User login
Baricitinib’s approval for alopecia areata: Considerations for starting patients on treatment
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
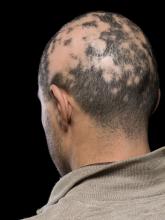
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mosquitoes and the vicious circle that’s gone viral
These viruses want mosquitoes with good taste
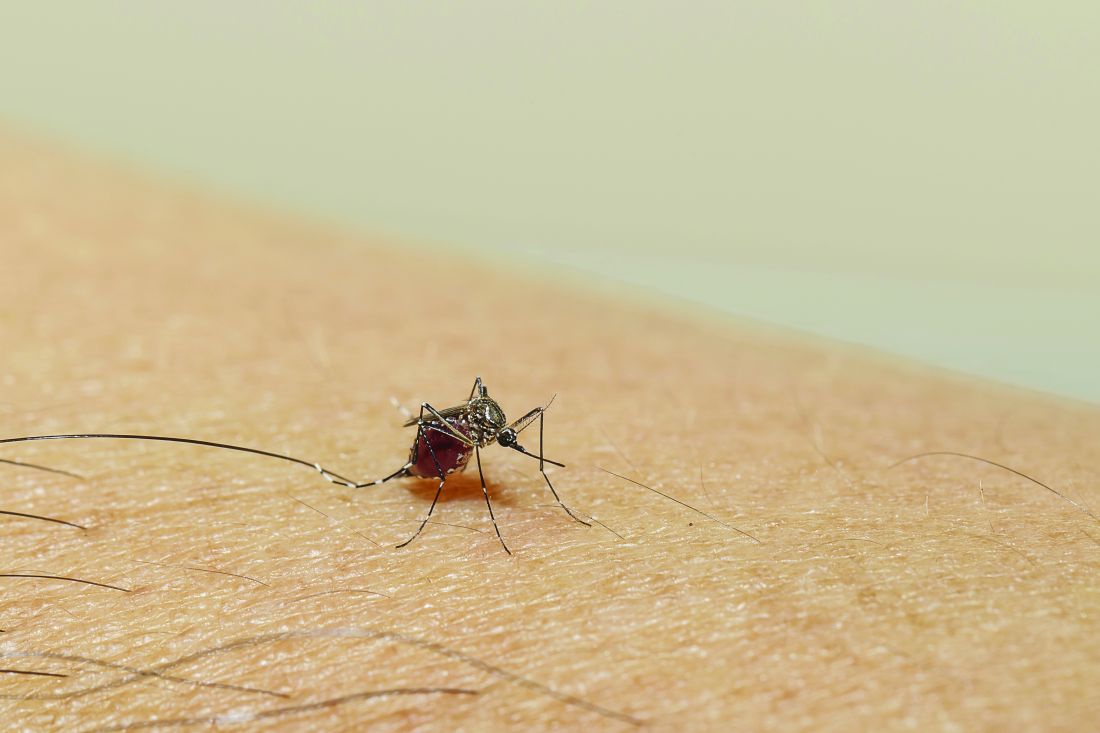
Taste can be a pretty subjective sense. Not everyone agrees on what tastes good and what tastes bad. Most people would agree that freshly baked cookies taste good, but what about lima beans? And what about mosquitoes? What tastes good to a mosquito?
The answer? Blood. Blood tastes good to a mosquito. That really wasn’t a very hard question, was it? You did know the answer, didn’t you? They don’t care about cookies, and they certainly don’t care about lima beans. It’s blood that they love.
That brings us back to subjectivity, because it is possible for blood to taste even better. The secret ingredient is dengue … and Zika.
A study just published in Cell demonstrates that mice infected with dengue and Zika viruses release a volatile compound called acetophenone. “We found that flavivirus [like dengue and Zika] can utilize the increased release of acetophenone to help itself achieve its lifecycles more effectively by making their hosts more attractive to mosquito vectors,” senior author Gong Cheng of Tsinghua University, Beijing, said in a written statement.
How do they do it? The viruses, he explained, promote the proliferation of acetophenone-producing skin bacteria. “As a result, some bacteria overreplicate and produce more acetophenone. Suddenly, these sick individuals smell as delicious to mosquitoes as a tray of freshly baked cookies to a group of five-year-old children,” the statement said.
And how do you stop a group of tiny, flying 5-year-olds? That’s right, with acne medication. Really? You knew that one but not the blood one before? The investigators fed isotretinoin to the infected mice, which led to reduced acetophenone release from skin bacteria and made the animals no more attractive to the mosquitoes than their uninfected counterparts.
The investigators are planning to take the next step – feeding isotretinoin to people with dengue and Zika – having gotten the official fictional taste-test approval of celebrity chef Gordon Ramsay, who said, “You’re going to feed this #$^% to sick people? ARE YOU &%*$@#& KIDDING ME?”
Okay, so maybe approval isn’t quite the right word.
Welcome to bladders of the rich and famous!
Don’t you hate it when you’re driving out to your multimillion-dollar second home in the Hamptons and traffic is so bad you absolutely have to find a place to “rest” along the way? But wouldn’t you know it, there just isn’t anywhere to stop! Geez, how do we live?
That’s where David Shusterman, MD, a urologist in New York City and a true American hero, comes in. He’s identified a market and positioned himself as the king of both bladder surgery and “bladder Botox” for the wealthy New Yorkers who regularly make long journeys from the city out to their second homes in the Hamptons. Traffic has increased dramatically on Long Island roads in recent years, and the journey can now taking upward of 4 hours. Some people just can’t make it that long without a bathroom break, and there are very few places to stop along the way.
Dr. Shusterman understands the plight of the Hamptons vacationer, as he told Insider.com: “I can’t tell you how many arguments I personally get into – I’ve lost three friends because I’m the driver and refuse to stop for them.” A tragedy worthy of Shakespeare himself.
During the summer season, Dr. Shusterman performs about 10 prostate artery embolizations a week, an hour-long procedure that shrinks the prostate, which is great for 50- to 60-year-old men with enlarged prostates that cause more frequent bathroom trips. He also performs Botox injections into the bladder once or twice a week for women, which reduces the need to urinate for roughly 6 months. The perfect amount of time to get them through the summer season.
These procedures are sometimes covered by insurance but can cost as much as $20,000 if paid out of pocket. That’s a lot of money to us, but if you’re the sort of person who has a second home in the Hamptons, $20,000 is chump change, especially if it means you won’t have to go 2 entire minutes out of your way to use a gas-station bathroom. Then again, having seen a more than a few gas-station bathrooms in our time, maybe they have a point.
Ditch the apples. Go for the avocados
We’ve all heard about “an apple a day,” but instead of apples you might want to go with avocados.
Avocados are generally thought to be a healthy fat. A study just published in the Journal of the American Heart Association proves that they actually don’t do anything for your waistline but will work wonders on your cholesterol level. The study involved 923 participants who were considered overweight/obese split into two groups: One was asked to consume an avocado a day, and the other continued their usual diets and were asked to consume fewer than two avocados a month.
At the end of the 6 months, the researchers found total cholesterol decreased by an additional 2.9 mg/dL and LDL cholesterol by 2.5 mg/dL in those who ate one avocado every day, compared with the usual-diet group. And even though avocados have a lot of calories, there was no clinical evidence that it impacted weight gain or any cardiometabolic risk factors, according to a statement from Penn State University.
Avocados, then, can be considered a guilt-free food. The findings from this study suggest it can give a substantial boost to your overall quality of diet, in turn lessening your risk of developing type 2 diabetes and some cancers, Kristina Peterson, PhD, assistant professor of nutritional sciences at Texas Tech University, said in the statement.
So get creative with your avocado recipes. You can only eat so much guacamole.
Your nose knows a good friend for you
You’ve probably noticed how dogs sniff other dogs and people before becoming friends. It would be pretty comical if people did the same thing, right? Just walked up to strangers and started sniffing them like dogs?
Well, apparently humans do go by smell when it comes to making friends, and they prefer people who smell like them. Maybe you’ve noticed that your friends look like you, share your values, and think the same way as you. You’re probably right, seeing as previous research has pointed to this.
For the current study, done to show how smell affects human behavior, researchers recruited people who befriended each other quickly, before knowing much about each other. They assumed that the relationships between these same-sex, nonromantic “click friends” relied more on physiological traits, including smell. After collecting samples from the click friends, researchers used an eNose to scan chemical signatures. In another experiment, human volunteers sniffed samples to determine if any were similar. Both experiments showed that click friends had more similar smells than pairs of random people.
“This is not to say that we act like goats or shrews – humans likely rely on other, far more dominant cues in their social decision-making. Nevertheless, our study’s results do suggest that our nose plays a bigger role than previously thought in our choice of friends,” said senior author Noam Sobel, PhD, of the Weizmann Institute of Science in Rehovot, Israel.
Lead author Inbal Ravreby, a graduate student at the institute, put it this way: “These results imply that, as the saying goes, there is chemistry in social chemistry.”
These viruses want mosquitoes with good taste
Taste can be a pretty subjective sense. Not everyone agrees on what tastes good and what tastes bad. Most people would agree that freshly baked cookies taste good, but what about lima beans? And what about mosquitoes? What tastes good to a mosquito?
The answer? Blood. Blood tastes good to a mosquito. That really wasn’t a very hard question, was it? You did know the answer, didn’t you? They don’t care about cookies, and they certainly don’t care about lima beans. It’s blood that they love.
That brings us back to subjectivity, because it is possible for blood to taste even better. The secret ingredient is dengue … and Zika.
A study just published in Cell demonstrates that mice infected with dengue and Zika viruses release a volatile compound called acetophenone. “We found that flavivirus [like dengue and Zika] can utilize the increased release of acetophenone to help itself achieve its lifecycles more effectively by making their hosts more attractive to mosquito vectors,” senior author Gong Cheng of Tsinghua University, Beijing, said in a written statement.
How do they do it? The viruses, he explained, promote the proliferation of acetophenone-producing skin bacteria. “As a result, some bacteria overreplicate and produce more acetophenone. Suddenly, these sick individuals smell as delicious to mosquitoes as a tray of freshly baked cookies to a group of five-year-old children,” the statement said.
And how do you stop a group of tiny, flying 5-year-olds? That’s right, with acne medication. Really? You knew that one but not the blood one before? The investigators fed isotretinoin to the infected mice, which led to reduced acetophenone release from skin bacteria and made the animals no more attractive to the mosquitoes than their uninfected counterparts.
The investigators are planning to take the next step – feeding isotretinoin to people with dengue and Zika – having gotten the official fictional taste-test approval of celebrity chef Gordon Ramsay, who said, “You’re going to feed this #$^% to sick people? ARE YOU &%*$@#& KIDDING ME?”
Okay, so maybe approval isn’t quite the right word.
Welcome to bladders of the rich and famous!
Don’t you hate it when you’re driving out to your multimillion-dollar second home in the Hamptons and traffic is so bad you absolutely have to find a place to “rest” along the way? But wouldn’t you know it, there just isn’t anywhere to stop! Geez, how do we live?
That’s where David Shusterman, MD, a urologist in New York City and a true American hero, comes in. He’s identified a market and positioned himself as the king of both bladder surgery and “bladder Botox” for the wealthy New Yorkers who regularly make long journeys from the city out to their second homes in the Hamptons. Traffic has increased dramatically on Long Island roads in recent years, and the journey can now taking upward of 4 hours. Some people just can’t make it that long without a bathroom break, and there are very few places to stop along the way.
Dr. Shusterman understands the plight of the Hamptons vacationer, as he told Insider.com: “I can’t tell you how many arguments I personally get into – I’ve lost three friends because I’m the driver and refuse to stop for them.” A tragedy worthy of Shakespeare himself.
During the summer season, Dr. Shusterman performs about 10 prostate artery embolizations a week, an hour-long procedure that shrinks the prostate, which is great for 50- to 60-year-old men with enlarged prostates that cause more frequent bathroom trips. He also performs Botox injections into the bladder once or twice a week for women, which reduces the need to urinate for roughly 6 months. The perfect amount of time to get them through the summer season.
These procedures are sometimes covered by insurance but can cost as much as $20,000 if paid out of pocket. That’s a lot of money to us, but if you’re the sort of person who has a second home in the Hamptons, $20,000 is chump change, especially if it means you won’t have to go 2 entire minutes out of your way to use a gas-station bathroom. Then again, having seen a more than a few gas-station bathrooms in our time, maybe they have a point.
Ditch the apples. Go for the avocados
We’ve all heard about “an apple a day,” but instead of apples you might want to go with avocados.
Avocados are generally thought to be a healthy fat. A study just published in the Journal of the American Heart Association proves that they actually don’t do anything for your waistline but will work wonders on your cholesterol level. The study involved 923 participants who were considered overweight/obese split into two groups: One was asked to consume an avocado a day, and the other continued their usual diets and were asked to consume fewer than two avocados a month.
At the end of the 6 months, the researchers found total cholesterol decreased by an additional 2.9 mg/dL and LDL cholesterol by 2.5 mg/dL in those who ate one avocado every day, compared with the usual-diet group. And even though avocados have a lot of calories, there was no clinical evidence that it impacted weight gain or any cardiometabolic risk factors, according to a statement from Penn State University.
Avocados, then, can be considered a guilt-free food. The findings from this study suggest it can give a substantial boost to your overall quality of diet, in turn lessening your risk of developing type 2 diabetes and some cancers, Kristina Peterson, PhD, assistant professor of nutritional sciences at Texas Tech University, said in the statement.
So get creative with your avocado recipes. You can only eat so much guacamole.
Your nose knows a good friend for you
You’ve probably noticed how dogs sniff other dogs and people before becoming friends. It would be pretty comical if people did the same thing, right? Just walked up to strangers and started sniffing them like dogs?
Well, apparently humans do go by smell when it comes to making friends, and they prefer people who smell like them. Maybe you’ve noticed that your friends look like you, share your values, and think the same way as you. You’re probably right, seeing as previous research has pointed to this.
For the current study, done to show how smell affects human behavior, researchers recruited people who befriended each other quickly, before knowing much about each other. They assumed that the relationships between these same-sex, nonromantic “click friends” relied more on physiological traits, including smell. After collecting samples from the click friends, researchers used an eNose to scan chemical signatures. In another experiment, human volunteers sniffed samples to determine if any were similar. Both experiments showed that click friends had more similar smells than pairs of random people.
“This is not to say that we act like goats or shrews – humans likely rely on other, far more dominant cues in their social decision-making. Nevertheless, our study’s results do suggest that our nose plays a bigger role than previously thought in our choice of friends,” said senior author Noam Sobel, PhD, of the Weizmann Institute of Science in Rehovot, Israel.
Lead author Inbal Ravreby, a graduate student at the institute, put it this way: “These results imply that, as the saying goes, there is chemistry in social chemistry.”
These viruses want mosquitoes with good taste
Taste can be a pretty subjective sense. Not everyone agrees on what tastes good and what tastes bad. Most people would agree that freshly baked cookies taste good, but what about lima beans? And what about mosquitoes? What tastes good to a mosquito?
The answer? Blood. Blood tastes good to a mosquito. That really wasn’t a very hard question, was it? You did know the answer, didn’t you? They don’t care about cookies, and they certainly don’t care about lima beans. It’s blood that they love.
That brings us back to subjectivity, because it is possible for blood to taste even better. The secret ingredient is dengue … and Zika.
A study just published in Cell demonstrates that mice infected with dengue and Zika viruses release a volatile compound called acetophenone. “We found that flavivirus [like dengue and Zika] can utilize the increased release of acetophenone to help itself achieve its lifecycles more effectively by making their hosts more attractive to mosquito vectors,” senior author Gong Cheng of Tsinghua University, Beijing, said in a written statement.
How do they do it? The viruses, he explained, promote the proliferation of acetophenone-producing skin bacteria. “As a result, some bacteria overreplicate and produce more acetophenone. Suddenly, these sick individuals smell as delicious to mosquitoes as a tray of freshly baked cookies to a group of five-year-old children,” the statement said.
And how do you stop a group of tiny, flying 5-year-olds? That’s right, with acne medication. Really? You knew that one but not the blood one before? The investigators fed isotretinoin to the infected mice, which led to reduced acetophenone release from skin bacteria and made the animals no more attractive to the mosquitoes than their uninfected counterparts.
The investigators are planning to take the next step – feeding isotretinoin to people with dengue and Zika – having gotten the official fictional taste-test approval of celebrity chef Gordon Ramsay, who said, “You’re going to feed this #$^% to sick people? ARE YOU &%*$@#& KIDDING ME?”
Okay, so maybe approval isn’t quite the right word.
Welcome to bladders of the rich and famous!
Don’t you hate it when you’re driving out to your multimillion-dollar second home in the Hamptons and traffic is so bad you absolutely have to find a place to “rest” along the way? But wouldn’t you know it, there just isn’t anywhere to stop! Geez, how do we live?
That’s where David Shusterman, MD, a urologist in New York City and a true American hero, comes in. He’s identified a market and positioned himself as the king of both bladder surgery and “bladder Botox” for the wealthy New Yorkers who regularly make long journeys from the city out to their second homes in the Hamptons. Traffic has increased dramatically on Long Island roads in recent years, and the journey can now taking upward of 4 hours. Some people just can’t make it that long without a bathroom break, and there are very few places to stop along the way.
Dr. Shusterman understands the plight of the Hamptons vacationer, as he told Insider.com: “I can’t tell you how many arguments I personally get into – I’ve lost three friends because I’m the driver and refuse to stop for them.” A tragedy worthy of Shakespeare himself.
During the summer season, Dr. Shusterman performs about 10 prostate artery embolizations a week, an hour-long procedure that shrinks the prostate, which is great for 50- to 60-year-old men with enlarged prostates that cause more frequent bathroom trips. He also performs Botox injections into the bladder once or twice a week for women, which reduces the need to urinate for roughly 6 months. The perfect amount of time to get them through the summer season.
These procedures are sometimes covered by insurance but can cost as much as $20,000 if paid out of pocket. That’s a lot of money to us, but if you’re the sort of person who has a second home in the Hamptons, $20,000 is chump change, especially if it means you won’t have to go 2 entire minutes out of your way to use a gas-station bathroom. Then again, having seen a more than a few gas-station bathrooms in our time, maybe they have a point.
Ditch the apples. Go for the avocados
We’ve all heard about “an apple a day,” but instead of apples you might want to go with avocados.
Avocados are generally thought to be a healthy fat. A study just published in the Journal of the American Heart Association proves that they actually don’t do anything for your waistline but will work wonders on your cholesterol level. The study involved 923 participants who were considered overweight/obese split into two groups: One was asked to consume an avocado a day, and the other continued their usual diets and were asked to consume fewer than two avocados a month.
At the end of the 6 months, the researchers found total cholesterol decreased by an additional 2.9 mg/dL and LDL cholesterol by 2.5 mg/dL in those who ate one avocado every day, compared with the usual-diet group. And even though avocados have a lot of calories, there was no clinical evidence that it impacted weight gain or any cardiometabolic risk factors, according to a statement from Penn State University.
Avocados, then, can be considered a guilt-free food. The findings from this study suggest it can give a substantial boost to your overall quality of diet, in turn lessening your risk of developing type 2 diabetes and some cancers, Kristina Peterson, PhD, assistant professor of nutritional sciences at Texas Tech University, said in the statement.
So get creative with your avocado recipes. You can only eat so much guacamole.
Your nose knows a good friend for you
You’ve probably noticed how dogs sniff other dogs and people before becoming friends. It would be pretty comical if people did the same thing, right? Just walked up to strangers and started sniffing them like dogs?
Well, apparently humans do go by smell when it comes to making friends, and they prefer people who smell like them. Maybe you’ve noticed that your friends look like you, share your values, and think the same way as you. You’re probably right, seeing as previous research has pointed to this.
For the current study, done to show how smell affects human behavior, researchers recruited people who befriended each other quickly, before knowing much about each other. They assumed that the relationships between these same-sex, nonromantic “click friends” relied more on physiological traits, including smell. After collecting samples from the click friends, researchers used an eNose to scan chemical signatures. In another experiment, human volunteers sniffed samples to determine if any were similar. Both experiments showed that click friends had more similar smells than pairs of random people.
“This is not to say that we act like goats or shrews – humans likely rely on other, far more dominant cues in their social decision-making. Nevertheless, our study’s results do suggest that our nose plays a bigger role than previously thought in our choice of friends,” said senior author Noam Sobel, PhD, of the Weizmann Institute of Science in Rehovot, Israel.
Lead author Inbal Ravreby, a graduate student at the institute, put it this way: “These results imply that, as the saying goes, there is chemistry in social chemistry.”
Will the headache field embrace rofecoxib?
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
Are headache clinical trials representative of the general patient population?
DENVER – In a debate over whether headache trials are representative of patients, one neurologist declared that they tend to leave out a variety of subjects with many types of headaches – the young, the old, the pregnant, and those without migraines, among others. But her counterpart defended migraine trials in particular, arguing that they’re evolving to become more valuable as researchers address their limitations.
At the core of the debate at the annual meeting of the American Headache Society were sharp divisions over how much the limitations of headache clinical trials matter. Both neurologists – Jan Brandes, MD, of Nashville (Tenn.) Neuroscience Group, and Amy Gelfand, MD, of the University of California at San Francisco, agree that they exist. But they diverged on how much they matter.
Exclusion/inclusion criteria are good
Dr. Brandes argued that randomized controlled trials “remain the single best study design,” and she said migraine headache trials have improved over the past couple of decades.
Eligibility criteria, for example, have expanded to allow patients with more subtypes of migraines to participate, she said. “Another change has been the establishment of guidelines or inclusion criteria that allow patients who have stable and treated hypertension, stable depression, and stable anxiety disorders that are controlled and treated and not interfering with the disease you’re studying.”
In essence, she said, “the exclusion/inclusion criteria are good.”
It’s also a positive change that longer patient-reported outcomes are included in trials, she said.
Exclusion/inclusion criteria are too restrictive
But Dr. Gelfand criticized the inclusion criteria in migraine trials, noting it includes “a lot of amazing complexity.” Trials often will limit participation to subjects aged 18-65, even though people have high rates of headaches, she said, and they frequently overrepresent men. Pregnant and lactating women are often omitted, too, even if a trial is examining a behavioral intervention. In some cases, lactating women may be breastfeeding for a year or two, she noted.
“The vast majority of births in the United States, 92%, are to females who are between the ages of 20 and 39. That is also the age range where migraine is most prevalent,” she said. Yes, certain new agents shouldn’t be tested for the first time in pregnant women because of the risk, she said, “but we need to grapple with the fact that migraine is affecting people who are also going to be pregnant and lactating.”
Many other criteria limit the subjects in headache trials, she said. The studies are “almost exclusively” of drugs for migraines, leaving out many people with other types such as adolescents with new persistent headaches. “Where are the trials for them?” she asked.
Other groups that are left out include those whose headaches that are due to a head injury, a viral infection such as COVID-19, or even vaccination against COVID-19, she said. “There are an infinite number of questions here that we are currently not even attempting to answer.”
Non-Whites are also poorly represented in trials, she said, and studies often don’t include data about non-Whites. “Race data exists. Where do we get off not even reporting it?”
Room for improvement
For her part, Dr. Brandes said less-common headache disorders are best studied in pragmatic trials until they can be better understood. “We need to understand pathophysiology better for some of these other disorders, particularly things like continuous headache and posttraumatic headache. Then we can begin to expand that.”
She added that randomized clinical trials are now underway regarding secondary headache related to COVID-19.
Dr. Brandes did not report disclosures. Dr. Gelfand had no disclosures.
DENVER – In a debate over whether headache trials are representative of patients, one neurologist declared that they tend to leave out a variety of subjects with many types of headaches – the young, the old, the pregnant, and those without migraines, among others. But her counterpart defended migraine trials in particular, arguing that they’re evolving to become more valuable as researchers address their limitations.
At the core of the debate at the annual meeting of the American Headache Society were sharp divisions over how much the limitations of headache clinical trials matter. Both neurologists – Jan Brandes, MD, of Nashville (Tenn.) Neuroscience Group, and Amy Gelfand, MD, of the University of California at San Francisco, agree that they exist. But they diverged on how much they matter.
Exclusion/inclusion criteria are good
Dr. Brandes argued that randomized controlled trials “remain the single best study design,” and she said migraine headache trials have improved over the past couple of decades.
Eligibility criteria, for example, have expanded to allow patients with more subtypes of migraines to participate, she said. “Another change has been the establishment of guidelines or inclusion criteria that allow patients who have stable and treated hypertension, stable depression, and stable anxiety disorders that are controlled and treated and not interfering with the disease you’re studying.”
In essence, she said, “the exclusion/inclusion criteria are good.”
It’s also a positive change that longer patient-reported outcomes are included in trials, she said.
Exclusion/inclusion criteria are too restrictive
But Dr. Gelfand criticized the inclusion criteria in migraine trials, noting it includes “a lot of amazing complexity.” Trials often will limit participation to subjects aged 18-65, even though people have high rates of headaches, she said, and they frequently overrepresent men. Pregnant and lactating women are often omitted, too, even if a trial is examining a behavioral intervention. In some cases, lactating women may be breastfeeding for a year or two, she noted.
“The vast majority of births in the United States, 92%, are to females who are between the ages of 20 and 39. That is also the age range where migraine is most prevalent,” she said. Yes, certain new agents shouldn’t be tested for the first time in pregnant women because of the risk, she said, “but we need to grapple with the fact that migraine is affecting people who are also going to be pregnant and lactating.”
Many other criteria limit the subjects in headache trials, she said. The studies are “almost exclusively” of drugs for migraines, leaving out many people with other types such as adolescents with new persistent headaches. “Where are the trials for them?” she asked.
Other groups that are left out include those whose headaches that are due to a head injury, a viral infection such as COVID-19, or even vaccination against COVID-19, she said. “There are an infinite number of questions here that we are currently not even attempting to answer.”
Non-Whites are also poorly represented in trials, she said, and studies often don’t include data about non-Whites. “Race data exists. Where do we get off not even reporting it?”
Room for improvement
For her part, Dr. Brandes said less-common headache disorders are best studied in pragmatic trials until they can be better understood. “We need to understand pathophysiology better for some of these other disorders, particularly things like continuous headache and posttraumatic headache. Then we can begin to expand that.”
She added that randomized clinical trials are now underway regarding secondary headache related to COVID-19.
Dr. Brandes did not report disclosures. Dr. Gelfand had no disclosures.
DENVER – In a debate over whether headache trials are representative of patients, one neurologist declared that they tend to leave out a variety of subjects with many types of headaches – the young, the old, the pregnant, and those without migraines, among others. But her counterpart defended migraine trials in particular, arguing that they’re evolving to become more valuable as researchers address their limitations.
At the core of the debate at the annual meeting of the American Headache Society were sharp divisions over how much the limitations of headache clinical trials matter. Both neurologists – Jan Brandes, MD, of Nashville (Tenn.) Neuroscience Group, and Amy Gelfand, MD, of the University of California at San Francisco, agree that they exist. But they diverged on how much they matter.
Exclusion/inclusion criteria are good
Dr. Brandes argued that randomized controlled trials “remain the single best study design,” and she said migraine headache trials have improved over the past couple of decades.
Eligibility criteria, for example, have expanded to allow patients with more subtypes of migraines to participate, she said. “Another change has been the establishment of guidelines or inclusion criteria that allow patients who have stable and treated hypertension, stable depression, and stable anxiety disorders that are controlled and treated and not interfering with the disease you’re studying.”
In essence, she said, “the exclusion/inclusion criteria are good.”
It’s also a positive change that longer patient-reported outcomes are included in trials, she said.
Exclusion/inclusion criteria are too restrictive
But Dr. Gelfand criticized the inclusion criteria in migraine trials, noting it includes “a lot of amazing complexity.” Trials often will limit participation to subjects aged 18-65, even though people have high rates of headaches, she said, and they frequently overrepresent men. Pregnant and lactating women are often omitted, too, even if a trial is examining a behavioral intervention. In some cases, lactating women may be breastfeeding for a year or two, she noted.
“The vast majority of births in the United States, 92%, are to females who are between the ages of 20 and 39. That is also the age range where migraine is most prevalent,” she said. Yes, certain new agents shouldn’t be tested for the first time in pregnant women because of the risk, she said, “but we need to grapple with the fact that migraine is affecting people who are also going to be pregnant and lactating.”
Many other criteria limit the subjects in headache trials, she said. The studies are “almost exclusively” of drugs for migraines, leaving out many people with other types such as adolescents with new persistent headaches. “Where are the trials for them?” she asked.
Other groups that are left out include those whose headaches that are due to a head injury, a viral infection such as COVID-19, or even vaccination against COVID-19, she said. “There are an infinite number of questions here that we are currently not even attempting to answer.”
Non-Whites are also poorly represented in trials, she said, and studies often don’t include data about non-Whites. “Race data exists. Where do we get off not even reporting it?”
Room for improvement
For her part, Dr. Brandes said less-common headache disorders are best studied in pragmatic trials until they can be better understood. “We need to understand pathophysiology better for some of these other disorders, particularly things like continuous headache and posttraumatic headache. Then we can begin to expand that.”
She added that randomized clinical trials are now underway regarding secondary headache related to COVID-19.
Dr. Brandes did not report disclosures. Dr. Gelfand had no disclosures.
AT AHS 2022
Nail dystrophy and foot pain
These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/

These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/
Evolving Treatment Options for Generalized Myasthenia Gravis
Myasthenia gravis (MG) is an autoimmune disease leading to muscle weakness and fatigue. Medical therapy for MG has traditionally focused on treatments to alleviate symptoms, but a range of new therapies are improving outcomes.
Dr Raghav Govindarajan, from HSHS Medical Group in O'Fallon, Illinois, reports on therapeutic advances for patients with MG presented at the American Academy of Neurology 2022 annual meeting.
First, Dr Govindarajan discusses interim results from the ADAPT+ study, an ongoing 3-year extension of ADAPT that evaluated the long-term safety, tolerability, and efficacy of efgartigimod.
Next, he highlights CHAMPION MG, an open-label extension trial that looked at the long-term efficacy and safety profile of ravulizumab in adults with anti-acetylcholine receptor antibody–positive generalized MG.
Dr Govindarajan concludes by reviewing a phase 2 study on nipocalimab, a monoclonal antibody that targets the IgG binding site on FcRn with high affinity, therefore reducing serum levels of total IgG and pathogenic IgG autoantibodies — the underlying cause of MG. This study evaluated efficacy data including myasthenia gravis activities of daily living score evaluated efficacy data including myasthenia gravis activities of daily living.
--
Raghav Govindarajan, MD, Staff neurologist, Stroke Director, HSHS Medical Group-St Elizabeth, O'Fallon, Illinois
Serve(d) as a speaker or a member of a speakers bureau for: Alexion
Received research grant from: Alexion
Received income in an amount equal to or greater than $250 from: Alexion
Myasthenia gravis (MG) is an autoimmune disease leading to muscle weakness and fatigue. Medical therapy for MG has traditionally focused on treatments to alleviate symptoms, but a range of new therapies are improving outcomes.
Dr Raghav Govindarajan, from HSHS Medical Group in O'Fallon, Illinois, reports on therapeutic advances for patients with MG presented at the American Academy of Neurology 2022 annual meeting.
First, Dr Govindarajan discusses interim results from the ADAPT+ study, an ongoing 3-year extension of ADAPT that evaluated the long-term safety, tolerability, and efficacy of efgartigimod.
Next, he highlights CHAMPION MG, an open-label extension trial that looked at the long-term efficacy and safety profile of ravulizumab in adults with anti-acetylcholine receptor antibody–positive generalized MG.
Dr Govindarajan concludes by reviewing a phase 2 study on nipocalimab, a monoclonal antibody that targets the IgG binding site on FcRn with high affinity, therefore reducing serum levels of total IgG and pathogenic IgG autoantibodies — the underlying cause of MG. This study evaluated efficacy data including myasthenia gravis activities of daily living score evaluated efficacy data including myasthenia gravis activities of daily living.
--
Raghav Govindarajan, MD, Staff neurologist, Stroke Director, HSHS Medical Group-St Elizabeth, O'Fallon, Illinois
Serve(d) as a speaker or a member of a speakers bureau for: Alexion
Received research grant from: Alexion
Received income in an amount equal to or greater than $250 from: Alexion
Myasthenia gravis (MG) is an autoimmune disease leading to muscle weakness and fatigue. Medical therapy for MG has traditionally focused on treatments to alleviate symptoms, but a range of new therapies are improving outcomes.
Dr Raghav Govindarajan, from HSHS Medical Group in O'Fallon, Illinois, reports on therapeutic advances for patients with MG presented at the American Academy of Neurology 2022 annual meeting.
First, Dr Govindarajan discusses interim results from the ADAPT+ study, an ongoing 3-year extension of ADAPT that evaluated the long-term safety, tolerability, and efficacy of efgartigimod.
Next, he highlights CHAMPION MG, an open-label extension trial that looked at the long-term efficacy and safety profile of ravulizumab in adults with anti-acetylcholine receptor antibody–positive generalized MG.
Dr Govindarajan concludes by reviewing a phase 2 study on nipocalimab, a monoclonal antibody that targets the IgG binding site on FcRn with high affinity, therefore reducing serum levels of total IgG and pathogenic IgG autoantibodies — the underlying cause of MG. This study evaluated efficacy data including myasthenia gravis activities of daily living score evaluated efficacy data including myasthenia gravis activities of daily living.
--
Raghav Govindarajan, MD, Staff neurologist, Stroke Director, HSHS Medical Group-St Elizabeth, O'Fallon, Illinois
Serve(d) as a speaker or a member of a speakers bureau for: Alexion
Received research grant from: Alexion
Received income in an amount equal to or greater than $250 from: Alexion

Commentary, Treatment of Refractory Migraine, June 2022
Many of our patients with refractory migraine do not respond to first-line acute or preventive treatments, and, almost by definition, first- and second-line treatments have failed in the majority of patients on calcitonin gene-related peptide (CGRP) antagonist medications. Three studies this month highlight the efficacy of CGRP monoclonal antibody (mAb) and small-molecule medications in this population specifically.
After an initial first dose of a CGRP mAb treatment, many patients ask whether a suboptimal response necessitates switching to another agent or whether a second (or third) dose should be given first. Eptinezumab is an intravenously administered mAb that is repeated every 12 weeks. Schim and colleagues present post hoc data for patients who initially had a minimally beneficial response to eptinezumab and received a second dose at week 13.
The authors define a suboptimal response as having less than a 50% decrease in monthly migraine days after 12 weeks. There were two pooled samples of patients—those who received 100 mg eptinezumab and those who received a 300 mg dose. Approximately 45% of patients in the pivotal trials of eptinezumab (PROMISE-1 and -2) were considered suboptimal responders, and 33%-37% of those suboptimal responders had a more than 50% decrease of their monthly migraine days after a second dose (week 24).
Further analysis determined predictive factors that favored a second dose response. The most prominent (and arguably most obvious) predictive factor was a favorable response after the first dose; the greater percent change in monthly migraine days after the first dose was proportional to the response after the second dose.Change in the Headache Impact Test (HIT-6) disability score after the first dose was also seen to be a strong predictive factor for improvement after the second dose.
When we discuss continuation of medications with our patients, especially when they have a suboptimal response, we should first keep in mind the degree of improvement that the patient initially had.There can be benefit from further treatment with the same medication; however, if the response truly was minimal, it may be better to consider another treatment option.
Practically every patient taking a preventive medication is taking at least one acute medication as well.Even the best preventive medication is not a guarantee that further exacerbations will not occur, and our patients will still need some acute treatment option even when their preventive medications are very effective. The study by Ambrosini and colleagues specifically shows how effective a preventive medication can be, specifically in allowing the patient to use fewer acute medications over time in a population of patients who have been resistant to two to four treatments.
Galcanezumab is a once-monthly mAb for the prevention of migraine.The authors of this study compared the acute use of medications for migraine in both the randomized and open-label stages of a study assessing treatment-refractory patients.A total of 462 patients were enrolled who were all resistant to two to four standard-of-care migraine-preventive medications that had been stopped either because of lack of efficacy or tolerance.The double-blind stage lasted 3 months; the open-label stage lasted another 3 months.
The treatment group was seen to use significantly fewer acute medications after just the first month and continued to improve through month 3.In the open-label phase, a similar improvement was noted in patients transitioning from placebo. In addition to acute medication use, emergency department use for migraine treatment was decreased significantly as well, by more than two thirds in month 3.
Migraine prevention will always remain the key ingredient for improvement for patients with higher frequencies of migraine, and adequate prevention will allow for the lower use of acute medications, and for less healthcare system use in general.
Most practitioners recommend migraine-specific medications for the acute treatment ofmigraine. Since the advent of sumatriptan, this has usually meant a triptan medication. However, a significant percentage of the population (up to 44% in one study) are either intolerant to, contraindicated for, or respond insufficiently to triptan medications. This can either be due to a strong triptan side effect (worsened nausea; tightness/soreness of the muscles of the chest, shoulders, and neck), having cardiovascular risk factors, or not responding adequately 2 hours after treatment.The study by Lipton and colleagues specifically assessed the efficacy of ubrogepant in this population.
Ubrogepant is a small-molecule CGRP antagonist for the acute treatment of migraine. Although somewhat controversial, most practitioners use ubrogepant in patients with some cardiovascular risk, a situation where they would be more likely to avoid the use of triptans.The study authors pooled post hoc data from the pivotal ubrogepant trials (ACHIEVE-1 and -2)to isolate patients with insufficient response to triptans, and their primary outcome was improvement in function 2 hours after medication dose.
Participants in the pivotal trials were separated into three groups: triptan responders, triptaninsufficient responders, and triptan-naive patients. Triptan response was defined as achieving pain freedom 2 hours after medication dose. Both those who had an insufficient response and those who no longer use the triptan owing to intolerance or contraindications were included in the group with insufficient triptan response. Function improvement was defined as the primary outcomeon the basis of a 4-point response scale (0 = no disability, 1 = mildly impaired, 2 = moderately impaired, 3 = severely impaired).In addition, patients were asked to report scores of satisfaction with the medication (yes or no) at 2 and 24 hours and their impression of overall change at 2 hours using a 7-point scale.
The population group of triptan insufficient responders (451 patients) had significant improvement in the primary outcome functional disability at 2, 4, and 7 hours after receipt of medications, but there was no statistical difference at 1 hour. This was similar when comparing those with intolerance to triptans, insufficient response to triptans, or contraindications for triptans. The secondary outcomes of satisfaction and global impression of change were also statistically improved in the insufficient-responders group. No additional tolerance issues or adverse events were noted in this group either.
It would certainly be worth considering the use of agepant acute medication, such as ubrogepant, in patients who are intolerant to or inadequately treated by triptan medications.There still is much to learn about cardiovascular risk and the use of CGRP antagonists, and although no adverse events were noted, more data may be necessary to widely prescribe this class in higher-risk patients.
Many of our patients with refractory migraine do not respond to first-line acute or preventive treatments, and, almost by definition, first- and second-line treatments have failed in the majority of patients on calcitonin gene-related peptide (CGRP) antagonist medications. Three studies this month highlight the efficacy of CGRP monoclonal antibody (mAb) and small-molecule medications in this population specifically.
After an initial first dose of a CGRP mAb treatment, many patients ask whether a suboptimal response necessitates switching to another agent or whether a second (or third) dose should be given first. Eptinezumab is an intravenously administered mAb that is repeated every 12 weeks. Schim and colleagues present post hoc data for patients who initially had a minimally beneficial response to eptinezumab and received a second dose at week 13.
The authors define a suboptimal response as having less than a 50% decrease in monthly migraine days after 12 weeks. There were two pooled samples of patients—those who received 100 mg eptinezumab and those who received a 300 mg dose. Approximately 45% of patients in the pivotal trials of eptinezumab (PROMISE-1 and -2) were considered suboptimal responders, and 33%-37% of those suboptimal responders had a more than 50% decrease of their monthly migraine days after a second dose (week 24).
Further analysis determined predictive factors that favored a second dose response. The most prominent (and arguably most obvious) predictive factor was a favorable response after the first dose; the greater percent change in monthly migraine days after the first dose was proportional to the response after the second dose.Change in the Headache Impact Test (HIT-6) disability score after the first dose was also seen to be a strong predictive factor for improvement after the second dose.
When we discuss continuation of medications with our patients, especially when they have a suboptimal response, we should first keep in mind the degree of improvement that the patient initially had.There can be benefit from further treatment with the same medication; however, if the response truly was minimal, it may be better to consider another treatment option.
Practically every patient taking a preventive medication is taking at least one acute medication as well.Even the best preventive medication is not a guarantee that further exacerbations will not occur, and our patients will still need some acute treatment option even when their preventive medications are very effective. The study by Ambrosini and colleagues specifically shows how effective a preventive medication can be, specifically in allowing the patient to use fewer acute medications over time in a population of patients who have been resistant to two to four treatments.
Galcanezumab is a once-monthly mAb for the prevention of migraine.The authors of this study compared the acute use of medications for migraine in both the randomized and open-label stages of a study assessing treatment-refractory patients.A total of 462 patients were enrolled who were all resistant to two to four standard-of-care migraine-preventive medications that had been stopped either because of lack of efficacy or tolerance.The double-blind stage lasted 3 months; the open-label stage lasted another 3 months.
The treatment group was seen to use significantly fewer acute medications after just the first month and continued to improve through month 3.In the open-label phase, a similar improvement was noted in patients transitioning from placebo. In addition to acute medication use, emergency department use for migraine treatment was decreased significantly as well, by more than two thirds in month 3.
Migraine prevention will always remain the key ingredient for improvement for patients with higher frequencies of migraine, and adequate prevention will allow for the lower use of acute medications, and for less healthcare system use in general.
Most practitioners recommend migraine-specific medications for the acute treatment ofmigraine. Since the advent of sumatriptan, this has usually meant a triptan medication. However, a significant percentage of the population (up to 44% in one study) are either intolerant to, contraindicated for, or respond insufficiently to triptan medications. This can either be due to a strong triptan side effect (worsened nausea; tightness/soreness of the muscles of the chest, shoulders, and neck), having cardiovascular risk factors, or not responding adequately 2 hours after treatment.The study by Lipton and colleagues specifically assessed the efficacy of ubrogepant in this population.
Ubrogepant is a small-molecule CGRP antagonist for the acute treatment of migraine. Although somewhat controversial, most practitioners use ubrogepant in patients with some cardiovascular risk, a situation where they would be more likely to avoid the use of triptans.The study authors pooled post hoc data from the pivotal ubrogepant trials (ACHIEVE-1 and -2)to isolate patients with insufficient response to triptans, and their primary outcome was improvement in function 2 hours after medication dose.
Participants in the pivotal trials were separated into three groups: triptan responders, triptaninsufficient responders, and triptan-naive patients. Triptan response was defined as achieving pain freedom 2 hours after medication dose. Both those who had an insufficient response and those who no longer use the triptan owing to intolerance or contraindications were included in the group with insufficient triptan response. Function improvement was defined as the primary outcomeon the basis of a 4-point response scale (0 = no disability, 1 = mildly impaired, 2 = moderately impaired, 3 = severely impaired).In addition, patients were asked to report scores of satisfaction with the medication (yes or no) at 2 and 24 hours and their impression of overall change at 2 hours using a 7-point scale.
The population group of triptan insufficient responders (451 patients) had significant improvement in the primary outcome functional disability at 2, 4, and 7 hours after receipt of medications, but there was no statistical difference at 1 hour. This was similar when comparing those with intolerance to triptans, insufficient response to triptans, or contraindications for triptans. The secondary outcomes of satisfaction and global impression of change were also statistically improved in the insufficient-responders group. No additional tolerance issues or adverse events were noted in this group either.
It would certainly be worth considering the use of agepant acute medication, such as ubrogepant, in patients who are intolerant to or inadequately treated by triptan medications.There still is much to learn about cardiovascular risk and the use of CGRP antagonists, and although no adverse events were noted, more data may be necessary to widely prescribe this class in higher-risk patients.
Many of our patients with refractory migraine do not respond to first-line acute or preventive treatments, and, almost by definition, first- and second-line treatments have failed in the majority of patients on calcitonin gene-related peptide (CGRP) antagonist medications. Three studies this month highlight the efficacy of CGRP monoclonal antibody (mAb) and small-molecule medications in this population specifically.
After an initial first dose of a CGRP mAb treatment, many patients ask whether a suboptimal response necessitates switching to another agent or whether a second (or third) dose should be given first. Eptinezumab is an intravenously administered mAb that is repeated every 12 weeks. Schim and colleagues present post hoc data for patients who initially had a minimally beneficial response to eptinezumab and received a second dose at week 13.
The authors define a suboptimal response as having less than a 50% decrease in monthly migraine days after 12 weeks. There were two pooled samples of patients—those who received 100 mg eptinezumab and those who received a 300 mg dose. Approximately 45% of patients in the pivotal trials of eptinezumab (PROMISE-1 and -2) were considered suboptimal responders, and 33%-37% of those suboptimal responders had a more than 50% decrease of their monthly migraine days after a second dose (week 24).
Further analysis determined predictive factors that favored a second dose response. The most prominent (and arguably most obvious) predictive factor was a favorable response after the first dose; the greater percent change in monthly migraine days after the first dose was proportional to the response after the second dose.Change in the Headache Impact Test (HIT-6) disability score after the first dose was also seen to be a strong predictive factor for improvement after the second dose.
When we discuss continuation of medications with our patients, especially when they have a suboptimal response, we should first keep in mind the degree of improvement that the patient initially had.There can be benefit from further treatment with the same medication; however, if the response truly was minimal, it may be better to consider another treatment option.
Practically every patient taking a preventive medication is taking at least one acute medication as well.Even the best preventive medication is not a guarantee that further exacerbations will not occur, and our patients will still need some acute treatment option even when their preventive medications are very effective. The study by Ambrosini and colleagues specifically shows how effective a preventive medication can be, specifically in allowing the patient to use fewer acute medications over time in a population of patients who have been resistant to two to four treatments.
Galcanezumab is a once-monthly mAb for the prevention of migraine.The authors of this study compared the acute use of medications for migraine in both the randomized and open-label stages of a study assessing treatment-refractory patients.A total of 462 patients were enrolled who were all resistant to two to four standard-of-care migraine-preventive medications that had been stopped either because of lack of efficacy or tolerance.The double-blind stage lasted 3 months; the open-label stage lasted another 3 months.
The treatment group was seen to use significantly fewer acute medications after just the first month and continued to improve through month 3.In the open-label phase, a similar improvement was noted in patients transitioning from placebo. In addition to acute medication use, emergency department use for migraine treatment was decreased significantly as well, by more than two thirds in month 3.
Migraine prevention will always remain the key ingredient for improvement for patients with higher frequencies of migraine, and adequate prevention will allow for the lower use of acute medications, and for less healthcare system use in general.
Most practitioners recommend migraine-specific medications for the acute treatment ofmigraine. Since the advent of sumatriptan, this has usually meant a triptan medication. However, a significant percentage of the population (up to 44% in one study) are either intolerant to, contraindicated for, or respond insufficiently to triptan medications. This can either be due to a strong triptan side effect (worsened nausea; tightness/soreness of the muscles of the chest, shoulders, and neck), having cardiovascular risk factors, or not responding adequately 2 hours after treatment.The study by Lipton and colleagues specifically assessed the efficacy of ubrogepant in this population.
Ubrogepant is a small-molecule CGRP antagonist for the acute treatment of migraine. Although somewhat controversial, most practitioners use ubrogepant in patients with some cardiovascular risk, a situation where they would be more likely to avoid the use of triptans.The study authors pooled post hoc data from the pivotal ubrogepant trials (ACHIEVE-1 and -2)to isolate patients with insufficient response to triptans, and their primary outcome was improvement in function 2 hours after medication dose.
Participants in the pivotal trials were separated into three groups: triptan responders, triptaninsufficient responders, and triptan-naive patients. Triptan response was defined as achieving pain freedom 2 hours after medication dose. Both those who had an insufficient response and those who no longer use the triptan owing to intolerance or contraindications were included in the group with insufficient triptan response. Function improvement was defined as the primary outcomeon the basis of a 4-point response scale (0 = no disability, 1 = mildly impaired, 2 = moderately impaired, 3 = severely impaired).In addition, patients were asked to report scores of satisfaction with the medication (yes or no) at 2 and 24 hours and their impression of overall change at 2 hours using a 7-point scale.
The population group of triptan insufficient responders (451 patients) had significant improvement in the primary outcome functional disability at 2, 4, and 7 hours after receipt of medications, but there was no statistical difference at 1 hour. This was similar when comparing those with intolerance to triptans, insufficient response to triptans, or contraindications for triptans. The secondary outcomes of satisfaction and global impression of change were also statistically improved in the insufficient-responders group. No additional tolerance issues or adverse events were noted in this group either.
It would certainly be worth considering the use of agepant acute medication, such as ubrogepant, in patients who are intolerant to or inadequately treated by triptan medications.There still is much to learn about cardiovascular risk and the use of CGRP antagonists, and although no adverse events were noted, more data may be necessary to widely prescribe this class in higher-risk patients.
Mepolizumab curbed corticosteroid use for severe asthma
Use of mepolizumab significantly reduced the need for maintenance oral corticosteroids in adults with severe asthma, based on data from more than 800 individuals.
Many patients with severe asthma require bursts of systemic corticosteroids (SCS) or maintenance oral corticosteroids (mOCS) for disease control, but these strategies are associated with side effects that can increase the disease burden, wrote Charles Pilette, MD, of Cliniques Universitaires Saint-Luc, Brussels, and colleagues.
Previous studies have shown that the humanized, monoclonal anti-interleukin (IL)–5 antibody mepolizumab, which is approved for the treatment of severe asthma, reduced use of SCS and has shown effectiveness in less homogeneous populations, but robust, real-world data on the occurrence and magnitude of these effects are lacking, the researchers said.
In a study known as REALITI-A, the researchers enrolled 822 adults with asthma diagnoses from 82 centers in Europe, Canada, and the United States who initiated mepolizumab at a subcutaneous dose of 100 mg. The study endpoints included daily use of oral corticosteroids at baseline and 1 year, percentage reduction in oral corticosteroid use from baseline, patients discontinuing oral corticosteroids; the primary outcome was the rate of clinically significant exacerbations (CSEs). CSEs were defined as the need for OCS for at least 3 days/parenteral administration, and/or an emergency department or hospital admission before and after treatment. The mean age of the participants was 54 years, 63% were women, and 60% were never-smokers. The mean asthma duration was 19.7 years.
A total of 319 patients (39%), used mOCS at baseline, and dose information was available for 298.
Real-world outcomes
At 1 year, the median mOCS dose in the study population was reduced by 75%, and 64% reduced their mOCS dose by at least 50% from baseline.
In addition, the proportion of patients who discontinued daily mOCS increased from 29% during week 25-28 to 43% during week 53-56.
Overall, 80% of patients remained on mepolizumab at 1 year. Lack of efficacy and patient decision were the top two reasons for discontinuation (6% and 4%, respectively).
The primary outcome of rate of CSE decreased by a clinically significant rate ratio of 0.29 (P < .001).
“The requirement for SCS bursts was also reduced, as observed by a decreased rate of CSEs,” the researchers wrote in their discussion. The results were consistent for patients receiving lower (less than 10 mg/day) or higher (10 mg/day or more) mOCS doses at baseline, they said. No unexpected safety signals were noted during the study period.
“Furthermore, , or those requiring hospitalization or an ER visit, improved symptom control, and lower work productivity and activity impairment,” they added.
The study findings were limited by several factors including the observational design, lack of mepolizumab comparator, and open-label data capture, the researchers noted. However, the results were consistent with similar studies, and support the use of mepolizumab as part of the standard of care for clinically effective disease control in severe asthma patients, they concluded.
The study was funded by GlaxoSmithKline. Lead author Dr. Pilette disclosed fees for advisory boards, speaker meetings, and 42 research grants from GSK, AstraZeneca, Chiesi, Novartis, Teva, and ALK-Abello.
Use of mepolizumab significantly reduced the need for maintenance oral corticosteroids in adults with severe asthma, based on data from more than 800 individuals.
Many patients with severe asthma require bursts of systemic corticosteroids (SCS) or maintenance oral corticosteroids (mOCS) for disease control, but these strategies are associated with side effects that can increase the disease burden, wrote Charles Pilette, MD, of Cliniques Universitaires Saint-Luc, Brussels, and colleagues.
Previous studies have shown that the humanized, monoclonal anti-interleukin (IL)–5 antibody mepolizumab, which is approved for the treatment of severe asthma, reduced use of SCS and has shown effectiveness in less homogeneous populations, but robust, real-world data on the occurrence and magnitude of these effects are lacking, the researchers said.
In a study known as REALITI-A, the researchers enrolled 822 adults with asthma diagnoses from 82 centers in Europe, Canada, and the United States who initiated mepolizumab at a subcutaneous dose of 100 mg. The study endpoints included daily use of oral corticosteroids at baseline and 1 year, percentage reduction in oral corticosteroid use from baseline, patients discontinuing oral corticosteroids; the primary outcome was the rate of clinically significant exacerbations (CSEs). CSEs were defined as the need for OCS for at least 3 days/parenteral administration, and/or an emergency department or hospital admission before and after treatment. The mean age of the participants was 54 years, 63% were women, and 60% were never-smokers. The mean asthma duration was 19.7 years.
A total of 319 patients (39%), used mOCS at baseline, and dose information was available for 298.
Real-world outcomes
At 1 year, the median mOCS dose in the study population was reduced by 75%, and 64% reduced their mOCS dose by at least 50% from baseline.
In addition, the proportion of patients who discontinued daily mOCS increased from 29% during week 25-28 to 43% during week 53-56.
Overall, 80% of patients remained on mepolizumab at 1 year. Lack of efficacy and patient decision were the top two reasons for discontinuation (6% and 4%, respectively).
The primary outcome of rate of CSE decreased by a clinically significant rate ratio of 0.29 (P < .001).
“The requirement for SCS bursts was also reduced, as observed by a decreased rate of CSEs,” the researchers wrote in their discussion. The results were consistent for patients receiving lower (less than 10 mg/day) or higher (10 mg/day or more) mOCS doses at baseline, they said. No unexpected safety signals were noted during the study period.
“Furthermore, , or those requiring hospitalization or an ER visit, improved symptom control, and lower work productivity and activity impairment,” they added.
The study findings were limited by several factors including the observational design, lack of mepolizumab comparator, and open-label data capture, the researchers noted. However, the results were consistent with similar studies, and support the use of mepolizumab as part of the standard of care for clinically effective disease control in severe asthma patients, they concluded.
The study was funded by GlaxoSmithKline. Lead author Dr. Pilette disclosed fees for advisory boards, speaker meetings, and 42 research grants from GSK, AstraZeneca, Chiesi, Novartis, Teva, and ALK-Abello.
Use of mepolizumab significantly reduced the need for maintenance oral corticosteroids in adults with severe asthma, based on data from more than 800 individuals.
Many patients with severe asthma require bursts of systemic corticosteroids (SCS) or maintenance oral corticosteroids (mOCS) for disease control, but these strategies are associated with side effects that can increase the disease burden, wrote Charles Pilette, MD, of Cliniques Universitaires Saint-Luc, Brussels, and colleagues.
Previous studies have shown that the humanized, monoclonal anti-interleukin (IL)–5 antibody mepolizumab, which is approved for the treatment of severe asthma, reduced use of SCS and has shown effectiveness in less homogeneous populations, but robust, real-world data on the occurrence and magnitude of these effects are lacking, the researchers said.
In a study known as REALITI-A, the researchers enrolled 822 adults with asthma diagnoses from 82 centers in Europe, Canada, and the United States who initiated mepolizumab at a subcutaneous dose of 100 mg. The study endpoints included daily use of oral corticosteroids at baseline and 1 year, percentage reduction in oral corticosteroid use from baseline, patients discontinuing oral corticosteroids; the primary outcome was the rate of clinically significant exacerbations (CSEs). CSEs were defined as the need for OCS for at least 3 days/parenteral administration, and/or an emergency department or hospital admission before and after treatment. The mean age of the participants was 54 years, 63% were women, and 60% were never-smokers. The mean asthma duration was 19.7 years.
A total of 319 patients (39%), used mOCS at baseline, and dose information was available for 298.
Real-world outcomes
At 1 year, the median mOCS dose in the study population was reduced by 75%, and 64% reduced their mOCS dose by at least 50% from baseline.
In addition, the proportion of patients who discontinued daily mOCS increased from 29% during week 25-28 to 43% during week 53-56.
Overall, 80% of patients remained on mepolizumab at 1 year. Lack of efficacy and patient decision were the top two reasons for discontinuation (6% and 4%, respectively).
The primary outcome of rate of CSE decreased by a clinically significant rate ratio of 0.29 (P < .001).
“The requirement for SCS bursts was also reduced, as observed by a decreased rate of CSEs,” the researchers wrote in their discussion. The results were consistent for patients receiving lower (less than 10 mg/day) or higher (10 mg/day or more) mOCS doses at baseline, they said. No unexpected safety signals were noted during the study period.
“Furthermore, , or those requiring hospitalization or an ER visit, improved symptom control, and lower work productivity and activity impairment,” they added.
The study findings were limited by several factors including the observational design, lack of mepolizumab comparator, and open-label data capture, the researchers noted. However, the results were consistent with similar studies, and support the use of mepolizumab as part of the standard of care for clinically effective disease control in severe asthma patients, they concluded.
The study was funded by GlaxoSmithKline. Lead author Dr. Pilette disclosed fees for advisory boards, speaker meetings, and 42 research grants from GSK, AstraZeneca, Chiesi, Novartis, Teva, and ALK-Abello.
FROM JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Commentary: Treating ER+ Breast Cancer and Brain Metastases, July 2022
The clinical benefit of fulvestrant as a second-line treatment after tumor progression with CDK4/6i-based therapy has been discouraging, highlighting an unmet need for a better SERD in this space.1 Multiple oral SERD are currently in trials. The EMERALD trial is a phase 3 study that randomly assigned 477 patients with ER+/HER2- mBC in a 1:1 ratio to elacestrant, an oral SERD, vs standard of care (SOC) endocrine therapy (ET). Enrolled patients had received one to two prior ET and one or less chemotherapy treatments in the metastatic disease settings. All patients had prior treatment with a CDK4/6i. Fulvestrant-naive patients were required to have fulvestrant as the SOC ET. In contrast, patients previously treated with fulvestrant received an AI, the selection of which was based on prior AI therapy. Primary endpoints were progression-free survival (PFS) in all patients and in patients with detectable ESR1 mutation. The median PFS in the elacestrant arm was 2.8 months vs 1.9 months in the control arm [hazard ratio (HR) 0.70; 95% CI 0.55-0.88; P = .002]. The 6-month PFS was 34% vs 20% in all patients and 41% vs 19% in patients with detectable ESR1 mutation, favoring the elacestrant arm. In the subgroup analysis among patients who received fulvestrant in the control arm, the 6-month PFS was 34% vs 21% in all patients and 41% vs 19% in patients with ESR1 mutation. Grade 3 or 4 adverse events developed in 27% patients in the elacestrant arm compared with 20% in the SOC arm. More patients in the elacestrant arm developed nausea, vomiting, and liver function abnormalities compared with patients in the SOC arm.
This is the first phase 3 trial demonstrating statistically significant prolongation of PFS associated with an oral SERD compared with SOC ET in patients with ER+/HER2- mBC who had prior treatment with a CDK4/6i. A new drug application has been submitted to the US Food and Drug Administration based on this data. If approved, elacestrant may be favored over fulvestrant as the standard ET for ER+/HER2- mBC after progression on a CDK4/6i-based therapy.
Patients with breast cancer brain metastasis (BrM) have a poor prognosis. Systemic therapies with good central nervous system (CNS) permeability and strong activity against BrM are much needed. An exploratory subset of the phase 3 BEACON trial demonstrated improvement in overall survival (OS) with etirinotecan pegol, a long-acting polymer conjugate of irinotecan, compared with physicians' choice chemotherapy in patients with mBC with treated and stable BrMs.2
Based on this data, a large phase 3 study (the ATTAIN study) was conducted. Patients with mBC with treated and stable BrM (n = 178) were randomly assigned to receive etirinotecan pegol vs physicians' choice of chemotherapy (eribulin, ixabepilone, vinorelbine, gemcitabine, paclitaxel, docetaxel, or nab-paclitaxel). The primary endpoint of OS was similar in both groups (etirinotecan pegol 7.8 months; chemotherapy 7.5 months; HR 0.90; 95% CI 0.61-1.33; P = .60). Median PFS for mBC with CNS metastases (etirinotecan pegol vs chemotherapy) were 3.9 vs 3.3 months (HR 0.59; 95% CI 0.33-1.05; P = .07) and for non-CNS metastases were 2.8 vs 1.9 months (HR 0.72; 95% CI 0.45-1.16; P = .18). Adverse events were grade 3 or 4 in 57% patients receiving etirinotecan pegol compared with 64% receiving SOC chemotherapy.
This trial failed to meet its primary endpoint. The possible explanations proposed by the investigators are a protocol amendment that reduced the power of the trial to 80%, some key differences in the patient population in the ATTAIN trial compared with the BEACON trial, and the possibility of the BEACON trial exploratory analysis result being a false positive. The OS of around 7 months highlights the unmet need for better systemic therapy for patients with BrM breast cancer, especially those with HER2- breast cancer.
Additional References
- Lindeman GJ, Fernando TM, Bowen R, et al. VERONICA: Randomized phase II study of fulvestrant and venetoclax in ER-positive metastatic breast cancer post-CDK4/6 inhibitors – Efficacy, safety, and biomarker results. Clin Cancer Res. 2022 (June 21). Doi: 10.1158/1078-0432.CCR-21-3811
- Cortés J, Rugo HS, Awada A, et al. Prolonged survival in patients with breast cancer and a history of brain metastases: Results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res Treat. 2017;165:329-341. Doi: 10.1007/s10549-017-4304-7
The clinical benefit of fulvestrant as a second-line treatment after tumor progression with CDK4/6i-based therapy has been discouraging, highlighting an unmet need for a better SERD in this space.1 Multiple oral SERD are currently in trials. The EMERALD trial is a phase 3 study that randomly assigned 477 patients with ER+/HER2- mBC in a 1:1 ratio to elacestrant, an oral SERD, vs standard of care (SOC) endocrine therapy (ET). Enrolled patients had received one to two prior ET and one or less chemotherapy treatments in the metastatic disease settings. All patients had prior treatment with a CDK4/6i. Fulvestrant-naive patients were required to have fulvestrant as the SOC ET. In contrast, patients previously treated with fulvestrant received an AI, the selection of which was based on prior AI therapy. Primary endpoints were progression-free survival (PFS) in all patients and in patients with detectable ESR1 mutation. The median PFS in the elacestrant arm was 2.8 months vs 1.9 months in the control arm [hazard ratio (HR) 0.70; 95% CI 0.55-0.88; P = .002]. The 6-month PFS was 34% vs 20% in all patients and 41% vs 19% in patients with detectable ESR1 mutation, favoring the elacestrant arm. In the subgroup analysis among patients who received fulvestrant in the control arm, the 6-month PFS was 34% vs 21% in all patients and 41% vs 19% in patients with ESR1 mutation. Grade 3 or 4 adverse events developed in 27% patients in the elacestrant arm compared with 20% in the SOC arm. More patients in the elacestrant arm developed nausea, vomiting, and liver function abnormalities compared with patients in the SOC arm.
This is the first phase 3 trial demonstrating statistically significant prolongation of PFS associated with an oral SERD compared with SOC ET in patients with ER+/HER2- mBC who had prior treatment with a CDK4/6i. A new drug application has been submitted to the US Food and Drug Administration based on this data. If approved, elacestrant may be favored over fulvestrant as the standard ET for ER+/HER2- mBC after progression on a CDK4/6i-based therapy.
Patients with breast cancer brain metastasis (BrM) have a poor prognosis. Systemic therapies with good central nervous system (CNS) permeability and strong activity against BrM are much needed. An exploratory subset of the phase 3 BEACON trial demonstrated improvement in overall survival (OS) with etirinotecan pegol, a long-acting polymer conjugate of irinotecan, compared with physicians' choice chemotherapy in patients with mBC with treated and stable BrMs.2
Based on this data, a large phase 3 study (the ATTAIN study) was conducted. Patients with mBC with treated and stable BrM (n = 178) were randomly assigned to receive etirinotecan pegol vs physicians' choice of chemotherapy (eribulin, ixabepilone, vinorelbine, gemcitabine, paclitaxel, docetaxel, or nab-paclitaxel). The primary endpoint of OS was similar in both groups (etirinotecan pegol 7.8 months; chemotherapy 7.5 months; HR 0.90; 95% CI 0.61-1.33; P = .60). Median PFS for mBC with CNS metastases (etirinotecan pegol vs chemotherapy) were 3.9 vs 3.3 months (HR 0.59; 95% CI 0.33-1.05; P = .07) and for non-CNS metastases were 2.8 vs 1.9 months (HR 0.72; 95% CI 0.45-1.16; P = .18). Adverse events were grade 3 or 4 in 57% patients receiving etirinotecan pegol compared with 64% receiving SOC chemotherapy.
This trial failed to meet its primary endpoint. The possible explanations proposed by the investigators are a protocol amendment that reduced the power of the trial to 80%, some key differences in the patient population in the ATTAIN trial compared with the BEACON trial, and the possibility of the BEACON trial exploratory analysis result being a false positive. The OS of around 7 months highlights the unmet need for better systemic therapy for patients with BrM breast cancer, especially those with HER2- breast cancer.
Additional References
- Lindeman GJ, Fernando TM, Bowen R, et al. VERONICA: Randomized phase II study of fulvestrant and venetoclax in ER-positive metastatic breast cancer post-CDK4/6 inhibitors – Efficacy, safety, and biomarker results. Clin Cancer Res. 2022 (June 21). Doi: 10.1158/1078-0432.CCR-21-3811
- Cortés J, Rugo HS, Awada A, et al. Prolonged survival in patients with breast cancer and a history of brain metastases: Results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res Treat. 2017;165:329-341. Doi: 10.1007/s10549-017-4304-7
The clinical benefit of fulvestrant as a second-line treatment after tumor progression with CDK4/6i-based therapy has been discouraging, highlighting an unmet need for a better SERD in this space.1 Multiple oral SERD are currently in trials. The EMERALD trial is a phase 3 study that randomly assigned 477 patients with ER+/HER2- mBC in a 1:1 ratio to elacestrant, an oral SERD, vs standard of care (SOC) endocrine therapy (ET). Enrolled patients had received one to two prior ET and one or less chemotherapy treatments in the metastatic disease settings. All patients had prior treatment with a CDK4/6i. Fulvestrant-naive patients were required to have fulvestrant as the SOC ET. In contrast, patients previously treated with fulvestrant received an AI, the selection of which was based on prior AI therapy. Primary endpoints were progression-free survival (PFS) in all patients and in patients with detectable ESR1 mutation. The median PFS in the elacestrant arm was 2.8 months vs 1.9 months in the control arm [hazard ratio (HR) 0.70; 95% CI 0.55-0.88; P = .002]. The 6-month PFS was 34% vs 20% in all patients and 41% vs 19% in patients with detectable ESR1 mutation, favoring the elacestrant arm. In the subgroup analysis among patients who received fulvestrant in the control arm, the 6-month PFS was 34% vs 21% in all patients and 41% vs 19% in patients with ESR1 mutation. Grade 3 or 4 adverse events developed in 27% patients in the elacestrant arm compared with 20% in the SOC arm. More patients in the elacestrant arm developed nausea, vomiting, and liver function abnormalities compared with patients in the SOC arm.
This is the first phase 3 trial demonstrating statistically significant prolongation of PFS associated with an oral SERD compared with SOC ET in patients with ER+/HER2- mBC who had prior treatment with a CDK4/6i. A new drug application has been submitted to the US Food and Drug Administration based on this data. If approved, elacestrant may be favored over fulvestrant as the standard ET for ER+/HER2- mBC after progression on a CDK4/6i-based therapy.
Patients with breast cancer brain metastasis (BrM) have a poor prognosis. Systemic therapies with good central nervous system (CNS) permeability and strong activity against BrM are much needed. An exploratory subset of the phase 3 BEACON trial demonstrated improvement in overall survival (OS) with etirinotecan pegol, a long-acting polymer conjugate of irinotecan, compared with physicians' choice chemotherapy in patients with mBC with treated and stable BrMs.2
Based on this data, a large phase 3 study (the ATTAIN study) was conducted. Patients with mBC with treated and stable BrM (n = 178) were randomly assigned to receive etirinotecan pegol vs physicians' choice of chemotherapy (eribulin, ixabepilone, vinorelbine, gemcitabine, paclitaxel, docetaxel, or nab-paclitaxel). The primary endpoint of OS was similar in both groups (etirinotecan pegol 7.8 months; chemotherapy 7.5 months; HR 0.90; 95% CI 0.61-1.33; P = .60). Median PFS for mBC with CNS metastases (etirinotecan pegol vs chemotherapy) were 3.9 vs 3.3 months (HR 0.59; 95% CI 0.33-1.05; P = .07) and for non-CNS metastases were 2.8 vs 1.9 months (HR 0.72; 95% CI 0.45-1.16; P = .18). Adverse events were grade 3 or 4 in 57% patients receiving etirinotecan pegol compared with 64% receiving SOC chemotherapy.
This trial failed to meet its primary endpoint. The possible explanations proposed by the investigators are a protocol amendment that reduced the power of the trial to 80%, some key differences in the patient population in the ATTAIN trial compared with the BEACON trial, and the possibility of the BEACON trial exploratory analysis result being a false positive. The OS of around 7 months highlights the unmet need for better systemic therapy for patients with BrM breast cancer, especially those with HER2- breast cancer.
Additional References
- Lindeman GJ, Fernando TM, Bowen R, et al. VERONICA: Randomized phase II study of fulvestrant and venetoclax in ER-positive metastatic breast cancer post-CDK4/6 inhibitors – Efficacy, safety, and biomarker results. Clin Cancer Res. 2022 (June 21). Doi: 10.1158/1078-0432.CCR-21-3811
- Cortés J, Rugo HS, Awada A, et al. Prolonged survival in patients with breast cancer and a history of brain metastases: Results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res Treat. 2017;165:329-341. Doi: 10.1007/s10549-017-4304-7
‘Not their fault:’ Obesity warrants long-term management
This transcript has been edited for clarity.
It’s important to remember and to think about the first time when patients with obesity come to see us: What have they faced? What have been their struggles? What shame and blame and bias have they faced?
One of the first things that I do when a patient comes to see me is invite them to share their weight journey with me. I ask them to tell me about their struggles, about what’s worked and what hasn’t worked, what they would like, and what their health goals are.
As they share their stories, I look for the opportunity to share with them that obesity is not their fault, but that it’s biology driving their body to carry extra weight and their body is super smart. Neither their body nor their brain want them to starve.
Our bodies evolved during a time where there was food scarcity and the potential of famine. We have a complex system that was designed to make sure that we always held on to extra weight, specifically extra fat, because that’s how we store energy. In the current obesogenic environment, what happens is our bodies carry extra weight, or specifically, extra fat.
Again, I say to them, this is biology. Your body’s doing exactly what it was designed to do. Your body’s very smart, but now we have to figure out how to help your body want to carry less fat because it is impacting your health. This is not your fault. Having obesity is not your fault any more than having diabetes or hypertension is anyone’s fault. Now it’s time for all of us to use highly effective tools that target the pathophysiology of obesity.
When a patient comes to me for weight management or to help them treat their obesity, I listen to them, and I look for clues as to what might help that specific patient. Every patient deserves to have individualized treatment. One medicine may be right for one person, another medicine may be right for another, and surgery may be right for another patient. I really try to listen and hear what that patient is telling me.
What we as providers really need is tools – different options – to be able to provide for our patients and basically present them with different options, and then guide them toward the best therapy for them. Whether it’s semaglutide or tirzepatide potentially in the future, these types of medications are excellent options for our patients. They’re highly effective tools with safe profiles.
A question that I often get from providers or patients is, “Well, Doctor, I’ve lost the weight now. How long should I take this medicine? Can I stop it now?”
Then, we have a conversation, and we actually usually have this conversation even before we start the medicine. Basically, we talk about the fact that obesity is a chronic disease. There’s no cure for obesity. Because it’s a chronic disease, we need to treat it like we would treat any other chronic disease.
The example that I often use is, if you have a patient who has hypertension and you start them on an antihypertensive medication, what happens? Their blood pressure goes down. It improves. Now, if their blood pressure is improved with a specific antihypertensive, would you stop that medicine? What would happen if you stopped that antihypertensive? Well, their blood pressure would go up, and we wouldn’t be surprised.
In the same way, if you have a patient who has obesity and you start that patient on an antiobesity medication, and their weight decreases, and their body fat mass at that point decreases, what would happen if you stop that medicine? They lost the weight, but you stop the medicine. Well, their weight gain comes back. They regain the weight.
We should not be surprised that weight gain occurs when we stop the treatment. That really underscores the fact that treatment needs to be continued. If a patient is started on an antiobesity medication and they lose weight, that medication needs to be continued to maintain that weight loss.
Basically, we eat food and our body responds by releasing these hormones. The hormones are made in our gut and in our pancreas and these hormones inform our brain. Are we hungry? Are we full? Where are we with our homeostatic set point of fat mass? Based on that, our brain is like the sensor or the thermostat.
Obesity is a chronic, treatable disease. We should treat obesity as we treat any other chronic disease, with effective and safe approaches that target underlying disease mechanisms. These results in the SURMOUNT-1 trial underscore that tirzepatide may be doing just that. Remarkably, 9 in 10 individuals with obesity lost weight while taking tirzepatide. These results are impressive. They’re an important step forward in potentially expanding effective therapeutic options for people with obesity.
Dr. Jastreboff is an associate professor of medicine and pediatrics at Yale University, New Haven, Conn., and director of weight management and obesity prevention at Yale Stress Center. She reported conducting trials with Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; serving on scientific advisory boards for Ely Lilly, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, and WW; and consulting for Boehringer Ingelheim and Scholar Rock.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
It’s important to remember and to think about the first time when patients with obesity come to see us: What have they faced? What have been their struggles? What shame and blame and bias have they faced?
One of the first things that I do when a patient comes to see me is invite them to share their weight journey with me. I ask them to tell me about their struggles, about what’s worked and what hasn’t worked, what they would like, and what their health goals are.
As they share their stories, I look for the opportunity to share with them that obesity is not their fault, but that it’s biology driving their body to carry extra weight and their body is super smart. Neither their body nor their brain want them to starve.
Our bodies evolved during a time where there was food scarcity and the potential of famine. We have a complex system that was designed to make sure that we always held on to extra weight, specifically extra fat, because that’s how we store energy. In the current obesogenic environment, what happens is our bodies carry extra weight, or specifically, extra fat.
Again, I say to them, this is biology. Your body’s doing exactly what it was designed to do. Your body’s very smart, but now we have to figure out how to help your body want to carry less fat because it is impacting your health. This is not your fault. Having obesity is not your fault any more than having diabetes or hypertension is anyone’s fault. Now it’s time for all of us to use highly effective tools that target the pathophysiology of obesity.
When a patient comes to me for weight management or to help them treat their obesity, I listen to them, and I look for clues as to what might help that specific patient. Every patient deserves to have individualized treatment. One medicine may be right for one person, another medicine may be right for another, and surgery may be right for another patient. I really try to listen and hear what that patient is telling me.
What we as providers really need is tools – different options – to be able to provide for our patients and basically present them with different options, and then guide them toward the best therapy for them. Whether it’s semaglutide or tirzepatide potentially in the future, these types of medications are excellent options for our patients. They’re highly effective tools with safe profiles.
A question that I often get from providers or patients is, “Well, Doctor, I’ve lost the weight now. How long should I take this medicine? Can I stop it now?”
Then, we have a conversation, and we actually usually have this conversation even before we start the medicine. Basically, we talk about the fact that obesity is a chronic disease. There’s no cure for obesity. Because it’s a chronic disease, we need to treat it like we would treat any other chronic disease.
The example that I often use is, if you have a patient who has hypertension and you start them on an antihypertensive medication, what happens? Their blood pressure goes down. It improves. Now, if their blood pressure is improved with a specific antihypertensive, would you stop that medicine? What would happen if you stopped that antihypertensive? Well, their blood pressure would go up, and we wouldn’t be surprised.
In the same way, if you have a patient who has obesity and you start that patient on an antiobesity medication, and their weight decreases, and their body fat mass at that point decreases, what would happen if you stop that medicine? They lost the weight, but you stop the medicine. Well, their weight gain comes back. They regain the weight.
We should not be surprised that weight gain occurs when we stop the treatment. That really underscores the fact that treatment needs to be continued. If a patient is started on an antiobesity medication and they lose weight, that medication needs to be continued to maintain that weight loss.
Basically, we eat food and our body responds by releasing these hormones. The hormones are made in our gut and in our pancreas and these hormones inform our brain. Are we hungry? Are we full? Where are we with our homeostatic set point of fat mass? Based on that, our brain is like the sensor or the thermostat.
Obesity is a chronic, treatable disease. We should treat obesity as we treat any other chronic disease, with effective and safe approaches that target underlying disease mechanisms. These results in the SURMOUNT-1 trial underscore that tirzepatide may be doing just that. Remarkably, 9 in 10 individuals with obesity lost weight while taking tirzepatide. These results are impressive. They’re an important step forward in potentially expanding effective therapeutic options for people with obesity.
Dr. Jastreboff is an associate professor of medicine and pediatrics at Yale University, New Haven, Conn., and director of weight management and obesity prevention at Yale Stress Center. She reported conducting trials with Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; serving on scientific advisory boards for Ely Lilly, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, and WW; and consulting for Boehringer Ingelheim and Scholar Rock.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
It’s important to remember and to think about the first time when patients with obesity come to see us: What have they faced? What have been their struggles? What shame and blame and bias have they faced?
One of the first things that I do when a patient comes to see me is invite them to share their weight journey with me. I ask them to tell me about their struggles, about what’s worked and what hasn’t worked, what they would like, and what their health goals are.
As they share their stories, I look for the opportunity to share with them that obesity is not their fault, but that it’s biology driving their body to carry extra weight and their body is super smart. Neither their body nor their brain want them to starve.
Our bodies evolved during a time where there was food scarcity and the potential of famine. We have a complex system that was designed to make sure that we always held on to extra weight, specifically extra fat, because that’s how we store energy. In the current obesogenic environment, what happens is our bodies carry extra weight, or specifically, extra fat.
Again, I say to them, this is biology. Your body’s doing exactly what it was designed to do. Your body’s very smart, but now we have to figure out how to help your body want to carry less fat because it is impacting your health. This is not your fault. Having obesity is not your fault any more than having diabetes or hypertension is anyone’s fault. Now it’s time for all of us to use highly effective tools that target the pathophysiology of obesity.
When a patient comes to me for weight management or to help them treat their obesity, I listen to them, and I look for clues as to what might help that specific patient. Every patient deserves to have individualized treatment. One medicine may be right for one person, another medicine may be right for another, and surgery may be right for another patient. I really try to listen and hear what that patient is telling me.
What we as providers really need is tools – different options – to be able to provide for our patients and basically present them with different options, and then guide them toward the best therapy for them. Whether it’s semaglutide or tirzepatide potentially in the future, these types of medications are excellent options for our patients. They’re highly effective tools with safe profiles.
A question that I often get from providers or patients is, “Well, Doctor, I’ve lost the weight now. How long should I take this medicine? Can I stop it now?”
Then, we have a conversation, and we actually usually have this conversation even before we start the medicine. Basically, we talk about the fact that obesity is a chronic disease. There’s no cure for obesity. Because it’s a chronic disease, we need to treat it like we would treat any other chronic disease.
The example that I often use is, if you have a patient who has hypertension and you start them on an antihypertensive medication, what happens? Their blood pressure goes down. It improves. Now, if their blood pressure is improved with a specific antihypertensive, would you stop that medicine? What would happen if you stopped that antihypertensive? Well, their blood pressure would go up, and we wouldn’t be surprised.
In the same way, if you have a patient who has obesity and you start that patient on an antiobesity medication, and their weight decreases, and their body fat mass at that point decreases, what would happen if you stop that medicine? They lost the weight, but you stop the medicine. Well, their weight gain comes back. They regain the weight.
We should not be surprised that weight gain occurs when we stop the treatment. That really underscores the fact that treatment needs to be continued. If a patient is started on an antiobesity medication and they lose weight, that medication needs to be continued to maintain that weight loss.
Basically, we eat food and our body responds by releasing these hormones. The hormones are made in our gut and in our pancreas and these hormones inform our brain. Are we hungry? Are we full? Where are we with our homeostatic set point of fat mass? Based on that, our brain is like the sensor or the thermostat.
Obesity is a chronic, treatable disease. We should treat obesity as we treat any other chronic disease, with effective and safe approaches that target underlying disease mechanisms. These results in the SURMOUNT-1 trial underscore that tirzepatide may be doing just that. Remarkably, 9 in 10 individuals with obesity lost weight while taking tirzepatide. These results are impressive. They’re an important step forward in potentially expanding effective therapeutic options for people with obesity.
Dr. Jastreboff is an associate professor of medicine and pediatrics at Yale University, New Haven, Conn., and director of weight management and obesity prevention at Yale Stress Center. She reported conducting trials with Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; serving on scientific advisory boards for Ely Lilly, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, and WW; and consulting for Boehringer Ingelheim and Scholar Rock.
A version of this article first appeared on Medscape.com.