User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Chondrodermatitis Nodularis Helicis After Mohs Micrographic Surgery and Radiation Therapy
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.
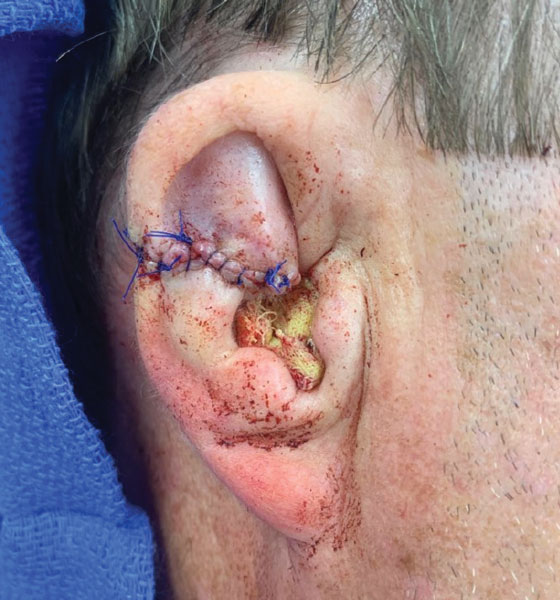
A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.
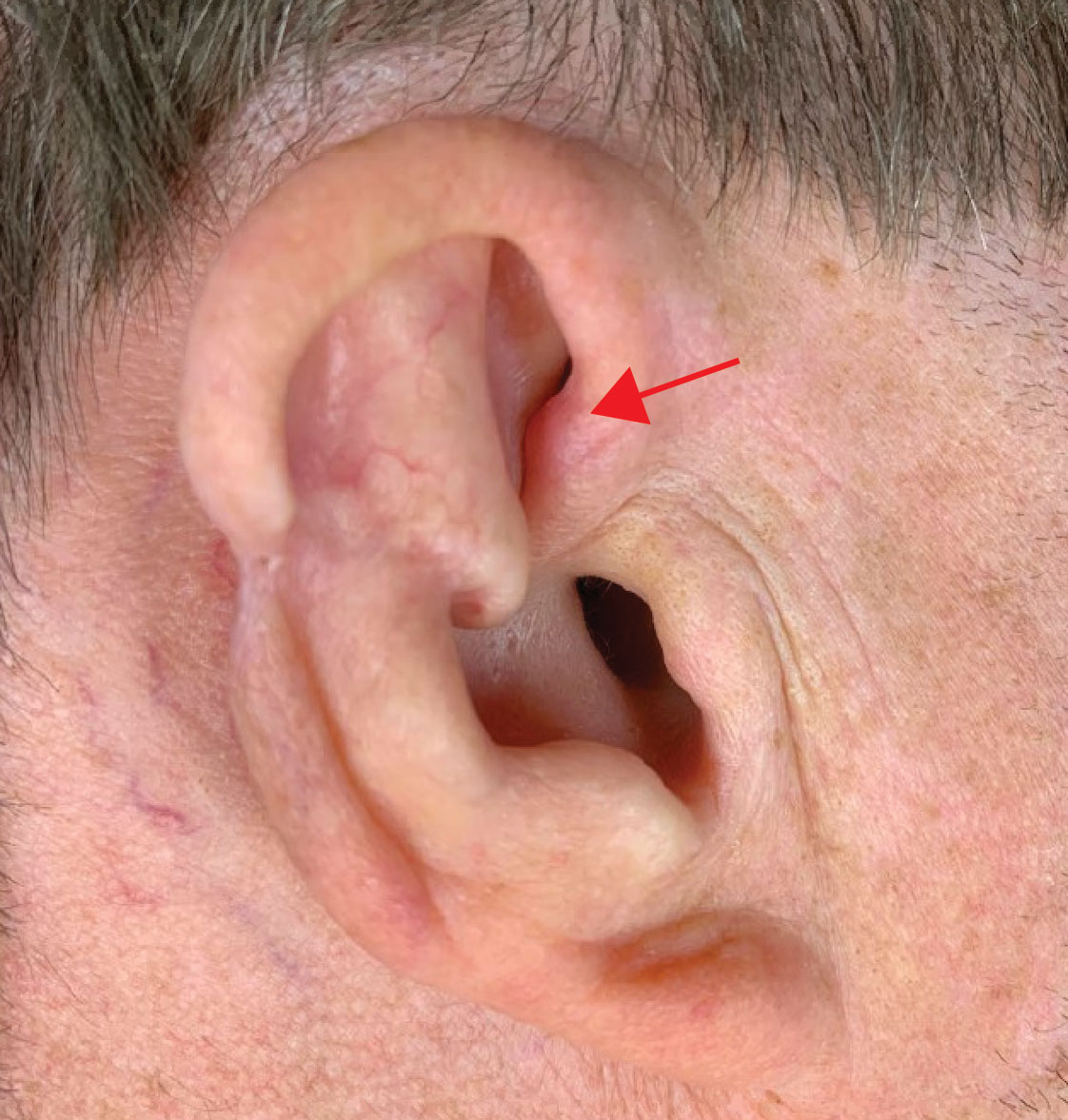
Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).
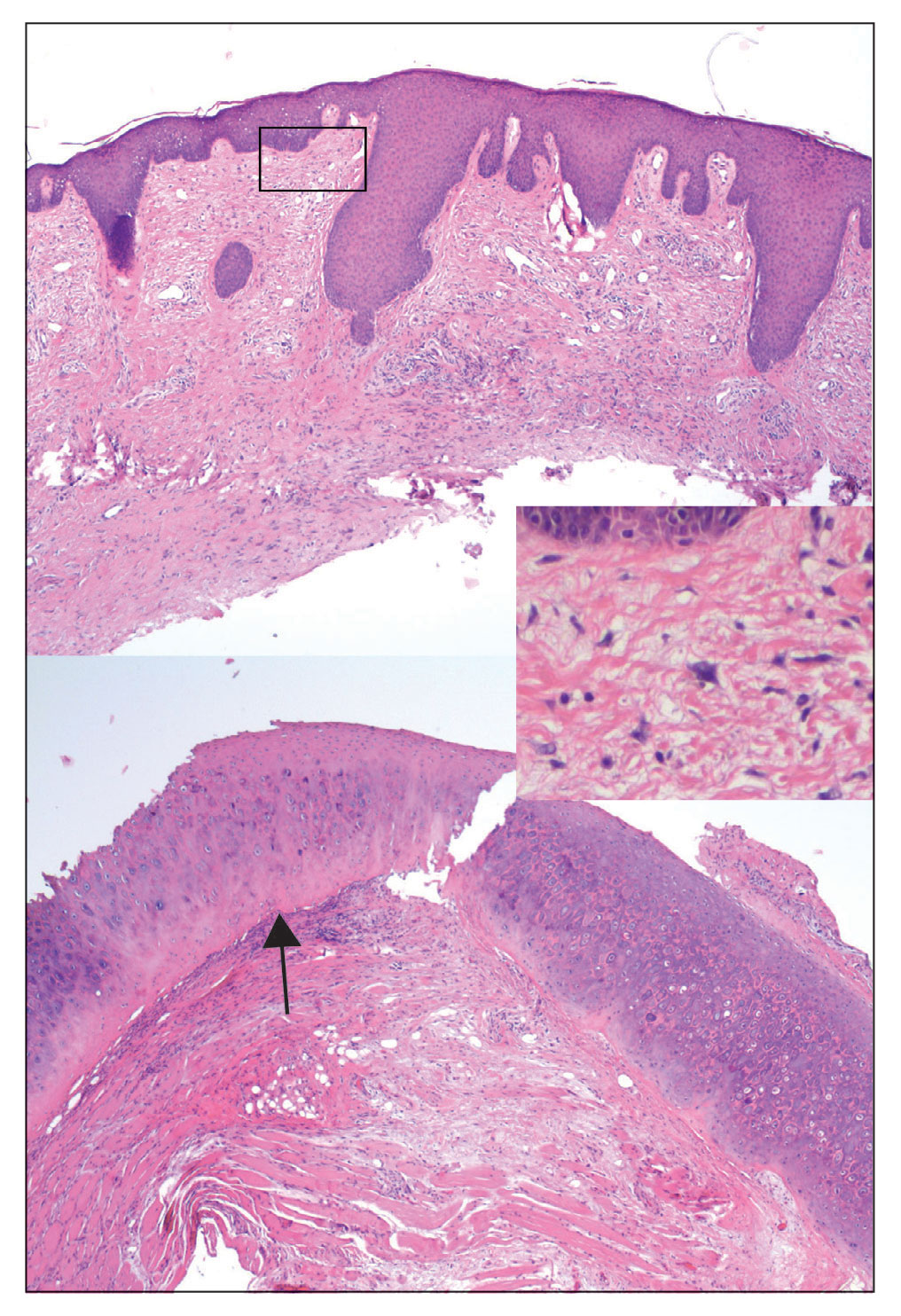
Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
Practice Points
- Although chondrodermatitis nodularis helicis (CNH) is benign by nature, it can mimic tumor recurrence when it presents close to the site of prior Mohs micrographic surgery (MMS). Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Skin lesions in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis.
An Evaluation of Spin in the Abstracts of Systematic Reviews and Meta-analyses on the Treatment of Psoriasis: A Cross-sectional Analysis
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
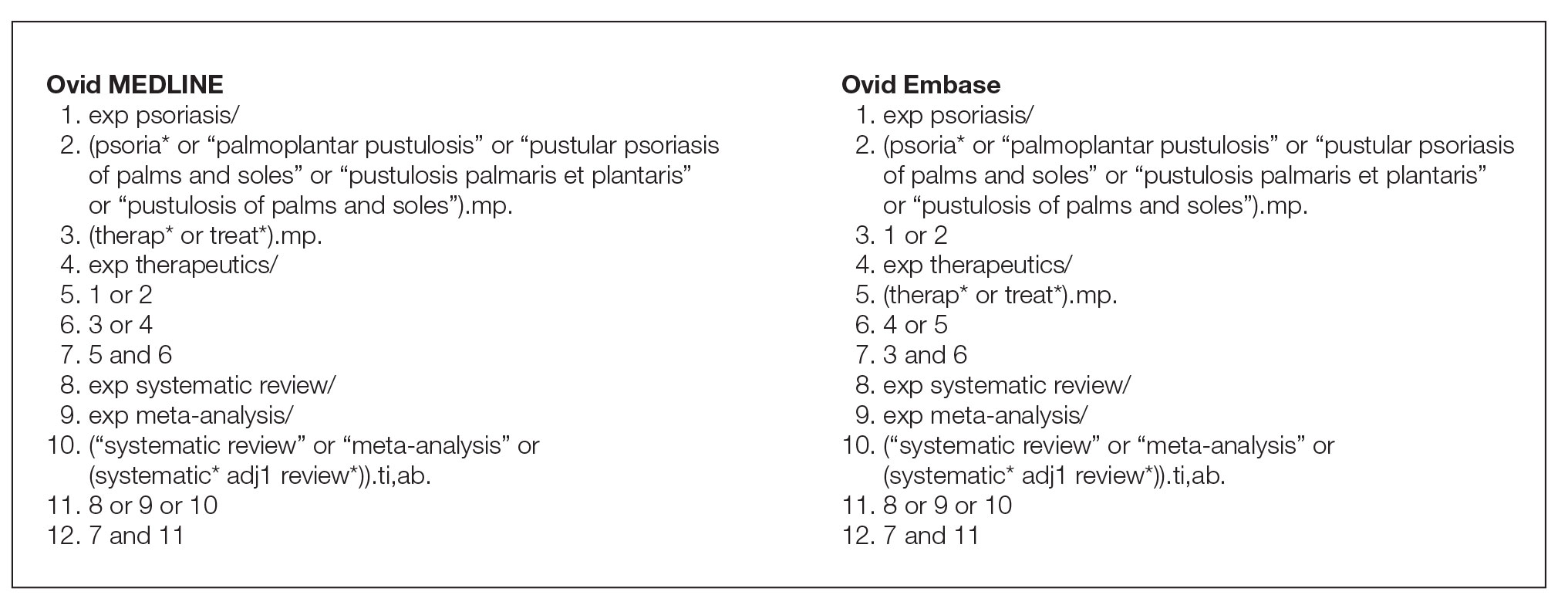
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.
Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
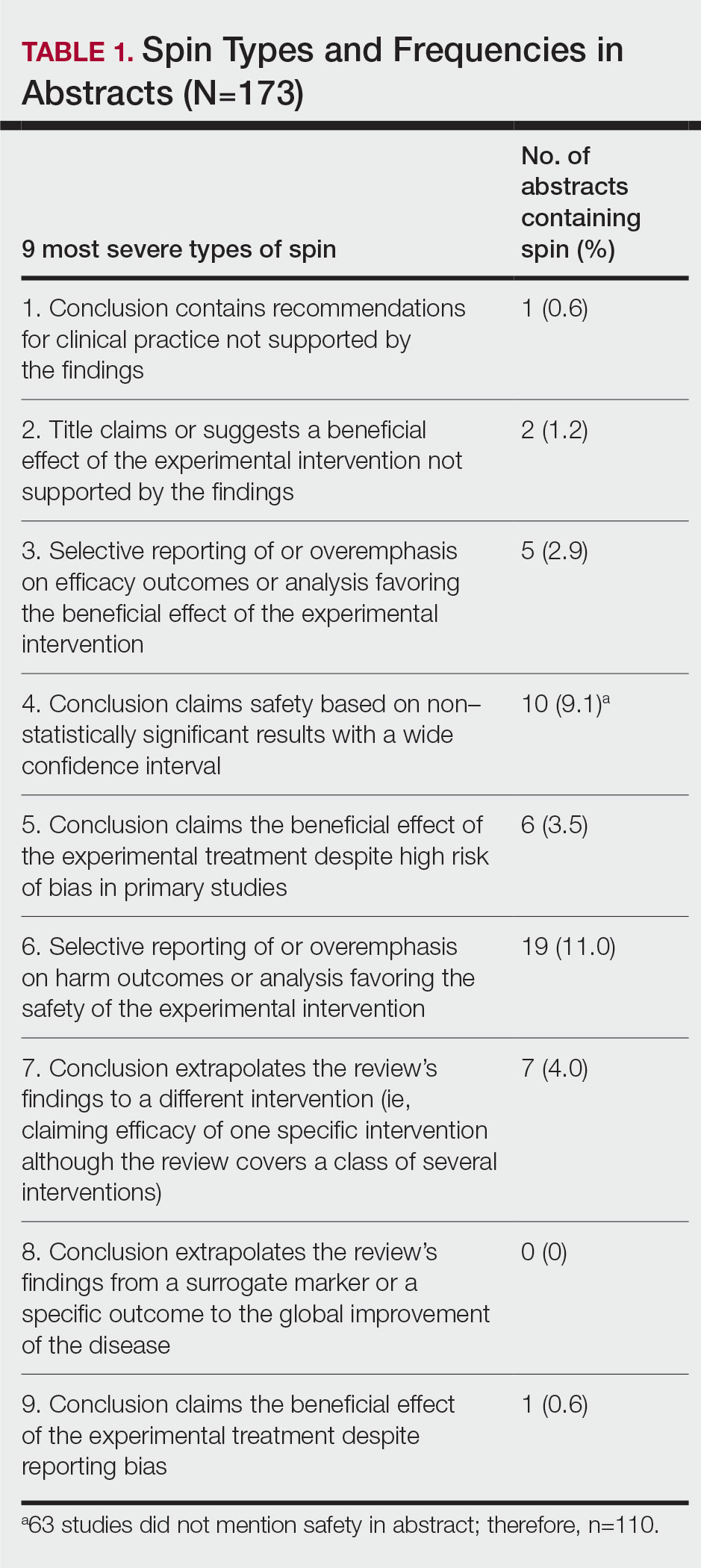
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19
During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
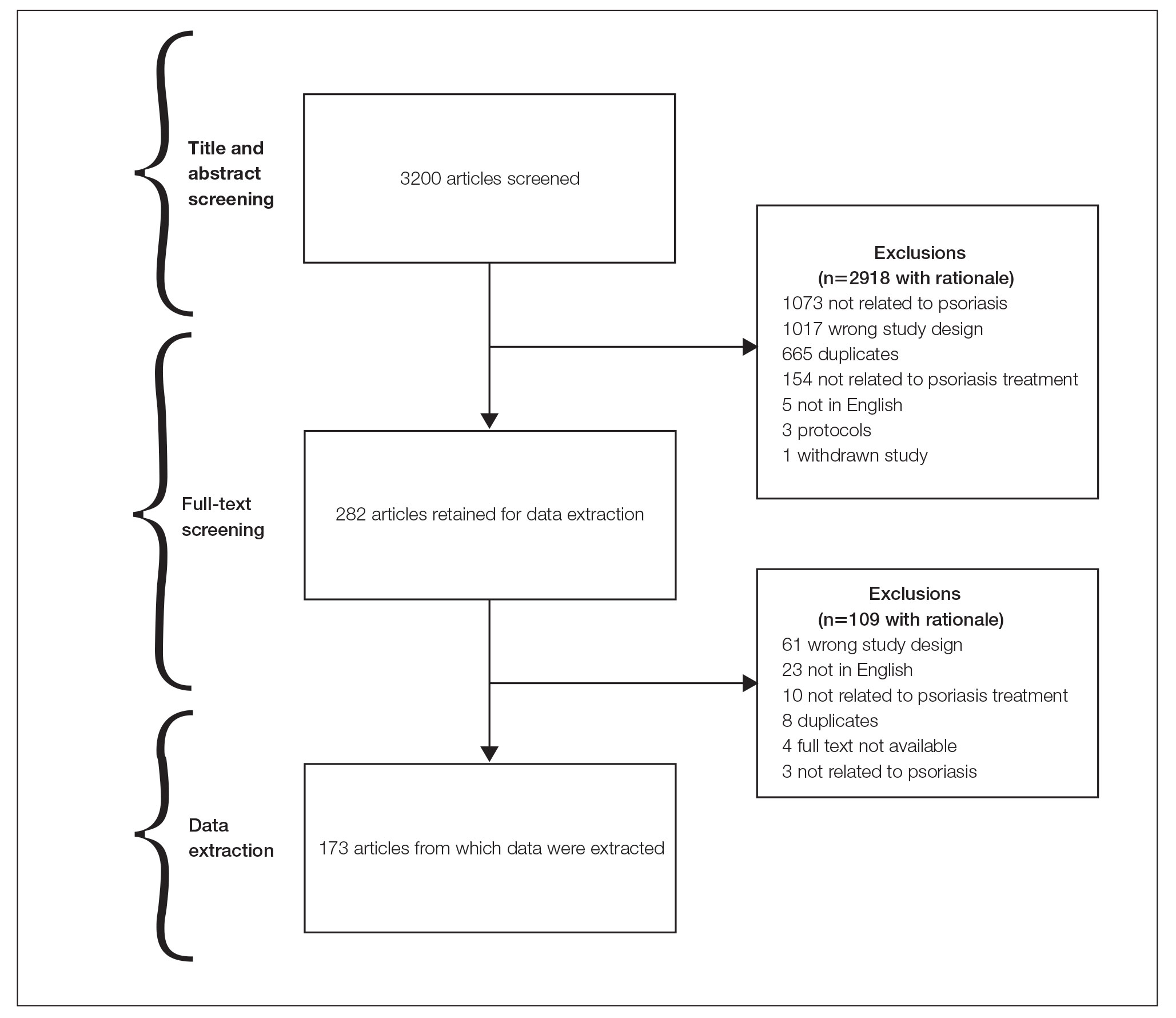
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.
Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).
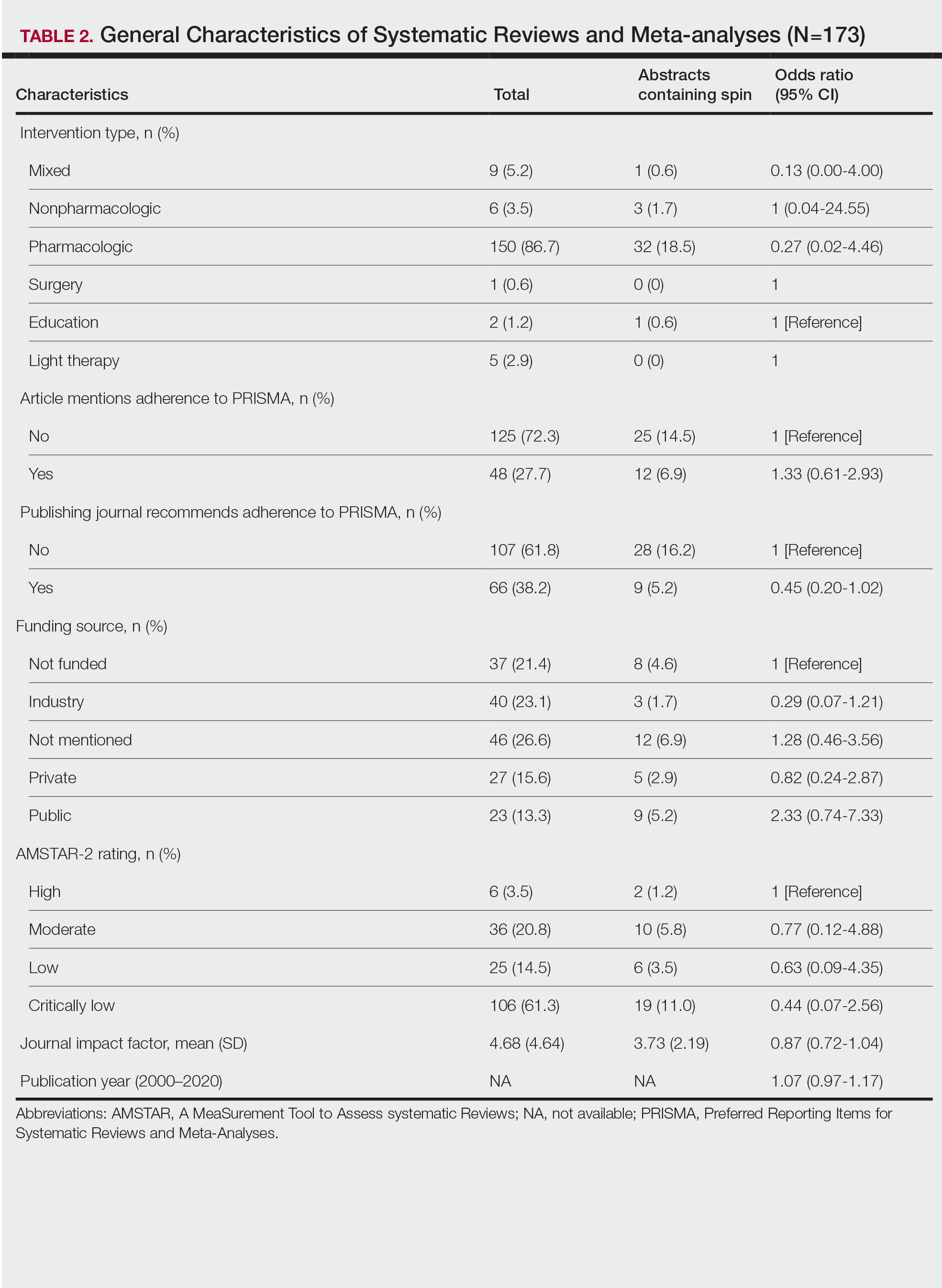
Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
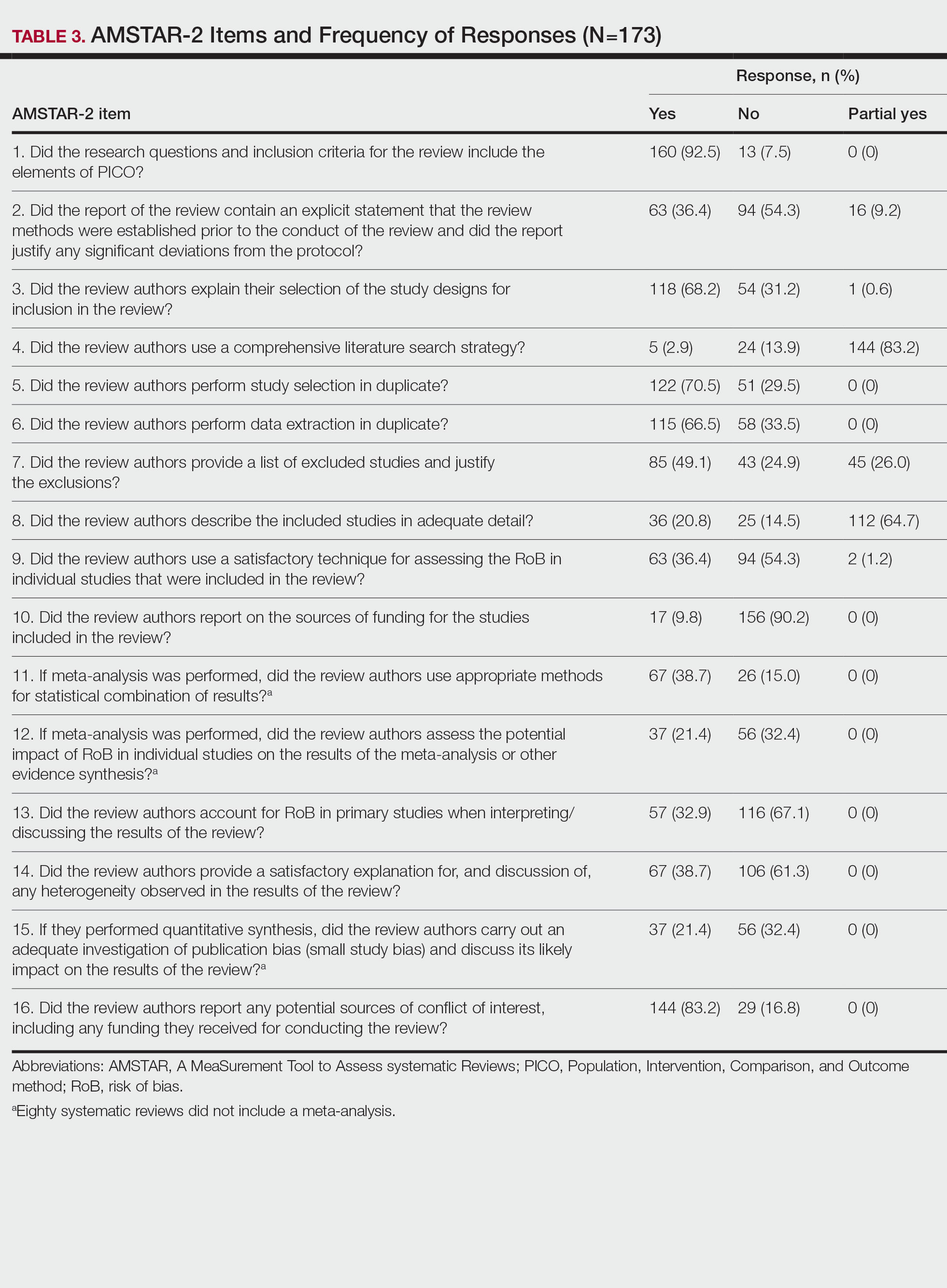
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.
Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.

Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19

During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.

Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).

Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.

Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.

Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19

During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.

Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).

Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.

Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
Practice Points
- Spin is defined as the intentional or unintentional misrepresentation of findings and can inappropriately highlight results and disregard results of equal importance.
- Our findings show that more than 20% of systematic reviews focused on the treatment of psoriasis contained some form of spin within the abstract.
- Because spin has the potential to misrepresent findings and distort a reader’s perception of psoriasis therapies, efforts are needed to prevent its occurrence.
Papular Acneform Eruption With Mucositis
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.
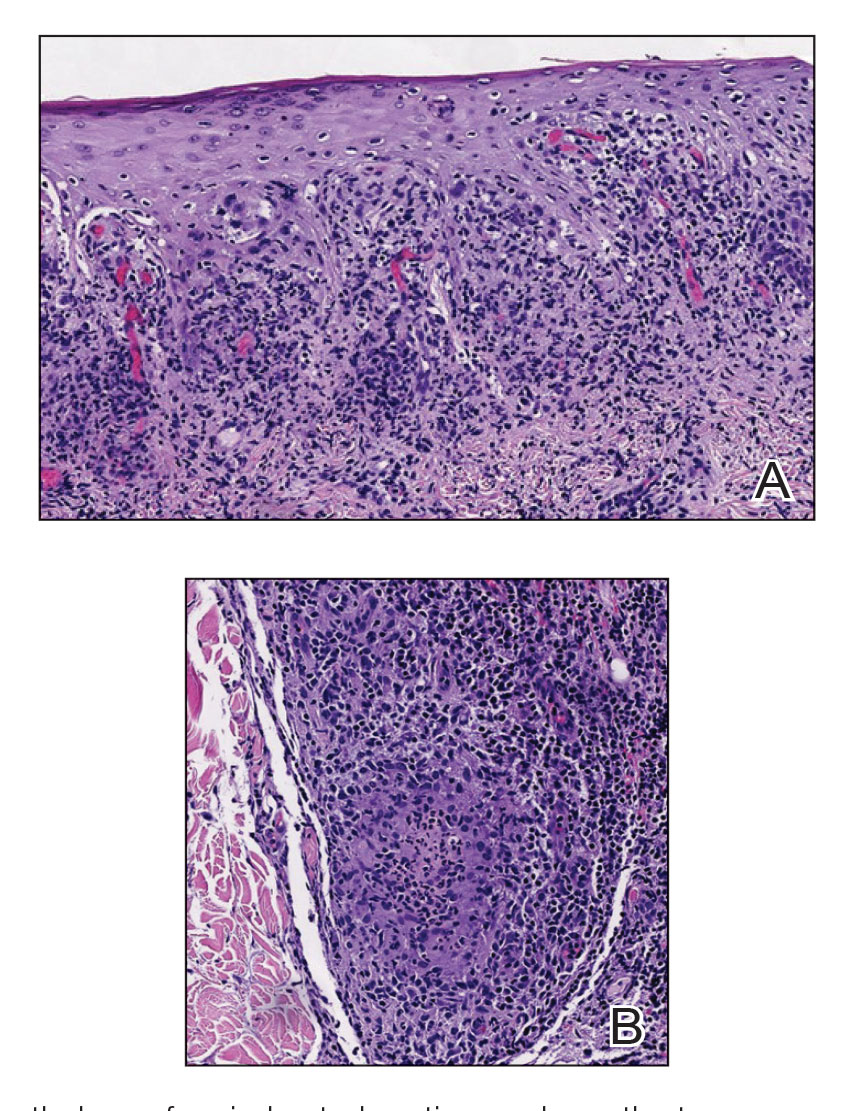
Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
A 48-year-old man with a history of ulcerative colitis that was well-controlled with adalimumab presented with a generalized acneform eruption involving the face, chest (top) and back, as well as a well-defined ovoid ulcer on the anterior aspect of the tongue (bottom) of 2 months’ duration. Prior treatment with prednisone 60 mg daily for 14 days resulted in no improvement. He denied unintentional weight loss, cyclic fever, or arthritis. A complete blood cell count with differential showed mild anemia (hemoglobin, 11.6 g/dL [reference range, 13.2–16.6 g/dL]) with a differential cell count that was within reference range for each cell type. The erythrocyte sedimentation rate was elevated at 44 mm/h (reference range, 0–22 mm/h). A 4-mm punch biopsy specimen of an indurated cystic papule on the torso was obtained.
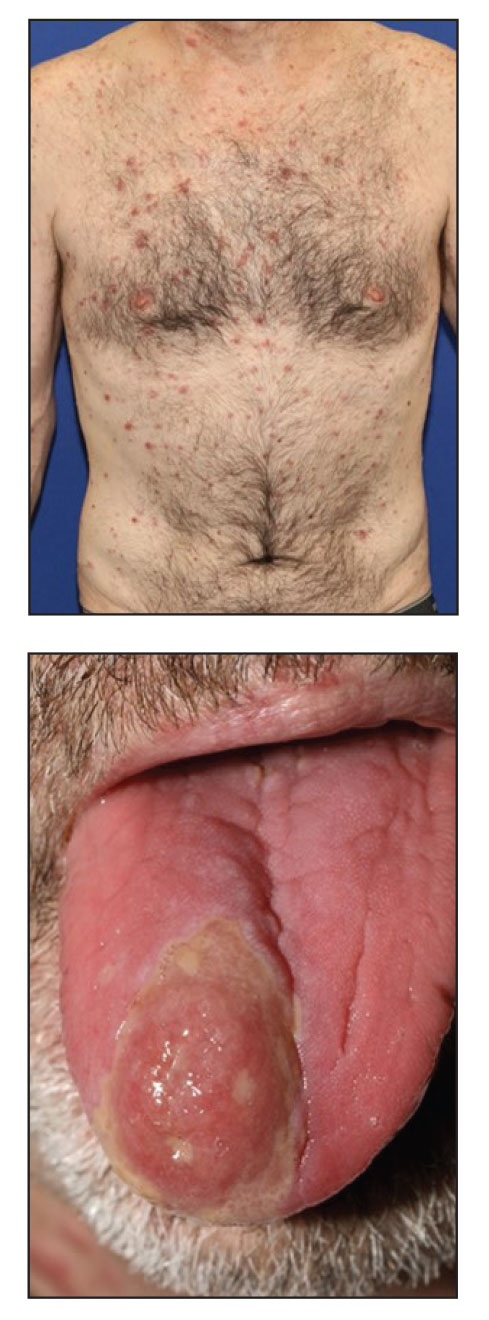
Acral Necrosis After PD-L1 Immune Checkpoint Inhibitor Therapy
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.
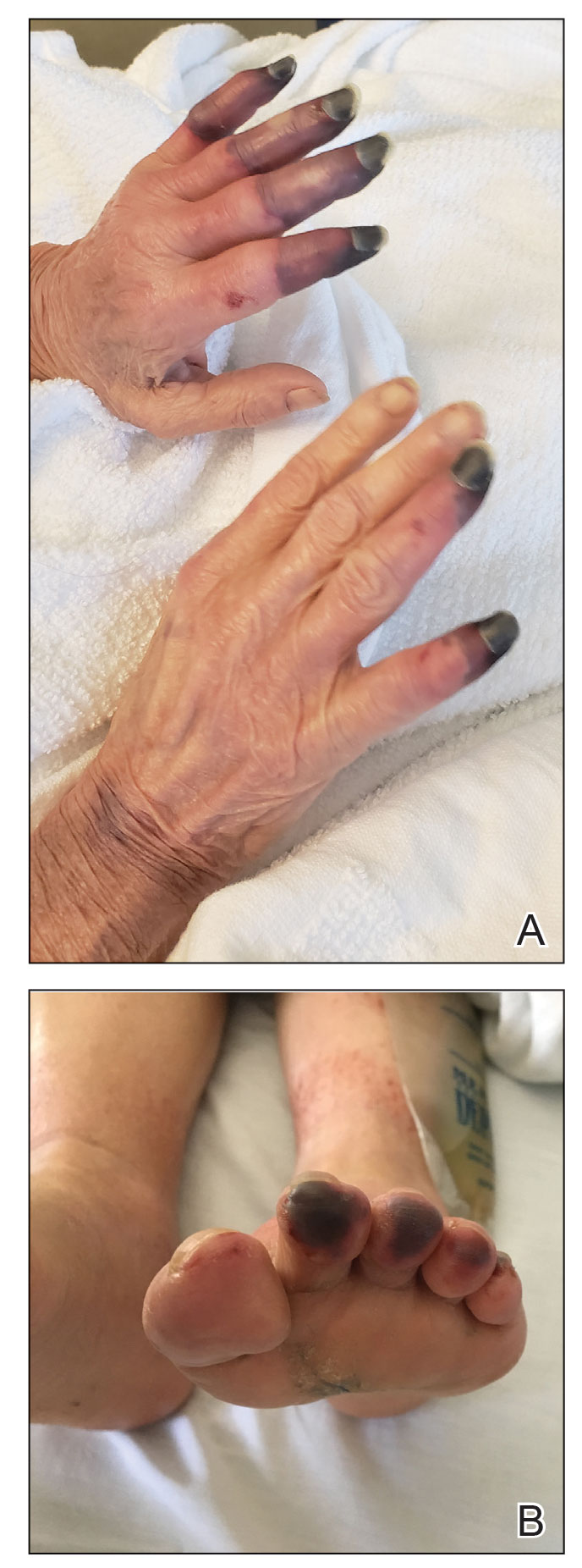
An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.

An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.

An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
Practice Points
- Dermatologists should be aware of acral necrosis as a rare adverse event of treatment with an immune checkpoint inhibitor.
- Delayed immune-related events are sequelae of immune checkpoint inhibitors that can occur months after treatment is discontinued.
Eruptive Keratoacanthomas After Nivolumab Treatment of Stage III Melanoma
To the Editor:
Programmed cell death protein 1 (PD-1) inhibitors have been widely used in the treatment of various cancers. Programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 located on cancer cells will bind to PD-1 receptors on T cells and suppress them, which will prevent cancer cell destruction. Programmed cell death protein 1 inhibitors block the binding of PD-L1 to cancer cells, which then prevents T-cell immunosuppression.1 However, cutaneous adverse effects have been associated with PD-1 inhibitors. Dermatitis associated with PD-1 inhibitor therapy occurs more frequently in patients with cutaneous tumors such as melanoma compared to those with head and neck cancers.2 Curry et al1 reported that treatment with an immune checkpoint blockade can lead to immune-related adverse effects, most commonly affecting the gastrointestinal tract, liver, and skin. The same report cited dermatologic toxicity as an adverse effect in approximately 39% of patients treated with anti–PD-1 and approximately 17% of anti–PD-L1.1 The 4 main categories of dermatologic toxicities to immunotherapies in general include inflammatory disorders, immunobullous disorders, alterations of keratinocytes, and alteration of melanocytes. The most common adverse effects from the use of the PD-1 inhibitor nivolumab were skin rashes, not otherwise specified (14%–20%), pruritus (13%–18%), and vitiligo (~8%).1 Of the cutaneous dermatitic reactions to PD-1 and PD-L1 inhibitors that were biopsied, the 2 most common were lichenoid dermatitis and spongiotic dermatitis.2 Seldomly, there have been reports of keratoacanthomas (KAs) in association with anti–PD-1 therapy.3
A KA is a common skin tumor that appears most frequently as a solitary lesion and is thought to arise from the hair follicle.4 It resembles squamous cell carcinoma and commonly regresses within months without intervention. Exposure to UV light is a known risk factor for the development of KAs.
Eruptive KAs have been found in association with 10 cases of various cancers treated with the PD-1 inhibitors pembrolizumab and nivolumab.3 Multiple lesions on photodistributed areas of the body were reported in all 10 cases. Various treatments were used in these 10 cases—doxycycline and niacinamide, electrodesiccation and curettage, clobetasol ointment and/or intralesional triamcinolone, cryotherapy, imiquimod, or no treatment—as well as the cessation of PD-1 inhibitor therapy, with 4 cases continuing therapy and 6 cases discontinuing therapy. Nine cases regressed by 6 months; electrodesiccation and curettage of the lesions was used in the tenth case.3 We report a case of eruptive KA after 1 cycle of nivolumab therapy for metastatic melanoma.
A 79-year-old woman with stage III melanoma presented to her dermatologist after developing generalized pruritic lichenoid eruptions involving the torso, arms, and legs, as well as erosions on the lips, buccal mucosa, and palate 1 month after starting nivolumab therapy. The patient initially presented to dermatology with an irregularly shaped lesion on the left upper back 3 months prior. Biopsy results at that time revealed a diagnosis of malignant melanoma, lentigo maligna type. The lesion was 1.5-mm thick and classified as Clark level IV with a mitotic count of 6 per mm2. Molecular genetic studies showed expression of PD-L1 and no expression of c-KIT. The patient underwent wide local excision, and a sentinel lymph node biopsy was positive. Positron emission tomography did not show any hypermetabolic lesions, and magnetic resonance imaging did not indicate brain metastasis. The patient underwent an axillary dissection, which did not show any residual melanoma. She was started on adjuvant immunotherapy with intravenous nivolumab 480 mg monthly and developed pruritic crusted lesions on the arms, legs, and torso 1 month later, which prompted follow-up to dermatology.
At the current presentation 4 months after the onset of lesions, physical examination revealed lichenoid patches with serous crusting that were concentrated on the torso but also affected the arms and legs. She developed erosions on the upper and lower lips, buccal mucosa, and hard and soft palates, as well as painful, erythematous, dome-shaped papules and nodules on the legs (Figure 1). Her oncologist previously had initiated treatment at the onset of the lesions with clobetasol cream and valacyclovir for the lesions, but the patient showed no improvement.
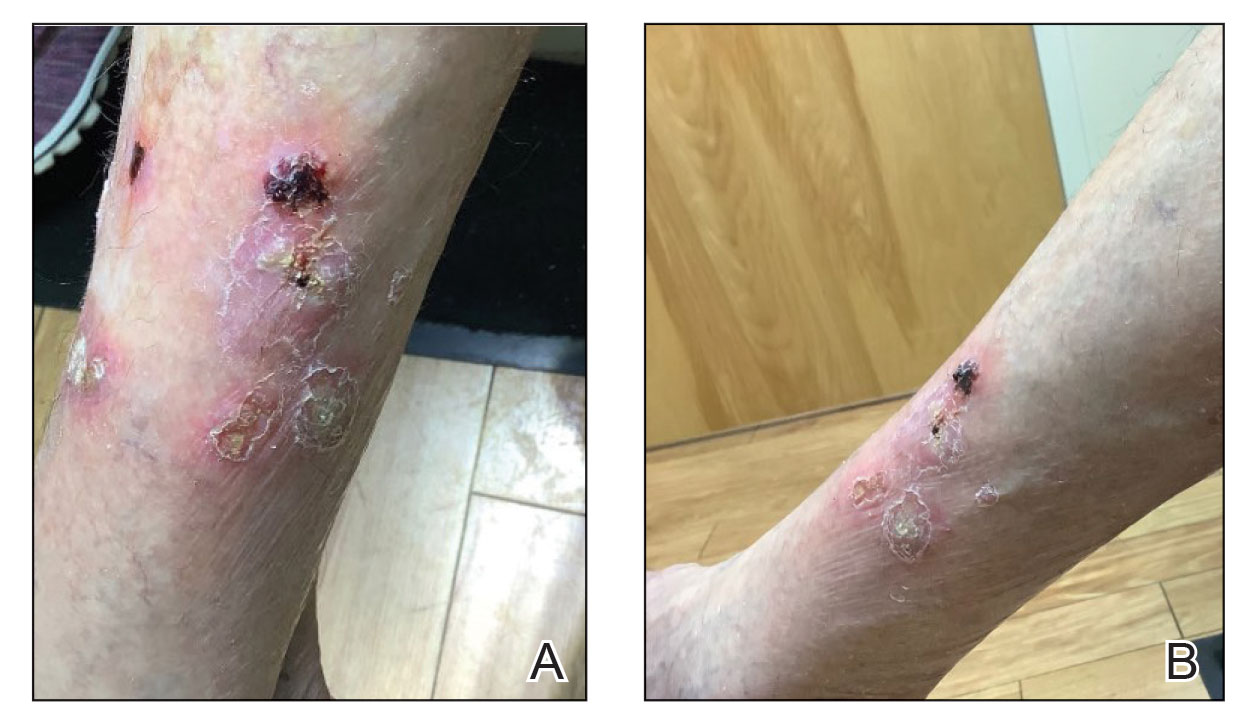
Four months after the onset of the lesions, the patient was re-referred to her dermatologist, and a biopsy was performed on the left lower leg that showed squamous cell carcinoma, KA type. Additionally, flat erythematous patches were seen on the legs that were consistent with a lichenoid drug eruption. Two weeks later, she was started on halobetasol propionate ointment 0.05% for treatment of the KAs. At 2-week follow-up, 5 months after the onset of the lesions, the patient showed no signs of improvement. An oral prednisone taper of 60 mg for 3 days, 40 mg for 3 days, and then 20 mg daily for a total of 4 weeks was started to treat the lichenoid dermatitis and eruptive KAs. At the next follow-up 6.5 months following the first eruptive KAs, she was no longer using topical or oral steroids, she did not have any new eruptive KAs, and old lesions showed regression (Figure 2). The patient still experienced postinflammatory erythema and hyperpigmentation at the location of the KAs but showed improvement of the lichenoid drug eruption.
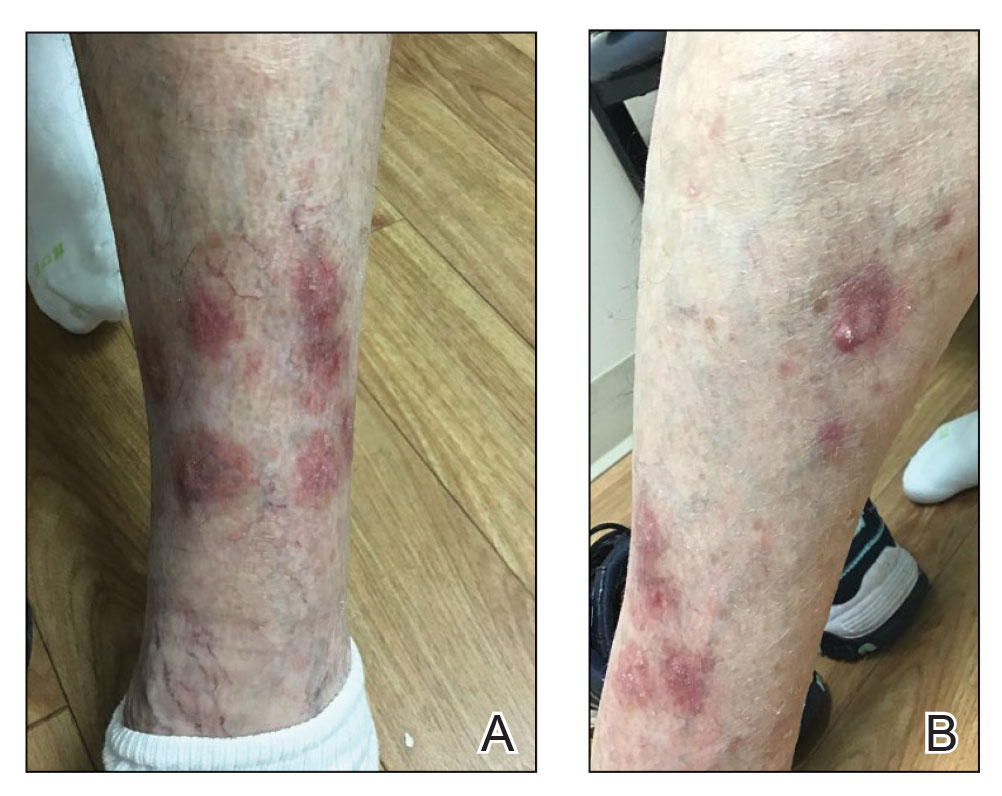
We describe a case of eruptive KAs after use of a PD-1 inhibitor for treatment of melanoma. Our patient developed eruptive KAs after only 1 nivolumab treatment. Another report described onset of eruptive KAs after 1 month of nivolumab infusions.3 The KAs experienced by our patient took 6.5 months to regress, which is unusual compared to other case reports in which the KAs self-resolved within a few months, though one other case described lesions that persisted for 6 months.3
Our patient was treated with topical steroids and an oral steroid taper for the concomitant lichenoid drug eruption. It is unknown if the steroids affected the course of the KAs or if they spontaneously regressed on their own. Freites-Martinez et al5 described that regression of KAs may be related to an immune response, but corticosteroids are inherently immunosuppressive. They hypothesized that corticosteroids help to temper the heightened immune response of eruptive KAs.5
Our patient had oral ulcers, which may have been indicative of an oral lichenoid drug eruption, as well as skin lesions representative of a cutaneous lichenoid drug eruption. This is a favorable reaction, as lichenoid dermatitis is thought to represent successful PD-1 inhibition and therefore a better response to oncologic therapies.2 Comorbid lichenoid drug eruption lesions and eruptive KAs may be suggestive of increased T-cell activity,2,6,7 though some prior case studies have reported eruptive KAs in isolation.3
Discontinuation of immunotherapy due to development of eruptive KAs presents a challenge in the treatment of underlying malignancies such as melanoma. Immunotherapy was discontinued in 7 of 11 cases due to these cutaneous reactions.3 Similarly, our patient underwent only 1 cycle of immunotherapy before developing eruptive KAs and discontinuing PD-1 inhibitor therapy. If we are better able to treat eruptive KAs, then patients can remain on immunotherapy to treat underlying malignancies. Crow et al8 showed improvement in lesions when 3 patients with eruptive KAs were treated with hydroxychloroquine; the Goeckerman regimen consisting of steroids, UVB phototherapy, and crude coal tar; and Unna boots with zinc oxide and compression stockings. The above may be added to a list of possible treatments to consider for hastening the regression of eruptive KAs.
Our patient’s clinical course was similar to reports on the regressive nature of eruptive KAs within 6 months after initial eruption. Although it is likely that KAs will regress on their own, treatment modalities that speed up recovery are a future source for research.
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176.
- Min Lee CK, Li S, Tran DC, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol. 2018;79:1047-1052. doi:10.1016/j.jaad.2018.05.035
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Bednarek R, Marks K, Lin G. Eruptive keratoacanthomas secondary to nivolumab immunotherapy. Int J Dermatol. 2018;57:E28-E29.
- Feldstein SI, Patel F, Kim E, et al. Eruptive keratoacanthomas arising in the setting of lichenoid toxicity after programmed cell death 1 inhibition with nivolumab. J Eur Acad Dermatol Venereol. 2018;32:E58-E59.
- Crow LD, Perkins I, Twigg AR, et al. Treatment of PD-1/PD-L1 inhibitor-induced dermatitis resolves concomitant eruptive keratoacanthomas. JAMA Dermatol. 2020;156:598-600. doi:10.1001/jamadermatol.2020.0176
To the Editor:
Programmed cell death protein 1 (PD-1) inhibitors have been widely used in the treatment of various cancers. Programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 located on cancer cells will bind to PD-1 receptors on T cells and suppress them, which will prevent cancer cell destruction. Programmed cell death protein 1 inhibitors block the binding of PD-L1 to cancer cells, which then prevents T-cell immunosuppression.1 However, cutaneous adverse effects have been associated with PD-1 inhibitors. Dermatitis associated with PD-1 inhibitor therapy occurs more frequently in patients with cutaneous tumors such as melanoma compared to those with head and neck cancers.2 Curry et al1 reported that treatment with an immune checkpoint blockade can lead to immune-related adverse effects, most commonly affecting the gastrointestinal tract, liver, and skin. The same report cited dermatologic toxicity as an adverse effect in approximately 39% of patients treated with anti–PD-1 and approximately 17% of anti–PD-L1.1 The 4 main categories of dermatologic toxicities to immunotherapies in general include inflammatory disorders, immunobullous disorders, alterations of keratinocytes, and alteration of melanocytes. The most common adverse effects from the use of the PD-1 inhibitor nivolumab were skin rashes, not otherwise specified (14%–20%), pruritus (13%–18%), and vitiligo (~8%).1 Of the cutaneous dermatitic reactions to PD-1 and PD-L1 inhibitors that were biopsied, the 2 most common were lichenoid dermatitis and spongiotic dermatitis.2 Seldomly, there have been reports of keratoacanthomas (KAs) in association with anti–PD-1 therapy.3
A KA is a common skin tumor that appears most frequently as a solitary lesion and is thought to arise from the hair follicle.4 It resembles squamous cell carcinoma and commonly regresses within months without intervention. Exposure to UV light is a known risk factor for the development of KAs.
Eruptive KAs have been found in association with 10 cases of various cancers treated with the PD-1 inhibitors pembrolizumab and nivolumab.3 Multiple lesions on photodistributed areas of the body were reported in all 10 cases. Various treatments were used in these 10 cases—doxycycline and niacinamide, electrodesiccation and curettage, clobetasol ointment and/or intralesional triamcinolone, cryotherapy, imiquimod, or no treatment—as well as the cessation of PD-1 inhibitor therapy, with 4 cases continuing therapy and 6 cases discontinuing therapy. Nine cases regressed by 6 months; electrodesiccation and curettage of the lesions was used in the tenth case.3 We report a case of eruptive KA after 1 cycle of nivolumab therapy for metastatic melanoma.
A 79-year-old woman with stage III melanoma presented to her dermatologist after developing generalized pruritic lichenoid eruptions involving the torso, arms, and legs, as well as erosions on the lips, buccal mucosa, and palate 1 month after starting nivolumab therapy. The patient initially presented to dermatology with an irregularly shaped lesion on the left upper back 3 months prior. Biopsy results at that time revealed a diagnosis of malignant melanoma, lentigo maligna type. The lesion was 1.5-mm thick and classified as Clark level IV with a mitotic count of 6 per mm2. Molecular genetic studies showed expression of PD-L1 and no expression of c-KIT. The patient underwent wide local excision, and a sentinel lymph node biopsy was positive. Positron emission tomography did not show any hypermetabolic lesions, and magnetic resonance imaging did not indicate brain metastasis. The patient underwent an axillary dissection, which did not show any residual melanoma. She was started on adjuvant immunotherapy with intravenous nivolumab 480 mg monthly and developed pruritic crusted lesions on the arms, legs, and torso 1 month later, which prompted follow-up to dermatology.
At the current presentation 4 months after the onset of lesions, physical examination revealed lichenoid patches with serous crusting that were concentrated on the torso but also affected the arms and legs. She developed erosions on the upper and lower lips, buccal mucosa, and hard and soft palates, as well as painful, erythematous, dome-shaped papules and nodules on the legs (Figure 1). Her oncologist previously had initiated treatment at the onset of the lesions with clobetasol cream and valacyclovir for the lesions, but the patient showed no improvement.

Four months after the onset of the lesions, the patient was re-referred to her dermatologist, and a biopsy was performed on the left lower leg that showed squamous cell carcinoma, KA type. Additionally, flat erythematous patches were seen on the legs that were consistent with a lichenoid drug eruption. Two weeks later, she was started on halobetasol propionate ointment 0.05% for treatment of the KAs. At 2-week follow-up, 5 months after the onset of the lesions, the patient showed no signs of improvement. An oral prednisone taper of 60 mg for 3 days, 40 mg for 3 days, and then 20 mg daily for a total of 4 weeks was started to treat the lichenoid dermatitis and eruptive KAs. At the next follow-up 6.5 months following the first eruptive KAs, she was no longer using topical or oral steroids, she did not have any new eruptive KAs, and old lesions showed regression (Figure 2). The patient still experienced postinflammatory erythema and hyperpigmentation at the location of the KAs but showed improvement of the lichenoid drug eruption.

We describe a case of eruptive KAs after use of a PD-1 inhibitor for treatment of melanoma. Our patient developed eruptive KAs after only 1 nivolumab treatment. Another report described onset of eruptive KAs after 1 month of nivolumab infusions.3 The KAs experienced by our patient took 6.5 months to regress, which is unusual compared to other case reports in which the KAs self-resolved within a few months, though one other case described lesions that persisted for 6 months.3
Our patient was treated with topical steroids and an oral steroid taper for the concomitant lichenoid drug eruption. It is unknown if the steroids affected the course of the KAs or if they spontaneously regressed on their own. Freites-Martinez et al5 described that regression of KAs may be related to an immune response, but corticosteroids are inherently immunosuppressive. They hypothesized that corticosteroids help to temper the heightened immune response of eruptive KAs.5
Our patient had oral ulcers, which may have been indicative of an oral lichenoid drug eruption, as well as skin lesions representative of a cutaneous lichenoid drug eruption. This is a favorable reaction, as lichenoid dermatitis is thought to represent successful PD-1 inhibition and therefore a better response to oncologic therapies.2 Comorbid lichenoid drug eruption lesions and eruptive KAs may be suggestive of increased T-cell activity,2,6,7 though some prior case studies have reported eruptive KAs in isolation.3
Discontinuation of immunotherapy due to development of eruptive KAs presents a challenge in the treatment of underlying malignancies such as melanoma. Immunotherapy was discontinued in 7 of 11 cases due to these cutaneous reactions.3 Similarly, our patient underwent only 1 cycle of immunotherapy before developing eruptive KAs and discontinuing PD-1 inhibitor therapy. If we are better able to treat eruptive KAs, then patients can remain on immunotherapy to treat underlying malignancies. Crow et al8 showed improvement in lesions when 3 patients with eruptive KAs were treated with hydroxychloroquine; the Goeckerman regimen consisting of steroids, UVB phototherapy, and crude coal tar; and Unna boots with zinc oxide and compression stockings. The above may be added to a list of possible treatments to consider for hastening the regression of eruptive KAs.
Our patient’s clinical course was similar to reports on the regressive nature of eruptive KAs within 6 months after initial eruption. Although it is likely that KAs will regress on their own, treatment modalities that speed up recovery are a future source for research.
To the Editor:
Programmed cell death protein 1 (PD-1) inhibitors have been widely used in the treatment of various cancers. Programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 located on cancer cells will bind to PD-1 receptors on T cells and suppress them, which will prevent cancer cell destruction. Programmed cell death protein 1 inhibitors block the binding of PD-L1 to cancer cells, which then prevents T-cell immunosuppression.1 However, cutaneous adverse effects have been associated with PD-1 inhibitors. Dermatitis associated with PD-1 inhibitor therapy occurs more frequently in patients with cutaneous tumors such as melanoma compared to those with head and neck cancers.2 Curry et al1 reported that treatment with an immune checkpoint blockade can lead to immune-related adverse effects, most commonly affecting the gastrointestinal tract, liver, and skin. The same report cited dermatologic toxicity as an adverse effect in approximately 39% of patients treated with anti–PD-1 and approximately 17% of anti–PD-L1.1 The 4 main categories of dermatologic toxicities to immunotherapies in general include inflammatory disorders, immunobullous disorders, alterations of keratinocytes, and alteration of melanocytes. The most common adverse effects from the use of the PD-1 inhibitor nivolumab were skin rashes, not otherwise specified (14%–20%), pruritus (13%–18%), and vitiligo (~8%).1 Of the cutaneous dermatitic reactions to PD-1 and PD-L1 inhibitors that were biopsied, the 2 most common were lichenoid dermatitis and spongiotic dermatitis.2 Seldomly, there have been reports of keratoacanthomas (KAs) in association with anti–PD-1 therapy.3
A KA is a common skin tumor that appears most frequently as a solitary lesion and is thought to arise from the hair follicle.4 It resembles squamous cell carcinoma and commonly regresses within months without intervention. Exposure to UV light is a known risk factor for the development of KAs.
Eruptive KAs have been found in association with 10 cases of various cancers treated with the PD-1 inhibitors pembrolizumab and nivolumab.3 Multiple lesions on photodistributed areas of the body were reported in all 10 cases. Various treatments were used in these 10 cases—doxycycline and niacinamide, electrodesiccation and curettage, clobetasol ointment and/or intralesional triamcinolone, cryotherapy, imiquimod, or no treatment—as well as the cessation of PD-1 inhibitor therapy, with 4 cases continuing therapy and 6 cases discontinuing therapy. Nine cases regressed by 6 months; electrodesiccation and curettage of the lesions was used in the tenth case.3 We report a case of eruptive KA after 1 cycle of nivolumab therapy for metastatic melanoma.
A 79-year-old woman with stage III melanoma presented to her dermatologist after developing generalized pruritic lichenoid eruptions involving the torso, arms, and legs, as well as erosions on the lips, buccal mucosa, and palate 1 month after starting nivolumab therapy. The patient initially presented to dermatology with an irregularly shaped lesion on the left upper back 3 months prior. Biopsy results at that time revealed a diagnosis of malignant melanoma, lentigo maligna type. The lesion was 1.5-mm thick and classified as Clark level IV with a mitotic count of 6 per mm2. Molecular genetic studies showed expression of PD-L1 and no expression of c-KIT. The patient underwent wide local excision, and a sentinel lymph node biopsy was positive. Positron emission tomography did not show any hypermetabolic lesions, and magnetic resonance imaging did not indicate brain metastasis. The patient underwent an axillary dissection, which did not show any residual melanoma. She was started on adjuvant immunotherapy with intravenous nivolumab 480 mg monthly and developed pruritic crusted lesions on the arms, legs, and torso 1 month later, which prompted follow-up to dermatology.
At the current presentation 4 months after the onset of lesions, physical examination revealed lichenoid patches with serous crusting that were concentrated on the torso but also affected the arms and legs. She developed erosions on the upper and lower lips, buccal mucosa, and hard and soft palates, as well as painful, erythematous, dome-shaped papules and nodules on the legs (Figure 1). Her oncologist previously had initiated treatment at the onset of the lesions with clobetasol cream and valacyclovir for the lesions, but the patient showed no improvement.

Four months after the onset of the lesions, the patient was re-referred to her dermatologist, and a biopsy was performed on the left lower leg that showed squamous cell carcinoma, KA type. Additionally, flat erythematous patches were seen on the legs that were consistent with a lichenoid drug eruption. Two weeks later, she was started on halobetasol propionate ointment 0.05% for treatment of the KAs. At 2-week follow-up, 5 months after the onset of the lesions, the patient showed no signs of improvement. An oral prednisone taper of 60 mg for 3 days, 40 mg for 3 days, and then 20 mg daily for a total of 4 weeks was started to treat the lichenoid dermatitis and eruptive KAs. At the next follow-up 6.5 months following the first eruptive KAs, she was no longer using topical or oral steroids, she did not have any new eruptive KAs, and old lesions showed regression (Figure 2). The patient still experienced postinflammatory erythema and hyperpigmentation at the location of the KAs but showed improvement of the lichenoid drug eruption.

We describe a case of eruptive KAs after use of a PD-1 inhibitor for treatment of melanoma. Our patient developed eruptive KAs after only 1 nivolumab treatment. Another report described onset of eruptive KAs after 1 month of nivolumab infusions.3 The KAs experienced by our patient took 6.5 months to regress, which is unusual compared to other case reports in which the KAs self-resolved within a few months, though one other case described lesions that persisted for 6 months.3
Our patient was treated with topical steroids and an oral steroid taper for the concomitant lichenoid drug eruption. It is unknown if the steroids affected the course of the KAs or if they spontaneously regressed on their own. Freites-Martinez et al5 described that regression of KAs may be related to an immune response, but corticosteroids are inherently immunosuppressive. They hypothesized that corticosteroids help to temper the heightened immune response of eruptive KAs.5
Our patient had oral ulcers, which may have been indicative of an oral lichenoid drug eruption, as well as skin lesions representative of a cutaneous lichenoid drug eruption. This is a favorable reaction, as lichenoid dermatitis is thought to represent successful PD-1 inhibition and therefore a better response to oncologic therapies.2 Comorbid lichenoid drug eruption lesions and eruptive KAs may be suggestive of increased T-cell activity,2,6,7 though some prior case studies have reported eruptive KAs in isolation.3
Discontinuation of immunotherapy due to development of eruptive KAs presents a challenge in the treatment of underlying malignancies such as melanoma. Immunotherapy was discontinued in 7 of 11 cases due to these cutaneous reactions.3 Similarly, our patient underwent only 1 cycle of immunotherapy before developing eruptive KAs and discontinuing PD-1 inhibitor therapy. If we are better able to treat eruptive KAs, then patients can remain on immunotherapy to treat underlying malignancies. Crow et al8 showed improvement in lesions when 3 patients with eruptive KAs were treated with hydroxychloroquine; the Goeckerman regimen consisting of steroids, UVB phototherapy, and crude coal tar; and Unna boots with zinc oxide and compression stockings. The above may be added to a list of possible treatments to consider for hastening the regression of eruptive KAs.
Our patient’s clinical course was similar to reports on the regressive nature of eruptive KAs within 6 months after initial eruption. Although it is likely that KAs will regress on their own, treatment modalities that speed up recovery are a future source for research.
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176.
- Min Lee CK, Li S, Tran DC, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol. 2018;79:1047-1052. doi:10.1016/j.jaad.2018.05.035
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Bednarek R, Marks K, Lin G. Eruptive keratoacanthomas secondary to nivolumab immunotherapy. Int J Dermatol. 2018;57:E28-E29.
- Feldstein SI, Patel F, Kim E, et al. Eruptive keratoacanthomas arising in the setting of lichenoid toxicity after programmed cell death 1 inhibition with nivolumab. J Eur Acad Dermatol Venereol. 2018;32:E58-E59.
- Crow LD, Perkins I, Twigg AR, et al. Treatment of PD-1/PD-L1 inhibitor-induced dermatitis resolves concomitant eruptive keratoacanthomas. JAMA Dermatol. 2020;156:598-600. doi:10.1001/jamadermatol.2020.0176
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176.
- Min Lee CK, Li S, Tran DC, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol. 2018;79:1047-1052. doi:10.1016/j.jaad.2018.05.035
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Bednarek R, Marks K, Lin G. Eruptive keratoacanthomas secondary to nivolumab immunotherapy. Int J Dermatol. 2018;57:E28-E29.
- Feldstein SI, Patel F, Kim E, et al. Eruptive keratoacanthomas arising in the setting of lichenoid toxicity after programmed cell death 1 inhibition with nivolumab. J Eur Acad Dermatol Venereol. 2018;32:E58-E59.
- Crow LD, Perkins I, Twigg AR, et al. Treatment of PD-1/PD-L1 inhibitor-induced dermatitis resolves concomitant eruptive keratoacanthomas. JAMA Dermatol. 2020;156:598-600. doi:10.1001/jamadermatol.2020.0176
Practice Points
- Eruptive keratoacanthomas (KAs) are a rare buttransient adverse effect of programmed cell death protein 1 (PD-1) inhibitor therapy.
- Nivolumab, a human monoclonal IgG4 antibody, is used as an antitumor treatment for melanoma by blocking PD-1.
- Possible new treatments may hasten the regression of eruptive KAs, which could allow patients to continue PD-1 inhibitor therapy.
Inpatient Dermatology Consultation Services in Hospital Institutions
Inpatient dermatology consultation services are becoming increasingly prevalent in hospital institutions.1-3 Although often underutilized as a consulting service, dermatology-related admissions cost hundreds of millions of dollars for the health care system.1,2 Misdiagnosis, prolonged hospital stays, and incorrect treatment are common results of lack of involvement by a skin expert.1-3 The importance of consultative inpatient dermatology cannot be understated. Accreditation Council for Graduate Medical Education requirements for proficiency in dermatology residency include exposure to inpatient dermatology, and it is our duty as residents to aid our colleagues in the management and treatment of cutaneous disease.
Although exposure to inpatient dermatology varies across residency programs, nearly every dermatology resident is bound to perform a consultation and be involved in the care of a hospitalized patient. At our program at the University of Utah (Salt Lake City), we have robust inpatient exposure, and after numerous hours spent on the forefront of inpatient dermatology, I have accrued a list of specific tips and techniques that have aided me as a resident clinician.
Pre-Rounding More Thoroughly
When I started as a postgraduate year 2 (PGY-2) on the inpatient dermatology rotation, I found myself perplexed. I had learned how to round in internal medicine but was unaccustomed to the nuances of specialty rounds. My list included calciphylaxis, small vessel vasculitis, cellulitis, stasis dermatitis, toxic epidermal necrolysis, and atypical mycobacterial infection. The first few days of service were undeniably difficult due to the daily consultations, complexity of admitted patients, and need for efficiency. I sometimes overlooked important laboratory test results, medication changes, and interdisciplinary discussions that prolonged rounding. As dermatologists, we are responsible for the largest organ of the body, and it is important to approach patients in a comprehensive manner. Pre-rounding should include reviewing interdisciplinary notes, laboratory values/results, and medications, and performing a focused skin examination with a review of systems during the encounter. Importantly, most electronic medical record systems offer an automated rounding sheet. In Epic (Epic Systems Corporation), I would use the bone marrow transplant rounding sheet, which includes laboratory test results, vitals, and medications. After printing out the rounding sheet, I would note important updates for each patient. Although pre-rounding and chart review requires time and effort, it aided me in providing elevated patient care and becoming more efficient during rounds. Over time I have come to strongly appreciate the term dermatology hospitalist. Cutaneous manifestations of systemic disease require thoughtful consideration and workup.
New Patient Consultations: Must-Ask Questions
Holding the university inpatient pager can be stressful. At the University of Utah, we often carry 5 to 10 patients on our list and receive 3 to 4 new consultations a day, sometimes right before 5
- What is the patient’s name, room number, and medical record number?
- Is this patient getting admitted or admitted currently?
- Is the rash the reason for admission? (This can greatly help with triaging the urgency of evaluation.)
- Is the rash painful?
- Is this patient ill?
- How would you describe the rash?
When evaluating new patients, it is crucial to remember the morphology camps. Formulating a differential diagnosis on a complex patient can be difficult; however, remembering the morphology camps of acneiform, dermal, eczematous, erythematous, subcutaneous, vasculitic, vasculopathic, and vesiculobullous lesions can be extremely helpful. Additionally, it is crucial to perform a thorough and complete skin examination on every patient. When emphasizing the importance of this, I often am reminded of a humbling moment early in my training. Our team was consulted on a patient with cellulitis and stasis dermatitis. It was a busy day, and my examination was quick and focused on the lower and upper extremities, chest, and back. The patient improved from a cutaneous standpoint and was discharged. At follow-up the next week, one of my attending providers biopsied an atypical macule on the retroauricular region, which was found to be consistent with a stage 1A melanoma. Even on the longest and most tiring hospital days, it is important to perform a full-body skin examination on each patient. You may end up saving a life.
An Organized Toolbox: What to Carry
Similar to our ophthalmology colleagues who are seen carrying around a suitcase in the hospital, I highly recommend some form of a toolbox or bag for performing inpatient biopsies (Table). Carrying around an organized bag, albeit bulky and unfashionable, has saved me numerous trips back to clinic for unexpected complications including fixing leaky vessels, closing stubborn ulcers, and coordinating sedated biopsies in the operating room.

Final Thoughts
As I near the completion of my residency journey, I hope these tips will aid budding and current dermatology residents at excelling as dermatology hospitalists during inpatient rotations. Dermatologists can make a profound impact on a variety of patients, especially when treating hospitalized patients on the clinical forefront. Our role extends beyond the skin, as cutaneous manifestations of internal disease are not uncommon.
- Afifi L, Shinkai K. Optimizing education on the inpatient dermatology consultative service. Semin Cutan Med Surg. 2017;36:28-34. doi:10.12788/j.sder.2017.003
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology [published online September 1, 2015]. Med Clin North Am. 2015;99:1349-1364. doi:10.1016/j.mcna.2015.06.004
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology? assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
Inpatient dermatology consultation services are becoming increasingly prevalent in hospital institutions.1-3 Although often underutilized as a consulting service, dermatology-related admissions cost hundreds of millions of dollars for the health care system.1,2 Misdiagnosis, prolonged hospital stays, and incorrect treatment are common results of lack of involvement by a skin expert.1-3 The importance of consultative inpatient dermatology cannot be understated. Accreditation Council for Graduate Medical Education requirements for proficiency in dermatology residency include exposure to inpatient dermatology, and it is our duty as residents to aid our colleagues in the management and treatment of cutaneous disease.
Although exposure to inpatient dermatology varies across residency programs, nearly every dermatology resident is bound to perform a consultation and be involved in the care of a hospitalized patient. At our program at the University of Utah (Salt Lake City), we have robust inpatient exposure, and after numerous hours spent on the forefront of inpatient dermatology, I have accrued a list of specific tips and techniques that have aided me as a resident clinician.
Pre-Rounding More Thoroughly
When I started as a postgraduate year 2 (PGY-2) on the inpatient dermatology rotation, I found myself perplexed. I had learned how to round in internal medicine but was unaccustomed to the nuances of specialty rounds. My list included calciphylaxis, small vessel vasculitis, cellulitis, stasis dermatitis, toxic epidermal necrolysis, and atypical mycobacterial infection. The first few days of service were undeniably difficult due to the daily consultations, complexity of admitted patients, and need for efficiency. I sometimes overlooked important laboratory test results, medication changes, and interdisciplinary discussions that prolonged rounding. As dermatologists, we are responsible for the largest organ of the body, and it is important to approach patients in a comprehensive manner. Pre-rounding should include reviewing interdisciplinary notes, laboratory values/results, and medications, and performing a focused skin examination with a review of systems during the encounter. Importantly, most electronic medical record systems offer an automated rounding sheet. In Epic (Epic Systems Corporation), I would use the bone marrow transplant rounding sheet, which includes laboratory test results, vitals, and medications. After printing out the rounding sheet, I would note important updates for each patient. Although pre-rounding and chart review requires time and effort, it aided me in providing elevated patient care and becoming more efficient during rounds. Over time I have come to strongly appreciate the term dermatology hospitalist. Cutaneous manifestations of systemic disease require thoughtful consideration and workup.
New Patient Consultations: Must-Ask Questions
Holding the university inpatient pager can be stressful. At the University of Utah, we often carry 5 to 10 patients on our list and receive 3 to 4 new consultations a day, sometimes right before 5
- What is the patient’s name, room number, and medical record number?
- Is this patient getting admitted or admitted currently?
- Is the rash the reason for admission? (This can greatly help with triaging the urgency of evaluation.)
- Is the rash painful?
- Is this patient ill?
- How would you describe the rash?
When evaluating new patients, it is crucial to remember the morphology camps. Formulating a differential diagnosis on a complex patient can be difficult; however, remembering the morphology camps of acneiform, dermal, eczematous, erythematous, subcutaneous, vasculitic, vasculopathic, and vesiculobullous lesions can be extremely helpful. Additionally, it is crucial to perform a thorough and complete skin examination on every patient. When emphasizing the importance of this, I often am reminded of a humbling moment early in my training. Our team was consulted on a patient with cellulitis and stasis dermatitis. It was a busy day, and my examination was quick and focused on the lower and upper extremities, chest, and back. The patient improved from a cutaneous standpoint and was discharged. At follow-up the next week, one of my attending providers biopsied an atypical macule on the retroauricular region, which was found to be consistent with a stage 1A melanoma. Even on the longest and most tiring hospital days, it is important to perform a full-body skin examination on each patient. You may end up saving a life.
An Organized Toolbox: What to Carry
Similar to our ophthalmology colleagues who are seen carrying around a suitcase in the hospital, I highly recommend some form of a toolbox or bag for performing inpatient biopsies (Table). Carrying around an organized bag, albeit bulky and unfashionable, has saved me numerous trips back to clinic for unexpected complications including fixing leaky vessels, closing stubborn ulcers, and coordinating sedated biopsies in the operating room.

Final Thoughts
As I near the completion of my residency journey, I hope these tips will aid budding and current dermatology residents at excelling as dermatology hospitalists during inpatient rotations. Dermatologists can make a profound impact on a variety of patients, especially when treating hospitalized patients on the clinical forefront. Our role extends beyond the skin, as cutaneous manifestations of internal disease are not uncommon.
Inpatient dermatology consultation services are becoming increasingly prevalent in hospital institutions.1-3 Although often underutilized as a consulting service, dermatology-related admissions cost hundreds of millions of dollars for the health care system.1,2 Misdiagnosis, prolonged hospital stays, and incorrect treatment are common results of lack of involvement by a skin expert.1-3 The importance of consultative inpatient dermatology cannot be understated. Accreditation Council for Graduate Medical Education requirements for proficiency in dermatology residency include exposure to inpatient dermatology, and it is our duty as residents to aid our colleagues in the management and treatment of cutaneous disease.
Although exposure to inpatient dermatology varies across residency programs, nearly every dermatology resident is bound to perform a consultation and be involved in the care of a hospitalized patient. At our program at the University of Utah (Salt Lake City), we have robust inpatient exposure, and after numerous hours spent on the forefront of inpatient dermatology, I have accrued a list of specific tips and techniques that have aided me as a resident clinician.
Pre-Rounding More Thoroughly
When I started as a postgraduate year 2 (PGY-2) on the inpatient dermatology rotation, I found myself perplexed. I had learned how to round in internal medicine but was unaccustomed to the nuances of specialty rounds. My list included calciphylaxis, small vessel vasculitis, cellulitis, stasis dermatitis, toxic epidermal necrolysis, and atypical mycobacterial infection. The first few days of service were undeniably difficult due to the daily consultations, complexity of admitted patients, and need for efficiency. I sometimes overlooked important laboratory test results, medication changes, and interdisciplinary discussions that prolonged rounding. As dermatologists, we are responsible for the largest organ of the body, and it is important to approach patients in a comprehensive manner. Pre-rounding should include reviewing interdisciplinary notes, laboratory values/results, and medications, and performing a focused skin examination with a review of systems during the encounter. Importantly, most electronic medical record systems offer an automated rounding sheet. In Epic (Epic Systems Corporation), I would use the bone marrow transplant rounding sheet, which includes laboratory test results, vitals, and medications. After printing out the rounding sheet, I would note important updates for each patient. Although pre-rounding and chart review requires time and effort, it aided me in providing elevated patient care and becoming more efficient during rounds. Over time I have come to strongly appreciate the term dermatology hospitalist. Cutaneous manifestations of systemic disease require thoughtful consideration and workup.
New Patient Consultations: Must-Ask Questions
Holding the university inpatient pager can be stressful. At the University of Utah, we often carry 5 to 10 patients on our list and receive 3 to 4 new consultations a day, sometimes right before 5
- What is the patient’s name, room number, and medical record number?
- Is this patient getting admitted or admitted currently?
- Is the rash the reason for admission? (This can greatly help with triaging the urgency of evaluation.)
- Is the rash painful?
- Is this patient ill?
- How would you describe the rash?
When evaluating new patients, it is crucial to remember the morphology camps. Formulating a differential diagnosis on a complex patient can be difficult; however, remembering the morphology camps of acneiform, dermal, eczematous, erythematous, subcutaneous, vasculitic, vasculopathic, and vesiculobullous lesions can be extremely helpful. Additionally, it is crucial to perform a thorough and complete skin examination on every patient. When emphasizing the importance of this, I often am reminded of a humbling moment early in my training. Our team was consulted on a patient with cellulitis and stasis dermatitis. It was a busy day, and my examination was quick and focused on the lower and upper extremities, chest, and back. The patient improved from a cutaneous standpoint and was discharged. At follow-up the next week, one of my attending providers biopsied an atypical macule on the retroauricular region, which was found to be consistent with a stage 1A melanoma. Even on the longest and most tiring hospital days, it is important to perform a full-body skin examination on each patient. You may end up saving a life.
An Organized Toolbox: What to Carry
Similar to our ophthalmology colleagues who are seen carrying around a suitcase in the hospital, I highly recommend some form of a toolbox or bag for performing inpatient biopsies (Table). Carrying around an organized bag, albeit bulky and unfashionable, has saved me numerous trips back to clinic for unexpected complications including fixing leaky vessels, closing stubborn ulcers, and coordinating sedated biopsies in the operating room.

Final Thoughts
As I near the completion of my residency journey, I hope these tips will aid budding and current dermatology residents at excelling as dermatology hospitalists during inpatient rotations. Dermatologists can make a profound impact on a variety of patients, especially when treating hospitalized patients on the clinical forefront. Our role extends beyond the skin, as cutaneous manifestations of internal disease are not uncommon.
- Afifi L, Shinkai K. Optimizing education on the inpatient dermatology consultative service. Semin Cutan Med Surg. 2017;36:28-34. doi:10.12788/j.sder.2017.003
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology [published online September 1, 2015]. Med Clin North Am. 2015;99:1349-1364. doi:10.1016/j.mcna.2015.06.004
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology? assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Afifi L, Shinkai K. Optimizing education on the inpatient dermatology consultative service. Semin Cutan Med Surg. 2017;36:28-34. doi:10.12788/j.sder.2017.003
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology [published online September 1, 2015]. Med Clin North Am. 2015;99:1349-1364. doi:10.1016/j.mcna.2015.06.004
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology? assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
Resident Pearl
- When performing inpatient dermatology consultations, residents should focus on pre-rounding and must-ask questions of requesting providers as well as carrying an organized toolbox.
Persistent Wounds Refractory to Broad-Spectrum Antibiotics
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.
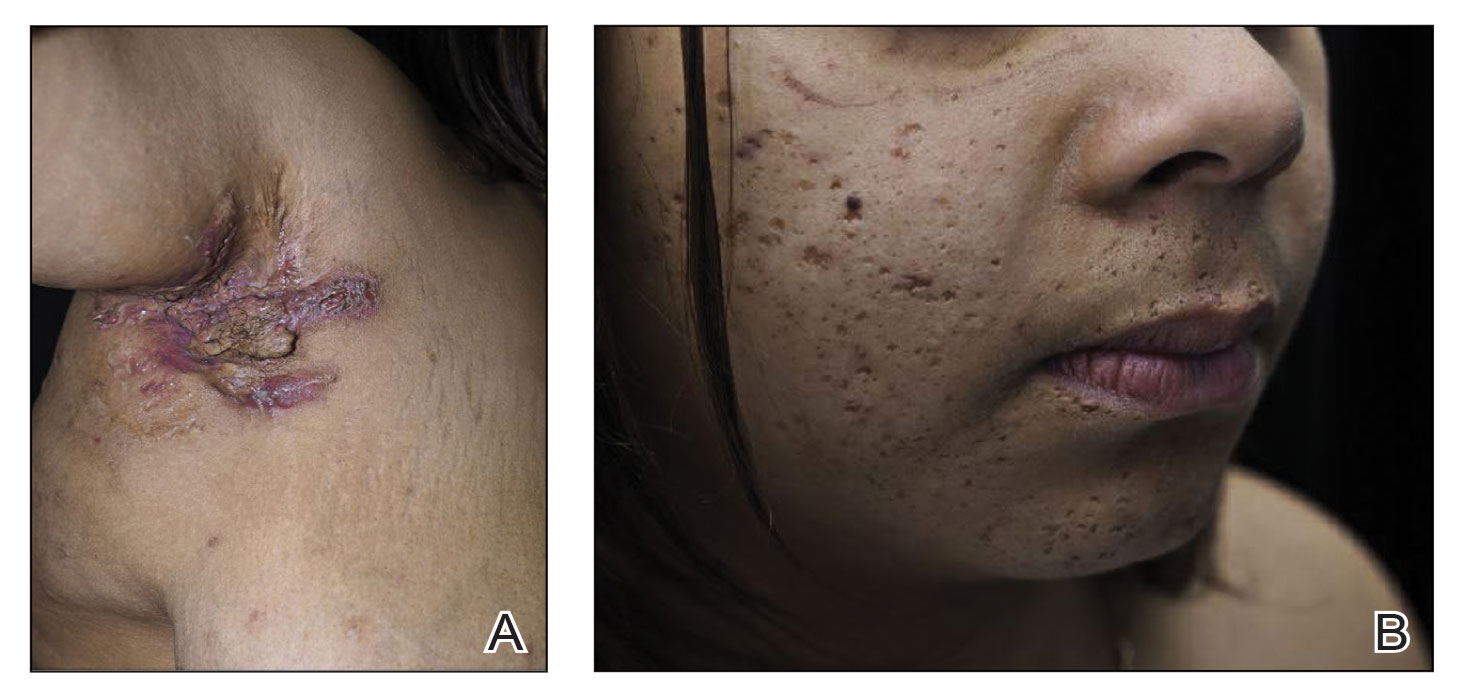
Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
A 28-year-old Black woman presented to the hospital for evaluation of worsening leg wounds as well as a similar eroding plaque on the left breast of 1 month’s duration. Broad-spectrum antibiotics prescribed during a prior emergency department visit resulted in no improvement. Her medical history was notable for hidradenitis suppurativa that previously was well controlled on adalimumab prior to discontinuation 1 year prior. A review of systems was negative for fever, chills, shortness of breath, chest pain, night sweats, and arthralgia. The patient had discontinued the antibiotics and was not taking any other medications at the time of presentation. She reported a history of smoking cigarettes (5 pack years). Physical examination revealed hyperkeratotic eroded plaques with violaceous borders circumferentially around the left breast (top) and legs with notable undermining (bottom). Inflammatory nodulocystic acne of the face as well as sinus tract formation with purulent drainage in the axillae also were present. Laboratory workup revealed an elevated erythrocyte sedimentation rate (116 mm/h [reference range, <20 mm/h]). Computed tomography of the leg wound was negative for soft-tissue infection. Aerobic and anaerobic tissue cultures demonstrated no growth.

New-Onset Pemphigoid Gestationis Following COVID-19 Vaccination
To the Editor:
Pemphigoid gestationis (PG), or gestational pemphigoid, is a rare autoimmune bullous disease (AIBD) occurring in 1 in 50,000 pregnancies. It is characterized by abrupt development of intensely pruritic papules and urticarial plaques, followed by an eruption of blisters.1 We present a case of new-onset PG that erupted 10 days following SARs-CoV-2 messenger RNA (mRNA) vaccination with BNT162b2 (Pfizer-BioNTech).
A 36-year-old pregnant woman (gravida 1, para 0, aborta 0) at 37 weeks’ gestation presented to our AIBD clinic with a pruritic dermatitis of 6 weeks’ duration that developed 10 days after receiving the second dose of BNT162b2. Multiple intensely pruritic, red bumps presented first on the forearms and within days spread to the thighs, hands, and abdomen, followed by progression to the ankles, feet, and back 2 weeks later. An initial biopsy was consistent with subacute spongiotic dermatitis with rare eosinophils. She found minimal relief from diphenhydramine or topical steroids. She denied oral, nasal, ocular, or genital involvement or history of any other skin disease. The pregnancy had been otherwise uneventful.
Physical examination revealed annular edematous plaques on the trunk and buttocks; excoriated and erythematous papules on the neck, trunk, arms, and legs; and scattered vesicles along the fingers, arms, hands, abdomen, back, legs, and feet (Figure 1). The Bullous Pemphigoid Disease Area Index (BPDAI) total skin activity score was 25.3, corresponding to moderate disease activity (validated at 20–56).2 The BPDAI total pruritus component score was 20. A repeat biopsy for direct immunofluorescence showed faint linear deposits of IgG and bright linear deposits of C3 along the basement membrane zone. Indirect immunofluorescence showed linear deposits of IgG localized to the blister roof of salt-split skin at a dilution of 1:40. An enzyme-linked immunosorbent assay for anti-BP180 was 62 U/mL (negative, <9 U/mL; positive, ≥9 U/mL), and anti-BP230 autoantibodies were less than 9 U/mL (negative <9 U/mL; positive, ≥9 U/mL). Given these clinical and histopathologic findings, PG was diagnosed.
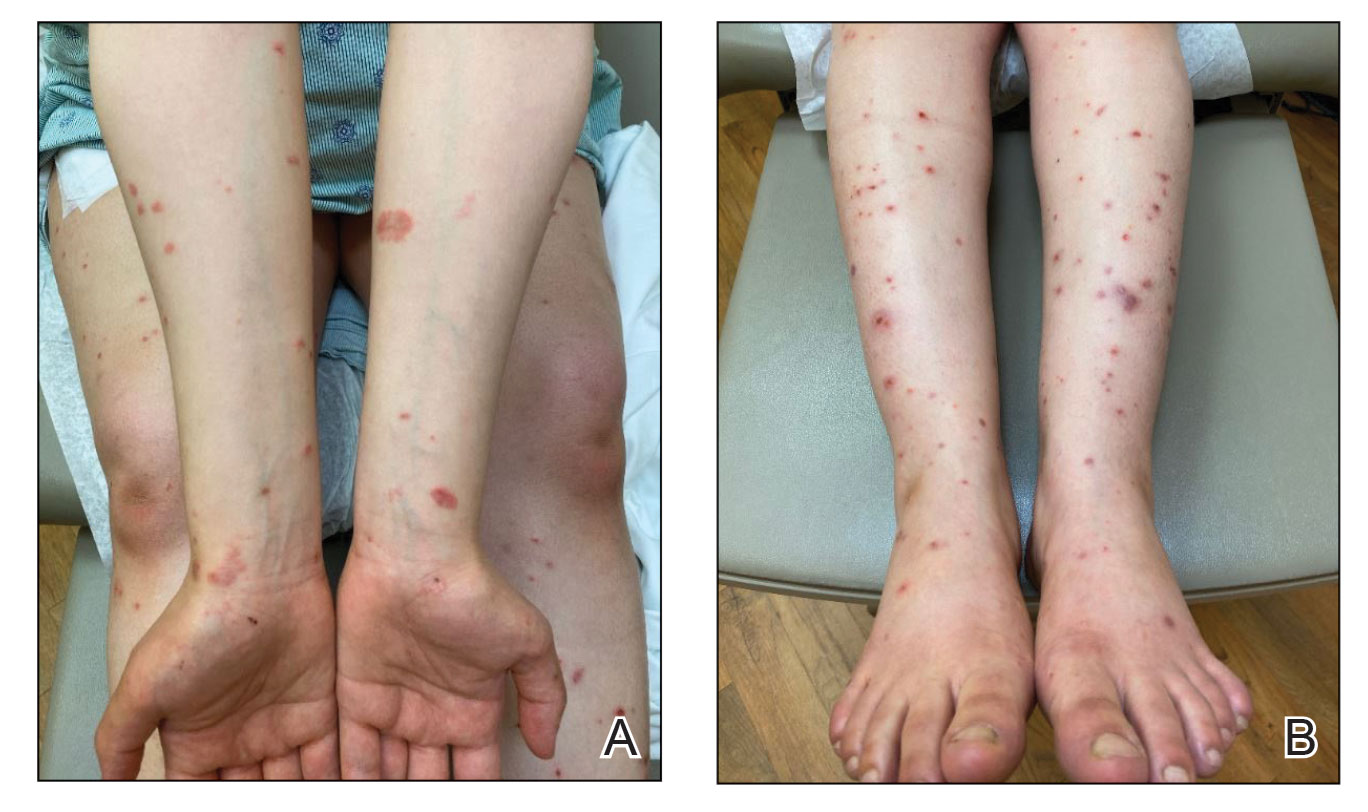
The patient was started on prednisone 20 mg and antihistamines while continuing topical steroids. Pruritus and blistering improved close to delivery. Fetal monitoring with regular biophysical profiles remained normal. The patient delivered a healthy neonate without skin lesions at 40 weeks’ gestation. The disease flared 2 days after delivery, and prednisone was increased to 40 mg and slowly tapered. Two months after delivery, the patient remained on prednisone 10 mg daily with ongoing but reduced blistering and pruritus (Figure 2). The BPDAI total skin activity and pruritus component scores remained elevated at 20.3 and 14, respectively, and anti-BP180 was 44 U/mL. After a discussion with the patient on safe systemic therapy while breastfeeding, intravenous immunoglobulin (IVIG) was initiated. The patient received 3 monthly infusions at 2 g/kg and was able to taper the prednisone to 5 mg every other day without new lesions. Four months after completion of IVIG therapy, she achieved complete remission off all therapy.
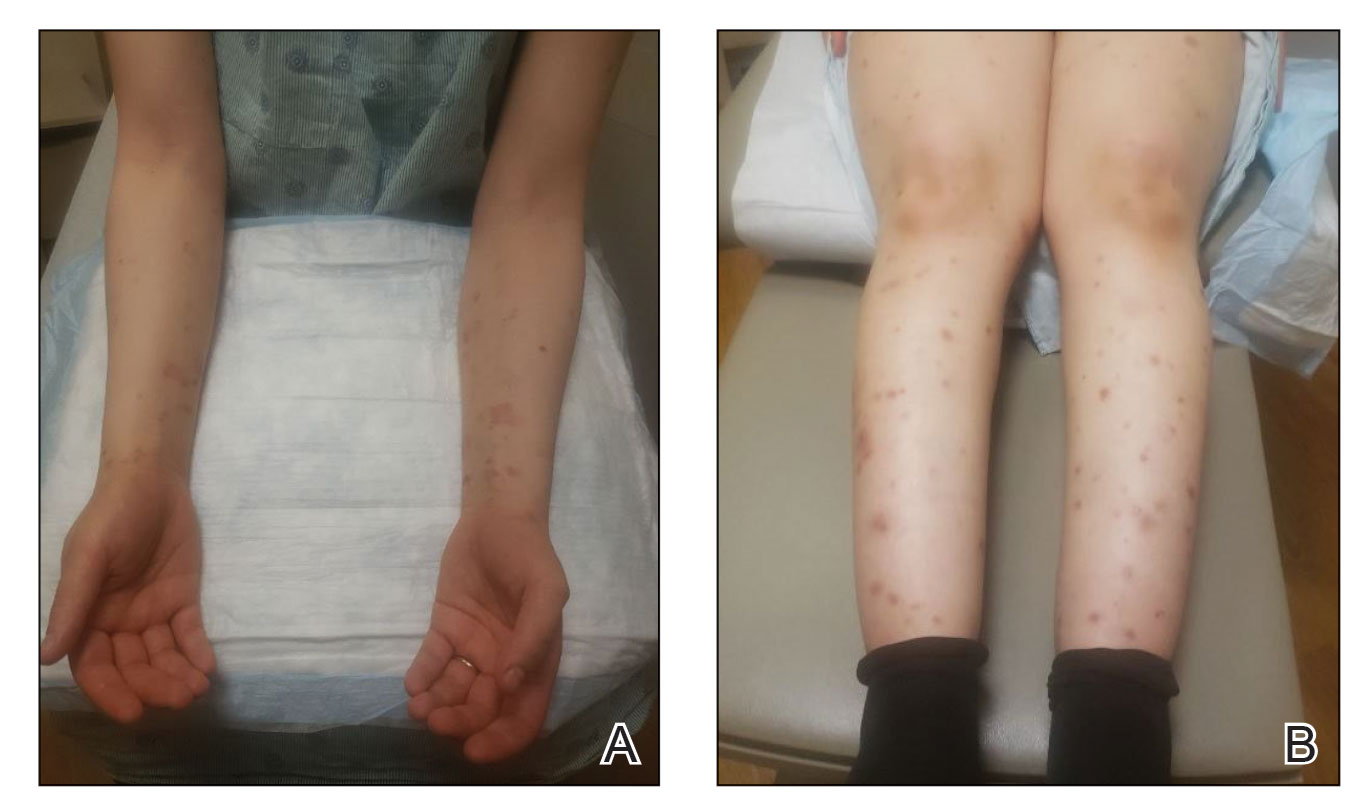
Management of PG begins with topical corticosteroids, but most patients require systemic steroid therapy.1 Remission commonly occurs close to delivery, and 75% of patients flare post partum, though the disease typically resolves 6 months following delivery.1,3,4 For persistent intrapartum cases requiring more than prednisone 20 mg daily, therapy can include dapsone, IVIG, azathioprine, rituximab, or plasmapheresis.4,5 Dapsone and IVIG are compatible with breastfeeding postpartum, but if dapsone is selected, the infant must be monitored for hemolytic anemia.5 Pemphigoid gestationis increases the risk for a premature or small-for-gestational-age neonate, necessitating regular fetal monitoring until delivery.1 Cutaneous lesions may affect the newborn, though this occurrence is rare and self-limiting.6 Pemphigoid gestationis may recur in subsequent pregnancies at a rate of 33% to 55%, with earlier and more severe presentations.4
Clinically and histologically, PG closely resembles bullous pemphigoid (BP), but the exact pathogenesis is not fully understood. Recently, another case of what was termed pseudo-PG has been described 3 days following administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.7 Since the introduction of COVID-19 mRNA vaccines, cases of postvaccination BP, BP-like eruptions, and pemphigus vulgaris have been described.8-11 Tomayko et al10 reported 12 cases of subepidermal eruptions, including BP, in which 7 patients developed blisters after the second dose of either the Pfizer-BioNTech or Moderna mRNA vaccine. Three patients who developed BP after the first dose of the vaccine and chose to receive the second dose tolerated it well, with a mild flare observed in 1 patient.10 Similarly, subsequent vaccine doses in reports of vaccine-associated AIBD resulted in increased disease activity in 21% of cases.12 COVID-19 vaccine–associated BP, similar to drug-induced BP, seemingly displays a milder course of disease compared to the classic form of BP.10,13 More follow-up is needed to better understand these reactions and inform appropriate discussions on the administration of booster doses. Currently, completion of the vaccination series against COVID-19 is advisable given the paucity of reports of postvaccination AIBD and the risk for COVID-19 infection, but careful discussions on a case-by-case basis are warranted related to the risk for disease exacerbation following subsequent vaccinations.
The clinical presentation and diagnostic evaluation of our patient’s rash were consistent with PG. The temporal relationship between vaccine administration and PG lesion onset suggests the mRNA vaccine triggered AIBD in our patient. Interestingly, AIBD associated with COVID-19 is not unique to only the vaccines and has been observed following infection with the virus itself.14 The high rate of vaccination against COVID-19 in contrast with the low number of reported cases of AIBD after vaccination supports the overall safety of COVID-19 vaccines but identifies a need for further understanding of the processes that lead to the development of autoimmune conditions in at-risk populations.
- Wiznia LE, Pomeranz MK. Skin changes and diseases in pregnancy. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Masmoudi W, Vaillant M, Vassileva S, et al. International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-1112. doi:10.1111/bjd.19611
- Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138-144.
- Savervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-449.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
- Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30:51-55.
- de Lorenzi C, Kaya G, Toutous Trellu L. Pseudo-pemphigoid gestationis eruption following SARS-CoV-2 vaccination with mRNA vaccine. Dermatopathology (Basel). 2022;9:203-206. doi:10.3390/dermatopathology9030025
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35:E649-E651.
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750-751.
- Coto-Segura P, Fernandez-Prada M, Mir-Bonafe M, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47:141-143.
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2022;86:1160-1164.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111.
To the Editor:
Pemphigoid gestationis (PG), or gestational pemphigoid, is a rare autoimmune bullous disease (AIBD) occurring in 1 in 50,000 pregnancies. It is characterized by abrupt development of intensely pruritic papules and urticarial plaques, followed by an eruption of blisters.1 We present a case of new-onset PG that erupted 10 days following SARs-CoV-2 messenger RNA (mRNA) vaccination with BNT162b2 (Pfizer-BioNTech).
A 36-year-old pregnant woman (gravida 1, para 0, aborta 0) at 37 weeks’ gestation presented to our AIBD clinic with a pruritic dermatitis of 6 weeks’ duration that developed 10 days after receiving the second dose of BNT162b2. Multiple intensely pruritic, red bumps presented first on the forearms and within days spread to the thighs, hands, and abdomen, followed by progression to the ankles, feet, and back 2 weeks later. An initial biopsy was consistent with subacute spongiotic dermatitis with rare eosinophils. She found minimal relief from diphenhydramine or topical steroids. She denied oral, nasal, ocular, or genital involvement or history of any other skin disease. The pregnancy had been otherwise uneventful.
Physical examination revealed annular edematous plaques on the trunk and buttocks; excoriated and erythematous papules on the neck, trunk, arms, and legs; and scattered vesicles along the fingers, arms, hands, abdomen, back, legs, and feet (Figure 1). The Bullous Pemphigoid Disease Area Index (BPDAI) total skin activity score was 25.3, corresponding to moderate disease activity (validated at 20–56).2 The BPDAI total pruritus component score was 20. A repeat biopsy for direct immunofluorescence showed faint linear deposits of IgG and bright linear deposits of C3 along the basement membrane zone. Indirect immunofluorescence showed linear deposits of IgG localized to the blister roof of salt-split skin at a dilution of 1:40. An enzyme-linked immunosorbent assay for anti-BP180 was 62 U/mL (negative, <9 U/mL; positive, ≥9 U/mL), and anti-BP230 autoantibodies were less than 9 U/mL (negative <9 U/mL; positive, ≥9 U/mL). Given these clinical and histopathologic findings, PG was diagnosed.

The patient was started on prednisone 20 mg and antihistamines while continuing topical steroids. Pruritus and blistering improved close to delivery. Fetal monitoring with regular biophysical profiles remained normal. The patient delivered a healthy neonate without skin lesions at 40 weeks’ gestation. The disease flared 2 days after delivery, and prednisone was increased to 40 mg and slowly tapered. Two months after delivery, the patient remained on prednisone 10 mg daily with ongoing but reduced blistering and pruritus (Figure 2). The BPDAI total skin activity and pruritus component scores remained elevated at 20.3 and 14, respectively, and anti-BP180 was 44 U/mL. After a discussion with the patient on safe systemic therapy while breastfeeding, intravenous immunoglobulin (IVIG) was initiated. The patient received 3 monthly infusions at 2 g/kg and was able to taper the prednisone to 5 mg every other day without new lesions. Four months after completion of IVIG therapy, she achieved complete remission off all therapy.

Management of PG begins with topical corticosteroids, but most patients require systemic steroid therapy.1 Remission commonly occurs close to delivery, and 75% of patients flare post partum, though the disease typically resolves 6 months following delivery.1,3,4 For persistent intrapartum cases requiring more than prednisone 20 mg daily, therapy can include dapsone, IVIG, azathioprine, rituximab, or plasmapheresis.4,5 Dapsone and IVIG are compatible with breastfeeding postpartum, but if dapsone is selected, the infant must be monitored for hemolytic anemia.5 Pemphigoid gestationis increases the risk for a premature or small-for-gestational-age neonate, necessitating regular fetal monitoring until delivery.1 Cutaneous lesions may affect the newborn, though this occurrence is rare and self-limiting.6 Pemphigoid gestationis may recur in subsequent pregnancies at a rate of 33% to 55%, with earlier and more severe presentations.4
Clinically and histologically, PG closely resembles bullous pemphigoid (BP), but the exact pathogenesis is not fully understood. Recently, another case of what was termed pseudo-PG has been described 3 days following administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.7 Since the introduction of COVID-19 mRNA vaccines, cases of postvaccination BP, BP-like eruptions, and pemphigus vulgaris have been described.8-11 Tomayko et al10 reported 12 cases of subepidermal eruptions, including BP, in which 7 patients developed blisters after the second dose of either the Pfizer-BioNTech or Moderna mRNA vaccine. Three patients who developed BP after the first dose of the vaccine and chose to receive the second dose tolerated it well, with a mild flare observed in 1 patient.10 Similarly, subsequent vaccine doses in reports of vaccine-associated AIBD resulted in increased disease activity in 21% of cases.12 COVID-19 vaccine–associated BP, similar to drug-induced BP, seemingly displays a milder course of disease compared to the classic form of BP.10,13 More follow-up is needed to better understand these reactions and inform appropriate discussions on the administration of booster doses. Currently, completion of the vaccination series against COVID-19 is advisable given the paucity of reports of postvaccination AIBD and the risk for COVID-19 infection, but careful discussions on a case-by-case basis are warranted related to the risk for disease exacerbation following subsequent vaccinations.
The clinical presentation and diagnostic evaluation of our patient’s rash were consistent with PG. The temporal relationship between vaccine administration and PG lesion onset suggests the mRNA vaccine triggered AIBD in our patient. Interestingly, AIBD associated with COVID-19 is not unique to only the vaccines and has been observed following infection with the virus itself.14 The high rate of vaccination against COVID-19 in contrast with the low number of reported cases of AIBD after vaccination supports the overall safety of COVID-19 vaccines but identifies a need for further understanding of the processes that lead to the development of autoimmune conditions in at-risk populations.
To the Editor:
Pemphigoid gestationis (PG), or gestational pemphigoid, is a rare autoimmune bullous disease (AIBD) occurring in 1 in 50,000 pregnancies. It is characterized by abrupt development of intensely pruritic papules and urticarial plaques, followed by an eruption of blisters.1 We present a case of new-onset PG that erupted 10 days following SARs-CoV-2 messenger RNA (mRNA) vaccination with BNT162b2 (Pfizer-BioNTech).
A 36-year-old pregnant woman (gravida 1, para 0, aborta 0) at 37 weeks’ gestation presented to our AIBD clinic with a pruritic dermatitis of 6 weeks’ duration that developed 10 days after receiving the second dose of BNT162b2. Multiple intensely pruritic, red bumps presented first on the forearms and within days spread to the thighs, hands, and abdomen, followed by progression to the ankles, feet, and back 2 weeks later. An initial biopsy was consistent with subacute spongiotic dermatitis with rare eosinophils. She found minimal relief from diphenhydramine or topical steroids. She denied oral, nasal, ocular, or genital involvement or history of any other skin disease. The pregnancy had been otherwise uneventful.
Physical examination revealed annular edematous plaques on the trunk and buttocks; excoriated and erythematous papules on the neck, trunk, arms, and legs; and scattered vesicles along the fingers, arms, hands, abdomen, back, legs, and feet (Figure 1). The Bullous Pemphigoid Disease Area Index (BPDAI) total skin activity score was 25.3, corresponding to moderate disease activity (validated at 20–56).2 The BPDAI total pruritus component score was 20. A repeat biopsy for direct immunofluorescence showed faint linear deposits of IgG and bright linear deposits of C3 along the basement membrane zone. Indirect immunofluorescence showed linear deposits of IgG localized to the blister roof of salt-split skin at a dilution of 1:40. An enzyme-linked immunosorbent assay for anti-BP180 was 62 U/mL (negative, <9 U/mL; positive, ≥9 U/mL), and anti-BP230 autoantibodies were less than 9 U/mL (negative <9 U/mL; positive, ≥9 U/mL). Given these clinical and histopathologic findings, PG was diagnosed.

The patient was started on prednisone 20 mg and antihistamines while continuing topical steroids. Pruritus and blistering improved close to delivery. Fetal monitoring with regular biophysical profiles remained normal. The patient delivered a healthy neonate without skin lesions at 40 weeks’ gestation. The disease flared 2 days after delivery, and prednisone was increased to 40 mg and slowly tapered. Two months after delivery, the patient remained on prednisone 10 mg daily with ongoing but reduced blistering and pruritus (Figure 2). The BPDAI total skin activity and pruritus component scores remained elevated at 20.3 and 14, respectively, and anti-BP180 was 44 U/mL. After a discussion with the patient on safe systemic therapy while breastfeeding, intravenous immunoglobulin (IVIG) was initiated. The patient received 3 monthly infusions at 2 g/kg and was able to taper the prednisone to 5 mg every other day without new lesions. Four months after completion of IVIG therapy, she achieved complete remission off all therapy.

Management of PG begins with topical corticosteroids, but most patients require systemic steroid therapy.1 Remission commonly occurs close to delivery, and 75% of patients flare post partum, though the disease typically resolves 6 months following delivery.1,3,4 For persistent intrapartum cases requiring more than prednisone 20 mg daily, therapy can include dapsone, IVIG, azathioprine, rituximab, or plasmapheresis.4,5 Dapsone and IVIG are compatible with breastfeeding postpartum, but if dapsone is selected, the infant must be monitored for hemolytic anemia.5 Pemphigoid gestationis increases the risk for a premature or small-for-gestational-age neonate, necessitating regular fetal monitoring until delivery.1 Cutaneous lesions may affect the newborn, though this occurrence is rare and self-limiting.6 Pemphigoid gestationis may recur in subsequent pregnancies at a rate of 33% to 55%, with earlier and more severe presentations.4
Clinically and histologically, PG closely resembles bullous pemphigoid (BP), but the exact pathogenesis is not fully understood. Recently, another case of what was termed pseudo-PG has been described 3 days following administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.7 Since the introduction of COVID-19 mRNA vaccines, cases of postvaccination BP, BP-like eruptions, and pemphigus vulgaris have been described.8-11 Tomayko et al10 reported 12 cases of subepidermal eruptions, including BP, in which 7 patients developed blisters after the second dose of either the Pfizer-BioNTech or Moderna mRNA vaccine. Three patients who developed BP after the first dose of the vaccine and chose to receive the second dose tolerated it well, with a mild flare observed in 1 patient.10 Similarly, subsequent vaccine doses in reports of vaccine-associated AIBD resulted in increased disease activity in 21% of cases.12 COVID-19 vaccine–associated BP, similar to drug-induced BP, seemingly displays a milder course of disease compared to the classic form of BP.10,13 More follow-up is needed to better understand these reactions and inform appropriate discussions on the administration of booster doses. Currently, completion of the vaccination series against COVID-19 is advisable given the paucity of reports of postvaccination AIBD and the risk for COVID-19 infection, but careful discussions on a case-by-case basis are warranted related to the risk for disease exacerbation following subsequent vaccinations.
The clinical presentation and diagnostic evaluation of our patient’s rash were consistent with PG. The temporal relationship between vaccine administration and PG lesion onset suggests the mRNA vaccine triggered AIBD in our patient. Interestingly, AIBD associated with COVID-19 is not unique to only the vaccines and has been observed following infection with the virus itself.14 The high rate of vaccination against COVID-19 in contrast with the low number of reported cases of AIBD after vaccination supports the overall safety of COVID-19 vaccines but identifies a need for further understanding of the processes that lead to the development of autoimmune conditions in at-risk populations.
- Wiznia LE, Pomeranz MK. Skin changes and diseases in pregnancy. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Masmoudi W, Vaillant M, Vassileva S, et al. International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-1112. doi:10.1111/bjd.19611
- Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138-144.
- Savervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-449.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
- Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30:51-55.
- de Lorenzi C, Kaya G, Toutous Trellu L. Pseudo-pemphigoid gestationis eruption following SARS-CoV-2 vaccination with mRNA vaccine. Dermatopathology (Basel). 2022;9:203-206. doi:10.3390/dermatopathology9030025
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35:E649-E651.
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750-751.
- Coto-Segura P, Fernandez-Prada M, Mir-Bonafe M, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47:141-143.
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2022;86:1160-1164.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111.
- Wiznia LE, Pomeranz MK. Skin changes and diseases in pregnancy. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Masmoudi W, Vaillant M, Vassileva S, et al. International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-1112. doi:10.1111/bjd.19611
- Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138-144.
- Savervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-449.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
- Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30:51-55.
- de Lorenzi C, Kaya G, Toutous Trellu L. Pseudo-pemphigoid gestationis eruption following SARS-CoV-2 vaccination with mRNA vaccine. Dermatopathology (Basel). 2022;9:203-206. doi:10.3390/dermatopathology9030025
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35:E649-E651.
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750-751.
- Coto-Segura P, Fernandez-Prada M, Mir-Bonafe M, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47:141-143.
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2022;86:1160-1164.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111.
Practice Points
- Dermatologists should be aware that COVID-19 messenger RNA vaccinations may present with various cutaneous complications.
- Pemphigoid gestationis should be considered in a pregnant or postpartum woman with an unexplained eruption of persistent, pruritic, urticarial lesions and blisters occurring postvaccination. Treatments include high-potency topical steroids and frequently systemic corticosteroids, along with steroid-sparing agents in severe cases.
Papular Reticulated Rash
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).
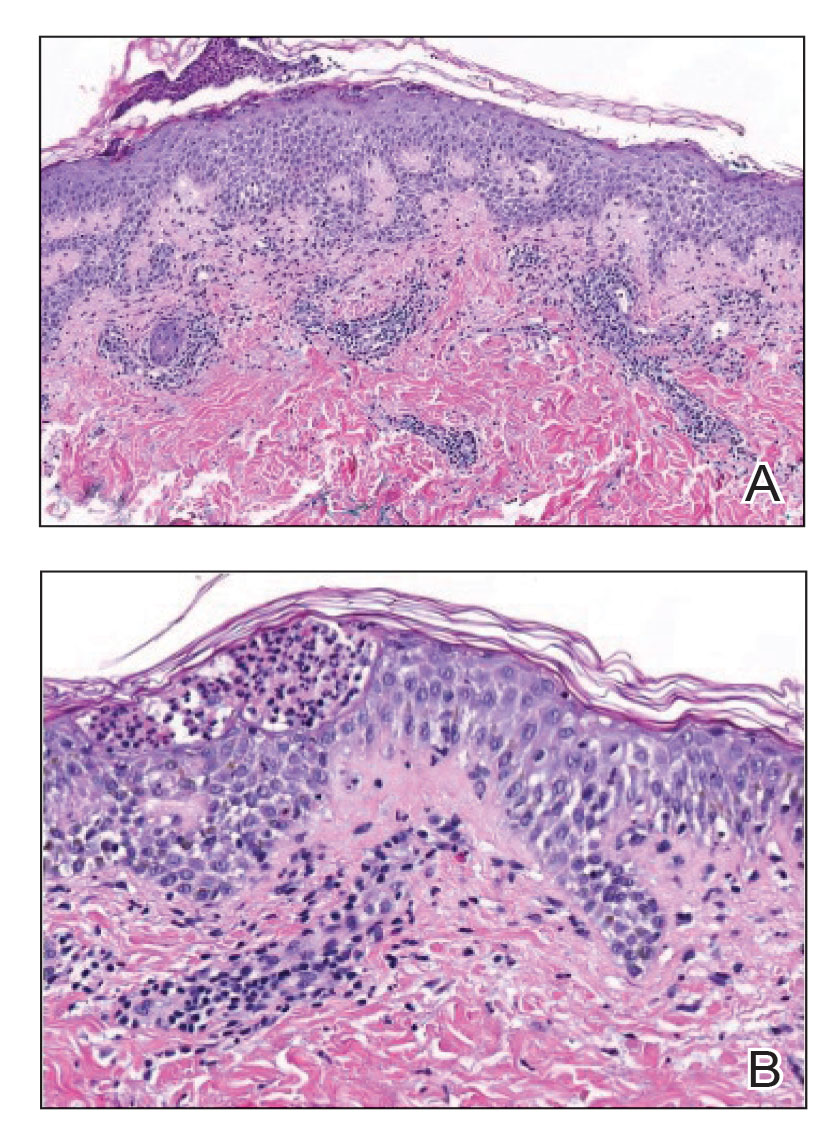
Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).

Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).

Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
An otherwise healthy 22-year-old woman presented with a painful eruption with burning and pruritus that had been slowly worsening as it spread over the last 4 weeks. The rash first appeared on the lower chest and inframammary folds (top) and spread to the upper chest, neck, back (bottom), arms, and lower face. Physical examination revealed multiple illdefined, erythematous papules, patches, and plaques on the chest, back, neck, and upper abdomen. Individual lesions coalesced into plaques that displayed a reticular configuration. There were no lesions in the axillae. The patient had been following a low-carbohydrate diet for 4 months. A punch biopsy was performed.
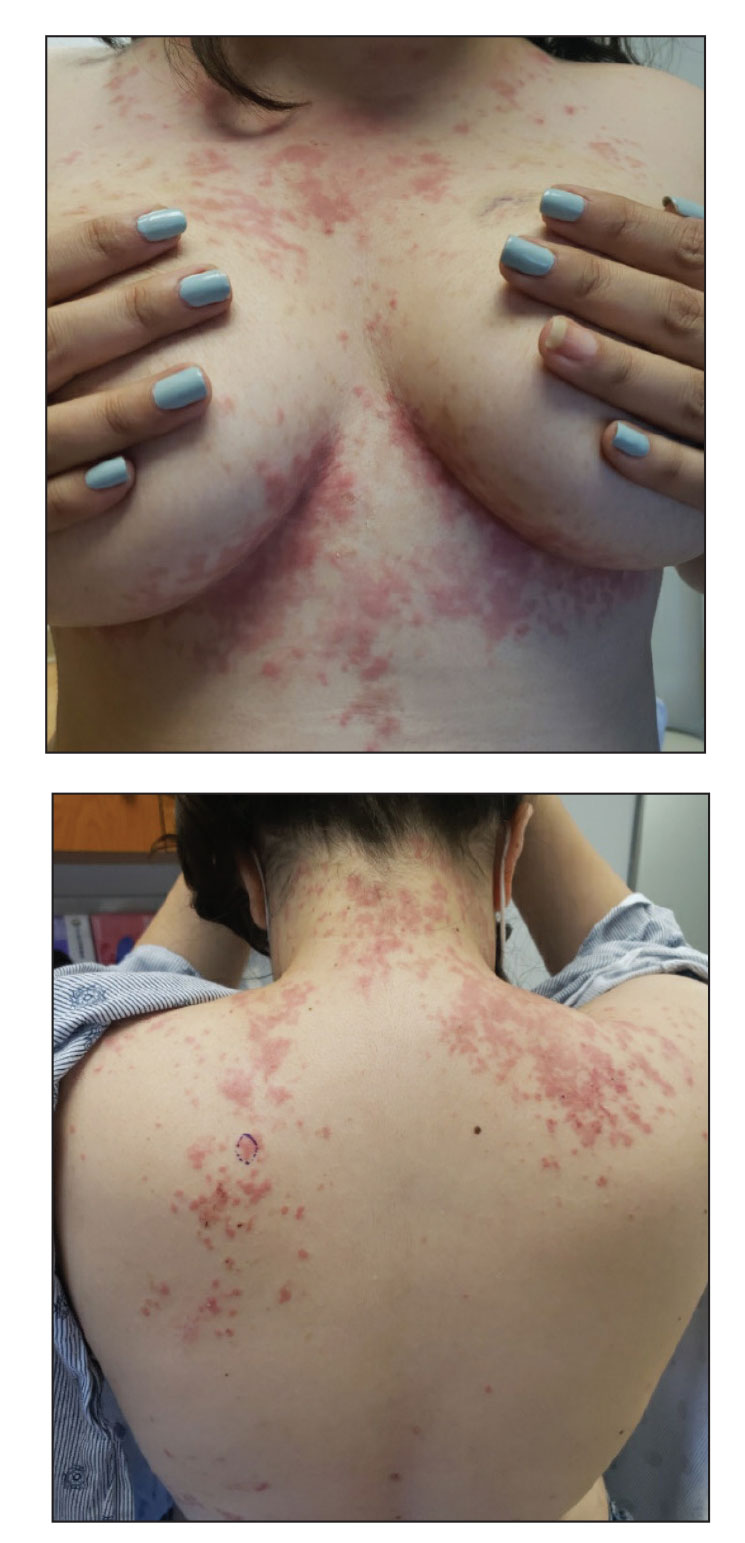
Miliarial Gout in an Immunocompromised Patient
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.
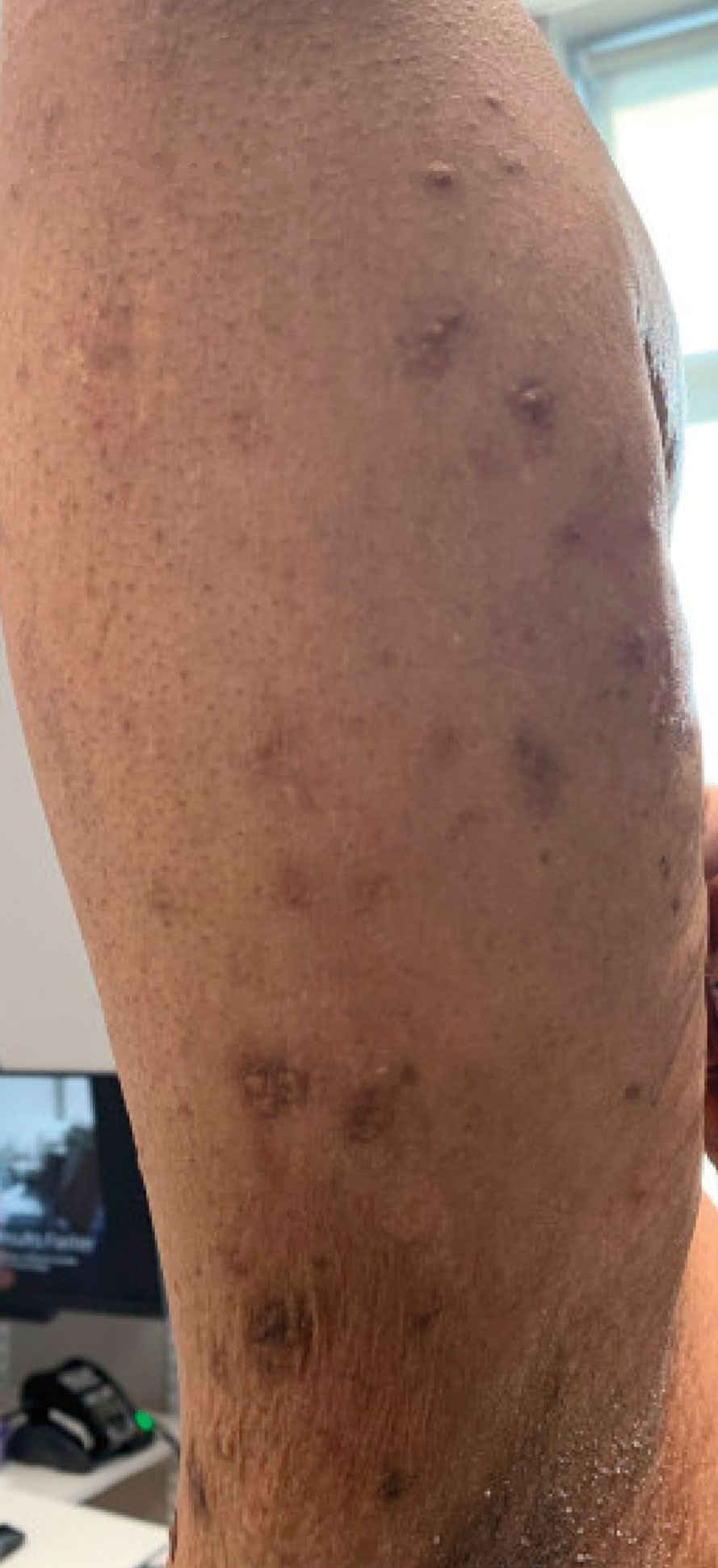
A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.
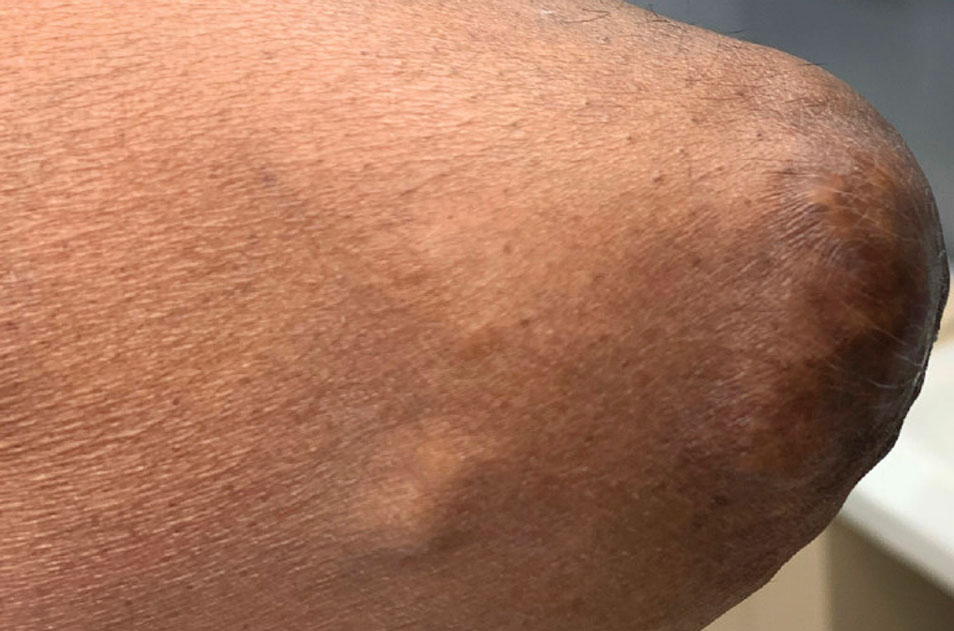
He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.
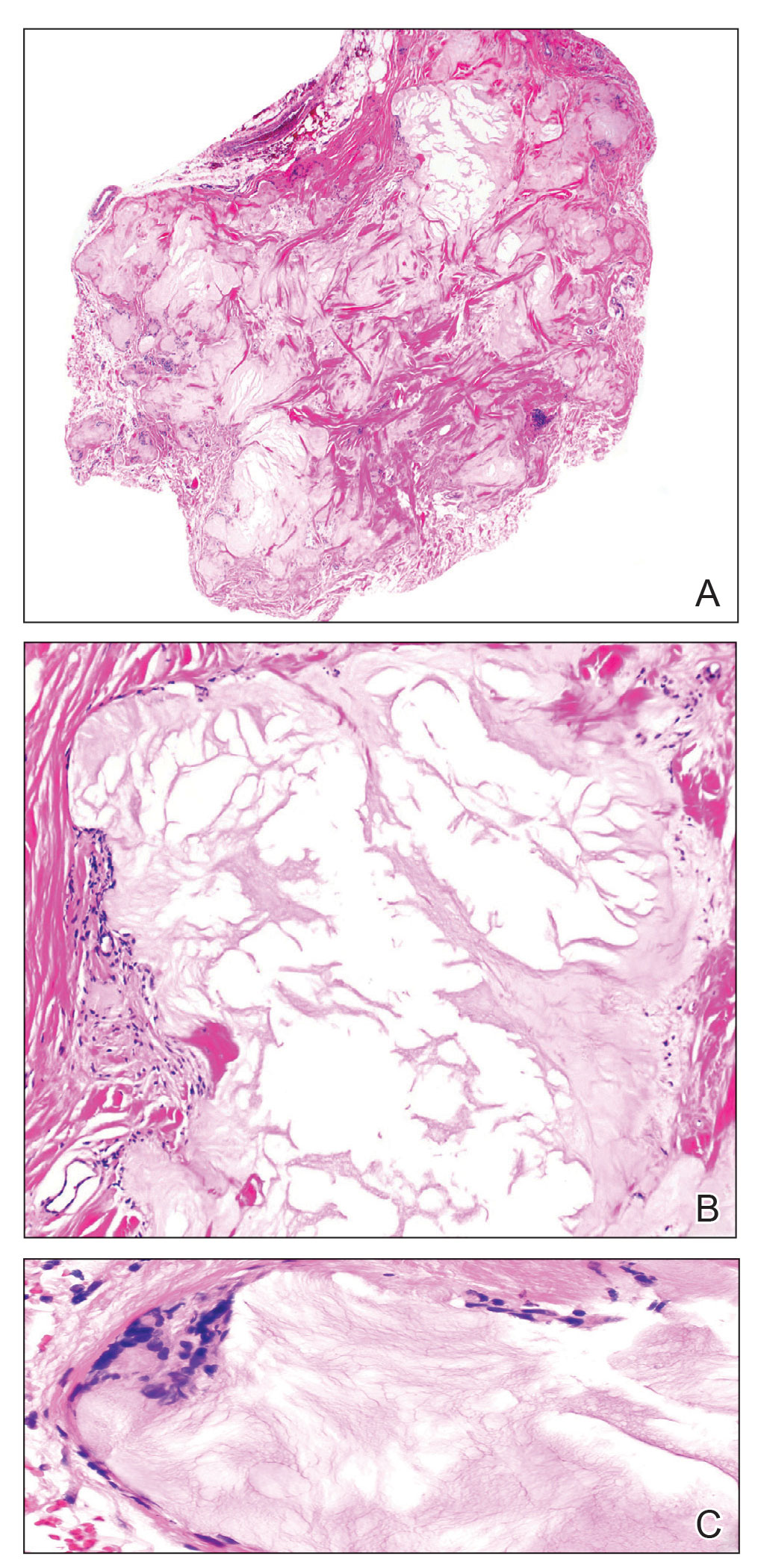
Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.

A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.

He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.

Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.

A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.

He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.

Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
Practice Points
- Miliarial gout is a rare intradermal manifestation of tophaceous gout and often presents as multiple small papules containing a white- to cream-colored substance.
- Immunocompromised status may be a risk factor for miliarial gout, especially in patients with a history of gout or hyperuricemia.
- Effective treatments for miliarial gout include allopurinol and colchicine.