User login
-
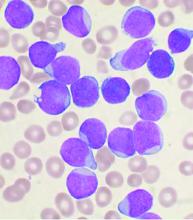
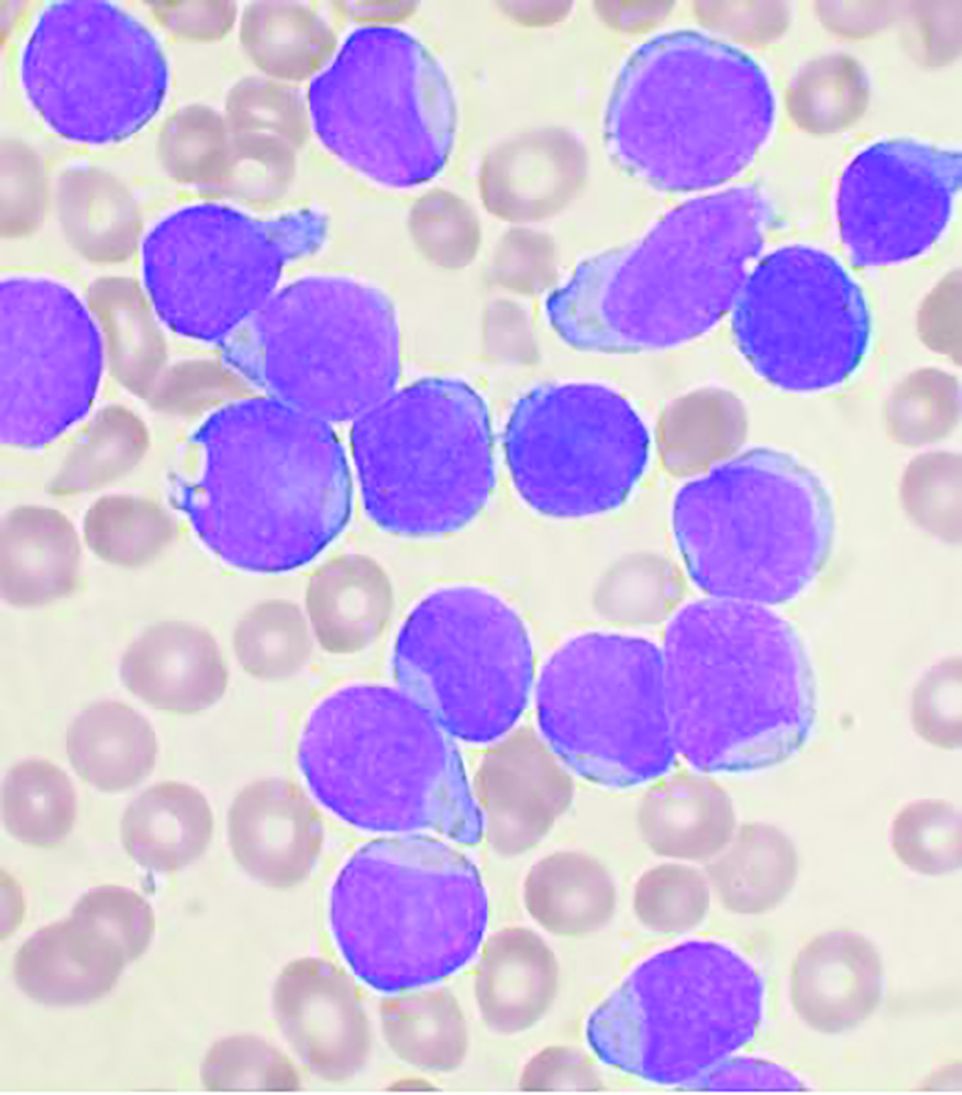
Omidubicel improves on umbilical cord blood transplants
Omidubicel, an investigational enriched umbilical cord blood product being developed by Gamida Cell for transplantation in patients with blood cancers, appears to have some advantages over standard umbilical cord blood.
The results come from a global phase 3 trial (NCT02730299) presented at the annual meeting of the European Society for Blood and Bone Marrow Transplantation.
“Transplantation with omidubicel, compared to standard cord blood transplantation, results in faster hematopoietic recovery, fewer infections, and fewer days in hospital,” said coinvestigator Guillermo F. Sanz, MD, PhD, from the Hospital Universitari i Politècnic la Fe in Valencia, Spain.
“Omidubicel should be considered as the new standard of care for patients eligible for umbilical cord blood transplantation,” Dr. Sanz concluded.
Zachariah DeFilipp, MD, from Mass General Cancer Center in Boston, a hematopoietic stem cell transplantation specialist who was not involved in the study, said in an interview that “omidubicel significantly improves the engraftment after transplant, as compared to standard cord blood transplant. For patients that lack an HLA-matched donor, this approach can help overcome the prolonged cytopenias that occur with standard cord blood transplants in adults.”
Gamida Cell plans to submit these data for approval of omidubicel by the Food and Drug Administration in the fourth quarter of 2021.
Omidubicel is also being evaluated in a phase 1/2 clinical study in patients with severe aplastic anemia (NCT03173937).
Expanding possibilities
Although umbilical cord blood stem cell grafts come from a readily available source and show greater tolerance across HLA barriers than other sources (such as bone marrow), the relatively low dose of stem cells in each unit results in delayed hematopoietic recovery, increased transplant-related morbidity and mortality, and longer hospitalizations, Dr. Sanz said.
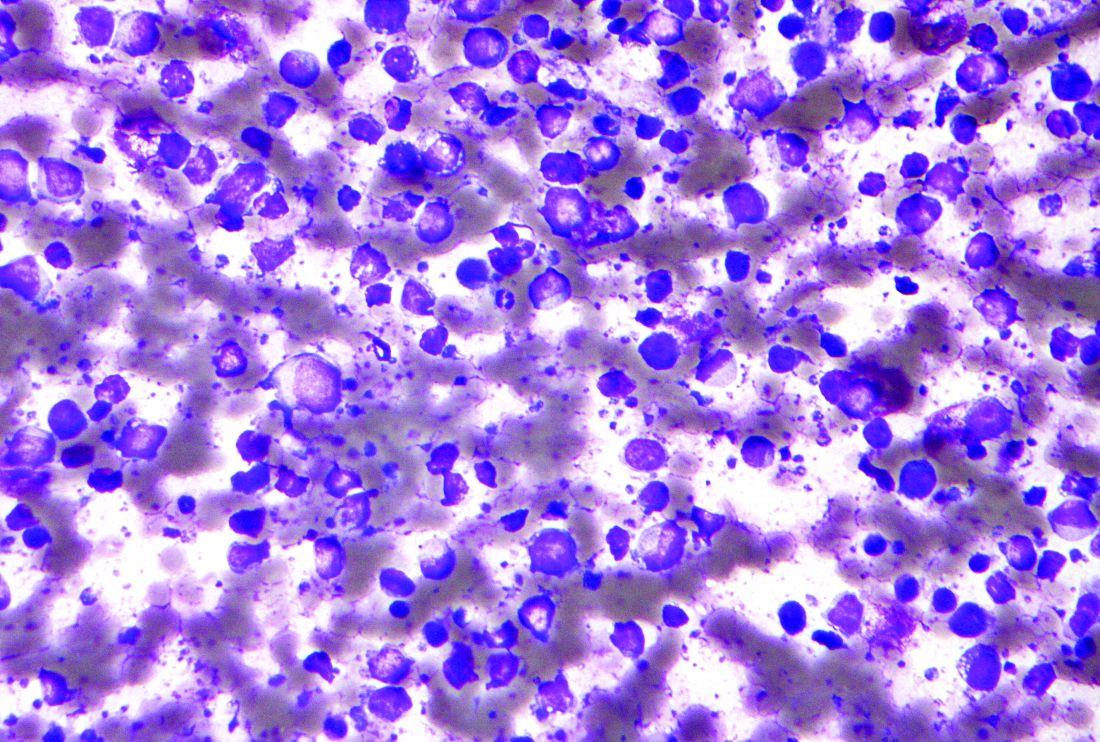
Omidubicel consists of two cryopreserved fractions from a single cord blood unit. The product contains both noncultured CD133-negative cells, including T cells, and CD133-positive cells that are then expanded ex vivo for 21 days in the presence of nicotinamide.
“Nicotinamide increases stem and progenitor cells, inhibits differentiation and increases migration, bone marrow homing, and engraftment efficiency while preserving cellular functionality and phenotype,” Dr. Sanz explained during his presentation.
In an earlier phase 1/2 trial in 36 patients with high-risk hematologic malignancies, omidubicel was associated with hematopoietic engraftment lasting at least 10 years.
Details of phase 3 trial results
The global phase 3 trial was conducted in 125 patients (aged 13-65 years) with high-risk malignancies, including acute myeloid and lymphoblastic leukemias, myelodysplastic syndrome, chronic myeloid leukemia, lymphomas, and rare leukemias. These patients were all eligible for allogeneic stem cell transplantation but did not have matched donors.
Patients were randomly assigned to receive hematopoietic reconstitution with either omidubicel (n = 52) or standard cord blood (n = 58).
At 42 days of follow-up, the median time to neutrophil engraftment in the intention-to-treat (ITT) population, the primary endpoint, was 12 days with omidubicel versus 22 days with standard cord blood (P < .001).
In the as-treated population – the 108 patients who actually received omidubicel or standard cord blood – median time to engraftment was 10.0 versus 20.5 days, respectively (P < .001).
Rates of neutrophil engraftment at 42 days were 96% with omidubicel versus 89% with standard cord blood.
The secondary endpoint of time-to-platelet engraftment in the ITT population also favored omidubicel, with a cumulative day 42 incidence rate of 55%, compared with 35% with standard cord blood (P = .028).
In the as-treated population, median times to platelet engraftment were 37 days and 50 days, respectively (P = .023). The cumulative rates of platelet engraftment at 100 days of follow-up were 83% and 73%, respectively.
The incidence of grade 2 or 3 bacterial or invasive fungal infections by day 100 in the ITT population was 37% among patients who received omidubicel, compared with 57% for patients who received standard cord blood (P = .027). Viral infections occurred in 10% versus 26% of patients, respectively.
The incidence of acute graft versus host disease at day 100 was similar between treatment groups, and there was no significant difference at 1 year.
Relapse and nonrelapse mortality rates, as well as disease-free and overall survival rates also did not differ between groups.
In the first 100 days post transplant, patients who received omidubicel were alive and out of the hospital for a median of 60.5 days, compared with 48 days for patients who received standard cord blood (P = .005).
The study was funded by Gamida Cell. Dr. Sanz reported receiving research funding from the company and several others, and consulting fees, honoraria, speakers bureau activity, and travel expenses from other companies. Dr. DeFilipp reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omidubicel, an investigational enriched umbilical cord blood product being developed by Gamida Cell for transplantation in patients with blood cancers, appears to have some advantages over standard umbilical cord blood.
The results come from a global phase 3 trial (NCT02730299) presented at the annual meeting of the European Society for Blood and Bone Marrow Transplantation.
“Transplantation with omidubicel, compared to standard cord blood transplantation, results in faster hematopoietic recovery, fewer infections, and fewer days in hospital,” said coinvestigator Guillermo F. Sanz, MD, PhD, from the Hospital Universitari i Politècnic la Fe in Valencia, Spain.
“Omidubicel should be considered as the new standard of care for patients eligible for umbilical cord blood transplantation,” Dr. Sanz concluded.
Zachariah DeFilipp, MD, from Mass General Cancer Center in Boston, a hematopoietic stem cell transplantation specialist who was not involved in the study, said in an interview that “omidubicel significantly improves the engraftment after transplant, as compared to standard cord blood transplant. For patients that lack an HLA-matched donor, this approach can help overcome the prolonged cytopenias that occur with standard cord blood transplants in adults.”
Gamida Cell plans to submit these data for approval of omidubicel by the Food and Drug Administration in the fourth quarter of 2021.
Omidubicel is also being evaluated in a phase 1/2 clinical study in patients with severe aplastic anemia (NCT03173937).
Expanding possibilities
Although umbilical cord blood stem cell grafts come from a readily available source and show greater tolerance across HLA barriers than other sources (such as bone marrow), the relatively low dose of stem cells in each unit results in delayed hematopoietic recovery, increased transplant-related morbidity and mortality, and longer hospitalizations, Dr. Sanz said.
Omidubicel consists of two cryopreserved fractions from a single cord blood unit. The product contains both noncultured CD133-negative cells, including T cells, and CD133-positive cells that are then expanded ex vivo for 21 days in the presence of nicotinamide.
“Nicotinamide increases stem and progenitor cells, inhibits differentiation and increases migration, bone marrow homing, and engraftment efficiency while preserving cellular functionality and phenotype,” Dr. Sanz explained during his presentation.
In an earlier phase 1/2 trial in 36 patients with high-risk hematologic malignancies, omidubicel was associated with hematopoietic engraftment lasting at least 10 years.
Details of phase 3 trial results
The global phase 3 trial was conducted in 125 patients (aged 13-65 years) with high-risk malignancies, including acute myeloid and lymphoblastic leukemias, myelodysplastic syndrome, chronic myeloid leukemia, lymphomas, and rare leukemias. These patients were all eligible for allogeneic stem cell transplantation but did not have matched donors.
Patients were randomly assigned to receive hematopoietic reconstitution with either omidubicel (n = 52) or standard cord blood (n = 58).
At 42 days of follow-up, the median time to neutrophil engraftment in the intention-to-treat (ITT) population, the primary endpoint, was 12 days with omidubicel versus 22 days with standard cord blood (P < .001).
In the as-treated population – the 108 patients who actually received omidubicel or standard cord blood – median time to engraftment was 10.0 versus 20.5 days, respectively (P < .001).
Rates of neutrophil engraftment at 42 days were 96% with omidubicel versus 89% with standard cord blood.
The secondary endpoint of time-to-platelet engraftment in the ITT population also favored omidubicel, with a cumulative day 42 incidence rate of 55%, compared with 35% with standard cord blood (P = .028).
In the as-treated population, median times to platelet engraftment were 37 days and 50 days, respectively (P = .023). The cumulative rates of platelet engraftment at 100 days of follow-up were 83% and 73%, respectively.
The incidence of grade 2 or 3 bacterial or invasive fungal infections by day 100 in the ITT population was 37% among patients who received omidubicel, compared with 57% for patients who received standard cord blood (P = .027). Viral infections occurred in 10% versus 26% of patients, respectively.
The incidence of acute graft versus host disease at day 100 was similar between treatment groups, and there was no significant difference at 1 year.
Relapse and nonrelapse mortality rates, as well as disease-free and overall survival rates also did not differ between groups.
In the first 100 days post transplant, patients who received omidubicel were alive and out of the hospital for a median of 60.5 days, compared with 48 days for patients who received standard cord blood (P = .005).
The study was funded by Gamida Cell. Dr. Sanz reported receiving research funding from the company and several others, and consulting fees, honoraria, speakers bureau activity, and travel expenses from other companies. Dr. DeFilipp reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omidubicel, an investigational enriched umbilical cord blood product being developed by Gamida Cell for transplantation in patients with blood cancers, appears to have some advantages over standard umbilical cord blood.
The results come from a global phase 3 trial (NCT02730299) presented at the annual meeting of the European Society for Blood and Bone Marrow Transplantation.
“Transplantation with omidubicel, compared to standard cord blood transplantation, results in faster hematopoietic recovery, fewer infections, and fewer days in hospital,” said coinvestigator Guillermo F. Sanz, MD, PhD, from the Hospital Universitari i Politècnic la Fe in Valencia, Spain.
“Omidubicel should be considered as the new standard of care for patients eligible for umbilical cord blood transplantation,” Dr. Sanz concluded.
Zachariah DeFilipp, MD, from Mass General Cancer Center in Boston, a hematopoietic stem cell transplantation specialist who was not involved in the study, said in an interview that “omidubicel significantly improves the engraftment after transplant, as compared to standard cord blood transplant. For patients that lack an HLA-matched donor, this approach can help overcome the prolonged cytopenias that occur with standard cord blood transplants in adults.”
Gamida Cell plans to submit these data for approval of omidubicel by the Food and Drug Administration in the fourth quarter of 2021.
Omidubicel is also being evaluated in a phase 1/2 clinical study in patients with severe aplastic anemia (NCT03173937).
Expanding possibilities
Although umbilical cord blood stem cell grafts come from a readily available source and show greater tolerance across HLA barriers than other sources (such as bone marrow), the relatively low dose of stem cells in each unit results in delayed hematopoietic recovery, increased transplant-related morbidity and mortality, and longer hospitalizations, Dr. Sanz said.
Omidubicel consists of two cryopreserved fractions from a single cord blood unit. The product contains both noncultured CD133-negative cells, including T cells, and CD133-positive cells that are then expanded ex vivo for 21 days in the presence of nicotinamide.
“Nicotinamide increases stem and progenitor cells, inhibits differentiation and increases migration, bone marrow homing, and engraftment efficiency while preserving cellular functionality and phenotype,” Dr. Sanz explained during his presentation.
In an earlier phase 1/2 trial in 36 patients with high-risk hematologic malignancies, omidubicel was associated with hematopoietic engraftment lasting at least 10 years.
Details of phase 3 trial results
The global phase 3 trial was conducted in 125 patients (aged 13-65 years) with high-risk malignancies, including acute myeloid and lymphoblastic leukemias, myelodysplastic syndrome, chronic myeloid leukemia, lymphomas, and rare leukemias. These patients were all eligible for allogeneic stem cell transplantation but did not have matched donors.
Patients were randomly assigned to receive hematopoietic reconstitution with either omidubicel (n = 52) or standard cord blood (n = 58).
At 42 days of follow-up, the median time to neutrophil engraftment in the intention-to-treat (ITT) population, the primary endpoint, was 12 days with omidubicel versus 22 days with standard cord blood (P < .001).
In the as-treated population – the 108 patients who actually received omidubicel or standard cord blood – median time to engraftment was 10.0 versus 20.5 days, respectively (P < .001).
Rates of neutrophil engraftment at 42 days were 96% with omidubicel versus 89% with standard cord blood.
The secondary endpoint of time-to-platelet engraftment in the ITT population also favored omidubicel, with a cumulative day 42 incidence rate of 55%, compared with 35% with standard cord blood (P = .028).
In the as-treated population, median times to platelet engraftment were 37 days and 50 days, respectively (P = .023). The cumulative rates of platelet engraftment at 100 days of follow-up were 83% and 73%, respectively.
The incidence of grade 2 or 3 bacterial or invasive fungal infections by day 100 in the ITT population was 37% among patients who received omidubicel, compared with 57% for patients who received standard cord blood (P = .027). Viral infections occurred in 10% versus 26% of patients, respectively.
The incidence of acute graft versus host disease at day 100 was similar between treatment groups, and there was no significant difference at 1 year.
Relapse and nonrelapse mortality rates, as well as disease-free and overall survival rates also did not differ between groups.
In the first 100 days post transplant, patients who received omidubicel were alive and out of the hospital for a median of 60.5 days, compared with 48 days for patients who received standard cord blood (P = .005).
The study was funded by Gamida Cell. Dr. Sanz reported receiving research funding from the company and several others, and consulting fees, honoraria, speakers bureau activity, and travel expenses from other companies. Dr. DeFilipp reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How to talk to patients reluctant to get a COVID-19 vaccine
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
2021 match sets records: Who matched and who didn’t?
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
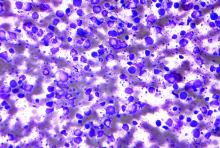
GVHD prophylaxis: Similar outcomes with PTCy and ATG
A newer regimen was no more effective than an older regimen when both were compared for graft versus host disease (GVHD) prophylaxis in patients who underwent reduced-intensity conditioning followed by a hematopoietic stem cell transplant (HSCT) from a 10/10 HLA-matched related or unrelated donor.
These results come from a multicenter randomized trial that compared posttransplant cyclophosphamide (PTCy) to antithymocyte globulin (ATG), which has been used for decades.
There were no significant differences between the two in either disease-free or overall survival, GVHD-free relapse-free survival (GRFS), or nonrelapse mortality, reported lead investigator Eolia Brissot, MD, of Hôpital Saint-Antoine, Sorbonne University, Paris.
Her presentation was judged ‘top abstract’ at the annual meeting of the European Society for Blood and Marrow Transplantation (EBMT), held virtually because of the pandemic.
ATG has been used for more than 30 years for GVHD prophylaxis in allogeneic HSCT. In contrast, PTCy is the new kid on the block, developed to facilitate haploidentical transplants using unmanipulated bone marrow cells to act as a method for selective allodepletion in vivo.
“PTCy [has proved] to be effective in preventing both acute and chronic GVHD,” Dr. Brissot said. “However, controversial outcome data remain when comparing PTCy and ATG according to the type of donors.”
Until now, she noted, there have been no prospective randomized data available for patients with donors (related or unrelated) that have 10 of 10 matched human leukocyte antigen (HLA) alleles. Hence, these were the patients studied in this latest trial, and in this population both regimens showed similar outcomes.
A bone marrow transplant specialist who was not involved in the study said that it’s a good first step.
“This is an important study to gain preliminary data to design a larger, subsequent phase 3 study,” said Zachariah DeFilipp, MD, of Mass General Cancer Center in Boston.
“The use of ATG as part of GVHD prophylaxis is common at many centers, especially in Europe, “ he explained. “The use of posttransplant cyclophosphamide is being expanded to more settings with transplant, beyond haploidentical transplant.
“Further investigations comparing the use of PTCy to ATG will help determine whether PTCy should be more broadly adopted as a standard-of-care GVHD prophylaxis approach, given currently available regimens,” he said in an interview.
Study details
The randomized phase 2b study (NCT02876679), conducted in centers in 11 cities in France, compared PTCy with ATG in patients with hematologic malignancies for whom a reduced-intensity allogeneic HSCT was indicated. This included patients aged 50 and older, and/or heavily pretreated patients who received an autologous HSCT or more than two prior lines of chemotherapy before allogeneic HSCT, as well as patients with poor performance status due to significant medical comorbidities.
Excluded from the trial were patients with creatinine clearance less than 30 mL/min; bilirubin or liver amino transferases more than three times the upper limit of normal; cardiac ejection fraction less than 40%; or pulmonary impairment with less than 50% lung carbon monoxide diffusing capacity.
Of 90 patients enrolled, 1 experienced a relapse before randomization, and the remaining 89 patients were assigned to either PTCy (experimental arm, 45 patients) or to ATG (control group, 44 patients).
Most patients had good performance status (Eastern Cooperative Oncology Group performance status 0 or 1). Diagnoses included acute myeloid and lymphoblastic leukemia, multiple myeloma, lymphomas, and myelodysplastic syndrome. The median age was 64 years, and the male to female ratio was about 2:1 in both groups.
All patients received “FB2” reduced-intensity conditioning with fludarabine30 mg/m2 per day for 4 days, and intravenous busulfan 130 mg/m2 per day for 2 days.
Patients in the experimental arm received cyclophosphamide 50 mg/kg per day on days 3 and 4 after transplant. Patients in the control group received ATG 2.5 mg/kg per day on days 3 and 2 prior to transplant.
All patients also received cyclosporine A, and those who had unrelated donors also received mycophenolate mofetil. In all, 39% of patients received cells from matched sibling donors, and 61% received cells from matched unrelated donors.
No significant differences seen
At 12 months of follow-up, there was no significant difference between the trial arms in the primary endpoint of GRFS, a composite of grade 3-4 acute GVHD, chronic GVHD requiring systemic treatment, relapse, or death. The rates of GRFS were 52.2% with PTCy vs. 45% with ATG.
Rates of disease-free survival were 68.5% with PTCy and 67.1% with ATG. The respective 12-month overall survival rates were 78.9% and 80.4%, respectively. The differences were not statistically significant.
The incidence of relapse at 1 year was 22.1% in the ATG group vs. 17.6% in the PTCy group. Respective nonrelapse mortality rates were 10.8% for the ATG group and 14% for the PTCy group. Neither difference was statistically significant.
There were also no significant between-group differences in the incidence at 12-month follow-up of either acute GVHD (34.9% PTCy arm vs. 24.3% ATG arm for grades II-IV combined, and 9.3% PTCy vs. 2.7% ATG for grades III or IV) or chronic GVHD (30.2% ATG vs. 26% PTCy).
The safety analysis showed no significant between-group differences in selected adverse events, including Epstein-Barr viral reactivation, cytomegalovirus reactivation, cardiac adverse events, or hemorrhagic cystitis.
The study was supported by Hospitals of Paris. Dr. Brissot and Dr. DeFilipp have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A newer regimen was no more effective than an older regimen when both were compared for graft versus host disease (GVHD) prophylaxis in patients who underwent reduced-intensity conditioning followed by a hematopoietic stem cell transplant (HSCT) from a 10/10 HLA-matched related or unrelated donor.
These results come from a multicenter randomized trial that compared posttransplant cyclophosphamide (PTCy) to antithymocyte globulin (ATG), which has been used for decades.
There were no significant differences between the two in either disease-free or overall survival, GVHD-free relapse-free survival (GRFS), or nonrelapse mortality, reported lead investigator Eolia Brissot, MD, of Hôpital Saint-Antoine, Sorbonne University, Paris.
Her presentation was judged ‘top abstract’ at the annual meeting of the European Society for Blood and Marrow Transplantation (EBMT), held virtually because of the pandemic.
ATG has been used for more than 30 years for GVHD prophylaxis in allogeneic HSCT. In contrast, PTCy is the new kid on the block, developed to facilitate haploidentical transplants using unmanipulated bone marrow cells to act as a method for selective allodepletion in vivo.
“PTCy [has proved] to be effective in preventing both acute and chronic GVHD,” Dr. Brissot said. “However, controversial outcome data remain when comparing PTCy and ATG according to the type of donors.”
Until now, she noted, there have been no prospective randomized data available for patients with donors (related or unrelated) that have 10 of 10 matched human leukocyte antigen (HLA) alleles. Hence, these were the patients studied in this latest trial, and in this population both regimens showed similar outcomes.
A bone marrow transplant specialist who was not involved in the study said that it’s a good first step.
“This is an important study to gain preliminary data to design a larger, subsequent phase 3 study,” said Zachariah DeFilipp, MD, of Mass General Cancer Center in Boston.
“The use of ATG as part of GVHD prophylaxis is common at many centers, especially in Europe, “ he explained. “The use of posttransplant cyclophosphamide is being expanded to more settings with transplant, beyond haploidentical transplant.
“Further investigations comparing the use of PTCy to ATG will help determine whether PTCy should be more broadly adopted as a standard-of-care GVHD prophylaxis approach, given currently available regimens,” he said in an interview.
Study details
The randomized phase 2b study (NCT02876679), conducted in centers in 11 cities in France, compared PTCy with ATG in patients with hematologic malignancies for whom a reduced-intensity allogeneic HSCT was indicated. This included patients aged 50 and older, and/or heavily pretreated patients who received an autologous HSCT or more than two prior lines of chemotherapy before allogeneic HSCT, as well as patients with poor performance status due to significant medical comorbidities.
Excluded from the trial were patients with creatinine clearance less than 30 mL/min; bilirubin or liver amino transferases more than three times the upper limit of normal; cardiac ejection fraction less than 40%; or pulmonary impairment with less than 50% lung carbon monoxide diffusing capacity.
Of 90 patients enrolled, 1 experienced a relapse before randomization, and the remaining 89 patients were assigned to either PTCy (experimental arm, 45 patients) or to ATG (control group, 44 patients).
Most patients had good performance status (Eastern Cooperative Oncology Group performance status 0 or 1). Diagnoses included acute myeloid and lymphoblastic leukemia, multiple myeloma, lymphomas, and myelodysplastic syndrome. The median age was 64 years, and the male to female ratio was about 2:1 in both groups.
All patients received “FB2” reduced-intensity conditioning with fludarabine30 mg/m2 per day for 4 days, and intravenous busulfan 130 mg/m2 per day for 2 days.
Patients in the experimental arm received cyclophosphamide 50 mg/kg per day on days 3 and 4 after transplant. Patients in the control group received ATG 2.5 mg/kg per day on days 3 and 2 prior to transplant.
All patients also received cyclosporine A, and those who had unrelated donors also received mycophenolate mofetil. In all, 39% of patients received cells from matched sibling donors, and 61% received cells from matched unrelated donors.
No significant differences seen
At 12 months of follow-up, there was no significant difference between the trial arms in the primary endpoint of GRFS, a composite of grade 3-4 acute GVHD, chronic GVHD requiring systemic treatment, relapse, or death. The rates of GRFS were 52.2% with PTCy vs. 45% with ATG.
Rates of disease-free survival were 68.5% with PTCy and 67.1% with ATG. The respective 12-month overall survival rates were 78.9% and 80.4%, respectively. The differences were not statistically significant.
The incidence of relapse at 1 year was 22.1% in the ATG group vs. 17.6% in the PTCy group. Respective nonrelapse mortality rates were 10.8% for the ATG group and 14% for the PTCy group. Neither difference was statistically significant.
There were also no significant between-group differences in the incidence at 12-month follow-up of either acute GVHD (34.9% PTCy arm vs. 24.3% ATG arm for grades II-IV combined, and 9.3% PTCy vs. 2.7% ATG for grades III or IV) or chronic GVHD (30.2% ATG vs. 26% PTCy).
The safety analysis showed no significant between-group differences in selected adverse events, including Epstein-Barr viral reactivation, cytomegalovirus reactivation, cardiac adverse events, or hemorrhagic cystitis.
The study was supported by Hospitals of Paris. Dr. Brissot and Dr. DeFilipp have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A newer regimen was no more effective than an older regimen when both were compared for graft versus host disease (GVHD) prophylaxis in patients who underwent reduced-intensity conditioning followed by a hematopoietic stem cell transplant (HSCT) from a 10/10 HLA-matched related or unrelated donor.
These results come from a multicenter randomized trial that compared posttransplant cyclophosphamide (PTCy) to antithymocyte globulin (ATG), which has been used for decades.
There were no significant differences between the two in either disease-free or overall survival, GVHD-free relapse-free survival (GRFS), or nonrelapse mortality, reported lead investigator Eolia Brissot, MD, of Hôpital Saint-Antoine, Sorbonne University, Paris.
Her presentation was judged ‘top abstract’ at the annual meeting of the European Society for Blood and Marrow Transplantation (EBMT), held virtually because of the pandemic.
ATG has been used for more than 30 years for GVHD prophylaxis in allogeneic HSCT. In contrast, PTCy is the new kid on the block, developed to facilitate haploidentical transplants using unmanipulated bone marrow cells to act as a method for selective allodepletion in vivo.
“PTCy [has proved] to be effective in preventing both acute and chronic GVHD,” Dr. Brissot said. “However, controversial outcome data remain when comparing PTCy and ATG according to the type of donors.”
Until now, she noted, there have been no prospective randomized data available for patients with donors (related or unrelated) that have 10 of 10 matched human leukocyte antigen (HLA) alleles. Hence, these were the patients studied in this latest trial, and in this population both regimens showed similar outcomes.
A bone marrow transplant specialist who was not involved in the study said that it’s a good first step.
“This is an important study to gain preliminary data to design a larger, subsequent phase 3 study,” said Zachariah DeFilipp, MD, of Mass General Cancer Center in Boston.
“The use of ATG as part of GVHD prophylaxis is common at many centers, especially in Europe, “ he explained. “The use of posttransplant cyclophosphamide is being expanded to more settings with transplant, beyond haploidentical transplant.
“Further investigations comparing the use of PTCy to ATG will help determine whether PTCy should be more broadly adopted as a standard-of-care GVHD prophylaxis approach, given currently available regimens,” he said in an interview.
Study details
The randomized phase 2b study (NCT02876679), conducted in centers in 11 cities in France, compared PTCy with ATG in patients with hematologic malignancies for whom a reduced-intensity allogeneic HSCT was indicated. This included patients aged 50 and older, and/or heavily pretreated patients who received an autologous HSCT or more than two prior lines of chemotherapy before allogeneic HSCT, as well as patients with poor performance status due to significant medical comorbidities.
Excluded from the trial were patients with creatinine clearance less than 30 mL/min; bilirubin or liver amino transferases more than three times the upper limit of normal; cardiac ejection fraction less than 40%; or pulmonary impairment with less than 50% lung carbon monoxide diffusing capacity.
Of 90 patients enrolled, 1 experienced a relapse before randomization, and the remaining 89 patients were assigned to either PTCy (experimental arm, 45 patients) or to ATG (control group, 44 patients).
Most patients had good performance status (Eastern Cooperative Oncology Group performance status 0 or 1). Diagnoses included acute myeloid and lymphoblastic leukemia, multiple myeloma, lymphomas, and myelodysplastic syndrome. The median age was 64 years, and the male to female ratio was about 2:1 in both groups.
All patients received “FB2” reduced-intensity conditioning with fludarabine30 mg/m2 per day for 4 days, and intravenous busulfan 130 mg/m2 per day for 2 days.
Patients in the experimental arm received cyclophosphamide 50 mg/kg per day on days 3 and 4 after transplant. Patients in the control group received ATG 2.5 mg/kg per day on days 3 and 2 prior to transplant.
All patients also received cyclosporine A, and those who had unrelated donors also received mycophenolate mofetil. In all, 39% of patients received cells from matched sibling donors, and 61% received cells from matched unrelated donors.
No significant differences seen
At 12 months of follow-up, there was no significant difference between the trial arms in the primary endpoint of GRFS, a composite of grade 3-4 acute GVHD, chronic GVHD requiring systemic treatment, relapse, or death. The rates of GRFS were 52.2% with PTCy vs. 45% with ATG.
Rates of disease-free survival were 68.5% with PTCy and 67.1% with ATG. The respective 12-month overall survival rates were 78.9% and 80.4%, respectively. The differences were not statistically significant.
The incidence of relapse at 1 year was 22.1% in the ATG group vs. 17.6% in the PTCy group. Respective nonrelapse mortality rates were 10.8% for the ATG group and 14% for the PTCy group. Neither difference was statistically significant.
There were also no significant between-group differences in the incidence at 12-month follow-up of either acute GVHD (34.9% PTCy arm vs. 24.3% ATG arm for grades II-IV combined, and 9.3% PTCy vs. 2.7% ATG for grades III or IV) or chronic GVHD (30.2% ATG vs. 26% PTCy).
The safety analysis showed no significant between-group differences in selected adverse events, including Epstein-Barr viral reactivation, cytomegalovirus reactivation, cardiac adverse events, or hemorrhagic cystitis.
The study was supported by Hospitals of Paris. Dr. Brissot and Dr. DeFilipp have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
High obesity rates in Southern states magnify COVID threats
In January, as Mississippi health officials planned for their incoming shipments of COVID-19 vaccine, they assessed the state’s most vulnerable: health care workers, of course, and elderly people in nursing homes. But among those who needed urgent protection from the virus ripping across the Magnolia State were 1 million Mississippians with obesity.
Obesity and weight-related illnesses have been deadly liabilities in the COVID era. A report released this month by the World Obesity Federation found that increased body weight is the second-greatest predictor of COVID-related hospitalization and death across the globe, trailing only old age as a risk factor.
As a fixture of life in the American South – home to 9 of the nation’s 12 heaviest states – obesity is playing a role not only in COVID outcomes, but in the calculus of the vaccination rollout. Mississippi was one of the first states to add a body mass index of 30 or more (a rough gauge of obesity tied to height and weight) to the list of qualifying medical conditions for a shot. About 40% of the state’s adults meet that definition, according to federal health survey data, and combined with the risk group already eligible for vaccination – residents 65 and older – that means fully half of Mississippi’s adults are entitled to vie for a restricted allotment of shots.
At least 29 states have green-lighted obesity for inclusion in the first phases of the vaccine rollout, according to KFF – a vast widening of eligibility that has the potential to overwhelm government efforts and heighten competition for scarce doses.
“We have a lifesaving intervention, and we don’t have enough of it,” said Jen Kates, PhD, director of global health and HIV policy for Kaiser Family Foundation. “Hard choices are being made about who should go first, and there is no right answer.”
The sheer prevalence of obesity in the nation – two in three Americans exceed what is considered a healthy weight – was a public health concern well before the pandemic. But COVID-19 dramatically fast-tracked the discussion from warnings about the long-term damage excess fat tissue can pose to heart, lung and metabolic functions to far more immediate threats.
In the United Kingdom, for example, overweight COVID patients were 67% more likely to require intensive care, and obese patients three times likelier, according to the World Obesity Federation report. A Centers for Disease Control and Prevention study released Monday found a similar trend among U.S. patients and noted that the risk of COVID-related hospitalization, ventilation and death increased with patients’ obesity level.
The counties that hug the southern Mississippi River are home to some of the most concentrated pockets of extreme obesity in the United States. Coronavirus infections began surging in Southern states early last summer, and hospitalizations rose in step.
Deaths in rural stretches of Arkansas, Louisiana, Mississippi, and Tennessee have been overshadowed by the sheer number of deaths in metropolitan areas like New York, Los Angeles, and Essex County, N.J. But as a share of the population, the coronavirus has been similarly unsparing in many Southern communities. In sparsely populated Claiborne County, Miss., on the floodplains of the Mississippi River, 30 residents – about 1 in 300 – had died as of early March. In East Feliciana Parish, La., north of Baton Rouge, with 106 deaths, about 1 in 180 had died by then.
“It’s just math. If the population is more obese and obesity clearly contributes to worse outcomes, then neighborhoods, cities, states and countries that are more obese will have a greater toll from COVID,” said Dr. James de Lemos, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas who led a study of hospitalized COVID patients published in the medical journal Circulation.
And, because in the U.S. obesity rates tend to be relatively high among African Americans and Latinos who are poor, with diminished access to health care, “it’s a triple whammy,” Dr. de Lemos said. “All these things intersect.”
Poverty and limited access to medical care are common features in the South, where residents like Michelle Antonyshyn, a former registered nurse and mother of seven in Salem, Ark., say they are afraid of the virus. Ms. Antonyshyn, 49, has obesity and debilitating pain in her knees and back, though she does not have high blood pressure or diabetes, two underlying conditions that federal health officials have determined are added risk factors for severe cases of COVID-19.
Still, she said, she “was very concerned just knowing that being obese puts you more at risk for bad outcomes such as being on a ventilator and death.” As a precaution, Ms. Antonyshyn said, she and her large brood locked down early and stopped attending church services in person, watching online instead.
“It’s not the same as having fellowship, but the risk for me was enough,” said Ms. Antonyshyn.
Governors throughout the South seem to recognize that weight can contribute to COVID-19 complications and have pushed for vaccine eligibility rules that prioritize obesity. But on the ground, local health officials are girding for having to tell newly eligible people who qualify as obese that there aren’t enough shots to go around.
In Port Gibson, Miss., Mheja Williams, MD, medical director of the Claiborne County Family Health Center, has been receiving barely enough doses to inoculate the health workers and oldest seniors in her county of 9,600. One week in early February, she received 100 doses.
Obesity and extreme obesity are endemic in Claiborne County, and health officials say the “normalization” of obesity means people often don’t register their weight as a risk factor, whether for COVID or other health issues. The risks are exacerbated by a general flouting of pandemic etiquette: Dr. Williams said that middle-aged and younger residents are not especially vigilant about physical distancing and that mask use is rare.
The rise of obesity in the United States is well documented over the past half-century, as the nation turned from a diet of fruits, vegetables and limited meats to one laden with ultra-processed foods and rich with salt, fat, sugar, and flavorings, along with copious amounts of meat, fast food, and soda. The U.S. has generally led the global obesity race, setting records as even toddlers and young children grew implausibly, dangerously overweight.
Well before COVID, obesity was a leading cause of preventable death in the United States. The National Institutes of Health declared it a disease in 1998, one that fosters heart disease, stroke, type 2 diabetes, and breast, colon, and other cancers.
Researchers say it is no coincidence that nations like the United States, the United Kingdom, and Italy, with relatively high obesity rates, have proved particularly vulnerable to the novel coronavirus.
They believe the virus may exploit underlying metabolic and physiological impairments that often exist in concert with obesity. Extra fat can lead to a cascade of metabolic disruptions, chronic systemic inflammation, and hormonal dysregulation that may thwart the body’s response to infection.
Other respiratory viruses, like influenza and SARS, which appeared in China in 2002, rely on cholesterol to spread enveloped RNA virus to neighboring cells, and researchers have proposed that a similar mechanism may play a role in the spread of the novel coronavirus.
There are also practical problems for coronavirus patients with obesity admitted to the hospital. They can be more difficult to intubate because of excess central weight pressing down on the diaphragm, making breathing with infected lungs even more difficult.
Physicians who specialize in treating patients with obesity say public health officials need to be more forthright and urgent in their messaging, telegraphing the risks of this COVID era.
“It should be explicit and direct,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General Hospital, Boston, and a Harvard Medical School instructor.
Dr. Stanford denounces the fat-shaming and bullying that people with obesity often experience. But telling patients – and the public – that obesity increases the risk of hospitalization and death is crucial, she said.
“I don’t think it’s stigmatizing,” she said. “If you tell them in that way, it’s not to scare you, it’s just giving information. Sometimes people are just unaware.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In January, as Mississippi health officials planned for their incoming shipments of COVID-19 vaccine, they assessed the state’s most vulnerable: health care workers, of course, and elderly people in nursing homes. But among those who needed urgent protection from the virus ripping across the Magnolia State were 1 million Mississippians with obesity.
Obesity and weight-related illnesses have been deadly liabilities in the COVID era. A report released this month by the World Obesity Federation found that increased body weight is the second-greatest predictor of COVID-related hospitalization and death across the globe, trailing only old age as a risk factor.
As a fixture of life in the American South – home to 9 of the nation’s 12 heaviest states – obesity is playing a role not only in COVID outcomes, but in the calculus of the vaccination rollout. Mississippi was one of the first states to add a body mass index of 30 or more (a rough gauge of obesity tied to height and weight) to the list of qualifying medical conditions for a shot. About 40% of the state’s adults meet that definition, according to federal health survey data, and combined with the risk group already eligible for vaccination – residents 65 and older – that means fully half of Mississippi’s adults are entitled to vie for a restricted allotment of shots.
At least 29 states have green-lighted obesity for inclusion in the first phases of the vaccine rollout, according to KFF – a vast widening of eligibility that has the potential to overwhelm government efforts and heighten competition for scarce doses.
“We have a lifesaving intervention, and we don’t have enough of it,” said Jen Kates, PhD, director of global health and HIV policy for Kaiser Family Foundation. “Hard choices are being made about who should go first, and there is no right answer.”
The sheer prevalence of obesity in the nation – two in three Americans exceed what is considered a healthy weight – was a public health concern well before the pandemic. But COVID-19 dramatically fast-tracked the discussion from warnings about the long-term damage excess fat tissue can pose to heart, lung and metabolic functions to far more immediate threats.
In the United Kingdom, for example, overweight COVID patients were 67% more likely to require intensive care, and obese patients three times likelier, according to the World Obesity Federation report. A Centers for Disease Control and Prevention study released Monday found a similar trend among U.S. patients and noted that the risk of COVID-related hospitalization, ventilation and death increased with patients’ obesity level.
The counties that hug the southern Mississippi River are home to some of the most concentrated pockets of extreme obesity in the United States. Coronavirus infections began surging in Southern states early last summer, and hospitalizations rose in step.
Deaths in rural stretches of Arkansas, Louisiana, Mississippi, and Tennessee have been overshadowed by the sheer number of deaths in metropolitan areas like New York, Los Angeles, and Essex County, N.J. But as a share of the population, the coronavirus has been similarly unsparing in many Southern communities. In sparsely populated Claiborne County, Miss., on the floodplains of the Mississippi River, 30 residents – about 1 in 300 – had died as of early March. In East Feliciana Parish, La., north of Baton Rouge, with 106 deaths, about 1 in 180 had died by then.
“It’s just math. If the population is more obese and obesity clearly contributes to worse outcomes, then neighborhoods, cities, states and countries that are more obese will have a greater toll from COVID,” said Dr. James de Lemos, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas who led a study of hospitalized COVID patients published in the medical journal Circulation.
And, because in the U.S. obesity rates tend to be relatively high among African Americans and Latinos who are poor, with diminished access to health care, “it’s a triple whammy,” Dr. de Lemos said. “All these things intersect.”
Poverty and limited access to medical care are common features in the South, where residents like Michelle Antonyshyn, a former registered nurse and mother of seven in Salem, Ark., say they are afraid of the virus. Ms. Antonyshyn, 49, has obesity and debilitating pain in her knees and back, though she does not have high blood pressure or diabetes, two underlying conditions that federal health officials have determined are added risk factors for severe cases of COVID-19.
Still, she said, she “was very concerned just knowing that being obese puts you more at risk for bad outcomes such as being on a ventilator and death.” As a precaution, Ms. Antonyshyn said, she and her large brood locked down early and stopped attending church services in person, watching online instead.
“It’s not the same as having fellowship, but the risk for me was enough,” said Ms. Antonyshyn.
Governors throughout the South seem to recognize that weight can contribute to COVID-19 complications and have pushed for vaccine eligibility rules that prioritize obesity. But on the ground, local health officials are girding for having to tell newly eligible people who qualify as obese that there aren’t enough shots to go around.
In Port Gibson, Miss., Mheja Williams, MD, medical director of the Claiborne County Family Health Center, has been receiving barely enough doses to inoculate the health workers and oldest seniors in her county of 9,600. One week in early February, she received 100 doses.
Obesity and extreme obesity are endemic in Claiborne County, and health officials say the “normalization” of obesity means people often don’t register their weight as a risk factor, whether for COVID or other health issues. The risks are exacerbated by a general flouting of pandemic etiquette: Dr. Williams said that middle-aged and younger residents are not especially vigilant about physical distancing and that mask use is rare.
The rise of obesity in the United States is well documented over the past half-century, as the nation turned from a diet of fruits, vegetables and limited meats to one laden with ultra-processed foods and rich with salt, fat, sugar, and flavorings, along with copious amounts of meat, fast food, and soda. The U.S. has generally led the global obesity race, setting records as even toddlers and young children grew implausibly, dangerously overweight.
Well before COVID, obesity was a leading cause of preventable death in the United States. The National Institutes of Health declared it a disease in 1998, one that fosters heart disease, stroke, type 2 diabetes, and breast, colon, and other cancers.
Researchers say it is no coincidence that nations like the United States, the United Kingdom, and Italy, with relatively high obesity rates, have proved particularly vulnerable to the novel coronavirus.
They believe the virus may exploit underlying metabolic and physiological impairments that often exist in concert with obesity. Extra fat can lead to a cascade of metabolic disruptions, chronic systemic inflammation, and hormonal dysregulation that may thwart the body’s response to infection.
Other respiratory viruses, like influenza and SARS, which appeared in China in 2002, rely on cholesterol to spread enveloped RNA virus to neighboring cells, and researchers have proposed that a similar mechanism may play a role in the spread of the novel coronavirus.
There are also practical problems for coronavirus patients with obesity admitted to the hospital. They can be more difficult to intubate because of excess central weight pressing down on the diaphragm, making breathing with infected lungs even more difficult.
Physicians who specialize in treating patients with obesity say public health officials need to be more forthright and urgent in their messaging, telegraphing the risks of this COVID era.
“It should be explicit and direct,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General Hospital, Boston, and a Harvard Medical School instructor.
Dr. Stanford denounces the fat-shaming and bullying that people with obesity often experience. But telling patients – and the public – that obesity increases the risk of hospitalization and death is crucial, she said.
“I don’t think it’s stigmatizing,” she said. “If you tell them in that way, it’s not to scare you, it’s just giving information. Sometimes people are just unaware.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In January, as Mississippi health officials planned for their incoming shipments of COVID-19 vaccine, they assessed the state’s most vulnerable: health care workers, of course, and elderly people in nursing homes. But among those who needed urgent protection from the virus ripping across the Magnolia State were 1 million Mississippians with obesity.
Obesity and weight-related illnesses have been deadly liabilities in the COVID era. A report released this month by the World Obesity Federation found that increased body weight is the second-greatest predictor of COVID-related hospitalization and death across the globe, trailing only old age as a risk factor.
As a fixture of life in the American South – home to 9 of the nation’s 12 heaviest states – obesity is playing a role not only in COVID outcomes, but in the calculus of the vaccination rollout. Mississippi was one of the first states to add a body mass index of 30 or more (a rough gauge of obesity tied to height and weight) to the list of qualifying medical conditions for a shot. About 40% of the state’s adults meet that definition, according to federal health survey data, and combined with the risk group already eligible for vaccination – residents 65 and older – that means fully half of Mississippi’s adults are entitled to vie for a restricted allotment of shots.
At least 29 states have green-lighted obesity for inclusion in the first phases of the vaccine rollout, according to KFF – a vast widening of eligibility that has the potential to overwhelm government efforts and heighten competition for scarce doses.
“We have a lifesaving intervention, and we don’t have enough of it,” said Jen Kates, PhD, director of global health and HIV policy for Kaiser Family Foundation. “Hard choices are being made about who should go first, and there is no right answer.”
The sheer prevalence of obesity in the nation – two in three Americans exceed what is considered a healthy weight – was a public health concern well before the pandemic. But COVID-19 dramatically fast-tracked the discussion from warnings about the long-term damage excess fat tissue can pose to heart, lung and metabolic functions to far more immediate threats.
In the United Kingdom, for example, overweight COVID patients were 67% more likely to require intensive care, and obese patients three times likelier, according to the World Obesity Federation report. A Centers for Disease Control and Prevention study released Monday found a similar trend among U.S. patients and noted that the risk of COVID-related hospitalization, ventilation and death increased with patients’ obesity level.
The counties that hug the southern Mississippi River are home to some of the most concentrated pockets of extreme obesity in the United States. Coronavirus infections began surging in Southern states early last summer, and hospitalizations rose in step.
Deaths in rural stretches of Arkansas, Louisiana, Mississippi, and Tennessee have been overshadowed by the sheer number of deaths in metropolitan areas like New York, Los Angeles, and Essex County, N.J. But as a share of the population, the coronavirus has been similarly unsparing in many Southern communities. In sparsely populated Claiborne County, Miss., on the floodplains of the Mississippi River, 30 residents – about 1 in 300 – had died as of early March. In East Feliciana Parish, La., north of Baton Rouge, with 106 deaths, about 1 in 180 had died by then.
“It’s just math. If the population is more obese and obesity clearly contributes to worse outcomes, then neighborhoods, cities, states and countries that are more obese will have a greater toll from COVID,” said Dr. James de Lemos, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas who led a study of hospitalized COVID patients published in the medical journal Circulation.
And, because in the U.S. obesity rates tend to be relatively high among African Americans and Latinos who are poor, with diminished access to health care, “it’s a triple whammy,” Dr. de Lemos said. “All these things intersect.”
Poverty and limited access to medical care are common features in the South, where residents like Michelle Antonyshyn, a former registered nurse and mother of seven in Salem, Ark., say they are afraid of the virus. Ms. Antonyshyn, 49, has obesity and debilitating pain in her knees and back, though she does not have high blood pressure or diabetes, two underlying conditions that federal health officials have determined are added risk factors for severe cases of COVID-19.
Still, she said, she “was very concerned just knowing that being obese puts you more at risk for bad outcomes such as being on a ventilator and death.” As a precaution, Ms. Antonyshyn said, she and her large brood locked down early and stopped attending church services in person, watching online instead.
“It’s not the same as having fellowship, but the risk for me was enough,” said Ms. Antonyshyn.
Governors throughout the South seem to recognize that weight can contribute to COVID-19 complications and have pushed for vaccine eligibility rules that prioritize obesity. But on the ground, local health officials are girding for having to tell newly eligible people who qualify as obese that there aren’t enough shots to go around.
In Port Gibson, Miss., Mheja Williams, MD, medical director of the Claiborne County Family Health Center, has been receiving barely enough doses to inoculate the health workers and oldest seniors in her county of 9,600. One week in early February, she received 100 doses.
Obesity and extreme obesity are endemic in Claiborne County, and health officials say the “normalization” of obesity means people often don’t register their weight as a risk factor, whether for COVID or other health issues. The risks are exacerbated by a general flouting of pandemic etiquette: Dr. Williams said that middle-aged and younger residents are not especially vigilant about physical distancing and that mask use is rare.
The rise of obesity in the United States is well documented over the past half-century, as the nation turned from a diet of fruits, vegetables and limited meats to one laden with ultra-processed foods and rich with salt, fat, sugar, and flavorings, along with copious amounts of meat, fast food, and soda. The U.S. has generally led the global obesity race, setting records as even toddlers and young children grew implausibly, dangerously overweight.
Well before COVID, obesity was a leading cause of preventable death in the United States. The National Institutes of Health declared it a disease in 1998, one that fosters heart disease, stroke, type 2 diabetes, and breast, colon, and other cancers.
Researchers say it is no coincidence that nations like the United States, the United Kingdom, and Italy, with relatively high obesity rates, have proved particularly vulnerable to the novel coronavirus.
They believe the virus may exploit underlying metabolic and physiological impairments that often exist in concert with obesity. Extra fat can lead to a cascade of metabolic disruptions, chronic systemic inflammation, and hormonal dysregulation that may thwart the body’s response to infection.
Other respiratory viruses, like influenza and SARS, which appeared in China in 2002, rely on cholesterol to spread enveloped RNA virus to neighboring cells, and researchers have proposed that a similar mechanism may play a role in the spread of the novel coronavirus.
There are also practical problems for coronavirus patients with obesity admitted to the hospital. They can be more difficult to intubate because of excess central weight pressing down on the diaphragm, making breathing with infected lungs even more difficult.
Physicians who specialize in treating patients with obesity say public health officials need to be more forthright and urgent in their messaging, telegraphing the risks of this COVID era.
“It should be explicit and direct,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General Hospital, Boston, and a Harvard Medical School instructor.
Dr. Stanford denounces the fat-shaming and bullying that people with obesity often experience. But telling patients – and the public – that obesity increases the risk of hospitalization and death is crucial, she said.
“I don’t think it’s stigmatizing,” she said. “If you tell them in that way, it’s not to scare you, it’s just giving information. Sometimes people are just unaware.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
FDA scrutinizes cancer therapies granted accelerated approval
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
Updated recommendations released on COVID-19 and pediatric ALL
The main threat to the vast majority of children with acute lymphoblastic leukemia still remains the ALL itself, according to updated recommendations released by the Leukemia Committee of the French Society for the Fight Against Cancers and Leukemias in Children and Adolescents (SFCE).
“The situation of the current COVID-19 pandemic is continuously evolving. We thus have taken the more recent knowledge into account to update the previous recommendations from the Leukemia Committee,” Jérémie Rouger-Gaudichon, MD, of Pediatric Hemato-Immuno-Oncology Unit, Centre Hospitalier Universitaire, Caen (France), and colleagues wrote on behalf of the SFCE.
The updated recommendations are based on data collected in a real-time prospective survey among the 30 SFCE centers since April 2020. As of December 2020, 127 cases of COVID-19 were reported, most of them being enrolled in the PEDONCOVID study (NCT04433871) according to the report. Of these, eight patients required hospitalization in intensive care unit and one patient with relapsed acute lymphoblastic leukemia (ALL) died from ARDS with multiorgan failure. This confirms earlier reports that SARS-CoV-2 infection can be severe in some children with cancer and/or having hematopoietic stem cell transplant (HSCT), according to the report, which was published online in Bulletin du Cancer.
Recommendations
General recommendations were provided in the report, including the following:
- Test for SARS-CoV-2 (preferably by PCR or at least by immunological tests, on nasopharyngeal swab) before starting intensive induction chemotherapy or other intensive phase of treatment, for ALL patients, with or without symptoms.
- Delay systemic treatment if possible (e.g., absence of major hyperleukocytosis) in positive patients. During later phases, if patients test positive, tests should be repeated over time until negativity, especially before the beginning of an intensive course.
- Isolate any COVID-19–negative child or adolescent to allow treatment to continue (facial mask, social distancing, barrier measures, no contact with individuals suspected of COVID-19 or COVID-19–positive), in particular for patients to be allografted.
- Limit visitation to parents and potentially siblings in patients slated for HSCT and follow all necessary sanitary procedures for those visits.
The report provides a lengthy discussion of more detailed recommendations, including the following for first-line treatment of ALL:
- For patients with high-risk ALL, an individualized decision regarding transplantation and its timing should weigh the risks of transplantation in an epidemic context of COVID-19 against the risk linked to ALL.
- Minimizing hospital visits by the use of home blood tests and partial use of telemedicine may be considered.
- A physical examination should be performed regularly to avoid any delay in the diagnosis of treatment complications or relapse and preventative measures for SARS-CoV-2 should be applied in the home.
Patients with relapsed ALL may be at more risk from the effects of COVID-19 disease, according to the others, so for ALL patients receiving second-line or more treatment the recommendations include the following:
- Testing must be performed before starting a chemotherapy block, and postponing chemotherapy in case of positive test should be discussed in accordance with each specific situation and benefits/risks ratio regarding the leukemia.
- First-relapse patients should follow the INTREALL treatment protocol as much as possible and those who reach appropriate complete remission should be considered promptly for allogeneic transplantation, despite the pandemic.
- Second relapse and refractory relapses require testing and negative results for inclusion in phase I-II trials being conducted by most if not all academic or industrial promoters.
- The indication for treatment with CAR-T cells must be weighed with the center that would perform the procedure to determine the feasibility of performing all necessary procedures including apheresis and manufacturing.
In the case of a SARS-CoV-2 infection diagnosis during the treatment of ALL, discussions should occur with regard to stopping and/or postponing all chemotherapies, according to the severity of the ALL, the stage of treatment and the severity of clinical and/or radiological signs. In addition, any specific anti-COVID-19 treatment must be discussed with the infectious diseases team, according to the report.
“Fortunately, SARS-CoV-2 infection appears nevertheless to be mild in most children with cancer/ALL. Thus, the main threat to the vast majority of children with ALL still remains the ALL itself. Long-term data including well-matched case-control studies will tell if treatment delays/modifications due to COVID-19 have impacted the outcome if children with ALL,” the authors stated. However, “despite extremely rapid advances obtained in less than one year, our knowledge of SARS-CoV-2 and its complications is still incomplete,” they concluded, adding that the recommendations will likely need to be updated within another few months.
The authors reported that they had no conflicts of interest.
The main threat to the vast majority of children with acute lymphoblastic leukemia still remains the ALL itself, according to updated recommendations released by the Leukemia Committee of the French Society for the Fight Against Cancers and Leukemias in Children and Adolescents (SFCE).
“The situation of the current COVID-19 pandemic is continuously evolving. We thus have taken the more recent knowledge into account to update the previous recommendations from the Leukemia Committee,” Jérémie Rouger-Gaudichon, MD, of Pediatric Hemato-Immuno-Oncology Unit, Centre Hospitalier Universitaire, Caen (France), and colleagues wrote on behalf of the SFCE.
The updated recommendations are based on data collected in a real-time prospective survey among the 30 SFCE centers since April 2020. As of December 2020, 127 cases of COVID-19 were reported, most of them being enrolled in the PEDONCOVID study (NCT04433871) according to the report. Of these, eight patients required hospitalization in intensive care unit and one patient with relapsed acute lymphoblastic leukemia (ALL) died from ARDS with multiorgan failure. This confirms earlier reports that SARS-CoV-2 infection can be severe in some children with cancer and/or having hematopoietic stem cell transplant (HSCT), according to the report, which was published online in Bulletin du Cancer.
Recommendations
General recommendations were provided in the report, including the following:
- Test for SARS-CoV-2 (preferably by PCR or at least by immunological tests, on nasopharyngeal swab) before starting intensive induction chemotherapy or other intensive phase of treatment, for ALL patients, with or without symptoms.
- Delay systemic treatment if possible (e.g., absence of major hyperleukocytosis) in positive patients. During later phases, if patients test positive, tests should be repeated over time until negativity, especially before the beginning of an intensive course.
- Isolate any COVID-19–negative child or adolescent to allow treatment to continue (facial mask, social distancing, barrier measures, no contact with individuals suspected of COVID-19 or COVID-19–positive), in particular for patients to be allografted.
- Limit visitation to parents and potentially siblings in patients slated for HSCT and follow all necessary sanitary procedures for those visits.
The report provides a lengthy discussion of more detailed recommendations, including the following for first-line treatment of ALL:
- For patients with high-risk ALL, an individualized decision regarding transplantation and its timing should weigh the risks of transplantation in an epidemic context of COVID-19 against the risk linked to ALL.
- Minimizing hospital visits by the use of home blood tests and partial use of telemedicine may be considered.
- A physical examination should be performed regularly to avoid any delay in the diagnosis of treatment complications or relapse and preventative measures for SARS-CoV-2 should be applied in the home.
Patients with relapsed ALL may be at more risk from the effects of COVID-19 disease, according to the others, so for ALL patients receiving second-line or more treatment the recommendations include the following:
- Testing must be performed before starting a chemotherapy block, and postponing chemotherapy in case of positive test should be discussed in accordance with each specific situation and benefits/risks ratio regarding the leukemia.
- First-relapse patients should follow the INTREALL treatment protocol as much as possible and those who reach appropriate complete remission should be considered promptly for allogeneic transplantation, despite the pandemic.
- Second relapse and refractory relapses require testing and negative results for inclusion in phase I-II trials being conducted by most if not all academic or industrial promoters.
- The indication for treatment with CAR-T cells must be weighed with the center that would perform the procedure to determine the feasibility of performing all necessary procedures including apheresis and manufacturing.
In the case of a SARS-CoV-2 infection diagnosis during the treatment of ALL, discussions should occur with regard to stopping and/or postponing all chemotherapies, according to the severity of the ALL, the stage of treatment and the severity of clinical and/or radiological signs. In addition, any specific anti-COVID-19 treatment must be discussed with the infectious diseases team, according to the report.
“Fortunately, SARS-CoV-2 infection appears nevertheless to be mild in most children with cancer/ALL. Thus, the main threat to the vast majority of children with ALL still remains the ALL itself. Long-term data including well-matched case-control studies will tell if treatment delays/modifications due to COVID-19 have impacted the outcome if children with ALL,” the authors stated. However, “despite extremely rapid advances obtained in less than one year, our knowledge of SARS-CoV-2 and its complications is still incomplete,” they concluded, adding that the recommendations will likely need to be updated within another few months.
The authors reported that they had no conflicts of interest.
The main threat to the vast majority of children with acute lymphoblastic leukemia still remains the ALL itself, according to updated recommendations released by the Leukemia Committee of the French Society for the Fight Against Cancers and Leukemias in Children and Adolescents (SFCE).
“The situation of the current COVID-19 pandemic is continuously evolving. We thus have taken the more recent knowledge into account to update the previous recommendations from the Leukemia Committee,” Jérémie Rouger-Gaudichon, MD, of Pediatric Hemato-Immuno-Oncology Unit, Centre Hospitalier Universitaire, Caen (France), and colleagues wrote on behalf of the SFCE.
The updated recommendations are based on data collected in a real-time prospective survey among the 30 SFCE centers since April 2020. As of December 2020, 127 cases of COVID-19 were reported, most of them being enrolled in the PEDONCOVID study (NCT04433871) according to the report. Of these, eight patients required hospitalization in intensive care unit and one patient with relapsed acute lymphoblastic leukemia (ALL) died from ARDS with multiorgan failure. This confirms earlier reports that SARS-CoV-2 infection can be severe in some children with cancer and/or having hematopoietic stem cell transplant (HSCT), according to the report, which was published online in Bulletin du Cancer.
Recommendations
General recommendations were provided in the report, including the following:
- Test for SARS-CoV-2 (preferably by PCR or at least by immunological tests, on nasopharyngeal swab) before starting intensive induction chemotherapy or other intensive phase of treatment, for ALL patients, with or without symptoms.
- Delay systemic treatment if possible (e.g., absence of major hyperleukocytosis) in positive patients. During later phases, if patients test positive, tests should be repeated over time until negativity, especially before the beginning of an intensive course.
- Isolate any COVID-19–negative child or adolescent to allow treatment to continue (facial mask, social distancing, barrier measures, no contact with individuals suspected of COVID-19 or COVID-19–positive), in particular for patients to be allografted.
- Limit visitation to parents and potentially siblings in patients slated for HSCT and follow all necessary sanitary procedures for those visits.
The report provides a lengthy discussion of more detailed recommendations, including the following for first-line treatment of ALL:
- For patients with high-risk ALL, an individualized decision regarding transplantation and its timing should weigh the risks of transplantation in an epidemic context of COVID-19 against the risk linked to ALL.
- Minimizing hospital visits by the use of home blood tests and partial use of telemedicine may be considered.
- A physical examination should be performed regularly to avoid any delay in the diagnosis of treatment complications or relapse and preventative measures for SARS-CoV-2 should be applied in the home.
Patients with relapsed ALL may be at more risk from the effects of COVID-19 disease, according to the others, so for ALL patients receiving second-line or more treatment the recommendations include the following:
- Testing must be performed before starting a chemotherapy block, and postponing chemotherapy in case of positive test should be discussed in accordance with each specific situation and benefits/risks ratio regarding the leukemia.
- First-relapse patients should follow the INTREALL treatment protocol as much as possible and those who reach appropriate complete remission should be considered promptly for allogeneic transplantation, despite the pandemic.
- Second relapse and refractory relapses require testing and negative results for inclusion in phase I-II trials being conducted by most if not all academic or industrial promoters.
- The indication for treatment with CAR-T cells must be weighed with the center that would perform the procedure to determine the feasibility of performing all necessary procedures including apheresis and manufacturing.
In the case of a SARS-CoV-2 infection diagnosis during the treatment of ALL, discussions should occur with regard to stopping and/or postponing all chemotherapies, according to the severity of the ALL, the stage of treatment and the severity of clinical and/or radiological signs. In addition, any specific anti-COVID-19 treatment must be discussed with the infectious diseases team, according to the report.
“Fortunately, SARS-CoV-2 infection appears nevertheless to be mild in most children with cancer/ALL. Thus, the main threat to the vast majority of children with ALL still remains the ALL itself. Long-term data including well-matched case-control studies will tell if treatment delays/modifications due to COVID-19 have impacted the outcome if children with ALL,” the authors stated. However, “despite extremely rapid advances obtained in less than one year, our knowledge of SARS-CoV-2 and its complications is still incomplete,” they concluded, adding that the recommendations will likely need to be updated within another few months.
The authors reported that they had no conflicts of interest.
FROM BULLETIN DU CANCER
High-dose chemo no better than standard dose for B-cell lymphoma
After 10 years of follow-up, event-free survival and overall survival were similar between conventional chemotherapy treated patients with aggressive B-cell lymphoma and those receiving high-dose chemotherapy followed by autologous hematopoietic stem-cell transplantation (HSCT), according to a report published online in the Lancet Hematology.
The open-label, randomized, phase 3 trial (NCT00129090) was conducted across 61 centers in Germany on patients aged 18-60 years who had newly diagnosed, high-risk, aggressive B-cell lymphoma, according to Fabian Frontzek, MD, of the University Hospital Münster (Germany) and colleagues.
Between March 2003 and April 2009, patients were randomly assigned to eight cycles of conventional chemotherapy (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone) plus rituximab (R-CHOEP-14) or four cycles of high-dose chemotherapy plus rituximab followed by autologous HSCT (R-MegaCHOEP). The intention-to-treat population comprised 130 patients in the R-CHOEP-14 group and 132 patients in the R-MegaCHOEP group. The median follow-up was 9.3 years.
Similar outcomes
The 10-year event-free survival was 51% in the R-MegaCHOEP group and 57% in the R-CHOEP-14 group, a nonsignificant difference (P = .23). Similarly, the 10-year progression-free survival was 59% in the
R-MegaCHOEP group and 60% (P = .64). The 10-year overall survival was 66% in the R-MegaCHOEP group and 72% in the R-CHOEP-14 group (P = .26). Among the 190 patients who had complete remission or unconfirmed complete remission, relapse occurred in 30 (16%); 17 (17%) of 100 patients in the R-CHOEP-14 group and 13 (14%) of 90 patients in the R-MegaCHOEP group.
In terms of secondary malignancies, 22 were reported in the intention-to-treat population; comprising 12 (9%) of 127 patients in the R-CHOEP-14 group and 10 (8%) of 126 patients in the R-MegaCHOEP group.
Patients who relapsed with aggressive histology and with CNS involvement in particular had worse outcomes and “represent a group with an unmet medical need, for which new molecular and cellular therapies should be studied,” the authors stated.
“This study shows that, in the rituximab era, high-dose therapy and autologous HSCT in first-line treatment does not improve long-term survival of younger high-risk patients with aggressive B-cell lymphoma. The R-CHOEP-14 regimen led to favorable outcomes, supporting its continued use in such patients,” the researchers concluded.
In an accompanying commentary, Gita Thanarajasingam, MD, of the Mayo Clinic, Rochester, Minn., and colleagues added that the issue of long-term outcomes is critical to evaluating these new regimens.
They applauded the inclusion of secondary malignancies in the long-term follow-up, but regretted the lack of the, admittedly resource-intensive, information on long-term nonneoplastic adverse events. They added that “the burden of late adverse events such as cardiotoxicity, cumulative neuropathy, delayed infections, or lasting cognitive effects, among others that might drive substantial morbidity, does matter to lymphoma survivors.”
They also commented on the importance of considering effects on fertility in these patients, noting that R-MegaCHOEP patients would be unable to conceive naturally, but that the effect of R-CHOEP-14 was less clear.
“We encourage ongoing emphasis on this type of longitudinal follow-up of secondary malignancies and other nonneoplastic late toxicities in phase 3 studies as well as in the real world in hematological malignancies, so that after prioritizing cure in the front-line setting, we do not neglect the life we have helped survivors achieve for years and decades to come,” they concluded.
The study was sponsored by the German High-Grade Non-Hodgkin’s Lymphoma Study Group. The authors reported grants, personal fees, and non-financial support from multiple pharmaceutical and biotechnology companies. Dr. Thanarajasingam and her colleagues reported that they had no competing interests.
After 10 years of follow-up, event-free survival and overall survival were similar between conventional chemotherapy treated patients with aggressive B-cell lymphoma and those receiving high-dose chemotherapy followed by autologous hematopoietic stem-cell transplantation (HSCT), according to a report published online in the Lancet Hematology.
The open-label, randomized, phase 3 trial (NCT00129090) was conducted across 61 centers in Germany on patients aged 18-60 years who had newly diagnosed, high-risk, aggressive B-cell lymphoma, according to Fabian Frontzek, MD, of the University Hospital Münster (Germany) and colleagues.
Between March 2003 and April 2009, patients were randomly assigned to eight cycles of conventional chemotherapy (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone) plus rituximab (R-CHOEP-14) or four cycles of high-dose chemotherapy plus rituximab followed by autologous HSCT (R-MegaCHOEP). The intention-to-treat population comprised 130 patients in the R-CHOEP-14 group and 132 patients in the R-MegaCHOEP group. The median follow-up was 9.3 years.
Similar outcomes
The 10-year event-free survival was 51% in the R-MegaCHOEP group and 57% in the R-CHOEP-14 group, a nonsignificant difference (P = .23). Similarly, the 10-year progression-free survival was 59% in the
R-MegaCHOEP group and 60% (P = .64). The 10-year overall survival was 66% in the R-MegaCHOEP group and 72% in the R-CHOEP-14 group (P = .26). Among the 190 patients who had complete remission or unconfirmed complete remission, relapse occurred in 30 (16%); 17 (17%) of 100 patients in the R-CHOEP-14 group and 13 (14%) of 90 patients in the R-MegaCHOEP group.
In terms of secondary malignancies, 22 were reported in the intention-to-treat population; comprising 12 (9%) of 127 patients in the R-CHOEP-14 group and 10 (8%) of 126 patients in the R-MegaCHOEP group.
Patients who relapsed with aggressive histology and with CNS involvement in particular had worse outcomes and “represent a group with an unmet medical need, for which new molecular and cellular therapies should be studied,” the authors stated.
“This study shows that, in the rituximab era, high-dose therapy and autologous HSCT in first-line treatment does not improve long-term survival of younger high-risk patients with aggressive B-cell lymphoma. The R-CHOEP-14 regimen led to favorable outcomes, supporting its continued use in such patients,” the researchers concluded.
In an accompanying commentary, Gita Thanarajasingam, MD, of the Mayo Clinic, Rochester, Minn., and colleagues added that the issue of long-term outcomes is critical to evaluating these new regimens.
They applauded the inclusion of secondary malignancies in the long-term follow-up, but regretted the lack of the, admittedly resource-intensive, information on long-term nonneoplastic adverse events. They added that “the burden of late adverse events such as cardiotoxicity, cumulative neuropathy, delayed infections, or lasting cognitive effects, among others that might drive substantial morbidity, does matter to lymphoma survivors.”
They also commented on the importance of considering effects on fertility in these patients, noting that R-MegaCHOEP patients would be unable to conceive naturally, but that the effect of R-CHOEP-14 was less clear.
“We encourage ongoing emphasis on this type of longitudinal follow-up of secondary malignancies and other nonneoplastic late toxicities in phase 3 studies as well as in the real world in hematological malignancies, so that after prioritizing cure in the front-line setting, we do not neglect the life we have helped survivors achieve for years and decades to come,” they concluded.
The study was sponsored by the German High-Grade Non-Hodgkin’s Lymphoma Study Group. The authors reported grants, personal fees, and non-financial support from multiple pharmaceutical and biotechnology companies. Dr. Thanarajasingam and her colleagues reported that they had no competing interests.
After 10 years of follow-up, event-free survival and overall survival were similar between conventional chemotherapy treated patients with aggressive B-cell lymphoma and those receiving high-dose chemotherapy followed by autologous hematopoietic stem-cell transplantation (HSCT), according to a report published online in the Lancet Hematology.
The open-label, randomized, phase 3 trial (NCT00129090) was conducted across 61 centers in Germany on patients aged 18-60 years who had newly diagnosed, high-risk, aggressive B-cell lymphoma, according to Fabian Frontzek, MD, of the University Hospital Münster (Germany) and colleagues.
Between March 2003 and April 2009, patients were randomly assigned to eight cycles of conventional chemotherapy (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone) plus rituximab (R-CHOEP-14) or four cycles of high-dose chemotherapy plus rituximab followed by autologous HSCT (R-MegaCHOEP). The intention-to-treat population comprised 130 patients in the R-CHOEP-14 group and 132 patients in the R-MegaCHOEP group. The median follow-up was 9.3 years.
Similar outcomes
The 10-year event-free survival was 51% in the R-MegaCHOEP group and 57% in the R-CHOEP-14 group, a nonsignificant difference (P = .23). Similarly, the 10-year progression-free survival was 59% in the
R-MegaCHOEP group and 60% (P = .64). The 10-year overall survival was 66% in the R-MegaCHOEP group and 72% in the R-CHOEP-14 group (P = .26). Among the 190 patients who had complete remission or unconfirmed complete remission, relapse occurred in 30 (16%); 17 (17%) of 100 patients in the R-CHOEP-14 group and 13 (14%) of 90 patients in the R-MegaCHOEP group.
In terms of secondary malignancies, 22 were reported in the intention-to-treat population; comprising 12 (9%) of 127 patients in the R-CHOEP-14 group and 10 (8%) of 126 patients in the R-MegaCHOEP group.
Patients who relapsed with aggressive histology and with CNS involvement in particular had worse outcomes and “represent a group with an unmet medical need, for which new molecular and cellular therapies should be studied,” the authors stated.
“This study shows that, in the rituximab era, high-dose therapy and autologous HSCT in first-line treatment does not improve long-term survival of younger high-risk patients with aggressive B-cell lymphoma. The R-CHOEP-14 regimen led to favorable outcomes, supporting its continued use in such patients,” the researchers concluded.
In an accompanying commentary, Gita Thanarajasingam, MD, of the Mayo Clinic, Rochester, Minn., and colleagues added that the issue of long-term outcomes is critical to evaluating these new regimens.
They applauded the inclusion of secondary malignancies in the long-term follow-up, but regretted the lack of the, admittedly resource-intensive, information on long-term nonneoplastic adverse events. They added that “the burden of late adverse events such as cardiotoxicity, cumulative neuropathy, delayed infections, or lasting cognitive effects, among others that might drive substantial morbidity, does matter to lymphoma survivors.”
They also commented on the importance of considering effects on fertility in these patients, noting that R-MegaCHOEP patients would be unable to conceive naturally, but that the effect of R-CHOEP-14 was less clear.
“We encourage ongoing emphasis on this type of longitudinal follow-up of secondary malignancies and other nonneoplastic late toxicities in phase 3 studies as well as in the real world in hematological malignancies, so that after prioritizing cure in the front-line setting, we do not neglect the life we have helped survivors achieve for years and decades to come,” they concluded.
The study was sponsored by the German High-Grade Non-Hodgkin’s Lymphoma Study Group. The authors reported grants, personal fees, and non-financial support from multiple pharmaceutical and biotechnology companies. Dr. Thanarajasingam and her colleagues reported that they had no competing interests.
FROM THE LANCET HEMATOLOGY
Blood cancer patients, survivors hesitate over COVID-19 vaccine
Nearly one in three patients with blood cancer, and survivors, say they are unlikely to get a COVID-19 vaccine or unsure about getting it if one were available. The findings come from a nationwide survey by The Leukemia & Lymphoma Society, which collected 6,517 responses.
“These findings are worrisome, to say the least,” Gwen Nichols, MD, chief medical officer of the society, said in a statement.
“We know cancer patients – and blood cancer patients in particular – are susceptible to the worst effects of the virus [and] all of us in the medical community need to help cancer patients understand the importance of getting vaccinated,” she added.
The survey – the largest ever done in which cancer patients and survivors were asked about their attitudes toward COVID-19 vaccines – was published online March 8 by The Leukemia & Lymphoma Society.
Survey sample
The survey asked patients with blood cancer, and survivors, about their attitudes regarding COVID-19 and COVID-19 vaccines.
“The main outcome [was] vaccine attitudes,” noted the authors, headed by Rena Conti, PhD, dean’s research scholar, Boston University.
Respondents were asked: “How likely are you to choose to get the vaccine?” Participants could indicate they were very unlikely, unlikely, neither likely nor unlikely, likely, or very likely to get vaccinated.
“We found that 17% of respondents indicate[d] that they [were] unlikely or very unlikely to take a vaccine,” Dr. Conti and colleagues observed.
Among the 17% – deemed to be “vaccine hesitant” – slightly over half (54%) stated they had concerns about the side effects associated with COVID-19 vaccination and believed neither of the two newly approved vaccines had been or would ever be tested properly.
The survey authors noted that there is no reason to believe COVID-19 vaccines are any less safe in patients with blood cancers, but concerns have been expressed that patients with some forms of blood cancer or those undergoing certain treatments may not achieve the same immune response to the vaccine as would noncancer controls.
Importantly, the survey was conducted Dec. 1-21, 2020, and responses differed depending on whether respondents answered the survey before or after the Pfizer-BioNTech and Moderna vaccines had been given emergency use authorization by the Food and Drug Administration starting Dec. 10, 2020.
There was a slight increase in positive responses after the vaccines were granted regulatory approval. (One-third of those who responded to the survey after the approval were 3.7% more likely to indicate they would get vaccinated). “This suggests that hesitancy may be influenced by emerging information dissemination, government action, and vaccine availability, transforming the hypothetical opportunity of vaccination to a real one,” the survey authors speculated.
Survey respondents who were vaccine hesitant were also over 14% more likely to indicate that they didn’t think they would require hospitalization should they contract COVID-19. But clinical data have suggested that approximately half of patients with a hematological malignancy who required hospitalization for COVID-19 die from the infection, the authors noted.
“Vaccine hesitant respondents [were] also significantly less likely to engage in protective health behaviors,” the survey authors pointed out. For example, they were almost 4% less likely to have worn a face mask and 1.6% less likely to have taken other protective measures to guard against COVID-19 infection.
Need for clear messaging
To counter vaccine hesitancy, the authors suggest there is a need for clear, consistent messaging targeting patients with cancer that emphasize the risks of COVID-19 and underscore vaccine benefits.
Dr. Conti pointed out that patients with blood cancer are, in fact, being given preferential access to vaccines in many communities, although this clearly doesn’t mean patients are willing to get vaccinated, as she also noted.
“We need both adequate supply and strong demand to keep this vulnerable population safe,” Dr. Conti emphasized.
The Leukemia & Lymphoma Society plans to repeat the survey in the near future to assess patients’ and survivors’ access to vaccines as well as their willingness to get vaccinated.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nearly one in three patients with blood cancer, and survivors, say they are unlikely to get a COVID-19 vaccine or unsure about getting it if one were available. The findings come from a nationwide survey by The Leukemia & Lymphoma Society, which collected 6,517 responses.
“These findings are worrisome, to say the least,” Gwen Nichols, MD, chief medical officer of the society, said in a statement.
“We know cancer patients – and blood cancer patients in particular – are susceptible to the worst effects of the virus [and] all of us in the medical community need to help cancer patients understand the importance of getting vaccinated,” she added.
The survey – the largest ever done in which cancer patients and survivors were asked about their attitudes toward COVID-19 vaccines – was published online March 8 by The Leukemia & Lymphoma Society.
Survey sample
The survey asked patients with blood cancer, and survivors, about their attitudes regarding COVID-19 and COVID-19 vaccines.
“The main outcome [was] vaccine attitudes,” noted the authors, headed by Rena Conti, PhD, dean’s research scholar, Boston University.
Respondents were asked: “How likely are you to choose to get the vaccine?” Participants could indicate they were very unlikely, unlikely, neither likely nor unlikely, likely, or very likely to get vaccinated.
“We found that 17% of respondents indicate[d] that they [were] unlikely or very unlikely to take a vaccine,” Dr. Conti and colleagues observed.
Among the 17% – deemed to be “vaccine hesitant” – slightly over half (54%) stated they had concerns about the side effects associated with COVID-19 vaccination and believed neither of the two newly approved vaccines had been or would ever be tested properly.
The survey authors noted that there is no reason to believe COVID-19 vaccines are any less safe in patients with blood cancers, but concerns have been expressed that patients with some forms of blood cancer or those undergoing certain treatments may not achieve the same immune response to the vaccine as would noncancer controls.
Importantly, the survey was conducted Dec. 1-21, 2020, and responses differed depending on whether respondents answered the survey before or after the Pfizer-BioNTech and Moderna vaccines had been given emergency use authorization by the Food and Drug Administration starting Dec. 10, 2020.
There was a slight increase in positive responses after the vaccines were granted regulatory approval. (One-third of those who responded to the survey after the approval were 3.7% more likely to indicate they would get vaccinated). “This suggests that hesitancy may be influenced by emerging information dissemination, government action, and vaccine availability, transforming the hypothetical opportunity of vaccination to a real one,” the survey authors speculated.
Survey respondents who were vaccine hesitant were also over 14% more likely to indicate that they didn’t think they would require hospitalization should they contract COVID-19. But clinical data have suggested that approximately half of patients with a hematological malignancy who required hospitalization for COVID-19 die from the infection, the authors noted.
“Vaccine hesitant respondents [were] also significantly less likely to engage in protective health behaviors,” the survey authors pointed out. For example, they were almost 4% less likely to have worn a face mask and 1.6% less likely to have taken other protective measures to guard against COVID-19 infection.
Need for clear messaging
To counter vaccine hesitancy, the authors suggest there is a need for clear, consistent messaging targeting patients with cancer that emphasize the risks of COVID-19 and underscore vaccine benefits.
Dr. Conti pointed out that patients with blood cancer are, in fact, being given preferential access to vaccines in many communities, although this clearly doesn’t mean patients are willing to get vaccinated, as she also noted.
“We need both adequate supply and strong demand to keep this vulnerable population safe,” Dr. Conti emphasized.
The Leukemia & Lymphoma Society plans to repeat the survey in the near future to assess patients’ and survivors’ access to vaccines as well as their willingness to get vaccinated.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nearly one in three patients with blood cancer, and survivors, say they are unlikely to get a COVID-19 vaccine or unsure about getting it if one were available. The findings come from a nationwide survey by The Leukemia & Lymphoma Society, which collected 6,517 responses.
“These findings are worrisome, to say the least,” Gwen Nichols, MD, chief medical officer of the society, said in a statement.
“We know cancer patients – and blood cancer patients in particular – are susceptible to the worst effects of the virus [and] all of us in the medical community need to help cancer patients understand the importance of getting vaccinated,” she added.
The survey – the largest ever done in which cancer patients and survivors were asked about their attitudes toward COVID-19 vaccines – was published online March 8 by The Leukemia & Lymphoma Society.
Survey sample
The survey asked patients with blood cancer, and survivors, about their attitudes regarding COVID-19 and COVID-19 vaccines.
“The main outcome [was] vaccine attitudes,” noted the authors, headed by Rena Conti, PhD, dean’s research scholar, Boston University.
Respondents were asked: “How likely are you to choose to get the vaccine?” Participants could indicate they were very unlikely, unlikely, neither likely nor unlikely, likely, or very likely to get vaccinated.
“We found that 17% of respondents indicate[d] that they [were] unlikely or very unlikely to take a vaccine,” Dr. Conti and colleagues observed.
Among the 17% – deemed to be “vaccine hesitant” – slightly over half (54%) stated they had concerns about the side effects associated with COVID-19 vaccination and believed neither of the two newly approved vaccines had been or would ever be tested properly.
The survey authors noted that there is no reason to believe COVID-19 vaccines are any less safe in patients with blood cancers, but concerns have been expressed that patients with some forms of blood cancer or those undergoing certain treatments may not achieve the same immune response to the vaccine as would noncancer controls.
Importantly, the survey was conducted Dec. 1-21, 2020, and responses differed depending on whether respondents answered the survey before or after the Pfizer-BioNTech and Moderna vaccines had been given emergency use authorization by the Food and Drug Administration starting Dec. 10, 2020.
There was a slight increase in positive responses after the vaccines were granted regulatory approval. (One-third of those who responded to the survey after the approval were 3.7% more likely to indicate they would get vaccinated). “This suggests that hesitancy may be influenced by emerging information dissemination, government action, and vaccine availability, transforming the hypothetical opportunity of vaccination to a real one,” the survey authors speculated.
Survey respondents who were vaccine hesitant were also over 14% more likely to indicate that they didn’t think they would require hospitalization should they contract COVID-19. But clinical data have suggested that approximately half of patients with a hematological malignancy who required hospitalization for COVID-19 die from the infection, the authors noted.
“Vaccine hesitant respondents [were] also significantly less likely to engage in protective health behaviors,” the survey authors pointed out. For example, they were almost 4% less likely to have worn a face mask and 1.6% less likely to have taken other protective measures to guard against COVID-19 infection.
Need for clear messaging
To counter vaccine hesitancy, the authors suggest there is a need for clear, consistent messaging targeting patients with cancer that emphasize the risks of COVID-19 and underscore vaccine benefits.
Dr. Conti pointed out that patients with blood cancer are, in fact, being given preferential access to vaccines in many communities, although this clearly doesn’t mean patients are willing to get vaccinated, as she also noted.
“We need both adequate supply and strong demand to keep this vulnerable population safe,” Dr. Conti emphasized.
The Leukemia & Lymphoma Society plans to repeat the survey in the near future to assess patients’ and survivors’ access to vaccines as well as their willingness to get vaccinated.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Don’t delay: Cancer patients need both doses of COVID vaccine
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, which are soon to be published as a preprint, cast doubt on the current U.K. policy of delaying the second dose of the vaccine.
Delaying the second dose can leave most patients with cancer wholly or partially unprotected, according to the researchers. Moreover, such a delay has implications for transmission of SARS-CoV-2 in the cancer patient’s environs as well as for the evolution of virus variants that could be of concern, the researchers concluded.
The data come from a British study that included 151 patients with cancer and 54 healthy control persons. All participants received the COVID-19 mRNA BNT162b2 vaccine (Pfizer-BioNTech).
This vaccine requires two doses. The first few participants in this study were given the second dose 21 days after they had received the first dose, but then national guidelines changed, and the remaining participants had to wait 12 weeks to receive their second dose.
The researchers reported that, among health controls, the immune efficacy of the first dose was very high (97% efficacious). By contrast, among patients with solid tumors, the immune efficacy of a single dose was strikingly low (39%), and it was even lower in patients with hematologic malignancies (13%).
The second dose of vaccine greatly and rapidly increased the immune efficacy in patients with solid tumors (95% within 2 weeks of receiving the second dose), the researchers added.
Too few patients with hematologic cancers had received the second dose before the study ended for clear conclusions to be drawn. Nevertheless, the available data suggest that 50% of patients with hematologic cancers who had received the booster at day 21 were seropositive at 5 weeks vs. only 8% of those who had not received the booster.
“Our data provide the first real-world evidence of immune efficacy following one dose of the Pfizer vaccine in immunocompromised patient populations [and] clearly show that the poor one-dose efficacy in cancer patients can be rescued with an early booster at day 21,” commented senior author Sheeba Irshad, MD, senior clinical lecturer, King’s College London.
“Based on our findings, we would recommend an urgent review of the vaccine strategy for clinically extremely vulnerable groups. Until then, it is important that cancer patients continue to observe all public health measures in place, such as social distancing and shielding when attending hospitals, even after vaccination,” Dr. Irshad added.
The paper, with first author Leticia Monin-Aldama, PhD, is scheduled to appear on the preprint server medRxiv. It has not undergone peer review. The paper was distributed to journalists, with comments from experts not involved in the study, by the UK Science Media Centre.
These data are “of immediate importance” to patients with cancer, commented Shoba Amarnath, PhD, Newcastle University research fellow, Laboratory of T-cell Regulation, Newcastle University Center for Cancer, Newcastle upon Tyne, England.
“These findings are consistent with our understanding. … We know that the immune system within cancer patients is compromised as compared to healthy controls,” Dr. Amarnath said. “The data in the study support the notion that, in solid cancer patients, a considerable delay in second dose will extend the period when cancer patients are at risk of SARS-CoV-2 infection.”
Although more data are required, “this study does raise the issue of whether patients with cancer, other diseases, or those undergoing therapies that affect the body’s immune response should be fast-tracked for their second vaccine dose,” commented Lawrence Young, PhD, professor of molecular oncology and director of the Warwick Cancer Research Center, University of Warwick, Coventry, England.
Stephen Evans, MSc, professor of pharmacoepidemiology, London School of Hygiene and Tropical Medicine, underlined that the study is “essentially” observational and “inevitable limitations must be taken into account.
“Nevertheless, these results do suggest that the vaccines may well not protect those patients with cancer as well as those without cancer,” Mr. Evans said. He added that it is “important that this population continues to observe all COVID-19–associated measures, such as social distancing and shielding when attending hospitals, even after vaccination.”
Study details
Previous studies have shown that some patients with cancer have prolonged responses to SARS-CoV-2 infection, with ongoing immune dysregulation, inefficient seroconversion, and prolonged viral shedding.
There are few data, however, on how these patients respond to COVID-19 vaccination. The authors point out that, among the 18,860 individuals who received the Pfizer vaccine during its development trials, “none with an active oncological diagnosis was included.”
To investigate this issue, they launched the SARS-CoV-2 for Cancer Patients (SOAP-02) study.
The 151 patients with cancer who participated in this study were mostly elderly, the authors noted (75% were older than 65 years; the median age was 73 years). The majority (63%) had solid-tumor malignancies. Of those, 8% had late-stage disease and had been living with their cancer for more than 24 months.
The healthy control persons were vaccine-eligible primary health care workers who were not age matched to the cancer patients.
All participants received the first dose of vaccine; 31 (of 151) patients with cancer and 16 (of 54) healthy control persons received the second dose on day 21.
The remaining participants were scheduled to receive their second dose 12 weeks later (after the study ended), in line with the changes in the national guidelines.
The team reported that, approximately 21 days after receiving the first vaccine dose, the immune efficacy of the vaccine was estimated to be 97% among healthy control persons vs. 39% for patients with solid tumors and only 13% for those with hematologic malignancies (P < .0001 for both).
T-cell responses, as assessed via interferon-gamma and/or interleukin-2 production, were observed in 82% of healthy control persons, 71% of patients with solid tumors, and 50% of those with hematologic cancers.
Vaccine boosting at day 21 resulted in immune efficacy of 100% for healthy control persons and 95% for patients with solid tumors. In contrast, only 43% of those who did not receive the second dose were seropositive 2 weeks later.
Further analysis suggested that participants who did not have a serologic response were “spread evenly” across different cancer types, but the reduced responses were more frequent among patients who had received the vaccine within 15 days of cancer treatment, especially chemotherapy, and had undergone intensive treatments.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas Trust Foundation NHS Trust. It is funded from grants from the KCL Charity, Cancer Research UK, and program grants from Breast Cancer Now. The investigators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.