User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
How Lp(a) can help improve ASCVD risk assessment
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).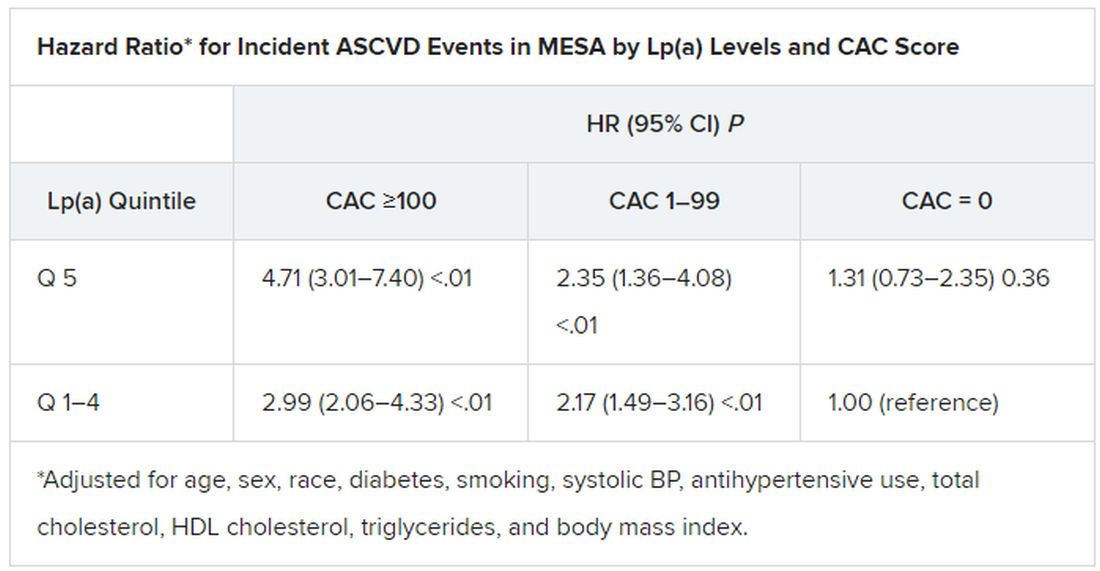
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).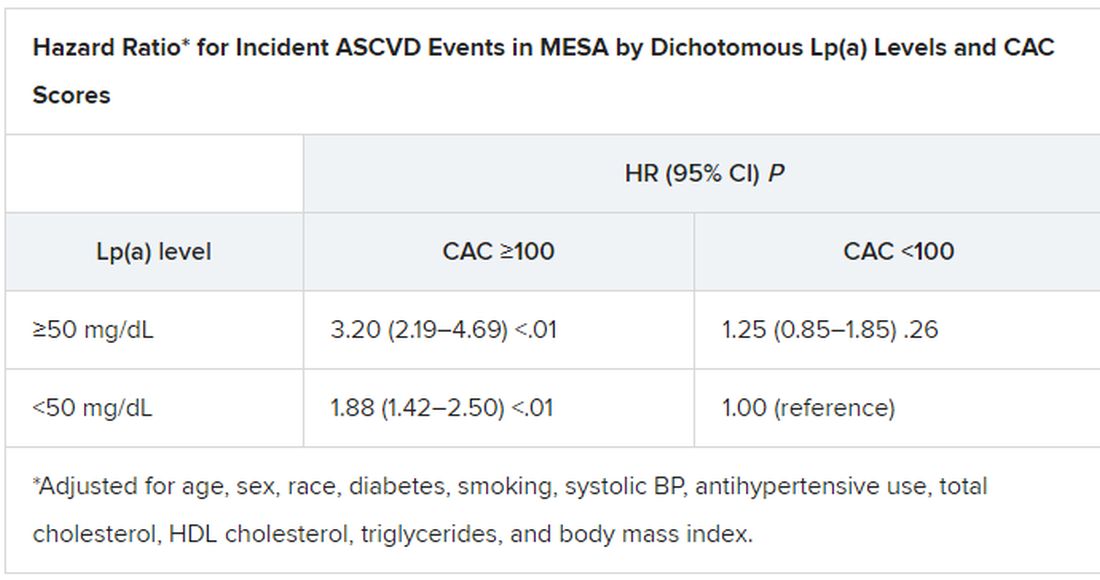
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
New data explore risk of magnetic interference with implantable devices
Building on several previous reports that the newest models of mobile telephones and other electronics that use magnets pose a threat to the function of defibrillators and other implantable cardiovascular devices, a new study implicates any device that emits a 10-gauss (G) magnetic field more than a couple of inches.
“Beside the devices described in our manuscript, this can be any portable consumer product [with magnets] like electric cigarettes or smart watches,” explained study author Sven Knecht, DSc, a research electrophysiologist associated with the department of cardiology, University Hospital Basel (Switzerland).
In the newly published article, the investigators evaluated earphones, earphone charging cases, and two electronic pens used to draw on electronic tablets. These particular devices are of interest because, like mobile phones, they are of a size and shape to fit in a breast pocket adjacent to where many cardiovascular devices are implanted.
The study joins several previous studies that have shown the same risk, but this study used three-dimensional (3D) mapping of the magnetic field rather than a one-axis sensor, which is a standard adopted by the U.S. Food and Drug Administration, according to the investigators.
3D mapping assessment used
Because of the 3D nature of magnetic fields, 3D mapping serves as a better tool to assess the risk of the magnetic force as the intensity gradient diminishes with distance from the source, the authors contended. The 3D maps used in this study have a resolution to 2 mm.
The ex vivo measurements of the magnetic field, which could be displayed in a configurable 3D volume in relation to the electronic products were performed on five different explanted cardioverter defibrillators from two manufacturers.
In the ex vivo setting, the ability of the earphones, earphone charging cases, and electronic pens to interfere with defibrillator function was compared to that of the Apple iPhone 12 Max, which was the subject of a small in vivo study published in 2021. When the iPhone 12 Max was placed on the skin over a cardiac implantable device in that study, clinically identifiable interference could be detected in all 3 patients evaluated.
Based on previous work, the International Organization for Standardization has established that a minimal field strength of 10 G is needed to interfere with an implantable device, but the actual risk from any specific device is determined by the distance at which this strength of magnetic field is projected.
In the 3D analysis, the 10-G intensity was found to project 20 mm from the surface of the ear phones, ear phone charging case, and one of the electronic pens and to project 29 mm from the other electronic pen. When tested against the five defibrillators, magnetic reversion mode was triggered by the portable electronics at distances ranging from 8 to 18 mm.
In an interview, Dr. Knecht explained that this study adds more devices to the list of those associated with potential for interfering with implantable cardiovascular devices, but added that the more important point is that any device that contains magnets emitting a force of 10 G or greater for more than a few inches can be expected to be associated with clinically meaningful interference. The devices tested in this study were produced by Apple and Microsoft, but a focus on specific devices obscures the main message.
“All portable electronics with an embedded permanent magnet creating a 10-G magnetic field have a theoretical capability of triggering implantable devices,” he said.
For pacemakers, the interference is likely to trigger constant pacing, which would not be expected to pose a significant health threat if detected with a reasonable period, according to Dr. Knecht. Interference is potentially more serious for defibrillators, which might fail during magnetic interference to provide the shock needed to terminate a serious arrhythmia.
The combination of events – interference at the time of an arrhythmia – make this risk “very low,” but Dr. Knecht said it is sufficient to mean that patients receiving an implantable cardiovascular device should be made aware of the risk and the need to avoid placing portable electronic products near the implanted device.
When in vivo evidence of a disturbance with the iPhone 12 was reported in 2021, it amplified existing concern. The American Heart Association maintains a list of electronic products with the potential to interfere with implantable devices on its website. But, again, understanding the potential for risk and the need to keep electronic products with magnets at a safe distance from cardiovascular implantable devices is more important than trying to memorize the ever-growing list of devices with this capability.
“Prudent education of patients receiving an implantable device is important,” said N.A. Mark Estes III, MD, professor of medicine in the division of cardiology at the University of Pittsburgh. However, in an interview, he warned that the growing list of implicated devices makes a complete survey impractical, and, even if achievable, likely to leave patients “feeling overwhelmed.”
In Dr. Estes’s practice, he does provide printed information about the risks of electronics to interfere with implantable devices as well as a list of dos and don’ts. He agreed that the absolute risk of interference from a device causing significant clinical complications is low, but the goal is to “bring it as close to zero as possible.”
“No clinical case of a meaningful interaction of an electronic product and dysfunction of an implantable device has ever been documented,” he said. Given the widespread use of the new generation of cellphones that contain magnets powerful enough to induce dysfunction in an implantable device, “this speaks to the fact that the risk continues to be very low.”
Dr. Knecht and coinvestigators, along with Dr. Estes, reported no potential conflicts of interest.
Building on several previous reports that the newest models of mobile telephones and other electronics that use magnets pose a threat to the function of defibrillators and other implantable cardiovascular devices, a new study implicates any device that emits a 10-gauss (G) magnetic field more than a couple of inches.
“Beside the devices described in our manuscript, this can be any portable consumer product [with magnets] like electric cigarettes or smart watches,” explained study author Sven Knecht, DSc, a research electrophysiologist associated with the department of cardiology, University Hospital Basel (Switzerland).
In the newly published article, the investigators evaluated earphones, earphone charging cases, and two electronic pens used to draw on electronic tablets. These particular devices are of interest because, like mobile phones, they are of a size and shape to fit in a breast pocket adjacent to where many cardiovascular devices are implanted.
The study joins several previous studies that have shown the same risk, but this study used three-dimensional (3D) mapping of the magnetic field rather than a one-axis sensor, which is a standard adopted by the U.S. Food and Drug Administration, according to the investigators.
3D mapping assessment used
Because of the 3D nature of magnetic fields, 3D mapping serves as a better tool to assess the risk of the magnetic force as the intensity gradient diminishes with distance from the source, the authors contended. The 3D maps used in this study have a resolution to 2 mm.
The ex vivo measurements of the magnetic field, which could be displayed in a configurable 3D volume in relation to the electronic products were performed on five different explanted cardioverter defibrillators from two manufacturers.
In the ex vivo setting, the ability of the earphones, earphone charging cases, and electronic pens to interfere with defibrillator function was compared to that of the Apple iPhone 12 Max, which was the subject of a small in vivo study published in 2021. When the iPhone 12 Max was placed on the skin over a cardiac implantable device in that study, clinically identifiable interference could be detected in all 3 patients evaluated.
Based on previous work, the International Organization for Standardization has established that a minimal field strength of 10 G is needed to interfere with an implantable device, but the actual risk from any specific device is determined by the distance at which this strength of magnetic field is projected.
In the 3D analysis, the 10-G intensity was found to project 20 mm from the surface of the ear phones, ear phone charging case, and one of the electronic pens and to project 29 mm from the other electronic pen. When tested against the five defibrillators, magnetic reversion mode was triggered by the portable electronics at distances ranging from 8 to 18 mm.
In an interview, Dr. Knecht explained that this study adds more devices to the list of those associated with potential for interfering with implantable cardiovascular devices, but added that the more important point is that any device that contains magnets emitting a force of 10 G or greater for more than a few inches can be expected to be associated with clinically meaningful interference. The devices tested in this study were produced by Apple and Microsoft, but a focus on specific devices obscures the main message.
“All portable electronics with an embedded permanent magnet creating a 10-G magnetic field have a theoretical capability of triggering implantable devices,” he said.
For pacemakers, the interference is likely to trigger constant pacing, which would not be expected to pose a significant health threat if detected with a reasonable period, according to Dr. Knecht. Interference is potentially more serious for defibrillators, which might fail during magnetic interference to provide the shock needed to terminate a serious arrhythmia.
The combination of events – interference at the time of an arrhythmia – make this risk “very low,” but Dr. Knecht said it is sufficient to mean that patients receiving an implantable cardiovascular device should be made aware of the risk and the need to avoid placing portable electronic products near the implanted device.
When in vivo evidence of a disturbance with the iPhone 12 was reported in 2021, it amplified existing concern. The American Heart Association maintains a list of electronic products with the potential to interfere with implantable devices on its website. But, again, understanding the potential for risk and the need to keep electronic products with magnets at a safe distance from cardiovascular implantable devices is more important than trying to memorize the ever-growing list of devices with this capability.
“Prudent education of patients receiving an implantable device is important,” said N.A. Mark Estes III, MD, professor of medicine in the division of cardiology at the University of Pittsburgh. However, in an interview, he warned that the growing list of implicated devices makes a complete survey impractical, and, even if achievable, likely to leave patients “feeling overwhelmed.”
In Dr. Estes’s practice, he does provide printed information about the risks of electronics to interfere with implantable devices as well as a list of dos and don’ts. He agreed that the absolute risk of interference from a device causing significant clinical complications is low, but the goal is to “bring it as close to zero as possible.”
“No clinical case of a meaningful interaction of an electronic product and dysfunction of an implantable device has ever been documented,” he said. Given the widespread use of the new generation of cellphones that contain magnets powerful enough to induce dysfunction in an implantable device, “this speaks to the fact that the risk continues to be very low.”
Dr. Knecht and coinvestigators, along with Dr. Estes, reported no potential conflicts of interest.
Building on several previous reports that the newest models of mobile telephones and other electronics that use magnets pose a threat to the function of defibrillators and other implantable cardiovascular devices, a new study implicates any device that emits a 10-gauss (G) magnetic field more than a couple of inches.
“Beside the devices described in our manuscript, this can be any portable consumer product [with magnets] like electric cigarettes or smart watches,” explained study author Sven Knecht, DSc, a research electrophysiologist associated with the department of cardiology, University Hospital Basel (Switzerland).
In the newly published article, the investigators evaluated earphones, earphone charging cases, and two electronic pens used to draw on electronic tablets. These particular devices are of interest because, like mobile phones, they are of a size and shape to fit in a breast pocket adjacent to where many cardiovascular devices are implanted.
The study joins several previous studies that have shown the same risk, but this study used three-dimensional (3D) mapping of the magnetic field rather than a one-axis sensor, which is a standard adopted by the U.S. Food and Drug Administration, according to the investigators.
3D mapping assessment used
Because of the 3D nature of magnetic fields, 3D mapping serves as a better tool to assess the risk of the magnetic force as the intensity gradient diminishes with distance from the source, the authors contended. The 3D maps used in this study have a resolution to 2 mm.
The ex vivo measurements of the magnetic field, which could be displayed in a configurable 3D volume in relation to the electronic products were performed on five different explanted cardioverter defibrillators from two manufacturers.
In the ex vivo setting, the ability of the earphones, earphone charging cases, and electronic pens to interfere with defibrillator function was compared to that of the Apple iPhone 12 Max, which was the subject of a small in vivo study published in 2021. When the iPhone 12 Max was placed on the skin over a cardiac implantable device in that study, clinically identifiable interference could be detected in all 3 patients evaluated.
Based on previous work, the International Organization for Standardization has established that a minimal field strength of 10 G is needed to interfere with an implantable device, but the actual risk from any specific device is determined by the distance at which this strength of magnetic field is projected.
In the 3D analysis, the 10-G intensity was found to project 20 mm from the surface of the ear phones, ear phone charging case, and one of the electronic pens and to project 29 mm from the other electronic pen. When tested against the five defibrillators, magnetic reversion mode was triggered by the portable electronics at distances ranging from 8 to 18 mm.
In an interview, Dr. Knecht explained that this study adds more devices to the list of those associated with potential for interfering with implantable cardiovascular devices, but added that the more important point is that any device that contains magnets emitting a force of 10 G or greater for more than a few inches can be expected to be associated with clinically meaningful interference. The devices tested in this study were produced by Apple and Microsoft, but a focus on specific devices obscures the main message.
“All portable electronics with an embedded permanent magnet creating a 10-G magnetic field have a theoretical capability of triggering implantable devices,” he said.
For pacemakers, the interference is likely to trigger constant pacing, which would not be expected to pose a significant health threat if detected with a reasonable period, according to Dr. Knecht. Interference is potentially more serious for defibrillators, which might fail during magnetic interference to provide the shock needed to terminate a serious arrhythmia.
The combination of events – interference at the time of an arrhythmia – make this risk “very low,” but Dr. Knecht said it is sufficient to mean that patients receiving an implantable cardiovascular device should be made aware of the risk and the need to avoid placing portable electronic products near the implanted device.
When in vivo evidence of a disturbance with the iPhone 12 was reported in 2021, it amplified existing concern. The American Heart Association maintains a list of electronic products with the potential to interfere with implantable devices on its website. But, again, understanding the potential for risk and the need to keep electronic products with magnets at a safe distance from cardiovascular implantable devices is more important than trying to memorize the ever-growing list of devices with this capability.
“Prudent education of patients receiving an implantable device is important,” said N.A. Mark Estes III, MD, professor of medicine in the division of cardiology at the University of Pittsburgh. However, in an interview, he warned that the growing list of implicated devices makes a complete survey impractical, and, even if achievable, likely to leave patients “feeling overwhelmed.”
In Dr. Estes’s practice, he does provide printed information about the risks of electronics to interfere with implantable devices as well as a list of dos and don’ts. He agreed that the absolute risk of interference from a device causing significant clinical complications is low, but the goal is to “bring it as close to zero as possible.”
“No clinical case of a meaningful interaction of an electronic product and dysfunction of an implantable device has ever been documented,” he said. Given the widespread use of the new generation of cellphones that contain magnets powerful enough to induce dysfunction in an implantable device, “this speaks to the fact that the risk continues to be very low.”
Dr. Knecht and coinvestigators, along with Dr. Estes, reported no potential conflicts of interest.
FROM CIRCULATION: ARRHYTHMIAS & ELECTROPHYSIOLOGY
Legionnaires’ disease shows steady increase in U.S. over 15+ years
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Autoantibodies may underpin clotting effects of COVID-19
Circulating antiphospholipid autoantibodies may contribute to endothelial cell activation and dysfunction in severe COVID-19, researchers report.
In 2020, the same researchers reported results from a preclinical study demonstrating that autoantibodies from patients with active COVID-19 caused clotting in mice.
The new study, published in Arthritis and Rheumatology, found higher-than-expected levels of antiphospholipid autoantibodies in the blood samples of 244 patients hospitalized with COVID-19.
“While endothelial dysfunction has been implicated in the widespread thromboinflammatory complications of COVID-19, the upstream mediators of endotheliopathy remain for the most part cryptic,” write Hui Shi, MD, PhD, and coauthors from the University of Michigan, Ann Arbor, and the National Heart, Lung, and Blood Institute.
When asked for comment on the study, Eline T. Luning Prak, MD, PhD, professor of pathology and laboratory medicine at the Hospital of the University of Pennsylvania in Philadelphia, said, “The autopsy cases for COVID-19 strongly point to thromboembolic complications in many individuals who succumbed to sequelae of the infection.
“Importantly, however, many factors can contribute to this pathology, including the inflammatory milieu, monocyte activation, neutrophil extracellular traps, immune complexes, complement, as well as effects on endothelial cells,” explained Dr. Luning Prak, who was not involved in the study.
“The findings in this paper nicely complement another study by Schmaier et al. that came out recently in JCI Insight that also suggests that endothelial cells can be activated by antibodies, she said.
‘Even stronger connection between autoantibody formation and clotting in COVID-19’
Dr. Shi and her team cultured human endothelial cells in serum or plasma from 244 patients hospitalized with COVID-19 and plasma from 100 patients with non-COVID sepsis. Using in-cell enzyme-linked immunosorbent assay, they measured levels of key cell adhesion molecules.
After analysis, the researchers found that serum from COVID-19 patients activated cultured endothelial cells to express surface adhesion molecules essential to inflammation and thrombosis, particularly E-selectin, ICAM-1, and VCAM-1.
“The presence of circulating antiphospholipid antibodies was a strong marker of the ability of COVID-19 serum to activate endothelium,” they explain.
Further analyses revealed that, for a subset of serum samples from patients with severe infection, this activation could be mitigated by depleting total immunoglobulin G.
In addition, supplementation of control serum with patient IgG was adequate to trigger endothelial activation.
On the basis of these results, the researchers hypothesize that antiphospholipid autoantibodies may characterize antibody profiles in severe COVID-19 that activate the endothelium and transition the usually quiescent blood-vessel wall interface toward inflammation and coagulation.
“[These findings] provide an even stronger connection between autoantibody formation and clotting in COVID-19,” Dr. Shi said in an accompanying press release.
Clinical implications
From a clinical perspective, Dr. Shi and her team question whether patients with severe COVID-19 should be tested for antiphospholipid antibodies to assess their risk of thrombosis and progression to respiratory failure.
Moreover, they question whether patients with high antiphospholipid antibody titers might benefit from therapies used in conventional cases of severe antiphospholipid syndrome, such as plasmapheresis, anticoagulation therapy, and complement inhibition, Dr. Shi added.
The researchers hope to answer these and other remaining questions in future studies. “Eventually, we may be able to repurpose treatments used in traditional cases of antiphospholipid syndrome for COVID-19.
“As we await definitive solutions to the pandemic, these findings add important context to the complex interplay between SARS-CoV-2 infection, the human immune system, and vascular immunobiology,” she concluded.
The study was supported by grants from the Rheumatology Research Foundation, the Michigan Medicine Frankel Cardiovascular Center, and the A. Alfred Taubman Medical Research Institute. One author is an inventor on an unrelated pending patent to the University of Michigan. The other authors and Dr. Luning Prak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Circulating antiphospholipid autoantibodies may contribute to endothelial cell activation and dysfunction in severe COVID-19, researchers report.
In 2020, the same researchers reported results from a preclinical study demonstrating that autoantibodies from patients with active COVID-19 caused clotting in mice.
The new study, published in Arthritis and Rheumatology, found higher-than-expected levels of antiphospholipid autoantibodies in the blood samples of 244 patients hospitalized with COVID-19.
“While endothelial dysfunction has been implicated in the widespread thromboinflammatory complications of COVID-19, the upstream mediators of endotheliopathy remain for the most part cryptic,” write Hui Shi, MD, PhD, and coauthors from the University of Michigan, Ann Arbor, and the National Heart, Lung, and Blood Institute.
When asked for comment on the study, Eline T. Luning Prak, MD, PhD, professor of pathology and laboratory medicine at the Hospital of the University of Pennsylvania in Philadelphia, said, “The autopsy cases for COVID-19 strongly point to thromboembolic complications in many individuals who succumbed to sequelae of the infection.
“Importantly, however, many factors can contribute to this pathology, including the inflammatory milieu, monocyte activation, neutrophil extracellular traps, immune complexes, complement, as well as effects on endothelial cells,” explained Dr. Luning Prak, who was not involved in the study.
“The findings in this paper nicely complement another study by Schmaier et al. that came out recently in JCI Insight that also suggests that endothelial cells can be activated by antibodies, she said.
‘Even stronger connection between autoantibody formation and clotting in COVID-19’
Dr. Shi and her team cultured human endothelial cells in serum or plasma from 244 patients hospitalized with COVID-19 and plasma from 100 patients with non-COVID sepsis. Using in-cell enzyme-linked immunosorbent assay, they measured levels of key cell adhesion molecules.
After analysis, the researchers found that serum from COVID-19 patients activated cultured endothelial cells to express surface adhesion molecules essential to inflammation and thrombosis, particularly E-selectin, ICAM-1, and VCAM-1.
“The presence of circulating antiphospholipid antibodies was a strong marker of the ability of COVID-19 serum to activate endothelium,” they explain.
Further analyses revealed that, for a subset of serum samples from patients with severe infection, this activation could be mitigated by depleting total immunoglobulin G.
In addition, supplementation of control serum with patient IgG was adequate to trigger endothelial activation.
On the basis of these results, the researchers hypothesize that antiphospholipid autoantibodies may characterize antibody profiles in severe COVID-19 that activate the endothelium and transition the usually quiescent blood-vessel wall interface toward inflammation and coagulation.
“[These findings] provide an even stronger connection between autoantibody formation and clotting in COVID-19,” Dr. Shi said in an accompanying press release.
Clinical implications
From a clinical perspective, Dr. Shi and her team question whether patients with severe COVID-19 should be tested for antiphospholipid antibodies to assess their risk of thrombosis and progression to respiratory failure.
Moreover, they question whether patients with high antiphospholipid antibody titers might benefit from therapies used in conventional cases of severe antiphospholipid syndrome, such as plasmapheresis, anticoagulation therapy, and complement inhibition, Dr. Shi added.
The researchers hope to answer these and other remaining questions in future studies. “Eventually, we may be able to repurpose treatments used in traditional cases of antiphospholipid syndrome for COVID-19.
“As we await definitive solutions to the pandemic, these findings add important context to the complex interplay between SARS-CoV-2 infection, the human immune system, and vascular immunobiology,” she concluded.
The study was supported by grants from the Rheumatology Research Foundation, the Michigan Medicine Frankel Cardiovascular Center, and the A. Alfred Taubman Medical Research Institute. One author is an inventor on an unrelated pending patent to the University of Michigan. The other authors and Dr. Luning Prak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Circulating antiphospholipid autoantibodies may contribute to endothelial cell activation and dysfunction in severe COVID-19, researchers report.
In 2020, the same researchers reported results from a preclinical study demonstrating that autoantibodies from patients with active COVID-19 caused clotting in mice.
The new study, published in Arthritis and Rheumatology, found higher-than-expected levels of antiphospholipid autoantibodies in the blood samples of 244 patients hospitalized with COVID-19.
“While endothelial dysfunction has been implicated in the widespread thromboinflammatory complications of COVID-19, the upstream mediators of endotheliopathy remain for the most part cryptic,” write Hui Shi, MD, PhD, and coauthors from the University of Michigan, Ann Arbor, and the National Heart, Lung, and Blood Institute.
When asked for comment on the study, Eline T. Luning Prak, MD, PhD, professor of pathology and laboratory medicine at the Hospital of the University of Pennsylvania in Philadelphia, said, “The autopsy cases for COVID-19 strongly point to thromboembolic complications in many individuals who succumbed to sequelae of the infection.
“Importantly, however, many factors can contribute to this pathology, including the inflammatory milieu, monocyte activation, neutrophil extracellular traps, immune complexes, complement, as well as effects on endothelial cells,” explained Dr. Luning Prak, who was not involved in the study.
“The findings in this paper nicely complement another study by Schmaier et al. that came out recently in JCI Insight that also suggests that endothelial cells can be activated by antibodies, she said.
‘Even stronger connection between autoantibody formation and clotting in COVID-19’
Dr. Shi and her team cultured human endothelial cells in serum or plasma from 244 patients hospitalized with COVID-19 and plasma from 100 patients with non-COVID sepsis. Using in-cell enzyme-linked immunosorbent assay, they measured levels of key cell adhesion molecules.
After analysis, the researchers found that serum from COVID-19 patients activated cultured endothelial cells to express surface adhesion molecules essential to inflammation and thrombosis, particularly E-selectin, ICAM-1, and VCAM-1.
“The presence of circulating antiphospholipid antibodies was a strong marker of the ability of COVID-19 serum to activate endothelium,” they explain.
Further analyses revealed that, for a subset of serum samples from patients with severe infection, this activation could be mitigated by depleting total immunoglobulin G.
In addition, supplementation of control serum with patient IgG was adequate to trigger endothelial activation.
On the basis of these results, the researchers hypothesize that antiphospholipid autoantibodies may characterize antibody profiles in severe COVID-19 that activate the endothelium and transition the usually quiescent blood-vessel wall interface toward inflammation and coagulation.
“[These findings] provide an even stronger connection between autoantibody formation and clotting in COVID-19,” Dr. Shi said in an accompanying press release.
Clinical implications
From a clinical perspective, Dr. Shi and her team question whether patients with severe COVID-19 should be tested for antiphospholipid antibodies to assess their risk of thrombosis and progression to respiratory failure.
Moreover, they question whether patients with high antiphospholipid antibody titers might benefit from therapies used in conventional cases of severe antiphospholipid syndrome, such as plasmapheresis, anticoagulation therapy, and complement inhibition, Dr. Shi added.
The researchers hope to answer these and other remaining questions in future studies. “Eventually, we may be able to repurpose treatments used in traditional cases of antiphospholipid syndrome for COVID-19.
“As we await definitive solutions to the pandemic, these findings add important context to the complex interplay between SARS-CoV-2 infection, the human immune system, and vascular immunobiology,” she concluded.
The study was supported by grants from the Rheumatology Research Foundation, the Michigan Medicine Frankel Cardiovascular Center, and the A. Alfred Taubman Medical Research Institute. One author is an inventor on an unrelated pending patent to the University of Michigan. The other authors and Dr. Luning Prak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ukrainian physicians ‘ready to die for their freedom’
Nasogastric tubes. Foley catheter kits. Hydrogel anti-burn bandages and transfusion bags. Heparin, atropine, tramadol.
These items are just a few of some two dozen critical medical supplies that physicians in Ukraine desperately need, according to Leo Wolansky, MD, a Ukrainian-American radiologist and president of the Ukrainian Medical Association of North America (UMANA).
Dr. Wolansky founded a teaching program with an organization called Friends of Radiology in Ukraine in 1996 and has been running courses for specialists there ever since. He last visited the country in 2019, before the COVID-19 pandemic, but has remained in contact with his medical colleagues by phone and email. Over the weekend of Feb. 26-27, UMANA held a fundraiser for Ukraine, raising more than $17,000.
Question: Where is your family from, and do you have relatives in the country now?
Dr. Wolansky: My family is from two different parts of Ukraine. My mother was from central Ukraine. Her father, Ivan Sharyj, was part of the students’ militia that fought at the famous battle of Kruty in 1918. Four hundred Ukrainian militia fought against 5,000 professional Russian soldiers and were massacred. He later wrote the first eye-witness account. Afterwards, he had the opportunity to flee Ukraine but chose to stay under a pseudonym. Eventually, during Stalin’s purges [1929-1933], the regime found him, arrested him, tortured him, and executed him. My mother was seven when she saw her father arrested, never to return home. My father was from Western Ukraine, which did not have a long history of Russian occupation. His mother’s family was very patriotic; her first cousin, Stepan Vytvytskyi, eventually became the president of Ukraine in exile from 1955-1964.
I have second and more distant cousins in Kyiv. My wife has first cousins in Western Ukraine. They and my doctor colleagues are suffering greatly but are ready to die for their freedom.
Question: The Russian invasion of Ukraine has put tremendous stress on the Ukrainian people, including the country’s medical professionals. How do doctors in these kinds of situations handle casualties they can’t prevent? How do they work around that sense that everything is out of their control?
Dr. Wolansky: A lot of infrastructural things are being disrupted; there are limitations that you wouldn’t normally encounter. Ukraine has been developing a lot of sophisticated medical technology, but it still has room to grow. Under these circumstances, when there are bombs going off and transportation is being disrupted, it creates very new and significant obstacles to surmount. It still has not risen to massive casualties, and we can just pray that it does not, but in times of war, a very different kind of medicine is practiced.
But remember, Ukraine has been at war since 2014, when Russia took Crimea and invaded the Eastern provinces. The doctors there are not unfamiliar with war injuries. At our conferences in Ukraine, I have seen radiological presentations of injuries sustained in war – gunshots, fractures, and amputations – as well as other kinds of traumatic injuries. You’re going for a kind of more emergent treatment: to transfuse, to maintain peoples’ blood pressure, put bandages on, sterilize and sanitize wounds to prevent infections. I imagine there will be many field hospitals set up between now and the next few weeks to deal with the acute injuries.
Question: Ukraine has struggled with high rates of HIV and multidrug-resistant tuberculosis, as well as a lack of resources for treating patients with mental illness. Meanwhile, the country has had more that 5 million cases of COVID-19 and an estimated 112,000 deaths from the disease. Are you concerned about an exacerbation of infection rates, including of COVID, particularly among refugees and those who become homeless?
Dr. Wolansky: Because COVID ran pretty rampant in Ukraine, I think that – at a high cost – there is a level of natural immunity in the population. And the weather is going to be getting warmer soon, and respiratory viruses are cyclic in nature, so I don’t know if that’s going to be a big complicating factor. However, people get sick all the time, and the prognosis for them is going to be much worse than it otherwise might be. If you have a heart attack, your chances were way better when the roads were clear and people weren’t shooting at you.
Right now, it’s very regional where the infrastructure is being destroyed. The West, where I used to go, is in much better shape than the East because it has not been the focus of Russian attacks. But Kyiv could turn into a very big humanitarian crisis very quickly if there’s no electricity, no water. All sorts of medical conditions could be greatly exacerbated, and some new health crises could arise from water contamination, bombs causing buildings to collapse, and other problems. Whatever the illness is, it’s going to be harder to take care of it.
Questions: Doctors Without Borders announced that it was suspending its operations in Ukraine because of the invasion – missions that included HIV care in Severodonetsk, tuberculosis care in Zhytomyr, and improving health care access in Donetsk in eastern Ukraine, according to the aid group. What do doctors in Ukraine need most acutely now, other than peace?
Dr. Wolansky: Obviously, money is valuable, and military protection, which would prevent additional damage to their infrastructure. One thing that bears mentioning. There’s been a fair amount of coverage of this, but I’ve witnessed it first-hand: The Ukrainian people are fiercely patriotic, and there’s really no way their spirit can be conquered. The USSR invaded Afghanistan, and after years of thinking they were in command, they left because they could no longer take the guerilla warfare and the constant sniper attacks. Ukraine’s population is many times larger than Afghanistan’s; there’s no way they can be subdued. And remember, the Ukrainian people have been free for 30 years – generations of young people have known no other way of life. They are not going to give that up.
A version of this article first appeared on Medscape.com.
Nasogastric tubes. Foley catheter kits. Hydrogel anti-burn bandages and transfusion bags. Heparin, atropine, tramadol.
These items are just a few of some two dozen critical medical supplies that physicians in Ukraine desperately need, according to Leo Wolansky, MD, a Ukrainian-American radiologist and president of the Ukrainian Medical Association of North America (UMANA).
Dr. Wolansky founded a teaching program with an organization called Friends of Radiology in Ukraine in 1996 and has been running courses for specialists there ever since. He last visited the country in 2019, before the COVID-19 pandemic, but has remained in contact with his medical colleagues by phone and email. Over the weekend of Feb. 26-27, UMANA held a fundraiser for Ukraine, raising more than $17,000.
Question: Where is your family from, and do you have relatives in the country now?
Dr. Wolansky: My family is from two different parts of Ukraine. My mother was from central Ukraine. Her father, Ivan Sharyj, was part of the students’ militia that fought at the famous battle of Kruty in 1918. Four hundred Ukrainian militia fought against 5,000 professional Russian soldiers and were massacred. He later wrote the first eye-witness account. Afterwards, he had the opportunity to flee Ukraine but chose to stay under a pseudonym. Eventually, during Stalin’s purges [1929-1933], the regime found him, arrested him, tortured him, and executed him. My mother was seven when she saw her father arrested, never to return home. My father was from Western Ukraine, which did not have a long history of Russian occupation. His mother’s family was very patriotic; her first cousin, Stepan Vytvytskyi, eventually became the president of Ukraine in exile from 1955-1964.
I have second and more distant cousins in Kyiv. My wife has first cousins in Western Ukraine. They and my doctor colleagues are suffering greatly but are ready to die for their freedom.
Question: The Russian invasion of Ukraine has put tremendous stress on the Ukrainian people, including the country’s medical professionals. How do doctors in these kinds of situations handle casualties they can’t prevent? How do they work around that sense that everything is out of their control?
Dr. Wolansky: A lot of infrastructural things are being disrupted; there are limitations that you wouldn’t normally encounter. Ukraine has been developing a lot of sophisticated medical technology, but it still has room to grow. Under these circumstances, when there are bombs going off and transportation is being disrupted, it creates very new and significant obstacles to surmount. It still has not risen to massive casualties, and we can just pray that it does not, but in times of war, a very different kind of medicine is practiced.
But remember, Ukraine has been at war since 2014, when Russia took Crimea and invaded the Eastern provinces. The doctors there are not unfamiliar with war injuries. At our conferences in Ukraine, I have seen radiological presentations of injuries sustained in war – gunshots, fractures, and amputations – as well as other kinds of traumatic injuries. You’re going for a kind of more emergent treatment: to transfuse, to maintain peoples’ blood pressure, put bandages on, sterilize and sanitize wounds to prevent infections. I imagine there will be many field hospitals set up between now and the next few weeks to deal with the acute injuries.
Question: Ukraine has struggled with high rates of HIV and multidrug-resistant tuberculosis, as well as a lack of resources for treating patients with mental illness. Meanwhile, the country has had more that 5 million cases of COVID-19 and an estimated 112,000 deaths from the disease. Are you concerned about an exacerbation of infection rates, including of COVID, particularly among refugees and those who become homeless?
Dr. Wolansky: Because COVID ran pretty rampant in Ukraine, I think that – at a high cost – there is a level of natural immunity in the population. And the weather is going to be getting warmer soon, and respiratory viruses are cyclic in nature, so I don’t know if that’s going to be a big complicating factor. However, people get sick all the time, and the prognosis for them is going to be much worse than it otherwise might be. If you have a heart attack, your chances were way better when the roads were clear and people weren’t shooting at you.
Right now, it’s very regional where the infrastructure is being destroyed. The West, where I used to go, is in much better shape than the East because it has not been the focus of Russian attacks. But Kyiv could turn into a very big humanitarian crisis very quickly if there’s no electricity, no water. All sorts of medical conditions could be greatly exacerbated, and some new health crises could arise from water contamination, bombs causing buildings to collapse, and other problems. Whatever the illness is, it’s going to be harder to take care of it.
Questions: Doctors Without Borders announced that it was suspending its operations in Ukraine because of the invasion – missions that included HIV care in Severodonetsk, tuberculosis care in Zhytomyr, and improving health care access in Donetsk in eastern Ukraine, according to the aid group. What do doctors in Ukraine need most acutely now, other than peace?
Dr. Wolansky: Obviously, money is valuable, and military protection, which would prevent additional damage to their infrastructure. One thing that bears mentioning. There’s been a fair amount of coverage of this, but I’ve witnessed it first-hand: The Ukrainian people are fiercely patriotic, and there’s really no way their spirit can be conquered. The USSR invaded Afghanistan, and after years of thinking they were in command, they left because they could no longer take the guerilla warfare and the constant sniper attacks. Ukraine’s population is many times larger than Afghanistan’s; there’s no way they can be subdued. And remember, the Ukrainian people have been free for 30 years – generations of young people have known no other way of life. They are not going to give that up.
A version of this article first appeared on Medscape.com.
Nasogastric tubes. Foley catheter kits. Hydrogel anti-burn bandages and transfusion bags. Heparin, atropine, tramadol.
These items are just a few of some two dozen critical medical supplies that physicians in Ukraine desperately need, according to Leo Wolansky, MD, a Ukrainian-American radiologist and president of the Ukrainian Medical Association of North America (UMANA).
Dr. Wolansky founded a teaching program with an organization called Friends of Radiology in Ukraine in 1996 and has been running courses for specialists there ever since. He last visited the country in 2019, before the COVID-19 pandemic, but has remained in contact with his medical colleagues by phone and email. Over the weekend of Feb. 26-27, UMANA held a fundraiser for Ukraine, raising more than $17,000.
Question: Where is your family from, and do you have relatives in the country now?
Dr. Wolansky: My family is from two different parts of Ukraine. My mother was from central Ukraine. Her father, Ivan Sharyj, was part of the students’ militia that fought at the famous battle of Kruty in 1918. Four hundred Ukrainian militia fought against 5,000 professional Russian soldiers and were massacred. He later wrote the first eye-witness account. Afterwards, he had the opportunity to flee Ukraine but chose to stay under a pseudonym. Eventually, during Stalin’s purges [1929-1933], the regime found him, arrested him, tortured him, and executed him. My mother was seven when she saw her father arrested, never to return home. My father was from Western Ukraine, which did not have a long history of Russian occupation. His mother’s family was very patriotic; her first cousin, Stepan Vytvytskyi, eventually became the president of Ukraine in exile from 1955-1964.
I have second and more distant cousins in Kyiv. My wife has first cousins in Western Ukraine. They and my doctor colleagues are suffering greatly but are ready to die for their freedom.
Question: The Russian invasion of Ukraine has put tremendous stress on the Ukrainian people, including the country’s medical professionals. How do doctors in these kinds of situations handle casualties they can’t prevent? How do they work around that sense that everything is out of their control?
Dr. Wolansky: A lot of infrastructural things are being disrupted; there are limitations that you wouldn’t normally encounter. Ukraine has been developing a lot of sophisticated medical technology, but it still has room to grow. Under these circumstances, when there are bombs going off and transportation is being disrupted, it creates very new and significant obstacles to surmount. It still has not risen to massive casualties, and we can just pray that it does not, but in times of war, a very different kind of medicine is practiced.
But remember, Ukraine has been at war since 2014, when Russia took Crimea and invaded the Eastern provinces. The doctors there are not unfamiliar with war injuries. At our conferences in Ukraine, I have seen radiological presentations of injuries sustained in war – gunshots, fractures, and amputations – as well as other kinds of traumatic injuries. You’re going for a kind of more emergent treatment: to transfuse, to maintain peoples’ blood pressure, put bandages on, sterilize and sanitize wounds to prevent infections. I imagine there will be many field hospitals set up between now and the next few weeks to deal with the acute injuries.
Question: Ukraine has struggled with high rates of HIV and multidrug-resistant tuberculosis, as well as a lack of resources for treating patients with mental illness. Meanwhile, the country has had more that 5 million cases of COVID-19 and an estimated 112,000 deaths from the disease. Are you concerned about an exacerbation of infection rates, including of COVID, particularly among refugees and those who become homeless?
Dr. Wolansky: Because COVID ran pretty rampant in Ukraine, I think that – at a high cost – there is a level of natural immunity in the population. And the weather is going to be getting warmer soon, and respiratory viruses are cyclic in nature, so I don’t know if that’s going to be a big complicating factor. However, people get sick all the time, and the prognosis for them is going to be much worse than it otherwise might be. If you have a heart attack, your chances were way better when the roads were clear and people weren’t shooting at you.
Right now, it’s very regional where the infrastructure is being destroyed. The West, where I used to go, is in much better shape than the East because it has not been the focus of Russian attacks. But Kyiv could turn into a very big humanitarian crisis very quickly if there’s no electricity, no water. All sorts of medical conditions could be greatly exacerbated, and some new health crises could arise from water contamination, bombs causing buildings to collapse, and other problems. Whatever the illness is, it’s going to be harder to take care of it.
Questions: Doctors Without Borders announced that it was suspending its operations in Ukraine because of the invasion – missions that included HIV care in Severodonetsk, tuberculosis care in Zhytomyr, and improving health care access in Donetsk in eastern Ukraine, according to the aid group. What do doctors in Ukraine need most acutely now, other than peace?
Dr. Wolansky: Obviously, money is valuable, and military protection, which would prevent additional damage to their infrastructure. One thing that bears mentioning. There’s been a fair amount of coverage of this, but I’ve witnessed it first-hand: The Ukrainian people are fiercely patriotic, and there’s really no way their spirit can be conquered. The USSR invaded Afghanistan, and after years of thinking they were in command, they left because they could no longer take the guerilla warfare and the constant sniper attacks. Ukraine’s population is many times larger than Afghanistan’s; there’s no way they can be subdued. And remember, the Ukrainian people have been free for 30 years – generations of young people have known no other way of life. They are not going to give that up.
A version of this article first appeared on Medscape.com.
Epidural may lower odds of severe maternal birth complications
Use of neuraxial analgesia for vaginal delivery is associated with a 14% decreased risk for severe maternal morbidity, in part from a reduction in postpartum hemorrhage, new research shows.
The findings indicate that increasing the use of epidural or combined spinal-epidural analgesia may improve maternal health outcomes, especially for Hispanic, Black, and uninsured women who are less likely than White women to receive these interventions, according to the researchers, who published their findings online in JAMA Network Open.
About 80% of non-Hispanic White women receive neuraxial analgesia during labor in the United States, compared with 70% of non-Hispanic Black women and 65% of Hispanic women, according to birth certificate data. Among women without health insurance, half receive epidurals.
Programs that inform pregnant women about epidural use, expand Medicaid, and provide in-house obstetric anesthesia teams “may improve patient participation in clinical decision making and access to care,” study author Guohua Li, MD, DrPH, of Columbia University, New York, said in a statement about the research.
Earlier data from France showed that neuraxial analgesia is associated with reduced risk for severe postpartum hemorrhage. To examine the association between labor neuraxial analgesia and severe maternal morbidity in the United States, Dr. Li and colleagues analyzed more than 575,000 vaginal deliveries in New York hospitals between 2010 and 2017; about half (47.4%) of the women received epidurals during labor.
The researchers focused on severe maternal morbidity, including 16 complications, such as heart failure and sepsis, and five procedures, including hysterectomy and ventilation.
They also considered patient characteristics and comorbidities and hospital-related factors to identify patients who were at higher risk for injury or death.
Severe maternal morbidity occurred in 1.3% of the women. Of the 7,712 women with severe morbidity, more than one in three (35.6%) experienced postpartum hemorrhage.
The overall incidence of severe maternal morbidity was 1.3% among women who received an epidural injection and 1.4% among those who did not. In a weighted analysis, the adjusted odds ratio of severe maternal morbidity associated with epidurals was 0.86 (95% confidence interval, 0.82-0.90).
The study is limited by its observational design and does not prove causation, the authors acknowledged.
“Labor neuraxial analgesia may facilitate early evaluation and management of the third stage of labor to avoid escalation of postpartum hemorrhaging into grave complications and death,” study author Jean Guglielminotti, MD, PhD, an anesthesiologist at Columbia University, said in a statement.
Concerning trends
The Department of Health & Human Services has labeled severe maternal morbidity a public health priority. Recent data from the Centers for Disease Control and Prevention show an increase in maternal mortality rates and worsening disparities by race and ethnicity.
According to the CDC, 861 women died of maternal causes in 2020, up from 754 in 2019. The rate of maternal mortality increased from 20.1 to 23.8 deaths per 100,000 live births.
For Black women, however, the maternal mortality rate was far higher: 55.3 deaths per 100,000 live births – nearly triple the figure of 19.1 per 100,000 for White women. Between 2019 and 2020, the mortality rate increased significantly for Black and Hispanic women, but not White mothers.
Researchers affiliated with University of Toronto and the Hospital for Sick Children agreed in an accompanying editorial that more access to neuraxial labor analgesia for vaginal delivery might improve maternal health outcomes and “may be a strategy well worth pursuing in public health policy.”
The intervention is relatively safe and can “alleviate discomfort and distress,” they wrote.
Neuraxial anesthesia in surgical procedures has been shown to decrease the risk for complications like deep vein thrombosis, pulmonary embolus, transfusion requirements, and kidney failure, said editorialists Evelina Pankiv, MD; Alan Yang, MSc; and Kazuyoshi Aoyama, MD, PhD.
Benefits potentially could stem from improving blood flow, mitigating hypercoagulation, or reducing surgical stress response. But there are rare risks to consider as well, including hemorrhage, infection, and neurologic injury, they added.
Guglielminotti disclosed grants from the National Institute on Minority Health and Health Disparities. Dr. Aoyama reported receiving grants from the Perioperative Services Facilitator Grant Program and Hospital for Sick Children. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of neuraxial analgesia for vaginal delivery is associated with a 14% decreased risk for severe maternal morbidity, in part from a reduction in postpartum hemorrhage, new research shows.
The findings indicate that increasing the use of epidural or combined spinal-epidural analgesia may improve maternal health outcomes, especially for Hispanic, Black, and uninsured women who are less likely than White women to receive these interventions, according to the researchers, who published their findings online in JAMA Network Open.
About 80% of non-Hispanic White women receive neuraxial analgesia during labor in the United States, compared with 70% of non-Hispanic Black women and 65% of Hispanic women, according to birth certificate data. Among women without health insurance, half receive epidurals.
Programs that inform pregnant women about epidural use, expand Medicaid, and provide in-house obstetric anesthesia teams “may improve patient participation in clinical decision making and access to care,” study author Guohua Li, MD, DrPH, of Columbia University, New York, said in a statement about the research.
Earlier data from France showed that neuraxial analgesia is associated with reduced risk for severe postpartum hemorrhage. To examine the association between labor neuraxial analgesia and severe maternal morbidity in the United States, Dr. Li and colleagues analyzed more than 575,000 vaginal deliveries in New York hospitals between 2010 and 2017; about half (47.4%) of the women received epidurals during labor.
The researchers focused on severe maternal morbidity, including 16 complications, such as heart failure and sepsis, and five procedures, including hysterectomy and ventilation.
They also considered patient characteristics and comorbidities and hospital-related factors to identify patients who were at higher risk for injury or death.
Severe maternal morbidity occurred in 1.3% of the women. Of the 7,712 women with severe morbidity, more than one in three (35.6%) experienced postpartum hemorrhage.
The overall incidence of severe maternal morbidity was 1.3% among women who received an epidural injection and 1.4% among those who did not. In a weighted analysis, the adjusted odds ratio of severe maternal morbidity associated with epidurals was 0.86 (95% confidence interval, 0.82-0.90).
The study is limited by its observational design and does not prove causation, the authors acknowledged.
“Labor neuraxial analgesia may facilitate early evaluation and management of the third stage of labor to avoid escalation of postpartum hemorrhaging into grave complications and death,” study author Jean Guglielminotti, MD, PhD, an anesthesiologist at Columbia University, said in a statement.
Concerning trends
The Department of Health & Human Services has labeled severe maternal morbidity a public health priority. Recent data from the Centers for Disease Control and Prevention show an increase in maternal mortality rates and worsening disparities by race and ethnicity.
According to the CDC, 861 women died of maternal causes in 2020, up from 754 in 2019. The rate of maternal mortality increased from 20.1 to 23.8 deaths per 100,000 live births.
For Black women, however, the maternal mortality rate was far higher: 55.3 deaths per 100,000 live births – nearly triple the figure of 19.1 per 100,000 for White women. Between 2019 and 2020, the mortality rate increased significantly for Black and Hispanic women, but not White mothers.
Researchers affiliated with University of Toronto and the Hospital for Sick Children agreed in an accompanying editorial that more access to neuraxial labor analgesia for vaginal delivery might improve maternal health outcomes and “may be a strategy well worth pursuing in public health policy.”
The intervention is relatively safe and can “alleviate discomfort and distress,” they wrote.
Neuraxial anesthesia in surgical procedures has been shown to decrease the risk for complications like deep vein thrombosis, pulmonary embolus, transfusion requirements, and kidney failure, said editorialists Evelina Pankiv, MD; Alan Yang, MSc; and Kazuyoshi Aoyama, MD, PhD.
Benefits potentially could stem from improving blood flow, mitigating hypercoagulation, or reducing surgical stress response. But there are rare risks to consider as well, including hemorrhage, infection, and neurologic injury, they added.
Guglielminotti disclosed grants from the National Institute on Minority Health and Health Disparities. Dr. Aoyama reported receiving grants from the Perioperative Services Facilitator Grant Program and Hospital for Sick Children. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of neuraxial analgesia for vaginal delivery is associated with a 14% decreased risk for severe maternal morbidity, in part from a reduction in postpartum hemorrhage, new research shows.
The findings indicate that increasing the use of epidural or combined spinal-epidural analgesia may improve maternal health outcomes, especially for Hispanic, Black, and uninsured women who are less likely than White women to receive these interventions, according to the researchers, who published their findings online in JAMA Network Open.
About 80% of non-Hispanic White women receive neuraxial analgesia during labor in the United States, compared with 70% of non-Hispanic Black women and 65% of Hispanic women, according to birth certificate data. Among women without health insurance, half receive epidurals.
Programs that inform pregnant women about epidural use, expand Medicaid, and provide in-house obstetric anesthesia teams “may improve patient participation in clinical decision making and access to care,” study author Guohua Li, MD, DrPH, of Columbia University, New York, said in a statement about the research.
Earlier data from France showed that neuraxial analgesia is associated with reduced risk for severe postpartum hemorrhage. To examine the association between labor neuraxial analgesia and severe maternal morbidity in the United States, Dr. Li and colleagues analyzed more than 575,000 vaginal deliveries in New York hospitals between 2010 and 2017; about half (47.4%) of the women received epidurals during labor.
The researchers focused on severe maternal morbidity, including 16 complications, such as heart failure and sepsis, and five procedures, including hysterectomy and ventilation.
They also considered patient characteristics and comorbidities and hospital-related factors to identify patients who were at higher risk for injury or death.
Severe maternal morbidity occurred in 1.3% of the women. Of the 7,712 women with severe morbidity, more than one in three (35.6%) experienced postpartum hemorrhage.
The overall incidence of severe maternal morbidity was 1.3% among women who received an epidural injection and 1.4% among those who did not. In a weighted analysis, the adjusted odds ratio of severe maternal morbidity associated with epidurals was 0.86 (95% confidence interval, 0.82-0.90).
The study is limited by its observational design and does not prove causation, the authors acknowledged.
“Labor neuraxial analgesia may facilitate early evaluation and management of the third stage of labor to avoid escalation of postpartum hemorrhaging into grave complications and death,” study author Jean Guglielminotti, MD, PhD, an anesthesiologist at Columbia University, said in a statement.
Concerning trends
The Department of Health & Human Services has labeled severe maternal morbidity a public health priority. Recent data from the Centers for Disease Control and Prevention show an increase in maternal mortality rates and worsening disparities by race and ethnicity.
According to the CDC, 861 women died of maternal causes in 2020, up from 754 in 2019. The rate of maternal mortality increased from 20.1 to 23.8 deaths per 100,000 live births.
For Black women, however, the maternal mortality rate was far higher: 55.3 deaths per 100,000 live births – nearly triple the figure of 19.1 per 100,000 for White women. Between 2019 and 2020, the mortality rate increased significantly for Black and Hispanic women, but not White mothers.
Researchers affiliated with University of Toronto and the Hospital for Sick Children agreed in an accompanying editorial that more access to neuraxial labor analgesia for vaginal delivery might improve maternal health outcomes and “may be a strategy well worth pursuing in public health policy.”
The intervention is relatively safe and can “alleviate discomfort and distress,” they wrote.
Neuraxial anesthesia in surgical procedures has been shown to decrease the risk for complications like deep vein thrombosis, pulmonary embolus, transfusion requirements, and kidney failure, said editorialists Evelina Pankiv, MD; Alan Yang, MSc; and Kazuyoshi Aoyama, MD, PhD.
Benefits potentially could stem from improving blood flow, mitigating hypercoagulation, or reducing surgical stress response. But there are rare risks to consider as well, including hemorrhage, infection, and neurologic injury, they added.
Guglielminotti disclosed grants from the National Institute on Minority Health and Health Disparities. Dr. Aoyama reported receiving grants from the Perioperative Services Facilitator Grant Program and Hospital for Sick Children. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
More than half of U.S. women enter pregnancy at higher CVD risk
Less than half of women in the United States enter pregnancy in favorable cardiovascular health, new research suggests.
In 2019, among women aged 20 to 44 years with live births in the United States, only 40.2% were in favorable cardiovascular health prior to pregnancy, defined as normal weight, no diabetes, and no hypertension.
Although all regions and states showed a decline in prepregnancy favorable cardiometabolic health, there were significant differences among geographic regions in the country, the authors report. “These data reveal critical deficiencies and geographic disparities in prepregnancy cardiometabolic health,” they conclude.
“One of the things that we know in the U.S. is that the maternal mortality rate has been increasing, and there are significant differences at the state level in both adverse maternal outcomes, such as maternal mortality, as well as adverse pregnancy outcomes,” corresponding author Sadiya S. Khan, MD, MS, FACC, Northwestern University Feinberg School of Medicine, Chicago, told this news organization.
“These outcomes are often related to health factors that predate pregnancy,” Dr. Khan explained, “and the processes that begin at the very, very beginning of conception are informed by health factors prior to pregnancy, in particular cardiometabolic factors like body mass index or obesity, high blood pressure, and diabetes.”
The results were published online on Feb. 14 in a special “Go Red for Women” spotlight issue of Circulation.
Cardiometabolic health factors
Using maternal birth records from live births in the Centers for Disease Control and Prevention Natality Database between 2016 and 2019, the authors analyzed data on 14,174,625 women with live births aged 20 to 44 years. The majority (81.4%) were 20 to 34 years of age, 22.7% were Hispanic or Latina, and 52.7% were non-Hispanic White.
Favorable cardiometabolic health was defined as a BMI of 18 to 24.9 kg/m2, absence of diabetes, and absence of hypertension.
Although all regions and states experienced a decline in favorable cardiometabolic health during the study period of 2016 to 2019, with a drop overall of 3.2% – from 43.5 to 40.2 per 100 live births – it was especially true of the South and Midwest regions.
In 2019, favorable prepregnancy cardiometabolic health was lowest in the South (38.1%) and Midwest (38.8%) and highest in the West (42.2%) and Northeast (43.6%).
State by state, the lowest prevalence of favorable cardiometabolic health was found in Mississippi, at 31.2%, and highest in Utah, at 47.2%.
They also found a correlation between favorable cardiometabolic health and state-level percentages of high-school education or less and enrollment in Medicaid in 2019.
Similar to what has been seen with cardiovascular disease, “we observe that the states with the lowest prevalence of favorable cardiometabolic health were in the Southeast United States,” said Dr. Khan, “and similar geographic variation was observed with some more patterns in education and Medicaid coverage for birth, and these were used as proxies for socioeconomic status in those areas.”
Although Dr. Khan notes that the relationships cannot be determined to be causal from this analysis, she said that “it does suggest that upstream social determinants of health are important determinants of cardiometabolic health.”
Socioeconomic intervention
Dr. Khan noted that policies at the federal and state level can identify ways to “ensure that individuals who are thinking about pregnancy have access to health care and have access to resources, too, from a broad range of health determinants, including housing stability, food security, as well as access to health care be optimized prior to pregnancy.”
The authors note that this analysis may actually overestimate the prevalence of favorable cardiometabolic health, and data on cholesterol, diet, a distinction between type 1 and type 2 diabetes, and physical activity were not available.
Only individuals with live births were included, which could result in the elimination of a potentially high-risk group; however, late pregnancy losses represent less than 0.3% of all pregnancies, they say.
The authors conclude that “future research is needed to equitably improve health prior to pregnancy and quantify the potential benefits in cardiovascular disease outcomes for birthing individuals and their offspring.”
This work was supported by grants from the National Heart, Lung, and Blood Institute and American Heart Association Transformational Project Award awarded to Sadiya S. Khan.
A version of this article first appeared on Medscape.com.
Less than half of women in the United States enter pregnancy in favorable cardiovascular health, new research suggests.
In 2019, among women aged 20 to 44 years with live births in the United States, only 40.2% were in favorable cardiovascular health prior to pregnancy, defined as normal weight, no diabetes, and no hypertension.
Although all regions and states showed a decline in prepregnancy favorable cardiometabolic health, there were significant differences among geographic regions in the country, the authors report. “These data reveal critical deficiencies and geographic disparities in prepregnancy cardiometabolic health,” they conclude.
“One of the things that we know in the U.S. is that the maternal mortality rate has been increasing, and there are significant differences at the state level in both adverse maternal outcomes, such as maternal mortality, as well as adverse pregnancy outcomes,” corresponding author Sadiya S. Khan, MD, MS, FACC, Northwestern University Feinberg School of Medicine, Chicago, told this news organization.
“These outcomes are often related to health factors that predate pregnancy,” Dr. Khan explained, “and the processes that begin at the very, very beginning of conception are informed by health factors prior to pregnancy, in particular cardiometabolic factors like body mass index or obesity, high blood pressure, and diabetes.”
The results were published online on Feb. 14 in a special “Go Red for Women” spotlight issue of Circulation.
Cardiometabolic health factors
Using maternal birth records from live births in the Centers for Disease Control and Prevention Natality Database between 2016 and 2019, the authors analyzed data on 14,174,625 women with live births aged 20 to 44 years. The majority (81.4%) were 20 to 34 years of age, 22.7% were Hispanic or Latina, and 52.7% were non-Hispanic White.
Favorable cardiometabolic health was defined as a BMI of 18 to 24.9 kg/m2, absence of diabetes, and absence of hypertension.
Although all regions and states experienced a decline in favorable cardiometabolic health during the study period of 2016 to 2019, with a drop overall of 3.2% – from 43.5 to 40.2 per 100 live births – it was especially true of the South and Midwest regions.
In 2019, favorable prepregnancy cardiometabolic health was lowest in the South (38.1%) and Midwest (38.8%) and highest in the West (42.2%) and Northeast (43.6%).
State by state, the lowest prevalence of favorable cardiometabolic health was found in Mississippi, at 31.2%, and highest in Utah, at 47.2%.
They also found a correlation between favorable cardiometabolic health and state-level percentages of high-school education or less and enrollment in Medicaid in 2019.
Similar to what has been seen with cardiovascular disease, “we observe that the states with the lowest prevalence of favorable cardiometabolic health were in the Southeast United States,” said Dr. Khan, “and similar geographic variation was observed with some more patterns in education and Medicaid coverage for birth, and these were used as proxies for socioeconomic status in those areas.”
Although Dr. Khan notes that the relationships cannot be determined to be causal from this analysis, she said that “it does suggest that upstream social determinants of health are important determinants of cardiometabolic health.”
Socioeconomic intervention
Dr. Khan noted that policies at the federal and state level can identify ways to “ensure that individuals who are thinking about pregnancy have access to health care and have access to resources, too, from a broad range of health determinants, including housing stability, food security, as well as access to health care be optimized prior to pregnancy.”
The authors note that this analysis may actually overestimate the prevalence of favorable cardiometabolic health, and data on cholesterol, diet, a distinction between type 1 and type 2 diabetes, and physical activity were not available.
Only individuals with live births were included, which could result in the elimination of a potentially high-risk group; however, late pregnancy losses represent less than 0.3% of all pregnancies, they say.
The authors conclude that “future research is needed to equitably improve health prior to pregnancy and quantify the potential benefits in cardiovascular disease outcomes for birthing individuals and their offspring.”
This work was supported by grants from the National Heart, Lung, and Blood Institute and American Heart Association Transformational Project Award awarded to Sadiya S. Khan.
A version of this article first appeared on Medscape.com.
Less than half of women in the United States enter pregnancy in favorable cardiovascular health, new research suggests.
In 2019, among women aged 20 to 44 years with live births in the United States, only 40.2% were in favorable cardiovascular health prior to pregnancy, defined as normal weight, no diabetes, and no hypertension.
Although all regions and states showed a decline in prepregnancy favorable cardiometabolic health, there were significant differences among geographic regions in the country, the authors report. “These data reveal critical deficiencies and geographic disparities in prepregnancy cardiometabolic health,” they conclude.
“One of the things that we know in the U.S. is that the maternal mortality rate has been increasing, and there are significant differences at the state level in both adverse maternal outcomes, such as maternal mortality, as well as adverse pregnancy outcomes,” corresponding author Sadiya S. Khan, MD, MS, FACC, Northwestern University Feinberg School of Medicine, Chicago, told this news organization.
“These outcomes are often related to health factors that predate pregnancy,” Dr. Khan explained, “and the processes that begin at the very, very beginning of conception are informed by health factors prior to pregnancy, in particular cardiometabolic factors like body mass index or obesity, high blood pressure, and diabetes.”
The results were published online on Feb. 14 in a special “Go Red for Women” spotlight issue of Circulation.
Cardiometabolic health factors
Using maternal birth records from live births in the Centers for Disease Control and Prevention Natality Database between 2016 and 2019, the authors analyzed data on 14,174,625 women with live births aged 20 to 44 years. The majority (81.4%) were 20 to 34 years of age, 22.7% were Hispanic or Latina, and 52.7% were non-Hispanic White.
Favorable cardiometabolic health was defined as a BMI of 18 to 24.9 kg/m2, absence of diabetes, and absence of hypertension.
Although all regions and states experienced a decline in favorable cardiometabolic health during the study period of 2016 to 2019, with a drop overall of 3.2% – from 43.5 to 40.2 per 100 live births – it was especially true of the South and Midwest regions.
In 2019, favorable prepregnancy cardiometabolic health was lowest in the South (38.1%) and Midwest (38.8%) and highest in the West (42.2%) and Northeast (43.6%).
State by state, the lowest prevalence of favorable cardiometabolic health was found in Mississippi, at 31.2%, and highest in Utah, at 47.2%.
They also found a correlation between favorable cardiometabolic health and state-level percentages of high-school education or less and enrollment in Medicaid in 2019.
Similar to what has been seen with cardiovascular disease, “we observe that the states with the lowest prevalence of favorable cardiometabolic health were in the Southeast United States,” said Dr. Khan, “and similar geographic variation was observed with some more patterns in education and Medicaid coverage for birth, and these were used as proxies for socioeconomic status in those areas.”
Although Dr. Khan notes that the relationships cannot be determined to be causal from this analysis, she said that “it does suggest that upstream social determinants of health are important determinants of cardiometabolic health.”
Socioeconomic intervention
Dr. Khan noted that policies at the federal and state level can identify ways to “ensure that individuals who are thinking about pregnancy have access to health care and have access to resources, too, from a broad range of health determinants, including housing stability, food security, as well as access to health care be optimized prior to pregnancy.”
The authors note that this analysis may actually overestimate the prevalence of favorable cardiometabolic health, and data on cholesterol, diet, a distinction between type 1 and type 2 diabetes, and physical activity were not available.
Only individuals with live births were included, which could result in the elimination of a potentially high-risk group; however, late pregnancy losses represent less than 0.3% of all pregnancies, they say.
The authors conclude that “future research is needed to equitably improve health prior to pregnancy and quantify the potential benefits in cardiovascular disease outcomes for birthing individuals and their offspring.”
This work was supported by grants from the National Heart, Lung, and Blood Institute and American Heart Association Transformational Project Award awarded to Sadiya S. Khan.
A version of this article first appeared on Medscape.com.
Excess sodium in soluble acetaminophen tied to CVD risk, death
a large observational study of more than 300,000 adults suggests.
“Numerous studies have reported that high sodium intake is associated with increased risks of cardiovascular disease,” Yuqing Zhang, DSc, with Massachusetts General Hospital and Harvard Medical School, Boston, told this news organization. “Given that the pain relief effect of non–sodium-containing acetaminophen is similar to that of sodium-containing acetaminophen, clinicians may prescribe non–sodium-containing acetaminophen to their patients to minimize the risk of CVD and mortality,” Dr. Zhang said.
The study was published online Feb. 24 in the European Heart Journal.
‘Compelling results’
Dr. Zhang and colleagues note that the effervescent and soluble formulations of 0.5 g acetaminophen contain 0.44 and 0.39 g of sodium, respectively.
Therefore, the intake of maximum daily dose (4 g/day) of sodium-containing acetaminophen corresponds to the ingestion of more than 3 g of sodium, a dose that alone exceeds the recommended total daily sodium intake allowance of the World Health Organization (2 g/day).
“This hidden extra sodium intake is often overlooked,” Dr. Zhang told this news organization.
Using data from the Health Improvement Network, a U.K. primary care database, the researchers examined 4,532 patients with hypertension taking sodium-containing acetaminophen and compared them with 146,866 patients with hypertension taking non–sodium-containing acetaminophen (tablet, capsule, or oral suspension formulations).
After 1 year, the risk of incident CVD (myocardial infarction, stroke, and heart failure) was 5.6% in those taking sodium-containing acetaminophen, compared with 4.6% in those taking non–sodium-containing acetaminophen (average weighted hazard ratio, 1.59; 95% confidence interval, 1.32-1.92).
A separate analysis of normotensive patients taking sodium-containing acetaminophen (n = 5,351) or non–sodium-containing acetaminophen (n = 141,948) gave similar results.
The 1-year risk of incident CVD was 4.4% in those taking sodium-containing acetaminophen vs. 3.7% among those taking non–sodium-containing acetaminophen (average weighted HR, 1.45; 95% CI, 1.18-1.79).
There was also evidence of a dose-response relationship.
In those with hypertension, CVD risk increased by roughly one-quarter (odds ratio, 1.26) for those with one prescription of sodium-containing acetaminophen and by nearly one half (OR, 1.45) for those with five or more prescriptions of sodium-containing acetaminophen. Similar findings were observed among adults without hypertension.
Mortality at 1 year was also higher in those taking sodium-containing acetaminophen than non–sodium-containing acetaminophen, in patients with hypertension (7.6% vs. 6.1%) and without hypertension (7.3% vs. 5.9%).
“The results are compelling,” write the authors of an editorial published with the study.
“The direct message from this study is clear – there are likely to be millions of people worldwide taking paracetamol on a daily basis in a ‘fast-acting’ effervescent or soluble formulation who are increasing their risks of cardiovascular disease and premature death,” say Aletta Schutte, PhD, and Bruce Neal, MBChB, PhD, of the George Institute for Global Health, Sydney.
“The weight of the evidence makes ongoing inaction on sodium-containing medications untenable. The widespread use of effervescent medication in the general population, and the enormous doses of sodium that can be consumed inadvertently by unsuspecting consumers requires urgent action,” Dr. Schutte and Dr. Neal say.
The study was supported by the National Natural Science Foundation of China, the National Key Research and Development Project, the Project Program of National Clinical Research Center for Geriatric Disorders, the Key Research and Development Program of Hunan Province, and the Science and Technology Program of Hunan Province. Dr. Zhang, Dr. Schutte, and Dr. Neal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a large observational study of more than 300,000 adults suggests.
“Numerous studies have reported that high sodium intake is associated with increased risks of cardiovascular disease,” Yuqing Zhang, DSc, with Massachusetts General Hospital and Harvard Medical School, Boston, told this news organization. “Given that the pain relief effect of non–sodium-containing acetaminophen is similar to that of sodium-containing acetaminophen, clinicians may prescribe non–sodium-containing acetaminophen to their patients to minimize the risk of CVD and mortality,” Dr. Zhang said.
The study was published online Feb. 24 in the European Heart Journal.
‘Compelling results’
Dr. Zhang and colleagues note that the effervescent and soluble formulations of 0.5 g acetaminophen contain 0.44 and 0.39 g of sodium, respectively.
Therefore, the intake of maximum daily dose (4 g/day) of sodium-containing acetaminophen corresponds to the ingestion of more than 3 g of sodium, a dose that alone exceeds the recommended total daily sodium intake allowance of the World Health Organization (2 g/day).
“This hidden extra sodium intake is often overlooked,” Dr. Zhang told this news organization.
Using data from the Health Improvement Network, a U.K. primary care database, the researchers examined 4,532 patients with hypertension taking sodium-containing acetaminophen and compared them with 146,866 patients with hypertension taking non–sodium-containing acetaminophen (tablet, capsule, or oral suspension formulations).
After 1 year, the risk of incident CVD (myocardial infarction, stroke, and heart failure) was 5.6% in those taking sodium-containing acetaminophen, compared with 4.6% in those taking non–sodium-containing acetaminophen (average weighted hazard ratio, 1.59; 95% confidence interval, 1.32-1.92).
A separate analysis of normotensive patients taking sodium-containing acetaminophen (n = 5,351) or non–sodium-containing acetaminophen (n = 141,948) gave similar results.
The 1-year risk of incident CVD was 4.4% in those taking sodium-containing acetaminophen vs. 3.7% among those taking non–sodium-containing acetaminophen (average weighted HR, 1.45; 95% CI, 1.18-1.79).
There was also evidence of a dose-response relationship.
In those with hypertension, CVD risk increased by roughly one-quarter (odds ratio, 1.26) for those with one prescription of sodium-containing acetaminophen and by nearly one half (OR, 1.45) for those with five or more prescriptions of sodium-containing acetaminophen. Similar findings were observed among adults without hypertension.
Mortality at 1 year was also higher in those taking sodium-containing acetaminophen than non–sodium-containing acetaminophen, in patients with hypertension (7.6% vs. 6.1%) and without hypertension (7.3% vs. 5.9%).
“The results are compelling,” write the authors of an editorial published with the study.
“The direct message from this study is clear – there are likely to be millions of people worldwide taking paracetamol on a daily basis in a ‘fast-acting’ effervescent or soluble formulation who are increasing their risks of cardiovascular disease and premature death,” say Aletta Schutte, PhD, and Bruce Neal, MBChB, PhD, of the George Institute for Global Health, Sydney.
“The weight of the evidence makes ongoing inaction on sodium-containing medications untenable. The widespread use of effervescent medication in the general population, and the enormous doses of sodium that can be consumed inadvertently by unsuspecting consumers requires urgent action,” Dr. Schutte and Dr. Neal say.
The study was supported by the National Natural Science Foundation of China, the National Key Research and Development Project, the Project Program of National Clinical Research Center for Geriatric Disorders, the Key Research and Development Program of Hunan Province, and the Science and Technology Program of Hunan Province. Dr. Zhang, Dr. Schutte, and Dr. Neal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a large observational study of more than 300,000 adults suggests.
“Numerous studies have reported that high sodium intake is associated with increased risks of cardiovascular disease,” Yuqing Zhang, DSc, with Massachusetts General Hospital and Harvard Medical School, Boston, told this news organization. “Given that the pain relief effect of non–sodium-containing acetaminophen is similar to that of sodium-containing acetaminophen, clinicians may prescribe non–sodium-containing acetaminophen to their patients to minimize the risk of CVD and mortality,” Dr. Zhang said.
The study was published online Feb. 24 in the European Heart Journal.
‘Compelling results’
Dr. Zhang and colleagues note that the effervescent and soluble formulations of 0.5 g acetaminophen contain 0.44 and 0.39 g of sodium, respectively.
Therefore, the intake of maximum daily dose (4 g/day) of sodium-containing acetaminophen corresponds to the ingestion of more than 3 g of sodium, a dose that alone exceeds the recommended total daily sodium intake allowance of the World Health Organization (2 g/day).
“This hidden extra sodium intake is often overlooked,” Dr. Zhang told this news organization.
Using data from the Health Improvement Network, a U.K. primary care database, the researchers examined 4,532 patients with hypertension taking sodium-containing acetaminophen and compared them with 146,866 patients with hypertension taking non–sodium-containing acetaminophen (tablet, capsule, or oral suspension formulations).
After 1 year, the risk of incident CVD (myocardial infarction, stroke, and heart failure) was 5.6% in those taking sodium-containing acetaminophen, compared with 4.6% in those taking non–sodium-containing acetaminophen (average weighted hazard ratio, 1.59; 95% confidence interval, 1.32-1.92).
A separate analysis of normotensive patients taking sodium-containing acetaminophen (n = 5,351) or non–sodium-containing acetaminophen (n = 141,948) gave similar results.
The 1-year risk of incident CVD was 4.4% in those taking sodium-containing acetaminophen vs. 3.7% among those taking non–sodium-containing acetaminophen (average weighted HR, 1.45; 95% CI, 1.18-1.79).
There was also evidence of a dose-response relationship.
In those with hypertension, CVD risk increased by roughly one-quarter (odds ratio, 1.26) for those with one prescription of sodium-containing acetaminophen and by nearly one half (OR, 1.45) for those with five or more prescriptions of sodium-containing acetaminophen. Similar findings were observed among adults without hypertension.
Mortality at 1 year was also higher in those taking sodium-containing acetaminophen than non–sodium-containing acetaminophen, in patients with hypertension (7.6% vs. 6.1%) and without hypertension (7.3% vs. 5.9%).
“The results are compelling,” write the authors of an editorial published with the study.
“The direct message from this study is clear – there are likely to be millions of people worldwide taking paracetamol on a daily basis in a ‘fast-acting’ effervescent or soluble formulation who are increasing their risks of cardiovascular disease and premature death,” say Aletta Schutte, PhD, and Bruce Neal, MBChB, PhD, of the George Institute for Global Health, Sydney.
“The weight of the evidence makes ongoing inaction on sodium-containing medications untenable. The widespread use of effervescent medication in the general population, and the enormous doses of sodium that can be consumed inadvertently by unsuspecting consumers requires urgent action,” Dr. Schutte and Dr. Neal say.
The study was supported by the National Natural Science Foundation of China, the National Key Research and Development Project, the Project Program of National Clinical Research Center for Geriatric Disorders, the Key Research and Development Program of Hunan Province, and the Science and Technology Program of Hunan Province. Dr. Zhang, Dr. Schutte, and Dr. Neal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN HEART JOURNAL
Violence in the workplace: The hidden dangers of a medical career
On Oct. 4, staff, patients, and medical students at my institution received word that a fatal shooting had occurred inside the campus hospital. For staff, this was a painful event compounding the already stressful pandemic times, while for students, it was a harsh introduction to the emerging dangers of practicing medicine.
Sure, . Unfortunately, acts of violence targeting health care workers occur at surprisingly high rates.
Reports following the shooting indicated that the gunman had a personal conflict with a coworker, and thankfully, larger numbers of people had not been targeted. While this may seem like a one-off incident, any shooting inside a hospital is a serious matter. Hospitals should be places of healing. Yes, they are inevitably places of suffering as well, but this pain should never be human-inflicted.
Health care workers are widely admired in the community, and increasingly so due to their sacrifices during the COVID-19 pandemic. Even though there is more attention to our health care spaces, the epidemic of occupational violence against our country’s health care workers has gone largely unrecognized, and this danger has only worsened since the onset of the pandemic.
Acts of violence against health care workers not only include fatal shootings or stabbings but may also include physical or verbal aggressions by frustrated patients and visitors. It is likely that students entering the health care field will encounter such danger during their careers.
Health care workers have four times the likelihood of being assaulted on the job, compared with those working in private industry. The World Health Organization reports that 38% of health workers can expect to experience physical violence at some point in their careers, while verbal threatening was reportedly even more common. It is plausible that the true rate of violence surpasses these rates, as reporting them is entirely voluntary.
In fact, the American Journal of Managed Care reported in 2019 that 75% of workplace assaults occur in health care, yet only 30% of nurses and 26% of emergency department physicians report such experiences.
Anecdotally, many of my own physician mentors have shared stories of troubling or threatening situations they have faced throughout their careers. These types of situations can be difficult to avoid, as providers are trained and naturally inclined to empathize with their patients and help as much as possible, making it difficult to turn away potentially violent individuals.
Since the start of the COVID-19 pandemic, as the public became more fatigued, incidents of violence rose. Facing staffing shortages, visitor restrictions, and high-acuity patients, health care workers found it increasingly difficult to manage large caseloads. High levels of stress were affecting not only patients, who were facing some of the toughest times of their lives, but also staff, who experienced rising demands.
Meanwhile, gun violence in our country has profoundly increased during the pandemic, creating an unstable backdrop to this tension.
Obviously, acts of violence against health care workers are unacceptable. Such events can pose real physical harms to providers, possibly resulting in irreversible injury, health problems, or even death. Additionally, they can yield long-term psychological harms, increase burnout, and impact job satisfaction.
Health care providers already make huge personal sacrifices to pursue their profession, and this threat of violence is an additional burden they unfortunately face.
In addition to the direct harm to employees, violence also has broader systemic detriments to patient outcomes and health care economics. Acts of violence against health care workers can lower the quality of care provided to patients – either directly, by virtue of being present during such dangerous situations, or indirectly, as stressed or burned-out providers may understandably be unable to provide optimal care. Rates of avoidable errors naturally rise in the presence of such stressors.
Unfortunately, regulations protecting health care workers from violence are sparse, and hospitals are not currently required to implement prevention plans for workplace violence. There are certainly some common-sense changes that institutions have begun implementing, including the use of metal detectors upon entry or the increased presence of security staff, but generally, it is questionable whether these measures alone can fully eliminate violence.
The first step in addressing this unacceptably common issue is to boost awareness and brainstorm creative solutions. Health care workers and medical students should at least be made aware of this widely prevalent threat, and safety training can be implemented to parallel that of our nation’s other schools, which have unfortunately faced a similar plight for decades.
However, similar to most issues in medicine, prevention is certainly the best strategy. By highlighting the unbelievably prevalent nature of this issue, along with its severe human and financial costs, hopefully we can draw the attention of policymakers to catalyze lasting change with a preventative focus.
The Thomas Jefferson University community responded to this tragic event with a message of resilience, offering mental health services, increasing its law enforcement presence, and promising to revisit physical security measures. This all-too-familiar pattern has been seen with previous acts of violence, but it has not yet yielded a true solution. Yet there’s not too much more an individual hospital can do without broader systemic change.
We must improve our awareness and understanding of the deep-rooted factors underlying this public health crisis and adapt how we communicate about them to achieve real progress.
Yash Shah is a first-year medical student at Thomas Jefferson University in Philadelphia. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On Oct. 4, staff, patients, and medical students at my institution received word that a fatal shooting had occurred inside the campus hospital. For staff, this was a painful event compounding the already stressful pandemic times, while for students, it was a harsh introduction to the emerging dangers of practicing medicine.
Sure, . Unfortunately, acts of violence targeting health care workers occur at surprisingly high rates.
Reports following the shooting indicated that the gunman had a personal conflict with a coworker, and thankfully, larger numbers of people had not been targeted. While this may seem like a one-off incident, any shooting inside a hospital is a serious matter. Hospitals should be places of healing. Yes, they are inevitably places of suffering as well, but this pain should never be human-inflicted.
Health care workers are widely admired in the community, and increasingly so due to their sacrifices during the COVID-19 pandemic. Even though there is more attention to our health care spaces, the epidemic of occupational violence against our country’s health care workers has gone largely unrecognized, and this danger has only worsened since the onset of the pandemic.
Acts of violence against health care workers not only include fatal shootings or stabbings but may also include physical or verbal aggressions by frustrated patients and visitors. It is likely that students entering the health care field will encounter such danger during their careers.
Health care workers have four times the likelihood of being assaulted on the job, compared with those working in private industry. The World Health Organization reports that 38% of health workers can expect to experience physical violence at some point in their careers, while verbal threatening was reportedly even more common. It is plausible that the true rate of violence surpasses these rates, as reporting them is entirely voluntary.
In fact, the American Journal of Managed Care reported in 2019 that 75% of workplace assaults occur in health care, yet only 30% of nurses and 26% of emergency department physicians report such experiences.
Anecdotally, many of my own physician mentors have shared stories of troubling or threatening situations they have faced throughout their careers. These types of situations can be difficult to avoid, as providers are trained and naturally inclined to empathize with their patients and help as much as possible, making it difficult to turn away potentially violent individuals.
Since the start of the COVID-19 pandemic, as the public became more fatigued, incidents of violence rose. Facing staffing shortages, visitor restrictions, and high-acuity patients, health care workers found it increasingly difficult to manage large caseloads. High levels of stress were affecting not only patients, who were facing some of the toughest times of their lives, but also staff, who experienced rising demands.
Meanwhile, gun violence in our country has profoundly increased during the pandemic, creating an unstable backdrop to this tension.
Obviously, acts of violence against health care workers are unacceptable. Such events can pose real physical harms to providers, possibly resulting in irreversible injury, health problems, or even death. Additionally, they can yield long-term psychological harms, increase burnout, and impact job satisfaction.
Health care providers already make huge personal sacrifices to pursue their profession, and this threat of violence is an additional burden they unfortunately face.
In addition to the direct harm to employees, violence also has broader systemic detriments to patient outcomes and health care economics. Acts of violence against health care workers can lower the quality of care provided to patients – either directly, by virtue of being present during such dangerous situations, or indirectly, as stressed or burned-out providers may understandably be unable to provide optimal care. Rates of avoidable errors naturally rise in the presence of such stressors.
Unfortunately, regulations protecting health care workers from violence are sparse, and hospitals are not currently required to implement prevention plans for workplace violence. There are certainly some common-sense changes that institutions have begun implementing, including the use of metal detectors upon entry or the increased presence of security staff, but generally, it is questionable whether these measures alone can fully eliminate violence.
The first step in addressing this unacceptably common issue is to boost awareness and brainstorm creative solutions. Health care workers and medical students should at least be made aware of this widely prevalent threat, and safety training can be implemented to parallel that of our nation’s other schools, which have unfortunately faced a similar plight for decades.
However, similar to most issues in medicine, prevention is certainly the best strategy. By highlighting the unbelievably prevalent nature of this issue, along with its severe human and financial costs, hopefully we can draw the attention of policymakers to catalyze lasting change with a preventative focus.
The Thomas Jefferson University community responded to this tragic event with a message of resilience, offering mental health services, increasing its law enforcement presence, and promising to revisit physical security measures. This all-too-familiar pattern has been seen with previous acts of violence, but it has not yet yielded a true solution. Yet there’s not too much more an individual hospital can do without broader systemic change.
We must improve our awareness and understanding of the deep-rooted factors underlying this public health crisis and adapt how we communicate about them to achieve real progress.
Yash Shah is a first-year medical student at Thomas Jefferson University in Philadelphia. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On Oct. 4, staff, patients, and medical students at my institution received word that a fatal shooting had occurred inside the campus hospital. For staff, this was a painful event compounding the already stressful pandemic times, while for students, it was a harsh introduction to the emerging dangers of practicing medicine.
Sure, . Unfortunately, acts of violence targeting health care workers occur at surprisingly high rates.
Reports following the shooting indicated that the gunman had a personal conflict with a coworker, and thankfully, larger numbers of people had not been targeted. While this may seem like a one-off incident, any shooting inside a hospital is a serious matter. Hospitals should be places of healing. Yes, they are inevitably places of suffering as well, but this pain should never be human-inflicted.
Health care workers are widely admired in the community, and increasingly so due to their sacrifices during the COVID-19 pandemic. Even though there is more attention to our health care spaces, the epidemic of occupational violence against our country’s health care workers has gone largely unrecognized, and this danger has only worsened since the onset of the pandemic.
Acts of violence against health care workers not only include fatal shootings or stabbings but may also include physical or verbal aggressions by frustrated patients and visitors. It is likely that students entering the health care field will encounter such danger during their careers.
Health care workers have four times the likelihood of being assaulted on the job, compared with those working in private industry. The World Health Organization reports that 38% of health workers can expect to experience physical violence at some point in their careers, while verbal threatening was reportedly even more common. It is plausible that the true rate of violence surpasses these rates, as reporting them is entirely voluntary.
In fact, the American Journal of Managed Care reported in 2019 that 75% of workplace assaults occur in health care, yet only 30% of nurses and 26% of emergency department physicians report such experiences.
Anecdotally, many of my own physician mentors have shared stories of troubling or threatening situations they have faced throughout their careers. These types of situations can be difficult to avoid, as providers are trained and naturally inclined to empathize with their patients and help as much as possible, making it difficult to turn away potentially violent individuals.
Since the start of the COVID-19 pandemic, as the public became more fatigued, incidents of violence rose. Facing staffing shortages, visitor restrictions, and high-acuity patients, health care workers found it increasingly difficult to manage large caseloads. High levels of stress were affecting not only patients, who were facing some of the toughest times of their lives, but also staff, who experienced rising demands.
Meanwhile, gun violence in our country has profoundly increased during the pandemic, creating an unstable backdrop to this tension.
Obviously, acts of violence against health care workers are unacceptable. Such events can pose real physical harms to providers, possibly resulting in irreversible injury, health problems, or even death. Additionally, they can yield long-term psychological harms, increase burnout, and impact job satisfaction.
Health care providers already make huge personal sacrifices to pursue their profession, and this threat of violence is an additional burden they unfortunately face.
In addition to the direct harm to employees, violence also has broader systemic detriments to patient outcomes and health care economics. Acts of violence against health care workers can lower the quality of care provided to patients – either directly, by virtue of being present during such dangerous situations, or indirectly, as stressed or burned-out providers may understandably be unable to provide optimal care. Rates of avoidable errors naturally rise in the presence of such stressors.
Unfortunately, regulations protecting health care workers from violence are sparse, and hospitals are not currently required to implement prevention plans for workplace violence. There are certainly some common-sense changes that institutions have begun implementing, including the use of metal detectors upon entry or the increased presence of security staff, but generally, it is questionable whether these measures alone can fully eliminate violence.
The first step in addressing this unacceptably common issue is to boost awareness and brainstorm creative solutions. Health care workers and medical students should at least be made aware of this widely prevalent threat, and safety training can be implemented to parallel that of our nation’s other schools, which have unfortunately faced a similar plight for decades.
However, similar to most issues in medicine, prevention is certainly the best strategy. By highlighting the unbelievably prevalent nature of this issue, along with its severe human and financial costs, hopefully we can draw the attention of policymakers to catalyze lasting change with a preventative focus.
The Thomas Jefferson University community responded to this tragic event with a message of resilience, offering mental health services, increasing its law enforcement presence, and promising to revisit physical security measures. This all-too-familiar pattern has been seen with previous acts of violence, but it has not yet yielded a true solution. Yet there’s not too much more an individual hospital can do without broader systemic change.
We must improve our awareness and understanding of the deep-rooted factors underlying this public health crisis and adapt how we communicate about them to achieve real progress.
Yash Shah is a first-year medical student at Thomas Jefferson University in Philadelphia. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AHA targets ‘low-value’ heart care in new scientific statement
Low-value health care services that provide little or no benefit to patients are “common, potentially harmful, and costly,” and there is a critical need to reduce this kind of care, the American Heart Association said in a newly released scientific statement.
Each year, nearly half of patients in the United States will receive at least one low-value test or procedure, with the attendant risk of avoidable complications from cascades of care and excess costs to individuals and society, the authors noted. Reducing low-value care is particularly important in cardiology, given the high prevalence and costs of cardiovascular disease in the United States.
The statement was published online Feb. 22, 2022, in Circulation: Cardiovascular Quality and Outcomes.
High burden with uncertain benefit
“Cardiovascular disease is common and can present suddenly, such as a heart attack or abnormal heart rhythm,” Vinay Kini, MD, chair of the statement writing group and assistant professor of medicine at Weill Cornell Medicine, New York, said in a news release.
“Our desire to be vigilant about treating and preventing cardiovascular disease may sometimes lead to use of tests and procedures where the benefits to patients may be uncertain,” Dr. Kini said. “This may impose burdens on patients, in the form of increased risk of physical harm from the low-value procedure or potential complications, as well as follow-up care and out-of-pocket financial costs.”
For example, studies have shown that up to one in five echocardiograms and up to half of all stress tests performed in the United States may be rated as rarely appropriate, based on established guidelines for their use.
In addition, up to 15% of percutaneous coronary interventions (PCIs) are classified as rarely appropriate, the writing group said.
Annually, among Medicare fee-for-service beneficiaries, low-value stress testing in patients with stable coronary artery disease is estimated to cost between $212 million and $2.1 billion, while costs of PCI for stable CAD range from $212 million to $2.8 billion, the writing group noted.
“At best, spending on low-value care potentially diverts resources from higher-value services that would benefit patients more effectively at the same or reduced cost. At worst, low-value care results in physical harm in the form of preventable morbidity and mortality,” they said.
“Thus, reducing low-value care is one of the few patient-centered solutions that directly address both the need to control health care spending and the societal imperative to devote its limited resources to beneficial health care services that improve health,” they added.
The group outlines several ways to reduce low-value cardiovascular care targeting patients, providers, and payers/policymakers.
For patients, education and shared decision-making may help reduce low-value care and dispel misconceptions about the intended purpose of test or treatment, they suggested.
For clinicians, a “layered” approach to reducing low-value care may be most effective, such as through education, audit and feedback, and behavioral science tools (“nudges”) to shift behaviors and practices, they said.
For payers and policy leaders, interventions to reduce low-value care include national insurance coverage determinations; prior authorization; alternative payment models that reward lower costs and higher-quality health care; value-based insurance designs that financially penalize low-value care; and medical liability reform to reduce defensive medical practices.
Low-value cardiovascular care is a complex problem, the writing group acknowledged, and achieving meaningful reductions in low-value cardiovascular care will require a multidisciplinary approach that includes continuous research, implementation, evaluation, and adjustment while ensuring equitable access to care.
“Each approach has benefits and drawbacks,” Dr. Kini said. “For example, prior authorization imposes a large burden on health care professionals to obtain insurance approval for tests and treatments. Prior authorization and some value-based payment models may unintentionally worsen existing racial and ethnic health care disparities.
“A one-size-fits-all approach to reducing low-value care is unlikely to succeed; rather, acting through multiple perspectives and frequently measuring impacts and potential unintended consequences is critical,” he concluded.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Council on Quality of Care and Outcomes Research.
The research had no commercial funding. Dr. Kini disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-value health care services that provide little or no benefit to patients are “common, potentially harmful, and costly,” and there is a critical need to reduce this kind of care, the American Heart Association said in a newly released scientific statement.
Each year, nearly half of patients in the United States will receive at least one low-value test or procedure, with the attendant risk of avoidable complications from cascades of care and excess costs to individuals and society, the authors noted. Reducing low-value care is particularly important in cardiology, given the high prevalence and costs of cardiovascular disease in the United States.
The statement was published online Feb. 22, 2022, in Circulation: Cardiovascular Quality and Outcomes.
High burden with uncertain benefit
“Cardiovascular disease is common and can present suddenly, such as a heart attack or abnormal heart rhythm,” Vinay Kini, MD, chair of the statement writing group and assistant professor of medicine at Weill Cornell Medicine, New York, said in a news release.
“Our desire to be vigilant about treating and preventing cardiovascular disease may sometimes lead to use of tests and procedures where the benefits to patients may be uncertain,” Dr. Kini said. “This may impose burdens on patients, in the form of increased risk of physical harm from the low-value procedure or potential complications, as well as follow-up care and out-of-pocket financial costs.”
For example, studies have shown that up to one in five echocardiograms and up to half of all stress tests performed in the United States may be rated as rarely appropriate, based on established guidelines for their use.
In addition, up to 15% of percutaneous coronary interventions (PCIs) are classified as rarely appropriate, the writing group said.
Annually, among Medicare fee-for-service beneficiaries, low-value stress testing in patients with stable coronary artery disease is estimated to cost between $212 million and $2.1 billion, while costs of PCI for stable CAD range from $212 million to $2.8 billion, the writing group noted.
“At best, spending on low-value care potentially diverts resources from higher-value services that would benefit patients more effectively at the same or reduced cost. At worst, low-value care results in physical harm in the form of preventable morbidity and mortality,” they said.
“Thus, reducing low-value care is one of the few patient-centered solutions that directly address both the need to control health care spending and the societal imperative to devote its limited resources to beneficial health care services that improve health,” they added.
The group outlines several ways to reduce low-value cardiovascular care targeting patients, providers, and payers/policymakers.
For patients, education and shared decision-making may help reduce low-value care and dispel misconceptions about the intended purpose of test or treatment, they suggested.
For clinicians, a “layered” approach to reducing low-value care may be most effective, such as through education, audit and feedback, and behavioral science tools (“nudges”) to shift behaviors and practices, they said.
For payers and policy leaders, interventions to reduce low-value care include national insurance coverage determinations; prior authorization; alternative payment models that reward lower costs and higher-quality health care; value-based insurance designs that financially penalize low-value care; and medical liability reform to reduce defensive medical practices.
Low-value cardiovascular care is a complex problem, the writing group acknowledged, and achieving meaningful reductions in low-value cardiovascular care will require a multidisciplinary approach that includes continuous research, implementation, evaluation, and adjustment while ensuring equitable access to care.
“Each approach has benefits and drawbacks,” Dr. Kini said. “For example, prior authorization imposes a large burden on health care professionals to obtain insurance approval for tests and treatments. Prior authorization and some value-based payment models may unintentionally worsen existing racial and ethnic health care disparities.
“A one-size-fits-all approach to reducing low-value care is unlikely to succeed; rather, acting through multiple perspectives and frequently measuring impacts and potential unintended consequences is critical,” he concluded.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Council on Quality of Care and Outcomes Research.
The research had no commercial funding. Dr. Kini disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-value health care services that provide little or no benefit to patients are “common, potentially harmful, and costly,” and there is a critical need to reduce this kind of care, the American Heart Association said in a newly released scientific statement.
Each year, nearly half of patients in the United States will receive at least one low-value test or procedure, with the attendant risk of avoidable complications from cascades of care and excess costs to individuals and society, the authors noted. Reducing low-value care is particularly important in cardiology, given the high prevalence and costs of cardiovascular disease in the United States.
The statement was published online Feb. 22, 2022, in Circulation: Cardiovascular Quality and Outcomes.
High burden with uncertain benefit
“Cardiovascular disease is common and can present suddenly, such as a heart attack or abnormal heart rhythm,” Vinay Kini, MD, chair of the statement writing group and assistant professor of medicine at Weill Cornell Medicine, New York, said in a news release.
“Our desire to be vigilant about treating and preventing cardiovascular disease may sometimes lead to use of tests and procedures where the benefits to patients may be uncertain,” Dr. Kini said. “This may impose burdens on patients, in the form of increased risk of physical harm from the low-value procedure or potential complications, as well as follow-up care and out-of-pocket financial costs.”
For example, studies have shown that up to one in five echocardiograms and up to half of all stress tests performed in the United States may be rated as rarely appropriate, based on established guidelines for their use.
In addition, up to 15% of percutaneous coronary interventions (PCIs) are classified as rarely appropriate, the writing group said.
Annually, among Medicare fee-for-service beneficiaries, low-value stress testing in patients with stable coronary artery disease is estimated to cost between $212 million and $2.1 billion, while costs of PCI for stable CAD range from $212 million to $2.8 billion, the writing group noted.
“At best, spending on low-value care potentially diverts resources from higher-value services that would benefit patients more effectively at the same or reduced cost. At worst, low-value care results in physical harm in the form of preventable morbidity and mortality,” they said.
“Thus, reducing low-value care is one of the few patient-centered solutions that directly address both the need to control health care spending and the societal imperative to devote its limited resources to beneficial health care services that improve health,” they added.
The group outlines several ways to reduce low-value cardiovascular care targeting patients, providers, and payers/policymakers.
For patients, education and shared decision-making may help reduce low-value care and dispel misconceptions about the intended purpose of test or treatment, they suggested.
For clinicians, a “layered” approach to reducing low-value care may be most effective, such as through education, audit and feedback, and behavioral science tools (“nudges”) to shift behaviors and practices, they said.
For payers and policy leaders, interventions to reduce low-value care include national insurance coverage determinations; prior authorization; alternative payment models that reward lower costs and higher-quality health care; value-based insurance designs that financially penalize low-value care; and medical liability reform to reduce defensive medical practices.
Low-value cardiovascular care is a complex problem, the writing group acknowledged, and achieving meaningful reductions in low-value cardiovascular care will require a multidisciplinary approach that includes continuous research, implementation, evaluation, and adjustment while ensuring equitable access to care.
“Each approach has benefits and drawbacks,” Dr. Kini said. “For example, prior authorization imposes a large burden on health care professionals to obtain insurance approval for tests and treatments. Prior authorization and some value-based payment models may unintentionally worsen existing racial and ethnic health care disparities.
“A one-size-fits-all approach to reducing low-value care is unlikely to succeed; rather, acting through multiple perspectives and frequently measuring impacts and potential unintended consequences is critical,” he concluded.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Council on Quality of Care and Outcomes Research.
The research had no commercial funding. Dr. Kini disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES







