User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Antiaffirmative action paper blasted on Twitter now retracted
An article published in March in the Journal of the American Heart Association that raised a ruckus on #medtwitter this week has now been retracted.
It’s unclear what prompted the public explosion of anger, sadness, and recrimination that ultimately led to the retraction of this article – which flew almost completely under the radar when it first appeared online and in print – but it’s crystal clear why it might offend.
To many readers, the paper, written by Norman C. Wang, MD, MSc, an electrophysiologist at the University of Pittsburgh Medical Center, is a “racist” rant that relies on half-truths (J Am Heart Assoc. 2020 Mar 24. doi: 10.1161/JAHA.120.015959).
Officially, the article, “Diversity, Inclusion, and Equity: Evolution of Race and Ethnicity Considerations for the Cardiology Workforce in the United States of America From 1969 to 2019,” was retracted after the American Heart Association “became aware of serious concerns after publication. The author’s institution, the University of Pittsburgh Medical Center, has notified the Editor‐in‐Chief that the article contains many misconceptions and misquotes and that together those inaccuracies, misstatements, and selective misreading of source materials strip the paper of its scientific validity,” the retraction reads (J Am Heart Assoc. 2020 Aug 6. doi: 10.1161/JAHA.119.014602).
The journal will be publishing a detailed rebuttal, the notice adds: “This retraction notice will be updated with a link to the rebuttal when it publishes.”
“The Editor‐in‐Chief deeply regrets publishing the article and offers his apologies,” it further reads. “The American Heart Association and the Editor‐in‐Chief have determined that the best interest of the public and the research community will be served by issuing this notice of retraction. The author does not agree to the retraction.”
In the paper, Dr. Wang argues that affirmative action policies designed to increase minority representation in medical schools and cardiovascular training programs result in unqualified applicants being admitted, where they will struggle to succeed.
The article itself is a dense review of the topic of diversity, inclusion, and equity, aiming to “critically assess current paradigms, and to consider potential solutions to anticipated challenges,” according to its author. Supported by 108 references, Dr. Wang concludes with a lengthy quote from tennis great Arthur Ashe, an opponent of affirmative action who died in 1993.
Affirmative action, said Mr. Ashe, is “an insult to the people it intended to help.” Dr. Wang suggests that “racial and ethnic preferences for undergraduate and medical school admissions should be gradually rolled back with a target end year of 2028.”
He cites the $16 billion in federal funding that cardiovascular disease training programs receive every year to support graduate medical education in support of this contention.
#Medtwitter explodes
“My entire lived experience contradicts everything in that racist @JAHA_AHA article, as does the experience of so many others. So, I know it’s just a bad opinion piece passed off as ‘research’ that shouldn’t have been published. Still the damage has been done. We MUST do better,” tweeted Bryan A. Smith, MD, University of Chicago Medicine.
According to its Altmetric score, the article received very little attention back in March and April. There were three tweets referencing it, including one from JAHA announcing its publication. Since Aug. 2, an additional 390-odd Tweets from 347 Twitter users have been registered. None appear to be complimentary. Several days into the Twitter storm, the article was officially retracted.
“This article is shocking and makes me sad,” Martha Gulati, MD, University of Arizona, Phoenix, said in an interview. “We are all working so hard to make cardiology more inclusive and diverse, and this takes us like 1,000 steps backwards.”
For her part, Dr. Gulati would have liked a retraction earlier in the week. “The analysis was selective and incorrect, and the statements made intimate that minority trainees were selected based on affirmative action rather than their merits,” she said. It also suggested that their presence was representative of a decline in standards in cardiology programs that take underrepresented minorities (URMs).
Standard arguments against affirmative action
According to Dr. Wang, who did not respond to a request to comment for this article, allowing minority students into medical school with academic records that are weaker than their classmates sets them up for failure.
“Many do not complete their intended programs or do not attain academic success to be attractive candidates for subsequent educational programs or employment,” he wrote.
This is a standard argument of opponents to affirmative action, said Quinn Capers IV, MD. Dr. Capers, a longtime advocate for diversity in medicine, acknowledges that, “on average,” test scores for Blacks, Hispanics, and Native Americans tend to be lower than for White applicants for a wide range of reasons, many of which are related to systemic racism.
“This is the strongest weapon opponents to affirmative action have, and they keep coming back to it, but it’s out of step with how many in academic medicine feel,” said Dr. Capers, who is an interventional cardiologist and the vice dean for faculty affairs at Ohio State University, Columbus.
This is why, he added, most medical schools have embraced the Association of American Medical Colleges’ concept of “holistic review,” which judges potential physicians on their academic records, their personal experiences, and their individual attributes.
“Standardized tests and academic records are important, but so are the experiences one has gone through and the individual attributes they may have. How resilient are you? How compassionate? Our embrace of this more holistic approach, I believe, is helping many medical schools move toward having a more diverse class that is closer to reflecting the needs of our multicultural and multiracial society,” Dr. Capers said.
To be clear, Dr. Capers is not afraid of having a discussion on this topic and denies that the uproar against this article represents “cancel culture.”
“Hey, I love to debate and I’m not against hearing divisive voices, but then let’s have a debate and hear both sides. But there are several problems with the way they did this. No. 1, they called it a ‘white paper,’ which to most people means it reflects the views of the organization, not a specific individual, and, secondly, it’s more than an opinion piece in that he manipulates facts to make his points, with no chance for rebuttal.”
Several have also questioned how this paper, which is written by a nonexpert in the field, passed peer review.
The article contains some accurate historical references, said Dr. Capers, but intertwined with this history the author editorializes in a fashion that is “charged with racism.” In other places, Dr. Wang is just outright wrong, he added.
“I can also tell you that, in one place where he quotes me specifically, what he says is quite damaging and completely wrong. He quotes something we wrote but cuts off the final sentence, making it seem as though we acknowledged that we had to artificially rank minority applicants high, just so we could say we have a diverse fellowship program.
“It’s frankly very hard to believe that was an accident,” Dr. Capers added.
AHA backs away, promises investigation
The article has been disowned by all levels of the AHA leadership – past, present, and future.
In an Editor’s Note, Barry London, MD, PhD, the Editor in chief of the Journal of the American Heart Association, apologized for his role and the role of his staff in publishing the article.
“JAHA will support all efforts to correct this error, including but not limited to the publication of alternate viewpoints, which we solicited at the time of publication but have not yet been submitted to the journal. In addition, we will work to improve our peer review system to prevent future missteps of this type,” Dr. London wrote. “I can only hope that igniting a discussion around diversity in cardiology will ultimately fuel new ideas and lead to real advances.”
“I want to emphasize in the strongest possible terms that this paper does not represent the views of the AHA as an organization or its leadership. This paper should never have been published. A thorough investigation is rightly being conducted,” tweeted Mitchell S.V. Elkind, MD, MPhil, who took over the AHA presidency last month.
“Author’s views are racist and not consistent with my values nor AHA,” tweeted Robert Harrington, MD, immediate past president of the AHA. ‘Investigation is underway into how it made it through the editorial process. Like you, I want to know what happened. I am angry, frustrated and disappointed that this piece was published; expect review soon.’
“Agree with @HeartBobH. It is impossible not to hear and feel the hurt and pain out there on a very personal level, especially among our young colleagues. You are valued, and worthy. Please stay tuned and then help all of us work to be better,” tweeted Donald Lloyd-Jones, MD, president-elect of AHA.
A version of this article originally appeared on Medscape.com.
An article published in March in the Journal of the American Heart Association that raised a ruckus on #medtwitter this week has now been retracted.
It’s unclear what prompted the public explosion of anger, sadness, and recrimination that ultimately led to the retraction of this article – which flew almost completely under the radar when it first appeared online and in print – but it’s crystal clear why it might offend.
To many readers, the paper, written by Norman C. Wang, MD, MSc, an electrophysiologist at the University of Pittsburgh Medical Center, is a “racist” rant that relies on half-truths (J Am Heart Assoc. 2020 Mar 24. doi: 10.1161/JAHA.120.015959).
Officially, the article, “Diversity, Inclusion, and Equity: Evolution of Race and Ethnicity Considerations for the Cardiology Workforce in the United States of America From 1969 to 2019,” was retracted after the American Heart Association “became aware of serious concerns after publication. The author’s institution, the University of Pittsburgh Medical Center, has notified the Editor‐in‐Chief that the article contains many misconceptions and misquotes and that together those inaccuracies, misstatements, and selective misreading of source materials strip the paper of its scientific validity,” the retraction reads (J Am Heart Assoc. 2020 Aug 6. doi: 10.1161/JAHA.119.014602).
The journal will be publishing a detailed rebuttal, the notice adds: “This retraction notice will be updated with a link to the rebuttal when it publishes.”
“The Editor‐in‐Chief deeply regrets publishing the article and offers his apologies,” it further reads. “The American Heart Association and the Editor‐in‐Chief have determined that the best interest of the public and the research community will be served by issuing this notice of retraction. The author does not agree to the retraction.”
In the paper, Dr. Wang argues that affirmative action policies designed to increase minority representation in medical schools and cardiovascular training programs result in unqualified applicants being admitted, where they will struggle to succeed.
The article itself is a dense review of the topic of diversity, inclusion, and equity, aiming to “critically assess current paradigms, and to consider potential solutions to anticipated challenges,” according to its author. Supported by 108 references, Dr. Wang concludes with a lengthy quote from tennis great Arthur Ashe, an opponent of affirmative action who died in 1993.
Affirmative action, said Mr. Ashe, is “an insult to the people it intended to help.” Dr. Wang suggests that “racial and ethnic preferences for undergraduate and medical school admissions should be gradually rolled back with a target end year of 2028.”
He cites the $16 billion in federal funding that cardiovascular disease training programs receive every year to support graduate medical education in support of this contention.
#Medtwitter explodes
“My entire lived experience contradicts everything in that racist @JAHA_AHA article, as does the experience of so many others. So, I know it’s just a bad opinion piece passed off as ‘research’ that shouldn’t have been published. Still the damage has been done. We MUST do better,” tweeted Bryan A. Smith, MD, University of Chicago Medicine.
According to its Altmetric score, the article received very little attention back in March and April. There were three tweets referencing it, including one from JAHA announcing its publication. Since Aug. 2, an additional 390-odd Tweets from 347 Twitter users have been registered. None appear to be complimentary. Several days into the Twitter storm, the article was officially retracted.
“This article is shocking and makes me sad,” Martha Gulati, MD, University of Arizona, Phoenix, said in an interview. “We are all working so hard to make cardiology more inclusive and diverse, and this takes us like 1,000 steps backwards.”
For her part, Dr. Gulati would have liked a retraction earlier in the week. “The analysis was selective and incorrect, and the statements made intimate that minority trainees were selected based on affirmative action rather than their merits,” she said. It also suggested that their presence was representative of a decline in standards in cardiology programs that take underrepresented minorities (URMs).
Standard arguments against affirmative action
According to Dr. Wang, who did not respond to a request to comment for this article, allowing minority students into medical school with academic records that are weaker than their classmates sets them up for failure.
“Many do not complete their intended programs or do not attain academic success to be attractive candidates for subsequent educational programs or employment,” he wrote.
This is a standard argument of opponents to affirmative action, said Quinn Capers IV, MD. Dr. Capers, a longtime advocate for diversity in medicine, acknowledges that, “on average,” test scores for Blacks, Hispanics, and Native Americans tend to be lower than for White applicants for a wide range of reasons, many of which are related to systemic racism.
“This is the strongest weapon opponents to affirmative action have, and they keep coming back to it, but it’s out of step with how many in academic medicine feel,” said Dr. Capers, who is an interventional cardiologist and the vice dean for faculty affairs at Ohio State University, Columbus.
This is why, he added, most medical schools have embraced the Association of American Medical Colleges’ concept of “holistic review,” which judges potential physicians on their academic records, their personal experiences, and their individual attributes.
“Standardized tests and academic records are important, but so are the experiences one has gone through and the individual attributes they may have. How resilient are you? How compassionate? Our embrace of this more holistic approach, I believe, is helping many medical schools move toward having a more diverse class that is closer to reflecting the needs of our multicultural and multiracial society,” Dr. Capers said.
To be clear, Dr. Capers is not afraid of having a discussion on this topic and denies that the uproar against this article represents “cancel culture.”
“Hey, I love to debate and I’m not against hearing divisive voices, but then let’s have a debate and hear both sides. But there are several problems with the way they did this. No. 1, they called it a ‘white paper,’ which to most people means it reflects the views of the organization, not a specific individual, and, secondly, it’s more than an opinion piece in that he manipulates facts to make his points, with no chance for rebuttal.”
Several have also questioned how this paper, which is written by a nonexpert in the field, passed peer review.
The article contains some accurate historical references, said Dr. Capers, but intertwined with this history the author editorializes in a fashion that is “charged with racism.” In other places, Dr. Wang is just outright wrong, he added.
“I can also tell you that, in one place where he quotes me specifically, what he says is quite damaging and completely wrong. He quotes something we wrote but cuts off the final sentence, making it seem as though we acknowledged that we had to artificially rank minority applicants high, just so we could say we have a diverse fellowship program.
“It’s frankly very hard to believe that was an accident,” Dr. Capers added.
AHA backs away, promises investigation
The article has been disowned by all levels of the AHA leadership – past, present, and future.
In an Editor’s Note, Barry London, MD, PhD, the Editor in chief of the Journal of the American Heart Association, apologized for his role and the role of his staff in publishing the article.
“JAHA will support all efforts to correct this error, including but not limited to the publication of alternate viewpoints, which we solicited at the time of publication but have not yet been submitted to the journal. In addition, we will work to improve our peer review system to prevent future missteps of this type,” Dr. London wrote. “I can only hope that igniting a discussion around diversity in cardiology will ultimately fuel new ideas and lead to real advances.”
“I want to emphasize in the strongest possible terms that this paper does not represent the views of the AHA as an organization or its leadership. This paper should never have been published. A thorough investigation is rightly being conducted,” tweeted Mitchell S.V. Elkind, MD, MPhil, who took over the AHA presidency last month.
“Author’s views are racist and not consistent with my values nor AHA,” tweeted Robert Harrington, MD, immediate past president of the AHA. ‘Investigation is underway into how it made it through the editorial process. Like you, I want to know what happened. I am angry, frustrated and disappointed that this piece was published; expect review soon.’
“Agree with @HeartBobH. It is impossible not to hear and feel the hurt and pain out there on a very personal level, especially among our young colleagues. You are valued, and worthy. Please stay tuned and then help all of us work to be better,” tweeted Donald Lloyd-Jones, MD, president-elect of AHA.
A version of this article originally appeared on Medscape.com.
An article published in March in the Journal of the American Heart Association that raised a ruckus on #medtwitter this week has now been retracted.
It’s unclear what prompted the public explosion of anger, sadness, and recrimination that ultimately led to the retraction of this article – which flew almost completely under the radar when it first appeared online and in print – but it’s crystal clear why it might offend.
To many readers, the paper, written by Norman C. Wang, MD, MSc, an electrophysiologist at the University of Pittsburgh Medical Center, is a “racist” rant that relies on half-truths (J Am Heart Assoc. 2020 Mar 24. doi: 10.1161/JAHA.120.015959).
Officially, the article, “Diversity, Inclusion, and Equity: Evolution of Race and Ethnicity Considerations for the Cardiology Workforce in the United States of America From 1969 to 2019,” was retracted after the American Heart Association “became aware of serious concerns after publication. The author’s institution, the University of Pittsburgh Medical Center, has notified the Editor‐in‐Chief that the article contains many misconceptions and misquotes and that together those inaccuracies, misstatements, and selective misreading of source materials strip the paper of its scientific validity,” the retraction reads (J Am Heart Assoc. 2020 Aug 6. doi: 10.1161/JAHA.119.014602).
The journal will be publishing a detailed rebuttal, the notice adds: “This retraction notice will be updated with a link to the rebuttal when it publishes.”
“The Editor‐in‐Chief deeply regrets publishing the article and offers his apologies,” it further reads. “The American Heart Association and the Editor‐in‐Chief have determined that the best interest of the public and the research community will be served by issuing this notice of retraction. The author does not agree to the retraction.”
In the paper, Dr. Wang argues that affirmative action policies designed to increase minority representation in medical schools and cardiovascular training programs result in unqualified applicants being admitted, where they will struggle to succeed.
The article itself is a dense review of the topic of diversity, inclusion, and equity, aiming to “critically assess current paradigms, and to consider potential solutions to anticipated challenges,” according to its author. Supported by 108 references, Dr. Wang concludes with a lengthy quote from tennis great Arthur Ashe, an opponent of affirmative action who died in 1993.
Affirmative action, said Mr. Ashe, is “an insult to the people it intended to help.” Dr. Wang suggests that “racial and ethnic preferences for undergraduate and medical school admissions should be gradually rolled back with a target end year of 2028.”
He cites the $16 billion in federal funding that cardiovascular disease training programs receive every year to support graduate medical education in support of this contention.
#Medtwitter explodes
“My entire lived experience contradicts everything in that racist @JAHA_AHA article, as does the experience of so many others. So, I know it’s just a bad opinion piece passed off as ‘research’ that shouldn’t have been published. Still the damage has been done. We MUST do better,” tweeted Bryan A. Smith, MD, University of Chicago Medicine.
According to its Altmetric score, the article received very little attention back in March and April. There were three tweets referencing it, including one from JAHA announcing its publication. Since Aug. 2, an additional 390-odd Tweets from 347 Twitter users have been registered. None appear to be complimentary. Several days into the Twitter storm, the article was officially retracted.
“This article is shocking and makes me sad,” Martha Gulati, MD, University of Arizona, Phoenix, said in an interview. “We are all working so hard to make cardiology more inclusive and diverse, and this takes us like 1,000 steps backwards.”
For her part, Dr. Gulati would have liked a retraction earlier in the week. “The analysis was selective and incorrect, and the statements made intimate that minority trainees were selected based on affirmative action rather than their merits,” she said. It also suggested that their presence was representative of a decline in standards in cardiology programs that take underrepresented minorities (URMs).
Standard arguments against affirmative action
According to Dr. Wang, who did not respond to a request to comment for this article, allowing minority students into medical school with academic records that are weaker than their classmates sets them up for failure.
“Many do not complete their intended programs or do not attain academic success to be attractive candidates for subsequent educational programs or employment,” he wrote.
This is a standard argument of opponents to affirmative action, said Quinn Capers IV, MD. Dr. Capers, a longtime advocate for diversity in medicine, acknowledges that, “on average,” test scores for Blacks, Hispanics, and Native Americans tend to be lower than for White applicants for a wide range of reasons, many of which are related to systemic racism.
“This is the strongest weapon opponents to affirmative action have, and they keep coming back to it, but it’s out of step with how many in academic medicine feel,” said Dr. Capers, who is an interventional cardiologist and the vice dean for faculty affairs at Ohio State University, Columbus.
This is why, he added, most medical schools have embraced the Association of American Medical Colleges’ concept of “holistic review,” which judges potential physicians on their academic records, their personal experiences, and their individual attributes.
“Standardized tests and academic records are important, but so are the experiences one has gone through and the individual attributes they may have. How resilient are you? How compassionate? Our embrace of this more holistic approach, I believe, is helping many medical schools move toward having a more diverse class that is closer to reflecting the needs of our multicultural and multiracial society,” Dr. Capers said.
To be clear, Dr. Capers is not afraid of having a discussion on this topic and denies that the uproar against this article represents “cancel culture.”
“Hey, I love to debate and I’m not against hearing divisive voices, but then let’s have a debate and hear both sides. But there are several problems with the way they did this. No. 1, they called it a ‘white paper,’ which to most people means it reflects the views of the organization, not a specific individual, and, secondly, it’s more than an opinion piece in that he manipulates facts to make his points, with no chance for rebuttal.”
Several have also questioned how this paper, which is written by a nonexpert in the field, passed peer review.
The article contains some accurate historical references, said Dr. Capers, but intertwined with this history the author editorializes in a fashion that is “charged with racism.” In other places, Dr. Wang is just outright wrong, he added.
“I can also tell you that, in one place where he quotes me specifically, what he says is quite damaging and completely wrong. He quotes something we wrote but cuts off the final sentence, making it seem as though we acknowledged that we had to artificially rank minority applicants high, just so we could say we have a diverse fellowship program.
“It’s frankly very hard to believe that was an accident,” Dr. Capers added.
AHA backs away, promises investigation
The article has been disowned by all levels of the AHA leadership – past, present, and future.
In an Editor’s Note, Barry London, MD, PhD, the Editor in chief of the Journal of the American Heart Association, apologized for his role and the role of his staff in publishing the article.
“JAHA will support all efforts to correct this error, including but not limited to the publication of alternate viewpoints, which we solicited at the time of publication but have not yet been submitted to the journal. In addition, we will work to improve our peer review system to prevent future missteps of this type,” Dr. London wrote. “I can only hope that igniting a discussion around diversity in cardiology will ultimately fuel new ideas and lead to real advances.”
“I want to emphasize in the strongest possible terms that this paper does not represent the views of the AHA as an organization or its leadership. This paper should never have been published. A thorough investigation is rightly being conducted,” tweeted Mitchell S.V. Elkind, MD, MPhil, who took over the AHA presidency last month.
“Author’s views are racist and not consistent with my values nor AHA,” tweeted Robert Harrington, MD, immediate past president of the AHA. ‘Investigation is underway into how it made it through the editorial process. Like you, I want to know what happened. I am angry, frustrated and disappointed that this piece was published; expect review soon.’
“Agree with @HeartBobH. It is impossible not to hear and feel the hurt and pain out there on a very personal level, especially among our young colleagues. You are valued, and worthy. Please stay tuned and then help all of us work to be better,” tweeted Donald Lloyd-Jones, MD, president-elect of AHA.
A version of this article originally appeared on Medscape.com.
ED visits for mental health, substance use doubled in 1 decade
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).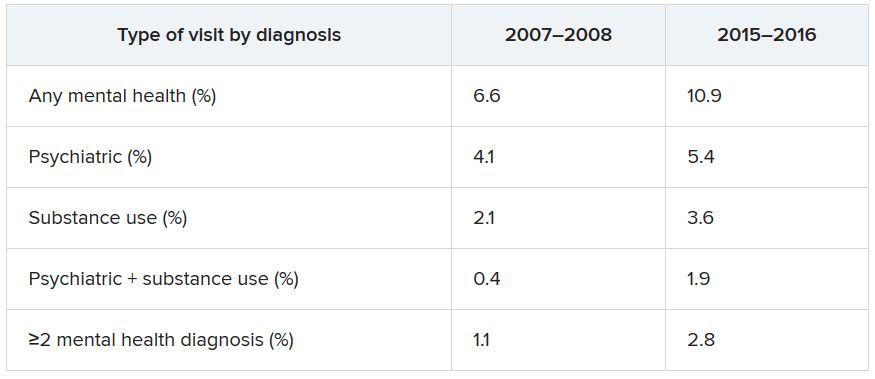
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Many children with COVID-19 present without classic symptoms
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
FROM HOSPITAL PEDIATRICS
Diabetic amputations soared amid Italian pandemic lockdown
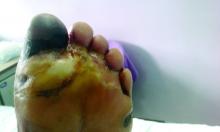
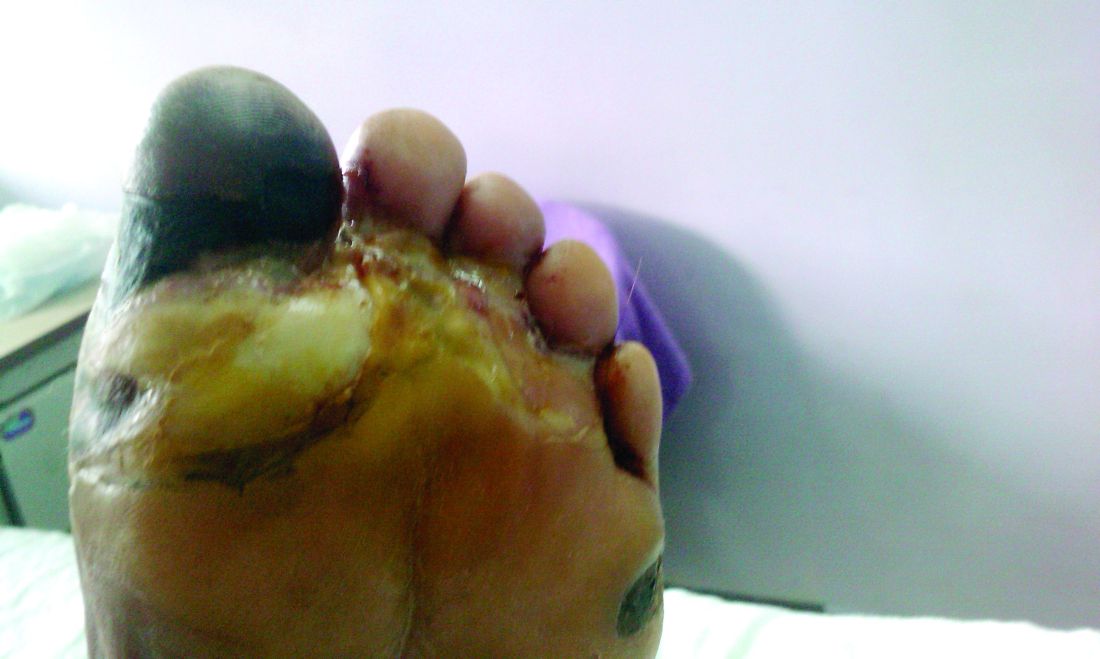
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
FROM DIABETES CARE
Diagnostic testing for COVID-19: A quick summary for PCPs
Information about COVID has evolved so quickly that it can be difficult for clinicians to feel confident that they are staying current. These summaries include links to our reference article on diagnosis of COVID-19, which is constantly updated to make sure you have the latest information.
Diagnostic testing for COVID-19 is critical. No one disputes that. But what is in dispute is whom to test, when to test, how to test, what to do while waiting for results, and how accurate those results are when you finally get them.
Here are the answers to those questions, based on the current information.
Whom to test. This is the (relatively) easy part. The ideal answer is that everyone should be tested. The Infectious Diseases Society of America issued tier-based recommendations way back in March, and they still apply. First priority continues to be patients who are ill, healthcare workers, and those with known exposure. But to truly figure out the amount of community spread in a given area, we need to test people who do not have a clear indication for testing. That is particularly true as more people return to work and the Centers for Disease Control and Prevention (CDC) has issued guidelines for workplaces to establish testing programs. Universal testing is recommended for some high-risk settings, such as nursing homes.
One key change: CDC no longer recommends testing to determine whether someone with a known infection is still infectious.
When to test. People with any symptoms suggestive of COVID should be tested, ideally as soon as feasible. But given the ongoing shortages of tests, that may not be possible, particularly for those requiring only symptomatic care. Rather, these patients should be treated as probable cases, with appropriate instructions regarding quarantine. Testing of those with known exposures ideally should be done about 5 days after exposure.
How to test. Only viral nucleic acid or antigen tests should be used to diagnose acute illness. CDC does not currently recommend using serologic assays, now broadly available, for diagnosis of acute infection, though they obviously play an important role in understanding the transmission dynamic of the virus in the general population.
Testing strategies vary from state to state and even within communities in a single state. It is recommended that clinicians check with their own local or state health department for specifics on tests available, indications for testing, and processing details. While often forgotten, it is worth emphasizing that no diagnostic tests have been approved by the US Food and Drug Administration (FDA). Rather, they are available under emergency use authorization (EUA), meaning that they have not been fully vetted by the FDA.
In late July, the FDA expanded authorization for real-time reverse transcription–polymerase chain reaction (rRT-PCR) molecular assays, utilizing nasal or nasopharyngeal swabs, to permit testing of all persons, regardless of exposure history or symptoms. The FDA maintains a list of all approved diagnostic tests and corresponding labs. Patients will have to get what is available via their health department or insurance plan.
Two point-of-care antigen tests using nasopharyngeal or nasal samples have been issued an EUA. These tests can be used only in settings with a valid CLIA certificate.
Several commercial laboratories have received approval to process diagnostic tests using patients’ self-collected saliva rather than swabs. One lab has now received authorization for in-home testing without any input from a clinician. These testing options can be a boon for patients who have symptoms or exposure and for whatever reason are unable to get to a diagnostic site. These samples are collected at home and mailed to a lab. Note that these tests are not yet widely available.
Waiting for results. If waiting for results meant a day or even a couple of days, the answer to this one would be easier. But if the wait extends to 1 and even sometimes 2 weeks, then the test is not able to meaningfully guide clinical decisions. The latest guidance from the CDC is that individuals with symptoms suggestive of COVID who do not require hospitalization should remain at home in self-quarantine for at least 10 days from symptom onset. Asymptomatic individuals with a known exposure to someone else with COVID, or participation in a high-risk event like an indoor gathering involving more than 10 persons, should self-quarantine either until they receive a negative test result or 14 days after the exposure.
Accuracy of results. A positive rRT-PCR antigen test is highly accurate, indicating presence of SARS-CoV-2 RNA. There appears to be no significant cross-reactivity with other respiratory viruses or even other coronaviruses. A small study conducted in Korea suggests that patients with persistent positive tests who are beyond 10 days from the initial positive test and are now symptom free are no longer infectious.
For patients with a high suspicion of COVID-19, a negative test should not rule out the infection. The number of false-negative results is not well known, though the resultant risk is “substantial.” A number of factors affect the likelihood of a false-negative test, including when the sample was collected relative to the timing of illness and the type of specimen collected; for example, nasopharyngeal swabs are more likely to be accurate vs nasal or throat specimens. Repeat or serial testing increases the sensitivity but may not always be available. Although rRT-PCR is the current criterion standard, more inclusive consensus-based criteria are likely to emerge because of the concern about these false-negative results.
This article first appeared on Medscape.com.
Information about COVID has evolved so quickly that it can be difficult for clinicians to feel confident that they are staying current. These summaries include links to our reference article on diagnosis of COVID-19, which is constantly updated to make sure you have the latest information.
Diagnostic testing for COVID-19 is critical. No one disputes that. But what is in dispute is whom to test, when to test, how to test, what to do while waiting for results, and how accurate those results are when you finally get them.
Here are the answers to those questions, based on the current information.
Whom to test. This is the (relatively) easy part. The ideal answer is that everyone should be tested. The Infectious Diseases Society of America issued tier-based recommendations way back in March, and they still apply. First priority continues to be patients who are ill, healthcare workers, and those with known exposure. But to truly figure out the amount of community spread in a given area, we need to test people who do not have a clear indication for testing. That is particularly true as more people return to work and the Centers for Disease Control and Prevention (CDC) has issued guidelines for workplaces to establish testing programs. Universal testing is recommended for some high-risk settings, such as nursing homes.
One key change: CDC no longer recommends testing to determine whether someone with a known infection is still infectious.
When to test. People with any symptoms suggestive of COVID should be tested, ideally as soon as feasible. But given the ongoing shortages of tests, that may not be possible, particularly for those requiring only symptomatic care. Rather, these patients should be treated as probable cases, with appropriate instructions regarding quarantine. Testing of those with known exposures ideally should be done about 5 days after exposure.
How to test. Only viral nucleic acid or antigen tests should be used to diagnose acute illness. CDC does not currently recommend using serologic assays, now broadly available, for diagnosis of acute infection, though they obviously play an important role in understanding the transmission dynamic of the virus in the general population.
Testing strategies vary from state to state and even within communities in a single state. It is recommended that clinicians check with their own local or state health department for specifics on tests available, indications for testing, and processing details. While often forgotten, it is worth emphasizing that no diagnostic tests have been approved by the US Food and Drug Administration (FDA). Rather, they are available under emergency use authorization (EUA), meaning that they have not been fully vetted by the FDA.
In late July, the FDA expanded authorization for real-time reverse transcription–polymerase chain reaction (rRT-PCR) molecular assays, utilizing nasal or nasopharyngeal swabs, to permit testing of all persons, regardless of exposure history or symptoms. The FDA maintains a list of all approved diagnostic tests and corresponding labs. Patients will have to get what is available via their health department or insurance plan.
Two point-of-care antigen tests using nasopharyngeal or nasal samples have been issued an EUA. These tests can be used only in settings with a valid CLIA certificate.
Several commercial laboratories have received approval to process diagnostic tests using patients’ self-collected saliva rather than swabs. One lab has now received authorization for in-home testing without any input from a clinician. These testing options can be a boon for patients who have symptoms or exposure and for whatever reason are unable to get to a diagnostic site. These samples are collected at home and mailed to a lab. Note that these tests are not yet widely available.
Waiting for results. If waiting for results meant a day or even a couple of days, the answer to this one would be easier. But if the wait extends to 1 and even sometimes 2 weeks, then the test is not able to meaningfully guide clinical decisions. The latest guidance from the CDC is that individuals with symptoms suggestive of COVID who do not require hospitalization should remain at home in self-quarantine for at least 10 days from symptom onset. Asymptomatic individuals with a known exposure to someone else with COVID, or participation in a high-risk event like an indoor gathering involving more than 10 persons, should self-quarantine either until they receive a negative test result or 14 days after the exposure.
Accuracy of results. A positive rRT-PCR antigen test is highly accurate, indicating presence of SARS-CoV-2 RNA. There appears to be no significant cross-reactivity with other respiratory viruses or even other coronaviruses. A small study conducted in Korea suggests that patients with persistent positive tests who are beyond 10 days from the initial positive test and are now symptom free are no longer infectious.
For patients with a high suspicion of COVID-19, a negative test should not rule out the infection. The number of false-negative results is not well known, though the resultant risk is “substantial.” A number of factors affect the likelihood of a false-negative test, including when the sample was collected relative to the timing of illness and the type of specimen collected; for example, nasopharyngeal swabs are more likely to be accurate vs nasal or throat specimens. Repeat or serial testing increases the sensitivity but may not always be available. Although rRT-PCR is the current criterion standard, more inclusive consensus-based criteria are likely to emerge because of the concern about these false-negative results.
This article first appeared on Medscape.com.
Information about COVID has evolved so quickly that it can be difficult for clinicians to feel confident that they are staying current. These summaries include links to our reference article on diagnosis of COVID-19, which is constantly updated to make sure you have the latest information.
Diagnostic testing for COVID-19 is critical. No one disputes that. But what is in dispute is whom to test, when to test, how to test, what to do while waiting for results, and how accurate those results are when you finally get them.
Here are the answers to those questions, based on the current information.
Whom to test. This is the (relatively) easy part. The ideal answer is that everyone should be tested. The Infectious Diseases Society of America issued tier-based recommendations way back in March, and they still apply. First priority continues to be patients who are ill, healthcare workers, and those with known exposure. But to truly figure out the amount of community spread in a given area, we need to test people who do not have a clear indication for testing. That is particularly true as more people return to work and the Centers for Disease Control and Prevention (CDC) has issued guidelines for workplaces to establish testing programs. Universal testing is recommended for some high-risk settings, such as nursing homes.
One key change: CDC no longer recommends testing to determine whether someone with a known infection is still infectious.
When to test. People with any symptoms suggestive of COVID should be tested, ideally as soon as feasible. But given the ongoing shortages of tests, that may not be possible, particularly for those requiring only symptomatic care. Rather, these patients should be treated as probable cases, with appropriate instructions regarding quarantine. Testing of those with known exposures ideally should be done about 5 days after exposure.
How to test. Only viral nucleic acid or antigen tests should be used to diagnose acute illness. CDC does not currently recommend using serologic assays, now broadly available, for diagnosis of acute infection, though they obviously play an important role in understanding the transmission dynamic of the virus in the general population.
Testing strategies vary from state to state and even within communities in a single state. It is recommended that clinicians check with their own local or state health department for specifics on tests available, indications for testing, and processing details. While often forgotten, it is worth emphasizing that no diagnostic tests have been approved by the US Food and Drug Administration (FDA). Rather, they are available under emergency use authorization (EUA), meaning that they have not been fully vetted by the FDA.
In late July, the FDA expanded authorization for real-time reverse transcription–polymerase chain reaction (rRT-PCR) molecular assays, utilizing nasal or nasopharyngeal swabs, to permit testing of all persons, regardless of exposure history or symptoms. The FDA maintains a list of all approved diagnostic tests and corresponding labs. Patients will have to get what is available via their health department or insurance plan.
Two point-of-care antigen tests using nasopharyngeal or nasal samples have been issued an EUA. These tests can be used only in settings with a valid CLIA certificate.
Several commercial laboratories have received approval to process diagnostic tests using patients’ self-collected saliva rather than swabs. One lab has now received authorization for in-home testing without any input from a clinician. These testing options can be a boon for patients who have symptoms or exposure and for whatever reason are unable to get to a diagnostic site. These samples are collected at home and mailed to a lab. Note that these tests are not yet widely available.
Waiting for results. If waiting for results meant a day or even a couple of days, the answer to this one would be easier. But if the wait extends to 1 and even sometimes 2 weeks, then the test is not able to meaningfully guide clinical decisions. The latest guidance from the CDC is that individuals with symptoms suggestive of COVID who do not require hospitalization should remain at home in self-quarantine for at least 10 days from symptom onset. Asymptomatic individuals with a known exposure to someone else with COVID, or participation in a high-risk event like an indoor gathering involving more than 10 persons, should self-quarantine either until they receive a negative test result or 14 days after the exposure.
Accuracy of results. A positive rRT-PCR antigen test is highly accurate, indicating presence of SARS-CoV-2 RNA. There appears to be no significant cross-reactivity with other respiratory viruses or even other coronaviruses. A small study conducted in Korea suggests that patients with persistent positive tests who are beyond 10 days from the initial positive test and are now symptom free are no longer infectious.
For patients with a high suspicion of COVID-19, a negative test should not rule out the infection. The number of false-negative results is not well known, though the resultant risk is “substantial.” A number of factors affect the likelihood of a false-negative test, including when the sample was collected relative to the timing of illness and the type of specimen collected; for example, nasopharyngeal swabs are more likely to be accurate vs nasal or throat specimens. Repeat or serial testing increases the sensitivity but may not always be available. Although rRT-PCR is the current criterion standard, more inclusive consensus-based criteria are likely to emerge because of the concern about these false-negative results.
This article first appeared on Medscape.com.
Cutaneous clues linked to COVID-19 coagulation risk
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
FROM JAMA DERMATOLOGY
When you see something ...
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Cardiorespiratory fitness may alter AFib ablation outcomes
Higher baseline cardiorespiratory fitness (CRF) is associated with better outcomes after atrial fibrillation (AFib) ablation, according to new research.
In a single-center, retrospective cohort study, patients with the highest level of baseline CRF had significantly lower rates of arrhythmia recurrence and death than did patients with lower levels of CRF.
“It is stunning how just a simple measure, in this case walking on a treadmill, can predict whether atrial fibrillation ablation will be a successful endeavor or if it will fail,” senior author Wael A. Jaber, MD, professor of medicine, Cleveland Clinic, said in an interview.
“We found that ablation was not successful in most patients who had poor functional class and, conversely, that it was successful in most patients who were in tip-top shape when they walked on the treadmill. Our results can help clinicians inform patients about what they can expect after the procedure, depending on the baseline fitness level,” Dr. Jaber said.
The study was published online Aug. 2 in Heart Rhythm.
Several studies have shown a reduction in AFib incidence among individuals who report a physically active lifestyle, but the extent to which baseline CRF influences arrhythmia rates after AFib ablation is unknown, the authors note.
For the study, Dr. Jaber and colleagues analyzed results in 591 consecutive patients (mean age, 66.5 years; 75% male) with symptomatic paroxysmal or persistent AFib who underwent de novo AFib ablation at their institution. Only patients who had undergone an exercise stress test in the 12 months before AFib ablation (average, 4.5 months) were included.
Age- and sex-specific predicted metabolic equivalents (METs) were calculated using the St. James model for women and the Veterans Affairs referral model for men. The number of METs achieved was then divided by the predicted METs, and the patients were categorized into low (<85% predicted; n = 152), adequate (85%-100% predicted; n = 115), and high (>100% predicted; n = 324) CRF groups. Functional capacity was characterized as poor in 56 patients (9.5%), fair in 94 (16.0%), average in 225 (38.1%), good in 169 (28.6%), and high in 47 (8.0%).
During a mean follow-up of 32 months, arrhythmia recurrence was observed in 79% of patients in the low-CRF group, 54% of patients in the adequate-CRF group, and 27.5% of patients in the high-CRF group (P < .0001). Rates of repeat arrhythmia-related hospitalization, repeat rhythm-control procedures, and the need for ongoing antiarrhythmic therapy (ATT) were significantly lower in the high-CRF group. Specifically, ATT was stopped in 56% of patients in the high-CRF group, compared with 24% in the adequate-CRF group and 11% in the low-CRF group (P < .0001). Rehospitalization for arrhythmia was required in 18.5%, 38.0%, and 60.5% of cases, respectively, and repeat direct-current cardioversion or ablation was performed in 26.0%, 49.0%, and 65.0%, respectively (P < .0001 for both).
Death occurred in 11% of the low-CRF group, compared with 4% in the adequate-CRF group and 2.5% in the high-CRF group. Most (70%) of the deaths were caused by cardiovascular events, including heart failure, cardiac arrest, and coronary artery disease. The most common cause of noncardiac death was respiratory failure (13%), followed by sepsis (10%), malignancy (3%), and complications of Parkinson’s disease (3%).
“Although there was a statistically significant association between higher CRF and lower mortality in this cohort, the findings are to be viewed through the prism of a small sample size and relatively low death rate,” the authors wrote.
Don’t “overpromise” results
“The important message for clinicians is that when, you are discussing what to expect after atrial fibrillation ablation with your patients, do not overpromise the results. You can inform them that the success of the procedure depends more on how they perform on the baseline exercise test, and less on the ablation itself,” Dr. Jaber said.
Clinicians might want to consider advising their patients to become more active and increase their fitness level before undergoing the procedure, but whether doing so will improve outcomes is still unknown.
“This is what we don’t know. It makes sense. Hopefully, our results will encourage people to be more active before they arrive here for the procedure,” he said. “Our study is retrospective and is hypothesis generating, but we are planning a prospective study where patients will be referred to cardiac rehab prior to having ablation to try to improve their functional class to see if this will improve outcomes.”
Survival of the fittest
In an accompanying editorial commentary, Eric Black-Maier, MD, and Jonathan P. Piccini Sr, MD, from Duke University Medical Center, Durham, N.C., wrote that the findings have “important implications for clinical practice and raise important additional questions.”
They note that catheter ablation as a first-line rhythm-control strategy, per current recommendations, “seems reasonable” in individuals with high baseline cardiorespiratory fitness, but that the benefit is less clear for patients with poor baseline CRF and uncontrolled risk factors.
“Significant limitations in functional status may be at least partially attributable to uncontrolled [AFib], and patients with limited exercise capacity may stand to gain most from successful catheter ablation,” the editorialists wrote.
“Furthermore, because shorter time from [AFib] diagnosis to catheter ablation has been associated with improved outcomes, the decision to postpone ablation in favor of lifestyle modification is not without potential adverse consequences,” they added.
Dr. Black-Maier and Dr. Piccini agree with the need for additional prospective randomized clinical trials to confirm that exercise training to improve cardiorespiratory fitness before AFib ablation is practical and effective for reducing arrhythmia recurrence.
“Over the past 50-plus years, our understanding of cardiorespiratory fitness, exercise capacity, and arrhythmia occurrence in patients with [AFib] continues to evolve,” the editorialists concluded. Data from the study “clearly demonstrate that arrhythmia-free survival is indeed survival of the fittest. Time will tell if exercise training and improvements in cardiorespiratory fitness can change outcomes after ablation.”
The study was sponsored by the Cleveland Clinic. Dr. Jaber and Dr. Black-Maier report no relevant financial relationships. Dr. Piccini receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and UpToDate.
A version of this story originally appeared on Medscape.com.
Higher baseline cardiorespiratory fitness (CRF) is associated with better outcomes after atrial fibrillation (AFib) ablation, according to new research.
In a single-center, retrospective cohort study, patients with the highest level of baseline CRF had significantly lower rates of arrhythmia recurrence and death than did patients with lower levels of CRF.
“It is stunning how just a simple measure, in this case walking on a treadmill, can predict whether atrial fibrillation ablation will be a successful endeavor or if it will fail,” senior author Wael A. Jaber, MD, professor of medicine, Cleveland Clinic, said in an interview.
“We found that ablation was not successful in most patients who had poor functional class and, conversely, that it was successful in most patients who were in tip-top shape when they walked on the treadmill. Our results can help clinicians inform patients about what they can expect after the procedure, depending on the baseline fitness level,” Dr. Jaber said.
The study was published online Aug. 2 in Heart Rhythm.
Several studies have shown a reduction in AFib incidence among individuals who report a physically active lifestyle, but the extent to which baseline CRF influences arrhythmia rates after AFib ablation is unknown, the authors note.
For the study, Dr. Jaber and colleagues analyzed results in 591 consecutive patients (mean age, 66.5 years; 75% male) with symptomatic paroxysmal or persistent AFib who underwent de novo AFib ablation at their institution. Only patients who had undergone an exercise stress test in the 12 months before AFib ablation (average, 4.5 months) were included.
Age- and sex-specific predicted metabolic equivalents (METs) were calculated using the St. James model for women and the Veterans Affairs referral model for men. The number of METs achieved was then divided by the predicted METs, and the patients were categorized into low (<85% predicted; n = 152), adequate (85%-100% predicted; n = 115), and high (>100% predicted; n = 324) CRF groups. Functional capacity was characterized as poor in 56 patients (9.5%), fair in 94 (16.0%), average in 225 (38.1%), good in 169 (28.6%), and high in 47 (8.0%).
During a mean follow-up of 32 months, arrhythmia recurrence was observed in 79% of patients in the low-CRF group, 54% of patients in the adequate-CRF group, and 27.5% of patients in the high-CRF group (P < .0001). Rates of repeat arrhythmia-related hospitalization, repeat rhythm-control procedures, and the need for ongoing antiarrhythmic therapy (ATT) were significantly lower in the high-CRF group. Specifically, ATT was stopped in 56% of patients in the high-CRF group, compared with 24% in the adequate-CRF group and 11% in the low-CRF group (P < .0001). Rehospitalization for arrhythmia was required in 18.5%, 38.0%, and 60.5% of cases, respectively, and repeat direct-current cardioversion or ablation was performed in 26.0%, 49.0%, and 65.0%, respectively (P < .0001 for both).
Death occurred in 11% of the low-CRF group, compared with 4% in the adequate-CRF group and 2.5% in the high-CRF group. Most (70%) of the deaths were caused by cardiovascular events, including heart failure, cardiac arrest, and coronary artery disease. The most common cause of noncardiac death was respiratory failure (13%), followed by sepsis (10%), malignancy (3%), and complications of Parkinson’s disease (3%).
“Although there was a statistically significant association between higher CRF and lower mortality in this cohort, the findings are to be viewed through the prism of a small sample size and relatively low death rate,” the authors wrote.
Don’t “overpromise” results
“The important message for clinicians is that when, you are discussing what to expect after atrial fibrillation ablation with your patients, do not overpromise the results. You can inform them that the success of the procedure depends more on how they perform on the baseline exercise test, and less on the ablation itself,” Dr. Jaber said.
Clinicians might want to consider advising their patients to become more active and increase their fitness level before undergoing the procedure, but whether doing so will improve outcomes is still unknown.
“This is what we don’t know. It makes sense. Hopefully, our results will encourage people to be more active before they arrive here for the procedure,” he said. “Our study is retrospective and is hypothesis generating, but we are planning a prospective study where patients will be referred to cardiac rehab prior to having ablation to try to improve their functional class to see if this will improve outcomes.”
Survival of the fittest
In an accompanying editorial commentary, Eric Black-Maier, MD, and Jonathan P. Piccini Sr, MD, from Duke University Medical Center, Durham, N.C., wrote that the findings have “important implications for clinical practice and raise important additional questions.”
They note that catheter ablation as a first-line rhythm-control strategy, per current recommendations, “seems reasonable” in individuals with high baseline cardiorespiratory fitness, but that the benefit is less clear for patients with poor baseline CRF and uncontrolled risk factors.
“Significant limitations in functional status may be at least partially attributable to uncontrolled [AFib], and patients with limited exercise capacity may stand to gain most from successful catheter ablation,” the editorialists wrote.
“Furthermore, because shorter time from [AFib] diagnosis to catheter ablation has been associated with improved outcomes, the decision to postpone ablation in favor of lifestyle modification is not without potential adverse consequences,” they added.
Dr. Black-Maier and Dr. Piccini agree with the need for additional prospective randomized clinical trials to confirm that exercise training to improve cardiorespiratory fitness before AFib ablation is practical and effective for reducing arrhythmia recurrence.
“Over the past 50-plus years, our understanding of cardiorespiratory fitness, exercise capacity, and arrhythmia occurrence in patients with [AFib] continues to evolve,” the editorialists concluded. Data from the study “clearly demonstrate that arrhythmia-free survival is indeed survival of the fittest. Time will tell if exercise training and improvements in cardiorespiratory fitness can change outcomes after ablation.”
The study was sponsored by the Cleveland Clinic. Dr. Jaber and Dr. Black-Maier report no relevant financial relationships. Dr. Piccini receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and UpToDate.
A version of this story originally appeared on Medscape.com.
Higher baseline cardiorespiratory fitness (CRF) is associated with better outcomes after atrial fibrillation (AFib) ablation, according to new research.
In a single-center, retrospective cohort study, patients with the highest level of baseline CRF had significantly lower rates of arrhythmia recurrence and death than did patients with lower levels of CRF.
“It is stunning how just a simple measure, in this case walking on a treadmill, can predict whether atrial fibrillation ablation will be a successful endeavor or if it will fail,” senior author Wael A. Jaber, MD, professor of medicine, Cleveland Clinic, said in an interview.
“We found that ablation was not successful in most patients who had poor functional class and, conversely, that it was successful in most patients who were in tip-top shape when they walked on the treadmill. Our results can help clinicians inform patients about what they can expect after the procedure, depending on the baseline fitness level,” Dr. Jaber said.
The study was published online Aug. 2 in Heart Rhythm.
Several studies have shown a reduction in AFib incidence among individuals who report a physically active lifestyle, but the extent to which baseline CRF influences arrhythmia rates after AFib ablation is unknown, the authors note.
For the study, Dr. Jaber and colleagues analyzed results in 591 consecutive patients (mean age, 66.5 years; 75% male) with symptomatic paroxysmal or persistent AFib who underwent de novo AFib ablation at their institution. Only patients who had undergone an exercise stress test in the 12 months before AFib ablation (average, 4.5 months) were included.
Age- and sex-specific predicted metabolic equivalents (METs) were calculated using the St. James model for women and the Veterans Affairs referral model for men. The number of METs achieved was then divided by the predicted METs, and the patients were categorized into low (<85% predicted; n = 152), adequate (85%-100% predicted; n = 115), and high (>100% predicted; n = 324) CRF groups. Functional capacity was characterized as poor in 56 patients (9.5%), fair in 94 (16.0%), average in 225 (38.1%), good in 169 (28.6%), and high in 47 (8.0%).
During a mean follow-up of 32 months, arrhythmia recurrence was observed in 79% of patients in the low-CRF group, 54% of patients in the adequate-CRF group, and 27.5% of patients in the high-CRF group (P < .0001). Rates of repeat arrhythmia-related hospitalization, repeat rhythm-control procedures, and the need for ongoing antiarrhythmic therapy (ATT) were significantly lower in the high-CRF group. Specifically, ATT was stopped in 56% of patients in the high-CRF group, compared with 24% in the adequate-CRF group and 11% in the low-CRF group (P < .0001). Rehospitalization for arrhythmia was required in 18.5%, 38.0%, and 60.5% of cases, respectively, and repeat direct-current cardioversion or ablation was performed in 26.0%, 49.0%, and 65.0%, respectively (P < .0001 for both).
Death occurred in 11% of the low-CRF group, compared with 4% in the adequate-CRF group and 2.5% in the high-CRF group. Most (70%) of the deaths were caused by cardiovascular events, including heart failure, cardiac arrest, and coronary artery disease. The most common cause of noncardiac death was respiratory failure (13%), followed by sepsis (10%), malignancy (3%), and complications of Parkinson’s disease (3%).
“Although there was a statistically significant association between higher CRF and lower mortality in this cohort, the findings are to be viewed through the prism of a small sample size and relatively low death rate,” the authors wrote.
Don’t “overpromise” results
“The important message for clinicians is that when, you are discussing what to expect after atrial fibrillation ablation with your patients, do not overpromise the results. You can inform them that the success of the procedure depends more on how they perform on the baseline exercise test, and less on the ablation itself,” Dr. Jaber said.
Clinicians might want to consider advising their patients to become more active and increase their fitness level before undergoing the procedure, but whether doing so will improve outcomes is still unknown.
“This is what we don’t know. It makes sense. Hopefully, our results will encourage people to be more active before they arrive here for the procedure,” he said. “Our study is retrospective and is hypothesis generating, but we are planning a prospective study where patients will be referred to cardiac rehab prior to having ablation to try to improve their functional class to see if this will improve outcomes.”
Survival of the fittest
In an accompanying editorial commentary, Eric Black-Maier, MD, and Jonathan P. Piccini Sr, MD, from Duke University Medical Center, Durham, N.C., wrote that the findings have “important implications for clinical practice and raise important additional questions.”
They note that catheter ablation as a first-line rhythm-control strategy, per current recommendations, “seems reasonable” in individuals with high baseline cardiorespiratory fitness, but that the benefit is less clear for patients with poor baseline CRF and uncontrolled risk factors.
“Significant limitations in functional status may be at least partially attributable to uncontrolled [AFib], and patients with limited exercise capacity may stand to gain most from successful catheter ablation,” the editorialists wrote.
“Furthermore, because shorter time from [AFib] diagnosis to catheter ablation has been associated with improved outcomes, the decision to postpone ablation in favor of lifestyle modification is not without potential adverse consequences,” they added.
Dr. Black-Maier and Dr. Piccini agree with the need for additional prospective randomized clinical trials to confirm that exercise training to improve cardiorespiratory fitness before AFib ablation is practical and effective for reducing arrhythmia recurrence.
“Over the past 50-plus years, our understanding of cardiorespiratory fitness, exercise capacity, and arrhythmia occurrence in patients with [AFib] continues to evolve,” the editorialists concluded. Data from the study “clearly demonstrate that arrhythmia-free survival is indeed survival of the fittest. Time will tell if exercise training and improvements in cardiorespiratory fitness can change outcomes after ablation.”
The study was sponsored by the Cleveland Clinic. Dr. Jaber and Dr. Black-Maier report no relevant financial relationships. Dr. Piccini receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and UpToDate.
A version of this story originally appeared on Medscape.com.
SGLT2 inhibitors have a breakout year
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
AHA on cannabis: No evidence of heart benefits, but potential harms
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
FROM CIRCULATION