User login
What explains poor adherence to eosinophilic esophagitis therapy?
Almost half of adult patients with eosinophilic esophagitis (EoE) reported poor adherence to long-term medical and dietary therapy, with age younger than 40 years and low necessity beliefs being the strongest predictors, a new study finds.
Clinicians need to spend more time discussing the need for EoE therapy with their patients, especially if they are younger, according to lead author Maria L. Haasnoot, MD, of Amsterdam University Medical Center (UMC), the Netherlands, and colleagues.
“Chronic treatment is necessary to maintain suppression of the inflammation and prevent negative outcomes in the long-term,” they write.
Until the recent approval of dupilumab (Dupixent) by the U.S. Food and Drug Administration, patients with EoE relied upon off-label options, including proton pump inhibitors and swallowed topical steroids, as well as dietary interventions for ongoing suppression of inflammation. But only about 1 in 6 patients achieve complete remission at 5 years, according to Dr. Haasnoot and colleagues.
“It is uncertain to what degree limited adherence to treatment [plays] a role in the limited long-term effects of treatment,” they write.
The findings were published online in American Journal of Gastroenterology.
Addressing a knowledge gap
The cross-sectional study involved 177 adult patients with EoE treated at Amsterdam UMC, who were prescribed dietary or medical maintenance therapy. Of note, some patients were treated with budesonide, which is approved for EoE in Europe but not in the United States.
Median participant age was 43 years, with a male-skewed distribution (71% men). Patients had been on EoE treatment for 2-6 years. Most (76%) were on medical treatments. Nearly half were on diets that avoided one to five food groups, with some on both medical treatments and elimination diets.
Using a link sent by mail, participants completed the online Medication Adherence Rating Scale, along with several other questionnaires, such as the Beliefs about Medicine Questionnaire, to measure secondary outcomes, including a patient’s view of how necessary or disruptive maintenance therapy is in their life.
The overall prevalence of poor adherence to therapy was high (41.8%), including a nonsignificant difference in adherence between medical and dietary therapies.
“It might come as a surprise that dietary-treated patients are certainly not less adherent to treatment than medically treated patients,” the authors write, noting that the opposite is usually true.
Multivariate logistic regression showed that patients younger than 40 years were more than twice as likely to be poorly adherent (odds ratio, 2.571; 95% confidence interval, 1.195-5.532). Those with low necessity beliefs were more than four times as likely to be poorly adherent (OR, 4.423; 95% CI, 2.169-9.016). Other factors linked to poor adherence were patients with longer disease duration and more severe symptoms.
“Clinicians should pay more attention to treatment adherence, particularly in younger patients,” the authors conclude. “The necessity of treatment should be actively discussed, and efforts should be done to take doubts away, as this may improve treatment adherence and subsequently may improve treatment effects and long-term outcomes.”
More patient education needed
According to Jennifer L. Horsley-Silva, MD, of Mayo Clinic, Scottsdale, Ariz., “This study is important, as it is one of the first studies to investigate the rate of treatment adherence in EoE patients and attempts to identify factors associated with adherence both in medically and dietary treated patients.”
Dr. Horsley-Silva commented that the findings align with recent research she and her colleagues conducted at the Mayo Clinic, where few patients successfully completed a six-food elimination diet, even when paired with a dietitian. As with the present study, success trended lower among younger adults. “These findings highlight the need for physicians treating EoE to motivate all patients, but especially younger patients, by discussing disease pathophysiology and explaining the reason for maintenance treatment early on,” Dr. Horsley-Silva said.
Conversations should also address the discordance between symptoms and histologic disease, patient doubts and concerns, and other barriers to adherence, she noted.
“Shared decisionmaking is of utmost importance when deciding upon a maintenance treatment strategy and should be readdressed continually,” she added.
Gary W. Falk, MD, of Penn Medicine, Philadelphia, said that patients with EoE may be poorly adherent because therapies tend to be complicated and people often forget to take their medications, especially when their symptoms improve, even though this is a poor indicator of underlying disease. The discordance between symptoms and histology is “not commonly appreciated by the EoE GI community,” he noted.
Patients may benefit from knowing that untreated or undertreated EoE increases the risk for strictures and stenoses, need for dilation, and frequency of food bolus impactions, Dr. Falk said.
“The other thing we know is that once someone is induced into remission, and they stay on therapy ... long-term remission can be maintained,” he added.
The impact of Dupilumab
John Leung, MD, of Boston Food Allergy Center, also cited the complexities of EoE therapies as reason for poor adherence, though he believes this paradigm will shift now that dupilumab has been approved. Dupilumab injections are “just once a week, so it’s much easier in terms of frequency,” Dr. Leung said. “I would expect that the compliance [for dupilumab] will be better” than for older therapies.
Dr. Leung, who helped conduct the dupilumab clinical trials contributing to its approval for EoE and receives speaking honoraria from manufacturer Regeneron/Sanofi, said that dupilumab also overcomes the challenges with elimination diets while offering relief for concomitant conditions, such as “asthma, eczema, food allergies, and seasonal allergies.”
But Dr. Falk, who also worked on the dupilumab clinical trials, said the situation is “not straightforward,” even with FDA approval.
“There are going to be significant costs with [prescribing dupilumab], because it’s a biologic,” Dr. Falk said.
Dr. Falk also pointed out that prior authorization will be required, and until more studies can be conducted, the true impact of once-weekly dosing versus daily dosing remains unknown.
“I would say [dupilumab] has the potential to improve adherence, but we need to see if that’s going to be the case or not,” Dr. Falk said.
The authors disclosed relationships with Dr. Falk Pharma, AstraZeneca, and Sanofi/Regeneron (the manufacturers of Dupixent [dupilumab]), among others. Dr. Horsley-Silva, Dr. Falk, and Dr. Leung conducted clinical trials for dupilumab on behalf of Sanofi/Regeneron, with Dr. Leung also disclosing speaking honoraria from Sanofi/Regeneron. Dr. Horsley-Silva has acted as a clinical trial site principal investigator for Allakos and Celgene/Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Almost half of adult patients with eosinophilic esophagitis (EoE) reported poor adherence to long-term medical and dietary therapy, with age younger than 40 years and low necessity beliefs being the strongest predictors, a new study finds.
Clinicians need to spend more time discussing the need for EoE therapy with their patients, especially if they are younger, according to lead author Maria L. Haasnoot, MD, of Amsterdam University Medical Center (UMC), the Netherlands, and colleagues.
“Chronic treatment is necessary to maintain suppression of the inflammation and prevent negative outcomes in the long-term,” they write.
Until the recent approval of dupilumab (Dupixent) by the U.S. Food and Drug Administration, patients with EoE relied upon off-label options, including proton pump inhibitors and swallowed topical steroids, as well as dietary interventions for ongoing suppression of inflammation. But only about 1 in 6 patients achieve complete remission at 5 years, according to Dr. Haasnoot and colleagues.
“It is uncertain to what degree limited adherence to treatment [plays] a role in the limited long-term effects of treatment,” they write.
The findings were published online in American Journal of Gastroenterology.
Addressing a knowledge gap
The cross-sectional study involved 177 adult patients with EoE treated at Amsterdam UMC, who were prescribed dietary or medical maintenance therapy. Of note, some patients were treated with budesonide, which is approved for EoE in Europe but not in the United States.
Median participant age was 43 years, with a male-skewed distribution (71% men). Patients had been on EoE treatment for 2-6 years. Most (76%) were on medical treatments. Nearly half were on diets that avoided one to five food groups, with some on both medical treatments and elimination diets.
Using a link sent by mail, participants completed the online Medication Adherence Rating Scale, along with several other questionnaires, such as the Beliefs about Medicine Questionnaire, to measure secondary outcomes, including a patient’s view of how necessary or disruptive maintenance therapy is in their life.
The overall prevalence of poor adherence to therapy was high (41.8%), including a nonsignificant difference in adherence between medical and dietary therapies.
“It might come as a surprise that dietary-treated patients are certainly not less adherent to treatment than medically treated patients,” the authors write, noting that the opposite is usually true.
Multivariate logistic regression showed that patients younger than 40 years were more than twice as likely to be poorly adherent (odds ratio, 2.571; 95% confidence interval, 1.195-5.532). Those with low necessity beliefs were more than four times as likely to be poorly adherent (OR, 4.423; 95% CI, 2.169-9.016). Other factors linked to poor adherence were patients with longer disease duration and more severe symptoms.
“Clinicians should pay more attention to treatment adherence, particularly in younger patients,” the authors conclude. “The necessity of treatment should be actively discussed, and efforts should be done to take doubts away, as this may improve treatment adherence and subsequently may improve treatment effects and long-term outcomes.”
More patient education needed
According to Jennifer L. Horsley-Silva, MD, of Mayo Clinic, Scottsdale, Ariz., “This study is important, as it is one of the first studies to investigate the rate of treatment adherence in EoE patients and attempts to identify factors associated with adherence both in medically and dietary treated patients.”
Dr. Horsley-Silva commented that the findings align with recent research she and her colleagues conducted at the Mayo Clinic, where few patients successfully completed a six-food elimination diet, even when paired with a dietitian. As with the present study, success trended lower among younger adults. “These findings highlight the need for physicians treating EoE to motivate all patients, but especially younger patients, by discussing disease pathophysiology and explaining the reason for maintenance treatment early on,” Dr. Horsley-Silva said.
Conversations should also address the discordance between symptoms and histologic disease, patient doubts and concerns, and other barriers to adherence, she noted.
“Shared decisionmaking is of utmost importance when deciding upon a maintenance treatment strategy and should be readdressed continually,” she added.
Gary W. Falk, MD, of Penn Medicine, Philadelphia, said that patients with EoE may be poorly adherent because therapies tend to be complicated and people often forget to take their medications, especially when their symptoms improve, even though this is a poor indicator of underlying disease. The discordance between symptoms and histology is “not commonly appreciated by the EoE GI community,” he noted.
Patients may benefit from knowing that untreated or undertreated EoE increases the risk for strictures and stenoses, need for dilation, and frequency of food bolus impactions, Dr. Falk said.
“The other thing we know is that once someone is induced into remission, and they stay on therapy ... long-term remission can be maintained,” he added.
The impact of Dupilumab
John Leung, MD, of Boston Food Allergy Center, also cited the complexities of EoE therapies as reason for poor adherence, though he believes this paradigm will shift now that dupilumab has been approved. Dupilumab injections are “just once a week, so it’s much easier in terms of frequency,” Dr. Leung said. “I would expect that the compliance [for dupilumab] will be better” than for older therapies.
Dr. Leung, who helped conduct the dupilumab clinical trials contributing to its approval for EoE and receives speaking honoraria from manufacturer Regeneron/Sanofi, said that dupilumab also overcomes the challenges with elimination diets while offering relief for concomitant conditions, such as “asthma, eczema, food allergies, and seasonal allergies.”
But Dr. Falk, who also worked on the dupilumab clinical trials, said the situation is “not straightforward,” even with FDA approval.
“There are going to be significant costs with [prescribing dupilumab], because it’s a biologic,” Dr. Falk said.
Dr. Falk also pointed out that prior authorization will be required, and until more studies can be conducted, the true impact of once-weekly dosing versus daily dosing remains unknown.
“I would say [dupilumab] has the potential to improve adherence, but we need to see if that’s going to be the case or not,” Dr. Falk said.
The authors disclosed relationships with Dr. Falk Pharma, AstraZeneca, and Sanofi/Regeneron (the manufacturers of Dupixent [dupilumab]), among others. Dr. Horsley-Silva, Dr. Falk, and Dr. Leung conducted clinical trials for dupilumab on behalf of Sanofi/Regeneron, with Dr. Leung also disclosing speaking honoraria from Sanofi/Regeneron. Dr. Horsley-Silva has acted as a clinical trial site principal investigator for Allakos and Celgene/Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Almost half of adult patients with eosinophilic esophagitis (EoE) reported poor adherence to long-term medical and dietary therapy, with age younger than 40 years and low necessity beliefs being the strongest predictors, a new study finds.
Clinicians need to spend more time discussing the need for EoE therapy with their patients, especially if they are younger, according to lead author Maria L. Haasnoot, MD, of Amsterdam University Medical Center (UMC), the Netherlands, and colleagues.
“Chronic treatment is necessary to maintain suppression of the inflammation and prevent negative outcomes in the long-term,” they write.
Until the recent approval of dupilumab (Dupixent) by the U.S. Food and Drug Administration, patients with EoE relied upon off-label options, including proton pump inhibitors and swallowed topical steroids, as well as dietary interventions for ongoing suppression of inflammation. But only about 1 in 6 patients achieve complete remission at 5 years, according to Dr. Haasnoot and colleagues.
“It is uncertain to what degree limited adherence to treatment [plays] a role in the limited long-term effects of treatment,” they write.
The findings were published online in American Journal of Gastroenterology.
Addressing a knowledge gap
The cross-sectional study involved 177 adult patients with EoE treated at Amsterdam UMC, who were prescribed dietary or medical maintenance therapy. Of note, some patients were treated with budesonide, which is approved for EoE in Europe but not in the United States.
Median participant age was 43 years, with a male-skewed distribution (71% men). Patients had been on EoE treatment for 2-6 years. Most (76%) were on medical treatments. Nearly half were on diets that avoided one to five food groups, with some on both medical treatments and elimination diets.
Using a link sent by mail, participants completed the online Medication Adherence Rating Scale, along with several other questionnaires, such as the Beliefs about Medicine Questionnaire, to measure secondary outcomes, including a patient’s view of how necessary or disruptive maintenance therapy is in their life.
The overall prevalence of poor adherence to therapy was high (41.8%), including a nonsignificant difference in adherence between medical and dietary therapies.
“It might come as a surprise that dietary-treated patients are certainly not less adherent to treatment than medically treated patients,” the authors write, noting that the opposite is usually true.
Multivariate logistic regression showed that patients younger than 40 years were more than twice as likely to be poorly adherent (odds ratio, 2.571; 95% confidence interval, 1.195-5.532). Those with low necessity beliefs were more than four times as likely to be poorly adherent (OR, 4.423; 95% CI, 2.169-9.016). Other factors linked to poor adherence were patients with longer disease duration and more severe symptoms.
“Clinicians should pay more attention to treatment adherence, particularly in younger patients,” the authors conclude. “The necessity of treatment should be actively discussed, and efforts should be done to take doubts away, as this may improve treatment adherence and subsequently may improve treatment effects and long-term outcomes.”
More patient education needed
According to Jennifer L. Horsley-Silva, MD, of Mayo Clinic, Scottsdale, Ariz., “This study is important, as it is one of the first studies to investigate the rate of treatment adherence in EoE patients and attempts to identify factors associated with adherence both in medically and dietary treated patients.”
Dr. Horsley-Silva commented that the findings align with recent research she and her colleagues conducted at the Mayo Clinic, where few patients successfully completed a six-food elimination diet, even when paired with a dietitian. As with the present study, success trended lower among younger adults. “These findings highlight the need for physicians treating EoE to motivate all patients, but especially younger patients, by discussing disease pathophysiology and explaining the reason for maintenance treatment early on,” Dr. Horsley-Silva said.
Conversations should also address the discordance between symptoms and histologic disease, patient doubts and concerns, and other barriers to adherence, she noted.
“Shared decisionmaking is of utmost importance when deciding upon a maintenance treatment strategy and should be readdressed continually,” she added.
Gary W. Falk, MD, of Penn Medicine, Philadelphia, said that patients with EoE may be poorly adherent because therapies tend to be complicated and people often forget to take their medications, especially when their symptoms improve, even though this is a poor indicator of underlying disease. The discordance between symptoms and histology is “not commonly appreciated by the EoE GI community,” he noted.
Patients may benefit from knowing that untreated or undertreated EoE increases the risk for strictures and stenoses, need for dilation, and frequency of food bolus impactions, Dr. Falk said.
“The other thing we know is that once someone is induced into remission, and they stay on therapy ... long-term remission can be maintained,” he added.
The impact of Dupilumab
John Leung, MD, of Boston Food Allergy Center, also cited the complexities of EoE therapies as reason for poor adherence, though he believes this paradigm will shift now that dupilumab has been approved. Dupilumab injections are “just once a week, so it’s much easier in terms of frequency,” Dr. Leung said. “I would expect that the compliance [for dupilumab] will be better” than for older therapies.
Dr. Leung, who helped conduct the dupilumab clinical trials contributing to its approval for EoE and receives speaking honoraria from manufacturer Regeneron/Sanofi, said that dupilumab also overcomes the challenges with elimination diets while offering relief for concomitant conditions, such as “asthma, eczema, food allergies, and seasonal allergies.”
But Dr. Falk, who also worked on the dupilumab clinical trials, said the situation is “not straightforward,” even with FDA approval.
“There are going to be significant costs with [prescribing dupilumab], because it’s a biologic,” Dr. Falk said.
Dr. Falk also pointed out that prior authorization will be required, and until more studies can be conducted, the true impact of once-weekly dosing versus daily dosing remains unknown.
“I would say [dupilumab] has the potential to improve adherence, but we need to see if that’s going to be the case or not,” Dr. Falk said.
The authors disclosed relationships with Dr. Falk Pharma, AstraZeneca, and Sanofi/Regeneron (the manufacturers of Dupixent [dupilumab]), among others. Dr. Horsley-Silva, Dr. Falk, and Dr. Leung conducted clinical trials for dupilumab on behalf of Sanofi/Regeneron, with Dr. Leung also disclosing speaking honoraria from Sanofi/Regeneron. Dr. Horsley-Silva has acted as a clinical trial site principal investigator for Allakos and Celgene/Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Telemedicine and Home Pregnancy Testing for iPLEDGE: A Survey of Clinician Perspectives
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).

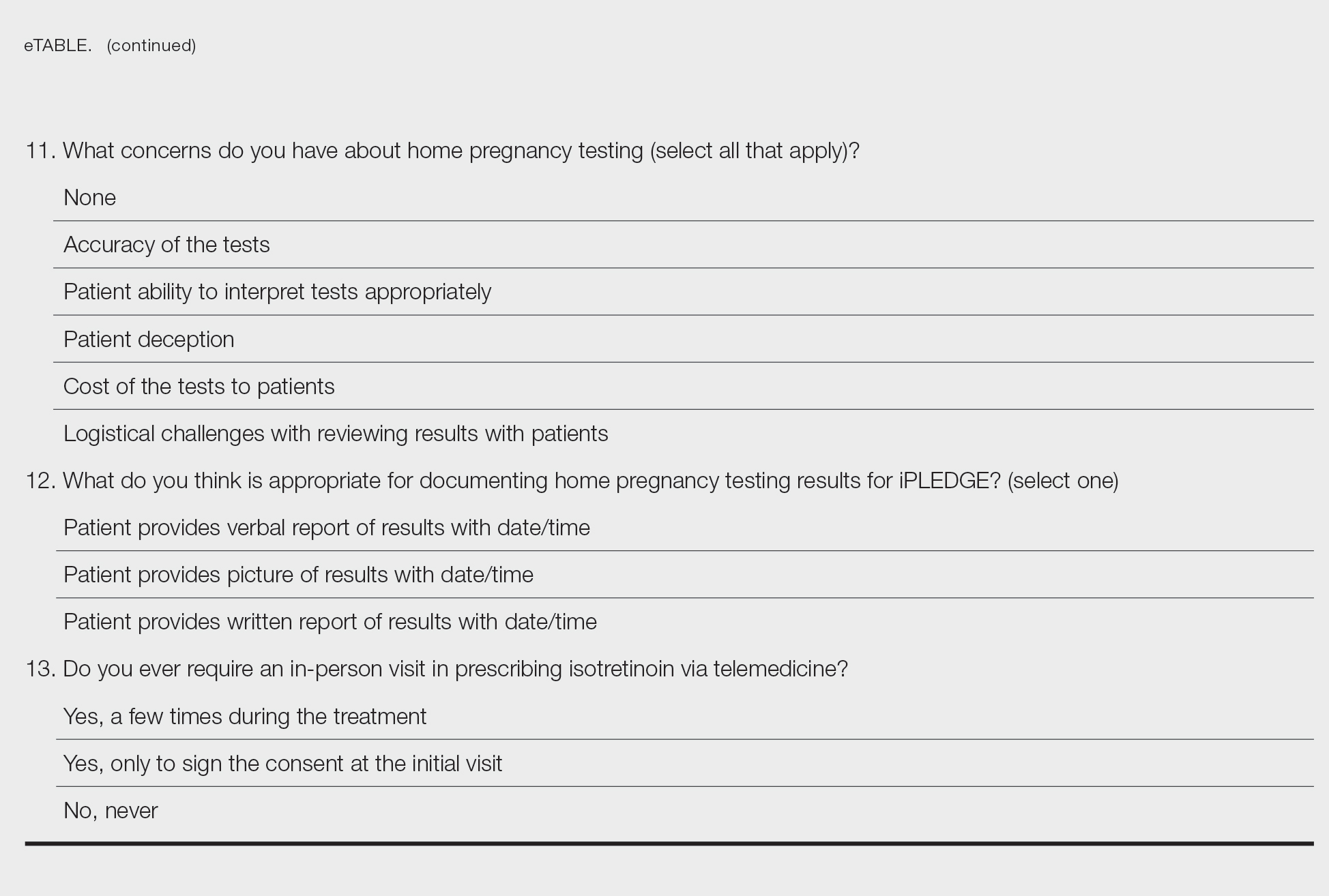
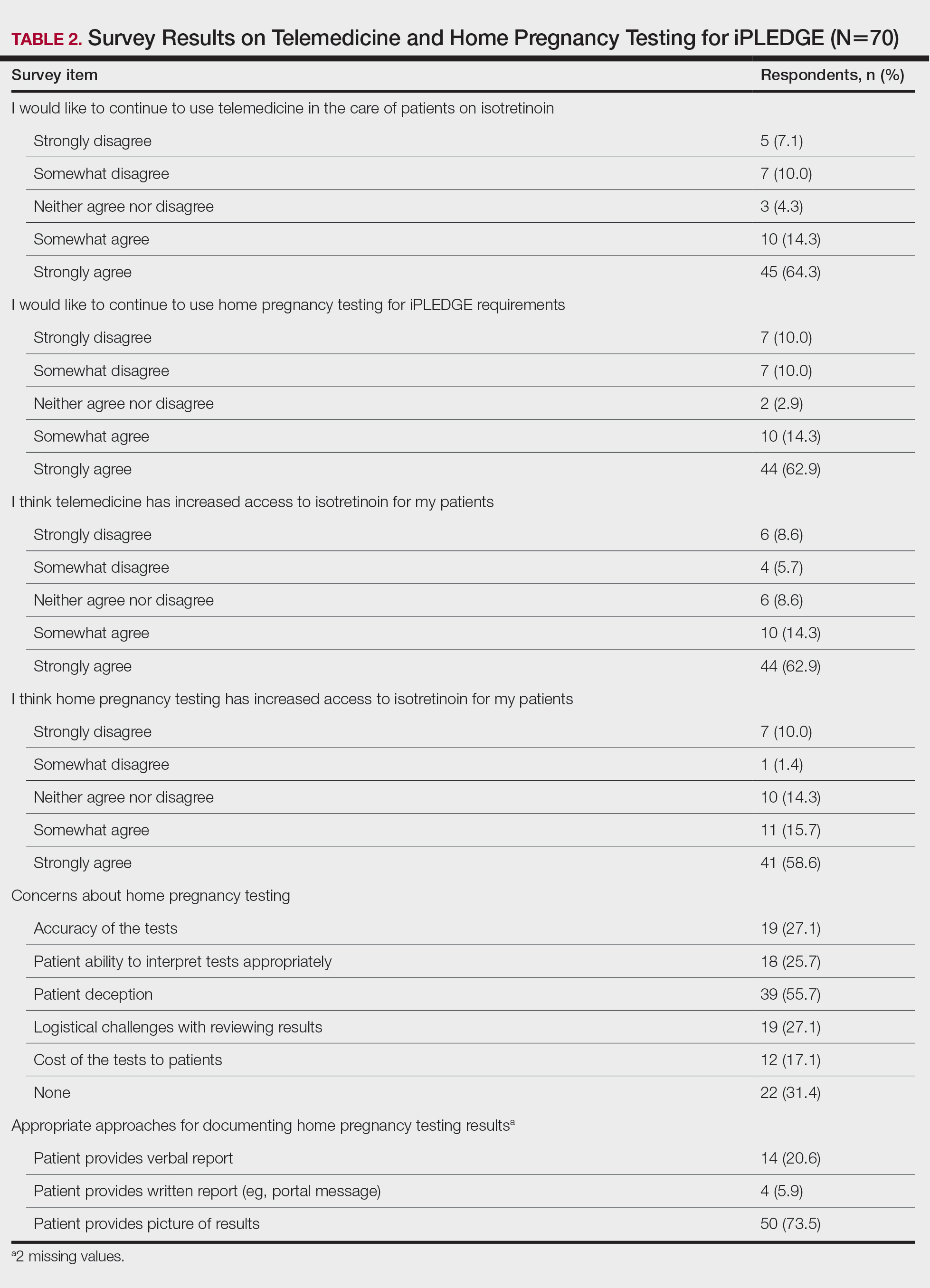
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
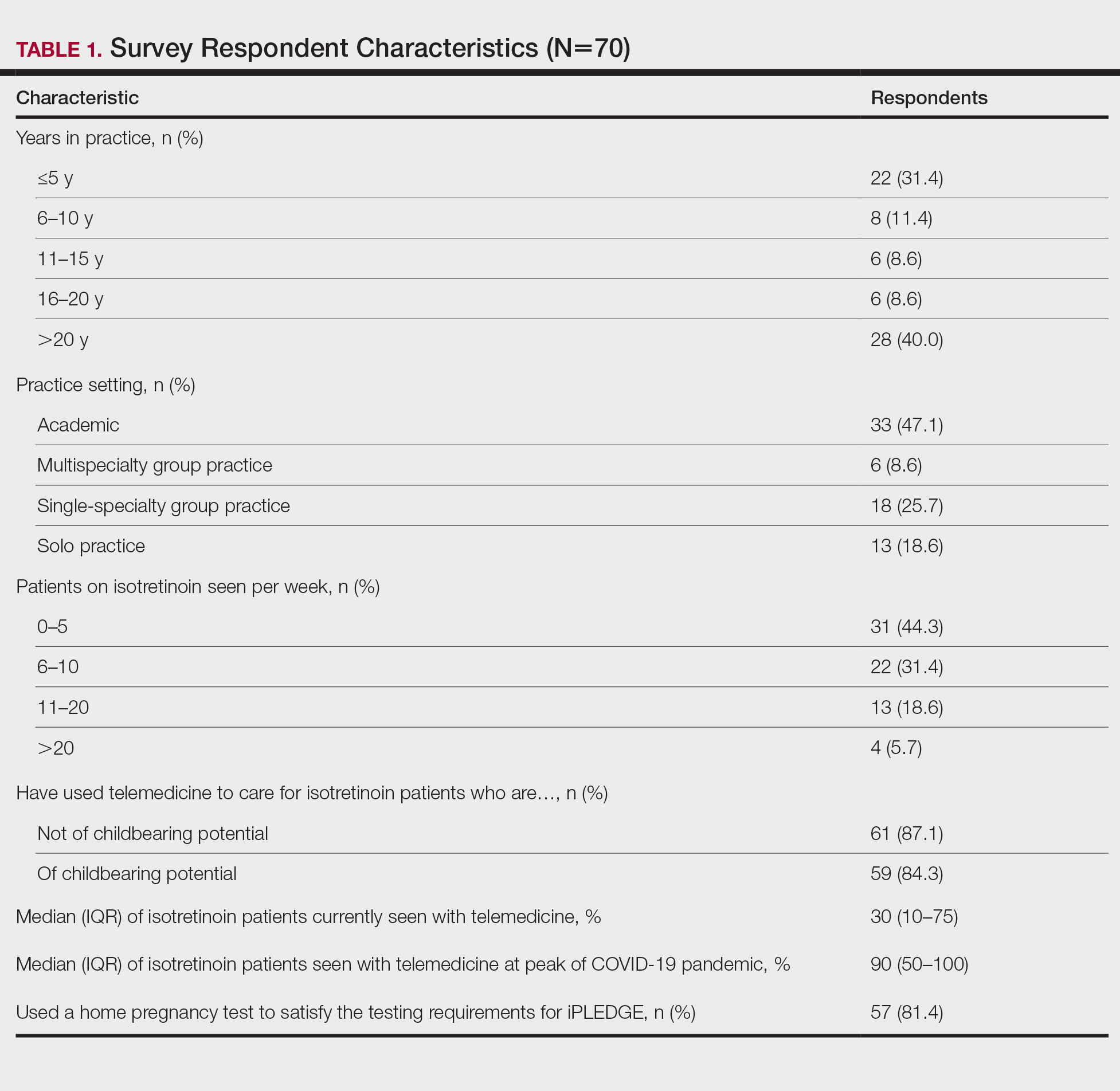
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
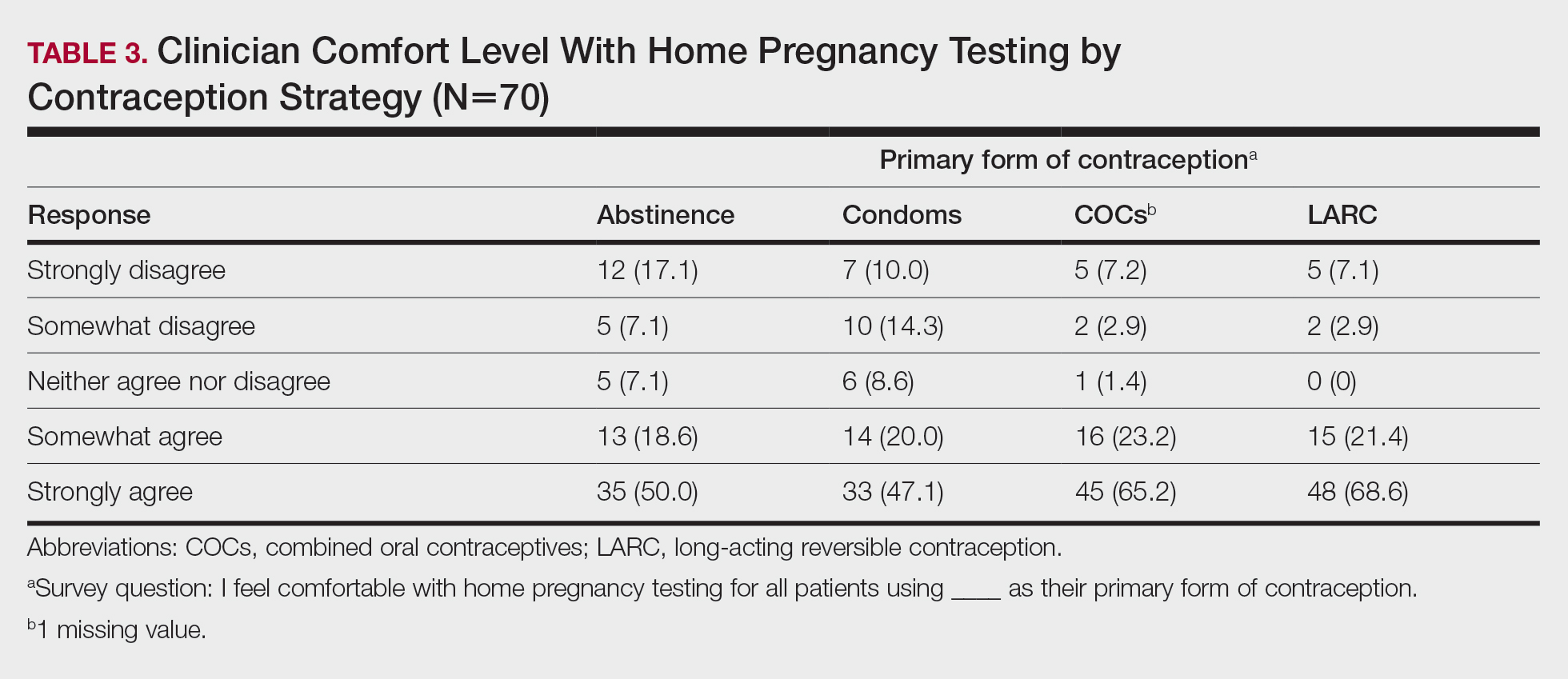
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).


Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).

The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).

In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2

Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).


Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).

The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).

In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2

Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
PRACTICE POINTS
- The majority of clinicians report that the use of telemedicine and home pregnancy testing for iPLEDGE has improved access to care and that they would like to continue these practices.
- Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care for patients treated with isotretinoin.
The toll of the unwanted pregnancy
In the wake of the Supreme Court’s June decision to repeal a federal right to abortion, many women will now be faced with the prospect of carrying an unwanted pregnancy to term.
One group of researchers has studied the fate of these women and their families for the last decade. Their findings show that women who were denied an abortion are worse off physically, mentally, and economically than those who underwent the procedure.
“There has been much hypothesizing about harms from abortion without considering what the consequences are when someone wants an abortion and can’t get one,” said Diana Greene Foster, PhD, professor of obstetrics and gynecology at University of California, San Francisco.
Dr. Foster leads the Turnaway Study, one of the first efforts to examine the physical and mental health effects of receiving or being denied abortions. The ongoing research also charts the economic and social outcomes of women and their families in either circumstance.
Dr. Foster and her colleagues have followed women through childbirth, examining their well-being through phone interviews months to years after the initial interviews.
The economic consequences of carrying an unwanted pregnancy are clear. Women who did not receive a wanted abortion were more likely to live under the poverty line and struggle to cover basic living expenses like food, housing, and transportation.
The physical toll is also significant.
A 2019 analysis from the Turnaway Study found that eight out of 1,132 participants died, two after delivery, during the five-year follow up period – a far greater proportion than what would be expected among women of reproductive age. The researchers also found that women who carry unwanted pregnancies have more comorbid conditions before and after delivery than other women.
Lauren J. Ralph, PhD, MPH, an epidemiologist and member of the Turnaway Study team, examined the physical well-being of women after delivering their unwanted pregnancies.
“They reported more chronic pain, more gestational hypertension, and were more likely to rate their health as fair or poor,” Dr. Ralph said. “Somewhat to our surprise, we also found that two women denied abortions died due to pregnancy-related causes. This is my biggest concern with the loss of abortion access, as all scientific evidence indicates we will see a rise in maternal deaths as a result.”
At least one preliminary study, released as a preprint and not yet peer reviewed, estimates that the number of women who will die each year from pregnancy complications will rise by 24%. For Black women, mortality could jump from 18% to 39% in every state, according to the researchers from the University of Colorado, Boulder.
State of denial
Regulations set in place at abortion clinics in each state individually determine who is able to obtain an abortion, dictated by a “gestational age limit” – how far along a woman is in her pregnancy from the end of her menstrual cycle. Some of the women from the Turnaway Study were unable to receive an abortion because of how far along they were. Others were granted the abortion because they were just under their state’s limit.
Before the latest Supreme Court ruling, this limit was 20 weeks in most states. Now, the cutoff can be as little as 6 weeks – before many women know they are pregnant – or zero weeks, under the most restrictive laws.
Over 70% of women who are denied an abortion carry the pregnancy to term, according to Dr. Foster’s analysis.
Interviews with nearly 1,000 women – in both the first and second trimester of pregnancy – in the Turnaway Study who sought abortions at 30 abortion clinics around the country revealed the main reasons for seeking the procedure were (a) not being able to afford a child; (b) the pregnancy coming at the wrong time in life; or (c) the partner involved not being suitable.
According to the U.S. Centers for Disease Control and Prevention, 59.7% of women seeking an abortion in the United States are already mothers. Having an unplanned child results in dramatically worse economic circumstances for their other children, who become nearly four times more likely to live below the poverty line than their peers. They also experience slower physical and mental development as a result of the arrival of the new sibling.
The latest efforts by states to ban abortion could make the situation much worse, said Liza Fuentes, DrPh, senior research scientist at the Guttmacher Institute. “We will need further research on what it means for women to be denied care in the context of the new restrictions,” Dr. Fuentes told this news organization.
Researchers cannot yet predict how many women will be unable to obtain an abortion in the coming months. But John Donahue, PhD, JD, an economist and professor of law at Stanford (Calif.) University, estimated that state laws would prevent roughly one-third of the 1 million abortions per year based on 2021 figures.
Dr. Ralph and her colleagues with the Turnaway Study know that restricting access to abortions will not make the need for abortions disappear. Rather, women will be forced to travel, potentially long distances at significant cost, for the procedure or will seek medication abortion by mail through virtual clinics.
But Dr. Ralph said she’s concerned about women who live in areas where telehealth abortions are banned, or who discover their pregnancies late, as medical abortions are only recommended for women who are 10 weeks pregnant or less.
“They may look to self-source the medications online or elsewhere, potentially putting themself at legal risk,” she said. “And, as my research has shown, others may turn to self-managing an abortion with herbs, other drugs or medications, or physical methods like hitting themselves in the abdomen; with this they put themselves at both legal and potentially medical risk.”
Constance Bohon, MD, an ob.gyn. in Washington, D.C., said further research should track what happens to women if they’re forced to leave a job to care for another child.
“Many of these women live paycheck to paycheck and cannot afford the cost of an additional child,” Dr. Bohon said. “They may also need to rely on social service agencies to help them find food and housing.”
Dr. Fuentes said she hopes the Turnaway Study will inspire other researchers to examine laws that outlaw abortion and the corresponding long-term effects on women.
“From a medical and a public health standpoint, these laws are unjust,” Dr. Fuentes said in an interview. “They’re not grounded in evidence, and they incur great costs not just to pregnant people but their families and their communities as well.”
A version of this article first appeared on Medscape.com.
In the wake of the Supreme Court’s June decision to repeal a federal right to abortion, many women will now be faced with the prospect of carrying an unwanted pregnancy to term.
One group of researchers has studied the fate of these women and their families for the last decade. Their findings show that women who were denied an abortion are worse off physically, mentally, and economically than those who underwent the procedure.
“There has been much hypothesizing about harms from abortion without considering what the consequences are when someone wants an abortion and can’t get one,” said Diana Greene Foster, PhD, professor of obstetrics and gynecology at University of California, San Francisco.
Dr. Foster leads the Turnaway Study, one of the first efforts to examine the physical and mental health effects of receiving or being denied abortions. The ongoing research also charts the economic and social outcomes of women and their families in either circumstance.
Dr. Foster and her colleagues have followed women through childbirth, examining their well-being through phone interviews months to years after the initial interviews.
The economic consequences of carrying an unwanted pregnancy are clear. Women who did not receive a wanted abortion were more likely to live under the poverty line and struggle to cover basic living expenses like food, housing, and transportation.
The physical toll is also significant.
A 2019 analysis from the Turnaway Study found that eight out of 1,132 participants died, two after delivery, during the five-year follow up period – a far greater proportion than what would be expected among women of reproductive age. The researchers also found that women who carry unwanted pregnancies have more comorbid conditions before and after delivery than other women.
Lauren J. Ralph, PhD, MPH, an epidemiologist and member of the Turnaway Study team, examined the physical well-being of women after delivering their unwanted pregnancies.
“They reported more chronic pain, more gestational hypertension, and were more likely to rate their health as fair or poor,” Dr. Ralph said. “Somewhat to our surprise, we also found that two women denied abortions died due to pregnancy-related causes. This is my biggest concern with the loss of abortion access, as all scientific evidence indicates we will see a rise in maternal deaths as a result.”
At least one preliminary study, released as a preprint and not yet peer reviewed, estimates that the number of women who will die each year from pregnancy complications will rise by 24%. For Black women, mortality could jump from 18% to 39% in every state, according to the researchers from the University of Colorado, Boulder.
State of denial
Regulations set in place at abortion clinics in each state individually determine who is able to obtain an abortion, dictated by a “gestational age limit” – how far along a woman is in her pregnancy from the end of her menstrual cycle. Some of the women from the Turnaway Study were unable to receive an abortion because of how far along they were. Others were granted the abortion because they were just under their state’s limit.
Before the latest Supreme Court ruling, this limit was 20 weeks in most states. Now, the cutoff can be as little as 6 weeks – before many women know they are pregnant – or zero weeks, under the most restrictive laws.
Over 70% of women who are denied an abortion carry the pregnancy to term, according to Dr. Foster’s analysis.
Interviews with nearly 1,000 women – in both the first and second trimester of pregnancy – in the Turnaway Study who sought abortions at 30 abortion clinics around the country revealed the main reasons for seeking the procedure were (a) not being able to afford a child; (b) the pregnancy coming at the wrong time in life; or (c) the partner involved not being suitable.
According to the U.S. Centers for Disease Control and Prevention, 59.7% of women seeking an abortion in the United States are already mothers. Having an unplanned child results in dramatically worse economic circumstances for their other children, who become nearly four times more likely to live below the poverty line than their peers. They also experience slower physical and mental development as a result of the arrival of the new sibling.
The latest efforts by states to ban abortion could make the situation much worse, said Liza Fuentes, DrPh, senior research scientist at the Guttmacher Institute. “We will need further research on what it means for women to be denied care in the context of the new restrictions,” Dr. Fuentes told this news organization.
Researchers cannot yet predict how many women will be unable to obtain an abortion in the coming months. But John Donahue, PhD, JD, an economist and professor of law at Stanford (Calif.) University, estimated that state laws would prevent roughly one-third of the 1 million abortions per year based on 2021 figures.
Dr. Ralph and her colleagues with the Turnaway Study know that restricting access to abortions will not make the need for abortions disappear. Rather, women will be forced to travel, potentially long distances at significant cost, for the procedure or will seek medication abortion by mail through virtual clinics.
But Dr. Ralph said she’s concerned about women who live in areas where telehealth abortions are banned, or who discover their pregnancies late, as medical abortions are only recommended for women who are 10 weeks pregnant or less.
“They may look to self-source the medications online or elsewhere, potentially putting themself at legal risk,” she said. “And, as my research has shown, others may turn to self-managing an abortion with herbs, other drugs or medications, or physical methods like hitting themselves in the abdomen; with this they put themselves at both legal and potentially medical risk.”
Constance Bohon, MD, an ob.gyn. in Washington, D.C., said further research should track what happens to women if they’re forced to leave a job to care for another child.
“Many of these women live paycheck to paycheck and cannot afford the cost of an additional child,” Dr. Bohon said. “They may also need to rely on social service agencies to help them find food and housing.”
Dr. Fuentes said she hopes the Turnaway Study will inspire other researchers to examine laws that outlaw abortion and the corresponding long-term effects on women.
“From a medical and a public health standpoint, these laws are unjust,” Dr. Fuentes said in an interview. “They’re not grounded in evidence, and they incur great costs not just to pregnant people but their families and their communities as well.”
A version of this article first appeared on Medscape.com.
In the wake of the Supreme Court’s June decision to repeal a federal right to abortion, many women will now be faced with the prospect of carrying an unwanted pregnancy to term.
One group of researchers has studied the fate of these women and their families for the last decade. Their findings show that women who were denied an abortion are worse off physically, mentally, and economically than those who underwent the procedure.
“There has been much hypothesizing about harms from abortion without considering what the consequences are when someone wants an abortion and can’t get one,” said Diana Greene Foster, PhD, professor of obstetrics and gynecology at University of California, San Francisco.
Dr. Foster leads the Turnaway Study, one of the first efforts to examine the physical and mental health effects of receiving or being denied abortions. The ongoing research also charts the economic and social outcomes of women and their families in either circumstance.
Dr. Foster and her colleagues have followed women through childbirth, examining their well-being through phone interviews months to years after the initial interviews.
The economic consequences of carrying an unwanted pregnancy are clear. Women who did not receive a wanted abortion were more likely to live under the poverty line and struggle to cover basic living expenses like food, housing, and transportation.
The physical toll is also significant.
A 2019 analysis from the Turnaway Study found that eight out of 1,132 participants died, two after delivery, during the five-year follow up period – a far greater proportion than what would be expected among women of reproductive age. The researchers also found that women who carry unwanted pregnancies have more comorbid conditions before and after delivery than other women.
Lauren J. Ralph, PhD, MPH, an epidemiologist and member of the Turnaway Study team, examined the physical well-being of women after delivering their unwanted pregnancies.
“They reported more chronic pain, more gestational hypertension, and were more likely to rate their health as fair or poor,” Dr. Ralph said. “Somewhat to our surprise, we also found that two women denied abortions died due to pregnancy-related causes. This is my biggest concern with the loss of abortion access, as all scientific evidence indicates we will see a rise in maternal deaths as a result.”
At least one preliminary study, released as a preprint and not yet peer reviewed, estimates that the number of women who will die each year from pregnancy complications will rise by 24%. For Black women, mortality could jump from 18% to 39% in every state, according to the researchers from the University of Colorado, Boulder.
State of denial
Regulations set in place at abortion clinics in each state individually determine who is able to obtain an abortion, dictated by a “gestational age limit” – how far along a woman is in her pregnancy from the end of her menstrual cycle. Some of the women from the Turnaway Study were unable to receive an abortion because of how far along they were. Others were granted the abortion because they were just under their state’s limit.
Before the latest Supreme Court ruling, this limit was 20 weeks in most states. Now, the cutoff can be as little as 6 weeks – before many women know they are pregnant – or zero weeks, under the most restrictive laws.
Over 70% of women who are denied an abortion carry the pregnancy to term, according to Dr. Foster’s analysis.
Interviews with nearly 1,000 women – in both the first and second trimester of pregnancy – in the Turnaway Study who sought abortions at 30 abortion clinics around the country revealed the main reasons for seeking the procedure were (a) not being able to afford a child; (b) the pregnancy coming at the wrong time in life; or (c) the partner involved not being suitable.
According to the U.S. Centers for Disease Control and Prevention, 59.7% of women seeking an abortion in the United States are already mothers. Having an unplanned child results in dramatically worse economic circumstances for their other children, who become nearly four times more likely to live below the poverty line than their peers. They also experience slower physical and mental development as a result of the arrival of the new sibling.
The latest efforts by states to ban abortion could make the situation much worse, said Liza Fuentes, DrPh, senior research scientist at the Guttmacher Institute. “We will need further research on what it means for women to be denied care in the context of the new restrictions,” Dr. Fuentes told this news organization.
Researchers cannot yet predict how many women will be unable to obtain an abortion in the coming months. But John Donahue, PhD, JD, an economist and professor of law at Stanford (Calif.) University, estimated that state laws would prevent roughly one-third of the 1 million abortions per year based on 2021 figures.
Dr. Ralph and her colleagues with the Turnaway Study know that restricting access to abortions will not make the need for abortions disappear. Rather, women will be forced to travel, potentially long distances at significant cost, for the procedure or will seek medication abortion by mail through virtual clinics.
But Dr. Ralph said she’s concerned about women who live in areas where telehealth abortions are banned, or who discover their pregnancies late, as medical abortions are only recommended for women who are 10 weeks pregnant or less.
“They may look to self-source the medications online or elsewhere, potentially putting themself at legal risk,” she said. “And, as my research has shown, others may turn to self-managing an abortion with herbs, other drugs or medications, or physical methods like hitting themselves in the abdomen; with this they put themselves at both legal and potentially medical risk.”
Constance Bohon, MD, an ob.gyn. in Washington, D.C., said further research should track what happens to women if they’re forced to leave a job to care for another child.
“Many of these women live paycheck to paycheck and cannot afford the cost of an additional child,” Dr. Bohon said. “They may also need to rely on social service agencies to help them find food and housing.”
Dr. Fuentes said she hopes the Turnaway Study will inspire other researchers to examine laws that outlaw abortion and the corresponding long-term effects on women.
“From a medical and a public health standpoint, these laws are unjust,” Dr. Fuentes said in an interview. “They’re not grounded in evidence, and they incur great costs not just to pregnant people but their families and their communities as well.”
A version of this article first appeared on Medscape.com.
No adverse impact of obesity in biologic-treated IBD
Patients with both inflammatory bowel disease (IBD) and obesity starting on new biologic therapies do not face an increased risk for hospitalization, IBD-related surgery, or serious infection, reveals a multicenter U.S. study published online in American Journal of Gastroenterology.
“Our findings were a bit surprising, since prior studies had suggested higher clinical disease activity and risk of flare and lower rates of endoscopic remission in obese patients treated with biologics,” Siddharth Singh, MD, MS, director of the IBD Center at the University of California, San Diego, told this news organization.
“However, in this study we focused on harder outcomes, including risk of hospitalization and surgery, and did not observe any detrimental effect,” he said.
Based on the findings, Dr. Singh believes that biologics are “completely safe and effective to use in obese patients.”
He clarified, however, that “examining the overall body of evidence, I still think obesity results in more rapid clearance of biologics, which negatively impacts the likelihood of achieving symptomatic and endoscopic remission.”
“Hence, there should be a low threshold to monitor and optimize biologic drug concentrations in obese patients. I preferentially use biologics that are dosed based on body weight in patients with class II or III obesity,” he said.
Research findings
Dr. Singh and colleagues write that, given that between 15% and 45% of patients with IBD are obese and a further 20%-40% are overweight, obesity is an “increasingly important consideration” in its management.
It is believed that obesity, largely via visceral adiposity, has a negative impact on IBD via increased production of adipokines, chemokines, and cytokines, such as tumor necrosis factor (TNF) alpha and interleukin-6, thus affecting treatment response as well as increasing the risk for complications and infections.
However, studies of the association between obesity and poorer treatment response, both large and small, have yielded conflicting results, potentially owing to methodological limitations.
To investigate further, Dr. Singh and colleagues gathered electronic health record data from five health systems in California on adults with IBD who were new users of TNF-alpha antagonists, or the monoclonal antibodies vedolizumab or ustekinumab, between Jan. 1, 2010, and June 30, 2017.
World Health Organization definitions were used to classify the patients as having normal BMI, overweight, or obesity, and the risk for all-cause hospitalization, IBD-related surgery, or serious infection was compared between the groups.
The team reviewed the cases of 3,038 patients with IBD, of whom 31.1% had ulcerative colitis. Among the participants, 28.2% were classified as overweight and 13.7% as obese. TNF-alpha antagonists were used by 76.3% of patients.
Patients with obesity were significantly older, were more likely to be of Hispanic ethnicity, had a higher burden of comorbidities, and were more likely to have elevated C-reactive protein levels at baseline.
However, there were no significant differences between obese and nonobese patients in terms of IBD type, class of biologic prescribed, prior surgery, or prior biologic exposure.
Within 1 year of starting a new biologic therapy, 22.9% of patients required hospitalization, whereas 3.3% required surgery and 5.8% were hospitalized with a serious infection.
Cox proportional hazard analyses showed that obesity was not associated with an increased risk for hospitalization versus normal body mass index (adjusted hazard ratio, 0.90; 95% confidence interval, 0.72-1.13), nor was it associated with IBD-related surgery (aHR, 0.62; 95% CI, 0.31-1.22) or serious infection (aHR, 1.11; 95% CI, 0.73-1.71).
The results were similar when the patients were stratified by IBD type and index biologic therapy, the researchers write.
When analyzed as a continuous variable, BMI was associated with a lower risk for hospitalization (aHR, 0.98 per 1 kg/m2; P = .044) but not with IBD-related surgery or serious infection.
Reassuring results for the standard of care
Discussing their findings, the authors note that “the discrepancy among studies potentially reflects the shortcomings of overall obesity measured using BMI to capture clinically meaningful adiposity.”
“A small but growing body of literature suggests visceral adipose tissue is a potentially superior prognostic measure of adiposity and better predicts adverse outcomes in IBD.”
Dr. Singh said that it would be “very interesting” to examine the relationship between visceral adiposity, as inferred from waist circumference, and IBD outcomes.
Approached for comment, Stephen B. Hanauer, MD, Clifford Joseph Barborka Professor, Northwestern University Feinberg School of Medicine, Chicago, said, “At the present time, there are no new clinical implications based on this study.”
He said in an interview that it “does not require any change in the current standard of care but rather attempts to reassure that the standard of care does not change for obese patients.”
“With that being said, the standard of care may require dosing adjustments for patients based on weight, as is already the case for infliximab/ustekinumab, and monitoring to treat to target in obese patients as well as in normal or underweight patients,” Dr. Hanauer concluded.
The study was supported by the ACG Junior Faculty Development Award and the Crohn’s and Colitis Foundation Career Development Award to Dr. Singh. Dr. Singh is supported by the National Institute of Diabetes and Digestive and Kidney Diseases and reports relationships with AbbVie, Janssen, and Pfizer. The other authors report numerous financial relationships. Dr. Hanauer reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with both inflammatory bowel disease (IBD) and obesity starting on new biologic therapies do not face an increased risk for hospitalization, IBD-related surgery, or serious infection, reveals a multicenter U.S. study published online in American Journal of Gastroenterology.
“Our findings were a bit surprising, since prior studies had suggested higher clinical disease activity and risk of flare and lower rates of endoscopic remission in obese patients treated with biologics,” Siddharth Singh, MD, MS, director of the IBD Center at the University of California, San Diego, told this news organization.
“However, in this study we focused on harder outcomes, including risk of hospitalization and surgery, and did not observe any detrimental effect,” he said.
Based on the findings, Dr. Singh believes that biologics are “completely safe and effective to use in obese patients.”
He clarified, however, that “examining the overall body of evidence, I still think obesity results in more rapid clearance of biologics, which negatively impacts the likelihood of achieving symptomatic and endoscopic remission.”
“Hence, there should be a low threshold to monitor and optimize biologic drug concentrations in obese patients. I preferentially use biologics that are dosed based on body weight in patients with class II or III obesity,” he said.
Research findings
Dr. Singh and colleagues write that, given that between 15% and 45% of patients with IBD are obese and a further 20%-40% are overweight, obesity is an “increasingly important consideration” in its management.
It is believed that obesity, largely via visceral adiposity, has a negative impact on IBD via increased production of adipokines, chemokines, and cytokines, such as tumor necrosis factor (TNF) alpha and interleukin-6, thus affecting treatment response as well as increasing the risk for complications and infections.
However, studies of the association between obesity and poorer treatment response, both large and small, have yielded conflicting results, potentially owing to methodological limitations.
To investigate further, Dr. Singh and colleagues gathered electronic health record data from five health systems in California on adults with IBD who were new users of TNF-alpha antagonists, or the monoclonal antibodies vedolizumab or ustekinumab, between Jan. 1, 2010, and June 30, 2017.
World Health Organization definitions were used to classify the patients as having normal BMI, overweight, or obesity, and the risk for all-cause hospitalization, IBD-related surgery, or serious infection was compared between the groups.
The team reviewed the cases of 3,038 patients with IBD, of whom 31.1% had ulcerative colitis. Among the participants, 28.2% were classified as overweight and 13.7% as obese. TNF-alpha antagonists were used by 76.3% of patients.
Patients with obesity were significantly older, were more likely to be of Hispanic ethnicity, had a higher burden of comorbidities, and were more likely to have elevated C-reactive protein levels at baseline.
However, there were no significant differences between obese and nonobese patients in terms of IBD type, class of biologic prescribed, prior surgery, or prior biologic exposure.
Within 1 year of starting a new biologic therapy, 22.9% of patients required hospitalization, whereas 3.3% required surgery and 5.8% were hospitalized with a serious infection.
Cox proportional hazard analyses showed that obesity was not associated with an increased risk for hospitalization versus normal body mass index (adjusted hazard ratio, 0.90; 95% confidence interval, 0.72-1.13), nor was it associated with IBD-related surgery (aHR, 0.62; 95% CI, 0.31-1.22) or serious infection (aHR, 1.11; 95% CI, 0.73-1.71).
The results were similar when the patients were stratified by IBD type and index biologic therapy, the researchers write.
When analyzed as a continuous variable, BMI was associated with a lower risk for hospitalization (aHR, 0.98 per 1 kg/m2; P = .044) but not with IBD-related surgery or serious infection.
Reassuring results for the standard of care
Discussing their findings, the authors note that “the discrepancy among studies potentially reflects the shortcomings of overall obesity measured using BMI to capture clinically meaningful adiposity.”
“A small but growing body of literature suggests visceral adipose tissue is a potentially superior prognostic measure of adiposity and better predicts adverse outcomes in IBD.”
Dr. Singh said that it would be “very interesting” to examine the relationship between visceral adiposity, as inferred from waist circumference, and IBD outcomes.
Approached for comment, Stephen B. Hanauer, MD, Clifford Joseph Barborka Professor, Northwestern University Feinberg School of Medicine, Chicago, said, “At the present time, there are no new clinical implications based on this study.”
He said in an interview that it “does not require any change in the current standard of care but rather attempts to reassure that the standard of care does not change for obese patients.”
“With that being said, the standard of care may require dosing adjustments for patients based on weight, as is already the case for infliximab/ustekinumab, and monitoring to treat to target in obese patients as well as in normal or underweight patients,” Dr. Hanauer concluded.
The study was supported by the ACG Junior Faculty Development Award and the Crohn’s and Colitis Foundation Career Development Award to Dr. Singh. Dr. Singh is supported by the National Institute of Diabetes and Digestive and Kidney Diseases and reports relationships with AbbVie, Janssen, and Pfizer. The other authors report numerous financial relationships. Dr. Hanauer reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with both inflammatory bowel disease (IBD) and obesity starting on new biologic therapies do not face an increased risk for hospitalization, IBD-related surgery, or serious infection, reveals a multicenter U.S. study published online in American Journal of Gastroenterology.
“Our findings were a bit surprising, since prior studies had suggested higher clinical disease activity and risk of flare and lower rates of endoscopic remission in obese patients treated with biologics,” Siddharth Singh, MD, MS, director of the IBD Center at the University of California, San Diego, told this news organization.
“However, in this study we focused on harder outcomes, including risk of hospitalization and surgery, and did not observe any detrimental effect,” he said.
Based on the findings, Dr. Singh believes that biologics are “completely safe and effective to use in obese patients.”
He clarified, however, that “examining the overall body of evidence, I still think obesity results in more rapid clearance of biologics, which negatively impacts the likelihood of achieving symptomatic and endoscopic remission.”
“Hence, there should be a low threshold to monitor and optimize biologic drug concentrations in obese patients. I preferentially use biologics that are dosed based on body weight in patients with class II or III obesity,” he said.
Research findings
Dr. Singh and colleagues write that, given that between 15% and 45% of patients with IBD are obese and a further 20%-40% are overweight, obesity is an “increasingly important consideration” in its management.
It is believed that obesity, largely via visceral adiposity, has a negative impact on IBD via increased production of adipokines, chemokines, and cytokines, such as tumor necrosis factor (TNF) alpha and interleukin-6, thus affecting treatment response as well as increasing the risk for complications and infections.
However, studies of the association between obesity and poorer treatment response, both large and small, have yielded conflicting results, potentially owing to methodological limitations.
To investigate further, Dr. Singh and colleagues gathered electronic health record data from five health systems in California on adults with IBD who were new users of TNF-alpha antagonists, or the monoclonal antibodies vedolizumab or ustekinumab, between Jan. 1, 2010, and June 30, 2017.
World Health Organization definitions were used to classify the patients as having normal BMI, overweight, or obesity, and the risk for all-cause hospitalization, IBD-related surgery, or serious infection was compared between the groups.
The team reviewed the cases of 3,038 patients with IBD, of whom 31.1% had ulcerative colitis. Among the participants, 28.2% were classified as overweight and 13.7% as obese. TNF-alpha antagonists were used by 76.3% of patients.
Patients with obesity were significantly older, were more likely to be of Hispanic ethnicity, had a higher burden of comorbidities, and were more likely to have elevated C-reactive protein levels at baseline.
However, there were no significant differences between obese and nonobese patients in terms of IBD type, class of biologic prescribed, prior surgery, or prior biologic exposure.
Within 1 year of starting a new biologic therapy, 22.9% of patients required hospitalization, whereas 3.3% required surgery and 5.8% were hospitalized with a serious infection.
Cox proportional hazard analyses showed that obesity was not associated with an increased risk for hospitalization versus normal body mass index (adjusted hazard ratio, 0.90; 95% confidence interval, 0.72-1.13), nor was it associated with IBD-related surgery (aHR, 0.62; 95% CI, 0.31-1.22) or serious infection (aHR, 1.11; 95% CI, 0.73-1.71).
The results were similar when the patients were stratified by IBD type and index biologic therapy, the researchers write.
When analyzed as a continuous variable, BMI was associated with a lower risk for hospitalization (aHR, 0.98 per 1 kg/m2; P = .044) but not with IBD-related surgery or serious infection.
Reassuring results for the standard of care
Discussing their findings, the authors note that “the discrepancy among studies potentially reflects the shortcomings of overall obesity measured using BMI to capture clinically meaningful adiposity.”
“A small but growing body of literature suggests visceral adipose tissue is a potentially superior prognostic measure of adiposity and better predicts adverse outcomes in IBD.”
Dr. Singh said that it would be “very interesting” to examine the relationship between visceral adiposity, as inferred from waist circumference, and IBD outcomes.
Approached for comment, Stephen B. Hanauer, MD, Clifford Joseph Barborka Professor, Northwestern University Feinberg School of Medicine, Chicago, said, “At the present time, there are no new clinical implications based on this study.”
He said in an interview that it “does not require any change in the current standard of care but rather attempts to reassure that the standard of care does not change for obese patients.”
“With that being said, the standard of care may require dosing adjustments for patients based on weight, as is already the case for infliximab/ustekinumab, and monitoring to treat to target in obese patients as well as in normal or underweight patients,” Dr. Hanauer concluded.
The study was supported by the ACG Junior Faculty Development Award and the Crohn’s and Colitis Foundation Career Development Award to Dr. Singh. Dr. Singh is supported by the National Institute of Diabetes and Digestive and Kidney Diseases and reports relationships with AbbVie, Janssen, and Pfizer. The other authors report numerous financial relationships. Dr. Hanauer reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What’s Diet Got to Do With It? Basic and Clinical Science Behind Diet and Acne
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
Practice Points
- Patients are frequently interested in the role that diet plays in acne, and dermatologists should be aware of the current evidence to answer these questions effectively.
- One of the primary pathways in acne pathogenesis, mTORC1 (mammalian target of rapamycin complex 1), is partially regulated by nutrient availability, insulin, and insulinlike growth factor 1.
- Dietary recommendations for acne based on available evidence may include a low glycemic index diet and avoidance of certain dairy products.
- Insulin resistance may underlie the pathogenesis of acne in a subset of patients, and assessing insulin resistance in acne patients should be considered.
Are social networks threatening adolescents’ mental health?
When it comes to the link between mental health and social networks, be careful of jumping to conclusions. This warning came from Margot Morgiève, PhD, sociology researcher at the French National Institute of Health and Medical Research and the Center for Research in Medicine, Science, Health, Mental Health, and Society (Inserm-Cermes 3). She delivered her remarks at the opening session of the Pediatric Societies Congress organized by the French Society of Pediatrics, based on an increasing amount of scientific literature on the subject.
In 2021, 4.2 billion people, or more than half the world’s population, used social networks, and 80.3% of French citizens had a social network account.
‘Facebook depression’
Between those who condemn social networks for causing problems in adolescents and those who, in contrast, view it as a lifeline, what do we really know about their impact on the mental health of young people?
Although several studies have found a significant association between the heavy use of social networks and anxiety, depressive symptoms, and stress, there have also been reports of decreased life satisfaction, as well as reduced general well-being and self-esteem.
“Due to an increased [concurrence] between mood disorders or depression and the use of social networks, researchers wanted to establish a new disorder: ‘Facebook Depression,’ ” commented Dr. Morgiève, who is also a clinical psychologist and coordinator of the chat and social network unit for the French national suicide prevention hotline 3114.
“But they quickly realized that it would be wrong to recognize it as a characterized disorder, because it would appear that the harmful effects of social networks on mental health are not linked to the social network itself, but rather to problematic social network use.”
Teens’ fantasy life
There are three major categories of problematic social network use, the first being social comparison. This refers to the spontaneous tendency of social beings to compare themselves to individuals who appear to be more attractive than them.
This is nothing new, but it is exacerbated on social networks. Users emphasize the positive aspects of their life and present themselves as balanced, popular, and satisfied.
However, this leads to strong normative constraints, which result in a negative self-assessment, thereby lowering self-esteem and promoting the emergence of depressive symptoms. “Thus, it isn’t the social network that creates depression, but rather the phenomenon of comparison, which it pushes to the extreme,” said Dr. Morgiève.
The second problem associated with social networks is their propensity to promote addictive behavior through [observational learning], which can give rise to compulsive and uncontrolled behavior, as illustrated by “FOMO,” or fear of missing out.
Hence the idea of defining a specific entity called “social network addiction,” which was also quickly abandoned. It is the very features of social networks that generate this fear and thus this tendency, just like news feeds (constant updating of a personalized news list).
“Substitutive” use is the third major category. This is when time spent in the online environment replaces that spent offline. Excessive users report a feeling of loneliness and an awareness of a lack of intimate connections.
Language of distress
Initial studies using artificial intelligence and machine learning tend to show that a digital language of distress exists. Authors noticed that themes associated with self-loathing, loneliness, suicide, death, and self-harm correlated with users who exhibited the highest levels of depression.
The very structure of the language (more words, more use of “I,” more references to death, and fewer verbs) correlated with users in distress.
According to the authors, the typical social network practice of vaguebooking – writing a post that may incite worry, such as “better days are coming” – is a significant predictive factor of suicidal ideation. A visual language of distress also reportedly exists – for example, the use of darker shades, like the black-and-white inkwell filter with no enhancements in Instagram.
Internet risks and dangers
Digital environments entail many risks and dangers. Suicide pacts and online suicides (like the suicide of a young girl on Periscope in 2016) remain rare but go viral. The same is true of challenges. In 2015, the Blue Whale Challenge consisted of a list of 50 challenges ranging from the benign to the dramatic, with the final challenge being to “hang yourself.”
Its huge media coverage might well have added to its viral success had the social networks not quickly reacted in a positive manner.
Trolling, for its part, consists of posting provocative content with the intent of either sparking conflict or causing distress.
Cyberbullying, the most common online risk adolescents face, is the repeated spreading of false, embarrassing, or hostile information.
A growing danger is sexting (sending, receiving, or passing on sexually explicit photographs, messages, or images). The serious potential consequences of sexting include revenge porn or cyber rape, which is defined as the distribution of illicit content without consent, the practice of which has been linked to depression and involvement in risky behavior.
The risk of suicide exposure should no longer be overlooked, in view of the hypothesis that some online content relating to suicide may produce a suggestive effect with respect to the idea or the method of suicide, as well as precipitating suicide attempts.
“People who post suicidal comments are in communities that are closely connected by bonds of affiliation (memberships, friendships) and activities (retweets, likes, comments),” explained Dr. Morgiève.
But in these communities, emotionally charged information that spreads rapidly and repetitively could promote corumination, hence the concept of “suicidocosme [suicide world]», developed in 2017 by Charles-Edouard Notredame, MD, of the child and adolescent psychiatry department at Lille (France) University Hospital. This, in turn, can produce and increase the suicide contagion based on the Werther effect model.
Just one of many examples is Marilyn Monroe’s suicide in 1962, which increased the suicide rate by 40% in Los Angeles. The Werther effect is especially significant because two biases are present: the prestige bias (identification with the person one admires) and similarity bias (identification with the person who resembles me).
Similarity bias is the most decisive in adolescence. It should be noted that the positive counterpart to the Werther effect is the Papageno effect. The Belgian singer-songwriter Stromae’s TV appearances earlier this year, in which he spoke about his suicidal ideations, enabling young people to recognize their suffering and seek help, is an example of the Papageno effect.
Support on social networks?
Social networks can increase connectedness, for example, the feeling of being connected to something meaningful outside oneself. Connectedness promotes psychological well-being and quality of life.
The very characteristics of social networks can enhance elements of connectedness, both objectively by increasing users’ social sphere, and subjectively by reinforcing the feeling of social belonging and subjective well-being.
Taking Facebook and its “anniversary” feature as an example, it has been shown that the greater the number of Facebook friends, the more individuals saw themselves as being connected to a community.
“Millennials, or people born between the beginning of the 1980s and the end of the 1990s, are thus more likely to take advantage of the digital social environment to establish a new relationship with psychological suffering and its attempts to ease it,” said Dr. Morgiève.
They are also more likely to naturally turn to the digital space to look for help. More and more of them are searching the Internet for information on mental health and sharing experiences to get support.”
An example is the It Gets Better Project, which is a good illustration of the structure of online peer communities, with stories from LGBTQ+ individuals who describe how they succeeded in coping with adversity during their adolescence. In this way, social media seems to help identify peers and positive resources that are usually unavailable outside of the digital space. As a result, thanks to normative models on extremely strong social networks that are easy to conform to, these online peer-support communities have the potential to facilitate social interactions and reinforce a feeling both of hope and of belonging to a group.”
Promoting access to care
In Dr. Morgiève’s opinion, “access to care, particularly in the area of adolescent mental health, is extremely critical, given the lack of support precisely when they need it the most, as [evidenced] by the number of suicide attempts.
“There are two types of barriers to seeking help which can explain this. The first is structural barriers: help is too expensive or too far away or the wait is too long. The second refers to personal barriers, including denying the need for help, which may involve a self-sufficiency bias, the feeling that one cannot be helped, refusal to bother close friends and family, fear of being stigmatized, and a feeling of shame.”
These types of barriers are particularly difficult to overcome because the beliefs regarding care and caregivers are limiting (doubts about caregiver confidentiality, reliability, and competence). This is observed especially in adolescents because of the desire for emancipation and development of identity. So [the help relationship] may be experienced as subordination or alienation.
On a positive note, it is the very properties of social networks that will enable these obstacles to seeking help to be overcome. The fact that they are available everywhere makes up for young people’s lack of mobility and regional disparities. In addition, it ensures discretion and freedom of use, while reducing inhibitions.
The fact that social networks are free of charge overcomes structural obstacles, such as financial and organizational costs, as well as personal obstacles, thereby facilitating engagement and lessening the motivational cost. The dissociative pseudonymity or anonymity reduces the feeling of vulnerability associated with revealing oneself, as well as fears of a breach of confidentiality.
Dr. Morgiève summed it up by saying: “While offline life is silent because young people don’t talk about their suicidal ideations, online life truly removes inhibitions about speaking, relationships, and sharing experiences. Thus, the internet offers adolescents new opportunities to express themselves, which they’re not doing in real life.”
Professionals go digital
France records one suicide every hour (8,885 deaths a year) and one suicide attempt every 4 minutes. Since the 1950s, government-funded telehealth prevention and assistance programs, such as S.O.S. Amitié, Suicide Écoute, SOS Suicide Phénix, etc., have been developed. Their values and principles are anonymity, nondirectivity, nonjudgment, and neutrality. In addition to these nonprofit offerings, a professional teleprevention program, the confidential suicide prevention hotline 3114 – with professionals who are available to listen 24 hours a day, 7 days a week – was launched by the Ministry of Health and Prevention in October 2021.
Its values and principles include confidentiality, proactivity, concern, and caring for others. To date, 13 of 17 centers have opened. In the space of 6 months, they have received 50,000 calls, with an average of 400-500 calls a day. The dedicated chat application was codesigned with users (suicide attempters). And now social networks are joining in. For example, the hotline number 3114 appears whenever a TikTok user types the word “suicide.”
Dr. Morgiève said she has no conflicts of interest regarding the subject presented.
This article was translated from the Medscape French edition. A version of this article first appeared on Medscape.com.
When it comes to the link between mental health and social networks, be careful of jumping to conclusions. This warning came from Margot Morgiève, PhD, sociology researcher at the French National Institute of Health and Medical Research and the Center for Research in Medicine, Science, Health, Mental Health, and Society (Inserm-Cermes 3). She delivered her remarks at the opening session of the Pediatric Societies Congress organized by the French Society of Pediatrics, based on an increasing amount of scientific literature on the subject.
In 2021, 4.2 billion people, or more than half the world’s population, used social networks, and 80.3% of French citizens had a social network account.
‘Facebook depression’
Between those who condemn social networks for causing problems in adolescents and those who, in contrast, view it as a lifeline, what do we really know about their impact on the mental health of young people?
Although several studies have found a significant association between the heavy use of social networks and anxiety, depressive symptoms, and stress, there have also been reports of decreased life satisfaction, as well as reduced general well-being and self-esteem.
“Due to an increased [concurrence] between mood disorders or depression and the use of social networks, researchers wanted to establish a new disorder: ‘Facebook Depression,’ ” commented Dr. Morgiève, who is also a clinical psychologist and coordinator of the chat and social network unit for the French national suicide prevention hotline 3114.
“But they quickly realized that it would be wrong to recognize it as a characterized disorder, because it would appear that the harmful effects of social networks on mental health are not linked to the social network itself, but rather to problematic social network use.”
Teens’ fantasy life
There are three major categories of problematic social network use, the first being social comparison. This refers to the spontaneous tendency of social beings to compare themselves to individuals who appear to be more attractive than them.
This is nothing new, but it is exacerbated on social networks. Users emphasize the positive aspects of their life and present themselves as balanced, popular, and satisfied.
However, this leads to strong normative constraints, which result in a negative self-assessment, thereby lowering self-esteem and promoting the emergence of depressive symptoms. “Thus, it isn’t the social network that creates depression, but rather the phenomenon of comparison, which it pushes to the extreme,” said Dr. Morgiève.
The second problem associated with social networks is their propensity to promote addictive behavior through [observational learning], which can give rise to compulsive and uncontrolled behavior, as illustrated by “FOMO,” or fear of missing out.
Hence the idea of defining a specific entity called “social network addiction,” which was also quickly abandoned. It is the very features of social networks that generate this fear and thus this tendency, just like news feeds (constant updating of a personalized news list).
“Substitutive” use is the third major category. This is when time spent in the online environment replaces that spent offline. Excessive users report a feeling of loneliness and an awareness of a lack of intimate connections.
Language of distress
Initial studies using artificial intelligence and machine learning tend to show that a digital language of distress exists. Authors noticed that themes associated with self-loathing, loneliness, suicide, death, and self-harm correlated with users who exhibited the highest levels of depression.
The very structure of the language (more words, more use of “I,” more references to death, and fewer verbs) correlated with users in distress.
According to the authors, the typical social network practice of vaguebooking – writing a post that may incite worry, such as “better days are coming” – is a significant predictive factor of suicidal ideation. A visual language of distress also reportedly exists – for example, the use of darker shades, like the black-and-white inkwell filter with no enhancements in Instagram.
Internet risks and dangers
Digital environments entail many risks and dangers. Suicide pacts and online suicides (like the suicide of a young girl on Periscope in 2016) remain rare but go viral. The same is true of challenges. In 2015, the Blue Whale Challenge consisted of a list of 50 challenges ranging from the benign to the dramatic, with the final challenge being to “hang yourself.”
Its huge media coverage might well have added to its viral success had the social networks not quickly reacted in a positive manner.
Trolling, for its part, consists of posting provocative content with the intent of either sparking conflict or causing distress.
Cyberbullying, the most common online risk adolescents face, is the repeated spreading of false, embarrassing, or hostile information.
A growing danger is sexting (sending, receiving, or passing on sexually explicit photographs, messages, or images). The serious potential consequences of sexting include revenge porn or cyber rape, which is defined as the distribution of illicit content without consent, the practice of which has been linked to depression and involvement in risky behavior.
The risk of suicide exposure should no longer be overlooked, in view of the hypothesis that some online content relating to suicide may produce a suggestive effect with respect to the idea or the method of suicide, as well as precipitating suicide attempts.
“People who post suicidal comments are in communities that are closely connected by bonds of affiliation (memberships, friendships) and activities (retweets, likes, comments),” explained Dr. Morgiève.
But in these communities, emotionally charged information that spreads rapidly and repetitively could promote corumination, hence the concept of “suicidocosme [suicide world]», developed in 2017 by Charles-Edouard Notredame, MD, of the child and adolescent psychiatry department at Lille (France) University Hospital. This, in turn, can produce and increase the suicide contagion based on the Werther effect model.
Just one of many examples is Marilyn Monroe’s suicide in 1962, which increased the suicide rate by 40% in Los Angeles. The Werther effect is especially significant because two biases are present: the prestige bias (identification with the person one admires) and similarity bias (identification with the person who resembles me).
Similarity bias is the most decisive in adolescence. It should be noted that the positive counterpart to the Werther effect is the Papageno effect. The Belgian singer-songwriter Stromae’s TV appearances earlier this year, in which he spoke about his suicidal ideations, enabling young people to recognize their suffering and seek help, is an example of the Papageno effect.
Support on social networks?
Social networks can increase connectedness, for example, the feeling of being connected to something meaningful outside oneself. Connectedness promotes psychological well-being and quality of life.
The very characteristics of social networks can enhance elements of connectedness, both objectively by increasing users’ social sphere, and subjectively by reinforcing the feeling of social belonging and subjective well-being.
Taking Facebook and its “anniversary” feature as an example, it has been shown that the greater the number of Facebook friends, the more individuals saw themselves as being connected to a community.
“Millennials, or people born between the beginning of the 1980s and the end of the 1990s, are thus more likely to take advantage of the digital social environment to establish a new relationship with psychological suffering and its attempts to ease it,” said Dr. Morgiève.
They are also more likely to naturally turn to the digital space to look for help. More and more of them are searching the Internet for information on mental health and sharing experiences to get support.”
An example is the It Gets Better Project, which is a good illustration of the structure of online peer communities, with stories from LGBTQ+ individuals who describe how they succeeded in coping with adversity during their adolescence. In this way, social media seems to help identify peers and positive resources that are usually unavailable outside of the digital space. As a result, thanks to normative models on extremely strong social networks that are easy to conform to, these online peer-support communities have the potential to facilitate social interactions and reinforce a feeling both of hope and of belonging to a group.”
Promoting access to care
In Dr. Morgiève’s opinion, “access to care, particularly in the area of adolescent mental health, is extremely critical, given the lack of support precisely when they need it the most, as [evidenced] by the number of suicide attempts.
“There are two types of barriers to seeking help which can explain this. The first is structural barriers: help is too expensive or too far away or the wait is too long. The second refers to personal barriers, including denying the need for help, which may involve a self-sufficiency bias, the feeling that one cannot be helped, refusal to bother close friends and family, fear of being stigmatized, and a feeling of shame.”
These types of barriers are particularly difficult to overcome because the beliefs regarding care and caregivers are limiting (doubts about caregiver confidentiality, reliability, and competence). This is observed especially in adolescents because of the desire for emancipation and development of identity. So [the help relationship] may be experienced as subordination or alienation.
On a positive note, it is the very properties of social networks that will enable these obstacles to seeking help to be overcome. The fact that they are available everywhere makes up for young people’s lack of mobility and regional disparities. In addition, it ensures discretion and freedom of use, while reducing inhibitions.
The fact that social networks are free of charge overcomes structural obstacles, such as financial and organizational costs, as well as personal obstacles, thereby facilitating engagement and lessening the motivational cost. The dissociative pseudonymity or anonymity reduces the feeling of vulnerability associated with revealing oneself, as well as fears of a breach of confidentiality.
Dr. Morgiève summed it up by saying: “While offline life is silent because young people don’t talk about their suicidal ideations, online life truly removes inhibitions about speaking, relationships, and sharing experiences. Thus, the internet offers adolescents new opportunities to express themselves, which they’re not doing in real life.”
Professionals go digital
France records one suicide every hour (8,885 deaths a year) and one suicide attempt every 4 minutes. Since the 1950s, government-funded telehealth prevention and assistance programs, such as S.O.S. Amitié, Suicide Écoute, SOS Suicide Phénix, etc., have been developed. Their values and principles are anonymity, nondirectivity, nonjudgment, and neutrality. In addition to these nonprofit offerings, a professional teleprevention program, the confidential suicide prevention hotline 3114 – with professionals who are available to listen 24 hours a day, 7 days a week – was launched by the Ministry of Health and Prevention in October 2021.
Its values and principles include confidentiality, proactivity, concern, and caring for others. To date, 13 of 17 centers have opened. In the space of 6 months, they have received 50,000 calls, with an average of 400-500 calls a day. The dedicated chat application was codesigned with users (suicide attempters). And now social networks are joining in. For example, the hotline number 3114 appears whenever a TikTok user types the word “suicide.”
Dr. Morgiève said she has no conflicts of interest regarding the subject presented.
This article was translated from the Medscape French edition. A version of this article first appeared on Medscape.com.
When it comes to the link between mental health and social networks, be careful of jumping to conclusions. This warning came from Margot Morgiève, PhD, sociology researcher at the French National Institute of Health and Medical Research and the Center for Research in Medicine, Science, Health, Mental Health, and Society (Inserm-Cermes 3). She delivered her remarks at the opening session of the Pediatric Societies Congress organized by the French Society of Pediatrics, based on an increasing amount of scientific literature on the subject.
In 2021, 4.2 billion people, or more than half the world’s population, used social networks, and 80.3% of French citizens had a social network account.
‘Facebook depression’
Between those who condemn social networks for causing problems in adolescents and those who, in contrast, view it as a lifeline, what do we really know about their impact on the mental health of young people?
Although several studies have found a significant association between the heavy use of social networks and anxiety, depressive symptoms, and stress, there have also been reports of decreased life satisfaction, as well as reduced general well-being and self-esteem.
“Due to an increased [concurrence] between mood disorders or depression and the use of social networks, researchers wanted to establish a new disorder: ‘Facebook Depression,’ ” commented Dr. Morgiève, who is also a clinical psychologist and coordinator of the chat and social network unit for the French national suicide prevention hotline 3114.
“But they quickly realized that it would be wrong to recognize it as a characterized disorder, because it would appear that the harmful effects of social networks on mental health are not linked to the social network itself, but rather to problematic social network use.”
Teens’ fantasy life
There are three major categories of problematic social network use, the first being social comparison. This refers to the spontaneous tendency of social beings to compare themselves to individuals who appear to be more attractive than them.
This is nothing new, but it is exacerbated on social networks. Users emphasize the positive aspects of their life and present themselves as balanced, popular, and satisfied.
However, this leads to strong normative constraints, which result in a negative self-assessment, thereby lowering self-esteem and promoting the emergence of depressive symptoms. “Thus, it isn’t the social network that creates depression, but rather the phenomenon of comparison, which it pushes to the extreme,” said Dr. Morgiève.
The second problem associated with social networks is their propensity to promote addictive behavior through [observational learning], which can give rise to compulsive and uncontrolled behavior, as illustrated by “FOMO,” or fear of missing out.
Hence the idea of defining a specific entity called “social network addiction,” which was also quickly abandoned. It is the very features of social networks that generate this fear and thus this tendency, just like news feeds (constant updating of a personalized news list).
“Substitutive” use is the third major category. This is when time spent in the online environment replaces that spent offline. Excessive users report a feeling of loneliness and an awareness of a lack of intimate connections.
Language of distress
Initial studies using artificial intelligence and machine learning tend to show that a digital language of distress exists. Authors noticed that themes associated with self-loathing, loneliness, suicide, death, and self-harm correlated with users who exhibited the highest levels of depression.
The very structure of the language (more words, more use of “I,” more references to death, and fewer verbs) correlated with users in distress.
According to the authors, the typical social network practice of vaguebooking – writing a post that may incite worry, such as “better days are coming” – is a significant predictive factor of suicidal ideation. A visual language of distress also reportedly exists – for example, the use of darker shades, like the black-and-white inkwell filter with no enhancements in Instagram.
Internet risks and dangers
Digital environments entail many risks and dangers. Suicide pacts and online suicides (like the suicide of a young girl on Periscope in 2016) remain rare but go viral. The same is true of challenges. In 2015, the Blue Whale Challenge consisted of a list of 50 challenges ranging from the benign to the dramatic, with the final challenge being to “hang yourself.”
Its huge media coverage might well have added to its viral success had the social networks not quickly reacted in a positive manner.
Trolling, for its part, consists of posting provocative content with the intent of either sparking conflict or causing distress.
Cyberbullying, the most common online risk adolescents face, is the repeated spreading of false, embarrassing, or hostile information.
A growing danger is sexting (sending, receiving, or passing on sexually explicit photographs, messages, or images). The serious potential consequences of sexting include revenge porn or cyber rape, which is defined as the distribution of illicit content without consent, the practice of which has been linked to depression and involvement in risky behavior.
The risk of suicide exposure should no longer be overlooked, in view of the hypothesis that some online content relating to suicide may produce a suggestive effect with respect to the idea or the method of suicide, as well as precipitating suicide attempts.
“People who post suicidal comments are in communities that are closely connected by bonds of affiliation (memberships, friendships) and activities (retweets, likes, comments),” explained Dr. Morgiève.
But in these communities, emotionally charged information that spreads rapidly and repetitively could promote corumination, hence the concept of “suicidocosme [suicide world]», developed in 2017 by Charles-Edouard Notredame, MD, of the child and adolescent psychiatry department at Lille (France) University Hospital. This, in turn, can produce and increase the suicide contagion based on the Werther effect model.
Just one of many examples is Marilyn Monroe’s suicide in 1962, which increased the suicide rate by 40% in Los Angeles. The Werther effect is especially significant because two biases are present: the prestige bias (identification with the person one admires) and similarity bias (identification with the person who resembles me).
Similarity bias is the most decisive in adolescence. It should be noted that the positive counterpart to the Werther effect is the Papageno effect. The Belgian singer-songwriter Stromae’s TV appearances earlier this year, in which he spoke about his suicidal ideations, enabling young people to recognize their suffering and seek help, is an example of the Papageno effect.
Support on social networks?
Social networks can increase connectedness, for example, the feeling of being connected to something meaningful outside oneself. Connectedness promotes psychological well-being and quality of life.
The very characteristics of social networks can enhance elements of connectedness, both objectively by increasing users’ social sphere, and subjectively by reinforcing the feeling of social belonging and subjective well-being.
Taking Facebook and its “anniversary” feature as an example, it has been shown that the greater the number of Facebook friends, the more individuals saw themselves as being connected to a community.
“Millennials, or people born between the beginning of the 1980s and the end of the 1990s, are thus more likely to take advantage of the digital social environment to establish a new relationship with psychological suffering and its attempts to ease it,” said Dr. Morgiève.
They are also more likely to naturally turn to the digital space to look for help. More and more of them are searching the Internet for information on mental health and sharing experiences to get support.”
An example is the It Gets Better Project, which is a good illustration of the structure of online peer communities, with stories from LGBTQ+ individuals who describe how they succeeded in coping with adversity during their adolescence. In this way, social media seems to help identify peers and positive resources that are usually unavailable outside of the digital space. As a result, thanks to normative models on extremely strong social networks that are easy to conform to, these online peer-support communities have the potential to facilitate social interactions and reinforce a feeling both of hope and of belonging to a group.”
Promoting access to care
In Dr. Morgiève’s opinion, “access to care, particularly in the area of adolescent mental health, is extremely critical, given the lack of support precisely when they need it the most, as [evidenced] by the number of suicide attempts.
“There are two types of barriers to seeking help which can explain this. The first is structural barriers: help is too expensive or too far away or the wait is too long. The second refers to personal barriers, including denying the need for help, which may involve a self-sufficiency bias, the feeling that one cannot be helped, refusal to bother close friends and family, fear of being stigmatized, and a feeling of shame.”
These types of barriers are particularly difficult to overcome because the beliefs regarding care and caregivers are limiting (doubts about caregiver confidentiality, reliability, and competence). This is observed especially in adolescents because of the desire for emancipation and development of identity. So [the help relationship] may be experienced as subordination or alienation.
On a positive note, it is the very properties of social networks that will enable these obstacles to seeking help to be overcome. The fact that they are available everywhere makes up for young people’s lack of mobility and regional disparities. In addition, it ensures discretion and freedom of use, while reducing inhibitions.
The fact that social networks are free of charge overcomes structural obstacles, such as financial and organizational costs, as well as personal obstacles, thereby facilitating engagement and lessening the motivational cost. The dissociative pseudonymity or anonymity reduces the feeling of vulnerability associated with revealing oneself, as well as fears of a breach of confidentiality.
Dr. Morgiève summed it up by saying: “While offline life is silent because young people don’t talk about their suicidal ideations, online life truly removes inhibitions about speaking, relationships, and sharing experiences. Thus, the internet offers adolescents new opportunities to express themselves, which they’re not doing in real life.”
Professionals go digital
France records one suicide every hour (8,885 deaths a year) and one suicide attempt every 4 minutes. Since the 1950s, government-funded telehealth prevention and assistance programs, such as S.O.S. Amitié, Suicide Écoute, SOS Suicide Phénix, etc., have been developed. Their values and principles are anonymity, nondirectivity, nonjudgment, and neutrality. In addition to these nonprofit offerings, a professional teleprevention program, the confidential suicide prevention hotline 3114 – with professionals who are available to listen 24 hours a day, 7 days a week – was launched by the Ministry of Health and Prevention in October 2021.
Its values and principles include confidentiality, proactivity, concern, and caring for others. To date, 13 of 17 centers have opened. In the space of 6 months, they have received 50,000 calls, with an average of 400-500 calls a day. The dedicated chat application was codesigned with users (suicide attempters). And now social networks are joining in. For example, the hotline number 3114 appears whenever a TikTok user types the word “suicide.”
Dr. Morgiève said she has no conflicts of interest regarding the subject presented.
This article was translated from the Medscape French edition. A version of this article first appeared on Medscape.com.
PTSD may accelerate cognitive decline over time
, new research suggests.
In an analysis of more than 12,000 middle-aged women who had experienced at least one trauma in their lives, those with PTSD symptoms showed an approximately twofold faster decline in cognition during follow-up compared with those who did not have PTSD symptoms.
These associations were not fully explained by other known cognition-related factors such as depression, the researchers noted.
“PTSD may increase the risk of dementia by accelerating cognitive decline at midlife,” coinvestigator Jiaxuan Liu, a doctoral candidate at Harvard TH Chan School of Public Health, Boston, said in an interview.
“Our findings may suggest the value of earlier cognitive screening among individuals with PTSD and the importance of PTSD prevention and treatment across the lifespan,” she added.
The results were published online in JAMA Network Open.
Vital public health issue
“Cognitive decline at midlife and older is of vital public health interest,” Ms. Liu said. “It is a risk factor for a variety of poor health outcomes and strongly predicts Alzheimer’s disease and other dementias.
Although PTSD has been linked to lower cognitive function and dementia incidence, it has not been known whether it is associated with decline in cognitive function, she added.
“In addition, both PTSD and dementia are more common in women than men, so it’s important to understand a possible link,” Ms. Liu said.
Because no large-scale study had examined whether PTSD is associated with cognitive decline in women, the current researchers examined PTSD symptoms and their association with repeated measures of cognitive function among a large civilian trauma-exposed cohort of women aged 50-70 years at study baseline.
Participants were drawn from the Nurses’ Health Study II, a longitudinal study of a cohort of 116,429 U.S. female nurses who were between 25 and 42 years old at enrollment in 1989. Participants completed biennial questionnaires, with follow-up on an ongoing basis.
The current analysis included 12,270 trauma-exposed women (mean age at baseline, 61.1 years) who completed assessments every 1 or 12 months for up to 24 months after baseline. The mean follow-up time was 0.9 years.
In the study population, 95.9% were non-Hispanic White, 1.3% were Hispanic, 1% were Asian, 0.6% were Black, and 1.2% were classified as “other.”
Higher depression scores
Lifetime trauma exposure and PTSD symptoms were assessed from March 1, 2008, to Feb. 28, 2010, using the Short Screening Scale for DSM-IV PTSD.
In total, 67% of the participants reported experiencing PTSD symptoms. The women were divided into four groups, on the basis of symptom number: no PTSD symptoms (n = 4,052), one to three PTSD symptoms (n = 5,058), four to five PTSD symptoms (n = 2,018), and six to seven PTSD symptoms (n = 1,052).
The Cogstate Brief Battery, a validated and self-administered online cognitive assessment, was completed by participants between Oct. 3, 2014, and July 30, 2019. The researchers measured cognitive function with two composite scores: psychomotor speed and attention, and learning and working memory.
Covariates potentially associated with cognitive decline included demographic, educational, and behavior-related health factors such as body mass index, physical activity, cigarette smoking, diet quality, and alcohol consumption.
The researchers conducted secondary analyses that adjusted for symptoms and history of depression as well as the consequences of potential practice effects of taking the test multiple times.
Behavior-related health factors “did not substantially differ by PTSD symptom level,” the investigators noted. However, compared with women who did not have PTSD symptoms, those who had such symptoms had higher depressive symptom scores and higher rates of clinician-diagnosed depression.
Both cognitive composite scores improved through the follow-up period, “likely because of practice effects,” the researchers wrote. But after adjusting for practice effects, they found a decline over time in both composite scores.
Dose-related trajectories
Results showed that having more PTSD symptoms was associated with dose-related poorer cognitive trajectories.
After adjustment for demographic characteristics, women with the highest symptom level (six to seven symptoms) had a significantly worse rate of change in both composite domains of learning and working memory (beta = −0.08 SD/y; 95% confidence interval [CI], −0.11 to −0.04 SD/y; P < .001) and of psychomotor speed and attention (beta = −0.05 SD/y; 95% CI, −0.09 to −0.01 SD/y; P = .02) compared with women with no PTSD symptoms.
Women with four to five PTSD symptoms showed a worse rate of change in learning and working memory compared with those who had no symptoms, but not in psychomotor speed and attention. Women with one to three PTSD symptoms had cognitive scores similar to those of women without PTSD symptoms.
Notably, the associations of PTSD with cognitive change remained evident after additional adjustment for behavioral factors and health conditions – and were only “partially attenuated but still evident” after further adjustment for practice effects and comorbid depression, the investigators wrote.
“We thought PTSD might be associated with worse cognitive decline through health behaviors like smoking and alcohol drinking and higher risk of other health conditions like hypertension and depression,” Ms. Liu said.
However, those factors did not account for the current study’s findings, she noted.
“We could not determine why women with PTSD had faster cognitive decline than those without PTSD,” she said.
Ms. Liu suggested that PTSD “may have effects on the brain, such as altering brain structures and affecting brain immune function.” However, more research is needed “to investigate these mechanisms that might underlie the association we found between PTSD and cognitive decline,” she said.
Neurotoxic effect
In a comment, Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation, said, “It is well known that stress is neurotoxic, and PTSD is a particularly serious form of stress.”
Dr. Fillit, clinical professor of geriatric medicine and palliative care, medicine, and neuroscience at Mount Sinai Hospital, New York, was not involved with the study.
“We tend to think of PTSD in postacute settings, such as soldiers returning from war,” he said. “This study contributes to our understanding of the long-term effects of PTSD on cognitive decline, measured objectively over time”
Dr. Fillit noted that an important implication is that, by increasing the risk for cognitive decline, PTSD also increases risk for Alzheimer’s disease. This leads to the “main take-home, which is that PTSD is a risk factor not only for cognitive decline but also for Alzheimer’s and related dementias,” he said.
However, this opens a potential therapeutic approach, Dr. Fillit added.
Because cortisol and other stress hormones drive the stress response, finding ways to block the neurotoxic effects of these hormones “might be a target to prevent cognitive decline and decrease Alzheimer’s disease risk,” he said.
The study was supported by grants from the National Institute of Mental Health and the National Institutes of Health. Ms. Liu and Dr. Fillit report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In an analysis of more than 12,000 middle-aged women who had experienced at least one trauma in their lives, those with PTSD symptoms showed an approximately twofold faster decline in cognition during follow-up compared with those who did not have PTSD symptoms.
These associations were not fully explained by other known cognition-related factors such as depression, the researchers noted.
“PTSD may increase the risk of dementia by accelerating cognitive decline at midlife,” coinvestigator Jiaxuan Liu, a doctoral candidate at Harvard TH Chan School of Public Health, Boston, said in an interview.
“Our findings may suggest the value of earlier cognitive screening among individuals with PTSD and the importance of PTSD prevention and treatment across the lifespan,” she added.
The results were published online in JAMA Network Open.
Vital public health issue
“Cognitive decline at midlife and older is of vital public health interest,” Ms. Liu said. “It is a risk factor for a variety of poor health outcomes and strongly predicts Alzheimer’s disease and other dementias.
Although PTSD has been linked to lower cognitive function and dementia incidence, it has not been known whether it is associated with decline in cognitive function, she added.
“In addition, both PTSD and dementia are more common in women than men, so it’s important to understand a possible link,” Ms. Liu said.
Because no large-scale study had examined whether PTSD is associated with cognitive decline in women, the current researchers examined PTSD symptoms and their association with repeated measures of cognitive function among a large civilian trauma-exposed cohort of women aged 50-70 years at study baseline.
Participants were drawn from the Nurses’ Health Study II, a longitudinal study of a cohort of 116,429 U.S. female nurses who were between 25 and 42 years old at enrollment in 1989. Participants completed biennial questionnaires, with follow-up on an ongoing basis.
The current analysis included 12,270 trauma-exposed women (mean age at baseline, 61.1 years) who completed assessments every 1 or 12 months for up to 24 months after baseline. The mean follow-up time was 0.9 years.
In the study population, 95.9% were non-Hispanic White, 1.3% were Hispanic, 1% were Asian, 0.6% were Black, and 1.2% were classified as “other.”
Higher depression scores
Lifetime trauma exposure and PTSD symptoms were assessed from March 1, 2008, to Feb. 28, 2010, using the Short Screening Scale for DSM-IV PTSD.
In total, 67% of the participants reported experiencing PTSD symptoms. The women were divided into four groups, on the basis of symptom number: no PTSD symptoms (n = 4,052), one to three PTSD symptoms (n = 5,058), four to five PTSD symptoms (n = 2,018), and six to seven PTSD symptoms (n = 1,052).
The Cogstate Brief Battery, a validated and self-administered online cognitive assessment, was completed by participants between Oct. 3, 2014, and July 30, 2019. The researchers measured cognitive function with two composite scores: psychomotor speed and attention, and learning and working memory.
Covariates potentially associated with cognitive decline included demographic, educational, and behavior-related health factors such as body mass index, physical activity, cigarette smoking, diet quality, and alcohol consumption.
The researchers conducted secondary analyses that adjusted for symptoms and history of depression as well as the consequences of potential practice effects of taking the test multiple times.
Behavior-related health factors “did not substantially differ by PTSD symptom level,” the investigators noted. However, compared with women who did not have PTSD symptoms, those who had such symptoms had higher depressive symptom scores and higher rates of clinician-diagnosed depression.
Both cognitive composite scores improved through the follow-up period, “likely because of practice effects,” the researchers wrote. But after adjusting for practice effects, they found a decline over time in both composite scores.
Dose-related trajectories
Results showed that having more PTSD symptoms was associated with dose-related poorer cognitive trajectories.
After adjustment for demographic characteristics, women with the highest symptom level (six to seven symptoms) had a significantly worse rate of change in both composite domains of learning and working memory (beta = −0.08 SD/y; 95% confidence interval [CI], −0.11 to −0.04 SD/y; P < .001) and of psychomotor speed and attention (beta = −0.05 SD/y; 95% CI, −0.09 to −0.01 SD/y; P = .02) compared with women with no PTSD symptoms.
Women with four to five PTSD symptoms showed a worse rate of change in learning and working memory compared with those who had no symptoms, but not in psychomotor speed and attention. Women with one to three PTSD symptoms had cognitive scores similar to those of women without PTSD symptoms.
Notably, the associations of PTSD with cognitive change remained evident after additional adjustment for behavioral factors and health conditions – and were only “partially attenuated but still evident” after further adjustment for practice effects and comorbid depression, the investigators wrote.
“We thought PTSD might be associated with worse cognitive decline through health behaviors like smoking and alcohol drinking and higher risk of other health conditions like hypertension and depression,” Ms. Liu said.
However, those factors did not account for the current study’s findings, she noted.
“We could not determine why women with PTSD had faster cognitive decline than those without PTSD,” she said.
Ms. Liu suggested that PTSD “may have effects on the brain, such as altering brain structures and affecting brain immune function.” However, more research is needed “to investigate these mechanisms that might underlie the association we found between PTSD and cognitive decline,” she said.
Neurotoxic effect
In a comment, Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation, said, “It is well known that stress is neurotoxic, and PTSD is a particularly serious form of stress.”
Dr. Fillit, clinical professor of geriatric medicine and palliative care, medicine, and neuroscience at Mount Sinai Hospital, New York, was not involved with the study.
“We tend to think of PTSD in postacute settings, such as soldiers returning from war,” he said. “This study contributes to our understanding of the long-term effects of PTSD on cognitive decline, measured objectively over time”
Dr. Fillit noted that an important implication is that, by increasing the risk for cognitive decline, PTSD also increases risk for Alzheimer’s disease. This leads to the “main take-home, which is that PTSD is a risk factor not only for cognitive decline but also for Alzheimer’s and related dementias,” he said.
However, this opens a potential therapeutic approach, Dr. Fillit added.
Because cortisol and other stress hormones drive the stress response, finding ways to block the neurotoxic effects of these hormones “might be a target to prevent cognitive decline and decrease Alzheimer’s disease risk,” he said.
The study was supported by grants from the National Institute of Mental Health and the National Institutes of Health. Ms. Liu and Dr. Fillit report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In an analysis of more than 12,000 middle-aged women who had experienced at least one trauma in their lives, those with PTSD symptoms showed an approximately twofold faster decline in cognition during follow-up compared with those who did not have PTSD symptoms.
These associations were not fully explained by other known cognition-related factors such as depression, the researchers noted.
“PTSD may increase the risk of dementia by accelerating cognitive decline at midlife,” coinvestigator Jiaxuan Liu, a doctoral candidate at Harvard TH Chan School of Public Health, Boston, said in an interview.
“Our findings may suggest the value of earlier cognitive screening among individuals with PTSD and the importance of PTSD prevention and treatment across the lifespan,” she added.
The results were published online in JAMA Network Open.
Vital public health issue
“Cognitive decline at midlife and older is of vital public health interest,” Ms. Liu said. “It is a risk factor for a variety of poor health outcomes and strongly predicts Alzheimer’s disease and other dementias.
Although PTSD has been linked to lower cognitive function and dementia incidence, it has not been known whether it is associated with decline in cognitive function, she added.
“In addition, both PTSD and dementia are more common in women than men, so it’s important to understand a possible link,” Ms. Liu said.
Because no large-scale study had examined whether PTSD is associated with cognitive decline in women, the current researchers examined PTSD symptoms and their association with repeated measures of cognitive function among a large civilian trauma-exposed cohort of women aged 50-70 years at study baseline.
Participants were drawn from the Nurses’ Health Study II, a longitudinal study of a cohort of 116,429 U.S. female nurses who were between 25 and 42 years old at enrollment in 1989. Participants completed biennial questionnaires, with follow-up on an ongoing basis.
The current analysis included 12,270 trauma-exposed women (mean age at baseline, 61.1 years) who completed assessments every 1 or 12 months for up to 24 months after baseline. The mean follow-up time was 0.9 years.
In the study population, 95.9% were non-Hispanic White, 1.3% were Hispanic, 1% were Asian, 0.6% were Black, and 1.2% were classified as “other.”
Higher depression scores
Lifetime trauma exposure and PTSD symptoms were assessed from March 1, 2008, to Feb. 28, 2010, using the Short Screening Scale for DSM-IV PTSD.
In total, 67% of the participants reported experiencing PTSD symptoms. The women were divided into four groups, on the basis of symptom number: no PTSD symptoms (n = 4,052), one to three PTSD symptoms (n = 5,058), four to five PTSD symptoms (n = 2,018), and six to seven PTSD symptoms (n = 1,052).
The Cogstate Brief Battery, a validated and self-administered online cognitive assessment, was completed by participants between Oct. 3, 2014, and July 30, 2019. The researchers measured cognitive function with two composite scores: psychomotor speed and attention, and learning and working memory.
Covariates potentially associated with cognitive decline included demographic, educational, and behavior-related health factors such as body mass index, physical activity, cigarette smoking, diet quality, and alcohol consumption.
The researchers conducted secondary analyses that adjusted for symptoms and history of depression as well as the consequences of potential practice effects of taking the test multiple times.
Behavior-related health factors “did not substantially differ by PTSD symptom level,” the investigators noted. However, compared with women who did not have PTSD symptoms, those who had such symptoms had higher depressive symptom scores and higher rates of clinician-diagnosed depression.
Both cognitive composite scores improved through the follow-up period, “likely because of practice effects,” the researchers wrote. But after adjusting for practice effects, they found a decline over time in both composite scores.
Dose-related trajectories
Results showed that having more PTSD symptoms was associated with dose-related poorer cognitive trajectories.
After adjustment for demographic characteristics, women with the highest symptom level (six to seven symptoms) had a significantly worse rate of change in both composite domains of learning and working memory (beta = −0.08 SD/y; 95% confidence interval [CI], −0.11 to −0.04 SD/y; P < .001) and of psychomotor speed and attention (beta = −0.05 SD/y; 95% CI, −0.09 to −0.01 SD/y; P = .02) compared with women with no PTSD symptoms.
Women with four to five PTSD symptoms showed a worse rate of change in learning and working memory compared with those who had no symptoms, but not in psychomotor speed and attention. Women with one to three PTSD symptoms had cognitive scores similar to those of women without PTSD symptoms.
Notably, the associations of PTSD with cognitive change remained evident after additional adjustment for behavioral factors and health conditions – and were only “partially attenuated but still evident” after further adjustment for practice effects and comorbid depression, the investigators wrote.
“We thought PTSD might be associated with worse cognitive decline through health behaviors like smoking and alcohol drinking and higher risk of other health conditions like hypertension and depression,” Ms. Liu said.
However, those factors did not account for the current study’s findings, she noted.
“We could not determine why women with PTSD had faster cognitive decline than those without PTSD,” she said.
Ms. Liu suggested that PTSD “may have effects on the brain, such as altering brain structures and affecting brain immune function.” However, more research is needed “to investigate these mechanisms that might underlie the association we found between PTSD and cognitive decline,” she said.
Neurotoxic effect
In a comment, Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation, said, “It is well known that stress is neurotoxic, and PTSD is a particularly serious form of stress.”
Dr. Fillit, clinical professor of geriatric medicine and palliative care, medicine, and neuroscience at Mount Sinai Hospital, New York, was not involved with the study.
“We tend to think of PTSD in postacute settings, such as soldiers returning from war,” he said. “This study contributes to our understanding of the long-term effects of PTSD on cognitive decline, measured objectively over time”
Dr. Fillit noted that an important implication is that, by increasing the risk for cognitive decline, PTSD also increases risk for Alzheimer’s disease. This leads to the “main take-home, which is that PTSD is a risk factor not only for cognitive decline but also for Alzheimer’s and related dementias,” he said.
However, this opens a potential therapeutic approach, Dr. Fillit added.
Because cortisol and other stress hormones drive the stress response, finding ways to block the neurotoxic effects of these hormones “might be a target to prevent cognitive decline and decrease Alzheimer’s disease risk,” he said.
The study was supported by grants from the National Institute of Mental Health and the National Institutes of Health. Ms. Liu and Dr. Fillit report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Adapting to Changes in Acne Management: Take One Step at a Time
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
New AHA checklist: Only one in five adults has optimal heart health
About 80% of American adults have low to moderate cardiovascular (CV) health based on the American Heart Association checklist for optimal heart health, which now includes healthy sleep as an essential component for heart health.
With the addition of sleep, “Life’s Essential 8” replaces the AHA’s “Life’s Simple 7” checklist.
“The new metric of sleep duration reflects the latest research findings: Sleep impacts overall health, and people who have healthier sleep patterns manage health factors such as weight, blood pressure, or risk for type 2 diabetes more effectively,” AHA President Donald M. Lloyd-Jones, MD, said in a news release.
“In addition, advances in ways to measure sleep, such as with wearable devices, now offer people the ability to reliably and routinely monitor their sleep habits at home,” said Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University in Chicago.
The AHA Presidential Advisory – Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct on Cardiovascular Health – was published online in the journal Circulation.
A companion paper published simultaneously in Circulation reports the first study using Life’s Essential 8.
Overall, the results show that CV health of the U.S. population is “suboptimal, and we see important differences across age and sociodemographic groups,” Dr. Lloyd-Jones said.
Refining Life’s Simple 7
The AHA first defined the seven metrics for optimal CV health in 2010. After 12 years and more than 2,400 scientific papers on the topic, new discoveries in CV health and ways to measure it provided an opportunity to revisit each health component in more detail and provide updates as needed, the AHA explains.
“We felt it was the right time to conduct a comprehensive review of the latest research to refine the existing metrics and consider any new metrics that add value to assessing cardiovascular health for all people,” Dr. Lloyd-Jones said.
Four of the original metrics have been redefined for consistency with newer clinical guidelines or compatibility with new measurement tools, and the scoring system can now also be applied to anyone ages 2 and older. Here is a snapshot of Life’s Essential 8 metrics, including updates.
1. Diet (updated)
The tool includes a new guide to assess diet quality for adults and children at the individual and population level. At the population level, dietary assessment is based on daily intake of elements in the Dietary Approaches to Stop Hypertension (DASH) eating pattern. For individuals, the Mediterranean Eating Pattern for Americans (MEPA) is used to assess and monitor cardiovascular health.
2. Physical activity (no changes)
Physical activity continues to be measured by the total number of minutes of moderate or vigorous physical activity per week, as defined by the U.S. Physical Activity Guidelines for Americans (2nd edition). The optimal level is 150 minutes (2.5 hours) of moderate physical activity or more per week or 75 minutes per week of vigorous-intensity physical activity for adults; 420 minutes (7 hours) or more per week for children ages 6 and older; and age-specific modifications for younger children.
3. Nicotine exposure (updated)
Use of inhaled nicotine-delivery systems, which includes e-cigarettes or vaping devices, has been added since the previous metric monitored only traditional, combustible cigarettes. This reflects use by adults and youth and their implications on long-term health. Second-hand smoke exposure for children and adults has also been added.
4. Sleep duration (new)
Sleep duration is associated with CV health. Measured by average hours of sleep per night, the ideal level is 7-9 hours daily for adults. Ideal daily sleep ranges for children are 10-16 hours per 24 hours for ages 5 and younger; 9-12 hours for ages 6-12 years; and 8-10 hours for ages 13-18 years.
5. Body mass index (no changes)
The AHA acknowledges that body mass index (BMI) is an imperfect metric. Yet, because it’s easily calculated and widely available, BMI continues as a “reasonable” gauge to assess weight categories that may lead to health problems. BMI of 18.5-24.9 is associated with the highest levels of CV health. The AHA notes that BMI ranges and the subsequent health risks associated with them may differ among people from diverse racial or ethnic backgrounds or ancestry. This aligns with the World Health Organization recommendations to adjust BMI ranges for people of Asian or Pacific Islander ancestry because recent evidence indicates their risk of conditions such as CVD or type 2 diabetes is higher at a lower BMI.
6. Blood lipids (updated)
The metric for blood lipids (cholesterol and triglycerides) is updated to use non-HDL cholesterol as the preferred number to monitor, rather than total cholesterol. This shift is made because non-HDL cholesterol can be measured without fasting beforehand (thereby increasing its availability at any time of day and implementation at more appointments) and reliably calculated among all people.
7. Blood glucose (updated)
This metric is expanded to include the option of hemoglobin A1c readings or blood glucose levels for people with or without type 1 or 2 diabetes or prediabetes.
8. Blood pressure (no changes)
Blood pressure criteria remain unchanged from 2017 guidance that established levels less than 120/80 mm Hg as optimal, and defined hypertension as 130-139 mm Hg systolic pressure or 80-89 mm Hg diastolic pressure.
‘Concerning’ new data
Results of the first study using Life’s Essential 8 show that the overall CV health of the U.S. population is “well below ideal,” with 80% of adults scoring at a low or moderate level, the researchers report.
Data for the analysis came from 2013-2018 U.S. National Health and Nutrition Examination surveys (NHANES) of more than 13,500 adults aged 20-79 years and nearly 9,900 children aged 2-19 years. Among the key findings:
- The average CV health score based on Life’s Essential 8 was 64.7 for adults and 65.5 for children – in the moderate range on the 0-100 scale.
- Only 0.45% of adults had a perfect score of 100; 20% had high CV health (score of 80 or higher), 63% moderate (score of 50-79), and 18% had low CV health (score of less than 50).
- Adult women had higher average CV health scores (67) compared with men (62.5).
- In general, adults scored lowest in the areas of diet, physical activity, and BMI.
- CV health scores were generally lower at older ages.
- Non-Hispanic Asian Americans had a higher average CV health score than other racial/ethnic groups. Non-Hispanic Whites had the second highest average CV health score, followed, in order, by Hispanic (other than Mexican), Mexican, and non-Hispanic Blacks.
- Children’s diet scores were low, at an average of 40.6.
- Adult sociodemographic groups varied notably in CV health scores for diet, nicotine exposure, blood glucose, and blood pressure.
“These data represent the first look at the cardiovascular health of the U.S. population using the AHA’s new Life’s Essential 8 scoring algorithm,” Dr. Lloyd-Jones said.
“Life’s Essential 8 is a major step forward in our ability to identify when cardiovascular health can be preserved and when it is suboptimal. It should energize efforts to improve cardiovascular health for all people and at every life stage,” Dr. Lloyd-Jones added.
“Analyses like this can help policymakers, communities, clinicians, and the public to understand the opportunities to intervene to improve and maintain optimal cardiovascular health across the life course,” he said.
This research had no commercial funding. The authors have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About 80% of American adults have low to moderate cardiovascular (CV) health based on the American Heart Association checklist for optimal heart health, which now includes healthy sleep as an essential component for heart health.
With the addition of sleep, “Life’s Essential 8” replaces the AHA’s “Life’s Simple 7” checklist.
“The new metric of sleep duration reflects the latest research findings: Sleep impacts overall health, and people who have healthier sleep patterns manage health factors such as weight, blood pressure, or risk for type 2 diabetes more effectively,” AHA President Donald M. Lloyd-Jones, MD, said in a news release.
“In addition, advances in ways to measure sleep, such as with wearable devices, now offer people the ability to reliably and routinely monitor their sleep habits at home,” said Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University in Chicago.
The AHA Presidential Advisory – Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct on Cardiovascular Health – was published online in the journal Circulation.
A companion paper published simultaneously in Circulation reports the first study using Life’s Essential 8.
Overall, the results show that CV health of the U.S. population is “suboptimal, and we see important differences across age and sociodemographic groups,” Dr. Lloyd-Jones said.
Refining Life’s Simple 7
The AHA first defined the seven metrics for optimal CV health in 2010. After 12 years and more than 2,400 scientific papers on the topic, new discoveries in CV health and ways to measure it provided an opportunity to revisit each health component in more detail and provide updates as needed, the AHA explains.
“We felt it was the right time to conduct a comprehensive review of the latest research to refine the existing metrics and consider any new metrics that add value to assessing cardiovascular health for all people,” Dr. Lloyd-Jones said.
Four of the original metrics have been redefined for consistency with newer clinical guidelines or compatibility with new measurement tools, and the scoring system can now also be applied to anyone ages 2 and older. Here is a snapshot of Life’s Essential 8 metrics, including updates.
1. Diet (updated)
The tool includes a new guide to assess diet quality for adults and children at the individual and population level. At the population level, dietary assessment is based on daily intake of elements in the Dietary Approaches to Stop Hypertension (DASH) eating pattern. For individuals, the Mediterranean Eating Pattern for Americans (MEPA) is used to assess and monitor cardiovascular health.
2. Physical activity (no changes)
Physical activity continues to be measured by the total number of minutes of moderate or vigorous physical activity per week, as defined by the U.S. Physical Activity Guidelines for Americans (2nd edition). The optimal level is 150 minutes (2.5 hours) of moderate physical activity or more per week or 75 minutes per week of vigorous-intensity physical activity for adults; 420 minutes (7 hours) or more per week for children ages 6 and older; and age-specific modifications for younger children.
3. Nicotine exposure (updated)
Use of inhaled nicotine-delivery systems, which includes e-cigarettes or vaping devices, has been added since the previous metric monitored only traditional, combustible cigarettes. This reflects use by adults and youth and their implications on long-term health. Second-hand smoke exposure for children and adults has also been added.
4. Sleep duration (new)
Sleep duration is associated with CV health. Measured by average hours of sleep per night, the ideal level is 7-9 hours daily for adults. Ideal daily sleep ranges for children are 10-16 hours per 24 hours for ages 5 and younger; 9-12 hours for ages 6-12 years; and 8-10 hours for ages 13-18 years.
5. Body mass index (no changes)
The AHA acknowledges that body mass index (BMI) is an imperfect metric. Yet, because it’s easily calculated and widely available, BMI continues as a “reasonable” gauge to assess weight categories that may lead to health problems. BMI of 18.5-24.9 is associated with the highest levels of CV health. The AHA notes that BMI ranges and the subsequent health risks associated with them may differ among people from diverse racial or ethnic backgrounds or ancestry. This aligns with the World Health Organization recommendations to adjust BMI ranges for people of Asian or Pacific Islander ancestry because recent evidence indicates their risk of conditions such as CVD or type 2 diabetes is higher at a lower BMI.
6. Blood lipids (updated)
The metric for blood lipids (cholesterol and triglycerides) is updated to use non-HDL cholesterol as the preferred number to monitor, rather than total cholesterol. This shift is made because non-HDL cholesterol can be measured without fasting beforehand (thereby increasing its availability at any time of day and implementation at more appointments) and reliably calculated among all people.
7. Blood glucose (updated)
This metric is expanded to include the option of hemoglobin A1c readings or blood glucose levels for people with or without type 1 or 2 diabetes or prediabetes.
8. Blood pressure (no changes)
Blood pressure criteria remain unchanged from 2017 guidance that established levels less than 120/80 mm Hg as optimal, and defined hypertension as 130-139 mm Hg systolic pressure or 80-89 mm Hg diastolic pressure.
‘Concerning’ new data
Results of the first study using Life’s Essential 8 show that the overall CV health of the U.S. population is “well below ideal,” with 80% of adults scoring at a low or moderate level, the researchers report.
Data for the analysis came from 2013-2018 U.S. National Health and Nutrition Examination surveys (NHANES) of more than 13,500 adults aged 20-79 years and nearly 9,900 children aged 2-19 years. Among the key findings:
- The average CV health score based on Life’s Essential 8 was 64.7 for adults and 65.5 for children – in the moderate range on the 0-100 scale.
- Only 0.45% of adults had a perfect score of 100; 20% had high CV health (score of 80 or higher), 63% moderate (score of 50-79), and 18% had low CV health (score of less than 50).
- Adult women had higher average CV health scores (67) compared with men (62.5).
- In general, adults scored lowest in the areas of diet, physical activity, and BMI.
- CV health scores were generally lower at older ages.
- Non-Hispanic Asian Americans had a higher average CV health score than other racial/ethnic groups. Non-Hispanic Whites had the second highest average CV health score, followed, in order, by Hispanic (other than Mexican), Mexican, and non-Hispanic Blacks.
- Children’s diet scores were low, at an average of 40.6.
- Adult sociodemographic groups varied notably in CV health scores for diet, nicotine exposure, blood glucose, and blood pressure.
“These data represent the first look at the cardiovascular health of the U.S. population using the AHA’s new Life’s Essential 8 scoring algorithm,” Dr. Lloyd-Jones said.
“Life’s Essential 8 is a major step forward in our ability to identify when cardiovascular health can be preserved and when it is suboptimal. It should energize efforts to improve cardiovascular health for all people and at every life stage,” Dr. Lloyd-Jones added.
“Analyses like this can help policymakers, communities, clinicians, and the public to understand the opportunities to intervene to improve and maintain optimal cardiovascular health across the life course,” he said.
This research had no commercial funding. The authors have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About 80% of American adults have low to moderate cardiovascular (CV) health based on the American Heart Association checklist for optimal heart health, which now includes healthy sleep as an essential component for heart health.
With the addition of sleep, “Life’s Essential 8” replaces the AHA’s “Life’s Simple 7” checklist.
“The new metric of sleep duration reflects the latest research findings: Sleep impacts overall health, and people who have healthier sleep patterns manage health factors such as weight, blood pressure, or risk for type 2 diabetes more effectively,” AHA President Donald M. Lloyd-Jones, MD, said in a news release.
“In addition, advances in ways to measure sleep, such as with wearable devices, now offer people the ability to reliably and routinely monitor their sleep habits at home,” said Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University in Chicago.
The AHA Presidential Advisory – Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct on Cardiovascular Health – was published online in the journal Circulation.
A companion paper published simultaneously in Circulation reports the first study using Life’s Essential 8.
Overall, the results show that CV health of the U.S. population is “suboptimal, and we see important differences across age and sociodemographic groups,” Dr. Lloyd-Jones said.
Refining Life’s Simple 7
The AHA first defined the seven metrics for optimal CV health in 2010. After 12 years and more than 2,400 scientific papers on the topic, new discoveries in CV health and ways to measure it provided an opportunity to revisit each health component in more detail and provide updates as needed, the AHA explains.
“We felt it was the right time to conduct a comprehensive review of the latest research to refine the existing metrics and consider any new metrics that add value to assessing cardiovascular health for all people,” Dr. Lloyd-Jones said.
Four of the original metrics have been redefined for consistency with newer clinical guidelines or compatibility with new measurement tools, and the scoring system can now also be applied to anyone ages 2 and older. Here is a snapshot of Life’s Essential 8 metrics, including updates.
1. Diet (updated)
The tool includes a new guide to assess diet quality for adults and children at the individual and population level. At the population level, dietary assessment is based on daily intake of elements in the Dietary Approaches to Stop Hypertension (DASH) eating pattern. For individuals, the Mediterranean Eating Pattern for Americans (MEPA) is used to assess and monitor cardiovascular health.
2. Physical activity (no changes)
Physical activity continues to be measured by the total number of minutes of moderate or vigorous physical activity per week, as defined by the U.S. Physical Activity Guidelines for Americans (2nd edition). The optimal level is 150 minutes (2.5 hours) of moderate physical activity or more per week or 75 minutes per week of vigorous-intensity physical activity for adults; 420 minutes (7 hours) or more per week for children ages 6 and older; and age-specific modifications for younger children.
3. Nicotine exposure (updated)
Use of inhaled nicotine-delivery systems, which includes e-cigarettes or vaping devices, has been added since the previous metric monitored only traditional, combustible cigarettes. This reflects use by adults and youth and their implications on long-term health. Second-hand smoke exposure for children and adults has also been added.
4. Sleep duration (new)
Sleep duration is associated with CV health. Measured by average hours of sleep per night, the ideal level is 7-9 hours daily for adults. Ideal daily sleep ranges for children are 10-16 hours per 24 hours for ages 5 and younger; 9-12 hours for ages 6-12 years; and 8-10 hours for ages 13-18 years.
5. Body mass index (no changes)
The AHA acknowledges that body mass index (BMI) is an imperfect metric. Yet, because it’s easily calculated and widely available, BMI continues as a “reasonable” gauge to assess weight categories that may lead to health problems. BMI of 18.5-24.9 is associated with the highest levels of CV health. The AHA notes that BMI ranges and the subsequent health risks associated with them may differ among people from diverse racial or ethnic backgrounds or ancestry. This aligns with the World Health Organization recommendations to adjust BMI ranges for people of Asian or Pacific Islander ancestry because recent evidence indicates their risk of conditions such as CVD or type 2 diabetes is higher at a lower BMI.
6. Blood lipids (updated)
The metric for blood lipids (cholesterol and triglycerides) is updated to use non-HDL cholesterol as the preferred number to monitor, rather than total cholesterol. This shift is made because non-HDL cholesterol can be measured without fasting beforehand (thereby increasing its availability at any time of day and implementation at more appointments) and reliably calculated among all people.
7. Blood glucose (updated)
This metric is expanded to include the option of hemoglobin A1c readings or blood glucose levels for people with or without type 1 or 2 diabetes or prediabetes.
8. Blood pressure (no changes)
Blood pressure criteria remain unchanged from 2017 guidance that established levels less than 120/80 mm Hg as optimal, and defined hypertension as 130-139 mm Hg systolic pressure or 80-89 mm Hg diastolic pressure.
‘Concerning’ new data
Results of the first study using Life’s Essential 8 show that the overall CV health of the U.S. population is “well below ideal,” with 80% of adults scoring at a low or moderate level, the researchers report.
Data for the analysis came from 2013-2018 U.S. National Health and Nutrition Examination surveys (NHANES) of more than 13,500 adults aged 20-79 years and nearly 9,900 children aged 2-19 years. Among the key findings:
- The average CV health score based on Life’s Essential 8 was 64.7 for adults and 65.5 for children – in the moderate range on the 0-100 scale.
- Only 0.45% of adults had a perfect score of 100; 20% had high CV health (score of 80 or higher), 63% moderate (score of 50-79), and 18% had low CV health (score of less than 50).
- Adult women had higher average CV health scores (67) compared with men (62.5).
- In general, adults scored lowest in the areas of diet, physical activity, and BMI.
- CV health scores were generally lower at older ages.
- Non-Hispanic Asian Americans had a higher average CV health score than other racial/ethnic groups. Non-Hispanic Whites had the second highest average CV health score, followed, in order, by Hispanic (other than Mexican), Mexican, and non-Hispanic Blacks.
- Children’s diet scores were low, at an average of 40.6.
- Adult sociodemographic groups varied notably in CV health scores for diet, nicotine exposure, blood glucose, and blood pressure.
“These data represent the first look at the cardiovascular health of the U.S. population using the AHA’s new Life’s Essential 8 scoring algorithm,” Dr. Lloyd-Jones said.
“Life’s Essential 8 is a major step forward in our ability to identify when cardiovascular health can be preserved and when it is suboptimal. It should energize efforts to improve cardiovascular health for all people and at every life stage,” Dr. Lloyd-Jones added.
“Analyses like this can help policymakers, communities, clinicians, and the public to understand the opportunities to intervene to improve and maintain optimal cardiovascular health across the life course,” he said.
This research had no commercial funding. The authors have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Administrative Burden of iPLEDGE Deters Isotretinoin Prescriptions: Results From a Survey of Dermatologists
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.
Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.

Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.

Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.

Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.

Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Practice Points
- Of clinicians who regularly prescribe isotretinoin, approximately 30% have at times chosen not to prescribe isotretinoin to patients with severe acne because of the burden of the iPLEDGE program.
- The US Food and Drug Administration should consider further streamlining the iPLEDGE program, as it is causing physician burden and therefore suboptimal treatment plans for patients.