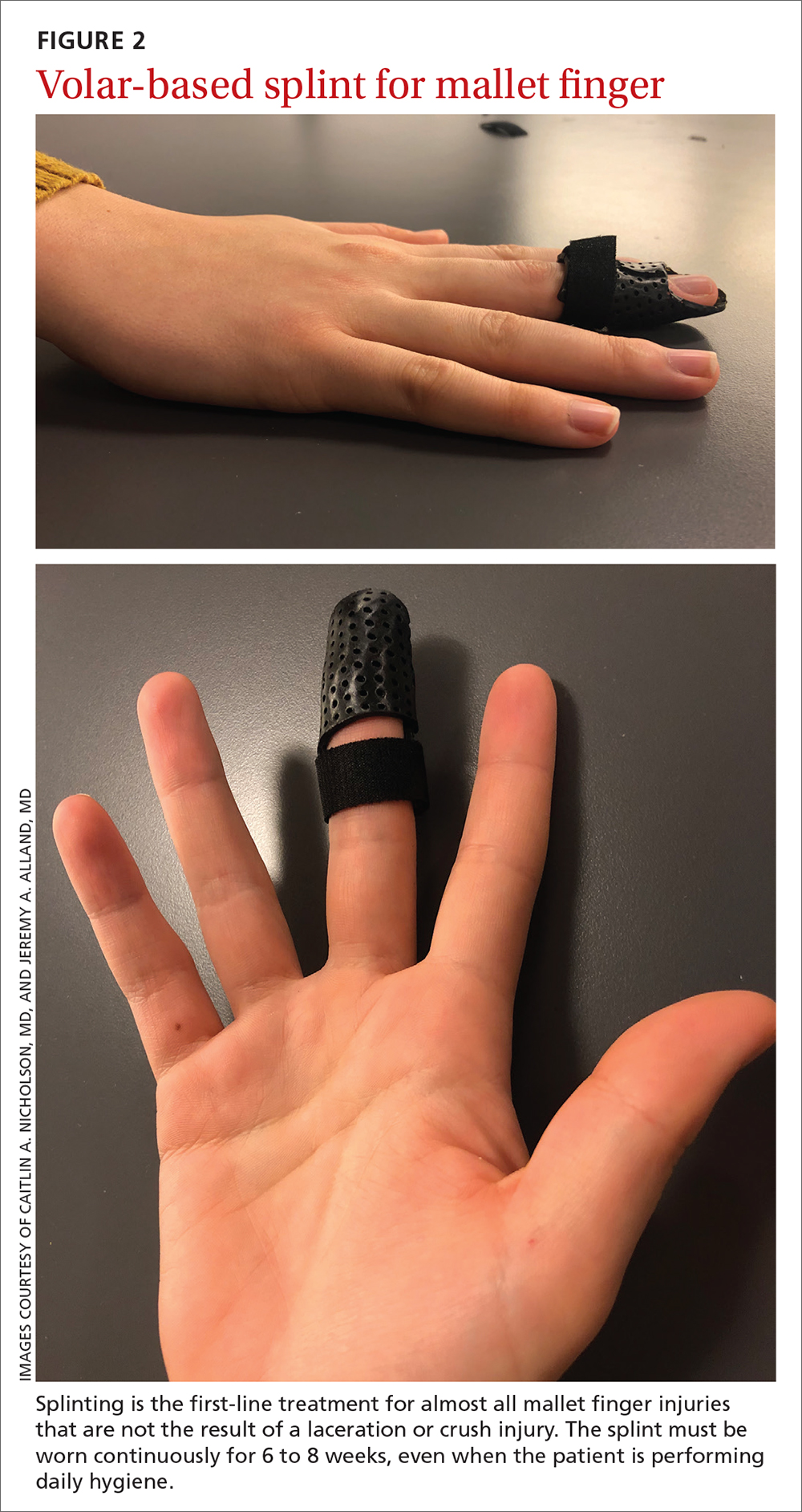
Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17
Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
PRACTICE RECOMMENDATIONS
› Treat trigger finger with a corticosteroid injection into the flexor tendon sheath. A
› Refer a case of jersey finger to a hand surgeon within 1 week after injury for flexor tendon repair. C
› Treat mallet finger with strict distal interphalangeal joint immobilization for 6 to 8 weeks. A
› Treat Grades 1 and 2 skier’s thumb with immobilization in a thumb spica splint or a cast for 4 to 6 weeks. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
2022 Billing and coding updates: Critical care services
The principal idea behind this article is to summarize comprehensively yet concisely the 2022 CMS updates regarding the critical care services. I would encourage and urge all the members to read this section attentively to stay abreast with all the recent developments.
As a general reminder the two critical care services billing codes for the evaluation and management of the critically ill injured patients are:
99291: First 30-74 minutes
99292: Each additional 30 minutes
And, the five major changes for 2022 as proposed by the CMS for critical care services are:
1. It is allowed for the physicians and APPs in the same specialty to bill concurrent critical care services.
Previously, same specialty practitioners were required to bill and were paid as “one” when multiple practitioners provided services on the same date. Now, they can bill for critical care services as subsequent care or as aggregate time, and they are highlighted below with examples:
Subsequent care
Initial visit by a provider for 65 minutes (bill as 99291 as the first claim)
Subsequent visit at a later time on the same day for 60 minutes (bill as 99292 x2 as the second claim)
Aggregate time
Time of multiple practitioners in the same specialty can be added to meet 99291 or 99292. If Practitioner A spends 15 minutes of critical care, then 99291 cannot be billed; but, if Practitioner B spends 30 minutes of critical care, they can bill 99291 with a total time of 45 minutes as one claim
The prerequisites are that the visits are medically necessary, and each visit meets the definition of critical care.
2. Modifier FS needs to be used for split sharing of critical care services.
Previously, critical care services could not be split shared, but it can be done in 2022. The practitioner who provides the significant portion of the visit needs to bill. A significant or substantive portion is considered to be more than half the cumulative total time of both providers.
Example: The APP spends 20 minutes in critical care services and the physician spends 30 minutes. Total time spent is 50 minutes, and the physician may bill 99291.
It is crucial to note that each provider needs to document a note for the medically necessary critical care that they personally performed and the time they spent. Additionally, upon review of the medical records, the two providers should be easily identifiable, and the medical record must be signed and dated by the provider who performed the substantive portion and billed.
Lastly, do not forget to submit the modifier FS.
3. Modifier 25 needs to be used to get paid for an ED visit or other E/M service on the same day as critical care.
Previously, hospital ED services were not paid on the same date as critical care by the same provider. But, in 2022, the practitioners may bill for ED visit at the hospital and also for other E/M services on the same day when there is supporting documentation. The practitioners will need to document that the E/M service was provided prior to the time when the patient did not require critical care, that the service was medically necessary, and that the service was separate and distinct with no duplication.
Of note, do not forget to submit the modifier 25.
4. Critical care visits will be separately billable from global surgery when unrelated with the use of modifier FT.
Previously pre- and postoperative critical care was included in the surgical package of many procedures with a global period of 10-90 days, and critical care visits would be paid only if the service was unrelated to the procedure. The concept remains the same in 2022 but, now, new modifier FT will need to be used to report critical care services unrelated to the procedure. Also, the service provided will need to meet the definition of critical care, which is usually above and beyond the procedure performed and should be unrelated to the specific injury or general surgical procedure performed.
5. There will be certain critical care medical record documentation requirements.
It is paramount that each practitioner must document the exact total critical care time and not a range or approximation of time. Additionally, it is equally as important for the documentation to indicate that the services provided were medically reasonable and necessary. In the setting of split/shared billing, the role of each practitioner should be clearly identifiable (the condition for which each practitioner treated the patient, how the care was concurrent either subsequent or aggregate, and the total time of each practitioner).
Hopefully, this review will provide a good perception for our members in regards to major updates for 2022, help them navigate the regulatory rules, and avoid any unnecessary setbacks. In the upcoming months, we will try to cover some more topics on practice management and administration, such as Medicare Physician Fee Schedule Rule, Hospital Outpatient Prospective Payment Rule, and coding/billing for teaching physicians, telehealth, and pulmonary rehabilitation services.
The principal idea behind this article is to summarize comprehensively yet concisely the 2022 CMS updates regarding the critical care services. I would encourage and urge all the members to read this section attentively to stay abreast with all the recent developments.
As a general reminder the two critical care services billing codes for the evaluation and management of the critically ill injured patients are:
99291: First 30-74 minutes
99292: Each additional 30 minutes
And, the five major changes for 2022 as proposed by the CMS for critical care services are:
1. It is allowed for the physicians and APPs in the same specialty to bill concurrent critical care services.
Previously, same specialty practitioners were required to bill and were paid as “one” when multiple practitioners provided services on the same date. Now, they can bill for critical care services as subsequent care or as aggregate time, and they are highlighted below with examples:
Subsequent care
Initial visit by a provider for 65 minutes (bill as 99291 as the first claim)
Subsequent visit at a later time on the same day for 60 minutes (bill as 99292 x2 as the second claim)
Aggregate time
Time of multiple practitioners in the same specialty can be added to meet 99291 or 99292. If Practitioner A spends 15 minutes of critical care, then 99291 cannot be billed; but, if Practitioner B spends 30 minutes of critical care, they can bill 99291 with a total time of 45 minutes as one claim
The prerequisites are that the visits are medically necessary, and each visit meets the definition of critical care.
2. Modifier FS needs to be used for split sharing of critical care services.
Previously, critical care services could not be split shared, but it can be done in 2022. The practitioner who provides the significant portion of the visit needs to bill. A significant or substantive portion is considered to be more than half the cumulative total time of both providers.
Example: The APP spends 20 minutes in critical care services and the physician spends 30 minutes. Total time spent is 50 minutes, and the physician may bill 99291.
It is crucial to note that each provider needs to document a note for the medically necessary critical care that they personally performed and the time they spent. Additionally, upon review of the medical records, the two providers should be easily identifiable, and the medical record must be signed and dated by the provider who performed the substantive portion and billed.
Lastly, do not forget to submit the modifier FS.
3. Modifier 25 needs to be used to get paid for an ED visit or other E/M service on the same day as critical care.
Previously, hospital ED services were not paid on the same date as critical care by the same provider. But, in 2022, the practitioners may bill for ED visit at the hospital and also for other E/M services on the same day when there is supporting documentation. The practitioners will need to document that the E/M service was provided prior to the time when the patient did not require critical care, that the service was medically necessary, and that the service was separate and distinct with no duplication.
Of note, do not forget to submit the modifier 25.
4. Critical care visits will be separately billable from global surgery when unrelated with the use of modifier FT.
Previously pre- and postoperative critical care was included in the surgical package of many procedures with a global period of 10-90 days, and critical care visits would be paid only if the service was unrelated to the procedure. The concept remains the same in 2022 but, now, new modifier FT will need to be used to report critical care services unrelated to the procedure. Also, the service provided will need to meet the definition of critical care, which is usually above and beyond the procedure performed and should be unrelated to the specific injury or general surgical procedure performed.
5. There will be certain critical care medical record documentation requirements.
It is paramount that each practitioner must document the exact total critical care time and not a range or approximation of time. Additionally, it is equally as important for the documentation to indicate that the services provided were medically reasonable and necessary. In the setting of split/shared billing, the role of each practitioner should be clearly identifiable (the condition for which each practitioner treated the patient, how the care was concurrent either subsequent or aggregate, and the total time of each practitioner).
Hopefully, this review will provide a good perception for our members in regards to major updates for 2022, help them navigate the regulatory rules, and avoid any unnecessary setbacks. In the upcoming months, we will try to cover some more topics on practice management and administration, such as Medicare Physician Fee Schedule Rule, Hospital Outpatient Prospective Payment Rule, and coding/billing for teaching physicians, telehealth, and pulmonary rehabilitation services.
The principal idea behind this article is to summarize comprehensively yet concisely the 2022 CMS updates regarding the critical care services. I would encourage and urge all the members to read this section attentively to stay abreast with all the recent developments.
As a general reminder the two critical care services billing codes for the evaluation and management of the critically ill injured patients are:
99291: First 30-74 minutes
99292: Each additional 30 minutes
And, the five major changes for 2022 as proposed by the CMS for critical care services are:
1. It is allowed for the physicians and APPs in the same specialty to bill concurrent critical care services.
Previously, same specialty practitioners were required to bill and were paid as “one” when multiple practitioners provided services on the same date. Now, they can bill for critical care services as subsequent care or as aggregate time, and they are highlighted below with examples:
Subsequent care
Initial visit by a provider for 65 minutes (bill as 99291 as the first claim)
Subsequent visit at a later time on the same day for 60 minutes (bill as 99292 x2 as the second claim)
Aggregate time
Time of multiple practitioners in the same specialty can be added to meet 99291 or 99292. If Practitioner A spends 15 minutes of critical care, then 99291 cannot be billed; but, if Practitioner B spends 30 minutes of critical care, they can bill 99291 with a total time of 45 minutes as one claim
The prerequisites are that the visits are medically necessary, and each visit meets the definition of critical care.
2. Modifier FS needs to be used for split sharing of critical care services.
Previously, critical care services could not be split shared, but it can be done in 2022. The practitioner who provides the significant portion of the visit needs to bill. A significant or substantive portion is considered to be more than half the cumulative total time of both providers.
Example: The APP spends 20 minutes in critical care services and the physician spends 30 minutes. Total time spent is 50 minutes, and the physician may bill 99291.
It is crucial to note that each provider needs to document a note for the medically necessary critical care that they personally performed and the time they spent. Additionally, upon review of the medical records, the two providers should be easily identifiable, and the medical record must be signed and dated by the provider who performed the substantive portion and billed.
Lastly, do not forget to submit the modifier FS.
3. Modifier 25 needs to be used to get paid for an ED visit or other E/M service on the same day as critical care.
Previously, hospital ED services were not paid on the same date as critical care by the same provider. But, in 2022, the practitioners may bill for ED visit at the hospital and also for other E/M services on the same day when there is supporting documentation. The practitioners will need to document that the E/M service was provided prior to the time when the patient did not require critical care, that the service was medically necessary, and that the service was separate and distinct with no duplication.
Of note, do not forget to submit the modifier 25.
4. Critical care visits will be separately billable from global surgery when unrelated with the use of modifier FT.
Previously pre- and postoperative critical care was included in the surgical package of many procedures with a global period of 10-90 days, and critical care visits would be paid only if the service was unrelated to the procedure. The concept remains the same in 2022 but, now, new modifier FT will need to be used to report critical care services unrelated to the procedure. Also, the service provided will need to meet the definition of critical care, which is usually above and beyond the procedure performed and should be unrelated to the specific injury or general surgical procedure performed.
5. There will be certain critical care medical record documentation requirements.
It is paramount that each practitioner must document the exact total critical care time and not a range or approximation of time. Additionally, it is equally as important for the documentation to indicate that the services provided were medically reasonable and necessary. In the setting of split/shared billing, the role of each practitioner should be clearly identifiable (the condition for which each practitioner treated the patient, how the care was concurrent either subsequent or aggregate, and the total time of each practitioner).
Hopefully, this review will provide a good perception for our members in regards to major updates for 2022, help them navigate the regulatory rules, and avoid any unnecessary setbacks. In the upcoming months, we will try to cover some more topics on practice management and administration, such as Medicare Physician Fee Schedule Rule, Hospital Outpatient Prospective Payment Rule, and coding/billing for teaching physicians, telehealth, and pulmonary rehabilitation services.
Pneumothorax, pneumomediastinum, and subcutaneous emphysema: The many faces of COVID-19 ARDS
I recall early in the pandemic being called to the bedside to examine an acutely decompensating patient with COVID-19. This was a 33-year-old, previously healthy woman, admitted to the medical ICU with hypoxemic respiratory failure requiring mechanical ventilation and undergoing treatment for severe acute respiratory distress syndrome (ARDS). I quickly realized she was seconds away from an arrest. As I examined her, one thing caught my eye. Her airway pressures had skyrocketed over the past few minutes. Could it be? I thought to myself as I reached for the ultrasound that confirmed my suspicions, tension pneumothorax. One emergent needle decompression and chest tube later and she survives, only to die a week later from overwhelming hypoxemia.
As we reflect on these past 26 months, we recall that caring for the critically ill patient with COVID-19 has posed numerous challenges. One challenge was the overwhelming incidence of the so-called “barotrauma-related complications.” However, we also recall seeing many patients develop such complications while receiving supplemental noninvasive forms of respiratory support. Perhaps, this is in agreement with prior literature that specifically discusses the presence of air outside the tracheobronchial tree and how it does not always correlate with high airway pressure and high tidal volumes, refuting the argument that these complications always fall under the umbrella of barotrauma. We will discuss these complications and attempt to shed light on the potential variables associated with their development.
The development of pneumothorax is a well-recognized complication associated with ventilator-dependent ARDS thought to be a form of barotrauma, with some reports indicating an incidence of 48.8% (Gattinoni L et al. JAMA. 1994;271[2]):1772-9) and a significantly increased mortality rate compared with postprocedural pneumothorax in the ICU (Chen K et al. Chest. 2002;122[2]:678-83). The incidence of such complication in COVID-19-related ARDS is significantly higher than in ARDS from other causes (Belletti A et al. Crit Care Med. 2022;50[3]:491-500), with a mortality rate approaching 100% (Chong WH et al. Heart Lung. 2021;50[5]:599-608).
So why are patients with COVID-19 developing these complications at a higher rate? When we examine the literature, we note that Leisman and colleagues (Am J Respir Crit Care Med. 2022;205[5]:507-19) describe higher baseline markers of alveolar damage, including RAGE (receptor for advanced glycation end-products) in mechanically ventilated patients with COVID-19 vs patients requiring mechanical ventilation for other causes. This poses a question that perhaps one of the main reasons patients with COVID-19 ARDS are at an increased risk for developing certain complications, such as pneumothorax, is inherent to the unique type of alveolar injury sustained with the infection. The authors also note that alveolar markers of injury had moderate to poor discrimination for invasive ventilation early in the disease and diminished over time in both ventilated patients receiving lung protective ventilation strategy and those spontaneously breathing. Likewise, this important finding suggests that the development of pneumothorax in patients with COVID-19 may not be entirely related to barotrauma.
Another phenomenon worth investigating is the development of pneumomediastinum and subcutaneous emphysema, with a reported seven-fold increased risk of development in patients with COVID-19. Lemmers and colleagues (ERJ Open Res. 2020;6[4]:00385-2020) found no statistically significant difference in PEEP, plateau pressure, ratio of tidal volume to ideal body weight, or compliance between patients who developed this complication and those who did not, again, signifying that perhaps there is more to the story here.
Belletti and colleagues (J Cardiothorac Vasc Anesth. 2021;35[12]:3642-51) published an article examining the predictors of pneumothorax and pneumomediastinum in patients with COVID-19. The authors found that the time from symptom onset to intubation and the total bilirubin level were the only two significant predictors for the development of these complications. They explain that longer time from symptom onset to intubation likely increased the risk for self-induced lung injury, inflammation, and fibrosis, contributing to the development of such complications. It is important to note that the authors did not find a significant difference in the ventilation parameters between patients who developed pneumothorax/pneumomediastinum and those who did not.
In our institute, we examined a total of 102 patients admitted to the ICU with COVID-19 ARDS over a 3-month period from March 2020 to May 2020. We identified a total of 36 patients who developed pneumothorax, pneumomediastinum, and/or subcutaneous emphysema. We compared these subjects to age- and gender-matched control subjects. Higher age was associated with an increased risk of development of these complications, whereas the presence of diabetes mellitus, hypertension, and chronic kidney disease at baseline was associated with lower risk. This translated into lower mSOFA scores in our subjects as opposed to the control subjects mainly due to higher creatinine levels at baseline in the control group, skewing our data and indicating that some predictive criteria may not reflect the underlying disease severity and risk for development of such complications. In analyzing our ventilator data and comparing the subjects to the control group, we found no differences in mode of ventilation, set tidal volumes, or PEEP levels between the two. The subjects had significantly higher peak airway pressures, lower compliance, and longer ventilator days. Intubation was needed significantly earlier in the subjects compared with the control group with a median of 2 days vs 6 days from admission. Our data are in concordance with prior published reports and are set to be presented in abstract form this May.
COVID-19 remains a challenging disease with the potential for morbid outcomes. As we phase out of the pandemic and move into an epidemic, future research direction will likely focus on some of the more unusually common complications, such as the ones presented here.
Dr. Abdullah is with the Henry Ford Health System, Detroit, Michigan.
I recall early in the pandemic being called to the bedside to examine an acutely decompensating patient with COVID-19. This was a 33-year-old, previously healthy woman, admitted to the medical ICU with hypoxemic respiratory failure requiring mechanical ventilation and undergoing treatment for severe acute respiratory distress syndrome (ARDS). I quickly realized she was seconds away from an arrest. As I examined her, one thing caught my eye. Her airway pressures had skyrocketed over the past few minutes. Could it be? I thought to myself as I reached for the ultrasound that confirmed my suspicions, tension pneumothorax. One emergent needle decompression and chest tube later and she survives, only to die a week later from overwhelming hypoxemia.
As we reflect on these past 26 months, we recall that caring for the critically ill patient with COVID-19 has posed numerous challenges. One challenge was the overwhelming incidence of the so-called “barotrauma-related complications.” However, we also recall seeing many patients develop such complications while receiving supplemental noninvasive forms of respiratory support. Perhaps, this is in agreement with prior literature that specifically discusses the presence of air outside the tracheobronchial tree and how it does not always correlate with high airway pressure and high tidal volumes, refuting the argument that these complications always fall under the umbrella of barotrauma. We will discuss these complications and attempt to shed light on the potential variables associated with their development.
The development of pneumothorax is a well-recognized complication associated with ventilator-dependent ARDS thought to be a form of barotrauma, with some reports indicating an incidence of 48.8% (Gattinoni L et al. JAMA. 1994;271[2]):1772-9) and a significantly increased mortality rate compared with postprocedural pneumothorax in the ICU (Chen K et al. Chest. 2002;122[2]:678-83). The incidence of such complication in COVID-19-related ARDS is significantly higher than in ARDS from other causes (Belletti A et al. Crit Care Med. 2022;50[3]:491-500), with a mortality rate approaching 100% (Chong WH et al. Heart Lung. 2021;50[5]:599-608).
So why are patients with COVID-19 developing these complications at a higher rate? When we examine the literature, we note that Leisman and colleagues (Am J Respir Crit Care Med. 2022;205[5]:507-19) describe higher baseline markers of alveolar damage, including RAGE (receptor for advanced glycation end-products) in mechanically ventilated patients with COVID-19 vs patients requiring mechanical ventilation for other causes. This poses a question that perhaps one of the main reasons patients with COVID-19 ARDS are at an increased risk for developing certain complications, such as pneumothorax, is inherent to the unique type of alveolar injury sustained with the infection. The authors also note that alveolar markers of injury had moderate to poor discrimination for invasive ventilation early in the disease and diminished over time in both ventilated patients receiving lung protective ventilation strategy and those spontaneously breathing. Likewise, this important finding suggests that the development of pneumothorax in patients with COVID-19 may not be entirely related to barotrauma.
Another phenomenon worth investigating is the development of pneumomediastinum and subcutaneous emphysema, with a reported seven-fold increased risk of development in patients with COVID-19. Lemmers and colleagues (ERJ Open Res. 2020;6[4]:00385-2020) found no statistically significant difference in PEEP, plateau pressure, ratio of tidal volume to ideal body weight, or compliance between patients who developed this complication and those who did not, again, signifying that perhaps there is more to the story here.
Belletti and colleagues (J Cardiothorac Vasc Anesth. 2021;35[12]:3642-51) published an article examining the predictors of pneumothorax and pneumomediastinum in patients with COVID-19. The authors found that the time from symptom onset to intubation and the total bilirubin level were the only two significant predictors for the development of these complications. They explain that longer time from symptom onset to intubation likely increased the risk for self-induced lung injury, inflammation, and fibrosis, contributing to the development of such complications. It is important to note that the authors did not find a significant difference in the ventilation parameters between patients who developed pneumothorax/pneumomediastinum and those who did not.
In our institute, we examined a total of 102 patients admitted to the ICU with COVID-19 ARDS over a 3-month period from March 2020 to May 2020. We identified a total of 36 patients who developed pneumothorax, pneumomediastinum, and/or subcutaneous emphysema. We compared these subjects to age- and gender-matched control subjects. Higher age was associated with an increased risk of development of these complications, whereas the presence of diabetes mellitus, hypertension, and chronic kidney disease at baseline was associated with lower risk. This translated into lower mSOFA scores in our subjects as opposed to the control subjects mainly due to higher creatinine levels at baseline in the control group, skewing our data and indicating that some predictive criteria may not reflect the underlying disease severity and risk for development of such complications. In analyzing our ventilator data and comparing the subjects to the control group, we found no differences in mode of ventilation, set tidal volumes, or PEEP levels between the two. The subjects had significantly higher peak airway pressures, lower compliance, and longer ventilator days. Intubation was needed significantly earlier in the subjects compared with the control group with a median of 2 days vs 6 days from admission. Our data are in concordance with prior published reports and are set to be presented in abstract form this May.
COVID-19 remains a challenging disease with the potential for morbid outcomes. As we phase out of the pandemic and move into an epidemic, future research direction will likely focus on some of the more unusually common complications, such as the ones presented here.
Dr. Abdullah is with the Henry Ford Health System, Detroit, Michigan.
I recall early in the pandemic being called to the bedside to examine an acutely decompensating patient with COVID-19. This was a 33-year-old, previously healthy woman, admitted to the medical ICU with hypoxemic respiratory failure requiring mechanical ventilation and undergoing treatment for severe acute respiratory distress syndrome (ARDS). I quickly realized she was seconds away from an arrest. As I examined her, one thing caught my eye. Her airway pressures had skyrocketed over the past few minutes. Could it be? I thought to myself as I reached for the ultrasound that confirmed my suspicions, tension pneumothorax. One emergent needle decompression and chest tube later and she survives, only to die a week later from overwhelming hypoxemia.
As we reflect on these past 26 months, we recall that caring for the critically ill patient with COVID-19 has posed numerous challenges. One challenge was the overwhelming incidence of the so-called “barotrauma-related complications.” However, we also recall seeing many patients develop such complications while receiving supplemental noninvasive forms of respiratory support. Perhaps, this is in agreement with prior literature that specifically discusses the presence of air outside the tracheobronchial tree and how it does not always correlate with high airway pressure and high tidal volumes, refuting the argument that these complications always fall under the umbrella of barotrauma. We will discuss these complications and attempt to shed light on the potential variables associated with their development.
The development of pneumothorax is a well-recognized complication associated with ventilator-dependent ARDS thought to be a form of barotrauma, with some reports indicating an incidence of 48.8% (Gattinoni L et al. JAMA. 1994;271[2]):1772-9) and a significantly increased mortality rate compared with postprocedural pneumothorax in the ICU (Chen K et al. Chest. 2002;122[2]:678-83). The incidence of such complication in COVID-19-related ARDS is significantly higher than in ARDS from other causes (Belletti A et al. Crit Care Med. 2022;50[3]:491-500), with a mortality rate approaching 100% (Chong WH et al. Heart Lung. 2021;50[5]:599-608).
So why are patients with COVID-19 developing these complications at a higher rate? When we examine the literature, we note that Leisman and colleagues (Am J Respir Crit Care Med. 2022;205[5]:507-19) describe higher baseline markers of alveolar damage, including RAGE (receptor for advanced glycation end-products) in mechanically ventilated patients with COVID-19 vs patients requiring mechanical ventilation for other causes. This poses a question that perhaps one of the main reasons patients with COVID-19 ARDS are at an increased risk for developing certain complications, such as pneumothorax, is inherent to the unique type of alveolar injury sustained with the infection. The authors also note that alveolar markers of injury had moderate to poor discrimination for invasive ventilation early in the disease and diminished over time in both ventilated patients receiving lung protective ventilation strategy and those spontaneously breathing. Likewise, this important finding suggests that the development of pneumothorax in patients with COVID-19 may not be entirely related to barotrauma.
Another phenomenon worth investigating is the development of pneumomediastinum and subcutaneous emphysema, with a reported seven-fold increased risk of development in patients with COVID-19. Lemmers and colleagues (ERJ Open Res. 2020;6[4]:00385-2020) found no statistically significant difference in PEEP, plateau pressure, ratio of tidal volume to ideal body weight, or compliance between patients who developed this complication and those who did not, again, signifying that perhaps there is more to the story here.
Belletti and colleagues (J Cardiothorac Vasc Anesth. 2021;35[12]:3642-51) published an article examining the predictors of pneumothorax and pneumomediastinum in patients with COVID-19. The authors found that the time from symptom onset to intubation and the total bilirubin level were the only two significant predictors for the development of these complications. They explain that longer time from symptom onset to intubation likely increased the risk for self-induced lung injury, inflammation, and fibrosis, contributing to the development of such complications. It is important to note that the authors did not find a significant difference in the ventilation parameters between patients who developed pneumothorax/pneumomediastinum and those who did not.
In our institute, we examined a total of 102 patients admitted to the ICU with COVID-19 ARDS over a 3-month period from March 2020 to May 2020. We identified a total of 36 patients who developed pneumothorax, pneumomediastinum, and/or subcutaneous emphysema. We compared these subjects to age- and gender-matched control subjects. Higher age was associated with an increased risk of development of these complications, whereas the presence of diabetes mellitus, hypertension, and chronic kidney disease at baseline was associated with lower risk. This translated into lower mSOFA scores in our subjects as opposed to the control subjects mainly due to higher creatinine levels at baseline in the control group, skewing our data and indicating that some predictive criteria may not reflect the underlying disease severity and risk for development of such complications. In analyzing our ventilator data and comparing the subjects to the control group, we found no differences in mode of ventilation, set tidal volumes, or PEEP levels between the two. The subjects had significantly higher peak airway pressures, lower compliance, and longer ventilator days. Intubation was needed significantly earlier in the subjects compared with the control group with a median of 2 days vs 6 days from admission. Our data are in concordance with prior published reports and are set to be presented in abstract form this May.
COVID-19 remains a challenging disease with the potential for morbid outcomes. As we phase out of the pandemic and move into an epidemic, future research direction will likely focus on some of the more unusually common complications, such as the ones presented here.
Dr. Abdullah is with the Henry Ford Health System, Detroit, Michigan.
Updates on eosinophilia in asthma
Our understanding of asthma endotypes and phenotypes has grown substantially in the last decade. Endotype-targeted therapy has become a foundation of management, and classification of patients during initial assessment is extremely important. The use of history, laboratory data, and pulmonary function testing together help to categorize our patients and help guide therapy. One lab test, that of sputum or blood eosinophils, facilitates categorization and has been evaluated for its ability to determine response to medications and predict exacerbations.
In particular, eosinophilia has been extensively studied in severe asthma and is associated with type 2 inflammation. The 2021 GINA guidelines describe type 2 inflammation as characterized by cytokines (especially IL-4, IL-5, and IL-13). “T2-high patients” tend to have elevated blood or sputum eosinophil counts and elevated fractional concentration of exhaled nitric oxide (FENO) and are more likely to respond to biologic therapy. (Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021).
However, what about patients with more mild-to-moderate asthma? Two recent studies have asked this question. In 2020, Pavord and colleagues performed a prespecified secondary subgroup analysis on an open-label randomized control trial comparing prn salbutamol alone to budesonide and as needed salbutamol to as needed budesonide-formoterol. The population was 675 adults with mild asthma receiving only as needed short acting beta-agonists (SABA) at baseline. The primary outcome was annual rate of asthma exacerbation, and whether it was different based on blood eosinophil count, FENO or a composite of both. They had several interesting findings. First, for patients only on an as needed SABA, the proportion having a severe exacerbation increased progressively with increasing blood eosinophil count. Second, inhaled corticosteroids (ICS) plus as needed SABA were more effective than SABA alone in patients with a blood eosinophil count of ≥300 cells/μL, both in terms of total exacerbations and severe exacerbations. The effects of budesonide-formoterol on exacerbations, however, was not associated with blood eosinophil count or FENO. This last point is particularly interesting in light of GINA guidelines that prioritize this combination (Pavord ID et al. Lancet Respir Med. 2020;8[7]:671-80).
More recently, a prespecified secondary analysis of the SIENA trial looked at 295 subjects with mild persistent asthma (237 adults aged 18+, and 58 adolescents aged 12-17). The primary outcome was a composite of asthma control (treatment failure, asthma control days, and FEV1). They found that sputum eosinophil levels, blood eosinophil levels, and FENO all predicted response to ICS in adults; however, the area under the receiver operative characteristic curve (AUC) was less than 0.7 for each of these findings, which was below the threshold for acceptability. A blood eosinophil count of ≥100 cells/μL offered 87% sensitivity and 17% specificity for response to ICS (Krishnan JA et al. Ann Am Thorac Soc. 2022;19[3]:372-80).
What does this tell us? Blood eosinophil count may help determine who will respond to ICS, and there remains utility in assessing blood eosinophil count in severe asthma for determining candidacy for biologic therapies. However, the overall utility of blood eosinophils in mild to moderate asthma is not as clear.
But, are we asking the right questions? Many studies look at a single blood eosinophil level, either at a single point in time, a baseline level, or a highest level over a specific time period. But do eosinophil counts vary over time?
A 2018 single-center study initially asked this question. The authors evaluated blood eosinophil levels in 219 adult patients at the NYU/Bellevue Hospital Asthma Clinic over a 5-year period. They found that individual patients had variable eosinophil levels. For example, only 6% (n=13) of patients had levels consistently above 300 cells/μL, but nearly 50% (n=104) had at least one level above 300. The degree of variability was then assessed by K-mean clustering yielding three clusters. Cluster 2 had the largest variability in blood eosinophil counts and a slightly higher absolute eosinophil level. While not significant, there was a suggestion of worse asthma control with more hospitalizations and more prescriptions for multiple controllers in this cluster with more variability. Clearly, this warranted further study (Rakowski E et al. Clin Exp Allergy. 2019;49[2]:163-70).
Variability was re-examined more recently in 2021. A post hoc analysis of two phase III clinical trials from the reslizumab BREATH program looked at eosinophil counts in the 476 patients randomized to receive placebo during the 52-week study. These patients did have eosinophilic asthma by definition and had to have an elevated eosinophil count >400 cells/μL over the 4-week enrollment period to enter the study. However, 124 patients (26.1%) had an eosinophil level <400 cells/μL immediately before the first dose of placebo. The primary outcome was variability in blood eosinophil count. Of patients who started with serum eosinophils <400, 27% to 56% of patients shifted to the ≥400 cells/μL category during the treatment period (this wide range is across three categories of low “baseline” blood eosinophil count; <150, 150 to 300, and 300 to 400). On the contrary, patients who started with eosinophils ≥400 cells/μL tended to stay at that level. The variability is reduced by taking two to three repeat measurements at baseline (Corren et al. J Allergy Clin Immunol Pract. 2021;9[3]:1224-31).
Does this variability have clinical significance? A recent retrospective cohort study looked at 10,059 stable adult patients with asthma from the MAJORICA cohort in Spain, compared with 8,557 control subjects. The primary outcome was total blood eosinophil count and an “eosinophil variability index” (EVI) where EVI=(Eosmax – Eosmin / Eosmax) x 100%. They found that an elevated EVI was associated with hospitalization, more so than maximum eosinophil count or any other eosinophil count variable, with an odds ratio of 3.18 by univariate regression (2.51 by multivariate). They also found that patients with an EVI ≥50% were twice as likely to be hospitalized or visit the ED than those with a lower EVI (Toledo-Pons N et al. Ann Am Thorac Soc. 2022;19[3]:407-14). These results are very interesting and merit further research.
So, what to do with this information? We know that patients with peripheral eosinophilia and severe asthma symptoms are candidates for biologic therapy. They are also more likely to respond to steroids, although the utility of this assessment alone in mild to moderate asthma is less clear. It does seem that more variability in eosinophils over time may be linked to more difficult-to-treat asthma.
Should you check eosinophils in your patients with asthma? GINA 2021 guidelines say to consider it, and list blood eosinophilia as a risk factor for future exacerbation, even if patients have few asthma symptoms. They also say to repeat blood eosinophils in patients with severe asthma, if the level is low at first assessment, based on the studies discussed above. We would agree. We also see the blood eosinophil count as one part of a clinical assessment of a patient’s overall asthma control – even if the patient has mild symptoms. More study on variability is welcome.
Dr. Haber and Dr. Jamieson are with Medstar Georgetown University Hospital, Washington, D.C.
Our understanding of asthma endotypes and phenotypes has grown substantially in the last decade. Endotype-targeted therapy has become a foundation of management, and classification of patients during initial assessment is extremely important. The use of history, laboratory data, and pulmonary function testing together help to categorize our patients and help guide therapy. One lab test, that of sputum or blood eosinophils, facilitates categorization and has been evaluated for its ability to determine response to medications and predict exacerbations.
In particular, eosinophilia has been extensively studied in severe asthma and is associated with type 2 inflammation. The 2021 GINA guidelines describe type 2 inflammation as characterized by cytokines (especially IL-4, IL-5, and IL-13). “T2-high patients” tend to have elevated blood or sputum eosinophil counts and elevated fractional concentration of exhaled nitric oxide (FENO) and are more likely to respond to biologic therapy. (Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021).
However, what about patients with more mild-to-moderate asthma? Two recent studies have asked this question. In 2020, Pavord and colleagues performed a prespecified secondary subgroup analysis on an open-label randomized control trial comparing prn salbutamol alone to budesonide and as needed salbutamol to as needed budesonide-formoterol. The population was 675 adults with mild asthma receiving only as needed short acting beta-agonists (SABA) at baseline. The primary outcome was annual rate of asthma exacerbation, and whether it was different based on blood eosinophil count, FENO or a composite of both. They had several interesting findings. First, for patients only on an as needed SABA, the proportion having a severe exacerbation increased progressively with increasing blood eosinophil count. Second, inhaled corticosteroids (ICS) plus as needed SABA were more effective than SABA alone in patients with a blood eosinophil count of ≥300 cells/μL, both in terms of total exacerbations and severe exacerbations. The effects of budesonide-formoterol on exacerbations, however, was not associated with blood eosinophil count or FENO. This last point is particularly interesting in light of GINA guidelines that prioritize this combination (Pavord ID et al. Lancet Respir Med. 2020;8[7]:671-80).
More recently, a prespecified secondary analysis of the SIENA trial looked at 295 subjects with mild persistent asthma (237 adults aged 18+, and 58 adolescents aged 12-17). The primary outcome was a composite of asthma control (treatment failure, asthma control days, and FEV1). They found that sputum eosinophil levels, blood eosinophil levels, and FENO all predicted response to ICS in adults; however, the area under the receiver operative characteristic curve (AUC) was less than 0.7 for each of these findings, which was below the threshold for acceptability. A blood eosinophil count of ≥100 cells/μL offered 87% sensitivity and 17% specificity for response to ICS (Krishnan JA et al. Ann Am Thorac Soc. 2022;19[3]:372-80).
What does this tell us? Blood eosinophil count may help determine who will respond to ICS, and there remains utility in assessing blood eosinophil count in severe asthma for determining candidacy for biologic therapies. However, the overall utility of blood eosinophils in mild to moderate asthma is not as clear.
But, are we asking the right questions? Many studies look at a single blood eosinophil level, either at a single point in time, a baseline level, or a highest level over a specific time period. But do eosinophil counts vary over time?
A 2018 single-center study initially asked this question. The authors evaluated blood eosinophil levels in 219 adult patients at the NYU/Bellevue Hospital Asthma Clinic over a 5-year period. They found that individual patients had variable eosinophil levels. For example, only 6% (n=13) of patients had levels consistently above 300 cells/μL, but nearly 50% (n=104) had at least one level above 300. The degree of variability was then assessed by K-mean clustering yielding three clusters. Cluster 2 had the largest variability in blood eosinophil counts and a slightly higher absolute eosinophil level. While not significant, there was a suggestion of worse asthma control with more hospitalizations and more prescriptions for multiple controllers in this cluster with more variability. Clearly, this warranted further study (Rakowski E et al. Clin Exp Allergy. 2019;49[2]:163-70).
Variability was re-examined more recently in 2021. A post hoc analysis of two phase III clinical trials from the reslizumab BREATH program looked at eosinophil counts in the 476 patients randomized to receive placebo during the 52-week study. These patients did have eosinophilic asthma by definition and had to have an elevated eosinophil count >400 cells/μL over the 4-week enrollment period to enter the study. However, 124 patients (26.1%) had an eosinophil level <400 cells/μL immediately before the first dose of placebo. The primary outcome was variability in blood eosinophil count. Of patients who started with serum eosinophils <400, 27% to 56% of patients shifted to the ≥400 cells/μL category during the treatment period (this wide range is across three categories of low “baseline” blood eosinophil count; <150, 150 to 300, and 300 to 400). On the contrary, patients who started with eosinophils ≥400 cells/μL tended to stay at that level. The variability is reduced by taking two to three repeat measurements at baseline (Corren et al. J Allergy Clin Immunol Pract. 2021;9[3]:1224-31).
Does this variability have clinical significance? A recent retrospective cohort study looked at 10,059 stable adult patients with asthma from the MAJORICA cohort in Spain, compared with 8,557 control subjects. The primary outcome was total blood eosinophil count and an “eosinophil variability index” (EVI) where EVI=(Eosmax – Eosmin / Eosmax) x 100%. They found that an elevated EVI was associated with hospitalization, more so than maximum eosinophil count or any other eosinophil count variable, with an odds ratio of 3.18 by univariate regression (2.51 by multivariate). They also found that patients with an EVI ≥50% were twice as likely to be hospitalized or visit the ED than those with a lower EVI (Toledo-Pons N et al. Ann Am Thorac Soc. 2022;19[3]:407-14). These results are very interesting and merit further research.
So, what to do with this information? We know that patients with peripheral eosinophilia and severe asthma symptoms are candidates for biologic therapy. They are also more likely to respond to steroids, although the utility of this assessment alone in mild to moderate asthma is less clear. It does seem that more variability in eosinophils over time may be linked to more difficult-to-treat asthma.
Should you check eosinophils in your patients with asthma? GINA 2021 guidelines say to consider it, and list blood eosinophilia as a risk factor for future exacerbation, even if patients have few asthma symptoms. They also say to repeat blood eosinophils in patients with severe asthma, if the level is low at first assessment, based on the studies discussed above. We would agree. We also see the blood eosinophil count as one part of a clinical assessment of a patient’s overall asthma control – even if the patient has mild symptoms. More study on variability is welcome.
Dr. Haber and Dr. Jamieson are with Medstar Georgetown University Hospital, Washington, D.C.
Our understanding of asthma endotypes and phenotypes has grown substantially in the last decade. Endotype-targeted therapy has become a foundation of management, and classification of patients during initial assessment is extremely important. The use of history, laboratory data, and pulmonary function testing together help to categorize our patients and help guide therapy. One lab test, that of sputum or blood eosinophils, facilitates categorization and has been evaluated for its ability to determine response to medications and predict exacerbations.
In particular, eosinophilia has been extensively studied in severe asthma and is associated with type 2 inflammation. The 2021 GINA guidelines describe type 2 inflammation as characterized by cytokines (especially IL-4, IL-5, and IL-13). “T2-high patients” tend to have elevated blood or sputum eosinophil counts and elevated fractional concentration of exhaled nitric oxide (FENO) and are more likely to respond to biologic therapy. (Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021).
However, what about patients with more mild-to-moderate asthma? Two recent studies have asked this question. In 2020, Pavord and colleagues performed a prespecified secondary subgroup analysis on an open-label randomized control trial comparing prn salbutamol alone to budesonide and as needed salbutamol to as needed budesonide-formoterol. The population was 675 adults with mild asthma receiving only as needed short acting beta-agonists (SABA) at baseline. The primary outcome was annual rate of asthma exacerbation, and whether it was different based on blood eosinophil count, FENO or a composite of both. They had several interesting findings. First, for patients only on an as needed SABA, the proportion having a severe exacerbation increased progressively with increasing blood eosinophil count. Second, inhaled corticosteroids (ICS) plus as needed SABA were more effective than SABA alone in patients with a blood eosinophil count of ≥300 cells/μL, both in terms of total exacerbations and severe exacerbations. The effects of budesonide-formoterol on exacerbations, however, was not associated with blood eosinophil count or FENO. This last point is particularly interesting in light of GINA guidelines that prioritize this combination (Pavord ID et al. Lancet Respir Med. 2020;8[7]:671-80).
More recently, a prespecified secondary analysis of the SIENA trial looked at 295 subjects with mild persistent asthma (237 adults aged 18+, and 58 adolescents aged 12-17). The primary outcome was a composite of asthma control (treatment failure, asthma control days, and FEV1). They found that sputum eosinophil levels, blood eosinophil levels, and FENO all predicted response to ICS in adults; however, the area under the receiver operative characteristic curve (AUC) was less than 0.7 for each of these findings, which was below the threshold for acceptability. A blood eosinophil count of ≥100 cells/μL offered 87% sensitivity and 17% specificity for response to ICS (Krishnan JA et al. Ann Am Thorac Soc. 2022;19[3]:372-80).
What does this tell us? Blood eosinophil count may help determine who will respond to ICS, and there remains utility in assessing blood eosinophil count in severe asthma for determining candidacy for biologic therapies. However, the overall utility of blood eosinophils in mild to moderate asthma is not as clear.
But, are we asking the right questions? Many studies look at a single blood eosinophil level, either at a single point in time, a baseline level, or a highest level over a specific time period. But do eosinophil counts vary over time?
A 2018 single-center study initially asked this question. The authors evaluated blood eosinophil levels in 219 adult patients at the NYU/Bellevue Hospital Asthma Clinic over a 5-year period. They found that individual patients had variable eosinophil levels. For example, only 6% (n=13) of patients had levels consistently above 300 cells/μL, but nearly 50% (n=104) had at least one level above 300. The degree of variability was then assessed by K-mean clustering yielding three clusters. Cluster 2 had the largest variability in blood eosinophil counts and a slightly higher absolute eosinophil level. While not significant, there was a suggestion of worse asthma control with more hospitalizations and more prescriptions for multiple controllers in this cluster with more variability. Clearly, this warranted further study (Rakowski E et al. Clin Exp Allergy. 2019;49[2]:163-70).
Variability was re-examined more recently in 2021. A post hoc analysis of two phase III clinical trials from the reslizumab BREATH program looked at eosinophil counts in the 476 patients randomized to receive placebo during the 52-week study. These patients did have eosinophilic asthma by definition and had to have an elevated eosinophil count >400 cells/μL over the 4-week enrollment period to enter the study. However, 124 patients (26.1%) had an eosinophil level <400 cells/μL immediately before the first dose of placebo. The primary outcome was variability in blood eosinophil count. Of patients who started with serum eosinophils <400, 27% to 56% of patients shifted to the ≥400 cells/μL category during the treatment period (this wide range is across three categories of low “baseline” blood eosinophil count; <150, 150 to 300, and 300 to 400). On the contrary, patients who started with eosinophils ≥400 cells/μL tended to stay at that level. The variability is reduced by taking two to three repeat measurements at baseline (Corren et al. J Allergy Clin Immunol Pract. 2021;9[3]:1224-31).
Does this variability have clinical significance? A recent retrospective cohort study looked at 10,059 stable adult patients with asthma from the MAJORICA cohort in Spain, compared with 8,557 control subjects. The primary outcome was total blood eosinophil count and an “eosinophil variability index” (EVI) where EVI=(Eosmax – Eosmin / Eosmax) x 100%. They found that an elevated EVI was associated with hospitalization, more so than maximum eosinophil count or any other eosinophil count variable, with an odds ratio of 3.18 by univariate regression (2.51 by multivariate). They also found that patients with an EVI ≥50% were twice as likely to be hospitalized or visit the ED than those with a lower EVI (Toledo-Pons N et al. Ann Am Thorac Soc. 2022;19[3]:407-14). These results are very interesting and merit further research.
So, what to do with this information? We know that patients with peripheral eosinophilia and severe asthma symptoms are candidates for biologic therapy. They are also more likely to respond to steroids, although the utility of this assessment alone in mild to moderate asthma is less clear. It does seem that more variability in eosinophils over time may be linked to more difficult-to-treat asthma.
Should you check eosinophils in your patients with asthma? GINA 2021 guidelines say to consider it, and list blood eosinophilia as a risk factor for future exacerbation, even if patients have few asthma symptoms. They also say to repeat blood eosinophils in patients with severe asthma, if the level is low at first assessment, based on the studies discussed above. We would agree. We also see the blood eosinophil count as one part of a clinical assessment of a patient’s overall asthma control – even if the patient has mild symptoms. More study on variability is welcome.
Dr. Haber and Dr. Jamieson are with Medstar Georgetown University Hospital, Washington, D.C.
In memoriam
CHEST has been informed of the following deaths of CHEST members.
We remember our colleagues and extend our sincere condolences.
Edward C. Rosenow III, MD, Master FCCP
Jack Stanko, MD, MS, FCCP
Arthur S. Turetsky, MD, FCCP
CHEST has been informed of the following deaths of CHEST members.
We remember our colleagues and extend our sincere condolences.
Edward C. Rosenow III, MD, Master FCCP
Jack Stanko, MD, MS, FCCP
Arthur S. Turetsky, MD, FCCP
CHEST has been informed of the following deaths of CHEST members.
We remember our colleagues and extend our sincere condolences.
Edward C. Rosenow III, MD, Master FCCP
Jack Stanko, MD, MS, FCCP
Arthur S. Turetsky, MD, FCCP
Living and leading with lung disease
Fred Schick and Betsy Glaeser use their diagnoses to help others
Receiving a chronic disease diagnosis can be paralyzing, with a wide range of associated emotions. A patient’s family, physicians, and other health care professionals can provide a source of support, but, often, the strongest support comes from those who can empathize.
Someone who has lived with a diagnosis can provide guidance and empathy at a more personal level because, to them, it is just that – personal. Fred Schick and Betsy Glaeser have done just that by taking their personal experiences and using them to help others navigate their diagnoses.
Improving patients’ lives is the core focus of the American College of Chest Physicians and the CHEST Foundation. Events like the Belmont Stakes Dinner and Auction provide an opportunity for us to recognize and celebrate powerful stories such as Fred and Betsy’s, while also raising funds to support important initiatives that will improve patient care. Please consider joining the fight against lung disease by making a donation to the CHEST Foundation today at chestfoundation.org/donate.
Patient Advocate – Fred Schick
Increasing awareness of pulmonary fibrosis
Fred Schick of the Chicagoland area was diagnosed with idiopathic pulmonary fibrosis (IPF) in 2017 after years of searching for the root cause of his worsening symptoms.
Fred started experiencing shortness of breath and labored breathing—once to the extent that he needed to be pulled out of the water on vacation despite being an active swimmer. Because Fred was a former cardiac patient, his doctors looked to his heart for a diagnosis.
It wasn’t until his primary care physician retired that he started seeing a new doctor who took a different look at his symptoms. In hearing about the strong changes in his exercise endurance, this particular doctor made the decision to refer Fred to a pulmonologist, which ultimately led Fred on the right path to his IPF diagnosis.
Helping others navigate the path
In his 5 years since being diagnosed with IPF, Fred uses his experience to advocate for others living with this illness. Active in support groups for those with IPF, he is especially focused on helping others navigate the first few months after receiving their diagnosis.
Fred knows from experience that receiving the IPF diagnosis is something to come to terms with but encourages others to look to him for an example of how to live with the illness.
“The first thing I say to someone who has been recently diagnosed with pulmonary fibrosis is, ‘Whatever you’ve read on the Internet, don’t believe it,’ because there are a lot of people who live well beyond the 3- to 5-year expectancy you’ll see in your Google search.”
“I also encourage everyone to be their own health advocate – tell your doctor if anything in your life is abnormal because you know your body better than anyone.”
Like Fred, many living with IPF wait years for a diagnosis because of the commonality in the way the symptoms present, including shortness of breath, fatigue, difficulty breathing, and others. To address this delay, the American College of Chest Physicians, supported by the CHEST Foundation, partnered with the Three Lakes Foundation to create an initiative led by a steering committee of pulmonologists and primary care physicians to join together to shorten the time to diagnosis for interstitial lung diseases like IPF. Among other activities, the steering committee will work to create tools for physicians to use during patient intake that can more quickly bring IPF into the conversation when it is pertinent.
Patient Advocate – Betsy Glaeser
Blazing the trail for NTM
Local to New York, Betsy Glaeser was diagnosed with pulmonary nontuberculous mycobacteria disease (NTM) more than 20 years ago.
Leading up to her diagnosis, Betsy was frequently short of breath with overwhelming fatigue and fevers. She was hospitalized multiple times for pneumonia and treated again and again with short-term standard antibiotics. At the time (1998), there were no clinical programs dedicated to NTM, and when her sputum was tested, it was only for pneumonia.
As a financial consultant required to travel 4 days per week for work, Betsy grew especially concerned about her illness when she developed hemoptysis and began coughing up blood. Lacking local resources, she sought care at the Mayo Clinic in Rochester, Minnesota, where she received her NTM diagnosis.
Based on the severity of her illness and her worsening symptoms, the recommendation of the Mayo Clinic was that she stop working. After 30 years of challenging jobs, quitting was very painful, but a Mayo doctor asked Betsy a very poignant question that resonated with her: “Are you planning to die for your employer?”
With that, she left her job and sought care for her illness. As her NTM developed a second, more resistant strain associated with her disease, requiring daily, constant treatment, Betsy was fortunate to be accepted into the National Institutes of Health NTM protocol, which has directed her care, coordinated with NYU-Langone.
Despite the challenges of having NTM, Betsy maintains an active and enriching life.
Leading with experience
Betsy uses her diagnosis and her experience with NTM to help others who are hearing their diagnoses for the first time. She serves as a charter member and co-leader of a New York NTM patient support group and serves as a member of the NTM Info & Research (NTMir) Board of Directors.
Her goal is to ensure that no one living with NTM feels alone or frightened.
“Not so long ago – and now, too, even – there were doctors who did not know how to treat NTM,” says Betsy. “But, it has really gotten better – as I’ve progressed through all of my medications and lived with this disease, NTM has progressed as well. I hope I helped expand NTM knowledge with my lived experiences, but I’ve been so fortunate to receive medical care from those doctors who knew the most about NTM.”
Fred Schick and Betsy Glaeser use their diagnoses to help others
Fred Schick and Betsy Glaeser use their diagnoses to help others
Receiving a chronic disease diagnosis can be paralyzing, with a wide range of associated emotions. A patient’s family, physicians, and other health care professionals can provide a source of support, but, often, the strongest support comes from those who can empathize.
Someone who has lived with a diagnosis can provide guidance and empathy at a more personal level because, to them, it is just that – personal. Fred Schick and Betsy Glaeser have done just that by taking their personal experiences and using them to help others navigate their diagnoses.
Improving patients’ lives is the core focus of the American College of Chest Physicians and the CHEST Foundation. Events like the Belmont Stakes Dinner and Auction provide an opportunity for us to recognize and celebrate powerful stories such as Fred and Betsy’s, while also raising funds to support important initiatives that will improve patient care. Please consider joining the fight against lung disease by making a donation to the CHEST Foundation today at chestfoundation.org/donate.
Patient Advocate – Fred Schick
Increasing awareness of pulmonary fibrosis
Fred Schick of the Chicagoland area was diagnosed with idiopathic pulmonary fibrosis (IPF) in 2017 after years of searching for the root cause of his worsening symptoms.
Fred started experiencing shortness of breath and labored breathing—once to the extent that he needed to be pulled out of the water on vacation despite being an active swimmer. Because Fred was a former cardiac patient, his doctors looked to his heart for a diagnosis.
It wasn’t until his primary care physician retired that he started seeing a new doctor who took a different look at his symptoms. In hearing about the strong changes in his exercise endurance, this particular doctor made the decision to refer Fred to a pulmonologist, which ultimately led Fred on the right path to his IPF diagnosis.
Helping others navigate the path
In his 5 years since being diagnosed with IPF, Fred uses his experience to advocate for others living with this illness. Active in support groups for those with IPF, he is especially focused on helping others navigate the first few months after receiving their diagnosis.
Fred knows from experience that receiving the IPF diagnosis is something to come to terms with but encourages others to look to him for an example of how to live with the illness.
“The first thing I say to someone who has been recently diagnosed with pulmonary fibrosis is, ‘Whatever you’ve read on the Internet, don’t believe it,’ because there are a lot of people who live well beyond the 3- to 5-year expectancy you’ll see in your Google search.”
“I also encourage everyone to be their own health advocate – tell your doctor if anything in your life is abnormal because you know your body better than anyone.”
Like Fred, many living with IPF wait years for a diagnosis because of the commonality in the way the symptoms present, including shortness of breath, fatigue, difficulty breathing, and others. To address this delay, the American College of Chest Physicians, supported by the CHEST Foundation, partnered with the Three Lakes Foundation to create an initiative led by a steering committee of pulmonologists and primary care physicians to join together to shorten the time to diagnosis for interstitial lung diseases like IPF. Among other activities, the steering committee will work to create tools for physicians to use during patient intake that can more quickly bring IPF into the conversation when it is pertinent.
Patient Advocate – Betsy Glaeser
Blazing the trail for NTM
Local to New York, Betsy Glaeser was diagnosed with pulmonary nontuberculous mycobacteria disease (NTM) more than 20 years ago.
Leading up to her diagnosis, Betsy was frequently short of breath with overwhelming fatigue and fevers. She was hospitalized multiple times for pneumonia and treated again and again with short-term standard antibiotics. At the time (1998), there were no clinical programs dedicated to NTM, and when her sputum was tested, it was only for pneumonia.
As a financial consultant required to travel 4 days per week for work, Betsy grew especially concerned about her illness when she developed hemoptysis and began coughing up blood. Lacking local resources, she sought care at the Mayo Clinic in Rochester, Minnesota, where she received her NTM diagnosis.
Based on the severity of her illness and her worsening symptoms, the recommendation of the Mayo Clinic was that she stop working. After 30 years of challenging jobs, quitting was very painful, but a Mayo doctor asked Betsy a very poignant question that resonated with her: “Are you planning to die for your employer?”
With that, she left her job and sought care for her illness. As her NTM developed a second, more resistant strain associated with her disease, requiring daily, constant treatment, Betsy was fortunate to be accepted into the National Institutes of Health NTM protocol, which has directed her care, coordinated with NYU-Langone.
Despite the challenges of having NTM, Betsy maintains an active and enriching life.
Leading with experience
Betsy uses her diagnosis and her experience with NTM to help others who are hearing their diagnoses for the first time. She serves as a charter member and co-leader of a New York NTM patient support group and serves as a member of the NTM Info & Research (NTMir) Board of Directors.
Her goal is to ensure that no one living with NTM feels alone or frightened.
“Not so long ago – and now, too, even – there were doctors who did not know how to treat NTM,” says Betsy. “But, it has really gotten better – as I’ve progressed through all of my medications and lived with this disease, NTM has progressed as well. I hope I helped expand NTM knowledge with my lived experiences, but I’ve been so fortunate to receive medical care from those doctors who knew the most about NTM.”
Receiving a chronic disease diagnosis can be paralyzing, with a wide range of associated emotions. A patient’s family, physicians, and other health care professionals can provide a source of support, but, often, the strongest support comes from those who can empathize.
Someone who has lived with a diagnosis can provide guidance and empathy at a more personal level because, to them, it is just that – personal. Fred Schick and Betsy Glaeser have done just that by taking their personal experiences and using them to help others navigate their diagnoses.
Improving patients’ lives is the core focus of the American College of Chest Physicians and the CHEST Foundation. Events like the Belmont Stakes Dinner and Auction provide an opportunity for us to recognize and celebrate powerful stories such as Fred and Betsy’s, while also raising funds to support important initiatives that will improve patient care. Please consider joining the fight against lung disease by making a donation to the CHEST Foundation today at chestfoundation.org/donate.
Patient Advocate – Fred Schick
Increasing awareness of pulmonary fibrosis
Fred Schick of the Chicagoland area was diagnosed with idiopathic pulmonary fibrosis (IPF) in 2017 after years of searching for the root cause of his worsening symptoms.
Fred started experiencing shortness of breath and labored breathing—once to the extent that he needed to be pulled out of the water on vacation despite being an active swimmer. Because Fred was a former cardiac patient, his doctors looked to his heart for a diagnosis.
It wasn’t until his primary care physician retired that he started seeing a new doctor who took a different look at his symptoms. In hearing about the strong changes in his exercise endurance, this particular doctor made the decision to refer Fred to a pulmonologist, which ultimately led Fred on the right path to his IPF diagnosis.
Helping others navigate the path
In his 5 years since being diagnosed with IPF, Fred uses his experience to advocate for others living with this illness. Active in support groups for those with IPF, he is especially focused on helping others navigate the first few months after receiving their diagnosis.
Fred knows from experience that receiving the IPF diagnosis is something to come to terms with but encourages others to look to him for an example of how to live with the illness.
“The first thing I say to someone who has been recently diagnosed with pulmonary fibrosis is, ‘Whatever you’ve read on the Internet, don’t believe it,’ because there are a lot of people who live well beyond the 3- to 5-year expectancy you’ll see in your Google search.”
“I also encourage everyone to be their own health advocate – tell your doctor if anything in your life is abnormal because you know your body better than anyone.”
Like Fred, many living with IPF wait years for a diagnosis because of the commonality in the way the symptoms present, including shortness of breath, fatigue, difficulty breathing, and others. To address this delay, the American College of Chest Physicians, supported by the CHEST Foundation, partnered with the Three Lakes Foundation to create an initiative led by a steering committee of pulmonologists and primary care physicians to join together to shorten the time to diagnosis for interstitial lung diseases like IPF. Among other activities, the steering committee will work to create tools for physicians to use during patient intake that can more quickly bring IPF into the conversation when it is pertinent.
Patient Advocate – Betsy Glaeser
Blazing the trail for NTM
Local to New York, Betsy Glaeser was diagnosed with pulmonary nontuberculous mycobacteria disease (NTM) more than 20 years ago.
Leading up to her diagnosis, Betsy was frequently short of breath with overwhelming fatigue and fevers. She was hospitalized multiple times for pneumonia and treated again and again with short-term standard antibiotics. At the time (1998), there were no clinical programs dedicated to NTM, and when her sputum was tested, it was only for pneumonia.
As a financial consultant required to travel 4 days per week for work, Betsy grew especially concerned about her illness when she developed hemoptysis and began coughing up blood. Lacking local resources, she sought care at the Mayo Clinic in Rochester, Minnesota, where she received her NTM diagnosis.
Based on the severity of her illness and her worsening symptoms, the recommendation of the Mayo Clinic was that she stop working. After 30 years of challenging jobs, quitting was very painful, but a Mayo doctor asked Betsy a very poignant question that resonated with her: “Are you planning to die for your employer?”
With that, she left her job and sought care for her illness. As her NTM developed a second, more resistant strain associated with her disease, requiring daily, constant treatment, Betsy was fortunate to be accepted into the National Institutes of Health NTM protocol, which has directed her care, coordinated with NYU-Langone.
Despite the challenges of having NTM, Betsy maintains an active and enriching life.
Leading with experience
Betsy uses her diagnosis and her experience with NTM to help others who are hearing their diagnoses for the first time. She serves as a charter member and co-leader of a New York NTM patient support group and serves as a member of the NTM Info & Research (NTMir) Board of Directors.
Her goal is to ensure that no one living with NTM feels alone or frightened.
“Not so long ago – and now, too, even – there were doctors who did not know how to treat NTM,” says Betsy. “But, it has really gotten better – as I’ve progressed through all of my medications and lived with this disease, NTM has progressed as well. I hope I helped expand NTM knowledge with my lived experiences, but I’ve been so fortunate to receive medical care from those doctors who knew the most about NTM.”
Bridging Specialties™: Timely diagnosis for patients with ILD
Experts in pulmonary and primary care medicine come together to reduce delays in diagnosing complex lung diseases.
Affecting around 400,000 people in the United States, interstitial lung diseases (ILD), like pulmonary fibrosis (PF), present with symptoms that are similar to other more common lung diseases, frequently resulting in misdiagnosis or delayed diagnosis. Some studies show that reaching a proper diagnosis for rarer lung diseases can take upwards of several years.
Despite scientific advancements and increased information available, timely and accurate diagnosis for PF remains a challenge. The course of the disease varies from person to person and can progress rapidly in some cases, increasing the necessity to have the condition diagnosed in its earliest stages. By the time patients learn they have PF, the condition may require reliance on oxygen use and hospitalizations, and it can lead to poor quality of life and a significantly shortened lifespan.
To address this issue, Three Lakes Foundation (TLF) and the American College of Chest Physicians (CHEST) recently announced their collaboration on a multiphase educational initiative led by a steering committee of medical experts aiming to reduce the time it takes to diagnose patients with ILDs like PF. Composed of pulmonologists, primary care physicians, and a nursing professional, the steering committee will work to create materials that will aid in identifying and diagnosing complex lung diseases quicker.
“As a catalyst for change in the PF community, Three Lakes Foundation spoke with patients, health care professionals, physicians, and advocacy groups to advance an understanding of the PF diagnostic experience,” said Dana Ball, executive director for Three Lakes Foundation. “We approached CHEST when it became apparent that primary care physicians could use specific tools to identify high-risk patients with pulmonary conditions. This collaboration is the result of our common need to increase awareness among health care professionals and to improve patient outcomes.”
Members of the expert steering committee include individuals from leading medical institutions, health systems, and organizations across the country:
- Daniel F. Dilling, MD, FCCP, Professor of Medicine, Division of Pulmonary and Critical Care, Loyola University Chicago, Stritch School of Medicine, Maywood, IL.
- Andrew Duggan, MPH, Patient Engagement and Innovation Leader representing Three Lakes Foundation, Boston, MA.
- Jessica Glennie, APRN, MSN, Nurse Practitioner, Interstitial Lung Disease Clinic, Cleveland Clinic, Cleveland, OH.
- Timothy Hernandez, MD, Family Medicine Physician, Chief Executive Officer of Entira Family Clinics, San Antonio, TX.
- Corey D. Kershaw, MD, FCCP, Associate Professor of Medicine, Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, Dallas, TX.
- Tejaswini Kulkarni, MD, MPH, FCCP, Assistant Professor, Director, Interstitial Lung Disease Program, Division of Pulmonary, Allergy and Critical Care Medicine, The University of Alabama at Birmingham, Birmingham, AL.
- William Lago, MD, Family Medicine Physician, Wooster Family Health Center, Cleveland Clinic Foundation, Wooster, OH.
- Andrew H. Limper, MD, FCCP, Annenberg Professor of Pulmonary Medicine, Professor of Biochemistry and Molecular Biology, Director – Thoracic Disease Research Unit, Mayo Clinic College of Medicine, Rochester, MN.
- Anoop M. Nambiar, MD, MS, FCCP, Professor of Medicine, Founding Director of the UT Health San Antonio Center for Interstitial Lung Diseases, Division of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Texas Health Science Center at San Antonio and South Texas Veterans Health Care System, San Antonio, TX.
- Mary Beth Scholand, MD, Associate Professor of Internal Medicine, Division of Pulmonary Diseases, Director, Interstitial Lung Program, University of Utah, Salt Lake City, UT
“While interstitial lung diseases do not affect a substantial amount of the population, those touched by the disease are impacted tremendously,” says steering committee member and pulmonologist, Dr. Andrew H. Limper. “Any delay in receiving a diagnosis is time that could be dedicated to finding a treatment therapy that can improve their quality of life. I look forward to the work of this committee helping to shape how patients with ILDs are diagnosed and treated in the future.”
Starting with data-gathering surveys sent to both primary care physicians and pulmonologists, the committee will evaluate the findings to develop tools that can be used to aid in diagnosing complex lung diseases.
“Having experts from both pulmonary and primary care medicine as members of the steering committee is critical,” says steering committee member and family medicine physician, Dr. William Lago. “Patients first see their family medicine or primary care clinicians and, all too often, the most complex lung diseases present in ways that are indistinguishable from more common conditions like asthma and COPD. Bringing together experts in both fields will yield the best results in creating a path to diagnosis.”
Three Lakes Foundation is providing the initial funding for CHEST to begin designing an educational intervention that addresses the gaps in knowledge and practice and will play an active role in overseeing the development of the program.
For more information on the Bridging Specialties™: Timely Diagnosis for Patients With ILD initiative and to sign up for updates, visit info.chestnet.org/bridging-specialties-timely-diagnosis-for-ild-patients.
Experts in pulmonary and primary care medicine come together to reduce delays in diagnosing complex lung diseases.
Experts in pulmonary and primary care medicine come together to reduce delays in diagnosing complex lung diseases.
Affecting around 400,000 people in the United States, interstitial lung diseases (ILD), like pulmonary fibrosis (PF), present with symptoms that are similar to other more common lung diseases, frequently resulting in misdiagnosis or delayed diagnosis. Some studies show that reaching a proper diagnosis for rarer lung diseases can take upwards of several years.
Despite scientific advancements and increased information available, timely and accurate diagnosis for PF remains a challenge. The course of the disease varies from person to person and can progress rapidly in some cases, increasing the necessity to have the condition diagnosed in its earliest stages. By the time patients learn they have PF, the condition may require reliance on oxygen use and hospitalizations, and it can lead to poor quality of life and a significantly shortened lifespan.
To address this issue, Three Lakes Foundation (TLF) and the American College of Chest Physicians (CHEST) recently announced their collaboration on a multiphase educational initiative led by a steering committee of medical experts aiming to reduce the time it takes to diagnose patients with ILDs like PF. Composed of pulmonologists, primary care physicians, and a nursing professional, the steering committee will work to create materials that will aid in identifying and diagnosing complex lung diseases quicker.
“As a catalyst for change in the PF community, Three Lakes Foundation spoke with patients, health care professionals, physicians, and advocacy groups to advance an understanding of the PF diagnostic experience,” said Dana Ball, executive director for Three Lakes Foundation. “We approached CHEST when it became apparent that primary care physicians could use specific tools to identify high-risk patients with pulmonary conditions. This collaboration is the result of our common need to increase awareness among health care professionals and to improve patient outcomes.”
Members of the expert steering committee include individuals from leading medical institutions, health systems, and organizations across the country:
- Daniel F. Dilling, MD, FCCP, Professor of Medicine, Division of Pulmonary and Critical Care, Loyola University Chicago, Stritch School of Medicine, Maywood, IL.
- Andrew Duggan, MPH, Patient Engagement and Innovation Leader representing Three Lakes Foundation, Boston, MA.
- Jessica Glennie, APRN, MSN, Nurse Practitioner, Interstitial Lung Disease Clinic, Cleveland Clinic, Cleveland, OH.
- Timothy Hernandez, MD, Family Medicine Physician, Chief Executive Officer of Entira Family Clinics, San Antonio, TX.
- Corey D. Kershaw, MD, FCCP, Associate Professor of Medicine, Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, Dallas, TX.
- Tejaswini Kulkarni, MD, MPH, FCCP, Assistant Professor, Director, Interstitial Lung Disease Program, Division of Pulmonary, Allergy and Critical Care Medicine, The University of Alabama at Birmingham, Birmingham, AL.
- William Lago, MD, Family Medicine Physician, Wooster Family Health Center, Cleveland Clinic Foundation, Wooster, OH.
- Andrew H. Limper, MD, FCCP, Annenberg Professor of Pulmonary Medicine, Professor of Biochemistry and Molecular Biology, Director – Thoracic Disease Research Unit, Mayo Clinic College of Medicine, Rochester, MN.
- Anoop M. Nambiar, MD, MS, FCCP, Professor of Medicine, Founding Director of the UT Health San Antonio Center for Interstitial Lung Diseases, Division of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Texas Health Science Center at San Antonio and South Texas Veterans Health Care System, San Antonio, TX.
- Mary Beth Scholand, MD, Associate Professor of Internal Medicine, Division of Pulmonary Diseases, Director, Interstitial Lung Program, University of Utah, Salt Lake City, UT
“While interstitial lung diseases do not affect a substantial amount of the population, those touched by the disease are impacted tremendously,” says steering committee member and pulmonologist, Dr. Andrew H. Limper. “Any delay in receiving a diagnosis is time that could be dedicated to finding a treatment therapy that can improve their quality of life. I look forward to the work of this committee helping to shape how patients with ILDs are diagnosed and treated in the future.”
Starting with data-gathering surveys sent to both primary care physicians and pulmonologists, the committee will evaluate the findings to develop tools that can be used to aid in diagnosing complex lung diseases.
“Having experts from both pulmonary and primary care medicine as members of the steering committee is critical,” says steering committee member and family medicine physician, Dr. William Lago. “Patients first see their family medicine or primary care clinicians and, all too often, the most complex lung diseases present in ways that are indistinguishable from more common conditions like asthma and COPD. Bringing together experts in both fields will yield the best results in creating a path to diagnosis.”
Three Lakes Foundation is providing the initial funding for CHEST to begin designing an educational intervention that addresses the gaps in knowledge and practice and will play an active role in overseeing the development of the program.
For more information on the Bridging Specialties™: Timely Diagnosis for Patients With ILD initiative and to sign up for updates, visit info.chestnet.org/bridging-specialties-timely-diagnosis-for-ild-patients.
Affecting around 400,000 people in the United States, interstitial lung diseases (ILD), like pulmonary fibrosis (PF), present with symptoms that are similar to other more common lung diseases, frequently resulting in misdiagnosis or delayed diagnosis. Some studies show that reaching a proper diagnosis for rarer lung diseases can take upwards of several years.
Despite scientific advancements and increased information available, timely and accurate diagnosis for PF remains a challenge. The course of the disease varies from person to person and can progress rapidly in some cases, increasing the necessity to have the condition diagnosed in its earliest stages. By the time patients learn they have PF, the condition may require reliance on oxygen use and hospitalizations, and it can lead to poor quality of life and a significantly shortened lifespan.
To address this issue, Three Lakes Foundation (TLF) and the American College of Chest Physicians (CHEST) recently announced their collaboration on a multiphase educational initiative led by a steering committee of medical experts aiming to reduce the time it takes to diagnose patients with ILDs like PF. Composed of pulmonologists, primary care physicians, and a nursing professional, the steering committee will work to create materials that will aid in identifying and diagnosing complex lung diseases quicker.
“As a catalyst for change in the PF community, Three Lakes Foundation spoke with patients, health care professionals, physicians, and advocacy groups to advance an understanding of the PF diagnostic experience,” said Dana Ball, executive director for Three Lakes Foundation. “We approached CHEST when it became apparent that primary care physicians could use specific tools to identify high-risk patients with pulmonary conditions. This collaboration is the result of our common need to increase awareness among health care professionals and to improve patient outcomes.”
Members of the expert steering committee include individuals from leading medical institutions, health systems, and organizations across the country:
- Daniel F. Dilling, MD, FCCP, Professor of Medicine, Division of Pulmonary and Critical Care, Loyola University Chicago, Stritch School of Medicine, Maywood, IL.
- Andrew Duggan, MPH, Patient Engagement and Innovation Leader representing Three Lakes Foundation, Boston, MA.
- Jessica Glennie, APRN, MSN, Nurse Practitioner, Interstitial Lung Disease Clinic, Cleveland Clinic, Cleveland, OH.
- Timothy Hernandez, MD, Family Medicine Physician, Chief Executive Officer of Entira Family Clinics, San Antonio, TX.
- Corey D. Kershaw, MD, FCCP, Associate Professor of Medicine, Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, Dallas, TX.
- Tejaswini Kulkarni, MD, MPH, FCCP, Assistant Professor, Director, Interstitial Lung Disease Program, Division of Pulmonary, Allergy and Critical Care Medicine, The University of Alabama at Birmingham, Birmingham, AL.
- William Lago, MD, Family Medicine Physician, Wooster Family Health Center, Cleveland Clinic Foundation, Wooster, OH.
- Andrew H. Limper, MD, FCCP, Annenberg Professor of Pulmonary Medicine, Professor of Biochemistry and Molecular Biology, Director – Thoracic Disease Research Unit, Mayo Clinic College of Medicine, Rochester, MN.
- Anoop M. Nambiar, MD, MS, FCCP, Professor of Medicine, Founding Director of the UT Health San Antonio Center for Interstitial Lung Diseases, Division of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Texas Health Science Center at San Antonio and South Texas Veterans Health Care System, San Antonio, TX.
- Mary Beth Scholand, MD, Associate Professor of Internal Medicine, Division of Pulmonary Diseases, Director, Interstitial Lung Program, University of Utah, Salt Lake City, UT
“While interstitial lung diseases do not affect a substantial amount of the population, those touched by the disease are impacted tremendously,” says steering committee member and pulmonologist, Dr. Andrew H. Limper. “Any delay in receiving a diagnosis is time that could be dedicated to finding a treatment therapy that can improve their quality of life. I look forward to the work of this committee helping to shape how patients with ILDs are diagnosed and treated in the future.”
Starting with data-gathering surveys sent to both primary care physicians and pulmonologists, the committee will evaluate the findings to develop tools that can be used to aid in diagnosing complex lung diseases.
“Having experts from both pulmonary and primary care medicine as members of the steering committee is critical,” says steering committee member and family medicine physician, Dr. William Lago. “Patients first see their family medicine or primary care clinicians and, all too often, the most complex lung diseases present in ways that are indistinguishable from more common conditions like asthma and COPD. Bringing together experts in both fields will yield the best results in creating a path to diagnosis.”
Three Lakes Foundation is providing the initial funding for CHEST to begin designing an educational intervention that addresses the gaps in knowledge and practice and will play an active role in overseeing the development of the program.
For more information on the Bridging Specialties™: Timely Diagnosis for Patients With ILD initiative and to sign up for updates, visit info.chestnet.org/bridging-specialties-timely-diagnosis-for-ild-patients.
Bronchiectasis, microplastics, and end-of-life
Airways disorders network, bronchiectasis section
Phenotyping bronchiectasis: Focus on eosinophilic bronchiectasis
Bronchiectasis has been often linked to neutrophilic inflammation; however, 20% may have a predominantly eosinophilic inflammation.
Eosinophilic bronchiectasis has been associated with a distinct airway microbiome. Shoemark and colleagues showed in an analysis of 1,007 patients from five countries that 22.6% of patients had blood eosinophil counts (BEC) of >300 cells/μL. BEC of <100 cells/μL were associated with higher bronchiectasis severity and increased mortality (Shoemark et al. Am J Respir Crit Care Med. 2022;205[8]:894-902).
BEC of >300 cells/μL were correlated with Streptococcus- and Pseudomonas-dominated microbiome profiles. Compared with patients with BEC of <100 cells/μL, patients with 100-299 cells/μL (hazard ratio [HR], 2.38; 95% confidence interval, 1.33–4.25; P = .003) and those with >300 cells/μL (HR, 3.99; 95% confidence interval, 2.20–7.85; P = .0001) were associated with shorter time to exacerbation.
Eosinophilic inflammation is a risk factor for exacerbations in patients with P. aeruginosa infection and may be considered as a treatable trait. Shoemark and colleagues’ data show that quality of life was improved with inhaled corticosteroid treatment in patients with bronchiectasis who had blood eosinophil counts of >3%, and eosinophils contribute to bronchiectasis exacerbations.
Dharani Narendra, MD
Navitha Ramesh, MD, FCCP
Diego Maselli Caceres, MD, FCCP
Section Members-at-Large
Diffuse lung disease and lung transplant network, occupational and environmental health section
A ubiquitous invasion: The rise of microplastics
About 6.3 billion tons of plastic waste were produced between 1950 and 2015.1 Their degradation into submillimeter fragments of 1 μm to 5 mm, is called microplastics (MP).2 MP are vectors of pollutants, pathologic microorganisms, and chemical additives used in their fabrication.3 Exposure to MP is unavoidable as they are bio-persistent and ubiquitous, even indoors.4 MP have been detected in the snow of large metropolitan areas and in remote locations.5 Humans are exposed to MP via oral ingestion and inhalation. A Brazilian study of human lung autopsy specimens revealed the presence of MP in 13 of 20 subjects.3
In vitro studies have suggested a causal role of polystyrene-MP in the development of chronic pulmonary disease through the formation of reactive oxygen species, inhibition of cell proliferation, and cellular morphology aberration.6 MP can cause local effects due to macrophage-induced inflammation, or alternatively, be transported distantly to the pleura and the systemic circulation.
In addition, MP may disrupt the endocrine pathway due to its estrogenic effects.7 Larger MPs of 8 to 10 µm, like nylon, have been associated with interstitial lung disease.8 Lung biopsies from workers exposed to airborne synthetic fibers (acrylic, polyester, and terylene) have revealed different degrees of inflammation, granulomas, and interstitial fibrosis.9 Factory workers exposed to polyvinyl chloride dust have increased risk of exertional dyspnea and decreased pulmonary function.10 Due to the pervasive nature of MP, it is essential to establish the global burden of airborne MP and to determine its role in lung health.
Bathmapriya Balakrishnan, MD
Member-at-Large
*Tyler Church, DO
Fellow-in-Training Member
*Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
References
1. Rhodes CJ. Plastic pollution and potential solutions. Sci Prog. 2018;101(3):207-60.
2. Danopoulos E et al. Microplastic contamination of drinking water: A systematic review. PLoS One. 2020;15(7):e0236838.
3. Amato-Lourenço LF et al. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126.
4. Al Horr Y et al. Occupant productivity and office indoor environment quality: A review of the literature. Building and Environment. 2016;105:369-89.
5. Bergmann M et al. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci Adv. 2019;5:eaax1157.
6. Dong CD et al. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575.
7. Amato-Lourenço LF et al. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health. Sci Total Environ. 2020;749:141676.
8. Kern DG et al. Flock worker’s lung: Chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129[4]:261-72. Erratum in: Ann Intern Med. 1999;130[3]:246.
9. Pimentel JC et al. Respiratory disease caused by synthetic fibers: a new occupational disease. Thorax. 1975;30:204-19.
10. Soutar CA et al. Epidemiological study of respiratory disease in workers exposed to polyvinyl chloride dust. Thorax. 1980;35:644-52.
Critical care network, palliative and end-of-life section
Discussing code status with families of critically ill patients
Discussing code status with patients is complex and emotional, especially when critically ill.
The complexity further increases when these conversations have to take place with family members.
Here are some practical tips to help have these conversations in a concise and compassionate manner.
Introduction
- Introduce yourself, and make sure to identify the correct decision-maker.
- Get to know the patient.
–What kind of person are they?
–What brings them joy?
- Find out what the family knows about the current clinical condition of their family member.
–What have you been hearing from the medical team?
–What are you worried about?
Update
- Fill in the gaps – update them on the clinical condition and ongoing management.
- Discuss how you think they will respond to current management and further management options.
- Allow them to process the information.
Provide a medical recommendation
- Example: We are worried he might die, and if his heart stops, interventions like CPR or intubation would not work, and we would not recommend them.
- Do not pressure for a decision right away. (You can say “We do not need a decision today, so please take time to process this information.”)
Respond to emotions
- I can’t image how hard this must be.
- Offer chaplain services if that is important to them.
Things to avoid
- Avoid aggressive language.
–We will have to pound on their chest, break ribs.
–They would be suffering.
- Blaming or judgmental language.
While this complex discussion r equires individualization, these tips will help set a framework for goals of care conversations that lead to high quality care for patients that aligns with their goals.
Reference
Goldfish and Rosielle. Language for Routine Code Status Discussions, Fast Facts and Concepts #365, Palliative Care Network of Wisconsin.
Syed Nazeer Mahmood, MD
Fellow-in-Training Member
Anne Kelemen, LCSW
Member-at-Large
Airways disorders network, bronchiectasis section
Phenotyping bronchiectasis: Focus on eosinophilic bronchiectasis
Bronchiectasis has been often linked to neutrophilic inflammation; however, 20% may have a predominantly eosinophilic inflammation.
Eosinophilic bronchiectasis has been associated with a distinct airway microbiome. Shoemark and colleagues showed in an analysis of 1,007 patients from five countries that 22.6% of patients had blood eosinophil counts (BEC) of >300 cells/μL. BEC of <100 cells/μL were associated with higher bronchiectasis severity and increased mortality (Shoemark et al. Am J Respir Crit Care Med. 2022;205[8]:894-902).
BEC of >300 cells/μL were correlated with Streptococcus- and Pseudomonas-dominated microbiome profiles. Compared with patients with BEC of <100 cells/μL, patients with 100-299 cells/μL (hazard ratio [HR], 2.38; 95% confidence interval, 1.33–4.25; P = .003) and those with >300 cells/μL (HR, 3.99; 95% confidence interval, 2.20–7.85; P = .0001) were associated with shorter time to exacerbation.
Eosinophilic inflammation is a risk factor for exacerbations in patients with P. aeruginosa infection and may be considered as a treatable trait. Shoemark and colleagues’ data show that quality of life was improved with inhaled corticosteroid treatment in patients with bronchiectasis who had blood eosinophil counts of >3%, and eosinophils contribute to bronchiectasis exacerbations.
Dharani Narendra, MD
Navitha Ramesh, MD, FCCP
Diego Maselli Caceres, MD, FCCP
Section Members-at-Large
Diffuse lung disease and lung transplant network, occupational and environmental health section
A ubiquitous invasion: The rise of microplastics
About 6.3 billion tons of plastic waste were produced between 1950 and 2015.1 Their degradation into submillimeter fragments of 1 μm to 5 mm, is called microplastics (MP).2 MP are vectors of pollutants, pathologic microorganisms, and chemical additives used in their fabrication.3 Exposure to MP is unavoidable as they are bio-persistent and ubiquitous, even indoors.4 MP have been detected in the snow of large metropolitan areas and in remote locations.5 Humans are exposed to MP via oral ingestion and inhalation. A Brazilian study of human lung autopsy specimens revealed the presence of MP in 13 of 20 subjects.3
In vitro studies have suggested a causal role of polystyrene-MP in the development of chronic pulmonary disease through the formation of reactive oxygen species, inhibition of cell proliferation, and cellular morphology aberration.6 MP can cause local effects due to macrophage-induced inflammation, or alternatively, be transported distantly to the pleura and the systemic circulation.
In addition, MP may disrupt the endocrine pathway due to its estrogenic effects.7 Larger MPs of 8 to 10 µm, like nylon, have been associated with interstitial lung disease.8 Lung biopsies from workers exposed to airborne synthetic fibers (acrylic, polyester, and terylene) have revealed different degrees of inflammation, granulomas, and interstitial fibrosis.9 Factory workers exposed to polyvinyl chloride dust have increased risk of exertional dyspnea and decreased pulmonary function.10 Due to the pervasive nature of MP, it is essential to establish the global burden of airborne MP and to determine its role in lung health.
Bathmapriya Balakrishnan, MD
Member-at-Large
*Tyler Church, DO
Fellow-in-Training Member
*Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
References
1. Rhodes CJ. Plastic pollution and potential solutions. Sci Prog. 2018;101(3):207-60.
2. Danopoulos E et al. Microplastic contamination of drinking water: A systematic review. PLoS One. 2020;15(7):e0236838.
3. Amato-Lourenço LF et al. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126.
4. Al Horr Y et al. Occupant productivity and office indoor environment quality: A review of the literature. Building and Environment. 2016;105:369-89.
5. Bergmann M et al. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci Adv. 2019;5:eaax1157.
6. Dong CD et al. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575.
7. Amato-Lourenço LF et al. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health. Sci Total Environ. 2020;749:141676.
8. Kern DG et al. Flock worker’s lung: Chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129[4]:261-72. Erratum in: Ann Intern Med. 1999;130[3]:246.
9. Pimentel JC et al. Respiratory disease caused by synthetic fibers: a new occupational disease. Thorax. 1975;30:204-19.
10. Soutar CA et al. Epidemiological study of respiratory disease in workers exposed to polyvinyl chloride dust. Thorax. 1980;35:644-52.
Critical care network, palliative and end-of-life section
Discussing code status with families of critically ill patients
Discussing code status with patients is complex and emotional, especially when critically ill.
The complexity further increases when these conversations have to take place with family members.
Here are some practical tips to help have these conversations in a concise and compassionate manner.
Introduction
- Introduce yourself, and make sure to identify the correct decision-maker.
- Get to know the patient.
–What kind of person are they?
–What brings them joy?
- Find out what the family knows about the current clinical condition of their family member.
–What have you been hearing from the medical team?
–What are you worried about?
Update
- Fill in the gaps – update them on the clinical condition and ongoing management.
- Discuss how you think they will respond to current management and further management options.
- Allow them to process the information.
Provide a medical recommendation
- Example: We are worried he might die, and if his heart stops, interventions like CPR or intubation would not work, and we would not recommend them.
- Do not pressure for a decision right away. (You can say “We do not need a decision today, so please take time to process this information.”)
Respond to emotions
- I can’t image how hard this must be.
- Offer chaplain services if that is important to them.
Things to avoid
- Avoid aggressive language.
–We will have to pound on their chest, break ribs.
–They would be suffering.
- Blaming or judgmental language.
While this complex discussion r equires individualization, these tips will help set a framework for goals of care conversations that lead to high quality care for patients that aligns with their goals.
Reference
Goldfish and Rosielle. Language for Routine Code Status Discussions, Fast Facts and Concepts #365, Palliative Care Network of Wisconsin.
Syed Nazeer Mahmood, MD
Fellow-in-Training Member
Anne Kelemen, LCSW
Member-at-Large
Airways disorders network, bronchiectasis section
Phenotyping bronchiectasis: Focus on eosinophilic bronchiectasis
Bronchiectasis has been often linked to neutrophilic inflammation; however, 20% may have a predominantly eosinophilic inflammation.
Eosinophilic bronchiectasis has been associated with a distinct airway microbiome. Shoemark and colleagues showed in an analysis of 1,007 patients from five countries that 22.6% of patients had blood eosinophil counts (BEC) of >300 cells/μL. BEC of <100 cells/μL were associated with higher bronchiectasis severity and increased mortality (Shoemark et al. Am J Respir Crit Care Med. 2022;205[8]:894-902).
BEC of >300 cells/μL were correlated with Streptococcus- and Pseudomonas-dominated microbiome profiles. Compared with patients with BEC of <100 cells/μL, patients with 100-299 cells/μL (hazard ratio [HR], 2.38; 95% confidence interval, 1.33–4.25; P = .003) and those with >300 cells/μL (HR, 3.99; 95% confidence interval, 2.20–7.85; P = .0001) were associated with shorter time to exacerbation.
Eosinophilic inflammation is a risk factor for exacerbations in patients with P. aeruginosa infection and may be considered as a treatable trait. Shoemark and colleagues’ data show that quality of life was improved with inhaled corticosteroid treatment in patients with bronchiectasis who had blood eosinophil counts of >3%, and eosinophils contribute to bronchiectasis exacerbations.
Dharani Narendra, MD
Navitha Ramesh, MD, FCCP
Diego Maselli Caceres, MD, FCCP
Section Members-at-Large
Diffuse lung disease and lung transplant network, occupational and environmental health section
A ubiquitous invasion: The rise of microplastics
About 6.3 billion tons of plastic waste were produced between 1950 and 2015.1 Their degradation into submillimeter fragments of 1 μm to 5 mm, is called microplastics (MP).2 MP are vectors of pollutants, pathologic microorganisms, and chemical additives used in their fabrication.3 Exposure to MP is unavoidable as they are bio-persistent and ubiquitous, even indoors.4 MP have been detected in the snow of large metropolitan areas and in remote locations.5 Humans are exposed to MP via oral ingestion and inhalation. A Brazilian study of human lung autopsy specimens revealed the presence of MP in 13 of 20 subjects.3
In vitro studies have suggested a causal role of polystyrene-MP in the development of chronic pulmonary disease through the formation of reactive oxygen species, inhibition of cell proliferation, and cellular morphology aberration.6 MP can cause local effects due to macrophage-induced inflammation, or alternatively, be transported distantly to the pleura and the systemic circulation.
In addition, MP may disrupt the endocrine pathway due to its estrogenic effects.7 Larger MPs of 8 to 10 µm, like nylon, have been associated with interstitial lung disease.8 Lung biopsies from workers exposed to airborne synthetic fibers (acrylic, polyester, and terylene) have revealed different degrees of inflammation, granulomas, and interstitial fibrosis.9 Factory workers exposed to polyvinyl chloride dust have increased risk of exertional dyspnea and decreased pulmonary function.10 Due to the pervasive nature of MP, it is essential to establish the global burden of airborne MP and to determine its role in lung health.
Bathmapriya Balakrishnan, MD
Member-at-Large
*Tyler Church, DO
Fellow-in-Training Member
*Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
References
1. Rhodes CJ. Plastic pollution and potential solutions. Sci Prog. 2018;101(3):207-60.
2. Danopoulos E et al. Microplastic contamination of drinking water: A systematic review. PLoS One. 2020;15(7):e0236838.
3. Amato-Lourenço LF et al. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126.
4. Al Horr Y et al. Occupant productivity and office indoor environment quality: A review of the literature. Building and Environment. 2016;105:369-89.
5. Bergmann M et al. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci Adv. 2019;5:eaax1157.
6. Dong CD et al. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575.
7. Amato-Lourenço LF et al. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health. Sci Total Environ. 2020;749:141676.
8. Kern DG et al. Flock worker’s lung: Chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129[4]:261-72. Erratum in: Ann Intern Med. 1999;130[3]:246.
9. Pimentel JC et al. Respiratory disease caused by synthetic fibers: a new occupational disease. Thorax. 1975;30:204-19.
10. Soutar CA et al. Epidemiological study of respiratory disease in workers exposed to polyvinyl chloride dust. Thorax. 1980;35:644-52.
Critical care network, palliative and end-of-life section
Discussing code status with families of critically ill patients
Discussing code status with patients is complex and emotional, especially when critically ill.
The complexity further increases when these conversations have to take place with family members.
Here are some practical tips to help have these conversations in a concise and compassionate manner.
Introduction
- Introduce yourself, and make sure to identify the correct decision-maker.
- Get to know the patient.
–What kind of person are they?
–What brings them joy?
- Find out what the family knows about the current clinical condition of their family member.
–What have you been hearing from the medical team?
–What are you worried about?
Update
- Fill in the gaps – update them on the clinical condition and ongoing management.
- Discuss how you think they will respond to current management and further management options.
- Allow them to process the information.
Provide a medical recommendation
- Example: We are worried he might die, and if his heart stops, interventions like CPR or intubation would not work, and we would not recommend them.
- Do not pressure for a decision right away. (You can say “We do not need a decision today, so please take time to process this information.”)
Respond to emotions
- I can’t image how hard this must be.
- Offer chaplain services if that is important to them.
Things to avoid
- Avoid aggressive language.
–We will have to pound on their chest, break ribs.
–They would be suffering.
- Blaming or judgmental language.
While this complex discussion r equires individualization, these tips will help set a framework for goals of care conversations that lead to high quality care for patients that aligns with their goals.
Reference
Goldfish and Rosielle. Language for Routine Code Status Discussions, Fast Facts and Concepts #365, Palliative Care Network of Wisconsin.
Syed Nazeer Mahmood, MD
Fellow-in-Training Member
Anne Kelemen, LCSW
Member-at-Large