User login
Painful Ulcers on the Elbows, Knees, and Ankles
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
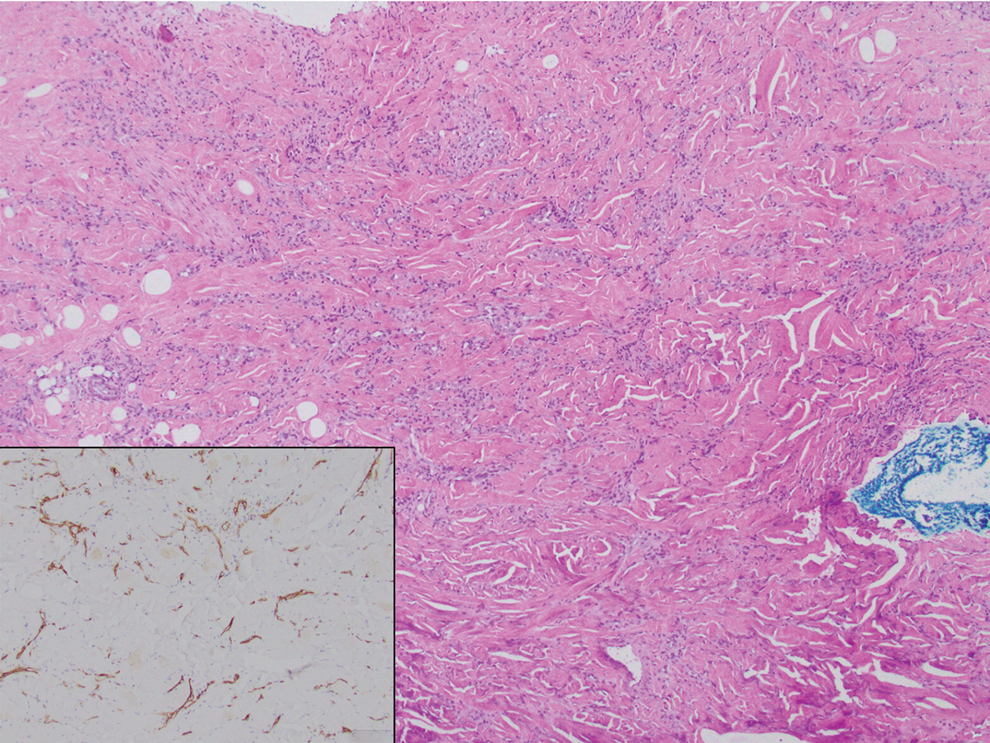
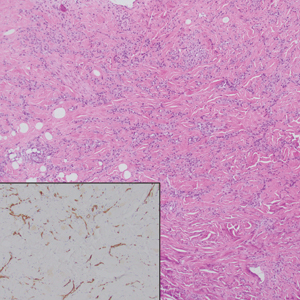
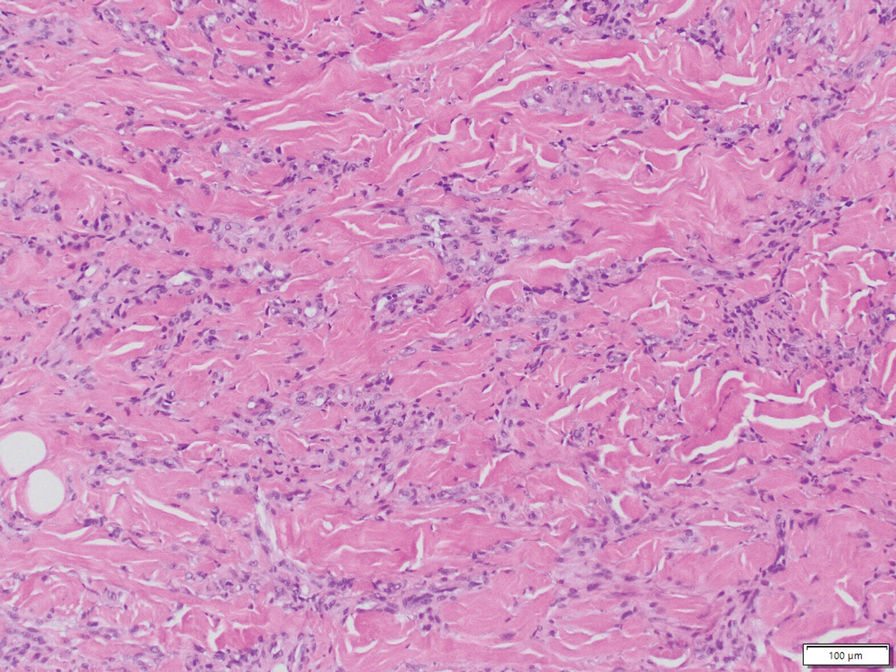
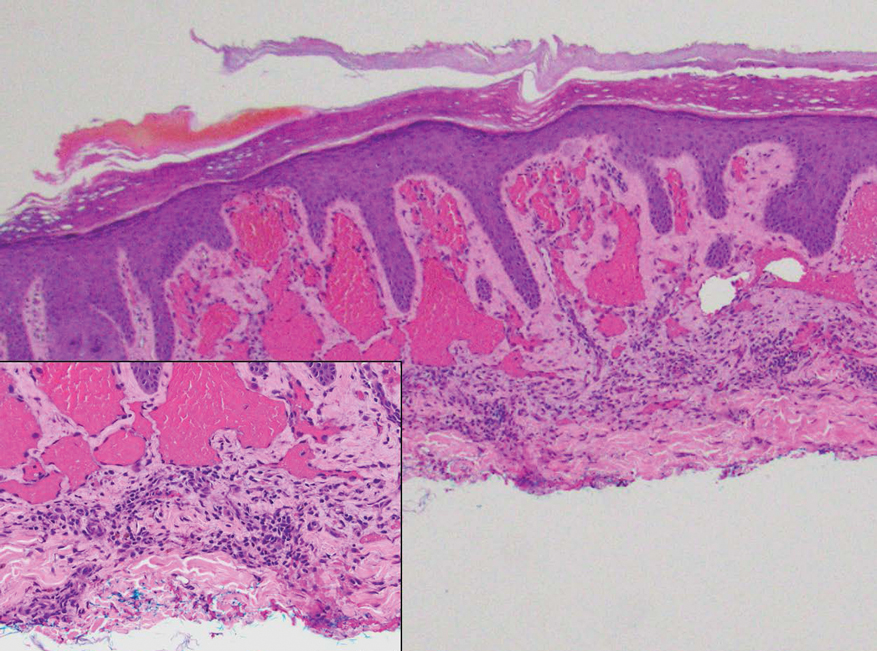
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.
The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6
Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9
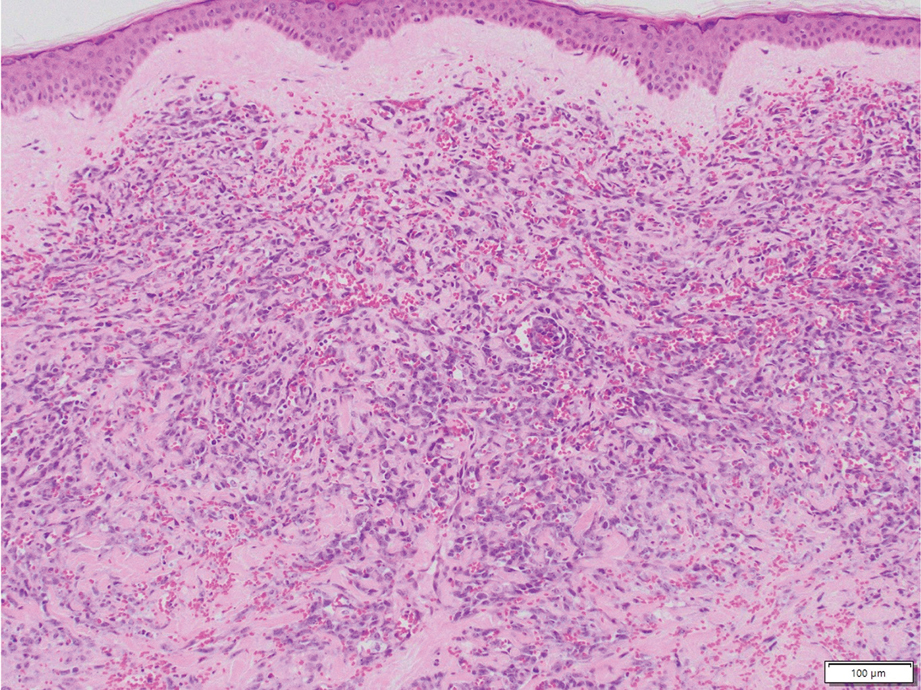
Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2
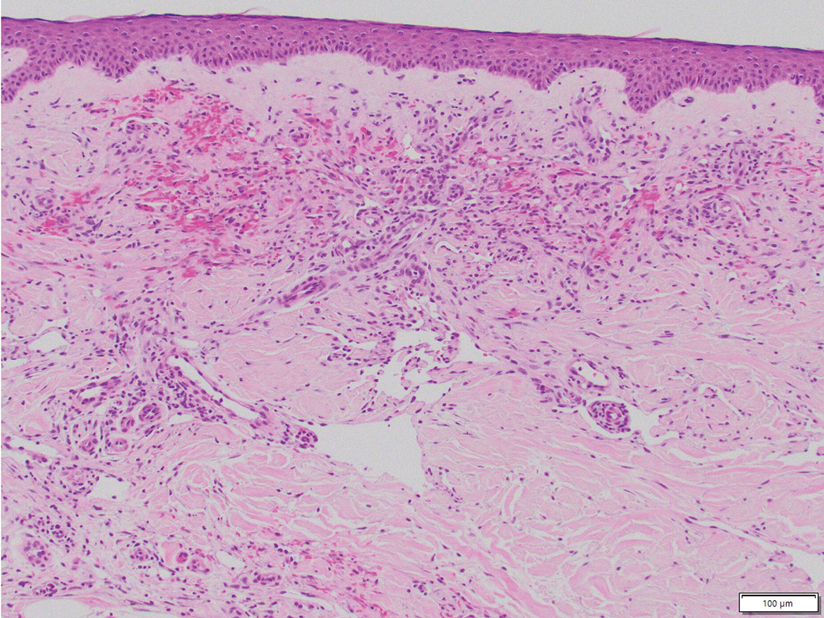
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
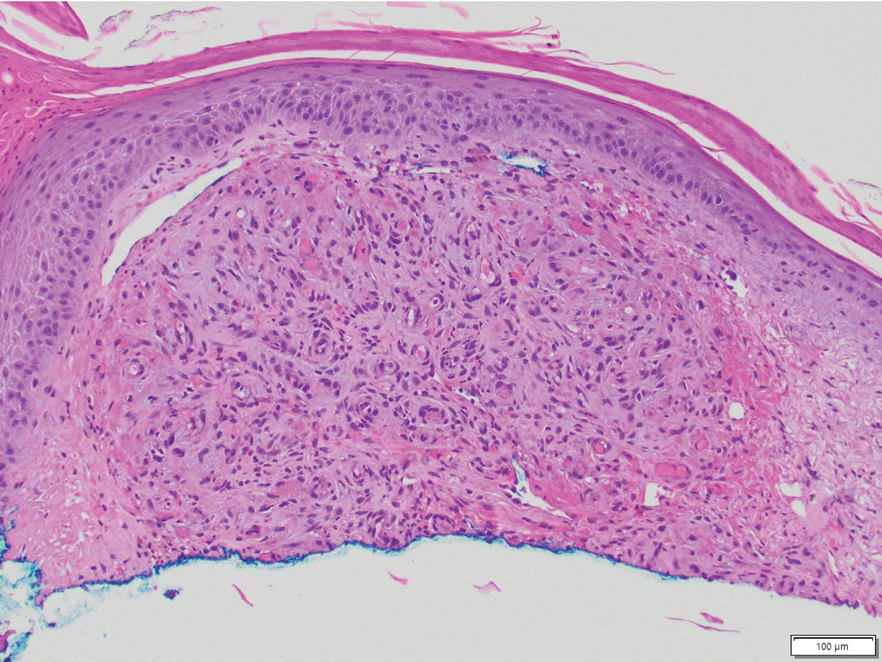
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
A 46-year-old woman with a history of systemic lupus erythematosus and end-stage renal disease presented to the dermatology department with painful ulcers on the extensor surfaces of the elbows, knees, and ankles of 2 months’ duration. Physical examination revealed angulated ulcers with surrounding pink erythema. A 4-mm punch biopsy and CD31 immunostaining of the left knee revealed dystrophic elastic fibers and purplish calciumlike depositions on connective tissue fibers in the mid to deep dermis.