User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
A Joint Effort to Save the Joints: What Dermatologists Need to Know About Psoriatic Arthritis
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Interacting With Dermatology Patients Online: Private Practice vs Academic Institute Website Content
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
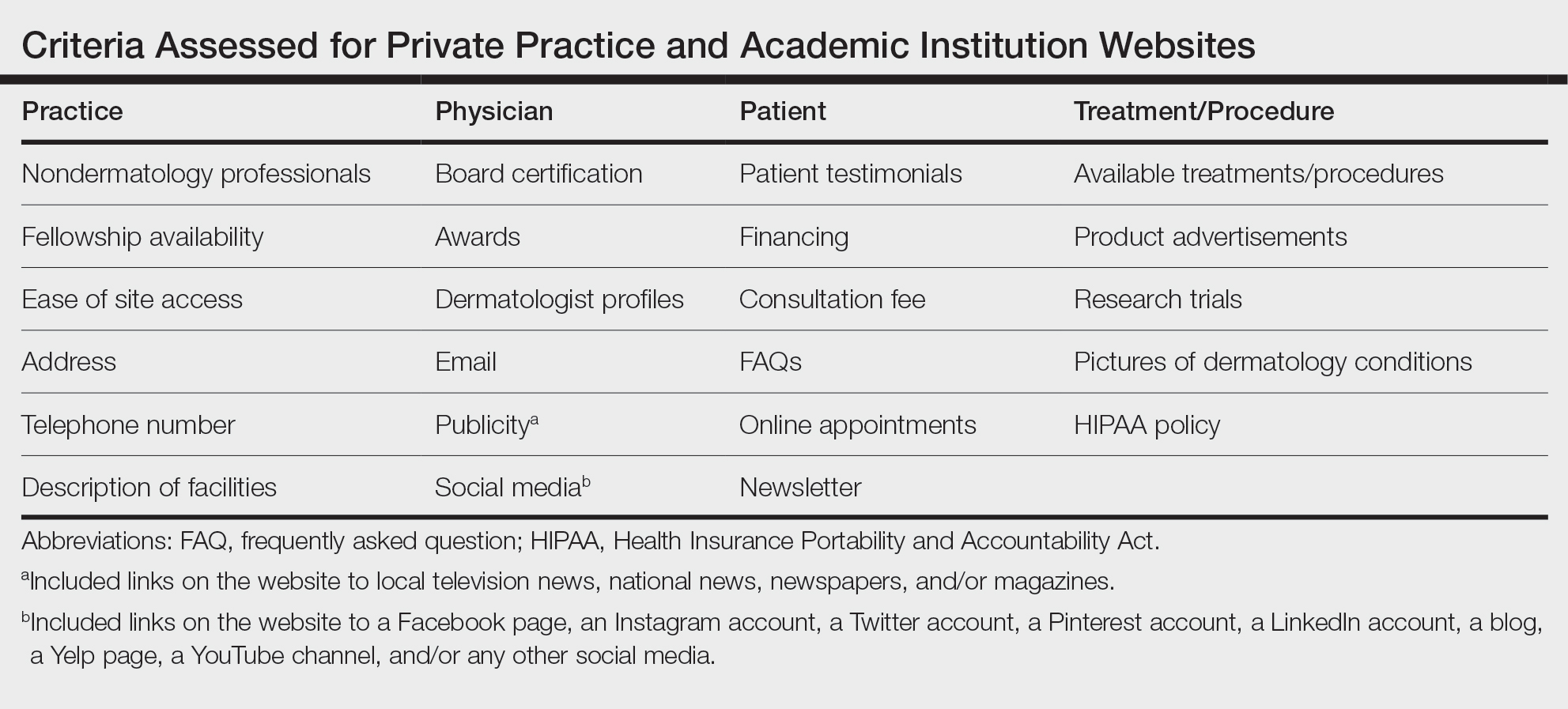
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.
Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).
When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.

Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).

When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.

Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).

When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
Practice Points
- Dermatologists at both private practices and academic institutions should understand that website content often may be the most accessible source of information about the practice available to patients and should be as specific and detailed as possible.
- When compared to private practices, academic institutions largely fail to have a social media presence, which may limit patient interaction with their websites.
Glitter Effects of Nail Art on Optical Coherence Tomography
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).
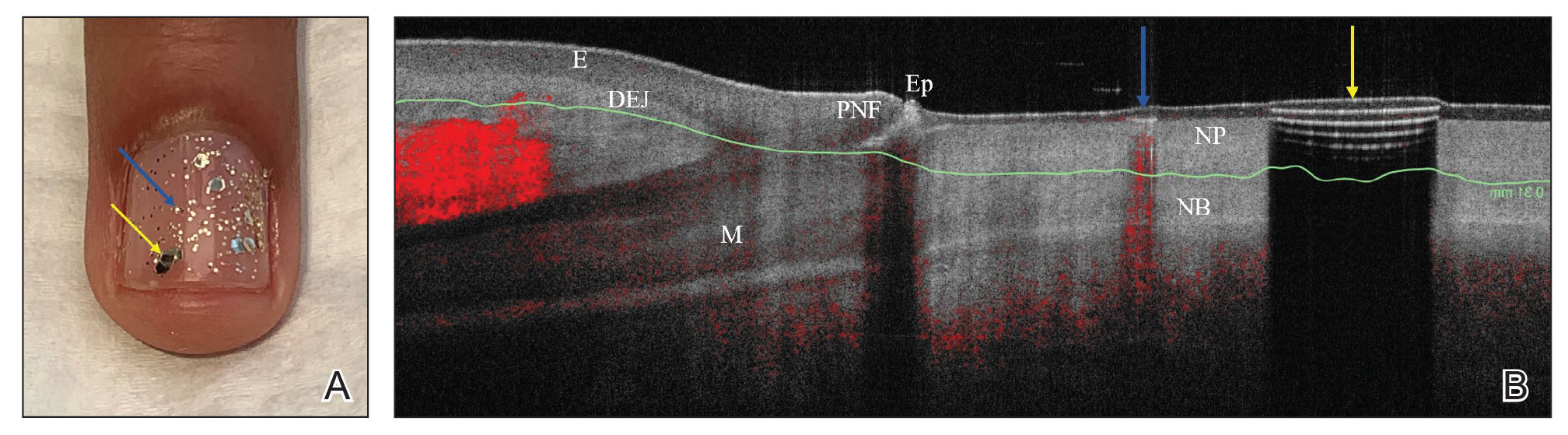
We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.
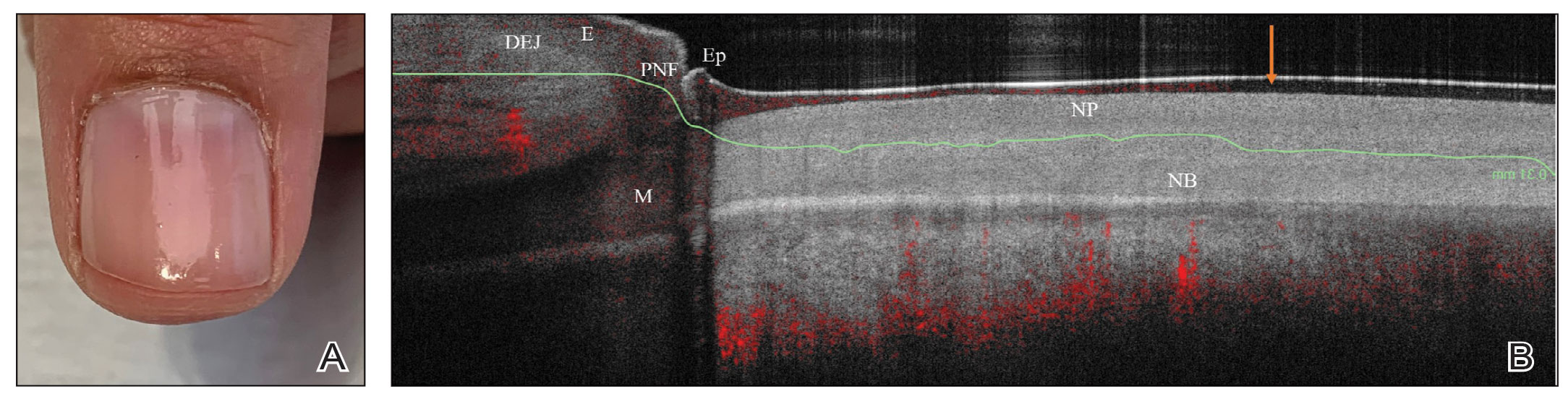
Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).

We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.

Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).

We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.

Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
Oval Brown Plaque on the Palm
The Diagnosis: Poroma
Histopathology showed an endophytic expansion of the epidermis by bland, uniform, basaloid epithelial cells with focal ductal differentiation and an abrupt transition with surrounding epidermal keratinocytes (Figure), consistent with a diagnosis of poroma. The patient elected to monitor the lesion rather than to have it excised.
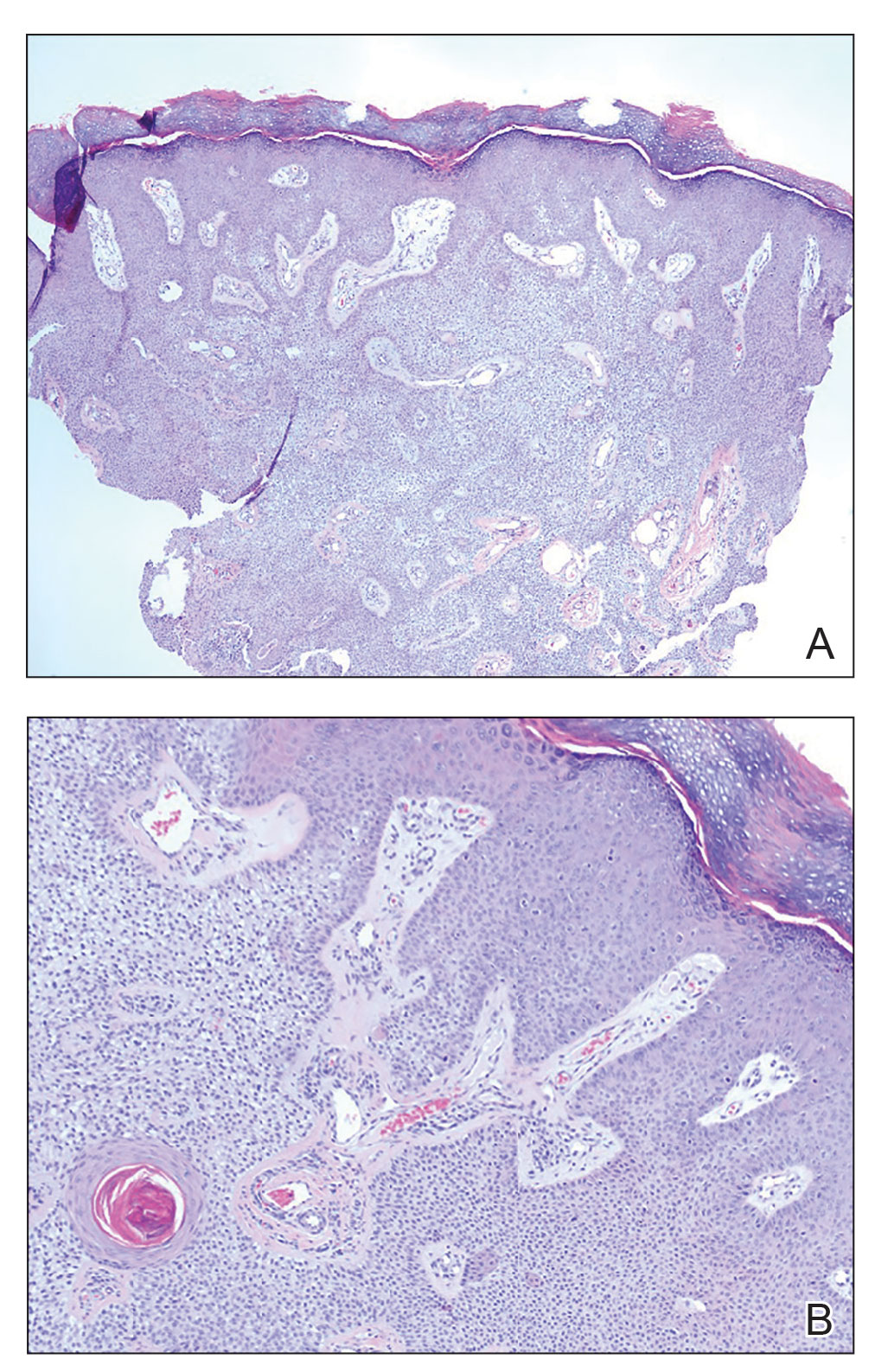
Eccrine poroma, used interchangeably with the term poroma, is a rare benign adnexal tumor of the eccrine sweat glands resulting from proliferation of the acrosyringium.1,2 It often occurs on the palms or soles, though it also can arise anywhere sweat glands are present.1 Eccrine poromas often appear in middle-aged individuals as singular, well-circumscribed, red-brown papules or nodules.3 A characteristic feature is a shallow, cup-shaped depression within the larger papule or nodule.1
Because the condition is benign and often asymptomatic, it can be safely monitored for progression.1 However, if the lesion is symptomatic or located in a sensitive area, complete excision is curative.4 Eccrine poromas can recur, making close monitoring following excision important.5 The development of bleeding, itching, or pain in a previously asymptomatic lesion may indicate possible malignant transformation, which occurs in only 18% of cases.6
The differential diagnosis includes basal cell carcinoma, circumscribed acral hypokeratosis, Kaposi sarcoma, and pyogenic granuloma. Basal cell carcinoma is the most common type of skin cancer.7 In rare cases it has been shown to present on the palms or soles as a slowgrowing, reddish-pink papule or plaque with central ulceration. It typically is asymptomatic. Histopathology shows dermal nests of basaloid cells with peripheral palisading, stromal mucin, and peritumoral clefts. Treatment is surgical excision.7
Circumscribed acral hypokeratosis presents on the palms or soles as a solitary, shallow, well-defined lesion with a flat base and raised border.8 It often is red-pink in color and most frequently occurs in middle-aged women. Although the cause of the condition is unknown, it is thought to be the result of trauma or human papillomavirus infection.8 Biopsy results characteristically show hypokeratosis demarcated by a sharp and frayed cutoff from uninvolved acral skin with discrete hypogranulosis, dilated blood vessels in the papillary dermis, and slightly thickened collagen fibers in the reticular dermis.9 Surgical excision is a potential treatment option, as topical corticosteroids, retinoids, and calcipotriene have not been shown to be effective; spontaneous resolution has been reported.8
Kaposi sarcoma is a vascular neoplasm that is associated with human herpesvirus 8 infection.10 It typically presents on mucocutaneous sites and the lower extremities. Palmar involvement has been reported in rare cases, occurring as a solitary, well-demarcated, violaceous macule or patch that may be painful.10-12 Characteristic histopathologic features include a proliferation in the dermis of slitlike vascular spaces and spindle cell proliferation.13 Treatment options include cryosurgery; pulsed dye laser; and topical, intralesional, or systemic chemotherapy agents, depending on the stage of the patient’s disease. Antiretroviral therapy is indicated for patients with Kaposi sarcoma secondary to AIDS.14
Pyogenic granuloma presents as a solitary red-brown or bluish-black papule or nodule that bleeds easily when manipulated.15 It commonly occurs following trauma, typically on the fingers, feet, and lips.6 Although benign, potential complications include ulceration and blood loss. Pyogenic granulomas can be treated via curettage and cautery, excision, cryosurgery, or pulsed dye laser.15
- Wankhade V, Singh R, Sadhwani V, et al. Eccrine poroma. Indian Dermatol Online J. 2015;6:304-305.
- Yorulmaz A, Aksoy GG, Ozhamam EU. A growing mass under the nail: subungual eccrine poroma. Skin Appendage Disord. 2020;6:254-257.
- Wang Y, Liu M, Zheng Y, et al. Eccrine poroma presented as spindleshaped plaque: a case report. Medicine (Baltimore). 2021;100:E25971. doi:10.1097/MD.0000000000025971
- Sharma M, Singh M, Gupta K, et al. Eccrine poroma of the eyelid. Indian J Ophthalmol. 2020;68:2522.
- Rasool MN, Hawary MB. Benign eccrine poroma in the palm of the hand. Ann Saudi Med. 2004;24:46-47.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms [published online April 2, 2014]. Int J Dermatol. 2014;53:1053-1061. doi:10.1111/ijd.12448
- López-Sánchez C, Ferguson P, Collgros H. Basal cell carcinoma of the palm: an unusual presentation of a common tumour [published online August 6, 2019]. Australas J Dermatol. 2020;61:69-70. doi:10.1111/ajd.13129
- Berk DR, Böer A, Bauschard FD, et al. Circumscribed acral hypokeratosis [published online April 6, 2007]. J Am Acad Dermatol. 2007;57:292-296. doi:10.1016/j.jaad.2007.02.022
- Majluf-Cáceres P, Vera-Kellet C, González-Bombardiere S. New dermoscopic keys for circumscribed acral hypokeratosis: report of four cases. Dermatol Pract Concept. 2021;11:E2021010. doi:10.5826/dpc.1102a10
- Simonart T, De Dobbeleer G, Stallenberg B. Classic Kaposi’s sarcoma of the palm in a metallurgist: role of iron filings in its development? Br J Dermatol. 2003;148:1061-1063. doi:10.1046/j.1365-2133.2003.05331.x
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294. doi:10.5858/arpa.2012-0101-RS
- Al Zolibani AA, Al Robaee AA. Primary palmoplantar Kaposi’s sarcoma: an unusual presentation. Skinmed. 2006;5:248-249. doi:10.1111/j.1540-9740.2006.04662.x
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Etemad SA, Dewan AK. Kaposi sarcoma updates [published online July 10, 2019]. Dermatol Clin. 2019;37:505-517. doi:10.1016/j. det.2019.05.008
- Murthy SC, Nagaraj A. Pyogenic granuloma. Indian Pediatr. 2012;49:855. doi:10.1007/s13312-012-0184-4
The Diagnosis: Poroma
Histopathology showed an endophytic expansion of the epidermis by bland, uniform, basaloid epithelial cells with focal ductal differentiation and an abrupt transition with surrounding epidermal keratinocytes (Figure), consistent with a diagnosis of poroma. The patient elected to monitor the lesion rather than to have it excised.

Eccrine poroma, used interchangeably with the term poroma, is a rare benign adnexal tumor of the eccrine sweat glands resulting from proliferation of the acrosyringium.1,2 It often occurs on the palms or soles, though it also can arise anywhere sweat glands are present.1 Eccrine poromas often appear in middle-aged individuals as singular, well-circumscribed, red-brown papules or nodules.3 A characteristic feature is a shallow, cup-shaped depression within the larger papule or nodule.1
Because the condition is benign and often asymptomatic, it can be safely monitored for progression.1 However, if the lesion is symptomatic or located in a sensitive area, complete excision is curative.4 Eccrine poromas can recur, making close monitoring following excision important.5 The development of bleeding, itching, or pain in a previously asymptomatic lesion may indicate possible malignant transformation, which occurs in only 18% of cases.6
The differential diagnosis includes basal cell carcinoma, circumscribed acral hypokeratosis, Kaposi sarcoma, and pyogenic granuloma. Basal cell carcinoma is the most common type of skin cancer.7 In rare cases it has been shown to present on the palms or soles as a slowgrowing, reddish-pink papule or plaque with central ulceration. It typically is asymptomatic. Histopathology shows dermal nests of basaloid cells with peripheral palisading, stromal mucin, and peritumoral clefts. Treatment is surgical excision.7
Circumscribed acral hypokeratosis presents on the palms or soles as a solitary, shallow, well-defined lesion with a flat base and raised border.8 It often is red-pink in color and most frequently occurs in middle-aged women. Although the cause of the condition is unknown, it is thought to be the result of trauma or human papillomavirus infection.8 Biopsy results characteristically show hypokeratosis demarcated by a sharp and frayed cutoff from uninvolved acral skin with discrete hypogranulosis, dilated blood vessels in the papillary dermis, and slightly thickened collagen fibers in the reticular dermis.9 Surgical excision is a potential treatment option, as topical corticosteroids, retinoids, and calcipotriene have not been shown to be effective; spontaneous resolution has been reported.8
Kaposi sarcoma is a vascular neoplasm that is associated with human herpesvirus 8 infection.10 It typically presents on mucocutaneous sites and the lower extremities. Palmar involvement has been reported in rare cases, occurring as a solitary, well-demarcated, violaceous macule or patch that may be painful.10-12 Characteristic histopathologic features include a proliferation in the dermis of slitlike vascular spaces and spindle cell proliferation.13 Treatment options include cryosurgery; pulsed dye laser; and topical, intralesional, or systemic chemotherapy agents, depending on the stage of the patient’s disease. Antiretroviral therapy is indicated for patients with Kaposi sarcoma secondary to AIDS.14
Pyogenic granuloma presents as a solitary red-brown or bluish-black papule or nodule that bleeds easily when manipulated.15 It commonly occurs following trauma, typically on the fingers, feet, and lips.6 Although benign, potential complications include ulceration and blood loss. Pyogenic granulomas can be treated via curettage and cautery, excision, cryosurgery, or pulsed dye laser.15
The Diagnosis: Poroma
Histopathology showed an endophytic expansion of the epidermis by bland, uniform, basaloid epithelial cells with focal ductal differentiation and an abrupt transition with surrounding epidermal keratinocytes (Figure), consistent with a diagnosis of poroma. The patient elected to monitor the lesion rather than to have it excised.

Eccrine poroma, used interchangeably with the term poroma, is a rare benign adnexal tumor of the eccrine sweat glands resulting from proliferation of the acrosyringium.1,2 It often occurs on the palms or soles, though it also can arise anywhere sweat glands are present.1 Eccrine poromas often appear in middle-aged individuals as singular, well-circumscribed, red-brown papules or nodules.3 A characteristic feature is a shallow, cup-shaped depression within the larger papule or nodule.1
Because the condition is benign and often asymptomatic, it can be safely monitored for progression.1 However, if the lesion is symptomatic or located in a sensitive area, complete excision is curative.4 Eccrine poromas can recur, making close monitoring following excision important.5 The development of bleeding, itching, or pain in a previously asymptomatic lesion may indicate possible malignant transformation, which occurs in only 18% of cases.6
The differential diagnosis includes basal cell carcinoma, circumscribed acral hypokeratosis, Kaposi sarcoma, and pyogenic granuloma. Basal cell carcinoma is the most common type of skin cancer.7 In rare cases it has been shown to present on the palms or soles as a slowgrowing, reddish-pink papule or plaque with central ulceration. It typically is asymptomatic. Histopathology shows dermal nests of basaloid cells with peripheral palisading, stromal mucin, and peritumoral clefts. Treatment is surgical excision.7
Circumscribed acral hypokeratosis presents on the palms or soles as a solitary, shallow, well-defined lesion with a flat base and raised border.8 It often is red-pink in color and most frequently occurs in middle-aged women. Although the cause of the condition is unknown, it is thought to be the result of trauma or human papillomavirus infection.8 Biopsy results characteristically show hypokeratosis demarcated by a sharp and frayed cutoff from uninvolved acral skin with discrete hypogranulosis, dilated blood vessels in the papillary dermis, and slightly thickened collagen fibers in the reticular dermis.9 Surgical excision is a potential treatment option, as topical corticosteroids, retinoids, and calcipotriene have not been shown to be effective; spontaneous resolution has been reported.8
Kaposi sarcoma is a vascular neoplasm that is associated with human herpesvirus 8 infection.10 It typically presents on mucocutaneous sites and the lower extremities. Palmar involvement has been reported in rare cases, occurring as a solitary, well-demarcated, violaceous macule or patch that may be painful.10-12 Characteristic histopathologic features include a proliferation in the dermis of slitlike vascular spaces and spindle cell proliferation.13 Treatment options include cryosurgery; pulsed dye laser; and topical, intralesional, or systemic chemotherapy agents, depending on the stage of the patient’s disease. Antiretroviral therapy is indicated for patients with Kaposi sarcoma secondary to AIDS.14
Pyogenic granuloma presents as a solitary red-brown or bluish-black papule or nodule that bleeds easily when manipulated.15 It commonly occurs following trauma, typically on the fingers, feet, and lips.6 Although benign, potential complications include ulceration and blood loss. Pyogenic granulomas can be treated via curettage and cautery, excision, cryosurgery, or pulsed dye laser.15
- Wankhade V, Singh R, Sadhwani V, et al. Eccrine poroma. Indian Dermatol Online J. 2015;6:304-305.
- Yorulmaz A, Aksoy GG, Ozhamam EU. A growing mass under the nail: subungual eccrine poroma. Skin Appendage Disord. 2020;6:254-257.
- Wang Y, Liu M, Zheng Y, et al. Eccrine poroma presented as spindleshaped plaque: a case report. Medicine (Baltimore). 2021;100:E25971. doi:10.1097/MD.0000000000025971
- Sharma M, Singh M, Gupta K, et al. Eccrine poroma of the eyelid. Indian J Ophthalmol. 2020;68:2522.
- Rasool MN, Hawary MB. Benign eccrine poroma in the palm of the hand. Ann Saudi Med. 2004;24:46-47.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms [published online April 2, 2014]. Int J Dermatol. 2014;53:1053-1061. doi:10.1111/ijd.12448
- López-Sánchez C, Ferguson P, Collgros H. Basal cell carcinoma of the palm: an unusual presentation of a common tumour [published online August 6, 2019]. Australas J Dermatol. 2020;61:69-70. doi:10.1111/ajd.13129
- Berk DR, Böer A, Bauschard FD, et al. Circumscribed acral hypokeratosis [published online April 6, 2007]. J Am Acad Dermatol. 2007;57:292-296. doi:10.1016/j.jaad.2007.02.022
- Majluf-Cáceres P, Vera-Kellet C, González-Bombardiere S. New dermoscopic keys for circumscribed acral hypokeratosis: report of four cases. Dermatol Pract Concept. 2021;11:E2021010. doi:10.5826/dpc.1102a10
- Simonart T, De Dobbeleer G, Stallenberg B. Classic Kaposi’s sarcoma of the palm in a metallurgist: role of iron filings in its development? Br J Dermatol. 2003;148:1061-1063. doi:10.1046/j.1365-2133.2003.05331.x
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294. doi:10.5858/arpa.2012-0101-RS
- Al Zolibani AA, Al Robaee AA. Primary palmoplantar Kaposi’s sarcoma: an unusual presentation. Skinmed. 2006;5:248-249. doi:10.1111/j.1540-9740.2006.04662.x
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Etemad SA, Dewan AK. Kaposi sarcoma updates [published online July 10, 2019]. Dermatol Clin. 2019;37:505-517. doi:10.1016/j. det.2019.05.008
- Murthy SC, Nagaraj A. Pyogenic granuloma. Indian Pediatr. 2012;49:855. doi:10.1007/s13312-012-0184-4
- Wankhade V, Singh R, Sadhwani V, et al. Eccrine poroma. Indian Dermatol Online J. 2015;6:304-305.
- Yorulmaz A, Aksoy GG, Ozhamam EU. A growing mass under the nail: subungual eccrine poroma. Skin Appendage Disord. 2020;6:254-257.
- Wang Y, Liu M, Zheng Y, et al. Eccrine poroma presented as spindleshaped plaque: a case report. Medicine (Baltimore). 2021;100:E25971. doi:10.1097/MD.0000000000025971
- Sharma M, Singh M, Gupta K, et al. Eccrine poroma of the eyelid. Indian J Ophthalmol. 2020;68:2522.
- Rasool MN, Hawary MB. Benign eccrine poroma in the palm of the hand. Ann Saudi Med. 2004;24:46-47.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms [published online April 2, 2014]. Int J Dermatol. 2014;53:1053-1061. doi:10.1111/ijd.12448
- López-Sánchez C, Ferguson P, Collgros H. Basal cell carcinoma of the palm: an unusual presentation of a common tumour [published online August 6, 2019]. Australas J Dermatol. 2020;61:69-70. doi:10.1111/ajd.13129
- Berk DR, Böer A, Bauschard FD, et al. Circumscribed acral hypokeratosis [published online April 6, 2007]. J Am Acad Dermatol. 2007;57:292-296. doi:10.1016/j.jaad.2007.02.022
- Majluf-Cáceres P, Vera-Kellet C, González-Bombardiere S. New dermoscopic keys for circumscribed acral hypokeratosis: report of four cases. Dermatol Pract Concept. 2021;11:E2021010. doi:10.5826/dpc.1102a10
- Simonart T, De Dobbeleer G, Stallenberg B. Classic Kaposi’s sarcoma of the palm in a metallurgist: role of iron filings in its development? Br J Dermatol. 2003;148:1061-1063. doi:10.1046/j.1365-2133.2003.05331.x
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294. doi:10.5858/arpa.2012-0101-RS
- Al Zolibani AA, Al Robaee AA. Primary palmoplantar Kaposi’s sarcoma: an unusual presentation. Skinmed. 2006;5:248-249. doi:10.1111/j.1540-9740.2006.04662.x
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Etemad SA, Dewan AK. Kaposi sarcoma updates [published online July 10, 2019]. Dermatol Clin. 2019;37:505-517. doi:10.1016/j. det.2019.05.008
- Murthy SC, Nagaraj A. Pyogenic granuloma. Indian Pediatr. 2012;49:855. doi:10.1007/s13312-012-0184-4
A 43-year-old woman presented with a painful lesion on the palm of 30 years’ duration that had grown in size. Physical examination revealed an oval, brown, lobulated plaque with a hyperkeratotic rim on the left palm. She reported bleeding and pain. A shallow cup-shaped depression was noted within the plaque. A 4-mm punch biopsy was performed.
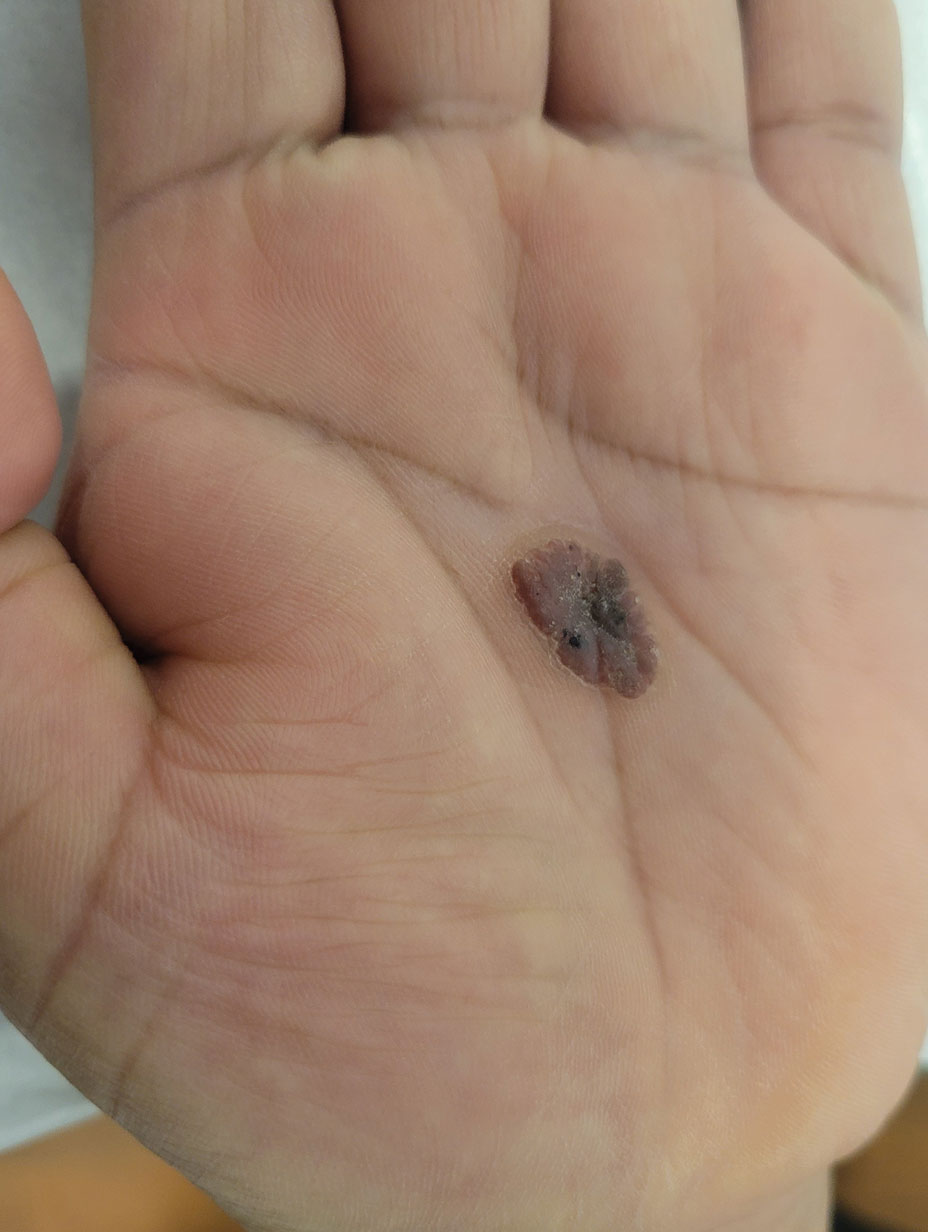
Crusted Scabies Presenting as Erythroderma in a Patient With Iatrogenic Immunosuppression for Treatment of Granulomatosis With Polyangiitis
Scabies is caused by cutaneous ectoparasitic infection by the mite Sarcoptes scabiei var hominis. The infection is highly contagious via direct skin-to-skin contact or indirectly through infested bedding, clothing or fomites.1,2 Scabies occurs at all ages, in all ethnic groups, and at all socioeconomic levels.1 Analysis by the Global Burden of Disease estimates that 200 million individuals have been infected with scabies worldwide. The World Health Organization has declared scabies a neglected tropical disease.3
Crusted scabies is a severe and rare form of scabies, with hyperinfestation of thousands to millions of mites, and more commonly is associated with immunosuppressed states, including HIV and hematologic malignancies.1,2,4 Crusted scabies has a high mortality rate due to sepsis when left untreated.3,5
Occasionally, iatrogenic immunosuppression contributes to the development of crusted scabies.1,2 Iatrogenic immunosuppression leading to crusted scabies most commonly occurs secondary to immunosuppression after bone marrow or solid organ transplantation.6 Less often, crusted scabies is caused by iatrogenic immunosuppression from other clinical scenarios.1,2
We describe a patient with iatrogenic immunosuppression due to azathioprine-induced myelosuppression for the treatment of granulomatosis with polyangiitis (GPA) who developed crusted scabies that clinically presented as erythroderma. Crusted scabies should be included in the differential diagnosis of erythroderma, especially in the setting of iatrogenic immunosuppression, for timely and appropriate management.
Case Report
An 84-year-old man presented with worsening pruritus, erythema, and thick yellow scale that progressed to erythroderma over the last 2 weeks. He was diagnosed with GPA 6 months prior to presentation and was treated with azathioprine 150 mg/d, prednisone 10 mg/d, and sulfamethoxazole 800 mg plus trimethoprim 160 mg twice weekly for prophylaxis against Pneumocystis jirovecii pneumonia.
Three weeks prior to presentation, the patient was hospitalized for pancytopenia attributed to azathioprine-induced myelosuppression (hemoglobin, 6.1 g/dL [reference range, 13.5–18.0 g/dL]; hematocrit, 17.5% [reference range, 42%–52%]; white blood cell count, 1.66×103/μL [reference range, 4.0–10.5×103/μL]; platelet count, 146×103/μL [reference range, 150–450×103/μL]; absolute neutrophil count, 1.29×103/μL [reference range, 1.4–6.5×103/μL]). He was transferred to a skilled nursing facility after discharge and referred to dermatology for evaluation of the worsening pruritic rash.
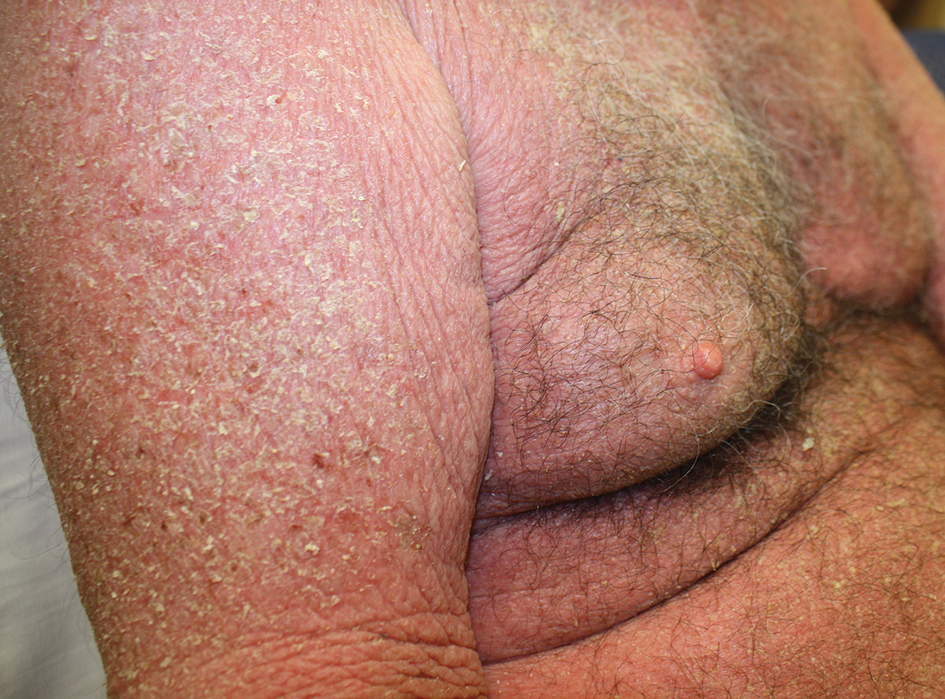
At the current presentation, the patient denied close contact with anyone who had a similar rash at home or at the skilled nursing facility. Physical examination revealed diffuse erythroderma with yellow scale on the scalp, trunk, arms, and legs (Figure 1). The palms showed scattered 2- to 3-mm pustules. The mucosal surfaces did not have lesions. A punch biopsy of a pustule from the right arm revealed focal spongiosis, parakeratosis, and acanthosis, as well as a perivascular and interstitial mixed inflammatory infiltrate with lymphocytes and eosinophils. Organisms morphologically compatible with scabies were found in the stratum corneum (Figure 2). Another punch biopsy of a pustule from the right arm was performed for direct immunofluorescence (DIF) and was negative for immunoglobulin deposition. Mineral oil preparation from pustules on the palm was positive for mites.
The patient was treated with permethrin cream 5% and oral ivermectin 200 μg/kg on day 1 and day 10. The prednisone dosage was increased from 10 mg/d to 50 mg/d and tapered over 2 weeks to treat the symptomatic rash and GPA. He remains on maintenance rituximab for GPA, without recurrence of scabies.
Comment
Pathogenesis—As an obligate parasite, S scabiei spends its entire life cycle within the host. Impregnated female mites burrow into the epidermis after mating and lay eggs daily for 1 to 2 months. Eggs hatch 2 or 3 days later. Larvae then migrate to the skin surface; burrow into the stratum corneum, where they mature into adults; and then mate on the skin surface.1,4
Clinical Presentation and Sequelae—Typically, scabies presents 2 to 6 weeks after initial exposure with generalized and intense itching and inflammatory pruritic papules on the finger webs, wrists, elbows, axillae, buttocks, umbilicus, genitalia, and areolae.1 Burrows are specific for scabies but may not always be present. Often, there are nonspecific secondary lesions, including excoriations, dermatitis, and impetiginization.
Complications of scabies can be severe, with initial colonization and infection of the skin resulting in impetigo and cellulitis. Systematic sequelae from local skin infection include post-streptococcal glomerulonephritis, rheumatic fever, and sepsis. Mortality from sepsis in scabies can be high.3,5
Classic Crusted Scabies and Other Variants—Crusted scabies presents with psoriasiform hyperkeratotic plaques involving the hands and feet with potential nail involvement that can become more generalized.1 Alterations in CD4+ T-cell function have been implicated in the development of crusted scabies, in which an excessive helper T cell (TH2) response is elicited against the ectoparasite, which may help explain the intense pruritus of scabies.6 Occasionally, iatrogenic immunosuppression contributes to development of crusted scabies,1 as was the case with our patient. However, it is rare for crusted scabies to present with erythroderma.7
Other atypical presentations of scabies include a seborrheic dermatitis–like presentation in infants, nodular lesions in the groin and axillae in more chronic scabies, and vesicles or bullous lesions.1
Diagnosis—Identification of mites, eggs, or feces is necessary for definitive diagnosis of scabies.8 These materials can be obtained through skin scrapings with mineral oil and observed under light microscopy or direct dermoscopy. Multiple scrapings on many lesions should be performed because failure to identify mites can be common and does not rule out scabies. Dermoscopic examination of active lesions under low power also can be helpful, given that identification of dark brown triangular structures can correspond to visualization of the pigmented anterior section of the mite.9-11 A skin biopsy can help identify mites, but histopathology often shows a nonspecific hypersensitivity reaction.12 Therefore, empiric treatment often is necessary.
Differential Diagnosis—The differential diagnosis of erythroderma is broad and includes a drug eruption; Sézary syndrome; and pre-existing skin diseases, including psoriasis, atopic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, and bullous pemphigoid. Histopathology is critical to differentiate these diagnoses. Bullous pemphigoid and pemphigus foliaceus are immunobullous diseases that typically are positive for immunoglobulin deposition on DIF. In rare cases, scabies also can present with bullae and positive DIF test results.13
Treatment—First-line treatment of crusted scabies in the United States is permethrin cream 5%, followed by oral ivermectin 200 μg/kg.4,5,14,15 Other scabicides include topicals such as benzyl benzoate 10% to 25%; precipitated sulfur 2% to 10%; crotamiton 10%; malathion 0.5%; and lindane 1%.5 The association of neurotoxicity with lindane has considerably reduced the drug’s use.1
During treatment of scabies, it is important to isolate patients to mitigate the possibility of spread.4 Pruritus can persist for a few weeks after completion of therapy.5 Patients should be closely monitored to ensure that this symptom is secondary to skin inflammation and not incomplete treatment.
Treatment of crusted scabies may require repeated treatments to decrease the notable mite burden as well as the associated crusting and scale. Adding a keratolytic such as 5% to 10% salicylic acid in petrolatum to the treatment regimen may be useful for breaking up thick scale.5
Immunosuppression—With numerous immunomodulatory drugs for treating autoimmunity comes an increased risk for iatrogenic immunosuppression that may contribute to the development of crusted scabies.16 In a number of autoimmune diseases such as rheumatoid arthritis,17-19 psoriasis,20,21 pemphigus vulgaris,22 systemic lupus erythematosus,23 systemic sclerosis,22,24 bullous pemphigoid,25,26 and dermatomyositis,27 patients have developed crusted scabies secondary to treatment-related immunosuppression. These immunosuppressive therapies include systemic steroids,22-24,26-31 methotrexate,23 infliximab,18 adalimumab,21 toclizumab,19 and etanercept.20 In a case of drug-induced Stevens-Johnson syndrome, the patient developed crusted scabies during long-term use of oral steroids.22
Patients with a malignancy who are being treated with chemotherapy also can develop crusted scabies.28 Crusted scabies has even been associated with long-term topical steroid32-34 and topical calcineurin inhibitor use.16
Iatrogenic immunosuppression in our patient resulted from treatment of GPA with azathioprine, an immunosuppressive drug that acts as an antagonist of the breakdown of purines, leading to inhibition of DNA, RNA, and protein synthesis.35 On occasion, azathioprine can induce immunosuppression in the form of myelosuppression and resulting pancytopenia, as was the case with our patient.
Conclusion
Although scabies is designated as a neglected tropical disease by the World Health Organization, it still causes a notable burden worldwide, regardless of the economics. Our case highlights an unusual presentation of scabies as erythroderma in the setting of iatrogenic immunosuppression from azathioprine use. Dermatologists should consider crusted scabies in the differential diagnosis of erythroderma, especially in immunocompromised patients, to avoid delays in diagnosis and treatment. Immunosuppressive therapy is an important mainstay in the treatment of many conditions, but it is important to consider that these medications can place patients at an increased risk for rare opportunistic infections. Therefore, patients receiving such treatment should be closely monitored.
- Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354:1718-1727. doi:10.1056/NEJMcp052784
- Salgado F, Elston DM. What’s eating you? scabies in the developing world. Cutis. 2017;100:287-289.
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Wang X-D, Shen H, Liu Z-H. Contagious erythroderma. J Emerg Med. 2016;51:180-181. doi:10.1016/j.jemermed.2016.05.027
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146. doi:10.1016/j.jaad.2010.03.010
- Bollea Garlatti LA, Torre AC, Bollea Garlatti ML, et al.. Dermoscopy aids the diagnosis of crusted scabies in an erythrodermic patient. J Am Acad Dermatol. 2015;73:E93-E95. doi:10.1016/j.jaad.2015.04.061
- Tang J, You Z, Ran Y. Simple methods to enhance the diagnosis of scabies. J Am Acad Dermatol. 2019;80:E99-E100. doi:10.1016/j.jaad.2017.07.038
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20:600-605. doi:10.1111/j.1365-4362.1981.tb00844.x
- Shahab RKA, Loo DS. Bullous scabies. J Am Acad Dermatol. 2003;49:346-350. doi:10.1067/s0190-9622(03)00876-4
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007:CD000320. doi:10.1002/14651858.CD000320.pub2
- Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane Review. JAMA Dermatol. 2019;155:730-732. doi:10.1001/jamadermatol.2019.0279
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol. 2006;23:299-300. doi:10.1111/j.1525-1470.2006.00241.x
- Bu X, Fan J, Hu X, et al. Norwegian scabies in a patient treated with Tripterygium glycoside for rheumatoid arthritis. An Bras Dermatol. 2017;92:556-558. doi:10.1590/abd1806-4841.20174946
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol. 2005;52:719-720. doi:10.1016/j.jaad.2004.12.039
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-404. doi:10.1016/j.jbspin.2011.02.008
- Saillard C, Darrieux L, Safa G. Crusted scabies complicates etanercept therapy in a patient with severe psoriasis. J Am Acad Dermatol. 2013;68:E138-E139. doi:10.1016/j.jaad.2012.09.049
- Belvisi V, Orsi GB, Del Borgo C, et al. Large nosocomial outbreakassociated with a Norwegian scabies index case undergoing TNF-α inhibitor treatment: management and control. Infect Control Hosp Epidemiol. 2015;36:1358-1360. doi:10.1017/ice.2015.188
- Nofal A. Variable response of crusted scabies to oral ivermectin: report on eight Egyptian patients. J Eur Acad Dermatol Venereol. 2009;23:793-797. doi:10.1111/j.1468-3083.2009.03177.x
- Yee BE, Carlos CA, Hata T. Crusted scabies of the scalp in a patient with systemic lupus erythematosus. Dermatol Online J. 2014;20:13030/qt9dm891gd.
- Bumb RA, Mehta RD. Crusted scabies in a patient of systemic sclerosis. Indian J Dermatol Venereol Leprol. 2000;66:143-144.
- Hylwa SA, Loss L, Grassi M. Crusted scabies and tinea corporis after treatment of presumed bullous pemphigoid. Cutis. 2013;92:193-198.
- Svecova D, Chmurova N, Pallova A, et al. Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction. Epidemiol Mikrobiol Imunol. 2009;58:121-123.
- Dourmishev AL, Serafimova DK, Dourmishev LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234. doi:10.1046/j.1365-4362.1998.00330.x
- Mortazavi H, Abedini R, Sadri F, et al. Crusted scabies in a patient with brain astrocytoma: report of a case. Int J Infect Dis. 2010;14:E526-E527. doi:10.1016/j.ijid.2009.06.011
- Lima FCDR, Cerqueira AMM, MBS, et al. Crusted scabies due to indiscriminate use of glucocorticoid therapy in infant. An Bras Dermatol. 2017;92:383-385. doi:10.1590/abd1806-4841.20174433
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2010;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: effective in crusted scabies induced by corticosteroid but ineffective in nail scabies. J Dermatol. 2003;30:411-416. doi:10.1111/j.1346-8138.2003.tb00408.x
- Bilan P, Colin-Gorski AM, Chapelon E, et al. Crusted scabies induced by topical corticosteroids: a case report [in French]. Arch Pediatr. 2015;22:1292-1294. doi:10.1016/j.arcped.2015.09.004
- Marlière V, Roul S, C, et al. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J Pediatr. 1999;135:122-124. doi:10.1016/s0022-3476(99)70342-2
- Jaramillo-Ayerbe F, J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol. 1998;134:143-145. doi:10.1001/archderm.134.2.143
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41-47. doi:10.1126/science.2649979
Scabies is caused by cutaneous ectoparasitic infection by the mite Sarcoptes scabiei var hominis. The infection is highly contagious via direct skin-to-skin contact or indirectly through infested bedding, clothing or fomites.1,2 Scabies occurs at all ages, in all ethnic groups, and at all socioeconomic levels.1 Analysis by the Global Burden of Disease estimates that 200 million individuals have been infected with scabies worldwide. The World Health Organization has declared scabies a neglected tropical disease.3
Crusted scabies is a severe and rare form of scabies, with hyperinfestation of thousands to millions of mites, and more commonly is associated with immunosuppressed states, including HIV and hematologic malignancies.1,2,4 Crusted scabies has a high mortality rate due to sepsis when left untreated.3,5
Occasionally, iatrogenic immunosuppression contributes to the development of crusted scabies.1,2 Iatrogenic immunosuppression leading to crusted scabies most commonly occurs secondary to immunosuppression after bone marrow or solid organ transplantation.6 Less often, crusted scabies is caused by iatrogenic immunosuppression from other clinical scenarios.1,2
We describe a patient with iatrogenic immunosuppression due to azathioprine-induced myelosuppression for the treatment of granulomatosis with polyangiitis (GPA) who developed crusted scabies that clinically presented as erythroderma. Crusted scabies should be included in the differential diagnosis of erythroderma, especially in the setting of iatrogenic immunosuppression, for timely and appropriate management.
Case Report
An 84-year-old man presented with worsening pruritus, erythema, and thick yellow scale that progressed to erythroderma over the last 2 weeks. He was diagnosed with GPA 6 months prior to presentation and was treated with azathioprine 150 mg/d, prednisone 10 mg/d, and sulfamethoxazole 800 mg plus trimethoprim 160 mg twice weekly for prophylaxis against Pneumocystis jirovecii pneumonia.
Three weeks prior to presentation, the patient was hospitalized for pancytopenia attributed to azathioprine-induced myelosuppression (hemoglobin, 6.1 g/dL [reference range, 13.5–18.0 g/dL]; hematocrit, 17.5% [reference range, 42%–52%]; white blood cell count, 1.66×103/μL [reference range, 4.0–10.5×103/μL]; platelet count, 146×103/μL [reference range, 150–450×103/μL]; absolute neutrophil count, 1.29×103/μL [reference range, 1.4–6.5×103/μL]). He was transferred to a skilled nursing facility after discharge and referred to dermatology for evaluation of the worsening pruritic rash.

At the current presentation, the patient denied close contact with anyone who had a similar rash at home or at the skilled nursing facility. Physical examination revealed diffuse erythroderma with yellow scale on the scalp, trunk, arms, and legs (Figure 1). The palms showed scattered 2- to 3-mm pustules. The mucosal surfaces did not have lesions. A punch biopsy of a pustule from the right arm revealed focal spongiosis, parakeratosis, and acanthosis, as well as a perivascular and interstitial mixed inflammatory infiltrate with lymphocytes and eosinophils. Organisms morphologically compatible with scabies were found in the stratum corneum (Figure 2). Another punch biopsy of a pustule from the right arm was performed for direct immunofluorescence (DIF) and was negative for immunoglobulin deposition. Mineral oil preparation from pustules on the palm was positive for mites.

The patient was treated with permethrin cream 5% and oral ivermectin 200 μg/kg on day 1 and day 10. The prednisone dosage was increased from 10 mg/d to 50 mg/d and tapered over 2 weeks to treat the symptomatic rash and GPA. He remains on maintenance rituximab for GPA, without recurrence of scabies.
Comment
Pathogenesis—As an obligate parasite, S scabiei spends its entire life cycle within the host. Impregnated female mites burrow into the epidermis after mating and lay eggs daily for 1 to 2 months. Eggs hatch 2 or 3 days later. Larvae then migrate to the skin surface; burrow into the stratum corneum, where they mature into adults; and then mate on the skin surface.1,4
Clinical Presentation and Sequelae—Typically, scabies presents 2 to 6 weeks after initial exposure with generalized and intense itching and inflammatory pruritic papules on the finger webs, wrists, elbows, axillae, buttocks, umbilicus, genitalia, and areolae.1 Burrows are specific for scabies but may not always be present. Often, there are nonspecific secondary lesions, including excoriations, dermatitis, and impetiginization.
Complications of scabies can be severe, with initial colonization and infection of the skin resulting in impetigo and cellulitis. Systematic sequelae from local skin infection include post-streptococcal glomerulonephritis, rheumatic fever, and sepsis. Mortality from sepsis in scabies can be high.3,5
Classic Crusted Scabies and Other Variants—Crusted scabies presents with psoriasiform hyperkeratotic plaques involving the hands and feet with potential nail involvement that can become more generalized.1 Alterations in CD4+ T-cell function have been implicated in the development of crusted scabies, in which an excessive helper T cell (TH2) response is elicited against the ectoparasite, which may help explain the intense pruritus of scabies.6 Occasionally, iatrogenic immunosuppression contributes to development of crusted scabies,1 as was the case with our patient. However, it is rare for crusted scabies to present with erythroderma.7
Other atypical presentations of scabies include a seborrheic dermatitis–like presentation in infants, nodular lesions in the groin and axillae in more chronic scabies, and vesicles or bullous lesions.1
Diagnosis—Identification of mites, eggs, or feces is necessary for definitive diagnosis of scabies.8 These materials can be obtained through skin scrapings with mineral oil and observed under light microscopy or direct dermoscopy. Multiple scrapings on many lesions should be performed because failure to identify mites can be common and does not rule out scabies. Dermoscopic examination of active lesions under low power also can be helpful, given that identification of dark brown triangular structures can correspond to visualization of the pigmented anterior section of the mite.9-11 A skin biopsy can help identify mites, but histopathology often shows a nonspecific hypersensitivity reaction.12 Therefore, empiric treatment often is necessary.
Differential Diagnosis—The differential diagnosis of erythroderma is broad and includes a drug eruption; Sézary syndrome; and pre-existing skin diseases, including psoriasis, atopic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, and bullous pemphigoid. Histopathology is critical to differentiate these diagnoses. Bullous pemphigoid and pemphigus foliaceus are immunobullous diseases that typically are positive for immunoglobulin deposition on DIF. In rare cases, scabies also can present with bullae and positive DIF test results.13
Treatment—First-line treatment of crusted scabies in the United States is permethrin cream 5%, followed by oral ivermectin 200 μg/kg.4,5,14,15 Other scabicides include topicals such as benzyl benzoate 10% to 25%; precipitated sulfur 2% to 10%; crotamiton 10%; malathion 0.5%; and lindane 1%.5 The association of neurotoxicity with lindane has considerably reduced the drug’s use.1
During treatment of scabies, it is important to isolate patients to mitigate the possibility of spread.4 Pruritus can persist for a few weeks after completion of therapy.5 Patients should be closely monitored to ensure that this symptom is secondary to skin inflammation and not incomplete treatment.
Treatment of crusted scabies may require repeated treatments to decrease the notable mite burden as well as the associated crusting and scale. Adding a keratolytic such as 5% to 10% salicylic acid in petrolatum to the treatment regimen may be useful for breaking up thick scale.5
Immunosuppression—With numerous immunomodulatory drugs for treating autoimmunity comes an increased risk for iatrogenic immunosuppression that may contribute to the development of crusted scabies.16 In a number of autoimmune diseases such as rheumatoid arthritis,17-19 psoriasis,20,21 pemphigus vulgaris,22 systemic lupus erythematosus,23 systemic sclerosis,22,24 bullous pemphigoid,25,26 and dermatomyositis,27 patients have developed crusted scabies secondary to treatment-related immunosuppression. These immunosuppressive therapies include systemic steroids,22-24,26-31 methotrexate,23 infliximab,18 adalimumab,21 toclizumab,19 and etanercept.20 In a case of drug-induced Stevens-Johnson syndrome, the patient developed crusted scabies during long-term use of oral steroids.22
Patients with a malignancy who are being treated with chemotherapy also can develop crusted scabies.28 Crusted scabies has even been associated with long-term topical steroid32-34 and topical calcineurin inhibitor use.16
Iatrogenic immunosuppression in our patient resulted from treatment of GPA with azathioprine, an immunosuppressive drug that acts as an antagonist of the breakdown of purines, leading to inhibition of DNA, RNA, and protein synthesis.35 On occasion, azathioprine can induce immunosuppression in the form of myelosuppression and resulting pancytopenia, as was the case with our patient.
Conclusion
Although scabies is designated as a neglected tropical disease by the World Health Organization, it still causes a notable burden worldwide, regardless of the economics. Our case highlights an unusual presentation of scabies as erythroderma in the setting of iatrogenic immunosuppression from azathioprine use. Dermatologists should consider crusted scabies in the differential diagnosis of erythroderma, especially in immunocompromised patients, to avoid delays in diagnosis and treatment. Immunosuppressive therapy is an important mainstay in the treatment of many conditions, but it is important to consider that these medications can place patients at an increased risk for rare opportunistic infections. Therefore, patients receiving such treatment should be closely monitored.
Scabies is caused by cutaneous ectoparasitic infection by the mite Sarcoptes scabiei var hominis. The infection is highly contagious via direct skin-to-skin contact or indirectly through infested bedding, clothing or fomites.1,2 Scabies occurs at all ages, in all ethnic groups, and at all socioeconomic levels.1 Analysis by the Global Burden of Disease estimates that 200 million individuals have been infected with scabies worldwide. The World Health Organization has declared scabies a neglected tropical disease.3
Crusted scabies is a severe and rare form of scabies, with hyperinfestation of thousands to millions of mites, and more commonly is associated with immunosuppressed states, including HIV and hematologic malignancies.1,2,4 Crusted scabies has a high mortality rate due to sepsis when left untreated.3,5
Occasionally, iatrogenic immunosuppression contributes to the development of crusted scabies.1,2 Iatrogenic immunosuppression leading to crusted scabies most commonly occurs secondary to immunosuppression after bone marrow or solid organ transplantation.6 Less often, crusted scabies is caused by iatrogenic immunosuppression from other clinical scenarios.1,2
We describe a patient with iatrogenic immunosuppression due to azathioprine-induced myelosuppression for the treatment of granulomatosis with polyangiitis (GPA) who developed crusted scabies that clinically presented as erythroderma. Crusted scabies should be included in the differential diagnosis of erythroderma, especially in the setting of iatrogenic immunosuppression, for timely and appropriate management.
Case Report
An 84-year-old man presented with worsening pruritus, erythema, and thick yellow scale that progressed to erythroderma over the last 2 weeks. He was diagnosed with GPA 6 months prior to presentation and was treated with azathioprine 150 mg/d, prednisone 10 mg/d, and sulfamethoxazole 800 mg plus trimethoprim 160 mg twice weekly for prophylaxis against Pneumocystis jirovecii pneumonia.
Three weeks prior to presentation, the patient was hospitalized for pancytopenia attributed to azathioprine-induced myelosuppression (hemoglobin, 6.1 g/dL [reference range, 13.5–18.0 g/dL]; hematocrit, 17.5% [reference range, 42%–52%]; white blood cell count, 1.66×103/μL [reference range, 4.0–10.5×103/μL]; platelet count, 146×103/μL [reference range, 150–450×103/μL]; absolute neutrophil count, 1.29×103/μL [reference range, 1.4–6.5×103/μL]). He was transferred to a skilled nursing facility after discharge and referred to dermatology for evaluation of the worsening pruritic rash.

At the current presentation, the patient denied close contact with anyone who had a similar rash at home or at the skilled nursing facility. Physical examination revealed diffuse erythroderma with yellow scale on the scalp, trunk, arms, and legs (Figure 1). The palms showed scattered 2- to 3-mm pustules. The mucosal surfaces did not have lesions. A punch biopsy of a pustule from the right arm revealed focal spongiosis, parakeratosis, and acanthosis, as well as a perivascular and interstitial mixed inflammatory infiltrate with lymphocytes and eosinophils. Organisms morphologically compatible with scabies were found in the stratum corneum (Figure 2). Another punch biopsy of a pustule from the right arm was performed for direct immunofluorescence (DIF) and was negative for immunoglobulin deposition. Mineral oil preparation from pustules on the palm was positive for mites.

The patient was treated with permethrin cream 5% and oral ivermectin 200 μg/kg on day 1 and day 10. The prednisone dosage was increased from 10 mg/d to 50 mg/d and tapered over 2 weeks to treat the symptomatic rash and GPA. He remains on maintenance rituximab for GPA, without recurrence of scabies.
Comment
Pathogenesis—As an obligate parasite, S scabiei spends its entire life cycle within the host. Impregnated female mites burrow into the epidermis after mating and lay eggs daily for 1 to 2 months. Eggs hatch 2 or 3 days later. Larvae then migrate to the skin surface; burrow into the stratum corneum, where they mature into adults; and then mate on the skin surface.1,4
Clinical Presentation and Sequelae—Typically, scabies presents 2 to 6 weeks after initial exposure with generalized and intense itching and inflammatory pruritic papules on the finger webs, wrists, elbows, axillae, buttocks, umbilicus, genitalia, and areolae.1 Burrows are specific for scabies but may not always be present. Often, there are nonspecific secondary lesions, including excoriations, dermatitis, and impetiginization.
Complications of scabies can be severe, with initial colonization and infection of the skin resulting in impetigo and cellulitis. Systematic sequelae from local skin infection include post-streptococcal glomerulonephritis, rheumatic fever, and sepsis. Mortality from sepsis in scabies can be high.3,5
Classic Crusted Scabies and Other Variants—Crusted scabies presents with psoriasiform hyperkeratotic plaques involving the hands and feet with potential nail involvement that can become more generalized.1 Alterations in CD4+ T-cell function have been implicated in the development of crusted scabies, in which an excessive helper T cell (TH2) response is elicited against the ectoparasite, which may help explain the intense pruritus of scabies.6 Occasionally, iatrogenic immunosuppression contributes to development of crusted scabies,1 as was the case with our patient. However, it is rare for crusted scabies to present with erythroderma.7
Other atypical presentations of scabies include a seborrheic dermatitis–like presentation in infants, nodular lesions in the groin and axillae in more chronic scabies, and vesicles or bullous lesions.1
Diagnosis—Identification of mites, eggs, or feces is necessary for definitive diagnosis of scabies.8 These materials can be obtained through skin scrapings with mineral oil and observed under light microscopy or direct dermoscopy. Multiple scrapings on many lesions should be performed because failure to identify mites can be common and does not rule out scabies. Dermoscopic examination of active lesions under low power also can be helpful, given that identification of dark brown triangular structures can correspond to visualization of the pigmented anterior section of the mite.9-11 A skin biopsy can help identify mites, but histopathology often shows a nonspecific hypersensitivity reaction.12 Therefore, empiric treatment often is necessary.
Differential Diagnosis—The differential diagnosis of erythroderma is broad and includes a drug eruption; Sézary syndrome; and pre-existing skin diseases, including psoriasis, atopic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, and bullous pemphigoid. Histopathology is critical to differentiate these diagnoses. Bullous pemphigoid and pemphigus foliaceus are immunobullous diseases that typically are positive for immunoglobulin deposition on DIF. In rare cases, scabies also can present with bullae and positive DIF test results.13
Treatment—First-line treatment of crusted scabies in the United States is permethrin cream 5%, followed by oral ivermectin 200 μg/kg.4,5,14,15 Other scabicides include topicals such as benzyl benzoate 10% to 25%; precipitated sulfur 2% to 10%; crotamiton 10%; malathion 0.5%; and lindane 1%.5 The association of neurotoxicity with lindane has considerably reduced the drug’s use.1
During treatment of scabies, it is important to isolate patients to mitigate the possibility of spread.4 Pruritus can persist for a few weeks after completion of therapy.5 Patients should be closely monitored to ensure that this symptom is secondary to skin inflammation and not incomplete treatment.
Treatment of crusted scabies may require repeated treatments to decrease the notable mite burden as well as the associated crusting and scale. Adding a keratolytic such as 5% to 10% salicylic acid in petrolatum to the treatment regimen may be useful for breaking up thick scale.5
Immunosuppression—With numerous immunomodulatory drugs for treating autoimmunity comes an increased risk for iatrogenic immunosuppression that may contribute to the development of crusted scabies.16 In a number of autoimmune diseases such as rheumatoid arthritis,17-19 psoriasis,20,21 pemphigus vulgaris,22 systemic lupus erythematosus,23 systemic sclerosis,22,24 bullous pemphigoid,25,26 and dermatomyositis,27 patients have developed crusted scabies secondary to treatment-related immunosuppression. These immunosuppressive therapies include systemic steroids,22-24,26-31 methotrexate,23 infliximab,18 adalimumab,21 toclizumab,19 and etanercept.20 In a case of drug-induced Stevens-Johnson syndrome, the patient developed crusted scabies during long-term use of oral steroids.22
Patients with a malignancy who are being treated with chemotherapy also can develop crusted scabies.28 Crusted scabies has even been associated with long-term topical steroid32-34 and topical calcineurin inhibitor use.16
Iatrogenic immunosuppression in our patient resulted from treatment of GPA with azathioprine, an immunosuppressive drug that acts as an antagonist of the breakdown of purines, leading to inhibition of DNA, RNA, and protein synthesis.35 On occasion, azathioprine can induce immunosuppression in the form of myelosuppression and resulting pancytopenia, as was the case with our patient.
Conclusion
Although scabies is designated as a neglected tropical disease by the World Health Organization, it still causes a notable burden worldwide, regardless of the economics. Our case highlights an unusual presentation of scabies as erythroderma in the setting of iatrogenic immunosuppression from azathioprine use. Dermatologists should consider crusted scabies in the differential diagnosis of erythroderma, especially in immunocompromised patients, to avoid delays in diagnosis and treatment. Immunosuppressive therapy is an important mainstay in the treatment of many conditions, but it is important to consider that these medications can place patients at an increased risk for rare opportunistic infections. Therefore, patients receiving such treatment should be closely monitored.
- Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354:1718-1727. doi:10.1056/NEJMcp052784
- Salgado F, Elston DM. What’s eating you? scabies in the developing world. Cutis. 2017;100:287-289.
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Wang X-D, Shen H, Liu Z-H. Contagious erythroderma. J Emerg Med. 2016;51:180-181. doi:10.1016/j.jemermed.2016.05.027
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146. doi:10.1016/j.jaad.2010.03.010
- Bollea Garlatti LA, Torre AC, Bollea Garlatti ML, et al.. Dermoscopy aids the diagnosis of crusted scabies in an erythrodermic patient. J Am Acad Dermatol. 2015;73:E93-E95. doi:10.1016/j.jaad.2015.04.061
- Tang J, You Z, Ran Y. Simple methods to enhance the diagnosis of scabies. J Am Acad Dermatol. 2019;80:E99-E100. doi:10.1016/j.jaad.2017.07.038
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20:600-605. doi:10.1111/j.1365-4362.1981.tb00844.x
- Shahab RKA, Loo DS. Bullous scabies. J Am Acad Dermatol. 2003;49:346-350. doi:10.1067/s0190-9622(03)00876-4
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007:CD000320. doi:10.1002/14651858.CD000320.pub2
- Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane Review. JAMA Dermatol. 2019;155:730-732. doi:10.1001/jamadermatol.2019.0279
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol. 2006;23:299-300. doi:10.1111/j.1525-1470.2006.00241.x
- Bu X, Fan J, Hu X, et al. Norwegian scabies in a patient treated with Tripterygium glycoside for rheumatoid arthritis. An Bras Dermatol. 2017;92:556-558. doi:10.1590/abd1806-4841.20174946
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol. 2005;52:719-720. doi:10.1016/j.jaad.2004.12.039
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-404. doi:10.1016/j.jbspin.2011.02.008
- Saillard C, Darrieux L, Safa G. Crusted scabies complicates etanercept therapy in a patient with severe psoriasis. J Am Acad Dermatol. 2013;68:E138-E139. doi:10.1016/j.jaad.2012.09.049
- Belvisi V, Orsi GB, Del Borgo C, et al. Large nosocomial outbreakassociated with a Norwegian scabies index case undergoing TNF-α inhibitor treatment: management and control. Infect Control Hosp Epidemiol. 2015;36:1358-1360. doi:10.1017/ice.2015.188
- Nofal A. Variable response of crusted scabies to oral ivermectin: report on eight Egyptian patients. J Eur Acad Dermatol Venereol. 2009;23:793-797. doi:10.1111/j.1468-3083.2009.03177.x
- Yee BE, Carlos CA, Hata T. Crusted scabies of the scalp in a patient with systemic lupus erythematosus. Dermatol Online J. 2014;20:13030/qt9dm891gd.
- Bumb RA, Mehta RD. Crusted scabies in a patient of systemic sclerosis. Indian J Dermatol Venereol Leprol. 2000;66:143-144.
- Hylwa SA, Loss L, Grassi M. Crusted scabies and tinea corporis after treatment of presumed bullous pemphigoid. Cutis. 2013;92:193-198.
- Svecova D, Chmurova N, Pallova A, et al. Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction. Epidemiol Mikrobiol Imunol. 2009;58:121-123.
- Dourmishev AL, Serafimova DK, Dourmishev LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234. doi:10.1046/j.1365-4362.1998.00330.x
- Mortazavi H, Abedini R, Sadri F, et al. Crusted scabies in a patient with brain astrocytoma: report of a case. Int J Infect Dis. 2010;14:E526-E527. doi:10.1016/j.ijid.2009.06.011
- Lima FCDR, Cerqueira AMM, MBS, et al. Crusted scabies due to indiscriminate use of glucocorticoid therapy in infant. An Bras Dermatol. 2017;92:383-385. doi:10.1590/abd1806-4841.20174433
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2010;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: effective in crusted scabies induced by corticosteroid but ineffective in nail scabies. J Dermatol. 2003;30:411-416. doi:10.1111/j.1346-8138.2003.tb00408.x
- Bilan P, Colin-Gorski AM, Chapelon E, et al. Crusted scabies induced by topical corticosteroids: a case report [in French]. Arch Pediatr. 2015;22:1292-1294. doi:10.1016/j.arcped.2015.09.004
- Marlière V, Roul S, C, et al. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J Pediatr. 1999;135:122-124. doi:10.1016/s0022-3476(99)70342-2
- Jaramillo-Ayerbe F, J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol. 1998;134:143-145. doi:10.1001/archderm.134.2.143
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41-47. doi:10.1126/science.2649979
- Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354:1718-1727. doi:10.1056/NEJMcp052784
- Salgado F, Elston DM. What’s eating you? scabies in the developing world. Cutis. 2017;100:287-289.
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Wang X-D, Shen H, Liu Z-H. Contagious erythroderma. J Emerg Med. 2016;51:180-181. doi:10.1016/j.jemermed.2016.05.027
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146. doi:10.1016/j.jaad.2010.03.010
- Bollea Garlatti LA, Torre AC, Bollea Garlatti ML, et al.. Dermoscopy aids the diagnosis of crusted scabies in an erythrodermic patient. J Am Acad Dermatol. 2015;73:E93-E95. doi:10.1016/j.jaad.2015.04.061
- Tang J, You Z, Ran Y. Simple methods to enhance the diagnosis of scabies. J Am Acad Dermatol. 2019;80:E99-E100. doi:10.1016/j.jaad.2017.07.038
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20:600-605. doi:10.1111/j.1365-4362.1981.tb00844.x
- Shahab RKA, Loo DS. Bullous scabies. J Am Acad Dermatol. 2003;49:346-350. doi:10.1067/s0190-9622(03)00876-4
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007:CD000320. doi:10.1002/14651858.CD000320.pub2
- Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane Review. JAMA Dermatol. 2019;155:730-732. doi:10.1001/jamadermatol.2019.0279
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol. 2006;23:299-300. doi:10.1111/j.1525-1470.2006.00241.x
- Bu X, Fan J, Hu X, et al. Norwegian scabies in a patient treated with Tripterygium glycoside for rheumatoid arthritis. An Bras Dermatol. 2017;92:556-558. doi:10.1590/abd1806-4841.20174946
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol. 2005;52:719-720. doi:10.1016/j.jaad.2004.12.039
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-404. doi:10.1016/j.jbspin.2011.02.008
- Saillard C, Darrieux L, Safa G. Crusted scabies complicates etanercept therapy in a patient with severe psoriasis. J Am Acad Dermatol. 2013;68:E138-E139. doi:10.1016/j.jaad.2012.09.049
- Belvisi V, Orsi GB, Del Borgo C, et al. Large nosocomial outbreakassociated with a Norwegian scabies index case undergoing TNF-α inhibitor treatment: management and control. Infect Control Hosp Epidemiol. 2015;36:1358-1360. doi:10.1017/ice.2015.188
- Nofal A. Variable response of crusted scabies to oral ivermectin: report on eight Egyptian patients. J Eur Acad Dermatol Venereol. 2009;23:793-797. doi:10.1111/j.1468-3083.2009.03177.x
- Yee BE, Carlos CA, Hata T. Crusted scabies of the scalp in a patient with systemic lupus erythematosus. Dermatol Online J. 2014;20:13030/qt9dm891gd.
- Bumb RA, Mehta RD. Crusted scabies in a patient of systemic sclerosis. Indian J Dermatol Venereol Leprol. 2000;66:143-144.
- Hylwa SA, Loss L, Grassi M. Crusted scabies and tinea corporis after treatment of presumed bullous pemphigoid. Cutis. 2013;92:193-198.
- Svecova D, Chmurova N, Pallova A, et al. Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction. Epidemiol Mikrobiol Imunol. 2009;58:121-123.
- Dourmishev AL, Serafimova DK, Dourmishev LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234. doi:10.1046/j.1365-4362.1998.00330.x
- Mortazavi H, Abedini R, Sadri F, et al. Crusted scabies in a patient with brain astrocytoma: report of a case. Int J Infect Dis. 2010;14:E526-E527. doi:10.1016/j.ijid.2009.06.011
- Lima FCDR, Cerqueira AMM, MBS, et al. Crusted scabies due to indiscriminate use of glucocorticoid therapy in infant. An Bras Dermatol. 2017;92:383-385. doi:10.1590/abd1806-4841.20174433
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2010;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: effective in crusted scabies induced by corticosteroid but ineffective in nail scabies. J Dermatol. 2003;30:411-416. doi:10.1111/j.1346-8138.2003.tb00408.x
- Bilan P, Colin-Gorski AM, Chapelon E, et al. Crusted scabies induced by topical corticosteroids: a case report [in French]. Arch Pediatr. 2015;22:1292-1294. doi:10.1016/j.arcped.2015.09.004
- Marlière V, Roul S, C, et al. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J Pediatr. 1999;135:122-124. doi:10.1016/s0022-3476(99)70342-2
- Jaramillo-Ayerbe F, J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol. 1998;134:143-145. doi:10.1001/archderm.134.2.143
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41-47. doi:10.1126/science.2649979
Practice Points
- Crusted scabies is a highly contagious, severe cutaneous ectoparasitic infection that can present atypically in the form of erythroderma.
- Immunomodulatory drugs for the treatment of autoimmune disease can predispose patients to infection, including ectoparasitic infection.
- Dermatologists should be familiar with the full scope of the clinical presentations of scabies and should especially consider this condition in the differential diagnosis of patients who present in an immunosuppressed state.
Primary Effusion Lymphoma: An Infiltrative Plaque in a Patient With HIV
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.
At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.
Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
Practice Points
- Extracavitary primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that occurs solely in the presence of human herpesvirus 8 infection and typically is associated with HIV/AIDS.
- Diagnosis necessitates a thorough workup and correlation of histologic, molecular, and immunophenotypic analysis.
- Antiretroviral therapy in HIV-positive patients and intensive chemotherapy regimens are the current recommended treatments. Despite newer targeted agents, the prognosis remains poor.
Pruritic Photosensitive Rash
The Diagnosis: Actinic Prurigo
Actinic prurigo is an idiopathic photodermatosis triggered by UV exposure that primarily affects sun-exposed areas of the skin.1,2 It typically presents as pruritic papules, plaques, and nodules, with most patients also experiencing oral tingling and pain.3 In more severe cases, it can progress to include conjunctival disease, scarring, and cheilitis.1 A study of ocular findings among children with actinic pruritus reported that photophobia was one of the most important features,4 which was present in our patient. The face, especially over the zygomatic arches, nasal bridge, and lower lip, commonly is affected.1 Secondary lichenification or eczematization may occur.5 In our patient, the combination of conjunctivitis, cheilitis, and an eruption on sun-exposed skin were crucial in making the diagnosis.
Most cases present in patients younger than 10 years. It most commonly is seen in American Indians in North America, Central America, and South America.2 After the diagnosis was considered in our patient, the family was asked about their ancestry and confirmed that both of the patient’s maternal and paternal grandparents were of American Indian descent. There also is a strong genetic component; the HLA-DR4 allele variant is present in 90% of cases, especially DRB1*0407, which is seen in 60% of cases.1,6 In our patient, testing revealed HLA-DR4, DRB1*04 positivity. We further hypothesized that his mother’s photosensitive rash may have been actinic prurigo as opposed to polymorphous light eruption, which could explain the lack of response to hydroxychloroquine.
The diagnosis of actinic prurigo usually is made clinically. A skin biopsy typically is not necessary but would show hyperkeratosis, spongiosis, and acanthosis with a lymphocytic perivascular infiltrate. Biopsies of the lip classically show lymphoid germinal centers in the lamina propria, which can help distinguish actinic prurigo from polymorphous light eruption.1
In our patient, the differential diagnosis included polymorphous light eruption, connective tissue disease such as lupus erythematosus or dermatomyositis, porphyria such as erythropoietic protoporphyria, and allergic contact dermatitis. Polymorphous light eruption was ruled out by the oral and ocular manifestations, which are not atypical for this diagnosis. The patient’s laboratory results displayed unremarkable antinuclear antibodies, creatine kinase, aldolase, and extractable nuclear antigens, which made connective tissue disease unlikely. Furthermore, a porphyria screen for total plasma porphyrins and whole blood protoporphyrin was negative, which helped rule out porphyria. Allergic contact dermatitis seemed less likely given the lack of improvement with topical steroids. Overall, the clinical presentation in a patient with relevant family ancestry and HLA-DR4 positivity supported a diagnosis of actinic prurigo.7
To manage the condition in our patient, strict photoprotection was recommended as well as the application of triamcinolone ointment 0.025% to the affected areas twice daily until the skin symptoms improved. For acute flares, other treatment considerations include topical tacrolimus, oral antihistamines, and oral corticosteroids. Some success has been reported with cyclosporine and azathioprine. For severe disease, thalidomide is the recommended treatment; it is effective in pediatric patients at dosages of 50 to 100 mg daily, but the dose has not yet been standardized for this age group.8,9 Many adult patients initially are controlled with 100 to 200 mg daily, which can be tapered down to a dosage of 25 to 50 mg weekly with few adverse effects; however, the overall substantial side effects of thalidomide limit its use in both pediatric and adult populations.1,2 Newer studies have suggested promising results with dupilumab, especially when actinic prurigo presents with high IgE levels or eosinophils on histology.7,10 In our patient, the IgE level was normal.
- Pile HD, Crane JS. Actinic prurigo. StatPearls. StatPearls Publishing; 2022.
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii.
- Vega Memije ME, Cuevas Gonzalez JC, Hojyo-Tomoka MT, et al. Actinic prurigo as a hypersensitivity reaction type 4. Int J Dermatol. 2017;56:E135-E136.
- Magaña M, Mendez Y, Rodriguez A, et al. The conjunctivitis of solar (actinic) prurigo. Pediatr Dermatol. 2000;17:432-435.
- Ross G, Foley P, Baker C. Actinic prurigo. Photodermatol Photoimmunol Photomed. 2008;24:272-275.
- Rodríguez-Carreón AA, Rodríguez-Lobato E, Rodríguez-Gutiérrez G, et al. Actinic prurigo. Skinmed. 2015;13:287-295.
- Balwani M, Bloomer J, Desnick R; Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network. Erythropoietic protoporphyria, autosomal recessive. GeneReviews. University of Washington; 1993.
- Crouch RB, Foley PA, Ng JCH, et al. Thalidomide experience of a major Australian teaching hospital. Australas J Dermatol. 2002;43:278-284.
- Watts-Santos A, Martinez-Rico JC, Gomez-Flores M, et al. Thalidomide: an option for the pediatric patient with actinic prurigo. Pediatr Dermatol. 2020;37:362-365.
- Eickstaedt JB, Starke S, Krakora D, et al. Clearance of pediatric actinic prurigo with dupilumab. Pediatr Dermatol. 2020;37:1176-1178.
The Diagnosis: Actinic Prurigo
Actinic prurigo is an idiopathic photodermatosis triggered by UV exposure that primarily affects sun-exposed areas of the skin.1,2 It typically presents as pruritic papules, plaques, and nodules, with most patients also experiencing oral tingling and pain.3 In more severe cases, it can progress to include conjunctival disease, scarring, and cheilitis.1 A study of ocular findings among children with actinic pruritus reported that photophobia was one of the most important features,4 which was present in our patient. The face, especially over the zygomatic arches, nasal bridge, and lower lip, commonly is affected.1 Secondary lichenification or eczematization may occur.5 In our patient, the combination of conjunctivitis, cheilitis, and an eruption on sun-exposed skin were crucial in making the diagnosis.
Most cases present in patients younger than 10 years. It most commonly is seen in American Indians in North America, Central America, and South America.2 After the diagnosis was considered in our patient, the family was asked about their ancestry and confirmed that both of the patient’s maternal and paternal grandparents were of American Indian descent. There also is a strong genetic component; the HLA-DR4 allele variant is present in 90% of cases, especially DRB1*0407, which is seen in 60% of cases.1,6 In our patient, testing revealed HLA-DR4, DRB1*04 positivity. We further hypothesized that his mother’s photosensitive rash may have been actinic prurigo as opposed to polymorphous light eruption, which could explain the lack of response to hydroxychloroquine.
The diagnosis of actinic prurigo usually is made clinically. A skin biopsy typically is not necessary but would show hyperkeratosis, spongiosis, and acanthosis with a lymphocytic perivascular infiltrate. Biopsies of the lip classically show lymphoid germinal centers in the lamina propria, which can help distinguish actinic prurigo from polymorphous light eruption.1
In our patient, the differential diagnosis included polymorphous light eruption, connective tissue disease such as lupus erythematosus or dermatomyositis, porphyria such as erythropoietic protoporphyria, and allergic contact dermatitis. Polymorphous light eruption was ruled out by the oral and ocular manifestations, which are not atypical for this diagnosis. The patient’s laboratory results displayed unremarkable antinuclear antibodies, creatine kinase, aldolase, and extractable nuclear antigens, which made connective tissue disease unlikely. Furthermore, a porphyria screen for total plasma porphyrins and whole blood protoporphyrin was negative, which helped rule out porphyria. Allergic contact dermatitis seemed less likely given the lack of improvement with topical steroids. Overall, the clinical presentation in a patient with relevant family ancestry and HLA-DR4 positivity supported a diagnosis of actinic prurigo.7
To manage the condition in our patient, strict photoprotection was recommended as well as the application of triamcinolone ointment 0.025% to the affected areas twice daily until the skin symptoms improved. For acute flares, other treatment considerations include topical tacrolimus, oral antihistamines, and oral corticosteroids. Some success has been reported with cyclosporine and azathioprine. For severe disease, thalidomide is the recommended treatment; it is effective in pediatric patients at dosages of 50 to 100 mg daily, but the dose has not yet been standardized for this age group.8,9 Many adult patients initially are controlled with 100 to 200 mg daily, which can be tapered down to a dosage of 25 to 50 mg weekly with few adverse effects; however, the overall substantial side effects of thalidomide limit its use in both pediatric and adult populations.1,2 Newer studies have suggested promising results with dupilumab, especially when actinic prurigo presents with high IgE levels or eosinophils on histology.7,10 In our patient, the IgE level was normal.
The Diagnosis: Actinic Prurigo
Actinic prurigo is an idiopathic photodermatosis triggered by UV exposure that primarily affects sun-exposed areas of the skin.1,2 It typically presents as pruritic papules, plaques, and nodules, with most patients also experiencing oral tingling and pain.3 In more severe cases, it can progress to include conjunctival disease, scarring, and cheilitis.1 A study of ocular findings among children with actinic pruritus reported that photophobia was one of the most important features,4 which was present in our patient. The face, especially over the zygomatic arches, nasal bridge, and lower lip, commonly is affected.1 Secondary lichenification or eczematization may occur.5 In our patient, the combination of conjunctivitis, cheilitis, and an eruption on sun-exposed skin were crucial in making the diagnosis.
Most cases present in patients younger than 10 years. It most commonly is seen in American Indians in North America, Central America, and South America.2 After the diagnosis was considered in our patient, the family was asked about their ancestry and confirmed that both of the patient’s maternal and paternal grandparents were of American Indian descent. There also is a strong genetic component; the HLA-DR4 allele variant is present in 90% of cases, especially DRB1*0407, which is seen in 60% of cases.1,6 In our patient, testing revealed HLA-DR4, DRB1*04 positivity. We further hypothesized that his mother’s photosensitive rash may have been actinic prurigo as opposed to polymorphous light eruption, which could explain the lack of response to hydroxychloroquine.
The diagnosis of actinic prurigo usually is made clinically. A skin biopsy typically is not necessary but would show hyperkeratosis, spongiosis, and acanthosis with a lymphocytic perivascular infiltrate. Biopsies of the lip classically show lymphoid germinal centers in the lamina propria, which can help distinguish actinic prurigo from polymorphous light eruption.1
In our patient, the differential diagnosis included polymorphous light eruption, connective tissue disease such as lupus erythematosus or dermatomyositis, porphyria such as erythropoietic protoporphyria, and allergic contact dermatitis. Polymorphous light eruption was ruled out by the oral and ocular manifestations, which are not atypical for this diagnosis. The patient’s laboratory results displayed unremarkable antinuclear antibodies, creatine kinase, aldolase, and extractable nuclear antigens, which made connective tissue disease unlikely. Furthermore, a porphyria screen for total plasma porphyrins and whole blood protoporphyrin was negative, which helped rule out porphyria. Allergic contact dermatitis seemed less likely given the lack of improvement with topical steroids. Overall, the clinical presentation in a patient with relevant family ancestry and HLA-DR4 positivity supported a diagnosis of actinic prurigo.7
To manage the condition in our patient, strict photoprotection was recommended as well as the application of triamcinolone ointment 0.025% to the affected areas twice daily until the skin symptoms improved. For acute flares, other treatment considerations include topical tacrolimus, oral antihistamines, and oral corticosteroids. Some success has been reported with cyclosporine and azathioprine. For severe disease, thalidomide is the recommended treatment; it is effective in pediatric patients at dosages of 50 to 100 mg daily, but the dose has not yet been standardized for this age group.8,9 Many adult patients initially are controlled with 100 to 200 mg daily, which can be tapered down to a dosage of 25 to 50 mg weekly with few adverse effects; however, the overall substantial side effects of thalidomide limit its use in both pediatric and adult populations.1,2 Newer studies have suggested promising results with dupilumab, especially when actinic prurigo presents with high IgE levels or eosinophils on histology.7,10 In our patient, the IgE level was normal.
- Pile HD, Crane JS. Actinic prurigo. StatPearls. StatPearls Publishing; 2022.
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii.
- Vega Memije ME, Cuevas Gonzalez JC, Hojyo-Tomoka MT, et al. Actinic prurigo as a hypersensitivity reaction type 4. Int J Dermatol. 2017;56:E135-E136.
- Magaña M, Mendez Y, Rodriguez A, et al. The conjunctivitis of solar (actinic) prurigo. Pediatr Dermatol. 2000;17:432-435.
- Ross G, Foley P, Baker C. Actinic prurigo. Photodermatol Photoimmunol Photomed. 2008;24:272-275.
- Rodríguez-Carreón AA, Rodríguez-Lobato E, Rodríguez-Gutiérrez G, et al. Actinic prurigo. Skinmed. 2015;13:287-295.
- Balwani M, Bloomer J, Desnick R; Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network. Erythropoietic protoporphyria, autosomal recessive. GeneReviews. University of Washington; 1993.
- Crouch RB, Foley PA, Ng JCH, et al. Thalidomide experience of a major Australian teaching hospital. Australas J Dermatol. 2002;43:278-284.
- Watts-Santos A, Martinez-Rico JC, Gomez-Flores M, et al. Thalidomide: an option for the pediatric patient with actinic prurigo. Pediatr Dermatol. 2020;37:362-365.
- Eickstaedt JB, Starke S, Krakora D, et al. Clearance of pediatric actinic prurigo with dupilumab. Pediatr Dermatol. 2020;37:1176-1178.
- Pile HD, Crane JS. Actinic prurigo. StatPearls. StatPearls Publishing; 2022.
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii.
- Vega Memije ME, Cuevas Gonzalez JC, Hojyo-Tomoka MT, et al. Actinic prurigo as a hypersensitivity reaction type 4. Int J Dermatol. 2017;56:E135-E136.
- Magaña M, Mendez Y, Rodriguez A, et al. The conjunctivitis of solar (actinic) prurigo. Pediatr Dermatol. 2000;17:432-435.
- Ross G, Foley P, Baker C. Actinic prurigo. Photodermatol Photoimmunol Photomed. 2008;24:272-275.
- Rodríguez-Carreón AA, Rodríguez-Lobato E, Rodríguez-Gutiérrez G, et al. Actinic prurigo. Skinmed. 2015;13:287-295.
- Balwani M, Bloomer J, Desnick R; Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network. Erythropoietic protoporphyria, autosomal recessive. GeneReviews. University of Washington; 1993.
- Crouch RB, Foley PA, Ng JCH, et al. Thalidomide experience of a major Australian teaching hospital. Australas J Dermatol. 2002;43:278-284.
- Watts-Santos A, Martinez-Rico JC, Gomez-Flores M, et al. Thalidomide: an option for the pediatric patient with actinic prurigo. Pediatr Dermatol. 2020;37:362-365.
- Eickstaedt JB, Starke S, Krakora D, et al. Clearance of pediatric actinic prurigo with dupilumab. Pediatr Dermatol. 2020;37:1176-1178.
A 6-year-old boy presented via telemedicine for evaluation of a recurring rash that first presented on the face 9 months prior to presentation and waxed and waned throughout the fall and winter seasons for about 5 months. His mother noted that on a warm and sunny day 5 months after its first appearance, the patient was at a dog park and developed the rash on the face and hands—the only areas that had been exposed to the sun—later that evening. The patient reported pruritus but no associated burning or stinging. He was evaluated by an allergist 1 month later and was treated with oral cefazolin and hydrocortisone ointment 2.5% for suspected impetiginized dermatitis without improvement. The rash persisted until evaluation by our clinic 2 months later. Photographs showed erythematous scaly plaques and papules scattered on the cheeks, nose, upper and lower lips, and vermilion borders, as well as the dorsal aspect of the hands. He also had conjunctival erythema, which his mother reported was particularly worse in the summer months and associated with photophobia. His mother also noted increased tear production when in the sun. There was no mucosal involvement. The patient had no notable medical history and was not taking any medications. His mother had a history of polymorphous light eruption that recently was treated with hydroxychloroquine but without benefit.
Extracellular Matrix–Based Collagen Dressings for Scalp Repair Following Mohs Micrographic Surgery
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.
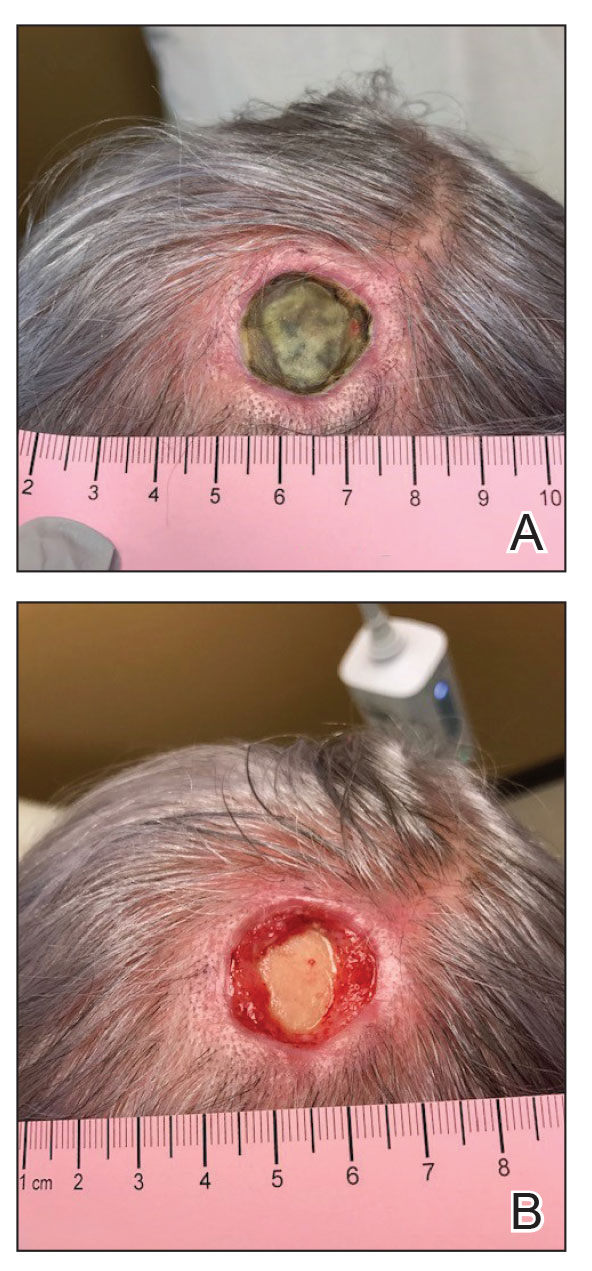
A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.

A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.

A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
Practice Points
- Patients who undergo Mohs micrographic surgery on the scalp are prone to developing complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.
- Use of extracellular matrix–based dressings may assist with deep wound healing on the scalp.
Nevus Sebaceus With Novel HRAS Sequence Variant Mutation Misdiagnosed as Alopecia Areata
To the Editor:
A 12-year-old girl presented to the dermatology clinic for evaluation of a congenital scalp lesion. The patient was diagnosed with alopecia areata by a dermatologist at 4 years of age, and she was treated with topical corticosteroids and minoxidil, which failed to resolve her condition. Physical examination revealed an 8×10-cm, well-demarcated, yellowish-pink plaque located over the vertex and right parietal scalp (Figure 1A), extending down to the right preauricular cheek (Figure 1B) in a linear configuration with blaschkoid features. The scalp plaque appeared bald and completely lacking in terminal hairs but contained numerous fine vellus hairs (Figure 1A). A 6-mm, oval-appearing, pigmented papule was present in the plaque, and a few smaller, scattered, pigmented papules were noted in the vertex region (Figure 1A).
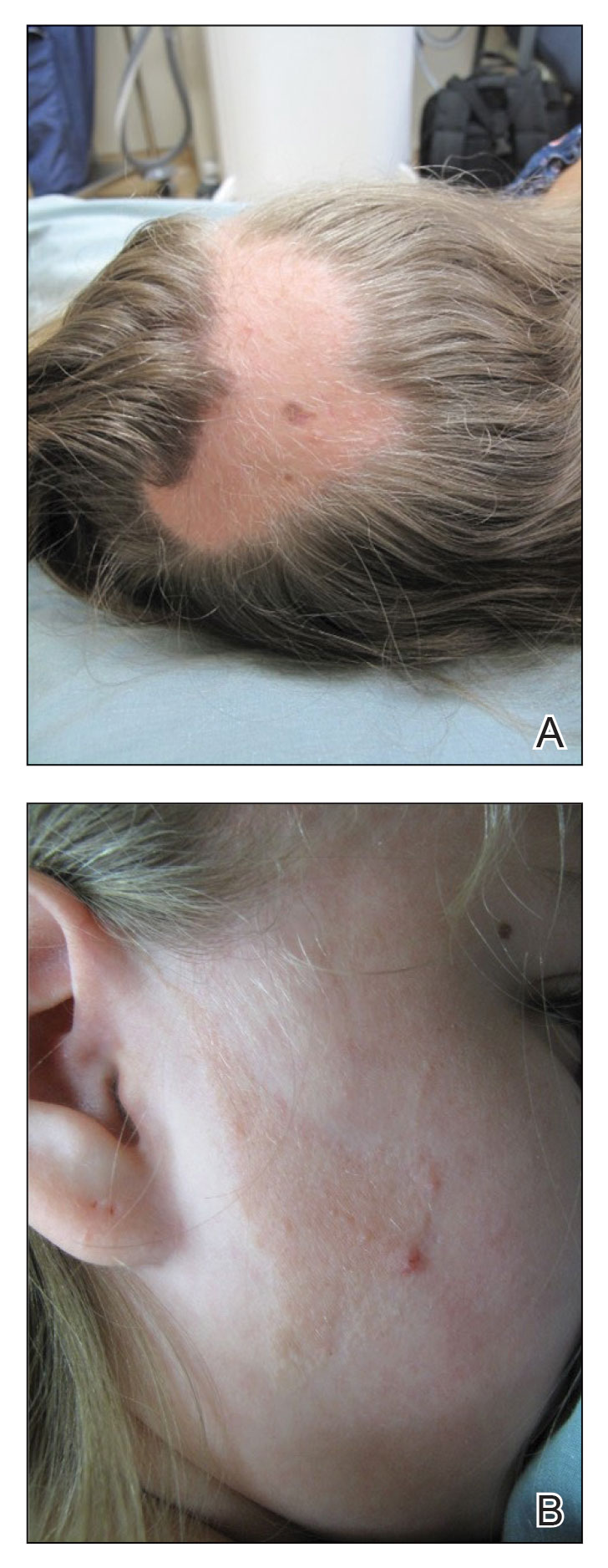
The cutaneous examination was otherwise unremarkable. A review of systems was negative, except for a history of attention-deficit/hyperactivity disorder. There was no history of seizures or other neurocognitive developmental abnormalities.
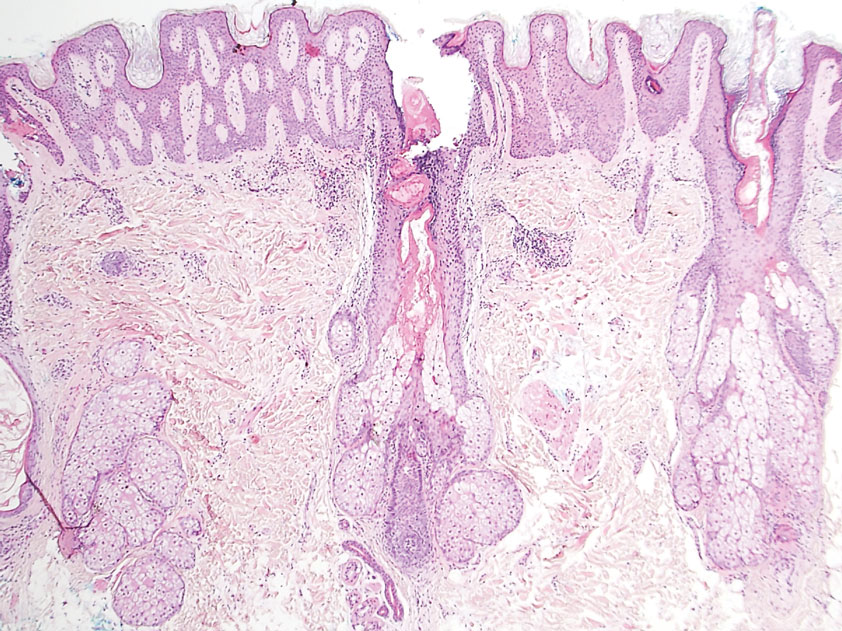
A 4-mm punch biopsy of the vertex scalp included the pigmented lesion but excluded an adnexal neoplasm. Epidermal acanthosis and mild papillomatosis were reported on microscopic examination. Multiple prominent sebaceous glands without associated hair follicles, which emptied directly onto the epidermal surface, were noted in the dermis (Figure 2). Several apocrine glands were observed (Figure 3). Epidermal and dermal melanocytic nests were highlighted with SOX-10 and Melan-A immunohistochemical stains, confirming the presence of a benign compound nevus. The punch biopsy analysis confirmed the diagnosis of a nevus sebaceus (NS) of Jadassohn (organoid nevus) with incidental compound nevus. Additional 4-mm punch biopsies were obtained for genetic testing, performed by the Genomics and Pathology Services at Washington University (St. Louis, Missouri). A missense HRAS p.G12V variant was observed in the tissue. A negative blood test result ruled out a germline mutation. The patient was managed with active observation of the lesion to evaluate for potential formation of neoplasms, as well as continuity of care with the dermatology clinic, considering the extent of the lesions, to monitor the development of any new medical conditions that would be concerning for syndromes associated with NS.
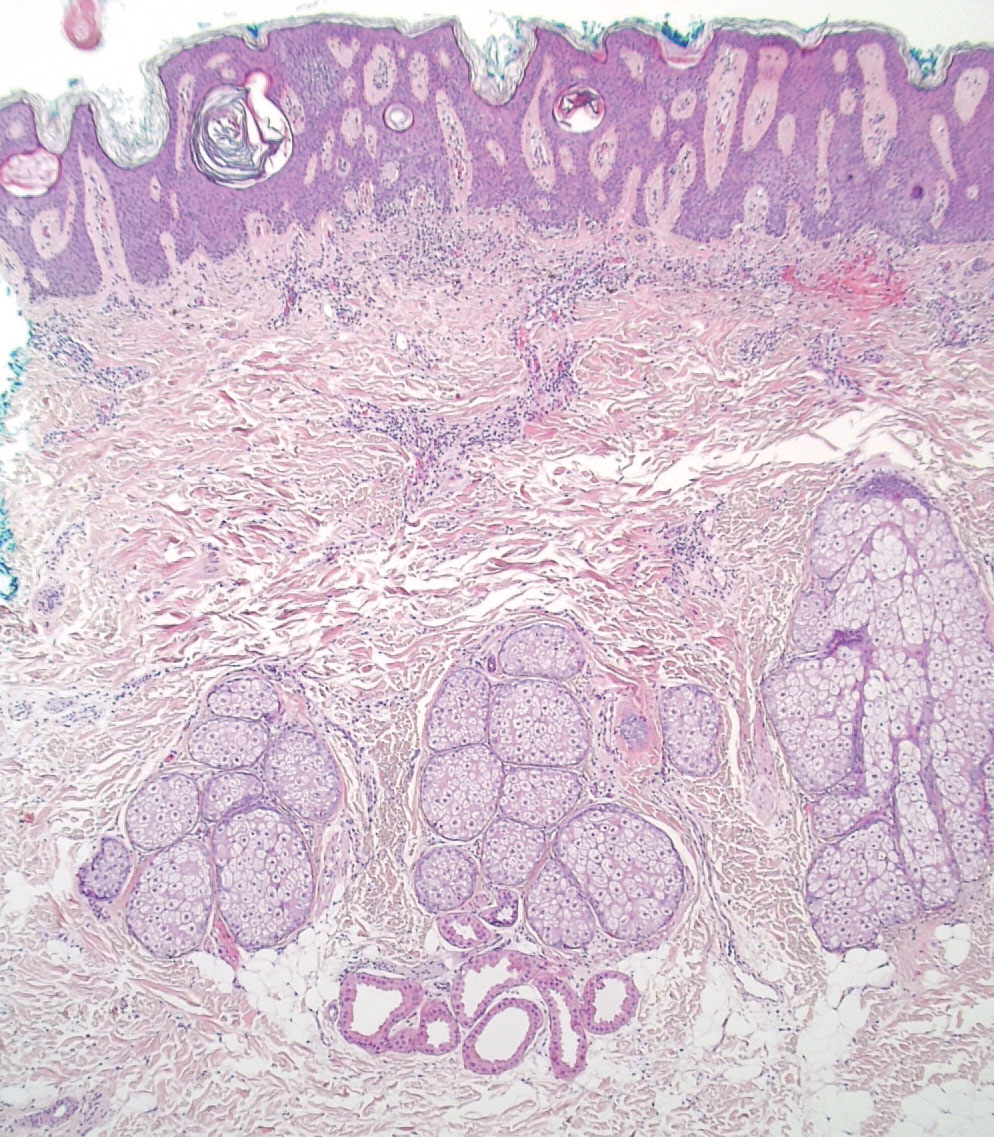
Nevus sebaceus is a benign skin hamartoma caused by a congenital defect in the pilosebaceous follicular unit and consists of epidermal, sebaceous, and apocrine elements.1,2 In dermatology patients, the prevalence of NS ranges from 0.05% to 1%.1 In 90% of cases, NS presents at birth as a 1- to 10-cm, round or linear, yellowish-orange, hairless plaque located on the scalp. It also may appear on the face, neck, trunk, oral mucosa, or labia minora.1,3 Although NS is a benign condition, secondary tumors may form within the lesion.3
The physical and histologic characteristics of NS evolve as the patient ages. In childhood, NS typically appears as a yellow-pink macule or patch with mild to moderate epidermal hyperplasia. Patients exhibit underdeveloped sebaceous glands, immature hair follicles, hyperkeratosis, and acanthosis.1,3,4 The development of early lesions can be quite subtle and can lead to diagnostic uncertainty, as described in our patient. During puberty, lesions thicken due to papillomatous hyperplasia in the epidermis, and the number and size of sebaceous and apocrine glands increase.4 In adults, the risk for secondary tumor formation increases. These physical and histologic transformations, including secondary tumor formation, are thought to be stimulated by the action of postpubertal androgens.1
Nevus sebaceus is associated with both benign and malignant secondary tumor formation; however, fewer than 1% of tumors are malignant.1 In a retrospective analysis, Idriss and Elston5 (N=707) reported that 21.4% of patients with NS had secondary neoplasms; 18.9% of the secondary neoplasms were benign, and 2.5% were malignant. Additionally, this study showed that secondary tumor formation can occur in children, though it typically occurs in adults. Benign neoplasms were reported in 5 children in the subset aged 0 to 10 years and 10 children in the subset aged 11 to 17 years; 1 child developed a malignant neoplasm in the latter subset.5 The most common NS-associated benign neoplasms include trichoblastoma and syringocystadenoma papilliferum. Others include trichilemmoma, apocrine/eccrine adenoma, and sebaceoma.1 Nevus sebaceus–associated malignant neoplasms include basal cell carcinoma, squamous cell carcinoma, adenocarcinoma, carcinosarcoma, and sebaceous carcinoma.3
Our patient was incorrectly diagnosed and treated for alopecia areata before an eventual diagnosis of NS was confirmed by biopsy. Additional genetic studies revealed a novel mutation in the HRAS gene, the most commonly affected gene in NS. The most common mutation location seen in more than 90% of NS lesions is HRAS c.37G>C (p.G13R), while KRAS mutations account for almost all the remaining cases.3 In our patient, a pathogenic missense HRAS p.G12V variant of somatic origin was detected with DNA extraction and sequencing from a fresh tissue sample acquired from two 4-mm punch biopsies performed on the lesion. The following genes were sequenced and found to be uninvolved: BRAF, FGFR1, FGFR2, FGFR3, GNA11, GNAQ, KRAS, MAP3K3, NRAS, PIK3CA, and TEK. The Sanger sequencing method for comparative analysis performed on peripheral blood was negative.
Nevus sebaceus typically is caused by a sporadic mutation, though familial cases have been reported.1 Additionally, germline HRAS mutations can lead to Costello syndrome, an autosomal-dominant disorder characterized by short stature; intellectual disabilities; coarse facial features; facial and perianal papillomata; cardiac defects; loose skin; joint hyperflexibility; and an increased risk for malignant tumors including rhabdomyosarcoma, neuroblastoma, and transitional cell carcinoma of the bladder.6
The diagnosis of NS often can be made clinically but can be difficult to confirm in underdeveloped lesions in young children. The differential diagnosis can include alopecia areata, aplasia cutis congenita, juvenile xanthogranuloma, epidermal nevus, de novo syringocystadenoma papilliferum, and solitary mastocytoma.1 Nevus sebaceus can be associated with 4 additional syndromes: Schimmelpenning syndrome; phacomatosis pigmentokeratotica; didymosis aplasticosebacea; and SCALP (sebaceus nevus, central nervous system malformations, aplasia cutis congenital, limbal dermoid, pigmented nevus) syndrome.1 Approximately 7% of NS cases may be associated with Schimmelpenning-Feuerstein-Mims (SFM) syndrome, a more severe condition that leads to systemic involvement and abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1,3 Phacomatosis pigmentokeratotica has speckled lentiginous nevi, as well as abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1 Didymosis aplasticosebacea is the concurrence of NS and aplasia cutis congenita.
The definitive treatment of NS is surgical excision. Alternative therapies include photodynamic therapy, fractional laser resurfacing, and dermabrasion; these are not definitive treatments, and patients must be monitored for the development of secondary neoplasms. Multiple variables must be considered when determining treatment, including patient age, risk potential for malignancy, and surgery-associated risks.1 In our patient, given the extent of the lesions, active observation and follow-up was agreed upon for management.
This case demonstrates the importance of considering NS as an alternative diagnosis when alopecia areata has been diagnosed in a child who is unresponsive to treatments. After the diagnosis of NS is confirmed, more serious associated syndromes should be ruled out, and treatment should be tailored to each case.
- Patel P, Malik K, Khachemoune A. Sebaceus and Becker’s nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197-204. doi:10.1007/s40257-015-0123-y
- Azzam MJ, Beutler BD, Calame A, et al. Osteoma cutis associated with nevus sebaceus: case report and review of cutaneous osteoma-associated skin tumors (COASTs). Cureus. 2019;11:E4959. doi:10.7759/cureus.4959
- Aslam A, Salam A, Griffiths CEM, et al. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39:1-6. doi:10.1111/ced.12209
- Basu P, Erickson CP, Calame A, et al. Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions. Dermatol Online J. 2020;26:13030/qt85k968bk.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Gripp KW, Rauen KA. Costello syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews [Internet]. University of Washington, Seattle; 1993-2020. August 29, 2006. Updated August 29, 2019. https://pubmed.ncbi.nlm.nih.gov/20301680
To the Editor:
A 12-year-old girl presented to the dermatology clinic for evaluation of a congenital scalp lesion. The patient was diagnosed with alopecia areata by a dermatologist at 4 years of age, and she was treated with topical corticosteroids and minoxidil, which failed to resolve her condition. Physical examination revealed an 8×10-cm, well-demarcated, yellowish-pink plaque located over the vertex and right parietal scalp (Figure 1A), extending down to the right preauricular cheek (Figure 1B) in a linear configuration with blaschkoid features. The scalp plaque appeared bald and completely lacking in terminal hairs but contained numerous fine vellus hairs (Figure 1A). A 6-mm, oval-appearing, pigmented papule was present in the plaque, and a few smaller, scattered, pigmented papules were noted in the vertex region (Figure 1A).

The cutaneous examination was otherwise unremarkable. A review of systems was negative, except for a history of attention-deficit/hyperactivity disorder. There was no history of seizures or other neurocognitive developmental abnormalities.

A 4-mm punch biopsy of the vertex scalp included the pigmented lesion but excluded an adnexal neoplasm. Epidermal acanthosis and mild papillomatosis were reported on microscopic examination. Multiple prominent sebaceous glands without associated hair follicles, which emptied directly onto the epidermal surface, were noted in the dermis (Figure 2). Several apocrine glands were observed (Figure 3). Epidermal and dermal melanocytic nests were highlighted with SOX-10 and Melan-A immunohistochemical stains, confirming the presence of a benign compound nevus. The punch biopsy analysis confirmed the diagnosis of a nevus sebaceus (NS) of Jadassohn (organoid nevus) with incidental compound nevus. Additional 4-mm punch biopsies were obtained for genetic testing, performed by the Genomics and Pathology Services at Washington University (St. Louis, Missouri). A missense HRAS p.G12V variant was observed in the tissue. A negative blood test result ruled out a germline mutation. The patient was managed with active observation of the lesion to evaluate for potential formation of neoplasms, as well as continuity of care with the dermatology clinic, considering the extent of the lesions, to monitor the development of any new medical conditions that would be concerning for syndromes associated with NS.

Nevus sebaceus is a benign skin hamartoma caused by a congenital defect in the pilosebaceous follicular unit and consists of epidermal, sebaceous, and apocrine elements.1,2 In dermatology patients, the prevalence of NS ranges from 0.05% to 1%.1 In 90% of cases, NS presents at birth as a 1- to 10-cm, round or linear, yellowish-orange, hairless plaque located on the scalp. It also may appear on the face, neck, trunk, oral mucosa, or labia minora.1,3 Although NS is a benign condition, secondary tumors may form within the lesion.3
The physical and histologic characteristics of NS evolve as the patient ages. In childhood, NS typically appears as a yellow-pink macule or patch with mild to moderate epidermal hyperplasia. Patients exhibit underdeveloped sebaceous glands, immature hair follicles, hyperkeratosis, and acanthosis.1,3,4 The development of early lesions can be quite subtle and can lead to diagnostic uncertainty, as described in our patient. During puberty, lesions thicken due to papillomatous hyperplasia in the epidermis, and the number and size of sebaceous and apocrine glands increase.4 In adults, the risk for secondary tumor formation increases. These physical and histologic transformations, including secondary tumor formation, are thought to be stimulated by the action of postpubertal androgens.1
Nevus sebaceus is associated with both benign and malignant secondary tumor formation; however, fewer than 1% of tumors are malignant.1 In a retrospective analysis, Idriss and Elston5 (N=707) reported that 21.4% of patients with NS had secondary neoplasms; 18.9% of the secondary neoplasms were benign, and 2.5% were malignant. Additionally, this study showed that secondary tumor formation can occur in children, though it typically occurs in adults. Benign neoplasms were reported in 5 children in the subset aged 0 to 10 years and 10 children in the subset aged 11 to 17 years; 1 child developed a malignant neoplasm in the latter subset.5 The most common NS-associated benign neoplasms include trichoblastoma and syringocystadenoma papilliferum. Others include trichilemmoma, apocrine/eccrine adenoma, and sebaceoma.1 Nevus sebaceus–associated malignant neoplasms include basal cell carcinoma, squamous cell carcinoma, adenocarcinoma, carcinosarcoma, and sebaceous carcinoma.3
Our patient was incorrectly diagnosed and treated for alopecia areata before an eventual diagnosis of NS was confirmed by biopsy. Additional genetic studies revealed a novel mutation in the HRAS gene, the most commonly affected gene in NS. The most common mutation location seen in more than 90% of NS lesions is HRAS c.37G>C (p.G13R), while KRAS mutations account for almost all the remaining cases.3 In our patient, a pathogenic missense HRAS p.G12V variant of somatic origin was detected with DNA extraction and sequencing from a fresh tissue sample acquired from two 4-mm punch biopsies performed on the lesion. The following genes were sequenced and found to be uninvolved: BRAF, FGFR1, FGFR2, FGFR3, GNA11, GNAQ, KRAS, MAP3K3, NRAS, PIK3CA, and TEK. The Sanger sequencing method for comparative analysis performed on peripheral blood was negative.
Nevus sebaceus typically is caused by a sporadic mutation, though familial cases have been reported.1 Additionally, germline HRAS mutations can lead to Costello syndrome, an autosomal-dominant disorder characterized by short stature; intellectual disabilities; coarse facial features; facial and perianal papillomata; cardiac defects; loose skin; joint hyperflexibility; and an increased risk for malignant tumors including rhabdomyosarcoma, neuroblastoma, and transitional cell carcinoma of the bladder.6
The diagnosis of NS often can be made clinically but can be difficult to confirm in underdeveloped lesions in young children. The differential diagnosis can include alopecia areata, aplasia cutis congenita, juvenile xanthogranuloma, epidermal nevus, de novo syringocystadenoma papilliferum, and solitary mastocytoma.1 Nevus sebaceus can be associated with 4 additional syndromes: Schimmelpenning syndrome; phacomatosis pigmentokeratotica; didymosis aplasticosebacea; and SCALP (sebaceus nevus, central nervous system malformations, aplasia cutis congenital, limbal dermoid, pigmented nevus) syndrome.1 Approximately 7% of NS cases may be associated with Schimmelpenning-Feuerstein-Mims (SFM) syndrome, a more severe condition that leads to systemic involvement and abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1,3 Phacomatosis pigmentokeratotica has speckled lentiginous nevi, as well as abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1 Didymosis aplasticosebacea is the concurrence of NS and aplasia cutis congenita.
The definitive treatment of NS is surgical excision. Alternative therapies include photodynamic therapy, fractional laser resurfacing, and dermabrasion; these are not definitive treatments, and patients must be monitored for the development of secondary neoplasms. Multiple variables must be considered when determining treatment, including patient age, risk potential for malignancy, and surgery-associated risks.1 In our patient, given the extent of the lesions, active observation and follow-up was agreed upon for management.
This case demonstrates the importance of considering NS as an alternative diagnosis when alopecia areata has been diagnosed in a child who is unresponsive to treatments. After the diagnosis of NS is confirmed, more serious associated syndromes should be ruled out, and treatment should be tailored to each case.
To the Editor:
A 12-year-old girl presented to the dermatology clinic for evaluation of a congenital scalp lesion. The patient was diagnosed with alopecia areata by a dermatologist at 4 years of age, and she was treated with topical corticosteroids and minoxidil, which failed to resolve her condition. Physical examination revealed an 8×10-cm, well-demarcated, yellowish-pink plaque located over the vertex and right parietal scalp (Figure 1A), extending down to the right preauricular cheek (Figure 1B) in a linear configuration with blaschkoid features. The scalp plaque appeared bald and completely lacking in terminal hairs but contained numerous fine vellus hairs (Figure 1A). A 6-mm, oval-appearing, pigmented papule was present in the plaque, and a few smaller, scattered, pigmented papules were noted in the vertex region (Figure 1A).

The cutaneous examination was otherwise unremarkable. A review of systems was negative, except for a history of attention-deficit/hyperactivity disorder. There was no history of seizures or other neurocognitive developmental abnormalities.

A 4-mm punch biopsy of the vertex scalp included the pigmented lesion but excluded an adnexal neoplasm. Epidermal acanthosis and mild papillomatosis were reported on microscopic examination. Multiple prominent sebaceous glands without associated hair follicles, which emptied directly onto the epidermal surface, were noted in the dermis (Figure 2). Several apocrine glands were observed (Figure 3). Epidermal and dermal melanocytic nests were highlighted with SOX-10 and Melan-A immunohistochemical stains, confirming the presence of a benign compound nevus. The punch biopsy analysis confirmed the diagnosis of a nevus sebaceus (NS) of Jadassohn (organoid nevus) with incidental compound nevus. Additional 4-mm punch biopsies were obtained for genetic testing, performed by the Genomics and Pathology Services at Washington University (St. Louis, Missouri). A missense HRAS p.G12V variant was observed in the tissue. A negative blood test result ruled out a germline mutation. The patient was managed with active observation of the lesion to evaluate for potential formation of neoplasms, as well as continuity of care with the dermatology clinic, considering the extent of the lesions, to monitor the development of any new medical conditions that would be concerning for syndromes associated with NS.

Nevus sebaceus is a benign skin hamartoma caused by a congenital defect in the pilosebaceous follicular unit and consists of epidermal, sebaceous, and apocrine elements.1,2 In dermatology patients, the prevalence of NS ranges from 0.05% to 1%.1 In 90% of cases, NS presents at birth as a 1- to 10-cm, round or linear, yellowish-orange, hairless plaque located on the scalp. It also may appear on the face, neck, trunk, oral mucosa, or labia minora.1,3 Although NS is a benign condition, secondary tumors may form within the lesion.3
The physical and histologic characteristics of NS evolve as the patient ages. In childhood, NS typically appears as a yellow-pink macule or patch with mild to moderate epidermal hyperplasia. Patients exhibit underdeveloped sebaceous glands, immature hair follicles, hyperkeratosis, and acanthosis.1,3,4 The development of early lesions can be quite subtle and can lead to diagnostic uncertainty, as described in our patient. During puberty, lesions thicken due to papillomatous hyperplasia in the epidermis, and the number and size of sebaceous and apocrine glands increase.4 In adults, the risk for secondary tumor formation increases. These physical and histologic transformations, including secondary tumor formation, are thought to be stimulated by the action of postpubertal androgens.1
Nevus sebaceus is associated with both benign and malignant secondary tumor formation; however, fewer than 1% of tumors are malignant.1 In a retrospective analysis, Idriss and Elston5 (N=707) reported that 21.4% of patients with NS had secondary neoplasms; 18.9% of the secondary neoplasms were benign, and 2.5% were malignant. Additionally, this study showed that secondary tumor formation can occur in children, though it typically occurs in adults. Benign neoplasms were reported in 5 children in the subset aged 0 to 10 years and 10 children in the subset aged 11 to 17 years; 1 child developed a malignant neoplasm in the latter subset.5 The most common NS-associated benign neoplasms include trichoblastoma and syringocystadenoma papilliferum. Others include trichilemmoma, apocrine/eccrine adenoma, and sebaceoma.1 Nevus sebaceus–associated malignant neoplasms include basal cell carcinoma, squamous cell carcinoma, adenocarcinoma, carcinosarcoma, and sebaceous carcinoma.3
Our patient was incorrectly diagnosed and treated for alopecia areata before an eventual diagnosis of NS was confirmed by biopsy. Additional genetic studies revealed a novel mutation in the HRAS gene, the most commonly affected gene in NS. The most common mutation location seen in more than 90% of NS lesions is HRAS c.37G>C (p.G13R), while KRAS mutations account for almost all the remaining cases.3 In our patient, a pathogenic missense HRAS p.G12V variant of somatic origin was detected with DNA extraction and sequencing from a fresh tissue sample acquired from two 4-mm punch biopsies performed on the lesion. The following genes were sequenced and found to be uninvolved: BRAF, FGFR1, FGFR2, FGFR3, GNA11, GNAQ, KRAS, MAP3K3, NRAS, PIK3CA, and TEK. The Sanger sequencing method for comparative analysis performed on peripheral blood was negative.
Nevus sebaceus typically is caused by a sporadic mutation, though familial cases have been reported.1 Additionally, germline HRAS mutations can lead to Costello syndrome, an autosomal-dominant disorder characterized by short stature; intellectual disabilities; coarse facial features; facial and perianal papillomata; cardiac defects; loose skin; joint hyperflexibility; and an increased risk for malignant tumors including rhabdomyosarcoma, neuroblastoma, and transitional cell carcinoma of the bladder.6
The diagnosis of NS often can be made clinically but can be difficult to confirm in underdeveloped lesions in young children. The differential diagnosis can include alopecia areata, aplasia cutis congenita, juvenile xanthogranuloma, epidermal nevus, de novo syringocystadenoma papilliferum, and solitary mastocytoma.1 Nevus sebaceus can be associated with 4 additional syndromes: Schimmelpenning syndrome; phacomatosis pigmentokeratotica; didymosis aplasticosebacea; and SCALP (sebaceus nevus, central nervous system malformations, aplasia cutis congenital, limbal dermoid, pigmented nevus) syndrome.1 Approximately 7% of NS cases may be associated with Schimmelpenning-Feuerstein-Mims (SFM) syndrome, a more severe condition that leads to systemic involvement and abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1,3 Phacomatosis pigmentokeratotica has speckled lentiginous nevi, as well as abnormalities in the neurological, ophthalmological, cardiovascular, genitourological, and skeletal systems.1 Didymosis aplasticosebacea is the concurrence of NS and aplasia cutis congenita.
The definitive treatment of NS is surgical excision. Alternative therapies include photodynamic therapy, fractional laser resurfacing, and dermabrasion; these are not definitive treatments, and patients must be monitored for the development of secondary neoplasms. Multiple variables must be considered when determining treatment, including patient age, risk potential for malignancy, and surgery-associated risks.1 In our patient, given the extent of the lesions, active observation and follow-up was agreed upon for management.
This case demonstrates the importance of considering NS as an alternative diagnosis when alopecia areata has been diagnosed in a child who is unresponsive to treatments. After the diagnosis of NS is confirmed, more serious associated syndromes should be ruled out, and treatment should be tailored to each case.
- Patel P, Malik K, Khachemoune A. Sebaceus and Becker’s nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197-204. doi:10.1007/s40257-015-0123-y
- Azzam MJ, Beutler BD, Calame A, et al. Osteoma cutis associated with nevus sebaceus: case report and review of cutaneous osteoma-associated skin tumors (COASTs). Cureus. 2019;11:E4959. doi:10.7759/cureus.4959
- Aslam A, Salam A, Griffiths CEM, et al. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39:1-6. doi:10.1111/ced.12209
- Basu P, Erickson CP, Calame A, et al. Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions. Dermatol Online J. 2020;26:13030/qt85k968bk.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Gripp KW, Rauen KA. Costello syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews [Internet]. University of Washington, Seattle; 1993-2020. August 29, 2006. Updated August 29, 2019. https://pubmed.ncbi.nlm.nih.gov/20301680
- Patel P, Malik K, Khachemoune A. Sebaceus and Becker’s nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197-204. doi:10.1007/s40257-015-0123-y
- Azzam MJ, Beutler BD, Calame A, et al. Osteoma cutis associated with nevus sebaceus: case report and review of cutaneous osteoma-associated skin tumors (COASTs). Cureus. 2019;11:E4959. doi:10.7759/cureus.4959
- Aslam A, Salam A, Griffiths CEM, et al. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39:1-6. doi:10.1111/ced.12209
- Basu P, Erickson CP, Calame A, et al. Nevus sebaceus with syringocystadenoma papilliferum, prurigo nodularis, apocrine cystadenoma, basaloid follicular proliferation, and sebaceoma: case report and review of nevus sebaceus-associated conditions. Dermatol Online J. 2020;26:13030/qt85k968bk.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Gripp KW, Rauen KA. Costello syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews [Internet]. University of Washington, Seattle; 1993-2020. August 29, 2006. Updated August 29, 2019. https://pubmed.ncbi.nlm.nih.gov/20301680
Practice Points
- Nevus sebaceus (NS), commonly referred to as NS of Jadassohn or organoid nevus, is a benign skin hamartoma that consists of epidermal, sebaceous, and apocrine elements and is caused by a congenital defect in the pilosebaceous follicular unit.
- Early stages of NS can be mistaken for alopecia areata.
- Once the diagnosis of NS is confirmed, the presence of associated syndromes should be evaluated.
- The definitive treatment of NS is surgical excision; however, multiple variables must be considered when determining treatment, including patient age, risk for developing malignancy, and surgery-associated risks.
Chondrodermatitis Nodularis Helicis After Mohs Micrographic Surgery and Radiation Therapy
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.
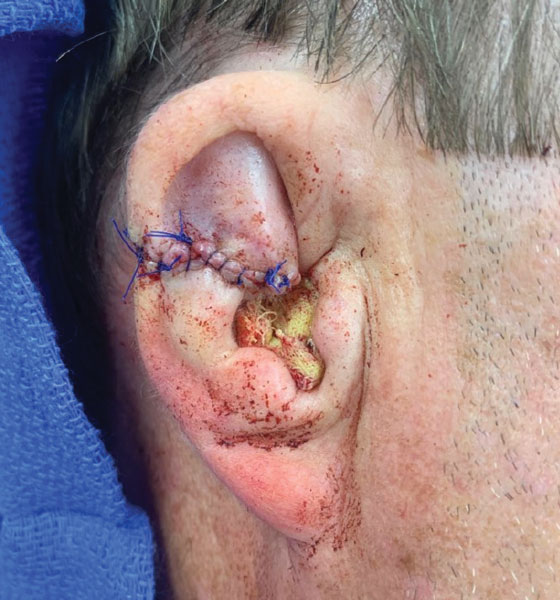
A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.
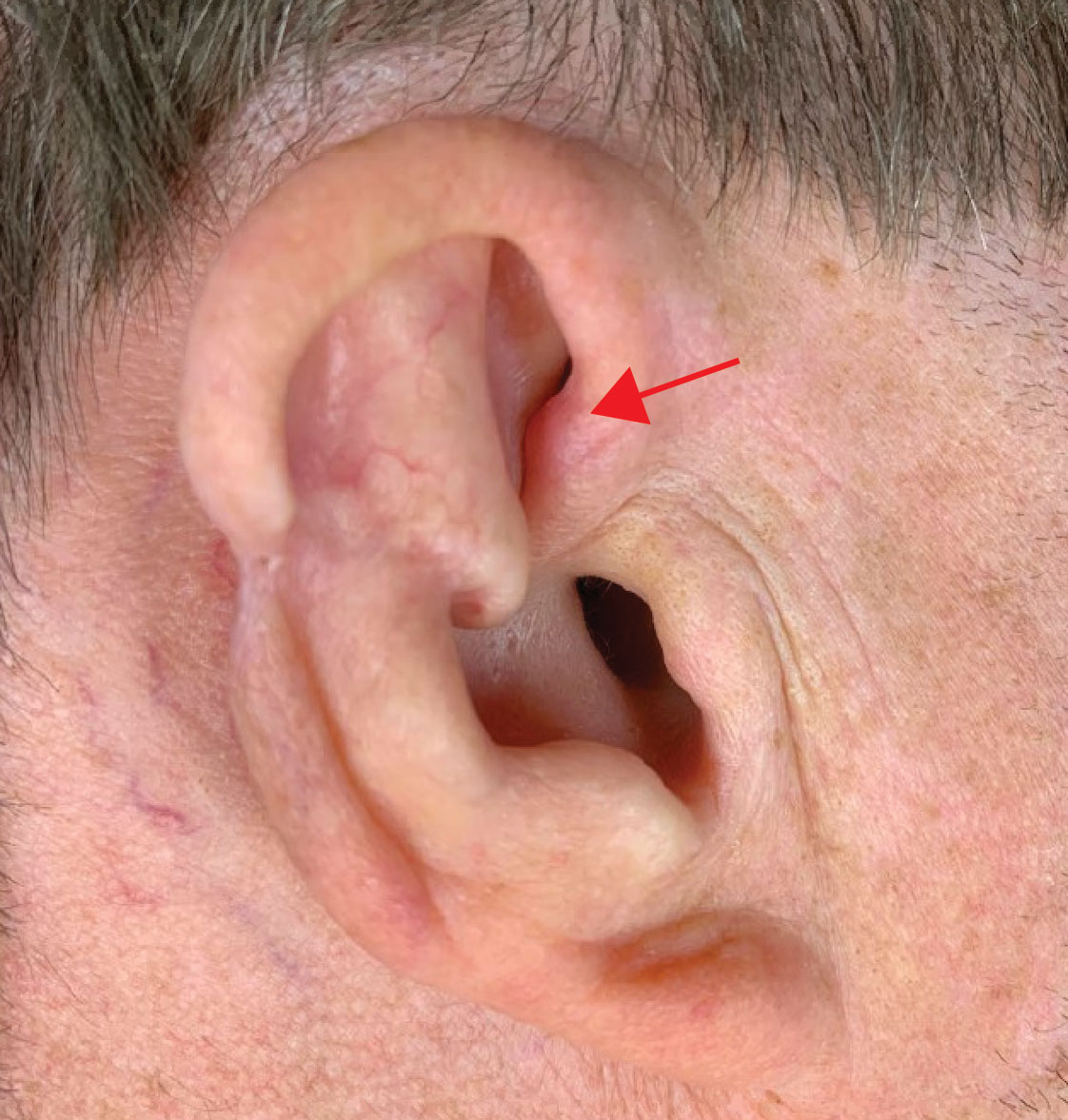
Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).
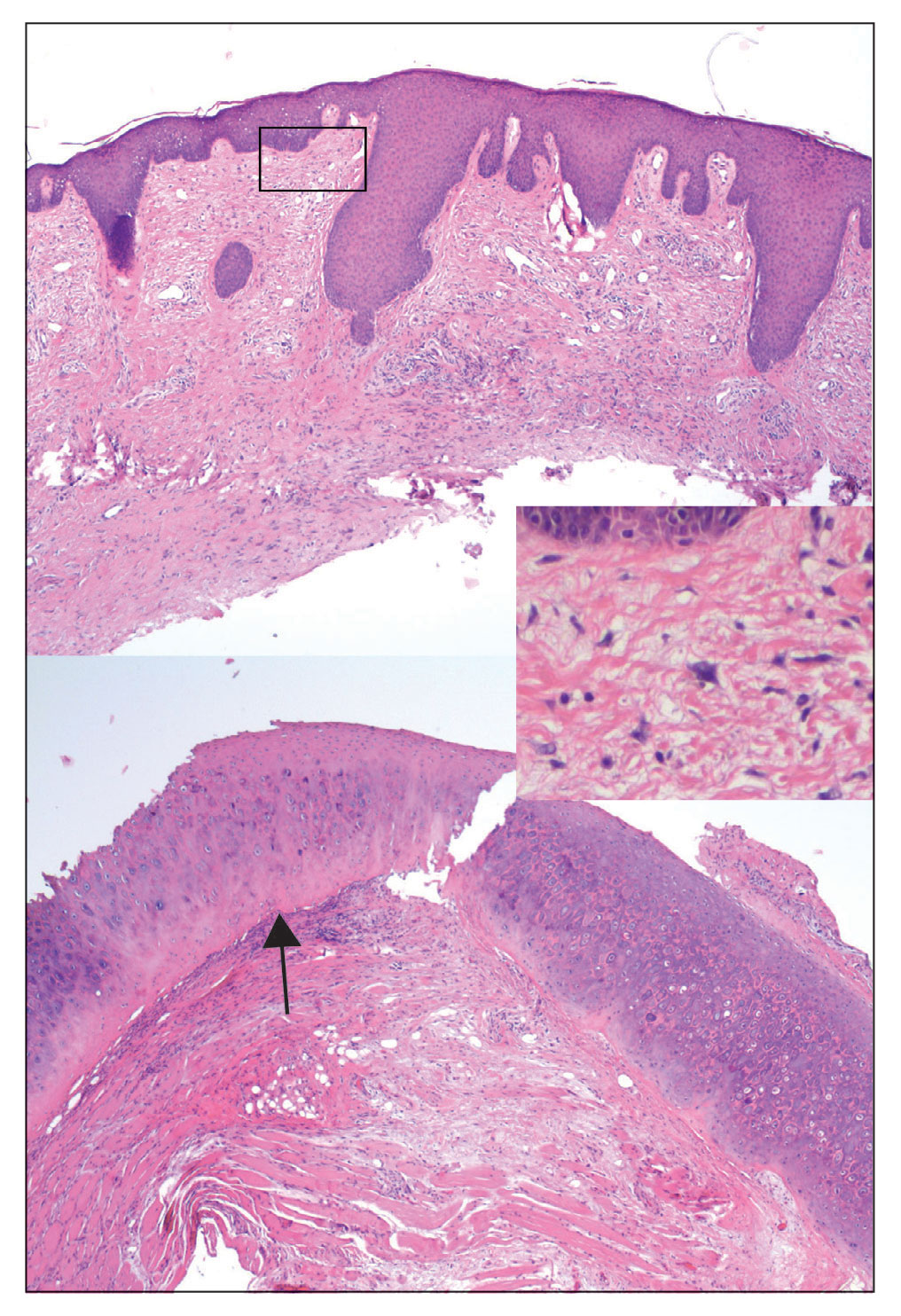
Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
Practice Points
- Although chondrodermatitis nodularis helicis (CNH) is benign by nature, it can mimic tumor recurrence when it presents close to the site of prior Mohs micrographic surgery (MMS). Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Skin lesions in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis.
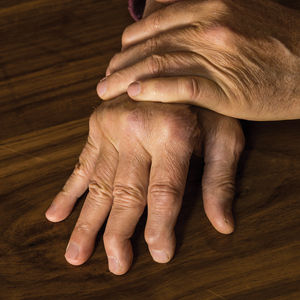


![An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_300x300.jpg)