User login
No more ‘escape hatch’: Post Roe, new worries about meds linked to birth defects
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
IBD: Frequent consumption of processed meat tied to increased mortality risk
Key clinical point: Patients with inflammatory bowel disease (IBD) who consumed processed meat more frequently had a higher mortality risk; however, no such association was observed for the consumption of other types of meat.
Major finding: The risk for all-cause mortality was higher in patients with IBD who consumed processed meat >4.0 vs. 0-0.9 times/week (hazard ratio 1.52; 95% CI 1.05-2.19; P trend for each 25 g increment = .075). However, the consumption of fish, unprocessed poultry, and unprocessed red meat had no significant association with mortality.
Study details: Findings are from a retrospective cohort analysis of 5763 patients with IBD from the UK Biobank cohort.
Disclosures: This study was funded by the National Natural Science Foundation of China. X Wang declared receiving research grants. The other authors declared no conflicts of interest.
Source: Chen H, Fu T. Dan L, et al. Meat consumption and all-cause mortality in 5763 patients with inflammatory bowel disease: A retrospective cohort study. EClinicalMedicine. 2022;47:101406 (Apr 21). Doi: 10.1016/j.eclinm.2022.101406
Key clinical point: Patients with inflammatory bowel disease (IBD) who consumed processed meat more frequently had a higher mortality risk; however, no such association was observed for the consumption of other types of meat.
Major finding: The risk for all-cause mortality was higher in patients with IBD who consumed processed meat >4.0 vs. 0-0.9 times/week (hazard ratio 1.52; 95% CI 1.05-2.19; P trend for each 25 g increment = .075). However, the consumption of fish, unprocessed poultry, and unprocessed red meat had no significant association with mortality.
Study details: Findings are from a retrospective cohort analysis of 5763 patients with IBD from the UK Biobank cohort.
Disclosures: This study was funded by the National Natural Science Foundation of China. X Wang declared receiving research grants. The other authors declared no conflicts of interest.
Source: Chen H, Fu T. Dan L, et al. Meat consumption and all-cause mortality in 5763 patients with inflammatory bowel disease: A retrospective cohort study. EClinicalMedicine. 2022;47:101406 (Apr 21). Doi: 10.1016/j.eclinm.2022.101406
Key clinical point: Patients with inflammatory bowel disease (IBD) who consumed processed meat more frequently had a higher mortality risk; however, no such association was observed for the consumption of other types of meat.
Major finding: The risk for all-cause mortality was higher in patients with IBD who consumed processed meat >4.0 vs. 0-0.9 times/week (hazard ratio 1.52; 95% CI 1.05-2.19; P trend for each 25 g increment = .075). However, the consumption of fish, unprocessed poultry, and unprocessed red meat had no significant association with mortality.
Study details: Findings are from a retrospective cohort analysis of 5763 patients with IBD from the UK Biobank cohort.
Disclosures: This study was funded by the National Natural Science Foundation of China. X Wang declared receiving research grants. The other authors declared no conflicts of interest.
Source: Chen H, Fu T. Dan L, et al. Meat consumption and all-cause mortality in 5763 patients with inflammatory bowel disease: A retrospective cohort study. EClinicalMedicine. 2022;47:101406 (Apr 21). Doi: 10.1016/j.eclinm.2022.101406
IBD: Intradermal HBV vaccination with topical imiquimod safe and effective
Key clinical point: Intradermal (ID) hepatitis B virus (HBV) vaccination with topical imiquimod pretreatment (ID-Imq) to the injection site was safe and provided superior seroprotection compared with intramuscular (IM) HBV vaccination with aqueous cream pretreatment (IM-Aq) in patients with inflammatory bowel disease (IBD).
Major finding: Patients who received ID-Imq vs. IM-Aq showed a significantly higher seroprotection rate at 12 months (91% vs. 69%; P = .005). Overall, 59% and 55% of patients in the ID-Imq and IM-Aq groups reported any adverse events, respectively, with local reactions being more common in the ID-Imq group.
Study details: Findings are from a phase 2 and 3 trial including 104 patients with IBD and no evidence of HBV infection or immunity who were randomly assigned to receive ID-Imq (n = 53) or IM-Aq (n = 51) at 0, 1, and 6 months.
Disclosures: This study was partly funded by a Young Investigator Research Grant from the Hong Kong College of Physicians. WK Leung reported serving as a speaker for Ferring, Janssen, and Takeda.
Source: Ko K-L et al. Clinical trial: Intra-dermal hepatitis B vaccination with topical imiquimod versus intra-muscular hepatitis B vaccination in inflammatory bowel disease patients. Aliment Pharmacol Ther. 2022 (May 11). Doi: 10.1111/apt.16970
Key clinical point: Intradermal (ID) hepatitis B virus (HBV) vaccination with topical imiquimod pretreatment (ID-Imq) to the injection site was safe and provided superior seroprotection compared with intramuscular (IM) HBV vaccination with aqueous cream pretreatment (IM-Aq) in patients with inflammatory bowel disease (IBD).
Major finding: Patients who received ID-Imq vs. IM-Aq showed a significantly higher seroprotection rate at 12 months (91% vs. 69%; P = .005). Overall, 59% and 55% of patients in the ID-Imq and IM-Aq groups reported any adverse events, respectively, with local reactions being more common in the ID-Imq group.
Study details: Findings are from a phase 2 and 3 trial including 104 patients with IBD and no evidence of HBV infection or immunity who were randomly assigned to receive ID-Imq (n = 53) or IM-Aq (n = 51) at 0, 1, and 6 months.
Disclosures: This study was partly funded by a Young Investigator Research Grant from the Hong Kong College of Physicians. WK Leung reported serving as a speaker for Ferring, Janssen, and Takeda.
Source: Ko K-L et al. Clinical trial: Intra-dermal hepatitis B vaccination with topical imiquimod versus intra-muscular hepatitis B vaccination in inflammatory bowel disease patients. Aliment Pharmacol Ther. 2022 (May 11). Doi: 10.1111/apt.16970
Key clinical point: Intradermal (ID) hepatitis B virus (HBV) vaccination with topical imiquimod pretreatment (ID-Imq) to the injection site was safe and provided superior seroprotection compared with intramuscular (IM) HBV vaccination with aqueous cream pretreatment (IM-Aq) in patients with inflammatory bowel disease (IBD).
Major finding: Patients who received ID-Imq vs. IM-Aq showed a significantly higher seroprotection rate at 12 months (91% vs. 69%; P = .005). Overall, 59% and 55% of patients in the ID-Imq and IM-Aq groups reported any adverse events, respectively, with local reactions being more common in the ID-Imq group.
Study details: Findings are from a phase 2 and 3 trial including 104 patients with IBD and no evidence of HBV infection or immunity who were randomly assigned to receive ID-Imq (n = 53) or IM-Aq (n = 51) at 0, 1, and 6 months.
Disclosures: This study was partly funded by a Young Investigator Research Grant from the Hong Kong College of Physicians. WK Leung reported serving as a speaker for Ferring, Janssen, and Takeda.
Source: Ko K-L et al. Clinical trial: Intra-dermal hepatitis B vaccination with topical imiquimod versus intra-muscular hepatitis B vaccination in inflammatory bowel disease patients. Aliment Pharmacol Ther. 2022 (May 11). Doi: 10.1111/apt.16970
Steroid excess associated with adverse clinical outcomes in inflammatory bowel disease
Key clinical point: Excess exposure to steroids has a significant negative impact on patients with inflammatory bowel disease (IBD).
Major finding: Steroid excess was observed in 15% of patients. The risks for ≥1 hospitalizations for IBD (odds ratio [OR] 12.33; 95% CI 8.89-17.11) and infections (OR 2.89; 95% CI 1.82-4.61) and ≥1 courses of general practitioner-prescribed antibiotics (OR 1.41; 95% CI 1.07-1.86) were significantly higher in patients with steroid excess vs. those without steroid exposure.
Study details: Findings are from a retrospective study including 2246 patients with IBD and primary care information.
Disclosures: This study was supported by a research grant from AbbVie. T Raine and CP Selinger declared receiving research/educational grants or speaker/consultation fees from various sources, including AbbVie.
Source: Rosiou K et al. Sources of excess steroid prescriptions and clinical adverse outcomes associated with steroid excess in patients with inflammatory bowel disease: The Leeds IBD Steroids study. Aliment Pharmacol Ther. 2022 (May 24). Doi: 10.1111/apt.17039
Key clinical point: Excess exposure to steroids has a significant negative impact on patients with inflammatory bowel disease (IBD).
Major finding: Steroid excess was observed in 15% of patients. The risks for ≥1 hospitalizations for IBD (odds ratio [OR] 12.33; 95% CI 8.89-17.11) and infections (OR 2.89; 95% CI 1.82-4.61) and ≥1 courses of general practitioner-prescribed antibiotics (OR 1.41; 95% CI 1.07-1.86) were significantly higher in patients with steroid excess vs. those without steroid exposure.
Study details: Findings are from a retrospective study including 2246 patients with IBD and primary care information.
Disclosures: This study was supported by a research grant from AbbVie. T Raine and CP Selinger declared receiving research/educational grants or speaker/consultation fees from various sources, including AbbVie.
Source: Rosiou K et al. Sources of excess steroid prescriptions and clinical adverse outcomes associated with steroid excess in patients with inflammatory bowel disease: The Leeds IBD Steroids study. Aliment Pharmacol Ther. 2022 (May 24). Doi: 10.1111/apt.17039
Key clinical point: Excess exposure to steroids has a significant negative impact on patients with inflammatory bowel disease (IBD).
Major finding: Steroid excess was observed in 15% of patients. The risks for ≥1 hospitalizations for IBD (odds ratio [OR] 12.33; 95% CI 8.89-17.11) and infections (OR 2.89; 95% CI 1.82-4.61) and ≥1 courses of general practitioner-prescribed antibiotics (OR 1.41; 95% CI 1.07-1.86) were significantly higher in patients with steroid excess vs. those without steroid exposure.
Study details: Findings are from a retrospective study including 2246 patients with IBD and primary care information.
Disclosures: This study was supported by a research grant from AbbVie. T Raine and CP Selinger declared receiving research/educational grants or speaker/consultation fees from various sources, including AbbVie.
Source: Rosiou K et al. Sources of excess steroid prescriptions and clinical adverse outcomes associated with steroid excess in patients with inflammatory bowel disease: The Leeds IBD Steroids study. Aliment Pharmacol Ther. 2022 (May 24). Doi: 10.1111/apt.17039
Sugar highs and royal meltdowns
I can dimly recall watching Queen Elizabeth’s coronation on a very small black and white television screen. Even in monochrome it was a riveting event. Recently, the Queen celebrated her Platinum Jubilee, marking her 70-year reign. Apparently it was a multiday event with all the trappings, floating above an undercurrent of scandal and intrigue. I had better things to do than I did as a 7-year-old entranced by the novelty of a neighbor’s television set.
But, it turns out that I had missed the opportunity to see live and in color a royal meltdown starring the Queen’s great-grandson, 4-year-old Prince Louis. Not to worry. It remains on video archives for our education and pleasure ad infinitum. His performance was no more dramatic than what you have seen numerous times in the checkout line of the grocery store. However, this meltdown was on the world stage in the front row of the royal box and performed in various venues on each day of a 4-day event.
As long as you weren’t his parents, Kate Middleton and Prince William, the meltdown had its moments of hilarity. Louis made full use of his youthful and plastic face, creating a wide variety of taunts and responses to his mother’s praiseworthy and understated attempts at regaining control. Of course, the British press and every armchair parent with a Twitter account had a field day contributing their explanations and advice.
For example, here’s the headline on an international news website that caught my eye: “Royal reveals why Prince Louis was so ‘mischievous’ during the Jubilee”. In the article, a fellow royal and former rugby star who was sitting directly behind the little Prince during one of his performances chalked up the 4-year-old’s behavior to a “sugar high” resulting from the ample supply of sweets available behind the royal box.
Nowhere in the article is there a question of whether the “sugar high” is a science-based phenomenon. In fact, the reporter assumes we all know it exists and writes that “parents across the globe can probably [read: definitely] relate.”
I’m curious: How do you respond when a parent in the office explains the child’s behavior as the result of a “sugar high”? Or when you’re at a cookout and someone makes a comment that makes it obvious that they believe that “sugar highs” are real? Do you immediately pause the conversation and launch into a short but tasteful observation that you know of no scientific studies that sugar can cause a high? Or, figuring that in the face of an overwhelming burden of old wives’ tales it’s not worth mounting a rebuttal, do you pretend you didn’t hear the comment?
Or am I completely off base because your experience has left you convinced that despite the lack of supporting studies the “sugar high” phenomenon exists? Maybe you even include it on your list of explanations and remedies for pediatric misbehaviors. I am ready to listen, but it will take some heavy lifting to convince me.
I suspect your response to offhand comments about “sugar highs” is similar to mine. It depends on the situation. If I think there are obvious and correctable causes for the child’s misbehavior such as sleep deprivation or a mismatch between parental expectation and the child’s tolerance for a stimulating environment I will include in my parenting advice the comment, “Sugar highs probably don’t exist.”
On the other hand, if I’m tired and think my observation will fall on deaf ears I let the conversation drift. I worry that my silence will be interpreted as a confirmation of an old wives’ tale. What I really don’t want to do is perpetuate a myth that may prevent some children from getting the care they need.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I can dimly recall watching Queen Elizabeth’s coronation on a very small black and white television screen. Even in monochrome it was a riveting event. Recently, the Queen celebrated her Platinum Jubilee, marking her 70-year reign. Apparently it was a multiday event with all the trappings, floating above an undercurrent of scandal and intrigue. I had better things to do than I did as a 7-year-old entranced by the novelty of a neighbor’s television set.
But, it turns out that I had missed the opportunity to see live and in color a royal meltdown starring the Queen’s great-grandson, 4-year-old Prince Louis. Not to worry. It remains on video archives for our education and pleasure ad infinitum. His performance was no more dramatic than what you have seen numerous times in the checkout line of the grocery store. However, this meltdown was on the world stage in the front row of the royal box and performed in various venues on each day of a 4-day event.
As long as you weren’t his parents, Kate Middleton and Prince William, the meltdown had its moments of hilarity. Louis made full use of his youthful and plastic face, creating a wide variety of taunts and responses to his mother’s praiseworthy and understated attempts at regaining control. Of course, the British press and every armchair parent with a Twitter account had a field day contributing their explanations and advice.
For example, here’s the headline on an international news website that caught my eye: “Royal reveals why Prince Louis was so ‘mischievous’ during the Jubilee”. In the article, a fellow royal and former rugby star who was sitting directly behind the little Prince during one of his performances chalked up the 4-year-old’s behavior to a “sugar high” resulting from the ample supply of sweets available behind the royal box.
Nowhere in the article is there a question of whether the “sugar high” is a science-based phenomenon. In fact, the reporter assumes we all know it exists and writes that “parents across the globe can probably [read: definitely] relate.”
I’m curious: How do you respond when a parent in the office explains the child’s behavior as the result of a “sugar high”? Or when you’re at a cookout and someone makes a comment that makes it obvious that they believe that “sugar highs” are real? Do you immediately pause the conversation and launch into a short but tasteful observation that you know of no scientific studies that sugar can cause a high? Or, figuring that in the face of an overwhelming burden of old wives’ tales it’s not worth mounting a rebuttal, do you pretend you didn’t hear the comment?
Or am I completely off base because your experience has left you convinced that despite the lack of supporting studies the “sugar high” phenomenon exists? Maybe you even include it on your list of explanations and remedies for pediatric misbehaviors. I am ready to listen, but it will take some heavy lifting to convince me.
I suspect your response to offhand comments about “sugar highs” is similar to mine. It depends on the situation. If I think there are obvious and correctable causes for the child’s misbehavior such as sleep deprivation or a mismatch between parental expectation and the child’s tolerance for a stimulating environment I will include in my parenting advice the comment, “Sugar highs probably don’t exist.”
On the other hand, if I’m tired and think my observation will fall on deaf ears I let the conversation drift. I worry that my silence will be interpreted as a confirmation of an old wives’ tale. What I really don’t want to do is perpetuate a myth that may prevent some children from getting the care they need.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I can dimly recall watching Queen Elizabeth’s coronation on a very small black and white television screen. Even in monochrome it was a riveting event. Recently, the Queen celebrated her Platinum Jubilee, marking her 70-year reign. Apparently it was a multiday event with all the trappings, floating above an undercurrent of scandal and intrigue. I had better things to do than I did as a 7-year-old entranced by the novelty of a neighbor’s television set.
But, it turns out that I had missed the opportunity to see live and in color a royal meltdown starring the Queen’s great-grandson, 4-year-old Prince Louis. Not to worry. It remains on video archives for our education and pleasure ad infinitum. His performance was no more dramatic than what you have seen numerous times in the checkout line of the grocery store. However, this meltdown was on the world stage in the front row of the royal box and performed in various venues on each day of a 4-day event.
As long as you weren’t his parents, Kate Middleton and Prince William, the meltdown had its moments of hilarity. Louis made full use of his youthful and plastic face, creating a wide variety of taunts and responses to his mother’s praiseworthy and understated attempts at regaining control. Of course, the British press and every armchair parent with a Twitter account had a field day contributing their explanations and advice.
For example, here’s the headline on an international news website that caught my eye: “Royal reveals why Prince Louis was so ‘mischievous’ during the Jubilee”. In the article, a fellow royal and former rugby star who was sitting directly behind the little Prince during one of his performances chalked up the 4-year-old’s behavior to a “sugar high” resulting from the ample supply of sweets available behind the royal box.
Nowhere in the article is there a question of whether the “sugar high” is a science-based phenomenon. In fact, the reporter assumes we all know it exists and writes that “parents across the globe can probably [read: definitely] relate.”
I’m curious: How do you respond when a parent in the office explains the child’s behavior as the result of a “sugar high”? Or when you’re at a cookout and someone makes a comment that makes it obvious that they believe that “sugar highs” are real? Do you immediately pause the conversation and launch into a short but tasteful observation that you know of no scientific studies that sugar can cause a high? Or, figuring that in the face of an overwhelming burden of old wives’ tales it’s not worth mounting a rebuttal, do you pretend you didn’t hear the comment?
Or am I completely off base because your experience has left you convinced that despite the lack of supporting studies the “sugar high” phenomenon exists? Maybe you even include it on your list of explanations and remedies for pediatric misbehaviors. I am ready to listen, but it will take some heavy lifting to convince me.
I suspect your response to offhand comments about “sugar highs” is similar to mine. It depends on the situation. If I think there are obvious and correctable causes for the child’s misbehavior such as sleep deprivation or a mismatch between parental expectation and the child’s tolerance for a stimulating environment I will include in my parenting advice the comment, “Sugar highs probably don’t exist.”
On the other hand, if I’m tired and think my observation will fall on deaf ears I let the conversation drift. I worry that my silence will be interpreted as a confirmation of an old wives’ tale. What I really don’t want to do is perpetuate a myth that may prevent some children from getting the care they need.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Drug-specific alteration of lipid levels in inflammatory bowel disease
Key clinical point: Induction therapy with tofacitinib or prednisone significantly increased serum lipid levels in patients with inflammatory bowel disease (IBD), whereas no changes were observed with immunomodulators or biologics.
Major finding: At 10 weeks, the total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels increased significantly in patients who initiated tofacitinib (+20%, +25%, and +26%, respectively; all P < .05) or prednisone (+26%, +31%, and +12%, respectively; all P < .05) therapy. The changes in lipid levels were not significant with other biologics or immunomodulators.
Study details: This was a prospective study that included 198 patients with ulcerative colitis (n = 61), Crohn’s disease (n = 137), or unclassified IBD (n = 8) who initiated prednisone, thiopurine, methotrexate, an anti-tumor necrosis factor alpha agent, ustekinumab, vedolizumab, or tofacitinib.
Disclosures: This study did not receive any funding. AC de Vries and CJ van der Woude declared receiving research funding and serving as advisory board members for various sources.
Source: Sleutjes JAM et al. Lipid changes after induction therapy in patients with inflammatory bowel disease: Effect of different drug classes and inflammation. Inflamm Bowel Dis. 2022 (May 19). Doi: 10.1093/ibd/izac100
Key clinical point: Induction therapy with tofacitinib or prednisone significantly increased serum lipid levels in patients with inflammatory bowel disease (IBD), whereas no changes were observed with immunomodulators or biologics.
Major finding: At 10 weeks, the total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels increased significantly in patients who initiated tofacitinib (+20%, +25%, and +26%, respectively; all P < .05) or prednisone (+26%, +31%, and +12%, respectively; all P < .05) therapy. The changes in lipid levels were not significant with other biologics or immunomodulators.
Study details: This was a prospective study that included 198 patients with ulcerative colitis (n = 61), Crohn’s disease (n = 137), or unclassified IBD (n = 8) who initiated prednisone, thiopurine, methotrexate, an anti-tumor necrosis factor alpha agent, ustekinumab, vedolizumab, or tofacitinib.
Disclosures: This study did not receive any funding. AC de Vries and CJ van der Woude declared receiving research funding and serving as advisory board members for various sources.
Source: Sleutjes JAM et al. Lipid changes after induction therapy in patients with inflammatory bowel disease: Effect of different drug classes and inflammation. Inflamm Bowel Dis. 2022 (May 19). Doi: 10.1093/ibd/izac100
Key clinical point: Induction therapy with tofacitinib or prednisone significantly increased serum lipid levels in patients with inflammatory bowel disease (IBD), whereas no changes were observed with immunomodulators or biologics.
Major finding: At 10 weeks, the total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels increased significantly in patients who initiated tofacitinib (+20%, +25%, and +26%, respectively; all P < .05) or prednisone (+26%, +31%, and +12%, respectively; all P < .05) therapy. The changes in lipid levels were not significant with other biologics or immunomodulators.
Study details: This was a prospective study that included 198 patients with ulcerative colitis (n = 61), Crohn’s disease (n = 137), or unclassified IBD (n = 8) who initiated prednisone, thiopurine, methotrexate, an anti-tumor necrosis factor alpha agent, ustekinumab, vedolizumab, or tofacitinib.
Disclosures: This study did not receive any funding. AC de Vries and CJ van der Woude declared receiving research funding and serving as advisory board members for various sources.
Source: Sleutjes JAM et al. Lipid changes after induction therapy in patients with inflammatory bowel disease: Effect of different drug classes and inflammation. Inflamm Bowel Dis. 2022 (May 19). Doi: 10.1093/ibd/izac100
Vedolizumab: A valid option for elderly patients with inflammatory bowel disease
Key clinical point: Vedolizumab can be considered a safe and valid option for elderly patients with inflammatory bowel disease, with efficacy being unaffected by age in Crohn’s disease (CD), but slightly reduced in elderly vs. nonelderly patients with ulcerative colitis (UC).
Major finding: Nonelderly vs. elderly patients with UC showed significantly higher clinical remission at 24 months (47.3% vs. 34.3%; P < .05) and persistence (67.6% vs. 51.4%; P = .02), both of which were not significantly different between elderly and nonelderly patients with CD. Adverse events were comparable among elderly and nonelderly patients.
Study details: This was a retrospective-prospective study that included 198 elderly patients aged ≥ 65 years with UC (n = 108) or CD (n = 90) and 396 matched nonelderly patients with UC (n = 205) or CD (n = 191), all of whom received vedolizumab.
Disclosures: This study was funded by Takeda. Some authors reported receiving consulting, lecture, or speakers’ fees, or serving as advisory board members for various sources, including Takeda.
Source: Pugliese D et al. Effectiveness and safety of vedolizumab in a matched cohort of elderly and nonelderly patients with inflammatory bowel disease: The IG-IBD LIVE study. Aliment Pharmacol Ther. 2022;56(1):95-109 (May 12). Doi: 10.1111/apt.16923
Key clinical point: Vedolizumab can be considered a safe and valid option for elderly patients with inflammatory bowel disease, with efficacy being unaffected by age in Crohn’s disease (CD), but slightly reduced in elderly vs. nonelderly patients with ulcerative colitis (UC).
Major finding: Nonelderly vs. elderly patients with UC showed significantly higher clinical remission at 24 months (47.3% vs. 34.3%; P < .05) and persistence (67.6% vs. 51.4%; P = .02), both of which were not significantly different between elderly and nonelderly patients with CD. Adverse events were comparable among elderly and nonelderly patients.
Study details: This was a retrospective-prospective study that included 198 elderly patients aged ≥ 65 years with UC (n = 108) or CD (n = 90) and 396 matched nonelderly patients with UC (n = 205) or CD (n = 191), all of whom received vedolizumab.
Disclosures: This study was funded by Takeda. Some authors reported receiving consulting, lecture, or speakers’ fees, or serving as advisory board members for various sources, including Takeda.
Source: Pugliese D et al. Effectiveness and safety of vedolizumab in a matched cohort of elderly and nonelderly patients with inflammatory bowel disease: The IG-IBD LIVE study. Aliment Pharmacol Ther. 2022;56(1):95-109 (May 12). Doi: 10.1111/apt.16923
Key clinical point: Vedolizumab can be considered a safe and valid option for elderly patients with inflammatory bowel disease, with efficacy being unaffected by age in Crohn’s disease (CD), but slightly reduced in elderly vs. nonelderly patients with ulcerative colitis (UC).
Major finding: Nonelderly vs. elderly patients with UC showed significantly higher clinical remission at 24 months (47.3% vs. 34.3%; P < .05) and persistence (67.6% vs. 51.4%; P = .02), both of which were not significantly different between elderly and nonelderly patients with CD. Adverse events were comparable among elderly and nonelderly patients.
Study details: This was a retrospective-prospective study that included 198 elderly patients aged ≥ 65 years with UC (n = 108) or CD (n = 90) and 396 matched nonelderly patients with UC (n = 205) or CD (n = 191), all of whom received vedolizumab.
Disclosures: This study was funded by Takeda. Some authors reported receiving consulting, lecture, or speakers’ fees, or serving as advisory board members for various sources, including Takeda.
Source: Pugliese D et al. Effectiveness and safety of vedolizumab in a matched cohort of elderly and nonelderly patients with inflammatory bowel disease: The IG-IBD LIVE study. Aliment Pharmacol Ther. 2022;56(1):95-109 (May 12). Doi: 10.1111/apt.16923
Crohn’s disease: Early postoperative biological therapy reduces disease recurrence and improves outcomes
Key clinical point: Early treatment with postoperative biological therapy decreased endoscopic recurrence rates and improved long-term outcomes in patients who underwent Crohn’s disease (CD)-related surgery.
Major finding: The rate of endoscopic recurrence was higher in patients not treated vs. treated with early postoperative biological therapy (80.8% vs. 45.2%; P < .000024), with the risk of experiencing hospitalization or surgery at 5 years being 23.3% higher (P = .02221) and the rate of medical therapy escalation being significantly higher (66.0% vs. 14.0%; P < .00001) in the no-treatment vs. treatment group.
Study details: Findings are from a retrospective cohort study including 141 patients with CD who underwent surgery and colonoscopy at 6-12 months postoperatively.
Disclosures: This study did not receive any funding. Some authors declared receiving consulting fees, lecture fees, speaker’s fees, grants, or serving as advisory board members for various sources.
Source: D'Amico F et al. Early biological therapy in operated Crohn’s disease patients is associated with a lower rate of endoscopic recurrence and improved long-term outcomes: A single-center experience. Inflamm Bowel Dis. 2022 (May 28). Doi: 10.1093/ibd/izac110
Key clinical point: Early treatment with postoperative biological therapy decreased endoscopic recurrence rates and improved long-term outcomes in patients who underwent Crohn’s disease (CD)-related surgery.
Major finding: The rate of endoscopic recurrence was higher in patients not treated vs. treated with early postoperative biological therapy (80.8% vs. 45.2%; P < .000024), with the risk of experiencing hospitalization or surgery at 5 years being 23.3% higher (P = .02221) and the rate of medical therapy escalation being significantly higher (66.0% vs. 14.0%; P < .00001) in the no-treatment vs. treatment group.
Study details: Findings are from a retrospective cohort study including 141 patients with CD who underwent surgery and colonoscopy at 6-12 months postoperatively.
Disclosures: This study did not receive any funding. Some authors declared receiving consulting fees, lecture fees, speaker’s fees, grants, or serving as advisory board members for various sources.
Source: D'Amico F et al. Early biological therapy in operated Crohn’s disease patients is associated with a lower rate of endoscopic recurrence and improved long-term outcomes: A single-center experience. Inflamm Bowel Dis. 2022 (May 28). Doi: 10.1093/ibd/izac110
Key clinical point: Early treatment with postoperative biological therapy decreased endoscopic recurrence rates and improved long-term outcomes in patients who underwent Crohn’s disease (CD)-related surgery.
Major finding: The rate of endoscopic recurrence was higher in patients not treated vs. treated with early postoperative biological therapy (80.8% vs. 45.2%; P < .000024), with the risk of experiencing hospitalization or surgery at 5 years being 23.3% higher (P = .02221) and the rate of medical therapy escalation being significantly higher (66.0% vs. 14.0%; P < .00001) in the no-treatment vs. treatment group.
Study details: Findings are from a retrospective cohort study including 141 patients with CD who underwent surgery and colonoscopy at 6-12 months postoperatively.
Disclosures: This study did not receive any funding. Some authors declared receiving consulting fees, lecture fees, speaker’s fees, grants, or serving as advisory board members for various sources.
Source: D'Amico F et al. Early biological therapy in operated Crohn’s disease patients is associated with a lower rate of endoscopic recurrence and improved long-term outcomes: A single-center experience. Inflamm Bowel Dis. 2022 (May 28). Doi: 10.1093/ibd/izac110
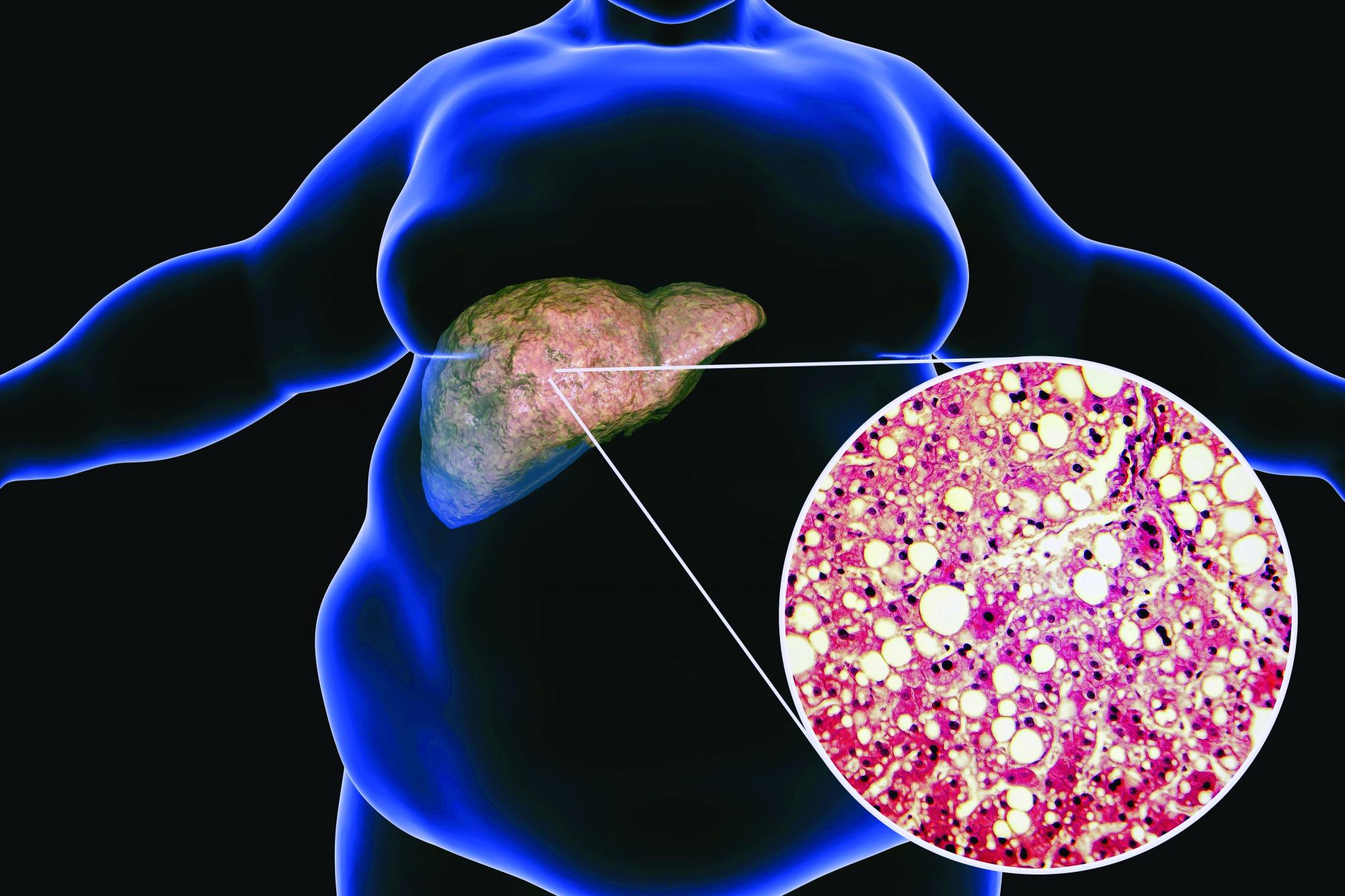
Low-carb, high-fat diet improves A1c, reduces liver fat
LONDON – A low-carbohydrate, high-fat (LCHF) diet reduced the progression of nonalcoholic fatty liver disease (NAFLD), and despite no calorie restriction, participants with both NAFLD and type 2 diabetes lost 5.8% of their body weight, according to a randomized controlled study.
“Based on these results, the LCHF diet may be recommended to people with NAFLD and type 2 diabetes,” said Camilla Dalby Hansen, MD, department of gastroenterology and hepatology, Odense University Hospital, Denmark, who presented the data at the International Liver Congress (ILC) 2022.
“Basically, if you have fat in your liver, you will benefit from eating fat,” she said.
The LCHF diet was compared with a low-fat, high-carbohydrate diet more typically followed for these conditions. The low-fat diet was also found to reduce the progression of NAFLD, but to a lesser extent than the LCHF diet.
Dr. Dalby Hansen called their study one of the most extensive investigations of the LCHF diet in patients with type 2 diabetes and fatty liver disease.
“Combining this [reduction in NAFLD score] with the huge weight loss, the lower HbA1c [blood sugar], the lowering of blood pressure in women, the rise in HDL levels, and reduction in triglycerides – all in all, this diet is very promising,” she said.
Stephen Harrison, MD, visiting professor, University of Oxford, United Kingdom, medical director of Pinnacle Clinical Research and president of Summit Clinical Research, San Antonio, commended Dr. Dalby Hansen on her methodology, which included before-and-after liver biopsies. “It’s a heinous effort to do paired liver biopsies in a lifestyle modification trial. That’s huge.”
“This study tells me that the way we manage patients doesn’t change – it is still lifestyle modification,” said Dr. Harrison, who was not involved with the study. “It’s eat less [rather] than more. It’s exercise and try to lose weight. In the long term, we give patients benefit, and we show that the disease has improved, and we offer something that means they can maintain a healthy life.”
He added that the relatively small and short trial was informative.
“They improved the NAFLD activity score [NAS],” he said. “I don’t know by how much. There was no change in fibrosis, but we wouldn’t expect this at 6 months.”
“It’s provocative work, and it gives us healthy information about how we can help manage our patients from a lifestyle perspective,” he concluded.
‘Do not lose weight. Eat until you are full’
In the study, 110 participants with type 2 diabetes and NAFLD, aged 18-78 years, were allocated to the LCHF diet, and 55 were allocated to the low-fat diet for 6 months.
The researchers performed liver biopsies at baseline and 6 months, which were blinded for scoring.
Participants had ongoing dietitian consultations, with follow-up visits at 3 and 6 months. Compliance was reported continuously through an online food diary platform.
The primary endpoint was change in glycemic control as measured by A1c level over 6 months. The secondary endpoints comprised the proportion of participants with changes in the NAS of at least 2 points over 6 months. Both these measures were compared between the two dietary groups.
The two groups were matched at baseline, with a mean age of 55-57 years, 58% were women, 89% with metabolic syndrome, and a mean BMI 34 kg/m2.
In baseline liver disease, F1 level fibrosis was the most common (58%), followed by hepatic steatosis (S1, 47%; S2, 32%), with a median NAS of 3, and 19% had nonalcoholic steatohepatitis.
The special thing about these diets was that participants were told to “not lose weight, but eat until you are full,” remarked Dr. Dalby Hansen.
Those on the LCHF diet consumed an average of 61% energy from fat, 13% from carbohydrates, and 23% from protein, compared with the low-fat diet, which comprised an average of 29% energy from fat, 46% from carbohydrates, and 21% from protein.
“It’s a lot of fat and corresponds to a quarter of a liter of olive oil per day,” said Dr. Dalby Hansen. “They really had to change their mindset a lot, because it was difficult for them to start eating all these fats, especially since we’ve all been told for decades that it isn’t good. But we supported them, and they got into it.”
The LCHF diet was primarily comprised of unsaturated fats – for example, avocado, oil, nuts, and seeds – but also included saturated fats, such as cheese, cream, and high-fat dairy products. Participants were free to eat unsaturated and saturated fats, but Dr. Dalby Hansen and her team advised participants that “good” unsaturated fats were preferable.
“Also, this diet contained vegetables but no bread, no potatoes, no rice, and no pasta. It was low in carbohydrates, below 20%,” she added.
Improved glycemic control, reduced liver fat
“We found that the LCHF diet improved diabetes control, it reduced the fat in the liver, and, even though they’re eating as many calories as they were used to until they were full, they lost 5.8% of body weight,” said Dr. Dalby Hansen in reporting the results. Participants in the low-fat group lost only 1.8% of body weight.
However, mean calorie intake dropped in both groups, by –2.2% in the LCHF group and –8.7% in the low-fat group.
“The LCHF diet improved the primary outcome of A1c by 9.5 mmol/mol, which is similar to some anti-diabetic medications, such as DPP-4 inhibitors and SGLT2 inhibitors,” she said.
The low-fat group reduced A1c by 3.4 mmol/mol, resulting in a between-group difference of 6.1 mmol/mol.
“Upon follow-up of 3 months, after stopping the diets, on average the participants in both groups returned their HbA1c levels to nearly baseline values,” she said. Results were adjusted for weight loss and baseline values.
Both diets also improved the NAS. The proportion of participants who improved their NAS score by 2 or more points was 22% in the LCHF group versus 17% in the low-fat group (P = 0.58). Additionally, in the LCHF group, 70% of participants improved their score by 1 or more points, compared with 49% in the low-fat group and fewer in the LCHF group experienced a worsening of their score (1% vs. 23%, respectively).
One participant on LCHF had high triglycerides of 12 mmol/L after 3 months. Overall, the low-density lipoprotein increased marginally by 0.2 mmol per liter in the high-fat group, said Dr. Dalby Hansen.
Dr. Dalby Hansen noted some limitations. The findings might not be applicable in more severe NAFLD, dietary assessment relied on self-reporting, no food was provided, and participants had to cook themselves. It was also an open-label study because of the nature of the intervention.
Some hope for more sustainable dieting
Many diets are difficult to adhere to, remarked Dr. Dalby Hansen. “We thought this [diet] might be easier to comply with in the longer term, and we hope that these results might provide patients with more options.”
She added that most people who started the diet adapted and complied with it. “However, it might not be for everyone, but I think we can say that if people try, and it fits into their lives, then they go for it.”
However, “it is not about going out and eating whatever fat and how much of it you want. It’s important that you cut the carbohydrates too,” she said. “With this approach, we really saw amazing results.”
Dr. Dalby Hansen added that having various diets available, including the LCHF one, meant that as clinicians they could empower patients to take control of their metabolic health.
“We can ask them directly, ‘What would fit into their life?’” she said. “We know that one size does not fit at all, and I believe that if we could engage patients more, then they can take control of their own situation.”
Asked whether these findings were enough to change guidelines, Zobair Younossi, MD, professor and chairman, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., remarked that it was the sugar at work here.
“Dietary fat – it’s not the same as fat in the liver, and this diet has more to do with the sugar levels,” he said.
“I’m always reluctant to take results from a short-term study without long-term follow-up,” Dr. Younossi said. “I want to know will patients live longer, and long-term data are needed for this. Until I have that strong evidence that outcomes are going to change, or at least some sign that the outcome is going to change, it is too early to change any guidelines.”
Dr. Dalby Hansen reports no relevant financial relationships. Dr. Harrison reported financial relationships with numerous pharmaceutical companies. Dr. Younossi reports the following financial relationships: research funds and/or consultant to Abbott, Allergan, Bristol Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
LONDON – A low-carbohydrate, high-fat (LCHF) diet reduced the progression of nonalcoholic fatty liver disease (NAFLD), and despite no calorie restriction, participants with both NAFLD and type 2 diabetes lost 5.8% of their body weight, according to a randomized controlled study.
“Based on these results, the LCHF diet may be recommended to people with NAFLD and type 2 diabetes,” said Camilla Dalby Hansen, MD, department of gastroenterology and hepatology, Odense University Hospital, Denmark, who presented the data at the International Liver Congress (ILC) 2022.
“Basically, if you have fat in your liver, you will benefit from eating fat,” she said.
The LCHF diet was compared with a low-fat, high-carbohydrate diet more typically followed for these conditions. The low-fat diet was also found to reduce the progression of NAFLD, but to a lesser extent than the LCHF diet.
Dr. Dalby Hansen called their study one of the most extensive investigations of the LCHF diet in patients with type 2 diabetes and fatty liver disease.
“Combining this [reduction in NAFLD score] with the huge weight loss, the lower HbA1c [blood sugar], the lowering of blood pressure in women, the rise in HDL levels, and reduction in triglycerides – all in all, this diet is very promising,” she said.
Stephen Harrison, MD, visiting professor, University of Oxford, United Kingdom, medical director of Pinnacle Clinical Research and president of Summit Clinical Research, San Antonio, commended Dr. Dalby Hansen on her methodology, which included before-and-after liver biopsies. “It’s a heinous effort to do paired liver biopsies in a lifestyle modification trial. That’s huge.”
“This study tells me that the way we manage patients doesn’t change – it is still lifestyle modification,” said Dr. Harrison, who was not involved with the study. “It’s eat less [rather] than more. It’s exercise and try to lose weight. In the long term, we give patients benefit, and we show that the disease has improved, and we offer something that means they can maintain a healthy life.”
He added that the relatively small and short trial was informative.
“They improved the NAFLD activity score [NAS],” he said. “I don’t know by how much. There was no change in fibrosis, but we wouldn’t expect this at 6 months.”
“It’s provocative work, and it gives us healthy information about how we can help manage our patients from a lifestyle perspective,” he concluded.
‘Do not lose weight. Eat until you are full’
In the study, 110 participants with type 2 diabetes and NAFLD, aged 18-78 years, were allocated to the LCHF diet, and 55 were allocated to the low-fat diet for 6 months.
The researchers performed liver biopsies at baseline and 6 months, which were blinded for scoring.
Participants had ongoing dietitian consultations, with follow-up visits at 3 and 6 months. Compliance was reported continuously through an online food diary platform.
The primary endpoint was change in glycemic control as measured by A1c level over 6 months. The secondary endpoints comprised the proportion of participants with changes in the NAS of at least 2 points over 6 months. Both these measures were compared between the two dietary groups.
The two groups were matched at baseline, with a mean age of 55-57 years, 58% were women, 89% with metabolic syndrome, and a mean BMI 34 kg/m2.
In baseline liver disease, F1 level fibrosis was the most common (58%), followed by hepatic steatosis (S1, 47%; S2, 32%), with a median NAS of 3, and 19% had nonalcoholic steatohepatitis.
The special thing about these diets was that participants were told to “not lose weight, but eat until you are full,” remarked Dr. Dalby Hansen.
Those on the LCHF diet consumed an average of 61% energy from fat, 13% from carbohydrates, and 23% from protein, compared with the low-fat diet, which comprised an average of 29% energy from fat, 46% from carbohydrates, and 21% from protein.
“It’s a lot of fat and corresponds to a quarter of a liter of olive oil per day,” said Dr. Dalby Hansen. “They really had to change their mindset a lot, because it was difficult for them to start eating all these fats, especially since we’ve all been told for decades that it isn’t good. But we supported them, and they got into it.”
The LCHF diet was primarily comprised of unsaturated fats – for example, avocado, oil, nuts, and seeds – but also included saturated fats, such as cheese, cream, and high-fat dairy products. Participants were free to eat unsaturated and saturated fats, but Dr. Dalby Hansen and her team advised participants that “good” unsaturated fats were preferable.
“Also, this diet contained vegetables but no bread, no potatoes, no rice, and no pasta. It was low in carbohydrates, below 20%,” she added.
Improved glycemic control, reduced liver fat
“We found that the LCHF diet improved diabetes control, it reduced the fat in the liver, and, even though they’re eating as many calories as they were used to until they were full, they lost 5.8% of body weight,” said Dr. Dalby Hansen in reporting the results. Participants in the low-fat group lost only 1.8% of body weight.
However, mean calorie intake dropped in both groups, by –2.2% in the LCHF group and –8.7% in the low-fat group.
“The LCHF diet improved the primary outcome of A1c by 9.5 mmol/mol, which is similar to some anti-diabetic medications, such as DPP-4 inhibitors and SGLT2 inhibitors,” she said.
The low-fat group reduced A1c by 3.4 mmol/mol, resulting in a between-group difference of 6.1 mmol/mol.
“Upon follow-up of 3 months, after stopping the diets, on average the participants in both groups returned their HbA1c levels to nearly baseline values,” she said. Results were adjusted for weight loss and baseline values.
Both diets also improved the NAS. The proportion of participants who improved their NAS score by 2 or more points was 22% in the LCHF group versus 17% in the low-fat group (P = 0.58). Additionally, in the LCHF group, 70% of participants improved their score by 1 or more points, compared with 49% in the low-fat group and fewer in the LCHF group experienced a worsening of their score (1% vs. 23%, respectively).
One participant on LCHF had high triglycerides of 12 mmol/L after 3 months. Overall, the low-density lipoprotein increased marginally by 0.2 mmol per liter in the high-fat group, said Dr. Dalby Hansen.
Dr. Dalby Hansen noted some limitations. The findings might not be applicable in more severe NAFLD, dietary assessment relied on self-reporting, no food was provided, and participants had to cook themselves. It was also an open-label study because of the nature of the intervention.
Some hope for more sustainable dieting
Many diets are difficult to adhere to, remarked Dr. Dalby Hansen. “We thought this [diet] might be easier to comply with in the longer term, and we hope that these results might provide patients with more options.”
She added that most people who started the diet adapted and complied with it. “However, it might not be for everyone, but I think we can say that if people try, and it fits into their lives, then they go for it.”
However, “it is not about going out and eating whatever fat and how much of it you want. It’s important that you cut the carbohydrates too,” she said. “With this approach, we really saw amazing results.”
Dr. Dalby Hansen added that having various diets available, including the LCHF one, meant that as clinicians they could empower patients to take control of their metabolic health.
“We can ask them directly, ‘What would fit into their life?’” she said. “We know that one size does not fit at all, and I believe that if we could engage patients more, then they can take control of their own situation.”
Asked whether these findings were enough to change guidelines, Zobair Younossi, MD, professor and chairman, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., remarked that it was the sugar at work here.
“Dietary fat – it’s not the same as fat in the liver, and this diet has more to do with the sugar levels,” he said.
“I’m always reluctant to take results from a short-term study without long-term follow-up,” Dr. Younossi said. “I want to know will patients live longer, and long-term data are needed for this. Until I have that strong evidence that outcomes are going to change, or at least some sign that the outcome is going to change, it is too early to change any guidelines.”
Dr. Dalby Hansen reports no relevant financial relationships. Dr. Harrison reported financial relationships with numerous pharmaceutical companies. Dr. Younossi reports the following financial relationships: research funds and/or consultant to Abbott, Allergan, Bristol Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
LONDON – A low-carbohydrate, high-fat (LCHF) diet reduced the progression of nonalcoholic fatty liver disease (NAFLD), and despite no calorie restriction, participants with both NAFLD and type 2 diabetes lost 5.8% of their body weight, according to a randomized controlled study.
“Based on these results, the LCHF diet may be recommended to people with NAFLD and type 2 diabetes,” said Camilla Dalby Hansen, MD, department of gastroenterology and hepatology, Odense University Hospital, Denmark, who presented the data at the International Liver Congress (ILC) 2022.
“Basically, if you have fat in your liver, you will benefit from eating fat,” she said.
The LCHF diet was compared with a low-fat, high-carbohydrate diet more typically followed for these conditions. The low-fat diet was also found to reduce the progression of NAFLD, but to a lesser extent than the LCHF diet.
Dr. Dalby Hansen called their study one of the most extensive investigations of the LCHF diet in patients with type 2 diabetes and fatty liver disease.
“Combining this [reduction in NAFLD score] with the huge weight loss, the lower HbA1c [blood sugar], the lowering of blood pressure in women, the rise in HDL levels, and reduction in triglycerides – all in all, this diet is very promising,” she said.
Stephen Harrison, MD, visiting professor, University of Oxford, United Kingdom, medical director of Pinnacle Clinical Research and president of Summit Clinical Research, San Antonio, commended Dr. Dalby Hansen on her methodology, which included before-and-after liver biopsies. “It’s a heinous effort to do paired liver biopsies in a lifestyle modification trial. That’s huge.”
“This study tells me that the way we manage patients doesn’t change – it is still lifestyle modification,” said Dr. Harrison, who was not involved with the study. “It’s eat less [rather] than more. It’s exercise and try to lose weight. In the long term, we give patients benefit, and we show that the disease has improved, and we offer something that means they can maintain a healthy life.”
He added that the relatively small and short trial was informative.
“They improved the NAFLD activity score [NAS],” he said. “I don’t know by how much. There was no change in fibrosis, but we wouldn’t expect this at 6 months.”
“It’s provocative work, and it gives us healthy information about how we can help manage our patients from a lifestyle perspective,” he concluded.
‘Do not lose weight. Eat until you are full’
In the study, 110 participants with type 2 diabetes and NAFLD, aged 18-78 years, were allocated to the LCHF diet, and 55 were allocated to the low-fat diet for 6 months.
The researchers performed liver biopsies at baseline and 6 months, which were blinded for scoring.
Participants had ongoing dietitian consultations, with follow-up visits at 3 and 6 months. Compliance was reported continuously through an online food diary platform.
The primary endpoint was change in glycemic control as measured by A1c level over 6 months. The secondary endpoints comprised the proportion of participants with changes in the NAS of at least 2 points over 6 months. Both these measures were compared between the two dietary groups.
The two groups were matched at baseline, with a mean age of 55-57 years, 58% were women, 89% with metabolic syndrome, and a mean BMI 34 kg/m2.
In baseline liver disease, F1 level fibrosis was the most common (58%), followed by hepatic steatosis (S1, 47%; S2, 32%), with a median NAS of 3, and 19% had nonalcoholic steatohepatitis.
The special thing about these diets was that participants were told to “not lose weight, but eat until you are full,” remarked Dr. Dalby Hansen.
Those on the LCHF diet consumed an average of 61% energy from fat, 13% from carbohydrates, and 23% from protein, compared with the low-fat diet, which comprised an average of 29% energy from fat, 46% from carbohydrates, and 21% from protein.
“It’s a lot of fat and corresponds to a quarter of a liter of olive oil per day,” said Dr. Dalby Hansen. “They really had to change their mindset a lot, because it was difficult for them to start eating all these fats, especially since we’ve all been told for decades that it isn’t good. But we supported them, and they got into it.”
The LCHF diet was primarily comprised of unsaturated fats – for example, avocado, oil, nuts, and seeds – but also included saturated fats, such as cheese, cream, and high-fat dairy products. Participants were free to eat unsaturated and saturated fats, but Dr. Dalby Hansen and her team advised participants that “good” unsaturated fats were preferable.
“Also, this diet contained vegetables but no bread, no potatoes, no rice, and no pasta. It was low in carbohydrates, below 20%,” she added.
Improved glycemic control, reduced liver fat
“We found that the LCHF diet improved diabetes control, it reduced the fat in the liver, and, even though they’re eating as many calories as they were used to until they were full, they lost 5.8% of body weight,” said Dr. Dalby Hansen in reporting the results. Participants in the low-fat group lost only 1.8% of body weight.
However, mean calorie intake dropped in both groups, by –2.2% in the LCHF group and –8.7% in the low-fat group.
“The LCHF diet improved the primary outcome of A1c by 9.5 mmol/mol, which is similar to some anti-diabetic medications, such as DPP-4 inhibitors and SGLT2 inhibitors,” she said.
The low-fat group reduced A1c by 3.4 mmol/mol, resulting in a between-group difference of 6.1 mmol/mol.
“Upon follow-up of 3 months, after stopping the diets, on average the participants in both groups returned their HbA1c levels to nearly baseline values,” she said. Results were adjusted for weight loss and baseline values.
Both diets also improved the NAS. The proportion of participants who improved their NAS score by 2 or more points was 22% in the LCHF group versus 17% in the low-fat group (P = 0.58). Additionally, in the LCHF group, 70% of participants improved their score by 1 or more points, compared with 49% in the low-fat group and fewer in the LCHF group experienced a worsening of their score (1% vs. 23%, respectively).
One participant on LCHF had high triglycerides of 12 mmol/L after 3 months. Overall, the low-density lipoprotein increased marginally by 0.2 mmol per liter in the high-fat group, said Dr. Dalby Hansen.
Dr. Dalby Hansen noted some limitations. The findings might not be applicable in more severe NAFLD, dietary assessment relied on self-reporting, no food was provided, and participants had to cook themselves. It was also an open-label study because of the nature of the intervention.
Some hope for more sustainable dieting
Many diets are difficult to adhere to, remarked Dr. Dalby Hansen. “We thought this [diet] might be easier to comply with in the longer term, and we hope that these results might provide patients with more options.”
She added that most people who started the diet adapted and complied with it. “However, it might not be for everyone, but I think we can say that if people try, and it fits into their lives, then they go for it.”
However, “it is not about going out and eating whatever fat and how much of it you want. It’s important that you cut the carbohydrates too,” she said. “With this approach, we really saw amazing results.”
Dr. Dalby Hansen added that having various diets available, including the LCHF one, meant that as clinicians they could empower patients to take control of their metabolic health.
“We can ask them directly, ‘What would fit into their life?’” she said. “We know that one size does not fit at all, and I believe that if we could engage patients more, then they can take control of their own situation.”
Asked whether these findings were enough to change guidelines, Zobair Younossi, MD, professor and chairman, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., remarked that it was the sugar at work here.
“Dietary fat – it’s not the same as fat in the liver, and this diet has more to do with the sugar levels,” he said.
“I’m always reluctant to take results from a short-term study without long-term follow-up,” Dr. Younossi said. “I want to know will patients live longer, and long-term data are needed for this. Until I have that strong evidence that outcomes are going to change, or at least some sign that the outcome is going to change, it is too early to change any guidelines.”
Dr. Dalby Hansen reports no relevant financial relationships. Dr. Harrison reported financial relationships with numerous pharmaceutical companies. Dr. Younossi reports the following financial relationships: research funds and/or consultant to Abbott, Allergan, Bristol Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
AT ILC 2022
IBD patients at higher risk for cancer and cancer-specific mortality
Key clinical point: Risk for overall cancer was 17% higher and cancer-specific mortality 26% higher in patients with inflammatory bowel disease (IBD) compared with non-IBD individuals.
Major finding: Risk for overall cancer (adjusted hazard ratio [aHR] 1.17; P < .001), particularly digestive cancer (aHR 1.33; P = .001), skin cancer (aHR 1.25; P < .001), and male genital cancer (aHR 1.29; P = .003), and cancer-specific mortality (aHR 1.26; P < .001) was significantly higher among patients with IBD vs. non-IBD individuals.
Study details: Findings are from a prospective study that included 5142 patients with ulcerative colitis (n = 3258), Crohn’s disease (n = 1449), or unclassified IBD (n = 435) and who were compared with 450,785 non-IBD reference individuals from the UK Biobank cohort.
Disclosures: The study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Wu S et al. Inflammatory bowel disease and long-term risk of cancer: A prospective cohort study among half a million adults in UK Biobank. Inflamm Bowel Dis. 2022 (May 27). Doi: 10.1093/ibd/izac096
Key clinical point: Risk for overall cancer was 17% higher and cancer-specific mortality 26% higher in patients with inflammatory bowel disease (IBD) compared with non-IBD individuals.
Major finding: Risk for overall cancer (adjusted hazard ratio [aHR] 1.17; P < .001), particularly digestive cancer (aHR 1.33; P = .001), skin cancer (aHR 1.25; P < .001), and male genital cancer (aHR 1.29; P = .003), and cancer-specific mortality (aHR 1.26; P < .001) was significantly higher among patients with IBD vs. non-IBD individuals.
Study details: Findings are from a prospective study that included 5142 patients with ulcerative colitis (n = 3258), Crohn’s disease (n = 1449), or unclassified IBD (n = 435) and who were compared with 450,785 non-IBD reference individuals from the UK Biobank cohort.
Disclosures: The study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Wu S et al. Inflammatory bowel disease and long-term risk of cancer: A prospective cohort study among half a million adults in UK Biobank. Inflamm Bowel Dis. 2022 (May 27). Doi: 10.1093/ibd/izac096
Key clinical point: Risk for overall cancer was 17% higher and cancer-specific mortality 26% higher in patients with inflammatory bowel disease (IBD) compared with non-IBD individuals.
Major finding: Risk for overall cancer (adjusted hazard ratio [aHR] 1.17; P < .001), particularly digestive cancer (aHR 1.33; P = .001), skin cancer (aHR 1.25; P < .001), and male genital cancer (aHR 1.29; P = .003), and cancer-specific mortality (aHR 1.26; P < .001) was significantly higher among patients with IBD vs. non-IBD individuals.
Study details: Findings are from a prospective study that included 5142 patients with ulcerative colitis (n = 3258), Crohn’s disease (n = 1449), or unclassified IBD (n = 435) and who were compared with 450,785 non-IBD reference individuals from the UK Biobank cohort.
Disclosures: The study was supported by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Wu S et al. Inflammatory bowel disease and long-term risk of cancer: A prospective cohort study among half a million adults in UK Biobank. Inflamm Bowel Dis. 2022 (May 27). Doi: 10.1093/ibd/izac096