User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Oral Biologics: The New Wave for Treating Psoriasis
Oral Biologics: The New Wave for Treating Psoriasis
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.
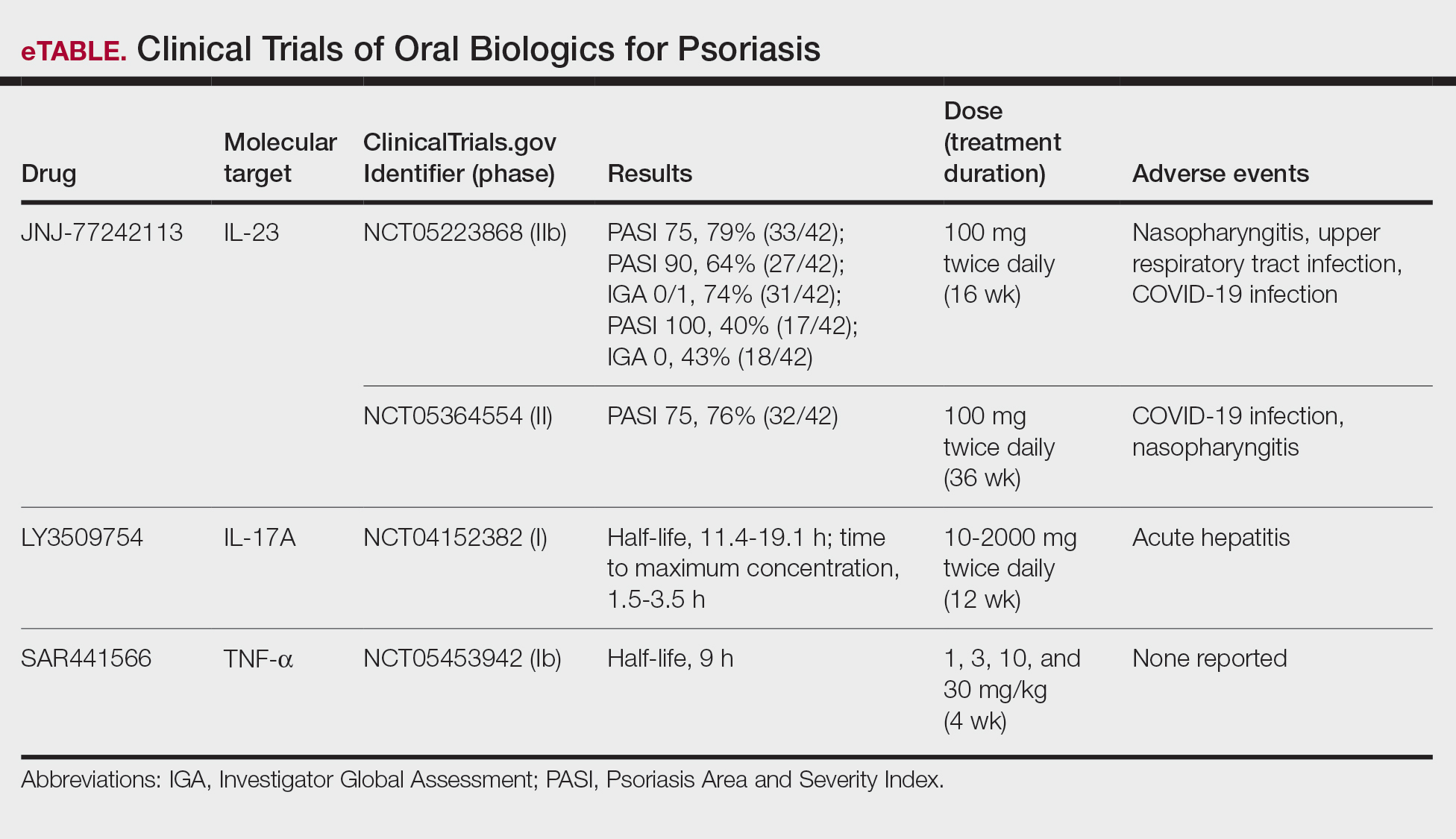
A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Oral Biologics: The New Wave for Treating Psoriasis
Oral Biologics: The New Wave for Treating Psoriasis
PRACTICE POINTS
- The biologics that currently are approved for psoriasis are expensive and must be administered via injection due to their large molecule size.
- Emerging small-molecule oral therapies for psoriasis are effective and affordable and may represent the future for psoriasis patients.
Legislative, Practice Management, and Coding Updates for 2025
Legislative, Practice Management, and Coding Updates for 2025
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
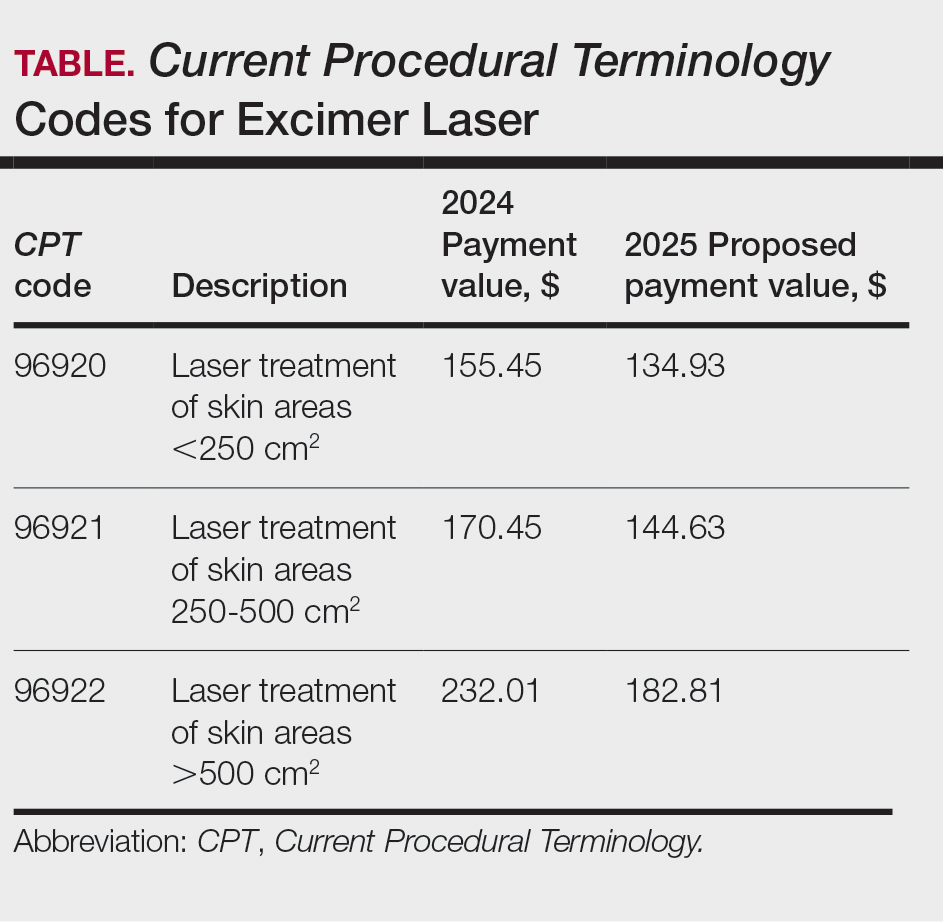
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11
Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
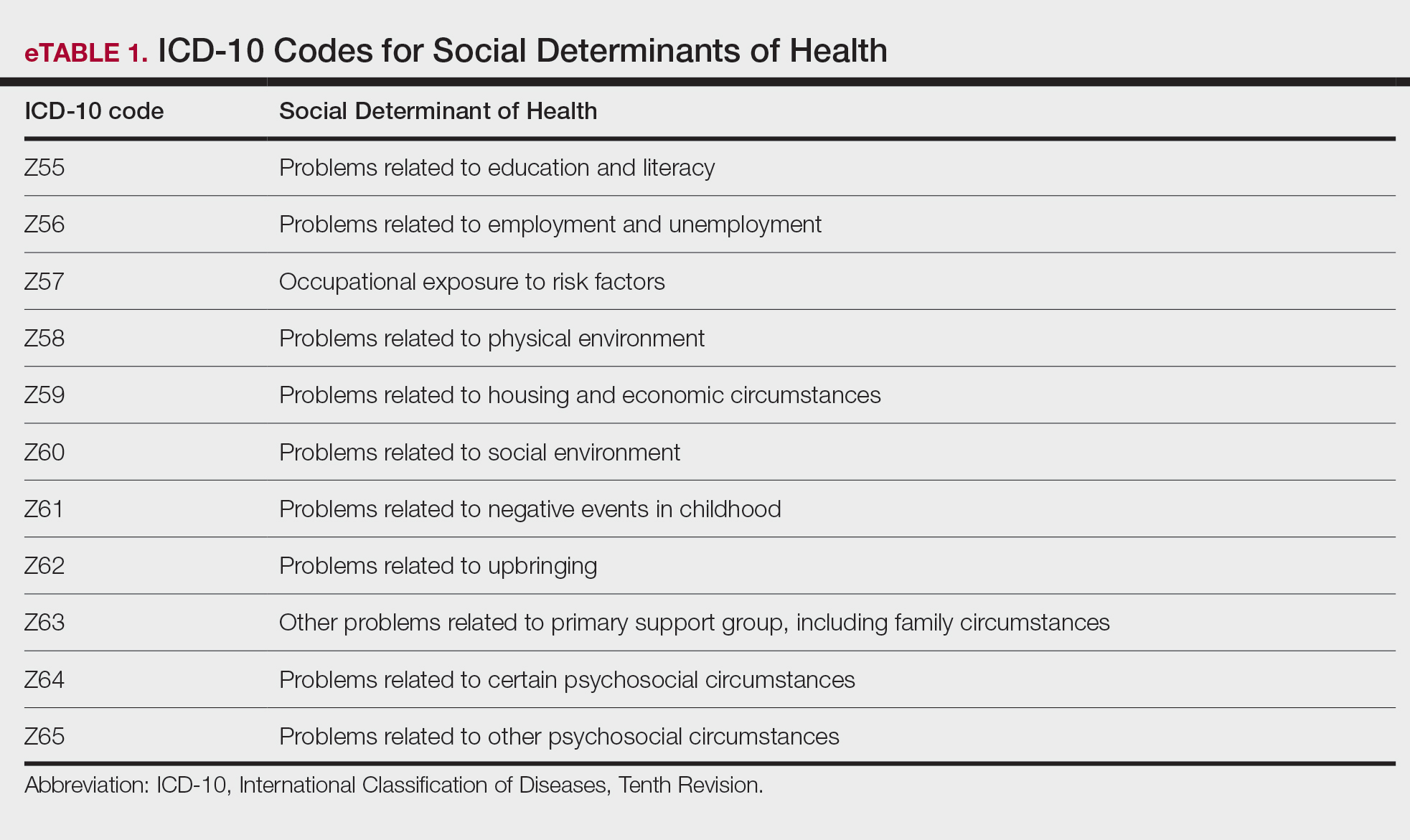
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13
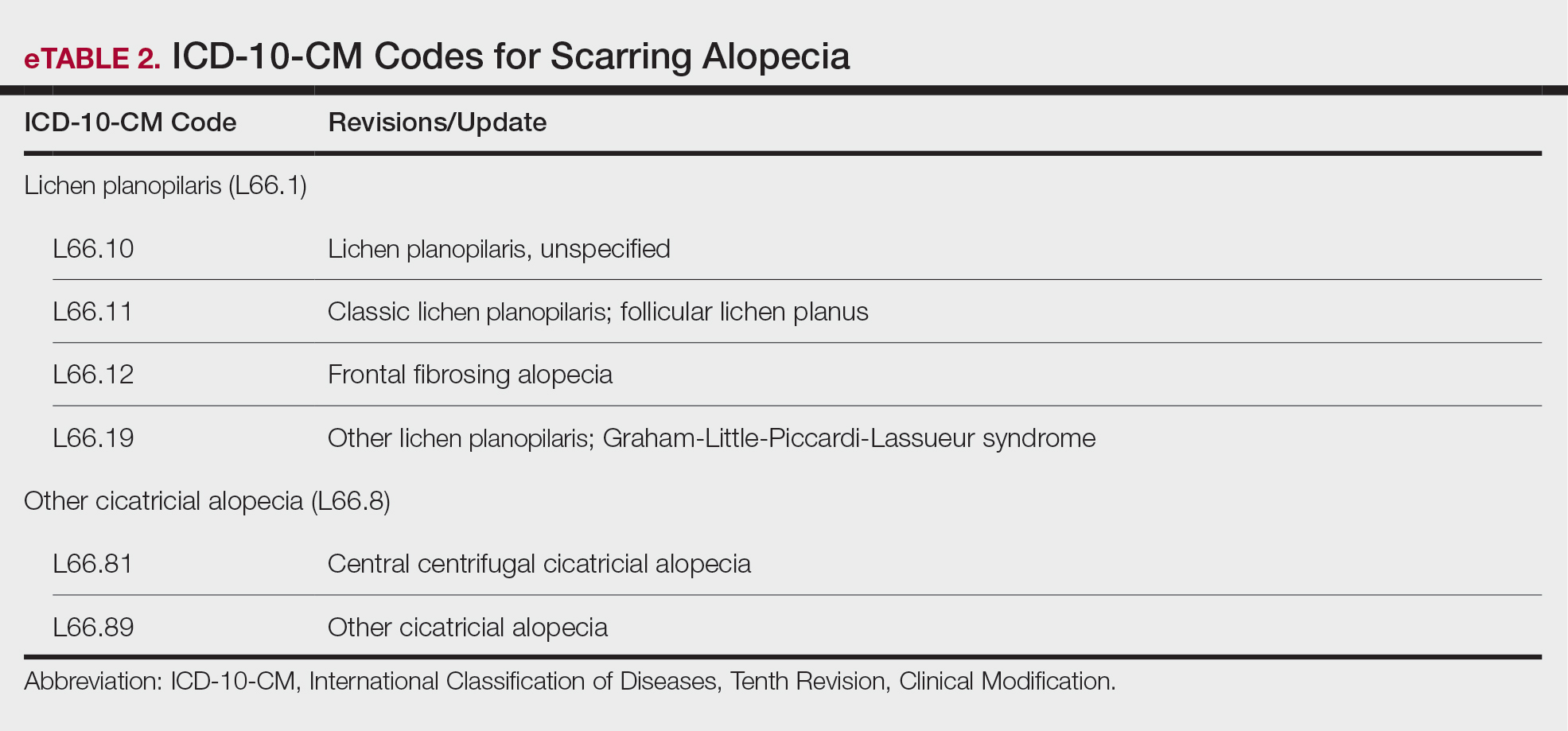
New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14
Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11

Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13

New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14

Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11

Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13

New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14

Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
Legislative, Practice Management, and Coding Updates for 2025
Legislative, Practice Management, and Coding Updates for 2025
PRACTICE POINTS
- The Centers for Medicare & Medicaid Services released the 2025 Medicare Physician Fee Schedule final rule on November 1, 2024, setting the 2025 conversion factor at $32.35—a 2.83% reduction from 2024.
- With this change, dermatology practices may see an overall 2.83% reduction in payments in 2025 compared to 2024, although individual outcomes will vary based on practice mix.
- The American Academy of Dermatology Association continues to advocate for change, and members need to urge their federal legislators to support critical bills aimed at reforming Medicare physician payment.
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.
The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6
Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9
Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
A 46-year-old woman with a history of systemic lupus erythematosus and end-stage renal disease presented to the dermatology department with painful ulcers on the extensor surfaces of the elbows, knees, and ankles of 2 months’ duration. Physical examination revealed angulated ulcers with surrounding pink erythema. A 4-mm punch biopsy and CD31 immunostaining of the left knee revealed dystrophic elastic fibers and purplish calciumlike depositions on connective tissue fibers in the mid to deep dermis.
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Dermatopathology education, which comprises approximately 30% of the dermatology residency curriculum, is crucial for the holistic training of dermatology residents to diagnose and manage a range of dermatologic conditions.1 Additionally, dermatopathology is the topic of one of the 4 American Board of Dermatology CORE Exam modules, further highlighting the need for comprehensive education in this area. A variety of resources including virtual dermatopathology and conventional microscopy training currently are used in residency programs for dermatopathology education.2,3 Although used less frequently, social media platforms such as Instagram also are used to aid in dermatopathology education for a wider audience.4 Other online resources, including the American Society of Dermatopathology website (www.asdp.org) and DermpathAtlas.com, are excellent tools for medical students, residents, and fellows to develop their knowledge.5 While these resources are accessible, they must be directly sought out by the student and utilized on their own time. Additionally, if medical students do not have a strong understanding of the basics of dermatopathology, they may not have the foundation required to benefit from these resources.
Dermatopathology education is critical for the overall practice of dermatology, yet most dermatology residency programs may not be incorporating dermatopathology education early enough in training. One study evaluating the timing and length of dermatopathology education during residency reported that fewer than 40% (20/51) of dermatology residency programs allocate 3 or more weeks to dermatopathology education during the second postgraduate year.1 Despite Ackerman6 advocating for early dermatopathology exposure to best prepare medical students to recognize and manage certain dermatologic conditions, the majority of exposure still seems to occur during postgraduate year 4.1 Furthermore, current primary care residents feel that their medical school training did not sufficiently prepare them to diagnose and manage dermatologic conditions, with only 37% (93/252) reporting feeling adequately prepared.7,8 Medical students also reported a lack of confidence in overall dermatology knowledge, with 89% (72/81) reporting they felt neutral, slightly confident, or not at all confident when asked to diagnose skin lesions.9 In the same study, the average score was 46.6% (7/15 questions answered correctly) when 74 participants were assessed via a multiple choice quiz on dermatologic diagnosis and treatment, further demonstrating the lack of general dermatology comfort among medical students.9 This likely stems from limited dermatology curriculum in medical schools, demonstrating the need for further dermatology education as a whole in medical school.10
Ensuring robust dermatopathology education in medical school and the first year of dermatology residency has the potential to better prepare medical students for the transition into dermatology residency and clinical practice. We created an introductory dermatopathology lecture and presented it to medical students and first year dermatology residents to improve dermatopathology knowledge and confidence in learners early in their dermatology training.
Structure of the Lecture
Participants included first-year dermatology residents and fourth-year medical students rotating with the Wayne State University Department of Dermatology (Detroit, Michigan). The same facilitator (H.O.) taught each of the lectures, and all lectures were conducted via Zoom at the beginning of the month from May 2024 through November 2024. A total of 7 lectures were given. The lecture was formatted so that a histologic image was shown, then learners expressed their thoughts about what the image was showing before the answer was given. This format allowed participants to view the images on their own device screen and allowed the facilitator to annotate the images. The lecture was divided into 3 sections: (1) cell types and basic structures, (2) anatomic slides, and (3) common diagnoses. Each session lasted approximately 45 minutes.
Section 1: Cell Types and Basic Structures—The first section covered the fundamental cell types (neutrophils, lymphocytes, plasma cells, melanocytes, and eosinophils) along with glandular structures (apocrine, eccrine, and sebaceous). The session was designed to follow a retention and allow learners to think through each slide. First, participants were shown histologic images of each cell type and were asked to identify what type of cell was being shown. On the following slide, key features of each cell type were highlighted. Next, participants similarly were shown images of the glandular structures followed by key features of each. The section concluded with a review of the layers of the skin (stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale). A histologic image was shown, and the facilitator discussed how to distinguish the layers.
Section 2: Anatomic Sites—This section focused on key pathologic features for differentiating body surfaces, including the scalp, face, eyelids, ears, areolae, palms and soles, and mucosae. Participants initially were shown an image of a hematoxylin and eosin–stained slide from a specific body surface and then were asked to identify structures that may serve as a clue to the anatomic location. If the participants were not sure, they were given hints; for example, when participants were shown an image of the ear and were unsure of the location, the facilitator circled cartilage and asked them to identify the structure. In most cases, once participants named this structure, they were able to recognize that the location was the ear.
Section 3: Common Diagnoses—This section addressed frequently encountered diagnoses in dermatopathology, including basal cell carcinoma, squamous cell carcinoma, squamous cell carcinoma in situ, epidermoid cyst, pilar cyst, seborrheic keratosis, solar lentigo, melanocytic nevus, melanoma, verruca vulgaris, spongiotic dermatitis, psoriasis, and lichen planus. It followed the same format of the first section: participants were shown an hemotoxyllin and eosin–stained image and then were asked to discuss what the diagnosis could be and why. Hints were given if participants struggled to come up with the correct diagnosis. A few slides also were dedicated to distinguishing benign nevi, dysplastic nevi, and melanoma.
Pretest and Posttest Results
Residents participated in the lecture as part of their first-year orientation, and medical students participated during their dermatology rotation. All participants were invited to complete a pretest and a posttest before and after the lecture, respectively. Both assessments were optional and anonymous. The pretest was completed electronically and consisted of 10 knowledge-based, multiple-choice questions that included a histopathologic image and asked, “What is the most likely diagnosis?,” “What is the predominant cell type?,” and “Where was this specimen taken from?” In addition to the knowledge-based questions, participants also were asked to rate their confidence in dermatopathology on a 5-point Likert scale ranging from 1 (not confident at all) to 5 (extremely confident). Participants completed the entire pretest before any information on the topic was provided. After the lecture, participants were asked to complete a posttest identical to the pretest and to rate their confidence in dermatopathology again on the same scale. The posttest included an additional question asking participants to rate the helpfulness of the lecture on a Likert scale ranging from 1 (not helpful at all) to 5 (extremely helpful). Participants completed the posttest within 48 hours of the lecture.
Overall, 15 learners participated in the pretest and 12 in the posttest. Of the 15 pretest participants, 3 were first-year residents and 12 were medical students. Similarly, in the posttest, 2 respondents were first-year residents and 10 were medical students. All responses contained complete pretests and posttests. The mean score on the pretest was 62%, whereas the mean score on the posttest was 75%. A paired t test indicated a statistically significant improvement (P=.017). In addition, the mean rating for confidence in dermatopathology knowledge before the lecture was 1.5 prior to the lecture and 2.6 after the lecture. A paired t test demonstrated statistical significance (P=.010). The mean rating of the helpfulness of the lecture was 4.67. The majority (91.7% [11/12]) of the participants gave a rating of 4 or 5.
Impact of the Lecture on Dermatopathology Knowledge
There is a gap in dermatopathology education early in medical training. Our introductory lecture led to higher post test scores and increased confidence in dermatopathology among medical students and dermatology residents, demonstrating the effectiveness of this kind of program in bridging this education gap. The majority of participants in our lecture said they found the session helpful. A previously published article called for early implementation of dermatology education as a whole in the medical curriculum due to lack of knowledge and confidence, and our introductory lecture may help to bridge this gap.8 Increasing dermatopathology content for medical students and first-year dermatology residents can expand knowledge, as shown by the increased scores on the posttest, and better supports learners transitioning to dermatology residency, where dermatopathology constitutes a large part of the overall curriculum.2 More comprehensive knowledge of dermatopathology early in dermatology training also may help to better prepare residents to accurately diagnose and manage dermatologic conditions.
Pretest scores showed that the average confidence rating in dermatopathology among participants in our lecture was 1.5, which is rather low. This is consistent with prior studies that have found that residents feel that medical school inadequately prepared them for dermatology residency.7,8 More than 87% (71/81) of medical students surveyed felt they received inadequate general dermatology training in medical school.9 This supports the proposed educational gap that is impacting confidence in overall dermatology knowledge, which includes dermatopathology. In our study, the average confidence rating increased by 1.1 points after the lecture, which was statistically significant (P=.010) and demonstrates that an introductory lecture serves as a feasible intervention to improve confidence in this area.
The feedback we received from participants in our lecture shows the benefits of an introductory interactive lecture with virtual dermatopathology images. Ngo et al2 highlighted how residents perceive virtual images to be superior to conventional microscopy for dermatopathology, which we utilized in our lecture. This method is not only cost effective but also provides a simple way for learners and facilitators to point out key findings on histopathology slides.2
Final Thoughts
Overall, implementing dermatopathology education early in training has a measurable impact on dermatopathology knowledge and confidence among medical students and first-year dermatology residents. An interactive lecture with virtual images similar to the one we describe here may better prepare learners for the transition to dermatology residency by addressing the educational gap in dermatopathology early in training.
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826, vii.
- Ngo TB, Niu W, Fang Z, et al. Dermatology residents’ perspectives on virtual dermatopathology education. J Cutan Pathol. 2024;51:530-537.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Hubbard G, Saal R, Wintringham J, et al. Utilizing Instagram as a novel method for dermatopathology instruction. Clin Exp Dermatol. 2023;49:89-91.
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14.
- Ackerman AB. Training residents in dermatopathology: why, when, where, and how. J Am Acad Dermatol. 1990;22(6 Pt 1):1104-1106.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.e1.
- Murase JE. Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept. 2015;5:95-96.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
Dermatopathology education, which comprises approximately 30% of the dermatology residency curriculum, is crucial for the holistic training of dermatology residents to diagnose and manage a range of dermatologic conditions.1 Additionally, dermatopathology is the topic of one of the 4 American Board of Dermatology CORE Exam modules, further highlighting the need for comprehensive education in this area. A variety of resources including virtual dermatopathology and conventional microscopy training currently are used in residency programs for dermatopathology education.2,3 Although used less frequently, social media platforms such as Instagram also are used to aid in dermatopathology education for a wider audience.4 Other online resources, including the American Society of Dermatopathology website (www.asdp.org) and DermpathAtlas.com, are excellent tools for medical students, residents, and fellows to develop their knowledge.5 While these resources are accessible, they must be directly sought out by the student and utilized on their own time. Additionally, if medical students do not have a strong understanding of the basics of dermatopathology, they may not have the foundation required to benefit from these resources.
Dermatopathology education is critical for the overall practice of dermatology, yet most dermatology residency programs may not be incorporating dermatopathology education early enough in training. One study evaluating the timing and length of dermatopathology education during residency reported that fewer than 40% (20/51) of dermatology residency programs allocate 3 or more weeks to dermatopathology education during the second postgraduate year.1 Despite Ackerman6 advocating for early dermatopathology exposure to best prepare medical students to recognize and manage certain dermatologic conditions, the majority of exposure still seems to occur during postgraduate year 4.1 Furthermore, current primary care residents feel that their medical school training did not sufficiently prepare them to diagnose and manage dermatologic conditions, with only 37% (93/252) reporting feeling adequately prepared.7,8 Medical students also reported a lack of confidence in overall dermatology knowledge, with 89% (72/81) reporting they felt neutral, slightly confident, or not at all confident when asked to diagnose skin lesions.9 In the same study, the average score was 46.6% (7/15 questions answered correctly) when 74 participants were assessed via a multiple choice quiz on dermatologic diagnosis and treatment, further demonstrating the lack of general dermatology comfort among medical students.9 This likely stems from limited dermatology curriculum in medical schools, demonstrating the need for further dermatology education as a whole in medical school.10
Ensuring robust dermatopathology education in medical school and the first year of dermatology residency has the potential to better prepare medical students for the transition into dermatology residency and clinical practice. We created an introductory dermatopathology lecture and presented it to medical students and first year dermatology residents to improve dermatopathology knowledge and confidence in learners early in their dermatology training.
Structure of the Lecture
Participants included first-year dermatology residents and fourth-year medical students rotating with the Wayne State University Department of Dermatology (Detroit, Michigan). The same facilitator (H.O.) taught each of the lectures, and all lectures were conducted via Zoom at the beginning of the month from May 2024 through November 2024. A total of 7 lectures were given. The lecture was formatted so that a histologic image was shown, then learners expressed their thoughts about what the image was showing before the answer was given. This format allowed participants to view the images on their own device screen and allowed the facilitator to annotate the images. The lecture was divided into 3 sections: (1) cell types and basic structures, (2) anatomic slides, and (3) common diagnoses. Each session lasted approximately 45 minutes.
Section 1: Cell Types and Basic Structures—The first section covered the fundamental cell types (neutrophils, lymphocytes, plasma cells, melanocytes, and eosinophils) along with glandular structures (apocrine, eccrine, and sebaceous). The session was designed to follow a retention and allow learners to think through each slide. First, participants were shown histologic images of each cell type and were asked to identify what type of cell was being shown. On the following slide, key features of each cell type were highlighted. Next, participants similarly were shown images of the glandular structures followed by key features of each. The section concluded with a review of the layers of the skin (stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale). A histologic image was shown, and the facilitator discussed how to distinguish the layers.
Section 2: Anatomic Sites—This section focused on key pathologic features for differentiating body surfaces, including the scalp, face, eyelids, ears, areolae, palms and soles, and mucosae. Participants initially were shown an image of a hematoxylin and eosin–stained slide from a specific body surface and then were asked to identify structures that may serve as a clue to the anatomic location. If the participants were not sure, they were given hints; for example, when participants were shown an image of the ear and were unsure of the location, the facilitator circled cartilage and asked them to identify the structure. In most cases, once participants named this structure, they were able to recognize that the location was the ear.
Section 3: Common Diagnoses—This section addressed frequently encountered diagnoses in dermatopathology, including basal cell carcinoma, squamous cell carcinoma, squamous cell carcinoma in situ, epidermoid cyst, pilar cyst, seborrheic keratosis, solar lentigo, melanocytic nevus, melanoma, verruca vulgaris, spongiotic dermatitis, psoriasis, and lichen planus. It followed the same format of the first section: participants were shown an hemotoxyllin and eosin–stained image and then were asked to discuss what the diagnosis could be and why. Hints were given if participants struggled to come up with the correct diagnosis. A few slides also were dedicated to distinguishing benign nevi, dysplastic nevi, and melanoma.
Pretest and Posttest Results
Residents participated in the lecture as part of their first-year orientation, and medical students participated during their dermatology rotation. All participants were invited to complete a pretest and a posttest before and after the lecture, respectively. Both assessments were optional and anonymous. The pretest was completed electronically and consisted of 10 knowledge-based, multiple-choice questions that included a histopathologic image and asked, “What is the most likely diagnosis?,” “What is the predominant cell type?,” and “Where was this specimen taken from?” In addition to the knowledge-based questions, participants also were asked to rate their confidence in dermatopathology on a 5-point Likert scale ranging from 1 (not confident at all) to 5 (extremely confident). Participants completed the entire pretest before any information on the topic was provided. After the lecture, participants were asked to complete a posttest identical to the pretest and to rate their confidence in dermatopathology again on the same scale. The posttest included an additional question asking participants to rate the helpfulness of the lecture on a Likert scale ranging from 1 (not helpful at all) to 5 (extremely helpful). Participants completed the posttest within 48 hours of the lecture.
Overall, 15 learners participated in the pretest and 12 in the posttest. Of the 15 pretest participants, 3 were first-year residents and 12 were medical students. Similarly, in the posttest, 2 respondents were first-year residents and 10 were medical students. All responses contained complete pretests and posttests. The mean score on the pretest was 62%, whereas the mean score on the posttest was 75%. A paired t test indicated a statistically significant improvement (P=.017). In addition, the mean rating for confidence in dermatopathology knowledge before the lecture was 1.5 prior to the lecture and 2.6 after the lecture. A paired t test demonstrated statistical significance (P=.010). The mean rating of the helpfulness of the lecture was 4.67. The majority (91.7% [11/12]) of the participants gave a rating of 4 or 5.
Impact of the Lecture on Dermatopathology Knowledge
There is a gap in dermatopathology education early in medical training. Our introductory lecture led to higher post test scores and increased confidence in dermatopathology among medical students and dermatology residents, demonstrating the effectiveness of this kind of program in bridging this education gap. The majority of participants in our lecture said they found the session helpful. A previously published article called for early implementation of dermatology education as a whole in the medical curriculum due to lack of knowledge and confidence, and our introductory lecture may help to bridge this gap.8 Increasing dermatopathology content for medical students and first-year dermatology residents can expand knowledge, as shown by the increased scores on the posttest, and better supports learners transitioning to dermatology residency, where dermatopathology constitutes a large part of the overall curriculum.2 More comprehensive knowledge of dermatopathology early in dermatology training also may help to better prepare residents to accurately diagnose and manage dermatologic conditions.
Pretest scores showed that the average confidence rating in dermatopathology among participants in our lecture was 1.5, which is rather low. This is consistent with prior studies that have found that residents feel that medical school inadequately prepared them for dermatology residency.7,8 More than 87% (71/81) of medical students surveyed felt they received inadequate general dermatology training in medical school.9 This supports the proposed educational gap that is impacting confidence in overall dermatology knowledge, which includes dermatopathology. In our study, the average confidence rating increased by 1.1 points after the lecture, which was statistically significant (P=.010) and demonstrates that an introductory lecture serves as a feasible intervention to improve confidence in this area.
The feedback we received from participants in our lecture shows the benefits of an introductory interactive lecture with virtual dermatopathology images. Ngo et al2 highlighted how residents perceive virtual images to be superior to conventional microscopy for dermatopathology, which we utilized in our lecture. This method is not only cost effective but also provides a simple way for learners and facilitators to point out key findings on histopathology slides.2
Final Thoughts
Overall, implementing dermatopathology education early in training has a measurable impact on dermatopathology knowledge and confidence among medical students and first-year dermatology residents. An interactive lecture with virtual images similar to the one we describe here may better prepare learners for the transition to dermatology residency by addressing the educational gap in dermatopathology early in training.
Dermatopathology education, which comprises approximately 30% of the dermatology residency curriculum, is crucial for the holistic training of dermatology residents to diagnose and manage a range of dermatologic conditions.1 Additionally, dermatopathology is the topic of one of the 4 American Board of Dermatology CORE Exam modules, further highlighting the need for comprehensive education in this area. A variety of resources including virtual dermatopathology and conventional microscopy training currently are used in residency programs for dermatopathology education.2,3 Although used less frequently, social media platforms such as Instagram also are used to aid in dermatopathology education for a wider audience.4 Other online resources, including the American Society of Dermatopathology website (www.asdp.org) and DermpathAtlas.com, are excellent tools for medical students, residents, and fellows to develop their knowledge.5 While these resources are accessible, they must be directly sought out by the student and utilized on their own time. Additionally, if medical students do not have a strong understanding of the basics of dermatopathology, they may not have the foundation required to benefit from these resources.
Dermatopathology education is critical for the overall practice of dermatology, yet most dermatology residency programs may not be incorporating dermatopathology education early enough in training. One study evaluating the timing and length of dermatopathology education during residency reported that fewer than 40% (20/51) of dermatology residency programs allocate 3 or more weeks to dermatopathology education during the second postgraduate year.1 Despite Ackerman6 advocating for early dermatopathology exposure to best prepare medical students to recognize and manage certain dermatologic conditions, the majority of exposure still seems to occur during postgraduate year 4.1 Furthermore, current primary care residents feel that their medical school training did not sufficiently prepare them to diagnose and manage dermatologic conditions, with only 37% (93/252) reporting feeling adequately prepared.7,8 Medical students also reported a lack of confidence in overall dermatology knowledge, with 89% (72/81) reporting they felt neutral, slightly confident, or not at all confident when asked to diagnose skin lesions.9 In the same study, the average score was 46.6% (7/15 questions answered correctly) when 74 participants were assessed via a multiple choice quiz on dermatologic diagnosis and treatment, further demonstrating the lack of general dermatology comfort among medical students.9 This likely stems from limited dermatology curriculum in medical schools, demonstrating the need for further dermatology education as a whole in medical school.10
Ensuring robust dermatopathology education in medical school and the first year of dermatology residency has the potential to better prepare medical students for the transition into dermatology residency and clinical practice. We created an introductory dermatopathology lecture and presented it to medical students and first year dermatology residents to improve dermatopathology knowledge and confidence in learners early in their dermatology training.
Structure of the Lecture
Participants included first-year dermatology residents and fourth-year medical students rotating with the Wayne State University Department of Dermatology (Detroit, Michigan). The same facilitator (H.O.) taught each of the lectures, and all lectures were conducted via Zoom at the beginning of the month from May 2024 through November 2024. A total of 7 lectures were given. The lecture was formatted so that a histologic image was shown, then learners expressed their thoughts about what the image was showing before the answer was given. This format allowed participants to view the images on their own device screen and allowed the facilitator to annotate the images. The lecture was divided into 3 sections: (1) cell types and basic structures, (2) anatomic slides, and (3) common diagnoses. Each session lasted approximately 45 minutes.
Section 1: Cell Types and Basic Structures—The first section covered the fundamental cell types (neutrophils, lymphocytes, plasma cells, melanocytes, and eosinophils) along with glandular structures (apocrine, eccrine, and sebaceous). The session was designed to follow a retention and allow learners to think through each slide. First, participants were shown histologic images of each cell type and were asked to identify what type of cell was being shown. On the following slide, key features of each cell type were highlighted. Next, participants similarly were shown images of the glandular structures followed by key features of each. The section concluded with a review of the layers of the skin (stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale). A histologic image was shown, and the facilitator discussed how to distinguish the layers.
Section 2: Anatomic Sites—This section focused on key pathologic features for differentiating body surfaces, including the scalp, face, eyelids, ears, areolae, palms and soles, and mucosae. Participants initially were shown an image of a hematoxylin and eosin–stained slide from a specific body surface and then were asked to identify structures that may serve as a clue to the anatomic location. If the participants were not sure, they were given hints; for example, when participants were shown an image of the ear and were unsure of the location, the facilitator circled cartilage and asked them to identify the structure. In most cases, once participants named this structure, they were able to recognize that the location was the ear.
Section 3: Common Diagnoses—This section addressed frequently encountered diagnoses in dermatopathology, including basal cell carcinoma, squamous cell carcinoma, squamous cell carcinoma in situ, epidermoid cyst, pilar cyst, seborrheic keratosis, solar lentigo, melanocytic nevus, melanoma, verruca vulgaris, spongiotic dermatitis, psoriasis, and lichen planus. It followed the same format of the first section: participants were shown an hemotoxyllin and eosin–stained image and then were asked to discuss what the diagnosis could be and why. Hints were given if participants struggled to come up with the correct diagnosis. A few slides also were dedicated to distinguishing benign nevi, dysplastic nevi, and melanoma.
Pretest and Posttest Results
Residents participated in the lecture as part of their first-year orientation, and medical students participated during their dermatology rotation. All participants were invited to complete a pretest and a posttest before and after the lecture, respectively. Both assessments were optional and anonymous. The pretest was completed electronically and consisted of 10 knowledge-based, multiple-choice questions that included a histopathologic image and asked, “What is the most likely diagnosis?,” “What is the predominant cell type?,” and “Where was this specimen taken from?” In addition to the knowledge-based questions, participants also were asked to rate their confidence in dermatopathology on a 5-point Likert scale ranging from 1 (not confident at all) to 5 (extremely confident). Participants completed the entire pretest before any information on the topic was provided. After the lecture, participants were asked to complete a posttest identical to the pretest and to rate their confidence in dermatopathology again on the same scale. The posttest included an additional question asking participants to rate the helpfulness of the lecture on a Likert scale ranging from 1 (not helpful at all) to 5 (extremely helpful). Participants completed the posttest within 48 hours of the lecture.
Overall, 15 learners participated in the pretest and 12 in the posttest. Of the 15 pretest participants, 3 were first-year residents and 12 were medical students. Similarly, in the posttest, 2 respondents were first-year residents and 10 were medical students. All responses contained complete pretests and posttests. The mean score on the pretest was 62%, whereas the mean score on the posttest was 75%. A paired t test indicated a statistically significant improvement (P=.017). In addition, the mean rating for confidence in dermatopathology knowledge before the lecture was 1.5 prior to the lecture and 2.6 after the lecture. A paired t test demonstrated statistical significance (P=.010). The mean rating of the helpfulness of the lecture was 4.67. The majority (91.7% [11/12]) of the participants gave a rating of 4 or 5.
Impact of the Lecture on Dermatopathology Knowledge
There is a gap in dermatopathology education early in medical training. Our introductory lecture led to higher post test scores and increased confidence in dermatopathology among medical students and dermatology residents, demonstrating the effectiveness of this kind of program in bridging this education gap. The majority of participants in our lecture said they found the session helpful. A previously published article called for early implementation of dermatology education as a whole in the medical curriculum due to lack of knowledge and confidence, and our introductory lecture may help to bridge this gap.8 Increasing dermatopathology content for medical students and first-year dermatology residents can expand knowledge, as shown by the increased scores on the posttest, and better supports learners transitioning to dermatology residency, where dermatopathology constitutes a large part of the overall curriculum.2 More comprehensive knowledge of dermatopathology early in dermatology training also may help to better prepare residents to accurately diagnose and manage dermatologic conditions.
Pretest scores showed that the average confidence rating in dermatopathology among participants in our lecture was 1.5, which is rather low. This is consistent with prior studies that have found that residents feel that medical school inadequately prepared them for dermatology residency.7,8 More than 87% (71/81) of medical students surveyed felt they received inadequate general dermatology training in medical school.9 This supports the proposed educational gap that is impacting confidence in overall dermatology knowledge, which includes dermatopathology. In our study, the average confidence rating increased by 1.1 points after the lecture, which was statistically significant (P=.010) and demonstrates that an introductory lecture serves as a feasible intervention to improve confidence in this area.
The feedback we received from participants in our lecture shows the benefits of an introductory interactive lecture with virtual dermatopathology images. Ngo et al2 highlighted how residents perceive virtual images to be superior to conventional microscopy for dermatopathology, which we utilized in our lecture. This method is not only cost effective but also provides a simple way for learners and facilitators to point out key findings on histopathology slides.2
Final Thoughts
Overall, implementing dermatopathology education early in training has a measurable impact on dermatopathology knowledge and confidence among medical students and first-year dermatology residents. An interactive lecture with virtual images similar to the one we describe here may better prepare learners for the transition to dermatology residency by addressing the educational gap in dermatopathology early in training.
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826, vii.
- Ngo TB, Niu W, Fang Z, et al. Dermatology residents’ perspectives on virtual dermatopathology education. J Cutan Pathol. 2024;51:530-537.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Hubbard G, Saal R, Wintringham J, et al. Utilizing Instagram as a novel method for dermatopathology instruction. Clin Exp Dermatol. 2023;49:89-91.
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14.
- Ackerman AB. Training residents in dermatopathology: why, when, where, and how. J Am Acad Dermatol. 1990;22(6 Pt 1):1104-1106.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.e1.
- Murase JE. Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept. 2015;5:95-96.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826, vii.
- Ngo TB, Niu W, Fang Z, et al. Dermatology residents’ perspectives on virtual dermatopathology education. J Cutan Pathol. 2024;51:530-537.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Hubbard G, Saal R, Wintringham J, et al. Utilizing Instagram as a novel method for dermatopathology instruction. Clin Exp Dermatol. 2023;49:89-91.
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14.
- Ackerman AB. Training residents in dermatopathology: why, when, where, and how. J Am Acad Dermatol. 1990;22(6 Pt 1):1104-1106.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.e1.
- Murase JE. Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept. 2015;5:95-96.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Ergonomics in Dermatologic Procedures: Mobility Exercises to Incorporate In and Out of the Office
Ergonomics in Dermatologic Procedures: Mobility Exercises to Incorporate In and Out of the Office
Practice Gap
Dermatology encompasses a wide range of procedures performed in both clinical and surgical settings. One comprehensive review of ergonomics in dermatologic surgery found a high prevalence of musculoskeletal injuries (MSIs).1 A survey conducted in 2010 revealed that 90% of dermatologic surgeons experienced MSIs, which commonly resulted in neck, shoulder, and/or back pain.2
Prolonged abnormal static postures and repetitive motions, which are common in dermatologic practice, can lead to muscle imbalances and focal muscular ischemia, increasing physicians’ susceptibility to MSIs. When muscle fibers experience enough repeated focal ischemia, they may enter a constant state of contraction leading to myofascial pain syndrome (MPS); these painful areas are known as trigger points and often are refractory to traditional stretching.3
Musculoskeletal injuries can potentially impact dermatologists’ career longevity and satisfaction. To date, the literature on techniques and exercises that may prevent or alleviate MSIs is limited.1,4 We collaborated with a colleague in physical therapy (R.P.) to present stretching, mobility, and strengthening techniques and exercises dermatologists can perform both in and outside the procedure room to potentially reduce pain and prevent future MSIs.
The Techniques
Stretching and Mobility Exercises—When dermatologists adopt abnormal static postures, they are at risk for muscular imbalances caused by repetitive flexion and/or rotation in one direction. Over time, these repetitive movements can result in loss of flexibility in the direction opposite to that in which they are consistently positioned.3 Regular stretching offers physiologic benefits such as maintaining joint range of motion, increasing blood flow to muscles, and increasing synovial fluid production—all of which contribute to reduced risk for MSIs.3 Multiple studies and a systematic review have found that regular stretching throughout the day serves as an effective method for preventing and mitigating MSI pain in health care providers.1,3-5
Considering the directional manner of MSIs induced by prolonged static positions, the most benefit will be derived from stretches or extension in the opposite direction of that in which the practitioner usually works. For most dermatologic surgeons, stretches should target the trapezius muscles, shoulders, and cervical musculature. Techniques such as the neck and shoulder combination stretch, the upper trapezius stretch, and the downward shoulder blade squeeze stretch can be performed regularly throughout the day.3,4 To perform the neck and shoulder combination stretch, place the arm in flexion to shoulder height and bend the elbow at a 90° angle. Gently pull the arm across the front of the body, point the head gazing in the direction of the shoulder being stretched, and hold for 10 to 20 seconds. Repeat with the other side (eFigure 1).
Some surgeons may experience pain that is refractory to stretching, potentially indicating the presence of MPS.3 Managing MPS via stretching alone may be a challenge. Physical therapists utilize various techniques to manually massage the tissue, but self-myofascial release—which involves the use of a tool such as a dense foam roller or massage ball, both of which can easily be purchased—may be convenient and effective for busy providers. To perform this technique, the operator lies with their back on a dense foam roller positioned perpendicular to the body and uses their legs to undulate or roll back and forth in a smooth motion (Figure 1). This may help to alleviate myofascial pain in the spinal intrinsic muscles, which often are prone to injury due to posture; it also warms the fascia and breaks up adhesions. Self-myofascial release may have similar acute analgesic effects to classic stretching while also helping to alleviate MPS.

Strengthening Exercises—Musculoskeletal injuries often begin with fatigue in postural stabilizing muscles of the trunk and shoulders, leading the dermatologist to assume a slouched posture. Dermatologists should perform strengthening exercises targeting the trunk and shoulder girdle, which help to promote good working posture while optimizing the function of the arms and hands. Ideally, dermatologists should incorporate strengthening exercises 3 to 4 times per week in combination with daily stretching.
The 4-point kneeling alternate arm and leg extensions technique targets many muscle groups that commonly are affected in dermatologists and dermatologic surgeons. While on all fours, the operator positions the hands under the shoulders and the knees under the hips. The neck remains in line with the back with the eyes facing the floor. The abdominal muscles are then pulled up and in while simultaneously extending the left arm and right leg until both are parallel to the floor. This position should be held for 5 seconds and then repeated with the opposite contralateral extremities (Figure 2). Exercises specific to each muscle group also can be performed, such as planks to enhance truncal stability or scapular wall clocks to strengthen the shoulder girdle (eFigure 2). To perform scapular wall clocks, wrap a single resistance band around both wrists. Next, press the hands and elbows gently into a wall pointing superiorly and imagine there is a clock on the wall with 12 o’clock at the top and 6 o’clock at the bottom. Press the wrists outward on the band, keep the elbows straight, and reach out with the right hand while keeping the left hand stable. Move the right hand to the 1-, 3-, and 5-o’clock positions. Repeat with the left hand while holding the right hand stable. Move the left hand to the 11-, 9-, 7-, and 6-o’clock positions. Repeat these steps for 3 to 5 sets.


It is important to note that a decreased flow of oxygen and nutrients to muscles contributes to MSIs. Aerobic exercises increase blood flow and improve the ability of the muscles to utilize oxygen. Engaging in an enjoyable aerobic activity (eg, walking, running, swimming, cycling) 3 to 4 times per week can help prevent MSIs; however, as with any new exercise regimen (including the strengthening techniques described here), it is important to consult your primary care physician before getting started.
Practice Implications
As dermatologists progress in their careers, implementation of these techniques can mitigate MSIs and their sequelae. The long-term benefits of stretching, mobility, and strengthening exercises are dependent on having ergonomically suitable environmental factors. In addition to their own mechanics and posture, dermatologists must consider all elements that may affect the ergonomics of their daily practice, including operating room layout, instrumentation and workflow, and patient positioning. Through a consistent approach to prevention using the techniques described here, dermatologists can minimize the risk for MSIs and foster sustainability in their careers.
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097 /DSS.0000000000002295
- Liang CA, Levine VJ, Dusza SW, et al. Musculoskeletal disorders and ergonomics in dermatologic surgery: a survey of Mohs surgeons in 2010. Dermatol Surg. 2012;38:240-248. doi:10.1111/j.1524-4725.2011.02237.x
- Valachi B, Valachi K. Preventing musculoskeletal disorders in clinical dentistry: strategies to address the mechanisms leading to musculoskeletal disorders. J Am Dent Assoc. 2003;134:1604-1612. doi:10.14219/jada.archive.2003.0106
- Carley SK, Strauss JD, Vidal NY. Ergonomic solutions for dermatologists. Int J Womens Dermatol. 2021;7(5 part B):863-866. doi:10.1016/j.ijwd.2021.08.006
- da Costa BR, Vieira ER. Stretching to reduce work-related musculoskeletal disorders: a systematic review. J Rehabil Med. 2008;40:321-328. doi:10.2340/16501977-0204
Practice Gap
Dermatology encompasses a wide range of procedures performed in both clinical and surgical settings. One comprehensive review of ergonomics in dermatologic surgery found a high prevalence of musculoskeletal injuries (MSIs).1 A survey conducted in 2010 revealed that 90% of dermatologic surgeons experienced MSIs, which commonly resulted in neck, shoulder, and/or back pain.2
Prolonged abnormal static postures and repetitive motions, which are common in dermatologic practice, can lead to muscle imbalances and focal muscular ischemia, increasing physicians’ susceptibility to MSIs. When muscle fibers experience enough repeated focal ischemia, they may enter a constant state of contraction leading to myofascial pain syndrome (MPS); these painful areas are known as trigger points and often are refractory to traditional stretching.3
Musculoskeletal injuries can potentially impact dermatologists’ career longevity and satisfaction. To date, the literature on techniques and exercises that may prevent or alleviate MSIs is limited.1,4 We collaborated with a colleague in physical therapy (R.P.) to present stretching, mobility, and strengthening techniques and exercises dermatologists can perform both in and outside the procedure room to potentially reduce pain and prevent future MSIs.
The Techniques
Stretching and Mobility Exercises—When dermatologists adopt abnormal static postures, they are at risk for muscular imbalances caused by repetitive flexion and/or rotation in one direction. Over time, these repetitive movements can result in loss of flexibility in the direction opposite to that in which they are consistently positioned.3 Regular stretching offers physiologic benefits such as maintaining joint range of motion, increasing blood flow to muscles, and increasing synovial fluid production—all of which contribute to reduced risk for MSIs.3 Multiple studies and a systematic review have found that regular stretching throughout the day serves as an effective method for preventing and mitigating MSI pain in health care providers.1,3-5
Considering the directional manner of MSIs induced by prolonged static positions, the most benefit will be derived from stretches or extension in the opposite direction of that in which the practitioner usually works. For most dermatologic surgeons, stretches should target the trapezius muscles, shoulders, and cervical musculature. Techniques such as the neck and shoulder combination stretch, the upper trapezius stretch, and the downward shoulder blade squeeze stretch can be performed regularly throughout the day.3,4 To perform the neck and shoulder combination stretch, place the arm in flexion to shoulder height and bend the elbow at a 90° angle. Gently pull the arm across the front of the body, point the head gazing in the direction of the shoulder being stretched, and hold for 10 to 20 seconds. Repeat with the other side (eFigure 1).

Some surgeons may experience pain that is refractory to stretching, potentially indicating the presence of MPS.3 Managing MPS via stretching alone may be a challenge. Physical therapists utilize various techniques to manually massage the tissue, but self-myofascial release—which involves the use of a tool such as a dense foam roller or massage ball, both of which can easily be purchased—may be convenient and effective for busy providers. To perform this technique, the operator lies with their back on a dense foam roller positioned perpendicular to the body and uses their legs to undulate or roll back and forth in a smooth motion (Figure 1). This may help to alleviate myofascial pain in the spinal intrinsic muscles, which often are prone to injury due to posture; it also warms the fascia and breaks up adhesions. Self-myofascial release may have similar acute analgesic effects to classic stretching while also helping to alleviate MPS.

Strengthening Exercises—Musculoskeletal injuries often begin with fatigue in postural stabilizing muscles of the trunk and shoulders, leading the dermatologist to assume a slouched posture. Dermatologists should perform strengthening exercises targeting the trunk and shoulder girdle, which help to promote good working posture while optimizing the function of the arms and hands. Ideally, dermatologists should incorporate strengthening exercises 3 to 4 times per week in combination with daily stretching.
The 4-point kneeling alternate arm and leg extensions technique targets many muscle groups that commonly are affected in dermatologists and dermatologic surgeons. While on all fours, the operator positions the hands under the shoulders and the knees under the hips. The neck remains in line with the back with the eyes facing the floor. The abdominal muscles are then pulled up and in while simultaneously extending the left arm and right leg until both are parallel to the floor. This position should be held for 5 seconds and then repeated with the opposite contralateral extremities (Figure 2). Exercises specific to each muscle group also can be performed, such as planks to enhance truncal stability or scapular wall clocks to strengthen the shoulder girdle (eFigure 2). To perform scapular wall clocks, wrap a single resistance band around both wrists. Next, press the hands and elbows gently into a wall pointing superiorly and imagine there is a clock on the wall with 12 o’clock at the top and 6 o’clock at the bottom. Press the wrists outward on the band, keep the elbows straight, and reach out with the right hand while keeping the left hand stable. Move the right hand to the 1-, 3-, and 5-o’clock positions. Repeat with the left hand while holding the right hand stable. Move the left hand to the 11-, 9-, 7-, and 6-o’clock positions. Repeat these steps for 3 to 5 sets.


It is important to note that a decreased flow of oxygen and nutrients to muscles contributes to MSIs. Aerobic exercises increase blood flow and improve the ability of the muscles to utilize oxygen. Engaging in an enjoyable aerobic activity (eg, walking, running, swimming, cycling) 3 to 4 times per week can help prevent MSIs; however, as with any new exercise regimen (including the strengthening techniques described here), it is important to consult your primary care physician before getting started.
Practice Implications
As dermatologists progress in their careers, implementation of these techniques can mitigate MSIs and their sequelae. The long-term benefits of stretching, mobility, and strengthening exercises are dependent on having ergonomically suitable environmental factors. In addition to their own mechanics and posture, dermatologists must consider all elements that may affect the ergonomics of their daily practice, including operating room layout, instrumentation and workflow, and patient positioning. Through a consistent approach to prevention using the techniques described here, dermatologists can minimize the risk for MSIs and foster sustainability in their careers.
Practice Gap
Dermatology encompasses a wide range of procedures performed in both clinical and surgical settings. One comprehensive review of ergonomics in dermatologic surgery found a high prevalence of musculoskeletal injuries (MSIs).1 A survey conducted in 2010 revealed that 90% of dermatologic surgeons experienced MSIs, which commonly resulted in neck, shoulder, and/or back pain.2
Prolonged abnormal static postures and repetitive motions, which are common in dermatologic practice, can lead to muscle imbalances and focal muscular ischemia, increasing physicians’ susceptibility to MSIs. When muscle fibers experience enough repeated focal ischemia, they may enter a constant state of contraction leading to myofascial pain syndrome (MPS); these painful areas are known as trigger points and often are refractory to traditional stretching.3
Musculoskeletal injuries can potentially impact dermatologists’ career longevity and satisfaction. To date, the literature on techniques and exercises that may prevent or alleviate MSIs is limited.1,4 We collaborated with a colleague in physical therapy (R.P.) to present stretching, mobility, and strengthening techniques and exercises dermatologists can perform both in and outside the procedure room to potentially reduce pain and prevent future MSIs.
The Techniques
Stretching and Mobility Exercises—When dermatologists adopt abnormal static postures, they are at risk for muscular imbalances caused by repetitive flexion and/or rotation in one direction. Over time, these repetitive movements can result in loss of flexibility in the direction opposite to that in which they are consistently positioned.3 Regular stretching offers physiologic benefits such as maintaining joint range of motion, increasing blood flow to muscles, and increasing synovial fluid production—all of which contribute to reduced risk for MSIs.3 Multiple studies and a systematic review have found that regular stretching throughout the day serves as an effective method for preventing and mitigating MSI pain in health care providers.1,3-5
Considering the directional manner of MSIs induced by prolonged static positions, the most benefit will be derived from stretches or extension in the opposite direction of that in which the practitioner usually works. For most dermatologic surgeons, stretches should target the trapezius muscles, shoulders, and cervical musculature. Techniques such as the neck and shoulder combination stretch, the upper trapezius stretch, and the downward shoulder blade squeeze stretch can be performed regularly throughout the day.3,4 To perform the neck and shoulder combination stretch, place the arm in flexion to shoulder height and bend the elbow at a 90° angle. Gently pull the arm across the front of the body, point the head gazing in the direction of the shoulder being stretched, and hold for 10 to 20 seconds. Repeat with the other side (eFigure 1).

Some surgeons may experience pain that is refractory to stretching, potentially indicating the presence of MPS.3 Managing MPS via stretching alone may be a challenge. Physical therapists utilize various techniques to manually massage the tissue, but self-myofascial release—which involves the use of a tool such as a dense foam roller or massage ball, both of which can easily be purchased—may be convenient and effective for busy providers. To perform this technique, the operator lies with their back on a dense foam roller positioned perpendicular to the body and uses their legs to undulate or roll back and forth in a smooth motion (Figure 1). This may help to alleviate myofascial pain in the spinal intrinsic muscles, which often are prone to injury due to posture; it also warms the fascia and breaks up adhesions. Self-myofascial release may have similar acute analgesic effects to classic stretching while also helping to alleviate MPS.

Strengthening Exercises—Musculoskeletal injuries often begin with fatigue in postural stabilizing muscles of the trunk and shoulders, leading the dermatologist to assume a slouched posture. Dermatologists should perform strengthening exercises targeting the trunk and shoulder girdle, which help to promote good working posture while optimizing the function of the arms and hands. Ideally, dermatologists should incorporate strengthening exercises 3 to 4 times per week in combination with daily stretching.
The 4-point kneeling alternate arm and leg extensions technique targets many muscle groups that commonly are affected in dermatologists and dermatologic surgeons. While on all fours, the operator positions the hands under the shoulders and the knees under the hips. The neck remains in line with the back with the eyes facing the floor. The abdominal muscles are then pulled up and in while simultaneously extending the left arm and right leg until both are parallel to the floor. This position should be held for 5 seconds and then repeated with the opposite contralateral extremities (Figure 2). Exercises specific to each muscle group also can be performed, such as planks to enhance truncal stability or scapular wall clocks to strengthen the shoulder girdle (eFigure 2). To perform scapular wall clocks, wrap a single resistance band around both wrists. Next, press the hands and elbows gently into a wall pointing superiorly and imagine there is a clock on the wall with 12 o’clock at the top and 6 o’clock at the bottom. Press the wrists outward on the band, keep the elbows straight, and reach out with the right hand while keeping the left hand stable. Move the right hand to the 1-, 3-, and 5-o’clock positions. Repeat with the left hand while holding the right hand stable. Move the left hand to the 11-, 9-, 7-, and 6-o’clock positions. Repeat these steps for 3 to 5 sets.


It is important to note that a decreased flow of oxygen and nutrients to muscles contributes to MSIs. Aerobic exercises increase blood flow and improve the ability of the muscles to utilize oxygen. Engaging in an enjoyable aerobic activity (eg, walking, running, swimming, cycling) 3 to 4 times per week can help prevent MSIs; however, as with any new exercise regimen (including the strengthening techniques described here), it is important to consult your primary care physician before getting started.
Practice Implications
As dermatologists progress in their careers, implementation of these techniques can mitigate MSIs and their sequelae. The long-term benefits of stretching, mobility, and strengthening exercises are dependent on having ergonomically suitable environmental factors. In addition to their own mechanics and posture, dermatologists must consider all elements that may affect the ergonomics of their daily practice, including operating room layout, instrumentation and workflow, and patient positioning. Through a consistent approach to prevention using the techniques described here, dermatologists can minimize the risk for MSIs and foster sustainability in their careers.
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097 /DSS.0000000000002295
- Liang CA, Levine VJ, Dusza SW, et al. Musculoskeletal disorders and ergonomics in dermatologic surgery: a survey of Mohs surgeons in 2010. Dermatol Surg. 2012;38:240-248. doi:10.1111/j.1524-4725.2011.02237.x
- Valachi B, Valachi K. Preventing musculoskeletal disorders in clinical dentistry: strategies to address the mechanisms leading to musculoskeletal disorders. J Am Dent Assoc. 2003;134:1604-1612. doi:10.14219/jada.archive.2003.0106
- Carley SK, Strauss JD, Vidal NY. Ergonomic solutions for dermatologists. Int J Womens Dermatol. 2021;7(5 part B):863-866. doi:10.1016/j.ijwd.2021.08.006
- da Costa BR, Vieira ER. Stretching to reduce work-related musculoskeletal disorders: a systematic review. J Rehabil Med. 2008;40:321-328. doi:10.2340/16501977-0204
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097 /DSS.0000000000002295
- Liang CA, Levine VJ, Dusza SW, et al. Musculoskeletal disorders and ergonomics in dermatologic surgery: a survey of Mohs surgeons in 2010. Dermatol Surg. 2012;38:240-248. doi:10.1111/j.1524-4725.2011.02237.x
- Valachi B, Valachi K. Preventing musculoskeletal disorders in clinical dentistry: strategies to address the mechanisms leading to musculoskeletal disorders. J Am Dent Assoc. 2003;134:1604-1612. doi:10.14219/jada.archive.2003.0106
- Carley SK, Strauss JD, Vidal NY. Ergonomic solutions for dermatologists. Int J Womens Dermatol. 2021;7(5 part B):863-866. doi:10.1016/j.ijwd.2021.08.006
- da Costa BR, Vieira ER. Stretching to reduce work-related musculoskeletal disorders: a systematic review. J Rehabil Med. 2008;40:321-328. doi:10.2340/16501977-0204
Ergonomics in Dermatologic Procedures: Mobility Exercises to Incorporate In and Out of the Office
Ergonomics in Dermatologic Procedures: Mobility Exercises to Incorporate In and Out of the Office
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
THE COMPARISON
- A A Black woman with dermatosis papulosa nigra manifesting as a cluster of light brown flat seborrheic keratoses that covered the cheeks and lateral face and extended to the neck.
- B A Black man with dermatosis papulosa nigra manifesting as small black papules on the cheeks and eyelids involving the central face.
Dermatosis papulosa nigra (DPN), a subvariant of seborrheic keratosis (SK), is characterized by benign pigmented epidermal neoplasms that typically manifest on the face, neck, and trunk in individuals with darker skin tones.1,2 While DPN meets the diagnostic criteria for SK, certain characteristics can help distinguish these lesions from other SK types. Treatment of DPN in patients with skin of color requires caution, particularly regarding the use of abrasive methods as well as cryotherapy, which generally should be avoided.
Epidemiology
The incidence of SKs increases with age.3,4 Although it can occur in patients of all skin tones, SK is more common in lighter skin tones, while DPN predominantly is diagnosed in darker skin types.1,4 The prevalence of DPN in Black patients ranges from 10% to 30%, and Black women are twice as likely to be diagnosed with DPN as men.2 One study reported a first-degree relative with DPN in 84% (42/50) of patients.5 The number and size of DPN papules increase with age.1
Key Clinical Features
Dermatosis papulosa nigra and SK have distinctive morphologies: DPN typically manifests as raised, round or filiform, sessile, brown to black, 1- to 5-mm papules.2 Seborrheic keratoses tend to be larger with a “stuck on” appearance and manifest as well-demarcated, pink to black papules or plaques that can range in size from millimeters to a few centimeters.3,4 In DPN, the lesions usually are asymptomatic but may be tender, pruritic, dry, or scaly and may become irritated.1,2 They develop symmetrically in sun-exposed areas, and the most common sites are the malar face, temporal region, neck, and trunk.1,2,6,7 Seborrheic keratoses can appear throughout the body, including in sun-exposed areas, but have varying textures (eg, greasy, waxy, verrucous).3,4
Worth Noting
Dermatosis papulosa nigra and SK can resemble each other histologically: DPN demonstrates a fibrous stroma, papillomatosis, hyperkeratosis, and acanthosis at the intraepidermal layer, which are diagnostic criteria for SK.2,4,8 However, other histologic features characteristic of SK that are not seen in DPN include pseudohorn cysts, spindle tumor cells, and basaloid cell nests.8
Dermoscopy can be useful in ruling out malignant skin cancers when evaluating pigmented lesions. The most common dermoscopic features of SK are cerebriform patterns such as fissures and ridges, comedolike openings, and pigmented fingerprintlike structures.3,4 To a lesser degree, milialike cysts, sharp demarcation, and hairpin-shaped vascular structures also may be present.4 The dermoscopic findings of DPN have not been well evaluated, but one study revealed that DPN had similar dermoscopic features to SK with some predominant features.6 Ridges and fissures were seen in 59% of patients diagnosed with DPN followed by comedolike openings seen in 27% of patients. The coexistence of a cerebriform pattern with comedolike openings was infrequent, and milialike cysts were rare.6
While DPN and SK are benign, patients often seek treatment for cosmetic reasons. Factors to consider when choosing a treatment modality include location of the lesions, the patient’s skin tone, and postprocedural outcomes (eg, depigmentation, wound healing). In general, treatments for SK include cryotherapy, electrodesiccation and curettage, and topical therapeutics such as hydrogen peroxide 40%, topical vitamin D3, and nitric-zinc 30%-50% solutions.4,8 Well-established treatment options for DPN include electrodesiccation, laser therapies, scissor excision, and cryotherapy, but topical options such as tazarotene also have been reported.1,9 Of the treatments for DPN, electrodesiccation and laser therapy routinely are used.10
The efficacy of electrodessication and potassium titanyl phosphate (KTP) laser were assessed in a randomized, investigator-blinded split-face study.11 Both modalities received high improvement ratings, with the results favoring the KTP laser. The patients (most of whom were Black) reported that KTP laser was more effective but more painful than electrodessication (P=.002).11 In another randomized study, patients received 3 treatments—electrodessication, pulsed dye laser, and curettage—for select DPN papules.10 There was no difference in the degree of clearance, cosmetic outcome, or postinflammatory hyperpigmentation between the 3 modalities, but patients found the laser to be the most painful.
It is important to exercise caution when using abrasive methods (eg, laser therapy, electrodesiccation, curettage) in patients with darker skin tones because of the increased risk for postinflammatory pigment alteration.1,2,12 Adverse effects of treatment are a top concern in the management of DPN.5,13 While cryotherapy is a preferred treatment of SK in lighter skin tones, it generally is avoided for DPN in darker skin types because melanocyte destruction can lead to cosmetically unsatisfactory and easily visible depigmentation.9
To mitigate postprocedural adverse effects, proper aftercare can promote wound healing and minimize postinflammatory pigment alteration. In one split-face study of Black patients, 2 DPN papules were removed from each side of the face using fine-curved surgical scissors.14 Next, a petrolatum-based ointment and an antibiotic ointment with polymyxin B sulfate/bacitracin zinc was applied twice daily for 21 days to opposite sides of the face. Patients did not develop infection, tolerated both treatments well, and demonstrated improved general wound appearance according to investigator- rated clinical assessment.14 Other reported postprocedural approaches include using topical agents with ingredients shown to improve hyperpigmentation (eg, niacinamide, azelaic acid) as well as photoprotection.12
Health Disparity Highlight
While DPN is benign, it can have adverse psychosocial effects on patients. A study in Senegal revealed that 60% (19/30) of patients with DPN experienced anxiety related to their condition, while others noted that DPN hindered their social relationships.13 In one US study of 50 Black patients with DPN, there was a moderate effect on quality of life, and 36% (18/50) of patients had the lesions removed. However, of the treated patients, 67% (12/18) reported few—if any—symptoms prior to removal.5 Although treatment of DPN is widely considered a cosmetic procedure, therapeutic management can address—and may improve—mental health in patients with skin of color.1,5,13 Despite the high prevalence of DPN in patients with darker skin tones, data on treatment frequency and insurance coverage are not widely available, thus limiting our understanding of treatment accessibility and economic burden.
- Frazier WT, Proddutur S, Swope K. Common dermatologic conditions in skin of color. Am Fam Physician.2023;107:26-34.
- Metin SA, Lee BW, Lambert WC, et al. Dermatosis papulosa nigra: a clinically and histopathologically distinct entity. Clin Dermatol. 2017;35:491-496.
- Braun RP, Ludwig S, Marghoob AA. Differential diagnosis of seborrheic keratosis: clinical and dermoscopic features. J Drugs Dermatol. 2017; 16: 835-842.
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217.
- Uwakwe LN, De Souza B, Subash J, et al. Dermatosis papulosa nigra: a quality of life survey study. J Clin Aesthet Dermatol. 2020;13:17-19.
- Bhat RM, Patrao N, Monteiro R, et al. A clinical, dermoscopic, and histopathological study of dermatosis papulosa nigra (DPN)—an Indian perspective. Int J Dermatol. 2017;56:957-960.
- Karampinis E, Georgopoulou KE, Kampra E, et al. Clinical and dermoscopic patterns of basal cell carcinoma and its mimickers in skin of color: a practical summary. Medicina (Kaunas). 2024;60:1386.
- Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:E15934.
- Jain S, Caire H, Haas CJ. Management of dermatosis papulosa nigra: a systematic review. Int J Dermatol. Published online October 4, 2024.
- Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968-1972.
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083.
- Markiewicz E, Karaman-Jurukovska N, Mammone T, et al. Postinflammatory hyperpigmentation in dark skin: molecular mechanism and skincare implications. Clin Cosmet Investig Dermatol. 2022;15: 2555-2565.
- Niang SO, Kane A, Diallo M, et al. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):45-47.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol. 2011;64(suppl 3):S30-S35.

THE COMPARISON
- A A Black woman with dermatosis papulosa nigra manifesting as a cluster of light brown flat seborrheic keratoses that covered the cheeks and lateral face and extended to the neck.
- B A Black man with dermatosis papulosa nigra manifesting as small black papules on the cheeks and eyelids involving the central face.
Dermatosis papulosa nigra (DPN), a subvariant of seborrheic keratosis (SK), is characterized by benign pigmented epidermal neoplasms that typically manifest on the face, neck, and trunk in individuals with darker skin tones.1,2 While DPN meets the diagnostic criteria for SK, certain characteristics can help distinguish these lesions from other SK types. Treatment of DPN in patients with skin of color requires caution, particularly regarding the use of abrasive methods as well as cryotherapy, which generally should be avoided.
Epidemiology
The incidence of SKs increases with age.3,4 Although it can occur in patients of all skin tones, SK is more common in lighter skin tones, while DPN predominantly is diagnosed in darker skin types.1,4 The prevalence of DPN in Black patients ranges from 10% to 30%, and Black women are twice as likely to be diagnosed with DPN as men.2 One study reported a first-degree relative with DPN in 84% (42/50) of patients.5 The number and size of DPN papules increase with age.1
Key Clinical Features
Dermatosis papulosa nigra and SK have distinctive morphologies: DPN typically manifests as raised, round or filiform, sessile, brown to black, 1- to 5-mm papules.2 Seborrheic keratoses tend to be larger with a “stuck on” appearance and manifest as well-demarcated, pink to black papules or plaques that can range in size from millimeters to a few centimeters.3,4 In DPN, the lesions usually are asymptomatic but may be tender, pruritic, dry, or scaly and may become irritated.1,2 They develop symmetrically in sun-exposed areas, and the most common sites are the malar face, temporal region, neck, and trunk.1,2,6,7 Seborrheic keratoses can appear throughout the body, including in sun-exposed areas, but have varying textures (eg, greasy, waxy, verrucous).3,4
Worth Noting
Dermatosis papulosa nigra and SK can resemble each other histologically: DPN demonstrates a fibrous stroma, papillomatosis, hyperkeratosis, and acanthosis at the intraepidermal layer, which are diagnostic criteria for SK.2,4,8 However, other histologic features characteristic of SK that are not seen in DPN include pseudohorn cysts, spindle tumor cells, and basaloid cell nests.8
Dermoscopy can be useful in ruling out malignant skin cancers when evaluating pigmented lesions. The most common dermoscopic features of SK are cerebriform patterns such as fissures and ridges, comedolike openings, and pigmented fingerprintlike structures.3,4 To a lesser degree, milialike cysts, sharp demarcation, and hairpin-shaped vascular structures also may be present.4 The dermoscopic findings of DPN have not been well evaluated, but one study revealed that DPN had similar dermoscopic features to SK with some predominant features.6 Ridges and fissures were seen in 59% of patients diagnosed with DPN followed by comedolike openings seen in 27% of patients. The coexistence of a cerebriform pattern with comedolike openings was infrequent, and milialike cysts were rare.6
While DPN and SK are benign, patients often seek treatment for cosmetic reasons. Factors to consider when choosing a treatment modality include location of the lesions, the patient’s skin tone, and postprocedural outcomes (eg, depigmentation, wound healing). In general, treatments for SK include cryotherapy, electrodesiccation and curettage, and topical therapeutics such as hydrogen peroxide 40%, topical vitamin D3, and nitric-zinc 30%-50% solutions.4,8 Well-established treatment options for DPN include electrodesiccation, laser therapies, scissor excision, and cryotherapy, but topical options such as tazarotene also have been reported.1,9 Of the treatments for DPN, electrodesiccation and laser therapy routinely are used.10
The efficacy of electrodessication and potassium titanyl phosphate (KTP) laser were assessed in a randomized, investigator-blinded split-face study.11 Both modalities received high improvement ratings, with the results favoring the KTP laser. The patients (most of whom were Black) reported that KTP laser was more effective but more painful than electrodessication (P=.002).11 In another randomized study, patients received 3 treatments—electrodessication, pulsed dye laser, and curettage—for select DPN papules.10 There was no difference in the degree of clearance, cosmetic outcome, or postinflammatory hyperpigmentation between the 3 modalities, but patients found the laser to be the most painful.
It is important to exercise caution when using abrasive methods (eg, laser therapy, electrodesiccation, curettage) in patients with darker skin tones because of the increased risk for postinflammatory pigment alteration.1,2,12 Adverse effects of treatment are a top concern in the management of DPN.5,13 While cryotherapy is a preferred treatment of SK in lighter skin tones, it generally is avoided for DPN in darker skin types because melanocyte destruction can lead to cosmetically unsatisfactory and easily visible depigmentation.9
To mitigate postprocedural adverse effects, proper aftercare can promote wound healing and minimize postinflammatory pigment alteration. In one split-face study of Black patients, 2 DPN papules were removed from each side of the face using fine-curved surgical scissors.14 Next, a petrolatum-based ointment and an antibiotic ointment with polymyxin B sulfate/bacitracin zinc was applied twice daily for 21 days to opposite sides of the face. Patients did not develop infection, tolerated both treatments well, and demonstrated improved general wound appearance according to investigator- rated clinical assessment.14 Other reported postprocedural approaches include using topical agents with ingredients shown to improve hyperpigmentation (eg, niacinamide, azelaic acid) as well as photoprotection.12
Health Disparity Highlight
While DPN is benign, it can have adverse psychosocial effects on patients. A study in Senegal revealed that 60% (19/30) of patients with DPN experienced anxiety related to their condition, while others noted that DPN hindered their social relationships.13 In one US study of 50 Black patients with DPN, there was a moderate effect on quality of life, and 36% (18/50) of patients had the lesions removed. However, of the treated patients, 67% (12/18) reported few—if any—symptoms prior to removal.5 Although treatment of DPN is widely considered a cosmetic procedure, therapeutic management can address—and may improve—mental health in patients with skin of color.1,5,13 Despite the high prevalence of DPN in patients with darker skin tones, data on treatment frequency and insurance coverage are not widely available, thus limiting our understanding of treatment accessibility and economic burden.

THE COMPARISON
- A A Black woman with dermatosis papulosa nigra manifesting as a cluster of light brown flat seborrheic keratoses that covered the cheeks and lateral face and extended to the neck.
- B A Black man with dermatosis papulosa nigra manifesting as small black papules on the cheeks and eyelids involving the central face.
Dermatosis papulosa nigra (DPN), a subvariant of seborrheic keratosis (SK), is characterized by benign pigmented epidermal neoplasms that typically manifest on the face, neck, and trunk in individuals with darker skin tones.1,2 While DPN meets the diagnostic criteria for SK, certain characteristics can help distinguish these lesions from other SK types. Treatment of DPN in patients with skin of color requires caution, particularly regarding the use of abrasive methods as well as cryotherapy, which generally should be avoided.
Epidemiology
The incidence of SKs increases with age.3,4 Although it can occur in patients of all skin tones, SK is more common in lighter skin tones, while DPN predominantly is diagnosed in darker skin types.1,4 The prevalence of DPN in Black patients ranges from 10% to 30%, and Black women are twice as likely to be diagnosed with DPN as men.2 One study reported a first-degree relative with DPN in 84% (42/50) of patients.5 The number and size of DPN papules increase with age.1
Key Clinical Features
Dermatosis papulosa nigra and SK have distinctive morphologies: DPN typically manifests as raised, round or filiform, sessile, brown to black, 1- to 5-mm papules.2 Seborrheic keratoses tend to be larger with a “stuck on” appearance and manifest as well-demarcated, pink to black papules or plaques that can range in size from millimeters to a few centimeters.3,4 In DPN, the lesions usually are asymptomatic but may be tender, pruritic, dry, or scaly and may become irritated.1,2 They develop symmetrically in sun-exposed areas, and the most common sites are the malar face, temporal region, neck, and trunk.1,2,6,7 Seborrheic keratoses can appear throughout the body, including in sun-exposed areas, but have varying textures (eg, greasy, waxy, verrucous).3,4
Worth Noting
Dermatosis papulosa nigra and SK can resemble each other histologically: DPN demonstrates a fibrous stroma, papillomatosis, hyperkeratosis, and acanthosis at the intraepidermal layer, which are diagnostic criteria for SK.2,4,8 However, other histologic features characteristic of SK that are not seen in DPN include pseudohorn cysts, spindle tumor cells, and basaloid cell nests.8
Dermoscopy can be useful in ruling out malignant skin cancers when evaluating pigmented lesions. The most common dermoscopic features of SK are cerebriform patterns such as fissures and ridges, comedolike openings, and pigmented fingerprintlike structures.3,4 To a lesser degree, milialike cysts, sharp demarcation, and hairpin-shaped vascular structures also may be present.4 The dermoscopic findings of DPN have not been well evaluated, but one study revealed that DPN had similar dermoscopic features to SK with some predominant features.6 Ridges and fissures were seen in 59% of patients diagnosed with DPN followed by comedolike openings seen in 27% of patients. The coexistence of a cerebriform pattern with comedolike openings was infrequent, and milialike cysts were rare.6
While DPN and SK are benign, patients often seek treatment for cosmetic reasons. Factors to consider when choosing a treatment modality include location of the lesions, the patient’s skin tone, and postprocedural outcomes (eg, depigmentation, wound healing). In general, treatments for SK include cryotherapy, electrodesiccation and curettage, and topical therapeutics such as hydrogen peroxide 40%, topical vitamin D3, and nitric-zinc 30%-50% solutions.4,8 Well-established treatment options for DPN include electrodesiccation, laser therapies, scissor excision, and cryotherapy, but topical options such as tazarotene also have been reported.1,9 Of the treatments for DPN, electrodesiccation and laser therapy routinely are used.10
The efficacy of electrodessication and potassium titanyl phosphate (KTP) laser were assessed in a randomized, investigator-blinded split-face study.11 Both modalities received high improvement ratings, with the results favoring the KTP laser. The patients (most of whom were Black) reported that KTP laser was more effective but more painful than electrodessication (P=.002).11 In another randomized study, patients received 3 treatments—electrodessication, pulsed dye laser, and curettage—for select DPN papules.10 There was no difference in the degree of clearance, cosmetic outcome, or postinflammatory hyperpigmentation between the 3 modalities, but patients found the laser to be the most painful.
It is important to exercise caution when using abrasive methods (eg, laser therapy, electrodesiccation, curettage) in patients with darker skin tones because of the increased risk for postinflammatory pigment alteration.1,2,12 Adverse effects of treatment are a top concern in the management of DPN.5,13 While cryotherapy is a preferred treatment of SK in lighter skin tones, it generally is avoided for DPN in darker skin types because melanocyte destruction can lead to cosmetically unsatisfactory and easily visible depigmentation.9
To mitigate postprocedural adverse effects, proper aftercare can promote wound healing and minimize postinflammatory pigment alteration. In one split-face study of Black patients, 2 DPN papules were removed from each side of the face using fine-curved surgical scissors.14 Next, a petrolatum-based ointment and an antibiotic ointment with polymyxin B sulfate/bacitracin zinc was applied twice daily for 21 days to opposite sides of the face. Patients did not develop infection, tolerated both treatments well, and demonstrated improved general wound appearance according to investigator- rated clinical assessment.14 Other reported postprocedural approaches include using topical agents with ingredients shown to improve hyperpigmentation (eg, niacinamide, azelaic acid) as well as photoprotection.12
Health Disparity Highlight
While DPN is benign, it can have adverse psychosocial effects on patients. A study in Senegal revealed that 60% (19/30) of patients with DPN experienced anxiety related to their condition, while others noted that DPN hindered their social relationships.13 In one US study of 50 Black patients with DPN, there was a moderate effect on quality of life, and 36% (18/50) of patients had the lesions removed. However, of the treated patients, 67% (12/18) reported few—if any—symptoms prior to removal.5 Although treatment of DPN is widely considered a cosmetic procedure, therapeutic management can address—and may improve—mental health in patients with skin of color.1,5,13 Despite the high prevalence of DPN in patients with darker skin tones, data on treatment frequency and insurance coverage are not widely available, thus limiting our understanding of treatment accessibility and economic burden.
- Frazier WT, Proddutur S, Swope K. Common dermatologic conditions in skin of color. Am Fam Physician.2023;107:26-34.
- Metin SA, Lee BW, Lambert WC, et al. Dermatosis papulosa nigra: a clinically and histopathologically distinct entity. Clin Dermatol. 2017;35:491-496.
- Braun RP, Ludwig S, Marghoob AA. Differential diagnosis of seborrheic keratosis: clinical and dermoscopic features. J Drugs Dermatol. 2017; 16: 835-842.
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217.
- Uwakwe LN, De Souza B, Subash J, et al. Dermatosis papulosa nigra: a quality of life survey study. J Clin Aesthet Dermatol. 2020;13:17-19.
- Bhat RM, Patrao N, Monteiro R, et al. A clinical, dermoscopic, and histopathological study of dermatosis papulosa nigra (DPN)—an Indian perspective. Int J Dermatol. 2017;56:957-960.
- Karampinis E, Georgopoulou KE, Kampra E, et al. Clinical and dermoscopic patterns of basal cell carcinoma and its mimickers in skin of color: a practical summary. Medicina (Kaunas). 2024;60:1386.
- Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:E15934.
- Jain S, Caire H, Haas CJ. Management of dermatosis papulosa nigra: a systematic review. Int J Dermatol. Published online October 4, 2024.
- Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968-1972.
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083.
- Markiewicz E, Karaman-Jurukovska N, Mammone T, et al. Postinflammatory hyperpigmentation in dark skin: molecular mechanism and skincare implications. Clin Cosmet Investig Dermatol. 2022;15: 2555-2565.
- Niang SO, Kane A, Diallo M, et al. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):45-47.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol. 2011;64(suppl 3):S30-S35.
- Frazier WT, Proddutur S, Swope K. Common dermatologic conditions in skin of color. Am Fam Physician.2023;107:26-34.
- Metin SA, Lee BW, Lambert WC, et al. Dermatosis papulosa nigra: a clinically and histopathologically distinct entity. Clin Dermatol. 2017;35:491-496.
- Braun RP, Ludwig S, Marghoob AA. Differential diagnosis of seborrheic keratosis: clinical and dermoscopic features. J Drugs Dermatol. 2017; 16: 835-842.
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217.
- Uwakwe LN, De Souza B, Subash J, et al. Dermatosis papulosa nigra: a quality of life survey study. J Clin Aesthet Dermatol. 2020;13:17-19.
- Bhat RM, Patrao N, Monteiro R, et al. A clinical, dermoscopic, and histopathological study of dermatosis papulosa nigra (DPN)—an Indian perspective. Int J Dermatol. 2017;56:957-960.
- Karampinis E, Georgopoulou KE, Kampra E, et al. Clinical and dermoscopic patterns of basal cell carcinoma and its mimickers in skin of color: a practical summary. Medicina (Kaunas). 2024;60:1386.
- Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:E15934.
- Jain S, Caire H, Haas CJ. Management of dermatosis papulosa nigra: a systematic review. Int J Dermatol. Published online October 4, 2024.
- Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968-1972.
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083.
- Markiewicz E, Karaman-Jurukovska N, Mammone T, et al. Postinflammatory hyperpigmentation in dark skin: molecular mechanism and skincare implications. Clin Cosmet Investig Dermatol. 2022;15: 2555-2565.
- Niang SO, Kane A, Diallo M, et al. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):45-47.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol. 2011;64(suppl 3):S30-S35.
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
Association Between Psoriasis and Sunburn Prevalence in US Adults
Association Between Psoriasis and Sunburn Prevalence in US Adults
To the Editor:
UV light plays an essential role in various environmental and biological processes.1 Excessive exposure to UV radiation can lead to sunburn, which is marked by skin erythema and pain.2 A study of more than 31,000 individuals found that 34.2% of adults aged 18 years and older reported at least 1 sunburn during the survey year.3 A lack of research regarding the incidence of sunburns in patients with psoriasis is particularly important considering the heightened incidence of skin cancer observed in this population.4 Thus, the aim of our study was to analyze the prevalence of sunburns among US adults with psoriasis utilizing data from the National Health and Nutrition Examination Survey (NHANES) database.5
Our analysis initially included 11,842 participants ranging in age from 20 to 59 years; 35 did not respond to questions assessing psoriasis and sunburn prevalence and thus were excluded. Multivariable logistic regression analyses were performed using Stata/SE 18 (StataCorp LLC) to assess the relationship between psoriasis and sunburns. Our models controlled for patient age, sex, income, race, education, diabetes status, tobacco use, and body mass index. A P value <.05 was considered statistically significant. The study period from January 2009 to December 2014 was chosen based on the availability of the most recent and comprehensive psoriasis data within the NHANES database.
In the NHANES data we evaluated, psoriasis status was assessed by asking, “Have you ever been told by a doctor or other health professional that you had psoriasis?” History of sunburns in the survey year was assessed by the question, “How many times in the past year have you had sunburn?” Patients who reported 1 or more sunburns were included in the sunburn cohort, while those who did not report a sunburn were included in the no sunburn cohort.
In our analysis, the prevalence of at least 1 sunburn in the survey year in patients with psoriasis was 55.4% (weighted), compared to 45.6% (weighted) among those without psoriasis (eTable 1). Although there was no statistically significant relationship between psoriasis and history of sunburn in patients aged 20 to 59 years, a subgroup analysis revealed a significant association between psoriasis and sunburn in adults aged 20 to 39 years after adjusting for potential confounding variables (adjusted OR, 1.57 [95% CI, 1.00-2.45]; P=.049)(eTable 2). Further analysis of subgroups showed no statistically significant results with adjustment of the logistic regression model. Characterizing response rates is important for assessing the validity of survey studies. The NHANES response rate from 2009 to 2014 was 72.9%, enhancing the reliability of our findings.
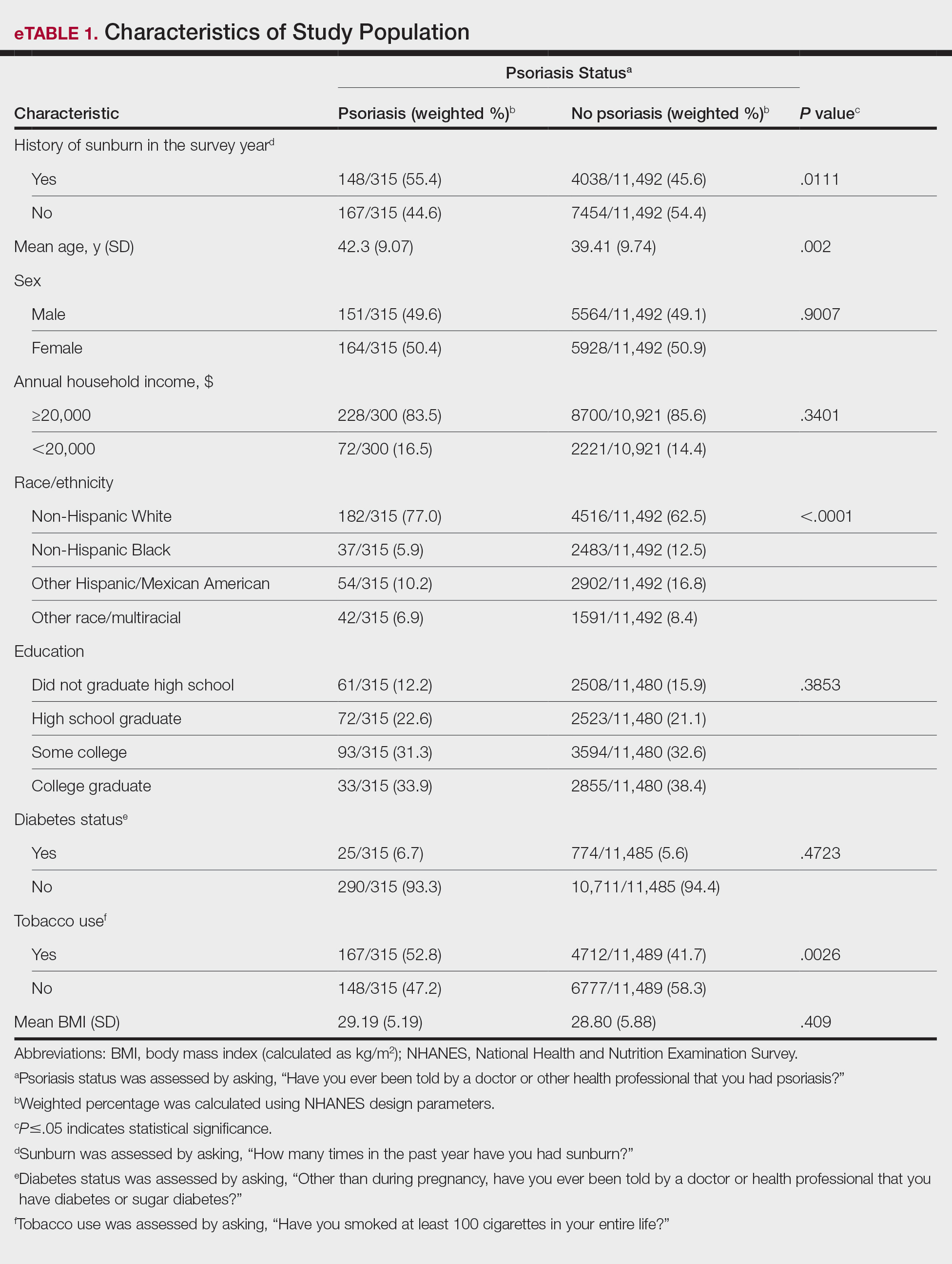
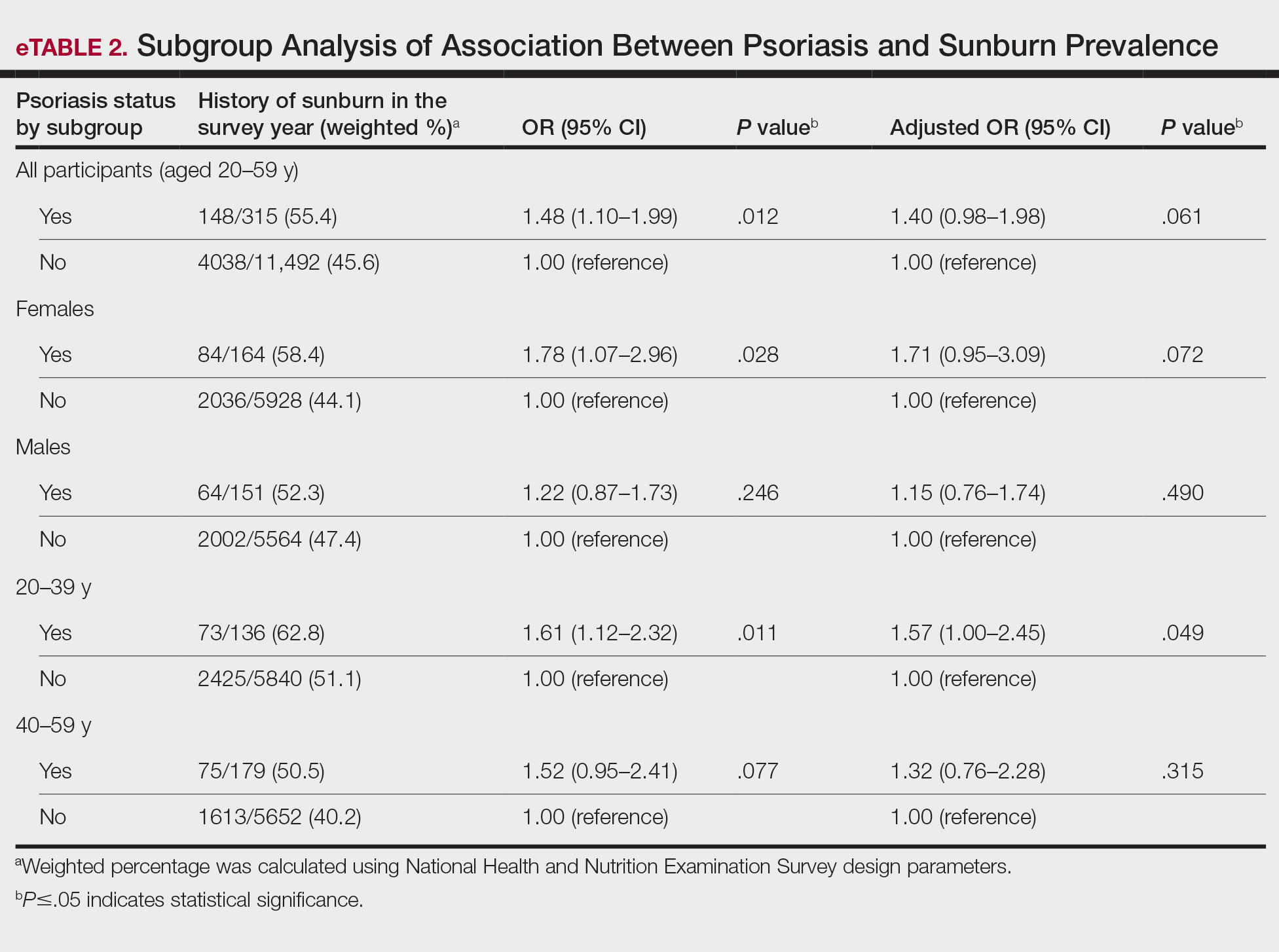
Our study revealed an increased prevalence of sunburn in US adults with psoriasis. A trend of increased sunburn prevalence among younger adults regardless of psoriasis status is corroborated by the literature. Surveys conducted in the United States in 2005, 2010, and 2015 showed that 43% to 50% of adults aged 18 to 39 years and 28% to 42% of those aged 40 to 59 years reported experiencing at least 1 sunburn within the respective survey year.6 Furthermore, in our study, patients with psoriasis reported higher rates of sunburn than their counterparts without psoriasis, both in those aged 20 to 39 years (psoriasis, 62.8% [73/136]; no psoriasis, 51.1% [2425/5840]) and those aged 40 to 59 years (psoriasis, 50.5% [n=75/179]; no psoriasis, 40.2% [1613/5652]), though it was only statistically significant in the 20-to-39 age group. This discrepancy may be attributed to differences in sun-protective behaviors in younger vs older adults. A study from the NHANES database found that, among individuals aged 20 to 39 years, 75.9% [4225/5493] reported staying in the shade, 50.0% [2346/5493] reported using sunscreen, and 31.2% [1874/5493] reported wearing sun-protective clothing.7 Interestingly, the likelihood of engaging in all 3 behaviors was 28% lower in the 20-to-39 age group vs the 40-to-59 age group (adjusted OR, 0.72; 95% CI, 0.62-0.83).7
While our analysis adjusted for age, race/ethnicity, and tobacco use to mitigate potential confounding, we acknowledge the statistically significant differences observed in these variables between study groups as presented in eTable 2. These differences may reflect inherent disparities in the study population. We employed multivariable regression analysis to control for these covariates in our primary analyses. Of note, there was a statistically significant difference associated with race/ethnicity when comparing non-Hispanic White individuals with psoriasis (77.0% [n=182/315]) and those without psoriasis (62.5% [n=4516/11,492])(P<.0001)(eTable 1). The higher proportion of non-Hispanic White patients in the psoriasis group may reflect an increased susceptibility to sunburn given their typically lighter skin pigmentation; however, our analysis controlled for race/ethnicity (eTable 2), thereby allowing us to isolate the effect of psoriasis on sunburn prevalence independent of racial/ethnic differences. There also were statistically significant differences in tobacco use (P=.0026) and age (P=.002) in our unadjusted findings (eTable 1). Again, our analysis controlled for these factors (eTable 2), thereby allowing us to isolate the effect of psoriasis on sunburn prevalence independent of tobacco use and age differences. This approach enhanced the reliability of our findings.
The association between psoriasis and skin cancer has previously been evaluated using the NHANES database—one study found that patients with psoriasis had a significantly higher prevalence of nonmelanoma skin cancer compared with those without psoriasis (3.0% vs 1.3%; relative risk, 2.29; P<.001).8 This difference remained significant after adjusting for confounding variables, as it was found that psoriasis was independently associated with a 1.5-fold increased risk for nonmelanoma skin cancer (adjusted relative risk, 2.06; P=.004).8
The relationship between psoriasis and sunburn may be due to behavioral choices, such as the use of phototherapy for managing psoriasis due to its recognized advantages.9 Patients may seek out both artificial and natural light sources more frequently, potentially increasing the risk for sunburn.10 Psoriasis-related sunburn susceptibility may stem from biological factors, including vitamin D insufficiency, as vitamin D is crucial for keratinocyte differentiation, immune function, and UV protection and repair.11 One study examined the effects of high-dose vitamin D3 on sunburn-induced inflammation.12 Patients who received high-dose vitamin D3 exhibited reduced skin inflammation, enhanced skin barrier repair, and increased anti-inflammatory response compared with those who did not receive the supplement. This improvement was associated with upregulation of arginase 1, an anti-inflammatory enzyme, leading to decreased levels of pro-inflammatory mediators such as tumor necrosis factor α and inducible nitric oxide synthase, thereby promoting tissue repair and reducing prolonged inflammation.12 These findings suggest that vitamin D insufficiency coupled with dysregulated immune responses may contribute to the heightened susceptibility of individuals with psoriasis to sunburn.
The established correlation between sunburn and skin cancer4,8 coupled with our findings of increased prevalence of sunburn in individuals with psoriasis underscores the need for additional research to clarify the underlying biological and behavioral factors that may contribute to a higher prevalence of sunburn in these patients, along with the implications for skin cancer development. Limitations of our study included potential recall bias, as individuals self-reported their clinical conditions and the inability to incorporate psoriasis severity into our analysis, as this was not consistently captured in the NHANES questionnaire during the study period.
- Blaustein AR, Searle C. Ultraviolet radiation. In: Levin SA, ed. Encyclopedia of Biodiversity. 2nd ed. Academic Press; 2013:296-303.
- D’Orazio J, Jarrett S, Amaro-Ortiz A, et al. UV radiation and the skin. Int J Mol Sci. 2013;14:12222-12248
- Holman DM, Ding H, Guy GP Jr, et al. Prevalence of sun protection use and sunburn and association of demographic and behavioral characteristics with sunburn among US adults. JAMA Dermatol. 2018;154:561-568.
- Balda A, Wani I, Roohi TF, et al. Psoriasis and skin cancer—is there a link? Int Immunopharmacol. 2023;121:110464.
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES questionnaires, datasets, and related documentation. Accessed December 4, 2024. https://wwwn.cdc.gov/nchs/nhanes/Default.aspx
- Holman DM, Ding H, Berkowitz Z, et al. Sunburn prevalence among US adults, National Health Interview Survey 2005, 2010, and 2015. J Am Acad Dermatol. 2019;80:817-820.
- Challapalli SD, Shetty KR, Bui Q, et al. Sun protective behaviors among adolescents and young adults in the United States. J Natl Med Assoc. 2023;115:353-361.
- Herbosa CM, Hodges W, Mann C, et al. Risk of cancer in psoriasis: study of a nationally representative sample of the US population with comparison to a single]institution cohort. J Am Acad Dermatol Venereol. 2020;34:E529-E531.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Åkerla P, Pukkala E, Helminen M, et al. Skin cancer risk of narrow-band UV-B (TL-01) phototherapy: a multi-center registry study with 4,815 patients. Acta Derm Venereol. 2024;104:adv39927.
- Filoni A, Vestita M, Congedo M, et al. Association between psoriasis and vitamin D: duration of disease correlates with decreased vitamin D serum levels: an observational case-control study. Medicine (Baltimore). 2018;97:E11185.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
To the Editor:
UV light plays an essential role in various environmental and biological processes.1 Excessive exposure to UV radiation can lead to sunburn, which is marked by skin erythema and pain.2 A study of more than 31,000 individuals found that 34.2% of adults aged 18 years and older reported at least 1 sunburn during the survey year.3 A lack of research regarding the incidence of sunburns in patients with psoriasis is particularly important considering the heightened incidence of skin cancer observed in this population.4 Thus, the aim of our study was to analyze the prevalence of sunburns among US adults with psoriasis utilizing data from the National Health and Nutrition Examination Survey (NHANES) database.5
Our analysis initially included 11,842 participants ranging in age from 20 to 59 years; 35 did not respond to questions assessing psoriasis and sunburn prevalence and thus were excluded. Multivariable logistic regression analyses were performed using Stata/SE 18 (StataCorp LLC) to assess the relationship between psoriasis and sunburns. Our models controlled for patient age, sex, income, race, education, diabetes status, tobacco use, and body mass index. A P value <.05 was considered statistically significant. The study period from January 2009 to December 2014 was chosen based on the availability of the most recent and comprehensive psoriasis data within the NHANES database.
In the NHANES data we evaluated, psoriasis status was assessed by asking, “Have you ever been told by a doctor or other health professional that you had psoriasis?” History of sunburns in the survey year was assessed by the question, “How many times in the past year have you had sunburn?” Patients who reported 1 or more sunburns were included in the sunburn cohort, while those who did not report a sunburn were included in the no sunburn cohort.
In our analysis, the prevalence of at least 1 sunburn in the survey year in patients with psoriasis was 55.4% (weighted), compared to 45.6% (weighted) among those without psoriasis (eTable 1). Although there was no statistically significant relationship between psoriasis and history of sunburn in patients aged 20 to 59 years, a subgroup analysis revealed a significant association between psoriasis and sunburn in adults aged 20 to 39 years after adjusting for potential confounding variables (adjusted OR, 1.57 [95% CI, 1.00-2.45]; P=.049)(eTable 2). Further analysis of subgroups showed no statistically significant results with adjustment of the logistic regression model. Characterizing response rates is important for assessing the validity of survey studies. The NHANES response rate from 2009 to 2014 was 72.9%, enhancing the reliability of our findings.


Our study revealed an increased prevalence of sunburn in US adults with psoriasis. A trend of increased sunburn prevalence among younger adults regardless of psoriasis status is corroborated by the literature. Surveys conducted in the United States in 2005, 2010, and 2015 showed that 43% to 50% of adults aged 18 to 39 years and 28% to 42% of those aged 40 to 59 years reported experiencing at least 1 sunburn within the respective survey year.6 Furthermore, in our study, patients with psoriasis reported higher rates of sunburn than their counterparts without psoriasis, both in those aged 20 to 39 years (psoriasis, 62.8% [73/136]; no psoriasis, 51.1% [2425/5840]) and those aged 40 to 59 years (psoriasis, 50.5% [n=75/179]; no psoriasis, 40.2% [1613/5652]), though it was only statistically significant in the 20-to-39 age group. This discrepancy may be attributed to differences in sun-protective behaviors in younger vs older adults. A study from the NHANES database found that, among individuals aged 20 to 39 years, 75.9% [4225/5493] reported staying in the shade, 50.0% [2346/5493] reported using sunscreen, and 31.2% [1874/5493] reported wearing sun-protective clothing.7 Interestingly, the likelihood of engaging in all 3 behaviors was 28% lower in the 20-to-39 age group vs the 40-to-59 age group (adjusted OR, 0.72; 95% CI, 0.62-0.83).7
While our analysis adjusted for age, race/ethnicity, and tobacco use to mitigate potential confounding, we acknowledge the statistically significant differences observed in these variables between study groups as presented in eTable 2. These differences may reflect inherent disparities in the study population. We employed multivariable regression analysis to control for these covariates in our primary analyses. Of note, there was a statistically significant difference associated with race/ethnicity when comparing non-Hispanic White individuals with psoriasis (77.0% [n=182/315]) and those without psoriasis (62.5% [n=4516/11,492])(P<.0001)(eTable 1). The higher proportion of non-Hispanic White patients in the psoriasis group may reflect an increased susceptibility to sunburn given their typically lighter skin pigmentation; however, our analysis controlled for race/ethnicity (eTable 2), thereby allowing us to isolate the effect of psoriasis on sunburn prevalence independent of racial/ethnic differences. There also were statistically significant differences in tobacco use (P=.0026) and age (P=.002) in our unadjusted findings (eTable 1). Again, our analysis controlled for these factors (eTable 2), thereby allowing us to isolate the effect of psoriasis on sunburn prevalence independent of tobacco use and age differences. This approach enhanced the reliability of our findings.
The association between psoriasis and skin cancer has previously been evaluated using the NHANES database—one study found that patients with psoriasis had a significantly higher prevalence of nonmelanoma skin cancer compared with those without psoriasis (3.0% vs 1.3%; relative risk, 2.29; P<.001).8 This difference remained significant after adjusting for confounding variables, as it was found that psoriasis was independently associated with a 1.5-fold increased risk for nonmelanoma skin cancer (adjusted relative risk, 2.06; P=.004).8
The relationship between psoriasis and sunburn may be due to behavioral choices, such as the use of phototherapy for managing psoriasis due to its recognized advantages.9 Patients may seek out both artificial and natural light sources more frequently, potentially increasing the risk for sunburn.10 Psoriasis-related sunburn susceptibility may stem from biological factors, including vitamin D insufficiency, as vitamin D is crucial for keratinocyte differentiation, immune function, and UV protection and repair.11 One study examined the effects of high-dose vitamin D3 on sunburn-induced inflammation.12 Patients who received high-dose vitamin D3 exhibited reduced skin inflammation, enhanced skin barrier repair, and increased anti-inflammatory response compared with those who did not receive the supplement. This improvement was associated with upregulation of arginase 1, an anti-inflammatory enzyme, leading to decreased levels of pro-inflammatory mediators such as tumor necrosis factor α and inducible nitric oxide synthase, thereby promoting tissue repair and reducing prolonged inflammation.12 These findings suggest that vitamin D insufficiency coupled with dysregulated immune responses may contribute to the heightened susceptibility of individuals with psoriasis to sunburn.
The established correlation between sunburn and skin cancer4,8 coupled with our findings of increased prevalence of sunburn in individuals with psoriasis underscores the need for additional research to clarify the underlying biological and behavioral factors that may contribute to a higher prevalence of sunburn in these patients, along with the implications for skin cancer development. Limitations of our study included potential recall bias, as individuals self-reported their clinical conditions and the inability to incorporate psoriasis severity into our analysis, as this was not consistently captured in the NHANES questionnaire during the study period.
To the Editor:
UV light plays an essential role in various environmental and biological processes.1 Excessive exposure to UV radiation can lead to sunburn, which is marked by skin erythema and pain.2 A study of more than 31,000 individuals found that 34.2% of adults aged 18 years and older reported at least 1 sunburn during the survey year.3 A lack of research regarding the incidence of sunburns in patients with psoriasis is particularly important considering the heightened incidence of skin cancer observed in this population.4 Thus, the aim of our study was to analyze the prevalence of sunburns among US adults with psoriasis utilizing data from the National Health and Nutrition Examination Survey (NHANES) database.5
Our analysis initially included 11,842 participants ranging in age from 20 to 59 years; 35 did not respond to questions assessing psoriasis and sunburn prevalence and thus were excluded. Multivariable logistic regression analyses were performed using Stata/SE 18 (StataCorp LLC) to assess the relationship between psoriasis and sunburns. Our models controlled for patient age, sex, income, race, education, diabetes status, tobacco use, and body mass index. A P value <.05 was considered statistically significant. The study period from January 2009 to December 2014 was chosen based on the availability of the most recent and comprehensive psoriasis data within the NHANES database.
In the NHANES data we evaluated, psoriasis status was assessed by asking, “Have you ever been told by a doctor or other health professional that you had psoriasis?” History of sunburns in the survey year was assessed by the question, “How many times in the past year have you had sunburn?” Patients who reported 1 or more sunburns were included in the sunburn cohort, while those who did not report a sunburn were included in the no sunburn cohort.
In our analysis, the prevalence of at least 1 sunburn in the survey year in patients with psoriasis was 55.4% (weighted), compared to 45.6% (weighted) among those without psoriasis (eTable 1). Although there was no statistically significant relationship between psoriasis and history of sunburn in patients aged 20 to 59 years, a subgroup analysis revealed a significant association between psoriasis and sunburn in adults aged 20 to 39 years after adjusting for potential confounding variables (adjusted OR, 1.57 [95% CI, 1.00-2.45]; P=.049)(eTable 2). Further analysis of subgroups showed no statistically significant results with adjustment of the logistic regression model. Characterizing response rates is important for assessing the validity of survey studies. The NHANES response rate from 2009 to 2014 was 72.9%, enhancing the reliability of our findings.


Our study revealed an increased prevalence of sunburn in US adults with psoriasis. A trend of increased sunburn prevalence among younger adults regardless of psoriasis status is corroborated by the literature. Surveys conducted in the United States in 2005, 2010, and 2015 showed that 43% to 50% of adults aged 18 to 39 years and 28% to 42% of those aged 40 to 59 years reported experiencing at least 1 sunburn within the respective survey year.6 Furthermore, in our study, patients with psoriasis reported higher rates of sunburn than their counterparts without psoriasis, both in those aged 20 to 39 years (psoriasis, 62.8% [73/136]; no psoriasis, 51.1% [2425/5840]) and those aged 40 to 59 years (psoriasis, 50.5% [n=75/179]; no psoriasis, 40.2% [1613/5652]), though it was only statistically significant in the 20-to-39 age group. This discrepancy may be attributed to differences in sun-protective behaviors in younger vs older adults. A study from the NHANES database found that, among individuals aged 20 to 39 years, 75.9% [4225/5493] reported staying in the shade, 50.0% [2346/5493] reported using sunscreen, and 31.2% [1874/5493] reported wearing sun-protective clothing.7 Interestingly, the likelihood of engaging in all 3 behaviors was 28% lower in the 20-to-39 age group vs the 40-to-59 age group (adjusted OR, 0.72; 95% CI, 0.62-0.83).7
While our analysis adjusted for age, race/ethnicity, and tobacco use to mitigate potential confounding, we acknowledge the statistically significant differences observed in these variables between study groups as presented in eTable 2. These differences may reflect inherent disparities in the study population. We employed multivariable regression analysis to control for these covariates in our primary analyses. Of note, there was a statistically significant difference associated with race/ethnicity when comparing non-Hispanic White individuals with psoriasis (77.0% [n=182/315]) and those without psoriasis (62.5% [n=4516/11,492])(P<.0001)(eTable 1). The higher proportion of non-Hispanic White patients in the psoriasis group may reflect an increased susceptibility to sunburn given their typically lighter skin pigmentation; however, our analysis controlled for race/ethnicity (eTable 2), thereby allowing us to isolate the effect of psoriasis on sunburn prevalence independent of racial/ethnic differences. There also were statistically significant differences in tobacco use (P=.0026) and age (P=.002) in our unadjusted findings (eTable 1). Again, our analysis controlled for these factors (eTable 2), thereby allowing us to isolate the effect of psoriasis on sunburn prevalence independent of tobacco use and age differences. This approach enhanced the reliability of our findings.
The association between psoriasis and skin cancer has previously been evaluated using the NHANES database—one study found that patients with psoriasis had a significantly higher prevalence of nonmelanoma skin cancer compared with those without psoriasis (3.0% vs 1.3%; relative risk, 2.29; P<.001).8 This difference remained significant after adjusting for confounding variables, as it was found that psoriasis was independently associated with a 1.5-fold increased risk for nonmelanoma skin cancer (adjusted relative risk, 2.06; P=.004).8
The relationship between psoriasis and sunburn may be due to behavioral choices, such as the use of phototherapy for managing psoriasis due to its recognized advantages.9 Patients may seek out both artificial and natural light sources more frequently, potentially increasing the risk for sunburn.10 Psoriasis-related sunburn susceptibility may stem from biological factors, including vitamin D insufficiency, as vitamin D is crucial for keratinocyte differentiation, immune function, and UV protection and repair.11 One study examined the effects of high-dose vitamin D3 on sunburn-induced inflammation.12 Patients who received high-dose vitamin D3 exhibited reduced skin inflammation, enhanced skin barrier repair, and increased anti-inflammatory response compared with those who did not receive the supplement. This improvement was associated with upregulation of arginase 1, an anti-inflammatory enzyme, leading to decreased levels of pro-inflammatory mediators such as tumor necrosis factor α and inducible nitric oxide synthase, thereby promoting tissue repair and reducing prolonged inflammation.12 These findings suggest that vitamin D insufficiency coupled with dysregulated immune responses may contribute to the heightened susceptibility of individuals with psoriasis to sunburn.
The established correlation between sunburn and skin cancer4,8 coupled with our findings of increased prevalence of sunburn in individuals with psoriasis underscores the need for additional research to clarify the underlying biological and behavioral factors that may contribute to a higher prevalence of sunburn in these patients, along with the implications for skin cancer development. Limitations of our study included potential recall bias, as individuals self-reported their clinical conditions and the inability to incorporate psoriasis severity into our analysis, as this was not consistently captured in the NHANES questionnaire during the study period.
- Blaustein AR, Searle C. Ultraviolet radiation. In: Levin SA, ed. Encyclopedia of Biodiversity. 2nd ed. Academic Press; 2013:296-303.
- D’Orazio J, Jarrett S, Amaro-Ortiz A, et al. UV radiation and the skin. Int J Mol Sci. 2013;14:12222-12248
- Holman DM, Ding H, Guy GP Jr, et al. Prevalence of sun protection use and sunburn and association of demographic and behavioral characteristics with sunburn among US adults. JAMA Dermatol. 2018;154:561-568.
- Balda A, Wani I, Roohi TF, et al. Psoriasis and skin cancer—is there a link? Int Immunopharmacol. 2023;121:110464.
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES questionnaires, datasets, and related documentation. Accessed December 4, 2024. https://wwwn.cdc.gov/nchs/nhanes/Default.aspx
- Holman DM, Ding H, Berkowitz Z, et al. Sunburn prevalence among US adults, National Health Interview Survey 2005, 2010, and 2015. J Am Acad Dermatol. 2019;80:817-820.
- Challapalli SD, Shetty KR, Bui Q, et al. Sun protective behaviors among adolescents and young adults in the United States. J Natl Med Assoc. 2023;115:353-361.
- Herbosa CM, Hodges W, Mann C, et al. Risk of cancer in psoriasis: study of a nationally representative sample of the US population with comparison to a single]institution cohort. J Am Acad Dermatol Venereol. 2020;34:E529-E531.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Åkerla P, Pukkala E, Helminen M, et al. Skin cancer risk of narrow-band UV-B (TL-01) phototherapy: a multi-center registry study with 4,815 patients. Acta Derm Venereol. 2024;104:adv39927.
- Filoni A, Vestita M, Congedo M, et al. Association between psoriasis and vitamin D: duration of disease correlates with decreased vitamin D serum levels: an observational case-control study. Medicine (Baltimore). 2018;97:E11185.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Blaustein AR, Searle C. Ultraviolet radiation. In: Levin SA, ed. Encyclopedia of Biodiversity. 2nd ed. Academic Press; 2013:296-303.
- D’Orazio J, Jarrett S, Amaro-Ortiz A, et al. UV radiation and the skin. Int J Mol Sci. 2013;14:12222-12248
- Holman DM, Ding H, Guy GP Jr, et al. Prevalence of sun protection use and sunburn and association of demographic and behavioral characteristics with sunburn among US adults. JAMA Dermatol. 2018;154:561-568.
- Balda A, Wani I, Roohi TF, et al. Psoriasis and skin cancer—is there a link? Int Immunopharmacol. 2023;121:110464.
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES questionnaires, datasets, and related documentation. Accessed December 4, 2024. https://wwwn.cdc.gov/nchs/nhanes/Default.aspx
- Holman DM, Ding H, Berkowitz Z, et al. Sunburn prevalence among US adults, National Health Interview Survey 2005, 2010, and 2015. J Am Acad Dermatol. 2019;80:817-820.
- Challapalli SD, Shetty KR, Bui Q, et al. Sun protective behaviors among adolescents and young adults in the United States. J Natl Med Assoc. 2023;115:353-361.
- Herbosa CM, Hodges W, Mann C, et al. Risk of cancer in psoriasis: study of a nationally representative sample of the US population with comparison to a single]institution cohort. J Am Acad Dermatol Venereol. 2020;34:E529-E531.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Åkerla P, Pukkala E, Helminen M, et al. Skin cancer risk of narrow-band UV-B (TL-01) phototherapy: a multi-center registry study with 4,815 patients. Acta Derm Venereol. 2024;104:adv39927.
- Filoni A, Vestita M, Congedo M, et al. Association between psoriasis and vitamin D: duration of disease correlates with decreased vitamin D serum levels: an observational case-control study. Medicine (Baltimore). 2018;97:E11185.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
Association Between Psoriasis and Sunburn Prevalence in US Adults
Association Between Psoriasis and Sunburn Prevalence in US Adults
PRACTICE POINTS
- It is important for dermatologists to encourage rigorous sun-safety practices in patients with psoriasis, particularly those aged 20 to 59 years.
- A thorough sunburn history should be taken for skin cancer risk assessment in patients with psoriasis.
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
Psoriasis treatments have come a long way in the past 20 years. We now have more than a dozen systemic targeted treatments for psoriatic disease, with more on the way; however, with each successive class of medications introduced, the gap has narrowed in terms of increasing efficacy. In an era of medications reporting complete clearance rates in the 70% range, the average improvement in Psoriasis Area and Severity Index (PASI) for most biologics has remained at 90% to 95% in the past half-decade. While this is a far cry from the mean PASI improvements of 70% seen with the first biologics,1 it is becoming more challenging to base our treatment decisions solely on PASI outcome measures.
How, then, do we approach rational selection of a systemic psoriasis treatment? We could try to delineate based on mechanism of action, but it may be disingenuous to dissect minor differences in pathways (eg, IL-17 vs IL-23) that are fundamentally related and on the same continuum in psoriasis pathophysiology. Therefore, the most meaningful way to select an appropriate therapeutic may be to adopt a patient-centered approach that accounts for both individual preferences and specific medical needs by evaluating for other comorbidities2 to exclude or select certain medicines or types of treatments. We have long known to avoid tumor necrosis factor (TNF) α inhibitors in patients with congestive heart failure or a history of demyelinating disorders while regularly considering the presence of psoriatic arthritis and family planning when making treatment decisions. Now, we can be more nuanced in our approaches to psoriasis biologics. Specifically, the most important comorbidities to consider broadly encompass cardiometabolic disorders, gastrointestinal conditions, and psychiatric conditions.
Cardiometabolic Disorders
Possibly the hottest topic in psoriasis for some years now, the relationship between cardiometabolic disorders and psoriasis is of great interest to clinicians, scientists, and patients alike. There is a clear link between development of atherosclerosis and Th17-related immune mechanisms that also are implicated in the pathogenesis of psoriasis.3 Furthermore, the incidence of cardiovascular disease is markedly increased in patients with psoriasis, which is an independent risk factor for myocardial infarction, particularly among younger patients.4,5 Although several retrospective studies6-8 have shown that TNF-α inhibitors are associated with a reduction in cardiovascular outcomes, it is yet to be seen whether biologic treatment actually has a direct impact on cardiovascular outcomes, multiple studies investigating the effect of biologics on arterial inflammation markers notwithstanding.9
There are some direct factors to keep in mind when considering cardiometabolic comorbidities in patients with psoriasis. Obesity is common in the psoriasis population and can have a direct negative effect on cardiovascular health.10 However, the data on obesity and psoriasis are somewhat mixed with regard to treatment outcomes. In general, with increased volumes of distribution for biologics in patients with obesity, it has been shown that treatment success is more difficult to achieve in those with a body mass index greater than 30.11 Rather surprisingly, a separate nationwide study in South Korea found that patients on biologics for psoriasis were more likely to experience weight gain, even after controlling for factors such as exercise, smoking, and drinking,12 but it is unclear whether this is driven mostly by a known connection between weight gain and TNF-α inhibitors.13 These contrasting results point to the need for further studies in this area, as our intuitive approach would involve promoting weight loss while starting on a systemic treatment for psoriasis—but perhaps it is important not to assume that one will come with the other in tow, reinforcing the need to discuss a healthy diet with our patients with psoriasis regardless of treatment decisions.
The data that we have do not directly answer the big questions about biologic treatment and cardiovascular health, but we are starting to see interesting signals. For example, in a report of tildrakizumab treatment in patients with and without metabolic syndrome, the rates of major adverse cardiovascular events as well as cardiac disorders were essentially the same in both groups after receiving treatment for up to 244 weeks.14 This is interesting, more because of the lack of an increase in cardiovascular adverse events in the metabolic syndrome group, who entered the trial on average 25 kg to 30 kg heavier than those without metabolic syndrome. There is an increased risk for adverse cardiovascular events among patients with metabolic syndrome, a roughly 2-fold relative risk in as few as 5 to 6 years of follow-up.15 While the cohorts in the tildrakizumab study14 were too small to draw firm conclusions, the data are interesting and a step in the right direction; we need much larger data sets for analysis. Among other agents, similar efficacy and safety have been reported for guselkumab in a long-term psoriasis study; as a class, IL-23 inhibitors also tend to perform well from an efficacy standpoint in patients with obesity.16
Overall, when assessing the evidence for cardiometabolic disorders, it is reasonable to consider starting a biologic from the IL-17 or IL-23 inhibitor classes— thus avoiding both the potential downside of weight gain and contraindication in patients with congestive heart failure associated with TNF-α inhibitors. It is important to counsel patients about weight loss in conjunction with these treatments, both to improve efficacy and reduce cardiovascular risk factors. There may be a preference for IL-23 inhibitors in patients with obesity, as this class of medications maintains efficacy particularly well in these patients. Patients with psoriasis should be counseled to follow up with a primary care physician given their higher risk for metabolic syndrome and adverse cardiovascular outcomes.
Gastrointestinal Conditions
Psoriasis and inflammatory bowel disease (IBD) have a bidirectional association, and patients with psoriasis are about 1.7 times more likely to have either Crohn disease or ulcerative colitis.17,18 This association may be related to a shared pathogenesis with regard to immune dysregulation and overactivated inflammatory pathways, but there are some important differences to consider from a therapeutic standpoint. Given the increased expression of IL-17 in patients with IBD,19 a phase II trial of secukinumab yielded surprising results—not only was secukinumab ineffective in treating Crohn disease, but there also were higher rates of adverse events20 (as noted on the product label for all IL-17 inhibitors). We have come to understand that there are regulatory subsets of IL-17 cells that are important in mucosal homeostasis and also regulate IL-10, which generally is considered an anti-inflammatory cytokine.21 Thus, while IL-17 inhibition can reduce some component of inflammatory signaling, it also can increase inflammatory signaling through indirect pathways while increasing intestinal permeability to microbes. Importantly, this process seems to occur via IL-23–independent pathways; as such, while direct inhibition of IL-17 can be deleterious, IL-23 inhibitors have become important therapeutics for IBD.22
IL-17 family, IL-17A clearly is the culprit for worsening colitis as evidenced by both human and animal models. On the contrary, IL-17F blockade has been shown to ameliorate colitis in a murine model, whereas IL-17A inhibition worsens it.23 Furthermore, dual blockade of IL-17A and IL-17F has a protective effect against colitis, suggesting that the IL-17F inhibition is dominant. This interesting finding has some mechanistic backing, since blockade of IL-17F induces Treg cells that serve to maintain gut epithelium homeostasis and integrity.24
Overall, IL-17A inhibitors should be avoided in patients with a history of IBD—namely, secukinumab and ixekizumab. While there may be theoretical reasons that brodalumab or bimekizumab may confer a somewhat different risk for IBD exacerbation, there may be better choices that would be expected to effectively treat both the psoriasis and IBD manifestations. Given the US Food and Drug Administration approval of IL-23 inhibitors for IBD and their high levels of efficacy in treating psoriasis, these likely will be the best choice for most patients. Another mainstay of IBD treatment is TNF-α inhibitors, but they come with other risks such as increased immunosuppression and increased risk for nonmelanoma skin cancer.
An important question remains: What about patients who do not have known IBD? Do we proactively change our treatment choice due to fear of IBD development given the higher incidence of both Crohn disease and ulcerative colitis in patients with psoriasis? What about patients with a family history of IBD? First-degree relatives of patients with Crohn disease and ulcerative colitis have an 8- and 4-fold higher risk for those same conditions, respectively.25 Postmarketing surveillance and database findings of low rates of IBD development with IL-17 inhibitors gives only modest reassurance, as dermatologists generally know to avoid these medications for patients with even questionable IBD symptoms. It is important to emphasize to our patients that in no case do we believe that a psoriasis medication actually will cause IBD—rather, someone with subclinical IBD could experience a flare and a first manifestation of colitis. The drug is not the culprit in inducing IBD but rather may serve to unmask existing disease.
One study suggested that for patients who move on to the IL-17 inhibitor secukinumab after being treated with TNF-α inhibitors for psoriasis, the rates of IBD development are higher (4.8%) than in those who start IL-17A inhibition without prior treatment (1%)(OR, 8.38; P=.018).26 This begs the question of whether subclinical IBD in many patients with psoriasis who are treated with TNF-α inhibitors can be unmasked later when they are transitioned to a treatment that either does not treat the IBD or could worsen it. There may be a mechanistic drive behind this sequencing of treatments that predisposes patients to colitis, which would suggest selecting an IL-23 inhibitor after failing/trying a TNF-α inhibitor. However, the data are very preliminary, and in real practice, other concerns such as severe psoriatic arthritis may outweigh these considerations, as the IL-17 inhibitor class still is considered to be more effective than IL-23 inhibition at treating psoriatic arthritis overall. For most patients with no personal history of IBD and no strong family history of IBD (ie, first-degree relatives), the choice of biologic should not be affected by concern over gastrointestinal issues.
Psychiatric Conditions
It has been well established that psoriasis is linked to higher rates of depression, anxiety, and suicidality.27 How do we take this into account when treating patients with psoriasis, especially when we have biologics with a warning label for suicidality and a Risk Evaluation and Mitigation Strategies program (brodalumab) and language around suicidal ideation in the label (bimekizumab)? While it is challenging to discuss mental health, it is not a conversation that we as dermatologists should shy away from. Appropriate treatment of psoriasis is an important tool to get our patients on the path to better mental health. A recent database study of more than 4000 patients showed that patients with psoriasis treated with biologics had a 17% lower risk for depression than those treated with conventional disease-modifying drugs such as methotrexate.28 The comparator of the conventional disease-modifying drug class is important as it serves as a control for disease severity. Too often, a higher rate of depression, anxiety, or suicidality can be attributed to a medication when we in fact may just be capturing the background of higher incidence of all 3 in patients with severe psoriasis.
Indeed, even with the medication that many worry about on this front (brodalumab), multiple studies have confirmed that the effect on mental health generally is a positive one, with decreases in depressive symptoms.29 In a cohort switched from TNF-α inhibitors to brodalumab, symptoms of depression actually improved,30 so attributing a direct treatment effect to negative mental health outcomes does not seem to be justified, especially in light of the low number of suicide events in global postmarketing surveillance for brodalumab, comparable to or lower than other biologics for psoriasis.31 Similarly, bimekizumab has language in the label about discussing suicidality with patients, although the rates of suicidal ideation and behavior are no different from other biologics and rates of depression improved with its use.32
Heightened awareness of our patients’ mental health is something that we as providers should embrace, even when it seems that we do not have much time to see each patient. The priority when a patient comes in with mental health symptoms should be to treat what is within our scope (ie, psoriasis) as quickly and effectively as possible— with a newer-generation biologic such as an IL-17 or IL-23 inhibitor—while encouraging the patient to seek care from a mental health professional. In these cases, one might even argue that the rapidity of action of IL-17 inhibitors may be of additional benefit.
Final Thoughts
We as dermatologists generally are tasked with seeing high volumes of patients, and an initial psoriasis consultation can be a lengthy visit; however, it is rewarding to establish this relationship with patients and a reminder of why we practice medicine to begin with. Psoriasis can be satisfying to treat, and we have so many highly effective medicines that can completely transform our patients’ lives. Applying an understanding of the interplay between psoriasis, its related comorbidities, and treatment choices can be a fulfilling exercise that captures the essence of shared decision-making, which can lead to better outcomes and satisfaction for both providers and patients.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022. doi:10.1056/NEJMoa030409
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22:425-442. doi:10.1007/s40257-021-00603-w
- Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5-22. doi:10.1007 /s00281-009-0153-8
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. doi:10.1001/jama.296.14.1735
- Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014-1024. doi:10.1016/j.jaad.2013.06.053
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90. doi:10.1016/j.jaad.2016.07.042
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250. doi:10.1001 /archdermatol.2012.2502
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68. doi:10.1016/j.jaad.2018.02.050
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi:10.3389/fphar.2021.774808
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:E984-E1010. doi:10.1161/CIR.0000000000000973
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41:917-925. doi:10.1007 /s40261-021-01080-z
- Kim H, Hong JY, Cheong S, et al. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: a nationwide population-based cohort study. Obes Res Clin Pract. 2023;17:210-217. doi:10.1016/j.orcp.2023.05.004
- Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290-295. doi:10.1016/j.phrs.2008.02.006
- Fernandez AP, Dauden E, Gerdes S, et al. Tildrakizumab efficacy and safety in patients with psoriasis and concomitant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. J Eur Acad Dermatol Venereol. 2022;36:1774-1783. doi:10.1111/jdv.18167
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. doi:10.1016/j.jacc.2010.05.034
- Ricceri F, Chiricozzi A, Peris K, et al. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: a multicentric retrospective study. Dermatol Ther. 2022;35:E15793. doi:10.1111/dth.15793
- Alinaghi F, Tekin HG, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease— a systematic review and meta-analysis. J Crohns Colitis. 2020;14:351-360. doi:10.1093/ecco-jcc/jjz152
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. doi:10.1136/gut.52.1.65
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693-1700. doi:10.1136 /gutjnl-2011-301668
- Brockmann L, Tran A, Huang Y, et al. Intestinal microbiotaspecific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719-2735 e7. doi:10.1016/j.immuni.2023.11.003
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727-738. doi:10.1016/j.immuni.2015.09.003
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. doi:10.1097 /MIB.0b013e318286fa1c
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T(reg) cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. doi:10.1038/s41590-018-0134-y
- El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27-35. doi:10.1007 /s40291-023-00678-7
- Albayrak F, Gür M, Karatas¸ A, et al. Is the use of secukinumab after anti-TNF therapy greater than expected for the risk of developing inflammatory bowel disease? Reumatol Clin (Engl Ed). 2024;20:123-127. doi:10.1016/j.reumae.2023.11.002
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Strober B, Soliman AM, Truong B, et al. Association between biologic exposure and the risk of depression in patients with psoriasis: a retrospective analysis of large US administrative claims data. Am J Clin Dermatol. 2024;25:853-856. doi:10.1007/s40257 -024-00877-w
- Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361-365.
- Andersch-Bjorkman Y, Micu E, Seifert O, et al. Effects of brodalumab on psoriasis and depressive symptoms in patients with insufficient response to TNF-alpha inhibitors. J Dermatol. 2023;50:1401-1414. doi:10.1111/1346-8138.16917
- Yeroushalmi S, Chung M, Bartholomew E, et al. Examining worldwide postmarketing suicides from biologics used for psoriasis with a focus on brodalumab: a cross-sectional analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS). JAAD Int. 2022;9:119-121. doi:10.1016/j.jdin.2022.08.010
- Blauvelt A, Armstrong A, Merola JF, et al. Mental health outcomes in patients with moderate to severe psoriasis treated with bimekizumab: analysis of phase 2/3 randomized trials. J Am Acad Dermatol. 2024;91:72-81. doi:10.1016/j.jaad.2024.02.039
Psoriasis treatments have come a long way in the past 20 years. We now have more than a dozen systemic targeted treatments for psoriatic disease, with more on the way; however, with each successive class of medications introduced, the gap has narrowed in terms of increasing efficacy. In an era of medications reporting complete clearance rates in the 70% range, the average improvement in Psoriasis Area and Severity Index (PASI) for most biologics has remained at 90% to 95% in the past half-decade. While this is a far cry from the mean PASI improvements of 70% seen with the first biologics,1 it is becoming more challenging to base our treatment decisions solely on PASI outcome measures.
How, then, do we approach rational selection of a systemic psoriasis treatment? We could try to delineate based on mechanism of action, but it may be disingenuous to dissect minor differences in pathways (eg, IL-17 vs IL-23) that are fundamentally related and on the same continuum in psoriasis pathophysiology. Therefore, the most meaningful way to select an appropriate therapeutic may be to adopt a patient-centered approach that accounts for both individual preferences and specific medical needs by evaluating for other comorbidities2 to exclude or select certain medicines or types of treatments. We have long known to avoid tumor necrosis factor (TNF) α inhibitors in patients with congestive heart failure or a history of demyelinating disorders while regularly considering the presence of psoriatic arthritis and family planning when making treatment decisions. Now, we can be more nuanced in our approaches to psoriasis biologics. Specifically, the most important comorbidities to consider broadly encompass cardiometabolic disorders, gastrointestinal conditions, and psychiatric conditions.
Cardiometabolic Disorders
Possibly the hottest topic in psoriasis for some years now, the relationship between cardiometabolic disorders and psoriasis is of great interest to clinicians, scientists, and patients alike. There is a clear link between development of atherosclerosis and Th17-related immune mechanisms that also are implicated in the pathogenesis of psoriasis.3 Furthermore, the incidence of cardiovascular disease is markedly increased in patients with psoriasis, which is an independent risk factor for myocardial infarction, particularly among younger patients.4,5 Although several retrospective studies6-8 have shown that TNF-α inhibitors are associated with a reduction in cardiovascular outcomes, it is yet to be seen whether biologic treatment actually has a direct impact on cardiovascular outcomes, multiple studies investigating the effect of biologics on arterial inflammation markers notwithstanding.9
There are some direct factors to keep in mind when considering cardiometabolic comorbidities in patients with psoriasis. Obesity is common in the psoriasis population and can have a direct negative effect on cardiovascular health.10 However, the data on obesity and psoriasis are somewhat mixed with regard to treatment outcomes. In general, with increased volumes of distribution for biologics in patients with obesity, it has been shown that treatment success is more difficult to achieve in those with a body mass index greater than 30.11 Rather surprisingly, a separate nationwide study in South Korea found that patients on biologics for psoriasis were more likely to experience weight gain, even after controlling for factors such as exercise, smoking, and drinking,12 but it is unclear whether this is driven mostly by a known connection between weight gain and TNF-α inhibitors.13 These contrasting results point to the need for further studies in this area, as our intuitive approach would involve promoting weight loss while starting on a systemic treatment for psoriasis—but perhaps it is important not to assume that one will come with the other in tow, reinforcing the need to discuss a healthy diet with our patients with psoriasis regardless of treatment decisions.
The data that we have do not directly answer the big questions about biologic treatment and cardiovascular health, but we are starting to see interesting signals. For example, in a report of tildrakizumab treatment in patients with and without metabolic syndrome, the rates of major adverse cardiovascular events as well as cardiac disorders were essentially the same in both groups after receiving treatment for up to 244 weeks.14 This is interesting, more because of the lack of an increase in cardiovascular adverse events in the metabolic syndrome group, who entered the trial on average 25 kg to 30 kg heavier than those without metabolic syndrome. There is an increased risk for adverse cardiovascular events among patients with metabolic syndrome, a roughly 2-fold relative risk in as few as 5 to 6 years of follow-up.15 While the cohorts in the tildrakizumab study14 were too small to draw firm conclusions, the data are interesting and a step in the right direction; we need much larger data sets for analysis. Among other agents, similar efficacy and safety have been reported for guselkumab in a long-term psoriasis study; as a class, IL-23 inhibitors also tend to perform well from an efficacy standpoint in patients with obesity.16
Overall, when assessing the evidence for cardiometabolic disorders, it is reasonable to consider starting a biologic from the IL-17 or IL-23 inhibitor classes— thus avoiding both the potential downside of weight gain and contraindication in patients with congestive heart failure associated with TNF-α inhibitors. It is important to counsel patients about weight loss in conjunction with these treatments, both to improve efficacy and reduce cardiovascular risk factors. There may be a preference for IL-23 inhibitors in patients with obesity, as this class of medications maintains efficacy particularly well in these patients. Patients with psoriasis should be counseled to follow up with a primary care physician given their higher risk for metabolic syndrome and adverse cardiovascular outcomes.
Gastrointestinal Conditions
Psoriasis and inflammatory bowel disease (IBD) have a bidirectional association, and patients with psoriasis are about 1.7 times more likely to have either Crohn disease or ulcerative colitis.17,18 This association may be related to a shared pathogenesis with regard to immune dysregulation and overactivated inflammatory pathways, but there are some important differences to consider from a therapeutic standpoint. Given the increased expression of IL-17 in patients with IBD,19 a phase II trial of secukinumab yielded surprising results—not only was secukinumab ineffective in treating Crohn disease, but there also were higher rates of adverse events20 (as noted on the product label for all IL-17 inhibitors). We have come to understand that there are regulatory subsets of IL-17 cells that are important in mucosal homeostasis and also regulate IL-10, which generally is considered an anti-inflammatory cytokine.21 Thus, while IL-17 inhibition can reduce some component of inflammatory signaling, it also can increase inflammatory signaling through indirect pathways while increasing intestinal permeability to microbes. Importantly, this process seems to occur via IL-23–independent pathways; as such, while direct inhibition of IL-17 can be deleterious, IL-23 inhibitors have become important therapeutics for IBD.22
IL-17 family, IL-17A clearly is the culprit for worsening colitis as evidenced by both human and animal models. On the contrary, IL-17F blockade has been shown to ameliorate colitis in a murine model, whereas IL-17A inhibition worsens it.23 Furthermore, dual blockade of IL-17A and IL-17F has a protective effect against colitis, suggesting that the IL-17F inhibition is dominant. This interesting finding has some mechanistic backing, since blockade of IL-17F induces Treg cells that serve to maintain gut epithelium homeostasis and integrity.24
Overall, IL-17A inhibitors should be avoided in patients with a history of IBD—namely, secukinumab and ixekizumab. While there may be theoretical reasons that brodalumab or bimekizumab may confer a somewhat different risk for IBD exacerbation, there may be better choices that would be expected to effectively treat both the psoriasis and IBD manifestations. Given the US Food and Drug Administration approval of IL-23 inhibitors for IBD and their high levels of efficacy in treating psoriasis, these likely will be the best choice for most patients. Another mainstay of IBD treatment is TNF-α inhibitors, but they come with other risks such as increased immunosuppression and increased risk for nonmelanoma skin cancer.
An important question remains: What about patients who do not have known IBD? Do we proactively change our treatment choice due to fear of IBD development given the higher incidence of both Crohn disease and ulcerative colitis in patients with psoriasis? What about patients with a family history of IBD? First-degree relatives of patients with Crohn disease and ulcerative colitis have an 8- and 4-fold higher risk for those same conditions, respectively.25 Postmarketing surveillance and database findings of low rates of IBD development with IL-17 inhibitors gives only modest reassurance, as dermatologists generally know to avoid these medications for patients with even questionable IBD symptoms. It is important to emphasize to our patients that in no case do we believe that a psoriasis medication actually will cause IBD—rather, someone with subclinical IBD could experience a flare and a first manifestation of colitis. The drug is not the culprit in inducing IBD but rather may serve to unmask existing disease.
One study suggested that for patients who move on to the IL-17 inhibitor secukinumab after being treated with TNF-α inhibitors for psoriasis, the rates of IBD development are higher (4.8%) than in those who start IL-17A inhibition without prior treatment (1%)(OR, 8.38; P=.018).26 This begs the question of whether subclinical IBD in many patients with psoriasis who are treated with TNF-α inhibitors can be unmasked later when they are transitioned to a treatment that either does not treat the IBD or could worsen it. There may be a mechanistic drive behind this sequencing of treatments that predisposes patients to colitis, which would suggest selecting an IL-23 inhibitor after failing/trying a TNF-α inhibitor. However, the data are very preliminary, and in real practice, other concerns such as severe psoriatic arthritis may outweigh these considerations, as the IL-17 inhibitor class still is considered to be more effective than IL-23 inhibition at treating psoriatic arthritis overall. For most patients with no personal history of IBD and no strong family history of IBD (ie, first-degree relatives), the choice of biologic should not be affected by concern over gastrointestinal issues.
Psychiatric Conditions
It has been well established that psoriasis is linked to higher rates of depression, anxiety, and suicidality.27 How do we take this into account when treating patients with psoriasis, especially when we have biologics with a warning label for suicidality and a Risk Evaluation and Mitigation Strategies program (brodalumab) and language around suicidal ideation in the label (bimekizumab)? While it is challenging to discuss mental health, it is not a conversation that we as dermatologists should shy away from. Appropriate treatment of psoriasis is an important tool to get our patients on the path to better mental health. A recent database study of more than 4000 patients showed that patients with psoriasis treated with biologics had a 17% lower risk for depression than those treated with conventional disease-modifying drugs such as methotrexate.28 The comparator of the conventional disease-modifying drug class is important as it serves as a control for disease severity. Too often, a higher rate of depression, anxiety, or suicidality can be attributed to a medication when we in fact may just be capturing the background of higher incidence of all 3 in patients with severe psoriasis.
Indeed, even with the medication that many worry about on this front (brodalumab), multiple studies have confirmed that the effect on mental health generally is a positive one, with decreases in depressive symptoms.29 In a cohort switched from TNF-α inhibitors to brodalumab, symptoms of depression actually improved,30 so attributing a direct treatment effect to negative mental health outcomes does not seem to be justified, especially in light of the low number of suicide events in global postmarketing surveillance for brodalumab, comparable to or lower than other biologics for psoriasis.31 Similarly, bimekizumab has language in the label about discussing suicidality with patients, although the rates of suicidal ideation and behavior are no different from other biologics and rates of depression improved with its use.32
Heightened awareness of our patients’ mental health is something that we as providers should embrace, even when it seems that we do not have much time to see each patient. The priority when a patient comes in with mental health symptoms should be to treat what is within our scope (ie, psoriasis) as quickly and effectively as possible— with a newer-generation biologic such as an IL-17 or IL-23 inhibitor—while encouraging the patient to seek care from a mental health professional. In these cases, one might even argue that the rapidity of action of IL-17 inhibitors may be of additional benefit.
Final Thoughts
We as dermatologists generally are tasked with seeing high volumes of patients, and an initial psoriasis consultation can be a lengthy visit; however, it is rewarding to establish this relationship with patients and a reminder of why we practice medicine to begin with. Psoriasis can be satisfying to treat, and we have so many highly effective medicines that can completely transform our patients’ lives. Applying an understanding of the interplay between psoriasis, its related comorbidities, and treatment choices can be a fulfilling exercise that captures the essence of shared decision-making, which can lead to better outcomes and satisfaction for both providers and patients.
Psoriasis treatments have come a long way in the past 20 years. We now have more than a dozen systemic targeted treatments for psoriatic disease, with more on the way; however, with each successive class of medications introduced, the gap has narrowed in terms of increasing efficacy. In an era of medications reporting complete clearance rates in the 70% range, the average improvement in Psoriasis Area and Severity Index (PASI) for most biologics has remained at 90% to 95% in the past half-decade. While this is a far cry from the mean PASI improvements of 70% seen with the first biologics,1 it is becoming more challenging to base our treatment decisions solely on PASI outcome measures.
How, then, do we approach rational selection of a systemic psoriasis treatment? We could try to delineate based on mechanism of action, but it may be disingenuous to dissect minor differences in pathways (eg, IL-17 vs IL-23) that are fundamentally related and on the same continuum in psoriasis pathophysiology. Therefore, the most meaningful way to select an appropriate therapeutic may be to adopt a patient-centered approach that accounts for both individual preferences and specific medical needs by evaluating for other comorbidities2 to exclude or select certain medicines or types of treatments. We have long known to avoid tumor necrosis factor (TNF) α inhibitors in patients with congestive heart failure or a history of demyelinating disorders while regularly considering the presence of psoriatic arthritis and family planning when making treatment decisions. Now, we can be more nuanced in our approaches to psoriasis biologics. Specifically, the most important comorbidities to consider broadly encompass cardiometabolic disorders, gastrointestinal conditions, and psychiatric conditions.
Cardiometabolic Disorders
Possibly the hottest topic in psoriasis for some years now, the relationship between cardiometabolic disorders and psoriasis is of great interest to clinicians, scientists, and patients alike. There is a clear link between development of atherosclerosis and Th17-related immune mechanisms that also are implicated in the pathogenesis of psoriasis.3 Furthermore, the incidence of cardiovascular disease is markedly increased in patients with psoriasis, which is an independent risk factor for myocardial infarction, particularly among younger patients.4,5 Although several retrospective studies6-8 have shown that TNF-α inhibitors are associated with a reduction in cardiovascular outcomes, it is yet to be seen whether biologic treatment actually has a direct impact on cardiovascular outcomes, multiple studies investigating the effect of biologics on arterial inflammation markers notwithstanding.9
There are some direct factors to keep in mind when considering cardiometabolic comorbidities in patients with psoriasis. Obesity is common in the psoriasis population and can have a direct negative effect on cardiovascular health.10 However, the data on obesity and psoriasis are somewhat mixed with regard to treatment outcomes. In general, with increased volumes of distribution for biologics in patients with obesity, it has been shown that treatment success is more difficult to achieve in those with a body mass index greater than 30.11 Rather surprisingly, a separate nationwide study in South Korea found that patients on biologics for psoriasis were more likely to experience weight gain, even after controlling for factors such as exercise, smoking, and drinking,12 but it is unclear whether this is driven mostly by a known connection between weight gain and TNF-α inhibitors.13 These contrasting results point to the need for further studies in this area, as our intuitive approach would involve promoting weight loss while starting on a systemic treatment for psoriasis—but perhaps it is important not to assume that one will come with the other in tow, reinforcing the need to discuss a healthy diet with our patients with psoriasis regardless of treatment decisions.
The data that we have do not directly answer the big questions about biologic treatment and cardiovascular health, but we are starting to see interesting signals. For example, in a report of tildrakizumab treatment in patients with and without metabolic syndrome, the rates of major adverse cardiovascular events as well as cardiac disorders were essentially the same in both groups after receiving treatment for up to 244 weeks.14 This is interesting, more because of the lack of an increase in cardiovascular adverse events in the metabolic syndrome group, who entered the trial on average 25 kg to 30 kg heavier than those without metabolic syndrome. There is an increased risk for adverse cardiovascular events among patients with metabolic syndrome, a roughly 2-fold relative risk in as few as 5 to 6 years of follow-up.15 While the cohorts in the tildrakizumab study14 were too small to draw firm conclusions, the data are interesting and a step in the right direction; we need much larger data sets for analysis. Among other agents, similar efficacy and safety have been reported for guselkumab in a long-term psoriasis study; as a class, IL-23 inhibitors also tend to perform well from an efficacy standpoint in patients with obesity.16
Overall, when assessing the evidence for cardiometabolic disorders, it is reasonable to consider starting a biologic from the IL-17 or IL-23 inhibitor classes— thus avoiding both the potential downside of weight gain and contraindication in patients with congestive heart failure associated with TNF-α inhibitors. It is important to counsel patients about weight loss in conjunction with these treatments, both to improve efficacy and reduce cardiovascular risk factors. There may be a preference for IL-23 inhibitors in patients with obesity, as this class of medications maintains efficacy particularly well in these patients. Patients with psoriasis should be counseled to follow up with a primary care physician given their higher risk for metabolic syndrome and adverse cardiovascular outcomes.
Gastrointestinal Conditions
Psoriasis and inflammatory bowel disease (IBD) have a bidirectional association, and patients with psoriasis are about 1.7 times more likely to have either Crohn disease or ulcerative colitis.17,18 This association may be related to a shared pathogenesis with regard to immune dysregulation and overactivated inflammatory pathways, but there are some important differences to consider from a therapeutic standpoint. Given the increased expression of IL-17 in patients with IBD,19 a phase II trial of secukinumab yielded surprising results—not only was secukinumab ineffective in treating Crohn disease, but there also were higher rates of adverse events20 (as noted on the product label for all IL-17 inhibitors). We have come to understand that there are regulatory subsets of IL-17 cells that are important in mucosal homeostasis and also regulate IL-10, which generally is considered an anti-inflammatory cytokine.21 Thus, while IL-17 inhibition can reduce some component of inflammatory signaling, it also can increase inflammatory signaling through indirect pathways while increasing intestinal permeability to microbes. Importantly, this process seems to occur via IL-23–independent pathways; as such, while direct inhibition of IL-17 can be deleterious, IL-23 inhibitors have become important therapeutics for IBD.22
IL-17 family, IL-17A clearly is the culprit for worsening colitis as evidenced by both human and animal models. On the contrary, IL-17F blockade has been shown to ameliorate colitis in a murine model, whereas IL-17A inhibition worsens it.23 Furthermore, dual blockade of IL-17A and IL-17F has a protective effect against colitis, suggesting that the IL-17F inhibition is dominant. This interesting finding has some mechanistic backing, since blockade of IL-17F induces Treg cells that serve to maintain gut epithelium homeostasis and integrity.24
Overall, IL-17A inhibitors should be avoided in patients with a history of IBD—namely, secukinumab and ixekizumab. While there may be theoretical reasons that brodalumab or bimekizumab may confer a somewhat different risk for IBD exacerbation, there may be better choices that would be expected to effectively treat both the psoriasis and IBD manifestations. Given the US Food and Drug Administration approval of IL-23 inhibitors for IBD and their high levels of efficacy in treating psoriasis, these likely will be the best choice for most patients. Another mainstay of IBD treatment is TNF-α inhibitors, but they come with other risks such as increased immunosuppression and increased risk for nonmelanoma skin cancer.
An important question remains: What about patients who do not have known IBD? Do we proactively change our treatment choice due to fear of IBD development given the higher incidence of both Crohn disease and ulcerative colitis in patients with psoriasis? What about patients with a family history of IBD? First-degree relatives of patients with Crohn disease and ulcerative colitis have an 8- and 4-fold higher risk for those same conditions, respectively.25 Postmarketing surveillance and database findings of low rates of IBD development with IL-17 inhibitors gives only modest reassurance, as dermatologists generally know to avoid these medications for patients with even questionable IBD symptoms. It is important to emphasize to our patients that in no case do we believe that a psoriasis medication actually will cause IBD—rather, someone with subclinical IBD could experience a flare and a first manifestation of colitis. The drug is not the culprit in inducing IBD but rather may serve to unmask existing disease.
One study suggested that for patients who move on to the IL-17 inhibitor secukinumab after being treated with TNF-α inhibitors for psoriasis, the rates of IBD development are higher (4.8%) than in those who start IL-17A inhibition without prior treatment (1%)(OR, 8.38; P=.018).26 This begs the question of whether subclinical IBD in many patients with psoriasis who are treated with TNF-α inhibitors can be unmasked later when they are transitioned to a treatment that either does not treat the IBD or could worsen it. There may be a mechanistic drive behind this sequencing of treatments that predisposes patients to colitis, which would suggest selecting an IL-23 inhibitor after failing/trying a TNF-α inhibitor. However, the data are very preliminary, and in real practice, other concerns such as severe psoriatic arthritis may outweigh these considerations, as the IL-17 inhibitor class still is considered to be more effective than IL-23 inhibition at treating psoriatic arthritis overall. For most patients with no personal history of IBD and no strong family history of IBD (ie, first-degree relatives), the choice of biologic should not be affected by concern over gastrointestinal issues.
Psychiatric Conditions
It has been well established that psoriasis is linked to higher rates of depression, anxiety, and suicidality.27 How do we take this into account when treating patients with psoriasis, especially when we have biologics with a warning label for suicidality and a Risk Evaluation and Mitigation Strategies program (brodalumab) and language around suicidal ideation in the label (bimekizumab)? While it is challenging to discuss mental health, it is not a conversation that we as dermatologists should shy away from. Appropriate treatment of psoriasis is an important tool to get our patients on the path to better mental health. A recent database study of more than 4000 patients showed that patients with psoriasis treated with biologics had a 17% lower risk for depression than those treated with conventional disease-modifying drugs such as methotrexate.28 The comparator of the conventional disease-modifying drug class is important as it serves as a control for disease severity. Too often, a higher rate of depression, anxiety, or suicidality can be attributed to a medication when we in fact may just be capturing the background of higher incidence of all 3 in patients with severe psoriasis.
Indeed, even with the medication that many worry about on this front (brodalumab), multiple studies have confirmed that the effect on mental health generally is a positive one, with decreases in depressive symptoms.29 In a cohort switched from TNF-α inhibitors to brodalumab, symptoms of depression actually improved,30 so attributing a direct treatment effect to negative mental health outcomes does not seem to be justified, especially in light of the low number of suicide events in global postmarketing surveillance for brodalumab, comparable to or lower than other biologics for psoriasis.31 Similarly, bimekizumab has language in the label about discussing suicidality with patients, although the rates of suicidal ideation and behavior are no different from other biologics and rates of depression improved with its use.32
Heightened awareness of our patients’ mental health is something that we as providers should embrace, even when it seems that we do not have much time to see each patient. The priority when a patient comes in with mental health symptoms should be to treat what is within our scope (ie, psoriasis) as quickly and effectively as possible— with a newer-generation biologic such as an IL-17 or IL-23 inhibitor—while encouraging the patient to seek care from a mental health professional. In these cases, one might even argue that the rapidity of action of IL-17 inhibitors may be of additional benefit.
Final Thoughts
We as dermatologists generally are tasked with seeing high volumes of patients, and an initial psoriasis consultation can be a lengthy visit; however, it is rewarding to establish this relationship with patients and a reminder of why we practice medicine to begin with. Psoriasis can be satisfying to treat, and we have so many highly effective medicines that can completely transform our patients’ lives. Applying an understanding of the interplay between psoriasis, its related comorbidities, and treatment choices can be a fulfilling exercise that captures the essence of shared decision-making, which can lead to better outcomes and satisfaction for both providers and patients.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022. doi:10.1056/NEJMoa030409
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22:425-442. doi:10.1007/s40257-021-00603-w
- Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5-22. doi:10.1007 /s00281-009-0153-8
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. doi:10.1001/jama.296.14.1735
- Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014-1024. doi:10.1016/j.jaad.2013.06.053
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90. doi:10.1016/j.jaad.2016.07.042
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250. doi:10.1001 /archdermatol.2012.2502
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68. doi:10.1016/j.jaad.2018.02.050
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi:10.3389/fphar.2021.774808
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:E984-E1010. doi:10.1161/CIR.0000000000000973
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41:917-925. doi:10.1007 /s40261-021-01080-z
- Kim H, Hong JY, Cheong S, et al. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: a nationwide population-based cohort study. Obes Res Clin Pract. 2023;17:210-217. doi:10.1016/j.orcp.2023.05.004
- Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290-295. doi:10.1016/j.phrs.2008.02.006
- Fernandez AP, Dauden E, Gerdes S, et al. Tildrakizumab efficacy and safety in patients with psoriasis and concomitant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. J Eur Acad Dermatol Venereol. 2022;36:1774-1783. doi:10.1111/jdv.18167
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. doi:10.1016/j.jacc.2010.05.034
- Ricceri F, Chiricozzi A, Peris K, et al. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: a multicentric retrospective study. Dermatol Ther. 2022;35:E15793. doi:10.1111/dth.15793
- Alinaghi F, Tekin HG, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease— a systematic review and meta-analysis. J Crohns Colitis. 2020;14:351-360. doi:10.1093/ecco-jcc/jjz152
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. doi:10.1136/gut.52.1.65
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693-1700. doi:10.1136 /gutjnl-2011-301668
- Brockmann L, Tran A, Huang Y, et al. Intestinal microbiotaspecific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719-2735 e7. doi:10.1016/j.immuni.2023.11.003
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727-738. doi:10.1016/j.immuni.2015.09.003
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. doi:10.1097 /MIB.0b013e318286fa1c
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T(reg) cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. doi:10.1038/s41590-018-0134-y
- El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27-35. doi:10.1007 /s40291-023-00678-7
- Albayrak F, Gür M, Karatas¸ A, et al. Is the use of secukinumab after anti-TNF therapy greater than expected for the risk of developing inflammatory bowel disease? Reumatol Clin (Engl Ed). 2024;20:123-127. doi:10.1016/j.reumae.2023.11.002
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Strober B, Soliman AM, Truong B, et al. Association between biologic exposure and the risk of depression in patients with psoriasis: a retrospective analysis of large US administrative claims data. Am J Clin Dermatol. 2024;25:853-856. doi:10.1007/s40257 -024-00877-w
- Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361-365.
- Andersch-Bjorkman Y, Micu E, Seifert O, et al. Effects of brodalumab on psoriasis and depressive symptoms in patients with insufficient response to TNF-alpha inhibitors. J Dermatol. 2023;50:1401-1414. doi:10.1111/1346-8138.16917
- Yeroushalmi S, Chung M, Bartholomew E, et al. Examining worldwide postmarketing suicides from biologics used for psoriasis with a focus on brodalumab: a cross-sectional analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS). JAAD Int. 2022;9:119-121. doi:10.1016/j.jdin.2022.08.010
- Blauvelt A, Armstrong A, Merola JF, et al. Mental health outcomes in patients with moderate to severe psoriasis treated with bimekizumab: analysis of phase 2/3 randomized trials. J Am Acad Dermatol. 2024;91:72-81. doi:10.1016/j.jaad.2024.02.039
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022. doi:10.1056/NEJMoa030409
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22:425-442. doi:10.1007/s40257-021-00603-w
- Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5-22. doi:10.1007 /s00281-009-0153-8
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. doi:10.1001/jama.296.14.1735
- Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014-1024. doi:10.1016/j.jaad.2013.06.053
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90. doi:10.1016/j.jaad.2016.07.042
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250. doi:10.1001 /archdermatol.2012.2502
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68. doi:10.1016/j.jaad.2018.02.050
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi:10.3389/fphar.2021.774808
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:E984-E1010. doi:10.1161/CIR.0000000000000973
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41:917-925. doi:10.1007 /s40261-021-01080-z
- Kim H, Hong JY, Cheong S, et al. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: a nationwide population-based cohort study. Obes Res Clin Pract. 2023;17:210-217. doi:10.1016/j.orcp.2023.05.004
- Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290-295. doi:10.1016/j.phrs.2008.02.006
- Fernandez AP, Dauden E, Gerdes S, et al. Tildrakizumab efficacy and safety in patients with psoriasis and concomitant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. J Eur Acad Dermatol Venereol. 2022;36:1774-1783. doi:10.1111/jdv.18167
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. doi:10.1016/j.jacc.2010.05.034
- Ricceri F, Chiricozzi A, Peris K, et al. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: a multicentric retrospective study. Dermatol Ther. 2022;35:E15793. doi:10.1111/dth.15793
- Alinaghi F, Tekin HG, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease— a systematic review and meta-analysis. J Crohns Colitis. 2020;14:351-360. doi:10.1093/ecco-jcc/jjz152
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. doi:10.1136/gut.52.1.65
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693-1700. doi:10.1136 /gutjnl-2011-301668
- Brockmann L, Tran A, Huang Y, et al. Intestinal microbiotaspecific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719-2735 e7. doi:10.1016/j.immuni.2023.11.003
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727-738. doi:10.1016/j.immuni.2015.09.003
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. doi:10.1097 /MIB.0b013e318286fa1c
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T(reg) cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. doi:10.1038/s41590-018-0134-y
- El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27-35. doi:10.1007 /s40291-023-00678-7
- Albayrak F, Gür M, Karatas¸ A, et al. Is the use of secukinumab after anti-TNF therapy greater than expected for the risk of developing inflammatory bowel disease? Reumatol Clin (Engl Ed). 2024;20:123-127. doi:10.1016/j.reumae.2023.11.002
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Strober B, Soliman AM, Truong B, et al. Association between biologic exposure and the risk of depression in patients with psoriasis: a retrospective analysis of large US administrative claims data. Am J Clin Dermatol. 2024;25:853-856. doi:10.1007/s40257 -024-00877-w
- Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361-365.
- Andersch-Bjorkman Y, Micu E, Seifert O, et al. Effects of brodalumab on psoriasis and depressive symptoms in patients with insufficient response to TNF-alpha inhibitors. J Dermatol. 2023;50:1401-1414. doi:10.1111/1346-8138.16917
- Yeroushalmi S, Chung M, Bartholomew E, et al. Examining worldwide postmarketing suicides from biologics used for psoriasis with a focus on brodalumab: a cross-sectional analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS). JAAD Int. 2022;9:119-121. doi:10.1016/j.jdin.2022.08.010
- Blauvelt A, Armstrong A, Merola JF, et al. Mental health outcomes in patients with moderate to severe psoriasis treated with bimekizumab: analysis of phase 2/3 randomized trials. J Am Acad Dermatol. 2024;91:72-81. doi:10.1016/j.jaad.2024.02.039
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
Cryotherapy for Treatment of Idiopathic Gingival Papillokeratosis With Crypt Formation
Cryotherapy for Treatment of Idiopathic Gingival Papillokeratosis With Crypt Formation
To the Editor:
Idiopathic gingival papillokeratosis with crypt formation (IGPC) is an uncommon benign condition that first was reported in 1967.1 The condition manifests as white plaques with a papillary appearance on the gingival tissue. While data on the prevalence of IGPC are limited, it is known to occur more frequently in younger patients (ie, 9-24 years1-3) and has been linked to use of orthodontic appliances.3,4 The lesions typically are asymptomatic with a bilateral appearance along the mucogingival junction. Research on IGPC has not identified the underlying mechanisms that trigger the hyperkeratinization and papillary alterations within the gingival tissue.
Management of IGPC can be challenging due to the rarity of the condition and its uncertain pathogenesis. Wiping or brushing the affected area offers only temporary improvement of symptoms and the appearance of the lesions. Surgical excision is another option; however, it can result in aesthetic and/or functional periodontal defects.2 Alternately, employing methods such as wiping or brushing the affected area offers only transient and temporary results in managing the condition. Additional investigative approaches and clinical studies are needed to identify more effective therapeutic modalities for the management of IGPC, particularly in pediatric patients, in whom aesthetic results may take on a heightened importance.1-3 We report a case of IGPC in which cryotherapy yielded satisfactory results with no recurrence of the lesions.
A 32-year-old woman presented to the dental clinic with white spots on the gingiva of 5 months’ duration. The patient reported a history of smoking cigarettes (3 packs per year) and drinking alcohol in social situations; her medical history was otherwise unremarkable. Clinical examination of the oral cavity revealed a bilateral, irregular, verrucouslike plaque throughout the vestibular upper attached gingiva. An incisional biopsy from the attached gingiva between teeth 13 and 23 was performed. Histopathologic analysis revealed parakeratosis and papillary acanthosis of the gingival mucosa associated with multifocal epithelial invaginations resembling crypts as well as long tapered epithelial ridges with no inflammation in the lamina propria. Based on the histopathologic findings, a diagnosis of IGPC was made (Figure 1).
Given the patient’s clinical presentation, we suggested treatment with cryotherapy as a minimally invasive option that would preserve the gingival architecture and aesthetics while avoiding the potential complications of surgical excision. The patient consented to the procedure, and liquid nitrogen was administered through a handheld device using a 0.6-mm aperture spray tip. During application, the spray tip was positioned at a distance of 0.5 to 1.0 cm from the labial marginal gingiva at about a 45° angle. The freeze/thaw cycle involved a continuous one-way spray application of liquid nitrogen onto the lesion until solid ice formed over the entire area, followed by a waiting period until gradual thawing occurred.
A total of 5 cryotherapy sessions were conducted over an 8-week period; no recurrence of the lesions was observed during a 2-year follow-up period (Figure 2).
We present our case to add to the body of knowledge regarding management options for IGPC, specifically cryotherapy. Historically, brushing with a toothbrush and surgical excision have been the most commonly used interventions.2 Gently brushing the affected areas can help stimulate local blood circulation, which can improve the health of the gingival tissue, promote oxygenation and delivery of nutrients to the cells, and aid in the removal of metabolic waste. Surgical excision is the most commonly used treatment method for IGPC to ensure that the lesions are safely and completely removed; however, this option can result in aesthetic and/or functional periodontal defects. There also is a risk for recurrence, although Noonan et al2 reported no recurrence 4 years after performing a surgical excision for IGPC.
Cryotherapy reduces tissue sensitivity, provides local anesthesia, and reduces inflammation in the oral mucosa. Moreover, cryotherapy accelerates healing by stimulating vasoconstriction and reactive vasodilation, thus enhancing blood flow, oxygenation, and nutrient delivery for faster cell regeneration of the oral mucosa.4,5 Cryotherapy generally is regarded as a simple noninvasive procedure that is relatively safe when performed by qualified professionals.4,5 It can provide benefits such as minimal patient discomfort, rapid recovery, and potential reduction of complications associated with more invasive procedures.5
The efficacy of cryotherapy for IGPC may vary based on lesion severity, individual patient response, and the need for repeated treatment sessions. Robust scientific evidence concerning the long-term efficacy of cryotherapy as a treatment for IGPC is limited due to the rarity of this condition.
The etiopathogenesis of IGPC has been hypothesized to involve both genetic and environmental factors with equal significance. This suggestion is based on reports of IGPC occurring in multiple members of the same family and animal model studies indicating that gingival tissue is sensitive to environmental influences, such as nutritional factors.1,6 However, it is important to emphasize that these hypotheses remain speculative, and the true etiopathogenesis of IGPC remains uncertain.6 Microscopically, biopsy fragments from suspected cases of IGPC reveal gingival mucosa characterized by parakeratosis and papillary acanthosis accompanied by multifocal epithelial invaginations resembling crypts.2 Additionally, elongated and tapered epithelial ridges without inflammation in the lamina propria may be observed (as in our case), favoring the diagnosis of IGPC.3 The absence of inflammation is noteworthy because it suggests that the observed alterations are not attributed to typical inflammatory processes seen in some gingival conditions.
The limited number of studies reporting successful treatment outcomes with long-term follow-up for IGPC cases underscores the need for further exploration of effective treatment options. Cryotherapy emerges as a promising minimally invasive therapeutic approach, with our case offering support for its potential application. Additional research and clinical trials are essential to validate its efficacy and improve our understanding of cryotherapy as a treatment modality for IGPC lesions.
- Bennett JS, Grupe HE. Epithelial adnexal formations in human gingiva. Oral Surg Oral Med Oral Pathol. 1967;23:789-795. doi:10.1016/0030-4220(67)90371-4
- Noonan VL, Woo SB, Sundararajan D, et al. Idiopathic gingival papillokeratosis with crypt formation, a report of 7 cases of a previously undescribed entity: possible unusual oral epithelial nevus? Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:358-364. doi:10.1016/j.oooo.2016.10.018
- Romo SA, de Arruda JAA, Nava FJT, et al. Idiopathic gingival papillokeratosis with crypt formation: a clinicopathological entity in the young population? Int J Dermatol. 2023;62:E291-E293. doi: 10.1111/ijd.16579
- Farah CS, Savage NW. Cryotherapy for treatment of oral lesions. Aust Dent J. 2006;51:2-5. doi:10.1111/j.1834-7819.2006.tb00392.x
- Nogueira VKC, Fernandes D, Navarro CM, et al. Cryotherapy for localized juvenile spongiotic gingival hyperplasia: preliminary findings on two cases. Int J Paediatr Dent. 2017;27:231-235. doi:10.1111/ipd.12278
- Bernick S, Bavetta LA. The development of gingival sebaceous-like glands and cysts in rats of the Holtzman strain. Oral Surg Oral Med Oral Pathol Oral Radiol. 1962;15:351-354. doi:10.1016/0030-4220(62)90116-0
To the Editor:
Idiopathic gingival papillokeratosis with crypt formation (IGPC) is an uncommon benign condition that first was reported in 1967.1 The condition manifests as white plaques with a papillary appearance on the gingival tissue. While data on the prevalence of IGPC are limited, it is known to occur more frequently in younger patients (ie, 9-24 years1-3) and has been linked to use of orthodontic appliances.3,4 The lesions typically are asymptomatic with a bilateral appearance along the mucogingival junction. Research on IGPC has not identified the underlying mechanisms that trigger the hyperkeratinization and papillary alterations within the gingival tissue.
Management of IGPC can be challenging due to the rarity of the condition and its uncertain pathogenesis. Wiping or brushing the affected area offers only temporary improvement of symptoms and the appearance of the lesions. Surgical excision is another option; however, it can result in aesthetic and/or functional periodontal defects.2 Alternately, employing methods such as wiping or brushing the affected area offers only transient and temporary results in managing the condition. Additional investigative approaches and clinical studies are needed to identify more effective therapeutic modalities for the management of IGPC, particularly in pediatric patients, in whom aesthetic results may take on a heightened importance.1-3 We report a case of IGPC in which cryotherapy yielded satisfactory results with no recurrence of the lesions.
A 32-year-old woman presented to the dental clinic with white spots on the gingiva of 5 months’ duration. The patient reported a history of smoking cigarettes (3 packs per year) and drinking alcohol in social situations; her medical history was otherwise unremarkable. Clinical examination of the oral cavity revealed a bilateral, irregular, verrucouslike plaque throughout the vestibular upper attached gingiva. An incisional biopsy from the attached gingiva between teeth 13 and 23 was performed. Histopathologic analysis revealed parakeratosis and papillary acanthosis of the gingival mucosa associated with multifocal epithelial invaginations resembling crypts as well as long tapered epithelial ridges with no inflammation in the lamina propria. Based on the histopathologic findings, a diagnosis of IGPC was made (Figure 1).

Given the patient’s clinical presentation, we suggested treatment with cryotherapy as a minimally invasive option that would preserve the gingival architecture and aesthetics while avoiding the potential complications of surgical excision. The patient consented to the procedure, and liquid nitrogen was administered through a handheld device using a 0.6-mm aperture spray tip. During application, the spray tip was positioned at a distance of 0.5 to 1.0 cm from the labial marginal gingiva at about a 45° angle. The freeze/thaw cycle involved a continuous one-way spray application of liquid nitrogen onto the lesion until solid ice formed over the entire area, followed by a waiting period until gradual thawing occurred.
A total of 5 cryotherapy sessions were conducted over an 8-week period; no recurrence of the lesions was observed during a 2-year follow-up period (Figure 2).

We present our case to add to the body of knowledge regarding management options for IGPC, specifically cryotherapy. Historically, brushing with a toothbrush and surgical excision have been the most commonly used interventions.2 Gently brushing the affected areas can help stimulate local blood circulation, which can improve the health of the gingival tissue, promote oxygenation and delivery of nutrients to the cells, and aid in the removal of metabolic waste. Surgical excision is the most commonly used treatment method for IGPC to ensure that the lesions are safely and completely removed; however, this option can result in aesthetic and/or functional periodontal defects. There also is a risk for recurrence, although Noonan et al2 reported no recurrence 4 years after performing a surgical excision for IGPC.
Cryotherapy reduces tissue sensitivity, provides local anesthesia, and reduces inflammation in the oral mucosa. Moreover, cryotherapy accelerates healing by stimulating vasoconstriction and reactive vasodilation, thus enhancing blood flow, oxygenation, and nutrient delivery for faster cell regeneration of the oral mucosa.4,5 Cryotherapy generally is regarded as a simple noninvasive procedure that is relatively safe when performed by qualified professionals.4,5 It can provide benefits such as minimal patient discomfort, rapid recovery, and potential reduction of complications associated with more invasive procedures.5
The efficacy of cryotherapy for IGPC may vary based on lesion severity, individual patient response, and the need for repeated treatment sessions. Robust scientific evidence concerning the long-term efficacy of cryotherapy as a treatment for IGPC is limited due to the rarity of this condition.
The etiopathogenesis of IGPC has been hypothesized to involve both genetic and environmental factors with equal significance. This suggestion is based on reports of IGPC occurring in multiple members of the same family and animal model studies indicating that gingival tissue is sensitive to environmental influences, such as nutritional factors.1,6 However, it is important to emphasize that these hypotheses remain speculative, and the true etiopathogenesis of IGPC remains uncertain.6 Microscopically, biopsy fragments from suspected cases of IGPC reveal gingival mucosa characterized by parakeratosis and papillary acanthosis accompanied by multifocal epithelial invaginations resembling crypts.2 Additionally, elongated and tapered epithelial ridges without inflammation in the lamina propria may be observed (as in our case), favoring the diagnosis of IGPC.3 The absence of inflammation is noteworthy because it suggests that the observed alterations are not attributed to typical inflammatory processes seen in some gingival conditions.
The limited number of studies reporting successful treatment outcomes with long-term follow-up for IGPC cases underscores the need for further exploration of effective treatment options. Cryotherapy emerges as a promising minimally invasive therapeutic approach, with our case offering support for its potential application. Additional research and clinical trials are essential to validate its efficacy and improve our understanding of cryotherapy as a treatment modality for IGPC lesions.
To the Editor:
Idiopathic gingival papillokeratosis with crypt formation (IGPC) is an uncommon benign condition that first was reported in 1967.1 The condition manifests as white plaques with a papillary appearance on the gingival tissue. While data on the prevalence of IGPC are limited, it is known to occur more frequently in younger patients (ie, 9-24 years1-3) and has been linked to use of orthodontic appliances.3,4 The lesions typically are asymptomatic with a bilateral appearance along the mucogingival junction. Research on IGPC has not identified the underlying mechanisms that trigger the hyperkeratinization and papillary alterations within the gingival tissue.
Management of IGPC can be challenging due to the rarity of the condition and its uncertain pathogenesis. Wiping or brushing the affected area offers only temporary improvement of symptoms and the appearance of the lesions. Surgical excision is another option; however, it can result in aesthetic and/or functional periodontal defects.2 Alternately, employing methods such as wiping or brushing the affected area offers only transient and temporary results in managing the condition. Additional investigative approaches and clinical studies are needed to identify more effective therapeutic modalities for the management of IGPC, particularly in pediatric patients, in whom aesthetic results may take on a heightened importance.1-3 We report a case of IGPC in which cryotherapy yielded satisfactory results with no recurrence of the lesions.
A 32-year-old woman presented to the dental clinic with white spots on the gingiva of 5 months’ duration. The patient reported a history of smoking cigarettes (3 packs per year) and drinking alcohol in social situations; her medical history was otherwise unremarkable. Clinical examination of the oral cavity revealed a bilateral, irregular, verrucouslike plaque throughout the vestibular upper attached gingiva. An incisional biopsy from the attached gingiva between teeth 13 and 23 was performed. Histopathologic analysis revealed parakeratosis and papillary acanthosis of the gingival mucosa associated with multifocal epithelial invaginations resembling crypts as well as long tapered epithelial ridges with no inflammation in the lamina propria. Based on the histopathologic findings, a diagnosis of IGPC was made (Figure 1).

Given the patient’s clinical presentation, we suggested treatment with cryotherapy as a minimally invasive option that would preserve the gingival architecture and aesthetics while avoiding the potential complications of surgical excision. The patient consented to the procedure, and liquid nitrogen was administered through a handheld device using a 0.6-mm aperture spray tip. During application, the spray tip was positioned at a distance of 0.5 to 1.0 cm from the labial marginal gingiva at about a 45° angle. The freeze/thaw cycle involved a continuous one-way spray application of liquid nitrogen onto the lesion until solid ice formed over the entire area, followed by a waiting period until gradual thawing occurred.
A total of 5 cryotherapy sessions were conducted over an 8-week period; no recurrence of the lesions was observed during a 2-year follow-up period (Figure 2).

We present our case to add to the body of knowledge regarding management options for IGPC, specifically cryotherapy. Historically, brushing with a toothbrush and surgical excision have been the most commonly used interventions.2 Gently brushing the affected areas can help stimulate local blood circulation, which can improve the health of the gingival tissue, promote oxygenation and delivery of nutrients to the cells, and aid in the removal of metabolic waste. Surgical excision is the most commonly used treatment method for IGPC to ensure that the lesions are safely and completely removed; however, this option can result in aesthetic and/or functional periodontal defects. There also is a risk for recurrence, although Noonan et al2 reported no recurrence 4 years after performing a surgical excision for IGPC.
Cryotherapy reduces tissue sensitivity, provides local anesthesia, and reduces inflammation in the oral mucosa. Moreover, cryotherapy accelerates healing by stimulating vasoconstriction and reactive vasodilation, thus enhancing blood flow, oxygenation, and nutrient delivery for faster cell regeneration of the oral mucosa.4,5 Cryotherapy generally is regarded as a simple noninvasive procedure that is relatively safe when performed by qualified professionals.4,5 It can provide benefits such as minimal patient discomfort, rapid recovery, and potential reduction of complications associated with more invasive procedures.5
The efficacy of cryotherapy for IGPC may vary based on lesion severity, individual patient response, and the need for repeated treatment sessions. Robust scientific evidence concerning the long-term efficacy of cryotherapy as a treatment for IGPC is limited due to the rarity of this condition.
The etiopathogenesis of IGPC has been hypothesized to involve both genetic and environmental factors with equal significance. This suggestion is based on reports of IGPC occurring in multiple members of the same family and animal model studies indicating that gingival tissue is sensitive to environmental influences, such as nutritional factors.1,6 However, it is important to emphasize that these hypotheses remain speculative, and the true etiopathogenesis of IGPC remains uncertain.6 Microscopically, biopsy fragments from suspected cases of IGPC reveal gingival mucosa characterized by parakeratosis and papillary acanthosis accompanied by multifocal epithelial invaginations resembling crypts.2 Additionally, elongated and tapered epithelial ridges without inflammation in the lamina propria may be observed (as in our case), favoring the diagnosis of IGPC.3 The absence of inflammation is noteworthy because it suggests that the observed alterations are not attributed to typical inflammatory processes seen in some gingival conditions.
The limited number of studies reporting successful treatment outcomes with long-term follow-up for IGPC cases underscores the need for further exploration of effective treatment options. Cryotherapy emerges as a promising minimally invasive therapeutic approach, with our case offering support for its potential application. Additional research and clinical trials are essential to validate its efficacy and improve our understanding of cryotherapy as a treatment modality for IGPC lesions.
- Bennett JS, Grupe HE. Epithelial adnexal formations in human gingiva. Oral Surg Oral Med Oral Pathol. 1967;23:789-795. doi:10.1016/0030-4220(67)90371-4
- Noonan VL, Woo SB, Sundararajan D, et al. Idiopathic gingival papillokeratosis with crypt formation, a report of 7 cases of a previously undescribed entity: possible unusual oral epithelial nevus? Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:358-364. doi:10.1016/j.oooo.2016.10.018
- Romo SA, de Arruda JAA, Nava FJT, et al. Idiopathic gingival papillokeratosis with crypt formation: a clinicopathological entity in the young population? Int J Dermatol. 2023;62:E291-E293. doi: 10.1111/ijd.16579
- Farah CS, Savage NW. Cryotherapy for treatment of oral lesions. Aust Dent J. 2006;51:2-5. doi:10.1111/j.1834-7819.2006.tb00392.x
- Nogueira VKC, Fernandes D, Navarro CM, et al. Cryotherapy for localized juvenile spongiotic gingival hyperplasia: preliminary findings on two cases. Int J Paediatr Dent. 2017;27:231-235. doi:10.1111/ipd.12278
- Bernick S, Bavetta LA. The development of gingival sebaceous-like glands and cysts in rats of the Holtzman strain. Oral Surg Oral Med Oral Pathol Oral Radiol. 1962;15:351-354. doi:10.1016/0030-4220(62)90116-0
- Bennett JS, Grupe HE. Epithelial adnexal formations in human gingiva. Oral Surg Oral Med Oral Pathol. 1967;23:789-795. doi:10.1016/0030-4220(67)90371-4
- Noonan VL, Woo SB, Sundararajan D, et al. Idiopathic gingival papillokeratosis with crypt formation, a report of 7 cases of a previously undescribed entity: possible unusual oral epithelial nevus? Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:358-364. doi:10.1016/j.oooo.2016.10.018
- Romo SA, de Arruda JAA, Nava FJT, et al. Idiopathic gingival papillokeratosis with crypt formation: a clinicopathological entity in the young population? Int J Dermatol. 2023;62:E291-E293. doi: 10.1111/ijd.16579
- Farah CS, Savage NW. Cryotherapy for treatment of oral lesions. Aust Dent J. 2006;51:2-5. doi:10.1111/j.1834-7819.2006.tb00392.x
- Nogueira VKC, Fernandes D, Navarro CM, et al. Cryotherapy for localized juvenile spongiotic gingival hyperplasia: preliminary findings on two cases. Int J Paediatr Dent. 2017;27:231-235. doi:10.1111/ipd.12278
- Bernick S, Bavetta LA. The development of gingival sebaceous-like glands and cysts in rats of the Holtzman strain. Oral Surg Oral Med Oral Pathol Oral Radiol. 1962;15:351-354. doi:10.1016/0030-4220(62)90116-0
Cryotherapy for Treatment of Idiopathic Gingival Papillokeratosis With Crypt Formation
Cryotherapy for Treatment of Idiopathic Gingival Papillokeratosis With Crypt Formation
PRACTICE POINTS
- Surgical excision is an effective treatment for idiopathic gingival papillokeratosis with crypt formation (IGPC) but may result in periodontal defects that impact the aesthetic outcome.
- Cryotherapy is a novel therapeutic intervention for IGPC.
Exophytic Scaly Nodule on the Wrist
Exophytic Scaly Nodule on the Wrist
THE DIAGNOSIS: Atypical Spitz Tumor
The shave biopsy revealed extensive dermal proliferation with spitzoid cytomorphology containing large, spindled nuclei; prominent nucleoli; and abundant homogenous cytoplasm arranged in haphazard fascicles. The proliferation was associated with prominent pseudoepitheliomatous hyperplasia of the overlying epidermis, and anaplastic lymphoma kinase immunohistochemistry showed diffuse strong positivity. Fluorescence in situ hybridization confirmed fusion of the tropomyosin 3 (TPM3) and anaplastic lymphoma kinase (ALK) genes, which finalized the diagnosis of an ALK-mutated atypical spitz tumor. Due to the location and size of the lesion, Mohs micrographic surgery was performed to excise the tumor and clear the margins.
Spitz nevi are uncommon benign melanocytic neoplasms that typically occur in pediatric populations.1 Atypical spitz nevi comprised fewer than 17% of all childhood melanocytic nevi in the United States and can be considered in the broader category of spitzoid tumors. Spitz nevi are divided into 3 classes: Spitz nevus, atypical Spitz nevus, and spitzoid melanoma. Atypical Spitz nevi have typical Spitz nevus and spitzoid melanoma features and often can be difficult to distinguish on dermoscopy. Malignant Spitz tumors typically occur in the fifth decade of life, though the age distribution can vary widely.1
Black patients are less likely to be diagnosed with Spitz nevi, potentially due to a lower prevalence in this population, thus limiting the clinician’s clinical exposure and leading to increased rates of misdiagnoses.2 Spitz nevi usually manifest as well-circumscribed, dome-shaped papules and frequently are described as pink to red due to increased vascularity and limited melanin content1; however, these lesions may appear more violaceous, dusky, or dark brown in darker skin types. Additionally, approximately 71% of patients in a clinical review of Spitz nevi had a pigmented lesion, ranging from light brown to black.3 It is important for dermatologists to understand that the contrast in color between the nevus and the surrounding skin may not be as striking, prominent, or clinically concerning, particularly in darker skin types, such as in our patient.
Spitz nevi frequently manifest as rapidly growing solitary lesions most frequently developing in the lower legs (shown in 41% of lesions in one report).4 However, a recent retrospective review indicated that Spitz nevi in Black patients most commonly were found on the upper extremities, as was seen in our patient.2 Compared to typical and common Spitz nevi, atypical Spitz nevi often are greater than 10 mm in diameter and have features of ulceration.
Diagnosing atypical spitzoid melanocytic lesions requires adequate clinical suspicion and confirmation via biopsy. Under dermoscopy, typical Spitz nevi often display a starburst or globular pattern with pinpoint vessels, though it can have variable manifestations of both patterns. Atypical Spitz nevi can be challenging to distinguish from melanoma on dermoscopy since both conditions can have atypical pigment networks or structureless homogenous areas.1 Consequently, there often is a lower threshold for biopsy and possible follow-up excision for atypical Spitz nevi. Histopathology of atypical Spitz nevi includes epithelioid and spindle melanocytes but can share features of melanomas, including areas of prominent pagetoid spread, asymmetry, and poor circumscription.5 Furthermore, atypical Spitz nevi with ALK gene fusion, as seen in our patient, have been shown in the literature to demonstrate distinct histopathologic features, such as wedge-shaped extension into the dermis or a bulbous lower border that can resemble pseudoepitheliomatous hyperplasia.6
The differential diagnosis for this rapidly growing scaly nodule also should include pyogenic granuloma, bacillary angiomatosis, Kaposi sarcoma, and amelanotic melanoma. Pyogenic granuloma is a rapidly growing, benign, vascular tumor that often becomes ulcerated and can occur in any age group.7 Pyogenic granuloma frequently appears at sites of trauma as a solitary, bright pink to red, friable, pedunculated papule and often manifests on the arms, hands, and face, similar to atypical Spitz nevi, though they can appear anywhere on the body. Histology shows a lobular capillary network with a central feeder vessel.7
Bacillary angiomatosis is an uncommon cutaneous infection associated with vascular proliferation and neovascularization due to the gram-negative organism Bartonella henselae.8 Bacillary nodules typically are reddish to purple and appear on the arms, sometimes with central ulceration and bleeding. Patients may present with multiple papules and nodules of varying sizes, as the lesions can arise in crops and follow a sporotrichoid pattern. Most patients with bacillary angiomatosis are immunosuppressed, though it rarely can affect immunocompetent patients. Histologically, bacillary angiomatosis is similar to pyogenic granuloma, though Gram or Warthin-Starry stains can help differentiate B henselae.8
Kaposi sarcoma is a malignant vascular neoplasm that often manifests in immunocompromised patients as violaceous, purple, or red patches, plaques, and nodules on the skin or oral mucosa. Histopathology shows spindle cell proliferation of irregular complex vascular channels dissecting through the dermis. Human herpesvirus 8 immunohistochemistry can be used to confirm diagnosis on histopathology.9 In contrast, amelanotic melanoma consists of lack of pigmentation, asymmetry with polymorphous vascular pattern, and high mitotic rate and is commonly found in sun-exposed areas. Dermoscopic features include irregular globules with blue-whitish veil.10
Treatment of atypical Spitz nevi depends mainly on the age of the patient and the histologic features of the nevus. Adults with atypical Spitz nevi frequently require excision, while the preferred choice for treatment in children with common Spitz nevi is regular clinical monitoring when there are no concerning clinical, dermoscopic, or histologic features.8 Compared to common Spitz nevi, atypical Spitz nevi have more melanoma-like features, resulting in a stronger recommendation for excision. Excision allows for a more thorough histologic evaluation and minimizes the likelihood of a recurrent atypical lesion.11 In all cases, close clinical follow-up is recommended to monitor for reoccurrence.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084. doi:10.1016/j.jaad.2011.04.040
- Farid YI, Honda KS. Spitz nevi in African Americans: a retrospective chart review of 11 patients. J Cutan Pathol. 2021;48:511-518. doi:10.1111 /cup.13903
- Dal Pozzo V, Benelli C, Restano L, et al. Clinical review of 247 case records of Spitz nevus (epithelioid cell and/or spindle cell nevus). Dermatology 1997;194:20-25. doi: 10.1159/000246051
- Berlingeri-Ramos AC, Morales-Burgos A, Sanchez JL, et al. Spitz nevus in a Hispanic population: a clinicopathological study of 130 cases. Am J Dermatopathol 2010;32:267-275. doi: 10.1097 /DAD.0b013e3181c52b99
- Brown A, Sawyer JD, Neumeister MW. Spitz nevus: review and update. Clin Plast Surg 2021;48:677-686. doi: 10.1016/j.cps.2021.06.002 [published Online First: 20210818]
- Yeh I, de la Fouchardiere A, Pissaloux D, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol 2015;39:581-91. doi: 10.1097/PAS.0000000000000387
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556077/
- Akram SM, Anwar MY, Thandra KC, et al. Bacillary angiomatosis. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 4, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448092/
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Pizzichetta MA, Talamini R, Stanganelli I, et al. Amelanotic/ hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol 2004;150(6):1117-1124. doi: 10.1111/j.1365-2133.2004.05928.x
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part II. natural history and management. J Am Acad Dermatol 2011;65:1087-1092. doi:10.1016/j.jaad.2011.06.045
THE DIAGNOSIS: Atypical Spitz Tumor
The shave biopsy revealed extensive dermal proliferation with spitzoid cytomorphology containing large, spindled nuclei; prominent nucleoli; and abundant homogenous cytoplasm arranged in haphazard fascicles. The proliferation was associated with prominent pseudoepitheliomatous hyperplasia of the overlying epidermis, and anaplastic lymphoma kinase immunohistochemistry showed diffuse strong positivity. Fluorescence in situ hybridization confirmed fusion of the tropomyosin 3 (TPM3) and anaplastic lymphoma kinase (ALK) genes, which finalized the diagnosis of an ALK-mutated atypical spitz tumor. Due to the location and size of the lesion, Mohs micrographic surgery was performed to excise the tumor and clear the margins.
Spitz nevi are uncommon benign melanocytic neoplasms that typically occur in pediatric populations.1 Atypical spitz nevi comprised fewer than 17% of all childhood melanocytic nevi in the United States and can be considered in the broader category of spitzoid tumors. Spitz nevi are divided into 3 classes: Spitz nevus, atypical Spitz nevus, and spitzoid melanoma. Atypical Spitz nevi have typical Spitz nevus and spitzoid melanoma features and often can be difficult to distinguish on dermoscopy. Malignant Spitz tumors typically occur in the fifth decade of life, though the age distribution can vary widely.1
Black patients are less likely to be diagnosed with Spitz nevi, potentially due to a lower prevalence in this population, thus limiting the clinician’s clinical exposure and leading to increased rates of misdiagnoses.2 Spitz nevi usually manifest as well-circumscribed, dome-shaped papules and frequently are described as pink to red due to increased vascularity and limited melanin content1; however, these lesions may appear more violaceous, dusky, or dark brown in darker skin types. Additionally, approximately 71% of patients in a clinical review of Spitz nevi had a pigmented lesion, ranging from light brown to black.3 It is important for dermatologists to understand that the contrast in color between the nevus and the surrounding skin may not be as striking, prominent, or clinically concerning, particularly in darker skin types, such as in our patient.
Spitz nevi frequently manifest as rapidly growing solitary lesions most frequently developing in the lower legs (shown in 41% of lesions in one report).4 However, a recent retrospective review indicated that Spitz nevi in Black patients most commonly were found on the upper extremities, as was seen in our patient.2 Compared to typical and common Spitz nevi, atypical Spitz nevi often are greater than 10 mm in diameter and have features of ulceration.
Diagnosing atypical spitzoid melanocytic lesions requires adequate clinical suspicion and confirmation via biopsy. Under dermoscopy, typical Spitz nevi often display a starburst or globular pattern with pinpoint vessels, though it can have variable manifestations of both patterns. Atypical Spitz nevi can be challenging to distinguish from melanoma on dermoscopy since both conditions can have atypical pigment networks or structureless homogenous areas.1 Consequently, there often is a lower threshold for biopsy and possible follow-up excision for atypical Spitz nevi. Histopathology of atypical Spitz nevi includes epithelioid and spindle melanocytes but can share features of melanomas, including areas of prominent pagetoid spread, asymmetry, and poor circumscription.5 Furthermore, atypical Spitz nevi with ALK gene fusion, as seen in our patient, have been shown in the literature to demonstrate distinct histopathologic features, such as wedge-shaped extension into the dermis or a bulbous lower border that can resemble pseudoepitheliomatous hyperplasia.6
The differential diagnosis for this rapidly growing scaly nodule also should include pyogenic granuloma, bacillary angiomatosis, Kaposi sarcoma, and amelanotic melanoma. Pyogenic granuloma is a rapidly growing, benign, vascular tumor that often becomes ulcerated and can occur in any age group.7 Pyogenic granuloma frequently appears at sites of trauma as a solitary, bright pink to red, friable, pedunculated papule and often manifests on the arms, hands, and face, similar to atypical Spitz nevi, though they can appear anywhere on the body. Histology shows a lobular capillary network with a central feeder vessel.7
Bacillary angiomatosis is an uncommon cutaneous infection associated with vascular proliferation and neovascularization due to the gram-negative organism Bartonella henselae.8 Bacillary nodules typically are reddish to purple and appear on the arms, sometimes with central ulceration and bleeding. Patients may present with multiple papules and nodules of varying sizes, as the lesions can arise in crops and follow a sporotrichoid pattern. Most patients with bacillary angiomatosis are immunosuppressed, though it rarely can affect immunocompetent patients. Histologically, bacillary angiomatosis is similar to pyogenic granuloma, though Gram or Warthin-Starry stains can help differentiate B henselae.8
Kaposi sarcoma is a malignant vascular neoplasm that often manifests in immunocompromised patients as violaceous, purple, or red patches, plaques, and nodules on the skin or oral mucosa. Histopathology shows spindle cell proliferation of irregular complex vascular channels dissecting through the dermis. Human herpesvirus 8 immunohistochemistry can be used to confirm diagnosis on histopathology.9 In contrast, amelanotic melanoma consists of lack of pigmentation, asymmetry with polymorphous vascular pattern, and high mitotic rate and is commonly found in sun-exposed areas. Dermoscopic features include irregular globules with blue-whitish veil.10
Treatment of atypical Spitz nevi depends mainly on the age of the patient and the histologic features of the nevus. Adults with atypical Spitz nevi frequently require excision, while the preferred choice for treatment in children with common Spitz nevi is regular clinical monitoring when there are no concerning clinical, dermoscopic, or histologic features.8 Compared to common Spitz nevi, atypical Spitz nevi have more melanoma-like features, resulting in a stronger recommendation for excision. Excision allows for a more thorough histologic evaluation and minimizes the likelihood of a recurrent atypical lesion.11 In all cases, close clinical follow-up is recommended to monitor for reoccurrence.
THE DIAGNOSIS: Atypical Spitz Tumor
The shave biopsy revealed extensive dermal proliferation with spitzoid cytomorphology containing large, spindled nuclei; prominent nucleoli; and abundant homogenous cytoplasm arranged in haphazard fascicles. The proliferation was associated with prominent pseudoepitheliomatous hyperplasia of the overlying epidermis, and anaplastic lymphoma kinase immunohistochemistry showed diffuse strong positivity. Fluorescence in situ hybridization confirmed fusion of the tropomyosin 3 (TPM3) and anaplastic lymphoma kinase (ALK) genes, which finalized the diagnosis of an ALK-mutated atypical spitz tumor. Due to the location and size of the lesion, Mohs micrographic surgery was performed to excise the tumor and clear the margins.
Spitz nevi are uncommon benign melanocytic neoplasms that typically occur in pediatric populations.1 Atypical spitz nevi comprised fewer than 17% of all childhood melanocytic nevi in the United States and can be considered in the broader category of spitzoid tumors. Spitz nevi are divided into 3 classes: Spitz nevus, atypical Spitz nevus, and spitzoid melanoma. Atypical Spitz nevi have typical Spitz nevus and spitzoid melanoma features and often can be difficult to distinguish on dermoscopy. Malignant Spitz tumors typically occur in the fifth decade of life, though the age distribution can vary widely.1
Black patients are less likely to be diagnosed with Spitz nevi, potentially due to a lower prevalence in this population, thus limiting the clinician’s clinical exposure and leading to increased rates of misdiagnoses.2 Spitz nevi usually manifest as well-circumscribed, dome-shaped papules and frequently are described as pink to red due to increased vascularity and limited melanin content1; however, these lesions may appear more violaceous, dusky, or dark brown in darker skin types. Additionally, approximately 71% of patients in a clinical review of Spitz nevi had a pigmented lesion, ranging from light brown to black.3 It is important for dermatologists to understand that the contrast in color between the nevus and the surrounding skin may not be as striking, prominent, or clinically concerning, particularly in darker skin types, such as in our patient.
Spitz nevi frequently manifest as rapidly growing solitary lesions most frequently developing in the lower legs (shown in 41% of lesions in one report).4 However, a recent retrospective review indicated that Spitz nevi in Black patients most commonly were found on the upper extremities, as was seen in our patient.2 Compared to typical and common Spitz nevi, atypical Spitz nevi often are greater than 10 mm in diameter and have features of ulceration.
Diagnosing atypical spitzoid melanocytic lesions requires adequate clinical suspicion and confirmation via biopsy. Under dermoscopy, typical Spitz nevi often display a starburst or globular pattern with pinpoint vessels, though it can have variable manifestations of both patterns. Atypical Spitz nevi can be challenging to distinguish from melanoma on dermoscopy since both conditions can have atypical pigment networks or structureless homogenous areas.1 Consequently, there often is a lower threshold for biopsy and possible follow-up excision for atypical Spitz nevi. Histopathology of atypical Spitz nevi includes epithelioid and spindle melanocytes but can share features of melanomas, including areas of prominent pagetoid spread, asymmetry, and poor circumscription.5 Furthermore, atypical Spitz nevi with ALK gene fusion, as seen in our patient, have been shown in the literature to demonstrate distinct histopathologic features, such as wedge-shaped extension into the dermis or a bulbous lower border that can resemble pseudoepitheliomatous hyperplasia.6
The differential diagnosis for this rapidly growing scaly nodule also should include pyogenic granuloma, bacillary angiomatosis, Kaposi sarcoma, and amelanotic melanoma. Pyogenic granuloma is a rapidly growing, benign, vascular tumor that often becomes ulcerated and can occur in any age group.7 Pyogenic granuloma frequently appears at sites of trauma as a solitary, bright pink to red, friable, pedunculated papule and often manifests on the arms, hands, and face, similar to atypical Spitz nevi, though they can appear anywhere on the body. Histology shows a lobular capillary network with a central feeder vessel.7
Bacillary angiomatosis is an uncommon cutaneous infection associated with vascular proliferation and neovascularization due to the gram-negative organism Bartonella henselae.8 Bacillary nodules typically are reddish to purple and appear on the arms, sometimes with central ulceration and bleeding. Patients may present with multiple papules and nodules of varying sizes, as the lesions can arise in crops and follow a sporotrichoid pattern. Most patients with bacillary angiomatosis are immunosuppressed, though it rarely can affect immunocompetent patients. Histologically, bacillary angiomatosis is similar to pyogenic granuloma, though Gram or Warthin-Starry stains can help differentiate B henselae.8
Kaposi sarcoma is a malignant vascular neoplasm that often manifests in immunocompromised patients as violaceous, purple, or red patches, plaques, and nodules on the skin or oral mucosa. Histopathology shows spindle cell proliferation of irregular complex vascular channels dissecting through the dermis. Human herpesvirus 8 immunohistochemistry can be used to confirm diagnosis on histopathology.9 In contrast, amelanotic melanoma consists of lack of pigmentation, asymmetry with polymorphous vascular pattern, and high mitotic rate and is commonly found in sun-exposed areas. Dermoscopic features include irregular globules with blue-whitish veil.10
Treatment of atypical Spitz nevi depends mainly on the age of the patient and the histologic features of the nevus. Adults with atypical Spitz nevi frequently require excision, while the preferred choice for treatment in children with common Spitz nevi is regular clinical monitoring when there are no concerning clinical, dermoscopic, or histologic features.8 Compared to common Spitz nevi, atypical Spitz nevi have more melanoma-like features, resulting in a stronger recommendation for excision. Excision allows for a more thorough histologic evaluation and minimizes the likelihood of a recurrent atypical lesion.11 In all cases, close clinical follow-up is recommended to monitor for reoccurrence.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084. doi:10.1016/j.jaad.2011.04.040
- Farid YI, Honda KS. Spitz nevi in African Americans: a retrospective chart review of 11 patients. J Cutan Pathol. 2021;48:511-518. doi:10.1111 /cup.13903
- Dal Pozzo V, Benelli C, Restano L, et al. Clinical review of 247 case records of Spitz nevus (epithelioid cell and/or spindle cell nevus). Dermatology 1997;194:20-25. doi: 10.1159/000246051
- Berlingeri-Ramos AC, Morales-Burgos A, Sanchez JL, et al. Spitz nevus in a Hispanic population: a clinicopathological study of 130 cases. Am J Dermatopathol 2010;32:267-275. doi: 10.1097 /DAD.0b013e3181c52b99
- Brown A, Sawyer JD, Neumeister MW. Spitz nevus: review and update. Clin Plast Surg 2021;48:677-686. doi: 10.1016/j.cps.2021.06.002 [published Online First: 20210818]
- Yeh I, de la Fouchardiere A, Pissaloux D, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol 2015;39:581-91. doi: 10.1097/PAS.0000000000000387
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556077/
- Akram SM, Anwar MY, Thandra KC, et al. Bacillary angiomatosis. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 4, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448092/
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Pizzichetta MA, Talamini R, Stanganelli I, et al. Amelanotic/ hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol 2004;150(6):1117-1124. doi: 10.1111/j.1365-2133.2004.05928.x
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part II. natural history and management. J Am Acad Dermatol 2011;65:1087-1092. doi:10.1016/j.jaad.2011.06.045
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084. doi:10.1016/j.jaad.2011.04.040
- Farid YI, Honda KS. Spitz nevi in African Americans: a retrospective chart review of 11 patients. J Cutan Pathol. 2021;48:511-518. doi:10.1111 /cup.13903
- Dal Pozzo V, Benelli C, Restano L, et al. Clinical review of 247 case records of Spitz nevus (epithelioid cell and/or spindle cell nevus). Dermatology 1997;194:20-25. doi: 10.1159/000246051
- Berlingeri-Ramos AC, Morales-Burgos A, Sanchez JL, et al. Spitz nevus in a Hispanic population: a clinicopathological study of 130 cases. Am J Dermatopathol 2010;32:267-275. doi: 10.1097 /DAD.0b013e3181c52b99
- Brown A, Sawyer JD, Neumeister MW. Spitz nevus: review and update. Clin Plast Surg 2021;48:677-686. doi: 10.1016/j.cps.2021.06.002 [published Online First: 20210818]
- Yeh I, de la Fouchardiere A, Pissaloux D, et al. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol 2015;39:581-91. doi: 10.1097/PAS.0000000000000387
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556077/
- Akram SM, Anwar MY, Thandra KC, et al. Bacillary angiomatosis. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 4, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448092/
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed December 4, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Pizzichetta MA, Talamini R, Stanganelli I, et al. Amelanotic/ hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol 2004;150(6):1117-1124. doi: 10.1111/j.1365-2133.2004.05928.x
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part II. natural history and management. J Am Acad Dermatol 2011;65:1087-1092. doi:10.1016/j.jaad.2011.06.045
Exophytic Scaly Nodule on the Wrist
Exophytic Scaly Nodule on the Wrist
A 30-year-old Black man presented to the dermatology clinic with a rapidly growing, exophytic, scaly nodule on the right volar wrist of 2 months’ duration. The patient’s medical history was otherwise unremarkable. Physical examination revealed an irregularly bordered, red to violaceous, scaly, eroded, exophytic nodule on the wrist that was 2 cm in diameter with a surrounding adherent white-yellow crust. The patient had presumed the nodule was a wart and had been self-treating with over-the-counter salicylic acid and cryotherapy with no relief. He denied any bleeding or pruritus. The rest of the skin examination was unremarkable. A shave biopsy was performed for further evaluation.