User login
Shingles vaccine deemed effective in people with autoimmune disease
The herpes zoster vaccine reduces the risk of shingles in older adults with autoimmune disease, even if they are taking immunosuppressants for their condition, but the protection begins to wane after about 5 years, a recent retrospective study found.
“There has been some concern that patients with autoimmune conditions might have a lower immunogenic response to herpes zoster vaccination, especially when treated with immunosuppressive medications such as glucocorticoids,” wrote Huifeng Yun, PhD, of the University of Alabama at Birmingham, and her colleagues.
The researchers used 2006-2013 Medicare data to calculate the risk of shingles among Medicare recipients who had an autoimmune disease and either did or did not receive the herpes zoster vaccine. All the patients had been enrolled in Medicare for at least 12 continuous months and had a diagnosis of ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis.
The researchers matched 59,627 patients who received the herpes zoster vaccine with 119,254 unvaccinated patients, based on age, sex, race, calendar year, autoimmune disease type, and use of autoimmune drugs (biologics, disease-modifying antirheumatic drugs, and glucocorticoids). During a follow-up of up to 7 years, the researchers additionally accounted for comorbid medical conditions and concurrent medications each year.
The cohort, with an average age of 73.5 years in both groups, included 53.1% of adults with rheumatoid arthritis, 31.6% with psoriasis, 20.9% with inflammatory bowel disease, 4.7% with psoriatic arthritis, and 1.4% with ankylosing spondylitis.
Those who received the vaccine had a rate of 0.75 herpes zoster cases per 100 people during the first year, which rose to 1.25 cases per 100 people per year at the seventh year after vaccination. The rate among unvaccinated individuals stayed steady at approximately 1.3-1.7 cases per 100 people per year throughout the study period. These rates, as expected, were approximately 50% higher than in the general population over age 70 without autoimmune disease.
Compared with unvaccinated individuals, vaccinated individuals had a reduced relative risk for shingles of 0.74-0.77 after adjustment for confounders, but the risk reduction only remained statistically significant for the first 5 years after vaccination.
The waning seen with the vaccine’s effectiveness “raises the possibility that patients might benefit from a booster vaccine at some point after initial vaccination, although no recommendation currently exists that would support such a practice,” the authors wrote.
Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
The herpes zoster vaccine reduces the risk of shingles in older adults with autoimmune disease, even if they are taking immunosuppressants for their condition, but the protection begins to wane after about 5 years, a recent retrospective study found.
“There has been some concern that patients with autoimmune conditions might have a lower immunogenic response to herpes zoster vaccination, especially when treated with immunosuppressive medications such as glucocorticoids,” wrote Huifeng Yun, PhD, of the University of Alabama at Birmingham, and her colleagues.
The researchers used 2006-2013 Medicare data to calculate the risk of shingles among Medicare recipients who had an autoimmune disease and either did or did not receive the herpes zoster vaccine. All the patients had been enrolled in Medicare for at least 12 continuous months and had a diagnosis of ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis.
The researchers matched 59,627 patients who received the herpes zoster vaccine with 119,254 unvaccinated patients, based on age, sex, race, calendar year, autoimmune disease type, and use of autoimmune drugs (biologics, disease-modifying antirheumatic drugs, and glucocorticoids). During a follow-up of up to 7 years, the researchers additionally accounted for comorbid medical conditions and concurrent medications each year.
The cohort, with an average age of 73.5 years in both groups, included 53.1% of adults with rheumatoid arthritis, 31.6% with psoriasis, 20.9% with inflammatory bowel disease, 4.7% with psoriatic arthritis, and 1.4% with ankylosing spondylitis.
Those who received the vaccine had a rate of 0.75 herpes zoster cases per 100 people during the first year, which rose to 1.25 cases per 100 people per year at the seventh year after vaccination. The rate among unvaccinated individuals stayed steady at approximately 1.3-1.7 cases per 100 people per year throughout the study period. These rates, as expected, were approximately 50% higher than in the general population over age 70 without autoimmune disease.
Compared with unvaccinated individuals, vaccinated individuals had a reduced relative risk for shingles of 0.74-0.77 after adjustment for confounders, but the risk reduction only remained statistically significant for the first 5 years after vaccination.
The waning seen with the vaccine’s effectiveness “raises the possibility that patients might benefit from a booster vaccine at some point after initial vaccination, although no recommendation currently exists that would support such a practice,” the authors wrote.
Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
The herpes zoster vaccine reduces the risk of shingles in older adults with autoimmune disease, even if they are taking immunosuppressants for their condition, but the protection begins to wane after about 5 years, a recent retrospective study found.
“There has been some concern that patients with autoimmune conditions might have a lower immunogenic response to herpes zoster vaccination, especially when treated with immunosuppressive medications such as glucocorticoids,” wrote Huifeng Yun, PhD, of the University of Alabama at Birmingham, and her colleagues.
The researchers used 2006-2013 Medicare data to calculate the risk of shingles among Medicare recipients who had an autoimmune disease and either did or did not receive the herpes zoster vaccine. All the patients had been enrolled in Medicare for at least 12 continuous months and had a diagnosis of ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis.
The researchers matched 59,627 patients who received the herpes zoster vaccine with 119,254 unvaccinated patients, based on age, sex, race, calendar year, autoimmune disease type, and use of autoimmune drugs (biologics, disease-modifying antirheumatic drugs, and glucocorticoids). During a follow-up of up to 7 years, the researchers additionally accounted for comorbid medical conditions and concurrent medications each year.
The cohort, with an average age of 73.5 years in both groups, included 53.1% of adults with rheumatoid arthritis, 31.6% with psoriasis, 20.9% with inflammatory bowel disease, 4.7% with psoriatic arthritis, and 1.4% with ankylosing spondylitis.
Those who received the vaccine had a rate of 0.75 herpes zoster cases per 100 people during the first year, which rose to 1.25 cases per 100 people per year at the seventh year after vaccination. The rate among unvaccinated individuals stayed steady at approximately 1.3-1.7 cases per 100 people per year throughout the study period. These rates, as expected, were approximately 50% higher than in the general population over age 70 without autoimmune disease.
Compared with unvaccinated individuals, vaccinated individuals had a reduced relative risk for shingles of 0.74-0.77 after adjustment for confounders, but the risk reduction only remained statistically significant for the first 5 years after vaccination.
The waning seen with the vaccine’s effectiveness “raises the possibility that patients might benefit from a booster vaccine at some point after initial vaccination, although no recommendation currently exists that would support such a practice,” the authors wrote.
Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
Key clinical point:
Major finding: Medicare patients with autoimmune disease had a 23%-26% reduced risk of shingles for 5 years after receiving the herpes zoster vaccine.
Data source: The findings are based on analysis of 2006-2013 Medicare data on 59,627 patients who received the herpes zoster vaccine and 119,254 patients who didn’t.
Disclosures: Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
Clinicians still seek the best uses for apremilast
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
Adalimumab for psoriasis: Blocking TNF-alpha had no effect on vascular inflammation
ORLANDO – Vascular inflammation was no different in patients with moderate to severe psoriasis after 16 weeks of treatment with adalimumab than in untreated controls, according to a study that evaluated the impact of blocking tumor necrosis factor–alpha on vascular inflammation.
Further, there was a modest increase in vascular inflammation in carotid arteries after 52 weeks of treatment with the tumor necrosis factor (TNF) alpha-antagonist adalimumab, Robert Bissonnette, MD, president of Innovaderm Research, Montreal, reported in a late-breaking session at the annual meeting of the American Academy of Dermatology.
At 16 weeks, there were no significant differences in vascular inflammation between the treatment and control arms, based on the change from baseline in the vessel wall target to background ratio from the ascending aorta (the primary endpoint). In the carotid arteries at 16 weeks, differences in vascular inflammation between the adalimumab group and control group were also not significant.
At 52 weeks, there was no significant change in target to background ratio from the ascending aorta between baseline and the start of adalimumab, although in the carotid arteries, there was a modest increase in vascular inflammation.
Several previous studies have suggested that reducing inflammation in psoriasis can also reduce the risk of some cardiovascular events. As to why this study did not demonstrate a correlation between vascular inflammation and treatment, Dr. Bissonnette said during the question and answer portion of the presentation that “either the dose of adalimumab that is used for psoriasis has no impact on vascular inflammation or it may be possible that levels of interleukin-6, a key cytokine correlated with vascular inflammation, were very low in our study.”
Interleukin-6 typically is increased in patients with psoriatic arthritis, he explained, noting that in this study, only 7.5% of patients in the treatment group and about 10% of those in the control group had a history of psoriatic arthritis.
Additionally, at baseline, high-sensitivity C-reactive protein levels were significantly higher in controls: 5.32, compared with 2.72 in the treatment arm (P = .003).
Another possible reason for the results could be the molecule size of adalimumab, session comoderator Joel Gelfand, MD, noted in an interview. “The drug may not penetrate the aorta. These are large molecules. It also might be that [TNF–alpha] is not the main driver of aortic inflammation in people with psoriasis. Increasingly, we think of people with psoriasis as an IL-23 and IL-17 disease, so maybe that is part of it,” said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. He also directs the psoriasis and phototherapy treatment center at the university.
In addition to being presented at the meeting, the results were published in the Journal of Investigative Dermatology (2017 Feb 7. doi: 10.1016/j.jid.2017.02.977).
Adalimumab is marketed as Humira by Abbvie; a biosimilar version, adalimumab-atto (Amjevita), was approved in the United States in 2016.
Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Vascular inflammation was no different in patients with moderate to severe psoriasis after 16 weeks of treatment with adalimumab than in untreated controls, according to a study that evaluated the impact of blocking tumor necrosis factor–alpha on vascular inflammation.
Further, there was a modest increase in vascular inflammation in carotid arteries after 52 weeks of treatment with the tumor necrosis factor (TNF) alpha-antagonist adalimumab, Robert Bissonnette, MD, president of Innovaderm Research, Montreal, reported in a late-breaking session at the annual meeting of the American Academy of Dermatology.
At 16 weeks, there were no significant differences in vascular inflammation between the treatment and control arms, based on the change from baseline in the vessel wall target to background ratio from the ascending aorta (the primary endpoint). In the carotid arteries at 16 weeks, differences in vascular inflammation between the adalimumab group and control group were also not significant.
At 52 weeks, there was no significant change in target to background ratio from the ascending aorta between baseline and the start of adalimumab, although in the carotid arteries, there was a modest increase in vascular inflammation.
Several previous studies have suggested that reducing inflammation in psoriasis can also reduce the risk of some cardiovascular events. As to why this study did not demonstrate a correlation between vascular inflammation and treatment, Dr. Bissonnette said during the question and answer portion of the presentation that “either the dose of adalimumab that is used for psoriasis has no impact on vascular inflammation or it may be possible that levels of interleukin-6, a key cytokine correlated with vascular inflammation, were very low in our study.”
Interleukin-6 typically is increased in patients with psoriatic arthritis, he explained, noting that in this study, only 7.5% of patients in the treatment group and about 10% of those in the control group had a history of psoriatic arthritis.
Additionally, at baseline, high-sensitivity C-reactive protein levels were significantly higher in controls: 5.32, compared with 2.72 in the treatment arm (P = .003).
Another possible reason for the results could be the molecule size of adalimumab, session comoderator Joel Gelfand, MD, noted in an interview. “The drug may not penetrate the aorta. These are large molecules. It also might be that [TNF–alpha] is not the main driver of aortic inflammation in people with psoriasis. Increasingly, we think of people with psoriasis as an IL-23 and IL-17 disease, so maybe that is part of it,” said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. He also directs the psoriasis and phototherapy treatment center at the university.
In addition to being presented at the meeting, the results were published in the Journal of Investigative Dermatology (2017 Feb 7. doi: 10.1016/j.jid.2017.02.977).
Adalimumab is marketed as Humira by Abbvie; a biosimilar version, adalimumab-atto (Amjevita), was approved in the United States in 2016.
Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Vascular inflammation was no different in patients with moderate to severe psoriasis after 16 weeks of treatment with adalimumab than in untreated controls, according to a study that evaluated the impact of blocking tumor necrosis factor–alpha on vascular inflammation.
Further, there was a modest increase in vascular inflammation in carotid arteries after 52 weeks of treatment with the tumor necrosis factor (TNF) alpha-antagonist adalimumab, Robert Bissonnette, MD, president of Innovaderm Research, Montreal, reported in a late-breaking session at the annual meeting of the American Academy of Dermatology.
At 16 weeks, there were no significant differences in vascular inflammation between the treatment and control arms, based on the change from baseline in the vessel wall target to background ratio from the ascending aorta (the primary endpoint). In the carotid arteries at 16 weeks, differences in vascular inflammation between the adalimumab group and control group were also not significant.
At 52 weeks, there was no significant change in target to background ratio from the ascending aorta between baseline and the start of adalimumab, although in the carotid arteries, there was a modest increase in vascular inflammation.
Several previous studies have suggested that reducing inflammation in psoriasis can also reduce the risk of some cardiovascular events. As to why this study did not demonstrate a correlation between vascular inflammation and treatment, Dr. Bissonnette said during the question and answer portion of the presentation that “either the dose of adalimumab that is used for psoriasis has no impact on vascular inflammation or it may be possible that levels of interleukin-6, a key cytokine correlated with vascular inflammation, were very low in our study.”
Interleukin-6 typically is increased in patients with psoriatic arthritis, he explained, noting that in this study, only 7.5% of patients in the treatment group and about 10% of those in the control group had a history of psoriatic arthritis.
Additionally, at baseline, high-sensitivity C-reactive protein levels were significantly higher in controls: 5.32, compared with 2.72 in the treatment arm (P = .003).
Another possible reason for the results could be the molecule size of adalimumab, session comoderator Joel Gelfand, MD, noted in an interview. “The drug may not penetrate the aorta. These are large molecules. It also might be that [TNF–alpha] is not the main driver of aortic inflammation in people with psoriasis. Increasingly, we think of people with psoriasis as an IL-23 and IL-17 disease, so maybe that is part of it,” said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. He also directs the psoriasis and phototherapy treatment center at the university.
In addition to being presented at the meeting, the results were published in the Journal of Investigative Dermatology (2017 Feb 7. doi: 10.1016/j.jid.2017.02.977).
Adalimumab is marketed as Humira by Abbvie; a biosimilar version, adalimumab-atto (Amjevita), was approved in the United States in 2016.
Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
[email protected]
On Twitter @whitneymcknight
Key clinical point:
Major finding: At 16 weeks, there were no significant differences in aortic and carotid inflammation in the change from baseline in moderate psoriasis patients given adalimumab and controls.
Data source: A randomized, double-blind multicenter study that used PET-CT scans to evaluate the impact of treatment on vascular inflammation in 107 adults with moderate to severe psoriasis.
Disclosures: Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
VIDEO: Stress alleviation strategies boost inflammatory skin treatment regimens
ORLANDO – “Consider the effects of the mind on the skin when treating patients with some skin diseases.” That was the message of several speakers during a session titled “The Skin and the Mind” at the annual meeting of the American Academy of Dermatology.
“I certainly believe that the time has come to use stress alleviation techniques, at least for selected patients, and this should certainly be part of our armamentarium in the clinic,” said Richard D. Granstein, MD, George W. Hambrick Jr. professor and chairman of the department of dermatology, Cornell University, New York. During the session, he spoke about the emerging science of stress in dermatology.
While it has long been believed that stress exacerbates different skin diseases, the connection has been difficult to prove scientifically, he pointed out. However, “the overwhelming number of reports and studies indicate that stress probably does exacerbate a number of inflammatory skin diseases,” he added.
In a video interview at the meeting, Dr. Granstein notes that a number of pathways that help explain this link have now been identified and discusses one of those pathways, which involves neuropeptides and future directions in understanding the relationship between stress and inflammatory skin diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Granstein disclosed serving as an advisor to Castle Biosciences, Elysium Health, Galderma Laboratories, and Velius.
[email protected]
On Twitter @whitneymcknight
ORLANDO – “Consider the effects of the mind on the skin when treating patients with some skin diseases.” That was the message of several speakers during a session titled “The Skin and the Mind” at the annual meeting of the American Academy of Dermatology.
“I certainly believe that the time has come to use stress alleviation techniques, at least for selected patients, and this should certainly be part of our armamentarium in the clinic,” said Richard D. Granstein, MD, George W. Hambrick Jr. professor and chairman of the department of dermatology, Cornell University, New York. During the session, he spoke about the emerging science of stress in dermatology.
While it has long been believed that stress exacerbates different skin diseases, the connection has been difficult to prove scientifically, he pointed out. However, “the overwhelming number of reports and studies indicate that stress probably does exacerbate a number of inflammatory skin diseases,” he added.
In a video interview at the meeting, Dr. Granstein notes that a number of pathways that help explain this link have now been identified and discusses one of those pathways, which involves neuropeptides and future directions in understanding the relationship between stress and inflammatory skin diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Granstein disclosed serving as an advisor to Castle Biosciences, Elysium Health, Galderma Laboratories, and Velius.
[email protected]
On Twitter @whitneymcknight
ORLANDO – “Consider the effects of the mind on the skin when treating patients with some skin diseases.” That was the message of several speakers during a session titled “The Skin and the Mind” at the annual meeting of the American Academy of Dermatology.
“I certainly believe that the time has come to use stress alleviation techniques, at least for selected patients, and this should certainly be part of our armamentarium in the clinic,” said Richard D. Granstein, MD, George W. Hambrick Jr. professor and chairman of the department of dermatology, Cornell University, New York. During the session, he spoke about the emerging science of stress in dermatology.
While it has long been believed that stress exacerbates different skin diseases, the connection has been difficult to prove scientifically, he pointed out. However, “the overwhelming number of reports and studies indicate that stress probably does exacerbate a number of inflammatory skin diseases,” he added.
In a video interview at the meeting, Dr. Granstein notes that a number of pathways that help explain this link have now been identified and discusses one of those pathways, which involves neuropeptides and future directions in understanding the relationship between stress and inflammatory skin diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Granstein disclosed serving as an advisor to Castle Biosciences, Elysium Health, Galderma Laboratories, and Velius.
[email protected]
On Twitter @whitneymcknight
AT AAD 17
Infection Risk With Biologic Therapy for Psoriasis: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CIMPASI-1 and -2 show certolizumab pegol benefits patients with severe plaque psoriasis
ORLANDO – Results from two phase III trials indicated the tumor necrosis factor alpha inhibitor certolizumab pegol led to significant improvements in moderate to severe chronic plaque psoriasis, compared with placebo, according to an oral presentation at this year’s annual Academy of Dermatology Meeting.
Across the CIMPASI-1 and CIMPASI-2 trials, at 16 weeks, both certolizumab pegol (Cimzia) 400 mg and 200 mg, administered subcutaneously every 2 weeks in groups of patients with moderate to severe chronic plaque psoriasis, demonstrated statistically significant, clinically meaningful improvements in Psoriasis Area and Severity Index (PASI) scores, and on the Physician Global Assessment (PGA) scores, when compared with placebo. The data were presented by Alice B. Gottlieb, MD, PhD, professor of dermatology at New York Medical College, Valhalla. Certolizumab pegol currently is approved in the United States for use in psoriatic arthritis, but not for psoriasis.
In CIMPASI-1, 234 mostly white male patients in their mid-40s who had moderate to severe chronic plaque psoriasis were randomly assigned to one of three groups. There were 88 participants in the arm given 400 mg every 2 weeks at weeks 0, 2, and 4; 95 given 200 mg at weeks 0, 2, and 4; and 51 given placebo. At week 16, the percentage of patients who achieved a PASI 75 was 75.8% in the first group, 66.5% in the second, and 6.5% for placebo. A PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 57.9% for group 1, 47.0% for the second group, and 4.2% for placebo.
Also at week 16, the percentage of patients who achieved a PASI 90 was 43.6% in the 400 mg dose group, 35.8% in the 200 mg dose group, and 0.4% in the placebo group.
In CIMPASI-2, 227 mostly white patients with moderate to severe chronic plaque psoriasis, most of whom were in their mid-40s, evenly distributed across the sexes, were randomly assigned to one of three groups. There were 87 participants in the 400 mg every 2 weeks at weeks 0, 2, and 4 group; 91 in the 200 mg every 2 weeks at weeks 0, 2, and 4 arm, and 49 in the placebo group. At week 16, 82.6% of patients in the first group and 81.4% in the second group had a PASI 75, compared with 11.6% of the placebo group. Also at 16 weeks, a PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 71.6% for group 1, 66.8% for the second group, and 2.0% for placebo. At week 16, 55.4% of patients receiving the 400 mg dose had a PASI 90, as did 52.6% of patients receiving the 200 mg dose every 2 weeks, compared with only 4.5% of the placebo group.
Additionally, Dr. Gottlieb said that at week 16, patients in the 400-mg and 200-mg groups showed significant improvements over baseline Dermatology Life Quality Index (DLQI) average scores, compared with placebo: a 10.2 decrease in the 400-mg group and a 9.3 decrease in the 200-mg group, compared with a 3.3 decrease in the placebo arm (P less than .001) in the CIMPASI-1 study. In the CIMPASI-2 study at week 16, there was a drop of 10.0 DLQI in average scores in the 400-mg group, 10.4 in the 200-mg group, and an average drop of only 3.8 in controls (P less than .001).
Dr. Gottlieb, who disclosed that her mother suffers from severe plaque psoriasis, told the audience that the patient improvements were on par with those seen with infliximab, which she said was “the best anti–TNF alpha in the pack to date, and that’s an intravenous drug. These are impressive drops in the DLQI.”
Adverse events were, said Dr. Gottlieb, “a whole lot of nothing. There’s nothing that stands out.” She said there were no cases of tuberculosis, one case of vulvovaginal candidiasis, and equal distribution of herpes zoster across the study.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Results from two phase III trials indicated the tumor necrosis factor alpha inhibitor certolizumab pegol led to significant improvements in moderate to severe chronic plaque psoriasis, compared with placebo, according to an oral presentation at this year’s annual Academy of Dermatology Meeting.
Across the CIMPASI-1 and CIMPASI-2 trials, at 16 weeks, both certolizumab pegol (Cimzia) 400 mg and 200 mg, administered subcutaneously every 2 weeks in groups of patients with moderate to severe chronic plaque psoriasis, demonstrated statistically significant, clinically meaningful improvements in Psoriasis Area and Severity Index (PASI) scores, and on the Physician Global Assessment (PGA) scores, when compared with placebo. The data were presented by Alice B. Gottlieb, MD, PhD, professor of dermatology at New York Medical College, Valhalla. Certolizumab pegol currently is approved in the United States for use in psoriatic arthritis, but not for psoriasis.
In CIMPASI-1, 234 mostly white male patients in their mid-40s who had moderate to severe chronic plaque psoriasis were randomly assigned to one of three groups. There were 88 participants in the arm given 400 mg every 2 weeks at weeks 0, 2, and 4; 95 given 200 mg at weeks 0, 2, and 4; and 51 given placebo. At week 16, the percentage of patients who achieved a PASI 75 was 75.8% in the first group, 66.5% in the second, and 6.5% for placebo. A PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 57.9% for group 1, 47.0% for the second group, and 4.2% for placebo.
Also at week 16, the percentage of patients who achieved a PASI 90 was 43.6% in the 400 mg dose group, 35.8% in the 200 mg dose group, and 0.4% in the placebo group.
In CIMPASI-2, 227 mostly white patients with moderate to severe chronic plaque psoriasis, most of whom were in their mid-40s, evenly distributed across the sexes, were randomly assigned to one of three groups. There were 87 participants in the 400 mg every 2 weeks at weeks 0, 2, and 4 group; 91 in the 200 mg every 2 weeks at weeks 0, 2, and 4 arm, and 49 in the placebo group. At week 16, 82.6% of patients in the first group and 81.4% in the second group had a PASI 75, compared with 11.6% of the placebo group. Also at 16 weeks, a PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 71.6% for group 1, 66.8% for the second group, and 2.0% for placebo. At week 16, 55.4% of patients receiving the 400 mg dose had a PASI 90, as did 52.6% of patients receiving the 200 mg dose every 2 weeks, compared with only 4.5% of the placebo group.
Additionally, Dr. Gottlieb said that at week 16, patients in the 400-mg and 200-mg groups showed significant improvements over baseline Dermatology Life Quality Index (DLQI) average scores, compared with placebo: a 10.2 decrease in the 400-mg group and a 9.3 decrease in the 200-mg group, compared with a 3.3 decrease in the placebo arm (P less than .001) in the CIMPASI-1 study. In the CIMPASI-2 study at week 16, there was a drop of 10.0 DLQI in average scores in the 400-mg group, 10.4 in the 200-mg group, and an average drop of only 3.8 in controls (P less than .001).
Dr. Gottlieb, who disclosed that her mother suffers from severe plaque psoriasis, told the audience that the patient improvements were on par with those seen with infliximab, which she said was “the best anti–TNF alpha in the pack to date, and that’s an intravenous drug. These are impressive drops in the DLQI.”
Adverse events were, said Dr. Gottlieb, “a whole lot of nothing. There’s nothing that stands out.” She said there were no cases of tuberculosis, one case of vulvovaginal candidiasis, and equal distribution of herpes zoster across the study.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Results from two phase III trials indicated the tumor necrosis factor alpha inhibitor certolizumab pegol led to significant improvements in moderate to severe chronic plaque psoriasis, compared with placebo, according to an oral presentation at this year’s annual Academy of Dermatology Meeting.
Across the CIMPASI-1 and CIMPASI-2 trials, at 16 weeks, both certolizumab pegol (Cimzia) 400 mg and 200 mg, administered subcutaneously every 2 weeks in groups of patients with moderate to severe chronic plaque psoriasis, demonstrated statistically significant, clinically meaningful improvements in Psoriasis Area and Severity Index (PASI) scores, and on the Physician Global Assessment (PGA) scores, when compared with placebo. The data were presented by Alice B. Gottlieb, MD, PhD, professor of dermatology at New York Medical College, Valhalla. Certolizumab pegol currently is approved in the United States for use in psoriatic arthritis, but not for psoriasis.
In CIMPASI-1, 234 mostly white male patients in their mid-40s who had moderate to severe chronic plaque psoriasis were randomly assigned to one of three groups. There were 88 participants in the arm given 400 mg every 2 weeks at weeks 0, 2, and 4; 95 given 200 mg at weeks 0, 2, and 4; and 51 given placebo. At week 16, the percentage of patients who achieved a PASI 75 was 75.8% in the first group, 66.5% in the second, and 6.5% for placebo. A PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 57.9% for group 1, 47.0% for the second group, and 4.2% for placebo.
Also at week 16, the percentage of patients who achieved a PASI 90 was 43.6% in the 400 mg dose group, 35.8% in the 200 mg dose group, and 0.4% in the placebo group.
In CIMPASI-2, 227 mostly white patients with moderate to severe chronic plaque psoriasis, most of whom were in their mid-40s, evenly distributed across the sexes, were randomly assigned to one of three groups. There were 87 participants in the 400 mg every 2 weeks at weeks 0, 2, and 4 group; 91 in the 200 mg every 2 weeks at weeks 0, 2, and 4 arm, and 49 in the placebo group. At week 16, 82.6% of patients in the first group and 81.4% in the second group had a PASI 75, compared with 11.6% of the placebo group. Also at 16 weeks, a PGA scale improvement of at least two points over baseline toward a final score of clear or almost clear skin at week 16 was 71.6% for group 1, 66.8% for the second group, and 2.0% for placebo. At week 16, 55.4% of patients receiving the 400 mg dose had a PASI 90, as did 52.6% of patients receiving the 200 mg dose every 2 weeks, compared with only 4.5% of the placebo group.
Additionally, Dr. Gottlieb said that at week 16, patients in the 400-mg and 200-mg groups showed significant improvements over baseline Dermatology Life Quality Index (DLQI) average scores, compared with placebo: a 10.2 decrease in the 400-mg group and a 9.3 decrease in the 200-mg group, compared with a 3.3 decrease in the placebo arm (P less than .001) in the CIMPASI-1 study. In the CIMPASI-2 study at week 16, there was a drop of 10.0 DLQI in average scores in the 400-mg group, 10.4 in the 200-mg group, and an average drop of only 3.8 in controls (P less than .001).
Dr. Gottlieb, who disclosed that her mother suffers from severe plaque psoriasis, told the audience that the patient improvements were on par with those seen with infliximab, which she said was “the best anti–TNF alpha in the pack to date, and that’s an intravenous drug. These are impressive drops in the DLQI.”
Adverse events were, said Dr. Gottlieb, “a whole lot of nothing. There’s nothing that stands out.” She said there were no cases of tuberculosis, one case of vulvovaginal candidiasis, and equal distribution of herpes zoster across the study.
[email protected]
On Twitter @whitneymcknight
AT AAD 17
Key clinical point:
Major finding: In CIMPASI-1, at week 16, the response rate for patients who achieved a PASI 75 was 75.8% in the 400-mg group, 66.5% in the 200-mg group, and 6.5% in the placebo group.
Data source: 461 adults with moderate to severe plaque arthritis randomly assigned to either 400 mg certolizumab pegol, 200 mg of the treatment, or placebo every other week at weeks 0, 2, and 4.
Disclosures: Dr. Gottlieb had relevant disclosures, including with Dermira and UCB, the sponsors of this trial.
Psoriasis and depression may pack a PsA punch
Patients with both psoriasis and major depressive disorder face an adjusted 37% greater risk of developing psoriatic arthritis (PsA) over a median follow-up of 5.1 years, a new study finds.
The findings don’t definitively prove that depression plays a role in PsA. Still, “,” study coauthor Cheryl Barnabe, MD, MSc, said in an interview.
For the new study published by the Journal of Investigative Dermatology, researchers tracked 73,447 people in the United Kingdom with psoriasis through a primary care medical records database for up to 25 years. The study statistics come from the years 1987-2012, reported Ryan T. Lewinson of the Cumming School of Medicine, in Calgary, Alta., and his associates (J Invest Dermatol. 2017. doi: 10.1016/j.jid.2016.11.032).
The median age at psoriasis diagnosis was 49.5 years (range, 20-90 years) and the median follow-up time was 5.1 years; 2% of the patients developed PsA and 7% developed major depression.
Via an unadjusted model, those with signs of depression were 1.56 times more likely to develop PsA (hazard ratio; 95% CI, 1.28-1.90; P less than .0001). In a model adjusted for factors such as age, gender, and obesity status, the extra risk of PsA was 1.37 (HR; 95% CI, 1.05-1.80; P = .021).
“The study draws into question the biological mechanisms by which depression increases the risk for developing psoriatic arthritis,” said Dr. Barnabe, an associate professor with the departments of medicine and community health sciences at the University of Calgary and a rheumatologist with Alberta Health Services.
The study notes that depression is linked to poor diet and lack of exercise, factors that could contribute to PsA. The authors also point out that researchers have linked depression to inflammation, a crucial component of both psoriasis and PsA, although they note that the study doesn’t examine systemic inflammation.
What’s next? “Mental health in chronic inflammatory diseases is not well addressed at the present time, in our system anyway, and should be a prime area of focus,” Dr. Barnabe noted. “Depression occurs at elevated rates in both psoriasis and psoriatic arthritis, and there is certainly a role for treatment to assist with disease management.”
The study authors reported no specific study funding and no relevant financial disclosures.
Patients with both psoriasis and major depressive disorder face an adjusted 37% greater risk of developing psoriatic arthritis (PsA) over a median follow-up of 5.1 years, a new study finds.
The findings don’t definitively prove that depression plays a role in PsA. Still, “,” study coauthor Cheryl Barnabe, MD, MSc, said in an interview.
For the new study published by the Journal of Investigative Dermatology, researchers tracked 73,447 people in the United Kingdom with psoriasis through a primary care medical records database for up to 25 years. The study statistics come from the years 1987-2012, reported Ryan T. Lewinson of the Cumming School of Medicine, in Calgary, Alta., and his associates (J Invest Dermatol. 2017. doi: 10.1016/j.jid.2016.11.032).
The median age at psoriasis diagnosis was 49.5 years (range, 20-90 years) and the median follow-up time was 5.1 years; 2% of the patients developed PsA and 7% developed major depression.
Via an unadjusted model, those with signs of depression were 1.56 times more likely to develop PsA (hazard ratio; 95% CI, 1.28-1.90; P less than .0001). In a model adjusted for factors such as age, gender, and obesity status, the extra risk of PsA was 1.37 (HR; 95% CI, 1.05-1.80; P = .021).
“The study draws into question the biological mechanisms by which depression increases the risk for developing psoriatic arthritis,” said Dr. Barnabe, an associate professor with the departments of medicine and community health sciences at the University of Calgary and a rheumatologist with Alberta Health Services.
The study notes that depression is linked to poor diet and lack of exercise, factors that could contribute to PsA. The authors also point out that researchers have linked depression to inflammation, a crucial component of both psoriasis and PsA, although they note that the study doesn’t examine systemic inflammation.
What’s next? “Mental health in chronic inflammatory diseases is not well addressed at the present time, in our system anyway, and should be a prime area of focus,” Dr. Barnabe noted. “Depression occurs at elevated rates in both psoriasis and psoriatic arthritis, and there is certainly a role for treatment to assist with disease management.”
The study authors reported no specific study funding and no relevant financial disclosures.
Patients with both psoriasis and major depressive disorder face an adjusted 37% greater risk of developing psoriatic arthritis (PsA) over a median follow-up of 5.1 years, a new study finds.
The findings don’t definitively prove that depression plays a role in PsA. Still, “,” study coauthor Cheryl Barnabe, MD, MSc, said in an interview.
For the new study published by the Journal of Investigative Dermatology, researchers tracked 73,447 people in the United Kingdom with psoriasis through a primary care medical records database for up to 25 years. The study statistics come from the years 1987-2012, reported Ryan T. Lewinson of the Cumming School of Medicine, in Calgary, Alta., and his associates (J Invest Dermatol. 2017. doi: 10.1016/j.jid.2016.11.032).
The median age at psoriasis diagnosis was 49.5 years (range, 20-90 years) and the median follow-up time was 5.1 years; 2% of the patients developed PsA and 7% developed major depression.
Via an unadjusted model, those with signs of depression were 1.56 times more likely to develop PsA (hazard ratio; 95% CI, 1.28-1.90; P less than .0001). In a model adjusted for factors such as age, gender, and obesity status, the extra risk of PsA was 1.37 (HR; 95% CI, 1.05-1.80; P = .021).
“The study draws into question the biological mechanisms by which depression increases the risk for developing psoriatic arthritis,” said Dr. Barnabe, an associate professor with the departments of medicine and community health sciences at the University of Calgary and a rheumatologist with Alberta Health Services.
The study notes that depression is linked to poor diet and lack of exercise, factors that could contribute to PsA. The authors also point out that researchers have linked depression to inflammation, a crucial component of both psoriasis and PsA, although they note that the study doesn’t examine systemic inflammation.
What’s next? “Mental health in chronic inflammatory diseases is not well addressed at the present time, in our system anyway, and should be a prime area of focus,” Dr. Barnabe noted. “Depression occurs at elevated rates in both psoriasis and psoriatic arthritis, and there is certainly a role for treatment to assist with disease management.”
The study authors reported no specific study funding and no relevant financial disclosures.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Depression is especially common in patients with psoriasis, and those with both conditions appear to face a higher risk of psoriatic arthritis.
Major finding: In an adjusted model, patients with psoriasis and signs of major depression were 37% more likely to develop PsA.
Data source: Retrospective study of 73,447 patients with psoriasis from the U.K. tracked for up to 25 years (median follow-up, 5.1 years).
Disclosures: The authors reported no specific study funding and no relevant financial disclosures.
Humira Pen topped per-person drug spending in 2016
Humira Pen (adalimumab) was the most expensive drug in 2016 when ranked by spending per person, according to pharmacy benefits manager Express Scripts.
Total spending per person with employer-sponsored insurance was $45.11 last year for Humira Pen, which is indicated for rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. Next in spending per person was Enbrel (etanercept) – another drug for arthritis, psoriatic arthritis, ankylosing spondylitis, and psoriasis – at $26.82, followed by the diabetes drug Lantus (insulin glargine) and two multiple sclerosis drugs: Tecfidera (dimethyl fumarate) and Copaxone (glatiramer), Express Scripts said in its “2016 Drug Trend Report.”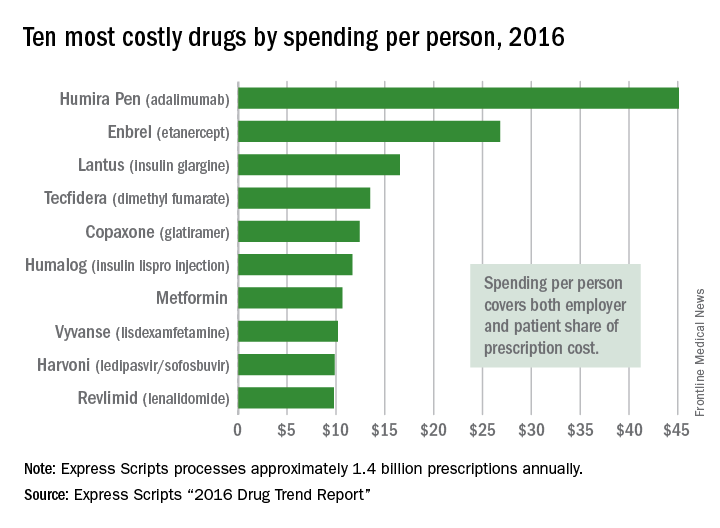
Humira Pen had the next-largest increase from 2015 – a mere 28% – while the hepatitis C drug Harvoni (ledipasvir/sofisbuvir) had the largest decrease in per-person spending among the top 10, dropping 54%, the report noted.
Express Scripts processes approximately 1.4 billion prescriptions annually for 85 million insured members from 3,000 client companies.
Humira Pen (adalimumab) was the most expensive drug in 2016 when ranked by spending per person, according to pharmacy benefits manager Express Scripts.
Total spending per person with employer-sponsored insurance was $45.11 last year for Humira Pen, which is indicated for rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. Next in spending per person was Enbrel (etanercept) – another drug for arthritis, psoriatic arthritis, ankylosing spondylitis, and psoriasis – at $26.82, followed by the diabetes drug Lantus (insulin glargine) and two multiple sclerosis drugs: Tecfidera (dimethyl fumarate) and Copaxone (glatiramer), Express Scripts said in its “2016 Drug Trend Report.”
Humira Pen had the next-largest increase from 2015 – a mere 28% – while the hepatitis C drug Harvoni (ledipasvir/sofisbuvir) had the largest decrease in per-person spending among the top 10, dropping 54%, the report noted.
Express Scripts processes approximately 1.4 billion prescriptions annually for 85 million insured members from 3,000 client companies.
Humira Pen (adalimumab) was the most expensive drug in 2016 when ranked by spending per person, according to pharmacy benefits manager Express Scripts.
Total spending per person with employer-sponsored insurance was $45.11 last year for Humira Pen, which is indicated for rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. Next in spending per person was Enbrel (etanercept) – another drug for arthritis, psoriatic arthritis, ankylosing spondylitis, and psoriasis – at $26.82, followed by the diabetes drug Lantus (insulin glargine) and two multiple sclerosis drugs: Tecfidera (dimethyl fumarate) and Copaxone (glatiramer), Express Scripts said in its “2016 Drug Trend Report.”
Humira Pen had the next-largest increase from 2015 – a mere 28% – while the hepatitis C drug Harvoni (ledipasvir/sofisbuvir) had the largest decrease in per-person spending among the top 10, dropping 54%, the report noted.
Express Scripts processes approximately 1.4 billion prescriptions annually for 85 million insured members from 3,000 client companies.
Brodalumab approved for psoriasis with REMS required
The Food and Drug Administration has approved brodalumab, an interleukin-17 receptor A-antagonist, for treating adults with moderate to severe plaque psoriasis, with a Risk Evaluation and Mitigation Strategy (REMS) that addresses the “observed risk of suicidal ideation and behavior” in clinical trials, the agency announced on Feb. 16.
In the three pivotal phase III studies of 4,373 adults with moderate to severe plaque psoriasis who were candidates for systemic therapy or phototherapy, 83%-86% of those treated with brodalumab achieved Psoriasis Area and Severity Index (PASI 75) scores at 12 weeks of treatment, compared with 3%-8% of those on placebo. In addition, 37%-44% of those on brodalumab achieved PASI 100 scores, compared with 1% or fewer of those on placebo. In the psoriasis clinical trials, suicidal ideation and behavior, which included four completed suicides, occurred in patients treated with brodalumab, according to the prescribing information
In the statement, the FDA points out that “a causal association between treatment with Siliq and increased risk of suicidal ideation and behavior has not been established,” but that “suicidal ideation and behavior, including completed suicides, have occurred in patients treated with Siliq during clinical trials. Siliq users with a history of suicidality or depression had an increased incidence of suicidal ideation and behavior, compared to users without this history.”
At a meeting in July 2016, the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee voted 18-0 in favor of approving brodalumab, with the majority (14) recommending risk management options beyond labeling to address these concerns.
Elements of the REMS include requirements for prescribers, pharmacy certification, and a medication guide for patients with information about the risks of suicidal ideation and behavior. Prescribers are required to counsel patients about this risk, and patients are required to sign a “Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety or other mood changes,” the FDA said. The prescribing information also includes a boxed warning about suicidal ideation and behavior.
The most common adverse events associated with brodalumab, the FDA statement noted, include arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, injection site reactions, neutropenia, and fungal infections. The recommended dose of brodalumab is 210 mg, administered subcutaneously, at weeks 0, 1, and 2, followed by 210 mg every 2 weeks.
The Food and Drug Administration has approved brodalumab, an interleukin-17 receptor A-antagonist, for treating adults with moderate to severe plaque psoriasis, with a Risk Evaluation and Mitigation Strategy (REMS) that addresses the “observed risk of suicidal ideation and behavior” in clinical trials, the agency announced on Feb. 16.
In the three pivotal phase III studies of 4,373 adults with moderate to severe plaque psoriasis who were candidates for systemic therapy or phototherapy, 83%-86% of those treated with brodalumab achieved Psoriasis Area and Severity Index (PASI 75) scores at 12 weeks of treatment, compared with 3%-8% of those on placebo. In addition, 37%-44% of those on brodalumab achieved PASI 100 scores, compared with 1% or fewer of those on placebo. In the psoriasis clinical trials, suicidal ideation and behavior, which included four completed suicides, occurred in patients treated with brodalumab, according to the prescribing information
In the statement, the FDA points out that “a causal association between treatment with Siliq and increased risk of suicidal ideation and behavior has not been established,” but that “suicidal ideation and behavior, including completed suicides, have occurred in patients treated with Siliq during clinical trials. Siliq users with a history of suicidality or depression had an increased incidence of suicidal ideation and behavior, compared to users without this history.”
At a meeting in July 2016, the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee voted 18-0 in favor of approving brodalumab, with the majority (14) recommending risk management options beyond labeling to address these concerns.
Elements of the REMS include requirements for prescribers, pharmacy certification, and a medication guide for patients with information about the risks of suicidal ideation and behavior. Prescribers are required to counsel patients about this risk, and patients are required to sign a “Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety or other mood changes,” the FDA said. The prescribing information also includes a boxed warning about suicidal ideation and behavior.
The most common adverse events associated with brodalumab, the FDA statement noted, include arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, injection site reactions, neutropenia, and fungal infections. The recommended dose of brodalumab is 210 mg, administered subcutaneously, at weeks 0, 1, and 2, followed by 210 mg every 2 weeks.
The Food and Drug Administration has approved brodalumab, an interleukin-17 receptor A-antagonist, for treating adults with moderate to severe plaque psoriasis, with a Risk Evaluation and Mitigation Strategy (REMS) that addresses the “observed risk of suicidal ideation and behavior” in clinical trials, the agency announced on Feb. 16.
In the three pivotal phase III studies of 4,373 adults with moderate to severe plaque psoriasis who were candidates for systemic therapy or phototherapy, 83%-86% of those treated with brodalumab achieved Psoriasis Area and Severity Index (PASI 75) scores at 12 weeks of treatment, compared with 3%-8% of those on placebo. In addition, 37%-44% of those on brodalumab achieved PASI 100 scores, compared with 1% or fewer of those on placebo. In the psoriasis clinical trials, suicidal ideation and behavior, which included four completed suicides, occurred in patients treated with brodalumab, according to the prescribing information
In the statement, the FDA points out that “a causal association between treatment with Siliq and increased risk of suicidal ideation and behavior has not been established,” but that “suicidal ideation and behavior, including completed suicides, have occurred in patients treated with Siliq during clinical trials. Siliq users with a history of suicidality or depression had an increased incidence of suicidal ideation and behavior, compared to users without this history.”
At a meeting in July 2016, the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee voted 18-0 in favor of approving brodalumab, with the majority (14) recommending risk management options beyond labeling to address these concerns.
Elements of the REMS include requirements for prescribers, pharmacy certification, and a medication guide for patients with information about the risks of suicidal ideation and behavior. Prescribers are required to counsel patients about this risk, and patients are required to sign a “Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety or other mood changes,” the FDA said. The prescribing information also includes a boxed warning about suicidal ideation and behavior.
The most common adverse events associated with brodalumab, the FDA statement noted, include arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, injection site reactions, neutropenia, and fungal infections. The recommended dose of brodalumab is 210 mg, administered subcutaneously, at weeks 0, 1, and 2, followed by 210 mg every 2 weeks.
Subcutaneous high-dose methotrexate controls psoriasis
Subcutaneous high-dose methotrexate can be safely initiated in people with moderate to severe psoriasis, and produces a rapid and sustained response, researchers found.
Although methotrexate is a first-line agent in moderate to severe psoriasis, and is considerably cheaper than biological agents, much remains unknown about its ideal dosage and route of administration.
Authors of a 2016 systematic review noted that, despite the fact that methotrexate has been used for more than 50 years in psoriasis, high-quality trial evidence remains wanting (PLoS One 2016 May 11. doi: 10.1371/journal.pone.0153740). Recent, well-designed trials have compared methotrexate to biological drugs used in psoriasis rather than placebo. These studies also have used oral formulations of methotrexate, in a range of starting doses as low as 5 mg, rather than subcutaneous formulations.
In their 52-week, multicenter trial conducted across 13 study sites in Europe, Dr. Warren and his colleagues randomized 120 patients to subcutaneous methotrexate at a dose of 17.5 mg/week (n = 91) or sham injections (n = 29) for 16 weeks. Patients in the intervention arm who did not achieve at least 50% improvement on the baseline Psoriasis Area and Severity Index (PASI) score at 8 weeks were increased to 22.5 mg methotrexate per week; 31% received this dose increase.
The study’s primary endpoint was reduction of the PASI score by 75% or more at 16 weeks, which 41% of the intervention arm achieved, compared with 10% of patients in the placebo arm (relative risk 3.93, P = .0026). After 16 weeks, all patients in the cohort were converted to open-label methotrexate for the remainder of the trial, following the same dosing schedule of between 17.5 and 22.5 mg, depending on response at 8 weeks after initiation.
At week 52, PASI 75 response rates were 45% in the methotrexate-methotrexate group and 34% in the placebo-methotrexate group. This compared favorably, the researchers wrote, with a previous study in which the PASI 75 response rate at week 52 was 24% with oral methotrexate at doses of up to 25 mg per week.
No serious adverse events were associated with methotrexate, although gastrointestinal problems (mostly nausea) and elevated liver enzymes were more common in patients receiving the treatment.
“Our findings encourage the use of subcutaneous methotrexate for treatment of psoriasis, and suggest long-term clinical outcomes better than previously reported for oral administration, although final confirmation will be needed in a direct head-to-head trial of subcutaneous versus oral dosing. Our findings might also help to guide future recommendations for the optimum dosing of methotrexate,” the investigators wrote.
Medac Pharma funded the study. Dr. Warren and six of his coauthors disclosed financial relationships with multiple pharmaceutical firms, including the study sponsor, while three coauthors declared no financial conflicts of interest.
The results from this study compare favorably with those of a previous 52-week study of oral methotrexate in this population, suggesting that subcutaneous administration is superior to oral administration in the management of psoriasis. However, response rates for methotrexate are still lower than those reported with biological therapy, especially with infliximab, adalimumab, ustekinumab, and, more recently, the anti-interleukin-17 drugs secukinumab and ixekizumab.
The question that remains is whether methotrexate should remain the first-line systemic therapy for moderate to severe psoriasis. Because we now know that psoriasis is not just skin deep, and that many of the comorbidities – including psoriatic arthritis, metabolic syndrome, and cardiovascular events, in addition to premature death – are related to the extent of skin involvement, perhaps drugs that effectively control inflammation should be used initially. This approach could be addressed only via long-term observations of prospective studies of patients treated with methotrexate, compared with those treated with biological therapy, with collection of information not only about clinical improvement of skin disease, but also about comorbidities.
Dafna D. Gladman, MD, is director of the psoriatic arthritis program at the Centre for Prognosis Studies in The Rheumatic Diseases at Toronto Western Hospital. This comment was excerpted and modified from an editorial (Lancet. 2017;389[10068]:482-3). that accompanied the study by Warren et al. Dr. Gladman disclosed financial relationships, mostly grants and fees related to clinical trials, with several pharmaceutical manufacturers.
The results from this study compare favorably with those of a previous 52-week study of oral methotrexate in this population, suggesting that subcutaneous administration is superior to oral administration in the management of psoriasis. However, response rates for methotrexate are still lower than those reported with biological therapy, especially with infliximab, adalimumab, ustekinumab, and, more recently, the anti-interleukin-17 drugs secukinumab and ixekizumab.
The question that remains is whether methotrexate should remain the first-line systemic therapy for moderate to severe psoriasis. Because we now know that psoriasis is not just skin deep, and that many of the comorbidities – including psoriatic arthritis, metabolic syndrome, and cardiovascular events, in addition to premature death – are related to the extent of skin involvement, perhaps drugs that effectively control inflammation should be used initially. This approach could be addressed only via long-term observations of prospective studies of patients treated with methotrexate, compared with those treated with biological therapy, with collection of information not only about clinical improvement of skin disease, but also about comorbidities.
Dafna D. Gladman, MD, is director of the psoriatic arthritis program at the Centre for Prognosis Studies in The Rheumatic Diseases at Toronto Western Hospital. This comment was excerpted and modified from an editorial (Lancet. 2017;389[10068]:482-3). that accompanied the study by Warren et al. Dr. Gladman disclosed financial relationships, mostly grants and fees related to clinical trials, with several pharmaceutical manufacturers.
The results from this study compare favorably with those of a previous 52-week study of oral methotrexate in this population, suggesting that subcutaneous administration is superior to oral administration in the management of psoriasis. However, response rates for methotrexate are still lower than those reported with biological therapy, especially with infliximab, adalimumab, ustekinumab, and, more recently, the anti-interleukin-17 drugs secukinumab and ixekizumab.
The question that remains is whether methotrexate should remain the first-line systemic therapy for moderate to severe psoriasis. Because we now know that psoriasis is not just skin deep, and that many of the comorbidities – including psoriatic arthritis, metabolic syndrome, and cardiovascular events, in addition to premature death – are related to the extent of skin involvement, perhaps drugs that effectively control inflammation should be used initially. This approach could be addressed only via long-term observations of prospective studies of patients treated with methotrexate, compared with those treated with biological therapy, with collection of information not only about clinical improvement of skin disease, but also about comorbidities.
Dafna D. Gladman, MD, is director of the psoriatic arthritis program at the Centre for Prognosis Studies in The Rheumatic Diseases at Toronto Western Hospital. This comment was excerpted and modified from an editorial (Lancet. 2017;389[10068]:482-3). that accompanied the study by Warren et al. Dr. Gladman disclosed financial relationships, mostly grants and fees related to clinical trials, with several pharmaceutical manufacturers.
Subcutaneous high-dose methotrexate can be safely initiated in people with moderate to severe psoriasis, and produces a rapid and sustained response, researchers found.
Although methotrexate is a first-line agent in moderate to severe psoriasis, and is considerably cheaper than biological agents, much remains unknown about its ideal dosage and route of administration.
Authors of a 2016 systematic review noted that, despite the fact that methotrexate has been used for more than 50 years in psoriasis, high-quality trial evidence remains wanting (PLoS One 2016 May 11. doi: 10.1371/journal.pone.0153740). Recent, well-designed trials have compared methotrexate to biological drugs used in psoriasis rather than placebo. These studies also have used oral formulations of methotrexate, in a range of starting doses as low as 5 mg, rather than subcutaneous formulations.
In their 52-week, multicenter trial conducted across 13 study sites in Europe, Dr. Warren and his colleagues randomized 120 patients to subcutaneous methotrexate at a dose of 17.5 mg/week (n = 91) or sham injections (n = 29) for 16 weeks. Patients in the intervention arm who did not achieve at least 50% improvement on the baseline Psoriasis Area and Severity Index (PASI) score at 8 weeks were increased to 22.5 mg methotrexate per week; 31% received this dose increase.
The study’s primary endpoint was reduction of the PASI score by 75% or more at 16 weeks, which 41% of the intervention arm achieved, compared with 10% of patients in the placebo arm (relative risk 3.93, P = .0026). After 16 weeks, all patients in the cohort were converted to open-label methotrexate for the remainder of the trial, following the same dosing schedule of between 17.5 and 22.5 mg, depending on response at 8 weeks after initiation.
At week 52, PASI 75 response rates were 45% in the methotrexate-methotrexate group and 34% in the placebo-methotrexate group. This compared favorably, the researchers wrote, with a previous study in which the PASI 75 response rate at week 52 was 24% with oral methotrexate at doses of up to 25 mg per week.
No serious adverse events were associated with methotrexate, although gastrointestinal problems (mostly nausea) and elevated liver enzymes were more common in patients receiving the treatment.
“Our findings encourage the use of subcutaneous methotrexate for treatment of psoriasis, and suggest long-term clinical outcomes better than previously reported for oral administration, although final confirmation will be needed in a direct head-to-head trial of subcutaneous versus oral dosing. Our findings might also help to guide future recommendations for the optimum dosing of methotrexate,” the investigators wrote.
Medac Pharma funded the study. Dr. Warren and six of his coauthors disclosed financial relationships with multiple pharmaceutical firms, including the study sponsor, while three coauthors declared no financial conflicts of interest.
Subcutaneous high-dose methotrexate can be safely initiated in people with moderate to severe psoriasis, and produces a rapid and sustained response, researchers found.
Although methotrexate is a first-line agent in moderate to severe psoriasis, and is considerably cheaper than biological agents, much remains unknown about its ideal dosage and route of administration.
Authors of a 2016 systematic review noted that, despite the fact that methotrexate has been used for more than 50 years in psoriasis, high-quality trial evidence remains wanting (PLoS One 2016 May 11. doi: 10.1371/journal.pone.0153740). Recent, well-designed trials have compared methotrexate to biological drugs used in psoriasis rather than placebo. These studies also have used oral formulations of methotrexate, in a range of starting doses as low as 5 mg, rather than subcutaneous formulations.
In their 52-week, multicenter trial conducted across 13 study sites in Europe, Dr. Warren and his colleagues randomized 120 patients to subcutaneous methotrexate at a dose of 17.5 mg/week (n = 91) or sham injections (n = 29) for 16 weeks. Patients in the intervention arm who did not achieve at least 50% improvement on the baseline Psoriasis Area and Severity Index (PASI) score at 8 weeks were increased to 22.5 mg methotrexate per week; 31% received this dose increase.
The study’s primary endpoint was reduction of the PASI score by 75% or more at 16 weeks, which 41% of the intervention arm achieved, compared with 10% of patients in the placebo arm (relative risk 3.93, P = .0026). After 16 weeks, all patients in the cohort were converted to open-label methotrexate for the remainder of the trial, following the same dosing schedule of between 17.5 and 22.5 mg, depending on response at 8 weeks after initiation.
At week 52, PASI 75 response rates were 45% in the methotrexate-methotrexate group and 34% in the placebo-methotrexate group. This compared favorably, the researchers wrote, with a previous study in which the PASI 75 response rate at week 52 was 24% with oral methotrexate at doses of up to 25 mg per week.
No serious adverse events were associated with methotrexate, although gastrointestinal problems (mostly nausea) and elevated liver enzymes were more common in patients receiving the treatment.
“Our findings encourage the use of subcutaneous methotrexate for treatment of psoriasis, and suggest long-term clinical outcomes better than previously reported for oral administration, although final confirmation will be needed in a direct head-to-head trial of subcutaneous versus oral dosing. Our findings might also help to guide future recommendations for the optimum dosing of methotrexate,” the investigators wrote.
Medac Pharma funded the study. Dr. Warren and six of his coauthors disclosed financial relationships with multiple pharmaceutical firms, including the study sponsor, while three coauthors declared no financial conflicts of interest.
FROM THE LANCET
Key clinical point:
Major finding: At 16 weeks, 41% of patients started on methotrexate achieved a 75% reduction in psoriasis severity scores, compared with 10% of patients in the placebo arm. At 1 year of treatment, 45% of patients saw this level of response.
Data source: A multisite, placebo-controlled trial randomizing 120 patients to subcutaneous methotrexate or placebo for 16 weeks, then converting all patients to open-label subcutaneous methotrexate through week 52.
Disclosures: Medac Pharma funded the study. Dr. Warren and six of his coauthors disclosed financial relationships with multiple pharmaceutical firms, including the study sponsor, while three coauthors declared no financial conflicts of interest.