User login
Resmetirom reduces liver, CV risk factors in NASH with cirrhosis
WASHINGTON – In patients with cirrhosis associated with nonalcoholic steatohepatitis, new research has found.
Two cohorts comprising a total of 180 patients with well-compensated NASH cirrhosis enrolled in an open-label arm of the phase 3 MAESTRO-NAFLD-1 trial. Researchers found that 52 weeks of treatment with resmetirom was associated with reductions in MRI proton density fat fraction (MRI-PDFF), FibroScan controlled attenuation parameter, FibroScan vibration-controlled transient elastography (VCTE), magnetic resonance elastography, liver and spleen volumes, liver enzyme levels, and lipids.
“Importantly, there was a statistically significant reduction in liver volume by an average of 20%, and also the potential to monitor spleen volume as a surrogate for portal hypertension, with the caveat that further research needs to be done in this area to understand that better,” said Stephen Harrison, MD, medical director for Pinnacle Clinical Research in San Antonio, Tex.
Dr. Harrison presented the findings at the annual meeting of the American Association for the Study of Liver Diseases.
Building on early findings
The thyroid hormone receptor–beta pathway helps to maintain liver health through control of de novo lipogenesis, fatty acid oxidation, mitophagy and mitochondrial biogenesis, cholesterol metabolism, and anti-inflammatory and antifibrotic effects, Dr. Harrison said.
Resmetirom (Madrigal Pharmaceuticals) is a selective thyroid hormone receptor-beta agonist that is reputed to offer optimal beneficial effects on the liver, while minimizing adverse cardiovascular and bone metabolic events that are mediated through a different pathway by the thyroid hormone receptor–alpha.
In 2019, Dr. Harrison and colleagues reported results of a phase 2, double-blind, placebo-controlled trial of resmetirom in adults with biopsy-confirmed NASH (fibrosis stages 1-3) and hepatic fat content greater than 10% as assessed by MRI-PDFF.
In that study, patients who received resmetirom had significantly greater reductions in relative hepatic fat content compared with patients who received placebo at both 12 weeks and 36 weeks of follow-up. Overall, 60% of patients who took resmetirom had at least a 30% fat reduction compared with 18% of those who took placebo.
In addition, the investigators presented data on a cohort of 105 patients with well-compensated NASH cirrhosis who were treated in an open-label study. Those data were presented at the International Liver Conference in London in June.
At the AASLD meeting, Dr. Harrison presented data on the same cohort combined with data on an additional cohort of 75 patients with well-compensated NASH cirrhosis and no prior history of decompensation.
‘Real-world’ conditions
In an attempt to mimic real-world conditions, patients in the trial did not receive a baseline biopsy but were determined to have NASH or presumed NASH with either results of a previous liver biopsy or noninvasive techniques, including FibroScan and MRI-PDFF.
Patients were started on oral resmetirom 80 mg daily, which could be titrated upward to 100 mg daily based on pharmacokinetic data from a 2-week sample.
The investigators first compared reductions in liver enzymes in both cohorts, with median reductions in ALT, AST, and gamma-glutamyl transferase of –20%, –18%, and –32%, respectively, in the original cohort, and –30%, –23%, and –37% in the more recent cohort.
Reductions in other parameters, including MRI-PDFF, liver volume, and lipids were also similar between the cohorts.
Given the similarities, researchers opted to treat the second cohort as a validation set, and combined data from the two cohorts to look at additional differences between baseline and 1-year follow-up.
Looking at imaging biomarkers in the combined cohorts of patients with responses, they saw that of patients with at least a 25% change over baseline in FibroScan VCTE, 48% of patients with baseline PDFF of 5% or less, and 42% of those with baseline PDFF greater than 5% had significant improvement at 1 year.
Among patients with changes in MR elastography of at least 15%, a fifth (22%) of those with baseline PDFF of 5% or less and about a quarter (26%) with baseline PDFF greater than 5% had improvement.
Independent of baseline cirrhosis severity, 73% of patients had a 15% or greater reduction in liver volume after 52 weeks of resmetirom. The investigators did not find a correlation of liver reduction with MRI-PDFF reduction among patients with PDFF of 5% or less at baseline.
The study found similar reductions in harmful lipids across all patient subgroups in both cohorts. Decreases in both systolic and diastolic blood pressure consistent with those seen in noncirrhotic NASH patients were also seen, independent of cirrhosis severity.
Among patients with at least a 10% change in spleen volume, 31% of those with low baseline PDFF readings and 45% of those with high readings had a decrease in volume.
The investigators found no differences in adverse events between cirrhosis severity groups or compared with noncirrhotic NASH patients.
The most common adverse events were intermittent loose stools or nausea at start of resmetirom therapy, and most were mild.
There were no changes in the central thyroid axis, apart from about a 10% decrease in prohormone FT4, which had been reported in other studies of resmetirom. No changes in active hormone FT3 or thyroid-stimulating hormone were found.
Although the study did not have a placebo control, it supports the rationale for the ongoing MAESTRO-NASH Outcomes trial, an event-driven trial comparing outcomes with resmetirom versus placebo in patients with well-compensated Child-Pugh A NASH cirrhosis, Dr. Harrison concluded.
Encouraging data
The data on resmetirom look promising as an approach to the treatment of NASH and related diseases, Cyrielle Caussy, MD, PhD, from the University Hospital of Lyon (France), said in an interview. Dr. Caussy, who was not involved in the study, was a moderator of the session where Dr. Harrison presented the data.
It does seem to be beneficial in NASH, she said. But we also have seen improvements in lipid metabolism with this drug; as shown in Dr. Harrison’s presentation, there is a difference in cardiovascular risk factors, Dr. Caussy added.
“I do think it could be one of the drugs that really improves outcomes for patients with NASH,” Dr. Caussy said.
The study was supported by Madrigal Pharmaceuticals. Dr. Harrison reported conflict of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
WASHINGTON – In patients with cirrhosis associated with nonalcoholic steatohepatitis, new research has found.
Two cohorts comprising a total of 180 patients with well-compensated NASH cirrhosis enrolled in an open-label arm of the phase 3 MAESTRO-NAFLD-1 trial. Researchers found that 52 weeks of treatment with resmetirom was associated with reductions in MRI proton density fat fraction (MRI-PDFF), FibroScan controlled attenuation parameter, FibroScan vibration-controlled transient elastography (VCTE), magnetic resonance elastography, liver and spleen volumes, liver enzyme levels, and lipids.
“Importantly, there was a statistically significant reduction in liver volume by an average of 20%, and also the potential to monitor spleen volume as a surrogate for portal hypertension, with the caveat that further research needs to be done in this area to understand that better,” said Stephen Harrison, MD, medical director for Pinnacle Clinical Research in San Antonio, Tex.
Dr. Harrison presented the findings at the annual meeting of the American Association for the Study of Liver Diseases.
Building on early findings
The thyroid hormone receptor–beta pathway helps to maintain liver health through control of de novo lipogenesis, fatty acid oxidation, mitophagy and mitochondrial biogenesis, cholesterol metabolism, and anti-inflammatory and antifibrotic effects, Dr. Harrison said.
Resmetirom (Madrigal Pharmaceuticals) is a selective thyroid hormone receptor-beta agonist that is reputed to offer optimal beneficial effects on the liver, while minimizing adverse cardiovascular and bone metabolic events that are mediated through a different pathway by the thyroid hormone receptor–alpha.
In 2019, Dr. Harrison and colleagues reported results of a phase 2, double-blind, placebo-controlled trial of resmetirom in adults with biopsy-confirmed NASH (fibrosis stages 1-3) and hepatic fat content greater than 10% as assessed by MRI-PDFF.
In that study, patients who received resmetirom had significantly greater reductions in relative hepatic fat content compared with patients who received placebo at both 12 weeks and 36 weeks of follow-up. Overall, 60% of patients who took resmetirom had at least a 30% fat reduction compared with 18% of those who took placebo.
In addition, the investigators presented data on a cohort of 105 patients with well-compensated NASH cirrhosis who were treated in an open-label study. Those data were presented at the International Liver Conference in London in June.
At the AASLD meeting, Dr. Harrison presented data on the same cohort combined with data on an additional cohort of 75 patients with well-compensated NASH cirrhosis and no prior history of decompensation.
‘Real-world’ conditions
In an attempt to mimic real-world conditions, patients in the trial did not receive a baseline biopsy but were determined to have NASH or presumed NASH with either results of a previous liver biopsy or noninvasive techniques, including FibroScan and MRI-PDFF.
Patients were started on oral resmetirom 80 mg daily, which could be titrated upward to 100 mg daily based on pharmacokinetic data from a 2-week sample.
The investigators first compared reductions in liver enzymes in both cohorts, with median reductions in ALT, AST, and gamma-glutamyl transferase of –20%, –18%, and –32%, respectively, in the original cohort, and –30%, –23%, and –37% in the more recent cohort.
Reductions in other parameters, including MRI-PDFF, liver volume, and lipids were also similar between the cohorts.
Given the similarities, researchers opted to treat the second cohort as a validation set, and combined data from the two cohorts to look at additional differences between baseline and 1-year follow-up.
Looking at imaging biomarkers in the combined cohorts of patients with responses, they saw that of patients with at least a 25% change over baseline in FibroScan VCTE, 48% of patients with baseline PDFF of 5% or less, and 42% of those with baseline PDFF greater than 5% had significant improvement at 1 year.
Among patients with changes in MR elastography of at least 15%, a fifth (22%) of those with baseline PDFF of 5% or less and about a quarter (26%) with baseline PDFF greater than 5% had improvement.
Independent of baseline cirrhosis severity, 73% of patients had a 15% or greater reduction in liver volume after 52 weeks of resmetirom. The investigators did not find a correlation of liver reduction with MRI-PDFF reduction among patients with PDFF of 5% or less at baseline.
The study found similar reductions in harmful lipids across all patient subgroups in both cohorts. Decreases in both systolic and diastolic blood pressure consistent with those seen in noncirrhotic NASH patients were also seen, independent of cirrhosis severity.
Among patients with at least a 10% change in spleen volume, 31% of those with low baseline PDFF readings and 45% of those with high readings had a decrease in volume.
The investigators found no differences in adverse events between cirrhosis severity groups or compared with noncirrhotic NASH patients.
The most common adverse events were intermittent loose stools or nausea at start of resmetirom therapy, and most were mild.
There were no changes in the central thyroid axis, apart from about a 10% decrease in prohormone FT4, which had been reported in other studies of resmetirom. No changes in active hormone FT3 or thyroid-stimulating hormone were found.
Although the study did not have a placebo control, it supports the rationale for the ongoing MAESTRO-NASH Outcomes trial, an event-driven trial comparing outcomes with resmetirom versus placebo in patients with well-compensated Child-Pugh A NASH cirrhosis, Dr. Harrison concluded.
Encouraging data
The data on resmetirom look promising as an approach to the treatment of NASH and related diseases, Cyrielle Caussy, MD, PhD, from the University Hospital of Lyon (France), said in an interview. Dr. Caussy, who was not involved in the study, was a moderator of the session where Dr. Harrison presented the data.
It does seem to be beneficial in NASH, she said. But we also have seen improvements in lipid metabolism with this drug; as shown in Dr. Harrison’s presentation, there is a difference in cardiovascular risk factors, Dr. Caussy added.
“I do think it could be one of the drugs that really improves outcomes for patients with NASH,” Dr. Caussy said.
The study was supported by Madrigal Pharmaceuticals. Dr. Harrison reported conflict of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
WASHINGTON – In patients with cirrhosis associated with nonalcoholic steatohepatitis, new research has found.
Two cohorts comprising a total of 180 patients with well-compensated NASH cirrhosis enrolled in an open-label arm of the phase 3 MAESTRO-NAFLD-1 trial. Researchers found that 52 weeks of treatment with resmetirom was associated with reductions in MRI proton density fat fraction (MRI-PDFF), FibroScan controlled attenuation parameter, FibroScan vibration-controlled transient elastography (VCTE), magnetic resonance elastography, liver and spleen volumes, liver enzyme levels, and lipids.
“Importantly, there was a statistically significant reduction in liver volume by an average of 20%, and also the potential to monitor spleen volume as a surrogate for portal hypertension, with the caveat that further research needs to be done in this area to understand that better,” said Stephen Harrison, MD, medical director for Pinnacle Clinical Research in San Antonio, Tex.
Dr. Harrison presented the findings at the annual meeting of the American Association for the Study of Liver Diseases.
Building on early findings
The thyroid hormone receptor–beta pathway helps to maintain liver health through control of de novo lipogenesis, fatty acid oxidation, mitophagy and mitochondrial biogenesis, cholesterol metabolism, and anti-inflammatory and antifibrotic effects, Dr. Harrison said.
Resmetirom (Madrigal Pharmaceuticals) is a selective thyroid hormone receptor-beta agonist that is reputed to offer optimal beneficial effects on the liver, while minimizing adverse cardiovascular and bone metabolic events that are mediated through a different pathway by the thyroid hormone receptor–alpha.
In 2019, Dr. Harrison and colleagues reported results of a phase 2, double-blind, placebo-controlled trial of resmetirom in adults with biopsy-confirmed NASH (fibrosis stages 1-3) and hepatic fat content greater than 10% as assessed by MRI-PDFF.
In that study, patients who received resmetirom had significantly greater reductions in relative hepatic fat content compared with patients who received placebo at both 12 weeks and 36 weeks of follow-up. Overall, 60% of patients who took resmetirom had at least a 30% fat reduction compared with 18% of those who took placebo.
In addition, the investigators presented data on a cohort of 105 patients with well-compensated NASH cirrhosis who were treated in an open-label study. Those data were presented at the International Liver Conference in London in June.
At the AASLD meeting, Dr. Harrison presented data on the same cohort combined with data on an additional cohort of 75 patients with well-compensated NASH cirrhosis and no prior history of decompensation.
‘Real-world’ conditions
In an attempt to mimic real-world conditions, patients in the trial did not receive a baseline biopsy but were determined to have NASH or presumed NASH with either results of a previous liver biopsy or noninvasive techniques, including FibroScan and MRI-PDFF.
Patients were started on oral resmetirom 80 mg daily, which could be titrated upward to 100 mg daily based on pharmacokinetic data from a 2-week sample.
The investigators first compared reductions in liver enzymes in both cohorts, with median reductions in ALT, AST, and gamma-glutamyl transferase of –20%, –18%, and –32%, respectively, in the original cohort, and –30%, –23%, and –37% in the more recent cohort.
Reductions in other parameters, including MRI-PDFF, liver volume, and lipids were also similar between the cohorts.
Given the similarities, researchers opted to treat the second cohort as a validation set, and combined data from the two cohorts to look at additional differences between baseline and 1-year follow-up.
Looking at imaging biomarkers in the combined cohorts of patients with responses, they saw that of patients with at least a 25% change over baseline in FibroScan VCTE, 48% of patients with baseline PDFF of 5% or less, and 42% of those with baseline PDFF greater than 5% had significant improvement at 1 year.
Among patients with changes in MR elastography of at least 15%, a fifth (22%) of those with baseline PDFF of 5% or less and about a quarter (26%) with baseline PDFF greater than 5% had improvement.
Independent of baseline cirrhosis severity, 73% of patients had a 15% or greater reduction in liver volume after 52 weeks of resmetirom. The investigators did not find a correlation of liver reduction with MRI-PDFF reduction among patients with PDFF of 5% or less at baseline.
The study found similar reductions in harmful lipids across all patient subgroups in both cohorts. Decreases in both systolic and diastolic blood pressure consistent with those seen in noncirrhotic NASH patients were also seen, independent of cirrhosis severity.
Among patients with at least a 10% change in spleen volume, 31% of those with low baseline PDFF readings and 45% of those with high readings had a decrease in volume.
The investigators found no differences in adverse events between cirrhosis severity groups or compared with noncirrhotic NASH patients.
The most common adverse events were intermittent loose stools or nausea at start of resmetirom therapy, and most were mild.
There were no changes in the central thyroid axis, apart from about a 10% decrease in prohormone FT4, which had been reported in other studies of resmetirom. No changes in active hormone FT3 or thyroid-stimulating hormone were found.
Although the study did not have a placebo control, it supports the rationale for the ongoing MAESTRO-NASH Outcomes trial, an event-driven trial comparing outcomes with resmetirom versus placebo in patients with well-compensated Child-Pugh A NASH cirrhosis, Dr. Harrison concluded.
Encouraging data
The data on resmetirom look promising as an approach to the treatment of NASH and related diseases, Cyrielle Caussy, MD, PhD, from the University Hospital of Lyon (France), said in an interview. Dr. Caussy, who was not involved in the study, was a moderator of the session where Dr. Harrison presented the data.
It does seem to be beneficial in NASH, she said. But we also have seen improvements in lipid metabolism with this drug; as shown in Dr. Harrison’s presentation, there is a difference in cardiovascular risk factors, Dr. Caussy added.
“I do think it could be one of the drugs that really improves outcomes for patients with NASH,” Dr. Caussy said.
The study was supported by Madrigal Pharmaceuticals. Dr. Harrison reported conflict of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
AT THE LIVER MEETING
End the year on a wine note at the final Viva La Vino event of 2022
Don’t miss your last chance to join the CHEST Foundation for a celebration of excellent initiatives – and equally excellent wines – at the last Viva La Vino event of 2022, happening on December 1 at 7 pm.
This event will focus on white and red varietals from Piedmont, a region of Northwest Italy. The Piedmont area is known for producing more wines classified as Denominazione di Origine Controllata e Garantita, the highest classification of quality for wines in Italy, than any other region.
Join CHEST CEO Bob Musacchio, PhD, as he guides attendees through a virtual and interactive exploration of the history, varietals, and techniques of Piedmont wines. Plus, hear from other CHEST leaders and friends of the Foundation about the important work currently being done and the evolution of the Foundation’s many initiatives since its inception.
With their ticket, attendees will receive one bottle of white wine and two bottles of red wine – including Paitin Starda Langhe Nebbiolo 2019, Michele Chiarlo Le Madri Roero Arneis 2020, and Massolino Barbera d’Alba 2019 – as well as an Italian-themed snack kit featuring cheese, salami, taralli, and other tasty treats, to complement their imbibes.
Funds raised from Viva La Vino benefit the Harold Amos Medical Faculty Development Program (AMFDP) and CHEST initiatives to improve patient care. The AMFDP offers 4-year postdoctoral research awards to physicians, dentists, and nurses from historically marginalized backgrounds. Learn more about the recipient of this year’s grant, George Alba, MD, in the September issue of CHEST Physician.
Don’t miss your last chance to join the CHEST Foundation for a celebration of excellent initiatives – and equally excellent wines – at the last Viva La Vino event of 2022, happening on December 1 at 7 pm.
This event will focus on white and red varietals from Piedmont, a region of Northwest Italy. The Piedmont area is known for producing more wines classified as Denominazione di Origine Controllata e Garantita, the highest classification of quality for wines in Italy, than any other region.
Join CHEST CEO Bob Musacchio, PhD, as he guides attendees through a virtual and interactive exploration of the history, varietals, and techniques of Piedmont wines. Plus, hear from other CHEST leaders and friends of the Foundation about the important work currently being done and the evolution of the Foundation’s many initiatives since its inception.
With their ticket, attendees will receive one bottle of white wine and two bottles of red wine – including Paitin Starda Langhe Nebbiolo 2019, Michele Chiarlo Le Madri Roero Arneis 2020, and Massolino Barbera d’Alba 2019 – as well as an Italian-themed snack kit featuring cheese, salami, taralli, and other tasty treats, to complement their imbibes.
Funds raised from Viva La Vino benefit the Harold Amos Medical Faculty Development Program (AMFDP) and CHEST initiatives to improve patient care. The AMFDP offers 4-year postdoctoral research awards to physicians, dentists, and nurses from historically marginalized backgrounds. Learn more about the recipient of this year’s grant, George Alba, MD, in the September issue of CHEST Physician.
Don’t miss your last chance to join the CHEST Foundation for a celebration of excellent initiatives – and equally excellent wines – at the last Viva La Vino event of 2022, happening on December 1 at 7 pm.
This event will focus on white and red varietals from Piedmont, a region of Northwest Italy. The Piedmont area is known for producing more wines classified as Denominazione di Origine Controllata e Garantita, the highest classification of quality for wines in Italy, than any other region.
Join CHEST CEO Bob Musacchio, PhD, as he guides attendees through a virtual and interactive exploration of the history, varietals, and techniques of Piedmont wines. Plus, hear from other CHEST leaders and friends of the Foundation about the important work currently being done and the evolution of the Foundation’s many initiatives since its inception.
With their ticket, attendees will receive one bottle of white wine and two bottles of red wine – including Paitin Starda Langhe Nebbiolo 2019, Michele Chiarlo Le Madri Roero Arneis 2020, and Massolino Barbera d’Alba 2019 – as well as an Italian-themed snack kit featuring cheese, salami, taralli, and other tasty treats, to complement their imbibes.
Funds raised from Viva La Vino benefit the Harold Amos Medical Faculty Development Program (AMFDP) and CHEST initiatives to improve patient care. The AMFDP offers 4-year postdoctoral research awards to physicians, dentists, and nurses from historically marginalized backgrounds. Learn more about the recipient of this year’s grant, George Alba, MD, in the September issue of CHEST Physician.
Chest Infections & Disaster Response Network
Disaster Response & Global Health Section
Responding to firearm violence in America
We think of disasters as sudden, calamitous events, but it does not take much imagination to recognize the loss of lives in America from firearm violence as a type of disaster. In 2020, 45,222 people died from gun-related injuries, an increase of 5,155 (14%) since 2019 (Kegler, et al. MMWR Morb Mortal Wkly Rep. 2022;71[19]:656). This is the highest death rate since 1994, and includes increases in both homicides and suicides. Mass shootings constitute a fraction of this total, but there have already been 530 deaths from mass shooting incidents in 2022.
Opinions about the appropriate degree of firearm regulations remain divided, but the need to improve our response as clinicians is clear. The National Center for Disaster Medicine and Public Health recently published consensus recommendations for healthcare response in mass shootings (Goolsby, et al. J Am Coll Surg. 2022; published online July 18, 2022). These recommendations address readiness training, triage, communications, public education, patient tracking, family reunification, and mental health services.
Stop the Bleed is a program originally based on the military’s Tactical Combat Casualty Care standards. It offers training on hemorrhage control for both the public and clinicians, similar to basic life support programs. It encourages bystanders to become trained and empowered to help in a bleeding emergency before professional help arrives. Opportunities for training are a frequent offering at the CHEST Annual Meeting, and additional information can be found at https://www.stopthebleed.org.
Stella Ogake, MD
Disaster Response & Global Health Section
Responding to firearm violence in America
We think of disasters as sudden, calamitous events, but it does not take much imagination to recognize the loss of lives in America from firearm violence as a type of disaster. In 2020, 45,222 people died from gun-related injuries, an increase of 5,155 (14%) since 2019 (Kegler, et al. MMWR Morb Mortal Wkly Rep. 2022;71[19]:656). This is the highest death rate since 1994, and includes increases in both homicides and suicides. Mass shootings constitute a fraction of this total, but there have already been 530 deaths from mass shooting incidents in 2022.
Opinions about the appropriate degree of firearm regulations remain divided, but the need to improve our response as clinicians is clear. The National Center for Disaster Medicine and Public Health recently published consensus recommendations for healthcare response in mass shootings (Goolsby, et al. J Am Coll Surg. 2022; published online July 18, 2022). These recommendations address readiness training, triage, communications, public education, patient tracking, family reunification, and mental health services.
Stop the Bleed is a program originally based on the military’s Tactical Combat Casualty Care standards. It offers training on hemorrhage control for both the public and clinicians, similar to basic life support programs. It encourages bystanders to become trained and empowered to help in a bleeding emergency before professional help arrives. Opportunities for training are a frequent offering at the CHEST Annual Meeting, and additional information can be found at https://www.stopthebleed.org.
Stella Ogake, MD
Disaster Response & Global Health Section
Responding to firearm violence in America
We think of disasters as sudden, calamitous events, but it does not take much imagination to recognize the loss of lives in America from firearm violence as a type of disaster. In 2020, 45,222 people died from gun-related injuries, an increase of 5,155 (14%) since 2019 (Kegler, et al. MMWR Morb Mortal Wkly Rep. 2022;71[19]:656). This is the highest death rate since 1994, and includes increases in both homicides and suicides. Mass shootings constitute a fraction of this total, but there have already been 530 deaths from mass shooting incidents in 2022.
Opinions about the appropriate degree of firearm regulations remain divided, but the need to improve our response as clinicians is clear. The National Center for Disaster Medicine and Public Health recently published consensus recommendations for healthcare response in mass shootings (Goolsby, et al. J Am Coll Surg. 2022; published online July 18, 2022). These recommendations address readiness training, triage, communications, public education, patient tracking, family reunification, and mental health services.
Stop the Bleed is a program originally based on the military’s Tactical Combat Casualty Care standards. It offers training on hemorrhage control for both the public and clinicians, similar to basic life support programs. It encourages bystanders to become trained and empowered to help in a bleeding emergency before professional help arrives. Opportunities for training are a frequent offering at the CHEST Annual Meeting, and additional information can be found at https://www.stopthebleed.org.
Stella Ogake, MD
Time travel and thoughts on leadership
This is an odd column for me to write. First, because of the nature of print publication, this writing for the November issue is being crafted just before the annual meeting is to be held in Nashville. Therefore, while I have a pretty good sense of what is in store for CHEST 2022, I have yet to see the final product, or the audience’s reaction to it. However, I will make some bold predictions as to what occurred therein:
- Even in the context of 3 years of separation, thousands CHEST members gathered in droves to rekindle friendships and to experience the best education in pulmonary, critical care, and sleep medicine that the world has to offer, leading to our second-biggest meeting ever.
- Neil Pasricha’s presentation helped attendees rekindle the “Art of Happiness.”
- Hundreds of attendees participated in, and successfully solved, our newest escape room, “Starship Relics.”
- Our valued CHEST members were able to successfully thwart Dr. Didactic and save the future of educational innovation.
- “CHEST After Hours” trended on social media and will become a normal and highly-anticipated part of the CHEST meeting moving forward.
- The most uncomfortable moment of the meeting centered on mayonnaise; for those of you who know what I am referencing, I am a little sorry…but only a little.
- Despite my best efforts, we were not able to recruit Neil Patrick Harris to participate.
Predicting the future of medical meetings is something we’ve spent a lot of time trying to do over the last year as we developed plans for CHEST 2022. But given the talented individuals involved in that planning, foreseeing the meeting’s success did not require any time travel; it was hardly a difficult task at all. Program Chair Subani Chandra and Vice-Chair Aneesa Das were exactly the people we needed at the helm for this all-important return to in-person meetings, and I cannot thank them enough for their creativity, effort, and leadership in bringing CHEST 2022 to fruition. And while I expect to have been seven-for-seven in my predictions above, I do hope I got that NPH one wrong.
The other reason that this column was a challenge to craft is because it represents my final formal presidential missive in these esteemed pages. And as I planned this final walk of the path, I gave careful consideration to the message with which I wanted to conclude my year. And as I put together my predictions for the future, my mind also turned to the past, considering things I wish I had known (or spent more time considering) as I started this journey. Some of this information may prove useful to the next generation of CHEST leaders, and some may be already well engrained for those of you with leadership experience. Here, in no particular order, are some thoughts for those of you in the audience who are considering future leadership opportunities at CHEST (or elsewhere in life; I suspect some of this advice is applicable to other venues). That said, the recommendations also come from yours truly, so take them with an appropriately large grain of salt, as your mileage may vary, and reasonable people could take issue here or there.
- The most important conversations should happen in person. The past 3 years have shown us the amazing things that modern technology can accomplish, but when it comes to providing important information, asking for input on a crucial issue, or providing feedback on a sensitive matter, there is no adequate substitute for a discussion in which all parties are in the same room.
- You are going to get things wrong sometimes; sometimes, this is because there wasn’t a way to get a right answer, and sometimes it will be because you tried something that didn’t work. You will learn far more from one of these experiences than from a dozen things that went as well as (or better than) expected.
- It is profoundly difficult to change someone’s mind if you aren’t interacting with them. I believe there is no gap so large that warrants breakdown of communication. Going that extra mile to talk to people who have a drastically different opinion than your own is the only way that you might be able to change someone’s mind and is a great way to ensure that your own opinion withstands pushback. With the growth of social media over the last decade, we’ve gotten very good at blocking people on social media; while this can sometimes be good (or even necessary) for emotional well-being, there can be value to interacting with such folks in a real-world environment.
- You do not have to bring everything to the table. The best leaders surround themselves with other really smart folks who, in aggregate, will provide support in areas in which you are deficient. That said, you need to know where these gaps in your knowledge and experience are, and when it is the right time to listen to those trusted advisors.
- When it comes time to identify folks for your “cabinet,” make sure to choose people who think differently than you and who may disagree with you on some fundamental things. Surrounding yourself with friends and close colleagues can lead to groupthink and poor decision making. The best results often stem from challenging and difficult decision-making processes.
- As a corollary to the above, every leader will bring their own sensibility and personality to the role. Make sure to bring yours, even if it involves silly jokes about holding a medical meeting in a former President’s basement or getting another former President to eat a big spoonful of the aforementioned condiment.
- Fun is important. Fun builds relationships, and teams, and trust. Make sure you are having it, as much as you possibly can, throughout your leadership tenure.
On that note, I will sign off for good, at least in these pages. I’ll still be bumbling around, proposing new educational experiences, hosting Pardon the Interruption, and serving as a sounding board for anyone who wants to chat. But I cannot wait to see what the next 3 years bring for our organization, under the leadership of Drs. Addrizzo-Harris, Buckley, and Howington. And for those of you who are just taking your first steps in leadership, and who will be following in their footsteps years down the road, I hope that you get just as much enjoyment from and fulfilment in the role of President as I have. #SchulmanOut
This is an odd column for me to write. First, because of the nature of print publication, this writing for the November issue is being crafted just before the annual meeting is to be held in Nashville. Therefore, while I have a pretty good sense of what is in store for CHEST 2022, I have yet to see the final product, or the audience’s reaction to it. However, I will make some bold predictions as to what occurred therein:
- Even in the context of 3 years of separation, thousands CHEST members gathered in droves to rekindle friendships and to experience the best education in pulmonary, critical care, and sleep medicine that the world has to offer, leading to our second-biggest meeting ever.
- Neil Pasricha’s presentation helped attendees rekindle the “Art of Happiness.”
- Hundreds of attendees participated in, and successfully solved, our newest escape room, “Starship Relics.”
- Our valued CHEST members were able to successfully thwart Dr. Didactic and save the future of educational innovation.
- “CHEST After Hours” trended on social media and will become a normal and highly-anticipated part of the CHEST meeting moving forward.
- The most uncomfortable moment of the meeting centered on mayonnaise; for those of you who know what I am referencing, I am a little sorry…but only a little.
- Despite my best efforts, we were not able to recruit Neil Patrick Harris to participate.
Predicting the future of medical meetings is something we’ve spent a lot of time trying to do over the last year as we developed plans for CHEST 2022. But given the talented individuals involved in that planning, foreseeing the meeting’s success did not require any time travel; it was hardly a difficult task at all. Program Chair Subani Chandra and Vice-Chair Aneesa Das were exactly the people we needed at the helm for this all-important return to in-person meetings, and I cannot thank them enough for their creativity, effort, and leadership in bringing CHEST 2022 to fruition. And while I expect to have been seven-for-seven in my predictions above, I do hope I got that NPH one wrong.
The other reason that this column was a challenge to craft is because it represents my final formal presidential missive in these esteemed pages. And as I planned this final walk of the path, I gave careful consideration to the message with which I wanted to conclude my year. And as I put together my predictions for the future, my mind also turned to the past, considering things I wish I had known (or spent more time considering) as I started this journey. Some of this information may prove useful to the next generation of CHEST leaders, and some may be already well engrained for those of you with leadership experience. Here, in no particular order, are some thoughts for those of you in the audience who are considering future leadership opportunities at CHEST (or elsewhere in life; I suspect some of this advice is applicable to other venues). That said, the recommendations also come from yours truly, so take them with an appropriately large grain of salt, as your mileage may vary, and reasonable people could take issue here or there.
- The most important conversations should happen in person. The past 3 years have shown us the amazing things that modern technology can accomplish, but when it comes to providing important information, asking for input on a crucial issue, or providing feedback on a sensitive matter, there is no adequate substitute for a discussion in which all parties are in the same room.
- You are going to get things wrong sometimes; sometimes, this is because there wasn’t a way to get a right answer, and sometimes it will be because you tried something that didn’t work. You will learn far more from one of these experiences than from a dozen things that went as well as (or better than) expected.
- It is profoundly difficult to change someone’s mind if you aren’t interacting with them. I believe there is no gap so large that warrants breakdown of communication. Going that extra mile to talk to people who have a drastically different opinion than your own is the only way that you might be able to change someone’s mind and is a great way to ensure that your own opinion withstands pushback. With the growth of social media over the last decade, we’ve gotten very good at blocking people on social media; while this can sometimes be good (or even necessary) for emotional well-being, there can be value to interacting with such folks in a real-world environment.
- You do not have to bring everything to the table. The best leaders surround themselves with other really smart folks who, in aggregate, will provide support in areas in which you are deficient. That said, you need to know where these gaps in your knowledge and experience are, and when it is the right time to listen to those trusted advisors.
- When it comes time to identify folks for your “cabinet,” make sure to choose people who think differently than you and who may disagree with you on some fundamental things. Surrounding yourself with friends and close colleagues can lead to groupthink and poor decision making. The best results often stem from challenging and difficult decision-making processes.
- As a corollary to the above, every leader will bring their own sensibility and personality to the role. Make sure to bring yours, even if it involves silly jokes about holding a medical meeting in a former President’s basement or getting another former President to eat a big spoonful of the aforementioned condiment.
- Fun is important. Fun builds relationships, and teams, and trust. Make sure you are having it, as much as you possibly can, throughout your leadership tenure.
On that note, I will sign off for good, at least in these pages. I’ll still be bumbling around, proposing new educational experiences, hosting Pardon the Interruption, and serving as a sounding board for anyone who wants to chat. But I cannot wait to see what the next 3 years bring for our organization, under the leadership of Drs. Addrizzo-Harris, Buckley, and Howington. And for those of you who are just taking your first steps in leadership, and who will be following in their footsteps years down the road, I hope that you get just as much enjoyment from and fulfilment in the role of President as I have. #SchulmanOut
This is an odd column for me to write. First, because of the nature of print publication, this writing for the November issue is being crafted just before the annual meeting is to be held in Nashville. Therefore, while I have a pretty good sense of what is in store for CHEST 2022, I have yet to see the final product, or the audience’s reaction to it. However, I will make some bold predictions as to what occurred therein:
- Even in the context of 3 years of separation, thousands CHEST members gathered in droves to rekindle friendships and to experience the best education in pulmonary, critical care, and sleep medicine that the world has to offer, leading to our second-biggest meeting ever.
- Neil Pasricha’s presentation helped attendees rekindle the “Art of Happiness.”
- Hundreds of attendees participated in, and successfully solved, our newest escape room, “Starship Relics.”
- Our valued CHEST members were able to successfully thwart Dr. Didactic and save the future of educational innovation.
- “CHEST After Hours” trended on social media and will become a normal and highly-anticipated part of the CHEST meeting moving forward.
- The most uncomfortable moment of the meeting centered on mayonnaise; for those of you who know what I am referencing, I am a little sorry…but only a little.
- Despite my best efforts, we were not able to recruit Neil Patrick Harris to participate.
Predicting the future of medical meetings is something we’ve spent a lot of time trying to do over the last year as we developed plans for CHEST 2022. But given the talented individuals involved in that planning, foreseeing the meeting’s success did not require any time travel; it was hardly a difficult task at all. Program Chair Subani Chandra and Vice-Chair Aneesa Das were exactly the people we needed at the helm for this all-important return to in-person meetings, and I cannot thank them enough for their creativity, effort, and leadership in bringing CHEST 2022 to fruition. And while I expect to have been seven-for-seven in my predictions above, I do hope I got that NPH one wrong.
The other reason that this column was a challenge to craft is because it represents my final formal presidential missive in these esteemed pages. And as I planned this final walk of the path, I gave careful consideration to the message with which I wanted to conclude my year. And as I put together my predictions for the future, my mind also turned to the past, considering things I wish I had known (or spent more time considering) as I started this journey. Some of this information may prove useful to the next generation of CHEST leaders, and some may be already well engrained for those of you with leadership experience. Here, in no particular order, are some thoughts for those of you in the audience who are considering future leadership opportunities at CHEST (or elsewhere in life; I suspect some of this advice is applicable to other venues). That said, the recommendations also come from yours truly, so take them with an appropriately large grain of salt, as your mileage may vary, and reasonable people could take issue here or there.
- The most important conversations should happen in person. The past 3 years have shown us the amazing things that modern technology can accomplish, but when it comes to providing important information, asking for input on a crucial issue, or providing feedback on a sensitive matter, there is no adequate substitute for a discussion in which all parties are in the same room.
- You are going to get things wrong sometimes; sometimes, this is because there wasn’t a way to get a right answer, and sometimes it will be because you tried something that didn’t work. You will learn far more from one of these experiences than from a dozen things that went as well as (or better than) expected.
- It is profoundly difficult to change someone’s mind if you aren’t interacting with them. I believe there is no gap so large that warrants breakdown of communication. Going that extra mile to talk to people who have a drastically different opinion than your own is the only way that you might be able to change someone’s mind and is a great way to ensure that your own opinion withstands pushback. With the growth of social media over the last decade, we’ve gotten very good at blocking people on social media; while this can sometimes be good (or even necessary) for emotional well-being, there can be value to interacting with such folks in a real-world environment.
- You do not have to bring everything to the table. The best leaders surround themselves with other really smart folks who, in aggregate, will provide support in areas in which you are deficient. That said, you need to know where these gaps in your knowledge and experience are, and when it is the right time to listen to those trusted advisors.
- When it comes time to identify folks for your “cabinet,” make sure to choose people who think differently than you and who may disagree with you on some fundamental things. Surrounding yourself with friends and close colleagues can lead to groupthink and poor decision making. The best results often stem from challenging and difficult decision-making processes.
- As a corollary to the above, every leader will bring their own sensibility and personality to the role. Make sure to bring yours, even if it involves silly jokes about holding a medical meeting in a former President’s basement or getting another former President to eat a big spoonful of the aforementioned condiment.
- Fun is important. Fun builds relationships, and teams, and trust. Make sure you are having it, as much as you possibly can, throughout your leadership tenure.
On that note, I will sign off for good, at least in these pages. I’ll still be bumbling around, proposing new educational experiences, hosting Pardon the Interruption, and serving as a sounding board for anyone who wants to chat. But I cannot wait to see what the next 3 years bring for our organization, under the leadership of Drs. Addrizzo-Harris, Buckley, and Howington. And for those of you who are just taking your first steps in leadership, and who will be following in their footsteps years down the road, I hope that you get just as much enjoyment from and fulfilment in the role of President as I have. #SchulmanOut
Novel drug eases Parkinson’s-related constipation in early trial
The findings are based on 135 patients who completed 7-25 days of treatment with a daily oral dose of the drug, ENT-01, or a placebo. Complete spontaneous bowel movements (CSBMs), the primary efficacy endpoint, increased from a mean of 0.7 per week to 3.2 in individuals who took ENT-01 versus 1.2 in the placebo group.
The phase 2, multicenter, randomized trial showed that the drug “is safe and that it rapidly normalized bowel function in a dose-dependent fashion, with an effect that seems to persist for several weeks beyond the treatment period,” the researchers wrote in their paper on the research, which was published in Annals of Internal Medicine.
The researchers hypothesized that displacing aggregated alpha-synuclein from nerve cells in the gastrointestinal tract may also “slow progression of neurologic symptoms” in patients with PD by arresting the abnormal development of alpha-nucleic aggregates in the brain.
Denise Barbut, MD, cofounder, president and chief medical officer of Enterin, the company developing ENT-01, said the next step is another phase 2 trial to determine whether the drug reverses dementia or psychosis in patients with PD, before conducting a phase 3 study.
“We want to treat all nonmotor symptoms of Parkinson’s disease, not just constipation,” she said.
Constipation is an early PD symptom
Constipation is a common and persistent symptom of PD that often emerges years earlier than other symptoms such as motor deficits. Recent research has linked it to aggregates of alpha-synuclein that bind to cells in the enteric nervous system and may spread to the brain via the vagus nerve.
According to the researchers, ENT-01, a synthetic derivative of the antimicrobial compound squalamine, improves neural signaling in the gut by displacing alpha-synuclein aggregates.
In their double-blinded study, patients were randomized 3:1 to receive ENT-01 or a placebo and stratified by constipation severity to one of two starting doses: 75 mg or three placebo pills or 150 mg or six placebo pills. Doses increased until a patient reached a “prokinetic” dose, a maximum of 250 mg or 10 placebo pills, or the individual’s tolerability limit.
Dosing was fixed for the remainder of the 25 days, after which all patients took a placebo for 2 weeks followed by a 4-week washout.
In addition to more CSBMs, the treatment group had greater improvements in secondary endpoints of weekly spontaneous bowel movements (P = .002), better stool consistency (P < .001), improved ease of passage (P = .006), and less laxative use (P = .041).
There were no significant differences between the groups in scores on the Patient Assessment of Constipation Symptoms or the Patient Assessment of Constipation Quality of Life.
No deaths occurred, and there were no serious adverse events attributed to ENT-01. However, adverse events occurred in 61 (65.6%) of patients who took the drug versus 27 (47.4%) of those who took a placebo.
The most common problems were nausea, experienced by 32 (34%) in the ENT-01 group and 3 (5.3%) in the placebo group, and diarrhea, which occurred in 18 (9.4%) of those in the ENT-01 group and three (5.3%) who took the placebo.
Of 93 patients randomized to the drug (25.8%), 24 discontinued treatment before therapy ended, mostly because of nausea or diarrhea. That compared with 8 of 57 (14.1%) patients in the placebo group who stopped taking their pills before the end of the therapy period.
The researchers suggested that nausea and diarrhea might be alleviated by more gradual dosing escalation and the use of antinausea medication.
Dr. Barbut noted that a previous open-label trial of 50 patients with PD showed that ENT-01 acts locally in the gastrointestinal tract, which means it would not be absorbed into the bloodstream or interfere with other medications.
Targeting the underlying disease
Researchers noted that, in small subsets of patients with dementia or psychosis, greater improvements in those symptoms occurred among those who took ENT-01 versus those who took a placebo.
According to the study, among 11 patients with psychosis, average scores on the Scale for the Assessment of Positive Symptoms adapted for PD dropped from 6.5 to 1.8 on a 45-point scale at the end of treatment in the ENT-01 group (n = 5) and from 6.3 to 3.4 in the placebo group (n = 6).
In 28 patients with dementia, scores on the Mini-Mental State Examination improved by 2.4 points on a 30-point scale, from 24.1 to 26.5, during the treatment period for the ENT-01 group (n = 14) versus an improvement of 0.9 points, from 24.8 to 25.7, in the placebo group (n = 14).
The researchers said the findings must be evaluated in future trials dedicated to studying ENT-01’s effects on PD-related psychosis and dementia.
He added that, if findings are reproduced in a large study, the drug could have “a major impact” not just in treating constipation, for which there are no PD-specific drugs, but also in addressing neurological dysfunctions that are cardinal features of PD. “That is what is exciting to me, because we’re now talking about reversing the disease itself,” he said.
However, Dr. Barbut said it’s been difficult to get across to the medical community and to investors that a drug that acts on nerve cells in the gut might reverse neurologic symptoms by improving direct gut-brain communication. “That’s a concept that is alien to most people’s thinking,” she said.
Enterin funded the study and was responsible for the design, data collection and analysis. Its employees also participated in the interpretation of data, writing of the report, and the decision to submit the manuscript for publication. Dr. Barbut reported stock options in Enterin and patent interests in ENT-01. Fifteen other study investigators reported financial ties to Enterin and/or ENT-01 including employment, stock options, research funding, consulting fees and patent application ownership. Dr. Rao reported receiving honoraria from multiple companies that market drugs for general constipation.
The findings are based on 135 patients who completed 7-25 days of treatment with a daily oral dose of the drug, ENT-01, or a placebo. Complete spontaneous bowel movements (CSBMs), the primary efficacy endpoint, increased from a mean of 0.7 per week to 3.2 in individuals who took ENT-01 versus 1.2 in the placebo group.
The phase 2, multicenter, randomized trial showed that the drug “is safe and that it rapidly normalized bowel function in a dose-dependent fashion, with an effect that seems to persist for several weeks beyond the treatment period,” the researchers wrote in their paper on the research, which was published in Annals of Internal Medicine.
The researchers hypothesized that displacing aggregated alpha-synuclein from nerve cells in the gastrointestinal tract may also “slow progression of neurologic symptoms” in patients with PD by arresting the abnormal development of alpha-nucleic aggregates in the brain.
Denise Barbut, MD, cofounder, president and chief medical officer of Enterin, the company developing ENT-01, said the next step is another phase 2 trial to determine whether the drug reverses dementia or psychosis in patients with PD, before conducting a phase 3 study.
“We want to treat all nonmotor symptoms of Parkinson’s disease, not just constipation,” she said.
Constipation is an early PD symptom
Constipation is a common and persistent symptom of PD that often emerges years earlier than other symptoms such as motor deficits. Recent research has linked it to aggregates of alpha-synuclein that bind to cells in the enteric nervous system and may spread to the brain via the vagus nerve.
According to the researchers, ENT-01, a synthetic derivative of the antimicrobial compound squalamine, improves neural signaling in the gut by displacing alpha-synuclein aggregates.
In their double-blinded study, patients were randomized 3:1 to receive ENT-01 or a placebo and stratified by constipation severity to one of two starting doses: 75 mg or three placebo pills or 150 mg or six placebo pills. Doses increased until a patient reached a “prokinetic” dose, a maximum of 250 mg or 10 placebo pills, or the individual’s tolerability limit.
Dosing was fixed for the remainder of the 25 days, after which all patients took a placebo for 2 weeks followed by a 4-week washout.
In addition to more CSBMs, the treatment group had greater improvements in secondary endpoints of weekly spontaneous bowel movements (P = .002), better stool consistency (P < .001), improved ease of passage (P = .006), and less laxative use (P = .041).
There were no significant differences between the groups in scores on the Patient Assessment of Constipation Symptoms or the Patient Assessment of Constipation Quality of Life.
No deaths occurred, and there were no serious adverse events attributed to ENT-01. However, adverse events occurred in 61 (65.6%) of patients who took the drug versus 27 (47.4%) of those who took a placebo.
The most common problems were nausea, experienced by 32 (34%) in the ENT-01 group and 3 (5.3%) in the placebo group, and diarrhea, which occurred in 18 (9.4%) of those in the ENT-01 group and three (5.3%) who took the placebo.
Of 93 patients randomized to the drug (25.8%), 24 discontinued treatment before therapy ended, mostly because of nausea or diarrhea. That compared with 8 of 57 (14.1%) patients in the placebo group who stopped taking their pills before the end of the therapy period.
The researchers suggested that nausea and diarrhea might be alleviated by more gradual dosing escalation and the use of antinausea medication.
Dr. Barbut noted that a previous open-label trial of 50 patients with PD showed that ENT-01 acts locally in the gastrointestinal tract, which means it would not be absorbed into the bloodstream or interfere with other medications.
Targeting the underlying disease
Researchers noted that, in small subsets of patients with dementia or psychosis, greater improvements in those symptoms occurred among those who took ENT-01 versus those who took a placebo.
According to the study, among 11 patients with psychosis, average scores on the Scale for the Assessment of Positive Symptoms adapted for PD dropped from 6.5 to 1.8 on a 45-point scale at the end of treatment in the ENT-01 group (n = 5) and from 6.3 to 3.4 in the placebo group (n = 6).
In 28 patients with dementia, scores on the Mini-Mental State Examination improved by 2.4 points on a 30-point scale, from 24.1 to 26.5, during the treatment period for the ENT-01 group (n = 14) versus an improvement of 0.9 points, from 24.8 to 25.7, in the placebo group (n = 14).
The researchers said the findings must be evaluated in future trials dedicated to studying ENT-01’s effects on PD-related psychosis and dementia.
He added that, if findings are reproduced in a large study, the drug could have “a major impact” not just in treating constipation, for which there are no PD-specific drugs, but also in addressing neurological dysfunctions that are cardinal features of PD. “That is what is exciting to me, because we’re now talking about reversing the disease itself,” he said.
However, Dr. Barbut said it’s been difficult to get across to the medical community and to investors that a drug that acts on nerve cells in the gut might reverse neurologic symptoms by improving direct gut-brain communication. “That’s a concept that is alien to most people’s thinking,” she said.
Enterin funded the study and was responsible for the design, data collection and analysis. Its employees also participated in the interpretation of data, writing of the report, and the decision to submit the manuscript for publication. Dr. Barbut reported stock options in Enterin and patent interests in ENT-01. Fifteen other study investigators reported financial ties to Enterin and/or ENT-01 including employment, stock options, research funding, consulting fees and patent application ownership. Dr. Rao reported receiving honoraria from multiple companies that market drugs for general constipation.
The findings are based on 135 patients who completed 7-25 days of treatment with a daily oral dose of the drug, ENT-01, or a placebo. Complete spontaneous bowel movements (CSBMs), the primary efficacy endpoint, increased from a mean of 0.7 per week to 3.2 in individuals who took ENT-01 versus 1.2 in the placebo group.
The phase 2, multicenter, randomized trial showed that the drug “is safe and that it rapidly normalized bowel function in a dose-dependent fashion, with an effect that seems to persist for several weeks beyond the treatment period,” the researchers wrote in their paper on the research, which was published in Annals of Internal Medicine.
The researchers hypothesized that displacing aggregated alpha-synuclein from nerve cells in the gastrointestinal tract may also “slow progression of neurologic symptoms” in patients with PD by arresting the abnormal development of alpha-nucleic aggregates in the brain.
Denise Barbut, MD, cofounder, president and chief medical officer of Enterin, the company developing ENT-01, said the next step is another phase 2 trial to determine whether the drug reverses dementia or psychosis in patients with PD, before conducting a phase 3 study.
“We want to treat all nonmotor symptoms of Parkinson’s disease, not just constipation,” she said.
Constipation is an early PD symptom
Constipation is a common and persistent symptom of PD that often emerges years earlier than other symptoms such as motor deficits. Recent research has linked it to aggregates of alpha-synuclein that bind to cells in the enteric nervous system and may spread to the brain via the vagus nerve.
According to the researchers, ENT-01, a synthetic derivative of the antimicrobial compound squalamine, improves neural signaling in the gut by displacing alpha-synuclein aggregates.
In their double-blinded study, patients were randomized 3:1 to receive ENT-01 or a placebo and stratified by constipation severity to one of two starting doses: 75 mg or three placebo pills or 150 mg or six placebo pills. Doses increased until a patient reached a “prokinetic” dose, a maximum of 250 mg or 10 placebo pills, or the individual’s tolerability limit.
Dosing was fixed for the remainder of the 25 days, after which all patients took a placebo for 2 weeks followed by a 4-week washout.
In addition to more CSBMs, the treatment group had greater improvements in secondary endpoints of weekly spontaneous bowel movements (P = .002), better stool consistency (P < .001), improved ease of passage (P = .006), and less laxative use (P = .041).
There were no significant differences between the groups in scores on the Patient Assessment of Constipation Symptoms or the Patient Assessment of Constipation Quality of Life.
No deaths occurred, and there were no serious adverse events attributed to ENT-01. However, adverse events occurred in 61 (65.6%) of patients who took the drug versus 27 (47.4%) of those who took a placebo.
The most common problems were nausea, experienced by 32 (34%) in the ENT-01 group and 3 (5.3%) in the placebo group, and diarrhea, which occurred in 18 (9.4%) of those in the ENT-01 group and three (5.3%) who took the placebo.
Of 93 patients randomized to the drug (25.8%), 24 discontinued treatment before therapy ended, mostly because of nausea or diarrhea. That compared with 8 of 57 (14.1%) patients in the placebo group who stopped taking their pills before the end of the therapy period.
The researchers suggested that nausea and diarrhea might be alleviated by more gradual dosing escalation and the use of antinausea medication.
Dr. Barbut noted that a previous open-label trial of 50 patients with PD showed that ENT-01 acts locally in the gastrointestinal tract, which means it would not be absorbed into the bloodstream or interfere with other medications.
Targeting the underlying disease
Researchers noted that, in small subsets of patients with dementia or psychosis, greater improvements in those symptoms occurred among those who took ENT-01 versus those who took a placebo.
According to the study, among 11 patients with psychosis, average scores on the Scale for the Assessment of Positive Symptoms adapted for PD dropped from 6.5 to 1.8 on a 45-point scale at the end of treatment in the ENT-01 group (n = 5) and from 6.3 to 3.4 in the placebo group (n = 6).
In 28 patients with dementia, scores on the Mini-Mental State Examination improved by 2.4 points on a 30-point scale, from 24.1 to 26.5, during the treatment period for the ENT-01 group (n = 14) versus an improvement of 0.9 points, from 24.8 to 25.7, in the placebo group (n = 14).
The researchers said the findings must be evaluated in future trials dedicated to studying ENT-01’s effects on PD-related psychosis and dementia.
He added that, if findings are reproduced in a large study, the drug could have “a major impact” not just in treating constipation, for which there are no PD-specific drugs, but also in addressing neurological dysfunctions that are cardinal features of PD. “That is what is exciting to me, because we’re now talking about reversing the disease itself,” he said.
However, Dr. Barbut said it’s been difficult to get across to the medical community and to investors that a drug that acts on nerve cells in the gut might reverse neurologic symptoms by improving direct gut-brain communication. “That’s a concept that is alien to most people’s thinking,” she said.
Enterin funded the study and was responsible for the design, data collection and analysis. Its employees also participated in the interpretation of data, writing of the report, and the decision to submit the manuscript for publication. Dr. Barbut reported stock options in Enterin and patent interests in ENT-01. Fifteen other study investigators reported financial ties to Enterin and/or ENT-01 including employment, stock options, research funding, consulting fees and patent application ownership. Dr. Rao reported receiving honoraria from multiple companies that market drugs for general constipation.
FROM ANNALS OF INTERNAL MEDICINE
Link between PCOS and increased risk of pancreatic cancer?
Women with polycystic ovary syndrome (PCOS) may be at a higher risk of developing pancreatic cancer, say researchers reporting a single-center case-control study.
A diagnosis of PCOS was associated with a 1.9-fold higher risk of pancreatic cancer after adjusting for age, race, ethnicity, estrogen level, and diabetes.
This is the second study to find such an association.
“Our study findings combined with those from the 2019 Swedish Registry study offer compelling evidence that PCOS may be a novel risk factor for pancreatic cancer,” said corresponding author Mengmeng Du, ScD, department of epidemiology and biostatistics, Memorial Sloan Kettering Cancer Center.
“These data suggest some individuals may have unknown metabolic derangements that may underlie the development of both conditions,” the team concluded.
The findings were published in JAMA Oncology.
Approached for comment, Srinivas Gaddam, MD, MPH, associate director of pancreatic biliary research medicine, Cedars-Sinai, suggested that the findings may pave the way for a better understanding of the two diseases, but he emphasized that more research is needed.
“I think there’s more research to be done because now we’re seeing more younger women get pancreatic cancer,” Dr. Gaddam said. “So that makes it interesting whether PCOS itself contributes to pancreatic cancer. I still think the jury is out there.”
Dr. Gaddam drew attention to the confidence interval for the finding – the adjusted odds ratio was 1.88 (95% confidence interval, 1.02-3.46). “Because their odds ratio includes 1, I’m left with the question as to whether or not this is truly associated. I’m not certain that we can draw any conclusions based on this,” he commented.
The investigators acknowledge that they did “not observe statistically significant interactions” and comment that “prospective studies are needed to examine underlying biologic mechanisms and confirm our findings.”
For the study, the team used data from the Memorial Sloan Kettering Cancer Center Pancreatic Tumor Registry. They identified patients with pancreatic cancer who also self-reported a diagnosis of PCOS.
The investigators compared data from 446 women with pathologically or cytologically confirmed pancreatic adenocarcinoma with 209 women who had no history of cancer. The mean age at cancer diagnosis or enrollment was 63.8 years among patients with pancreatic cancer and 57.7 years in the control group.
The study found that having PCOS nearly doubled a person’s risk of developing pancreatic cancer.
When adjusted for type 2 diabetes diagnosis, the odds ratio fell slightly to 1.78 (95% CI, 0.95-3.34).
Dr. Du, along with lead author Noah Peeri, PhD, were surprised that even after adjusting for body mass index and the presence of type 2 diabetes, PCOS remained strongly associated with pancreatic cancer risk.
“We originally thought type 2 diabetes may drive this association, given more than half of those with PCOS develop type 2 diabetes by age 40, according to the CDC, and type 2 diabetes has also been linked with increased pancreatic cancer risk,” said Dr. Du.
“While the association was slightly weaker and no longer statistically significant after we controlled for type 2 diabetes, the magnitude of the association remained largely unchanged,” he said.
Dr. Peeri believes that some of the factors that have been causally related to PCOS may increase an individual’s pancreatic cancer risk.
“PCOS itself does not likely cause pancreatic cancer, but metabolic problems (for example, improper breakdown of insulin) and chronic inflammation can contribute to both PCOS and pancreatic cancer risk,” Dr. Peeri said.
He concluded that the study results “suggest other underlying metabolic dysfunction may increase an individual’s pancreatic cancer risk.”
An important limitation of this study was that women in the study self-reported PCOS and may have incorrectly recalled their diagnosis. However, the authors believe it is unlikely that that had a bearing on the study findings.
The study was supported by National Cancer Institute grants, the Geoffrey Beene Foundation, and the Arnold and Arlene Goldstein Family Foundation.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) may be at a higher risk of developing pancreatic cancer, say researchers reporting a single-center case-control study.
A diagnosis of PCOS was associated with a 1.9-fold higher risk of pancreatic cancer after adjusting for age, race, ethnicity, estrogen level, and diabetes.
This is the second study to find such an association.
“Our study findings combined with those from the 2019 Swedish Registry study offer compelling evidence that PCOS may be a novel risk factor for pancreatic cancer,” said corresponding author Mengmeng Du, ScD, department of epidemiology and biostatistics, Memorial Sloan Kettering Cancer Center.
“These data suggest some individuals may have unknown metabolic derangements that may underlie the development of both conditions,” the team concluded.
The findings were published in JAMA Oncology.
Approached for comment, Srinivas Gaddam, MD, MPH, associate director of pancreatic biliary research medicine, Cedars-Sinai, suggested that the findings may pave the way for a better understanding of the two diseases, but he emphasized that more research is needed.
“I think there’s more research to be done because now we’re seeing more younger women get pancreatic cancer,” Dr. Gaddam said. “So that makes it interesting whether PCOS itself contributes to pancreatic cancer. I still think the jury is out there.”
Dr. Gaddam drew attention to the confidence interval for the finding – the adjusted odds ratio was 1.88 (95% confidence interval, 1.02-3.46). “Because their odds ratio includes 1, I’m left with the question as to whether or not this is truly associated. I’m not certain that we can draw any conclusions based on this,” he commented.
The investigators acknowledge that they did “not observe statistically significant interactions” and comment that “prospective studies are needed to examine underlying biologic mechanisms and confirm our findings.”
For the study, the team used data from the Memorial Sloan Kettering Cancer Center Pancreatic Tumor Registry. They identified patients with pancreatic cancer who also self-reported a diagnosis of PCOS.
The investigators compared data from 446 women with pathologically or cytologically confirmed pancreatic adenocarcinoma with 209 women who had no history of cancer. The mean age at cancer diagnosis or enrollment was 63.8 years among patients with pancreatic cancer and 57.7 years in the control group.
The study found that having PCOS nearly doubled a person’s risk of developing pancreatic cancer.
When adjusted for type 2 diabetes diagnosis, the odds ratio fell slightly to 1.78 (95% CI, 0.95-3.34).
Dr. Du, along with lead author Noah Peeri, PhD, were surprised that even after adjusting for body mass index and the presence of type 2 diabetes, PCOS remained strongly associated with pancreatic cancer risk.
“We originally thought type 2 diabetes may drive this association, given more than half of those with PCOS develop type 2 diabetes by age 40, according to the CDC, and type 2 diabetes has also been linked with increased pancreatic cancer risk,” said Dr. Du.
“While the association was slightly weaker and no longer statistically significant after we controlled for type 2 diabetes, the magnitude of the association remained largely unchanged,” he said.
Dr. Peeri believes that some of the factors that have been causally related to PCOS may increase an individual’s pancreatic cancer risk.
“PCOS itself does not likely cause pancreatic cancer, but metabolic problems (for example, improper breakdown of insulin) and chronic inflammation can contribute to both PCOS and pancreatic cancer risk,” Dr. Peeri said.
He concluded that the study results “suggest other underlying metabolic dysfunction may increase an individual’s pancreatic cancer risk.”
An important limitation of this study was that women in the study self-reported PCOS and may have incorrectly recalled their diagnosis. However, the authors believe it is unlikely that that had a bearing on the study findings.
The study was supported by National Cancer Institute grants, the Geoffrey Beene Foundation, and the Arnold and Arlene Goldstein Family Foundation.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) may be at a higher risk of developing pancreatic cancer, say researchers reporting a single-center case-control study.
A diagnosis of PCOS was associated with a 1.9-fold higher risk of pancreatic cancer after adjusting for age, race, ethnicity, estrogen level, and diabetes.
This is the second study to find such an association.
“Our study findings combined with those from the 2019 Swedish Registry study offer compelling evidence that PCOS may be a novel risk factor for pancreatic cancer,” said corresponding author Mengmeng Du, ScD, department of epidemiology and biostatistics, Memorial Sloan Kettering Cancer Center.
“These data suggest some individuals may have unknown metabolic derangements that may underlie the development of both conditions,” the team concluded.
The findings were published in JAMA Oncology.
Approached for comment, Srinivas Gaddam, MD, MPH, associate director of pancreatic biliary research medicine, Cedars-Sinai, suggested that the findings may pave the way for a better understanding of the two diseases, but he emphasized that more research is needed.
“I think there’s more research to be done because now we’re seeing more younger women get pancreatic cancer,” Dr. Gaddam said. “So that makes it interesting whether PCOS itself contributes to pancreatic cancer. I still think the jury is out there.”
Dr. Gaddam drew attention to the confidence interval for the finding – the adjusted odds ratio was 1.88 (95% confidence interval, 1.02-3.46). “Because their odds ratio includes 1, I’m left with the question as to whether or not this is truly associated. I’m not certain that we can draw any conclusions based on this,” he commented.
The investigators acknowledge that they did “not observe statistically significant interactions” and comment that “prospective studies are needed to examine underlying biologic mechanisms and confirm our findings.”
For the study, the team used data from the Memorial Sloan Kettering Cancer Center Pancreatic Tumor Registry. They identified patients with pancreatic cancer who also self-reported a diagnosis of PCOS.
The investigators compared data from 446 women with pathologically or cytologically confirmed pancreatic adenocarcinoma with 209 women who had no history of cancer. The mean age at cancer diagnosis or enrollment was 63.8 years among patients with pancreatic cancer and 57.7 years in the control group.
The study found that having PCOS nearly doubled a person’s risk of developing pancreatic cancer.
When adjusted for type 2 diabetes diagnosis, the odds ratio fell slightly to 1.78 (95% CI, 0.95-3.34).
Dr. Du, along with lead author Noah Peeri, PhD, were surprised that even after adjusting for body mass index and the presence of type 2 diabetes, PCOS remained strongly associated with pancreatic cancer risk.
“We originally thought type 2 diabetes may drive this association, given more than half of those with PCOS develop type 2 diabetes by age 40, according to the CDC, and type 2 diabetes has also been linked with increased pancreatic cancer risk,” said Dr. Du.
“While the association was slightly weaker and no longer statistically significant after we controlled for type 2 diabetes, the magnitude of the association remained largely unchanged,” he said.
Dr. Peeri believes that some of the factors that have been causally related to PCOS may increase an individual’s pancreatic cancer risk.
“PCOS itself does not likely cause pancreatic cancer, but metabolic problems (for example, improper breakdown of insulin) and chronic inflammation can contribute to both PCOS and pancreatic cancer risk,” Dr. Peeri said.
He concluded that the study results “suggest other underlying metabolic dysfunction may increase an individual’s pancreatic cancer risk.”
An important limitation of this study was that women in the study self-reported PCOS and may have incorrectly recalled their diagnosis. However, the authors believe it is unlikely that that had a bearing on the study findings.
The study was supported by National Cancer Institute grants, the Geoffrey Beene Foundation, and the Arnold and Arlene Goldstein Family Foundation.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
CDC warns of early uptick in respiratory disease
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
DTC telemedicine expands access to gender-affirming therapy
Direct-to-consumer telemedicine services that provide gender-affirming hormone therapy appear to follow evidence-based guidelines and charge about the same as brick-and-mortar medical centers, according to researchers who reviewed the platforms’ websites.
The findings suggest that virtual care “may be a good option” for transgender, nonbinary, and intersex people, who often report difficulty finding physicians they trust, Erin Jesse, MD, a fifth-year urology resident at University Hospitals Cleveland Medical Center, who is the first author of the study, told this news organization.
Dr. Jesse’s group presented their findings at a joint scientific meeting of the Sexual Medicine Society of North America and the International Society for Sexual Medicine in Miami. The results have not been published in a peer-reviewed journal.
New direct-to-consumer telemedicine companies have emerged with gender-diverse staff and services tailored to the needs of these individuals. They offer “a more inclusive feel” than might be encountered at a physician’s office, Dr. Jesse said.
Confirming that these companies adhere to standards of care and cost-effectiveness “is especially important considering the reduced access to care and potentially increased vulnerability of the gender-diverse population,” she and her colleagues wrote.
From a Google search in March, the team identified six U.S.-based platforms that offer gender-affirming medical therapy: FOLX, True U Clinic, QueerDoc, Queer Med, TransClinique, and Plume.
From information posted on the companies’ websites, the researchers determined that all aligned with the World Professional Association for Transgender Health’s Standards of Care in two areas: use of an informed consent model to ensure that patients have sufficient information and understanding to decide on their own treatment and endorsement of frequent laboratory monitoring of hormone levels in early stages of treatment.
The team also compared the costs listed on the websites for the first year of therapy to the costs of similar care at a tertiary center, as determined using University Hospitals Cleveland Medical Center’s online estimator.
The platforms offered various pricing models, including fee-for-service and monthly membership plans ranging from $59 to $139. For individuals without insurance, estimates ranged from $1,022 to $1,428 for oral estradiol and from $1,184 to $1,668 for intramuscular testosterone from the online companies, compared with $1,184 and $1,216, respectively, at the tertiary center.
Although some platforms accept insurance, the researchers were not able to evaluate the cost of using private insurance or Medicaid, Dr. Jesse said. She noted that transgender individuals are more likely to lack insurance than are cisgender patients.
The team also assessed the scope of services. All companies offered legal help with changes to names and gender markers, such as “M” and “F.” Three or more companies offered preexposure prophylaxis to prevent HIV infection, treatment for erectile dysfunction, referrals for surgery, and medical letters of support for surgery.
Two offered puberty blockers, although the researchers were unable to determine the risk of adolescents obtaining treatment without proper assessments, because details of those services are not disclosed on websites, Dr. Jesse said.
An avenue of further research would be to interview patients to learn how platforms operate in practice and whether patients are properly assessed before treatment. “Those sorts of questions we can’t answer just by looking at the websites,” she said.
However, Charlotte Hoffman, JD, senior policy counsel for the National Center for Transgender Equality, an advocacy group, said she does not harbor concerns about patients being treated inappropriately simply because care is virtual. All clinicians who provide gender-affirming care face potential repercussions, such as malpractice lawsuits or state disciplinary action, if they veer from treatment guidelines, she said.
“I don’t necessarily take the premise that telehealth is inherently worse than in-person care as a given,” Ms. Hoffman said.
During the COVID-19 pandemic, Ms. Hoffman added, direct-to-consumer telemedicine has expanded access for individuals in rural areas, people with disabilities, and those who live in places where in-person providers of transgender care face public hostility, although individuals without the resources to pay may still be left out.
What might happen to that access if telemedicine restrictions that were loosened during the pandemic are reinstated is unclear, she said.
The researchers and Ms. Hoffman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Direct-to-consumer telemedicine services that provide gender-affirming hormone therapy appear to follow evidence-based guidelines and charge about the same as brick-and-mortar medical centers, according to researchers who reviewed the platforms’ websites.
The findings suggest that virtual care “may be a good option” for transgender, nonbinary, and intersex people, who often report difficulty finding physicians they trust, Erin Jesse, MD, a fifth-year urology resident at University Hospitals Cleveland Medical Center, who is the first author of the study, told this news organization.
Dr. Jesse’s group presented their findings at a joint scientific meeting of the Sexual Medicine Society of North America and the International Society for Sexual Medicine in Miami. The results have not been published in a peer-reviewed journal.
New direct-to-consumer telemedicine companies have emerged with gender-diverse staff and services tailored to the needs of these individuals. They offer “a more inclusive feel” than might be encountered at a physician’s office, Dr. Jesse said.
Confirming that these companies adhere to standards of care and cost-effectiveness “is especially important considering the reduced access to care and potentially increased vulnerability of the gender-diverse population,” she and her colleagues wrote.
From a Google search in March, the team identified six U.S.-based platforms that offer gender-affirming medical therapy: FOLX, True U Clinic, QueerDoc, Queer Med, TransClinique, and Plume.
From information posted on the companies’ websites, the researchers determined that all aligned with the World Professional Association for Transgender Health’s Standards of Care in two areas: use of an informed consent model to ensure that patients have sufficient information and understanding to decide on their own treatment and endorsement of frequent laboratory monitoring of hormone levels in early stages of treatment.
The team also compared the costs listed on the websites for the first year of therapy to the costs of similar care at a tertiary center, as determined using University Hospitals Cleveland Medical Center’s online estimator.
The platforms offered various pricing models, including fee-for-service and monthly membership plans ranging from $59 to $139. For individuals without insurance, estimates ranged from $1,022 to $1,428 for oral estradiol and from $1,184 to $1,668 for intramuscular testosterone from the online companies, compared with $1,184 and $1,216, respectively, at the tertiary center.
Although some platforms accept insurance, the researchers were not able to evaluate the cost of using private insurance or Medicaid, Dr. Jesse said. She noted that transgender individuals are more likely to lack insurance than are cisgender patients.
The team also assessed the scope of services. All companies offered legal help with changes to names and gender markers, such as “M” and “F.” Three or more companies offered preexposure prophylaxis to prevent HIV infection, treatment for erectile dysfunction, referrals for surgery, and medical letters of support for surgery.
Two offered puberty blockers, although the researchers were unable to determine the risk of adolescents obtaining treatment without proper assessments, because details of those services are not disclosed on websites, Dr. Jesse said.
An avenue of further research would be to interview patients to learn how platforms operate in practice and whether patients are properly assessed before treatment. “Those sorts of questions we can’t answer just by looking at the websites,” she said.
However, Charlotte Hoffman, JD, senior policy counsel for the National Center for Transgender Equality, an advocacy group, said she does not harbor concerns about patients being treated inappropriately simply because care is virtual. All clinicians who provide gender-affirming care face potential repercussions, such as malpractice lawsuits or state disciplinary action, if they veer from treatment guidelines, she said.
“I don’t necessarily take the premise that telehealth is inherently worse than in-person care as a given,” Ms. Hoffman said.
During the COVID-19 pandemic, Ms. Hoffman added, direct-to-consumer telemedicine has expanded access for individuals in rural areas, people with disabilities, and those who live in places where in-person providers of transgender care face public hostility, although individuals without the resources to pay may still be left out.
What might happen to that access if telemedicine restrictions that were loosened during the pandemic are reinstated is unclear, she said.
The researchers and Ms. Hoffman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Direct-to-consumer telemedicine services that provide gender-affirming hormone therapy appear to follow evidence-based guidelines and charge about the same as brick-and-mortar medical centers, according to researchers who reviewed the platforms’ websites.
The findings suggest that virtual care “may be a good option” for transgender, nonbinary, and intersex people, who often report difficulty finding physicians they trust, Erin Jesse, MD, a fifth-year urology resident at University Hospitals Cleveland Medical Center, who is the first author of the study, told this news organization.
Dr. Jesse’s group presented their findings at a joint scientific meeting of the Sexual Medicine Society of North America and the International Society for Sexual Medicine in Miami. The results have not been published in a peer-reviewed journal.
New direct-to-consumer telemedicine companies have emerged with gender-diverse staff and services tailored to the needs of these individuals. They offer “a more inclusive feel” than might be encountered at a physician’s office, Dr. Jesse said.
Confirming that these companies adhere to standards of care and cost-effectiveness “is especially important considering the reduced access to care and potentially increased vulnerability of the gender-diverse population,” she and her colleagues wrote.
From a Google search in March, the team identified six U.S.-based platforms that offer gender-affirming medical therapy: FOLX, True U Clinic, QueerDoc, Queer Med, TransClinique, and Plume.
From information posted on the companies’ websites, the researchers determined that all aligned with the World Professional Association for Transgender Health’s Standards of Care in two areas: use of an informed consent model to ensure that patients have sufficient information and understanding to decide on their own treatment and endorsement of frequent laboratory monitoring of hormone levels in early stages of treatment.
The team also compared the costs listed on the websites for the first year of therapy to the costs of similar care at a tertiary center, as determined using University Hospitals Cleveland Medical Center’s online estimator.
The platforms offered various pricing models, including fee-for-service and monthly membership plans ranging from $59 to $139. For individuals without insurance, estimates ranged from $1,022 to $1,428 for oral estradiol and from $1,184 to $1,668 for intramuscular testosterone from the online companies, compared with $1,184 and $1,216, respectively, at the tertiary center.
Although some platforms accept insurance, the researchers were not able to evaluate the cost of using private insurance or Medicaid, Dr. Jesse said. She noted that transgender individuals are more likely to lack insurance than are cisgender patients.
The team also assessed the scope of services. All companies offered legal help with changes to names and gender markers, such as “M” and “F.” Three or more companies offered preexposure prophylaxis to prevent HIV infection, treatment for erectile dysfunction, referrals for surgery, and medical letters of support for surgery.
Two offered puberty blockers, although the researchers were unable to determine the risk of adolescents obtaining treatment without proper assessments, because details of those services are not disclosed on websites, Dr. Jesse said.
An avenue of further research would be to interview patients to learn how platforms operate in practice and whether patients are properly assessed before treatment. “Those sorts of questions we can’t answer just by looking at the websites,” she said.
However, Charlotte Hoffman, JD, senior policy counsel for the National Center for Transgender Equality, an advocacy group, said she does not harbor concerns about patients being treated inappropriately simply because care is virtual. All clinicians who provide gender-affirming care face potential repercussions, such as malpractice lawsuits or state disciplinary action, if they veer from treatment guidelines, she said.
“I don’t necessarily take the premise that telehealth is inherently worse than in-person care as a given,” Ms. Hoffman said.
During the COVID-19 pandemic, Ms. Hoffman added, direct-to-consumer telemedicine has expanded access for individuals in rural areas, people with disabilities, and those who live in places where in-person providers of transgender care face public hostility, although individuals without the resources to pay may still be left out.
What might happen to that access if telemedicine restrictions that were loosened during the pandemic are reinstated is unclear, she said.
The researchers and Ms. Hoffman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More evidence for EBV’s role in MS
In 2022, two studies received quite a bit of attention. One showed that EBV seroconversion occurs in the years prior to MS diagnosis in virtually every patient, and that serum levels of the neuronal damage biomarker neurofilament light (NfL) rose following EBV infection. Another paper showed anti-EBNA (Epstein-Barr nuclear antigen) antibodies in the cerebrospinal fluid cross-react with the central nervous system antigen GlialCAM in some MS patients.
Based on those studies, “it’s tempting to speculate that primary EBV infection could be a trigger to the autoimmune process suspected for MS,” said Tilman Schneider-Hohendorf, PhD, during a presentation at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Dr. Schneider-Hohendorf, who is a postdoctoral fellow at the University of Münster, Germany, presented a new study that added more evidence that EBV may be a key player in MS pathogenesis. He and colleagues conducted a genetic analysis of patient T cells and found evidence that EBV viral activity may be occurring during MS.
A viral pathway to MS
Asked for comment, Bruce Cree, MD, PhD, said: “I think it is a very interesting one, because what we know about EBV is that it’s a risk factor for MS. So many studies performed over the last 20 years have shown a very strong association between EBV infection and the occurrence of MS. Studies have shown quite conclusively that EBV infection precedes MS in almost every patient, and that EBV infection is followed by a rise in serum NfL, which is a biomarker of neuronal damage. You have EBV infection, and then typically several years later a rise in serum concentrations of this marker of neuronal injury, and this is all in a presymptomatic state. Then that is followed by the onset of clinical symptoms in MS. That temporal sequence, I think, is very convincing,” said Dr. Cree, who is a professor of clinical neurology at the University of California, San Francisco.
He pointed out that EBV is not the sole causal pathway of MS, since genetic and environmental factors are known to be involved. “Nonetheless, it’s very strong evidence to indicate that this virus is involved in disease pathogenesis,” said Dr. Cree.
The new research takes the work a step further by revealing a population of T cells in MS patients that appear to be responding directly to EBV during active viral disease. That could be telling because most people who experience an EBV infection and experience mononucleosis recover, and some never even realize they have been infected. As a herpes virus, EBV remains in a latent state in B cells and other immune cells. “We know that you need an EBV infection (to trigger MS), but is EBV in some way continuing to be active in MS?” said Dr. Cree.
Other groups have looked for such evidence, but results have been mixed. Dr. Cree’s own group looked for evidence of EBV in spinal fluid of MS patients when they first present with symptoms, and could find no evidence. On the other hand, an autopsy study of MS patients has found evidence of chronic EBV infection in and around the brain, including the meninges, which could implicate the B cells found in that region. Another study found EBV-targeting antibodies that cross react with neuronal antigens in the cerebral spinal fluid of MS patients. “So depending on the assay used and the types of investigation, there is variable evidence to indicate that EBV has a role in ongoing MS pathogenesis – that it isn’t just a risk factor for MS that triggers the disease but potentially has a role in determining the course of MS,” said Dr. Cree.
IS EBV part of MS pathogenesis?
The new study presented at ECTRIMS by Dr. Schneider-Hohendorf offered evidence that MS patients have excess CD8-positive T cells that recognize EBV antigens typically shed during active viral infection. The results suggest “that the immune system is responding to that chronic infection,” said Dr. Cree.
The findings have some implications for a clinical study now in progress, called EMBOLD, which is looking at whether a heterologous infusion of T cells that have been primed to attack EBV could improve symptoms of progressive MS. “The hypothesis there is that chronically infected cells within the body are causing progressive MS and that if we could eradicate those cells, both within the central nervous system and within the periphery, perhaps we could see improvement in MS functional outcomes,” said Dr. Cree, who is a co-investigator for the EMBOLD study. The trial is using T cells from donors that are matched for the human leukocyte antigen complex, which is hoped will target and kill EBV-infected cells.
The study presented by Dr. Schneider-Hohendorf supports the approach. “There is an implication from this study that the trial that that’s currently being conducted might actually possibly have a benefit in the sense that there’s now another piece of evidence to indicate that EBV is not only a risk factor for MS, but may actually participate during the course of the disease as part of the pathogenesis,” said Dr. Cree.
In the new study, the researchers sequenced the T-cell receptor variable beta-chain (TRBV) peripheral repertoire among three cohorts of MS patients: A discovery cohort with 1,336 patients with MS and 229 controls; a validation cohort with 59 patients with MS and 51 controls; and 35 monozygotic twins who were discordant for MS. They identified sequences known to bind to EBV, SARS-CoV-2, cytomegalovirus, and influenza A, and used the latter three viruses as a proof of concept to demonstrate the validity of the approach. EBV-specific MHC-1 restricted CD8 TRBV in the serum of MS patients, with large effect sizes in the discovery (+2.2), validation (+2.1), and MS twin (+1.6) populations. The findings in the twin population rule out a genetic or environmental explanation for the findings in the discovery and validation cohorts, according to Dr. Schneider-Hohendorf.
The researchers also sequenced CSF among six healthy donors and five patients with MS and found significant differences. The T-cell populations had more lytic properties that suggested ongoing immune surveillance. “We can conclude that we found a broader response that could indicate an aberrant immune response. This could be a remnant of disease triggering an event or it could indicate an ongoing immune response to EBV. Is this EBV activity? We really don’t know. To find out, we would expand our pathogen-specific sequences, we would assess CNS tissue and lesions, and we would define the primary response in pediatric cohorts to better understand what might go wrong,” Dr. Schneider-Hohendorf concluded.
Dr. Cree has a financial relationship with Biogen and is a co-investigator for the EMBOLD trial. Dr. Schneider-Hohendorf has financial relationships with Biogen, Novartis, and Roche.
In 2022, two studies received quite a bit of attention. One showed that EBV seroconversion occurs in the years prior to MS diagnosis in virtually every patient, and that serum levels of the neuronal damage biomarker neurofilament light (NfL) rose following EBV infection. Another paper showed anti-EBNA (Epstein-Barr nuclear antigen) antibodies in the cerebrospinal fluid cross-react with the central nervous system antigen GlialCAM in some MS patients.
Based on those studies, “it’s tempting to speculate that primary EBV infection could be a trigger to the autoimmune process suspected for MS,” said Tilman Schneider-Hohendorf, PhD, during a presentation at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Dr. Schneider-Hohendorf, who is a postdoctoral fellow at the University of Münster, Germany, presented a new study that added more evidence that EBV may be a key player in MS pathogenesis. He and colleagues conducted a genetic analysis of patient T cells and found evidence that EBV viral activity may be occurring during MS.
A viral pathway to MS
Asked for comment, Bruce Cree, MD, PhD, said: “I think it is a very interesting one, because what we know about EBV is that it’s a risk factor for MS. So many studies performed over the last 20 years have shown a very strong association between EBV infection and the occurrence of MS. Studies have shown quite conclusively that EBV infection precedes MS in almost every patient, and that EBV infection is followed by a rise in serum NfL, which is a biomarker of neuronal damage. You have EBV infection, and then typically several years later a rise in serum concentrations of this marker of neuronal injury, and this is all in a presymptomatic state. Then that is followed by the onset of clinical symptoms in MS. That temporal sequence, I think, is very convincing,” said Dr. Cree, who is a professor of clinical neurology at the University of California, San Francisco.
He pointed out that EBV is not the sole causal pathway of MS, since genetic and environmental factors are known to be involved. “Nonetheless, it’s very strong evidence to indicate that this virus is involved in disease pathogenesis,” said Dr. Cree.
The new research takes the work a step further by revealing a population of T cells in MS patients that appear to be responding directly to EBV during active viral disease. That could be telling because most people who experience an EBV infection and experience mononucleosis recover, and some never even realize they have been infected. As a herpes virus, EBV remains in a latent state in B cells and other immune cells. “We know that you need an EBV infection (to trigger MS), but is EBV in some way continuing to be active in MS?” said Dr. Cree.
Other groups have looked for such evidence, but results have been mixed. Dr. Cree’s own group looked for evidence of EBV in spinal fluid of MS patients when they first present with symptoms, and could find no evidence. On the other hand, an autopsy study of MS patients has found evidence of chronic EBV infection in and around the brain, including the meninges, which could implicate the B cells found in that region. Another study found EBV-targeting antibodies that cross react with neuronal antigens in the cerebral spinal fluid of MS patients. “So depending on the assay used and the types of investigation, there is variable evidence to indicate that EBV has a role in ongoing MS pathogenesis – that it isn’t just a risk factor for MS that triggers the disease but potentially has a role in determining the course of MS,” said Dr. Cree.
IS EBV part of MS pathogenesis?
The new study presented at ECTRIMS by Dr. Schneider-Hohendorf offered evidence that MS patients have excess CD8-positive T cells that recognize EBV antigens typically shed during active viral infection. The results suggest “that the immune system is responding to that chronic infection,” said Dr. Cree.
The findings have some implications for a clinical study now in progress, called EMBOLD, which is looking at whether a heterologous infusion of T cells that have been primed to attack EBV could improve symptoms of progressive MS. “The hypothesis there is that chronically infected cells within the body are causing progressive MS and that if we could eradicate those cells, both within the central nervous system and within the periphery, perhaps we could see improvement in MS functional outcomes,” said Dr. Cree, who is a co-investigator for the EMBOLD study. The trial is using T cells from donors that are matched for the human leukocyte antigen complex, which is hoped will target and kill EBV-infected cells.
The study presented by Dr. Schneider-Hohendorf supports the approach. “There is an implication from this study that the trial that that’s currently being conducted might actually possibly have a benefit in the sense that there’s now another piece of evidence to indicate that EBV is not only a risk factor for MS, but may actually participate during the course of the disease as part of the pathogenesis,” said Dr. Cree.
In the new study, the researchers sequenced the T-cell receptor variable beta-chain (TRBV) peripheral repertoire among three cohorts of MS patients: A discovery cohort with 1,336 patients with MS and 229 controls; a validation cohort with 59 patients with MS and 51 controls; and 35 monozygotic twins who were discordant for MS. They identified sequences known to bind to EBV, SARS-CoV-2, cytomegalovirus, and influenza A, and used the latter three viruses as a proof of concept to demonstrate the validity of the approach. EBV-specific MHC-1 restricted CD8 TRBV in the serum of MS patients, with large effect sizes in the discovery (+2.2), validation (+2.1), and MS twin (+1.6) populations. The findings in the twin population rule out a genetic or environmental explanation for the findings in the discovery and validation cohorts, according to Dr. Schneider-Hohendorf.
The researchers also sequenced CSF among six healthy donors and five patients with MS and found significant differences. The T-cell populations had more lytic properties that suggested ongoing immune surveillance. “We can conclude that we found a broader response that could indicate an aberrant immune response. This could be a remnant of disease triggering an event or it could indicate an ongoing immune response to EBV. Is this EBV activity? We really don’t know. To find out, we would expand our pathogen-specific sequences, we would assess CNS tissue and lesions, and we would define the primary response in pediatric cohorts to better understand what might go wrong,” Dr. Schneider-Hohendorf concluded.
Dr. Cree has a financial relationship with Biogen and is a co-investigator for the EMBOLD trial. Dr. Schneider-Hohendorf has financial relationships with Biogen, Novartis, and Roche.
In 2022, two studies received quite a bit of attention. One showed that EBV seroconversion occurs in the years prior to MS diagnosis in virtually every patient, and that serum levels of the neuronal damage biomarker neurofilament light (NfL) rose following EBV infection. Another paper showed anti-EBNA (Epstein-Barr nuclear antigen) antibodies in the cerebrospinal fluid cross-react with the central nervous system antigen GlialCAM in some MS patients.
Based on those studies, “it’s tempting to speculate that primary EBV infection could be a trigger to the autoimmune process suspected for MS,” said Tilman Schneider-Hohendorf, PhD, during a presentation at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Dr. Schneider-Hohendorf, who is a postdoctoral fellow at the University of Münster, Germany, presented a new study that added more evidence that EBV may be a key player in MS pathogenesis. He and colleagues conducted a genetic analysis of patient T cells and found evidence that EBV viral activity may be occurring during MS.
A viral pathway to MS
Asked for comment, Bruce Cree, MD, PhD, said: “I think it is a very interesting one, because what we know about EBV is that it’s a risk factor for MS. So many studies performed over the last 20 years have shown a very strong association between EBV infection and the occurrence of MS. Studies have shown quite conclusively that EBV infection precedes MS in almost every patient, and that EBV infection is followed by a rise in serum NfL, which is a biomarker of neuronal damage. You have EBV infection, and then typically several years later a rise in serum concentrations of this marker of neuronal injury, and this is all in a presymptomatic state. Then that is followed by the onset of clinical symptoms in MS. That temporal sequence, I think, is very convincing,” said Dr. Cree, who is a professor of clinical neurology at the University of California, San Francisco.
He pointed out that EBV is not the sole causal pathway of MS, since genetic and environmental factors are known to be involved. “Nonetheless, it’s very strong evidence to indicate that this virus is involved in disease pathogenesis,” said Dr. Cree.
The new research takes the work a step further by revealing a population of T cells in MS patients that appear to be responding directly to EBV during active viral disease. That could be telling because most people who experience an EBV infection and experience mononucleosis recover, and some never even realize they have been infected. As a herpes virus, EBV remains in a latent state in B cells and other immune cells. “We know that you need an EBV infection (to trigger MS), but is EBV in some way continuing to be active in MS?” said Dr. Cree.
Other groups have looked for such evidence, but results have been mixed. Dr. Cree’s own group looked for evidence of EBV in spinal fluid of MS patients when they first present with symptoms, and could find no evidence. On the other hand, an autopsy study of MS patients has found evidence of chronic EBV infection in and around the brain, including the meninges, which could implicate the B cells found in that region. Another study found EBV-targeting antibodies that cross react with neuronal antigens in the cerebral spinal fluid of MS patients. “So depending on the assay used and the types of investigation, there is variable evidence to indicate that EBV has a role in ongoing MS pathogenesis – that it isn’t just a risk factor for MS that triggers the disease but potentially has a role in determining the course of MS,” said Dr. Cree.
IS EBV part of MS pathogenesis?
The new study presented at ECTRIMS by Dr. Schneider-Hohendorf offered evidence that MS patients have excess CD8-positive T cells that recognize EBV antigens typically shed during active viral infection. The results suggest “that the immune system is responding to that chronic infection,” said Dr. Cree.
The findings have some implications for a clinical study now in progress, called EMBOLD, which is looking at whether a heterologous infusion of T cells that have been primed to attack EBV could improve symptoms of progressive MS. “The hypothesis there is that chronically infected cells within the body are causing progressive MS and that if we could eradicate those cells, both within the central nervous system and within the periphery, perhaps we could see improvement in MS functional outcomes,” said Dr. Cree, who is a co-investigator for the EMBOLD study. The trial is using T cells from donors that are matched for the human leukocyte antigen complex, which is hoped will target and kill EBV-infected cells.
The study presented by Dr. Schneider-Hohendorf supports the approach. “There is an implication from this study that the trial that that’s currently being conducted might actually possibly have a benefit in the sense that there’s now another piece of evidence to indicate that EBV is not only a risk factor for MS, but may actually participate during the course of the disease as part of the pathogenesis,” said Dr. Cree.
In the new study, the researchers sequenced the T-cell receptor variable beta-chain (TRBV) peripheral repertoire among three cohorts of MS patients: A discovery cohort with 1,336 patients with MS and 229 controls; a validation cohort with 59 patients with MS and 51 controls; and 35 monozygotic twins who were discordant for MS. They identified sequences known to bind to EBV, SARS-CoV-2, cytomegalovirus, and influenza A, and used the latter three viruses as a proof of concept to demonstrate the validity of the approach. EBV-specific MHC-1 restricted CD8 TRBV in the serum of MS patients, with large effect sizes in the discovery (+2.2), validation (+2.1), and MS twin (+1.6) populations. The findings in the twin population rule out a genetic or environmental explanation for the findings in the discovery and validation cohorts, according to Dr. Schneider-Hohendorf.
The researchers also sequenced CSF among six healthy donors and five patients with MS and found significant differences. The T-cell populations had more lytic properties that suggested ongoing immune surveillance. “We can conclude that we found a broader response that could indicate an aberrant immune response. This could be a remnant of disease triggering an event or it could indicate an ongoing immune response to EBV. Is this EBV activity? We really don’t know. To find out, we would expand our pathogen-specific sequences, we would assess CNS tissue and lesions, and we would define the primary response in pediatric cohorts to better understand what might go wrong,” Dr. Schneider-Hohendorf concluded.
Dr. Cree has a financial relationship with Biogen and is a co-investigator for the EMBOLD trial. Dr. Schneider-Hohendorf has financial relationships with Biogen, Novartis, and Roche.
FROM ECTRIMS 2022
Academic dermatology: Gender diversity advances as some gaps persist
, according to a recent cross-sectional study.
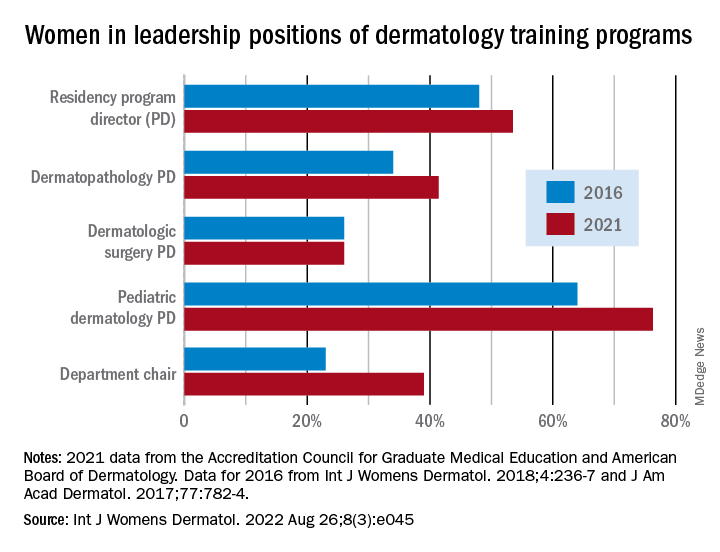
Although women made up more than half of the dermatology residency program directors (53.5%), associate directors (62.6%), and assistant directors (58.3%) in 2021, those numbers fall short of women’s majority (65% in 2018) among the trainees themselves, Yasmine Abushukur of Oakland University in Rochester, Mich., and associates said in a research letter.
Advancements were “made in gender diversity within academic dermatology from 2016 to 2021, [but] women remain underrepresented, particularly in leadership of dermatopathology and dermatologic surgery fellowships,” the investigators wrote.
Data gathered from 142 dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education show that progress has been made since 2016, at least among program directors (PDs), of whom 48% were women, according to a previous study. Data on associate and assistant PDs from 2016 were not available to Ms. Abushukur and associates.
At the fellowship program level, women made gains as PDs in dermatopathology (34% in 2016 and 41% in 2021) and pediatric dermatology (64% in 2016 and 76% in 2021), but not in dermatologic surgery, where the proportion held at 26% over the study period. “This disparity is reflective of the general trend in surgery and pathology leadership nationally,” the researchers noted.
Taking a couple of steps up the ladder of authority shows that 39% of dermatology chairs were women in 2021, compared with 23% in 2016. A study published in 2016 demonstrated decreased diversity among academic faculty members as faculty rank increased, and “our data mirror this sentiment by demonstrating a majority of women in assistant and associate PD positions, with a minority of women chairs,” they wrote.
The investigators said that they had no conflicts of interest and no outside funding. Ms. Abushukur’s coauthors were from the departments of dermatology at the Henry Ford Health System, Detroit, and Wayne State University, Dearborn, Mich.
, according to a recent cross-sectional study.

Although women made up more than half of the dermatology residency program directors (53.5%), associate directors (62.6%), and assistant directors (58.3%) in 2021, those numbers fall short of women’s majority (65% in 2018) among the trainees themselves, Yasmine Abushukur of Oakland University in Rochester, Mich., and associates said in a research letter.
Advancements were “made in gender diversity within academic dermatology from 2016 to 2021, [but] women remain underrepresented, particularly in leadership of dermatopathology and dermatologic surgery fellowships,” the investigators wrote.
Data gathered from 142 dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education show that progress has been made since 2016, at least among program directors (PDs), of whom 48% were women, according to a previous study. Data on associate and assistant PDs from 2016 were not available to Ms. Abushukur and associates.
At the fellowship program level, women made gains as PDs in dermatopathology (34% in 2016 and 41% in 2021) and pediatric dermatology (64% in 2016 and 76% in 2021), but not in dermatologic surgery, where the proportion held at 26% over the study period. “This disparity is reflective of the general trend in surgery and pathology leadership nationally,” the researchers noted.
Taking a couple of steps up the ladder of authority shows that 39% of dermatology chairs were women in 2021, compared with 23% in 2016. A study published in 2016 demonstrated decreased diversity among academic faculty members as faculty rank increased, and “our data mirror this sentiment by demonstrating a majority of women in assistant and associate PD positions, with a minority of women chairs,” they wrote.
The investigators said that they had no conflicts of interest and no outside funding. Ms. Abushukur’s coauthors were from the departments of dermatology at the Henry Ford Health System, Detroit, and Wayne State University, Dearborn, Mich.
, according to a recent cross-sectional study.

Although women made up more than half of the dermatology residency program directors (53.5%), associate directors (62.6%), and assistant directors (58.3%) in 2021, those numbers fall short of women’s majority (65% in 2018) among the trainees themselves, Yasmine Abushukur of Oakland University in Rochester, Mich., and associates said in a research letter.
Advancements were “made in gender diversity within academic dermatology from 2016 to 2021, [but] women remain underrepresented, particularly in leadership of dermatopathology and dermatologic surgery fellowships,” the investigators wrote.
Data gathered from 142 dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education show that progress has been made since 2016, at least among program directors (PDs), of whom 48% were women, according to a previous study. Data on associate and assistant PDs from 2016 were not available to Ms. Abushukur and associates.
At the fellowship program level, women made gains as PDs in dermatopathology (34% in 2016 and 41% in 2021) and pediatric dermatology (64% in 2016 and 76% in 2021), but not in dermatologic surgery, where the proportion held at 26% over the study period. “This disparity is reflective of the general trend in surgery and pathology leadership nationally,” the researchers noted.
Taking a couple of steps up the ladder of authority shows that 39% of dermatology chairs were women in 2021, compared with 23% in 2016. A study published in 2016 demonstrated decreased diversity among academic faculty members as faculty rank increased, and “our data mirror this sentiment by demonstrating a majority of women in assistant and associate PD positions, with a minority of women chairs,” they wrote.
The investigators said that they had no conflicts of interest and no outside funding. Ms. Abushukur’s coauthors were from the departments of dermatology at the Henry Ford Health System, Detroit, and Wayne State University, Dearborn, Mich.
FROM INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY







