User login
Genetic ‘taste score’ could help us eat healthier and reduce disease risk
Addicted to cookies? Can’t stand broccoli? You may be able to blame Mom and Dad.
That’s because our taste preferences are influenced by our genes. And this may play an important role in determining our food choices and, in turn, our health, according to early study findings presented at this year’s annual meeting of the American Society for Nutrition.
“Our genetic predispositions to perceive certain tastes might be one of many reasons why some of us struggle to make healthy food choices,” says the study’s lead researcher, Julie Gervis, a doctoral degree candidate at the Tufts Jean Mayer USDA Human Nutrition Research Center on Aging.
As the field of personalized nutrition – a branch of science that uses technology to help people figure out what to eat for good health – advances, the findings could bring us closer to more effective personalized nutrition advice, better diets, and less risk for things like obesity, type 2 diabetes, and heart disease.
What’s your ‘polygenic taste score’?
We know genes influence our taste, but little is known about how taste-related genes impact diet quality and health. To investigate this, the researchers used data from “genome-wide association studies,” which scientists use to find gene variations associated with a trait, to create something called a polygenic taste score.
Your polygenic taste score shows how your genes impact your unique perception of taste – be it bitter, salty, sweet, sour, or savory (umami). If you have a high score for, say, sweet, that means you may be more sensitive to sweetness than someone with a moderate or low sweet score.
In the study sample of more than 6,000 adults, those with a high “bitter” score tended to eat fewer whole grains (two fewer servings a week), while those scoring high for savory ate fewer vegetables, especially orange and red types like carrots and bell peppers. That matters because whole grains have been shown to reduce heart disease risk, while a higher veggie intake is linked to lower risk of type 2 diabetes.
Meanwhile, genes related to sweet seemed key for health related to your heart and metabolism, as a higher sweet score was linked with lower triglycerides, a type of fat found in the blood.
From lab to shopping list
While we have a long way to go before dietitians and consumers can use polygenic taste scores, the tool could one day help us use – or minimize – the influence our genes has on our food choices, Ms. Gervis says. That may help us improve personalized nutrition advice aimed at reducing disease risk.
But first, other research needs to repeat the findings, Ms. Gervis says. And more large-scale, genome-wide studies on taste perception should be done.
“I hope these preliminary data convey the potential benefit of incorporating taste-related genes, and taste perception, into personalized nutrition,” she says. “After all, while we don’t always choose what foods are good for us, we do always choose what foods taste good to us.”
A version of this article first appeared on WebMD.com.
Addicted to cookies? Can’t stand broccoli? You may be able to blame Mom and Dad.
That’s because our taste preferences are influenced by our genes. And this may play an important role in determining our food choices and, in turn, our health, according to early study findings presented at this year’s annual meeting of the American Society for Nutrition.
“Our genetic predispositions to perceive certain tastes might be one of many reasons why some of us struggle to make healthy food choices,” says the study’s lead researcher, Julie Gervis, a doctoral degree candidate at the Tufts Jean Mayer USDA Human Nutrition Research Center on Aging.
As the field of personalized nutrition – a branch of science that uses technology to help people figure out what to eat for good health – advances, the findings could bring us closer to more effective personalized nutrition advice, better diets, and less risk for things like obesity, type 2 diabetes, and heart disease.
What’s your ‘polygenic taste score’?
We know genes influence our taste, but little is known about how taste-related genes impact diet quality and health. To investigate this, the researchers used data from “genome-wide association studies,” which scientists use to find gene variations associated with a trait, to create something called a polygenic taste score.
Your polygenic taste score shows how your genes impact your unique perception of taste – be it bitter, salty, sweet, sour, or savory (umami). If you have a high score for, say, sweet, that means you may be more sensitive to sweetness than someone with a moderate or low sweet score.
In the study sample of more than 6,000 adults, those with a high “bitter” score tended to eat fewer whole grains (two fewer servings a week), while those scoring high for savory ate fewer vegetables, especially orange and red types like carrots and bell peppers. That matters because whole grains have been shown to reduce heart disease risk, while a higher veggie intake is linked to lower risk of type 2 diabetes.
Meanwhile, genes related to sweet seemed key for health related to your heart and metabolism, as a higher sweet score was linked with lower triglycerides, a type of fat found in the blood.
From lab to shopping list
While we have a long way to go before dietitians and consumers can use polygenic taste scores, the tool could one day help us use – or minimize – the influence our genes has on our food choices, Ms. Gervis says. That may help us improve personalized nutrition advice aimed at reducing disease risk.
But first, other research needs to repeat the findings, Ms. Gervis says. And more large-scale, genome-wide studies on taste perception should be done.
“I hope these preliminary data convey the potential benefit of incorporating taste-related genes, and taste perception, into personalized nutrition,” she says. “After all, while we don’t always choose what foods are good for us, we do always choose what foods taste good to us.”
A version of this article first appeared on WebMD.com.
Addicted to cookies? Can’t stand broccoli? You may be able to blame Mom and Dad.
That’s because our taste preferences are influenced by our genes. And this may play an important role in determining our food choices and, in turn, our health, according to early study findings presented at this year’s annual meeting of the American Society for Nutrition.
“Our genetic predispositions to perceive certain tastes might be one of many reasons why some of us struggle to make healthy food choices,” says the study’s lead researcher, Julie Gervis, a doctoral degree candidate at the Tufts Jean Mayer USDA Human Nutrition Research Center on Aging.
As the field of personalized nutrition – a branch of science that uses technology to help people figure out what to eat for good health – advances, the findings could bring us closer to more effective personalized nutrition advice, better diets, and less risk for things like obesity, type 2 diabetes, and heart disease.
What’s your ‘polygenic taste score’?
We know genes influence our taste, but little is known about how taste-related genes impact diet quality and health. To investigate this, the researchers used data from “genome-wide association studies,” which scientists use to find gene variations associated with a trait, to create something called a polygenic taste score.
Your polygenic taste score shows how your genes impact your unique perception of taste – be it bitter, salty, sweet, sour, or savory (umami). If you have a high score for, say, sweet, that means you may be more sensitive to sweetness than someone with a moderate or low sweet score.
In the study sample of more than 6,000 adults, those with a high “bitter” score tended to eat fewer whole grains (two fewer servings a week), while those scoring high for savory ate fewer vegetables, especially orange and red types like carrots and bell peppers. That matters because whole grains have been shown to reduce heart disease risk, while a higher veggie intake is linked to lower risk of type 2 diabetes.
Meanwhile, genes related to sweet seemed key for health related to your heart and metabolism, as a higher sweet score was linked with lower triglycerides, a type of fat found in the blood.
From lab to shopping list
While we have a long way to go before dietitians and consumers can use polygenic taste scores, the tool could one day help us use – or minimize – the influence our genes has on our food choices, Ms. Gervis says. That may help us improve personalized nutrition advice aimed at reducing disease risk.
But first, other research needs to repeat the findings, Ms. Gervis says. And more large-scale, genome-wide studies on taste perception should be done.
“I hope these preliminary data convey the potential benefit of incorporating taste-related genes, and taste perception, into personalized nutrition,” she says. “After all, while we don’t always choose what foods are good for us, we do always choose what foods taste good to us.”
A version of this article first appeared on WebMD.com.
Cancer may increase risk of type 2 diabetes
most notably pancreatic malignancies.
“Our study demonstrates that there is an elevated risk of developing diabetes if a person is affected by lung, pancreatic, breast, brain, urinary tract, or uterine cancers,” said Lykke Sylow, PhD, associate professor in the Molecular Metabolism in Cancer and Ageing Group at the University of Copenhagen, in a statement.
“It is great to see such a large, well-designed study confirm the findings of previous smaller studies and observations,” said Elias S. Siraj, MD, the David L. Bernd Distinguished Chair for EVMS-Sentara Cardiovascular Diabetes Program at Eastern Virginia Medical School in Norfolk, when asked for comment by this news organization. Dr. Siraj also noted that “in clinical care we do observe that many patients develop diabetes after being diagnosed with cancer although one needs a well-designed study to confirm that observation.”
Diabetes risk highest with pancreatic cancer
Type 2 diabetes at the time of cancer diagnosis is known to increase cancer-specific and all-cause mortality, but not much is known about whether cancer is a risk factor for type 2 diabetes, the researchers state in their study, published in Diabetes Care.
Dr. Sylow and colleagues from the Steno Diabetes Center Copenhagen, Rigshospitalet, analyzed a database consisting of 112 million blood samples from 1.3 million Danes from 2000 to 2015. They looked at cancer cases with an incidence of more than 1,000 and excluded individuals with diabetes prior to cancer diagnosis.
They found an increased risk of new-onset type 2 diabetes for all cancers (hazard ratio, 1.09; 95% confidence interval, 1.03-1.14). For pancreatic cancer, the hazard ratio rose to 5.0 (95% CI, 3.62-6.90), for brain and nervous system cancers the hazard ratio was 1.54 (95% CI, 1.22-1.95), and for uterine cancer the hazard ratio was 1.41 (95% CI, 1.10-1.84).
The link with pancreatic cancer was not surprising, said Dr. Sylow.
Dr. Siraj agreed, noting that a few studies have shown a strong association. “It has also been observed for years that many patients with pancreatic cancer may present with new-onset diabetes,” he said. “The mechanism is not clearly understood but could include a direct damage of the beta cells by the pancreatic cancer or could be due to a paraneoplastic secretion of special factors by the cancer that can affect beta-cell function or insulin resistance,” said Dr. Siraj, who is also professor and chief of endocrinology and director of the Strelitz Diabetes Center at Eastern Virginia Medical School.
The higher diabetes risk associated with brain and nervous system cancers has not been previously described and is “an intriguing finding,” he said.
In their statement, the Danish investigators said there is nothing in their research to suggest why some cancers are associated with a higher risk of new-onset type 2 diabetes, but they offered some theories, including that chemotherapeutics and perhaps the cancer, itself, may contribute.
“We know that cancer cells are able to secrete substances that can affect organs and possibility contribute to an increased incidence of diabetes,” said Dr. Sylow in the statement.
Increased mortality risk in those with cancer and type 2 diabetes
Dr. Sylow and colleagues also analyzed mortality in a subset of 28,308 patients with cancer who were still alive 2 years after diagnosis. They documented a 21% higher rate of all-cause mortality in these patients compared with those who did not have new-onset type 2 diabetes.
“We do not know enough about the patients who were diagnosed with type 2 diabetes, but we think our findings illustrate a potential new area of intervention in the cancer clinic,” Dr. Sylow said. However, the findings still require replication before drawing any definite conclusions, she added.
Christoffer Johansen, MD, PhD, DMSc, of Rigshospitalet, said in the statement that it might be prudent to screen patients with lung, breast, brain, uterine, and urinary tract cancers for diabetes. “Early intervention could have an impact on certain cancer patients,” said Dr. Johansen.
Dr. Siraj said he would urge oncologists to routinely monitor blood glucose levels during cancer treatment and as part of long-term surveillance, and to consider the potential risk of new-onset diabetes when choosing a cancer therapy. If diabetes is diagnosed, clinicians should be sure that it’s managed by a primary care physician or endocrinologist, “as proper treatment may contribute to better outcomes of the cancer,” said Dr. Siraj.
Endocrinologists should consider the possibility of pancreatic cancer if someone with few risk factors for type 2 diabetes has a new-onset diagnosis, he said. And they should aim for good glycemic control in those with new-onset type 2 diabetes, as it may lead to better cancer outcomes, he said.
Dr. Sylow has reported grant support from the Novo Nordisk Foundation and Independent Research Fund Denmark. Dr. Johansen has reported serving as an educator for Janssen and Pfizer. Coauthors have received grant support from the Danish Cancer Society and served as consultants, on advisory boards, or as educators for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Incyte, GSK, MSD, Mundipharma, Novartis, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
most notably pancreatic malignancies.
“Our study demonstrates that there is an elevated risk of developing diabetes if a person is affected by lung, pancreatic, breast, brain, urinary tract, or uterine cancers,” said Lykke Sylow, PhD, associate professor in the Molecular Metabolism in Cancer and Ageing Group at the University of Copenhagen, in a statement.
“It is great to see such a large, well-designed study confirm the findings of previous smaller studies and observations,” said Elias S. Siraj, MD, the David L. Bernd Distinguished Chair for EVMS-Sentara Cardiovascular Diabetes Program at Eastern Virginia Medical School in Norfolk, when asked for comment by this news organization. Dr. Siraj also noted that “in clinical care we do observe that many patients develop diabetes after being diagnosed with cancer although one needs a well-designed study to confirm that observation.”
Diabetes risk highest with pancreatic cancer
Type 2 diabetes at the time of cancer diagnosis is known to increase cancer-specific and all-cause mortality, but not much is known about whether cancer is a risk factor for type 2 diabetes, the researchers state in their study, published in Diabetes Care.
Dr. Sylow and colleagues from the Steno Diabetes Center Copenhagen, Rigshospitalet, analyzed a database consisting of 112 million blood samples from 1.3 million Danes from 2000 to 2015. They looked at cancer cases with an incidence of more than 1,000 and excluded individuals with diabetes prior to cancer diagnosis.
They found an increased risk of new-onset type 2 diabetes for all cancers (hazard ratio, 1.09; 95% confidence interval, 1.03-1.14). For pancreatic cancer, the hazard ratio rose to 5.0 (95% CI, 3.62-6.90), for brain and nervous system cancers the hazard ratio was 1.54 (95% CI, 1.22-1.95), and for uterine cancer the hazard ratio was 1.41 (95% CI, 1.10-1.84).
The link with pancreatic cancer was not surprising, said Dr. Sylow.
Dr. Siraj agreed, noting that a few studies have shown a strong association. “It has also been observed for years that many patients with pancreatic cancer may present with new-onset diabetes,” he said. “The mechanism is not clearly understood but could include a direct damage of the beta cells by the pancreatic cancer or could be due to a paraneoplastic secretion of special factors by the cancer that can affect beta-cell function or insulin resistance,” said Dr. Siraj, who is also professor and chief of endocrinology and director of the Strelitz Diabetes Center at Eastern Virginia Medical School.
The higher diabetes risk associated with brain and nervous system cancers has not been previously described and is “an intriguing finding,” he said.
In their statement, the Danish investigators said there is nothing in their research to suggest why some cancers are associated with a higher risk of new-onset type 2 diabetes, but they offered some theories, including that chemotherapeutics and perhaps the cancer, itself, may contribute.
“We know that cancer cells are able to secrete substances that can affect organs and possibility contribute to an increased incidence of diabetes,” said Dr. Sylow in the statement.
Increased mortality risk in those with cancer and type 2 diabetes
Dr. Sylow and colleagues also analyzed mortality in a subset of 28,308 patients with cancer who were still alive 2 years after diagnosis. They documented a 21% higher rate of all-cause mortality in these patients compared with those who did not have new-onset type 2 diabetes.
“We do not know enough about the patients who were diagnosed with type 2 diabetes, but we think our findings illustrate a potential new area of intervention in the cancer clinic,” Dr. Sylow said. However, the findings still require replication before drawing any definite conclusions, she added.
Christoffer Johansen, MD, PhD, DMSc, of Rigshospitalet, said in the statement that it might be prudent to screen patients with lung, breast, brain, uterine, and urinary tract cancers for diabetes. “Early intervention could have an impact on certain cancer patients,” said Dr. Johansen.
Dr. Siraj said he would urge oncologists to routinely monitor blood glucose levels during cancer treatment and as part of long-term surveillance, and to consider the potential risk of new-onset diabetes when choosing a cancer therapy. If diabetes is diagnosed, clinicians should be sure that it’s managed by a primary care physician or endocrinologist, “as proper treatment may contribute to better outcomes of the cancer,” said Dr. Siraj.
Endocrinologists should consider the possibility of pancreatic cancer if someone with few risk factors for type 2 diabetes has a new-onset diagnosis, he said. And they should aim for good glycemic control in those with new-onset type 2 diabetes, as it may lead to better cancer outcomes, he said.
Dr. Sylow has reported grant support from the Novo Nordisk Foundation and Independent Research Fund Denmark. Dr. Johansen has reported serving as an educator for Janssen and Pfizer. Coauthors have received grant support from the Danish Cancer Society and served as consultants, on advisory boards, or as educators for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Incyte, GSK, MSD, Mundipharma, Novartis, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
most notably pancreatic malignancies.
“Our study demonstrates that there is an elevated risk of developing diabetes if a person is affected by lung, pancreatic, breast, brain, urinary tract, or uterine cancers,” said Lykke Sylow, PhD, associate professor in the Molecular Metabolism in Cancer and Ageing Group at the University of Copenhagen, in a statement.
“It is great to see such a large, well-designed study confirm the findings of previous smaller studies and observations,” said Elias S. Siraj, MD, the David L. Bernd Distinguished Chair for EVMS-Sentara Cardiovascular Diabetes Program at Eastern Virginia Medical School in Norfolk, when asked for comment by this news organization. Dr. Siraj also noted that “in clinical care we do observe that many patients develop diabetes after being diagnosed with cancer although one needs a well-designed study to confirm that observation.”
Diabetes risk highest with pancreatic cancer
Type 2 diabetes at the time of cancer diagnosis is known to increase cancer-specific and all-cause mortality, but not much is known about whether cancer is a risk factor for type 2 diabetes, the researchers state in their study, published in Diabetes Care.
Dr. Sylow and colleagues from the Steno Diabetes Center Copenhagen, Rigshospitalet, analyzed a database consisting of 112 million blood samples from 1.3 million Danes from 2000 to 2015. They looked at cancer cases with an incidence of more than 1,000 and excluded individuals with diabetes prior to cancer diagnosis.
They found an increased risk of new-onset type 2 diabetes for all cancers (hazard ratio, 1.09; 95% confidence interval, 1.03-1.14). For pancreatic cancer, the hazard ratio rose to 5.0 (95% CI, 3.62-6.90), for brain and nervous system cancers the hazard ratio was 1.54 (95% CI, 1.22-1.95), and for uterine cancer the hazard ratio was 1.41 (95% CI, 1.10-1.84).
The link with pancreatic cancer was not surprising, said Dr. Sylow.
Dr. Siraj agreed, noting that a few studies have shown a strong association. “It has also been observed for years that many patients with pancreatic cancer may present with new-onset diabetes,” he said. “The mechanism is not clearly understood but could include a direct damage of the beta cells by the pancreatic cancer or could be due to a paraneoplastic secretion of special factors by the cancer that can affect beta-cell function or insulin resistance,” said Dr. Siraj, who is also professor and chief of endocrinology and director of the Strelitz Diabetes Center at Eastern Virginia Medical School.
The higher diabetes risk associated with brain and nervous system cancers has not been previously described and is “an intriguing finding,” he said.
In their statement, the Danish investigators said there is nothing in their research to suggest why some cancers are associated with a higher risk of new-onset type 2 diabetes, but they offered some theories, including that chemotherapeutics and perhaps the cancer, itself, may contribute.
“We know that cancer cells are able to secrete substances that can affect organs and possibility contribute to an increased incidence of diabetes,” said Dr. Sylow in the statement.
Increased mortality risk in those with cancer and type 2 diabetes
Dr. Sylow and colleagues also analyzed mortality in a subset of 28,308 patients with cancer who were still alive 2 years after diagnosis. They documented a 21% higher rate of all-cause mortality in these patients compared with those who did not have new-onset type 2 diabetes.
“We do not know enough about the patients who were diagnosed with type 2 diabetes, but we think our findings illustrate a potential new area of intervention in the cancer clinic,” Dr. Sylow said. However, the findings still require replication before drawing any definite conclusions, she added.
Christoffer Johansen, MD, PhD, DMSc, of Rigshospitalet, said in the statement that it might be prudent to screen patients with lung, breast, brain, uterine, and urinary tract cancers for diabetes. “Early intervention could have an impact on certain cancer patients,” said Dr. Johansen.
Dr. Siraj said he would urge oncologists to routinely monitor blood glucose levels during cancer treatment and as part of long-term surveillance, and to consider the potential risk of new-onset diabetes when choosing a cancer therapy. If diabetes is diagnosed, clinicians should be sure that it’s managed by a primary care physician or endocrinologist, “as proper treatment may contribute to better outcomes of the cancer,” said Dr. Siraj.
Endocrinologists should consider the possibility of pancreatic cancer if someone with few risk factors for type 2 diabetes has a new-onset diagnosis, he said. And they should aim for good glycemic control in those with new-onset type 2 diabetes, as it may lead to better cancer outcomes, he said.
Dr. Sylow has reported grant support from the Novo Nordisk Foundation and Independent Research Fund Denmark. Dr. Johansen has reported serving as an educator for Janssen and Pfizer. Coauthors have received grant support from the Danish Cancer Society and served as consultants, on advisory boards, or as educators for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Incyte, GSK, MSD, Mundipharma, Novartis, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Vasectomy requests increase after Roe ruling
Urologists told The Washington Post that more men are seeking the procedure to prevent pregnancies and avoid abortion-related concerns.
“It was very, very noticeable [June 24], and then the number that came in over the weekend was huge, and the number that is still coming in far exceeds what we have experienced in the past,” Doug Stein, MD, a Florida urologist known as the “Vasectomy King” for his advocacy of the procedure, told the newspaper.
Before June 24, Dr. Stein received four or five vasectomy requests per day. But since then, that number has increased to 12 to 18 requests per day.
“Many of the guys are saying that they have been thinking about a vasectomy for a while, and the Roe v. Wade decision was just that final factor that tipped them over the edge and made them submit the online registration,” he said.
Urologists in California, Iowa, and New York also told the Post that they’ve seen a massive increase in the number of vasectomy consultations, as well as an increase in website traffic on their pages that offer information about vasectomies.
About 2 decades ago, Americans said the main reason they relied on a vasectomy as a form of birth control was that they or their partners had all the children they wanted. In the past decade, other reasons became more common, such as medical issues and problems with other types of birth control, the newspaper reported.
In anticipation of Roe v. Wade being overturned and anti-abortion legislation taking effect in states, advocates for vasectomies have encouraged people to get the procedure.
Dr. Stein said his practice is now booked through the end of August with vasectomy appointments, so he’s opening more days in his schedule to accommodate patients who submitted recent requests. He and his associate, John Curington, MD, said men under age 30 without children are requesting the procedure in greater numbers than before, with some citing the concurring opinion by Justice Clarence Thomas, which said the Supreme Court should reconsider other landmark cases that protect rights under the 14th Amendment, such as access to contraceptives.
“I’d say at least 60 or 70% are mentioning the Supreme Court decision,” Dr. Curington said, according to the Post. “And a few of them have such sophistication as young men that they actually are thinking about Justice Thomas and his opinion that contraception may fall next. And that’s shocking. That’s something that doesn’t enter into our conversations ever, until this week.”
A version of this article first appeared on WebMD.com.
Urologists told The Washington Post that more men are seeking the procedure to prevent pregnancies and avoid abortion-related concerns.
“It was very, very noticeable [June 24], and then the number that came in over the weekend was huge, and the number that is still coming in far exceeds what we have experienced in the past,” Doug Stein, MD, a Florida urologist known as the “Vasectomy King” for his advocacy of the procedure, told the newspaper.
Before June 24, Dr. Stein received four or five vasectomy requests per day. But since then, that number has increased to 12 to 18 requests per day.
“Many of the guys are saying that they have been thinking about a vasectomy for a while, and the Roe v. Wade decision was just that final factor that tipped them over the edge and made them submit the online registration,” he said.
Urologists in California, Iowa, and New York also told the Post that they’ve seen a massive increase in the number of vasectomy consultations, as well as an increase in website traffic on their pages that offer information about vasectomies.
About 2 decades ago, Americans said the main reason they relied on a vasectomy as a form of birth control was that they or their partners had all the children they wanted. In the past decade, other reasons became more common, such as medical issues and problems with other types of birth control, the newspaper reported.
In anticipation of Roe v. Wade being overturned and anti-abortion legislation taking effect in states, advocates for vasectomies have encouraged people to get the procedure.
Dr. Stein said his practice is now booked through the end of August with vasectomy appointments, so he’s opening more days in his schedule to accommodate patients who submitted recent requests. He and his associate, John Curington, MD, said men under age 30 without children are requesting the procedure in greater numbers than before, with some citing the concurring opinion by Justice Clarence Thomas, which said the Supreme Court should reconsider other landmark cases that protect rights under the 14th Amendment, such as access to contraceptives.
“I’d say at least 60 or 70% are mentioning the Supreme Court decision,” Dr. Curington said, according to the Post. “And a few of them have such sophistication as young men that they actually are thinking about Justice Thomas and his opinion that contraception may fall next. And that’s shocking. That’s something that doesn’t enter into our conversations ever, until this week.”
A version of this article first appeared on WebMD.com.
Urologists told The Washington Post that more men are seeking the procedure to prevent pregnancies and avoid abortion-related concerns.
“It was very, very noticeable [June 24], and then the number that came in over the weekend was huge, and the number that is still coming in far exceeds what we have experienced in the past,” Doug Stein, MD, a Florida urologist known as the “Vasectomy King” for his advocacy of the procedure, told the newspaper.
Before June 24, Dr. Stein received four or five vasectomy requests per day. But since then, that number has increased to 12 to 18 requests per day.
“Many of the guys are saying that they have been thinking about a vasectomy for a while, and the Roe v. Wade decision was just that final factor that tipped them over the edge and made them submit the online registration,” he said.
Urologists in California, Iowa, and New York also told the Post that they’ve seen a massive increase in the number of vasectomy consultations, as well as an increase in website traffic on their pages that offer information about vasectomies.
About 2 decades ago, Americans said the main reason they relied on a vasectomy as a form of birth control was that they or their partners had all the children they wanted. In the past decade, other reasons became more common, such as medical issues and problems with other types of birth control, the newspaper reported.
In anticipation of Roe v. Wade being overturned and anti-abortion legislation taking effect in states, advocates for vasectomies have encouraged people to get the procedure.
Dr. Stein said his practice is now booked through the end of August with vasectomy appointments, so he’s opening more days in his schedule to accommodate patients who submitted recent requests. He and his associate, John Curington, MD, said men under age 30 without children are requesting the procedure in greater numbers than before, with some citing the concurring opinion by Justice Clarence Thomas, which said the Supreme Court should reconsider other landmark cases that protect rights under the 14th Amendment, such as access to contraceptives.
“I’d say at least 60 or 70% are mentioning the Supreme Court decision,” Dr. Curington said, according to the Post. “And a few of them have such sophistication as young men that they actually are thinking about Justice Thomas and his opinion that contraception may fall next. And that’s shocking. That’s something that doesn’t enter into our conversations ever, until this week.”
A version of this article first appeared on WebMD.com.
CRC screening disparities greatest among those under 55
Adults younger than 55 years were least likely to get screened for colorectal cancer over the past 2 decades, particularly if they were Hispanic or Asian or had a low income, lower education level, or no health insurance, according to a new study published online in Cancer Epidemiology, Biomarkers & Prevention.
The findings have raised concerns that disparities in screening rates will be even greater in adults aged 45-49 years, prompting the need for increased awareness and outreach to ensure that underserved groups have access to screenings.
“Differences in prevalence of screening by race and ethnicity, educational attainment, household income, and health insurance were most pronounced for those ages 50-54 years, whereas older adults experienced larger increases in prevalence across these groups,” wrote Po-Hong Liu, MD, MPH, a clinical investigator at Harvard University, Boston, and his colleagues. “The persistent and worsening disparities we observed in adults 50-54 years may extend to those ages 45-49 as they become eligible for screening.”
The U.S. Preventive Services Task Force shifted their recommendation for colorectal cancer screening in May 2021 to 5 years earlier, advising people to start screenings at 45 instead of 50, which aligns with the recommendations the American Cancer Society made 3 years earlier.
Both organizations made the change because of increasing rates of colorectal cancer in adults under age 50 and research indicating that beginning screenings at age 45 results in fewer cases, fewer deaths, and more life years gained.
“Across all age groups, colorectal cancer screening participation remains below national goals, and the benefits of screening are not equally realized across populations,” senior author Caitlin Murphy, PhD, MPH, associate professor, UTHealth School of Public Health, Houston, said in a prepared statement. “Extra care must be taken to ensure that expanding screening to younger ages does not negatively impact efforts to eliminate disparities in colorectal screening and outcomes nor jeopardize efforts to increase screening initiation among older adults who remain unscreened.”
Data analyzed from 8 years over 2 decades
The researchers analyzed data from the CDC’s cross-sectional National Health Interview Survey during 8 years over the past 2 decades: 2000, 2003, 2005, 2008, 2010, 2013, 2015, and 2018.
The number of participants each year ranged from a low of 21,781 in 2008 to a high of 34,557 in 2013. After excluding participants with a history of colorectal cancer or missing information on screenings, the total population sample included 80,220 participants 50-75 years old.
The researchers considered a person as having been screened if they received at least one recommended screening test within the year covered by the survey, regardless of why they underwent the test.
Recommended tests included sigmoidoscopy, colonoscopy, and stool-based tests for all survey years. In addition, the surveys for 2010, 2015, and 2018 included CT colonography, and the 2018 survey included FIT-DNA.
Screening across population groups
Colorectal cancer screening rates have doubled in the past 2 decades, from 36.7% in 2000 to 66.1% in 2018.
Rates are considerably lower, however, for several key groups, including the youngest group. Less than half (47.6%) of those aged 50-54 years received screenings in 2018, though this was still a nearly 20-point improvement over the 28.2% in this age group who were screened in 2000.
Separate from age, several other groups continue to have low screening rates in general, including Hispanics (56.5%, up from 25.9% in 2000), Asians (57.1%, up from 22.6% in 2000), those who have not received a high school degree (53.6%, up from 26.8% in 2000), and those from low income families (56.6%, up from 30.2% in 2000).
The group with the greatest need for more outreach and screenings are people without insurance, only 39.7% of whom were screened in 2018, a modest increase from 30.2% in 2000.
The biggest increase in screenings over time occurred in those aged 70-75 years, from 46.4% in 2000 to 78% in 2018 overall.
Racial/ethnic, economic, education, and insurance-based disparities were particularly evident the younger people were, including in terms of progress made over time.
For example, screenings of non-Hispanic White people aged 50-54 years improved 21 points (30.3% to 51%) between 2000 and 2018, compared with 19 points in Hispanics (16.7% to 35.5%) and 15 points in Asians (17.3% to 32.3%). Fortunately, Black Americans made even greater strides than White Americans with a 27-point increase during that time (23.4% to 50%).
Similarly, income correlated with expansion in screening rates for 50- to 54-year-olds: Those earning at least 400% over the federal poverty line improved 20 points (from 33.5% to 53.8%), compared with a 16-point improvement in those earning less than 200% above the poverty line (from 19.3% to 35%).
Those with private insurance likewise improved 21 points (from 30.7% to 51.7%), while those in this age group without insurance declined, with just 21.2% getting screened in 2018, compared with 28.2% in 2000. Those on public insurance saw a 15-point improvement, from 27.8% in 2000 to 43.1% in 2018.
“The individual and societal burden of colorectal cancer is especially great among younger adults,” the authors wrote.
The reasons for the much lower prevalence of screening in those under 55, the authors suggested, is likely due to less concern about colorectal cancer, less access to medical care (including being underinsured or uninsured), and the barriers created by competing priorities, such as work schedules, family responsibilities, and caregiving. The latter may be particularly true in underserved populations, the authors noted.
“Screening programs must consider the barriers unique to younger adults, ensuring the benefits of screening are equally realized by all population groups,” the authors concluded.
The research was funded by the National Institutes of Health and the Cancer Prevention and Research Institute of Texas. One author reported grants from Epigenomics and Freenome and personal fees from Guardant Health. Another author reported personal fees from Freenome, and a third author reported personal fees from Exact Sciences. No other authors had industry disclosures.
A version of this article first appeared on Medscape.com.
Adults younger than 55 years were least likely to get screened for colorectal cancer over the past 2 decades, particularly if they were Hispanic or Asian or had a low income, lower education level, or no health insurance, according to a new study published online in Cancer Epidemiology, Biomarkers & Prevention.
The findings have raised concerns that disparities in screening rates will be even greater in adults aged 45-49 years, prompting the need for increased awareness and outreach to ensure that underserved groups have access to screenings.
“Differences in prevalence of screening by race and ethnicity, educational attainment, household income, and health insurance were most pronounced for those ages 50-54 years, whereas older adults experienced larger increases in prevalence across these groups,” wrote Po-Hong Liu, MD, MPH, a clinical investigator at Harvard University, Boston, and his colleagues. “The persistent and worsening disparities we observed in adults 50-54 years may extend to those ages 45-49 as they become eligible for screening.”
The U.S. Preventive Services Task Force shifted their recommendation for colorectal cancer screening in May 2021 to 5 years earlier, advising people to start screenings at 45 instead of 50, which aligns with the recommendations the American Cancer Society made 3 years earlier.
Both organizations made the change because of increasing rates of colorectal cancer in adults under age 50 and research indicating that beginning screenings at age 45 results in fewer cases, fewer deaths, and more life years gained.
“Across all age groups, colorectal cancer screening participation remains below national goals, and the benefits of screening are not equally realized across populations,” senior author Caitlin Murphy, PhD, MPH, associate professor, UTHealth School of Public Health, Houston, said in a prepared statement. “Extra care must be taken to ensure that expanding screening to younger ages does not negatively impact efforts to eliminate disparities in colorectal screening and outcomes nor jeopardize efforts to increase screening initiation among older adults who remain unscreened.”
Data analyzed from 8 years over 2 decades
The researchers analyzed data from the CDC’s cross-sectional National Health Interview Survey during 8 years over the past 2 decades: 2000, 2003, 2005, 2008, 2010, 2013, 2015, and 2018.
The number of participants each year ranged from a low of 21,781 in 2008 to a high of 34,557 in 2013. After excluding participants with a history of colorectal cancer or missing information on screenings, the total population sample included 80,220 participants 50-75 years old.
The researchers considered a person as having been screened if they received at least one recommended screening test within the year covered by the survey, regardless of why they underwent the test.
Recommended tests included sigmoidoscopy, colonoscopy, and stool-based tests for all survey years. In addition, the surveys for 2010, 2015, and 2018 included CT colonography, and the 2018 survey included FIT-DNA.
Screening across population groups
Colorectal cancer screening rates have doubled in the past 2 decades, from 36.7% in 2000 to 66.1% in 2018.
Rates are considerably lower, however, for several key groups, including the youngest group. Less than half (47.6%) of those aged 50-54 years received screenings in 2018, though this was still a nearly 20-point improvement over the 28.2% in this age group who were screened in 2000.
Separate from age, several other groups continue to have low screening rates in general, including Hispanics (56.5%, up from 25.9% in 2000), Asians (57.1%, up from 22.6% in 2000), those who have not received a high school degree (53.6%, up from 26.8% in 2000), and those from low income families (56.6%, up from 30.2% in 2000).
The group with the greatest need for more outreach and screenings are people without insurance, only 39.7% of whom were screened in 2018, a modest increase from 30.2% in 2000.
The biggest increase in screenings over time occurred in those aged 70-75 years, from 46.4% in 2000 to 78% in 2018 overall.
Racial/ethnic, economic, education, and insurance-based disparities were particularly evident the younger people were, including in terms of progress made over time.
For example, screenings of non-Hispanic White people aged 50-54 years improved 21 points (30.3% to 51%) between 2000 and 2018, compared with 19 points in Hispanics (16.7% to 35.5%) and 15 points in Asians (17.3% to 32.3%). Fortunately, Black Americans made even greater strides than White Americans with a 27-point increase during that time (23.4% to 50%).
Similarly, income correlated with expansion in screening rates for 50- to 54-year-olds: Those earning at least 400% over the federal poverty line improved 20 points (from 33.5% to 53.8%), compared with a 16-point improvement in those earning less than 200% above the poverty line (from 19.3% to 35%).
Those with private insurance likewise improved 21 points (from 30.7% to 51.7%), while those in this age group without insurance declined, with just 21.2% getting screened in 2018, compared with 28.2% in 2000. Those on public insurance saw a 15-point improvement, from 27.8% in 2000 to 43.1% in 2018.
“The individual and societal burden of colorectal cancer is especially great among younger adults,” the authors wrote.
The reasons for the much lower prevalence of screening in those under 55, the authors suggested, is likely due to less concern about colorectal cancer, less access to medical care (including being underinsured or uninsured), and the barriers created by competing priorities, such as work schedules, family responsibilities, and caregiving. The latter may be particularly true in underserved populations, the authors noted.
“Screening programs must consider the barriers unique to younger adults, ensuring the benefits of screening are equally realized by all population groups,” the authors concluded.
The research was funded by the National Institutes of Health and the Cancer Prevention and Research Institute of Texas. One author reported grants from Epigenomics and Freenome and personal fees from Guardant Health. Another author reported personal fees from Freenome, and a third author reported personal fees from Exact Sciences. No other authors had industry disclosures.
A version of this article first appeared on Medscape.com.
Adults younger than 55 years were least likely to get screened for colorectal cancer over the past 2 decades, particularly if they were Hispanic or Asian or had a low income, lower education level, or no health insurance, according to a new study published online in Cancer Epidemiology, Biomarkers & Prevention.
The findings have raised concerns that disparities in screening rates will be even greater in adults aged 45-49 years, prompting the need for increased awareness and outreach to ensure that underserved groups have access to screenings.
“Differences in prevalence of screening by race and ethnicity, educational attainment, household income, and health insurance were most pronounced for those ages 50-54 years, whereas older adults experienced larger increases in prevalence across these groups,” wrote Po-Hong Liu, MD, MPH, a clinical investigator at Harvard University, Boston, and his colleagues. “The persistent and worsening disparities we observed in adults 50-54 years may extend to those ages 45-49 as they become eligible for screening.”
The U.S. Preventive Services Task Force shifted their recommendation for colorectal cancer screening in May 2021 to 5 years earlier, advising people to start screenings at 45 instead of 50, which aligns with the recommendations the American Cancer Society made 3 years earlier.
Both organizations made the change because of increasing rates of colorectal cancer in adults under age 50 and research indicating that beginning screenings at age 45 results in fewer cases, fewer deaths, and more life years gained.
“Across all age groups, colorectal cancer screening participation remains below national goals, and the benefits of screening are not equally realized across populations,” senior author Caitlin Murphy, PhD, MPH, associate professor, UTHealth School of Public Health, Houston, said in a prepared statement. “Extra care must be taken to ensure that expanding screening to younger ages does not negatively impact efforts to eliminate disparities in colorectal screening and outcomes nor jeopardize efforts to increase screening initiation among older adults who remain unscreened.”
Data analyzed from 8 years over 2 decades
The researchers analyzed data from the CDC’s cross-sectional National Health Interview Survey during 8 years over the past 2 decades: 2000, 2003, 2005, 2008, 2010, 2013, 2015, and 2018.
The number of participants each year ranged from a low of 21,781 in 2008 to a high of 34,557 in 2013. After excluding participants with a history of colorectal cancer or missing information on screenings, the total population sample included 80,220 participants 50-75 years old.
The researchers considered a person as having been screened if they received at least one recommended screening test within the year covered by the survey, regardless of why they underwent the test.
Recommended tests included sigmoidoscopy, colonoscopy, and stool-based tests for all survey years. In addition, the surveys for 2010, 2015, and 2018 included CT colonography, and the 2018 survey included FIT-DNA.
Screening across population groups
Colorectal cancer screening rates have doubled in the past 2 decades, from 36.7% in 2000 to 66.1% in 2018.
Rates are considerably lower, however, for several key groups, including the youngest group. Less than half (47.6%) of those aged 50-54 years received screenings in 2018, though this was still a nearly 20-point improvement over the 28.2% in this age group who were screened in 2000.
Separate from age, several other groups continue to have low screening rates in general, including Hispanics (56.5%, up from 25.9% in 2000), Asians (57.1%, up from 22.6% in 2000), those who have not received a high school degree (53.6%, up from 26.8% in 2000), and those from low income families (56.6%, up from 30.2% in 2000).
The group with the greatest need for more outreach and screenings are people without insurance, only 39.7% of whom were screened in 2018, a modest increase from 30.2% in 2000.
The biggest increase in screenings over time occurred in those aged 70-75 years, from 46.4% in 2000 to 78% in 2018 overall.
Racial/ethnic, economic, education, and insurance-based disparities were particularly evident the younger people were, including in terms of progress made over time.
For example, screenings of non-Hispanic White people aged 50-54 years improved 21 points (30.3% to 51%) between 2000 and 2018, compared with 19 points in Hispanics (16.7% to 35.5%) and 15 points in Asians (17.3% to 32.3%). Fortunately, Black Americans made even greater strides than White Americans with a 27-point increase during that time (23.4% to 50%).
Similarly, income correlated with expansion in screening rates for 50- to 54-year-olds: Those earning at least 400% over the federal poverty line improved 20 points (from 33.5% to 53.8%), compared with a 16-point improvement in those earning less than 200% above the poverty line (from 19.3% to 35%).
Those with private insurance likewise improved 21 points (from 30.7% to 51.7%), while those in this age group without insurance declined, with just 21.2% getting screened in 2018, compared with 28.2% in 2000. Those on public insurance saw a 15-point improvement, from 27.8% in 2000 to 43.1% in 2018.
“The individual and societal burden of colorectal cancer is especially great among younger adults,” the authors wrote.
The reasons for the much lower prevalence of screening in those under 55, the authors suggested, is likely due to less concern about colorectal cancer, less access to medical care (including being underinsured or uninsured), and the barriers created by competing priorities, such as work schedules, family responsibilities, and caregiving. The latter may be particularly true in underserved populations, the authors noted.
“Screening programs must consider the barriers unique to younger adults, ensuring the benefits of screening are equally realized by all population groups,” the authors concluded.
The research was funded by the National Institutes of Health and the Cancer Prevention and Research Institute of Texas. One author reported grants from Epigenomics and Freenome and personal fees from Guardant Health. Another author reported personal fees from Freenome, and a third author reported personal fees from Exact Sciences. No other authors had industry disclosures.
A version of this article first appeared on Medscape.com.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS AN PREVENTION
Generalized anxiety disorder: 8 studies of biological interventions
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
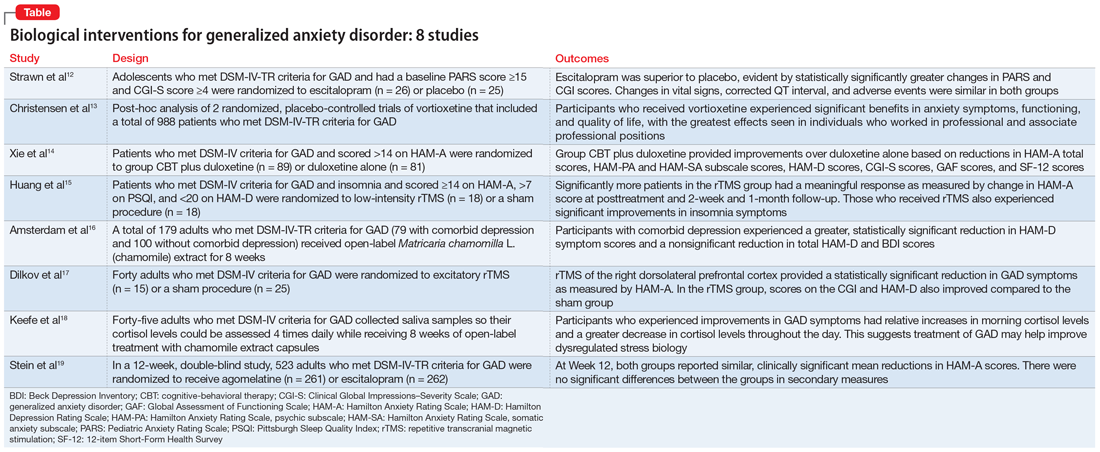
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).
1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).

1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).

1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 3)
Optimal diagnosis and treatment of psychiatric illness requires clinicians to be able to connect mental and physical symptoms. Direct brain neurotransmitter testing is presently in its infancy and the science of defining the underlying mechanisms of psychiatric disorders lags behind the obvious clinical needs. We are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. In this article series, we suggest an indirect way of judging neurotransmitter activity by recognizing specific mental and physical symptoms connected by common biology. Here we present hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. The descriptions we present in this series do not reflect the entire set of symptoms caused by the neurotransmitters we discuss; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypothesis we present. We argue that in cases of multiple psychiatric disorders and chronic pain, the development and approval of medications currently is based on an umbrella descriptive diagnoses, and disregards the various underlying causes of such conditions. Similar to how the many types of pneumonias are treated differently depending on the infective agent, we suggested the same possible causative approach to various types of depression and pain.
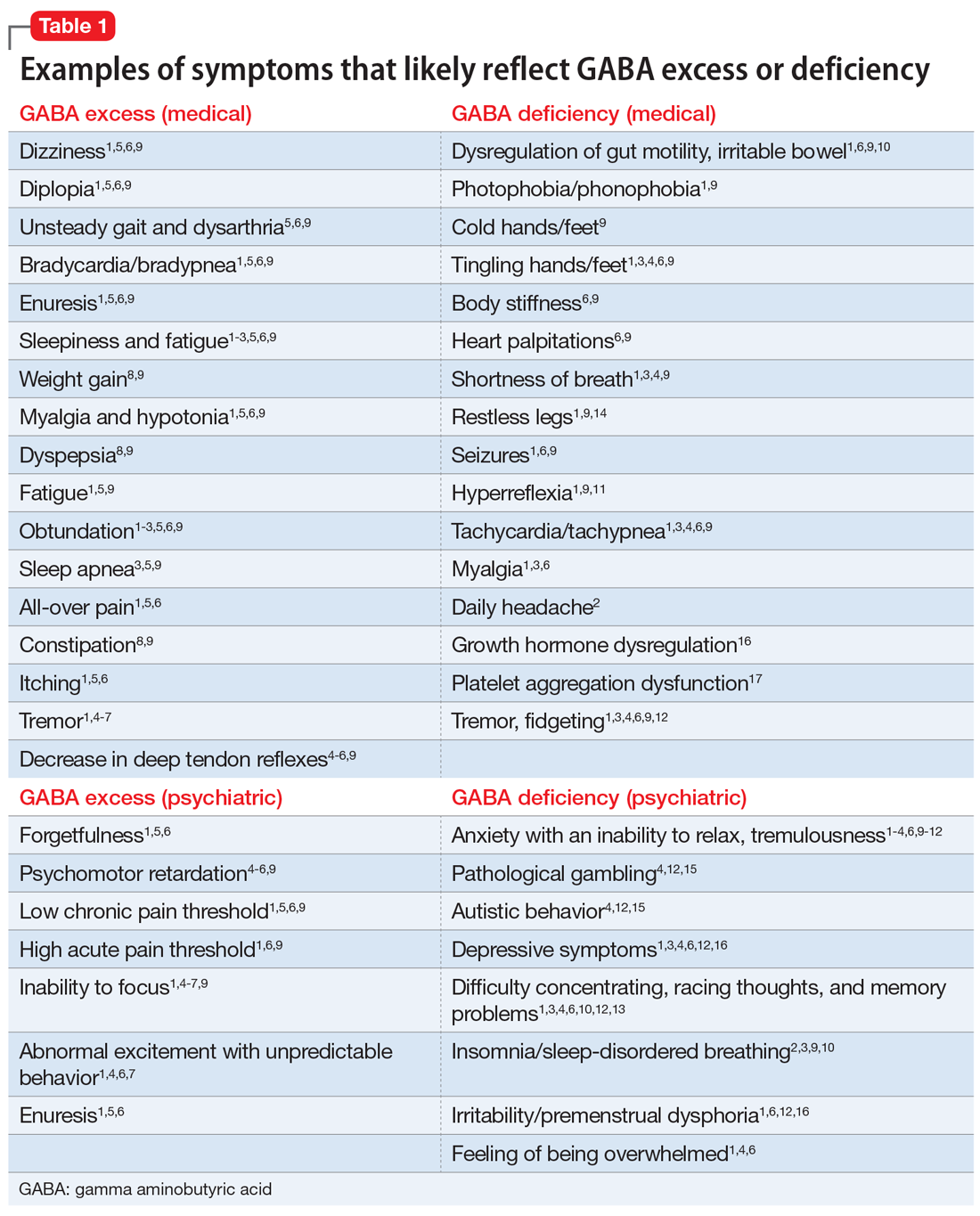
Part 1 of this series (
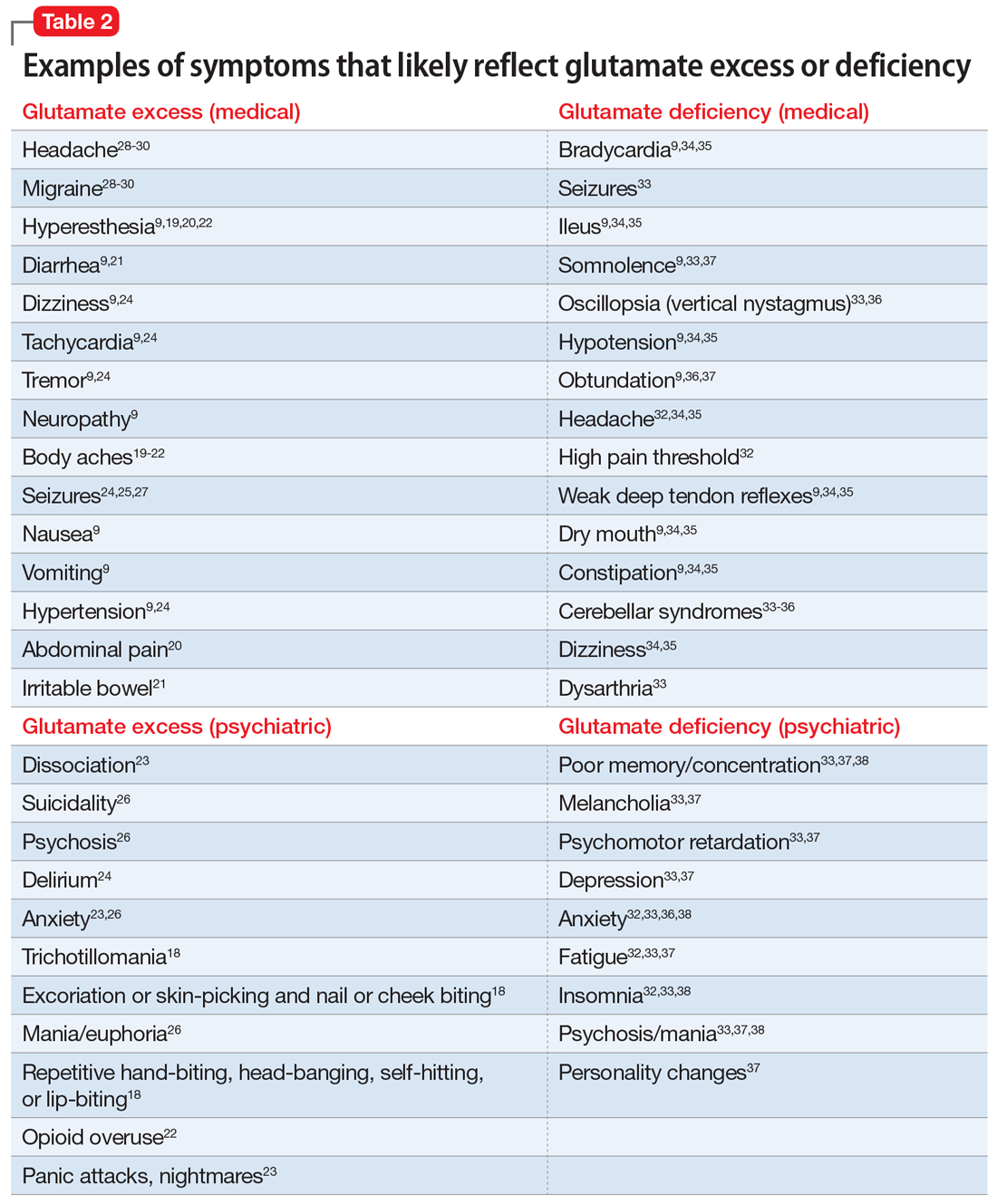
GABA excess (Table 11-9)
Ms. V is brought to your office by a friend. She complains of pain all over her body, itchiness, inability to focus, and dizziness.1,5,6,9 She is puzzled by how little pain she feels when she cuts her finger but by how much pain she is in every day, though her clinicians have not discovered a reason for her pain.1,6,9 She states that her fatigue is so severe that she can sleep 15 hours a day.1-6,9 Her obstructive and central sleep apnea have been treated, but this did not improve her fatigue.3,5,9 She is forgetful and has been diagnosed with depression, though she says she does not feel depressed.1,5,6 Nothing is pleasant to her, but she is prone to abnormal excitement and unpredictable behavior.1,4,6,7
A physical exam shows slow breathing, bradycardia, decreased deep tendon reflexes, and decreased muscle tone.1,5,6,9 Ms. V complains of double vision1,5,6,9 and problems with gait and balance,5,6,9 as well as tremors.1,4-7 She experienced enuresis well into adulthood1,5,6,9 and is prone to weight gain, dyspepsia, and constipation.8,9 She cannot understand people who have anxiety, and is prone to melancholy.4-6,9 Ms. V had been treated with electroconvulsive therapy in the past but states that she “had to have so much electricity, they gave up on me.”
Impression. Ms. V exhibits multiple symptoms associated with GABA excess. Dopaminergic medications such as methylphenidate or amphetamines may be helpful, as they suppress GABA. GABAergic medications and supplements should be avoided in such a patient. Noradrenergic medications including antidepressants with corresponding activity or vasopressors may be beneficial. Suppression of glutamate increases GABA, which is why ketamine in any formulation should be avoided in a patient such as Ms. V.
GABA deficiency (Table 11-4,6,9-17)
Mr. N complains of depression,1,3,4,6,12,16 pain all over his body, tingling in his hands and feet,1,6,9 a constant dull headache,2 and severe insomnia.2,3,9,10 He cannot control his anxiety and, in general, has problems relaxing. In the office, he is jumpy, tremulous, and fidgety during the interview and examination.1,3,4,6,9,12 His muscle tone is high1,9,11 and he feels stiff.6,9 Mr. N’s pupils are narrow1,9; he is hyper-reflexive1,9,11 and reports “Klonopin withdrawal seizures.”1,6,9 He loves alcohol because “it makes me feel good” and helps with his mind, which otherwise “never stops.”1,6,13 Mr. N is frequently anxious and very sensitive to pain, especially when he is upset. He was diagnosed with fibromyalgia by his primary care doctor, who says that irritable bowel is common in patients like him.1,6 His anxiety disables him.1-4,6,9-12 His sister reports that in addition to having difficulty relaxing, Mr. N is easily frustrated and sleeps poorly because he says he has racing thoughts.10 She mentions that her brother’s gambling addiction endangered his finances on several occasions4,12,15 and he was suspected of having autism spectrum disorder.4,12 Mr. N is frequently overwhelmed, including during your interview.1,3,4,6 He is sensitive to light and noise1,9 and complains of palpitations1,3,4,6,9 and frequent shortness of breath.1,3,4,9 He mentions his hands and feet often are cold, especially when he is anxious.1,3,4,6,9 Not eating at regular times makes his symptoms even worse. Mr. N commonly feels depressed, but his anxiety is more bothersome.1,3,4,6,12,16 His ongoing complaints include difficulty concentrating and memory problems,3,4,12,13 as well as a constant feeling of being overwhelmed.1,3,4,6 His restless leg syndrome requires ongoing treatment.1,9,14 Though uncommon, Mr. N has episodes of slowing and weakness, which are associated with growth hormone problems.16 In the past, he experienced gut motility dysregulation9,10 and prolonged bleeding that worried his doctors.17
Impression. Mr. N shows multiple symptoms associated with GABA deficiency. The deficiency of GABA activity ultimately causes an increase in norepinephrine and dopamine firing; therefore, symptoms of GABA deficiency are partially aligned with symptoms of dopamine and norepinephrine excess. GABAergic medications would be most beneficial for such patients. Anticonvulsants (eg, gabapentin and pregabalin) are preferable. Acamprostate may be considered. For long-term use, benzodiapines are as problematic as opioids and should be avoided, if possible. The use of opioids in such patients is especially counterproductive. Some supplements and vitamins may enhance GABA activity. Avoiding bupropion and stimulants would be wise. Ketamine in any formulation would be a good choice in this scenario. Sedating antipsychotic medications have a promise for patients such as Mr. N. The muscle relaxant baclofen frequently helps with these patients’ pain, anxiety, and sleep.
Continue to: Glutamate excess
Glutamate excess (Table 29,18-30)
Mr. B is anxious and bites his fingernails and cheek while you interview him.18 He has scars on his lower arms that were caused by years of picking his skin.18 He complains of headache28-30 and deep muscle, whole body,19-23 and abdominal pain.20 Both hyperesthesia (he calls it “fibromyalgia”)9,19,20,22 and irritable bowel syndrome flare up if he eats Chinese food that contains monosodium glutamate.21 This also increases nausea, vomiting, and hypertensive episodes.9,19,20,22,24,26 Mr. B developed and received treatment for opioid use disorder after being prescribed morphine for the treatment of fibromyalgia.22 He is being treated for posttraumatic stress disorder at the VA hospital and is bitter that his flashbacks are not controlled.23 Once, he experienced a frank psychosis.26 He commonly experiences dissociative symptoms and suicidality.23,26 The sensations of crawling skin,18 panic attacks, and nightmares complicate his life.23 Mr. B is angry that his “incompetent” psychiatrist stopped his diazepam and that it “almost killed him” by causing delirium.24 He suffers from severe neuropathic pain in his feet and says that his pain, depression, and anxiety respond especially well to ketamine treatment.9,23,26 He is prone to euphoria and has had several manic episodes.26 In childhood, his parents brought him to a psychiatrist to address episodes of head-banging and self-hitting.18 Mr. B developed seizures; presently, they are controlled, but he remains chronically dizzy.9,24,25,27 He claims that his headaches and migraines respond only to methadone and that sumatriptan makes them worse, especially in prolonged treatment.28-30 He is tachycardic, tremulous, and makes you feel deeply uneasy.9,24
Impression. Mr. B has many symptoms of glutamate hyperactivity. The use of N-methyl-
Glutamate deficiency (Table 29,32-38)
Mr. Z feels dull, fatigued, and unhappy.32,33,37 He is overweight and moves slowly. Sometimes he is so slow and clumsy that he seems obtunded.9,36,37 He states that his peripheral neuropathy does not cause him pain, though his neurodiagnostic results are unfavorable.32 Mr. Z’s overall pain threshold is high, and he is unhappy with people who complain about pain because “who cares?”32 His memory and concentration were never good.33,37,38 He suffers from insomnia and is frequently miserable and disheartened.32,33,38 People view him as melancholic.33,37 Mr. Z is mildly depressed, but he experiences aggressive outbursts37,38 and bouts of anxiety,32,33,36,38 psychosis, and mania.33,37,38 He is visibly confused37 and says it is easy for him to get disoriented and lost.37,38 His medical history includes long-term constipation and several episodes of ileus.9,34,35 His childhood-onset seizures are controlled presently.33 He complains of frequent bouts of dizziness and headache.32,34,35 On physical exam, Mr. Z has dry mouth, hypotension, diminished deep tendon reflexes, and bradycardia.9,34,35 He sought a consultation from an ophthalmologist to evaluate an eye movement problem.33,36 No cause was found, but the ophthalmologist thought this problem might have the same underlying mechanism as his dysarthria.33 Mr. Z’s balance is bothersome, but his podiatrist was unable to help him to correct his abnormal gait.33-36 A friend who came with Mr. Z mentioned she had noticed personality changes in him over the last several months.37
Impression. Mr. Z exhibits multiple signs of low glutamatergic function. Amino acid taurine has been shown in rodents to increase brain levels of both GABA and glutamate. Glutamate is metabolized into GABA, so low glutamate and low GABA symptoms overlap. Glutamine, which is present in meat, fish, eggs, dairy, wheat, and some vegetables, is converted in the body into glutamate and may be considered for a patient with low glutamate function. The medication approach to such a patient would be similar to the treatment of a low GABA patient and includes glutamate-enhancing magnesium and dextromethorphan.
Rarely is just 1 neurotransmitter involved
Most real-world patients have mixed presentations with more than 1 neurotransmitter implicated in the pathology of their symptoms. A clinician’s ability to dissect the clinical picture and select an appropriate treatment must be based on history and observed behavior because no lab results or reliable tests are presently available.
Continue to: The most studied...
The most studied neurotransmitter in depression and anxiety is serotonin, and for many years psychiatrists have paid too much attention to it. Similarly, pain physicians have been overly focused on the opioid system. Excessive attention to these neurochemicals has overshadowed multiple other (no less impactful) neurotransmitters. Dopamine is frequently not attended to by many physicians who treat chronic pain. Psychiatrists also may overlook underlying endorphin or glutamate dysfunction in patients with psychiatric illness.
Nonpharmacologic approaches can affect neurotransmitters
With all the emphasis on pharmacologic treatments, it is important to remember that nonpharmacologic modalities such as exercise, diet, hydrotherapy, acupuncture, and psychotherapy can help normalize neurotransmitter function in the brain and ultimately help patients with chronic conditions. Careful use of nutritional supplements and vitamins may also be beneficial.
A hypothesis for future research
Multiple peripheral and central mechanisms define various chronic pain and psychiatric symptoms and disorders, including depression, anxiety, and fibromyalgia. The variety of mechanisms of pathologic mood and pain perception may be expressed to a different extent and in countless combinations in individual patients. This, in part, explains the variable responses to the same treatment observed in similar patients, or even in the same patient.
Clinicians should always remember that depression and anxiety as well as chronic pain (including fibromyalgia and chronic headache) are not a representation of a single condition but are the result of an assembly of different syndromes; therefore, 1 treatment does not fit all patients. Pain is ultimately recognized and comprehended centrally, making it very much a neuropsychiatric field. The optimal treatment for 2 patients with similar pain or psychiatric symptoms may be drastically different due to different underlying mechanisms that can be distinguished by looking at the symptoms other than “pain” or “depression.”
Remembering that every neurotransmitter deficiency or excess has an identifiable clinical correlation is important. Basing a treatment approach on a specific clinical presentation in a particular depressed or chronic pain patient would assure a more successful and reliable outcome.
Continue to: This 3-part series...
This 3-part series was designed to bring attention to a notion that diagnosis and treatment of diverse conditions such as “depression,” “anxiety,” or “chronic pain” should be based on clinically identifiable symptoms that may suggest specific neurotransmitter(s) involved in a specific type of each of these conditions. However, there are no well-recognized, well-established, reliable, or validated syndromes described in this series. The collection of symptoms associated with the various neurotransmitters described in this series is not complete. We have assembled what is described in the literature as a suggestion for future research.
Bottom Line
Both high and low levels of gamma aminobutyric acid (GABA) and glutamate may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 2). Current Psychiatry. 2022;21(6):28-33. doi:10.12788/cp.0253
Drug Brand Names
Acamprostate • Campral
Amantadine • Gocovri
Bupropion • Wellbutrin
Clonazepam • Klonopin
Clonidine • Catapres
Diazepam • Valium
Gabapentin • Neurontin
Ketamine • Ketalar
Memantine • Namenda
Methylphenidate • Concerta
Morphine • Kadian
Pregabalin • Lyrica
Sumatriptan • Imitrex
Tizanidine • Zanaflex
1. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
2. Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31(11):1499-1506.
3. Pereira AC, Mao X, Jiang CS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114(38):10250-10255.
4. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352.
5. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
6. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229-233.
7. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445-461.
8. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability, and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
9. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Elsevier; 2011:550-551,692-693.
10. Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239-244.
11. Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23(4):145-151.
12. Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-120.
13. Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in the brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-2705.
14. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Neurochemical features of idiopathic restless legs syndrome. Sleep Med Rev. 2019;45:70-87.
15. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
16. Brambilla P, Perez J, Barale F, et al. Gabaergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721-737.
17. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20(5):328-333.
18. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702.
19. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
20. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98(1-2):69-78.
21. Holton KF, Taren DL, Thomson CA, et al. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin Exp Rheumatol. 2012;30(6 Suppl 74):10-70.
22. Sekiya Y, Nakagawa T, Ozawa T, et al. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxy aspartate in rats. Eur J Pharmacol. 2004;485(1-3):201-210.
23. Bestha D, Soliman L, Blankenship K. et al. The walking wounded: emerging treatments for PTSD. Curr Psychiatry Rep. 2018;20(10):94.
24. Tsuda M, Shimizu N, Suzuki T. Contribution of glutamate receptors to benzodiazepine withdrawal signs. Jpn J Pharmacol. 1999;81(1):1-6.
25. Spravato [package insert]. Janssen Pharmaceuticals, Inc; 2019.
26. Mattingly GW, Anderson RH. Intranasal ketamine. Current Psychiatry. 2019;18(5):31-38.
27. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269-1275.
28. Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatment of migraines lower plasma glutamate levels. Cephalalgia. 2009;29(4):423-429.
29. Vieira DS, Naffah-Mazzacoratti Mda G, Zukerman E, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache. 2007;47(6):842-847.
30. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165-1176.
31. Pappa S, Tsouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
32. Kraal AZ, Arvanitis NR, Jaeger AP, et al. Could dietary glutamate play a role in psychiatric distress? Neuro Psych. 2020;79:13-19.
33. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 2014;121(8):1029-1075.
34. Lancaster E. CNS syndromes associated with antibodies against metabotropic receptors. Curr Opin Neurol. 2017;30:354-360.
35. Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27.
36. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627-630.
37. Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698-1701.
38. Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80(14):1349-1350.
Optimal diagnosis and treatment of psychiatric illness requires clinicians to be able to connect mental and physical symptoms. Direct brain neurotransmitter testing is presently in its infancy and the science of defining the underlying mechanisms of psychiatric disorders lags behind the obvious clinical needs. We are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. In this article series, we suggest an indirect way of judging neurotransmitter activity by recognizing specific mental and physical symptoms connected by common biology. Here we present hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. The descriptions we present in this series do not reflect the entire set of symptoms caused by the neurotransmitters we discuss; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypothesis we present. We argue that in cases of multiple psychiatric disorders and chronic pain, the development and approval of medications currently is based on an umbrella descriptive diagnoses, and disregards the various underlying causes of such conditions. Similar to how the many types of pneumonias are treated differently depending on the infective agent, we suggested the same possible causative approach to various types of depression and pain.

Part 1 of this series (

GABA excess (Table 11-9)
Ms. V is brought to your office by a friend. She complains of pain all over her body, itchiness, inability to focus, and dizziness.1,5,6,9 She is puzzled by how little pain she feels when she cuts her finger but by how much pain she is in every day, though her clinicians have not discovered a reason for her pain.1,6,9 She states that her fatigue is so severe that she can sleep 15 hours a day.1-6,9 Her obstructive and central sleep apnea have been treated, but this did not improve her fatigue.3,5,9 She is forgetful and has been diagnosed with depression, though she says she does not feel depressed.1,5,6 Nothing is pleasant to her, but she is prone to abnormal excitement and unpredictable behavior.1,4,6,7
A physical exam shows slow breathing, bradycardia, decreased deep tendon reflexes, and decreased muscle tone.1,5,6,9 Ms. V complains of double vision1,5,6,9 and problems with gait and balance,5,6,9 as well as tremors.1,4-7 She experienced enuresis well into adulthood1,5,6,9 and is prone to weight gain, dyspepsia, and constipation.8,9 She cannot understand people who have anxiety, and is prone to melancholy.4-6,9 Ms. V had been treated with electroconvulsive therapy in the past but states that she “had to have so much electricity, they gave up on me.”
Impression. Ms. V exhibits multiple symptoms associated with GABA excess. Dopaminergic medications such as methylphenidate or amphetamines may be helpful, as they suppress GABA. GABAergic medications and supplements should be avoided in such a patient. Noradrenergic medications including antidepressants with corresponding activity or vasopressors may be beneficial. Suppression of glutamate increases GABA, which is why ketamine in any formulation should be avoided in a patient such as Ms. V.
GABA deficiency (Table 11-4,6,9-17)
Mr. N complains of depression,1,3,4,6,12,16 pain all over his body, tingling in his hands and feet,1,6,9 a constant dull headache,2 and severe insomnia.2,3,9,10 He cannot control his anxiety and, in general, has problems relaxing. In the office, he is jumpy, tremulous, and fidgety during the interview and examination.1,3,4,6,9,12 His muscle tone is high1,9,11 and he feels stiff.6,9 Mr. N’s pupils are narrow1,9; he is hyper-reflexive1,9,11 and reports “Klonopin withdrawal seizures.”1,6,9 He loves alcohol because “it makes me feel good” and helps with his mind, which otherwise “never stops.”1,6,13 Mr. N is frequently anxious and very sensitive to pain, especially when he is upset. He was diagnosed with fibromyalgia by his primary care doctor, who says that irritable bowel is common in patients like him.1,6 His anxiety disables him.1-4,6,9-12 His sister reports that in addition to having difficulty relaxing, Mr. N is easily frustrated and sleeps poorly because he says he has racing thoughts.10 She mentions that her brother’s gambling addiction endangered his finances on several occasions4,12,15 and he was suspected of having autism spectrum disorder.4,12 Mr. N is frequently overwhelmed, including during your interview.1,3,4,6 He is sensitive to light and noise1,9 and complains of palpitations1,3,4,6,9 and frequent shortness of breath.1,3,4,9 He mentions his hands and feet often are cold, especially when he is anxious.1,3,4,6,9 Not eating at regular times makes his symptoms even worse. Mr. N commonly feels depressed, but his anxiety is more bothersome.1,3,4,6,12,16 His ongoing complaints include difficulty concentrating and memory problems,3,4,12,13 as well as a constant feeling of being overwhelmed.1,3,4,6 His restless leg syndrome requires ongoing treatment.1,9,14 Though uncommon, Mr. N has episodes of slowing and weakness, which are associated with growth hormone problems.16 In the past, he experienced gut motility dysregulation9,10 and prolonged bleeding that worried his doctors.17
Impression. Mr. N shows multiple symptoms associated with GABA deficiency. The deficiency of GABA activity ultimately causes an increase in norepinephrine and dopamine firing; therefore, symptoms of GABA deficiency are partially aligned with symptoms of dopamine and norepinephrine excess. GABAergic medications would be most beneficial for such patients. Anticonvulsants (eg, gabapentin and pregabalin) are preferable. Acamprostate may be considered. For long-term use, benzodiapines are as problematic as opioids and should be avoided, if possible. The use of opioids in such patients is especially counterproductive. Some supplements and vitamins may enhance GABA activity. Avoiding bupropion and stimulants would be wise. Ketamine in any formulation would be a good choice in this scenario. Sedating antipsychotic medications have a promise for patients such as Mr. N. The muscle relaxant baclofen frequently helps with these patients’ pain, anxiety, and sleep.
Continue to: Glutamate excess
Glutamate excess (Table 29,18-30)
Mr. B is anxious and bites his fingernails and cheek while you interview him.18 He has scars on his lower arms that were caused by years of picking his skin.18 He complains of headache28-30 and deep muscle, whole body,19-23 and abdominal pain.20 Both hyperesthesia (he calls it “fibromyalgia”)9,19,20,22 and irritable bowel syndrome flare up if he eats Chinese food that contains monosodium glutamate.21 This also increases nausea, vomiting, and hypertensive episodes.9,19,20,22,24,26 Mr. B developed and received treatment for opioid use disorder after being prescribed morphine for the treatment of fibromyalgia.22 He is being treated for posttraumatic stress disorder at the VA hospital and is bitter that his flashbacks are not controlled.23 Once, he experienced a frank psychosis.26 He commonly experiences dissociative symptoms and suicidality.23,26 The sensations of crawling skin,18 panic attacks, and nightmares complicate his life.23 Mr. B is angry that his “incompetent” psychiatrist stopped his diazepam and that it “almost killed him” by causing delirium.24 He suffers from severe neuropathic pain in his feet and says that his pain, depression, and anxiety respond especially well to ketamine treatment.9,23,26 He is prone to euphoria and has had several manic episodes.26 In childhood, his parents brought him to a psychiatrist to address episodes of head-banging and self-hitting.18 Mr. B developed seizures; presently, they are controlled, but he remains chronically dizzy.9,24,25,27 He claims that his headaches and migraines respond only to methadone and that sumatriptan makes them worse, especially in prolonged treatment.28-30 He is tachycardic, tremulous, and makes you feel deeply uneasy.9,24
Impression. Mr. B has many symptoms of glutamate hyperactivity. The use of N-methyl-
Glutamate deficiency (Table 29,32-38)
Mr. Z feels dull, fatigued, and unhappy.32,33,37 He is overweight and moves slowly. Sometimes he is so slow and clumsy that he seems obtunded.9,36,37 He states that his peripheral neuropathy does not cause him pain, though his neurodiagnostic results are unfavorable.32 Mr. Z’s overall pain threshold is high, and he is unhappy with people who complain about pain because “who cares?”32 His memory and concentration were never good.33,37,38 He suffers from insomnia and is frequently miserable and disheartened.32,33,38 People view him as melancholic.33,37 Mr. Z is mildly depressed, but he experiences aggressive outbursts37,38 and bouts of anxiety,32,33,36,38 psychosis, and mania.33,37,38 He is visibly confused37 and says it is easy for him to get disoriented and lost.37,38 His medical history includes long-term constipation and several episodes of ileus.9,34,35 His childhood-onset seizures are controlled presently.33 He complains of frequent bouts of dizziness and headache.32,34,35 On physical exam, Mr. Z has dry mouth, hypotension, diminished deep tendon reflexes, and bradycardia.9,34,35 He sought a consultation from an ophthalmologist to evaluate an eye movement problem.33,36 No cause was found, but the ophthalmologist thought this problem might have the same underlying mechanism as his dysarthria.33 Mr. Z’s balance is bothersome, but his podiatrist was unable to help him to correct his abnormal gait.33-36 A friend who came with Mr. Z mentioned she had noticed personality changes in him over the last several months.37
Impression. Mr. Z exhibits multiple signs of low glutamatergic function. Amino acid taurine has been shown in rodents to increase brain levels of both GABA and glutamate. Glutamate is metabolized into GABA, so low glutamate and low GABA symptoms overlap. Glutamine, which is present in meat, fish, eggs, dairy, wheat, and some vegetables, is converted in the body into glutamate and may be considered for a patient with low glutamate function. The medication approach to such a patient would be similar to the treatment of a low GABA patient and includes glutamate-enhancing magnesium and dextromethorphan.
Rarely is just 1 neurotransmitter involved
Most real-world patients have mixed presentations with more than 1 neurotransmitter implicated in the pathology of their symptoms. A clinician’s ability to dissect the clinical picture and select an appropriate treatment must be based on history and observed behavior because no lab results or reliable tests are presently available.
Continue to: The most studied...
The most studied neurotransmitter in depression and anxiety is serotonin, and for many years psychiatrists have paid too much attention to it. Similarly, pain physicians have been overly focused on the opioid system. Excessive attention to these neurochemicals has overshadowed multiple other (no less impactful) neurotransmitters. Dopamine is frequently not attended to by many physicians who treat chronic pain. Psychiatrists also may overlook underlying endorphin or glutamate dysfunction in patients with psychiatric illness.
Nonpharmacologic approaches can affect neurotransmitters
With all the emphasis on pharmacologic treatments, it is important to remember that nonpharmacologic modalities such as exercise, diet, hydrotherapy, acupuncture, and psychotherapy can help normalize neurotransmitter function in the brain and ultimately help patients with chronic conditions. Careful use of nutritional supplements and vitamins may also be beneficial.
A hypothesis for future research
Multiple peripheral and central mechanisms define various chronic pain and psychiatric symptoms and disorders, including depression, anxiety, and fibromyalgia. The variety of mechanisms of pathologic mood and pain perception may be expressed to a different extent and in countless combinations in individual patients. This, in part, explains the variable responses to the same treatment observed in similar patients, or even in the same patient.
Clinicians should always remember that depression and anxiety as well as chronic pain (including fibromyalgia and chronic headache) are not a representation of a single condition but are the result of an assembly of different syndromes; therefore, 1 treatment does not fit all patients. Pain is ultimately recognized and comprehended centrally, making it very much a neuropsychiatric field. The optimal treatment for 2 patients with similar pain or psychiatric symptoms may be drastically different due to different underlying mechanisms that can be distinguished by looking at the symptoms other than “pain” or “depression.”
Remembering that every neurotransmitter deficiency or excess has an identifiable clinical correlation is important. Basing a treatment approach on a specific clinical presentation in a particular depressed or chronic pain patient would assure a more successful and reliable outcome.
Continue to: This 3-part series...
This 3-part series was designed to bring attention to a notion that diagnosis and treatment of diverse conditions such as “depression,” “anxiety,” or “chronic pain” should be based on clinically identifiable symptoms that may suggest specific neurotransmitter(s) involved in a specific type of each of these conditions. However, there are no well-recognized, well-established, reliable, or validated syndromes described in this series. The collection of symptoms associated with the various neurotransmitters described in this series is not complete. We have assembled what is described in the literature as a suggestion for future research.
Bottom Line
Both high and low levels of gamma aminobutyric acid (GABA) and glutamate may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 2). Current Psychiatry. 2022;21(6):28-33. doi:10.12788/cp.0253
Drug Brand Names
Acamprostate • Campral
Amantadine • Gocovri
Bupropion • Wellbutrin
Clonazepam • Klonopin
Clonidine • Catapres
Diazepam • Valium
Gabapentin • Neurontin
Ketamine • Ketalar
Memantine • Namenda
Methylphenidate • Concerta
Morphine • Kadian
Pregabalin • Lyrica
Sumatriptan • Imitrex
Tizanidine • Zanaflex
Optimal diagnosis and treatment of psychiatric illness requires clinicians to be able to connect mental and physical symptoms. Direct brain neurotransmitter testing is presently in its infancy and the science of defining the underlying mechanisms of psychiatric disorders lags behind the obvious clinical needs. We are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. In this article series, we suggest an indirect way of judging neurotransmitter activity by recognizing specific mental and physical symptoms connected by common biology. Here we present hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. The descriptions we present in this series do not reflect the entire set of symptoms caused by the neurotransmitters we discuss; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypothesis we present. We argue that in cases of multiple psychiatric disorders and chronic pain, the development and approval of medications currently is based on an umbrella descriptive diagnoses, and disregards the various underlying causes of such conditions. Similar to how the many types of pneumonias are treated differently depending on the infective agent, we suggested the same possible causative approach to various types of depression and pain.

Part 1 of this series (

GABA excess (Table 11-9)
Ms. V is brought to your office by a friend. She complains of pain all over her body, itchiness, inability to focus, and dizziness.1,5,6,9 She is puzzled by how little pain she feels when she cuts her finger but by how much pain she is in every day, though her clinicians have not discovered a reason for her pain.1,6,9 She states that her fatigue is so severe that she can sleep 15 hours a day.1-6,9 Her obstructive and central sleep apnea have been treated, but this did not improve her fatigue.3,5,9 She is forgetful and has been diagnosed with depression, though she says she does not feel depressed.1,5,6 Nothing is pleasant to her, but she is prone to abnormal excitement and unpredictable behavior.1,4,6,7
A physical exam shows slow breathing, bradycardia, decreased deep tendon reflexes, and decreased muscle tone.1,5,6,9 Ms. V complains of double vision1,5,6,9 and problems with gait and balance,5,6,9 as well as tremors.1,4-7 She experienced enuresis well into adulthood1,5,6,9 and is prone to weight gain, dyspepsia, and constipation.8,9 She cannot understand people who have anxiety, and is prone to melancholy.4-6,9 Ms. V had been treated with electroconvulsive therapy in the past but states that she “had to have so much electricity, they gave up on me.”
Impression. Ms. V exhibits multiple symptoms associated with GABA excess. Dopaminergic medications such as methylphenidate or amphetamines may be helpful, as they suppress GABA. GABAergic medications and supplements should be avoided in such a patient. Noradrenergic medications including antidepressants with corresponding activity or vasopressors may be beneficial. Suppression of glutamate increases GABA, which is why ketamine in any formulation should be avoided in a patient such as Ms. V.
GABA deficiency (Table 11-4,6,9-17)
Mr. N complains of depression,1,3,4,6,12,16 pain all over his body, tingling in his hands and feet,1,6,9 a constant dull headache,2 and severe insomnia.2,3,9,10 He cannot control his anxiety and, in general, has problems relaxing. In the office, he is jumpy, tremulous, and fidgety during the interview and examination.1,3,4,6,9,12 His muscle tone is high1,9,11 and he feels stiff.6,9 Mr. N’s pupils are narrow1,9; he is hyper-reflexive1,9,11 and reports “Klonopin withdrawal seizures.”1,6,9 He loves alcohol because “it makes me feel good” and helps with his mind, which otherwise “never stops.”1,6,13 Mr. N is frequently anxious and very sensitive to pain, especially when he is upset. He was diagnosed with fibromyalgia by his primary care doctor, who says that irritable bowel is common in patients like him.1,6 His anxiety disables him.1-4,6,9-12 His sister reports that in addition to having difficulty relaxing, Mr. N is easily frustrated and sleeps poorly because he says he has racing thoughts.10 She mentions that her brother’s gambling addiction endangered his finances on several occasions4,12,15 and he was suspected of having autism spectrum disorder.4,12 Mr. N is frequently overwhelmed, including during your interview.1,3,4,6 He is sensitive to light and noise1,9 and complains of palpitations1,3,4,6,9 and frequent shortness of breath.1,3,4,9 He mentions his hands and feet often are cold, especially when he is anxious.1,3,4,6,9 Not eating at regular times makes his symptoms even worse. Mr. N commonly feels depressed, but his anxiety is more bothersome.1,3,4,6,12,16 His ongoing complaints include difficulty concentrating and memory problems,3,4,12,13 as well as a constant feeling of being overwhelmed.1,3,4,6 His restless leg syndrome requires ongoing treatment.1,9,14 Though uncommon, Mr. N has episodes of slowing and weakness, which are associated with growth hormone problems.16 In the past, he experienced gut motility dysregulation9,10 and prolonged bleeding that worried his doctors.17
Impression. Mr. N shows multiple symptoms associated with GABA deficiency. The deficiency of GABA activity ultimately causes an increase in norepinephrine and dopamine firing; therefore, symptoms of GABA deficiency are partially aligned with symptoms of dopamine and norepinephrine excess. GABAergic medications would be most beneficial for such patients. Anticonvulsants (eg, gabapentin and pregabalin) are preferable. Acamprostate may be considered. For long-term use, benzodiapines are as problematic as opioids and should be avoided, if possible. The use of opioids in such patients is especially counterproductive. Some supplements and vitamins may enhance GABA activity. Avoiding bupropion and stimulants would be wise. Ketamine in any formulation would be a good choice in this scenario. Sedating antipsychotic medications have a promise for patients such as Mr. N. The muscle relaxant baclofen frequently helps with these patients’ pain, anxiety, and sleep.
Continue to: Glutamate excess
Glutamate excess (Table 29,18-30)
Mr. B is anxious and bites his fingernails and cheek while you interview him.18 He has scars on his lower arms that were caused by years of picking his skin.18 He complains of headache28-30 and deep muscle, whole body,19-23 and abdominal pain.20 Both hyperesthesia (he calls it “fibromyalgia”)9,19,20,22 and irritable bowel syndrome flare up if he eats Chinese food that contains monosodium glutamate.21 This also increases nausea, vomiting, and hypertensive episodes.9,19,20,22,24,26 Mr. B developed and received treatment for opioid use disorder after being prescribed morphine for the treatment of fibromyalgia.22 He is being treated for posttraumatic stress disorder at the VA hospital and is bitter that his flashbacks are not controlled.23 Once, he experienced a frank psychosis.26 He commonly experiences dissociative symptoms and suicidality.23,26 The sensations of crawling skin,18 panic attacks, and nightmares complicate his life.23 Mr. B is angry that his “incompetent” psychiatrist stopped his diazepam and that it “almost killed him” by causing delirium.24 He suffers from severe neuropathic pain in his feet and says that his pain, depression, and anxiety respond especially well to ketamine treatment.9,23,26 He is prone to euphoria and has had several manic episodes.26 In childhood, his parents brought him to a psychiatrist to address episodes of head-banging and self-hitting.18 Mr. B developed seizures; presently, they are controlled, but he remains chronically dizzy.9,24,25,27 He claims that his headaches and migraines respond only to methadone and that sumatriptan makes them worse, especially in prolonged treatment.28-30 He is tachycardic, tremulous, and makes you feel deeply uneasy.9,24
Impression. Mr. B has many symptoms of glutamate hyperactivity. The use of N-methyl-
Glutamate deficiency (Table 29,32-38)
Mr. Z feels dull, fatigued, and unhappy.32,33,37 He is overweight and moves slowly. Sometimes he is so slow and clumsy that he seems obtunded.9,36,37 He states that his peripheral neuropathy does not cause him pain, though his neurodiagnostic results are unfavorable.32 Mr. Z’s overall pain threshold is high, and he is unhappy with people who complain about pain because “who cares?”32 His memory and concentration were never good.33,37,38 He suffers from insomnia and is frequently miserable and disheartened.32,33,38 People view him as melancholic.33,37 Mr. Z is mildly depressed, but he experiences aggressive outbursts37,38 and bouts of anxiety,32,33,36,38 psychosis, and mania.33,37,38 He is visibly confused37 and says it is easy for him to get disoriented and lost.37,38 His medical history includes long-term constipation and several episodes of ileus.9,34,35 His childhood-onset seizures are controlled presently.33 He complains of frequent bouts of dizziness and headache.32,34,35 On physical exam, Mr. Z has dry mouth, hypotension, diminished deep tendon reflexes, and bradycardia.9,34,35 He sought a consultation from an ophthalmologist to evaluate an eye movement problem.33,36 No cause was found, but the ophthalmologist thought this problem might have the same underlying mechanism as his dysarthria.33 Mr. Z’s balance is bothersome, but his podiatrist was unable to help him to correct his abnormal gait.33-36 A friend who came with Mr. Z mentioned she had noticed personality changes in him over the last several months.37
Impression. Mr. Z exhibits multiple signs of low glutamatergic function. Amino acid taurine has been shown in rodents to increase brain levels of both GABA and glutamate. Glutamate is metabolized into GABA, so low glutamate and low GABA symptoms overlap. Glutamine, which is present in meat, fish, eggs, dairy, wheat, and some vegetables, is converted in the body into glutamate and may be considered for a patient with low glutamate function. The medication approach to such a patient would be similar to the treatment of a low GABA patient and includes glutamate-enhancing magnesium and dextromethorphan.
Rarely is just 1 neurotransmitter involved
Most real-world patients have mixed presentations with more than 1 neurotransmitter implicated in the pathology of their symptoms. A clinician’s ability to dissect the clinical picture and select an appropriate treatment must be based on history and observed behavior because no lab results or reliable tests are presently available.
Continue to: The most studied...
The most studied neurotransmitter in depression and anxiety is serotonin, and for many years psychiatrists have paid too much attention to it. Similarly, pain physicians have been overly focused on the opioid system. Excessive attention to these neurochemicals has overshadowed multiple other (no less impactful) neurotransmitters. Dopamine is frequently not attended to by many physicians who treat chronic pain. Psychiatrists also may overlook underlying endorphin or glutamate dysfunction in patients with psychiatric illness.
Nonpharmacologic approaches can affect neurotransmitters
With all the emphasis on pharmacologic treatments, it is important to remember that nonpharmacologic modalities such as exercise, diet, hydrotherapy, acupuncture, and psychotherapy can help normalize neurotransmitter function in the brain and ultimately help patients with chronic conditions. Careful use of nutritional supplements and vitamins may also be beneficial.
A hypothesis for future research
Multiple peripheral and central mechanisms define various chronic pain and psychiatric symptoms and disorders, including depression, anxiety, and fibromyalgia. The variety of mechanisms of pathologic mood and pain perception may be expressed to a different extent and in countless combinations in individual patients. This, in part, explains the variable responses to the same treatment observed in similar patients, or even in the same patient.
Clinicians should always remember that depression and anxiety as well as chronic pain (including fibromyalgia and chronic headache) are not a representation of a single condition but are the result of an assembly of different syndromes; therefore, 1 treatment does not fit all patients. Pain is ultimately recognized and comprehended centrally, making it very much a neuropsychiatric field. The optimal treatment for 2 patients with similar pain or psychiatric symptoms may be drastically different due to different underlying mechanisms that can be distinguished by looking at the symptoms other than “pain” or “depression.”
Remembering that every neurotransmitter deficiency or excess has an identifiable clinical correlation is important. Basing a treatment approach on a specific clinical presentation in a particular depressed or chronic pain patient would assure a more successful and reliable outcome.
Continue to: This 3-part series...
This 3-part series was designed to bring attention to a notion that diagnosis and treatment of diverse conditions such as “depression,” “anxiety,” or “chronic pain” should be based on clinically identifiable symptoms that may suggest specific neurotransmitter(s) involved in a specific type of each of these conditions. However, there are no well-recognized, well-established, reliable, or validated syndromes described in this series. The collection of symptoms associated with the various neurotransmitters described in this series is not complete. We have assembled what is described in the literature as a suggestion for future research.
Bottom Line
Both high and low levels of gamma aminobutyric acid (GABA) and glutamate may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 2). Current Psychiatry. 2022;21(6):28-33. doi:10.12788/cp.0253
Drug Brand Names
Acamprostate • Campral
Amantadine • Gocovri
Bupropion • Wellbutrin
Clonazepam • Klonopin
Clonidine • Catapres
Diazepam • Valium
Gabapentin • Neurontin
Ketamine • Ketalar
Memantine • Namenda
Methylphenidate • Concerta
Morphine • Kadian
Pregabalin • Lyrica
Sumatriptan • Imitrex
Tizanidine • Zanaflex
1. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
2. Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31(11):1499-1506.
3. Pereira AC, Mao X, Jiang CS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114(38):10250-10255.
4. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352.
5. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
6. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229-233.
7. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445-461.
8. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability, and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
9. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Elsevier; 2011:550-551,692-693.
10. Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239-244.
11. Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23(4):145-151.
12. Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-120.
13. Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in the brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-2705.
14. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Neurochemical features of idiopathic restless legs syndrome. Sleep Med Rev. 2019;45:70-87.
15. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
16. Brambilla P, Perez J, Barale F, et al. Gabaergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721-737.
17. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20(5):328-333.
18. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702.
19. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
20. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98(1-2):69-78.
21. Holton KF, Taren DL, Thomson CA, et al. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin Exp Rheumatol. 2012;30(6 Suppl 74):10-70.
22. Sekiya Y, Nakagawa T, Ozawa T, et al. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxy aspartate in rats. Eur J Pharmacol. 2004;485(1-3):201-210.
23. Bestha D, Soliman L, Blankenship K. et al. The walking wounded: emerging treatments for PTSD. Curr Psychiatry Rep. 2018;20(10):94.
24. Tsuda M, Shimizu N, Suzuki T. Contribution of glutamate receptors to benzodiazepine withdrawal signs. Jpn J Pharmacol. 1999;81(1):1-6.
25. Spravato [package insert]. Janssen Pharmaceuticals, Inc; 2019.
26. Mattingly GW, Anderson RH. Intranasal ketamine. Current Psychiatry. 2019;18(5):31-38.
27. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269-1275.
28. Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatment of migraines lower plasma glutamate levels. Cephalalgia. 2009;29(4):423-429.
29. Vieira DS, Naffah-Mazzacoratti Mda G, Zukerman E, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache. 2007;47(6):842-847.
30. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165-1176.
31. Pappa S, Tsouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
32. Kraal AZ, Arvanitis NR, Jaeger AP, et al. Could dietary glutamate play a role in psychiatric distress? Neuro Psych. 2020;79:13-19.
33. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 2014;121(8):1029-1075.
34. Lancaster E. CNS syndromes associated with antibodies against metabotropic receptors. Curr Opin Neurol. 2017;30:354-360.
35. Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27.
36. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627-630.
37. Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698-1701.
38. Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80(14):1349-1350.
1. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
2. Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31(11):1499-1506.
3. Pereira AC, Mao X, Jiang CS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114(38):10250-10255.
4. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352.
5. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
6. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229-233.
7. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445-461.
8. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability, and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
9. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Elsevier; 2011:550-551,692-693.
10. Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239-244.
11. Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23(4):145-151.
12. Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-120.
13. Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in the brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-2705.
14. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Neurochemical features of idiopathic restless legs syndrome. Sleep Med Rev. 2019;45:70-87.
15. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
16. Brambilla P, Perez J, Barale F, et al. Gabaergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721-737.
17. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20(5):328-333.
18. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702.
19. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
20. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98(1-2):69-78.
21. Holton KF, Taren DL, Thomson CA, et al. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin Exp Rheumatol. 2012;30(6 Suppl 74):10-70.
22. Sekiya Y, Nakagawa T, Ozawa T, et al. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxy aspartate in rats. Eur J Pharmacol. 2004;485(1-3):201-210.
23. Bestha D, Soliman L, Blankenship K. et al. The walking wounded: emerging treatments for PTSD. Curr Psychiatry Rep. 2018;20(10):94.
24. Tsuda M, Shimizu N, Suzuki T. Contribution of glutamate receptors to benzodiazepine withdrawal signs. Jpn J Pharmacol. 1999;81(1):1-6.
25. Spravato [package insert]. Janssen Pharmaceuticals, Inc; 2019.
26. Mattingly GW, Anderson RH. Intranasal ketamine. Current Psychiatry. 2019;18(5):31-38.
27. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269-1275.
28. Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatment of migraines lower plasma glutamate levels. Cephalalgia. 2009;29(4):423-429.
29. Vieira DS, Naffah-Mazzacoratti Mda G, Zukerman E, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache. 2007;47(6):842-847.
30. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165-1176.
31. Pappa S, Tsouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
32. Kraal AZ, Arvanitis NR, Jaeger AP, et al. Could dietary glutamate play a role in psychiatric distress? Neuro Psych. 2020;79:13-19.
33. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 2014;121(8):1029-1075.
34. Lancaster E. CNS syndromes associated with antibodies against metabotropic receptors. Curr Opin Neurol. 2017;30:354-360.
35. Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27.
36. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627-630.
37. Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698-1701.
38. Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80(14):1349-1350.
3 steps to bend the curve of schizophrenia
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24

Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24

Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24

Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
Smoking cessation: Varenicline and the risk of neuropsychiatric adverse events
Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.
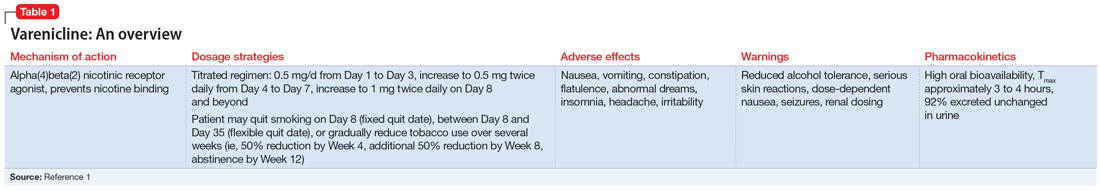
Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.
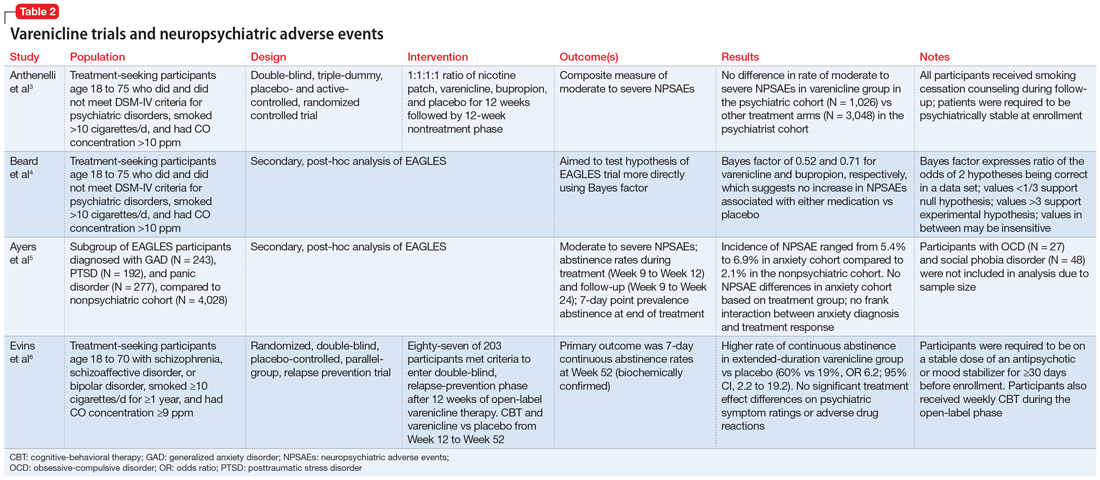
The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES
Further analysis of EAGLES
Beard et al4 used Bayes factor testing for additional analysis of EAGLES data to determine whether the data were insensitive to neuropsychiatric effects secondary to a lack of statistical power. In the psychiatric cohort, the varenicline and bupropion groups exhibited suggestive but not conclusive data that there was no increase in NPSAEs compared to placebo (Bayes factor 0.52 and 0.71, respectively).4
Another EAGLES analysis by Ayers et al5 evaluated participants with anxiety disorders (N = 712), including PTSD (N = 192), generalized anxiety disorder (GAD) (N = 243), and panic disorder (N = 277).Of those with PTSD who received varenicline, there were no statistically significant differences in CAR from Week 9 to Week 12 vs placebo.5 However, there was a significant difference in individuals with GAD (OR 4.53; 95% CI, 1.20 to 17.10), and panic disorder (OR 8.49; 95% CI, 1.57 to 45.78).5 In contrast to CAR from Week 9 to Week 12, 7-day point prevalence abstinence at Week 12 for participants with PTSD was significant (OR 4.04; 95% CI, 1.39 to 11.74) when comparing varenicline to placebo. Within the anxiety disorder cohort, there were no significant differences in moderate to severe NPSAE rates based on treatment group. Calculated risk differences comparing varenicline to placebo were: PTSD group -7.73 (95% CI, -21.95 to 6.49), GAD group 2.80 (95% CI, -6.63 to 12.23), and panic disorder group -0.18 (95% CI, -9.57 to 9.21).5
Other studies
Evins et al6 conducted a randomized controlled trial to evaluate the safety of varenicline maintenance therapy in patients with schizophrenia or bipolar disorder. To be deemed clinically stable, participants in this study needed to be taking a stable dose of an antipsychotic or mood-stabilizing agent(s) for ≥30 days, compared to the 3-month requirement of the EAGLES trial.3,6 Participants received 12 weeks of open-label varenicline; those who achieved abstinence (N = 87) entered the relapse-prevention phase and were randomized to varenicline 1 mg twice a day or placebo for 40 weeks. Of those who entered relapse-prevention, 5 in the placebo group and 2 in the varenicline group were psychiatrically hospitalized (risk ratio 0.45; 95% CI, 0.04 to 2.9).6 These researchers concluded that varenicline maintenance therapy prolonged abstinence rates with no significant increase in neuropsychiatric events.6
Although treatment options for smoking cessation have advanced, individuals with SMI are still disproportionately affected by the negative outcomes of cigarette smoking. Current literature suggests that varenicline does not confer an appreciable risk of neuropsychiatric events in otherwise stable patients and is the preferred first-line treatment. However, there is a gap in understanding the impact of this medication on individuals with unstable psychiatric illness. Health care professionals should be encouraged to use varenicline with careful monitoring for appropriate patients with psychiatric disorders as a standard of care to help them quit smoking.
CASE CONTINUED
After consulting with the psychiatric pharmacist and discussing the risks and benefits of varenicline, Mr. T is started on the appropriate titration schedule (Table 11). A pharmacist provides varenicline education, including the possibility of psychiatric adverse effects, and tells Mr. T to report any worsening psychiatric symptoms. Mr. T is scheduled for frequent follow-up visits to monitor possible adverse effects and his tobacco use. He says he understands the potential adverse effects of varenicline and agrees to frequent follow-up appointments while taking it.
Related Resources
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005.1982ST
- Cieslak K, Freudenreich O. 4 Ways to help your patients with schizophrenia quit smoking. Current Psychiatry. 2018; 17(2):28,33.
Drug Brand Names
Bupropion • Wellbutrin
Varenicline • Chantix
1. Chantix [package insert]. New York, NY: Pfizer Inc; 2019.
2. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120. doi:10.1177/0004867417726176
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/s0140-6736(16)30272-0
4. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824. doi:10.1111/add.15440
5. Ayers CR, Heffner JL, Russ C, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depress Anxiety. 2020;37(3)247-260. doi:10.1002/da.22982
6. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi:10.1001/jama.2013.285113
Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.

Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.

The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES
Further analysis of EAGLES
Beard et al4 used Bayes factor testing for additional analysis of EAGLES data to determine whether the data were insensitive to neuropsychiatric effects secondary to a lack of statistical power. In the psychiatric cohort, the varenicline and bupropion groups exhibited suggestive but not conclusive data that there was no increase in NPSAEs compared to placebo (Bayes factor 0.52 and 0.71, respectively).4
Another EAGLES analysis by Ayers et al5 evaluated participants with anxiety disorders (N = 712), including PTSD (N = 192), generalized anxiety disorder (GAD) (N = 243), and panic disorder (N = 277).Of those with PTSD who received varenicline, there were no statistically significant differences in CAR from Week 9 to Week 12 vs placebo.5 However, there was a significant difference in individuals with GAD (OR 4.53; 95% CI, 1.20 to 17.10), and panic disorder (OR 8.49; 95% CI, 1.57 to 45.78).5 In contrast to CAR from Week 9 to Week 12, 7-day point prevalence abstinence at Week 12 for participants with PTSD was significant (OR 4.04; 95% CI, 1.39 to 11.74) when comparing varenicline to placebo. Within the anxiety disorder cohort, there were no significant differences in moderate to severe NPSAE rates based on treatment group. Calculated risk differences comparing varenicline to placebo were: PTSD group -7.73 (95% CI, -21.95 to 6.49), GAD group 2.80 (95% CI, -6.63 to 12.23), and panic disorder group -0.18 (95% CI, -9.57 to 9.21).5
Other studies
Evins et al6 conducted a randomized controlled trial to evaluate the safety of varenicline maintenance therapy in patients with schizophrenia or bipolar disorder. To be deemed clinically stable, participants in this study needed to be taking a stable dose of an antipsychotic or mood-stabilizing agent(s) for ≥30 days, compared to the 3-month requirement of the EAGLES trial.3,6 Participants received 12 weeks of open-label varenicline; those who achieved abstinence (N = 87) entered the relapse-prevention phase and were randomized to varenicline 1 mg twice a day or placebo for 40 weeks. Of those who entered relapse-prevention, 5 in the placebo group and 2 in the varenicline group were psychiatrically hospitalized (risk ratio 0.45; 95% CI, 0.04 to 2.9).6 These researchers concluded that varenicline maintenance therapy prolonged abstinence rates with no significant increase in neuropsychiatric events.6
Although treatment options for smoking cessation have advanced, individuals with SMI are still disproportionately affected by the negative outcomes of cigarette smoking. Current literature suggests that varenicline does not confer an appreciable risk of neuropsychiatric events in otherwise stable patients and is the preferred first-line treatment. However, there is a gap in understanding the impact of this medication on individuals with unstable psychiatric illness. Health care professionals should be encouraged to use varenicline with careful monitoring for appropriate patients with psychiatric disorders as a standard of care to help them quit smoking.
CASE CONTINUED
After consulting with the psychiatric pharmacist and discussing the risks and benefits of varenicline, Mr. T is started on the appropriate titration schedule (Table 11). A pharmacist provides varenicline education, including the possibility of psychiatric adverse effects, and tells Mr. T to report any worsening psychiatric symptoms. Mr. T is scheduled for frequent follow-up visits to monitor possible adverse effects and his tobacco use. He says he understands the potential adverse effects of varenicline and agrees to frequent follow-up appointments while taking it.
Related Resources
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005.1982ST
- Cieslak K, Freudenreich O. 4 Ways to help your patients with schizophrenia quit smoking. Current Psychiatry. 2018; 17(2):28,33.
Drug Brand Names
Bupropion • Wellbutrin
Varenicline • Chantix
Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.

Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.

The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES
Further analysis of EAGLES
Beard et al4 used Bayes factor testing for additional analysis of EAGLES data to determine whether the data were insensitive to neuropsychiatric effects secondary to a lack of statistical power. In the psychiatric cohort, the varenicline and bupropion groups exhibited suggestive but not conclusive data that there was no increase in NPSAEs compared to placebo (Bayes factor 0.52 and 0.71, respectively).4
Another EAGLES analysis by Ayers et al5 evaluated participants with anxiety disorders (N = 712), including PTSD (N = 192), generalized anxiety disorder (GAD) (N = 243), and panic disorder (N = 277).Of those with PTSD who received varenicline, there were no statistically significant differences in CAR from Week 9 to Week 12 vs placebo.5 However, there was a significant difference in individuals with GAD (OR 4.53; 95% CI, 1.20 to 17.10), and panic disorder (OR 8.49; 95% CI, 1.57 to 45.78).5 In contrast to CAR from Week 9 to Week 12, 7-day point prevalence abstinence at Week 12 for participants with PTSD was significant (OR 4.04; 95% CI, 1.39 to 11.74) when comparing varenicline to placebo. Within the anxiety disorder cohort, there were no significant differences in moderate to severe NPSAE rates based on treatment group. Calculated risk differences comparing varenicline to placebo were: PTSD group -7.73 (95% CI, -21.95 to 6.49), GAD group 2.80 (95% CI, -6.63 to 12.23), and panic disorder group -0.18 (95% CI, -9.57 to 9.21).5
Other studies
Evins et al6 conducted a randomized controlled trial to evaluate the safety of varenicline maintenance therapy in patients with schizophrenia or bipolar disorder. To be deemed clinically stable, participants in this study needed to be taking a stable dose of an antipsychotic or mood-stabilizing agent(s) for ≥30 days, compared to the 3-month requirement of the EAGLES trial.3,6 Participants received 12 weeks of open-label varenicline; those who achieved abstinence (N = 87) entered the relapse-prevention phase and were randomized to varenicline 1 mg twice a day or placebo for 40 weeks. Of those who entered relapse-prevention, 5 in the placebo group and 2 in the varenicline group were psychiatrically hospitalized (risk ratio 0.45; 95% CI, 0.04 to 2.9).6 These researchers concluded that varenicline maintenance therapy prolonged abstinence rates with no significant increase in neuropsychiatric events.6
Although treatment options for smoking cessation have advanced, individuals with SMI are still disproportionately affected by the negative outcomes of cigarette smoking. Current literature suggests that varenicline does not confer an appreciable risk of neuropsychiatric events in otherwise stable patients and is the preferred first-line treatment. However, there is a gap in understanding the impact of this medication on individuals with unstable psychiatric illness. Health care professionals should be encouraged to use varenicline with careful monitoring for appropriate patients with psychiatric disorders as a standard of care to help them quit smoking.
CASE CONTINUED
After consulting with the psychiatric pharmacist and discussing the risks and benefits of varenicline, Mr. T is started on the appropriate titration schedule (Table 11). A pharmacist provides varenicline education, including the possibility of psychiatric adverse effects, and tells Mr. T to report any worsening psychiatric symptoms. Mr. T is scheduled for frequent follow-up visits to monitor possible adverse effects and his tobacco use. He says he understands the potential adverse effects of varenicline and agrees to frequent follow-up appointments while taking it.
Related Resources
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005.1982ST
- Cieslak K, Freudenreich O. 4 Ways to help your patients with schizophrenia quit smoking. Current Psychiatry. 2018; 17(2):28,33.
Drug Brand Names
Bupropion • Wellbutrin
Varenicline • Chantix
1. Chantix [package insert]. New York, NY: Pfizer Inc; 2019.
2. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120. doi:10.1177/0004867417726176
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/s0140-6736(16)30272-0
4. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824. doi:10.1111/add.15440
5. Ayers CR, Heffner JL, Russ C, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depress Anxiety. 2020;37(3)247-260. doi:10.1002/da.22982
6. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi:10.1001/jama.2013.285113
1. Chantix [package insert]. New York, NY: Pfizer Inc; 2019.
2. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120. doi:10.1177/0004867417726176
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/s0140-6736(16)30272-0
4. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824. doi:10.1111/add.15440
5. Ayers CR, Heffner JL, Russ C, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depress Anxiety. 2020;37(3)247-260. doi:10.1002/da.22982
6. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi:10.1001/jama.2013.285113
Adaptive changes to antipsychotics: How to avoid the consequences
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
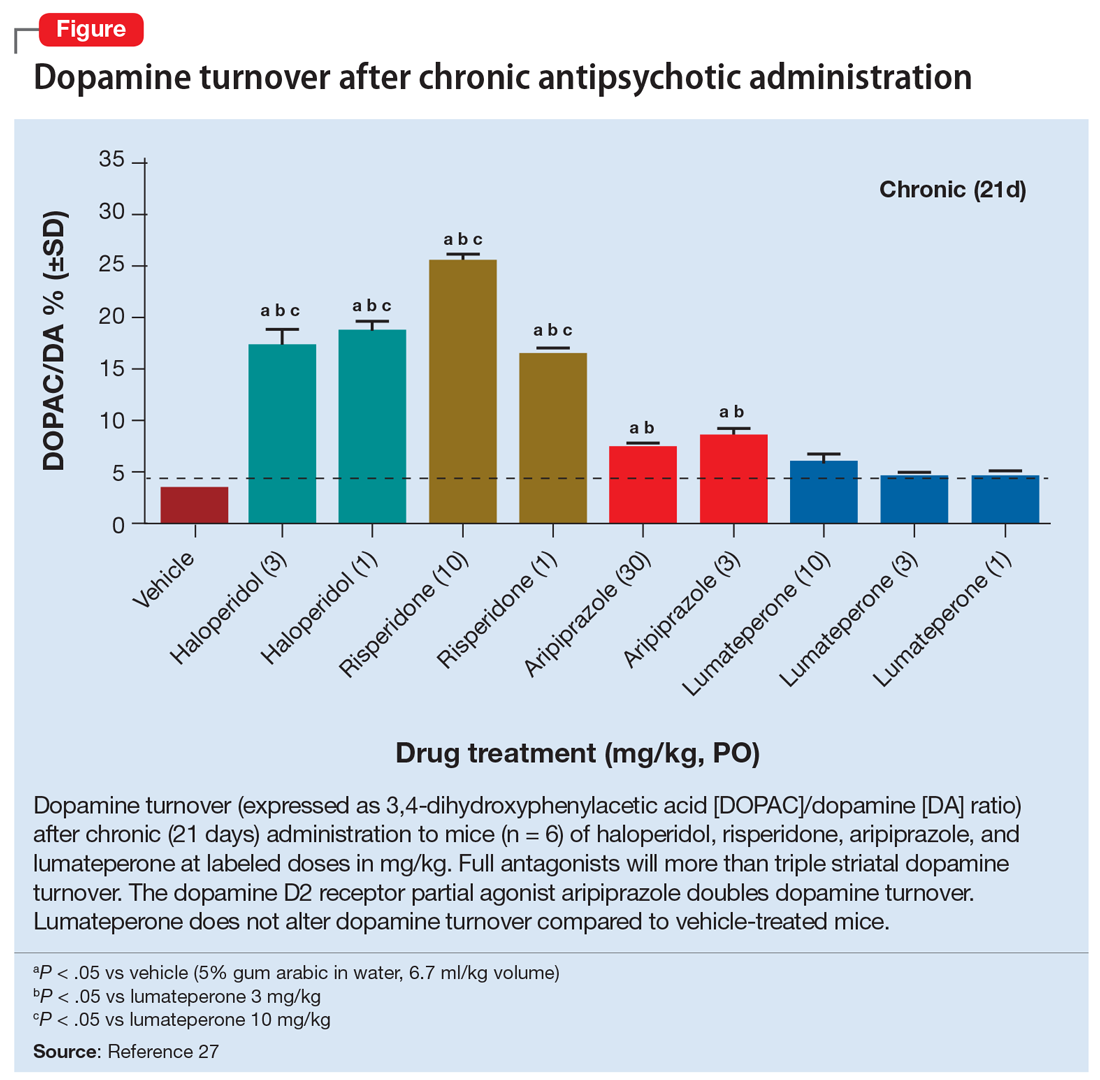
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28

Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28

Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
Termination of pregnancy for medical reasons: A mental health perspective
Termination of pregnancy for medical reasons (TFMR) occurs when a pregnancy is ended due to medical complications that threaten the health of a pregnant individual and/or fetus, or when a fetus has a poor prognosis or life-limiting diagnosis. It is distinct from the American College of Obstetricians and Gynecologists identification of all abortions as medically indicated. Common indications for TFMR include life-threatening pregnancy complications (eg, placental abruption, hyperemesis gravidarum, exacerbation of psychiatric illness), chromosomal abnormalities (eg, Trisomy 13, 18, and 21; Klinefelter syndrome), and fetal anomalies (eg, neural tube defects, cardiac defects, renal agenesis). In this article, we discuss the negative psychological outcomes of TFMR, and how to screen and intervene to best help women who experience TFMR.
Psychiatric sequelae of TFMR
Unlike abortions in general, negative psychological outcomes are common among women who experience TFMR.1 Nearly one-half of women develop symptoms of posttraumatic stress disorder (PTSD), and approximately one-fourth show signs of depression at 4 months after termination.2 Such symptoms usually improve with time but may return around trauma anniversaries (date of diagnosis or termination). Women with a history of trauma, a prior psychiatric diagnosis, and/or no living children are at greater risk. Self-blame, doubt, and high levels of distress are also risk factors.2-4 Protective factors include positive coping strategies (such as acceptance or reframing), higher perceived social support, and high self-efficacy.3,4
Screening: What to ask, and how
Use open-ended questions to ask about a patient’s obstetric history:
- Have you ever been pregnant?
- If you’re comfortable sharing, what were the outcomes of these pregnancies?
If a woman discloses that she has experienced a TFMR, screen for and normalize psychiatric outcomes by asking:
- Symptoms of grief, depression, and anxiety are common after TFMR. Have you experienced such symptoms?
- What impact has terminating your pregnancy for medical reasons had on your mental health?
Screening tools such as the General Self-Efficacy Scale can help assess predictive factors, while other scales can assess specific diagnoses (eg, Patient Health Questionaire-9 for depression, Impact of Event Scale-Revised and PTSD Checklist for DSM-5 for trauma-related symptoms, Traumatic Grief Inventory Self Report Version for pathological grief). The Edinburgh Postnatal Depression Scale can assess for depression, but if you use this instrument, exclude statements that reference a current pregnancy or recent delivery.
How to best help
Interventions should be specific and targeted. Thus, consider the individual nature of the experience and variation in attachment that can occur over time.5 OB-GYN and perinatal psychiatry clinicians can recommend local resources and support groups that specifically focus on TFMR, rather than on general pregnancy loss. Refer patients to therapists who specialize in pregnancy loss, reproductive trauma, and/or TFMR. Cognitive-behavioral therapy and acceptance and commitment therapy may be appropriate and effective.3 Online support groups (such as Termination of Pregnancy for Medical Reasons; www.facebook.com/groups/TFMRgroup/) can supplement or fill gaps in local resources. Suggest books that discuss TFMR, such
1. González-Ramos Z, Zuriguel-Pérez E, Albacar-Riobóo N, et al. The emotional responses of women when terminating a pregnancy for medical reasons: a scoping review. Midwifery. 2021;103:103095. doi:10.1016/j.midw.2021.103095
2. Korenromp MJ, Page-Christiaens GCML, van den Bout J, et al. Adjustment to termination of pregnancy for fetal anomaly: a longitudinal study in women at 4, 8, and 16 months. Am J Obstet Gynecol. 2009;201(2):160.e1-7.
3. Lafarge C, Mitchell K, Fox P. Perinatal grief following a termination of pregnancy for foetal abnormality: the impact of coping strategies. Prenat Diagn. 2013;33(12):1173-1182.
4. Korenromp MJ, Christiaens GC, van den Bout J, et al. Long-term psychological consequences of pregnancy termination for fetal abnormality: a cross-sectional study. Prenat Diagn. 2005;25(3):253-260.
5. Lou S, Hvidtjørn D, Jørgensen ML, Vogel I. “I had to think: this is not a child.” A qualitative exploration of how women/couples articulate their relation to the fetus/child following termination of a wanted pregnancy due to Down syndrome. Sex Reprod Healthc. 2021;28:100606. doi: 10.1016/j.srhc.2021.100606
6. Brooks C (ed.). Our Heartbreaking Choices: Forty-Six Women Share Their Stories of Interrupting a Much-Wanted Pregnancy. iUniverse; 2008.
Termination of pregnancy for medical reasons (TFMR) occurs when a pregnancy is ended due to medical complications that threaten the health of a pregnant individual and/or fetus, or when a fetus has a poor prognosis or life-limiting diagnosis. It is distinct from the American College of Obstetricians and Gynecologists identification of all abortions as medically indicated. Common indications for TFMR include life-threatening pregnancy complications (eg, placental abruption, hyperemesis gravidarum, exacerbation of psychiatric illness), chromosomal abnormalities (eg, Trisomy 13, 18, and 21; Klinefelter syndrome), and fetal anomalies (eg, neural tube defects, cardiac defects, renal agenesis). In this article, we discuss the negative psychological outcomes of TFMR, and how to screen and intervene to best help women who experience TFMR.
Psychiatric sequelae of TFMR
Unlike abortions in general, negative psychological outcomes are common among women who experience TFMR.1 Nearly one-half of women develop symptoms of posttraumatic stress disorder (PTSD), and approximately one-fourth show signs of depression at 4 months after termination.2 Such symptoms usually improve with time but may return around trauma anniversaries (date of diagnosis or termination). Women with a history of trauma, a prior psychiatric diagnosis, and/or no living children are at greater risk. Self-blame, doubt, and high levels of distress are also risk factors.2-4 Protective factors include positive coping strategies (such as acceptance or reframing), higher perceived social support, and high self-efficacy.3,4
Screening: What to ask, and how
Use open-ended questions to ask about a patient’s obstetric history:
- Have you ever been pregnant?
- If you’re comfortable sharing, what were the outcomes of these pregnancies?
If a woman discloses that she has experienced a TFMR, screen for and normalize psychiatric outcomes by asking:
- Symptoms of grief, depression, and anxiety are common after TFMR. Have you experienced such symptoms?
- What impact has terminating your pregnancy for medical reasons had on your mental health?
Screening tools such as the General Self-Efficacy Scale can help assess predictive factors, while other scales can assess specific diagnoses (eg, Patient Health Questionaire-9 for depression, Impact of Event Scale-Revised and PTSD Checklist for DSM-5 for trauma-related symptoms, Traumatic Grief Inventory Self Report Version for pathological grief). The Edinburgh Postnatal Depression Scale can assess for depression, but if you use this instrument, exclude statements that reference a current pregnancy or recent delivery.
How to best help
Interventions should be specific and targeted. Thus, consider the individual nature of the experience and variation in attachment that can occur over time.5 OB-GYN and perinatal psychiatry clinicians can recommend local resources and support groups that specifically focus on TFMR, rather than on general pregnancy loss. Refer patients to therapists who specialize in pregnancy loss, reproductive trauma, and/or TFMR. Cognitive-behavioral therapy and acceptance and commitment therapy may be appropriate and effective.3 Online support groups (such as Termination of Pregnancy for Medical Reasons; www.facebook.com/groups/TFMRgroup/) can supplement or fill gaps in local resources. Suggest books that discuss TFMR, such
Termination of pregnancy for medical reasons (TFMR) occurs when a pregnancy is ended due to medical complications that threaten the health of a pregnant individual and/or fetus, or when a fetus has a poor prognosis or life-limiting diagnosis. It is distinct from the American College of Obstetricians and Gynecologists identification of all abortions as medically indicated. Common indications for TFMR include life-threatening pregnancy complications (eg, placental abruption, hyperemesis gravidarum, exacerbation of psychiatric illness), chromosomal abnormalities (eg, Trisomy 13, 18, and 21; Klinefelter syndrome), and fetal anomalies (eg, neural tube defects, cardiac defects, renal agenesis). In this article, we discuss the negative psychological outcomes of TFMR, and how to screen and intervene to best help women who experience TFMR.
Psychiatric sequelae of TFMR
Unlike abortions in general, negative psychological outcomes are common among women who experience TFMR.1 Nearly one-half of women develop symptoms of posttraumatic stress disorder (PTSD), and approximately one-fourth show signs of depression at 4 months after termination.2 Such symptoms usually improve with time but may return around trauma anniversaries (date of diagnosis or termination). Women with a history of trauma, a prior psychiatric diagnosis, and/or no living children are at greater risk. Self-blame, doubt, and high levels of distress are also risk factors.2-4 Protective factors include positive coping strategies (such as acceptance or reframing), higher perceived social support, and high self-efficacy.3,4
Screening: What to ask, and how
Use open-ended questions to ask about a patient’s obstetric history:
- Have you ever been pregnant?
- If you’re comfortable sharing, what were the outcomes of these pregnancies?
If a woman discloses that she has experienced a TFMR, screen for and normalize psychiatric outcomes by asking:
- Symptoms of grief, depression, and anxiety are common after TFMR. Have you experienced such symptoms?
- What impact has terminating your pregnancy for medical reasons had on your mental health?
Screening tools such as the General Self-Efficacy Scale can help assess predictive factors, while other scales can assess specific diagnoses (eg, Patient Health Questionaire-9 for depression, Impact of Event Scale-Revised and PTSD Checklist for DSM-5 for trauma-related symptoms, Traumatic Grief Inventory Self Report Version for pathological grief). The Edinburgh Postnatal Depression Scale can assess for depression, but if you use this instrument, exclude statements that reference a current pregnancy or recent delivery.
How to best help
Interventions should be specific and targeted. Thus, consider the individual nature of the experience and variation in attachment that can occur over time.5 OB-GYN and perinatal psychiatry clinicians can recommend local resources and support groups that specifically focus on TFMR, rather than on general pregnancy loss. Refer patients to therapists who specialize in pregnancy loss, reproductive trauma, and/or TFMR. Cognitive-behavioral therapy and acceptance and commitment therapy may be appropriate and effective.3 Online support groups (such as Termination of Pregnancy for Medical Reasons; www.facebook.com/groups/TFMRgroup/) can supplement or fill gaps in local resources. Suggest books that discuss TFMR, such
1. González-Ramos Z, Zuriguel-Pérez E, Albacar-Riobóo N, et al. The emotional responses of women when terminating a pregnancy for medical reasons: a scoping review. Midwifery. 2021;103:103095. doi:10.1016/j.midw.2021.103095
2. Korenromp MJ, Page-Christiaens GCML, van den Bout J, et al. Adjustment to termination of pregnancy for fetal anomaly: a longitudinal study in women at 4, 8, and 16 months. Am J Obstet Gynecol. 2009;201(2):160.e1-7.
3. Lafarge C, Mitchell K, Fox P. Perinatal grief following a termination of pregnancy for foetal abnormality: the impact of coping strategies. Prenat Diagn. 2013;33(12):1173-1182.
4. Korenromp MJ, Christiaens GC, van den Bout J, et al. Long-term psychological consequences of pregnancy termination for fetal abnormality: a cross-sectional study. Prenat Diagn. 2005;25(3):253-260.
5. Lou S, Hvidtjørn D, Jørgensen ML, Vogel I. “I had to think: this is not a child.” A qualitative exploration of how women/couples articulate their relation to the fetus/child following termination of a wanted pregnancy due to Down syndrome. Sex Reprod Healthc. 2021;28:100606. doi: 10.1016/j.srhc.2021.100606
6. Brooks C (ed.). Our Heartbreaking Choices: Forty-Six Women Share Their Stories of Interrupting a Much-Wanted Pregnancy. iUniverse; 2008.
1. González-Ramos Z, Zuriguel-Pérez E, Albacar-Riobóo N, et al. The emotional responses of women when terminating a pregnancy for medical reasons: a scoping review. Midwifery. 2021;103:103095. doi:10.1016/j.midw.2021.103095
2. Korenromp MJ, Page-Christiaens GCML, van den Bout J, et al. Adjustment to termination of pregnancy for fetal anomaly: a longitudinal study in women at 4, 8, and 16 months. Am J Obstet Gynecol. 2009;201(2):160.e1-7.
3. Lafarge C, Mitchell K, Fox P. Perinatal grief following a termination of pregnancy for foetal abnormality: the impact of coping strategies. Prenat Diagn. 2013;33(12):1173-1182.
4. Korenromp MJ, Christiaens GC, van den Bout J, et al. Long-term psychological consequences of pregnancy termination for fetal abnormality: a cross-sectional study. Prenat Diagn. 2005;25(3):253-260.
5. Lou S, Hvidtjørn D, Jørgensen ML, Vogel I. “I had to think: this is not a child.” A qualitative exploration of how women/couples articulate their relation to the fetus/child following termination of a wanted pregnancy due to Down syndrome. Sex Reprod Healthc. 2021;28:100606. doi: 10.1016/j.srhc.2021.100606
6. Brooks C (ed.). Our Heartbreaking Choices: Forty-Six Women Share Their Stories of Interrupting a Much-Wanted Pregnancy. iUniverse; 2008.