User login
Oral Biologics: The New Wave for Treating Psoriasis
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.
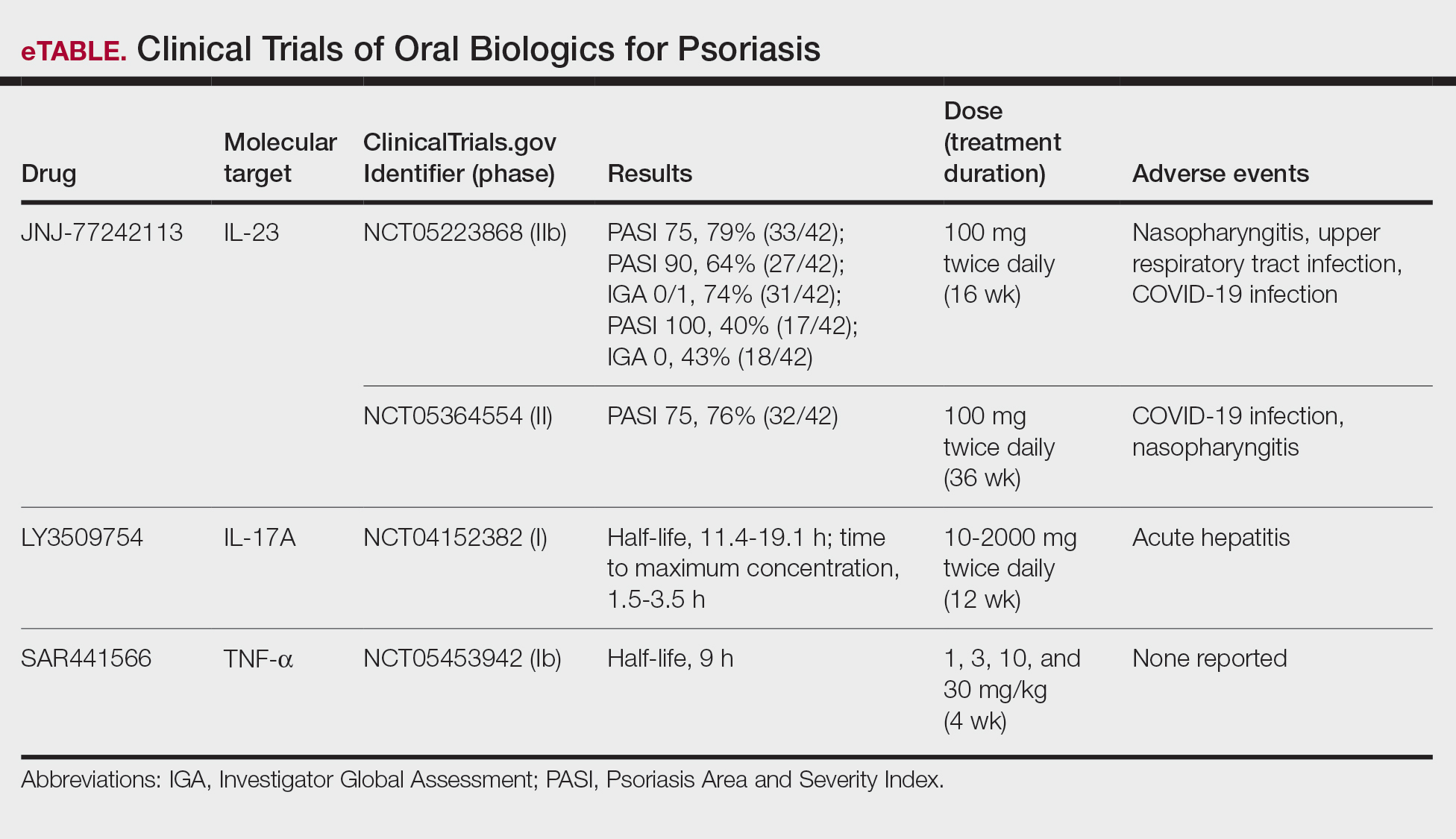
A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Oral Biologics: The New Wave for Treating Psoriasis
Oral Biologics: The New Wave for Treating Psoriasis
PRACTICE POINTS
- The biologics that currently are approved for psoriasis are expensive and must be administered via injection due to their large molecule size.
- Emerging small-molecule oral therapies for psoriasis are effective and affordable and may represent the future for psoriasis patients.