User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
U.S. is poised to produce a COVID-19 vaccine, but don’t expect it soon
Manufacturers will begin producing COVID-19 vaccine doses in anticipation of approval so that if a product gets the okay for usage, distribution can begin quickly, according to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
“We will be producing vaccine at risk, which means we’ll be [investing] considerable resources in developing doses even before we know any given candidate or candidates work,” he testified during a May 12, 2020, hearing of the Senate Health, Education, Labor, and Pensions Committee.
During the hearing, Dr. Fauci did not elaborate on how the production at risk would be undertaken, what criteria would be in place for selecting which candidates would be in the pipeline, or how much would be spent on the advanced production of these vaccines.
And while Dr. Fauci, a member of the White House coronavirus task force, remained optimistic that one or more vaccine candidates would ultimately be viable, he cautioned that there remain many unknowns that could slow the development of a vaccine for COVID-19.
“I must warn that there’s also the possibility of negative consequences that certain vaccines can actually enhance the negative effect of the infection,” he said. “The big unknown is efficacy. Will it be present or absent and how durable will it be?”
It’s unlikely that either a vaccine or an effective treatment will be available in the next 3 months, Dr. Fauci told the committee.
Sen. Lamar Alexander (R-Tenn.), the committee chairman, asked Dr. Fauci what he would say to college, primary, and secondary school administrators about how the availability of treatments and vaccines could influence the ability to reopen campuses to students. Dr. Fauci replied that the idea of having treatments or a vaccine available to facilitate the reentry of students in the fall term would be “a bit of a bridge too far.”
The emphasis in the coming months should be on testing, contact tracing, and isolation of those infected with the virus, Dr. Fauci said.
Manufacturers will begin producing COVID-19 vaccine doses in anticipation of approval so that if a product gets the okay for usage, distribution can begin quickly, according to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
“We will be producing vaccine at risk, which means we’ll be [investing] considerable resources in developing doses even before we know any given candidate or candidates work,” he testified during a May 12, 2020, hearing of the Senate Health, Education, Labor, and Pensions Committee.
During the hearing, Dr. Fauci did not elaborate on how the production at risk would be undertaken, what criteria would be in place for selecting which candidates would be in the pipeline, or how much would be spent on the advanced production of these vaccines.
And while Dr. Fauci, a member of the White House coronavirus task force, remained optimistic that one or more vaccine candidates would ultimately be viable, he cautioned that there remain many unknowns that could slow the development of a vaccine for COVID-19.
“I must warn that there’s also the possibility of negative consequences that certain vaccines can actually enhance the negative effect of the infection,” he said. “The big unknown is efficacy. Will it be present or absent and how durable will it be?”
It’s unlikely that either a vaccine or an effective treatment will be available in the next 3 months, Dr. Fauci told the committee.
Sen. Lamar Alexander (R-Tenn.), the committee chairman, asked Dr. Fauci what he would say to college, primary, and secondary school administrators about how the availability of treatments and vaccines could influence the ability to reopen campuses to students. Dr. Fauci replied that the idea of having treatments or a vaccine available to facilitate the reentry of students in the fall term would be “a bit of a bridge too far.”
The emphasis in the coming months should be on testing, contact tracing, and isolation of those infected with the virus, Dr. Fauci said.
Manufacturers will begin producing COVID-19 vaccine doses in anticipation of approval so that if a product gets the okay for usage, distribution can begin quickly, according to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
“We will be producing vaccine at risk, which means we’ll be [investing] considerable resources in developing doses even before we know any given candidate or candidates work,” he testified during a May 12, 2020, hearing of the Senate Health, Education, Labor, and Pensions Committee.
During the hearing, Dr. Fauci did not elaborate on how the production at risk would be undertaken, what criteria would be in place for selecting which candidates would be in the pipeline, or how much would be spent on the advanced production of these vaccines.
And while Dr. Fauci, a member of the White House coronavirus task force, remained optimistic that one or more vaccine candidates would ultimately be viable, he cautioned that there remain many unknowns that could slow the development of a vaccine for COVID-19.
“I must warn that there’s also the possibility of negative consequences that certain vaccines can actually enhance the negative effect of the infection,” he said. “The big unknown is efficacy. Will it be present or absent and how durable will it be?”
It’s unlikely that either a vaccine or an effective treatment will be available in the next 3 months, Dr. Fauci told the committee.
Sen. Lamar Alexander (R-Tenn.), the committee chairman, asked Dr. Fauci what he would say to college, primary, and secondary school administrators about how the availability of treatments and vaccines could influence the ability to reopen campuses to students. Dr. Fauci replied that the idea of having treatments or a vaccine available to facilitate the reentry of students in the fall term would be “a bit of a bridge too far.”
The emphasis in the coming months should be on testing, contact tracing, and isolation of those infected with the virus, Dr. Fauci said.
ACR gives guidance on rheumatic disease management during pandemic
When COVID-19 is suspected or confirmed in a patient with a rheumatic disease, treatment with hydroxychloroquine may be continued, but other treatments may need to be stopped or held temporarily, according to new guidance issued by the American College of Rheumatology.
That includes disease-modifying treatment with antirheumatic drugs such as sulfasalazine, methotrexate, leflunomide, and the Janus kinase (JAK) inhibitors, as well as immunosuppressants and non-interleukin (IL)-6 biologics, and this is regardless of how severe the COVID-19 illness is. NSAIDs should also be stopped if there are respiratory symptoms.
The advice is slightly less drastic if someone with stable rheumatic disease has probably been exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are asymptomatic. In those patients, DMARDs may be continued, although there is uncertainty over whether there is a need to temporarily stop methotrexate or leflunomide. Interruption of immunosuppressive, non–IL-6, and JAK inhibitor treatment is advised pending a negative SARS-CoV-2 test result, assuming the patient’s rheumatic disease is stable.
Impetus for ACR COVID-19 guidance
“One of the earliest challenges for rheumatologists during the COVID-19 pandemic was determining how to advise our patients who were taking immunosuppressive medications and were concerned as to whether or not to discontinue their therapy,” ACR President Ellen Gravallese, MD, said in an interview about the ACR Clinical Guidance Document, which is published online in Arthritis & Rheumatology.
“A second challenge was keeping our patients safe from exposure to the virus, while still seeing those patients in person who required office visits,” added Dr. Gravallese, who is chief of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital in Boston.
She continued: “The ACR Clinical Guidance Document was prepared in order to assist rheumatologists with decisions as to how to handle current medications during different phases of a patient’s exposure to the SARS-CoV-2 virus.”
But with very little evidence available on how to manage COVID-19 patients generally, let alone specifically in those with rheumatic diseases, “it became evident that any recommendations made would need to be done in a thoughtful and organized manner, evaluating the evidence that was available and obtaining the advice of experts in infectious disease, epidemiology, and in the use of biologic and nonbiologic agents for rheumatic disease,” she said.
As such, the ACR convened a task force of 10 rheumatologists and 4 infectious disease specialists from North America to look at how best to manage patients with rheumatic disease during the COVID-19 pandemic.
“Our charge was to develop a guidance document for the care of adult rheumatic disease patients in the context of COVID-19 and not per se to provide guidance for the treatment of COVID-19,” explained task force member and the corresponding author for the guidance, Ted R. Mikuls, MD, MSPH, of the University of Nebraska Medical Center, Omaha.
Dr. Mikuls, who was speaking at a virtual town hall meeting hosted by the ACR on May 6, noted that the guidance was obviously based on the best consensus of the available data and as such represented a “living document” that “would change and be added to” as necessary.
General recommendations for adult rheumatic disease management
In terms of general recommendations for the management of adult rheumatic disease patients, Dr. Mikuls said that six statements had been made “specific to risk assessment, prevention of infection, and best practices related to glucocorticoid use and the use of ACE [angiotensin-converting enzyme] inhibitors and ARBs [angiotensin II receptor blockers] during the pandemic.”
For example, general advice is to counsel patients to keep up general preventive measures such as social distancing and regular hand washing, reducing the number of in-person health care visits, and undertaking other means to try to prevent potential SARS-CoV-2 exposure. As for general treatment advice, glucocorticoids should be used at their lowest doses possible and should not be abruptly stopped, and antihypertensive treatment should be used as indicated.
Additional guidance statements include those that address the treatment of patients with stable rheumatic disease in the absence of infection or known exposure to SARS-CoV-2, with guidance specific to the treatment of systemic lupus erythematosus (SLE), and those with newly diagnosed or active rheumatic disease.
SLE and inflammatory arthritis recommendations
“There are several sections within the guidance document that address the treatment of patients with systemic lupus erythematosus during this pandemic,” Dr. Gravallese pointed out. “In general, it is recommended that lupus patients who are currently taking hydroxychloroquine can remain on the therapy prior to and during infection and that newly diagnosed patients with lupus can be placed on this medication at full dose. It is recommended that pregnant patients with lupus remain on therapy with this drug.”
She also observed that, for the treatment of active inflammatory arthritis, “the recommendations were written to address specific medications that could be used in this setting. In general, the task force recommendations were guided by the importance of controlling inflammation prior to exposure to the virus, even during this pandemic.
Guidance raises questions
During the ACR’s town hall meeting, the task force answered several questions raised by the guidance, such as the reasoning behind recommending that the use of traditional DMARDs be discontinued in patients with confirmed SARS-CoV-2 infection.
Dr. Mikuls observed: “Maybe if you just read the guidance statements it isn’t terribly intuitive.” There was a lot of discussion about whether or not conventional DMARDs were immunosuppressive, and even though they may not have such effects, it was decided to err on the side of caution.
“I think the task force felt that, with a COVID-19–positive patient, there is a concern of potentially confusing adverse effects related to medicines or conflate those with problems from the infection,” he said. Although rare, examples of those issues could be drug-induced hypersensitivity, hypersensitivity pneumonitis, or gastrointestinal side effects of hepatitis, all of which have been described in COVID-19. “Not only could it cause confusion, but it could maybe worsen those sequalae of COVID-19,” he said.
“I think the other part of this answer was that the panel really felt that the risk in terms of the flaring of the underlying rheumatic disease was likely to be pretty low given the finite time frame you’d be taking about – usually a time frame of 2-3 weeks you’d be holding the agent – so I think that is really why the task force ended up with that recommendation.”
Similarly, for the JAK inhibitors, the decision was to err on the side of caution when COVID-19 was suspected or confirmed. “Not so much because of the risk of thromboembolic disease, but concerns over immunosuppression that these drugs carry with them and also the fact the JAK inhibitors are probably inhibitors of type 1 interferons, which play a significant role in viral immunity and could potentially have a negative impact,” said Stanley Cohen, MD, who practices rheumatology in the Dallas area.
“On the flipside, there is interest in some of the JAK inhibitors as a potential treatment for COVID-19,” Dr. Cohen said, referring to anecdotal evidence for baricitinib (Olumiant).
Michael Weinblatt, MD, of Brigham and Women’s Hospital, addressed the recent concern over the use of NSAIDs by the public.
“There’s been a lot in the lay press that NSAIDs – because of the effects on receptors in the lung – could lead to deleterious outcomes in patients with COVID and there’s very little data to support this.
“We did recommend that NSAIDs be held in the hospitalized patient and that wasn’t because of the COVID-19 issue, it really was just medical practice, and we didn’t want to confound the care of these really sick patients with potential toxicities from NSAIDs. But as far as routine rheumatological care in your outpatients, we did not recommend that nonsteroidals be stopped if they were tolerated.”
One part of the guidance that might already need revision is the recommendation on the continued use of hydroxychloroquine in patients who develop COVID-19.
“Our guidance document says it’s OK; we were all in very strong agreement to continue hydroxychloroquine in our patients with COVID-19 because at that point, just a couple of weeks ago, we thought it was part of the potential treatment,” Karen Costenbader, MD, MPH, of Brigham and Women’s Hospital, said during the town hall meeting.
“Now the pendulum has swung the other way, and we’re worried about maybe we shouldn’t be continuing it because COVID-19 patients will be getting many other medications,” Dr. Costenbader said, and these may affect the QT-interval. “They will not be getting azithromycin because the pendulum swung the other way on that one too, but definitely on many other medications when they are sick.”
Potentially, she added, “if the rheumatic disease is under good control the inpatient physicians could decide whether they should continue [hydroxychloroquine] or not. If the COVID-19 is a mild disease, I would say we probably could continue in accordance with what we put in the document, but we will have to revisit this as well.”
Guidance is a ‘living document’
“We will be providing updates to the Clinical Guidance Document as the need arises,” Dr. Gravallese emphasized. While the general recommendations are unlikely to change very much, “the task force will be interested in seeing the results of all new data, but the results of randomized, clinical trials will be particularly important as they become available,” she said. In particular, randomized, controlled trials of glucocorticoids and IL-6 receptor blockade for use in COVID-19 will be of great importance.
“In this initial document, we could not take on all of the medical scenarios our members will face. For example, we could not take on recommendations for the pediatric population as this group of patients has a very different response than adults to the SARS-CoV-2 virus,” Dr. Gravallese acknowledged. The plan is to provide guidance for that group of patients soon.
In addition, the ACR Executive Committee has appointed a Practice and Advocacy Task Force that will “address issues rheumatologists face on the practice side, including advice regarding how to effectively use telemedicine, address the frequency and safety of infusions, determine urgent versus nonurgent issues that would or would not require face-to-face visits, and help with financial challenges.”
The American College of Rheumatology supported the guidance-development process. Dr. Mikuls, Dr. Weinblatt, Dr. Cohen, and Dr. Costenbader each disclosed research support or consultancies with multiple pharmaceutical companies. Dr. Gravallese had no disclosures.
SOURCE: Mikuls TR et al. Arthritis Rheumatol. 2020 Apr 29. doi: 10.1002/art.41301.
When COVID-19 is suspected or confirmed in a patient with a rheumatic disease, treatment with hydroxychloroquine may be continued, but other treatments may need to be stopped or held temporarily, according to new guidance issued by the American College of Rheumatology.
That includes disease-modifying treatment with antirheumatic drugs such as sulfasalazine, methotrexate, leflunomide, and the Janus kinase (JAK) inhibitors, as well as immunosuppressants and non-interleukin (IL)-6 biologics, and this is regardless of how severe the COVID-19 illness is. NSAIDs should also be stopped if there are respiratory symptoms.
The advice is slightly less drastic if someone with stable rheumatic disease has probably been exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are asymptomatic. In those patients, DMARDs may be continued, although there is uncertainty over whether there is a need to temporarily stop methotrexate or leflunomide. Interruption of immunosuppressive, non–IL-6, and JAK inhibitor treatment is advised pending a negative SARS-CoV-2 test result, assuming the patient’s rheumatic disease is stable.
Impetus for ACR COVID-19 guidance
“One of the earliest challenges for rheumatologists during the COVID-19 pandemic was determining how to advise our patients who were taking immunosuppressive medications and were concerned as to whether or not to discontinue their therapy,” ACR President Ellen Gravallese, MD, said in an interview about the ACR Clinical Guidance Document, which is published online in Arthritis & Rheumatology.
“A second challenge was keeping our patients safe from exposure to the virus, while still seeing those patients in person who required office visits,” added Dr. Gravallese, who is chief of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital in Boston.
She continued: “The ACR Clinical Guidance Document was prepared in order to assist rheumatologists with decisions as to how to handle current medications during different phases of a patient’s exposure to the SARS-CoV-2 virus.”
But with very little evidence available on how to manage COVID-19 patients generally, let alone specifically in those with rheumatic diseases, “it became evident that any recommendations made would need to be done in a thoughtful and organized manner, evaluating the evidence that was available and obtaining the advice of experts in infectious disease, epidemiology, and in the use of biologic and nonbiologic agents for rheumatic disease,” she said.
As such, the ACR convened a task force of 10 rheumatologists and 4 infectious disease specialists from North America to look at how best to manage patients with rheumatic disease during the COVID-19 pandemic.
“Our charge was to develop a guidance document for the care of adult rheumatic disease patients in the context of COVID-19 and not per se to provide guidance for the treatment of COVID-19,” explained task force member and the corresponding author for the guidance, Ted R. Mikuls, MD, MSPH, of the University of Nebraska Medical Center, Omaha.
Dr. Mikuls, who was speaking at a virtual town hall meeting hosted by the ACR on May 6, noted that the guidance was obviously based on the best consensus of the available data and as such represented a “living document” that “would change and be added to” as necessary.
General recommendations for adult rheumatic disease management
In terms of general recommendations for the management of adult rheumatic disease patients, Dr. Mikuls said that six statements had been made “specific to risk assessment, prevention of infection, and best practices related to glucocorticoid use and the use of ACE [angiotensin-converting enzyme] inhibitors and ARBs [angiotensin II receptor blockers] during the pandemic.”
For example, general advice is to counsel patients to keep up general preventive measures such as social distancing and regular hand washing, reducing the number of in-person health care visits, and undertaking other means to try to prevent potential SARS-CoV-2 exposure. As for general treatment advice, glucocorticoids should be used at their lowest doses possible and should not be abruptly stopped, and antihypertensive treatment should be used as indicated.
Additional guidance statements include those that address the treatment of patients with stable rheumatic disease in the absence of infection or known exposure to SARS-CoV-2, with guidance specific to the treatment of systemic lupus erythematosus (SLE), and those with newly diagnosed or active rheumatic disease.
SLE and inflammatory arthritis recommendations
“There are several sections within the guidance document that address the treatment of patients with systemic lupus erythematosus during this pandemic,” Dr. Gravallese pointed out. “In general, it is recommended that lupus patients who are currently taking hydroxychloroquine can remain on the therapy prior to and during infection and that newly diagnosed patients with lupus can be placed on this medication at full dose. It is recommended that pregnant patients with lupus remain on therapy with this drug.”
She also observed that, for the treatment of active inflammatory arthritis, “the recommendations were written to address specific medications that could be used in this setting. In general, the task force recommendations were guided by the importance of controlling inflammation prior to exposure to the virus, even during this pandemic.
Guidance raises questions
During the ACR’s town hall meeting, the task force answered several questions raised by the guidance, such as the reasoning behind recommending that the use of traditional DMARDs be discontinued in patients with confirmed SARS-CoV-2 infection.
Dr. Mikuls observed: “Maybe if you just read the guidance statements it isn’t terribly intuitive.” There was a lot of discussion about whether or not conventional DMARDs were immunosuppressive, and even though they may not have such effects, it was decided to err on the side of caution.
“I think the task force felt that, with a COVID-19–positive patient, there is a concern of potentially confusing adverse effects related to medicines or conflate those with problems from the infection,” he said. Although rare, examples of those issues could be drug-induced hypersensitivity, hypersensitivity pneumonitis, or gastrointestinal side effects of hepatitis, all of which have been described in COVID-19. “Not only could it cause confusion, but it could maybe worsen those sequalae of COVID-19,” he said.
“I think the other part of this answer was that the panel really felt that the risk in terms of the flaring of the underlying rheumatic disease was likely to be pretty low given the finite time frame you’d be taking about – usually a time frame of 2-3 weeks you’d be holding the agent – so I think that is really why the task force ended up with that recommendation.”
Similarly, for the JAK inhibitors, the decision was to err on the side of caution when COVID-19 was suspected or confirmed. “Not so much because of the risk of thromboembolic disease, but concerns over immunosuppression that these drugs carry with them and also the fact the JAK inhibitors are probably inhibitors of type 1 interferons, which play a significant role in viral immunity and could potentially have a negative impact,” said Stanley Cohen, MD, who practices rheumatology in the Dallas area.
“On the flipside, there is interest in some of the JAK inhibitors as a potential treatment for COVID-19,” Dr. Cohen said, referring to anecdotal evidence for baricitinib (Olumiant).
Michael Weinblatt, MD, of Brigham and Women’s Hospital, addressed the recent concern over the use of NSAIDs by the public.
“There’s been a lot in the lay press that NSAIDs – because of the effects on receptors in the lung – could lead to deleterious outcomes in patients with COVID and there’s very little data to support this.
“We did recommend that NSAIDs be held in the hospitalized patient and that wasn’t because of the COVID-19 issue, it really was just medical practice, and we didn’t want to confound the care of these really sick patients with potential toxicities from NSAIDs. But as far as routine rheumatological care in your outpatients, we did not recommend that nonsteroidals be stopped if they were tolerated.”
One part of the guidance that might already need revision is the recommendation on the continued use of hydroxychloroquine in patients who develop COVID-19.
“Our guidance document says it’s OK; we were all in very strong agreement to continue hydroxychloroquine in our patients with COVID-19 because at that point, just a couple of weeks ago, we thought it was part of the potential treatment,” Karen Costenbader, MD, MPH, of Brigham and Women’s Hospital, said during the town hall meeting.
“Now the pendulum has swung the other way, and we’re worried about maybe we shouldn’t be continuing it because COVID-19 patients will be getting many other medications,” Dr. Costenbader said, and these may affect the QT-interval. “They will not be getting azithromycin because the pendulum swung the other way on that one too, but definitely on many other medications when they are sick.”
Potentially, she added, “if the rheumatic disease is under good control the inpatient physicians could decide whether they should continue [hydroxychloroquine] or not. If the COVID-19 is a mild disease, I would say we probably could continue in accordance with what we put in the document, but we will have to revisit this as well.”
Guidance is a ‘living document’
“We will be providing updates to the Clinical Guidance Document as the need arises,” Dr. Gravallese emphasized. While the general recommendations are unlikely to change very much, “the task force will be interested in seeing the results of all new data, but the results of randomized, clinical trials will be particularly important as they become available,” she said. In particular, randomized, controlled trials of glucocorticoids and IL-6 receptor blockade for use in COVID-19 will be of great importance.
“In this initial document, we could not take on all of the medical scenarios our members will face. For example, we could not take on recommendations for the pediatric population as this group of patients has a very different response than adults to the SARS-CoV-2 virus,” Dr. Gravallese acknowledged. The plan is to provide guidance for that group of patients soon.
In addition, the ACR Executive Committee has appointed a Practice and Advocacy Task Force that will “address issues rheumatologists face on the practice side, including advice regarding how to effectively use telemedicine, address the frequency and safety of infusions, determine urgent versus nonurgent issues that would or would not require face-to-face visits, and help with financial challenges.”
The American College of Rheumatology supported the guidance-development process. Dr. Mikuls, Dr. Weinblatt, Dr. Cohen, and Dr. Costenbader each disclosed research support or consultancies with multiple pharmaceutical companies. Dr. Gravallese had no disclosures.
SOURCE: Mikuls TR et al. Arthritis Rheumatol. 2020 Apr 29. doi: 10.1002/art.41301.
When COVID-19 is suspected or confirmed in a patient with a rheumatic disease, treatment with hydroxychloroquine may be continued, but other treatments may need to be stopped or held temporarily, according to new guidance issued by the American College of Rheumatology.
That includes disease-modifying treatment with antirheumatic drugs such as sulfasalazine, methotrexate, leflunomide, and the Janus kinase (JAK) inhibitors, as well as immunosuppressants and non-interleukin (IL)-6 biologics, and this is regardless of how severe the COVID-19 illness is. NSAIDs should also be stopped if there are respiratory symptoms.
The advice is slightly less drastic if someone with stable rheumatic disease has probably been exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are asymptomatic. In those patients, DMARDs may be continued, although there is uncertainty over whether there is a need to temporarily stop methotrexate or leflunomide. Interruption of immunosuppressive, non–IL-6, and JAK inhibitor treatment is advised pending a negative SARS-CoV-2 test result, assuming the patient’s rheumatic disease is stable.
Impetus for ACR COVID-19 guidance
“One of the earliest challenges for rheumatologists during the COVID-19 pandemic was determining how to advise our patients who were taking immunosuppressive medications and were concerned as to whether or not to discontinue their therapy,” ACR President Ellen Gravallese, MD, said in an interview about the ACR Clinical Guidance Document, which is published online in Arthritis & Rheumatology.
“A second challenge was keeping our patients safe from exposure to the virus, while still seeing those patients in person who required office visits,” added Dr. Gravallese, who is chief of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital in Boston.
She continued: “The ACR Clinical Guidance Document was prepared in order to assist rheumatologists with decisions as to how to handle current medications during different phases of a patient’s exposure to the SARS-CoV-2 virus.”
But with very little evidence available on how to manage COVID-19 patients generally, let alone specifically in those with rheumatic diseases, “it became evident that any recommendations made would need to be done in a thoughtful and organized manner, evaluating the evidence that was available and obtaining the advice of experts in infectious disease, epidemiology, and in the use of biologic and nonbiologic agents for rheumatic disease,” she said.
As such, the ACR convened a task force of 10 rheumatologists and 4 infectious disease specialists from North America to look at how best to manage patients with rheumatic disease during the COVID-19 pandemic.
“Our charge was to develop a guidance document for the care of adult rheumatic disease patients in the context of COVID-19 and not per se to provide guidance for the treatment of COVID-19,” explained task force member and the corresponding author for the guidance, Ted R. Mikuls, MD, MSPH, of the University of Nebraska Medical Center, Omaha.
Dr. Mikuls, who was speaking at a virtual town hall meeting hosted by the ACR on May 6, noted that the guidance was obviously based on the best consensus of the available data and as such represented a “living document” that “would change and be added to” as necessary.
General recommendations for adult rheumatic disease management
In terms of general recommendations for the management of adult rheumatic disease patients, Dr. Mikuls said that six statements had been made “specific to risk assessment, prevention of infection, and best practices related to glucocorticoid use and the use of ACE [angiotensin-converting enzyme] inhibitors and ARBs [angiotensin II receptor blockers] during the pandemic.”
For example, general advice is to counsel patients to keep up general preventive measures such as social distancing and regular hand washing, reducing the number of in-person health care visits, and undertaking other means to try to prevent potential SARS-CoV-2 exposure. As for general treatment advice, glucocorticoids should be used at their lowest doses possible and should not be abruptly stopped, and antihypertensive treatment should be used as indicated.
Additional guidance statements include those that address the treatment of patients with stable rheumatic disease in the absence of infection or known exposure to SARS-CoV-2, with guidance specific to the treatment of systemic lupus erythematosus (SLE), and those with newly diagnosed or active rheumatic disease.
SLE and inflammatory arthritis recommendations
“There are several sections within the guidance document that address the treatment of patients with systemic lupus erythematosus during this pandemic,” Dr. Gravallese pointed out. “In general, it is recommended that lupus patients who are currently taking hydroxychloroquine can remain on the therapy prior to and during infection and that newly diagnosed patients with lupus can be placed on this medication at full dose. It is recommended that pregnant patients with lupus remain on therapy with this drug.”
She also observed that, for the treatment of active inflammatory arthritis, “the recommendations were written to address specific medications that could be used in this setting. In general, the task force recommendations were guided by the importance of controlling inflammation prior to exposure to the virus, even during this pandemic.
Guidance raises questions
During the ACR’s town hall meeting, the task force answered several questions raised by the guidance, such as the reasoning behind recommending that the use of traditional DMARDs be discontinued in patients with confirmed SARS-CoV-2 infection.
Dr. Mikuls observed: “Maybe if you just read the guidance statements it isn’t terribly intuitive.” There was a lot of discussion about whether or not conventional DMARDs were immunosuppressive, and even though they may not have such effects, it was decided to err on the side of caution.
“I think the task force felt that, with a COVID-19–positive patient, there is a concern of potentially confusing adverse effects related to medicines or conflate those with problems from the infection,” he said. Although rare, examples of those issues could be drug-induced hypersensitivity, hypersensitivity pneumonitis, or gastrointestinal side effects of hepatitis, all of which have been described in COVID-19. “Not only could it cause confusion, but it could maybe worsen those sequalae of COVID-19,” he said.
“I think the other part of this answer was that the panel really felt that the risk in terms of the flaring of the underlying rheumatic disease was likely to be pretty low given the finite time frame you’d be taking about – usually a time frame of 2-3 weeks you’d be holding the agent – so I think that is really why the task force ended up with that recommendation.”
Similarly, for the JAK inhibitors, the decision was to err on the side of caution when COVID-19 was suspected or confirmed. “Not so much because of the risk of thromboembolic disease, but concerns over immunosuppression that these drugs carry with them and also the fact the JAK inhibitors are probably inhibitors of type 1 interferons, which play a significant role in viral immunity and could potentially have a negative impact,” said Stanley Cohen, MD, who practices rheumatology in the Dallas area.
“On the flipside, there is interest in some of the JAK inhibitors as a potential treatment for COVID-19,” Dr. Cohen said, referring to anecdotal evidence for baricitinib (Olumiant).
Michael Weinblatt, MD, of Brigham and Women’s Hospital, addressed the recent concern over the use of NSAIDs by the public.
“There’s been a lot in the lay press that NSAIDs – because of the effects on receptors in the lung – could lead to deleterious outcomes in patients with COVID and there’s very little data to support this.
“We did recommend that NSAIDs be held in the hospitalized patient and that wasn’t because of the COVID-19 issue, it really was just medical practice, and we didn’t want to confound the care of these really sick patients with potential toxicities from NSAIDs. But as far as routine rheumatological care in your outpatients, we did not recommend that nonsteroidals be stopped if they were tolerated.”
One part of the guidance that might already need revision is the recommendation on the continued use of hydroxychloroquine in patients who develop COVID-19.
“Our guidance document says it’s OK; we were all in very strong agreement to continue hydroxychloroquine in our patients with COVID-19 because at that point, just a couple of weeks ago, we thought it was part of the potential treatment,” Karen Costenbader, MD, MPH, of Brigham and Women’s Hospital, said during the town hall meeting.
“Now the pendulum has swung the other way, and we’re worried about maybe we shouldn’t be continuing it because COVID-19 patients will be getting many other medications,” Dr. Costenbader said, and these may affect the QT-interval. “They will not be getting azithromycin because the pendulum swung the other way on that one too, but definitely on many other medications when they are sick.”
Potentially, she added, “if the rheumatic disease is under good control the inpatient physicians could decide whether they should continue [hydroxychloroquine] or not. If the COVID-19 is a mild disease, I would say we probably could continue in accordance with what we put in the document, but we will have to revisit this as well.”
Guidance is a ‘living document’
“We will be providing updates to the Clinical Guidance Document as the need arises,” Dr. Gravallese emphasized. While the general recommendations are unlikely to change very much, “the task force will be interested in seeing the results of all new data, but the results of randomized, clinical trials will be particularly important as they become available,” she said. In particular, randomized, controlled trials of glucocorticoids and IL-6 receptor blockade for use in COVID-19 will be of great importance.
“In this initial document, we could not take on all of the medical scenarios our members will face. For example, we could not take on recommendations for the pediatric population as this group of patients has a very different response than adults to the SARS-CoV-2 virus,” Dr. Gravallese acknowledged. The plan is to provide guidance for that group of patients soon.
In addition, the ACR Executive Committee has appointed a Practice and Advocacy Task Force that will “address issues rheumatologists face on the practice side, including advice regarding how to effectively use telemedicine, address the frequency and safety of infusions, determine urgent versus nonurgent issues that would or would not require face-to-face visits, and help with financial challenges.”
The American College of Rheumatology supported the guidance-development process. Dr. Mikuls, Dr. Weinblatt, Dr. Cohen, and Dr. Costenbader each disclosed research support or consultancies with multiple pharmaceutical companies. Dr. Gravallese had no disclosures.
SOURCE: Mikuls TR et al. Arthritis Rheumatol. 2020 Apr 29. doi: 10.1002/art.41301.
FROM ARTHRITIS & RHEUMATOLOGY
Bronchoscopy guideline for COVID-19 pandemic: Use sparingly
With little evidence available on the role of bronchoscopy during the COVID-19 pandemic, an expert panel has published a guideline recommending its spare use in COVID-19 patients and those with suspected COVID-19 infection.
The panel stated that in the context of the COVID-19 crisis, bronchoscopy and other aerosol-generating procedures put health care workers (HCWs) at particularly high risk of exposure and infection. They recommended deferring bronchoscopy in nonurgent cases, and advised practitioners to wear personal protective equipment when performing bronchoscopy, even on asymptomatic patients.
The guideline and expert panel report have been published online in the journal Chest. CHEST and the American Association for Bronchology and Interventional Pulmonology participated in selecting the 14 panelists. “The recommendation and suggestions outlined in this document were specifically created to address what were felt to be clinically common and urgent questions that frontline clinicians are likely to face,” wrote lead author and panel cochair Momen M. Wahidi, MD, MBA, of Duke University, Durham, N.C., and colleagues.
Only one of the six recommendations is based on graded evidence; the remainder are ungraded consensus-based statements. The guideline consists of the following recommendations for performing or using bronchoscopy:
- HCWs in the procedure or recovery rooms should wear either an N-95 respirator or powered air-purifying respirator (PAPR) when performing bronchoscopy on patients suspected or confirmed to have COVID-19. They should wear personal protective equipment (PPE) that includes a face shield, gown, and gloves, and they should discard N-95 respirators after performing bronchoscopy.
- A nasopharyngeal specimen in COVID-19 suspects should be obtained before performing bronchoscopy. If the patient has severe or progressive disease that requires intubation but an additional specimen is needed to confirm COVID-19 or another diagnosis that could change the treatment course, an option would be lower-respiratory specimen from the endotracheal aspirate or bronchoscopy with bronchoalveolar lavage.
- HCWs should wear an N-95 or PAPR when doing bronchoscopy on asymptomatic patients in an area with community spread of COVID-19 – again, with the PPE designated in the first recommendation.
- Test for COVID-19 before doing bronchoscopy on asymptomatic patients. Defer nonurgent bronchoscopy if the test is positive. If it’s negative, follow the recommendations regarding respirators and PPE when doing bronchoscopy.
- Perform timely bronchoscopy when indicated even in an area with known community spread of COVID-19. This is the only graded recommendation among the six (Grade 2C) and may be the most nuanced. Local teams should develop strategies for using bronchoscopy in their setting, taking into account local resources and availability of PPE, and they should send noninfected cancer patients from resource-depleted hospitals to other centers.
- Base the timing of bronchoscopy in patients recovering after COVID-19 on the indication for the procedure, disease severity, and time duration since symptoms resolved. The recommendation noted that the exact timing is still unknown, but that a wait of at least 30 days after symptoms recede is “reasonable.”
The expert panel added a noteworthy caveat to the recommendations. “We would like to stress that these protective strategies can be rendered completely ineffective if proper training on donning and doffing is not provided to HCW,” Dr. Wahidi and colleagues wrote. “Proper personnel instruction and practice for wearing PPE should receive as much attention by health facilities as the chosen strategy for protection.”
Dr. Wahidi and colleagues have no financial relationships to disclose.
SOURCE: Wahidi MM et al. CHEST. 2020 Apr 30. doi: 10.1016/j.chest.2020.04.036.
With little evidence available on the role of bronchoscopy during the COVID-19 pandemic, an expert panel has published a guideline recommending its spare use in COVID-19 patients and those with suspected COVID-19 infection.
The panel stated that in the context of the COVID-19 crisis, bronchoscopy and other aerosol-generating procedures put health care workers (HCWs) at particularly high risk of exposure and infection. They recommended deferring bronchoscopy in nonurgent cases, and advised practitioners to wear personal protective equipment when performing bronchoscopy, even on asymptomatic patients.
The guideline and expert panel report have been published online in the journal Chest. CHEST and the American Association for Bronchology and Interventional Pulmonology participated in selecting the 14 panelists. “The recommendation and suggestions outlined in this document were specifically created to address what were felt to be clinically common and urgent questions that frontline clinicians are likely to face,” wrote lead author and panel cochair Momen M. Wahidi, MD, MBA, of Duke University, Durham, N.C., and colleagues.
Only one of the six recommendations is based on graded evidence; the remainder are ungraded consensus-based statements. The guideline consists of the following recommendations for performing or using bronchoscopy:
- HCWs in the procedure or recovery rooms should wear either an N-95 respirator or powered air-purifying respirator (PAPR) when performing bronchoscopy on patients suspected or confirmed to have COVID-19. They should wear personal protective equipment (PPE) that includes a face shield, gown, and gloves, and they should discard N-95 respirators after performing bronchoscopy.
- A nasopharyngeal specimen in COVID-19 suspects should be obtained before performing bronchoscopy. If the patient has severe or progressive disease that requires intubation but an additional specimen is needed to confirm COVID-19 or another diagnosis that could change the treatment course, an option would be lower-respiratory specimen from the endotracheal aspirate or bronchoscopy with bronchoalveolar lavage.
- HCWs should wear an N-95 or PAPR when doing bronchoscopy on asymptomatic patients in an area with community spread of COVID-19 – again, with the PPE designated in the first recommendation.
- Test for COVID-19 before doing bronchoscopy on asymptomatic patients. Defer nonurgent bronchoscopy if the test is positive. If it’s negative, follow the recommendations regarding respirators and PPE when doing bronchoscopy.
- Perform timely bronchoscopy when indicated even in an area with known community spread of COVID-19. This is the only graded recommendation among the six (Grade 2C) and may be the most nuanced. Local teams should develop strategies for using bronchoscopy in their setting, taking into account local resources and availability of PPE, and they should send noninfected cancer patients from resource-depleted hospitals to other centers.
- Base the timing of bronchoscopy in patients recovering after COVID-19 on the indication for the procedure, disease severity, and time duration since symptoms resolved. The recommendation noted that the exact timing is still unknown, but that a wait of at least 30 days after symptoms recede is “reasonable.”
The expert panel added a noteworthy caveat to the recommendations. “We would like to stress that these protective strategies can be rendered completely ineffective if proper training on donning and doffing is not provided to HCW,” Dr. Wahidi and colleagues wrote. “Proper personnel instruction and practice for wearing PPE should receive as much attention by health facilities as the chosen strategy for protection.”
Dr. Wahidi and colleagues have no financial relationships to disclose.
SOURCE: Wahidi MM et al. CHEST. 2020 Apr 30. doi: 10.1016/j.chest.2020.04.036.
With little evidence available on the role of bronchoscopy during the COVID-19 pandemic, an expert panel has published a guideline recommending its spare use in COVID-19 patients and those with suspected COVID-19 infection.
The panel stated that in the context of the COVID-19 crisis, bronchoscopy and other aerosol-generating procedures put health care workers (HCWs) at particularly high risk of exposure and infection. They recommended deferring bronchoscopy in nonurgent cases, and advised practitioners to wear personal protective equipment when performing bronchoscopy, even on asymptomatic patients.
The guideline and expert panel report have been published online in the journal Chest. CHEST and the American Association for Bronchology and Interventional Pulmonology participated in selecting the 14 panelists. “The recommendation and suggestions outlined in this document were specifically created to address what were felt to be clinically common and urgent questions that frontline clinicians are likely to face,” wrote lead author and panel cochair Momen M. Wahidi, MD, MBA, of Duke University, Durham, N.C., and colleagues.
Only one of the six recommendations is based on graded evidence; the remainder are ungraded consensus-based statements. The guideline consists of the following recommendations for performing or using bronchoscopy:
- HCWs in the procedure or recovery rooms should wear either an N-95 respirator or powered air-purifying respirator (PAPR) when performing bronchoscopy on patients suspected or confirmed to have COVID-19. They should wear personal protective equipment (PPE) that includes a face shield, gown, and gloves, and they should discard N-95 respirators after performing bronchoscopy.
- A nasopharyngeal specimen in COVID-19 suspects should be obtained before performing bronchoscopy. If the patient has severe or progressive disease that requires intubation but an additional specimen is needed to confirm COVID-19 or another diagnosis that could change the treatment course, an option would be lower-respiratory specimen from the endotracheal aspirate or bronchoscopy with bronchoalveolar lavage.
- HCWs should wear an N-95 or PAPR when doing bronchoscopy on asymptomatic patients in an area with community spread of COVID-19 – again, with the PPE designated in the first recommendation.
- Test for COVID-19 before doing bronchoscopy on asymptomatic patients. Defer nonurgent bronchoscopy if the test is positive. If it’s negative, follow the recommendations regarding respirators and PPE when doing bronchoscopy.
- Perform timely bronchoscopy when indicated even in an area with known community spread of COVID-19. This is the only graded recommendation among the six (Grade 2C) and may be the most nuanced. Local teams should develop strategies for using bronchoscopy in their setting, taking into account local resources and availability of PPE, and they should send noninfected cancer patients from resource-depleted hospitals to other centers.
- Base the timing of bronchoscopy in patients recovering after COVID-19 on the indication for the procedure, disease severity, and time duration since symptoms resolved. The recommendation noted that the exact timing is still unknown, but that a wait of at least 30 days after symptoms recede is “reasonable.”
The expert panel added a noteworthy caveat to the recommendations. “We would like to stress that these protective strategies can be rendered completely ineffective if proper training on donning and doffing is not provided to HCW,” Dr. Wahidi and colleagues wrote. “Proper personnel instruction and practice for wearing PPE should receive as much attention by health facilities as the chosen strategy for protection.”
Dr. Wahidi and colleagues have no financial relationships to disclose.
SOURCE: Wahidi MM et al. CHEST. 2020 Apr 30. doi: 10.1016/j.chest.2020.04.036.
FROM CHEST
Many hydroxychloroquine COVID-19 prophylaxis trials lack ECG screening
Many planned randomized trials to test the efficacy of hydroxychloroquine or related drugs for preventing COVID-19 infection have, as of the end of April 2020, failed to include ECG assessment to either exclude people at the highest risk for possibly developing a life-threatening cardiac arrhythmia or to flag people who achieve a dangerous QTc interval on treatment, according to an analysis of the posted designs of several dozen studies.
Hydroxychloroquine, the related agent chloroquine, and azithromycin have all recently received attention as potentially effective but unproven agents for both reducing the severity and duration of established COVID-19 infection as well as possibly preventing or mitigating an incident infection. As of April 30, 155 randomized, control trials listed on a major index for pending and in-progress trials, clinicaltrials.gov, had designs that intended to randomized an overall total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis, Michael H. Gollob, MD, said in an article posted by the Journal of the American College of Cardiology (2020 May 11. doi: 10.1016/j.jacc.2020.05.008).
The problem is that all three agents are documented to potentially produce lengthening of the corrected QT interval (QTc), and if this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation from drug treatment could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob, a cardiac arrhythmia researcher at Toronto General Hospital and the University of Toronto. As a consequence, he recommended excluding from these prophylaxis trials anyone with a resting QTc at baseline assessment of greater than 450 msec, as well as discontinuing treatment from anyone who develops a resting QTc of more than 480 ms while on treatment.
“Though this may seem like a conservative value for subject withdrawal from a study, this is a prudent QTc cut-off, particularly when the severity of the adverse event, sudden death, may be worse than the study endpoint” of reduced incidence of COVID-19 infection, he wrote in his opinion piece.
“We cannot provide an accurate number for elevated risk” faced by people whose QTc climbs above these thresholds, “but we know that events will occur, which is why most trials that involve QT-prolonging drugs typically have an ECG exclusion criterion of QTc greater than 450 msec,” Dr. Gollob said in an interview.
His analysis of the 155 planned randomized prophylaxis trials on clinicaltrials.gov that he examined in detail had enrollment goals that would translate into more than 85,000 uninfected people who would receive hydroxychloroquine or chloroquine plus, in come cases, azithromycin. Only six relatively small studies from among these 155 included a plan for ECG screening and monitoring in its design, he noted. “It is reasonable to estimate that among the 80,000 patients randomized to a QT-prolonging drug [without ECG screening or monitoring] there will certainly be arrhythmic events.” If some of these people were to then die from a drug-induced arrhythmic event that could have been prevented by ECG screening or monitoring, it would be a “tragedy,” Dr. Gollob said.
“It is not only inexplicable, but also inexcusable that clinical investigators would dare to include healthy individuals in a clinical trial involving QT-prolonging medications without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. “The fact that we needed Dr. Gollob to ring this alarm is, itself, shocking,” he said in an interview.
“ECG screening is a good option to minimize the risk. You don’t eliminate the risk, but you can minimize it,” commented Arthur Wilde, MD, a cardiac electrophysiologist and professor of medicine at the Academic Medical Center in Amsterdam. Both Dr. Viskin and Dr. Wilde agreed with the QTc interval thresholds Dr. Gollob recommended using for excluding or discontinuing study participants.
In his commentary, Dr. Gollob estimated that if 85,000 otherwise healthy adults were randomized to received a drug that can increase the QTc interval, as many as about 3,400 people (4%) in the group could statistically be expected to have an especially high vulnerability to QT prolongation because of genetic variants they might carry that collectively have roughly this prevalence. In some people of African heritage, the prevalence of genetic risk for excessive QTc lengthening can be even higher, approaching about 10%, noted Dr. Wilde.
Dr. Gollob hoped the concerns he raised will prompt the organizers of many of these studies to revise their design, and he said he already knew of one study based in Toronto that recently added an ECG-monitoring strategy in response to the concerns he raised. He expressed optimism that more studies will follow.
“It’s a real issue to have these trials designed without ECG exclusions or monitoring. I’m glad that Dr. Gollob sent this warning, because he is right. ECG monitoring during treatment is important so you can stop the treatment in time,” Dr. Wilde said. Dr. Wilde also noted that many, if not most, of the studies listed on clinicaltrials.gov may not actually launch.
In April, representatives from several cardiology societies coauthored a document of considerations when using hydroxychloroquine, chloroquine, or azithromycin to treat patients with a diagnosed COVID-19 infection, and highlighted a QTc interval of 500 msec or greater as flagging patients who should no longer receive these drugs (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). For patients who do not yet have COVID-19 disease and the goal from treatment is prevention the potential efficacy of these drugs is reasonable to explore, but “does not exclude the need to minimize risk to research participants, especially when enrolling healthy subjects,” Dr. Gollob said.
Dr. Gollob, Dr. Viskin, and Dr. Wilde had no relevant financial disclosures.
Many planned randomized trials to test the efficacy of hydroxychloroquine or related drugs for preventing COVID-19 infection have, as of the end of April 2020, failed to include ECG assessment to either exclude people at the highest risk for possibly developing a life-threatening cardiac arrhythmia or to flag people who achieve a dangerous QTc interval on treatment, according to an analysis of the posted designs of several dozen studies.
Hydroxychloroquine, the related agent chloroquine, and azithromycin have all recently received attention as potentially effective but unproven agents for both reducing the severity and duration of established COVID-19 infection as well as possibly preventing or mitigating an incident infection. As of April 30, 155 randomized, control trials listed on a major index for pending and in-progress trials, clinicaltrials.gov, had designs that intended to randomized an overall total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis, Michael H. Gollob, MD, said in an article posted by the Journal of the American College of Cardiology (2020 May 11. doi: 10.1016/j.jacc.2020.05.008).
The problem is that all three agents are documented to potentially produce lengthening of the corrected QT interval (QTc), and if this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation from drug treatment could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob, a cardiac arrhythmia researcher at Toronto General Hospital and the University of Toronto. As a consequence, he recommended excluding from these prophylaxis trials anyone with a resting QTc at baseline assessment of greater than 450 msec, as well as discontinuing treatment from anyone who develops a resting QTc of more than 480 ms while on treatment.
“Though this may seem like a conservative value for subject withdrawal from a study, this is a prudent QTc cut-off, particularly when the severity of the adverse event, sudden death, may be worse than the study endpoint” of reduced incidence of COVID-19 infection, he wrote in his opinion piece.
“We cannot provide an accurate number for elevated risk” faced by people whose QTc climbs above these thresholds, “but we know that events will occur, which is why most trials that involve QT-prolonging drugs typically have an ECG exclusion criterion of QTc greater than 450 msec,” Dr. Gollob said in an interview.
His analysis of the 155 planned randomized prophylaxis trials on clinicaltrials.gov that he examined in detail had enrollment goals that would translate into more than 85,000 uninfected people who would receive hydroxychloroquine or chloroquine plus, in come cases, azithromycin. Only six relatively small studies from among these 155 included a plan for ECG screening and monitoring in its design, he noted. “It is reasonable to estimate that among the 80,000 patients randomized to a QT-prolonging drug [without ECG screening or monitoring] there will certainly be arrhythmic events.” If some of these people were to then die from a drug-induced arrhythmic event that could have been prevented by ECG screening or monitoring, it would be a “tragedy,” Dr. Gollob said.
“It is not only inexplicable, but also inexcusable that clinical investigators would dare to include healthy individuals in a clinical trial involving QT-prolonging medications without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. “The fact that we needed Dr. Gollob to ring this alarm is, itself, shocking,” he said in an interview.
“ECG screening is a good option to minimize the risk. You don’t eliminate the risk, but you can minimize it,” commented Arthur Wilde, MD, a cardiac electrophysiologist and professor of medicine at the Academic Medical Center in Amsterdam. Both Dr. Viskin and Dr. Wilde agreed with the QTc interval thresholds Dr. Gollob recommended using for excluding or discontinuing study participants.
In his commentary, Dr. Gollob estimated that if 85,000 otherwise healthy adults were randomized to received a drug that can increase the QTc interval, as many as about 3,400 people (4%) in the group could statistically be expected to have an especially high vulnerability to QT prolongation because of genetic variants they might carry that collectively have roughly this prevalence. In some people of African heritage, the prevalence of genetic risk for excessive QTc lengthening can be even higher, approaching about 10%, noted Dr. Wilde.
Dr. Gollob hoped the concerns he raised will prompt the organizers of many of these studies to revise their design, and he said he already knew of one study based in Toronto that recently added an ECG-monitoring strategy in response to the concerns he raised. He expressed optimism that more studies will follow.
“It’s a real issue to have these trials designed without ECG exclusions or monitoring. I’m glad that Dr. Gollob sent this warning, because he is right. ECG monitoring during treatment is important so you can stop the treatment in time,” Dr. Wilde said. Dr. Wilde also noted that many, if not most, of the studies listed on clinicaltrials.gov may not actually launch.
In April, representatives from several cardiology societies coauthored a document of considerations when using hydroxychloroquine, chloroquine, or azithromycin to treat patients with a diagnosed COVID-19 infection, and highlighted a QTc interval of 500 msec or greater as flagging patients who should no longer receive these drugs (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). For patients who do not yet have COVID-19 disease and the goal from treatment is prevention the potential efficacy of these drugs is reasonable to explore, but “does not exclude the need to minimize risk to research participants, especially when enrolling healthy subjects,” Dr. Gollob said.
Dr. Gollob, Dr. Viskin, and Dr. Wilde had no relevant financial disclosures.
Many planned randomized trials to test the efficacy of hydroxychloroquine or related drugs for preventing COVID-19 infection have, as of the end of April 2020, failed to include ECG assessment to either exclude people at the highest risk for possibly developing a life-threatening cardiac arrhythmia or to flag people who achieve a dangerous QTc interval on treatment, according to an analysis of the posted designs of several dozen studies.
Hydroxychloroquine, the related agent chloroquine, and azithromycin have all recently received attention as potentially effective but unproven agents for both reducing the severity and duration of established COVID-19 infection as well as possibly preventing or mitigating an incident infection. As of April 30, 155 randomized, control trials listed on a major index for pending and in-progress trials, clinicaltrials.gov, had designs that intended to randomized an overall total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis, Michael H. Gollob, MD, said in an article posted by the Journal of the American College of Cardiology (2020 May 11. doi: 10.1016/j.jacc.2020.05.008).
The problem is that all three agents are documented to potentially produce lengthening of the corrected QT interval (QTc), and if this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation from drug treatment could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob, a cardiac arrhythmia researcher at Toronto General Hospital and the University of Toronto. As a consequence, he recommended excluding from these prophylaxis trials anyone with a resting QTc at baseline assessment of greater than 450 msec, as well as discontinuing treatment from anyone who develops a resting QTc of more than 480 ms while on treatment.
“Though this may seem like a conservative value for subject withdrawal from a study, this is a prudent QTc cut-off, particularly when the severity of the adverse event, sudden death, may be worse than the study endpoint” of reduced incidence of COVID-19 infection, he wrote in his opinion piece.
“We cannot provide an accurate number for elevated risk” faced by people whose QTc climbs above these thresholds, “but we know that events will occur, which is why most trials that involve QT-prolonging drugs typically have an ECG exclusion criterion of QTc greater than 450 msec,” Dr. Gollob said in an interview.
His analysis of the 155 planned randomized prophylaxis trials on clinicaltrials.gov that he examined in detail had enrollment goals that would translate into more than 85,000 uninfected people who would receive hydroxychloroquine or chloroquine plus, in come cases, azithromycin. Only six relatively small studies from among these 155 included a plan for ECG screening and monitoring in its design, he noted. “It is reasonable to estimate that among the 80,000 patients randomized to a QT-prolonging drug [without ECG screening or monitoring] there will certainly be arrhythmic events.” If some of these people were to then die from a drug-induced arrhythmic event that could have been prevented by ECG screening or monitoring, it would be a “tragedy,” Dr. Gollob said.
“It is not only inexplicable, but also inexcusable that clinical investigators would dare to include healthy individuals in a clinical trial involving QT-prolonging medications without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. “The fact that we needed Dr. Gollob to ring this alarm is, itself, shocking,” he said in an interview.
“ECG screening is a good option to minimize the risk. You don’t eliminate the risk, but you can minimize it,” commented Arthur Wilde, MD, a cardiac electrophysiologist and professor of medicine at the Academic Medical Center in Amsterdam. Both Dr. Viskin and Dr. Wilde agreed with the QTc interval thresholds Dr. Gollob recommended using for excluding or discontinuing study participants.
In his commentary, Dr. Gollob estimated that if 85,000 otherwise healthy adults were randomized to received a drug that can increase the QTc interval, as many as about 3,400 people (4%) in the group could statistically be expected to have an especially high vulnerability to QT prolongation because of genetic variants they might carry that collectively have roughly this prevalence. In some people of African heritage, the prevalence of genetic risk for excessive QTc lengthening can be even higher, approaching about 10%, noted Dr. Wilde.
Dr. Gollob hoped the concerns he raised will prompt the organizers of many of these studies to revise their design, and he said he already knew of one study based in Toronto that recently added an ECG-monitoring strategy in response to the concerns he raised. He expressed optimism that more studies will follow.
“It’s a real issue to have these trials designed without ECG exclusions or monitoring. I’m glad that Dr. Gollob sent this warning, because he is right. ECG monitoring during treatment is important so you can stop the treatment in time,” Dr. Wilde said. Dr. Wilde also noted that many, if not most, of the studies listed on clinicaltrials.gov may not actually launch.
In April, representatives from several cardiology societies coauthored a document of considerations when using hydroxychloroquine, chloroquine, or azithromycin to treat patients with a diagnosed COVID-19 infection, and highlighted a QTc interval of 500 msec or greater as flagging patients who should no longer receive these drugs (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). For patients who do not yet have COVID-19 disease and the goal from treatment is prevention the potential efficacy of these drugs is reasonable to explore, but “does not exclude the need to minimize risk to research participants, especially when enrolling healthy subjects,” Dr. Gollob said.
Dr. Gollob, Dr. Viskin, and Dr. Wilde had no relevant financial disclosures.
REPORTING FROM JACC
COVID-19: Telehealth at the forefront of the pandemic
On Jan. 20, 2020, the first confirmed case of the 2019 novel coronavirus in the United States was admitted to Providence Regional Medical Center in Everett, Wash. Less than 3 months later, the COVID-19 pandemic has put enormous stress on the U.S. health care system, which is confronting acute resource shortage because of the surge of acute and critically ill patients, health care provider safety and burnout, and an ongoing need for managing vulnerable populations while minimizing the infection spread.
With the onset of these unprecedented challenges, telehealth has emerged as a powerful new resource for health care providers, hospitals, and health care systems across the country. This article offers a summary of government regulations that enabled telehealth expansion, and provides an overview of how two health care organizations, Providence St. Joseph Health and Sound Physicians, are employing telehealth services to combat the COVID-19 health care crisis.
The government response: Telehealth expansion
In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) have significantly increased access to telehealth services for Medicare and Medicaid beneficiaries. CMS swiftly put measures in place such as:
- Expanding telehealth beyond rural areas.
- Adding 80 services that can be provided in all settings, including patient homes
- Allowing providers to bill for telehealth visits at the same rate as in-person visits.
The U.S. Department of Health and Human Services also aided this effort by:
- Waiving requirements that physicians or other health care professionals must have licenses in the state in which they provide services, if they have an equivalent license from another state.
- Waving penalties for HIPAA violations against health care providers that serve patients in good faith through everyday communications technologies, such as FaceTime or Skype
Without prior regulatory and reimbursement restrictions, telehealth rapidly became a powerful tool in helping to solve some of the problems brought about by the COVID-19 pandemic.
Providence Telehealth for COVID-19
Providence St. Joseph Health is a not-for-profit health care system operating 51 hospitals and 1,085 clinics across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Providence has developed an enterprise telemedicine network with more than 100 virtual programs. Several of these services – including Telestroke, Telepsychiatry, TeleICU, and Telehospitalist – have been scaled across several states as a clinical cloud. More than 400 telemedicine endpoints are deployed, such as robotic carts and fixed InTouch TVs. In fact, the first U.S. COVID-19 patient was treated at Providence Regional Medical Center in Everett, Wash., using the telemedical robot Vici from InTouch Health.
According to Todd Czartoski, MD, chief medical technology officer at Providence, “while telehealth has been around for many years, COVID-19 opened a lot of people’s eyes to the value of virtual care delivery.”
Providence’s telehealth response to COVID-19 has encompassed five main areas: COVID-19 home care, COVID-19 acute care, ambulatory virtual visits, behavioral health concierge (BHC) expansion, and additional support for outside partnerships.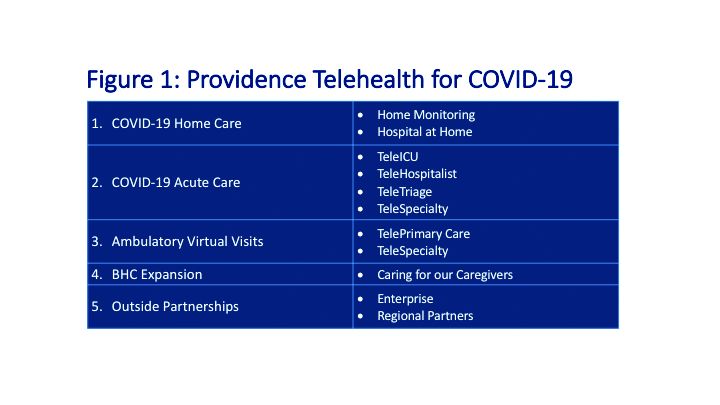
COVID-19 Home Care
Providence rapidly deployed home monitoring for nearly 2,000 positive or presumptive COVID-19 patients. Those symptomatic, clinically stable patients are given a thermometer and a pulse oximeter, and are monitored from home by a central team of nurses and physicians using the Xealth and Twistle programs.
Providence is evaluating expansion of home monitoring to other diagnoses, including higher acuity conditions.
COVID-19 Acute Care
TeleTriage expedites the triage of suspected COVID-19 patients and reduces the use of personal protective equipment (PPE) by 50% per patient per day. To date, TeleTriage has resulted in the conservation of more than 90,000 PPE units.
TeleHospitalist services expanded from traditional night coverage to caring for patients in COVID-19 units around the clock. Currently, there are 25 telehospitalists who practice both in-person and virtual medicine.
TeleICU offers remote management of more than 180 ICU beds across 17 hospitals from two central command centers in Washington state and Alaska. The services include night-time intensivist and ICU nurse coverage, including medication and ventilator management, and family conferences. COVID-19 increased the demand for TeleICU, with anticipated expansion to more than 300 beds.
Core TeleSpecialty services include TeleStroke and TelePsychiatry across 135 remote sites.
Ambulatory Virtual Visits
Providence launched the COVID-19 hub microsite to help educate patients by providing accurate and timely information. A chatbot named Grace helps screen patients who are worried about COVID-19. Grace also suggests next steps, such as a video visit with a patient’s primary care provider or a visit using Express Care/Virtual team, a direct-to-consumer service available to patients within and outside of the health care system.
In less than 2 weeks, Providence enabled virtual visits for more than 7,000 outpatient providers, with more than 14,000 alternative visits now occurring daily. This has allowed primary and specialty providers to continue to manage their patient panels remotely. The number of Express Care/Virtual visits increased from 60 to more than 1,000 per day.
BHC Expansion
In the effort to improve care for its caregivers, Providence launched a behavioral health concierge (BHC) service that offers employees and their dependents virtual access to licensed mental health professionals. Over the last half of 2019, BHC provided more than 1,000 phone and virtual visits, depending on the individual preference of patients. Notably, 21% percent of users were physicians; 65% of users were seen the same day and 100% of users were seen within 48 hours.
COVID-19 increased demand for services that initially started in Seattle and rapidly expanded to Montana, Oregon, and California.
Outside Partnerships
Providence has established partnerships with outside facilities by providing services to 135 sites across eight states. COVID-19 accelerated the employment of new services, including TeleICU.
Telemedicine at Sound Physicians
Sound Physicians is a national physician-founded and -led organization that provides emergency medicine, critical care, hospital medicine, population health, and physician advisory services. Five years ago, Sound launched a telemedicine service line. I spoke with Brian Carpenter, MD, national medical director for TeleHospitalist Services at Sound, to learn about his experience implementing Telehospitalist programs across 22 hospitals and 22 skilled nursing facilities.
Prior to COVID-19, Sound offered a spectrum of telemedicine services including night-time telephonic cross coverage, as well as video-assisted admissions, transfers, and rapid responses. In 2019, Sound Telehospitalists received 88,000 connect requests, including 6,400 video-assisted new admissions and 82 rapid responses. Typically, one physician covers four to eight hospitals with back-up available for surges. The team uses a predictive model for staffing and developed an acuity-based algorithm to ensure that patients in distress are evaluated immediately, new stable admissions on average are seen within 12 minutes, and order clarifications are provided within 30 minutes.
The COVID-19 pandemic created an urgent demand for providers to support an overwhelmed health care system. Without the traditional barriers to implementation – such as lack of acceptance by medical staff, nurses and patients, strict state licensing and technology requirements, lack of reimbursement, and delays in hospital credentialing – Sound was able to develop a rapid implementation model for telemedicine services. Currently, four new hospitals are in the active implementation phase, with 40 more hospitals in the pipeline.
Implementing a telemedicine program at your hospital
In order to successfully launch a telemedicine program, Dr. Carpenter outlined the following critical implementation steps:
- In collaboration with local leadership, define the problem you are trying to solve, which helps inform the scope of the telemedicine practice and technology requirements (for example, night-time cross-coverage vs. full telemedicine service).
- Complete a discovery process (for example, existing workflow for patient admission and transfer) with the end-goal of developing a workflow and rules of engagement.
- Obtain hospital credentialing/privileges and EMR access.
- Train end-users, including physicians and nurse telepresenters.
Dr. Carpenter offered this advice to those considering a telemedicine program: “Telemedicine is not just about technology; a true telemedicine program encompasses change management, workflow development, end-user training, compliance, and mechanisms for continuous process improvement. We want to make things better for the physicians, nurses, and patients.”
Telehealth is offering support to health care providers on the front lines, patients in need of care, and health care systems managing the unprecedented surges in volume.
Dr. Farah is a hospitalist, physician adviser, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
On Jan. 20, 2020, the first confirmed case of the 2019 novel coronavirus in the United States was admitted to Providence Regional Medical Center in Everett, Wash. Less than 3 months later, the COVID-19 pandemic has put enormous stress on the U.S. health care system, which is confronting acute resource shortage because of the surge of acute and critically ill patients, health care provider safety and burnout, and an ongoing need for managing vulnerable populations while minimizing the infection spread.
With the onset of these unprecedented challenges, telehealth has emerged as a powerful new resource for health care providers, hospitals, and health care systems across the country. This article offers a summary of government regulations that enabled telehealth expansion, and provides an overview of how two health care organizations, Providence St. Joseph Health and Sound Physicians, are employing telehealth services to combat the COVID-19 health care crisis.
The government response: Telehealth expansion
In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) have significantly increased access to telehealth services for Medicare and Medicaid beneficiaries. CMS swiftly put measures in place such as:
- Expanding telehealth beyond rural areas.
- Adding 80 services that can be provided in all settings, including patient homes
- Allowing providers to bill for telehealth visits at the same rate as in-person visits.
The U.S. Department of Health and Human Services also aided this effort by:
- Waiving requirements that physicians or other health care professionals must have licenses in the state in which they provide services, if they have an equivalent license from another state.
- Waving penalties for HIPAA violations against health care providers that serve patients in good faith through everyday communications technologies, such as FaceTime or Skype
Without prior regulatory and reimbursement restrictions, telehealth rapidly became a powerful tool in helping to solve some of the problems brought about by the COVID-19 pandemic.
Providence Telehealth for COVID-19
Providence St. Joseph Health is a not-for-profit health care system operating 51 hospitals and 1,085 clinics across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Providence has developed an enterprise telemedicine network with more than 100 virtual programs. Several of these services – including Telestroke, Telepsychiatry, TeleICU, and Telehospitalist – have been scaled across several states as a clinical cloud. More than 400 telemedicine endpoints are deployed, such as robotic carts and fixed InTouch TVs. In fact, the first U.S. COVID-19 patient was treated at Providence Regional Medical Center in Everett, Wash., using the telemedical robot Vici from InTouch Health.
According to Todd Czartoski, MD, chief medical technology officer at Providence, “while telehealth has been around for many years, COVID-19 opened a lot of people’s eyes to the value of virtual care delivery.”
Providence’s telehealth response to COVID-19 has encompassed five main areas: COVID-19 home care, COVID-19 acute care, ambulatory virtual visits, behavioral health concierge (BHC) expansion, and additional support for outside partnerships.
COVID-19 Home Care
Providence rapidly deployed home monitoring for nearly 2,000 positive or presumptive COVID-19 patients. Those symptomatic, clinically stable patients are given a thermometer and a pulse oximeter, and are monitored from home by a central team of nurses and physicians using the Xealth and Twistle programs.
Providence is evaluating expansion of home monitoring to other diagnoses, including higher acuity conditions.
COVID-19 Acute Care
TeleTriage expedites the triage of suspected COVID-19 patients and reduces the use of personal protective equipment (PPE) by 50% per patient per day. To date, TeleTriage has resulted in the conservation of more than 90,000 PPE units.
TeleHospitalist services expanded from traditional night coverage to caring for patients in COVID-19 units around the clock. Currently, there are 25 telehospitalists who practice both in-person and virtual medicine.
TeleICU offers remote management of more than 180 ICU beds across 17 hospitals from two central command centers in Washington state and Alaska. The services include night-time intensivist and ICU nurse coverage, including medication and ventilator management, and family conferences. COVID-19 increased the demand for TeleICU, with anticipated expansion to more than 300 beds.
Core TeleSpecialty services include TeleStroke and TelePsychiatry across 135 remote sites.
Ambulatory Virtual Visits
Providence launched the COVID-19 hub microsite to help educate patients by providing accurate and timely information. A chatbot named Grace helps screen patients who are worried about COVID-19. Grace also suggests next steps, such as a video visit with a patient’s primary care provider or a visit using Express Care/Virtual team, a direct-to-consumer service available to patients within and outside of the health care system.
In less than 2 weeks, Providence enabled virtual visits for more than 7,000 outpatient providers, with more than 14,000 alternative visits now occurring daily. This has allowed primary and specialty providers to continue to manage their patient panels remotely. The number of Express Care/Virtual visits increased from 60 to more than 1,000 per day.
BHC Expansion
In the effort to improve care for its caregivers, Providence launched a behavioral health concierge (BHC) service that offers employees and their dependents virtual access to licensed mental health professionals. Over the last half of 2019, BHC provided more than 1,000 phone and virtual visits, depending on the individual preference of patients. Notably, 21% percent of users were physicians; 65% of users were seen the same day and 100% of users were seen within 48 hours.
COVID-19 increased demand for services that initially started in Seattle and rapidly expanded to Montana, Oregon, and California.
Outside Partnerships
Providence has established partnerships with outside facilities by providing services to 135 sites across eight states. COVID-19 accelerated the employment of new services, including TeleICU.
Telemedicine at Sound Physicians
Sound Physicians is a national physician-founded and -led organization that provides emergency medicine, critical care, hospital medicine, population health, and physician advisory services. Five years ago, Sound launched a telemedicine service line. I spoke with Brian Carpenter, MD, national medical director for TeleHospitalist Services at Sound, to learn about his experience implementing Telehospitalist programs across 22 hospitals and 22 skilled nursing facilities.
Prior to COVID-19, Sound offered a spectrum of telemedicine services including night-time telephonic cross coverage, as well as video-assisted admissions, transfers, and rapid responses. In 2019, Sound Telehospitalists received 88,000 connect requests, including 6,400 video-assisted new admissions and 82 rapid responses. Typically, one physician covers four to eight hospitals with back-up available for surges. The team uses a predictive model for staffing and developed an acuity-based algorithm to ensure that patients in distress are evaluated immediately, new stable admissions on average are seen within 12 minutes, and order clarifications are provided within 30 minutes.
The COVID-19 pandemic created an urgent demand for providers to support an overwhelmed health care system. Without the traditional barriers to implementation – such as lack of acceptance by medical staff, nurses and patients, strict state licensing and technology requirements, lack of reimbursement, and delays in hospital credentialing – Sound was able to develop a rapid implementation model for telemedicine services. Currently, four new hospitals are in the active implementation phase, with 40 more hospitals in the pipeline.
Implementing a telemedicine program at your hospital
In order to successfully launch a telemedicine program, Dr. Carpenter outlined the following critical implementation steps:
- In collaboration with local leadership, define the problem you are trying to solve, which helps inform the scope of the telemedicine practice and technology requirements (for example, night-time cross-coverage vs. full telemedicine service).
- Complete a discovery process (for example, existing workflow for patient admission and transfer) with the end-goal of developing a workflow and rules of engagement.
- Obtain hospital credentialing/privileges and EMR access.
- Train end-users, including physicians and nurse telepresenters.
Dr. Carpenter offered this advice to those considering a telemedicine program: “Telemedicine is not just about technology; a true telemedicine program encompasses change management, workflow development, end-user training, compliance, and mechanisms for continuous process improvement. We want to make things better for the physicians, nurses, and patients.”
Telehealth is offering support to health care providers on the front lines, patients in need of care, and health care systems managing the unprecedented surges in volume.
Dr. Farah is a hospitalist, physician adviser, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
On Jan. 20, 2020, the first confirmed case of the 2019 novel coronavirus in the United States was admitted to Providence Regional Medical Center in Everett, Wash. Less than 3 months later, the COVID-19 pandemic has put enormous stress on the U.S. health care system, which is confronting acute resource shortage because of the surge of acute and critically ill patients, health care provider safety and burnout, and an ongoing need for managing vulnerable populations while minimizing the infection spread.
With the onset of these unprecedented challenges, telehealth has emerged as a powerful new resource for health care providers, hospitals, and health care systems across the country. This article offers a summary of government regulations that enabled telehealth expansion, and provides an overview of how two health care organizations, Providence St. Joseph Health and Sound Physicians, are employing telehealth services to combat the COVID-19 health care crisis.
The government response: Telehealth expansion
In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) have significantly increased access to telehealth services for Medicare and Medicaid beneficiaries. CMS swiftly put measures in place such as:
- Expanding telehealth beyond rural areas.
- Adding 80 services that can be provided in all settings, including patient homes
- Allowing providers to bill for telehealth visits at the same rate as in-person visits.
The U.S. Department of Health and Human Services also aided this effort by:
- Waiving requirements that physicians or other health care professionals must have licenses in the state in which they provide services, if they have an equivalent license from another state.
- Waving penalties for HIPAA violations against health care providers that serve patients in good faith through everyday communications technologies, such as FaceTime or Skype
Without prior regulatory and reimbursement restrictions, telehealth rapidly became a powerful tool in helping to solve some of the problems brought about by the COVID-19 pandemic.
Providence Telehealth for COVID-19
Providence St. Joseph Health is a not-for-profit health care system operating 51 hospitals and 1,085 clinics across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Providence has developed an enterprise telemedicine network with more than 100 virtual programs. Several of these services – including Telestroke, Telepsychiatry, TeleICU, and Telehospitalist – have been scaled across several states as a clinical cloud. More than 400 telemedicine endpoints are deployed, such as robotic carts and fixed InTouch TVs. In fact, the first U.S. COVID-19 patient was treated at Providence Regional Medical Center in Everett, Wash., using the telemedical robot Vici from InTouch Health.
According to Todd Czartoski, MD, chief medical technology officer at Providence, “while telehealth has been around for many years, COVID-19 opened a lot of people’s eyes to the value of virtual care delivery.”
Providence’s telehealth response to COVID-19 has encompassed five main areas: COVID-19 home care, COVID-19 acute care, ambulatory virtual visits, behavioral health concierge (BHC) expansion, and additional support for outside partnerships.
COVID-19 Home Care
Providence rapidly deployed home monitoring for nearly 2,000 positive or presumptive COVID-19 patients. Those symptomatic, clinically stable patients are given a thermometer and a pulse oximeter, and are monitored from home by a central team of nurses and physicians using the Xealth and Twistle programs.
Providence is evaluating expansion of home monitoring to other diagnoses, including higher acuity conditions.
COVID-19 Acute Care
TeleTriage expedites the triage of suspected COVID-19 patients and reduces the use of personal protective equipment (PPE) by 50% per patient per day. To date, TeleTriage has resulted in the conservation of more than 90,000 PPE units.
TeleHospitalist services expanded from traditional night coverage to caring for patients in COVID-19 units around the clock. Currently, there are 25 telehospitalists who practice both in-person and virtual medicine.
TeleICU offers remote management of more than 180 ICU beds across 17 hospitals from two central command centers in Washington state and Alaska. The services include night-time intensivist and ICU nurse coverage, including medication and ventilator management, and family conferences. COVID-19 increased the demand for TeleICU, with anticipated expansion to more than 300 beds.
Core TeleSpecialty services include TeleStroke and TelePsychiatry across 135 remote sites.
Ambulatory Virtual Visits
Providence launched the COVID-19 hub microsite to help educate patients by providing accurate and timely information. A chatbot named Grace helps screen patients who are worried about COVID-19. Grace also suggests next steps, such as a video visit with a patient’s primary care provider or a visit using Express Care/Virtual team, a direct-to-consumer service available to patients within and outside of the health care system.
In less than 2 weeks, Providence enabled virtual visits for more than 7,000 outpatient providers, with more than 14,000 alternative visits now occurring daily. This has allowed primary and specialty providers to continue to manage their patient panels remotely. The number of Express Care/Virtual visits increased from 60 to more than 1,000 per day.
BHC Expansion
In the effort to improve care for its caregivers, Providence launched a behavioral health concierge (BHC) service that offers employees and their dependents virtual access to licensed mental health professionals. Over the last half of 2019, BHC provided more than 1,000 phone and virtual visits, depending on the individual preference of patients. Notably, 21% percent of users were physicians; 65% of users were seen the same day and 100% of users were seen within 48 hours.
COVID-19 increased demand for services that initially started in Seattle and rapidly expanded to Montana, Oregon, and California.
Outside Partnerships
Providence has established partnerships with outside facilities by providing services to 135 sites across eight states. COVID-19 accelerated the employment of new services, including TeleICU.
Telemedicine at Sound Physicians
Sound Physicians is a national physician-founded and -led organization that provides emergency medicine, critical care, hospital medicine, population health, and physician advisory services. Five years ago, Sound launched a telemedicine service line. I spoke with Brian Carpenter, MD, national medical director for TeleHospitalist Services at Sound, to learn about his experience implementing Telehospitalist programs across 22 hospitals and 22 skilled nursing facilities.
Prior to COVID-19, Sound offered a spectrum of telemedicine services including night-time telephonic cross coverage, as well as video-assisted admissions, transfers, and rapid responses. In 2019, Sound Telehospitalists received 88,000 connect requests, including 6,400 video-assisted new admissions and 82 rapid responses. Typically, one physician covers four to eight hospitals with back-up available for surges. The team uses a predictive model for staffing and developed an acuity-based algorithm to ensure that patients in distress are evaluated immediately, new stable admissions on average are seen within 12 minutes, and order clarifications are provided within 30 minutes.
The COVID-19 pandemic created an urgent demand for providers to support an overwhelmed health care system. Without the traditional barriers to implementation – such as lack of acceptance by medical staff, nurses and patients, strict state licensing and technology requirements, lack of reimbursement, and delays in hospital credentialing – Sound was able to develop a rapid implementation model for telemedicine services. Currently, four new hospitals are in the active implementation phase, with 40 more hospitals in the pipeline.
Implementing a telemedicine program at your hospital
In order to successfully launch a telemedicine program, Dr. Carpenter outlined the following critical implementation steps:
- In collaboration with local leadership, define the problem you are trying to solve, which helps inform the scope of the telemedicine practice and technology requirements (for example, night-time cross-coverage vs. full telemedicine service).
- Complete a discovery process (for example, existing workflow for patient admission and transfer) with the end-goal of developing a workflow and rules of engagement.
- Obtain hospital credentialing/privileges and EMR access.
- Train end-users, including physicians and nurse telepresenters.
Dr. Carpenter offered this advice to those considering a telemedicine program: “Telemedicine is not just about technology; a true telemedicine program encompasses change management, workflow development, end-user training, compliance, and mechanisms for continuous process improvement. We want to make things better for the physicians, nurses, and patients.”
Telehealth is offering support to health care providers on the front lines, patients in need of care, and health care systems managing the unprecedented surges in volume.
Dr. Farah is a hospitalist, physician adviser, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
Triple-antiviral combo speeds COVID-19 recovery
A triple-antiviral therapy regimen of interferon-beta1, lopinavir/ritonavir, and ribavirin shortened median time to COVID-19 viral negativity by 5 days in a small trial from Hong Kong.
In an open-label, randomized phase 2 trial in patients with mild or moderate COVID-19 infections, the median time to viral negativity by nasopharyngeal swab was 7 days for 86 patients assigned to receive a 14-day course of lopinavir 400 mg and ritonavir 100 mg every 12 hours, ribavirin 400 mg every 12 hours, and three doses of 8 million international units of interferon beta-1b on alternate days, compared with a median time to negativity of 12 days for patients treated with lopinavir/ritonavir alone (P = .0010), wrote Ivan Fan-Ngai Hung, MD, from Gleaneagles Hospital in Hong Kong, and colleagues.
“Triple-antiviral therapy with interferon beta-1b, lopinavir/ritonavir, and ribavirin were safe and superior to lopinavir/ritonavir alone in shortening virus shedding, alleviating symptoms, and facilitating discharge of patients with mild to moderate COVID-19,” they wrote in a study published online in The Lancet.
Patients who received the combination also had significantly shorter time to complete alleviation of symptoms as assessed by a National Early Warning Score 2 (NEWS2, a system for detecting clinical deterioration in patients with acute illnesses) score of 0 (4 vs. 8 days, respectively; hazard ratio 3.92, P < .0001), and to a Sequential Organ Failure Assessment (SOFA) score of 0 (3 vs. 8 days, HR 1.89, P = .041).
The median hospital stay was 9 days for patients treated with the combination, compared with 14.5 days for controls (HR 2.72, P = .016).
In most patients treated with the combination, SARS-CoV-2 viral load was effectively suppressed in all clinical specimens, including nasopharyngeal swabs, throat and posterior oropharyngeal saliva, and stool.
In addition, serum levels of interleukin 6 (IL-6) – an inflammatory cytokine implicated in the cytokine storm frequently seen in patients with severe COVID-19 infections – were significantly lower on treatment days 2, 6, and 8 in patients treated with the combination, compared with those treated with lopinavir/ritonavir alone.
“Our trial demonstrates that early treatment of mild to moderate COVID-19 with a triple combination of antiviral drugs may rapidly suppress the amount of virus in a patient’s body, relieve symptoms, and reduce the risk to health care workers by reducing the duration and quantity of viral shedding (when the virus is detectable and potentially transmissible). Furthermore, the treatment combination appeared safe and well tolerated by patients,” said lead investigator Professor Kwok-Yung Yuen from the University of Hong Kong, in a statement.
“Despite these encouraging findings,” he continued, “we must confirm in larger phase 3 trials that interferon beta-1b alone or in combination with other drugs is effective in patients with more severe illness (in whom the virus has had more time to replicate).”
Plausible rationale
Benjamin Medoff, MD, chief of the division of pulmonary and critical care medicine at Massachusetts General Hospital in Boston, who was not involved in the study, said in an interview that the biologic rationale for the combination is plausible.
“I think this is a promising study that suggests that a regimen of interferon beta-1b, lopinavir/ritonavir, and ribavirin can shorten the duration of infection and improve symptoms in COVID-19 patients especially if started early in disease, in less than 7 days of symptom onset,” he said in reply to a request for expert analysis.
“The open-label nature and small size of the study limits the broad use of the regimen as noted by the authors, and it’s important to emphasize that the subjects enrolled did not have very severe disease (not in the ICU). However, the study does suggest that a larger truly randomized study is warranted,” he said.
AIDS drugs repurposed
Lopinavir/ritonavir is commonly used to treat HIV/AIDS throughout the world, and the investigators had previously reported that the antiviral agents combined with ribavirin reduced deaths and the need for intensive ventilator support among patients with SARS-CoV, the betacoronavirus that causes severe acute respiratory syndrome (SARS), and antivirals have shown in vitro activity against both SARS-CoV and MERS-CoV, the closely related pathogen that causes Middle East respiratory syndrome.
“ However the viral load of SARS and MERS peaks at around day 7-10 after symptom onset, whereas the viral load of COVID-19 peaks at the time of presentation, similar to influenza. Experience from the treatment of patients with influenza who are admitted to hospital suggested that a combination of multiple antiviral drugs is more effective than single-drug treatments in this setting of patients with a high viral load at presentation,” the investigators wrote.
To test this, they enrolled adults patients admitted to one of six Hong Kong Hospitals for virologically confirmed COVID-19 infections from Feb. 10 through March 20, 2020.
A total of 86 patients were randomly assigned to the combination and 41 to lopinavir/ritonavir alone as controls, at doses described above.
Patients who entered the trial within less than 7 days of symptom onset received the triple combination, with interferon dosing adjusted according to the day that treatment started. Patients recruited 1 or 2 days after symptom onset received three doses of interferon, patients started on day 3 or 4 received two doses, and those started on days 5 or 6 received one interferon dose. Patients recruited 7 days or later from symptom onset did not receive interferon beta-1b because of its proinflammatory effects.
In post-hoc analysis by day of treatment initiation, clinical and virological outcomes (except stool samples) were superior in patients admitted less than 7 days after symptom onset for the 52 patients who received a least one interferon dose plus lopinavir/ritonavir and ribavirin, compared with 24 patients randomized to the control arm (lopinavir/ritonavir only). In contrast, among patients admitted and started on treatment at day 7 or later after symptom onset, there were no differences between those who received lopinavir/ritonavir alone or combined with ribavirin.
Adverse events were reported in 41 of 86 patients in the combination group and 20 of 41 patients in the control arm. The most common adverse events were diarrhea, occurring in 52 of all 127 patients, fever in 48, nausea in 43, and elevated alanine transaminase level in 18. The side effects generally resolved within 3 days of the start of treatments.
There were no serious adverse events reported in the combination group. One patient in the control group had impaired hepatic enzymes requiring discontinuation of treatment. No patients died during the study.
The study was funded by the Shaw Foundation, Richard and Carol Yu, May Tam Mak Mei Yin, and Sanming Project of Medicine. The authors and Dr. Medoff declared no competing interests.
SOURCE: Hung IFN et al. Lancet. 2020 May 8. doi: 10.1016/S0140-6736(20)31101-6.
A triple-antiviral therapy regimen of interferon-beta1, lopinavir/ritonavir, and ribavirin shortened median time to COVID-19 viral negativity by 5 days in a small trial from Hong Kong.
In an open-label, randomized phase 2 trial in patients with mild or moderate COVID-19 infections, the median time to viral negativity by nasopharyngeal swab was 7 days for 86 patients assigned to receive a 14-day course of lopinavir 400 mg and ritonavir 100 mg every 12 hours, ribavirin 400 mg every 12 hours, and three doses of 8 million international units of interferon beta-1b on alternate days, compared with a median time to negativity of 12 days for patients treated with lopinavir/ritonavir alone (P = .0010), wrote Ivan Fan-Ngai Hung, MD, from Gleaneagles Hospital in Hong Kong, and colleagues.
“Triple-antiviral therapy with interferon beta-1b, lopinavir/ritonavir, and ribavirin were safe and superior to lopinavir/ritonavir alone in shortening virus shedding, alleviating symptoms, and facilitating discharge of patients with mild to moderate COVID-19,” they wrote in a study published online in The Lancet.
Patients who received the combination also had significantly shorter time to complete alleviation of symptoms as assessed by a National Early Warning Score 2 (NEWS2, a system for detecting clinical deterioration in patients with acute illnesses) score of 0 (4 vs. 8 days, respectively; hazard ratio 3.92, P < .0001), and to a Sequential Organ Failure Assessment (SOFA) score of 0 (3 vs. 8 days, HR 1.89, P = .041).
The median hospital stay was 9 days for patients treated with the combination, compared with 14.5 days for controls (HR 2.72, P = .016).
In most patients treated with the combination, SARS-CoV-2 viral load was effectively suppressed in all clinical specimens, including nasopharyngeal swabs, throat and posterior oropharyngeal saliva, and stool.
In addition, serum levels of interleukin 6 (IL-6) – an inflammatory cytokine implicated in the cytokine storm frequently seen in patients with severe COVID-19 infections – were significantly lower on treatment days 2, 6, and 8 in patients treated with the combination, compared with those treated with lopinavir/ritonavir alone.
“Our trial demonstrates that early treatment of mild to moderate COVID-19 with a triple combination of antiviral drugs may rapidly suppress the amount of virus in a patient’s body, relieve symptoms, and reduce the risk to health care workers by reducing the duration and quantity of viral shedding (when the virus is detectable and potentially transmissible). Furthermore, the treatment combination appeared safe and well tolerated by patients,” said lead investigator Professor Kwok-Yung Yuen from the University of Hong Kong, in a statement.
“Despite these encouraging findings,” he continued, “we must confirm in larger phase 3 trials that interferon beta-1b alone or in combination with other drugs is effective in patients with more severe illness (in whom the virus has had more time to replicate).”
Plausible rationale
Benjamin Medoff, MD, chief of the division of pulmonary and critical care medicine at Massachusetts General Hospital in Boston, who was not involved in the study, said in an interview that the biologic rationale for the combination is plausible.
“I think this is a promising study that suggests that a regimen of interferon beta-1b, lopinavir/ritonavir, and ribavirin can shorten the duration of infection and improve symptoms in COVID-19 patients especially if started early in disease, in less than 7 days of symptom onset,” he said in reply to a request for expert analysis.
“The open-label nature and small size of the study limits the broad use of the regimen as noted by the authors, and it’s important to emphasize that the subjects enrolled did not have very severe disease (not in the ICU). However, the study does suggest that a larger truly randomized study is warranted,” he said.
AIDS drugs repurposed
Lopinavir/ritonavir is commonly used to treat HIV/AIDS throughout the world, and the investigators had previously reported that the antiviral agents combined with ribavirin reduced deaths and the need for intensive ventilator support among patients with SARS-CoV, the betacoronavirus that causes severe acute respiratory syndrome (SARS), and antivirals have shown in vitro activity against both SARS-CoV and MERS-CoV, the closely related pathogen that causes Middle East respiratory syndrome.
“ However the viral load of SARS and MERS peaks at around day 7-10 after symptom onset, whereas the viral load of COVID-19 peaks at the time of presentation, similar to influenza. Experience from the treatment of patients with influenza who are admitted to hospital suggested that a combination of multiple antiviral drugs is more effective than single-drug treatments in this setting of patients with a high viral load at presentation,” the investigators wrote.
To test this, they enrolled adults patients admitted to one of six Hong Kong Hospitals for virologically confirmed COVID-19 infections from Feb. 10 through March 20, 2020.
A total of 86 patients were randomly assigned to the combination and 41 to lopinavir/ritonavir alone as controls, at doses described above.
Patients who entered the trial within less than 7 days of symptom onset received the triple combination, with interferon dosing adjusted according to the day that treatment started. Patients recruited 1 or 2 days after symptom onset received three doses of interferon, patients started on day 3 or 4 received two doses, and those started on days 5 or 6 received one interferon dose. Patients recruited 7 days or later from symptom onset did not receive interferon beta-1b because of its proinflammatory effects.
In post-hoc analysis by day of treatment initiation, clinical and virological outcomes (except stool samples) were superior in patients admitted less than 7 days after symptom onset for the 52 patients who received a least one interferon dose plus lopinavir/ritonavir and ribavirin, compared with 24 patients randomized to the control arm (lopinavir/ritonavir only). In contrast, among patients admitted and started on treatment at day 7 or later after symptom onset, there were no differences between those who received lopinavir/ritonavir alone or combined with ribavirin.
Adverse events were reported in 41 of 86 patients in the combination group and 20 of 41 patients in the control arm. The most common adverse events were diarrhea, occurring in 52 of all 127 patients, fever in 48, nausea in 43, and elevated alanine transaminase level in 18. The side effects generally resolved within 3 days of the start of treatments.
There were no serious adverse events reported in the combination group. One patient in the control group had impaired hepatic enzymes requiring discontinuation of treatment. No patients died during the study.
The study was funded by the Shaw Foundation, Richard and Carol Yu, May Tam Mak Mei Yin, and Sanming Project of Medicine. The authors and Dr. Medoff declared no competing interests.
SOURCE: Hung IFN et al. Lancet. 2020 May 8. doi: 10.1016/S0140-6736(20)31101-6.
A triple-antiviral therapy regimen of interferon-beta1, lopinavir/ritonavir, and ribavirin shortened median time to COVID-19 viral negativity by 5 days in a small trial from Hong Kong.
In an open-label, randomized phase 2 trial in patients with mild or moderate COVID-19 infections, the median time to viral negativity by nasopharyngeal swab was 7 days for 86 patients assigned to receive a 14-day course of lopinavir 400 mg and ritonavir 100 mg every 12 hours, ribavirin 400 mg every 12 hours, and three doses of 8 million international units of interferon beta-1b on alternate days, compared with a median time to negativity of 12 days for patients treated with lopinavir/ritonavir alone (P = .0010), wrote Ivan Fan-Ngai Hung, MD, from Gleaneagles Hospital in Hong Kong, and colleagues.
“Triple-antiviral therapy with interferon beta-1b, lopinavir/ritonavir, and ribavirin were safe and superior to lopinavir/ritonavir alone in shortening virus shedding, alleviating symptoms, and facilitating discharge of patients with mild to moderate COVID-19,” they wrote in a study published online in The Lancet.
Patients who received the combination also had significantly shorter time to complete alleviation of symptoms as assessed by a National Early Warning Score 2 (NEWS2, a system for detecting clinical deterioration in patients with acute illnesses) score of 0 (4 vs. 8 days, respectively; hazard ratio 3.92, P < .0001), and to a Sequential Organ Failure Assessment (SOFA) score of 0 (3 vs. 8 days, HR 1.89, P = .041).
The median hospital stay was 9 days for patients treated with the combination, compared with 14.5 days for controls (HR 2.72, P = .016).
In most patients treated with the combination, SARS-CoV-2 viral load was effectively suppressed in all clinical specimens, including nasopharyngeal swabs, throat and posterior oropharyngeal saliva, and stool.
In addition, serum levels of interleukin 6 (IL-6) – an inflammatory cytokine implicated in the cytokine storm frequently seen in patients with severe COVID-19 infections – were significantly lower on treatment days 2, 6, and 8 in patients treated with the combination, compared with those treated with lopinavir/ritonavir alone.
“Our trial demonstrates that early treatment of mild to moderate COVID-19 with a triple combination of antiviral drugs may rapidly suppress the amount of virus in a patient’s body, relieve symptoms, and reduce the risk to health care workers by reducing the duration and quantity of viral shedding (when the virus is detectable and potentially transmissible). Furthermore, the treatment combination appeared safe and well tolerated by patients,” said lead investigator Professor Kwok-Yung Yuen from the University of Hong Kong, in a statement.
“Despite these encouraging findings,” he continued, “we must confirm in larger phase 3 trials that interferon beta-1b alone or in combination with other drugs is effective in patients with more severe illness (in whom the virus has had more time to replicate).”
Plausible rationale
Benjamin Medoff, MD, chief of the division of pulmonary and critical care medicine at Massachusetts General Hospital in Boston, who was not involved in the study, said in an interview that the biologic rationale for the combination is plausible.
“I think this is a promising study that suggests that a regimen of interferon beta-1b, lopinavir/ritonavir, and ribavirin can shorten the duration of infection and improve symptoms in COVID-19 patients especially if started early in disease, in less than 7 days of symptom onset,” he said in reply to a request for expert analysis.
“The open-label nature and small size of the study limits the broad use of the regimen as noted by the authors, and it’s important to emphasize that the subjects enrolled did not have very severe disease (not in the ICU). However, the study does suggest that a larger truly randomized study is warranted,” he said.
AIDS drugs repurposed
Lopinavir/ritonavir is commonly used to treat HIV/AIDS throughout the world, and the investigators had previously reported that the antiviral agents combined with ribavirin reduced deaths and the need for intensive ventilator support among patients with SARS-CoV, the betacoronavirus that causes severe acute respiratory syndrome (SARS), and antivirals have shown in vitro activity against both SARS-CoV and MERS-CoV, the closely related pathogen that causes Middle East respiratory syndrome.
“ However the viral load of SARS and MERS peaks at around day 7-10 after symptom onset, whereas the viral load of COVID-19 peaks at the time of presentation, similar to influenza. Experience from the treatment of patients with influenza who are admitted to hospital suggested that a combination of multiple antiviral drugs is more effective than single-drug treatments in this setting of patients with a high viral load at presentation,” the investigators wrote.
To test this, they enrolled adults patients admitted to one of six Hong Kong Hospitals for virologically confirmed COVID-19 infections from Feb. 10 through March 20, 2020.
A total of 86 patients were randomly assigned to the combination and 41 to lopinavir/ritonavir alone as controls, at doses described above.
Patients who entered the trial within less than 7 days of symptom onset received the triple combination, with interferon dosing adjusted according to the day that treatment started. Patients recruited 1 or 2 days after symptom onset received three doses of interferon, patients started on day 3 or 4 received two doses, and those started on days 5 or 6 received one interferon dose. Patients recruited 7 days or later from symptom onset did not receive interferon beta-1b because of its proinflammatory effects.
In post-hoc analysis by day of treatment initiation, clinical and virological outcomes (except stool samples) were superior in patients admitted less than 7 days after symptom onset for the 52 patients who received a least one interferon dose plus lopinavir/ritonavir and ribavirin, compared with 24 patients randomized to the control arm (lopinavir/ritonavir only). In contrast, among patients admitted and started on treatment at day 7 or later after symptom onset, there were no differences between those who received lopinavir/ritonavir alone or combined with ribavirin.
Adverse events were reported in 41 of 86 patients in the combination group and 20 of 41 patients in the control arm. The most common adverse events were diarrhea, occurring in 52 of all 127 patients, fever in 48, nausea in 43, and elevated alanine transaminase level in 18. The side effects generally resolved within 3 days of the start of treatments.
There were no serious adverse events reported in the combination group. One patient in the control group had impaired hepatic enzymes requiring discontinuation of treatment. No patients died during the study.
The study was funded by the Shaw Foundation, Richard and Carol Yu, May Tam Mak Mei Yin, and Sanming Project of Medicine. The authors and Dr. Medoff declared no competing interests.
SOURCE: Hung IFN et al. Lancet. 2020 May 8. doi: 10.1016/S0140-6736(20)31101-6.
FROM THE LANCET
ASCO goes ahead online, as conference center is used as hospital
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Societies offer advice on treating osteoporosis patients during pandemic
Five leading bone health organizations have gotten together to provide new recommendations for managing patients with osteoporosis during the COVID-19 pandemic.
The joint guidance – released by the American Society for Bone and Mineral Research (ASBMR), the American Association of Clinical Endocrinologists, the Endocrine Society, the European Calcified Tissue Society, and the National Osteoporosis Foundation – offered both general and specific recommendations for patients whose osteoporosis treatment plan is either continuing or has been disrupted during the COVID-19 pandemic.
Among the general recommendations are to initiate oral bisphosphonate therapy over either the telephone or through a video visit, with no delays for patients at high risk of fracture. They also noted that, as elective procedures, bone mineral density examinations may need to be postponed.
For patients already on osteoporosis medications – such as oral and IV bisphosphonates, denosumab, estrogen, raloxifene, teriparatide, abaloparatide, and romosozumab – they recommend continuing treatment whenever possible. “There is no evidence that any osteoporosis therapy increases the risk or severity of COVID-19 infection or alters the disease course,” they wrote. They did add, however, that COVID-19 may increase the risk of hypercoagulable complications and so caution should be exercised when treating patients with estrogen or raloxifene.
Separately, in a letter to the editor published in the Journal of Clinical Endocrinology and Metabolism (doi: 10.1210/clinem/dgaa254), Ruban Dhaliwal, MD, MPH, of the State University of New York, Syracuse, and coauthors concur in regard to raloxifene. They wrote that, because of the increased risk of thromboembolic events related to COVID-19, “it is best to discontinue raloxifene, which is also associated with such risk.”
The joint statement recognizes current social distancing policies and therefore recommends avoiding standard pretreatment labs prior to IV bisphosphonate and/or denosumab administration if previous labs were normal and the patient’s recent health has been deemed “stable.” Lab evaluation is recommended, however, for patients with fluctuating renal function and for those at higher risk of developing hypocalcemia.
The statement also provides potential alternative methods for delivering parenteral osteoporosis treatments, including off-site clinics, home delivery and administration, self-injection of denosumab and/or romosozumab, and drive-through administration of denosumab and/or romosozumab. They acknowledged the complications surrounding each alternative, including residents of “socioeconomically challenged communities” being unable to reach clinics if public transportation is not available and the “important medicolegal issues” to consider around self-injection.
For all patients whose treatments have been disrupted, the authors recommend frequent reevaluation “with the goal to resume the original osteoporosis treatment plan once circumstances allow.” As for specific recommendations, patients on denosumab who will not be treatable within 7 months of their previous injection should be transitioned to oral bisphosphonate if at all possible. For patients with underlying gastrointestinal disorders, they recommend monthly ibandronate or weekly/monthly risedronate; for patients with chronic renal insufficiency, they recommend an off-label regimen of lower dose oral bisphosphonate.
For patients on teriparatide or abaloparatide who will be unable to receive continued treatment, they recommend a delay in treatment. If that delay goes beyond several months, they recommend a temporary transition to oral bisphosphonate. For patients on romosozumab who will be unable to receive continued treatment, they also recommend a delay in treatment and a temporary transition to oral bisphosphonate. Finally, they expressed confidence that patients on IV bisphosphonates will not be harmed by treatment delays, even those of several months.
“I think we could fall into a trap during this era of the pandemic and fail to address patients’ underlying chronic conditions, even though those comorbidities will end up greatly affecting their overall health,” said incoming ASBMR president Suzanne Jan de Beur, MD, of the Johns Hopkins University, Baltimore. “As we continue to care for our patients, we need to keep chronic conditions like osteoporosis on the radar screen and not stop diagnosing people at risk or those who present with fractures. Even when we can’t perform full screening tests due to distancing policies, we need to be vigilant for those patients who need treatment and administer the treatments we have available as needed.”
The statement’s authors acknowledged the limitations of their recommendations, noting that “there is a paucity of data to provide clear guidance” and as such they were “based primarily on expert opinion.”
The authors from the five organizations did not disclose any conflicts of interest.
Five leading bone health organizations have gotten together to provide new recommendations for managing patients with osteoporosis during the COVID-19 pandemic.
The joint guidance – released by the American Society for Bone and Mineral Research (ASBMR), the American Association of Clinical Endocrinologists, the Endocrine Society, the European Calcified Tissue Society, and the National Osteoporosis Foundation – offered both general and specific recommendations for patients whose osteoporosis treatment plan is either continuing or has been disrupted during the COVID-19 pandemic.
Among the general recommendations are to initiate oral bisphosphonate therapy over either the telephone or through a video visit, with no delays for patients at high risk of fracture. They also noted that, as elective procedures, bone mineral density examinations may need to be postponed.
For patients already on osteoporosis medications – such as oral and IV bisphosphonates, denosumab, estrogen, raloxifene, teriparatide, abaloparatide, and romosozumab – they recommend continuing treatment whenever possible. “There is no evidence that any osteoporosis therapy increases the risk or severity of COVID-19 infection or alters the disease course,” they wrote. They did add, however, that COVID-19 may increase the risk of hypercoagulable complications and so caution should be exercised when treating patients with estrogen or raloxifene.
Separately, in a letter to the editor published in the Journal of Clinical Endocrinology and Metabolism (doi: 10.1210/clinem/dgaa254), Ruban Dhaliwal, MD, MPH, of the State University of New York, Syracuse, and coauthors concur in regard to raloxifene. They wrote that, because of the increased risk of thromboembolic events related to COVID-19, “it is best to discontinue raloxifene, which is also associated with such risk.”
The joint statement recognizes current social distancing policies and therefore recommends avoiding standard pretreatment labs prior to IV bisphosphonate and/or denosumab administration if previous labs were normal and the patient’s recent health has been deemed “stable.” Lab evaluation is recommended, however, for patients with fluctuating renal function and for those at higher risk of developing hypocalcemia.
The statement also provides potential alternative methods for delivering parenteral osteoporosis treatments, including off-site clinics, home delivery and administration, self-injection of denosumab and/or romosozumab, and drive-through administration of denosumab and/or romosozumab. They acknowledged the complications surrounding each alternative, including residents of “socioeconomically challenged communities” being unable to reach clinics if public transportation is not available and the “important medicolegal issues” to consider around self-injection.
For all patients whose treatments have been disrupted, the authors recommend frequent reevaluation “with the goal to resume the original osteoporosis treatment plan once circumstances allow.” As for specific recommendations, patients on denosumab who will not be treatable within 7 months of their previous injection should be transitioned to oral bisphosphonate if at all possible. For patients with underlying gastrointestinal disorders, they recommend monthly ibandronate or weekly/monthly risedronate; for patients with chronic renal insufficiency, they recommend an off-label regimen of lower dose oral bisphosphonate.
For patients on teriparatide or abaloparatide who will be unable to receive continued treatment, they recommend a delay in treatment. If that delay goes beyond several months, they recommend a temporary transition to oral bisphosphonate. For patients on romosozumab who will be unable to receive continued treatment, they also recommend a delay in treatment and a temporary transition to oral bisphosphonate. Finally, they expressed confidence that patients on IV bisphosphonates will not be harmed by treatment delays, even those of several months.
“I think we could fall into a trap during this era of the pandemic and fail to address patients’ underlying chronic conditions, even though those comorbidities will end up greatly affecting their overall health,” said incoming ASBMR president Suzanne Jan de Beur, MD, of the Johns Hopkins University, Baltimore. “As we continue to care for our patients, we need to keep chronic conditions like osteoporosis on the radar screen and not stop diagnosing people at risk or those who present with fractures. Even when we can’t perform full screening tests due to distancing policies, we need to be vigilant for those patients who need treatment and administer the treatments we have available as needed.”
The statement’s authors acknowledged the limitations of their recommendations, noting that “there is a paucity of data to provide clear guidance” and as such they were “based primarily on expert opinion.”
The authors from the five organizations did not disclose any conflicts of interest.
Five leading bone health organizations have gotten together to provide new recommendations for managing patients with osteoporosis during the COVID-19 pandemic.
The joint guidance – released by the American Society for Bone and Mineral Research (ASBMR), the American Association of Clinical Endocrinologists, the Endocrine Society, the European Calcified Tissue Society, and the National Osteoporosis Foundation – offered both general and specific recommendations for patients whose osteoporosis treatment plan is either continuing or has been disrupted during the COVID-19 pandemic.
Among the general recommendations are to initiate oral bisphosphonate therapy over either the telephone or through a video visit, with no delays for patients at high risk of fracture. They also noted that, as elective procedures, bone mineral density examinations may need to be postponed.
For patients already on osteoporosis medications – such as oral and IV bisphosphonates, denosumab, estrogen, raloxifene, teriparatide, abaloparatide, and romosozumab – they recommend continuing treatment whenever possible. “There is no evidence that any osteoporosis therapy increases the risk or severity of COVID-19 infection or alters the disease course,” they wrote. They did add, however, that COVID-19 may increase the risk of hypercoagulable complications and so caution should be exercised when treating patients with estrogen or raloxifene.
Separately, in a letter to the editor published in the Journal of Clinical Endocrinology and Metabolism (doi: 10.1210/clinem/dgaa254), Ruban Dhaliwal, MD, MPH, of the State University of New York, Syracuse, and coauthors concur in regard to raloxifene. They wrote that, because of the increased risk of thromboembolic events related to COVID-19, “it is best to discontinue raloxifene, which is also associated with such risk.”
The joint statement recognizes current social distancing policies and therefore recommends avoiding standard pretreatment labs prior to IV bisphosphonate and/or denosumab administration if previous labs were normal and the patient’s recent health has been deemed “stable.” Lab evaluation is recommended, however, for patients with fluctuating renal function and for those at higher risk of developing hypocalcemia.
The statement also provides potential alternative methods for delivering parenteral osteoporosis treatments, including off-site clinics, home delivery and administration, self-injection of denosumab and/or romosozumab, and drive-through administration of denosumab and/or romosozumab. They acknowledged the complications surrounding each alternative, including residents of “socioeconomically challenged communities” being unable to reach clinics if public transportation is not available and the “important medicolegal issues” to consider around self-injection.
For all patients whose treatments have been disrupted, the authors recommend frequent reevaluation “with the goal to resume the original osteoporosis treatment plan once circumstances allow.” As for specific recommendations, patients on denosumab who will not be treatable within 7 months of their previous injection should be transitioned to oral bisphosphonate if at all possible. For patients with underlying gastrointestinal disorders, they recommend monthly ibandronate or weekly/monthly risedronate; for patients with chronic renal insufficiency, they recommend an off-label regimen of lower dose oral bisphosphonate.
For patients on teriparatide or abaloparatide who will be unable to receive continued treatment, they recommend a delay in treatment. If that delay goes beyond several months, they recommend a temporary transition to oral bisphosphonate. For patients on romosozumab who will be unable to receive continued treatment, they also recommend a delay in treatment and a temporary transition to oral bisphosphonate. Finally, they expressed confidence that patients on IV bisphosphonates will not be harmed by treatment delays, even those of several months.
“I think we could fall into a trap during this era of the pandemic and fail to address patients’ underlying chronic conditions, even though those comorbidities will end up greatly affecting their overall health,” said incoming ASBMR president Suzanne Jan de Beur, MD, of the Johns Hopkins University, Baltimore. “As we continue to care for our patients, we need to keep chronic conditions like osteoporosis on the radar screen and not stop diagnosing people at risk or those who present with fractures. Even when we can’t perform full screening tests due to distancing policies, we need to be vigilant for those patients who need treatment and administer the treatments we have available as needed.”
The statement’s authors acknowledged the limitations of their recommendations, noting that “there is a paucity of data to provide clear guidance” and as such they were “based primarily on expert opinion.”
The authors from the five organizations did not disclose any conflicts of interest.
Plan now to address the COVID-19 mental health fallout
COVID-19 affects the physical, psychological, and social health of people around the world. In the United States, newly reported cases are rising at alarming rates.
As of early May, more than 1.3 million people were confirmed to be COVID-19 infected in the United States and more than 4 million cases were reported globally.1
According to new internal projections from the Centers for Disease Control and Prevention, by June 1, the number of daily deaths could reach about 3,000. By the end of June, a draft CDC report projects that the United States will see 200,000 new cases each day.2
COVID-19 undeniably harms mental health. It gravely instills uncertainty and anxiety, sometimes compounded by the grief of losing loved ones and not being able to mourn those losses in traditional ways. The pandemic also has led to occupational and/or financial losses. Physical distancing and shelter-in-place practices make it even harder to cope with those stresses, although those practices mitigate the dangers. The fears tied to those practices are thought to be keeping some patients with health problems from seeking needed care from hospital EDs.3 In light of the mental health crisis emerging because of the profound impact of this pandemic on all aspects of life, clinicians should start working with public health and political leaders to develop plans to address these issues now.
Known impact of previous outbreaks
Previous disease outbreaks evidence a similar pattern of heightened anxiety as the patterns seen with COVID-19. For example, during the 2009 swine flu outbreak, 36 surveys of more than 3,000 participants in the United Kingdom found that 9.6%-32.9% of the participants were “very” or “fairly” worried about the possibility of contracting swine flu.4 The 1995 Ebola outbreak in the Democratic Republic of the Congo produced stigmatization tied to the illness. That outbreak provided many lessons for physicians.5
The metaphors ascribed to different diseases affect communities’ responses to it. The SARS virus has been particularly insidious and has been thought of as a “plague.”6 Epidemics of all kinds cause fears, not only of contracting the disease and dying, but also of social exclusion.7 The emotional responses to COVID-19 can precipitate anxiety, depression, insomnia, and somatic symptoms.
Repeated exposure to news media about the disease adds to theses stresss.10 Constant news consumption can result in panicky hoarding of resources, such as masks; gloves; first-aid kits; alcohol hand rubs; and daily necessities such as food, water, and toilet paper.
Who is most affected by outbreaks?
Those most affected after a disease outbreak are patients, their families, and medical personnel. In one study, researchers who conducted an online survey of 1,210 respondents in 194 cities in China during the early phase of the outbreak found that the psychological effects were worst among women, students, and vulnerable populations.11
Meanwhile, a 2003 cross-sectional survey of 1,115 ethnic Chinese adults in Hong Kong who responded to the SARS outbreak found that the respondents most likely to heed precautionary measures against the infection were “older, female, more educated people as well as those with a positive contact history and SARS-like symptoms.”12
Negative mental health consequences of a disease outbreak might persist long after the infection has dissipated. An increased association has been found between people with mental illness and posttraumatic stress following many disasters.13,14,15
Political and health care leaders should develop plans aimed at helping people copewith pandemics.16 Such strategies should include prioritizing treatment of the physical and mental health needs of patients infected with COVID-19 and of the general population. Screening for anxiety, depression, and suicidal thoughts ought to be implemented, and specialized psychiatric care teams should be assigned.17 We know that psychiatrists and other physicians turned to telemedicine to provide support, psychotherapy, and medical attention to patients soon after physical distancing measures were put into place. Those kinds of quick responses are important for our patients.
Fear of contagious diseases often creates social divisions. Governments should offer accurate information to reduce the detrimental effect of rumors and false propaganda.18 “Social distancing” is a misleading term; these practices should be referred to as “physical distancing.” We should encourage patients to maintain interpersonal contacts – albeit at a distance – to reach out to those in need, and to support one another during these troubled times.19
References
1. World Health Organization. Situation Report–107. 2020 May 6.
2. Centers for Disease Control and Prevention. Situation Update. 2020 Apr 30.
3. O’Brien M. “Are Americans in medical crisis avoiding the ER due to coronavirus?” PBS Newshour. 2020 May 6.
4. Rubin G et al. Health Technol Assess. 2010 Jul;14(340):183-266.
5. Hall R et al. Gen Hosp Psychiatry. 2008 Sep-Oct;30(5):466-52.
6. Verghese A. Clin Infect Dis. 2004;38:932-3.
7. Interagency Standing Committee. Briefing note on addressing health and psychosocial aspects of COVID-19 Outbreak – Version 11. 2020 Feb.
8. Sim K et al. J Psychosom Res. 2010;68:195-202.
9. Shigemura J et al. Psychiatry Clin Neurosci. 2020;74:281-2.
10. Garfin DR et al. Health Psychol. 2020 May;39(5):355-7.
11. Wang C et al. Int J Environ Res Public Health. 2020 Mar 6. doi: 10.3390/ijerph1751729.
12. Leung GM et al. J Epidemiol Community Health. 2003 Nov;57(1):857-63.
13. Xiang Y et al. Int J Biol Sci. 2020;16:1741-4.
14. Alvarez J, Hunt M. J Trauma Stress. 2005 Oct 18(5);18:497-505.
15. Cukor J et al. Depress Anxiety. 2011 Mar;28(3):210-7.
16. Horton R. Lancet. 2020 Feb;395(10222):400.
17. Xiang Y-T et al. Lancet Psychiatry. 2020 Feb 4;7:228-9.
18. World Health Organization. “Rational use of personal protective equipment (PPE) for coronavirus (COVID-19).” Interim Guidance. 2020 Mar.
19. Brooks S et al. Lancet 2020 Mar 14;395:912-20.
Dr. Doppalapudi is affiliated with Griffin Memorial Hospital in Norman, Okla. Dr. Lippmann is emeritus professor of psychiatry and also in family medicine at the University of Louisville (Ky.) Dr. Doppalapudi and Dr. Lippmann disclosed no conflicts of interest.
COVID-19 affects the physical, psychological, and social health of people around the world. In the United States, newly reported cases are rising at alarming rates.
As of early May, more than 1.3 million people were confirmed to be COVID-19 infected in the United States and more than 4 million cases were reported globally.1
According to new internal projections from the Centers for Disease Control and Prevention, by June 1, the number of daily deaths could reach about 3,000. By the end of June, a draft CDC report projects that the United States will see 200,000 new cases each day.2
COVID-19 undeniably harms mental health. It gravely instills uncertainty and anxiety, sometimes compounded by the grief of losing loved ones and not being able to mourn those losses in traditional ways. The pandemic also has led to occupational and/or financial losses. Physical distancing and shelter-in-place practices make it even harder to cope with those stresses, although those practices mitigate the dangers. The fears tied to those practices are thought to be keeping some patients with health problems from seeking needed care from hospital EDs.3 In light of the mental health crisis emerging because of the profound impact of this pandemic on all aspects of life, clinicians should start working with public health and political leaders to develop plans to address these issues now.
Known impact of previous outbreaks
Previous disease outbreaks evidence a similar pattern of heightened anxiety as the patterns seen with COVID-19. For example, during the 2009 swine flu outbreak, 36 surveys of more than 3,000 participants in the United Kingdom found that 9.6%-32.9% of the participants were “very” or “fairly” worried about the possibility of contracting swine flu.4 The 1995 Ebola outbreak in the Democratic Republic of the Congo produced stigmatization tied to the illness. That outbreak provided many lessons for physicians.5
The metaphors ascribed to different diseases affect communities’ responses to it. The SARS virus has been particularly insidious and has been thought of as a “plague.”6 Epidemics of all kinds cause fears, not only of contracting the disease and dying, but also of social exclusion.7 The emotional responses to COVID-19 can precipitate anxiety, depression, insomnia, and somatic symptoms.
Repeated exposure to news media about the disease adds to theses stresss.10 Constant news consumption can result in panicky hoarding of resources, such as masks; gloves; first-aid kits; alcohol hand rubs; and daily necessities such as food, water, and toilet paper.
Who is most affected by outbreaks?
Those most affected after a disease outbreak are patients, their families, and medical personnel. In one study, researchers who conducted an online survey of 1,210 respondents in 194 cities in China during the early phase of the outbreak found that the psychological effects were worst among women, students, and vulnerable populations.11
Meanwhile, a 2003 cross-sectional survey of 1,115 ethnic Chinese adults in Hong Kong who responded to the SARS outbreak found that the respondents most likely to heed precautionary measures against the infection were “older, female, more educated people as well as those with a positive contact history and SARS-like symptoms.”12
Negative mental health consequences of a disease outbreak might persist long after the infection has dissipated. An increased association has been found between people with mental illness and posttraumatic stress following many disasters.13,14,15
Political and health care leaders should develop plans aimed at helping people copewith pandemics.16 Such strategies should include prioritizing treatment of the physical and mental health needs of patients infected with COVID-19 and of the general population. Screening for anxiety, depression, and suicidal thoughts ought to be implemented, and specialized psychiatric care teams should be assigned.17 We know that psychiatrists and other physicians turned to telemedicine to provide support, psychotherapy, and medical attention to patients soon after physical distancing measures were put into place. Those kinds of quick responses are important for our patients.
Fear of contagious diseases often creates social divisions. Governments should offer accurate information to reduce the detrimental effect of rumors and false propaganda.18 “Social distancing” is a misleading term; these practices should be referred to as “physical distancing.” We should encourage patients to maintain interpersonal contacts – albeit at a distance – to reach out to those in need, and to support one another during these troubled times.19
References
1. World Health Organization. Situation Report–107. 2020 May 6.
2. Centers for Disease Control and Prevention. Situation Update. 2020 Apr 30.
3. O’Brien M. “Are Americans in medical crisis avoiding the ER due to coronavirus?” PBS Newshour. 2020 May 6.
4. Rubin G et al. Health Technol Assess. 2010 Jul;14(340):183-266.
5. Hall R et al. Gen Hosp Psychiatry. 2008 Sep-Oct;30(5):466-52.
6. Verghese A. Clin Infect Dis. 2004;38:932-3.
7. Interagency Standing Committee. Briefing note on addressing health and psychosocial aspects of COVID-19 Outbreak – Version 11. 2020 Feb.
8. Sim K et al. J Psychosom Res. 2010;68:195-202.
9. Shigemura J et al. Psychiatry Clin Neurosci. 2020;74:281-2.
10. Garfin DR et al. Health Psychol. 2020 May;39(5):355-7.
11. Wang C et al. Int J Environ Res Public Health. 2020 Mar 6. doi: 10.3390/ijerph1751729.
12. Leung GM et al. J Epidemiol Community Health. 2003 Nov;57(1):857-63.
13. Xiang Y et al. Int J Biol Sci. 2020;16:1741-4.
14. Alvarez J, Hunt M. J Trauma Stress. 2005 Oct 18(5);18:497-505.
15. Cukor J et al. Depress Anxiety. 2011 Mar;28(3):210-7.
16. Horton R. Lancet. 2020 Feb;395(10222):400.
17. Xiang Y-T et al. Lancet Psychiatry. 2020 Feb 4;7:228-9.
18. World Health Organization. “Rational use of personal protective equipment (PPE) for coronavirus (COVID-19).” Interim Guidance. 2020 Mar.
19. Brooks S et al. Lancet 2020 Mar 14;395:912-20.
Dr. Doppalapudi is affiliated with Griffin Memorial Hospital in Norman, Okla. Dr. Lippmann is emeritus professor of psychiatry and also in family medicine at the University of Louisville (Ky.) Dr. Doppalapudi and Dr. Lippmann disclosed no conflicts of interest.
COVID-19 affects the physical, psychological, and social health of people around the world. In the United States, newly reported cases are rising at alarming rates.
As of early May, more than 1.3 million people were confirmed to be COVID-19 infected in the United States and more than 4 million cases were reported globally.1
According to new internal projections from the Centers for Disease Control and Prevention, by June 1, the number of daily deaths could reach about 3,000. By the end of June, a draft CDC report projects that the United States will see 200,000 new cases each day.2
COVID-19 undeniably harms mental health. It gravely instills uncertainty and anxiety, sometimes compounded by the grief of losing loved ones and not being able to mourn those losses in traditional ways. The pandemic also has led to occupational and/or financial losses. Physical distancing and shelter-in-place practices make it even harder to cope with those stresses, although those practices mitigate the dangers. The fears tied to those practices are thought to be keeping some patients with health problems from seeking needed care from hospital EDs.3 In light of the mental health crisis emerging because of the profound impact of this pandemic on all aspects of life, clinicians should start working with public health and political leaders to develop plans to address these issues now.
Known impact of previous outbreaks
Previous disease outbreaks evidence a similar pattern of heightened anxiety as the patterns seen with COVID-19. For example, during the 2009 swine flu outbreak, 36 surveys of more than 3,000 participants in the United Kingdom found that 9.6%-32.9% of the participants were “very” or “fairly” worried about the possibility of contracting swine flu.4 The 1995 Ebola outbreak in the Democratic Republic of the Congo produced stigmatization tied to the illness. That outbreak provided many lessons for physicians.5
The metaphors ascribed to different diseases affect communities’ responses to it. The SARS virus has been particularly insidious and has been thought of as a “plague.”6 Epidemics of all kinds cause fears, not only of contracting the disease and dying, but also of social exclusion.7 The emotional responses to COVID-19 can precipitate anxiety, depression, insomnia, and somatic symptoms.
Repeated exposure to news media about the disease adds to theses stresss.10 Constant news consumption can result in panicky hoarding of resources, such as masks; gloves; first-aid kits; alcohol hand rubs; and daily necessities such as food, water, and toilet paper.
Who is most affected by outbreaks?
Those most affected after a disease outbreak are patients, their families, and medical personnel. In one study, researchers who conducted an online survey of 1,210 respondents in 194 cities in China during the early phase of the outbreak found that the psychological effects were worst among women, students, and vulnerable populations.11
Meanwhile, a 2003 cross-sectional survey of 1,115 ethnic Chinese adults in Hong Kong who responded to the SARS outbreak found that the respondents most likely to heed precautionary measures against the infection were “older, female, more educated people as well as those with a positive contact history and SARS-like symptoms.”12
Negative mental health consequences of a disease outbreak might persist long after the infection has dissipated. An increased association has been found between people with mental illness and posttraumatic stress following many disasters.13,14,15
Political and health care leaders should develop plans aimed at helping people copewith pandemics.16 Such strategies should include prioritizing treatment of the physical and mental health needs of patients infected with COVID-19 and of the general population. Screening for anxiety, depression, and suicidal thoughts ought to be implemented, and specialized psychiatric care teams should be assigned.17 We know that psychiatrists and other physicians turned to telemedicine to provide support, psychotherapy, and medical attention to patients soon after physical distancing measures were put into place. Those kinds of quick responses are important for our patients.
Fear of contagious diseases often creates social divisions. Governments should offer accurate information to reduce the detrimental effect of rumors and false propaganda.18 “Social distancing” is a misleading term; these practices should be referred to as “physical distancing.” We should encourage patients to maintain interpersonal contacts – albeit at a distance – to reach out to those in need, and to support one another during these troubled times.19
References
1. World Health Organization. Situation Report–107. 2020 May 6.
2. Centers for Disease Control and Prevention. Situation Update. 2020 Apr 30.
3. O’Brien M. “Are Americans in medical crisis avoiding the ER due to coronavirus?” PBS Newshour. 2020 May 6.
4. Rubin G et al. Health Technol Assess. 2010 Jul;14(340):183-266.
5. Hall R et al. Gen Hosp Psychiatry. 2008 Sep-Oct;30(5):466-52.
6. Verghese A. Clin Infect Dis. 2004;38:932-3.
7. Interagency Standing Committee. Briefing note on addressing health and psychosocial aspects of COVID-19 Outbreak – Version 11. 2020 Feb.
8. Sim K et al. J Psychosom Res. 2010;68:195-202.
9. Shigemura J et al. Psychiatry Clin Neurosci. 2020;74:281-2.
10. Garfin DR et al. Health Psychol. 2020 May;39(5):355-7.
11. Wang C et al. Int J Environ Res Public Health. 2020 Mar 6. doi: 10.3390/ijerph1751729.
12. Leung GM et al. J Epidemiol Community Health. 2003 Nov;57(1):857-63.
13. Xiang Y et al. Int J Biol Sci. 2020;16:1741-4.
14. Alvarez J, Hunt M. J Trauma Stress. 2005 Oct 18(5);18:497-505.
15. Cukor J et al. Depress Anxiety. 2011 Mar;28(3):210-7.
16. Horton R. Lancet. 2020 Feb;395(10222):400.
17. Xiang Y-T et al. Lancet Psychiatry. 2020 Feb 4;7:228-9.
18. World Health Organization. “Rational use of personal protective equipment (PPE) for coronavirus (COVID-19).” Interim Guidance. 2020 Mar.
19. Brooks S et al. Lancet 2020 Mar 14;395:912-20.
Dr. Doppalapudi is affiliated with Griffin Memorial Hospital in Norman, Okla. Dr. Lippmann is emeritus professor of psychiatry and also in family medicine at the University of Louisville (Ky.) Dr. Doppalapudi and Dr. Lippmann disclosed no conflicts of interest.
Silent brain infarcts found in 3% of AFib patients, tied to cognitive decline
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
FROM HEART RHYTHM 2020



















