User login
COVID bivalent booster better vs. recent Omicron subvariants: Pfizer
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
Avoid routine early ECMO in severe cardiogenic shock: ECMO-CS
CHICAGO – Routine early, expeditious use of extracorporeal membrane oxygenation (ECMO) is a common strategy in patients with severe cardiogenic shock, but a less aggressive initial approach may be just as effective, a randomized trial suggests.
In the study that assigned patients with “rapidly deteriorating or severe” cardiogenic shock to one or the other approach, clinical outcomes were no better for those who received immediate ECMO than for those initially managed with inotropes and vasopressors, researchers said.
The conservative strategy, importantly, allowed for downstream ECMO in the event of hemodynamic deterioration, which occurred in a substantial 39% of cases, observed Petr Ostadal, MD, PhD, when presenting the results at the American Heart Association scientific sessions.
Dr. Ostadal of Na Homolce Hospital, Prague, is also first author on the published report of the study, called Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS), which was published the same day in Circulation.
The trial makes a firm case for preferring the conservative initial approach over routine early ECMO in the kind of patients it entered, Larry A. Allen, MD, MHS, University of Coloradoat Denver, Aurora, told this news organization.
More than 60% of the trial’s 117 patients had shock secondary to an acute coronary syndrome; another 23% were in heart failure decompensation.
A preference for the conservative initial approach would be welcome, he said. The early aggressive ECMO approach is resource intensive and carries some important risks, such as stroke or coagulopathy, said Dr. Allen, who is not connected with ECMO-CS. Yet it is increasingly the go-to approach in such patients, based primarily on observational data.
Although early ECMO apparently didn’t benefit patients in this study in their specific stage of cardiogenic shock, Dr. Allen observed, it would presumably help some, but identifying them in practice presents challenges. “Defining where people are in the spectrum of early versus middle versus late cardiogenic shock is actually very tricky.”
It will therefore be important, he said, to identify ways to predict which conservatively managed patients do well with the strategy, and which are most at risk for hemodynamic deterioration and for whom ECMO should be readily available.
“I think part of what ECMO-CS tells us is that, if a patient is stable on intravenous inotropic and vasopressor support, you can defer ECMO while you’re thinking about the patient – about their larger context and the right medical decision-making for them.”
The trial randomly assigned 122 patients with rapidly deteriorating or severe cardiogenic shock to the immediate-ECMO or the conservative strategy at four centers in the Czech Republic. The 117 patients for whom informed consent could be obtained were included in the analysis, 58 and 59 patients, respectively. Their mean age was about 65 years and three-fourths were male.
The primary endpoint, the only endpoint for which the study was powered, consisted of death from any cause, resuscitated circulatory arrest, or use of a different form of mechanical circulatory support (MCS) by 30 days.
It occurred in 63.8% of patients assigned to immediate ECMO and 71.2% of those in the conservative strategy group, for a hazard ratio of 0.72 (95% confidence interval, 0.46-1.12; P = .21).
As individual endpoints, rates of death from any cause and resuscitated arrest did not significantly differ between the groups, but conservatively managed patients more often used another form of MCS. The HRs were 1.11 (95% CI, 0.66-1.87) for death from any cause, 0.79 (95% CI, 0.27-2.28) for resuscitated cardiac arrest, and 0.38 (95% CI, 0.18-0.79) for use of another MCS device.
The rates for serious adverse events – including bleeding, ischemia, stroke, pneumonia, or sepsis – were similar at 60.3% in the early-ECMO group and 61% in group with conservative initial management, Dr. Ostadal reported.
Other than the 23 patients in the conservative initial strategy group who went on to receive ECMO (1.9 days after randomization, on average), 1 went on to undergo implantation with a HeartMate (Abbott) ventricular assist device and 3 received an Impella pump (Abiomed).
Six patients in the early-ECMO group were already receiving intra-aortic balloon pump (IABP) support at randomization, two underwent temporary implantation with a Centrimag device (Abbott), and three went on to receive a HeartMate device, the published report notes.
ECMO is the optimal first choice for MCS in such patients with cardiogenic shock who need a circulatory support device, especially because it also oxygenates the blood, Dr. Ostadal told this news organization.
But ECMO doesn’t help with ventricular unloading. Indeed, it can sometimes reduce ventricular preload, especially if right-heart pressures are low. So MCS devices that unload the ventricle, typically an IABP, can complement ECMO.
Dr. Ostadal speculates, however, that there may be a better pairing option. “Impella plus ECMO, I think, is the combination which has a future,” he said, for patients in cardiogenic shock who need a short-term percutaneous hemodynamic support device. Impella “supports the whole circulation” and unloads the left ventricle.
“A balloon pump in combination with ECMO is still not a bad choice. It’s very cheap in comparison with Impella.” But in his opinion, Dr. Ostadal said, “The combination of Impella plus ECMO is more efficient from a hemodynamic point of view.”
As the published report notes, ongoing randomized trials looking at ECMO plus other MCS devices in cardiogenic shock include ECLS-SHOCK, with a projected enrollment of 420 patients, and EURO-SHOCK, aiming for a similar number of patients; both compare routine ECMO to conservative management.
In addition, ANCHOR, in which ECMO is combined with IABP, and DanShock, which looks at early use of Impella rather than ECMO, are enrolling patients with shock secondary to acute coronary syndromes.
Dr. Ostadal disclosed consulting for Getinge, Edwards, Medtronic, Biomedica, and Xenios/Fresenius, and receiving research support from Xenios/Fresenius. Dr. Allen disclosed modest or significant relationships with ACI Clinical, Novartis, UpToDate, Boston Scientific, and Cytokinetics.
A version of this article first appeared on Medscape.com.
CHICAGO – Routine early, expeditious use of extracorporeal membrane oxygenation (ECMO) is a common strategy in patients with severe cardiogenic shock, but a less aggressive initial approach may be just as effective, a randomized trial suggests.
In the study that assigned patients with “rapidly deteriorating or severe” cardiogenic shock to one or the other approach, clinical outcomes were no better for those who received immediate ECMO than for those initially managed with inotropes and vasopressors, researchers said.
The conservative strategy, importantly, allowed for downstream ECMO in the event of hemodynamic deterioration, which occurred in a substantial 39% of cases, observed Petr Ostadal, MD, PhD, when presenting the results at the American Heart Association scientific sessions.
Dr. Ostadal of Na Homolce Hospital, Prague, is also first author on the published report of the study, called Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS), which was published the same day in Circulation.
The trial makes a firm case for preferring the conservative initial approach over routine early ECMO in the kind of patients it entered, Larry A. Allen, MD, MHS, University of Coloradoat Denver, Aurora, told this news organization.
More than 60% of the trial’s 117 patients had shock secondary to an acute coronary syndrome; another 23% were in heart failure decompensation.
A preference for the conservative initial approach would be welcome, he said. The early aggressive ECMO approach is resource intensive and carries some important risks, such as stroke or coagulopathy, said Dr. Allen, who is not connected with ECMO-CS. Yet it is increasingly the go-to approach in such patients, based primarily on observational data.
Although early ECMO apparently didn’t benefit patients in this study in their specific stage of cardiogenic shock, Dr. Allen observed, it would presumably help some, but identifying them in practice presents challenges. “Defining where people are in the spectrum of early versus middle versus late cardiogenic shock is actually very tricky.”
It will therefore be important, he said, to identify ways to predict which conservatively managed patients do well with the strategy, and which are most at risk for hemodynamic deterioration and for whom ECMO should be readily available.
“I think part of what ECMO-CS tells us is that, if a patient is stable on intravenous inotropic and vasopressor support, you can defer ECMO while you’re thinking about the patient – about their larger context and the right medical decision-making for them.”
The trial randomly assigned 122 patients with rapidly deteriorating or severe cardiogenic shock to the immediate-ECMO or the conservative strategy at four centers in the Czech Republic. The 117 patients for whom informed consent could be obtained were included in the analysis, 58 and 59 patients, respectively. Their mean age was about 65 years and three-fourths were male.
The primary endpoint, the only endpoint for which the study was powered, consisted of death from any cause, resuscitated circulatory arrest, or use of a different form of mechanical circulatory support (MCS) by 30 days.
It occurred in 63.8% of patients assigned to immediate ECMO and 71.2% of those in the conservative strategy group, for a hazard ratio of 0.72 (95% confidence interval, 0.46-1.12; P = .21).
As individual endpoints, rates of death from any cause and resuscitated arrest did not significantly differ between the groups, but conservatively managed patients more often used another form of MCS. The HRs were 1.11 (95% CI, 0.66-1.87) for death from any cause, 0.79 (95% CI, 0.27-2.28) for resuscitated cardiac arrest, and 0.38 (95% CI, 0.18-0.79) for use of another MCS device.
The rates for serious adverse events – including bleeding, ischemia, stroke, pneumonia, or sepsis – were similar at 60.3% in the early-ECMO group and 61% in group with conservative initial management, Dr. Ostadal reported.
Other than the 23 patients in the conservative initial strategy group who went on to receive ECMO (1.9 days after randomization, on average), 1 went on to undergo implantation with a HeartMate (Abbott) ventricular assist device and 3 received an Impella pump (Abiomed).
Six patients in the early-ECMO group were already receiving intra-aortic balloon pump (IABP) support at randomization, two underwent temporary implantation with a Centrimag device (Abbott), and three went on to receive a HeartMate device, the published report notes.
ECMO is the optimal first choice for MCS in such patients with cardiogenic shock who need a circulatory support device, especially because it also oxygenates the blood, Dr. Ostadal told this news organization.
But ECMO doesn’t help with ventricular unloading. Indeed, it can sometimes reduce ventricular preload, especially if right-heart pressures are low. So MCS devices that unload the ventricle, typically an IABP, can complement ECMO.
Dr. Ostadal speculates, however, that there may be a better pairing option. “Impella plus ECMO, I think, is the combination which has a future,” he said, for patients in cardiogenic shock who need a short-term percutaneous hemodynamic support device. Impella “supports the whole circulation” and unloads the left ventricle.
“A balloon pump in combination with ECMO is still not a bad choice. It’s very cheap in comparison with Impella.” But in his opinion, Dr. Ostadal said, “The combination of Impella plus ECMO is more efficient from a hemodynamic point of view.”
As the published report notes, ongoing randomized trials looking at ECMO plus other MCS devices in cardiogenic shock include ECLS-SHOCK, with a projected enrollment of 420 patients, and EURO-SHOCK, aiming for a similar number of patients; both compare routine ECMO to conservative management.
In addition, ANCHOR, in which ECMO is combined with IABP, and DanShock, which looks at early use of Impella rather than ECMO, are enrolling patients with shock secondary to acute coronary syndromes.
Dr. Ostadal disclosed consulting for Getinge, Edwards, Medtronic, Biomedica, and Xenios/Fresenius, and receiving research support from Xenios/Fresenius. Dr. Allen disclosed modest or significant relationships with ACI Clinical, Novartis, UpToDate, Boston Scientific, and Cytokinetics.
A version of this article first appeared on Medscape.com.
CHICAGO – Routine early, expeditious use of extracorporeal membrane oxygenation (ECMO) is a common strategy in patients with severe cardiogenic shock, but a less aggressive initial approach may be just as effective, a randomized trial suggests.
In the study that assigned patients with “rapidly deteriorating or severe” cardiogenic shock to one or the other approach, clinical outcomes were no better for those who received immediate ECMO than for those initially managed with inotropes and vasopressors, researchers said.
The conservative strategy, importantly, allowed for downstream ECMO in the event of hemodynamic deterioration, which occurred in a substantial 39% of cases, observed Petr Ostadal, MD, PhD, when presenting the results at the American Heart Association scientific sessions.
Dr. Ostadal of Na Homolce Hospital, Prague, is also first author on the published report of the study, called Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS), which was published the same day in Circulation.
The trial makes a firm case for preferring the conservative initial approach over routine early ECMO in the kind of patients it entered, Larry A. Allen, MD, MHS, University of Coloradoat Denver, Aurora, told this news organization.
More than 60% of the trial’s 117 patients had shock secondary to an acute coronary syndrome; another 23% were in heart failure decompensation.
A preference for the conservative initial approach would be welcome, he said. The early aggressive ECMO approach is resource intensive and carries some important risks, such as stroke or coagulopathy, said Dr. Allen, who is not connected with ECMO-CS. Yet it is increasingly the go-to approach in such patients, based primarily on observational data.
Although early ECMO apparently didn’t benefit patients in this study in their specific stage of cardiogenic shock, Dr. Allen observed, it would presumably help some, but identifying them in practice presents challenges. “Defining where people are in the spectrum of early versus middle versus late cardiogenic shock is actually very tricky.”
It will therefore be important, he said, to identify ways to predict which conservatively managed patients do well with the strategy, and which are most at risk for hemodynamic deterioration and for whom ECMO should be readily available.
“I think part of what ECMO-CS tells us is that, if a patient is stable on intravenous inotropic and vasopressor support, you can defer ECMO while you’re thinking about the patient – about their larger context and the right medical decision-making for them.”
The trial randomly assigned 122 patients with rapidly deteriorating or severe cardiogenic shock to the immediate-ECMO or the conservative strategy at four centers in the Czech Republic. The 117 patients for whom informed consent could be obtained were included in the analysis, 58 and 59 patients, respectively. Their mean age was about 65 years and three-fourths were male.
The primary endpoint, the only endpoint for which the study was powered, consisted of death from any cause, resuscitated circulatory arrest, or use of a different form of mechanical circulatory support (MCS) by 30 days.
It occurred in 63.8% of patients assigned to immediate ECMO and 71.2% of those in the conservative strategy group, for a hazard ratio of 0.72 (95% confidence interval, 0.46-1.12; P = .21).
As individual endpoints, rates of death from any cause and resuscitated arrest did not significantly differ between the groups, but conservatively managed patients more often used another form of MCS. The HRs were 1.11 (95% CI, 0.66-1.87) for death from any cause, 0.79 (95% CI, 0.27-2.28) for resuscitated cardiac arrest, and 0.38 (95% CI, 0.18-0.79) for use of another MCS device.
The rates for serious adverse events – including bleeding, ischemia, stroke, pneumonia, or sepsis – were similar at 60.3% in the early-ECMO group and 61% in group with conservative initial management, Dr. Ostadal reported.
Other than the 23 patients in the conservative initial strategy group who went on to receive ECMO (1.9 days after randomization, on average), 1 went on to undergo implantation with a HeartMate (Abbott) ventricular assist device and 3 received an Impella pump (Abiomed).
Six patients in the early-ECMO group were already receiving intra-aortic balloon pump (IABP) support at randomization, two underwent temporary implantation with a Centrimag device (Abbott), and three went on to receive a HeartMate device, the published report notes.
ECMO is the optimal first choice for MCS in such patients with cardiogenic shock who need a circulatory support device, especially because it also oxygenates the blood, Dr. Ostadal told this news organization.
But ECMO doesn’t help with ventricular unloading. Indeed, it can sometimes reduce ventricular preload, especially if right-heart pressures are low. So MCS devices that unload the ventricle, typically an IABP, can complement ECMO.
Dr. Ostadal speculates, however, that there may be a better pairing option. “Impella plus ECMO, I think, is the combination which has a future,” he said, for patients in cardiogenic shock who need a short-term percutaneous hemodynamic support device. Impella “supports the whole circulation” and unloads the left ventricle.
“A balloon pump in combination with ECMO is still not a bad choice. It’s very cheap in comparison with Impella.” But in his opinion, Dr. Ostadal said, “The combination of Impella plus ECMO is more efficient from a hemodynamic point of view.”
As the published report notes, ongoing randomized trials looking at ECMO plus other MCS devices in cardiogenic shock include ECLS-SHOCK, with a projected enrollment of 420 patients, and EURO-SHOCK, aiming for a similar number of patients; both compare routine ECMO to conservative management.
In addition, ANCHOR, in which ECMO is combined with IABP, and DanShock, which looks at early use of Impella rather than ECMO, are enrolling patients with shock secondary to acute coronary syndromes.
Dr. Ostadal disclosed consulting for Getinge, Edwards, Medtronic, Biomedica, and Xenios/Fresenius, and receiving research support from Xenios/Fresenius. Dr. Allen disclosed modest or significant relationships with ACI Clinical, Novartis, UpToDate, Boston Scientific, and Cytokinetics.
A version of this article first appeared on Medscape.com.
AT AHA 2022
ISCHEMIA-EXTEND: Conservative stable CAD management holds up
CHICAGO – The case for survival equipoise between an invasive or conservative strategy for managing patients with stable coronary disease and moderate or severe cardiac ischemia grew stronger with an additional 2.5 years of median follow-up of the landmark ISCHEMIA trial.
During a median follow-up of 5.7 years in ISCHEMIA-EXTEND – and as long as 7 years – patients randomized to an upfront invasive strategy regardless of their symptoms had an all-cause mortality rate of 12.7%, compared with a 13.4% rate in the patients randomized to the conservative, medication-based management strategy that employed revascularization only when the medical approach failed to resolve their angina. This survival difference fell far short of significance (adjusted hazard ratio, 1.00; 95% confidence interval, 0.85-1.18), solidifying a finding first seen in the main ISCHEMIA results when they came out 3 years before, in late 2019, Judith S. Hochman, MD, said at the American Heart Association scientific sessions.
The new results “provide evidence for patients with chronic coronary disease and their physicians as they decide whether to add invasive management to guideline-directed medical therapy,” concluded Dr. Hochman, professor and senior associate dean for clinical sciences at New York University Langone Health. Simultaneous with her report, the extended follow-up results also appeared in an article published online in Circulation.
Nil probability of a survival benefit
“The probability over 5.7 years that a patient’s risk of dying is lower with the invasive strategy is nil, which means: Go with the patient’s preference. Not undergoing revascularization is a reasonable strategy because there is no excess mortality,” Dr. Hochman said in an interview. The trial’s extended follow-up provides “much more robust evidence” for the neutral effect on survival. The investigators plan to further follow-up out to a maximum of 10 years to continue to monitor for a signal of a mortality difference.
“These findings might help physicians in shared decision-making as to whether to add invasive management to guideline-directed medical management in selected patients with chronic coronary artery disease and moderate or severe ischemia,” commented M. Cecilia Bahit, MD, designated discussant for the report and chief of cardiology for INECO Neurosciences in Rosario, Argentina.
The original ISCHEMIA results had also shown that invasive intervention can improve the quality of life in patients who have angina as a result of their coronary disease, but also showed “minimal benefits” from an invasive approach in asymptomatic patients, who comprised 35% of the study cohort of 5,179 patients.
While ISCHEMIA enrolled patients with moderate to severe coronary ischemia identified with noninvasive testing, it excluded certain patients for whom an invasive strategy is recommended, including those with unprotected left main coronary stenoses of at least 50%, a recent acute coronary syndrome event, a left ventricular ejection fraction of less than 35%, more advanced functional limitations from heart failure, or advanced chronic kidney disease.
Follow-up without adjudication
The extended follow-up included 4,825 patients from the initial cohort, with data collected from 4,540 patients. One limitation of the follow-up was that the cause of death was not adjudicated as it had been during the initial follow-up phase. It instead relied on unconfirmed information collected either from patients’ families or national databases. The demographics and clinical profiles of the study participants available for extended follow-up closely matched the entire original study cohort.
The additional follow-up also revealed a significant survival benefit from the invasive approach for cardiovascular deaths, with an incidence of 8.6% in the conservative arm and 6.4% in the invasive group, an adjusted 22% relative reduction in this outcome favoring the invasive strategy (95% CI, 0.63-0.96). This difference had appeared as a nonsignificant signal in the initial 3.2-year median follow-up.
However, this significant benefit from the invasive strategy was counterbalanced by a surprising and inexplicable increase in deaths from noncardiovascular causes in those managed with the invasive strategy. Noncardiovascular deaths occurred in 5.5% of those in the invasive arm and in 4.4% of those in the conservative arm, a significant adjusted 44% relative increase in this outcome associated with invasive management. Again, this difference was not as clearly apparent after the initial follow-up phase.
“The increase in noncardiovascular deaths with the invasive strategy surprisingly persisted over time and offset” the cardiovascular survival benefit from upfront invasive treatment, explained Dr. Hochman. A prior report from the investigators looked in depth at the noncardiovascular deaths during the initial follow-up phase and found that most of the excess was caused by malignancies, although why this happened in the invasively treated patients remains a mystery.
Staying alive is what patients care about
“I think that interventional cardiologists who favor an invasive strategy will be excited to see this significant reduction in cardiovascular deaths, but patients don’t care what they die from. What patients care about is whether they are dead or alive,” Dr. Hochman noted.
But B. Hadley Wilson, MD, an interventional cardiologist and vice president of the American College of Cardiology, had a somewhat different take on these findings.
“We need to consider the significant decrease in cardiovascular mortality, as we sort out the conundrum” of the increase in noncardiovascular deaths,” he said in an interview. “Hopefully, the 10-year outcomes will help answer this.”
But until more information is available, the ISCHEMIA and ISCHEMIA-EXTEND results have already helped advance the conversation that patients with stable coronary disease and their families have with clinicians about management decisions.
“I love that ISCHEMIA highlighted the importance of shared decision making and a heart team approach,” said Dr. Wilson, executive vice chair of the Sanger Heart & Vascular Institute of Atrium Health in Charlotte, N.C.
Anecdotally, ISCHEMIA reduced invasive management
After the initial ISCHEMIA results were published nearly 3 years ago, “I think use of invasive treatment for these patients has decreased, although I have seen no numbers” that document this, said Dr. Wilson. “I think most interventional cardiologists would say that ISCHEMIA has had an impact,” with fewer patients who match the trial’s enrollment criteria undergoing invasive management.
“Anecdotally, cardiologists are reviewing the ISCHEMIA data with their patients,” agreed Dr. Hochman, who added that no actual data have yet appeared to document this, nor do data yet document a change in the use of invasive management. “It takes time to measure the impact.”
To expedite the shared decision-making process for these patients, the ISCHEMIA researchers are planning to make available an app that will allow patients and physicians to enter clinical and demographic data and see a calculated estimate of their future cardiovascular disease risk and how amenable it may be to modification by invasive management, Dr. Hochman said. The app would be available on the ISCHEMIA study website in 2023.
ISCHEMIA and ISCHEMIA EXTEND received no commercial funding. Dr. Hochman and Dr. Wilson had no disclosures. Dr. Bahit has received honoraria from Behring, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, MSD, and Pfizer.
CHICAGO – The case for survival equipoise between an invasive or conservative strategy for managing patients with stable coronary disease and moderate or severe cardiac ischemia grew stronger with an additional 2.5 years of median follow-up of the landmark ISCHEMIA trial.
During a median follow-up of 5.7 years in ISCHEMIA-EXTEND – and as long as 7 years – patients randomized to an upfront invasive strategy regardless of their symptoms had an all-cause mortality rate of 12.7%, compared with a 13.4% rate in the patients randomized to the conservative, medication-based management strategy that employed revascularization only when the medical approach failed to resolve their angina. This survival difference fell far short of significance (adjusted hazard ratio, 1.00; 95% confidence interval, 0.85-1.18), solidifying a finding first seen in the main ISCHEMIA results when they came out 3 years before, in late 2019, Judith S. Hochman, MD, said at the American Heart Association scientific sessions.
The new results “provide evidence for patients with chronic coronary disease and their physicians as they decide whether to add invasive management to guideline-directed medical therapy,” concluded Dr. Hochman, professor and senior associate dean for clinical sciences at New York University Langone Health. Simultaneous with her report, the extended follow-up results also appeared in an article published online in Circulation.
Nil probability of a survival benefit
“The probability over 5.7 years that a patient’s risk of dying is lower with the invasive strategy is nil, which means: Go with the patient’s preference. Not undergoing revascularization is a reasonable strategy because there is no excess mortality,” Dr. Hochman said in an interview. The trial’s extended follow-up provides “much more robust evidence” for the neutral effect on survival. The investigators plan to further follow-up out to a maximum of 10 years to continue to monitor for a signal of a mortality difference.
“These findings might help physicians in shared decision-making as to whether to add invasive management to guideline-directed medical management in selected patients with chronic coronary artery disease and moderate or severe ischemia,” commented M. Cecilia Bahit, MD, designated discussant for the report and chief of cardiology for INECO Neurosciences in Rosario, Argentina.
The original ISCHEMIA results had also shown that invasive intervention can improve the quality of life in patients who have angina as a result of their coronary disease, but also showed “minimal benefits” from an invasive approach in asymptomatic patients, who comprised 35% of the study cohort of 5,179 patients.
While ISCHEMIA enrolled patients with moderate to severe coronary ischemia identified with noninvasive testing, it excluded certain patients for whom an invasive strategy is recommended, including those with unprotected left main coronary stenoses of at least 50%, a recent acute coronary syndrome event, a left ventricular ejection fraction of less than 35%, more advanced functional limitations from heart failure, or advanced chronic kidney disease.
Follow-up without adjudication
The extended follow-up included 4,825 patients from the initial cohort, with data collected from 4,540 patients. One limitation of the follow-up was that the cause of death was not adjudicated as it had been during the initial follow-up phase. It instead relied on unconfirmed information collected either from patients’ families or national databases. The demographics and clinical profiles of the study participants available for extended follow-up closely matched the entire original study cohort.
The additional follow-up also revealed a significant survival benefit from the invasive approach for cardiovascular deaths, with an incidence of 8.6% in the conservative arm and 6.4% in the invasive group, an adjusted 22% relative reduction in this outcome favoring the invasive strategy (95% CI, 0.63-0.96). This difference had appeared as a nonsignificant signal in the initial 3.2-year median follow-up.
However, this significant benefit from the invasive strategy was counterbalanced by a surprising and inexplicable increase in deaths from noncardiovascular causes in those managed with the invasive strategy. Noncardiovascular deaths occurred in 5.5% of those in the invasive arm and in 4.4% of those in the conservative arm, a significant adjusted 44% relative increase in this outcome associated with invasive management. Again, this difference was not as clearly apparent after the initial follow-up phase.
“The increase in noncardiovascular deaths with the invasive strategy surprisingly persisted over time and offset” the cardiovascular survival benefit from upfront invasive treatment, explained Dr. Hochman. A prior report from the investigators looked in depth at the noncardiovascular deaths during the initial follow-up phase and found that most of the excess was caused by malignancies, although why this happened in the invasively treated patients remains a mystery.
Staying alive is what patients care about
“I think that interventional cardiologists who favor an invasive strategy will be excited to see this significant reduction in cardiovascular deaths, but patients don’t care what they die from. What patients care about is whether they are dead or alive,” Dr. Hochman noted.
But B. Hadley Wilson, MD, an interventional cardiologist and vice president of the American College of Cardiology, had a somewhat different take on these findings.
“We need to consider the significant decrease in cardiovascular mortality, as we sort out the conundrum” of the increase in noncardiovascular deaths,” he said in an interview. “Hopefully, the 10-year outcomes will help answer this.”
But until more information is available, the ISCHEMIA and ISCHEMIA-EXTEND results have already helped advance the conversation that patients with stable coronary disease and their families have with clinicians about management decisions.
“I love that ISCHEMIA highlighted the importance of shared decision making and a heart team approach,” said Dr. Wilson, executive vice chair of the Sanger Heart & Vascular Institute of Atrium Health in Charlotte, N.C.
Anecdotally, ISCHEMIA reduced invasive management
After the initial ISCHEMIA results were published nearly 3 years ago, “I think use of invasive treatment for these patients has decreased, although I have seen no numbers” that document this, said Dr. Wilson. “I think most interventional cardiologists would say that ISCHEMIA has had an impact,” with fewer patients who match the trial’s enrollment criteria undergoing invasive management.
“Anecdotally, cardiologists are reviewing the ISCHEMIA data with their patients,” agreed Dr. Hochman, who added that no actual data have yet appeared to document this, nor do data yet document a change in the use of invasive management. “It takes time to measure the impact.”
To expedite the shared decision-making process for these patients, the ISCHEMIA researchers are planning to make available an app that will allow patients and physicians to enter clinical and demographic data and see a calculated estimate of their future cardiovascular disease risk and how amenable it may be to modification by invasive management, Dr. Hochman said. The app would be available on the ISCHEMIA study website in 2023.
ISCHEMIA and ISCHEMIA EXTEND received no commercial funding. Dr. Hochman and Dr. Wilson had no disclosures. Dr. Bahit has received honoraria from Behring, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, MSD, and Pfizer.
CHICAGO – The case for survival equipoise between an invasive or conservative strategy for managing patients with stable coronary disease and moderate or severe cardiac ischemia grew stronger with an additional 2.5 years of median follow-up of the landmark ISCHEMIA trial.
During a median follow-up of 5.7 years in ISCHEMIA-EXTEND – and as long as 7 years – patients randomized to an upfront invasive strategy regardless of their symptoms had an all-cause mortality rate of 12.7%, compared with a 13.4% rate in the patients randomized to the conservative, medication-based management strategy that employed revascularization only when the medical approach failed to resolve their angina. This survival difference fell far short of significance (adjusted hazard ratio, 1.00; 95% confidence interval, 0.85-1.18), solidifying a finding first seen in the main ISCHEMIA results when they came out 3 years before, in late 2019, Judith S. Hochman, MD, said at the American Heart Association scientific sessions.
The new results “provide evidence for patients with chronic coronary disease and their physicians as they decide whether to add invasive management to guideline-directed medical therapy,” concluded Dr. Hochman, professor and senior associate dean for clinical sciences at New York University Langone Health. Simultaneous with her report, the extended follow-up results also appeared in an article published online in Circulation.
Nil probability of a survival benefit
“The probability over 5.7 years that a patient’s risk of dying is lower with the invasive strategy is nil, which means: Go with the patient’s preference. Not undergoing revascularization is a reasonable strategy because there is no excess mortality,” Dr. Hochman said in an interview. The trial’s extended follow-up provides “much more robust evidence” for the neutral effect on survival. The investigators plan to further follow-up out to a maximum of 10 years to continue to monitor for a signal of a mortality difference.
“These findings might help physicians in shared decision-making as to whether to add invasive management to guideline-directed medical management in selected patients with chronic coronary artery disease and moderate or severe ischemia,” commented M. Cecilia Bahit, MD, designated discussant for the report and chief of cardiology for INECO Neurosciences in Rosario, Argentina.
The original ISCHEMIA results had also shown that invasive intervention can improve the quality of life in patients who have angina as a result of their coronary disease, but also showed “minimal benefits” from an invasive approach in asymptomatic patients, who comprised 35% of the study cohort of 5,179 patients.
While ISCHEMIA enrolled patients with moderate to severe coronary ischemia identified with noninvasive testing, it excluded certain patients for whom an invasive strategy is recommended, including those with unprotected left main coronary stenoses of at least 50%, a recent acute coronary syndrome event, a left ventricular ejection fraction of less than 35%, more advanced functional limitations from heart failure, or advanced chronic kidney disease.
Follow-up without adjudication
The extended follow-up included 4,825 patients from the initial cohort, with data collected from 4,540 patients. One limitation of the follow-up was that the cause of death was not adjudicated as it had been during the initial follow-up phase. It instead relied on unconfirmed information collected either from patients’ families or national databases. The demographics and clinical profiles of the study participants available for extended follow-up closely matched the entire original study cohort.
The additional follow-up also revealed a significant survival benefit from the invasive approach for cardiovascular deaths, with an incidence of 8.6% in the conservative arm and 6.4% in the invasive group, an adjusted 22% relative reduction in this outcome favoring the invasive strategy (95% CI, 0.63-0.96). This difference had appeared as a nonsignificant signal in the initial 3.2-year median follow-up.
However, this significant benefit from the invasive strategy was counterbalanced by a surprising and inexplicable increase in deaths from noncardiovascular causes in those managed with the invasive strategy. Noncardiovascular deaths occurred in 5.5% of those in the invasive arm and in 4.4% of those in the conservative arm, a significant adjusted 44% relative increase in this outcome associated with invasive management. Again, this difference was not as clearly apparent after the initial follow-up phase.
“The increase in noncardiovascular deaths with the invasive strategy surprisingly persisted over time and offset” the cardiovascular survival benefit from upfront invasive treatment, explained Dr. Hochman. A prior report from the investigators looked in depth at the noncardiovascular deaths during the initial follow-up phase and found that most of the excess was caused by malignancies, although why this happened in the invasively treated patients remains a mystery.
Staying alive is what patients care about
“I think that interventional cardiologists who favor an invasive strategy will be excited to see this significant reduction in cardiovascular deaths, but patients don’t care what they die from. What patients care about is whether they are dead or alive,” Dr. Hochman noted.
But B. Hadley Wilson, MD, an interventional cardiologist and vice president of the American College of Cardiology, had a somewhat different take on these findings.
“We need to consider the significant decrease in cardiovascular mortality, as we sort out the conundrum” of the increase in noncardiovascular deaths,” he said in an interview. “Hopefully, the 10-year outcomes will help answer this.”
But until more information is available, the ISCHEMIA and ISCHEMIA-EXTEND results have already helped advance the conversation that patients with stable coronary disease and their families have with clinicians about management decisions.
“I love that ISCHEMIA highlighted the importance of shared decision making and a heart team approach,” said Dr. Wilson, executive vice chair of the Sanger Heart & Vascular Institute of Atrium Health in Charlotte, N.C.
Anecdotally, ISCHEMIA reduced invasive management
After the initial ISCHEMIA results were published nearly 3 years ago, “I think use of invasive treatment for these patients has decreased, although I have seen no numbers” that document this, said Dr. Wilson. “I think most interventional cardiologists would say that ISCHEMIA has had an impact,” with fewer patients who match the trial’s enrollment criteria undergoing invasive management.
“Anecdotally, cardiologists are reviewing the ISCHEMIA data with their patients,” agreed Dr. Hochman, who added that no actual data have yet appeared to document this, nor do data yet document a change in the use of invasive management. “It takes time to measure the impact.”
To expedite the shared decision-making process for these patients, the ISCHEMIA researchers are planning to make available an app that will allow patients and physicians to enter clinical and demographic data and see a calculated estimate of their future cardiovascular disease risk and how amenable it may be to modification by invasive management, Dr. Hochman said. The app would be available on the ISCHEMIA study website in 2023.
ISCHEMIA and ISCHEMIA EXTEND received no commercial funding. Dr. Hochman and Dr. Wilson had no disclosures. Dr. Bahit has received honoraria from Behring, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, MSD, and Pfizer.
AT AHA 2022
Inpatient sleep medicine: An invaluable service for hospital medicine
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.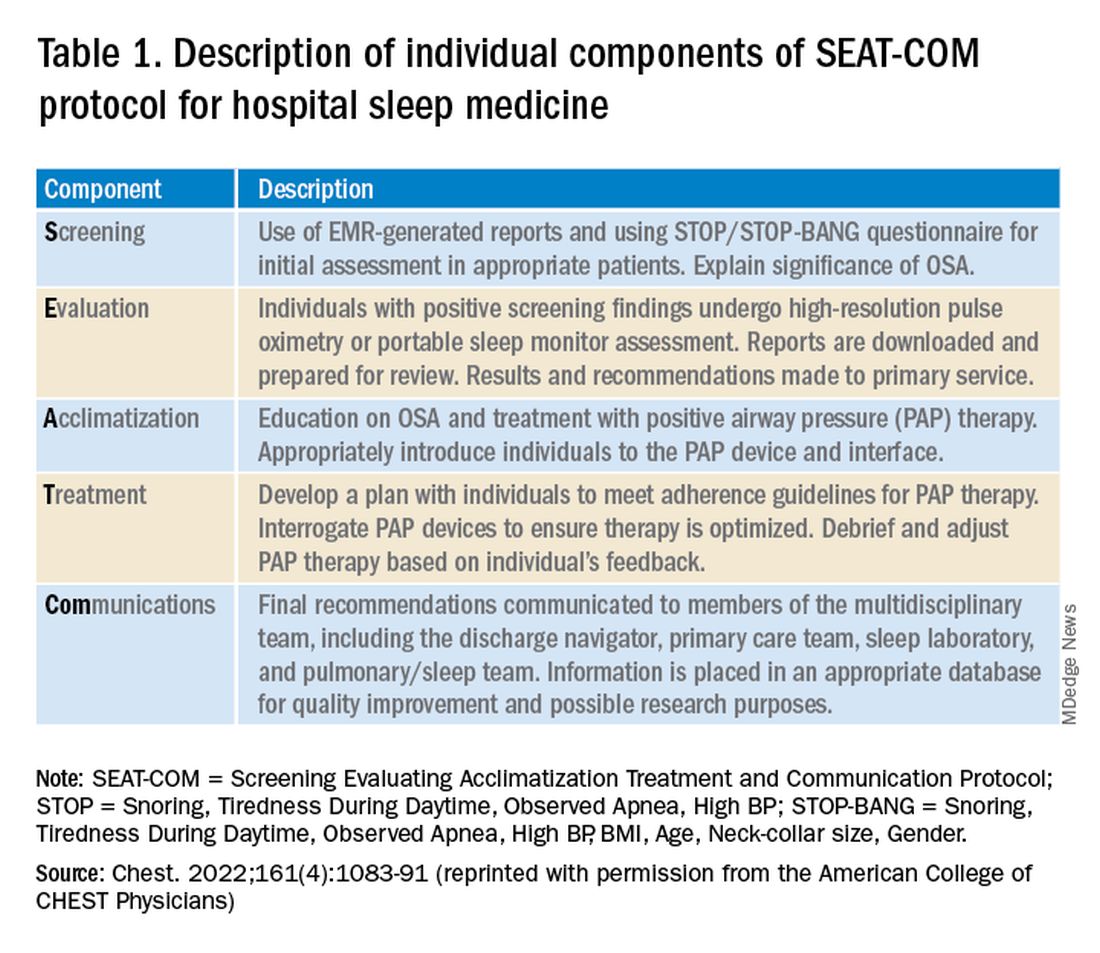
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Study sheds new light on RAS inhibitors’ role for advanced CKD
ORLANDO – Treatment with a renin-angiotensin system (RAS) inhibitor is widely accepted as standard practice for slowing progression of chronic kidney disease (CKD), but data have been inconsistent as to whether there is benefit to continuing RAS inhibition when patients develop advanced CKD, defined as an estimated glomerular filtration rate (eGFR) of less than 30 mL/min per 1.73 m2.
Now, in STOP ACEi, a new multicenter, randomized trial of 411 patients, , for 3 years.
People who continued RAS inhibitor treatment did not develop a significant or clinically relevant decrease in eGFR, the study’s primary outcome, both overall as well as in several prespecified subgroups compared with those who discontinued treatment, said Sunil Bhandari, MBChB, PhD, and associates, who presented the research in a poster at the annual meeting of the American Society of Nephrology.
“I hope these results will reassure clinicians to continue ACE inhibitors or ARBs” in patients with advanced CKD, “with their known beneficial cardiovascular effects,” Dr. Bhandari said in an interview.
The results were simultaneously published in the New England Journal of Medicine.
Similar eGFR levels after 3 years
While it’s clear that in patients with mild or moderate CKD, treatment with a RAS inhibitor, which includes angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs), reduces blood pressure, slows decline in eGFR, reduces proteinuria, and delays progression to advanced CKD, there has been little evidence that the use of RAS inhibitors benefits patients with advanced CKD.
Data from previous trials have been inconsistent regarding whether the use of RAS inhibitors is nephroprotective in patients with advanced CKD, say Dr. Bhandari, a nephrologist and professor at Hull York Medical School, Hull, England, and colleagues.
“Current guidelines do not provide specific advice on whether to continue or stop ACE inhibitors or ARBs for advanced chronic kidney disease,” they also note.
And so they decided to assess whether discontinuation of ACE inhibitors/ARBs could slow progression of CKD in patients with advanced CKD.
Three years after 206 study participants stopped RAS inhibitor treatment, the least-squares mean eGFR was 12.6 mL/min per 1.73m2 in the discontinuation group and 13.3 mL/min per 1.73 m2 in the 205 patients in the continuation group, a difference that was not significant.
In addition to the primary outcome, 62% of patients who stopped RAS inhibitor treatment and 56% of those who continued developed end-stage kidney disease or required renal-replacement therapy, which translated into an adjusted hazard ratio of 1.28 for this outcome among those who discontinued compared with those who continued, which was just short of significance (95% CI, 0.99-1.65).
The two study groups also showed no significant differences in the 3-year incidence of hospitalization for any reason, cardiovascular events, or deaths. The two groups also showed no meaningful differences in various domains of quality of life and no differences in serious adverse effects.
Participants had an eGFR less than 30 mL/min per 1.73 m2
The study ran at 39 United Kingdom centers in 2014-2019. Investigators enrolled adults with an eGFR of less than 30 mL/min per 1.73 m2 who were not on dialysis and had not received a kidney transplant. In addition, all enrolled patients had to have an annual drop in eGFR of more than 2 mL/min per 1.73 m2 during the prior 2 years and had to have been on treatment with at least one RAS inhibitor for more than 6 months.
The randomization protocol insured balanced distribution of subjects between the two study arms by age, eGFR, presence of diabetes, and level of proteinuria, among other factors. The study design also mandated that participants maintain a blood pressure of no more than 140/85 mm Hg.
Those who discontinued RAS-inhibitor treatment could receive any guideline-recommended antihypertensive agent that was not a RAS inhibitor, although adding a RAS inhibitor was permitted as a last treatment resort.
People in the maintenance group could receive whichever additional antihypertensive agents their treating clinicians deemed necessary for maintaining the target blood pressure.
The enrolled population was a median age of 63 years old and 68% were men. Their average eGFR at baseline was 18 mL/min per 1.73 m2, and 118 (29%) had an eGFR of less than 15 mL/min per 1.73 m2. Their median level of proteinuria was 115 mg/mmol (about 1,018 mg/g). Diabetes was prevalent in 37%, and 58% of participants were taking at least three antihypertensive medications at entry.
Among the study’s limitations, the researchers cited the open-label design, which may have affected clinical care and the tally of subjective endpoints, including quality of life and exercise capacity. Also, because the study enrolled people who were on a RAS inhibitor at the time of randomization, it did not include anyone who had already discontinued these agents.
Continue RAS inhibitors in advanced CKD for best outcomes
Dr. Bhandari and colleagues note that in a large observational trial published in January 2021, Swedish researchers found an increase in the incidence of major cardiovascular events and death among patients with advanced CKD who had discontinued RAS inhibitors.
But they observe, “Our trial did not have sufficient power to investigate the effect of the discontinuation of RAS inhibitors on cardiovascular events or mortality. However, because our findings are consistent with a lack of advantage for such discontinuation with respect to kidney function, there is little rationale to conduct a larger randomized trial to investigate cardiovascular safety.”
“Our findings do not support the hypothesis that the discontinuation of RAS inhibitors in patients with advanced and progressive chronic kidney disease would improve kidney function, quality of life, or exercise capacity.”
“The results of this trial will inform future clinical practice worldwide and guideline recommendations,” they conclude.
STOP ACEi received no commercial funding. Dr. Bhandari has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – Treatment with a renin-angiotensin system (RAS) inhibitor is widely accepted as standard practice for slowing progression of chronic kidney disease (CKD), but data have been inconsistent as to whether there is benefit to continuing RAS inhibition when patients develop advanced CKD, defined as an estimated glomerular filtration rate (eGFR) of less than 30 mL/min per 1.73 m2.
Now, in STOP ACEi, a new multicenter, randomized trial of 411 patients, , for 3 years.
People who continued RAS inhibitor treatment did not develop a significant or clinically relevant decrease in eGFR, the study’s primary outcome, both overall as well as in several prespecified subgroups compared with those who discontinued treatment, said Sunil Bhandari, MBChB, PhD, and associates, who presented the research in a poster at the annual meeting of the American Society of Nephrology.
“I hope these results will reassure clinicians to continue ACE inhibitors or ARBs” in patients with advanced CKD, “with their known beneficial cardiovascular effects,” Dr. Bhandari said in an interview.
The results were simultaneously published in the New England Journal of Medicine.
Similar eGFR levels after 3 years
While it’s clear that in patients with mild or moderate CKD, treatment with a RAS inhibitor, which includes angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs), reduces blood pressure, slows decline in eGFR, reduces proteinuria, and delays progression to advanced CKD, there has been little evidence that the use of RAS inhibitors benefits patients with advanced CKD.
Data from previous trials have been inconsistent regarding whether the use of RAS inhibitors is nephroprotective in patients with advanced CKD, say Dr. Bhandari, a nephrologist and professor at Hull York Medical School, Hull, England, and colleagues.
“Current guidelines do not provide specific advice on whether to continue or stop ACE inhibitors or ARBs for advanced chronic kidney disease,” they also note.
And so they decided to assess whether discontinuation of ACE inhibitors/ARBs could slow progression of CKD in patients with advanced CKD.
Three years after 206 study participants stopped RAS inhibitor treatment, the least-squares mean eGFR was 12.6 mL/min per 1.73m2 in the discontinuation group and 13.3 mL/min per 1.73 m2 in the 205 patients in the continuation group, a difference that was not significant.
In addition to the primary outcome, 62% of patients who stopped RAS inhibitor treatment and 56% of those who continued developed end-stage kidney disease or required renal-replacement therapy, which translated into an adjusted hazard ratio of 1.28 for this outcome among those who discontinued compared with those who continued, which was just short of significance (95% CI, 0.99-1.65).
The two study groups also showed no significant differences in the 3-year incidence of hospitalization for any reason, cardiovascular events, or deaths. The two groups also showed no meaningful differences in various domains of quality of life and no differences in serious adverse effects.
Participants had an eGFR less than 30 mL/min per 1.73 m2
The study ran at 39 United Kingdom centers in 2014-2019. Investigators enrolled adults with an eGFR of less than 30 mL/min per 1.73 m2 who were not on dialysis and had not received a kidney transplant. In addition, all enrolled patients had to have an annual drop in eGFR of more than 2 mL/min per 1.73 m2 during the prior 2 years and had to have been on treatment with at least one RAS inhibitor for more than 6 months.
The randomization protocol insured balanced distribution of subjects between the two study arms by age, eGFR, presence of diabetes, and level of proteinuria, among other factors. The study design also mandated that participants maintain a blood pressure of no more than 140/85 mm Hg.
Those who discontinued RAS-inhibitor treatment could receive any guideline-recommended antihypertensive agent that was not a RAS inhibitor, although adding a RAS inhibitor was permitted as a last treatment resort.
People in the maintenance group could receive whichever additional antihypertensive agents their treating clinicians deemed necessary for maintaining the target blood pressure.
The enrolled population was a median age of 63 years old and 68% were men. Their average eGFR at baseline was 18 mL/min per 1.73 m2, and 118 (29%) had an eGFR of less than 15 mL/min per 1.73 m2. Their median level of proteinuria was 115 mg/mmol (about 1,018 mg/g). Diabetes was prevalent in 37%, and 58% of participants were taking at least three antihypertensive medications at entry.
Among the study’s limitations, the researchers cited the open-label design, which may have affected clinical care and the tally of subjective endpoints, including quality of life and exercise capacity. Also, because the study enrolled people who were on a RAS inhibitor at the time of randomization, it did not include anyone who had already discontinued these agents.
Continue RAS inhibitors in advanced CKD for best outcomes
Dr. Bhandari and colleagues note that in a large observational trial published in January 2021, Swedish researchers found an increase in the incidence of major cardiovascular events and death among patients with advanced CKD who had discontinued RAS inhibitors.
But they observe, “Our trial did not have sufficient power to investigate the effect of the discontinuation of RAS inhibitors on cardiovascular events or mortality. However, because our findings are consistent with a lack of advantage for such discontinuation with respect to kidney function, there is little rationale to conduct a larger randomized trial to investigate cardiovascular safety.”
“Our findings do not support the hypothesis that the discontinuation of RAS inhibitors in patients with advanced and progressive chronic kidney disease would improve kidney function, quality of life, or exercise capacity.”
“The results of this trial will inform future clinical practice worldwide and guideline recommendations,” they conclude.
STOP ACEi received no commercial funding. Dr. Bhandari has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – Treatment with a renin-angiotensin system (RAS) inhibitor is widely accepted as standard practice for slowing progression of chronic kidney disease (CKD), but data have been inconsistent as to whether there is benefit to continuing RAS inhibition when patients develop advanced CKD, defined as an estimated glomerular filtration rate (eGFR) of less than 30 mL/min per 1.73 m2.
Now, in STOP ACEi, a new multicenter, randomized trial of 411 patients, , for 3 years.
People who continued RAS inhibitor treatment did not develop a significant or clinically relevant decrease in eGFR, the study’s primary outcome, both overall as well as in several prespecified subgroups compared with those who discontinued treatment, said Sunil Bhandari, MBChB, PhD, and associates, who presented the research in a poster at the annual meeting of the American Society of Nephrology.
“I hope these results will reassure clinicians to continue ACE inhibitors or ARBs” in patients with advanced CKD, “with their known beneficial cardiovascular effects,” Dr. Bhandari said in an interview.
The results were simultaneously published in the New England Journal of Medicine.
Similar eGFR levels after 3 years
While it’s clear that in patients with mild or moderate CKD, treatment with a RAS inhibitor, which includes angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs), reduces blood pressure, slows decline in eGFR, reduces proteinuria, and delays progression to advanced CKD, there has been little evidence that the use of RAS inhibitors benefits patients with advanced CKD.
Data from previous trials have been inconsistent regarding whether the use of RAS inhibitors is nephroprotective in patients with advanced CKD, say Dr. Bhandari, a nephrologist and professor at Hull York Medical School, Hull, England, and colleagues.
“Current guidelines do not provide specific advice on whether to continue or stop ACE inhibitors or ARBs for advanced chronic kidney disease,” they also note.
And so they decided to assess whether discontinuation of ACE inhibitors/ARBs could slow progression of CKD in patients with advanced CKD.
Three years after 206 study participants stopped RAS inhibitor treatment, the least-squares mean eGFR was 12.6 mL/min per 1.73m2 in the discontinuation group and 13.3 mL/min per 1.73 m2 in the 205 patients in the continuation group, a difference that was not significant.
In addition to the primary outcome, 62% of patients who stopped RAS inhibitor treatment and 56% of those who continued developed end-stage kidney disease or required renal-replacement therapy, which translated into an adjusted hazard ratio of 1.28 for this outcome among those who discontinued compared with those who continued, which was just short of significance (95% CI, 0.99-1.65).
The two study groups also showed no significant differences in the 3-year incidence of hospitalization for any reason, cardiovascular events, or deaths. The two groups also showed no meaningful differences in various domains of quality of life and no differences in serious adverse effects.
Participants had an eGFR less than 30 mL/min per 1.73 m2
The study ran at 39 United Kingdom centers in 2014-2019. Investigators enrolled adults with an eGFR of less than 30 mL/min per 1.73 m2 who were not on dialysis and had not received a kidney transplant. In addition, all enrolled patients had to have an annual drop in eGFR of more than 2 mL/min per 1.73 m2 during the prior 2 years and had to have been on treatment with at least one RAS inhibitor for more than 6 months.
The randomization protocol insured balanced distribution of subjects between the two study arms by age, eGFR, presence of diabetes, and level of proteinuria, among other factors. The study design also mandated that participants maintain a blood pressure of no more than 140/85 mm Hg.
Those who discontinued RAS-inhibitor treatment could receive any guideline-recommended antihypertensive agent that was not a RAS inhibitor, although adding a RAS inhibitor was permitted as a last treatment resort.
People in the maintenance group could receive whichever additional antihypertensive agents their treating clinicians deemed necessary for maintaining the target blood pressure.
The enrolled population was a median age of 63 years old and 68% were men. Their average eGFR at baseline was 18 mL/min per 1.73 m2, and 118 (29%) had an eGFR of less than 15 mL/min per 1.73 m2. Their median level of proteinuria was 115 mg/mmol (about 1,018 mg/g). Diabetes was prevalent in 37%, and 58% of participants were taking at least three antihypertensive medications at entry.
Among the study’s limitations, the researchers cited the open-label design, which may have affected clinical care and the tally of subjective endpoints, including quality of life and exercise capacity. Also, because the study enrolled people who were on a RAS inhibitor at the time of randomization, it did not include anyone who had already discontinued these agents.
Continue RAS inhibitors in advanced CKD for best outcomes
Dr. Bhandari and colleagues note that in a large observational trial published in January 2021, Swedish researchers found an increase in the incidence of major cardiovascular events and death among patients with advanced CKD who had discontinued RAS inhibitors.
But they observe, “Our trial did not have sufficient power to investigate the effect of the discontinuation of RAS inhibitors on cardiovascular events or mortality. However, because our findings are consistent with a lack of advantage for such discontinuation with respect to kidney function, there is little rationale to conduct a larger randomized trial to investigate cardiovascular safety.”
“Our findings do not support the hypothesis that the discontinuation of RAS inhibitors in patients with advanced and progressive chronic kidney disease would improve kidney function, quality of life, or exercise capacity.”
“The results of this trial will inform future clinical practice worldwide and guideline recommendations,” they conclude.
STOP ACEi received no commercial funding. Dr. Bhandari has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT KIDNEY WEEK 2022
Therapeutic drug monitoring pays off for arthritis patients
Therapeutic drug monitoring allowed patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis to reduce their dosage of tumor necrosis factor–alpha (TNF) inhibitors, based on data from 239 individuals.
Use of TNF-alpha inhibitors improves treatment response for many arthritis patients but dosage is rarely adjusted on an individual level, which may lead to unnecessary overdosing in some patients, Mogens Pfeiffer-Jensen, MD, of Aarhus (Denmark) University Hospital, and colleagues wrote.
Data from previous studies suggest that therapeutic drug monitoring (TDM) based on serum trough levels may allow for dose optimization and dose reduction in inflammatory bowel disease patients, but data in patients with arthritis are lacking, they wrote.
In a study published in the Scandinavian Journal of Rheumatology, the researchers recruited 99 patients with RA, 48 with psoriatic arthritis (PsA), and 92 with spondyloarthritis (SpA). The participants were randomized to standard care or standard care plus TDM. Serum trough levels were assessed at baseline and at every 4 months, and prescription changes or drug switches were implemented based on these levels. At baseline, 81 patients were being treated with infliximab (Remicade and biosimilars), 79 with etanercept (Enbrel), and 79 with adalimumab (Humira).
The primary endpoint was reduced drug prescription after 48 weeks.
Overall, TDM significantly reduced prescription of infliximab by 12% (P = .001) and prescription of etanercept by 15% (P = .01), compared with standard care. TDM also prolonged the interdosing intervals of etanercept by 235% (P = .02) and of adalimumab by 28% (P = .04), compared with standard care.
TDM patients taking infliximab had more frequent dose reduction and less frequent dose increases during and after the study when compared with patients who stayed with standard care; similar trends were seen with adalimumab. TDM also accelerated the switch to other biologics for patients on all three medications.
No significant differences occurred in adverse events or hospitalizations between the TDM and standard care patients.
Clinical composite scores (Disease Activity Score based on 28 joints with C-reactive protein) were reduced in patients with RA and PsA who were taking adalimumab and randomized to TDM, but no other clinical outcome differences were noted. Scores on the Health Assessment Questionnaire and global Visual Analog Scale for pain were significantly lower in patients in the TDM group who were taking infliximab and adalimumab, “indicating equally or superior sustained remission across diagnoses,” the researchers emphasized.
The findings were limited by several factors, including the variations in pathophysiology and open-label design. “However, since the TDM was based on an objective serum value and decision procedures were clear, we do not consider the potential of unconscious bias to outweigh the benefits of dose-changing abilities,” they wrote.
The researchers expressed surprise that the reduced use of TNF-alpha inhibitors did not significantly reduce adverse events or serious adverse events, compared with standard care, but they proposed that standard of care may have taken adverse events into account, because all patients had received prescriptions at least 3 months before the study.
As for clinical implications, the current costs of the biochemical assays necessary for TDM may be a barrier to implementing TDM as a standard part of daily clinical practice, the researchers added. However, the study was strengthened by the inclusion of patients with RA, PsA, and SpA, and is the first known to include patients receiving etanercept or adalimumab in an examination of TDM.
“Our data support TDM based solely on serum trough levels in [TNF-alpha inhibitors] with different pharmacokinetics as a future key player in personalized medicine for chronic rheumatoid diseases treated with biologics,” they concluded.
The study was supported by Spydspidspuljen, Region Midt, Denmark, and Department of Rheumatology, Aarhus University Hospital. The researchers had no financial conflicts to disclose.
Therapeutic drug monitoring allowed patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis to reduce their dosage of tumor necrosis factor–alpha (TNF) inhibitors, based on data from 239 individuals.
Use of TNF-alpha inhibitors improves treatment response for many arthritis patients but dosage is rarely adjusted on an individual level, which may lead to unnecessary overdosing in some patients, Mogens Pfeiffer-Jensen, MD, of Aarhus (Denmark) University Hospital, and colleagues wrote.
Data from previous studies suggest that therapeutic drug monitoring (TDM) based on serum trough levels may allow for dose optimization and dose reduction in inflammatory bowel disease patients, but data in patients with arthritis are lacking, they wrote.
In a study published in the Scandinavian Journal of Rheumatology, the researchers recruited 99 patients with RA, 48 with psoriatic arthritis (PsA), and 92 with spondyloarthritis (SpA). The participants were randomized to standard care or standard care plus TDM. Serum trough levels were assessed at baseline and at every 4 months, and prescription changes or drug switches were implemented based on these levels. At baseline, 81 patients were being treated with infliximab (Remicade and biosimilars), 79 with etanercept (Enbrel), and 79 with adalimumab (Humira).
The primary endpoint was reduced drug prescription after 48 weeks.
Overall, TDM significantly reduced prescription of infliximab by 12% (P = .001) and prescription of etanercept by 15% (P = .01), compared with standard care. TDM also prolonged the interdosing intervals of etanercept by 235% (P = .02) and of adalimumab by 28% (P = .04), compared with standard care.
TDM patients taking infliximab had more frequent dose reduction and less frequent dose increases during and after the study when compared with patients who stayed with standard care; similar trends were seen with adalimumab. TDM also accelerated the switch to other biologics for patients on all three medications.
No significant differences occurred in adverse events or hospitalizations between the TDM and standard care patients.
Clinical composite scores (Disease Activity Score based on 28 joints with C-reactive protein) were reduced in patients with RA and PsA who were taking adalimumab and randomized to TDM, but no other clinical outcome differences were noted. Scores on the Health Assessment Questionnaire and global Visual Analog Scale for pain were significantly lower in patients in the TDM group who were taking infliximab and adalimumab, “indicating equally or superior sustained remission across diagnoses,” the researchers emphasized.
The findings were limited by several factors, including the variations in pathophysiology and open-label design. “However, since the TDM was based on an objective serum value and decision procedures were clear, we do not consider the potential of unconscious bias to outweigh the benefits of dose-changing abilities,” they wrote.
The researchers expressed surprise that the reduced use of TNF-alpha inhibitors did not significantly reduce adverse events or serious adverse events, compared with standard care, but they proposed that standard of care may have taken adverse events into account, because all patients had received prescriptions at least 3 months before the study.
As for clinical implications, the current costs of the biochemical assays necessary for TDM may be a barrier to implementing TDM as a standard part of daily clinical practice, the researchers added. However, the study was strengthened by the inclusion of patients with RA, PsA, and SpA, and is the first known to include patients receiving etanercept or adalimumab in an examination of TDM.
“Our data support TDM based solely on serum trough levels in [TNF-alpha inhibitors] with different pharmacokinetics as a future key player in personalized medicine for chronic rheumatoid diseases treated with biologics,” they concluded.
The study was supported by Spydspidspuljen, Region Midt, Denmark, and Department of Rheumatology, Aarhus University Hospital. The researchers had no financial conflicts to disclose.
Therapeutic drug monitoring allowed patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis to reduce their dosage of tumor necrosis factor–alpha (TNF) inhibitors, based on data from 239 individuals.
Use of TNF-alpha inhibitors improves treatment response for many arthritis patients but dosage is rarely adjusted on an individual level, which may lead to unnecessary overdosing in some patients, Mogens Pfeiffer-Jensen, MD, of Aarhus (Denmark) University Hospital, and colleagues wrote.
Data from previous studies suggest that therapeutic drug monitoring (TDM) based on serum trough levels may allow for dose optimization and dose reduction in inflammatory bowel disease patients, but data in patients with arthritis are lacking, they wrote.
In a study published in the Scandinavian Journal of Rheumatology, the researchers recruited 99 patients with RA, 48 with psoriatic arthritis (PsA), and 92 with spondyloarthritis (SpA). The participants were randomized to standard care or standard care plus TDM. Serum trough levels were assessed at baseline and at every 4 months, and prescription changes or drug switches were implemented based on these levels. At baseline, 81 patients were being treated with infliximab (Remicade and biosimilars), 79 with etanercept (Enbrel), and 79 with adalimumab (Humira).
The primary endpoint was reduced drug prescription after 48 weeks.
Overall, TDM significantly reduced prescription of infliximab by 12% (P = .001) and prescription of etanercept by 15% (P = .01), compared with standard care. TDM also prolonged the interdosing intervals of etanercept by 235% (P = .02) and of adalimumab by 28% (P = .04), compared with standard care.
TDM patients taking infliximab had more frequent dose reduction and less frequent dose increases during and after the study when compared with patients who stayed with standard care; similar trends were seen with adalimumab. TDM also accelerated the switch to other biologics for patients on all three medications.
No significant differences occurred in adverse events or hospitalizations between the TDM and standard care patients.
Clinical composite scores (Disease Activity Score based on 28 joints with C-reactive protein) were reduced in patients with RA and PsA who were taking adalimumab and randomized to TDM, but no other clinical outcome differences were noted. Scores on the Health Assessment Questionnaire and global Visual Analog Scale for pain were significantly lower in patients in the TDM group who were taking infliximab and adalimumab, “indicating equally or superior sustained remission across diagnoses,” the researchers emphasized.
The findings were limited by several factors, including the variations in pathophysiology and open-label design. “However, since the TDM was based on an objective serum value and decision procedures were clear, we do not consider the potential of unconscious bias to outweigh the benefits of dose-changing abilities,” they wrote.
The researchers expressed surprise that the reduced use of TNF-alpha inhibitors did not significantly reduce adverse events or serious adverse events, compared with standard care, but they proposed that standard of care may have taken adverse events into account, because all patients had received prescriptions at least 3 months before the study.
As for clinical implications, the current costs of the biochemical assays necessary for TDM may be a barrier to implementing TDM as a standard part of daily clinical practice, the researchers added. However, the study was strengthened by the inclusion of patients with RA, PsA, and SpA, and is the first known to include patients receiving etanercept or adalimumab in an examination of TDM.
“Our data support TDM based solely on serum trough levels in [TNF-alpha inhibitors] with different pharmacokinetics as a future key player in personalized medicine for chronic rheumatoid diseases treated with biologics,” they concluded.
The study was supported by Spydspidspuljen, Region Midt, Denmark, and Department of Rheumatology, Aarhus University Hospital. The researchers had no financial conflicts to disclose.
FROM THE SCANDINAVIAN JOURNAL OF RHEUMATOLOGY
Moving the needle: SGLT2 inhibitor role for isolated kidney disease
ORLANDO – in a pivotal trial with more than 6,600 patients.
This confirms the efficacy for this population that was previously seen with dapagliflozin, another agent from the same class, in the DAPA-CKD trial.
In the new trial, EMPA-Kidney, treatment with empagliflozin 10 mg daily for a median of 2.0 years led to a significant 28% relative risk reduction in the primary combined endpoint in comparison with placebo, William G. Herrington, MD, reported at the annual meeting of the American Society of Nephrology.
The results were simultaneously published in the New England Journal of Medicine.
In 2020, a different team of researchers running DAPA-CKD reported that during a median of 2.4 years, treatment of 4,304 patients with dapagliflozin 10 mg daily resulted in a significant 39% relative risk reduction, compared with placebo for an identical combined primary endpoint. Enrollment criteria for the DAPA-CKD trial were mostly similar to that of the current trial.
‘Remarkably similar’ findings
Results from EMPA-Kidney and DAPA-CKD are “remarkably similar,” said Dr. Herrington during a press briefing at the meeting.
He also noted that when the EMPA-Kidney study began – before results from DAPA-CKD were known – “we never imagined such a large effect” on important endpoints in people with CKD.
In addition to cardiovascular death, the combined primary endpoint included the incidence of renal death, incident end-stage kidney disease, a sustained decrease in estimated glomerular filtration rate to less than 10 mL/min per 1.73m2, or a sustained decrease in eGFR of at least 40% from baseline.
Having similar evidence from both trials “will hopefully provide people with the confidence to start to use SGLT2 inhibitors as standard care in people with CKD” who match enrollment criteria of the two trials, added Dr. Herrington, a nephrologist at the University of Oxford (England).
The analyses he reported also showed that empagliflozin had similar efficacy for the primary endpoint regardless of whether patients had type 2 diabetes at the time of enrollment and regardless of their eGFR at entry.
To enter EMPA-Kidney, people needed to have either an eGFR of 20-44 mL/min per 1.73m2 with no minimum level of albuminuria or an eGFR of 45-89 mL/min per 1.73m2 with a urine albumin-to-creatinine ratio (UACR) of at least 200 mg/g.
In contrast, to enroll in DAPA-CKD, patients had to have a UACR of at least 200 mg/g. This means that for the first time, EMPA-Kidney produced data on the relationship between albuminuria severity and the impact of treatment with an SGLT2 inhibitor in the enrolled population.
A signal of greater efficacy with higher UACR
A total of 6,609 patients underwent randomization in EMPA-Kidney. During a median of 2.0 years of follow-up, the primary endpoint – progression of kidney disease or death from cardiovascular causes – occurred in 432 of 3,304 patients (13.1%) in the empagliflozin group and in 558 of 3,305 patients (16.9%) in the placebo group (hazard ratio, 0.72; P < .001).
The results “suggested that the effects [of empagliflozin] are greater in patients with higher levels of albuminuria, with statistically significant heterogeneity between this subgroup and those with a UACR of less than 200 mg/g (P = .02),” Dr. Herrington said.
Of the study population, 54% had no evidence of diabetes at enrollment.
Having data from a second large trial of an SGLT2 inhibitor that included people with isolated CKD who did not have diabetes or heart failure “will start to move the needle” on using this class of drugs in these types of patients, commented F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn.
On the basis of the DAPA-CKD results, in April 2021 the Food and Drug Administration expanded dapagliflozin’s indications to include CKD, yet, “a lot of nephrologists consider SGLT2 inhibitors to be agents for people with diabetes or heart failure, and they defer prescribing them to endocrinologists and cardiologists,” Dr. Wilson said in an interview.
‘Flozinators’ rising
But Pascale H. Lane, MD, a pediatric nephrologist at the University of Oklahoma Health Sciences Center, Oklahoma City, commented that many nephrologists she knows have been prescribing dapagliflozin “widely” to their patients with CKD.
“I know many adult nephrologists who use it almost universally now,” Dr. Lane said. “They call themselves ‘flozinators.’ ”
EMPA-Kidney was sponsored by Boehringer Ingelheim, the company that along with Lilly markets empagliflozin (Jardiance). Dr. Herrington, Dr. Wilson, and Dr. Lane disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – in a pivotal trial with more than 6,600 patients.
This confirms the efficacy for this population that was previously seen with dapagliflozin, another agent from the same class, in the DAPA-CKD trial.
In the new trial, EMPA-Kidney, treatment with empagliflozin 10 mg daily for a median of 2.0 years led to a significant 28% relative risk reduction in the primary combined endpoint in comparison with placebo, William G. Herrington, MD, reported at the annual meeting of the American Society of Nephrology.
The results were simultaneously published in the New England Journal of Medicine.
In 2020, a different team of researchers running DAPA-CKD reported that during a median of 2.4 years, treatment of 4,304 patients with dapagliflozin 10 mg daily resulted in a significant 39% relative risk reduction, compared with placebo for an identical combined primary endpoint. Enrollment criteria for the DAPA-CKD trial were mostly similar to that of the current trial.
‘Remarkably similar’ findings
Results from EMPA-Kidney and DAPA-CKD are “remarkably similar,” said Dr. Herrington during a press briefing at the meeting.
He also noted that when the EMPA-Kidney study began – before results from DAPA-CKD were known – “we never imagined such a large effect” on important endpoints in people with CKD.
In addition to cardiovascular death, the combined primary endpoint included the incidence of renal death, incident end-stage kidney disease, a sustained decrease in estimated glomerular filtration rate to less than 10 mL/min per 1.73m2, or a sustained decrease in eGFR of at least 40% from baseline.
Having similar evidence from both trials “will hopefully provide people with the confidence to start to use SGLT2 inhibitors as standard care in people with CKD” who match enrollment criteria of the two trials, added Dr. Herrington, a nephrologist at the University of Oxford (England).
The analyses he reported also showed that empagliflozin had similar efficacy for the primary endpoint regardless of whether patients had type 2 diabetes at the time of enrollment and regardless of their eGFR at entry.
To enter EMPA-Kidney, people needed to have either an eGFR of 20-44 mL/min per 1.73m2 with no minimum level of albuminuria or an eGFR of 45-89 mL/min per 1.73m2 with a urine albumin-to-creatinine ratio (UACR) of at least 200 mg/g.
In contrast, to enroll in DAPA-CKD, patients had to have a UACR of at least 200 mg/g. This means that for the first time, EMPA-Kidney produced data on the relationship between albuminuria severity and the impact of treatment with an SGLT2 inhibitor in the enrolled population.
A signal of greater efficacy with higher UACR
A total of 6,609 patients underwent randomization in EMPA-Kidney. During a median of 2.0 years of follow-up, the primary endpoint – progression of kidney disease or death from cardiovascular causes – occurred in 432 of 3,304 patients (13.1%) in the empagliflozin group and in 558 of 3,305 patients (16.9%) in the placebo group (hazard ratio, 0.72; P < .001).
The results “suggested that the effects [of empagliflozin] are greater in patients with higher levels of albuminuria, with statistically significant heterogeneity between this subgroup and those with a UACR of less than 200 mg/g (P = .02),” Dr. Herrington said.
Of the study population, 54% had no evidence of diabetes at enrollment.
Having data from a second large trial of an SGLT2 inhibitor that included people with isolated CKD who did not have diabetes or heart failure “will start to move the needle” on using this class of drugs in these types of patients, commented F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn.
On the basis of the DAPA-CKD results, in April 2021 the Food and Drug Administration expanded dapagliflozin’s indications to include CKD, yet, “a lot of nephrologists consider SGLT2 inhibitors to be agents for people with diabetes or heart failure, and they defer prescribing them to endocrinologists and cardiologists,” Dr. Wilson said in an interview.
‘Flozinators’ rising
But Pascale H. Lane, MD, a pediatric nephrologist at the University of Oklahoma Health Sciences Center, Oklahoma City, commented that many nephrologists she knows have been prescribing dapagliflozin “widely” to their patients with CKD.
“I know many adult nephrologists who use it almost universally now,” Dr. Lane said. “They call themselves ‘flozinators.’ ”
EMPA-Kidney was sponsored by Boehringer Ingelheim, the company that along with Lilly markets empagliflozin (Jardiance). Dr. Herrington, Dr. Wilson, and Dr. Lane disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – in a pivotal trial with more than 6,600 patients.
This confirms the efficacy for this population that was previously seen with dapagliflozin, another agent from the same class, in the DAPA-CKD trial.
In the new trial, EMPA-Kidney, treatment with empagliflozin 10 mg daily for a median of 2.0 years led to a significant 28% relative risk reduction in the primary combined endpoint in comparison with placebo, William G. Herrington, MD, reported at the annual meeting of the American Society of Nephrology.
The results were simultaneously published in the New England Journal of Medicine.
In 2020, a different team of researchers running DAPA-CKD reported that during a median of 2.4 years, treatment of 4,304 patients with dapagliflozin 10 mg daily resulted in a significant 39% relative risk reduction, compared with placebo for an identical combined primary endpoint. Enrollment criteria for the DAPA-CKD trial were mostly similar to that of the current trial.
‘Remarkably similar’ findings
Results from EMPA-Kidney and DAPA-CKD are “remarkably similar,” said Dr. Herrington during a press briefing at the meeting.
He also noted that when the EMPA-Kidney study began – before results from DAPA-CKD were known – “we never imagined such a large effect” on important endpoints in people with CKD.
In addition to cardiovascular death, the combined primary endpoint included the incidence of renal death, incident end-stage kidney disease, a sustained decrease in estimated glomerular filtration rate to less than 10 mL/min per 1.73m2, or a sustained decrease in eGFR of at least 40% from baseline.
Having similar evidence from both trials “will hopefully provide people with the confidence to start to use SGLT2 inhibitors as standard care in people with CKD” who match enrollment criteria of the two trials, added Dr. Herrington, a nephrologist at the University of Oxford (England).
The analyses he reported also showed that empagliflozin had similar efficacy for the primary endpoint regardless of whether patients had type 2 diabetes at the time of enrollment and regardless of their eGFR at entry.
To enter EMPA-Kidney, people needed to have either an eGFR of 20-44 mL/min per 1.73m2 with no minimum level of albuminuria or an eGFR of 45-89 mL/min per 1.73m2 with a urine albumin-to-creatinine ratio (UACR) of at least 200 mg/g.
In contrast, to enroll in DAPA-CKD, patients had to have a UACR of at least 200 mg/g. This means that for the first time, EMPA-Kidney produced data on the relationship between albuminuria severity and the impact of treatment with an SGLT2 inhibitor in the enrolled population.
A signal of greater efficacy with higher UACR
A total of 6,609 patients underwent randomization in EMPA-Kidney. During a median of 2.0 years of follow-up, the primary endpoint – progression of kidney disease or death from cardiovascular causes – occurred in 432 of 3,304 patients (13.1%) in the empagliflozin group and in 558 of 3,305 patients (16.9%) in the placebo group (hazard ratio, 0.72; P < .001).
The results “suggested that the effects [of empagliflozin] are greater in patients with higher levels of albuminuria, with statistically significant heterogeneity between this subgroup and those with a UACR of less than 200 mg/g (P = .02),” Dr. Herrington said.
Of the study population, 54% had no evidence of diabetes at enrollment.
Having data from a second large trial of an SGLT2 inhibitor that included people with isolated CKD who did not have diabetes or heart failure “will start to move the needle” on using this class of drugs in these types of patients, commented F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn.
On the basis of the DAPA-CKD results, in April 2021 the Food and Drug Administration expanded dapagliflozin’s indications to include CKD, yet, “a lot of nephrologists consider SGLT2 inhibitors to be agents for people with diabetes or heart failure, and they defer prescribing them to endocrinologists and cardiologists,” Dr. Wilson said in an interview.
‘Flozinators’ rising
But Pascale H. Lane, MD, a pediatric nephrologist at the University of Oklahoma Health Sciences Center, Oklahoma City, commented that many nephrologists she knows have been prescribing dapagliflozin “widely” to their patients with CKD.
“I know many adult nephrologists who use it almost universally now,” Dr. Lane said. “They call themselves ‘flozinators.’ ”
EMPA-Kidney was sponsored by Boehringer Ingelheim, the company that along with Lilly markets empagliflozin (Jardiance). Dr. Herrington, Dr. Wilson, and Dr. Lane disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT KIDNEY WEEK 2022
Low-carb diet aids weight loss in liver transplant recipients with obesity
A low-carbohydrate diet appears to be an effective weight-loss intervention in liver transplant recipients with obesity as compared with a calorie-restrictive diet, according to interim findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
In particular, the intervention showed significant improvements in the metabophenotype profile, including visceral adipose tissue and abdominal subcutaneous adipose tissue, said Mohammad Siddiqui, MD, a gastroenterologist and liver transplant specialist at Virginia Commonwealth University, Richmond.
“Weight gain and obesity after liver transplantation is common,” he said. “Posttransplant obesity is associated with increased cardiometabolic risk burden, increased risk of cardiovascular disease and mortality, and overall mortality.”
Previously, Dr. Siddiqui and colleagues have shown that posttransplant weight loss is difficult because of metabolic inflexibility and mitochondrial inefficiency. By specifically targeting carbohydrate utilization, metabolic flexibility could be restored in liver transplant recipients, he noted.
Dr. Siddiqui and colleagues conducted a randomized controlled trial of 27 adult liver transplant recipients with obesity for 24 weeks. The primary endpoint was change in weight, and the secondary endpoints involved metabophenotype, metabolic flexibility, mitochondrial function, and metabolic risk. The research team excluded patients with end-stage disease, terminal disease, use of weight-loss medications, pregnancy, or uncontrolled psychiatric illness that could interfere with adherence.
Among the participants, 13 were randomized to a calorie restrictive diet of less than 1,200-1,500 calories per day, and 14 were randomized to a low-carbohydrate diet of 20 grams or less per day. At enrollment, the participants underwent dietary, activity, skeletal muscle, and body composition assessments, as well as metabophenotype measurements of visceral adipose tissue, abdominal subcutaneous adipose tissue, muscle fat infiltration, fat-free muscle volume, and proton density fat fraction.
All participants were advised to maintain the same level of physical activity, which was measured through 7-day accelerometry. In addition, the patients were contacted every 2 weeks throughout the 24-week study period.
“We wanted to reinforce the dietary advice. We wanted to identify factors that may lead to compliance,” Dr. Siddiqui said. “Multiple studies have documented that the more contact that patients have during weight-loss studies with medical personnel, the more effective those strategies are.”
Overall, the dietary interventions were well tolerated, and neither group showed a significant change in renal function.
The average weight loss was –7.6 kg over 6 months in the low-carbohydrate group, as compared with –0.6 kg in the calorie-restrictive group.
The low carbohydrate diet also positively affected participants’ metabophenotype profile, particularly fat deposits. As compared with the calorie-restrictive group, the low-carbohydrate group showed statistically significant improvements in visceral adipose tissue, abdominal subcutaneous adipose tissue, and muscle fat infiltration.
The liver proton density fat fraction, which is associated with fatty liver disease, decreased by 0.53% in the low-carbohydrate group and increased by 0.46% in the calorie-restrictive group, but the difference didn’t reach statistical significance.
The fat-free muscle volume decreased by about 5% in the low-carbohydrate group. Dr. Siddiqui noted that the researchers don’t know yet whether this translates to a decrease in muscle function.
In terms of metabolic risk, the low-carbohydrate diet did not affect serum lipids (such as triglycerides or cholesterol measures), renal function (such as serum creatinine, glomerular filtration rate, or blood urea nitrogen), or insulin resistance (through glucose or hemoglobin A1c). At the same time, among patients taking insulin at the time of enrollment, about 90% of patients randomized to the low-carbohydrate group were able to reduce insulin to zero during the study.
Upon completion of the current study, Dr. Siddiqui and colleagues hope to provide foundational safety and efficacy data for carbohydrate restriction in liver transplant recipients. In the ongoing study, the researchers are further investigating the dietary intervention impacts on metabolic flexibility, skeletal muscle mitochondrial function, atherogenic lipoproteins, and vascular function.
“Are we actually, on a molecular level, fixing the fundamental problem that liver transplant recipients have to improve outcomes?” he said. “We’re doing very detailed profiling of these patients, so we will have data that shows how this actually affects them.”
Dr. Siddiqui was asked about the sustainability of the low-carbohydrate diet, particularly with a restrictive parameter of 20 grams per day. During the COVID-19 pandemic, Dr. Siddiqui noted, the study was slowed and the research team was able to collect follow-up data.
“Surprisingly, we have a high rate of compliance, even after 6 months of therapy, and I think this has to do with a patient population that’s been through cirrhosis and has almost died,” he said. “They’re far more compliant, and we’re seeing that. We’re also changing the physiology and improving mitochondrial function, which improves the weight loss and weight maintenance, though I don’t know how long that’s going to last.”
The study sponsorship was not disclosed. Dr. Siddiqui reported no relevant conflicts of interest.
A low-carbohydrate diet appears to be an effective weight-loss intervention in liver transplant recipients with obesity as compared with a calorie-restrictive diet, according to interim findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
In particular, the intervention showed significant improvements in the metabophenotype profile, including visceral adipose tissue and abdominal subcutaneous adipose tissue, said Mohammad Siddiqui, MD, a gastroenterologist and liver transplant specialist at Virginia Commonwealth University, Richmond.
“Weight gain and obesity after liver transplantation is common,” he said. “Posttransplant obesity is associated with increased cardiometabolic risk burden, increased risk of cardiovascular disease and mortality, and overall mortality.”
Previously, Dr. Siddiqui and colleagues have shown that posttransplant weight loss is difficult because of metabolic inflexibility and mitochondrial inefficiency. By specifically targeting carbohydrate utilization, metabolic flexibility could be restored in liver transplant recipients, he noted.
Dr. Siddiqui and colleagues conducted a randomized controlled trial of 27 adult liver transplant recipients with obesity for 24 weeks. The primary endpoint was change in weight, and the secondary endpoints involved metabophenotype, metabolic flexibility, mitochondrial function, and metabolic risk. The research team excluded patients with end-stage disease, terminal disease, use of weight-loss medications, pregnancy, or uncontrolled psychiatric illness that could interfere with adherence.
Among the participants, 13 were randomized to a calorie restrictive diet of less than 1,200-1,500 calories per day, and 14 were randomized to a low-carbohydrate diet of 20 grams or less per day. At enrollment, the participants underwent dietary, activity, skeletal muscle, and body composition assessments, as well as metabophenotype measurements of visceral adipose tissue, abdominal subcutaneous adipose tissue, muscle fat infiltration, fat-free muscle volume, and proton density fat fraction.
All participants were advised to maintain the same level of physical activity, which was measured through 7-day accelerometry. In addition, the patients were contacted every 2 weeks throughout the 24-week study period.
“We wanted to reinforce the dietary advice. We wanted to identify factors that may lead to compliance,” Dr. Siddiqui said. “Multiple studies have documented that the more contact that patients have during weight-loss studies with medical personnel, the more effective those strategies are.”
Overall, the dietary interventions were well tolerated, and neither group showed a significant change in renal function.
The average weight loss was –7.6 kg over 6 months in the low-carbohydrate group, as compared with –0.6 kg in the calorie-restrictive group.
The low carbohydrate diet also positively affected participants’ metabophenotype profile, particularly fat deposits. As compared with the calorie-restrictive group, the low-carbohydrate group showed statistically significant improvements in visceral adipose tissue, abdominal subcutaneous adipose tissue, and muscle fat infiltration.
The liver proton density fat fraction, which is associated with fatty liver disease, decreased by 0.53% in the low-carbohydrate group and increased by 0.46% in the calorie-restrictive group, but the difference didn’t reach statistical significance.
The fat-free muscle volume decreased by about 5% in the low-carbohydrate group. Dr. Siddiqui noted that the researchers don’t know yet whether this translates to a decrease in muscle function.
In terms of metabolic risk, the low-carbohydrate diet did not affect serum lipids (such as triglycerides or cholesterol measures), renal function (such as serum creatinine, glomerular filtration rate, or blood urea nitrogen), or insulin resistance (through glucose or hemoglobin A1c). At the same time, among patients taking insulin at the time of enrollment, about 90% of patients randomized to the low-carbohydrate group were able to reduce insulin to zero during the study.
Upon completion of the current study, Dr. Siddiqui and colleagues hope to provide foundational safety and efficacy data for carbohydrate restriction in liver transplant recipients. In the ongoing study, the researchers are further investigating the dietary intervention impacts on metabolic flexibility, skeletal muscle mitochondrial function, atherogenic lipoproteins, and vascular function.
“Are we actually, on a molecular level, fixing the fundamental problem that liver transplant recipients have to improve outcomes?” he said. “We’re doing very detailed profiling of these patients, so we will have data that shows how this actually affects them.”
Dr. Siddiqui was asked about the sustainability of the low-carbohydrate diet, particularly with a restrictive parameter of 20 grams per day. During the COVID-19 pandemic, Dr. Siddiqui noted, the study was slowed and the research team was able to collect follow-up data.
“Surprisingly, we have a high rate of compliance, even after 6 months of therapy, and I think this has to do with a patient population that’s been through cirrhosis and has almost died,” he said. “They’re far more compliant, and we’re seeing that. We’re also changing the physiology and improving mitochondrial function, which improves the weight loss and weight maintenance, though I don’t know how long that’s going to last.”
The study sponsorship was not disclosed. Dr. Siddiqui reported no relevant conflicts of interest.
A low-carbohydrate diet appears to be an effective weight-loss intervention in liver transplant recipients with obesity as compared with a calorie-restrictive diet, according to interim findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
In particular, the intervention showed significant improvements in the metabophenotype profile, including visceral adipose tissue and abdominal subcutaneous adipose tissue, said Mohammad Siddiqui, MD, a gastroenterologist and liver transplant specialist at Virginia Commonwealth University, Richmond.
“Weight gain and obesity after liver transplantation is common,” he said. “Posttransplant obesity is associated with increased cardiometabolic risk burden, increased risk of cardiovascular disease and mortality, and overall mortality.”
Previously, Dr. Siddiqui and colleagues have shown that posttransplant weight loss is difficult because of metabolic inflexibility and mitochondrial inefficiency. By specifically targeting carbohydrate utilization, metabolic flexibility could be restored in liver transplant recipients, he noted.
Dr. Siddiqui and colleagues conducted a randomized controlled trial of 27 adult liver transplant recipients with obesity for 24 weeks. The primary endpoint was change in weight, and the secondary endpoints involved metabophenotype, metabolic flexibility, mitochondrial function, and metabolic risk. The research team excluded patients with end-stage disease, terminal disease, use of weight-loss medications, pregnancy, or uncontrolled psychiatric illness that could interfere with adherence.
Among the participants, 13 were randomized to a calorie restrictive diet of less than 1,200-1,500 calories per day, and 14 were randomized to a low-carbohydrate diet of 20 grams or less per day. At enrollment, the participants underwent dietary, activity, skeletal muscle, and body composition assessments, as well as metabophenotype measurements of visceral adipose tissue, abdominal subcutaneous adipose tissue, muscle fat infiltration, fat-free muscle volume, and proton density fat fraction.
All participants were advised to maintain the same level of physical activity, which was measured through 7-day accelerometry. In addition, the patients were contacted every 2 weeks throughout the 24-week study period.
“We wanted to reinforce the dietary advice. We wanted to identify factors that may lead to compliance,” Dr. Siddiqui said. “Multiple studies have documented that the more contact that patients have during weight-loss studies with medical personnel, the more effective those strategies are.”
Overall, the dietary interventions were well tolerated, and neither group showed a significant change in renal function.
The average weight loss was –7.6 kg over 6 months in the low-carbohydrate group, as compared with –0.6 kg in the calorie-restrictive group.
The low carbohydrate diet also positively affected participants’ metabophenotype profile, particularly fat deposits. As compared with the calorie-restrictive group, the low-carbohydrate group showed statistically significant improvements in visceral adipose tissue, abdominal subcutaneous adipose tissue, and muscle fat infiltration.
The liver proton density fat fraction, which is associated with fatty liver disease, decreased by 0.53% in the low-carbohydrate group and increased by 0.46% in the calorie-restrictive group, but the difference didn’t reach statistical significance.
The fat-free muscle volume decreased by about 5% in the low-carbohydrate group. Dr. Siddiqui noted that the researchers don’t know yet whether this translates to a decrease in muscle function.
In terms of metabolic risk, the low-carbohydrate diet did not affect serum lipids (such as triglycerides or cholesterol measures), renal function (such as serum creatinine, glomerular filtration rate, or blood urea nitrogen), or insulin resistance (through glucose or hemoglobin A1c). At the same time, among patients taking insulin at the time of enrollment, about 90% of patients randomized to the low-carbohydrate group were able to reduce insulin to zero during the study.
Upon completion of the current study, Dr. Siddiqui and colleagues hope to provide foundational safety and efficacy data for carbohydrate restriction in liver transplant recipients. In the ongoing study, the researchers are further investigating the dietary intervention impacts on metabolic flexibility, skeletal muscle mitochondrial function, atherogenic lipoproteins, and vascular function.
“Are we actually, on a molecular level, fixing the fundamental problem that liver transplant recipients have to improve outcomes?” he said. “We’re doing very detailed profiling of these patients, so we will have data that shows how this actually affects them.”
Dr. Siddiqui was asked about the sustainability of the low-carbohydrate diet, particularly with a restrictive parameter of 20 grams per day. During the COVID-19 pandemic, Dr. Siddiqui noted, the study was slowed and the research team was able to collect follow-up data.
“Surprisingly, we have a high rate of compliance, even after 6 months of therapy, and I think this has to do with a patient population that’s been through cirrhosis and has almost died,” he said. “They’re far more compliant, and we’re seeing that. We’re also changing the physiology and improving mitochondrial function, which improves the weight loss and weight maintenance, though I don’t know how long that’s going to last.”
The study sponsorship was not disclosed. Dr. Siddiqui reported no relevant conflicts of interest.
FROM THE LIVER MEETING
FDA expands tenofovir alafenamide (Vemlidy) use to adolescents with chronic HBV
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
Education about OTC tools key for patients with acne and rosacea
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY



