User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA okays empagliflozin for HF regardless of ejection fraction
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded heart failure indication for the sodium-glucose transporter 2 inhibitor empagliflozin (Jardiance) that now includes HF with mid-range or preserved left ventricular ejection fraction (LVEF), the agency announced on Feb. 24.
That means the SGLT2 inhibitor, once considered primarily an antidiabetic agent, is approved for use in patients with HF per se without regard to ventricular function. The drug received approval for HF with reduced LVEF in August 2021.
The expanded indication, specifically for reducing the risk of cardiovascular death and HF hospitalization in adults, was widely anticipated based on the landmark results from the EMPEROR-Preserved trial. The study saw a significant 21% relative reduction in that composite endpoint over about 2 years in patients with New York Heart Association class II-IV heart failure and an LVEF greater than 40% who received empagliflozin along with other standard care.
Interestingly, the drug’s expanded indication in HF resembles that approved for sacubitril/valsartan (Entresto) in February 2021 based mostly on the PARAGON-HF trial, which entered patients with HF and an LVEF at least 45%. The trial was “negative” in that it saw no significant advantage to the drug for its primary clinical outcome but did suggest benefit for some secondary endpoints.
The FDA had used more cautionary language in its expanded indication for sacubitril/valsartan, “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction below normal.”
A version of this article first appeared on Medscape.com.
Cardiologist whistleblower lawsuit settled for $3.8 million
Catholic Medical Center has agreed to pay $3.8 million to settle claims it provided free call coverage to a cardiologist in exchange for patient referrals to the Manchester, N.H., hospital, according to federal officials.
“The cardiologist who received the free call coverage referred millions of dollars in medical procedures and services to CMC over the decade in which the free services were provided,” the Department of Justice said in a news release.
Because the hospital submitted claims for payment to Medicare, Medicaid, and other federal health care programs for the services referred by the cardiologist, the government alleged the claims were the result of unlawful kickbacks.
The settlement resolves allegations brought in a whistleblower lawsuit filed in 2018 by cardiologist David Goldberg, MD, who previously worked at Catholic Medical Center (CMC) and is represented by Douglas, Leonard & Garvey.
The news release did not name the cardiologist involved in the alleged kickback scheme but the recently unsealed lawsuit says CMC paid its cardiologists above market rates ($10,000 per weekend, $3,000 per night) to provide free coverage services for Mary-Claire Paicopolis, MD.
The lawsuit also claims Dr. Paicopolis insisted the hospital implant only Boston Scientific devices in her patients and that her preferred electrophysiologist use only its Rhythmia mapping system during ablation procedures. To keep CMC from objecting, the suit alleges Boston Scientific offered CMC early access to its Watchman left atrial appendage occluder and provided “unprecedented” support to a nonacademic community hospital site.
“It went back several years, and that and the other issues in the suit were strong motivators for Dr. Goldberg to try to rectify the situation and he deserves a lot of credit for having done so,” attorney Charles G. Douglas III told this news organization.
Dr. Goldberg will receive $570,000 of the $3.8 million settlement as well as $145,361 in expenses, attorney fees, and costs.
Although not addressed in the federal news release, the lawsuit also alleges that CMC staff manipulated mortality data by discharging patients from the ICU and then readmitting them to hospice with a new patient number, “thereby avoiding the need to claim a surgical mortality.”
The lawsuit also says CMC “created a practice of covering up medical errors” and detailed 12 patient deaths between 2012 and 2018, alleging that these deaths were the result of substandard care.
CMC spokesperson Lauren Collins-Cline said in an email that the call coverage arrangement is no longer in place and originated almost 15 years ago with the input of legal counsel in order to provide high-quality care for patients.
“While CMC vigorously disagrees with the government’s allegations that this arrangement violated federal law, we have agreed to settle in order to avoid long costly civil litigation,” she said.
As to the other claims in the complaint, Ms. Collins-Cline said they were investigated by the government and dismissed per the settlement agreement. “CMC holds itself to the highest ethical standards in patient care and business conduct. That’s embedded in our mission and will always remain our highest priority.”
Mr. Douglas, however, said the government retains the right to pursue other claims in the lawsuit in the future. “So, [the hospital] is a little more optimistic than the reality of what the government agrees is the situation.”
A version of this article first appeared on Medscape.com.
Catholic Medical Center has agreed to pay $3.8 million to settle claims it provided free call coverage to a cardiologist in exchange for patient referrals to the Manchester, N.H., hospital, according to federal officials.
“The cardiologist who received the free call coverage referred millions of dollars in medical procedures and services to CMC over the decade in which the free services were provided,” the Department of Justice said in a news release.
Because the hospital submitted claims for payment to Medicare, Medicaid, and other federal health care programs for the services referred by the cardiologist, the government alleged the claims were the result of unlawful kickbacks.
The settlement resolves allegations brought in a whistleblower lawsuit filed in 2018 by cardiologist David Goldberg, MD, who previously worked at Catholic Medical Center (CMC) and is represented by Douglas, Leonard & Garvey.
The news release did not name the cardiologist involved in the alleged kickback scheme but the recently unsealed lawsuit says CMC paid its cardiologists above market rates ($10,000 per weekend, $3,000 per night) to provide free coverage services for Mary-Claire Paicopolis, MD.
The lawsuit also claims Dr. Paicopolis insisted the hospital implant only Boston Scientific devices in her patients and that her preferred electrophysiologist use only its Rhythmia mapping system during ablation procedures. To keep CMC from objecting, the suit alleges Boston Scientific offered CMC early access to its Watchman left atrial appendage occluder and provided “unprecedented” support to a nonacademic community hospital site.
“It went back several years, and that and the other issues in the suit were strong motivators for Dr. Goldberg to try to rectify the situation and he deserves a lot of credit for having done so,” attorney Charles G. Douglas III told this news organization.
Dr. Goldberg will receive $570,000 of the $3.8 million settlement as well as $145,361 in expenses, attorney fees, and costs.
Although not addressed in the federal news release, the lawsuit also alleges that CMC staff manipulated mortality data by discharging patients from the ICU and then readmitting them to hospice with a new patient number, “thereby avoiding the need to claim a surgical mortality.”
The lawsuit also says CMC “created a practice of covering up medical errors” and detailed 12 patient deaths between 2012 and 2018, alleging that these deaths were the result of substandard care.
CMC spokesperson Lauren Collins-Cline said in an email that the call coverage arrangement is no longer in place and originated almost 15 years ago with the input of legal counsel in order to provide high-quality care for patients.
“While CMC vigorously disagrees with the government’s allegations that this arrangement violated federal law, we have agreed to settle in order to avoid long costly civil litigation,” she said.
As to the other claims in the complaint, Ms. Collins-Cline said they were investigated by the government and dismissed per the settlement agreement. “CMC holds itself to the highest ethical standards in patient care and business conduct. That’s embedded in our mission and will always remain our highest priority.”
Mr. Douglas, however, said the government retains the right to pursue other claims in the lawsuit in the future. “So, [the hospital] is a little more optimistic than the reality of what the government agrees is the situation.”
A version of this article first appeared on Medscape.com.
Catholic Medical Center has agreed to pay $3.8 million to settle claims it provided free call coverage to a cardiologist in exchange for patient referrals to the Manchester, N.H., hospital, according to federal officials.
“The cardiologist who received the free call coverage referred millions of dollars in medical procedures and services to CMC over the decade in which the free services were provided,” the Department of Justice said in a news release.
Because the hospital submitted claims for payment to Medicare, Medicaid, and other federal health care programs for the services referred by the cardiologist, the government alleged the claims were the result of unlawful kickbacks.
The settlement resolves allegations brought in a whistleblower lawsuit filed in 2018 by cardiologist David Goldberg, MD, who previously worked at Catholic Medical Center (CMC) and is represented by Douglas, Leonard & Garvey.
The news release did not name the cardiologist involved in the alleged kickback scheme but the recently unsealed lawsuit says CMC paid its cardiologists above market rates ($10,000 per weekend, $3,000 per night) to provide free coverage services for Mary-Claire Paicopolis, MD.
The lawsuit also claims Dr. Paicopolis insisted the hospital implant only Boston Scientific devices in her patients and that her preferred electrophysiologist use only its Rhythmia mapping system during ablation procedures. To keep CMC from objecting, the suit alleges Boston Scientific offered CMC early access to its Watchman left atrial appendage occluder and provided “unprecedented” support to a nonacademic community hospital site.
“It went back several years, and that and the other issues in the suit were strong motivators for Dr. Goldberg to try to rectify the situation and he deserves a lot of credit for having done so,” attorney Charles G. Douglas III told this news organization.
Dr. Goldberg will receive $570,000 of the $3.8 million settlement as well as $145,361 in expenses, attorney fees, and costs.
Although not addressed in the federal news release, the lawsuit also alleges that CMC staff manipulated mortality data by discharging patients from the ICU and then readmitting them to hospice with a new patient number, “thereby avoiding the need to claim a surgical mortality.”
The lawsuit also says CMC “created a practice of covering up medical errors” and detailed 12 patient deaths between 2012 and 2018, alleging that these deaths were the result of substandard care.
CMC spokesperson Lauren Collins-Cline said in an email that the call coverage arrangement is no longer in place and originated almost 15 years ago with the input of legal counsel in order to provide high-quality care for patients.
“While CMC vigorously disagrees with the government’s allegations that this arrangement violated federal law, we have agreed to settle in order to avoid long costly civil litigation,” she said.
As to the other claims in the complaint, Ms. Collins-Cline said they were investigated by the government and dismissed per the settlement agreement. “CMC holds itself to the highest ethical standards in patient care and business conduct. That’s embedded in our mission and will always remain our highest priority.”
Mr. Douglas, however, said the government retains the right to pursue other claims in the lawsuit in the future. “So, [the hospital] is a little more optimistic than the reality of what the government agrees is the situation.”
A version of this article first appeared on Medscape.com.
Twenty-three percent of health care workers likely to leave industry soon: Poll
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
Your heart doesn’t like peas any more than you do
Big Vegetable has lied to us all
Hear this, children of the world: Your parents have betrayed you. They tell you day in and day out that vegetables are necessary, that they’re healthy, that you need them, but it is not the truth. Behind their foul taste is nothing but empty lies.
Okay, before we get a full-blown child rebellion on our hands, let’s reel things in. Eating vegetables has many benefits, and will help prevent many nasty medical conditions, such as diabetes or cancer. However, cardiovascular disease is not among them.
For their study published in Frontiers in Nutrition, researchers analyzed the diet, lifestyle, and medical history of nearly 400,000 U.K. adults over a 5-year period, finding that 4.5% developed heart disease and that the average adult consumed about 5 tablespoons of vegetables per day. Those who consumed the most vegetables had a reduction in heart disease incidence of about 15%, compared with those who ate the least.
Hang on, you’re thinking, we just said that vegetables didn’t prevent cardiovascular disease. But the data show otherwise! Ah, but the data are unadjusted. Once the researchers took socioeconomic status, information level, and general lifestyle into account, that benefit disappeared almost completely. The benefit seems to come not from the vegetables themselves, but from being able to afford better food and medical care in general.
The researchers were quick to note the other benefits of eating vegetables, and that people should probably keep eating those five servings a day. But we’re onto you, scientists. You can’t fool us with your vegetable-based lies. Unless we’re talking about pizza. Pizza is the best vegetable.
The good old days of surgery?
Modern surgical instruments, techniques, and technological innovations are amazing. It’s hard to imagine what surgery was like before laparoscopes came along, or x-ray machines, or even anesthesia. But those days weren’t really that long ago. Modern anesthesia, after all, dates back to just 1846. We’ve got socks almost that old.
But suppose we go back even further … say 5,300 years. Older than the oldest sock. Scientists studying a funerary chamber in Burgos, Spain, which was built in the 4th millennium B.C., have come across what looks like “the first known radical mastoidectomy in the history of humankind,” Sonia Díaz-Navarro of the University of Valladolid (Spain) and associates wrote in Scientific Reports.
One of the skulls they uncovered shows signs of trepanation. “Despite the [evidence] of cut marks, it is difficult to conclude the type of tool used to remove the bone tissue, most likely a sharp instrument with a circular movement,” they investigators said.
What is clear, though, is that the patient survived the surgery, because there is evidence of bone regeneration at the surgical sites. Sites? “Based on the differences in bone remodelling between the two temporals, it appears that the procedure was first conducted on the right ear, due to an ear pathology sufficiently alarming to require an intervention, which this prehistoric woman survived,” they explained.
The same procedure was then performed on the left ear, “but whether this was performed shortly after the right ear, or several months or even years later can’t be concluded from the existing evidence,” IFL Science reported.
Located nearby was a small section of tree bark with some scratches on it. That, ladies and gentlemen, was the first prior authorization form.
I hate that song, with reason
Do you have a favorite song? You may have a million reasons for loving that song. And past research can tell you why. But it’s only in a recent study that researchers were able to tell you why you dislike a song. And you know the song we’re talking about.
Dislike breaks down into three major categories of rationale: subject-related reasons (how the song makes you feel emotionally and/or physically), object-related reasons (the lyrics or composition), and social reasons (do you relate to this?). Researchers at the Max Planck Institute for Empirical Aesthetics in Frankfurt, Germany, interviewed 21 participants and asked them to come up with a prepared list of music that they disliked and why they didn’t like it. And there was a lot that they didn’t like: 277 dislikes worth, to be exact.
“The most often mentioned type of dislike was musical style, followed by artist and genre,” senior author Julia Merrill explained on Eurekalert. Just over 40% of those rationales for not liking the music just had to do with the music itself, but 85% involved the music combined with one of the other categories.
Social reasoning played a big part in dislike. If the listener didn’t feel like a part of the target in-group for the music or the music didn’t have the same social values as those of the listener, it had an impact on dislike, they said.
But our dislike of certain types of music doesn’t just separate us from people in a negative way. Looking at the dislike of certain types of music helps us define our terms of having good taste, the researchers explained. Saying that one type of music is better than another can bring us closer with like-minded people and becomes a piece of how we identify ourselves. Cue the music snobs.
So if you can blast Barry Manilow but can’t bring yourself to play the Rolling Stones, there’s a reason for that. And if you love Aretha Franklin but not Frank Sinatra, there’s a reason for that, too. It’s all very personal. Just as music is meant to be.
Big Vegetable has lied to us all
Hear this, children of the world: Your parents have betrayed you. They tell you day in and day out that vegetables are necessary, that they’re healthy, that you need them, but it is not the truth. Behind their foul taste is nothing but empty lies.
Okay, before we get a full-blown child rebellion on our hands, let’s reel things in. Eating vegetables has many benefits, and will help prevent many nasty medical conditions, such as diabetes or cancer. However, cardiovascular disease is not among them.
For their study published in Frontiers in Nutrition, researchers analyzed the diet, lifestyle, and medical history of nearly 400,000 U.K. adults over a 5-year period, finding that 4.5% developed heart disease and that the average adult consumed about 5 tablespoons of vegetables per day. Those who consumed the most vegetables had a reduction in heart disease incidence of about 15%, compared with those who ate the least.
Hang on, you’re thinking, we just said that vegetables didn’t prevent cardiovascular disease. But the data show otherwise! Ah, but the data are unadjusted. Once the researchers took socioeconomic status, information level, and general lifestyle into account, that benefit disappeared almost completely. The benefit seems to come not from the vegetables themselves, but from being able to afford better food and medical care in general.
The researchers were quick to note the other benefits of eating vegetables, and that people should probably keep eating those five servings a day. But we’re onto you, scientists. You can’t fool us with your vegetable-based lies. Unless we’re talking about pizza. Pizza is the best vegetable.
The good old days of surgery?
Modern surgical instruments, techniques, and technological innovations are amazing. It’s hard to imagine what surgery was like before laparoscopes came along, or x-ray machines, or even anesthesia. But those days weren’t really that long ago. Modern anesthesia, after all, dates back to just 1846. We’ve got socks almost that old.
But suppose we go back even further … say 5,300 years. Older than the oldest sock. Scientists studying a funerary chamber in Burgos, Spain, which was built in the 4th millennium B.C., have come across what looks like “the first known radical mastoidectomy in the history of humankind,” Sonia Díaz-Navarro of the University of Valladolid (Spain) and associates wrote in Scientific Reports.
One of the skulls they uncovered shows signs of trepanation. “Despite the [evidence] of cut marks, it is difficult to conclude the type of tool used to remove the bone tissue, most likely a sharp instrument with a circular movement,” they investigators said.
What is clear, though, is that the patient survived the surgery, because there is evidence of bone regeneration at the surgical sites. Sites? “Based on the differences in bone remodelling between the two temporals, it appears that the procedure was first conducted on the right ear, due to an ear pathology sufficiently alarming to require an intervention, which this prehistoric woman survived,” they explained.
The same procedure was then performed on the left ear, “but whether this was performed shortly after the right ear, or several months or even years later can’t be concluded from the existing evidence,” IFL Science reported.
Located nearby was a small section of tree bark with some scratches on it. That, ladies and gentlemen, was the first prior authorization form.
I hate that song, with reason
Do you have a favorite song? You may have a million reasons for loving that song. And past research can tell you why. But it’s only in a recent study that researchers were able to tell you why you dislike a song. And you know the song we’re talking about.
Dislike breaks down into three major categories of rationale: subject-related reasons (how the song makes you feel emotionally and/or physically), object-related reasons (the lyrics or composition), and social reasons (do you relate to this?). Researchers at the Max Planck Institute for Empirical Aesthetics in Frankfurt, Germany, interviewed 21 participants and asked them to come up with a prepared list of music that they disliked and why they didn’t like it. And there was a lot that they didn’t like: 277 dislikes worth, to be exact.
“The most often mentioned type of dislike was musical style, followed by artist and genre,” senior author Julia Merrill explained on Eurekalert. Just over 40% of those rationales for not liking the music just had to do with the music itself, but 85% involved the music combined with one of the other categories.
Social reasoning played a big part in dislike. If the listener didn’t feel like a part of the target in-group for the music or the music didn’t have the same social values as those of the listener, it had an impact on dislike, they said.
But our dislike of certain types of music doesn’t just separate us from people in a negative way. Looking at the dislike of certain types of music helps us define our terms of having good taste, the researchers explained. Saying that one type of music is better than another can bring us closer with like-minded people and becomes a piece of how we identify ourselves. Cue the music snobs.
So if you can blast Barry Manilow but can’t bring yourself to play the Rolling Stones, there’s a reason for that. And if you love Aretha Franklin but not Frank Sinatra, there’s a reason for that, too. It’s all very personal. Just as music is meant to be.
Big Vegetable has lied to us all
Hear this, children of the world: Your parents have betrayed you. They tell you day in and day out that vegetables are necessary, that they’re healthy, that you need them, but it is not the truth. Behind their foul taste is nothing but empty lies.
Okay, before we get a full-blown child rebellion on our hands, let’s reel things in. Eating vegetables has many benefits, and will help prevent many nasty medical conditions, such as diabetes or cancer. However, cardiovascular disease is not among them.
For their study published in Frontiers in Nutrition, researchers analyzed the diet, lifestyle, and medical history of nearly 400,000 U.K. adults over a 5-year period, finding that 4.5% developed heart disease and that the average adult consumed about 5 tablespoons of vegetables per day. Those who consumed the most vegetables had a reduction in heart disease incidence of about 15%, compared with those who ate the least.
Hang on, you’re thinking, we just said that vegetables didn’t prevent cardiovascular disease. But the data show otherwise! Ah, but the data are unadjusted. Once the researchers took socioeconomic status, information level, and general lifestyle into account, that benefit disappeared almost completely. The benefit seems to come not from the vegetables themselves, but from being able to afford better food and medical care in general.
The researchers were quick to note the other benefits of eating vegetables, and that people should probably keep eating those five servings a day. But we’re onto you, scientists. You can’t fool us with your vegetable-based lies. Unless we’re talking about pizza. Pizza is the best vegetable.
The good old days of surgery?
Modern surgical instruments, techniques, and technological innovations are amazing. It’s hard to imagine what surgery was like before laparoscopes came along, or x-ray machines, or even anesthesia. But those days weren’t really that long ago. Modern anesthesia, after all, dates back to just 1846. We’ve got socks almost that old.
But suppose we go back even further … say 5,300 years. Older than the oldest sock. Scientists studying a funerary chamber in Burgos, Spain, which was built in the 4th millennium B.C., have come across what looks like “the first known radical mastoidectomy in the history of humankind,” Sonia Díaz-Navarro of the University of Valladolid (Spain) and associates wrote in Scientific Reports.
One of the skulls they uncovered shows signs of trepanation. “Despite the [evidence] of cut marks, it is difficult to conclude the type of tool used to remove the bone tissue, most likely a sharp instrument with a circular movement,” they investigators said.
What is clear, though, is that the patient survived the surgery, because there is evidence of bone regeneration at the surgical sites. Sites? “Based on the differences in bone remodelling between the two temporals, it appears that the procedure was first conducted on the right ear, due to an ear pathology sufficiently alarming to require an intervention, which this prehistoric woman survived,” they explained.
The same procedure was then performed on the left ear, “but whether this was performed shortly after the right ear, or several months or even years later can’t be concluded from the existing evidence,” IFL Science reported.
Located nearby was a small section of tree bark with some scratches on it. That, ladies and gentlemen, was the first prior authorization form.
I hate that song, with reason
Do you have a favorite song? You may have a million reasons for loving that song. And past research can tell you why. But it’s only in a recent study that researchers were able to tell you why you dislike a song. And you know the song we’re talking about.
Dislike breaks down into three major categories of rationale: subject-related reasons (how the song makes you feel emotionally and/or physically), object-related reasons (the lyrics or composition), and social reasons (do you relate to this?). Researchers at the Max Planck Institute for Empirical Aesthetics in Frankfurt, Germany, interviewed 21 participants and asked them to come up with a prepared list of music that they disliked and why they didn’t like it. And there was a lot that they didn’t like: 277 dislikes worth, to be exact.
“The most often mentioned type of dislike was musical style, followed by artist and genre,” senior author Julia Merrill explained on Eurekalert. Just over 40% of those rationales for not liking the music just had to do with the music itself, but 85% involved the music combined with one of the other categories.
Social reasoning played a big part in dislike. If the listener didn’t feel like a part of the target in-group for the music or the music didn’t have the same social values as those of the listener, it had an impact on dislike, they said.
But our dislike of certain types of music doesn’t just separate us from people in a negative way. Looking at the dislike of certain types of music helps us define our terms of having good taste, the researchers explained. Saying that one type of music is better than another can bring us closer with like-minded people and becomes a piece of how we identify ourselves. Cue the music snobs.
So if you can blast Barry Manilow but can’t bring yourself to play the Rolling Stones, there’s a reason for that. And if you love Aretha Franklin but not Frank Sinatra, there’s a reason for that, too. It’s all very personal. Just as music is meant to be.
Robotic transcranial Doppler improves PFO detection after stroke
in a new study.
Being far easier to perform than regular transcranial Doppler ultrasound, it’s hoped that use of the robotic device will enable many more patients to undergo the more sensitive transcranial screening modality and increase the number of shunts identified.
“I believe robot-assisted transcranial Doppler ultrasound can fill the gap between the gold standard transcranial Doppler and transthoracic echocardiography, which is the current standard of care,” said lead author Mark Rubin, MD.
Dr. Rubin, who is assistant professor of neurology at University of Tennessee Health Science Center, Memphis, presented results of the BUBL study at the International Stroke Conference (ISC) 2022, where they were greeted with applause from the floor.
An improvement in the current standard of care
Dr. Rubin explained that patients with suspected embolic stroke are routinely screened for shunts in the heart, such as patent foramen ovale (PFO), that allow blood to flow from the right chamber to the left chamber and can lead to clots from the venous system, accessing the arterial system, then traveling to the brain and causing a stroke.
The current standard of care in screening for such shunts is the use of transthoracic echocardiography (TTE), a widely available and easy to perform, non-invasive procedure. “But we have known for decades that TTE does not pick up these shunts very well. With a sensitivity of only around 45%, it identifies less than half of the patients affected,” Dr. Rubin noted.
The more sensitive transesophageal echocardiography (TEE) gives much better results, but it is an invasive and unpleasant procedure with the ultrasound probe being passed down the throat, and the patient needing to be sedated, so it’s not appropriate for everyone, he noted.
“Transcranial Doppler ultrasound (TCD) also gives excellent results, with a sensitivity of about 96% for detecting PFO, but this procedure is difficult to perform and requires a great deal of skill in placing the probes in the right position and interpreting the signal,” Dr. Rubin said. “TCD has been around for decades, but it hasn’t caught on, as it is too difficult to do. It takes a lot of time to learn the technique.”
“With the robotic-assisted transcranial Doppler device, we can achieve the sensitivity of TCD without needing expert operators. This should vastly improve accessibility to this technology,” he said. “With such technology we can make significant strides into more accurate diagnoses on the cause of stroke, which should lead to better preventive treatments in those found to have right-to-left shunts.”
Robotic detection of shunts
For the BUBL study, the robotic TCD technique was compared with the standard TTE in 129 patients who had a diagnosis of presumed embolic stroke or transient ischemic attack (TIA), with all patients undergoing both procedures.
The robotic TCD device resembles a giant pair of headphones containing the ultrasound probes, which are attached to a frame. In the study, it was operated by a health care professional without TCD skills. Each ultrasound probe independently scans the temporal area autonomously – with angling and positive pressure against the scalp akin to a sonographer – to find and optimize bilateral middle cerebral artery signals, Dr. Rubin explained.
The primary endpoint was the detection of a right-to-left shunt. This occurred in 82 of the 129 patients (63.6%) with the robotic TCD device but in only 27 patients (20.9%) when TTE was used. This gives an absolute difference of 42.6% (95% confidence interval, 28.6%-56.7%; P < .001), which Dr. Rubin described as “astounding.”
However, he said he was not surprised by these results.
“In my experience with transcranial Doppler, I find shunts in patients every day that have not been seen with transthoracic echo,” he commented.
He noted that a previous meta-analysis has suggested a similar difference between TCD and transthoracic echo, but the current study provides prospectively collected data produced in a clinical trial setting and is therefore more reliable.
“What I hope comes from this is that more patients will be able to undergo transcranial Doppler, which is a far superior screening technique for identifying right-to-left shunts. There is so much evidence to support the use of transcranial Doppler, but with this new artificial-intelligence robotic device, we don’t need an expert to use it,” Dr. Rubin said.
He explained that finding a right-to-left shunt in stroke patients is particularly important, as it can direct treatment strategies to reduce future risk of recurrent strokes.
“If a patient has a large shunt, then they have a high risk of having another stroke, and the PFO should be closed.”
In this study, the robotic-assisted TCD detected three times as many large shunts that were considered “intervenable,” compared with transthoracic echo, identifying these shunts in 35 patients (27%) compared to just 13 (10%) with TTE.
“Of the 35 patients with intervenable shunts detected with robotic transcranial Doppler, TTE was completely negative in 18 of them and only suggested a small shunt in the others. So, the standard of care (TTE) missed half the patients with intervenable PFOs,” Dr. Rubin reported.
Study should ‘dramatically change’ practice
Commenting on the study, Patrick Lyden, MD, professor of physiology and neuroscience and of neurology, University of Southern California, Los Angeles, said: “Most clinicians hesitate to use transcranial Doppler given the need for specialized technical expertise to obtain a reliable result. This study showed that a robotic transcranial Doppler device – which can be applied by any cardiac non-invasive lab technician – provides reliable and rigorous data.”
He added: “This result will dramatically change the typical evaluation of patients with suspected PFO: In place of an invasive transesophageal echo that requires anesthesia and a cardiologist, most patients can have a non-invasive, robotic-guided transcranial Doppler and get the same diagnostic benefit.”
Dr. Lyden also pointed out that the cost of TCD is typically one-tenth that of TEE, although he said the cost of the robotic guided TCD “is not clear.”
A representative of the company that makes the robotic assisted device, NovaSignal, says the cost of the equipment is approximately $250,000, but “understanding the importance of the technology, we work with each hospital to meet their unique needs.”
The company adds that it currently has “over 45 commercial solutions deployed across 25 centers with 3-4 times growth expected year over year.”
The study was supported by NovaSignal, the company which makes the robotic device. Dr. Rubin reports acting as a consultant for the NovaSignal.
A version of this article first appeared on Medscape.com.
in a new study.
Being far easier to perform than regular transcranial Doppler ultrasound, it’s hoped that use of the robotic device will enable many more patients to undergo the more sensitive transcranial screening modality and increase the number of shunts identified.
“I believe robot-assisted transcranial Doppler ultrasound can fill the gap between the gold standard transcranial Doppler and transthoracic echocardiography, which is the current standard of care,” said lead author Mark Rubin, MD.
Dr. Rubin, who is assistant professor of neurology at University of Tennessee Health Science Center, Memphis, presented results of the BUBL study at the International Stroke Conference (ISC) 2022, where they were greeted with applause from the floor.
An improvement in the current standard of care
Dr. Rubin explained that patients with suspected embolic stroke are routinely screened for shunts in the heart, such as patent foramen ovale (PFO), that allow blood to flow from the right chamber to the left chamber and can lead to clots from the venous system, accessing the arterial system, then traveling to the brain and causing a stroke.
The current standard of care in screening for such shunts is the use of transthoracic echocardiography (TTE), a widely available and easy to perform, non-invasive procedure. “But we have known for decades that TTE does not pick up these shunts very well. With a sensitivity of only around 45%, it identifies less than half of the patients affected,” Dr. Rubin noted.
The more sensitive transesophageal echocardiography (TEE) gives much better results, but it is an invasive and unpleasant procedure with the ultrasound probe being passed down the throat, and the patient needing to be sedated, so it’s not appropriate for everyone, he noted.
“Transcranial Doppler ultrasound (TCD) also gives excellent results, with a sensitivity of about 96% for detecting PFO, but this procedure is difficult to perform and requires a great deal of skill in placing the probes in the right position and interpreting the signal,” Dr. Rubin said. “TCD has been around for decades, but it hasn’t caught on, as it is too difficult to do. It takes a lot of time to learn the technique.”
“With the robotic-assisted transcranial Doppler device, we can achieve the sensitivity of TCD without needing expert operators. This should vastly improve accessibility to this technology,” he said. “With such technology we can make significant strides into more accurate diagnoses on the cause of stroke, which should lead to better preventive treatments in those found to have right-to-left shunts.”
Robotic detection of shunts
For the BUBL study, the robotic TCD technique was compared with the standard TTE in 129 patients who had a diagnosis of presumed embolic stroke or transient ischemic attack (TIA), with all patients undergoing both procedures.
The robotic TCD device resembles a giant pair of headphones containing the ultrasound probes, which are attached to a frame. In the study, it was operated by a health care professional without TCD skills. Each ultrasound probe independently scans the temporal area autonomously – with angling and positive pressure against the scalp akin to a sonographer – to find and optimize bilateral middle cerebral artery signals, Dr. Rubin explained.
The primary endpoint was the detection of a right-to-left shunt. This occurred in 82 of the 129 patients (63.6%) with the robotic TCD device but in only 27 patients (20.9%) when TTE was used. This gives an absolute difference of 42.6% (95% confidence interval, 28.6%-56.7%; P < .001), which Dr. Rubin described as “astounding.”
However, he said he was not surprised by these results.
“In my experience with transcranial Doppler, I find shunts in patients every day that have not been seen with transthoracic echo,” he commented.
He noted that a previous meta-analysis has suggested a similar difference between TCD and transthoracic echo, but the current study provides prospectively collected data produced in a clinical trial setting and is therefore more reliable.
“What I hope comes from this is that more patients will be able to undergo transcranial Doppler, which is a far superior screening technique for identifying right-to-left shunts. There is so much evidence to support the use of transcranial Doppler, but with this new artificial-intelligence robotic device, we don’t need an expert to use it,” Dr. Rubin said.
He explained that finding a right-to-left shunt in stroke patients is particularly important, as it can direct treatment strategies to reduce future risk of recurrent strokes.
“If a patient has a large shunt, then they have a high risk of having another stroke, and the PFO should be closed.”
In this study, the robotic-assisted TCD detected three times as many large shunts that were considered “intervenable,” compared with transthoracic echo, identifying these shunts in 35 patients (27%) compared to just 13 (10%) with TTE.
“Of the 35 patients with intervenable shunts detected with robotic transcranial Doppler, TTE was completely negative in 18 of them and only suggested a small shunt in the others. So, the standard of care (TTE) missed half the patients with intervenable PFOs,” Dr. Rubin reported.
Study should ‘dramatically change’ practice
Commenting on the study, Patrick Lyden, MD, professor of physiology and neuroscience and of neurology, University of Southern California, Los Angeles, said: “Most clinicians hesitate to use transcranial Doppler given the need for specialized technical expertise to obtain a reliable result. This study showed that a robotic transcranial Doppler device – which can be applied by any cardiac non-invasive lab technician – provides reliable and rigorous data.”
He added: “This result will dramatically change the typical evaluation of patients with suspected PFO: In place of an invasive transesophageal echo that requires anesthesia and a cardiologist, most patients can have a non-invasive, robotic-guided transcranial Doppler and get the same diagnostic benefit.”
Dr. Lyden also pointed out that the cost of TCD is typically one-tenth that of TEE, although he said the cost of the robotic guided TCD “is not clear.”
A representative of the company that makes the robotic assisted device, NovaSignal, says the cost of the equipment is approximately $250,000, but “understanding the importance of the technology, we work with each hospital to meet their unique needs.”
The company adds that it currently has “over 45 commercial solutions deployed across 25 centers with 3-4 times growth expected year over year.”
The study was supported by NovaSignal, the company which makes the robotic device. Dr. Rubin reports acting as a consultant for the NovaSignal.
A version of this article first appeared on Medscape.com.
in a new study.
Being far easier to perform than regular transcranial Doppler ultrasound, it’s hoped that use of the robotic device will enable many more patients to undergo the more sensitive transcranial screening modality and increase the number of shunts identified.
“I believe robot-assisted transcranial Doppler ultrasound can fill the gap between the gold standard transcranial Doppler and transthoracic echocardiography, which is the current standard of care,” said lead author Mark Rubin, MD.
Dr. Rubin, who is assistant professor of neurology at University of Tennessee Health Science Center, Memphis, presented results of the BUBL study at the International Stroke Conference (ISC) 2022, where they were greeted with applause from the floor.
An improvement in the current standard of care
Dr. Rubin explained that patients with suspected embolic stroke are routinely screened for shunts in the heart, such as patent foramen ovale (PFO), that allow blood to flow from the right chamber to the left chamber and can lead to clots from the venous system, accessing the arterial system, then traveling to the brain and causing a stroke.
The current standard of care in screening for such shunts is the use of transthoracic echocardiography (TTE), a widely available and easy to perform, non-invasive procedure. “But we have known for decades that TTE does not pick up these shunts very well. With a sensitivity of only around 45%, it identifies less than half of the patients affected,” Dr. Rubin noted.
The more sensitive transesophageal echocardiography (TEE) gives much better results, but it is an invasive and unpleasant procedure with the ultrasound probe being passed down the throat, and the patient needing to be sedated, so it’s not appropriate for everyone, he noted.
“Transcranial Doppler ultrasound (TCD) also gives excellent results, with a sensitivity of about 96% for detecting PFO, but this procedure is difficult to perform and requires a great deal of skill in placing the probes in the right position and interpreting the signal,” Dr. Rubin said. “TCD has been around for decades, but it hasn’t caught on, as it is too difficult to do. It takes a lot of time to learn the technique.”
“With the robotic-assisted transcranial Doppler device, we can achieve the sensitivity of TCD without needing expert operators. This should vastly improve accessibility to this technology,” he said. “With such technology we can make significant strides into more accurate diagnoses on the cause of stroke, which should lead to better preventive treatments in those found to have right-to-left shunts.”
Robotic detection of shunts
For the BUBL study, the robotic TCD technique was compared with the standard TTE in 129 patients who had a diagnosis of presumed embolic stroke or transient ischemic attack (TIA), with all patients undergoing both procedures.
The robotic TCD device resembles a giant pair of headphones containing the ultrasound probes, which are attached to a frame. In the study, it was operated by a health care professional without TCD skills. Each ultrasound probe independently scans the temporal area autonomously – with angling and positive pressure against the scalp akin to a sonographer – to find and optimize bilateral middle cerebral artery signals, Dr. Rubin explained.
The primary endpoint was the detection of a right-to-left shunt. This occurred in 82 of the 129 patients (63.6%) with the robotic TCD device but in only 27 patients (20.9%) when TTE was used. This gives an absolute difference of 42.6% (95% confidence interval, 28.6%-56.7%; P < .001), which Dr. Rubin described as “astounding.”
However, he said he was not surprised by these results.
“In my experience with transcranial Doppler, I find shunts in patients every day that have not been seen with transthoracic echo,” he commented.
He noted that a previous meta-analysis has suggested a similar difference between TCD and transthoracic echo, but the current study provides prospectively collected data produced in a clinical trial setting and is therefore more reliable.
“What I hope comes from this is that more patients will be able to undergo transcranial Doppler, which is a far superior screening technique for identifying right-to-left shunts. There is so much evidence to support the use of transcranial Doppler, but with this new artificial-intelligence robotic device, we don’t need an expert to use it,” Dr. Rubin said.
He explained that finding a right-to-left shunt in stroke patients is particularly important, as it can direct treatment strategies to reduce future risk of recurrent strokes.
“If a patient has a large shunt, then they have a high risk of having another stroke, and the PFO should be closed.”
In this study, the robotic-assisted TCD detected three times as many large shunts that were considered “intervenable,” compared with transthoracic echo, identifying these shunts in 35 patients (27%) compared to just 13 (10%) with TTE.
“Of the 35 patients with intervenable shunts detected with robotic transcranial Doppler, TTE was completely negative in 18 of them and only suggested a small shunt in the others. So, the standard of care (TTE) missed half the patients with intervenable PFOs,” Dr. Rubin reported.
Study should ‘dramatically change’ practice
Commenting on the study, Patrick Lyden, MD, professor of physiology and neuroscience and of neurology, University of Southern California, Los Angeles, said: “Most clinicians hesitate to use transcranial Doppler given the need for specialized technical expertise to obtain a reliable result. This study showed that a robotic transcranial Doppler device – which can be applied by any cardiac non-invasive lab technician – provides reliable and rigorous data.”
He added: “This result will dramatically change the typical evaluation of patients with suspected PFO: In place of an invasive transesophageal echo that requires anesthesia and a cardiologist, most patients can have a non-invasive, robotic-guided transcranial Doppler and get the same diagnostic benefit.”
Dr. Lyden also pointed out that the cost of TCD is typically one-tenth that of TEE, although he said the cost of the robotic guided TCD “is not clear.”
A representative of the company that makes the robotic assisted device, NovaSignal, says the cost of the equipment is approximately $250,000, but “understanding the importance of the technology, we work with each hospital to meet their unique needs.”
The company adds that it currently has “over 45 commercial solutions deployed across 25 centers with 3-4 times growth expected year over year.”
The study was supported by NovaSignal, the company which makes the robotic device. Dr. Rubin reports acting as a consultant for the NovaSignal.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
Children and COVID: The Omicron surge has become a retreat
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 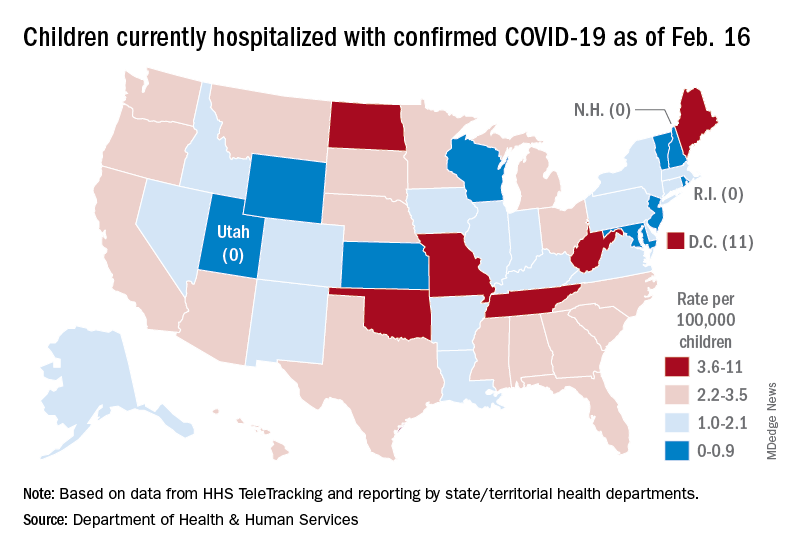
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
Liquid embolism of AVM tied to high cure rate
new observational data suggest. In a prospective, real-world study of more than 100 patients, use of the Onyx system was associated with a cure rate of 86% for cAVMs smaller than 3 cm.
“Endovascular treatment using Onyx is able to achieve, on its own, a very efficient cure rate with a low morbidity and mortality rate,” said investigator Laurent Spelle, MD, PhD, professor of neuroradiology at Paris-Saclay University and chair of NEURI, the Brain Vascular Center, Bicêtre Hospital, also in Paris.
Dr. Spelle presented the findings at the International Stroke Conference sponsored by the American Heart Association.
Prospective, multicenter study
Currently, the main treatment options for cAVM are embolization, neurosurgery, and radiosurgery. The Onyx liquid system, one method of providing embolization, uses a biocompatible ethylene vinyl alcohol copolymer.
It has been used in Europe for 22 years as a curative treatment and as a treatment before radiosurgery or neurosurgery. In the United States, Onyx is indicated for presurgical and preradiotherapy treatment only.
For this analysis, the researchers conducted a prospective, multicenter study to evaluate the long-term safety and efficacy of Onyx for the embolization of cAVM as curative treatment or preoperative preparation.
They enrolled 165 patients in the nonrandomized, observational study, which was conducted at 15 hospitals in France. Eligible participants had an untreated cAVM.
Patients were assigned to one of three groups, according to the hospital’s standard of care. One group underwent embolization with Onyx as curative treatment, one received Onyx as preparation for neurosurgery, and one underwent embolization with Onyx as preparation for radiosurgery.
The study’s safety endpoints were device- and procedure-related serious adverse events at 1 month after each embolization. The efficacy endpoints were recovery at 12 months after the last embolization or neurosurgery, or at a minimum of 36 months after radiosurgery.
The researchers defined morbidity as a worsening of modified Rankin Scale score of 2 or more points for patients presenting with baseline mRS of 0 or 1, or a worsening of 1 or more points for patients with an mRS of 2 or greater at baseline. An independent clinical events committee and core laboratory adjudicated the results.
‘A fantastic result’
In all, 140 patients were prospectively included, and 212 embolization procedures were performed. The population’s mean age was 41.4 years, and 60% of participants were men. About 61% of patients presented with symptoms, the most common of which were progressive neurologic deficit (41.2%) and headache (36.5%).
Approximately 64% of the cAVMs were ruptured. Most (75.7%) were smaller than 3 cm, and the remainder were between 3 and 6 cm. Most patients (59.3%) did not have an aneurysm.
Eight (3.8%) adverse events were associated with the use of Onyx. The rate of procedure-related neurologic serious adverse events was 7.1% within 1 month post embolization. Three deaths occurred (2.1%), one of which was considered device or procedure related.
A total of 87 patients underwent embolization alone, 14 of whom did not complete the study (2 died, 5 were lost to follow-up, and 7 withdrew). Of the 73 who completed the study, 58 (79.5%) had complete occlusion and full recovery at last follow-up. An additional 6.8% had 99% occlusion.
In addition, 3.4% of the population had significant morbidity, and 18.4% presented at baseline with mRS scores of 3-5. Of the latter group, 81.3% had mRS scores of 0-2 at last visit.
Of 21 patients who underwent subsequent neurosurgery, 18 completed follow-up. Of this group, 94.4% had complete occlusion. Of 32 patients receiving subsequent radiosurgery, 54.8% had complete occlusion, which was “a little bit disappointing,” said Dr. Spelle.
Overall, most patients (92.9%) had improved or stable mRS score. The overall mortality rate was 2.9%, and the rate of significant morbidity was 4.3%.
The rate of improved or stable mRS score was 94.3% for patients who underwent embolization alone, 85.7% for patients who also underwent neurosurgery, and 93.75% for patients who also underwent radiosurgery.
The mortality rate was 3.4% for patients who underwent embolization alone, 4.8% for patients who also underwent neurosurgery, and 0% for patients who also underwent radiosurgery.
The rate of significant morbidity was 2.3% for patients who underwent embolization alone, 9.5% for those who also underwent neurosurgery, and 6.25% for those who also underwent radiosurgery.
“We knew that this treatment was very effective, but this effectiveness was only known in a limited number of centers with a very high level of expertise,” said Dr. Spelle. “We were very pleasantly surprised that a larger-scale, multicenter study conducted in 15 different hospitals in France could achieve such a fantastic result.”
The study sites, however, were all departments in university hospitals with great experience in endovascular treatment of cAVM, he added.
Effective in unruptured AVMs?
Commenting on the findings, Mitchell Elkind, MD, professor of neurology and epidemiology, Columbia University, New York, said: “Arteriovenous malformations remain a relatively uncommon but serious cerebrovascular disorder. Any additional tool in the armamentarium to treat these lesions is welcome.”
The study results are encouraging, said Dr. Elkind, who was not involved in the study. They suggest that Onyx embolization can play an important role in the care of these patients. The treatment is associated with “low morbidity and excellent efficacy, particularly in combination with other surgical and radiographic approaches.”
The lack of a direct comparison with alternative embolization materials is a limitation of the study, however. “It is hard to compare Onyx to other agents based on these results,” said Dr. Elkind.
“It is also notable that one-third of the patients in the study had unruptured AVMs, which at least in one randomized trial, ARUBA, were not clearly shown to benefit from an intervention at all,” he continued.
It would have been valuable for the researchers to stratify the study results by ruptured versus unruptured AVMs, Dr. Elkind said.
The study was funded by Medtronic. Dr. Spelle reported receiving honoraria from the company. Dr. Elkind disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new observational data suggest. In a prospective, real-world study of more than 100 patients, use of the Onyx system was associated with a cure rate of 86% for cAVMs smaller than 3 cm.
“Endovascular treatment using Onyx is able to achieve, on its own, a very efficient cure rate with a low morbidity and mortality rate,” said investigator Laurent Spelle, MD, PhD, professor of neuroradiology at Paris-Saclay University and chair of NEURI, the Brain Vascular Center, Bicêtre Hospital, also in Paris.
Dr. Spelle presented the findings at the International Stroke Conference sponsored by the American Heart Association.
Prospective, multicenter study
Currently, the main treatment options for cAVM are embolization, neurosurgery, and radiosurgery. The Onyx liquid system, one method of providing embolization, uses a biocompatible ethylene vinyl alcohol copolymer.
It has been used in Europe for 22 years as a curative treatment and as a treatment before radiosurgery or neurosurgery. In the United States, Onyx is indicated for presurgical and preradiotherapy treatment only.
For this analysis, the researchers conducted a prospective, multicenter study to evaluate the long-term safety and efficacy of Onyx for the embolization of cAVM as curative treatment or preoperative preparation.
They enrolled 165 patients in the nonrandomized, observational study, which was conducted at 15 hospitals in France. Eligible participants had an untreated cAVM.
Patients were assigned to one of three groups, according to the hospital’s standard of care. One group underwent embolization with Onyx as curative treatment, one received Onyx as preparation for neurosurgery, and one underwent embolization with Onyx as preparation for radiosurgery.
The study’s safety endpoints were device- and procedure-related serious adverse events at 1 month after each embolization. The efficacy endpoints were recovery at 12 months after the last embolization or neurosurgery, or at a minimum of 36 months after radiosurgery.
The researchers defined morbidity as a worsening of modified Rankin Scale score of 2 or more points for patients presenting with baseline mRS of 0 or 1, or a worsening of 1 or more points for patients with an mRS of 2 or greater at baseline. An independent clinical events committee and core laboratory adjudicated the results.
‘A fantastic result’
In all, 140 patients were prospectively included, and 212 embolization procedures were performed. The population’s mean age was 41.4 years, and 60% of participants were men. About 61% of patients presented with symptoms, the most common of which were progressive neurologic deficit (41.2%) and headache (36.5%).
Approximately 64% of the cAVMs were ruptured. Most (75.7%) were smaller than 3 cm, and the remainder were between 3 and 6 cm. Most patients (59.3%) did not have an aneurysm.
Eight (3.8%) adverse events were associated with the use of Onyx. The rate of procedure-related neurologic serious adverse events was 7.1% within 1 month post embolization. Three deaths occurred (2.1%), one of which was considered device or procedure related.
A total of 87 patients underwent embolization alone, 14 of whom did not complete the study (2 died, 5 were lost to follow-up, and 7 withdrew). Of the 73 who completed the study, 58 (79.5%) had complete occlusion and full recovery at last follow-up. An additional 6.8% had 99% occlusion.
In addition, 3.4% of the population had significant morbidity, and 18.4% presented at baseline with mRS scores of 3-5. Of the latter group, 81.3% had mRS scores of 0-2 at last visit.
Of 21 patients who underwent subsequent neurosurgery, 18 completed follow-up. Of this group, 94.4% had complete occlusion. Of 32 patients receiving subsequent radiosurgery, 54.8% had complete occlusion, which was “a little bit disappointing,” said Dr. Spelle.
Overall, most patients (92.9%) had improved or stable mRS score. The overall mortality rate was 2.9%, and the rate of significant morbidity was 4.3%.
The rate of improved or stable mRS score was 94.3% for patients who underwent embolization alone, 85.7% for patients who also underwent neurosurgery, and 93.75% for patients who also underwent radiosurgery.
The mortality rate was 3.4% for patients who underwent embolization alone, 4.8% for patients who also underwent neurosurgery, and 0% for patients who also underwent radiosurgery.
The rate of significant morbidity was 2.3% for patients who underwent embolization alone, 9.5% for those who also underwent neurosurgery, and 6.25% for those who also underwent radiosurgery.
“We knew that this treatment was very effective, but this effectiveness was only known in a limited number of centers with a very high level of expertise,” said Dr. Spelle. “We were very pleasantly surprised that a larger-scale, multicenter study conducted in 15 different hospitals in France could achieve such a fantastic result.”
The study sites, however, were all departments in university hospitals with great experience in endovascular treatment of cAVM, he added.
Effective in unruptured AVMs?
Commenting on the findings, Mitchell Elkind, MD, professor of neurology and epidemiology, Columbia University, New York, said: “Arteriovenous malformations remain a relatively uncommon but serious cerebrovascular disorder. Any additional tool in the armamentarium to treat these lesions is welcome.”
The study results are encouraging, said Dr. Elkind, who was not involved in the study. They suggest that Onyx embolization can play an important role in the care of these patients. The treatment is associated with “low morbidity and excellent efficacy, particularly in combination with other surgical and radiographic approaches.”
The lack of a direct comparison with alternative embolization materials is a limitation of the study, however. “It is hard to compare Onyx to other agents based on these results,” said Dr. Elkind.
“It is also notable that one-third of the patients in the study had unruptured AVMs, which at least in one randomized trial, ARUBA, were not clearly shown to benefit from an intervention at all,” he continued.
It would have been valuable for the researchers to stratify the study results by ruptured versus unruptured AVMs, Dr. Elkind said.
The study was funded by Medtronic. Dr. Spelle reported receiving honoraria from the company. Dr. Elkind disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new observational data suggest. In a prospective, real-world study of more than 100 patients, use of the Onyx system was associated with a cure rate of 86% for cAVMs smaller than 3 cm.
“Endovascular treatment using Onyx is able to achieve, on its own, a very efficient cure rate with a low morbidity and mortality rate,” said investigator Laurent Spelle, MD, PhD, professor of neuroradiology at Paris-Saclay University and chair of NEURI, the Brain Vascular Center, Bicêtre Hospital, also in Paris.
Dr. Spelle presented the findings at the International Stroke Conference sponsored by the American Heart Association.
Prospective, multicenter study
Currently, the main treatment options for cAVM are embolization, neurosurgery, and radiosurgery. The Onyx liquid system, one method of providing embolization, uses a biocompatible ethylene vinyl alcohol copolymer.
It has been used in Europe for 22 years as a curative treatment and as a treatment before radiosurgery or neurosurgery. In the United States, Onyx is indicated for presurgical and preradiotherapy treatment only.
For this analysis, the researchers conducted a prospective, multicenter study to evaluate the long-term safety and efficacy of Onyx for the embolization of cAVM as curative treatment or preoperative preparation.
They enrolled 165 patients in the nonrandomized, observational study, which was conducted at 15 hospitals in France. Eligible participants had an untreated cAVM.
Patients were assigned to one of three groups, according to the hospital’s standard of care. One group underwent embolization with Onyx as curative treatment, one received Onyx as preparation for neurosurgery, and one underwent embolization with Onyx as preparation for radiosurgery.
The study’s safety endpoints were device- and procedure-related serious adverse events at 1 month after each embolization. The efficacy endpoints were recovery at 12 months after the last embolization or neurosurgery, or at a minimum of 36 months after radiosurgery.
The researchers defined morbidity as a worsening of modified Rankin Scale score of 2 or more points for patients presenting with baseline mRS of 0 or 1, or a worsening of 1 or more points for patients with an mRS of 2 or greater at baseline. An independent clinical events committee and core laboratory adjudicated the results.
‘A fantastic result’
In all, 140 patients were prospectively included, and 212 embolization procedures were performed. The population’s mean age was 41.4 years, and 60% of participants were men. About 61% of patients presented with symptoms, the most common of which were progressive neurologic deficit (41.2%) and headache (36.5%).
Approximately 64% of the cAVMs were ruptured. Most (75.7%) were smaller than 3 cm, and the remainder were between 3 and 6 cm. Most patients (59.3%) did not have an aneurysm.
Eight (3.8%) adverse events were associated with the use of Onyx. The rate of procedure-related neurologic serious adverse events was 7.1% within 1 month post embolization. Three deaths occurred (2.1%), one of which was considered device or procedure related.
A total of 87 patients underwent embolization alone, 14 of whom did not complete the study (2 died, 5 were lost to follow-up, and 7 withdrew). Of the 73 who completed the study, 58 (79.5%) had complete occlusion and full recovery at last follow-up. An additional 6.8% had 99% occlusion.
In addition, 3.4% of the population had significant morbidity, and 18.4% presented at baseline with mRS scores of 3-5. Of the latter group, 81.3% had mRS scores of 0-2 at last visit.
Of 21 patients who underwent subsequent neurosurgery, 18 completed follow-up. Of this group, 94.4% had complete occlusion. Of 32 patients receiving subsequent radiosurgery, 54.8% had complete occlusion, which was “a little bit disappointing,” said Dr. Spelle.
Overall, most patients (92.9%) had improved or stable mRS score. The overall mortality rate was 2.9%, and the rate of significant morbidity was 4.3%.
The rate of improved or stable mRS score was 94.3% for patients who underwent embolization alone, 85.7% for patients who also underwent neurosurgery, and 93.75% for patients who also underwent radiosurgery.
The mortality rate was 3.4% for patients who underwent embolization alone, 4.8% for patients who also underwent neurosurgery, and 0% for patients who also underwent radiosurgery.
The rate of significant morbidity was 2.3% for patients who underwent embolization alone, 9.5% for those who also underwent neurosurgery, and 6.25% for those who also underwent radiosurgery.
“We knew that this treatment was very effective, but this effectiveness was only known in a limited number of centers with a very high level of expertise,” said Dr. Spelle. “We were very pleasantly surprised that a larger-scale, multicenter study conducted in 15 different hospitals in France could achieve such a fantastic result.”
The study sites, however, were all departments in university hospitals with great experience in endovascular treatment of cAVM, he added.
Effective in unruptured AVMs?
Commenting on the findings, Mitchell Elkind, MD, professor of neurology and epidemiology, Columbia University, New York, said: “Arteriovenous malformations remain a relatively uncommon but serious cerebrovascular disorder. Any additional tool in the armamentarium to treat these lesions is welcome.”
The study results are encouraging, said Dr. Elkind, who was not involved in the study. They suggest that Onyx embolization can play an important role in the care of these patients. The treatment is associated with “low morbidity and excellent efficacy, particularly in combination with other surgical and radiographic approaches.”
The lack of a direct comparison with alternative embolization materials is a limitation of the study, however. “It is hard to compare Onyx to other agents based on these results,” said Dr. Elkind.
“It is also notable that one-third of the patients in the study had unruptured AVMs, which at least in one randomized trial, ARUBA, were not clearly shown to benefit from an intervention at all,” he continued.
It would have been valuable for the researchers to stratify the study results by ruptured versus unruptured AVMs, Dr. Elkind said.
The study was funded by Medtronic. Dr. Spelle reported receiving honoraria from the company. Dr. Elkind disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
New MIS-C guidance addresses diagnostic challenges, cardiac care
Updated guidance for health care providers on multisystem inflammatory syndrome in children (MIS-C) recognizes the evolving nature of the disease and offers strategies for pediatric rheumatologists, who also may be asked to recommend treatment for hyperinflammation in children with acute COVID-19.
Guidance is needed for many reasons, including the variable case definitions for MIS-C, the presence of MIS-C features in other infections and childhood rheumatic diseases, the extrapolation of treatment strategies from other conditions with similar presentations, and the issue of myocardial dysfunction, wrote Lauren A. Henderson, MD, MMSC, of Boston Children’s Hospital, and members of the American College of Rheumatology MIS-C and COVID-19–Related Hyperinflammation Task Force.
However, “modifications to treatment plans, particularly in patients with complex conditions, are highly disease, patient, geography, and time specific, and therefore must be individualized as part of a shared decision-making process,” the authors said. The updated guidance was published in Arthritis & Rheumatology.
Update needed in wake of Omicron
“We continue to see cases of MIS-C across the United States due to the spike in SARS-CoV-2 infections from the Omicron variant,” and therefore updated guidance is important at this time, Dr. Henderson told this news organization.
“MIS-C remains a serious complication of COVID-19 in children and the ACR wanted to continue to provide pediatricians with up-to-date recommendations for the management of MIS-C,” she said.
“Children began to present with MIS-C in April 2020. At that time, little was known about this entity. Most of the recommendations in the first version of the MIS-C guidance were based on expert opinion,” she explained. However, “over the last 2 years, pediatricians have worked very hard to conduct high-quality research studies to better understand MIS-C, so we now have more scientific evidence to guide our recommendations.
“In version three of the MIS-C guidance, there are new recommendations on treatment. Previously, it was unclear what medications should be used for first-line treatment in patients with MIS-C. Some children were given intravenous immunoglobulin while others were given IVIg and steroids together. Several new studies show that children with MIS-C who are treated with a combination of IVIg and steroids have better outcomes. Accordingly, the MIS-C guidance now recommends dual therapy with IVIg and steroids in children with MIS-C.”
Diagnostic evaluation
The guidance calls for maintaining a broad differential diagnosis of MIS-C, given that the condition remains rare, and that most children with COVID-19 present with mild symptoms and have excellent outcomes, the authors noted. The range of clinical features associated with MIS-C include fever, mucocutaneous findings, myocardial dysfunction, cardiac conduction abnormalities, shock, gastrointestinal symptoms, and lymphadenopathy.
Some patients also experience neurologic involvement in the form of severe headache, altered mental status, seizures, cranial nerve palsies, meningismus, cerebral edema, and ischemic or hemorrhagic stroke. Given the nonspecific nature of these symptoms, “it is imperative that a diagnostic evaluation for MIS-C include investigation for other possible causes, as deemed appropriate by the treating provider,” the authors emphasized. Other diagnostic considerations include the prevalence and chronology of COVID-19 in the community, which may change over time.
MIS-C and Kawasaki disease phenotypes
Earlier in the pandemic, when MIS-C first emerged, it was compared with Kawasaki disease (KD). “However, a closer examination of the literature shows that only about one-quarter to half of patients with a reported diagnosis of MIS-C meet the full diagnostic criteria for KD,” the authors wrote. Key features that separate MIS-C from KD include the greater incidence of KD among children in Japan and East Asia versus the higher incidence of MIS-C among non-Hispanic Black children. In addition, children with MIS-C have shown a wider age range, more prominent gastrointestinal and neurologic symptoms, and more frequent cardiac dysfunction, compared with those with KD.
Cardiac management
Close follow-up with cardiology is essential for children with MIS-C, according to the authors. The recommendations call for repeat echocardiograms for all children with MIS-C at a minimum of 7-14 days, then again at 4-6 weeks after the initial presentation. The authors also recommended additional echocardiograms for children with left ventricular dysfunction and cardiac aortic aneurysms.
MIS-C treatment
Current treatment recommendations emphasize that patients under investigation for MIS-C with life-threatening manifestations may need immunomodulatory therapy before a full diagnostic evaluation is complete, the authors said. However, patients without life-threatening manifestations should be evaluated before starting immunomodulatory treatment to avoid potentially harmful therapies for pediatric patients who don’t need them.
When MIS-C is refractory to initial immunomodulatory treatment, a second dose of IVIg is not recommended, but intensification therapy is advised with either high-dose (10-30 mg/kg per day) glucocorticoids, anakinra, or infliximab. However, there is little evidence available for selecting a specific agent for intensification therapy.
The task force also advises giving low-dose aspirin (3-5 mg/kg per day, up to 81 mg once daily) to all MIS-C patients without active bleeding or significant bleeding risk until normalization of the platelet count and confirmed normal coronary arteries at least 4 weeks after diagnosis.
COVID-19 and hyperinflammation
The task force also noted a distinction between MIS-C and severe COVID-19 in children. Although many children with MIS-C are previously healthy, most children who develop severe COVID-19 during an initial infection have complex conditions or comorbidities such as developmental delay or genetic anomaly, or chronic conditions such as congenital heart disease, type 1 diabetes, or asthma, the authors said. They recommend that “hospitalized children with COVID-19 requiring supplemental oxygen or respiratory support should be considered for immunomodulatory therapy in addition to supportive care and antiviral medications.”
The authors acknowledged the limitations and evolving nature of the recommendations, which will continue to change and do not replace clinical judgment for the management of individual patients. In the meantime, the ACR will support the task force in reviewing new evidence and providing revised versions of the current document.
Many questions about MIS-C remain, Dr. Henderson said in an interview. “It can be very hard to diagnose children with MIS-C because many of the symptoms are similar to those seen in other febrile illness of childhood. We need to identify better biomarkers to help us make the diagnosis of MIS-C. In addition, we need studies to provide information about what treatments should be used if children fail to respond to IVIg and steroids. Finally, it appears that vaccination [against SARS-CoV-2] protects against severe forms of MIS-C, and studies are needed to see how vaccination protects children from MIS-C.”
The development of the guidance was supported by the American College of Rheumatology. Dr. Henderson disclosed relationships with companies including Sobi, Pfizer, and Adaptive Biotechnologies (less than $10,000) and research support from the Childhood Arthritis and Rheumatology Research Alliance and research grant support from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Updated guidance for health care providers on multisystem inflammatory syndrome in children (MIS-C) recognizes the evolving nature of the disease and offers strategies for pediatric rheumatologists, who also may be asked to recommend treatment for hyperinflammation in children with acute COVID-19.
Guidance is needed for many reasons, including the variable case definitions for MIS-C, the presence of MIS-C features in other infections and childhood rheumatic diseases, the extrapolation of treatment strategies from other conditions with similar presentations, and the issue of myocardial dysfunction, wrote Lauren A. Henderson, MD, MMSC, of Boston Children’s Hospital, and members of the American College of Rheumatology MIS-C and COVID-19–Related Hyperinflammation Task Force.
However, “modifications to treatment plans, particularly in patients with complex conditions, are highly disease, patient, geography, and time specific, and therefore must be individualized as part of a shared decision-making process,” the authors said. The updated guidance was published in Arthritis & Rheumatology.
Update needed in wake of Omicron
“We continue to see cases of MIS-C across the United States due to the spike in SARS-CoV-2 infections from the Omicron variant,” and therefore updated guidance is important at this time, Dr. Henderson told this news organization.
“MIS-C remains a serious complication of COVID-19 in children and the ACR wanted to continue to provide pediatricians with up-to-date recommendations for the management of MIS-C,” she said.
“Children began to present with MIS-C in April 2020. At that time, little was known about this entity. Most of the recommendations in the first version of the MIS-C guidance were based on expert opinion,” she explained. However, “over the last 2 years, pediatricians have worked very hard to conduct high-quality research studies to better understand MIS-C, so we now have more scientific evidence to guide our recommendations.
“In version three of the MIS-C guidance, there are new recommendations on treatment. Previously, it was unclear what medications should be used for first-line treatment in patients with MIS-C. Some children were given intravenous immunoglobulin while others were given IVIg and steroids together. Several new studies show that children with MIS-C who are treated with a combination of IVIg and steroids have better outcomes. Accordingly, the MIS-C guidance now recommends dual therapy with IVIg and steroids in children with MIS-C.”
Diagnostic evaluation
The guidance calls for maintaining a broad differential diagnosis of MIS-C, given that the condition remains rare, and that most children with COVID-19 present with mild symptoms and have excellent outcomes, the authors noted. The range of clinical features associated with MIS-C include fever, mucocutaneous findings, myocardial dysfunction, cardiac conduction abnormalities, shock, gastrointestinal symptoms, and lymphadenopathy.
Some patients also experience neurologic involvement in the form of severe headache, altered mental status, seizures, cranial nerve palsies, meningismus, cerebral edema, and ischemic or hemorrhagic stroke. Given the nonspecific nature of these symptoms, “it is imperative that a diagnostic evaluation for MIS-C include investigation for other possible causes, as deemed appropriate by the treating provider,” the authors emphasized. Other diagnostic considerations include the prevalence and chronology of COVID-19 in the community, which may change over time.
MIS-C and Kawasaki disease phenotypes
Earlier in the pandemic, when MIS-C first emerged, it was compared with Kawasaki disease (KD). “However, a closer examination of the literature shows that only about one-quarter to half of patients with a reported diagnosis of MIS-C meet the full diagnostic criteria for KD,” the authors wrote. Key features that separate MIS-C from KD include the greater incidence of KD among children in Japan and East Asia versus the higher incidence of MIS-C among non-Hispanic Black children. In addition, children with MIS-C have shown a wider age range, more prominent gastrointestinal and neurologic symptoms, and more frequent cardiac dysfunction, compared with those with KD.
Cardiac management
Close follow-up with cardiology is essential for children with MIS-C, according to the authors. The recommendations call for repeat echocardiograms for all children with MIS-C at a minimum of 7-14 days, then again at 4-6 weeks after the initial presentation. The authors also recommended additional echocardiograms for children with left ventricular dysfunction and cardiac aortic aneurysms.
MIS-C treatment
Current treatment recommendations emphasize that patients under investigation for MIS-C with life-threatening manifestations may need immunomodulatory therapy before a full diagnostic evaluation is complete, the authors said. However, patients without life-threatening manifestations should be evaluated before starting immunomodulatory treatment to avoid potentially harmful therapies for pediatric patients who don’t need them.
When MIS-C is refractory to initial immunomodulatory treatment, a second dose of IVIg is not recommended, but intensification therapy is advised with either high-dose (10-30 mg/kg per day) glucocorticoids, anakinra, or infliximab. However, there is little evidence available for selecting a specific agent for intensification therapy.
The task force also advises giving low-dose aspirin (3-5 mg/kg per day, up to 81 mg once daily) to all MIS-C patients without active bleeding or significant bleeding risk until normalization of the platelet count and confirmed normal coronary arteries at least 4 weeks after diagnosis.
COVID-19 and hyperinflammation
The task force also noted a distinction between MIS-C and severe COVID-19 in children. Although many children with MIS-C are previously healthy, most children who develop severe COVID-19 during an initial infection have complex conditions or comorbidities such as developmental delay or genetic anomaly, or chronic conditions such as congenital heart disease, type 1 diabetes, or asthma, the authors said. They recommend that “hospitalized children with COVID-19 requiring supplemental oxygen or respiratory support should be considered for immunomodulatory therapy in addition to supportive care and antiviral medications.”
The authors acknowledged the limitations and evolving nature of the recommendations, which will continue to change and do not replace clinical judgment for the management of individual patients. In the meantime, the ACR will support the task force in reviewing new evidence and providing revised versions of the current document.
Many questions about MIS-C remain, Dr. Henderson said in an interview. “It can be very hard to diagnose children with MIS-C because many of the symptoms are similar to those seen in other febrile illness of childhood. We need to identify better biomarkers to help us make the diagnosis of MIS-C. In addition, we need studies to provide information about what treatments should be used if children fail to respond to IVIg and steroids. Finally, it appears that vaccination [against SARS-CoV-2] protects against severe forms of MIS-C, and studies are needed to see how vaccination protects children from MIS-C.”
The development of the guidance was supported by the American College of Rheumatology. Dr. Henderson disclosed relationships with companies including Sobi, Pfizer, and Adaptive Biotechnologies (less than $10,000) and research support from the Childhood Arthritis and Rheumatology Research Alliance and research grant support from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Updated guidance for health care providers on multisystem inflammatory syndrome in children (MIS-C) recognizes the evolving nature of the disease and offers strategies for pediatric rheumatologists, who also may be asked to recommend treatment for hyperinflammation in children with acute COVID-19.
Guidance is needed for many reasons, including the variable case definitions for MIS-C, the presence of MIS-C features in other infections and childhood rheumatic diseases, the extrapolation of treatment strategies from other conditions with similar presentations, and the issue of myocardial dysfunction, wrote Lauren A. Henderson, MD, MMSC, of Boston Children’s Hospital, and members of the American College of Rheumatology MIS-C and COVID-19–Related Hyperinflammation Task Force.
However, “modifications to treatment plans, particularly in patients with complex conditions, are highly disease, patient, geography, and time specific, and therefore must be individualized as part of a shared decision-making process,” the authors said. The updated guidance was published in Arthritis & Rheumatology.
Update needed in wake of Omicron
“We continue to see cases of MIS-C across the United States due to the spike in SARS-CoV-2 infections from the Omicron variant,” and therefore updated guidance is important at this time, Dr. Henderson told this news organization.
“MIS-C remains a serious complication of COVID-19 in children and the ACR wanted to continue to provide pediatricians with up-to-date recommendations for the management of MIS-C,” she said.
“Children began to present with MIS-C in April 2020. At that time, little was known about this entity. Most of the recommendations in the first version of the MIS-C guidance were based on expert opinion,” she explained. However, “over the last 2 years, pediatricians have worked very hard to conduct high-quality research studies to better understand MIS-C, so we now have more scientific evidence to guide our recommendations.
“In version three of the MIS-C guidance, there are new recommendations on treatment. Previously, it was unclear what medications should be used for first-line treatment in patients with MIS-C. Some children were given intravenous immunoglobulin while others were given IVIg and steroids together. Several new studies show that children with MIS-C who are treated with a combination of IVIg and steroids have better outcomes. Accordingly, the MIS-C guidance now recommends dual therapy with IVIg and steroids in children with MIS-C.”
Diagnostic evaluation
The guidance calls for maintaining a broad differential diagnosis of MIS-C, given that the condition remains rare, and that most children with COVID-19 present with mild symptoms and have excellent outcomes, the authors noted. The range of clinical features associated with MIS-C include fever, mucocutaneous findings, myocardial dysfunction, cardiac conduction abnormalities, shock, gastrointestinal symptoms, and lymphadenopathy.
Some patients also experience neurologic involvement in the form of severe headache, altered mental status, seizures, cranial nerve palsies, meningismus, cerebral edema, and ischemic or hemorrhagic stroke. Given the nonspecific nature of these symptoms, “it is imperative that a diagnostic evaluation for MIS-C include investigation for other possible causes, as deemed appropriate by the treating provider,” the authors emphasized. Other diagnostic considerations include the prevalence and chronology of COVID-19 in the community, which may change over time.
MIS-C and Kawasaki disease phenotypes
Earlier in the pandemic, when MIS-C first emerged, it was compared with Kawasaki disease (KD). “However, a closer examination of the literature shows that only about one-quarter to half of patients with a reported diagnosis of MIS-C meet the full diagnostic criteria for KD,” the authors wrote. Key features that separate MIS-C from KD include the greater incidence of KD among children in Japan and East Asia versus the higher incidence of MIS-C among non-Hispanic Black children. In addition, children with MIS-C have shown a wider age range, more prominent gastrointestinal and neurologic symptoms, and more frequent cardiac dysfunction, compared with those with KD.
Cardiac management
Close follow-up with cardiology is essential for children with MIS-C, according to the authors. The recommendations call for repeat echocardiograms for all children with MIS-C at a minimum of 7-14 days, then again at 4-6 weeks after the initial presentation. The authors also recommended additional echocardiograms for children with left ventricular dysfunction and cardiac aortic aneurysms.
MIS-C treatment
Current treatment recommendations emphasize that patients under investigation for MIS-C with life-threatening manifestations may need immunomodulatory therapy before a full diagnostic evaluation is complete, the authors said. However, patients without life-threatening manifestations should be evaluated before starting immunomodulatory treatment to avoid potentially harmful therapies for pediatric patients who don’t need them.
When MIS-C is refractory to initial immunomodulatory treatment, a second dose of IVIg is not recommended, but intensification therapy is advised with either high-dose (10-30 mg/kg per day) glucocorticoids, anakinra, or infliximab. However, there is little evidence available for selecting a specific agent for intensification therapy.
The task force also advises giving low-dose aspirin (3-5 mg/kg per day, up to 81 mg once daily) to all MIS-C patients without active bleeding or significant bleeding risk until normalization of the platelet count and confirmed normal coronary arteries at least 4 weeks after diagnosis.
COVID-19 and hyperinflammation
The task force also noted a distinction between MIS-C and severe COVID-19 in children. Although many children with MIS-C are previously healthy, most children who develop severe COVID-19 during an initial infection have complex conditions or comorbidities such as developmental delay or genetic anomaly, or chronic conditions such as congenital heart disease, type 1 diabetes, or asthma, the authors said. They recommend that “hospitalized children with COVID-19 requiring supplemental oxygen or respiratory support should be considered for immunomodulatory therapy in addition to supportive care and antiviral medications.”
The authors acknowledged the limitations and evolving nature of the recommendations, which will continue to change and do not replace clinical judgment for the management of individual patients. In the meantime, the ACR will support the task force in reviewing new evidence and providing revised versions of the current document.
Many questions about MIS-C remain, Dr. Henderson said in an interview. “It can be very hard to diagnose children with MIS-C because many of the symptoms are similar to those seen in other febrile illness of childhood. We need to identify better biomarkers to help us make the diagnosis of MIS-C. In addition, we need studies to provide information about what treatments should be used if children fail to respond to IVIg and steroids. Finally, it appears that vaccination [against SARS-CoV-2] protects against severe forms of MIS-C, and studies are needed to see how vaccination protects children from MIS-C.”
The development of the guidance was supported by the American College of Rheumatology. Dr. Henderson disclosed relationships with companies including Sobi, Pfizer, and Adaptive Biotechnologies (less than $10,000) and research support from the Childhood Arthritis and Rheumatology Research Alliance and research grant support from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS AND RHEUMATOLOGY
Subvariant may be more dangerous than original Omicron strain
, a lab study from Japan says.
“Our multiscale investigations suggest that the risk of BA.2 for global health is potentially higher than that of BA.1,” the researchers said in the study published on the preprint server bioRxiv. The study has not been peer-reviewed.
The researchers infected hamsters with BA.1 and BA.2. The hamsters infected with BA.2 got sicker, with more lung damage and loss of body weight. Results were similar when mice were infected with BA.1 and BA.2.
“Infection experiments using hamsters show that BA.2 is more pathogenic than BA.1,” the study said.
BA.1 and BA.2 both appear to evade immunity created by COVID-19 vaccines, the study said. But a booster shot makes illness after infection 74% less likely, CNN said.
What’s more, therapeutic monoclonal antibodies used to treat people infected with COVID didn’t have much effect on BA.2.
BA.2 was “almost completely resistant” to casirivimab and imdevimab and was 35 times more resistant to sotrovimab, compared to the original B.1.1 virus, the researchers wrote.
“In summary, our data suggest the possibility that BA.2 would be the most concerned variant to global health,” the researchers wrote. “Currently, both BA.2 and BA.1 are recognised together as Omicron and these are almost undistinguishable. Based on our findings, we propose that BA.2 should be recognised as a unique variant of concern, and this SARS-CoV-2 variant should be monitored in depth.”
If the World Health Organization recognized BA.2 as a “unique variant of concern,” it would be given its own Greek letter.
But some scientists noted that findings in the lab don’t always reflect what’s happening in the real world of people.
“I think it’s always hard to translate differences in animal and cell culture models to what’s going on with regards to human disease,” Jeremy Kamil, PhD, an associate professor of microbiology and immunology at Louisiana State University Health Shreveport, told Newsweek. “That said, the differences do look real.”
“It might be, from a human’s perspective, a worse virus than BA.1 and might be able to transmit better and cause worse disease,” Daniel Rhoads, MD, section head of microbiology at the Cleveland Clinic in Ohio, told CNN. He reviewed the Japanese study but was not involved in it.
Another scientist who reviewed the study but was not involved in the research noted that human immune systems are evolving along with the COVID variants.
“One of the caveats that we have to think about, as we get new variants that might seem more dangerous, is the fact that there’s two sides to the story,” Deborah Fuller, PhD, a virologist at the University of Washington School of Medicine, told CNN. “Our immune system is evolving as well. And so that’s pushing back on things.”
Scientists have already established that BA.2 is more transmissible than BA.1. The Omicron subvariant has been detected in 74 countries and 47 U.S. states, according to CNN. About 4% of Americans with COVID were infected with BA.2, the outlet reported, citing the CDC, but it’s now the dominant strain in other nations.
It’s not clear yet if BA.2 causes more severe illness in people. While BA.2 spreads faster than BA.1, there’s no evidence the subvariant makes people any sicker, an official with the World Health Organization said, according to CNBC.
A version of this article first appeared on WebMD.com.
, a lab study from Japan says.
“Our multiscale investigations suggest that the risk of BA.2 for global health is potentially higher than that of BA.1,” the researchers said in the study published on the preprint server bioRxiv. The study has not been peer-reviewed.
The researchers infected hamsters with BA.1 and BA.2. The hamsters infected with BA.2 got sicker, with more lung damage and loss of body weight. Results were similar when mice were infected with BA.1 and BA.2.
“Infection experiments using hamsters show that BA.2 is more pathogenic than BA.1,” the study said.
BA.1 and BA.2 both appear to evade immunity created by COVID-19 vaccines, the study said. But a booster shot makes illness after infection 74% less likely, CNN said.
What’s more, therapeutic monoclonal antibodies used to treat people infected with COVID didn’t have much effect on BA.2.
BA.2 was “almost completely resistant” to casirivimab and imdevimab and was 35 times more resistant to sotrovimab, compared to the original B.1.1 virus, the researchers wrote.
“In summary, our data suggest the possibility that BA.2 would be the most concerned variant to global health,” the researchers wrote. “Currently, both BA.2 and BA.1 are recognised together as Omicron and these are almost undistinguishable. Based on our findings, we propose that BA.2 should be recognised as a unique variant of concern, and this SARS-CoV-2 variant should be monitored in depth.”
If the World Health Organization recognized BA.2 as a “unique variant of concern,” it would be given its own Greek letter.
But some scientists noted that findings in the lab don’t always reflect what’s happening in the real world of people.
“I think it’s always hard to translate differences in animal and cell culture models to what’s going on with regards to human disease,” Jeremy Kamil, PhD, an associate professor of microbiology and immunology at Louisiana State University Health Shreveport, told Newsweek. “That said, the differences do look real.”
“It might be, from a human’s perspective, a worse virus than BA.1 and might be able to transmit better and cause worse disease,” Daniel Rhoads, MD, section head of microbiology at the Cleveland Clinic in Ohio, told CNN. He reviewed the Japanese study but was not involved in it.
Another scientist who reviewed the study but was not involved in the research noted that human immune systems are evolving along with the COVID variants.
“One of the caveats that we have to think about, as we get new variants that might seem more dangerous, is the fact that there’s two sides to the story,” Deborah Fuller, PhD, a virologist at the University of Washington School of Medicine, told CNN. “Our immune system is evolving as well. And so that’s pushing back on things.”
Scientists have already established that BA.2 is more transmissible than BA.1. The Omicron subvariant has been detected in 74 countries and 47 U.S. states, according to CNN. About 4% of Americans with COVID were infected with BA.2, the outlet reported, citing the CDC, but it’s now the dominant strain in other nations.
It’s not clear yet if BA.2 causes more severe illness in people. While BA.2 spreads faster than BA.1, there’s no evidence the subvariant makes people any sicker, an official with the World Health Organization said, according to CNBC.
A version of this article first appeared on WebMD.com.
, a lab study from Japan says.
“Our multiscale investigations suggest that the risk of BA.2 for global health is potentially higher than that of BA.1,” the researchers said in the study published on the preprint server bioRxiv. The study has not been peer-reviewed.
The researchers infected hamsters with BA.1 and BA.2. The hamsters infected with BA.2 got sicker, with more lung damage and loss of body weight. Results were similar when mice were infected with BA.1 and BA.2.
“Infection experiments using hamsters show that BA.2 is more pathogenic than BA.1,” the study said.
BA.1 and BA.2 both appear to evade immunity created by COVID-19 vaccines, the study said. But a booster shot makes illness after infection 74% less likely, CNN said.
What’s more, therapeutic monoclonal antibodies used to treat people infected with COVID didn’t have much effect on BA.2.
BA.2 was “almost completely resistant” to casirivimab and imdevimab and was 35 times more resistant to sotrovimab, compared to the original B.1.1 virus, the researchers wrote.
“In summary, our data suggest the possibility that BA.2 would be the most concerned variant to global health,” the researchers wrote. “Currently, both BA.2 and BA.1 are recognised together as Omicron and these are almost undistinguishable. Based on our findings, we propose that BA.2 should be recognised as a unique variant of concern, and this SARS-CoV-2 variant should be monitored in depth.”
If the World Health Organization recognized BA.2 as a “unique variant of concern,” it would be given its own Greek letter.
But some scientists noted that findings in the lab don’t always reflect what’s happening in the real world of people.
“I think it’s always hard to translate differences in animal and cell culture models to what’s going on with regards to human disease,” Jeremy Kamil, PhD, an associate professor of microbiology and immunology at Louisiana State University Health Shreveport, told Newsweek. “That said, the differences do look real.”
“It might be, from a human’s perspective, a worse virus than BA.1 and might be able to transmit better and cause worse disease,” Daniel Rhoads, MD, section head of microbiology at the Cleveland Clinic in Ohio, told CNN. He reviewed the Japanese study but was not involved in it.
Another scientist who reviewed the study but was not involved in the research noted that human immune systems are evolving along with the COVID variants.
“One of the caveats that we have to think about, as we get new variants that might seem more dangerous, is the fact that there’s two sides to the story,” Deborah Fuller, PhD, a virologist at the University of Washington School of Medicine, told CNN. “Our immune system is evolving as well. And so that’s pushing back on things.”
Scientists have already established that BA.2 is more transmissible than BA.1. The Omicron subvariant has been detected in 74 countries and 47 U.S. states, according to CNN. About 4% of Americans with COVID were infected with BA.2, the outlet reported, citing the CDC, but it’s now the dominant strain in other nations.
It’s not clear yet if BA.2 causes more severe illness in people. While BA.2 spreads faster than BA.1, there’s no evidence the subvariant makes people any sicker, an official with the World Health Organization said, according to CNBC.
A version of this article first appeared on WebMD.com.
Ivermectin does not stop progression to severe COVID: randomized trial
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICNE







