User login
Endometrial receptivity testing before IVF seen as unnecessary
Endometrial receptivity testing (ERT) did not increase the chances of achieving live birth in patients undergoing in vitro fertilization.
The study was promoted by widespread use of the tests in reproductive medicine and earlier conflicting studies regarding its effectiveness. The procedure, in which doctors extract cells from a woman’s endometrial lining in an effort to determine the best day to perform in vitro fertilization, requires a biopsy, can take a month to generate results, and costs up to $1,000.
But the new study, published online December 6 in the Journal of the American Medical Association, found that embryo transfer based on the timing of ERT was no better than that based on the standard protocol.
“Endometrial receptivity testing ended up not being beneficial in the population of interest, a good-prognosis IVF patient population,” said Nicole Doyle, MD, PhD, of Shady Grove Fertility, in Arlington, Va., who led the study. “For this particular patient population I would not recommend ERT based on the results of the trial.”
“Unfortunately, as is our history in reproductive medicine, we may embrace technology prematurely given patient desperation and physician eagerness to improve pregnancy outcomes,” said Mark P. Trolice, MD, director of the IVF Center in Orlando, who was not involved in the new research.
The double-blind, randomized clinical trial enrolled 726 women treated at Dr. Doyle’s clinic between May 2018 and September 2020.
All the women underwent ERT. Of those who received adjusted progesterone exposure after the test, live birth occurred in 58.5% of transfers (223 of 381). Among those in a control group who did not have their progesterone adjusted after ERT and underwent IVF on a standardized schedule, 61.9% of transfers (239 of 386) resulted in live birth, according to the researchers.
The differences in rates of clinical (77.2% vs. 79.5% [95% confidence interval, −10.4% to 2.4%]) and biochemical pregnancy (68.8% vs. 72.8% [95% CI, −8.2% to 3.5%]) were not statistically significant between the two groups, Dr. Doyle and her colleagues reported.
Women who experienced recurrent implantation failure (RIF), defined as more than two failed embryo transfers, were excluded from the study. “We can’t assess the benefit of an endometrial receptivity testing in this particular patient population,” Dr. Doyle said.
However, she noted that the number of women who undergo RIF is “a very small fraction of all IVF patients, less than 5%.” Of those, half are expected to have embryos that are not suitable for implantation, Dr. Doyle said.
As a result, she said, “it’s really only about 2.5% of IVF patients for which we don’t yet have an answer regarding the utility of ERT.”
Dr. Trolice, also a professor at the University of Central Florida, Orlando, expressed certainty that the “one-size-fits-all approach” for ERT has been disproven by the study’s failure to find a benefit from the procedure in women with a “good prognosis.” But, he added, whether ERT is of value in a subset of patients, such as those with recurrent implantation failure, remains “a question of vital importance.”
Dr. Doyle and Dr. Trolice reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Endometrial receptivity testing (ERT) did not increase the chances of achieving live birth in patients undergoing in vitro fertilization.
The study was promoted by widespread use of the tests in reproductive medicine and earlier conflicting studies regarding its effectiveness. The procedure, in which doctors extract cells from a woman’s endometrial lining in an effort to determine the best day to perform in vitro fertilization, requires a biopsy, can take a month to generate results, and costs up to $1,000.
But the new study, published online December 6 in the Journal of the American Medical Association, found that embryo transfer based on the timing of ERT was no better than that based on the standard protocol.
“Endometrial receptivity testing ended up not being beneficial in the population of interest, a good-prognosis IVF patient population,” said Nicole Doyle, MD, PhD, of Shady Grove Fertility, in Arlington, Va., who led the study. “For this particular patient population I would not recommend ERT based on the results of the trial.”
“Unfortunately, as is our history in reproductive medicine, we may embrace technology prematurely given patient desperation and physician eagerness to improve pregnancy outcomes,” said Mark P. Trolice, MD, director of the IVF Center in Orlando, who was not involved in the new research.
The double-blind, randomized clinical trial enrolled 726 women treated at Dr. Doyle’s clinic between May 2018 and September 2020.
All the women underwent ERT. Of those who received adjusted progesterone exposure after the test, live birth occurred in 58.5% of transfers (223 of 381). Among those in a control group who did not have their progesterone adjusted after ERT and underwent IVF on a standardized schedule, 61.9% of transfers (239 of 386) resulted in live birth, according to the researchers.
The differences in rates of clinical (77.2% vs. 79.5% [95% confidence interval, −10.4% to 2.4%]) and biochemical pregnancy (68.8% vs. 72.8% [95% CI, −8.2% to 3.5%]) were not statistically significant between the two groups, Dr. Doyle and her colleagues reported.
Women who experienced recurrent implantation failure (RIF), defined as more than two failed embryo transfers, were excluded from the study. “We can’t assess the benefit of an endometrial receptivity testing in this particular patient population,” Dr. Doyle said.
However, she noted that the number of women who undergo RIF is “a very small fraction of all IVF patients, less than 5%.” Of those, half are expected to have embryos that are not suitable for implantation, Dr. Doyle said.
As a result, she said, “it’s really only about 2.5% of IVF patients for which we don’t yet have an answer regarding the utility of ERT.”
Dr. Trolice, also a professor at the University of Central Florida, Orlando, expressed certainty that the “one-size-fits-all approach” for ERT has been disproven by the study’s failure to find a benefit from the procedure in women with a “good prognosis.” But, he added, whether ERT is of value in a subset of patients, such as those with recurrent implantation failure, remains “a question of vital importance.”
Dr. Doyle and Dr. Trolice reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Endometrial receptivity testing (ERT) did not increase the chances of achieving live birth in patients undergoing in vitro fertilization.
The study was promoted by widespread use of the tests in reproductive medicine and earlier conflicting studies regarding its effectiveness. The procedure, in which doctors extract cells from a woman’s endometrial lining in an effort to determine the best day to perform in vitro fertilization, requires a biopsy, can take a month to generate results, and costs up to $1,000.
But the new study, published online December 6 in the Journal of the American Medical Association, found that embryo transfer based on the timing of ERT was no better than that based on the standard protocol.
“Endometrial receptivity testing ended up not being beneficial in the population of interest, a good-prognosis IVF patient population,” said Nicole Doyle, MD, PhD, of Shady Grove Fertility, in Arlington, Va., who led the study. “For this particular patient population I would not recommend ERT based on the results of the trial.”
“Unfortunately, as is our history in reproductive medicine, we may embrace technology prematurely given patient desperation and physician eagerness to improve pregnancy outcomes,” said Mark P. Trolice, MD, director of the IVF Center in Orlando, who was not involved in the new research.
The double-blind, randomized clinical trial enrolled 726 women treated at Dr. Doyle’s clinic between May 2018 and September 2020.
All the women underwent ERT. Of those who received adjusted progesterone exposure after the test, live birth occurred in 58.5% of transfers (223 of 381). Among those in a control group who did not have their progesterone adjusted after ERT and underwent IVF on a standardized schedule, 61.9% of transfers (239 of 386) resulted in live birth, according to the researchers.
The differences in rates of clinical (77.2% vs. 79.5% [95% confidence interval, −10.4% to 2.4%]) and biochemical pregnancy (68.8% vs. 72.8% [95% CI, −8.2% to 3.5%]) were not statistically significant between the two groups, Dr. Doyle and her colleagues reported.
Women who experienced recurrent implantation failure (RIF), defined as more than two failed embryo transfers, were excluded from the study. “We can’t assess the benefit of an endometrial receptivity testing in this particular patient population,” Dr. Doyle said.
However, she noted that the number of women who undergo RIF is “a very small fraction of all IVF patients, less than 5%.” Of those, half are expected to have embryos that are not suitable for implantation, Dr. Doyle said.
As a result, she said, “it’s really only about 2.5% of IVF patients for which we don’t yet have an answer regarding the utility of ERT.”
Dr. Trolice, also a professor at the University of Central Florida, Orlando, expressed certainty that the “one-size-fits-all approach” for ERT has been disproven by the study’s failure to find a benefit from the procedure in women with a “good prognosis.” But, he added, whether ERT is of value in a subset of patients, such as those with recurrent implantation failure, remains “a question of vital importance.”
Dr. Doyle and Dr. Trolice reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Improving Patient Access to the My HealtheVet Electronic Patient Portal for Veterans
Patient portals are secure online website tools that provide patient access to personal health information (PHI). Access to online PHI improves health equity and satisfies the meaningful use objectives of the Medicare electronic health record (EHR) incentive program.1,2 Through patient portals, individuals can access PHI records and current diagnoses, request and reschedule appointments, locate test results, track trends for vital signs and laboratory values, refill medications, and communicate directly with the health care team through secure messaging. This alternative method of communication with the team is associated with increased patient satisfaction.3 Patients reported improved patient engagement in health care self-management and decision making, as well as strengthened relationships with their health care team.4
Background
One well-documented strategy to improve portal use includes the development of a nurse champion to facilitate enrollment during the clinic visit.5 Patient perceptions of portal value increased after education by a health care professional (HCP) and assistance in enrollment to familiarize patients with the platform for ongoing use.5 Use of patient portals has been associated with favorable outcomes in chronic disease management. Patients with diabetes mellitus who regularly use patient portals for prescription refills and secure messaging have demonstrated improved glycemic control, medication adherence, and associated health parameters compared with nonusers.5-7 In patients with congestive heart failure, meaningful patient portal use results in fewer emergency department visits, fewer hospital admissions, lower readmission rates, and reduced unscheduled and no-show visits.8-11
Patient portal access is a quality improvement (QI) measure that meets Medicare and Medicaid meaningful use requirements that is designed to improve collaboration between HCPs and patients through EHRs. Despite legislation, uptake of patient portal access has been slow, especially among older adults.10,12,13 Barriers to patient portal registration and use include patient lack of awareness, perceived or actual digital illiteracy, mistrust in privacy precautions, lack of user-friendly interfaces, lack of internet or technology, HCP bias and workload, and misperceptions of usefulness.9,10,12,14 The HCPs most likely to facilitate the use of patient portals, typically include nurse practitioners (NPs), nurses, and medical residents.10,15 Patient portal platforms promote the partnership of these disciplines with the veteran to help the patient better manage their health. Despite the benefits and widespread integration of patient portals in health care systems, socioeconomic inequalities and HCP attitudes contribute to persistent disparities in its adoption by underserved populations. The veteran population is often faced with additional barriers to health care access with regard to geographic location, advanced age, trauma, disabilities, mental health challenges, and homelessness.10,16 These barriers require unique approaches to maximize the use of technologic advances.17 Advanced age contributes to low rates of patient portal enrollment and lack of digital platform use, thus creating a digital divide.11,12
The digital divide is described as the gap between those persons who use technology including computers and internet, and those persons who do not because of social and geographic barriers.16 It contributes to a growing health disparity in both access to care and quality of care especially for rural veterans. About 25% of the US population lacks fixed broadband at home; these individuals are more likely to be racial minorities, older, widowed, or to have lower levels of education.18,19 Veterans are disproportionately represented in these demographic categories.20 According to the US Department of Veterans Affairs (VA) Office of Rural Health, the percentage of rural veterans enrolled in the VA health care system (58%) is significantly higher than enrollment of urban veterans (38%); additionally, 27% of rural veterans do not access the internet at home.21
My HealtheVet
The VA plays an integral part in increasing virtual access to care, from the introduction of My Healthevet (MHV) in 2003 to the distribution of iPad tablets to vulnerable veterans during the COVID-19 pandemic.22,23 Due to COVID-19, the need for VA patient access to the internet and VA Video-Connect (VVC) telehealth services increased significantly.22 Access to internet and hardware supporting use of VVC and MHV has been facilitated by the Digital Divide Consult, a VA program launched in 2020 to increase access to telehealth services.24 The VA has distributed > 26,000 cellular-enabled tablets and provided > 50,000 veterans with connectivity in collaboration with various private sector companies.22 Patients report that MHV facilitates engagement in health care through improved access to EHRs and expedited communication with the health care team.4
MHV is a secure online tool that provides patients access to PHI. MHV aims to empower veterans to take charge of their health by improving communication with HCPs, setting patient goals, and offering health and well-being resources.25,26 In a study of outpatients at a large urban multisite health care system, < 35% of patients on 16 medical resident panels were enrolled in a patient portal.15 MHV internal national metrics show increasing registration and active users of the patient portal, yet locally, disparities in the use of the portal by rural and older veterans exist.
The Local Problem
A review of the registration process at a rural VA clinic revealed barriers to facilitating the veteran registration process at the point of care. Clinical reminders exist within the EHR to prompt clinicians at the point of care to improve quality of care. At the New England Healthcare System (Veterans Integrated Service Network [VISN] 1), a patient portal clinical reminder prompts staff to encourage veterans to register. Anecdotal data obtained from primary care staff interviews at a rural VA primary care clinic in Vermont revealed low clinician confidence in completing the clinical reminder, a lack of knowledge of MHV, and lack of time to educate veterans about the benefits of MHV.
Despite availability of a registration process at the point of care and clinical staff assigned to provide registration information to the veteran, access to the patient portal among veterans at this clinic remained low. This QI project aimed to increase patient portal enrollment of veterans in MHV in a single NP patient panel of 100 patients from a baseline of 33% by 10% in a 3-month time frame.
Implementation
Before implementing the first Plan-Do-Study-Act (PDSA) cycle, we established the baseline data for 1 patient panel to be 33%. A retrospective review of the NP resident’s panel of 100 revealed 33 veterans were enrolled in MHV, providing a setting for process improvement. Evaluation of potential enrollment data for the panel population revealed unenrolled veterans were primarily aged ≥ 65 years. A rapid cycle QI (RCQI) strategy using the PDSA method was used to identify, implement, and measure changes over a 3-month time frame in 1 NP patient panel.14
The RCQI process included establishing baseline data and 3 PDSA cycles that evaluated the current state of patient access to the electronic patient portal, elucidated patient barriers to registration, assessed the processes for point-of-care enrollment, and developed strategies to improve the process and increase veteran enrollment. The QI project team included an NP resident as the project manager and MHV champion, a clinical faculty mentor at the site, a telehealth coordinator, an MHV coordinator, clinic registered nurse (RN), and clinic licensed nursing assistant (LNA). The RN and LNA additionally served as MHV champions as the project progressed.
PDSA Cycles
The objective for PDSA cycle 1 was to evaluate the process of patient registration and assess the impact on NP workload and clinic workflow over a 4-week period to improve veteran enrollment. Data were collected in a spreadsheet to track the number of veterans enrolled, time frame to enroll, and field notes that the NP resident recorded about the experience. The NP resident was trained in registration methods by the MHV coordinator. Several barriers to the registration process were identified: The process resulted in a change of the clinic visit closure focus, the clinic room was blocked for use by another patient, veterans had difficulty generating a unique username and password, veterans were unfamiliar with basic tablet accessibility and use, and additional time was required if incorrect information was entered. The veterans displayed low confidence in using tablet technology and were unaware of the patient portal or its usefulness. After discussion of the process with the project team, recommendations were made to address challenges, including an RN-led registration process. The first PDSA cycle increased the total patient panel enrollment by 4 veterans to 37%.
In PDSA cycle 2 after the NP visit, patients who agreed to register for the MHV portal were introduced to the tablet. The registration process was completed by the patient with the RN prior to the patient checkout. Once patient registration was completed, the veteran met the MHV coordinator and upgraded to a premium account, which provided full access to portal features. Electronic messaging was tested by the MHV coordinator and veteran to validate patient understanding. Although preloading demographic information improved accessibility issues, time was still required for the RN to orient the veteran to the tablet, provide additional directions, and answer questions.
The registration process reduced NP time commitment but added to the RN time burden and disrupted workflow; and clinic room access continued to be an issue. The wait time for the veteran to register in the clinic remained dependent on the availability of the RN. The decision was to move the registration process to the initial patient rooming assignment in the clinic and was transitioned from RN to LNA, prior to the NP-veteran encounter. Four additional veterans registered in the second PDSA cycle, and total enrollment increased to 41%, an overall 8% increase from baseline.
In the third PDSA cycle the patient enrollment process was managed by the clinic LNA using scripted information about MHV prior to the veteran encounter. A partially preloaded tablet was offered to the veteran to register with MHV during the rooming process, and written and oral instruction were provided to the veteran. The time required for each veteran to register for MHV averaged 10 minutes, and the veteran was able to register while waiting for the NP to enter the room. Typical LNA tasks included greeting patients, updating health records, completing clinical reminders with the veteran, obtaining vital signs, and addressing questions. The LNA introduced the veteran to MHV using scripted information and supported them in registering for MHV prior to the NP-veteran encounter. Registration at point of care during the rooming process was well received by both the LNA and veterans. The LNA reported the process was efficient and did not add excessive time to the LNA workflow. The LNA reported verbal patient satisfaction and registration was facilitated for 6 veterans during the 4-week period.
Registration during point of care was reported as feasible and sustainable by the LNA. Upgrading the patient to a premium MHV account was transitioned to the MHV coordinator. All veterans seen during the 4-week period were approached about registration; if the veteran declined, written at-home step-by-step instructions were provided. A replacement electronic clinical reminder was proposed to the VISN clinical reminders team for review and was pilot tested by the primary care clinical team. The third PDSA cycle increased the total patient panel enrollment to 47%, an overall 14% increase from baseline. Six new veteran users were added during PDSA cycle 3.
Discussion
The project team successfully used a RCQI method with a PDSA strategy to improve patient access to the MHV portal and increased veteran enrollment by 14% on 1 NP resident patient panel. The project evaluated clinic workflow regarding veteran patient portal registration, uncovered inefficiencies, and developed improved processes to increase veteran access to the patient portal. Results were positively impacted through the recognition of inefficiencies and initiation of new processes to engage veterans in the portal registration process. Familiarizing the entire clinical team with the clinical reminder and registration process raised the awareness of a digital divide consult and the utility of the portal in patient care. The project provided an opportunity to evaluate veterans’ digital literacy, digital access to send and receive messages, and to provide coaching as needed. Sequential PDSA cycles employed audit and feedback, information preloading, multimodal teaching strategies (verbal, print, hands-on tablet learning), scripting, staff interviews, time studies, and workflow evaluation to improve processes. An MHV champion led the team, monitored the progress, set deadlines, and effectively communicated project performance.
Limitations
Project limitations included the single-site location, its small sample size, and the short 3-month implementation time frame. The patient panel was representative of other NP resident patient panels at the facility but may not be representative of other VA facilities.
Ethical Considerations
Patient confidentiality was maintained throughout the registration and data collection process. The project team (NP, RN, LNA) received training and written instructions on protection of patient confidentiality by the MHV coordinator prior to assisting veterans with the registration process. Privacy was maintained, no patient identifiers were collected or viewed, and no assistance was provided for username, password, or security questions. The tablet was password protected and secured, used only by the project team when veteran was interested in point-of-care portal registration.
Sustainability
QI projects require ongoing systemic efforts to enhance sustainability.26,27 The project team used the PDSA methodology to stimulate the design of new workflow processes to engage staff and veterans in portal registration. Several actions were taken to promote sustainability for veteran portal registration and improve access to health care for rural and underserved veterans. First, printed instructions and website link are available in the clinic intake and examination rooms. Staff are equipped with patient education discussion points about the portal. A tablet is available in the clinic to encourage veterans to sign up. A clinical reminder is in place to encourage portal registration. A designated super-user is available to help new patient portal users register and navigate the system. Outcomes of the QI project were presented at 2 separate VISN 1 nursing grand rounds and reported to the MHV coordinator and telehealth coordinator to promote dialogue among staff and raise awareness of challenges to veteran MHV access.
Conclusions
Reviewing patient portal registration processes at the local level is essential to improve veteran access. This QI project proposed a realistic and scalable solution to implementing and improving patient enrollment to MHV in primary care clinics. Integrating measurement of patient registration into the daily routine of the clinic empowers the entire clinical team to improve the quality of access to patient portal.
The project team worked together to accomplish a shared goal, using errors as opportunities to improve the process, while using available staff without compromising significant time or resources. Engaging the entire team to audit processes and designating one member of the team as an MHV champion to provide feedback is critical to the sustainability of point-of-care registration in the MHV patient portal. Multifaceted approaches to maximizing the use of technology lessens the digital divide for veterans who are faced with geographical and social barriers to health care access.
Acknowledgments
We thank the Office of Academic Affiliations and the US Department of Veterans Affairs Nursing Academic Partnerships in Graduate Education Nurse Practitioner residency program and clinical faculty and the affiliated University of Vermont faculty mentor/quality improvement coach for the support of the project.
1. Centers for Medicare and Medicaid Services. Promoting interoperability programs. Updated October 6, 2022. Accessed November 3, 2022. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms
2. American Hospital Association. Goals of the Medicare and Medicaid electronic health records programs. Accessed November 3, 2022. https://www.aha.org/websites/2009-12-11-goals-medicare-and-medicaid-electronic-health-records-programs
3. Rozenblum R, Donzé J, Hockey PM, et al. The impact of medical informatics on patient satisfaction: a USA-based literature review. Int J Med Inform. 2013;82(3):141-158. doi:10.1016/j.ijmedinf.2012.12.008
4. Stewart MT, Hogan TP, Nicklas J, et al. The promise of patient portals for individuals living with chronic illness: qualitative study identifying pathways of patient engagement. J Med Internet Res. 2020;22(7):e17744. Published 2020 Jul 17. doi:10.2196/17744
5. Harris LT, Haneuse SJ, Martin DP, Ralston JD. Diabetes quality of care and outpatient utilization associated with electronic patient-provider messaging: a cross-sectional analysis. Diabetes Care. 2009;32(7):1182-1187. doi:10.2337/dc08-1771
6. Robinson SA, Zocchi MS, Netherton D, et al. Secure messaging, diabetes self-management, and the importance of patient autonomy: a mixed methods study. J Gen Intern Med. 2020;35(10):2955-2962. doi:10.1007/s11606-020-05834-x
7. Zocchi MS, Robinson SA, Ash AS, et al. Patient portal engagement and diabetes management among new portal users in the Veterans Health Administration. J Am Med Inform Assoc. 2021;28(10):2176-2183. doi:10.1093/jamia/ocab115
8. Bao C, Bardhan IR, Singh H, Meyer BA, Kirksey K. Patient-provider engagement and its impact on health outcomes: a longitudinal study of patient portal use. MIS Quarterly. 2020;44(2):699-723. doi:10.25300/MISQ/2020/14180
9. Grossman LV, Masterson Creber RM, Benda NC, Wright D, Vawdrey DK, Ancker JS. Interventions to increase patient portal use in vulnerable populations: a systematic review. J Am Med Informs Assoc. 2019;26(8-9):855-870. doi:10.1093/jamia/ocz023
10. Zhao JY, Song B, Anand E, et al. Barriers, facilitators, and solutions to optimal patient portal and personal health record use: a systematic review of the literature. AMIA Annu Symp Proc. 2018;2017:1913-1922. Published 2018 Apr 16.
11. Zhong X, Park J, Liang M, et al. Characteristics of patients using different patient portal functions and the impact on primary care service utilization and appointment adherence: retrospective observational study. J Med Internet Res. 2020;22(2):e14410. Published 2020 Feb 25. doi:10.2196/14410
12. Krishnaswami A, Beavers C, Dorsch MP, et al. Gerotechnology for older adults with cardiovascular diseases. J Am Coll Cardiol. 2020;76(22):2650-2670. doi:10.1016/j.jacc.2020.09.606
13. Fix GM, Hogan TP, Amante DJ, McInnes DK, Nazi KM, Simon SR. Encouraging patient portal use in the patient-centered medical home: three stakeholder perspectives. J Med Internet Res. 2016;18(11):e308. Published 2016 Nov 22. doi:10.2196/jmir.6488
14. Ancker JS, Nosal S, Hauser D, Way C, Calman N. Access policy and the digital divide in patient access to medical records. Health Policy Technol. 2016;6(3-11). doi:10.1016/j.hlpt.2016.11.004
15. Rhudy C, Broxterman J, Stewart S, et al. Improving patient portal enrolment in an academic resident continuity clinic: quality improvement made simple. BMJ Open Qual. 2019;8(2):e000430. Published 2019 Apr 25. doi:10.1136/bmjoq-2018-000430
16. Kontos E, Blake KD, Chou WY, Prestin A. Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res. 2014;16(7):e172. Published 2014 Jul 16. doi:10.2196/jmir.3117
17. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice. The state of health disparities in the United States. In: Baciu A, Negussie Y, Geller A, et al, eds. Communities in Action: Pathways to Health Equity. National Academies Press (US); January 11, 2017. Accessed November 3, 2022. https://www.ncbi.nlm.nih.gov/books/NBK425848/
18. Pew Research Center. Internet/broadband fact sheet. Updated April 7, 2021. Accessed November 3, 2022. https://www.pewresearch.org/internet/fact-sheet/internet-broadband
19. Roberts ET, Mehrotra A. Assessment of disparities in digital access among Medicare beneficiaries and implications for telemedicine. JAMA Intern Med. 2020;180(10):1386-1389. doi:10.1001/jamainternmed.2020.2666
20. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed November 3, 2022. https://www.va.gov/vetdata/veteran_population.asp
21. US Department of Veterans Affairs, Office of Rural Health. Rural veterans health care challenges. Updated March 31, 2022. Accessed November 3, 2022. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
22. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands veteran access to telehealth with iPad services. Press release. September 15, 2020. Accessed November 3, 2022. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5521
23. Zulman DM, Wong EP, Slightam C, et al. Making connections: National implementation of video telehealth tablets to address access barriers in veterans. JAMIA Open. 2019;2(3):323-329. doi:10.1093/jamiaopen/ooz024
24. Malone NC, Williams MM, Smith Fawzi MC, et al. Mobile health clinics in the United States. Int J Equity Health. 2020;19(1):40. doi:10.1186/s12939-020-1135-7
25. US Department of Veterans Affairs. How to use My HealtheVet. Accessed November 3, 2022. https://www.myhealth.va.gov/mhv-portal-web/how-to-use-mhv
26. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Whole health for life. 2017. Accessed November 3, 2022. https://www.va.gov/wholehealth/docs/2017-AR-Vet-Facing_FNL-W508.pdf27. Mortimer F, Isherwood J, Wilkinson A, Vaux E. Sustainability in quality improvement: redefining value. Future Healthc J. 2018;5(2):88-93. doi:10.7861/futurehosp.5-2-88
Patient portals are secure online website tools that provide patient access to personal health information (PHI). Access to online PHI improves health equity and satisfies the meaningful use objectives of the Medicare electronic health record (EHR) incentive program.1,2 Through patient portals, individuals can access PHI records and current diagnoses, request and reschedule appointments, locate test results, track trends for vital signs and laboratory values, refill medications, and communicate directly with the health care team through secure messaging. This alternative method of communication with the team is associated with increased patient satisfaction.3 Patients reported improved patient engagement in health care self-management and decision making, as well as strengthened relationships with their health care team.4
Background
One well-documented strategy to improve portal use includes the development of a nurse champion to facilitate enrollment during the clinic visit.5 Patient perceptions of portal value increased after education by a health care professional (HCP) and assistance in enrollment to familiarize patients with the platform for ongoing use.5 Use of patient portals has been associated with favorable outcomes in chronic disease management. Patients with diabetes mellitus who regularly use patient portals for prescription refills and secure messaging have demonstrated improved glycemic control, medication adherence, and associated health parameters compared with nonusers.5-7 In patients with congestive heart failure, meaningful patient portal use results in fewer emergency department visits, fewer hospital admissions, lower readmission rates, and reduced unscheduled and no-show visits.8-11
Patient portal access is a quality improvement (QI) measure that meets Medicare and Medicaid meaningful use requirements that is designed to improve collaboration between HCPs and patients through EHRs. Despite legislation, uptake of patient portal access has been slow, especially among older adults.10,12,13 Barriers to patient portal registration and use include patient lack of awareness, perceived or actual digital illiteracy, mistrust in privacy precautions, lack of user-friendly interfaces, lack of internet or technology, HCP bias and workload, and misperceptions of usefulness.9,10,12,14 The HCPs most likely to facilitate the use of patient portals, typically include nurse practitioners (NPs), nurses, and medical residents.10,15 Patient portal platforms promote the partnership of these disciplines with the veteran to help the patient better manage their health. Despite the benefits and widespread integration of patient portals in health care systems, socioeconomic inequalities and HCP attitudes contribute to persistent disparities in its adoption by underserved populations. The veteran population is often faced with additional barriers to health care access with regard to geographic location, advanced age, trauma, disabilities, mental health challenges, and homelessness.10,16 These barriers require unique approaches to maximize the use of technologic advances.17 Advanced age contributes to low rates of patient portal enrollment and lack of digital platform use, thus creating a digital divide.11,12
The digital divide is described as the gap between those persons who use technology including computers and internet, and those persons who do not because of social and geographic barriers.16 It contributes to a growing health disparity in both access to care and quality of care especially for rural veterans. About 25% of the US population lacks fixed broadband at home; these individuals are more likely to be racial minorities, older, widowed, or to have lower levels of education.18,19 Veterans are disproportionately represented in these demographic categories.20 According to the US Department of Veterans Affairs (VA) Office of Rural Health, the percentage of rural veterans enrolled in the VA health care system (58%) is significantly higher than enrollment of urban veterans (38%); additionally, 27% of rural veterans do not access the internet at home.21
My HealtheVet
The VA plays an integral part in increasing virtual access to care, from the introduction of My Healthevet (MHV) in 2003 to the distribution of iPad tablets to vulnerable veterans during the COVID-19 pandemic.22,23 Due to COVID-19, the need for VA patient access to the internet and VA Video-Connect (VVC) telehealth services increased significantly.22 Access to internet and hardware supporting use of VVC and MHV has been facilitated by the Digital Divide Consult, a VA program launched in 2020 to increase access to telehealth services.24 The VA has distributed > 26,000 cellular-enabled tablets and provided > 50,000 veterans with connectivity in collaboration with various private sector companies.22 Patients report that MHV facilitates engagement in health care through improved access to EHRs and expedited communication with the health care team.4
MHV is a secure online tool that provides patients access to PHI. MHV aims to empower veterans to take charge of their health by improving communication with HCPs, setting patient goals, and offering health and well-being resources.25,26 In a study of outpatients at a large urban multisite health care system, < 35% of patients on 16 medical resident panels were enrolled in a patient portal.15 MHV internal national metrics show increasing registration and active users of the patient portal, yet locally, disparities in the use of the portal by rural and older veterans exist.
The Local Problem
A review of the registration process at a rural VA clinic revealed barriers to facilitating the veteran registration process at the point of care. Clinical reminders exist within the EHR to prompt clinicians at the point of care to improve quality of care. At the New England Healthcare System (Veterans Integrated Service Network [VISN] 1), a patient portal clinical reminder prompts staff to encourage veterans to register. Anecdotal data obtained from primary care staff interviews at a rural VA primary care clinic in Vermont revealed low clinician confidence in completing the clinical reminder, a lack of knowledge of MHV, and lack of time to educate veterans about the benefits of MHV.
Despite availability of a registration process at the point of care and clinical staff assigned to provide registration information to the veteran, access to the patient portal among veterans at this clinic remained low. This QI project aimed to increase patient portal enrollment of veterans in MHV in a single NP patient panel of 100 patients from a baseline of 33% by 10% in a 3-month time frame.
Implementation
Before implementing the first Plan-Do-Study-Act (PDSA) cycle, we established the baseline data for 1 patient panel to be 33%. A retrospective review of the NP resident’s panel of 100 revealed 33 veterans were enrolled in MHV, providing a setting for process improvement. Evaluation of potential enrollment data for the panel population revealed unenrolled veterans were primarily aged ≥ 65 years. A rapid cycle QI (RCQI) strategy using the PDSA method was used to identify, implement, and measure changes over a 3-month time frame in 1 NP patient panel.14
The RCQI process included establishing baseline data and 3 PDSA cycles that evaluated the current state of patient access to the electronic patient portal, elucidated patient barriers to registration, assessed the processes for point-of-care enrollment, and developed strategies to improve the process and increase veteran enrollment. The QI project team included an NP resident as the project manager and MHV champion, a clinical faculty mentor at the site, a telehealth coordinator, an MHV coordinator, clinic registered nurse (RN), and clinic licensed nursing assistant (LNA). The RN and LNA additionally served as MHV champions as the project progressed.
PDSA Cycles
The objective for PDSA cycle 1 was to evaluate the process of patient registration and assess the impact on NP workload and clinic workflow over a 4-week period to improve veteran enrollment. Data were collected in a spreadsheet to track the number of veterans enrolled, time frame to enroll, and field notes that the NP resident recorded about the experience. The NP resident was trained in registration methods by the MHV coordinator. Several barriers to the registration process were identified: The process resulted in a change of the clinic visit closure focus, the clinic room was blocked for use by another patient, veterans had difficulty generating a unique username and password, veterans were unfamiliar with basic tablet accessibility and use, and additional time was required if incorrect information was entered. The veterans displayed low confidence in using tablet technology and were unaware of the patient portal or its usefulness. After discussion of the process with the project team, recommendations were made to address challenges, including an RN-led registration process. The first PDSA cycle increased the total patient panel enrollment by 4 veterans to 37%.
In PDSA cycle 2 after the NP visit, patients who agreed to register for the MHV portal were introduced to the tablet. The registration process was completed by the patient with the RN prior to the patient checkout. Once patient registration was completed, the veteran met the MHV coordinator and upgraded to a premium account, which provided full access to portal features. Electronic messaging was tested by the MHV coordinator and veteran to validate patient understanding. Although preloading demographic information improved accessibility issues, time was still required for the RN to orient the veteran to the tablet, provide additional directions, and answer questions.
The registration process reduced NP time commitment but added to the RN time burden and disrupted workflow; and clinic room access continued to be an issue. The wait time for the veteran to register in the clinic remained dependent on the availability of the RN. The decision was to move the registration process to the initial patient rooming assignment in the clinic and was transitioned from RN to LNA, prior to the NP-veteran encounter. Four additional veterans registered in the second PDSA cycle, and total enrollment increased to 41%, an overall 8% increase from baseline.
In the third PDSA cycle the patient enrollment process was managed by the clinic LNA using scripted information about MHV prior to the veteran encounter. A partially preloaded tablet was offered to the veteran to register with MHV during the rooming process, and written and oral instruction were provided to the veteran. The time required for each veteran to register for MHV averaged 10 minutes, and the veteran was able to register while waiting for the NP to enter the room. Typical LNA tasks included greeting patients, updating health records, completing clinical reminders with the veteran, obtaining vital signs, and addressing questions. The LNA introduced the veteran to MHV using scripted information and supported them in registering for MHV prior to the NP-veteran encounter. Registration at point of care during the rooming process was well received by both the LNA and veterans. The LNA reported the process was efficient and did not add excessive time to the LNA workflow. The LNA reported verbal patient satisfaction and registration was facilitated for 6 veterans during the 4-week period.
Registration during point of care was reported as feasible and sustainable by the LNA. Upgrading the patient to a premium MHV account was transitioned to the MHV coordinator. All veterans seen during the 4-week period were approached about registration; if the veteran declined, written at-home step-by-step instructions were provided. A replacement electronic clinical reminder was proposed to the VISN clinical reminders team for review and was pilot tested by the primary care clinical team. The third PDSA cycle increased the total patient panel enrollment to 47%, an overall 14% increase from baseline. Six new veteran users were added during PDSA cycle 3.
Discussion
The project team successfully used a RCQI method with a PDSA strategy to improve patient access to the MHV portal and increased veteran enrollment by 14% on 1 NP resident patient panel. The project evaluated clinic workflow regarding veteran patient portal registration, uncovered inefficiencies, and developed improved processes to increase veteran access to the patient portal. Results were positively impacted through the recognition of inefficiencies and initiation of new processes to engage veterans in the portal registration process. Familiarizing the entire clinical team with the clinical reminder and registration process raised the awareness of a digital divide consult and the utility of the portal in patient care. The project provided an opportunity to evaluate veterans’ digital literacy, digital access to send and receive messages, and to provide coaching as needed. Sequential PDSA cycles employed audit and feedback, information preloading, multimodal teaching strategies (verbal, print, hands-on tablet learning), scripting, staff interviews, time studies, and workflow evaluation to improve processes. An MHV champion led the team, monitored the progress, set deadlines, and effectively communicated project performance.
Limitations
Project limitations included the single-site location, its small sample size, and the short 3-month implementation time frame. The patient panel was representative of other NP resident patient panels at the facility but may not be representative of other VA facilities.
Ethical Considerations
Patient confidentiality was maintained throughout the registration and data collection process. The project team (NP, RN, LNA) received training and written instructions on protection of patient confidentiality by the MHV coordinator prior to assisting veterans with the registration process. Privacy was maintained, no patient identifiers were collected or viewed, and no assistance was provided for username, password, or security questions. The tablet was password protected and secured, used only by the project team when veteran was interested in point-of-care portal registration.
Sustainability
QI projects require ongoing systemic efforts to enhance sustainability.26,27 The project team used the PDSA methodology to stimulate the design of new workflow processes to engage staff and veterans in portal registration. Several actions were taken to promote sustainability for veteran portal registration and improve access to health care for rural and underserved veterans. First, printed instructions and website link are available in the clinic intake and examination rooms. Staff are equipped with patient education discussion points about the portal. A tablet is available in the clinic to encourage veterans to sign up. A clinical reminder is in place to encourage portal registration. A designated super-user is available to help new patient portal users register and navigate the system. Outcomes of the QI project were presented at 2 separate VISN 1 nursing grand rounds and reported to the MHV coordinator and telehealth coordinator to promote dialogue among staff and raise awareness of challenges to veteran MHV access.
Conclusions
Reviewing patient portal registration processes at the local level is essential to improve veteran access. This QI project proposed a realistic and scalable solution to implementing and improving patient enrollment to MHV in primary care clinics. Integrating measurement of patient registration into the daily routine of the clinic empowers the entire clinical team to improve the quality of access to patient portal.
The project team worked together to accomplish a shared goal, using errors as opportunities to improve the process, while using available staff without compromising significant time or resources. Engaging the entire team to audit processes and designating one member of the team as an MHV champion to provide feedback is critical to the sustainability of point-of-care registration in the MHV patient portal. Multifaceted approaches to maximizing the use of technology lessens the digital divide for veterans who are faced with geographical and social barriers to health care access.
Acknowledgments
We thank the Office of Academic Affiliations and the US Department of Veterans Affairs Nursing Academic Partnerships in Graduate Education Nurse Practitioner residency program and clinical faculty and the affiliated University of Vermont faculty mentor/quality improvement coach for the support of the project.
Patient portals are secure online website tools that provide patient access to personal health information (PHI). Access to online PHI improves health equity and satisfies the meaningful use objectives of the Medicare electronic health record (EHR) incentive program.1,2 Through patient portals, individuals can access PHI records and current diagnoses, request and reschedule appointments, locate test results, track trends for vital signs and laboratory values, refill medications, and communicate directly with the health care team through secure messaging. This alternative method of communication with the team is associated with increased patient satisfaction.3 Patients reported improved patient engagement in health care self-management and decision making, as well as strengthened relationships with their health care team.4
Background
One well-documented strategy to improve portal use includes the development of a nurse champion to facilitate enrollment during the clinic visit.5 Patient perceptions of portal value increased after education by a health care professional (HCP) and assistance in enrollment to familiarize patients with the platform for ongoing use.5 Use of patient portals has been associated with favorable outcomes in chronic disease management. Patients with diabetes mellitus who regularly use patient portals for prescription refills and secure messaging have demonstrated improved glycemic control, medication adherence, and associated health parameters compared with nonusers.5-7 In patients with congestive heart failure, meaningful patient portal use results in fewer emergency department visits, fewer hospital admissions, lower readmission rates, and reduced unscheduled and no-show visits.8-11
Patient portal access is a quality improvement (QI) measure that meets Medicare and Medicaid meaningful use requirements that is designed to improve collaboration between HCPs and patients through EHRs. Despite legislation, uptake of patient portal access has been slow, especially among older adults.10,12,13 Barriers to patient portal registration and use include patient lack of awareness, perceived or actual digital illiteracy, mistrust in privacy precautions, lack of user-friendly interfaces, lack of internet or technology, HCP bias and workload, and misperceptions of usefulness.9,10,12,14 The HCPs most likely to facilitate the use of patient portals, typically include nurse practitioners (NPs), nurses, and medical residents.10,15 Patient portal platforms promote the partnership of these disciplines with the veteran to help the patient better manage their health. Despite the benefits and widespread integration of patient portals in health care systems, socioeconomic inequalities and HCP attitudes contribute to persistent disparities in its adoption by underserved populations. The veteran population is often faced with additional barriers to health care access with regard to geographic location, advanced age, trauma, disabilities, mental health challenges, and homelessness.10,16 These barriers require unique approaches to maximize the use of technologic advances.17 Advanced age contributes to low rates of patient portal enrollment and lack of digital platform use, thus creating a digital divide.11,12
The digital divide is described as the gap between those persons who use technology including computers and internet, and those persons who do not because of social and geographic barriers.16 It contributes to a growing health disparity in both access to care and quality of care especially for rural veterans. About 25% of the US population lacks fixed broadband at home; these individuals are more likely to be racial minorities, older, widowed, or to have lower levels of education.18,19 Veterans are disproportionately represented in these demographic categories.20 According to the US Department of Veterans Affairs (VA) Office of Rural Health, the percentage of rural veterans enrolled in the VA health care system (58%) is significantly higher than enrollment of urban veterans (38%); additionally, 27% of rural veterans do not access the internet at home.21
My HealtheVet
The VA plays an integral part in increasing virtual access to care, from the introduction of My Healthevet (MHV) in 2003 to the distribution of iPad tablets to vulnerable veterans during the COVID-19 pandemic.22,23 Due to COVID-19, the need for VA patient access to the internet and VA Video-Connect (VVC) telehealth services increased significantly.22 Access to internet and hardware supporting use of VVC and MHV has been facilitated by the Digital Divide Consult, a VA program launched in 2020 to increase access to telehealth services.24 The VA has distributed > 26,000 cellular-enabled tablets and provided > 50,000 veterans with connectivity in collaboration with various private sector companies.22 Patients report that MHV facilitates engagement in health care through improved access to EHRs and expedited communication with the health care team.4
MHV is a secure online tool that provides patients access to PHI. MHV aims to empower veterans to take charge of their health by improving communication with HCPs, setting patient goals, and offering health and well-being resources.25,26 In a study of outpatients at a large urban multisite health care system, < 35% of patients on 16 medical resident panels were enrolled in a patient portal.15 MHV internal national metrics show increasing registration and active users of the patient portal, yet locally, disparities in the use of the portal by rural and older veterans exist.
The Local Problem
A review of the registration process at a rural VA clinic revealed barriers to facilitating the veteran registration process at the point of care. Clinical reminders exist within the EHR to prompt clinicians at the point of care to improve quality of care. At the New England Healthcare System (Veterans Integrated Service Network [VISN] 1), a patient portal clinical reminder prompts staff to encourage veterans to register. Anecdotal data obtained from primary care staff interviews at a rural VA primary care clinic in Vermont revealed low clinician confidence in completing the clinical reminder, a lack of knowledge of MHV, and lack of time to educate veterans about the benefits of MHV.
Despite availability of a registration process at the point of care and clinical staff assigned to provide registration information to the veteran, access to the patient portal among veterans at this clinic remained low. This QI project aimed to increase patient portal enrollment of veterans in MHV in a single NP patient panel of 100 patients from a baseline of 33% by 10% in a 3-month time frame.
Implementation
Before implementing the first Plan-Do-Study-Act (PDSA) cycle, we established the baseline data for 1 patient panel to be 33%. A retrospective review of the NP resident’s panel of 100 revealed 33 veterans were enrolled in MHV, providing a setting for process improvement. Evaluation of potential enrollment data for the panel population revealed unenrolled veterans were primarily aged ≥ 65 years. A rapid cycle QI (RCQI) strategy using the PDSA method was used to identify, implement, and measure changes over a 3-month time frame in 1 NP patient panel.14
The RCQI process included establishing baseline data and 3 PDSA cycles that evaluated the current state of patient access to the electronic patient portal, elucidated patient barriers to registration, assessed the processes for point-of-care enrollment, and developed strategies to improve the process and increase veteran enrollment. The QI project team included an NP resident as the project manager and MHV champion, a clinical faculty mentor at the site, a telehealth coordinator, an MHV coordinator, clinic registered nurse (RN), and clinic licensed nursing assistant (LNA). The RN and LNA additionally served as MHV champions as the project progressed.
PDSA Cycles
The objective for PDSA cycle 1 was to evaluate the process of patient registration and assess the impact on NP workload and clinic workflow over a 4-week period to improve veteran enrollment. Data were collected in a spreadsheet to track the number of veterans enrolled, time frame to enroll, and field notes that the NP resident recorded about the experience. The NP resident was trained in registration methods by the MHV coordinator. Several barriers to the registration process were identified: The process resulted in a change of the clinic visit closure focus, the clinic room was blocked for use by another patient, veterans had difficulty generating a unique username and password, veterans were unfamiliar with basic tablet accessibility and use, and additional time was required if incorrect information was entered. The veterans displayed low confidence in using tablet technology and were unaware of the patient portal or its usefulness. After discussion of the process with the project team, recommendations were made to address challenges, including an RN-led registration process. The first PDSA cycle increased the total patient panel enrollment by 4 veterans to 37%.
In PDSA cycle 2 after the NP visit, patients who agreed to register for the MHV portal were introduced to the tablet. The registration process was completed by the patient with the RN prior to the patient checkout. Once patient registration was completed, the veteran met the MHV coordinator and upgraded to a premium account, which provided full access to portal features. Electronic messaging was tested by the MHV coordinator and veteran to validate patient understanding. Although preloading demographic information improved accessibility issues, time was still required for the RN to orient the veteran to the tablet, provide additional directions, and answer questions.
The registration process reduced NP time commitment but added to the RN time burden and disrupted workflow; and clinic room access continued to be an issue. The wait time for the veteran to register in the clinic remained dependent on the availability of the RN. The decision was to move the registration process to the initial patient rooming assignment in the clinic and was transitioned from RN to LNA, prior to the NP-veteran encounter. Four additional veterans registered in the second PDSA cycle, and total enrollment increased to 41%, an overall 8% increase from baseline.
In the third PDSA cycle the patient enrollment process was managed by the clinic LNA using scripted information about MHV prior to the veteran encounter. A partially preloaded tablet was offered to the veteran to register with MHV during the rooming process, and written and oral instruction were provided to the veteran. The time required for each veteran to register for MHV averaged 10 minutes, and the veteran was able to register while waiting for the NP to enter the room. Typical LNA tasks included greeting patients, updating health records, completing clinical reminders with the veteran, obtaining vital signs, and addressing questions. The LNA introduced the veteran to MHV using scripted information and supported them in registering for MHV prior to the NP-veteran encounter. Registration at point of care during the rooming process was well received by both the LNA and veterans. The LNA reported the process was efficient and did not add excessive time to the LNA workflow. The LNA reported verbal patient satisfaction and registration was facilitated for 6 veterans during the 4-week period.
Registration during point of care was reported as feasible and sustainable by the LNA. Upgrading the patient to a premium MHV account was transitioned to the MHV coordinator. All veterans seen during the 4-week period were approached about registration; if the veteran declined, written at-home step-by-step instructions were provided. A replacement electronic clinical reminder was proposed to the VISN clinical reminders team for review and was pilot tested by the primary care clinical team. The third PDSA cycle increased the total patient panel enrollment to 47%, an overall 14% increase from baseline. Six new veteran users were added during PDSA cycle 3.
Discussion
The project team successfully used a RCQI method with a PDSA strategy to improve patient access to the MHV portal and increased veteran enrollment by 14% on 1 NP resident patient panel. The project evaluated clinic workflow regarding veteran patient portal registration, uncovered inefficiencies, and developed improved processes to increase veteran access to the patient portal. Results were positively impacted through the recognition of inefficiencies and initiation of new processes to engage veterans in the portal registration process. Familiarizing the entire clinical team with the clinical reminder and registration process raised the awareness of a digital divide consult and the utility of the portal in patient care. The project provided an opportunity to evaluate veterans’ digital literacy, digital access to send and receive messages, and to provide coaching as needed. Sequential PDSA cycles employed audit and feedback, information preloading, multimodal teaching strategies (verbal, print, hands-on tablet learning), scripting, staff interviews, time studies, and workflow evaluation to improve processes. An MHV champion led the team, monitored the progress, set deadlines, and effectively communicated project performance.
Limitations
Project limitations included the single-site location, its small sample size, and the short 3-month implementation time frame. The patient panel was representative of other NP resident patient panels at the facility but may not be representative of other VA facilities.
Ethical Considerations
Patient confidentiality was maintained throughout the registration and data collection process. The project team (NP, RN, LNA) received training and written instructions on protection of patient confidentiality by the MHV coordinator prior to assisting veterans with the registration process. Privacy was maintained, no patient identifiers were collected or viewed, and no assistance was provided for username, password, or security questions. The tablet was password protected and secured, used only by the project team when veteran was interested in point-of-care portal registration.
Sustainability
QI projects require ongoing systemic efforts to enhance sustainability.26,27 The project team used the PDSA methodology to stimulate the design of new workflow processes to engage staff and veterans in portal registration. Several actions were taken to promote sustainability for veteran portal registration and improve access to health care for rural and underserved veterans. First, printed instructions and website link are available in the clinic intake and examination rooms. Staff are equipped with patient education discussion points about the portal. A tablet is available in the clinic to encourage veterans to sign up. A clinical reminder is in place to encourage portal registration. A designated super-user is available to help new patient portal users register and navigate the system. Outcomes of the QI project were presented at 2 separate VISN 1 nursing grand rounds and reported to the MHV coordinator and telehealth coordinator to promote dialogue among staff and raise awareness of challenges to veteran MHV access.
Conclusions
Reviewing patient portal registration processes at the local level is essential to improve veteran access. This QI project proposed a realistic and scalable solution to implementing and improving patient enrollment to MHV in primary care clinics. Integrating measurement of patient registration into the daily routine of the clinic empowers the entire clinical team to improve the quality of access to patient portal.
The project team worked together to accomplish a shared goal, using errors as opportunities to improve the process, while using available staff without compromising significant time or resources. Engaging the entire team to audit processes and designating one member of the team as an MHV champion to provide feedback is critical to the sustainability of point-of-care registration in the MHV patient portal. Multifaceted approaches to maximizing the use of technology lessens the digital divide for veterans who are faced with geographical and social barriers to health care access.
Acknowledgments
We thank the Office of Academic Affiliations and the US Department of Veterans Affairs Nursing Academic Partnerships in Graduate Education Nurse Practitioner residency program and clinical faculty and the affiliated University of Vermont faculty mentor/quality improvement coach for the support of the project.
1. Centers for Medicare and Medicaid Services. Promoting interoperability programs. Updated October 6, 2022. Accessed November 3, 2022. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms
2. American Hospital Association. Goals of the Medicare and Medicaid electronic health records programs. Accessed November 3, 2022. https://www.aha.org/websites/2009-12-11-goals-medicare-and-medicaid-electronic-health-records-programs
3. Rozenblum R, Donzé J, Hockey PM, et al. The impact of medical informatics on patient satisfaction: a USA-based literature review. Int J Med Inform. 2013;82(3):141-158. doi:10.1016/j.ijmedinf.2012.12.008
4. Stewart MT, Hogan TP, Nicklas J, et al. The promise of patient portals for individuals living with chronic illness: qualitative study identifying pathways of patient engagement. J Med Internet Res. 2020;22(7):e17744. Published 2020 Jul 17. doi:10.2196/17744
5. Harris LT, Haneuse SJ, Martin DP, Ralston JD. Diabetes quality of care and outpatient utilization associated with electronic patient-provider messaging: a cross-sectional analysis. Diabetes Care. 2009;32(7):1182-1187. doi:10.2337/dc08-1771
6. Robinson SA, Zocchi MS, Netherton D, et al. Secure messaging, diabetes self-management, and the importance of patient autonomy: a mixed methods study. J Gen Intern Med. 2020;35(10):2955-2962. doi:10.1007/s11606-020-05834-x
7. Zocchi MS, Robinson SA, Ash AS, et al. Patient portal engagement and diabetes management among new portal users in the Veterans Health Administration. J Am Med Inform Assoc. 2021;28(10):2176-2183. doi:10.1093/jamia/ocab115
8. Bao C, Bardhan IR, Singh H, Meyer BA, Kirksey K. Patient-provider engagement and its impact on health outcomes: a longitudinal study of patient portal use. MIS Quarterly. 2020;44(2):699-723. doi:10.25300/MISQ/2020/14180
9. Grossman LV, Masterson Creber RM, Benda NC, Wright D, Vawdrey DK, Ancker JS. Interventions to increase patient portal use in vulnerable populations: a systematic review. J Am Med Informs Assoc. 2019;26(8-9):855-870. doi:10.1093/jamia/ocz023
10. Zhao JY, Song B, Anand E, et al. Barriers, facilitators, and solutions to optimal patient portal and personal health record use: a systematic review of the literature. AMIA Annu Symp Proc. 2018;2017:1913-1922. Published 2018 Apr 16.
11. Zhong X, Park J, Liang M, et al. Characteristics of patients using different patient portal functions and the impact on primary care service utilization and appointment adherence: retrospective observational study. J Med Internet Res. 2020;22(2):e14410. Published 2020 Feb 25. doi:10.2196/14410
12. Krishnaswami A, Beavers C, Dorsch MP, et al. Gerotechnology for older adults with cardiovascular diseases. J Am Coll Cardiol. 2020;76(22):2650-2670. doi:10.1016/j.jacc.2020.09.606
13. Fix GM, Hogan TP, Amante DJ, McInnes DK, Nazi KM, Simon SR. Encouraging patient portal use in the patient-centered medical home: three stakeholder perspectives. J Med Internet Res. 2016;18(11):e308. Published 2016 Nov 22. doi:10.2196/jmir.6488
14. Ancker JS, Nosal S, Hauser D, Way C, Calman N. Access policy and the digital divide in patient access to medical records. Health Policy Technol. 2016;6(3-11). doi:10.1016/j.hlpt.2016.11.004
15. Rhudy C, Broxterman J, Stewart S, et al. Improving patient portal enrolment in an academic resident continuity clinic: quality improvement made simple. BMJ Open Qual. 2019;8(2):e000430. Published 2019 Apr 25. doi:10.1136/bmjoq-2018-000430
16. Kontos E, Blake KD, Chou WY, Prestin A. Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res. 2014;16(7):e172. Published 2014 Jul 16. doi:10.2196/jmir.3117
17. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice. The state of health disparities in the United States. In: Baciu A, Negussie Y, Geller A, et al, eds. Communities in Action: Pathways to Health Equity. National Academies Press (US); January 11, 2017. Accessed November 3, 2022. https://www.ncbi.nlm.nih.gov/books/NBK425848/
18. Pew Research Center. Internet/broadband fact sheet. Updated April 7, 2021. Accessed November 3, 2022. https://www.pewresearch.org/internet/fact-sheet/internet-broadband
19. Roberts ET, Mehrotra A. Assessment of disparities in digital access among Medicare beneficiaries and implications for telemedicine. JAMA Intern Med. 2020;180(10):1386-1389. doi:10.1001/jamainternmed.2020.2666
20. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed November 3, 2022. https://www.va.gov/vetdata/veteran_population.asp
21. US Department of Veterans Affairs, Office of Rural Health. Rural veterans health care challenges. Updated March 31, 2022. Accessed November 3, 2022. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
22. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands veteran access to telehealth with iPad services. Press release. September 15, 2020. Accessed November 3, 2022. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5521
23. Zulman DM, Wong EP, Slightam C, et al. Making connections: National implementation of video telehealth tablets to address access barriers in veterans. JAMIA Open. 2019;2(3):323-329. doi:10.1093/jamiaopen/ooz024
24. Malone NC, Williams MM, Smith Fawzi MC, et al. Mobile health clinics in the United States. Int J Equity Health. 2020;19(1):40. doi:10.1186/s12939-020-1135-7
25. US Department of Veterans Affairs. How to use My HealtheVet. Accessed November 3, 2022. https://www.myhealth.va.gov/mhv-portal-web/how-to-use-mhv
26. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Whole health for life. 2017. Accessed November 3, 2022. https://www.va.gov/wholehealth/docs/2017-AR-Vet-Facing_FNL-W508.pdf27. Mortimer F, Isherwood J, Wilkinson A, Vaux E. Sustainability in quality improvement: redefining value. Future Healthc J. 2018;5(2):88-93. doi:10.7861/futurehosp.5-2-88
1. Centers for Medicare and Medicaid Services. Promoting interoperability programs. Updated October 6, 2022. Accessed November 3, 2022. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms
2. American Hospital Association. Goals of the Medicare and Medicaid electronic health records programs. Accessed November 3, 2022. https://www.aha.org/websites/2009-12-11-goals-medicare-and-medicaid-electronic-health-records-programs
3. Rozenblum R, Donzé J, Hockey PM, et al. The impact of medical informatics on patient satisfaction: a USA-based literature review. Int J Med Inform. 2013;82(3):141-158. doi:10.1016/j.ijmedinf.2012.12.008
4. Stewart MT, Hogan TP, Nicklas J, et al. The promise of patient portals for individuals living with chronic illness: qualitative study identifying pathways of patient engagement. J Med Internet Res. 2020;22(7):e17744. Published 2020 Jul 17. doi:10.2196/17744
5. Harris LT, Haneuse SJ, Martin DP, Ralston JD. Diabetes quality of care and outpatient utilization associated with electronic patient-provider messaging: a cross-sectional analysis. Diabetes Care. 2009;32(7):1182-1187. doi:10.2337/dc08-1771
6. Robinson SA, Zocchi MS, Netherton D, et al. Secure messaging, diabetes self-management, and the importance of patient autonomy: a mixed methods study. J Gen Intern Med. 2020;35(10):2955-2962. doi:10.1007/s11606-020-05834-x
7. Zocchi MS, Robinson SA, Ash AS, et al. Patient portal engagement and diabetes management among new portal users in the Veterans Health Administration. J Am Med Inform Assoc. 2021;28(10):2176-2183. doi:10.1093/jamia/ocab115
8. Bao C, Bardhan IR, Singh H, Meyer BA, Kirksey K. Patient-provider engagement and its impact on health outcomes: a longitudinal study of patient portal use. MIS Quarterly. 2020;44(2):699-723. doi:10.25300/MISQ/2020/14180
9. Grossman LV, Masterson Creber RM, Benda NC, Wright D, Vawdrey DK, Ancker JS. Interventions to increase patient portal use in vulnerable populations: a systematic review. J Am Med Informs Assoc. 2019;26(8-9):855-870. doi:10.1093/jamia/ocz023
10. Zhao JY, Song B, Anand E, et al. Barriers, facilitators, and solutions to optimal patient portal and personal health record use: a systematic review of the literature. AMIA Annu Symp Proc. 2018;2017:1913-1922. Published 2018 Apr 16.
11. Zhong X, Park J, Liang M, et al. Characteristics of patients using different patient portal functions and the impact on primary care service utilization and appointment adherence: retrospective observational study. J Med Internet Res. 2020;22(2):e14410. Published 2020 Feb 25. doi:10.2196/14410
12. Krishnaswami A, Beavers C, Dorsch MP, et al. Gerotechnology for older adults with cardiovascular diseases. J Am Coll Cardiol. 2020;76(22):2650-2670. doi:10.1016/j.jacc.2020.09.606
13. Fix GM, Hogan TP, Amante DJ, McInnes DK, Nazi KM, Simon SR. Encouraging patient portal use in the patient-centered medical home: three stakeholder perspectives. J Med Internet Res. 2016;18(11):e308. Published 2016 Nov 22. doi:10.2196/jmir.6488
14. Ancker JS, Nosal S, Hauser D, Way C, Calman N. Access policy and the digital divide in patient access to medical records. Health Policy Technol. 2016;6(3-11). doi:10.1016/j.hlpt.2016.11.004
15. Rhudy C, Broxterman J, Stewart S, et al. Improving patient portal enrolment in an academic resident continuity clinic: quality improvement made simple. BMJ Open Qual. 2019;8(2):e000430. Published 2019 Apr 25. doi:10.1136/bmjoq-2018-000430
16. Kontos E, Blake KD, Chou WY, Prestin A. Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res. 2014;16(7):e172. Published 2014 Jul 16. doi:10.2196/jmir.3117
17. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice. The state of health disparities in the United States. In: Baciu A, Negussie Y, Geller A, et al, eds. Communities in Action: Pathways to Health Equity. National Academies Press (US); January 11, 2017. Accessed November 3, 2022. https://www.ncbi.nlm.nih.gov/books/NBK425848/
18. Pew Research Center. Internet/broadband fact sheet. Updated April 7, 2021. Accessed November 3, 2022. https://www.pewresearch.org/internet/fact-sheet/internet-broadband
19. Roberts ET, Mehrotra A. Assessment of disparities in digital access among Medicare beneficiaries and implications for telemedicine. JAMA Intern Med. 2020;180(10):1386-1389. doi:10.1001/jamainternmed.2020.2666
20. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed November 3, 2022. https://www.va.gov/vetdata/veteran_population.asp
21. US Department of Veterans Affairs, Office of Rural Health. Rural veterans health care challenges. Updated March 31, 2022. Accessed November 3, 2022. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
22. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands veteran access to telehealth with iPad services. Press release. September 15, 2020. Accessed November 3, 2022. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5521
23. Zulman DM, Wong EP, Slightam C, et al. Making connections: National implementation of video telehealth tablets to address access barriers in veterans. JAMIA Open. 2019;2(3):323-329. doi:10.1093/jamiaopen/ooz024
24. Malone NC, Williams MM, Smith Fawzi MC, et al. Mobile health clinics in the United States. Int J Equity Health. 2020;19(1):40. doi:10.1186/s12939-020-1135-7
25. US Department of Veterans Affairs. How to use My HealtheVet. Accessed November 3, 2022. https://www.myhealth.va.gov/mhv-portal-web/how-to-use-mhv
26. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Whole health for life. 2017. Accessed November 3, 2022. https://www.va.gov/wholehealth/docs/2017-AR-Vet-Facing_FNL-W508.pdf27. Mortimer F, Isherwood J, Wilkinson A, Vaux E. Sustainability in quality improvement: redefining value. Future Healthc J. 2018;5(2):88-93. doi:10.7861/futurehosp.5-2-88
A 17-year-old male was referred by his pediatrician for evaluation of a year-long rash
A biopsy of the edge of one of lesions on the torso was performed. Histopathology demonstrated hyperkeratosis of the stratum corneum with focal thickening of the granular cell layer, basal layer degeneration of the epidermis, and a band-like subepidermal lymphocytic infiltrate with Civatte bodies consistent with lichen planus. There was some reduction in the elastic fibers on the papillary dermis.
Given the morphology of the lesions and the histopathologic presentation, he was diagnosed with annular atrophic lichen planus (AALP). Lichen planus is a chronic inflammatory condition that can affect the skin, nails, hair, and mucosa. Lichen planus is seen in less than 1% of the population, occurring mainly in middle-aged adults and rarely seen in children. Though, there appears to be no clear racial predilection, a small study in the United States showed a higher incidence of lichen planus in Black children. Lesions with classic characteristics are pruritic, polygonal, violaceous, flat-topped papules and plaques.
There are different subtypes of lichen planus, which include papular or classic form, hypertrophic, vesiculobullous, actinic, annular, atrophic, annular atrophic, linear, follicular, lichen planus pigmentosus, lichen pigmentosa pigmentosus-inversus, lichen planus–lupus erythematosus overlap syndrome, and lichen planus pemphigoides. The annular atrophic form is the least common of all, and there are few reports in the pediatric population. AALP presents as annular papules and plaques with atrophic centers that resolve within a few months leaving postinflammatory hypo- or hyperpigmentation and, in some patients, permanent atrophic scarring.
In histopathology, the lesions show the classic characteristics of lichen planus including vacuolar interface changes and necrotic keratinocytes, hypergranulosis, band-like infiltrate in the dermis, melanin incontinence, and Civatte bodies. In AALP, the center of the lesion shows an atrophic epidermis, and there is also a characteristic partial reduction to complete destruction of elastic fibers in the papillary dermis in the center of the lesion and sometimes in the periphery as well, which helps differentiate AALP from other forms of lichen planus.
The differential diagnosis for AALP includes tinea corporis, which can present with annular lesions, but they are usually scaly and rarely resolve on their own. Pityriasis rosea lesions can also look very similar to AALP lesions, but the difference is the presence of an inner collaret of scale and a lack of atrophy in pityriasis rosea. Pityriasis rosea is a rash that can be triggered by viral infections, medications, and vaccines and self-resolves within 10-12 weeks. Secondary syphilis can also be annular and resemble lesions of AALP. Syphilis patients are usually sexually active and may have lesions present on the palms and soles, which were not seen in our patient.
Granuloma annulare should also be included in the differential diagnosis of AALP. Granuloma annulare lesions present as annular papules or plaques with raised borders and a slightly hyperpigmented center that may appear more depressed compared to the edges of the lesion, though not atrophic as seen in AALP. Pityriasis lichenoides chronica is an inflammatory condition of the skin in which patients present with erythematous to brown papules in different stages which may have a mica-like scale, usually not seen on AALP. Sometimes a skin biopsy will be needed to differentiate between these conditions.
It is very important to make a timely diagnosis of AALP and treat the lesions early as it may leave long-lasting dyspigmentation and scarring. Though AAPL lesions can be resistant to treatment with topical medications, there are reports of improvement with superpotent topical corticosteroids and calcineurin inhibitors. In recalcitrant cases, systemic therapy with isotretinoin, acitretin, methotrexate, systemic corticosteroids, dapsone, and hydroxychloroquine can be considered. Our patient was treated with clobetasol propionate ointment 0.05% with good response.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Bowers S and Warshaw EM. J Am Acad Dermatol. 2006 Oct;55(4):557-72; quiz 573-6.
Gorouhi F et al. Scientific World Journal. 2014 Jan 30;2014:742826.
Santhosh P and George M. Int J Dermatol. 2022.61:1213-7.
Sears S et al. Pediatr Dermatol. 2021;38:1283-7.
Weston G and Payette M. Int J Womens Dermatol. 2015 Sep 16;1(3):140-9.
A biopsy of the edge of one of lesions on the torso was performed. Histopathology demonstrated hyperkeratosis of the stratum corneum with focal thickening of the granular cell layer, basal layer degeneration of the epidermis, and a band-like subepidermal lymphocytic infiltrate with Civatte bodies consistent with lichen planus. There was some reduction in the elastic fibers on the papillary dermis.
Given the morphology of the lesions and the histopathologic presentation, he was diagnosed with annular atrophic lichen planus (AALP). Lichen planus is a chronic inflammatory condition that can affect the skin, nails, hair, and mucosa. Lichen planus is seen in less than 1% of the population, occurring mainly in middle-aged adults and rarely seen in children. Though, there appears to be no clear racial predilection, a small study in the United States showed a higher incidence of lichen planus in Black children. Lesions with classic characteristics are pruritic, polygonal, violaceous, flat-topped papules and plaques.
There are different subtypes of lichen planus, which include papular or classic form, hypertrophic, vesiculobullous, actinic, annular, atrophic, annular atrophic, linear, follicular, lichen planus pigmentosus, lichen pigmentosa pigmentosus-inversus, lichen planus–lupus erythematosus overlap syndrome, and lichen planus pemphigoides. The annular atrophic form is the least common of all, and there are few reports in the pediatric population. AALP presents as annular papules and plaques with atrophic centers that resolve within a few months leaving postinflammatory hypo- or hyperpigmentation and, in some patients, permanent atrophic scarring.
In histopathology, the lesions show the classic characteristics of lichen planus including vacuolar interface changes and necrotic keratinocytes, hypergranulosis, band-like infiltrate in the dermis, melanin incontinence, and Civatte bodies. In AALP, the center of the lesion shows an atrophic epidermis, and there is also a characteristic partial reduction to complete destruction of elastic fibers in the papillary dermis in the center of the lesion and sometimes in the periphery as well, which helps differentiate AALP from other forms of lichen planus.
The differential diagnosis for AALP includes tinea corporis, which can present with annular lesions, but they are usually scaly and rarely resolve on their own. Pityriasis rosea lesions can also look very similar to AALP lesions, but the difference is the presence of an inner collaret of scale and a lack of atrophy in pityriasis rosea. Pityriasis rosea is a rash that can be triggered by viral infections, medications, and vaccines and self-resolves within 10-12 weeks. Secondary syphilis can also be annular and resemble lesions of AALP. Syphilis patients are usually sexually active and may have lesions present on the palms and soles, which were not seen in our patient.
Granuloma annulare should also be included in the differential diagnosis of AALP. Granuloma annulare lesions present as annular papules or plaques with raised borders and a slightly hyperpigmented center that may appear more depressed compared to the edges of the lesion, though not atrophic as seen in AALP. Pityriasis lichenoides chronica is an inflammatory condition of the skin in which patients present with erythematous to brown papules in different stages which may have a mica-like scale, usually not seen on AALP. Sometimes a skin biopsy will be needed to differentiate between these conditions.
It is very important to make a timely diagnosis of AALP and treat the lesions early as it may leave long-lasting dyspigmentation and scarring. Though AAPL lesions can be resistant to treatment with topical medications, there are reports of improvement with superpotent topical corticosteroids and calcineurin inhibitors. In recalcitrant cases, systemic therapy with isotretinoin, acitretin, methotrexate, systemic corticosteroids, dapsone, and hydroxychloroquine can be considered. Our patient was treated with clobetasol propionate ointment 0.05% with good response.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Bowers S and Warshaw EM. J Am Acad Dermatol. 2006 Oct;55(4):557-72; quiz 573-6.
Gorouhi F et al. Scientific World Journal. 2014 Jan 30;2014:742826.
Santhosh P and George M. Int J Dermatol. 2022.61:1213-7.
Sears S et al. Pediatr Dermatol. 2021;38:1283-7.
Weston G and Payette M. Int J Womens Dermatol. 2015 Sep 16;1(3):140-9.
A biopsy of the edge of one of lesions on the torso was performed. Histopathology demonstrated hyperkeratosis of the stratum corneum with focal thickening of the granular cell layer, basal layer degeneration of the epidermis, and a band-like subepidermal lymphocytic infiltrate with Civatte bodies consistent with lichen planus. There was some reduction in the elastic fibers on the papillary dermis.
Given the morphology of the lesions and the histopathologic presentation, he was diagnosed with annular atrophic lichen planus (AALP). Lichen planus is a chronic inflammatory condition that can affect the skin, nails, hair, and mucosa. Lichen planus is seen in less than 1% of the population, occurring mainly in middle-aged adults and rarely seen in children. Though, there appears to be no clear racial predilection, a small study in the United States showed a higher incidence of lichen planus in Black children. Lesions with classic characteristics are pruritic, polygonal, violaceous, flat-topped papules and plaques.
There are different subtypes of lichen planus, which include papular or classic form, hypertrophic, vesiculobullous, actinic, annular, atrophic, annular atrophic, linear, follicular, lichen planus pigmentosus, lichen pigmentosa pigmentosus-inversus, lichen planus–lupus erythematosus overlap syndrome, and lichen planus pemphigoides. The annular atrophic form is the least common of all, and there are few reports in the pediatric population. AALP presents as annular papules and plaques with atrophic centers that resolve within a few months leaving postinflammatory hypo- or hyperpigmentation and, in some patients, permanent atrophic scarring.
In histopathology, the lesions show the classic characteristics of lichen planus including vacuolar interface changes and necrotic keratinocytes, hypergranulosis, band-like infiltrate in the dermis, melanin incontinence, and Civatte bodies. In AALP, the center of the lesion shows an atrophic epidermis, and there is also a characteristic partial reduction to complete destruction of elastic fibers in the papillary dermis in the center of the lesion and sometimes in the periphery as well, which helps differentiate AALP from other forms of lichen planus.
The differential diagnosis for AALP includes tinea corporis, which can present with annular lesions, but they are usually scaly and rarely resolve on their own. Pityriasis rosea lesions can also look very similar to AALP lesions, but the difference is the presence of an inner collaret of scale and a lack of atrophy in pityriasis rosea. Pityriasis rosea is a rash that can be triggered by viral infections, medications, and vaccines and self-resolves within 10-12 weeks. Secondary syphilis can also be annular and resemble lesions of AALP. Syphilis patients are usually sexually active and may have lesions present on the palms and soles, which were not seen in our patient.
Granuloma annulare should also be included in the differential diagnosis of AALP. Granuloma annulare lesions present as annular papules or plaques with raised borders and a slightly hyperpigmented center that may appear more depressed compared to the edges of the lesion, though not atrophic as seen in AALP. Pityriasis lichenoides chronica is an inflammatory condition of the skin in which patients present with erythematous to brown papules in different stages which may have a mica-like scale, usually not seen on AALP. Sometimes a skin biopsy will be needed to differentiate between these conditions.
It is very important to make a timely diagnosis of AALP and treat the lesions early as it may leave long-lasting dyspigmentation and scarring. Though AAPL lesions can be resistant to treatment with topical medications, there are reports of improvement with superpotent topical corticosteroids and calcineurin inhibitors. In recalcitrant cases, systemic therapy with isotretinoin, acitretin, methotrexate, systemic corticosteroids, dapsone, and hydroxychloroquine can be considered. Our patient was treated with clobetasol propionate ointment 0.05% with good response.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Bowers S and Warshaw EM. J Am Acad Dermatol. 2006 Oct;55(4):557-72; quiz 573-6.
Gorouhi F et al. Scientific World Journal. 2014 Jan 30;2014:742826.
Santhosh P and George M. Int J Dermatol. 2022.61:1213-7.
Sears S et al. Pediatr Dermatol. 2021;38:1283-7.
Weston G and Payette M. Int J Womens Dermatol. 2015 Sep 16;1(3):140-9.
A 17-year-old healthy male was referred by his pediatrician for evaluation of a rash on the skin which has been present on and off for a year. During the initial presentation, the lesions were clustered on the back, were slightly itchy, and resolved after 3 months. Several new lesions have developed on the neck, torso, and extremities, leaving hypopigmented marks on the skin. He has previously been treated with topical antifungal creams, oral fluconazole, and triamcinolone ointment without resolution of the lesions.
He is not involved in any contact sports, he has not traveled outside the country, and is not taking any other medications. He is not sexually active. He also has a diagnosis of mild acne that he is currently treating with over-the-counter medications.
On physical exam he had several annular plaques with central atrophic centers and no scale. He also had some hypo- and hyperpigmented macules at the sites of prior skin lesions
How to advocate in a post-Roe world, no matter your zip code
For many, the recent Supreme Court decision in the Dobbs v Jackson case that removed the constitutional right to an abortion has introduced outrage, fear, and confusion throughout the country. While the American College of Obstetricians and Gynecologists (ACOG) clearly has established that abortion is essential health care and has published resources regarding the issue (www.acog.org/advocacy/abortion-is-essential), and many providers know what to do medically, they do not know what they can do legally. In a country where 45% of pregnancies are unplanned and 25% of women will access abortion services in their lifetime, this decision will completely change the landscape of providing and receiving abortion care. This decision will affect every provider and their patients and will affect them differently in each state. The country likely will be divided into 24 destination states that will protect the right to abortion and another 26 states that have or will soon ban abortion or severely restrict access to it.
Regardless of the state you practice in, it is clear that our voices, actions, and advocacy are essential during these challenging times. It can feel difficult to find ways to advocate, especially if you are in a state or have an employer that supports anti-abortion legislation or has been silent after the Dobbs decision was released. We have created a guide to help and encourage all ObGyn providers to find ways to advocate, no matter their zip code.
1. Donate
Many of our patients will need to travel out of state to seek abortion care. The cost of abortion care can be expensive, and travel, child care, and time off of work add to the costs of the procedure itself, making access to abortion care financially out of reach for some. There are many well-established abortion funds throughout the country; consider donating to one of them or organizing a fundraiser in your community. Go to abortionfunds.org/funds to find an abortion fund that will support patients in your community, or donate generally to support them all.
2. Save your stories
We already are hearing the devastating impact abortion bans have on patient care around the country. If you had to deny or delay care because of the new legal landscape surrounding abortion, write down or record the experience. Your stories can be critical in discussing the impact of legislation. If you choose to share on social media, ask the involved patients if they are comfortable with their story being shared online (as long as their identity is protected).
3. Talk about it
Talking about abortion is a critical step in destigmatizing it and supporting our patients as well as our field. These conversations can be challenging, but ACOG has provided an important guide that includes key phrases and statements to help shape the conversation and avoid polarizing language (https://www.acog.org/advocacy/abortion-is-essential/come-prepared). This guide also can be helpful to keep in mind when talking to members of the media.
Continue to: 4. Write about it...
4. Write about it
There are many opportunities to write about the impact of the Dobbs decision, especially locally. As a clinician and trusted member of the community, you can uniquely share your and your patients’ experiences. Your article does not have to appear in a major publication; you can still have an important impact in your local paper. See resources on how to write an op-ed and letter to the editor (https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community/legislative-rx-op-eds-and-letters-to-the-editor).
5. Teach about it
These legislative changes uniquely impact our ObGyn residents; 44% of residents likely will be in a training program in a state that will ban or severely restrict abortion access. Abortion is health care, and a vast majority of our residents could graduate without important skills to save lives. As we strategize to ensure all ObGyn residents are able to receive this important training, work on incorporating an advocacy curriculum into your residents’ educational experience. Teaching about how to advocate is an important skill for supporting our patients and ensuring critical health policy. ACOG has published guides focused on education and training (www.acog.org/advocacy/abortion-is-essential/education-and-training). We also have included our own medical center’s advocacy curriculum (https://docs.google.com/document/d/1STxLzE0j55mlDEbF0_wZbo9O QryAcs6RpfZ47Mwfs4I/edit).
6. Get involved and seek out allies
It’s important that ObGyns be at the table for all discussions surrounding abortion care and reproductive health. Join hospital committees and help influence policy within your own institution. Refer back to those abortion talking points—this will help in some of these challenging conversations.
7. Get on social media
Using social media can be a powerful tool for advocacy. You can help elevate issues and encourage others to get active as well. Using a common hashtag, such as #AbortionisHealthcare, on different platforms can help connect you to other advocates. Share simple and important graphics provided by ACOG on important topics in our field (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state/social-media) and review ACOG’s recommendation for professionalism in social media (https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/10/professional-use-of-digital-and-social-media).
8. Get active locally
We have seen the introduction of hundreds of bills in states around the country not only on abortion but also on other legislation that directly impacts the care we provide. It is critical that we get involved in advocating for important reproductive health legislation and against bills that cause harm and interfere with the doctor-patient relationship. Stay up to date on legislative issues with your local ACOG and medical chapters (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state). Consider testifying at your State house, providing written or oral testimony. Connect with ACOG or your state medical chapter to help with talking points!
9. Read up
There have been many new policies at the federal level that could impact the care you provide. Take some time to read up on these new changes. Patients also may ask you about self-managed abortion. There are guides and resources (https://www.acog.org/advocacy/abortion-is-essential/practice-management) for patients that may seek medication online, and we want to ensure that patients have the resources to make informed decisions.
10. Hit the Capitol
Consider making time to come to the annual Congressional Leadership Conference in Washington, DC (https://www.acog.org/education-and-events/meetings/acog-congressional-leadership-conference), or other advocacy events offered through the American Medical Association or other subspecialty organizations. When we all come together as an organization, a field, and a community, it sends a powerful message that we are standing up together for our patients and our colleagues.
Make a difference
There is no advocacy too big or too small. It is critical that we continue to use our voices and our platforms to stand up for health care and access to critical services, including abortion care. ●
For many, the recent Supreme Court decision in the Dobbs v Jackson case that removed the constitutional right to an abortion has introduced outrage, fear, and confusion throughout the country. While the American College of Obstetricians and Gynecologists (ACOG) clearly has established that abortion is essential health care and has published resources regarding the issue (www.acog.org/advocacy/abortion-is-essential), and many providers know what to do medically, they do not know what they can do legally. In a country where 45% of pregnancies are unplanned and 25% of women will access abortion services in their lifetime, this decision will completely change the landscape of providing and receiving abortion care. This decision will affect every provider and their patients and will affect them differently in each state. The country likely will be divided into 24 destination states that will protect the right to abortion and another 26 states that have or will soon ban abortion or severely restrict access to it.
Regardless of the state you practice in, it is clear that our voices, actions, and advocacy are essential during these challenging times. It can feel difficult to find ways to advocate, especially if you are in a state or have an employer that supports anti-abortion legislation or has been silent after the Dobbs decision was released. We have created a guide to help and encourage all ObGyn providers to find ways to advocate, no matter their zip code.
1. Donate
Many of our patients will need to travel out of state to seek abortion care. The cost of abortion care can be expensive, and travel, child care, and time off of work add to the costs of the procedure itself, making access to abortion care financially out of reach for some. There are many well-established abortion funds throughout the country; consider donating to one of them or organizing a fundraiser in your community. Go to abortionfunds.org/funds to find an abortion fund that will support patients in your community, or donate generally to support them all.
2. Save your stories
We already are hearing the devastating impact abortion bans have on patient care around the country. If you had to deny or delay care because of the new legal landscape surrounding abortion, write down or record the experience. Your stories can be critical in discussing the impact of legislation. If you choose to share on social media, ask the involved patients if they are comfortable with their story being shared online (as long as their identity is protected).
3. Talk about it
Talking about abortion is a critical step in destigmatizing it and supporting our patients as well as our field. These conversations can be challenging, but ACOG has provided an important guide that includes key phrases and statements to help shape the conversation and avoid polarizing language (https://www.acog.org/advocacy/abortion-is-essential/come-prepared). This guide also can be helpful to keep in mind when talking to members of the media.
Continue to: 4. Write about it...
4. Write about it
There are many opportunities to write about the impact of the Dobbs decision, especially locally. As a clinician and trusted member of the community, you can uniquely share your and your patients’ experiences. Your article does not have to appear in a major publication; you can still have an important impact in your local paper. See resources on how to write an op-ed and letter to the editor (https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community/legislative-rx-op-eds-and-letters-to-the-editor).
5. Teach about it
These legislative changes uniquely impact our ObGyn residents; 44% of residents likely will be in a training program in a state that will ban or severely restrict abortion access. Abortion is health care, and a vast majority of our residents could graduate without important skills to save lives. As we strategize to ensure all ObGyn residents are able to receive this important training, work on incorporating an advocacy curriculum into your residents’ educational experience. Teaching about how to advocate is an important skill for supporting our patients and ensuring critical health policy. ACOG has published guides focused on education and training (www.acog.org/advocacy/abortion-is-essential/education-and-training). We also have included our own medical center’s advocacy curriculum (https://docs.google.com/document/d/1STxLzE0j55mlDEbF0_wZbo9O QryAcs6RpfZ47Mwfs4I/edit).
6. Get involved and seek out allies
It’s important that ObGyns be at the table for all discussions surrounding abortion care and reproductive health. Join hospital committees and help influence policy within your own institution. Refer back to those abortion talking points—this will help in some of these challenging conversations.
7. Get on social media
Using social media can be a powerful tool for advocacy. You can help elevate issues and encourage others to get active as well. Using a common hashtag, such as #AbortionisHealthcare, on different platforms can help connect you to other advocates. Share simple and important graphics provided by ACOG on important topics in our field (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state/social-media) and review ACOG’s recommendation for professionalism in social media (https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/10/professional-use-of-digital-and-social-media).
8. Get active locally
We have seen the introduction of hundreds of bills in states around the country not only on abortion but also on other legislation that directly impacts the care we provide. It is critical that we get involved in advocating for important reproductive health legislation and against bills that cause harm and interfere with the doctor-patient relationship. Stay up to date on legislative issues with your local ACOG and medical chapters (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state). Consider testifying at your State house, providing written or oral testimony. Connect with ACOG or your state medical chapter to help with talking points!
9. Read up
There have been many new policies at the federal level that could impact the care you provide. Take some time to read up on these new changes. Patients also may ask you about self-managed abortion. There are guides and resources (https://www.acog.org/advocacy/abortion-is-essential/practice-management) for patients that may seek medication online, and we want to ensure that patients have the resources to make informed decisions.
10. Hit the Capitol
Consider making time to come to the annual Congressional Leadership Conference in Washington, DC (https://www.acog.org/education-and-events/meetings/acog-congressional-leadership-conference), or other advocacy events offered through the American Medical Association or other subspecialty organizations. When we all come together as an organization, a field, and a community, it sends a powerful message that we are standing up together for our patients and our colleagues.
Make a difference
There is no advocacy too big or too small. It is critical that we continue to use our voices and our platforms to stand up for health care and access to critical services, including abortion care. ●
For many, the recent Supreme Court decision in the Dobbs v Jackson case that removed the constitutional right to an abortion has introduced outrage, fear, and confusion throughout the country. While the American College of Obstetricians and Gynecologists (ACOG) clearly has established that abortion is essential health care and has published resources regarding the issue (www.acog.org/advocacy/abortion-is-essential), and many providers know what to do medically, they do not know what they can do legally. In a country where 45% of pregnancies are unplanned and 25% of women will access abortion services in their lifetime, this decision will completely change the landscape of providing and receiving abortion care. This decision will affect every provider and their patients and will affect them differently in each state. The country likely will be divided into 24 destination states that will protect the right to abortion and another 26 states that have or will soon ban abortion or severely restrict access to it.
Regardless of the state you practice in, it is clear that our voices, actions, and advocacy are essential during these challenging times. It can feel difficult to find ways to advocate, especially if you are in a state or have an employer that supports anti-abortion legislation or has been silent after the Dobbs decision was released. We have created a guide to help and encourage all ObGyn providers to find ways to advocate, no matter their zip code.
1. Donate
Many of our patients will need to travel out of state to seek abortion care. The cost of abortion care can be expensive, and travel, child care, and time off of work add to the costs of the procedure itself, making access to abortion care financially out of reach for some. There are many well-established abortion funds throughout the country; consider donating to one of them or organizing a fundraiser in your community. Go to abortionfunds.org/funds to find an abortion fund that will support patients in your community, or donate generally to support them all.
2. Save your stories
We already are hearing the devastating impact abortion bans have on patient care around the country. If you had to deny or delay care because of the new legal landscape surrounding abortion, write down or record the experience. Your stories can be critical in discussing the impact of legislation. If you choose to share on social media, ask the involved patients if they are comfortable with their story being shared online (as long as their identity is protected).
3. Talk about it
Talking about abortion is a critical step in destigmatizing it and supporting our patients as well as our field. These conversations can be challenging, but ACOG has provided an important guide that includes key phrases and statements to help shape the conversation and avoid polarizing language (https://www.acog.org/advocacy/abortion-is-essential/come-prepared). This guide also can be helpful to keep in mind when talking to members of the media.
Continue to: 4. Write about it...
4. Write about it
There are many opportunities to write about the impact of the Dobbs decision, especially locally. As a clinician and trusted member of the community, you can uniquely share your and your patients’ experiences. Your article does not have to appear in a major publication; you can still have an important impact in your local paper. See resources on how to write an op-ed and letter to the editor (https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community/legislative-rx-op-eds-and-letters-to-the-editor).
5. Teach about it
These legislative changes uniquely impact our ObGyn residents; 44% of residents likely will be in a training program in a state that will ban or severely restrict abortion access. Abortion is health care, and a vast majority of our residents could graduate without important skills to save lives. As we strategize to ensure all ObGyn residents are able to receive this important training, work on incorporating an advocacy curriculum into your residents’ educational experience. Teaching about how to advocate is an important skill for supporting our patients and ensuring critical health policy. ACOG has published guides focused on education and training (www.acog.org/advocacy/abortion-is-essential/education-and-training). We also have included our own medical center’s advocacy curriculum (https://docs.google.com/document/d/1STxLzE0j55mlDEbF0_wZbo9O QryAcs6RpfZ47Mwfs4I/edit).
6. Get involved and seek out allies
It’s important that ObGyns be at the table for all discussions surrounding abortion care and reproductive health. Join hospital committees and help influence policy within your own institution. Refer back to those abortion talking points—this will help in some of these challenging conversations.
7. Get on social media
Using social media can be a powerful tool for advocacy. You can help elevate issues and encourage others to get active as well. Using a common hashtag, such as #AbortionisHealthcare, on different platforms can help connect you to other advocates. Share simple and important graphics provided by ACOG on important topics in our field (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state/social-media) and review ACOG’s recommendation for professionalism in social media (https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/10/professional-use-of-digital-and-social-media).
8. Get active locally
We have seen the introduction of hundreds of bills in states around the country not only on abortion but also on other legislation that directly impacts the care we provide. It is critical that we get involved in advocating for important reproductive health legislation and against bills that cause harm and interfere with the doctor-patient relationship. Stay up to date on legislative issues with your local ACOG and medical chapters (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state). Consider testifying at your State house, providing written or oral testimony. Connect with ACOG or your state medical chapter to help with talking points!
9. Read up
There have been many new policies at the federal level that could impact the care you provide. Take some time to read up on these new changes. Patients also may ask you about self-managed abortion. There are guides and resources (https://www.acog.org/advocacy/abortion-is-essential/practice-management) for patients that may seek medication online, and we want to ensure that patients have the resources to make informed decisions.
10. Hit the Capitol
Consider making time to come to the annual Congressional Leadership Conference in Washington, DC (https://www.acog.org/education-and-events/meetings/acog-congressional-leadership-conference), or other advocacy events offered through the American Medical Association or other subspecialty organizations. When we all come together as an organization, a field, and a community, it sends a powerful message that we are standing up together for our patients and our colleagues.
Make a difference
There is no advocacy too big or too small. It is critical that we continue to use our voices and our platforms to stand up for health care and access to critical services, including abortion care. ●
Simplify your approach to the diagnosis and treatment of PCOS
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).
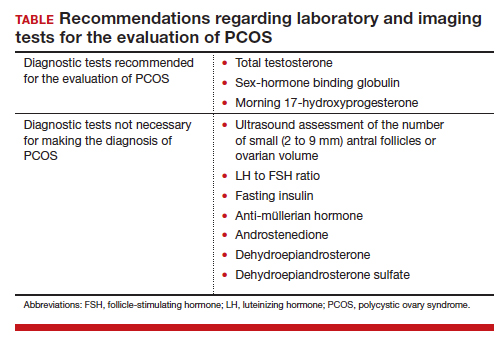
The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.
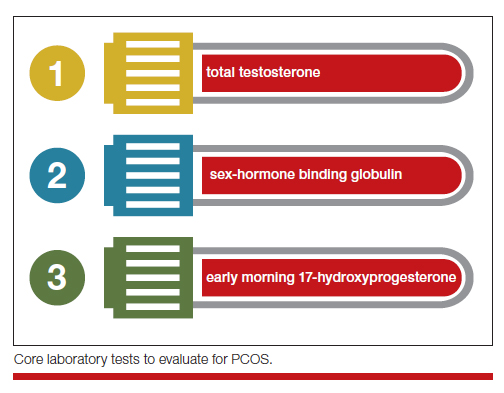
Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
U.S. sees most flu hospitalizations in a decade
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.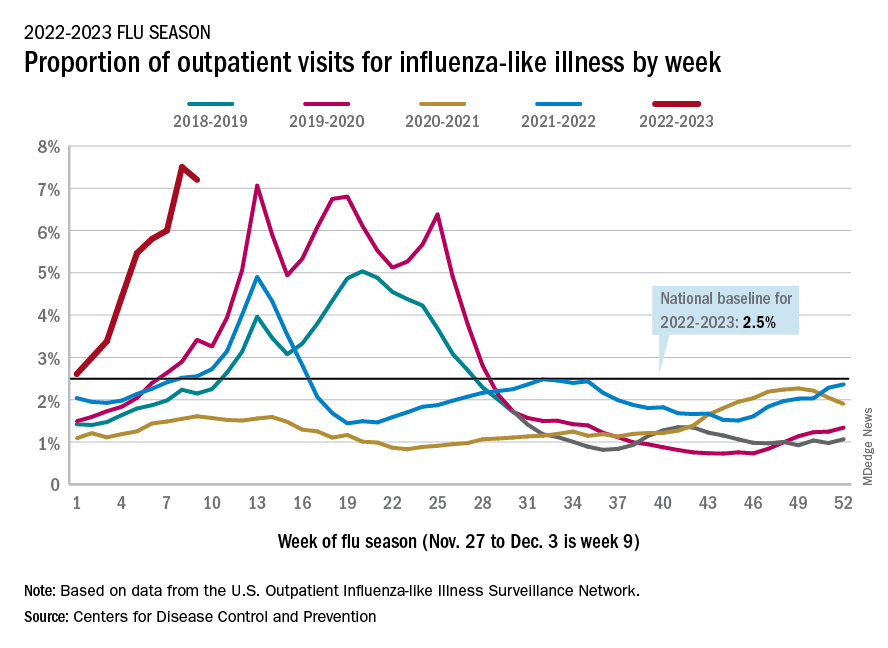
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
Improving sleep boosts cognition in refractory temporal lobe epilepsy
NASHVILLE, TENN. – Targeting relevant sleep problems for patients with refractory temporal lobe epilepsy (TLE) improves cognition, results of a new, double-blind, randomized controlled trial suggest.
Study findings show significant improvement in REM sleep and language scores for patients with TLE who took the cholinesterase inhibitor donepezil and better slow-wave sleep and memory scores for those who took the sleep aid zolpidem.
, study investigator Garima Shukla, MBBS, MD, DM, professor, division of neurology, department of medicine, Queens University, Kingston, Ont., told this news organization.
Daytime sleepiness could be a red flag in these patients, although it could mean they just have treatable sleep apnea, said Dr. Shukla. “But if they have very poor slow-wave sleep, we could try increasing its percentage by prescribing zolpidem.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Sleep, cognitive disturbances common
Sleep disturbances and cognitive disturbances are common among patients with TLE. Executive function is affected in almost all patients with refractory epilepsy, and it’s “super common” that TLE patients have memory disturbances, said Dr. Shukla.
The study included 108 patients with refractory TLE who were awaiting surgery. The patients, who had no severe comorbidities, were randomly assigned to three groups; the final number in each group was 36.
Patients in group 1 received donepezil 10 mg in the morning and a placebo at night. (Donepezil is used to treat memory loss associated with Alzheimer’s disease.)
Those in group 2 received a placebo in the morning and zolpidem 6.25 mg at night. Group 3 patients received a placebo in the morning and again at night.
The mean age of the patients was 25.4, 27.1, and 27.6 years, and the percentage of men was 63.8%, 72.2%, and 63.8% in groups 1, 2, and 3, respectively.
In all groups, patients had been experiencing about three seizures per month. The median number of antiseizure medications was two in group 1 and three in both groups 2 and 3.
Researchers evaluated sleep using the Pittsburgh Sleep Quality Index, the Epsworth Sleepiness Scale, and video polysomnography and electroencephalography.
To assess executive function, they used the Trail A & B, Stroop, and forward and backward Digit Span tests. For memory, they used the Weschler Memory Scale, and for language, the Western Aphasia Battery. They conducted follow-up evaluations at 6 months.
The results showed significant improvement in the percentage of rapid eye movement (REM) sleep in group 1 (from 14.81 at baseline to 18.21 at 6 months). In this group, the number of patients whose REM sleep percentage was less than 15 dropped significantly from 29 (of 36) to 10.
In group 2, sleep-onset latency significantly improved, and the percentage of N3 (slow-wave) sleep stage increased significantly from 25.27 to 28.74.
Regarding cognitive outcomes, backward Digit Span was significantly improved for patients in group 1. In this group, there was also a significant reduction in the time taken for Stroop A test, and there was significant improvement in language.
In group 2, there was a significant improvement in verbal and visual memory scores. There were no significant changes in group 3.
The increase in REM sleep percentage in group 1 strongly correlated with increased language and executive function scores. Similarly, in group 2, the increase in N3 sleep percentage strongly correlated with an increase in verbal memory scores.
On the basis of these observations, giving a small dose of zolpidem to a patient with “acceptable” REM sleep but very little slow-wave sleep may boost the patient’s non-REM sleep, said Dr. Shukla. “By improving non-REM sleep percentage, we will possibly help memory consolidation.”
Dr. Shukla sees this study as “a stepping-stone” to larger, multicenter trials testing “the effect of zolpidem through its impact on improving non-REM sleep percentage consolidation and its impact on memory.”
This idea veers somewhat from the traditional idea that REM sleep plays a greater role in memory consolidation, she said. “We actually found it correlates very well with language, which we have also seen in some of our anecdotal case reports.”
Patients whose language scores are very poor are “the population I would pick to target REM sleep through donepezil,” said Dr. Shukla.
‘Encouraging’ findings
Commenting for this news organization, Daniel Goldenholz, MD, PhD, assistant professor, Harvard Beth Israel Deaconess Medical Center, Boston, praised the study design.
“It allows for comparison between different treatments, as well as a placebo control group,” said Dr. Goldenholz, who added, “There appears to be good follow-up” as well.
The fact that medication may provide some cognitive benefit for patients with TLE is “very encouraging,” he said.
He noted many patients with TLE complain of memory or language problems. “So, this is a major concern.”
However, he cautioned about side effects. “Putting all temporal lobe epilepsy patients who say that they have memory problems or language problems on these medications could have some serious consequences.”
The study was funded by a Department of Health Research grant from the government of India. Dr. Goldenholz is on the advisory board for epilepsy AI, Eyzs, and Magic Leap.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – Targeting relevant sleep problems for patients with refractory temporal lobe epilepsy (TLE) improves cognition, results of a new, double-blind, randomized controlled trial suggest.
Study findings show significant improvement in REM sleep and language scores for patients with TLE who took the cholinesterase inhibitor donepezil and better slow-wave sleep and memory scores for those who took the sleep aid zolpidem.
, study investigator Garima Shukla, MBBS, MD, DM, professor, division of neurology, department of medicine, Queens University, Kingston, Ont., told this news organization.
Daytime sleepiness could be a red flag in these patients, although it could mean they just have treatable sleep apnea, said Dr. Shukla. “But if they have very poor slow-wave sleep, we could try increasing its percentage by prescribing zolpidem.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Sleep, cognitive disturbances common
Sleep disturbances and cognitive disturbances are common among patients with TLE. Executive function is affected in almost all patients with refractory epilepsy, and it’s “super common” that TLE patients have memory disturbances, said Dr. Shukla.
The study included 108 patients with refractory TLE who were awaiting surgery. The patients, who had no severe comorbidities, were randomly assigned to three groups; the final number in each group was 36.
Patients in group 1 received donepezil 10 mg in the morning and a placebo at night. (Donepezil is used to treat memory loss associated with Alzheimer’s disease.)
Those in group 2 received a placebo in the morning and zolpidem 6.25 mg at night. Group 3 patients received a placebo in the morning and again at night.
The mean age of the patients was 25.4, 27.1, and 27.6 years, and the percentage of men was 63.8%, 72.2%, and 63.8% in groups 1, 2, and 3, respectively.
In all groups, patients had been experiencing about three seizures per month. The median number of antiseizure medications was two in group 1 and three in both groups 2 and 3.
Researchers evaluated sleep using the Pittsburgh Sleep Quality Index, the Epsworth Sleepiness Scale, and video polysomnography and electroencephalography.
To assess executive function, they used the Trail A & B, Stroop, and forward and backward Digit Span tests. For memory, they used the Weschler Memory Scale, and for language, the Western Aphasia Battery. They conducted follow-up evaluations at 6 months.
The results showed significant improvement in the percentage of rapid eye movement (REM) sleep in group 1 (from 14.81 at baseline to 18.21 at 6 months). In this group, the number of patients whose REM sleep percentage was less than 15 dropped significantly from 29 (of 36) to 10.
In group 2, sleep-onset latency significantly improved, and the percentage of N3 (slow-wave) sleep stage increased significantly from 25.27 to 28.74.
Regarding cognitive outcomes, backward Digit Span was significantly improved for patients in group 1. In this group, there was also a significant reduction in the time taken for Stroop A test, and there was significant improvement in language.
In group 2, there was a significant improvement in verbal and visual memory scores. There were no significant changes in group 3.
The increase in REM sleep percentage in group 1 strongly correlated with increased language and executive function scores. Similarly, in group 2, the increase in N3 sleep percentage strongly correlated with an increase in verbal memory scores.
On the basis of these observations, giving a small dose of zolpidem to a patient with “acceptable” REM sleep but very little slow-wave sleep may boost the patient’s non-REM sleep, said Dr. Shukla. “By improving non-REM sleep percentage, we will possibly help memory consolidation.”
Dr. Shukla sees this study as “a stepping-stone” to larger, multicenter trials testing “the effect of zolpidem through its impact on improving non-REM sleep percentage consolidation and its impact on memory.”
This idea veers somewhat from the traditional idea that REM sleep plays a greater role in memory consolidation, she said. “We actually found it correlates very well with language, which we have also seen in some of our anecdotal case reports.”
Patients whose language scores are very poor are “the population I would pick to target REM sleep through donepezil,” said Dr. Shukla.
‘Encouraging’ findings
Commenting for this news organization, Daniel Goldenholz, MD, PhD, assistant professor, Harvard Beth Israel Deaconess Medical Center, Boston, praised the study design.
“It allows for comparison between different treatments, as well as a placebo control group,” said Dr. Goldenholz, who added, “There appears to be good follow-up” as well.
The fact that medication may provide some cognitive benefit for patients with TLE is “very encouraging,” he said.
He noted many patients with TLE complain of memory or language problems. “So, this is a major concern.”
However, he cautioned about side effects. “Putting all temporal lobe epilepsy patients who say that they have memory problems or language problems on these medications could have some serious consequences.”
The study was funded by a Department of Health Research grant from the government of India. Dr. Goldenholz is on the advisory board for epilepsy AI, Eyzs, and Magic Leap.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – Targeting relevant sleep problems for patients with refractory temporal lobe epilepsy (TLE) improves cognition, results of a new, double-blind, randomized controlled trial suggest.
Study findings show significant improvement in REM sleep and language scores for patients with TLE who took the cholinesterase inhibitor donepezil and better slow-wave sleep and memory scores for those who took the sleep aid zolpidem.
, study investigator Garima Shukla, MBBS, MD, DM, professor, division of neurology, department of medicine, Queens University, Kingston, Ont., told this news organization.
Daytime sleepiness could be a red flag in these patients, although it could mean they just have treatable sleep apnea, said Dr. Shukla. “But if they have very poor slow-wave sleep, we could try increasing its percentage by prescribing zolpidem.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Sleep, cognitive disturbances common
Sleep disturbances and cognitive disturbances are common among patients with TLE. Executive function is affected in almost all patients with refractory epilepsy, and it’s “super common” that TLE patients have memory disturbances, said Dr. Shukla.
The study included 108 patients with refractory TLE who were awaiting surgery. The patients, who had no severe comorbidities, were randomly assigned to three groups; the final number in each group was 36.
Patients in group 1 received donepezil 10 mg in the morning and a placebo at night. (Donepezil is used to treat memory loss associated with Alzheimer’s disease.)
Those in group 2 received a placebo in the morning and zolpidem 6.25 mg at night. Group 3 patients received a placebo in the morning and again at night.
The mean age of the patients was 25.4, 27.1, and 27.6 years, and the percentage of men was 63.8%, 72.2%, and 63.8% in groups 1, 2, and 3, respectively.
In all groups, patients had been experiencing about three seizures per month. The median number of antiseizure medications was two in group 1 and three in both groups 2 and 3.
Researchers evaluated sleep using the Pittsburgh Sleep Quality Index, the Epsworth Sleepiness Scale, and video polysomnography and electroencephalography.
To assess executive function, they used the Trail A & B, Stroop, and forward and backward Digit Span tests. For memory, they used the Weschler Memory Scale, and for language, the Western Aphasia Battery. They conducted follow-up evaluations at 6 months.
The results showed significant improvement in the percentage of rapid eye movement (REM) sleep in group 1 (from 14.81 at baseline to 18.21 at 6 months). In this group, the number of patients whose REM sleep percentage was less than 15 dropped significantly from 29 (of 36) to 10.
In group 2, sleep-onset latency significantly improved, and the percentage of N3 (slow-wave) sleep stage increased significantly from 25.27 to 28.74.
Regarding cognitive outcomes, backward Digit Span was significantly improved for patients in group 1. In this group, there was also a significant reduction in the time taken for Stroop A test, and there was significant improvement in language.
In group 2, there was a significant improvement in verbal and visual memory scores. There were no significant changes in group 3.
The increase in REM sleep percentage in group 1 strongly correlated with increased language and executive function scores. Similarly, in group 2, the increase in N3 sleep percentage strongly correlated with an increase in verbal memory scores.
On the basis of these observations, giving a small dose of zolpidem to a patient with “acceptable” REM sleep but very little slow-wave sleep may boost the patient’s non-REM sleep, said Dr. Shukla. “By improving non-REM sleep percentage, we will possibly help memory consolidation.”
Dr. Shukla sees this study as “a stepping-stone” to larger, multicenter trials testing “the effect of zolpidem through its impact on improving non-REM sleep percentage consolidation and its impact on memory.”
This idea veers somewhat from the traditional idea that REM sleep plays a greater role in memory consolidation, she said. “We actually found it correlates very well with language, which we have also seen in some of our anecdotal case reports.”
Patients whose language scores are very poor are “the population I would pick to target REM sleep through donepezil,” said Dr. Shukla.
‘Encouraging’ findings
Commenting for this news organization, Daniel Goldenholz, MD, PhD, assistant professor, Harvard Beth Israel Deaconess Medical Center, Boston, praised the study design.
“It allows for comparison between different treatments, as well as a placebo control group,” said Dr. Goldenholz, who added, “There appears to be good follow-up” as well.
The fact that medication may provide some cognitive benefit for patients with TLE is “very encouraging,” he said.
He noted many patients with TLE complain of memory or language problems. “So, this is a major concern.”
However, he cautioned about side effects. “Putting all temporal lobe epilepsy patients who say that they have memory problems or language problems on these medications could have some serious consequences.”
The study was funded by a Department of Health Research grant from the government of India. Dr. Goldenholz is on the advisory board for epilepsy AI, Eyzs, and Magic Leap.
A version of this article first appeared on Medscape.com.
AT AES 2022
Lifestyle choices could curb genetic risk for thyroid cancer
A healthier lifestyle mitigated the impact of genetic factors on the risk of thyroid cancer, in a study based on data from more than 260,000 individuals.
Thyroid cancer has increased globally in recent years and ranks 9th among 36 cancers worldwide, at a considerable cost to health care systems, wrote Xiuming Feng of Guangxi Medical University, Nanning, Guangxi, China, and colleagues.
Both genetic and lifestyle factors are related to thyroid cancer; previous research suggests a heritability of about 50%, but data on the impact of modifiable lifestyle factors on thyroid cancer are limited, the researchers said.
In a prospective cohort study published in JAMA Network Open, the researchers used data from the UK Biobank and recruited adults aged 40-69 years during March 2006–October 2010. The final study population included 264,956 individuals of European descent. The median age of the participants was 57 years, and 52% were women.
Data on lifestyle behaviors were collected using interviews and questionnaires. The researchers constructed a total lifestyle score based on five variables: diet, physical activity, weight, smoking, and alcohol consumption. Each variable was assigned a score of 0 or 1, with 1 being favorable lifestyle behavior. Lifestyle was divided into three categories: unfavorable (scores 0-1), intermediate (score 2), and favorable (scores 3-5).
Each individual’s polygenic risk score (PRS) was categorized as low, intermediate, or high based on a meta–genome-wide association study of three cohorts.
The main outcome was the development of thyroid cancer.
The researchers identified 423 incident thyroid cancer cases over a median follow-up of 11.1 years.
Overall, higher PRSs were significantly associated with thyroid cancer (hazard ratio, 2.25; 95% confidence interval [CI], 1.91-2.64; P < .00001) as was an unfavorable lifestyle score (HR, 1.93; 95% CI, 1.50-2.49; P < .001 for trend).
An unfavorable lifestyle was significantly associated with thyroid cancer in the highest PRS group, and individuals with high PRS and unfavorable lifestyle had a nearly fivefold increased risk of thyroid cancer (HR, 4.89; 95% CI, 3.03-7.91; P < .001). By extension, “Adherence to a healthier lifestyle could decrease the incidence of thyroid cancer in individuals with a higher PRS,” the researchers wrote in their discussion.
The findings were limited by several factors, including the availability of only baseline lifestyle data, and lack of data on iodine intake, radiation exposure, experience, and family history, the researchers noted. Other limitations include the potential lack of generalizability to populations other than the individuals of European descent in the current study, they said.
However, the study is the first known to address the association among lifestyle, genetic factors, and risk of thyroid cancer, and was strengthened by the large study population, and the results suggest that lifestyle interventions may help reduce the risk of thyroid cancer in those with a genetic predisposition, they concluded.
Healthy living can make a difference
The incidence of thyroid cancer has increased annually, and exploring the possible risk factors could prevent the occurrence of thyroid cancer, corresponding author Xiaobo Yang, PhD, said in an interview.
Previous studies have reported that thyroid cancer is related to genetics and lifestyle, said Dr. Yang. “However, whether healthy lifestyle was associated with thyroid cancer risk and could attenuate the impact of genetic variants on thyroid cancer remains equivocal; therefore, it is crucial to determine the associations between genetic and lifestyle with thyroid cancer,” he said.
“To our surprise, we found that adherence to healthier lifestyle also could reduce the risk of thyroid cancer in those with high genetic predispositions,” said Dr. Yang. “The findings highlight the potential role of lifestyle interventions on thyroid cancer, especially in those with high genetic risk, because the heritability of thyroid cancer was very high, approximately 50%,” he said. “More attention should be paid to the role of healthier lifestyle in the prevention of cancer,” he added.
“Adherence to a healthier lifestyle could decrease the risk of thyroid cancer, which is the important message for clinicians,” said Dr. Yang. “It is not too soon to comment on implications for clinical practice, because many studies have maintained the consistent comment that healthier lifestyle could prevent the occurrence of cancer,” he said.
The relationship between sex-specific lifestyle factors such as smoking and alcohol use and thyroid cancer remains uncertain, and more research is needed to validate these associations, Dr. Yang said. More research also is needed to confirm the complex mechanism between lifestyle and genetics in thyroid cancer, he added.
The study was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
A healthier lifestyle mitigated the impact of genetic factors on the risk of thyroid cancer, in a study based on data from more than 260,000 individuals.
Thyroid cancer has increased globally in recent years and ranks 9th among 36 cancers worldwide, at a considerable cost to health care systems, wrote Xiuming Feng of Guangxi Medical University, Nanning, Guangxi, China, and colleagues.
Both genetic and lifestyle factors are related to thyroid cancer; previous research suggests a heritability of about 50%, but data on the impact of modifiable lifestyle factors on thyroid cancer are limited, the researchers said.
In a prospective cohort study published in JAMA Network Open, the researchers used data from the UK Biobank and recruited adults aged 40-69 years during March 2006–October 2010. The final study population included 264,956 individuals of European descent. The median age of the participants was 57 years, and 52% were women.
Data on lifestyle behaviors were collected using interviews and questionnaires. The researchers constructed a total lifestyle score based on five variables: diet, physical activity, weight, smoking, and alcohol consumption. Each variable was assigned a score of 0 or 1, with 1 being favorable lifestyle behavior. Lifestyle was divided into three categories: unfavorable (scores 0-1), intermediate (score 2), and favorable (scores 3-5).
Each individual’s polygenic risk score (PRS) was categorized as low, intermediate, or high based on a meta–genome-wide association study of three cohorts.
The main outcome was the development of thyroid cancer.
The researchers identified 423 incident thyroid cancer cases over a median follow-up of 11.1 years.
Overall, higher PRSs were significantly associated with thyroid cancer (hazard ratio, 2.25; 95% confidence interval [CI], 1.91-2.64; P < .00001) as was an unfavorable lifestyle score (HR, 1.93; 95% CI, 1.50-2.49; P < .001 for trend).
An unfavorable lifestyle was significantly associated with thyroid cancer in the highest PRS group, and individuals with high PRS and unfavorable lifestyle had a nearly fivefold increased risk of thyroid cancer (HR, 4.89; 95% CI, 3.03-7.91; P < .001). By extension, “Adherence to a healthier lifestyle could decrease the incidence of thyroid cancer in individuals with a higher PRS,” the researchers wrote in their discussion.
The findings were limited by several factors, including the availability of only baseline lifestyle data, and lack of data on iodine intake, radiation exposure, experience, and family history, the researchers noted. Other limitations include the potential lack of generalizability to populations other than the individuals of European descent in the current study, they said.
However, the study is the first known to address the association among lifestyle, genetic factors, and risk of thyroid cancer, and was strengthened by the large study population, and the results suggest that lifestyle interventions may help reduce the risk of thyroid cancer in those with a genetic predisposition, they concluded.
Healthy living can make a difference
The incidence of thyroid cancer has increased annually, and exploring the possible risk factors could prevent the occurrence of thyroid cancer, corresponding author Xiaobo Yang, PhD, said in an interview.
Previous studies have reported that thyroid cancer is related to genetics and lifestyle, said Dr. Yang. “However, whether healthy lifestyle was associated with thyroid cancer risk and could attenuate the impact of genetic variants on thyroid cancer remains equivocal; therefore, it is crucial to determine the associations between genetic and lifestyle with thyroid cancer,” he said.
“To our surprise, we found that adherence to healthier lifestyle also could reduce the risk of thyroid cancer in those with high genetic predispositions,” said Dr. Yang. “The findings highlight the potential role of lifestyle interventions on thyroid cancer, especially in those with high genetic risk, because the heritability of thyroid cancer was very high, approximately 50%,” he said. “More attention should be paid to the role of healthier lifestyle in the prevention of cancer,” he added.
“Adherence to a healthier lifestyle could decrease the risk of thyroid cancer, which is the important message for clinicians,” said Dr. Yang. “It is not too soon to comment on implications for clinical practice, because many studies have maintained the consistent comment that healthier lifestyle could prevent the occurrence of cancer,” he said.
The relationship between sex-specific lifestyle factors such as smoking and alcohol use and thyroid cancer remains uncertain, and more research is needed to validate these associations, Dr. Yang said. More research also is needed to confirm the complex mechanism between lifestyle and genetics in thyroid cancer, he added.
The study was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
A healthier lifestyle mitigated the impact of genetic factors on the risk of thyroid cancer, in a study based on data from more than 260,000 individuals.
Thyroid cancer has increased globally in recent years and ranks 9th among 36 cancers worldwide, at a considerable cost to health care systems, wrote Xiuming Feng of Guangxi Medical University, Nanning, Guangxi, China, and colleagues.
Both genetic and lifestyle factors are related to thyroid cancer; previous research suggests a heritability of about 50%, but data on the impact of modifiable lifestyle factors on thyroid cancer are limited, the researchers said.
In a prospective cohort study published in JAMA Network Open, the researchers used data from the UK Biobank and recruited adults aged 40-69 years during March 2006–October 2010. The final study population included 264,956 individuals of European descent. The median age of the participants was 57 years, and 52% were women.
Data on lifestyle behaviors were collected using interviews and questionnaires. The researchers constructed a total lifestyle score based on five variables: diet, physical activity, weight, smoking, and alcohol consumption. Each variable was assigned a score of 0 or 1, with 1 being favorable lifestyle behavior. Lifestyle was divided into three categories: unfavorable (scores 0-1), intermediate (score 2), and favorable (scores 3-5).
Each individual’s polygenic risk score (PRS) was categorized as low, intermediate, or high based on a meta–genome-wide association study of three cohorts.
The main outcome was the development of thyroid cancer.
The researchers identified 423 incident thyroid cancer cases over a median follow-up of 11.1 years.
Overall, higher PRSs were significantly associated with thyroid cancer (hazard ratio, 2.25; 95% confidence interval [CI], 1.91-2.64; P < .00001) as was an unfavorable lifestyle score (HR, 1.93; 95% CI, 1.50-2.49; P < .001 for trend).
An unfavorable lifestyle was significantly associated with thyroid cancer in the highest PRS group, and individuals with high PRS and unfavorable lifestyle had a nearly fivefold increased risk of thyroid cancer (HR, 4.89; 95% CI, 3.03-7.91; P < .001). By extension, “Adherence to a healthier lifestyle could decrease the incidence of thyroid cancer in individuals with a higher PRS,” the researchers wrote in their discussion.
The findings were limited by several factors, including the availability of only baseline lifestyle data, and lack of data on iodine intake, radiation exposure, experience, and family history, the researchers noted. Other limitations include the potential lack of generalizability to populations other than the individuals of European descent in the current study, they said.
However, the study is the first known to address the association among lifestyle, genetic factors, and risk of thyroid cancer, and was strengthened by the large study population, and the results suggest that lifestyle interventions may help reduce the risk of thyroid cancer in those with a genetic predisposition, they concluded.
Healthy living can make a difference
The incidence of thyroid cancer has increased annually, and exploring the possible risk factors could prevent the occurrence of thyroid cancer, corresponding author Xiaobo Yang, PhD, said in an interview.
Previous studies have reported that thyroid cancer is related to genetics and lifestyle, said Dr. Yang. “However, whether healthy lifestyle was associated with thyroid cancer risk and could attenuate the impact of genetic variants on thyroid cancer remains equivocal; therefore, it is crucial to determine the associations between genetic and lifestyle with thyroid cancer,” he said.
“To our surprise, we found that adherence to healthier lifestyle also could reduce the risk of thyroid cancer in those with high genetic predispositions,” said Dr. Yang. “The findings highlight the potential role of lifestyle interventions on thyroid cancer, especially in those with high genetic risk, because the heritability of thyroid cancer was very high, approximately 50%,” he said. “More attention should be paid to the role of healthier lifestyle in the prevention of cancer,” he added.
“Adherence to a healthier lifestyle could decrease the risk of thyroid cancer, which is the important message for clinicians,” said Dr. Yang. “It is not too soon to comment on implications for clinical practice, because many studies have maintained the consistent comment that healthier lifestyle could prevent the occurrence of cancer,” he said.
The relationship between sex-specific lifestyle factors such as smoking and alcohol use and thyroid cancer remains uncertain, and more research is needed to validate these associations, Dr. Yang said. More research also is needed to confirm the complex mechanism between lifestyle and genetics in thyroid cancer, he added.
The study was supported by the National Key R&D Program of China and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Can a Scholarship Program Fill VA’s Staffing Gaps?
In a new attempt to replenish the constantly draining pool of mental health professionals, the US Department of Veterans Affairs (VA) is establishing a scholarship program for students pursuing graduate degrees in psychology, social work, marriage and family therapy, or mental health counseling.
Staffing shortages in mental health have reached crisis proportions across the country, driven in part by 3 years of the pandemic. The VA is not immune. VA shortages go back a long way and have never really been resolved. In 2012, for instance, the VA announced that it planned to expand its mental health staff by nearly 10%, hiring about 1600 additional psychiatrists, psychologists, social workers, and other mental health clinicians to reduce long wait times at many VA medical centers. And indeed, between 2018 and 2021, the number of severe shortages reported declined from 3,068 to 2,152.
However, in 2021, the VA Office of the Inspector General (OIG) released its eighth report in a series on occupational staffing shortages for the 139 facilities. According to the OIG report, 136 facilities reported at least 1 severe occupational staffing shortage, an increase from 132 in fiscal year 2020. Psychiatry was the most frequently reported clinical occupation with severe staffing shortages.
In July 2022, the OIG released its ninth report and the fifth to identify “severe occupational staffing shortages” for VA facilities. The OIG found severe shortages were widespread: Facilities identified 2,622 severe occupational staffing shortages across 285 occupations, which ended a downward trend. Of the 139 facilities, 73 identified severe shortage in psychology, 71 listed psychiatry, 44 listed social work, and 30 listed registered nurse staff for inpatient mental health sections.
In fact, although the Veterans Health Administration has been increasing the number of staff since 2017, psychology and psychiatry have remained in the top 10 most frequently reported severe shortages annually.
The scholarship program, expected to start in summer 2023, will fund up to 2 years of graduate studies. After completing their degrees, the mental health professionals will serve full time for 6 years at one of the VA’s Vet Centers, specifically in underserved areas and in states with a per capita population of more than 5% veterans. Vet Centers are community-based outpatient counseling centers that provide a wide range of social and psychological services.
“In 300 communities across the country, Vet Centers provide veterans, service members, and their families with quick and easy access to the mental health care they need and deserve,” said VA Secretary Denis McDonough. “These scholarships will help VA ensure all veterans and service members—including those in historically underserved areas—have access to Vet Centers with highly qualified, trained and compassionate staff.”
The VA has posted a final rule for public inspection in the Federal Register 86 FR 81094 to create the scholarship program.
In a new attempt to replenish the constantly draining pool of mental health professionals, the US Department of Veterans Affairs (VA) is establishing a scholarship program for students pursuing graduate degrees in psychology, social work, marriage and family therapy, or mental health counseling.
Staffing shortages in mental health have reached crisis proportions across the country, driven in part by 3 years of the pandemic. The VA is not immune. VA shortages go back a long way and have never really been resolved. In 2012, for instance, the VA announced that it planned to expand its mental health staff by nearly 10%, hiring about 1600 additional psychiatrists, psychologists, social workers, and other mental health clinicians to reduce long wait times at many VA medical centers. And indeed, between 2018 and 2021, the number of severe shortages reported declined from 3,068 to 2,152.
However, in 2021, the VA Office of the Inspector General (OIG) released its eighth report in a series on occupational staffing shortages for the 139 facilities. According to the OIG report, 136 facilities reported at least 1 severe occupational staffing shortage, an increase from 132 in fiscal year 2020. Psychiatry was the most frequently reported clinical occupation with severe staffing shortages.
In July 2022, the OIG released its ninth report and the fifth to identify “severe occupational staffing shortages” for VA facilities. The OIG found severe shortages were widespread: Facilities identified 2,622 severe occupational staffing shortages across 285 occupations, which ended a downward trend. Of the 139 facilities, 73 identified severe shortage in psychology, 71 listed psychiatry, 44 listed social work, and 30 listed registered nurse staff for inpatient mental health sections.
In fact, although the Veterans Health Administration has been increasing the number of staff since 2017, psychology and psychiatry have remained in the top 10 most frequently reported severe shortages annually.
The scholarship program, expected to start in summer 2023, will fund up to 2 years of graduate studies. After completing their degrees, the mental health professionals will serve full time for 6 years at one of the VA’s Vet Centers, specifically in underserved areas and in states with a per capita population of more than 5% veterans. Vet Centers are community-based outpatient counseling centers that provide a wide range of social and psychological services.
“In 300 communities across the country, Vet Centers provide veterans, service members, and their families with quick and easy access to the mental health care they need and deserve,” said VA Secretary Denis McDonough. “These scholarships will help VA ensure all veterans and service members—including those in historically underserved areas—have access to Vet Centers with highly qualified, trained and compassionate staff.”
The VA has posted a final rule for public inspection in the Federal Register 86 FR 81094 to create the scholarship program.
In a new attempt to replenish the constantly draining pool of mental health professionals, the US Department of Veterans Affairs (VA) is establishing a scholarship program for students pursuing graduate degrees in psychology, social work, marriage and family therapy, or mental health counseling.
Staffing shortages in mental health have reached crisis proportions across the country, driven in part by 3 years of the pandemic. The VA is not immune. VA shortages go back a long way and have never really been resolved. In 2012, for instance, the VA announced that it planned to expand its mental health staff by nearly 10%, hiring about 1600 additional psychiatrists, psychologists, social workers, and other mental health clinicians to reduce long wait times at many VA medical centers. And indeed, between 2018 and 2021, the number of severe shortages reported declined from 3,068 to 2,152.
However, in 2021, the VA Office of the Inspector General (OIG) released its eighth report in a series on occupational staffing shortages for the 139 facilities. According to the OIG report, 136 facilities reported at least 1 severe occupational staffing shortage, an increase from 132 in fiscal year 2020. Psychiatry was the most frequently reported clinical occupation with severe staffing shortages.
In July 2022, the OIG released its ninth report and the fifth to identify “severe occupational staffing shortages” for VA facilities. The OIG found severe shortages were widespread: Facilities identified 2,622 severe occupational staffing shortages across 285 occupations, which ended a downward trend. Of the 139 facilities, 73 identified severe shortage in psychology, 71 listed psychiatry, 44 listed social work, and 30 listed registered nurse staff for inpatient mental health sections.
In fact, although the Veterans Health Administration has been increasing the number of staff since 2017, psychology and psychiatry have remained in the top 10 most frequently reported severe shortages annually.
The scholarship program, expected to start in summer 2023, will fund up to 2 years of graduate studies. After completing their degrees, the mental health professionals will serve full time for 6 years at one of the VA’s Vet Centers, specifically in underserved areas and in states with a per capita population of more than 5% veterans. Vet Centers are community-based outpatient counseling centers that provide a wide range of social and psychological services.
“In 300 communities across the country, Vet Centers provide veterans, service members, and their families with quick and easy access to the mental health care they need and deserve,” said VA Secretary Denis McDonough. “These scholarships will help VA ensure all veterans and service members—including those in historically underserved areas—have access to Vet Centers with highly qualified, trained and compassionate staff.”
The VA has posted a final rule for public inspection in the Federal Register 86 FR 81094 to create the scholarship program.
Upadacitinib curbed UC symptoms from Day 1 in study
Patients with ulcerative colitis who were treated with upadacitinib showed significant improvement in symptoms compared to placebo within days of starting treatment, according to results of a new study.
Immunosuppressants and biologics used to treat moderate to severe disease can vary in their response times from weeks to months, and some ambulatory patients fail to respond to these treatments, wrote Edward V. Loftus Jr., MD, of the Mayo Clinic, Rochester, Minn., and colleagues.
“The lack of effective, quick-acting therapies may lead patients to experience prolonged relapses, reduced quality of life, and a significant burden of illness,” the researchers said.
Upadacitinib, an oral, once-daily reversible JAK inhibitor, is approved by the Food and Drug Administration for patients with moderate to severe ulcerative colitis (UC) who have not responded to at least one tumor necrosis factor (TNF) blocker, but the time frame for response to upadacitinib has not been well studied, they said.
In a study published online in Clinical Gastroenterology and Hepatology, the researchers reviewed data from two phase 3 randomized, placebo-controlled induction trials, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026), that assessed the safety and efficacy of a 45-mg daily dose of upadacitinib for managing symptoms of moderate to severe UC over an 8-week period.
The intent-to-treat analysis included 660 patients who had been randomized to received upadacitinib and 328 in the placebo group. Demographics were similar between the groups. Symptom improvement was based on changes in inflammatory markers high-sensitivity C-reactive protein (hs-CRP) and fecal calprotectin (FCP) measured at week 2. Quality of life was assessed at 2 and 8 weeks.
Between 1 and 3 days after starting treatment, significantly greater percentages of patients in the treatment arm achieved a reduction of at least 50% from baseline in hs-CRP (75.7% vs. 21.9%) and FCP (48.2% vs. 20.2%).
The significant differences in symptom improvement persisted for 14 days from the first dose.
Patients in the upadacitinib group also showed increased rates of clinical remission/response (26.9%/59.4%) based on Partial Mayo scores at week 2 compared with placebo patients (4.3%/22.3%). Treated patients showed significant improvements in quality of life at weeks 2 and 8.
In addition, patient-reported UC symptoms of stool frequency, rectal bleeding, abdominal pain, and bowel urgency improved significantly compared with placebo by day 3, the researchers noted. “In this study, upadacitinib led to absence of rectal bleeding by day 1 and absence of abdominal pain and bowel urgency by day 3 in a significant proportion of patients vs. placebo (all P < .05),” they said.
The findings were limited by the post hoc design, but reflect results of other studies, including those of the JAK inhibitor tofacitinib, the researchers noted. The results of the current study demonstrate the ability of upadacitinib to alleviate key UC symptoms as early as 1 day after the first dose, they concluded.
Need for rapid onset treatment
A majority of biologics used to treat IBD take weeks to months to exert an effect, “so it is crucial that a drug with more rapid onset of action become available,” Atsushi Sakuraba, MD, director of clinical trial/IBD research at the University of Chicago, said in an interview.
Dr. Sakuraba, who was not involved in the study, said he was surprised by the findings.
“It is amazing that upadacitinib started to show response in 1-3 days and showed superior rates of remission/response versus placebo by week 2,” he said.
“Clinicians treating IBD patients should take the time to response into consideration, because it leads to rapid improvement in quality of life and reduced need for steroids,” Dr. Sakuraba said. The current study findings need to be confirmed in real life studies, he cautioned.
Expanding clinical options
Jeffrey Berinstein, MD, of the University of Michigan, Ann Arbor, who was not involved with the study, said in an interview that he was not surprised by upadacitinib’s quick action, which supports what he has seen in clinical practice. “We now have post hoc data from the phase 3, multicenter induction trials ... to provide us with hard evidence to support our observations,” he noted.
The current study is important because many patients with UC can be very symptomatic, with rectal bleeding, diarrhea, urgency, and abdominal pain, he said in an interview.
“It is often critical to have a fast-acting medication to avoid worsening symptoms, hospitalization, and severe complications. This study shows that upadacitinib, a new oral small molecule that selectively inhibits JAK-1, works within 1 day to improve symptoms and within 2 weeks to improve CRP and fecal calprotectin,” he said.
The takeaway message to clinicians is that upadacitinib is effective, relatively safe, and fast-acting option for patients with UC who have previously failed an anti-TNF agent, he said.
Challenges remain, however.
“Despite this exciting new therapeutic, there is still significant research needed to break our current therapeutic efficacy ceiling and get more IBD patients into a stable and durable remission,” Dr. Berinstein said. “Additional research is needed to develop personalized treatment strategies to address the unique needs of individual patients and to guide optimal medical management.”
Dr. Berinstein and his team are exploring the use of upadacitinib on the sickest IBD patients, “those admitted to the hospital with acute severe ulcerative colitis,” he said. Emerging data from multiple academic centers suggest that “upadacitinib induction may be a viable treatment strategy for these very high-risk patients. Of course, large prospective trials will be needed to confirm this,” he added.
The study was funded by AbbVie, which markets upadacitinib (Rinvoq). Lead author Dr. Loftus and several study authors disclosed financial relationships with AbbVie and other companies. Dr. Sakuraba and Dr. Berinstein reported having no relevant financial conflicts.
Patients with ulcerative colitis who were treated with upadacitinib showed significant improvement in symptoms compared to placebo within days of starting treatment, according to results of a new study.
Immunosuppressants and biologics used to treat moderate to severe disease can vary in their response times from weeks to months, and some ambulatory patients fail to respond to these treatments, wrote Edward V. Loftus Jr., MD, of the Mayo Clinic, Rochester, Minn., and colleagues.
“The lack of effective, quick-acting therapies may lead patients to experience prolonged relapses, reduced quality of life, and a significant burden of illness,” the researchers said.
Upadacitinib, an oral, once-daily reversible JAK inhibitor, is approved by the Food and Drug Administration for patients with moderate to severe ulcerative colitis (UC) who have not responded to at least one tumor necrosis factor (TNF) blocker, but the time frame for response to upadacitinib has not been well studied, they said.
In a study published online in Clinical Gastroenterology and Hepatology, the researchers reviewed data from two phase 3 randomized, placebo-controlled induction trials, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026), that assessed the safety and efficacy of a 45-mg daily dose of upadacitinib for managing symptoms of moderate to severe UC over an 8-week period.
The intent-to-treat analysis included 660 patients who had been randomized to received upadacitinib and 328 in the placebo group. Demographics were similar between the groups. Symptom improvement was based on changes in inflammatory markers high-sensitivity C-reactive protein (hs-CRP) and fecal calprotectin (FCP) measured at week 2. Quality of life was assessed at 2 and 8 weeks.
Between 1 and 3 days after starting treatment, significantly greater percentages of patients in the treatment arm achieved a reduction of at least 50% from baseline in hs-CRP (75.7% vs. 21.9%) and FCP (48.2% vs. 20.2%).
The significant differences in symptom improvement persisted for 14 days from the first dose.
Patients in the upadacitinib group also showed increased rates of clinical remission/response (26.9%/59.4%) based on Partial Mayo scores at week 2 compared with placebo patients (4.3%/22.3%). Treated patients showed significant improvements in quality of life at weeks 2 and 8.
In addition, patient-reported UC symptoms of stool frequency, rectal bleeding, abdominal pain, and bowel urgency improved significantly compared with placebo by day 3, the researchers noted. “In this study, upadacitinib led to absence of rectal bleeding by day 1 and absence of abdominal pain and bowel urgency by day 3 in a significant proportion of patients vs. placebo (all P < .05),” they said.
The findings were limited by the post hoc design, but reflect results of other studies, including those of the JAK inhibitor tofacitinib, the researchers noted. The results of the current study demonstrate the ability of upadacitinib to alleviate key UC symptoms as early as 1 day after the first dose, they concluded.
Need for rapid onset treatment
A majority of biologics used to treat IBD take weeks to months to exert an effect, “so it is crucial that a drug with more rapid onset of action become available,” Atsushi Sakuraba, MD, director of clinical trial/IBD research at the University of Chicago, said in an interview.
Dr. Sakuraba, who was not involved in the study, said he was surprised by the findings.
“It is amazing that upadacitinib started to show response in 1-3 days and showed superior rates of remission/response versus placebo by week 2,” he said.
“Clinicians treating IBD patients should take the time to response into consideration, because it leads to rapid improvement in quality of life and reduced need for steroids,” Dr. Sakuraba said. The current study findings need to be confirmed in real life studies, he cautioned.
Expanding clinical options
Jeffrey Berinstein, MD, of the University of Michigan, Ann Arbor, who was not involved with the study, said in an interview that he was not surprised by upadacitinib’s quick action, which supports what he has seen in clinical practice. “We now have post hoc data from the phase 3, multicenter induction trials ... to provide us with hard evidence to support our observations,” he noted.
The current study is important because many patients with UC can be very symptomatic, with rectal bleeding, diarrhea, urgency, and abdominal pain, he said in an interview.
“It is often critical to have a fast-acting medication to avoid worsening symptoms, hospitalization, and severe complications. This study shows that upadacitinib, a new oral small molecule that selectively inhibits JAK-1, works within 1 day to improve symptoms and within 2 weeks to improve CRP and fecal calprotectin,” he said.
The takeaway message to clinicians is that upadacitinib is effective, relatively safe, and fast-acting option for patients with UC who have previously failed an anti-TNF agent, he said.
Challenges remain, however.
“Despite this exciting new therapeutic, there is still significant research needed to break our current therapeutic efficacy ceiling and get more IBD patients into a stable and durable remission,” Dr. Berinstein said. “Additional research is needed to develop personalized treatment strategies to address the unique needs of individual patients and to guide optimal medical management.”
Dr. Berinstein and his team are exploring the use of upadacitinib on the sickest IBD patients, “those admitted to the hospital with acute severe ulcerative colitis,” he said. Emerging data from multiple academic centers suggest that “upadacitinib induction may be a viable treatment strategy for these very high-risk patients. Of course, large prospective trials will be needed to confirm this,” he added.
The study was funded by AbbVie, which markets upadacitinib (Rinvoq). Lead author Dr. Loftus and several study authors disclosed financial relationships with AbbVie and other companies. Dr. Sakuraba and Dr. Berinstein reported having no relevant financial conflicts.
Patients with ulcerative colitis who were treated with upadacitinib showed significant improvement in symptoms compared to placebo within days of starting treatment, according to results of a new study.
Immunosuppressants and biologics used to treat moderate to severe disease can vary in their response times from weeks to months, and some ambulatory patients fail to respond to these treatments, wrote Edward V. Loftus Jr., MD, of the Mayo Clinic, Rochester, Minn., and colleagues.
“The lack of effective, quick-acting therapies may lead patients to experience prolonged relapses, reduced quality of life, and a significant burden of illness,” the researchers said.
Upadacitinib, an oral, once-daily reversible JAK inhibitor, is approved by the Food and Drug Administration for patients with moderate to severe ulcerative colitis (UC) who have not responded to at least one tumor necrosis factor (TNF) blocker, but the time frame for response to upadacitinib has not been well studied, they said.
In a study published online in Clinical Gastroenterology and Hepatology, the researchers reviewed data from two phase 3 randomized, placebo-controlled induction trials, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026), that assessed the safety and efficacy of a 45-mg daily dose of upadacitinib for managing symptoms of moderate to severe UC over an 8-week period.
The intent-to-treat analysis included 660 patients who had been randomized to received upadacitinib and 328 in the placebo group. Demographics were similar between the groups. Symptom improvement was based on changes in inflammatory markers high-sensitivity C-reactive protein (hs-CRP) and fecal calprotectin (FCP) measured at week 2. Quality of life was assessed at 2 and 8 weeks.
Between 1 and 3 days after starting treatment, significantly greater percentages of patients in the treatment arm achieved a reduction of at least 50% from baseline in hs-CRP (75.7% vs. 21.9%) and FCP (48.2% vs. 20.2%).
The significant differences in symptom improvement persisted for 14 days from the first dose.
Patients in the upadacitinib group also showed increased rates of clinical remission/response (26.9%/59.4%) based on Partial Mayo scores at week 2 compared with placebo patients (4.3%/22.3%). Treated patients showed significant improvements in quality of life at weeks 2 and 8.
In addition, patient-reported UC symptoms of stool frequency, rectal bleeding, abdominal pain, and bowel urgency improved significantly compared with placebo by day 3, the researchers noted. “In this study, upadacitinib led to absence of rectal bleeding by day 1 and absence of abdominal pain and bowel urgency by day 3 in a significant proportion of patients vs. placebo (all P < .05),” they said.
The findings were limited by the post hoc design, but reflect results of other studies, including those of the JAK inhibitor tofacitinib, the researchers noted. The results of the current study demonstrate the ability of upadacitinib to alleviate key UC symptoms as early as 1 day after the first dose, they concluded.
Need for rapid onset treatment
A majority of biologics used to treat IBD take weeks to months to exert an effect, “so it is crucial that a drug with more rapid onset of action become available,” Atsushi Sakuraba, MD, director of clinical trial/IBD research at the University of Chicago, said in an interview.
Dr. Sakuraba, who was not involved in the study, said he was surprised by the findings.
“It is amazing that upadacitinib started to show response in 1-3 days and showed superior rates of remission/response versus placebo by week 2,” he said.
“Clinicians treating IBD patients should take the time to response into consideration, because it leads to rapid improvement in quality of life and reduced need for steroids,” Dr. Sakuraba said. The current study findings need to be confirmed in real life studies, he cautioned.
Expanding clinical options
Jeffrey Berinstein, MD, of the University of Michigan, Ann Arbor, who was not involved with the study, said in an interview that he was not surprised by upadacitinib’s quick action, which supports what he has seen in clinical practice. “We now have post hoc data from the phase 3, multicenter induction trials ... to provide us with hard evidence to support our observations,” he noted.
The current study is important because many patients with UC can be very symptomatic, with rectal bleeding, diarrhea, urgency, and abdominal pain, he said in an interview.
“It is often critical to have a fast-acting medication to avoid worsening symptoms, hospitalization, and severe complications. This study shows that upadacitinib, a new oral small molecule that selectively inhibits JAK-1, works within 1 day to improve symptoms and within 2 weeks to improve CRP and fecal calprotectin,” he said.
The takeaway message to clinicians is that upadacitinib is effective, relatively safe, and fast-acting option for patients with UC who have previously failed an anti-TNF agent, he said.
Challenges remain, however.
“Despite this exciting new therapeutic, there is still significant research needed to break our current therapeutic efficacy ceiling and get more IBD patients into a stable and durable remission,” Dr. Berinstein said. “Additional research is needed to develop personalized treatment strategies to address the unique needs of individual patients and to guide optimal medical management.”
Dr. Berinstein and his team are exploring the use of upadacitinib on the sickest IBD patients, “those admitted to the hospital with acute severe ulcerative colitis,” he said. Emerging data from multiple academic centers suggest that “upadacitinib induction may be a viable treatment strategy for these very high-risk patients. Of course, large prospective trials will be needed to confirm this,” he added.
The study was funded by AbbVie, which markets upadacitinib (Rinvoq). Lead author Dr. Loftus and several study authors disclosed financial relationships with AbbVie and other companies. Dr. Sakuraba and Dr. Berinstein reported having no relevant financial conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY