User login
Vaccine update for the 2022-23 influenza season
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.
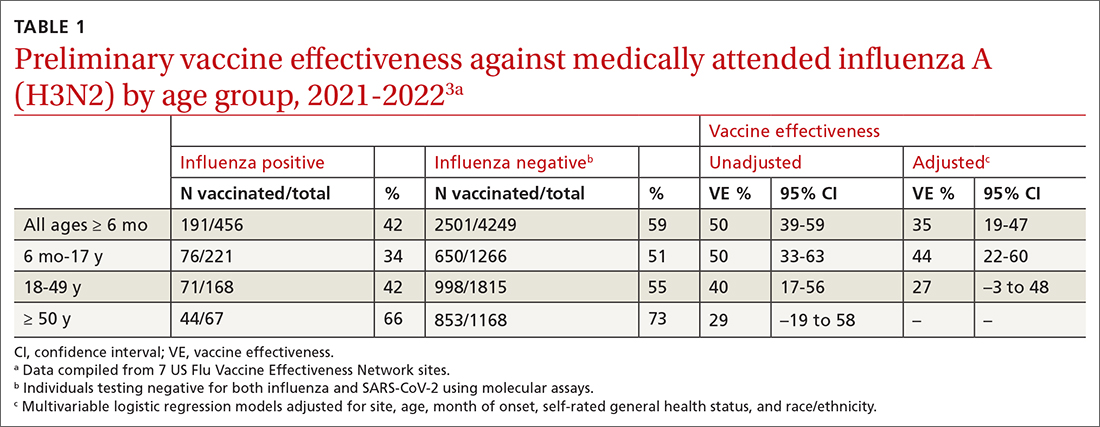
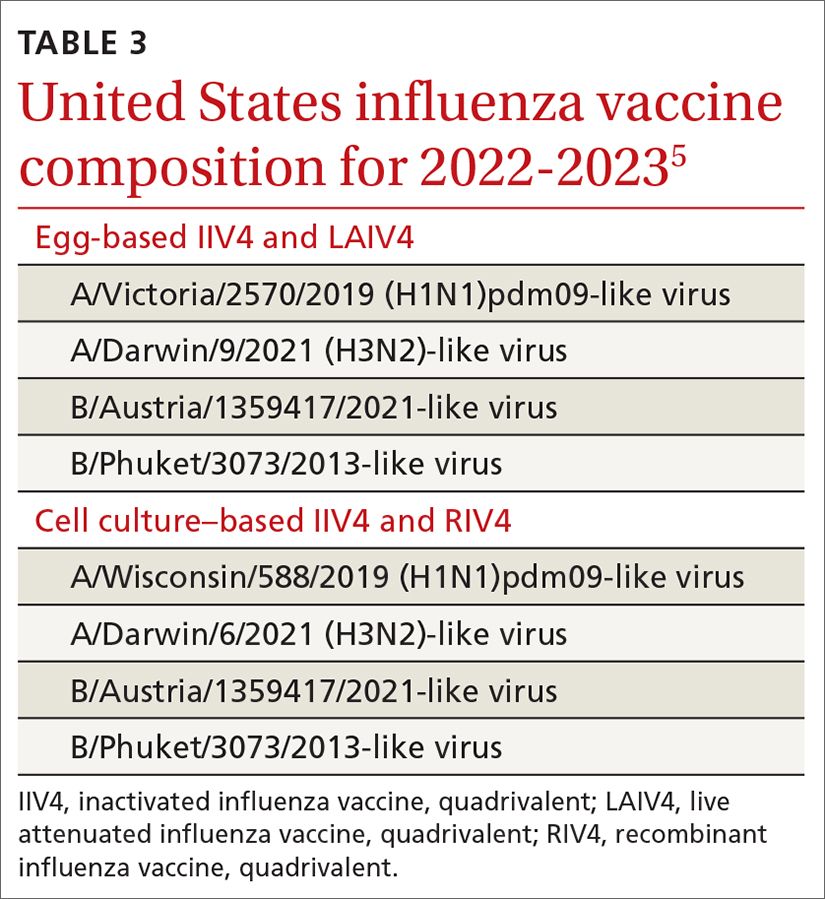
The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).
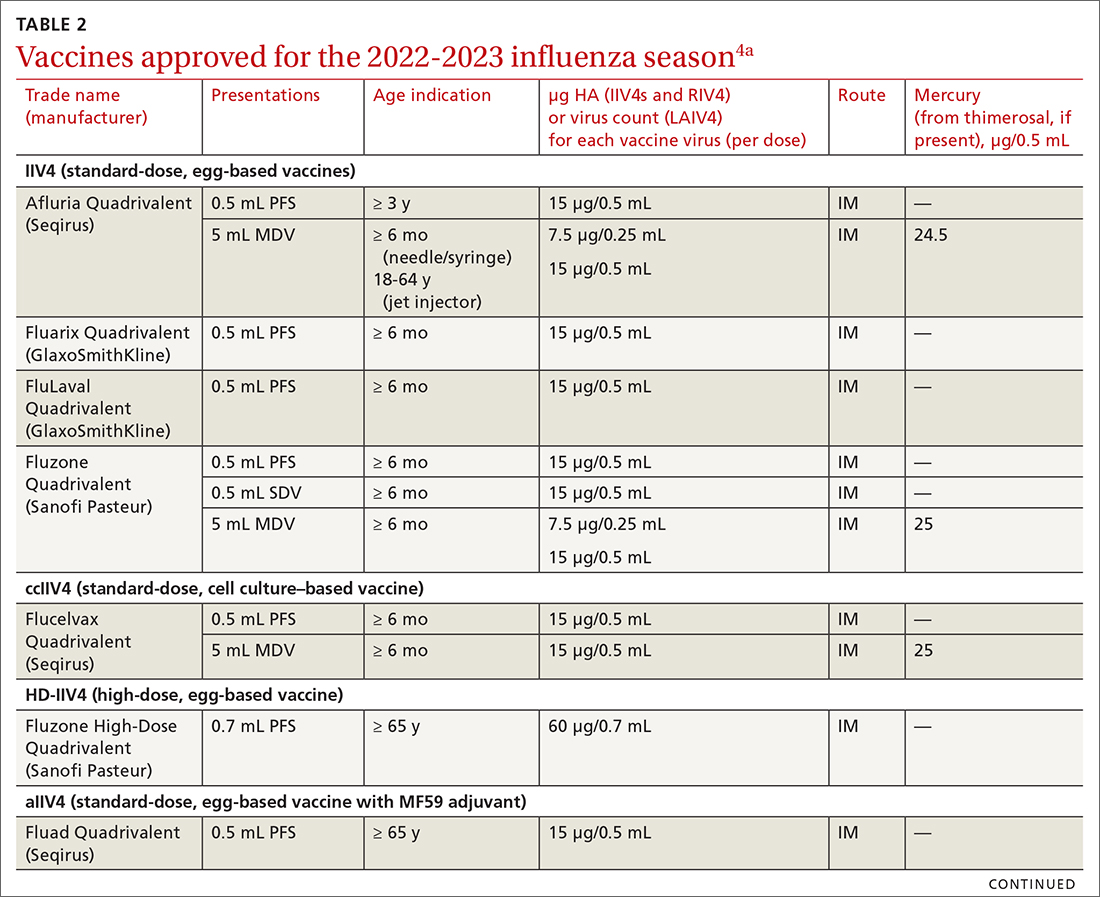
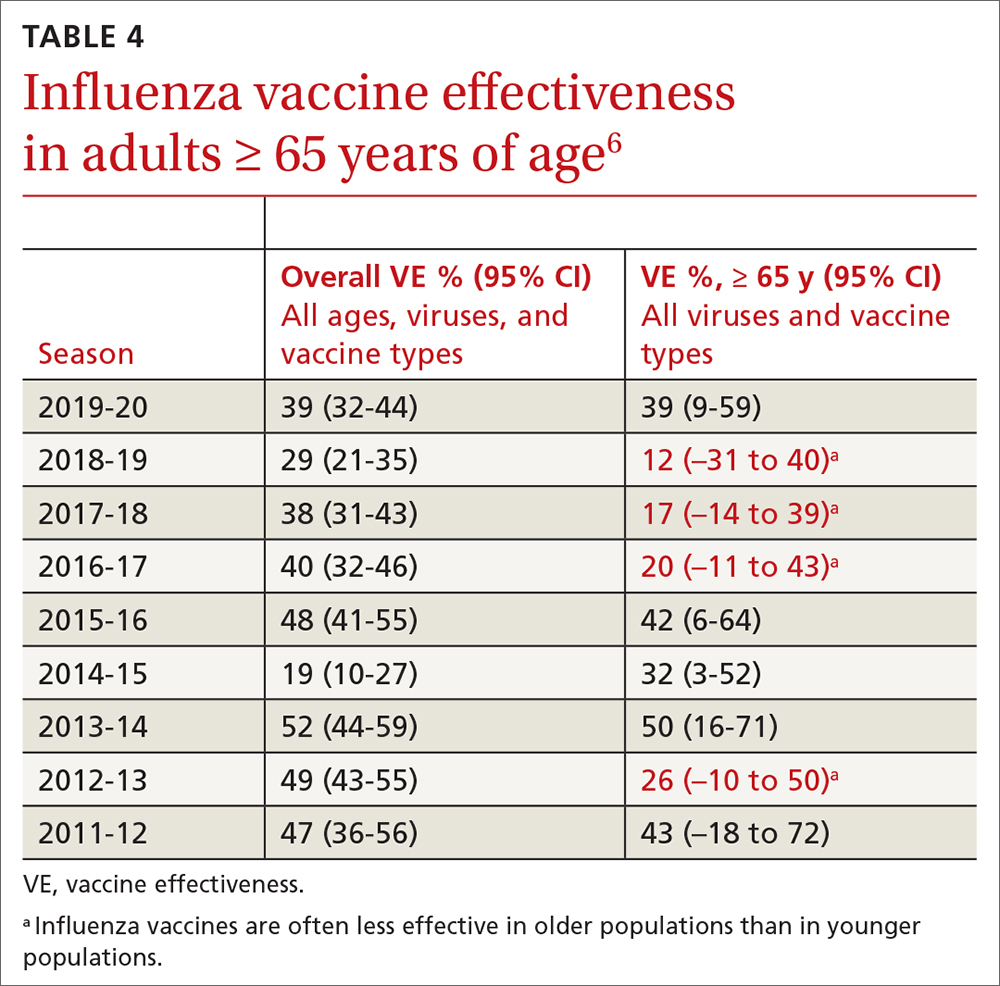
Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).
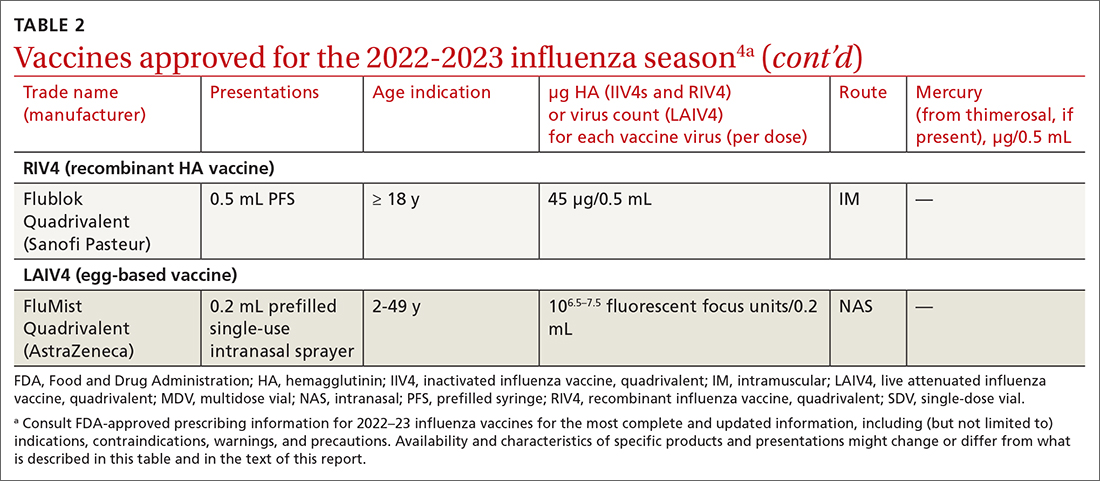
Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.
All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.
One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
Maximizing lifestyle changes to manage type 2 diabetes
Type 2 diabetes has been increasing in incidence and prevalence over the past 20 years, with worldwide prevalence estimated at 6.28%.1 The estimated cost of diagnosed diabetes in the United States was $327 billion in 2017; this included direct medical costs and reduced productivity.2 Type 2 diabetes can be prevented in most patients, given that it is a metabolic derangement caused by a complicated interaction between a patient’s genetic predisposition and lifestyle. A consensus statement by the American Academy of Clinical Endocrinologists (AACE) and American College of Endocrinology indicates that the recommended lifestyle modifications for diabetes include medical nutrition therapy with healthy eating patterns, regular physical activity, adequate sleep, behavioral support/counseling, and smoking cessation.3 Evidence shows that adherence to these lifestyle changes alone yields a relative reduction in type 2 diabetes mortality of 57%.4

In the discussion that follows, we review the current guideline recommendations for dietary modifications and physical activity and summarize their effectiveness in the treatment of type 2 diabetes. We also describe practical clinical strategies to promote change in patient behavior, and examine current literature supporting intensive lifestyle changes that, if achieved, may induce disease remission.5
Dietary strategies
Low, or very low, carbohydrate diet
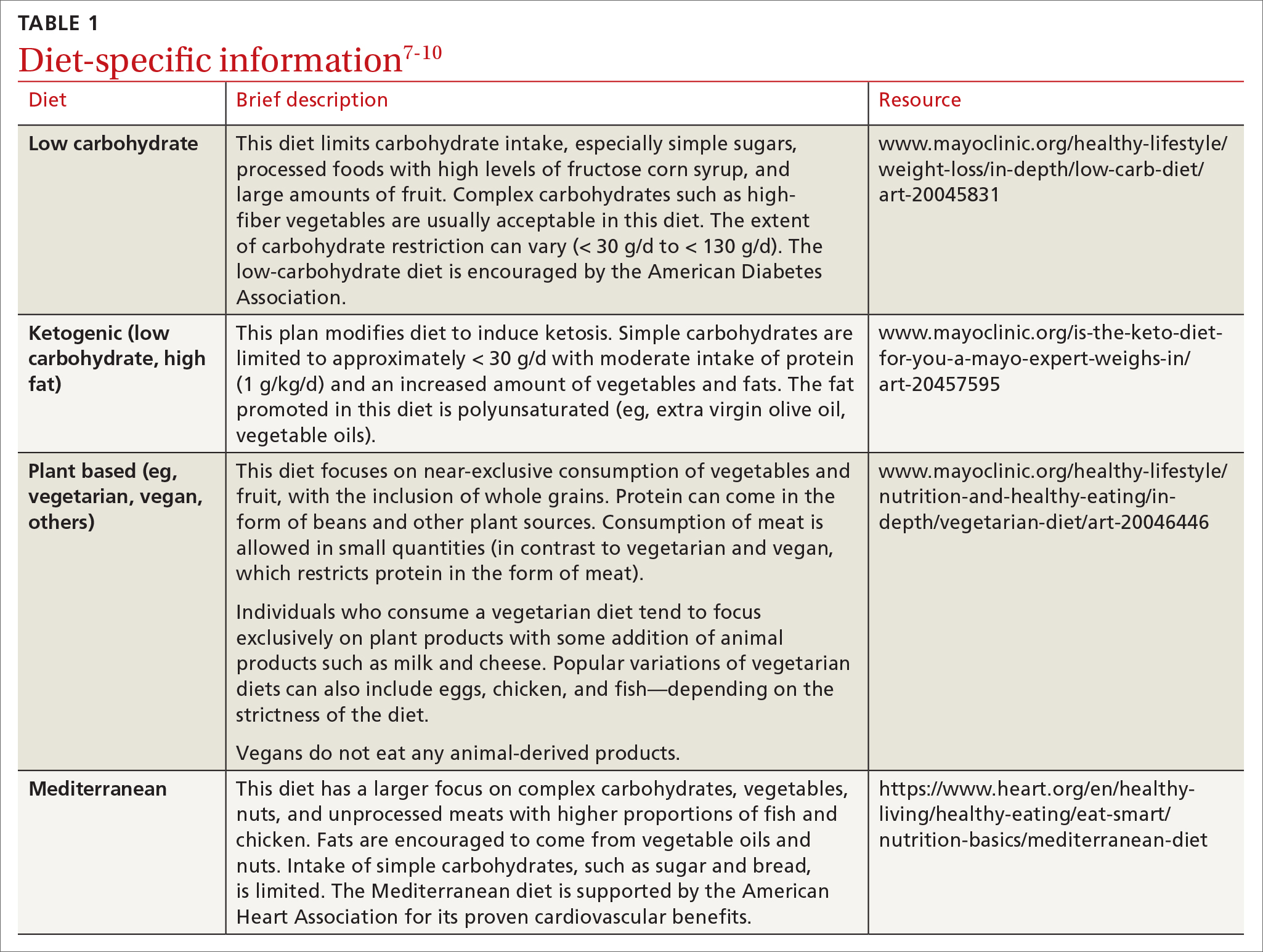
Carbohydrates can affect blood glucose levels in varying degrees depending on their intrinsic properties such as fiber content, sugars, and starches . 6 According to the American Diabetes Association’s (ADA) 2019 consensus report, 6 the carbohydrate quality that generally should be recommended is high in fiber, vitamins, and minerals, and low in added sugars, fats, and sodium (processed carbohydrates) ( TABLE 1 7-10 ). A low-carbohydrate diet (LCD) typically has a carbohydrate content < 130 g/d or < 26% of a 2000 kcal/d diet. 11 A very low–carbohydrate diet (VLCD) is 20-50 g/d or < 10% of the 2000 kcal/day diet. 11
In a meta-analysis by Goldenberg et al11, the LCD was shown to reduce A1C by 0.47% at 6 months (95% CI, –0.6 to –0.34) and by 0.23% at 12 months when compared with control diets. A review of multiple meta-analyses also showed a significant reduction in A1C especially with VLCD patterns; however, the results waned at the 12-month follow-up.5 In addition, confounding factors were seen when comparing adherence between LCD and VLCD, with patients in the latter group having larger problems with adherence, which decreased the benefit seen in the overall group comparison.11
Very low–carbohydrate/high-fat (ketogenic) diet
Ketogenic diets generally follow a VLCD with the carbohydrate portion set at 5% to 10% of total caloric intake (generally < 30 g/d) and the rest of the calories taken up by protein (typically 1 g/kg/d) and fat (TABLE 17-10).12 The fat content recommended is primarily polyunsaturated fat such as olive oil, while saturated fats such as butter and lard (animal fat) should be limited.
A recent meta-analysis by Choi et al12 showed that in overweight or obese patients with type 2 diabetes, the average A1C reduction was 0.62% (95% CI, –0.89 to –0.35) in the ketogenic intervention group. Another meta-analysis showed an even more significant A1C reduction at 1.07% (95% CI, –1.37 to –0.78).13 Concerns have been raised about the ketogenic diet, particularly as it relates to lipid metabolism and cholesterol levels; however, in the 2 referenced meta-analyses, the total cholesterol and triglyceride levels actually declined in the ketogenic intervention groups with minimal effect on LDL-C.12,13 This may alleviate some of the concerns of lipid management with this diet.
Plant-based diet
Popularized by Dr. T. Colin Campbell, a plant-based diet refers to a low-fat, high-fiber, whole-foods diet (whole fruits, vegetables, and naturally occurring carbohydrates, as opposed to processed foods). Examples of this type of diet include the popular vegan diet, which restricts all animal-derived products, and the vegetarian diet, which is generally limited to foods in the plant category with some addition of animal products, such as milk and cheese. Other variations of these diets exist and include other sources of protein (eg, chicken, eggs, or fish) (TABLE 17-10).
Continue to: A review by...
A review by Salas-Salvadó et al14 showed that a vegan diet yields an average A1C reduction of 0.41% (95% CI, –0.58 to –0.23).Several meta-analyses report similar effects on A1C with vegetarian and vegan eating patterns.6,15,16 The ADA review notes that weight loss was more significant in the vegan group and concluded that this diet should be studied further while controlling for weight loss.6
Mediterranean diet
The Mediterranean diet emphasizes vegetables, whole grains, fruits, lean meats, nuts, and olive oil. The benefits of the Mediterranean diet are well known and, as a result, the diet is recommended by organizations including the American Heart Association as part of a strategy to reduce cardiovascular risk (TABLE 17-10).
Mediterranean diet interventions have generally shown mixed effects on A1C reduction, weight management, and lipid control in type 2 diabetes. 6 The PREDIMED trial is the largest and longest randomized controlled trial to date comparing the Mediterranean diet to a low-fat diet. 17 This trial has reliably shown a reduced risk for type 2 diabetes and a trend to reduced A1C. 17 A reduction in the need for glucose-lowering medications was demonstrated in a subgroup analysis of the intervention group (adjusted hazard ratio = 0.78; 95% CI, 0.62-0.98). 18 Also, the Mediterranean diet has shown a significant reduction in the incidence of cardiovascular disease in patients with type 2 diabetes. 6
Physical activity and exercise
What do current guidelines recommend?
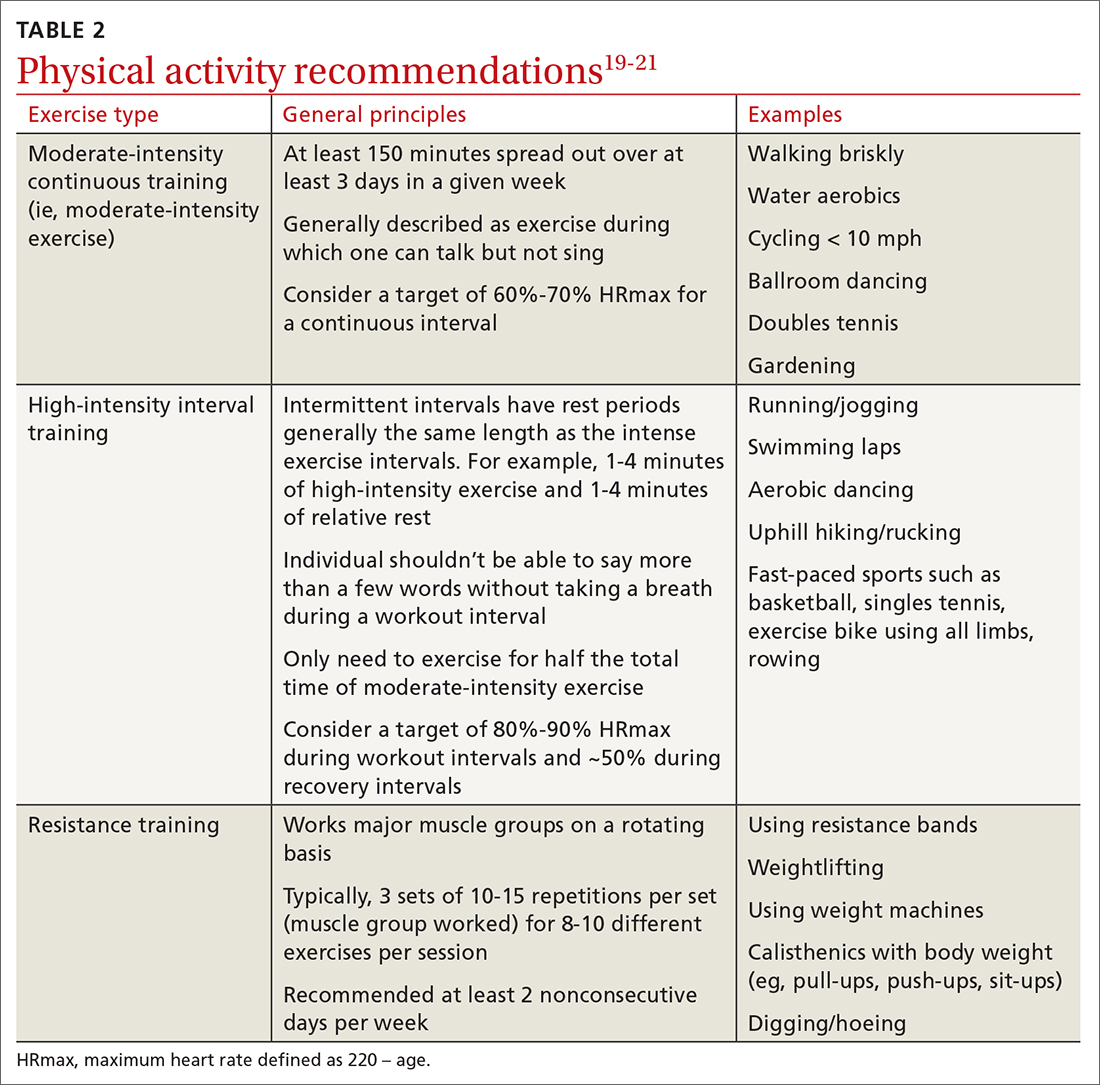
For most adults with type 2 diabetes, current guidelines by the ADA and by the National Institute of Diabetes and Digestive and Kidney Diseases recommend at least 150 minutes of moderate-to-vigorous intensity exercise every week spread out over at least 3 days, with no more than 2 consecutive days without exercise; and resistance training at least 2 other days per week which should balance all major muscle groups (TABLE 219-21). The benefits of exercise for type 2 diabetes have been well reviewed: positive effects on glucose control, insulin sensitivity, cardiovascular disease, lipid profiles, skeletal muscle metabolism, and solid-organ functioning.19,22,23
Grace et al24 showed in a meta-analysis that moderate aerobic exercise reduced A1C by 0.69% (95% CI, –1.09 to –0.3) at 13 weeks, and a Cochrane review showed an average A1C reduction of 0.6% with moderate-intensity exercise.25 Borror et al26 demonstrated in a systematic review that postprandial moderate-intensity aerobic exercise starting 1 hour after meals results in a reduced 24-hour prevalence of hyperglycemia (33.5% reduction vs control). A meta-analysis in China showed an average A1C reduction of 0.68% for patients performing a Tai Chi physical activity intervention.27
Continue to: Consider high-intensity interval training
Consider high-intensity interval training
Multiple randomized controlled trials highlight the benefits of high-intensity interval training (HIIT) (TABLE 219-21) compared with moderate-intensity continuous training (MICT) on improving A1C. A meta-analysis showed a weighted mean difference in A1C of 0.23% (95% CI, –0.43 to –0.02%).28 Also, a patient could spend less time performing HIIT as opposed to MICT to achieve the same benefits. For example, a patient typically performing 30 minutes of MICT may only need to perform 15 minutes of HIIT,a time-saving option for patients.20,22
Interrupt sedentary behavior
Risk for incident type 2 diabetes increases when someone is sedentary for more than 6 to 8 hours daily or watches TV for 3 to 4 hours (relative risk [RR] = 1.12).29 Recommendations for interrupting a sedentary lifestyle include standing from a seated position at least every 30 minutes and engaging in a light activity during the break interval for at least 3 minutes.19 Most studies have reliably shown that interrupting sedentary behavior reduces postprandial and 24-hour average blood glucose levels.19 Interrupted sitting/sedentary behavior has also been shown to reduce resting blood pressure in patients with type 2 diabetes.30
O ther important lifestyle factors
Encourage 7 to 8 hours of sleep
There is a U-shaped association between glycemic control and sleep quantity based on a meta-analysis by Lee et al 31 that showed a 0.23% increase in A1C in patients with insufficient sleep (< 4.5-6 hours/night) and a 0.13% increase in patients with ≥ 8 hours of sleep per night. Patients should be encouraged to obtain 7 to 8 hours of sleep per night to help maximize their diabetes control.
Address stress reduction
Although evidence for stress reduction interventions on glycemic control is mixed, there does seem to be a benefit in diminishing emotional distress in patients with diabetes. A systematic review by Noordali et al32 demonstrated that patients who received mindfulness-based interventions had improvements in stress, anxiety, and depression symptoms which resulted in improved quality of life. These psychological benefits may subsequently lead to positive behavioral changes.
Assist patients with smoking cessation
A large meta-analysis showed that active smoking increases the risk of cardiovascular events in patients with type 2 diabetes (RR = 1.44; 95% CI, 1.34-1.54).33 Former smokers still have an increased risk (RR = 1.09; 95% CI, 1.05-1.13), but it is lower than that of current smokers, so patients should be encouraged to quit smoking.3,33
Continue to: How can I get my patient to change?
H ow can I get my patient to change?
The AACE recommends using motivational interviewing, behavioral therapy consultation, and wearable feedback devices (eg, accelerometers/pedometers) to stimulate behavioral change in patients.3 Motivational interviewing is the principal counseling strategy and is supported by multiple studies showing the benefits of using this technique in a clinical encounter to induce behavioral changes.34 In general, offer receptive patients intensive behavioral interventions and provide them with resources to accomplish their goals.35 For example, a 7-step yearly intensive behavioral counseling intervention over 3 years showed significant improvements in activity of any intensity, reduced sedentary time, and led to favorable metabolic outcomes.36 Wearable devices result in up to a 1 hour increase in physical activity per week for the wearers vs control, although there was no appreciable effect on A1C.37
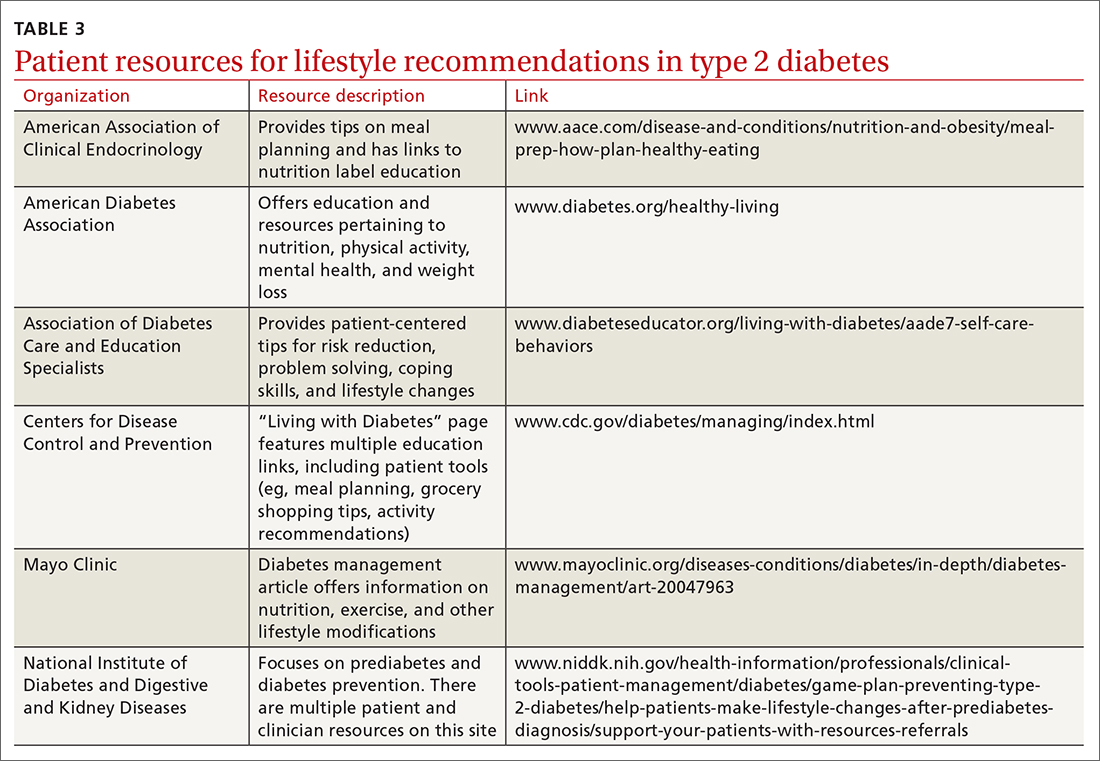
One systematic review showed a 0.5% reduction in A1C (95% CI, –0.65 to –0.34) by focusing on environmental changes related to the diet, with the most effective intervention being full meal replacement for calorie control (ie, each meal was pre-made and provided to the patients based on macronutrient and caloric goals).38 Additionally, diabetes self-management education includes coping strategies, problem solving, self-advocacy, and health care system navigation, which have been shown to reduce A1C by an average of 0.6%.21 Patient resources are available for further assistance with lifestyle modifications (TABLE 3).
C an your patient achieve remission?
Emerging evidence suggests that patients may achieve remission from type 2 diabetes with intensive lifestyle interventions.39 This is supported by the American College of Lifestyle Medicine.5 Although there is no consensus definition for remission, in general it is reasonable to presume remission if a patient achieves normo-glycemia (A1C < 5.7%) for at least 1 year without any medication therapy.5 These intensive lifestyle interventions would include a mostly plant-based diet with moderate calorie restriction, appropriate and sustained physical activity, adequate sleep, and stress-reduction techniques.5 One study found that 46% of patients in a weight-management program across multiple primary care clinics achieved remission at 12 months.40 A meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients (although the result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used).11 Thus far, most studies have focused on short-term follow-up intervals, but evidence is emerging that with intensive lifestyle interventions the effects are sustained at the 2-year mark.41
This evidence could reframe our understanding of type 2 diabetes therapy and could change the conversations we have with patients regarding their treatment. Instead of focusing on an A1C goal that is adequate for control of type 2 diabetes, we would instead focus on achieving remission.
CORRESPONDENCE
Stephen McMullan, MD, Mayo Clinic College of Medicine and Science, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected]
1. Kahn MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. doi: 10.2991/jegh.k.191028.001
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. doi:10.2337/dci18-0007
3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107-139. doi:10.4158/CS-2019-0472
4. Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020;74:481-487. doi: 10.1136/jech-2019-213415
5. Kelly J, Karlsen M, Steinke G. Type 2 Diabetes Remission and Lifestyle Medicine: A Position Statement from the American College of Lifestyle Medicine. Am J Lifestyle Med. 2020;14:406-419. doi: 10.1177/1559827620930962
6. Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
7. Mayo Clinic. Low-carb diet: Can it help you lose weight? Accessed August 22, 2022. www.mayoclinic.org/healthylifestyle/weight-loss/in-depth/low-carb-diet/art-20045831
8. Mayo Clinic. Is the keto diet for You? A Mayo expert weighs in. Accessed September 16, 2022. www.mayoclinic.org/is-the-keto-diet-for-you-a-mayo-expert-weighs-in/art-20457595
9. Mayo Clinic. Vegetarian diet: How to get the best nutrition. Accessed August 22, 2022. www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
10. AHA. What is the Mediterranean diet? Accessed September 16, 2022. www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/mediterranean-diet
11. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743
12. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005
13. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z
14. Salas-Salvadó J, Becerra-Tomás N, Papandreou C, et al. Dietary patterns emphasizing the consumption of plant foods in the management of type 2 diabetes: a narrative review. Adv Nutr. 2019;10(suppl_4):S320-S331. doi: 10.1093/advances/nmy102
15. Viguiliouk E, Kendall CW, Kahleová H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;38:1133-1145. doi: 10.1016/j.clnu.2018.05.032
16. Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04
17. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389
18. Basterra-Gortari FJ, Ruiz-Canela M, Martínez-González MA, et al. Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the PREDIMED trial. Diabetes Care. 2019;42:1390-1397. doi: 10.2337/dc18-2475
19. Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. doi:10.2337/dc16-1728
20. Hwang CL, Lim J, Yoo JK, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46-53. doi:10.1016/j.exger.2018.12.013
21. Zangeneh F, Boltri J, Dallas A, et al. National Institute of Diabetes and Digestive and Kidney Diseases. Guiding principles for the care of people with or at risk for diabetes. Accessed September 16, 2022. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/guiding-principles-care-people-risk-diabetes
22. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 suppl 1):S15-S21. doi: 10.3949/ccjm.84.s1.03
23. Zanuso S, Sacchetti M, Sundberg CJ, et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. a review of the evidence. Br J Sports Med. 2017;51:1533-1538. doi: 10.1136/bjsports-2016-096724
24. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. Published 2017 Mar 14. doi: 10.1186/s12933-017-0518-6
25. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968. doi: 10.1002/14651858.CD002968.pub2
26. Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018;48:1479-1491. doi: 10.1007/s40279-018-0864-x
27. Xia TW, Yang Y, Li WH, et al. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19:63. doi: 10.1186/s12906-019-2475-y
28. Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769-781. doi: 10.1007/s00592-016-0870-0
29. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-829. doi: 10.1007/s10654-018-0380-1
30. Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376-2382. doi: 10.1097/HJH.0000000000001101
31. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. doi: 10.1016/j.smrv.2016.02.001.
32. Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based intervention on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol. 2017;22:965-983. doi: 10.1177/1359105315620293
33. Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795-1804. doi:10.116/circulationaha.115.017926
34. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. doi:10.1007/s10865-013-9527-4
35. Koenigsberg MR, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96:362-370.
36. Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care. 2017;40:1444-1452. doi: 10.2337/dc17-0594
37. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34:612-620. doi:10.1111/dme.13331
38. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
39. Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11040766
40. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. doi: 10.1016/S0140-6736(17)33102-1
41. Sbroma Tomaro E, Pippi R, Reginato E, et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:688-694. doi:10.1016/j.numecd.2017.06.009
Type 2 diabetes has been increasing in incidence and prevalence over the past 20 years, with worldwide prevalence estimated at 6.28%.1 The estimated cost of diagnosed diabetes in the United States was $327 billion in 2017; this included direct medical costs and reduced productivity.2 Type 2 diabetes can be prevented in most patients, given that it is a metabolic derangement caused by a complicated interaction between a patient’s genetic predisposition and lifestyle. A consensus statement by the American Academy of Clinical Endocrinologists (AACE) and American College of Endocrinology indicates that the recommended lifestyle modifications for diabetes include medical nutrition therapy with healthy eating patterns, regular physical activity, adequate sleep, behavioral support/counseling, and smoking cessation.3 Evidence shows that adherence to these lifestyle changes alone yields a relative reduction in type 2 diabetes mortality of 57%.4

In the discussion that follows, we review the current guideline recommendations for dietary modifications and physical activity and summarize their effectiveness in the treatment of type 2 diabetes. We also describe practical clinical strategies to promote change in patient behavior, and examine current literature supporting intensive lifestyle changes that, if achieved, may induce disease remission.5
Dietary strategies
Low, or very low, carbohydrate diet
Carbohydrates can affect blood glucose levels in varying degrees depending on their intrinsic properties such as fiber content, sugars, and starches . 6 According to the American Diabetes Association’s (ADA) 2019 consensus report, 6 the carbohydrate quality that generally should be recommended is high in fiber, vitamins, and minerals, and low in added sugars, fats, and sodium (processed carbohydrates) ( TABLE 1 7-10 ). A low-carbohydrate diet (LCD) typically has a carbohydrate content < 130 g/d or < 26% of a 2000 kcal/d diet. 11 A very low–carbohydrate diet (VLCD) is 20-50 g/d or < 10% of the 2000 kcal/day diet. 11

In a meta-analysis by Goldenberg et al11, the LCD was shown to reduce A1C by 0.47% at 6 months (95% CI, –0.6 to –0.34) and by 0.23% at 12 months when compared with control diets. A review of multiple meta-analyses also showed a significant reduction in A1C especially with VLCD patterns; however, the results waned at the 12-month follow-up.5 In addition, confounding factors were seen when comparing adherence between LCD and VLCD, with patients in the latter group having larger problems with adherence, which decreased the benefit seen in the overall group comparison.11
Very low–carbohydrate/high-fat (ketogenic) diet
Ketogenic diets generally follow a VLCD with the carbohydrate portion set at 5% to 10% of total caloric intake (generally < 30 g/d) and the rest of the calories taken up by protein (typically 1 g/kg/d) and fat (TABLE 17-10).12 The fat content recommended is primarily polyunsaturated fat such as olive oil, while saturated fats such as butter and lard (animal fat) should be limited.
A recent meta-analysis by Choi et al12 showed that in overweight or obese patients with type 2 diabetes, the average A1C reduction was 0.62% (95% CI, –0.89 to –0.35) in the ketogenic intervention group. Another meta-analysis showed an even more significant A1C reduction at 1.07% (95% CI, –1.37 to –0.78).13 Concerns have been raised about the ketogenic diet, particularly as it relates to lipid metabolism and cholesterol levels; however, in the 2 referenced meta-analyses, the total cholesterol and triglyceride levels actually declined in the ketogenic intervention groups with minimal effect on LDL-C.12,13 This may alleviate some of the concerns of lipid management with this diet.
Plant-based diet
Popularized by Dr. T. Colin Campbell, a plant-based diet refers to a low-fat, high-fiber, whole-foods diet (whole fruits, vegetables, and naturally occurring carbohydrates, as opposed to processed foods). Examples of this type of diet include the popular vegan diet, which restricts all animal-derived products, and the vegetarian diet, which is generally limited to foods in the plant category with some addition of animal products, such as milk and cheese. Other variations of these diets exist and include other sources of protein (eg, chicken, eggs, or fish) (TABLE 17-10).
Continue to: A review by...
A review by Salas-Salvadó et al14 showed that a vegan diet yields an average A1C reduction of 0.41% (95% CI, –0.58 to –0.23).Several meta-analyses report similar effects on A1C with vegetarian and vegan eating patterns.6,15,16 The ADA review notes that weight loss was more significant in the vegan group and concluded that this diet should be studied further while controlling for weight loss.6
Mediterranean diet
The Mediterranean diet emphasizes vegetables, whole grains, fruits, lean meats, nuts, and olive oil. The benefits of the Mediterranean diet are well known and, as a result, the diet is recommended by organizations including the American Heart Association as part of a strategy to reduce cardiovascular risk (TABLE 17-10).
Mediterranean diet interventions have generally shown mixed effects on A1C reduction, weight management, and lipid control in type 2 diabetes. 6 The PREDIMED trial is the largest and longest randomized controlled trial to date comparing the Mediterranean diet to a low-fat diet. 17 This trial has reliably shown a reduced risk for type 2 diabetes and a trend to reduced A1C. 17 A reduction in the need for glucose-lowering medications was demonstrated in a subgroup analysis of the intervention group (adjusted hazard ratio = 0.78; 95% CI, 0.62-0.98). 18 Also, the Mediterranean diet has shown a significant reduction in the incidence of cardiovascular disease in patients with type 2 diabetes. 6
Physical activity and exercise
What do current guidelines recommend?
For most adults with type 2 diabetes, current guidelines by the ADA and by the National Institute of Diabetes and Digestive and Kidney Diseases recommend at least 150 minutes of moderate-to-vigorous intensity exercise every week spread out over at least 3 days, with no more than 2 consecutive days without exercise; and resistance training at least 2 other days per week which should balance all major muscle groups (TABLE 219-21). The benefits of exercise for type 2 diabetes have been well reviewed: positive effects on glucose control, insulin sensitivity, cardiovascular disease, lipid profiles, skeletal muscle metabolism, and solid-organ functioning.19,22,23

Grace et al24 showed in a meta-analysis that moderate aerobic exercise reduced A1C by 0.69% (95% CI, –1.09 to –0.3) at 13 weeks, and a Cochrane review showed an average A1C reduction of 0.6% with moderate-intensity exercise.25 Borror et al26 demonstrated in a systematic review that postprandial moderate-intensity aerobic exercise starting 1 hour after meals results in a reduced 24-hour prevalence of hyperglycemia (33.5% reduction vs control). A meta-analysis in China showed an average A1C reduction of 0.68% for patients performing a Tai Chi physical activity intervention.27
Continue to: Consider high-intensity interval training
Consider high-intensity interval training
Multiple randomized controlled trials highlight the benefits of high-intensity interval training (HIIT) (TABLE 219-21) compared with moderate-intensity continuous training (MICT) on improving A1C. A meta-analysis showed a weighted mean difference in A1C of 0.23% (95% CI, –0.43 to –0.02%).28 Also, a patient could spend less time performing HIIT as opposed to MICT to achieve the same benefits. For example, a patient typically performing 30 minutes of MICT may only need to perform 15 minutes of HIIT,a time-saving option for patients.20,22
Interrupt sedentary behavior
Risk for incident type 2 diabetes increases when someone is sedentary for more than 6 to 8 hours daily or watches TV for 3 to 4 hours (relative risk [RR] = 1.12).29 Recommendations for interrupting a sedentary lifestyle include standing from a seated position at least every 30 minutes and engaging in a light activity during the break interval for at least 3 minutes.19 Most studies have reliably shown that interrupting sedentary behavior reduces postprandial and 24-hour average blood glucose levels.19 Interrupted sitting/sedentary behavior has also been shown to reduce resting blood pressure in patients with type 2 diabetes.30
O ther important lifestyle factors
Encourage 7 to 8 hours of sleep
There is a U-shaped association between glycemic control and sleep quantity based on a meta-analysis by Lee et al 31 that showed a 0.23% increase in A1C in patients with insufficient sleep (< 4.5-6 hours/night) and a 0.13% increase in patients with ≥ 8 hours of sleep per night. Patients should be encouraged to obtain 7 to 8 hours of sleep per night to help maximize their diabetes control.
Address stress reduction
Although evidence for stress reduction interventions on glycemic control is mixed, there does seem to be a benefit in diminishing emotional distress in patients with diabetes. A systematic review by Noordali et al32 demonstrated that patients who received mindfulness-based interventions had improvements in stress, anxiety, and depression symptoms which resulted in improved quality of life. These psychological benefits may subsequently lead to positive behavioral changes.
Assist patients with smoking cessation
A large meta-analysis showed that active smoking increases the risk of cardiovascular events in patients with type 2 diabetes (RR = 1.44; 95% CI, 1.34-1.54).33 Former smokers still have an increased risk (RR = 1.09; 95% CI, 1.05-1.13), but it is lower than that of current smokers, so patients should be encouraged to quit smoking.3,33
Continue to: How can I get my patient to change?
H ow can I get my patient to change?
The AACE recommends using motivational interviewing, behavioral therapy consultation, and wearable feedback devices (eg, accelerometers/pedometers) to stimulate behavioral change in patients.3 Motivational interviewing is the principal counseling strategy and is supported by multiple studies showing the benefits of using this technique in a clinical encounter to induce behavioral changes.34 In general, offer receptive patients intensive behavioral interventions and provide them with resources to accomplish their goals.35 For example, a 7-step yearly intensive behavioral counseling intervention over 3 years showed significant improvements in activity of any intensity, reduced sedentary time, and led to favorable metabolic outcomes.36 Wearable devices result in up to a 1 hour increase in physical activity per week for the wearers vs control, although there was no appreciable effect on A1C.37
One systematic review showed a 0.5% reduction in A1C (95% CI, –0.65 to –0.34) by focusing on environmental changes related to the diet, with the most effective intervention being full meal replacement for calorie control (ie, each meal was pre-made and provided to the patients based on macronutrient and caloric goals).38 Additionally, diabetes self-management education includes coping strategies, problem solving, self-advocacy, and health care system navigation, which have been shown to reduce A1C by an average of 0.6%.21 Patient resources are available for further assistance with lifestyle modifications (TABLE 3).

C an your patient achieve remission?
Emerging evidence suggests that patients may achieve remission from type 2 diabetes with intensive lifestyle interventions.39 This is supported by the American College of Lifestyle Medicine.5 Although there is no consensus definition for remission, in general it is reasonable to presume remission if a patient achieves normo-glycemia (A1C < 5.7%) for at least 1 year without any medication therapy.5 These intensive lifestyle interventions would include a mostly plant-based diet with moderate calorie restriction, appropriate and sustained physical activity, adequate sleep, and stress-reduction techniques.5 One study found that 46% of patients in a weight-management program across multiple primary care clinics achieved remission at 12 months.40 A meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients (although the result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used).11 Thus far, most studies have focused on short-term follow-up intervals, but evidence is emerging that with intensive lifestyle interventions the effects are sustained at the 2-year mark.41
This evidence could reframe our understanding of type 2 diabetes therapy and could change the conversations we have with patients regarding their treatment. Instead of focusing on an A1C goal that is adequate for control of type 2 diabetes, we would instead focus on achieving remission.
CORRESPONDENCE
Stephen McMullan, MD, Mayo Clinic College of Medicine and Science, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected]
Type 2 diabetes has been increasing in incidence and prevalence over the past 20 years, with worldwide prevalence estimated at 6.28%.1 The estimated cost of diagnosed diabetes in the United States was $327 billion in 2017; this included direct medical costs and reduced productivity.2 Type 2 diabetes can be prevented in most patients, given that it is a metabolic derangement caused by a complicated interaction between a patient’s genetic predisposition and lifestyle. A consensus statement by the American Academy of Clinical Endocrinologists (AACE) and American College of Endocrinology indicates that the recommended lifestyle modifications for diabetes include medical nutrition therapy with healthy eating patterns, regular physical activity, adequate sleep, behavioral support/counseling, and smoking cessation.3 Evidence shows that adherence to these lifestyle changes alone yields a relative reduction in type 2 diabetes mortality of 57%.4

In the discussion that follows, we review the current guideline recommendations for dietary modifications and physical activity and summarize their effectiveness in the treatment of type 2 diabetes. We also describe practical clinical strategies to promote change in patient behavior, and examine current literature supporting intensive lifestyle changes that, if achieved, may induce disease remission.5
Dietary strategies
Low, or very low, carbohydrate diet
Carbohydrates can affect blood glucose levels in varying degrees depending on their intrinsic properties such as fiber content, sugars, and starches . 6 According to the American Diabetes Association’s (ADA) 2019 consensus report, 6 the carbohydrate quality that generally should be recommended is high in fiber, vitamins, and minerals, and low in added sugars, fats, and sodium (processed carbohydrates) ( TABLE 1 7-10 ). A low-carbohydrate diet (LCD) typically has a carbohydrate content < 130 g/d or < 26% of a 2000 kcal/d diet. 11 A very low–carbohydrate diet (VLCD) is 20-50 g/d or < 10% of the 2000 kcal/day diet. 11

In a meta-analysis by Goldenberg et al11, the LCD was shown to reduce A1C by 0.47% at 6 months (95% CI, –0.6 to –0.34) and by 0.23% at 12 months when compared with control diets. A review of multiple meta-analyses also showed a significant reduction in A1C especially with VLCD patterns; however, the results waned at the 12-month follow-up.5 In addition, confounding factors were seen when comparing adherence between LCD and VLCD, with patients in the latter group having larger problems with adherence, which decreased the benefit seen in the overall group comparison.11
Very low–carbohydrate/high-fat (ketogenic) diet
Ketogenic diets generally follow a VLCD with the carbohydrate portion set at 5% to 10% of total caloric intake (generally < 30 g/d) and the rest of the calories taken up by protein (typically 1 g/kg/d) and fat (TABLE 17-10).12 The fat content recommended is primarily polyunsaturated fat such as olive oil, while saturated fats such as butter and lard (animal fat) should be limited.
A recent meta-analysis by Choi et al12 showed that in overweight or obese patients with type 2 diabetes, the average A1C reduction was 0.62% (95% CI, –0.89 to –0.35) in the ketogenic intervention group. Another meta-analysis showed an even more significant A1C reduction at 1.07% (95% CI, –1.37 to –0.78).13 Concerns have been raised about the ketogenic diet, particularly as it relates to lipid metabolism and cholesterol levels; however, in the 2 referenced meta-analyses, the total cholesterol and triglyceride levels actually declined in the ketogenic intervention groups with minimal effect on LDL-C.12,13 This may alleviate some of the concerns of lipid management with this diet.
Plant-based diet
Popularized by Dr. T. Colin Campbell, a plant-based diet refers to a low-fat, high-fiber, whole-foods diet (whole fruits, vegetables, and naturally occurring carbohydrates, as opposed to processed foods). Examples of this type of diet include the popular vegan diet, which restricts all animal-derived products, and the vegetarian diet, which is generally limited to foods in the plant category with some addition of animal products, such as milk and cheese. Other variations of these diets exist and include other sources of protein (eg, chicken, eggs, or fish) (TABLE 17-10).
Continue to: A review by...
A review by Salas-Salvadó et al14 showed that a vegan diet yields an average A1C reduction of 0.41% (95% CI, –0.58 to –0.23).Several meta-analyses report similar effects on A1C with vegetarian and vegan eating patterns.6,15,16 The ADA review notes that weight loss was more significant in the vegan group and concluded that this diet should be studied further while controlling for weight loss.6
Mediterranean diet
The Mediterranean diet emphasizes vegetables, whole grains, fruits, lean meats, nuts, and olive oil. The benefits of the Mediterranean diet are well known and, as a result, the diet is recommended by organizations including the American Heart Association as part of a strategy to reduce cardiovascular risk (TABLE 17-10).
Mediterranean diet interventions have generally shown mixed effects on A1C reduction, weight management, and lipid control in type 2 diabetes. 6 The PREDIMED trial is the largest and longest randomized controlled trial to date comparing the Mediterranean diet to a low-fat diet. 17 This trial has reliably shown a reduced risk for type 2 diabetes and a trend to reduced A1C. 17 A reduction in the need for glucose-lowering medications was demonstrated in a subgroup analysis of the intervention group (adjusted hazard ratio = 0.78; 95% CI, 0.62-0.98). 18 Also, the Mediterranean diet has shown a significant reduction in the incidence of cardiovascular disease in patients with type 2 diabetes. 6
Physical activity and exercise
What do current guidelines recommend?
For most adults with type 2 diabetes, current guidelines by the ADA and by the National Institute of Diabetes and Digestive and Kidney Diseases recommend at least 150 minutes of moderate-to-vigorous intensity exercise every week spread out over at least 3 days, with no more than 2 consecutive days without exercise; and resistance training at least 2 other days per week which should balance all major muscle groups (TABLE 219-21). The benefits of exercise for type 2 diabetes have been well reviewed: positive effects on glucose control, insulin sensitivity, cardiovascular disease, lipid profiles, skeletal muscle metabolism, and solid-organ functioning.19,22,23

Grace et al24 showed in a meta-analysis that moderate aerobic exercise reduced A1C by 0.69% (95% CI, –1.09 to –0.3) at 13 weeks, and a Cochrane review showed an average A1C reduction of 0.6% with moderate-intensity exercise.25 Borror et al26 demonstrated in a systematic review that postprandial moderate-intensity aerobic exercise starting 1 hour after meals results in a reduced 24-hour prevalence of hyperglycemia (33.5% reduction vs control). A meta-analysis in China showed an average A1C reduction of 0.68% for patients performing a Tai Chi physical activity intervention.27
Continue to: Consider high-intensity interval training
Consider high-intensity interval training
Multiple randomized controlled trials highlight the benefits of high-intensity interval training (HIIT) (TABLE 219-21) compared with moderate-intensity continuous training (MICT) on improving A1C. A meta-analysis showed a weighted mean difference in A1C of 0.23% (95% CI, –0.43 to –0.02%).28 Also, a patient could spend less time performing HIIT as opposed to MICT to achieve the same benefits. For example, a patient typically performing 30 minutes of MICT may only need to perform 15 minutes of HIIT,a time-saving option for patients.20,22
Interrupt sedentary behavior
Risk for incident type 2 diabetes increases when someone is sedentary for more than 6 to 8 hours daily or watches TV for 3 to 4 hours (relative risk [RR] = 1.12).29 Recommendations for interrupting a sedentary lifestyle include standing from a seated position at least every 30 minutes and engaging in a light activity during the break interval for at least 3 minutes.19 Most studies have reliably shown that interrupting sedentary behavior reduces postprandial and 24-hour average blood glucose levels.19 Interrupted sitting/sedentary behavior has also been shown to reduce resting blood pressure in patients with type 2 diabetes.30
O ther important lifestyle factors
Encourage 7 to 8 hours of sleep
There is a U-shaped association between glycemic control and sleep quantity based on a meta-analysis by Lee et al 31 that showed a 0.23% increase in A1C in patients with insufficient sleep (< 4.5-6 hours/night) and a 0.13% increase in patients with ≥ 8 hours of sleep per night. Patients should be encouraged to obtain 7 to 8 hours of sleep per night to help maximize their diabetes control.
Address stress reduction
Although evidence for stress reduction interventions on glycemic control is mixed, there does seem to be a benefit in diminishing emotional distress in patients with diabetes. A systematic review by Noordali et al32 demonstrated that patients who received mindfulness-based interventions had improvements in stress, anxiety, and depression symptoms which resulted in improved quality of life. These psychological benefits may subsequently lead to positive behavioral changes.
Assist patients with smoking cessation
A large meta-analysis showed that active smoking increases the risk of cardiovascular events in patients with type 2 diabetes (RR = 1.44; 95% CI, 1.34-1.54).33 Former smokers still have an increased risk (RR = 1.09; 95% CI, 1.05-1.13), but it is lower than that of current smokers, so patients should be encouraged to quit smoking.3,33
Continue to: How can I get my patient to change?
H ow can I get my patient to change?
The AACE recommends using motivational interviewing, behavioral therapy consultation, and wearable feedback devices (eg, accelerometers/pedometers) to stimulate behavioral change in patients.3 Motivational interviewing is the principal counseling strategy and is supported by multiple studies showing the benefits of using this technique in a clinical encounter to induce behavioral changes.34 In general, offer receptive patients intensive behavioral interventions and provide them with resources to accomplish their goals.35 For example, a 7-step yearly intensive behavioral counseling intervention over 3 years showed significant improvements in activity of any intensity, reduced sedentary time, and led to favorable metabolic outcomes.36 Wearable devices result in up to a 1 hour increase in physical activity per week for the wearers vs control, although there was no appreciable effect on A1C.37
One systematic review showed a 0.5% reduction in A1C (95% CI, –0.65 to –0.34) by focusing on environmental changes related to the diet, with the most effective intervention being full meal replacement for calorie control (ie, each meal was pre-made and provided to the patients based on macronutrient and caloric goals).38 Additionally, diabetes self-management education includes coping strategies, problem solving, self-advocacy, and health care system navigation, which have been shown to reduce A1C by an average of 0.6%.21 Patient resources are available for further assistance with lifestyle modifications (TABLE 3).

C an your patient achieve remission?
Emerging evidence suggests that patients may achieve remission from type 2 diabetes with intensive lifestyle interventions.39 This is supported by the American College of Lifestyle Medicine.5 Although there is no consensus definition for remission, in general it is reasonable to presume remission if a patient achieves normo-glycemia (A1C < 5.7%) for at least 1 year without any medication therapy.5 These intensive lifestyle interventions would include a mostly plant-based diet with moderate calorie restriction, appropriate and sustained physical activity, adequate sleep, and stress-reduction techniques.5 One study found that 46% of patients in a weight-management program across multiple primary care clinics achieved remission at 12 months.40 A meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients (although the result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used).11 Thus far, most studies have focused on short-term follow-up intervals, but evidence is emerging that with intensive lifestyle interventions the effects are sustained at the 2-year mark.41
This evidence could reframe our understanding of type 2 diabetes therapy and could change the conversations we have with patients regarding their treatment. Instead of focusing on an A1C goal that is adequate for control of type 2 diabetes, we would instead focus on achieving remission.
CORRESPONDENCE
Stephen McMullan, MD, Mayo Clinic College of Medicine and Science, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected]
1. Kahn MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. doi: 10.2991/jegh.k.191028.001
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. doi:10.2337/dci18-0007
3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107-139. doi:10.4158/CS-2019-0472
4. Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020;74:481-487. doi: 10.1136/jech-2019-213415
5. Kelly J, Karlsen M, Steinke G. Type 2 Diabetes Remission and Lifestyle Medicine: A Position Statement from the American College of Lifestyle Medicine. Am J Lifestyle Med. 2020;14:406-419. doi: 10.1177/1559827620930962
6. Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
7. Mayo Clinic. Low-carb diet: Can it help you lose weight? Accessed August 22, 2022. www.mayoclinic.org/healthylifestyle/weight-loss/in-depth/low-carb-diet/art-20045831
8. Mayo Clinic. Is the keto diet for You? A Mayo expert weighs in. Accessed September 16, 2022. www.mayoclinic.org/is-the-keto-diet-for-you-a-mayo-expert-weighs-in/art-20457595
9. Mayo Clinic. Vegetarian diet: How to get the best nutrition. Accessed August 22, 2022. www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
10. AHA. What is the Mediterranean diet? Accessed September 16, 2022. www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/mediterranean-diet
11. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743
12. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005
13. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z
14. Salas-Salvadó J, Becerra-Tomás N, Papandreou C, et al. Dietary patterns emphasizing the consumption of plant foods in the management of type 2 diabetes: a narrative review. Adv Nutr. 2019;10(suppl_4):S320-S331. doi: 10.1093/advances/nmy102
15. Viguiliouk E, Kendall CW, Kahleová H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;38:1133-1145. doi: 10.1016/j.clnu.2018.05.032
16. Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04
17. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389
18. Basterra-Gortari FJ, Ruiz-Canela M, Martínez-González MA, et al. Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the PREDIMED trial. Diabetes Care. 2019;42:1390-1397. doi: 10.2337/dc18-2475
19. Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. doi:10.2337/dc16-1728
20. Hwang CL, Lim J, Yoo JK, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46-53. doi:10.1016/j.exger.2018.12.013
21. Zangeneh F, Boltri J, Dallas A, et al. National Institute of Diabetes and Digestive and Kidney Diseases. Guiding principles for the care of people with or at risk for diabetes. Accessed September 16, 2022. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/guiding-principles-care-people-risk-diabetes
22. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 suppl 1):S15-S21. doi: 10.3949/ccjm.84.s1.03
23. Zanuso S, Sacchetti M, Sundberg CJ, et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. a review of the evidence. Br J Sports Med. 2017;51:1533-1538. doi: 10.1136/bjsports-2016-096724
24. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. Published 2017 Mar 14. doi: 10.1186/s12933-017-0518-6
25. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968. doi: 10.1002/14651858.CD002968.pub2
26. Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018;48:1479-1491. doi: 10.1007/s40279-018-0864-x
27. Xia TW, Yang Y, Li WH, et al. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19:63. doi: 10.1186/s12906-019-2475-y
28. Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769-781. doi: 10.1007/s00592-016-0870-0
29. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-829. doi: 10.1007/s10654-018-0380-1
30. Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376-2382. doi: 10.1097/HJH.0000000000001101
31. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. doi: 10.1016/j.smrv.2016.02.001.
32. Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based intervention on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol. 2017;22:965-983. doi: 10.1177/1359105315620293
33. Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795-1804. doi:10.116/circulationaha.115.017926
34. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. doi:10.1007/s10865-013-9527-4
35. Koenigsberg MR, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96:362-370.
36. Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care. 2017;40:1444-1452. doi: 10.2337/dc17-0594
37. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34:612-620. doi:10.1111/dme.13331
38. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
39. Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11040766
40. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. doi: 10.1016/S0140-6736(17)33102-1
41. Sbroma Tomaro E, Pippi R, Reginato E, et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:688-694. doi:10.1016/j.numecd.2017.06.009
1. Kahn MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. doi: 10.2991/jegh.k.191028.001
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. doi:10.2337/dci18-0007
3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107-139. doi:10.4158/CS-2019-0472
4. Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020;74:481-487. doi: 10.1136/jech-2019-213415
5. Kelly J, Karlsen M, Steinke G. Type 2 Diabetes Remission and Lifestyle Medicine: A Position Statement from the American College of Lifestyle Medicine. Am J Lifestyle Med. 2020;14:406-419. doi: 10.1177/1559827620930962
6. Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
7. Mayo Clinic. Low-carb diet: Can it help you lose weight? Accessed August 22, 2022. www.mayoclinic.org/healthylifestyle/weight-loss/in-depth/low-carb-diet/art-20045831
8. Mayo Clinic. Is the keto diet for You? A Mayo expert weighs in. Accessed September 16, 2022. www.mayoclinic.org/is-the-keto-diet-for-you-a-mayo-expert-weighs-in/art-20457595
9. Mayo Clinic. Vegetarian diet: How to get the best nutrition. Accessed August 22, 2022. www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
10. AHA. What is the Mediterranean diet? Accessed September 16, 2022. www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/mediterranean-diet
11. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743
12. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005
13. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z
14. Salas-Salvadó J, Becerra-Tomás N, Papandreou C, et al. Dietary patterns emphasizing the consumption of plant foods in the management of type 2 diabetes: a narrative review. Adv Nutr. 2019;10(suppl_4):S320-S331. doi: 10.1093/advances/nmy102
15. Viguiliouk E, Kendall CW, Kahleová H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;38:1133-1145. doi: 10.1016/j.clnu.2018.05.032
16. Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04
17. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389
18. Basterra-Gortari FJ, Ruiz-Canela M, Martínez-González MA, et al. Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the PREDIMED trial. Diabetes Care. 2019;42:1390-1397. doi: 10.2337/dc18-2475
19. Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. doi:10.2337/dc16-1728
20. Hwang CL, Lim J, Yoo JK, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46-53. doi:10.1016/j.exger.2018.12.013
21. Zangeneh F, Boltri J, Dallas A, et al. National Institute of Diabetes and Digestive and Kidney Diseases. Guiding principles for the care of people with or at risk for diabetes. Accessed September 16, 2022. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/guiding-principles-care-people-risk-diabetes
22. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 suppl 1):S15-S21. doi: 10.3949/ccjm.84.s1.03
23. Zanuso S, Sacchetti M, Sundberg CJ, et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. a review of the evidence. Br J Sports Med. 2017;51:1533-1538. doi: 10.1136/bjsports-2016-096724
24. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. Published 2017 Mar 14. doi: 10.1186/s12933-017-0518-6
25. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968. doi: 10.1002/14651858.CD002968.pub2
26. Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018;48:1479-1491. doi: 10.1007/s40279-018-0864-x
27. Xia TW, Yang Y, Li WH, et al. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19:63. doi: 10.1186/s12906-019-2475-y
28. Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769-781. doi: 10.1007/s00592-016-0870-0
29. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-829. doi: 10.1007/s10654-018-0380-1
30. Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376-2382. doi: 10.1097/HJH.0000000000001101
31. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. doi: 10.1016/j.smrv.2016.02.001.
32. Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based intervention on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol. 2017;22:965-983. doi: 10.1177/1359105315620293
33. Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795-1804. doi:10.116/circulationaha.115.017926
34. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. doi:10.1007/s10865-013-9527-4
35. Koenigsberg MR, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96:362-370.
36. Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care. 2017;40:1444-1452. doi: 10.2337/dc17-0594
37. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34:612-620. doi:10.1111/dme.13331
38. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
39. Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11040766
40. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. doi: 10.1016/S0140-6736(17)33102-1
41. Sbroma Tomaro E, Pippi R, Reginato E, et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:688-694. doi:10.1016/j.numecd.2017.06.009
PRACTICE RECOMMENDATIONS
› Recommend a reduced-calorie diet that is generally plant based and low in carbohydrates as part of the treatment plan for type 2 diabetes. B
› Counsel all patients with type 2 diabetes to engage in physical activity for at least 150 minutes per week at moderate intensity and to add resistance training on at least 2 days to improve glycemic control. B
› Teach patients techniques to reduce stress and improve sleep quality. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The truth about the ‘happy hormone’: Why we shouldn’t mess with dopamine
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Crohn Disease Medication Overview
Sigmoidoscopy screening cuts CRC mortality, incidence
Although endoscopic screening provides an opportunity for early identification and removal of premalignant polyps, data quantifying the long-term effects of sigmoidoscopy screening are lacking, corresponding author Frederik E. Juul, MD, said in an interview.
“Sigmoidoscopy screening have been shown to reduce colorectal cancer incidence and mortality, but it was unknown how long-lasting the effects were, and whether the effect differed by sex or age,” Dr. Juul said.
“For the first time, we were able to pool data from all four randomized sigmoidoscopy screening trials and include data from recent updates from two of the trials (U.S. and Italy), which means that we were able to answer these questions better than ever before,” he said.
In the pooled analysis, published in Annals of Internal Medicine, researchers from Norway, the United States, Italy, and the United Kingdom reviewed data from four studies with at least 15 years of follow-up. The analysis included 137,493 individuals randomized to at least one sigmoidoscopy screening and 137,459 randomized to usual care.
The primary outcomes were the incidence and mortality of CRC after sigmoidoscopy screening, compared with usual care, in adults with average CRC risk aged 55-64 years. Secondary outcomes included CRC incidence and mortality based on distal versus proximal colon, sex, and older versus younger age group (55-59 years vs. 60-64 years at study enrollment).
After 15 years’ follow-up, the pooled cumulative incidence of CRC was 1.84 cases per 100 persons in the screening group versus 2.35 cases per 100 persons in the usual-care group, representing a 21% reduction in incidence among those who were screened.
The pooled cumulative CRC mortality was 0.51 deaths per 100 persons in the screening group versus 0.65 deaths per 100 persons in the usual-care group, representing a 20% reduction in CRC mortality for those who were screened, the researchers noted. The all-cause mortality was reduced by 2% in the screening group compared with usual care; the pooled cumulative all-cause mortality was 14.3 deaths per 100 persons in the screening group versus 14.6 deaths per 100 persons in the usual-care group.
In terms of secondary outcomes, the significant reductions in CRC incidence and mortality were confined to the distal colon, with no significant differences observed in the proximal colon, the researchers noted. The reasons for this difference are unclear. Previous studies of three of the four trials showed a small reduction in CRC in the proximal colon, but may be related to the longer follow-up in the analysis of four trials.
The incidence of CRC varied by gender, with an incidence reduction of 25% for men versus 16% for women. The reasons for the gender difference are yet to be undetermined, but may include differences in the quality of bowel preparation, the greater technical challenge of screening women, and the higher incidence and proportion of proximal colon cancer versus distal colon cancer in women, the researchers noted.
“The long-term benefit of one single procedure was probably what surprised us the most,” Dr. Juul said in an interview. “Not only were the cumulative incidence and mortality lower in screened individuals 15 years after the procedure, but the yearly incidence was consistently lower in screened individuals compared to usual care, even at the end of the follow-up period.
“Although a previous study in Norway had indicated a sex difference in effect, we were surprised to see this in a pooled analysis across trials in four different countries,” he added.
Data may drive screening guidelines
The main finding of the study is that sigmoidoscopy screening with investigation of the distal colon provides at least 15 years of protection against colorectal cancer; “this may have an impact on how often average-risk individuals needs to be screened,” Dr. Juul said in an interview.
As for additional research, ongoing studies are examining primary colonoscopy screening, including a study recently published in the New England Journal of Medicine, Dr. Juul said.“Our study investigating sigmoidoscopy screening has a longer follow-up and it will be interesting to see if primary colonoscopy screening is equally or more effective as sigmoidoscopy at 15-years follow-up.”
More research is needed on direct comparisons of different colorectal cancer screening methods such as sigmoidoscopy and colonoscopy, said Dr. Juul. In addition, “The optimal surveillance interval in individuals identified at screening to be low- or high-risk of developing colorectal cancer are unknown,” he said.
“Our research group is involved in trials [the EPoS trials] looking into this question, but there are still years until we have the final results,” he added.
The findings were limited by several factors including the variation in methodology among the four trials and the lower number of individuals referred for colonoscopy in the U.K. and Italian trials, lack of analysis of potential confounding variables, and less granular data from the U.K. trial because of privacy regulations, the researchers wrote.
However, the findings were strengthened by the large study population, long-term follow-up, and detailed data, and they indicate a “significant and sustained” effect of screening sigmoidoscopy for the long-term reduction of CRC incidence and mortality, the authors concluded.
Findings can inform shared decision-making
“Colon cancer is the third-leading cause of death in the United States in men and women, and the second-leading cause of cancer deaths if we were to combine both genders,” Noel Deep, MD, said in an interview. “Sigmoidoscopy is more acceptable as a screening tool compared to a colonoscopy because of the lower risk of bowel injury, fewer side effects and less of a bowel prep, and also less need for sedation. This current study confirms prior data, including the 2012 PLCO trial, that it [sigmoidoscopy] reduces the incidence and mortality from colorectal cancer.”
The study findings were not surprising, given the prior knowledge and evidence of the benefits of sigmoidoscopy, Dr. Deep said, who was not involved in the study. However, “the fact that a single sigmoidoscopy led to decreased incidence and decreased mortality at 15 years was surprising to me, as current models suggest increasing incidence of proximal colon adenomas and cancers, which did not seem to be the case in this study.”
The current study can help primary care physicians and advance practice clinicians in patient counseling by supporting sigmoidoscopy as an option for patients who are unwilling to commit to a full colonoscopy, Dr. Deep said. However, “the patients should be advised that abnormal findings on the sigmoidoscopy would necessitate them being referred for a colonoscopy, and also the limitations of a sigmoidoscopy in detecting polyps or cancers in the cecum, ascending colon, transverse colon and descending colon.”
Looking ahead, “I would like to see research into the appropriate age for colorectal cancer screening using sigmoidoscopy and any benefit in offering this option at an earlier age,” Dr. Deep said. He also expressed a wish to know more about the reasons for the decreased benefit of screening sigmoidoscopy in women, and the reasons for the observed difference in all-cause mortality between genders.
“I would also like to see what the results of screening colonoscopies in a general population would reveal, and if it would reveal similar benefits, and also if there would be a gender difference or age-based difference in outcomes,” he said.
The study was supported by the Health Fund of South-East Norway. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Although endoscopic screening provides an opportunity for early identification and removal of premalignant polyps, data quantifying the long-term effects of sigmoidoscopy screening are lacking, corresponding author Frederik E. Juul, MD, said in an interview.
“Sigmoidoscopy screening have been shown to reduce colorectal cancer incidence and mortality, but it was unknown how long-lasting the effects were, and whether the effect differed by sex or age,” Dr. Juul said.
“For the first time, we were able to pool data from all four randomized sigmoidoscopy screening trials and include data from recent updates from two of the trials (U.S. and Italy), which means that we were able to answer these questions better than ever before,” he said.
In the pooled analysis, published in Annals of Internal Medicine, researchers from Norway, the United States, Italy, and the United Kingdom reviewed data from four studies with at least 15 years of follow-up. The analysis included 137,493 individuals randomized to at least one sigmoidoscopy screening and 137,459 randomized to usual care.
The primary outcomes were the incidence and mortality of CRC after sigmoidoscopy screening, compared with usual care, in adults with average CRC risk aged 55-64 years. Secondary outcomes included CRC incidence and mortality based on distal versus proximal colon, sex, and older versus younger age group (55-59 years vs. 60-64 years at study enrollment).
After 15 years’ follow-up, the pooled cumulative incidence of CRC was 1.84 cases per 100 persons in the screening group versus 2.35 cases per 100 persons in the usual-care group, representing a 21% reduction in incidence among those who were screened.
The pooled cumulative CRC mortality was 0.51 deaths per 100 persons in the screening group versus 0.65 deaths per 100 persons in the usual-care group, representing a 20% reduction in CRC mortality for those who were screened, the researchers noted. The all-cause mortality was reduced by 2% in the screening group compared with usual care; the pooled cumulative all-cause mortality was 14.3 deaths per 100 persons in the screening group versus 14.6 deaths per 100 persons in the usual-care group.
In terms of secondary outcomes, the significant reductions in CRC incidence and mortality were confined to the distal colon, with no significant differences observed in the proximal colon, the researchers noted. The reasons for this difference are unclear. Previous studies of three of the four trials showed a small reduction in CRC in the proximal colon, but may be related to the longer follow-up in the analysis of four trials.
The incidence of CRC varied by gender, with an incidence reduction of 25% for men versus 16% for women. The reasons for the gender difference are yet to be undetermined, but may include differences in the quality of bowel preparation, the greater technical challenge of screening women, and the higher incidence and proportion of proximal colon cancer versus distal colon cancer in women, the researchers noted.
“The long-term benefit of one single procedure was probably what surprised us the most,” Dr. Juul said in an interview. “Not only were the cumulative incidence and mortality lower in screened individuals 15 years after the procedure, but the yearly incidence was consistently lower in screened individuals compared to usual care, even at the end of the follow-up period.
“Although a previous study in Norway had indicated a sex difference in effect, we were surprised to see this in a pooled analysis across trials in four different countries,” he added.
Data may drive screening guidelines
The main finding of the study is that sigmoidoscopy screening with investigation of the distal colon provides at least 15 years of protection against colorectal cancer; “this may have an impact on how often average-risk individuals needs to be screened,” Dr. Juul said in an interview.
As for additional research, ongoing studies are examining primary colonoscopy screening, including a study recently published in the New England Journal of Medicine, Dr. Juul said.“Our study investigating sigmoidoscopy screening has a longer follow-up and it will be interesting to see if primary colonoscopy screening is equally or more effective as sigmoidoscopy at 15-years follow-up.”
More research is needed on direct comparisons of different colorectal cancer screening methods such as sigmoidoscopy and colonoscopy, said Dr. Juul. In addition, “The optimal surveillance interval in individuals identified at screening to be low- or high-risk of developing colorectal cancer are unknown,” he said.
“Our research group is involved in trials [the EPoS trials] looking into this question, but there are still years until we have the final results,” he added.
The findings were limited by several factors including the variation in methodology among the four trials and the lower number of individuals referred for colonoscopy in the U.K. and Italian trials, lack of analysis of potential confounding variables, and less granular data from the U.K. trial because of privacy regulations, the researchers wrote.
However, the findings were strengthened by the large study population, long-term follow-up, and detailed data, and they indicate a “significant and sustained” effect of screening sigmoidoscopy for the long-term reduction of CRC incidence and mortality, the authors concluded.
Findings can inform shared decision-making
“Colon cancer is the third-leading cause of death in the United States in men and women, and the second-leading cause of cancer deaths if we were to combine both genders,” Noel Deep, MD, said in an interview. “Sigmoidoscopy is more acceptable as a screening tool compared to a colonoscopy because of the lower risk of bowel injury, fewer side effects and less of a bowel prep, and also less need for sedation. This current study confirms prior data, including the 2012 PLCO trial, that it [sigmoidoscopy] reduces the incidence and mortality from colorectal cancer.”
The study findings were not surprising, given the prior knowledge and evidence of the benefits of sigmoidoscopy, Dr. Deep said, who was not involved in the study. However, “the fact that a single sigmoidoscopy led to decreased incidence and decreased mortality at 15 years was surprising to me, as current models suggest increasing incidence of proximal colon adenomas and cancers, which did not seem to be the case in this study.”
The current study can help primary care physicians and advance practice clinicians in patient counseling by supporting sigmoidoscopy as an option for patients who are unwilling to commit to a full colonoscopy, Dr. Deep said. However, “the patients should be advised that abnormal findings on the sigmoidoscopy would necessitate them being referred for a colonoscopy, and also the limitations of a sigmoidoscopy in detecting polyps or cancers in the cecum, ascending colon, transverse colon and descending colon.”
Looking ahead, “I would like to see research into the appropriate age for colorectal cancer screening using sigmoidoscopy and any benefit in offering this option at an earlier age,” Dr. Deep said. He also expressed a wish to know more about the reasons for the decreased benefit of screening sigmoidoscopy in women, and the reasons for the observed difference in all-cause mortality between genders.
“I would also like to see what the results of screening colonoscopies in a general population would reveal, and if it would reveal similar benefits, and also if there would be a gender difference or age-based difference in outcomes,” he said.
The study was supported by the Health Fund of South-East Norway. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Although endoscopic screening provides an opportunity for early identification and removal of premalignant polyps, data quantifying the long-term effects of sigmoidoscopy screening are lacking, corresponding author Frederik E. Juul, MD, said in an interview.
“Sigmoidoscopy screening have been shown to reduce colorectal cancer incidence and mortality, but it was unknown how long-lasting the effects were, and whether the effect differed by sex or age,” Dr. Juul said.
“For the first time, we were able to pool data from all four randomized sigmoidoscopy screening trials and include data from recent updates from two of the trials (U.S. and Italy), which means that we were able to answer these questions better than ever before,” he said.
In the pooled analysis, published in Annals of Internal Medicine, researchers from Norway, the United States, Italy, and the United Kingdom reviewed data from four studies with at least 15 years of follow-up. The analysis included 137,493 individuals randomized to at least one sigmoidoscopy screening and 137,459 randomized to usual care.
The primary outcomes were the incidence and mortality of CRC after sigmoidoscopy screening, compared with usual care, in adults with average CRC risk aged 55-64 years. Secondary outcomes included CRC incidence and mortality based on distal versus proximal colon, sex, and older versus younger age group (55-59 years vs. 60-64 years at study enrollment).
After 15 years’ follow-up, the pooled cumulative incidence of CRC was 1.84 cases per 100 persons in the screening group versus 2.35 cases per 100 persons in the usual-care group, representing a 21% reduction in incidence among those who were screened.
The pooled cumulative CRC mortality was 0.51 deaths per 100 persons in the screening group versus 0.65 deaths per 100 persons in the usual-care group, representing a 20% reduction in CRC mortality for those who were screened, the researchers noted. The all-cause mortality was reduced by 2% in the screening group compared with usual care; the pooled cumulative all-cause mortality was 14.3 deaths per 100 persons in the screening group versus 14.6 deaths per 100 persons in the usual-care group.
In terms of secondary outcomes, the significant reductions in CRC incidence and mortality were confined to the distal colon, with no significant differences observed in the proximal colon, the researchers noted. The reasons for this difference are unclear. Previous studies of three of the four trials showed a small reduction in CRC in the proximal colon, but may be related to the longer follow-up in the analysis of four trials.
The incidence of CRC varied by gender, with an incidence reduction of 25% for men versus 16% for women. The reasons for the gender difference are yet to be undetermined, but may include differences in the quality of bowel preparation, the greater technical challenge of screening women, and the higher incidence and proportion of proximal colon cancer versus distal colon cancer in women, the researchers noted.
“The long-term benefit of one single procedure was probably what surprised us the most,” Dr. Juul said in an interview. “Not only were the cumulative incidence and mortality lower in screened individuals 15 years after the procedure, but the yearly incidence was consistently lower in screened individuals compared to usual care, even at the end of the follow-up period.
“Although a previous study in Norway had indicated a sex difference in effect, we were surprised to see this in a pooled analysis across trials in four different countries,” he added.
Data may drive screening guidelines
The main finding of the study is that sigmoidoscopy screening with investigation of the distal colon provides at least 15 years of protection against colorectal cancer; “this may have an impact on how often average-risk individuals needs to be screened,” Dr. Juul said in an interview.
As for additional research, ongoing studies are examining primary colonoscopy screening, including a study recently published in the New England Journal of Medicine, Dr. Juul said.“Our study investigating sigmoidoscopy screening has a longer follow-up and it will be interesting to see if primary colonoscopy screening is equally or more effective as sigmoidoscopy at 15-years follow-up.”
More research is needed on direct comparisons of different colorectal cancer screening methods such as sigmoidoscopy and colonoscopy, said Dr. Juul. In addition, “The optimal surveillance interval in individuals identified at screening to be low- or high-risk of developing colorectal cancer are unknown,” he said.
“Our research group is involved in trials [the EPoS trials] looking into this question, but there are still years until we have the final results,” he added.
The findings were limited by several factors including the variation in methodology among the four trials and the lower number of individuals referred for colonoscopy in the U.K. and Italian trials, lack of analysis of potential confounding variables, and less granular data from the U.K. trial because of privacy regulations, the researchers wrote.
However, the findings were strengthened by the large study population, long-term follow-up, and detailed data, and they indicate a “significant and sustained” effect of screening sigmoidoscopy for the long-term reduction of CRC incidence and mortality, the authors concluded.
Findings can inform shared decision-making
“Colon cancer is the third-leading cause of death in the United States in men and women, and the second-leading cause of cancer deaths if we were to combine both genders,” Noel Deep, MD, said in an interview. “Sigmoidoscopy is more acceptable as a screening tool compared to a colonoscopy because of the lower risk of bowel injury, fewer side effects and less of a bowel prep, and also less need for sedation. This current study confirms prior data, including the 2012 PLCO trial, that it [sigmoidoscopy] reduces the incidence and mortality from colorectal cancer.”
The study findings were not surprising, given the prior knowledge and evidence of the benefits of sigmoidoscopy, Dr. Deep said, who was not involved in the study. However, “the fact that a single sigmoidoscopy led to decreased incidence and decreased mortality at 15 years was surprising to me, as current models suggest increasing incidence of proximal colon adenomas and cancers, which did not seem to be the case in this study.”
The current study can help primary care physicians and advance practice clinicians in patient counseling by supporting sigmoidoscopy as an option for patients who are unwilling to commit to a full colonoscopy, Dr. Deep said. However, “the patients should be advised that abnormal findings on the sigmoidoscopy would necessitate them being referred for a colonoscopy, and also the limitations of a sigmoidoscopy in detecting polyps or cancers in the cecum, ascending colon, transverse colon and descending colon.”
Looking ahead, “I would like to see research into the appropriate age for colorectal cancer screening using sigmoidoscopy and any benefit in offering this option at an earlier age,” Dr. Deep said. He also expressed a wish to know more about the reasons for the decreased benefit of screening sigmoidoscopy in women, and the reasons for the observed difference in all-cause mortality between genders.
“I would also like to see what the results of screening colonoscopies in a general population would reveal, and if it would reveal similar benefits, and also if there would be a gender difference or age-based difference in outcomes,” he said.
The study was supported by the Health Fund of South-East Norway. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
FROM ANNALS OF INTERNAL MEDICINE
Longer boarding times predict patient processing in ED
on the basis of data from nearly 900 facilities presented at the annual meeting of the American College of Emergency Physicians.
The study was important to conduct at this time because ED boarding is significantly limiting ED physicians to provide optimal care, said Camila Tyminski, MD, of Brown University, Providence, R.I., who presented the findings at the meeting.
“Boarding had steadily been rising prior to the COVID-19 pandemic due to increased ED use. As our data show, boarding had a detrimental impact on ED throughput measures, including increased door to provider time, increased length of stay of the patient discharged from the ED, and increased rate of patients that left before completion of treatment,” she said.
“It was important to understand these trends prior to 2019-2020 because the COVID-19 pandemic and national nursing shortage have drastically worsened boarding. This study provided a framework for future studies on boarding across ED’s nationally since the start of the pandemic,” she added.
“Post-pandemic, we have hit a crisis point,” lead author Anthony Napoli, MD, also of Brown University, said in an interview. “Boarding is largely a hospital capacity problem, but one key fix germane to EM [emergency medicine] is the provider in triage model (PIT). While PIT has been shown to improve efficiency of ED care, a single institution study demonstrated that it was unable to mitigate the effects of boarding. The study of the association of boarding and efficiency of ED operations and intake needed to be shown on a national scale,” he said.
The researchers reviewed cross-sectional ED operational data from the ED Department Benchmarking Alliance (EDBA), a voluntary database that includes self-reports of operational metrics from approximately half of EDs in the United States.
The data set included 892 EDs; freestanding and pediatric EDs, as well as those with missing boarding data, were excluded.
The primary outcome was boarding time, door-to-provider time (D2P), length of stay for discharged patients (LOSD) and the percentage of patients who left the hospital before treatment was complete (LBTC).
In a multivariate analysis, increased boarding time was significantly associated with longer D2P time, LOSD time, and rates of LBTC.
Overall, D2P and LOSD increased by 0.8 minutes and 2.8 minutes, respectively, for each additional 10 minutes of boarding time. LBTC rates increased by 0.1% for each additional 10 minutes of boarding time.
However, boarding did not have a significant impact on operational metrics among hospitals with fewer than 20,000 visits per year.
Although more research is needed, the results indicate that boarding reduces the throughput of nonboarded patients at a ratio of approximately 4:1. The limited impact of ED efficiency measures on operations highlights the need for hospital-based solutions to boarding, Dr. Tyminski concluded.
“Overall, we expected that there would be an association between boarding and reductions in ED intake and operational efficiency,” said Dr. Napoli in an interview. “However, we were surprised the relationship continued to be as strong in a national study of nearly a quarter of all EDs, as it did in our prior local study,” he said. “Every 10 minutes of boarding in an ED is associated with an approximate 0.1% increase in LWBS and a 3-minute increase in LOSD. Extrapolating this association across the country, we predicted that nearly one million patients may have potentially not received ED care due to boarding,” he explained. “Not only does this potentially have a huge impact on hospital finances but also the overall health of our patients,” he added.
The key takeaway from the study is that boarding is a hospital capacity management issue, said Dr. Napoli. Hospital leadership must be directly involved in plans to mitigate or eliminate it to the extent possible; until then, boarding will continue to result in inefficient ED operations, he explained.
“As ED providers, we are limited in what we can do, but one area where we might be able to make the most impact is to optimize the care and throughput of the LOSD patients,” Dr. Tyminski said. More research is needed to see if interventions to reduce boarding correspond with equivalent improvements in emergency department intake and improved ED throughput, she noted.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
on the basis of data from nearly 900 facilities presented at the annual meeting of the American College of Emergency Physicians.
The study was important to conduct at this time because ED boarding is significantly limiting ED physicians to provide optimal care, said Camila Tyminski, MD, of Brown University, Providence, R.I., who presented the findings at the meeting.
“Boarding had steadily been rising prior to the COVID-19 pandemic due to increased ED use. As our data show, boarding had a detrimental impact on ED throughput measures, including increased door to provider time, increased length of stay of the patient discharged from the ED, and increased rate of patients that left before completion of treatment,” she said.
“It was important to understand these trends prior to 2019-2020 because the COVID-19 pandemic and national nursing shortage have drastically worsened boarding. This study provided a framework for future studies on boarding across ED’s nationally since the start of the pandemic,” she added.
“Post-pandemic, we have hit a crisis point,” lead author Anthony Napoli, MD, also of Brown University, said in an interview. “Boarding is largely a hospital capacity problem, but one key fix germane to EM [emergency medicine] is the provider in triage model (PIT). While PIT has been shown to improve efficiency of ED care, a single institution study demonstrated that it was unable to mitigate the effects of boarding. The study of the association of boarding and efficiency of ED operations and intake needed to be shown on a national scale,” he said.
The researchers reviewed cross-sectional ED operational data from the ED Department Benchmarking Alliance (EDBA), a voluntary database that includes self-reports of operational metrics from approximately half of EDs in the United States.
The data set included 892 EDs; freestanding and pediatric EDs, as well as those with missing boarding data, were excluded.
The primary outcome was boarding time, door-to-provider time (D2P), length of stay for discharged patients (LOSD) and the percentage of patients who left the hospital before treatment was complete (LBTC).
In a multivariate analysis, increased boarding time was significantly associated with longer D2P time, LOSD time, and rates of LBTC.
Overall, D2P and LOSD increased by 0.8 minutes and 2.8 minutes, respectively, for each additional 10 minutes of boarding time. LBTC rates increased by 0.1% for each additional 10 minutes of boarding time.
However, boarding did not have a significant impact on operational metrics among hospitals with fewer than 20,000 visits per year.
Although more research is needed, the results indicate that boarding reduces the throughput of nonboarded patients at a ratio of approximately 4:1. The limited impact of ED efficiency measures on operations highlights the need for hospital-based solutions to boarding, Dr. Tyminski concluded.
“Overall, we expected that there would be an association between boarding and reductions in ED intake and operational efficiency,” said Dr. Napoli in an interview. “However, we were surprised the relationship continued to be as strong in a national study of nearly a quarter of all EDs, as it did in our prior local study,” he said. “Every 10 minutes of boarding in an ED is associated with an approximate 0.1% increase in LWBS and a 3-minute increase in LOSD. Extrapolating this association across the country, we predicted that nearly one million patients may have potentially not received ED care due to boarding,” he explained. “Not only does this potentially have a huge impact on hospital finances but also the overall health of our patients,” he added.
The key takeaway from the study is that boarding is a hospital capacity management issue, said Dr. Napoli. Hospital leadership must be directly involved in plans to mitigate or eliminate it to the extent possible; until then, boarding will continue to result in inefficient ED operations, he explained.
“As ED providers, we are limited in what we can do, but one area where we might be able to make the most impact is to optimize the care and throughput of the LOSD patients,” Dr. Tyminski said. More research is needed to see if interventions to reduce boarding correspond with equivalent improvements in emergency department intake and improved ED throughput, she noted.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
on the basis of data from nearly 900 facilities presented at the annual meeting of the American College of Emergency Physicians.
The study was important to conduct at this time because ED boarding is significantly limiting ED physicians to provide optimal care, said Camila Tyminski, MD, of Brown University, Providence, R.I., who presented the findings at the meeting.
“Boarding had steadily been rising prior to the COVID-19 pandemic due to increased ED use. As our data show, boarding had a detrimental impact on ED throughput measures, including increased door to provider time, increased length of stay of the patient discharged from the ED, and increased rate of patients that left before completion of treatment,” she said.
“It was important to understand these trends prior to 2019-2020 because the COVID-19 pandemic and national nursing shortage have drastically worsened boarding. This study provided a framework for future studies on boarding across ED’s nationally since the start of the pandemic,” she added.
“Post-pandemic, we have hit a crisis point,” lead author Anthony Napoli, MD, also of Brown University, said in an interview. “Boarding is largely a hospital capacity problem, but one key fix germane to EM [emergency medicine] is the provider in triage model (PIT). While PIT has been shown to improve efficiency of ED care, a single institution study demonstrated that it was unable to mitigate the effects of boarding. The study of the association of boarding and efficiency of ED operations and intake needed to be shown on a national scale,” he said.
The researchers reviewed cross-sectional ED operational data from the ED Department Benchmarking Alliance (EDBA), a voluntary database that includes self-reports of operational metrics from approximately half of EDs in the United States.
The data set included 892 EDs; freestanding and pediatric EDs, as well as those with missing boarding data, were excluded.
The primary outcome was boarding time, door-to-provider time (D2P), length of stay for discharged patients (LOSD) and the percentage of patients who left the hospital before treatment was complete (LBTC).
In a multivariate analysis, increased boarding time was significantly associated with longer D2P time, LOSD time, and rates of LBTC.
Overall, D2P and LOSD increased by 0.8 minutes and 2.8 minutes, respectively, for each additional 10 minutes of boarding time. LBTC rates increased by 0.1% for each additional 10 minutes of boarding time.
However, boarding did not have a significant impact on operational metrics among hospitals with fewer than 20,000 visits per year.
Although more research is needed, the results indicate that boarding reduces the throughput of nonboarded patients at a ratio of approximately 4:1. The limited impact of ED efficiency measures on operations highlights the need for hospital-based solutions to boarding, Dr. Tyminski concluded.
“Overall, we expected that there would be an association between boarding and reductions in ED intake and operational efficiency,” said Dr. Napoli in an interview. “However, we were surprised the relationship continued to be as strong in a national study of nearly a quarter of all EDs, as it did in our prior local study,” he said. “Every 10 minutes of boarding in an ED is associated with an approximate 0.1% increase in LWBS and a 3-minute increase in LOSD. Extrapolating this association across the country, we predicted that nearly one million patients may have potentially not received ED care due to boarding,” he explained. “Not only does this potentially have a huge impact on hospital finances but also the overall health of our patients,” he added.
The key takeaway from the study is that boarding is a hospital capacity management issue, said Dr. Napoli. Hospital leadership must be directly involved in plans to mitigate or eliminate it to the extent possible; until then, boarding will continue to result in inefficient ED operations, he explained.
“As ED providers, we are limited in what we can do, but one area where we might be able to make the most impact is to optimize the care and throughput of the LOSD patients,” Dr. Tyminski said. More research is needed to see if interventions to reduce boarding correspond with equivalent improvements in emergency department intake and improved ED throughput, she noted.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Like texting and driving: The human cost of AI
A recent medical meeting I attended included multiple sessions on the use of artificial intelligence (AI), a mere preview, I suspect, of what is to come for both patients and physicians.
I vow not to be a contrarian, but I have concerns. If we’d known how cell phones would permeate nearly every waking moment of our lives, would we have built in more protections from the onset?
Although anyone can see the enormous potential of AI in medicine, harnessing the wonders of it without guarding against the dangers could be paramount to texting and driving.
A palpable disruption in the common work-a-day human interaction is a given. CEOs who mind the bottom line will seek every opportunity to cut personnel whenever machine learning can deliver. As our dependence on algorithms increases, our need to understand electrocardiogram interpretation and echocardiographic calculations will wane. Subtle case information will go undetected. Nuanced subconscious alerts regarding the patient condition will go unnoticed.
These realities are never reflected in the pronouncements of companies who promote and develop AI.
The 2-minute echo
In September 2020, Carolyn Lam, MBBS, PhD, and James Hare, MBA, founders of the AI tech company US2.AI, told Healthcare Transformers that AI advances in echocardiology will turn “a manual process of 30 minutes, 250 clicks, with up to 21% variability among fully trained sonographers analyzing the same exam, into an AI-automated process taking 2 minutes, 1 click, with 0% variability.”
Let’s contrast this 2-minute human-machine interaction with the standard 20- to 30-minute human-to-human echocardiography procedure.
Take Mrs. Smith, for instance. She is referred for echocardiography for shortness of breath. She’s shown to a room and instructed to lie down on a table, where she undergoes a brief AI-directed acquisition of images and then a cheery dismissal from the imaging lab. Medical corporate chief financial officers will salivate at the efficiency, the decrease in cost for personnel, and the sharp increase in put-through for the echo lab schedule.
But what if Mrs. Smith gets a standard 30-minute sonographer-directed exam and the astute echocardiographer notes a left ventricular ejection fraction of 38%. A conversation with the patient reveals that she lost her son a few weeks ago. Upon completion of the study, the patient stands up and then adds, “I hope I can sleep in my bed tonight.” Thinking there may be more to the patient’s insomnia than grief-driven anxiety, the sonographer asks her to explain. “I had to sleep in a chair last night because I couldn’t breathe,” Mrs. Smith replies.
The sonographer reasons correctly that Mrs. Smith is likely a few weeks past an acute coronary syndrome for which she didn’t seek attention and is now in heart failure. The consulting cardiologist is alerted. Mrs. Smith is worked into the office schedule a week earlier than planned, and a costly in-patient stay for acute heart failure or worse is avoided.
Here’s a true-life example (some details have been changed to protect the patient’s identity): Mr. Rodriquez was referred for echocardiography because of dizziness. The sonographer notes significant mitral regurgitation and a decline in left ventricular ejection fraction from moderately impaired to severely reduced. When the sonographer inquires about a fresh bruise over Mr. Rodriguez’s left eye, he replies that he “must have fallen, but can’t remember.” The sonographer also notes runs of nonsustained ventricular tachycardia on the echo telemetry, and after a phone call from the echo lab to the ordering physician, Mr. Rodriquez is admitted. Instead of chancing a sudden death at home while awaiting follow-up, he undergoes catheterization and gets an implantable cardioverter defibrillator.
These scenarios illustrate that a 2-minute visit for AI-directed acquisition of echocardiogram images will never garner the protections of a conversation with a human. Any attempts at downplaying the importance of these human interactions are misguided.
Sometimes we embrace the latest advances in medicine while failing to tend to the most rudimentary necessities of data analysis and reporting. Catherine M. Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle, is a fan of the basics.
At the recent annual congress of the European Society of Cardiology, she commented on the AI-ENHANCED trial, which used an AI decision support algorithm to identify patients with moderate to severe aortic stenosis, which is associated with poor survival if left untreated. She correctly highlighted that while we are discussing the merits of AI-driven assessment of aortic stenosis, we are doing so in an era when many echo interpreters exclude critical information. The vital findings of aortic valve area, Vmax, and ejection fraction are often nowhere to be seen on reports. We should attend to our basic flaws in interpretation and reporting before we shift our focus to AI.
Flawed algorithms
Incorrect AI algorithms that are broadly adopted could negatively affect the health of millions.
Perhaps the most unsettling claim is made by causaLens: “Causal AI is the only technology that can reason and make choices like humans do,” the website states. A tantalizing tag line that is categorically untrue.
Our mysterious and complex neurophysiological function of reasoning still eludes understanding, but one thing is certain: medical reasoning originates with listening, seeing, and touching.
As AI infiltrates mainstream medicine, opportunities for hearing, observing, and palpating will be greatly reduced.
Folkert Asselbergs from University Medical Center Utrecht, the Netherlands, who has cautioned against overhyping AI, was the discussant for an ESC study on the use of causal AI to improve cardiovascular risk estimation.
He flashed a slide of a 2019 Science article on racial bias in an algorithm that U.S. health care systems use. Remedying that bias “would increase the percentage of Black people receiving additional help from 17.7% to 46.5%,” according to the authors.
Successful integration of AI-driven technology will come only if we build human interaction into every patient encounter.
I hope I don’t live to see the rise of the physician cyborg.
Artificial intelligence could be the greatest boon since the invention of the stethoscope, but it will be our downfall if we stop administering a healthy dose of humanity to every patient encounter.
Melissa Walton-Shirley, MD, is a clinical cardiologist in Nashville, Tenn., who has retired from full-time invasive cardiology. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent medical meeting I attended included multiple sessions on the use of artificial intelligence (AI), a mere preview, I suspect, of what is to come for both patients and physicians.
I vow not to be a contrarian, but I have concerns. If we’d known how cell phones would permeate nearly every waking moment of our lives, would we have built in more protections from the onset?
Although anyone can see the enormous potential of AI in medicine, harnessing the wonders of it without guarding against the dangers could be paramount to texting and driving.
A palpable disruption in the common work-a-day human interaction is a given. CEOs who mind the bottom line will seek every opportunity to cut personnel whenever machine learning can deliver. As our dependence on algorithms increases, our need to understand electrocardiogram interpretation and echocardiographic calculations will wane. Subtle case information will go undetected. Nuanced subconscious alerts regarding the patient condition will go unnoticed.
These realities are never reflected in the pronouncements of companies who promote and develop AI.
The 2-minute echo
In September 2020, Carolyn Lam, MBBS, PhD, and James Hare, MBA, founders of the AI tech company US2.AI, told Healthcare Transformers that AI advances in echocardiology will turn “a manual process of 30 minutes, 250 clicks, with up to 21% variability among fully trained sonographers analyzing the same exam, into an AI-automated process taking 2 minutes, 1 click, with 0% variability.”
Let’s contrast this 2-minute human-machine interaction with the standard 20- to 30-minute human-to-human echocardiography procedure.
Take Mrs. Smith, for instance. She is referred for echocardiography for shortness of breath. She’s shown to a room and instructed to lie down on a table, where she undergoes a brief AI-directed acquisition of images and then a cheery dismissal from the imaging lab. Medical corporate chief financial officers will salivate at the efficiency, the decrease in cost for personnel, and the sharp increase in put-through for the echo lab schedule.
But what if Mrs. Smith gets a standard 30-minute sonographer-directed exam and the astute echocardiographer notes a left ventricular ejection fraction of 38%. A conversation with the patient reveals that she lost her son a few weeks ago. Upon completion of the study, the patient stands up and then adds, “I hope I can sleep in my bed tonight.” Thinking there may be more to the patient’s insomnia than grief-driven anxiety, the sonographer asks her to explain. “I had to sleep in a chair last night because I couldn’t breathe,” Mrs. Smith replies.
The sonographer reasons correctly that Mrs. Smith is likely a few weeks past an acute coronary syndrome for which she didn’t seek attention and is now in heart failure. The consulting cardiologist is alerted. Mrs. Smith is worked into the office schedule a week earlier than planned, and a costly in-patient stay for acute heart failure or worse is avoided.
Here’s a true-life example (some details have been changed to protect the patient’s identity): Mr. Rodriquez was referred for echocardiography because of dizziness. The sonographer notes significant mitral regurgitation and a decline in left ventricular ejection fraction from moderately impaired to severely reduced. When the sonographer inquires about a fresh bruise over Mr. Rodriguez’s left eye, he replies that he “must have fallen, but can’t remember.” The sonographer also notes runs of nonsustained ventricular tachycardia on the echo telemetry, and after a phone call from the echo lab to the ordering physician, Mr. Rodriquez is admitted. Instead of chancing a sudden death at home while awaiting follow-up, he undergoes catheterization and gets an implantable cardioverter defibrillator.
These scenarios illustrate that a 2-minute visit for AI-directed acquisition of echocardiogram images will never garner the protections of a conversation with a human. Any attempts at downplaying the importance of these human interactions are misguided.
Sometimes we embrace the latest advances in medicine while failing to tend to the most rudimentary necessities of data analysis and reporting. Catherine M. Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle, is a fan of the basics.
At the recent annual congress of the European Society of Cardiology, she commented on the AI-ENHANCED trial, which used an AI decision support algorithm to identify patients with moderate to severe aortic stenosis, which is associated with poor survival if left untreated. She correctly highlighted that while we are discussing the merits of AI-driven assessment of aortic stenosis, we are doing so in an era when many echo interpreters exclude critical information. The vital findings of aortic valve area, Vmax, and ejection fraction are often nowhere to be seen on reports. We should attend to our basic flaws in interpretation and reporting before we shift our focus to AI.
Flawed algorithms
Incorrect AI algorithms that are broadly adopted could negatively affect the health of millions.
Perhaps the most unsettling claim is made by causaLens: “Causal AI is the only technology that can reason and make choices like humans do,” the website states. A tantalizing tag line that is categorically untrue.
Our mysterious and complex neurophysiological function of reasoning still eludes understanding, but one thing is certain: medical reasoning originates with listening, seeing, and touching.
As AI infiltrates mainstream medicine, opportunities for hearing, observing, and palpating will be greatly reduced.
Folkert Asselbergs from University Medical Center Utrecht, the Netherlands, who has cautioned against overhyping AI, was the discussant for an ESC study on the use of causal AI to improve cardiovascular risk estimation.
He flashed a slide of a 2019 Science article on racial bias in an algorithm that U.S. health care systems use. Remedying that bias “would increase the percentage of Black people receiving additional help from 17.7% to 46.5%,” according to the authors.
Successful integration of AI-driven technology will come only if we build human interaction into every patient encounter.
I hope I don’t live to see the rise of the physician cyborg.
Artificial intelligence could be the greatest boon since the invention of the stethoscope, but it will be our downfall if we stop administering a healthy dose of humanity to every patient encounter.
Melissa Walton-Shirley, MD, is a clinical cardiologist in Nashville, Tenn., who has retired from full-time invasive cardiology. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent medical meeting I attended included multiple sessions on the use of artificial intelligence (AI), a mere preview, I suspect, of what is to come for both patients and physicians.
I vow not to be a contrarian, but I have concerns. If we’d known how cell phones would permeate nearly every waking moment of our lives, would we have built in more protections from the onset?
Although anyone can see the enormous potential of AI in medicine, harnessing the wonders of it without guarding against the dangers could be paramount to texting and driving.
A palpable disruption in the common work-a-day human interaction is a given. CEOs who mind the bottom line will seek every opportunity to cut personnel whenever machine learning can deliver. As our dependence on algorithms increases, our need to understand electrocardiogram interpretation and echocardiographic calculations will wane. Subtle case information will go undetected. Nuanced subconscious alerts regarding the patient condition will go unnoticed.
These realities are never reflected in the pronouncements of companies who promote and develop AI.
The 2-minute echo
In September 2020, Carolyn Lam, MBBS, PhD, and James Hare, MBA, founders of the AI tech company US2.AI, told Healthcare Transformers that AI advances in echocardiology will turn “a manual process of 30 minutes, 250 clicks, with up to 21% variability among fully trained sonographers analyzing the same exam, into an AI-automated process taking 2 minutes, 1 click, with 0% variability.”
Let’s contrast this 2-minute human-machine interaction with the standard 20- to 30-minute human-to-human echocardiography procedure.
Take Mrs. Smith, for instance. She is referred for echocardiography for shortness of breath. She’s shown to a room and instructed to lie down on a table, where she undergoes a brief AI-directed acquisition of images and then a cheery dismissal from the imaging lab. Medical corporate chief financial officers will salivate at the efficiency, the decrease in cost for personnel, and the sharp increase in put-through for the echo lab schedule.
But what if Mrs. Smith gets a standard 30-minute sonographer-directed exam and the astute echocardiographer notes a left ventricular ejection fraction of 38%. A conversation with the patient reveals that she lost her son a few weeks ago. Upon completion of the study, the patient stands up and then adds, “I hope I can sleep in my bed tonight.” Thinking there may be more to the patient’s insomnia than grief-driven anxiety, the sonographer asks her to explain. “I had to sleep in a chair last night because I couldn’t breathe,” Mrs. Smith replies.
The sonographer reasons correctly that Mrs. Smith is likely a few weeks past an acute coronary syndrome for which she didn’t seek attention and is now in heart failure. The consulting cardiologist is alerted. Mrs. Smith is worked into the office schedule a week earlier than planned, and a costly in-patient stay for acute heart failure or worse is avoided.
Here’s a true-life example (some details have been changed to protect the patient’s identity): Mr. Rodriquez was referred for echocardiography because of dizziness. The sonographer notes significant mitral regurgitation and a decline in left ventricular ejection fraction from moderately impaired to severely reduced. When the sonographer inquires about a fresh bruise over Mr. Rodriguez’s left eye, he replies that he “must have fallen, but can’t remember.” The sonographer also notes runs of nonsustained ventricular tachycardia on the echo telemetry, and after a phone call from the echo lab to the ordering physician, Mr. Rodriquez is admitted. Instead of chancing a sudden death at home while awaiting follow-up, he undergoes catheterization and gets an implantable cardioverter defibrillator.
These scenarios illustrate that a 2-minute visit for AI-directed acquisition of echocardiogram images will never garner the protections of a conversation with a human. Any attempts at downplaying the importance of these human interactions are misguided.
Sometimes we embrace the latest advances in medicine while failing to tend to the most rudimentary necessities of data analysis and reporting. Catherine M. Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle, is a fan of the basics.
At the recent annual congress of the European Society of Cardiology, she commented on the AI-ENHANCED trial, which used an AI decision support algorithm to identify patients with moderate to severe aortic stenosis, which is associated with poor survival if left untreated. She correctly highlighted that while we are discussing the merits of AI-driven assessment of aortic stenosis, we are doing so in an era when many echo interpreters exclude critical information. The vital findings of aortic valve area, Vmax, and ejection fraction are often nowhere to be seen on reports. We should attend to our basic flaws in interpretation and reporting before we shift our focus to AI.
Flawed algorithms
Incorrect AI algorithms that are broadly adopted could negatively affect the health of millions.
Perhaps the most unsettling claim is made by causaLens: “Causal AI is the only technology that can reason and make choices like humans do,” the website states. A tantalizing tag line that is categorically untrue.
Our mysterious and complex neurophysiological function of reasoning still eludes understanding, but one thing is certain: medical reasoning originates with listening, seeing, and touching.
As AI infiltrates mainstream medicine, opportunities for hearing, observing, and palpating will be greatly reduced.
Folkert Asselbergs from University Medical Center Utrecht, the Netherlands, who has cautioned against overhyping AI, was the discussant for an ESC study on the use of causal AI to improve cardiovascular risk estimation.
He flashed a slide of a 2019 Science article on racial bias in an algorithm that U.S. health care systems use. Remedying that bias “would increase the percentage of Black people receiving additional help from 17.7% to 46.5%,” according to the authors.
Successful integration of AI-driven technology will come only if we build human interaction into every patient encounter.
I hope I don’t live to see the rise of the physician cyborg.
Artificial intelligence could be the greatest boon since the invention of the stethoscope, but it will be our downfall if we stop administering a healthy dose of humanity to every patient encounter.
Melissa Walton-Shirley, MD, is a clinical cardiologist in Nashville, Tenn., who has retired from full-time invasive cardiology. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Dapagliflozin DELIVERs regardless of systolic pressure in HFpEF
Whatever the mechanism of benefit from dapagliflozin (Farxiga) in patients with heart failure (HF) – and potentially also other sodium-glucose cotransporter 2 (SGLT2) inhibitors – its blood pressure lowering effects aren’t likely to contribute much.
Indeed, at least in patients with HF and non-reduced ejection fractions, dapagliflozin has only a modest BP-lowering effect and cuts cardiovascular (CV) risk regardless of baseline pressure or change in systolic BP, suggests a secondary analysis from the large placebo-controlled DELIVER trial.
Systolic BP fell over 1 month by just under 2 mmHg, on average, in trial patients with either mildly reduced or preserved ejection fraction (HFmrEF or HFpEF, respectively) assigned to take dapagliflozin versus placebo.
The effect was achieved without increasing the risk for adverse events from dapagliflozin, even among patients with the lowest baseline systolic pressures. Adverse outcomes overall, however, were more common at the lowest systolic BP level than at higher pressures, researchers reported.
They say the findings should help alleviate long-standing concerns that initiating SGLT2 inhibitors, with their recognized diuretic effects, might present a hazard in patients with HF and low systolic BP.
“It is a consistent theme in heart failure trials that the blood pressure–lowering effect of SGLT2 inhibitors is more modest than it is in non–heart-failure populations,” Senthil Selvaraj, MD, Duke University, Durham, N.C., told this news organization.
Changes to antihypertensive drug therapy throughout the trial, which presumably enhanced BP responses and “might occur more frequently in the placebo group,” Dr. Selvaraj said, “might explain why the blood pressure effect is a little bit more modest in this population.”
Dr. Selvaraj presented the analysis at the Annual Scientific Meeting of the Heart Failure Society of America, held in National Harbor, Md., and is lead author on its same-day publication in JACC: Heart Failure.
The findings “reinforce the clinical benefits of SGLT2 inhibitors in patients with heart failure across the full spectrum of ejection fractions and large range of systolic blood pressures,” said Gregg C. Fonarow, MD, University of California, Los Angeles Medical Center, who was not part of the DELIVER analysis.
The study’s greater adjusted risks for CV and all-cause mortality risks at the lowest baseline systolic pressures “parallels a series of observational analyses from registries, including OPTIMIZE-HF,” Dr. Fonarow observed.
In those prior studies of patients with established HFpEF, “systolic BP less than 120 mmHg or even 130 mmHg was associated with worse outcomes than those with higher systolic BP.”
The current findings, therefore, “highlight how optimal blood pressure targets in patients with established heart failure have not been well established,” Dr. Fonarow said.
The analysis included all 6,263 participants in DELIVER, outpatients or patients hospitalized for worsening HF who were in NYHA class 2-4 with a left ventricular ejection fraction (LVEF) greater than 40%. They averaged 72 in age, and 44% were women. Their mean baseline systolic BP was 128 mmHg.
After 1 month, mean systolic BP had fallen by 1.8 mmHg (P < .001) in patients who had been randomly assigned to dapagliflozin versus placebo. The effect was consistent (interaction P = .16) across all systolic BP categories (less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and 140 mmHg or higher).
The effect was similarly independent of estimated glomerular filtration rate (eGFR) and LVEF (interaction P = .30 and P = .33, respectively), Dr. Selvaraj reported.
In an analysis adjusted for both baseline and 1-month change in systolic BP, the effect of dapagliflozin on the primary endpoint was “minimally attenuated,” compared with the primary analysis, he said. That suggests the clinical benefits “did not significantly relate to the blood pressure–lowering effect” of the SGLT2 inhibitor.
In that analysis, the hazard ratio for CV death or worsening HF for dapagliflozin versus placebo was 0.85 (95% confidence interval, 0.75-0.96; P = .010). The HR had been 0.82 (95% CI, 0.73-0.92; P < .001) overall in the DELIVER primary analysis.
The current study doesn’t shed further light on the main SGLT2 inhibitor mechanism of clinical benefit in nondiabetics with HF, which remains a mystery.
“There is a diuretic effect, but it’s not incredibly robust,” Dr. Selvaraj observed. It may contribute to the drugs’ benefits, “but it’s definitely more than that – a lot more than that.”
DELIVER was funded by AstraZeneca. Dr. Selvaraj reported no relevant conflicts. Disclosures for the other authors are in the report. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Whatever the mechanism of benefit from dapagliflozin (Farxiga) in patients with heart failure (HF) – and potentially also other sodium-glucose cotransporter 2 (SGLT2) inhibitors – its blood pressure lowering effects aren’t likely to contribute much.
Indeed, at least in patients with HF and non-reduced ejection fractions, dapagliflozin has only a modest BP-lowering effect and cuts cardiovascular (CV) risk regardless of baseline pressure or change in systolic BP, suggests a secondary analysis from the large placebo-controlled DELIVER trial.
Systolic BP fell over 1 month by just under 2 mmHg, on average, in trial patients with either mildly reduced or preserved ejection fraction (HFmrEF or HFpEF, respectively) assigned to take dapagliflozin versus placebo.
The effect was achieved without increasing the risk for adverse events from dapagliflozin, even among patients with the lowest baseline systolic pressures. Adverse outcomes overall, however, were more common at the lowest systolic BP level than at higher pressures, researchers reported.
They say the findings should help alleviate long-standing concerns that initiating SGLT2 inhibitors, with their recognized diuretic effects, might present a hazard in patients with HF and low systolic BP.
“It is a consistent theme in heart failure trials that the blood pressure–lowering effect of SGLT2 inhibitors is more modest than it is in non–heart-failure populations,” Senthil Selvaraj, MD, Duke University, Durham, N.C., told this news organization.
Changes to antihypertensive drug therapy throughout the trial, which presumably enhanced BP responses and “might occur more frequently in the placebo group,” Dr. Selvaraj said, “might explain why the blood pressure effect is a little bit more modest in this population.”
Dr. Selvaraj presented the analysis at the Annual Scientific Meeting of the Heart Failure Society of America, held in National Harbor, Md., and is lead author on its same-day publication in JACC: Heart Failure.
The findings “reinforce the clinical benefits of SGLT2 inhibitors in patients with heart failure across the full spectrum of ejection fractions and large range of systolic blood pressures,” said Gregg C. Fonarow, MD, University of California, Los Angeles Medical Center, who was not part of the DELIVER analysis.
The study’s greater adjusted risks for CV and all-cause mortality risks at the lowest baseline systolic pressures “parallels a series of observational analyses from registries, including OPTIMIZE-HF,” Dr. Fonarow observed.
In those prior studies of patients with established HFpEF, “systolic BP less than 120 mmHg or even 130 mmHg was associated with worse outcomes than those with higher systolic BP.”
The current findings, therefore, “highlight how optimal blood pressure targets in patients with established heart failure have not been well established,” Dr. Fonarow said.
The analysis included all 6,263 participants in DELIVER, outpatients or patients hospitalized for worsening HF who were in NYHA class 2-4 with a left ventricular ejection fraction (LVEF) greater than 40%. They averaged 72 in age, and 44% were women. Their mean baseline systolic BP was 128 mmHg.
After 1 month, mean systolic BP had fallen by 1.8 mmHg (P < .001) in patients who had been randomly assigned to dapagliflozin versus placebo. The effect was consistent (interaction P = .16) across all systolic BP categories (less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and 140 mmHg or higher).
The effect was similarly independent of estimated glomerular filtration rate (eGFR) and LVEF (interaction P = .30 and P = .33, respectively), Dr. Selvaraj reported.
In an analysis adjusted for both baseline and 1-month change in systolic BP, the effect of dapagliflozin on the primary endpoint was “minimally attenuated,” compared with the primary analysis, he said. That suggests the clinical benefits “did not significantly relate to the blood pressure–lowering effect” of the SGLT2 inhibitor.
In that analysis, the hazard ratio for CV death or worsening HF for dapagliflozin versus placebo was 0.85 (95% confidence interval, 0.75-0.96; P = .010). The HR had been 0.82 (95% CI, 0.73-0.92; P < .001) overall in the DELIVER primary analysis.
The current study doesn’t shed further light on the main SGLT2 inhibitor mechanism of clinical benefit in nondiabetics with HF, which remains a mystery.
“There is a diuretic effect, but it’s not incredibly robust,” Dr. Selvaraj observed. It may contribute to the drugs’ benefits, “but it’s definitely more than that – a lot more than that.”
DELIVER was funded by AstraZeneca. Dr. Selvaraj reported no relevant conflicts. Disclosures for the other authors are in the report. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Whatever the mechanism of benefit from dapagliflozin (Farxiga) in patients with heart failure (HF) – and potentially also other sodium-glucose cotransporter 2 (SGLT2) inhibitors – its blood pressure lowering effects aren’t likely to contribute much.
Indeed, at least in patients with HF and non-reduced ejection fractions, dapagliflozin has only a modest BP-lowering effect and cuts cardiovascular (CV) risk regardless of baseline pressure or change in systolic BP, suggests a secondary analysis from the large placebo-controlled DELIVER trial.
Systolic BP fell over 1 month by just under 2 mmHg, on average, in trial patients with either mildly reduced or preserved ejection fraction (HFmrEF or HFpEF, respectively) assigned to take dapagliflozin versus placebo.
The effect was achieved without increasing the risk for adverse events from dapagliflozin, even among patients with the lowest baseline systolic pressures. Adverse outcomes overall, however, were more common at the lowest systolic BP level than at higher pressures, researchers reported.
They say the findings should help alleviate long-standing concerns that initiating SGLT2 inhibitors, with their recognized diuretic effects, might present a hazard in patients with HF and low systolic BP.
“It is a consistent theme in heart failure trials that the blood pressure–lowering effect of SGLT2 inhibitors is more modest than it is in non–heart-failure populations,” Senthil Selvaraj, MD, Duke University, Durham, N.C., told this news organization.
Changes to antihypertensive drug therapy throughout the trial, which presumably enhanced BP responses and “might occur more frequently in the placebo group,” Dr. Selvaraj said, “might explain why the blood pressure effect is a little bit more modest in this population.”
Dr. Selvaraj presented the analysis at the Annual Scientific Meeting of the Heart Failure Society of America, held in National Harbor, Md., and is lead author on its same-day publication in JACC: Heart Failure.
The findings “reinforce the clinical benefits of SGLT2 inhibitors in patients with heart failure across the full spectrum of ejection fractions and large range of systolic blood pressures,” said Gregg C. Fonarow, MD, University of California, Los Angeles Medical Center, who was not part of the DELIVER analysis.
The study’s greater adjusted risks for CV and all-cause mortality risks at the lowest baseline systolic pressures “parallels a series of observational analyses from registries, including OPTIMIZE-HF,” Dr. Fonarow observed.
In those prior studies of patients with established HFpEF, “systolic BP less than 120 mmHg or even 130 mmHg was associated with worse outcomes than those with higher systolic BP.”
The current findings, therefore, “highlight how optimal blood pressure targets in patients with established heart failure have not been well established,” Dr. Fonarow said.
The analysis included all 6,263 participants in DELIVER, outpatients or patients hospitalized for worsening HF who were in NYHA class 2-4 with a left ventricular ejection fraction (LVEF) greater than 40%. They averaged 72 in age, and 44% were women. Their mean baseline systolic BP was 128 mmHg.
After 1 month, mean systolic BP had fallen by 1.8 mmHg (P < .001) in patients who had been randomly assigned to dapagliflozin versus placebo. The effect was consistent (interaction P = .16) across all systolic BP categories (less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and 140 mmHg or higher).
The effect was similarly independent of estimated glomerular filtration rate (eGFR) and LVEF (interaction P = .30 and P = .33, respectively), Dr. Selvaraj reported.
In an analysis adjusted for both baseline and 1-month change in systolic BP, the effect of dapagliflozin on the primary endpoint was “minimally attenuated,” compared with the primary analysis, he said. That suggests the clinical benefits “did not significantly relate to the blood pressure–lowering effect” of the SGLT2 inhibitor.
In that analysis, the hazard ratio for CV death or worsening HF for dapagliflozin versus placebo was 0.85 (95% confidence interval, 0.75-0.96; P = .010). The HR had been 0.82 (95% CI, 0.73-0.92; P < .001) overall in the DELIVER primary analysis.
The current study doesn’t shed further light on the main SGLT2 inhibitor mechanism of clinical benefit in nondiabetics with HF, which remains a mystery.
“There is a diuretic effect, but it’s not incredibly robust,” Dr. Selvaraj observed. It may contribute to the drugs’ benefits, “but it’s definitely more than that – a lot more than that.”
DELIVER was funded by AstraZeneca. Dr. Selvaraj reported no relevant conflicts. Disclosures for the other authors are in the report. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Pandemic drove drop in rheumatology payments from industry
Payments to rheumatologists from industry declined early in the COVID-19 pandemic but showed some rebound in 2021, based on information from the Open Payments Database (OPD).
The OPD was established in 2013 to improve transparency in financial relationships between industry and health care professionals in the United States, although many physicians and much of the general public is unaware of the OPD, Anju Murayama of the Medical Governance Research Institute, Tokyo, and colleagues wrote.
The COVID-19 pandemic may have limited rheumatologists’ involvement with industry, but potential changes in financial relationships during the pandemic have not been well studied, they wrote.
In a study published in the Journal of Rheumatology, the researchers reviewed data from 6,047 rheumatologists who received at least one general payment from industry between August 2013 and December 2021. The total value of the payments was $288,326,257.
The data set included all general payments made to the physicians whose primary specialty was categorized as rheumatology in the National Plan and Provider Enumeration System profile. The payment information came from the OPD and included payments between August 2013 and December 2021.
In this analysis, the periods before and after March 2020 were considered as before and after the pandemic, respectively.
At the onset of the pandemic, monthly payments to rheumatologists overall decreased by 65.1%, and the number of rheumatologists who received payments decreased by 39.8%; a decrease occurred across all levels of payment.
“However, the recovery trend in payments during the pandemic was higher among the rheumatologists with lower payments,” the researchers noted.
The most significant decreases across payment types occurred in travel and accommodation, which dropped by 98.2% at the start of the pandemic. Payments for speaking engagements and meals decreased by 72.3% and 72.0%, respectively, at the start of the pandemic; consulting payments decreased by 23.3%.
The number of rheumatologists with payments ranged from 3,547 in 2020 to 4,444 in 2015, and did not change significantly between 2014 and 2019. However, the median total payments increased from $730 in 2014 to $812 in 2019.
Compared with the 2014-2019 period, the number of rheumatologists with payments in 2020-2021 decreased by 21.7% and the payments per rheumatologist decreased by 41.9% (P < .001 for both).
In 2021, general payments to rheumatologists were still below levels from the 2014-2019 period.
The study findings were limited by the exclusion of rheumatologist without payments and the lack of data on confounding factors, the researchers noted. However, the study is the first to show the impact of the COVID-19 pandemic on the financial relationships between U.S. rheumatologists and industry.
“Although there were recovering trends in general payments right after the onset of the COVID-19 pandemic, we observed general payments remaining at low levels between 2020 and 2021,” they noted.
A previous study showed that general payments to rheumatologists between 2013 and 2015 were significantly associated with increased prescription of brand-name rheumatology drugs and health care use. But more long-term studies are needed “to investigate whether this downward trend in general payments [observed in the current study] has contributed to reducing undue influence on rheumatologists’ clinical practice,” the researchers concluded.
The study received no outside funding. One coauthor disclosed personal fees from Medical Network Systems unrelated to the current study. The study authors had no financial conflicts related to the current study, but continue to research financial and nonfinancial conflicts of interest among health care professionals and pharmaceutical companies in Japan and the United States.
Payments to rheumatologists from industry declined early in the COVID-19 pandemic but showed some rebound in 2021, based on information from the Open Payments Database (OPD).
The OPD was established in 2013 to improve transparency in financial relationships between industry and health care professionals in the United States, although many physicians and much of the general public is unaware of the OPD, Anju Murayama of the Medical Governance Research Institute, Tokyo, and colleagues wrote.
The COVID-19 pandemic may have limited rheumatologists’ involvement with industry, but potential changes in financial relationships during the pandemic have not been well studied, they wrote.
In a study published in the Journal of Rheumatology, the researchers reviewed data from 6,047 rheumatologists who received at least one general payment from industry between August 2013 and December 2021. The total value of the payments was $288,326,257.
The data set included all general payments made to the physicians whose primary specialty was categorized as rheumatology in the National Plan and Provider Enumeration System profile. The payment information came from the OPD and included payments between August 2013 and December 2021.
In this analysis, the periods before and after March 2020 were considered as before and after the pandemic, respectively.
At the onset of the pandemic, monthly payments to rheumatologists overall decreased by 65.1%, and the number of rheumatologists who received payments decreased by 39.8%; a decrease occurred across all levels of payment.
“However, the recovery trend in payments during the pandemic was higher among the rheumatologists with lower payments,” the researchers noted.
The most significant decreases across payment types occurred in travel and accommodation, which dropped by 98.2% at the start of the pandemic. Payments for speaking engagements and meals decreased by 72.3% and 72.0%, respectively, at the start of the pandemic; consulting payments decreased by 23.3%.
The number of rheumatologists with payments ranged from 3,547 in 2020 to 4,444 in 2015, and did not change significantly between 2014 and 2019. However, the median total payments increased from $730 in 2014 to $812 in 2019.
Compared with the 2014-2019 period, the number of rheumatologists with payments in 2020-2021 decreased by 21.7% and the payments per rheumatologist decreased by 41.9% (P < .001 for both).
In 2021, general payments to rheumatologists were still below levels from the 2014-2019 period.
The study findings were limited by the exclusion of rheumatologist without payments and the lack of data on confounding factors, the researchers noted. However, the study is the first to show the impact of the COVID-19 pandemic on the financial relationships between U.S. rheumatologists and industry.
“Although there were recovering trends in general payments right after the onset of the COVID-19 pandemic, we observed general payments remaining at low levels between 2020 and 2021,” they noted.
A previous study showed that general payments to rheumatologists between 2013 and 2015 were significantly associated with increased prescription of brand-name rheumatology drugs and health care use. But more long-term studies are needed “to investigate whether this downward trend in general payments [observed in the current study] has contributed to reducing undue influence on rheumatologists’ clinical practice,” the researchers concluded.
The study received no outside funding. One coauthor disclosed personal fees from Medical Network Systems unrelated to the current study. The study authors had no financial conflicts related to the current study, but continue to research financial and nonfinancial conflicts of interest among health care professionals and pharmaceutical companies in Japan and the United States.
Payments to rheumatologists from industry declined early in the COVID-19 pandemic but showed some rebound in 2021, based on information from the Open Payments Database (OPD).
The OPD was established in 2013 to improve transparency in financial relationships between industry and health care professionals in the United States, although many physicians and much of the general public is unaware of the OPD, Anju Murayama of the Medical Governance Research Institute, Tokyo, and colleagues wrote.
The COVID-19 pandemic may have limited rheumatologists’ involvement with industry, but potential changes in financial relationships during the pandemic have not been well studied, they wrote.
In a study published in the Journal of Rheumatology, the researchers reviewed data from 6,047 rheumatologists who received at least one general payment from industry between August 2013 and December 2021. The total value of the payments was $288,326,257.
The data set included all general payments made to the physicians whose primary specialty was categorized as rheumatology in the National Plan and Provider Enumeration System profile. The payment information came from the OPD and included payments between August 2013 and December 2021.
In this analysis, the periods before and after March 2020 were considered as before and after the pandemic, respectively.
At the onset of the pandemic, monthly payments to rheumatologists overall decreased by 65.1%, and the number of rheumatologists who received payments decreased by 39.8%; a decrease occurred across all levels of payment.
“However, the recovery trend in payments during the pandemic was higher among the rheumatologists with lower payments,” the researchers noted.
The most significant decreases across payment types occurred in travel and accommodation, which dropped by 98.2% at the start of the pandemic. Payments for speaking engagements and meals decreased by 72.3% and 72.0%, respectively, at the start of the pandemic; consulting payments decreased by 23.3%.
The number of rheumatologists with payments ranged from 3,547 in 2020 to 4,444 in 2015, and did not change significantly between 2014 and 2019. However, the median total payments increased from $730 in 2014 to $812 in 2019.
Compared with the 2014-2019 period, the number of rheumatologists with payments in 2020-2021 decreased by 21.7% and the payments per rheumatologist decreased by 41.9% (P < .001 for both).
In 2021, general payments to rheumatologists were still below levels from the 2014-2019 period.
The study findings were limited by the exclusion of rheumatologist without payments and the lack of data on confounding factors, the researchers noted. However, the study is the first to show the impact of the COVID-19 pandemic on the financial relationships between U.S. rheumatologists and industry.
“Although there were recovering trends in general payments right after the onset of the COVID-19 pandemic, we observed general payments remaining at low levels between 2020 and 2021,” they noted.
A previous study showed that general payments to rheumatologists between 2013 and 2015 were significantly associated with increased prescription of brand-name rheumatology drugs and health care use. But more long-term studies are needed “to investigate whether this downward trend in general payments [observed in the current study] has contributed to reducing undue influence on rheumatologists’ clinical practice,” the researchers concluded.
The study received no outside funding. One coauthor disclosed personal fees from Medical Network Systems unrelated to the current study. The study authors had no financial conflicts related to the current study, but continue to research financial and nonfinancial conflicts of interest among health care professionals and pharmaceutical companies in Japan and the United States.
FROM THE JOURNAL OF RHEUMATOLOGY