User login
Loan forgiveness and med school debt: What about me?
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I run the division of medical ethics at New York University Grossman School of Medicine.
Many of you know that President Biden created a loan forgiveness program, forgiving up to $10,000 against federal student loans, including graduate and undergraduate education. The Department of Education is supposed to provide up to $20,000 in debt cancellation to Pell Grant recipients who have loans that are held by the Department of Education. Borrowers can get this relief if their income is less than $125,000 for an individual or $250,000 for married couples.
Many people have looked at this and said, “Hey, wait a minute. I paid off my loans. I didn’t get any reimbursement. That isn’t fair.”
who often still have huge amounts of debt, and either because of the income limits or because they don’t qualify because this debt was accrued long in the past, they’re saying, “What about me? Don’t you want to give any relief to me?”
This is a topic near and dear to my heart because I happen to be at a medical school, NYU, that has decided for the two medical schools it runs – our main campus, NYU in Manhattan and NYU Langone out on Long Island – that we’re going to go tuition free. We’ve done it for a couple of years.
We did it because I think all the administrators and faculty understood the tremendous burden that debt poses on people who both carry forward their undergraduate debt and then have medical school debt. This really leads to very difficult situations – which we have great empathy for – about what specialty you’re going to go into, whether you have to moonlight, and how you’re going to manage a huge burden of debt.
Many people don’t have sympathy out in the public. They say doctors make a large amount of money and they live a nice lifestyle, so we’re not going to relieve their debt. The reality is that, whoever you are, short of Bill Gates or Elon Musk, having hundreds of thousands of dollars of debt is no easy task to live with and to work off.
Still, when we created free tuition at NYU for our medical school, there were many people who paid high tuition fees in the past. Some of them said to us, “What about me?” We decided not to try to do anything retrospectively. The plan was to build up enough money so that we could handle no-cost tuition going forward. We didn’t really have it in our pocketbook to help people who’d already paid their debts or were saddled with NYU debt. Is it fair? No, it’s probably not fair, but it’s an improvement.
That’s what I want people to think about who are saying, “What about my medical school debt? What about my undergraduate plus medical school debt?” I think we should be grateful when efforts are being made to reduce very burdensome student loans that people have. It’s good to give that benefit and move it forward.
Does that mean no one should get anything unless everyone with any kind of debt from school is covered? I don’t think so. I don’t think that’s fair either.
It is possible that we could continue to agitate politically and say, let’s go after some of the health care debt. Let’s go after some of the things that are still driving people to have to work more than they would or to choose specialties that they really don’t want to be in because they have to make up that debt.
It doesn’t mean the last word has been said about the politics of debt relief or, for that matter, the price of going to medical school in the first place and trying to see whether that can be driven down.
I don’t think it’s right to say, “If I can’t benefit, given the huge burden that I’m carrying, then I’m not going to try to give relief to others.” I think we’re relieving debt to the extent that we can do it. The nation can afford it. Going forward is a good thing. It’s wrong to create those gigantic debts in the first place.
What are we going to do about the past? We may decide that we need some sort of forgiveness or reparations for loans that were built up for others going backwards. I wouldn’t hold hostage the future and our children to what was probably a very poor, unethical practice about saddling doctors and others in the past with huge debt.
I’m Art Caplan at the division of medical ethics at New York University Grossman School of Medicine. Thank you for watching.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I run the division of medical ethics at New York University Grossman School of Medicine.
Many of you know that President Biden created a loan forgiveness program, forgiving up to $10,000 against federal student loans, including graduate and undergraduate education. The Department of Education is supposed to provide up to $20,000 in debt cancellation to Pell Grant recipients who have loans that are held by the Department of Education. Borrowers can get this relief if their income is less than $125,000 for an individual or $250,000 for married couples.
Many people have looked at this and said, “Hey, wait a minute. I paid off my loans. I didn’t get any reimbursement. That isn’t fair.”
who often still have huge amounts of debt, and either because of the income limits or because they don’t qualify because this debt was accrued long in the past, they’re saying, “What about me? Don’t you want to give any relief to me?”
This is a topic near and dear to my heart because I happen to be at a medical school, NYU, that has decided for the two medical schools it runs – our main campus, NYU in Manhattan and NYU Langone out on Long Island – that we’re going to go tuition free. We’ve done it for a couple of years.
We did it because I think all the administrators and faculty understood the tremendous burden that debt poses on people who both carry forward their undergraduate debt and then have medical school debt. This really leads to very difficult situations – which we have great empathy for – about what specialty you’re going to go into, whether you have to moonlight, and how you’re going to manage a huge burden of debt.
Many people don’t have sympathy out in the public. They say doctors make a large amount of money and they live a nice lifestyle, so we’re not going to relieve their debt. The reality is that, whoever you are, short of Bill Gates or Elon Musk, having hundreds of thousands of dollars of debt is no easy task to live with and to work off.
Still, when we created free tuition at NYU for our medical school, there were many people who paid high tuition fees in the past. Some of them said to us, “What about me?” We decided not to try to do anything retrospectively. The plan was to build up enough money so that we could handle no-cost tuition going forward. We didn’t really have it in our pocketbook to help people who’d already paid their debts or were saddled with NYU debt. Is it fair? No, it’s probably not fair, but it’s an improvement.
That’s what I want people to think about who are saying, “What about my medical school debt? What about my undergraduate plus medical school debt?” I think we should be grateful when efforts are being made to reduce very burdensome student loans that people have. It’s good to give that benefit and move it forward.
Does that mean no one should get anything unless everyone with any kind of debt from school is covered? I don’t think so. I don’t think that’s fair either.
It is possible that we could continue to agitate politically and say, let’s go after some of the health care debt. Let’s go after some of the things that are still driving people to have to work more than they would or to choose specialties that they really don’t want to be in because they have to make up that debt.
It doesn’t mean the last word has been said about the politics of debt relief or, for that matter, the price of going to medical school in the first place and trying to see whether that can be driven down.
I don’t think it’s right to say, “If I can’t benefit, given the huge burden that I’m carrying, then I’m not going to try to give relief to others.” I think we’re relieving debt to the extent that we can do it. The nation can afford it. Going forward is a good thing. It’s wrong to create those gigantic debts in the first place.
What are we going to do about the past? We may decide that we need some sort of forgiveness or reparations for loans that were built up for others going backwards. I wouldn’t hold hostage the future and our children to what was probably a very poor, unethical practice about saddling doctors and others in the past with huge debt.
I’m Art Caplan at the division of medical ethics at New York University Grossman School of Medicine. Thank you for watching.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I run the division of medical ethics at New York University Grossman School of Medicine.
Many of you know that President Biden created a loan forgiveness program, forgiving up to $10,000 against federal student loans, including graduate and undergraduate education. The Department of Education is supposed to provide up to $20,000 in debt cancellation to Pell Grant recipients who have loans that are held by the Department of Education. Borrowers can get this relief if their income is less than $125,000 for an individual or $250,000 for married couples.
Many people have looked at this and said, “Hey, wait a minute. I paid off my loans. I didn’t get any reimbursement. That isn’t fair.”
who often still have huge amounts of debt, and either because of the income limits or because they don’t qualify because this debt was accrued long in the past, they’re saying, “What about me? Don’t you want to give any relief to me?”
This is a topic near and dear to my heart because I happen to be at a medical school, NYU, that has decided for the two medical schools it runs – our main campus, NYU in Manhattan and NYU Langone out on Long Island – that we’re going to go tuition free. We’ve done it for a couple of years.
We did it because I think all the administrators and faculty understood the tremendous burden that debt poses on people who both carry forward their undergraduate debt and then have medical school debt. This really leads to very difficult situations – which we have great empathy for – about what specialty you’re going to go into, whether you have to moonlight, and how you’re going to manage a huge burden of debt.
Many people don’t have sympathy out in the public. They say doctors make a large amount of money and they live a nice lifestyle, so we’re not going to relieve their debt. The reality is that, whoever you are, short of Bill Gates or Elon Musk, having hundreds of thousands of dollars of debt is no easy task to live with and to work off.
Still, when we created free tuition at NYU for our medical school, there were many people who paid high tuition fees in the past. Some of them said to us, “What about me?” We decided not to try to do anything retrospectively. The plan was to build up enough money so that we could handle no-cost tuition going forward. We didn’t really have it in our pocketbook to help people who’d already paid their debts or were saddled with NYU debt. Is it fair? No, it’s probably not fair, but it’s an improvement.
That’s what I want people to think about who are saying, “What about my medical school debt? What about my undergraduate plus medical school debt?” I think we should be grateful when efforts are being made to reduce very burdensome student loans that people have. It’s good to give that benefit and move it forward.
Does that mean no one should get anything unless everyone with any kind of debt from school is covered? I don’t think so. I don’t think that’s fair either.
It is possible that we could continue to agitate politically and say, let’s go after some of the health care debt. Let’s go after some of the things that are still driving people to have to work more than they would or to choose specialties that they really don’t want to be in because they have to make up that debt.
It doesn’t mean the last word has been said about the politics of debt relief or, for that matter, the price of going to medical school in the first place and trying to see whether that can be driven down.
I don’t think it’s right to say, “If I can’t benefit, given the huge burden that I’m carrying, then I’m not going to try to give relief to others.” I think we’re relieving debt to the extent that we can do it. The nation can afford it. Going forward is a good thing. It’s wrong to create those gigantic debts in the first place.
What are we going to do about the past? We may decide that we need some sort of forgiveness or reparations for loans that were built up for others going backwards. I wouldn’t hold hostage the future and our children to what was probably a very poor, unethical practice about saddling doctors and others in the past with huge debt.
I’m Art Caplan at the division of medical ethics at New York University Grossman School of Medicine. Thank you for watching.
A version of this article first appeared on Medscape.com.
The marked contrast in pandemic outcomes between Japan and the United States
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
Tirzepatide’s benefits expand: Lean mass up, serum lipids down
STOCKHOLM – New insights into the benefits of treatment with the “twincretin” tirzepatide for people with overweight or obesity – with or without diabetes – come from new findings reported at the annual meeting of the European Association for the Study of Diabetes.
Additional results from the SURMOUNT-1 trial, which matched tirzepatide against placebo in people with overweight or obesity, provide further details on the favorable changes produced by 72 weeks of tirzepatide treatment on outcomes that included fat and lean mass, insulin sensitivity, and patient-reported outcomes related to functional health and well being, reported Ania M. Jastreboff, MD, PhD.
And results from a meta-analysis of six trials that compared tirzepatide (Mounjaro) against several different comparators in patients with type 2 diabetes further confirm the drug’s ability to reliably produce positive changes in blood lipids, especially by significantly lowering levels of triglycerides, LDL cholesterol, and very LDL (VLDL) cholesterol, said Thomas Karagiannis, MD, PhD, in a separate report at the meeting.
Tirzepatide works as an agonist on receptors for both the glucagonlike peptide–1 (GLP-1), and for the glucose-dependent insulinotropic polypeptide, and received Food and Drug Administration approval for treating people with type 2 diabetes in May 2022. On the basis of results from SURMOUNT-1, the FDA on Oct. 6 granted tirzepatide fast-track designation for a proposed labeling of the agent for treating people with overweight or obesity. This FDA decision will likely remain pending at least until results from a second trial in people with overweight or obesity but without diabetes, SURMOUNT-2, become available in 2023.
SURMOUNT-1 randomized 2,539 people with obesity or overweight and at least one weight-related complication to a weekly injection of tirzepatide or placebo for 72 weeks. The study’s primary efficacy endpoints were the average reduction in weight from baseline, and the percentage of people in each treatment arm achieving weight loss of at least 5% from baseline.
For both endpoints, the outcomes with tirzepatide significantly surpassed placebo effects. Average weight loss ranged from 15%-21% from baseline, depending on dose, compared with 3% on placebo. The rate of participants with at least a 5% weight loss ranged from 85% to 91%, compared with 35% with placebo, as reported in July 2022 in the New England Journal of Medicine.
Cutting fat mass, boosting lean mass
New results from the trial reported by Dr. Jastreboff included a cut in fat mass from 46.2% of total body mass at baseline to 38.5% after 72 weeks, compared with a change from 46.8% at baseline to 44.7% after 72 weeks in the placebo group. Concurrently, lean mass increased with tirzepatide treatment from 51.0% at baseline to 58.1% after 72 weeks.
Participants who received tirzepatide, compared with those who received placebo, had “proportionately greater decrease in fat mass and proportionately greater increase in lean mass” compared with those who received placebo, said Dr. Jastreboff, an endocrinologist and obesity medicine specialist with Yale Medicine in New Haven, Conn. “I was impressed by the amount of visceral fat lost.”
These effects translated into a significant reduction in fat mass-to-lean mass ratio among the people treated with tirzepatide, with the greatest reduction in those who lost at least 15% of their starting weight. In that subgroup the fat-to-lean mass ratio dropped from 0.94 at baseline to 0.64 after 72 weeks of treatment, she said.
Focus on diet quality
People treated with tirzepatide “eat so little food that we need to improve the quality of what they eat to protect their muscle,” commented Carel le Roux, MBChB, PhD, a professor in the Diabetes Complications Research Centre of University College Dublin. “You no longer need a dietitian to help people lose weight, because the drug does that. You need dietitians to look after the nutritional health of patients while they lose weight,” Dr. le Roux said in a separate session at the meeting.
Additional tests showed that blood glucose and insulin levels were all significantly lower among trial participants on all three doses of tirzepatide compared with those on placebo, and the tirzepatide-treated subjects also had significant, roughly twofold elevations in their insulin sensitivity measured by the Matsuda Index.
The impact of tirzepatide on glucose and insulin levels and on insulin sensitivity was similar regardless of whether study participants had normoglycemia or prediabetes at entry. By design, no study participants had diabetes.
The trial assessed patient-reported quality-of-life outcomes using the 36-Item Short Form Survey (SF-36). Participants had significant increases in all eight domains within the SF-36 at all three tirzepatide doses, compared with placebo, at 72 weeks, Dr. Jastreboff reported. Improvements in the physical function domain increased most notably among study participants on tirzepatide who had functional limitations at baseline. Heart rate rose among participants who received either of the two highest tirzepatide doses by 2.3-2.5 beats/min, comparable with the effect of other injected incretin-based treatments.
Lipids improve in those with type 2 diabetes
Tirzepatide treatment also results in a “secondary effect” of improving levels of several lipids in people with type 2 diabetes, according to a meta-analysis of findings from six randomized trials. The meta-analysis collectively involved 4,502 participants treated for numerous weeks with one of three doses of tirzepatide and 2,144 people in comparator groups, reported Dr. Karagiannis, a diabetes researcher at Aristotle University of Thessaloniki (Greece).
Among the significant lipid changes linked with tirzepatide treatment, compared with placebo, were an average 13 mg/dL decrease in LDL cholesterol, an average 6 mg/dL decrease in VLDL cholesterol, and an average 50 mg/dL decrease in triglycerides. In comparison to a GLP-1 receptor agonist, an average 25 mg/dL decrease in triglycerides and an average 4 mg/dL reduction in VLDL cholesterol were seen. And trials comparing tirzepatide with basal insulin saw average reductions of 7% in LDL cholesterol, 15% in VLDL cholesterol, 15% in triglycerides, and an 8% increase in HDL cholesterol.
Dr. Karagiannis highlighted that the clinical impact of these effects is unclear, although he noted that the average reduction in LDL cholesterol relative to placebo is of a magnitude that could have a modest effect on long-term outcomes.
These lipid effects of tirzepatide “should be considered alongside” tirzepatide’s “key metabolic effects” on weight and hemoglobin A1c as well as the drug’s safety, concluded Dr. Karagiannis.
The tirzepatide trials were all funded by Eli Lilly, which markets tirzepatide (Mounjaro). Dr. Jastreboff has been an adviser and consultant to Eli Lilly, as well as to Intellihealth, Novo Nordisk, Pfizer, Rhythm Scholars, Roche, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Karagiannis had no disclosures. Dr. le Roux has had financial relationships with Eli Lilly, as well as with Boehringer Ingelheim, Consilient Health, Covidion, Fractyl, GL Dynamics, Herbalife, Johnson & Johnson, Keyron, and Novo Nordisk.
STOCKHOLM – New insights into the benefits of treatment with the “twincretin” tirzepatide for people with overweight or obesity – with or without diabetes – come from new findings reported at the annual meeting of the European Association for the Study of Diabetes.
Additional results from the SURMOUNT-1 trial, which matched tirzepatide against placebo in people with overweight or obesity, provide further details on the favorable changes produced by 72 weeks of tirzepatide treatment on outcomes that included fat and lean mass, insulin sensitivity, and patient-reported outcomes related to functional health and well being, reported Ania M. Jastreboff, MD, PhD.
And results from a meta-analysis of six trials that compared tirzepatide (Mounjaro) against several different comparators in patients with type 2 diabetes further confirm the drug’s ability to reliably produce positive changes in blood lipids, especially by significantly lowering levels of triglycerides, LDL cholesterol, and very LDL (VLDL) cholesterol, said Thomas Karagiannis, MD, PhD, in a separate report at the meeting.
Tirzepatide works as an agonist on receptors for both the glucagonlike peptide–1 (GLP-1), and for the glucose-dependent insulinotropic polypeptide, and received Food and Drug Administration approval for treating people with type 2 diabetes in May 2022. On the basis of results from SURMOUNT-1, the FDA on Oct. 6 granted tirzepatide fast-track designation for a proposed labeling of the agent for treating people with overweight or obesity. This FDA decision will likely remain pending at least until results from a second trial in people with overweight or obesity but without diabetes, SURMOUNT-2, become available in 2023.
SURMOUNT-1 randomized 2,539 people with obesity or overweight and at least one weight-related complication to a weekly injection of tirzepatide or placebo for 72 weeks. The study’s primary efficacy endpoints were the average reduction in weight from baseline, and the percentage of people in each treatment arm achieving weight loss of at least 5% from baseline.
For both endpoints, the outcomes with tirzepatide significantly surpassed placebo effects. Average weight loss ranged from 15%-21% from baseline, depending on dose, compared with 3% on placebo. The rate of participants with at least a 5% weight loss ranged from 85% to 91%, compared with 35% with placebo, as reported in July 2022 in the New England Journal of Medicine.
Cutting fat mass, boosting lean mass
New results from the trial reported by Dr. Jastreboff included a cut in fat mass from 46.2% of total body mass at baseline to 38.5% after 72 weeks, compared with a change from 46.8% at baseline to 44.7% after 72 weeks in the placebo group. Concurrently, lean mass increased with tirzepatide treatment from 51.0% at baseline to 58.1% after 72 weeks.
Participants who received tirzepatide, compared with those who received placebo, had “proportionately greater decrease in fat mass and proportionately greater increase in lean mass” compared with those who received placebo, said Dr. Jastreboff, an endocrinologist and obesity medicine specialist with Yale Medicine in New Haven, Conn. “I was impressed by the amount of visceral fat lost.”
These effects translated into a significant reduction in fat mass-to-lean mass ratio among the people treated with tirzepatide, with the greatest reduction in those who lost at least 15% of their starting weight. In that subgroup the fat-to-lean mass ratio dropped from 0.94 at baseline to 0.64 after 72 weeks of treatment, she said.
Focus on diet quality
People treated with tirzepatide “eat so little food that we need to improve the quality of what they eat to protect their muscle,” commented Carel le Roux, MBChB, PhD, a professor in the Diabetes Complications Research Centre of University College Dublin. “You no longer need a dietitian to help people lose weight, because the drug does that. You need dietitians to look after the nutritional health of patients while they lose weight,” Dr. le Roux said in a separate session at the meeting.
Additional tests showed that blood glucose and insulin levels were all significantly lower among trial participants on all three doses of tirzepatide compared with those on placebo, and the tirzepatide-treated subjects also had significant, roughly twofold elevations in their insulin sensitivity measured by the Matsuda Index.
The impact of tirzepatide on glucose and insulin levels and on insulin sensitivity was similar regardless of whether study participants had normoglycemia or prediabetes at entry. By design, no study participants had diabetes.
The trial assessed patient-reported quality-of-life outcomes using the 36-Item Short Form Survey (SF-36). Participants had significant increases in all eight domains within the SF-36 at all three tirzepatide doses, compared with placebo, at 72 weeks, Dr. Jastreboff reported. Improvements in the physical function domain increased most notably among study participants on tirzepatide who had functional limitations at baseline. Heart rate rose among participants who received either of the two highest tirzepatide doses by 2.3-2.5 beats/min, comparable with the effect of other injected incretin-based treatments.
Lipids improve in those with type 2 diabetes
Tirzepatide treatment also results in a “secondary effect” of improving levels of several lipids in people with type 2 diabetes, according to a meta-analysis of findings from six randomized trials. The meta-analysis collectively involved 4,502 participants treated for numerous weeks with one of three doses of tirzepatide and 2,144 people in comparator groups, reported Dr. Karagiannis, a diabetes researcher at Aristotle University of Thessaloniki (Greece).
Among the significant lipid changes linked with tirzepatide treatment, compared with placebo, were an average 13 mg/dL decrease in LDL cholesterol, an average 6 mg/dL decrease in VLDL cholesterol, and an average 50 mg/dL decrease in triglycerides. In comparison to a GLP-1 receptor agonist, an average 25 mg/dL decrease in triglycerides and an average 4 mg/dL reduction in VLDL cholesterol were seen. And trials comparing tirzepatide with basal insulin saw average reductions of 7% in LDL cholesterol, 15% in VLDL cholesterol, 15% in triglycerides, and an 8% increase in HDL cholesterol.
Dr. Karagiannis highlighted that the clinical impact of these effects is unclear, although he noted that the average reduction in LDL cholesterol relative to placebo is of a magnitude that could have a modest effect on long-term outcomes.
These lipid effects of tirzepatide “should be considered alongside” tirzepatide’s “key metabolic effects” on weight and hemoglobin A1c as well as the drug’s safety, concluded Dr. Karagiannis.
The tirzepatide trials were all funded by Eli Lilly, which markets tirzepatide (Mounjaro). Dr. Jastreboff has been an adviser and consultant to Eli Lilly, as well as to Intellihealth, Novo Nordisk, Pfizer, Rhythm Scholars, Roche, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Karagiannis had no disclosures. Dr. le Roux has had financial relationships with Eli Lilly, as well as with Boehringer Ingelheim, Consilient Health, Covidion, Fractyl, GL Dynamics, Herbalife, Johnson & Johnson, Keyron, and Novo Nordisk.
STOCKHOLM – New insights into the benefits of treatment with the “twincretin” tirzepatide for people with overweight or obesity – with or without diabetes – come from new findings reported at the annual meeting of the European Association for the Study of Diabetes.
Additional results from the SURMOUNT-1 trial, which matched tirzepatide against placebo in people with overweight or obesity, provide further details on the favorable changes produced by 72 weeks of tirzepatide treatment on outcomes that included fat and lean mass, insulin sensitivity, and patient-reported outcomes related to functional health and well being, reported Ania M. Jastreboff, MD, PhD.
And results from a meta-analysis of six trials that compared tirzepatide (Mounjaro) against several different comparators in patients with type 2 diabetes further confirm the drug’s ability to reliably produce positive changes in blood lipids, especially by significantly lowering levels of triglycerides, LDL cholesterol, and very LDL (VLDL) cholesterol, said Thomas Karagiannis, MD, PhD, in a separate report at the meeting.
Tirzepatide works as an agonist on receptors for both the glucagonlike peptide–1 (GLP-1), and for the glucose-dependent insulinotropic polypeptide, and received Food and Drug Administration approval for treating people with type 2 diabetes in May 2022. On the basis of results from SURMOUNT-1, the FDA on Oct. 6 granted tirzepatide fast-track designation for a proposed labeling of the agent for treating people with overweight or obesity. This FDA decision will likely remain pending at least until results from a second trial in people with overweight or obesity but without diabetes, SURMOUNT-2, become available in 2023.
SURMOUNT-1 randomized 2,539 people with obesity or overweight and at least one weight-related complication to a weekly injection of tirzepatide or placebo for 72 weeks. The study’s primary efficacy endpoints were the average reduction in weight from baseline, and the percentage of people in each treatment arm achieving weight loss of at least 5% from baseline.
For both endpoints, the outcomes with tirzepatide significantly surpassed placebo effects. Average weight loss ranged from 15%-21% from baseline, depending on dose, compared with 3% on placebo. The rate of participants with at least a 5% weight loss ranged from 85% to 91%, compared with 35% with placebo, as reported in July 2022 in the New England Journal of Medicine.
Cutting fat mass, boosting lean mass
New results from the trial reported by Dr. Jastreboff included a cut in fat mass from 46.2% of total body mass at baseline to 38.5% after 72 weeks, compared with a change from 46.8% at baseline to 44.7% after 72 weeks in the placebo group. Concurrently, lean mass increased with tirzepatide treatment from 51.0% at baseline to 58.1% after 72 weeks.
Participants who received tirzepatide, compared with those who received placebo, had “proportionately greater decrease in fat mass and proportionately greater increase in lean mass” compared with those who received placebo, said Dr. Jastreboff, an endocrinologist and obesity medicine specialist with Yale Medicine in New Haven, Conn. “I was impressed by the amount of visceral fat lost.”
These effects translated into a significant reduction in fat mass-to-lean mass ratio among the people treated with tirzepatide, with the greatest reduction in those who lost at least 15% of their starting weight. In that subgroup the fat-to-lean mass ratio dropped from 0.94 at baseline to 0.64 after 72 weeks of treatment, she said.
Focus on diet quality
People treated with tirzepatide “eat so little food that we need to improve the quality of what they eat to protect their muscle,” commented Carel le Roux, MBChB, PhD, a professor in the Diabetes Complications Research Centre of University College Dublin. “You no longer need a dietitian to help people lose weight, because the drug does that. You need dietitians to look after the nutritional health of patients while they lose weight,” Dr. le Roux said in a separate session at the meeting.
Additional tests showed that blood glucose and insulin levels were all significantly lower among trial participants on all three doses of tirzepatide compared with those on placebo, and the tirzepatide-treated subjects also had significant, roughly twofold elevations in their insulin sensitivity measured by the Matsuda Index.
The impact of tirzepatide on glucose and insulin levels and on insulin sensitivity was similar regardless of whether study participants had normoglycemia or prediabetes at entry. By design, no study participants had diabetes.
The trial assessed patient-reported quality-of-life outcomes using the 36-Item Short Form Survey (SF-36). Participants had significant increases in all eight domains within the SF-36 at all three tirzepatide doses, compared with placebo, at 72 weeks, Dr. Jastreboff reported. Improvements in the physical function domain increased most notably among study participants on tirzepatide who had functional limitations at baseline. Heart rate rose among participants who received either of the two highest tirzepatide doses by 2.3-2.5 beats/min, comparable with the effect of other injected incretin-based treatments.
Lipids improve in those with type 2 diabetes
Tirzepatide treatment also results in a “secondary effect” of improving levels of several lipids in people with type 2 diabetes, according to a meta-analysis of findings from six randomized trials. The meta-analysis collectively involved 4,502 participants treated for numerous weeks with one of three doses of tirzepatide and 2,144 people in comparator groups, reported Dr. Karagiannis, a diabetes researcher at Aristotle University of Thessaloniki (Greece).
Among the significant lipid changes linked with tirzepatide treatment, compared with placebo, were an average 13 mg/dL decrease in LDL cholesterol, an average 6 mg/dL decrease in VLDL cholesterol, and an average 50 mg/dL decrease in triglycerides. In comparison to a GLP-1 receptor agonist, an average 25 mg/dL decrease in triglycerides and an average 4 mg/dL reduction in VLDL cholesterol were seen. And trials comparing tirzepatide with basal insulin saw average reductions of 7% in LDL cholesterol, 15% in VLDL cholesterol, 15% in triglycerides, and an 8% increase in HDL cholesterol.
Dr. Karagiannis highlighted that the clinical impact of these effects is unclear, although he noted that the average reduction in LDL cholesterol relative to placebo is of a magnitude that could have a modest effect on long-term outcomes.
These lipid effects of tirzepatide “should be considered alongside” tirzepatide’s “key metabolic effects” on weight and hemoglobin A1c as well as the drug’s safety, concluded Dr. Karagiannis.
The tirzepatide trials were all funded by Eli Lilly, which markets tirzepatide (Mounjaro). Dr. Jastreboff has been an adviser and consultant to Eli Lilly, as well as to Intellihealth, Novo Nordisk, Pfizer, Rhythm Scholars, Roche, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Karagiannis had no disclosures. Dr. le Roux has had financial relationships with Eli Lilly, as well as with Boehringer Ingelheim, Consilient Health, Covidion, Fractyl, GL Dynamics, Herbalife, Johnson & Johnson, Keyron, and Novo Nordisk.
AT EASD 2022
Playing the fat shame game in medicine: It needs to stop
I will remember that there is art to medicine as well as science, and that warmth, sympathy, and understanding may outweigh the surgeon’s knife or the chemist’s drug.
Upon finishing medical school, many of us recited this passage from a modernized version of the Hippocratic Oath. Though there has been controversy regarding the current relevancy of this oath, it can still serve as a reminder of the promises we made on behalf of our patients: To treat them ethically, with empathy and respect, and without pretension. Though I hadn’t thought about the Hippocratic Oath in ages, it came to mind recently after I read an article about weight trends in adults during the COVID pandemic.
No surprise – we gained weight during the initial surge at a rate of roughly a pound and a half per month following the initial shelter-in-place period. For some of us, that trend in weight gain worsened as the pandemic persisted. A survey conducted in February 2021 suggested that over 40% of adults who experienced undesired weight changes since the start of the pandemic gained an average of 29 pounds (significantly more than the typical gain of 15 pounds, often referred to as the “Quarantine 15” or “COVID-15”).
Updated data, obtained via a review of electronic health records for over 15 million patients, shows that 39% of patients gained weight during the pandemic (10% of them gained more than 12.5 pounds, while 2% gained over 27.5 pounds). Though these recent numbers may be lower than previously reported, they still aren’t reassuring.
Research has already confirmed that sizeism has a negative impact on both a patient’s physical health and psychological well-being, and as medical providers, we’re part of the problem. We cause distress in our patients through disrespectful treatment and medical fat shaming, which can lead to cycles of disordered eating, reduced physical activity, and more weight gain. We discriminate based on weight, causing our patients to delay health care visits and other provider interactions, resulting in increased risks for morbidity and even mortality. We make assumptions that a patient’s presenting complaints are due to weight rather than other causes, resulting in missed diagnoses. And we recommend different treatments for obese patients with the same condition as nonobese patients simply because of their weight.
One study has suggested that over 40% of adults in the United States have suffered from weight stigma, and physicians and coworkers are listed as some of the most common sources. Another study suggests that nearly 70% of overweight or obese patients report feeling stigmatized by physicians, whether through expressed biases or purposeful avoidance (patients have previously reported that their providers addressed weight loss in fewer than 20% of their examinations).
As health care providers, we need to do better. We should all be willing to consider our own biases about body size, and there are self-assessments to help with this, including the Implicit Associations Test: Weight Bias. By becoming more self-aware, hopefully we can change the doctor-patient conversation about weight management.
Studies have shown that meaningful conversations with physicians can have a significant impact on patients’ attempts to change behaviors related to weight. Yet, many medical providers are not trained in how to counsel patients on nutrition, weight loss, and physical activity (if we bring it up at all). We need to better educate ourselves about weight science and treatments.
In the meantime, we can work on how we interact with our patients:
- Make sure that your practice space is accommodating and nondiscriminatory, with appropriately sized furniture in the waiting and exam rooms, large blood pressure cuffs and gowns, and size-inclusive reading materials.
- Ensure that your workplace has an antiharassment policy that includes sizeism.
- Be an ally and speak up against weight discrimination.
- Educate your office staff about weight stigma and ensure that they avoid commenting on the weight or body size of others (being recognized only for losing weight isn’t a compliment, and sharing “fat jokes” isn’t funny).
- Remember that a person’s body size tells you nothing about that person’s health behaviors. Stop assuming that larger body sizes are related to laziness, overeating, or a lack of motivation.
- Ask your overweight or obese patients if they are willing to talk about their weight before jumping into the topic.
- Practice (patients are more likely to report changing their exercise routine and attempting to lose weight with these techniques).
- Be mindful of your word choices; for example, it can be more helpful to focus on comorbidities (such as high blood pressure or prediabetes) rather than body weight, nutrition rather than dieting, and physical activity rather than specific exercises.
Regardless of how you feel about reciting the Hippocratic Oath, our patients, no matter their body size, deserve to be treated with respect and dignity, as others have said in more eloquent ways than I. Let’s stop playing the fat shame game and help fight weight bias in medicine.
Dr. Devlin is president, Locum Infectious Disease Services, and an independent contractor for Weatherby Healthcare. She reported no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I will remember that there is art to medicine as well as science, and that warmth, sympathy, and understanding may outweigh the surgeon’s knife or the chemist’s drug.
Upon finishing medical school, many of us recited this passage from a modernized version of the Hippocratic Oath. Though there has been controversy regarding the current relevancy of this oath, it can still serve as a reminder of the promises we made on behalf of our patients: To treat them ethically, with empathy and respect, and without pretension. Though I hadn’t thought about the Hippocratic Oath in ages, it came to mind recently after I read an article about weight trends in adults during the COVID pandemic.
No surprise – we gained weight during the initial surge at a rate of roughly a pound and a half per month following the initial shelter-in-place period. For some of us, that trend in weight gain worsened as the pandemic persisted. A survey conducted in February 2021 suggested that over 40% of adults who experienced undesired weight changes since the start of the pandemic gained an average of 29 pounds (significantly more than the typical gain of 15 pounds, often referred to as the “Quarantine 15” or “COVID-15”).
Updated data, obtained via a review of electronic health records for over 15 million patients, shows that 39% of patients gained weight during the pandemic (10% of them gained more than 12.5 pounds, while 2% gained over 27.5 pounds). Though these recent numbers may be lower than previously reported, they still aren’t reassuring.
Research has already confirmed that sizeism has a negative impact on both a patient’s physical health and psychological well-being, and as medical providers, we’re part of the problem. We cause distress in our patients through disrespectful treatment and medical fat shaming, which can lead to cycles of disordered eating, reduced physical activity, and more weight gain. We discriminate based on weight, causing our patients to delay health care visits and other provider interactions, resulting in increased risks for morbidity and even mortality. We make assumptions that a patient’s presenting complaints are due to weight rather than other causes, resulting in missed diagnoses. And we recommend different treatments for obese patients with the same condition as nonobese patients simply because of their weight.
One study has suggested that over 40% of adults in the United States have suffered from weight stigma, and physicians and coworkers are listed as some of the most common sources. Another study suggests that nearly 70% of overweight or obese patients report feeling stigmatized by physicians, whether through expressed biases or purposeful avoidance (patients have previously reported that their providers addressed weight loss in fewer than 20% of their examinations).
As health care providers, we need to do better. We should all be willing to consider our own biases about body size, and there are self-assessments to help with this, including the Implicit Associations Test: Weight Bias. By becoming more self-aware, hopefully we can change the doctor-patient conversation about weight management.
Studies have shown that meaningful conversations with physicians can have a significant impact on patients’ attempts to change behaviors related to weight. Yet, many medical providers are not trained in how to counsel patients on nutrition, weight loss, and physical activity (if we bring it up at all). We need to better educate ourselves about weight science and treatments.
In the meantime, we can work on how we interact with our patients:
- Make sure that your practice space is accommodating and nondiscriminatory, with appropriately sized furniture in the waiting and exam rooms, large blood pressure cuffs and gowns, and size-inclusive reading materials.
- Ensure that your workplace has an antiharassment policy that includes sizeism.
- Be an ally and speak up against weight discrimination.
- Educate your office staff about weight stigma and ensure that they avoid commenting on the weight or body size of others (being recognized only for losing weight isn’t a compliment, and sharing “fat jokes” isn’t funny).
- Remember that a person’s body size tells you nothing about that person’s health behaviors. Stop assuming that larger body sizes are related to laziness, overeating, or a lack of motivation.
- Ask your overweight or obese patients if they are willing to talk about their weight before jumping into the topic.
- Practice (patients are more likely to report changing their exercise routine and attempting to lose weight with these techniques).
- Be mindful of your word choices; for example, it can be more helpful to focus on comorbidities (such as high blood pressure or prediabetes) rather than body weight, nutrition rather than dieting, and physical activity rather than specific exercises.
Regardless of how you feel about reciting the Hippocratic Oath, our patients, no matter their body size, deserve to be treated with respect and dignity, as others have said in more eloquent ways than I. Let’s stop playing the fat shame game and help fight weight bias in medicine.
Dr. Devlin is president, Locum Infectious Disease Services, and an independent contractor for Weatherby Healthcare. She reported no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I will remember that there is art to medicine as well as science, and that warmth, sympathy, and understanding may outweigh the surgeon’s knife or the chemist’s drug.
Upon finishing medical school, many of us recited this passage from a modernized version of the Hippocratic Oath. Though there has been controversy regarding the current relevancy of this oath, it can still serve as a reminder of the promises we made on behalf of our patients: To treat them ethically, with empathy and respect, and without pretension. Though I hadn’t thought about the Hippocratic Oath in ages, it came to mind recently after I read an article about weight trends in adults during the COVID pandemic.
No surprise – we gained weight during the initial surge at a rate of roughly a pound and a half per month following the initial shelter-in-place period. For some of us, that trend in weight gain worsened as the pandemic persisted. A survey conducted in February 2021 suggested that over 40% of adults who experienced undesired weight changes since the start of the pandemic gained an average of 29 pounds (significantly more than the typical gain of 15 pounds, often referred to as the “Quarantine 15” or “COVID-15”).
Updated data, obtained via a review of electronic health records for over 15 million patients, shows that 39% of patients gained weight during the pandemic (10% of them gained more than 12.5 pounds, while 2% gained over 27.5 pounds). Though these recent numbers may be lower than previously reported, they still aren’t reassuring.
Research has already confirmed that sizeism has a negative impact on both a patient’s physical health and psychological well-being, and as medical providers, we’re part of the problem. We cause distress in our patients through disrespectful treatment and medical fat shaming, which can lead to cycles of disordered eating, reduced physical activity, and more weight gain. We discriminate based on weight, causing our patients to delay health care visits and other provider interactions, resulting in increased risks for morbidity and even mortality. We make assumptions that a patient’s presenting complaints are due to weight rather than other causes, resulting in missed diagnoses. And we recommend different treatments for obese patients with the same condition as nonobese patients simply because of their weight.
One study has suggested that over 40% of adults in the United States have suffered from weight stigma, and physicians and coworkers are listed as some of the most common sources. Another study suggests that nearly 70% of overweight or obese patients report feeling stigmatized by physicians, whether through expressed biases or purposeful avoidance (patients have previously reported that their providers addressed weight loss in fewer than 20% of their examinations).
As health care providers, we need to do better. We should all be willing to consider our own biases about body size, and there are self-assessments to help with this, including the Implicit Associations Test: Weight Bias. By becoming more self-aware, hopefully we can change the doctor-patient conversation about weight management.
Studies have shown that meaningful conversations with physicians can have a significant impact on patients’ attempts to change behaviors related to weight. Yet, many medical providers are not trained in how to counsel patients on nutrition, weight loss, and physical activity (if we bring it up at all). We need to better educate ourselves about weight science and treatments.
In the meantime, we can work on how we interact with our patients:
- Make sure that your practice space is accommodating and nondiscriminatory, with appropriately sized furniture in the waiting and exam rooms, large blood pressure cuffs and gowns, and size-inclusive reading materials.
- Ensure that your workplace has an antiharassment policy that includes sizeism.
- Be an ally and speak up against weight discrimination.
- Educate your office staff about weight stigma and ensure that they avoid commenting on the weight or body size of others (being recognized only for losing weight isn’t a compliment, and sharing “fat jokes” isn’t funny).
- Remember that a person’s body size tells you nothing about that person’s health behaviors. Stop assuming that larger body sizes are related to laziness, overeating, or a lack of motivation.
- Ask your overweight or obese patients if they are willing to talk about their weight before jumping into the topic.
- Practice (patients are more likely to report changing their exercise routine and attempting to lose weight with these techniques).
- Be mindful of your word choices; for example, it can be more helpful to focus on comorbidities (such as high blood pressure or prediabetes) rather than body weight, nutrition rather than dieting, and physical activity rather than specific exercises.
Regardless of how you feel about reciting the Hippocratic Oath, our patients, no matter their body size, deserve to be treated with respect and dignity, as others have said in more eloquent ways than I. Let’s stop playing the fat shame game and help fight weight bias in medicine.
Dr. Devlin is president, Locum Infectious Disease Services, and an independent contractor for Weatherby Healthcare. She reported no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
Headache for inpatients with COVID-19 may predict better survival
, according to recent research published in the journal Headache.
In the systematic review and meta-analysis, Víctor J. Gallardo, MSc, of the headache and neurologic pain research group, Vall d’Hebron Research Institute at the Universitat Autònoma de Barcelona, and colleagues performed a search of studies in PubMed involving headache symptoms, disease survival, and inpatient COVID-19 cases published between December 2019 and December 2020. Overall, 48 studies were identified, consisting of 43,169 inpatients with COVID-19. Using random-effects pooling models, Mr. Gallardo and colleagues estimated the prevalence of headache for inpatients who survived COVID-19, compared with those who did not survive.
Within those studies, 35,132 inpatients (81.4%) survived, while 8,037 inpatients (18.6%) died from COVID-19. The researchers found that inpatients with COVID-19 and headache symptoms had a significantly higher survival rate compared with inpatients with COVID-19 without headache symptoms (risk ratio, 1.90; 95% confidence interval, 1.46-2.47; P < .0001). There was an overall pooled prevalence of headache as a COVID-19 symptom in 10.4% of inpatients, which was reduced to an estimated pooled prevalence of 9.7% after the researchers removed outlier studies in a sensitivity analysis.
Other COVID-19 symptoms that led to improved rates of survival among inpatients were anosmia (RR, 2.94; 95% CI, 1.94-4.45) and myalgia (RR, 1.57; 95% CI, 1.34-1.83) as well as nausea or vomiting (RR, 1.41; 95% CI, 1.08-1.82), while symptoms such as dyspnea, diabetes, chronic liver diseases, chronic respiratory diseases, and chronic kidney diseases were more likely to increase the risk of dying from COVID-19.
The researchers noted several limitations in their meta-analysis that may make their findings less generalizable to future SARS-CoV-2 variants, such as including only studies that were published before COVID-19 vaccines were available and before more infectious SARS-CoV-2 variants like the B.1.617.2 (Delta) variant emerged. They also included studies where inpatients were not tested for COVID-19 because access to testing was not widely available.
“Our meta-analysis points toward a novel possibility: Headache arising secondary to an infection is not a ‘nonspecific’ symptom, but rather it may be a marker of enhanced likelihood of survival. That is, we find that patients reporting headache in the setting of COVID-19 are at reduced risk of death,” Mr. Gallardo and colleagues wrote.
More data needed on association between headache and COVID-19
While headache appeared to affect a small proportion of overall inpatients with COVID-19, the researchers noted this might be because individuals with COVID-19 and headache symptoms are less likely to require hospitalization or a visit to the ED. Another potential explanation is that “people with primary headache disorders, including migraine, may be more likely to report symptoms of COVID-19, but they also may be relatively less likely to experience a life-threatening COVID-19 disease course.”
The researchers said this potential association should be explored in future studies as well as in other viral infections or postviral syndromes such as long COVID. “Defining specific headache mechanisms that could enhance survival from viral infections represents an opportunity for the potential discovery of improved viral therapeutics, as well as for understanding whether, and how, primary headache disorders may be adaptive.”
In a comment, Morris Levin, MD, director of the University of California San Francisco Headache Center, said the findings “of this very thought-provoking review suggest that reporting a headache during a COVID-19 infection seems to be associated with better recovery in hospitalized patients.”
Dr. Levin, who was not involved with the study, acknowledged the researchers’ explanation for the overall low rate of headache in these inpatients as one possible explanation.
“Another could be that sick COVID patients were much more troubled by other symptoms like respiratory distress, which overshadowed their headache symptoms, particularly if they were very ill or if the headache pain was of only mild to moderate severity,” he said. “That could also be an alternate explanation for why less dangerously ill hospitalized patients seemed to have more headaches.”
One limitation he saw in the meta-analysis was how clearly the clinicians characterized headache symptoms in each reviewed study. Dr. Levin suggested a retrospective assessment of premorbid migraine history in hospitalized patients with COVID-19, including survivors and fatalities, might have helped clarify this issue. “The headaches themselves were not characterized so drawing conclusions regarding migraine is challenging.”
Dr. Levin noted it is still not well understood how acute and persistent headaches and other neurological symptoms like mental fog occur in patients with COVID-19. We also do not fully understand the natural history of post-COVID headaches and other neurologic sequelae and the management options for acute and persistent neurological sequelae.
Three authors reported personal and institutional relationships in the form of grants, consultancies, speaker’s bureau positions, guidelines committee member appointments, and editorial board positions for a variety of pharmaceutical companies, agencies, societies, and other organizations. Mr. Gallardo reported no relevant financial disclosures. Dr. Levin reported no relevant financial disclosures.
, according to recent research published in the journal Headache.
In the systematic review and meta-analysis, Víctor J. Gallardo, MSc, of the headache and neurologic pain research group, Vall d’Hebron Research Institute at the Universitat Autònoma de Barcelona, and colleagues performed a search of studies in PubMed involving headache symptoms, disease survival, and inpatient COVID-19 cases published between December 2019 and December 2020. Overall, 48 studies were identified, consisting of 43,169 inpatients with COVID-19. Using random-effects pooling models, Mr. Gallardo and colleagues estimated the prevalence of headache for inpatients who survived COVID-19, compared with those who did not survive.
Within those studies, 35,132 inpatients (81.4%) survived, while 8,037 inpatients (18.6%) died from COVID-19. The researchers found that inpatients with COVID-19 and headache symptoms had a significantly higher survival rate compared with inpatients with COVID-19 without headache symptoms (risk ratio, 1.90; 95% confidence interval, 1.46-2.47; P < .0001). There was an overall pooled prevalence of headache as a COVID-19 symptom in 10.4% of inpatients, which was reduced to an estimated pooled prevalence of 9.7% after the researchers removed outlier studies in a sensitivity analysis.
Other COVID-19 symptoms that led to improved rates of survival among inpatients were anosmia (RR, 2.94; 95% CI, 1.94-4.45) and myalgia (RR, 1.57; 95% CI, 1.34-1.83) as well as nausea or vomiting (RR, 1.41; 95% CI, 1.08-1.82), while symptoms such as dyspnea, diabetes, chronic liver diseases, chronic respiratory diseases, and chronic kidney diseases were more likely to increase the risk of dying from COVID-19.
The researchers noted several limitations in their meta-analysis that may make their findings less generalizable to future SARS-CoV-2 variants, such as including only studies that were published before COVID-19 vaccines were available and before more infectious SARS-CoV-2 variants like the B.1.617.2 (Delta) variant emerged. They also included studies where inpatients were not tested for COVID-19 because access to testing was not widely available.
“Our meta-analysis points toward a novel possibility: Headache arising secondary to an infection is not a ‘nonspecific’ symptom, but rather it may be a marker of enhanced likelihood of survival. That is, we find that patients reporting headache in the setting of COVID-19 are at reduced risk of death,” Mr. Gallardo and colleagues wrote.
More data needed on association between headache and COVID-19
While headache appeared to affect a small proportion of overall inpatients with COVID-19, the researchers noted this might be because individuals with COVID-19 and headache symptoms are less likely to require hospitalization or a visit to the ED. Another potential explanation is that “people with primary headache disorders, including migraine, may be more likely to report symptoms of COVID-19, but they also may be relatively less likely to experience a life-threatening COVID-19 disease course.”
The researchers said this potential association should be explored in future studies as well as in other viral infections or postviral syndromes such as long COVID. “Defining specific headache mechanisms that could enhance survival from viral infections represents an opportunity for the potential discovery of improved viral therapeutics, as well as for understanding whether, and how, primary headache disorders may be adaptive.”
In a comment, Morris Levin, MD, director of the University of California San Francisco Headache Center, said the findings “of this very thought-provoking review suggest that reporting a headache during a COVID-19 infection seems to be associated with better recovery in hospitalized patients.”
Dr. Levin, who was not involved with the study, acknowledged the researchers’ explanation for the overall low rate of headache in these inpatients as one possible explanation.
“Another could be that sick COVID patients were much more troubled by other symptoms like respiratory distress, which overshadowed their headache symptoms, particularly if they were very ill or if the headache pain was of only mild to moderate severity,” he said. “That could also be an alternate explanation for why less dangerously ill hospitalized patients seemed to have more headaches.”
One limitation he saw in the meta-analysis was how clearly the clinicians characterized headache symptoms in each reviewed study. Dr. Levin suggested a retrospective assessment of premorbid migraine history in hospitalized patients with COVID-19, including survivors and fatalities, might have helped clarify this issue. “The headaches themselves were not characterized so drawing conclusions regarding migraine is challenging.”
Dr. Levin noted it is still not well understood how acute and persistent headaches and other neurological symptoms like mental fog occur in patients with COVID-19. We also do not fully understand the natural history of post-COVID headaches and other neurologic sequelae and the management options for acute and persistent neurological sequelae.
Three authors reported personal and institutional relationships in the form of grants, consultancies, speaker’s bureau positions, guidelines committee member appointments, and editorial board positions for a variety of pharmaceutical companies, agencies, societies, and other organizations. Mr. Gallardo reported no relevant financial disclosures. Dr. Levin reported no relevant financial disclosures.
, according to recent research published in the journal Headache.
In the systematic review and meta-analysis, Víctor J. Gallardo, MSc, of the headache and neurologic pain research group, Vall d’Hebron Research Institute at the Universitat Autònoma de Barcelona, and colleagues performed a search of studies in PubMed involving headache symptoms, disease survival, and inpatient COVID-19 cases published between December 2019 and December 2020. Overall, 48 studies were identified, consisting of 43,169 inpatients with COVID-19. Using random-effects pooling models, Mr. Gallardo and colleagues estimated the prevalence of headache for inpatients who survived COVID-19, compared with those who did not survive.
Within those studies, 35,132 inpatients (81.4%) survived, while 8,037 inpatients (18.6%) died from COVID-19. The researchers found that inpatients with COVID-19 and headache symptoms had a significantly higher survival rate compared with inpatients with COVID-19 without headache symptoms (risk ratio, 1.90; 95% confidence interval, 1.46-2.47; P < .0001). There was an overall pooled prevalence of headache as a COVID-19 symptom in 10.4% of inpatients, which was reduced to an estimated pooled prevalence of 9.7% after the researchers removed outlier studies in a sensitivity analysis.
Other COVID-19 symptoms that led to improved rates of survival among inpatients were anosmia (RR, 2.94; 95% CI, 1.94-4.45) and myalgia (RR, 1.57; 95% CI, 1.34-1.83) as well as nausea or vomiting (RR, 1.41; 95% CI, 1.08-1.82), while symptoms such as dyspnea, diabetes, chronic liver diseases, chronic respiratory diseases, and chronic kidney diseases were more likely to increase the risk of dying from COVID-19.
The researchers noted several limitations in their meta-analysis that may make their findings less generalizable to future SARS-CoV-2 variants, such as including only studies that were published before COVID-19 vaccines were available and before more infectious SARS-CoV-2 variants like the B.1.617.2 (Delta) variant emerged. They also included studies where inpatients were not tested for COVID-19 because access to testing was not widely available.
“Our meta-analysis points toward a novel possibility: Headache arising secondary to an infection is not a ‘nonspecific’ symptom, but rather it may be a marker of enhanced likelihood of survival. That is, we find that patients reporting headache in the setting of COVID-19 are at reduced risk of death,” Mr. Gallardo and colleagues wrote.
More data needed on association between headache and COVID-19
While headache appeared to affect a small proportion of overall inpatients with COVID-19, the researchers noted this might be because individuals with COVID-19 and headache symptoms are less likely to require hospitalization or a visit to the ED. Another potential explanation is that “people with primary headache disorders, including migraine, may be more likely to report symptoms of COVID-19, but they also may be relatively less likely to experience a life-threatening COVID-19 disease course.”
The researchers said this potential association should be explored in future studies as well as in other viral infections or postviral syndromes such as long COVID. “Defining specific headache mechanisms that could enhance survival from viral infections represents an opportunity for the potential discovery of improved viral therapeutics, as well as for understanding whether, and how, primary headache disorders may be adaptive.”
In a comment, Morris Levin, MD, director of the University of California San Francisco Headache Center, said the findings “of this very thought-provoking review suggest that reporting a headache during a COVID-19 infection seems to be associated with better recovery in hospitalized patients.”
Dr. Levin, who was not involved with the study, acknowledged the researchers’ explanation for the overall low rate of headache in these inpatients as one possible explanation.
“Another could be that sick COVID patients were much more troubled by other symptoms like respiratory distress, which overshadowed their headache symptoms, particularly if they were very ill or if the headache pain was of only mild to moderate severity,” he said. “That could also be an alternate explanation for why less dangerously ill hospitalized patients seemed to have more headaches.”
One limitation he saw in the meta-analysis was how clearly the clinicians characterized headache symptoms in each reviewed study. Dr. Levin suggested a retrospective assessment of premorbid migraine history in hospitalized patients with COVID-19, including survivors and fatalities, might have helped clarify this issue. “The headaches themselves were not characterized so drawing conclusions regarding migraine is challenging.”
Dr. Levin noted it is still not well understood how acute and persistent headaches and other neurological symptoms like mental fog occur in patients with COVID-19. We also do not fully understand the natural history of post-COVID headaches and other neurologic sequelae and the management options for acute and persistent neurological sequelae.
Three authors reported personal and institutional relationships in the form of grants, consultancies, speaker’s bureau positions, guidelines committee member appointments, and editorial board positions for a variety of pharmaceutical companies, agencies, societies, and other organizations. Mr. Gallardo reported no relevant financial disclosures. Dr. Levin reported no relevant financial disclosures.
FROM HEADACHE
For many, long COVID’s impacts go on and on, major study says
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
Mentorship key to improving GI, hepatology workforce diversity
Increasing mentorship opportunities for gastroenterology and hepatology residents and medical students from populations underrepresented in medicine is essential to increase diversity in the specialty and improve health disparities among patients, according to a special report published simultaneously in Gastroenterology and three other journals.
“This study helps to establish priorities for diversity, equity and inclusion in our field and informs future interventions to improve workforce diversity and eliminate health care disparities among the patients we serve,” Folasade P. May, MD, PhD, MPhil, the study’s corresponding author and an associate professor of medicine at the University of California, Los Angeles, said in a prepared statement.
The report, the result of a partnership between researchers at UCLA and the Intersociety Group on Diversity, reveals the findings of a survey aimed at assessing current perspectives on individuals underrepresented in medicine and health equity within gastroenterology and hepatology. The collaboration involved five gastroenterology professional societies: the American Association for the Study of Liver Disease; American College of Gastroenterology; American Gastroenterological Association; American Society of Gastrointestinal Endoscopy; and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition.
”The current racial and ethnic composition of the GI and hepatology workforce does not reflect the population of patients served or the current matriculants in medicine,” Harman K. Rahal, MD, of UCLA and Cedars-Sinai Medical Center, Los Angeles, and James H. Tabibian, MD, PhD, of UCLA and Olive View–UCLA Medical Center, and colleagues wrote. “As there are several conditions in GI and hepatology with disparities in incidence, treatment, and outcomes, representation of UIM [underrepresented in medicine] individuals is critical to address health disparities.”
The term “underrepresented in medicine” is defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.” The authors explained that these groups “have traditionally included Latino (i.e., Latino/a/x), Black (or African American), Native American (namely, American Indian, Alaska Native, and Native Hawaiian), Pacific Islander, and mainland Puerto Rican individuals.”
The five gastroenterology and hepatology societies partnered with investigators at UCLA to develop a 33-question electronic survey “to determine perspectives of current racial, ethnic, and gender diversity within GI and hepatology; to assess current views on interventions needed to increase racial, ethnic, and gender diversity in the field; and to collect data on the experiences of UIM individuals and women in our field,” according to the report’s authors. The survey was then distributed to members of those societies, with 1,219 respondents.
The report found that inadequate representation of people from those underrepresented groups in the education and training pipeline was the most frequently reported barrier to improving racial and ethnic diversity in the field (35.4%), followed by insufficient racial and ethnic minority group representation in professional leadership (27.9%) and insufficient racial and ethnic minority group representation among practicing GI and hepatology professionals in the workplace (26.6%). Only 9% of fellows in GI and hepatology are from groups underrepresented in medicine, according to data from the Accreditation Council for Graduate Medical Education. Furthermore, one study has shown that the proportion of UIM in academic faculty has never exceeded 10% at each academic rank; there has even been a decline recently among junior academic faculty positions. That study also found that only 9% of academic gastroenterologists in the United states identify as underrepresented in medicine, with little change over the last decade.
Potential contributors to this low level of representation, the authors wrote, include “lack of racial and ethnic diversity in the medical training pipeline, nondiverse leadership, bias, racial discrimination, and the notion that UIM physicians may be less likely to promote themselves or be promoted.”
Another potential contributor, however, may be complacency within the field about the need to improve diversity and taking actions to do so.
A majority of White physicians (78%) were very or somewhat satisfied with current levels of workforce diversity, compared with a majority of Black physicians (63%) feeling very or somewhat unsatisfied.
This disconnect was not surprising to Aja McCutchen, MD, a partner at Atlanta Gastroenterology Associates who was not involved in the survey.
“One cannot discount the lived experience of a [person underrepresented in medicine] as it relates to recognizing conscious and unconscious biases, microaggression recognition, and absence of [underrepresented clinicians] in key positions. This is a reality that I do see on a daily basis,” Dr. McCutchen said in an interview.
Only 35% of respondents felt there is “insufficient racial and ethnic representation in education and training,” and just over a quarter (28%) felt the same about representation in leadership. In fact, most respondents (59.7%) thought that racial and ethnic diversity had increased over the past 5 years even though data show no change, the authors noted.
Although Dr. McCutchen appreciated the broad recognition from respondents, regardless of background, to improve diversity in the pipeline, she noted that “retention of current talent and future talent would also require cultural shifts in understanding the challenges of the [underrepresented] members,” Dr. McCutchen said.
Again, however, the majority of the respondents (64.6%) were themselves not members of underrepresented groups. Nearly half the respondents (48.7%) were non-Hispanic White, and one in five (22.5%) were Asian, Native Hawaiian, or Pacific Islander. The remaining respondents, making up less than a third of the total, were Hispanic (10.6%), Black (9.1%), American Indian or Alaskan Native (0.2%), another race/ethnicity (3.3%), or preferred not to answer (5.7%).
Dr. McCutchen said she had mixed feelings about the survey overall.
“On the one hand, I was eager to read the perceptions of survey respondents as it relates to diversity, equity and inclusion in the GI space as very little cross-organizational data exists,” said Dr. McCutchen. “On the other hand, the responses reminded me that there is a lot of work to be done as I expected more dissatisfaction with the current GI workforce in both academia and private practice respondents.”
She was surprised, for example, that nearly three-quarters of the respondents were somewhat or very satisfied, and that a majority thought racial and ethnic diversity had increased.
Studies on provider-patient concordance have shown that patients feel it’s important to share common ground with their physicians particularly in terms of race, ethnicity and language, the authors noted.
“This patient preference underscores the need to recruit and train a more diverse cohort of trainees into GI and hepatology fellowships if the desired goal is to optimize patient care and combat health disparities,” they wrote. They pointed out that cultural understanding can influence how patients perceive their health, symptoms, and concerns, which can then affect providers’ diagnostic accuracy and treatment recommendations. In turn, patients may have better adherence to treatment recommendations when they share a similar background as their clinician.
“Diversity in medicine also leads to greater diversity in thoughts, better returns on investments, increased scholarly activities related to health equity to name a few,” Dr. McCutchen said.
The top recommendations from respondents for improving representation of currently underrepresented individuals in GI and hepatology were to increase mentorship opportunities for residents (45%) and medical students (43%) from these groups and to increase representation of professionals from these backgrounds in program and professional society leadership (39%). A third of respondents also recommended increasing shadowing opportunities for undergraduate students from these underrepresented populations.
Dr. McCutchen expressed optimism regarding the initiatives to improve diversity, equity and inclusion across the gastroenterology spectrum.
“It is incumbent upon all of us to continue to be the driving force of change, which will be a journey and not a destination,” McCutchen said. “In the future, diversity, equity and inclusion will be the expectation, and we will ultimately move closer to the goal of completely eliminating health care inequities.”
The research was funded by the National Cancer Institute, the UCLA Jonsson Comprehensive Cancer Center, and Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Ablon Scholars Program. The authors reported no conflicts of interest. Dr. McCutchen disclosed relationships with Bristol-Myers Squibb and Redhill Biopharmaceuticals.
Increasing mentorship opportunities for gastroenterology and hepatology residents and medical students from populations underrepresented in medicine is essential to increase diversity in the specialty and improve health disparities among patients, according to a special report published simultaneously in Gastroenterology and three other journals.
“This study helps to establish priorities for diversity, equity and inclusion in our field and informs future interventions to improve workforce diversity and eliminate health care disparities among the patients we serve,” Folasade P. May, MD, PhD, MPhil, the study’s corresponding author and an associate professor of medicine at the University of California, Los Angeles, said in a prepared statement.
The report, the result of a partnership between researchers at UCLA and the Intersociety Group on Diversity, reveals the findings of a survey aimed at assessing current perspectives on individuals underrepresented in medicine and health equity within gastroenterology and hepatology. The collaboration involved five gastroenterology professional societies: the American Association for the Study of Liver Disease; American College of Gastroenterology; American Gastroenterological Association; American Society of Gastrointestinal Endoscopy; and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition.
”The current racial and ethnic composition of the GI and hepatology workforce does not reflect the population of patients served or the current matriculants in medicine,” Harman K. Rahal, MD, of UCLA and Cedars-Sinai Medical Center, Los Angeles, and James H. Tabibian, MD, PhD, of UCLA and Olive View–UCLA Medical Center, and colleagues wrote. “As there are several conditions in GI and hepatology with disparities in incidence, treatment, and outcomes, representation of UIM [underrepresented in medicine] individuals is critical to address health disparities.”
The term “underrepresented in medicine” is defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.” The authors explained that these groups “have traditionally included Latino (i.e., Latino/a/x), Black (or African American), Native American (namely, American Indian, Alaska Native, and Native Hawaiian), Pacific Islander, and mainland Puerto Rican individuals.”
The five gastroenterology and hepatology societies partnered with investigators at UCLA to develop a 33-question electronic survey “to determine perspectives of current racial, ethnic, and gender diversity within GI and hepatology; to assess current views on interventions needed to increase racial, ethnic, and gender diversity in the field; and to collect data on the experiences of UIM individuals and women in our field,” according to the report’s authors. The survey was then distributed to members of those societies, with 1,219 respondents.
The report found that inadequate representation of people from those underrepresented groups in the education and training pipeline was the most frequently reported barrier to improving racial and ethnic diversity in the field (35.4%), followed by insufficient racial and ethnic minority group representation in professional leadership (27.9%) and insufficient racial and ethnic minority group representation among practicing GI and hepatology professionals in the workplace (26.6%). Only 9% of fellows in GI and hepatology are from groups underrepresented in medicine, according to data from the Accreditation Council for Graduate Medical Education. Furthermore, one study has shown that the proportion of UIM in academic faculty has never exceeded 10% at each academic rank; there has even been a decline recently among junior academic faculty positions. That study also found that only 9% of academic gastroenterologists in the United states identify as underrepresented in medicine, with little change over the last decade.
Potential contributors to this low level of representation, the authors wrote, include “lack of racial and ethnic diversity in the medical training pipeline, nondiverse leadership, bias, racial discrimination, and the notion that UIM physicians may be less likely to promote themselves or be promoted.”
Another potential contributor, however, may be complacency within the field about the need to improve diversity and taking actions to do so.
A majority of White physicians (78%) were very or somewhat satisfied with current levels of workforce diversity, compared with a majority of Black physicians (63%) feeling very or somewhat unsatisfied.
This disconnect was not surprising to Aja McCutchen, MD, a partner at Atlanta Gastroenterology Associates who was not involved in the survey.
“One cannot discount the lived experience of a [person underrepresented in medicine] as it relates to recognizing conscious and unconscious biases, microaggression recognition, and absence of [underrepresented clinicians] in key positions. This is a reality that I do see on a daily basis,” Dr. McCutchen said in an interview.
Only 35% of respondents felt there is “insufficient racial and ethnic representation in education and training,” and just over a quarter (28%) felt the same about representation in leadership. In fact, most respondents (59.7%) thought that racial and ethnic diversity had increased over the past 5 years even though data show no change, the authors noted.
Although Dr. McCutchen appreciated the broad recognition from respondents, regardless of background, to improve diversity in the pipeline, she noted that “retention of current talent and future talent would also require cultural shifts in understanding the challenges of the [underrepresented] members,” Dr. McCutchen said.
Again, however, the majority of the respondents (64.6%) were themselves not members of underrepresented groups. Nearly half the respondents (48.7%) were non-Hispanic White, and one in five (22.5%) were Asian, Native Hawaiian, or Pacific Islander. The remaining respondents, making up less than a third of the total, were Hispanic (10.6%), Black (9.1%), American Indian or Alaskan Native (0.2%), another race/ethnicity (3.3%), or preferred not to answer (5.7%).
Dr. McCutchen said she had mixed feelings about the survey overall.
“On the one hand, I was eager to read the perceptions of survey respondents as it relates to diversity, equity and inclusion in the GI space as very little cross-organizational data exists,” said Dr. McCutchen. “On the other hand, the responses reminded me that there is a lot of work to be done as I expected more dissatisfaction with the current GI workforce in both academia and private practice respondents.”
She was surprised, for example, that nearly three-quarters of the respondents were somewhat or very satisfied, and that a majority thought racial and ethnic diversity had increased.
Studies on provider-patient concordance have shown that patients feel it’s important to share common ground with their physicians particularly in terms of race, ethnicity and language, the authors noted.
“This patient preference underscores the need to recruit and train a more diverse cohort of trainees into GI and hepatology fellowships if the desired goal is to optimize patient care and combat health disparities,” they wrote. They pointed out that cultural understanding can influence how patients perceive their health, symptoms, and concerns, which can then affect providers’ diagnostic accuracy and treatment recommendations. In turn, patients may have better adherence to treatment recommendations when they share a similar background as their clinician.
“Diversity in medicine also leads to greater diversity in thoughts, better returns on investments, increased scholarly activities related to health equity to name a few,” Dr. McCutchen said.
The top recommendations from respondents for improving representation of currently underrepresented individuals in GI and hepatology were to increase mentorship opportunities for residents (45%) and medical students (43%) from these groups and to increase representation of professionals from these backgrounds in program and professional society leadership (39%). A third of respondents also recommended increasing shadowing opportunities for undergraduate students from these underrepresented populations.
Dr. McCutchen expressed optimism regarding the initiatives to improve diversity, equity and inclusion across the gastroenterology spectrum.
“It is incumbent upon all of us to continue to be the driving force of change, which will be a journey and not a destination,” McCutchen said. “In the future, diversity, equity and inclusion will be the expectation, and we will ultimately move closer to the goal of completely eliminating health care inequities.”
The research was funded by the National Cancer Institute, the UCLA Jonsson Comprehensive Cancer Center, and Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Ablon Scholars Program. The authors reported no conflicts of interest. Dr. McCutchen disclosed relationships with Bristol-Myers Squibb and Redhill Biopharmaceuticals.
Increasing mentorship opportunities for gastroenterology and hepatology residents and medical students from populations underrepresented in medicine is essential to increase diversity in the specialty and improve health disparities among patients, according to a special report published simultaneously in Gastroenterology and three other journals.
“This study helps to establish priorities for diversity, equity and inclusion in our field and informs future interventions to improve workforce diversity and eliminate health care disparities among the patients we serve,” Folasade P. May, MD, PhD, MPhil, the study’s corresponding author and an associate professor of medicine at the University of California, Los Angeles, said in a prepared statement.
The report, the result of a partnership between researchers at UCLA and the Intersociety Group on Diversity, reveals the findings of a survey aimed at assessing current perspectives on individuals underrepresented in medicine and health equity within gastroenterology and hepatology. The collaboration involved five gastroenterology professional societies: the American Association for the Study of Liver Disease; American College of Gastroenterology; American Gastroenterological Association; American Society of Gastrointestinal Endoscopy; and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition.
”The current racial and ethnic composition of the GI and hepatology workforce does not reflect the population of patients served or the current matriculants in medicine,” Harman K. Rahal, MD, of UCLA and Cedars-Sinai Medical Center, Los Angeles, and James H. Tabibian, MD, PhD, of UCLA and Olive View–UCLA Medical Center, and colleagues wrote. “As there are several conditions in GI and hepatology with disparities in incidence, treatment, and outcomes, representation of UIM [underrepresented in medicine] individuals is critical to address health disparities.”
The term “underrepresented in medicine” is defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.” The authors explained that these groups “have traditionally included Latino (i.e., Latino/a/x), Black (or African American), Native American (namely, American Indian, Alaska Native, and Native Hawaiian), Pacific Islander, and mainland Puerto Rican individuals.”
The five gastroenterology and hepatology societies partnered with investigators at UCLA to develop a 33-question electronic survey “to determine perspectives of current racial, ethnic, and gender diversity within GI and hepatology; to assess current views on interventions needed to increase racial, ethnic, and gender diversity in the field; and to collect data on the experiences of UIM individuals and women in our field,” according to the report’s authors. The survey was then distributed to members of those societies, with 1,219 respondents.
The report found that inadequate representation of people from those underrepresented groups in the education and training pipeline was the most frequently reported barrier to improving racial and ethnic diversity in the field (35.4%), followed by insufficient racial and ethnic minority group representation in professional leadership (27.9%) and insufficient racial and ethnic minority group representation among practicing GI and hepatology professionals in the workplace (26.6%). Only 9% of fellows in GI and hepatology are from groups underrepresented in medicine, according to data from the Accreditation Council for Graduate Medical Education. Furthermore, one study has shown that the proportion of UIM in academic faculty has never exceeded 10% at each academic rank; there has even been a decline recently among junior academic faculty positions. That study also found that only 9% of academic gastroenterologists in the United states identify as underrepresented in medicine, with little change over the last decade.
Potential contributors to this low level of representation, the authors wrote, include “lack of racial and ethnic diversity in the medical training pipeline, nondiverse leadership, bias, racial discrimination, and the notion that UIM physicians may be less likely to promote themselves or be promoted.”
Another potential contributor, however, may be complacency within the field about the need to improve diversity and taking actions to do so.
A majority of White physicians (78%) were very or somewhat satisfied with current levels of workforce diversity, compared with a majority of Black physicians (63%) feeling very or somewhat unsatisfied.
This disconnect was not surprising to Aja McCutchen, MD, a partner at Atlanta Gastroenterology Associates who was not involved in the survey.
“One cannot discount the lived experience of a [person underrepresented in medicine] as it relates to recognizing conscious and unconscious biases, microaggression recognition, and absence of [underrepresented clinicians] in key positions. This is a reality that I do see on a daily basis,” Dr. McCutchen said in an interview.
Only 35% of respondents felt there is “insufficient racial and ethnic representation in education and training,” and just over a quarter (28%) felt the same about representation in leadership. In fact, most respondents (59.7%) thought that racial and ethnic diversity had increased over the past 5 years even though data show no change, the authors noted.
Although Dr. McCutchen appreciated the broad recognition from respondents, regardless of background, to improve diversity in the pipeline, she noted that “retention of current talent and future talent would also require cultural shifts in understanding the challenges of the [underrepresented] members,” Dr. McCutchen said.
Again, however, the majority of the respondents (64.6%) were themselves not members of underrepresented groups. Nearly half the respondents (48.7%) were non-Hispanic White, and one in five (22.5%) were Asian, Native Hawaiian, or Pacific Islander. The remaining respondents, making up less than a third of the total, were Hispanic (10.6%), Black (9.1%), American Indian or Alaskan Native (0.2%), another race/ethnicity (3.3%), or preferred not to answer (5.7%).
Dr. McCutchen said she had mixed feelings about the survey overall.
“On the one hand, I was eager to read the perceptions of survey respondents as it relates to diversity, equity and inclusion in the GI space as very little cross-organizational data exists,” said Dr. McCutchen. “On the other hand, the responses reminded me that there is a lot of work to be done as I expected more dissatisfaction with the current GI workforce in both academia and private practice respondents.”
She was surprised, for example, that nearly three-quarters of the respondents were somewhat or very satisfied, and that a majority thought racial and ethnic diversity had increased.
Studies on provider-patient concordance have shown that patients feel it’s important to share common ground with their physicians particularly in terms of race, ethnicity and language, the authors noted.
“This patient preference underscores the need to recruit and train a more diverse cohort of trainees into GI and hepatology fellowships if the desired goal is to optimize patient care and combat health disparities,” they wrote. They pointed out that cultural understanding can influence how patients perceive their health, symptoms, and concerns, which can then affect providers’ diagnostic accuracy and treatment recommendations. In turn, patients may have better adherence to treatment recommendations when they share a similar background as their clinician.
“Diversity in medicine also leads to greater diversity in thoughts, better returns on investments, increased scholarly activities related to health equity to name a few,” Dr. McCutchen said.
The top recommendations from respondents for improving representation of currently underrepresented individuals in GI and hepatology were to increase mentorship opportunities for residents (45%) and medical students (43%) from these groups and to increase representation of professionals from these backgrounds in program and professional society leadership (39%). A third of respondents also recommended increasing shadowing opportunities for undergraduate students from these underrepresented populations.
Dr. McCutchen expressed optimism regarding the initiatives to improve diversity, equity and inclusion across the gastroenterology spectrum.
“It is incumbent upon all of us to continue to be the driving force of change, which will be a journey and not a destination,” McCutchen said. “In the future, diversity, equity and inclusion will be the expectation, and we will ultimately move closer to the goal of completely eliminating health care inequities.”
The research was funded by the National Cancer Institute, the UCLA Jonsson Comprehensive Cancer Center, and Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Ablon Scholars Program. The authors reported no conflicts of interest. Dr. McCutchen disclosed relationships with Bristol-Myers Squibb and Redhill Biopharmaceuticals.
FROM GASTROENTEROLOGY
Keep menstrual cramps away the dietary prevention way
Foods for thought: Menstrual cramp prevention
For those who menstruate, it’s typical for that time of the month to bring cravings for things that may give a serotonin boost that eases the rise in stress hormones. Chocolate and other foods high in sugar fall into that category, but they could actually be adding to the problem.
About 90% of adolescent girls have menstrual pain, and it’s the leading cause of school absences for the demographic. Muscle relaxers and PMS pills are usually the recommended solution to alleviating menstrual cramps, but what if the patient doesn’t want to take any medicine?
Serah Sannoh of Rutgers University wanted to find another way to relieve her menstrual pains. The literature review she presented at the annual meeting of the North American Menopause Society found multiple studies that examined dietary patterns that resulted in menstrual pain.
In Ms. Sannoh’s analysis, she looked at how certain foods have an effect on cramps. Do they contribute to the pain or reduce it? Diets high in processed foods, oils, sugars, salt, and omega-6 fatty acids promote inflammation in the muscles around the uterus. Thus, cramps.
The answer, sometimes, is not to add a medicine but to change our daily practices, she suggested. Foods high in omega-3 fatty acids helped reduce pain, and those who practiced a vegan diet had the lowest muscle inflammation rates. So more salmon and fewer Swedish Fish.
Stage 1 of the robot apocalypse is already upon us
The mere mention of a robot apocalypse is enough to conjure images of terrifying robot soldiers with Austrian accents harvesting and killing humanity while the survivors live blissfully in a simulation and do low-gravity kung fu with high-profile Hollywood actors. They’ll even take over the navy.
Reality is often less exciting than the movies, but rest assured, the robots will not be denied their dominion of Earth. Our future robot overlords are simply taking a more subtle, less dramatic route toward their ultimate subjugation of mankind: They’re making us all sad and burned out.
The research pulls from work conducted in multiple countries to paint a picture of a humanity filled with anxiety about jobs as robotic automation grows more common. In India, a survey of automobile manufacturing works showed that working alongside industrial robots was linked with greater reports of burnout and workplace incivility. In Singapore, a group of college students randomly assigned to read one of three articles – one about the use of robots in business, a generic article about robots, or an article unrelated to robots – were then surveyed about their job security concerns. Three guesses as to which group was most worried.
In addition, the researchers analyzed 185 U.S. metropolitan areas for robot prevalence alongside use of job-recruiting websites and found that the more robots a city used, the more common job searches were. Unemployment rates weren’t affected, suggesting people had job insecurity because of robots. Sure, there could be other, nonrobotic reasons for this, but that’s no fun. We’re here because we fear our future android rulers.
It’s not all doom and gloom, fortunately. In an online experiment, the study authors found that self-affirmation exercises, such as writing down characteristics or values important to us, can overcome the existential fears and lessen concern about robots in the workplace. One of the authors noted that, while some fear is justified, “media reports on new technologies like robots and algorithms tend to be apocalyptic in nature, so people may develop an irrational fear about them.”
Oops. Our bad.
Apocalypse, stage 2: Leaping oral superorganisms
The terms of our secret agreement with the shadowy-but-powerful dental-industrial complex stipulate that LOTME can only cover tooth-related news once a year. This is that once a year.
Since we’ve already dealt with a robot apocalypse, how about a sci-fi horror story? A story with a “cross-kingdom partnership” in which assemblages of bacteria and fungi perform feats greater than either could achieve on its own. A story in which new microscopy technologies allow “scientists to visualize the behavior of living microbes in real time,” according to a statement from the University of Pennsylvania, Philadelphia.
While looking at saliva samples from toddlers with severe tooth decay, lead author Zhi Ren and associates “noticed the bacteria and fungi forming these assemblages and developing motions we never thought they would possess: a ‘walking-like’ and ‘leaping-like’ mobility. … It’s almost like a new organism – a superorganism – with new functions,” said senior author Hyun Koo, DDS, PhD, of Penn Dental Medicine.
Did he say “mobility”? He did, didn’t he?
To study these alleged superorganisms, they set up a laboratory system “using the bacteria, fungi, and a tooth-like material, all incubated in human saliva,” the university explained.
“Incubated in human saliva.” There’s a phrase you don’t see every day.
It only took a few hours for the investigators to observe the bacterial/fungal assemblages making leaps of more than 100 microns across the tooth-like material. “That is more than 200 times their own body length,” Dr. Ren said, “making them even better than most vertebrates, relative to body size. For example, tree frogs and grasshoppers can leap forward about 50 times and 20 times their own body length, respectively.”
So, will it be the robots or the evil superorganisms? Let us give you a word of advice: Always bet on bacteria.
Foods for thought: Menstrual cramp prevention
For those who menstruate, it’s typical for that time of the month to bring cravings for things that may give a serotonin boost that eases the rise in stress hormones. Chocolate and other foods high in sugar fall into that category, but they could actually be adding to the problem.
About 90% of adolescent girls have menstrual pain, and it’s the leading cause of school absences for the demographic. Muscle relaxers and PMS pills are usually the recommended solution to alleviating menstrual cramps, but what if the patient doesn’t want to take any medicine?
Serah Sannoh of Rutgers University wanted to find another way to relieve her menstrual pains. The literature review she presented at the annual meeting of the North American Menopause Society found multiple studies that examined dietary patterns that resulted in menstrual pain.
In Ms. Sannoh’s analysis, she looked at how certain foods have an effect on cramps. Do they contribute to the pain or reduce it? Diets high in processed foods, oils, sugars, salt, and omega-6 fatty acids promote inflammation in the muscles around the uterus. Thus, cramps.
The answer, sometimes, is not to add a medicine but to change our daily practices, she suggested. Foods high in omega-3 fatty acids helped reduce pain, and those who practiced a vegan diet had the lowest muscle inflammation rates. So more salmon and fewer Swedish Fish.
Stage 1 of the robot apocalypse is already upon us
The mere mention of a robot apocalypse is enough to conjure images of terrifying robot soldiers with Austrian accents harvesting and killing humanity while the survivors live blissfully in a simulation and do low-gravity kung fu with high-profile Hollywood actors. They’ll even take over the navy.
Reality is often less exciting than the movies, but rest assured, the robots will not be denied their dominion of Earth. Our future robot overlords are simply taking a more subtle, less dramatic route toward their ultimate subjugation of mankind: They’re making us all sad and burned out.
The research pulls from work conducted in multiple countries to paint a picture of a humanity filled with anxiety about jobs as robotic automation grows more common. In India, a survey of automobile manufacturing works showed that working alongside industrial robots was linked with greater reports of burnout and workplace incivility. In Singapore, a group of college students randomly assigned to read one of three articles – one about the use of robots in business, a generic article about robots, or an article unrelated to robots – were then surveyed about their job security concerns. Three guesses as to which group was most worried.
In addition, the researchers analyzed 185 U.S. metropolitan areas for robot prevalence alongside use of job-recruiting websites and found that the more robots a city used, the more common job searches were. Unemployment rates weren’t affected, suggesting people had job insecurity because of robots. Sure, there could be other, nonrobotic reasons for this, but that’s no fun. We’re here because we fear our future android rulers.
It’s not all doom and gloom, fortunately. In an online experiment, the study authors found that self-affirmation exercises, such as writing down characteristics or values important to us, can overcome the existential fears and lessen concern about robots in the workplace. One of the authors noted that, while some fear is justified, “media reports on new technologies like robots and algorithms tend to be apocalyptic in nature, so people may develop an irrational fear about them.”
Oops. Our bad.
Apocalypse, stage 2: Leaping oral superorganisms
The terms of our secret agreement with the shadowy-but-powerful dental-industrial complex stipulate that LOTME can only cover tooth-related news once a year. This is that once a year.
Since we’ve already dealt with a robot apocalypse, how about a sci-fi horror story? A story with a “cross-kingdom partnership” in which assemblages of bacteria and fungi perform feats greater than either could achieve on its own. A story in which new microscopy technologies allow “scientists to visualize the behavior of living microbes in real time,” according to a statement from the University of Pennsylvania, Philadelphia.
While looking at saliva samples from toddlers with severe tooth decay, lead author Zhi Ren and associates “noticed the bacteria and fungi forming these assemblages and developing motions we never thought they would possess: a ‘walking-like’ and ‘leaping-like’ mobility. … It’s almost like a new organism – a superorganism – with new functions,” said senior author Hyun Koo, DDS, PhD, of Penn Dental Medicine.
Did he say “mobility”? He did, didn’t he?
To study these alleged superorganisms, they set up a laboratory system “using the bacteria, fungi, and a tooth-like material, all incubated in human saliva,” the university explained.
“Incubated in human saliva.” There’s a phrase you don’t see every day.
It only took a few hours for the investigators to observe the bacterial/fungal assemblages making leaps of more than 100 microns across the tooth-like material. “That is more than 200 times their own body length,” Dr. Ren said, “making them even better than most vertebrates, relative to body size. For example, tree frogs and grasshoppers can leap forward about 50 times and 20 times their own body length, respectively.”
So, will it be the robots or the evil superorganisms? Let us give you a word of advice: Always bet on bacteria.
Foods for thought: Menstrual cramp prevention
For those who menstruate, it’s typical for that time of the month to bring cravings for things that may give a serotonin boost that eases the rise in stress hormones. Chocolate and other foods high in sugar fall into that category, but they could actually be adding to the problem.
About 90% of adolescent girls have menstrual pain, and it’s the leading cause of school absences for the demographic. Muscle relaxers and PMS pills are usually the recommended solution to alleviating menstrual cramps, but what if the patient doesn’t want to take any medicine?
Serah Sannoh of Rutgers University wanted to find another way to relieve her menstrual pains. The literature review she presented at the annual meeting of the North American Menopause Society found multiple studies that examined dietary patterns that resulted in menstrual pain.
In Ms. Sannoh’s analysis, she looked at how certain foods have an effect on cramps. Do they contribute to the pain or reduce it? Diets high in processed foods, oils, sugars, salt, and omega-6 fatty acids promote inflammation in the muscles around the uterus. Thus, cramps.
The answer, sometimes, is not to add a medicine but to change our daily practices, she suggested. Foods high in omega-3 fatty acids helped reduce pain, and those who practiced a vegan diet had the lowest muscle inflammation rates. So more salmon and fewer Swedish Fish.
Stage 1 of the robot apocalypse is already upon us
The mere mention of a robot apocalypse is enough to conjure images of terrifying robot soldiers with Austrian accents harvesting and killing humanity while the survivors live blissfully in a simulation and do low-gravity kung fu with high-profile Hollywood actors. They’ll even take over the navy.
Reality is often less exciting than the movies, but rest assured, the robots will not be denied their dominion of Earth. Our future robot overlords are simply taking a more subtle, less dramatic route toward their ultimate subjugation of mankind: They’re making us all sad and burned out.
The research pulls from work conducted in multiple countries to paint a picture of a humanity filled with anxiety about jobs as robotic automation grows more common. In India, a survey of automobile manufacturing works showed that working alongside industrial robots was linked with greater reports of burnout and workplace incivility. In Singapore, a group of college students randomly assigned to read one of three articles – one about the use of robots in business, a generic article about robots, or an article unrelated to robots – were then surveyed about their job security concerns. Three guesses as to which group was most worried.
In addition, the researchers analyzed 185 U.S. metropolitan areas for robot prevalence alongside use of job-recruiting websites and found that the more robots a city used, the more common job searches were. Unemployment rates weren’t affected, suggesting people had job insecurity because of robots. Sure, there could be other, nonrobotic reasons for this, but that’s no fun. We’re here because we fear our future android rulers.
It’s not all doom and gloom, fortunately. In an online experiment, the study authors found that self-affirmation exercises, such as writing down characteristics or values important to us, can overcome the existential fears and lessen concern about robots in the workplace. One of the authors noted that, while some fear is justified, “media reports on new technologies like robots and algorithms tend to be apocalyptic in nature, so people may develop an irrational fear about them.”
Oops. Our bad.
Apocalypse, stage 2: Leaping oral superorganisms
The terms of our secret agreement with the shadowy-but-powerful dental-industrial complex stipulate that LOTME can only cover tooth-related news once a year. This is that once a year.
Since we’ve already dealt with a robot apocalypse, how about a sci-fi horror story? A story with a “cross-kingdom partnership” in which assemblages of bacteria and fungi perform feats greater than either could achieve on its own. A story in which new microscopy technologies allow “scientists to visualize the behavior of living microbes in real time,” according to a statement from the University of Pennsylvania, Philadelphia.
While looking at saliva samples from toddlers with severe tooth decay, lead author Zhi Ren and associates “noticed the bacteria and fungi forming these assemblages and developing motions we never thought they would possess: a ‘walking-like’ and ‘leaping-like’ mobility. … It’s almost like a new organism – a superorganism – with new functions,” said senior author Hyun Koo, DDS, PhD, of Penn Dental Medicine.
Did he say “mobility”? He did, didn’t he?
To study these alleged superorganisms, they set up a laboratory system “using the bacteria, fungi, and a tooth-like material, all incubated in human saliva,” the university explained.
“Incubated in human saliva.” There’s a phrase you don’t see every day.
It only took a few hours for the investigators to observe the bacterial/fungal assemblages making leaps of more than 100 microns across the tooth-like material. “That is more than 200 times their own body length,” Dr. Ren said, “making them even better than most vertebrates, relative to body size. For example, tree frogs and grasshoppers can leap forward about 50 times and 20 times their own body length, respectively.”
So, will it be the robots or the evil superorganisms? Let us give you a word of advice: Always bet on bacteria.
Randomized, Double-Blind Placebo-Controlled Trial to Assess the Effect of Probiotics on Irritable Bowel Syndrome in Veterans With Gulf War Illness
About 700,000 US military personnel were deployed in Operation Desert Storm (August 1990 to March 1991).1 Almost 30 years since the war, a large number of these veterans continue to experience a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly affects health and quality of life (QOL). The lack of clear etiology of the illness has impaired research to find specific treatments and has further exacerbated the stress among veterans. GWI typically includes a mixture of chronic headache, cognitive difficulties, widespread pain, unexplained fatigue, memory and concentration problems, as well as chronic respiratory and gastrointestinal (GI) symptoms.2 Abdominal pain and alteration of bowel habits are also symptoms typical of irritable bowel syndrome (IBS). It has been estimated that IBS occurs in up to 30% of Gulf War veterans.3
The etiology of IBS is unknown. Possible mechanisms include visceral hypersensitivity, altered gut motor function, aberrant brain-gut interaction, and psychological factors, perhaps with a genetic predisposition.4 Gastroenteritis has been reported as a triggering mechanism in up to one-third of patients with IBS.5 Gastroenteritis can alter the gut microbiota and has been reported to be a significant risk factor for the development of IBS.6 In one study of Operation Desert Shield soldiers, > 50% of military personnel developed acute gastroenteritis while on duty.7 A high prevalence of extra-intestinal symptoms also has been reported, including fatigue, headache, joint pains, and anxiety, in Gulf War veterans with IBS. These extra-intestinal symptoms of IBS are consistent with the reported GWI symptoms. Change in gut microbiota also has been associated with many of the extra-intestinal symptoms of IBS, especially fatigue.8,9 Gut microbiota are known to change with travel, stress, and a change in diet, all potential factors that are relevant to Gulf War veterans. This would suggest that an imbalance in the gut microbiota, ie, dysbiosis, may play a role in the pathogenesis of both IBS and GWI. Dysbiosis could be a risk factor for or alternatively a consequence of GWI.
A systematic review highlighted the heterogeneity of the gut microbiota in patients with IBS.10 Overall, Enterobacteriaceae, Lactobacillaceae, and Bacteroides were increased, whereas Clostridiales, Faecalibacterium, and Bifidobacterium were decreased in patients with IBS compared with controls. Gut microbiota also has been associated with cognitive changes, anxiety, and depression—symptoms associated with IBS and are part of the GWI.
If altered gut microbiota contributes to the etiopathogenesis of IBS, its restoration of with probiotics should help. Probiotics are live organisms that when ingested may improve health by promoting the growth of naturally occurring flora and establishing a healthy gut flora. Probiotics have several mechanisms of actions. Probiotics work in the lumen of the gut by producing antibacterial molecules and enhancing the mucosal barrier.11 Probiotics also may produce metabolic compounds that alter the intestinal microbiota and improve intestinal barrier function.12 Probiotics also have been shown to activate receptors in the enteric nervous system with the potential to promote pain relief in the setting of visceral hyperalgesia.13,14 The anti-inflammatory properties of probiotics potentially could modulate the basic pathophysiology of IBS and improve motility, visceral hypersensitivity, and brain-gut interaction.15 Furthermore, significant gut dysbiosis has been shown with GWI; suggesting that probiotics may have a role in its management.16,17
Probiotics have not been studied in Gulf War veterans with IBS. We performed a prospective, double-blind placebo-controlled study to determine the efficacy of a commercially available probiotic containing 8 strains of bacteria (De Simone Formulation; formally known as VSL#3 and Visbiome) on symptoms of IBS and GWI. This probiotic was selected as the overall literature suggested benefit of combination probiotics in IBS, and VSL#3 has been shown to be efficacious in ulcerative colitis and microscopic colitis.18-20
Methods
Veterans who served in Operation Desert Storm (August 1990 to March 1991) and enrolled at the George E. Wahlen Veterans Affairs (VA) Medical Center (GEWVAMC), Salt Lake City, Utah, were eligible for the study. The inclusion criteria were: veterans aged ≥ 35 years; ≥ 2 nonintestinal GWI symptoms (eg, fatigue, joint pains, insomnia, general stiffness, and headache); IBS diagnosis based on the Rome III criteria; IBS symptoms > 6 months; normal gross appearance of the colonic mucosa; negative markers for celiac disease and inflammatory bowel disease (IBD); normal thyroid function; and serum calcium levels.21 Those who had a clinically significant cardiac, pulmonary, hepatic or renal dysfunction; history of/or presence of systemic malignancy; current evidence of celiac disease or IBD; unstable/significant psychiatric disease; recent change in GI medications; current pregnancy; or use of antibiotics or probiotics within the past 1 month were excluded. Subjects were enrolled from a list of veterans with GWI from the GEWVAMC Gulf War registry; referrals to gastroenterology clinics for IBS from internal medicine clinics; and posted advertisements.
Protocol
After written informed consent was obtained, each veteran was verified to have IBS and ≥ 2 GWI symptoms. All veterans had the following tests and panels: complete blood count, erythrocyte sedimentation rate, serum comprehensive metabolic panel, thyroid-stimulating hormone, tissue transglutaminase, stool test for ova and parasite, giardia antigen, and clostridia toxins to exclude organic cause of GI symptoms. Colonoscopy was performed in all veterans to exclude IBD, and to rule out microscopic or lymphocytic colitis.
Randomization was computer generated and maintained by the study pharmacist so that study personnel and patients were blinded to the trial groups. All investigators were blinded and allocation was concealed. The medication was supplied in a numbered container by the pharmacist after patient enrollment. After a 2-week run-in period, veterans were randomized (1:1) to receive either 1 sachet of probiotic (De Simone Formulation; formally known as VSL#3 and Visbiome) or placebo once daily for 8 weeks.
Each probiotic packet contains 900 billion probiotic bacteria per sachet.11 This formulation contained 8 viable strains of bacteria: 4 strains of Lactobacillus (L acidophilus, L plantarum, L paracasei, L delbrueckii subsp. bulgaricus); 3 strains of Bifidobacteria (Bifidobacterium breve, B lactis, B infantis); and 1 strain of Streptococcus thermophilus. This formulation had been commercialized and studied as VSL#3 and is currently available in the United States under the Visbiome trade name. While branding changed during the study, the formulation did not. The investigational medicine (VSL#3, Visbiome, and placebo) were shipped from the manufacturer Dupont/Danisco in Madison, Wisconsin. The subjects received placebo or probiotic (VSL#3/Visbiome) and both were identical in appearance. The medication was supplied in a numbered container by the pharmacist after patient enrollment.
Measures
Veterans completed the bowel disease questionnaire to record baseline bowel habits.22 All veterans recorded daily bowel symptoms to confirm the presence of IBS during the 2-week pretreatment period, at baseline, and at the end of the 8-week treatment. The symptoms assessed included severity of abdominal pain (0, none to 100, severe); severity of bloating (0, none to 100, severe); stool frequency; Bristol stool scale (1, very hard to 7, watery); severity of diarrhea (0, none to 100, severe); severity of constipation (0, none to 100, severe); satisfaction with bowel habits (0, none to 100, severe); and IBS affecting or interfering with life (0, none to 100, severe). The bowel symptom score is the sum of the 5 symptom scores.23,24
IBS-specific QOL (IBS-QOL) was recorded at baseline and at the end of treatment.25 The IBS-QOL consists of a 34-item validated disease-specific questionnaire that measures 8 domains relevant to subjects with IBS: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual life, and relationships. We used the Somatic Symptom Checklist to detect the following extra-intestinal symptoms that are common among veterans with GWI: headache, backache, wheeziness, insomnia, bad breath, fatigue, general stiffness, dizziness, weakness, sensitivity to hot and cold, palpitation, and tightness in chest. Subjects rated symptoms on a scale of 1 to 5: how often (1, none; 2, monthly; 3, once weekly; 4, several times weekly; 5, daily), and how bothersome (1, not at all to 5, extremely).26
Subjects completed the Posttraumatic Stress Disorder (PTSD) Checklist–Military, which is specific to military experience with 17 items on a 1 to 5 scale (1, not at all to 5, extremely). Scores were summed to produce a total symptom severity score (range, 17-85).27 Subjects also completed the Brief Symptom Inventory 18 (BSI-18) during the baseline evaluation.28 BSI-18 measures subjects’ reported overall psychological distress. It assesses 3 symptoms dimensions (somatization, depression, and anxiety) and a global severity index. The raw scores were transferred to normative T scores based on samples of nonpatient normal men and women.
Symptom data were compared after 8 weeks of treatment. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, extra-intestinal symptoms, and PTSD score. The study was approved by the Salt Lake City Veterans Affairs Medical Center and the University of Utah Institutional Review Board and registered in ClinicalTrials.gov (NCT03078530).
Statistical Methods
Comparisons of the probiotic vs placebo groups for demographic variable were analyzed using a 2-sample t test for continuous variables, and with a χ2 test or Fisher exact test for categorical variables. The primary and secondary outcome variables were recorded daily for 2 weeks as pretreatment baseline and for 2 weeks at the end of treatment. These symptoms were recorded as ordered categorical variables, which were then averaged across the week to produce a continuous measurement for statistical analysis. For the primary outcome of GI symptoms, posttreatment comparisons were made between the study groups using a 2-sample t test of the baseline vs posttreatment values. All P values were calculated for 2-sided comparisons. The planned sample size in our study protocol was to recruit 40 individuals per group in order to achieve 80% power to detect a 30% improvement between baseline and end of treatment in the primary bowel symptom score. This study recruited 53 subjects. With this sample size, the study had 80% power to detect a 0.8 SD in any of the outcomes.
Results
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.
An individual’s response to probiotics may depend on the subtype of IBS. However, most of the studies, like ours, included groups of all subtypes. It may be that probiotics are more effective in patients with moderate-to-severe symptoms. Most of our patients had milder symptoms, and we cannot discount how subjects with more severe disease may have responded to the drug. Interestingly, one study demonstrated that Lactobacillus was more effective in patients with moderately severe abdominal pain compared with mild symptoms.44
In our study, the probiotic did not improve PTSD symptoms or other extra-intestinal symptoms common in IBS and GWI. Similar to our study, Wong and colleagues did not find significant improvement of psychological and sleep scores after treatment with VSL#3.6 Similarly, there is evidence that alteration in gut microbiota is associated with health and diseases, but what specific alterations occur and whether they can be improved with probiotics remains unknown.45
Limitations
The inconsistent response to probiotics in various studies may be due to IBS heterogeneity. Furthermore, there are demographic differences between Gulf War veterans and patients enrolled in other studies: Gulf War veterans are predominantly male, many were deployed abroad and had a history of gastroenteritis during deployment, and were exposed to stressful situations.46 These factors may be involved in triggering or maintaining IBS in Gulf War veterans. A further limitation of our randomized trial is the relatively small sample size.
Conclusions
This study did not demonstrate statistically significant improvement in symptoms of IBS or improvement in QOL after treatment with a multistrain probiotic. We also did not find any improvement in symptoms of GWI or PTSD. There was no difference in psychological scores between the placebo and treatment groups, and it is unlikely that psychological factors confounded the response to treatment in this study.
The effectiveness of a probiotic may depend on the baseline gut microbiome of the individual and depend on the strain, amount, and frequency of bacteria used. A lack of response of the probiotics does not exclude gut viruses and fungi having a role in exacerbating GWI symptoms. It is also possible that the bacteria present or the dose of the probiotic used was not sufficient to improve symptoms. So far, the definitive benefit of probiotics has been demonstrated for only a few preparations, and none are approved by the US Food and Drug Administration for any disease. More research is needed to determine whether probiotics have any role in the treatment of IBS and GWI.
Acknowledgments
AKT received grant support from the US Department of Veterans Affairs and the US Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). We thank Keith G. Tolman, MD, for assistance in editing the initial proposal and for periodic consultation. We thank the manufacturer of the probiotic for supplying the active drug and the placebo. The manufacture of the probiotic had no role in the design and conduct of the study, analysis and interpretation of the data, and in the preparation of the manuscript.
1. O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025.
2. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987.
3. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.066100
4. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001.
5. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142.
6. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8.
7. Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325(20):1423-1428. doi:10.1056/NEJM199111143252006 8. Clancy RL, Gleeson M, Cox A, et al. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40(4):351-354. doi:10.1136/bjsm.2005.024364
9. Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi:10.1186/1475-2891-8-4
10. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108. doi:10.1053/j.gastro.2019.03.049
11. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9(2):99-107. doi:10.2174/1573401311309020004
12. O´Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025
13. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987
14. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.06610015. O´Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. doi:10.1053/j.gastro.2004.11.050
16. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.17. Janulewicz PA, Seth RK, Carlson JM, et al. The gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193751
18. Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846. doi:10.1371/journal.pone.0228846
19. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561; quiz 1546, 1562. doi:10.1038/ajg.2014.202
20. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2(1):e000018. doi:10.1136/bmjgast-2014-000018
21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. doi:10.1053/j.gastro.2005.11.061
22. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111(8):671-674. doi:10.7326/0003-4819-111-8-671
23. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585-1589. doi:10.1001/jama.280.18.1585
24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395-402. doi:10.1046/j.1365-2036.1997.142318000.x
25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400-411. doi:10.1023/a:1018831127942
26. Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7(2):247-257. doi:10.1007/BF00845390
27. Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Accessed August 25, 2022. https://www.researchgate.net/publication/291448760_The_PTSD_Checklist_PCL_Reliability_validity_and_diagnostic_utility
28. Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. National Computer Systems; 2000.
29. Stamps BW, Lyon WJ, Irvin AP, Kelley-Loughnane N, Goodson MS. A pilot study of the effect of deployment on the gut microbiome and traveler´s diarrhea susceptibility. Front Cell Infect Microbiol. 2020;10:589297. doi:10.3389/fcimb.2020.589297
30. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. doi:10.1046/j.1365-2036.2003.01543.x
31. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687-696. doi:10.1111/j.1365-2982.2005.00695.x32. Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of vsl#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. doi:10.1007/s12602-010-9059-y
33. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-332. doi:10.1136/gut.2008.167270

34. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001
35. Dale HF, Rasmussen SH, Asiller OO, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9). doi:10.3390/nu11092048
36. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072-84. doi:10.3748/wjg.v21.i10.3072
37. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054-1070. doi:10.1111/apt.14539
38. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142
39. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8
40. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26; quiz S27. doi: 10.1038/ajg.2014.187
41. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76. doi:10.1136/gutjnl-2012-302167
42. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. doi:10.1097/MCG.0b013e31823712b1
43. Connell M, Shin A, James-Stevenson T, Xu H, Imperiale TF, Herron J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(12):e13427. doi:10.1111/nmo.13427
44. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. doi:10.3748/wjg.v22.i48.10631
45. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796. doi:10.1136/gutjnl-2012-302504
46. Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56(11):3109-3111. doi:10.1007/s10620-011-1856-y
About 700,000 US military personnel were deployed in Operation Desert Storm (August 1990 to March 1991).1 Almost 30 years since the war, a large number of these veterans continue to experience a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly affects health and quality of life (QOL). The lack of clear etiology of the illness has impaired research to find specific treatments and has further exacerbated the stress among veterans. GWI typically includes a mixture of chronic headache, cognitive difficulties, widespread pain, unexplained fatigue, memory and concentration problems, as well as chronic respiratory and gastrointestinal (GI) symptoms.2 Abdominal pain and alteration of bowel habits are also symptoms typical of irritable bowel syndrome (IBS). It has been estimated that IBS occurs in up to 30% of Gulf War veterans.3
The etiology of IBS is unknown. Possible mechanisms include visceral hypersensitivity, altered gut motor function, aberrant brain-gut interaction, and psychological factors, perhaps with a genetic predisposition.4 Gastroenteritis has been reported as a triggering mechanism in up to one-third of patients with IBS.5 Gastroenteritis can alter the gut microbiota and has been reported to be a significant risk factor for the development of IBS.6 In one study of Operation Desert Shield soldiers, > 50% of military personnel developed acute gastroenteritis while on duty.7 A high prevalence of extra-intestinal symptoms also has been reported, including fatigue, headache, joint pains, and anxiety, in Gulf War veterans with IBS. These extra-intestinal symptoms of IBS are consistent with the reported GWI symptoms. Change in gut microbiota also has been associated with many of the extra-intestinal symptoms of IBS, especially fatigue.8,9 Gut microbiota are known to change with travel, stress, and a change in diet, all potential factors that are relevant to Gulf War veterans. This would suggest that an imbalance in the gut microbiota, ie, dysbiosis, may play a role in the pathogenesis of both IBS and GWI. Dysbiosis could be a risk factor for or alternatively a consequence of GWI.
A systematic review highlighted the heterogeneity of the gut microbiota in patients with IBS.10 Overall, Enterobacteriaceae, Lactobacillaceae, and Bacteroides were increased, whereas Clostridiales, Faecalibacterium, and Bifidobacterium were decreased in patients with IBS compared with controls. Gut microbiota also has been associated with cognitive changes, anxiety, and depression—symptoms associated with IBS and are part of the GWI.
If altered gut microbiota contributes to the etiopathogenesis of IBS, its restoration of with probiotics should help. Probiotics are live organisms that when ingested may improve health by promoting the growth of naturally occurring flora and establishing a healthy gut flora. Probiotics have several mechanisms of actions. Probiotics work in the lumen of the gut by producing antibacterial molecules and enhancing the mucosal barrier.11 Probiotics also may produce metabolic compounds that alter the intestinal microbiota and improve intestinal barrier function.12 Probiotics also have been shown to activate receptors in the enteric nervous system with the potential to promote pain relief in the setting of visceral hyperalgesia.13,14 The anti-inflammatory properties of probiotics potentially could modulate the basic pathophysiology of IBS and improve motility, visceral hypersensitivity, and brain-gut interaction.15 Furthermore, significant gut dysbiosis has been shown with GWI; suggesting that probiotics may have a role in its management.16,17
Probiotics have not been studied in Gulf War veterans with IBS. We performed a prospective, double-blind placebo-controlled study to determine the efficacy of a commercially available probiotic containing 8 strains of bacteria (De Simone Formulation; formally known as VSL#3 and Visbiome) on symptoms of IBS and GWI. This probiotic was selected as the overall literature suggested benefit of combination probiotics in IBS, and VSL#3 has been shown to be efficacious in ulcerative colitis and microscopic colitis.18-20
Methods
Veterans who served in Operation Desert Storm (August 1990 to March 1991) and enrolled at the George E. Wahlen Veterans Affairs (VA) Medical Center (GEWVAMC), Salt Lake City, Utah, were eligible for the study. The inclusion criteria were: veterans aged ≥ 35 years; ≥ 2 nonintestinal GWI symptoms (eg, fatigue, joint pains, insomnia, general stiffness, and headache); IBS diagnosis based on the Rome III criteria; IBS symptoms > 6 months; normal gross appearance of the colonic mucosa; negative markers for celiac disease and inflammatory bowel disease (IBD); normal thyroid function; and serum calcium levels.21 Those who had a clinically significant cardiac, pulmonary, hepatic or renal dysfunction; history of/or presence of systemic malignancy; current evidence of celiac disease or IBD; unstable/significant psychiatric disease; recent change in GI medications; current pregnancy; or use of antibiotics or probiotics within the past 1 month were excluded. Subjects were enrolled from a list of veterans with GWI from the GEWVAMC Gulf War registry; referrals to gastroenterology clinics for IBS from internal medicine clinics; and posted advertisements.
Protocol
After written informed consent was obtained, each veteran was verified to have IBS and ≥ 2 GWI symptoms. All veterans had the following tests and panels: complete blood count, erythrocyte sedimentation rate, serum comprehensive metabolic panel, thyroid-stimulating hormone, tissue transglutaminase, stool test for ova and parasite, giardia antigen, and clostridia toxins to exclude organic cause of GI symptoms. Colonoscopy was performed in all veterans to exclude IBD, and to rule out microscopic or lymphocytic colitis.
Randomization was computer generated and maintained by the study pharmacist so that study personnel and patients were blinded to the trial groups. All investigators were blinded and allocation was concealed. The medication was supplied in a numbered container by the pharmacist after patient enrollment. After a 2-week run-in period, veterans were randomized (1:1) to receive either 1 sachet of probiotic (De Simone Formulation; formally known as VSL#3 and Visbiome) or placebo once daily for 8 weeks.
Each probiotic packet contains 900 billion probiotic bacteria per sachet.11 This formulation contained 8 viable strains of bacteria: 4 strains of Lactobacillus (L acidophilus, L plantarum, L paracasei, L delbrueckii subsp. bulgaricus); 3 strains of Bifidobacteria (Bifidobacterium breve, B lactis, B infantis); and 1 strain of Streptococcus thermophilus. This formulation had been commercialized and studied as VSL#3 and is currently available in the United States under the Visbiome trade name. While branding changed during the study, the formulation did not. The investigational medicine (VSL#3, Visbiome, and placebo) were shipped from the manufacturer Dupont/Danisco in Madison, Wisconsin. The subjects received placebo or probiotic (VSL#3/Visbiome) and both were identical in appearance. The medication was supplied in a numbered container by the pharmacist after patient enrollment.
Measures
Veterans completed the bowel disease questionnaire to record baseline bowel habits.22 All veterans recorded daily bowel symptoms to confirm the presence of IBS during the 2-week pretreatment period, at baseline, and at the end of the 8-week treatment. The symptoms assessed included severity of abdominal pain (0, none to 100, severe); severity of bloating (0, none to 100, severe); stool frequency; Bristol stool scale (1, very hard to 7, watery); severity of diarrhea (0, none to 100, severe); severity of constipation (0, none to 100, severe); satisfaction with bowel habits (0, none to 100, severe); and IBS affecting or interfering with life (0, none to 100, severe). The bowel symptom score is the sum of the 5 symptom scores.23,24
IBS-specific QOL (IBS-QOL) was recorded at baseline and at the end of treatment.25 The IBS-QOL consists of a 34-item validated disease-specific questionnaire that measures 8 domains relevant to subjects with IBS: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual life, and relationships. We used the Somatic Symptom Checklist to detect the following extra-intestinal symptoms that are common among veterans with GWI: headache, backache, wheeziness, insomnia, bad breath, fatigue, general stiffness, dizziness, weakness, sensitivity to hot and cold, palpitation, and tightness in chest. Subjects rated symptoms on a scale of 1 to 5: how often (1, none; 2, monthly; 3, once weekly; 4, several times weekly; 5, daily), and how bothersome (1, not at all to 5, extremely).26
Subjects completed the Posttraumatic Stress Disorder (PTSD) Checklist–Military, which is specific to military experience with 17 items on a 1 to 5 scale (1, not at all to 5, extremely). Scores were summed to produce a total symptom severity score (range, 17-85).27 Subjects also completed the Brief Symptom Inventory 18 (BSI-18) during the baseline evaluation.28 BSI-18 measures subjects’ reported overall psychological distress. It assesses 3 symptoms dimensions (somatization, depression, and anxiety) and a global severity index. The raw scores were transferred to normative T scores based on samples of nonpatient normal men and women.
Symptom data were compared after 8 weeks of treatment. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, extra-intestinal symptoms, and PTSD score. The study was approved by the Salt Lake City Veterans Affairs Medical Center and the University of Utah Institutional Review Board and registered in ClinicalTrials.gov (NCT03078530).
Statistical Methods
Comparisons of the probiotic vs placebo groups for demographic variable were analyzed using a 2-sample t test for continuous variables, and with a χ2 test or Fisher exact test for categorical variables. The primary and secondary outcome variables were recorded daily for 2 weeks as pretreatment baseline and for 2 weeks at the end of treatment. These symptoms were recorded as ordered categorical variables, which were then averaged across the week to produce a continuous measurement for statistical analysis. For the primary outcome of GI symptoms, posttreatment comparisons were made between the study groups using a 2-sample t test of the baseline vs posttreatment values. All P values were calculated for 2-sided comparisons. The planned sample size in our study protocol was to recruit 40 individuals per group in order to achieve 80% power to detect a 30% improvement between baseline and end of treatment in the primary bowel symptom score. This study recruited 53 subjects. With this sample size, the study had 80% power to detect a 0.8 SD in any of the outcomes.
Results
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.
An individual’s response to probiotics may depend on the subtype of IBS. However, most of the studies, like ours, included groups of all subtypes. It may be that probiotics are more effective in patients with moderate-to-severe symptoms. Most of our patients had milder symptoms, and we cannot discount how subjects with more severe disease may have responded to the drug. Interestingly, one study demonstrated that Lactobacillus was more effective in patients with moderately severe abdominal pain compared with mild symptoms.44
In our study, the probiotic did not improve PTSD symptoms or other extra-intestinal symptoms common in IBS and GWI. Similar to our study, Wong and colleagues did not find significant improvement of psychological and sleep scores after treatment with VSL#3.6 Similarly, there is evidence that alteration in gut microbiota is associated with health and diseases, but what specific alterations occur and whether they can be improved with probiotics remains unknown.45
Limitations
The inconsistent response to probiotics in various studies may be due to IBS heterogeneity. Furthermore, there are demographic differences between Gulf War veterans and patients enrolled in other studies: Gulf War veterans are predominantly male, many were deployed abroad and had a history of gastroenteritis during deployment, and were exposed to stressful situations.46 These factors may be involved in triggering or maintaining IBS in Gulf War veterans. A further limitation of our randomized trial is the relatively small sample size.
Conclusions
This study did not demonstrate statistically significant improvement in symptoms of IBS or improvement in QOL after treatment with a multistrain probiotic. We also did not find any improvement in symptoms of GWI or PTSD. There was no difference in psychological scores between the placebo and treatment groups, and it is unlikely that psychological factors confounded the response to treatment in this study.
The effectiveness of a probiotic may depend on the baseline gut microbiome of the individual and depend on the strain, amount, and frequency of bacteria used. A lack of response of the probiotics does not exclude gut viruses and fungi having a role in exacerbating GWI symptoms. It is also possible that the bacteria present or the dose of the probiotic used was not sufficient to improve symptoms. So far, the definitive benefit of probiotics has been demonstrated for only a few preparations, and none are approved by the US Food and Drug Administration for any disease. More research is needed to determine whether probiotics have any role in the treatment of IBS and GWI.
Acknowledgments
AKT received grant support from the US Department of Veterans Affairs and the US Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). We thank Keith G. Tolman, MD, for assistance in editing the initial proposal and for periodic consultation. We thank the manufacturer of the probiotic for supplying the active drug and the placebo. The manufacture of the probiotic had no role in the design and conduct of the study, analysis and interpretation of the data, and in the preparation of the manuscript.
About 700,000 US military personnel were deployed in Operation Desert Storm (August 1990 to March 1991).1 Almost 30 years since the war, a large number of these veterans continue to experience a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly affects health and quality of life (QOL). The lack of clear etiology of the illness has impaired research to find specific treatments and has further exacerbated the stress among veterans. GWI typically includes a mixture of chronic headache, cognitive difficulties, widespread pain, unexplained fatigue, memory and concentration problems, as well as chronic respiratory and gastrointestinal (GI) symptoms.2 Abdominal pain and alteration of bowel habits are also symptoms typical of irritable bowel syndrome (IBS). It has been estimated that IBS occurs in up to 30% of Gulf War veterans.3
The etiology of IBS is unknown. Possible mechanisms include visceral hypersensitivity, altered gut motor function, aberrant brain-gut interaction, and psychological factors, perhaps with a genetic predisposition.4 Gastroenteritis has been reported as a triggering mechanism in up to one-third of patients with IBS.5 Gastroenteritis can alter the gut microbiota and has been reported to be a significant risk factor for the development of IBS.6 In one study of Operation Desert Shield soldiers, > 50% of military personnel developed acute gastroenteritis while on duty.7 A high prevalence of extra-intestinal symptoms also has been reported, including fatigue, headache, joint pains, and anxiety, in Gulf War veterans with IBS. These extra-intestinal symptoms of IBS are consistent with the reported GWI symptoms. Change in gut microbiota also has been associated with many of the extra-intestinal symptoms of IBS, especially fatigue.8,9 Gut microbiota are known to change with travel, stress, and a change in diet, all potential factors that are relevant to Gulf War veterans. This would suggest that an imbalance in the gut microbiota, ie, dysbiosis, may play a role in the pathogenesis of both IBS and GWI. Dysbiosis could be a risk factor for or alternatively a consequence of GWI.
A systematic review highlighted the heterogeneity of the gut microbiota in patients with IBS.10 Overall, Enterobacteriaceae, Lactobacillaceae, and Bacteroides were increased, whereas Clostridiales, Faecalibacterium, and Bifidobacterium were decreased in patients with IBS compared with controls. Gut microbiota also has been associated with cognitive changes, anxiety, and depression—symptoms associated with IBS and are part of the GWI.
If altered gut microbiota contributes to the etiopathogenesis of IBS, its restoration of with probiotics should help. Probiotics are live organisms that when ingested may improve health by promoting the growth of naturally occurring flora and establishing a healthy gut flora. Probiotics have several mechanisms of actions. Probiotics work in the lumen of the gut by producing antibacterial molecules and enhancing the mucosal barrier.11 Probiotics also may produce metabolic compounds that alter the intestinal microbiota and improve intestinal barrier function.12 Probiotics also have been shown to activate receptors in the enteric nervous system with the potential to promote pain relief in the setting of visceral hyperalgesia.13,14 The anti-inflammatory properties of probiotics potentially could modulate the basic pathophysiology of IBS and improve motility, visceral hypersensitivity, and brain-gut interaction.15 Furthermore, significant gut dysbiosis has been shown with GWI; suggesting that probiotics may have a role in its management.16,17
Probiotics have not been studied in Gulf War veterans with IBS. We performed a prospective, double-blind placebo-controlled study to determine the efficacy of a commercially available probiotic containing 8 strains of bacteria (De Simone Formulation; formally known as VSL#3 and Visbiome) on symptoms of IBS and GWI. This probiotic was selected as the overall literature suggested benefit of combination probiotics in IBS, and VSL#3 has been shown to be efficacious in ulcerative colitis and microscopic colitis.18-20
Methods
Veterans who served in Operation Desert Storm (August 1990 to March 1991) and enrolled at the George E. Wahlen Veterans Affairs (VA) Medical Center (GEWVAMC), Salt Lake City, Utah, were eligible for the study. The inclusion criteria were: veterans aged ≥ 35 years; ≥ 2 nonintestinal GWI symptoms (eg, fatigue, joint pains, insomnia, general stiffness, and headache); IBS diagnosis based on the Rome III criteria; IBS symptoms > 6 months; normal gross appearance of the colonic mucosa; negative markers for celiac disease and inflammatory bowel disease (IBD); normal thyroid function; and serum calcium levels.21 Those who had a clinically significant cardiac, pulmonary, hepatic or renal dysfunction; history of/or presence of systemic malignancy; current evidence of celiac disease or IBD; unstable/significant psychiatric disease; recent change in GI medications; current pregnancy; or use of antibiotics or probiotics within the past 1 month were excluded. Subjects were enrolled from a list of veterans with GWI from the GEWVAMC Gulf War registry; referrals to gastroenterology clinics for IBS from internal medicine clinics; and posted advertisements.
Protocol
After written informed consent was obtained, each veteran was verified to have IBS and ≥ 2 GWI symptoms. All veterans had the following tests and panels: complete blood count, erythrocyte sedimentation rate, serum comprehensive metabolic panel, thyroid-stimulating hormone, tissue transglutaminase, stool test for ova and parasite, giardia antigen, and clostridia toxins to exclude organic cause of GI symptoms. Colonoscopy was performed in all veterans to exclude IBD, and to rule out microscopic or lymphocytic colitis.
Randomization was computer generated and maintained by the study pharmacist so that study personnel and patients were blinded to the trial groups. All investigators were blinded and allocation was concealed. The medication was supplied in a numbered container by the pharmacist after patient enrollment. After a 2-week run-in period, veterans were randomized (1:1) to receive either 1 sachet of probiotic (De Simone Formulation; formally known as VSL#3 and Visbiome) or placebo once daily for 8 weeks.
Each probiotic packet contains 900 billion probiotic bacteria per sachet.11 This formulation contained 8 viable strains of bacteria: 4 strains of Lactobacillus (L acidophilus, L plantarum, L paracasei, L delbrueckii subsp. bulgaricus); 3 strains of Bifidobacteria (Bifidobacterium breve, B lactis, B infantis); and 1 strain of Streptococcus thermophilus. This formulation had been commercialized and studied as VSL#3 and is currently available in the United States under the Visbiome trade name. While branding changed during the study, the formulation did not. The investigational medicine (VSL#3, Visbiome, and placebo) were shipped from the manufacturer Dupont/Danisco in Madison, Wisconsin. The subjects received placebo or probiotic (VSL#3/Visbiome) and both were identical in appearance. The medication was supplied in a numbered container by the pharmacist after patient enrollment.
Measures
Veterans completed the bowel disease questionnaire to record baseline bowel habits.22 All veterans recorded daily bowel symptoms to confirm the presence of IBS during the 2-week pretreatment period, at baseline, and at the end of the 8-week treatment. The symptoms assessed included severity of abdominal pain (0, none to 100, severe); severity of bloating (0, none to 100, severe); stool frequency; Bristol stool scale (1, very hard to 7, watery); severity of diarrhea (0, none to 100, severe); severity of constipation (0, none to 100, severe); satisfaction with bowel habits (0, none to 100, severe); and IBS affecting or interfering with life (0, none to 100, severe). The bowel symptom score is the sum of the 5 symptom scores.23,24
IBS-specific QOL (IBS-QOL) was recorded at baseline and at the end of treatment.25 The IBS-QOL consists of a 34-item validated disease-specific questionnaire that measures 8 domains relevant to subjects with IBS: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual life, and relationships. We used the Somatic Symptom Checklist to detect the following extra-intestinal symptoms that are common among veterans with GWI: headache, backache, wheeziness, insomnia, bad breath, fatigue, general stiffness, dizziness, weakness, sensitivity to hot and cold, palpitation, and tightness in chest. Subjects rated symptoms on a scale of 1 to 5: how often (1, none; 2, monthly; 3, once weekly; 4, several times weekly; 5, daily), and how bothersome (1, not at all to 5, extremely).26
Subjects completed the Posttraumatic Stress Disorder (PTSD) Checklist–Military, which is specific to military experience with 17 items on a 1 to 5 scale (1, not at all to 5, extremely). Scores were summed to produce a total symptom severity score (range, 17-85).27 Subjects also completed the Brief Symptom Inventory 18 (BSI-18) during the baseline evaluation.28 BSI-18 measures subjects’ reported overall psychological distress. It assesses 3 symptoms dimensions (somatization, depression, and anxiety) and a global severity index. The raw scores were transferred to normative T scores based on samples of nonpatient normal men and women.
Symptom data were compared after 8 weeks of treatment. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, extra-intestinal symptoms, and PTSD score. The study was approved by the Salt Lake City Veterans Affairs Medical Center and the University of Utah Institutional Review Board and registered in ClinicalTrials.gov (NCT03078530).
Statistical Methods
Comparisons of the probiotic vs placebo groups for demographic variable were analyzed using a 2-sample t test for continuous variables, and with a χ2 test or Fisher exact test for categorical variables. The primary and secondary outcome variables were recorded daily for 2 weeks as pretreatment baseline and for 2 weeks at the end of treatment. These symptoms were recorded as ordered categorical variables, which were then averaged across the week to produce a continuous measurement for statistical analysis. For the primary outcome of GI symptoms, posttreatment comparisons were made between the study groups using a 2-sample t test of the baseline vs posttreatment values. All P values were calculated for 2-sided comparisons. The planned sample size in our study protocol was to recruit 40 individuals per group in order to achieve 80% power to detect a 30% improvement between baseline and end of treatment in the primary bowel symptom score. This study recruited 53 subjects. With this sample size, the study had 80% power to detect a 0.8 SD in any of the outcomes.
Results
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.
An individual’s response to probiotics may depend on the subtype of IBS. However, most of the studies, like ours, included groups of all subtypes. It may be that probiotics are more effective in patients with moderate-to-severe symptoms. Most of our patients had milder symptoms, and we cannot discount how subjects with more severe disease may have responded to the drug. Interestingly, one study demonstrated that Lactobacillus was more effective in patients with moderately severe abdominal pain compared with mild symptoms.44
In our study, the probiotic did not improve PTSD symptoms or other extra-intestinal symptoms common in IBS and GWI. Similar to our study, Wong and colleagues did not find significant improvement of psychological and sleep scores after treatment with VSL#3.6 Similarly, there is evidence that alteration in gut microbiota is associated with health and diseases, but what specific alterations occur and whether they can be improved with probiotics remains unknown.45
Limitations
The inconsistent response to probiotics in various studies may be due to IBS heterogeneity. Furthermore, there are demographic differences between Gulf War veterans and patients enrolled in other studies: Gulf War veterans are predominantly male, many were deployed abroad and had a history of gastroenteritis during deployment, and were exposed to stressful situations.46 These factors may be involved in triggering or maintaining IBS in Gulf War veterans. A further limitation of our randomized trial is the relatively small sample size.
Conclusions
This study did not demonstrate statistically significant improvement in symptoms of IBS or improvement in QOL after treatment with a multistrain probiotic. We also did not find any improvement in symptoms of GWI or PTSD. There was no difference in psychological scores between the placebo and treatment groups, and it is unlikely that psychological factors confounded the response to treatment in this study.
The effectiveness of a probiotic may depend on the baseline gut microbiome of the individual and depend on the strain, amount, and frequency of bacteria used. A lack of response of the probiotics does not exclude gut viruses and fungi having a role in exacerbating GWI symptoms. It is also possible that the bacteria present or the dose of the probiotic used was not sufficient to improve symptoms. So far, the definitive benefit of probiotics has been demonstrated for only a few preparations, and none are approved by the US Food and Drug Administration for any disease. More research is needed to determine whether probiotics have any role in the treatment of IBS and GWI.
Acknowledgments
AKT received grant support from the US Department of Veterans Affairs and the US Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). We thank Keith G. Tolman, MD, for assistance in editing the initial proposal and for periodic consultation. We thank the manufacturer of the probiotic for supplying the active drug and the placebo. The manufacture of the probiotic had no role in the design and conduct of the study, analysis and interpretation of the data, and in the preparation of the manuscript.
1. O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025.
2. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987.
3. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.066100
4. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001.
5. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142.
6. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8.
7. Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325(20):1423-1428. doi:10.1056/NEJM199111143252006 8. Clancy RL, Gleeson M, Cox A, et al. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40(4):351-354. doi:10.1136/bjsm.2005.024364
9. Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi:10.1186/1475-2891-8-4
10. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108. doi:10.1053/j.gastro.2019.03.049
11. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9(2):99-107. doi:10.2174/1573401311309020004
12. O´Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025
13. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987
14. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.06610015. O´Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. doi:10.1053/j.gastro.2004.11.050
16. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.17. Janulewicz PA, Seth RK, Carlson JM, et al. The gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193751
18. Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846. doi:10.1371/journal.pone.0228846
19. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561; quiz 1546, 1562. doi:10.1038/ajg.2014.202
20. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2(1):e000018. doi:10.1136/bmjgast-2014-000018
21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. doi:10.1053/j.gastro.2005.11.061
22. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111(8):671-674. doi:10.7326/0003-4819-111-8-671
23. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585-1589. doi:10.1001/jama.280.18.1585
24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395-402. doi:10.1046/j.1365-2036.1997.142318000.x
25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400-411. doi:10.1023/a:1018831127942
26. Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7(2):247-257. doi:10.1007/BF00845390
27. Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Accessed August 25, 2022. https://www.researchgate.net/publication/291448760_The_PTSD_Checklist_PCL_Reliability_validity_and_diagnostic_utility
28. Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. National Computer Systems; 2000.
29. Stamps BW, Lyon WJ, Irvin AP, Kelley-Loughnane N, Goodson MS. A pilot study of the effect of deployment on the gut microbiome and traveler´s diarrhea susceptibility. Front Cell Infect Microbiol. 2020;10:589297. doi:10.3389/fcimb.2020.589297
30. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. doi:10.1046/j.1365-2036.2003.01543.x
31. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687-696. doi:10.1111/j.1365-2982.2005.00695.x32. Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of vsl#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. doi:10.1007/s12602-010-9059-y
33. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-332. doi:10.1136/gut.2008.167270

34. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001
35. Dale HF, Rasmussen SH, Asiller OO, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9). doi:10.3390/nu11092048
36. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072-84. doi:10.3748/wjg.v21.i10.3072
37. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054-1070. doi:10.1111/apt.14539
38. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142
39. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8
40. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26; quiz S27. doi: 10.1038/ajg.2014.187
41. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76. doi:10.1136/gutjnl-2012-302167
42. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. doi:10.1097/MCG.0b013e31823712b1
43. Connell M, Shin A, James-Stevenson T, Xu H, Imperiale TF, Herron J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(12):e13427. doi:10.1111/nmo.13427
44. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. doi:10.3748/wjg.v22.i48.10631
45. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796. doi:10.1136/gutjnl-2012-302504
46. Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56(11):3109-3111. doi:10.1007/s10620-011-1856-y
1. O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025.
2. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987.
3. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.066100
4. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001.
5. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142.
6. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8.
7. Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325(20):1423-1428. doi:10.1056/NEJM199111143252006 8. Clancy RL, Gleeson M, Cox A, et al. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40(4):351-354. doi:10.1136/bjsm.2005.024364
9. Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi:10.1186/1475-2891-8-4
10. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108. doi:10.1053/j.gastro.2019.03.049
11. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9(2):99-107. doi:10.2174/1573401311309020004
12. O´Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025
13. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987
14. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.06610015. O´Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. doi:10.1053/j.gastro.2004.11.050
16. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.17. Janulewicz PA, Seth RK, Carlson JM, et al. The gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193751
18. Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846. doi:10.1371/journal.pone.0228846
19. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561; quiz 1546, 1562. doi:10.1038/ajg.2014.202
20. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2(1):e000018. doi:10.1136/bmjgast-2014-000018
21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. doi:10.1053/j.gastro.2005.11.061
22. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111(8):671-674. doi:10.7326/0003-4819-111-8-671
23. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585-1589. doi:10.1001/jama.280.18.1585
24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395-402. doi:10.1046/j.1365-2036.1997.142318000.x
25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400-411. doi:10.1023/a:1018831127942
26. Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7(2):247-257. doi:10.1007/BF00845390
27. Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Accessed August 25, 2022. https://www.researchgate.net/publication/291448760_The_PTSD_Checklist_PCL_Reliability_validity_and_diagnostic_utility
28. Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. National Computer Systems; 2000.
29. Stamps BW, Lyon WJ, Irvin AP, Kelley-Loughnane N, Goodson MS. A pilot study of the effect of deployment on the gut microbiome and traveler´s diarrhea susceptibility. Front Cell Infect Microbiol. 2020;10:589297. doi:10.3389/fcimb.2020.589297
30. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. doi:10.1046/j.1365-2036.2003.01543.x
31. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687-696. doi:10.1111/j.1365-2982.2005.00695.x32. Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of vsl#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. doi:10.1007/s12602-010-9059-y
33. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-332. doi:10.1136/gut.2008.167270

34. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001
35. Dale HF, Rasmussen SH, Asiller OO, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9). doi:10.3390/nu11092048
36. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072-84. doi:10.3748/wjg.v21.i10.3072
37. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054-1070. doi:10.1111/apt.14539
38. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142
39. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8
40. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26; quiz S27. doi: 10.1038/ajg.2014.187
41. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76. doi:10.1136/gutjnl-2012-302167
42. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. doi:10.1097/MCG.0b013e31823712b1
43. Connell M, Shin A, James-Stevenson T, Xu H, Imperiale TF, Herron J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(12):e13427. doi:10.1111/nmo.13427
44. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. doi:10.3748/wjg.v22.i48.10631
45. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796. doi:10.1136/gutjnl-2012-302504
46. Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56(11):3109-3111. doi:10.1007/s10620-011-1856-y
Spontaneous ecchymoses
A 65-YEAR-OLD WOMAN was brought into the emergency department by her daughter for spontaneous bruising, fatigue, and weakness of several weeks’ duration. She denied taking any medications or illicit drugs and had not experienced any falls or trauma. On a daily basis, she smoked 5 to 7 cigarettes and drank 6 or 7 beers, as had been her custom for several years. The patient lived alone and was grieving the death of her beloved dog, who had died a month earlier. She reported that since the death of her dog, her diet, which hadn’t been especially good to begin with, had deteriorated; it now consisted of beer and crackers.
On admission, she was mildly tachycardic (105 beats/min) with a blood pressure of 125/66 mm Hg. Physical examination revealed a frail-appearing woman who was in no acute distress but was unable to stand without assistance. She had diffuse ecchymoses and perifollicular, purpuric, hyperkeratotic papules and plaques on both of her legs (FIGURES 1A and 1B). In addition, she had faint perifollicular purpuric macules on her upper back. An oral examination revealed poor dentition.
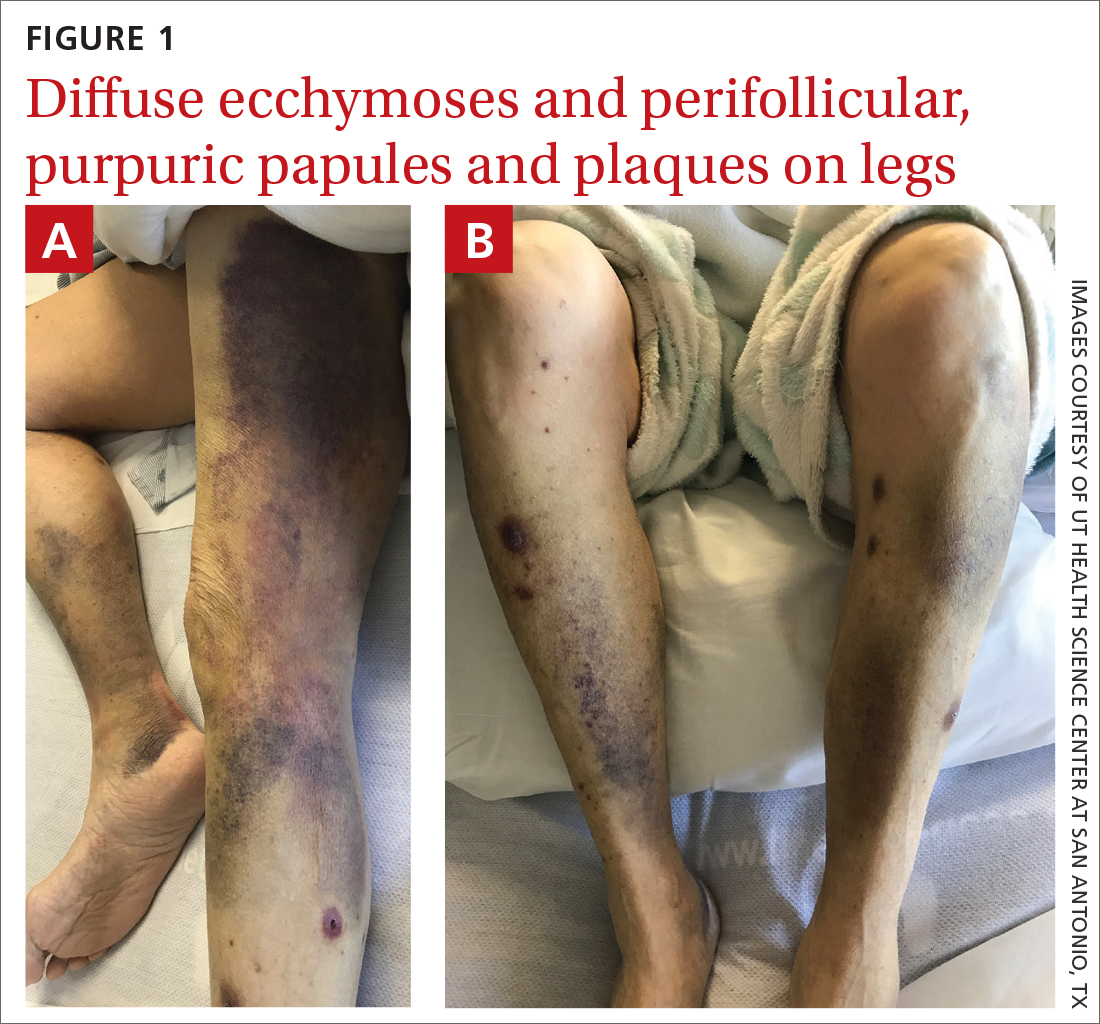
A punch biopsy was performed on her leg, and it revealed noninflammatory dermal hemorrhage without evidence of vasculitis or vasculopathy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scurvy
Based on the patient’s appearance and her dietary history, we suspected scurvy, so a serum vitamin C level was ordered. The results took several days to return. In the meantime, additional lab work revealed hyponatremia (sodium, 129 mmol/L; normal range, 135-145 mmol/L), hypokalemia (potassium, 3 mmol/L; normal range, 3.5-5.2 mmol/L), hypophosphatemia (phosphorus, 2.3 mg/dL; normal range, 2.8-4.5 mg/dL); low serum vitamin D (6 ng/mL; normal range, 20-40 ng/mL); and macrocytic anemia (hemoglobin, 7.4 g/dL; normal range, 11-18 g/dL) with a mean corpuscular volume of 101.1 fL (normal range, 80-100 fL). Her iron panel showed normal serum iron and total iron binding capacity with a normal ferritin level (294 ng/mL; normal range, 30-300 ng/mL). A peripheral blood smear test uncovered mild anisocytosis and polychromasia, with no schistocytes. A fecal immunochemical test was negative.
Several days after admission, the results of the patient’s vitamin C test came back. Her levels were undetectable (< 5 µmol/L; normal range, 11-23 µmol/L), confirming that the patient had scurvy.
A health hazard to marinersthat is still around today
Scurvy is a condition that arises from a deficiency of vitamin C, or ascorbic acid. The first known case of scurvy was in 1550 BC.1 Hippocrates termed the condition “ileos ematitis” and stated that “the mouth feels bad; the gums are detached from the teeth; blood runs from the nostrils … ulcerations on the legs … skin is thin.”1 Scurvy was a major health hazard of mariners between the 15thand 18th centuries.2 Today, the deficiency is uncommon in industrialized countries because there are many sources of vitamin C available through diet and vitamin supplements.3 In the United States, the prevalence of vitamin C deficiency is approximately 7%.4
An essential nutrient in humans, vitamin C is required as a cofactor in the synthesis of mature collagen.3 Collagen is found in skin, bone, and endothelium. Inadequate collagen levels can result in poor dermal support of vessels and tissue fragility, leading to hemorrhage, which can occur in nearly any organ system.
Vitamin C deficiency occurs when serum concentration falls below 11.4
Continue to: Scurvy manifests after 8 to 12 weeks
Scurvy manifests after 8 to 12 weeks of inadequate vitamin C intake.1 Patients may initially experience malaise and irritability. Anemia is common. Dermatologic findings include hyperkeratotic lesions, ecchymoses, poor wound healing, gingival swelling with loss of teeth, petechiae, and corkscrew hairs. Perifollicular hemorrhage is a characteristic finding of scurvy, generally seen on the lower extremities, where the capillaries are under higher hydrostatic pressure.3 Patients may also have musculoskeletal involvement with osteopenia or hemarthroses, which may be seen on imaging.3,5 Cardiorespiratory, gastrointestinal, ophthalmologic, and neurologic findings have also been reported.3
Differential is broad; zero in on patient’s history
The differential diagnosis for hemorrhagic skin lesions is extensive and includes scurvy, coagulopathies, trauma, vasculitis, and vasculopathies.
The presence of perifollicular hemorrhage with corkscrew hairs and a dietary history of inadequate vitamin C intake can differentiate scurvy from other conditions. Serum testing revealing low plasma vitamin C will support the diagnosis, but this is an insensitive test, as values increase with recent intake. Leukocyte ascorbic acid concentrations are more representative of total body stores, but impractical for routine use.6 Skin biopsy is not necessary but may help to rule out other conditions.
Ascorbic acid will facilitate a speedy recovery
Treatment of scurvy includes vitamin C replacement. Response is rapid, with improvement to lethargy within several days and disappearance of other manifestations within several weeks.3 Recommendations on supplementation doses and forms vary, but adults require 300 to 1000 mg/d of ascorbic acid for at least 1 week or until clinical symptoms resolve and stores are repleted.3,5,7
During our patient’s hospital stay, she remained stable and improved clinically with vitamin supplementation (ascorbic acid 1 g/d for 3 days, 500 mg/d after that) and physical therapy. She was counseled on a healthy diet, which would include citrus fruits, tomatoes, and leafy vegetables. The patient was also advised to refrain from drinking alcohol and was given information on an alcohol abstinence program.
At her 1-month follow-up, her condition had improved with near resolution of the skin lesions. She reported that she had given up cigarettes and alcohol. She said she’d also begun eating more citrus fruits and leafy vegetables.
1. Maxfield L, Crane JS. Vitamin C deficiency (scurvy). In: StatPearls. StatPearls Publishing; 2020. Accessed on September 13, 2022. www.ncbi.nlm.nih.gov/books/NBK493187/
2. Worral S. A nightmare disease haunted ships during age of discovery. National Geographic. January 15, 2017. Accessed September 21, 2022. www.nationalgeographic.com/science/article/scurvy-disease-discovery-jonathan-lamb
3. Hirschmann JV, Raugi GJ. Adult Scurvy. J Am Acad Dermatol. 1999;41:895-906. doi: 10.1016/s0190-9622(99)70244-6
4. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. doi: 10.3945/ajcn.2008.27016
5. Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group – A disease often forgotten? J Clin Orthop Trauma. 2015;6:101-107. doi: 10.1016/j.jcot.2014.12.003
6. Scurvy and its prevention and control in major emergencies. World Health Organization. February 23, 1999. Accessed September 13, 2022. www.who.int/publications/i/item/WHO-NHD-99.11
7. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. doi: 10.1542/peds.108.3.e55
A 65-YEAR-OLD WOMAN was brought into the emergency department by her daughter for spontaneous bruising, fatigue, and weakness of several weeks’ duration. She denied taking any medications or illicit drugs and had not experienced any falls or trauma. On a daily basis, she smoked 5 to 7 cigarettes and drank 6 or 7 beers, as had been her custom for several years. The patient lived alone and was grieving the death of her beloved dog, who had died a month earlier. She reported that since the death of her dog, her diet, which hadn’t been especially good to begin with, had deteriorated; it now consisted of beer and crackers.
On admission, she was mildly tachycardic (105 beats/min) with a blood pressure of 125/66 mm Hg. Physical examination revealed a frail-appearing woman who was in no acute distress but was unable to stand without assistance. She had diffuse ecchymoses and perifollicular, purpuric, hyperkeratotic papules and plaques on both of her legs (FIGURES 1A and 1B). In addition, she had faint perifollicular purpuric macules on her upper back. An oral examination revealed poor dentition.

A punch biopsy was performed on her leg, and it revealed noninflammatory dermal hemorrhage without evidence of vasculitis or vasculopathy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scurvy
Based on the patient’s appearance and her dietary history, we suspected scurvy, so a serum vitamin C level was ordered. The results took several days to return. In the meantime, additional lab work revealed hyponatremia (sodium, 129 mmol/L; normal range, 135-145 mmol/L), hypokalemia (potassium, 3 mmol/L; normal range, 3.5-5.2 mmol/L), hypophosphatemia (phosphorus, 2.3 mg/dL; normal range, 2.8-4.5 mg/dL); low serum vitamin D (6 ng/mL; normal range, 20-40 ng/mL); and macrocytic anemia (hemoglobin, 7.4 g/dL; normal range, 11-18 g/dL) with a mean corpuscular volume of 101.1 fL (normal range, 80-100 fL). Her iron panel showed normal serum iron and total iron binding capacity with a normal ferritin level (294 ng/mL; normal range, 30-300 ng/mL). A peripheral blood smear test uncovered mild anisocytosis and polychromasia, with no schistocytes. A fecal immunochemical test was negative.
Several days after admission, the results of the patient’s vitamin C test came back. Her levels were undetectable (< 5 µmol/L; normal range, 11-23 µmol/L), confirming that the patient had scurvy.
A health hazard to marinersthat is still around today
Scurvy is a condition that arises from a deficiency of vitamin C, or ascorbic acid. The first known case of scurvy was in 1550 BC.1 Hippocrates termed the condition “ileos ematitis” and stated that “the mouth feels bad; the gums are detached from the teeth; blood runs from the nostrils … ulcerations on the legs … skin is thin.”1 Scurvy was a major health hazard of mariners between the 15thand 18th centuries.2 Today, the deficiency is uncommon in industrialized countries because there are many sources of vitamin C available through diet and vitamin supplements.3 In the United States, the prevalence of vitamin C deficiency is approximately 7%.4
An essential nutrient in humans, vitamin C is required as a cofactor in the synthesis of mature collagen.3 Collagen is found in skin, bone, and endothelium. Inadequate collagen levels can result in poor dermal support of vessels and tissue fragility, leading to hemorrhage, which can occur in nearly any organ system.
Vitamin C deficiency occurs when serum concentration falls below 11.4
Continue to: Scurvy manifests after 8 to 12 weeks
Scurvy manifests after 8 to 12 weeks of inadequate vitamin C intake.1 Patients may initially experience malaise and irritability. Anemia is common. Dermatologic findings include hyperkeratotic lesions, ecchymoses, poor wound healing, gingival swelling with loss of teeth, petechiae, and corkscrew hairs. Perifollicular hemorrhage is a characteristic finding of scurvy, generally seen on the lower extremities, where the capillaries are under higher hydrostatic pressure.3 Patients may also have musculoskeletal involvement with osteopenia or hemarthroses, which may be seen on imaging.3,5 Cardiorespiratory, gastrointestinal, ophthalmologic, and neurologic findings have also been reported.3
Differential is broad; zero in on patient’s history
The differential diagnosis for hemorrhagic skin lesions is extensive and includes scurvy, coagulopathies, trauma, vasculitis, and vasculopathies.
The presence of perifollicular hemorrhage with corkscrew hairs and a dietary history of inadequate vitamin C intake can differentiate scurvy from other conditions. Serum testing revealing low plasma vitamin C will support the diagnosis, but this is an insensitive test, as values increase with recent intake. Leukocyte ascorbic acid concentrations are more representative of total body stores, but impractical for routine use.6 Skin biopsy is not necessary but may help to rule out other conditions.
Ascorbic acid will facilitate a speedy recovery
Treatment of scurvy includes vitamin C replacement. Response is rapid, with improvement to lethargy within several days and disappearance of other manifestations within several weeks.3 Recommendations on supplementation doses and forms vary, but adults require 300 to 1000 mg/d of ascorbic acid for at least 1 week or until clinical symptoms resolve and stores are repleted.3,5,7
During our patient’s hospital stay, she remained stable and improved clinically with vitamin supplementation (ascorbic acid 1 g/d for 3 days, 500 mg/d after that) and physical therapy. She was counseled on a healthy diet, which would include citrus fruits, tomatoes, and leafy vegetables. The patient was also advised to refrain from drinking alcohol and was given information on an alcohol abstinence program.
At her 1-month follow-up, her condition had improved with near resolution of the skin lesions. She reported that she had given up cigarettes and alcohol. She said she’d also begun eating more citrus fruits and leafy vegetables.
A 65-YEAR-OLD WOMAN was brought into the emergency department by her daughter for spontaneous bruising, fatigue, and weakness of several weeks’ duration. She denied taking any medications or illicit drugs and had not experienced any falls or trauma. On a daily basis, she smoked 5 to 7 cigarettes and drank 6 or 7 beers, as had been her custom for several years. The patient lived alone and was grieving the death of her beloved dog, who had died a month earlier. She reported that since the death of her dog, her diet, which hadn’t been especially good to begin with, had deteriorated; it now consisted of beer and crackers.
On admission, she was mildly tachycardic (105 beats/min) with a blood pressure of 125/66 mm Hg. Physical examination revealed a frail-appearing woman who was in no acute distress but was unable to stand without assistance. She had diffuse ecchymoses and perifollicular, purpuric, hyperkeratotic papules and plaques on both of her legs (FIGURES 1A and 1B). In addition, she had faint perifollicular purpuric macules on her upper back. An oral examination revealed poor dentition.

A punch biopsy was performed on her leg, and it revealed noninflammatory dermal hemorrhage without evidence of vasculitis or vasculopathy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scurvy
Based on the patient’s appearance and her dietary history, we suspected scurvy, so a serum vitamin C level was ordered. The results took several days to return. In the meantime, additional lab work revealed hyponatremia (sodium, 129 mmol/L; normal range, 135-145 mmol/L), hypokalemia (potassium, 3 mmol/L; normal range, 3.5-5.2 mmol/L), hypophosphatemia (phosphorus, 2.3 mg/dL; normal range, 2.8-4.5 mg/dL); low serum vitamin D (6 ng/mL; normal range, 20-40 ng/mL); and macrocytic anemia (hemoglobin, 7.4 g/dL; normal range, 11-18 g/dL) with a mean corpuscular volume of 101.1 fL (normal range, 80-100 fL). Her iron panel showed normal serum iron and total iron binding capacity with a normal ferritin level (294 ng/mL; normal range, 30-300 ng/mL). A peripheral blood smear test uncovered mild anisocytosis and polychromasia, with no schistocytes. A fecal immunochemical test was negative.
Several days after admission, the results of the patient’s vitamin C test came back. Her levels were undetectable (< 5 µmol/L; normal range, 11-23 µmol/L), confirming that the patient had scurvy.
A health hazard to marinersthat is still around today
Scurvy is a condition that arises from a deficiency of vitamin C, or ascorbic acid. The first known case of scurvy was in 1550 BC.1 Hippocrates termed the condition “ileos ematitis” and stated that “the mouth feels bad; the gums are detached from the teeth; blood runs from the nostrils … ulcerations on the legs … skin is thin.”1 Scurvy was a major health hazard of mariners between the 15thand 18th centuries.2 Today, the deficiency is uncommon in industrialized countries because there are many sources of vitamin C available through diet and vitamin supplements.3 In the United States, the prevalence of vitamin C deficiency is approximately 7%.4
An essential nutrient in humans, vitamin C is required as a cofactor in the synthesis of mature collagen.3 Collagen is found in skin, bone, and endothelium. Inadequate collagen levels can result in poor dermal support of vessels and tissue fragility, leading to hemorrhage, which can occur in nearly any organ system.
Vitamin C deficiency occurs when serum concentration falls below 11.4
Continue to: Scurvy manifests after 8 to 12 weeks
Scurvy manifests after 8 to 12 weeks of inadequate vitamin C intake.1 Patients may initially experience malaise and irritability. Anemia is common. Dermatologic findings include hyperkeratotic lesions, ecchymoses, poor wound healing, gingival swelling with loss of teeth, petechiae, and corkscrew hairs. Perifollicular hemorrhage is a characteristic finding of scurvy, generally seen on the lower extremities, where the capillaries are under higher hydrostatic pressure.3 Patients may also have musculoskeletal involvement with osteopenia or hemarthroses, which may be seen on imaging.3,5 Cardiorespiratory, gastrointestinal, ophthalmologic, and neurologic findings have also been reported.3
Differential is broad; zero in on patient’s history
The differential diagnosis for hemorrhagic skin lesions is extensive and includes scurvy, coagulopathies, trauma, vasculitis, and vasculopathies.
The presence of perifollicular hemorrhage with corkscrew hairs and a dietary history of inadequate vitamin C intake can differentiate scurvy from other conditions. Serum testing revealing low plasma vitamin C will support the diagnosis, but this is an insensitive test, as values increase with recent intake. Leukocyte ascorbic acid concentrations are more representative of total body stores, but impractical for routine use.6 Skin biopsy is not necessary but may help to rule out other conditions.
Ascorbic acid will facilitate a speedy recovery
Treatment of scurvy includes vitamin C replacement. Response is rapid, with improvement to lethargy within several days and disappearance of other manifestations within several weeks.3 Recommendations on supplementation doses and forms vary, but adults require 300 to 1000 mg/d of ascorbic acid for at least 1 week or until clinical symptoms resolve and stores are repleted.3,5,7
During our patient’s hospital stay, she remained stable and improved clinically with vitamin supplementation (ascorbic acid 1 g/d for 3 days, 500 mg/d after that) and physical therapy. She was counseled on a healthy diet, which would include citrus fruits, tomatoes, and leafy vegetables. The patient was also advised to refrain from drinking alcohol and was given information on an alcohol abstinence program.
At her 1-month follow-up, her condition had improved with near resolution of the skin lesions. She reported that she had given up cigarettes and alcohol. She said she’d also begun eating more citrus fruits and leafy vegetables.
1. Maxfield L, Crane JS. Vitamin C deficiency (scurvy). In: StatPearls. StatPearls Publishing; 2020. Accessed on September 13, 2022. www.ncbi.nlm.nih.gov/books/NBK493187/
2. Worral S. A nightmare disease haunted ships during age of discovery. National Geographic. January 15, 2017. Accessed September 21, 2022. www.nationalgeographic.com/science/article/scurvy-disease-discovery-jonathan-lamb
3. Hirschmann JV, Raugi GJ. Adult Scurvy. J Am Acad Dermatol. 1999;41:895-906. doi: 10.1016/s0190-9622(99)70244-6
4. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. doi: 10.3945/ajcn.2008.27016
5. Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group – A disease often forgotten? J Clin Orthop Trauma. 2015;6:101-107. doi: 10.1016/j.jcot.2014.12.003
6. Scurvy and its prevention and control in major emergencies. World Health Organization. February 23, 1999. Accessed September 13, 2022. www.who.int/publications/i/item/WHO-NHD-99.11
7. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. doi: 10.1542/peds.108.3.e55
1. Maxfield L, Crane JS. Vitamin C deficiency (scurvy). In: StatPearls. StatPearls Publishing; 2020. Accessed on September 13, 2022. www.ncbi.nlm.nih.gov/books/NBK493187/
2. Worral S. A nightmare disease haunted ships during age of discovery. National Geographic. January 15, 2017. Accessed September 21, 2022. www.nationalgeographic.com/science/article/scurvy-disease-discovery-jonathan-lamb
3. Hirschmann JV, Raugi GJ. Adult Scurvy. J Am Acad Dermatol. 1999;41:895-906. doi: 10.1016/s0190-9622(99)70244-6
4. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. doi: 10.3945/ajcn.2008.27016
5. Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group – A disease often forgotten? J Clin Orthop Trauma. 2015;6:101-107. doi: 10.1016/j.jcot.2014.12.003
6. Scurvy and its prevention and control in major emergencies. World Health Organization. February 23, 1999. Accessed September 13, 2022. www.who.int/publications/i/item/WHO-NHD-99.11
7. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. doi: 10.1542/peds.108.3.e55