User login
Commentary: Evaluating New Treatments and Cardiovascular Risk in PsA, July 2022
Advanced targeted therapies have proven safety and efficacy over conventional therapies, often dramatically improving signs and symptoms. However, it is also desirable that such expensive therapies also show benefit in other outcomes, such as work productivity and quality of life. To evaluate work productivity and daily activity impairment and health-related quality of life in patients with inflammatory arthritis (rheumatoid arthritis, n = 95; PsA, n = 69, and axial spondyloarthritis, n = 95) treated with golimumab, Dejaco and colleagues conducted a prospective, multicenter study in Austria. A total of 110 of these patients were followed for 24 months. At 24 months after golimumab initiation, there was significant improvement in total work productivity, presenteeism, activity impairment, and quality-of-life scores. Thus, golimumab, in addition to reducing disease activity, improved work productivity, activity, and health-related quality of life in patients with inflammatory arthritis, including PsA.
Cardiovascular disease (CVD) remains a major comorbidity in patients with PsA. This observation was once again confirmed in an observational, cross-sectional, case-control study including 207 patients with PsA and 414 matched controls from France. Degboe and colleagues demonstrated that patients with PsA had a higher prevalence of cardiovascular events and cardiovascular risk factors, such as high body mass index, triglyceride level, and hypertension, compared with controls. The proportion of patients with PsA who were estimated to have very high cardiovascular risk factors (≥ 10%) increased when SCORE (European Society of Cardiology Systematic Coronary Risk Evaluation) and QRISK2 (British Heart Foundation) equations considered the additional risk attributable to PsA. However, risk predictions scores such as SCORE and QRISK2 perform poorly in patients with PsA. To identify novel inflammatory and metabolic parameters associated with cardiovascular disease, Schwartz and colleagues looked at 18F-fluorodeoxyglucose (FDG) PET-CT uptake in a cross-sectional analysis of a prospective study including 39 patients with biologic-treatment-naive PsA and 56 age-sex matched controls without PsA. They found that coronary artery disease (CAD) was significantly associated with visceral adiposity and FDG uptake in the bone marrow, liver, spleen, and subcutaneous adipose tissue. Thus, inflammatory and metabolic parameters, including visceral adiposity, potentially contribute to subclinical CAD in patients with PsA and may in the future be used to refine CVD risk and be targets for CAD preventive treatments.
Advanced targeted therapies have proven safety and efficacy over conventional therapies, often dramatically improving signs and symptoms. However, it is also desirable that such expensive therapies also show benefit in other outcomes, such as work productivity and quality of life. To evaluate work productivity and daily activity impairment and health-related quality of life in patients with inflammatory arthritis (rheumatoid arthritis, n = 95; PsA, n = 69, and axial spondyloarthritis, n = 95) treated with golimumab, Dejaco and colleagues conducted a prospective, multicenter study in Austria. A total of 110 of these patients were followed for 24 months. At 24 months after golimumab initiation, there was significant improvement in total work productivity, presenteeism, activity impairment, and quality-of-life scores. Thus, golimumab, in addition to reducing disease activity, improved work productivity, activity, and health-related quality of life in patients with inflammatory arthritis, including PsA.
Cardiovascular disease (CVD) remains a major comorbidity in patients with PsA. This observation was once again confirmed in an observational, cross-sectional, case-control study including 207 patients with PsA and 414 matched controls from France. Degboe and colleagues demonstrated that patients with PsA had a higher prevalence of cardiovascular events and cardiovascular risk factors, such as high body mass index, triglyceride level, and hypertension, compared with controls. The proportion of patients with PsA who were estimated to have very high cardiovascular risk factors (≥ 10%) increased when SCORE (European Society of Cardiology Systematic Coronary Risk Evaluation) and QRISK2 (British Heart Foundation) equations considered the additional risk attributable to PsA. However, risk predictions scores such as SCORE and QRISK2 perform poorly in patients with PsA. To identify novel inflammatory and metabolic parameters associated with cardiovascular disease, Schwartz and colleagues looked at 18F-fluorodeoxyglucose (FDG) PET-CT uptake in a cross-sectional analysis of a prospective study including 39 patients with biologic-treatment-naive PsA and 56 age-sex matched controls without PsA. They found that coronary artery disease (CAD) was significantly associated with visceral adiposity and FDG uptake in the bone marrow, liver, spleen, and subcutaneous adipose tissue. Thus, inflammatory and metabolic parameters, including visceral adiposity, potentially contribute to subclinical CAD in patients with PsA and may in the future be used to refine CVD risk and be targets for CAD preventive treatments.
Advanced targeted therapies have proven safety and efficacy over conventional therapies, often dramatically improving signs and symptoms. However, it is also desirable that such expensive therapies also show benefit in other outcomes, such as work productivity and quality of life. To evaluate work productivity and daily activity impairment and health-related quality of life in patients with inflammatory arthritis (rheumatoid arthritis, n = 95; PsA, n = 69, and axial spondyloarthritis, n = 95) treated with golimumab, Dejaco and colleagues conducted a prospective, multicenter study in Austria. A total of 110 of these patients were followed for 24 months. At 24 months after golimumab initiation, there was significant improvement in total work productivity, presenteeism, activity impairment, and quality-of-life scores. Thus, golimumab, in addition to reducing disease activity, improved work productivity, activity, and health-related quality of life in patients with inflammatory arthritis, including PsA.
Cardiovascular disease (CVD) remains a major comorbidity in patients with PsA. This observation was once again confirmed in an observational, cross-sectional, case-control study including 207 patients with PsA and 414 matched controls from France. Degboe and colleagues demonstrated that patients with PsA had a higher prevalence of cardiovascular events and cardiovascular risk factors, such as high body mass index, triglyceride level, and hypertension, compared with controls. The proportion of patients with PsA who were estimated to have very high cardiovascular risk factors (≥ 10%) increased when SCORE (European Society of Cardiology Systematic Coronary Risk Evaluation) and QRISK2 (British Heart Foundation) equations considered the additional risk attributable to PsA. However, risk predictions scores such as SCORE and QRISK2 perform poorly in patients with PsA. To identify novel inflammatory and metabolic parameters associated with cardiovascular disease, Schwartz and colleagues looked at 18F-fluorodeoxyglucose (FDG) PET-CT uptake in a cross-sectional analysis of a prospective study including 39 patients with biologic-treatment-naive PsA and 56 age-sex matched controls without PsA. They found that coronary artery disease (CAD) was significantly associated with visceral adiposity and FDG uptake in the bone marrow, liver, spleen, and subcutaneous adipose tissue. Thus, inflammatory and metabolic parameters, including visceral adiposity, potentially contribute to subclinical CAD in patients with PsA and may in the future be used to refine CVD risk and be targets for CAD preventive treatments.
ASCO outlines optimal treatments for patients with metastatic clear cell renal cell carcinoma
.
This year in the U.S., it is estimated that 79,000 men and women will be diagnosed with kidney cancer. Clear cell renal cell carcinoma (ccRCC) is the most common subtype and is a leading source of morbidity and mortality. It is also commonly used to study new targeted molecular therapies. The resulting influx of phase III trial results has transformed ccRCC care. “We have an array of different treatment options, and the structure in which these treatments would be applied needed to have expert input. It is an exciting time for kidney cancer, and optimizing therapy now has immediate and meaningful impact into patients longevity,” guideline lead author W. Kimryn Rathmell, MD, PhD, said in an interview.
“The key developments are the emergence of targeted therapies in addition to immune therapies as well as combinations. The order of treatments matters and different patients will have different needs,” said Dr. Rathmell, professor of medicine at Vanderbilt University, Nashville, Tenn.
Dr. Rathmell highlighted the section of the guideline that discusses the need for robust tissue-based diagnosis, as well as active surveillance or cytoreductive nephrectomy. She also emphasized sections on differentiating courses of treatment and plans the use of International Metastatic RCC Database Consortium (IMDC) risk model.
Cytoreductive nephrectomy is an option for patients with one risk factor in which a significant majority of the tumor is within the kidney. The patients should also have good Eastern Cooperative Oncology Group (ECOG) performance status, and no brain, bone, or liver metastases.
Some with metastatic ccRCC can be offered active surveillance. Defining characteristics include favorable or immediate risk, few or no symptoms, a favorable histologic profile, a long interval between nephrectomy and onset of metastasis, or low burden of metastatic disease.
The guidelines also discuss the need to stratify patients within risk groups. Patients rated as intermediate or poor risk in the first line setting should be treated with two immune checkpoint inhibitors (ICI) or an ICI combined with a vascular endothelial growth factor tyrosine kinase inhibitor (VEGFR TKI). ICI combined with a VEGFR TKI can be appropriate for patients with favorable risk but requiring systemic therapy. Those with favorable risk or another medical condition can be offered monotherapy with a VEGFR TKI or an ICI. Another first-line option is high-dose interleukin 2, but there are no established criteria for determining which patients are most likely to benefit.
The guideline discusses second- or later-line therapy options, metastasis-directed therapies, and metastatic subsets including bone, brain, and sarcomatoid carcinomas.
The authors pointed out that significant disparity exists among patients with ccRCC, with some patients having much less access to health care because of racial, geographic, or socioeconomic inequities. There are also known biases within ccRCC care: Females and African Americans are less likely than are males to receive systemic therapy and more likely to receive no treatment; African Americans are less likely to receive systemic therapy; and non-Hispanic African Americans and Hispanics less often undergo cytoreductive nephropathy. African Americans with ccRCC have a pattern of worse survival outcomes than do Whites.
The recommendations cannot be applied to renal cell carcinoma with non-clear cell histology.
“It is important to be comfortable with all of the treatment options for ccRCC, because applying them in the best order, and with the most informed ability to determine efficacy, will have a real impact on patient survival. We are near a goal to offer cure to an increasing number of patients, so choosing therapies that offer that option when it may be possible is important, and when cure is not on the table, we can rationally select therapies that allow patients to have more time with their families, with side effects that are manageable,” Dr. Rathmell said.
The IMDC risk stratification methodology needs to be more widely used in routine practice, Dr. Rathmell said.
“The impact was not significant in patient care until we reached a point of having multiple competing options for treatment. The stakes are higher now, so using this resource is important until we get to the next level with biological classifications,” he said.
Similarly, since the stakes are so high, having an accurate diagnosis is important. Even experts in the field are fooled by imaging findings, and over- or undertreatment of patients has a major impact on outcomes. “This is a message that we need to share for establishing best practices,” he said.
“Just because we have agents, the time to use them is as important as the selection of agent. Similarly, for the cytoreductive nephrectomy issue, new data both clarified and caused some confusion. Not every patient has the luxury of a comprehensive and multidisciplinary tumor board, so we felt it was important to provide some guidance that help making those complex decisions,” Dr. Rathmell said.
Dr. Rathmell has no relevant financial disclosures.
.
This year in the U.S., it is estimated that 79,000 men and women will be diagnosed with kidney cancer. Clear cell renal cell carcinoma (ccRCC) is the most common subtype and is a leading source of morbidity and mortality. It is also commonly used to study new targeted molecular therapies. The resulting influx of phase III trial results has transformed ccRCC care. “We have an array of different treatment options, and the structure in which these treatments would be applied needed to have expert input. It is an exciting time for kidney cancer, and optimizing therapy now has immediate and meaningful impact into patients longevity,” guideline lead author W. Kimryn Rathmell, MD, PhD, said in an interview.
“The key developments are the emergence of targeted therapies in addition to immune therapies as well as combinations. The order of treatments matters and different patients will have different needs,” said Dr. Rathmell, professor of medicine at Vanderbilt University, Nashville, Tenn.
Dr. Rathmell highlighted the section of the guideline that discusses the need for robust tissue-based diagnosis, as well as active surveillance or cytoreductive nephrectomy. She also emphasized sections on differentiating courses of treatment and plans the use of International Metastatic RCC Database Consortium (IMDC) risk model.
Cytoreductive nephrectomy is an option for patients with one risk factor in which a significant majority of the tumor is within the kidney. The patients should also have good Eastern Cooperative Oncology Group (ECOG) performance status, and no brain, bone, or liver metastases.
Some with metastatic ccRCC can be offered active surveillance. Defining characteristics include favorable or immediate risk, few or no symptoms, a favorable histologic profile, a long interval between nephrectomy and onset of metastasis, or low burden of metastatic disease.
The guidelines also discuss the need to stratify patients within risk groups. Patients rated as intermediate or poor risk in the first line setting should be treated with two immune checkpoint inhibitors (ICI) or an ICI combined with a vascular endothelial growth factor tyrosine kinase inhibitor (VEGFR TKI). ICI combined with a VEGFR TKI can be appropriate for patients with favorable risk but requiring systemic therapy. Those with favorable risk or another medical condition can be offered monotherapy with a VEGFR TKI or an ICI. Another first-line option is high-dose interleukin 2, but there are no established criteria for determining which patients are most likely to benefit.
The guideline discusses second- or later-line therapy options, metastasis-directed therapies, and metastatic subsets including bone, brain, and sarcomatoid carcinomas.
The authors pointed out that significant disparity exists among patients with ccRCC, with some patients having much less access to health care because of racial, geographic, or socioeconomic inequities. There are also known biases within ccRCC care: Females and African Americans are less likely than are males to receive systemic therapy and more likely to receive no treatment; African Americans are less likely to receive systemic therapy; and non-Hispanic African Americans and Hispanics less often undergo cytoreductive nephropathy. African Americans with ccRCC have a pattern of worse survival outcomes than do Whites.
The recommendations cannot be applied to renal cell carcinoma with non-clear cell histology.
“It is important to be comfortable with all of the treatment options for ccRCC, because applying them in the best order, and with the most informed ability to determine efficacy, will have a real impact on patient survival. We are near a goal to offer cure to an increasing number of patients, so choosing therapies that offer that option when it may be possible is important, and when cure is not on the table, we can rationally select therapies that allow patients to have more time with their families, with side effects that are manageable,” Dr. Rathmell said.
The IMDC risk stratification methodology needs to be more widely used in routine practice, Dr. Rathmell said.
“The impact was not significant in patient care until we reached a point of having multiple competing options for treatment. The stakes are higher now, so using this resource is important until we get to the next level with biological classifications,” he said.
Similarly, since the stakes are so high, having an accurate diagnosis is important. Even experts in the field are fooled by imaging findings, and over- or undertreatment of patients has a major impact on outcomes. “This is a message that we need to share for establishing best practices,” he said.
“Just because we have agents, the time to use them is as important as the selection of agent. Similarly, for the cytoreductive nephrectomy issue, new data both clarified and caused some confusion. Not every patient has the luxury of a comprehensive and multidisciplinary tumor board, so we felt it was important to provide some guidance that help making those complex decisions,” Dr. Rathmell said.
Dr. Rathmell has no relevant financial disclosures.
.
This year in the U.S., it is estimated that 79,000 men and women will be diagnosed with kidney cancer. Clear cell renal cell carcinoma (ccRCC) is the most common subtype and is a leading source of morbidity and mortality. It is also commonly used to study new targeted molecular therapies. The resulting influx of phase III trial results has transformed ccRCC care. “We have an array of different treatment options, and the structure in which these treatments would be applied needed to have expert input. It is an exciting time for kidney cancer, and optimizing therapy now has immediate and meaningful impact into patients longevity,” guideline lead author W. Kimryn Rathmell, MD, PhD, said in an interview.
“The key developments are the emergence of targeted therapies in addition to immune therapies as well as combinations. The order of treatments matters and different patients will have different needs,” said Dr. Rathmell, professor of medicine at Vanderbilt University, Nashville, Tenn.
Dr. Rathmell highlighted the section of the guideline that discusses the need for robust tissue-based diagnosis, as well as active surveillance or cytoreductive nephrectomy. She also emphasized sections on differentiating courses of treatment and plans the use of International Metastatic RCC Database Consortium (IMDC) risk model.
Cytoreductive nephrectomy is an option for patients with one risk factor in which a significant majority of the tumor is within the kidney. The patients should also have good Eastern Cooperative Oncology Group (ECOG) performance status, and no brain, bone, or liver metastases.
Some with metastatic ccRCC can be offered active surveillance. Defining characteristics include favorable or immediate risk, few or no symptoms, a favorable histologic profile, a long interval between nephrectomy and onset of metastasis, or low burden of metastatic disease.
The guidelines also discuss the need to stratify patients within risk groups. Patients rated as intermediate or poor risk in the first line setting should be treated with two immune checkpoint inhibitors (ICI) or an ICI combined with a vascular endothelial growth factor tyrosine kinase inhibitor (VEGFR TKI). ICI combined with a VEGFR TKI can be appropriate for patients with favorable risk but requiring systemic therapy. Those with favorable risk or another medical condition can be offered monotherapy with a VEGFR TKI or an ICI. Another first-line option is high-dose interleukin 2, but there are no established criteria for determining which patients are most likely to benefit.
The guideline discusses second- or later-line therapy options, metastasis-directed therapies, and metastatic subsets including bone, brain, and sarcomatoid carcinomas.
The authors pointed out that significant disparity exists among patients with ccRCC, with some patients having much less access to health care because of racial, geographic, or socioeconomic inequities. There are also known biases within ccRCC care: Females and African Americans are less likely than are males to receive systemic therapy and more likely to receive no treatment; African Americans are less likely to receive systemic therapy; and non-Hispanic African Americans and Hispanics less often undergo cytoreductive nephropathy. African Americans with ccRCC have a pattern of worse survival outcomes than do Whites.
The recommendations cannot be applied to renal cell carcinoma with non-clear cell histology.
“It is important to be comfortable with all of the treatment options for ccRCC, because applying them in the best order, and with the most informed ability to determine efficacy, will have a real impact on patient survival. We are near a goal to offer cure to an increasing number of patients, so choosing therapies that offer that option when it may be possible is important, and when cure is not on the table, we can rationally select therapies that allow patients to have more time with their families, with side effects that are manageable,” Dr. Rathmell said.
The IMDC risk stratification methodology needs to be more widely used in routine practice, Dr. Rathmell said.
“The impact was not significant in patient care until we reached a point of having multiple competing options for treatment. The stakes are higher now, so using this resource is important until we get to the next level with biological classifications,” he said.
Similarly, since the stakes are so high, having an accurate diagnosis is important. Even experts in the field are fooled by imaging findings, and over- or undertreatment of patients has a major impact on outcomes. “This is a message that we need to share for establishing best practices,” he said.
“Just because we have agents, the time to use them is as important as the selection of agent. Similarly, for the cytoreductive nephrectomy issue, new data both clarified and caused some confusion. Not every patient has the luxury of a comprehensive and multidisciplinary tumor board, so we felt it was important to provide some guidance that help making those complex decisions,” Dr. Rathmell said.
Dr. Rathmell has no relevant financial disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
‘The Rock’ assumes the presidency of AGA
We’re honored to announce that John M. Carethers, MD, AGAF, affectionately nicknamed ‘The Rock,’ will begin his term as the 117th president of the AGA Institute on June 1, 2022.
He currently serves as John G. Searle professor of internal medicine and chair of the department of internal medicine at the University of Michigan Health System, a position he has held since 2009.
Dr. Carethers’ research programs focus on familial colon cancer and polyposis syndromes. His research encompasses Lynch syndrome, juvenile polyposis, hyperplastic polyposis, and colorectal cancer. He has published more than 182 articles.
A native of Detroit, Dr. Carethers earned his undergraduate degree in molecular biology and biophysics at Wayne State University. He remained there for medical school, where he graduated at the top of his class. His ability to stay focused on his work earned him the moniker ‘The Rock’. It’s a strength that’s made him an outstanding role model and exemplary leader.
An active member of AGA for more than 20 years, Dr. Carethers received the AGA Gastrointestinal Oncology Section Research Mentor Award as well as the AGA Distinguished Mentor Award in 2017. He has served on several AGA committees, including the AGA Nominating Committee, AGA Underrepresented Minorities Committee, AGA Research Policy Committee, AGA Institute Council and the AGA Trainee & Young GI Committee. He has also served as senior associate editor of Gastroenterology.
His academic career began at the University of California, San Diego, preceded by a gastroenterology fellowship at University of Michigan in Ann Arbor and residency at Massachusetts General Hospital in Boston. From the beginning, he has inspired others with his strong work ethic and intense dedication.
Dr. Carethers joined the AGA Governing Board in June 2020 as vice president and served as president-elect prior to assuming the top leadership role.
We’re honored to announce that John M. Carethers, MD, AGAF, affectionately nicknamed ‘The Rock,’ will begin his term as the 117th president of the AGA Institute on June 1, 2022.
He currently serves as John G. Searle professor of internal medicine and chair of the department of internal medicine at the University of Michigan Health System, a position he has held since 2009.
Dr. Carethers’ research programs focus on familial colon cancer and polyposis syndromes. His research encompasses Lynch syndrome, juvenile polyposis, hyperplastic polyposis, and colorectal cancer. He has published more than 182 articles.
A native of Detroit, Dr. Carethers earned his undergraduate degree in molecular biology and biophysics at Wayne State University. He remained there for medical school, where he graduated at the top of his class. His ability to stay focused on his work earned him the moniker ‘The Rock’. It’s a strength that’s made him an outstanding role model and exemplary leader.
An active member of AGA for more than 20 years, Dr. Carethers received the AGA Gastrointestinal Oncology Section Research Mentor Award as well as the AGA Distinguished Mentor Award in 2017. He has served on several AGA committees, including the AGA Nominating Committee, AGA Underrepresented Minorities Committee, AGA Research Policy Committee, AGA Institute Council and the AGA Trainee & Young GI Committee. He has also served as senior associate editor of Gastroenterology.
His academic career began at the University of California, San Diego, preceded by a gastroenterology fellowship at University of Michigan in Ann Arbor and residency at Massachusetts General Hospital in Boston. From the beginning, he has inspired others with his strong work ethic and intense dedication.
Dr. Carethers joined the AGA Governing Board in June 2020 as vice president and served as president-elect prior to assuming the top leadership role.
We’re honored to announce that John M. Carethers, MD, AGAF, affectionately nicknamed ‘The Rock,’ will begin his term as the 117th president of the AGA Institute on June 1, 2022.
He currently serves as John G. Searle professor of internal medicine and chair of the department of internal medicine at the University of Michigan Health System, a position he has held since 2009.
Dr. Carethers’ research programs focus on familial colon cancer and polyposis syndromes. His research encompasses Lynch syndrome, juvenile polyposis, hyperplastic polyposis, and colorectal cancer. He has published more than 182 articles.
A native of Detroit, Dr. Carethers earned his undergraduate degree in molecular biology and biophysics at Wayne State University. He remained there for medical school, where he graduated at the top of his class. His ability to stay focused on his work earned him the moniker ‘The Rock’. It’s a strength that’s made him an outstanding role model and exemplary leader.
An active member of AGA for more than 20 years, Dr. Carethers received the AGA Gastrointestinal Oncology Section Research Mentor Award as well as the AGA Distinguished Mentor Award in 2017. He has served on several AGA committees, including the AGA Nominating Committee, AGA Underrepresented Minorities Committee, AGA Research Policy Committee, AGA Institute Council and the AGA Trainee & Young GI Committee. He has also served as senior associate editor of Gastroenterology.
His academic career began at the University of California, San Diego, preceded by a gastroenterology fellowship at University of Michigan in Ann Arbor and residency at Massachusetts General Hospital in Boston. From the beginning, he has inspired others with his strong work ethic and intense dedication.
Dr. Carethers joined the AGA Governing Board in June 2020 as vice president and served as president-elect prior to assuming the top leadership role.
First-line nivolumab monotherapy effective in some kidney cancers
The anti–PD-1 agent nivolumab may have some utility as monotherapy in the treatment of naive advanced clear cell renal cell carcinoma (ccRCC), according results from a new phase 2 clinical trial (HCRN GU16-260).
Patients received nivolumab (Opdivo, Bristol-Myers Squibb) monotherapy in the first phase of the study, with qualifying patients moving on to dual therapy with nivolumab and the anti–CTLA-4 agent ipilimumab (Yervoy, Bristol-Myers Squibb).
“At the moment, monotherapy with anti–PD-1 is not FDA approved in patients with treatment-naive ccRCC. Based on the results of this study, it might be applicable in patients with favorable risk disease where only tyrosine kinase inhibitors or TKI-containing combinations are approved,” lead investigator Michael Atkins, MD, said in an interview. The study was published online in the Journal of Clinical Oncology.
“It shows the contribution of [nivolumab] to the combination in treatment-naive patients. This data was not previously available as nivolumab monotherapy had only been formally tested in patients with prior history of TKI therapy. It also gives valuable information on the role of PD-L1 as a biomarker in response and landmark progression-free survival in patients treated with anti–PD-1 monotherapy,” said Dr. Atkins, who serves as deputy director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, D.C.
Patients with higher expression rates of PD-L1 had a higher overall response rate and higher probability of progression-free survival at 1 year.
“ but there is concern about risk of toxicity if ipilimumab is added. These might include elderly patients and those with a history or autoimmune conditions,” Dr. Atkins said.
Next on the agenda for the researchers are more extensive biomarker studies of the tissues obtained in the phase 2 study. They hope to identify factors that predict response and resistance in the absence of confounders like combination therapy, primary versus metastatic tumors, and use of nonimmunotherapy endpoints. They will also track treatment-free survival following cessation of therapy at 2 years.
The study “provides much needed first-line data for check point inhibitor monotherapy in an era that is heavily invested in combination strategies,” Martin Voss, MD, wrote in an accompanying editorial. Further studies currently in progress should shed even more light on monotherapy in this patient population. These include the phase 3 CheckMate 8Y8 trial comparing nivolumab with ipilimumab/nivolumab in untreated patients, and the phase 3 PDIGREE23 trial, which is comparing upfront, response-adaptive ipilimumab/nivolumab with escalation through addition of TKIs in patients with progressive disease and de-escalation to nivolumab monotherapy in patients who achieve a complete response, and randomization between the two strategies for nonprogressive disease and non–complete response patients.
However, these phase 3 studies are limited to intermediate- to poor-risk patients. For favorable-risk patients, small cohort studies like HCRN GU16-260 represent the best currently available guide to treatment, Dr. Voss wrote.
In part A of the study, 123 patients received nivolumab for 96 weeks. 35 patients who experienced progressive disease or had a best response of stable disease at 48 weeks became eligible for part B, in which they received the combination of nivolumab and ipilimumab for 12 weeks, and then nivolumab monotherapy for 48 weeks. All patients underwent PD-L1 testing, and tumors were categorized by percentage of tumor cells expressing PD-L1 as 0%, 1%-5%, greater than 5%-20%, and greater than 20%.
The overall objective response rate was 34.1%. 6.5% had a complete response. Patients categorized by International Metastatic RCC Database Consortium category as intermediate or poor risk had an ORR of 25.0%. Those categorized as favorable risk had an ORR of 57.1%, with a complete response rate of 11.4%. Just one patient with favorable risk had progressive disease at their 12-week CT scan. The ORR was higher with increasing percentages of PD-L1 expression, from 26.9% in PD-L1 0 to 50% in PD-L1 1-20 and 75.0% in PD-L1 greater than 20% (P = .002). Five of 7 (71.4%) patients with PD-L1 values of 1 or more had a tumor response to nivolumab monotherapy, but 14 of 23 (60.9%) of those with favorable risk disease and PD-L1 of zero also responded.
The median duration of response was 27.6 months, and progression-free survival was 34.6% at 1 year in the PD-L1 0 and 75.0% in the PD-L1 greater than 20 categories (P = .050). Among patients eligible for part B, the ORR was 11.4%.
Dr. Atkins has consulted or been on the advisory board for Bristol-Myers Squibb.
The anti–PD-1 agent nivolumab may have some utility as monotherapy in the treatment of naive advanced clear cell renal cell carcinoma (ccRCC), according results from a new phase 2 clinical trial (HCRN GU16-260).
Patients received nivolumab (Opdivo, Bristol-Myers Squibb) monotherapy in the first phase of the study, with qualifying patients moving on to dual therapy with nivolumab and the anti–CTLA-4 agent ipilimumab (Yervoy, Bristol-Myers Squibb).
“At the moment, monotherapy with anti–PD-1 is not FDA approved in patients with treatment-naive ccRCC. Based on the results of this study, it might be applicable in patients with favorable risk disease where only tyrosine kinase inhibitors or TKI-containing combinations are approved,” lead investigator Michael Atkins, MD, said in an interview. The study was published online in the Journal of Clinical Oncology.
“It shows the contribution of [nivolumab] to the combination in treatment-naive patients. This data was not previously available as nivolumab monotherapy had only been formally tested in patients with prior history of TKI therapy. It also gives valuable information on the role of PD-L1 as a biomarker in response and landmark progression-free survival in patients treated with anti–PD-1 monotherapy,” said Dr. Atkins, who serves as deputy director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, D.C.
Patients with higher expression rates of PD-L1 had a higher overall response rate and higher probability of progression-free survival at 1 year.
“ but there is concern about risk of toxicity if ipilimumab is added. These might include elderly patients and those with a history or autoimmune conditions,” Dr. Atkins said.
Next on the agenda for the researchers are more extensive biomarker studies of the tissues obtained in the phase 2 study. They hope to identify factors that predict response and resistance in the absence of confounders like combination therapy, primary versus metastatic tumors, and use of nonimmunotherapy endpoints. They will also track treatment-free survival following cessation of therapy at 2 years.
The study “provides much needed first-line data for check point inhibitor monotherapy in an era that is heavily invested in combination strategies,” Martin Voss, MD, wrote in an accompanying editorial. Further studies currently in progress should shed even more light on monotherapy in this patient population. These include the phase 3 CheckMate 8Y8 trial comparing nivolumab with ipilimumab/nivolumab in untreated patients, and the phase 3 PDIGREE23 trial, which is comparing upfront, response-adaptive ipilimumab/nivolumab with escalation through addition of TKIs in patients with progressive disease and de-escalation to nivolumab monotherapy in patients who achieve a complete response, and randomization between the two strategies for nonprogressive disease and non–complete response patients.
However, these phase 3 studies are limited to intermediate- to poor-risk patients. For favorable-risk patients, small cohort studies like HCRN GU16-260 represent the best currently available guide to treatment, Dr. Voss wrote.
In part A of the study, 123 patients received nivolumab for 96 weeks. 35 patients who experienced progressive disease or had a best response of stable disease at 48 weeks became eligible for part B, in which they received the combination of nivolumab and ipilimumab for 12 weeks, and then nivolumab monotherapy for 48 weeks. All patients underwent PD-L1 testing, and tumors were categorized by percentage of tumor cells expressing PD-L1 as 0%, 1%-5%, greater than 5%-20%, and greater than 20%.
The overall objective response rate was 34.1%. 6.5% had a complete response. Patients categorized by International Metastatic RCC Database Consortium category as intermediate or poor risk had an ORR of 25.0%. Those categorized as favorable risk had an ORR of 57.1%, with a complete response rate of 11.4%. Just one patient with favorable risk had progressive disease at their 12-week CT scan. The ORR was higher with increasing percentages of PD-L1 expression, from 26.9% in PD-L1 0 to 50% in PD-L1 1-20 and 75.0% in PD-L1 greater than 20% (P = .002). Five of 7 (71.4%) patients with PD-L1 values of 1 or more had a tumor response to nivolumab monotherapy, but 14 of 23 (60.9%) of those with favorable risk disease and PD-L1 of zero also responded.
The median duration of response was 27.6 months, and progression-free survival was 34.6% at 1 year in the PD-L1 0 and 75.0% in the PD-L1 greater than 20 categories (P = .050). Among patients eligible for part B, the ORR was 11.4%.
Dr. Atkins has consulted or been on the advisory board for Bristol-Myers Squibb.
The anti–PD-1 agent nivolumab may have some utility as monotherapy in the treatment of naive advanced clear cell renal cell carcinoma (ccRCC), according results from a new phase 2 clinical trial (HCRN GU16-260).
Patients received nivolumab (Opdivo, Bristol-Myers Squibb) monotherapy in the first phase of the study, with qualifying patients moving on to dual therapy with nivolumab and the anti–CTLA-4 agent ipilimumab (Yervoy, Bristol-Myers Squibb).
“At the moment, monotherapy with anti–PD-1 is not FDA approved in patients with treatment-naive ccRCC. Based on the results of this study, it might be applicable in patients with favorable risk disease where only tyrosine kinase inhibitors or TKI-containing combinations are approved,” lead investigator Michael Atkins, MD, said in an interview. The study was published online in the Journal of Clinical Oncology.
“It shows the contribution of [nivolumab] to the combination in treatment-naive patients. This data was not previously available as nivolumab monotherapy had only been formally tested in patients with prior history of TKI therapy. It also gives valuable information on the role of PD-L1 as a biomarker in response and landmark progression-free survival in patients treated with anti–PD-1 monotherapy,” said Dr. Atkins, who serves as deputy director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, D.C.
Patients with higher expression rates of PD-L1 had a higher overall response rate and higher probability of progression-free survival at 1 year.
“ but there is concern about risk of toxicity if ipilimumab is added. These might include elderly patients and those with a history or autoimmune conditions,” Dr. Atkins said.
Next on the agenda for the researchers are more extensive biomarker studies of the tissues obtained in the phase 2 study. They hope to identify factors that predict response and resistance in the absence of confounders like combination therapy, primary versus metastatic tumors, and use of nonimmunotherapy endpoints. They will also track treatment-free survival following cessation of therapy at 2 years.
The study “provides much needed first-line data for check point inhibitor monotherapy in an era that is heavily invested in combination strategies,” Martin Voss, MD, wrote in an accompanying editorial. Further studies currently in progress should shed even more light on monotherapy in this patient population. These include the phase 3 CheckMate 8Y8 trial comparing nivolumab with ipilimumab/nivolumab in untreated patients, and the phase 3 PDIGREE23 trial, which is comparing upfront, response-adaptive ipilimumab/nivolumab with escalation through addition of TKIs in patients with progressive disease and de-escalation to nivolumab monotherapy in patients who achieve a complete response, and randomization between the two strategies for nonprogressive disease and non–complete response patients.
However, these phase 3 studies are limited to intermediate- to poor-risk patients. For favorable-risk patients, small cohort studies like HCRN GU16-260 represent the best currently available guide to treatment, Dr. Voss wrote.
In part A of the study, 123 patients received nivolumab for 96 weeks. 35 patients who experienced progressive disease or had a best response of stable disease at 48 weeks became eligible for part B, in which they received the combination of nivolumab and ipilimumab for 12 weeks, and then nivolumab monotherapy for 48 weeks. All patients underwent PD-L1 testing, and tumors were categorized by percentage of tumor cells expressing PD-L1 as 0%, 1%-5%, greater than 5%-20%, and greater than 20%.
The overall objective response rate was 34.1%. 6.5% had a complete response. Patients categorized by International Metastatic RCC Database Consortium category as intermediate or poor risk had an ORR of 25.0%. Those categorized as favorable risk had an ORR of 57.1%, with a complete response rate of 11.4%. Just one patient with favorable risk had progressive disease at their 12-week CT scan. The ORR was higher with increasing percentages of PD-L1 expression, from 26.9% in PD-L1 0 to 50% in PD-L1 1-20 and 75.0% in PD-L1 greater than 20% (P = .002). Five of 7 (71.4%) patients with PD-L1 values of 1 or more had a tumor response to nivolumab monotherapy, but 14 of 23 (60.9%) of those with favorable risk disease and PD-L1 of zero also responded.
The median duration of response was 27.6 months, and progression-free survival was 34.6% at 1 year in the PD-L1 0 and 75.0% in the PD-L1 greater than 20 categories (P = .050). Among patients eligible for part B, the ORR was 11.4%.
Dr. Atkins has consulted or been on the advisory board for Bristol-Myers Squibb.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
New AGA Research Foundation Executive Board members
We’re pleased to share that Michael Camilleri, MD, AGAF, will be taking over the AGA Research Foundation chair role beginning this month. He has recruited five members to be part of the 2022-2024 AGA Research Foundation Executive Board.
Meet the new Foundation Executive Board members
- Michael Camilleri, MD, AGAF, Mayo Clinic, Rochester, MN.
- Aline Charabaty, MD, AGAF, Johns Hopkins School of Medicine, Washington, D.C.
- Eric Esrailian, MD, MPH, AGAF, David Geffen School of Medicine at UCLA, Los Angeles, CA
- Robert A. Ganz, MD, MASGE, MNGI Digestive Health, Minnetonka, MN
- Aja S. McCutchen, MD, Atlanta Gastroenterology Associates, Hoschton, GA
- Michael L. Kochman, MD, AGAF, University of Pennsylvania, Philadelphia, PA
We’re pleased to share that Michael Camilleri, MD, AGAF, will be taking over the AGA Research Foundation chair role beginning this month. He has recruited five members to be part of the 2022-2024 AGA Research Foundation Executive Board.
Meet the new Foundation Executive Board members
- Michael Camilleri, MD, AGAF, Mayo Clinic, Rochester, MN.
- Aline Charabaty, MD, AGAF, Johns Hopkins School of Medicine, Washington, D.C.
- Eric Esrailian, MD, MPH, AGAF, David Geffen School of Medicine at UCLA, Los Angeles, CA
- Robert A. Ganz, MD, MASGE, MNGI Digestive Health, Minnetonka, MN
- Aja S. McCutchen, MD, Atlanta Gastroenterology Associates, Hoschton, GA
- Michael L. Kochman, MD, AGAF, University of Pennsylvania, Philadelphia, PA
We’re pleased to share that Michael Camilleri, MD, AGAF, will be taking over the AGA Research Foundation chair role beginning this month. He has recruited five members to be part of the 2022-2024 AGA Research Foundation Executive Board.
Meet the new Foundation Executive Board members
- Michael Camilleri, MD, AGAF, Mayo Clinic, Rochester, MN.
- Aline Charabaty, MD, AGAF, Johns Hopkins School of Medicine, Washington, D.C.
- Eric Esrailian, MD, MPH, AGAF, David Geffen School of Medicine at UCLA, Los Angeles, CA
- Robert A. Ganz, MD, MASGE, MNGI Digestive Health, Minnetonka, MN
- Aja S. McCutchen, MD, Atlanta Gastroenterology Associates, Hoschton, GA
- Michael L. Kochman, MD, AGAF, University of Pennsylvania, Philadelphia, PA
Murder of physician raises the stress level for all clinicians
As if it weren’t enough that doctors work in a profession where it’s almost more a question of when they’ll be sued than if they’ll be sued – where COVID, staff shortages, long hours, and patients frustrated over canceled procedures have caused unrelenting fatigue and stress – they now have to worry that an unhappy patient is going to buy a gun, walk into their office, and kill them.
That’s exactly what happened in Tulsa, Okla., where a patient complaining of pain after back surgery murdered his doctor and several others who happened to be in the wrong place at the wrong time.
The temptation in the aftermath of such tragedies is to think about preventive measures: Make medical facilities “hardened” targets, like schools have become, with armed guards, metal detectors, automatically locking doors, physical barriers within, security cameras, and buzzers for entry – although hardening a large medical center where members of the community routinely come and go would be challenging.
What about the enormous stress on doctors, nurses, and others in the medical workplace? Physicians who have been sued for malpractice often describe how it changes the way they interact with patients: They now size patients up and make judgments about their potential litigiousness. Will the physicians now look over their patients’ shoulders at the video feed from a security camera when they’re taking a history? Will medical professionals be forced to make snap judgments about patients’ psychological state before deciding whether to treat them?
Remember, there was a time when school shootings were unimaginable. Once one person crosses that line, others inevitably follow.
It could be a drug-seeking patient complaining of ongoing pain, angry because he can’t get a new prescription. It could be a patient whose unpaid bill was turned over to a collection agency, angry because he’s now getting calls from collectors. It could be someone who blames a physician for the loss of a loved one. It could be someone who would otherwise have filed a lawsuit, who now thinks he has a more effective option for exacting retribution.
Most of us would find it unbearable to live and work under the kind of stress faced by medical professionals today. And unfortunately, there is no short-term, systemic relief on the horizon. But there are methods of relieving at least some of the psychological burden being carried by these dedicated individuals.
For starters, the government should provide funds to improve safety and security at medical facilities. It’s sad but it’s a fact of life. The physical structure of schools, along with emergency procedures, have been changed since Columbine and Sandy Hook, and our children and their teachers undergo active shooter drills. Health care facilities will need to adopt similar strategies.
But if we don’t also support the individuals who work in health care, we’ll no longer have even partially staffed health care facilities. Hospitals and medical groups need to be conscious of the effects stress may have on them. Medical staff and administrators need to recognize changes in their colleagues’ behavior and refer those cohorts to professional stress coaches who can get them back on track.
Medical personnel should be picking up on warning signs, like irritability, depression, sudden weight gain or loss, lack of motivation and job satisfaction, obsessiveness, unusual levels of fatigue, alcohol or drug use, and, of course, avoidable medical errors.
In addition, colleagues in the medical workplace need to know each other well. They are usually the first ones to notice if something is off and may be in the best position to refer coworkers for help. Also, medical malpractice insurance carriers should consider encouraging and covering coaching sessions, because helping physicians cope with this heightened stress will prevent medical errors and the lawsuits that inevitably accompany mistakes.
This needn’t be a long-term process like ongoing psychotherapy; a few sessions with a well-trained coach may help psychologically challenged peers restore their focus and perspective. It won’t eliminate the threat any more than litigation stress coaching eliminates the threat of being sued, but it can prevent that stress from leading to avoidable errors. It also can prevent physicians’ personal lives and relationships from going off the rails and driving them out of the medical profession.
None of us can afford to ignore the impacts that these new stressors are having and simply act as if it’s business as usual. The people in the trenches need our help.
Ms. Fiore is President of Winning Focus in Murrysville, Pa. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As if it weren’t enough that doctors work in a profession where it’s almost more a question of when they’ll be sued than if they’ll be sued – where COVID, staff shortages, long hours, and patients frustrated over canceled procedures have caused unrelenting fatigue and stress – they now have to worry that an unhappy patient is going to buy a gun, walk into their office, and kill them.
That’s exactly what happened in Tulsa, Okla., where a patient complaining of pain after back surgery murdered his doctor and several others who happened to be in the wrong place at the wrong time.
The temptation in the aftermath of such tragedies is to think about preventive measures: Make medical facilities “hardened” targets, like schools have become, with armed guards, metal detectors, automatically locking doors, physical barriers within, security cameras, and buzzers for entry – although hardening a large medical center where members of the community routinely come and go would be challenging.
What about the enormous stress on doctors, nurses, and others in the medical workplace? Physicians who have been sued for malpractice often describe how it changes the way they interact with patients: They now size patients up and make judgments about their potential litigiousness. Will the physicians now look over their patients’ shoulders at the video feed from a security camera when they’re taking a history? Will medical professionals be forced to make snap judgments about patients’ psychological state before deciding whether to treat them?
Remember, there was a time when school shootings were unimaginable. Once one person crosses that line, others inevitably follow.
It could be a drug-seeking patient complaining of ongoing pain, angry because he can’t get a new prescription. It could be a patient whose unpaid bill was turned over to a collection agency, angry because he’s now getting calls from collectors. It could be someone who blames a physician for the loss of a loved one. It could be someone who would otherwise have filed a lawsuit, who now thinks he has a more effective option for exacting retribution.
Most of us would find it unbearable to live and work under the kind of stress faced by medical professionals today. And unfortunately, there is no short-term, systemic relief on the horizon. But there are methods of relieving at least some of the psychological burden being carried by these dedicated individuals.
For starters, the government should provide funds to improve safety and security at medical facilities. It’s sad but it’s a fact of life. The physical structure of schools, along with emergency procedures, have been changed since Columbine and Sandy Hook, and our children and their teachers undergo active shooter drills. Health care facilities will need to adopt similar strategies.
But if we don’t also support the individuals who work in health care, we’ll no longer have even partially staffed health care facilities. Hospitals and medical groups need to be conscious of the effects stress may have on them. Medical staff and administrators need to recognize changes in their colleagues’ behavior and refer those cohorts to professional stress coaches who can get them back on track.
Medical personnel should be picking up on warning signs, like irritability, depression, sudden weight gain or loss, lack of motivation and job satisfaction, obsessiveness, unusual levels of fatigue, alcohol or drug use, and, of course, avoidable medical errors.
In addition, colleagues in the medical workplace need to know each other well. They are usually the first ones to notice if something is off and may be in the best position to refer coworkers for help. Also, medical malpractice insurance carriers should consider encouraging and covering coaching sessions, because helping physicians cope with this heightened stress will prevent medical errors and the lawsuits that inevitably accompany mistakes.
This needn’t be a long-term process like ongoing psychotherapy; a few sessions with a well-trained coach may help psychologically challenged peers restore their focus and perspective. It won’t eliminate the threat any more than litigation stress coaching eliminates the threat of being sued, but it can prevent that stress from leading to avoidable errors. It also can prevent physicians’ personal lives and relationships from going off the rails and driving them out of the medical profession.
None of us can afford to ignore the impacts that these new stressors are having and simply act as if it’s business as usual. The people in the trenches need our help.
Ms. Fiore is President of Winning Focus in Murrysville, Pa. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As if it weren’t enough that doctors work in a profession where it’s almost more a question of when they’ll be sued than if they’ll be sued – where COVID, staff shortages, long hours, and patients frustrated over canceled procedures have caused unrelenting fatigue and stress – they now have to worry that an unhappy patient is going to buy a gun, walk into their office, and kill them.
That’s exactly what happened in Tulsa, Okla., where a patient complaining of pain after back surgery murdered his doctor and several others who happened to be in the wrong place at the wrong time.
The temptation in the aftermath of such tragedies is to think about preventive measures: Make medical facilities “hardened” targets, like schools have become, with armed guards, metal detectors, automatically locking doors, physical barriers within, security cameras, and buzzers for entry – although hardening a large medical center where members of the community routinely come and go would be challenging.
What about the enormous stress on doctors, nurses, and others in the medical workplace? Physicians who have been sued for malpractice often describe how it changes the way they interact with patients: They now size patients up and make judgments about their potential litigiousness. Will the physicians now look over their patients’ shoulders at the video feed from a security camera when they’re taking a history? Will medical professionals be forced to make snap judgments about patients’ psychological state before deciding whether to treat them?
Remember, there was a time when school shootings were unimaginable. Once one person crosses that line, others inevitably follow.
It could be a drug-seeking patient complaining of ongoing pain, angry because he can’t get a new prescription. It could be a patient whose unpaid bill was turned over to a collection agency, angry because he’s now getting calls from collectors. It could be someone who blames a physician for the loss of a loved one. It could be someone who would otherwise have filed a lawsuit, who now thinks he has a more effective option for exacting retribution.
Most of us would find it unbearable to live and work under the kind of stress faced by medical professionals today. And unfortunately, there is no short-term, systemic relief on the horizon. But there are methods of relieving at least some of the psychological burden being carried by these dedicated individuals.
For starters, the government should provide funds to improve safety and security at medical facilities. It’s sad but it’s a fact of life. The physical structure of schools, along with emergency procedures, have been changed since Columbine and Sandy Hook, and our children and their teachers undergo active shooter drills. Health care facilities will need to adopt similar strategies.
But if we don’t also support the individuals who work in health care, we’ll no longer have even partially staffed health care facilities. Hospitals and medical groups need to be conscious of the effects stress may have on them. Medical staff and administrators need to recognize changes in their colleagues’ behavior and refer those cohorts to professional stress coaches who can get them back on track.
Medical personnel should be picking up on warning signs, like irritability, depression, sudden weight gain or loss, lack of motivation and job satisfaction, obsessiveness, unusual levels of fatigue, alcohol or drug use, and, of course, avoidable medical errors.
In addition, colleagues in the medical workplace need to know each other well. They are usually the first ones to notice if something is off and may be in the best position to refer coworkers for help. Also, medical malpractice insurance carriers should consider encouraging and covering coaching sessions, because helping physicians cope with this heightened stress will prevent medical errors and the lawsuits that inevitably accompany mistakes.
This needn’t be a long-term process like ongoing psychotherapy; a few sessions with a well-trained coach may help psychologically challenged peers restore their focus and perspective. It won’t eliminate the threat any more than litigation stress coaching eliminates the threat of being sued, but it can prevent that stress from leading to avoidable errors. It also can prevent physicians’ personal lives and relationships from going off the rails and driving them out of the medical profession.
None of us can afford to ignore the impacts that these new stressors are having and simply act as if it’s business as usual. The people in the trenches need our help.
Ms. Fiore is President of Winning Focus in Murrysville, Pa. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
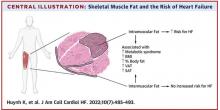
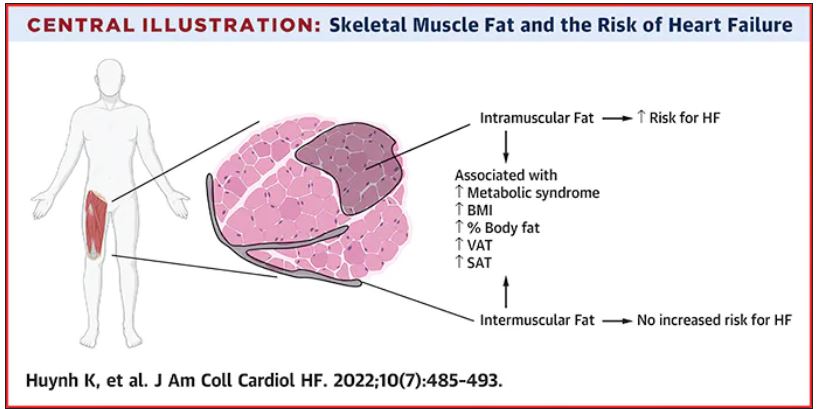
Thigh muscle fat predicts risk of developing heart failure
in a new study. The association was independent of other cardiometabolic risk factors and measures of adiposity such as body mass index.
The observation raises the possibility of new avenues of research aimed at modifying intramuscular fat levels as a strategy to reduce the risk of developing heart failure.
The study was published online in JACC: Heart Failure.
The authors, led by Kevin Huynh, MD, University of Texas Southwestern Medical Center, Dallas, explained that obesity is a known risk for heart failure, and has been incorporated into risk calculators for heart failure.
However, obesity is a complex and heterogeneous disease with substantial regional variability of adipose deposition in body tissues, they noted. For example, variability in visceral adipose tissue and subcutaneous adipose tissue has been shown to have a differential impact on both cardiovascular risk factors and clinical cardiovascular disease outcomes.
The fat deposition around and within nonadipose tissues (termed “ectopic fat”), such as skeletal muscle, is also a known risk factor for cardiovascular disease, independent of adiposity. However, the impact of peripheral skeletal muscle fat deposition on heart failure risk is not as well studied.
The researchers noted that ectopic fat in skeletal muscle can be measured through imaging and categorized as either intermuscular or intramuscular fat according to the location of muscle fat around or within skeletal muscle, respectively.
The researchers conducted the current study to characterize the association of both intermuscular and intramuscular fat deposition with heart failure risk in a large cohort of older adults.
They used data from 2,399 individuals aged 70-79 years without heart failure at baseline who participated in the Health ABC (Health, Aging and Body Composition) study. Measures of intramuscular and intermuscular fat in the thigh were determined by CT, and the participants were followed for an average of 12 years.
During the follow-up period, there were 485 incident heart failure events. Higher sex-specific tertiles of intramuscular and intermuscular fat were each associated with heart failure risk.
After multivariable adjustment for age, sex, race, education, blood pressure, fasting blood sugar, current smoking, prevalent coronary disease, and creatinine, higher intramuscular fat, but not intermuscular fat, was significantly associated with higher risk for heart failure.
Individuals in the highest tertile of intramuscular fat had a 34% increased risk of developing heart failure, compared with those in the lowest tertile. This finding was independent of other cardiometabolic risk factors, measures of adiposity including body mass index and percent fat, muscle strength, and muscle mass.
The association was slightly attenuated when adjusted for inflammatory markers, suggesting that inflammation may be a contributor.
The association between higher intramuscular fat and heart failure appeared specific to higher risk of incident heart failure with reduced ejection fraction, but not with heart failure with preserved ejection fraction.
The researchers noted that skeletal muscle is a pivotal endocrine organ in addition to the role it plays in the production of mechanical power.
They pointed out that there are differences in the biology of intermuscular and intramuscular fat deposition, and that excess intramuscular fat deposition is a result of dysregulated lipid metabolism and is associated with insulin resistance (a known risk factor for the development of heart failure), inflammation, and muscle wasting conditions.
They concluded that, in patients with heart failure, alterations in skeletal muscle function are most likely affected by multiple contributors, including inflammation, oxidative stress, and neurohormonal factors. “As these factors are also implicated in the pathogenesis of heart failure, intramuscular fat deposition may indicate a biological milieu that increases the risk of heart failure.”
New approaches to reduce heart failure risk?
In an accompanying editorial, Salvatore Carbone, PhD, Virginia Commonwealth University, Richmond, said the findings of the study are “exceptionally novel,” providing novel evidence that noncardiac body composition compartments, particularly intramuscular adipose tissue, can predict the risk for heart failure in a diverse population of older adults.
He called for further research to understand the mechanisms involved and to assess if this risk factor can be effectively modified to reduce the risk of developing heart failure.
Dr. Carbone reported that intramuscular adipose tissue can be influenced by dietary fat intake and can be worsened by accumulation of saturated fatty acids, which also contribute to insulin resistance.
He noted that saturated fatty acid–induced insulin resistance in the skeletal muscle appears to be mediated by proinflammatory pathways within the skeletal muscle itself, which can be reversed by monounsaturated fatty acids, like oleic acid, that can be found in the largest amount in food like olive oil, canola oil, and avocados, among others.
He added that sodium-glucose transporter 2 inhibitors, drugs used in the treatment of diabetes that have also been shown to prevent heart failure in individuals at risk, can also improve the composition of intramuscular adipose tissue by reducing its content of saturated fatty acids and increase the content of monosaturated fatty acids.
The study results suggest that the quality of intramuscular adipose tissue might also play an important role and could be targeted by therapeutic strategies, he commented.
Dr. Carbone concluded that “studies testing novel modalities of exercise training, intentional weight loss, diet quality improvements with and without weight loss (i.e., increase of dietary monounsaturated fatty acids, such as oleic acid), as well as pharmacological anti-inflammatory strategies should be encouraged in this population to test whether the reduction in intramuscular adipose tissue or improvements of its quality can ultimately reduce the risk for heart failure in this population.”
This research was supported by the National Institute on Aging and the National Institute of Nursing Research. Dr. Huynh and Dr. Carbone disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a new study. The association was independent of other cardiometabolic risk factors and measures of adiposity such as body mass index.
The observation raises the possibility of new avenues of research aimed at modifying intramuscular fat levels as a strategy to reduce the risk of developing heart failure.
The study was published online in JACC: Heart Failure.
The authors, led by Kevin Huynh, MD, University of Texas Southwestern Medical Center, Dallas, explained that obesity is a known risk for heart failure, and has been incorporated into risk calculators for heart failure.
However, obesity is a complex and heterogeneous disease with substantial regional variability of adipose deposition in body tissues, they noted. For example, variability in visceral adipose tissue and subcutaneous adipose tissue has been shown to have a differential impact on both cardiovascular risk factors and clinical cardiovascular disease outcomes.
The fat deposition around and within nonadipose tissues (termed “ectopic fat”), such as skeletal muscle, is also a known risk factor for cardiovascular disease, independent of adiposity. However, the impact of peripheral skeletal muscle fat deposition on heart failure risk is not as well studied.
The researchers noted that ectopic fat in skeletal muscle can be measured through imaging and categorized as either intermuscular or intramuscular fat according to the location of muscle fat around or within skeletal muscle, respectively.
The researchers conducted the current study to characterize the association of both intermuscular and intramuscular fat deposition with heart failure risk in a large cohort of older adults.
They used data from 2,399 individuals aged 70-79 years without heart failure at baseline who participated in the Health ABC (Health, Aging and Body Composition) study. Measures of intramuscular and intermuscular fat in the thigh were determined by CT, and the participants were followed for an average of 12 years.
During the follow-up period, there were 485 incident heart failure events. Higher sex-specific tertiles of intramuscular and intermuscular fat were each associated with heart failure risk.
After multivariable adjustment for age, sex, race, education, blood pressure, fasting blood sugar, current smoking, prevalent coronary disease, and creatinine, higher intramuscular fat, but not intermuscular fat, was significantly associated with higher risk for heart failure.
Individuals in the highest tertile of intramuscular fat had a 34% increased risk of developing heart failure, compared with those in the lowest tertile. This finding was independent of other cardiometabolic risk factors, measures of adiposity including body mass index and percent fat, muscle strength, and muscle mass.
The association was slightly attenuated when adjusted for inflammatory markers, suggesting that inflammation may be a contributor.
The association between higher intramuscular fat and heart failure appeared specific to higher risk of incident heart failure with reduced ejection fraction, but not with heart failure with preserved ejection fraction.
The researchers noted that skeletal muscle is a pivotal endocrine organ in addition to the role it plays in the production of mechanical power.
They pointed out that there are differences in the biology of intermuscular and intramuscular fat deposition, and that excess intramuscular fat deposition is a result of dysregulated lipid metabolism and is associated with insulin resistance (a known risk factor for the development of heart failure), inflammation, and muscle wasting conditions.
They concluded that, in patients with heart failure, alterations in skeletal muscle function are most likely affected by multiple contributors, including inflammation, oxidative stress, and neurohormonal factors. “As these factors are also implicated in the pathogenesis of heart failure, intramuscular fat deposition may indicate a biological milieu that increases the risk of heart failure.”
New approaches to reduce heart failure risk?
In an accompanying editorial, Salvatore Carbone, PhD, Virginia Commonwealth University, Richmond, said the findings of the study are “exceptionally novel,” providing novel evidence that noncardiac body composition compartments, particularly intramuscular adipose tissue, can predict the risk for heart failure in a diverse population of older adults.
He called for further research to understand the mechanisms involved and to assess if this risk factor can be effectively modified to reduce the risk of developing heart failure.
Dr. Carbone reported that intramuscular adipose tissue can be influenced by dietary fat intake and can be worsened by accumulation of saturated fatty acids, which also contribute to insulin resistance.
He noted that saturated fatty acid–induced insulin resistance in the skeletal muscle appears to be mediated by proinflammatory pathways within the skeletal muscle itself, which can be reversed by monounsaturated fatty acids, like oleic acid, that can be found in the largest amount in food like olive oil, canola oil, and avocados, among others.
He added that sodium-glucose transporter 2 inhibitors, drugs used in the treatment of diabetes that have also been shown to prevent heart failure in individuals at risk, can also improve the composition of intramuscular adipose tissue by reducing its content of saturated fatty acids and increase the content of monosaturated fatty acids.
The study results suggest that the quality of intramuscular adipose tissue might also play an important role and could be targeted by therapeutic strategies, he commented.
Dr. Carbone concluded that “studies testing novel modalities of exercise training, intentional weight loss, diet quality improvements with and without weight loss (i.e., increase of dietary monounsaturated fatty acids, such as oleic acid), as well as pharmacological anti-inflammatory strategies should be encouraged in this population to test whether the reduction in intramuscular adipose tissue or improvements of its quality can ultimately reduce the risk for heart failure in this population.”
This research was supported by the National Institute on Aging and the National Institute of Nursing Research. Dr. Huynh and Dr. Carbone disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a new study. The association was independent of other cardiometabolic risk factors and measures of adiposity such as body mass index.
The observation raises the possibility of new avenues of research aimed at modifying intramuscular fat levels as a strategy to reduce the risk of developing heart failure.
The study was published online in JACC: Heart Failure.
The authors, led by Kevin Huynh, MD, University of Texas Southwestern Medical Center, Dallas, explained that obesity is a known risk for heart failure, and has been incorporated into risk calculators for heart failure.
However, obesity is a complex and heterogeneous disease with substantial regional variability of adipose deposition in body tissues, they noted. For example, variability in visceral adipose tissue and subcutaneous adipose tissue has been shown to have a differential impact on both cardiovascular risk factors and clinical cardiovascular disease outcomes.
The fat deposition around and within nonadipose tissues (termed “ectopic fat”), such as skeletal muscle, is also a known risk factor for cardiovascular disease, independent of adiposity. However, the impact of peripheral skeletal muscle fat deposition on heart failure risk is not as well studied.
The researchers noted that ectopic fat in skeletal muscle can be measured through imaging and categorized as either intermuscular or intramuscular fat according to the location of muscle fat around or within skeletal muscle, respectively.
The researchers conducted the current study to characterize the association of both intermuscular and intramuscular fat deposition with heart failure risk in a large cohort of older adults.
They used data from 2,399 individuals aged 70-79 years without heart failure at baseline who participated in the Health ABC (Health, Aging and Body Composition) study. Measures of intramuscular and intermuscular fat in the thigh were determined by CT, and the participants were followed for an average of 12 years.
During the follow-up period, there were 485 incident heart failure events. Higher sex-specific tertiles of intramuscular and intermuscular fat were each associated with heart failure risk.
After multivariable adjustment for age, sex, race, education, blood pressure, fasting blood sugar, current smoking, prevalent coronary disease, and creatinine, higher intramuscular fat, but not intermuscular fat, was significantly associated with higher risk for heart failure.
Individuals in the highest tertile of intramuscular fat had a 34% increased risk of developing heart failure, compared with those in the lowest tertile. This finding was independent of other cardiometabolic risk factors, measures of adiposity including body mass index and percent fat, muscle strength, and muscle mass.
The association was slightly attenuated when adjusted for inflammatory markers, suggesting that inflammation may be a contributor.
The association between higher intramuscular fat and heart failure appeared specific to higher risk of incident heart failure with reduced ejection fraction, but not with heart failure with preserved ejection fraction.
The researchers noted that skeletal muscle is a pivotal endocrine organ in addition to the role it plays in the production of mechanical power.
They pointed out that there are differences in the biology of intermuscular and intramuscular fat deposition, and that excess intramuscular fat deposition is a result of dysregulated lipid metabolism and is associated with insulin resistance (a known risk factor for the development of heart failure), inflammation, and muscle wasting conditions.
They concluded that, in patients with heart failure, alterations in skeletal muscle function are most likely affected by multiple contributors, including inflammation, oxidative stress, and neurohormonal factors. “As these factors are also implicated in the pathogenesis of heart failure, intramuscular fat deposition may indicate a biological milieu that increases the risk of heart failure.”
New approaches to reduce heart failure risk?
In an accompanying editorial, Salvatore Carbone, PhD, Virginia Commonwealth University, Richmond, said the findings of the study are “exceptionally novel,” providing novel evidence that noncardiac body composition compartments, particularly intramuscular adipose tissue, can predict the risk for heart failure in a diverse population of older adults.
He called for further research to understand the mechanisms involved and to assess if this risk factor can be effectively modified to reduce the risk of developing heart failure.
Dr. Carbone reported that intramuscular adipose tissue can be influenced by dietary fat intake and can be worsened by accumulation of saturated fatty acids, which also contribute to insulin resistance.
He noted that saturated fatty acid–induced insulin resistance in the skeletal muscle appears to be mediated by proinflammatory pathways within the skeletal muscle itself, which can be reversed by monounsaturated fatty acids, like oleic acid, that can be found in the largest amount in food like olive oil, canola oil, and avocados, among others.
He added that sodium-glucose transporter 2 inhibitors, drugs used in the treatment of diabetes that have also been shown to prevent heart failure in individuals at risk, can also improve the composition of intramuscular adipose tissue by reducing its content of saturated fatty acids and increase the content of monosaturated fatty acids.
The study results suggest that the quality of intramuscular adipose tissue might also play an important role and could be targeted by therapeutic strategies, he commented.
Dr. Carbone concluded that “studies testing novel modalities of exercise training, intentional weight loss, diet quality improvements with and without weight loss (i.e., increase of dietary monounsaturated fatty acids, such as oleic acid), as well as pharmacological anti-inflammatory strategies should be encouraged in this population to test whether the reduction in intramuscular adipose tissue or improvements of its quality can ultimately reduce the risk for heart failure in this population.”
This research was supported by the National Institute on Aging and the National Institute of Nursing Research. Dr. Huynh and Dr. Carbone disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
Nurse who won’t give Viagra to White conservative men resigns
the day after her now-viral post.
The discriminatory tweet with political overtones comes just days after the U.S. Supreme Court handed down its decision to overturn Roe v. Wade, which permitted abortions.
Libs of TikTok, which featured the tweet, identified the nurse practitioner as Shawna Harris. More than a dozen visitors to WebMD’s healthcare directory, which indicates Ms. Harris specialized in family medicine, gave her a 1-star (out of 5 stars) review after the posting. Among the comments left on the site:
“By threatening patients that hold views she is against, she has broken the bond of trust between patient and doctor.” Still another visitor voiced: “If you are White and conservative I’d be careful going here because she tweeted she withholds medication based on race and political affiliation. That’s scary.”
Meanwhile, the health system where she worked, Sarah Bush Lincoln in Sullivan, Ill., in a since-deleted bio listed Ms. Harris’ rating as 4.8 out of 5 stars. The bio stated she was a certified family nurse practitioner and was board certified by the American Academy of Nurse Practitioners.
Sarah Bush Lincoln posted the APRN’s apology and resignation on Twitter. “I am deeply sorry for my posts on social media,” she wrote, according to the health system’s tweet. “I allowed my personal feelings to spill out. Those hateful words are not aligned with how I have provided care to my patients.”
Jerry Esker, the health system’s president and CEO, also stated in the post: “Our mission is to provide exceptional care to all. That means we provide care to everyone regardless of race, religion, gender, sexual orientation, disability, income, national origin, cultural personal values, beliefs, and preferences.”
Mr. Esker added that he wanted to talk with the APRN before taking any action and that “everyone is entitled to due process,” according to the health system post.
Sarah Bush Lincoln is a 145-bed, not-for-profit, regional hospital in east central Illinois, according to its website.
A version of this article first appeared on Medscape.com.
the day after her now-viral post.
The discriminatory tweet with political overtones comes just days after the U.S. Supreme Court handed down its decision to overturn Roe v. Wade, which permitted abortions.
Libs of TikTok, which featured the tweet, identified the nurse practitioner as Shawna Harris. More than a dozen visitors to WebMD’s healthcare directory, which indicates Ms. Harris specialized in family medicine, gave her a 1-star (out of 5 stars) review after the posting. Among the comments left on the site:
“By threatening patients that hold views she is against, she has broken the bond of trust between patient and doctor.” Still another visitor voiced: “If you are White and conservative I’d be careful going here because she tweeted she withholds medication based on race and political affiliation. That’s scary.”
Meanwhile, the health system where she worked, Sarah Bush Lincoln in Sullivan, Ill., in a since-deleted bio listed Ms. Harris’ rating as 4.8 out of 5 stars. The bio stated she was a certified family nurse practitioner and was board certified by the American Academy of Nurse Practitioners.
Sarah Bush Lincoln posted the APRN’s apology and resignation on Twitter. “I am deeply sorry for my posts on social media,” she wrote, according to the health system’s tweet. “I allowed my personal feelings to spill out. Those hateful words are not aligned with how I have provided care to my patients.”
Jerry Esker, the health system’s president and CEO, also stated in the post: “Our mission is to provide exceptional care to all. That means we provide care to everyone regardless of race, religion, gender, sexual orientation, disability, income, national origin, cultural personal values, beliefs, and preferences.”
Mr. Esker added that he wanted to talk with the APRN before taking any action and that “everyone is entitled to due process,” according to the health system post.
Sarah Bush Lincoln is a 145-bed, not-for-profit, regional hospital in east central Illinois, according to its website.
A version of this article first appeared on Medscape.com.
the day after her now-viral post.
The discriminatory tweet with political overtones comes just days after the U.S. Supreme Court handed down its decision to overturn Roe v. Wade, which permitted abortions.
Libs of TikTok, which featured the tweet, identified the nurse practitioner as Shawna Harris. More than a dozen visitors to WebMD’s healthcare directory, which indicates Ms. Harris specialized in family medicine, gave her a 1-star (out of 5 stars) review after the posting. Among the comments left on the site:
“By threatening patients that hold views she is against, she has broken the bond of trust between patient and doctor.” Still another visitor voiced: “If you are White and conservative I’d be careful going here because she tweeted she withholds medication based on race and political affiliation. That’s scary.”
Meanwhile, the health system where she worked, Sarah Bush Lincoln in Sullivan, Ill., in a since-deleted bio listed Ms. Harris’ rating as 4.8 out of 5 stars. The bio stated she was a certified family nurse practitioner and was board certified by the American Academy of Nurse Practitioners.
Sarah Bush Lincoln posted the APRN’s apology and resignation on Twitter. “I am deeply sorry for my posts on social media,” she wrote, according to the health system’s tweet. “I allowed my personal feelings to spill out. Those hateful words are not aligned with how I have provided care to my patients.”
Jerry Esker, the health system’s president and CEO, also stated in the post: “Our mission is to provide exceptional care to all. That means we provide care to everyone regardless of race, religion, gender, sexual orientation, disability, income, national origin, cultural personal values, beliefs, and preferences.”
Mr. Esker added that he wanted to talk with the APRN before taking any action and that “everyone is entitled to due process,” according to the health system post.
Sarah Bush Lincoln is a 145-bed, not-for-profit, regional hospital in east central Illinois, according to its website.
A version of this article first appeared on Medscape.com.
Alabama cites Roe decision in call to ban transgender health care
Alabama urged a federal court on June 28 to drop its block on the state’s ban on gender-affirming care for transgender youth, citing the Supreme Court’s recent decision to overturn Roe v. Wade.
Alabama Attorney General Steve Marshall said the high court ruled that abortion isn’t protected under the 14th Amendment because it’s not “deeply rooted” in the nation’s history, which he noted could be said about access to gender-affirming care as well, according to Axios.
“No one – adult or child – has a right to transitioning treatments that is deeply rooted in our Nation’s history and tradition,” he wrote in a court document.
“The State can thus regulate or prohibit those interventions for children, even if an adult wants the drugs for his child,” he wrote.
In May, a federal judge blocked part of Alabama’s Senate Bill 184, which makes it a felony for someone to “engage in or cause” certain types of medical care for transgender youths. The law, which was put in place in April, allows for criminal prosecution against doctors, parents, guardians, and anyone else who provides care to a minor. The penalties could result in up to 10 years in prison and up to $15,000 in fines.
At that time, U.S. District Judge Liles Burke issued an injunction to stop Alabama from enforcing the law and allow challenges, including one filed by the Department of Justice. Mr. Burke said the state provided “no credible evidence to show that transitioning medications are ‘experimental.’ ”
“While Defendants offer some evidence that transitioning medications pose certain risks, the uncontradicted record evidence is that at least twenty-two major medical associations in the United States endorse transitioning medications as well-established, evidence-based treatments for gender dysphoria in minors,” he wrote in the ruling.
Medical organizations such as the American Academy of Pediatrics, American Psychological Association, and American Medical Association have urged governors to oppose legislation this year that would restrict gender-affirming medical care, saying that such laws could have negative effects on the mental health of transgender youths.
But on June 28, Mr. Marshall focused on the Constitution and what he believes the recent overturn of Roe implies.
“Just as the parental relationship does not unlock a Due Process right allowing parents to obtain medical marijuana or abortions for their children, neither does it unlock a right to transitioning treatments,” he wrote.
“The Constitution reserves to the State – not courts or medical interest groups – the authority to determine that these sterilizing interventions are too dangerous for minors,” he said.
Since the Supreme Court overturned Roe, people have expressed concerns that lawsuits could now target several rights that are protected under the 14th Amendment, including same-sex relationships, marriage equality, and access to contraceptives.
Justice Clarence Thomas, who wrote a concurring opinion to the majority decision, said the Supreme Court, “in future cases” should reconsider “substantive due process precedents” under previous landmark cases such as Griswold v. Connecticut, Lawrence v. Texas, and Obergefell v. Hodges.
At the same time, Justice Brett Kavanaugh, who also wrote a concurring opinion, said the decision to overturn Roe was only focused on abortion, saying it “does not mean the overruling of those precedents, and does not threaten or cast doubt on those precedents.”
A version of this article first appeared on WebMD.com.
Alabama urged a federal court on June 28 to drop its block on the state’s ban on gender-affirming care for transgender youth, citing the Supreme Court’s recent decision to overturn Roe v. Wade.
Alabama Attorney General Steve Marshall said the high court ruled that abortion isn’t protected under the 14th Amendment because it’s not “deeply rooted” in the nation’s history, which he noted could be said about access to gender-affirming care as well, according to Axios.
“No one – adult or child – has a right to transitioning treatments that is deeply rooted in our Nation’s history and tradition,” he wrote in a court document.
“The State can thus regulate or prohibit those interventions for children, even if an adult wants the drugs for his child,” he wrote.
In May, a federal judge blocked part of Alabama’s Senate Bill 184, which makes it a felony for someone to “engage in or cause” certain types of medical care for transgender youths. The law, which was put in place in April, allows for criminal prosecution against doctors, parents, guardians, and anyone else who provides care to a minor. The penalties could result in up to 10 years in prison and up to $15,000 in fines.
At that time, U.S. District Judge Liles Burke issued an injunction to stop Alabama from enforcing the law and allow challenges, including one filed by the Department of Justice. Mr. Burke said the state provided “no credible evidence to show that transitioning medications are ‘experimental.’ ”
“While Defendants offer some evidence that transitioning medications pose certain risks, the uncontradicted record evidence is that at least twenty-two major medical associations in the United States endorse transitioning medications as well-established, evidence-based treatments for gender dysphoria in minors,” he wrote in the ruling.
Medical organizations such as the American Academy of Pediatrics, American Psychological Association, and American Medical Association have urged governors to oppose legislation this year that would restrict gender-affirming medical care, saying that such laws could have negative effects on the mental health of transgender youths.
But on June 28, Mr. Marshall focused on the Constitution and what he believes the recent overturn of Roe implies.
“Just as the parental relationship does not unlock a Due Process right allowing parents to obtain medical marijuana or abortions for their children, neither does it unlock a right to transitioning treatments,” he wrote.
“The Constitution reserves to the State – not courts or medical interest groups – the authority to determine that these sterilizing interventions are too dangerous for minors,” he said.
Since the Supreme Court overturned Roe, people have expressed concerns that lawsuits could now target several rights that are protected under the 14th Amendment, including same-sex relationships, marriage equality, and access to contraceptives.
Justice Clarence Thomas, who wrote a concurring opinion to the majority decision, said the Supreme Court, “in future cases” should reconsider “substantive due process precedents” under previous landmark cases such as Griswold v. Connecticut, Lawrence v. Texas, and Obergefell v. Hodges.
At the same time, Justice Brett Kavanaugh, who also wrote a concurring opinion, said the decision to overturn Roe was only focused on abortion, saying it “does not mean the overruling of those precedents, and does not threaten or cast doubt on those precedents.”
A version of this article first appeared on WebMD.com.
Alabama urged a federal court on June 28 to drop its block on the state’s ban on gender-affirming care for transgender youth, citing the Supreme Court’s recent decision to overturn Roe v. Wade.
Alabama Attorney General Steve Marshall said the high court ruled that abortion isn’t protected under the 14th Amendment because it’s not “deeply rooted” in the nation’s history, which he noted could be said about access to gender-affirming care as well, according to Axios.
“No one – adult or child – has a right to transitioning treatments that is deeply rooted in our Nation’s history and tradition,” he wrote in a court document.
“The State can thus regulate or prohibit those interventions for children, even if an adult wants the drugs for his child,” he wrote.
In May, a federal judge blocked part of Alabama’s Senate Bill 184, which makes it a felony for someone to “engage in or cause” certain types of medical care for transgender youths. The law, which was put in place in April, allows for criminal prosecution against doctors, parents, guardians, and anyone else who provides care to a minor. The penalties could result in up to 10 years in prison and up to $15,000 in fines.
At that time, U.S. District Judge Liles Burke issued an injunction to stop Alabama from enforcing the law and allow challenges, including one filed by the Department of Justice. Mr. Burke said the state provided “no credible evidence to show that transitioning medications are ‘experimental.’ ”
“While Defendants offer some evidence that transitioning medications pose certain risks, the uncontradicted record evidence is that at least twenty-two major medical associations in the United States endorse transitioning medications as well-established, evidence-based treatments for gender dysphoria in minors,” he wrote in the ruling.
Medical organizations such as the American Academy of Pediatrics, American Psychological Association, and American Medical Association have urged governors to oppose legislation this year that would restrict gender-affirming medical care, saying that such laws could have negative effects on the mental health of transgender youths.
But on June 28, Mr. Marshall focused on the Constitution and what he believes the recent overturn of Roe implies.
“Just as the parental relationship does not unlock a Due Process right allowing parents to obtain medical marijuana or abortions for their children, neither does it unlock a right to transitioning treatments,” he wrote.
“The Constitution reserves to the State – not courts or medical interest groups – the authority to determine that these sterilizing interventions are too dangerous for minors,” he said.
Since the Supreme Court overturned Roe, people have expressed concerns that lawsuits could now target several rights that are protected under the 14th Amendment, including same-sex relationships, marriage equality, and access to contraceptives.
Justice Clarence Thomas, who wrote a concurring opinion to the majority decision, said the Supreme Court, “in future cases” should reconsider “substantive due process precedents” under previous landmark cases such as Griswold v. Connecticut, Lawrence v. Texas, and Obergefell v. Hodges.
At the same time, Justice Brett Kavanaugh, who also wrote a concurring opinion, said the decision to overturn Roe was only focused on abortion, saying it “does not mean the overruling of those precedents, and does not threaten or cast doubt on those precedents.”
A version of this article first appeared on WebMD.com.
First-ever Huntington staging system may jump-start drug development for early-stage disease
Researchers liken the Huntington’s disease Integrated Staging System (HD-ISS) to the system currently used to stage cancer. It groups patients according to their underlying biological, clinical, and functional characteristics.
It also includes criteria to biologically define Huntington’s disease stages across the entire disease spectrum, from birth to death, which is something that has not been done before. For now, the HD-ISS is only intended for research, but it could one day be modified for use in the clinic, investigators wrote.
“This systematization is of critical importance to select the most appropriate target population for clinical trials and studies,” said co-investigator Cristina Sampaio, MD, chief medical officer at the CHDI Foundation, Princeton, N.J.
“By providing a methodology to precisely define cases early in the neurodegenerative process, the HD-ISS will be instrumental in conducting trials in the very early disease stages,” Dr. Sampaio added.
The position paper was published in the July issue of the Lancet Neurology.
New approach needed
There is no approved therapy to slow Huntington’s disease progression. Clinical trials currently enroll patients with demonstrable symptoms, which limits the ability to test therapeutics that could delay or prevent neurodegeneration.
Huntington’s disease is rare, occurring in about 2.7 per 100,000 individuals worldwide. It is caused by a mutation in the HTT gene involving a DNA segment known as a CAG trinucleotide repeat.
Currently, Huntington’s disease is diagnosed on the basis of clinical signs that emerge late in the disease course, an approach developed before the discovery of the HTT gene and the development of the genetic test for the CAG mutation.
The disease phase prior to diagnosis has been described as presymptomatic, premanifest, or prodromal. However, the three terms have varying definitions that make it difficult to compare study results across trials.
Because drug development had focused on the overt motor sign phase of the disease, there was no real need for an evidence-based staging system that classified disease phases from birth, the investigators noted.
“Now, the research community and regulators recognize that it is critical to conduct trials early in the disease when no signs or overt symptoms are measurable,” Dr. Sampaio said.
Defining disease stages
Work on the staging system was done through the Huntington’s Disease Regulatory Science Consortium, an international project begun in 2018 among biotech and pharma companies, academic institutions, and nonprofit research and advocacy organizations.
Overall, more than 50 clinicians and researchers were involved in developing the HD-ISS.
Using modeling data from four large observational studies that included patients with Huntington’s disease and control groups, researchers identified four different stages of Huntington’s disease:
- Stage 0: Begins at birth with identification of HTT gene mutations but no detectable pathologic changes.
- Stage 1: Begins when biomarker changes are detected via MRI by a volume decrease in six brain areas.
- Stage 2: Begins when clinical signs of Huntington’s disease are present, as determined through motor and cognitive assessments.
- Stage 3: Begins when functional decline is evident, with worsening on the Independence Scale and the Total Functional Capacity of the Unified Huntington’s Disease Rating Scale.
Applying the HD-ISS to clinical trials requires the collection of information routinely recorded in Huntington’s disease research, as well as some additional data, but researchers say its application is straightforward.
The HD-ISS uses a numerical staging system similar to that used in the U.S. Food and Drug Administration’s guidance for Alzheimer’s disease (AD) and integrates the prodromal, presymptomatic, or premanifest phase of the disease. This distinguishes it from earlier classification systems.
The HD-ISS can be adapted if new Huntington’s disease biomarkers are identified.
“As research results are generated, this will further validate the HD-ISS and potentially lead to the development of a derivative, and possibly simplified, system for clinical practice,” Dr. Sampaio said.
The new system goes further than a more recent proposal from the Movement Disorder Society task force, which addresses earlier stages in Huntington’s disease but doesn’t consider objective biomarker data.
Question of timing
Commenting on the findings, Erin Furr-Stimming, MD, neurologist and director of the Huntington’s Disease Society of America Center of Excellence with McGovern Medical School, UTHealth, Houston, said targeting early-stage disease will be key.
“Similar to more common neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, there is a period of at least a decade when changes are occurring in the nervous system, prior to the manifestation of clinical symptoms and signs significant enough to warrant a clinical diagnosis,” Dr. Furr-Stimming said.
She noted that multiple trials of disease-modifying agents for Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease have failed for a multitude of reasons, “but one consistent question that is relevant to all these diseases is that of timing: Should we intervene and test these therapies earlier?
“The premanifest or prodromal period may be the ideal time to intervene with a disease-modifying therapy, prior to onset of any neurodegeneration,” Dr. Furr-Stimming said.
The CHDI Foundation provided financial support to the Critical Path Institute for the Huntington’s Disease Regulatory Science Consortium, including all working group efforts. Dr. Sampio is an employee of and receives salary from CHDI Management. She has also received consultancy honorariums (unrelated to HD) from Pfizer, Kyowa Kirin, vTv Therapeutics, GW Pharmaceuticals, Neuraly, Neuroderm, Green Valley Pharmaceuticals, and Pinteon Pharmaceuticals. A full list of disclosures for the other researchers is in the original article. Dr. Furr-Stimming reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers liken the Huntington’s disease Integrated Staging System (HD-ISS) to the system currently used to stage cancer. It groups patients according to their underlying biological, clinical, and functional characteristics.
It also includes criteria to biologically define Huntington’s disease stages across the entire disease spectrum, from birth to death, which is something that has not been done before. For now, the HD-ISS is only intended for research, but it could one day be modified for use in the clinic, investigators wrote.
“This systematization is of critical importance to select the most appropriate target population for clinical trials and studies,” said co-investigator Cristina Sampaio, MD, chief medical officer at the CHDI Foundation, Princeton, N.J.
“By providing a methodology to precisely define cases early in the neurodegenerative process, the HD-ISS will be instrumental in conducting trials in the very early disease stages,” Dr. Sampaio added.
The position paper was published in the July issue of the Lancet Neurology.
New approach needed
There is no approved therapy to slow Huntington’s disease progression. Clinical trials currently enroll patients with demonstrable symptoms, which limits the ability to test therapeutics that could delay or prevent neurodegeneration.
Huntington’s disease is rare, occurring in about 2.7 per 100,000 individuals worldwide. It is caused by a mutation in the HTT gene involving a DNA segment known as a CAG trinucleotide repeat.
Currently, Huntington’s disease is diagnosed on the basis of clinical signs that emerge late in the disease course, an approach developed before the discovery of the HTT gene and the development of the genetic test for the CAG mutation.
The disease phase prior to diagnosis has been described as presymptomatic, premanifest, or prodromal. However, the three terms have varying definitions that make it difficult to compare study results across trials.
Because drug development had focused on the overt motor sign phase of the disease, there was no real need for an evidence-based staging system that classified disease phases from birth, the investigators noted.
“Now, the research community and regulators recognize that it is critical to conduct trials early in the disease when no signs or overt symptoms are measurable,” Dr. Sampaio said.
Defining disease stages
Work on the staging system was done through the Huntington’s Disease Regulatory Science Consortium, an international project begun in 2018 among biotech and pharma companies, academic institutions, and nonprofit research and advocacy organizations.
Overall, more than 50 clinicians and researchers were involved in developing the HD-ISS.
Using modeling data from four large observational studies that included patients with Huntington’s disease and control groups, researchers identified four different stages of Huntington’s disease:
- Stage 0: Begins at birth with identification of HTT gene mutations but no detectable pathologic changes.
- Stage 1: Begins when biomarker changes are detected via MRI by a volume decrease in six brain areas.
- Stage 2: Begins when clinical signs of Huntington’s disease are present, as determined through motor and cognitive assessments.
- Stage 3: Begins when functional decline is evident, with worsening on the Independence Scale and the Total Functional Capacity of the Unified Huntington’s Disease Rating Scale.
Applying the HD-ISS to clinical trials requires the collection of information routinely recorded in Huntington’s disease research, as well as some additional data, but researchers say its application is straightforward.
The HD-ISS uses a numerical staging system similar to that used in the U.S. Food and Drug Administration’s guidance for Alzheimer’s disease (AD) and integrates the prodromal, presymptomatic, or premanifest phase of the disease. This distinguishes it from earlier classification systems.
The HD-ISS can be adapted if new Huntington’s disease biomarkers are identified.
“As research results are generated, this will further validate the HD-ISS and potentially lead to the development of a derivative, and possibly simplified, system for clinical practice,” Dr. Sampaio said.
The new system goes further than a more recent proposal from the Movement Disorder Society task force, which addresses earlier stages in Huntington’s disease but doesn’t consider objective biomarker data.
Question of timing
Commenting on the findings, Erin Furr-Stimming, MD, neurologist and director of the Huntington’s Disease Society of America Center of Excellence with McGovern Medical School, UTHealth, Houston, said targeting early-stage disease will be key.
“Similar to more common neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, there is a period of at least a decade when changes are occurring in the nervous system, prior to the manifestation of clinical symptoms and signs significant enough to warrant a clinical diagnosis,” Dr. Furr-Stimming said.
She noted that multiple trials of disease-modifying agents for Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease have failed for a multitude of reasons, “but one consistent question that is relevant to all these diseases is that of timing: Should we intervene and test these therapies earlier?
“The premanifest or prodromal period may be the ideal time to intervene with a disease-modifying therapy, prior to onset of any neurodegeneration,” Dr. Furr-Stimming said.
The CHDI Foundation provided financial support to the Critical Path Institute for the Huntington’s Disease Regulatory Science Consortium, including all working group efforts. Dr. Sampio is an employee of and receives salary from CHDI Management. She has also received consultancy honorariums (unrelated to HD) from Pfizer, Kyowa Kirin, vTv Therapeutics, GW Pharmaceuticals, Neuraly, Neuroderm, Green Valley Pharmaceuticals, and Pinteon Pharmaceuticals. A full list of disclosures for the other researchers is in the original article. Dr. Furr-Stimming reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers liken the Huntington’s disease Integrated Staging System (HD-ISS) to the system currently used to stage cancer. It groups patients according to their underlying biological, clinical, and functional characteristics.
It also includes criteria to biologically define Huntington’s disease stages across the entire disease spectrum, from birth to death, which is something that has not been done before. For now, the HD-ISS is only intended for research, but it could one day be modified for use in the clinic, investigators wrote.
“This systematization is of critical importance to select the most appropriate target population for clinical trials and studies,” said co-investigator Cristina Sampaio, MD, chief medical officer at the CHDI Foundation, Princeton, N.J.
“By providing a methodology to precisely define cases early in the neurodegenerative process, the HD-ISS will be instrumental in conducting trials in the very early disease stages,” Dr. Sampaio added.
The position paper was published in the July issue of the Lancet Neurology.
New approach needed
There is no approved therapy to slow Huntington’s disease progression. Clinical trials currently enroll patients with demonstrable symptoms, which limits the ability to test therapeutics that could delay or prevent neurodegeneration.
Huntington’s disease is rare, occurring in about 2.7 per 100,000 individuals worldwide. It is caused by a mutation in the HTT gene involving a DNA segment known as a CAG trinucleotide repeat.
Currently, Huntington’s disease is diagnosed on the basis of clinical signs that emerge late in the disease course, an approach developed before the discovery of the HTT gene and the development of the genetic test for the CAG mutation.
The disease phase prior to diagnosis has been described as presymptomatic, premanifest, or prodromal. However, the three terms have varying definitions that make it difficult to compare study results across trials.
Because drug development had focused on the overt motor sign phase of the disease, there was no real need for an evidence-based staging system that classified disease phases from birth, the investigators noted.
“Now, the research community and regulators recognize that it is critical to conduct trials early in the disease when no signs or overt symptoms are measurable,” Dr. Sampaio said.
Defining disease stages
Work on the staging system was done through the Huntington’s Disease Regulatory Science Consortium, an international project begun in 2018 among biotech and pharma companies, academic institutions, and nonprofit research and advocacy organizations.
Overall, more than 50 clinicians and researchers were involved in developing the HD-ISS.
Using modeling data from four large observational studies that included patients with Huntington’s disease and control groups, researchers identified four different stages of Huntington’s disease:
- Stage 0: Begins at birth with identification of HTT gene mutations but no detectable pathologic changes.
- Stage 1: Begins when biomarker changes are detected via MRI by a volume decrease in six brain areas.
- Stage 2: Begins when clinical signs of Huntington’s disease are present, as determined through motor and cognitive assessments.
- Stage 3: Begins when functional decline is evident, with worsening on the Independence Scale and the Total Functional Capacity of the Unified Huntington’s Disease Rating Scale.
Applying the HD-ISS to clinical trials requires the collection of information routinely recorded in Huntington’s disease research, as well as some additional data, but researchers say its application is straightforward.
The HD-ISS uses a numerical staging system similar to that used in the U.S. Food and Drug Administration’s guidance for Alzheimer’s disease (AD) and integrates the prodromal, presymptomatic, or premanifest phase of the disease. This distinguishes it from earlier classification systems.
The HD-ISS can be adapted if new Huntington’s disease biomarkers are identified.
“As research results are generated, this will further validate the HD-ISS and potentially lead to the development of a derivative, and possibly simplified, system for clinical practice,” Dr. Sampaio said.
The new system goes further than a more recent proposal from the Movement Disorder Society task force, which addresses earlier stages in Huntington’s disease but doesn’t consider objective biomarker data.
Question of timing
Commenting on the findings, Erin Furr-Stimming, MD, neurologist and director of the Huntington’s Disease Society of America Center of Excellence with McGovern Medical School, UTHealth, Houston, said targeting early-stage disease will be key.
“Similar to more common neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, there is a period of at least a decade when changes are occurring in the nervous system, prior to the manifestation of clinical symptoms and signs significant enough to warrant a clinical diagnosis,” Dr. Furr-Stimming said.
She noted that multiple trials of disease-modifying agents for Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease have failed for a multitude of reasons, “but one consistent question that is relevant to all these diseases is that of timing: Should we intervene and test these therapies earlier?
“The premanifest or prodromal period may be the ideal time to intervene with a disease-modifying therapy, prior to onset of any neurodegeneration,” Dr. Furr-Stimming said.
The CHDI Foundation provided financial support to the Critical Path Institute for the Huntington’s Disease Regulatory Science Consortium, including all working group efforts. Dr. Sampio is an employee of and receives salary from CHDI Management. She has also received consultancy honorariums (unrelated to HD) from Pfizer, Kyowa Kirin, vTv Therapeutics, GW Pharmaceuticals, Neuraly, Neuroderm, Green Valley Pharmaceuticals, and Pinteon Pharmaceuticals. A full list of disclosures for the other researchers is in the original article. Dr. Furr-Stimming reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET NEUROLOGY