User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
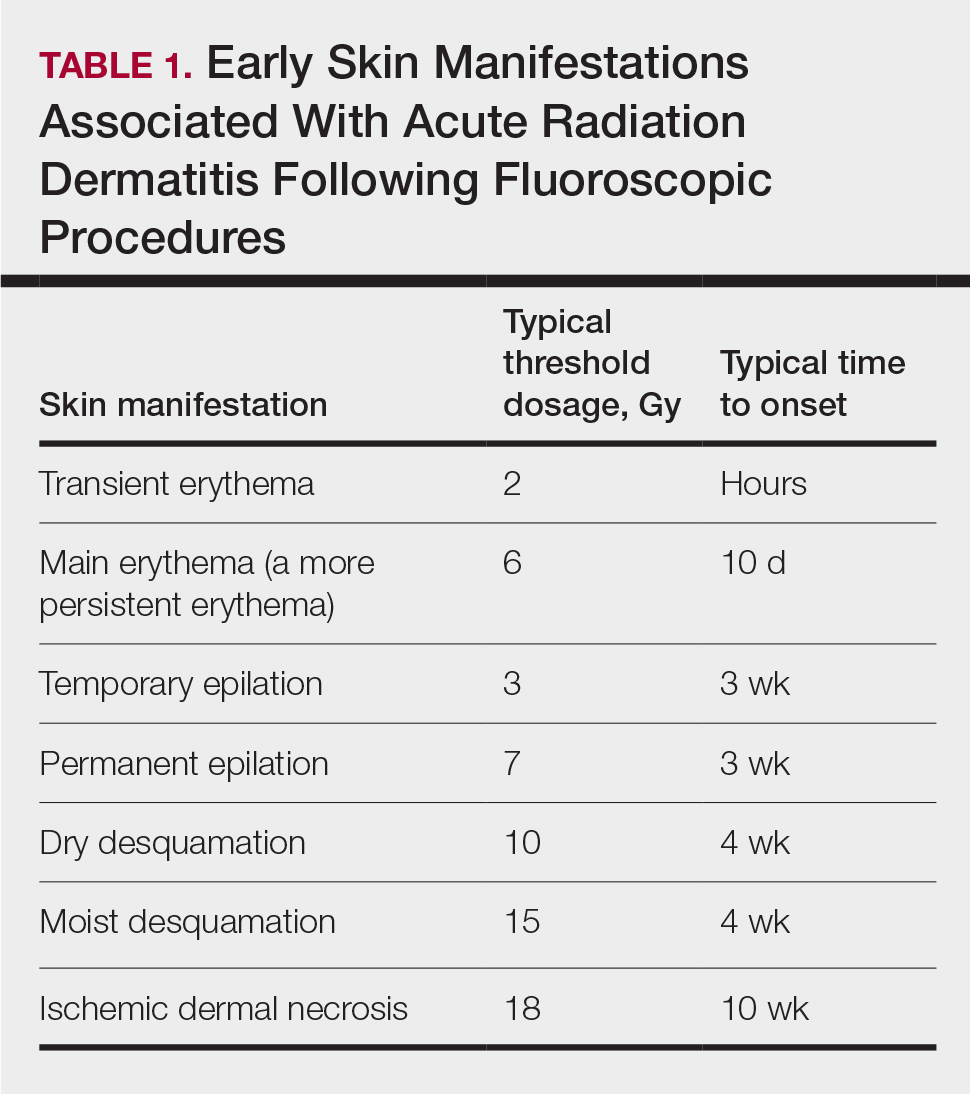
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).
Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
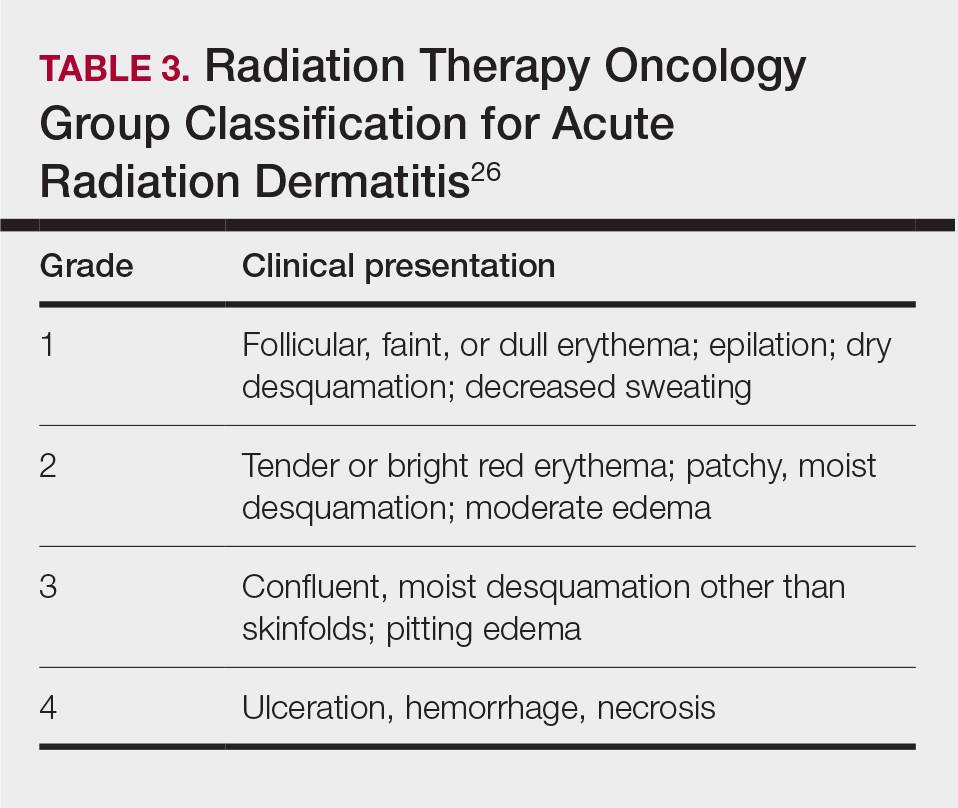
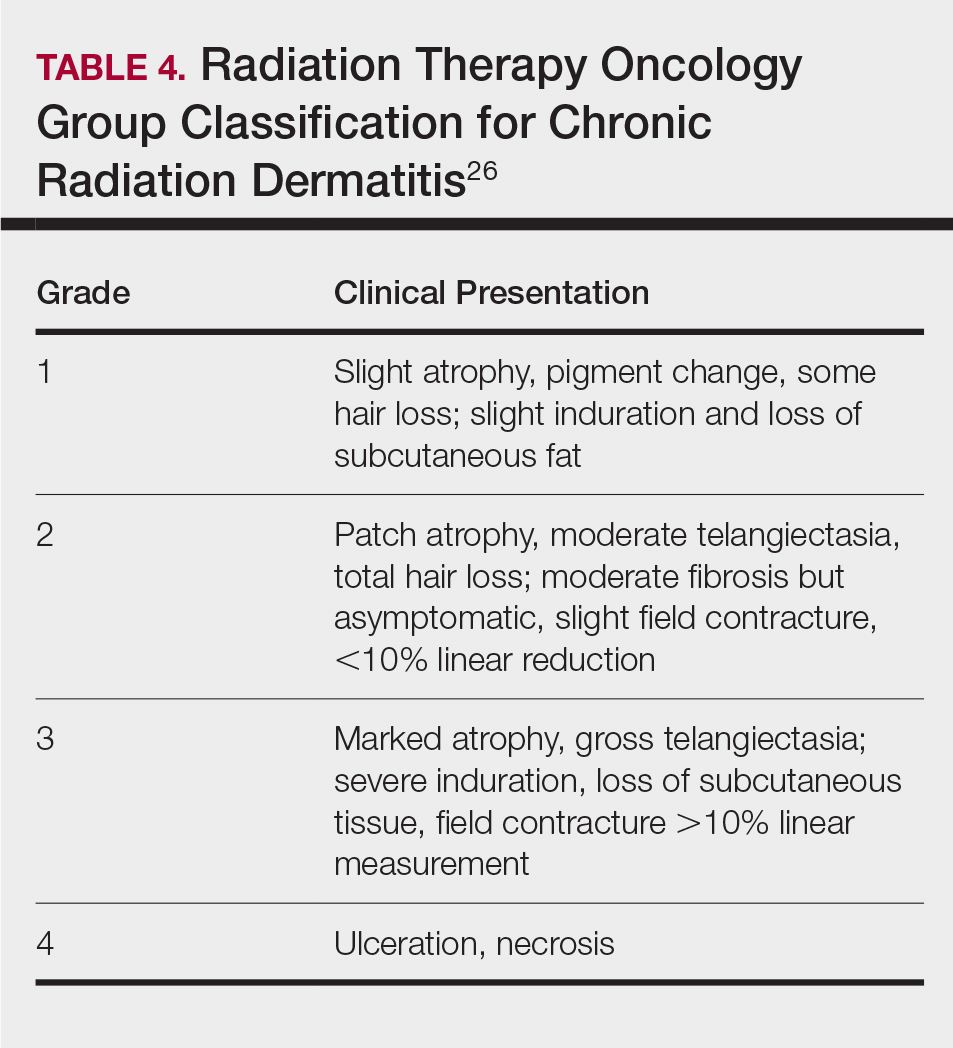
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27

Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45
Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
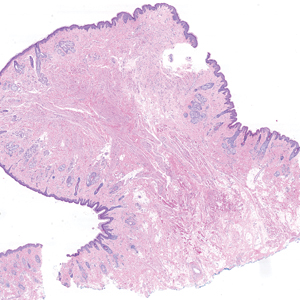
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
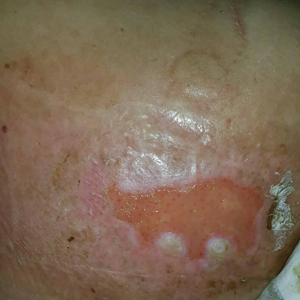
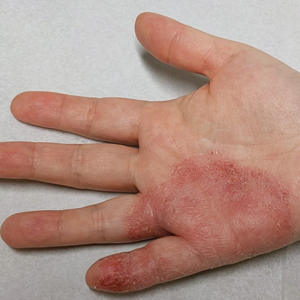
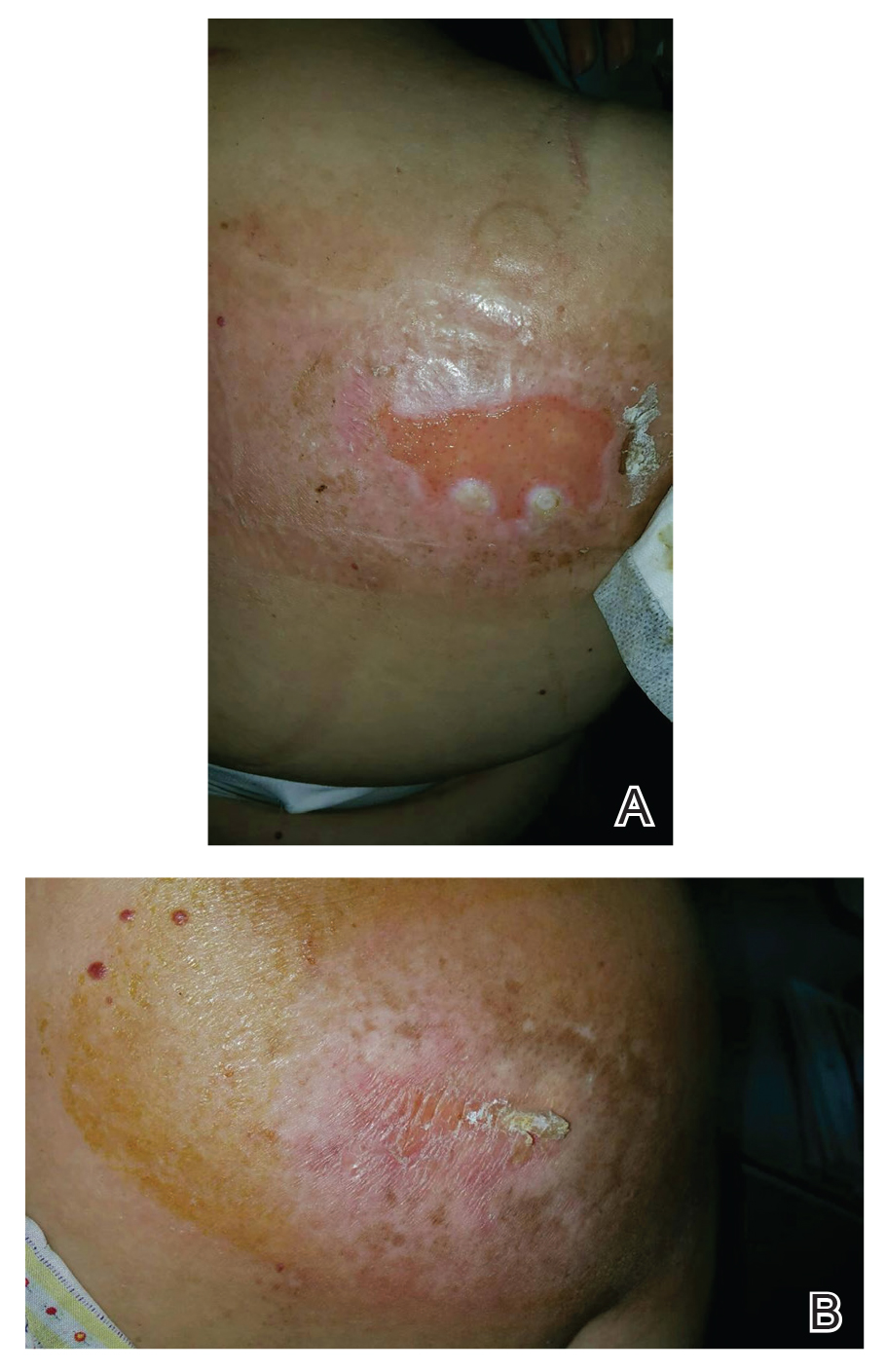
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
PRACTICE POINTS
- Fluoroscopy-induced chronic radiation dermatitis poses diagnostic challenges, as patients often are unable to associate a history of fluoroscopic procedures with the development of skin lesions.
- Scapular and subscapular lesions as well as those on the anterolateral chest and mid back should prompt clinicians to inquire about the patient’s history of fluoroscopic procedures.
- Because lesions can remain refractory to treatment, longterm monitoring is necessary if they are not excised.
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).
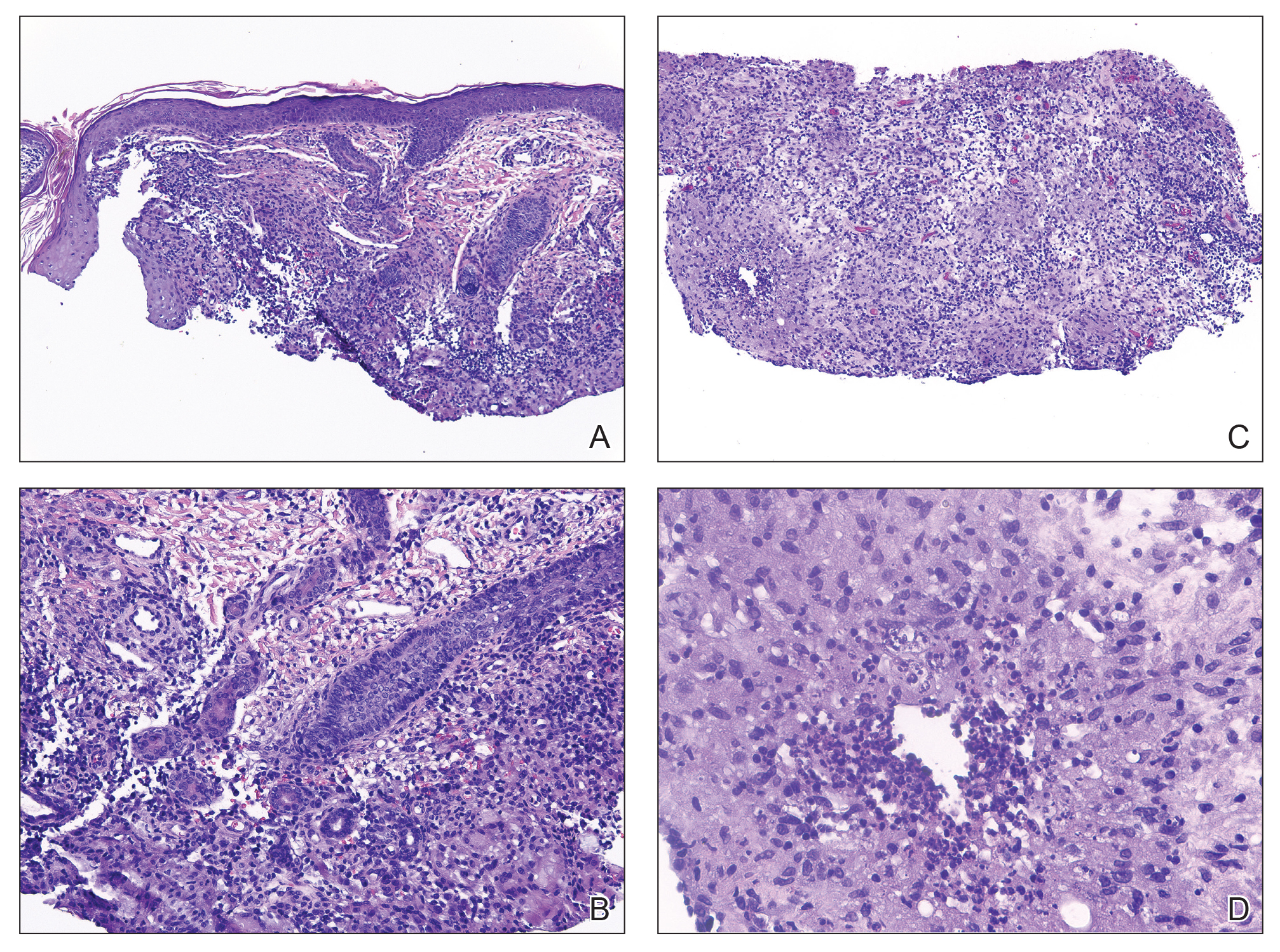
First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
A 4-year-old girl presented to the dermatology clinic with a painless, red to golden-yellowish nodule on the right lower eyelid of 4 months’ duration. The patient had no history of skin disease and was otherwise healthy. Physical examination revealed a single 1-cm, soft, erythematous and yellowish plaque on the right lower eyelid that was subtly fluctuant on palpation. She had no associated systemic symptoms or lymphadenopathy. A punch biopsy of the lesion was performed.
Exophytic Papule on the Chin of a Child
Exophytic Papule on the Chin of a Child
THE DIAGNOSIS: Rhabdomyomatous Mesenchymal Hamartoma
Histopathologic examination of the excised tissue revealed haphazardly arranged bundles of mature striated muscle within the dermis and subcutaneous tissue admixed with adipose tissue, adnexal structures, blood vessels, and nerves. The presence of the lesion since birth, midline clinical presentation, and histologic findings were consistent with a diagnosis of rhabdomyomatous mesenchymal hamartoma (RMH).
Also referred to as striated muscle hamartoma, RMH is a rare benign lesion thought to have embryonic origin due to its midline presentation.1 The etiology of RMH is unknown but is hypothesized to be due to abnormal migration or growth of embryonic mesenchymal tissue. Rhabdomyomatous mesenchymal hamartoma typically manifests in infancy or early childhood as a solitary midline papule on the head or neck, although there have been rare reports of development in adulthood.2-4 Lesions often are polypoid or exophytic but may manifest as smooth papules or subcutaneous nodules.2 Although benign, RMH may be associated with other congenital abnormalities and conditions, such as Delleman syndrome, which is caused by a sporadic genetic abnormality and results in defects of the eye, central nervous system, and skin.5 Treatment for RMH is not needed, but surgical excision for cosmetic purposes can be performed with low risk for recurrence. Histologically, RMH demonstrates a normal epidermis overlying disorganized bundles of skeletal muscle accompanied by varying amounts of other mature dermal and subcutaneous tissues including nerves, blood vessels, adipose tissue, and other adnexal structures.2,6 Myoglobin and desmin are positive within the skeletal muscle bundles.7
Fibrous hamartoma of infancy (FHI) often manifests as a movable, ill-defined nodule within the subcutaneous tissue. While also occurring in young children—typically within the first 2 years of life—FHI primarily is found on the upper arms, back, and axillae, in contrast to FHI.8 The classic histopathologic presentation of FHI consists of a triphasic morphology consisting of undifferentiated mesenchymal cells and dense fibroblastic/myofibroblastic tissue with mature adipose tissue woven throughout in islands (Figure 1).9 Skeletal muscle is not a component of this tumor.
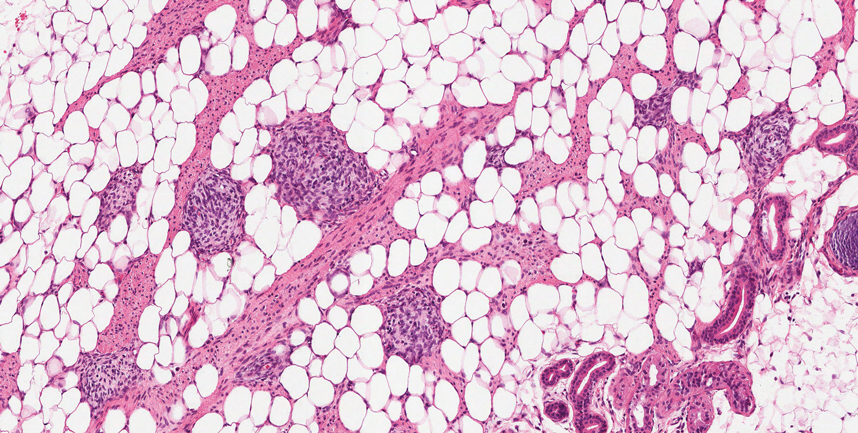
Neurofibromas also may manifest clinically as papules or nodules and arise from the peripheral nerve sheath. There are 3 major subtypes of neurofibromas—localized, diffuse, and plexiform—with the last being strongly associated with neurofibromatosis type 1.10 The plexiform type has a rare risk for malignant transformation. The typical histopathologic finding of a localized cutaneous neurofibroma is a dermal proliferation of spindle cells with wavy nuclei within a variably myxoid stroma (Figure 2).11 Interspersed mast cells also can be seen. A plexiform neurofibroma typically involves multiple nerve fascicles and comprises multinodular or tortuous bundles of cytologically bland spindle cells. Compared to RMH, skeletal muscle is not a component of this tumor.
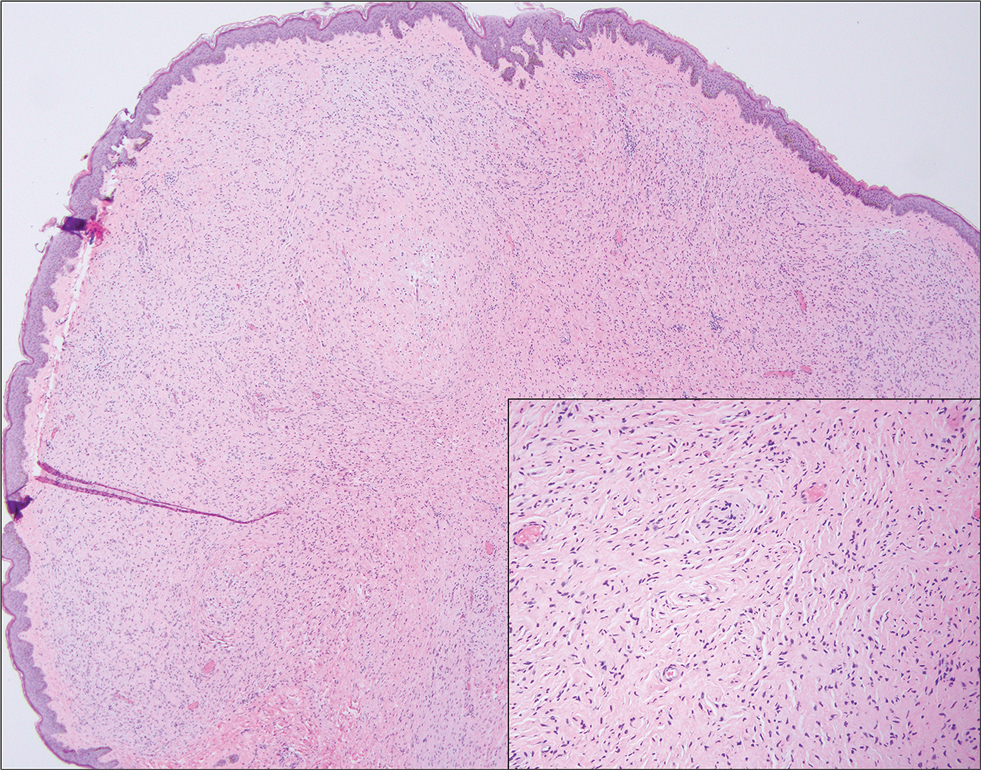
Nevus lipomatosus superficialis is a benign hamartoma that can manifest as a pedunculated or exophytic papule. The lesions may be solitary or multiple and, unlike RMH, are most common on the buttocks, upper thighs, and trunk.12 The histopathologic features of nevus lipomatosus superficialis include clusters of mature adipose tissue in the superficial dermis admixed with collagen fibers and variably increased vasculature (Figure 3).13 Nevus lipomatosus superficialis does not contain skeletal muscle within the tumor in comparison to RMH.
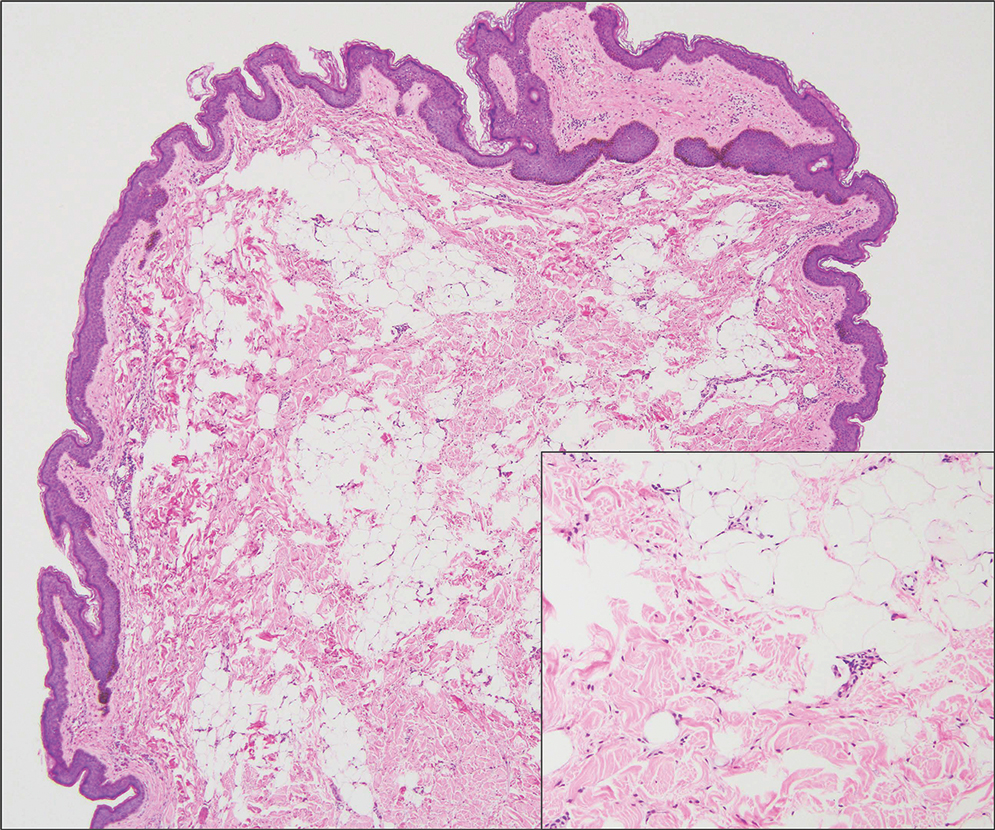
It is important to distinguish rhabdomyosarcoma (RMS) from RMH, as it is associated with increased mortality and morbidity. Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and is derived from mesenchyme with variable degrees of skeletal muscle differentiation.14 Due to its mesenchymal origin, these tumors can manifest in a variety of places but most commonly on the head and neck and in the genital region.15 The most common subtype is embryonal rhabdomyosarcoma. Histologically, embryonal RMS shows a moderately cellular tumor composed of sheets of spindle-shaped or round cells with scant or eosinophilic cytoplasm (Figure 4). The absence of genetic translocation in the paired box-forkhead box protein 01 (PAX-FOXO1) gene helps distinguish it from solid alveolar RMS, the second most common and more aggressive subtype.12 Positive immunohistochemical staining for desmin, myoblast determination protein 1 (MyoD1), and myogenin supports myogenic differentiation.14
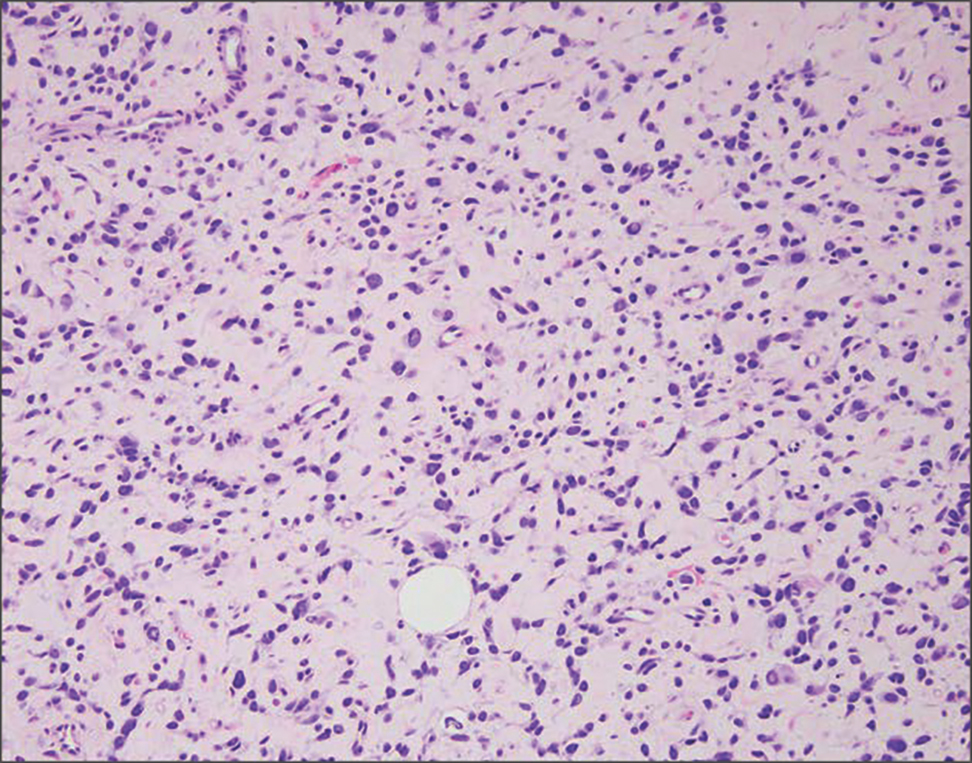
- Bernal-Mañas CM, Isaac-Montero MA, Vargas-Uribe MC, et al. Hamartoma mesenquimal rabdomiomatoso [rhabdomyomatous mesenchymal hamartoma]. An Pediatr (Barc). 2013;78:260-262. doi:10.1016/j.anpedi.2012.08.005
- Al Amri R, De Stefano DV, Wang Q, et al. Morphologic spectrum of rhabdomyomatous mesenchymal hamartomas (striated muscle hamartomas) in pediatric dermatopathology. Am J Dermatopathol. 2022;44:170-173. doi:10.1097/DAD.0000000000002062
- Carboni A, Fomin D. A rare adult presentation of a congenital tumor discovered incidentally after trauma. JAAD Case Rep. 2022;31:121-123. doi:10.1016/j.jdcr.2022.10.024
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005; 21(4):185-188. doi:10.1016/S1607-551X(09)70299-2
- Bahmani M, Naseri R, Iraniparast A, et al. Oculocerebrocutaneous syndrome (Delleman syndrome): a case with a novel presentation of orbital involvement. Case Rep Pediatr. 2021;2021:5524131. doi:10.1155/2021/5524131
- Kim H, Chung JH, Sung HM, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a midline mass on a chin. Arch Craniofac Surg. 2017;18:292-295. doi:10.7181/acfs.2017.18.4.292.
- Lin CP, Nguyen JM, Aboutalebi S, et al. Incidental rhabdomyomatous mesenchymal hamartoma. Proc (Bayl Univ Med Cent). 2020;34:161-162. doi:10.1080/08998280.2020.1801087
- Ji Y, Hu P, Zhang C, et al. Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord. 2019;20:356. doi:10.1186/s12891-019-2743-5
- Yu G, Wang Y, Wang G, et al. Fibrous hamartoma of infancy: a clinical pathological analysis of seventeen cases. Int J Clin Exp Pathol. 2015;8:3374-3377.
- Ferner RE, O’Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679-684. doi:10.1097/01.wco.0000044763.39452.aa
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. doi:10.1016/j.humpath.2017.05.010
- Kim RH, Stevenson ML, Hale CS, et al. Nevus lipomatosus superficialis. Dermatol Online J. 2014;20:13030/qt2cb3c5t3.
- Singh P, Anandani GM. Nevus lipomatosus superficialis, an unusual case report. J Family Med Prim Care. 2022;11:4045-4047. doi:10.4103/jfmpc.jfmpc_2352_21
- Shern JF, Yohe ME, Khan J. Pediatric rhabdomyosarcoma. Crit Rev Oncog. 2015;20:227-243. doi:10.1615/critrevoncog.2015013800
- Rogers TN, Dasgupta R. Management of rhabdomyosarcoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:339-353. doi:10.1016/j.soc.2020.11.003
- Machado I, Mayordomo-Aranda E, Giner F, et al. The role of immunohistochemistry in rhabdomyosarcoma diagnosis using tissue microarray technology and a xenograft model. Fetal Pediatr Pathol. 2015;34:271-281. doi:10.3109/15513815.2015.1042604
THE DIAGNOSIS: Rhabdomyomatous Mesenchymal Hamartoma
Histopathologic examination of the excised tissue revealed haphazardly arranged bundles of mature striated muscle within the dermis and subcutaneous tissue admixed with adipose tissue, adnexal structures, blood vessels, and nerves. The presence of the lesion since birth, midline clinical presentation, and histologic findings were consistent with a diagnosis of rhabdomyomatous mesenchymal hamartoma (RMH).
Also referred to as striated muscle hamartoma, RMH is a rare benign lesion thought to have embryonic origin due to its midline presentation.1 The etiology of RMH is unknown but is hypothesized to be due to abnormal migration or growth of embryonic mesenchymal tissue. Rhabdomyomatous mesenchymal hamartoma typically manifests in infancy or early childhood as a solitary midline papule on the head or neck, although there have been rare reports of development in adulthood.2-4 Lesions often are polypoid or exophytic but may manifest as smooth papules or subcutaneous nodules.2 Although benign, RMH may be associated with other congenital abnormalities and conditions, such as Delleman syndrome, which is caused by a sporadic genetic abnormality and results in defects of the eye, central nervous system, and skin.5 Treatment for RMH is not needed, but surgical excision for cosmetic purposes can be performed with low risk for recurrence. Histologically, RMH demonstrates a normal epidermis overlying disorganized bundles of skeletal muscle accompanied by varying amounts of other mature dermal and subcutaneous tissues including nerves, blood vessels, adipose tissue, and other adnexal structures.2,6 Myoglobin and desmin are positive within the skeletal muscle bundles.7
Fibrous hamartoma of infancy (FHI) often manifests as a movable, ill-defined nodule within the subcutaneous tissue. While also occurring in young children—typically within the first 2 years of life—FHI primarily is found on the upper arms, back, and axillae, in contrast to FHI.8 The classic histopathologic presentation of FHI consists of a triphasic morphology consisting of undifferentiated mesenchymal cells and dense fibroblastic/myofibroblastic tissue with mature adipose tissue woven throughout in islands (Figure 1).9 Skeletal muscle is not a component of this tumor.

Neurofibromas also may manifest clinically as papules or nodules and arise from the peripheral nerve sheath. There are 3 major subtypes of neurofibromas—localized, diffuse, and plexiform—with the last being strongly associated with neurofibromatosis type 1.10 The plexiform type has a rare risk for malignant transformation. The typical histopathologic finding of a localized cutaneous neurofibroma is a dermal proliferation of spindle cells with wavy nuclei within a variably myxoid stroma (Figure 2).11 Interspersed mast cells also can be seen. A plexiform neurofibroma typically involves multiple nerve fascicles and comprises multinodular or tortuous bundles of cytologically bland spindle cells. Compared to RMH, skeletal muscle is not a component of this tumor.

Nevus lipomatosus superficialis is a benign hamartoma that can manifest as a pedunculated or exophytic papule. The lesions may be solitary or multiple and, unlike RMH, are most common on the buttocks, upper thighs, and trunk.12 The histopathologic features of nevus lipomatosus superficialis include clusters of mature adipose tissue in the superficial dermis admixed with collagen fibers and variably increased vasculature (Figure 3).13 Nevus lipomatosus superficialis does not contain skeletal muscle within the tumor in comparison to RMH.

It is important to distinguish rhabdomyosarcoma (RMS) from RMH, as it is associated with increased mortality and morbidity. Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and is derived from mesenchyme with variable degrees of skeletal muscle differentiation.14 Due to its mesenchymal origin, these tumors can manifest in a variety of places but most commonly on the head and neck and in the genital region.15 The most common subtype is embryonal rhabdomyosarcoma. Histologically, embryonal RMS shows a moderately cellular tumor composed of sheets of spindle-shaped or round cells with scant or eosinophilic cytoplasm (Figure 4). The absence of genetic translocation in the paired box-forkhead box protein 01 (PAX-FOXO1) gene helps distinguish it from solid alveolar RMS, the second most common and more aggressive subtype.12 Positive immunohistochemical staining for desmin, myoblast determination protein 1 (MyoD1), and myogenin supports myogenic differentiation.14

THE DIAGNOSIS: Rhabdomyomatous Mesenchymal Hamartoma
Histopathologic examination of the excised tissue revealed haphazardly arranged bundles of mature striated muscle within the dermis and subcutaneous tissue admixed with adipose tissue, adnexal structures, blood vessels, and nerves. The presence of the lesion since birth, midline clinical presentation, and histologic findings were consistent with a diagnosis of rhabdomyomatous mesenchymal hamartoma (RMH).
Also referred to as striated muscle hamartoma, RMH is a rare benign lesion thought to have embryonic origin due to its midline presentation.1 The etiology of RMH is unknown but is hypothesized to be due to abnormal migration or growth of embryonic mesenchymal tissue. Rhabdomyomatous mesenchymal hamartoma typically manifests in infancy or early childhood as a solitary midline papule on the head or neck, although there have been rare reports of development in adulthood.2-4 Lesions often are polypoid or exophytic but may manifest as smooth papules or subcutaneous nodules.2 Although benign, RMH may be associated with other congenital abnormalities and conditions, such as Delleman syndrome, which is caused by a sporadic genetic abnormality and results in defects of the eye, central nervous system, and skin.5 Treatment for RMH is not needed, but surgical excision for cosmetic purposes can be performed with low risk for recurrence. Histologically, RMH demonstrates a normal epidermis overlying disorganized bundles of skeletal muscle accompanied by varying amounts of other mature dermal and subcutaneous tissues including nerves, blood vessels, adipose tissue, and other adnexal structures.2,6 Myoglobin and desmin are positive within the skeletal muscle bundles.7
Fibrous hamartoma of infancy (FHI) often manifests as a movable, ill-defined nodule within the subcutaneous tissue. While also occurring in young children—typically within the first 2 years of life—FHI primarily is found on the upper arms, back, and axillae, in contrast to FHI.8 The classic histopathologic presentation of FHI consists of a triphasic morphology consisting of undifferentiated mesenchymal cells and dense fibroblastic/myofibroblastic tissue with mature adipose tissue woven throughout in islands (Figure 1).9 Skeletal muscle is not a component of this tumor.

Neurofibromas also may manifest clinically as papules or nodules and arise from the peripheral nerve sheath. There are 3 major subtypes of neurofibromas—localized, diffuse, and plexiform—with the last being strongly associated with neurofibromatosis type 1.10 The plexiform type has a rare risk for malignant transformation. The typical histopathologic finding of a localized cutaneous neurofibroma is a dermal proliferation of spindle cells with wavy nuclei within a variably myxoid stroma (Figure 2).11 Interspersed mast cells also can be seen. A plexiform neurofibroma typically involves multiple nerve fascicles and comprises multinodular or tortuous bundles of cytologically bland spindle cells. Compared to RMH, skeletal muscle is not a component of this tumor.

Nevus lipomatosus superficialis is a benign hamartoma that can manifest as a pedunculated or exophytic papule. The lesions may be solitary or multiple and, unlike RMH, are most common on the buttocks, upper thighs, and trunk.12 The histopathologic features of nevus lipomatosus superficialis include clusters of mature adipose tissue in the superficial dermis admixed with collagen fibers and variably increased vasculature (Figure 3).13 Nevus lipomatosus superficialis does not contain skeletal muscle within the tumor in comparison to RMH.

It is important to distinguish rhabdomyosarcoma (RMS) from RMH, as it is associated with increased mortality and morbidity. Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and is derived from mesenchyme with variable degrees of skeletal muscle differentiation.14 Due to its mesenchymal origin, these tumors can manifest in a variety of places but most commonly on the head and neck and in the genital region.15 The most common subtype is embryonal rhabdomyosarcoma. Histologically, embryonal RMS shows a moderately cellular tumor composed of sheets of spindle-shaped or round cells with scant or eosinophilic cytoplasm (Figure 4). The absence of genetic translocation in the paired box-forkhead box protein 01 (PAX-FOXO1) gene helps distinguish it from solid alveolar RMS, the second most common and more aggressive subtype.12 Positive immunohistochemical staining for desmin, myoblast determination protein 1 (MyoD1), and myogenin supports myogenic differentiation.14

- Bernal-Mañas CM, Isaac-Montero MA, Vargas-Uribe MC, et al. Hamartoma mesenquimal rabdomiomatoso [rhabdomyomatous mesenchymal hamartoma]. An Pediatr (Barc). 2013;78:260-262. doi:10.1016/j.anpedi.2012.08.005
- Al Amri R, De Stefano DV, Wang Q, et al. Morphologic spectrum of rhabdomyomatous mesenchymal hamartomas (striated muscle hamartomas) in pediatric dermatopathology. Am J Dermatopathol. 2022;44:170-173. doi:10.1097/DAD.0000000000002062
- Carboni A, Fomin D. A rare adult presentation of a congenital tumor discovered incidentally after trauma. JAAD Case Rep. 2022;31:121-123. doi:10.1016/j.jdcr.2022.10.024
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005; 21(4):185-188. doi:10.1016/S1607-551X(09)70299-2
- Bahmani M, Naseri R, Iraniparast A, et al. Oculocerebrocutaneous syndrome (Delleman syndrome): a case with a novel presentation of orbital involvement. Case Rep Pediatr. 2021;2021:5524131. doi:10.1155/2021/5524131
- Kim H, Chung JH, Sung HM, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a midline mass on a chin. Arch Craniofac Surg. 2017;18:292-295. doi:10.7181/acfs.2017.18.4.292.
- Lin CP, Nguyen JM, Aboutalebi S, et al. Incidental rhabdomyomatous mesenchymal hamartoma. Proc (Bayl Univ Med Cent). 2020;34:161-162. doi:10.1080/08998280.2020.1801087
- Ji Y, Hu P, Zhang C, et al. Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord. 2019;20:356. doi:10.1186/s12891-019-2743-5
- Yu G, Wang Y, Wang G, et al. Fibrous hamartoma of infancy: a clinical pathological analysis of seventeen cases. Int J Clin Exp Pathol. 2015;8:3374-3377.
- Ferner RE, O’Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679-684. doi:10.1097/01.wco.0000044763.39452.aa
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. doi:10.1016/j.humpath.2017.05.010
- Kim RH, Stevenson ML, Hale CS, et al. Nevus lipomatosus superficialis. Dermatol Online J. 2014;20:13030/qt2cb3c5t3.
- Singh P, Anandani GM. Nevus lipomatosus superficialis, an unusual case report. J Family Med Prim Care. 2022;11:4045-4047. doi:10.4103/jfmpc.jfmpc_2352_21
- Shern JF, Yohe ME, Khan J. Pediatric rhabdomyosarcoma. Crit Rev Oncog. 2015;20:227-243. doi:10.1615/critrevoncog.2015013800
- Rogers TN, Dasgupta R. Management of rhabdomyosarcoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:339-353. doi:10.1016/j.soc.2020.11.003
- Machado I, Mayordomo-Aranda E, Giner F, et al. The role of immunohistochemistry in rhabdomyosarcoma diagnosis using tissue microarray technology and a xenograft model. Fetal Pediatr Pathol. 2015;34:271-281. doi:10.3109/15513815.2015.1042604
- Bernal-Mañas CM, Isaac-Montero MA, Vargas-Uribe MC, et al. Hamartoma mesenquimal rabdomiomatoso [rhabdomyomatous mesenchymal hamartoma]. An Pediatr (Barc). 2013;78:260-262. doi:10.1016/j.anpedi.2012.08.005
- Al Amri R, De Stefano DV, Wang Q, et al. Morphologic spectrum of rhabdomyomatous mesenchymal hamartomas (striated muscle hamartomas) in pediatric dermatopathology. Am J Dermatopathol. 2022;44:170-173. doi:10.1097/DAD.0000000000002062
- Carboni A, Fomin D. A rare adult presentation of a congenital tumor discovered incidentally after trauma. JAAD Case Rep. 2022;31:121-123. doi:10.1016/j.jdcr.2022.10.024
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005; 21(4):185-188. doi:10.1016/S1607-551X(09)70299-2
- Bahmani M, Naseri R, Iraniparast A, et al. Oculocerebrocutaneous syndrome (Delleman syndrome): a case with a novel presentation of orbital involvement. Case Rep Pediatr. 2021;2021:5524131. doi:10.1155/2021/5524131
- Kim H, Chung JH, Sung HM, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a midline mass on a chin. Arch Craniofac Surg. 2017;18:292-295. doi:10.7181/acfs.2017.18.4.292.
- Lin CP, Nguyen JM, Aboutalebi S, et al. Incidental rhabdomyomatous mesenchymal hamartoma. Proc (Bayl Univ Med Cent). 2020;34:161-162. doi:10.1080/08998280.2020.1801087
- Ji Y, Hu P, Zhang C, et al. Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord. 2019;20:356. doi:10.1186/s12891-019-2743-5
- Yu G, Wang Y, Wang G, et al. Fibrous hamartoma of infancy: a clinical pathological analysis of seventeen cases. Int J Clin Exp Pathol. 2015;8:3374-3377.
- Ferner RE, O’Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679-684. doi:10.1097/01.wco.0000044763.39452.aa
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. doi:10.1016/j.humpath.2017.05.010
- Kim RH, Stevenson ML, Hale CS, et al. Nevus lipomatosus superficialis. Dermatol Online J. 2014;20:13030/qt2cb3c5t3.
- Singh P, Anandani GM. Nevus lipomatosus superficialis, an unusual case report. J Family Med Prim Care. 2022;11:4045-4047. doi:10.4103/jfmpc.jfmpc_2352_21
- Shern JF, Yohe ME, Khan J. Pediatric rhabdomyosarcoma. Crit Rev Oncog. 2015;20:227-243. doi:10.1615/critrevoncog.2015013800
- Rogers TN, Dasgupta R. Management of rhabdomyosarcoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:339-353. doi:10.1016/j.soc.2020.11.003
- Machado I, Mayordomo-Aranda E, Giner F, et al. The role of immunohistochemistry in rhabdomyosarcoma diagnosis using tissue microarray technology and a xenograft model. Fetal Pediatr Pathol. 2015;34:271-281. doi:10.3109/15513815.2015.1042604
Exophytic Papule on the Chin of a Child
Exophytic Papule on the Chin of a Child
A 3-year-old boy presented to the dermatology department for evaluation of an asymptomatic papule on the chin that had been present since birth. His medical history was otherwise unremarkable. Physical examination revealed a 4×2-mm, flesh-colored, exophytic papule on the midline chin. An excisional biopsy was performed.
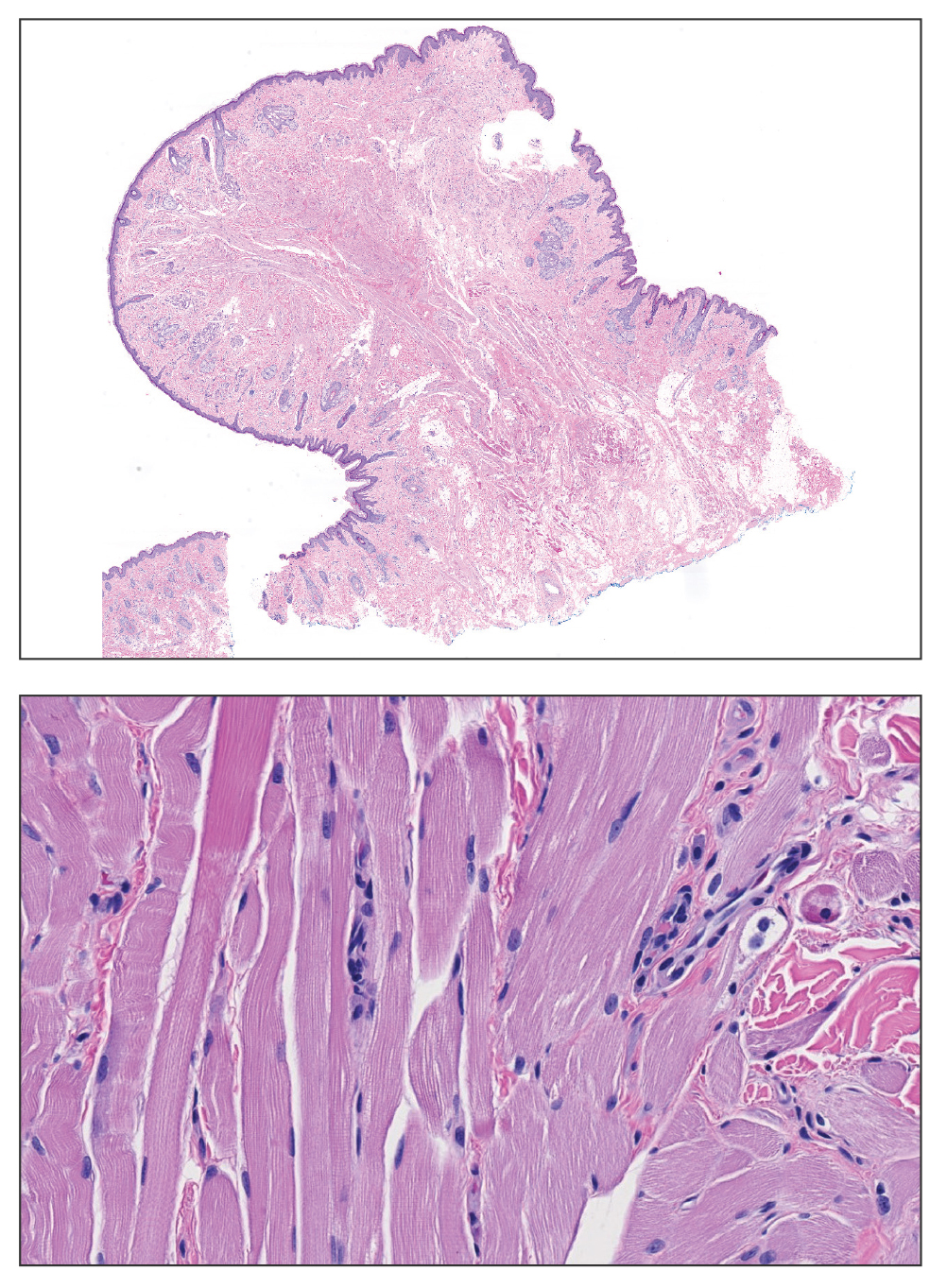
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
Cosmetic laser procedures as well as energy-based fat reduction and body-contouring devices are increasingly popular among individuals with skin of color (SOC). Innovations in cosmetic devices and procedures tailored for SOC have allowed for the optimization of outcomes in this patient population. In this article, SOC is defined as darker skin types, including Fitzpatrick skin types (FSTs) IV to VI and ethnic backgrounds such as LatinX, African American, Southeast Asian, Native American, Pacific Islander, Middle Eastern, Asian, and African. Indications for laser treatment include dermatosis papulosa nigrans (DPN), acne scars, skin rejuvenation, and hyperpigmentation. There currently are 6 procedures for nonsurgical fat reduction that are approved by the US Food and Drug Administration (FDA): high-frequency focused ultrasound, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring (Supplementary Table S1).1
In this review, our initial focus is cosmetic laser procedures, encompassing FDA-cleared indications along with the associated risks and benefits in SOC populations. Subsequently, we delve into the realms of energy-based fat reduction and body contouring, offering a comprehensive overview of these noninvasive therapies and addressing considerations for efficacy and safety in these patients.
Dermatosis Papulosa Nigra
In patients with SOC, scissor excision, curettage, or electrodesiccation are the mainstay treatments for removal of DPN (Figure 1). Curettage and electrodesiccation can cause temporary postinflammatory hyperpigmentation (PIH) in these populations, while cryotherapy is not a preferred method in patients with SOC due to the possibility of cryotherapy-induced depigmentation. In a 14-patient split-face study comparing the 532-nm potassium titanyl phosphate (KTP) laser vs electrodesiccation in FSTs IV to VI, the KTP-treated side showed an improvement rate of 96%, while the electrodesiccation side showed an improvement rate of 79%. There was a statistically significant favorable experience for KTP with regard to pain tolerability (P=.002).2 Complete resolution of lesions may be seen after 3 to 4 sessions at 4-week intervals. Additionally, the 1064-nm Nd:YAG laser was assessed for treatment of DPN in 2 patients, with 70% to 90% of lesions resolved after a single treatment with no complications.3
Most dermatologists still rely on curettage and electrodesiccation instead of laser therapy to remove DPNs in patients with SOC. The use of the Nd:YAG laser is promising yet expensive for the provider both to purchase and maintain. Electrodesiccation has been used by dermatology practices for decades and can be used without permanent discoloration. To minimize the risk for PIH, we recommend application of a healing ointment such as petroleum jelly or aloe vera gel to the treated lesions as well as lightening agents for PIH and daily use of sunscreen. Overall, providers do not need to purchase an expensive laser device for DPN removal.
Acne Scars
The invention of fractional technology in the early 2000s and its favorable safety profile have changed how dermatologists treat scarring in patients with SOC.
In one study of the short-pulsed nonablative Nd:YAG laser, 9 patients with FSTs I to V and 2 patients with FSTs IV to V underwent 8 treatments at 2-week intervals. Three blinded observers found a 29% improvement in the Global Acne Scar Severity score, while 89% (8/9) of patients self-reported subjective improvement in their acne scars.10
The 755-nm picosecond laser and diffractive lens array also have been shown to reduce the appearance of acne scars in patients with SOC, as shown via serial photography in a retrospective study of 56 patients with FSTs IV to VI. Transient hyperpigmentation, erythema, and edema were reported.11
Nonablative laser therapy is preferred for skin rejuvenation in patients with SOC due to a reduced risk for postprocedural hyperpigmentation.11 Ablative resurfacing (eg, CO2 laser) poses major risks for postprocedural hyperpigmentation, hypopigmentation, and scar formation and therefore should be avoided in these populations.12,13 A study involving 30 Asian patients (FSTs III-IV) demonstrated that the 1550-nm fractional laser was well tolerated, though higher treatment densities and fluences may lead to temporary adverse effects such as increased redness, swelling, and pain (P<.01).14 Furthermore, greater density was shown to cause higher levels of redness, hyperpigmentation, and swelling in comparison to higher fluence settings. Of note, patient satisfaction was markedly higher in patients who underwent treatment with higher fluence settings but not in patients with higher densities (P<.05). Postprocedural hyperpigmentation was noted in 6.7% (2/30) of patients studied.14 In another study, 8 patients with FSTs II to V were treated with either the 1064-nm long-pulsed Nd:YAG laser or the grid fractional monopolar radiofrequency laser.15 All participants experienced a significant decrease in mean wrinkle count using the Lemperle wrinkle assessment (P<.05). A significant decrease in mean wrinkle assessment score from 3.5 to 3.17 in clinical assessment and a decrease from 3.165 to 2.33 for photographic assessment was noted in patients treated with the grid laser (P<.05). A similar decrease in mean wrinkle assessment score was observed in the Nd:YAG group, with a mean decrease of 3.665 to 2.83 after 2 months for clinical assessment and 3.5 to 2.67 for photographic assessment. Among all patients in the study, 68% (6/8) experienced erythema, 25% (2/8) had a burning sensation, and 25% (2/8) experienced urticaria immediately postprocedure.15
Nonablative fractional resurfacing is preferred for the management of acne scars in patients with SOC. Adverse effects such as hyperpigmentation typically are transient, and the risk may be minimized with strict photoprotective practices following the procedure. Furthermore, avoidance of topicals containing exfoliants or α-hydroxy acids applied to the treated area following the procedure also may mitigate the risk for postprocedural hyperpigmentation.16 If hyperpigmentation does occur, use of topical melanogenesis inhibitors such as hydroquinone, kojic acid, or azelaic acid has shown some utility in practice.
Skin Rejuvenation
Nonablative fractional lasers (NAFLs) continue to be popular for treatment of photoaging. One study including 10 Asian patients (FSTs III-V) assessed the 1440-nm diode-based fractional laser for facial rejuvenation.17 After 4 sessions at 2-week intervals, 80% (8/10) of patients reported decreased skin roughness after both the second and third treatments, while 90% (9/10) had improved texture 1 month after the final procedure. Adverse effects included moderate facial edema and one case of transient hyperpigmentation.17 Another study reported a significant reduction in pore score (P<.002), with patients noting an overall improvement in skin appearance with minimal erythema, dryness, and flaking following 6 sessions at 2-week intervals using the 1440-nm diode-based fractional laser.18
The 1550-nm diode fractional laser significantly improved skin pigmentation (P<.001) and texture (P<.001) in 10 patients with FSTs II to IV following 5 sessions at 2- to 3-week intervals, with self-resolving erythema and edema posttreatment (Supplementary Table S2).19 Overall, NAFLs for the treatment of photoaging are effective with minimal adverse effects (eg, facial edema), which can be reduced with application of cold compression to the face and elevation of the head following treatment as well as the use of additional pillows during overnight sleep.
Laser Treatment for Hyperpigmentation Disorders
Melasma—The FDA recently approved fractional photothermolysis for the treatment of melasma; however, due to the risk for hyperpigmentation given its pathogenesis linked to hyperactive melanocytes, this laser is not considered a first-line therapy for melasma.20 In a split-face, randomized study, 22 patients with FSTs III to V who were diagnosed with either dermal or mixed-type melasma were treated with a low-fluence Q-switched Nd:YAG laser combined with hydroquinone 2% vs hydroquinone 2% alone (Supplementary Table S3).21 Each patient was treated weekly for 5 consecutive weeks. The laser-treated side was found to reach an average of 92.5% improvement compared with 19.7% on the hydroquinone-only side. Three of the 22 (13.6%) patients developed mottled hypopigmentation after 5 laser treatments, and 8 (36.4%) developed confetti-type hypopigmentation. Four (18.2%) patients developed rebound hyperpigmentation, and all 22 patients experienced recurrence of melasma by 12 weeks posttreatment.21
First-line treatment for melasma involves the application of topical lightening agents such as hydroquinone, azelaic acid, kojic acid, retinoids, or mild topical steroids. Combining laser technology with topical medications can enhance treatment outcomes, particularly yielding positive results for patients with persistent pigmentation concerns. Notably, utilization of 650-microsecond technology with the 1064-nm Nd:YAG laser is considered superior in clinical practice, especially for patients with FSTs IV through VI.
Postinflammatory Hyperpigmentation—A retrospective evaluation of 61 patients with FSTs IV to VI with PIH treated with a 1927-nm NAFL showed a mean improvement of 43.24%, as assessed by 2 dermatologists.22 Additionally, the Nd:YAG 1064-nm 650-microsecond pulse duration laser is an emerging treatment that delivers high and low fluences between 4 J/cm2 and 255 J/cm2 within a single 650-microsecond pulse duration.23 The short-pulse duration avoids overheating the skin, mitigating procedural discomfort and the risk for adverse effects commonly seen with the previous generation of low-pulsed lasers. In addition to PIH, this laser has been successfully used to treat pseudofolliculitis barbae.24
Solar Lentigos—In a split-face study treating solar lentigos in Asian patients, 4 treatments with a low-pulsed KTP 532-nm laser were administered with and without a second treatment with a low-pulsed 1064-nm Nd:YAG laser.25 Scoring of a modified pigment severity index and measurement of the melanin index showed that skin treated with the low-pulsed 532-nm laser alone and in combination with the low-pulsed 1064-nm Nd:YAG laser resulted in improvement at 3 months’ follow-up. However, there was no difference between the 2 sides of the face, leading the researchers to conclude that the low-pulsed 532-nm laser appears to be safe and effective for treatment of solar lentigos in Asian patients and does not require the addition of the low-pulsed 1064-nm laser.25
To avoid hyperpigmentation in patients with SOC, strict photoprotection to the treated areas should be advised. Proper cooling of the laser-treated area is required to minimize PIH, as cooling decreases tissue damage and excessive thermal injury. Test spots should be considered prior to initiation of the full laser treatment. Hydroquinone in a 4% concentration applied daily for 2 weeks preprocedure commonly is employed to reduce the risk for postprocedural hyperpigmentation in clinical practice.26,27
Skin Tightening and Body Contouring
In general, skin-tightening and body-contouring devices are among the most sought-after procedures. Studies performed in patients with SOC are limited. Herein, we provide background on why these devices are favorable for patients with SOC and our experiences in using them. A summary of these devices can be found in Supplementary Table S4.
Radiofrequency Skin Tightening—Radiofrequency devices are utilized for skin tightening as well as mild fat reduction; they commonly are used on the abdomen, thighs, buttocks, and face.28 People with SOC are more responsive to radiofrequency skin-tightening therapy due to higher baseline collagen content and dermal thickness, more sebaceous activity and skin elasticity, and more melanin content which offers protective thermal buffering.29,30 As the radiofrequency device emits heat, penetrating deep into the dermis, it generates collagen remodeling and synthesis within 4 to 6 months posttreatment.
Nonsurgical Fat Reduction
Procedures for nonsurgical fat reduction are favorable due to minimal recovery time, manageable cost, and an in-office procedure setting. As noted previously, there are 6 FDA-indicated interventions for nonsurgical fat reduction: ultrasonography, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring.31
Ultrasonography—Ultrasound devices designed for body contouring are used for skin tightening and mild fat reduction through the use of acoustic energy.32 These devices can be divided into 2 categories: high frequency and low frequency, with the high-frequency devices being the most popular. High-frequency ultrasound energy produces heat at target sites, which induces necrosis of adipocytes and stimulates collagen remodeling within the tissue matrix.33 Tissue temperatures above 56°C stimulate adipocyte necrosis while sparing nearby nerves and vessels.28 Because of the short duration of the procedure, the risk for epidermal damage is minimal. Contrary to high-frequency ultrasonography, focus-pulsed ultrasonography employs low-frequency waves to induce the mechanical disruption of adipocytes, which is generally better tolerated due to its nonthermal mechanism. The latter may be advantageous in patients with SOC due to a reduced risk for thermal injury to the epidermis. Multiple treatments often are needed at 3- to 4-week intervals, resulting in gradual improvement observed over 2 to 6 months. One study of microfocused ultrasonography in 25 Asian patients for treatment of face and neck laxity reported that skin laxity was improved or much improved in 84% (21/25) of patients following treatment.34 Adverse effects were reported as mild and transient, resolving within 90 days.34 Ultrasound devices also were shown to improve wrinkles, texture, and overall appearance of the skin in a 71-year-old African American woman 4 months following treatment (Figure 2). These photographs highlight the clinical utility of a microfocused ultrasound skin-tightening treatment in African American patients.
Cryolipolysis—Cryolipolysis is a noninvasive body contouring procedure that employs controlled cooling to induce subcutaneous panniculitis. Through cold-induced apoptosis of adipocytes, this procedure selectively reduces adipose tissue in localized areas such as the flank, abdomen, thighs, buttocks, back, submental area, and upper arms. The temperature used in cryolipolysis is approximately –10°C.35 The lethal temperature for melanocytes is –4 °C, below which melanocyte apoptosis may be induced, resulting in depigmentation. Given the prolonged contact of the skin with a cryolipolysis device for up to 60 minutes during a body-contouring procedure, there is a risk for resultant depigmentation in darker skin types. Controlled studies are needed to fully evaluate the safety and efficacy of cryolipolysis in patients with SOC. One retrospective study of cryolipolysis applied to the abdomen and upper arm of 4122 Asian patients reported a significant (P<.05) reduction in the circumference of the abdomen and the upper-arm areas. No long-term adverse effects were reported.36
Laser Lipolysis—The 1060-nm diode laser for body contouring selectively destroys adipose tissue, resulting in body contouring via thermally induced inflammation. Hyperthermic exposure for 15 minutes selectively elevates adipocyte temperature between 42°C to 47°C, which triggers apoptosis and the eventual clearance of destroyed cells from the interstitial space.37 The selectivity of the 1060-nm wavelength coupled with the device’s contact cooling system preserves the overlying skin and adnexa during the procedure,37 which would minimize epidermal damage that may induce dyspigmentation in patients with SOC. No notable adverse effects or dyspigmentation have been reported using this device.
Injection Lipolysis—Deoxycholic acid is an injectable adipocytolytic for the reduction of submental fat. It nonselectively lyses muscle and other adjacent nonfatty tissue. One study of 50 Indian patients demonstrated a substantial reduction of submental fat in 90% (45/50).38 For each treatment, 5 mL of 30 mg/mL deoxycholic acid was injected. Serial sessions were conducted at 2-month intervals, and most (64% [32/50]) patients required 3 sessions to see a treatment effect. Adverse effects included transient swelling, lumpiness, and tenderness. A phase 2a investigation of the novel injectable small-molecule drug CBL-514 in 43 Asian and White participants found a significant improvement in the reduction in abdominal fat volume (P<.00001) and thickness (P<.0001) relative to baseline at higher doses (unit dose, 2.0 mg/cm2 and 1.6 mg/cm2).39 In addition to the adverse effects mentioned previously, pruritus, repeated urticaria, body rash, and fever also were reported.39
Radiofrequency Lipolysis—Radiofrequency is used for adipolysis through heat-induced apoptosis. To achieve this effect, adipose tissue must sustain a temperature of 42 °C to 45 °C for at least 15 minutes.40 In one study, 4 treatments performed at 7-day intervals resulted in a statistically significant reduction in circumference to the treated areas of the inner and outer thighs without any reported adverse effects (P<0.001).41 Of note, there was 1 cm of distance between the applicator and the skin. The absence of direct contact with the skin is likely to reduce the risk for postprocedural complications in patients with SOC.
Magnetic Resonance Contouring—Magnetic resonance contouring with high-intensity focused electromagnetic technology is an emerging treatment modality for noninvasive body contouring. One distinguishing characteristic from other currently available noninvasive fat-reduction therapies is that magnetic resonance may improve strength, tone, and muscle thickness.42 This modality is FDA approved for contouring of the buttocks and abdomen and employs electromagnetic energy to stimulate approximately 20,000 muscle contractions within a time frame of 30 minutes. Though the mechanisms causing benefits to muscular and adipose tissue have not been elucidated, current findings suggest that the contractions stimulate substantial lipolysis of adipocytes, resulting in the release of large amounts of free fatty acids that cause damage to nearby adipose tissue.43 Multiple treatments are required over time to maintain effect. No major adverse effects have been reported. The likely mechanism of action of magnetic resonance contouring does not appear to pose an increased risk to patients with SOC.
Final Thoughts
One of the major roadblocks in distilling indications along with associated risks and benefits for nonsurgical cosmetic practices for patients with SOC is a void in the primary literature involving these populations. Clinical experience serves to address this deficit in combination with a thorough review of the literature. The 1064-nm Nd:YAG laser has shown clinical utility in the treatment of DPN, melanoma, and acne scars, but it poses financial constraints to the provider in comparison to modalities used for many years. Notably, NAF resurfacing is preferred for the management of acne scars in patients with SOC and continues to gain popularity for the treatment of photoaging. Regarding skin-tightening and body-contouring devices, studies performed in patients with SOC are limited and affected by factors such as small sample sizes, underrepresentation of FSTs IV through VI, short follow-up durations, and a lack of standardized outcome measures. Additionally, few studies assess pigmentary adverse effects or stratify results by skin type, which is critical given the higher risk for PIH in SOC. Ultrasound devices showed clinical utility in improvement of skin laxity, texture, and overall improvement. Patients with SOC respond well to skin-tightening devices due to the increased collagen synthesis. Regarding emerging devices for reduction of adipocytes, deoxycholic acid when injected showed notable improvement in fat reduction but also had adverse effects. As additional studies on cosmetic procedures in SOC emerge, an expansion of treatment options could be offered to this demographic group with confidence, provided proper treatment and follow-up protocols are in place.
Cosmetic laser procedures as well as energy-based fat reduction and body-contouring devices are increasingly popular among individuals with skin of color (SOC). Innovations in cosmetic devices and procedures tailored for SOC have allowed for the optimization of outcomes in this patient population. In this article, SOC is defined as darker skin types, including Fitzpatrick skin types (FSTs) IV to VI and ethnic backgrounds such as LatinX, African American, Southeast Asian, Native American, Pacific Islander, Middle Eastern, Asian, and African. Indications for laser treatment include dermatosis papulosa nigrans (DPN), acne scars, skin rejuvenation, and hyperpigmentation. There currently are 6 procedures for nonsurgical fat reduction that are approved by the US Food and Drug Administration (FDA): high-frequency focused ultrasound, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring (Supplementary Table S1).1
In this review, our initial focus is cosmetic laser procedures, encompassing FDA-cleared indications along with the associated risks and benefits in SOC populations. Subsequently, we delve into the realms of energy-based fat reduction and body contouring, offering a comprehensive overview of these noninvasive therapies and addressing considerations for efficacy and safety in these patients.
Dermatosis Papulosa Nigra
In patients with SOC, scissor excision, curettage, or electrodesiccation are the mainstay treatments for removal of DPN (Figure 1). Curettage and electrodesiccation can cause temporary postinflammatory hyperpigmentation (PIH) in these populations, while cryotherapy is not a preferred method in patients with SOC due to the possibility of cryotherapy-induced depigmentation. In a 14-patient split-face study comparing the 532-nm potassium titanyl phosphate (KTP) laser vs electrodesiccation in FSTs IV to VI, the KTP-treated side showed an improvement rate of 96%, while the electrodesiccation side showed an improvement rate of 79%. There was a statistically significant favorable experience for KTP with regard to pain tolerability (P=.002).2 Complete resolution of lesions may be seen after 3 to 4 sessions at 4-week intervals. Additionally, the 1064-nm Nd:YAG laser was assessed for treatment of DPN in 2 patients, with 70% to 90% of lesions resolved after a single treatment with no complications.3

Most dermatologists still rely on curettage and electrodesiccation instead of laser therapy to remove DPNs in patients with SOC. The use of the Nd:YAG laser is promising yet expensive for the provider both to purchase and maintain. Electrodesiccation has been used by dermatology practices for decades and can be used without permanent discoloration. To minimize the risk for PIH, we recommend application of a healing ointment such as petroleum jelly or aloe vera gel to the treated lesions as well as lightening agents for PIH and daily use of sunscreen. Overall, providers do not need to purchase an expensive laser device for DPN removal.
Acne Scars
The invention of fractional technology in the early 2000s and its favorable safety profile have changed how dermatologists treat scarring in patients with SOC.
In one study of the short-pulsed nonablative Nd:YAG laser, 9 patients with FSTs I to V and 2 patients with FSTs IV to V underwent 8 treatments at 2-week intervals. Three blinded observers found a 29% improvement in the Global Acne Scar Severity score, while 89% (8/9) of patients self-reported subjective improvement in their acne scars.10
The 755-nm picosecond laser and diffractive lens array also have been shown to reduce the appearance of acne scars in patients with SOC, as shown via serial photography in a retrospective study of 56 patients with FSTs IV to VI. Transient hyperpigmentation, erythema, and edema were reported.11
Nonablative laser therapy is preferred for skin rejuvenation in patients with SOC due to a reduced risk for postprocedural hyperpigmentation.11 Ablative resurfacing (eg, CO2 laser) poses major risks for postprocedural hyperpigmentation, hypopigmentation, and scar formation and therefore should be avoided in these populations.12,13 A study involving 30 Asian patients (FSTs III-IV) demonstrated that the 1550-nm fractional laser was well tolerated, though higher treatment densities and fluences may lead to temporary adverse effects such as increased redness, swelling, and pain (P<.01).14 Furthermore, greater density was shown to cause higher levels of redness, hyperpigmentation, and swelling in comparison to higher fluence settings. Of note, patient satisfaction was markedly higher in patients who underwent treatment with higher fluence settings but not in patients with higher densities (P<.05). Postprocedural hyperpigmentation was noted in 6.7% (2/30) of patients studied.14 In another study, 8 patients with FSTs II to V were treated with either the 1064-nm long-pulsed Nd:YAG laser or the grid fractional monopolar radiofrequency laser.15 All participants experienced a significant decrease in mean wrinkle count using the Lemperle wrinkle assessment (P<.05). A significant decrease in mean wrinkle assessment score from 3.5 to 3.17 in clinical assessment and a decrease from 3.165 to 2.33 for photographic assessment was noted in patients treated with the grid laser (P<.05). A similar decrease in mean wrinkle assessment score was observed in the Nd:YAG group, with a mean decrease of 3.665 to 2.83 after 2 months for clinical assessment and 3.5 to 2.67 for photographic assessment. Among all patients in the study, 68% (6/8) experienced erythema, 25% (2/8) had a burning sensation, and 25% (2/8) experienced urticaria immediately postprocedure.15
Nonablative fractional resurfacing is preferred for the management of acne scars in patients with SOC. Adverse effects such as hyperpigmentation typically are transient, and the risk may be minimized with strict photoprotective practices following the procedure. Furthermore, avoidance of topicals containing exfoliants or α-hydroxy acids applied to the treated area following the procedure also may mitigate the risk for postprocedural hyperpigmentation.16 If hyperpigmentation does occur, use of topical melanogenesis inhibitors such as hydroquinone, kojic acid, or azelaic acid has shown some utility in practice.
Skin Rejuvenation
Nonablative fractional lasers (NAFLs) continue to be popular for treatment of photoaging. One study including 10 Asian patients (FSTs III-V) assessed the 1440-nm diode-based fractional laser for facial rejuvenation.17 After 4 sessions at 2-week intervals, 80% (8/10) of patients reported decreased skin roughness after both the second and third treatments, while 90% (9/10) had improved texture 1 month after the final procedure. Adverse effects included moderate facial edema and one case of transient hyperpigmentation.17 Another study reported a significant reduction in pore score (P<.002), with patients noting an overall improvement in skin appearance with minimal erythema, dryness, and flaking following 6 sessions at 2-week intervals using the 1440-nm diode-based fractional laser.18
The 1550-nm diode fractional laser significantly improved skin pigmentation (P<.001) and texture (P<.001) in 10 patients with FSTs II to IV following 5 sessions at 2- to 3-week intervals, with self-resolving erythema and edema posttreatment (Supplementary Table S2).19 Overall, NAFLs for the treatment of photoaging are effective with minimal adverse effects (eg, facial edema), which can be reduced with application of cold compression to the face and elevation of the head following treatment as well as the use of additional pillows during overnight sleep.
Laser Treatment for Hyperpigmentation Disorders
Melasma—The FDA recently approved fractional photothermolysis for the treatment of melasma; however, due to the risk for hyperpigmentation given its pathogenesis linked to hyperactive melanocytes, this laser is not considered a first-line therapy for melasma.20 In a split-face, randomized study, 22 patients with FSTs III to V who were diagnosed with either dermal or mixed-type melasma were treated with a low-fluence Q-switched Nd:YAG laser combined with hydroquinone 2% vs hydroquinone 2% alone (Supplementary Table S3).21 Each patient was treated weekly for 5 consecutive weeks. The laser-treated side was found to reach an average of 92.5% improvement compared with 19.7% on the hydroquinone-only side. Three of the 22 (13.6%) patients developed mottled hypopigmentation after 5 laser treatments, and 8 (36.4%) developed confetti-type hypopigmentation. Four (18.2%) patients developed rebound hyperpigmentation, and all 22 patients experienced recurrence of melasma by 12 weeks posttreatment.21
First-line treatment for melasma involves the application of topical lightening agents such as hydroquinone, azelaic acid, kojic acid, retinoids, or mild topical steroids. Combining laser technology with topical medications can enhance treatment outcomes, particularly yielding positive results for patients with persistent pigmentation concerns. Notably, utilization of 650-microsecond technology with the 1064-nm Nd:YAG laser is considered superior in clinical practice, especially for patients with FSTs IV through VI.
Postinflammatory Hyperpigmentation—A retrospective evaluation of 61 patients with FSTs IV to VI with PIH treated with a 1927-nm NAFL showed a mean improvement of 43.24%, as assessed by 2 dermatologists.22 Additionally, the Nd:YAG 1064-nm 650-microsecond pulse duration laser is an emerging treatment that delivers high and low fluences between 4 J/cm2 and 255 J/cm2 within a single 650-microsecond pulse duration.23 The short-pulse duration avoids overheating the skin, mitigating procedural discomfort and the risk for adverse effects commonly seen with the previous generation of low-pulsed lasers. In addition to PIH, this laser has been successfully used to treat pseudofolliculitis barbae.24
Solar Lentigos—In a split-face study treating solar lentigos in Asian patients, 4 treatments with a low-pulsed KTP 532-nm laser were administered with and without a second treatment with a low-pulsed 1064-nm Nd:YAG laser.25 Scoring of a modified pigment severity index and measurement of the melanin index showed that skin treated with the low-pulsed 532-nm laser alone and in combination with the low-pulsed 1064-nm Nd:YAG laser resulted in improvement at 3 months’ follow-up. However, there was no difference between the 2 sides of the face, leading the researchers to conclude that the low-pulsed 532-nm laser appears to be safe and effective for treatment of solar lentigos in Asian patients and does not require the addition of the low-pulsed 1064-nm laser.25
To avoid hyperpigmentation in patients with SOC, strict photoprotection to the treated areas should be advised. Proper cooling of the laser-treated area is required to minimize PIH, as cooling decreases tissue damage and excessive thermal injury. Test spots should be considered prior to initiation of the full laser treatment. Hydroquinone in a 4% concentration applied daily for 2 weeks preprocedure commonly is employed to reduce the risk for postprocedural hyperpigmentation in clinical practice.26,27
Skin Tightening and Body Contouring
In general, skin-tightening and body-contouring devices are among the most sought-after procedures. Studies performed in patients with SOC are limited. Herein, we provide background on why these devices are favorable for patients with SOC and our experiences in using them. A summary of these devices can be found in Supplementary Table S4.
Radiofrequency Skin Tightening—Radiofrequency devices are utilized for skin tightening as well as mild fat reduction; they commonly are used on the abdomen, thighs, buttocks, and face.28 People with SOC are more responsive to radiofrequency skin-tightening therapy due to higher baseline collagen content and dermal thickness, more sebaceous activity and skin elasticity, and more melanin content which offers protective thermal buffering.29,30 As the radiofrequency device emits heat, penetrating deep into the dermis, it generates collagen remodeling and synthesis within 4 to 6 months posttreatment.
Nonsurgical Fat Reduction
Procedures for nonsurgical fat reduction are favorable due to minimal recovery time, manageable cost, and an in-office procedure setting. As noted previously, there are 6 FDA-indicated interventions for nonsurgical fat reduction: ultrasonography, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring.31
Ultrasonography—Ultrasound devices designed for body contouring are used for skin tightening and mild fat reduction through the use of acoustic energy.32 These devices can be divided into 2 categories: high frequency and low frequency, with the high-frequency devices being the most popular. High-frequency ultrasound energy produces heat at target sites, which induces necrosis of adipocytes and stimulates collagen remodeling within the tissue matrix.33 Tissue temperatures above 56°C stimulate adipocyte necrosis while sparing nearby nerves and vessels.28 Because of the short duration of the procedure, the risk for epidermal damage is minimal. Contrary to high-frequency ultrasonography, focus-pulsed ultrasonography employs low-frequency waves to induce the mechanical disruption of adipocytes, which is generally better tolerated due to its nonthermal mechanism. The latter may be advantageous in patients with SOC due to a reduced risk for thermal injury to the epidermis. Multiple treatments often are needed at 3- to 4-week intervals, resulting in gradual improvement observed over 2 to 6 months. One study of microfocused ultrasonography in 25 Asian patients for treatment of face and neck laxity reported that skin laxity was improved or much improved in 84% (21/25) of patients following treatment.34 Adverse effects were reported as mild and transient, resolving within 90 days.34 Ultrasound devices also were shown to improve wrinkles, texture, and overall appearance of the skin in a 71-year-old African American woman 4 months following treatment (Figure 2). These photographs highlight the clinical utility of a microfocused ultrasound skin-tightening treatment in African American patients.

Cryolipolysis—Cryolipolysis is a noninvasive body contouring procedure that employs controlled cooling to induce subcutaneous panniculitis. Through cold-induced apoptosis of adipocytes, this procedure selectively reduces adipose tissue in localized areas such as the flank, abdomen, thighs, buttocks, back, submental area, and upper arms. The temperature used in cryolipolysis is approximately –10°C.35 The lethal temperature for melanocytes is –4 °C, below which melanocyte apoptosis may be induced, resulting in depigmentation. Given the prolonged contact of the skin with a cryolipolysis device for up to 60 minutes during a body-contouring procedure, there is a risk for resultant depigmentation in darker skin types. Controlled studies are needed to fully evaluate the safety and efficacy of cryolipolysis in patients with SOC. One retrospective study of cryolipolysis applied to the abdomen and upper arm of 4122 Asian patients reported a significant (P<.05) reduction in the circumference of the abdomen and the upper-arm areas. No long-term adverse effects were reported.36
Laser Lipolysis—The 1060-nm diode laser for body contouring selectively destroys adipose tissue, resulting in body contouring via thermally induced inflammation. Hyperthermic exposure for 15 minutes selectively elevates adipocyte temperature between 42°C to 47°C, which triggers apoptosis and the eventual clearance of destroyed cells from the interstitial space.37 The selectivity of the 1060-nm wavelength coupled with the device’s contact cooling system preserves the overlying skin and adnexa during the procedure,37 which would minimize epidermal damage that may induce dyspigmentation in patients with SOC. No notable adverse effects or dyspigmentation have been reported using this device.
Injection Lipolysis—Deoxycholic acid is an injectable adipocytolytic for the reduction of submental fat. It nonselectively lyses muscle and other adjacent nonfatty tissue. One study of 50 Indian patients demonstrated a substantial reduction of submental fat in 90% (45/50).38 For each treatment, 5 mL of 30 mg/mL deoxycholic acid was injected. Serial sessions were conducted at 2-month intervals, and most (64% [32/50]) patients required 3 sessions to see a treatment effect. Adverse effects included transient swelling, lumpiness, and tenderness. A phase 2a investigation of the novel injectable small-molecule drug CBL-514 in 43 Asian and White participants found a significant improvement in the reduction in abdominal fat volume (P<.00001) and thickness (P<.0001) relative to baseline at higher doses (unit dose, 2.0 mg/cm2 and 1.6 mg/cm2).39 In addition to the adverse effects mentioned previously, pruritus, repeated urticaria, body rash, and fever also were reported.39
Radiofrequency Lipolysis—Radiofrequency is used for adipolysis through heat-induced apoptosis. To achieve this effect, adipose tissue must sustain a temperature of 42 °C to 45 °C for at least 15 minutes.40 In one study, 4 treatments performed at 7-day intervals resulted in a statistically significant reduction in circumference to the treated areas of the inner and outer thighs without any reported adverse effects (P<0.001).41 Of note, there was 1 cm of distance between the applicator and the skin. The absence of direct contact with the skin is likely to reduce the risk for postprocedural complications in patients with SOC.
Magnetic Resonance Contouring—Magnetic resonance contouring with high-intensity focused electromagnetic technology is an emerging treatment modality for noninvasive body contouring. One distinguishing characteristic from other currently available noninvasive fat-reduction therapies is that magnetic resonance may improve strength, tone, and muscle thickness.42 This modality is FDA approved for contouring of the buttocks and abdomen and employs electromagnetic energy to stimulate approximately 20,000 muscle contractions within a time frame of 30 minutes. Though the mechanisms causing benefits to muscular and adipose tissue have not been elucidated, current findings suggest that the contractions stimulate substantial lipolysis of adipocytes, resulting in the release of large amounts of free fatty acids that cause damage to nearby adipose tissue.43 Multiple treatments are required over time to maintain effect. No major adverse effects have been reported. The likely mechanism of action of magnetic resonance contouring does not appear to pose an increased risk to patients with SOC.
Final Thoughts
One of the major roadblocks in distilling indications along with associated risks and benefits for nonsurgical cosmetic practices for patients with SOC is a void in the primary literature involving these populations. Clinical experience serves to address this deficit in combination with a thorough review of the literature. The 1064-nm Nd:YAG laser has shown clinical utility in the treatment of DPN, melanoma, and acne scars, but it poses financial constraints to the provider in comparison to modalities used for many years. Notably, NAF resurfacing is preferred for the management of acne scars in patients with SOC and continues to gain popularity for the treatment of photoaging. Regarding skin-tightening and body-contouring devices, studies performed in patients with SOC are limited and affected by factors such as small sample sizes, underrepresentation of FSTs IV through VI, short follow-up durations, and a lack of standardized outcome measures. Additionally, few studies assess pigmentary adverse effects or stratify results by skin type, which is critical given the higher risk for PIH in SOC. Ultrasound devices showed clinical utility in improvement of skin laxity, texture, and overall improvement. Patients with SOC respond well to skin-tightening devices due to the increased collagen synthesis. Regarding emerging devices for reduction of adipocytes, deoxycholic acid when injected showed notable improvement in fat reduction but also had adverse effects. As additional studies on cosmetic procedures in SOC emerge, an expansion of treatment options could be offered to this demographic group with confidence, provided proper treatment and follow-up protocols are in place.
Cosmetic laser procedures as well as energy-based fat reduction and body-contouring devices are increasingly popular among individuals with skin of color (SOC). Innovations in cosmetic devices and procedures tailored for SOC have allowed for the optimization of outcomes in this patient population. In this article, SOC is defined as darker skin types, including Fitzpatrick skin types (FSTs) IV to VI and ethnic backgrounds such as LatinX, African American, Southeast Asian, Native American, Pacific Islander, Middle Eastern, Asian, and African. Indications for laser treatment include dermatosis papulosa nigrans (DPN), acne scars, skin rejuvenation, and hyperpigmentation. There currently are 6 procedures for nonsurgical fat reduction that are approved by the US Food and Drug Administration (FDA): high-frequency focused ultrasound, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring (Supplementary Table S1).1
In this review, our initial focus is cosmetic laser procedures, encompassing FDA-cleared indications along with the associated risks and benefits in SOC populations. Subsequently, we delve into the realms of energy-based fat reduction and body contouring, offering a comprehensive overview of these noninvasive therapies and addressing considerations for efficacy and safety in these patients.
Dermatosis Papulosa Nigra
In patients with SOC, scissor excision, curettage, or electrodesiccation are the mainstay treatments for removal of DPN (Figure 1). Curettage and electrodesiccation can cause temporary postinflammatory hyperpigmentation (PIH) in these populations, while cryotherapy is not a preferred method in patients with SOC due to the possibility of cryotherapy-induced depigmentation. In a 14-patient split-face study comparing the 532-nm potassium titanyl phosphate (KTP) laser vs electrodesiccation in FSTs IV to VI, the KTP-treated side showed an improvement rate of 96%, while the electrodesiccation side showed an improvement rate of 79%. There was a statistically significant favorable experience for KTP with regard to pain tolerability (P=.002).2 Complete resolution of lesions may be seen after 3 to 4 sessions at 4-week intervals. Additionally, the 1064-nm Nd:YAG laser was assessed for treatment of DPN in 2 patients, with 70% to 90% of lesions resolved after a single treatment with no complications.3

Most dermatologists still rely on curettage and electrodesiccation instead of laser therapy to remove DPNs in patients with SOC. The use of the Nd:YAG laser is promising yet expensive for the provider both to purchase and maintain. Electrodesiccation has been used by dermatology practices for decades and can be used without permanent discoloration. To minimize the risk for PIH, we recommend application of a healing ointment such as petroleum jelly or aloe vera gel to the treated lesions as well as lightening agents for PIH and daily use of sunscreen. Overall, providers do not need to purchase an expensive laser device for DPN removal.
Acne Scars
The invention of fractional technology in the early 2000s and its favorable safety profile have changed how dermatologists treat scarring in patients with SOC.
In one study of the short-pulsed nonablative Nd:YAG laser, 9 patients with FSTs I to V and 2 patients with FSTs IV to V underwent 8 treatments at 2-week intervals. Three blinded observers found a 29% improvement in the Global Acne Scar Severity score, while 89% (8/9) of patients self-reported subjective improvement in their acne scars.10
The 755-nm picosecond laser and diffractive lens array also have been shown to reduce the appearance of acne scars in patients with SOC, as shown via serial photography in a retrospective study of 56 patients with FSTs IV to VI. Transient hyperpigmentation, erythema, and edema were reported.11
Nonablative laser therapy is preferred for skin rejuvenation in patients with SOC due to a reduced risk for postprocedural hyperpigmentation.11 Ablative resurfacing (eg, CO2 laser) poses major risks for postprocedural hyperpigmentation, hypopigmentation, and scar formation and therefore should be avoided in these populations.12,13 A study involving 30 Asian patients (FSTs III-IV) demonstrated that the 1550-nm fractional laser was well tolerated, though higher treatment densities and fluences may lead to temporary adverse effects such as increased redness, swelling, and pain (P<.01).14 Furthermore, greater density was shown to cause higher levels of redness, hyperpigmentation, and swelling in comparison to higher fluence settings. Of note, patient satisfaction was markedly higher in patients who underwent treatment with higher fluence settings but not in patients with higher densities (P<.05). Postprocedural hyperpigmentation was noted in 6.7% (2/30) of patients studied.14 In another study, 8 patients with FSTs II to V were treated with either the 1064-nm long-pulsed Nd:YAG laser or the grid fractional monopolar radiofrequency laser.15 All participants experienced a significant decrease in mean wrinkle count using the Lemperle wrinkle assessment (P<.05). A significant decrease in mean wrinkle assessment score from 3.5 to 3.17 in clinical assessment and a decrease from 3.165 to 2.33 for photographic assessment was noted in patients treated with the grid laser (P<.05). A similar decrease in mean wrinkle assessment score was observed in the Nd:YAG group, with a mean decrease of 3.665 to 2.83 after 2 months for clinical assessment and 3.5 to 2.67 for photographic assessment. Among all patients in the study, 68% (6/8) experienced erythema, 25% (2/8) had a burning sensation, and 25% (2/8) experienced urticaria immediately postprocedure.15
Nonablative fractional resurfacing is preferred for the management of acne scars in patients with SOC. Adverse effects such as hyperpigmentation typically are transient, and the risk may be minimized with strict photoprotective practices following the procedure. Furthermore, avoidance of topicals containing exfoliants or α-hydroxy acids applied to the treated area following the procedure also may mitigate the risk for postprocedural hyperpigmentation.16 If hyperpigmentation does occur, use of topical melanogenesis inhibitors such as hydroquinone, kojic acid, or azelaic acid has shown some utility in practice.
Skin Rejuvenation
Nonablative fractional lasers (NAFLs) continue to be popular for treatment of photoaging. One study including 10 Asian patients (FSTs III-V) assessed the 1440-nm diode-based fractional laser for facial rejuvenation.17 After 4 sessions at 2-week intervals, 80% (8/10) of patients reported decreased skin roughness after both the second and third treatments, while 90% (9/10) had improved texture 1 month after the final procedure. Adverse effects included moderate facial edema and one case of transient hyperpigmentation.17 Another study reported a significant reduction in pore score (P<.002), with patients noting an overall improvement in skin appearance with minimal erythema, dryness, and flaking following 6 sessions at 2-week intervals using the 1440-nm diode-based fractional laser.18
The 1550-nm diode fractional laser significantly improved skin pigmentation (P<.001) and texture (P<.001) in 10 patients with FSTs II to IV following 5 sessions at 2- to 3-week intervals, with self-resolving erythema and edema posttreatment (Supplementary Table S2).19 Overall, NAFLs for the treatment of photoaging are effective with minimal adverse effects (eg, facial edema), which can be reduced with application of cold compression to the face and elevation of the head following treatment as well as the use of additional pillows during overnight sleep.
Laser Treatment for Hyperpigmentation Disorders
Melasma—The FDA recently approved fractional photothermolysis for the treatment of melasma; however, due to the risk for hyperpigmentation given its pathogenesis linked to hyperactive melanocytes, this laser is not considered a first-line therapy for melasma.20 In a split-face, randomized study, 22 patients with FSTs III to V who were diagnosed with either dermal or mixed-type melasma were treated with a low-fluence Q-switched Nd:YAG laser combined with hydroquinone 2% vs hydroquinone 2% alone (Supplementary Table S3).21 Each patient was treated weekly for 5 consecutive weeks. The laser-treated side was found to reach an average of 92.5% improvement compared with 19.7% on the hydroquinone-only side. Three of the 22 (13.6%) patients developed mottled hypopigmentation after 5 laser treatments, and 8 (36.4%) developed confetti-type hypopigmentation. Four (18.2%) patients developed rebound hyperpigmentation, and all 22 patients experienced recurrence of melasma by 12 weeks posttreatment.21
First-line treatment for melasma involves the application of topical lightening agents such as hydroquinone, azelaic acid, kojic acid, retinoids, or mild topical steroids. Combining laser technology with topical medications can enhance treatment outcomes, particularly yielding positive results for patients with persistent pigmentation concerns. Notably, utilization of 650-microsecond technology with the 1064-nm Nd:YAG laser is considered superior in clinical practice, especially for patients with FSTs IV through VI.
Postinflammatory Hyperpigmentation—A retrospective evaluation of 61 patients with FSTs IV to VI with PIH treated with a 1927-nm NAFL showed a mean improvement of 43.24%, as assessed by 2 dermatologists.22 Additionally, the Nd:YAG 1064-nm 650-microsecond pulse duration laser is an emerging treatment that delivers high and low fluences between 4 J/cm2 and 255 J/cm2 within a single 650-microsecond pulse duration.23 The short-pulse duration avoids overheating the skin, mitigating procedural discomfort and the risk for adverse effects commonly seen with the previous generation of low-pulsed lasers. In addition to PIH, this laser has been successfully used to treat pseudofolliculitis barbae.24
Solar Lentigos—In a split-face study treating solar lentigos in Asian patients, 4 treatments with a low-pulsed KTP 532-nm laser were administered with and without a second treatment with a low-pulsed 1064-nm Nd:YAG laser.25 Scoring of a modified pigment severity index and measurement of the melanin index showed that skin treated with the low-pulsed 532-nm laser alone and in combination with the low-pulsed 1064-nm Nd:YAG laser resulted in improvement at 3 months’ follow-up. However, there was no difference between the 2 sides of the face, leading the researchers to conclude that the low-pulsed 532-nm laser appears to be safe and effective for treatment of solar lentigos in Asian patients and does not require the addition of the low-pulsed 1064-nm laser.25
To avoid hyperpigmentation in patients with SOC, strict photoprotection to the treated areas should be advised. Proper cooling of the laser-treated area is required to minimize PIH, as cooling decreases tissue damage and excessive thermal injury. Test spots should be considered prior to initiation of the full laser treatment. Hydroquinone in a 4% concentration applied daily for 2 weeks preprocedure commonly is employed to reduce the risk for postprocedural hyperpigmentation in clinical practice.26,27
Skin Tightening and Body Contouring
In general, skin-tightening and body-contouring devices are among the most sought-after procedures. Studies performed in patients with SOC are limited. Herein, we provide background on why these devices are favorable for patients with SOC and our experiences in using them. A summary of these devices can be found in Supplementary Table S4.
Radiofrequency Skin Tightening—Radiofrequency devices are utilized for skin tightening as well as mild fat reduction; they commonly are used on the abdomen, thighs, buttocks, and face.28 People with SOC are more responsive to radiofrequency skin-tightening therapy due to higher baseline collagen content and dermal thickness, more sebaceous activity and skin elasticity, and more melanin content which offers protective thermal buffering.29,30 As the radiofrequency device emits heat, penetrating deep into the dermis, it generates collagen remodeling and synthesis within 4 to 6 months posttreatment.
Nonsurgical Fat Reduction
Procedures for nonsurgical fat reduction are favorable due to minimal recovery time, manageable cost, and an in-office procedure setting. As noted previously, there are 6 FDA-indicated interventions for nonsurgical fat reduction: ultrasonography, cryolipolysis, laser lipolysis, injection lipolysis, radiofrequency lipolysis, and magnetic resonance contouring.31
Ultrasonography—Ultrasound devices designed for body contouring are used for skin tightening and mild fat reduction through the use of acoustic energy.32 These devices can be divided into 2 categories: high frequency and low frequency, with the high-frequency devices being the most popular. High-frequency ultrasound energy produces heat at target sites, which induces necrosis of adipocytes and stimulates collagen remodeling within the tissue matrix.33 Tissue temperatures above 56°C stimulate adipocyte necrosis while sparing nearby nerves and vessels.28 Because of the short duration of the procedure, the risk for epidermal damage is minimal. Contrary to high-frequency ultrasonography, focus-pulsed ultrasonography employs low-frequency waves to induce the mechanical disruption of adipocytes, which is generally better tolerated due to its nonthermal mechanism. The latter may be advantageous in patients with SOC due to a reduced risk for thermal injury to the epidermis. Multiple treatments often are needed at 3- to 4-week intervals, resulting in gradual improvement observed over 2 to 6 months. One study of microfocused ultrasonography in 25 Asian patients for treatment of face and neck laxity reported that skin laxity was improved or much improved in 84% (21/25) of patients following treatment.34 Adverse effects were reported as mild and transient, resolving within 90 days.34 Ultrasound devices also were shown to improve wrinkles, texture, and overall appearance of the skin in a 71-year-old African American woman 4 months following treatment (Figure 2). These photographs highlight the clinical utility of a microfocused ultrasound skin-tightening treatment in African American patients.

Cryolipolysis—Cryolipolysis is a noninvasive body contouring procedure that employs controlled cooling to induce subcutaneous panniculitis. Through cold-induced apoptosis of adipocytes, this procedure selectively reduces adipose tissue in localized areas such as the flank, abdomen, thighs, buttocks, back, submental area, and upper arms. The temperature used in cryolipolysis is approximately –10°C.35 The lethal temperature for melanocytes is –4 °C, below which melanocyte apoptosis may be induced, resulting in depigmentation. Given the prolonged contact of the skin with a cryolipolysis device for up to 60 minutes during a body-contouring procedure, there is a risk for resultant depigmentation in darker skin types. Controlled studies are needed to fully evaluate the safety and efficacy of cryolipolysis in patients with SOC. One retrospective study of cryolipolysis applied to the abdomen and upper arm of 4122 Asian patients reported a significant (P<.05) reduction in the circumference of the abdomen and the upper-arm areas. No long-term adverse effects were reported.36
Laser Lipolysis—The 1060-nm diode laser for body contouring selectively destroys adipose tissue, resulting in body contouring via thermally induced inflammation. Hyperthermic exposure for 15 minutes selectively elevates adipocyte temperature between 42°C to 47°C, which triggers apoptosis and the eventual clearance of destroyed cells from the interstitial space.37 The selectivity of the 1060-nm wavelength coupled with the device’s contact cooling system preserves the overlying skin and adnexa during the procedure,37 which would minimize epidermal damage that may induce dyspigmentation in patients with SOC. No notable adverse effects or dyspigmentation have been reported using this device.
Injection Lipolysis—Deoxycholic acid is an injectable adipocytolytic for the reduction of submental fat. It nonselectively lyses muscle and other adjacent nonfatty tissue. One study of 50 Indian patients demonstrated a substantial reduction of submental fat in 90% (45/50).38 For each treatment, 5 mL of 30 mg/mL deoxycholic acid was injected. Serial sessions were conducted at 2-month intervals, and most (64% [32/50]) patients required 3 sessions to see a treatment effect. Adverse effects included transient swelling, lumpiness, and tenderness. A phase 2a investigation of the novel injectable small-molecule drug CBL-514 in 43 Asian and White participants found a significant improvement in the reduction in abdominal fat volume (P<.00001) and thickness (P<.0001) relative to baseline at higher doses (unit dose, 2.0 mg/cm2 and 1.6 mg/cm2).39 In addition to the adverse effects mentioned previously, pruritus, repeated urticaria, body rash, and fever also were reported.39
Radiofrequency Lipolysis—Radiofrequency is used for adipolysis through heat-induced apoptosis. To achieve this effect, adipose tissue must sustain a temperature of 42 °C to 45 °C for at least 15 minutes.40 In one study, 4 treatments performed at 7-day intervals resulted in a statistically significant reduction in circumference to the treated areas of the inner and outer thighs without any reported adverse effects (P<0.001).41 Of note, there was 1 cm of distance between the applicator and the skin. The absence of direct contact with the skin is likely to reduce the risk for postprocedural complications in patients with SOC.
Magnetic Resonance Contouring—Magnetic resonance contouring with high-intensity focused electromagnetic technology is an emerging treatment modality for noninvasive body contouring. One distinguishing characteristic from other currently available noninvasive fat-reduction therapies is that magnetic resonance may improve strength, tone, and muscle thickness.42 This modality is FDA approved for contouring of the buttocks and abdomen and employs electromagnetic energy to stimulate approximately 20,000 muscle contractions within a time frame of 30 minutes. Though the mechanisms causing benefits to muscular and adipose tissue have not been elucidated, current findings suggest that the contractions stimulate substantial lipolysis of adipocytes, resulting in the release of large amounts of free fatty acids that cause damage to nearby adipose tissue.43 Multiple treatments are required over time to maintain effect. No major adverse effects have been reported. The likely mechanism of action of magnetic resonance contouring does not appear to pose an increased risk to patients with SOC.
Final Thoughts
One of the major roadblocks in distilling indications along with associated risks and benefits for nonsurgical cosmetic practices for patients with SOC is a void in the primary literature involving these populations. Clinical experience serves to address this deficit in combination with a thorough review of the literature. The 1064-nm Nd:YAG laser has shown clinical utility in the treatment of DPN, melanoma, and acne scars, but it poses financial constraints to the provider in comparison to modalities used for many years. Notably, NAF resurfacing is preferred for the management of acne scars in patients with SOC and continues to gain popularity for the treatment of photoaging. Regarding skin-tightening and body-contouring devices, studies performed in patients with SOC are limited and affected by factors such as small sample sizes, underrepresentation of FSTs IV through VI, short follow-up durations, and a lack of standardized outcome measures. Additionally, few studies assess pigmentary adverse effects or stratify results by skin type, which is critical given the higher risk for PIH in SOC. Ultrasound devices showed clinical utility in improvement of skin laxity, texture, and overall improvement. Patients with SOC respond well to skin-tightening devices due to the increased collagen synthesis. Regarding emerging devices for reduction of adipocytes, deoxycholic acid when injected showed notable improvement in fat reduction but also had adverse effects. As additional studies on cosmetic procedures in SOC emerge, an expansion of treatment options could be offered to this demographic group with confidence, provided proper treatment and follow-up protocols are in place.
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
Cosmetic Laser Procedures and Nonsurgical Body Contouring in Patients With Skin of Color
- Mazzoni D, Lin MJ, Dubin DP, et al. Review of non-invasive body contouring devices for fat reduction, skin tightening and muscle definition. Australas J Dermatol. 2019;60:278-283. doi:10.1111/ajd.13090
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083. doi:10.1111/j.1524-4725.2009.01186.x&
- Schweiger ES, Kwasniak L, Aires DJ. Treatment of dermatosis papulosa nigra with a 1064 nm Nd:YAG laser: report of two cases. J Cosmet Laser Ther. 2008;10:120-122. doi:10.1080/14764170801950070
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426-438. doi:10.1002/lsm.20048
- Alajlan AM, Alsuwaidan SN. Acne scars in ethnic skin treated with both non-ablative fractional 1,550 nm and ablative fractional CO2 lasers: comparative retrospective analysis with recommended guidelines. Lasers Surg Med. 2011;43effi:787-791. doi:10.1002/lsm.21092
- Ke R, Cai B, Ni X, et al. Efficacy and safety of non-ablative vs. ablative lasers for acne scarring: a meta-analysis. J Deutschen Dermatologischen Gesellschaft. Published online March 11, 2025. doi: 10.1111/ddg.15651
- Goel A, Krupashankar DS, Aurangabadkar S, et al. Fractional lasers in dermatology—current status and recommendations. Indian J Dermatol Venereol Leprol. 2011;77:369. doi:10.4103/0378-6323.79732
- Lee HS, Lee JH, Ahn GY, et al. Fractional photothermolysis for the treatment of acne scars: a report of 27 Korean patients. J Dermatolog Treat. 2008;19:45-49. doi:10.1080/09546630701691244
- Zhang AD, Clovie J, Lazar M, et al. Treatment of benign pigmented lesions using lasers: a scoping review. J Clin Med. 2025;14li:3985. doi:10.3390/jcm14113985
- Lipper GM, Perez M. Nonablative acne scar reduction after a series of treatments with a short-pulsed 1,064-nm neodymium:YAG laser. Dermatol Surg. 2006;32:998-1006. doi:10.1111/j.1524-4725.2006.32222.x
- Mar K, Khalid B, Maazi M, et al. Treatment of post-inflammatory hyperpigmentation in skin of colour: a systematic review. J Cutan Med Surg. 2024;28:473-480. doi:10.1177/12034754241265716
- Kono T, Chan HH, Groff WF, et al. Prospective direct comparison study of fractional resurfacing using different fluences and densities for skin rejuvenation in Asians. Lasers Surg Med. 2007;39:311-314. doi:10.1002/lsm.20484
- Sharkey JR, Sharf BF, St John JA. “Una persona derechita (staying right in the mind)”: perceptions of Spanish-speaking Mexican American older adults in South Texas colonias. Gerontologist. 2009;49 suppl 1:S79-85. doi:10.1093/geront/gnp086
- Wu X, Cen Q, Jin J, et al. An effective and safe laser treatment strategy of fractional carbon dioxide laser for Chinese populations with periorbital wrinkles: a randomized split-face trial. Dermatol Therapy. 2025;15:1307-1317.
- Milante RR, Doria-Ruiz MJ, Beloso MB, et al. Split-face comparison of grid fractional radiofrequency vs 1064-nm Nd-YAG laser treatment of periorbital rhytides among Filipino patients. Dermatol Ther. 2020;33:e14031. doi:10.1111/dth.14031
- Alexis AF, Andriessen A, Beach RA, et al. Periprocedural skincare for nonenergy and nonablative energy-based aesthetic procedures in patients with skin of color. J Cosmet Dermatol. 2025;24:E16712. doi:10.1111/jocd.16712
- Marmon S, Shek SYN, Yeung CK, et al. Evaluating the safety and efficacy of the 1,440-nm laser in the treatment of photodamage in Asian skin. Lasers Surg Med. 2014;46:375-379. doi:10.1002/lsm.22242
- Saedi N, Petrell K, Arndt K, et al. Evaluating facial pores and skin texture after low-energy nonablative fractional 1440-nm laser treatments. J Am Acad Dermatol. 2013;68:113-118. doi:10.1016/j.jaad.2012.08.041
- Jih MH, Goldberg LH, Kimyai-Asadi A. Fractional photothermolysis for photoaging of hands. Dermatol Surg. 2008;34:73-78. doi:10.1111/j.1524-4725.2007.34011.x
- Prohaska J, Hohman MH. Laser complications. StatPearls. Updated August 28, 2023. Accessed July 23, 2025. http://www.ncbi.nlm.nih.gov/books/NBK532248/
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20. doi:10.1016/j.ijwd.2017.01.004
- Brauer JA, Kazlouskaya V, Alabdulrazzaq H, et al. Use of a picosecond pulse duration laser with specialized optic for treatment of facial acne scarring. JAMA Dermatol. 2015;151:278-284. doi:10.1001/jamadermatol.2014.3045
- Greywal T, Ortiz A. Treating melasma with the 1064 nm Nd:YAG laser with a 650-microsecond pulse duration: a clinical evaluation. J Cosmet Dermatol. 2021;20:3889-3892. doi:10.1111/jocd.14558
- Weaver SM, Sagaral EC. Treatment of pseudofolliculitis barbae using the long-pulse Nd:YAG laser on skin types V and VI. Dermatol Surg. 2003;29:1187-1191. doi:10.1111/j.1524-4725.2003.29387.x
- Negishi K, Tanaka S, Tobita S. Prospective, randomized, evaluator-blinded study of the long pulse 532-nm KTP laser alone or in combination with the long pulse 1064-nm Nd:YAG laser on facial rejuvenation in Asian skin. Lasers Surg Med. 2016;48:844-851. doi:10.1002/lsm.22582
- Kaushik S, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthetic Dermatol. 2017;10:51-67.
- Garg S, Vashisht KR, Garg D, et al. Advancements in laser therapies for dermal hyperpigmentation in skin of color: a comprehensive literature review and experience of sequential laser treatments in a cohort of 122 Indian patients. J Clin Med. 2024;13:2116. doi:10.3390/jcm13072116
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:e36727. doi:10.5812/ijem.36727
- Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28:79-93. doi:10.1111/j.1467-2494.2006.00302.x
- El-Domyati M, El-Ammawi TS, Medhat W, et al. Radiofrequency facial rejuvenation: Evidence-based effect. J Am Acad Dermatol. 2011;64:524-535. doi:10.1016/j.jaad.2010.06.045
- US Food and Drug Administration. Non-invasive body contouring technologies. Published December 7, 2022. Accessed July 23, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/non-invasive-body-contouring-technologies
- Robinson DM, Kaminer MS, Baumann L, et al. High-intensity focused ultrasound for the reduction of subcutaneous adipose tissue using multiple treatment techniques. Dermatol Surg. 2014;40:641-651. doi:10.1111/dsu.0000000000000022
- Biskanaki F, Tertipi N, Sfyri E, et al. Complications and risks of high-intensity focused ultrasound (HIFU) in esthetic procedures: a review. Applied Sciences. 2025;15:4958. doi:10.3390/app15094958
- Lu PH, Yang CH, Chang YC. Quantitative analysis of face and neck skin tightening by microfocused ultrasound with visualization in Asians. Dermatol Surg. 2017;43:1332-1338. doi:10.1097/DSS.0000000000001181
- Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41:703-708. doi:10.1002/lsm.20864
- Nishikawa A, Aikawa Y. Quantitative assessment of the cryolipolysis method for body contouring in Asian patients. Clin Cosmet Investig Dermatol. 2021;14:1773-1781. doi:10.2147/CCID.S337487
- Bass LS, Doherty ST. Safety and efficacy of a non-invasive 1060 nm diode laser for fat reduction of the abdomen. J Drugs Dermatol. 2018;17:106-112
- Shome D, Khare S, Kapoor R. The use of deoxycholic acid for the clinical reduction of excess submental fat in Indian patients. J Drugs Dermatol. 2019;18:266-272.
- Goodman GJ, Ho WWS, Chang KJ, et al. Efficacy of a novel injection lipolysis to induce targeted adipocyte apoptosis: a randomized, phase IIa study of CBL-514 injection on abdominal subcutaneous fat reduction. Aesthetic Surg J. 2022;42:NP662-NP674. doi:10.1093/asj/sjac162
- McDaniel D, Lozanova P. Human adipocyte apoptosis immediately following high frequency focused field radio frequency: case study.J Drugs Dermatol. 2015;14:622-623.
- Fritz K, Samková P, Salavastru C, et al. A novel selective RF applicator for reducing thigh circumference: a clinical evaluation. Dermatol Ther. 2016;29:92-95. doi:10.1111/dth.12304
- Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: safety and efficacy study of a dual tissue effect based non-invasive abdominal body shaping. Lasers Surg Med. 2019;51:40-46. doi:10.1002/lsm.23024
- Negosanti F, Cannarozzo G, Zingoni T, et al. Is it possible to reshape the body and tone it at the same time? Schwarzy: the new technology for body sculpting. Bioengineering (Basel). 2022;9:284. doi:10.3390/bioengineering9070284
PRACTICE POINTS
- Nonablative fractional lasers are preferred for acne scars in skin of color (SOC), minimizing hyperpigmentation risk.
- The 1064-nm Nd:YAG and picosecond lasers are safe and effective when used with SOC-appropriate settings.
- Photoprotection and topical lightening agents reduce postprocedure pigmentation risks.
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
Personal electronic devices have become more common as consumer-driven health and entertainment practices continue to increase in popularity. A wide variety of devices including smartphones, headphones and earbuds, fitness watches, and continuous glucose monitors (CGMs) allow consumers to collect data and personalize their daily activities and health practices. The global market for fitness tracking devices alone was valued at $62.03 billion in 2024 and is projected to grow to $290.85 billion by 2032.1 Accordingly, the growing demand for continuous data tracking has led to new and prolonged skin contact with these devices, which have become emerging sources of allergic contact dermatitis (ACD). In this article, we provide a summary of the potential allergenicity of personal electronic devices with a focus on wearable devices, including clinical manifestations, reported allergens, and patch testing and management considerations (Table2-28).
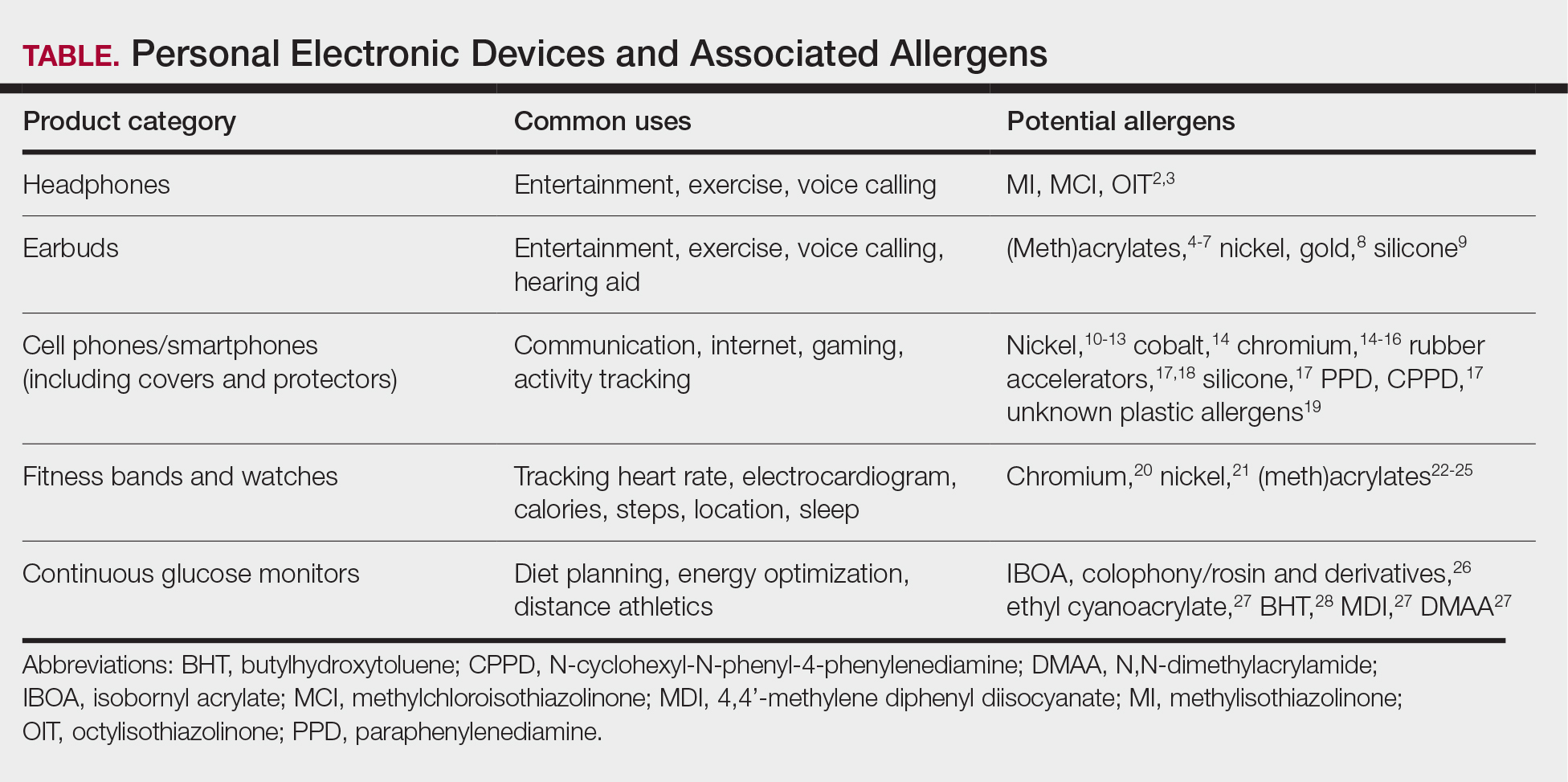
Earbuds and Headphones
Wireless earbuds and headphones are used for listening to media and may contain microphones for voice calls. Earbuds are inserted into the ears while headphones are worn over the ears with a connecting band across the scalp. These devices frequently are worn during physical activity and thus in the setting of moist sweaty environments and mechanical friction on the skin. Depending on the style of the earbuds or headphones, associated ACD may manifest as acute or chronic pruritic eczema involving the inner and/or outer ears and potentially the periauricular areas or scalp.2 In a reported case of earbud ACD, the patient first presented to an otolaryngologist before being referred to a dermatologist for further evaluation and patch testing.9 Clinicians may be unfamiliar with these devices as a source of ACD or may potentially overlook inner ear canal manifestations, which may delay diagnosis.
Allergens reported in earbuds include (meth)acrylates,4-6 nickel, gold,8 and silicone.9 Apple AirPods and Samsung Galaxy Buds disclose the presence of acrylates and nickel.5,6 Cases also have been reported of ACD to gold earbud microphones8 and unknown allergens within silicone tips.4,9 Acrylates, named the 2012 Allergen of the Year by the American Contact Dermatitis Society,29 are used in a wide variety of consumer products as adhesives and coatings and are among the most frequently suspected headphone allergens.4 While fully polymerized acrylates theoretically are nonallergenic, residual acrylic monomers are potent allergens that may be found in in these products due to incomplete curing or polymer breakdown.29 It remains unclear whether earbud allergen concentrations are sufficient to induce sensitization or merely elicit ACD in previously sensitized users.29 Among patients with earbud ACD, the finding of inconsistent patch test reactions/cross-reactions led to the hypothesis that these headphones may contain an unidentified proprietary (meth)acrylate.4
Headphones, often utilized by runners and gymgoers for their comfort and fit, also have gained recent attention for their unique allergen profiles. In 2024, a case series described primary sensitization to octylisothiazolinone causing severe headphone-related ACD.3 This preservative, which is in the same family as methylchloroisothiazolinone/methylisothiazolinone, is used as a biocide in the leather or faux leather that encases the foam padding of headphones.3 Another case report highlighted ACD caused by methylisothiazolinone, methylchloroisothiazolinone, and octylisothiazolinone present in various components of a pair of headphones.2 These cases are notable, as European legislation limiting the use of methylchloroisothiazolinone/methylisothiazolinone in personal care products does not apply to inclusion of isothiazolinones in other product categories, such as detergents, paints, glues, and personal electronic devices.
Mobile Phones
Mobile phones are a staple in modern society, used for a multitude of tasks including communication, internet browsing, entertainment, and activity tracking. In the early 2000s, mobile phone ACD primarily manifested on the lateral face, ears, and periauricular regions,12 as well as the thighs from carriage in pants pockets. Early cases of mobile phone ACD were attributed to metals including chromium16 and nickel.14 At that time, lengthy and frequent phone calls with the device against the ear were thought to increase exposure to metal allergens.30 More recently, as the utility of these devices has evolved, ACD has been reported to manifest on the fingers and hands associated with contact with cell phone cases, accessories, and screen protectors (Figure). In one report, a 17-year-old boy with chronic eczema of the palms was diagnosed with ACD to the rubber-related chemicals paraphenylenediamine and N-cyclohexyl-N-phenyl-4-phenylenediamine, confirmed via chemical analysis to be present in a phone case the patient used during daily gaming.17 Similarly, another case of palmar ACD resulted from thiuram rubber accelerators in a phone case.18 Most recently, a Japanese patient with a history of skin reactions to costume jewelry developed ACD involving the proximal middle finger due to exposure to nickel in a ring-grip phone case.11 While the European Union has enacted regulations regarding maximum nickel leaching in products that come into direct and prolonged contact with the skin, such regulations have not been implemented in Japan or the United States.11 International e-commerce makes these grips widely available, even in regions where strict metal regulations are in place. As screen time increases, it is important to consider all phone-related exposures including components of the case, screen protector, and main device body.
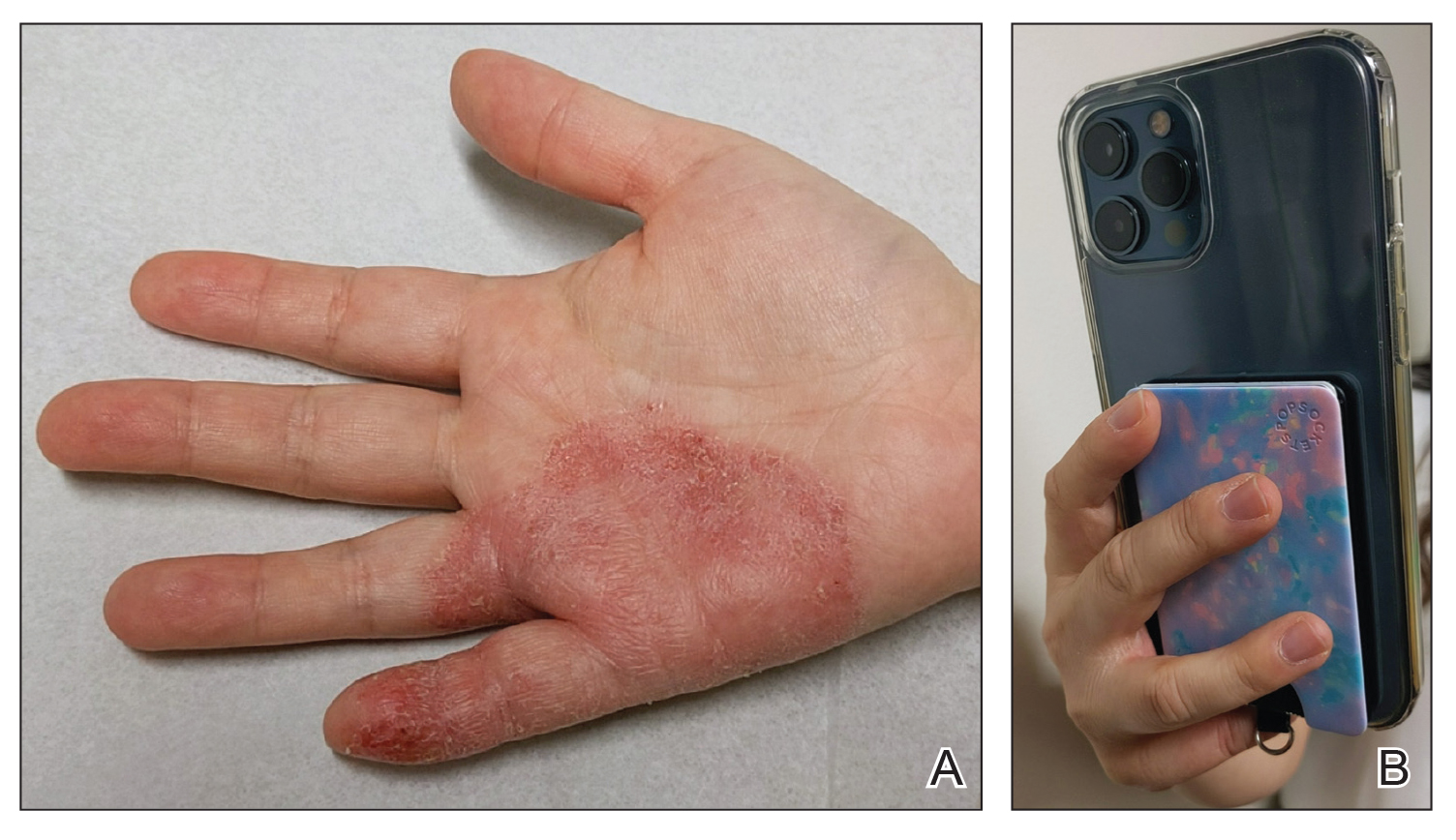
Watches
Smart watches and fitness bands are widely available to consumers and serve a variety of health and lifestyle functions. Features include fitness tracking, notification management, mobile payment, electrocardiography, navigation, and sleep and oxygen sensors. Multiple companies have produced hand- and wrist-based sensors for detailed wellness tracking within these categories. Allergic contact dermatitis to smart watches and wristbands manifests as eczematous lesions on the wrist (dorsal,21,22 volar,20 or circumferential involvement23,24).
(Meth)acrylates used to adhere screen protectors, house lithium ion batteries, and bind metal to plastic have been reported to cause ACD in smart watch users.22,25 In addition, there are at least 2 published reports of ACD to nickel in Apple Watches.21,31 Apple, having sold more than 229 million watches worldwide, has acknowledged the presence of trace acrylates and nickel in their watches (the latter falling below European Registration, Evaluation, Authorization, and Restriction of Chemicals limits).32 Hosoki et al20 identified ACD resulting from chromium exposure in the clasp of an Apple Watch band, which remains unreported by the manufacturer as a potential allergen.
Continuous Glucose Monitors
Continuous glucose monitoring systems provide users with dynamic information on their glycemic status and are associated with lower glycated hemoglobin and reduced episodes of hypoglycemia in patients with diabetes.33 Recently, growing interest in personalized health monitoring and performance optimization has expanded CGM use to individuals without diabetes; there are 2 over-the-counter CGM options currently available in the United States.34
Allergic contact dermatitis to CGMs in patients with diabetes is well characterized, manifesting as pruritic acute or chronic dermatitis at the sensor site.27 To date, we are unaware of published cases of ACD associated with use of CGM in individuals without diabetes; however, wearing a CGM during athletic activities and sweating could potentially increase adhesive degradation and/or penetration of allergens in the skin.6
Isobornyl acrylate, named the 2020 Allergen of the Year,35 is the most well-known contact allergen in glucose sensors.36,33 Initially suspected as a component of the CGM skin adhesive, isobornyl acrylate was found to leach from the device body onto the skin in users of one CGM device.36 Other reported allergens in CGM devices include colophony and related rosin derivatives, ethyl cyanoacrylate, and several chemicals that are not available as commercial patch test substances.27 Understanding these potential allergens is important for patch testing considerations as CGM use increases in individuals without diabetes.
Final Thoughts
Allergic contact dermatitis to personal electronic devices including wearables, sensors, and fitness trackers is an emerging problem that should be considered in cases of dermatitis of the wrists, hands, face, ears, or in any area that comes into contact with such devices. Although in-depth studies are lacking, certain wearable devices appear to introduce continuous, low-level allergen exposure that may be below the sensitization threshold but still is capable of eliciting ACD in previously sensitized users.21,26 Furthermore, increased allergen exposure is facilitated by prolonged skin contact, mechanical friction, and sweat.
Comprehensive patch testing often is necessary to diagnose cases of ACD to personal electronic devices.33 The thin-layer rapid use epicutaneous (T.R.U.E.) test does not include (meth)acrylates, which repeatedly have come up as culprit allergens.37 Isobornyl acrylate, a key allergen related to CGMs, is absent from standard patch test series.26 Nickel remains a common culprit in these devices despite adherence to European regulations.21 Since there is no obligation for manufacturers to declare all possible ingredients, chemical analysis can be useful in identifying potential allergens and directing the patch test strategy, but this is not feasible in general clinical practice outside the research setting.2
Following patch testing, patient education is essential to managing personal electronic device—induced ACD. Informed patients should switch to products that do not contain their triggers—although this may be more easily said than done, since incomplete ingredient disclosure from manufacturers may necessitate a frustrating and expensive trial-and-error approach. As wearable technology proliferates, device composition and potential contact allergen transparency must be prioritized by manufacturers and regulatory bodies. Until then, clinicians should stay on their toes regarding new and emerging clinical presentations and contact allergens in hopes of improving patient outcomes.
- Fitness tracker market size, share & industry analysis, by device type (smart watches, fitness bands, smart glasses, smart clothing, and others), by application (heart rate tracking, sleep measurement, glucose measurement, sports, running, and cycling tracking), by distribution channel (online, retail, and others), and regional forecast, 2025-2032. Fortune Business Insights. Updated June 9, 2025. Accessed June 25, 2025. https://www.fortunebusinessinsights.com/fitness-trackermarket-103358
- Caroppo ES, Stingeni L, Goracci L, et al. Wireless over-ear headphones: a new source of allergic contact dermatitis to isothiazolinones. Contact Dermatitis. 2024;90:621-625. doi:10.1111/cod.14528
- Menanteau M, Fenech G, Adam B, et al. Severe allergic contact dermatitis from octylisothiazolinone in over-ear headphones: a case series. Contact Dermatitis. 2025;92:291-298. doi:10.1111/cod.14733
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461. doi:10.1111/ijd.15954
- Fontane Hoyos CN, Goldminz AM. I’m all ears: common allergens in wireless in-ear headphones. Dermatitis. 2024;35:513-514. doi:10.1089/derm.2023.0251
- Lee LJ, Koh WL, Lim SPR. Allergic contact dermatitis to Apple AirPods Pro. Contact Dermatitis. 2022;86:127-129. doi:10.1111/cod.13987
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112. doi:10.1097/der.0000000000000735
- Hayakawa M, Suzuki C, Zhu Y, et al. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermatitis. 2022;86:328-330. doi:10.1111/cod.14036
- Hua W, Jin Y, Yao X, et al. Allergic contact dermatitis to in-ear headphones occurring in the external ear. Contact Dermatitis. 2024;91:83-85. doi:10.1111/cod.14556
- Guarneri F, Guarneri C, Cannavò SP. An unusual case of cell phone dermatitis. Contact Dermatitis. 2010;62:117. doi:10.1111 /j.1600-0536.2009.01674.x
- Ueda S, Akashi K, Washio K. A case of contact dermatitis caused by a cell phone grip ring. Contact Dermatitis. 2025;92:155-156. doi:10.1111/cod.14719
- Roberts H, Tate B. Nickel allergy presenting as mobile phone contact dermatitis. Australas J Dermatol. 2010;51:23-25. doi:10.1111 /j.1440-0960.2009.00580.x
- Livideanu C, Giordano-Labadie F, Paul C. Cellular phone addiction and allergic contact dermatitis to nickel. Contact Dermatitis. 2007;57:130- 131. doi:10.1111/j.1600-0536.2007.01090.x
- Rajpara A, Feldman SR. Cell phone allergic contact dermatitis: case report and review. Dermatol Online J. 2010;16:9.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Seishima M, Oyama Z, Yamamura M. Cellular phone dermatitis. Arch Dermatol. 2002;2:272-273.
- Corazza M, Schettini N, Catani M, et al. Pediatric allergic contact dermatitis due to rubber additives in a cellphone case. Dermatitis. 2021;32:E140-E141. doi:10.1097/der.0000000000000797
- Hamann D, Sköld MB, Hamann CR, et al. Thiuram allergic contact dermatitis on the hands after skin contact with a rubber cellphone case. Contact Dermatitis. 2019;80:130-131. doi:10.1111/cod.13140
- Williams PJ, King C, Arslanian V. Allergic contact dermatitis caused by a cell phone cover. Australas J Dermatol. 2012;53:76-77. doi:10.1111 /j.1440-0960.2011.00801.x
- Hosoki M, Tajima T, Miyagi M, et al. This report details a case of allergic contact dermatitis resulting from exposure to chromium in the clasp of an Apple Watch band. Dermatitis. Published online December 23, 2024. doi:10.1089/derm.2024.0171
- Levian B, Chan GC, Adler BL. Out of REACH: allergic contact dermatitis to nickel in an Apple Watch. Contact Dermatitis. 2024;90:99-101. doi:10.1111 /cod.14444
- Davies A, Stone N. Watch out! potential allergic contact dermatitis to acrylates in a smart watch. Contact Dermatitis. Published online December 26, 2024. doi:10.1111/cod.14749
- Gatica-Ortega ME, Mowitz M, Navarro-Triviño FJ, et al. Nonoccupational allergic contact dermatitis to 4-acryloylmorpholine in smartwatch screen protectors glue. Dermatitis. 2022;33:429-434. doi:10.1097 /der.0000000000000888
- Otero-Alonso A, Rodríguez-Vázquez V, López-Pesado I, et al. Smartwatch protective cover´s glue: a new non-occupational acrylate allergy. Contact Dermatitis. 2020;83:159-161. doi:10.1111/cod.13586
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Mowitz M, Hosseini S, Siemund I, et al. New device, ‘old’ allergens. allergic contact dermatitis caused by the Dexcom G7 glucose sensor. Contact Dermatitis. 2024;90:495-500. doi:10.1111/cod.14514
- de Groot A, van Oers EM, Ipenburg NA, et al. Allergic contact dermatitis caused by glucose sensors and insulin pumps: a full review: part 1: sensors and pumps, adverse cutaneous reactions, allergens, and diabetes devices causing allergic contact dermatitis. Contact Dermatitis. 2025;92:87-112. doi:10.1111/cod.14698
- Oppel E, Kamann S, Heinemann L, et al. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis. 2020;83:429-431. doi:10.1111/cod.13638
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- Tan S, Nixon R. Allergic contact dermatitis caused by chromium in a mobile phone. Contact Dermatitis. 2011;65:246-247. doi:10.1111 /j.1600-0536.2011.01955.x
- Ko WC, Yu J. Nickel allergy elicited by an Apple Watch. Dermatitis. 2022;33:E11-E12. doi:10.1097/der.0000000000000848
- Apple Support. Wearing your Apple Watch: for people who are sensitive to certain materials. Accessed June 27, 2025. https://support.apple.com/en-us/118234
- Seibold A. Minimizing adverse skin reactions to wearable continuous glucose monitoring sensors in patients with diabetes. J Diabetes Sci Technol. 2021;15:713-714. doi:10.1177/1932296820984763
- Klonoff DC, Nguyen KT, Xu NY, et al. Use of continuous glucose monitors by people without diabetes: an idea whose time has come? J Diabetes Sci Technol. 2023;17:1686-1697. doi:10.1177/19322968221110830
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12. doi:10.1097/der.0000000000000549
- Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. 2020;31:283-286. doi:10.1097/der.0000000000000575
- SmartPractice. Contact dermatitis products. SmartPractice. Accessed April 24, 2025. https://www.smartpractice.com/shop/category?id=581719&m=SPA
Personal electronic devices have become more common as consumer-driven health and entertainment practices continue to increase in popularity. A wide variety of devices including smartphones, headphones and earbuds, fitness watches, and continuous glucose monitors (CGMs) allow consumers to collect data and personalize their daily activities and health practices. The global market for fitness tracking devices alone was valued at $62.03 billion in 2024 and is projected to grow to $290.85 billion by 2032.1 Accordingly, the growing demand for continuous data tracking has led to new and prolonged skin contact with these devices, which have become emerging sources of allergic contact dermatitis (ACD). In this article, we provide a summary of the potential allergenicity of personal electronic devices with a focus on wearable devices, including clinical manifestations, reported allergens, and patch testing and management considerations (Table2-28).

Earbuds and Headphones
Wireless earbuds and headphones are used for listening to media and may contain microphones for voice calls. Earbuds are inserted into the ears while headphones are worn over the ears with a connecting band across the scalp. These devices frequently are worn during physical activity and thus in the setting of moist sweaty environments and mechanical friction on the skin. Depending on the style of the earbuds or headphones, associated ACD may manifest as acute or chronic pruritic eczema involving the inner and/or outer ears and potentially the periauricular areas or scalp.2 In a reported case of earbud ACD, the patient first presented to an otolaryngologist before being referred to a dermatologist for further evaluation and patch testing.9 Clinicians may be unfamiliar with these devices as a source of ACD or may potentially overlook inner ear canal manifestations, which may delay diagnosis.
Allergens reported in earbuds include (meth)acrylates,4-6 nickel, gold,8 and silicone.9 Apple AirPods and Samsung Galaxy Buds disclose the presence of acrylates and nickel.5,6 Cases also have been reported of ACD to gold earbud microphones8 and unknown allergens within silicone tips.4,9 Acrylates, named the 2012 Allergen of the Year by the American Contact Dermatitis Society,29 are used in a wide variety of consumer products as adhesives and coatings and are among the most frequently suspected headphone allergens.4 While fully polymerized acrylates theoretically are nonallergenic, residual acrylic monomers are potent allergens that may be found in in these products due to incomplete curing or polymer breakdown.29 It remains unclear whether earbud allergen concentrations are sufficient to induce sensitization or merely elicit ACD in previously sensitized users.29 Among patients with earbud ACD, the finding of inconsistent patch test reactions/cross-reactions led to the hypothesis that these headphones may contain an unidentified proprietary (meth)acrylate.4
Headphones, often utilized by runners and gymgoers for their comfort and fit, also have gained recent attention for their unique allergen profiles. In 2024, a case series described primary sensitization to octylisothiazolinone causing severe headphone-related ACD.3 This preservative, which is in the same family as methylchloroisothiazolinone/methylisothiazolinone, is used as a biocide in the leather or faux leather that encases the foam padding of headphones.3 Another case report highlighted ACD caused by methylisothiazolinone, methylchloroisothiazolinone, and octylisothiazolinone present in various components of a pair of headphones.2 These cases are notable, as European legislation limiting the use of methylchloroisothiazolinone/methylisothiazolinone in personal care products does not apply to inclusion of isothiazolinones in other product categories, such as detergents, paints, glues, and personal electronic devices.
Mobile Phones
Mobile phones are a staple in modern society, used for a multitude of tasks including communication, internet browsing, entertainment, and activity tracking. In the early 2000s, mobile phone ACD primarily manifested on the lateral face, ears, and periauricular regions,12 as well as the thighs from carriage in pants pockets. Early cases of mobile phone ACD were attributed to metals including chromium16 and nickel.14 At that time, lengthy and frequent phone calls with the device against the ear were thought to increase exposure to metal allergens.30 More recently, as the utility of these devices has evolved, ACD has been reported to manifest on the fingers and hands associated with contact with cell phone cases, accessories, and screen protectors (Figure). In one report, a 17-year-old boy with chronic eczema of the palms was diagnosed with ACD to the rubber-related chemicals paraphenylenediamine and N-cyclohexyl-N-phenyl-4-phenylenediamine, confirmed via chemical analysis to be present in a phone case the patient used during daily gaming.17 Similarly, another case of palmar ACD resulted from thiuram rubber accelerators in a phone case.18 Most recently, a Japanese patient with a history of skin reactions to costume jewelry developed ACD involving the proximal middle finger due to exposure to nickel in a ring-grip phone case.11 While the European Union has enacted regulations regarding maximum nickel leaching in products that come into direct and prolonged contact with the skin, such regulations have not been implemented in Japan or the United States.11 International e-commerce makes these grips widely available, even in regions where strict metal regulations are in place. As screen time increases, it is important to consider all phone-related exposures including components of the case, screen protector, and main device body.

Watches
Smart watches and fitness bands are widely available to consumers and serve a variety of health and lifestyle functions. Features include fitness tracking, notification management, mobile payment, electrocardiography, navigation, and sleep and oxygen sensors. Multiple companies have produced hand- and wrist-based sensors for detailed wellness tracking within these categories. Allergic contact dermatitis to smart watches and wristbands manifests as eczematous lesions on the wrist (dorsal,21,22 volar,20 or circumferential involvement23,24).
(Meth)acrylates used to adhere screen protectors, house lithium ion batteries, and bind metal to plastic have been reported to cause ACD in smart watch users.22,25 In addition, there are at least 2 published reports of ACD to nickel in Apple Watches.21,31 Apple, having sold more than 229 million watches worldwide, has acknowledged the presence of trace acrylates and nickel in their watches (the latter falling below European Registration, Evaluation, Authorization, and Restriction of Chemicals limits).32 Hosoki et al20 identified ACD resulting from chromium exposure in the clasp of an Apple Watch band, which remains unreported by the manufacturer as a potential allergen.
Continuous Glucose Monitors
Continuous glucose monitoring systems provide users with dynamic information on their glycemic status and are associated with lower glycated hemoglobin and reduced episodes of hypoglycemia in patients with diabetes.33 Recently, growing interest in personalized health monitoring and performance optimization has expanded CGM use to individuals without diabetes; there are 2 over-the-counter CGM options currently available in the United States.34
Allergic contact dermatitis to CGMs in patients with diabetes is well characterized, manifesting as pruritic acute or chronic dermatitis at the sensor site.27 To date, we are unaware of published cases of ACD associated with use of CGM in individuals without diabetes; however, wearing a CGM during athletic activities and sweating could potentially increase adhesive degradation and/or penetration of allergens in the skin.6
Isobornyl acrylate, named the 2020 Allergen of the Year,35 is the most well-known contact allergen in glucose sensors.36,33 Initially suspected as a component of the CGM skin adhesive, isobornyl acrylate was found to leach from the device body onto the skin in users of one CGM device.36 Other reported allergens in CGM devices include colophony and related rosin derivatives, ethyl cyanoacrylate, and several chemicals that are not available as commercial patch test substances.27 Understanding these potential allergens is important for patch testing considerations as CGM use increases in individuals without diabetes.
Final Thoughts
Allergic contact dermatitis to personal electronic devices including wearables, sensors, and fitness trackers is an emerging problem that should be considered in cases of dermatitis of the wrists, hands, face, ears, or in any area that comes into contact with such devices. Although in-depth studies are lacking, certain wearable devices appear to introduce continuous, low-level allergen exposure that may be below the sensitization threshold but still is capable of eliciting ACD in previously sensitized users.21,26 Furthermore, increased allergen exposure is facilitated by prolonged skin contact, mechanical friction, and sweat.
Comprehensive patch testing often is necessary to diagnose cases of ACD to personal electronic devices.33 The thin-layer rapid use epicutaneous (T.R.U.E.) test does not include (meth)acrylates, which repeatedly have come up as culprit allergens.37 Isobornyl acrylate, a key allergen related to CGMs, is absent from standard patch test series.26 Nickel remains a common culprit in these devices despite adherence to European regulations.21 Since there is no obligation for manufacturers to declare all possible ingredients, chemical analysis can be useful in identifying potential allergens and directing the patch test strategy, but this is not feasible in general clinical practice outside the research setting.2
Following patch testing, patient education is essential to managing personal electronic device—induced ACD. Informed patients should switch to products that do not contain their triggers—although this may be more easily said than done, since incomplete ingredient disclosure from manufacturers may necessitate a frustrating and expensive trial-and-error approach. As wearable technology proliferates, device composition and potential contact allergen transparency must be prioritized by manufacturers and regulatory bodies. Until then, clinicians should stay on their toes regarding new and emerging clinical presentations and contact allergens in hopes of improving patient outcomes.
Personal electronic devices have become more common as consumer-driven health and entertainment practices continue to increase in popularity. A wide variety of devices including smartphones, headphones and earbuds, fitness watches, and continuous glucose monitors (CGMs) allow consumers to collect data and personalize their daily activities and health practices. The global market for fitness tracking devices alone was valued at $62.03 billion in 2024 and is projected to grow to $290.85 billion by 2032.1 Accordingly, the growing demand for continuous data tracking has led to new and prolonged skin contact with these devices, which have become emerging sources of allergic contact dermatitis (ACD). In this article, we provide a summary of the potential allergenicity of personal electronic devices with a focus on wearable devices, including clinical manifestations, reported allergens, and patch testing and management considerations (Table2-28).

Earbuds and Headphones
Wireless earbuds and headphones are used for listening to media and may contain microphones for voice calls. Earbuds are inserted into the ears while headphones are worn over the ears with a connecting band across the scalp. These devices frequently are worn during physical activity and thus in the setting of moist sweaty environments and mechanical friction on the skin. Depending on the style of the earbuds or headphones, associated ACD may manifest as acute or chronic pruritic eczema involving the inner and/or outer ears and potentially the periauricular areas or scalp.2 In a reported case of earbud ACD, the patient first presented to an otolaryngologist before being referred to a dermatologist for further evaluation and patch testing.9 Clinicians may be unfamiliar with these devices as a source of ACD or may potentially overlook inner ear canal manifestations, which may delay diagnosis.
Allergens reported in earbuds include (meth)acrylates,4-6 nickel, gold,8 and silicone.9 Apple AirPods and Samsung Galaxy Buds disclose the presence of acrylates and nickel.5,6 Cases also have been reported of ACD to gold earbud microphones8 and unknown allergens within silicone tips.4,9 Acrylates, named the 2012 Allergen of the Year by the American Contact Dermatitis Society,29 are used in a wide variety of consumer products as adhesives and coatings and are among the most frequently suspected headphone allergens.4 While fully polymerized acrylates theoretically are nonallergenic, residual acrylic monomers are potent allergens that may be found in in these products due to incomplete curing or polymer breakdown.29 It remains unclear whether earbud allergen concentrations are sufficient to induce sensitization or merely elicit ACD in previously sensitized users.29 Among patients with earbud ACD, the finding of inconsistent patch test reactions/cross-reactions led to the hypothesis that these headphones may contain an unidentified proprietary (meth)acrylate.4
Headphones, often utilized by runners and gymgoers for their comfort and fit, also have gained recent attention for their unique allergen profiles. In 2024, a case series described primary sensitization to octylisothiazolinone causing severe headphone-related ACD.3 This preservative, which is in the same family as methylchloroisothiazolinone/methylisothiazolinone, is used as a biocide in the leather or faux leather that encases the foam padding of headphones.3 Another case report highlighted ACD caused by methylisothiazolinone, methylchloroisothiazolinone, and octylisothiazolinone present in various components of a pair of headphones.2 These cases are notable, as European legislation limiting the use of methylchloroisothiazolinone/methylisothiazolinone in personal care products does not apply to inclusion of isothiazolinones in other product categories, such as detergents, paints, glues, and personal electronic devices.
Mobile Phones
Mobile phones are a staple in modern society, used for a multitude of tasks including communication, internet browsing, entertainment, and activity tracking. In the early 2000s, mobile phone ACD primarily manifested on the lateral face, ears, and periauricular regions,12 as well as the thighs from carriage in pants pockets. Early cases of mobile phone ACD were attributed to metals including chromium16 and nickel.14 At that time, lengthy and frequent phone calls with the device against the ear were thought to increase exposure to metal allergens.30 More recently, as the utility of these devices has evolved, ACD has been reported to manifest on the fingers and hands associated with contact with cell phone cases, accessories, and screen protectors (Figure). In one report, a 17-year-old boy with chronic eczema of the palms was diagnosed with ACD to the rubber-related chemicals paraphenylenediamine and N-cyclohexyl-N-phenyl-4-phenylenediamine, confirmed via chemical analysis to be present in a phone case the patient used during daily gaming.17 Similarly, another case of palmar ACD resulted from thiuram rubber accelerators in a phone case.18 Most recently, a Japanese patient with a history of skin reactions to costume jewelry developed ACD involving the proximal middle finger due to exposure to nickel in a ring-grip phone case.11 While the European Union has enacted regulations regarding maximum nickel leaching in products that come into direct and prolonged contact with the skin, such regulations have not been implemented in Japan or the United States.11 International e-commerce makes these grips widely available, even in regions where strict metal regulations are in place. As screen time increases, it is important to consider all phone-related exposures including components of the case, screen protector, and main device body.

Watches
Smart watches and fitness bands are widely available to consumers and serve a variety of health and lifestyle functions. Features include fitness tracking, notification management, mobile payment, electrocardiography, navigation, and sleep and oxygen sensors. Multiple companies have produced hand- and wrist-based sensors for detailed wellness tracking within these categories. Allergic contact dermatitis to smart watches and wristbands manifests as eczematous lesions on the wrist (dorsal,21,22 volar,20 or circumferential involvement23,24).
(Meth)acrylates used to adhere screen protectors, house lithium ion batteries, and bind metal to plastic have been reported to cause ACD in smart watch users.22,25 In addition, there are at least 2 published reports of ACD to nickel in Apple Watches.21,31 Apple, having sold more than 229 million watches worldwide, has acknowledged the presence of trace acrylates and nickel in their watches (the latter falling below European Registration, Evaluation, Authorization, and Restriction of Chemicals limits).32 Hosoki et al20 identified ACD resulting from chromium exposure in the clasp of an Apple Watch band, which remains unreported by the manufacturer as a potential allergen.
Continuous Glucose Monitors
Continuous glucose monitoring systems provide users with dynamic information on their glycemic status and are associated with lower glycated hemoglobin and reduced episodes of hypoglycemia in patients with diabetes.33 Recently, growing interest in personalized health monitoring and performance optimization has expanded CGM use to individuals without diabetes; there are 2 over-the-counter CGM options currently available in the United States.34
Allergic contact dermatitis to CGMs in patients with diabetes is well characterized, manifesting as pruritic acute or chronic dermatitis at the sensor site.27 To date, we are unaware of published cases of ACD associated with use of CGM in individuals without diabetes; however, wearing a CGM during athletic activities and sweating could potentially increase adhesive degradation and/or penetration of allergens in the skin.6
Isobornyl acrylate, named the 2020 Allergen of the Year,35 is the most well-known contact allergen in glucose sensors.36,33 Initially suspected as a component of the CGM skin adhesive, isobornyl acrylate was found to leach from the device body onto the skin in users of one CGM device.36 Other reported allergens in CGM devices include colophony and related rosin derivatives, ethyl cyanoacrylate, and several chemicals that are not available as commercial patch test substances.27 Understanding these potential allergens is important for patch testing considerations as CGM use increases in individuals without diabetes.
Final Thoughts
Allergic contact dermatitis to personal electronic devices including wearables, sensors, and fitness trackers is an emerging problem that should be considered in cases of dermatitis of the wrists, hands, face, ears, or in any area that comes into contact with such devices. Although in-depth studies are lacking, certain wearable devices appear to introduce continuous, low-level allergen exposure that may be below the sensitization threshold but still is capable of eliciting ACD in previously sensitized users.21,26 Furthermore, increased allergen exposure is facilitated by prolonged skin contact, mechanical friction, and sweat.
Comprehensive patch testing often is necessary to diagnose cases of ACD to personal electronic devices.33 The thin-layer rapid use epicutaneous (T.R.U.E.) test does not include (meth)acrylates, which repeatedly have come up as culprit allergens.37 Isobornyl acrylate, a key allergen related to CGMs, is absent from standard patch test series.26 Nickel remains a common culprit in these devices despite adherence to European regulations.21 Since there is no obligation for manufacturers to declare all possible ingredients, chemical analysis can be useful in identifying potential allergens and directing the patch test strategy, but this is not feasible in general clinical practice outside the research setting.2
Following patch testing, patient education is essential to managing personal electronic device—induced ACD. Informed patients should switch to products that do not contain their triggers—although this may be more easily said than done, since incomplete ingredient disclosure from manufacturers may necessitate a frustrating and expensive trial-and-error approach. As wearable technology proliferates, device composition and potential contact allergen transparency must be prioritized by manufacturers and regulatory bodies. Until then, clinicians should stay on their toes regarding new and emerging clinical presentations and contact allergens in hopes of improving patient outcomes.
- Fitness tracker market size, share & industry analysis, by device type (smart watches, fitness bands, smart glasses, smart clothing, and others), by application (heart rate tracking, sleep measurement, glucose measurement, sports, running, and cycling tracking), by distribution channel (online, retail, and others), and regional forecast, 2025-2032. Fortune Business Insights. Updated June 9, 2025. Accessed June 25, 2025. https://www.fortunebusinessinsights.com/fitness-trackermarket-103358
- Caroppo ES, Stingeni L, Goracci L, et al. Wireless over-ear headphones: a new source of allergic contact dermatitis to isothiazolinones. Contact Dermatitis. 2024;90:621-625. doi:10.1111/cod.14528
- Menanteau M, Fenech G, Adam B, et al. Severe allergic contact dermatitis from octylisothiazolinone in over-ear headphones: a case series. Contact Dermatitis. 2025;92:291-298. doi:10.1111/cod.14733
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461. doi:10.1111/ijd.15954
- Fontane Hoyos CN, Goldminz AM. I’m all ears: common allergens in wireless in-ear headphones. Dermatitis. 2024;35:513-514. doi:10.1089/derm.2023.0251
- Lee LJ, Koh WL, Lim SPR. Allergic contact dermatitis to Apple AirPods Pro. Contact Dermatitis. 2022;86:127-129. doi:10.1111/cod.13987
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112. doi:10.1097/der.0000000000000735
- Hayakawa M, Suzuki C, Zhu Y, et al. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermatitis. 2022;86:328-330. doi:10.1111/cod.14036
- Hua W, Jin Y, Yao X, et al. Allergic contact dermatitis to in-ear headphones occurring in the external ear. Contact Dermatitis. 2024;91:83-85. doi:10.1111/cod.14556
- Guarneri F, Guarneri C, Cannavò SP. An unusual case of cell phone dermatitis. Contact Dermatitis. 2010;62:117. doi:10.1111 /j.1600-0536.2009.01674.x
- Ueda S, Akashi K, Washio K. A case of contact dermatitis caused by a cell phone grip ring. Contact Dermatitis. 2025;92:155-156. doi:10.1111/cod.14719
- Roberts H, Tate B. Nickel allergy presenting as mobile phone contact dermatitis. Australas J Dermatol. 2010;51:23-25. doi:10.1111 /j.1440-0960.2009.00580.x
- Livideanu C, Giordano-Labadie F, Paul C. Cellular phone addiction and allergic contact dermatitis to nickel. Contact Dermatitis. 2007;57:130- 131. doi:10.1111/j.1600-0536.2007.01090.x
- Rajpara A, Feldman SR. Cell phone allergic contact dermatitis: case report and review. Dermatol Online J. 2010;16:9.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Seishima M, Oyama Z, Yamamura M. Cellular phone dermatitis. Arch Dermatol. 2002;2:272-273.
- Corazza M, Schettini N, Catani M, et al. Pediatric allergic contact dermatitis due to rubber additives in a cellphone case. Dermatitis. 2021;32:E140-E141. doi:10.1097/der.0000000000000797
- Hamann D, Sköld MB, Hamann CR, et al. Thiuram allergic contact dermatitis on the hands after skin contact with a rubber cellphone case. Contact Dermatitis. 2019;80:130-131. doi:10.1111/cod.13140
- Williams PJ, King C, Arslanian V. Allergic contact dermatitis caused by a cell phone cover. Australas J Dermatol. 2012;53:76-77. doi:10.1111 /j.1440-0960.2011.00801.x
- Hosoki M, Tajima T, Miyagi M, et al. This report details a case of allergic contact dermatitis resulting from exposure to chromium in the clasp of an Apple Watch band. Dermatitis. Published online December 23, 2024. doi:10.1089/derm.2024.0171
- Levian B, Chan GC, Adler BL. Out of REACH: allergic contact dermatitis to nickel in an Apple Watch. Contact Dermatitis. 2024;90:99-101. doi:10.1111 /cod.14444
- Davies A, Stone N. Watch out! potential allergic contact dermatitis to acrylates in a smart watch. Contact Dermatitis. Published online December 26, 2024. doi:10.1111/cod.14749
- Gatica-Ortega ME, Mowitz M, Navarro-Triviño FJ, et al. Nonoccupational allergic contact dermatitis to 4-acryloylmorpholine in smartwatch screen protectors glue. Dermatitis. 2022;33:429-434. doi:10.1097 /der.0000000000000888
- Otero-Alonso A, Rodríguez-Vázquez V, López-Pesado I, et al. Smartwatch protective cover´s glue: a new non-occupational acrylate allergy. Contact Dermatitis. 2020;83:159-161. doi:10.1111/cod.13586
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Mowitz M, Hosseini S, Siemund I, et al. New device, ‘old’ allergens. allergic contact dermatitis caused by the Dexcom G7 glucose sensor. Contact Dermatitis. 2024;90:495-500. doi:10.1111/cod.14514
- de Groot A, van Oers EM, Ipenburg NA, et al. Allergic contact dermatitis caused by glucose sensors and insulin pumps: a full review: part 1: sensors and pumps, adverse cutaneous reactions, allergens, and diabetes devices causing allergic contact dermatitis. Contact Dermatitis. 2025;92:87-112. doi:10.1111/cod.14698
- Oppel E, Kamann S, Heinemann L, et al. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis. 2020;83:429-431. doi:10.1111/cod.13638
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- Tan S, Nixon R. Allergic contact dermatitis caused by chromium in a mobile phone. Contact Dermatitis. 2011;65:246-247. doi:10.1111 /j.1600-0536.2011.01955.x
- Ko WC, Yu J. Nickel allergy elicited by an Apple Watch. Dermatitis. 2022;33:E11-E12. doi:10.1097/der.0000000000000848
- Apple Support. Wearing your Apple Watch: for people who are sensitive to certain materials. Accessed June 27, 2025. https://support.apple.com/en-us/118234
- Seibold A. Minimizing adverse skin reactions to wearable continuous glucose monitoring sensors in patients with diabetes. J Diabetes Sci Technol. 2021;15:713-714. doi:10.1177/1932296820984763
- Klonoff DC, Nguyen KT, Xu NY, et al. Use of continuous glucose monitors by people without diabetes: an idea whose time has come? J Diabetes Sci Technol. 2023;17:1686-1697. doi:10.1177/19322968221110830
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12. doi:10.1097/der.0000000000000549
- Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. 2020;31:283-286. doi:10.1097/der.0000000000000575
- SmartPractice. Contact dermatitis products. SmartPractice. Accessed April 24, 2025. https://www.smartpractice.com/shop/category?id=581719&m=SPA
- Fitness tracker market size, share & industry analysis, by device type (smart watches, fitness bands, smart glasses, smart clothing, and others), by application (heart rate tracking, sleep measurement, glucose measurement, sports, running, and cycling tracking), by distribution channel (online, retail, and others), and regional forecast, 2025-2032. Fortune Business Insights. Updated June 9, 2025. Accessed June 25, 2025. https://www.fortunebusinessinsights.com/fitness-trackermarket-103358
- Caroppo ES, Stingeni L, Goracci L, et al. Wireless over-ear headphones: a new source of allergic contact dermatitis to isothiazolinones. Contact Dermatitis. 2024;90:621-625. doi:10.1111/cod.14528
- Menanteau M, Fenech G, Adam B, et al. Severe allergic contact dermatitis from octylisothiazolinone in over-ear headphones: a case series. Contact Dermatitis. 2025;92:291-298. doi:10.1111/cod.14733
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461. doi:10.1111/ijd.15954
- Fontane Hoyos CN, Goldminz AM. I’m all ears: common allergens in wireless in-ear headphones. Dermatitis. 2024;35:513-514. doi:10.1089/derm.2023.0251
- Lee LJ, Koh WL, Lim SPR. Allergic contact dermatitis to Apple AirPods Pro. Contact Dermatitis. 2022;86:127-129. doi:10.1111/cod.13987
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112. doi:10.1097/der.0000000000000735
- Hayakawa M, Suzuki C, Zhu Y, et al. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermatitis. 2022;86:328-330. doi:10.1111/cod.14036
- Hua W, Jin Y, Yao X, et al. Allergic contact dermatitis to in-ear headphones occurring in the external ear. Contact Dermatitis. 2024;91:83-85. doi:10.1111/cod.14556
- Guarneri F, Guarneri C, Cannavò SP. An unusual case of cell phone dermatitis. Contact Dermatitis. 2010;62:117. doi:10.1111 /j.1600-0536.2009.01674.x
- Ueda S, Akashi K, Washio K. A case of contact dermatitis caused by a cell phone grip ring. Contact Dermatitis. 2025;92:155-156. doi:10.1111/cod.14719
- Roberts H, Tate B. Nickel allergy presenting as mobile phone contact dermatitis. Australas J Dermatol. 2010;51:23-25. doi:10.1111 /j.1440-0960.2009.00580.x
- Livideanu C, Giordano-Labadie F, Paul C. Cellular phone addiction and allergic contact dermatitis to nickel. Contact Dermatitis. 2007;57:130- 131. doi:10.1111/j.1600-0536.2007.01090.x
- Rajpara A, Feldman SR. Cell phone allergic contact dermatitis: case report and review. Dermatol Online J. 2010;16:9.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Seishima M, Oyama Z, Yamamura M. Cellular phone dermatitis. Arch Dermatol. 2002;2:272-273.
- Corazza M, Schettini N, Catani M, et al. Pediatric allergic contact dermatitis due to rubber additives in a cellphone case. Dermatitis. 2021;32:E140-E141. doi:10.1097/der.0000000000000797
- Hamann D, Sköld MB, Hamann CR, et al. Thiuram allergic contact dermatitis on the hands after skin contact with a rubber cellphone case. Contact Dermatitis. 2019;80:130-131. doi:10.1111/cod.13140
- Williams PJ, King C, Arslanian V. Allergic contact dermatitis caused by a cell phone cover. Australas J Dermatol. 2012;53:76-77. doi:10.1111 /j.1440-0960.2011.00801.x
- Hosoki M, Tajima T, Miyagi M, et al. This report details a case of allergic contact dermatitis resulting from exposure to chromium in the clasp of an Apple Watch band. Dermatitis. Published online December 23, 2024. doi:10.1089/derm.2024.0171
- Levian B, Chan GC, Adler BL. Out of REACH: allergic contact dermatitis to nickel in an Apple Watch. Contact Dermatitis. 2024;90:99-101. doi:10.1111 /cod.14444
- Davies A, Stone N. Watch out! potential allergic contact dermatitis to acrylates in a smart watch. Contact Dermatitis. Published online December 26, 2024. doi:10.1111/cod.14749
- Gatica-Ortega ME, Mowitz M, Navarro-Triviño FJ, et al. Nonoccupational allergic contact dermatitis to 4-acryloylmorpholine in smartwatch screen protectors glue. Dermatitis. 2022;33:429-434. doi:10.1097 /der.0000000000000888
- Otero-Alonso A, Rodríguez-Vázquez V, López-Pesado I, et al. Smartwatch protective cover´s glue: a new non-occupational acrylate allergy. Contact Dermatitis. 2020;83:159-161. doi:10.1111/cod.13586
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Mowitz M, Hosseini S, Siemund I, et al. New device, ‘old’ allergens. allergic contact dermatitis caused by the Dexcom G7 glucose sensor. Contact Dermatitis. 2024;90:495-500. doi:10.1111/cod.14514
- de Groot A, van Oers EM, Ipenburg NA, et al. Allergic contact dermatitis caused by glucose sensors and insulin pumps: a full review: part 1: sensors and pumps, adverse cutaneous reactions, allergens, and diabetes devices causing allergic contact dermatitis. Contact Dermatitis. 2025;92:87-112. doi:10.1111/cod.14698
- Oppel E, Kamann S, Heinemann L, et al. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis. 2020;83:429-431. doi:10.1111/cod.13638
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- Tan S, Nixon R. Allergic contact dermatitis caused by chromium in a mobile phone. Contact Dermatitis. 2011;65:246-247. doi:10.1111 /j.1600-0536.2011.01955.x
- Ko WC, Yu J. Nickel allergy elicited by an Apple Watch. Dermatitis. 2022;33:E11-E12. doi:10.1097/der.0000000000000848
- Apple Support. Wearing your Apple Watch: for people who are sensitive to certain materials. Accessed June 27, 2025. https://support.apple.com/en-us/118234
- Seibold A. Minimizing adverse skin reactions to wearable continuous glucose monitoring sensors in patients with diabetes. J Diabetes Sci Technol. 2021;15:713-714. doi:10.1177/1932296820984763
- Klonoff DC, Nguyen KT, Xu NY, et al. Use of continuous glucose monitors by people without diabetes: an idea whose time has come? J Diabetes Sci Technol. 2023;17:1686-1697. doi:10.1177/19322968221110830
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12. doi:10.1097/der.0000000000000549
- Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. 2020;31:283-286. doi:10.1097/der.0000000000000575
- SmartPractice. Contact dermatitis products. SmartPractice. Accessed April 24, 2025. https://www.smartpractice.com/shop/category?id=581719&m=SPA
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
PRACTICE POINTS
- Personal electronic devices including smart phones, headphones, watches, and continuous glucose monitors represent an emerging source of allergic contact dermatitis.
- Reactions often are localized to areas of skin contact including the face, ears, wrists, and hands.
- Reported allergens in personal electronic devices include (meth)acrylates, metals, and rubber compounds.
- Patch testing is key in detecting and avoiding culprit allergens, but a major challenge is lack of transparency regarding device composition and ingredients.
Choosing the Best Formalin-Resistant Ink for Biopsy Specimen Labeling
Choosing the Best Formalin-Resistant Ink for Biopsy Specimen Labeling
Practice Gap
Many dermatology practices utilize pens and markers to label biopsy specimen containers, but the ink may have variable susceptibility to fading and smearing when exposed to moisture before processing. Specimen containers often are placed in plastic bags for transport. If formalin accidentally spills into the bag during this time, the labels may be exposed to moisture for hours, overnight, or even over a weekend. Effective labeling with formalin-resistant ink is crucial for maintaining the clarity of anatomic location and planning treatment, especially when multiple samples are obtained.
The Technique
We tested 12 pens and markers commonly used when labeling specimen containers to determine their susceptibility to fading due to accidental formalin exposure (Figure). Various inks were allowed to dry on sample specimen labels for 5 minutes before a thin layer of 10% buffered formalin was evenly distributed over the dried ink. Photographs of the labels were taken at baseline as well as 15 minutes, 1 hour, 3 hours, and 24 hours after formalin exposure.
Fading was observed in both the skin marker and gel panes after 15 minutes and peaked after 1 hour. Gel pens were most susceptible to fading on exposure to formalin, and the level of fading varied by ink color, with certain colors disappearing almost entirely (Figure). The solvent-resistant marker had a robust defense to formalin, as did both ballpoint pens.
Practice Implications
Given our findings, dermatology practices should avoid using gel pens to label specimen containers. Solvent-resistant markers performed as expected; however, ballpoint pens appeared to withstand formalin exposure to a similar degree and often are more readily available. Labeling biopsy specimens with an appropriate ink ensures that each sample is clearly identified with the appropriate anatomic location and any other relevant patient information.
Practice Gap
Many dermatology practices utilize pens and markers to label biopsy specimen containers, but the ink may have variable susceptibility to fading and smearing when exposed to moisture before processing. Specimen containers often are placed in plastic bags for transport. If formalin accidentally spills into the bag during this time, the labels may be exposed to moisture for hours, overnight, or even over a weekend. Effective labeling with formalin-resistant ink is crucial for maintaining the clarity of anatomic location and planning treatment, especially when multiple samples are obtained.
The Technique
We tested 12 pens and markers commonly used when labeling specimen containers to determine their susceptibility to fading due to accidental formalin exposure (Figure). Various inks were allowed to dry on sample specimen labels for 5 minutes before a thin layer of 10% buffered formalin was evenly distributed over the dried ink. Photographs of the labels were taken at baseline as well as 15 minutes, 1 hour, 3 hours, and 24 hours after formalin exposure.

Fading was observed in both the skin marker and gel panes after 15 minutes and peaked after 1 hour. Gel pens were most susceptible to fading on exposure to formalin, and the level of fading varied by ink color, with certain colors disappearing almost entirely (Figure). The solvent-resistant marker had a robust defense to formalin, as did both ballpoint pens.
Practice Implications
Given our findings, dermatology practices should avoid using gel pens to label specimen containers. Solvent-resistant markers performed as expected; however, ballpoint pens appeared to withstand formalin exposure to a similar degree and often are more readily available. Labeling biopsy specimens with an appropriate ink ensures that each sample is clearly identified with the appropriate anatomic location and any other relevant patient information.
Practice Gap
Many dermatology practices utilize pens and markers to label biopsy specimen containers, but the ink may have variable susceptibility to fading and smearing when exposed to moisture before processing. Specimen containers often are placed in plastic bags for transport. If formalin accidentally spills into the bag during this time, the labels may be exposed to moisture for hours, overnight, or even over a weekend. Effective labeling with formalin-resistant ink is crucial for maintaining the clarity of anatomic location and planning treatment, especially when multiple samples are obtained.
The Technique
We tested 12 pens and markers commonly used when labeling specimen containers to determine their susceptibility to fading due to accidental formalin exposure (Figure). Various inks were allowed to dry on sample specimen labels for 5 minutes before a thin layer of 10% buffered formalin was evenly distributed over the dried ink. Photographs of the labels were taken at baseline as well as 15 minutes, 1 hour, 3 hours, and 24 hours after formalin exposure.

Fading was observed in both the skin marker and gel panes after 15 minutes and peaked after 1 hour. Gel pens were most susceptible to fading on exposure to formalin, and the level of fading varied by ink color, with certain colors disappearing almost entirely (Figure). The solvent-resistant marker had a robust defense to formalin, as did both ballpoint pens.
Practice Implications
Given our findings, dermatology practices should avoid using gel pens to label specimen containers. Solvent-resistant markers performed as expected; however, ballpoint pens appeared to withstand formalin exposure to a similar degree and often are more readily available. Labeling biopsy specimens with an appropriate ink ensures that each sample is clearly identified with the appropriate anatomic location and any other relevant patient information.
Choosing the Best Formalin-Resistant Ink for Biopsy Specimen Labeling
Choosing the Best Formalin-Resistant Ink for Biopsy Specimen Labeling
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
The umbrella term skin of color (SOC) includes individuals identifying as Black/African, Hispanic, Asian, Native American, Middle Eastern, and Mediterranean as well as multiracial groups. While the Fitzpatrick skin typing system is not an accurate proxy for describing skin tone, SOC populations typically correspond to Fitzpatrick skin types IV to VI, and clinical researchers often report the Fitzpatrick skin type of their study populations.1
Over the past several decades, the underrepresentation of diverse skin tones in educational resources has limited clinical training.2 For example, only 10.3% of conditions featured in contemporary dermatology textbooks are shown in darker skin tones.3 This educational resource gap has spurred a transformative movement toward inclusivity in dermatologic education, research, and clinical practice. Notable examples include VisualDx4 and Dermatology for Skin of Color.5 In addition, Cutis began publishing the Dx Across the Skin Color Spectrum fact sheet series in 2022 to highlight differences in how cutaneous conditions manifest in various skin tones (https://www.mdedge.com/cutis/dx-across-skin-color-spectrum).
These resources play a critical role in advancing dermatologic knowledge, ensuring that dermatologists and other health care professionals are well equipped to diagnose and treat dermatologic conditions in SOC populations with accuracy and cultural humility. These innovations also have enhanced our understanding of how common dermatologic conditions manifest and respond to treatment in SOC populations. Herein, we highlight advances in diagnostic and therapeutic approaches for the most common concerns among SOC populations in the United States, including acne vulgaris, atopic dermatitis (AD), seborrheic dermatitis (SD), melasma, postinflammatory hyperpigmentation, psoriasis, and seborrheic keratosis.
Chief Concerns Common Among SOC Populations in the United States
Acne Vulgaris—In patients with SOC, acne frequently results in pigmentary changes and scarring that can manifest as both hypertrophic and keloidal scars.6 Clinical evidence from randomized controlled studies supports the use of topical dapsone gel as a safe and effective frontline treatment for acne in patients with SOC.7,8 Notably, the US Food and Drug Administration–approved 1726-nm laser with a contact-cooling sapphire window has demonstrated safety and efficacy in the management of acne across Fitzpatrick skin types II to VI.9-11 To manage atrophic acne scars, cutting-edge laser and radiofrequency devices including erbium-doped yttrium aluminum garnet, fractional CO2, and picosecond lasers have been effectively employed in SOC populations. When these energy-based treatments are combined with cooling systems, they substantially reduce the risk for thermal damage in darker skin tones.12,13
Atopic Dermatitis—While epidemiologic data indicate that Black patients experience a higher prevalence (19.3%) of AD than Asian (17.8%), White (16.1%), or Hispanic (7.8%) groups in the United States, this disparity may be influenced by factors such as access to care and environmental stressors, which require further study.14-16 The pathogenesis of AD involves a complex interaction between skin barrier dysfunction, immune dysregulation, and environmental triggers, with patients with SOC exhibiting distinct endotypes.14,17 For example, East Asian individuals have elevated TH17-related cytokines and a blended TH17/TH2 AD-psoriasis endotype,14,18 while Black individuals have greater TH2 skewing and filaggrin variations and higher serum IgE levels.17 Diagnostic advancements, including a modified Eczema Area and Severity Index using grayscale rather than erythema-based assessments for patients with SOC as well as a novel SOC dermatology atlas that includes AD have increased equity in disease evaluation.19,20 Recent clinical trials support the efficacy of topical crisaborole, topical ruxolitinib, and biologics such as dupilumab, tralokinumab, lebrikizumab, and fezakinumab for AD in SOC populations, with dupilumab also improving postinflammatory hyperpigmentation.20-22
Seborrheic Dermatitis—Seborrheic dermatitis is common in patients with SOC, though its manifestations vary by racial/ethnic background.23 In Black patients, petaloid SD is more prevalent and can resemble secondary syphilis, making accurate diagnosis essential to rule out potential mimickers.24 Effective treatments remain limited, as current therapies often fail to address both the underlying yeast-driven inflammation and the resulting pigmentary changes that commonly affect SOC populations.25 Roflumilast foam 0.3%, a phosphodiesterase 4 inhibitor, has emerged as a promising option, offering both anti-inflammatory benefits and improvements in pigmentary alterations—making it particularly valuable for treatment of SD in patients with SOC.26
Melasma—Melasma is more prevalent in women with darker skin types, particularly those of African descent and those from East and Southeast Asia or Latin America.27,28 Standard treatments including hydroquinone, retinoids, azelaic acid, kojic acid, ascorbic acid, arbutin, alpha hydroxy acids, niacinamide, and the Kligman formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) remain therapeutic foundations in patients with SOC.29 Newer alternatives that are effective in SOC populations include topical metformin 30%30; topical isobutylamido thiazolyl resorcinol or thiamidol31; and tranexamic acid cream 5%, which has comparable efficacy to hydroquinone 4% with fewer adverse effects.32 Laser therapies such as the 675-nm and 1064-nm Q-switched neodymium-doped yttrium aluminum garnet lasers, offer effective pigment reduction and are safe in darker skin tones.33,34
Postinflammatory Hyperpigmentation—Postinflammatory hyperpigmentation, often triggered by acne in SOC populations,23 manifests as brown, tan, or gray discoloration and is managed using similar topical agents as melasma, with the 1927-nm laser providing an additional treatment option for patients with SOC.27,35,36
Psoriasis—In patients with SOC, psoriasis often manifests with thicker plaques, increased scaling, and greater body surface area involvement, leading to considerable quality-of-life implications.37 Although prevalence is highest in White populations (3.6%), Asian (2.5%) and Hispanic/Latino (1.9%) patients experience increased disease severity, potentially explaining why psoriasis is among the top chief complaints for these racial/ ethnic groups in the United States.23,38 Greater diversity in clinical trials has improved our understanding of the efficacy of biologics for psoriasis in SOC populations. The VISIBLE trial—the first SOC-exclusive psoriasis trial—demonstrated a Psoriasis Area and Severity Index 90 response in 57.1% (44/77) of participants receiving guselkumab vs 3.8% (1/26) of participants receiving placebo by week 16 (P<.001).39 Other biologics such as risankizumab, secukinumab, and brodalumab also have shown efficacy in SOC populations.40-42 Additionally, topical therapies such as calcipotriene-betamethasone dipropionate cream/aerosol foam and halobetasol propionatetazarotene lotion have proven effective, with minimal adverse effects and low discontinuation rates in patients with SOC.43-46
Seborrheic Keratosis—In SOC, seborrheic keratosis (SK) often appears as a variant known as dermatosis papulosa nigra (DPN), manifesting as small, benign, hyperpigmented papules, particularly on the face and neck.47 Dermatosis papulosa nigra is common in Black, Hispanic, and some Asian populations, with variations in color and distribution among different racial/ethnic groups.48 For example, in Korean populations, SKs commonly affect males, and in contrast to the dark brown color common in White populations, SKs in Korean patients often appear lighter brown or sometimes pink.49 In contrast to the verrucous and stuck-on appearance often seen in White populations, South Asian populations more often have variants including pedunculated SKs, flat SKs, and stucco keratoses.50 High-resolution dermoscopy improves differentiation from malignant lesions; however, a sudden SK eruption in any population warrants evaluation for underlying malignancy. Cryotherapy, though effective for removal of SKs, can cause pigmentary changes in SOC populations, making laser therapy and electrosurgery preferable for these patients due to the lower risk for pigmentary sequela. If hyperpigmentation occurs, topical treatments such as hydroquinone, tretinoin, or azelaic acid can help. New laser technologies and hydrogen-peroxide–based therapies offer safer and more effective removal options while minimizing pigmentary risks in SOC populations.47,50 While DPNs are common in patients with darker skin tones, there are limited data on optimal treatment frequency, insurance coverage, and efficacy. This literature gap hinders our understanding of treatment accessibility and economic impact on our patients.51
Final Thoughts
Innovations such as standardized scoring systems and customized therapeutic strategies for conditions including acne, pigmentary disorders, and atopic dermatitis have markedly enhanced patient care and outcomes for the most common chief concerns in SOC populations. In addition, population-specific advancements have addressed unique diagnostic and therapeutic developments in Black, Asian/Pacific Islander, and Hispanic groups, from the nuanced presentations of atopic and seborrheic dermatitis in Black patients, to those of psoriasis in Asian/Pacific Islander and Hispanic populations. Finally, updated epidemiologic studies are essential to capture the current and evolving dermatologic concerns pertinent to patients with SOC, ensuring that future clinical and research efforts align with the unique needs of these populations.
- Taylor SC. Diagnosing skin diseases in skin of color. Dermatol Clin. 2023;41:xiii-xv. doi:10.1016/j.det.2023.03.001
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a crosssectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016 /j.jaad.2020.06.041
- An ongoing commitment to equity in medicine. VisualDx. Accessed April 30, 2025. https://www.visualdx.com/about-visualdx/diversity/
- Kelly A, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023;24:681-694. doi:10.1007/s40257-023-00789-1
- Piette WW, Taylor S, Pariser D, et al. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564-1570. doi:10.1001/archdermatol.2008.518
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 suppl):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Jean-Pierre P, Tordjman L, Ghodasara A, et al. Emerging lasers and light-based therapies in the management of acne: a review. Lasers Med Sci. 2024;39:245. doi:10.1007/s10103-024-04196-8
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Alexiades M, Kothare A, Goldberg D, et al. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J Am Acad Dermatol. 2023;89:703-710. doi:10.1016/j.jaad.2023.05.085
- Battle EF Jr, Soden CE Jr. The use of lasers in darker skin types. Semin Cutan Med Surg. 2009;28:130-140. doi:10.1016/j.sder.2009.04.003
- Teymour S, Kania B, Lal K, et al. Energy-based devices in the treatment of acne scars in skin of color. J Cosmet Dermatol. 2023;22:1177-1184. doi:10.1111/jocd.15572
- Adawi W, Cornman H, Kambala A, et al. Diagnosing atopic dermatitis in skin of color. Dermatol Clin. 2023;41:417-429. doi:10.1016/j.det.2023.02.003
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840-1852. doi:10.1016/j.jaip.2020.02.022
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35:173-177. doi:10.1089/derm.2023.0051
- Gan C, Mahil S, Pink A, et al. Atopic dermatitis in skin of colour. part 2: considerations in clinical presentation and treatment options. Clin Exp Dermatol. 2023;48:1091-1101. doi:10.1093 /ced/llad162
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/ jamadermatol.2021.5596
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and postinflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/JDD.2020.4
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Wu T, Frommeyer TC, Rohan CA, et al. Uncommon petaloid form of seborrheic dermatitis seen in Fitzpatrick skin types V-VI. J Clin Investig Dermatol. 2023;11:10.13188/2373-1044.1000086. doi:10.13188/2373 -1044.1000086
- Jackson JM, Alexis A, Zirwas M, et al. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol. 2024;90:597-604. doi:10.1016/j.jaad.2022.12.017
- Alexis AF, Zirwas M, Bukhalo M, et al. Long-term safety and efficacy of roflumilast foam 0.3% in patients with seborrheic dermatitis in a 24–52-week, open-label phase 2 trial. Headache. 2022;13:3-3.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405. doi:10.1016 /j.det.2023.02.005
- Vashi NA, Wirya SA, Inyang M, et al. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol. 2017;18:215-230. doi:10.1007/s40257-016-0239-8
- Kania B, Lolis M, Goldberg D. Melasma management: a comprehensive review of treatment strategies including BTX-A. J Cosmet Dermatol. 2025;24:E16669. doi:10.1111/jocd.16669
- AboAlsoud ES, Eldahshan RM, AbouKhodair MH, et al. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman’s formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21:2508-2515. doi:10.1111/jocd.14953
- Roggenkamp D, Sammain A, Fürstenau M, et al. Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase. J Dermatol. 2021;48:1871-1876. doi:10.1111/1346-8138.16080
- El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:E14240. doi:10.1111/dth.14240
- Coricciati L, Gabellone M, Donne PD, et al. The 675-nm wavelength for treating facial melasma. Skin Res Technol. 2023;29:E13434.
- Ertam Sagduyu I, Marakli O, Oraloglu G, et al. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022;35:E15970.
- Obeng-Nyarko CN, Puerta Durango KS, Jackson S, et al. Innovations in hyperpigmentation. Dermatol Clin. 2025;43:111-121. doi:10.1016/j.det.2024.08.009
- Bae YC, Rettig S, Weiss E, et al. Treatment of post-inflammatory hyperpigmentation in patients with darker skin types using a low energy 1,927 nm non-ablative fractional laser: a retrospective photographic review analysis. Laser Surg Med. 2020;52:7-12.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Janssen Scientific Affairs. Tremfya: overview of VISIBLE clinical trial. Updated January 4, 2025. Accessed April 30, 2025. https://www.janssenscience.com/products/tremfya/medical-content/tremfya-overview-of-visible-clinical-trial
- Alexis AF, Gooderham M, Kwatra SG, et al. A descriptive, post hoc analysis of efficacy and safety of risankizumab in diverse racial and ethnic patient populations with moderate-to-severe psoriasis. Dermatol Ther (Heidelb). 2024;14:2877-2887. doi:10.1007 /s13555-024-01268-z
- El-Kashlan N, Cices A, Kaufman B, et al. Efficacy and safety of secukinumab in the treatment of psoriasis in patients with skin phototypes IV to VI. J Drugs Dermatol. 2024;23:600-606. doi:10.36849JDD.8128
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20:267-276. doi:10.1007 /s40257-018-0408-z
- Kontzias CL, Curcio A, Gorodokin B, et al. Efficacy, convenience, and safety of calcipotriene-betamethasone dipropionate cream in skin of color patients with plaque psoriasis. J Drugs Dermatol. 2023;22:668-672. doi:10.36849/JDD.7497
- Liu J, Cices A, Kaufman B, et al. Efficacy and safety of calcipotriene/betamethasone dipropionate foam in the treatment of psoriasis in skin of color. J Drugs Dermatol. 2023;22:165-173. doi:10.36849/JDD.6910
- Alexis AF, Desai SR, Han G, et al. Fixed-combination halobetasol propionate and tazarotene lotion for psoriasis in patients with skin of color. J Drugs Dermatol. 2021;20:744. doi:10.36849/JDD.735
- Desai SR, Alexis AF, Jacobson A. Successful management of a black male with psoriasis and dyspigmentation treated with halobetasol propionate 0.01%/tazarotene 0.045% lotion: case report. J Drugs Dermatol. 2020;19:1000-1004. doi:10.36849/JDD.2020.5347
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315:1089-1097. doi:10.1007/s00403-022-02493-2
- Xiao A, Muse ME, Ettefagh L. Dermatosis papulosa nigra. In: StatPearls. StatPearls Publishing; 2022.
- Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73-80. doi:10.1034/j.1600-0781.2003.00025.x
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488. doi:10.4103/0378-6323.82408
- Duncan N, Usatine RP, Heath CR. Key features of dermatosis papulosa nigra vs seborrheic keratosis. Cutis. 2025;115:70-71. doi:10.12788/cutis.1170
The umbrella term skin of color (SOC) includes individuals identifying as Black/African, Hispanic, Asian, Native American, Middle Eastern, and Mediterranean as well as multiracial groups. While the Fitzpatrick skin typing system is not an accurate proxy for describing skin tone, SOC populations typically correspond to Fitzpatrick skin types IV to VI, and clinical researchers often report the Fitzpatrick skin type of their study populations.1
Over the past several decades, the underrepresentation of diverse skin tones in educational resources has limited clinical training.2 For example, only 10.3% of conditions featured in contemporary dermatology textbooks are shown in darker skin tones.3 This educational resource gap has spurred a transformative movement toward inclusivity in dermatologic education, research, and clinical practice. Notable examples include VisualDx4 and Dermatology for Skin of Color.5 In addition, Cutis began publishing the Dx Across the Skin Color Spectrum fact sheet series in 2022 to highlight differences in how cutaneous conditions manifest in various skin tones (https://www.mdedge.com/cutis/dx-across-skin-color-spectrum).
These resources play a critical role in advancing dermatologic knowledge, ensuring that dermatologists and other health care professionals are well equipped to diagnose and treat dermatologic conditions in SOC populations with accuracy and cultural humility. These innovations also have enhanced our understanding of how common dermatologic conditions manifest and respond to treatment in SOC populations. Herein, we highlight advances in diagnostic and therapeutic approaches for the most common concerns among SOC populations in the United States, including acne vulgaris, atopic dermatitis (AD), seborrheic dermatitis (SD), melasma, postinflammatory hyperpigmentation, psoriasis, and seborrheic keratosis.
Chief Concerns Common Among SOC Populations in the United States
Acne Vulgaris—In patients with SOC, acne frequently results in pigmentary changes and scarring that can manifest as both hypertrophic and keloidal scars.6 Clinical evidence from randomized controlled studies supports the use of topical dapsone gel as a safe and effective frontline treatment for acne in patients with SOC.7,8 Notably, the US Food and Drug Administration–approved 1726-nm laser with a contact-cooling sapphire window has demonstrated safety and efficacy in the management of acne across Fitzpatrick skin types II to VI.9-11 To manage atrophic acne scars, cutting-edge laser and radiofrequency devices including erbium-doped yttrium aluminum garnet, fractional CO2, and picosecond lasers have been effectively employed in SOC populations. When these energy-based treatments are combined with cooling systems, they substantially reduce the risk for thermal damage in darker skin tones.12,13
Atopic Dermatitis—While epidemiologic data indicate that Black patients experience a higher prevalence (19.3%) of AD than Asian (17.8%), White (16.1%), or Hispanic (7.8%) groups in the United States, this disparity may be influenced by factors such as access to care and environmental stressors, which require further study.14-16 The pathogenesis of AD involves a complex interaction between skin barrier dysfunction, immune dysregulation, and environmental triggers, with patients with SOC exhibiting distinct endotypes.14,17 For example, East Asian individuals have elevated TH17-related cytokines and a blended TH17/TH2 AD-psoriasis endotype,14,18 while Black individuals have greater TH2 skewing and filaggrin variations and higher serum IgE levels.17 Diagnostic advancements, including a modified Eczema Area and Severity Index using grayscale rather than erythema-based assessments for patients with SOC as well as a novel SOC dermatology atlas that includes AD have increased equity in disease evaluation.19,20 Recent clinical trials support the efficacy of topical crisaborole, topical ruxolitinib, and biologics such as dupilumab, tralokinumab, lebrikizumab, and fezakinumab for AD in SOC populations, with dupilumab also improving postinflammatory hyperpigmentation.20-22
Seborrheic Dermatitis—Seborrheic dermatitis is common in patients with SOC, though its manifestations vary by racial/ethnic background.23 In Black patients, petaloid SD is more prevalent and can resemble secondary syphilis, making accurate diagnosis essential to rule out potential mimickers.24 Effective treatments remain limited, as current therapies often fail to address both the underlying yeast-driven inflammation and the resulting pigmentary changes that commonly affect SOC populations.25 Roflumilast foam 0.3%, a phosphodiesterase 4 inhibitor, has emerged as a promising option, offering both anti-inflammatory benefits and improvements in pigmentary alterations—making it particularly valuable for treatment of SD in patients with SOC.26
Melasma—Melasma is more prevalent in women with darker skin types, particularly those of African descent and those from East and Southeast Asia or Latin America.27,28 Standard treatments including hydroquinone, retinoids, azelaic acid, kojic acid, ascorbic acid, arbutin, alpha hydroxy acids, niacinamide, and the Kligman formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) remain therapeutic foundations in patients with SOC.29 Newer alternatives that are effective in SOC populations include topical metformin 30%30; topical isobutylamido thiazolyl resorcinol or thiamidol31; and tranexamic acid cream 5%, which has comparable efficacy to hydroquinone 4% with fewer adverse effects.32 Laser therapies such as the 675-nm and 1064-nm Q-switched neodymium-doped yttrium aluminum garnet lasers, offer effective pigment reduction and are safe in darker skin tones.33,34
Postinflammatory Hyperpigmentation—Postinflammatory hyperpigmentation, often triggered by acne in SOC populations,23 manifests as brown, tan, or gray discoloration and is managed using similar topical agents as melasma, with the 1927-nm laser providing an additional treatment option for patients with SOC.27,35,36
Psoriasis—In patients with SOC, psoriasis often manifests with thicker plaques, increased scaling, and greater body surface area involvement, leading to considerable quality-of-life implications.37 Although prevalence is highest in White populations (3.6%), Asian (2.5%) and Hispanic/Latino (1.9%) patients experience increased disease severity, potentially explaining why psoriasis is among the top chief complaints for these racial/ ethnic groups in the United States.23,38 Greater diversity in clinical trials has improved our understanding of the efficacy of biologics for psoriasis in SOC populations. The VISIBLE trial—the first SOC-exclusive psoriasis trial—demonstrated a Psoriasis Area and Severity Index 90 response in 57.1% (44/77) of participants receiving guselkumab vs 3.8% (1/26) of participants receiving placebo by week 16 (P<.001).39 Other biologics such as risankizumab, secukinumab, and brodalumab also have shown efficacy in SOC populations.40-42 Additionally, topical therapies such as calcipotriene-betamethasone dipropionate cream/aerosol foam and halobetasol propionatetazarotene lotion have proven effective, with minimal adverse effects and low discontinuation rates in patients with SOC.43-46
Seborrheic Keratosis—In SOC, seborrheic keratosis (SK) often appears as a variant known as dermatosis papulosa nigra (DPN), manifesting as small, benign, hyperpigmented papules, particularly on the face and neck.47 Dermatosis papulosa nigra is common in Black, Hispanic, and some Asian populations, with variations in color and distribution among different racial/ethnic groups.48 For example, in Korean populations, SKs commonly affect males, and in contrast to the dark brown color common in White populations, SKs in Korean patients often appear lighter brown or sometimes pink.49 In contrast to the verrucous and stuck-on appearance often seen in White populations, South Asian populations more often have variants including pedunculated SKs, flat SKs, and stucco keratoses.50 High-resolution dermoscopy improves differentiation from malignant lesions; however, a sudden SK eruption in any population warrants evaluation for underlying malignancy. Cryotherapy, though effective for removal of SKs, can cause pigmentary changes in SOC populations, making laser therapy and electrosurgery preferable for these patients due to the lower risk for pigmentary sequela. If hyperpigmentation occurs, topical treatments such as hydroquinone, tretinoin, or azelaic acid can help. New laser technologies and hydrogen-peroxide–based therapies offer safer and more effective removal options while minimizing pigmentary risks in SOC populations.47,50 While DPNs are common in patients with darker skin tones, there are limited data on optimal treatment frequency, insurance coverage, and efficacy. This literature gap hinders our understanding of treatment accessibility and economic impact on our patients.51
Final Thoughts
Innovations such as standardized scoring systems and customized therapeutic strategies for conditions including acne, pigmentary disorders, and atopic dermatitis have markedly enhanced patient care and outcomes for the most common chief concerns in SOC populations. In addition, population-specific advancements have addressed unique diagnostic and therapeutic developments in Black, Asian/Pacific Islander, and Hispanic groups, from the nuanced presentations of atopic and seborrheic dermatitis in Black patients, to those of psoriasis in Asian/Pacific Islander and Hispanic populations. Finally, updated epidemiologic studies are essential to capture the current and evolving dermatologic concerns pertinent to patients with SOC, ensuring that future clinical and research efforts align with the unique needs of these populations.
The umbrella term skin of color (SOC) includes individuals identifying as Black/African, Hispanic, Asian, Native American, Middle Eastern, and Mediterranean as well as multiracial groups. While the Fitzpatrick skin typing system is not an accurate proxy for describing skin tone, SOC populations typically correspond to Fitzpatrick skin types IV to VI, and clinical researchers often report the Fitzpatrick skin type of their study populations.1
Over the past several decades, the underrepresentation of diverse skin tones in educational resources has limited clinical training.2 For example, only 10.3% of conditions featured in contemporary dermatology textbooks are shown in darker skin tones.3 This educational resource gap has spurred a transformative movement toward inclusivity in dermatologic education, research, and clinical practice. Notable examples include VisualDx4 and Dermatology for Skin of Color.5 In addition, Cutis began publishing the Dx Across the Skin Color Spectrum fact sheet series in 2022 to highlight differences in how cutaneous conditions manifest in various skin tones (https://www.mdedge.com/cutis/dx-across-skin-color-spectrum).
These resources play a critical role in advancing dermatologic knowledge, ensuring that dermatologists and other health care professionals are well equipped to diagnose and treat dermatologic conditions in SOC populations with accuracy and cultural humility. These innovations also have enhanced our understanding of how common dermatologic conditions manifest and respond to treatment in SOC populations. Herein, we highlight advances in diagnostic and therapeutic approaches for the most common concerns among SOC populations in the United States, including acne vulgaris, atopic dermatitis (AD), seborrheic dermatitis (SD), melasma, postinflammatory hyperpigmentation, psoriasis, and seborrheic keratosis.
Chief Concerns Common Among SOC Populations in the United States
Acne Vulgaris—In patients with SOC, acne frequently results in pigmentary changes and scarring that can manifest as both hypertrophic and keloidal scars.6 Clinical evidence from randomized controlled studies supports the use of topical dapsone gel as a safe and effective frontline treatment for acne in patients with SOC.7,8 Notably, the US Food and Drug Administration–approved 1726-nm laser with a contact-cooling sapphire window has demonstrated safety and efficacy in the management of acne across Fitzpatrick skin types II to VI.9-11 To manage atrophic acne scars, cutting-edge laser and radiofrequency devices including erbium-doped yttrium aluminum garnet, fractional CO2, and picosecond lasers have been effectively employed in SOC populations. When these energy-based treatments are combined with cooling systems, they substantially reduce the risk for thermal damage in darker skin tones.12,13
Atopic Dermatitis—While epidemiologic data indicate that Black patients experience a higher prevalence (19.3%) of AD than Asian (17.8%), White (16.1%), or Hispanic (7.8%) groups in the United States, this disparity may be influenced by factors such as access to care and environmental stressors, which require further study.14-16 The pathogenesis of AD involves a complex interaction between skin barrier dysfunction, immune dysregulation, and environmental triggers, with patients with SOC exhibiting distinct endotypes.14,17 For example, East Asian individuals have elevated TH17-related cytokines and a blended TH17/TH2 AD-psoriasis endotype,14,18 while Black individuals have greater TH2 skewing and filaggrin variations and higher serum IgE levels.17 Diagnostic advancements, including a modified Eczema Area and Severity Index using grayscale rather than erythema-based assessments for patients with SOC as well as a novel SOC dermatology atlas that includes AD have increased equity in disease evaluation.19,20 Recent clinical trials support the efficacy of topical crisaborole, topical ruxolitinib, and biologics such as dupilumab, tralokinumab, lebrikizumab, and fezakinumab for AD in SOC populations, with dupilumab also improving postinflammatory hyperpigmentation.20-22
Seborrheic Dermatitis—Seborrheic dermatitis is common in patients with SOC, though its manifestations vary by racial/ethnic background.23 In Black patients, petaloid SD is more prevalent and can resemble secondary syphilis, making accurate diagnosis essential to rule out potential mimickers.24 Effective treatments remain limited, as current therapies often fail to address both the underlying yeast-driven inflammation and the resulting pigmentary changes that commonly affect SOC populations.25 Roflumilast foam 0.3%, a phosphodiesterase 4 inhibitor, has emerged as a promising option, offering both anti-inflammatory benefits and improvements in pigmentary alterations—making it particularly valuable for treatment of SD in patients with SOC.26
Melasma—Melasma is more prevalent in women with darker skin types, particularly those of African descent and those from East and Southeast Asia or Latin America.27,28 Standard treatments including hydroquinone, retinoids, azelaic acid, kojic acid, ascorbic acid, arbutin, alpha hydroxy acids, niacinamide, and the Kligman formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) remain therapeutic foundations in patients with SOC.29 Newer alternatives that are effective in SOC populations include topical metformin 30%30; topical isobutylamido thiazolyl resorcinol or thiamidol31; and tranexamic acid cream 5%, which has comparable efficacy to hydroquinone 4% with fewer adverse effects.32 Laser therapies such as the 675-nm and 1064-nm Q-switched neodymium-doped yttrium aluminum garnet lasers, offer effective pigment reduction and are safe in darker skin tones.33,34
Postinflammatory Hyperpigmentation—Postinflammatory hyperpigmentation, often triggered by acne in SOC populations,23 manifests as brown, tan, or gray discoloration and is managed using similar topical agents as melasma, with the 1927-nm laser providing an additional treatment option for patients with SOC.27,35,36
Psoriasis—In patients with SOC, psoriasis often manifests with thicker plaques, increased scaling, and greater body surface area involvement, leading to considerable quality-of-life implications.37 Although prevalence is highest in White populations (3.6%), Asian (2.5%) and Hispanic/Latino (1.9%) patients experience increased disease severity, potentially explaining why psoriasis is among the top chief complaints for these racial/ ethnic groups in the United States.23,38 Greater diversity in clinical trials has improved our understanding of the efficacy of biologics for psoriasis in SOC populations. The VISIBLE trial—the first SOC-exclusive psoriasis trial—demonstrated a Psoriasis Area and Severity Index 90 response in 57.1% (44/77) of participants receiving guselkumab vs 3.8% (1/26) of participants receiving placebo by week 16 (P<.001).39 Other biologics such as risankizumab, secukinumab, and brodalumab also have shown efficacy in SOC populations.40-42 Additionally, topical therapies such as calcipotriene-betamethasone dipropionate cream/aerosol foam and halobetasol propionatetazarotene lotion have proven effective, with minimal adverse effects and low discontinuation rates in patients with SOC.43-46
Seborrheic Keratosis—In SOC, seborrheic keratosis (SK) often appears as a variant known as dermatosis papulosa nigra (DPN), manifesting as small, benign, hyperpigmented papules, particularly on the face and neck.47 Dermatosis papulosa nigra is common in Black, Hispanic, and some Asian populations, with variations in color and distribution among different racial/ethnic groups.48 For example, in Korean populations, SKs commonly affect males, and in contrast to the dark brown color common in White populations, SKs in Korean patients often appear lighter brown or sometimes pink.49 In contrast to the verrucous and stuck-on appearance often seen in White populations, South Asian populations more often have variants including pedunculated SKs, flat SKs, and stucco keratoses.50 High-resolution dermoscopy improves differentiation from malignant lesions; however, a sudden SK eruption in any population warrants evaluation for underlying malignancy. Cryotherapy, though effective for removal of SKs, can cause pigmentary changes in SOC populations, making laser therapy and electrosurgery preferable for these patients due to the lower risk for pigmentary sequela. If hyperpigmentation occurs, topical treatments such as hydroquinone, tretinoin, or azelaic acid can help. New laser technologies and hydrogen-peroxide–based therapies offer safer and more effective removal options while minimizing pigmentary risks in SOC populations.47,50 While DPNs are common in patients with darker skin tones, there are limited data on optimal treatment frequency, insurance coverage, and efficacy. This literature gap hinders our understanding of treatment accessibility and economic impact on our patients.51
Final Thoughts
Innovations such as standardized scoring systems and customized therapeutic strategies for conditions including acne, pigmentary disorders, and atopic dermatitis have markedly enhanced patient care and outcomes for the most common chief concerns in SOC populations. In addition, population-specific advancements have addressed unique diagnostic and therapeutic developments in Black, Asian/Pacific Islander, and Hispanic groups, from the nuanced presentations of atopic and seborrheic dermatitis in Black patients, to those of psoriasis in Asian/Pacific Islander and Hispanic populations. Finally, updated epidemiologic studies are essential to capture the current and evolving dermatologic concerns pertinent to patients with SOC, ensuring that future clinical and research efforts align with the unique needs of these populations.
- Taylor SC. Diagnosing skin diseases in skin of color. Dermatol Clin. 2023;41:xiii-xv. doi:10.1016/j.det.2023.03.001
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a crosssectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016 /j.jaad.2020.06.041
- An ongoing commitment to equity in medicine. VisualDx. Accessed April 30, 2025. https://www.visualdx.com/about-visualdx/diversity/
- Kelly A, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023;24:681-694. doi:10.1007/s40257-023-00789-1
- Piette WW, Taylor S, Pariser D, et al. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564-1570. doi:10.1001/archdermatol.2008.518
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 suppl):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Jean-Pierre P, Tordjman L, Ghodasara A, et al. Emerging lasers and light-based therapies in the management of acne: a review. Lasers Med Sci. 2024;39:245. doi:10.1007/s10103-024-04196-8
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Alexiades M, Kothare A, Goldberg D, et al. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J Am Acad Dermatol. 2023;89:703-710. doi:10.1016/j.jaad.2023.05.085
- Battle EF Jr, Soden CE Jr. The use of lasers in darker skin types. Semin Cutan Med Surg. 2009;28:130-140. doi:10.1016/j.sder.2009.04.003
- Teymour S, Kania B, Lal K, et al. Energy-based devices in the treatment of acne scars in skin of color. J Cosmet Dermatol. 2023;22:1177-1184. doi:10.1111/jocd.15572
- Adawi W, Cornman H, Kambala A, et al. Diagnosing atopic dermatitis in skin of color. Dermatol Clin. 2023;41:417-429. doi:10.1016/j.det.2023.02.003
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840-1852. doi:10.1016/j.jaip.2020.02.022
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35:173-177. doi:10.1089/derm.2023.0051
- Gan C, Mahil S, Pink A, et al. Atopic dermatitis in skin of colour. part 2: considerations in clinical presentation and treatment options. Clin Exp Dermatol. 2023;48:1091-1101. doi:10.1093 /ced/llad162
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/ jamadermatol.2021.5596
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and postinflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/JDD.2020.4
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Wu T, Frommeyer TC, Rohan CA, et al. Uncommon petaloid form of seborrheic dermatitis seen in Fitzpatrick skin types V-VI. J Clin Investig Dermatol. 2023;11:10.13188/2373-1044.1000086. doi:10.13188/2373 -1044.1000086
- Jackson JM, Alexis A, Zirwas M, et al. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol. 2024;90:597-604. doi:10.1016/j.jaad.2022.12.017
- Alexis AF, Zirwas M, Bukhalo M, et al. Long-term safety and efficacy of roflumilast foam 0.3% in patients with seborrheic dermatitis in a 24–52-week, open-label phase 2 trial. Headache. 2022;13:3-3.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405. doi:10.1016 /j.det.2023.02.005
- Vashi NA, Wirya SA, Inyang M, et al. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol. 2017;18:215-230. doi:10.1007/s40257-016-0239-8
- Kania B, Lolis M, Goldberg D. Melasma management: a comprehensive review of treatment strategies including BTX-A. J Cosmet Dermatol. 2025;24:E16669. doi:10.1111/jocd.16669
- AboAlsoud ES, Eldahshan RM, AbouKhodair MH, et al. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman’s formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21:2508-2515. doi:10.1111/jocd.14953
- Roggenkamp D, Sammain A, Fürstenau M, et al. Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase. J Dermatol. 2021;48:1871-1876. doi:10.1111/1346-8138.16080
- El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:E14240. doi:10.1111/dth.14240
- Coricciati L, Gabellone M, Donne PD, et al. The 675-nm wavelength for treating facial melasma. Skin Res Technol. 2023;29:E13434.
- Ertam Sagduyu I, Marakli O, Oraloglu G, et al. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022;35:E15970.
- Obeng-Nyarko CN, Puerta Durango KS, Jackson S, et al. Innovations in hyperpigmentation. Dermatol Clin. 2025;43:111-121. doi:10.1016/j.det.2024.08.009
- Bae YC, Rettig S, Weiss E, et al. Treatment of post-inflammatory hyperpigmentation in patients with darker skin types using a low energy 1,927 nm non-ablative fractional laser: a retrospective photographic review analysis. Laser Surg Med. 2020;52:7-12.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Janssen Scientific Affairs. Tremfya: overview of VISIBLE clinical trial. Updated January 4, 2025. Accessed April 30, 2025. https://www.janssenscience.com/products/tremfya/medical-content/tremfya-overview-of-visible-clinical-trial
- Alexis AF, Gooderham M, Kwatra SG, et al. A descriptive, post hoc analysis of efficacy and safety of risankizumab in diverse racial and ethnic patient populations with moderate-to-severe psoriasis. Dermatol Ther (Heidelb). 2024;14:2877-2887. doi:10.1007 /s13555-024-01268-z
- El-Kashlan N, Cices A, Kaufman B, et al. Efficacy and safety of secukinumab in the treatment of psoriasis in patients with skin phototypes IV to VI. J Drugs Dermatol. 2024;23:600-606. doi:10.36849JDD.8128
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20:267-276. doi:10.1007 /s40257-018-0408-z
- Kontzias CL, Curcio A, Gorodokin B, et al. Efficacy, convenience, and safety of calcipotriene-betamethasone dipropionate cream in skin of color patients with plaque psoriasis. J Drugs Dermatol. 2023;22:668-672. doi:10.36849/JDD.7497
- Liu J, Cices A, Kaufman B, et al. Efficacy and safety of calcipotriene/betamethasone dipropionate foam in the treatment of psoriasis in skin of color. J Drugs Dermatol. 2023;22:165-173. doi:10.36849/JDD.6910
- Alexis AF, Desai SR, Han G, et al. Fixed-combination halobetasol propionate and tazarotene lotion for psoriasis in patients with skin of color. J Drugs Dermatol. 2021;20:744. doi:10.36849/JDD.735
- Desai SR, Alexis AF, Jacobson A. Successful management of a black male with psoriasis and dyspigmentation treated with halobetasol propionate 0.01%/tazarotene 0.045% lotion: case report. J Drugs Dermatol. 2020;19:1000-1004. doi:10.36849/JDD.2020.5347
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315:1089-1097. doi:10.1007/s00403-022-02493-2
- Xiao A, Muse ME, Ettefagh L. Dermatosis papulosa nigra. In: StatPearls. StatPearls Publishing; 2022.
- Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73-80. doi:10.1034/j.1600-0781.2003.00025.x
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488. doi:10.4103/0378-6323.82408
- Duncan N, Usatine RP, Heath CR. Key features of dermatosis papulosa nigra vs seborrheic keratosis. Cutis. 2025;115:70-71. doi:10.12788/cutis.1170
- Taylor SC. Diagnosing skin diseases in skin of color. Dermatol Clin. 2023;41:xiii-xv. doi:10.1016/j.det.2023.03.001
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a crosssectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016 /j.jaad.2020.06.041
- An ongoing commitment to equity in medicine. VisualDx. Accessed April 30, 2025. https://www.visualdx.com/about-visualdx/diversity/
- Kelly A, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023;24:681-694. doi:10.1007/s40257-023-00789-1
- Piette WW, Taylor S, Pariser D, et al. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch Dermatol. 2008;144:1564-1570. doi:10.1001/archdermatol.2008.518
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(1 suppl):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Jean-Pierre P, Tordjman L, Ghodasara A, et al. Emerging lasers and light-based therapies in the management of acne: a review. Lasers Med Sci. 2024;39:245. doi:10.1007/s10103-024-04196-8
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Alexiades M, Kothare A, Goldberg D, et al. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J Am Acad Dermatol. 2023;89:703-710. doi:10.1016/j.jaad.2023.05.085
- Battle EF Jr, Soden CE Jr. The use of lasers in darker skin types. Semin Cutan Med Surg. 2009;28:130-140. doi:10.1016/j.sder.2009.04.003
- Teymour S, Kania B, Lal K, et al. Energy-based devices in the treatment of acne scars in skin of color. J Cosmet Dermatol. 2023;22:1177-1184. doi:10.1111/jocd.15572
- Adawi W, Cornman H, Kambala A, et al. Diagnosing atopic dermatitis in skin of color. Dermatol Clin. 2023;41:417-429. doi:10.1016/j.det.2023.02.003
- Fu T, Keiser E, Linos E, et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population-based study. Pediatr Dermatol. 2014;31:21-26. doi:10.1111/pde.12237
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8:1840-1852. doi:10.1016/j.jaip.2020.02.022
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35:173-177. doi:10.1089/derm.2023.0051
- Gan C, Mahil S, Pink A, et al. Atopic dermatitis in skin of colour. part 2: considerations in clinical presentation and treatment options. Clin Exp Dermatol. 2023;48:1091-1101. doi:10.1093 /ced/llad162
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/ jamadermatol.2021.5596
- Grayson C, Heath CR. Dupilumab improves atopic dermatitis and postinflammatory hyperpigmentation in patient with skin of color. J Drugs Dermatol. 2020;19:776-778. doi:10.36849/JDD.2020.4
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Wu T, Frommeyer TC, Rohan CA, et al. Uncommon petaloid form of seborrheic dermatitis seen in Fitzpatrick skin types V-VI. J Clin Investig Dermatol. 2023;11:10.13188/2373-1044.1000086. doi:10.13188/2373 -1044.1000086
- Jackson JM, Alexis A, Zirwas M, et al. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol. 2024;90:597-604. doi:10.1016/j.jaad.2022.12.017
- Alexis AF, Zirwas M, Bukhalo M, et al. Long-term safety and efficacy of roflumilast foam 0.3% in patients with seborrheic dermatitis in a 24–52-week, open-label phase 2 trial. Headache. 2022;13:3-3.
- Syder NC, Quarshie C, Elbuluk N. Disorders of facial hyperpigmentation. Dermatol Clin. 2023;41:393-405. doi:10.1016 /j.det.2023.02.005
- Vashi NA, Wirya SA, Inyang M, et al. Facial hyperpigmentation in skin of color: special considerations and treatment. Am J Clin Dermatol. 2017;18:215-230. doi:10.1007/s40257-016-0239-8
- Kania B, Lolis M, Goldberg D. Melasma management: a comprehensive review of treatment strategies including BTX-A. J Cosmet Dermatol. 2025;24:E16669. doi:10.1111/jocd.16669
- AboAlsoud ES, Eldahshan RM, AbouKhodair MH, et al. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman’s formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21:2508-2515. doi:10.1111/jocd.14953
- Roggenkamp D, Sammain A, Fürstenau M, et al. Thiamidol® in moderate-to-severe melasma: 24-week, randomized, double-blind, vehicle-controlled clinical study with subsequent regression phase. J Dermatol. 2021;48:1871-1876. doi:10.1111/1346-8138.16080
- El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33:E14240. doi:10.1111/dth.14240
- Coricciati L, Gabellone M, Donne PD, et al. The 675-nm wavelength for treating facial melasma. Skin Res Technol. 2023;29:E13434.
- Ertam Sagduyu I, Marakli O, Oraloglu G, et al. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022;35:E15970.
- Obeng-Nyarko CN, Puerta Durango KS, Jackson S, et al. Innovations in hyperpigmentation. Dermatol Clin. 2025;43:111-121. doi:10.1016/j.det.2024.08.009
- Bae YC, Rettig S, Weiss E, et al. Treatment of post-inflammatory hyperpigmentation in patients with darker skin types using a low energy 1,927 nm non-ablative fractional laser: a retrospective photographic review analysis. Laser Surg Med. 2020;52:7-12.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Janssen Scientific Affairs. Tremfya: overview of VISIBLE clinical trial. Updated January 4, 2025. Accessed April 30, 2025. https://www.janssenscience.com/products/tremfya/medical-content/tremfya-overview-of-visible-clinical-trial
- Alexis AF, Gooderham M, Kwatra SG, et al. A descriptive, post hoc analysis of efficacy and safety of risankizumab in diverse racial and ethnic patient populations with moderate-to-severe psoriasis. Dermatol Ther (Heidelb). 2024;14:2877-2887. doi:10.1007 /s13555-024-01268-z
- El-Kashlan N, Cices A, Kaufman B, et al. Efficacy and safety of secukinumab in the treatment of psoriasis in patients with skin phototypes IV to VI. J Drugs Dermatol. 2024;23:600-606. doi:10.36849JDD.8128
- McMichael A, Desai SR, Qureshi A, et al. Efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis and skin of color: results from the pooled AMAGINE-2/-3 randomized trials. Am J Clin Dermatol. 2019;20:267-276. doi:10.1007 /s40257-018-0408-z
- Kontzias CL, Curcio A, Gorodokin B, et al. Efficacy, convenience, and safety of calcipotriene-betamethasone dipropionate cream in skin of color patients with plaque psoriasis. J Drugs Dermatol. 2023;22:668-672. doi:10.36849/JDD.7497
- Liu J, Cices A, Kaufman B, et al. Efficacy and safety of calcipotriene/betamethasone dipropionate foam in the treatment of psoriasis in skin of color. J Drugs Dermatol. 2023;22:165-173. doi:10.36849/JDD.6910
- Alexis AF, Desai SR, Han G, et al. Fixed-combination halobetasol propionate and tazarotene lotion for psoriasis in patients with skin of color. J Drugs Dermatol. 2021;20:744. doi:10.36849/JDD.735
- Desai SR, Alexis AF, Jacobson A. Successful management of a black male with psoriasis and dyspigmentation treated with halobetasol propionate 0.01%/tazarotene 0.045% lotion: case report. J Drugs Dermatol. 2020;19:1000-1004. doi:10.36849/JDD.2020.5347
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315:1089-1097. doi:10.1007/s00403-022-02493-2
- Xiao A, Muse ME, Ettefagh L. Dermatosis papulosa nigra. In: StatPearls. StatPearls Publishing; 2022.
- Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19:73-80. doi:10.1034/j.1600-0781.2003.00025.x
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488. doi:10.4103/0378-6323.82408
- Duncan N, Usatine RP, Heath CR. Key features of dermatosis papulosa nigra vs seborrheic keratosis. Cutis. 2025;115:70-71. doi:10.12788/cutis.1170
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
Common Chief Concerns in Skin of Color Populations and Advancements in Diagnostics and Therapeutics
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that involve widespread necrosis of the skin and mucous membranes.1 Guidelines for SJS and TEN recommend management in hospitals with access to inpatient dermatology to provide immediate interventions that are necessary for achieving optimal patient outcomes.2 A delay in admission of 5 days or more after onset of symptoms has been associated with increases in overall mortality, bacteremia, intensive care unit (ICU) admission, and length of stay.3 Patients who are not directly admitted to specialized facilities and require transfer from other hospitals may experience delays in receiving critical interventions, further increasing the risk for mortality and complications. In this study, we analyzed the clinical outcomes of patients with SJS/TEN in relation to their admission pathway.
Methods
A single-center retrospective chart review was performed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) in Winston-Salem, North Carolina. Participants were identified using i2b2, an informatics tool compliant with the Health Insurance Portability and Accountability Act for integrating biology and the bedside. Inclusion criteria were having a diagnosis of SJS (International Classification of Diseases, Tenth Revision, code L51.1; International Classification of Diseases, Ninth Revision, code 695.13), TEN (International Classification of Diseases, Tenth Revision, code L51.2; International Classification of Diseases, Ninth Revision, code 695.15) or Lyell syndrome from January 2012 to December 2024. Patients with erythema multiforme or bullous drug eruption were excluded, as these conditions initially were misdiagnosed as SJS or TEN. Patients with only a reported history of prior SJS or TEN also were excluded.
The following clinical outcomes were assessed: demographics, comorbidities, age at disease onset, outside hospital transfer status, complications during admission, inpatient length of stay in days, age of mortality (if applicable), culprit medications, interventions received, Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) upon admission, site of admission (eg, floor bed, ICU, medical ICU, burn unit), and length of disease process prior to hospital admission. Patients then were categorized as either direct or transfer admissions based on the initial point of care and admission process. Direct admissions included patients who presented to the AHWFBMC emergency department and were subsequently admitted. Transfer patients included patients who initially presented to an outside hospital and were transferred to AHWFBMC. Data regarding the wait time for Physician Access Line requests and the time elapsed from the initial transfer call to arrival at the tertiary hospital also were collected—this is a method that outside hospitals can use to contact physicians at the tertiary hospital for a possible transfer. Statistical analysis was performed using unpaired t tests and X2 tests as necessary using GraphPad By Dotmatics Prism.
Results
A total of 112 patients were included in the analysis; of these, 71 had a diagnosis with biopsy confirmation of SJS, SJS/TEN overlap, or TEN (Table 1). Forty-one patients were excluded due to having a diagnosis of erythema multiforme or bullous drug eruption or a reported history of prior SJS or TEN without hospitalization. All biopsies were performed at AHWFBMC. Of the 71 confirmed patients with SJS/TEN, 54 (76%) were female with a mean age of 44 years. The majority of patients identified as Black (35 [49%]) or White (27 [38%]), along with Asian (7 [10%]) and other (2 [3%]). The most common comorbidity was cardiovascular disease in 42 (59%) patients, followed by type 2 diabetes in 36 (51%) patients. Among these 71 patients with SJS/TEN, 29 (41%) were directly admitted to the tertiary hospital, while 42 (59%) were transferred from outside hospitals.
Of the 71 confirmed patients with SJS/TEN, sulfonamides were identified as the most common inciting drug in 25 (41%) patients, followed by beta-lactam antibiotics in 16 (23%) patients (Table 2). This is consistent with previous literature of sulfamethoxazole with trimethoprim as the primary causative drug for SJS and TEN in the United States.1
Clinical Outcomes—Of the 71 patients, there were 23 (32%) cases of SJS, 29 (41%) cases of SJS/TEN overlap, and 19 (27%) cases of TEN (eTable). The initial and maximum affected body surface area (BSA) was higher in transfer admissions, with a mean maximum BSA of 38.55% in the transfer group compared to 19.14% in the direct admissions. The mean SCORTEN (range, 0-5) was 1.6 overall, with a higher mean score of 1.92 in the transfer group compared to 1.07 in the direct admissions.
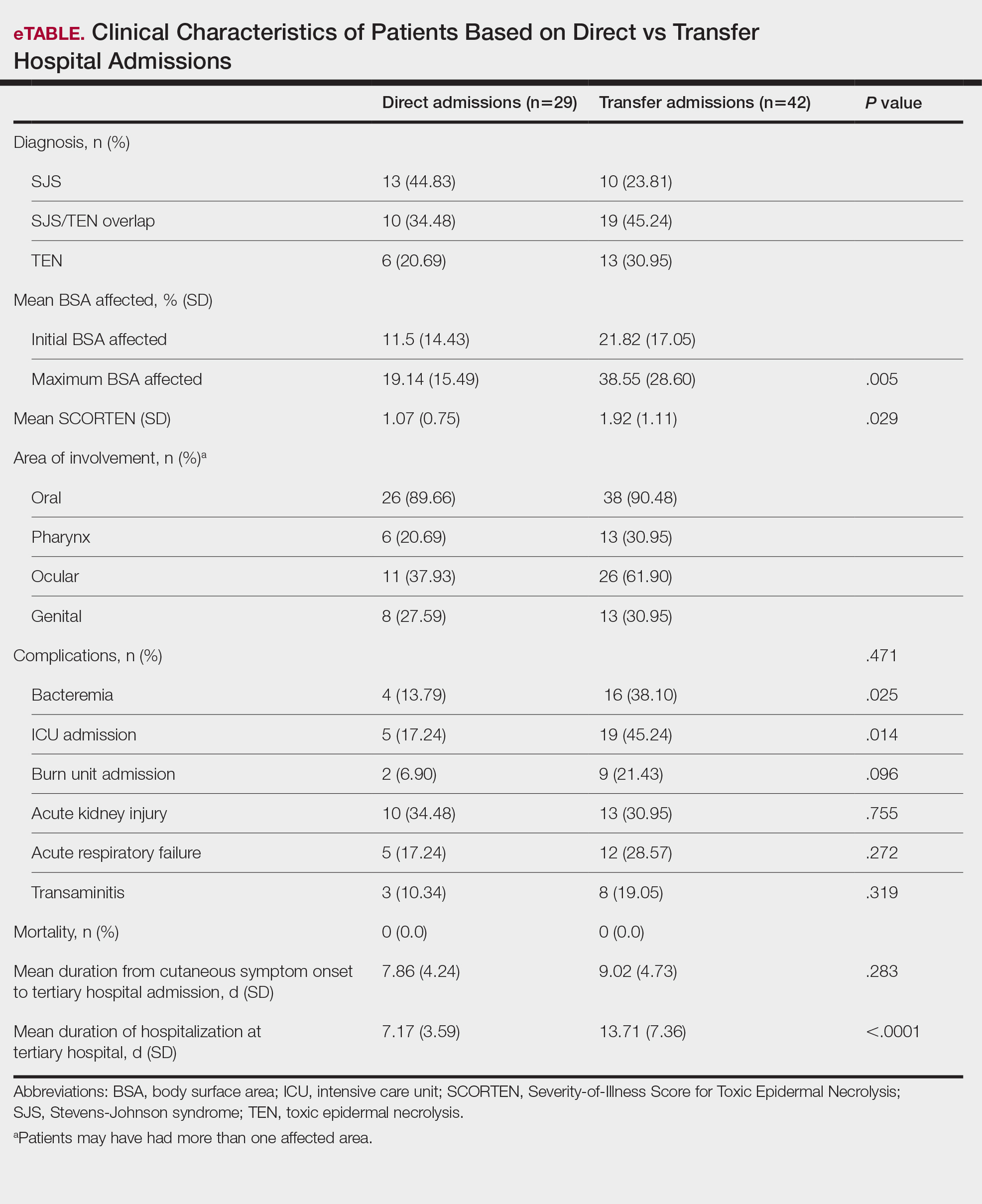
Transfer patients had a longer mean stay at the tertiary hospital (13.71 d) compared to direct admissions (7.17 d). The mean time from symptom onset until tertiary hospital admission was 8.5 days; transfer and direct admission patients had similar mean time from symptom onset of 9.02 days and 7.86 days, respectively. Although the duration of cutaneous symptoms from onset until tertiary hospital admission was similar (P=.283) between direct admissions (7.86 d) and transfer patients (9.02 d), the transfer group presented with greater disease severity at the time of admission. Transfer patients had a higher mean maximum BSA involvement (38.55% vs 19.14% [P=.005]), elevated SCORTEN (1.92 vs 1.07 [P=.029]), and longer mean hospital stays (13.71 d vs 7.17 d [P<.0001]) compared to direct admissions.
Despite the absence of mortality in both groups, transfer patients showed a higher number of ICU admissions (19 vs 5 [P=.014]) and burn unit admissions (9 vs 2 [P=.096]), bacteremia (16 vs 4 [P=.025]), acute kidney injury (13 vs 10 [P=.755]), acute respiratory failure (12 vs 5 [P=.272]), and transaminitis (8 vs 3 [P=.319]).
Outside Hospital Treatments—All outside hospitals provided supportive care with intravenous fluids and acetaminophen; however, further care provided at outside hospitals varied (Table 3), with transfer patients most frequently being treated with diphenhydramine (69% [29/42]), antimicrobial medications (57% [24/42]), steroids (40%), and epinephrine (10% [4/42]). Some patients may have received more than one of these treatments. Based on outside hospital treatments, the primary care teams’ main clinical concerns were allergic reactions and infection, as 33 (79%) patients received diphenhydramine (29 [89%]) or epinephrine (4 [12%]) and 24 (52%) received antimicrobial medications. Of the 42 transfer patients, 24 (57%) received or continued these medications before transfer; the medications were promptly discontinued upon tertiary hospital admission.
Once the outside hospitals contacted the tertiary hospital for a referral, the mean length of time between the transfer request and Physician Access Line call was 17.13 minutes (Table 4). Following the transfer request, the mean length of time for arrival at the tertiary hospital was 6.22 hours. The mean length of stay at the outside hospital prior to the patient being transferred was 3.84 days.

Comment
This retrospective study examined 71 patients with biopsy-confirmed SJS, SJS/TEN overlap, or TEN to evaluate differences in clinical outcomes between direct and transfer admissions. Transfer patients had a higher mean maximum affected BSA (38.55% vs 19.14% [P=.005]) and elevated SCORTEN (1.92 vs 1.07 [P=.029]); a higher number of transfer patients were admitted to the ICU (19 vs 5 [P=.014]) and burn unit (9 vs 2 [P=.096]), and this group also demonstrated longer hospitalization stays (13.71 vs 7.17 [P<.0001]). There were more complications among transfer patients, including bacteremia (16 vs 4 [P=.025]), which is consistent with findings from the existing literature.3
Once the decision for transfer of the patients included in our study was initiated and accepted, there was a prompt response and transfer of care; the mean length of time for Physician Access Line request was 17.13 minutes, and the mean transfer time to arrive at the tertiary hospital was 6.22 hours; however, patients spent an average of 3.84 days at outside hospitals, reflecting that transfer calls frequently were initiated due to urgent clinical decline of the patient rather than as an early intervention strategy. The management at outside hospitals often included the continuation of antimicrobial medications, which were discontinued upon transfer to AHWFBMC. Causative agents were either previously prescribed for a new medical condition or initiated for the management of suspected infections at outside hospitals. This may reflect the difficulty in correctly diagnosing SJS/TEN and initiating appropriate management at hospital facilities without an inpatient dermatologist.
The presence of inpatient dermatologists can improve the diagnostic accuracy and treatment of various conditions.4,5 Dermatology consultations added or changed 77% of treatment plans for 271 hospitalized patients.4 The impact of this intervention is reflected by the success of early dermatology consultations in reducing the length of hospitalization and use of inappropriate treatments in the care of skin diseases.6-8
Access to dermatologic care has been an identified need in inpatient hospitals that may limit the ability of hospitals to promptly treat serious conditions such as SJS/TEN.9 From an inpatient dermatology study from 2013 through 2019, 98.2% of 782 inpatient dermatologists reside in metropolitan areas, limiting the availability of care for rural patients; this study also found a decreasing number of facilities with inpatient dermatologists.10
The limitations of our study include a small sample size of 71 patients, which restricted the generalizability of our results. Our study also was based at a single tertiary center, which thereby limited the findings to this geographic area. It also was difficult to match patients by their demographic and comorbid conditions. The retrospective study design depended on the accuracy and completeness of medical records, which can introduce information bias. Future studies should compare the clinical outcomes of SJS/TEN based on burn unit and ICU admissions.
Conclusion
Prompt identification of SJS/TEN and rapid transfer to hospitals with inpatient dermatology are essential to optimize patient outcomes. Developing and validating SJS/TEN diagnosis and transfer protocols across multiple institutions may be helpful.
- Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157:1182-1190. doi:10.1001/jamadermatol.2021.3154
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016 /j.jaad.2020.02.066
- Clark AE, Fook-Chong S, Choo K, et al. Delayed admission to a specialist referral center for Stevens-Johnson syndrome and toxic epidermal necrolysis is associated with increased mortality: a retrospective cohort study. JAAD Int. 2021;4:10-12. doi:10.1016/j.jdin.2021.03.008
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi:10.1186/1750-1172-5-39
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Messenger E, Kovarik CL, Lipoff JB. Access to inpatient dermatology care in Pennsylvania hospitals. Cutis. 2016;97:49-51.
- Hydol-Smith JA, Gallardo MA, Korman A, et al. The United States dermatology inpatient workforce between 2013 and 2019: a Medicare analysis reveals contraction of the workforce and vast access desertsa cross-sectional analysis. Arch Dermatol Res. 2024;316:103. doi:10.1007 /s00403-024-02845-0
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that involve widespread necrosis of the skin and mucous membranes.1 Guidelines for SJS and TEN recommend management in hospitals with access to inpatient dermatology to provide immediate interventions that are necessary for achieving optimal patient outcomes.2 A delay in admission of 5 days or more after onset of symptoms has been associated with increases in overall mortality, bacteremia, intensive care unit (ICU) admission, and length of stay.3 Patients who are not directly admitted to specialized facilities and require transfer from other hospitals may experience delays in receiving critical interventions, further increasing the risk for mortality and complications. In this study, we analyzed the clinical outcomes of patients with SJS/TEN in relation to their admission pathway.
Methods
A single-center retrospective chart review was performed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) in Winston-Salem, North Carolina. Participants were identified using i2b2, an informatics tool compliant with the Health Insurance Portability and Accountability Act for integrating biology and the bedside. Inclusion criteria were having a diagnosis of SJS (International Classification of Diseases, Tenth Revision, code L51.1; International Classification of Diseases, Ninth Revision, code 695.13), TEN (International Classification of Diseases, Tenth Revision, code L51.2; International Classification of Diseases, Ninth Revision, code 695.15) or Lyell syndrome from January 2012 to December 2024. Patients with erythema multiforme or bullous drug eruption were excluded, as these conditions initially were misdiagnosed as SJS or TEN. Patients with only a reported history of prior SJS or TEN also were excluded.
The following clinical outcomes were assessed: demographics, comorbidities, age at disease onset, outside hospital transfer status, complications during admission, inpatient length of stay in days, age of mortality (if applicable), culprit medications, interventions received, Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) upon admission, site of admission (eg, floor bed, ICU, medical ICU, burn unit), and length of disease process prior to hospital admission. Patients then were categorized as either direct or transfer admissions based on the initial point of care and admission process. Direct admissions included patients who presented to the AHWFBMC emergency department and were subsequently admitted. Transfer patients included patients who initially presented to an outside hospital and were transferred to AHWFBMC. Data regarding the wait time for Physician Access Line requests and the time elapsed from the initial transfer call to arrival at the tertiary hospital also were collected—this is a method that outside hospitals can use to contact physicians at the tertiary hospital for a possible transfer. Statistical analysis was performed using unpaired t tests and X2 tests as necessary using GraphPad By Dotmatics Prism.
Results
A total of 112 patients were included in the analysis; of these, 71 had a diagnosis with biopsy confirmation of SJS, SJS/TEN overlap, or TEN (Table 1). Forty-one patients were excluded due to having a diagnosis of erythema multiforme or bullous drug eruption or a reported history of prior SJS or TEN without hospitalization. All biopsies were performed at AHWFBMC. Of the 71 confirmed patients with SJS/TEN, 54 (76%) were female with a mean age of 44 years. The majority of patients identified as Black (35 [49%]) or White (27 [38%]), along with Asian (7 [10%]) and other (2 [3%]). The most common comorbidity was cardiovascular disease in 42 (59%) patients, followed by type 2 diabetes in 36 (51%) patients. Among these 71 patients with SJS/TEN, 29 (41%) were directly admitted to the tertiary hospital, while 42 (59%) were transferred from outside hospitals.

Of the 71 confirmed patients with SJS/TEN, sulfonamides were identified as the most common inciting drug in 25 (41%) patients, followed by beta-lactam antibiotics in 16 (23%) patients (Table 2). This is consistent with previous literature of sulfamethoxazole with trimethoprim as the primary causative drug for SJS and TEN in the United States.1

Clinical Outcomes—Of the 71 patients, there were 23 (32%) cases of SJS, 29 (41%) cases of SJS/TEN overlap, and 19 (27%) cases of TEN (eTable). The initial and maximum affected body surface area (BSA) was higher in transfer admissions, with a mean maximum BSA of 38.55% in the transfer group compared to 19.14% in the direct admissions. The mean SCORTEN (range, 0-5) was 1.6 overall, with a higher mean score of 1.92 in the transfer group compared to 1.07 in the direct admissions.

Transfer patients had a longer mean stay at the tertiary hospital (13.71 d) compared to direct admissions (7.17 d). The mean time from symptom onset until tertiary hospital admission was 8.5 days; transfer and direct admission patients had similar mean time from symptom onset of 9.02 days and 7.86 days, respectively. Although the duration of cutaneous symptoms from onset until tertiary hospital admission was similar (P=.283) between direct admissions (7.86 d) and transfer patients (9.02 d), the transfer group presented with greater disease severity at the time of admission. Transfer patients had a higher mean maximum BSA involvement (38.55% vs 19.14% [P=.005]), elevated SCORTEN (1.92 vs 1.07 [P=.029]), and longer mean hospital stays (13.71 d vs 7.17 d [P<.0001]) compared to direct admissions.
Despite the absence of mortality in both groups, transfer patients showed a higher number of ICU admissions (19 vs 5 [P=.014]) and burn unit admissions (9 vs 2 [P=.096]), bacteremia (16 vs 4 [P=.025]), acute kidney injury (13 vs 10 [P=.755]), acute respiratory failure (12 vs 5 [P=.272]), and transaminitis (8 vs 3 [P=.319]).
Outside Hospital Treatments—All outside hospitals provided supportive care with intravenous fluids and acetaminophen; however, further care provided at outside hospitals varied (Table 3), with transfer patients most frequently being treated with diphenhydramine (69% [29/42]), antimicrobial medications (57% [24/42]), steroids (40%), and epinephrine (10% [4/42]). Some patients may have received more than one of these treatments. Based on outside hospital treatments, the primary care teams’ main clinical concerns were allergic reactions and infection, as 33 (79%) patients received diphenhydramine (29 [89%]) or epinephrine (4 [12%]) and 24 (52%) received antimicrobial medications. Of the 42 transfer patients, 24 (57%) received or continued these medications before transfer; the medications were promptly discontinued upon tertiary hospital admission.

Once the outside hospitals contacted the tertiary hospital for a referral, the mean length of time between the transfer request and Physician Access Line call was 17.13 minutes (Table 4). Following the transfer request, the mean length of time for arrival at the tertiary hospital was 6.22 hours. The mean length of stay at the outside hospital prior to the patient being transferred was 3.84 days.

Comment
This retrospective study examined 71 patients with biopsy-confirmed SJS, SJS/TEN overlap, or TEN to evaluate differences in clinical outcomes between direct and transfer admissions. Transfer patients had a higher mean maximum affected BSA (38.55% vs 19.14% [P=.005]) and elevated SCORTEN (1.92 vs 1.07 [P=.029]); a higher number of transfer patients were admitted to the ICU (19 vs 5 [P=.014]) and burn unit (9 vs 2 [P=.096]), and this group also demonstrated longer hospitalization stays (13.71 vs 7.17 [P<.0001]). There were more complications among transfer patients, including bacteremia (16 vs 4 [P=.025]), which is consistent with findings from the existing literature.3
Once the decision for transfer of the patients included in our study was initiated and accepted, there was a prompt response and transfer of care; the mean length of time for Physician Access Line request was 17.13 minutes, and the mean transfer time to arrive at the tertiary hospital was 6.22 hours; however, patients spent an average of 3.84 days at outside hospitals, reflecting that transfer calls frequently were initiated due to urgent clinical decline of the patient rather than as an early intervention strategy. The management at outside hospitals often included the continuation of antimicrobial medications, which were discontinued upon transfer to AHWFBMC. Causative agents were either previously prescribed for a new medical condition or initiated for the management of suspected infections at outside hospitals. This may reflect the difficulty in correctly diagnosing SJS/TEN and initiating appropriate management at hospital facilities without an inpatient dermatologist.
The presence of inpatient dermatologists can improve the diagnostic accuracy and treatment of various conditions.4,5 Dermatology consultations added or changed 77% of treatment plans for 271 hospitalized patients.4 The impact of this intervention is reflected by the success of early dermatology consultations in reducing the length of hospitalization and use of inappropriate treatments in the care of skin diseases.6-8
Access to dermatologic care has been an identified need in inpatient hospitals that may limit the ability of hospitals to promptly treat serious conditions such as SJS/TEN.9 From an inpatient dermatology study from 2013 through 2019, 98.2% of 782 inpatient dermatologists reside in metropolitan areas, limiting the availability of care for rural patients; this study also found a decreasing number of facilities with inpatient dermatologists.10
The limitations of our study include a small sample size of 71 patients, which restricted the generalizability of our results. Our study also was based at a single tertiary center, which thereby limited the findings to this geographic area. It also was difficult to match patients by their demographic and comorbid conditions. The retrospective study design depended on the accuracy and completeness of medical records, which can introduce information bias. Future studies should compare the clinical outcomes of SJS/TEN based on burn unit and ICU admissions.
Conclusion
Prompt identification of SJS/TEN and rapid transfer to hospitals with inpatient dermatology are essential to optimize patient outcomes. Developing and validating SJS/TEN diagnosis and transfer protocols across multiple institutions may be helpful.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that involve widespread necrosis of the skin and mucous membranes.1 Guidelines for SJS and TEN recommend management in hospitals with access to inpatient dermatology to provide immediate interventions that are necessary for achieving optimal patient outcomes.2 A delay in admission of 5 days or more after onset of symptoms has been associated with increases in overall mortality, bacteremia, intensive care unit (ICU) admission, and length of stay.3 Patients who are not directly admitted to specialized facilities and require transfer from other hospitals may experience delays in receiving critical interventions, further increasing the risk for mortality and complications. In this study, we analyzed the clinical outcomes of patients with SJS/TEN in relation to their admission pathway.
Methods
A single-center retrospective chart review was performed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) in Winston-Salem, North Carolina. Participants were identified using i2b2, an informatics tool compliant with the Health Insurance Portability and Accountability Act for integrating biology and the bedside. Inclusion criteria were having a diagnosis of SJS (International Classification of Diseases, Tenth Revision, code L51.1; International Classification of Diseases, Ninth Revision, code 695.13), TEN (International Classification of Diseases, Tenth Revision, code L51.2; International Classification of Diseases, Ninth Revision, code 695.15) or Lyell syndrome from January 2012 to December 2024. Patients with erythema multiforme or bullous drug eruption were excluded, as these conditions initially were misdiagnosed as SJS or TEN. Patients with only a reported history of prior SJS or TEN also were excluded.
The following clinical outcomes were assessed: demographics, comorbidities, age at disease onset, outside hospital transfer status, complications during admission, inpatient length of stay in days, age of mortality (if applicable), culprit medications, interventions received, Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) upon admission, site of admission (eg, floor bed, ICU, medical ICU, burn unit), and length of disease process prior to hospital admission. Patients then were categorized as either direct or transfer admissions based on the initial point of care and admission process. Direct admissions included patients who presented to the AHWFBMC emergency department and were subsequently admitted. Transfer patients included patients who initially presented to an outside hospital and were transferred to AHWFBMC. Data regarding the wait time for Physician Access Line requests and the time elapsed from the initial transfer call to arrival at the tertiary hospital also were collected—this is a method that outside hospitals can use to contact physicians at the tertiary hospital for a possible transfer. Statistical analysis was performed using unpaired t tests and X2 tests as necessary using GraphPad By Dotmatics Prism.
Results
A total of 112 patients were included in the analysis; of these, 71 had a diagnosis with biopsy confirmation of SJS, SJS/TEN overlap, or TEN (Table 1). Forty-one patients were excluded due to having a diagnosis of erythema multiforme or bullous drug eruption or a reported history of prior SJS or TEN without hospitalization. All biopsies were performed at AHWFBMC. Of the 71 confirmed patients with SJS/TEN, 54 (76%) were female with a mean age of 44 years. The majority of patients identified as Black (35 [49%]) or White (27 [38%]), along with Asian (7 [10%]) and other (2 [3%]). The most common comorbidity was cardiovascular disease in 42 (59%) patients, followed by type 2 diabetes in 36 (51%) patients. Among these 71 patients with SJS/TEN, 29 (41%) were directly admitted to the tertiary hospital, while 42 (59%) were transferred from outside hospitals.

Of the 71 confirmed patients with SJS/TEN, sulfonamides were identified as the most common inciting drug in 25 (41%) patients, followed by beta-lactam antibiotics in 16 (23%) patients (Table 2). This is consistent with previous literature of sulfamethoxazole with trimethoprim as the primary causative drug for SJS and TEN in the United States.1

Clinical Outcomes—Of the 71 patients, there were 23 (32%) cases of SJS, 29 (41%) cases of SJS/TEN overlap, and 19 (27%) cases of TEN (eTable). The initial and maximum affected body surface area (BSA) was higher in transfer admissions, with a mean maximum BSA of 38.55% in the transfer group compared to 19.14% in the direct admissions. The mean SCORTEN (range, 0-5) was 1.6 overall, with a higher mean score of 1.92 in the transfer group compared to 1.07 in the direct admissions.

Transfer patients had a longer mean stay at the tertiary hospital (13.71 d) compared to direct admissions (7.17 d). The mean time from symptom onset until tertiary hospital admission was 8.5 days; transfer and direct admission patients had similar mean time from symptom onset of 9.02 days and 7.86 days, respectively. Although the duration of cutaneous symptoms from onset until tertiary hospital admission was similar (P=.283) between direct admissions (7.86 d) and transfer patients (9.02 d), the transfer group presented with greater disease severity at the time of admission. Transfer patients had a higher mean maximum BSA involvement (38.55% vs 19.14% [P=.005]), elevated SCORTEN (1.92 vs 1.07 [P=.029]), and longer mean hospital stays (13.71 d vs 7.17 d [P<.0001]) compared to direct admissions.
Despite the absence of mortality in both groups, transfer patients showed a higher number of ICU admissions (19 vs 5 [P=.014]) and burn unit admissions (9 vs 2 [P=.096]), bacteremia (16 vs 4 [P=.025]), acute kidney injury (13 vs 10 [P=.755]), acute respiratory failure (12 vs 5 [P=.272]), and transaminitis (8 vs 3 [P=.319]).
Outside Hospital Treatments—All outside hospitals provided supportive care with intravenous fluids and acetaminophen; however, further care provided at outside hospitals varied (Table 3), with transfer patients most frequently being treated with diphenhydramine (69% [29/42]), antimicrobial medications (57% [24/42]), steroids (40%), and epinephrine (10% [4/42]). Some patients may have received more than one of these treatments. Based on outside hospital treatments, the primary care teams’ main clinical concerns were allergic reactions and infection, as 33 (79%) patients received diphenhydramine (29 [89%]) or epinephrine (4 [12%]) and 24 (52%) received antimicrobial medications. Of the 42 transfer patients, 24 (57%) received or continued these medications before transfer; the medications were promptly discontinued upon tertiary hospital admission.

Once the outside hospitals contacted the tertiary hospital for a referral, the mean length of time between the transfer request and Physician Access Line call was 17.13 minutes (Table 4). Following the transfer request, the mean length of time for arrival at the tertiary hospital was 6.22 hours. The mean length of stay at the outside hospital prior to the patient being transferred was 3.84 days.

Comment
This retrospective study examined 71 patients with biopsy-confirmed SJS, SJS/TEN overlap, or TEN to evaluate differences in clinical outcomes between direct and transfer admissions. Transfer patients had a higher mean maximum affected BSA (38.55% vs 19.14% [P=.005]) and elevated SCORTEN (1.92 vs 1.07 [P=.029]); a higher number of transfer patients were admitted to the ICU (19 vs 5 [P=.014]) and burn unit (9 vs 2 [P=.096]), and this group also demonstrated longer hospitalization stays (13.71 vs 7.17 [P<.0001]). There were more complications among transfer patients, including bacteremia (16 vs 4 [P=.025]), which is consistent with findings from the existing literature.3
Once the decision for transfer of the patients included in our study was initiated and accepted, there was a prompt response and transfer of care; the mean length of time for Physician Access Line request was 17.13 minutes, and the mean transfer time to arrive at the tertiary hospital was 6.22 hours; however, patients spent an average of 3.84 days at outside hospitals, reflecting that transfer calls frequently were initiated due to urgent clinical decline of the patient rather than as an early intervention strategy. The management at outside hospitals often included the continuation of antimicrobial medications, which were discontinued upon transfer to AHWFBMC. Causative agents were either previously prescribed for a new medical condition or initiated for the management of suspected infections at outside hospitals. This may reflect the difficulty in correctly diagnosing SJS/TEN and initiating appropriate management at hospital facilities without an inpatient dermatologist.
The presence of inpatient dermatologists can improve the diagnostic accuracy and treatment of various conditions.4,5 Dermatology consultations added or changed 77% of treatment plans for 271 hospitalized patients.4 The impact of this intervention is reflected by the success of early dermatology consultations in reducing the length of hospitalization and use of inappropriate treatments in the care of skin diseases.6-8
Access to dermatologic care has been an identified need in inpatient hospitals that may limit the ability of hospitals to promptly treat serious conditions such as SJS/TEN.9 From an inpatient dermatology study from 2013 through 2019, 98.2% of 782 inpatient dermatologists reside in metropolitan areas, limiting the availability of care for rural patients; this study also found a decreasing number of facilities with inpatient dermatologists.10
The limitations of our study include a small sample size of 71 patients, which restricted the generalizability of our results. Our study also was based at a single tertiary center, which thereby limited the findings to this geographic area. It also was difficult to match patients by their demographic and comorbid conditions. The retrospective study design depended on the accuracy and completeness of medical records, which can introduce information bias. Future studies should compare the clinical outcomes of SJS/TEN based on burn unit and ICU admissions.
Conclusion
Prompt identification of SJS/TEN and rapid transfer to hospitals with inpatient dermatology are essential to optimize patient outcomes. Developing and validating SJS/TEN diagnosis and transfer protocols across multiple institutions may be helpful.
- Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157:1182-1190. doi:10.1001/jamadermatol.2021.3154
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016 /j.jaad.2020.02.066
- Clark AE, Fook-Chong S, Choo K, et al. Delayed admission to a specialist referral center for Stevens-Johnson syndrome and toxic epidermal necrolysis is associated with increased mortality: a retrospective cohort study. JAAD Int. 2021;4:10-12. doi:10.1016/j.jdin.2021.03.008
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi:10.1186/1750-1172-5-39
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Messenger E, Kovarik CL, Lipoff JB. Access to inpatient dermatology care in Pennsylvania hospitals. Cutis. 2016;97:49-51.
- Hydol-Smith JA, Gallardo MA, Korman A, et al. The United States dermatology inpatient workforce between 2013 and 2019: a Medicare analysis reveals contraction of the workforce and vast access desertsa cross-sectional analysis. Arch Dermatol Res. 2024;316:103. doi:10.1007 /s00403-024-02845-0
- Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157:1182-1190. doi:10.1001/jamadermatol.2021.3154
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016 /j.jaad.2020.02.066
- Clark AE, Fook-Chong S, Choo K, et al. Delayed admission to a specialist referral center for Stevens-Johnson syndrome and toxic epidermal necrolysis is associated with increased mortality: a retrospective cohort study. JAAD Int. 2021;4:10-12. doi:10.1016/j.jdin.2021.03.008
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi:10.1186/1750-1172-5-39
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Messenger E, Kovarik CL, Lipoff JB. Access to inpatient dermatology care in Pennsylvania hospitals. Cutis. 2016;97:49-51.
- Hydol-Smith JA, Gallardo MA, Korman A, et al. The United States dermatology inpatient workforce between 2013 and 2019: a Medicare analysis reveals contraction of the workforce and vast access desertsa cross-sectional analysis. Arch Dermatol Res. 2024;316:103. doi:10.1007 /s00403-024-02845-0
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
PRACTICE POINTS
- Early identification and diagnosis of Stevens-Johnson syndrome and toxic epidermal necrolysis are essential to improving patient outcomes.
- Patients transferred from outside hospitals often present with more severe disease due to delays in diagnosis and initiation of appropriate treatment.
- Inpatient dermatology consultation plays a vital role in accurately diagnosing and managing life-threatening dermatologic conditions.
- Establishing timely interhospital transfer protocols may help expedite access to specialized treatment and improve patient outcomes.
Don’t Miss These Signs of Rosacea in Darker Skin Types
Don’t Miss These Signs of Rosacea in Darker Skin Types
THE COMPARISON:
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2
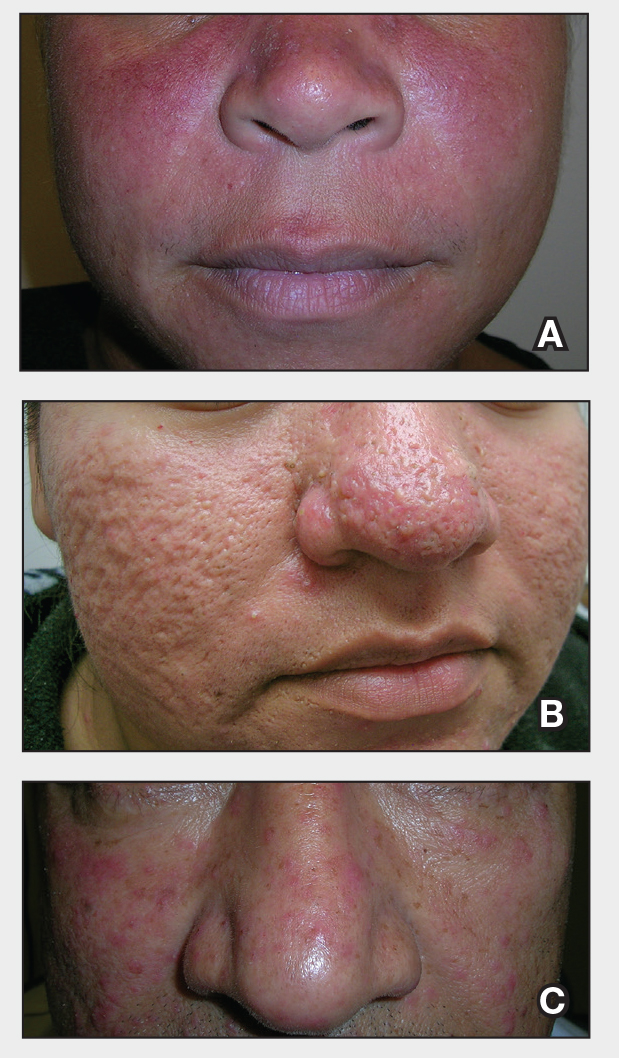
Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
THE COMPARISON:
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2

Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
THE COMPARISON:
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2

Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
Don’t Miss These Signs of Rosacea in Darker Skin Types
Don’t Miss These Signs of Rosacea in Darker Skin Types